User login
Chondromyxoid Fibroma of the Radial Shaft Treated With Nonvascularized Fibular Autograft
A Review of the Anti-inflammatory Properties of Clindamycin in the Treatment of Acne Vulgaris
What's Eating You? Cat Flea (Ctenocephalides felis)
CPT changes for ObGyns are minor in 2010; the big news is Medicare’s toss of consult codes
Current Procedural Terminology (CPT) 2010, which took effect January 1, doesn’t bring many changes for ObGyn practice, but there’s been a major backpedaling in Medicare coverage of consultations that you must be aware of. In conjunction with this move by the Centers for Medicare & Medicaid Services (CMS), CPT has added a definition of “transfer of care” and established two possible reasons for providing a consultation. I’ll have more to report about these important developments later in this article.
Among the changes to billing codes for the work performed in ObGyn: rebundling of commonly performed urodynamics procedures and new codes for revision of a vaginal graft. There is also a new (and unpublished) code for administering the H1N1 influenza vaccine.
Last, CPT has revised the explanation of non–face-to-face prolonged services. Read on!
New codes bundle urodynamic studies—a product of joint CMS and CPT input
The biggest changes in coding for ObGyn procedures are urodynamics study codes. The American Medical Association (AMA) has 1) created three new codes that represent test bundles and, in the process, 2) deleted the stand-alone urodynamics codes 51772 (urethral pressure profile studies [UPP] [urethral closure pressure profile], any technique) and 51795 (voiding pressure studies; bladder voiding pressure, any technique).
These changes were made because the most commonly reported codes for a female patient were billed together 90% of the time (51726, 51772, 51795, and 51797); the AMA reasoned that the most frequent combinations were considered overvalued when billed separately—that is, there was no repeat of pre-test and post-test work when these combinations were performed and there was no duplication in the cost of supplies and staff time.
The new bundles were therefore considered to better reflect current medical practice, and the Relative Value Update Committee (RUC) recommended, and CMS accepted, the relative value units (RVU) for the combination codes to reflect the true physician work value and practice expense of the combined procedures.
New and revised codes are:
51726 Complex cystometrogram (i.e., calibrated electronic equipment)
51727 …with urethral pressure profile studies (i.e., urethral closure pressure profile), any technique
51728 …with voiding pressure studies (i.e., bladder voiding pressure), any technique
51729 …with voiding pressure studies (i.e., bladder voiding pressure) and urethral pressure profile studies (i.e., urethral closure pressure profile), any technique.
According to the clinical vignette submitted to the AMA for code 51727, this procedure will include a sustained Valsalva maneuver as part of the urethral closure pressure profile. CPT did, however, retain the add-on code +51797 (voiding pressure studies, intra-abdominal [i.e., rectal, gastric, intraperitoneal]) and has clarified that 51797 may be billed in addition to 51728 and 51729 if a rectal catheter is placed to determine if the patient is straining during the voiding event.
In other words, the add-on code may be reported only when the primary procedure includes a voiding pressure study.
RVU for these new procedures have also been revised (see the TABLE ). Notable is the seeming discrepancy in RVU between code 51726 (cystometrogram alone) and the bundled tests. This is the case because the practice expense for 51726 has not reached its final level (the practice expense RVU are being increased or decreased in increments over several years); for 2010 only, therefore, this code will have a higher total RVU value than the new codes (51727, 51728, 51729), despite having a lower physician work relative value.
The discrepancy will be corrected in 2011, when 51726 will have lower RVU than the other urodynamics combination test codes.
TABLE
Changes in 2010 to RVU for urodynamic studies
| 2009 | 2010 | |||
|---|---|---|---|---|
| CPT code | Work RVU | Total RVU | Work RVU | Total RVU |
| 51726 | 1.71 | 9.02 | 1.71 | 8.71 |
| 51727 | Not applicable (NA) | NA | 2.11 | 8.07 |
| 51728 | NA | NA | 2.11 | 8.06 |
| 51729 | NA | NA | 2.11 | 8.14 |
Laparoscopic revision of a vaginal graft
In 2006, the AMA added the code for a vaginal approach to revising a graft (57295, revision [including removal] of prosthetic vaginal graft; vaginal approach). Then, in 2007, it added a code for an abdominal approach (57296, revision [including removal] of prosthetic vaginal graft; open abdominal approach).
Now, you have a code for a laparoscopic approach, completing the code set for this procedure. As with 57295 and 57296, report the new code when the graft is either revised or removed entirely.
57426 Revision (including removal) of prosthetic vaginal graft, laparoscopic approach
Other, miscellaneous changes take effect
OBSTETRIC PANEL
Although code 80055 comprises a battery of tests that are performed routinely on obstetric patients, a new code, 86780, was created to report syphilis screening using a treponemal antibody method, in which IgM and IgG antibodies are measured. This test is not the same syphilis test that is now part of the 80055 panel. CPT has therefore cautioned that, when you use code 86780 instead of the standard syphilis test code 86592, you should not report the obstetrics panel but, instead, separately report each test performed.
REPRODUCTIVE MEDICINE
New code 89398 (unlisted reproductive medicine laboratory procedure) has been added, but CPT still directs billers to use the unlisted miscellaneous pathology test code 89240 to report cryopreservation of reproductive ovarian tissues.
BILLING FOR THE H1N1 INFLUENZA VACCINE
Because of the urgency of collecting data on the H1N1 influenza epidemic, CPT has revised code 90663 to include the H1N1 formulation of the flu vaccine product. In addition, CPT has created a new code, 90470, for administering the H1N1 flu vaccine, which became valid in September (but which isn’t included in the hard-copy version of CPT 2010). The new code is to be used for intramuscular injection or intranasal administration, and includes any time spent counseling.
In addition:
- Do not report established code 90471 (immunization administration [includes percutaneous, intradermal, subcutaneous, or intramuscular injections]; one vaccine [single or combination vaccine/toxoid]) when you administer the H1N1 flu vaccine
- Report the vaccine product code only when your practice has purchased the vaccine, or when the payer requires the code with a 0 charge to match the administration code.
- Medicare coding for administering the H1N1 flu vaccine is different than what I’ve just described. Do not use CPT codes for Medicare patients; instead, code H1N1 flu immunization as:
G9141 Influenza A (H1N1) immunization administration (includes the physician counseling the patient/family)
G9142 Influenza A (H1N1) vaccine, any route of administration
Medicare will not reimburse for the vaccine product because it is being given to its providers without cost. Some carriers may require that the new vaccine product code be listed with a 0 charge.
Prolonged inpatient E/M services
CPT has revised guidelines for prolonged services that do not involve direct face-to-face contact with a patient. Keep in mind, however, that, although these changes are welcome, many payers don’t reimburse separately for work that isn’t performed face to face.
These codes are no longer considered add-on codes; they can be reported on a different date than the related E/M service.
According to CPT, codes 99358 and 99359 are reported when the prolonged time:
- is greater than would be expected for normal pre-service and post-service work associated with the E/M service
- exceeds 30 minutes
- is related to an E/M service that has already occurred, or to one that will occur and represents ongoing patient management (for example, your review of extensive patient records that weren’t available at the time of the visit)
- is in addition to any telephone services codes (99441–99443)—but not with more specific codes, such as medical team conferences, online medical evaluation, or care plan oversight services, which have no upper limit to the time required to accomplish the service.
Consultation codes and clarifications
Two changes of note, from a CPT perspective, have been made in the area of consultations. CPT has:
- added a definition for a transfer of care
- defined two circumstances under which a consultation can be coded. These revisions come at the same time Medicare has made the decision to no longer pay for consultations other than tele-health consults (see following section).
For 2010, CPT defines transfer of care as
…the process whereby a physician who is providing management for some or all of a patient’s problems relinquishes this responsibility to another physician who agrees to accept this responsibility and who, from the initial encounter, is not providing consultative services.
The guidelines also explain that 1) a transferring physician is no longer responsible for caring for the problem for which the patient was referred and 2) the consultation codes should not be reported by the physician who accepts care.
Two alternative conditions must now apply for a consultation to be considered provided:
- A physician requested an opinion or advice for a specific condition or problem, or
- The consulting physician saw the patient first to determine whether to accept ongoing management of her entire care or of a specific condition or problem (i.e., transfer of care).
The second condition is new; it remains to be seen if payers will accept it as a valid reason to bill for consultation.
As with all billable services, you should ensure that the criteria required by the payer you are billing have been met. CPT also directs that the written request for consultation can be documented by either the requesting or the receiving physician—something that was unacceptable under Medicare guidelines.
Last, CPT has added instructions to clarify the type of consultation code to bill under certain circumstances:
- When the patient is admitted after an outpatient consultation but the physician does not see the patient on the unit on the date of admission, bill only for outpatient consultation
- When the patient is seen for an office visit, emergency room visit, or outpatient consult on the date of admission and the physician then sees the patient on the unit that day, bill only the inpatient consultation or initial hospital care code, whichever applies. All services that day are used to determine the final level of service.
Medicare tilts the playing field on consultations
Although CPT has retained all consultation codes, and although the hope is that commercial payers will continue to reimburse for such services in the near future, the big news is that Medicare has announced that it will no longer recognize (or reimburse for) codes for outpatient or inpatient consultations. (Note: This story is still unfolding, however. The changes announced by Medicare that I discuss below are still before Congress as this article goes to press. Although Medicare has, in fact, released the transmittal letter to all carriers instructing them about the changes, Senator Arlen Specter [D-Pa] has introduced an amendment to the Patient Protection and Affordable Care Act [H.R. 3590] to postpone the policy change for 1 year. If Congress has not passed this bill before the end of 2009, the changes go through as planned. Stay tuned for developments!)
Assuming the changes go through, here is what is expected of you in the circumstances of providing consultations and billing Medicare (Medicaid payers aren’t required to follow this policy change but may opt to do so).
Outpatients. Document, and report, the appropriate level of visit for a new or established Medicare patient using outpatient codes 99201–99215
Inpatients. If you are a non-admitting physician asked to see a patient for the first time, report the appropriate level of initial hospital care (codes 99221–99223). Note the following three points:
- Initial hospital care includes only three levels of service—not the five levels from which you choose for consultation codes
- The lowest level of history and exam for these initial visit codes is a detailed history and examination—no matter the level of medical decision-making. If the level of history or exam is documented lower than “detailed”—say, as “expanded problem-focused”—you are required to report the unlisted E/M code 99499.
- The admitting physician adds the new Healthcare Common Procedure Coding System (HCPCS) modifier –AI (that is, “‘A’ upper-case ‘i’”) to the initial visit code, so that Medicare can distinguish the admitting physician from others providing care for the patient.
- All subsequent visits with the inpatient continue to be billed with the subsequent care inpatient codes (99231–99233).
Fallout from this change? Medicare is studying the implications of its new policy on secondary payments—that is, when Medicare is the primary payer and there is a supplemental carrier, or when Medicare is the secondary payer. Note: Medicare strongly advises all providers to check with their primary payers, because 1) Medicare will not accept a consultation code when a primary insurer has paid on that code and 2) it’s doubtful that a commercial payer will accept a consultation code when Medicare has paid for a new or established patient service.
To add to the turmoil…
The CMS has announced that, as a result of the changes in Medicare policy on consultations, it is increasing the relative values for all new and established patient services and initial hospital care. CMS is doing this, however, by reducing the relative values of some consultation codes.
In addition, all surgical procedure codes that carry a 10- or 90-day global period will see an increase in work RVU because of the increase in E/M services that are a part of all global care. Keep in mind that payers who use the Resource-Based Relative Value Scale (RBRVS) to reimburse services will probably adopt the new values when contracts are up for renewal, although many will be unable to do so in the short term.
It also remains to be seen if any commercial payers adopt Medicare policy or continue to pay for consultations. This area might be a contract issue with payers.
Current Procedural Terminology (CPT) 2010, which took effect January 1, doesn’t bring many changes for ObGyn practice, but there’s been a major backpedaling in Medicare coverage of consultations that you must be aware of. In conjunction with this move by the Centers for Medicare & Medicaid Services (CMS), CPT has added a definition of “transfer of care” and established two possible reasons for providing a consultation. I’ll have more to report about these important developments later in this article.
Among the changes to billing codes for the work performed in ObGyn: rebundling of commonly performed urodynamics procedures and new codes for revision of a vaginal graft. There is also a new (and unpublished) code for administering the H1N1 influenza vaccine.
Last, CPT has revised the explanation of non–face-to-face prolonged services. Read on!
New codes bundle urodynamic studies—a product of joint CMS and CPT input
The biggest changes in coding for ObGyn procedures are urodynamics study codes. The American Medical Association (AMA) has 1) created three new codes that represent test bundles and, in the process, 2) deleted the stand-alone urodynamics codes 51772 (urethral pressure profile studies [UPP] [urethral closure pressure profile], any technique) and 51795 (voiding pressure studies; bladder voiding pressure, any technique).
These changes were made because the most commonly reported codes for a female patient were billed together 90% of the time (51726, 51772, 51795, and 51797); the AMA reasoned that the most frequent combinations were considered overvalued when billed separately—that is, there was no repeat of pre-test and post-test work when these combinations were performed and there was no duplication in the cost of supplies and staff time.
The new bundles were therefore considered to better reflect current medical practice, and the Relative Value Update Committee (RUC) recommended, and CMS accepted, the relative value units (RVU) for the combination codes to reflect the true physician work value and practice expense of the combined procedures.
New and revised codes are:
51726 Complex cystometrogram (i.e., calibrated electronic equipment)
51727 …with urethral pressure profile studies (i.e., urethral closure pressure profile), any technique
51728 …with voiding pressure studies (i.e., bladder voiding pressure), any technique
51729 …with voiding pressure studies (i.e., bladder voiding pressure) and urethral pressure profile studies (i.e., urethral closure pressure profile), any technique.
According to the clinical vignette submitted to the AMA for code 51727, this procedure will include a sustained Valsalva maneuver as part of the urethral closure pressure profile. CPT did, however, retain the add-on code +51797 (voiding pressure studies, intra-abdominal [i.e., rectal, gastric, intraperitoneal]) and has clarified that 51797 may be billed in addition to 51728 and 51729 if a rectal catheter is placed to determine if the patient is straining during the voiding event.
In other words, the add-on code may be reported only when the primary procedure includes a voiding pressure study.
RVU for these new procedures have also been revised (see the TABLE ). Notable is the seeming discrepancy in RVU between code 51726 (cystometrogram alone) and the bundled tests. This is the case because the practice expense for 51726 has not reached its final level (the practice expense RVU are being increased or decreased in increments over several years); for 2010 only, therefore, this code will have a higher total RVU value than the new codes (51727, 51728, 51729), despite having a lower physician work relative value.
The discrepancy will be corrected in 2011, when 51726 will have lower RVU than the other urodynamics combination test codes.
TABLE
Changes in 2010 to RVU for urodynamic studies
| 2009 | 2010 | |||
|---|---|---|---|---|
| CPT code | Work RVU | Total RVU | Work RVU | Total RVU |
| 51726 | 1.71 | 9.02 | 1.71 | 8.71 |
| 51727 | Not applicable (NA) | NA | 2.11 | 8.07 |
| 51728 | NA | NA | 2.11 | 8.06 |
| 51729 | NA | NA | 2.11 | 8.14 |
Laparoscopic revision of a vaginal graft
In 2006, the AMA added the code for a vaginal approach to revising a graft (57295, revision [including removal] of prosthetic vaginal graft; vaginal approach). Then, in 2007, it added a code for an abdominal approach (57296, revision [including removal] of prosthetic vaginal graft; open abdominal approach).
Now, you have a code for a laparoscopic approach, completing the code set for this procedure. As with 57295 and 57296, report the new code when the graft is either revised or removed entirely.
57426 Revision (including removal) of prosthetic vaginal graft, laparoscopic approach
Other, miscellaneous changes take effect
OBSTETRIC PANEL
Although code 80055 comprises a battery of tests that are performed routinely on obstetric patients, a new code, 86780, was created to report syphilis screening using a treponemal antibody method, in which IgM and IgG antibodies are measured. This test is not the same syphilis test that is now part of the 80055 panel. CPT has therefore cautioned that, when you use code 86780 instead of the standard syphilis test code 86592, you should not report the obstetrics panel but, instead, separately report each test performed.
REPRODUCTIVE MEDICINE
New code 89398 (unlisted reproductive medicine laboratory procedure) has been added, but CPT still directs billers to use the unlisted miscellaneous pathology test code 89240 to report cryopreservation of reproductive ovarian tissues.
BILLING FOR THE H1N1 INFLUENZA VACCINE
Because of the urgency of collecting data on the H1N1 influenza epidemic, CPT has revised code 90663 to include the H1N1 formulation of the flu vaccine product. In addition, CPT has created a new code, 90470, for administering the H1N1 flu vaccine, which became valid in September (but which isn’t included in the hard-copy version of CPT 2010). The new code is to be used for intramuscular injection or intranasal administration, and includes any time spent counseling.
In addition:
- Do not report established code 90471 (immunization administration [includes percutaneous, intradermal, subcutaneous, or intramuscular injections]; one vaccine [single or combination vaccine/toxoid]) when you administer the H1N1 flu vaccine
- Report the vaccine product code only when your practice has purchased the vaccine, or when the payer requires the code with a 0 charge to match the administration code.
- Medicare coding for administering the H1N1 flu vaccine is different than what I’ve just described. Do not use CPT codes for Medicare patients; instead, code H1N1 flu immunization as:
G9141 Influenza A (H1N1) immunization administration (includes the physician counseling the patient/family)
G9142 Influenza A (H1N1) vaccine, any route of administration
Medicare will not reimburse for the vaccine product because it is being given to its providers without cost. Some carriers may require that the new vaccine product code be listed with a 0 charge.
Prolonged inpatient E/M services
CPT has revised guidelines for prolonged services that do not involve direct face-to-face contact with a patient. Keep in mind, however, that, although these changes are welcome, many payers don’t reimburse separately for work that isn’t performed face to face.
These codes are no longer considered add-on codes; they can be reported on a different date than the related E/M service.
According to CPT, codes 99358 and 99359 are reported when the prolonged time:
- is greater than would be expected for normal pre-service and post-service work associated with the E/M service
- exceeds 30 minutes
- is related to an E/M service that has already occurred, or to one that will occur and represents ongoing patient management (for example, your review of extensive patient records that weren’t available at the time of the visit)
- is in addition to any telephone services codes (99441–99443)—but not with more specific codes, such as medical team conferences, online medical evaluation, or care plan oversight services, which have no upper limit to the time required to accomplish the service.
Consultation codes and clarifications
Two changes of note, from a CPT perspective, have been made in the area of consultations. CPT has:
- added a definition for a transfer of care
- defined two circumstances under which a consultation can be coded. These revisions come at the same time Medicare has made the decision to no longer pay for consultations other than tele-health consults (see following section).
For 2010, CPT defines transfer of care as
…the process whereby a physician who is providing management for some or all of a patient’s problems relinquishes this responsibility to another physician who agrees to accept this responsibility and who, from the initial encounter, is not providing consultative services.
The guidelines also explain that 1) a transferring physician is no longer responsible for caring for the problem for which the patient was referred and 2) the consultation codes should not be reported by the physician who accepts care.
Two alternative conditions must now apply for a consultation to be considered provided:
- A physician requested an opinion or advice for a specific condition or problem, or
- The consulting physician saw the patient first to determine whether to accept ongoing management of her entire care or of a specific condition or problem (i.e., transfer of care).
The second condition is new; it remains to be seen if payers will accept it as a valid reason to bill for consultation.
As with all billable services, you should ensure that the criteria required by the payer you are billing have been met. CPT also directs that the written request for consultation can be documented by either the requesting or the receiving physician—something that was unacceptable under Medicare guidelines.
Last, CPT has added instructions to clarify the type of consultation code to bill under certain circumstances:
- When the patient is admitted after an outpatient consultation but the physician does not see the patient on the unit on the date of admission, bill only for outpatient consultation
- When the patient is seen for an office visit, emergency room visit, or outpatient consult on the date of admission and the physician then sees the patient on the unit that day, bill only the inpatient consultation or initial hospital care code, whichever applies. All services that day are used to determine the final level of service.
Medicare tilts the playing field on consultations
Although CPT has retained all consultation codes, and although the hope is that commercial payers will continue to reimburse for such services in the near future, the big news is that Medicare has announced that it will no longer recognize (or reimburse for) codes for outpatient or inpatient consultations. (Note: This story is still unfolding, however. The changes announced by Medicare that I discuss below are still before Congress as this article goes to press. Although Medicare has, in fact, released the transmittal letter to all carriers instructing them about the changes, Senator Arlen Specter [D-Pa] has introduced an amendment to the Patient Protection and Affordable Care Act [H.R. 3590] to postpone the policy change for 1 year. If Congress has not passed this bill before the end of 2009, the changes go through as planned. Stay tuned for developments!)
Assuming the changes go through, here is what is expected of you in the circumstances of providing consultations and billing Medicare (Medicaid payers aren’t required to follow this policy change but may opt to do so).
Outpatients. Document, and report, the appropriate level of visit for a new or established Medicare patient using outpatient codes 99201–99215
Inpatients. If you are a non-admitting physician asked to see a patient for the first time, report the appropriate level of initial hospital care (codes 99221–99223). Note the following three points:
- Initial hospital care includes only three levels of service—not the five levels from which you choose for consultation codes
- The lowest level of history and exam for these initial visit codes is a detailed history and examination—no matter the level of medical decision-making. If the level of history or exam is documented lower than “detailed”—say, as “expanded problem-focused”—you are required to report the unlisted E/M code 99499.
- The admitting physician adds the new Healthcare Common Procedure Coding System (HCPCS) modifier –AI (that is, “‘A’ upper-case ‘i’”) to the initial visit code, so that Medicare can distinguish the admitting physician from others providing care for the patient.
- All subsequent visits with the inpatient continue to be billed with the subsequent care inpatient codes (99231–99233).
Fallout from this change? Medicare is studying the implications of its new policy on secondary payments—that is, when Medicare is the primary payer and there is a supplemental carrier, or when Medicare is the secondary payer. Note: Medicare strongly advises all providers to check with their primary payers, because 1) Medicare will not accept a consultation code when a primary insurer has paid on that code and 2) it’s doubtful that a commercial payer will accept a consultation code when Medicare has paid for a new or established patient service.
To add to the turmoil…
The CMS has announced that, as a result of the changes in Medicare policy on consultations, it is increasing the relative values for all new and established patient services and initial hospital care. CMS is doing this, however, by reducing the relative values of some consultation codes.
In addition, all surgical procedure codes that carry a 10- or 90-day global period will see an increase in work RVU because of the increase in E/M services that are a part of all global care. Keep in mind that payers who use the Resource-Based Relative Value Scale (RBRVS) to reimburse services will probably adopt the new values when contracts are up for renewal, although many will be unable to do so in the short term.
It also remains to be seen if any commercial payers adopt Medicare policy or continue to pay for consultations. This area might be a contract issue with payers.
Current Procedural Terminology (CPT) 2010, which took effect January 1, doesn’t bring many changes for ObGyn practice, but there’s been a major backpedaling in Medicare coverage of consultations that you must be aware of. In conjunction with this move by the Centers for Medicare & Medicaid Services (CMS), CPT has added a definition of “transfer of care” and established two possible reasons for providing a consultation. I’ll have more to report about these important developments later in this article.
Among the changes to billing codes for the work performed in ObGyn: rebundling of commonly performed urodynamics procedures and new codes for revision of a vaginal graft. There is also a new (and unpublished) code for administering the H1N1 influenza vaccine.
Last, CPT has revised the explanation of non–face-to-face prolonged services. Read on!
New codes bundle urodynamic studies—a product of joint CMS and CPT input
The biggest changes in coding for ObGyn procedures are urodynamics study codes. The American Medical Association (AMA) has 1) created three new codes that represent test bundles and, in the process, 2) deleted the stand-alone urodynamics codes 51772 (urethral pressure profile studies [UPP] [urethral closure pressure profile], any technique) and 51795 (voiding pressure studies; bladder voiding pressure, any technique).
These changes were made because the most commonly reported codes for a female patient were billed together 90% of the time (51726, 51772, 51795, and 51797); the AMA reasoned that the most frequent combinations were considered overvalued when billed separately—that is, there was no repeat of pre-test and post-test work when these combinations were performed and there was no duplication in the cost of supplies and staff time.
The new bundles were therefore considered to better reflect current medical practice, and the Relative Value Update Committee (RUC) recommended, and CMS accepted, the relative value units (RVU) for the combination codes to reflect the true physician work value and practice expense of the combined procedures.
New and revised codes are:
51726 Complex cystometrogram (i.e., calibrated electronic equipment)
51727 …with urethral pressure profile studies (i.e., urethral closure pressure profile), any technique
51728 …with voiding pressure studies (i.e., bladder voiding pressure), any technique
51729 …with voiding pressure studies (i.e., bladder voiding pressure) and urethral pressure profile studies (i.e., urethral closure pressure profile), any technique.
According to the clinical vignette submitted to the AMA for code 51727, this procedure will include a sustained Valsalva maneuver as part of the urethral closure pressure profile. CPT did, however, retain the add-on code +51797 (voiding pressure studies, intra-abdominal [i.e., rectal, gastric, intraperitoneal]) and has clarified that 51797 may be billed in addition to 51728 and 51729 if a rectal catheter is placed to determine if the patient is straining during the voiding event.
In other words, the add-on code may be reported only when the primary procedure includes a voiding pressure study.
RVU for these new procedures have also been revised (see the TABLE ). Notable is the seeming discrepancy in RVU between code 51726 (cystometrogram alone) and the bundled tests. This is the case because the practice expense for 51726 has not reached its final level (the practice expense RVU are being increased or decreased in increments over several years); for 2010 only, therefore, this code will have a higher total RVU value than the new codes (51727, 51728, 51729), despite having a lower physician work relative value.
The discrepancy will be corrected in 2011, when 51726 will have lower RVU than the other urodynamics combination test codes.
TABLE
Changes in 2010 to RVU for urodynamic studies
| 2009 | 2010 | |||
|---|---|---|---|---|
| CPT code | Work RVU | Total RVU | Work RVU | Total RVU |
| 51726 | 1.71 | 9.02 | 1.71 | 8.71 |
| 51727 | Not applicable (NA) | NA | 2.11 | 8.07 |
| 51728 | NA | NA | 2.11 | 8.06 |
| 51729 | NA | NA | 2.11 | 8.14 |
Laparoscopic revision of a vaginal graft
In 2006, the AMA added the code for a vaginal approach to revising a graft (57295, revision [including removal] of prosthetic vaginal graft; vaginal approach). Then, in 2007, it added a code for an abdominal approach (57296, revision [including removal] of prosthetic vaginal graft; open abdominal approach).
Now, you have a code for a laparoscopic approach, completing the code set for this procedure. As with 57295 and 57296, report the new code when the graft is either revised or removed entirely.
57426 Revision (including removal) of prosthetic vaginal graft, laparoscopic approach
Other, miscellaneous changes take effect
OBSTETRIC PANEL
Although code 80055 comprises a battery of tests that are performed routinely on obstetric patients, a new code, 86780, was created to report syphilis screening using a treponemal antibody method, in which IgM and IgG antibodies are measured. This test is not the same syphilis test that is now part of the 80055 panel. CPT has therefore cautioned that, when you use code 86780 instead of the standard syphilis test code 86592, you should not report the obstetrics panel but, instead, separately report each test performed.
REPRODUCTIVE MEDICINE
New code 89398 (unlisted reproductive medicine laboratory procedure) has been added, but CPT still directs billers to use the unlisted miscellaneous pathology test code 89240 to report cryopreservation of reproductive ovarian tissues.
BILLING FOR THE H1N1 INFLUENZA VACCINE
Because of the urgency of collecting data on the H1N1 influenza epidemic, CPT has revised code 90663 to include the H1N1 formulation of the flu vaccine product. In addition, CPT has created a new code, 90470, for administering the H1N1 flu vaccine, which became valid in September (but which isn’t included in the hard-copy version of CPT 2010). The new code is to be used for intramuscular injection or intranasal administration, and includes any time spent counseling.
In addition:
- Do not report established code 90471 (immunization administration [includes percutaneous, intradermal, subcutaneous, or intramuscular injections]; one vaccine [single or combination vaccine/toxoid]) when you administer the H1N1 flu vaccine
- Report the vaccine product code only when your practice has purchased the vaccine, or when the payer requires the code with a 0 charge to match the administration code.
- Medicare coding for administering the H1N1 flu vaccine is different than what I’ve just described. Do not use CPT codes for Medicare patients; instead, code H1N1 flu immunization as:
G9141 Influenza A (H1N1) immunization administration (includes the physician counseling the patient/family)
G9142 Influenza A (H1N1) vaccine, any route of administration
Medicare will not reimburse for the vaccine product because it is being given to its providers without cost. Some carriers may require that the new vaccine product code be listed with a 0 charge.
Prolonged inpatient E/M services
CPT has revised guidelines for prolonged services that do not involve direct face-to-face contact with a patient. Keep in mind, however, that, although these changes are welcome, many payers don’t reimburse separately for work that isn’t performed face to face.
These codes are no longer considered add-on codes; they can be reported on a different date than the related E/M service.
According to CPT, codes 99358 and 99359 are reported when the prolonged time:
- is greater than would be expected for normal pre-service and post-service work associated with the E/M service
- exceeds 30 minutes
- is related to an E/M service that has already occurred, or to one that will occur and represents ongoing patient management (for example, your review of extensive patient records that weren’t available at the time of the visit)
- is in addition to any telephone services codes (99441–99443)—but not with more specific codes, such as medical team conferences, online medical evaluation, or care plan oversight services, which have no upper limit to the time required to accomplish the service.
Consultation codes and clarifications
Two changes of note, from a CPT perspective, have been made in the area of consultations. CPT has:
- added a definition for a transfer of care
- defined two circumstances under which a consultation can be coded. These revisions come at the same time Medicare has made the decision to no longer pay for consultations other than tele-health consults (see following section).
For 2010, CPT defines transfer of care as
…the process whereby a physician who is providing management for some or all of a patient’s problems relinquishes this responsibility to another physician who agrees to accept this responsibility and who, from the initial encounter, is not providing consultative services.
The guidelines also explain that 1) a transferring physician is no longer responsible for caring for the problem for which the patient was referred and 2) the consultation codes should not be reported by the physician who accepts care.
Two alternative conditions must now apply for a consultation to be considered provided:
- A physician requested an opinion or advice for a specific condition or problem, or
- The consulting physician saw the patient first to determine whether to accept ongoing management of her entire care or of a specific condition or problem (i.e., transfer of care).
The second condition is new; it remains to be seen if payers will accept it as a valid reason to bill for consultation.
As with all billable services, you should ensure that the criteria required by the payer you are billing have been met. CPT also directs that the written request for consultation can be documented by either the requesting or the receiving physician—something that was unacceptable under Medicare guidelines.
Last, CPT has added instructions to clarify the type of consultation code to bill under certain circumstances:
- When the patient is admitted after an outpatient consultation but the physician does not see the patient on the unit on the date of admission, bill only for outpatient consultation
- When the patient is seen for an office visit, emergency room visit, or outpatient consult on the date of admission and the physician then sees the patient on the unit that day, bill only the inpatient consultation or initial hospital care code, whichever applies. All services that day are used to determine the final level of service.
Medicare tilts the playing field on consultations
Although CPT has retained all consultation codes, and although the hope is that commercial payers will continue to reimburse for such services in the near future, the big news is that Medicare has announced that it will no longer recognize (or reimburse for) codes for outpatient or inpatient consultations. (Note: This story is still unfolding, however. The changes announced by Medicare that I discuss below are still before Congress as this article goes to press. Although Medicare has, in fact, released the transmittal letter to all carriers instructing them about the changes, Senator Arlen Specter [D-Pa] has introduced an amendment to the Patient Protection and Affordable Care Act [H.R. 3590] to postpone the policy change for 1 year. If Congress has not passed this bill before the end of 2009, the changes go through as planned. Stay tuned for developments!)
Assuming the changes go through, here is what is expected of you in the circumstances of providing consultations and billing Medicare (Medicaid payers aren’t required to follow this policy change but may opt to do so).
Outpatients. Document, and report, the appropriate level of visit for a new or established Medicare patient using outpatient codes 99201–99215
Inpatients. If you are a non-admitting physician asked to see a patient for the first time, report the appropriate level of initial hospital care (codes 99221–99223). Note the following three points:
- Initial hospital care includes only three levels of service—not the five levels from which you choose for consultation codes
- The lowest level of history and exam for these initial visit codes is a detailed history and examination—no matter the level of medical decision-making. If the level of history or exam is documented lower than “detailed”—say, as “expanded problem-focused”—you are required to report the unlisted E/M code 99499.
- The admitting physician adds the new Healthcare Common Procedure Coding System (HCPCS) modifier –AI (that is, “‘A’ upper-case ‘i’”) to the initial visit code, so that Medicare can distinguish the admitting physician from others providing care for the patient.
- All subsequent visits with the inpatient continue to be billed with the subsequent care inpatient codes (99231–99233).
Fallout from this change? Medicare is studying the implications of its new policy on secondary payments—that is, when Medicare is the primary payer and there is a supplemental carrier, or when Medicare is the secondary payer. Note: Medicare strongly advises all providers to check with their primary payers, because 1) Medicare will not accept a consultation code when a primary insurer has paid on that code and 2) it’s doubtful that a commercial payer will accept a consultation code when Medicare has paid for a new or established patient service.
To add to the turmoil…
The CMS has announced that, as a result of the changes in Medicare policy on consultations, it is increasing the relative values for all new and established patient services and initial hospital care. CMS is doing this, however, by reducing the relative values of some consultation codes.
In addition, all surgical procedure codes that carry a 10- or 90-day global period will see an increase in work RVU because of the increase in E/M services that are a part of all global care. Keep in mind that payers who use the Resource-Based Relative Value Scale (RBRVS) to reimburse services will probably adopt the new values when contracts are up for renewal, although many will be unable to do so in the short term.
It also remains to be seen if any commercial payers adopt Medicare policy or continue to pay for consultations. This area might be a contract issue with payers.
Guanfacine extended release for ADHD
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants
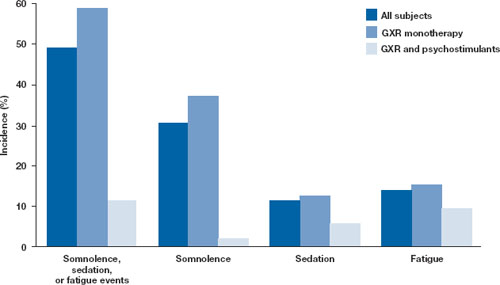
In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
Nursemaid’s elbow: Its diagnostic clues and preferred means of reduction
• Nursemaid’s elbow typically occurs with a sudden pull on a child’s arm. Reserve radiography for uncertain cases in which you need to exclude more severe injuries. B
• Consider reducing nursemaid’s elbow by rapid pronation of the forearm, which has been shown to be less painful and more effective than supination. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Nursemaid’s elbow—sudden subluxation of the radial head—usually results from forcible traction to a child’s pronated hand or wrist, with the elbow extended.1 Ironically, this can occur when a parent tries to maneuver a child away from perceived danger, and the child experiences pain and acute loss of function of the affected limb.2
Nursemaid’s elbow reportedly occurs frequently among children,3,4 and thus we would expect to encounter it often in primary care. However, this condition receives little attention in medical training or in the literature, and many physicians do not recognize it.4-6
In this article we describe the epidemiology, underlying pathology, diagnosis, and treatment of nursemaid’s elbow, based on a systematic review of the current literature.
Methods
Literature search
Using PubMed and Embase, we conducted a literature search for articles published in Dutch, English, German, or French from 1966 through July 2007 on the topic of nursemaid’s elbow in children. We used as search terms all known synonyms for nursemaid’s elbow—eg, radial head subluxation, partial epiphyseal separation of the radial head, pulled elbow, babysitter’s elbow, curbstone fracture, etc. Publications cited in our initial search were also checked for relevance. Articles were reviewed and judged independently by 2 authors (M.K. and J.C.v.d.W.).
Articles we selected focused on proximal radial subluxation. We excluded articles on distal radial subluxation and luxation of the radius.
The 2 reviewers assessed the quality of articles on treatment using the validated Jadad score,7 wherein a maximum of 5 points may be awarded:
- 1 point if the study is described as randomized:
- 1 point if the study is described as double-blind:
- 1 point for a description of withdrawals or dropouts.
No cutoff limit for Jadad scores was planned as a criterion for exclusion. As it is not possible to treat nursemaid’s elbow in a double-blind fashion, 3 was the highest possible score in our study.
Results
Our literature search produced 368 potentially relevant papers; of these, 60 met our inclusion criteria. The reference lists of selected studies and reviews yielded an additional 25 acceptable papers, each covering various aspects of the topic (epidemiology, 19; pathology, 10; diagnosis, 10; treatment, 9;). Thirty-seven of the 85 selected papers were review articles.
Epidemiology
Most reports agree that nursemaid’s elbow is a frequent injury among children.4,8-10 Unfortunately, published population-based incidence rates are scarce; only 1 article gives an occurrence rate in the total population—1.2%.11 Most epidemiologic data are derived from case series, which show a predominance of injury among girls and to the left arm. Most cases occur at a median age of about 2 years.2-5,8
Pathology
The many synonyms of nursemaid’s elbow reflect a once obscure understanding of its pathology. Among initial reports from the 1800s, the focus was on determining whether the injury occurred at the wrist or the elbow.12 Subsequent studies showed that the mechanism of injury usually is a tug on the pronated arm5,13-16 of young children (who have relatively lax tissue), thereby pulling the radius through the annular ligament,13-15 which may partially tear and (with the meniscoid synovial fold) become entrapped between the radial head and the capitellum.15 Most commonly a parent or other caregiver is holding the child by the hand while walking and suddenly pulls the child away from a dangerous situation or merely drags the child up a curb or a step.1
Diagnosis
We found no clinical studies that assessed the value of physical examination or history taking. The only studies relevant to diagnosis discussed radiography.
Nursemaid’s elbow is an easily recognized diagnosis based on the history and physical examination.17 Still, it seems many physicians do not recognize the condition.4-6 Typically, a parent reports that the child cried out after a pull on the arm and then refused to use the arm, holding it slightly flexed and pronated.18 Pain may be felt only at the wrist or shoulder.3,18 Occasionally, a snap or click is heard when the accident happens.5 The elbow can usually be flexed and extended, but the child resists supination of the forearm, which causes pain in the elbow. There is no swelling or bruising.19
Children are often referred for radiographic examination with the observation, “refuses to use arm; please x-ray from shoulder to wrist.”20 Radiography is of little help, however, and exposes the child to a dose of ionizing radiation. Although some studies show small significant differences between nursemaid’s elbow and the normal elbow,21-23 radiographic results generally are reported as normal.4,6,8,24 (Some commentators assume this may occur if the radiology technician repositions the arm in an attempt to obtain a true anteroposterior projection of the elbow.1,18,25) Restrict radiography, therefore, to cases with an unclear history or a history of trauma other than arm pull, to exclude more severe injuries.
The role of sonography is not yet clear, but it may turn out to be a fast and harmless technique for diagnosing uncertain cases.20,25,26
Treatment
Although no articles have described the natural course of nursemaid’s elbow, most authors report that it resolves on its own when a child moves the arm in supination or pronation. It is so easily treated that parents of children with recurrent episodes have even been instructed by phone how to perform the reduction.27
Most articles and textbooks recommend reducing nursemaid’s elbow by a rapid supination of the forearm, followed by flexion or extension.9,28 However, some articles have described a pronation method.29 We found 2 high-quality trials that compared the success rate of the supination method with the pronation method.30,31 Researchers conducting 1 medium-quality trial assessed the difference in pain experienced with these 2 methods.32 And researchers conducting 1 low-quality trial tried to assess whether splinting after manipulation helps to prevent recurrences of nursemaid’s elbow.10
These trials indicate the pronation method is more successful. In addition, some studies report that the pronation method is less painful for the child and less frightening for a parent to watch.29-31 Green et al confirmed this in their randomized trial.32
Most compelling finding
The highest quality studies were those devoted to treatment,28,30-33 and the clear conclusion from their findings—in contrast to what textbooks recommend—is that reduction with a pronation maneuver is more often successful than the supination method. Of course more studies will be needed before textbooks change their recommendations. But at least these studies provide helpful guidance now.
CORRESPONDENCE
Marjolein Krul, MD, Department of General Practice, Room Ff304, Erasmus MC-University Medical Center Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands; [email protected]
1. Salter RB, Zaltz C. Anatomic investigations of the mechanism of injury and pathologic anatomy of “nursemaid’s elbow” in young children. Clin Orthop Relat Res. 1971;77:134-143.
2. Hagroo GA, Zaki HM, Choudhary MT, et al. Nursemaid’s elbow-not the effect of hypermobility of joints. Injury. 1995;26:687-690.
3. Griffin ME. Subluxation of the head of the radius in young children. Pediatrics. 1955;15:103-106.
4. Illingworth CM. Pulled elbow: a study of 100 patients. Br Med J. 1975;2:672-674.
5. Magill HK, Aitken AP. Nursemaid’s elbow. Surg Gynecol Obstet. 1954;98:753-756.
6. Bobrow RS. Childhood radial head subluxation. Physician unfamiliarity with “nursemaid’s” or “pulled” elbow. NY State J Med. 1977;77:908-909.
7. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12.
8. Quan L, Marcuse EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194-1197.
9. Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150:164-166.
10. Lyver MB. Radial head subluxation. J Emerg Med. 1991;9:154-156.
11. Jongschaap HC, Youngson GG, Beattie TF. The epidemiology of radial head subluxation (‘nursemaid’s elbow’) in the Aberdeen city area. Health Bull (Edinb). 1990;48:58-61.
12. Hutchinson J. On certain obscure sprains of the elbow occurring in young children. Ann Surg. 1885;2:91-97.
13. Stone CA. Subluxation of the head of the radius. JAMA. 1916;1:28-29.
14. Miles KA, Finlay DBI. Disruption of the radiocapitellar line in the normal elbow. Injury. 1989;20:365-367.
15. Matles AL, Eliopoulos K. Internal derangement of the elbow in children. Int Surg. 1967;48:259-263.
16. Walcher K. Beobachtungen zur Ätiologie der Pronatio Dolorosa. Arch Orthop Unfall-Chir. 1972;74:197-203.
17. Dimon JH. Pulled elbow or babysitter’s elbow. Ona J. 1979;6:72.-
18. Asher MA. Dislocations of the upper extremity in children. Orthop Clin North Am. 1976;7:583-591.
19. Hardy RH. Nursemaid’s elbow. J R Coll Gen Pract. 1978;28:224-226.
20. Outzen S. Chassaignac-Im Zweifel Diagnose per Sonographie? Chir Praxis. 2002;59:119-126.
21. Mehara AK, Bhan S. A radiological sign in nursemaid’s elbows. Int Orthop. 1995;19:174-175.
22. Snyder HS. Radiographic changes with radial head subluxation in children. J Emerg Med. 1990;8:265-269.
23. Frumkin K. Nursemaid’s elbow: a radiographic demonstration. Ann Emerg Med. 1985;14:690-693.
24. Salkind MR. Pulled elbow. Lancet. 1957;272:192-193.
25. Shabat S, Folman Y, Mann G, et al. The role of sonography in detecting radial head subluxation in a child. J Clin Ultrasound. 2005;33:187-189.
26. Kosuwon W, Mahaisavariya B, Saengnipanthkul S, et al. Ultrasonography of nursemaid’s elbow. J Bone Joint Surg Br. 1993;75:421-422.
27. Kaplan RE, Lillis KA. Recurrent nursemaid’s elbow (annular ligament displacement) treatment via telephone. Pediatrics. 2002;110:171-174.
28. Taha AM. The treatment of pulled elbow: a prospective randomized study. Arch Orthop Trauma Surg. 2000;120:336-337.
29. Nichols HH. Nursemaid’s elbow: reducing it to simple terms. Contemp Pediatr. 1988;5:50-55.
30. Macias CG, Bothner J, Wiebe R. A comparison of supination/flexion to hyperpronation in the reduction of radial head subluxations. Pediatrics. 1998;102(1):e10.-
31. McDonald J, Whitelaw C, Goldsmith LJ. Radial head subluxation: comparing two methods of reduction. Acad Emerg Med. 1999;6:715-718.
32. Green DA, Linares MY, Garcia Peña BM, et al. Randomized comparison of pain during radial head subluxation reduction using supination-flexion or forced pronation. Acad Emerg Med. 2006;22:235-239.
33. Krul M, van der Wouden JC, van Suijlekom-Smit LW, et al. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2009;(4):CD007759.-
• Nursemaid’s elbow typically occurs with a sudden pull on a child’s arm. Reserve radiography for uncertain cases in which you need to exclude more severe injuries. B
• Consider reducing nursemaid’s elbow by rapid pronation of the forearm, which has been shown to be less painful and more effective than supination. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Nursemaid’s elbow—sudden subluxation of the radial head—usually results from forcible traction to a child’s pronated hand or wrist, with the elbow extended.1 Ironically, this can occur when a parent tries to maneuver a child away from perceived danger, and the child experiences pain and acute loss of function of the affected limb.2
Nursemaid’s elbow reportedly occurs frequently among children,3,4 and thus we would expect to encounter it often in primary care. However, this condition receives little attention in medical training or in the literature, and many physicians do not recognize it.4-6
In this article we describe the epidemiology, underlying pathology, diagnosis, and treatment of nursemaid’s elbow, based on a systematic review of the current literature.
Methods
Literature search
Using PubMed and Embase, we conducted a literature search for articles published in Dutch, English, German, or French from 1966 through July 2007 on the topic of nursemaid’s elbow in children. We used as search terms all known synonyms for nursemaid’s elbow—eg, radial head subluxation, partial epiphyseal separation of the radial head, pulled elbow, babysitter’s elbow, curbstone fracture, etc. Publications cited in our initial search were also checked for relevance. Articles were reviewed and judged independently by 2 authors (M.K. and J.C.v.d.W.).
Articles we selected focused on proximal radial subluxation. We excluded articles on distal radial subluxation and luxation of the radius.
The 2 reviewers assessed the quality of articles on treatment using the validated Jadad score,7 wherein a maximum of 5 points may be awarded:
- 1 point if the study is described as randomized:
- 1 point if the study is described as double-blind:
- 1 point for a description of withdrawals or dropouts.
No cutoff limit for Jadad scores was planned as a criterion for exclusion. As it is not possible to treat nursemaid’s elbow in a double-blind fashion, 3 was the highest possible score in our study.
Results
Our literature search produced 368 potentially relevant papers; of these, 60 met our inclusion criteria. The reference lists of selected studies and reviews yielded an additional 25 acceptable papers, each covering various aspects of the topic (epidemiology, 19; pathology, 10; diagnosis, 10; treatment, 9;). Thirty-seven of the 85 selected papers were review articles.
Epidemiology
Most reports agree that nursemaid’s elbow is a frequent injury among children.4,8-10 Unfortunately, published population-based incidence rates are scarce; only 1 article gives an occurrence rate in the total population—1.2%.11 Most epidemiologic data are derived from case series, which show a predominance of injury among girls and to the left arm. Most cases occur at a median age of about 2 years.2-5,8
Pathology
The many synonyms of nursemaid’s elbow reflect a once obscure understanding of its pathology. Among initial reports from the 1800s, the focus was on determining whether the injury occurred at the wrist or the elbow.12 Subsequent studies showed that the mechanism of injury usually is a tug on the pronated arm5,13-16 of young children (who have relatively lax tissue), thereby pulling the radius through the annular ligament,13-15 which may partially tear and (with the meniscoid synovial fold) become entrapped between the radial head and the capitellum.15 Most commonly a parent or other caregiver is holding the child by the hand while walking and suddenly pulls the child away from a dangerous situation or merely drags the child up a curb or a step.1
Diagnosis
We found no clinical studies that assessed the value of physical examination or history taking. The only studies relevant to diagnosis discussed radiography.
Nursemaid’s elbow is an easily recognized diagnosis based on the history and physical examination.17 Still, it seems many physicians do not recognize the condition.4-6 Typically, a parent reports that the child cried out after a pull on the arm and then refused to use the arm, holding it slightly flexed and pronated.18 Pain may be felt only at the wrist or shoulder.3,18 Occasionally, a snap or click is heard when the accident happens.5 The elbow can usually be flexed and extended, but the child resists supination of the forearm, which causes pain in the elbow. There is no swelling or bruising.19
Children are often referred for radiographic examination with the observation, “refuses to use arm; please x-ray from shoulder to wrist.”20 Radiography is of little help, however, and exposes the child to a dose of ionizing radiation. Although some studies show small significant differences between nursemaid’s elbow and the normal elbow,21-23 radiographic results generally are reported as normal.4,6,8,24 (Some commentators assume this may occur if the radiology technician repositions the arm in an attempt to obtain a true anteroposterior projection of the elbow.1,18,25) Restrict radiography, therefore, to cases with an unclear history or a history of trauma other than arm pull, to exclude more severe injuries.
The role of sonography is not yet clear, but it may turn out to be a fast and harmless technique for diagnosing uncertain cases.20,25,26
Treatment
Although no articles have described the natural course of nursemaid’s elbow, most authors report that it resolves on its own when a child moves the arm in supination or pronation. It is so easily treated that parents of children with recurrent episodes have even been instructed by phone how to perform the reduction.27
Most articles and textbooks recommend reducing nursemaid’s elbow by a rapid supination of the forearm, followed by flexion or extension.9,28 However, some articles have described a pronation method.29 We found 2 high-quality trials that compared the success rate of the supination method with the pronation method.30,31 Researchers conducting 1 medium-quality trial assessed the difference in pain experienced with these 2 methods.32 And researchers conducting 1 low-quality trial tried to assess whether splinting after manipulation helps to prevent recurrences of nursemaid’s elbow.10
These trials indicate the pronation method is more successful. In addition, some studies report that the pronation method is less painful for the child and less frightening for a parent to watch.29-31 Green et al confirmed this in their randomized trial.32
Most compelling finding
The highest quality studies were those devoted to treatment,28,30-33 and the clear conclusion from their findings—in contrast to what textbooks recommend—is that reduction with a pronation maneuver is more often successful than the supination method. Of course more studies will be needed before textbooks change their recommendations. But at least these studies provide helpful guidance now.
CORRESPONDENCE
Marjolein Krul, MD, Department of General Practice, Room Ff304, Erasmus MC-University Medical Center Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands; [email protected]
• Nursemaid’s elbow typically occurs with a sudden pull on a child’s arm. Reserve radiography for uncertain cases in which you need to exclude more severe injuries. B
• Consider reducing nursemaid’s elbow by rapid pronation of the forearm, which has been shown to be less painful and more effective than supination. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Nursemaid’s elbow—sudden subluxation of the radial head—usually results from forcible traction to a child’s pronated hand or wrist, with the elbow extended.1 Ironically, this can occur when a parent tries to maneuver a child away from perceived danger, and the child experiences pain and acute loss of function of the affected limb.2
Nursemaid’s elbow reportedly occurs frequently among children,3,4 and thus we would expect to encounter it often in primary care. However, this condition receives little attention in medical training or in the literature, and many physicians do not recognize it.4-6
In this article we describe the epidemiology, underlying pathology, diagnosis, and treatment of nursemaid’s elbow, based on a systematic review of the current literature.
Methods
Literature search
Using PubMed and Embase, we conducted a literature search for articles published in Dutch, English, German, or French from 1966 through July 2007 on the topic of nursemaid’s elbow in children. We used as search terms all known synonyms for nursemaid’s elbow—eg, radial head subluxation, partial epiphyseal separation of the radial head, pulled elbow, babysitter’s elbow, curbstone fracture, etc. Publications cited in our initial search were also checked for relevance. Articles were reviewed and judged independently by 2 authors (M.K. and J.C.v.d.W.).
Articles we selected focused on proximal radial subluxation. We excluded articles on distal radial subluxation and luxation of the radius.
The 2 reviewers assessed the quality of articles on treatment using the validated Jadad score,7 wherein a maximum of 5 points may be awarded:
- 1 point if the study is described as randomized:
- 1 point if the study is described as double-blind:
- 1 point for a description of withdrawals or dropouts.
No cutoff limit for Jadad scores was planned as a criterion for exclusion. As it is not possible to treat nursemaid’s elbow in a double-blind fashion, 3 was the highest possible score in our study.
Results
Our literature search produced 368 potentially relevant papers; of these, 60 met our inclusion criteria. The reference lists of selected studies and reviews yielded an additional 25 acceptable papers, each covering various aspects of the topic (epidemiology, 19; pathology, 10; diagnosis, 10; treatment, 9;). Thirty-seven of the 85 selected papers were review articles.
Epidemiology
Most reports agree that nursemaid’s elbow is a frequent injury among children.4,8-10 Unfortunately, published population-based incidence rates are scarce; only 1 article gives an occurrence rate in the total population—1.2%.11 Most epidemiologic data are derived from case series, which show a predominance of injury among girls and to the left arm. Most cases occur at a median age of about 2 years.2-5,8
Pathology
The many synonyms of nursemaid’s elbow reflect a once obscure understanding of its pathology. Among initial reports from the 1800s, the focus was on determining whether the injury occurred at the wrist or the elbow.12 Subsequent studies showed that the mechanism of injury usually is a tug on the pronated arm5,13-16 of young children (who have relatively lax tissue), thereby pulling the radius through the annular ligament,13-15 which may partially tear and (with the meniscoid synovial fold) become entrapped between the radial head and the capitellum.15 Most commonly a parent or other caregiver is holding the child by the hand while walking and suddenly pulls the child away from a dangerous situation or merely drags the child up a curb or a step.1
Diagnosis
We found no clinical studies that assessed the value of physical examination or history taking. The only studies relevant to diagnosis discussed radiography.
Nursemaid’s elbow is an easily recognized diagnosis based on the history and physical examination.17 Still, it seems many physicians do not recognize the condition.4-6 Typically, a parent reports that the child cried out after a pull on the arm and then refused to use the arm, holding it slightly flexed and pronated.18 Pain may be felt only at the wrist or shoulder.3,18 Occasionally, a snap or click is heard when the accident happens.5 The elbow can usually be flexed and extended, but the child resists supination of the forearm, which causes pain in the elbow. There is no swelling or bruising.19
Children are often referred for radiographic examination with the observation, “refuses to use arm; please x-ray from shoulder to wrist.”20 Radiography is of little help, however, and exposes the child to a dose of ionizing radiation. Although some studies show small significant differences between nursemaid’s elbow and the normal elbow,21-23 radiographic results generally are reported as normal.4,6,8,24 (Some commentators assume this may occur if the radiology technician repositions the arm in an attempt to obtain a true anteroposterior projection of the elbow.1,18,25) Restrict radiography, therefore, to cases with an unclear history or a history of trauma other than arm pull, to exclude more severe injuries.
The role of sonography is not yet clear, but it may turn out to be a fast and harmless technique for diagnosing uncertain cases.20,25,26
Treatment
Although no articles have described the natural course of nursemaid’s elbow, most authors report that it resolves on its own when a child moves the arm in supination or pronation. It is so easily treated that parents of children with recurrent episodes have even been instructed by phone how to perform the reduction.27
Most articles and textbooks recommend reducing nursemaid’s elbow by a rapid supination of the forearm, followed by flexion or extension.9,28 However, some articles have described a pronation method.29 We found 2 high-quality trials that compared the success rate of the supination method with the pronation method.30,31 Researchers conducting 1 medium-quality trial assessed the difference in pain experienced with these 2 methods.32 And researchers conducting 1 low-quality trial tried to assess whether splinting after manipulation helps to prevent recurrences of nursemaid’s elbow.10
These trials indicate the pronation method is more successful. In addition, some studies report that the pronation method is less painful for the child and less frightening for a parent to watch.29-31 Green et al confirmed this in their randomized trial.32
Most compelling finding
The highest quality studies were those devoted to treatment,28,30-33 and the clear conclusion from their findings—in contrast to what textbooks recommend—is that reduction with a pronation maneuver is more often successful than the supination method. Of course more studies will be needed before textbooks change their recommendations. But at least these studies provide helpful guidance now.
CORRESPONDENCE
Marjolein Krul, MD, Department of General Practice, Room Ff304, Erasmus MC-University Medical Center Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands; [email protected]
1. Salter RB, Zaltz C. Anatomic investigations of the mechanism of injury and pathologic anatomy of “nursemaid’s elbow” in young children. Clin Orthop Relat Res. 1971;77:134-143.
2. Hagroo GA, Zaki HM, Choudhary MT, et al. Nursemaid’s elbow-not the effect of hypermobility of joints. Injury. 1995;26:687-690.
3. Griffin ME. Subluxation of the head of the radius in young children. Pediatrics. 1955;15:103-106.
4. Illingworth CM. Pulled elbow: a study of 100 patients. Br Med J. 1975;2:672-674.
5. Magill HK, Aitken AP. Nursemaid’s elbow. Surg Gynecol Obstet. 1954;98:753-756.
6. Bobrow RS. Childhood radial head subluxation. Physician unfamiliarity with “nursemaid’s” or “pulled” elbow. NY State J Med. 1977;77:908-909.
7. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12.
8. Quan L, Marcuse EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194-1197.
9. Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150:164-166.
10. Lyver MB. Radial head subluxation. J Emerg Med. 1991;9:154-156.
11. Jongschaap HC, Youngson GG, Beattie TF. The epidemiology of radial head subluxation (‘nursemaid’s elbow’) in the Aberdeen city area. Health Bull (Edinb). 1990;48:58-61.
12. Hutchinson J. On certain obscure sprains of the elbow occurring in young children. Ann Surg. 1885;2:91-97.
13. Stone CA. Subluxation of the head of the radius. JAMA. 1916;1:28-29.
14. Miles KA, Finlay DBI. Disruption of the radiocapitellar line in the normal elbow. Injury. 1989;20:365-367.
15. Matles AL, Eliopoulos K. Internal derangement of the elbow in children. Int Surg. 1967;48:259-263.
16. Walcher K. Beobachtungen zur Ätiologie der Pronatio Dolorosa. Arch Orthop Unfall-Chir. 1972;74:197-203.
17. Dimon JH. Pulled elbow or babysitter’s elbow. Ona J. 1979;6:72.-
18. Asher MA. Dislocations of the upper extremity in children. Orthop Clin North Am. 1976;7:583-591.
19. Hardy RH. Nursemaid’s elbow. J R Coll Gen Pract. 1978;28:224-226.
20. Outzen S. Chassaignac-Im Zweifel Diagnose per Sonographie? Chir Praxis. 2002;59:119-126.
21. Mehara AK, Bhan S. A radiological sign in nursemaid’s elbows. Int Orthop. 1995;19:174-175.
22. Snyder HS. Radiographic changes with radial head subluxation in children. J Emerg Med. 1990;8:265-269.
23. Frumkin K. Nursemaid’s elbow: a radiographic demonstration. Ann Emerg Med. 1985;14:690-693.
24. Salkind MR. Pulled elbow. Lancet. 1957;272:192-193.
25. Shabat S, Folman Y, Mann G, et al. The role of sonography in detecting radial head subluxation in a child. J Clin Ultrasound. 2005;33:187-189.
26. Kosuwon W, Mahaisavariya B, Saengnipanthkul S, et al. Ultrasonography of nursemaid’s elbow. J Bone Joint Surg Br. 1993;75:421-422.
27. Kaplan RE, Lillis KA. Recurrent nursemaid’s elbow (annular ligament displacement) treatment via telephone. Pediatrics. 2002;110:171-174.
28. Taha AM. The treatment of pulled elbow: a prospective randomized study. Arch Orthop Trauma Surg. 2000;120:336-337.
29. Nichols HH. Nursemaid’s elbow: reducing it to simple terms. Contemp Pediatr. 1988;5:50-55.
30. Macias CG, Bothner J, Wiebe R. A comparison of supination/flexion to hyperpronation in the reduction of radial head subluxations. Pediatrics. 1998;102(1):e10.-
31. McDonald J, Whitelaw C, Goldsmith LJ. Radial head subluxation: comparing two methods of reduction. Acad Emerg Med. 1999;6:715-718.
32. Green DA, Linares MY, Garcia Peña BM, et al. Randomized comparison of pain during radial head subluxation reduction using supination-flexion or forced pronation. Acad Emerg Med. 2006;22:235-239.
33. Krul M, van der Wouden JC, van Suijlekom-Smit LW, et al. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2009;(4):CD007759.-
1. Salter RB, Zaltz C. Anatomic investigations of the mechanism of injury and pathologic anatomy of “nursemaid’s elbow” in young children. Clin Orthop Relat Res. 1971;77:134-143.
2. Hagroo GA, Zaki HM, Choudhary MT, et al. Nursemaid’s elbow-not the effect of hypermobility of joints. Injury. 1995;26:687-690.
3. Griffin ME. Subluxation of the head of the radius in young children. Pediatrics. 1955;15:103-106.
4. Illingworth CM. Pulled elbow: a study of 100 patients. Br Med J. 1975;2:672-674.
5. Magill HK, Aitken AP. Nursemaid’s elbow. Surg Gynecol Obstet. 1954;98:753-756.
6. Bobrow RS. Childhood radial head subluxation. Physician unfamiliarity with “nursemaid’s” or “pulled” elbow. NY State J Med. 1977;77:908-909.
7. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12.
8. Quan L, Marcuse EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194-1197.
9. Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150:164-166.
10. Lyver MB. Radial head subluxation. J Emerg Med. 1991;9:154-156.
11. Jongschaap HC, Youngson GG, Beattie TF. The epidemiology of radial head subluxation (‘nursemaid’s elbow’) in the Aberdeen city area. Health Bull (Edinb). 1990;48:58-61.
12. Hutchinson J. On certain obscure sprains of the elbow occurring in young children. Ann Surg. 1885;2:91-97.
13. Stone CA. Subluxation of the head of the radius. JAMA. 1916;1:28-29.
14. Miles KA, Finlay DBI. Disruption of the radiocapitellar line in the normal elbow. Injury. 1989;20:365-367.
15. Matles AL, Eliopoulos K. Internal derangement of the elbow in children. Int Surg. 1967;48:259-263.
16. Walcher K. Beobachtungen zur Ätiologie der Pronatio Dolorosa. Arch Orthop Unfall-Chir. 1972;74:197-203.
17. Dimon JH. Pulled elbow or babysitter’s elbow. Ona J. 1979;6:72.-
18. Asher MA. Dislocations of the upper extremity in children. Orthop Clin North Am. 1976;7:583-591.
19. Hardy RH. Nursemaid’s elbow. J R Coll Gen Pract. 1978;28:224-226.
20. Outzen S. Chassaignac-Im Zweifel Diagnose per Sonographie? Chir Praxis. 2002;59:119-126.
21. Mehara AK, Bhan S. A radiological sign in nursemaid’s elbows. Int Orthop. 1995;19:174-175.
22. Snyder HS. Radiographic changes with radial head subluxation in children. J Emerg Med. 1990;8:265-269.
23. Frumkin K. Nursemaid’s elbow: a radiographic demonstration. Ann Emerg Med. 1985;14:690-693.
24. Salkind MR. Pulled elbow. Lancet. 1957;272:192-193.
25. Shabat S, Folman Y, Mann G, et al. The role of sonography in detecting radial head subluxation in a child. J Clin Ultrasound. 2005;33:187-189.
26. Kosuwon W, Mahaisavariya B, Saengnipanthkul S, et al. Ultrasonography of nursemaid’s elbow. J Bone Joint Surg Br. 1993;75:421-422.
27. Kaplan RE, Lillis KA. Recurrent nursemaid’s elbow (annular ligament displacement) treatment via telephone. Pediatrics. 2002;110:171-174.
28. Taha AM. The treatment of pulled elbow: a prospective randomized study. Arch Orthop Trauma Surg. 2000;120:336-337.
29. Nichols HH. Nursemaid’s elbow: reducing it to simple terms. Contemp Pediatr. 1988;5:50-55.
30. Macias CG, Bothner J, Wiebe R. A comparison of supination/flexion to hyperpronation in the reduction of radial head subluxations. Pediatrics. 1998;102(1):e10.-
31. McDonald J, Whitelaw C, Goldsmith LJ. Radial head subluxation: comparing two methods of reduction. Acad Emerg Med. 1999;6:715-718.
32. Green DA, Linares MY, Garcia Peña BM, et al. Randomized comparison of pain during radial head subluxation reduction using supination-flexion or forced pronation. Acad Emerg Med. 2006;22:235-239.
33. Krul M, van der Wouden JC, van Suijlekom-Smit LW, et al. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2009;(4):CD007759.-
Sudden onset of amnesia in a healthy woman
CASE A 63-year-old woman came to our emergency department with her fiancé following an abrupt onset of confusion that began 1 hour earlier. The patient had been working outside in the yard when she approached her fiancé, repeatedly asking where she was and what she was doing. She remained conscious of her identity, however, and exhibited no other neurologic symptoms, such as muscle weakness, gait imbalance, sensory loss, vision changes, slurred speech, or facial droop. The fiancé did not witness any loss of consciousness, head trauma, or seizure-like activity.
Before the event, the patient was feeling well, without any fever, headache, emesis, or vertigo. She denied using tobacco, alcohol, or illicit drugs. Her medical history was unremarkable, including an absence of diabetes, hypertension, and hyperlipidemia. The only significant finding in her family history was a stroke her mother experienced at an advanced age. During our interview, the patient remained confused about where she was and what was happening. She was aware of her confusion and distressed by it.
On examination, the patient was alert and oriented to self and year. She appeared appropriately anxious about her situation. She was afebrile and slightly hypertensive. Her other vital signs were normal. She could not recall events immediately preceding her arrival at the emergency department, but could recall events of the day before and earlier. There was no evidence of trauma. Head, neck, cardiovascular, lung, and abdominal exams were within normal limits.
Her neurologic exam revealed intact cranial nerves, symmetric face, 5/5 muscle strength in all extremities, intact sensation, and normal gait. Grossly, visual fields were intact. There was no Babinski sign, clonus, or pronator drift. She had 3/3 immediate recall of named objects, but 0/3 recall at 5 minutes. Results for complete blood count, basic metabolic panel, and urinalysis were within normal limits, including a blood glucose level of 77 mg/dL and a low-density lipoprotein level of 161 mg/dL. The result for cardiac enzymes was negative. Noncontrast computed tomography of the head revealed a remote pontine lacunar infarct.
WHAT IS THE MOST LIKELY EXPLANATION FOR HER CONDITION?
Transient global amnesia
We admitted the patient for further evaluation with a presumptive diagnosis of transient global amnesia (TGA).
With a chief complaint of amnesia, the differential diagnosis is broad (TABLE 1).1-3 In this case, a stroke was unlikely given the absence of neurologic deficits, specifically the lack of visual field defects. The elapsed time of her symptoms was too long for a transient ischemic attack or seizure. There was no supporting evidence for encephalitis, intracranial bleed, or hypoglycemia. While delirium could be considered, its characteristic features of inattention and a waxing and waning course were not present, nor was there any obvious underlying cause, such as infection or polypharmacy. The patient had no loss of self-identity that would suggest a psychogenic cause. The time course and the patient’s symptoms were congruent with the clinical criteria for TGA, and we confidently based our diagnosis on this.
TABLE 1
Rule out these disorders with acute anterograde amnesia1-3
| Transient ischemic attack |
| Delirium |
| Intoxication or alcohol/drug withdrawal |
| Concussion |
| Intracranial bleed |
| Complex partial seizures |
| Postictal state |
| Hypoglycemia |
| Encephalitis |
| Transient global amnesia |
| Psychogenic amnesia |
| Wernicke’s encephalopathy |
Type of memory loss as a clue to cause
Amnesia occurs when memory and learning in an alert person are impaired to a degree out of proportion to the person’s overall neurologic status. It may affect the formation of new memories (anterograde amnesia) or the recall of past memories (retrograde amnesia).
How memory works. Memory can be broken down into categories (TABLE 2).1 Explicit memory requires a conscious effort to recall. An example is episodic memory, in which memories are framed within a context, such as recalling what was served for dinner the night before. Its function is critical to creating new memories. Other forms of explicit memory are semantic memory—memorized facts that are independent of a context—and working memory, in which focused attention is used to manipulate information. Implicit memory operates subconsciously. The prime example is procedural memory, involving the ability to learn new skills and perform them without total concentration.
Memory function affected in TGA. In TGA, episodic memory—critical in the laying down of new memories—is most affected. Episodic memory relies heavily on the hippocampus to function correctly. When it dysfunctions, a person cannot consolidate and retain new information, thus resulting in anterograde amnesia.1
Retrograde amnesia generally requires dysfunction of the frontal lobe in addition to the temporal lobe.3 However, it may be present concurrently with anterograde amnesia when a lesion is isolated to the hippocampus; it is usually limited to more recent memories. That recent memories tend to be the more vulnerable is known as Ribot’s law. If retrograde amnesia is present, it usually resolves before anterograde amnesia.4
In TGA, procedural memory is unaffected. Thus, activities of daily living and instrumental activities of daily living remain intact—eg, the patient retains the necessary skills to drive a car.
TABLE 2
Categories of memory function1
Explicit memory: requiring conscious effort to recall information.
|
Implicit memory: recall is done subconsciously.
|
Most often the prognosis is good
TGA is an unusual manifestation of anterograde amnesia that is self-limited and tends not to recur.5 An episode typically lasts 1 to 8 hours.6 Although the disorder was first described in 1956, a set of clinical criteria (TABLE 3) was not defined until 1990.7 The highlights of these criteria are that self-identity is preserved and no evidence exists for neurologic deficit or seizure activity.6 The incidence of TGA is 3 to 10 in 100,000.5 TGA usually affects patients in their early 60s,2 and men and women are affected equally.
Interestingly, more than half of patients with TGA report a precipitating event, usually involving physical activity or a Valsalva maneuver.6 Classically, the patient repeatedly asks the same questions. The most common associated symptoms are headache, dizziness, and nausea.2,6
Generally, the patient’s prognosis is good, without long-term sequelae. Importantly, reassure patients and their families that there will be no memories of the event itself, as their memory-making ability was impaired.2
TABLE 3
Clinical criteria for transient global amnesia, as defined by Hodges and Warlow7
| Amnesia must be witnessed by another |
| Acute onset of anterograde amnesia |
| Patient is alert—no change in consciousness |
| No loss of personal identity |
| No focal neurologic deficits |
| No recent history of head trauma or seizure |
| Amnesia resolves in 24 hours |
If episodes do recur
A small subset of people may have recurrent episodes. Recurrence rates over a 5-year span have been reported as 3% to 26%; however, this range includes cases and studies recorded before the diagnostic criteria were developed in 1990.6 Although the clinical criteria for TGA can be helpful in diagnosing the disorder, there is no standardized workup because TGA has no clear etiology or known underlying mechanism. Many causal theories exist, however, and have evidence to support them.
Possible underlying conditions. One proposed explanation is ischemia of the hippocampus. This raises questions of whether vascular risk factors place people at higher risk.8 Recent studies have not confirmed this theory, and patients with diabetes, hypertension, or hyperlipidemia appear not to be at higher risk of TGA. Still, it is interesting that TGA is a disease affecting older adults and that evidence of small-vessel ischemia is often discovered incidentally.6,8
On the other hand, some experts take into account the high association of TGA with migraines documented in multiple studies, and therefore propose a spreading depression as the cause.5 Another hypothesis is a valvular insufficiency of the jugular veins that allows reflux, resulting in venous ischemia of the hippocampal area, especially during a Valsalva maneuver.9 Indeed, jugular valve insufficiency has been noted in up to two-thirds of TGA patients. However, if valvular insufficiency is truly the mechanism of disease, why do recurrence rates remain so low?10
MRI may be helpful. Given the many theories of TGA origin, several imaging mechanisms have been tried with mixed results: single photon emission computed tomography, magnetic resonance imaging (MRI) with diffusion-weighted imaging, and positron emission tomography.
The lack of reliable results makes it difficult to establish diagnostic criteria. Some generalized guidelines are as follows:
If there are any neurologic findings or concern about a transient ischemic attack or cerebrovascular accident, obtain an MRI. This should include diffusion-weighted imaging, which may reveal a transient lesion in the hippocampus.6 If the patient has recurrent episodes, or has episodes that last less than 1 hour, suspect the possibility of seizure and consider arranging for an electroencephalogram.4,6 Likewise, recurrence may also be due to a patent foramen ovale (PFO) causing paradoxical emboli and transient ischemia of the hippocampus. In 1 study, the rate of PFO in the TGA arm was 55%; it was 50% in those with recurrent episodes.11
- Order an MRI if your patient with a suspected case of TGA has any neurologic findings or if you are concerned about transient ischemic attack or cerebrovascular accident.
- If the patient has had recurrent episodes, or has episodes that last less than 1 hour, suspect the possibility of seizure and consider an electroencephalogram.
- Reassure TGA patients that there will be no memories of the event itself, as their memory-making ability was impaired, and that there are no long-term sequelae.
Our patient’s outcome
In the 24 hours after admission, the patient’s anterograde amnesia gradually resolved. She was able to remember the medical staff caring for her and retain orientation to her situation. However, she was unable to regain memories of the events immediately surrounding the onset of amnesia. During her hospitalization, the patient underwent a thorough work-up, including carotid artery Doppler ultrasound and echocardiogram with agitated saline (bubble study), both of which yielded normal results. Her MR angiography showed patent cerebral vessels. As mentioned, an MRI of the head showed a remote lacunar infarct of her left upper pons and nonspecific subcortical white matter disease was noted, consistent with chronic small vessel disease. The patient was discharged with reassurance, and she has done well.
CORRESPONDENCE
Chris Bernheisel, MD, director, Family Medicine Inpatient Service, The University of Cincinnati, 2123 Auburn Ave, Suite 340, Cincinnati, OH 45219; [email protected]
1. Budson AE, Price BH. Memory dysfunction. N Engl J Med. 2005;352:692-699.
2. Owen D, Paranandi B, Sivakumar R, et al. Classical diseases revisited: transient global amnesia. Postgrad Med J. 2007;83:236-239.
3. Kopelman MD. Disorders of memory. Brain. 2002;125:2152-2190.
4. Guillery-Girard B, Desgrandes B, Urban C, et al. The dynamic time course of memory recovery in transient global amnesia. J Neurol Neurosurg Psychiatry. 2004;75:1532-1540.
5. Pantoni L, Lamassa M, Inzitari D. Transient global amnesia: a review emphasizing pathogenic aspects. Acta Neurol Scand. 2000;102:275-283.
6. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640-1658.
7. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53:834-843.
8. Sander K, Sander D. New insights into transient global amnesia: recent imaging and clinical findings. Lancet Neurol. 2005;4:437-444.
9. Menendez Gonzalez M, Rivera MM. Transient global amnesia: Increasing evidence of a venous etiology. Arch Neurol. 2006;63:1334-1335.
10. Bettermann K. Transient global amnesia: the continuing quest for a source. Arch Neurol. 2006;63:1336-1338.
11. Klotzsch C, Sliwka U, Berlit P, et al. An increased frequency of patent foramen ovale in patients with transient global amnesia. Arch Neurol. 1996;53:504-508.
CASE A 63-year-old woman came to our emergency department with her fiancé following an abrupt onset of confusion that began 1 hour earlier. The patient had been working outside in the yard when she approached her fiancé, repeatedly asking where she was and what she was doing. She remained conscious of her identity, however, and exhibited no other neurologic symptoms, such as muscle weakness, gait imbalance, sensory loss, vision changes, slurred speech, or facial droop. The fiancé did not witness any loss of consciousness, head trauma, or seizure-like activity.
Before the event, the patient was feeling well, without any fever, headache, emesis, or vertigo. She denied using tobacco, alcohol, or illicit drugs. Her medical history was unremarkable, including an absence of diabetes, hypertension, and hyperlipidemia. The only significant finding in her family history was a stroke her mother experienced at an advanced age. During our interview, the patient remained confused about where she was and what was happening. She was aware of her confusion and distressed by it.
On examination, the patient was alert and oriented to self and year. She appeared appropriately anxious about her situation. She was afebrile and slightly hypertensive. Her other vital signs were normal. She could not recall events immediately preceding her arrival at the emergency department, but could recall events of the day before and earlier. There was no evidence of trauma. Head, neck, cardiovascular, lung, and abdominal exams were within normal limits.
Her neurologic exam revealed intact cranial nerves, symmetric face, 5/5 muscle strength in all extremities, intact sensation, and normal gait. Grossly, visual fields were intact. There was no Babinski sign, clonus, or pronator drift. She had 3/3 immediate recall of named objects, but 0/3 recall at 5 minutes. Results for complete blood count, basic metabolic panel, and urinalysis were within normal limits, including a blood glucose level of 77 mg/dL and a low-density lipoprotein level of 161 mg/dL. The result for cardiac enzymes was negative. Noncontrast computed tomography of the head revealed a remote pontine lacunar infarct.
WHAT IS THE MOST LIKELY EXPLANATION FOR HER CONDITION?
Transient global amnesia
We admitted the patient for further evaluation with a presumptive diagnosis of transient global amnesia (TGA).
With a chief complaint of amnesia, the differential diagnosis is broad (TABLE 1).1-3 In this case, a stroke was unlikely given the absence of neurologic deficits, specifically the lack of visual field defects. The elapsed time of her symptoms was too long for a transient ischemic attack or seizure. There was no supporting evidence for encephalitis, intracranial bleed, or hypoglycemia. While delirium could be considered, its characteristic features of inattention and a waxing and waning course were not present, nor was there any obvious underlying cause, such as infection or polypharmacy. The patient had no loss of self-identity that would suggest a psychogenic cause. The time course and the patient’s symptoms were congruent with the clinical criteria for TGA, and we confidently based our diagnosis on this.
TABLE 1
Rule out these disorders with acute anterograde amnesia1-3
| Transient ischemic attack |
| Delirium |
| Intoxication or alcohol/drug withdrawal |
| Concussion |
| Intracranial bleed |
| Complex partial seizures |
| Postictal state |
| Hypoglycemia |
| Encephalitis |
| Transient global amnesia |
| Psychogenic amnesia |
| Wernicke’s encephalopathy |
Type of memory loss as a clue to cause
Amnesia occurs when memory and learning in an alert person are impaired to a degree out of proportion to the person’s overall neurologic status. It may affect the formation of new memories (anterograde amnesia) or the recall of past memories (retrograde amnesia).
How memory works. Memory can be broken down into categories (TABLE 2).1 Explicit memory requires a conscious effort to recall. An example is episodic memory, in which memories are framed within a context, such as recalling what was served for dinner the night before. Its function is critical to creating new memories. Other forms of explicit memory are semantic memory—memorized facts that are independent of a context—and working memory, in which focused attention is used to manipulate information. Implicit memory operates subconsciously. The prime example is procedural memory, involving the ability to learn new skills and perform them without total concentration.
Memory function affected in TGA. In TGA, episodic memory—critical in the laying down of new memories—is most affected. Episodic memory relies heavily on the hippocampus to function correctly. When it dysfunctions, a person cannot consolidate and retain new information, thus resulting in anterograde amnesia.1
Retrograde amnesia generally requires dysfunction of the frontal lobe in addition to the temporal lobe.3 However, it may be present concurrently with anterograde amnesia when a lesion is isolated to the hippocampus; it is usually limited to more recent memories. That recent memories tend to be the more vulnerable is known as Ribot’s law. If retrograde amnesia is present, it usually resolves before anterograde amnesia.4
In TGA, procedural memory is unaffected. Thus, activities of daily living and instrumental activities of daily living remain intact—eg, the patient retains the necessary skills to drive a car.
TABLE 2
Categories of memory function1
Explicit memory: requiring conscious effort to recall information.
|
Implicit memory: recall is done subconsciously.
|
Most often the prognosis is good
TGA is an unusual manifestation of anterograde amnesia that is self-limited and tends not to recur.5 An episode typically lasts 1 to 8 hours.6 Although the disorder was first described in 1956, a set of clinical criteria (TABLE 3) was not defined until 1990.7 The highlights of these criteria are that self-identity is preserved and no evidence exists for neurologic deficit or seizure activity.6 The incidence of TGA is 3 to 10 in 100,000.5 TGA usually affects patients in their early 60s,2 and men and women are affected equally.
Interestingly, more than half of patients with TGA report a precipitating event, usually involving physical activity or a Valsalva maneuver.6 Classically, the patient repeatedly asks the same questions. The most common associated symptoms are headache, dizziness, and nausea.2,6
Generally, the patient’s prognosis is good, without long-term sequelae. Importantly, reassure patients and their families that there will be no memories of the event itself, as their memory-making ability was impaired.2
TABLE 3
Clinical criteria for transient global amnesia, as defined by Hodges and Warlow7
| Amnesia must be witnessed by another |
| Acute onset of anterograde amnesia |
| Patient is alert—no change in consciousness |
| No loss of personal identity |
| No focal neurologic deficits |
| No recent history of head trauma or seizure |
| Amnesia resolves in 24 hours |
If episodes do recur
A small subset of people may have recurrent episodes. Recurrence rates over a 5-year span have been reported as 3% to 26%; however, this range includes cases and studies recorded before the diagnostic criteria were developed in 1990.6 Although the clinical criteria for TGA can be helpful in diagnosing the disorder, there is no standardized workup because TGA has no clear etiology or known underlying mechanism. Many causal theories exist, however, and have evidence to support them.
Possible underlying conditions. One proposed explanation is ischemia of the hippocampus. This raises questions of whether vascular risk factors place people at higher risk.8 Recent studies have not confirmed this theory, and patients with diabetes, hypertension, or hyperlipidemia appear not to be at higher risk of TGA. Still, it is interesting that TGA is a disease affecting older adults and that evidence of small-vessel ischemia is often discovered incidentally.6,8
On the other hand, some experts take into account the high association of TGA with migraines documented in multiple studies, and therefore propose a spreading depression as the cause.5 Another hypothesis is a valvular insufficiency of the jugular veins that allows reflux, resulting in venous ischemia of the hippocampal area, especially during a Valsalva maneuver.9 Indeed, jugular valve insufficiency has been noted in up to two-thirds of TGA patients. However, if valvular insufficiency is truly the mechanism of disease, why do recurrence rates remain so low?10
MRI may be helpful. Given the many theories of TGA origin, several imaging mechanisms have been tried with mixed results: single photon emission computed tomography, magnetic resonance imaging (MRI) with diffusion-weighted imaging, and positron emission tomography.
The lack of reliable results makes it difficult to establish diagnostic criteria. Some generalized guidelines are as follows:
If there are any neurologic findings or concern about a transient ischemic attack or cerebrovascular accident, obtain an MRI. This should include diffusion-weighted imaging, which may reveal a transient lesion in the hippocampus.6 If the patient has recurrent episodes, or has episodes that last less than 1 hour, suspect the possibility of seizure and consider arranging for an electroencephalogram.4,6 Likewise, recurrence may also be due to a patent foramen ovale (PFO) causing paradoxical emboli and transient ischemia of the hippocampus. In 1 study, the rate of PFO in the TGA arm was 55%; it was 50% in those with recurrent episodes.11
- Order an MRI if your patient with a suspected case of TGA has any neurologic findings or if you are concerned about transient ischemic attack or cerebrovascular accident.
- If the patient has had recurrent episodes, or has episodes that last less than 1 hour, suspect the possibility of seizure and consider an electroencephalogram.
- Reassure TGA patients that there will be no memories of the event itself, as their memory-making ability was impaired, and that there are no long-term sequelae.
Our patient’s outcome
In the 24 hours after admission, the patient’s anterograde amnesia gradually resolved. She was able to remember the medical staff caring for her and retain orientation to her situation. However, she was unable to regain memories of the events immediately surrounding the onset of amnesia. During her hospitalization, the patient underwent a thorough work-up, including carotid artery Doppler ultrasound and echocardiogram with agitated saline (bubble study), both of which yielded normal results. Her MR angiography showed patent cerebral vessels. As mentioned, an MRI of the head showed a remote lacunar infarct of her left upper pons and nonspecific subcortical white matter disease was noted, consistent with chronic small vessel disease. The patient was discharged with reassurance, and she has done well.
CORRESPONDENCE
Chris Bernheisel, MD, director, Family Medicine Inpatient Service, The University of Cincinnati, 2123 Auburn Ave, Suite 340, Cincinnati, OH 45219; [email protected]
CASE A 63-year-old woman came to our emergency department with her fiancé following an abrupt onset of confusion that began 1 hour earlier. The patient had been working outside in the yard when she approached her fiancé, repeatedly asking where she was and what she was doing. She remained conscious of her identity, however, and exhibited no other neurologic symptoms, such as muscle weakness, gait imbalance, sensory loss, vision changes, slurred speech, or facial droop. The fiancé did not witness any loss of consciousness, head trauma, or seizure-like activity.
Before the event, the patient was feeling well, without any fever, headache, emesis, or vertigo. She denied using tobacco, alcohol, or illicit drugs. Her medical history was unremarkable, including an absence of diabetes, hypertension, and hyperlipidemia. The only significant finding in her family history was a stroke her mother experienced at an advanced age. During our interview, the patient remained confused about where she was and what was happening. She was aware of her confusion and distressed by it.
On examination, the patient was alert and oriented to self and year. She appeared appropriately anxious about her situation. She was afebrile and slightly hypertensive. Her other vital signs were normal. She could not recall events immediately preceding her arrival at the emergency department, but could recall events of the day before and earlier. There was no evidence of trauma. Head, neck, cardiovascular, lung, and abdominal exams were within normal limits.
Her neurologic exam revealed intact cranial nerves, symmetric face, 5/5 muscle strength in all extremities, intact sensation, and normal gait. Grossly, visual fields were intact. There was no Babinski sign, clonus, or pronator drift. She had 3/3 immediate recall of named objects, but 0/3 recall at 5 minutes. Results for complete blood count, basic metabolic panel, and urinalysis were within normal limits, including a blood glucose level of 77 mg/dL and a low-density lipoprotein level of 161 mg/dL. The result for cardiac enzymes was negative. Noncontrast computed tomography of the head revealed a remote pontine lacunar infarct.
WHAT IS THE MOST LIKELY EXPLANATION FOR HER CONDITION?
Transient global amnesia
We admitted the patient for further evaluation with a presumptive diagnosis of transient global amnesia (TGA).
With a chief complaint of amnesia, the differential diagnosis is broad (TABLE 1).1-3 In this case, a stroke was unlikely given the absence of neurologic deficits, specifically the lack of visual field defects. The elapsed time of her symptoms was too long for a transient ischemic attack or seizure. There was no supporting evidence for encephalitis, intracranial bleed, or hypoglycemia. While delirium could be considered, its characteristic features of inattention and a waxing and waning course were not present, nor was there any obvious underlying cause, such as infection or polypharmacy. The patient had no loss of self-identity that would suggest a psychogenic cause. The time course and the patient’s symptoms were congruent with the clinical criteria for TGA, and we confidently based our diagnosis on this.
TABLE 1
Rule out these disorders with acute anterograde amnesia1-3
| Transient ischemic attack |
| Delirium |
| Intoxication or alcohol/drug withdrawal |
| Concussion |
| Intracranial bleed |
| Complex partial seizures |
| Postictal state |
| Hypoglycemia |
| Encephalitis |
| Transient global amnesia |
| Psychogenic amnesia |
| Wernicke’s encephalopathy |
Type of memory loss as a clue to cause
Amnesia occurs when memory and learning in an alert person are impaired to a degree out of proportion to the person’s overall neurologic status. It may affect the formation of new memories (anterograde amnesia) or the recall of past memories (retrograde amnesia).
How memory works. Memory can be broken down into categories (TABLE 2).1 Explicit memory requires a conscious effort to recall. An example is episodic memory, in which memories are framed within a context, such as recalling what was served for dinner the night before. Its function is critical to creating new memories. Other forms of explicit memory are semantic memory—memorized facts that are independent of a context—and working memory, in which focused attention is used to manipulate information. Implicit memory operates subconsciously. The prime example is procedural memory, involving the ability to learn new skills and perform them without total concentration.
Memory function affected in TGA. In TGA, episodic memory—critical in the laying down of new memories—is most affected. Episodic memory relies heavily on the hippocampus to function correctly. When it dysfunctions, a person cannot consolidate and retain new information, thus resulting in anterograde amnesia.1
Retrograde amnesia generally requires dysfunction of the frontal lobe in addition to the temporal lobe.3 However, it may be present concurrently with anterograde amnesia when a lesion is isolated to the hippocampus; it is usually limited to more recent memories. That recent memories tend to be the more vulnerable is known as Ribot’s law. If retrograde amnesia is present, it usually resolves before anterograde amnesia.4
In TGA, procedural memory is unaffected. Thus, activities of daily living and instrumental activities of daily living remain intact—eg, the patient retains the necessary skills to drive a car.
TABLE 2
Categories of memory function1
Explicit memory: requiring conscious effort to recall information.
|
Implicit memory: recall is done subconsciously.
|
Most often the prognosis is good
TGA is an unusual manifestation of anterograde amnesia that is self-limited and tends not to recur.5 An episode typically lasts 1 to 8 hours.6 Although the disorder was first described in 1956, a set of clinical criteria (TABLE 3) was not defined until 1990.7 The highlights of these criteria are that self-identity is preserved and no evidence exists for neurologic deficit or seizure activity.6 The incidence of TGA is 3 to 10 in 100,000.5 TGA usually affects patients in their early 60s,2 and men and women are affected equally.
Interestingly, more than half of patients with TGA report a precipitating event, usually involving physical activity or a Valsalva maneuver.6 Classically, the patient repeatedly asks the same questions. The most common associated symptoms are headache, dizziness, and nausea.2,6
Generally, the patient’s prognosis is good, without long-term sequelae. Importantly, reassure patients and their families that there will be no memories of the event itself, as their memory-making ability was impaired.2
TABLE 3
Clinical criteria for transient global amnesia, as defined by Hodges and Warlow7
| Amnesia must be witnessed by another |
| Acute onset of anterograde amnesia |
| Patient is alert—no change in consciousness |
| No loss of personal identity |
| No focal neurologic deficits |
| No recent history of head trauma or seizure |
| Amnesia resolves in 24 hours |
If episodes do recur
A small subset of people may have recurrent episodes. Recurrence rates over a 5-year span have been reported as 3% to 26%; however, this range includes cases and studies recorded before the diagnostic criteria were developed in 1990.6 Although the clinical criteria for TGA can be helpful in diagnosing the disorder, there is no standardized workup because TGA has no clear etiology or known underlying mechanism. Many causal theories exist, however, and have evidence to support them.
Possible underlying conditions. One proposed explanation is ischemia of the hippocampus. This raises questions of whether vascular risk factors place people at higher risk.8 Recent studies have not confirmed this theory, and patients with diabetes, hypertension, or hyperlipidemia appear not to be at higher risk of TGA. Still, it is interesting that TGA is a disease affecting older adults and that evidence of small-vessel ischemia is often discovered incidentally.6,8
On the other hand, some experts take into account the high association of TGA with migraines documented in multiple studies, and therefore propose a spreading depression as the cause.5 Another hypothesis is a valvular insufficiency of the jugular veins that allows reflux, resulting in venous ischemia of the hippocampal area, especially during a Valsalva maneuver.9 Indeed, jugular valve insufficiency has been noted in up to two-thirds of TGA patients. However, if valvular insufficiency is truly the mechanism of disease, why do recurrence rates remain so low?10
MRI may be helpful. Given the many theories of TGA origin, several imaging mechanisms have been tried with mixed results: single photon emission computed tomography, magnetic resonance imaging (MRI) with diffusion-weighted imaging, and positron emission tomography.
The lack of reliable results makes it difficult to establish diagnostic criteria. Some generalized guidelines are as follows:
If there are any neurologic findings or concern about a transient ischemic attack or cerebrovascular accident, obtain an MRI. This should include diffusion-weighted imaging, which may reveal a transient lesion in the hippocampus.6 If the patient has recurrent episodes, or has episodes that last less than 1 hour, suspect the possibility of seizure and consider arranging for an electroencephalogram.4,6 Likewise, recurrence may also be due to a patent foramen ovale (PFO) causing paradoxical emboli and transient ischemia of the hippocampus. In 1 study, the rate of PFO in the TGA arm was 55%; it was 50% in those with recurrent episodes.11
- Order an MRI if your patient with a suspected case of TGA has any neurologic findings or if you are concerned about transient ischemic attack or cerebrovascular accident.
- If the patient has had recurrent episodes, or has episodes that last less than 1 hour, suspect the possibility of seizure and consider an electroencephalogram.
- Reassure TGA patients that there will be no memories of the event itself, as their memory-making ability was impaired, and that there are no long-term sequelae.
Our patient’s outcome
In the 24 hours after admission, the patient’s anterograde amnesia gradually resolved. She was able to remember the medical staff caring for her and retain orientation to her situation. However, she was unable to regain memories of the events immediately surrounding the onset of amnesia. During her hospitalization, the patient underwent a thorough work-up, including carotid artery Doppler ultrasound and echocardiogram with agitated saline (bubble study), both of which yielded normal results. Her MR angiography showed patent cerebral vessels. As mentioned, an MRI of the head showed a remote lacunar infarct of her left upper pons and nonspecific subcortical white matter disease was noted, consistent with chronic small vessel disease. The patient was discharged with reassurance, and she has done well.
CORRESPONDENCE
Chris Bernheisel, MD, director, Family Medicine Inpatient Service, The University of Cincinnati, 2123 Auburn Ave, Suite 340, Cincinnati, OH 45219; [email protected]
1. Budson AE, Price BH. Memory dysfunction. N Engl J Med. 2005;352:692-699.
2. Owen D, Paranandi B, Sivakumar R, et al. Classical diseases revisited: transient global amnesia. Postgrad Med J. 2007;83:236-239.
3. Kopelman MD. Disorders of memory. Brain. 2002;125:2152-2190.
4. Guillery-Girard B, Desgrandes B, Urban C, et al. The dynamic time course of memory recovery in transient global amnesia. J Neurol Neurosurg Psychiatry. 2004;75:1532-1540.
5. Pantoni L, Lamassa M, Inzitari D. Transient global amnesia: a review emphasizing pathogenic aspects. Acta Neurol Scand. 2000;102:275-283.
6. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640-1658.
7. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53:834-843.
8. Sander K, Sander D. New insights into transient global amnesia: recent imaging and clinical findings. Lancet Neurol. 2005;4:437-444.
9. Menendez Gonzalez M, Rivera MM. Transient global amnesia: Increasing evidence of a venous etiology. Arch Neurol. 2006;63:1334-1335.
10. Bettermann K. Transient global amnesia: the continuing quest for a source. Arch Neurol. 2006;63:1336-1338.
11. Klotzsch C, Sliwka U, Berlit P, et al. An increased frequency of patent foramen ovale in patients with transient global amnesia. Arch Neurol. 1996;53:504-508.
1. Budson AE, Price BH. Memory dysfunction. N Engl J Med. 2005;352:692-699.
2. Owen D, Paranandi B, Sivakumar R, et al. Classical diseases revisited: transient global amnesia. Postgrad Med J. 2007;83:236-239.
3. Kopelman MD. Disorders of memory. Brain. 2002;125:2152-2190.
4. Guillery-Girard B, Desgrandes B, Urban C, et al. The dynamic time course of memory recovery in transient global amnesia. J Neurol Neurosurg Psychiatry. 2004;75:1532-1540.
5. Pantoni L, Lamassa M, Inzitari D. Transient global amnesia: a review emphasizing pathogenic aspects. Acta Neurol Scand. 2000;102:275-283.
6. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640-1658.
7. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53:834-843.
8. Sander K, Sander D. New insights into transient global amnesia: recent imaging and clinical findings. Lancet Neurol. 2005;4:437-444.
9. Menendez Gonzalez M, Rivera MM. Transient global amnesia: Increasing evidence of a venous etiology. Arch Neurol. 2006;63:1334-1335.
10. Bettermann K. Transient global amnesia: the continuing quest for a source. Arch Neurol. 2006;63:1336-1338.
11. Klotzsch C, Sliwka U, Berlit P, et al. An increased frequency of patent foramen ovale in patients with transient global amnesia. Arch Neurol. 1996;53:504-508.
Inadequate follow-up ends in kidney transplant … Teenager dies of undiagnosed pneumonia … more
Inadequate follow-up ends in a kidney transplant
SMALL AMOUNTS OF PROTEIN AND BLOOD appeared in urine samples obtained during routine screenings of a 34-year-old man by his primary care physician. The doctor never told the patient about the proteinuria and reassured him that the presence of blood was normal for some adults and nothing to worry about.
The physician requested a urology consult on 1 occasion, but no cause was found for the blood and protein in the urine. After a further workup, the primary care physician concluded that it was benign. The urologist maintained that it wasn’t his job to do a workup for kidney disease or proteinuria; a kidney specialist would normally do such a work-up.
The blood and protein in the patient’s urine increased during subsequent years. The primary care physician didn’t order additional testing or consult a kidney specialist.
At a routine physical exam 5 years after the initial finding of proteinuria and hematuria, the patient’s blood and urine screening tests were grossly abnormal; he had anemia and kidney failure and needed immediate hospitalization. The primary care physician didn’t tell the patient about the abnormal test results because he didn’t see them—a lapse he blamed on a system error and office staff.
Several weeks after his latest doctor visit, the patient became acutely ill. His kidneys stopped functioning, and he went into hypertensive crisis. He was hospitalized and IgA nephropathy was diagnosed. His kidneys never recovered. The patient was placed on hemodialysis and received a kidney transplant 6 months later.
PLAINTIFF’S CLAIM Although IgA nephropathy has no known cause or cure, it can be treated with diet modification, lifestyle change, blood pressure control, and medication. With proper diagnosis and treatment, the patient would have retained kidney function for another 2½ years or more.
DOCTORS’ DEFENSE Earlier diagnosis would have prolonged kidney function for only about 6 months.
VERDICT $400,000 Massachusetts settlement.
COMMENT Blaming a bad outcome on “a system error and office staff ” is unlikely to be a winning defense in a court of law.
Teenager dies of undiagnosed pneumonia
A 16-YEAR-OLD GIRL was taken to the emergency room with diarrhea, fever, a nonproductive cough, chest pain, and rhinorrhea. The pediatrician and nurse who examined her found no abnormalities of the lungs, respiration, or oxygenation. A viral syndrome and/or infection of the upper respiratory tract was diagnosed. The girl was discharged with instructions to see her primary physician and return to the ER if her condition worsened.
The patient saw her pediatrician 3 days later after becoming increasingly weak. The pediatrician noted abnormalities in her respiration. He diagnosed a virus but prescribed antibiotics, and told the girl to return if her condition became worse. The girl didn’t return and died 3 days later. Her death was attributed to pneumonia.
PLAINTIFF’S CLAIM The pediatrician and nurse in the ER should have diagnosed pneumonia. The differential diagnosis in the ER should have included pneumonia, and the patient shouldn’t have been released until pneumonia had been ruled out. The patient’s pediatrician should have given IV antibiotics and ordered a chest radiograph and white blood cell count.
DOCTORS’ DEFENSE The patient’s symptoms were characteristic of a viral infection and not typical of a bacterial infection. The pneumonia originated after the patient was last seen and was an aggressive form.
VERDICT $3.9 million New York verdict reduced to $500,000 under a high/low agreement.
COMMENT Our worst nightmare: treating a patient appropriately by withholding antibiotics (in the case of the emergency room staff ) followed by a catastrophic outcome. This case is a great example of why we practice defensive medicine and what’s wrong with our tort system.
Serious symptoms and history fail to prompt stroke workup
A MAN WITH DIABETES AND HYPERTENSION went to his primary care physician’s office complaining of right-sided headache, dizziness, some weakness and tingling on his left side, and difficulty picking up his left foot. The 56-year-old patient was seen by a nurse practitioner. The nurse consulted the physician twice during the visit, but the physician didn’t examine the patient personally.
An electrocardiogram was performed. The nurse found no neurologic indications of a transient ischemic attack. The patient was sent home with prescriptions for aspirin and atenolol and instructions to return in a week.
The patient’s condition deteriorated, and he went to the emergency department, where he was treated for a stroke. The symptoms progressed, however, leading to significant physical and cognitive disabilities.
PLAINTIFF’S CLAIM The physician and nurse practitioner failed to appreciate the patient’s risk of a stroke and recognize that his symptoms suggested a serious neurologic event. Immediate referral to an ED for a stroke work-up and treatment would have prevented progression of the stroke and the resulting disabilities. The physician should have evaluated the patient personally. The patient had not received proper treatment for hypertension, diabetes, and high cholesterol for many years before the stroke.
THE DEFENSE The treatment given was proper; earlier admission wouldn’t have made a difference.
VERDICT $750,000 Massachusetts settlement.
COMMENT Supervision of midlevel employees carries its own risks. When in doubt, see the patient!
Inadequate follow-up ends in a kidney transplant
SMALL AMOUNTS OF PROTEIN AND BLOOD appeared in urine samples obtained during routine screenings of a 34-year-old man by his primary care physician. The doctor never told the patient about the proteinuria and reassured him that the presence of blood was normal for some adults and nothing to worry about.
The physician requested a urology consult on 1 occasion, but no cause was found for the blood and protein in the urine. After a further workup, the primary care physician concluded that it was benign. The urologist maintained that it wasn’t his job to do a workup for kidney disease or proteinuria; a kidney specialist would normally do such a work-up.
The blood and protein in the patient’s urine increased during subsequent years. The primary care physician didn’t order additional testing or consult a kidney specialist.
At a routine physical exam 5 years after the initial finding of proteinuria and hematuria, the patient’s blood and urine screening tests were grossly abnormal; he had anemia and kidney failure and needed immediate hospitalization. The primary care physician didn’t tell the patient about the abnormal test results because he didn’t see them—a lapse he blamed on a system error and office staff.
Several weeks after his latest doctor visit, the patient became acutely ill. His kidneys stopped functioning, and he went into hypertensive crisis. He was hospitalized and IgA nephropathy was diagnosed. His kidneys never recovered. The patient was placed on hemodialysis and received a kidney transplant 6 months later.
PLAINTIFF’S CLAIM Although IgA nephropathy has no known cause or cure, it can be treated with diet modification, lifestyle change, blood pressure control, and medication. With proper diagnosis and treatment, the patient would have retained kidney function for another 2½ years or more.
DOCTORS’ DEFENSE Earlier diagnosis would have prolonged kidney function for only about 6 months.
VERDICT $400,000 Massachusetts settlement.
COMMENT Blaming a bad outcome on “a system error and office staff ” is unlikely to be a winning defense in a court of law.
Teenager dies of undiagnosed pneumonia
A 16-YEAR-OLD GIRL was taken to the emergency room with diarrhea, fever, a nonproductive cough, chest pain, and rhinorrhea. The pediatrician and nurse who examined her found no abnormalities of the lungs, respiration, or oxygenation. A viral syndrome and/or infection of the upper respiratory tract was diagnosed. The girl was discharged with instructions to see her primary physician and return to the ER if her condition worsened.
The patient saw her pediatrician 3 days later after becoming increasingly weak. The pediatrician noted abnormalities in her respiration. He diagnosed a virus but prescribed antibiotics, and told the girl to return if her condition became worse. The girl didn’t return and died 3 days later. Her death was attributed to pneumonia.
PLAINTIFF’S CLAIM The pediatrician and nurse in the ER should have diagnosed pneumonia. The differential diagnosis in the ER should have included pneumonia, and the patient shouldn’t have been released until pneumonia had been ruled out. The patient’s pediatrician should have given IV antibiotics and ordered a chest radiograph and white blood cell count.
DOCTORS’ DEFENSE The patient’s symptoms were characteristic of a viral infection and not typical of a bacterial infection. The pneumonia originated after the patient was last seen and was an aggressive form.
VERDICT $3.9 million New York verdict reduced to $500,000 under a high/low agreement.
COMMENT Our worst nightmare: treating a patient appropriately by withholding antibiotics (in the case of the emergency room staff ) followed by a catastrophic outcome. This case is a great example of why we practice defensive medicine and what’s wrong with our tort system.
Serious symptoms and history fail to prompt stroke workup
A MAN WITH DIABETES AND HYPERTENSION went to his primary care physician’s office complaining of right-sided headache, dizziness, some weakness and tingling on his left side, and difficulty picking up his left foot. The 56-year-old patient was seen by a nurse practitioner. The nurse consulted the physician twice during the visit, but the physician didn’t examine the patient personally.
An electrocardiogram was performed. The nurse found no neurologic indications of a transient ischemic attack. The patient was sent home with prescriptions for aspirin and atenolol and instructions to return in a week.
The patient’s condition deteriorated, and he went to the emergency department, where he was treated for a stroke. The symptoms progressed, however, leading to significant physical and cognitive disabilities.
PLAINTIFF’S CLAIM The physician and nurse practitioner failed to appreciate the patient’s risk of a stroke and recognize that his symptoms suggested a serious neurologic event. Immediate referral to an ED for a stroke work-up and treatment would have prevented progression of the stroke and the resulting disabilities. The physician should have evaluated the patient personally. The patient had not received proper treatment for hypertension, diabetes, and high cholesterol for many years before the stroke.
THE DEFENSE The treatment given was proper; earlier admission wouldn’t have made a difference.
VERDICT $750,000 Massachusetts settlement.
COMMENT Supervision of midlevel employees carries its own risks. When in doubt, see the patient!
Inadequate follow-up ends in a kidney transplant
SMALL AMOUNTS OF PROTEIN AND BLOOD appeared in urine samples obtained during routine screenings of a 34-year-old man by his primary care physician. The doctor never told the patient about the proteinuria and reassured him that the presence of blood was normal for some adults and nothing to worry about.
The physician requested a urology consult on 1 occasion, but no cause was found for the blood and protein in the urine. After a further workup, the primary care physician concluded that it was benign. The urologist maintained that it wasn’t his job to do a workup for kidney disease or proteinuria; a kidney specialist would normally do such a work-up.
The blood and protein in the patient’s urine increased during subsequent years. The primary care physician didn’t order additional testing or consult a kidney specialist.
At a routine physical exam 5 years after the initial finding of proteinuria and hematuria, the patient’s blood and urine screening tests were grossly abnormal; he had anemia and kidney failure and needed immediate hospitalization. The primary care physician didn’t tell the patient about the abnormal test results because he didn’t see them—a lapse he blamed on a system error and office staff.
Several weeks after his latest doctor visit, the patient became acutely ill. His kidneys stopped functioning, and he went into hypertensive crisis. He was hospitalized and IgA nephropathy was diagnosed. His kidneys never recovered. The patient was placed on hemodialysis and received a kidney transplant 6 months later.
PLAINTIFF’S CLAIM Although IgA nephropathy has no known cause or cure, it can be treated with diet modification, lifestyle change, blood pressure control, and medication. With proper diagnosis and treatment, the patient would have retained kidney function for another 2½ years or more.
DOCTORS’ DEFENSE Earlier diagnosis would have prolonged kidney function for only about 6 months.
VERDICT $400,000 Massachusetts settlement.
COMMENT Blaming a bad outcome on “a system error and office staff ” is unlikely to be a winning defense in a court of law.
Teenager dies of undiagnosed pneumonia
A 16-YEAR-OLD GIRL was taken to the emergency room with diarrhea, fever, a nonproductive cough, chest pain, and rhinorrhea. The pediatrician and nurse who examined her found no abnormalities of the lungs, respiration, or oxygenation. A viral syndrome and/or infection of the upper respiratory tract was diagnosed. The girl was discharged with instructions to see her primary physician and return to the ER if her condition worsened.
The patient saw her pediatrician 3 days later after becoming increasingly weak. The pediatrician noted abnormalities in her respiration. He diagnosed a virus but prescribed antibiotics, and told the girl to return if her condition became worse. The girl didn’t return and died 3 days later. Her death was attributed to pneumonia.
PLAINTIFF’S CLAIM The pediatrician and nurse in the ER should have diagnosed pneumonia. The differential diagnosis in the ER should have included pneumonia, and the patient shouldn’t have been released until pneumonia had been ruled out. The patient’s pediatrician should have given IV antibiotics and ordered a chest radiograph and white blood cell count.
DOCTORS’ DEFENSE The patient’s symptoms were characteristic of a viral infection and not typical of a bacterial infection. The pneumonia originated after the patient was last seen and was an aggressive form.
VERDICT $3.9 million New York verdict reduced to $500,000 under a high/low agreement.
COMMENT Our worst nightmare: treating a patient appropriately by withholding antibiotics (in the case of the emergency room staff ) followed by a catastrophic outcome. This case is a great example of why we practice defensive medicine and what’s wrong with our tort system.
Serious symptoms and history fail to prompt stroke workup
A MAN WITH DIABETES AND HYPERTENSION went to his primary care physician’s office complaining of right-sided headache, dizziness, some weakness and tingling on his left side, and difficulty picking up his left foot. The 56-year-old patient was seen by a nurse practitioner. The nurse consulted the physician twice during the visit, but the physician didn’t examine the patient personally.
An electrocardiogram was performed. The nurse found no neurologic indications of a transient ischemic attack. The patient was sent home with prescriptions for aspirin and atenolol and instructions to return in a week.
The patient’s condition deteriorated, and he went to the emergency department, where he was treated for a stroke. The symptoms progressed, however, leading to significant physical and cognitive disabilities.
PLAINTIFF’S CLAIM The physician and nurse practitioner failed to appreciate the patient’s risk of a stroke and recognize that his symptoms suggested a serious neurologic event. Immediate referral to an ED for a stroke work-up and treatment would have prevented progression of the stroke and the resulting disabilities. The physician should have evaluated the patient personally. The patient had not received proper treatment for hypertension, diabetes, and high cholesterol for many years before the stroke.
THE DEFENSE The treatment given was proper; earlier admission wouldn’t have made a difference.
VERDICT $750,000 Massachusetts settlement.
COMMENT Supervision of midlevel employees carries its own risks. When in doubt, see the patient!
Is it stroke, or something else?
• Arrange for urgent transport to the hospital when a patient presents with stroke-like symptoms of acute onset, especially within the 3- to 6-hour therapeutic window. B
• Use a validated prehospital stroke identification algorithm such as the Face Arms Speech Time (FAST) test to identify possible acute stroke patients requiring urgent transport to the hospital. B
• Obtain a CBC and basic metabolic panel for all patients with signs and symptoms suggestive of stroke—and a blood alcohol, hepatic function, and toxicology screen, in select patients—to help rule out stroke mimics. C
• Ensure that patients undergo brain imaging to rule out stroke mimics before treatment for acute ischemic stroke is initiated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke is the third leading cause of death (claiming the life of 1 person every 3 to 4 minutes) and the No. 1 cause of adult disability in the United States.1 Advances in thrombolysis and clot removal can improve outcomes, but are dependent on swift and certain diagnosis. Amid the rush to ensure that treatment is initiated within the therapeutic window for cerebral reperfusion, “stroke mimics”—so called because of their ability to cause signs and symptoms similar to stroke—are sometimes mistaken for the real thing.
The prevalence of misdiagnosis ranges from about 4% of patients who receive tissue plasminogen activator (tPA) for reperfusion2 to 25% of patients who are rushed to the hospital because they are thought to be having a stroke.3 Seizures, migraine, sepsis, and peripheral vestibular disorders are among the many conditions that can masquerade as stroke.
Misdiagnosis can subject patients to unnecessary, and potentially harmful, invasive stroke therapies, and significantly delay the treatment they need. To prevent such outcomes, it is essential for primary care physicians, as well as emergency responders and emergency department (ED) physicians, to be aware of—and on the lookout for—the clues that can distinguish stroke from stroke mimics.
Suspect stroke? Establish a baseline
Despite a nationwide effort to increase public awareness of stroke and the importance of getting to the hospital within the therapeutic window for treatment,4 too few patients arrive within the time frame for cerebral reperfusion therapy. Primary care physicians can help by educating patients about stroke signs and symptoms.
When a patient presents with possible stroke, determine whether symptoms began gradually or abruptly. An acute ischemic stroke is heralded by the sudden onset of a focal neurologic deficit in a vascular pattern. Duration of symptoms can help distinguish stroke from a transient ischemic attack (TIA). Although TIAs were defined by the National Institutes of Health in 1975 as neurologic deficits that resolve within 24 hours of their onset, we now know that they typically last only 2 to 15 minutes, with the vast majority resolving within an hour.5
If the onset was sudden, find out when the patient was last seen at his or her neurologic baseline—information a family member, friend, or caregiver can often provide. This information is crucial because the neurologic baseline, rather than the time at which the symptoms were first noticed, is the basis for the therapeutic window for thrombolysis (3 hours for intravenous tPA and 6 hours for intra-arterial tPA). (Clot extraction with a mechanical embolus retrieval device [MERCI, Concentric Medical, Mountain View, Calif] has a 9-hour window.6,7)
Use a rapid stroke screening tool. To rapidly evaluate a patient with stroke-like signs and symptoms in a clinic or other outpatient setting, use a stroke screening tool with a high sensitivity,8 such as the Cincinnati Prehospital Stroke Scale (CPSS), the Face Arms Speech Time (FAST) test, or the Los Angeles Prehospital Stroke Screen (LAPSS) (TABLE 1). All 3 have a high positive predictive value (CPSS: 88%, FAST: 89%, LAPSS: 87%), but there is greater variation in the negative predictive value: 75%, 73%, and 55%, respectively.9
Patients with positive results typically require rapid transport to the ED—even if you notice red flags that may signal that you’re dealing with a stroke mimic.
TABLE 1
Stroke screening tools for outpatient use*30-32
| Cincinnati Prehospital Stroke Scale (CPSS) (www.strokecenter.org/trials/scales/cincinnati.html), which assesses the unilateral presence of any (or all) of the 3 key indicators—facial droop, arm drift, or slurred speech |
| Face Arms Speech Time (FAST) (www.stroke.org/site/PageServer?pagename=symp), a modification of CPSS based on the same criteria, has been validated in primary care clinics as well as emergency departments |
| Los Angeles Prehospital Stroke Screen (LAPSS) (www.strokecenter.org/trials/scales/lapss.html), a 1-page instrument that uses 5 criteria—age (>45 years), seizure history (none), onset of neurologic symptoms (within 24 hours), ambulatory status (ambulatory prior to event), and blood glucose level (60-400 mg/dL)—and 3 physical characteristics (facial smile/grimace, grip, and arm weakness) to screen for possible stroke |
| * A positive test is based on the presence of 1 or more key features for CPSS or FAST, and on a Yes (or Unknown) response to all the screening criteria in LAPSS. |
Be alert to the signs of conditions masquerading as stroke
Seizure at the onset of the episode; isolated mild neurological deficits, such as ataxia, sensory loss, or dysarthria alone; and/or minimal weakness are contraindications to thrombolytic therapy, according to the American Academy of Neurology (AAN).10
Rapidly improving neurological status is a probable indicator of a TIA or nonstroke etiology. Decreased level of consciousness with normal eye movements increases the likelihood that the patient has a condition that mimics stroke.11 Additional symptoms that strongly suggest a disorder other than stroke are convulsions (odds ratio [OR]: 0.1), loss of consciousness (OR: 0.1), confusion (OR: 0.2), headache (OR: 0.8), nausea (OR: 0.5), vomiting (OR: 0.6), and dizziness (OR: 0.3).9
Age is another consideration. The vast majority of patients with conditions that turn out to be stroke mimics are younger than 50 years of age. In patients older than 50, the prevalence of stroke misdiagnosis is just 3%.12
Watch for these stroke mimics
Seizures, either unwitnessed or unrecognized, and complex migraine are the most common stroke masqueraders. Other conditions frequently misdiagnosed as stroke include: systemic infections and early sepsis, central nervous system (CNS) tumors, and toxic-metabolic syndromes (including intoxication, hypoglycemia, hypercalcemia, and hyperosmolar nonketotic coma) (TABLE 2). Patients with cranial or peripheral neuropathy; dementia; labyrinthitis/benign positional vertigo; psychiatric disorders, in particular, conversion reaction; syncope; and transient global amnesia may also present with neurological symptoms suggestive of stroke. (For more on transient global amnesia, see this month’s Hospitalist Rounds at http://www.jfponline.com/CollectionContent.asp?CollectionID=286.) Characteristics of some of the more common mimics are detailed below.
Seizures. Neurologic deficits associated with seizures are reversible, with no structural CNS abnormalities. Postictal hemiparesis, also known as Todd’s paralysis—a focal weakness after a seizure, typically localized to 1 side of the body—occurs in approximately 13% of all seizures.13 Todd’s paralysis, which can be seen after either partial complex or generalized tonic-clonic seizures, may also affect speech and vision, producing a range of signs and symptoms easily mistaken for stroke. Duration ranges from minutes to 48 hours,14 but generally lasts only 3 to 22 minutes.13
Differentiating Todd’s paralysis from stroke is complicated by the fact that some strokes trigger focal seizures during the acute phase. However, a history of seizures or witnessed seizure activity points to Todd’s paralysis rather than stroke.
Complex migraine. Like Todd’s paralysis, complex migraine may result in hemiparesis. The presentation may also include vision loss, aphasia, or vertigo and other basilar symptoms—neurologic changes that can outlast the headache. Complex migraine is a diagnosis of exclusion, arrived at after a full neurologic assessment, including stroke work-up. Indeed, you can be certain of a diagnosis of complex migraine only after the patient has had recurrent complex migraine attacks.
Some basilar TIAs can also present with headache, but the onset is typically sudden, as opposed to the more gradual onset of migraine aura with posterior circulation symptoms.15 Age is a factor as well: Complex migraines usually develop well before the age of 40, while the mean age for ischemic stroke is 70. Although complex migraine is a risk factor for ischemic stroke, in most patients migraine is a benign condition.15,16
Systemic infections. Sepsis from almost any infectious agent can cause delirium, altered speech, weakness, and less specific stroke-like symptoms. Microbial seeding of the CNS can result in focal lesions (eg, the lesions shown in FIGURE 1B are associated with cryptococcal meningoencephalitis) or abscess formation with focal neurologic deficits.
Mass lesions. Primary CNS tumors, metastatic tumors, and cerebral abscesses are among the lesions that can cause symptoms that mimic stroke. In most cases, symptoms develop gradually as the lesion enlarges, but a small subset of patients have symptoms lasting less than 1 day. This is thought to be due to hemorrhage into the tumor or the acute development of obstructive hydrocephalus.17
Metabolic disorders. Diabetic hypoglycemia, among other metabolic disorders, is a classic stroke mimic, as well as a cause of seizures, so early evaluation of blood glucose is a crucial step in evaluating a patient with neurologic signs and symptoms. Patients with diabetic hypoglycemia may present with hemiplegia and aphasia; similar symptoms may occur in patients with hypoglycemia secondary to alcoholism, among other causes. Those with hyperglycemic nonketotic hyperosmolar states, severe hyponatremia, and hepatic encephalopathy may also present with focal stroke-like symptoms. Neurologic changes associated with metabolic disorders generally resolve rapidly with the administration of IV glucose, but on rare occasions may take several hours to resolve.14
Psychiatric illness. Patients with certain psychiatric disorders—including conversion reaction, a psychological condition that presents as an alteration in, or loss of, physical function—may present with dramatic focal problems and apparent deficits that mimic neurologic disease. Subtle disparities in the physical exam, such as Hoover’s sign, give-away weakness,18 and “la belle indifference,” as well as negative neuroimaging, will establish this difficult-to-treat stroke mimic.19 Grand mal pseudo-seizures can be differentiated from actual grand mal seizures by the failure of a prolactin level (drawn 10 to 20 minutes post-event) to rise at least 2-fold.20
Transient global amnesia. The rare, sudden development of dense anterograde amnesia occurs without alteration in level of consciousness, focal neurologic deficits, or seizure activity. It is self-limiting and mainly affects those older than 50. Transient global amnesia has an uncertain etiology, although atypical migraine, seizure discharge, and venous congestion with hippocampal ischemia are viewed as possible causes. Reported triggers include severe physical or emotional stress, strenuous physical activity, and orgasmic sexual intercourse.21
TABLE 2
Common stroke mimics9,11,12,14,22
| Condition | Misdiagnosed as stroke (%) |
|---|---|
| Brain tumor | 7-15 |
| Labyrinthitis | 5-6 |
| Metabolic disorder | 3-13 |
| Migraine | 11-47 |
| Psychiatric disorder | 1-40 |
| Seizures | 11-40 |
| Sepsis | 14-17 |
| Syncope | 5-22 |
| Transient global amnesia | 3-10 |
| Other | 11-37 |
In the ED: Evaluation is guided by a timeline
Current guidelines from the American Heart Association and American Stroke Association recommend that a possible stroke patient be evaluated by the physician in the ED within 10 minutes of his or her arrival—and that a decision on how to proceed be reached within 60 minutes of arrival. The guidelines call for the initial computed tomography (CT) to be completed within 25 minutes of the patient’s arrival and interpreted by a physician with expertise in reading CT studies within 45 minutes of arrival.6,24
In the ED, the National Institutes of Health Stroke Scale (NIHSS)25 (TABLE 3) is an ideal way to focus and record the neurological exam.6 The scale assesses 6 separate neurologic functions (level of consciousness, vision, motor function, sensory function, language, and cerebellar function) and can be performed within 5 to 8 minutes. It yields a score from 0 to 42, with the higher numbers indicating worse neurologic function.26 Although a score ≤10 is generally considered to be predictive of a stroke mimic, a recent study found that 19% of patients with an NIHSS score >10 also had conditions masquerading as stroke.27
Imaging leads to accurate diagnosis. The rate at which stroke mimics are mistaken for actual strokes varies with the population studied and the diagnostic tests performed. While stroke is largely a clinical diagnosis and a history and physical exam focused on onset, duration, and symptoms are key elements in differentiating stroke from a stroke mimic, studies have found that the incidence of misdiagnosis (19% with history, physical, and lab work alone) drops to 5% when noncontrast CT is added. When diffusion-weighted magnetic resonance imaging (MRI) is used instead, misdiagnosis drops to just 2%.11,12,14,22,23
Basic lab tests—a complete blood count and basic metabolic panel, with blood alcohol, hepatic function tests, and toxicology screens in select cases—help rule out stroke mimics. Radiographic imaging of the brain provides further clarification (FIGURE 1A AND 1B), serving 2 main purposes: to (1) evaluate diagnoses other than stroke and (2) identify the presence of any acute intracranial bleeding. Noncontrast CT scans detect acute hemorrhage with a sensitivity of 89% and specificity of 100%.27 CT angiography (which can identify the location of a clot) and CT perfusion (which allows an assessment of any existing penumbra) can also be obtained in a timely fashion with newer multislice scanners.
Some institutions, however, evaluate acute stroke patients with MRI. Depending on the sequences used, MRI has the advantage of being able to detect early ischemic changes, diffusion and perfusion mismatches, and abnormalities of the posterior fossa.29 In acute ischemic stroke, diffusion-weighted MRI has a sensitivity of 83% and specificity of 96%, compared with a sensitivity of 16% and specificity of 98% for noncontrast CT.28
TABLE 3
National Institutes of Health Stroke Scale25
| Item | Response score* |
|---|---|
| 1a. Level of consciousness | 0 = alert 1 = not alert 2 = obtunded 3 = unresponsive |
| 1b. Level of consciousness Questions | 0 = answers both correctly 1 = answers one correctly 2 = answers neither correctly |
| 1c. Level of consciousness Commands | 0 = performs both tasks correctly 1 = performs one task correctly 2 = performs neither task correctly |
| 2. Gaze | 0 = normal 1 = partial gaze palsy 2 = total gaze palsy |
| 3. Visual fields | 0 = no visual loss 1 = partial hemianopsia 2 = complete hemianopsia 3 = bilateral hemianopsia |
| 4. Facial palsy | 0 = normal 1 = minor paralysis 2 = partial paralysis 3 = complete paralysis |
| 5. Motor arm a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 6. Motor leg a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 7. Ataxia | 0 = absent 1 = 1 limb 2 = 2 limbs |
| 8. Sensory | 0 = normal 1 = mild loss 2 = severe loss |
| 9. Language | 0 = normal 1 = mild aphasia 2 = severe aphasia 3 = mute or global aphasia |
| 10. Dysarthria | 0 = normal 1 = mild 2 = severe |
| 11. Extinction/inattention | 0 = normal 1 = mild 2 = severe |
| * Yields a score from 0 to 42 (higher numbers indicate worse neurologic function). | |
FIGURE 1
2 patients with common symptoms, vastly different diagnoses
The markedly abnormal perfusion (arrows) this CT image reveals corresponds to an acute occlusion of the left vertebral artery and a subsequent infarct.
An axial postcontrast MRI reveals multiple lesions in the left temporal lobe (arrows) in a patient with rapid-onset mental changes. The diagnosis: cryptococcal meningoencephalitis.
CORRESPONDENCE
Konrad C. Nau, MD, West Virginia University Department of Family Medicine-Eastern Division, 171 Taylor Street, Harpers Ferry, WV 25425; [email protected]
1. Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480-486.
2. Scott PA, Silbergeit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med. 2003;42:611-618.
3. Morgenstern LB, Lisabeth LD, Mecozzi AC, et al. A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62:895-900.
4. Nicol MB, Thrift AG. Knowledge of risk factors and warning signs of stroke. Vasc Health Risk Manag. 2005;1:137-147.
5. Albers GW. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347:1713-1716.
6. Adams HP, del Zoppo G, Alberts MJ, et al. Guidelines for early management of adults with ischemic stroke. Circulation. 2007;115:e478-e534.
7. Rosamond WD, Reeves MJ, Johnson A, et al. Paul Coverdell National Acute Stroke Registry Prototype Investigators. Documentation of stroke onset time: challenges and recommendations. Am J Prev Med. 2006;6(suppl 2):S230-S234.
8. Crocco TJ. Streamlining stroke care: from symptom onset to emergency department. J Emerg Med. 2007;33:255-260.
9. Nor AM, Davis J, Sen B, et al. The recognition of stroke in the emergency room scale: development and validation of a stroke recognition scale. Lancet Neurol. 2005;4:727-734.
10. Practice Advisory: Thrombolytic therapy for acute ischemic stroke-summary statement. Report of the Quality standards subcommittee of the American Academy of Neurology. Neurology. 1996;47:835-839.
11. Libman RB, Wirkowski E, Alvir J, et al. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119-1122.
12. Vroomen P, Buddingh MK, Kuijckx G, et al. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis. 2008;17:418-422.
13. Gallmetzer P, Leutmezer F, Serles W, et al. Postictal paresis in focal epilepsies: incidence, duration, and causes. Neurology. 2004;12:2160-2164.
14. Huff JS. Stroke mimics and chameleons. Emerg Med Clin N Am. 2002;20:583-595.
15. Bousser MG, Welch KM. Relation between migraine and stroke. Lancet Neurol. 2005;4:533-542.
16. Bigal ME, Kurth T, Hu H, et al. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864-1871.
17. Snyder H, Robinson K, Shah D, et al. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med. 1993;11:253-258.
18. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatr. 2002;73:241-245.
19. Phoebe SC, Tobiano PS, Wang HE, et al. Case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286.
20. Chen DK, So YT, Fischer RS. Use of serum prolactin in diagnosing epileptic seizures. Report of the therapeutics and technology subcommittee of the American Academy of Neurology. Neurology. 2005;65:668-675.
21. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640-1658.
22. Kothari RU, Brott T, Broderick JP, et al. Emergency physicians: accuracy in diagnosis of stroke. Stroke. 1995;26:2238-2241.
23. Ay H, Buonanno FS, Rordorf G, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52:1784-1792.
24. Bock BF. Response system for patients presenting with acute stroke. In: Marler JR, Jones PM, Emr M, ed. Proceeding of a National Symposium on Rapid Identification and Treatment of Acute Stroke: 1997. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health; 1997.
25. National Institutes of Health. Know stroke. Available at: http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale_Booklet.pdf. Accessed December 10, 2009.
26. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603-612.
27. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: the Brain Attack Study. Stroke. 2006;36:769-775.
28. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computerized tomography in emergency assessment of patients with suspected acute stroke—a prospective comparison. Lancet. 2007;369:293-298.
29. Kohrmann M, Jüttler E, Huttner HB, et al. Acute stroke imaging for thrombolytic therapy—an update. Cerebrovasc Dis. 2007;24:161-169.
30. Kothari RU, Panciolo A, Liu T, et al. Cincinnati prehospital stroke scale: reproducibility and validity. Ann Emerg Med. 1999;33:373-378.
31. Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71-76.
32. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field: prospective validation of the Los Angeles prehospital stroke screen (LAPPS). Stroke. 2000;31:71-76.
• Arrange for urgent transport to the hospital when a patient presents with stroke-like symptoms of acute onset, especially within the 3- to 6-hour therapeutic window. B
• Use a validated prehospital stroke identification algorithm such as the Face Arms Speech Time (FAST) test to identify possible acute stroke patients requiring urgent transport to the hospital. B
• Obtain a CBC and basic metabolic panel for all patients with signs and symptoms suggestive of stroke—and a blood alcohol, hepatic function, and toxicology screen, in select patients—to help rule out stroke mimics. C
• Ensure that patients undergo brain imaging to rule out stroke mimics before treatment for acute ischemic stroke is initiated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke is the third leading cause of death (claiming the life of 1 person every 3 to 4 minutes) and the No. 1 cause of adult disability in the United States.1 Advances in thrombolysis and clot removal can improve outcomes, but are dependent on swift and certain diagnosis. Amid the rush to ensure that treatment is initiated within the therapeutic window for cerebral reperfusion, “stroke mimics”—so called because of their ability to cause signs and symptoms similar to stroke—are sometimes mistaken for the real thing.
The prevalence of misdiagnosis ranges from about 4% of patients who receive tissue plasminogen activator (tPA) for reperfusion2 to 25% of patients who are rushed to the hospital because they are thought to be having a stroke.3 Seizures, migraine, sepsis, and peripheral vestibular disorders are among the many conditions that can masquerade as stroke.
Misdiagnosis can subject patients to unnecessary, and potentially harmful, invasive stroke therapies, and significantly delay the treatment they need. To prevent such outcomes, it is essential for primary care physicians, as well as emergency responders and emergency department (ED) physicians, to be aware of—and on the lookout for—the clues that can distinguish stroke from stroke mimics.
Suspect stroke? Establish a baseline
Despite a nationwide effort to increase public awareness of stroke and the importance of getting to the hospital within the therapeutic window for treatment,4 too few patients arrive within the time frame for cerebral reperfusion therapy. Primary care physicians can help by educating patients about stroke signs and symptoms.
When a patient presents with possible stroke, determine whether symptoms began gradually or abruptly. An acute ischemic stroke is heralded by the sudden onset of a focal neurologic deficit in a vascular pattern. Duration of symptoms can help distinguish stroke from a transient ischemic attack (TIA). Although TIAs were defined by the National Institutes of Health in 1975 as neurologic deficits that resolve within 24 hours of their onset, we now know that they typically last only 2 to 15 minutes, with the vast majority resolving within an hour.5
If the onset was sudden, find out when the patient was last seen at his or her neurologic baseline—information a family member, friend, or caregiver can often provide. This information is crucial because the neurologic baseline, rather than the time at which the symptoms were first noticed, is the basis for the therapeutic window for thrombolysis (3 hours for intravenous tPA and 6 hours for intra-arterial tPA). (Clot extraction with a mechanical embolus retrieval device [MERCI, Concentric Medical, Mountain View, Calif] has a 9-hour window.6,7)
Use a rapid stroke screening tool. To rapidly evaluate a patient with stroke-like signs and symptoms in a clinic or other outpatient setting, use a stroke screening tool with a high sensitivity,8 such as the Cincinnati Prehospital Stroke Scale (CPSS), the Face Arms Speech Time (FAST) test, or the Los Angeles Prehospital Stroke Screen (LAPSS) (TABLE 1). All 3 have a high positive predictive value (CPSS: 88%, FAST: 89%, LAPSS: 87%), but there is greater variation in the negative predictive value: 75%, 73%, and 55%, respectively.9
Patients with positive results typically require rapid transport to the ED—even if you notice red flags that may signal that you’re dealing with a stroke mimic.
TABLE 1
Stroke screening tools for outpatient use*30-32
| Cincinnati Prehospital Stroke Scale (CPSS) (www.strokecenter.org/trials/scales/cincinnati.html), which assesses the unilateral presence of any (or all) of the 3 key indicators—facial droop, arm drift, or slurred speech |
| Face Arms Speech Time (FAST) (www.stroke.org/site/PageServer?pagename=symp), a modification of CPSS based on the same criteria, has been validated in primary care clinics as well as emergency departments |
| Los Angeles Prehospital Stroke Screen (LAPSS) (www.strokecenter.org/trials/scales/lapss.html), a 1-page instrument that uses 5 criteria—age (>45 years), seizure history (none), onset of neurologic symptoms (within 24 hours), ambulatory status (ambulatory prior to event), and blood glucose level (60-400 mg/dL)—and 3 physical characteristics (facial smile/grimace, grip, and arm weakness) to screen for possible stroke |
| * A positive test is based on the presence of 1 or more key features for CPSS or FAST, and on a Yes (or Unknown) response to all the screening criteria in LAPSS. |
Be alert to the signs of conditions masquerading as stroke
Seizure at the onset of the episode; isolated mild neurological deficits, such as ataxia, sensory loss, or dysarthria alone; and/or minimal weakness are contraindications to thrombolytic therapy, according to the American Academy of Neurology (AAN).10
Rapidly improving neurological status is a probable indicator of a TIA or nonstroke etiology. Decreased level of consciousness with normal eye movements increases the likelihood that the patient has a condition that mimics stroke.11 Additional symptoms that strongly suggest a disorder other than stroke are convulsions (odds ratio [OR]: 0.1), loss of consciousness (OR: 0.1), confusion (OR: 0.2), headache (OR: 0.8), nausea (OR: 0.5), vomiting (OR: 0.6), and dizziness (OR: 0.3).9
Age is another consideration. The vast majority of patients with conditions that turn out to be stroke mimics are younger than 50 years of age. In patients older than 50, the prevalence of stroke misdiagnosis is just 3%.12
Watch for these stroke mimics
Seizures, either unwitnessed or unrecognized, and complex migraine are the most common stroke masqueraders. Other conditions frequently misdiagnosed as stroke include: systemic infections and early sepsis, central nervous system (CNS) tumors, and toxic-metabolic syndromes (including intoxication, hypoglycemia, hypercalcemia, and hyperosmolar nonketotic coma) (TABLE 2). Patients with cranial or peripheral neuropathy; dementia; labyrinthitis/benign positional vertigo; psychiatric disorders, in particular, conversion reaction; syncope; and transient global amnesia may also present with neurological symptoms suggestive of stroke. (For more on transient global amnesia, see this month’s Hospitalist Rounds at http://www.jfponline.com/CollectionContent.asp?CollectionID=286.) Characteristics of some of the more common mimics are detailed below.
Seizures. Neurologic deficits associated with seizures are reversible, with no structural CNS abnormalities. Postictal hemiparesis, also known as Todd’s paralysis—a focal weakness after a seizure, typically localized to 1 side of the body—occurs in approximately 13% of all seizures.13 Todd’s paralysis, which can be seen after either partial complex or generalized tonic-clonic seizures, may also affect speech and vision, producing a range of signs and symptoms easily mistaken for stroke. Duration ranges from minutes to 48 hours,14 but generally lasts only 3 to 22 minutes.13
Differentiating Todd’s paralysis from stroke is complicated by the fact that some strokes trigger focal seizures during the acute phase. However, a history of seizures or witnessed seizure activity points to Todd’s paralysis rather than stroke.
Complex migraine. Like Todd’s paralysis, complex migraine may result in hemiparesis. The presentation may also include vision loss, aphasia, or vertigo and other basilar symptoms—neurologic changes that can outlast the headache. Complex migraine is a diagnosis of exclusion, arrived at after a full neurologic assessment, including stroke work-up. Indeed, you can be certain of a diagnosis of complex migraine only after the patient has had recurrent complex migraine attacks.
Some basilar TIAs can also present with headache, but the onset is typically sudden, as opposed to the more gradual onset of migraine aura with posterior circulation symptoms.15 Age is a factor as well: Complex migraines usually develop well before the age of 40, while the mean age for ischemic stroke is 70. Although complex migraine is a risk factor for ischemic stroke, in most patients migraine is a benign condition.15,16
Systemic infections. Sepsis from almost any infectious agent can cause delirium, altered speech, weakness, and less specific stroke-like symptoms. Microbial seeding of the CNS can result in focal lesions (eg, the lesions shown in FIGURE 1B are associated with cryptococcal meningoencephalitis) or abscess formation with focal neurologic deficits.
Mass lesions. Primary CNS tumors, metastatic tumors, and cerebral abscesses are among the lesions that can cause symptoms that mimic stroke. In most cases, symptoms develop gradually as the lesion enlarges, but a small subset of patients have symptoms lasting less than 1 day. This is thought to be due to hemorrhage into the tumor or the acute development of obstructive hydrocephalus.17
Metabolic disorders. Diabetic hypoglycemia, among other metabolic disorders, is a classic stroke mimic, as well as a cause of seizures, so early evaluation of blood glucose is a crucial step in evaluating a patient with neurologic signs and symptoms. Patients with diabetic hypoglycemia may present with hemiplegia and aphasia; similar symptoms may occur in patients with hypoglycemia secondary to alcoholism, among other causes. Those with hyperglycemic nonketotic hyperosmolar states, severe hyponatremia, and hepatic encephalopathy may also present with focal stroke-like symptoms. Neurologic changes associated with metabolic disorders generally resolve rapidly with the administration of IV glucose, but on rare occasions may take several hours to resolve.14
Psychiatric illness. Patients with certain psychiatric disorders—including conversion reaction, a psychological condition that presents as an alteration in, or loss of, physical function—may present with dramatic focal problems and apparent deficits that mimic neurologic disease. Subtle disparities in the physical exam, such as Hoover’s sign, give-away weakness,18 and “la belle indifference,” as well as negative neuroimaging, will establish this difficult-to-treat stroke mimic.19 Grand mal pseudo-seizures can be differentiated from actual grand mal seizures by the failure of a prolactin level (drawn 10 to 20 minutes post-event) to rise at least 2-fold.20
Transient global amnesia. The rare, sudden development of dense anterograde amnesia occurs without alteration in level of consciousness, focal neurologic deficits, or seizure activity. It is self-limiting and mainly affects those older than 50. Transient global amnesia has an uncertain etiology, although atypical migraine, seizure discharge, and venous congestion with hippocampal ischemia are viewed as possible causes. Reported triggers include severe physical or emotional stress, strenuous physical activity, and orgasmic sexual intercourse.21
TABLE 2
Common stroke mimics9,11,12,14,22
| Condition | Misdiagnosed as stroke (%) |
|---|---|
| Brain tumor | 7-15 |
| Labyrinthitis | 5-6 |
| Metabolic disorder | 3-13 |
| Migraine | 11-47 |
| Psychiatric disorder | 1-40 |
| Seizures | 11-40 |
| Sepsis | 14-17 |
| Syncope | 5-22 |
| Transient global amnesia | 3-10 |
| Other | 11-37 |
In the ED: Evaluation is guided by a timeline
Current guidelines from the American Heart Association and American Stroke Association recommend that a possible stroke patient be evaluated by the physician in the ED within 10 minutes of his or her arrival—and that a decision on how to proceed be reached within 60 minutes of arrival. The guidelines call for the initial computed tomography (CT) to be completed within 25 minutes of the patient’s arrival and interpreted by a physician with expertise in reading CT studies within 45 minutes of arrival.6,24
In the ED, the National Institutes of Health Stroke Scale (NIHSS)25 (TABLE 3) is an ideal way to focus and record the neurological exam.6 The scale assesses 6 separate neurologic functions (level of consciousness, vision, motor function, sensory function, language, and cerebellar function) and can be performed within 5 to 8 minutes. It yields a score from 0 to 42, with the higher numbers indicating worse neurologic function.26 Although a score ≤10 is generally considered to be predictive of a stroke mimic, a recent study found that 19% of patients with an NIHSS score >10 also had conditions masquerading as stroke.27
Imaging leads to accurate diagnosis. The rate at which stroke mimics are mistaken for actual strokes varies with the population studied and the diagnostic tests performed. While stroke is largely a clinical diagnosis and a history and physical exam focused on onset, duration, and symptoms are key elements in differentiating stroke from a stroke mimic, studies have found that the incidence of misdiagnosis (19% with history, physical, and lab work alone) drops to 5% when noncontrast CT is added. When diffusion-weighted magnetic resonance imaging (MRI) is used instead, misdiagnosis drops to just 2%.11,12,14,22,23
Basic lab tests—a complete blood count and basic metabolic panel, with blood alcohol, hepatic function tests, and toxicology screens in select cases—help rule out stroke mimics. Radiographic imaging of the brain provides further clarification (FIGURE 1A AND 1B), serving 2 main purposes: to (1) evaluate diagnoses other than stroke and (2) identify the presence of any acute intracranial bleeding. Noncontrast CT scans detect acute hemorrhage with a sensitivity of 89% and specificity of 100%.27 CT angiography (which can identify the location of a clot) and CT perfusion (which allows an assessment of any existing penumbra) can also be obtained in a timely fashion with newer multislice scanners.
Some institutions, however, evaluate acute stroke patients with MRI. Depending on the sequences used, MRI has the advantage of being able to detect early ischemic changes, diffusion and perfusion mismatches, and abnormalities of the posterior fossa.29 In acute ischemic stroke, diffusion-weighted MRI has a sensitivity of 83% and specificity of 96%, compared with a sensitivity of 16% and specificity of 98% for noncontrast CT.28
TABLE 3
National Institutes of Health Stroke Scale25
| Item | Response score* |
|---|---|
| 1a. Level of consciousness | 0 = alert 1 = not alert 2 = obtunded 3 = unresponsive |
| 1b. Level of consciousness Questions | 0 = answers both correctly 1 = answers one correctly 2 = answers neither correctly |
| 1c. Level of consciousness Commands | 0 = performs both tasks correctly 1 = performs one task correctly 2 = performs neither task correctly |
| 2. Gaze | 0 = normal 1 = partial gaze palsy 2 = total gaze palsy |
| 3. Visual fields | 0 = no visual loss 1 = partial hemianopsia 2 = complete hemianopsia 3 = bilateral hemianopsia |
| 4. Facial palsy | 0 = normal 1 = minor paralysis 2 = partial paralysis 3 = complete paralysis |
| 5. Motor arm a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 6. Motor leg a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 7. Ataxia | 0 = absent 1 = 1 limb 2 = 2 limbs |
| 8. Sensory | 0 = normal 1 = mild loss 2 = severe loss |
| 9. Language | 0 = normal 1 = mild aphasia 2 = severe aphasia 3 = mute or global aphasia |
| 10. Dysarthria | 0 = normal 1 = mild 2 = severe |
| 11. Extinction/inattention | 0 = normal 1 = mild 2 = severe |
| * Yields a score from 0 to 42 (higher numbers indicate worse neurologic function). | |
FIGURE 1
2 patients with common symptoms, vastly different diagnoses
The markedly abnormal perfusion (arrows) this CT image reveals corresponds to an acute occlusion of the left vertebral artery and a subsequent infarct.
An axial postcontrast MRI reveals multiple lesions in the left temporal lobe (arrows) in a patient with rapid-onset mental changes. The diagnosis: cryptococcal meningoencephalitis.
CORRESPONDENCE
Konrad C. Nau, MD, West Virginia University Department of Family Medicine-Eastern Division, 171 Taylor Street, Harpers Ferry, WV 25425; [email protected]
• Arrange for urgent transport to the hospital when a patient presents with stroke-like symptoms of acute onset, especially within the 3- to 6-hour therapeutic window. B
• Use a validated prehospital stroke identification algorithm such as the Face Arms Speech Time (FAST) test to identify possible acute stroke patients requiring urgent transport to the hospital. B
• Obtain a CBC and basic metabolic panel for all patients with signs and symptoms suggestive of stroke—and a blood alcohol, hepatic function, and toxicology screen, in select patients—to help rule out stroke mimics. C
• Ensure that patients undergo brain imaging to rule out stroke mimics before treatment for acute ischemic stroke is initiated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke is the third leading cause of death (claiming the life of 1 person every 3 to 4 minutes) and the No. 1 cause of adult disability in the United States.1 Advances in thrombolysis and clot removal can improve outcomes, but are dependent on swift and certain diagnosis. Amid the rush to ensure that treatment is initiated within the therapeutic window for cerebral reperfusion, “stroke mimics”—so called because of their ability to cause signs and symptoms similar to stroke—are sometimes mistaken for the real thing.
The prevalence of misdiagnosis ranges from about 4% of patients who receive tissue plasminogen activator (tPA) for reperfusion2 to 25% of patients who are rushed to the hospital because they are thought to be having a stroke.3 Seizures, migraine, sepsis, and peripheral vestibular disorders are among the many conditions that can masquerade as stroke.
Misdiagnosis can subject patients to unnecessary, and potentially harmful, invasive stroke therapies, and significantly delay the treatment they need. To prevent such outcomes, it is essential for primary care physicians, as well as emergency responders and emergency department (ED) physicians, to be aware of—and on the lookout for—the clues that can distinguish stroke from stroke mimics.
Suspect stroke? Establish a baseline
Despite a nationwide effort to increase public awareness of stroke and the importance of getting to the hospital within the therapeutic window for treatment,4 too few patients arrive within the time frame for cerebral reperfusion therapy. Primary care physicians can help by educating patients about stroke signs and symptoms.
When a patient presents with possible stroke, determine whether symptoms began gradually or abruptly. An acute ischemic stroke is heralded by the sudden onset of a focal neurologic deficit in a vascular pattern. Duration of symptoms can help distinguish stroke from a transient ischemic attack (TIA). Although TIAs were defined by the National Institutes of Health in 1975 as neurologic deficits that resolve within 24 hours of their onset, we now know that they typically last only 2 to 15 minutes, with the vast majority resolving within an hour.5
If the onset was sudden, find out when the patient was last seen at his or her neurologic baseline—information a family member, friend, or caregiver can often provide. This information is crucial because the neurologic baseline, rather than the time at which the symptoms were first noticed, is the basis for the therapeutic window for thrombolysis (3 hours for intravenous tPA and 6 hours for intra-arterial tPA). (Clot extraction with a mechanical embolus retrieval device [MERCI, Concentric Medical, Mountain View, Calif] has a 9-hour window.6,7)
Use a rapid stroke screening tool. To rapidly evaluate a patient with stroke-like signs and symptoms in a clinic or other outpatient setting, use a stroke screening tool with a high sensitivity,8 such as the Cincinnati Prehospital Stroke Scale (CPSS), the Face Arms Speech Time (FAST) test, or the Los Angeles Prehospital Stroke Screen (LAPSS) (TABLE 1). All 3 have a high positive predictive value (CPSS: 88%, FAST: 89%, LAPSS: 87%), but there is greater variation in the negative predictive value: 75%, 73%, and 55%, respectively.9
Patients with positive results typically require rapid transport to the ED—even if you notice red flags that may signal that you’re dealing with a stroke mimic.
TABLE 1
Stroke screening tools for outpatient use*30-32
| Cincinnati Prehospital Stroke Scale (CPSS) (www.strokecenter.org/trials/scales/cincinnati.html), which assesses the unilateral presence of any (or all) of the 3 key indicators—facial droop, arm drift, or slurred speech |
| Face Arms Speech Time (FAST) (www.stroke.org/site/PageServer?pagename=symp), a modification of CPSS based on the same criteria, has been validated in primary care clinics as well as emergency departments |
| Los Angeles Prehospital Stroke Screen (LAPSS) (www.strokecenter.org/trials/scales/lapss.html), a 1-page instrument that uses 5 criteria—age (>45 years), seizure history (none), onset of neurologic symptoms (within 24 hours), ambulatory status (ambulatory prior to event), and blood glucose level (60-400 mg/dL)—and 3 physical characteristics (facial smile/grimace, grip, and arm weakness) to screen for possible stroke |
| * A positive test is based on the presence of 1 or more key features for CPSS or FAST, and on a Yes (or Unknown) response to all the screening criteria in LAPSS. |
Be alert to the signs of conditions masquerading as stroke
Seizure at the onset of the episode; isolated mild neurological deficits, such as ataxia, sensory loss, or dysarthria alone; and/or minimal weakness are contraindications to thrombolytic therapy, according to the American Academy of Neurology (AAN).10
Rapidly improving neurological status is a probable indicator of a TIA or nonstroke etiology. Decreased level of consciousness with normal eye movements increases the likelihood that the patient has a condition that mimics stroke.11 Additional symptoms that strongly suggest a disorder other than stroke are convulsions (odds ratio [OR]: 0.1), loss of consciousness (OR: 0.1), confusion (OR: 0.2), headache (OR: 0.8), nausea (OR: 0.5), vomiting (OR: 0.6), and dizziness (OR: 0.3).9
Age is another consideration. The vast majority of patients with conditions that turn out to be stroke mimics are younger than 50 years of age. In patients older than 50, the prevalence of stroke misdiagnosis is just 3%.12
Watch for these stroke mimics
Seizures, either unwitnessed or unrecognized, and complex migraine are the most common stroke masqueraders. Other conditions frequently misdiagnosed as stroke include: systemic infections and early sepsis, central nervous system (CNS) tumors, and toxic-metabolic syndromes (including intoxication, hypoglycemia, hypercalcemia, and hyperosmolar nonketotic coma) (TABLE 2). Patients with cranial or peripheral neuropathy; dementia; labyrinthitis/benign positional vertigo; psychiatric disorders, in particular, conversion reaction; syncope; and transient global amnesia may also present with neurological symptoms suggestive of stroke. (For more on transient global amnesia, see this month’s Hospitalist Rounds at http://www.jfponline.com/CollectionContent.asp?CollectionID=286.) Characteristics of some of the more common mimics are detailed below.
Seizures. Neurologic deficits associated with seizures are reversible, with no structural CNS abnormalities. Postictal hemiparesis, also known as Todd’s paralysis—a focal weakness after a seizure, typically localized to 1 side of the body—occurs in approximately 13% of all seizures.13 Todd’s paralysis, which can be seen after either partial complex or generalized tonic-clonic seizures, may also affect speech and vision, producing a range of signs and symptoms easily mistaken for stroke. Duration ranges from minutes to 48 hours,14 but generally lasts only 3 to 22 minutes.13
Differentiating Todd’s paralysis from stroke is complicated by the fact that some strokes trigger focal seizures during the acute phase. However, a history of seizures or witnessed seizure activity points to Todd’s paralysis rather than stroke.
Complex migraine. Like Todd’s paralysis, complex migraine may result in hemiparesis. The presentation may also include vision loss, aphasia, or vertigo and other basilar symptoms—neurologic changes that can outlast the headache. Complex migraine is a diagnosis of exclusion, arrived at after a full neurologic assessment, including stroke work-up. Indeed, you can be certain of a diagnosis of complex migraine only after the patient has had recurrent complex migraine attacks.
Some basilar TIAs can also present with headache, but the onset is typically sudden, as opposed to the more gradual onset of migraine aura with posterior circulation symptoms.15 Age is a factor as well: Complex migraines usually develop well before the age of 40, while the mean age for ischemic stroke is 70. Although complex migraine is a risk factor for ischemic stroke, in most patients migraine is a benign condition.15,16
Systemic infections. Sepsis from almost any infectious agent can cause delirium, altered speech, weakness, and less specific stroke-like symptoms. Microbial seeding of the CNS can result in focal lesions (eg, the lesions shown in FIGURE 1B are associated with cryptococcal meningoencephalitis) or abscess formation with focal neurologic deficits.
Mass lesions. Primary CNS tumors, metastatic tumors, and cerebral abscesses are among the lesions that can cause symptoms that mimic stroke. In most cases, symptoms develop gradually as the lesion enlarges, but a small subset of patients have symptoms lasting less than 1 day. This is thought to be due to hemorrhage into the tumor or the acute development of obstructive hydrocephalus.17
Metabolic disorders. Diabetic hypoglycemia, among other metabolic disorders, is a classic stroke mimic, as well as a cause of seizures, so early evaluation of blood glucose is a crucial step in evaluating a patient with neurologic signs and symptoms. Patients with diabetic hypoglycemia may present with hemiplegia and aphasia; similar symptoms may occur in patients with hypoglycemia secondary to alcoholism, among other causes. Those with hyperglycemic nonketotic hyperosmolar states, severe hyponatremia, and hepatic encephalopathy may also present with focal stroke-like symptoms. Neurologic changes associated with metabolic disorders generally resolve rapidly with the administration of IV glucose, but on rare occasions may take several hours to resolve.14
Psychiatric illness. Patients with certain psychiatric disorders—including conversion reaction, a psychological condition that presents as an alteration in, or loss of, physical function—may present with dramatic focal problems and apparent deficits that mimic neurologic disease. Subtle disparities in the physical exam, such as Hoover’s sign, give-away weakness,18 and “la belle indifference,” as well as negative neuroimaging, will establish this difficult-to-treat stroke mimic.19 Grand mal pseudo-seizures can be differentiated from actual grand mal seizures by the failure of a prolactin level (drawn 10 to 20 minutes post-event) to rise at least 2-fold.20
Transient global amnesia. The rare, sudden development of dense anterograde amnesia occurs without alteration in level of consciousness, focal neurologic deficits, or seizure activity. It is self-limiting and mainly affects those older than 50. Transient global amnesia has an uncertain etiology, although atypical migraine, seizure discharge, and venous congestion with hippocampal ischemia are viewed as possible causes. Reported triggers include severe physical or emotional stress, strenuous physical activity, and orgasmic sexual intercourse.21
TABLE 2
Common stroke mimics9,11,12,14,22
| Condition | Misdiagnosed as stroke (%) |
|---|---|
| Brain tumor | 7-15 |
| Labyrinthitis | 5-6 |
| Metabolic disorder | 3-13 |
| Migraine | 11-47 |
| Psychiatric disorder | 1-40 |
| Seizures | 11-40 |
| Sepsis | 14-17 |
| Syncope | 5-22 |
| Transient global amnesia | 3-10 |
| Other | 11-37 |
In the ED: Evaluation is guided by a timeline
Current guidelines from the American Heart Association and American Stroke Association recommend that a possible stroke patient be evaluated by the physician in the ED within 10 minutes of his or her arrival—and that a decision on how to proceed be reached within 60 minutes of arrival. The guidelines call for the initial computed tomography (CT) to be completed within 25 minutes of the patient’s arrival and interpreted by a physician with expertise in reading CT studies within 45 minutes of arrival.6,24
In the ED, the National Institutes of Health Stroke Scale (NIHSS)25 (TABLE 3) is an ideal way to focus and record the neurological exam.6 The scale assesses 6 separate neurologic functions (level of consciousness, vision, motor function, sensory function, language, and cerebellar function) and can be performed within 5 to 8 minutes. It yields a score from 0 to 42, with the higher numbers indicating worse neurologic function.26 Although a score ≤10 is generally considered to be predictive of a stroke mimic, a recent study found that 19% of patients with an NIHSS score >10 also had conditions masquerading as stroke.27
Imaging leads to accurate diagnosis. The rate at which stroke mimics are mistaken for actual strokes varies with the population studied and the diagnostic tests performed. While stroke is largely a clinical diagnosis and a history and physical exam focused on onset, duration, and symptoms are key elements in differentiating stroke from a stroke mimic, studies have found that the incidence of misdiagnosis (19% with history, physical, and lab work alone) drops to 5% when noncontrast CT is added. When diffusion-weighted magnetic resonance imaging (MRI) is used instead, misdiagnosis drops to just 2%.11,12,14,22,23
Basic lab tests—a complete blood count and basic metabolic panel, with blood alcohol, hepatic function tests, and toxicology screens in select cases—help rule out stroke mimics. Radiographic imaging of the brain provides further clarification (FIGURE 1A AND 1B), serving 2 main purposes: to (1) evaluate diagnoses other than stroke and (2) identify the presence of any acute intracranial bleeding. Noncontrast CT scans detect acute hemorrhage with a sensitivity of 89% and specificity of 100%.27 CT angiography (which can identify the location of a clot) and CT perfusion (which allows an assessment of any existing penumbra) can also be obtained in a timely fashion with newer multislice scanners.
Some institutions, however, evaluate acute stroke patients with MRI. Depending on the sequences used, MRI has the advantage of being able to detect early ischemic changes, diffusion and perfusion mismatches, and abnormalities of the posterior fossa.29 In acute ischemic stroke, diffusion-weighted MRI has a sensitivity of 83% and specificity of 96%, compared with a sensitivity of 16% and specificity of 98% for noncontrast CT.28
TABLE 3
National Institutes of Health Stroke Scale25
| Item | Response score* |
|---|---|
| 1a. Level of consciousness | 0 = alert 1 = not alert 2 = obtunded 3 = unresponsive |
| 1b. Level of consciousness Questions | 0 = answers both correctly 1 = answers one correctly 2 = answers neither correctly |
| 1c. Level of consciousness Commands | 0 = performs both tasks correctly 1 = performs one task correctly 2 = performs neither task correctly |
| 2. Gaze | 0 = normal 1 = partial gaze palsy 2 = total gaze palsy |
| 3. Visual fields | 0 = no visual loss 1 = partial hemianopsia 2 = complete hemianopsia 3 = bilateral hemianopsia |
| 4. Facial palsy | 0 = normal 1 = minor paralysis 2 = partial paralysis 3 = complete paralysis |
| 5. Motor arm a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 6. Motor leg a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 7. Ataxia | 0 = absent 1 = 1 limb 2 = 2 limbs |
| 8. Sensory | 0 = normal 1 = mild loss 2 = severe loss |
| 9. Language | 0 = normal 1 = mild aphasia 2 = severe aphasia 3 = mute or global aphasia |
| 10. Dysarthria | 0 = normal 1 = mild 2 = severe |
| 11. Extinction/inattention | 0 = normal 1 = mild 2 = severe |
| * Yields a score from 0 to 42 (higher numbers indicate worse neurologic function). | |
FIGURE 1
2 patients with common symptoms, vastly different diagnoses
The markedly abnormal perfusion (arrows) this CT image reveals corresponds to an acute occlusion of the left vertebral artery and a subsequent infarct.
An axial postcontrast MRI reveals multiple lesions in the left temporal lobe (arrows) in a patient with rapid-onset mental changes. The diagnosis: cryptococcal meningoencephalitis.
CORRESPONDENCE
Konrad C. Nau, MD, West Virginia University Department of Family Medicine-Eastern Division, 171 Taylor Street, Harpers Ferry, WV 25425; [email protected]
1. Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480-486.
2. Scott PA, Silbergeit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med. 2003;42:611-618.
3. Morgenstern LB, Lisabeth LD, Mecozzi AC, et al. A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62:895-900.
4. Nicol MB, Thrift AG. Knowledge of risk factors and warning signs of stroke. Vasc Health Risk Manag. 2005;1:137-147.
5. Albers GW. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347:1713-1716.
6. Adams HP, del Zoppo G, Alberts MJ, et al. Guidelines for early management of adults with ischemic stroke. Circulation. 2007;115:e478-e534.
7. Rosamond WD, Reeves MJ, Johnson A, et al. Paul Coverdell National Acute Stroke Registry Prototype Investigators. Documentation of stroke onset time: challenges and recommendations. Am J Prev Med. 2006;6(suppl 2):S230-S234.
8. Crocco TJ. Streamlining stroke care: from symptom onset to emergency department. J Emerg Med. 2007;33:255-260.
9. Nor AM, Davis J, Sen B, et al. The recognition of stroke in the emergency room scale: development and validation of a stroke recognition scale. Lancet Neurol. 2005;4:727-734.
10. Practice Advisory: Thrombolytic therapy for acute ischemic stroke-summary statement. Report of the Quality standards subcommittee of the American Academy of Neurology. Neurology. 1996;47:835-839.
11. Libman RB, Wirkowski E, Alvir J, et al. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119-1122.
12. Vroomen P, Buddingh MK, Kuijckx G, et al. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis. 2008;17:418-422.
13. Gallmetzer P, Leutmezer F, Serles W, et al. Postictal paresis in focal epilepsies: incidence, duration, and causes. Neurology. 2004;12:2160-2164.
14. Huff JS. Stroke mimics and chameleons. Emerg Med Clin N Am. 2002;20:583-595.
15. Bousser MG, Welch KM. Relation between migraine and stroke. Lancet Neurol. 2005;4:533-542.
16. Bigal ME, Kurth T, Hu H, et al. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864-1871.
17. Snyder H, Robinson K, Shah D, et al. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med. 1993;11:253-258.
18. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatr. 2002;73:241-245.
19. Phoebe SC, Tobiano PS, Wang HE, et al. Case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286.
20. Chen DK, So YT, Fischer RS. Use of serum prolactin in diagnosing epileptic seizures. Report of the therapeutics and technology subcommittee of the American Academy of Neurology. Neurology. 2005;65:668-675.
21. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640-1658.
22. Kothari RU, Brott T, Broderick JP, et al. Emergency physicians: accuracy in diagnosis of stroke. Stroke. 1995;26:2238-2241.
23. Ay H, Buonanno FS, Rordorf G, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52:1784-1792.
24. Bock BF. Response system for patients presenting with acute stroke. In: Marler JR, Jones PM, Emr M, ed. Proceeding of a National Symposium on Rapid Identification and Treatment of Acute Stroke: 1997. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health; 1997.
25. National Institutes of Health. Know stroke. Available at: http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale_Booklet.pdf. Accessed December 10, 2009.
26. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603-612.
27. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: the Brain Attack Study. Stroke. 2006;36:769-775.
28. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computerized tomography in emergency assessment of patients with suspected acute stroke—a prospective comparison. Lancet. 2007;369:293-298.
29. Kohrmann M, Jüttler E, Huttner HB, et al. Acute stroke imaging for thrombolytic therapy—an update. Cerebrovasc Dis. 2007;24:161-169.
30. Kothari RU, Panciolo A, Liu T, et al. Cincinnati prehospital stroke scale: reproducibility and validity. Ann Emerg Med. 1999;33:373-378.
31. Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71-76.
32. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field: prospective validation of the Los Angeles prehospital stroke screen (LAPPS). Stroke. 2000;31:71-76.
1. Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480-486.
2. Scott PA, Silbergeit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med. 2003;42:611-618.
3. Morgenstern LB, Lisabeth LD, Mecozzi AC, et al. A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62:895-900.
4. Nicol MB, Thrift AG. Knowledge of risk factors and warning signs of stroke. Vasc Health Risk Manag. 2005;1:137-147.
5. Albers GW. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347:1713-1716.
6. Adams HP, del Zoppo G, Alberts MJ, et al. Guidelines for early management of adults with ischemic stroke. Circulation. 2007;115:e478-e534.
7. Rosamond WD, Reeves MJ, Johnson A, et al. Paul Coverdell National Acute Stroke Registry Prototype Investigators. Documentation of stroke onset time: challenges and recommendations. Am J Prev Med. 2006;6(suppl 2):S230-S234.
8. Crocco TJ. Streamlining stroke care: from symptom onset to emergency department. J Emerg Med. 2007;33:255-260.
9. Nor AM, Davis J, Sen B, et al. The recognition of stroke in the emergency room scale: development and validation of a stroke recognition scale. Lancet Neurol. 2005;4:727-734.
10. Practice Advisory: Thrombolytic therapy for acute ischemic stroke-summary statement. Report of the Quality standards subcommittee of the American Academy of Neurology. Neurology. 1996;47:835-839.
11. Libman RB, Wirkowski E, Alvir J, et al. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119-1122.
12. Vroomen P, Buddingh MK, Kuijckx G, et al. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis. 2008;17:418-422.
13. Gallmetzer P, Leutmezer F, Serles W, et al. Postictal paresis in focal epilepsies: incidence, duration, and causes. Neurology. 2004;12:2160-2164.
14. Huff JS. Stroke mimics and chameleons. Emerg Med Clin N Am. 2002;20:583-595.
15. Bousser MG, Welch KM. Relation between migraine and stroke. Lancet Neurol. 2005;4:533-542.
16. Bigal ME, Kurth T, Hu H, et al. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864-1871.
17. Snyder H, Robinson K, Shah D, et al. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med. 1993;11:253-258.
18. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatr. 2002;73:241-245.
19. Phoebe SC, Tobiano PS, Wang HE, et al. Case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286.
20. Chen DK, So YT, Fischer RS. Use of serum prolactin in diagnosing epileptic seizures. Report of the therapeutics and technology subcommittee of the American Academy of Neurology. Neurology. 2005;65:668-675.
21. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640-1658.
22. Kothari RU, Brott T, Broderick JP, et al. Emergency physicians: accuracy in diagnosis of stroke. Stroke. 1995;26:2238-2241.
23. Ay H, Buonanno FS, Rordorf G, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52:1784-1792.
24. Bock BF. Response system for patients presenting with acute stroke. In: Marler JR, Jones PM, Emr M, ed. Proceeding of a National Symposium on Rapid Identification and Treatment of Acute Stroke: 1997. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health; 1997.
25. National Institutes of Health. Know stroke. Available at: http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale_Booklet.pdf. Accessed December 10, 2009.
26. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603-612.
27. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: the Brain Attack Study. Stroke. 2006;36:769-775.
28. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computerized tomography in emergency assessment of patients with suspected acute stroke—a prospective comparison. Lancet. 2007;369:293-298.
29. Kohrmann M, Jüttler E, Huttner HB, et al. Acute stroke imaging for thrombolytic therapy—an update. Cerebrovasc Dis. 2007;24:161-169.
30. Kothari RU, Panciolo A, Liu T, et al. Cincinnati prehospital stroke scale: reproducibility and validity. Ann Emerg Med. 1999;33:373-378.
31. Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71-76.
32. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field: prospective validation of the Los Angeles prehospital stroke screen (LAPPS). Stroke. 2000;31:71-76.
Anemia and chronic kidney disease: What’s the connection?
• Evaluate for chronic kidney disease (CKD) anemia when a patient has a serum creatinine ≥2 mg/dL and hemoglobin <12 g/dL (adult males and postmenopausal females) or <11 g/dL (premenopausal females). A
• Before you treat CKD anemia, correct any underlying iron deficiency. A
• Start anemia therapy with erythropoietin-stimulating agents when hemoglobin is ≤10 g/dL, and maintain target hemoglobin levels between 11 and 12 g/dL, in accordance with National Kidney Foundation guidelines. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Mary J, a 65-year-old woman with stage 3 chronic kidney disease (CKD), is in your office for a follow-up appointment. Over the past 6 months, she has noticed a decrease in her energy level. On her routine blood work, you see that her hemoglobin has been slowly declining over the past year. It is now 9 g/dL and her estimated glomerular filtration rate (GFR) is 40 mL/min.
How would you evaluate Mary’s anemia, and would you suspect that it was related to her CKD?
Most physicians are aware that CKD—which affects approximately 10% of the US population1—has a deleterious effect on cardiovascular disease, but many fail to recognize the impact it has on the hematopoietic system. Managing the anemia that accompanies CKD in patients like Mary requires a finely tuned diagnostic approach and treatment strategy. This article will help toward that end.
Anemia of CKD: A common problem
Anemia of CKD is one of the first signs of kidney dysfunction, yet it often goes undetected because of its insidious onset. Anemia develops gradually as kidney function declines and the GFR drops to 70 mL/min in male patients and 50 mL/min in females.2 Epidemiologic data indicate that two-thirds of patients in the early stages of kidney failure are also anemic, with a hemoglobin level of less than 11 g/dL, yet only one-third of these patients have ever received erythropoietin-stimulating agents (ESAs) to treat their anemia.1 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend that the evaluation of anemia of CKD begin in patients with a serum creatinine ≥2 mg/dL when the hemoglobin is <12 g/dL in adult males and postmenopausal females and <11 g/dL in premenopausal females.3
How kidney failure leads to anemia
Patients like Mary develop anemia of CKD because failing kidneys produce less erythropoietin (EPO) than the body requires for the production of red blood cells. EPO is an endogenous hormone produced by peritubular fibroblasts in the renal cortex.4 Most of this hormone (90%) is produced in the kidney, with the remainder manufactured by hepatocytes.
Erythropoiesis is stimulated by blood loss, decreased oxygen tension, and an increase in oxygen affinity, which leads to an increase in EPO production via upregulation of the EPO gene. In healthy individuals, detection of hypoxia by the kidney can result in a 1000-fold increase in EPO production.5 Patients with CKD don’t have that kind of robust response, and their EPO levels remain normal or below normal even when challenged by lack of oxygen. Anemia in CKD can also be caused by nutritional deficiencies, decreased red blood cell survival because of uremic toxins, oxidative stress, inflammation, and the use of angiotensin-converting enzyme (ACE) inhibitors.
Chronic anemia, CKD, and CV disease: A deadly triad
The leading cause of death in patients with CKD is cardiovascular disease. Patients with cardiorenal anemia syndrome develop a self-perpetuating triad that increases the risk of death when all 3 conditions are present. Anemic patients double their relative risk of death when CKD is present and triple their risk if they have anemia, CKD, and cardiovascular disease.6
Epidemiologic studies suggest an association among anemia, left ventricular hypertrophy (LVH), mortality, and cardiovascular outcomes. One study evaluated 2423 stage 3 and 4 CKD patients with anemia, defined as hemoglobin <13 g/dL in males and <12 g/dL in females. The results showed an increase in composite outcomes of myocardial infarction, stroke, and death.7 A prospective study evaluating 246 people with stages 2 to 4 CKD reported anemia to be an independent risk factor for the development of LVH.8 The stages of CKD are shown in the TABLE.
Suspected mechanisms of cardiovascular disease progression due to chronic anemia include tissue hypoxia, free radical formation, endothelial dysfunction, and vascular damage. Compensatory neurohumeral adaptations result in an increased sympathetic response and upregulation of the reninangiotensin-aldosterone system.9
TABLE
Stages of chronic kidney disease
| Stage | Description | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | ≥90 |
| 2 | Kidney damage with mildly decreased GFR | 60-89 |
| 3 | Moderately decreased GFR | 30-59 |
| 4 | Severely decreased GFR | 15-29 |
| 5 | Kidney failure | <15 or dialysis |
| GFR, glomerular filtration rate. | ||
| Source: KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007.3 | ||
Anemia of CKD: A diagnosis of exclusion
Because anemia can have many causes, other possibilities must be ruled out before a diagnosis of CKD anemia can be made. Testing should be tailored to each individual situation, determined by a thorough history and physical. Steps in the diagnosis are shown in the FLOW CHART. A basic work-up should include complete blood count with differential, iron studies (ferritin, serum Fe, and total iron binding capacity), reticulocyte count, and a guaiac test. Other blood tests, such as thyroid-stimulating hormone (TSH), B12, and folate levels, and a hemolysis panel (lactate dehydrogenase, haptoglobin), should be obtained if the history suggests these disorders. A peripheral blood smear showing normocytic red blood cells with a normochromic pattern would favor the diagnosis of anemia of CKD.
FLOW CHART
A step-by-step guide to CKD anemia diagnosis and treatment
CBC, complete blood count; CKD, chronic kidney disease; ESA, erythropoietin-stimulating agents; R/O, rule out; TIBC/TSAT, total iron-binding capacity/transferrin saturation.
A look at the iron connection
Many patients with CKD anemia have iron deficiency and are unable to produce adequate numbers of red blood cells. Iron deficiency can have many causes: not enough iron-rich food in the diet, chronic bleeding, malabsorption, or an occult gastrointestinal malignancy. Once iron deficiency anemia is diagnosed, a colonoscopy is warranted to rule out occult malignancy. Ferritin, a protein found mostly in macrophages and hepatocytes, stores iron and serves as a marker for total iron stores. Using stored iron requires transferrin, a transporting protein, to shuttle iron from the reticuloendothelial system and gut to the bone marrow. CKD is a pro-inflammatory state that results in a limited ability to use iron stores. For this reason, patients with CKD require higher levels of iron.
Absolute iron deficiency. Iron deficiency in CKD patients with serum ferritin <100 ng/mL and transferrin saturation (TSAT) <20% is characterized as absolute iron deficiency. The TSAT represents the percent of iron bound to transferrin and is a good indicator of the body’s functional capacity to use stored iron.
Relative iron deficiency and iron block. Patients who do not respond to ESA therapy even though they have adequate iron stores are said to have a functional or relative iron deficiency. Iron block is a condition that results in anemia from a chronic inflammatory state such as infection, autoimmune disorders, or malignancies. It resolves once the inflammatory process abates. Both conditions have similar anemia profiles, with a serum ferritin >100 ng/mL and a TSAT <20%. Differentiating between these conditions requires dynamic testing using serial iron studies and observing responses to ESAs and iron supplementation.
Options for correcting iron deficiency
After a thorough history and physical with appropriate screening, you find that Mary has an iron deficiency that must be corrected before her anemia can be treated effectively. Treatment for iron deficiency is usually initiated with oral therapy, at the recommended dose of 200 mg oral elemental iron a day in 3 divided doses.
If the oral therapy does not correct iron deficiency within 3 months, or a patient cannot tolerate the constipation that is often a side effect of this therapy, IV iron administration can be considered. Because CKD patients do not have the ongoing iron losses seen in patients with end-stage renal disease (ESRD), a conservative approach using a single IV dose followed by repeat testing is warranted. The goal is to achieve ferritin levels >100 ng/dL and TSAT >20%. A number of products for IV iron administration are available. The most widely used are iron dextran (INFeD), ferric gluconate (Ferrlecit), and iron sucrose (Venofer).
Iron stores are replenished? Time to treat the anemia
When ferritin levels and TSAT show that iron deficiency has been corrected, ESA treatment for anemia can begin. Two major brands of ESAs currently in use in the United States are a recombinant human erythropoietin (rHuEPO) known as epoetin alfa (Procrit, Epogen), and darbepoetin alpha (Aranesp). Both medications are effective and can be given intravenously or subcutaneously. Subcutaneous darbepoetin alpha has a longer half-life compared with epoetin alpha (70 vs 24 hours), so dosing intervals can be longer.10,11 ESAs should not be started in patients with uncontrolled hypertension until the blood pressure is controlled, or in patients with an active malignancy unless the treatment is directly supervised by an oncologist.
Aim for complete anemia resolution? That’s controversial
Treatment of CKD anemia with ESAs is widely practiced, but controversy over whether it is beneficial to aim for complete resolution of anemia is ongoing. The CREATE (Cardiovascular Risk Reduction by Early Anemia Treatment) and CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trials published in 2006 failed to resolve the issue.12,13
In the CREATE trial, patients targeted to achieve normal hemoglobin levels did no better in avoiding cardiovascular events than patients targeted for lower levels. The CHOIR trial was stopped early because of an increased trend toward death and hospitalization for congestive heart failure in the group with therapy targeted to achieve normal hemoglobin levels.
The recently published TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of patients with type 2 diabetes and CKD showed no reduction in all-cause mortality, cardiovascular morbidity, or ESRD in patients receiving Aranesp targeted to achieve a hemoglobin level of approximately 13 g/dL, compared with placebo.14 The study did demonstrate, however, that patients receiving Aranesp were about twice as likely to have a stroke than the placebo subjects (101 vs 53)—which might lead clinicians to ponder whether the gains, if any, were worth the risk.
Revised labeling. Late last year, the US Food and Drug Administration approved a label change for Procrit and Aranesp, warning that patients with renal failure “experienced greater risks for death and serious cardiovascular events when administered ESAs to target higher vs lower hemoglobin levels” and advising physicians to “individualize dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL.”10,11 The 2007 NKF KDOQI guidelines suggest maintaining a hemoglobin level between 11 and 12 g/dL and have not incorporated the results of the TREAT trial.
Some patients don’t respond to ESAs
Inadequate response to ESAs is most commonly caused by underdosing or inadequate iron stores. NKF KDOQI guidelines recommend checking TSAT and ferritin prior to initiating therapy and monitoring these levels every 3 months.3 True nonresponders are individuals with good iron stores who are unable to achieve target hemoglobin within 4 to 6 months despite receiving subcutaneous epoetin 300 IU/kg per week. Inadequate response to ESAs can be caused by ongoing occult blood loss, infection, inflammation, nutritional deficiencies, hemolysis, hemoglobinemias, aluminum toxicity, anti-EPO antibody, hyperparathyroidism, multiple myeloma, and bone marrow dysfunction.10,11 If patients do not respond to ESA therapy, the NKF KDOQI guidelines recommend referral to a nephrologist or hematologist.3
How did Mary fare?
Mary did well taking oral iron supplementation. Once her iron deficiency was corrected, you were able to begin treating her anemia. After appropriate titration of her ESA, she was able to maintain a hemoglobin level between 11 and 12 g/dL 4 months into therapy. On a follow-up visit, she had no side effects from the medication and reported an increase in her energy level.
CORRESPONDENCE
Jonathan Taliercio, DO, Cleveland Clinic, Department of Nephrology and Hypertension, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. United States Renal Data System, USRDS. 2009 Annual Data Report. Atlas of Chronic Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
2. Hsu CJ, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504-510.
3. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471-530.
4. Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis. 2001;38:415-425.
5. Ebert B, Franklin H. Regulation of the erythropoietin gene. Blood. 1999;94:1864-1877.
6. Silverberg D, Wexler D, Blum M, et al. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(suppl 8):viii 7-viii 12.
7. Weiner D, Tighiouart H, Vlagopoulos P, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803-1810.
8. Levin A, Thompson C, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125-134.
9. Rao M, Pereira B. Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int. 2005;68:1432-1438.
10. Amgen. Aranesp (Darbepoetin Alpha) package insert. Available at www.aranesp.com/professional/crf/full_prescribing_info/pi.jsp. Accessed November 16, 2009.
11. Amgen. Procrit (Epoetin Alpha) package insert. Available at www.procrit.com/sites/default/files/shared/OBI/PI/ProcritBooklet.pdf#page=1. Accessed November 16, 2009.
12. Drueke T, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with CKD and anemia. N Engl J Med. 2006;355:2071-2084.
13. Singh A, Szczech L, Tang K. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085-2098.
14. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032.
• Evaluate for chronic kidney disease (CKD) anemia when a patient has a serum creatinine ≥2 mg/dL and hemoglobin <12 g/dL (adult males and postmenopausal females) or <11 g/dL (premenopausal females). A
• Before you treat CKD anemia, correct any underlying iron deficiency. A
• Start anemia therapy with erythropoietin-stimulating agents when hemoglobin is ≤10 g/dL, and maintain target hemoglobin levels between 11 and 12 g/dL, in accordance with National Kidney Foundation guidelines. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Mary J, a 65-year-old woman with stage 3 chronic kidney disease (CKD), is in your office for a follow-up appointment. Over the past 6 months, she has noticed a decrease in her energy level. On her routine blood work, you see that her hemoglobin has been slowly declining over the past year. It is now 9 g/dL and her estimated glomerular filtration rate (GFR) is 40 mL/min.
How would you evaluate Mary’s anemia, and would you suspect that it was related to her CKD?
Most physicians are aware that CKD—which affects approximately 10% of the US population1—has a deleterious effect on cardiovascular disease, but many fail to recognize the impact it has on the hematopoietic system. Managing the anemia that accompanies CKD in patients like Mary requires a finely tuned diagnostic approach and treatment strategy. This article will help toward that end.
Anemia of CKD: A common problem
Anemia of CKD is one of the first signs of kidney dysfunction, yet it often goes undetected because of its insidious onset. Anemia develops gradually as kidney function declines and the GFR drops to 70 mL/min in male patients and 50 mL/min in females.2 Epidemiologic data indicate that two-thirds of patients in the early stages of kidney failure are also anemic, with a hemoglobin level of less than 11 g/dL, yet only one-third of these patients have ever received erythropoietin-stimulating agents (ESAs) to treat their anemia.1 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend that the evaluation of anemia of CKD begin in patients with a serum creatinine ≥2 mg/dL when the hemoglobin is <12 g/dL in adult males and postmenopausal females and <11 g/dL in premenopausal females.3
How kidney failure leads to anemia
Patients like Mary develop anemia of CKD because failing kidneys produce less erythropoietin (EPO) than the body requires for the production of red blood cells. EPO is an endogenous hormone produced by peritubular fibroblasts in the renal cortex.4 Most of this hormone (90%) is produced in the kidney, with the remainder manufactured by hepatocytes.
Erythropoiesis is stimulated by blood loss, decreased oxygen tension, and an increase in oxygen affinity, which leads to an increase in EPO production via upregulation of the EPO gene. In healthy individuals, detection of hypoxia by the kidney can result in a 1000-fold increase in EPO production.5 Patients with CKD don’t have that kind of robust response, and their EPO levels remain normal or below normal even when challenged by lack of oxygen. Anemia in CKD can also be caused by nutritional deficiencies, decreased red blood cell survival because of uremic toxins, oxidative stress, inflammation, and the use of angiotensin-converting enzyme (ACE) inhibitors.
Chronic anemia, CKD, and CV disease: A deadly triad
The leading cause of death in patients with CKD is cardiovascular disease. Patients with cardiorenal anemia syndrome develop a self-perpetuating triad that increases the risk of death when all 3 conditions are present. Anemic patients double their relative risk of death when CKD is present and triple their risk if they have anemia, CKD, and cardiovascular disease.6
Epidemiologic studies suggest an association among anemia, left ventricular hypertrophy (LVH), mortality, and cardiovascular outcomes. One study evaluated 2423 stage 3 and 4 CKD patients with anemia, defined as hemoglobin <13 g/dL in males and <12 g/dL in females. The results showed an increase in composite outcomes of myocardial infarction, stroke, and death.7 A prospective study evaluating 246 people with stages 2 to 4 CKD reported anemia to be an independent risk factor for the development of LVH.8 The stages of CKD are shown in the TABLE.
Suspected mechanisms of cardiovascular disease progression due to chronic anemia include tissue hypoxia, free radical formation, endothelial dysfunction, and vascular damage. Compensatory neurohumeral adaptations result in an increased sympathetic response and upregulation of the reninangiotensin-aldosterone system.9
TABLE
Stages of chronic kidney disease
| Stage | Description | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | ≥90 |
| 2 | Kidney damage with mildly decreased GFR | 60-89 |
| 3 | Moderately decreased GFR | 30-59 |
| 4 | Severely decreased GFR | 15-29 |
| 5 | Kidney failure | <15 or dialysis |
| GFR, glomerular filtration rate. | ||
| Source: KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007.3 | ||
Anemia of CKD: A diagnosis of exclusion
Because anemia can have many causes, other possibilities must be ruled out before a diagnosis of CKD anemia can be made. Testing should be tailored to each individual situation, determined by a thorough history and physical. Steps in the diagnosis are shown in the FLOW CHART. A basic work-up should include complete blood count with differential, iron studies (ferritin, serum Fe, and total iron binding capacity), reticulocyte count, and a guaiac test. Other blood tests, such as thyroid-stimulating hormone (TSH), B12, and folate levels, and a hemolysis panel (lactate dehydrogenase, haptoglobin), should be obtained if the history suggests these disorders. A peripheral blood smear showing normocytic red blood cells with a normochromic pattern would favor the diagnosis of anemia of CKD.
FLOW CHART
A step-by-step guide to CKD anemia diagnosis and treatment
CBC, complete blood count; CKD, chronic kidney disease; ESA, erythropoietin-stimulating agents; R/O, rule out; TIBC/TSAT, total iron-binding capacity/transferrin saturation.
A look at the iron connection
Many patients with CKD anemia have iron deficiency and are unable to produce adequate numbers of red blood cells. Iron deficiency can have many causes: not enough iron-rich food in the diet, chronic bleeding, malabsorption, or an occult gastrointestinal malignancy. Once iron deficiency anemia is diagnosed, a colonoscopy is warranted to rule out occult malignancy. Ferritin, a protein found mostly in macrophages and hepatocytes, stores iron and serves as a marker for total iron stores. Using stored iron requires transferrin, a transporting protein, to shuttle iron from the reticuloendothelial system and gut to the bone marrow. CKD is a pro-inflammatory state that results in a limited ability to use iron stores. For this reason, patients with CKD require higher levels of iron.
Absolute iron deficiency. Iron deficiency in CKD patients with serum ferritin <100 ng/mL and transferrin saturation (TSAT) <20% is characterized as absolute iron deficiency. The TSAT represents the percent of iron bound to transferrin and is a good indicator of the body’s functional capacity to use stored iron.
Relative iron deficiency and iron block. Patients who do not respond to ESA therapy even though they have adequate iron stores are said to have a functional or relative iron deficiency. Iron block is a condition that results in anemia from a chronic inflammatory state such as infection, autoimmune disorders, or malignancies. It resolves once the inflammatory process abates. Both conditions have similar anemia profiles, with a serum ferritin >100 ng/mL and a TSAT <20%. Differentiating between these conditions requires dynamic testing using serial iron studies and observing responses to ESAs and iron supplementation.
Options for correcting iron deficiency
After a thorough history and physical with appropriate screening, you find that Mary has an iron deficiency that must be corrected before her anemia can be treated effectively. Treatment for iron deficiency is usually initiated with oral therapy, at the recommended dose of 200 mg oral elemental iron a day in 3 divided doses.
If the oral therapy does not correct iron deficiency within 3 months, or a patient cannot tolerate the constipation that is often a side effect of this therapy, IV iron administration can be considered. Because CKD patients do not have the ongoing iron losses seen in patients with end-stage renal disease (ESRD), a conservative approach using a single IV dose followed by repeat testing is warranted. The goal is to achieve ferritin levels >100 ng/dL and TSAT >20%. A number of products for IV iron administration are available. The most widely used are iron dextran (INFeD), ferric gluconate (Ferrlecit), and iron sucrose (Venofer).
Iron stores are replenished? Time to treat the anemia
When ferritin levels and TSAT show that iron deficiency has been corrected, ESA treatment for anemia can begin. Two major brands of ESAs currently in use in the United States are a recombinant human erythropoietin (rHuEPO) known as epoetin alfa (Procrit, Epogen), and darbepoetin alpha (Aranesp). Both medications are effective and can be given intravenously or subcutaneously. Subcutaneous darbepoetin alpha has a longer half-life compared with epoetin alpha (70 vs 24 hours), so dosing intervals can be longer.10,11 ESAs should not be started in patients with uncontrolled hypertension until the blood pressure is controlled, or in patients with an active malignancy unless the treatment is directly supervised by an oncologist.
Aim for complete anemia resolution? That’s controversial
Treatment of CKD anemia with ESAs is widely practiced, but controversy over whether it is beneficial to aim for complete resolution of anemia is ongoing. The CREATE (Cardiovascular Risk Reduction by Early Anemia Treatment) and CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trials published in 2006 failed to resolve the issue.12,13
In the CREATE trial, patients targeted to achieve normal hemoglobin levels did no better in avoiding cardiovascular events than patients targeted for lower levels. The CHOIR trial was stopped early because of an increased trend toward death and hospitalization for congestive heart failure in the group with therapy targeted to achieve normal hemoglobin levels.
The recently published TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of patients with type 2 diabetes and CKD showed no reduction in all-cause mortality, cardiovascular morbidity, or ESRD in patients receiving Aranesp targeted to achieve a hemoglobin level of approximately 13 g/dL, compared with placebo.14 The study did demonstrate, however, that patients receiving Aranesp were about twice as likely to have a stroke than the placebo subjects (101 vs 53)—which might lead clinicians to ponder whether the gains, if any, were worth the risk.
Revised labeling. Late last year, the US Food and Drug Administration approved a label change for Procrit and Aranesp, warning that patients with renal failure “experienced greater risks for death and serious cardiovascular events when administered ESAs to target higher vs lower hemoglobin levels” and advising physicians to “individualize dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL.”10,11 The 2007 NKF KDOQI guidelines suggest maintaining a hemoglobin level between 11 and 12 g/dL and have not incorporated the results of the TREAT trial.
Some patients don’t respond to ESAs
Inadequate response to ESAs is most commonly caused by underdosing or inadequate iron stores. NKF KDOQI guidelines recommend checking TSAT and ferritin prior to initiating therapy and monitoring these levels every 3 months.3 True nonresponders are individuals with good iron stores who are unable to achieve target hemoglobin within 4 to 6 months despite receiving subcutaneous epoetin 300 IU/kg per week. Inadequate response to ESAs can be caused by ongoing occult blood loss, infection, inflammation, nutritional deficiencies, hemolysis, hemoglobinemias, aluminum toxicity, anti-EPO antibody, hyperparathyroidism, multiple myeloma, and bone marrow dysfunction.10,11 If patients do not respond to ESA therapy, the NKF KDOQI guidelines recommend referral to a nephrologist or hematologist.3
How did Mary fare?
Mary did well taking oral iron supplementation. Once her iron deficiency was corrected, you were able to begin treating her anemia. After appropriate titration of her ESA, she was able to maintain a hemoglobin level between 11 and 12 g/dL 4 months into therapy. On a follow-up visit, she had no side effects from the medication and reported an increase in her energy level.
CORRESPONDENCE
Jonathan Taliercio, DO, Cleveland Clinic, Department of Nephrology and Hypertension, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
• Evaluate for chronic kidney disease (CKD) anemia when a patient has a serum creatinine ≥2 mg/dL and hemoglobin <12 g/dL (adult males and postmenopausal females) or <11 g/dL (premenopausal females). A
• Before you treat CKD anemia, correct any underlying iron deficiency. A
• Start anemia therapy with erythropoietin-stimulating agents when hemoglobin is ≤10 g/dL, and maintain target hemoglobin levels between 11 and 12 g/dL, in accordance with National Kidney Foundation guidelines. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Mary J, a 65-year-old woman with stage 3 chronic kidney disease (CKD), is in your office for a follow-up appointment. Over the past 6 months, she has noticed a decrease in her energy level. On her routine blood work, you see that her hemoglobin has been slowly declining over the past year. It is now 9 g/dL and her estimated glomerular filtration rate (GFR) is 40 mL/min.
How would you evaluate Mary’s anemia, and would you suspect that it was related to her CKD?
Most physicians are aware that CKD—which affects approximately 10% of the US population1—has a deleterious effect on cardiovascular disease, but many fail to recognize the impact it has on the hematopoietic system. Managing the anemia that accompanies CKD in patients like Mary requires a finely tuned diagnostic approach and treatment strategy. This article will help toward that end.
Anemia of CKD: A common problem
Anemia of CKD is one of the first signs of kidney dysfunction, yet it often goes undetected because of its insidious onset. Anemia develops gradually as kidney function declines and the GFR drops to 70 mL/min in male patients and 50 mL/min in females.2 Epidemiologic data indicate that two-thirds of patients in the early stages of kidney failure are also anemic, with a hemoglobin level of less than 11 g/dL, yet only one-third of these patients have ever received erythropoietin-stimulating agents (ESAs) to treat their anemia.1 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend that the evaluation of anemia of CKD begin in patients with a serum creatinine ≥2 mg/dL when the hemoglobin is <12 g/dL in adult males and postmenopausal females and <11 g/dL in premenopausal females.3
How kidney failure leads to anemia
Patients like Mary develop anemia of CKD because failing kidneys produce less erythropoietin (EPO) than the body requires for the production of red blood cells. EPO is an endogenous hormone produced by peritubular fibroblasts in the renal cortex.4 Most of this hormone (90%) is produced in the kidney, with the remainder manufactured by hepatocytes.
Erythropoiesis is stimulated by blood loss, decreased oxygen tension, and an increase in oxygen affinity, which leads to an increase in EPO production via upregulation of the EPO gene. In healthy individuals, detection of hypoxia by the kidney can result in a 1000-fold increase in EPO production.5 Patients with CKD don’t have that kind of robust response, and their EPO levels remain normal or below normal even when challenged by lack of oxygen. Anemia in CKD can also be caused by nutritional deficiencies, decreased red blood cell survival because of uremic toxins, oxidative stress, inflammation, and the use of angiotensin-converting enzyme (ACE) inhibitors.
Chronic anemia, CKD, and CV disease: A deadly triad
The leading cause of death in patients with CKD is cardiovascular disease. Patients with cardiorenal anemia syndrome develop a self-perpetuating triad that increases the risk of death when all 3 conditions are present. Anemic patients double their relative risk of death when CKD is present and triple their risk if they have anemia, CKD, and cardiovascular disease.6
Epidemiologic studies suggest an association among anemia, left ventricular hypertrophy (LVH), mortality, and cardiovascular outcomes. One study evaluated 2423 stage 3 and 4 CKD patients with anemia, defined as hemoglobin <13 g/dL in males and <12 g/dL in females. The results showed an increase in composite outcomes of myocardial infarction, stroke, and death.7 A prospective study evaluating 246 people with stages 2 to 4 CKD reported anemia to be an independent risk factor for the development of LVH.8 The stages of CKD are shown in the TABLE.
Suspected mechanisms of cardiovascular disease progression due to chronic anemia include tissue hypoxia, free radical formation, endothelial dysfunction, and vascular damage. Compensatory neurohumeral adaptations result in an increased sympathetic response and upregulation of the reninangiotensin-aldosterone system.9
TABLE
Stages of chronic kidney disease
| Stage | Description | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | ≥90 |
| 2 | Kidney damage with mildly decreased GFR | 60-89 |
| 3 | Moderately decreased GFR | 30-59 |
| 4 | Severely decreased GFR | 15-29 |
| 5 | Kidney failure | <15 or dialysis |
| GFR, glomerular filtration rate. | ||
| Source: KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007.3 | ||
Anemia of CKD: A diagnosis of exclusion
Because anemia can have many causes, other possibilities must be ruled out before a diagnosis of CKD anemia can be made. Testing should be tailored to each individual situation, determined by a thorough history and physical. Steps in the diagnosis are shown in the FLOW CHART. A basic work-up should include complete blood count with differential, iron studies (ferritin, serum Fe, and total iron binding capacity), reticulocyte count, and a guaiac test. Other blood tests, such as thyroid-stimulating hormone (TSH), B12, and folate levels, and a hemolysis panel (lactate dehydrogenase, haptoglobin), should be obtained if the history suggests these disorders. A peripheral blood smear showing normocytic red blood cells with a normochromic pattern would favor the diagnosis of anemia of CKD.
FLOW CHART
A step-by-step guide to CKD anemia diagnosis and treatment
CBC, complete blood count; CKD, chronic kidney disease; ESA, erythropoietin-stimulating agents; R/O, rule out; TIBC/TSAT, total iron-binding capacity/transferrin saturation.
A look at the iron connection
Many patients with CKD anemia have iron deficiency and are unable to produce adequate numbers of red blood cells. Iron deficiency can have many causes: not enough iron-rich food in the diet, chronic bleeding, malabsorption, or an occult gastrointestinal malignancy. Once iron deficiency anemia is diagnosed, a colonoscopy is warranted to rule out occult malignancy. Ferritin, a protein found mostly in macrophages and hepatocytes, stores iron and serves as a marker for total iron stores. Using stored iron requires transferrin, a transporting protein, to shuttle iron from the reticuloendothelial system and gut to the bone marrow. CKD is a pro-inflammatory state that results in a limited ability to use iron stores. For this reason, patients with CKD require higher levels of iron.
Absolute iron deficiency. Iron deficiency in CKD patients with serum ferritin <100 ng/mL and transferrin saturation (TSAT) <20% is characterized as absolute iron deficiency. The TSAT represents the percent of iron bound to transferrin and is a good indicator of the body’s functional capacity to use stored iron.
Relative iron deficiency and iron block. Patients who do not respond to ESA therapy even though they have adequate iron stores are said to have a functional or relative iron deficiency. Iron block is a condition that results in anemia from a chronic inflammatory state such as infection, autoimmune disorders, or malignancies. It resolves once the inflammatory process abates. Both conditions have similar anemia profiles, with a serum ferritin >100 ng/mL and a TSAT <20%. Differentiating between these conditions requires dynamic testing using serial iron studies and observing responses to ESAs and iron supplementation.
Options for correcting iron deficiency
After a thorough history and physical with appropriate screening, you find that Mary has an iron deficiency that must be corrected before her anemia can be treated effectively. Treatment for iron deficiency is usually initiated with oral therapy, at the recommended dose of 200 mg oral elemental iron a day in 3 divided doses.
If the oral therapy does not correct iron deficiency within 3 months, or a patient cannot tolerate the constipation that is often a side effect of this therapy, IV iron administration can be considered. Because CKD patients do not have the ongoing iron losses seen in patients with end-stage renal disease (ESRD), a conservative approach using a single IV dose followed by repeat testing is warranted. The goal is to achieve ferritin levels >100 ng/dL and TSAT >20%. A number of products for IV iron administration are available. The most widely used are iron dextran (INFeD), ferric gluconate (Ferrlecit), and iron sucrose (Venofer).
Iron stores are replenished? Time to treat the anemia
When ferritin levels and TSAT show that iron deficiency has been corrected, ESA treatment for anemia can begin. Two major brands of ESAs currently in use in the United States are a recombinant human erythropoietin (rHuEPO) known as epoetin alfa (Procrit, Epogen), and darbepoetin alpha (Aranesp). Both medications are effective and can be given intravenously or subcutaneously. Subcutaneous darbepoetin alpha has a longer half-life compared with epoetin alpha (70 vs 24 hours), so dosing intervals can be longer.10,11 ESAs should not be started in patients with uncontrolled hypertension until the blood pressure is controlled, or in patients with an active malignancy unless the treatment is directly supervised by an oncologist.
Aim for complete anemia resolution? That’s controversial
Treatment of CKD anemia with ESAs is widely practiced, but controversy over whether it is beneficial to aim for complete resolution of anemia is ongoing. The CREATE (Cardiovascular Risk Reduction by Early Anemia Treatment) and CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trials published in 2006 failed to resolve the issue.12,13
In the CREATE trial, patients targeted to achieve normal hemoglobin levels did no better in avoiding cardiovascular events than patients targeted for lower levels. The CHOIR trial was stopped early because of an increased trend toward death and hospitalization for congestive heart failure in the group with therapy targeted to achieve normal hemoglobin levels.
The recently published TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) study of patients with type 2 diabetes and CKD showed no reduction in all-cause mortality, cardiovascular morbidity, or ESRD in patients receiving Aranesp targeted to achieve a hemoglobin level of approximately 13 g/dL, compared with placebo.14 The study did demonstrate, however, that patients receiving Aranesp were about twice as likely to have a stroke than the placebo subjects (101 vs 53)—which might lead clinicians to ponder whether the gains, if any, were worth the risk.
Revised labeling. Late last year, the US Food and Drug Administration approved a label change for Procrit and Aranesp, warning that patients with renal failure “experienced greater risks for death and serious cardiovascular events when administered ESAs to target higher vs lower hemoglobin levels” and advising physicians to “individualize dosing to achieve and maintain hemoglobin levels within the range of 10 to 12 g/dL.”10,11 The 2007 NKF KDOQI guidelines suggest maintaining a hemoglobin level between 11 and 12 g/dL and have not incorporated the results of the TREAT trial.
Some patients don’t respond to ESAs
Inadequate response to ESAs is most commonly caused by underdosing or inadequate iron stores. NKF KDOQI guidelines recommend checking TSAT and ferritin prior to initiating therapy and monitoring these levels every 3 months.3 True nonresponders are individuals with good iron stores who are unable to achieve target hemoglobin within 4 to 6 months despite receiving subcutaneous epoetin 300 IU/kg per week. Inadequate response to ESAs can be caused by ongoing occult blood loss, infection, inflammation, nutritional deficiencies, hemolysis, hemoglobinemias, aluminum toxicity, anti-EPO antibody, hyperparathyroidism, multiple myeloma, and bone marrow dysfunction.10,11 If patients do not respond to ESA therapy, the NKF KDOQI guidelines recommend referral to a nephrologist or hematologist.3
How did Mary fare?
Mary did well taking oral iron supplementation. Once her iron deficiency was corrected, you were able to begin treating her anemia. After appropriate titration of her ESA, she was able to maintain a hemoglobin level between 11 and 12 g/dL 4 months into therapy. On a follow-up visit, she had no side effects from the medication and reported an increase in her energy level.
CORRESPONDENCE
Jonathan Taliercio, DO, Cleveland Clinic, Department of Nephrology and Hypertension, 9500 Euclid Avenue, Cleveland, OH 44195; [email protected]
1. United States Renal Data System, USRDS. 2009 Annual Data Report. Atlas of Chronic Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
2. Hsu CJ, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504-510.
3. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471-530.
4. Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis. 2001;38:415-425.
5. Ebert B, Franklin H. Regulation of the erythropoietin gene. Blood. 1999;94:1864-1877.
6. Silverberg D, Wexler D, Blum M, et al. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(suppl 8):viii 7-viii 12.
7. Weiner D, Tighiouart H, Vlagopoulos P, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803-1810.
8. Levin A, Thompson C, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125-134.
9. Rao M, Pereira B. Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int. 2005;68:1432-1438.
10. Amgen. Aranesp (Darbepoetin Alpha) package insert. Available at www.aranesp.com/professional/crf/full_prescribing_info/pi.jsp. Accessed November 16, 2009.
11. Amgen. Procrit (Epoetin Alpha) package insert. Available at www.procrit.com/sites/default/files/shared/OBI/PI/ProcritBooklet.pdf#page=1. Accessed November 16, 2009.
12. Drueke T, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with CKD and anemia. N Engl J Med. 2006;355:2071-2084.
13. Singh A, Szczech L, Tang K. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085-2098.
14. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032.
1. United States Renal Data System, USRDS. 2009 Annual Data Report. Atlas of Chronic Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
2. Hsu CJ, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504-510.
3. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471-530.
4. Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis. 2001;38:415-425.
5. Ebert B, Franklin H. Regulation of the erythropoietin gene. Blood. 1999;94:1864-1877.
6. Silverberg D, Wexler D, Blum M, et al. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(suppl 8):viii 7-viii 12.
7. Weiner D, Tighiouart H, Vlagopoulos P, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803-1810.
8. Levin A, Thompson C, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125-134.
9. Rao M, Pereira B. Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int. 2005;68:1432-1438.
10. Amgen. Aranesp (Darbepoetin Alpha) package insert. Available at www.aranesp.com/professional/crf/full_prescribing_info/pi.jsp. Accessed November 16, 2009.
11. Amgen. Procrit (Epoetin Alpha) package insert. Available at www.procrit.com/sites/default/files/shared/OBI/PI/ProcritBooklet.pdf#page=1. Accessed November 16, 2009.
12. Drueke T, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with CKD and anemia. N Engl J Med. 2006;355:2071-2084.
13. Singh A, Szczech L, Tang K. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085-2098.
14. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032.