User login
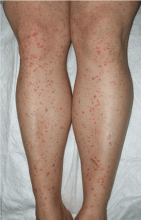
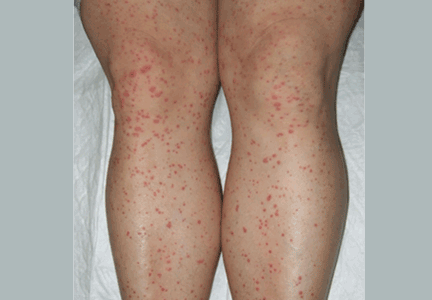
Palpable purpura
Q: Which is the most likely diagnosis?
- Idiopathic thrombocytopenic purpura
- Vitamin C deficiency (scurvy)
- Kaposi sarcoma not related to human immunodeficiency virus (HIV) infection
- Henoch-Schönlein purpura
- Polyarteritis nodosa
A: The correct answer is Henoch-Schönlein purpura.
Idiopathic thrombocytopenic purpura is an autoimmune disease caused by specific antibodies against platelet-membrane glycoproteins. It is characterized by thrombocytopenia not explainable by contact with toxic substances or by other causes. Along with nonpalpable purpura, other common signs are epistaxis, gingival bleeding, menorrhagia, and retinal hemorrhage.
Scurvy is an uncommon deficiency of ascorbic acid (vitamin C). The elderly and alcoholics are at higher risk, as they do not take in enough vitamin C in the diet. Patients usually show perifollicular hemorrhages of the skin and mucous membranes, typically petechial hemorrhage or ecchymosis of the gums around the upper incisors. Other cutaneous signs are follicular hyperkeratosis on the forearms, small corkscrew hairs, and sicca syndrome, which is more common in adults.
Non-HIV Kaposi sarcoma usually affects elderly patients, with pink, red, or brown papules or nodules on the legs and, less commonly, on the head and neck. Histopathologic examination shows newly formed irregular blood vessels with an inflammatory infiltrate of plasma cells and lymphocytes; immunohistochemical human herpes virus staining is usually positive.
Polyarteritis nodosa is a systemic vasculitis that affects medium or small arteries with necrotizing inflammation; renal glomeruli and arterioles, capillaries, and venules are unaffected. Skin manifestations include palpable purpura, livedo reticularis, ulcers, and distal gangrene. The condition also usually affects the kidneys, the heart, and the musculoskeletal and nervous systems.
A SYSTEMIC VASCULITIS
Henoch-Schönlein purpura is a systemic vasculitis affecting the skin, gastrointestinal tract, kidneys, and joints. Palpable purpura and joint pain are the most common and consistent presenting symptoms. The kidneys are affected in about one-third of children and in 60% of adults, and this is the major factor determining the long-term outcome.1
In our patient, laboratory testing that included a complete blood cell count, biochemical testing (including IgA levels), urinary sediment, and coagulation studies showed no abnormalities except elevations of the erythrocyte sedimentation rate and the concentration of C-reactive protein (an acute-phase reactant). These can be normal in some patients. Renal involvement was also not present.
DIAGNOSIS
The diagnosis relies on clinical manifestations. Because Henoch-Schönlein purpura is less common in adults, biopsy plays a more important role in establishing the diagnosis in this age group, and it does this by demonstrating leukocytoclastic vasculitis with a predominance of IgA deposition under immunofluorescence. Recent studies in children showed that an elevated IgA concentration along with reduced IgM levels was associated with a higher rate of severe complications.2 However, depending on the age of the biopsied lesion, IgA may not be detected.
TREATMENT DIRECTED AT SYMPTOMS
Our patient received oral corticosteroids 0.5 mg/kg per day for 20 days, and the lesions resolved by 4 weeks.
Management of Henoch-Schönlein purpura is mainly directed at the symptoms, with oral hydration and nonsteroidal anti-inflammatory drugs. For severe cases, a short course of corticosteroids (0.5–1 mg/kg) may be used.
Although no controlled clinical trial has proven that Henoch-Schönlein purpura responds to corticosteroids, colchicine, or other drugs, corticosteroids are used most often, especially in patients with renal disease. Patients with severe renal insufficiency, abdominal pain, joint involvement, or bleeding should be hospitalized. Plasmapheresis3 has been used in severe cases.
HENOCH-SCHÖNLEIN PURPURA AND MALIGNANCY
During a follow-up evaluation 1 month later, our patient was diagnosed with adenocarcinoma of the breast. This highlights the value of a workup for cancer in adults with cutaneous vasculitis.
Cutaneous vasculitis can represent a paraneoplastic syndrome associated with a malignant tumor. The pathophysiology of this association is unclear, but one proposed mechanism is the exaggerated production of antibodies that react against tumor neoantigens, leading to the formation of immune complexes, or that occasionally recognize endothelial cells because of similarities with tumor antigens. Another theory is that abnormally high levels of inflammatory cytokines are produced by neoplastic cells or in response to decreased immune complex clearance.
Yet another theory is that hyperviscosity of the blood, seen in some cancers, increases the contact time for deposition of immune complexes and causes endothelial damage. Drugs used to treat cancer have also been reported to produce Henoch-Schönlein purpura.4
Although hematologic malignancy is three to five times more common than solid tumors in patients with small-vessel vasculitis, the disease has been associated with solid tumors of the liver, skin, colon, and breast in adults over age 40.5 An evaluation for neoplasm is therefore reasonable in adults with Henoch-Schönlein purpura, as is an evaluation for tumor recurrence or metastasis if the patient has been previously treated for a malignant tumor.
- Rieu P, Noël LH. Henoch-Schönlein nephritis in children and adults. Morphological features and clinicopathological correlations. Ann Med Interne (Paris) 1999; 150:151–159.
- Fretzayas A, Sionti I, Moustaki M, Nicolaidou P. Clinical impact of altered immunoglobulin levels in Henoch-Schönlein purpura. Pediatr Int 2009; 51:381–384.
- Donghi D, Schanz U, Sahrbacher U, et al. Life-threatening or organimpairing Henoch-Schönlein purpura: plasmapheresis may save lives and limit organ damage. Dermatology 2009; 219:167–170.
- Mitsui H, Shibagaki N, Kawamura T, Matsue H, Shimada S. A clinical study of Henoch-Schönlein purpura associated with malignancy. J Eur Acad Dermatol Venereol 2009; 23:394–401.
- Maestri A, Malacarne P, Santini A. Henoch-Schönlein syndrome associated with breast cancer. A case report. Angiology 1995; 46:625–627.
Q: Which is the most likely diagnosis?
- Idiopathic thrombocytopenic purpura
- Vitamin C deficiency (scurvy)
- Kaposi sarcoma not related to human immunodeficiency virus (HIV) infection
- Henoch-Schönlein purpura
- Polyarteritis nodosa
A: The correct answer is Henoch-Schönlein purpura.
Idiopathic thrombocytopenic purpura is an autoimmune disease caused by specific antibodies against platelet-membrane glycoproteins. It is characterized by thrombocytopenia not explainable by contact with toxic substances or by other causes. Along with nonpalpable purpura, other common signs are epistaxis, gingival bleeding, menorrhagia, and retinal hemorrhage.
Scurvy is an uncommon deficiency of ascorbic acid (vitamin C). The elderly and alcoholics are at higher risk, as they do not take in enough vitamin C in the diet. Patients usually show perifollicular hemorrhages of the skin and mucous membranes, typically petechial hemorrhage or ecchymosis of the gums around the upper incisors. Other cutaneous signs are follicular hyperkeratosis on the forearms, small corkscrew hairs, and sicca syndrome, which is more common in adults.
Non-HIV Kaposi sarcoma usually affects elderly patients, with pink, red, or brown papules or nodules on the legs and, less commonly, on the head and neck. Histopathologic examination shows newly formed irregular blood vessels with an inflammatory infiltrate of plasma cells and lymphocytes; immunohistochemical human herpes virus staining is usually positive.
Polyarteritis nodosa is a systemic vasculitis that affects medium or small arteries with necrotizing inflammation; renal glomeruli and arterioles, capillaries, and venules are unaffected. Skin manifestations include palpable purpura, livedo reticularis, ulcers, and distal gangrene. The condition also usually affects the kidneys, the heart, and the musculoskeletal and nervous systems.
A SYSTEMIC VASCULITIS
Henoch-Schönlein purpura is a systemic vasculitis affecting the skin, gastrointestinal tract, kidneys, and joints. Palpable purpura and joint pain are the most common and consistent presenting symptoms. The kidneys are affected in about one-third of children and in 60% of adults, and this is the major factor determining the long-term outcome.1
In our patient, laboratory testing that included a complete blood cell count, biochemical testing (including IgA levels), urinary sediment, and coagulation studies showed no abnormalities except elevations of the erythrocyte sedimentation rate and the concentration of C-reactive protein (an acute-phase reactant). These can be normal in some patients. Renal involvement was also not present.
DIAGNOSIS
The diagnosis relies on clinical manifestations. Because Henoch-Schönlein purpura is less common in adults, biopsy plays a more important role in establishing the diagnosis in this age group, and it does this by demonstrating leukocytoclastic vasculitis with a predominance of IgA deposition under immunofluorescence. Recent studies in children showed that an elevated IgA concentration along with reduced IgM levels was associated with a higher rate of severe complications.2 However, depending on the age of the biopsied lesion, IgA may not be detected.
TREATMENT DIRECTED AT SYMPTOMS
Our patient received oral corticosteroids 0.5 mg/kg per day for 20 days, and the lesions resolved by 4 weeks.
Management of Henoch-Schönlein purpura is mainly directed at the symptoms, with oral hydration and nonsteroidal anti-inflammatory drugs. For severe cases, a short course of corticosteroids (0.5–1 mg/kg) may be used.
Although no controlled clinical trial has proven that Henoch-Schönlein purpura responds to corticosteroids, colchicine, or other drugs, corticosteroids are used most often, especially in patients with renal disease. Patients with severe renal insufficiency, abdominal pain, joint involvement, or bleeding should be hospitalized. Plasmapheresis3 has been used in severe cases.
HENOCH-SCHÖNLEIN PURPURA AND MALIGNANCY
During a follow-up evaluation 1 month later, our patient was diagnosed with adenocarcinoma of the breast. This highlights the value of a workup for cancer in adults with cutaneous vasculitis.
Cutaneous vasculitis can represent a paraneoplastic syndrome associated with a malignant tumor. The pathophysiology of this association is unclear, but one proposed mechanism is the exaggerated production of antibodies that react against tumor neoantigens, leading to the formation of immune complexes, or that occasionally recognize endothelial cells because of similarities with tumor antigens. Another theory is that abnormally high levels of inflammatory cytokines are produced by neoplastic cells or in response to decreased immune complex clearance.
Yet another theory is that hyperviscosity of the blood, seen in some cancers, increases the contact time for deposition of immune complexes and causes endothelial damage. Drugs used to treat cancer have also been reported to produce Henoch-Schönlein purpura.4
Although hematologic malignancy is three to five times more common than solid tumors in patients with small-vessel vasculitis, the disease has been associated with solid tumors of the liver, skin, colon, and breast in adults over age 40.5 An evaluation for neoplasm is therefore reasonable in adults with Henoch-Schönlein purpura, as is an evaluation for tumor recurrence or metastasis if the patient has been previously treated for a malignant tumor.
Q: Which is the most likely diagnosis?
- Idiopathic thrombocytopenic purpura
- Vitamin C deficiency (scurvy)
- Kaposi sarcoma not related to human immunodeficiency virus (HIV) infection
- Henoch-Schönlein purpura
- Polyarteritis nodosa
A: The correct answer is Henoch-Schönlein purpura.
Idiopathic thrombocytopenic purpura is an autoimmune disease caused by specific antibodies against platelet-membrane glycoproteins. It is characterized by thrombocytopenia not explainable by contact with toxic substances or by other causes. Along with nonpalpable purpura, other common signs are epistaxis, gingival bleeding, menorrhagia, and retinal hemorrhage.
Scurvy is an uncommon deficiency of ascorbic acid (vitamin C). The elderly and alcoholics are at higher risk, as they do not take in enough vitamin C in the diet. Patients usually show perifollicular hemorrhages of the skin and mucous membranes, typically petechial hemorrhage or ecchymosis of the gums around the upper incisors. Other cutaneous signs are follicular hyperkeratosis on the forearms, small corkscrew hairs, and sicca syndrome, which is more common in adults.
Non-HIV Kaposi sarcoma usually affects elderly patients, with pink, red, or brown papules or nodules on the legs and, less commonly, on the head and neck. Histopathologic examination shows newly formed irregular blood vessels with an inflammatory infiltrate of plasma cells and lymphocytes; immunohistochemical human herpes virus staining is usually positive.
Polyarteritis nodosa is a systemic vasculitis that affects medium or small arteries with necrotizing inflammation; renal glomeruli and arterioles, capillaries, and venules are unaffected. Skin manifestations include palpable purpura, livedo reticularis, ulcers, and distal gangrene. The condition also usually affects the kidneys, the heart, and the musculoskeletal and nervous systems.
A SYSTEMIC VASCULITIS
Henoch-Schönlein purpura is a systemic vasculitis affecting the skin, gastrointestinal tract, kidneys, and joints. Palpable purpura and joint pain are the most common and consistent presenting symptoms. The kidneys are affected in about one-third of children and in 60% of adults, and this is the major factor determining the long-term outcome.1
In our patient, laboratory testing that included a complete blood cell count, biochemical testing (including IgA levels), urinary sediment, and coagulation studies showed no abnormalities except elevations of the erythrocyte sedimentation rate and the concentration of C-reactive protein (an acute-phase reactant). These can be normal in some patients. Renal involvement was also not present.
DIAGNOSIS
The diagnosis relies on clinical manifestations. Because Henoch-Schönlein purpura is less common in adults, biopsy plays a more important role in establishing the diagnosis in this age group, and it does this by demonstrating leukocytoclastic vasculitis with a predominance of IgA deposition under immunofluorescence. Recent studies in children showed that an elevated IgA concentration along with reduced IgM levels was associated with a higher rate of severe complications.2 However, depending on the age of the biopsied lesion, IgA may not be detected.
TREATMENT DIRECTED AT SYMPTOMS
Our patient received oral corticosteroids 0.5 mg/kg per day for 20 days, and the lesions resolved by 4 weeks.
Management of Henoch-Schönlein purpura is mainly directed at the symptoms, with oral hydration and nonsteroidal anti-inflammatory drugs. For severe cases, a short course of corticosteroids (0.5–1 mg/kg) may be used.
Although no controlled clinical trial has proven that Henoch-Schönlein purpura responds to corticosteroids, colchicine, or other drugs, corticosteroids are used most often, especially in patients with renal disease. Patients with severe renal insufficiency, abdominal pain, joint involvement, or bleeding should be hospitalized. Plasmapheresis3 has been used in severe cases.
HENOCH-SCHÖNLEIN PURPURA AND MALIGNANCY
During a follow-up evaluation 1 month later, our patient was diagnosed with adenocarcinoma of the breast. This highlights the value of a workup for cancer in adults with cutaneous vasculitis.
Cutaneous vasculitis can represent a paraneoplastic syndrome associated with a malignant tumor. The pathophysiology of this association is unclear, but one proposed mechanism is the exaggerated production of antibodies that react against tumor neoantigens, leading to the formation of immune complexes, or that occasionally recognize endothelial cells because of similarities with tumor antigens. Another theory is that abnormally high levels of inflammatory cytokines are produced by neoplastic cells or in response to decreased immune complex clearance.
Yet another theory is that hyperviscosity of the blood, seen in some cancers, increases the contact time for deposition of immune complexes and causes endothelial damage. Drugs used to treat cancer have also been reported to produce Henoch-Schönlein purpura.4
Although hematologic malignancy is three to five times more common than solid tumors in patients with small-vessel vasculitis, the disease has been associated with solid tumors of the liver, skin, colon, and breast in adults over age 40.5 An evaluation for neoplasm is therefore reasonable in adults with Henoch-Schönlein purpura, as is an evaluation for tumor recurrence or metastasis if the patient has been previously treated for a malignant tumor.
- Rieu P, Noël LH. Henoch-Schönlein nephritis in children and adults. Morphological features and clinicopathological correlations. Ann Med Interne (Paris) 1999; 150:151–159.
- Fretzayas A, Sionti I, Moustaki M, Nicolaidou P. Clinical impact of altered immunoglobulin levels in Henoch-Schönlein purpura. Pediatr Int 2009; 51:381–384.
- Donghi D, Schanz U, Sahrbacher U, et al. Life-threatening or organimpairing Henoch-Schönlein purpura: plasmapheresis may save lives and limit organ damage. Dermatology 2009; 219:167–170.
- Mitsui H, Shibagaki N, Kawamura T, Matsue H, Shimada S. A clinical study of Henoch-Schönlein purpura associated with malignancy. J Eur Acad Dermatol Venereol 2009; 23:394–401.
- Maestri A, Malacarne P, Santini A. Henoch-Schönlein syndrome associated with breast cancer. A case report. Angiology 1995; 46:625–627.
- Rieu P, Noël LH. Henoch-Schönlein nephritis in children and adults. Morphological features and clinicopathological correlations. Ann Med Interne (Paris) 1999; 150:151–159.
- Fretzayas A, Sionti I, Moustaki M, Nicolaidou P. Clinical impact of altered immunoglobulin levels in Henoch-Schönlein purpura. Pediatr Int 2009; 51:381–384.
- Donghi D, Schanz U, Sahrbacher U, et al. Life-threatening or organimpairing Henoch-Schönlein purpura: plasmapheresis may save lives and limit organ damage. Dermatology 2009; 219:167–170.
- Mitsui H, Shibagaki N, Kawamura T, Matsue H, Shimada S. A clinical study of Henoch-Schönlein purpura associated with malignancy. J Eur Acad Dermatol Venereol 2009; 23:394–401.
- Maestri A, Malacarne P, Santini A. Henoch-Schönlein syndrome associated with breast cancer. A case report. Angiology 1995; 46:625–627.
Interpreting The JUPITER Trial: Statins can prevent VTE, but more study is needed
A major placebo-controlled trial has found that a statin can reduce the risk of venous thromboembolism (VTE).1
We do not recommend prescribing this class of drugs for this purpose until much more research has been done, and we certainly do not recommend substituting a statin for anticoagulant therapy in a patient at risk of VTE.
Nevertheless, we are excited by the latest findings, and we find comfort in knowing that if a patient is taking a statin for an approved indication, ie, reducing the risk of cardiovascular disease in a patient with hyperlipidemia or a previous cardiovascular event, the drug will also reduce the risk of VTE.
In the pages that follow, we describe and comment on what is known about the effect of statins on the risk of VTE.
ARTERIAL AND VENOUS THROMBOSIS: HOW ARE THEY LINKED?
The causes of arterial thrombosis may not be entirely distinct from those of deep vein thrombosis and pulmonary embolism, collectively referred to as VTE. Some studies have found that risk factors for arterial thrombosis overlap with those for VTE.2–4 However, other studies have shown no association between venous and arterial events.5–10
Hyperlipidemia, in particular, has been evaluated to see if it is a risk factor for VTE. As with other risk factors for arterial thrombosis, the data have been mixed, with some reports favoring an association with VTE and others not.4,5,11 Even so, preventive strategies targeting arterial risk factors have shown promise in reducing VTE events.12
Although commonly used to treat hyperlipidemia, statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are believed to reduce the incidence of thrombosis by a number of mechanisms13:
- Decreasing platelet aggregation
- Inhibiting expression of tissue factor and plasminogen activator inhibitor 1
- Increasing expression of tissue plasminogen activator
- Increasing expression of thrombomodulin, which can activate protein C and prevent thrombin-induced platelet and factor V activation and fibrinogen clotting.
STATINS AND VTE IN OBSERVATIONAL AND CASE-CONTROL STUDIES
In view of the multiple effects of statins, several studies have looked at whether these drugs reduce the occurrence of both arterial thrombosis and VTE.14–19
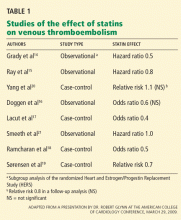
Two prospective observational studies and four case-control studies found that statins reduced the risk of VTE by 20% to 60%.14–19 Interestingly, two of the case-control studies found that antiplatelet therapy did not reduce the risk of VTE.18,19
THE JUPITER STUDY
The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study primarily sought to determine if rosuvastatin (Crestor) 20 mg/day, compared with placebo, would reduce the rate of first major cardiovascular events.22 A prespecified secondary end point of the trial was VTE, making JUPITER the first randomized, placebo-controlled trial to specifically test whether statins prevent VTE.1
Inclusion criteria: Normal LDL, high CRP
The study included men age 50 and older and women age 60 and older with no history of cardiovascular disease. In addition, their lowdensity lipoprotein (LDL) cholesterol levels had to be lower than 130 mg/dL (3.4 mmol/L), their triglyceride levels had to be lower than 500 mg/dL (5.6 mmol/L), and their highsensitivity C-reactive protein (hs-CRP) levels had to be 2.0 mg/L or higher.
Since high levels of hs-CRP, a marker of inflammation, predict cardiovascular events and since statins lower hs-CRP levels, the investigators hypothesized that people with elevated hs-CRP but without hyperlipidemia might benefit from statin treatment.21
Patients were excluded if they had received lipid-lowering therapy within 6 weeks of the trial screening, had diabetes mellitus or uncontrolled hypertension, were currently using postmenopausal hormone-replacement therapy, or had had cancer within the previous 5 years, except for certain skin cancers.
Candidates who complied well during a 4-week placebo run-in phase were randomly assigned to receive either rosuvastatin 20 mg daily (an intermediate dose) or a matching placebo. In all, 17,802 people were randomized. The two assigned groups appeared to be well matched.
Patients were to come in for visits twice a year for 60 months after randomization to be assessed for symptomatic deep venous thrombosis and pulmonary embolism. New cases of VTE were confirmed by imaging studies, by the initiation of anticoagulation therapy, or by death ascribed to pulmonary embolism.
Idiopathic VTE was classified as unprovoked if it occurred in the absence of trauma, hospitalization, or surgery within 3 months before the event, and in the absence of any diagnosed cancer within 3 months before and after the event. Provoked VTE events were those that occurred in a participant with cancer or when a precipitating event was associated with trauma, hospitalization, or surgery.
Rosuvastatin prevents heart attack, stroke
On the recommendation of the trial’s independent data and safety monitoring board, JUPITER was stopped early because the trial drug showed evidence of efficacy in preventing the combined primary end point of a first major cardiovascular event—ie, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from a cardiovascular cause.22 (The cardiovascular outcomes of the JUPITER study were reviewed by Shishehbor and Hazen23 in the January 2009 issue of the Cleveland Clinic Journal of Medicine; see doi:10.3949/ccjm.75a.08105).
Formal follow-up for the trial's primary and secondary efficacy end points ended then, but data on VTE continued to be collected until each patient’s closeout visit as part of a safety monitoring protocol. The last closeout visit occurred on August 20, 2008. The primary analysis focused on events occurring up to March 30, 2008, the date the study was stopped.
Secondary end point results: Rosuvastatin prevents VTE
At a median follow-up of 1.9 years, an episode of VTE had occurred in 94 (0.53%) of the 17,802 patients—34 in the rosuvastatin group and 60 in the placebo group.1 This translates to 0.18 and 0.32 events per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio for the rosuvastatin group 0.57, 95% confidence interval [CI] 0.37–0.86, P = .007).
Forty-four cases of VTE were classified as provoked and 50 cases were categorized as unprovoked. The risk reduction was statistically significant for provoked cases (hazard ratio 0.52, 95% CI 0.28–0.96, P = .03), but not for unprovoked events (hazard ratio 0.61, 95% CI 0.35–1.09, P = .09).
Subgroup analysis revealed no significant association between patient characteristics and the impact of rosuvastatin on the risk of a VTE event, but, as expected, more benefit was associated with higher baseline lipid levels.
STILL TOO SOON TO ADVISE ROUTINE STATIN USE TO PREVENT VTE
While the JUPITER trial data show an apparent benefit of statin use on the rate of VTE events, advising routine use of statins to prevent VTE is premature, for three main reasons.
Many must be treated to prevent one case of VTE. The number needed to treat (NNT) with rosuvastatin for 5 years to prevent either a case of VTE or a cardiovascular event was 21, and the NNT to prevent one cardiovascular event was 25. In a review of the two most recent case-control studies investigating the effects of statins on VTE,18,19 Cushman24 calculated that the NNT to prevent one VTE event each year was 333 for those age 75 and older. Though the Jupiter data did not provide the specific incidence of VTE at 1 year, except graphically, we can estimate that the NNT to prevent one VTE event at 1 year in the study is also very high.
Practically speaking, the perceived benefits of VTE prevention require large numbers to be treated, and the net clinical gain is still largely in preventing arterial events such as heart attack and stroke rather than VTE.
Statins, though safe, can still have adverse effects. The JUPITER study found a trend (albeit nonsignificant) toward more muscle complaints and elevations on liver function testing in apparently healthy persons taking a statin.22 Although severe complications of statin therapy such as rhabdomyolysis and elevations of creatine phosphokinase are rare, patients taking a statin have a 39% higher risk of an adverse event, most commonly myalgias or abnormalities on liver function testing.25 Were statins to be given routinely to even more people than they are now, more adverse outcomes would be likely.
More study is needed. The JUPITER study did not address a high risk of VTE. In fact, the investigators provided no information as to the VTE history of those enrolled.
Clearly, statins should not be substituted for proven prophylaxis and anticoagulation without further investigation, especially for patients with recurrent deep venous thrombosis, hospitalized patients, postoperative patients, and other patients prone to VTE.
OUR VIEW
The JUPITER study is an important leap forward in adding to our knowledge of how to prevent VTE. For people with another indication for taking a statin (eg, a previous cardiovascular event, hyperlipidemia), it is helpful to know that their risk of VTE may be reduced without exposure to the risks of other kinds of conventional thromboprophylaxis.
We look forward to additional studies to elaborate on the benefits of statins in both the prevention and treatment of VTE for averagerisk and VTE-prone populations.
- Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009; 360:1851–1861.
- Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med 2003; 348:1435–1441.
- Prandoni P, Ghirarduzzi A, Prins MH, et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost 2006; 4:1891–1896.
- Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromso study. J Thromb Haemost 2008; 6:1851–1857.
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 2002; 162:1182–1189.
- van der Hagen PB, Folsom AR, Jenny NS, et al. Subclinical atherosclerosis and the risk of future venous thrombosis in the Cardiovascular Health Study. J Thromb Haemost 2006; 4:1903–1908.
- Reich LM, Folsom AR, Key NS, et al. Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost 2006; 4:1909–1913.
- Huerta C, Johansson S, Wallander MA, Rodriguez LA. Risk of myocardial infarction and overall mortality in survivors of venous thromboembolism. Thromb J 2008; 6:10.
- Linnemann B, Schindewolf M, Zgouras D, Erbe M, Jarosch-Preusche M, Lindhoff-Last E. Are patients with thrombophilia and previous venous thromboembolism at higher risk to arterial thrombosis? Thromb Res 2008; 121:743–750.
- Schwaiger J, Kiechl S, Stockner H, et al. Burden of atherosclerosis and risk of venous thromboembolism in patients with migraine. Neurology 2008; 71:937–943.
- Linnemann B, Zgouras D, Schindewolf M, Schwonberg J, Jarosch-Preusche M, Lindhoff-Last E. Impact of sex and traditional cardiovascular risk factors on the risk of recurrent venous thromboembolism: results from the German MAISTHRO Registry. Blood Coagul Fibrinolysis 2008; 19:159–165.
- Steffen LM, Folsom AR, Cushman M, Jacobs DR, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation 2007; 115:188–195.
- Arslan F, Pasterkamp G, de Kleijn DP. Unraveling pleiotropic effects of statins: bit by bit, a slow case with perspective. Circ Res 2008; 103:334–336.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2000; 132:689–696.
- Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med 2001; 161:1405–1410.
- Doggen CJ, Lemaitre RN, Smith NL, Heckbert SR, Psaty BM. HMG CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost 2004; 2:700–701.
- Lacut K, Oger E, Le Gal G, et al. Statins but not fibrates are associated with a reduced risk of venous thromboembolism: a hospitalbased case-control study. Fundam Clin Pharmacol 2004; 18:477–482.
- Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost 2009; 7:514–520.
- Sørensen HT, Horvath-Puho E, Sogaard KK, et al. Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 2009; 7:521–528.
- Yang CC, Jick SS, Jick H. Statins and the risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 2002; 53:101–105.
- Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009; 67:99–109.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shishehbor MH, Hazen SL. JUPITER to Earth: A statin helps peole with normal LDL-C and high hs-CRP, but what does it mean? Cleve Clin J Med 2009; 76:37–44.
- Cushman M. A new indication for statins to prevent venous thromboembolism? Not yet. J Thromb Haemost 2009; 7:511–513.
- Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther 2006; 28:26–35.
A major placebo-controlled trial has found that a statin can reduce the risk of venous thromboembolism (VTE).1
We do not recommend prescribing this class of drugs for this purpose until much more research has been done, and we certainly do not recommend substituting a statin for anticoagulant therapy in a patient at risk of VTE.
Nevertheless, we are excited by the latest findings, and we find comfort in knowing that if a patient is taking a statin for an approved indication, ie, reducing the risk of cardiovascular disease in a patient with hyperlipidemia or a previous cardiovascular event, the drug will also reduce the risk of VTE.
In the pages that follow, we describe and comment on what is known about the effect of statins on the risk of VTE.
ARTERIAL AND VENOUS THROMBOSIS: HOW ARE THEY LINKED?
The causes of arterial thrombosis may not be entirely distinct from those of deep vein thrombosis and pulmonary embolism, collectively referred to as VTE. Some studies have found that risk factors for arterial thrombosis overlap with those for VTE.2–4 However, other studies have shown no association between venous and arterial events.5–10
Hyperlipidemia, in particular, has been evaluated to see if it is a risk factor for VTE. As with other risk factors for arterial thrombosis, the data have been mixed, with some reports favoring an association with VTE and others not.4,5,11 Even so, preventive strategies targeting arterial risk factors have shown promise in reducing VTE events.12
Although commonly used to treat hyperlipidemia, statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are believed to reduce the incidence of thrombosis by a number of mechanisms13:
- Decreasing platelet aggregation
- Inhibiting expression of tissue factor and plasminogen activator inhibitor 1
- Increasing expression of tissue plasminogen activator
- Increasing expression of thrombomodulin, which can activate protein C and prevent thrombin-induced platelet and factor V activation and fibrinogen clotting.
STATINS AND VTE IN OBSERVATIONAL AND CASE-CONTROL STUDIES
In view of the multiple effects of statins, several studies have looked at whether these drugs reduce the occurrence of both arterial thrombosis and VTE.14–19
Two prospective observational studies and four case-control studies found that statins reduced the risk of VTE by 20% to 60%.14–19 Interestingly, two of the case-control studies found that antiplatelet therapy did not reduce the risk of VTE.18,19
THE JUPITER STUDY
The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study primarily sought to determine if rosuvastatin (Crestor) 20 mg/day, compared with placebo, would reduce the rate of first major cardiovascular events.22 A prespecified secondary end point of the trial was VTE, making JUPITER the first randomized, placebo-controlled trial to specifically test whether statins prevent VTE.1
Inclusion criteria: Normal LDL, high CRP
The study included men age 50 and older and women age 60 and older with no history of cardiovascular disease. In addition, their lowdensity lipoprotein (LDL) cholesterol levels had to be lower than 130 mg/dL (3.4 mmol/L), their triglyceride levels had to be lower than 500 mg/dL (5.6 mmol/L), and their highsensitivity C-reactive protein (hs-CRP) levels had to be 2.0 mg/L or higher.
Since high levels of hs-CRP, a marker of inflammation, predict cardiovascular events and since statins lower hs-CRP levels, the investigators hypothesized that people with elevated hs-CRP but without hyperlipidemia might benefit from statin treatment.21
Patients were excluded if they had received lipid-lowering therapy within 6 weeks of the trial screening, had diabetes mellitus or uncontrolled hypertension, were currently using postmenopausal hormone-replacement therapy, or had had cancer within the previous 5 years, except for certain skin cancers.
Candidates who complied well during a 4-week placebo run-in phase were randomly assigned to receive either rosuvastatin 20 mg daily (an intermediate dose) or a matching placebo. In all, 17,802 people were randomized. The two assigned groups appeared to be well matched.
Patients were to come in for visits twice a year for 60 months after randomization to be assessed for symptomatic deep venous thrombosis and pulmonary embolism. New cases of VTE were confirmed by imaging studies, by the initiation of anticoagulation therapy, or by death ascribed to pulmonary embolism.
Idiopathic VTE was classified as unprovoked if it occurred in the absence of trauma, hospitalization, or surgery within 3 months before the event, and in the absence of any diagnosed cancer within 3 months before and after the event. Provoked VTE events were those that occurred in a participant with cancer or when a precipitating event was associated with trauma, hospitalization, or surgery.
Rosuvastatin prevents heart attack, stroke
On the recommendation of the trial’s independent data and safety monitoring board, JUPITER was stopped early because the trial drug showed evidence of efficacy in preventing the combined primary end point of a first major cardiovascular event—ie, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from a cardiovascular cause.22 (The cardiovascular outcomes of the JUPITER study were reviewed by Shishehbor and Hazen23 in the January 2009 issue of the Cleveland Clinic Journal of Medicine; see doi:10.3949/ccjm.75a.08105).
Formal follow-up for the trial's primary and secondary efficacy end points ended then, but data on VTE continued to be collected until each patient’s closeout visit as part of a safety monitoring protocol. The last closeout visit occurred on August 20, 2008. The primary analysis focused on events occurring up to March 30, 2008, the date the study was stopped.
Secondary end point results: Rosuvastatin prevents VTE
At a median follow-up of 1.9 years, an episode of VTE had occurred in 94 (0.53%) of the 17,802 patients—34 in the rosuvastatin group and 60 in the placebo group.1 This translates to 0.18 and 0.32 events per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio for the rosuvastatin group 0.57, 95% confidence interval [CI] 0.37–0.86, P = .007).
Forty-four cases of VTE were classified as provoked and 50 cases were categorized as unprovoked. The risk reduction was statistically significant for provoked cases (hazard ratio 0.52, 95% CI 0.28–0.96, P = .03), but not for unprovoked events (hazard ratio 0.61, 95% CI 0.35–1.09, P = .09).
Subgroup analysis revealed no significant association between patient characteristics and the impact of rosuvastatin on the risk of a VTE event, but, as expected, more benefit was associated with higher baseline lipid levels.
STILL TOO SOON TO ADVISE ROUTINE STATIN USE TO PREVENT VTE
While the JUPITER trial data show an apparent benefit of statin use on the rate of VTE events, advising routine use of statins to prevent VTE is premature, for three main reasons.
Many must be treated to prevent one case of VTE. The number needed to treat (NNT) with rosuvastatin for 5 years to prevent either a case of VTE or a cardiovascular event was 21, and the NNT to prevent one cardiovascular event was 25. In a review of the two most recent case-control studies investigating the effects of statins on VTE,18,19 Cushman24 calculated that the NNT to prevent one VTE event each year was 333 for those age 75 and older. Though the Jupiter data did not provide the specific incidence of VTE at 1 year, except graphically, we can estimate that the NNT to prevent one VTE event at 1 year in the study is also very high.
Practically speaking, the perceived benefits of VTE prevention require large numbers to be treated, and the net clinical gain is still largely in preventing arterial events such as heart attack and stroke rather than VTE.
Statins, though safe, can still have adverse effects. The JUPITER study found a trend (albeit nonsignificant) toward more muscle complaints and elevations on liver function testing in apparently healthy persons taking a statin.22 Although severe complications of statin therapy such as rhabdomyolysis and elevations of creatine phosphokinase are rare, patients taking a statin have a 39% higher risk of an adverse event, most commonly myalgias or abnormalities on liver function testing.25 Were statins to be given routinely to even more people than they are now, more adverse outcomes would be likely.
More study is needed. The JUPITER study did not address a high risk of VTE. In fact, the investigators provided no information as to the VTE history of those enrolled.
Clearly, statins should not be substituted for proven prophylaxis and anticoagulation without further investigation, especially for patients with recurrent deep venous thrombosis, hospitalized patients, postoperative patients, and other patients prone to VTE.
OUR VIEW
The JUPITER study is an important leap forward in adding to our knowledge of how to prevent VTE. For people with another indication for taking a statin (eg, a previous cardiovascular event, hyperlipidemia), it is helpful to know that their risk of VTE may be reduced without exposure to the risks of other kinds of conventional thromboprophylaxis.
We look forward to additional studies to elaborate on the benefits of statins in both the prevention and treatment of VTE for averagerisk and VTE-prone populations.
A major placebo-controlled trial has found that a statin can reduce the risk of venous thromboembolism (VTE).1
We do not recommend prescribing this class of drugs for this purpose until much more research has been done, and we certainly do not recommend substituting a statin for anticoagulant therapy in a patient at risk of VTE.
Nevertheless, we are excited by the latest findings, and we find comfort in knowing that if a patient is taking a statin for an approved indication, ie, reducing the risk of cardiovascular disease in a patient with hyperlipidemia or a previous cardiovascular event, the drug will also reduce the risk of VTE.
In the pages that follow, we describe and comment on what is known about the effect of statins on the risk of VTE.
ARTERIAL AND VENOUS THROMBOSIS: HOW ARE THEY LINKED?
The causes of arterial thrombosis may not be entirely distinct from those of deep vein thrombosis and pulmonary embolism, collectively referred to as VTE. Some studies have found that risk factors for arterial thrombosis overlap with those for VTE.2–4 However, other studies have shown no association between venous and arterial events.5–10
Hyperlipidemia, in particular, has been evaluated to see if it is a risk factor for VTE. As with other risk factors for arterial thrombosis, the data have been mixed, with some reports favoring an association with VTE and others not.4,5,11 Even so, preventive strategies targeting arterial risk factors have shown promise in reducing VTE events.12
Although commonly used to treat hyperlipidemia, statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are believed to reduce the incidence of thrombosis by a number of mechanisms13:
- Decreasing platelet aggregation
- Inhibiting expression of tissue factor and plasminogen activator inhibitor 1
- Increasing expression of tissue plasminogen activator
- Increasing expression of thrombomodulin, which can activate protein C and prevent thrombin-induced platelet and factor V activation and fibrinogen clotting.
STATINS AND VTE IN OBSERVATIONAL AND CASE-CONTROL STUDIES
In view of the multiple effects of statins, several studies have looked at whether these drugs reduce the occurrence of both arterial thrombosis and VTE.14–19
Two prospective observational studies and four case-control studies found that statins reduced the risk of VTE by 20% to 60%.14–19 Interestingly, two of the case-control studies found that antiplatelet therapy did not reduce the risk of VTE.18,19
THE JUPITER STUDY
The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study primarily sought to determine if rosuvastatin (Crestor) 20 mg/day, compared with placebo, would reduce the rate of first major cardiovascular events.22 A prespecified secondary end point of the trial was VTE, making JUPITER the first randomized, placebo-controlled trial to specifically test whether statins prevent VTE.1
Inclusion criteria: Normal LDL, high CRP
The study included men age 50 and older and women age 60 and older with no history of cardiovascular disease. In addition, their lowdensity lipoprotein (LDL) cholesterol levels had to be lower than 130 mg/dL (3.4 mmol/L), their triglyceride levels had to be lower than 500 mg/dL (5.6 mmol/L), and their highsensitivity C-reactive protein (hs-CRP) levels had to be 2.0 mg/L or higher.
Since high levels of hs-CRP, a marker of inflammation, predict cardiovascular events and since statins lower hs-CRP levels, the investigators hypothesized that people with elevated hs-CRP but without hyperlipidemia might benefit from statin treatment.21
Patients were excluded if they had received lipid-lowering therapy within 6 weeks of the trial screening, had diabetes mellitus or uncontrolled hypertension, were currently using postmenopausal hormone-replacement therapy, or had had cancer within the previous 5 years, except for certain skin cancers.
Candidates who complied well during a 4-week placebo run-in phase were randomly assigned to receive either rosuvastatin 20 mg daily (an intermediate dose) or a matching placebo. In all, 17,802 people were randomized. The two assigned groups appeared to be well matched.
Patients were to come in for visits twice a year for 60 months after randomization to be assessed for symptomatic deep venous thrombosis and pulmonary embolism. New cases of VTE were confirmed by imaging studies, by the initiation of anticoagulation therapy, or by death ascribed to pulmonary embolism.
Idiopathic VTE was classified as unprovoked if it occurred in the absence of trauma, hospitalization, or surgery within 3 months before the event, and in the absence of any diagnosed cancer within 3 months before and after the event. Provoked VTE events were those that occurred in a participant with cancer or when a precipitating event was associated with trauma, hospitalization, or surgery.
Rosuvastatin prevents heart attack, stroke
On the recommendation of the trial’s independent data and safety monitoring board, JUPITER was stopped early because the trial drug showed evidence of efficacy in preventing the combined primary end point of a first major cardiovascular event—ie, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from a cardiovascular cause.22 (The cardiovascular outcomes of the JUPITER study were reviewed by Shishehbor and Hazen23 in the January 2009 issue of the Cleveland Clinic Journal of Medicine; see doi:10.3949/ccjm.75a.08105).
Formal follow-up for the trial's primary and secondary efficacy end points ended then, but data on VTE continued to be collected until each patient’s closeout visit as part of a safety monitoring protocol. The last closeout visit occurred on August 20, 2008. The primary analysis focused on events occurring up to March 30, 2008, the date the study was stopped.
Secondary end point results: Rosuvastatin prevents VTE
At a median follow-up of 1.9 years, an episode of VTE had occurred in 94 (0.53%) of the 17,802 patients—34 in the rosuvastatin group and 60 in the placebo group.1 This translates to 0.18 and 0.32 events per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio for the rosuvastatin group 0.57, 95% confidence interval [CI] 0.37–0.86, P = .007).
Forty-four cases of VTE were classified as provoked and 50 cases were categorized as unprovoked. The risk reduction was statistically significant for provoked cases (hazard ratio 0.52, 95% CI 0.28–0.96, P = .03), but not for unprovoked events (hazard ratio 0.61, 95% CI 0.35–1.09, P = .09).
Subgroup analysis revealed no significant association between patient characteristics and the impact of rosuvastatin on the risk of a VTE event, but, as expected, more benefit was associated with higher baseline lipid levels.
STILL TOO SOON TO ADVISE ROUTINE STATIN USE TO PREVENT VTE
While the JUPITER trial data show an apparent benefit of statin use on the rate of VTE events, advising routine use of statins to prevent VTE is premature, for three main reasons.
Many must be treated to prevent one case of VTE. The number needed to treat (NNT) with rosuvastatin for 5 years to prevent either a case of VTE or a cardiovascular event was 21, and the NNT to prevent one cardiovascular event was 25. In a review of the two most recent case-control studies investigating the effects of statins on VTE,18,19 Cushman24 calculated that the NNT to prevent one VTE event each year was 333 for those age 75 and older. Though the Jupiter data did not provide the specific incidence of VTE at 1 year, except graphically, we can estimate that the NNT to prevent one VTE event at 1 year in the study is also very high.
Practically speaking, the perceived benefits of VTE prevention require large numbers to be treated, and the net clinical gain is still largely in preventing arterial events such as heart attack and stroke rather than VTE.
Statins, though safe, can still have adverse effects. The JUPITER study found a trend (albeit nonsignificant) toward more muscle complaints and elevations on liver function testing in apparently healthy persons taking a statin.22 Although severe complications of statin therapy such as rhabdomyolysis and elevations of creatine phosphokinase are rare, patients taking a statin have a 39% higher risk of an adverse event, most commonly myalgias or abnormalities on liver function testing.25 Were statins to be given routinely to even more people than they are now, more adverse outcomes would be likely.
More study is needed. The JUPITER study did not address a high risk of VTE. In fact, the investigators provided no information as to the VTE history of those enrolled.
Clearly, statins should not be substituted for proven prophylaxis and anticoagulation without further investigation, especially for patients with recurrent deep venous thrombosis, hospitalized patients, postoperative patients, and other patients prone to VTE.
OUR VIEW
The JUPITER study is an important leap forward in adding to our knowledge of how to prevent VTE. For people with another indication for taking a statin (eg, a previous cardiovascular event, hyperlipidemia), it is helpful to know that their risk of VTE may be reduced without exposure to the risks of other kinds of conventional thromboprophylaxis.
We look forward to additional studies to elaborate on the benefits of statins in both the prevention and treatment of VTE for averagerisk and VTE-prone populations.
- Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009; 360:1851–1861.
- Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med 2003; 348:1435–1441.
- Prandoni P, Ghirarduzzi A, Prins MH, et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost 2006; 4:1891–1896.
- Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromso study. J Thromb Haemost 2008; 6:1851–1857.
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 2002; 162:1182–1189.
- van der Hagen PB, Folsom AR, Jenny NS, et al. Subclinical atherosclerosis and the risk of future venous thrombosis in the Cardiovascular Health Study. J Thromb Haemost 2006; 4:1903–1908.
- Reich LM, Folsom AR, Key NS, et al. Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost 2006; 4:1909–1913.
- Huerta C, Johansson S, Wallander MA, Rodriguez LA. Risk of myocardial infarction and overall mortality in survivors of venous thromboembolism. Thromb J 2008; 6:10.
- Linnemann B, Schindewolf M, Zgouras D, Erbe M, Jarosch-Preusche M, Lindhoff-Last E. Are patients with thrombophilia and previous venous thromboembolism at higher risk to arterial thrombosis? Thromb Res 2008; 121:743–750.
- Schwaiger J, Kiechl S, Stockner H, et al. Burden of atherosclerosis and risk of venous thromboembolism in patients with migraine. Neurology 2008; 71:937–943.
- Linnemann B, Zgouras D, Schindewolf M, Schwonberg J, Jarosch-Preusche M, Lindhoff-Last E. Impact of sex and traditional cardiovascular risk factors on the risk of recurrent venous thromboembolism: results from the German MAISTHRO Registry. Blood Coagul Fibrinolysis 2008; 19:159–165.
- Steffen LM, Folsom AR, Cushman M, Jacobs DR, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation 2007; 115:188–195.
- Arslan F, Pasterkamp G, de Kleijn DP. Unraveling pleiotropic effects of statins: bit by bit, a slow case with perspective. Circ Res 2008; 103:334–336.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2000; 132:689–696.
- Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med 2001; 161:1405–1410.
- Doggen CJ, Lemaitre RN, Smith NL, Heckbert SR, Psaty BM. HMG CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost 2004; 2:700–701.
- Lacut K, Oger E, Le Gal G, et al. Statins but not fibrates are associated with a reduced risk of venous thromboembolism: a hospitalbased case-control study. Fundam Clin Pharmacol 2004; 18:477–482.
- Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost 2009; 7:514–520.
- Sørensen HT, Horvath-Puho E, Sogaard KK, et al. Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 2009; 7:521–528.
- Yang CC, Jick SS, Jick H. Statins and the risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 2002; 53:101–105.
- Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009; 67:99–109.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shishehbor MH, Hazen SL. JUPITER to Earth: A statin helps peole with normal LDL-C and high hs-CRP, but what does it mean? Cleve Clin J Med 2009; 76:37–44.
- Cushman M. A new indication for statins to prevent venous thromboembolism? Not yet. J Thromb Haemost 2009; 7:511–513.
- Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther 2006; 28:26–35.
- Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009; 360:1851–1861.
- Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med 2003; 348:1435–1441.
- Prandoni P, Ghirarduzzi A, Prins MH, et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost 2006; 4:1891–1896.
- Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromso study. J Thromb Haemost 2008; 6:1851–1857.
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 2002; 162:1182–1189.
- van der Hagen PB, Folsom AR, Jenny NS, et al. Subclinical atherosclerosis and the risk of future venous thrombosis in the Cardiovascular Health Study. J Thromb Haemost 2006; 4:1903–1908.
- Reich LM, Folsom AR, Key NS, et al. Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost 2006; 4:1909–1913.
- Huerta C, Johansson S, Wallander MA, Rodriguez LA. Risk of myocardial infarction and overall mortality in survivors of venous thromboembolism. Thromb J 2008; 6:10.
- Linnemann B, Schindewolf M, Zgouras D, Erbe M, Jarosch-Preusche M, Lindhoff-Last E. Are patients with thrombophilia and previous venous thromboembolism at higher risk to arterial thrombosis? Thromb Res 2008; 121:743–750.
- Schwaiger J, Kiechl S, Stockner H, et al. Burden of atherosclerosis and risk of venous thromboembolism in patients with migraine. Neurology 2008; 71:937–943.
- Linnemann B, Zgouras D, Schindewolf M, Schwonberg J, Jarosch-Preusche M, Lindhoff-Last E. Impact of sex and traditional cardiovascular risk factors on the risk of recurrent venous thromboembolism: results from the German MAISTHRO Registry. Blood Coagul Fibrinolysis 2008; 19:159–165.
- Steffen LM, Folsom AR, Cushman M, Jacobs DR, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation 2007; 115:188–195.
- Arslan F, Pasterkamp G, de Kleijn DP. Unraveling pleiotropic effects of statins: bit by bit, a slow case with perspective. Circ Res 2008; 103:334–336.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2000; 132:689–696.
- Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med 2001; 161:1405–1410.
- Doggen CJ, Lemaitre RN, Smith NL, Heckbert SR, Psaty BM. HMG CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost 2004; 2:700–701.
- Lacut K, Oger E, Le Gal G, et al. Statins but not fibrates are associated with a reduced risk of venous thromboembolism: a hospitalbased case-control study. Fundam Clin Pharmacol 2004; 18:477–482.
- Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost 2009; 7:514–520.
- Sørensen HT, Horvath-Puho E, Sogaard KK, et al. Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 2009; 7:521–528.
- Yang CC, Jick SS, Jick H. Statins and the risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 2002; 53:101–105.
- Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009; 67:99–109.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shishehbor MH, Hazen SL. JUPITER to Earth: A statin helps peole with normal LDL-C and high hs-CRP, but what does it mean? Cleve Clin J Med 2009; 76:37–44.
- Cushman M. A new indication for statins to prevent venous thromboembolism? Not yet. J Thromb Haemost 2009; 7:511–513.
- Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther 2006; 28:26–35.
KEY POINTS
- Risk factors for VTE overlap with those for arterial thrombosis, although the data are mixed.
- The statin drugs have a number of effects on factors other than lipid levels, notably on markers of inflammation and on clotting factors.
- In the JUPITER trial, the incidence of VTE in people taking rosuvastatin (Crestor) 20 mg/day was about half that in people taking placebo. This was a relatively healthy population, and the incidence in both groups was low.
- Further study is needed in patients at risk of VTE.
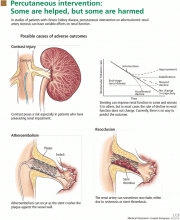
Stenting for atherosclerotic renal artery stenosis: One poorly designed trial after another
The role of stenting for atherosclerotic renal artery stenosis is hotly debated among different specialties.1,2 If we may generalize a bit, interventionalists (cardiologists, interventional radiologists, vascular surgeons, and vascular medicine specialists) have been in favor of liberal use of stenting, and nephrologists often favor medical therapy alone. And as with all controversial issues, each group feels rather strongly about its position.
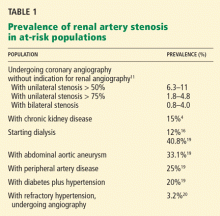
Because few prospective randomized trials have been completed, the management of atherosclerotic renal artery stenosis has been guided by retrospective studies and case series. 3
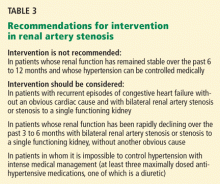
In this issue of the Cleveland Clinic Journal of Medicine, Dr. James Simon4 provides an excellent overview of the prevalence, natural history, and clinical presentation of atherosclerotic renal artery stenosis. In addition, he does an admirable job of reviewing the available prospective randomized trials and providing editorial commentary about the role of the various specialists in the management of renal artery disease. And while the title of his paper says that it is “time to be less aggressive,” Dr. Simon ultimately comes to the same conclusions that we do5 on the indications for renal artery stenting (see Table 3 of Dr. Simon’s article), which are those of the multidisciplinary 2006 American College of Cardiology/American Heart Association guidelines on the management of peripheral artery disease.3
So what then is all the controversy about? We all agree that prospective randomized trials that provide class I, level A evidence impart the only unbiased scientific information on the best treatment strategy for patients with renal artery disease. The basic controversial issue is the interpretation of these trials. We contend that the three randomized trials of stenting vs medical therapy published so far6–8 (see below) are so seriously flawed that it is impossible to make treatment decisions based on their results.
Since these trials were published in wellrespected journals, their results are often taken as gospel. However, careful review of each of these will reveal the flaws in study design and implementation.
THE DRASTIC TRIAL
In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial,6 106 patients with renal artery stenosis and hypertension (diastolic blood pressure > 95 mm Hg) despite treatment with two antihypertensive medications were randomly assigned to either renal angioplasty (n = 56) or drug therapy (n = 50).
Authors’ conclusions
“In the treatment of patients with hypertension and renal-artery stenosis, angioplasty has little advantage over antihypertensive-drug therapy.”6
Four serious problems
As we discussed in a letter to the editor of the New England Journal of Medicine on August 10, 2000, this study had four serious problems that invalidate its authors’ conclusions.9
The sample size was insufficient to detect a significant difference between treatment groups. In other words, the chance of a type 2 statistical error is high.
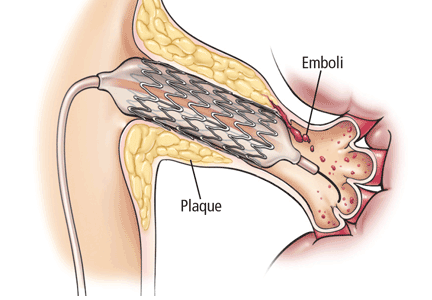
Balloon angioplasty without stenting was used as the method of revascularization. Experts now recognize that stenting is required for renal artery intervention to have a durable result.3,5
Renal artery stenosis was defined as greater than 50% stenosis. This allowed a large number of patients to enter the trial who had hemodynamically and clinically insignificant lesions. Most clinicians believe that stenosis of less than 70% is not hemodynamically important.5,10,11
Twenty-two of the 50 patients randomized to medical therapy crossed over to the angioplasty group because their blood pressure became difficult to control. In other words, 44% of the patients in the medical group underwent angioplasty, an astounding percentage in an intention-to-treat analysis comparing one therapy with another.
Despite these serious flaws, the results of DRASTIC influenced therapy for years after its publication.
THE STAR TRIAL
In the Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,7 140 patients with a creatinine clearance of less than 80 mL/min/1.73m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy (n = 64) or medical therapy alone (n = 76). The primary end point was a 20% or greater decrease in creatinine clearance. Secondary end points included measures of safety and cardiovascular morbidity and mortality.
Authors’ conclusions
“Stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. The study findings favor a conservative approach to patients with [atherosclerotic renal artery stenosis], focused on cardiovascular risk factor management and avoiding stenting.”7
Serious flaws
A number of serious flaws render this study uninterpretable.
Mild renal artery stenosis. At least 33% of the patients in the study had mild renal artery stenosis (50%–70%), and 12 (19%) of the 64 patients in the group randomized to stenting actually had stenosis of less than 50%. How can one expect there to be a benefit to stenting in patients with mild (and hemodynamically insignificant) renal artery stenosis? This is especially true when the primary end point is a change in renal function.
More than half of the patients had unilateral disease. It seems intuitive that if one were to plan a trial with a primary end point of a change in renal function, only patients with bilateral renal artery stenosis of greater than 70% or with stenosis of greater than 70% to a solitary functioning kidney would be included. One would not expect that patients with unilateral disease and a stenosis of less than 70% would derive any benefit from revascularization.
Not all “stent” patients received stents. All of the patients in the medical group received medication and there were no crossovers. However, only 46 (72%) of the 64 patients randomized to stenting actually received a stent, while 18 (28%) did not. There were two technical failures, and 12 patients should not have been randomized because they had less than 50% stenosis on angiography and thus were not stented. Yet all 64 patients were analyzed (by intention to treat) in the stent group. With these numbers, one could predict that the results would be negative.
Like DRASTIC, this trial was underpowered, meaning that the chance of a type 2 statistical error is high. In fact, the editors of the Annals of Internal Medicine, in a note accompanying the article, cautioned that the study “was underpowered to provide a definitive estimate of efficacy.”7 If the study was underpowered to answer the question at hand, why was it deemed worthy of publication?
High complication rates. The periprocedural complication and death rates were much higher than in many other reports on renal artery stenting (see details below).5
THE ASTRAL TRIAL
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial,8 the primary outcome measure was the change in renal function over time as assessed by the mean slope of the reciprocal of the serum creatinine. In this trial, 806 patients with atherosclerotic renal artery stenosis were randomized to either stent-based revascularization combined with medical therapy or medical therapy alone.
Authors’ conclusions
“We found substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.”8
Despite size, flaws remain
Unlike the other trials, ASTRAL had a sample size large enough to provide an answer. However, numerous flaws in study design and implementation invalidate its results for the overall population of patients with renal artery stenosis. The major flaws in ASTRAL were:
Selection bias. For a patient to be enrolled, the treating physician had to be undecided on whether the patient should undergo revascularization or medical management alone. However, the treatment of atherosclerotic renal artery stenosis is so controversial that physicians of different specialties cannot agree on the most effective treatment strategy for most patients.1,2 Therefore, to exclude patients when their physicians were sure they needed or did not need renal artery revascularization is incomprehensible and introduces considerable selection bias into the trial design.
Normal renal function at baseline. The primary outcome was a change in renal function over time. Yet 25% of patients had normal renal function at the outset of the trial. In addition, a significant number had unilateral disease, and 41% had a stenosis less than 70%. What made the investigators think that stent implantation could possibly be shown to be beneficial if they entered patients into a renal function study who had near-normal renal function, unilateral disease, and mild renal artery stenosis? These are patients whose condition would not be expected to worsen with medical therapy nor to improve with stenting. Most clinicians would not consider stenting a patient to preserve renal function if the patient has unilateral mild renal artery stenosis.
There was no core laboratory to adjudicate the interpretation of the imaging studies. To determine the degree of stenosis of an artery in an accurate and unbiased fashion, a core laboratory must be used.
The reason this is so important is that visual assessment of the degree of stenosis on angiography is not accurate and almost always overestimates the degree of stenosis.12,13 In a study assessing the physiologic importance of renal artery lesions, the lesion severity by visual estimation was 74.9% ± 11.5% (range 50%–90%), which exceeded the quantitative vascular angiographic lesion severity of 56.6% ± 10.8% (range 45%–76%).13
Therefore, in ASTRAL, some patients in the 50%–70% stenosis group (about 40% of patients entered) actually had a stenosis of less than 50%. And some patients in the group with stenosis greater than 70% had stenosis of less than 70%. This further illustrates that, for the most part, the patients in ASTRAL had mild to moderate renal artery stenosis.
A high adverse event rate. The major adverse event rate in the first 24 hours was 9%, whereas the usual rate is 2% or less.14–18 Of the 280 patients in the revascularization group for whom data on adverse events were available at 1 month, 55 (20%) suffered a serious adverse event (including two patients who died) between 24 hours and 1 month after the procedure. This is in contrast to a major complication rate of 1.99% in five reports involving 727 patients.5
The trial centers were not high-volume centers. During the 7 years of recruitment, 24 centers (42% of all participating centers) randomized between one and five patients, and 32 centers (61% of all participating centers) randomized nine patients or fewer. This means that many participating centers randomized, on average, less than one patient per year! This was not a group of high-volume operators.
WILL CORAL GIVE US THE ANSWER?
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial is under way.19 Enrollment was to have ended on January 31, 2010, and it will be several years before the data are available for analysis.
CORAL, a multicenter study funded in 2004 by the National Institutes of Health, will have randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting. Inclusion criteria are a documented history of hypertension on two or more antihypertensive drugs or renal dysfunction, defined as stage 3 or greater chronic kidney disease based on the National Kidney Foundation classification (estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the modified Modification of Diet in Renal Disease [MDRD] formula) and stenosis of 60% or greater but less than 100%, as assessed by a core laboratory. The primary end point is survival free of cardiovascular and renal adverse events, defined as a composite of cardiovascular or renal death, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or need for permanent renal replacement therapy.
We hope this trial will give us a clear answer to the question of whether renal artery stenting is beneficial in the patient population studied. One note of caution: recruitment for this trial was difficult and slow. Thus, there were a number of protocol amendments throughout the trial in order to make recruitment easier. Hopefully, this will not be a problem when analyzing the results.
WE ALL AGREE ON THE INDICATIONS FOR STENTING
So, are we really so far apart in our thinking? And is it really “time to be less aggressive” if we follow the precepts below?
All renal arteries with stenosis do not need to be (and should not be) stented.
There must be a good clinical indicationandhemodynamically significant stenosis. This means the degree of stenosis should be more than 70% on angiography or intravascular ultrasonography.
Indications for stenting. Until more data from compelling randomized trials become available, adherence to the American College of Cardiology/American Heart Association guidelines on indications for renal artery stenting is advised3:
- Hypertension: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with hemodynamically significant renal artery stenosis and accelerated hypertension, resistant hypertension, and malignant hypertension.
- Preservation of renal function: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with renal artery stenosis and progressive chronic kidney disease with bilateral renal artery stenosis or a stenosis to a solitary functioning kidney.
- Congestive heart failure: class I, level of evidence B. Percutaneous revascularization is indicated for patients with hemodynamically significant renal artery stenosis (ie, > 70% stenosis on angiography or intravascular ultrasonography) and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema.
- Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? The case for renal artery stenting for treatment of renal artery stenosis. Circulation 2007; 115:263–269.
- Dworkin LD, Jamerson KA. Is renal artery stenting the correct treatment of renal artery stenosis? Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation 2007; 115:271–276.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006; 113:e463–e654.
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med 2010; 77:178–189.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–841.
- Wheatley K, Ives N, Gray R, et al Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Tan WA, Wholey MH, Olin JW. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis [letter]. N Engl J Med 2000; 343:438.
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation 2008; 118:2873–2878.
- Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Intervent 2009; 2:175–182.
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92:2333–2342.
- Subramanian R, White CJ, Rosenfield K, et al Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv 2005; 64:480–486.
- Burket MW, Cooper CJ, Kennedy DJ, et al Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000; 139:64–71.
- Dorros G, Jaff M, Mathiak L, et al Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998; 98:642–647.
- Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 2005; 46:776–783.
- Tuttle KR, Chouinard RF, Webber JT, et al Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32:611–622.
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol 1997; 30:1445–1450.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
The role of stenting for atherosclerotic renal artery stenosis is hotly debated among different specialties.1,2 If we may generalize a bit, interventionalists (cardiologists, interventional radiologists, vascular surgeons, and vascular medicine specialists) have been in favor of liberal use of stenting, and nephrologists often favor medical therapy alone. And as with all controversial issues, each group feels rather strongly about its position.
Because few prospective randomized trials have been completed, the management of atherosclerotic renal artery stenosis has been guided by retrospective studies and case series. 3
In this issue of the Cleveland Clinic Journal of Medicine, Dr. James Simon4 provides an excellent overview of the prevalence, natural history, and clinical presentation of atherosclerotic renal artery stenosis. In addition, he does an admirable job of reviewing the available prospective randomized trials and providing editorial commentary about the role of the various specialists in the management of renal artery disease. And while the title of his paper says that it is “time to be less aggressive,” Dr. Simon ultimately comes to the same conclusions that we do5 on the indications for renal artery stenting (see Table 3 of Dr. Simon’s article), which are those of the multidisciplinary 2006 American College of Cardiology/American Heart Association guidelines on the management of peripheral artery disease.3
So what then is all the controversy about? We all agree that prospective randomized trials that provide class I, level A evidence impart the only unbiased scientific information on the best treatment strategy for patients with renal artery disease. The basic controversial issue is the interpretation of these trials. We contend that the three randomized trials of stenting vs medical therapy published so far6–8 (see below) are so seriously flawed that it is impossible to make treatment decisions based on their results.
Since these trials were published in wellrespected journals, their results are often taken as gospel. However, careful review of each of these will reveal the flaws in study design and implementation.
THE DRASTIC TRIAL
In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial,6 106 patients with renal artery stenosis and hypertension (diastolic blood pressure > 95 mm Hg) despite treatment with two antihypertensive medications were randomly assigned to either renal angioplasty (n = 56) or drug therapy (n = 50).
Authors’ conclusions
“In the treatment of patients with hypertension and renal-artery stenosis, angioplasty has little advantage over antihypertensive-drug therapy.”6
Four serious problems
As we discussed in a letter to the editor of the New England Journal of Medicine on August 10, 2000, this study had four serious problems that invalidate its authors’ conclusions.9
The sample size was insufficient to detect a significant difference between treatment groups. In other words, the chance of a type 2 statistical error is high.
Balloon angioplasty without stenting was used as the method of revascularization. Experts now recognize that stenting is required for renal artery intervention to have a durable result.3,5
Renal artery stenosis was defined as greater than 50% stenosis. This allowed a large number of patients to enter the trial who had hemodynamically and clinically insignificant lesions. Most clinicians believe that stenosis of less than 70% is not hemodynamically important.5,10,11
Twenty-two of the 50 patients randomized to medical therapy crossed over to the angioplasty group because their blood pressure became difficult to control. In other words, 44% of the patients in the medical group underwent angioplasty, an astounding percentage in an intention-to-treat analysis comparing one therapy with another.
Despite these serious flaws, the results of DRASTIC influenced therapy for years after its publication.
THE STAR TRIAL
In the Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,7 140 patients with a creatinine clearance of less than 80 mL/min/1.73m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy (n = 64) or medical therapy alone (n = 76). The primary end point was a 20% or greater decrease in creatinine clearance. Secondary end points included measures of safety and cardiovascular morbidity and mortality.
Authors’ conclusions
“Stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. The study findings favor a conservative approach to patients with [atherosclerotic renal artery stenosis], focused on cardiovascular risk factor management and avoiding stenting.”7
Serious flaws
A number of serious flaws render this study uninterpretable.
Mild renal artery stenosis. At least 33% of the patients in the study had mild renal artery stenosis (50%–70%), and 12 (19%) of the 64 patients in the group randomized to stenting actually had stenosis of less than 50%. How can one expect there to be a benefit to stenting in patients with mild (and hemodynamically insignificant) renal artery stenosis? This is especially true when the primary end point is a change in renal function.
More than half of the patients had unilateral disease. It seems intuitive that if one were to plan a trial with a primary end point of a change in renal function, only patients with bilateral renal artery stenosis of greater than 70% or with stenosis of greater than 70% to a solitary functioning kidney would be included. One would not expect that patients with unilateral disease and a stenosis of less than 70% would derive any benefit from revascularization.
Not all “stent” patients received stents. All of the patients in the medical group received medication and there were no crossovers. However, only 46 (72%) of the 64 patients randomized to stenting actually received a stent, while 18 (28%) did not. There were two technical failures, and 12 patients should not have been randomized because they had less than 50% stenosis on angiography and thus were not stented. Yet all 64 patients were analyzed (by intention to treat) in the stent group. With these numbers, one could predict that the results would be negative.
Like DRASTIC, this trial was underpowered, meaning that the chance of a type 2 statistical error is high. In fact, the editors of the Annals of Internal Medicine, in a note accompanying the article, cautioned that the study “was underpowered to provide a definitive estimate of efficacy.”7 If the study was underpowered to answer the question at hand, why was it deemed worthy of publication?
High complication rates. The periprocedural complication and death rates were much higher than in many other reports on renal artery stenting (see details below).5
THE ASTRAL TRIAL
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial,8 the primary outcome measure was the change in renal function over time as assessed by the mean slope of the reciprocal of the serum creatinine. In this trial, 806 patients with atherosclerotic renal artery stenosis were randomized to either stent-based revascularization combined with medical therapy or medical therapy alone.
Authors’ conclusions
“We found substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.”8
Despite size, flaws remain
Unlike the other trials, ASTRAL had a sample size large enough to provide an answer. However, numerous flaws in study design and implementation invalidate its results for the overall population of patients with renal artery stenosis. The major flaws in ASTRAL were:
Selection bias. For a patient to be enrolled, the treating physician had to be undecided on whether the patient should undergo revascularization or medical management alone. However, the treatment of atherosclerotic renal artery stenosis is so controversial that physicians of different specialties cannot agree on the most effective treatment strategy for most patients.1,2 Therefore, to exclude patients when their physicians were sure they needed or did not need renal artery revascularization is incomprehensible and introduces considerable selection bias into the trial design.
Normal renal function at baseline. The primary outcome was a change in renal function over time. Yet 25% of patients had normal renal function at the outset of the trial. In addition, a significant number had unilateral disease, and 41% had a stenosis less than 70%. What made the investigators think that stent implantation could possibly be shown to be beneficial if they entered patients into a renal function study who had near-normal renal function, unilateral disease, and mild renal artery stenosis? These are patients whose condition would not be expected to worsen with medical therapy nor to improve with stenting. Most clinicians would not consider stenting a patient to preserve renal function if the patient has unilateral mild renal artery stenosis.
There was no core laboratory to adjudicate the interpretation of the imaging studies. To determine the degree of stenosis of an artery in an accurate and unbiased fashion, a core laboratory must be used.
The reason this is so important is that visual assessment of the degree of stenosis on angiography is not accurate and almost always overestimates the degree of stenosis.12,13 In a study assessing the physiologic importance of renal artery lesions, the lesion severity by visual estimation was 74.9% ± 11.5% (range 50%–90%), which exceeded the quantitative vascular angiographic lesion severity of 56.6% ± 10.8% (range 45%–76%).13
Therefore, in ASTRAL, some patients in the 50%–70% stenosis group (about 40% of patients entered) actually had a stenosis of less than 50%. And some patients in the group with stenosis greater than 70% had stenosis of less than 70%. This further illustrates that, for the most part, the patients in ASTRAL had mild to moderate renal artery stenosis.
A high adverse event rate. The major adverse event rate in the first 24 hours was 9%, whereas the usual rate is 2% or less.14–18 Of the 280 patients in the revascularization group for whom data on adverse events were available at 1 month, 55 (20%) suffered a serious adverse event (including two patients who died) between 24 hours and 1 month after the procedure. This is in contrast to a major complication rate of 1.99% in five reports involving 727 patients.5
The trial centers were not high-volume centers. During the 7 years of recruitment, 24 centers (42% of all participating centers) randomized between one and five patients, and 32 centers (61% of all participating centers) randomized nine patients or fewer. This means that many participating centers randomized, on average, less than one patient per year! This was not a group of high-volume operators.
WILL CORAL GIVE US THE ANSWER?
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial is under way.19 Enrollment was to have ended on January 31, 2010, and it will be several years before the data are available for analysis.
CORAL, a multicenter study funded in 2004 by the National Institutes of Health, will have randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting. Inclusion criteria are a documented history of hypertension on two or more antihypertensive drugs or renal dysfunction, defined as stage 3 or greater chronic kidney disease based on the National Kidney Foundation classification (estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the modified Modification of Diet in Renal Disease [MDRD] formula) and stenosis of 60% or greater but less than 100%, as assessed by a core laboratory. The primary end point is survival free of cardiovascular and renal adverse events, defined as a composite of cardiovascular or renal death, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or need for permanent renal replacement therapy.
We hope this trial will give us a clear answer to the question of whether renal artery stenting is beneficial in the patient population studied. One note of caution: recruitment for this trial was difficult and slow. Thus, there were a number of protocol amendments throughout the trial in order to make recruitment easier. Hopefully, this will not be a problem when analyzing the results.
WE ALL AGREE ON THE INDICATIONS FOR STENTING
So, are we really so far apart in our thinking? And is it really “time to be less aggressive” if we follow the precepts below?
All renal arteries with stenosis do not need to be (and should not be) stented.
There must be a good clinical indicationandhemodynamically significant stenosis. This means the degree of stenosis should be more than 70% on angiography or intravascular ultrasonography.
Indications for stenting. Until more data from compelling randomized trials become available, adherence to the American College of Cardiology/American Heart Association guidelines on indications for renal artery stenting is advised3:
- Hypertension: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with hemodynamically significant renal artery stenosis and accelerated hypertension, resistant hypertension, and malignant hypertension.
- Preservation of renal function: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with renal artery stenosis and progressive chronic kidney disease with bilateral renal artery stenosis or a stenosis to a solitary functioning kidney.
- Congestive heart failure: class I, level of evidence B. Percutaneous revascularization is indicated for patients with hemodynamically significant renal artery stenosis (ie, > 70% stenosis on angiography or intravascular ultrasonography) and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema.
The role of stenting for atherosclerotic renal artery stenosis is hotly debated among different specialties.1,2 If we may generalize a bit, interventionalists (cardiologists, interventional radiologists, vascular surgeons, and vascular medicine specialists) have been in favor of liberal use of stenting, and nephrologists often favor medical therapy alone. And as with all controversial issues, each group feels rather strongly about its position.
Because few prospective randomized trials have been completed, the management of atherosclerotic renal artery stenosis has been guided by retrospective studies and case series. 3
In this issue of the Cleveland Clinic Journal of Medicine, Dr. James Simon4 provides an excellent overview of the prevalence, natural history, and clinical presentation of atherosclerotic renal artery stenosis. In addition, he does an admirable job of reviewing the available prospective randomized trials and providing editorial commentary about the role of the various specialists in the management of renal artery disease. And while the title of his paper says that it is “time to be less aggressive,” Dr. Simon ultimately comes to the same conclusions that we do5 on the indications for renal artery stenting (see Table 3 of Dr. Simon’s article), which are those of the multidisciplinary 2006 American College of Cardiology/American Heart Association guidelines on the management of peripheral artery disease.3
So what then is all the controversy about? We all agree that prospective randomized trials that provide class I, level A evidence impart the only unbiased scientific information on the best treatment strategy for patients with renal artery disease. The basic controversial issue is the interpretation of these trials. We contend that the three randomized trials of stenting vs medical therapy published so far6–8 (see below) are so seriously flawed that it is impossible to make treatment decisions based on their results.
Since these trials were published in wellrespected journals, their results are often taken as gospel. However, careful review of each of these will reveal the flaws in study design and implementation.
THE DRASTIC TRIAL
In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial,6 106 patients with renal artery stenosis and hypertension (diastolic blood pressure > 95 mm Hg) despite treatment with two antihypertensive medications were randomly assigned to either renal angioplasty (n = 56) or drug therapy (n = 50).
Authors’ conclusions
“In the treatment of patients with hypertension and renal-artery stenosis, angioplasty has little advantage over antihypertensive-drug therapy.”6
Four serious problems
As we discussed in a letter to the editor of the New England Journal of Medicine on August 10, 2000, this study had four serious problems that invalidate its authors’ conclusions.9
The sample size was insufficient to detect a significant difference between treatment groups. In other words, the chance of a type 2 statistical error is high.
Balloon angioplasty without stenting was used as the method of revascularization. Experts now recognize that stenting is required for renal artery intervention to have a durable result.3,5
Renal artery stenosis was defined as greater than 50% stenosis. This allowed a large number of patients to enter the trial who had hemodynamically and clinically insignificant lesions. Most clinicians believe that stenosis of less than 70% is not hemodynamically important.5,10,11
Twenty-two of the 50 patients randomized to medical therapy crossed over to the angioplasty group because their blood pressure became difficult to control. In other words, 44% of the patients in the medical group underwent angioplasty, an astounding percentage in an intention-to-treat analysis comparing one therapy with another.
Despite these serious flaws, the results of DRASTIC influenced therapy for years after its publication.
THE STAR TRIAL
In the Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,7 140 patients with a creatinine clearance of less than 80 mL/min/1.73m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy (n = 64) or medical therapy alone (n = 76). The primary end point was a 20% or greater decrease in creatinine clearance. Secondary end points included measures of safety and cardiovascular morbidity and mortality.
Authors’ conclusions
“Stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. The study findings favor a conservative approach to patients with [atherosclerotic renal artery stenosis], focused on cardiovascular risk factor management and avoiding stenting.”7
Serious flaws
A number of serious flaws render this study uninterpretable.
Mild renal artery stenosis. At least 33% of the patients in the study had mild renal artery stenosis (50%–70%), and 12 (19%) of the 64 patients in the group randomized to stenting actually had stenosis of less than 50%. How can one expect there to be a benefit to stenting in patients with mild (and hemodynamically insignificant) renal artery stenosis? This is especially true when the primary end point is a change in renal function.
More than half of the patients had unilateral disease. It seems intuitive that if one were to plan a trial with a primary end point of a change in renal function, only patients with bilateral renal artery stenosis of greater than 70% or with stenosis of greater than 70% to a solitary functioning kidney would be included. One would not expect that patients with unilateral disease and a stenosis of less than 70% would derive any benefit from revascularization.
Not all “stent” patients received stents. All of the patients in the medical group received medication and there were no crossovers. However, only 46 (72%) of the 64 patients randomized to stenting actually received a stent, while 18 (28%) did not. There were two technical failures, and 12 patients should not have been randomized because they had less than 50% stenosis on angiography and thus were not stented. Yet all 64 patients were analyzed (by intention to treat) in the stent group. With these numbers, one could predict that the results would be negative.
Like DRASTIC, this trial was underpowered, meaning that the chance of a type 2 statistical error is high. In fact, the editors of the Annals of Internal Medicine, in a note accompanying the article, cautioned that the study “was underpowered to provide a definitive estimate of efficacy.”7 If the study was underpowered to answer the question at hand, why was it deemed worthy of publication?
High complication rates. The periprocedural complication and death rates were much higher than in many other reports on renal artery stenting (see details below).5
THE ASTRAL TRIAL
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial,8 the primary outcome measure was the change in renal function over time as assessed by the mean slope of the reciprocal of the serum creatinine. In this trial, 806 patients with atherosclerotic renal artery stenosis were randomized to either stent-based revascularization combined with medical therapy or medical therapy alone.
Authors’ conclusions
“We found substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.”8
Despite size, flaws remain
Unlike the other trials, ASTRAL had a sample size large enough to provide an answer. However, numerous flaws in study design and implementation invalidate its results for the overall population of patients with renal artery stenosis. The major flaws in ASTRAL were:
Selection bias. For a patient to be enrolled, the treating physician had to be undecided on whether the patient should undergo revascularization or medical management alone. However, the treatment of atherosclerotic renal artery stenosis is so controversial that physicians of different specialties cannot agree on the most effective treatment strategy for most patients.1,2 Therefore, to exclude patients when their physicians were sure they needed or did not need renal artery revascularization is incomprehensible and introduces considerable selection bias into the trial design.
Normal renal function at baseline. The primary outcome was a change in renal function over time. Yet 25% of patients had normal renal function at the outset of the trial. In addition, a significant number had unilateral disease, and 41% had a stenosis less than 70%. What made the investigators think that stent implantation could possibly be shown to be beneficial if they entered patients into a renal function study who had near-normal renal function, unilateral disease, and mild renal artery stenosis? These are patients whose condition would not be expected to worsen with medical therapy nor to improve with stenting. Most clinicians would not consider stenting a patient to preserve renal function if the patient has unilateral mild renal artery stenosis.
There was no core laboratory to adjudicate the interpretation of the imaging studies. To determine the degree of stenosis of an artery in an accurate and unbiased fashion, a core laboratory must be used.
The reason this is so important is that visual assessment of the degree of stenosis on angiography is not accurate and almost always overestimates the degree of stenosis.12,13 In a study assessing the physiologic importance of renal artery lesions, the lesion severity by visual estimation was 74.9% ± 11.5% (range 50%–90%), which exceeded the quantitative vascular angiographic lesion severity of 56.6% ± 10.8% (range 45%–76%).13
Therefore, in ASTRAL, some patients in the 50%–70% stenosis group (about 40% of patients entered) actually had a stenosis of less than 50%. And some patients in the group with stenosis greater than 70% had stenosis of less than 70%. This further illustrates that, for the most part, the patients in ASTRAL had mild to moderate renal artery stenosis.
A high adverse event rate. The major adverse event rate in the first 24 hours was 9%, whereas the usual rate is 2% or less.14–18 Of the 280 patients in the revascularization group for whom data on adverse events were available at 1 month, 55 (20%) suffered a serious adverse event (including two patients who died) between 24 hours and 1 month after the procedure. This is in contrast to a major complication rate of 1.99% in five reports involving 727 patients.5
The trial centers were not high-volume centers. During the 7 years of recruitment, 24 centers (42% of all participating centers) randomized between one and five patients, and 32 centers (61% of all participating centers) randomized nine patients or fewer. This means that many participating centers randomized, on average, less than one patient per year! This was not a group of high-volume operators.
WILL CORAL GIVE US THE ANSWER?
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial is under way.19 Enrollment was to have ended on January 31, 2010, and it will be several years before the data are available for analysis.
CORAL, a multicenter study funded in 2004 by the National Institutes of Health, will have randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting. Inclusion criteria are a documented history of hypertension on two or more antihypertensive drugs or renal dysfunction, defined as stage 3 or greater chronic kidney disease based on the National Kidney Foundation classification (estimated glomerular filtration rate < 60 mL/min/1.73 m2 calculated by the modified Modification of Diet in Renal Disease [MDRD] formula) and stenosis of 60% or greater but less than 100%, as assessed by a core laboratory. The primary end point is survival free of cardiovascular and renal adverse events, defined as a composite of cardiovascular or renal death, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive renal insufficiency, or need for permanent renal replacement therapy.
We hope this trial will give us a clear answer to the question of whether renal artery stenting is beneficial in the patient population studied. One note of caution: recruitment for this trial was difficult and slow. Thus, there were a number of protocol amendments throughout the trial in order to make recruitment easier. Hopefully, this will not be a problem when analyzing the results.
WE ALL AGREE ON THE INDICATIONS FOR STENTING
So, are we really so far apart in our thinking? And is it really “time to be less aggressive” if we follow the precepts below?
All renal arteries with stenosis do not need to be (and should not be) stented.
There must be a good clinical indicationandhemodynamically significant stenosis. This means the degree of stenosis should be more than 70% on angiography or intravascular ultrasonography.
Indications for stenting. Until more data from compelling randomized trials become available, adherence to the American College of Cardiology/American Heart Association guidelines on indications for renal artery stenting is advised3:
- Hypertension: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with hemodynamically significant renal artery stenosis and accelerated hypertension, resistant hypertension, and malignant hypertension.
- Preservation of renal function: class IIa, level of evidence B. Percutaneous revascularization is reasonable for patients with renal artery stenosis and progressive chronic kidney disease with bilateral renal artery stenosis or a stenosis to a solitary functioning kidney.
- Congestive heart failure: class I, level of evidence B. Percutaneous revascularization is indicated for patients with hemodynamically significant renal artery stenosis (ie, > 70% stenosis on angiography or intravascular ultrasonography) and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema.
- Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? The case for renal artery stenting for treatment of renal artery stenosis. Circulation 2007; 115:263–269.
- Dworkin LD, Jamerson KA. Is renal artery stenting the correct treatment of renal artery stenosis? Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation 2007; 115:271–276.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006; 113:e463–e654.
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med 2010; 77:178–189.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–841.
- Wheatley K, Ives N, Gray R, et al Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Tan WA, Wholey MH, Olin JW. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis [letter]. N Engl J Med 2000; 343:438.
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation 2008; 118:2873–2878.
- Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Intervent 2009; 2:175–182.
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92:2333–2342.
- Subramanian R, White CJ, Rosenfield K, et al Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv 2005; 64:480–486.
- Burket MW, Cooper CJ, Kennedy DJ, et al Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000; 139:64–71.
- Dorros G, Jaff M, Mathiak L, et al Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998; 98:642–647.
- Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 2005; 46:776–783.
- Tuttle KR, Chouinard RF, Webber JT, et al Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32:611–622.
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol 1997; 30:1445–1450.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
- Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? The case for renal artery stenting for treatment of renal artery stenosis. Circulation 2007; 115:263–269.
- Dworkin LD, Jamerson KA. Is renal artery stenting the correct treatment of renal artery stenosis? Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation 2007; 115:271–276.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006; 113:e463–e654.
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve Clin J Med 2010; 77:178–189.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–841.
- Wheatley K, Ives N, Gray R, et al Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Tan WA, Wholey MH, Olin JW. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis [letter]. N Engl J Med 2000; 343:438.
- Rocha-Singh KJ, Eisenhauer AC, Textor SC, et al Atherosclerotic Peripheral Vascular Disease Symposium II: intervention for renal artery disease. Circulation 2008; 118:2873–2878.
- Textor SC, Lerman L, McKusick M. The uncertain value of renal artery interventions: where are we now? JACC Cardiovasc Intervent 2009; 2:175–182.
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92:2333–2342.
- Subramanian R, White CJ, Rosenfield K, et al Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv 2005; 64:480–486.
- Burket MW, Cooper CJ, Kennedy DJ, et al Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000; 139:64–71.
- Dorros G, Jaff M, Mathiak L, et al Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998; 98:642–647.
- Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 2005; 46:776–783.
- Tuttle KR, Chouinard RF, Webber JT, et al Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 1998; 32:611–622.
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J Am Coll Cardiol 1997; 30:1445–1450.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
Stenting atherosclerotic renal arteries: Time to be less aggressive
Author’s note: Atherosclerosis accounts for about 90% of cases of renal artery stenosis in people over age 40.1 Fibromuscular dysplasia, the other major cause, is a separate topic; in this paper “renal artery stenosis” refers to atherosclerotic disease only.
Renal artery stenosis is very common, and the number of angioplasty-stenting procedures performed every year is on the rise. Yet there is no overwhelming evidence that intervention yields clinical benefits—ie, better blood pressure control or renal function— than does medical therapy.
Earlier randomized controlled trials comparing angioplasty without stents and medical management showed no impressive difference in blood pressure.2,3 The data on renal function were even more questionable, with some studies suggesting that, with stenting, the chance of worsening renal function is equal to that of improvement.4
Two large randomized trials comparing renal intervention with medical therapy failed to show any benefit of intervention.5–7 A third study is under way.8
It is time to strongly reconsider the current aggressive approach to revascularization of stenotic renal arteries and take a more coordinated, critical approach.
RENAL INTERVENTIONS ON THE RISE
Renal angioplasty began replacing surgical revascularization in the 1990s, as this less-invasive procedure became more readily available and was shown to have similar clinical outcomes.9 In the last decade, stent placement during angioplasty has become standard, improving the rates of technical success.
As these procedures have become easier to perform and their radiographic outcomes have become more consistent, interventionalists have become more likely, if they see stenosis in a renal artery, to intervene and insert a stent, regardless of proven benefit. In addition, interventionalists from at least three different specialties now compete for these procedures, often by looking at the renal arteries during angiography of other vascular beds (the “drive-by”).
As a result, the number of renal interventions has been rising. Medicare received 21,660 claims for renal artery interventions (surgery or angioplasty) in 2000, compared with 13,380 in 1996—an increase of 62%. However, the number of surgeries actually decreased by 45% during this time, while the number of percutaneous procedures increased by 240%. The number of endovascular claims for renal artery stenosis by cardiologists alone rose 390%.10 Since then, the reports on intervention have been mixed, with one report citing a continued increase in 2005 to 35,000 claims,11 and another suggesting a decrease back to 1997 levels.12
HOW COMMON IS RENAL ARTERY STENOSIS?
The prevalence of renal artery stenosis depends on the definition used and the population screened. It is more common in older patients who have risk factors for other vascular diseases than in the general population.
Renal Doppler ultrasonography can detect stenosis only if the artery is narrowed by more than 60%. Hansen et al13 used ultrasonography to screen 870 people over age 65 and found a lesion (a narrowing of more than 60%) in 6.8%.
Angiography (direct, computed tomographic, or magnetic resonance) can detect less-severe stenosis. Thus, most angiographic studies define renal artery stenosis as a narrowing of more than 50%, and severe disease as a narrowing of more than 70%. Many experts believe that unilateral stenosis needs to be more than 70% to pose a risk to the kidney.14,15
Angiographic prevalence studies have been performed only in patients who were undergoing angiography for another reason such as coronary or peripheral arterial disease that inherently places them at higher risk of renal artery stenosis. For instance, renal artery stenosis is found in 11% to 28% of patients undergoing diagnostic cardiac catheterization. 16
No studies of the prevalence of renal artery stenosis have been performed in the general population. Medicare data indicate that from 1999 to 2001 the incidence of diagnosed renal artery stenosis was 3.7 per 1,000 patientyears. 17 Holley et al,18 in an autopsy series, found renal artery stenosis of greater than 50% in 27% of patients over age 50 and in 56.4% of hypertensive patients. The prevalence was 10% in normotensive patients.
WHO IS AT RISK?
Factors associated with a higher risk of finding renal artery stenosis on a radiographic study include14:
- Older age
- Female sex
- Hypertension
- Three-vessel coronary artery disease
- Peripheral artery disease
- Chronic kidney disease
- Diabetes
- A low level of high-density lipoprotein cholesterol
- The use of at least two cardiovascular drugs.
The prevalence of renal artery stenosis in at-risk populations ranges from 3% to 75% (Table 1).2,4,6,19,20
HOW OFTEN DOES STENOSIS PROGRESS?
The reported rates of progression of atherosclerotic renal artery lesions vary depending on the type of imaging test used and the reason for doing it.
In studies that used duplex ultrasonography, roughly half of lesions smaller than 60% grew to greater than 60% over 3 years.21,22 The risk of total occlusion of an artery was relatively low and depended on the severity of stenosis: 0.7% if the baseline stenosis was less than 60% and 2.3% to 7% if it was greater.21,22
In a seminal study in 1984, Schreiber and colleagues23 compared serial angiograms obtained a mean of 52 months apart in 85 patients who did not undergo intervention. Stenosis had progressed in 37 (44%), and to the point of total occlusion in 14 (16%). In contrast, a 1998 study found progression in 11.1% over 2.6 years, with older patients, women, and those with baseline coronary artery disease at higher risk.24
The the rates of progression differed in these two studies probably because the indications for screening were different (clinical suspicion23 vs routine screening during coronary angiography24), as was the severity of stenosis at the time of diagnosis. Also, when these studies were done, fewer people were taking statins. Thus, similar studies, if repeated, might show even lower rates of progression.
Finally, progression of renal artery stenosis has not been correlated with worsening renal function.
FOUR CLINICAL PRESENTATIONS OF RENAL ARTERY STENOSIS
Renal artery stenosis can present in one of four ways:
Clinically silent stenosis. Because renal artery stenosis is most often found in older patients, who are more likely to have essential hypertension and chronic kidney disease due to other causes, it can be an incidental finding that is completely clinically silent.16,25
Renovascular hypertension is defined as high blood pressure due to up-regulation of neurohormonal activity in response to decreased perfusion from renal artery stenosis. Renal artery stenosis is estimated to be the cause of hypertension in only 0.5% to 4.0% of hypertensive patients, or in 26% of patients with secondary hypertension.3
Ischemic nephropathy is more difficult to define because ischemia alone rarely explains the damage done to the kidneys. Activation of neurohormonal pathways and microvascular injury are thought to contribute to oxidative stress and fibrosis.26 These phenomena may explain why similar degrees of stenosis lead to varying degrees of kidney damage in different patients and why the severity of stenosis does not correlate with the degree of renal dysfunction.27
Furthermore, stenosis may lead to irreversible but stable kidney damage. It is therefore not surprising that, in studies in unselected populations (ie, studies that included patients with all presentations of renal artery stenosis, not just those more likely to benefit), up to two-thirds of renal interventions yielded no clinical benefit.25
As a result, if we define ischemic nephropathy as renal artery stenosis with renal dysfunction not attributable to another cause, we probably will overestimate the prevalence of ischemic nephropathy, leading to overly optimistic expectations about the response to revascularization.
Recurrent “flash” pulmonary edema is a less common presentation, usually occurring in patients with critical bilateral renal artery stenosis or unilateral stenosis in an artery supplying a solitary functioning kidney. Most have severe hypertension (average systolic blood pressure 174–207 mm Hg) and poor renal function.28–30
The association between pulmonary edema and bilateral renal artery stenosis was first noted in 1998 by Pickering et al,31 who in several case series showed that 82% to 92% of patients with recurrent pulmonary edema and renal artery stenosis had bilateral stenosis, compared with 20% to 65% of those with other presentations. Later case series corroborated this finding: 85% to 100% of patients with renal artery stenosis and pulmonary edema had bilateral stenosis.28–30
STENTING IS NOW STANDARD
Stenting has become standard in the endovascular treatment of renal artery stenosis.
Most atherosclerotic renal artery lesions are located in the ostium (ie, where the artery branches off from the aorta), and many are extensions of calcified aortic plaque.26,32 These hard lesions tend to rebound to their original shape more often with balloon angioplasty alone. Stenting provides the additional force needed to permanently disrupt the lesion, leading to a longer-lasting result.
Rates of technical success (dilating the artery during the intervention) are higher with stents than without them (98% vs 46%– 77%).33,34 If the lesion is ostial, this difference is even more impressive (75% vs 29%). In addition, restenosis rates at 6 months are lower with stents (14% vs 26%–48%).34
GOALS: LOWER THE BLOOD PRESSURE, SAVE THE KIDNEY
Because endovascular procedures pose some risk to the patient, it is critical to intervene only in patients most likely to respond clinically. The decision to intervene depends largely on the clinical goal, which should depend on the clinical presentation.
However, if renal artery stenosis is clinically silent, most of the evidence suggests that intervention has no benefit. Furthermore, although retrospective studies have indicated that intervention may improve survival rates,35,36 prospective studies have not. Similarly, studies have not shown that intervention generally improves cardiovascular outcomes, even though renal artery stenosis is associated with cardiovascular risk.
Hypertension plus stenosis is not necessarily renovascular hypertension
Essential hypertension and clinically silent renal artery stenosis often coexist, which is why blood pressure control often does not improve after stenting. Also, essential hypertension often coexists with renovascular hypertension.37 In this situation, stenting may not eliminate the need for antihypertensive drugs, although it may improve blood pressure control and decrease the drug burden.
Before stents came into use, several randomized controlled trials found that blood pressure was no better controlled after angioplasty, 2,3,38 except in cases of bilateral stenosis.2 This may be because stenosis tended to recur after angioplasty without stents.
The 2000 Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) study was the first randomized controlled trial to examine the effect of angioplasty on blood pressure control in renal artery stenosis.38 It had significant design flaws. For example, many patients crossed over from the medical management group to the intervention group because their hypertension was resistant to medical therapy. Overall, intervention (balloon angioplasty without stents in 54 of 56 patients, with stents in the other 2) carried no benefit. However, in subgroup analysis, the patients who crossed over because of resistant hypertension (failure of a three-drug regimen) were more likely to benefit from angioplasty. This suggested that risk stratification should take place early on, before proceeding with revascularization.
With stents, Zeller,39 in a prospective nonrandomized study, found that the mean arterial pressure decreased by 10 mm Hg. Randomized trials (see below) have failed to demonstrate such a benefit.
Stenting may not improve renal function
Coincidental renal artery stenosis in a patient with unrelated chronic kidney disease is very hard to differentiate from true ischemic nephropathy. Furthermore, most patients with ischemic nephropathy do not benefit from revascularization, making it challenging to identify those few whose renal function may respond.
Given that patients with chronic kidney disease tend to have a higher risk of cardiovascular disease, it is not surprising that 15% of them may have renal artery stenosis,4 most often incidental.
Chábová40 examined the outcomes of 68 patients who had chronic kidney disease and a renal artery lesion larger than 70% who did not undergo angioplasty. In only 10 (15%) of the patients did the glomerular filtration rate (GFR) decline by more than 50% of its baseline value during the study period of 3 years. Given the retrospective nature of the study, it cannot be determined (and is rather unlikely) that ischemic nephropathy was the cause of the decline in kidney function in all 10 patients.
In a prospective cohort study in 304 patients with chronic kidney disease and renal artery stenosis who underwent surgical revascularization, Textor4 reported that the serum creatinine level showed a meaningful improvement afterward in 28%, worsened in 19.7%, and remained unchanged in 160 52.6%. (A “meaningful” change was defined as > 1.0 mg/dL.) Findings were similar in a cohort that underwent stenting.33
Davies et al41 found that 20% of patients who underwent renal stenting had a persistent increase in serum creatinine of 0.5 mg/dL or more. These patients were nearly three times more likely (19% vs 7%) to eventually require dialysis, and they had a lower 5-year survival rate (41% vs 71%).
Zeller et al39 found that renal function improved slightly in 52% of patients who received stents. The mean decrease in serum creatinine in this group was 0.22 mg/dL. However, the other 48% had a mean increase in serum creatinine of 1.1 mg/dL.
From these data we can conclude that, in an unselected population with renal artery stenosis, stenting provides no benefit to renal function, and that the risk of a worsening of renal function after intervention is roughly equal to the likelihood of achieving any benefit.
Other predictors of improvement in renal function have been proposed, but the evidence supporting them has not been consistent. For example, although Radermacher et al42 reported that a renal resistive index (which reflects arterial stiffness downstream of the stenosis) lower than 0.8 predicted a response in renal function, this finding has not been reliably reproduced.43,44 Similarly, while several studies suggest that patients with milder renal dysfunction have a higher likelihood of a renal response,45,46 other studies suggest either that the opposite is true39 or that baseline renal function alone has no impact on outcome.47
In addition, once significant renal atrophy occurs, revascularization may not help much, since irreversible sclerosis has developed. Thus, the goal is to identify kidneys being harmed by renal artery stenosis during the ischemic phase, when the tissue is still viable.
Unfortunately, we still lack a good renal stress test—eg, analogous to the cardiac stress test—to diagnose reversible ischemia in the kidney. The captopril renal scan has that capability but is not accurate in patients with bilateral stenosis or a GFR less than 50 mL/min, severely limiting its applicability.26 Newer technologies such as blood-oxygen-level-dependent (BOLD) magnetic resonance imaging are being investigated for such a role.48
Cohort studies in patients with declining renal function
In several case series, patients whose renal function had been declining before intervention had impressive rates of better renal function afterward.33,39,47,49–54 In a prospective cohort study by Muray et al,47 a rise in serum creatinine of more than 0.1 mg/mL/month before intervention seemed to predict an improvement in renal function afterward.
One would expect that, for renal function to respond to intervention, severe bilateral stenosis or unilateral stenosis to a solitary functioning kidney would need to be present. However, this was an inconsistent finding in these case series.33,39,47,52,53 The Angioplasty and Stent for Renal Artery Lesions (ASTRAL) trial,6,7 discussed later, sheds a bit more light on this.
Stenting usually improves flash pulmonary edema
Acute pulmonary edema in the setting of bilateral renal artery stenosis seems to be a unique case in which improvement in clinical status can be expected in most patients after intervention. Blood pressure improves in 94% to 100% of patients,28,31 renal function either improves or stabilizes in 77% to 91%,28–31 and pulmonary edema resolves without recurrence in 77% to 100%.28–30
NEW RANDOMIZED TRIALS: STAR, ASTRAL, AND CORAL
Despite the lack of evidence supporting revascularization of renal artery stenosis, many interventionalists practice under the assumption that the radiographic finding of renal artery stenosis alone is an indication for renal revascularization. Only three randomized controlled trials in the modern era attempt to examine this hypothesis: STAR, ASTRAL, and CORAL.
STAR: No clear benefit
The Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial5 was a European multicenter trial that enrolled 140 patients with ostial renal artery stenosis greater than 50%, blood pressure controlled to less than 140/90 mm Hg, and creatinine clearance 15 to 80 mL/min.
Patients were randomized to undergo stenting or medical therapy alone. High blood pressure was treated according to a protocol in which angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers were relegated to second-line use. All patients received a statin, regardless of lipid levels.
At 2 years, the primary end point (a decline in creatinine clearance of 20% or greater) had been reached in 10 (16%) of the 64 patients in the stent group and 16 (22%) of the 76 patients in the medication group; the difference was not statistically significant (hazard ratio 0.73, 95% confidence interval 0.33–1.61). No difference was seen in the secondary end points of the degree of blood pressure control or the rates of cardiovascular morbidity and death.5
ASTRAL: Also no clear benefit
In the international, multicenter ASTRAL trial,6,7 806 patients with at least one stenotic renal artery considered suitable for balloon angioplasty, stenting, or both7 were randomized to undergo intervention or medical management. Hypertension treatment was not specified by a protocol. The mean estimated GFR was 40 mL/min. Most patients (95%–96%) were on statin therapy. The primary outcome was the rate of decline of renal function over time. Secondary outcomes included blood pressure control, renal events, cardiovascular events, and death.
Results. At a mean follow-up of 33.6 months (range 1–4 years), no difference was noted between treatment groups in decline in renal function or blood pressure control at 1 year. Renal function worsened slightly in both groups.
The decline in renal function over time, calculated as the mean slope of the reciprocal of the serum creatinine level over time, was slightly slower in the revascularization group, but the difference was not statistically significant (−0.07 × 10−3 vs −0.13 × 10−3 L/μmol/year, P = .06). This difference did not appear until the last year of the study. There was no difference in the number of patients whose renal function improved or declined during the study.
There was no difference in the rate of any secondary outcome. The medical management group required a slightly higher number of antihypertensive drugs, reaching statistical but not clinical significance (2.97 vs 2.77 drugs, P = .03). More people in the revascularization group were taking ACE inhibitors or angiotensin receptor blockers. There was no difference in the number of patients on any antihypertensive therapy (97% vs 99%). Interestingly, amputations were more common in the revascularization group, occurring in 42 (12%) of the 386 patients in the revascularization group vs 29 (7%) of the 395 patients in the medical group (P = .04).
Seventeen percent of patients randomized to intervention did not have the procedure done. An as-treated analysis of the 317 (83%) patients randomized to revascularization who did receive it showed no differences in outcomes.
There were no differences in outcomes among specific, predefined subgroups based on severity of stenosis at baseline, renal length, baseline estimated GFR, baseline serum creatinine, and rate of progression of renal dysfunction before randomization.7
Comments. ASTRAL contradicts previous nonrandomized studies that suggested that rapidly declining renal function (loss of 20% in 1 year) predicts response to intervention. Considering the large number of patients with unilateral disease in the study, it would be interesting to see what effect stenting had on patients with both severe disease and declining renal function.
ASTRAL has been criticized because it lacked a central laboratory to interpret the severity of stenosis, it did not use a standardized intervention technique (5% of patients underwent angioplasty without stents, although this did not affect outcomes7), and patients were enrolled only if the clinician involved in the case was uncertain of the appropriate management.
This last issue raises the concern for selection bias toward inclusion of more difficult cases that may not respond to intervention. But these shortcomings are not serious enough to negate the fact that preliminary results from the largest randomized controlled trial to date confirm conclusions of other randomized trials, ie, that intervention in renal artery stenosis yields no benefits over medical management in most patients.
Based on the results of STAR and ASTRAL, the practice of indiscriminately revascularizing stenosed renal arteries without strong evidence that the procedure will provide a clinical benefit is no longer tenable. The challenge is to identify those few patients who will respond, and to intervene only on them. Unfortunately, none of the subgroups from ASTRAL helped characterize this population.
CORAL: A large trial is ongoing
The Cardiovascular Outcomes in Renal Artherosclerotic Lesions (CORAL) trial,8 an ongoing multicenter randomized controlled trial in the United States, may be of additional help.
Unlike ASTRAL, CORAL is studying patients who have difficult-to-control hypertension (systolic blood pressure ≥ 155 mm Hg on two or more drugs).8 Chronic kidney disease is not an exclusion criterion unless the serum creatinine concentration is greater than 3.0 mg/dL.
CORAL is using a standardized medical protocol to control blood pressure. In addition, use of embolic protection devices during stenting is encouraged. Hopefully, the large size (a goal of 1,080 patients) and the inclusion of patients with more marked hypertension will address the utility of intervention in higher-risk populations with renal artery stenosis.
RECOMMENDED APPROACH TO INTERVENTION IN RENAL ARTERY STENOSIS
As we wait for CORAL to be completed, we have two modern-era randomized controlled trials that leave us with fewer indications for renal intervention. Table 2 lists commonly cited indications for intervention in renal artery stenosis and the evidence to support them. As most of these are based on retrospective data or have conflicting support in the literature, their utility remains in question. At this point we can safely recommend:
- Patients with preserved or even decreased but stable renal function will not likely have a benefit in renal function after intervention.
- Patients with resistant hypertension may benefit.
- The best evidence supporting intervention is for bilateral stenosis with flash pulmonary edema, but the evidence is from retrospective studies.
- Stenting in bilateral disease without another indication has no apparent benefit.
- Declining renal function is not a guarantee of success.
- It is unclear if patients with severe bilateral stenosis or severe stenosis to a solitary functioning kidney with declining renal function will benefit. Anecdotally, they do respond more often, but as with many other indications for intervention that have gone by the wayside, this may not bear out when studied properly.
As the utility of intervention narrows, the scope of practice for such interventions should narrow accordingly. Attention should now be focusing on clinical, rather than radiographic, indications for intervening on renal artery stenosis.
Therefore, the decision to intervene must not be made solely by the interventionalist. A multidisciplinary approach should be adopted that at the very least includes the input of a nephrologist well versed in renal artery stenosis. In this way, the clinical risks and benefits of renal intervention can be discussed with the patient by providers who are likely to be involved in their care should renal function or hypertension fail to improve afterward.
RISK OF ATHEROEMBOLISM
While renal stenting yields improved technical success in the treatment of renal artery stenosis, it carries with it an increasingly common risk to kidney function: atheroembolism as the stent crushes the plaque against the vessel wall. This may lead to obstruction of the renal microvasculature, increasing the risk of irreversible damage to renal function.
Atheroembolic kidney disease can manifest as progressive renal failure occurring over weeks to months, commonly misdiagnosed as permanent damage from contrast nephropathy.55
Embolic protection devices, inserted downstream of the lesion before stenting to catch any debris that may break loose, have been developed to help address this problem.
Holden et al 57 prospectively studied 63 patients with renal artery stenosis and deteriorating renal function (undefined) who underwent stenting with an embolic protection device. At 6 months after the intervention, renal function had either improved or stabilized in 97% of patients, suggesting that many of the deleterious effects of stenting on renal function are related to atheroembolism.
The Prospective Randomized Study Comparing Renal Artery Stenting With or Without Distal Protection (RESIST) trial, in which renal dysfunction was mild and the GFR was not declining (average estimated GFR 59.3 mL/min), found contrary results.57 In a two-by-two factorial study, patients were randomized to undergo stenting alone, stenting with the antiplatelet agent abciximab (ReoPro), stenting with an embolic protection device, or stenting with both abciximab and an embolic protection device. Interestingly, renal function declined in the first three groups, but remained stable in the group that received both abciximab and an embolic protection device.
ANTIPLATELET THERAPY AFTER RENAL STENTING: HOW LONG?
We have no data on the optimal duration of antiplatelet therapy after renal stenting, and guidelines from professional societies do not comment on it.58 As a result, practice patterns vary significantly among practitioners.
While in-stent restenosis rates are acceptably low after renal stenting, the risks and side effects of antiplatelet therapy often lead to arbitrary withdrawal of these drugs. The effect on stent patency is yet to be determined.
FUTURE DEVELOPMENTS
Results of STAR and ASTRAL confirm the growing suspicion that the surge seen in the last decade in renal artery stenting should be coming to an end. We await results either from CORAL or possibly a post hoc analysis of ASTRAL that might identify potential high-risk groups that will benefit from renal intervention. And as embolic protection devices become more agile and suitable to different renal lesions, there remains the possibility that, due to lower rates of unidentified atheroembolic kidney disease, CORAL may demonstrate improved renal outcomes after stenting. If not, the search for the best means to predict who should have renal intervention will continue.
We know through experience that stenting does provide great benefits for some patients with renal artery stenosis. Furthermore, the clinical problem is too intriguing, and too profitable, to die altogether.
- Choncol M, Linas S. Diagnosis and management of ischemic nephropathy. Clin J Am Soc Nephrol 2006; 1:172–181.
- Webster J, Marshall F, Abdalla M, et al Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998; 12:329–335.
- Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998; 31:823–829.
- Textor SC. Revascularization in atherosclerotic renal artery disease. Kidney Int 1998; 53:799–811.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848.
- Mistry S, Ives N, Harding J, et al Angioplasty and STent for Renal Artery Lesions (ASTRAL trial): rationale, methods and results so far. J Hum Hypertens 2007; 21:511–515.
- Wheatley K, Ives N, Kalra P, Moss J. Revascularization versus medical therapy for renal-artery stenosis (ASTRAL). N Engl J Med 2009; 361:1953–1962.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
- Galaria II, Surowiec SM, Rhodes JM, et al Percutaneous and open renal revascularizations have equivalent long-term functional outcomes. Ann Vasc Surg 2005; 19:218–228.
- Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by Medicare beneficiaries 1996–2000. AJR Am J Roentgenol 2004; 183:561–568.
- Textor SC. Atherosclerotic renal artery stenosis: overtreated but underrated? J Am Soc Nephrol 2008; 19:656–659.
- Kalra PA, Guo H, Gilbertson DT, et al Atherosclerotic renovascular disease in the United States. Kidney Int 2010; 77:37–43.
- Hansen KJ, Edwards MS, Craven TE, et al Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Cohen MG, Pascua JA, Garcia-Ben M, et al A simple prediction rule for significant renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 2005; 150:1204–1211.
- Buller CE, Nogareda JG, Ramanathan K, et al The profile of cardiac patients with renal artery stenosis. J Am Coll Cardiol 2004; 43:1606–1613.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- Kalra PA, Guo H, Kausz AT, et al Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 69:293–301.
- Holley KE, Hunt JC, Brown AL, Kincaid OW, Sheps SG. Renal artery stenosis. A clinical-pathologic study in normotensive and hypertensive patients. Am J Med 1964; 37:14–22.
- de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systemic literature review. J Hypertens 2009; 27:1333–1340.
- Kuczera P, Włoszczynska E, Adamczak M, Pencak P, Chudek J, Wiecek A. Frequency of renal artery stenosis and variants of renal vascularization in hypertensive patients: analysis of 1550 angiographies in one centre. J Hum Hypertens 2009; 23:396–401.
- Caps MT, Perissinotto C, Zierler RE, et al Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998; 98:2866–2872.
- Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens 1996; 9:1055–1061.
- Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol Clin North Am 1984; 11:383–392.
- Crowley JJ, Santos RM, Peter RH, et al Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 1998; 136:913–918.
- Textor SC. Renovascular hypertension update. Curr Hypertens Rep 2006; 8:521–527.
- Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol 2004; 15:1974–1982.
- Wright JR, Shurrab AE, Cheung C, et al A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis 2002; 39:1153–1161.
- Messina LM, Zelenock GB, Yao KA, Stanley JC. Renal revascularization for recurrent pulmonary edema in patients with poorly controlled hypertension and renal insufficiency: a distinct subgroup of patients with arteriosclerotic renal artery occlusive disease. J Vasc Surg 1992; 15:73–80.
- Bloch MJ, Trost DW, Pickering TG, Sos TA, August P. Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens 1999; 12:1–7.
- Gray BH, Olin JW, Childs MB, Sullivan TM, Bacharach JM. Clinical benefit of renal artery angioplasty with stenting for the control of recurrent and refractory congestive heart failure. Vasc Med 2002; 7:275–279.
- Pickering TG, Herman L, Devereux RB, et al Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet 1988; 2:551–552.
- Kennedy DJ, Colyer WR, Brewster PS, et al Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis 2003; 14:926–935.
- Beutler JJ, Van Ampting JM, Van De Ven PJ, et al Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol 2001; 12:1475–1481.
- Van de Ven PJ, Kaatee R, Beutler JJ, et al Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomized trial. Lancet 1999; 353:282–286.
- Isles C, Main J, O’Connell J, et al Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scott Med J 1990; 35:70–73.
- Hunt JC, Sheps SG, Harrison EG, Strong CG, Bernatz PE. Renal and renovascular hypertension. A reasoned approach to diagnosis and management. Arch Intern Med 1974; 133:988–999.
- Textor SC. Atherosclerotic renal artery stenosis: how big is the problem, and what happens if nothing is done? J Hypertens Suppl 2005; 23:S5–S13.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Zeller T, Frank U, Müller C, et al Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation 2003; 108;2244–2249.
- Chábová V, Schirger A, Stanson AW, McKusick MA, Textor SC. Outcomes of atherosclerotic renal artery stenosis managed without revascularization. Mayo Clin Proc 2000; 75:437–444.
- Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. Implications of acute functional injury following percutaneous renal artery intervention. Ann Vasc Surg 2008; 22:783–789.
- Radermacher J, Chavin A, Bleck J, et al Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Eng J Med 2001; 344:410–417.
- García-Criado A, Gilabert R, Nicolau C, et al Value of Doppler sonography for predicting clinical outcome after renal artery revascularization in atherosclerotic renal artery stenosis. J Ultrasound Med 2005; 24:1641–1647.
- Zeller T, Müller C, Frank U, et al Stent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc Interv 2003; 58:510–515.
- Harden PN, MacLeod MJ, Rodger RSC, et al Effect of renal-artery stenting on progression of renovascular renal failure. Lancet 1997; 349:1133–1136.
- Isles CG, Robertson S, Hill D. Management of renovascular disease: a review of renal artery stenting in ten studies. QJM 1999; 92:159–167.
- Muray S, Martın M, Amoedo ML, et al Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis 2002; 39:60–66.
- Textor SC, Glockner JF, Lerman LO, et al The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 2008; 19:780–788.
- Paraskevas KI, Perrea D, Briana DD, Liapis CD. Management of atherosclerotic renovascular disease: the effect of renal artery stenting on renal function and blood pressure. Int Urol Nephrol 2006; 38:683–691.
- Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation 2000; 102:1671–1677.
- Dean RH, Kieffer RW, Smith BM, et al Renovascular hypertension: anatomic and renal function changes during drug therapy. Arch Surg 1981; 116:1408–1415.
- Zhang Q, Shen W, Zhang R, Zhang J, Hu J, Zhang X. Effects of renal artery stenting on renal function and blood pressure in patients with atherosclerotic renovascular disease. Chin Med J (Engl) 2003; 116:1451–1454.
- Ramos F, Kotliar C, Alvarez D, et al Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int 2003; 63:276–282.
- Rocha-Singh KJ, Ahuja RK, Sung CH, Rutherford J. Long-term renal function preservation after renal artery stenting in patients with progressive ischemic nephropathy. Catheter Cardiovasc Interv 2002; 57:135–141.
- Thadhani RI, Camargo CA, Xavier RJ, Fang LS, Bazari H. Atheroembolic renal failure after invasive procedures. Natural history based on 52 histologically proven cases. Medicine (Baltimore) 1995; 74:350–358.
- Holden A, Hill A, Jaff MR, Pilmore H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int 2006; 70:948–955.
- Cooper CJ, Haller ST, Colyer W, et al Embolic protection and platelet inhibition during renal artery stenting. Circulation 2008; 117:2752–2760.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
Author’s note: Atherosclerosis accounts for about 90% of cases of renal artery stenosis in people over age 40.1 Fibromuscular dysplasia, the other major cause, is a separate topic; in this paper “renal artery stenosis” refers to atherosclerotic disease only.
Renal artery stenosis is very common, and the number of angioplasty-stenting procedures performed every year is on the rise. Yet there is no overwhelming evidence that intervention yields clinical benefits—ie, better blood pressure control or renal function— than does medical therapy.
Earlier randomized controlled trials comparing angioplasty without stents and medical management showed no impressive difference in blood pressure.2,3 The data on renal function were even more questionable, with some studies suggesting that, with stenting, the chance of worsening renal function is equal to that of improvement.4
Two large randomized trials comparing renal intervention with medical therapy failed to show any benefit of intervention.5–7 A third study is under way.8
It is time to strongly reconsider the current aggressive approach to revascularization of stenotic renal arteries and take a more coordinated, critical approach.
RENAL INTERVENTIONS ON THE RISE
Renal angioplasty began replacing surgical revascularization in the 1990s, as this less-invasive procedure became more readily available and was shown to have similar clinical outcomes.9 In the last decade, stent placement during angioplasty has become standard, improving the rates of technical success.
As these procedures have become easier to perform and their radiographic outcomes have become more consistent, interventionalists have become more likely, if they see stenosis in a renal artery, to intervene and insert a stent, regardless of proven benefit. In addition, interventionalists from at least three different specialties now compete for these procedures, often by looking at the renal arteries during angiography of other vascular beds (the “drive-by”).
As a result, the number of renal interventions has been rising. Medicare received 21,660 claims for renal artery interventions (surgery or angioplasty) in 2000, compared with 13,380 in 1996—an increase of 62%. However, the number of surgeries actually decreased by 45% during this time, while the number of percutaneous procedures increased by 240%. The number of endovascular claims for renal artery stenosis by cardiologists alone rose 390%.10 Since then, the reports on intervention have been mixed, with one report citing a continued increase in 2005 to 35,000 claims,11 and another suggesting a decrease back to 1997 levels.12
HOW COMMON IS RENAL ARTERY STENOSIS?
The prevalence of renal artery stenosis depends on the definition used and the population screened. It is more common in older patients who have risk factors for other vascular diseases than in the general population.
Renal Doppler ultrasonography can detect stenosis only if the artery is narrowed by more than 60%. Hansen et al13 used ultrasonography to screen 870 people over age 65 and found a lesion (a narrowing of more than 60%) in 6.8%.
Angiography (direct, computed tomographic, or magnetic resonance) can detect less-severe stenosis. Thus, most angiographic studies define renal artery stenosis as a narrowing of more than 50%, and severe disease as a narrowing of more than 70%. Many experts believe that unilateral stenosis needs to be more than 70% to pose a risk to the kidney.14,15
Angiographic prevalence studies have been performed only in patients who were undergoing angiography for another reason such as coronary or peripheral arterial disease that inherently places them at higher risk of renal artery stenosis. For instance, renal artery stenosis is found in 11% to 28% of patients undergoing diagnostic cardiac catheterization. 16
No studies of the prevalence of renal artery stenosis have been performed in the general population. Medicare data indicate that from 1999 to 2001 the incidence of diagnosed renal artery stenosis was 3.7 per 1,000 patientyears. 17 Holley et al,18 in an autopsy series, found renal artery stenosis of greater than 50% in 27% of patients over age 50 and in 56.4% of hypertensive patients. The prevalence was 10% in normotensive patients.
WHO IS AT RISK?
Factors associated with a higher risk of finding renal artery stenosis on a radiographic study include14:
- Older age
- Female sex
- Hypertension
- Three-vessel coronary artery disease
- Peripheral artery disease
- Chronic kidney disease
- Diabetes
- A low level of high-density lipoprotein cholesterol
- The use of at least two cardiovascular drugs.
The prevalence of renal artery stenosis in at-risk populations ranges from 3% to 75% (Table 1).2,4,6,19,20
HOW OFTEN DOES STENOSIS PROGRESS?
The reported rates of progression of atherosclerotic renal artery lesions vary depending on the type of imaging test used and the reason for doing it.
In studies that used duplex ultrasonography, roughly half of lesions smaller than 60% grew to greater than 60% over 3 years.21,22 The risk of total occlusion of an artery was relatively low and depended on the severity of stenosis: 0.7% if the baseline stenosis was less than 60% and 2.3% to 7% if it was greater.21,22
In a seminal study in 1984, Schreiber and colleagues23 compared serial angiograms obtained a mean of 52 months apart in 85 patients who did not undergo intervention. Stenosis had progressed in 37 (44%), and to the point of total occlusion in 14 (16%). In contrast, a 1998 study found progression in 11.1% over 2.6 years, with older patients, women, and those with baseline coronary artery disease at higher risk.24
The the rates of progression differed in these two studies probably because the indications for screening were different (clinical suspicion23 vs routine screening during coronary angiography24), as was the severity of stenosis at the time of diagnosis. Also, when these studies were done, fewer people were taking statins. Thus, similar studies, if repeated, might show even lower rates of progression.
Finally, progression of renal artery stenosis has not been correlated with worsening renal function.
FOUR CLINICAL PRESENTATIONS OF RENAL ARTERY STENOSIS
Renal artery stenosis can present in one of four ways:
Clinically silent stenosis. Because renal artery stenosis is most often found in older patients, who are more likely to have essential hypertension and chronic kidney disease due to other causes, it can be an incidental finding that is completely clinically silent.16,25
Renovascular hypertension is defined as high blood pressure due to up-regulation of neurohormonal activity in response to decreased perfusion from renal artery stenosis. Renal artery stenosis is estimated to be the cause of hypertension in only 0.5% to 4.0% of hypertensive patients, or in 26% of patients with secondary hypertension.3
Ischemic nephropathy is more difficult to define because ischemia alone rarely explains the damage done to the kidneys. Activation of neurohormonal pathways and microvascular injury are thought to contribute to oxidative stress and fibrosis.26 These phenomena may explain why similar degrees of stenosis lead to varying degrees of kidney damage in different patients and why the severity of stenosis does not correlate with the degree of renal dysfunction.27
Furthermore, stenosis may lead to irreversible but stable kidney damage. It is therefore not surprising that, in studies in unselected populations (ie, studies that included patients with all presentations of renal artery stenosis, not just those more likely to benefit), up to two-thirds of renal interventions yielded no clinical benefit.25
As a result, if we define ischemic nephropathy as renal artery stenosis with renal dysfunction not attributable to another cause, we probably will overestimate the prevalence of ischemic nephropathy, leading to overly optimistic expectations about the response to revascularization.
Recurrent “flash” pulmonary edema is a less common presentation, usually occurring in patients with critical bilateral renal artery stenosis or unilateral stenosis in an artery supplying a solitary functioning kidney. Most have severe hypertension (average systolic blood pressure 174–207 mm Hg) and poor renal function.28–30
The association between pulmonary edema and bilateral renal artery stenosis was first noted in 1998 by Pickering et al,31 who in several case series showed that 82% to 92% of patients with recurrent pulmonary edema and renal artery stenosis had bilateral stenosis, compared with 20% to 65% of those with other presentations. Later case series corroborated this finding: 85% to 100% of patients with renal artery stenosis and pulmonary edema had bilateral stenosis.28–30
STENTING IS NOW STANDARD
Stenting has become standard in the endovascular treatment of renal artery stenosis.
Most atherosclerotic renal artery lesions are located in the ostium (ie, where the artery branches off from the aorta), and many are extensions of calcified aortic plaque.26,32 These hard lesions tend to rebound to their original shape more often with balloon angioplasty alone. Stenting provides the additional force needed to permanently disrupt the lesion, leading to a longer-lasting result.
Rates of technical success (dilating the artery during the intervention) are higher with stents than without them (98% vs 46%– 77%).33,34 If the lesion is ostial, this difference is even more impressive (75% vs 29%). In addition, restenosis rates at 6 months are lower with stents (14% vs 26%–48%).34
GOALS: LOWER THE BLOOD PRESSURE, SAVE THE KIDNEY
Because endovascular procedures pose some risk to the patient, it is critical to intervene only in patients most likely to respond clinically. The decision to intervene depends largely on the clinical goal, which should depend on the clinical presentation.
However, if renal artery stenosis is clinically silent, most of the evidence suggests that intervention has no benefit. Furthermore, although retrospective studies have indicated that intervention may improve survival rates,35,36 prospective studies have not. Similarly, studies have not shown that intervention generally improves cardiovascular outcomes, even though renal artery stenosis is associated with cardiovascular risk.
Hypertension plus stenosis is not necessarily renovascular hypertension
Essential hypertension and clinically silent renal artery stenosis often coexist, which is why blood pressure control often does not improve after stenting. Also, essential hypertension often coexists with renovascular hypertension.37 In this situation, stenting may not eliminate the need for antihypertensive drugs, although it may improve blood pressure control and decrease the drug burden.
Before stents came into use, several randomized controlled trials found that blood pressure was no better controlled after angioplasty, 2,3,38 except in cases of bilateral stenosis.2 This may be because stenosis tended to recur after angioplasty without stents.
The 2000 Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) study was the first randomized controlled trial to examine the effect of angioplasty on blood pressure control in renal artery stenosis.38 It had significant design flaws. For example, many patients crossed over from the medical management group to the intervention group because their hypertension was resistant to medical therapy. Overall, intervention (balloon angioplasty without stents in 54 of 56 patients, with stents in the other 2) carried no benefit. However, in subgroup analysis, the patients who crossed over because of resistant hypertension (failure of a three-drug regimen) were more likely to benefit from angioplasty. This suggested that risk stratification should take place early on, before proceeding with revascularization.
With stents, Zeller,39 in a prospective nonrandomized study, found that the mean arterial pressure decreased by 10 mm Hg. Randomized trials (see below) have failed to demonstrate such a benefit.
Stenting may not improve renal function
Coincidental renal artery stenosis in a patient with unrelated chronic kidney disease is very hard to differentiate from true ischemic nephropathy. Furthermore, most patients with ischemic nephropathy do not benefit from revascularization, making it challenging to identify those few whose renal function may respond.
Given that patients with chronic kidney disease tend to have a higher risk of cardiovascular disease, it is not surprising that 15% of them may have renal artery stenosis,4 most often incidental.
Chábová40 examined the outcomes of 68 patients who had chronic kidney disease and a renal artery lesion larger than 70% who did not undergo angioplasty. In only 10 (15%) of the patients did the glomerular filtration rate (GFR) decline by more than 50% of its baseline value during the study period of 3 years. Given the retrospective nature of the study, it cannot be determined (and is rather unlikely) that ischemic nephropathy was the cause of the decline in kidney function in all 10 patients.
In a prospective cohort study in 304 patients with chronic kidney disease and renal artery stenosis who underwent surgical revascularization, Textor4 reported that the serum creatinine level showed a meaningful improvement afterward in 28%, worsened in 19.7%, and remained unchanged in 160 52.6%. (A “meaningful” change was defined as > 1.0 mg/dL.) Findings were similar in a cohort that underwent stenting.33
Davies et al41 found that 20% of patients who underwent renal stenting had a persistent increase in serum creatinine of 0.5 mg/dL or more. These patients were nearly three times more likely (19% vs 7%) to eventually require dialysis, and they had a lower 5-year survival rate (41% vs 71%).
Zeller et al39 found that renal function improved slightly in 52% of patients who received stents. The mean decrease in serum creatinine in this group was 0.22 mg/dL. However, the other 48% had a mean increase in serum creatinine of 1.1 mg/dL.
From these data we can conclude that, in an unselected population with renal artery stenosis, stenting provides no benefit to renal function, and that the risk of a worsening of renal function after intervention is roughly equal to the likelihood of achieving any benefit.
Other predictors of improvement in renal function have been proposed, but the evidence supporting them has not been consistent. For example, although Radermacher et al42 reported that a renal resistive index (which reflects arterial stiffness downstream of the stenosis) lower than 0.8 predicted a response in renal function, this finding has not been reliably reproduced.43,44 Similarly, while several studies suggest that patients with milder renal dysfunction have a higher likelihood of a renal response,45,46 other studies suggest either that the opposite is true39 or that baseline renal function alone has no impact on outcome.47
In addition, once significant renal atrophy occurs, revascularization may not help much, since irreversible sclerosis has developed. Thus, the goal is to identify kidneys being harmed by renal artery stenosis during the ischemic phase, when the tissue is still viable.
Unfortunately, we still lack a good renal stress test—eg, analogous to the cardiac stress test—to diagnose reversible ischemia in the kidney. The captopril renal scan has that capability but is not accurate in patients with bilateral stenosis or a GFR less than 50 mL/min, severely limiting its applicability.26 Newer technologies such as blood-oxygen-level-dependent (BOLD) magnetic resonance imaging are being investigated for such a role.48
Cohort studies in patients with declining renal function
In several case series, patients whose renal function had been declining before intervention had impressive rates of better renal function afterward.33,39,47,49–54 In a prospective cohort study by Muray et al,47 a rise in serum creatinine of more than 0.1 mg/mL/month before intervention seemed to predict an improvement in renal function afterward.
One would expect that, for renal function to respond to intervention, severe bilateral stenosis or unilateral stenosis to a solitary functioning kidney would need to be present. However, this was an inconsistent finding in these case series.33,39,47,52,53 The Angioplasty and Stent for Renal Artery Lesions (ASTRAL) trial,6,7 discussed later, sheds a bit more light on this.
Stenting usually improves flash pulmonary edema
Acute pulmonary edema in the setting of bilateral renal artery stenosis seems to be a unique case in which improvement in clinical status can be expected in most patients after intervention. Blood pressure improves in 94% to 100% of patients,28,31 renal function either improves or stabilizes in 77% to 91%,28–31 and pulmonary edema resolves without recurrence in 77% to 100%.28–30
NEW RANDOMIZED TRIALS: STAR, ASTRAL, AND CORAL
Despite the lack of evidence supporting revascularization of renal artery stenosis, many interventionalists practice under the assumption that the radiographic finding of renal artery stenosis alone is an indication for renal revascularization. Only three randomized controlled trials in the modern era attempt to examine this hypothesis: STAR, ASTRAL, and CORAL.
STAR: No clear benefit
The Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial5 was a European multicenter trial that enrolled 140 patients with ostial renal artery stenosis greater than 50%, blood pressure controlled to less than 140/90 mm Hg, and creatinine clearance 15 to 80 mL/min.
Patients were randomized to undergo stenting or medical therapy alone. High blood pressure was treated according to a protocol in which angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers were relegated to second-line use. All patients received a statin, regardless of lipid levels.
At 2 years, the primary end point (a decline in creatinine clearance of 20% or greater) had been reached in 10 (16%) of the 64 patients in the stent group and 16 (22%) of the 76 patients in the medication group; the difference was not statistically significant (hazard ratio 0.73, 95% confidence interval 0.33–1.61). No difference was seen in the secondary end points of the degree of blood pressure control or the rates of cardiovascular morbidity and death.5
ASTRAL: Also no clear benefit
In the international, multicenter ASTRAL trial,6,7 806 patients with at least one stenotic renal artery considered suitable for balloon angioplasty, stenting, or both7 were randomized to undergo intervention or medical management. Hypertension treatment was not specified by a protocol. The mean estimated GFR was 40 mL/min. Most patients (95%–96%) were on statin therapy. The primary outcome was the rate of decline of renal function over time. Secondary outcomes included blood pressure control, renal events, cardiovascular events, and death.
Results. At a mean follow-up of 33.6 months (range 1–4 years), no difference was noted between treatment groups in decline in renal function or blood pressure control at 1 year. Renal function worsened slightly in both groups.
The decline in renal function over time, calculated as the mean slope of the reciprocal of the serum creatinine level over time, was slightly slower in the revascularization group, but the difference was not statistically significant (−0.07 × 10−3 vs −0.13 × 10−3 L/μmol/year, P = .06). This difference did not appear until the last year of the study. There was no difference in the number of patients whose renal function improved or declined during the study.
There was no difference in the rate of any secondary outcome. The medical management group required a slightly higher number of antihypertensive drugs, reaching statistical but not clinical significance (2.97 vs 2.77 drugs, P = .03). More people in the revascularization group were taking ACE inhibitors or angiotensin receptor blockers. There was no difference in the number of patients on any antihypertensive therapy (97% vs 99%). Interestingly, amputations were more common in the revascularization group, occurring in 42 (12%) of the 386 patients in the revascularization group vs 29 (7%) of the 395 patients in the medical group (P = .04).
Seventeen percent of patients randomized to intervention did not have the procedure done. An as-treated analysis of the 317 (83%) patients randomized to revascularization who did receive it showed no differences in outcomes.
There were no differences in outcomes among specific, predefined subgroups based on severity of stenosis at baseline, renal length, baseline estimated GFR, baseline serum creatinine, and rate of progression of renal dysfunction before randomization.7
Comments. ASTRAL contradicts previous nonrandomized studies that suggested that rapidly declining renal function (loss of 20% in 1 year) predicts response to intervention. Considering the large number of patients with unilateral disease in the study, it would be interesting to see what effect stenting had on patients with both severe disease and declining renal function.
ASTRAL has been criticized because it lacked a central laboratory to interpret the severity of stenosis, it did not use a standardized intervention technique (5% of patients underwent angioplasty without stents, although this did not affect outcomes7), and patients were enrolled only if the clinician involved in the case was uncertain of the appropriate management.
This last issue raises the concern for selection bias toward inclusion of more difficult cases that may not respond to intervention. But these shortcomings are not serious enough to negate the fact that preliminary results from the largest randomized controlled trial to date confirm conclusions of other randomized trials, ie, that intervention in renal artery stenosis yields no benefits over medical management in most patients.
Based on the results of STAR and ASTRAL, the practice of indiscriminately revascularizing stenosed renal arteries without strong evidence that the procedure will provide a clinical benefit is no longer tenable. The challenge is to identify those few patients who will respond, and to intervene only on them. Unfortunately, none of the subgroups from ASTRAL helped characterize this population.
CORAL: A large trial is ongoing
The Cardiovascular Outcomes in Renal Artherosclerotic Lesions (CORAL) trial,8 an ongoing multicenter randomized controlled trial in the United States, may be of additional help.
Unlike ASTRAL, CORAL is studying patients who have difficult-to-control hypertension (systolic blood pressure ≥ 155 mm Hg on two or more drugs).8 Chronic kidney disease is not an exclusion criterion unless the serum creatinine concentration is greater than 3.0 mg/dL.
CORAL is using a standardized medical protocol to control blood pressure. In addition, use of embolic protection devices during stenting is encouraged. Hopefully, the large size (a goal of 1,080 patients) and the inclusion of patients with more marked hypertension will address the utility of intervention in higher-risk populations with renal artery stenosis.
RECOMMENDED APPROACH TO INTERVENTION IN RENAL ARTERY STENOSIS
As we wait for CORAL to be completed, we have two modern-era randomized controlled trials that leave us with fewer indications for renal intervention. Table 2 lists commonly cited indications for intervention in renal artery stenosis and the evidence to support them. As most of these are based on retrospective data or have conflicting support in the literature, their utility remains in question. At this point we can safely recommend:
- Patients with preserved or even decreased but stable renal function will not likely have a benefit in renal function after intervention.
- Patients with resistant hypertension may benefit.
- The best evidence supporting intervention is for bilateral stenosis with flash pulmonary edema, but the evidence is from retrospective studies.
- Stenting in bilateral disease without another indication has no apparent benefit.
- Declining renal function is not a guarantee of success.
- It is unclear if patients with severe bilateral stenosis or severe stenosis to a solitary functioning kidney with declining renal function will benefit. Anecdotally, they do respond more often, but as with many other indications for intervention that have gone by the wayside, this may not bear out when studied properly.
As the utility of intervention narrows, the scope of practice for such interventions should narrow accordingly. Attention should now be focusing on clinical, rather than radiographic, indications for intervening on renal artery stenosis.
Therefore, the decision to intervene must not be made solely by the interventionalist. A multidisciplinary approach should be adopted that at the very least includes the input of a nephrologist well versed in renal artery stenosis. In this way, the clinical risks and benefits of renal intervention can be discussed with the patient by providers who are likely to be involved in their care should renal function or hypertension fail to improve afterward.
RISK OF ATHEROEMBOLISM
While renal stenting yields improved technical success in the treatment of renal artery stenosis, it carries with it an increasingly common risk to kidney function: atheroembolism as the stent crushes the plaque against the vessel wall. This may lead to obstruction of the renal microvasculature, increasing the risk of irreversible damage to renal function.
Atheroembolic kidney disease can manifest as progressive renal failure occurring over weeks to months, commonly misdiagnosed as permanent damage from contrast nephropathy.55
Embolic protection devices, inserted downstream of the lesion before stenting to catch any debris that may break loose, have been developed to help address this problem.
Holden et al 57 prospectively studied 63 patients with renal artery stenosis and deteriorating renal function (undefined) who underwent stenting with an embolic protection device. At 6 months after the intervention, renal function had either improved or stabilized in 97% of patients, suggesting that many of the deleterious effects of stenting on renal function are related to atheroembolism.
The Prospective Randomized Study Comparing Renal Artery Stenting With or Without Distal Protection (RESIST) trial, in which renal dysfunction was mild and the GFR was not declining (average estimated GFR 59.3 mL/min), found contrary results.57 In a two-by-two factorial study, patients were randomized to undergo stenting alone, stenting with the antiplatelet agent abciximab (ReoPro), stenting with an embolic protection device, or stenting with both abciximab and an embolic protection device. Interestingly, renal function declined in the first three groups, but remained stable in the group that received both abciximab and an embolic protection device.
ANTIPLATELET THERAPY AFTER RENAL STENTING: HOW LONG?
We have no data on the optimal duration of antiplatelet therapy after renal stenting, and guidelines from professional societies do not comment on it.58 As a result, practice patterns vary significantly among practitioners.
While in-stent restenosis rates are acceptably low after renal stenting, the risks and side effects of antiplatelet therapy often lead to arbitrary withdrawal of these drugs. The effect on stent patency is yet to be determined.
FUTURE DEVELOPMENTS
Results of STAR and ASTRAL confirm the growing suspicion that the surge seen in the last decade in renal artery stenting should be coming to an end. We await results either from CORAL or possibly a post hoc analysis of ASTRAL that might identify potential high-risk groups that will benefit from renal intervention. And as embolic protection devices become more agile and suitable to different renal lesions, there remains the possibility that, due to lower rates of unidentified atheroembolic kidney disease, CORAL may demonstrate improved renal outcomes after stenting. If not, the search for the best means to predict who should have renal intervention will continue.
We know through experience that stenting does provide great benefits for some patients with renal artery stenosis. Furthermore, the clinical problem is too intriguing, and too profitable, to die altogether.
Author’s note: Atherosclerosis accounts for about 90% of cases of renal artery stenosis in people over age 40.1 Fibromuscular dysplasia, the other major cause, is a separate topic; in this paper “renal artery stenosis” refers to atherosclerotic disease only.
Renal artery stenosis is very common, and the number of angioplasty-stenting procedures performed every year is on the rise. Yet there is no overwhelming evidence that intervention yields clinical benefits—ie, better blood pressure control or renal function— than does medical therapy.
Earlier randomized controlled trials comparing angioplasty without stents and medical management showed no impressive difference in blood pressure.2,3 The data on renal function were even more questionable, with some studies suggesting that, with stenting, the chance of worsening renal function is equal to that of improvement.4
Two large randomized trials comparing renal intervention with medical therapy failed to show any benefit of intervention.5–7 A third study is under way.8
It is time to strongly reconsider the current aggressive approach to revascularization of stenotic renal arteries and take a more coordinated, critical approach.
RENAL INTERVENTIONS ON THE RISE
Renal angioplasty began replacing surgical revascularization in the 1990s, as this less-invasive procedure became more readily available and was shown to have similar clinical outcomes.9 In the last decade, stent placement during angioplasty has become standard, improving the rates of technical success.
As these procedures have become easier to perform and their radiographic outcomes have become more consistent, interventionalists have become more likely, if they see stenosis in a renal artery, to intervene and insert a stent, regardless of proven benefit. In addition, interventionalists from at least three different specialties now compete for these procedures, often by looking at the renal arteries during angiography of other vascular beds (the “drive-by”).
As a result, the number of renal interventions has been rising. Medicare received 21,660 claims for renal artery interventions (surgery or angioplasty) in 2000, compared with 13,380 in 1996—an increase of 62%. However, the number of surgeries actually decreased by 45% during this time, while the number of percutaneous procedures increased by 240%. The number of endovascular claims for renal artery stenosis by cardiologists alone rose 390%.10 Since then, the reports on intervention have been mixed, with one report citing a continued increase in 2005 to 35,000 claims,11 and another suggesting a decrease back to 1997 levels.12
HOW COMMON IS RENAL ARTERY STENOSIS?
The prevalence of renal artery stenosis depends on the definition used and the population screened. It is more common in older patients who have risk factors for other vascular diseases than in the general population.
Renal Doppler ultrasonography can detect stenosis only if the artery is narrowed by more than 60%. Hansen et al13 used ultrasonography to screen 870 people over age 65 and found a lesion (a narrowing of more than 60%) in 6.8%.
Angiography (direct, computed tomographic, or magnetic resonance) can detect less-severe stenosis. Thus, most angiographic studies define renal artery stenosis as a narrowing of more than 50%, and severe disease as a narrowing of more than 70%. Many experts believe that unilateral stenosis needs to be more than 70% to pose a risk to the kidney.14,15
Angiographic prevalence studies have been performed only in patients who were undergoing angiography for another reason such as coronary or peripheral arterial disease that inherently places them at higher risk of renal artery stenosis. For instance, renal artery stenosis is found in 11% to 28% of patients undergoing diagnostic cardiac catheterization. 16
No studies of the prevalence of renal artery stenosis have been performed in the general population. Medicare data indicate that from 1999 to 2001 the incidence of diagnosed renal artery stenosis was 3.7 per 1,000 patientyears. 17 Holley et al,18 in an autopsy series, found renal artery stenosis of greater than 50% in 27% of patients over age 50 and in 56.4% of hypertensive patients. The prevalence was 10% in normotensive patients.
WHO IS AT RISK?
Factors associated with a higher risk of finding renal artery stenosis on a radiographic study include14:
- Older age
- Female sex
- Hypertension
- Three-vessel coronary artery disease
- Peripheral artery disease
- Chronic kidney disease
- Diabetes
- A low level of high-density lipoprotein cholesterol
- The use of at least two cardiovascular drugs.
The prevalence of renal artery stenosis in at-risk populations ranges from 3% to 75% (Table 1).2,4,6,19,20
HOW OFTEN DOES STENOSIS PROGRESS?
The reported rates of progression of atherosclerotic renal artery lesions vary depending on the type of imaging test used and the reason for doing it.
In studies that used duplex ultrasonography, roughly half of lesions smaller than 60% grew to greater than 60% over 3 years.21,22 The risk of total occlusion of an artery was relatively low and depended on the severity of stenosis: 0.7% if the baseline stenosis was less than 60% and 2.3% to 7% if it was greater.21,22
In a seminal study in 1984, Schreiber and colleagues23 compared serial angiograms obtained a mean of 52 months apart in 85 patients who did not undergo intervention. Stenosis had progressed in 37 (44%), and to the point of total occlusion in 14 (16%). In contrast, a 1998 study found progression in 11.1% over 2.6 years, with older patients, women, and those with baseline coronary artery disease at higher risk.24
The the rates of progression differed in these two studies probably because the indications for screening were different (clinical suspicion23 vs routine screening during coronary angiography24), as was the severity of stenosis at the time of diagnosis. Also, when these studies were done, fewer people were taking statins. Thus, similar studies, if repeated, might show even lower rates of progression.
Finally, progression of renal artery stenosis has not been correlated with worsening renal function.
FOUR CLINICAL PRESENTATIONS OF RENAL ARTERY STENOSIS
Renal artery stenosis can present in one of four ways:
Clinically silent stenosis. Because renal artery stenosis is most often found in older patients, who are more likely to have essential hypertension and chronic kidney disease due to other causes, it can be an incidental finding that is completely clinically silent.16,25
Renovascular hypertension is defined as high blood pressure due to up-regulation of neurohormonal activity in response to decreased perfusion from renal artery stenosis. Renal artery stenosis is estimated to be the cause of hypertension in only 0.5% to 4.0% of hypertensive patients, or in 26% of patients with secondary hypertension.3
Ischemic nephropathy is more difficult to define because ischemia alone rarely explains the damage done to the kidneys. Activation of neurohormonal pathways and microvascular injury are thought to contribute to oxidative stress and fibrosis.26 These phenomena may explain why similar degrees of stenosis lead to varying degrees of kidney damage in different patients and why the severity of stenosis does not correlate with the degree of renal dysfunction.27
Furthermore, stenosis may lead to irreversible but stable kidney damage. It is therefore not surprising that, in studies in unselected populations (ie, studies that included patients with all presentations of renal artery stenosis, not just those more likely to benefit), up to two-thirds of renal interventions yielded no clinical benefit.25
As a result, if we define ischemic nephropathy as renal artery stenosis with renal dysfunction not attributable to another cause, we probably will overestimate the prevalence of ischemic nephropathy, leading to overly optimistic expectations about the response to revascularization.
Recurrent “flash” pulmonary edema is a less common presentation, usually occurring in patients with critical bilateral renal artery stenosis or unilateral stenosis in an artery supplying a solitary functioning kidney. Most have severe hypertension (average systolic blood pressure 174–207 mm Hg) and poor renal function.28–30
The association between pulmonary edema and bilateral renal artery stenosis was first noted in 1998 by Pickering et al,31 who in several case series showed that 82% to 92% of patients with recurrent pulmonary edema and renal artery stenosis had bilateral stenosis, compared with 20% to 65% of those with other presentations. Later case series corroborated this finding: 85% to 100% of patients with renal artery stenosis and pulmonary edema had bilateral stenosis.28–30
STENTING IS NOW STANDARD
Stenting has become standard in the endovascular treatment of renal artery stenosis.
Most atherosclerotic renal artery lesions are located in the ostium (ie, where the artery branches off from the aorta), and many are extensions of calcified aortic plaque.26,32 These hard lesions tend to rebound to their original shape more often with balloon angioplasty alone. Stenting provides the additional force needed to permanently disrupt the lesion, leading to a longer-lasting result.
Rates of technical success (dilating the artery during the intervention) are higher with stents than without them (98% vs 46%– 77%).33,34 If the lesion is ostial, this difference is even more impressive (75% vs 29%). In addition, restenosis rates at 6 months are lower with stents (14% vs 26%–48%).34
GOALS: LOWER THE BLOOD PRESSURE, SAVE THE KIDNEY
Because endovascular procedures pose some risk to the patient, it is critical to intervene only in patients most likely to respond clinically. The decision to intervene depends largely on the clinical goal, which should depend on the clinical presentation.
However, if renal artery stenosis is clinically silent, most of the evidence suggests that intervention has no benefit. Furthermore, although retrospective studies have indicated that intervention may improve survival rates,35,36 prospective studies have not. Similarly, studies have not shown that intervention generally improves cardiovascular outcomes, even though renal artery stenosis is associated with cardiovascular risk.
Hypertension plus stenosis is not necessarily renovascular hypertension
Essential hypertension and clinically silent renal artery stenosis often coexist, which is why blood pressure control often does not improve after stenting. Also, essential hypertension often coexists with renovascular hypertension.37 In this situation, stenting may not eliminate the need for antihypertensive drugs, although it may improve blood pressure control and decrease the drug burden.
Before stents came into use, several randomized controlled trials found that blood pressure was no better controlled after angioplasty, 2,3,38 except in cases of bilateral stenosis.2 This may be because stenosis tended to recur after angioplasty without stents.
The 2000 Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) study was the first randomized controlled trial to examine the effect of angioplasty on blood pressure control in renal artery stenosis.38 It had significant design flaws. For example, many patients crossed over from the medical management group to the intervention group because their hypertension was resistant to medical therapy. Overall, intervention (balloon angioplasty without stents in 54 of 56 patients, with stents in the other 2) carried no benefit. However, in subgroup analysis, the patients who crossed over because of resistant hypertension (failure of a three-drug regimen) were more likely to benefit from angioplasty. This suggested that risk stratification should take place early on, before proceeding with revascularization.
With stents, Zeller,39 in a prospective nonrandomized study, found that the mean arterial pressure decreased by 10 mm Hg. Randomized trials (see below) have failed to demonstrate such a benefit.
Stenting may not improve renal function
Coincidental renal artery stenosis in a patient with unrelated chronic kidney disease is very hard to differentiate from true ischemic nephropathy. Furthermore, most patients with ischemic nephropathy do not benefit from revascularization, making it challenging to identify those few whose renal function may respond.
Given that patients with chronic kidney disease tend to have a higher risk of cardiovascular disease, it is not surprising that 15% of them may have renal artery stenosis,4 most often incidental.
Chábová40 examined the outcomes of 68 patients who had chronic kidney disease and a renal artery lesion larger than 70% who did not undergo angioplasty. In only 10 (15%) of the patients did the glomerular filtration rate (GFR) decline by more than 50% of its baseline value during the study period of 3 years. Given the retrospective nature of the study, it cannot be determined (and is rather unlikely) that ischemic nephropathy was the cause of the decline in kidney function in all 10 patients.
In a prospective cohort study in 304 patients with chronic kidney disease and renal artery stenosis who underwent surgical revascularization, Textor4 reported that the serum creatinine level showed a meaningful improvement afterward in 28%, worsened in 19.7%, and remained unchanged in 160 52.6%. (A “meaningful” change was defined as > 1.0 mg/dL.) Findings were similar in a cohort that underwent stenting.33
Davies et al41 found that 20% of patients who underwent renal stenting had a persistent increase in serum creatinine of 0.5 mg/dL or more. These patients were nearly three times more likely (19% vs 7%) to eventually require dialysis, and they had a lower 5-year survival rate (41% vs 71%).
Zeller et al39 found that renal function improved slightly in 52% of patients who received stents. The mean decrease in serum creatinine in this group was 0.22 mg/dL. However, the other 48% had a mean increase in serum creatinine of 1.1 mg/dL.
From these data we can conclude that, in an unselected population with renal artery stenosis, stenting provides no benefit to renal function, and that the risk of a worsening of renal function after intervention is roughly equal to the likelihood of achieving any benefit.
Other predictors of improvement in renal function have been proposed, but the evidence supporting them has not been consistent. For example, although Radermacher et al42 reported that a renal resistive index (which reflects arterial stiffness downstream of the stenosis) lower than 0.8 predicted a response in renal function, this finding has not been reliably reproduced.43,44 Similarly, while several studies suggest that patients with milder renal dysfunction have a higher likelihood of a renal response,45,46 other studies suggest either that the opposite is true39 or that baseline renal function alone has no impact on outcome.47
In addition, once significant renal atrophy occurs, revascularization may not help much, since irreversible sclerosis has developed. Thus, the goal is to identify kidneys being harmed by renal artery stenosis during the ischemic phase, when the tissue is still viable.
Unfortunately, we still lack a good renal stress test—eg, analogous to the cardiac stress test—to diagnose reversible ischemia in the kidney. The captopril renal scan has that capability but is not accurate in patients with bilateral stenosis or a GFR less than 50 mL/min, severely limiting its applicability.26 Newer technologies such as blood-oxygen-level-dependent (BOLD) magnetic resonance imaging are being investigated for such a role.48
Cohort studies in patients with declining renal function
In several case series, patients whose renal function had been declining before intervention had impressive rates of better renal function afterward.33,39,47,49–54 In a prospective cohort study by Muray et al,47 a rise in serum creatinine of more than 0.1 mg/mL/month before intervention seemed to predict an improvement in renal function afterward.
One would expect that, for renal function to respond to intervention, severe bilateral stenosis or unilateral stenosis to a solitary functioning kidney would need to be present. However, this was an inconsistent finding in these case series.33,39,47,52,53 The Angioplasty and Stent for Renal Artery Lesions (ASTRAL) trial,6,7 discussed later, sheds a bit more light on this.
Stenting usually improves flash pulmonary edema
Acute pulmonary edema in the setting of bilateral renal artery stenosis seems to be a unique case in which improvement in clinical status can be expected in most patients after intervention. Blood pressure improves in 94% to 100% of patients,28,31 renal function either improves or stabilizes in 77% to 91%,28–31 and pulmonary edema resolves without recurrence in 77% to 100%.28–30
NEW RANDOMIZED TRIALS: STAR, ASTRAL, AND CORAL
Despite the lack of evidence supporting revascularization of renal artery stenosis, many interventionalists practice under the assumption that the radiographic finding of renal artery stenosis alone is an indication for renal revascularization. Only three randomized controlled trials in the modern era attempt to examine this hypothesis: STAR, ASTRAL, and CORAL.
STAR: No clear benefit
The Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial5 was a European multicenter trial that enrolled 140 patients with ostial renal artery stenosis greater than 50%, blood pressure controlled to less than 140/90 mm Hg, and creatinine clearance 15 to 80 mL/min.
Patients were randomized to undergo stenting or medical therapy alone. High blood pressure was treated according to a protocol in which angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers were relegated to second-line use. All patients received a statin, regardless of lipid levels.
At 2 years, the primary end point (a decline in creatinine clearance of 20% or greater) had been reached in 10 (16%) of the 64 patients in the stent group and 16 (22%) of the 76 patients in the medication group; the difference was not statistically significant (hazard ratio 0.73, 95% confidence interval 0.33–1.61). No difference was seen in the secondary end points of the degree of blood pressure control or the rates of cardiovascular morbidity and death.5
ASTRAL: Also no clear benefit
In the international, multicenter ASTRAL trial,6,7 806 patients with at least one stenotic renal artery considered suitable for balloon angioplasty, stenting, or both7 were randomized to undergo intervention or medical management. Hypertension treatment was not specified by a protocol. The mean estimated GFR was 40 mL/min. Most patients (95%–96%) were on statin therapy. The primary outcome was the rate of decline of renal function over time. Secondary outcomes included blood pressure control, renal events, cardiovascular events, and death.
Results. At a mean follow-up of 33.6 months (range 1–4 years), no difference was noted between treatment groups in decline in renal function or blood pressure control at 1 year. Renal function worsened slightly in both groups.
The decline in renal function over time, calculated as the mean slope of the reciprocal of the serum creatinine level over time, was slightly slower in the revascularization group, but the difference was not statistically significant (−0.07 × 10−3 vs −0.13 × 10−3 L/μmol/year, P = .06). This difference did not appear until the last year of the study. There was no difference in the number of patients whose renal function improved or declined during the study.
There was no difference in the rate of any secondary outcome. The medical management group required a slightly higher number of antihypertensive drugs, reaching statistical but not clinical significance (2.97 vs 2.77 drugs, P = .03). More people in the revascularization group were taking ACE inhibitors or angiotensin receptor blockers. There was no difference in the number of patients on any antihypertensive therapy (97% vs 99%). Interestingly, amputations were more common in the revascularization group, occurring in 42 (12%) of the 386 patients in the revascularization group vs 29 (7%) of the 395 patients in the medical group (P = .04).
Seventeen percent of patients randomized to intervention did not have the procedure done. An as-treated analysis of the 317 (83%) patients randomized to revascularization who did receive it showed no differences in outcomes.
There were no differences in outcomes among specific, predefined subgroups based on severity of stenosis at baseline, renal length, baseline estimated GFR, baseline serum creatinine, and rate of progression of renal dysfunction before randomization.7
Comments. ASTRAL contradicts previous nonrandomized studies that suggested that rapidly declining renal function (loss of 20% in 1 year) predicts response to intervention. Considering the large number of patients with unilateral disease in the study, it would be interesting to see what effect stenting had on patients with both severe disease and declining renal function.
ASTRAL has been criticized because it lacked a central laboratory to interpret the severity of stenosis, it did not use a standardized intervention technique (5% of patients underwent angioplasty without stents, although this did not affect outcomes7), and patients were enrolled only if the clinician involved in the case was uncertain of the appropriate management.
This last issue raises the concern for selection bias toward inclusion of more difficult cases that may not respond to intervention. But these shortcomings are not serious enough to negate the fact that preliminary results from the largest randomized controlled trial to date confirm conclusions of other randomized trials, ie, that intervention in renal artery stenosis yields no benefits over medical management in most patients.
Based on the results of STAR and ASTRAL, the practice of indiscriminately revascularizing stenosed renal arteries without strong evidence that the procedure will provide a clinical benefit is no longer tenable. The challenge is to identify those few patients who will respond, and to intervene only on them. Unfortunately, none of the subgroups from ASTRAL helped characterize this population.
CORAL: A large trial is ongoing
The Cardiovascular Outcomes in Renal Artherosclerotic Lesions (CORAL) trial,8 an ongoing multicenter randomized controlled trial in the United States, may be of additional help.
Unlike ASTRAL, CORAL is studying patients who have difficult-to-control hypertension (systolic blood pressure ≥ 155 mm Hg on two or more drugs).8 Chronic kidney disease is not an exclusion criterion unless the serum creatinine concentration is greater than 3.0 mg/dL.
CORAL is using a standardized medical protocol to control blood pressure. In addition, use of embolic protection devices during stenting is encouraged. Hopefully, the large size (a goal of 1,080 patients) and the inclusion of patients with more marked hypertension will address the utility of intervention in higher-risk populations with renal artery stenosis.
RECOMMENDED APPROACH TO INTERVENTION IN RENAL ARTERY STENOSIS
As we wait for CORAL to be completed, we have two modern-era randomized controlled trials that leave us with fewer indications for renal intervention. Table 2 lists commonly cited indications for intervention in renal artery stenosis and the evidence to support them. As most of these are based on retrospective data or have conflicting support in the literature, their utility remains in question. At this point we can safely recommend:
- Patients with preserved or even decreased but stable renal function will not likely have a benefit in renal function after intervention.
- Patients with resistant hypertension may benefit.
- The best evidence supporting intervention is for bilateral stenosis with flash pulmonary edema, but the evidence is from retrospective studies.
- Stenting in bilateral disease without another indication has no apparent benefit.
- Declining renal function is not a guarantee of success.
- It is unclear if patients with severe bilateral stenosis or severe stenosis to a solitary functioning kidney with declining renal function will benefit. Anecdotally, they do respond more often, but as with many other indications for intervention that have gone by the wayside, this may not bear out when studied properly.
As the utility of intervention narrows, the scope of practice for such interventions should narrow accordingly. Attention should now be focusing on clinical, rather than radiographic, indications for intervening on renal artery stenosis.
Therefore, the decision to intervene must not be made solely by the interventionalist. A multidisciplinary approach should be adopted that at the very least includes the input of a nephrologist well versed in renal artery stenosis. In this way, the clinical risks and benefits of renal intervention can be discussed with the patient by providers who are likely to be involved in their care should renal function or hypertension fail to improve afterward.
RISK OF ATHEROEMBOLISM
While renal stenting yields improved technical success in the treatment of renal artery stenosis, it carries with it an increasingly common risk to kidney function: atheroembolism as the stent crushes the plaque against the vessel wall. This may lead to obstruction of the renal microvasculature, increasing the risk of irreversible damage to renal function.
Atheroembolic kidney disease can manifest as progressive renal failure occurring over weeks to months, commonly misdiagnosed as permanent damage from contrast nephropathy.55
Embolic protection devices, inserted downstream of the lesion before stenting to catch any debris that may break loose, have been developed to help address this problem.
Holden et al 57 prospectively studied 63 patients with renal artery stenosis and deteriorating renal function (undefined) who underwent stenting with an embolic protection device. At 6 months after the intervention, renal function had either improved or stabilized in 97% of patients, suggesting that many of the deleterious effects of stenting on renal function are related to atheroembolism.
The Prospective Randomized Study Comparing Renal Artery Stenting With or Without Distal Protection (RESIST) trial, in which renal dysfunction was mild and the GFR was not declining (average estimated GFR 59.3 mL/min), found contrary results.57 In a two-by-two factorial study, patients were randomized to undergo stenting alone, stenting with the antiplatelet agent abciximab (ReoPro), stenting with an embolic protection device, or stenting with both abciximab and an embolic protection device. Interestingly, renal function declined in the first three groups, but remained stable in the group that received both abciximab and an embolic protection device.
ANTIPLATELET THERAPY AFTER RENAL STENTING: HOW LONG?
We have no data on the optimal duration of antiplatelet therapy after renal stenting, and guidelines from professional societies do not comment on it.58 As a result, practice patterns vary significantly among practitioners.
While in-stent restenosis rates are acceptably low after renal stenting, the risks and side effects of antiplatelet therapy often lead to arbitrary withdrawal of these drugs. The effect on stent patency is yet to be determined.
FUTURE DEVELOPMENTS
Results of STAR and ASTRAL confirm the growing suspicion that the surge seen in the last decade in renal artery stenting should be coming to an end. We await results either from CORAL or possibly a post hoc analysis of ASTRAL that might identify potential high-risk groups that will benefit from renal intervention. And as embolic protection devices become more agile and suitable to different renal lesions, there remains the possibility that, due to lower rates of unidentified atheroembolic kidney disease, CORAL may demonstrate improved renal outcomes after stenting. If not, the search for the best means to predict who should have renal intervention will continue.
We know through experience that stenting does provide great benefits for some patients with renal artery stenosis. Furthermore, the clinical problem is too intriguing, and too profitable, to die altogether.
- Choncol M, Linas S. Diagnosis and management of ischemic nephropathy. Clin J Am Soc Nephrol 2006; 1:172–181.
- Webster J, Marshall F, Abdalla M, et al Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998; 12:329–335.
- Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998; 31:823–829.
- Textor SC. Revascularization in atherosclerotic renal artery disease. Kidney Int 1998; 53:799–811.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848.
- Mistry S, Ives N, Harding J, et al Angioplasty and STent for Renal Artery Lesions (ASTRAL trial): rationale, methods and results so far. J Hum Hypertens 2007; 21:511–515.
- Wheatley K, Ives N, Kalra P, Moss J. Revascularization versus medical therapy for renal-artery stenosis (ASTRAL). N Engl J Med 2009; 361:1953–1962.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
- Galaria II, Surowiec SM, Rhodes JM, et al Percutaneous and open renal revascularizations have equivalent long-term functional outcomes. Ann Vasc Surg 2005; 19:218–228.
- Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by Medicare beneficiaries 1996–2000. AJR Am J Roentgenol 2004; 183:561–568.
- Textor SC. Atherosclerotic renal artery stenosis: overtreated but underrated? J Am Soc Nephrol 2008; 19:656–659.
- Kalra PA, Guo H, Gilbertson DT, et al Atherosclerotic renovascular disease in the United States. Kidney Int 2010; 77:37–43.
- Hansen KJ, Edwards MS, Craven TE, et al Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Cohen MG, Pascua JA, Garcia-Ben M, et al A simple prediction rule for significant renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 2005; 150:1204–1211.
- Buller CE, Nogareda JG, Ramanathan K, et al The profile of cardiac patients with renal artery stenosis. J Am Coll Cardiol 2004; 43:1606–1613.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- Kalra PA, Guo H, Kausz AT, et al Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 69:293–301.
- Holley KE, Hunt JC, Brown AL, Kincaid OW, Sheps SG. Renal artery stenosis. A clinical-pathologic study in normotensive and hypertensive patients. Am J Med 1964; 37:14–22.
- de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systemic literature review. J Hypertens 2009; 27:1333–1340.
- Kuczera P, Włoszczynska E, Adamczak M, Pencak P, Chudek J, Wiecek A. Frequency of renal artery stenosis and variants of renal vascularization in hypertensive patients: analysis of 1550 angiographies in one centre. J Hum Hypertens 2009; 23:396–401.
- Caps MT, Perissinotto C, Zierler RE, et al Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998; 98:2866–2872.
- Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens 1996; 9:1055–1061.
- Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol Clin North Am 1984; 11:383–392.
- Crowley JJ, Santos RM, Peter RH, et al Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 1998; 136:913–918.
- Textor SC. Renovascular hypertension update. Curr Hypertens Rep 2006; 8:521–527.
- Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol 2004; 15:1974–1982.
- Wright JR, Shurrab AE, Cheung C, et al A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis 2002; 39:1153–1161.
- Messina LM, Zelenock GB, Yao KA, Stanley JC. Renal revascularization for recurrent pulmonary edema in patients with poorly controlled hypertension and renal insufficiency: a distinct subgroup of patients with arteriosclerotic renal artery occlusive disease. J Vasc Surg 1992; 15:73–80.
- Bloch MJ, Trost DW, Pickering TG, Sos TA, August P. Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens 1999; 12:1–7.
- Gray BH, Olin JW, Childs MB, Sullivan TM, Bacharach JM. Clinical benefit of renal artery angioplasty with stenting for the control of recurrent and refractory congestive heart failure. Vasc Med 2002; 7:275–279.
- Pickering TG, Herman L, Devereux RB, et al Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet 1988; 2:551–552.
- Kennedy DJ, Colyer WR, Brewster PS, et al Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis 2003; 14:926–935.
- Beutler JJ, Van Ampting JM, Van De Ven PJ, et al Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol 2001; 12:1475–1481.
- Van de Ven PJ, Kaatee R, Beutler JJ, et al Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomized trial. Lancet 1999; 353:282–286.
- Isles C, Main J, O’Connell J, et al Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scott Med J 1990; 35:70–73.
- Hunt JC, Sheps SG, Harrison EG, Strong CG, Bernatz PE. Renal and renovascular hypertension. A reasoned approach to diagnosis and management. Arch Intern Med 1974; 133:988–999.
- Textor SC. Atherosclerotic renal artery stenosis: how big is the problem, and what happens if nothing is done? J Hypertens Suppl 2005; 23:S5–S13.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Zeller T, Frank U, Müller C, et al Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation 2003; 108;2244–2249.
- Chábová V, Schirger A, Stanson AW, McKusick MA, Textor SC. Outcomes of atherosclerotic renal artery stenosis managed without revascularization. Mayo Clin Proc 2000; 75:437–444.
- Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. Implications of acute functional injury following percutaneous renal artery intervention. Ann Vasc Surg 2008; 22:783–789.
- Radermacher J, Chavin A, Bleck J, et al Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Eng J Med 2001; 344:410–417.
- García-Criado A, Gilabert R, Nicolau C, et al Value of Doppler sonography for predicting clinical outcome after renal artery revascularization in atherosclerotic renal artery stenosis. J Ultrasound Med 2005; 24:1641–1647.
- Zeller T, Müller C, Frank U, et al Stent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc Interv 2003; 58:510–515.
- Harden PN, MacLeod MJ, Rodger RSC, et al Effect of renal-artery stenting on progression of renovascular renal failure. Lancet 1997; 349:1133–1136.
- Isles CG, Robertson S, Hill D. Management of renovascular disease: a review of renal artery stenting in ten studies. QJM 1999; 92:159–167.
- Muray S, Martın M, Amoedo ML, et al Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis 2002; 39:60–66.
- Textor SC, Glockner JF, Lerman LO, et al The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 2008; 19:780–788.
- Paraskevas KI, Perrea D, Briana DD, Liapis CD. Management of atherosclerotic renovascular disease: the effect of renal artery stenting on renal function and blood pressure. Int Urol Nephrol 2006; 38:683–691.
- Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation 2000; 102:1671–1677.
- Dean RH, Kieffer RW, Smith BM, et al Renovascular hypertension: anatomic and renal function changes during drug therapy. Arch Surg 1981; 116:1408–1415.
- Zhang Q, Shen W, Zhang R, Zhang J, Hu J, Zhang X. Effects of renal artery stenting on renal function and blood pressure in patients with atherosclerotic renovascular disease. Chin Med J (Engl) 2003; 116:1451–1454.
- Ramos F, Kotliar C, Alvarez D, et al Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int 2003; 63:276–282.
- Rocha-Singh KJ, Ahuja RK, Sung CH, Rutherford J. Long-term renal function preservation after renal artery stenting in patients with progressive ischemic nephropathy. Catheter Cardiovasc Interv 2002; 57:135–141.
- Thadhani RI, Camargo CA, Xavier RJ, Fang LS, Bazari H. Atheroembolic renal failure after invasive procedures. Natural history based on 52 histologically proven cases. Medicine (Baltimore) 1995; 74:350–358.
- Holden A, Hill A, Jaff MR, Pilmore H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int 2006; 70:948–955.
- Cooper CJ, Haller ST, Colyer W, et al Embolic protection and platelet inhibition during renal artery stenting. Circulation 2008; 117:2752–2760.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Choncol M, Linas S. Diagnosis and management of ischemic nephropathy. Clin J Am Soc Nephrol 2006; 1:172–181.
- Webster J, Marshall F, Abdalla M, et al Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998; 12:329–335.
- Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998; 31:823–829.
- Textor SC. Revascularization in atherosclerotic renal artery disease. Kidney Int 1998; 53:799–811.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848.
- Mistry S, Ives N, Harding J, et al Angioplasty and STent for Renal Artery Lesions (ASTRAL trial): rationale, methods and results so far. J Hum Hypertens 2007; 21:511–515.
- Wheatley K, Ives N, Kalra P, Moss J. Revascularization versus medical therapy for renal-artery stenosis (ASTRAL). N Engl J Med 2009; 361:1953–1962.
- Cooper CJ, Murphy TP, Matsumoto A, et al Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J 2006; 152:59–66.
- Galaria II, Surowiec SM, Rhodes JM, et al Percutaneous and open renal revascularizations have equivalent long-term functional outcomes. Ann Vasc Surg 2005; 19:218–228.
- Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by Medicare beneficiaries 1996–2000. AJR Am J Roentgenol 2004; 183:561–568.
- Textor SC. Atherosclerotic renal artery stenosis: overtreated but underrated? J Am Soc Nephrol 2008; 19:656–659.
- Kalra PA, Guo H, Gilbertson DT, et al Atherosclerotic renovascular disease in the United States. Kidney Int 2010; 77:37–43.
- Hansen KJ, Edwards MS, Craven TE, et al Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Cohen MG, Pascua JA, Garcia-Ben M, et al A simple prediction rule for significant renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 2005; 150:1204–1211.
- Buller CE, Nogareda JG, Ramanathan K, et al The profile of cardiac patients with renal artery stenosis. J Am Coll Cardiol 2004; 43:1606–1613.
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 2009; 6:176–190.
- Kalra PA, Guo H, Kausz AT, et al Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 69:293–301.
- Holley KE, Hunt JC, Brown AL, Kincaid OW, Sheps SG. Renal artery stenosis. A clinical-pathologic study in normotensive and hypertensive patients. Am J Med 1964; 37:14–22.
- de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systemic literature review. J Hypertens 2009; 27:1333–1340.
- Kuczera P, Włoszczynska E, Adamczak M, Pencak P, Chudek J, Wiecek A. Frequency of renal artery stenosis and variants of renal vascularization in hypertensive patients: analysis of 1550 angiographies in one centre. J Hum Hypertens 2009; 23:396–401.
- Caps MT, Perissinotto C, Zierler RE, et al Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998; 98:2866–2872.
- Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens 1996; 9:1055–1061.
- Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol Clin North Am 1984; 11:383–392.
- Crowley JJ, Santos RM, Peter RH, et al Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 1998; 136:913–918.
- Textor SC. Renovascular hypertension update. Curr Hypertens Rep 2006; 8:521–527.
- Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol 2004; 15:1974–1982.
- Wright JR, Shurrab AE, Cheung C, et al A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis 2002; 39:1153–1161.
- Messina LM, Zelenock GB, Yao KA, Stanley JC. Renal revascularization for recurrent pulmonary edema in patients with poorly controlled hypertension and renal insufficiency: a distinct subgroup of patients with arteriosclerotic renal artery occlusive disease. J Vasc Surg 1992; 15:73–80.
- Bloch MJ, Trost DW, Pickering TG, Sos TA, August P. Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens 1999; 12:1–7.
- Gray BH, Olin JW, Childs MB, Sullivan TM, Bacharach JM. Clinical benefit of renal artery angioplasty with stenting for the control of recurrent and refractory congestive heart failure. Vasc Med 2002; 7:275–279.
- Pickering TG, Herman L, Devereux RB, et al Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet 1988; 2:551–552.
- Kennedy DJ, Colyer WR, Brewster PS, et al Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis 2003; 14:926–935.
- Beutler JJ, Van Ampting JM, Van De Ven PJ, et al Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol 2001; 12:1475–1481.
- Van de Ven PJ, Kaatee R, Beutler JJ, et al Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomized trial. Lancet 1999; 353:282–286.
- Isles C, Main J, O’Connell J, et al Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scott Med J 1990; 35:70–73.
- Hunt JC, Sheps SG, Harrison EG, Strong CG, Bernatz PE. Renal and renovascular hypertension. A reasoned approach to diagnosis and management. Arch Intern Med 1974; 133:988–999.
- Textor SC. Atherosclerotic renal artery stenosis: how big is the problem, and what happens if nothing is done? J Hypertens Suppl 2005; 23:S5–S13.
- van Jaarsveld BC, Krijnen P, Pieterman H, et al The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342:1007–1014.
- Zeller T, Frank U, Müller C, et al Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation 2003; 108;2244–2249.
- Chábová V, Schirger A, Stanson AW, McKusick MA, Textor SC. Outcomes of atherosclerotic renal artery stenosis managed without revascularization. Mayo Clin Proc 2000; 75:437–444.
- Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. Implications of acute functional injury following percutaneous renal artery intervention. Ann Vasc Surg 2008; 22:783–789.
- Radermacher J, Chavin A, Bleck J, et al Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Eng J Med 2001; 344:410–417.
- García-Criado A, Gilabert R, Nicolau C, et al Value of Doppler sonography for predicting clinical outcome after renal artery revascularization in atherosclerotic renal artery stenosis. J Ultrasound Med 2005; 24:1641–1647.
- Zeller T, Müller C, Frank U, et al Stent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc Interv 2003; 58:510–515.
- Harden PN, MacLeod MJ, Rodger RSC, et al Effect of renal-artery stenting on progression of renovascular renal failure. Lancet 1997; 349:1133–1136.
- Isles CG, Robertson S, Hill D. Management of renovascular disease: a review of renal artery stenting in ten studies. QJM 1999; 92:159–167.
- Muray S, Martın M, Amoedo ML, et al Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis 2002; 39:60–66.
- Textor SC, Glockner JF, Lerman LO, et al The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 2008; 19:780–788.
- Paraskevas KI, Perrea D, Briana DD, Liapis CD. Management of atherosclerotic renovascular disease: the effect of renal artery stenting on renal function and blood pressure. Int Urol Nephrol 2006; 38:683–691.
- Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation 2000; 102:1671–1677.
- Dean RH, Kieffer RW, Smith BM, et al Renovascular hypertension: anatomic and renal function changes during drug therapy. Arch Surg 1981; 116:1408–1415.
- Zhang Q, Shen W, Zhang R, Zhang J, Hu J, Zhang X. Effects of renal artery stenting on renal function and blood pressure in patients with atherosclerotic renovascular disease. Chin Med J (Engl) 2003; 116:1451–1454.
- Ramos F, Kotliar C, Alvarez D, et al Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int 2003; 63:276–282.
- Rocha-Singh KJ, Ahuja RK, Sung CH, Rutherford J. Long-term renal function preservation after renal artery stenting in patients with progressive ischemic nephropathy. Catheter Cardiovasc Interv 2002; 57:135–141.
- Thadhani RI, Camargo CA, Xavier RJ, Fang LS, Bazari H. Atheroembolic renal failure after invasive procedures. Natural history based on 52 histologically proven cases. Medicine (Baltimore) 1995; 74:350–358.
- Holden A, Hill A, Jaff MR, Pilmore H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int 2006; 70:948–955.
- Cooper CJ, Haller ST, Colyer W, et al Embolic protection and platelet inhibition during renal artery stenting. Circulation 2008; 117:2752–2760.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
KEY POINTS
- Two large randomized trials of intervention vs medical therapy showed negative results for intervention. A third trial is under way.
- Intervention is not recommended if renal function has remained stable over the past 6 to 12 months and if hypertension can be controlled medically.
- The best evidence supporting intervention is for bilateral stenosis with “flash” pulmonary edema, but the evidence is from retrospective studies.
- Stenosis by itself, even if bilateral, is not an indication for renal artery stenting.
Treating silent reflux disease does not improve poorly controlled asthma
Should patients with poorly controlled asthma be treated empirically for gastroesphageal reflux disease (GERD)?
Current guidelines1 indicate that trying a proton pump inhibitor may be worthwhile. However, the results of a recent multicenter trial2 indicate that this does not help control asthma symptoms and that we need to reevaluate the guidelines and focus on other factors that can worsen asthma control.
REFLUX DISEASE IS LINKED TO ASTHMA
GERD’s association with asthma has long been recognized. Asthma patients have a higher prevalence of GERD than the general population, with reported rates of 20% to 80%.3–8
GERD may worsen asthma via several mechanisms. If stomach acid gets into the airway, it can induce bronchoconstriction, vagal reflexes, and chronic airway inflammation, all of which can increase airway reactivity.9–16 Chronic reflux can also cause inflammation of the esophagus, which can exacerbate cough and possibly bronchospasm via neurogenic mechanisms.17
In turn, asthma may worsen GERD. Airway restriction can lead to hyperinflation and increased negative inspiratory pleural pressure, both of which may reduce the effectiveness of the lower esophageal sphincter.18 In addition, the beta-agonists and methylxanthines used to treat asthma may impair function of the lower esophageal sphincter and exacerbate reflux.18–20
CURRENT GUIDELINES ARE BASED ON LIMITED INFORMATION
The symptoms of GERD and asthma are nonspecific and can be similar (chest tightness, chest discomfort), which can make it challenging for clinicians or patients to distinguish asthma from GERD.2 Moreover, in asthma patients, GERD often presents without classic symptoms such as heartburn, and thus has been labeled “silent” GERD.
However, these studies all had significant limitations, such as small sample size. Also, the definitions of asthma and GERD differed from study to study. In some cases, the definition of GERD included self-reported GERD, which often fails to correlate with GERD documented with esophageal pH monitoring in asthma patients.1 These limitations were highlighted in a Cochrane review,30 which found that asthma patients with GERD showed no overall improvement in asthma after treatment of reflux. It concluded that small groups of patients may benefit, but that predicting who will respond is difficult.
Larger randomized controlled trials28,29 attempted to address some of these limitations, with varying results.
Littner et al29 gave lansoprazole (Prevacid) 30 mg twice daily or placebo to 207 patients with moderate to severe asthma and symptomatic GERD and saw no improvement in daily asthma symptoms, ie, asthma control in the active-treatment group. While these patients had an improvement in symptoms of severe reflux, their overall quality-of-life scores were similar to those of the placebo group. Of note, patients needing more than one type of drug for asthma control had a lower rate of asthma exacerbations.
Kiljander et al28 gave esomeprazole (Nexium) 40 mg twice daily or placebo to 770 patients who had mild to moderate asthma and symptoms of nocturnal asthma with or without symptoms of GERD. The only benefit was a slight improvement in peak expiratory flow in those with symptoms of both GERD and nocturnal asthma, and this was most significant in patients taking long-acting beta-agonists. Other measures—eg, the forced expiratory volume in the first second (FEV1), use of a beta-agonist, symptom scores, and nocturnal awakenings—did not improve.
In both of these studies,28,29 patients reported symptoms of GERD, so they did not have silent GERD.
THE DESIGN OF SARA
In SARA, 412 patients age 18 and older with inadequately controlled asthma were randomized to receive esomeprazole 40 mg twice a day or placebo for 24 weeks. Inadequate control was defined as a score of 1.5 or higher on the Juniper Asthma Control Questionnaire31 despite treatment with inhaled corticosteroids. Patients had no symptoms of GERD. The 40-mg twice-daily dosage of esomeprazole was chosen because it is known to suppress more than 90% of acid reflux.24,32
All patients completed a baseline asthma diary, recording peak expiratory flow rates, asthma symptoms, nighttime symptoms, and beta-agonist use. This information was collected every 4 weeks throughout the trial.
All participants also underwent esophageal pH monitoring for an objective confirmation of GERD. Patients were randomized independently of the results of the pH probe; in fact, investigators and patients were blinded to these results.
The primary outcome measure was the rate of episodes of poor asthma control, with poor control defined as any of the following:
- A decrease of 30% or more in the morning peak expiratory flow rate on 2 consecutive days, compared with the patient’s best rate during the run-in period
- An urgent visit, defined as an unscheduled health care visit, for asthma symptoms
- The need for a course of oral prednisone for treatment of asthma.
Asthma was defined as doctor-diagnosed, plus either a positive methacholine challenge test (a concentration of methacholine causing a 20% reduction in FEV1 [PC20] < 16 mg/mL) or a positive bronchodilator response (a 12% increase in FEV1) to an inhaled beta-agonist. Participants had no other indication for acid suppression, including symptoms of GERD or previously diagnosed erosive esophageal or gastric disease.
Acid reflux was evaluated by ambulatory pH monitoring, which had to last at least 16 hours and span one meal and 2 hours in the recumbent position. Reflux was present if the pH was less than 4.0 for more than 5.8% of total time, 8.2% of time upright, or 3.5% of time lying down.33 Episodes and severity were measured by the Gastroesophageal Reflux Disease Symptom Assessment Scale.34
SARA RESULTS: NO IMPROVEMENT IN ASTHMA WITH GERD TREATMENT
The SARA treatment and control groups had similar baseline characteristics, with similar asthma symptoms. Most of the patients were women: 72% of the placebo group and 64% of the esomeprazole group. Most had baseline spirometric results at the lower end of normal (the mean FEV1 was 76% ± 16 SD in the treatment group and 78% ± 15 in the placebo group) and had very poor asthma control, with an average Juniper Asthma Control Questionnaire score of 1.9 (> 1.5 is considered poor control).31 GERD was documented with esophageal pH monitoring in 40% of patients, showing that a significant number had silent GERD.
Episodes of poor asthma control occurred with similar frequency in the esomeprazole and placebo groups (2.5 vs 2.3 events per personyear, P = .66). Treatment made no difference in this end point regardless of the baseline results of pH monitoring. No treatment effect was noted in the individual components of the episodes of poor asthma control or in secondary outcomes, including pulmonary function, airway reactivity, asthma control, symptom scores, nocturnal awakening, or quality of life.
In addition, subgroup analysis failed to identify any group—including those with documented reflux on pH probe testing or those receiving a long-acting beta-agonist—who benefited from proton pump inhibitor therapy.
The investigators concluded that these data suggest treatment of silent GERD does not improve asthma control and thus that a reevaluation of current guidelines and clinical practice is warranted.2
ISSUES REMAIN
This large clinical trial, in which asthma and GERD were well defined and objectively measured, was robustly negative in terms of showing any benefit of treatment of silent GERD on asthma control. The study population was representative of those for whom such a treatment is recommended in the current NIH guidelines, which are based on data published prior to SARA.
However, while SARA was well designed and had clear results, it had some limitations, and some issues regarding GERD and asthma remain unanswered.
Is acid the only problem in GERD? SARA focused on acidic GERD. Aspiration of substances such as pancreatic enzymes, pepsin, and bile has also been shown to induce symptoms in asthma patients.2,32,35 In addition, distention of the esophagus and stimulation of neurogenically mediated reflexes can cause symptoms or neurogenic airway inflammation that is not mitigated by drugs that target acid reflux.32
Indirectly supporting this theory is evidence that surgical interventions such as fundoplication can improve asthma symptoms. 36 However, this evidence is only from small studies with significant limitations.
Is proximal GERD worse than distal GERD? SARA did not address whether proximal and distal reflux may affect asthma differently. The importance of proximal reflux in asthma has not been clearly established, but there is evidence that patients with proximal GERD have a higher incidence of nocturnal cough than patients who have only distal reflux. 37
Dimango et al38 recently reported additional data from SARA in which patients with poorly controlled asthma underwent both proximal and distal esophageal pH monitoring to see if proximal GERD was associated with poor asthma control: 304 patients underwent dual pH-probe assessment and 38% of them had proximal reflux. The authors found no difference between those with and without proximal GERD with regard to nocturnal awakenings, need to use a rescue inhaler, inhaled controller medication dose, lung function, or airway reactivity by methacholine challenge. However, they did find that those with proximal GERD had worse asthma quality-of-life scores, and worse health-related quality-of-life scores and were more likely to complain of cough.
Thus, it appears that proximal GERD may worsen quality of life in asthmatic patients but does not worsen asthma control.
SARA RESULTS: IMPLICATIONS FOR MANAGEMENT
The SARA results suggest that patients with poorly controlled asthma who are on adequate controller medications should not be treated empirically for silent GERD in the expectation that the asthma will improve. Rather, they suggest that the focus should be on other factors that can worsen asthma control, such as the ability to properly use an inhaler, the ability to afford medications, compliance with drug treatment, and adequate control of other significant comorbidities such as allergic bronchopulmonary aspergillosis, sinusitis, allergic rhinitis, vocal cord dysfunction, and occult heart disease. The most recent NIH guidelines also suggest considering referral to an asthma specialist if symptoms persist despite adequate controller therapy.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120( suppl 5):S94–S138.
- Mastronarde JG, Anthonisen NR, Castro M, et al., American Lung Association Asthma Clinical Research Centers Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009; 360:1487–1499.
- Harding SM, Guzzo MR, Richter JE. 24-h Esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest 1999; 115:654–659.
- Sontag SJ, O’Connell S, Khandelwal S, et al Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology 1990; 99:613–620.
- Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am J Respir Crit Care Med 2000; 162:34–39.
- Vincent D, Cohen-Jonathan AM, Leport J, et al Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J 1997; 10:2255–2259.
- Simpson WG. Gastroesophageal reflux disease and asthma. Diagnosis and management. Arch Intern Med 1995; 155:798–803.
- Irwin RS, Curley FJ, French CL. Difficult-to-control asthma. Contributing factors and outcome of a systematic management protocol. Chest 1993; 103:1662–1669.
- Harding SM, Richter JE. The role of gastroesophageal reflux in chronic cough and asthma. Chest 1997; 111:1389–1402.
- Richter JE. Asthma and gastroesophageal reflux disease: the truth is difficult to define. Chest 1999; 116:1150–1152.
- Ekstrom T, Tibbling L. Esophageal acid perfusion, airway function, and symptoms in asthmatic patients with marked bronchial hyperreactivity. Chest 1989; 96:995–998.
- Herve P, Denjean A, Jian R, Simonneau G, Duroux P. Intraesophageal perfusion of acid increases the bronchomotor response to methacholine and to isocapnic hyperventilation in asthmatic subjects. Am Rev Respir Dis 1986; 134:986–989.
- Wu DN, Tanifuji Y, Kobayashi H, et al Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest 2000; 118:1553–1556.
- Cuttitta G, Cibella F, Visconti A, Scichilone N, Bellia V, Bonsignore G. Spontaneous gastroesophageal reflux and airway patency during the night in adult asthmatics. Am J Respir Crit Care Med 2000; 161:177–181.
- Jack CI, Calverley PM, Donnelly RJ, et al Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastrooesophageal reflux. Thorax 1995; 50:201–204.
- Harding SM, Schan CA, Guzzo MR, Alexander RW, Bradley LA, Richter JE. Gastroesophageal reflux-induced bronchoconstriction. Is microaspiration a factor? Chest 1995; 108:1220–1227.
- Irwin RS, Madison JM, Fraire AE. The cough reflex and its relation to gastroesophageal reflux. Am J Med 2000; 108( suppl 4a):73S–78S.
- Choy D, Leung R. Gastro-oesophageal reflux disease and asthma. Respirology 1997; 2:163–168.
- Zerbib F, Guisset O, Lamouliatte H, Quinton A, Galmiche JP, Tunon-De-Lara JM. Effects of bronchial obstruction on lower esophageal sphincter motility and gastroesophageal reflux in patients with asthma. Am J Respir Crit Care Med 2002; 166:1206–1211.
- Lacy BE, Mathis C, DesBiens J, Liu MC. The effects of nebulized albuterol on esophageal function in asthmatic patients. Dig Dis Sci 2008; 53:2627–2633.
- Ford GA, Oliver PS, Prior JS, Butland RJ, Wilkinson SP. Omeprazole in the treatment of asthmatics with nocturnal symptoms and gastrooesophageal reflux: a placebo-controlled cross-over study. Postgrad Med J 1994; 70:350–354.
- Teichtahl H, Kronborg IJ, Yeomans ND, Robinson P. Adult asthma and gastro-oesophageal reflux: the effects of omeprazole therapy on asthma. Aust N Z J Med 1996; 26:671–676.
- Meier JH, McNally PR, Punja M, et al Does omeprazole (Prilosec) improve respiratory function in asthmatics with gastroesophageal reflux? A double-blind, placebo-controlled crossover study. Dig Dis Sci 1994; 39:2127–2133.
- Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med 1996; 100:395–405.
- Levin TR, Sperling RM, McQuaid KR. Omeprazole improves peak expiratory flow rate and quality of life in asthmatics with gastroesophageal reflux. Am J Gastroenterol 1998; 93:1060–1063.
- Boeree MJ, Peters FT, Postma DS, Kleibeuker JH. No effects of highdose omeprazole in patients with severe airway hyperresponsiveness and (a)symptomatic gastro-oesophageal reflux. Eur Respir J 1998; 11:1070–1074.
- Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Gastroesophageal reflux in asthmatics: a double-blind, placebo-controlled crossover study with omeprazole. Chest 1999; 116:1257–1264.
- Kiljander TO, Harding SM, Field SK, et al Effects of eso-meprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2006; 173:1091–1097.
- Littner MR, Leung FW, Ballard ED, Huang B, Samra NKLansoprazole Asthma Study Group. Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest 2005; 128:1128–1135.
- Gibson PG, Henry R, Coughlan JJL. Gastro-oesophageal reflux treatment for asthma in adults and children. Coch-rane Database Syst Rev 2000;CD001496. Also available online at www.cochrane.org/reviews/en/ab001496.html. Accessed January 28, 2010.
- Juniper EF, Bousquet J, Abetz L, Bateman EDGOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006; 100:616–621.
- Canning BJ, Mazzone SB. Reflex mechanisms in gastro-esophageal reflux disease and asthma. Am J Med 2003; 115( suppl 3A):45S–48S.
- Richter JE, Bradley LA, DeMeester TR, Wu WC. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig Dis Sci 1992; 37:849–856.
- Damiano A, Handley K, Adler E, Siddique R, Bhattacharyja A. Measuring symptom distress and health-related quality of life in clinical trials of gastroesophageal reflux disease treatment: further validation of the Gastroesophageal Reflux Disease Symptom Assessment Scale (GSAS). Dig Dis Sci 2002; 47:1530–1537.
- Asano K, Suzuki H. Silent acid reflux and asthma control [editorial]. N Engl J Med 2009; 360:1551–1553.
- Rakita S, Villadolid D, Thomas A, et al Laparoscopic Nissen fundoplication offers high patient satisfaction with relief of extraesophageal symptoms of gastroesophageal reflux disease. Am Surg 2006; 72:207–212.
- Tomonaga T, Awad ZT, Filipi CJ, et al Symptom predictability of reflux-induced respiratory disease. Dig Dis Sci 2002; 47:9–14.
- Dimango E, Holbrook JT, Simpson E, et al., American Lung Association Asthma Clinical Research Centers. Effects of asymptomatic proximal and distal gastroesophageal reflux on asthma severity. Am J Respir Crit Care Med 2009; 180:809–816.
Should patients with poorly controlled asthma be treated empirically for gastroesphageal reflux disease (GERD)?
Current guidelines1 indicate that trying a proton pump inhibitor may be worthwhile. However, the results of a recent multicenter trial2 indicate that this does not help control asthma symptoms and that we need to reevaluate the guidelines and focus on other factors that can worsen asthma control.
REFLUX DISEASE IS LINKED TO ASTHMA
GERD’s association with asthma has long been recognized. Asthma patients have a higher prevalence of GERD than the general population, with reported rates of 20% to 80%.3–8
GERD may worsen asthma via several mechanisms. If stomach acid gets into the airway, it can induce bronchoconstriction, vagal reflexes, and chronic airway inflammation, all of which can increase airway reactivity.9–16 Chronic reflux can also cause inflammation of the esophagus, which can exacerbate cough and possibly bronchospasm via neurogenic mechanisms.17
In turn, asthma may worsen GERD. Airway restriction can lead to hyperinflation and increased negative inspiratory pleural pressure, both of which may reduce the effectiveness of the lower esophageal sphincter.18 In addition, the beta-agonists and methylxanthines used to treat asthma may impair function of the lower esophageal sphincter and exacerbate reflux.18–20
CURRENT GUIDELINES ARE BASED ON LIMITED INFORMATION
The symptoms of GERD and asthma are nonspecific and can be similar (chest tightness, chest discomfort), which can make it challenging for clinicians or patients to distinguish asthma from GERD.2 Moreover, in asthma patients, GERD often presents without classic symptoms such as heartburn, and thus has been labeled “silent” GERD.
However, these studies all had significant limitations, such as small sample size. Also, the definitions of asthma and GERD differed from study to study. In some cases, the definition of GERD included self-reported GERD, which often fails to correlate with GERD documented with esophageal pH monitoring in asthma patients.1 These limitations were highlighted in a Cochrane review,30 which found that asthma patients with GERD showed no overall improvement in asthma after treatment of reflux. It concluded that small groups of patients may benefit, but that predicting who will respond is difficult.
Larger randomized controlled trials28,29 attempted to address some of these limitations, with varying results.
Littner et al29 gave lansoprazole (Prevacid) 30 mg twice daily or placebo to 207 patients with moderate to severe asthma and symptomatic GERD and saw no improvement in daily asthma symptoms, ie, asthma control in the active-treatment group. While these patients had an improvement in symptoms of severe reflux, their overall quality-of-life scores were similar to those of the placebo group. Of note, patients needing more than one type of drug for asthma control had a lower rate of asthma exacerbations.
Kiljander et al28 gave esomeprazole (Nexium) 40 mg twice daily or placebo to 770 patients who had mild to moderate asthma and symptoms of nocturnal asthma with or without symptoms of GERD. The only benefit was a slight improvement in peak expiratory flow in those with symptoms of both GERD and nocturnal asthma, and this was most significant in patients taking long-acting beta-agonists. Other measures—eg, the forced expiratory volume in the first second (FEV1), use of a beta-agonist, symptom scores, and nocturnal awakenings—did not improve.
In both of these studies,28,29 patients reported symptoms of GERD, so they did not have silent GERD.
THE DESIGN OF SARA
In SARA, 412 patients age 18 and older with inadequately controlled asthma were randomized to receive esomeprazole 40 mg twice a day or placebo for 24 weeks. Inadequate control was defined as a score of 1.5 or higher on the Juniper Asthma Control Questionnaire31 despite treatment with inhaled corticosteroids. Patients had no symptoms of GERD. The 40-mg twice-daily dosage of esomeprazole was chosen because it is known to suppress more than 90% of acid reflux.24,32
All patients completed a baseline asthma diary, recording peak expiratory flow rates, asthma symptoms, nighttime symptoms, and beta-agonist use. This information was collected every 4 weeks throughout the trial.
All participants also underwent esophageal pH monitoring for an objective confirmation of GERD. Patients were randomized independently of the results of the pH probe; in fact, investigators and patients were blinded to these results.
The primary outcome measure was the rate of episodes of poor asthma control, with poor control defined as any of the following:
- A decrease of 30% or more in the morning peak expiratory flow rate on 2 consecutive days, compared with the patient’s best rate during the run-in period
- An urgent visit, defined as an unscheduled health care visit, for asthma symptoms
- The need for a course of oral prednisone for treatment of asthma.
Asthma was defined as doctor-diagnosed, plus either a positive methacholine challenge test (a concentration of methacholine causing a 20% reduction in FEV1 [PC20] < 16 mg/mL) or a positive bronchodilator response (a 12% increase in FEV1) to an inhaled beta-agonist. Participants had no other indication for acid suppression, including symptoms of GERD or previously diagnosed erosive esophageal or gastric disease.
Acid reflux was evaluated by ambulatory pH monitoring, which had to last at least 16 hours and span one meal and 2 hours in the recumbent position. Reflux was present if the pH was less than 4.0 for more than 5.8% of total time, 8.2% of time upright, or 3.5% of time lying down.33 Episodes and severity were measured by the Gastroesophageal Reflux Disease Symptom Assessment Scale.34
SARA RESULTS: NO IMPROVEMENT IN ASTHMA WITH GERD TREATMENT
The SARA treatment and control groups had similar baseline characteristics, with similar asthma symptoms. Most of the patients were women: 72% of the placebo group and 64% of the esomeprazole group. Most had baseline spirometric results at the lower end of normal (the mean FEV1 was 76% ± 16 SD in the treatment group and 78% ± 15 in the placebo group) and had very poor asthma control, with an average Juniper Asthma Control Questionnaire score of 1.9 (> 1.5 is considered poor control).31 GERD was documented with esophageal pH monitoring in 40% of patients, showing that a significant number had silent GERD.
Episodes of poor asthma control occurred with similar frequency in the esomeprazole and placebo groups (2.5 vs 2.3 events per personyear, P = .66). Treatment made no difference in this end point regardless of the baseline results of pH monitoring. No treatment effect was noted in the individual components of the episodes of poor asthma control or in secondary outcomes, including pulmonary function, airway reactivity, asthma control, symptom scores, nocturnal awakening, or quality of life.
In addition, subgroup analysis failed to identify any group—including those with documented reflux on pH probe testing or those receiving a long-acting beta-agonist—who benefited from proton pump inhibitor therapy.
The investigators concluded that these data suggest treatment of silent GERD does not improve asthma control and thus that a reevaluation of current guidelines and clinical practice is warranted.2
ISSUES REMAIN
This large clinical trial, in which asthma and GERD were well defined and objectively measured, was robustly negative in terms of showing any benefit of treatment of silent GERD on asthma control. The study population was representative of those for whom such a treatment is recommended in the current NIH guidelines, which are based on data published prior to SARA.
However, while SARA was well designed and had clear results, it had some limitations, and some issues regarding GERD and asthma remain unanswered.
Is acid the only problem in GERD? SARA focused on acidic GERD. Aspiration of substances such as pancreatic enzymes, pepsin, and bile has also been shown to induce symptoms in asthma patients.2,32,35 In addition, distention of the esophagus and stimulation of neurogenically mediated reflexes can cause symptoms or neurogenic airway inflammation that is not mitigated by drugs that target acid reflux.32
Indirectly supporting this theory is evidence that surgical interventions such as fundoplication can improve asthma symptoms. 36 However, this evidence is only from small studies with significant limitations.
Is proximal GERD worse than distal GERD? SARA did not address whether proximal and distal reflux may affect asthma differently. The importance of proximal reflux in asthma has not been clearly established, but there is evidence that patients with proximal GERD have a higher incidence of nocturnal cough than patients who have only distal reflux. 37
Dimango et al38 recently reported additional data from SARA in which patients with poorly controlled asthma underwent both proximal and distal esophageal pH monitoring to see if proximal GERD was associated with poor asthma control: 304 patients underwent dual pH-probe assessment and 38% of them had proximal reflux. The authors found no difference between those with and without proximal GERD with regard to nocturnal awakenings, need to use a rescue inhaler, inhaled controller medication dose, lung function, or airway reactivity by methacholine challenge. However, they did find that those with proximal GERD had worse asthma quality-of-life scores, and worse health-related quality-of-life scores and were more likely to complain of cough.
Thus, it appears that proximal GERD may worsen quality of life in asthmatic patients but does not worsen asthma control.
SARA RESULTS: IMPLICATIONS FOR MANAGEMENT
The SARA results suggest that patients with poorly controlled asthma who are on adequate controller medications should not be treated empirically for silent GERD in the expectation that the asthma will improve. Rather, they suggest that the focus should be on other factors that can worsen asthma control, such as the ability to properly use an inhaler, the ability to afford medications, compliance with drug treatment, and adequate control of other significant comorbidities such as allergic bronchopulmonary aspergillosis, sinusitis, allergic rhinitis, vocal cord dysfunction, and occult heart disease. The most recent NIH guidelines also suggest considering referral to an asthma specialist if symptoms persist despite adequate controller therapy.
Should patients with poorly controlled asthma be treated empirically for gastroesphageal reflux disease (GERD)?
Current guidelines1 indicate that trying a proton pump inhibitor may be worthwhile. However, the results of a recent multicenter trial2 indicate that this does not help control asthma symptoms and that we need to reevaluate the guidelines and focus on other factors that can worsen asthma control.
REFLUX DISEASE IS LINKED TO ASTHMA
GERD’s association with asthma has long been recognized. Asthma patients have a higher prevalence of GERD than the general population, with reported rates of 20% to 80%.3–8
GERD may worsen asthma via several mechanisms. If stomach acid gets into the airway, it can induce bronchoconstriction, vagal reflexes, and chronic airway inflammation, all of which can increase airway reactivity.9–16 Chronic reflux can also cause inflammation of the esophagus, which can exacerbate cough and possibly bronchospasm via neurogenic mechanisms.17
In turn, asthma may worsen GERD. Airway restriction can lead to hyperinflation and increased negative inspiratory pleural pressure, both of which may reduce the effectiveness of the lower esophageal sphincter.18 In addition, the beta-agonists and methylxanthines used to treat asthma may impair function of the lower esophageal sphincter and exacerbate reflux.18–20
CURRENT GUIDELINES ARE BASED ON LIMITED INFORMATION
The symptoms of GERD and asthma are nonspecific and can be similar (chest tightness, chest discomfort), which can make it challenging for clinicians or patients to distinguish asthma from GERD.2 Moreover, in asthma patients, GERD often presents without classic symptoms such as heartburn, and thus has been labeled “silent” GERD.
However, these studies all had significant limitations, such as small sample size. Also, the definitions of asthma and GERD differed from study to study. In some cases, the definition of GERD included self-reported GERD, which often fails to correlate with GERD documented with esophageal pH monitoring in asthma patients.1 These limitations were highlighted in a Cochrane review,30 which found that asthma patients with GERD showed no overall improvement in asthma after treatment of reflux. It concluded that small groups of patients may benefit, but that predicting who will respond is difficult.
Larger randomized controlled trials28,29 attempted to address some of these limitations, with varying results.
Littner et al29 gave lansoprazole (Prevacid) 30 mg twice daily or placebo to 207 patients with moderate to severe asthma and symptomatic GERD and saw no improvement in daily asthma symptoms, ie, asthma control in the active-treatment group. While these patients had an improvement in symptoms of severe reflux, their overall quality-of-life scores were similar to those of the placebo group. Of note, patients needing more than one type of drug for asthma control had a lower rate of asthma exacerbations.
Kiljander et al28 gave esomeprazole (Nexium) 40 mg twice daily or placebo to 770 patients who had mild to moderate asthma and symptoms of nocturnal asthma with or without symptoms of GERD. The only benefit was a slight improvement in peak expiratory flow in those with symptoms of both GERD and nocturnal asthma, and this was most significant in patients taking long-acting beta-agonists. Other measures—eg, the forced expiratory volume in the first second (FEV1), use of a beta-agonist, symptom scores, and nocturnal awakenings—did not improve.
In both of these studies,28,29 patients reported symptoms of GERD, so they did not have silent GERD.
THE DESIGN OF SARA
In SARA, 412 patients age 18 and older with inadequately controlled asthma were randomized to receive esomeprazole 40 mg twice a day or placebo for 24 weeks. Inadequate control was defined as a score of 1.5 or higher on the Juniper Asthma Control Questionnaire31 despite treatment with inhaled corticosteroids. Patients had no symptoms of GERD. The 40-mg twice-daily dosage of esomeprazole was chosen because it is known to suppress more than 90% of acid reflux.24,32
All patients completed a baseline asthma diary, recording peak expiratory flow rates, asthma symptoms, nighttime symptoms, and beta-agonist use. This information was collected every 4 weeks throughout the trial.
All participants also underwent esophageal pH monitoring for an objective confirmation of GERD. Patients were randomized independently of the results of the pH probe; in fact, investigators and patients were blinded to these results.
The primary outcome measure was the rate of episodes of poor asthma control, with poor control defined as any of the following:
- A decrease of 30% or more in the morning peak expiratory flow rate on 2 consecutive days, compared with the patient’s best rate during the run-in period
- An urgent visit, defined as an unscheduled health care visit, for asthma symptoms
- The need for a course of oral prednisone for treatment of asthma.
Asthma was defined as doctor-diagnosed, plus either a positive methacholine challenge test (a concentration of methacholine causing a 20% reduction in FEV1 [PC20] < 16 mg/mL) or a positive bronchodilator response (a 12% increase in FEV1) to an inhaled beta-agonist. Participants had no other indication for acid suppression, including symptoms of GERD or previously diagnosed erosive esophageal or gastric disease.
Acid reflux was evaluated by ambulatory pH monitoring, which had to last at least 16 hours and span one meal and 2 hours in the recumbent position. Reflux was present if the pH was less than 4.0 for more than 5.8% of total time, 8.2% of time upright, or 3.5% of time lying down.33 Episodes and severity were measured by the Gastroesophageal Reflux Disease Symptom Assessment Scale.34
SARA RESULTS: NO IMPROVEMENT IN ASTHMA WITH GERD TREATMENT
The SARA treatment and control groups had similar baseline characteristics, with similar asthma symptoms. Most of the patients were women: 72% of the placebo group and 64% of the esomeprazole group. Most had baseline spirometric results at the lower end of normal (the mean FEV1 was 76% ± 16 SD in the treatment group and 78% ± 15 in the placebo group) and had very poor asthma control, with an average Juniper Asthma Control Questionnaire score of 1.9 (> 1.5 is considered poor control).31 GERD was documented with esophageal pH monitoring in 40% of patients, showing that a significant number had silent GERD.
Episodes of poor asthma control occurred with similar frequency in the esomeprazole and placebo groups (2.5 vs 2.3 events per personyear, P = .66). Treatment made no difference in this end point regardless of the baseline results of pH monitoring. No treatment effect was noted in the individual components of the episodes of poor asthma control or in secondary outcomes, including pulmonary function, airway reactivity, asthma control, symptom scores, nocturnal awakening, or quality of life.
In addition, subgroup analysis failed to identify any group—including those with documented reflux on pH probe testing or those receiving a long-acting beta-agonist—who benefited from proton pump inhibitor therapy.
The investigators concluded that these data suggest treatment of silent GERD does not improve asthma control and thus that a reevaluation of current guidelines and clinical practice is warranted.2
ISSUES REMAIN
This large clinical trial, in which asthma and GERD were well defined and objectively measured, was robustly negative in terms of showing any benefit of treatment of silent GERD on asthma control. The study population was representative of those for whom such a treatment is recommended in the current NIH guidelines, which are based on data published prior to SARA.
However, while SARA was well designed and had clear results, it had some limitations, and some issues regarding GERD and asthma remain unanswered.
Is acid the only problem in GERD? SARA focused on acidic GERD. Aspiration of substances such as pancreatic enzymes, pepsin, and bile has also been shown to induce symptoms in asthma patients.2,32,35 In addition, distention of the esophagus and stimulation of neurogenically mediated reflexes can cause symptoms or neurogenic airway inflammation that is not mitigated by drugs that target acid reflux.32
Indirectly supporting this theory is evidence that surgical interventions such as fundoplication can improve asthma symptoms. 36 However, this evidence is only from small studies with significant limitations.
Is proximal GERD worse than distal GERD? SARA did not address whether proximal and distal reflux may affect asthma differently. The importance of proximal reflux in asthma has not been clearly established, but there is evidence that patients with proximal GERD have a higher incidence of nocturnal cough than patients who have only distal reflux. 37
Dimango et al38 recently reported additional data from SARA in which patients with poorly controlled asthma underwent both proximal and distal esophageal pH monitoring to see if proximal GERD was associated with poor asthma control: 304 patients underwent dual pH-probe assessment and 38% of them had proximal reflux. The authors found no difference between those with and without proximal GERD with regard to nocturnal awakenings, need to use a rescue inhaler, inhaled controller medication dose, lung function, or airway reactivity by methacholine challenge. However, they did find that those with proximal GERD had worse asthma quality-of-life scores, and worse health-related quality-of-life scores and were more likely to complain of cough.
Thus, it appears that proximal GERD may worsen quality of life in asthmatic patients but does not worsen asthma control.
SARA RESULTS: IMPLICATIONS FOR MANAGEMENT
The SARA results suggest that patients with poorly controlled asthma who are on adequate controller medications should not be treated empirically for silent GERD in the expectation that the asthma will improve. Rather, they suggest that the focus should be on other factors that can worsen asthma control, such as the ability to properly use an inhaler, the ability to afford medications, compliance with drug treatment, and adequate control of other significant comorbidities such as allergic bronchopulmonary aspergillosis, sinusitis, allergic rhinitis, vocal cord dysfunction, and occult heart disease. The most recent NIH guidelines also suggest considering referral to an asthma specialist if symptoms persist despite adequate controller therapy.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120( suppl 5):S94–S138.
- Mastronarde JG, Anthonisen NR, Castro M, et al., American Lung Association Asthma Clinical Research Centers Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009; 360:1487–1499.
- Harding SM, Guzzo MR, Richter JE. 24-h Esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest 1999; 115:654–659.
- Sontag SJ, O’Connell S, Khandelwal S, et al Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology 1990; 99:613–620.
- Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am J Respir Crit Care Med 2000; 162:34–39.
- Vincent D, Cohen-Jonathan AM, Leport J, et al Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J 1997; 10:2255–2259.
- Simpson WG. Gastroesophageal reflux disease and asthma. Diagnosis and management. Arch Intern Med 1995; 155:798–803.
- Irwin RS, Curley FJ, French CL. Difficult-to-control asthma. Contributing factors and outcome of a systematic management protocol. Chest 1993; 103:1662–1669.
- Harding SM, Richter JE. The role of gastroesophageal reflux in chronic cough and asthma. Chest 1997; 111:1389–1402.
- Richter JE. Asthma and gastroesophageal reflux disease: the truth is difficult to define. Chest 1999; 116:1150–1152.
- Ekstrom T, Tibbling L. Esophageal acid perfusion, airway function, and symptoms in asthmatic patients with marked bronchial hyperreactivity. Chest 1989; 96:995–998.
- Herve P, Denjean A, Jian R, Simonneau G, Duroux P. Intraesophageal perfusion of acid increases the bronchomotor response to methacholine and to isocapnic hyperventilation in asthmatic subjects. Am Rev Respir Dis 1986; 134:986–989.
- Wu DN, Tanifuji Y, Kobayashi H, et al Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest 2000; 118:1553–1556.
- Cuttitta G, Cibella F, Visconti A, Scichilone N, Bellia V, Bonsignore G. Spontaneous gastroesophageal reflux and airway patency during the night in adult asthmatics. Am J Respir Crit Care Med 2000; 161:177–181.
- Jack CI, Calverley PM, Donnelly RJ, et al Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastrooesophageal reflux. Thorax 1995; 50:201–204.
- Harding SM, Schan CA, Guzzo MR, Alexander RW, Bradley LA, Richter JE. Gastroesophageal reflux-induced bronchoconstriction. Is microaspiration a factor? Chest 1995; 108:1220–1227.
- Irwin RS, Madison JM, Fraire AE. The cough reflex and its relation to gastroesophageal reflux. Am J Med 2000; 108( suppl 4a):73S–78S.
- Choy D, Leung R. Gastro-oesophageal reflux disease and asthma. Respirology 1997; 2:163–168.
- Zerbib F, Guisset O, Lamouliatte H, Quinton A, Galmiche JP, Tunon-De-Lara JM. Effects of bronchial obstruction on lower esophageal sphincter motility and gastroesophageal reflux in patients with asthma. Am J Respir Crit Care Med 2002; 166:1206–1211.
- Lacy BE, Mathis C, DesBiens J, Liu MC. The effects of nebulized albuterol on esophageal function in asthmatic patients. Dig Dis Sci 2008; 53:2627–2633.
- Ford GA, Oliver PS, Prior JS, Butland RJ, Wilkinson SP. Omeprazole in the treatment of asthmatics with nocturnal symptoms and gastrooesophageal reflux: a placebo-controlled cross-over study. Postgrad Med J 1994; 70:350–354.
- Teichtahl H, Kronborg IJ, Yeomans ND, Robinson P. Adult asthma and gastro-oesophageal reflux: the effects of omeprazole therapy on asthma. Aust N Z J Med 1996; 26:671–676.
- Meier JH, McNally PR, Punja M, et al Does omeprazole (Prilosec) improve respiratory function in asthmatics with gastroesophageal reflux? A double-blind, placebo-controlled crossover study. Dig Dis Sci 1994; 39:2127–2133.
- Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med 1996; 100:395–405.
- Levin TR, Sperling RM, McQuaid KR. Omeprazole improves peak expiratory flow rate and quality of life in asthmatics with gastroesophageal reflux. Am J Gastroenterol 1998; 93:1060–1063.
- Boeree MJ, Peters FT, Postma DS, Kleibeuker JH. No effects of highdose omeprazole in patients with severe airway hyperresponsiveness and (a)symptomatic gastro-oesophageal reflux. Eur Respir J 1998; 11:1070–1074.
- Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Gastroesophageal reflux in asthmatics: a double-blind, placebo-controlled crossover study with omeprazole. Chest 1999; 116:1257–1264.
- Kiljander TO, Harding SM, Field SK, et al Effects of eso-meprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2006; 173:1091–1097.
- Littner MR, Leung FW, Ballard ED, Huang B, Samra NKLansoprazole Asthma Study Group. Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest 2005; 128:1128–1135.
- Gibson PG, Henry R, Coughlan JJL. Gastro-oesophageal reflux treatment for asthma in adults and children. Coch-rane Database Syst Rev 2000;CD001496. Also available online at www.cochrane.org/reviews/en/ab001496.html. Accessed January 28, 2010.
- Juniper EF, Bousquet J, Abetz L, Bateman EDGOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006; 100:616–621.
- Canning BJ, Mazzone SB. Reflex mechanisms in gastro-esophageal reflux disease and asthma. Am J Med 2003; 115( suppl 3A):45S–48S.
- Richter JE, Bradley LA, DeMeester TR, Wu WC. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig Dis Sci 1992; 37:849–856.
- Damiano A, Handley K, Adler E, Siddique R, Bhattacharyja A. Measuring symptom distress and health-related quality of life in clinical trials of gastroesophageal reflux disease treatment: further validation of the Gastroesophageal Reflux Disease Symptom Assessment Scale (GSAS). Dig Dis Sci 2002; 47:1530–1537.
- Asano K, Suzuki H. Silent acid reflux and asthma control [editorial]. N Engl J Med 2009; 360:1551–1553.
- Rakita S, Villadolid D, Thomas A, et al Laparoscopic Nissen fundoplication offers high patient satisfaction with relief of extraesophageal symptoms of gastroesophageal reflux disease. Am Surg 2006; 72:207–212.
- Tomonaga T, Awad ZT, Filipi CJ, et al Symptom predictability of reflux-induced respiratory disease. Dig Dis Sci 2002; 47:9–14.
- Dimango E, Holbrook JT, Simpson E, et al., American Lung Association Asthma Clinical Research Centers. Effects of asymptomatic proximal and distal gastroesophageal reflux on asthma severity. Am J Respir Crit Care Med 2009; 180:809–816.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120( suppl 5):S94–S138.
- Mastronarde JG, Anthonisen NR, Castro M, et al., American Lung Association Asthma Clinical Research Centers Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009; 360:1487–1499.
- Harding SM, Guzzo MR, Richter JE. 24-h Esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest 1999; 115:654–659.
- Sontag SJ, O’Connell S, Khandelwal S, et al Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology 1990; 99:613–620.
- Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am J Respir Crit Care Med 2000; 162:34–39.
- Vincent D, Cohen-Jonathan AM, Leport J, et al Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J 1997; 10:2255–2259.
- Simpson WG. Gastroesophageal reflux disease and asthma. Diagnosis and management. Arch Intern Med 1995; 155:798–803.
- Irwin RS, Curley FJ, French CL. Difficult-to-control asthma. Contributing factors and outcome of a systematic management protocol. Chest 1993; 103:1662–1669.
- Harding SM, Richter JE. The role of gastroesophageal reflux in chronic cough and asthma. Chest 1997; 111:1389–1402.
- Richter JE. Asthma and gastroesophageal reflux disease: the truth is difficult to define. Chest 1999; 116:1150–1152.
- Ekstrom T, Tibbling L. Esophageal acid perfusion, airway function, and symptoms in asthmatic patients with marked bronchial hyperreactivity. Chest 1989; 96:995–998.
- Herve P, Denjean A, Jian R, Simonneau G, Duroux P. Intraesophageal perfusion of acid increases the bronchomotor response to methacholine and to isocapnic hyperventilation in asthmatic subjects. Am Rev Respir Dis 1986; 134:986–989.
- Wu DN, Tanifuji Y, Kobayashi H, et al Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest 2000; 118:1553–1556.
- Cuttitta G, Cibella F, Visconti A, Scichilone N, Bellia V, Bonsignore G. Spontaneous gastroesophageal reflux and airway patency during the night in adult asthmatics. Am J Respir Crit Care Med 2000; 161:177–181.
- Jack CI, Calverley PM, Donnelly RJ, et al Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastrooesophageal reflux. Thorax 1995; 50:201–204.
- Harding SM, Schan CA, Guzzo MR, Alexander RW, Bradley LA, Richter JE. Gastroesophageal reflux-induced bronchoconstriction. Is microaspiration a factor? Chest 1995; 108:1220–1227.
- Irwin RS, Madison JM, Fraire AE. The cough reflex and its relation to gastroesophageal reflux. Am J Med 2000; 108( suppl 4a):73S–78S.
- Choy D, Leung R. Gastro-oesophageal reflux disease and asthma. Respirology 1997; 2:163–168.
- Zerbib F, Guisset O, Lamouliatte H, Quinton A, Galmiche JP, Tunon-De-Lara JM. Effects of bronchial obstruction on lower esophageal sphincter motility and gastroesophageal reflux in patients with asthma. Am J Respir Crit Care Med 2002; 166:1206–1211.
- Lacy BE, Mathis C, DesBiens J, Liu MC. The effects of nebulized albuterol on esophageal function in asthmatic patients. Dig Dis Sci 2008; 53:2627–2633.
- Ford GA, Oliver PS, Prior JS, Butland RJ, Wilkinson SP. Omeprazole in the treatment of asthmatics with nocturnal symptoms and gastrooesophageal reflux: a placebo-controlled cross-over study. Postgrad Med J 1994; 70:350–354.
- Teichtahl H, Kronborg IJ, Yeomans ND, Robinson P. Adult asthma and gastro-oesophageal reflux: the effects of omeprazole therapy on asthma. Aust N Z J Med 1996; 26:671–676.
- Meier JH, McNally PR, Punja M, et al Does omeprazole (Prilosec) improve respiratory function in asthmatics with gastroesophageal reflux? A double-blind, placebo-controlled crossover study. Dig Dis Sci 1994; 39:2127–2133.
- Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med 1996; 100:395–405.
- Levin TR, Sperling RM, McQuaid KR. Omeprazole improves peak expiratory flow rate and quality of life in asthmatics with gastroesophageal reflux. Am J Gastroenterol 1998; 93:1060–1063.
- Boeree MJ, Peters FT, Postma DS, Kleibeuker JH. No effects of highdose omeprazole in patients with severe airway hyperresponsiveness and (a)symptomatic gastro-oesophageal reflux. Eur Respir J 1998; 11:1070–1074.
- Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Gastroesophageal reflux in asthmatics: a double-blind, placebo-controlled crossover study with omeprazole. Chest 1999; 116:1257–1264.
- Kiljander TO, Harding SM, Field SK, et al Effects of eso-meprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2006; 173:1091–1097.
- Littner MR, Leung FW, Ballard ED, Huang B, Samra NKLansoprazole Asthma Study Group. Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest 2005; 128:1128–1135.
- Gibson PG, Henry R, Coughlan JJL. Gastro-oesophageal reflux treatment for asthma in adults and children. Coch-rane Database Syst Rev 2000;CD001496. Also available online at www.cochrane.org/reviews/en/ab001496.html. Accessed January 28, 2010.
- Juniper EF, Bousquet J, Abetz L, Bateman EDGOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006; 100:616–621.
- Canning BJ, Mazzone SB. Reflex mechanisms in gastro-esophageal reflux disease and asthma. Am J Med 2003; 115( suppl 3A):45S–48S.
- Richter JE, Bradley LA, DeMeester TR, Wu WC. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig Dis Sci 1992; 37:849–856.
- Damiano A, Handley K, Adler E, Siddique R, Bhattacharyja A. Measuring symptom distress and health-related quality of life in clinical trials of gastroesophageal reflux disease treatment: further validation of the Gastroesophageal Reflux Disease Symptom Assessment Scale (GSAS). Dig Dis Sci 2002; 47:1530–1537.
- Asano K, Suzuki H. Silent acid reflux and asthma control [editorial]. N Engl J Med 2009; 360:1551–1553.
- Rakita S, Villadolid D, Thomas A, et al Laparoscopic Nissen fundoplication offers high patient satisfaction with relief of extraesophageal symptoms of gastroesophageal reflux disease. Am Surg 2006; 72:207–212.
- Tomonaga T, Awad ZT, Filipi CJ, et al Symptom predictability of reflux-induced respiratory disease. Dig Dis Sci 2002; 47:9–14.
- Dimango E, Holbrook JT, Simpson E, et al., American Lung Association Asthma Clinical Research Centers. Effects of asymptomatic proximal and distal gastroesophageal reflux on asthma severity. Am J Respir Crit Care Med 2009; 180:809–816.
KEY POINTS
- Acid reflux is more prevalent in patients with asthma, and it often occurs without classic symptoms such as heartburn.
- Current guidelines, based on data from older studies with significant limitations, recommend considering treatment for reflux disease, even without the classic symptoms, in patients with uncontrolled asthma.
- The recent Study of Acid Reflux in Asthma found not only that treating silent acid reflux does not improve asthma control, but also that esophageal pH monitoring does not detect a subgroup of asthma patients who might respond to a proton pump inhibitor. These data suggest that we should reconsider clinical practice based on current guidelines.
Vaccination: An option not to be ignored
We have previously discussed in the Journal the recrudescence of pertussis in adults and the challenges to its diagnosis, which include the misperception that it is a rare disease. 1 Adult pertussis infection is usually due to the waning effect of childhood vaccination, and in adults it is more often an extreme annoyance than a life-threatening illness.
In this issue, Dr. Camille Sabella2 discusses measles, an infection thought to be all but eradicated in the United States by vaccination, predominantly using the live-attenuated measles virus contained in the measles-mumps-rubella (MMR) vaccine.
But measles outbreaks are seemingly on the rise, and because measles is extremely contagious, it poses a real risk to closed communities such as college dormitories, churches, and health care facilities. Measles infection can have significant adverse outcomes, particularly in immunosuppressed patients.
Although outbreaks have been attributed to virus imported from locations outside the United States, the spread of infection has been blamed on an increased number of unvaccinated children and adults. The reasons for decreased vaccination rates are many, and include parental fears that the vaccine will cause problems such as autism.
This autism link is a goblin that refuses to go away, despite strongly worded debunking by the US Centers for Disease Control and Prevention, the Institute of Medicine,1 and many peer-reviewed publications. The very recent retraction by the Lancet4—based on ethical and nondisclosure concerns—of the 1998 paper by Wakefield et al5 (which suggested a link in 12 children between MMR vaccine and chronic gastrointestinal problems and autism spectrum disorders) may further diffuse this concern. But I fear it will not.
So at present, we should reeducate ourselves on the clinical features, natural history, and potential complications of this eradicable disease, particularly if we treat patients who work in closed communities or have defects in cellular immunity.
- Sabella C. Pertussis: old foe, persistent problem. Cleve Clin J Med 2005; 72:601–608.
- Sabella C. Measles: not just a childhood rash. Cleve Clin J Med 2010; 77:207–213.
- Board on Population Health and Public Health Practice, Institute of Medicine of the National Academies. Immunization safety review: vaccines and autism. http://www.iom.edu/Reports/2004/Immunization-Safety-Review-Vaccines-and-Autism.aspx. Accessed February 11, 2010.
- The Editors of The Lancet. Retraction—ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet (published online February 2, 2010) DOI:10.1016/S0140-6736(10)60175-4. Accessed February 11, 2010.
- Wakefield AJ, Murch SH, Anthony A, et al Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998; 351:637–641.
We have previously discussed in the Journal the recrudescence of pertussis in adults and the challenges to its diagnosis, which include the misperception that it is a rare disease. 1 Adult pertussis infection is usually due to the waning effect of childhood vaccination, and in adults it is more often an extreme annoyance than a life-threatening illness.
In this issue, Dr. Camille Sabella2 discusses measles, an infection thought to be all but eradicated in the United States by vaccination, predominantly using the live-attenuated measles virus contained in the measles-mumps-rubella (MMR) vaccine.
But measles outbreaks are seemingly on the rise, and because measles is extremely contagious, it poses a real risk to closed communities such as college dormitories, churches, and health care facilities. Measles infection can have significant adverse outcomes, particularly in immunosuppressed patients.
Although outbreaks have been attributed to virus imported from locations outside the United States, the spread of infection has been blamed on an increased number of unvaccinated children and adults. The reasons for decreased vaccination rates are many, and include parental fears that the vaccine will cause problems such as autism.
This autism link is a goblin that refuses to go away, despite strongly worded debunking by the US Centers for Disease Control and Prevention, the Institute of Medicine,1 and many peer-reviewed publications. The very recent retraction by the Lancet4—based on ethical and nondisclosure concerns—of the 1998 paper by Wakefield et al5 (which suggested a link in 12 children between MMR vaccine and chronic gastrointestinal problems and autism spectrum disorders) may further diffuse this concern. But I fear it will not.
So at present, we should reeducate ourselves on the clinical features, natural history, and potential complications of this eradicable disease, particularly if we treat patients who work in closed communities or have defects in cellular immunity.
We have previously discussed in the Journal the recrudescence of pertussis in adults and the challenges to its diagnosis, which include the misperception that it is a rare disease. 1 Adult pertussis infection is usually due to the waning effect of childhood vaccination, and in adults it is more often an extreme annoyance than a life-threatening illness.
In this issue, Dr. Camille Sabella2 discusses measles, an infection thought to be all but eradicated in the United States by vaccination, predominantly using the live-attenuated measles virus contained in the measles-mumps-rubella (MMR) vaccine.
But measles outbreaks are seemingly on the rise, and because measles is extremely contagious, it poses a real risk to closed communities such as college dormitories, churches, and health care facilities. Measles infection can have significant adverse outcomes, particularly in immunosuppressed patients.
Although outbreaks have been attributed to virus imported from locations outside the United States, the spread of infection has been blamed on an increased number of unvaccinated children and adults. The reasons for decreased vaccination rates are many, and include parental fears that the vaccine will cause problems such as autism.
This autism link is a goblin that refuses to go away, despite strongly worded debunking by the US Centers for Disease Control and Prevention, the Institute of Medicine,1 and many peer-reviewed publications. The very recent retraction by the Lancet4—based on ethical and nondisclosure concerns—of the 1998 paper by Wakefield et al5 (which suggested a link in 12 children between MMR vaccine and chronic gastrointestinal problems and autism spectrum disorders) may further diffuse this concern. But I fear it will not.
So at present, we should reeducate ourselves on the clinical features, natural history, and potential complications of this eradicable disease, particularly if we treat patients who work in closed communities or have defects in cellular immunity.
- Sabella C. Pertussis: old foe, persistent problem. Cleve Clin J Med 2005; 72:601–608.
- Sabella C. Measles: not just a childhood rash. Cleve Clin J Med 2010; 77:207–213.
- Board on Population Health and Public Health Practice, Institute of Medicine of the National Academies. Immunization safety review: vaccines and autism. http://www.iom.edu/Reports/2004/Immunization-Safety-Review-Vaccines-and-Autism.aspx. Accessed February 11, 2010.
- The Editors of The Lancet. Retraction—ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet (published online February 2, 2010) DOI:10.1016/S0140-6736(10)60175-4. Accessed February 11, 2010.
- Wakefield AJ, Murch SH, Anthony A, et al Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998; 351:637–641.
- Sabella C. Pertussis: old foe, persistent problem. Cleve Clin J Med 2005; 72:601–608.
- Sabella C. Measles: not just a childhood rash. Cleve Clin J Med 2010; 77:207–213.
- Board on Population Health and Public Health Practice, Institute of Medicine of the National Academies. Immunization safety review: vaccines and autism. http://www.iom.edu/Reports/2004/Immunization-Safety-Review-Vaccines-and-Autism.aspx. Accessed February 11, 2010.
- The Editors of The Lancet. Retraction—ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet (published online February 2, 2010) DOI:10.1016/S0140-6736(10)60175-4. Accessed February 11, 2010.
- Wakefield AJ, Murch SH, Anthony A, et al Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998; 351:637–641.
Measles: Not just a childhood rash
Although measles is generally considered a disease of children, it affects people of all ages. While the incidence of measles in the United States is significantly lower than in 1963, when an effective measles vaccine was first introduced, recent increases in the number of sporadic cases and community outbreaks in adults show that measles is still a significant health problem.
PATHOGENESIS OF MEASLES
Measles is a highly contagious viral infection, whose manifestations have been recognized since the 7th century. The measles virus is an RNA virus of the Paramyxoviridae family. It is very difficult to isolate from clinical specimens, requiring special cell lines for in vitro propagation.
After acquisition, the measles virus establishes localized infection of the respiratory epithelium and then spreads to the regional lymphatics. A primary viremia then occurs, in which the virus replicates at the site of inoculation and in the reticuloendothelial tissues. A secondary viremia follows, in which the virus infects and replicates in the skin, conjunctiva, respiratory tract, and other distant organs.
The measles rash is thought to be due to a hypersensitivity reaction.1 Cell-mediated responses are the main line of defense against measles, as evidenced by the fact that people with cell-mediated deficiencies develop severe measles infection.2 Immunity to wild-type measles is believed to be lifelong.3,4
MEASLES IS HIGHLY CONTAGIOUS
Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts. 4 The fact that emergency departments and physicians’ offices have become sites of measles transmission in recent years underscores the transmissibility of the virus.5–7
Although the virus is very labile, it can remain infective in respiratory droplets from the air for many hours. Thus, measles virus spreads from person to person by direct contact with droplets from respiratory secretions of infected persons.
The period of maximal contagion is the late prodrome, ie, 2 to 4 days before the onset of the rash. People who are generally in good health are contagious through 4 days after the onset of the rash, whereas people with compromised immunity can continue to shed the virus for the entire duration of the illness.
Airborne transmission precautions are required for 4 days after the onset of the rash in hospitalized, non-immunocompromised patients with measles, and for the duration of the illness for immunocompromised patients.
In the absence of widespread measles vaccination, measles infection peaks in late winter and early spring.
EPIDEMIOLOGIC TRENDS: CAUSE FOR CONCERN
Since an effective vaccine became available in 1963, the annual incidence of measles cases in the United States has decreased by more than 99%. A significant resurgence from 1989 to 1991 affected mainly unvaccinated preschoolers and resulted in more than 55,000 cases and 130 deaths.8 This resurgence prompted widespread, intensive immunization efforts and the recommendation that a second dose of measles vaccine be given to school-aged children. This led to the effective elimination of endemic transmission of measles in the United States.9
Although 90% of these cases either were directly imported or were associated with importation from other countries,10 the reason for the large number of cases was clearly the greater transmission after importation of the virus into the United States. This transmission was the direct result of the fact that 91% of the cases occurred in unvaccinated people or people whose vaccination status was not known or was not documented. A high proportion— at least 61 (47%)—of the 131 measles cases in 2008 were in school-aged children whose parents chose not to have them vaccinated. Although no deaths were reported in these 131 patients, 15 required hospitalization.
Although most reported measles cases are still in young and school-aged children, recent cases and outbreaks have also occurred in isolated communities of adults. Approximately 25% of the cases reported in 2008 were in people age 20 and older. Most adults who contracted measles had unknown or undocumented vaccination status. Similarly, a small measles outbreak occurred in Indiana in 2005, when an adolescent US citizen traveling in Europe became infected in Romania and exposed 500 people at a church gathering upon her return. Thirty-four cases of measles were reported from this exposure, and many were in adults.11
The recent increase in the number of cases reported and the continued reports of outbreaks highlight the fact that measles outbreaks can occur in communities with a high number of unvaccinated people, and underscore the need for high overall measles vaccination coverage to limit the spread of this infection.12
CLINICAL FEATURES OF MEASLES
The first sign of measles is a distinct prodrome, which occurs after an incubation period of 10 to 12 days. The prodrome is characterized by fever, malaise, anorexia, conjunctivitis, coryza, and cough and may resemble an upper respiratory tract infection; it lasts 2 to 4 days.
Towards the end of the prodrome, the body temperature can rise to as high as 40°C, and Koplik spots, pathognomonic for measles, appear. Koplik spots, bluish-gray specks on an erythematous base, usually appear on the buccal mucosa opposite the second molars 1 to 2 days before the onset of the rash, and last for 1 to 2 days after the onset of the rash. Thus, it is not unusual for Koplik spots to have disappeared at the time the diagnosis of measles is entertained.
The classic measles rash is an erythematous maculopapular eruption that begins on the head and face and spreads to involve the entire body. It usually persists for 4 to 5 days and is most confluent on the face and upper body. The rash fades in order of appearance, and may desquamate. People with measles appear ill, especially 1 to 2 days after the rash appears.
The entire course of measles usually lasts 7 to 10 days in patients with a healthy immune system. The cough, a manifestation of tracheobronchitis, is usually the last symptom to resolve. Patients are contagious 2 to 4 days before the onset of the rash, and remain so through 4 days after the onset of the rash.
COMPLICATIONS
Respiratory complications
Pneumonia is responsible for 60% of deaths associated with measles.13 Although radiographic evidence of pneumonia is found in measles patients with no complications, symptomatic pneumonia occurs in 1% to 6% of patients. It is the result of either direct invasion by the virus or secondary bacterial infection,17 most often with Staphylococcus aureus and Streptococcus pneumoniae. Other respiratory complications include otitis media, sinusitis, and laryngotracheobronchitis.
Neurologic complications
Acute measles encephalitis is more common in adults than in children. Occuring in 1 in 1,000 to 2,000 patients,18 it is characterized by the resurgence of fever during the convalescent phase of the illness, along with headaches, seizures, and altered consciousness. These manifestations may be mild or severe, but they lead to permanent neurologic sequelae in a substantial proportion of affected patients. It is not clear whether acute measles encephalitis represents direct invasion of the virus or a postinfectious process from a hypersensitivity to the virus.19
Subacute sclerosing panencephalitis is a rare, chronic, degenerative central nervous system disease that occurs secondary to persistent infection with a defective measles virus.20 The prevalence is estimated at 1 per 100,000 cases. Signs and symptoms appear an average of 7 years after the initial infection and include personality changes, myoclonic seizures, and motor disturbances. Often, coma and death follow.
This condition occurs particularly in those who had measles at a very young age, ie, before the age of 2 years, and it occurs despite a vigorous host-immune response to the virus. Patients have high titers of measles-specific antibody in the sera and cerebrospinal fluid.
Other complications
Diarrhea and stomatitis account for much of the sickness and death from measles in developing countries.
Subclinical hepatitis occurs in at least 30% of adult measles patients.
Less common complications include thrombocytopenia, appendicitis, ileocolitis, pericarditis, myocarditis, and hypocalcemia.
MEASLES DURING PREGNANCY
Measles during pregnancy may be severe, mainly due to primary measles pneumonia.21 Measles is associated with a risk of miscarriage and prematurity, but congenital anomalies of the fetus have not been described, as they have for rubella infection.22
MEASLES IN COMPROMISED IMMUNITY
Measles patients with deficiencies of cellmediated immunity have a prolonged, severe, and often fatal course.2,23,24 This includes patients with:
- Human immunodeficiency virus (HIV) infection
- Congenital immunodeficiencies
- Disorders requiring chemotherapeutic and immunosuppressive therapy.
These patients are particularly susceptible to acute progressive encephalitis and measles pneumonitis. Case-fatality rates of 70% in cancer patients and 40% in HIV-infected patients have been reported.24
The diagnosis of measles may be difficult in patients without cell-mediated immunity, as 25% to 40% of them do not develop the characteristic rash.2,23 The absence of rash supports the theory that the rash is a hypersensitivity reaction to the virus.
MODIFIED AND ATYPICAL MEASLES
Modified measles
A modified form of measles can occur in people with some degree of passive immunity to the virus, including those previously vaccinated. It occurs mostly in patients who recently received immunoglobulin products, or in young infants who have residual maternal antibody. A modified measles illness can also follow vaccination with live-virus vaccine (see later discussion).
The clinical manifestations vary, and the illness may not have the classic features of prodrome, rash, and Koplik spots.
Atypical measles
Atypical measles is an unusual form that can occur when a person previously vaccinated with a killed-virus measles vaccine (used from 1963 to 1967) is exposed to wild-type measles.25 Features include a shorter prodrome (1 to 2 days), followed by appearance of a rash that begins on the distal extremities and spreads centripetally, usually sparing the neck, face, and head. The rash may be petechial, maculopapular, urticarial, vesicular, or a combination. The rash is accompanied by high fever and edema of the extremities. Complications such as pneumonia and hepatitis may occur.
The course of atypical measles is more prolonged than with classic measles, but because these patients are thought to have partial protection against the virus, they do not transmit it and are not considered contagious.26
DIAGNOSIS OF MEASLES
The classic clinical features are usually enough to distinguish measles from other febrile illnesses with similar clinical manifestions, such as rubella, dengue, parvovirus B19 infection, erythema multiforme, Stevens-Johnson syndrome, and streptococcal scarlet fever. The distinctive measles prodrome, Koplik spots, the progression of the rash from the head and neck to the trunk and the extremities, and the severity of disease are distinctive features of measles.
Laboratory tests to confirm the diagnosis are often used in areas where measles is rare, and laboratory confirmation is currently recommended in the United States. Because viral isolation is technically difficult and is not widely available, serologic testing is the method most commonly used. The measles-specific immunoglobulin M (IgM) antibody assay, the test used most often, is almost 100% sensitive when done 2 to 3 days after the onset of the rash.27,28 Measles IgM antibody peaks at 4 weeks after the infection and disappears by 6 to 8 weeks.
It is important to remember that false-positive measles IgM antibody may occur with other viral infections, such as parvovirus B19 and rubella. Because measles-specific IgG antibody is produced with the onset of infection and peaks at 4 weeks, a fourfold rise in the IgG titer is useful in confirming the diagnosis. Measles IgG antibody after infection is sustained for life.
Reverse transcription-polymerase chain reaction testing can also detect measles virus in the blood and urine when direct evidence of the virus is necessary, such as in immunocompromised patients.29
TREATMENT IS SUPPORTIVE
Treatment of measles mainly involves supportive measures, such as fluids and antipyretics. Antiviral agents such as ribavirin and interferon have in vitro activity against the measles virus and have been used to treat severe measles infection in immunocompromised patients. However, their clinical efficacy is unproven.30
Routine use of antibacterial agents to prevent secondary bacterial infection is not recommended.
CURRENT RECOMMENDATIONS FOR ACTIVE IMMUNIZATION
Active immunization for measles has been available since 1963. Between 1963 and 1967, both killed-virus and live-virus vaccines were available. As atypical measles cases became recognized, the killed-virus vaccine was withdrawn.
The vaccine currently available in the United States is a live-attenuated strain prepared in chicken embryo cell culture and combined with mumps and rubella vaccine (MMR) or mumps, rubella, and varicella vaccine (MMRV).
Two doses of live-virus measles vaccine are recommended for all healthy children before they begin school, with the first dose given at 12 to 15 months of age. A second dose is needed because the failure rate with one dose is 5%. More than 99% of people who receive two doses separated by 4 weeks develop serologic evidence of measles.
Waning immunity after vaccination occurs very rarely, with approximately 5% of children developing secondary vaccine failure 10 to 15 years after vaccination.3,31
Although rates of vaccination in the United States are high, cases of measles continue to occur in unvaccinated infants and in children who are either too young to be vaccinated or whose parents claimed exemption because of religious or personal beliefs.
Because of the occurrence of measles cases in adolescents, young adults, and adults, potentially susceptible people should be identified and vaccinated according to current guidelines. People should be considered susceptible unless they have documentation of at least two doses of measles vaccine given at least 28 days apart, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. All adults who are susceptible should receive at least one dose of measles vaccine.10 Adults at higher risk of contracting measles include:
- Students in high school and college
- International travelers
- Health care personnel.
For these adults, two doses of measles vaccine, at least 28 days apart, are recommended.32
Postexposure prophylaxis
Measles vaccination given to susceptible contacts within 72 hours of exposure as postexposure prophylaxis may protect against infection and induces protection against subsequent exposures to measles.33,34 Vaccination is the intervention of choice for susceptible individuals older than 12 months of age who are exposed to measles and who do not have a contraindication to measles vaccination.35 Active rather than passive immunization is also the strategy of choice for controlling measles outbreaks.
Passive immunization with intramuscular immune globulin within 6 days of exposure can be used in selected circumstances to prevent transmission or to modify the clinical course of the infection.36 Immune globulin therapy is recommended for susceptible individuals who are exposed to measles and who are at high risk of developing severe or fatal measles. This includes individuals who are being treated with immunosuppressive agents, those with HIV infection, pregnant women, and infants less than 1 year of age. Immune globulin should not be used to control measles outbreaks.
ADVERSE EFFECTS OF MEASLES VACCINE
Live-virus measles vaccine has an excellent safety record. A transient fever, which may be accompanied by a measles-like rash, occurs in 5% to 15% of people 5 to 12 days after vaccination. The rash may be discrete or confluent and is self-limited.
Although measles vaccine is a live-attenuated vaccine, vaccinated people do not transmit the virus to susceptible contacts and are not considered contagious, even if they develop a vaccine-associated rash. Thus, the vaccine can be safely given to close contacts of immunocompromised and other susceptible people. Encephalitis is exceedingly rare following vaccination.
There is no scientific evidence that the risk of autism is higher in children who receive measles or MMR vaccine than in unvaccinated children.37 An Institute of Medicine report in 2001 rejected a causal relationship between MMR vaccine and autism spectrum disorders.38
CONTRAINDICATIONS TO MEASLES VACCINATION
Measles vaccine is contraindicated for:
- People who have cell-mediated immune deficiencies (except patients wtih HIV infection—see discussion just below)
- Pregnant women
- Those who had a severe allergic reaction to a vaccine component after a previous dose
- Those with moderate or severe acute illness
- Those who have recently received immune globulin products.
HIV-infected patients with severe immunosuppression should not receive the liveattenuated measles vaccine. However, because patients with HIV are at risk of severe measles, and because the vaccine has been shown to be safe in HIV patients who do not have severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression. 39
After receiving immune globulin
Anyone who has recently received immune globulin should not receive measles vaccine until sufficient time has passed, since passively acquired antibodies interfere with the immune response to live-virus vaccines. How long to wait depends on the type of immune globulin, the indication, the amount, and the route of administration. In general, the waiting period is:
- At least 3 months after intramuscular immune globulin or tetanus, hepatitis A, or hepatitis B prophylaxis
- At least 4 months after intramuscular immune globulin for rabies, or 6 months after intravenous immune globulin for cytomegalovirus (dose, 150 mg/kg)
- At least 8 months after intravenous immune globulin as replacement or therapy for immune deficiencies (dose, 400 mg/kg), or after intravenous immune globulin for immune thrombocytopenic purpura (400 mg/kg)
- At least 10 months after intravenous immune globulin for immune thrombocytopenic pupura at a dose of 1 g/kg.39
Egg allergy is not a contraindication
Although measles vaccine is produced in chick embryo cell culture, the vaccine has been shown to be safe in people with egg allergy, so they may be vaccinated without first being tested for egg allergy.39,40
- Lachmann PJ. Immunopathology of measles. Proc R Soc Med 1974; 67:1120–1122.
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in case of giant cell pneumonia without rash. N Engl J Med 1959; 261:875–881.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stokes J, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Farizo KM, Stehr-Green PA, Simpson DM, Markowitz LE. Pediatric emergency room visits: a risk factor for acquiring measles. Pediatrics 1991; 87:74–79.
- Bloch AB, Orenstein W, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics 1985; 75:676–683.
- Remington PL, Hall WN, Davis IH, et al. Airborne transmission of measles in a physician’s office. JAMA 1985; 253:1574–1577.
- US Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. MMWR 1994; 43:57–60.
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189 (suppl 1):S1–S3.
- US Centers for Disease Control and Prevention. Update: Measles—United States, January–July 2008. MMWR 2008; 57:893–896.
- Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–455.
- Mulholland EK. Measles in the United States, 2006. N Engl J Med 2006; 355:440–443.
- Barkin RM. Measles mortality. Analysis of the primary cause of death. Am J Dis Child 1975; 129:307–309.
- Barkin RM. Measles mortality: a retrospective look at the vaccine era. Am J Epidemiol 1975; 102:341–349.
- US Centers for Disease Control and Prevention. Public-sector vaccination efforts in response to the resurgence of measles among preschool-aged children—United States, 1989–1991. MMWR 1992; 41:522–525.
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. an analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189( suppl 1):S4–S16.
- Johnson RT, Griffin DE, Hirsch RL, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Gershon AA. Chickenpox, measles, and mumps. In:Remington J, Klein J, eds. Infectious Diseases of the Fetus and Newborn Infant. Elsevier Saunders: Philadelphia, 2006:693–738.
- Mitus A, Enders JF, Craig JM, Holloway A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med 1959; 261:882–889.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Mayo DR, Brennan T, Cormier DP, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol 1991; 29:2865–2867.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187( suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Forni Al, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994; 19:454–462.
- Anders JF, Jacobsen RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studes. Pediar Infect Dis J 1996; 15:62–66.
- US Centers for Disease Control and Prevention. Measles—United States, January 1–April 25, 2008. MMWR 2008; 57:494–498.
- Berkovitz S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Strebel PM, Papania MJ, Halsey NA. Measles vaccine. In:Plotkin SA, Orenstein WA, eds. Vaccines. Saunders: New York, 2004:389–440.
- US Centers for Disease Control and Prevention. Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998; 47( RR-8):1–57. http://www.cdc.gov/mmwr/PDF/rr/rr4708.pdf. Accessed December 30, 2009.
- Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–1482.
- Straton K, Gable A, Shetty P, McCormick M; for the Institute of Medicine. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. National Academy Press: Washington, DC, 2001.
- Pickerington LK, Baker CJ, Long SS, McMillan JA, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–455.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med 1995; 332:1262–1266.
Although measles is generally considered a disease of children, it affects people of all ages. While the incidence of measles in the United States is significantly lower than in 1963, when an effective measles vaccine was first introduced, recent increases in the number of sporadic cases and community outbreaks in adults show that measles is still a significant health problem.
PATHOGENESIS OF MEASLES
Measles is a highly contagious viral infection, whose manifestations have been recognized since the 7th century. The measles virus is an RNA virus of the Paramyxoviridae family. It is very difficult to isolate from clinical specimens, requiring special cell lines for in vitro propagation.
After acquisition, the measles virus establishes localized infection of the respiratory epithelium and then spreads to the regional lymphatics. A primary viremia then occurs, in which the virus replicates at the site of inoculation and in the reticuloendothelial tissues. A secondary viremia follows, in which the virus infects and replicates in the skin, conjunctiva, respiratory tract, and other distant organs.
The measles rash is thought to be due to a hypersensitivity reaction.1 Cell-mediated responses are the main line of defense against measles, as evidenced by the fact that people with cell-mediated deficiencies develop severe measles infection.2 Immunity to wild-type measles is believed to be lifelong.3,4
MEASLES IS HIGHLY CONTAGIOUS
Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts. 4 The fact that emergency departments and physicians’ offices have become sites of measles transmission in recent years underscores the transmissibility of the virus.5–7
Although the virus is very labile, it can remain infective in respiratory droplets from the air for many hours. Thus, measles virus spreads from person to person by direct contact with droplets from respiratory secretions of infected persons.
The period of maximal contagion is the late prodrome, ie, 2 to 4 days before the onset of the rash. People who are generally in good health are contagious through 4 days after the onset of the rash, whereas people with compromised immunity can continue to shed the virus for the entire duration of the illness.
Airborne transmission precautions are required for 4 days after the onset of the rash in hospitalized, non-immunocompromised patients with measles, and for the duration of the illness for immunocompromised patients.
In the absence of widespread measles vaccination, measles infection peaks in late winter and early spring.
EPIDEMIOLOGIC TRENDS: CAUSE FOR CONCERN
Since an effective vaccine became available in 1963, the annual incidence of measles cases in the United States has decreased by more than 99%. A significant resurgence from 1989 to 1991 affected mainly unvaccinated preschoolers and resulted in more than 55,000 cases and 130 deaths.8 This resurgence prompted widespread, intensive immunization efforts and the recommendation that a second dose of measles vaccine be given to school-aged children. This led to the effective elimination of endemic transmission of measles in the United States.9
Although 90% of these cases either were directly imported or were associated with importation from other countries,10 the reason for the large number of cases was clearly the greater transmission after importation of the virus into the United States. This transmission was the direct result of the fact that 91% of the cases occurred in unvaccinated people or people whose vaccination status was not known or was not documented. A high proportion— at least 61 (47%)—of the 131 measles cases in 2008 were in school-aged children whose parents chose not to have them vaccinated. Although no deaths were reported in these 131 patients, 15 required hospitalization.
Although most reported measles cases are still in young and school-aged children, recent cases and outbreaks have also occurred in isolated communities of adults. Approximately 25% of the cases reported in 2008 were in people age 20 and older. Most adults who contracted measles had unknown or undocumented vaccination status. Similarly, a small measles outbreak occurred in Indiana in 2005, when an adolescent US citizen traveling in Europe became infected in Romania and exposed 500 people at a church gathering upon her return. Thirty-four cases of measles were reported from this exposure, and many were in adults.11
The recent increase in the number of cases reported and the continued reports of outbreaks highlight the fact that measles outbreaks can occur in communities with a high number of unvaccinated people, and underscore the need for high overall measles vaccination coverage to limit the spread of this infection.12
CLINICAL FEATURES OF MEASLES
The first sign of measles is a distinct prodrome, which occurs after an incubation period of 10 to 12 days. The prodrome is characterized by fever, malaise, anorexia, conjunctivitis, coryza, and cough and may resemble an upper respiratory tract infection; it lasts 2 to 4 days.
Towards the end of the prodrome, the body temperature can rise to as high as 40°C, and Koplik spots, pathognomonic for measles, appear. Koplik spots, bluish-gray specks on an erythematous base, usually appear on the buccal mucosa opposite the second molars 1 to 2 days before the onset of the rash, and last for 1 to 2 days after the onset of the rash. Thus, it is not unusual for Koplik spots to have disappeared at the time the diagnosis of measles is entertained.
The classic measles rash is an erythematous maculopapular eruption that begins on the head and face and spreads to involve the entire body. It usually persists for 4 to 5 days and is most confluent on the face and upper body. The rash fades in order of appearance, and may desquamate. People with measles appear ill, especially 1 to 2 days after the rash appears.
The entire course of measles usually lasts 7 to 10 days in patients with a healthy immune system. The cough, a manifestation of tracheobronchitis, is usually the last symptom to resolve. Patients are contagious 2 to 4 days before the onset of the rash, and remain so through 4 days after the onset of the rash.
COMPLICATIONS
Respiratory complications
Pneumonia is responsible for 60% of deaths associated with measles.13 Although radiographic evidence of pneumonia is found in measles patients with no complications, symptomatic pneumonia occurs in 1% to 6% of patients. It is the result of either direct invasion by the virus or secondary bacterial infection,17 most often with Staphylococcus aureus and Streptococcus pneumoniae. Other respiratory complications include otitis media, sinusitis, and laryngotracheobronchitis.
Neurologic complications
Acute measles encephalitis is more common in adults than in children. Occuring in 1 in 1,000 to 2,000 patients,18 it is characterized by the resurgence of fever during the convalescent phase of the illness, along with headaches, seizures, and altered consciousness. These manifestations may be mild or severe, but they lead to permanent neurologic sequelae in a substantial proportion of affected patients. It is not clear whether acute measles encephalitis represents direct invasion of the virus or a postinfectious process from a hypersensitivity to the virus.19
Subacute sclerosing panencephalitis is a rare, chronic, degenerative central nervous system disease that occurs secondary to persistent infection with a defective measles virus.20 The prevalence is estimated at 1 per 100,000 cases. Signs and symptoms appear an average of 7 years after the initial infection and include personality changes, myoclonic seizures, and motor disturbances. Often, coma and death follow.
This condition occurs particularly in those who had measles at a very young age, ie, before the age of 2 years, and it occurs despite a vigorous host-immune response to the virus. Patients have high titers of measles-specific antibody in the sera and cerebrospinal fluid.
Other complications
Diarrhea and stomatitis account for much of the sickness and death from measles in developing countries.
Subclinical hepatitis occurs in at least 30% of adult measles patients.
Less common complications include thrombocytopenia, appendicitis, ileocolitis, pericarditis, myocarditis, and hypocalcemia.
MEASLES DURING PREGNANCY
Measles during pregnancy may be severe, mainly due to primary measles pneumonia.21 Measles is associated with a risk of miscarriage and prematurity, but congenital anomalies of the fetus have not been described, as they have for rubella infection.22
MEASLES IN COMPROMISED IMMUNITY
Measles patients with deficiencies of cellmediated immunity have a prolonged, severe, and often fatal course.2,23,24 This includes patients with:
- Human immunodeficiency virus (HIV) infection
- Congenital immunodeficiencies
- Disorders requiring chemotherapeutic and immunosuppressive therapy.
These patients are particularly susceptible to acute progressive encephalitis and measles pneumonitis. Case-fatality rates of 70% in cancer patients and 40% in HIV-infected patients have been reported.24
The diagnosis of measles may be difficult in patients without cell-mediated immunity, as 25% to 40% of them do not develop the characteristic rash.2,23 The absence of rash supports the theory that the rash is a hypersensitivity reaction to the virus.
MODIFIED AND ATYPICAL MEASLES
Modified measles
A modified form of measles can occur in people with some degree of passive immunity to the virus, including those previously vaccinated. It occurs mostly in patients who recently received immunoglobulin products, or in young infants who have residual maternal antibody. A modified measles illness can also follow vaccination with live-virus vaccine (see later discussion).
The clinical manifestations vary, and the illness may not have the classic features of prodrome, rash, and Koplik spots.
Atypical measles
Atypical measles is an unusual form that can occur when a person previously vaccinated with a killed-virus measles vaccine (used from 1963 to 1967) is exposed to wild-type measles.25 Features include a shorter prodrome (1 to 2 days), followed by appearance of a rash that begins on the distal extremities and spreads centripetally, usually sparing the neck, face, and head. The rash may be petechial, maculopapular, urticarial, vesicular, or a combination. The rash is accompanied by high fever and edema of the extremities. Complications such as pneumonia and hepatitis may occur.
The course of atypical measles is more prolonged than with classic measles, but because these patients are thought to have partial protection against the virus, they do not transmit it and are not considered contagious.26
DIAGNOSIS OF MEASLES
The classic clinical features are usually enough to distinguish measles from other febrile illnesses with similar clinical manifestions, such as rubella, dengue, parvovirus B19 infection, erythema multiforme, Stevens-Johnson syndrome, and streptococcal scarlet fever. The distinctive measles prodrome, Koplik spots, the progression of the rash from the head and neck to the trunk and the extremities, and the severity of disease are distinctive features of measles.
Laboratory tests to confirm the diagnosis are often used in areas where measles is rare, and laboratory confirmation is currently recommended in the United States. Because viral isolation is technically difficult and is not widely available, serologic testing is the method most commonly used. The measles-specific immunoglobulin M (IgM) antibody assay, the test used most often, is almost 100% sensitive when done 2 to 3 days after the onset of the rash.27,28 Measles IgM antibody peaks at 4 weeks after the infection and disappears by 6 to 8 weeks.
It is important to remember that false-positive measles IgM antibody may occur with other viral infections, such as parvovirus B19 and rubella. Because measles-specific IgG antibody is produced with the onset of infection and peaks at 4 weeks, a fourfold rise in the IgG titer is useful in confirming the diagnosis. Measles IgG antibody after infection is sustained for life.
Reverse transcription-polymerase chain reaction testing can also detect measles virus in the blood and urine when direct evidence of the virus is necessary, such as in immunocompromised patients.29
TREATMENT IS SUPPORTIVE
Treatment of measles mainly involves supportive measures, such as fluids and antipyretics. Antiviral agents such as ribavirin and interferon have in vitro activity against the measles virus and have been used to treat severe measles infection in immunocompromised patients. However, their clinical efficacy is unproven.30
Routine use of antibacterial agents to prevent secondary bacterial infection is not recommended.
CURRENT RECOMMENDATIONS FOR ACTIVE IMMUNIZATION
Active immunization for measles has been available since 1963. Between 1963 and 1967, both killed-virus and live-virus vaccines were available. As atypical measles cases became recognized, the killed-virus vaccine was withdrawn.
The vaccine currently available in the United States is a live-attenuated strain prepared in chicken embryo cell culture and combined with mumps and rubella vaccine (MMR) or mumps, rubella, and varicella vaccine (MMRV).
Two doses of live-virus measles vaccine are recommended for all healthy children before they begin school, with the first dose given at 12 to 15 months of age. A second dose is needed because the failure rate with one dose is 5%. More than 99% of people who receive two doses separated by 4 weeks develop serologic evidence of measles.
Waning immunity after vaccination occurs very rarely, with approximately 5% of children developing secondary vaccine failure 10 to 15 years after vaccination.3,31
Although rates of vaccination in the United States are high, cases of measles continue to occur in unvaccinated infants and in children who are either too young to be vaccinated or whose parents claimed exemption because of religious or personal beliefs.
Because of the occurrence of measles cases in adolescents, young adults, and adults, potentially susceptible people should be identified and vaccinated according to current guidelines. People should be considered susceptible unless they have documentation of at least two doses of measles vaccine given at least 28 days apart, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. All adults who are susceptible should receive at least one dose of measles vaccine.10 Adults at higher risk of contracting measles include:
- Students in high school and college
- International travelers
- Health care personnel.
For these adults, two doses of measles vaccine, at least 28 days apart, are recommended.32
Postexposure prophylaxis
Measles vaccination given to susceptible contacts within 72 hours of exposure as postexposure prophylaxis may protect against infection and induces protection against subsequent exposures to measles.33,34 Vaccination is the intervention of choice for susceptible individuals older than 12 months of age who are exposed to measles and who do not have a contraindication to measles vaccination.35 Active rather than passive immunization is also the strategy of choice for controlling measles outbreaks.
Passive immunization with intramuscular immune globulin within 6 days of exposure can be used in selected circumstances to prevent transmission or to modify the clinical course of the infection.36 Immune globulin therapy is recommended for susceptible individuals who are exposed to measles and who are at high risk of developing severe or fatal measles. This includes individuals who are being treated with immunosuppressive agents, those with HIV infection, pregnant women, and infants less than 1 year of age. Immune globulin should not be used to control measles outbreaks.
ADVERSE EFFECTS OF MEASLES VACCINE
Live-virus measles vaccine has an excellent safety record. A transient fever, which may be accompanied by a measles-like rash, occurs in 5% to 15% of people 5 to 12 days after vaccination. The rash may be discrete or confluent and is self-limited.
Although measles vaccine is a live-attenuated vaccine, vaccinated people do not transmit the virus to susceptible contacts and are not considered contagious, even if they develop a vaccine-associated rash. Thus, the vaccine can be safely given to close contacts of immunocompromised and other susceptible people. Encephalitis is exceedingly rare following vaccination.
There is no scientific evidence that the risk of autism is higher in children who receive measles or MMR vaccine than in unvaccinated children.37 An Institute of Medicine report in 2001 rejected a causal relationship between MMR vaccine and autism spectrum disorders.38
CONTRAINDICATIONS TO MEASLES VACCINATION
Measles vaccine is contraindicated for:
- People who have cell-mediated immune deficiencies (except patients wtih HIV infection—see discussion just below)
- Pregnant women
- Those who had a severe allergic reaction to a vaccine component after a previous dose
- Those with moderate or severe acute illness
- Those who have recently received immune globulin products.
HIV-infected patients with severe immunosuppression should not receive the liveattenuated measles vaccine. However, because patients with HIV are at risk of severe measles, and because the vaccine has been shown to be safe in HIV patients who do not have severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression. 39
After receiving immune globulin
Anyone who has recently received immune globulin should not receive measles vaccine until sufficient time has passed, since passively acquired antibodies interfere with the immune response to live-virus vaccines. How long to wait depends on the type of immune globulin, the indication, the amount, and the route of administration. In general, the waiting period is:
- At least 3 months after intramuscular immune globulin or tetanus, hepatitis A, or hepatitis B prophylaxis
- At least 4 months after intramuscular immune globulin for rabies, or 6 months after intravenous immune globulin for cytomegalovirus (dose, 150 mg/kg)
- At least 8 months after intravenous immune globulin as replacement or therapy for immune deficiencies (dose, 400 mg/kg), or after intravenous immune globulin for immune thrombocytopenic purpura (400 mg/kg)
- At least 10 months after intravenous immune globulin for immune thrombocytopenic pupura at a dose of 1 g/kg.39
Egg allergy is not a contraindication
Although measles vaccine is produced in chick embryo cell culture, the vaccine has been shown to be safe in people with egg allergy, so they may be vaccinated without first being tested for egg allergy.39,40
Although measles is generally considered a disease of children, it affects people of all ages. While the incidence of measles in the United States is significantly lower than in 1963, when an effective measles vaccine was first introduced, recent increases in the number of sporadic cases and community outbreaks in adults show that measles is still a significant health problem.
PATHOGENESIS OF MEASLES
Measles is a highly contagious viral infection, whose manifestations have been recognized since the 7th century. The measles virus is an RNA virus of the Paramyxoviridae family. It is very difficult to isolate from clinical specimens, requiring special cell lines for in vitro propagation.
After acquisition, the measles virus establishes localized infection of the respiratory epithelium and then spreads to the regional lymphatics. A primary viremia then occurs, in which the virus replicates at the site of inoculation and in the reticuloendothelial tissues. A secondary viremia follows, in which the virus infects and replicates in the skin, conjunctiva, respiratory tract, and other distant organs.
The measles rash is thought to be due to a hypersensitivity reaction.1 Cell-mediated responses are the main line of defense against measles, as evidenced by the fact that people with cell-mediated deficiencies develop severe measles infection.2 Immunity to wild-type measles is believed to be lifelong.3,4
MEASLES IS HIGHLY CONTAGIOUS
Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts. 4 The fact that emergency departments and physicians’ offices have become sites of measles transmission in recent years underscores the transmissibility of the virus.5–7
Although the virus is very labile, it can remain infective in respiratory droplets from the air for many hours. Thus, measles virus spreads from person to person by direct contact with droplets from respiratory secretions of infected persons.
The period of maximal contagion is the late prodrome, ie, 2 to 4 days before the onset of the rash. People who are generally in good health are contagious through 4 days after the onset of the rash, whereas people with compromised immunity can continue to shed the virus for the entire duration of the illness.
Airborne transmission precautions are required for 4 days after the onset of the rash in hospitalized, non-immunocompromised patients with measles, and for the duration of the illness for immunocompromised patients.
In the absence of widespread measles vaccination, measles infection peaks in late winter and early spring.
EPIDEMIOLOGIC TRENDS: CAUSE FOR CONCERN
Since an effective vaccine became available in 1963, the annual incidence of measles cases in the United States has decreased by more than 99%. A significant resurgence from 1989 to 1991 affected mainly unvaccinated preschoolers and resulted in more than 55,000 cases and 130 deaths.8 This resurgence prompted widespread, intensive immunization efforts and the recommendation that a second dose of measles vaccine be given to school-aged children. This led to the effective elimination of endemic transmission of measles in the United States.9
Although 90% of these cases either were directly imported or were associated with importation from other countries,10 the reason for the large number of cases was clearly the greater transmission after importation of the virus into the United States. This transmission was the direct result of the fact that 91% of the cases occurred in unvaccinated people or people whose vaccination status was not known or was not documented. A high proportion— at least 61 (47%)—of the 131 measles cases in 2008 were in school-aged children whose parents chose not to have them vaccinated. Although no deaths were reported in these 131 patients, 15 required hospitalization.
Although most reported measles cases are still in young and school-aged children, recent cases and outbreaks have also occurred in isolated communities of adults. Approximately 25% of the cases reported in 2008 were in people age 20 and older. Most adults who contracted measles had unknown or undocumented vaccination status. Similarly, a small measles outbreak occurred in Indiana in 2005, when an adolescent US citizen traveling in Europe became infected in Romania and exposed 500 people at a church gathering upon her return. Thirty-four cases of measles were reported from this exposure, and many were in adults.11
The recent increase in the number of cases reported and the continued reports of outbreaks highlight the fact that measles outbreaks can occur in communities with a high number of unvaccinated people, and underscore the need for high overall measles vaccination coverage to limit the spread of this infection.12
CLINICAL FEATURES OF MEASLES
The first sign of measles is a distinct prodrome, which occurs after an incubation period of 10 to 12 days. The prodrome is characterized by fever, malaise, anorexia, conjunctivitis, coryza, and cough and may resemble an upper respiratory tract infection; it lasts 2 to 4 days.
Towards the end of the prodrome, the body temperature can rise to as high as 40°C, and Koplik spots, pathognomonic for measles, appear. Koplik spots, bluish-gray specks on an erythematous base, usually appear on the buccal mucosa opposite the second molars 1 to 2 days before the onset of the rash, and last for 1 to 2 days after the onset of the rash. Thus, it is not unusual for Koplik spots to have disappeared at the time the diagnosis of measles is entertained.
The classic measles rash is an erythematous maculopapular eruption that begins on the head and face and spreads to involve the entire body. It usually persists for 4 to 5 days and is most confluent on the face and upper body. The rash fades in order of appearance, and may desquamate. People with measles appear ill, especially 1 to 2 days after the rash appears.
The entire course of measles usually lasts 7 to 10 days in patients with a healthy immune system. The cough, a manifestation of tracheobronchitis, is usually the last symptom to resolve. Patients are contagious 2 to 4 days before the onset of the rash, and remain so through 4 days after the onset of the rash.
COMPLICATIONS
Respiratory complications
Pneumonia is responsible for 60% of deaths associated with measles.13 Although radiographic evidence of pneumonia is found in measles patients with no complications, symptomatic pneumonia occurs in 1% to 6% of patients. It is the result of either direct invasion by the virus or secondary bacterial infection,17 most often with Staphylococcus aureus and Streptococcus pneumoniae. Other respiratory complications include otitis media, sinusitis, and laryngotracheobronchitis.
Neurologic complications
Acute measles encephalitis is more common in adults than in children. Occuring in 1 in 1,000 to 2,000 patients,18 it is characterized by the resurgence of fever during the convalescent phase of the illness, along with headaches, seizures, and altered consciousness. These manifestations may be mild or severe, but they lead to permanent neurologic sequelae in a substantial proportion of affected patients. It is not clear whether acute measles encephalitis represents direct invasion of the virus or a postinfectious process from a hypersensitivity to the virus.19
Subacute sclerosing panencephalitis is a rare, chronic, degenerative central nervous system disease that occurs secondary to persistent infection with a defective measles virus.20 The prevalence is estimated at 1 per 100,000 cases. Signs and symptoms appear an average of 7 years after the initial infection and include personality changes, myoclonic seizures, and motor disturbances. Often, coma and death follow.
This condition occurs particularly in those who had measles at a very young age, ie, before the age of 2 years, and it occurs despite a vigorous host-immune response to the virus. Patients have high titers of measles-specific antibody in the sera and cerebrospinal fluid.
Other complications
Diarrhea and stomatitis account for much of the sickness and death from measles in developing countries.
Subclinical hepatitis occurs in at least 30% of adult measles patients.
Less common complications include thrombocytopenia, appendicitis, ileocolitis, pericarditis, myocarditis, and hypocalcemia.
MEASLES DURING PREGNANCY
Measles during pregnancy may be severe, mainly due to primary measles pneumonia.21 Measles is associated with a risk of miscarriage and prematurity, but congenital anomalies of the fetus have not been described, as they have for rubella infection.22
MEASLES IN COMPROMISED IMMUNITY
Measles patients with deficiencies of cellmediated immunity have a prolonged, severe, and often fatal course.2,23,24 This includes patients with:
- Human immunodeficiency virus (HIV) infection
- Congenital immunodeficiencies
- Disorders requiring chemotherapeutic and immunosuppressive therapy.
These patients are particularly susceptible to acute progressive encephalitis and measles pneumonitis. Case-fatality rates of 70% in cancer patients and 40% in HIV-infected patients have been reported.24
The diagnosis of measles may be difficult in patients without cell-mediated immunity, as 25% to 40% of them do not develop the characteristic rash.2,23 The absence of rash supports the theory that the rash is a hypersensitivity reaction to the virus.
MODIFIED AND ATYPICAL MEASLES
Modified measles
A modified form of measles can occur in people with some degree of passive immunity to the virus, including those previously vaccinated. It occurs mostly in patients who recently received immunoglobulin products, or in young infants who have residual maternal antibody. A modified measles illness can also follow vaccination with live-virus vaccine (see later discussion).
The clinical manifestations vary, and the illness may not have the classic features of prodrome, rash, and Koplik spots.
Atypical measles
Atypical measles is an unusual form that can occur when a person previously vaccinated with a killed-virus measles vaccine (used from 1963 to 1967) is exposed to wild-type measles.25 Features include a shorter prodrome (1 to 2 days), followed by appearance of a rash that begins on the distal extremities and spreads centripetally, usually sparing the neck, face, and head. The rash may be petechial, maculopapular, urticarial, vesicular, or a combination. The rash is accompanied by high fever and edema of the extremities. Complications such as pneumonia and hepatitis may occur.
The course of atypical measles is more prolonged than with classic measles, but because these patients are thought to have partial protection against the virus, they do not transmit it and are not considered contagious.26
DIAGNOSIS OF MEASLES
The classic clinical features are usually enough to distinguish measles from other febrile illnesses with similar clinical manifestions, such as rubella, dengue, parvovirus B19 infection, erythema multiforme, Stevens-Johnson syndrome, and streptococcal scarlet fever. The distinctive measles prodrome, Koplik spots, the progression of the rash from the head and neck to the trunk and the extremities, and the severity of disease are distinctive features of measles.
Laboratory tests to confirm the diagnosis are often used in areas where measles is rare, and laboratory confirmation is currently recommended in the United States. Because viral isolation is technically difficult and is not widely available, serologic testing is the method most commonly used. The measles-specific immunoglobulin M (IgM) antibody assay, the test used most often, is almost 100% sensitive when done 2 to 3 days after the onset of the rash.27,28 Measles IgM antibody peaks at 4 weeks after the infection and disappears by 6 to 8 weeks.
It is important to remember that false-positive measles IgM antibody may occur with other viral infections, such as parvovirus B19 and rubella. Because measles-specific IgG antibody is produced with the onset of infection and peaks at 4 weeks, a fourfold rise in the IgG titer is useful in confirming the diagnosis. Measles IgG antibody after infection is sustained for life.
Reverse transcription-polymerase chain reaction testing can also detect measles virus in the blood and urine when direct evidence of the virus is necessary, such as in immunocompromised patients.29
TREATMENT IS SUPPORTIVE
Treatment of measles mainly involves supportive measures, such as fluids and antipyretics. Antiviral agents such as ribavirin and interferon have in vitro activity against the measles virus and have been used to treat severe measles infection in immunocompromised patients. However, their clinical efficacy is unproven.30
Routine use of antibacterial agents to prevent secondary bacterial infection is not recommended.
CURRENT RECOMMENDATIONS FOR ACTIVE IMMUNIZATION
Active immunization for measles has been available since 1963. Between 1963 and 1967, both killed-virus and live-virus vaccines were available. As atypical measles cases became recognized, the killed-virus vaccine was withdrawn.
The vaccine currently available in the United States is a live-attenuated strain prepared in chicken embryo cell culture and combined with mumps and rubella vaccine (MMR) or mumps, rubella, and varicella vaccine (MMRV).
Two doses of live-virus measles vaccine are recommended for all healthy children before they begin school, with the first dose given at 12 to 15 months of age. A second dose is needed because the failure rate with one dose is 5%. More than 99% of people who receive two doses separated by 4 weeks develop serologic evidence of measles.
Waning immunity after vaccination occurs very rarely, with approximately 5% of children developing secondary vaccine failure 10 to 15 years after vaccination.3,31
Although rates of vaccination in the United States are high, cases of measles continue to occur in unvaccinated infants and in children who are either too young to be vaccinated or whose parents claimed exemption because of religious or personal beliefs.
Because of the occurrence of measles cases in adolescents, young adults, and adults, potentially susceptible people should be identified and vaccinated according to current guidelines. People should be considered susceptible unless they have documentation of at least two doses of measles vaccine given at least 28 days apart, physician-diagnosed measles, laboratory evidence of immunity to measles, or were born before 1957. All adults who are susceptible should receive at least one dose of measles vaccine.10 Adults at higher risk of contracting measles include:
- Students in high school and college
- International travelers
- Health care personnel.
For these adults, two doses of measles vaccine, at least 28 days apart, are recommended.32
Postexposure prophylaxis
Measles vaccination given to susceptible contacts within 72 hours of exposure as postexposure prophylaxis may protect against infection and induces protection against subsequent exposures to measles.33,34 Vaccination is the intervention of choice for susceptible individuals older than 12 months of age who are exposed to measles and who do not have a contraindication to measles vaccination.35 Active rather than passive immunization is also the strategy of choice for controlling measles outbreaks.
Passive immunization with intramuscular immune globulin within 6 days of exposure can be used in selected circumstances to prevent transmission or to modify the clinical course of the infection.36 Immune globulin therapy is recommended for susceptible individuals who are exposed to measles and who are at high risk of developing severe or fatal measles. This includes individuals who are being treated with immunosuppressive agents, those with HIV infection, pregnant women, and infants less than 1 year of age. Immune globulin should not be used to control measles outbreaks.
ADVERSE EFFECTS OF MEASLES VACCINE
Live-virus measles vaccine has an excellent safety record. A transient fever, which may be accompanied by a measles-like rash, occurs in 5% to 15% of people 5 to 12 days after vaccination. The rash may be discrete or confluent and is self-limited.
Although measles vaccine is a live-attenuated vaccine, vaccinated people do not transmit the virus to susceptible contacts and are not considered contagious, even if they develop a vaccine-associated rash. Thus, the vaccine can be safely given to close contacts of immunocompromised and other susceptible people. Encephalitis is exceedingly rare following vaccination.
There is no scientific evidence that the risk of autism is higher in children who receive measles or MMR vaccine than in unvaccinated children.37 An Institute of Medicine report in 2001 rejected a causal relationship between MMR vaccine and autism spectrum disorders.38
CONTRAINDICATIONS TO MEASLES VACCINATION
Measles vaccine is contraindicated for:
- People who have cell-mediated immune deficiencies (except patients wtih HIV infection—see discussion just below)
- Pregnant women
- Those who had a severe allergic reaction to a vaccine component after a previous dose
- Those with moderate or severe acute illness
- Those who have recently received immune globulin products.
HIV-infected patients with severe immunosuppression should not receive the liveattenuated measles vaccine. However, because patients with HIV are at risk of severe measles, and because the vaccine has been shown to be safe in HIV patients who do not have severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression. 39
After receiving immune globulin
Anyone who has recently received immune globulin should not receive measles vaccine until sufficient time has passed, since passively acquired antibodies interfere with the immune response to live-virus vaccines. How long to wait depends on the type of immune globulin, the indication, the amount, and the route of administration. In general, the waiting period is:
- At least 3 months after intramuscular immune globulin or tetanus, hepatitis A, or hepatitis B prophylaxis
- At least 4 months after intramuscular immune globulin for rabies, or 6 months after intravenous immune globulin for cytomegalovirus (dose, 150 mg/kg)
- At least 8 months after intravenous immune globulin as replacement or therapy for immune deficiencies (dose, 400 mg/kg), or after intravenous immune globulin for immune thrombocytopenic purpura (400 mg/kg)
- At least 10 months after intravenous immune globulin for immune thrombocytopenic pupura at a dose of 1 g/kg.39
Egg allergy is not a contraindication
Although measles vaccine is produced in chick embryo cell culture, the vaccine has been shown to be safe in people with egg allergy, so they may be vaccinated without first being tested for egg allergy.39,40
- Lachmann PJ. Immunopathology of measles. Proc R Soc Med 1974; 67:1120–1122.
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in case of giant cell pneumonia without rash. N Engl J Med 1959; 261:875–881.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stokes J, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Farizo KM, Stehr-Green PA, Simpson DM, Markowitz LE. Pediatric emergency room visits: a risk factor for acquiring measles. Pediatrics 1991; 87:74–79.
- Bloch AB, Orenstein W, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics 1985; 75:676–683.
- Remington PL, Hall WN, Davis IH, et al. Airborne transmission of measles in a physician’s office. JAMA 1985; 253:1574–1577.
- US Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. MMWR 1994; 43:57–60.
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189 (suppl 1):S1–S3.
- US Centers for Disease Control and Prevention. Update: Measles—United States, January–July 2008. MMWR 2008; 57:893–896.
- Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–455.
- Mulholland EK. Measles in the United States, 2006. N Engl J Med 2006; 355:440–443.
- Barkin RM. Measles mortality. Analysis of the primary cause of death. Am J Dis Child 1975; 129:307–309.
- Barkin RM. Measles mortality: a retrospective look at the vaccine era. Am J Epidemiol 1975; 102:341–349.
- US Centers for Disease Control and Prevention. Public-sector vaccination efforts in response to the resurgence of measles among preschool-aged children—United States, 1989–1991. MMWR 1992; 41:522–525.
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. an analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189( suppl 1):S4–S16.
- Johnson RT, Griffin DE, Hirsch RL, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Gershon AA. Chickenpox, measles, and mumps. In:Remington J, Klein J, eds. Infectious Diseases of the Fetus and Newborn Infant. Elsevier Saunders: Philadelphia, 2006:693–738.
- Mitus A, Enders JF, Craig JM, Holloway A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med 1959; 261:882–889.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Mayo DR, Brennan T, Cormier DP, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol 1991; 29:2865–2867.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187( suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Forni Al, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994; 19:454–462.
- Anders JF, Jacobsen RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studes. Pediar Infect Dis J 1996; 15:62–66.
- US Centers for Disease Control and Prevention. Measles—United States, January 1–April 25, 2008. MMWR 2008; 57:494–498.
- Berkovitz S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Strebel PM, Papania MJ, Halsey NA. Measles vaccine. In:Plotkin SA, Orenstein WA, eds. Vaccines. Saunders: New York, 2004:389–440.
- US Centers for Disease Control and Prevention. Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998; 47( RR-8):1–57. http://www.cdc.gov/mmwr/PDF/rr/rr4708.pdf. Accessed December 30, 2009.
- Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–1482.
- Straton K, Gable A, Shetty P, McCormick M; for the Institute of Medicine. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. National Academy Press: Washington, DC, 2001.
- Pickerington LK, Baker CJ, Long SS, McMillan JA, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–455.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med 1995; 332:1262–1266.
- Lachmann PJ. Immunopathology of measles. Proc R Soc Med 1974; 67:1120–1122.
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in case of giant cell pneumonia without rash. N Engl J Med 1959; 261:875–881.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stokes J, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Farizo KM, Stehr-Green PA, Simpson DM, Markowitz LE. Pediatric emergency room visits: a risk factor for acquiring measles. Pediatrics 1991; 87:74–79.
- Bloch AB, Orenstein W, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics 1985; 75:676–683.
- Remington PL, Hall WN, Davis IH, et al. Airborne transmission of measles in a physician’s office. JAMA 1985; 253:1574–1577.
- US Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. MMWR 1994; 43:57–60.
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189 (suppl 1):S1–S3.
- US Centers for Disease Control and Prevention. Update: Measles—United States, January–July 2008. MMWR 2008; 57:893–896.
- Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–455.
- Mulholland EK. Measles in the United States, 2006. N Engl J Med 2006; 355:440–443.
- Barkin RM. Measles mortality. Analysis of the primary cause of death. Am J Dis Child 1975; 129:307–309.
- Barkin RM. Measles mortality: a retrospective look at the vaccine era. Am J Epidemiol 1975; 102:341–349.
- US Centers for Disease Control and Prevention. Public-sector vaccination efforts in response to the resurgence of measles among preschool-aged children—United States, 1989–1991. MMWR 1992; 41:522–525.
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. an analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189( suppl 1):S4–S16.
- Johnson RT, Griffin DE, Hirsch RL, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Gershon AA. Chickenpox, measles, and mumps. In:Remington J, Klein J, eds. Infectious Diseases of the Fetus and Newborn Infant. Elsevier Saunders: Philadelphia, 2006:693–738.
- Mitus A, Enders JF, Craig JM, Holloway A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med 1959; 261:882–889.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Mayo DR, Brennan T, Cormier DP, Hadler J, Lamb P. Evaluation of a commercial measles virus immunoglobulin M enzyme immunoassay. J Clin Microbiol 1991; 29:2865–2867.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187( suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Forni Al, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994; 19:454–462.
- Anders JF, Jacobsen RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studes. Pediar Infect Dis J 1996; 15:62–66.
- US Centers for Disease Control and Prevention. Measles—United States, January 1–April 25, 2008. MMWR 2008; 57:494–498.
- Berkovitz S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Strebel PM, Papania MJ, Halsey NA. Measles vaccine. In:Plotkin SA, Orenstein WA, eds. Vaccines. Saunders: New York, 2004:389–440.
- US Centers for Disease Control and Prevention. Measles, mumps and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998; 47( RR-8):1–57. http://www.cdc.gov/mmwr/PDF/rr/rr4708.pdf. Accessed December 30, 2009.
- Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–1482.
- Straton K, Gable A, Shetty P, McCormick M; for the Institute of Medicine. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. National Academy Press: Washington, DC, 2001.
- Pickerington LK, Baker CJ, Long SS, McMillan JA, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–455.
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med 1995; 332:1262–1266.
KEY POINTS
- Measles is one of the most contagious infectious diseases, with a secondary attack rate of at least 90% in susceptible household contacts.
- Since 1993, most reported cases of measles have been directly or indirectly linked to international travel, and many have occurred in adults.
- Acute measles encephalitis, a neurologic complication of measles, is more common in adults than in children and is characterized by the resurgence of fever during the convalescent phase, along with headaches, seizures, and altered consciousness.
News Roundup: New and Noteworthy Information
Untreated poor vision may be a contributing factor to late-life dementia and Alzheimer’s disease, a study in the February 11 online American Journal of Epidemiology found. Using data from the Health and Retirement Study and Medicare files from 1992 to 2005, researchers tracked the visual health of 625 elderly subjects with normal cognition for an average span of 8.5 years. Subjects with very good or excellent vision at baseline had a 63% reduced risk of dementia. Those with poorer vision who did not seek ophthalmologic treatment had a 9.5-fold increased risk of developing Alzheimer’s disease and a fivefold greater risk of cognitive impairment without dementia. Poorer vision without a previous eye procedure increased the risk of Alzheimer’s disease fivefold. In study subjects ages 90 years or older, 77.9% who maintained normal cognition had at least one previous eye procedure, compared with 51% of those who developed Alzheimer’s disease.
Behavioral signs of autism are not present from birth to age 6 months, but emerge with significantly declining trajectories over time, according to a study in the March issue of the Journal of the American Academy of Child and Adolescent Psychiatry. In a prospective longitudinal study, researchers compared 25 infants, who were later diagnosed with an autism spectrum disorder (ASD), with 25 gender-matched, low-risk control children, later determined to have typical development. Subjects were evaluated via videos taken at 6, 12, 18, 24, and 36 months. Researchers rated children based on frequency of gaze to faces, social smiles, and directed vocalizations. Both groups were similar at 6 months of age, but those with ASD declined significantly by 12 months of age. “Although repeated evaluation documented loss of skills in most infants with ASD, most parents did not report a regression in their child’s development,” the study authors wrote. “More children may present with a regressive course than previously thought, but parent report methods do not capture this phenomenon well.”
Treating children who have intractable epilepsy with a ketogenic diet appears to have no long-term adverse effects, researchers reported in the February 1 online Epilepsia. The investigators recruited questionnaires and laboratory reports from patients who were treated with the diet at Johns Hopkins Hospital between November 1993 and December 2008. Of the 101 responders (median age, 13), 96% would recommend the diet to others; however, just slightly more than half would have started the diet before trying anticonvulsants. The respondents’ mean total cholesterol was normal at 158 mg/dL, although most lipid levels were abnormal during the diet.
Elderly individuals who experience spontaneous alterations in cognition, attention, and arousal are 4.6 times more likely to have dementia, according to research published in the January 19 Neurology. In a study of 511 subjects (mean age, 78.1) at the Washington University Alzheimer Disease Research Center, investigators assessed patients for dementia using the Clinical Dementia Rating (CDR) and a neuropsychologic test battery. Participants also filled out the Mayo Fluctuations Questionnaire to assess cognitive changes and the Mayo Sleep Questionnaire to determine daytime alertness levels. Those presenting with disorganized, illogical thinking were 7.8 times more likely to have a CDR rating greater than 0. The incidence of a CDR rating of 0.5 was 13.4 times greater in patients with fluctuations than in those without, and a CDR 1 rating was associated with a 34-fold increase in patients with fluctuations.
An increase in brain magnesium enhances both short-term synaptic facilitation and longer-term potentiation and improves learning and memory functions, according to data published in the January 28 Neuron. In a study of young and old rats, researchers found that increasing brain magnesium using magnesium-L-threonate (MgT), a novel magnesium compound, enhanced learning ability, working memory, and short- and long-term memory in rats. “MgT treated rats had higher density of synaptophysin-/synaptobrevin-positive puncta in DG and CA1 subregions of the hypocampus that were correlated with memory improvement,” the authors wrote. “Functionally, magnesium increased the number of functional presynaptic release sites, while it reduced their release probability.” In addition, the researchers noted that when combined with upregulation of NR2B-containing NMDA receptors, MgT further enhanced synaptic plasticity.
A defect in the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) signaling in Cftr-deficient mice can be corrected with rosiglitazone, which helped reduce the severity of the cystic fibrosis phenotype, investigators reported in the February 14 online Nature Medicine. Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator. Treatment with the synthetic PPAR-gamma ligand rosiglitazone partially normalized the altered gene expression patterned, reducing disease severity. Although the drug had no effect on chloride secretion in the colon, it increased expression of the genes encoding carbonic anhydrases 4 and 2, increased bicarbonate secretion, and reduced mucus retention.
Untreated poor vision may be a contributing factor to late-life dementia and Alzheimer’s disease, a study in the February 11 online American Journal of Epidemiology found. Using data from the Health and Retirement Study and Medicare files from 1992 to 2005, researchers tracked the visual health of 625 elderly subjects with normal cognition for an average span of 8.5 years. Subjects with very good or excellent vision at baseline had a 63% reduced risk of dementia. Those with poorer vision who did not seek ophthalmologic treatment had a 9.5-fold increased risk of developing Alzheimer’s disease and a fivefold greater risk of cognitive impairment without dementia. Poorer vision without a previous eye procedure increased the risk of Alzheimer’s disease fivefold. In study subjects ages 90 years or older, 77.9% who maintained normal cognition had at least one previous eye procedure, compared with 51% of those who developed Alzheimer’s disease.
Behavioral signs of autism are not present from birth to age 6 months, but emerge with significantly declining trajectories over time, according to a study in the March issue of the Journal of the American Academy of Child and Adolescent Psychiatry. In a prospective longitudinal study, researchers compared 25 infants, who were later diagnosed with an autism spectrum disorder (ASD), with 25 gender-matched, low-risk control children, later determined to have typical development. Subjects were evaluated via videos taken at 6, 12, 18, 24, and 36 months. Researchers rated children based on frequency of gaze to faces, social smiles, and directed vocalizations. Both groups were similar at 6 months of age, but those with ASD declined significantly by 12 months of age. “Although repeated evaluation documented loss of skills in most infants with ASD, most parents did not report a regression in their child’s development,” the study authors wrote. “More children may present with a regressive course than previously thought, but parent report methods do not capture this phenomenon well.”
Treating children who have intractable epilepsy with a ketogenic diet appears to have no long-term adverse effects, researchers reported in the February 1 online Epilepsia. The investigators recruited questionnaires and laboratory reports from patients who were treated with the diet at Johns Hopkins Hospital between November 1993 and December 2008. Of the 101 responders (median age, 13), 96% would recommend the diet to others; however, just slightly more than half would have started the diet before trying anticonvulsants. The respondents’ mean total cholesterol was normal at 158 mg/dL, although most lipid levels were abnormal during the diet.
Elderly individuals who experience spontaneous alterations in cognition, attention, and arousal are 4.6 times more likely to have dementia, according to research published in the January 19 Neurology. In a study of 511 subjects (mean age, 78.1) at the Washington University Alzheimer Disease Research Center, investigators assessed patients for dementia using the Clinical Dementia Rating (CDR) and a neuropsychologic test battery. Participants also filled out the Mayo Fluctuations Questionnaire to assess cognitive changes and the Mayo Sleep Questionnaire to determine daytime alertness levels. Those presenting with disorganized, illogical thinking were 7.8 times more likely to have a CDR rating greater than 0. The incidence of a CDR rating of 0.5 was 13.4 times greater in patients with fluctuations than in those without, and a CDR 1 rating was associated with a 34-fold increase in patients with fluctuations.
An increase in brain magnesium enhances both short-term synaptic facilitation and longer-term potentiation and improves learning and memory functions, according to data published in the January 28 Neuron. In a study of young and old rats, researchers found that increasing brain magnesium using magnesium-L-threonate (MgT), a novel magnesium compound, enhanced learning ability, working memory, and short- and long-term memory in rats. “MgT treated rats had higher density of synaptophysin-/synaptobrevin-positive puncta in DG and CA1 subregions of the hypocampus that were correlated with memory improvement,” the authors wrote. “Functionally, magnesium increased the number of functional presynaptic release sites, while it reduced their release probability.” In addition, the researchers noted that when combined with upregulation of NR2B-containing NMDA receptors, MgT further enhanced synaptic plasticity.
A defect in the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) signaling in Cftr-deficient mice can be corrected with rosiglitazone, which helped reduce the severity of the cystic fibrosis phenotype, investigators reported in the February 14 online Nature Medicine. Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator. Treatment with the synthetic PPAR-gamma ligand rosiglitazone partially normalized the altered gene expression patterned, reducing disease severity. Although the drug had no effect on chloride secretion in the colon, it increased expression of the genes encoding carbonic anhydrases 4 and 2, increased bicarbonate secretion, and reduced mucus retention.
Untreated poor vision may be a contributing factor to late-life dementia and Alzheimer’s disease, a study in the February 11 online American Journal of Epidemiology found. Using data from the Health and Retirement Study and Medicare files from 1992 to 2005, researchers tracked the visual health of 625 elderly subjects with normal cognition for an average span of 8.5 years. Subjects with very good or excellent vision at baseline had a 63% reduced risk of dementia. Those with poorer vision who did not seek ophthalmologic treatment had a 9.5-fold increased risk of developing Alzheimer’s disease and a fivefold greater risk of cognitive impairment without dementia. Poorer vision without a previous eye procedure increased the risk of Alzheimer’s disease fivefold. In study subjects ages 90 years or older, 77.9% who maintained normal cognition had at least one previous eye procedure, compared with 51% of those who developed Alzheimer’s disease.
Behavioral signs of autism are not present from birth to age 6 months, but emerge with significantly declining trajectories over time, according to a study in the March issue of the Journal of the American Academy of Child and Adolescent Psychiatry. In a prospective longitudinal study, researchers compared 25 infants, who were later diagnosed with an autism spectrum disorder (ASD), with 25 gender-matched, low-risk control children, later determined to have typical development. Subjects were evaluated via videos taken at 6, 12, 18, 24, and 36 months. Researchers rated children based on frequency of gaze to faces, social smiles, and directed vocalizations. Both groups were similar at 6 months of age, but those with ASD declined significantly by 12 months of age. “Although repeated evaluation documented loss of skills in most infants with ASD, most parents did not report a regression in their child’s development,” the study authors wrote. “More children may present with a regressive course than previously thought, but parent report methods do not capture this phenomenon well.”
Treating children who have intractable epilepsy with a ketogenic diet appears to have no long-term adverse effects, researchers reported in the February 1 online Epilepsia. The investigators recruited questionnaires and laboratory reports from patients who were treated with the diet at Johns Hopkins Hospital between November 1993 and December 2008. Of the 101 responders (median age, 13), 96% would recommend the diet to others; however, just slightly more than half would have started the diet before trying anticonvulsants. The respondents’ mean total cholesterol was normal at 158 mg/dL, although most lipid levels were abnormal during the diet.
Elderly individuals who experience spontaneous alterations in cognition, attention, and arousal are 4.6 times more likely to have dementia, according to research published in the January 19 Neurology. In a study of 511 subjects (mean age, 78.1) at the Washington University Alzheimer Disease Research Center, investigators assessed patients for dementia using the Clinical Dementia Rating (CDR) and a neuropsychologic test battery. Participants also filled out the Mayo Fluctuations Questionnaire to assess cognitive changes and the Mayo Sleep Questionnaire to determine daytime alertness levels. Those presenting with disorganized, illogical thinking were 7.8 times more likely to have a CDR rating greater than 0. The incidence of a CDR rating of 0.5 was 13.4 times greater in patients with fluctuations than in those without, and a CDR 1 rating was associated with a 34-fold increase in patients with fluctuations.
An increase in brain magnesium enhances both short-term synaptic facilitation and longer-term potentiation and improves learning and memory functions, according to data published in the January 28 Neuron. In a study of young and old rats, researchers found that increasing brain magnesium using magnesium-L-threonate (MgT), a novel magnesium compound, enhanced learning ability, working memory, and short- and long-term memory in rats. “MgT treated rats had higher density of synaptophysin-/synaptobrevin-positive puncta in DG and CA1 subregions of the hypocampus that were correlated with memory improvement,” the authors wrote. “Functionally, magnesium increased the number of functional presynaptic release sites, while it reduced their release probability.” In addition, the researchers noted that when combined with upregulation of NR2B-containing NMDA receptors, MgT further enhanced synaptic plasticity.
A defect in the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) signaling in Cftr-deficient mice can be corrected with rosiglitazone, which helped reduce the severity of the cystic fibrosis phenotype, investigators reported in the February 14 online Nature Medicine. Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator. Treatment with the synthetic PPAR-gamma ligand rosiglitazone partially normalized the altered gene expression patterned, reducing disease severity. Although the drug had no effect on chloride secretion in the colon, it increased expression of the genes encoding carbonic anhydrases 4 and 2, increased bicarbonate secretion, and reduced mucus retention.