User login
Time to prescribe sauna bathing for cardiovascular health?
Is it time to start recommending regular sauna bathing to improve heart health?
While a post-workout sauna can compound the benefits of exercise, the hormetic effects of heat therapy alone can produce significant gains for microvascular and endothelial function, no workout required.
“There’s enough evidence to say that regular sauna use improves cardiovascular health,” Matthew S. Ganio, PhD, a professor of exercise science at the University of Arkansas in Fayetteville, who studies thermoregulatory responses and cardiovascular health, said.
“The more they used it, the greater the reduction in cardiovascular events like heart attack. But you don’t need to be in there more than 20-30 minutes. That’s where it seemed to have the best effect,” Dr. Ganio said, adding that studies have shown a dose-response.
A prospective cohort study published in 2015 in JAMA Internal Medicine included 20 years of data on more than 2,300 Finnish men who regularly sauna bathed. The researchers found that among participants who sat in saunas more frequently, rates of death from heart disease and stroke were lower than among those who did so less often.
Cutaneous vasodilation
The body experiences several physiologic changes when exposed to heat therapy of any kind, including sauna, hot water submerging, shortwave diathermy, and heat wrapping. Many of these changes involve elements of the cardiovascular system, said Earric Lee, PhD, an exercise physiologist and postdoctoral researcher at the University of Jyväskylä in Finland, who has studied the effects of sauna on cardiovascular health.
The mechanisms by which heat therapy improves cardiovascular fitness have not been determined, as few studies of sauna bathing have been conducted to this degree. One driver appears to be cutaneous vasodilation. To cool the body when exposed to extreme external heat, cutaneous vessels dilate and push blood to the skin, which lowers body temperature, increases heart rate, and delivers oxygen to muscles in the limbs in a way similar to aerobic exercise.
Sauna bathing has similar effects on heart rate and cardiac output. Studies have shown it can improve the circulation of blood through the body, as well as vascular endothelial function, which is closely tied to vascular tone.
“Increased cardiac output is one of the physiologic reasons sauna is good for heart health,” Dr. Ganio said.
During a sauna session, cardiac output can increase by as much as 70% in relation to elevated heart rate. And while heart rate and cardiac output rise, stroke volume remains stable. As stroke volume increases, the effort that muscle must exert increases. When heart rate rises, stroke volume often falls, which subjects the heart to less of a workout and reduces the amount of oxygen and blood circulating throughout the body.
Heat therapy also temporarily increases blood pressure, but in a way similar to exercise, which supports better long-term heart health, said Christopher Minson, PhD, the Kenneth M. and Kenda H. Singer Endowed Professor of Human Physiology at the University of Oregon in Eugene.
A small study of 19 healthy adults that was published in Complementary Therapies in Medicine in 2019 found that blood pressure and heart rate rose during a 25-minute sauna session as they might during moderate exercise, equivalent to an exercise load of about 60-100 watts. These parameters then steadily decreased for 30 minutes after the sauna. An earlier study found that in the long term, blood pressure was lower after a sauna than before a sauna.
Upregulated heat shock proteins
Both aerobic exercise and heat stress from sauna bathing increase the activity of heat shock proteins. A 2021 review published in Experimental Gerontology found that heat shock proteins become elevated in cells within 30 minutes of exposure to heat and remain elevated over time – an effect similar to exercise.
“Saunas increase heat shock proteins that break down old, dysfunctional proteins and then protect new proteins from becoming dysfunctional,” Hunter S. Waldman, PhD, an assistant professor of exercise science at the University of North Alabama in Florence, said. This effect is one way sauna bathing may quell systemic inflammation, Dr. Waldman said.
According to a 2018 review published in BioMed Research International, an abundance of heat shock proteins may increase exercise tolerance. The researchers concluded that the positive stress associated with elevated body temperature could help people be physically active for longer periods.
Added stress, especially heat-related strain, is not good for everyone, however. Dr. Waldman cautioned that heat exposure, be it through a sauna, hot tub, or other source, can be harmful for pregnant women and children and can be dangerous for people who have low blood pressure, since blood pressure often drops to rates that are lower than before taking a sauna. It also can impair semen quality for months after exposure, so people who are trying to conceive should avoid sauna bathing.
Anyone who has been diagnosed with a heart condition, including cardiac arrhythmia, coronary artery disease, and congestive heart failure, should always consult their physician prior to using sauna for the first time or before using it habitually, Dr. Lee said.
Effects compounded by exercise
Dr. Minson stressed that any type of heat therapy should be part of a lifestyle that includes mostly healthy habits overall, especially a regular exercise regime when possible.
“You have to have everything else working as well: finding time to relax, not being overly stressed, staying hydrated – all those things are critical with any exercise training and heat therapy program,” he said.
Dr. Lee said it’s easy to overhype the benefits of sauna bathing and agreed the practice should be used in tandem with other therapies, not as a replacement. So far, stacking research has shown it to be an effective extension of aerobic exercise.
A June 2023 review published in Mayo Clinic Proceedings found that while sauna bathing can produce benefits on its own, a post-workout sauna can extend the benefits of exercise. As a result, the researchers concluded, saunas likely provide the most benefit when combined with aerobic and strength training.
While some of the benefits of exercise overlap those associated with sauna bathing, “you’re going to get some benefits with exercise that you’re never going to get with sauna,” Dr. Ganio said.
For instance, strength training or aerobic exercise usually results in muscle contractions, which sauna bathing does not produce.
If a person is impaired in a way that makes exercise difficult, taking a sauna after aerobic activity can extend the cardiovascular benefits of the workout, even if muscle-building does not occur, Dr. Lee said.
“All other things considered, especially with aerobic exercise, it is very comparable, so we can look at adding sauna bathing post exercise as a way to lengthen the aerobic exercise workout,” he said. “It’s not to the same degree, but you can get many of the ranging benefits of exercising simply by going into the sauna.”
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Is it time to start recommending regular sauna bathing to improve heart health?
While a post-workout sauna can compound the benefits of exercise, the hormetic effects of heat therapy alone can produce significant gains for microvascular and endothelial function, no workout required.
“There’s enough evidence to say that regular sauna use improves cardiovascular health,” Matthew S. Ganio, PhD, a professor of exercise science at the University of Arkansas in Fayetteville, who studies thermoregulatory responses and cardiovascular health, said.
“The more they used it, the greater the reduction in cardiovascular events like heart attack. But you don’t need to be in there more than 20-30 minutes. That’s where it seemed to have the best effect,” Dr. Ganio said, adding that studies have shown a dose-response.
A prospective cohort study published in 2015 in JAMA Internal Medicine included 20 years of data on more than 2,300 Finnish men who regularly sauna bathed. The researchers found that among participants who sat in saunas more frequently, rates of death from heart disease and stroke were lower than among those who did so less often.
Cutaneous vasodilation
The body experiences several physiologic changes when exposed to heat therapy of any kind, including sauna, hot water submerging, shortwave diathermy, and heat wrapping. Many of these changes involve elements of the cardiovascular system, said Earric Lee, PhD, an exercise physiologist and postdoctoral researcher at the University of Jyväskylä in Finland, who has studied the effects of sauna on cardiovascular health.
The mechanisms by which heat therapy improves cardiovascular fitness have not been determined, as few studies of sauna bathing have been conducted to this degree. One driver appears to be cutaneous vasodilation. To cool the body when exposed to extreme external heat, cutaneous vessels dilate and push blood to the skin, which lowers body temperature, increases heart rate, and delivers oxygen to muscles in the limbs in a way similar to aerobic exercise.
Sauna bathing has similar effects on heart rate and cardiac output. Studies have shown it can improve the circulation of blood through the body, as well as vascular endothelial function, which is closely tied to vascular tone.
“Increased cardiac output is one of the physiologic reasons sauna is good for heart health,” Dr. Ganio said.
During a sauna session, cardiac output can increase by as much as 70% in relation to elevated heart rate. And while heart rate and cardiac output rise, stroke volume remains stable. As stroke volume increases, the effort that muscle must exert increases. When heart rate rises, stroke volume often falls, which subjects the heart to less of a workout and reduces the amount of oxygen and blood circulating throughout the body.
Heat therapy also temporarily increases blood pressure, but in a way similar to exercise, which supports better long-term heart health, said Christopher Minson, PhD, the Kenneth M. and Kenda H. Singer Endowed Professor of Human Physiology at the University of Oregon in Eugene.
A small study of 19 healthy adults that was published in Complementary Therapies in Medicine in 2019 found that blood pressure and heart rate rose during a 25-minute sauna session as they might during moderate exercise, equivalent to an exercise load of about 60-100 watts. These parameters then steadily decreased for 30 minutes after the sauna. An earlier study found that in the long term, blood pressure was lower after a sauna than before a sauna.
Upregulated heat shock proteins
Both aerobic exercise and heat stress from sauna bathing increase the activity of heat shock proteins. A 2021 review published in Experimental Gerontology found that heat shock proteins become elevated in cells within 30 minutes of exposure to heat and remain elevated over time – an effect similar to exercise.
“Saunas increase heat shock proteins that break down old, dysfunctional proteins and then protect new proteins from becoming dysfunctional,” Hunter S. Waldman, PhD, an assistant professor of exercise science at the University of North Alabama in Florence, said. This effect is one way sauna bathing may quell systemic inflammation, Dr. Waldman said.
According to a 2018 review published in BioMed Research International, an abundance of heat shock proteins may increase exercise tolerance. The researchers concluded that the positive stress associated with elevated body temperature could help people be physically active for longer periods.
Added stress, especially heat-related strain, is not good for everyone, however. Dr. Waldman cautioned that heat exposure, be it through a sauna, hot tub, or other source, can be harmful for pregnant women and children and can be dangerous for people who have low blood pressure, since blood pressure often drops to rates that are lower than before taking a sauna. It also can impair semen quality for months after exposure, so people who are trying to conceive should avoid sauna bathing.
Anyone who has been diagnosed with a heart condition, including cardiac arrhythmia, coronary artery disease, and congestive heart failure, should always consult their physician prior to using sauna for the first time or before using it habitually, Dr. Lee said.
Effects compounded by exercise
Dr. Minson stressed that any type of heat therapy should be part of a lifestyle that includes mostly healthy habits overall, especially a regular exercise regime when possible.
“You have to have everything else working as well: finding time to relax, not being overly stressed, staying hydrated – all those things are critical with any exercise training and heat therapy program,” he said.
Dr. Lee said it’s easy to overhype the benefits of sauna bathing and agreed the practice should be used in tandem with other therapies, not as a replacement. So far, stacking research has shown it to be an effective extension of aerobic exercise.
A June 2023 review published in Mayo Clinic Proceedings found that while sauna bathing can produce benefits on its own, a post-workout sauna can extend the benefits of exercise. As a result, the researchers concluded, saunas likely provide the most benefit when combined with aerobic and strength training.
While some of the benefits of exercise overlap those associated with sauna bathing, “you’re going to get some benefits with exercise that you’re never going to get with sauna,” Dr. Ganio said.
For instance, strength training or aerobic exercise usually results in muscle contractions, which sauna bathing does not produce.
If a person is impaired in a way that makes exercise difficult, taking a sauna after aerobic activity can extend the cardiovascular benefits of the workout, even if muscle-building does not occur, Dr. Lee said.
“All other things considered, especially with aerobic exercise, it is very comparable, so we can look at adding sauna bathing post exercise as a way to lengthen the aerobic exercise workout,” he said. “It’s not to the same degree, but you can get many of the ranging benefits of exercising simply by going into the sauna.”
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Is it time to start recommending regular sauna bathing to improve heart health?
While a post-workout sauna can compound the benefits of exercise, the hormetic effects of heat therapy alone can produce significant gains for microvascular and endothelial function, no workout required.
“There’s enough evidence to say that regular sauna use improves cardiovascular health,” Matthew S. Ganio, PhD, a professor of exercise science at the University of Arkansas in Fayetteville, who studies thermoregulatory responses and cardiovascular health, said.
“The more they used it, the greater the reduction in cardiovascular events like heart attack. But you don’t need to be in there more than 20-30 minutes. That’s where it seemed to have the best effect,” Dr. Ganio said, adding that studies have shown a dose-response.
A prospective cohort study published in 2015 in JAMA Internal Medicine included 20 years of data on more than 2,300 Finnish men who regularly sauna bathed. The researchers found that among participants who sat in saunas more frequently, rates of death from heart disease and stroke were lower than among those who did so less often.
Cutaneous vasodilation
The body experiences several physiologic changes when exposed to heat therapy of any kind, including sauna, hot water submerging, shortwave diathermy, and heat wrapping. Many of these changes involve elements of the cardiovascular system, said Earric Lee, PhD, an exercise physiologist and postdoctoral researcher at the University of Jyväskylä in Finland, who has studied the effects of sauna on cardiovascular health.
The mechanisms by which heat therapy improves cardiovascular fitness have not been determined, as few studies of sauna bathing have been conducted to this degree. One driver appears to be cutaneous vasodilation. To cool the body when exposed to extreme external heat, cutaneous vessels dilate and push blood to the skin, which lowers body temperature, increases heart rate, and delivers oxygen to muscles in the limbs in a way similar to aerobic exercise.
Sauna bathing has similar effects on heart rate and cardiac output. Studies have shown it can improve the circulation of blood through the body, as well as vascular endothelial function, which is closely tied to vascular tone.
“Increased cardiac output is one of the physiologic reasons sauna is good for heart health,” Dr. Ganio said.
During a sauna session, cardiac output can increase by as much as 70% in relation to elevated heart rate. And while heart rate and cardiac output rise, stroke volume remains stable. As stroke volume increases, the effort that muscle must exert increases. When heart rate rises, stroke volume often falls, which subjects the heart to less of a workout and reduces the amount of oxygen and blood circulating throughout the body.
Heat therapy also temporarily increases blood pressure, but in a way similar to exercise, which supports better long-term heart health, said Christopher Minson, PhD, the Kenneth M. and Kenda H. Singer Endowed Professor of Human Physiology at the University of Oregon in Eugene.
A small study of 19 healthy adults that was published in Complementary Therapies in Medicine in 2019 found that blood pressure and heart rate rose during a 25-minute sauna session as they might during moderate exercise, equivalent to an exercise load of about 60-100 watts. These parameters then steadily decreased for 30 minutes after the sauna. An earlier study found that in the long term, blood pressure was lower after a sauna than before a sauna.
Upregulated heat shock proteins
Both aerobic exercise and heat stress from sauna bathing increase the activity of heat shock proteins. A 2021 review published in Experimental Gerontology found that heat shock proteins become elevated in cells within 30 minutes of exposure to heat and remain elevated over time – an effect similar to exercise.
“Saunas increase heat shock proteins that break down old, dysfunctional proteins and then protect new proteins from becoming dysfunctional,” Hunter S. Waldman, PhD, an assistant professor of exercise science at the University of North Alabama in Florence, said. This effect is one way sauna bathing may quell systemic inflammation, Dr. Waldman said.
According to a 2018 review published in BioMed Research International, an abundance of heat shock proteins may increase exercise tolerance. The researchers concluded that the positive stress associated with elevated body temperature could help people be physically active for longer periods.
Added stress, especially heat-related strain, is not good for everyone, however. Dr. Waldman cautioned that heat exposure, be it through a sauna, hot tub, or other source, can be harmful for pregnant women and children and can be dangerous for people who have low blood pressure, since blood pressure often drops to rates that are lower than before taking a sauna. It also can impair semen quality for months after exposure, so people who are trying to conceive should avoid sauna bathing.
Anyone who has been diagnosed with a heart condition, including cardiac arrhythmia, coronary artery disease, and congestive heart failure, should always consult their physician prior to using sauna for the first time or before using it habitually, Dr. Lee said.
Effects compounded by exercise
Dr. Minson stressed that any type of heat therapy should be part of a lifestyle that includes mostly healthy habits overall, especially a regular exercise regime when possible.
“You have to have everything else working as well: finding time to relax, not being overly stressed, staying hydrated – all those things are critical with any exercise training and heat therapy program,” he said.
Dr. Lee said it’s easy to overhype the benefits of sauna bathing and agreed the practice should be used in tandem with other therapies, not as a replacement. So far, stacking research has shown it to be an effective extension of aerobic exercise.
A June 2023 review published in Mayo Clinic Proceedings found that while sauna bathing can produce benefits on its own, a post-workout sauna can extend the benefits of exercise. As a result, the researchers concluded, saunas likely provide the most benefit when combined with aerobic and strength training.
While some of the benefits of exercise overlap those associated with sauna bathing, “you’re going to get some benefits with exercise that you’re never going to get with sauna,” Dr. Ganio said.
For instance, strength training or aerobic exercise usually results in muscle contractions, which sauna bathing does not produce.
If a person is impaired in a way that makes exercise difficult, taking a sauna after aerobic activity can extend the cardiovascular benefits of the workout, even if muscle-building does not occur, Dr. Lee said.
“All other things considered, especially with aerobic exercise, it is very comparable, so we can look at adding sauna bathing post exercise as a way to lengthen the aerobic exercise workout,” he said. “It’s not to the same degree, but you can get many of the ranging benefits of exercising simply by going into the sauna.”
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
West Nile virus cases rising nationwide amid mosquito season
In the past 2 weeks, new cases have been reported in Iowa and Nebraska, adding to previous 2023 reports from Arizona, Georgia, Illinois, Louisiana, Oregon, Pennsylvania, and Wyoming. A mosquito at a monitoring site near Houston tested positive last week for the potentially fatal virus, prompting local health officials to begin evening spray operations in the area where the mosquito was found, according to an announcement from Harris County Public Health.
According to the CDC, which compiles local reports, there have been 13 human cases of West Nile virus in 2023. In 2022, there were 1,126 cases, including 90 deaths.
Among this year’s 13 cases reported to the CDC so far, eight people add severe neuroinvasive disease, which means the disease spread to the nervous system. Such severe symptoms typically occur in 1 in every 150 cases of West Nile virus and can include encephalitis, which is inflammation of the brain, or meningitis, which is inflammation of the membranes that surround the brain and spinal cord. Three of the neuroinvasive cases occurred earlier this year amid an outbreak in Maricopa County, Arizona, where the disease is considered endemic, according to an April 28 report from the CDC.
The CDC says West Nile virus is the most common disease spread by mosquitoes in the continental United States. Local health officials sample mosquitoes to guide mosquito control strategies. So far this year, the CDC has received 28 reports of mosquitoes testing positive. Those mosquito testing reports came from Arizona, California, Florida, Indiana, Louisiana, and Texas.
West Nile virus is transmitted to people by the bite of an infected mosquito, but it can also be spread to humans if they handle a dead bird that is infected. The CDC says there are no medications to treat the virus in people. Most people who are infected do not feel sick, and 1 in 5 people infected develop a fever and other symptoms like headache, body ache, or a rash.
Prevention strategies are to wear insect repellent and to wear long-sleeved shirts and long pants to avoid mosquito bites.
A version of this article originally appeared on WebMD.com.
In the past 2 weeks, new cases have been reported in Iowa and Nebraska, adding to previous 2023 reports from Arizona, Georgia, Illinois, Louisiana, Oregon, Pennsylvania, and Wyoming. A mosquito at a monitoring site near Houston tested positive last week for the potentially fatal virus, prompting local health officials to begin evening spray operations in the area where the mosquito was found, according to an announcement from Harris County Public Health.
According to the CDC, which compiles local reports, there have been 13 human cases of West Nile virus in 2023. In 2022, there were 1,126 cases, including 90 deaths.
Among this year’s 13 cases reported to the CDC so far, eight people add severe neuroinvasive disease, which means the disease spread to the nervous system. Such severe symptoms typically occur in 1 in every 150 cases of West Nile virus and can include encephalitis, which is inflammation of the brain, or meningitis, which is inflammation of the membranes that surround the brain and spinal cord. Three of the neuroinvasive cases occurred earlier this year amid an outbreak in Maricopa County, Arizona, where the disease is considered endemic, according to an April 28 report from the CDC.
The CDC says West Nile virus is the most common disease spread by mosquitoes in the continental United States. Local health officials sample mosquitoes to guide mosquito control strategies. So far this year, the CDC has received 28 reports of mosquitoes testing positive. Those mosquito testing reports came from Arizona, California, Florida, Indiana, Louisiana, and Texas.
West Nile virus is transmitted to people by the bite of an infected mosquito, but it can also be spread to humans if they handle a dead bird that is infected. The CDC says there are no medications to treat the virus in people. Most people who are infected do not feel sick, and 1 in 5 people infected develop a fever and other symptoms like headache, body ache, or a rash.
Prevention strategies are to wear insect repellent and to wear long-sleeved shirts and long pants to avoid mosquito bites.
A version of this article originally appeared on WebMD.com.
In the past 2 weeks, new cases have been reported in Iowa and Nebraska, adding to previous 2023 reports from Arizona, Georgia, Illinois, Louisiana, Oregon, Pennsylvania, and Wyoming. A mosquito at a monitoring site near Houston tested positive last week for the potentially fatal virus, prompting local health officials to begin evening spray operations in the area where the mosquito was found, according to an announcement from Harris County Public Health.
According to the CDC, which compiles local reports, there have been 13 human cases of West Nile virus in 2023. In 2022, there were 1,126 cases, including 90 deaths.
Among this year’s 13 cases reported to the CDC so far, eight people add severe neuroinvasive disease, which means the disease spread to the nervous system. Such severe symptoms typically occur in 1 in every 150 cases of West Nile virus and can include encephalitis, which is inflammation of the brain, or meningitis, which is inflammation of the membranes that surround the brain and spinal cord. Three of the neuroinvasive cases occurred earlier this year amid an outbreak in Maricopa County, Arizona, where the disease is considered endemic, according to an April 28 report from the CDC.
The CDC says West Nile virus is the most common disease spread by mosquitoes in the continental United States. Local health officials sample mosquitoes to guide mosquito control strategies. So far this year, the CDC has received 28 reports of mosquitoes testing positive. Those mosquito testing reports came from Arizona, California, Florida, Indiana, Louisiana, and Texas.
West Nile virus is transmitted to people by the bite of an infected mosquito, but it can also be spread to humans if they handle a dead bird that is infected. The CDC says there are no medications to treat the virus in people. Most people who are infected do not feel sick, and 1 in 5 people infected develop a fever and other symptoms like headache, body ache, or a rash.
Prevention strategies are to wear insect repellent and to wear long-sleeved shirts and long pants to avoid mosquito bites.
A version of this article originally appeared on WebMD.com.
Raising the bar (and the OR table):Ergonomics in MIGS
Work-related musculoskeletal disorders (WMSDs) are “musculoskeletal disorders (injuries or disorders of the muscles, nerves, tendons, joints, cartilage, and spinal discs) in which the work environment and performance of work contribute significantly to the condition; and/or the condition is made worse or persists longer due to work conditions.”1 The health care industry has one of the highest rates of WMSDs, even when compared with traditional labor-intensive occupations, such as coal mining. In 2017, the health care industry reported more than a half million incidents of work-related injury and illness.2,3 In particular, surgeons are at increased risk for WMSDs, since they repetitively perform the classic tenets of poor ergonomics, including operating in static, extreme, and awkward positions and for prolonged periods of time.3
Gynecologic surgeons face unique ergonomic challenges. Operating in the pelvis requires an oblique approach that adds complexity and inhibits appropriate ergonomic positioning.4 All modalities of surgery incur their own challenges and risks to the surgeon, including minimally invasive gynecologic surgery (MIGS), which has become the standard of care for most conditions. Although MIGS has several benefits for the patient, a survey of gynecologic oncologists found that 88% of respondents reported discomfort related to MIGS.5 Several factors contribute to the development of WMSDs in surgery, including lack of ergonomic awareness, suboptimal ergonomic education and training,5,6 and ergonomically poor operating room (OR) equipment and instrument design.7 Furthermore, surgical culture does not generally prioritize ergonomics in the OR or requests for ergonomic accommodations.7,8
Within 5 years, a physician workforce shortage is projected for the United States.9 WMSDs contribute to workforce issues as they are associated with decreased productivity; time off needed for pain and treatment, including short-term disability; and possibly early retirement (as those who are older and have more work experience may be more likely to seek medical attention).10 In a 2013 study of vaginal surgeons, 14% missed work; 21% modified their work hours, work type, or amount of surgery; and 29% modified their surgical technique because of injury.10 Work-related pain also can negatively affect mental health, sleep, relationships, and quality of life.6
Recently, awareness has increased regarding WMSDs and their consequences, which has led to significant strides in the study of ergonomics among surgeons, a growing body of research on the topic, and guidance for optimizing ergonomics in the OR.
Risk factors for ergonomic strain
Several factors contribute to ergonomic strain and, subsequently, the development of WMSDs. Recognizing these factors can direct strategies for injury prevention.
Patient factors
The prevalence of obesity in the United States increased from 30.5% in 1999–2000 to 41.9% between 2017 and 2020.11 As the average patient’s body mass index (BMI) has increased, there is concern for a parallel increase in the ergonomic strain on laparoscopic surgeons.
A study of simulated laparoscopic tasks at varying model BMI levels demonstrated increased surgeon postural stress and workload at higher model BMIs (50 kg/m2) when compared with lower model BMIs (20 and 30 kg/m2).11 This result was supported in another study, which demonstrated both increased muscle activity and increased time needed to complete a surgical task with laparoscopic surgery; interestingly, when the same study measured these parameters for robotic surgery, this association was not seen.12 This suggests that a robotic rather than a laparoscopic approach may avoid some of the ergonomic strain associated with increased patient BMI.
Continue to: Surgeon factors...
Surgeon factors
Various surgeon characteristics have been shown to influence ergonomics in the OR. Surgeons with smaller hand sizes, for example, reported greater physical discomfort and demonstrated greater ergonomic workload when operating laparoscopically.13-15 In particular, those with a glove size of 6.5 or smaller have more difficulty using laparoscopic instruments, and those with a glove size smaller than 7 demonstrate a larger decline in grip strength when using laparoscopic instruments repeatedly.14,16
Surgeon height also can affect the amount of time spent in high-risk, nonergonomic positions. In a study that evaluated video recordings of surgeon posture during gynecologic laparoscopy, shorter surgeons were noted to use greater degrees of neck rotation to look at the monitor.17 Furthermore, surgeons with shorter arm lengths experienced more “extreme positions” of the nondominant shoulder and elbow.17 This trend also was seen in open and robotic surgery, where surgeons with a height of 66 cm or less reported increased pain scores after operating.18
Surgical instruments and OR setup
Surgical instrument characteristics can contribute to ergonomic strain, especially when the instruments have been designed with a one-size-fits-all mentality.8,19 In an examination of the anthropometric measurements of surgeon hand sizes and their correlation with difficulty when using a “standard” laparoscopic instrument, surgeons with smaller finger and hand spans had trouble using these instruments.19 Another study compared surgeon grip strength and ergonomic workloads after using 3 laparoscopic advanced bipolar instruments.16 Gender and hand size aside, the authors found that use of several of the laparoscopic devices led to greater decline in grip strength.16
The setup of the OR also can have a profound effect on the surgeon’s ergonomics. Monitor placement, for example, is crucial to ergonomic success. One study found that positioning the monitor directly in front of the surgeon at eye level was associated with the lowest neck muscle activity during a simulated task.20
Route of surgery
Each surgical approach has intrinsic ergonomic risks. With laparoscopy, surgeons often remain in straight head and back positions without much trunk motion, especially when compared with open surgery.21 In one study, laparoscopic surgeons spent more than 60% of a case in a static position and more than 80% of a case in a high-risk, “demanding” neck position.22
Robotic surgery, in contrast to laparoscopy, often has been cited as being more “ergonomic.” While robotic surgery has less of an effect on the neck, shoulders, arms, and legs than laparoscopy23 and often is associated with less physical discomfort than either open or laparoscopic surgery,23,24 robotic surgery still maintains its own innate ergonomic risks. Of robotic surgeons surveyed, 56.1% reported neck stiffness, finger fatigue, and eye symptoms in one study.25 In another survey study, more robotic surgeons (72%) reported physical symptoms than laparoscopic (57%) and open (49%) surgeons.26Vaginal surgery also puts surgeons at ergonomic risk. A majority of surgeons (87.2%) who completed more than 50% of their cases vaginally reported a history of WMSDs.10 Vaginal surgery places surgeons in awkward positions of the neck, shoulder, and trunk frequently and for longer durations.27
Continue to: Strategies for preventing WMSDs...
Strategies for preventing WMSDs
As factors that contribute to the development of WMSDs are identified, preventive strategies can be targeted to these individual factors. Research has focused on appropriate setup of the OR, surgeon posture, intraoperative microbreaks, and stretching both in and outside of the OR.
1. OR setup and positioning of the surgeon by MIGS route
The route of MIGS affects OR setup and surgeon posture. Ergonomic recommendations for laparoscopy, robotic surgery, and vaginal surgery are all unique to the risks posed by each particular approach.
Laparoscopic surgery. Laparoscopic monitors should face the surgeon directly, with the screen just below eye level to maintain the surgeon’s neck in a neutral position.28 The table height should be set for the tallest surgeon, and shorter surgeons should stand on steps as needed.28 The table height also should allow for the surgeon’s hands to be at elbow height, with the elbows bent at 90 degrees with the wrists straight.29 Foot pedals should be placed at the surgeons’ foot level and should be reached easily.28 Additionally, the patient’s arms should be tucked at their sides to allow surgeons a larger operative space.29 When using laparoscopic instruments, locking and ratcheting features should be used whenever possible to reduce prolonged grip or squeeze forces.28 The laparoscopic camera should be held in the palm with the wrist in a neutral position.29
Robotic surgery. Positioning and setup of the robotic console is a main focus of ergonomic recommendations. The surgeon’s chair should be brought as close to the console as possible, and the knees positioned in a 90-degree angle.30 The foot pedals should be brought toward the surgeon to maintain this angle of the knees.30 The console should be rotated toward the surgeon and then the height adjusted so that the surgeon can look through the eyepiece while sitting upright and can maintain the neck in a neutral position.28,30 The surgeon’s forehead should rest comfortably on the headrest.29 The forearms should rest on the armrest while the arms are maintained in a neutral position and the shoulders remain relaxed while the surgeon holds the robotic controls.30 It is important to utilize the armrest often to relieve stress on the arm while operating.28 Frequent use of the clutch function can keep the robotic controls in the center of the workspace.28
Vaginal surgery. Both seated and standing positions are associated with high-risk positioning of the trunk and bilateral shoulders, respectively, in vaginal surgery.31 However, surgeons who stand while operating vaginally reported more discomfort in the bilateral wrists, thighs, and lower legs than those who operated while seated.31 This suggests a potential ergonomic advantage to the seated position for vaginal surgery. Chair height should be adjusted so the surgeon can look straight ahead with the neck in a neutral position.32 Surgeons should consider using a headlamp, as this may prevent repetitive awkward movements to adjust overhead lights.32 For standing surgery, the table height should be adjusted for the tallest surgeon, and shorter surgeons or assistants should use steps as needed.3
Surgical assistants should switch sides during the course of the case to avoid excessive unilateral upper-extremity strain.32 The addition of a table-mounted vaginal retractor system may be useful in relieving physical strain for surgical assistants, but data currently are lacking to demonstrate this ergonomic benefit.33 Further studies are needed, especially since many surgeons take on the role of surgical assist in the teaching environment and subsequently report more WMSDs than their colleagues who do not work in teaching environments.10,34
2. Pain relief from individual ergonomic positioning devices
Apart from adjusting how the OR equipment is arranged or how the surgeons adjust their positioning, several devices that assist with surgeon positioning—including gel mats or insoles, exoskeletons, and “augmented reality” glasses—are being studied.
The use of gel mats or insoles in the OR has mixed evidence in the literature.35-37
Exoskeletons, external devices that support a surgeon’s posture and positioning, have been studied thus far in simulated nonsterile surgical environments. Preliminarily, it appears that use of an exoskeleton can decrease muscle activity and time spent in static positions, with a reported decrease in post-task user discomfort.38,39 More data are needed to determine if exoskeletons can be used in the sterile setting and for longer durations as may occur in actual OR cases.
Augmented reality glasses project the laparoscopic monitor image to the glasses, which frees the surgeon to place the “monitor” in a more neutral, ergonomic position. In one study, use of augmented reality glasses was associated with decreased muscle activity and a reduction in Rapid Entire Body Assessment (REBA) scores when compared with use of the conventional laparoscopic monitor.40More data are needed on these emerging technologies to determine whether adverse effects occur with prolonged use.
Continue to: 3. Implementing intraoperative microbreaks and stretching...
3. Implementing intraoperative microbreaks and stretching
The American College of Surgeons (ACS) recommends that surgeons avoid prolonged static postures during procedures.28 One strategy for preventing sustained positioning is to incorporate breaks with associated stretching routinely during surgery.28
Microbreaks. In a landmark study by Park and colleagues in 2017, 120-second long targeted stretching microbreaks (TSMBs) were completed every 20 to 40 minutes during a surgery, and results demonstrated improved postoperative surgeon pain scores without an associated increase in the length of the case.41 These surgeons reported improved pain in the neck, bilateral shoulders, bilateral hands, and lower back. Eighty-eight percent of surgeons reported either improvement or “no change” in their mental focus, and 100% reported improvement or “no change” in their physical performance after TSMBs were implemented.42 Of surveyed surgeons, 87% wanted TSMBs incorporated routinely.41,42
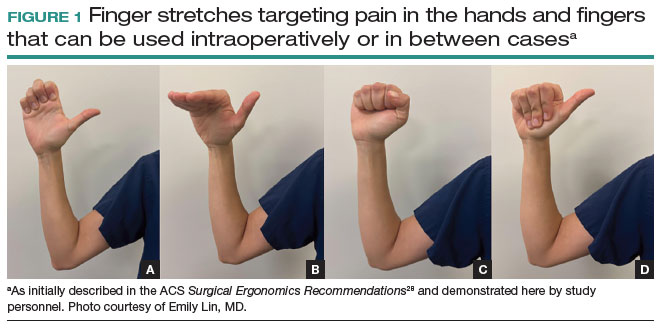
Stretches. Multiple resources, such as the ACS and the Mayo Clinic, for intraoperative stretches are available. The ACS recommends performing neck and shoulder stretches during intraoperative microbreaks, including a range-of-movement neck exercise, deep cervical flexor training, and standing scapular retraction.28 The ACS also demonstrates lumbrical stretches for the fingers and passive wrist extension exercises to be used intraoperatively (or between cases) (FIGURE 1).28 The Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR Stretch Instructional Video” in which the surgeon is guided through several different short stretches, including shoulder shrugging and side bends, that can be used during surgery.43
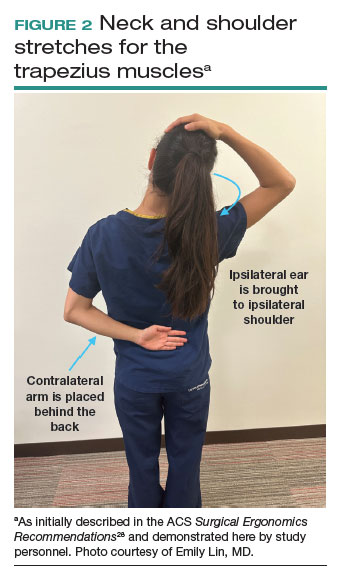
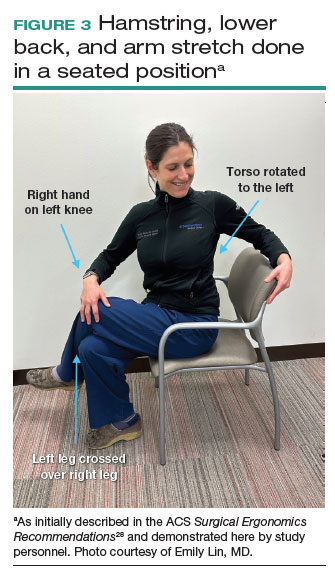
Both the ACS and the Mayo Clinic provide examples of pertinent stretch exercises for use when not in the sterile environment, between cases or after cases are complete. The ACS recommends several neck and shoulder stretches for the trapezius, levator scapulae, and pectoralis and recommends the use of a foam roller to improve thoracic mobility (FIGURE 2).28 As above, the Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR-Stretch Between Surgery Stretches Video” in which the surgeon is guided through several short stretches that are done in a seated position, including stretches for the hamstring, lower back, and arms (FIGURE 3).43
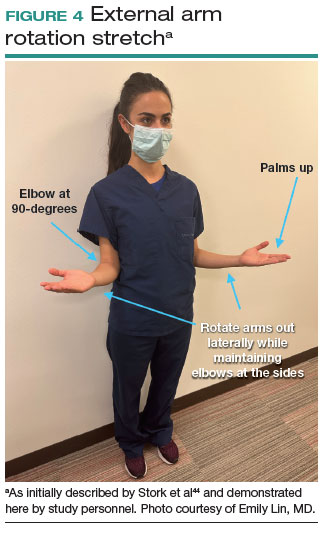
Many of the above-mentioned stretches were designed for use in the context of open, laparoscopic, or robotic surgery. For the vaginal surgeon, the intraoperative ergonomic stressors differ from those of other routes of surgery, and thus stretches tailored to the positioning during vaginal surgery are necessary. In a video recently published by the Society of Gynecologic Surgeons, several stretches are reviewed that target high-risk positions often held by the surgeon or assistant when operating vaginally.44 These stretches include cervical retraction, thoracic extension, external arm rotation, cervical side bending, and lumbar extension (FIGURE 4).44 The recommendation is to complete these exercises 2 times per day, with 8 to 10 repetitions per set.44
Prioritizing ergonomic awareness and training
As caregivers, it is not uncommon for us to prioritize the needs of others before those of ourselves. However, WMSDs are prevalent, and their downstream effects may cause catastrophic professional and personal losses. Cumulatively, the global impact of WMSDs is a significant issue for the health care workforce and its longevity.
To prevent WMSDs, it is imperative that surgeons are aware of the factors that contribute to injury development and the appropriate, accessible modifications for these factors. While each surgical modality confers its own ergonomic challenges, these risks can be mitigated through increased awareness of OR setup, surgeon positioning, and incorporation of microbreaks and stretching exercises during and after surgical procedures.
Formal training in surgical ergonomics is lacking across specialties, including gynecology.45 Multiple educational interventions have been proposed and studied to help fill this training gap.30,46-49When used, these interventions have been associated with increased knowledge of surgical ergonomic principles or reduction in surgeon pain scores, including trainees.50 As we become more cognizant of WMSDs, standardized resident curricula should be developed in an effort to reduce the prevalence of these potentially career-ending injuries.
In addition to education, cultivating a culture in which ergonomics is prioritized is essential. Although most surgeons report work-related pain, very few report their injuries to occupational health. For example, while 29% of gynecologic oncologists reported seeking treatment for a WMSD, only 1% had reported their injury to their employer.5 In a study of ACS members, only 19% of injuries were reported, 30% of surgeons stated that they did not know how to report an injury, and 21% felt that the resources for surgeons during and after an injury were inadequate.6
As we prioritize the health and safety of our patients, we also need to promote ergonomic awareness in the OR, respect the need for accommodations, encourage injury reporting, support surgeons who need to take time away for medical treatment, and partner with industry to develop new instruments and technology with effective ergonomic features. ●
- Workplace health glossary. Reviewed February 12, 2020. Centers for Disease Control and Prevention. Accessed May 18, 2023. https://www.cdc.gov/workplacehealthpromotion/tools-resources /glossary/glossary.html#W
- Epstein S, Sparer EH, Tran BN, et al. Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists: a systematic review and meta-analysis. JAMA Surg. 2018;153:e174947.
- Yurteri-Kaplan LA, Park AJ. Surgical ergonomics and preventing workrelated musculoskeletal disorders. Obstet Gynecol. 2023;141:455-462.
- Symer MM, Keller DS. Human factors in pelvic surgery. Eur J Surg Oncol. 2022;48:2346-2351.
- Franasiak J, Ko EM, Kidd J, et al. Physical strain and urgent need for ergonomic training among gynecologic oncologists who perform minimally invasive surgery. Gynecol Oncol. 2012;126:437-442.
- Davis WT, Fletcher SA, Guillamondegui OD. Musculoskeletal occupational injury among surgeons: effects for patients, providers, and institutions. J Surg Res. 2014;189:207-212.e6.
- Fox M. Surgeons face unique ergonomic challenges. American College of Surgeons. September 1, 2022. Accessed May 22, 2023. https://www.facs.org/for-medical-professionals/news-publications /news-and-articles/bulletin/september-2022-volume-107-issue-9 /surgeons-face-unique-ergonomic-challenges/
- Wong JMK, Carey ET, King C, et al. A call to action for ergonomic surgical devices designed for diverse surgeon end users. Obstet Gynecol. 2023;141:463-466.
- IHS Inc. The Complexities of Physician Supply and Demand: Projections from 2014 to 2025. Association of American Medical Colleges. April 5, 2016.
- Kim-Fine S, Woolley SM, Weaver AL, et al. Work-related musculoskeletal disorders among vaginal surgeons. Int Urogynecol J. 2013;24:1191-1200.
- Sers R, Forrester S, Zecca M, et al. The ergonomic impact of patient body mass index on surgeon posture during simulated laparoscopy. Appl Ergon. 2021;97:103501.
- Moss EL, Sarhanis P, Ind T, et al. Impact of obesity on surgeon ergonomics in robotic and straight-stick laparoscopic surgery. J Minim Invasive Gynecol. 2020;27:1063-1069.
- Sutton E, Irvin M, Zeigler C, et al. The ergonomics of women in surgery. Surg Endosc. 2014;28:1051-1055.
- Berguer R, Hreljac A. The relationship between hand size and difficulty using surgical instruments: a survey of 726 laparoscopic surgeons. Surg Endosc. 2004;18:508-512.
- Bellini MI, Amabile MI, Saullo P, et al. A woman’s place is in theatre, but are theatres designed with women in mind? A systematic review of ergonomics for women in surgery. J Clin Med. 2022;11:3496.
- Wong JMK, Moore KJ, Lewis P, et al. Ergonomic assessment of surgeon characteristics and laparoscopic device strain in gynecologic surgery. J Minim Invasive Gynecol. 2022;29:1357-1363.
- Aitchison LP, Cui CK, Arnold A, et al. The ergonomics of laparoscopic surgery: a quantitative study of the time and motion of laparoscopic surgeons in live surgical environments. Surg Endosc. 2016;30:5068-5076.
- Stewart C, Raoof M, Fong Y, et al. Who is hurting? A prospective study of surgeon ergonomics. Surg Endosc. 2022;36:292-299.
- Green SV, Morris DE, Naumann DN, et al. One size does not fit all: impact of hand size on ease of use of instruments for minimally invasive surgery. Surgeon. 2022;S1479-666X(22)00131-7.
- Matern U, Faist M, Kehl K, et al. Monitor position in laparoscopic surgery. Surg Endosc. 2005;19:436-440.
- Berguer R, Rab GT, Abu-Ghaida H, et al. A comparison of surgeons’ posture during laparoscopic and open surgical procedures. Surg Endosc. 1997;11:139-142.
- Athanasiadis DI, Monfared S, Asadi H, et al. An analysis of the ergonomic risk of surgical trainees and experienced surgeons during laparoscopic procedures. Surgery. 2021;169:496-501.
- Hotton J, Bogart E, Le Deley MC, et al. Ergonomic assessment of the surgeon’s physical workload during robot-assisted versus standard laparoscopy in a French multicenter randomized trial (ROBOGYN-1004 Trial). Ann Surg Oncol. 2023;30:916-923.
- Plerhoples TA, Hernandez-Boussard T, Wren SM. The aching surgeon: a survey of physical discomfort and symptoms following open, laparoscopic, and robotic surgery. J Robot Surg. 2012;6:65-72.
- Lee GI, Lee MR, Green I, et al. Surgeons’ physical discomfort and symptoms during robotic surgery: a comprehensive ergonomic survey study. Surg Endosc. 2017;31:1697-1706.
- McDonald ME, Ramirez PT, Munsell MF, et al. Physician pain and discomfort during minimally invasive gynecologic cancer surgery. Gynecol Oncol. 2014;134:243-247.
- Zhu X, Yurteri-Kaplan LA, Gutman RE, et al. Postural stress experienced by vaginal surgeons. Proc Hum Factors Ergonomics Soc Annu Meet. 2014;58:763-767.
- American College of Surgeons Division of Education and Surgical Ergonomics Committee. Surgical Ergonomics Recommendations. ACS Education. 2022.
- Cardenas-Trowers O, Kjellsson K, Hatch K. Ergonomics: making the OR a comfortable place. Int Urogynecol J. 2018;29:1065-1066.
- Hokenstad ED, Hallbeck MS, Lowndes BR, et al. Ergonomic robotic console configuration in gynecologic surgery: an interventional study. J Minim Invasive Gynecol. 2021;28:850-859.
- Singh R, Yurteri-Kaplan LA, Morrow MM, et al. Sitting versus standing makes a difference in musculoskeletal discomfort and postural load for surgeons performing vaginal surgery. Int Urogynecol J. 2019;30:231-237.
- Hullfish KL, Trowbridge ER, Bodine G. Ergonomics and gynecologic surgery: “surgeon protect thyself.” J Pelvic Med Surg. 2009;15:435-439.
- Woodburn KL, Kho RM. Vaginal surgery: don’t get bent out of shape. Am J Obstet Gynecol. 2020;223:762-763.
- Hobson DTG, Meriwether KV, Gaskins JT, et al. Learner satisfaction and experience with a high-definition telescopic camera during vaginal procedures: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2021;27:105-111.
- Speed G, Harris K, Keegel T. The effect of cushioning materials on musculoskeletal discomfort and fatigue during prolonged standing at work: a systematic review. Appl Ergon. 2018;70:300-334.
- Haramis G, Rosales JC, Palacios JM, et al. Prospective randomized evaluation of FOOT gel pads for operating room staff COMFORT during laparoscopic renal surgery. Urology. 2010;76:1405-1408.
- Voss RK, Chiang YJ, Cromwell KD, et al. Do no harm, except to ourselves? A survey of symptoms and injuries in oncologic surgeons and pilot study of an intraoperative ergonomic intervention. J Am Coll Surg. 2017;224:16-25.e1.
- Marquetand J, Gabriel J, Seibt R, et al. Ergonomics for surgeons—prototype of an external surgeon support system reduces muscular activity and fatigue. J Electromyogr Kinesiol. 2021;60:102586.
- Tetteh E, Hallbeck MS, Mirka GA. Effects of passive exoskeleton support on EMG measures of the neck, shoulder and trunk muscles while holding simulated surgical postures and performing a simulated surgical procedure. Appl Ergon. 2022;100:103646.
- Lim AK, Ryu J, Yoon HM, et al. Ergonomic effects of medical augmented reality glasses in video-assisted surgery. Surg Endosc. 2022;36:988-998.
- Park AE, Zahiri HR, Hallbeck MS, et al. Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: a multicenter cohort study. Ann Surg. 2017;265:340-346.
- Hallbeck MS, Lowndes BR, Bingener J, et al. The impact of intraoperative microbreaks with exercises on surgeons: a multi-center cohort study. Appl Ergon. 2017;60:334-341.
- Hallbeck Human Factors Engineering Laboratories. OR Stretch Videos. Mayo Clinic, 2018. Accessed May 19, 2023. https://www.mayo .edu/research/labs/human-factors-engineering/or-stretch /or-stretch-videos
- Stork A, Bacon T, Corton M. Prevention of Work-Related Musculoskeletal Disorders in Vaginal Surgery. Video presentation at: Society of Gynecologic Surgeons’ Annual Scientific Meeting 2023, Tucson, AZ. Accessed April 3, 2023. https://sgs.eng.us/category.php?cat=2023 -video-presentations
- Aaron KA, Vaughan J, Gupta R, et al. The risk of ergonomic injury across surgical specialties. PLoS One. 2021;16:e0244868.
- Smith TG, Lowndes BR, Schmida E, et al. Course design and learning outcomes of a practical online ergonomics course for surgical residents. J Surg Educ. 2022;79:1489-1499.
- Franasiak J, Craven R, Mosaly P, et al. Feasibility and acceptance of a robotic surgery ergonomic training program. JSLS. 2014;18:e2014.00166.
- Cerier E, Hu A, Goldring A, et al. Ergonomics workshop improves musculoskeletal symptoms in general surgery residents. J Surg Res. 2022;280:567-574.
- Giagio S, Volpe G, Pillastrini P, et al. A preventive program for workrelated musculoskeletal disorders among surgeons: outcomes of a randomized controlled clinical trial. Ann Surg. 2019;270:969-975.
- Jensen MJ, Liao J, Van Gorp B, et al. Incorporating surgical ergonomics education into surgical residency curriculum. J Surg Educ. 2021;78:1209-1215.
Work-related musculoskeletal disorders (WMSDs) are “musculoskeletal disorders (injuries or disorders of the muscles, nerves, tendons, joints, cartilage, and spinal discs) in which the work environment and performance of work contribute significantly to the condition; and/or the condition is made worse or persists longer due to work conditions.”1 The health care industry has one of the highest rates of WMSDs, even when compared with traditional labor-intensive occupations, such as coal mining. In 2017, the health care industry reported more than a half million incidents of work-related injury and illness.2,3 In particular, surgeons are at increased risk for WMSDs, since they repetitively perform the classic tenets of poor ergonomics, including operating in static, extreme, and awkward positions and for prolonged periods of time.3
Gynecologic surgeons face unique ergonomic challenges. Operating in the pelvis requires an oblique approach that adds complexity and inhibits appropriate ergonomic positioning.4 All modalities of surgery incur their own challenges and risks to the surgeon, including minimally invasive gynecologic surgery (MIGS), which has become the standard of care for most conditions. Although MIGS has several benefits for the patient, a survey of gynecologic oncologists found that 88% of respondents reported discomfort related to MIGS.5 Several factors contribute to the development of WMSDs in surgery, including lack of ergonomic awareness, suboptimal ergonomic education and training,5,6 and ergonomically poor operating room (OR) equipment and instrument design.7 Furthermore, surgical culture does not generally prioritize ergonomics in the OR or requests for ergonomic accommodations.7,8
Within 5 years, a physician workforce shortage is projected for the United States.9 WMSDs contribute to workforce issues as they are associated with decreased productivity; time off needed for pain and treatment, including short-term disability; and possibly early retirement (as those who are older and have more work experience may be more likely to seek medical attention).10 In a 2013 study of vaginal surgeons, 14% missed work; 21% modified their work hours, work type, or amount of surgery; and 29% modified their surgical technique because of injury.10 Work-related pain also can negatively affect mental health, sleep, relationships, and quality of life.6
Recently, awareness has increased regarding WMSDs and their consequences, which has led to significant strides in the study of ergonomics among surgeons, a growing body of research on the topic, and guidance for optimizing ergonomics in the OR.
Risk factors for ergonomic strain
Several factors contribute to ergonomic strain and, subsequently, the development of WMSDs. Recognizing these factors can direct strategies for injury prevention.
Patient factors
The prevalence of obesity in the United States increased from 30.5% in 1999–2000 to 41.9% between 2017 and 2020.11 As the average patient’s body mass index (BMI) has increased, there is concern for a parallel increase in the ergonomic strain on laparoscopic surgeons.
A study of simulated laparoscopic tasks at varying model BMI levels demonstrated increased surgeon postural stress and workload at higher model BMIs (50 kg/m2) when compared with lower model BMIs (20 and 30 kg/m2).11 This result was supported in another study, which demonstrated both increased muscle activity and increased time needed to complete a surgical task with laparoscopic surgery; interestingly, when the same study measured these parameters for robotic surgery, this association was not seen.12 This suggests that a robotic rather than a laparoscopic approach may avoid some of the ergonomic strain associated with increased patient BMI.
Continue to: Surgeon factors...
Surgeon factors
Various surgeon characteristics have been shown to influence ergonomics in the OR. Surgeons with smaller hand sizes, for example, reported greater physical discomfort and demonstrated greater ergonomic workload when operating laparoscopically.13-15 In particular, those with a glove size of 6.5 or smaller have more difficulty using laparoscopic instruments, and those with a glove size smaller than 7 demonstrate a larger decline in grip strength when using laparoscopic instruments repeatedly.14,16
Surgeon height also can affect the amount of time spent in high-risk, nonergonomic positions. In a study that evaluated video recordings of surgeon posture during gynecologic laparoscopy, shorter surgeons were noted to use greater degrees of neck rotation to look at the monitor.17 Furthermore, surgeons with shorter arm lengths experienced more “extreme positions” of the nondominant shoulder and elbow.17 This trend also was seen in open and robotic surgery, where surgeons with a height of 66 cm or less reported increased pain scores after operating.18
Surgical instruments and OR setup
Surgical instrument characteristics can contribute to ergonomic strain, especially when the instruments have been designed with a one-size-fits-all mentality.8,19 In an examination of the anthropometric measurements of surgeon hand sizes and their correlation with difficulty when using a “standard” laparoscopic instrument, surgeons with smaller finger and hand spans had trouble using these instruments.19 Another study compared surgeon grip strength and ergonomic workloads after using 3 laparoscopic advanced bipolar instruments.16 Gender and hand size aside, the authors found that use of several of the laparoscopic devices led to greater decline in grip strength.16
The setup of the OR also can have a profound effect on the surgeon’s ergonomics. Monitor placement, for example, is crucial to ergonomic success. One study found that positioning the monitor directly in front of the surgeon at eye level was associated with the lowest neck muscle activity during a simulated task.20
Route of surgery
Each surgical approach has intrinsic ergonomic risks. With laparoscopy, surgeons often remain in straight head and back positions without much trunk motion, especially when compared with open surgery.21 In one study, laparoscopic surgeons spent more than 60% of a case in a static position and more than 80% of a case in a high-risk, “demanding” neck position.22
Robotic surgery, in contrast to laparoscopy, often has been cited as being more “ergonomic.” While robotic surgery has less of an effect on the neck, shoulders, arms, and legs than laparoscopy23 and often is associated with less physical discomfort than either open or laparoscopic surgery,23,24 robotic surgery still maintains its own innate ergonomic risks. Of robotic surgeons surveyed, 56.1% reported neck stiffness, finger fatigue, and eye symptoms in one study.25 In another survey study, more robotic surgeons (72%) reported physical symptoms than laparoscopic (57%) and open (49%) surgeons.26Vaginal surgery also puts surgeons at ergonomic risk. A majority of surgeons (87.2%) who completed more than 50% of their cases vaginally reported a history of WMSDs.10 Vaginal surgery places surgeons in awkward positions of the neck, shoulder, and trunk frequently and for longer durations.27
Continue to: Strategies for preventing WMSDs...
Strategies for preventing WMSDs
As factors that contribute to the development of WMSDs are identified, preventive strategies can be targeted to these individual factors. Research has focused on appropriate setup of the OR, surgeon posture, intraoperative microbreaks, and stretching both in and outside of the OR.
1. OR setup and positioning of the surgeon by MIGS route
The route of MIGS affects OR setup and surgeon posture. Ergonomic recommendations for laparoscopy, robotic surgery, and vaginal surgery are all unique to the risks posed by each particular approach.
Laparoscopic surgery. Laparoscopic monitors should face the surgeon directly, with the screen just below eye level to maintain the surgeon’s neck in a neutral position.28 The table height should be set for the tallest surgeon, and shorter surgeons should stand on steps as needed.28 The table height also should allow for the surgeon’s hands to be at elbow height, with the elbows bent at 90 degrees with the wrists straight.29 Foot pedals should be placed at the surgeons’ foot level and should be reached easily.28 Additionally, the patient’s arms should be tucked at their sides to allow surgeons a larger operative space.29 When using laparoscopic instruments, locking and ratcheting features should be used whenever possible to reduce prolonged grip or squeeze forces.28 The laparoscopic camera should be held in the palm with the wrist in a neutral position.29
Robotic surgery. Positioning and setup of the robotic console is a main focus of ergonomic recommendations. The surgeon’s chair should be brought as close to the console as possible, and the knees positioned in a 90-degree angle.30 The foot pedals should be brought toward the surgeon to maintain this angle of the knees.30 The console should be rotated toward the surgeon and then the height adjusted so that the surgeon can look through the eyepiece while sitting upright and can maintain the neck in a neutral position.28,30 The surgeon’s forehead should rest comfortably on the headrest.29 The forearms should rest on the armrest while the arms are maintained in a neutral position and the shoulders remain relaxed while the surgeon holds the robotic controls.30 It is important to utilize the armrest often to relieve stress on the arm while operating.28 Frequent use of the clutch function can keep the robotic controls in the center of the workspace.28
Vaginal surgery. Both seated and standing positions are associated with high-risk positioning of the trunk and bilateral shoulders, respectively, in vaginal surgery.31 However, surgeons who stand while operating vaginally reported more discomfort in the bilateral wrists, thighs, and lower legs than those who operated while seated.31 This suggests a potential ergonomic advantage to the seated position for vaginal surgery. Chair height should be adjusted so the surgeon can look straight ahead with the neck in a neutral position.32 Surgeons should consider using a headlamp, as this may prevent repetitive awkward movements to adjust overhead lights.32 For standing surgery, the table height should be adjusted for the tallest surgeon, and shorter surgeons or assistants should use steps as needed.3
Surgical assistants should switch sides during the course of the case to avoid excessive unilateral upper-extremity strain.32 The addition of a table-mounted vaginal retractor system may be useful in relieving physical strain for surgical assistants, but data currently are lacking to demonstrate this ergonomic benefit.33 Further studies are needed, especially since many surgeons take on the role of surgical assist in the teaching environment and subsequently report more WMSDs than their colleagues who do not work in teaching environments.10,34
2. Pain relief from individual ergonomic positioning devices
Apart from adjusting how the OR equipment is arranged or how the surgeons adjust their positioning, several devices that assist with surgeon positioning—including gel mats or insoles, exoskeletons, and “augmented reality” glasses—are being studied.
The use of gel mats or insoles in the OR has mixed evidence in the literature.35-37
Exoskeletons, external devices that support a surgeon’s posture and positioning, have been studied thus far in simulated nonsterile surgical environments. Preliminarily, it appears that use of an exoskeleton can decrease muscle activity and time spent in static positions, with a reported decrease in post-task user discomfort.38,39 More data are needed to determine if exoskeletons can be used in the sterile setting and for longer durations as may occur in actual OR cases.
Augmented reality glasses project the laparoscopic monitor image to the glasses, which frees the surgeon to place the “monitor” in a more neutral, ergonomic position. In one study, use of augmented reality glasses was associated with decreased muscle activity and a reduction in Rapid Entire Body Assessment (REBA) scores when compared with use of the conventional laparoscopic monitor.40More data are needed on these emerging technologies to determine whether adverse effects occur with prolonged use.
Continue to: 3. Implementing intraoperative microbreaks and stretching...
3. Implementing intraoperative microbreaks and stretching
The American College of Surgeons (ACS) recommends that surgeons avoid prolonged static postures during procedures.28 One strategy for preventing sustained positioning is to incorporate breaks with associated stretching routinely during surgery.28
Microbreaks. In a landmark study by Park and colleagues in 2017, 120-second long targeted stretching microbreaks (TSMBs) were completed every 20 to 40 minutes during a surgery, and results demonstrated improved postoperative surgeon pain scores without an associated increase in the length of the case.41 These surgeons reported improved pain in the neck, bilateral shoulders, bilateral hands, and lower back. Eighty-eight percent of surgeons reported either improvement or “no change” in their mental focus, and 100% reported improvement or “no change” in their physical performance after TSMBs were implemented.42 Of surveyed surgeons, 87% wanted TSMBs incorporated routinely.41,42
Stretches. Multiple resources, such as the ACS and the Mayo Clinic, for intraoperative stretches are available. The ACS recommends performing neck and shoulder stretches during intraoperative microbreaks, including a range-of-movement neck exercise, deep cervical flexor training, and standing scapular retraction.28 The ACS also demonstrates lumbrical stretches for the fingers and passive wrist extension exercises to be used intraoperatively (or between cases) (FIGURE 1).28 The Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR Stretch Instructional Video” in which the surgeon is guided through several different short stretches, including shoulder shrugging and side bends, that can be used during surgery.43

Both the ACS and the Mayo Clinic provide examples of pertinent stretch exercises for use when not in the sterile environment, between cases or after cases are complete. The ACS recommends several neck and shoulder stretches for the trapezius, levator scapulae, and pectoralis and recommends the use of a foam roller to improve thoracic mobility (FIGURE 2).28 As above, the Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR-Stretch Between Surgery Stretches Video” in which the surgeon is guided through several short stretches that are done in a seated position, including stretches for the hamstring, lower back, and arms (FIGURE 3).43
Many of the above-mentioned stretches were designed for use in the context of open, laparoscopic, or robotic surgery. For the vaginal surgeon, the intraoperative ergonomic stressors differ from those of other routes of surgery, and thus stretches tailored to the positioning during vaginal surgery are necessary. In a video recently published by the Society of Gynecologic Surgeons, several stretches are reviewed that target high-risk positions often held by the surgeon or assistant when operating vaginally.44 These stretches include cervical retraction, thoracic extension, external arm rotation, cervical side bending, and lumbar extension (FIGURE 4).44 The recommendation is to complete these exercises 2 times per day, with 8 to 10 repetitions per set.44



Prioritizing ergonomic awareness and training
As caregivers, it is not uncommon for us to prioritize the needs of others before those of ourselves. However, WMSDs are prevalent, and their downstream effects may cause catastrophic professional and personal losses. Cumulatively, the global impact of WMSDs is a significant issue for the health care workforce and its longevity.
To prevent WMSDs, it is imperative that surgeons are aware of the factors that contribute to injury development and the appropriate, accessible modifications for these factors. While each surgical modality confers its own ergonomic challenges, these risks can be mitigated through increased awareness of OR setup, surgeon positioning, and incorporation of microbreaks and stretching exercises during and after surgical procedures.
Formal training in surgical ergonomics is lacking across specialties, including gynecology.45 Multiple educational interventions have been proposed and studied to help fill this training gap.30,46-49When used, these interventions have been associated with increased knowledge of surgical ergonomic principles or reduction in surgeon pain scores, including trainees.50 As we become more cognizant of WMSDs, standardized resident curricula should be developed in an effort to reduce the prevalence of these potentially career-ending injuries.
In addition to education, cultivating a culture in which ergonomics is prioritized is essential. Although most surgeons report work-related pain, very few report their injuries to occupational health. For example, while 29% of gynecologic oncologists reported seeking treatment for a WMSD, only 1% had reported their injury to their employer.5 In a study of ACS members, only 19% of injuries were reported, 30% of surgeons stated that they did not know how to report an injury, and 21% felt that the resources for surgeons during and after an injury were inadequate.6
As we prioritize the health and safety of our patients, we also need to promote ergonomic awareness in the OR, respect the need for accommodations, encourage injury reporting, support surgeons who need to take time away for medical treatment, and partner with industry to develop new instruments and technology with effective ergonomic features. ●
Work-related musculoskeletal disorders (WMSDs) are “musculoskeletal disorders (injuries or disorders of the muscles, nerves, tendons, joints, cartilage, and spinal discs) in which the work environment and performance of work contribute significantly to the condition; and/or the condition is made worse or persists longer due to work conditions.”1 The health care industry has one of the highest rates of WMSDs, even when compared with traditional labor-intensive occupations, such as coal mining. In 2017, the health care industry reported more than a half million incidents of work-related injury and illness.2,3 In particular, surgeons are at increased risk for WMSDs, since they repetitively perform the classic tenets of poor ergonomics, including operating in static, extreme, and awkward positions and for prolonged periods of time.3
Gynecologic surgeons face unique ergonomic challenges. Operating in the pelvis requires an oblique approach that adds complexity and inhibits appropriate ergonomic positioning.4 All modalities of surgery incur their own challenges and risks to the surgeon, including minimally invasive gynecologic surgery (MIGS), which has become the standard of care for most conditions. Although MIGS has several benefits for the patient, a survey of gynecologic oncologists found that 88% of respondents reported discomfort related to MIGS.5 Several factors contribute to the development of WMSDs in surgery, including lack of ergonomic awareness, suboptimal ergonomic education and training,5,6 and ergonomically poor operating room (OR) equipment and instrument design.7 Furthermore, surgical culture does not generally prioritize ergonomics in the OR or requests for ergonomic accommodations.7,8
Within 5 years, a physician workforce shortage is projected for the United States.9 WMSDs contribute to workforce issues as they are associated with decreased productivity; time off needed for pain and treatment, including short-term disability; and possibly early retirement (as those who are older and have more work experience may be more likely to seek medical attention).10 In a 2013 study of vaginal surgeons, 14% missed work; 21% modified their work hours, work type, or amount of surgery; and 29% modified their surgical technique because of injury.10 Work-related pain also can negatively affect mental health, sleep, relationships, and quality of life.6
Recently, awareness has increased regarding WMSDs and their consequences, which has led to significant strides in the study of ergonomics among surgeons, a growing body of research on the topic, and guidance for optimizing ergonomics in the OR.
Risk factors for ergonomic strain
Several factors contribute to ergonomic strain and, subsequently, the development of WMSDs. Recognizing these factors can direct strategies for injury prevention.
Patient factors
The prevalence of obesity in the United States increased from 30.5% in 1999–2000 to 41.9% between 2017 and 2020.11 As the average patient’s body mass index (BMI) has increased, there is concern for a parallel increase in the ergonomic strain on laparoscopic surgeons.
A study of simulated laparoscopic tasks at varying model BMI levels demonstrated increased surgeon postural stress and workload at higher model BMIs (50 kg/m2) when compared with lower model BMIs (20 and 30 kg/m2).11 This result was supported in another study, which demonstrated both increased muscle activity and increased time needed to complete a surgical task with laparoscopic surgery; interestingly, when the same study measured these parameters for robotic surgery, this association was not seen.12 This suggests that a robotic rather than a laparoscopic approach may avoid some of the ergonomic strain associated with increased patient BMI.
Continue to: Surgeon factors...
Surgeon factors
Various surgeon characteristics have been shown to influence ergonomics in the OR. Surgeons with smaller hand sizes, for example, reported greater physical discomfort and demonstrated greater ergonomic workload when operating laparoscopically.13-15 In particular, those with a glove size of 6.5 or smaller have more difficulty using laparoscopic instruments, and those with a glove size smaller than 7 demonstrate a larger decline in grip strength when using laparoscopic instruments repeatedly.14,16
Surgeon height also can affect the amount of time spent in high-risk, nonergonomic positions. In a study that evaluated video recordings of surgeon posture during gynecologic laparoscopy, shorter surgeons were noted to use greater degrees of neck rotation to look at the monitor.17 Furthermore, surgeons with shorter arm lengths experienced more “extreme positions” of the nondominant shoulder and elbow.17 This trend also was seen in open and robotic surgery, where surgeons with a height of 66 cm or less reported increased pain scores after operating.18
Surgical instruments and OR setup
Surgical instrument characteristics can contribute to ergonomic strain, especially when the instruments have been designed with a one-size-fits-all mentality.8,19 In an examination of the anthropometric measurements of surgeon hand sizes and their correlation with difficulty when using a “standard” laparoscopic instrument, surgeons with smaller finger and hand spans had trouble using these instruments.19 Another study compared surgeon grip strength and ergonomic workloads after using 3 laparoscopic advanced bipolar instruments.16 Gender and hand size aside, the authors found that use of several of the laparoscopic devices led to greater decline in grip strength.16
The setup of the OR also can have a profound effect on the surgeon’s ergonomics. Monitor placement, for example, is crucial to ergonomic success. One study found that positioning the monitor directly in front of the surgeon at eye level was associated with the lowest neck muscle activity during a simulated task.20
Route of surgery
Each surgical approach has intrinsic ergonomic risks. With laparoscopy, surgeons often remain in straight head and back positions without much trunk motion, especially when compared with open surgery.21 In one study, laparoscopic surgeons spent more than 60% of a case in a static position and more than 80% of a case in a high-risk, “demanding” neck position.22
Robotic surgery, in contrast to laparoscopy, often has been cited as being more “ergonomic.” While robotic surgery has less of an effect on the neck, shoulders, arms, and legs than laparoscopy23 and often is associated with less physical discomfort than either open or laparoscopic surgery,23,24 robotic surgery still maintains its own innate ergonomic risks. Of robotic surgeons surveyed, 56.1% reported neck stiffness, finger fatigue, and eye symptoms in one study.25 In another survey study, more robotic surgeons (72%) reported physical symptoms than laparoscopic (57%) and open (49%) surgeons.26Vaginal surgery also puts surgeons at ergonomic risk. A majority of surgeons (87.2%) who completed more than 50% of their cases vaginally reported a history of WMSDs.10 Vaginal surgery places surgeons in awkward positions of the neck, shoulder, and trunk frequently and for longer durations.27
Continue to: Strategies for preventing WMSDs...
Strategies for preventing WMSDs
As factors that contribute to the development of WMSDs are identified, preventive strategies can be targeted to these individual factors. Research has focused on appropriate setup of the OR, surgeon posture, intraoperative microbreaks, and stretching both in and outside of the OR.
1. OR setup and positioning of the surgeon by MIGS route
The route of MIGS affects OR setup and surgeon posture. Ergonomic recommendations for laparoscopy, robotic surgery, and vaginal surgery are all unique to the risks posed by each particular approach.
Laparoscopic surgery. Laparoscopic monitors should face the surgeon directly, with the screen just below eye level to maintain the surgeon’s neck in a neutral position.28 The table height should be set for the tallest surgeon, and shorter surgeons should stand on steps as needed.28 The table height also should allow for the surgeon’s hands to be at elbow height, with the elbows bent at 90 degrees with the wrists straight.29 Foot pedals should be placed at the surgeons’ foot level and should be reached easily.28 Additionally, the patient’s arms should be tucked at their sides to allow surgeons a larger operative space.29 When using laparoscopic instruments, locking and ratcheting features should be used whenever possible to reduce prolonged grip or squeeze forces.28 The laparoscopic camera should be held in the palm with the wrist in a neutral position.29
Robotic surgery. Positioning and setup of the robotic console is a main focus of ergonomic recommendations. The surgeon’s chair should be brought as close to the console as possible, and the knees positioned in a 90-degree angle.30 The foot pedals should be brought toward the surgeon to maintain this angle of the knees.30 The console should be rotated toward the surgeon and then the height adjusted so that the surgeon can look through the eyepiece while sitting upright and can maintain the neck in a neutral position.28,30 The surgeon’s forehead should rest comfortably on the headrest.29 The forearms should rest on the armrest while the arms are maintained in a neutral position and the shoulders remain relaxed while the surgeon holds the robotic controls.30 It is important to utilize the armrest often to relieve stress on the arm while operating.28 Frequent use of the clutch function can keep the robotic controls in the center of the workspace.28
Vaginal surgery. Both seated and standing positions are associated with high-risk positioning of the trunk and bilateral shoulders, respectively, in vaginal surgery.31 However, surgeons who stand while operating vaginally reported more discomfort in the bilateral wrists, thighs, and lower legs than those who operated while seated.31 This suggests a potential ergonomic advantage to the seated position for vaginal surgery. Chair height should be adjusted so the surgeon can look straight ahead with the neck in a neutral position.32 Surgeons should consider using a headlamp, as this may prevent repetitive awkward movements to adjust overhead lights.32 For standing surgery, the table height should be adjusted for the tallest surgeon, and shorter surgeons or assistants should use steps as needed.3
Surgical assistants should switch sides during the course of the case to avoid excessive unilateral upper-extremity strain.32 The addition of a table-mounted vaginal retractor system may be useful in relieving physical strain for surgical assistants, but data currently are lacking to demonstrate this ergonomic benefit.33 Further studies are needed, especially since many surgeons take on the role of surgical assist in the teaching environment and subsequently report more WMSDs than their colleagues who do not work in teaching environments.10,34
2. Pain relief from individual ergonomic positioning devices
Apart from adjusting how the OR equipment is arranged or how the surgeons adjust their positioning, several devices that assist with surgeon positioning—including gel mats or insoles, exoskeletons, and “augmented reality” glasses—are being studied.
The use of gel mats or insoles in the OR has mixed evidence in the literature.35-37
Exoskeletons, external devices that support a surgeon’s posture and positioning, have been studied thus far in simulated nonsterile surgical environments. Preliminarily, it appears that use of an exoskeleton can decrease muscle activity and time spent in static positions, with a reported decrease in post-task user discomfort.38,39 More data are needed to determine if exoskeletons can be used in the sterile setting and for longer durations as may occur in actual OR cases.
Augmented reality glasses project the laparoscopic monitor image to the glasses, which frees the surgeon to place the “monitor” in a more neutral, ergonomic position. In one study, use of augmented reality glasses was associated with decreased muscle activity and a reduction in Rapid Entire Body Assessment (REBA) scores when compared with use of the conventional laparoscopic monitor.40More data are needed on these emerging technologies to determine whether adverse effects occur with prolonged use.
Continue to: 3. Implementing intraoperative microbreaks and stretching...
3. Implementing intraoperative microbreaks and stretching
The American College of Surgeons (ACS) recommends that surgeons avoid prolonged static postures during procedures.28 One strategy for preventing sustained positioning is to incorporate breaks with associated stretching routinely during surgery.28
Microbreaks. In a landmark study by Park and colleagues in 2017, 120-second long targeted stretching microbreaks (TSMBs) were completed every 20 to 40 minutes during a surgery, and results demonstrated improved postoperative surgeon pain scores without an associated increase in the length of the case.41 These surgeons reported improved pain in the neck, bilateral shoulders, bilateral hands, and lower back. Eighty-eight percent of surgeons reported either improvement or “no change” in their mental focus, and 100% reported improvement or “no change” in their physical performance after TSMBs were implemented.42 Of surveyed surgeons, 87% wanted TSMBs incorporated routinely.41,42
Stretches. Multiple resources, such as the ACS and the Mayo Clinic, for intraoperative stretches are available. The ACS recommends performing neck and shoulder stretches during intraoperative microbreaks, including a range-of-movement neck exercise, deep cervical flexor training, and standing scapular retraction.28 The ACS also demonstrates lumbrical stretches for the fingers and passive wrist extension exercises to be used intraoperatively (or between cases) (FIGURE 1).28 The Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR Stretch Instructional Video” in which the surgeon is guided through several different short stretches, including shoulder shrugging and side bends, that can be used during surgery.43

Both the ACS and the Mayo Clinic provide examples of pertinent stretch exercises for use when not in the sterile environment, between cases or after cases are complete. The ACS recommends several neck and shoulder stretches for the trapezius, levator scapulae, and pectoralis and recommends the use of a foam roller to improve thoracic mobility (FIGURE 2).28 As above, the Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR-Stretch Between Surgery Stretches Video” in which the surgeon is guided through several short stretches that are done in a seated position, including stretches for the hamstring, lower back, and arms (FIGURE 3).43
Many of the above-mentioned stretches were designed for use in the context of open, laparoscopic, or robotic surgery. For the vaginal surgeon, the intraoperative ergonomic stressors differ from those of other routes of surgery, and thus stretches tailored to the positioning during vaginal surgery are necessary. In a video recently published by the Society of Gynecologic Surgeons, several stretches are reviewed that target high-risk positions often held by the surgeon or assistant when operating vaginally.44 These stretches include cervical retraction, thoracic extension, external arm rotation, cervical side bending, and lumbar extension (FIGURE 4).44 The recommendation is to complete these exercises 2 times per day, with 8 to 10 repetitions per set.44



Prioritizing ergonomic awareness and training
As caregivers, it is not uncommon for us to prioritize the needs of others before those of ourselves. However, WMSDs are prevalent, and their downstream effects may cause catastrophic professional and personal losses. Cumulatively, the global impact of WMSDs is a significant issue for the health care workforce and its longevity.
To prevent WMSDs, it is imperative that surgeons are aware of the factors that contribute to injury development and the appropriate, accessible modifications for these factors. While each surgical modality confers its own ergonomic challenges, these risks can be mitigated through increased awareness of OR setup, surgeon positioning, and incorporation of microbreaks and stretching exercises during and after surgical procedures.
Formal training in surgical ergonomics is lacking across specialties, including gynecology.45 Multiple educational interventions have been proposed and studied to help fill this training gap.30,46-49When used, these interventions have been associated with increased knowledge of surgical ergonomic principles or reduction in surgeon pain scores, including trainees.50 As we become more cognizant of WMSDs, standardized resident curricula should be developed in an effort to reduce the prevalence of these potentially career-ending injuries.
In addition to education, cultivating a culture in which ergonomics is prioritized is essential. Although most surgeons report work-related pain, very few report their injuries to occupational health. For example, while 29% of gynecologic oncologists reported seeking treatment for a WMSD, only 1% had reported their injury to their employer.5 In a study of ACS members, only 19% of injuries were reported, 30% of surgeons stated that they did not know how to report an injury, and 21% felt that the resources for surgeons during and after an injury were inadequate.6
As we prioritize the health and safety of our patients, we also need to promote ergonomic awareness in the OR, respect the need for accommodations, encourage injury reporting, support surgeons who need to take time away for medical treatment, and partner with industry to develop new instruments and technology with effective ergonomic features. ●
- Workplace health glossary. Reviewed February 12, 2020. Centers for Disease Control and Prevention. Accessed May 18, 2023. https://www.cdc.gov/workplacehealthpromotion/tools-resources /glossary/glossary.html#W
- Epstein S, Sparer EH, Tran BN, et al. Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists: a systematic review and meta-analysis. JAMA Surg. 2018;153:e174947.
- Yurteri-Kaplan LA, Park AJ. Surgical ergonomics and preventing workrelated musculoskeletal disorders. Obstet Gynecol. 2023;141:455-462.
- Symer MM, Keller DS. Human factors in pelvic surgery. Eur J Surg Oncol. 2022;48:2346-2351.
- Franasiak J, Ko EM, Kidd J, et al. Physical strain and urgent need for ergonomic training among gynecologic oncologists who perform minimally invasive surgery. Gynecol Oncol. 2012;126:437-442.
- Davis WT, Fletcher SA, Guillamondegui OD. Musculoskeletal occupational injury among surgeons: effects for patients, providers, and institutions. J Surg Res. 2014;189:207-212.e6.
- Fox M. Surgeons face unique ergonomic challenges. American College of Surgeons. September 1, 2022. Accessed May 22, 2023. https://www.facs.org/for-medical-professionals/news-publications /news-and-articles/bulletin/september-2022-volume-107-issue-9 /surgeons-face-unique-ergonomic-challenges/
- Wong JMK, Carey ET, King C, et al. A call to action for ergonomic surgical devices designed for diverse surgeon end users. Obstet Gynecol. 2023;141:463-466.
- IHS Inc. The Complexities of Physician Supply and Demand: Projections from 2014 to 2025. Association of American Medical Colleges. April 5, 2016.
- Kim-Fine S, Woolley SM, Weaver AL, et al. Work-related musculoskeletal disorders among vaginal surgeons. Int Urogynecol J. 2013;24:1191-1200.
- Sers R, Forrester S, Zecca M, et al. The ergonomic impact of patient body mass index on surgeon posture during simulated laparoscopy. Appl Ergon. 2021;97:103501.
- Moss EL, Sarhanis P, Ind T, et al. Impact of obesity on surgeon ergonomics in robotic and straight-stick laparoscopic surgery. J Minim Invasive Gynecol. 2020;27:1063-1069.
- Sutton E, Irvin M, Zeigler C, et al. The ergonomics of women in surgery. Surg Endosc. 2014;28:1051-1055.
- Berguer R, Hreljac A. The relationship between hand size and difficulty using surgical instruments: a survey of 726 laparoscopic surgeons. Surg Endosc. 2004;18:508-512.
- Bellini MI, Amabile MI, Saullo P, et al. A woman’s place is in theatre, but are theatres designed with women in mind? A systematic review of ergonomics for women in surgery. J Clin Med. 2022;11:3496.
- Wong JMK, Moore KJ, Lewis P, et al. Ergonomic assessment of surgeon characteristics and laparoscopic device strain in gynecologic surgery. J Minim Invasive Gynecol. 2022;29:1357-1363.
- Aitchison LP, Cui CK, Arnold A, et al. The ergonomics of laparoscopic surgery: a quantitative study of the time and motion of laparoscopic surgeons in live surgical environments. Surg Endosc. 2016;30:5068-5076.
- Stewart C, Raoof M, Fong Y, et al. Who is hurting? A prospective study of surgeon ergonomics. Surg Endosc. 2022;36:292-299.
- Green SV, Morris DE, Naumann DN, et al. One size does not fit all: impact of hand size on ease of use of instruments for minimally invasive surgery. Surgeon. 2022;S1479-666X(22)00131-7.
- Matern U, Faist M, Kehl K, et al. Monitor position in laparoscopic surgery. Surg Endosc. 2005;19:436-440.
- Berguer R, Rab GT, Abu-Ghaida H, et al. A comparison of surgeons’ posture during laparoscopic and open surgical procedures. Surg Endosc. 1997;11:139-142.
- Athanasiadis DI, Monfared S, Asadi H, et al. An analysis of the ergonomic risk of surgical trainees and experienced surgeons during laparoscopic procedures. Surgery. 2021;169:496-501.
- Hotton J, Bogart E, Le Deley MC, et al. Ergonomic assessment of the surgeon’s physical workload during robot-assisted versus standard laparoscopy in a French multicenter randomized trial (ROBOGYN-1004 Trial). Ann Surg Oncol. 2023;30:916-923.
- Plerhoples TA, Hernandez-Boussard T, Wren SM. The aching surgeon: a survey of physical discomfort and symptoms following open, laparoscopic, and robotic surgery. J Robot Surg. 2012;6:65-72.
- Lee GI, Lee MR, Green I, et al. Surgeons’ physical discomfort and symptoms during robotic surgery: a comprehensive ergonomic survey study. Surg Endosc. 2017;31:1697-1706.
- McDonald ME, Ramirez PT, Munsell MF, et al. Physician pain and discomfort during minimally invasive gynecologic cancer surgery. Gynecol Oncol. 2014;134:243-247.
- Zhu X, Yurteri-Kaplan LA, Gutman RE, et al. Postural stress experienced by vaginal surgeons. Proc Hum Factors Ergonomics Soc Annu Meet. 2014;58:763-767.
- American College of Surgeons Division of Education and Surgical Ergonomics Committee. Surgical Ergonomics Recommendations. ACS Education. 2022.
- Cardenas-Trowers O, Kjellsson K, Hatch K. Ergonomics: making the OR a comfortable place. Int Urogynecol J. 2018;29:1065-1066.
- Hokenstad ED, Hallbeck MS, Lowndes BR, et al. Ergonomic robotic console configuration in gynecologic surgery: an interventional study. J Minim Invasive Gynecol. 2021;28:850-859.
- Singh R, Yurteri-Kaplan LA, Morrow MM, et al. Sitting versus standing makes a difference in musculoskeletal discomfort and postural load for surgeons performing vaginal surgery. Int Urogynecol J. 2019;30:231-237.
- Hullfish KL, Trowbridge ER, Bodine G. Ergonomics and gynecologic surgery: “surgeon protect thyself.” J Pelvic Med Surg. 2009;15:435-439.
- Woodburn KL, Kho RM. Vaginal surgery: don’t get bent out of shape. Am J Obstet Gynecol. 2020;223:762-763.
- Hobson DTG, Meriwether KV, Gaskins JT, et al. Learner satisfaction and experience with a high-definition telescopic camera during vaginal procedures: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2021;27:105-111.
- Speed G, Harris K, Keegel T. The effect of cushioning materials on musculoskeletal discomfort and fatigue during prolonged standing at work: a systematic review. Appl Ergon. 2018;70:300-334.
- Haramis G, Rosales JC, Palacios JM, et al. Prospective randomized evaluation of FOOT gel pads for operating room staff COMFORT during laparoscopic renal surgery. Urology. 2010;76:1405-1408.
- Voss RK, Chiang YJ, Cromwell KD, et al. Do no harm, except to ourselves? A survey of symptoms and injuries in oncologic surgeons and pilot study of an intraoperative ergonomic intervention. J Am Coll Surg. 2017;224:16-25.e1.
- Marquetand J, Gabriel J, Seibt R, et al. Ergonomics for surgeons—prototype of an external surgeon support system reduces muscular activity and fatigue. J Electromyogr Kinesiol. 2021;60:102586.
- Tetteh E, Hallbeck MS, Mirka GA. Effects of passive exoskeleton support on EMG measures of the neck, shoulder and trunk muscles while holding simulated surgical postures and performing a simulated surgical procedure. Appl Ergon. 2022;100:103646.
- Lim AK, Ryu J, Yoon HM, et al. Ergonomic effects of medical augmented reality glasses in video-assisted surgery. Surg Endosc. 2022;36:988-998.
- Park AE, Zahiri HR, Hallbeck MS, et al. Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: a multicenter cohort study. Ann Surg. 2017;265:340-346.
- Hallbeck MS, Lowndes BR, Bingener J, et al. The impact of intraoperative microbreaks with exercises on surgeons: a multi-center cohort study. Appl Ergon. 2017;60:334-341.
- Hallbeck Human Factors Engineering Laboratories. OR Stretch Videos. Mayo Clinic, 2018. Accessed May 19, 2023. https://www.mayo .edu/research/labs/human-factors-engineering/or-stretch /or-stretch-videos
- Stork A, Bacon T, Corton M. Prevention of Work-Related Musculoskeletal Disorders in Vaginal Surgery. Video presentation at: Society of Gynecologic Surgeons’ Annual Scientific Meeting 2023, Tucson, AZ. Accessed April 3, 2023. https://sgs.eng.us/category.php?cat=2023 -video-presentations
- Aaron KA, Vaughan J, Gupta R, et al. The risk of ergonomic injury across surgical specialties. PLoS One. 2021;16:e0244868.
- Smith TG, Lowndes BR, Schmida E, et al. Course design and learning outcomes of a practical online ergonomics course for surgical residents. J Surg Educ. 2022;79:1489-1499.
- Franasiak J, Craven R, Mosaly P, et al. Feasibility and acceptance of a robotic surgery ergonomic training program. JSLS. 2014;18:e2014.00166.
- Cerier E, Hu A, Goldring A, et al. Ergonomics workshop improves musculoskeletal symptoms in general surgery residents. J Surg Res. 2022;280:567-574.
- Giagio S, Volpe G, Pillastrini P, et al. A preventive program for workrelated musculoskeletal disorders among surgeons: outcomes of a randomized controlled clinical trial. Ann Surg. 2019;270:969-975.
- Jensen MJ, Liao J, Van Gorp B, et al. Incorporating surgical ergonomics education into surgical residency curriculum. J Surg Educ. 2021;78:1209-1215.
- Workplace health glossary. Reviewed February 12, 2020. Centers for Disease Control and Prevention. Accessed May 18, 2023. https://www.cdc.gov/workplacehealthpromotion/tools-resources /glossary/glossary.html#W
- Epstein S, Sparer EH, Tran BN, et al. Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists: a systematic review and meta-analysis. JAMA Surg. 2018;153:e174947.
- Yurteri-Kaplan LA, Park AJ. Surgical ergonomics and preventing workrelated musculoskeletal disorders. Obstet Gynecol. 2023;141:455-462.
- Symer MM, Keller DS. Human factors in pelvic surgery. Eur J Surg Oncol. 2022;48:2346-2351.
- Franasiak J, Ko EM, Kidd J, et al. Physical strain and urgent need for ergonomic training among gynecologic oncologists who perform minimally invasive surgery. Gynecol Oncol. 2012;126:437-442.
- Davis WT, Fletcher SA, Guillamondegui OD. Musculoskeletal occupational injury among surgeons: effects for patients, providers, and institutions. J Surg Res. 2014;189:207-212.e6.
- Fox M. Surgeons face unique ergonomic challenges. American College of Surgeons. September 1, 2022. Accessed May 22, 2023. https://www.facs.org/for-medical-professionals/news-publications /news-and-articles/bulletin/september-2022-volume-107-issue-9 /surgeons-face-unique-ergonomic-challenges/
- Wong JMK, Carey ET, King C, et al. A call to action for ergonomic surgical devices designed for diverse surgeon end users. Obstet Gynecol. 2023;141:463-466.
- IHS Inc. The Complexities of Physician Supply and Demand: Projections from 2014 to 2025. Association of American Medical Colleges. April 5, 2016.
- Kim-Fine S, Woolley SM, Weaver AL, et al. Work-related musculoskeletal disorders among vaginal surgeons. Int Urogynecol J. 2013;24:1191-1200.
- Sers R, Forrester S, Zecca M, et al. The ergonomic impact of patient body mass index on surgeon posture during simulated laparoscopy. Appl Ergon. 2021;97:103501.
- Moss EL, Sarhanis P, Ind T, et al. Impact of obesity on surgeon ergonomics in robotic and straight-stick laparoscopic surgery. J Minim Invasive Gynecol. 2020;27:1063-1069.
- Sutton E, Irvin M, Zeigler C, et al. The ergonomics of women in surgery. Surg Endosc. 2014;28:1051-1055.
- Berguer R, Hreljac A. The relationship between hand size and difficulty using surgical instruments: a survey of 726 laparoscopic surgeons. Surg Endosc. 2004;18:508-512.
- Bellini MI, Amabile MI, Saullo P, et al. A woman’s place is in theatre, but are theatres designed with women in mind? A systematic review of ergonomics for women in surgery. J Clin Med. 2022;11:3496.
- Wong JMK, Moore KJ, Lewis P, et al. Ergonomic assessment of surgeon characteristics and laparoscopic device strain in gynecologic surgery. J Minim Invasive Gynecol. 2022;29:1357-1363.
- Aitchison LP, Cui CK, Arnold A, et al. The ergonomics of laparoscopic surgery: a quantitative study of the time and motion of laparoscopic surgeons in live surgical environments. Surg Endosc. 2016;30:5068-5076.
- Stewart C, Raoof M, Fong Y, et al. Who is hurting? A prospective study of surgeon ergonomics. Surg Endosc. 2022;36:292-299.
- Green SV, Morris DE, Naumann DN, et al. One size does not fit all: impact of hand size on ease of use of instruments for minimally invasive surgery. Surgeon. 2022;S1479-666X(22)00131-7.
- Matern U, Faist M, Kehl K, et al. Monitor position in laparoscopic surgery. Surg Endosc. 2005;19:436-440.
- Berguer R, Rab GT, Abu-Ghaida H, et al. A comparison of surgeons’ posture during laparoscopic and open surgical procedures. Surg Endosc. 1997;11:139-142.
- Athanasiadis DI, Monfared S, Asadi H, et al. An analysis of the ergonomic risk of surgical trainees and experienced surgeons during laparoscopic procedures. Surgery. 2021;169:496-501.
- Hotton J, Bogart E, Le Deley MC, et al. Ergonomic assessment of the surgeon’s physical workload during robot-assisted versus standard laparoscopy in a French multicenter randomized trial (ROBOGYN-1004 Trial). Ann Surg Oncol. 2023;30:916-923.
- Plerhoples TA, Hernandez-Boussard T, Wren SM. The aching surgeon: a survey of physical discomfort and symptoms following open, laparoscopic, and robotic surgery. J Robot Surg. 2012;6:65-72.
- Lee GI, Lee MR, Green I, et al. Surgeons’ physical discomfort and symptoms during robotic surgery: a comprehensive ergonomic survey study. Surg Endosc. 2017;31:1697-1706.
- McDonald ME, Ramirez PT, Munsell MF, et al. Physician pain and discomfort during minimally invasive gynecologic cancer surgery. Gynecol Oncol. 2014;134:243-247.
- Zhu X, Yurteri-Kaplan LA, Gutman RE, et al. Postural stress experienced by vaginal surgeons. Proc Hum Factors Ergonomics Soc Annu Meet. 2014;58:763-767.
- American College of Surgeons Division of Education and Surgical Ergonomics Committee. Surgical Ergonomics Recommendations. ACS Education. 2022.
- Cardenas-Trowers O, Kjellsson K, Hatch K. Ergonomics: making the OR a comfortable place. Int Urogynecol J. 2018;29:1065-1066.
- Hokenstad ED, Hallbeck MS, Lowndes BR, et al. Ergonomic robotic console configuration in gynecologic surgery: an interventional study. J Minim Invasive Gynecol. 2021;28:850-859.
- Singh R, Yurteri-Kaplan LA, Morrow MM, et al. Sitting versus standing makes a difference in musculoskeletal discomfort and postural load for surgeons performing vaginal surgery. Int Urogynecol J. 2019;30:231-237.
- Hullfish KL, Trowbridge ER, Bodine G. Ergonomics and gynecologic surgery: “surgeon protect thyself.” J Pelvic Med Surg. 2009;15:435-439.
- Woodburn KL, Kho RM. Vaginal surgery: don’t get bent out of shape. Am J Obstet Gynecol. 2020;223:762-763.
- Hobson DTG, Meriwether KV, Gaskins JT, et al. Learner satisfaction and experience with a high-definition telescopic camera during vaginal procedures: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2021;27:105-111.
- Speed G, Harris K, Keegel T. The effect of cushioning materials on musculoskeletal discomfort and fatigue during prolonged standing at work: a systematic review. Appl Ergon. 2018;70:300-334.
- Haramis G, Rosales JC, Palacios JM, et al. Prospective randomized evaluation of FOOT gel pads for operating room staff COMFORT during laparoscopic renal surgery. Urology. 2010;76:1405-1408.
- Voss RK, Chiang YJ, Cromwell KD, et al. Do no harm, except to ourselves? A survey of symptoms and injuries in oncologic surgeons and pilot study of an intraoperative ergonomic intervention. J Am Coll Surg. 2017;224:16-25.e1.
- Marquetand J, Gabriel J, Seibt R, et al. Ergonomics for surgeons—prototype of an external surgeon support system reduces muscular activity and fatigue. J Electromyogr Kinesiol. 2021;60:102586.
- Tetteh E, Hallbeck MS, Mirka GA. Effects of passive exoskeleton support on EMG measures of the neck, shoulder and trunk muscles while holding simulated surgical postures and performing a simulated surgical procedure. Appl Ergon. 2022;100:103646.
- Lim AK, Ryu J, Yoon HM, et al. Ergonomic effects of medical augmented reality glasses in video-assisted surgery. Surg Endosc. 2022;36:988-998.
- Park AE, Zahiri HR, Hallbeck MS, et al. Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: a multicenter cohort study. Ann Surg. 2017;265:340-346.
- Hallbeck MS, Lowndes BR, Bingener J, et al. The impact of intraoperative microbreaks with exercises on surgeons: a multi-center cohort study. Appl Ergon. 2017;60:334-341.
- Hallbeck Human Factors Engineering Laboratories. OR Stretch Videos. Mayo Clinic, 2018. Accessed May 19, 2023. https://www.mayo .edu/research/labs/human-factors-engineering/or-stretch /or-stretch-videos
- Stork A, Bacon T, Corton M. Prevention of Work-Related Musculoskeletal Disorders in Vaginal Surgery. Video presentation at: Society of Gynecologic Surgeons’ Annual Scientific Meeting 2023, Tucson, AZ. Accessed April 3, 2023. https://sgs.eng.us/category.php?cat=2023 -video-presentations
- Aaron KA, Vaughan J, Gupta R, et al. The risk of ergonomic injury across surgical specialties. PLoS One. 2021;16:e0244868.
- Smith TG, Lowndes BR, Schmida E, et al. Course design and learning outcomes of a practical online ergonomics course for surgical residents. J Surg Educ. 2022;79:1489-1499.
- Franasiak J, Craven R, Mosaly P, et al. Feasibility and acceptance of a robotic surgery ergonomic training program. JSLS. 2014;18:e2014.00166.
- Cerier E, Hu A, Goldring A, et al. Ergonomics workshop improves musculoskeletal symptoms in general surgery residents. J Surg Res. 2022;280:567-574.
- Giagio S, Volpe G, Pillastrini P, et al. A preventive program for workrelated musculoskeletal disorders among surgeons: outcomes of a randomized controlled clinical trial. Ann Surg. 2019;270:969-975.
- Jensen MJ, Liao J, Van Gorp B, et al. Incorporating surgical ergonomics education into surgical residency curriculum. J Surg Educ. 2021;78:1209-1215.
Reducing risk for thrombosis in patients with lung cancer
CHICAGO – Having cancer is a known risk factor for thrombosis. A patient with cancer has a fourfold risk of developing venous thromboembolism (VTE).
This risk may be increased by certain cancer drugs, which seems to be the case for the combination of innovative targeted therapies lazertinib-amivantamab, as shown in patients with advanced or metastatic non–small cell lung cancer.
A study of this increased risk of VTE was presented by Nicolas Girard, MD, a respiratory medicine specialist at Curie-Montsouris Chest Center in Paris, during the annual meeting of the American Society of Clinical Oncology.
Combination therapies
Amivantamab is an EGFR and cMET bispecific antibody, and lazertinib is a third-generation EGFR tyrosine kinase inhibitor. Prescribed after osimertinib or after osimertinib plus chemotherapy, this combination of amivantamab and lazertinib has been evaluated in several cohorts of patients with EGFR-mutated advanced non–small cell lung cancer in whom targeted therapy and chemotherapy has failed.
The antitumor activity appears to be improved when both therapies are given in combination. The side effects generally are acceptable. It is these side effects, particularly the rate of VTEs, that Dr. Girard and his colleagues are interested in.
The researchers collated the data from the ongoing CHRYSALIS, CHRYSALIS-2, and LASER201 clinical trials, which assess the efficacy of these new agents as monotherapy or in combination. They initially investigated all reported thrombotic events and ruled out those that occurred during or after the 30 days before disease progression.
Increased thrombosis risk
The analysis included 560 patients who had been given amivantamab as monotherapy, 536 patients who had received amivantamab plus lazertinib in combination, and 252 who had taken lazertinib as monotherapy. The incidence of thromboembolic events was higher among patients who received amivantamab plus lazertinib in combination (21%) than in those who were given amivantamab (11%) or lazertinib (11%) as monotherapy.
The first thromboembolic event occurred an average of 84.5 days after starting treatment with amivantamab, 79 days after starting the combination therapy, and 170 days after starting treatment with lazertinib. For the amivantamab plus lazertinib combination, most VTEs developed in the first 4 months of treatment. The most common VTEs were pulmonary embolism and deep vein thrombosis.
The incidence of severe thrombotic events (grade ≥ 3), which was relatively low (amivantamab, 5%; amivantamab plus lazertinib, 6%; lazertinib, 6%) was similar regardless of the treatment, and there were no grade 5 thrombotic events among patients treated with the combination of both targeted therapies.
The significant risk factors for VTEs identified in this study were being age 60 years or older, having a score of 1 on the ECOG Performance Status Scale, and response to treatment (P < .05).
At a press conference organized by the Institut Curie before the ASCO conference, Dr. Girard said, “There has been shown to be an increased risk of blood clots with the use of this combination of targeted therapies. Preventive measures should therefore also be put forward, such as adding anticoagulant medication. This is an important study for the further development of these therapies.”
In a press release, the respiratory medicine specialist noted, “Institut Curie is particularly alert to the issue of the risk of thrombosis arising in cancer patients.” The DASTO project, dedicated to this issue and headed by the Institut Curie, “aims to cross-reference data sourced from several French cancer centers with data from the social security system to understand the risk factors, improve patient treatment, make changes to the care pathway, and prevent this unwanted occurrence from arising.”
Dr. Girard has direct links with Amgen, AstraZeneca, AbbVie, BMS, Daiichi Sankyo, Ipsen, Janssen, Roche, Lilly, Medtronic, MSD, Novartis, OSE Pharma, Pfizer, Sanofi, and Sivan.
This article was translated from the Medscape French Edition. A version of this article appeared on Medscape.com.
CHICAGO – Having cancer is a known risk factor for thrombosis. A patient with cancer has a fourfold risk of developing venous thromboembolism (VTE).
This risk may be increased by certain cancer drugs, which seems to be the case for the combination of innovative targeted therapies lazertinib-amivantamab, as shown in patients with advanced or metastatic non–small cell lung cancer.
A study of this increased risk of VTE was presented by Nicolas Girard, MD, a respiratory medicine specialist at Curie-Montsouris Chest Center in Paris, during the annual meeting of the American Society of Clinical Oncology.
Combination therapies
Amivantamab is an EGFR and cMET bispecific antibody, and lazertinib is a third-generation EGFR tyrosine kinase inhibitor. Prescribed after osimertinib or after osimertinib plus chemotherapy, this combination of amivantamab and lazertinib has been evaluated in several cohorts of patients with EGFR-mutated advanced non–small cell lung cancer in whom targeted therapy and chemotherapy has failed.
The antitumor activity appears to be improved when both therapies are given in combination. The side effects generally are acceptable. It is these side effects, particularly the rate of VTEs, that Dr. Girard and his colleagues are interested in.
The researchers collated the data from the ongoing CHRYSALIS, CHRYSALIS-2, and LASER201 clinical trials, which assess the efficacy of these new agents as monotherapy or in combination. They initially investigated all reported thrombotic events and ruled out those that occurred during or after the 30 days before disease progression.
Increased thrombosis risk
The analysis included 560 patients who had been given amivantamab as monotherapy, 536 patients who had received amivantamab plus lazertinib in combination, and 252 who had taken lazertinib as monotherapy. The incidence of thromboembolic events was higher among patients who received amivantamab plus lazertinib in combination (21%) than in those who were given amivantamab (11%) or lazertinib (11%) as monotherapy.
The first thromboembolic event occurred an average of 84.5 days after starting treatment with amivantamab, 79 days after starting the combination therapy, and 170 days after starting treatment with lazertinib. For the amivantamab plus lazertinib combination, most VTEs developed in the first 4 months of treatment. The most common VTEs were pulmonary embolism and deep vein thrombosis.
The incidence of severe thrombotic events (grade ≥ 3), which was relatively low (amivantamab, 5%; amivantamab plus lazertinib, 6%; lazertinib, 6%) was similar regardless of the treatment, and there were no grade 5 thrombotic events among patients treated with the combination of both targeted therapies.
The significant risk factors for VTEs identified in this study were being age 60 years or older, having a score of 1 on the ECOG Performance Status Scale, and response to treatment (P < .05).
At a press conference organized by the Institut Curie before the ASCO conference, Dr. Girard said, “There has been shown to be an increased risk of blood clots with the use of this combination of targeted therapies. Preventive measures should therefore also be put forward, such as adding anticoagulant medication. This is an important study for the further development of these therapies.”
In a press release, the respiratory medicine specialist noted, “Institut Curie is particularly alert to the issue of the risk of thrombosis arising in cancer patients.” The DASTO project, dedicated to this issue and headed by the Institut Curie, “aims to cross-reference data sourced from several French cancer centers with data from the social security system to understand the risk factors, improve patient treatment, make changes to the care pathway, and prevent this unwanted occurrence from arising.”
Dr. Girard has direct links with Amgen, AstraZeneca, AbbVie, BMS, Daiichi Sankyo, Ipsen, Janssen, Roche, Lilly, Medtronic, MSD, Novartis, OSE Pharma, Pfizer, Sanofi, and Sivan.
This article was translated from the Medscape French Edition. A version of this article appeared on Medscape.com.
CHICAGO – Having cancer is a known risk factor for thrombosis. A patient with cancer has a fourfold risk of developing venous thromboembolism (VTE).
This risk may be increased by certain cancer drugs, which seems to be the case for the combination of innovative targeted therapies lazertinib-amivantamab, as shown in patients with advanced or metastatic non–small cell lung cancer.
A study of this increased risk of VTE was presented by Nicolas Girard, MD, a respiratory medicine specialist at Curie-Montsouris Chest Center in Paris, during the annual meeting of the American Society of Clinical Oncology.
Combination therapies
Amivantamab is an EGFR and cMET bispecific antibody, and lazertinib is a third-generation EGFR tyrosine kinase inhibitor. Prescribed after osimertinib or after osimertinib plus chemotherapy, this combination of amivantamab and lazertinib has been evaluated in several cohorts of patients with EGFR-mutated advanced non–small cell lung cancer in whom targeted therapy and chemotherapy has failed.
The antitumor activity appears to be improved when both therapies are given in combination. The side effects generally are acceptable. It is these side effects, particularly the rate of VTEs, that Dr. Girard and his colleagues are interested in.
The researchers collated the data from the ongoing CHRYSALIS, CHRYSALIS-2, and LASER201 clinical trials, which assess the efficacy of these new agents as monotherapy or in combination. They initially investigated all reported thrombotic events and ruled out those that occurred during or after the 30 days before disease progression.
Increased thrombosis risk
The analysis included 560 patients who had been given amivantamab as monotherapy, 536 patients who had received amivantamab plus lazertinib in combination, and 252 who had taken lazertinib as monotherapy. The incidence of thromboembolic events was higher among patients who received amivantamab plus lazertinib in combination (21%) than in those who were given amivantamab (11%) or lazertinib (11%) as monotherapy.
The first thromboembolic event occurred an average of 84.5 days after starting treatment with amivantamab, 79 days after starting the combination therapy, and 170 days after starting treatment with lazertinib. For the amivantamab plus lazertinib combination, most VTEs developed in the first 4 months of treatment. The most common VTEs were pulmonary embolism and deep vein thrombosis.
The incidence of severe thrombotic events (grade ≥ 3), which was relatively low (amivantamab, 5%; amivantamab plus lazertinib, 6%; lazertinib, 6%) was similar regardless of the treatment, and there were no grade 5 thrombotic events among patients treated with the combination of both targeted therapies.
The significant risk factors for VTEs identified in this study were being age 60 years or older, having a score of 1 on the ECOG Performance Status Scale, and response to treatment (P < .05).
At a press conference organized by the Institut Curie before the ASCO conference, Dr. Girard said, “There has been shown to be an increased risk of blood clots with the use of this combination of targeted therapies. Preventive measures should therefore also be put forward, such as adding anticoagulant medication. This is an important study for the further development of these therapies.”
In a press release, the respiratory medicine specialist noted, “Institut Curie is particularly alert to the issue of the risk of thrombosis arising in cancer patients.” The DASTO project, dedicated to this issue and headed by the Institut Curie, “aims to cross-reference data sourced from several French cancer centers with data from the social security system to understand the risk factors, improve patient treatment, make changes to the care pathway, and prevent this unwanted occurrence from arising.”
Dr. Girard has direct links with Amgen, AstraZeneca, AbbVie, BMS, Daiichi Sankyo, Ipsen, Janssen, Roche, Lilly, Medtronic, MSD, Novartis, OSE Pharma, Pfizer, Sanofi, and Sivan.
This article was translated from the Medscape French Edition. A version of this article appeared on Medscape.com.
AT ASCO 2023
SGS showcases gyn surgeons’ impact on innovation, education, equity, and enterprise
The theme of the 49th Annual Scientific Meeting of the Society of Gynecologic Surgeons was Impact Factor—an allusion to scientific journal impact factor, as well as how we as gynecologic surgeons have a societal impact through our innovation, education, equity, and enterprise-level efforts. This theme and the diverse roster of speakers and presentations on contemporary and controversial issues impacting today’s gynecologic surgeons clearly resonated, breaking the prior registration record with more than 200 additional attendees than the previous year.
As always, the preconference postgraduate courses delivered relevant content that spanned the educational and surgical spectrum, including: “Innovations in training gynecologic surgeons”; “Urologic surgery for the gynecologic surgeon”; the social media workshop “Gynfluencing: Using social media to find your digital voice”; and “The sim factor: Making an impact in surgical education.” This also marked the first year of offering a specific SGS Fellows/Young Attendings’ course. The featured speaker of the SGS Equity Council was Patty Brisben, philanthropist, CEO, and founder of Pure Romance.
Dr. Beri Ridgeway, Cleveland Clinic Chief of Staff, delivered the Mark D. Walters Lecture, “Surgeon in the C-suite,” on leading approximately 5,000 physicians and the importance of surgeons and specifically ObGyns having a seat at the table. The TeLinde lecturer, Dr. Pam Moalli, Professor and Division Director for Urogynecology at the University of Pittsburgh Magee Womens Hospital, spoke on “Biomaterials for gynecologic surgeons: Toward bioinspired biomimetic devices.” The panel on the “Ergonomics of gynecologic surgery” was moderated by Dr. Amanda Fader and Dr. Kim Kho, who shared their experiences with work-related musculoskeletal injury, and featured esteemed panelists Dr. Noor Abu-Alnadi from UNC, Dr. Sue Hallbeck from Mayo Clinic, and Dr. Ladin Yurteri-Kaplan from Columbia University.
The conference also featured a new format of Ted Med Talks:
- Dr. Jason Wright, Editor-in-Chief, Obstetrics & Gynecology, and Division Director of Gynecologic Oncology at Columbia University, who spoke on “Surgical volume and outcomes for gynecologic surgery: Is more always better?”
- Dr. Kelly Wright, Division Director, Minimally Invasive Gynecologic Surgery, Cedars Sinai, on “Climate change starts at 7:15”
- Dr. Ebony Carter, Associate Editor, Equity, Obstetrics & Gynecology, and Division Director, Maternal Fetal Medicine, Washington University, on “Centering equity in reproductive health research.”
In this special section, several of these talks are presented. Additionally, Dr. Laura Homewood and her coauthors will discuss gender and racial biases in a large multi-institutional sample of more than 15,000 Press Ganey patient satisfaction surveys.
Dr. Cheryl Iglesia, SGS former president, and I hope that you will consider attending #SGS2024 in Orlando, Florida, led by Dr. Suzie As-Sanie, program chair, and Dr. Rosanne Kho, current SGS president, which promises to be another exciting meeting. ●
The theme of the 49th Annual Scientific Meeting of the Society of Gynecologic Surgeons was Impact Factor—an allusion to scientific journal impact factor, as well as how we as gynecologic surgeons have a societal impact through our innovation, education, equity, and enterprise-level efforts. This theme and the diverse roster of speakers and presentations on contemporary and controversial issues impacting today’s gynecologic surgeons clearly resonated, breaking the prior registration record with more than 200 additional attendees than the previous year.
As always, the preconference postgraduate courses delivered relevant content that spanned the educational and surgical spectrum, including: “Innovations in training gynecologic surgeons”; “Urologic surgery for the gynecologic surgeon”; the social media workshop “Gynfluencing: Using social media to find your digital voice”; and “The sim factor: Making an impact in surgical education.” This also marked the first year of offering a specific SGS Fellows/Young Attendings’ course. The featured speaker of the SGS Equity Council was Patty Brisben, philanthropist, CEO, and founder of Pure Romance.
Dr. Beri Ridgeway, Cleveland Clinic Chief of Staff, delivered the Mark D. Walters Lecture, “Surgeon in the C-suite,” on leading approximately 5,000 physicians and the importance of surgeons and specifically ObGyns having a seat at the table. The TeLinde lecturer, Dr. Pam Moalli, Professor and Division Director for Urogynecology at the University of Pittsburgh Magee Womens Hospital, spoke on “Biomaterials for gynecologic surgeons: Toward bioinspired biomimetic devices.” The panel on the “Ergonomics of gynecologic surgery” was moderated by Dr. Amanda Fader and Dr. Kim Kho, who shared their experiences with work-related musculoskeletal injury, and featured esteemed panelists Dr. Noor Abu-Alnadi from UNC, Dr. Sue Hallbeck from Mayo Clinic, and Dr. Ladin Yurteri-Kaplan from Columbia University.
The conference also featured a new format of Ted Med Talks:
- Dr. Jason Wright, Editor-in-Chief, Obstetrics & Gynecology, and Division Director of Gynecologic Oncology at Columbia University, who spoke on “Surgical volume and outcomes for gynecologic surgery: Is more always better?”
- Dr. Kelly Wright, Division Director, Minimally Invasive Gynecologic Surgery, Cedars Sinai, on “Climate change starts at 7:15”
- Dr. Ebony Carter, Associate Editor, Equity, Obstetrics & Gynecology, and Division Director, Maternal Fetal Medicine, Washington University, on “Centering equity in reproductive health research.”
In this special section, several of these talks are presented. Additionally, Dr. Laura Homewood and her coauthors will discuss gender and racial biases in a large multi-institutional sample of more than 15,000 Press Ganey patient satisfaction surveys.
Dr. Cheryl Iglesia, SGS former president, and I hope that you will consider attending #SGS2024 in Orlando, Florida, led by Dr. Suzie As-Sanie, program chair, and Dr. Rosanne Kho, current SGS president, which promises to be another exciting meeting. ●
The theme of the 49th Annual Scientific Meeting of the Society of Gynecologic Surgeons was Impact Factor—an allusion to scientific journal impact factor, as well as how we as gynecologic surgeons have a societal impact through our innovation, education, equity, and enterprise-level efforts. This theme and the diverse roster of speakers and presentations on contemporary and controversial issues impacting today’s gynecologic surgeons clearly resonated, breaking the prior registration record with more than 200 additional attendees than the previous year.
As always, the preconference postgraduate courses delivered relevant content that spanned the educational and surgical spectrum, including: “Innovations in training gynecologic surgeons”; “Urologic surgery for the gynecologic surgeon”; the social media workshop “Gynfluencing: Using social media to find your digital voice”; and “The sim factor: Making an impact in surgical education.” This also marked the first year of offering a specific SGS Fellows/Young Attendings’ course. The featured speaker of the SGS Equity Council was Patty Brisben, philanthropist, CEO, and founder of Pure Romance.
Dr. Beri Ridgeway, Cleveland Clinic Chief of Staff, delivered the Mark D. Walters Lecture, “Surgeon in the C-suite,” on leading approximately 5,000 physicians and the importance of surgeons and specifically ObGyns having a seat at the table. The TeLinde lecturer, Dr. Pam Moalli, Professor and Division Director for Urogynecology at the University of Pittsburgh Magee Womens Hospital, spoke on “Biomaterials for gynecologic surgeons: Toward bioinspired biomimetic devices.” The panel on the “Ergonomics of gynecologic surgery” was moderated by Dr. Amanda Fader and Dr. Kim Kho, who shared their experiences with work-related musculoskeletal injury, and featured esteemed panelists Dr. Noor Abu-Alnadi from UNC, Dr. Sue Hallbeck from Mayo Clinic, and Dr. Ladin Yurteri-Kaplan from Columbia University.
The conference also featured a new format of Ted Med Talks:
- Dr. Jason Wright, Editor-in-Chief, Obstetrics & Gynecology, and Division Director of Gynecologic Oncology at Columbia University, who spoke on “Surgical volume and outcomes for gynecologic surgery: Is more always better?”
- Dr. Kelly Wright, Division Director, Minimally Invasive Gynecologic Surgery, Cedars Sinai, on “Climate change starts at 7:15”
- Dr. Ebony Carter, Associate Editor, Equity, Obstetrics & Gynecology, and Division Director, Maternal Fetal Medicine, Washington University, on “Centering equity in reproductive health research.”
In this special section, several of these talks are presented. Additionally, Dr. Laura Homewood and her coauthors will discuss gender and racial biases in a large multi-institutional sample of more than 15,000 Press Ganey patient satisfaction surveys.
Dr. Cheryl Iglesia, SGS former president, and I hope that you will consider attending #SGS2024 in Orlando, Florida, led by Dr. Suzie As-Sanie, program chair, and Dr. Rosanne Kho, current SGS president, which promises to be another exciting meeting. ●
FDA OKs empagliflozin for children with type 2 diabetes
aged 10 years and older.
This approval represents only the second oral treatment option for children and adolescents with type 2 diabetes after metformin; the latter appears to be less effective for pediatric patients than for adults.
Injectable glucagonlike peptide–1 (GLP-1) agonists are also available for youth with type 2 diabetes. These include daily liraglutide (Victoza) and once-weekly extended-release exenatide (Bydureon/Bydureon BCise).
Jardiance has been approved for adults with type 2 diabetes since 2014, and Synjardy has been approved since 2015.
“Compared to adults, children with type 2 diabetes have limited treatment options, even though the disease and symptom onset generally progress more rapidly in children,” said Michelle Carey, MD, MPH.
“Today’s approvals provide much-needed additional treatment options for children with type 2 diabetes,” added Dr. Carey, associate director for therapeutic review for the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research.
Type 2 diabetes rising exponentially in children, mainly non-Whites
Type 2 diabetes is rising exponentially in children and adolescents in the United States.
Data from the SEARCH for Diabetes in Youth study show that the incidence of type 2 diabetes among youth rose by about 5% per year between 2002 and 2015, and it continues to rise.
A more recent study found that a doubling of cases occurred during the pandemic, with youth often presenting with more severe disease. The majority of cases are among non-White racial groups.
Safety and efficacy data for empagliflozin for children came from the Diabetes Study of Linagliptin and Empagliflozin in Children and Adolescents (DINAMO) trial. That trial included 157 patients aged 10-17 years with A1c of 7% or above. Patients were randomly assigned to receive empagliflozin 10 mg or 25 mg daily, linagliptin (a DPP-4 inhibitor) 5 mg, or placebo for 26 weeks. Over 90% were also taking metformin, 40% in combination with insulin. All patients were given diet and exercise advice.
At week 26, the children treated with empagliflozin showed an average 0.2 percentage point decrease in A1c, compared with a 0.7-point increase among those taking placebo. Use of empagliflozin was also associated with lower fasting plasma glucose levels compared with placebo.
Side effects were similar to those seen in adults except for a higher risk of hypoglycemia, regardless of other glucose-lowering therapies that were being taken.
Reduction in A1c for participants treated with linagliptin was not statistically significant in comparison with placebo. There was a numerical reduction of 0.34% (P = .2935).
“Across the lifespan, we know that people living with type 2 diabetes have a high risk for many diabetes complications, so it’s important to recognize and treat diabetes early in its course,” Lori Laffel, MD, lead investigator of the DINAMO study, said in a press release from BI.
“These findings are particularly important given the need for more therapeutic options, especially oral agents, to manage type 2 diabetes in young people as, to date, metformin [has been] the only globally available oral treatment for youth,” added Dr. Laffel, chief of the pediatric, adolescent, and young adult section at the Joslin Diabetes Center and professor of pediatrics at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
aged 10 years and older.
This approval represents only the second oral treatment option for children and adolescents with type 2 diabetes after metformin; the latter appears to be less effective for pediatric patients than for adults.
Injectable glucagonlike peptide–1 (GLP-1) agonists are also available for youth with type 2 diabetes. These include daily liraglutide (Victoza) and once-weekly extended-release exenatide (Bydureon/Bydureon BCise).
Jardiance has been approved for adults with type 2 diabetes since 2014, and Synjardy has been approved since 2015.
“Compared to adults, children with type 2 diabetes have limited treatment options, even though the disease and symptom onset generally progress more rapidly in children,” said Michelle Carey, MD, MPH.
“Today’s approvals provide much-needed additional treatment options for children with type 2 diabetes,” added Dr. Carey, associate director for therapeutic review for the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research.
Type 2 diabetes rising exponentially in children, mainly non-Whites
Type 2 diabetes is rising exponentially in children and adolescents in the United States.
Data from the SEARCH for Diabetes in Youth study show that the incidence of type 2 diabetes among youth rose by about 5% per year between 2002 and 2015, and it continues to rise.
A more recent study found that a doubling of cases occurred during the pandemic, with youth often presenting with more severe disease. The majority of cases are among non-White racial groups.
Safety and efficacy data for empagliflozin for children came from the Diabetes Study of Linagliptin and Empagliflozin in Children and Adolescents (DINAMO) trial. That trial included 157 patients aged 10-17 years with A1c of 7% or above. Patients were randomly assigned to receive empagliflozin 10 mg or 25 mg daily, linagliptin (a DPP-4 inhibitor) 5 mg, or placebo for 26 weeks. Over 90% were also taking metformin, 40% in combination with insulin. All patients were given diet and exercise advice.
At week 26, the children treated with empagliflozin showed an average 0.2 percentage point decrease in A1c, compared with a 0.7-point increase among those taking placebo. Use of empagliflozin was also associated with lower fasting plasma glucose levels compared with placebo.
Side effects were similar to those seen in adults except for a higher risk of hypoglycemia, regardless of other glucose-lowering therapies that were being taken.
Reduction in A1c for participants treated with linagliptin was not statistically significant in comparison with placebo. There was a numerical reduction of 0.34% (P = .2935).
“Across the lifespan, we know that people living with type 2 diabetes have a high risk for many diabetes complications, so it’s important to recognize and treat diabetes early in its course,” Lori Laffel, MD, lead investigator of the DINAMO study, said in a press release from BI.
“These findings are particularly important given the need for more therapeutic options, especially oral agents, to manage type 2 diabetes in young people as, to date, metformin [has been] the only globally available oral treatment for youth,” added Dr. Laffel, chief of the pediatric, adolescent, and young adult section at the Joslin Diabetes Center and professor of pediatrics at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
aged 10 years and older.
This approval represents only the second oral treatment option for children and adolescents with type 2 diabetes after metformin; the latter appears to be less effective for pediatric patients than for adults.
Injectable glucagonlike peptide–1 (GLP-1) agonists are also available for youth with type 2 diabetes. These include daily liraglutide (Victoza) and once-weekly extended-release exenatide (Bydureon/Bydureon BCise).
Jardiance has been approved for adults with type 2 diabetes since 2014, and Synjardy has been approved since 2015.
“Compared to adults, children with type 2 diabetes have limited treatment options, even though the disease and symptom onset generally progress more rapidly in children,” said Michelle Carey, MD, MPH.
“Today’s approvals provide much-needed additional treatment options for children with type 2 diabetes,” added Dr. Carey, associate director for therapeutic review for the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research.
Type 2 diabetes rising exponentially in children, mainly non-Whites
Type 2 diabetes is rising exponentially in children and adolescents in the United States.
Data from the SEARCH for Diabetes in Youth study show that the incidence of type 2 diabetes among youth rose by about 5% per year between 2002 and 2015, and it continues to rise.
A more recent study found that a doubling of cases occurred during the pandemic, with youth often presenting with more severe disease. The majority of cases are among non-White racial groups.
Safety and efficacy data for empagliflozin for children came from the Diabetes Study of Linagliptin and Empagliflozin in Children and Adolescents (DINAMO) trial. That trial included 157 patients aged 10-17 years with A1c of 7% or above. Patients were randomly assigned to receive empagliflozin 10 mg or 25 mg daily, linagliptin (a DPP-4 inhibitor) 5 mg, or placebo for 26 weeks. Over 90% were also taking metformin, 40% in combination with insulin. All patients were given diet and exercise advice.
At week 26, the children treated with empagliflozin showed an average 0.2 percentage point decrease in A1c, compared with a 0.7-point increase among those taking placebo. Use of empagliflozin was also associated with lower fasting plasma glucose levels compared with placebo.
Side effects were similar to those seen in adults except for a higher risk of hypoglycemia, regardless of other glucose-lowering therapies that were being taken.
Reduction in A1c for participants treated with linagliptin was not statistically significant in comparison with placebo. There was a numerical reduction of 0.34% (P = .2935).
“Across the lifespan, we know that people living with type 2 diabetes have a high risk for many diabetes complications, so it’s important to recognize and treat diabetes early in its course,” Lori Laffel, MD, lead investigator of the DINAMO study, said in a press release from BI.
“These findings are particularly important given the need for more therapeutic options, especially oral agents, to manage type 2 diabetes in young people as, to date, metformin [has been] the only globally available oral treatment for youth,” added Dr. Laffel, chief of the pediatric, adolescent, and young adult section at the Joslin Diabetes Center and professor of pediatrics at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
Few of those eligible get lung cancer screening, despite USPSTF recommendations
Only 12.8% of eligible adults get CT screening for lung cancer, despite recommendations from the U.S. Preventive Services Task Force.
Kristin G. Maki, PhD, with Karmanos Cancer Institute, Wayne State University, Detroit, led a team that estimated lung cancer screening (LCS) from the 2021 Behavioral Risk Factor Surveillance System in four states (Maine, Michigan, New Jersey, and Rhode Island).
“Increasing LCS among eligible adults is a national priority,” the authors wrote in the study, published online in JAMA Network Open. Lung cancer remains the top cause of cancer in the United States and smoking accounts for approximately 90% of cases.
Screening much higher for other cancers
The authors pointed out that screening rates for eligible people are much higher for other cancers. Melzer and colleagues wrote in a 2021 editorial that breast and colon cancer screening rates are near 70% “despite combined annual death rates less than two-thirds that of lung cancer.”
The USPSTF updated its recommendations for lung cancer screening in March 2021.
Eligibility now includes anyone aged between 50 and 80 years who has smoked at least 20 pack-years and either still smokes or quit within the last 15 years.
The researchers found that, when comparing screening by health status, the highest odds for screening were seen in those who reported they were in poor health, which is concerning, the authors note, because those patients may not be healthy enough to benefit from treatment for their lung cancer.
The odds ratio for getting screening was 2.88 (95% confidence interval, 0.85-9.77) times higher than that of the reference group, which reported excellent health.
Rates differ by state
Consistent with previous studies, this analysis found that screening rates differed by state. Their analysis, for example, showed a higher likelihood of screening for respondents in Rhode Island, compared with Maine (OR, 1.96; 95% CI, 1.05-3.67; P = .03).
Patients who reported having a primary health professional were more than five times more likely to undergo screening, compared with those without one (OR, 5.62; 95% CI, 1.19-26.49).
The authors said their results also highlight the need for Medicare coverage for screening as those with public insurance had lower odds of screening than those with private insurance (OR, 0.81; 95% CI, 0.42-1.56).
Neelima Navuluri, MD, assistant professor in the division of pulmonary, allergy, and critical care at Duke University and the Duke Global Health Institute, both in Durham, N.C., pointed out that the study highlights age, smoking status, and health care access as key factors associated with lack of uptake.
Work needed on all levels
Dr. Navuluri said in an interview that multifaceted patient-, provider- and system-level interventions are needed to improve screening rates.
“For example, we need more community engagement to increase knowledge and awareness of eligibility for lung cancer screening,” she said.
She highlighted the need for interventions around improving and streamlining shared decision-making conversations about screening (a CMS requirement that does not exist for other cancer screening).
Emphasis is needed on younger age groups, people who currently smoke, and communities of color as well as policy to improve insurance coverage of screening, she said.
Dr. Navuluri, who also works with the Durham Veterans Affairs Medical Center, was lead author on a study published in JAMA Network Open on racial disparities in screening among veterans.
“We demonstrate similar findings related to age, smoking status, and poor health status,” she said. “We discuss the need for more qualitative studies to better understand the role of these factors as well as implementation studies to assess effectiveness of various interventions to improve disparities in lung cancer screening rates.”
“Research to identify facilitators for LCS among persons who currently smoke is needed, including a focus on the role of stigma as a barrier to screening,” they wrote.
One coauthor is supported by the cancer prevention and research training program at the University of Texas MD Anderson Cancer Center and the Cancer Prevention and Research Institute of Texas. No other disclosures were reported. Dr. Navuluri receives funding from the National Comprehensive Cancer Network for work on lung cancer screening.
Only 12.8% of eligible adults get CT screening for lung cancer, despite recommendations from the U.S. Preventive Services Task Force.
Kristin G. Maki, PhD, with Karmanos Cancer Institute, Wayne State University, Detroit, led a team that estimated lung cancer screening (LCS) from the 2021 Behavioral Risk Factor Surveillance System in four states (Maine, Michigan, New Jersey, and Rhode Island).
“Increasing LCS among eligible adults is a national priority,” the authors wrote in the study, published online in JAMA Network Open. Lung cancer remains the top cause of cancer in the United States and smoking accounts for approximately 90% of cases.
Screening much higher for other cancers
The authors pointed out that screening rates for eligible people are much higher for other cancers. Melzer and colleagues wrote in a 2021 editorial that breast and colon cancer screening rates are near 70% “despite combined annual death rates less than two-thirds that of lung cancer.”
The USPSTF updated its recommendations for lung cancer screening in March 2021.
Eligibility now includes anyone aged between 50 and 80 years who has smoked at least 20 pack-years and either still smokes or quit within the last 15 years.
The researchers found that, when comparing screening by health status, the highest odds for screening were seen in those who reported they were in poor health, which is concerning, the authors note, because those patients may not be healthy enough to benefit from treatment for their lung cancer.
The odds ratio for getting screening was 2.88 (95% confidence interval, 0.85-9.77) times higher than that of the reference group, which reported excellent health.
Rates differ by state
Consistent with previous studies, this analysis found that screening rates differed by state. Their analysis, for example, showed a higher likelihood of screening for respondents in Rhode Island, compared with Maine (OR, 1.96; 95% CI, 1.05-3.67; P = .03).
Patients who reported having a primary health professional were more than five times more likely to undergo screening, compared with those without one (OR, 5.62; 95% CI, 1.19-26.49).
The authors said their results also highlight the need for Medicare coverage for screening as those with public insurance had lower odds of screening than those with private insurance (OR, 0.81; 95% CI, 0.42-1.56).
Neelima Navuluri, MD, assistant professor in the division of pulmonary, allergy, and critical care at Duke University and the Duke Global Health Institute, both in Durham, N.C., pointed out that the study highlights age, smoking status, and health care access as key factors associated with lack of uptake.
Work needed on all levels
Dr. Navuluri said in an interview that multifaceted patient-, provider- and system-level interventions are needed to improve screening rates.
“For example, we need more community engagement to increase knowledge and awareness of eligibility for lung cancer screening,” she said.
She highlighted the need for interventions around improving and streamlining shared decision-making conversations about screening (a CMS requirement that does not exist for other cancer screening).
Emphasis is needed on younger age groups, people who currently smoke, and communities of color as well as policy to improve insurance coverage of screening, she said.
Dr. Navuluri, who also works with the Durham Veterans Affairs Medical Center, was lead author on a study published in JAMA Network Open on racial disparities in screening among veterans.
“We demonstrate similar findings related to age, smoking status, and poor health status,” she said. “We discuss the need for more qualitative studies to better understand the role of these factors as well as implementation studies to assess effectiveness of various interventions to improve disparities in lung cancer screening rates.”
“Research to identify facilitators for LCS among persons who currently smoke is needed, including a focus on the role of stigma as a barrier to screening,” they wrote.
One coauthor is supported by the cancer prevention and research training program at the University of Texas MD Anderson Cancer Center and the Cancer Prevention and Research Institute of Texas. No other disclosures were reported. Dr. Navuluri receives funding from the National Comprehensive Cancer Network for work on lung cancer screening.
Only 12.8% of eligible adults get CT screening for lung cancer, despite recommendations from the U.S. Preventive Services Task Force.
Kristin G. Maki, PhD, with Karmanos Cancer Institute, Wayne State University, Detroit, led a team that estimated lung cancer screening (LCS) from the 2021 Behavioral Risk Factor Surveillance System in four states (Maine, Michigan, New Jersey, and Rhode Island).
“Increasing LCS among eligible adults is a national priority,” the authors wrote in the study, published online in JAMA Network Open. Lung cancer remains the top cause of cancer in the United States and smoking accounts for approximately 90% of cases.
Screening much higher for other cancers
The authors pointed out that screening rates for eligible people are much higher for other cancers. Melzer and colleagues wrote in a 2021 editorial that breast and colon cancer screening rates are near 70% “despite combined annual death rates less than two-thirds that of lung cancer.”
The USPSTF updated its recommendations for lung cancer screening in March 2021.
Eligibility now includes anyone aged between 50 and 80 years who has smoked at least 20 pack-years and either still smokes or quit within the last 15 years.
The researchers found that, when comparing screening by health status, the highest odds for screening were seen in those who reported they were in poor health, which is concerning, the authors note, because those patients may not be healthy enough to benefit from treatment for their lung cancer.
The odds ratio for getting screening was 2.88 (95% confidence interval, 0.85-9.77) times higher than that of the reference group, which reported excellent health.
Rates differ by state
Consistent with previous studies, this analysis found that screening rates differed by state. Their analysis, for example, showed a higher likelihood of screening for respondents in Rhode Island, compared with Maine (OR, 1.96; 95% CI, 1.05-3.67; P = .03).
Patients who reported having a primary health professional were more than five times more likely to undergo screening, compared with those without one (OR, 5.62; 95% CI, 1.19-26.49).
The authors said their results also highlight the need for Medicare coverage for screening as those with public insurance had lower odds of screening than those with private insurance (OR, 0.81; 95% CI, 0.42-1.56).
Neelima Navuluri, MD, assistant professor in the division of pulmonary, allergy, and critical care at Duke University and the Duke Global Health Institute, both in Durham, N.C., pointed out that the study highlights age, smoking status, and health care access as key factors associated with lack of uptake.
Work needed on all levels
Dr. Navuluri said in an interview that multifaceted patient-, provider- and system-level interventions are needed to improve screening rates.
“For example, we need more community engagement to increase knowledge and awareness of eligibility for lung cancer screening,” she said.
She highlighted the need for interventions around improving and streamlining shared decision-making conversations about screening (a CMS requirement that does not exist for other cancer screening).
Emphasis is needed on younger age groups, people who currently smoke, and communities of color as well as policy to improve insurance coverage of screening, she said.
Dr. Navuluri, who also works with the Durham Veterans Affairs Medical Center, was lead author on a study published in JAMA Network Open on racial disparities in screening among veterans.
“We demonstrate similar findings related to age, smoking status, and poor health status,” she said. “We discuss the need for more qualitative studies to better understand the role of these factors as well as implementation studies to assess effectiveness of various interventions to improve disparities in lung cancer screening rates.”
“Research to identify facilitators for LCS among persons who currently smoke is needed, including a focus on the role of stigma as a barrier to screening,” they wrote.
One coauthor is supported by the cancer prevention and research training program at the University of Texas MD Anderson Cancer Center and the Cancer Prevention and Research Institute of Texas. No other disclosures were reported. Dr. Navuluri receives funding from the National Comprehensive Cancer Network for work on lung cancer screening.
FROM JAMA NETWORK OPEN
Final USPSTF recommendations on anxiety, depression, suicide risk
In line with draft recommendations, the task force for the first time has endorsed screening for anxiety disorders in all adults younger than age 65 without recognized signs or symptoms of anxiety.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit. There currently is not enough evidence to recommend for or against screening for anxiety disorders in adults 65 and older, the task force said.
The USPSTF final recommendation statements and corresponding evidence summaries were published online in the Journal of the American Medical Association, as well as on the task force website.
Jury out on screening for suicide risk
The task force continues to recommend screening all adults for depression. This “B” recommendation reflects moderate-certainty evidence that screening for major depression in adults has a moderate net benefit.
However, there is not enough evidence to recommend for or against screening for suicide risk in all adults. Therefore, the task issued an “I” statement, indicating that the balance of benefits and harms cannot be determined at present.
“We are urgently calling for more research to determine the effectiveness of screening all adults for suicide risk and screening adults 65 and older for anxiety disorders,” task force member Gbenga Ogedegbe, MD, MPH, founding director of the Institute for Excellence in Health Equity at NYU Langone Health, New York, said in a statement.
The authors of an accompanying editorial noted that a positive screen result for anxiety “should be immediately followed with clinical evaluation for suicidality”.
Murray Stein, MD, MPH, and Linda Hill, MD, MPH, both with University of California, San Diego, also noted that a positive screen for anxiety could be indicative of posttraumatic stress disorder (PTSD) and clinicians should “be prepared to follow up with requisite questions about traumatic experiences that will be needed to home in on a diagnosis of PTSD that may require additional follow-up, referral, or both.
“Anxiety disorders can be distressing and disabling, and appropriate recognition and treatment can be life-altering and, in some cases, lifesaving, for patients,” Dr. Stein and Dr. Hill pointed out.
Effective, evidence-based psychological and pharmacologic treatments for anxiety disorders are available, they added. But the recommendation to routinely screen for anxiety disorder “must be accompanied by the recognition that there are too few mental health specialists available to manage the care of all patients with anxiety disorders, and even fewer who provide services for low-income and non-English-speaking populations,” they wrote.
This research report received no commercial funding. Disclosures for task force members and editorial writers are listed with the original articles.
A version of this article originally appeared on Medscape.com.
In line with draft recommendations, the task force for the first time has endorsed screening for anxiety disorders in all adults younger than age 65 without recognized signs or symptoms of anxiety.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit. There currently is not enough evidence to recommend for or against screening for anxiety disorders in adults 65 and older, the task force said.
The USPSTF final recommendation statements and corresponding evidence summaries were published online in the Journal of the American Medical Association, as well as on the task force website.
Jury out on screening for suicide risk
The task force continues to recommend screening all adults for depression. This “B” recommendation reflects moderate-certainty evidence that screening for major depression in adults has a moderate net benefit.
However, there is not enough evidence to recommend for or against screening for suicide risk in all adults. Therefore, the task issued an “I” statement, indicating that the balance of benefits and harms cannot be determined at present.
“We are urgently calling for more research to determine the effectiveness of screening all adults for suicide risk and screening adults 65 and older for anxiety disorders,” task force member Gbenga Ogedegbe, MD, MPH, founding director of the Institute for Excellence in Health Equity at NYU Langone Health, New York, said in a statement.
The authors of an accompanying editorial noted that a positive screen result for anxiety “should be immediately followed with clinical evaluation for suicidality”.
Murray Stein, MD, MPH, and Linda Hill, MD, MPH, both with University of California, San Diego, also noted that a positive screen for anxiety could be indicative of posttraumatic stress disorder (PTSD) and clinicians should “be prepared to follow up with requisite questions about traumatic experiences that will be needed to home in on a diagnosis of PTSD that may require additional follow-up, referral, or both.
“Anxiety disorders can be distressing and disabling, and appropriate recognition and treatment can be life-altering and, in some cases, lifesaving, for patients,” Dr. Stein and Dr. Hill pointed out.
Effective, evidence-based psychological and pharmacologic treatments for anxiety disorders are available, they added. But the recommendation to routinely screen for anxiety disorder “must be accompanied by the recognition that there are too few mental health specialists available to manage the care of all patients with anxiety disorders, and even fewer who provide services for low-income and non-English-speaking populations,” they wrote.
This research report received no commercial funding. Disclosures for task force members and editorial writers are listed with the original articles.
A version of this article originally appeared on Medscape.com.
In line with draft recommendations, the task force for the first time has endorsed screening for anxiety disorders in all adults younger than age 65 without recognized signs or symptoms of anxiety.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit. There currently is not enough evidence to recommend for or against screening for anxiety disorders in adults 65 and older, the task force said.
The USPSTF final recommendation statements and corresponding evidence summaries were published online in the Journal of the American Medical Association, as well as on the task force website.
Jury out on screening for suicide risk
The task force continues to recommend screening all adults for depression. This “B” recommendation reflects moderate-certainty evidence that screening for major depression in adults has a moderate net benefit.
However, there is not enough evidence to recommend for or against screening for suicide risk in all adults. Therefore, the task issued an “I” statement, indicating that the balance of benefits and harms cannot be determined at present.
“We are urgently calling for more research to determine the effectiveness of screening all adults for suicide risk and screening adults 65 and older for anxiety disorders,” task force member Gbenga Ogedegbe, MD, MPH, founding director of the Institute for Excellence in Health Equity at NYU Langone Health, New York, said in a statement.
The authors of an accompanying editorial noted that a positive screen result for anxiety “should be immediately followed with clinical evaluation for suicidality”.
Murray Stein, MD, MPH, and Linda Hill, MD, MPH, both with University of California, San Diego, also noted that a positive screen for anxiety could be indicative of posttraumatic stress disorder (PTSD) and clinicians should “be prepared to follow up with requisite questions about traumatic experiences that will be needed to home in on a diagnosis of PTSD that may require additional follow-up, referral, or both.
“Anxiety disorders can be distressing and disabling, and appropriate recognition and treatment can be life-altering and, in some cases, lifesaving, for patients,” Dr. Stein and Dr. Hill pointed out.
Effective, evidence-based psychological and pharmacologic treatments for anxiety disorders are available, they added. But the recommendation to routinely screen for anxiety disorder “must be accompanied by the recognition that there are too few mental health specialists available to manage the care of all patients with anxiety disorders, and even fewer who provide services for low-income and non-English-speaking populations,” they wrote.
This research report received no commercial funding. Disclosures for task force members and editorial writers are listed with the original articles.
A version of this article originally appeared on Medscape.com.
Race and ethnicity loom large in CRC screening
Disparities in colorectal screening represent a serious public health challenge, say the authors of a new literature review that describes specific areas of concern and recommendations for improvement.
For their research, published in Techniques and Innovations in Gastrointestinal Endoscopy, gastroenterologists Abraham Segura, MD, and Shazia Mehmood Siddique, MD, of the University of Pennsylvania, Philadelphia, sought to identify studies that shed light on ethnicity or race-based differences in screening uptake, as well as known barriers and facilitators to screening.
Significant racial and ethnic disparities can be seen in rates of colonoscopy selection as a screening method, and of screening completion, Dr. Segura and Dr. Siddique noted, with White individuals who chose the method three times more likely to complete screening as Asian, Hispanic, or Black individuals. Disparities were also seen reflected in people’s choice of screening method, with non–English-speaking Hispanic individuals less likely to choose colonoscopy compared with other groups.
Use of stool-based screening methods, such as the fecal occult blood test (FOBT) and fecal immunochemical test (FIT), has risen over time across ethnic and racial groups. However, Hispanic and Asian individuals were more likely to complete and adhere to the FOBT, compared with non-Hispanic White individuals. Follow-up colonoscopy rates after FOBT or FIT also differ along ethnic and racial lines, Dr. Segura and Dr. Siddique noted, with Asian and American Indian groups less likely to complete follow-up after an abnormal result.
The study authors pointed to structural racism at the root of some observed disparities, citing barriers to healthcare access and quality that include higher rates of noninsurance among Black and Hispanic populations and a lower likelihood of the same populations to receive physician counseling regarding screening.
Barriers to economic stability, including living in impoverished neighborhoods, were also cited as contributors to lower colorectal screening. Patients covered by Medicaid were more than twice as likely as non-Medicaid patients to have suboptimal bowel preparation at screening, the authors noted. Access to transportation remained another frequently observed barrier to completing recommended testing and follow-up.
Mistrust of doctors has been linked to lower screening uptake among Black men. “Longstanding conscious and implicit racism, differences in communication, and socioeconomic context ... engender medical mistrust among racial and ethnic groups,” the authors wrote. Reversing it “ultimately requires vast societal change, and we as physicians can facilitate this by encouraging patient-centered discussions that humanize and empower traditionally marginalized populations.”
Dr. Segura and Dr. Siddique described strategies that have been shown to result in better uptake in specific populations, including removing out-of-pocket costs for screening and follow-up, and designing faith-based or culturally specific outreach delivered through churches and local businesses.
They recommended that researchers change how they study the disparities that bear on colorectal screening and outcomes. “Collection and use of data on race and ethnicity must be optimized and standardized to ensure that all groups are adequately captured,” they wrote. Standardizing self-reporting of race and ethnicity would help address issues of misclassification.
The authors also advised designing studies with longer follow-up, noting that “we must better understand the mechanisms of long-term adherence.” Additional research is needed, they said, to evaluate the efficacy of older outreach strategies after societal changes resulting from the COVID-19 pandemic. Efforts to increase the number of Black, Hispanic, Asian, and Alaskan Native/American Indian groups in CRC screening interventions and studies “must be prioritized.”
Dr. Segura’s and Dr. Siddique’s study was funded with grants from the National Institutes of Health. They disclosed no conflicts of interest.
Understanding disparities in medicine is the requisite first step toward achieving health equity. The review by Segura and Siddique highlight reasons for health disparities in colorectal cancer (CRC) screening, and propose some solutions.
Issues such as structural racism, socioeconomic status and lack of health insurance need to be addressed at the societal level. Recent elimination of cost-sharing for colonoscopy after a positive noninvasive screening test, and elimination of cost-sharing for screening exams with polypectomy, reduce financial barriers for those patients who have health care insurance and Medicare.
How can practitioners apply this information? Recognition of implicit bias among health care workers is an essential first step toward achieving equity. Providing equitable access to CRC screening works. In a study from Kaiser Permanente, disparities in CRC outcomes between non-Hispanic White versus Black patients, were eliminated within 10 years after implementing an annual mailed fecal immunochemical test kit. This is an exciting proof of principle – physicians and health care organizations can reduce health disparities.
David Lieberman, MD, professor of medicine and formerly chief of the division of gastroenterology and hepatology (1997-2021), Oregon Health and Science University, Portland. Dr. Lieberman does not have any relevant disclosures.
Understanding disparities in medicine is the requisite first step toward achieving health equity. The review by Segura and Siddique highlight reasons for health disparities in colorectal cancer (CRC) screening, and propose some solutions.
Issues such as structural racism, socioeconomic status and lack of health insurance need to be addressed at the societal level. Recent elimination of cost-sharing for colonoscopy after a positive noninvasive screening test, and elimination of cost-sharing for screening exams with polypectomy, reduce financial barriers for those patients who have health care insurance and Medicare.
How can practitioners apply this information? Recognition of implicit bias among health care workers is an essential first step toward achieving equity. Providing equitable access to CRC screening works. In a study from Kaiser Permanente, disparities in CRC outcomes between non-Hispanic White versus Black patients, were eliminated within 10 years after implementing an annual mailed fecal immunochemical test kit. This is an exciting proof of principle – physicians and health care organizations can reduce health disparities.
David Lieberman, MD, professor of medicine and formerly chief of the division of gastroenterology and hepatology (1997-2021), Oregon Health and Science University, Portland. Dr. Lieberman does not have any relevant disclosures.
Understanding disparities in medicine is the requisite first step toward achieving health equity. The review by Segura and Siddique highlight reasons for health disparities in colorectal cancer (CRC) screening, and propose some solutions.
Issues such as structural racism, socioeconomic status and lack of health insurance need to be addressed at the societal level. Recent elimination of cost-sharing for colonoscopy after a positive noninvasive screening test, and elimination of cost-sharing for screening exams with polypectomy, reduce financial barriers for those patients who have health care insurance and Medicare.
How can practitioners apply this information? Recognition of implicit bias among health care workers is an essential first step toward achieving equity. Providing equitable access to CRC screening works. In a study from Kaiser Permanente, disparities in CRC outcomes between non-Hispanic White versus Black patients, were eliminated within 10 years after implementing an annual mailed fecal immunochemical test kit. This is an exciting proof of principle – physicians and health care organizations can reduce health disparities.
David Lieberman, MD, professor of medicine and formerly chief of the division of gastroenterology and hepatology (1997-2021), Oregon Health and Science University, Portland. Dr. Lieberman does not have any relevant disclosures.
Disparities in colorectal screening represent a serious public health challenge, say the authors of a new literature review that describes specific areas of concern and recommendations for improvement.
For their research, published in Techniques and Innovations in Gastrointestinal Endoscopy, gastroenterologists Abraham Segura, MD, and Shazia Mehmood Siddique, MD, of the University of Pennsylvania, Philadelphia, sought to identify studies that shed light on ethnicity or race-based differences in screening uptake, as well as known barriers and facilitators to screening.
Significant racial and ethnic disparities can be seen in rates of colonoscopy selection as a screening method, and of screening completion, Dr. Segura and Dr. Siddique noted, with White individuals who chose the method three times more likely to complete screening as Asian, Hispanic, or Black individuals. Disparities were also seen reflected in people’s choice of screening method, with non–English-speaking Hispanic individuals less likely to choose colonoscopy compared with other groups.
Use of stool-based screening methods, such as the fecal occult blood test (FOBT) and fecal immunochemical test (FIT), has risen over time across ethnic and racial groups. However, Hispanic and Asian individuals were more likely to complete and adhere to the FOBT, compared with non-Hispanic White individuals. Follow-up colonoscopy rates after FOBT or FIT also differ along ethnic and racial lines, Dr. Segura and Dr. Siddique noted, with Asian and American Indian groups less likely to complete follow-up after an abnormal result.
The study authors pointed to structural racism at the root of some observed disparities, citing barriers to healthcare access and quality that include higher rates of noninsurance among Black and Hispanic populations and a lower likelihood of the same populations to receive physician counseling regarding screening.
Barriers to economic stability, including living in impoverished neighborhoods, were also cited as contributors to lower colorectal screening. Patients covered by Medicaid were more than twice as likely as non-Medicaid patients to have suboptimal bowel preparation at screening, the authors noted. Access to transportation remained another frequently observed barrier to completing recommended testing and follow-up.
Mistrust of doctors has been linked to lower screening uptake among Black men. “Longstanding conscious and implicit racism, differences in communication, and socioeconomic context ... engender medical mistrust among racial and ethnic groups,” the authors wrote. Reversing it “ultimately requires vast societal change, and we as physicians can facilitate this by encouraging patient-centered discussions that humanize and empower traditionally marginalized populations.”
Dr. Segura and Dr. Siddique described strategies that have been shown to result in better uptake in specific populations, including removing out-of-pocket costs for screening and follow-up, and designing faith-based or culturally specific outreach delivered through churches and local businesses.
They recommended that researchers change how they study the disparities that bear on colorectal screening and outcomes. “Collection and use of data on race and ethnicity must be optimized and standardized to ensure that all groups are adequately captured,” they wrote. Standardizing self-reporting of race and ethnicity would help address issues of misclassification.
The authors also advised designing studies with longer follow-up, noting that “we must better understand the mechanisms of long-term adherence.” Additional research is needed, they said, to evaluate the efficacy of older outreach strategies after societal changes resulting from the COVID-19 pandemic. Efforts to increase the number of Black, Hispanic, Asian, and Alaskan Native/American Indian groups in CRC screening interventions and studies “must be prioritized.”
Dr. Segura’s and Dr. Siddique’s study was funded with grants from the National Institutes of Health. They disclosed no conflicts of interest.
Disparities in colorectal screening represent a serious public health challenge, say the authors of a new literature review that describes specific areas of concern and recommendations for improvement.
For their research, published in Techniques and Innovations in Gastrointestinal Endoscopy, gastroenterologists Abraham Segura, MD, and Shazia Mehmood Siddique, MD, of the University of Pennsylvania, Philadelphia, sought to identify studies that shed light on ethnicity or race-based differences in screening uptake, as well as known barriers and facilitators to screening.
Significant racial and ethnic disparities can be seen in rates of colonoscopy selection as a screening method, and of screening completion, Dr. Segura and Dr. Siddique noted, with White individuals who chose the method three times more likely to complete screening as Asian, Hispanic, or Black individuals. Disparities were also seen reflected in people’s choice of screening method, with non–English-speaking Hispanic individuals less likely to choose colonoscopy compared with other groups.
Use of stool-based screening methods, such as the fecal occult blood test (FOBT) and fecal immunochemical test (FIT), has risen over time across ethnic and racial groups. However, Hispanic and Asian individuals were more likely to complete and adhere to the FOBT, compared with non-Hispanic White individuals. Follow-up colonoscopy rates after FOBT or FIT also differ along ethnic and racial lines, Dr. Segura and Dr. Siddique noted, with Asian and American Indian groups less likely to complete follow-up after an abnormal result.
The study authors pointed to structural racism at the root of some observed disparities, citing barriers to healthcare access and quality that include higher rates of noninsurance among Black and Hispanic populations and a lower likelihood of the same populations to receive physician counseling regarding screening.
Barriers to economic stability, including living in impoverished neighborhoods, were also cited as contributors to lower colorectal screening. Patients covered by Medicaid were more than twice as likely as non-Medicaid patients to have suboptimal bowel preparation at screening, the authors noted. Access to transportation remained another frequently observed barrier to completing recommended testing and follow-up.
Mistrust of doctors has been linked to lower screening uptake among Black men. “Longstanding conscious and implicit racism, differences in communication, and socioeconomic context ... engender medical mistrust among racial and ethnic groups,” the authors wrote. Reversing it “ultimately requires vast societal change, and we as physicians can facilitate this by encouraging patient-centered discussions that humanize and empower traditionally marginalized populations.”
Dr. Segura and Dr. Siddique described strategies that have been shown to result in better uptake in specific populations, including removing out-of-pocket costs for screening and follow-up, and designing faith-based or culturally specific outreach delivered through churches and local businesses.
They recommended that researchers change how they study the disparities that bear on colorectal screening and outcomes. “Collection and use of data on race and ethnicity must be optimized and standardized to ensure that all groups are adequately captured,” they wrote. Standardizing self-reporting of race and ethnicity would help address issues of misclassification.
The authors also advised designing studies with longer follow-up, noting that “we must better understand the mechanisms of long-term adherence.” Additional research is needed, they said, to evaluate the efficacy of older outreach strategies after societal changes resulting from the COVID-19 pandemic. Efforts to increase the number of Black, Hispanic, Asian, and Alaskan Native/American Indian groups in CRC screening interventions and studies “must be prioritized.”
Dr. Segura’s and Dr. Siddique’s study was funded with grants from the National Institutes of Health. They disclosed no conflicts of interest.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Gilteritinib maintenance reduces relapse in MRD+ AML
The research was presented at the European Hematology Association Hybrid Congress 2023.
For the study, AML patients with the most common form of mutation in the proto-oncogene fms-like tyrosine kinase 3 (FLT3), known as the internal tandem duplication (ITD), were randomized to 24 months of maintenance therapy with either the FLT3 inhibitor gilteritinib or placebo.
The trial did not meet its primary endpoint, as there was no significant difference in relapse-free survival (RFS) between those assigned to the active drug and those given placebo, and there was no difference in overall survival rates.
However, subgroup analysis revealed that FLT3/ITD AML patients who were MRD+ after transplant, which represented approximately half of the participants, experienced a significant 48% improvement in RFS with gilteritinib versus placebo, while no benefit was seen in MRD– patients.
While acknowledging that the trial did not meet its primary endpoint, presenter Mark J. Levis, MD, PhD, program leader, hematologic malignancies and bone marrow transplant program, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, said it was nevertheless “a successful study.”
“We learned how to use these drugs and in whom,” he continued, adding: “No, not everybody needs and should get a FLT3 inhibitor post-transplant, but we can use this [MRD] assay to identify who.”
Consequently, Dr. Levis believes that gilteritinib “should be a standard of care for those who are MRD positive,” although the decision to use it “should be balanced against the potential for toxicity,” compared with not adding an additional treatment after HCT.
He told a press conference that “we’re going to certainly make sure that patients who are MRD positive get [gilteritinib],” although the MRD negative patients “are going to be more questionable,” especially because the assay that they used in the study is not “perfect.”
Dr. Levis also suggested that the trial did not meet its endpoint because of regional differences in the clinical practice, such as in the number of treatment cycles prior to HCT, the time to transplant, and the previous use of a FLT3 inhibitor, all of which may have skewed the findings.
“Everybody in the world is convinced that they’re the best transplanter,” he said, and yet “they all do it differently, and the heterogeneity is astounding.”
He added: “If we’d restricted everybody [to a] pretransplant regimen, I suspect we would have had a different result than what we’re getting here, but this is releasing the drug into the world and saying: ‘Here, transplant however you want, however it’s practiced in the real world. Tell us how this works.’ ”
Approached for comment, Claudio Brunstein, MD, PhD, vice-chair of the department of hematology and oncology in the Cleveland Clinic Taussig Cancer Institute, said that while there was “some disappointment” with the results, he was “not surprised” that the trial did not meet its primary endpoint.
He said in an interview that the patient population was not of “high enough risk” to demonstrate an overall difference between gilteritinib and placebo, although he conceded that it is “hard to get to high-risk patients in a timely way” and so conduct a trial with them.
As to the notion that variations in clinical practice could have been responsible, Dr. Brunstein pointed out that it was a randomized trial, so the issue would have applied equally to both sides.
He nevertheless believes that it is “a very important study,” and “just the fact that it was done in the context of a number of drugs coming and being approved by the [U.S. Food and Drug Administration] in AML is quite remarkable.”
This is especially the case given that “many centers are already using [gilteritinib] as off-label maintenance therapy.”
Dr. Brunstein added that it is “good news” that the drug was effective in MRD+ patients, as it shows “you can overcome that with maintenance therapy rather than keeping giving more and more chemotherapy, especially as there are patients you’re worried about giving more intensive chemotherapy to make them MRD negative.”
He pointed out, however, that the assay used in the trial was “research grade” and very sensitive to MRD and “is not available everywhere, so there is an adjustment that the community will have to do to in order to apply this data.”
“But for those who are more obviously MRD positive with less sensitive assays, gilteritinib is already something that can be used,” Dr. Brunstein said.
Presenting the findings, Dr. Levis stated: “We all know that patients with FLT3/ITD AML have a high risk of relapse and are routinely referred for transplant. And we know that the detection of measurable residual disease pretransplant is highly predictive of outcome post-transplant.”
He continued that FLT3 inhibitors are “routinely given as post-transplant maintenance ... based on some prior trials, mostly with sorafenib.”
“But uncertainty exists as to the broad applicability of these trials,” Dr. Levis said. Moreover, the use of sorafenib in this context is “off label and can be difficult to tolerate,” and “we know that most patients are cured with allogeneic transplant alone.”
Gilteritinib is already known to be well tolerated as a monotherapy, and was approved by the FDA for the treatment of adult patients with FLT3 mutation–positive relapsed or refractory AML in 2018.
The investigators therefore examined whether it would be beneficial as a post-HCT maintenance therapy in FLT3-ITD AML. Patients were required to be in morphologic remission after one or two courses of induction therapy, with Dr. Levis underlining: “We did not allow patients who had been salvaged onto the study.”
They subsequently had a marrow aspirate sample taken for MRD analysis before undergoing allogeneic transplant, with any conditioning regimen, donor, or graft-versus-host disease (GVHD) prophylaxis allowed.
Between 30 and 90 days later, patients with successful engraftment who were able to take oral medication were then randomized to 24 months of maintenance therapy with either gilteritinib or placebo.
Dr. Levis showed that, among 620 patients screened at 110 centers in 16 countries, 356 were randomized between Aug. 15, 2017, and July 8, 2020. The median age was 53 years, and 49% of gilteritinib patients and 48% of those given placebo were female.
He noted that there was a “fairly even global distribution” of patients from North America, Europe, and the Asia/Pacific region, and that 60% of patients underwent a myeloablative conditioning regimen. Approximately the same proportion had received an FLT3 inhibitor prior to HCT.
MRD positivity, assessed at a cell count of ≥ 10-6, was observed pre-HCT in 47% of patients in both treatment groups, and in 50% of gilteritinib patients and 51% of placebo patients at both pre- and post-transplant assessments.
The treatment regimen was completed by 52.8% of patients assigned to gilteritinib and 53.9% in the placebo arm. Dr. Levis said that 18.5% and 20.3% of patients, respectively, experienced a grade 3/4 treatment emergent acute GVHD event, while 32.6% and 21.5%, respectively, had a grade ≥ 3 treatment emergent infection.
He noted that “adverse events were clearly more common in the gilteritinib arm and often led to either dose reduction or interruption, or withdrawal of treatment.”
The most common grade ≥ 3 treatment emergent adverse event was a decrease in neutrophil count, seen in 24.7% of gilteritinib patients and 7.9% of those given placebo, followed by reduced platelet count, in 15.2% and 5.6%, respectively, and anemia, in 6.2% and 1.7%, respectively.
Turning to the efficacy outcomes, Dr. Levis reported that the trial did not meet its primary endpoint, with no significant difference in RFS between the gilteritinib and placebo arms, at a hazard ratio of 0.679 (P = .0518). There was also no significant difference in the key secondary objective of overall survival, at a hazard ratio of 0.846 (P = .4394).
However, Dr. Levis noted that there was a “clear difference in the benefit of gilteritinib by region,” and, “at every level,” MRD predicted a benefit from gilteritinib, which he said was a “big surprise” and “really leapt out in the subgroup analysis.”
He explained that the researchers used a modified version of a two-step assay that has been used in previous studies, and was able to detect MRD at a sensitivity of approximately 1x10-6. “In our study, 98% of participants had samples pre- and post-[transplant].”
Regardless of treatment arm, MRD positivity measured at that sensitivity was associated with a significant reduction in overall survival, at a hazard ratio versus MRD– status of 0.514 (P = .0025).
When stratifying the patients by MRD status, the researchers found that, among MRD+ participants, gilteritinib was associated with a significant improvement in RFS, at a hazard ratio versus placebo of 0.515 (P = .0065), while there was no significant difference in MRD– patients.
Stratifying the patients by their conditioning regimen prior to HCT also revealed differences, with those undergoing myeloablative conditioning having significantly greater overall survival than those who underwent reduced-intensity conditioning, at a hazard ratio for death of 0.529 (P = .0027).
Dr. Levis said there is “no surprise there,” and the result could reflect the selection of fitter, younger patients to undergo the more intensive regimen.
He then showed that MRD+ patients who had undergone myeloablative conditioning had better overall survival with gilteritinib than placebo, at a hazard ratio for death of 0.418 (P = .0087). Again, the difference disappeared when looking at MRD– patients.
“So conditioning doesn’t help you in the setting of MRD,” Dr. Levis said.
Finally, he took a deeper dive into the regional differences in outcomes, noting that patients in the Asia/Pacific region, where gilteritinib showed no benefit over placebo, “were 10 years younger” than those in other regions, “tended to get myeloablative conditioning, and hardly ever used FLT3 inhibitors.”
In contrast, North American patients, who experienced a significant gilteritinib benefit in terms of RFS, underwent HCT an average of 26 days earlier than those elsewhere, and received fewer courses of chemotherapy pre-HCT. Moreover, 93.5% received an FLT3 inhibitor pretransplant.
The study was funded by Astellas Pharma Global Development. Dr. Levis declares relationships with Abbvie, Amgen, Astellas, Bristol-Myers-Squibb, Daiichi-Sankyo, GlaxoSmithKline, Jazz, Menarini, Pfizer, Sumitomo-Dainippon, Syndax, Takeda. Dr. Brunstein declares no relevant relationships.
The research was presented at the European Hematology Association Hybrid Congress 2023.
For the study, AML patients with the most common form of mutation in the proto-oncogene fms-like tyrosine kinase 3 (FLT3), known as the internal tandem duplication (ITD), were randomized to 24 months of maintenance therapy with either the FLT3 inhibitor gilteritinib or placebo.
The trial did not meet its primary endpoint, as there was no significant difference in relapse-free survival (RFS) between those assigned to the active drug and those given placebo, and there was no difference in overall survival rates.
However, subgroup analysis revealed that FLT3/ITD AML patients who were MRD+ after transplant, which represented approximately half of the participants, experienced a significant 48% improvement in RFS with gilteritinib versus placebo, while no benefit was seen in MRD– patients.
While acknowledging that the trial did not meet its primary endpoint, presenter Mark J. Levis, MD, PhD, program leader, hematologic malignancies and bone marrow transplant program, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, said it was nevertheless “a successful study.”
“We learned how to use these drugs and in whom,” he continued, adding: “No, not everybody needs and should get a FLT3 inhibitor post-transplant, but we can use this [MRD] assay to identify who.”
Consequently, Dr. Levis believes that gilteritinib “should be a standard of care for those who are MRD positive,” although the decision to use it “should be balanced against the potential for toxicity,” compared with not adding an additional treatment after HCT.
He told a press conference that “we’re going to certainly make sure that patients who are MRD positive get [gilteritinib],” although the MRD negative patients “are going to be more questionable,” especially because the assay that they used in the study is not “perfect.”
Dr. Levis also suggested that the trial did not meet its endpoint because of regional differences in the clinical practice, such as in the number of treatment cycles prior to HCT, the time to transplant, and the previous use of a FLT3 inhibitor, all of which may have skewed the findings.
“Everybody in the world is convinced that they’re the best transplanter,” he said, and yet “they all do it differently, and the heterogeneity is astounding.”
He added: “If we’d restricted everybody [to a] pretransplant regimen, I suspect we would have had a different result than what we’re getting here, but this is releasing the drug into the world and saying: ‘Here, transplant however you want, however it’s practiced in the real world. Tell us how this works.’ ”
Approached for comment, Claudio Brunstein, MD, PhD, vice-chair of the department of hematology and oncology in the Cleveland Clinic Taussig Cancer Institute, said that while there was “some disappointment” with the results, he was “not surprised” that the trial did not meet its primary endpoint.
He said in an interview that the patient population was not of “high enough risk” to demonstrate an overall difference between gilteritinib and placebo, although he conceded that it is “hard to get to high-risk patients in a timely way” and so conduct a trial with them.
As to the notion that variations in clinical practice could have been responsible, Dr. Brunstein pointed out that it was a randomized trial, so the issue would have applied equally to both sides.
He nevertheless believes that it is “a very important study,” and “just the fact that it was done in the context of a number of drugs coming and being approved by the [U.S. Food and Drug Administration] in AML is quite remarkable.”
This is especially the case given that “many centers are already using [gilteritinib] as off-label maintenance therapy.”
Dr. Brunstein added that it is “good news” that the drug was effective in MRD+ patients, as it shows “you can overcome that with maintenance therapy rather than keeping giving more and more chemotherapy, especially as there are patients you’re worried about giving more intensive chemotherapy to make them MRD negative.”
He pointed out, however, that the assay used in the trial was “research grade” and very sensitive to MRD and “is not available everywhere, so there is an adjustment that the community will have to do to in order to apply this data.”
“But for those who are more obviously MRD positive with less sensitive assays, gilteritinib is already something that can be used,” Dr. Brunstein said.
Presenting the findings, Dr. Levis stated: “We all know that patients with FLT3/ITD AML have a high risk of relapse and are routinely referred for transplant. And we know that the detection of measurable residual disease pretransplant is highly predictive of outcome post-transplant.”
He continued that FLT3 inhibitors are “routinely given as post-transplant maintenance ... based on some prior trials, mostly with sorafenib.”
“But uncertainty exists as to the broad applicability of these trials,” Dr. Levis said. Moreover, the use of sorafenib in this context is “off label and can be difficult to tolerate,” and “we know that most patients are cured with allogeneic transplant alone.”
Gilteritinib is already known to be well tolerated as a monotherapy, and was approved by the FDA for the treatment of adult patients with FLT3 mutation–positive relapsed or refractory AML in 2018.
The investigators therefore examined whether it would be beneficial as a post-HCT maintenance therapy in FLT3-ITD AML. Patients were required to be in morphologic remission after one or two courses of induction therapy, with Dr. Levis underlining: “We did not allow patients who had been salvaged onto the study.”
They subsequently had a marrow aspirate sample taken for MRD analysis before undergoing allogeneic transplant, with any conditioning regimen, donor, or graft-versus-host disease (GVHD) prophylaxis allowed.
Between 30 and 90 days later, patients with successful engraftment who were able to take oral medication were then randomized to 24 months of maintenance therapy with either gilteritinib or placebo.
Dr. Levis showed that, among 620 patients screened at 110 centers in 16 countries, 356 were randomized between Aug. 15, 2017, and July 8, 2020. The median age was 53 years, and 49% of gilteritinib patients and 48% of those given placebo were female.
He noted that there was a “fairly even global distribution” of patients from North America, Europe, and the Asia/Pacific region, and that 60% of patients underwent a myeloablative conditioning regimen. Approximately the same proportion had received an FLT3 inhibitor prior to HCT.
MRD positivity, assessed at a cell count of ≥ 10-6, was observed pre-HCT in 47% of patients in both treatment groups, and in 50% of gilteritinib patients and 51% of placebo patients at both pre- and post-transplant assessments.
The treatment regimen was completed by 52.8% of patients assigned to gilteritinib and 53.9% in the placebo arm. Dr. Levis said that 18.5% and 20.3% of patients, respectively, experienced a grade 3/4 treatment emergent acute GVHD event, while 32.6% and 21.5%, respectively, had a grade ≥ 3 treatment emergent infection.
He noted that “adverse events were clearly more common in the gilteritinib arm and often led to either dose reduction or interruption, or withdrawal of treatment.”
The most common grade ≥ 3 treatment emergent adverse event was a decrease in neutrophil count, seen in 24.7% of gilteritinib patients and 7.9% of those given placebo, followed by reduced platelet count, in 15.2% and 5.6%, respectively, and anemia, in 6.2% and 1.7%, respectively.
Turning to the efficacy outcomes, Dr. Levis reported that the trial did not meet its primary endpoint, with no significant difference in RFS between the gilteritinib and placebo arms, at a hazard ratio of 0.679 (P = .0518). There was also no significant difference in the key secondary objective of overall survival, at a hazard ratio of 0.846 (P = .4394).
However, Dr. Levis noted that there was a “clear difference in the benefit of gilteritinib by region,” and, “at every level,” MRD predicted a benefit from gilteritinib, which he said was a “big surprise” and “really leapt out in the subgroup analysis.”
He explained that the researchers used a modified version of a two-step assay that has been used in previous studies, and was able to detect MRD at a sensitivity of approximately 1x10-6. “In our study, 98% of participants had samples pre- and post-[transplant].”
Regardless of treatment arm, MRD positivity measured at that sensitivity was associated with a significant reduction in overall survival, at a hazard ratio versus MRD– status of 0.514 (P = .0025).
When stratifying the patients by MRD status, the researchers found that, among MRD+ participants, gilteritinib was associated with a significant improvement in RFS, at a hazard ratio versus placebo of 0.515 (P = .0065), while there was no significant difference in MRD– patients.
Stratifying the patients by their conditioning regimen prior to HCT also revealed differences, with those undergoing myeloablative conditioning having significantly greater overall survival than those who underwent reduced-intensity conditioning, at a hazard ratio for death of 0.529 (P = .0027).
Dr. Levis said there is “no surprise there,” and the result could reflect the selection of fitter, younger patients to undergo the more intensive regimen.
He then showed that MRD+ patients who had undergone myeloablative conditioning had better overall survival with gilteritinib than placebo, at a hazard ratio for death of 0.418 (P = .0087). Again, the difference disappeared when looking at MRD– patients.
“So conditioning doesn’t help you in the setting of MRD,” Dr. Levis said.
Finally, he took a deeper dive into the regional differences in outcomes, noting that patients in the Asia/Pacific region, where gilteritinib showed no benefit over placebo, “were 10 years younger” than those in other regions, “tended to get myeloablative conditioning, and hardly ever used FLT3 inhibitors.”
In contrast, North American patients, who experienced a significant gilteritinib benefit in terms of RFS, underwent HCT an average of 26 days earlier than those elsewhere, and received fewer courses of chemotherapy pre-HCT. Moreover, 93.5% received an FLT3 inhibitor pretransplant.
The study was funded by Astellas Pharma Global Development. Dr. Levis declares relationships with Abbvie, Amgen, Astellas, Bristol-Myers-Squibb, Daiichi-Sankyo, GlaxoSmithKline, Jazz, Menarini, Pfizer, Sumitomo-Dainippon, Syndax, Takeda. Dr. Brunstein declares no relevant relationships.
The research was presented at the European Hematology Association Hybrid Congress 2023.
For the study, AML patients with the most common form of mutation in the proto-oncogene fms-like tyrosine kinase 3 (FLT3), known as the internal tandem duplication (ITD), were randomized to 24 months of maintenance therapy with either the FLT3 inhibitor gilteritinib or placebo.
The trial did not meet its primary endpoint, as there was no significant difference in relapse-free survival (RFS) between those assigned to the active drug and those given placebo, and there was no difference in overall survival rates.
However, subgroup analysis revealed that FLT3/ITD AML patients who were MRD+ after transplant, which represented approximately half of the participants, experienced a significant 48% improvement in RFS with gilteritinib versus placebo, while no benefit was seen in MRD– patients.
While acknowledging that the trial did not meet its primary endpoint, presenter Mark J. Levis, MD, PhD, program leader, hematologic malignancies and bone marrow transplant program, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, said it was nevertheless “a successful study.”
“We learned how to use these drugs and in whom,” he continued, adding: “No, not everybody needs and should get a FLT3 inhibitor post-transplant, but we can use this [MRD] assay to identify who.”
Consequently, Dr. Levis believes that gilteritinib “should be a standard of care for those who are MRD positive,” although the decision to use it “should be balanced against the potential for toxicity,” compared with not adding an additional treatment after HCT.
He told a press conference that “we’re going to certainly make sure that patients who are MRD positive get [gilteritinib],” although the MRD negative patients “are going to be more questionable,” especially because the assay that they used in the study is not “perfect.”
Dr. Levis also suggested that the trial did not meet its endpoint because of regional differences in the clinical practice, such as in the number of treatment cycles prior to HCT, the time to transplant, and the previous use of a FLT3 inhibitor, all of which may have skewed the findings.
“Everybody in the world is convinced that they’re the best transplanter,” he said, and yet “they all do it differently, and the heterogeneity is astounding.”
He added: “If we’d restricted everybody [to a] pretransplant regimen, I suspect we would have had a different result than what we’re getting here, but this is releasing the drug into the world and saying: ‘Here, transplant however you want, however it’s practiced in the real world. Tell us how this works.’ ”
Approached for comment, Claudio Brunstein, MD, PhD, vice-chair of the department of hematology and oncology in the Cleveland Clinic Taussig Cancer Institute, said that while there was “some disappointment” with the results, he was “not surprised” that the trial did not meet its primary endpoint.
He said in an interview that the patient population was not of “high enough risk” to demonstrate an overall difference between gilteritinib and placebo, although he conceded that it is “hard to get to high-risk patients in a timely way” and so conduct a trial with them.
As to the notion that variations in clinical practice could have been responsible, Dr. Brunstein pointed out that it was a randomized trial, so the issue would have applied equally to both sides.
He nevertheless believes that it is “a very important study,” and “just the fact that it was done in the context of a number of drugs coming and being approved by the [U.S. Food and Drug Administration] in AML is quite remarkable.”
This is especially the case given that “many centers are already using [gilteritinib] as off-label maintenance therapy.”
Dr. Brunstein added that it is “good news” that the drug was effective in MRD+ patients, as it shows “you can overcome that with maintenance therapy rather than keeping giving more and more chemotherapy, especially as there are patients you’re worried about giving more intensive chemotherapy to make them MRD negative.”
He pointed out, however, that the assay used in the trial was “research grade” and very sensitive to MRD and “is not available everywhere, so there is an adjustment that the community will have to do to in order to apply this data.”
“But for those who are more obviously MRD positive with less sensitive assays, gilteritinib is already something that can be used,” Dr. Brunstein said.
Presenting the findings, Dr. Levis stated: “We all know that patients with FLT3/ITD AML have a high risk of relapse and are routinely referred for transplant. And we know that the detection of measurable residual disease pretransplant is highly predictive of outcome post-transplant.”
He continued that FLT3 inhibitors are “routinely given as post-transplant maintenance ... based on some prior trials, mostly with sorafenib.”
“But uncertainty exists as to the broad applicability of these trials,” Dr. Levis said. Moreover, the use of sorafenib in this context is “off label and can be difficult to tolerate,” and “we know that most patients are cured with allogeneic transplant alone.”
Gilteritinib is already known to be well tolerated as a monotherapy, and was approved by the FDA for the treatment of adult patients with FLT3 mutation–positive relapsed or refractory AML in 2018.
The investigators therefore examined whether it would be beneficial as a post-HCT maintenance therapy in FLT3-ITD AML. Patients were required to be in morphologic remission after one or two courses of induction therapy, with Dr. Levis underlining: “We did not allow patients who had been salvaged onto the study.”
They subsequently had a marrow aspirate sample taken for MRD analysis before undergoing allogeneic transplant, with any conditioning regimen, donor, or graft-versus-host disease (GVHD) prophylaxis allowed.
Between 30 and 90 days later, patients with successful engraftment who were able to take oral medication were then randomized to 24 months of maintenance therapy with either gilteritinib or placebo.
Dr. Levis showed that, among 620 patients screened at 110 centers in 16 countries, 356 were randomized between Aug. 15, 2017, and July 8, 2020. The median age was 53 years, and 49% of gilteritinib patients and 48% of those given placebo were female.
He noted that there was a “fairly even global distribution” of patients from North America, Europe, and the Asia/Pacific region, and that 60% of patients underwent a myeloablative conditioning regimen. Approximately the same proportion had received an FLT3 inhibitor prior to HCT.
MRD positivity, assessed at a cell count of ≥ 10-6, was observed pre-HCT in 47% of patients in both treatment groups, and in 50% of gilteritinib patients and 51% of placebo patients at both pre- and post-transplant assessments.
The treatment regimen was completed by 52.8% of patients assigned to gilteritinib and 53.9% in the placebo arm. Dr. Levis said that 18.5% and 20.3% of patients, respectively, experienced a grade 3/4 treatment emergent acute GVHD event, while 32.6% and 21.5%, respectively, had a grade ≥ 3 treatment emergent infection.
He noted that “adverse events were clearly more common in the gilteritinib arm and often led to either dose reduction or interruption, or withdrawal of treatment.”
The most common grade ≥ 3 treatment emergent adverse event was a decrease in neutrophil count, seen in 24.7% of gilteritinib patients and 7.9% of those given placebo, followed by reduced platelet count, in 15.2% and 5.6%, respectively, and anemia, in 6.2% and 1.7%, respectively.
Turning to the efficacy outcomes, Dr. Levis reported that the trial did not meet its primary endpoint, with no significant difference in RFS between the gilteritinib and placebo arms, at a hazard ratio of 0.679 (P = .0518). There was also no significant difference in the key secondary objective of overall survival, at a hazard ratio of 0.846 (P = .4394).
However, Dr. Levis noted that there was a “clear difference in the benefit of gilteritinib by region,” and, “at every level,” MRD predicted a benefit from gilteritinib, which he said was a “big surprise” and “really leapt out in the subgroup analysis.”
He explained that the researchers used a modified version of a two-step assay that has been used in previous studies, and was able to detect MRD at a sensitivity of approximately 1x10-6. “In our study, 98% of participants had samples pre- and post-[transplant].”
Regardless of treatment arm, MRD positivity measured at that sensitivity was associated with a significant reduction in overall survival, at a hazard ratio versus MRD– status of 0.514 (P = .0025).
When stratifying the patients by MRD status, the researchers found that, among MRD+ participants, gilteritinib was associated with a significant improvement in RFS, at a hazard ratio versus placebo of 0.515 (P = .0065), while there was no significant difference in MRD– patients.
Stratifying the patients by their conditioning regimen prior to HCT also revealed differences, with those undergoing myeloablative conditioning having significantly greater overall survival than those who underwent reduced-intensity conditioning, at a hazard ratio for death of 0.529 (P = .0027).
Dr. Levis said there is “no surprise there,” and the result could reflect the selection of fitter, younger patients to undergo the more intensive regimen.
He then showed that MRD+ patients who had undergone myeloablative conditioning had better overall survival with gilteritinib than placebo, at a hazard ratio for death of 0.418 (P = .0087). Again, the difference disappeared when looking at MRD– patients.
“So conditioning doesn’t help you in the setting of MRD,” Dr. Levis said.
Finally, he took a deeper dive into the regional differences in outcomes, noting that patients in the Asia/Pacific region, where gilteritinib showed no benefit over placebo, “were 10 years younger” than those in other regions, “tended to get myeloablative conditioning, and hardly ever used FLT3 inhibitors.”
In contrast, North American patients, who experienced a significant gilteritinib benefit in terms of RFS, underwent HCT an average of 26 days earlier than those elsewhere, and received fewer courses of chemotherapy pre-HCT. Moreover, 93.5% received an FLT3 inhibitor pretransplant.
The study was funded by Astellas Pharma Global Development. Dr. Levis declares relationships with Abbvie, Amgen, Astellas, Bristol-Myers-Squibb, Daiichi-Sankyo, GlaxoSmithKline, Jazz, Menarini, Pfizer, Sumitomo-Dainippon, Syndax, Takeda. Dr. Brunstein declares no relevant relationships.
AT EHA 2023



