User login
A middle-aged man with progressive fatigue
A 61-year-old white man presents with progressive fatigue, which began several months ago and has accelerated in severity over the past week. He says he has had no shortness of breath, chest pain, or symptoms of heart failure, but he has noticed a decrease in exertional capacity and now has trouble completing his daily 5-mile walk.
He saw his primary physician, who obtained an electrocardiogram that showed a new left bundle branch block. Transthoracic echocardiography indicated that his left ventricular ejection fraction, which was 60% a year earlier, was now 35%.
He has hypertension, dyslipidemia, type 2 diabetes, and chronic kidney disease. Although he was previously morbidly obese, he has lost more than 100 pounds with diet and exercise over the past 10 years. He also used to smoke; in fact, he has a 30-pack-year history, but he quit in 1987. He has a family history of premature coronary artery disease.
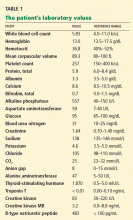
Physical examination. His heart rate is 75 beats per minute, blood pressure 142/85 mm Hg, and blood oxygen saturation 96% while breathing room air. He weighs 169 pounds (76.6 kg) and he is 6 feet tall (182.9 cm), so his body mass index is 22.9 kg/m2.
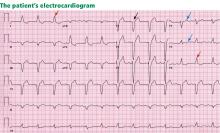
Electrocardiography reveals sinus rhythm with a left bundle branch block and left axis deviation (Figure 1), which were not present 1 year ago.
Chest roentgenography is normal.
A WORRISOME PICTURE
1. Which of the following is associated with left bundle branch block?
- Myocardial injury
- Hypertension
- Aortic stenosis
- Intrinsic conduction system disease
- All of the above
All of the above are true. For left bundle branch block to be diagnosed, the rhythm must be supraventricular and the QRS duration must be 120 ms or more. There should be a QS or RS complex in V1 and a monophasic R wave in I and V6. Also, the T wave should be deflected opposite the terminal deflection of the QRS complex. This is known as appropriate T-wave discordance with bundle branch block. A concordant T wave is nonspecific but suggests ischemia or myocardial infarction.
Potential causes of a new left bundle branch block include hypertension, acute myocardial infarction, aortic stenosis, and conduction system disease. A new left bundle branch block with a concomitant decrease in ejection fraction, especially in a patient with cardiac risk factors, is very worrisome, raising the possibility of ischemic heart disease.
MORE CARDIAC TESTING
The patient undergoes more cardiac testing.
Transthoracic echocardiography is done again. The left ventricle is normal in size, but the ejection fraction is 35%. In addition, stage 1 diastolic dysfunction (abnormal relaxation) and evidence of mechanical dyssynchrony (disruption in the normal sequence of activation and contraction of segments of the left ventricular wall) are seen. The right ventricle is normal in size and function. There is trivial mitral regurgitation and mild tricuspid regurgitation with normal right-sided pressures.
A gated rubidium-82 dipyridamole stress test yields no evidence of a fixed or reversible perfusion defect.
Left heart catheterization reveals angiographically normal coronary arteries.
Magnetic resonance imaging (MRI) shows a moderately hypertrophied left ventricle with moderately to severely depressed systolic function (left ventricular ejection fraction 27%). The left ventricle appears dyssynchronous. Delayed-enhancement imaging reveals patchy delayed enhancement in the basal septum and the basal inferolateral walls. These findings suggest cardiac sarcoidosis, with a sensitivity of nearly 100% and a specificity of approximately 78%.1
SARCOIDOSIS IS A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem disease characterized by noncaseating granulomas. Almost any organ can be affected, but it most commonly involves the respiratory and lymphatic systems.2 Although infectious, environmental, and genetic factors have been implicated, the cause remains unknown. The prevalence is approximately 20 per 100,000, being higher in black3 and Japanese 4 populations.
CARDIAC SARCOIDOSIS
2. What percentage of patients with sarcoidosis have cardiac involvement?
- 10%–20%
- 20%–30%
- 50%
- 80%
Cardiac involvement is seen in 20% to 30% of patients with sarcoidosis.5–7 However, most cases are subclinical, and symptomatic cardiac involvement is present in only about 5% of patients with systemic sarcoidosis.8 Isolated cardiac sarcoidosis has been described in case reports but is rare.9
The clinical manifestations of cardiac sarcoidosis depend on the location and extent of granulomatous inflammation. In a necropsy study of 113 patients with cardiac sarcoidosis, the left ventricular free wall was the most common location, followed by the interventricular septum.10
3. How does cardiac sarcoidosis most commonly present?
- Conduction abnormalities
- Ventricular tachycardia
- Cardiomyopathy
- Sudden death
- None of the above
Common presentations of cardiac sarcoidosis include conduction system disease and arrhythmias (which can sometimes result in sudden death), and heart failure.
Conduction abnormalities due to granulomas (in the active phase of sarcoidosis) and fibrosis (in the fibrotic phase) in the atrioventricular node or bundle of His are the most common presentation of cardiac sarcoidosis.9 These lesions may result in relatively benign first-degree heart block or may be as potentially devastating as complete heart block.
In almost all patients with conduction abnormalities, the basal interventricular septum is involved.11 Patients who develop complete heart block from sarcoidosis tend to be younger than those with idiopathic heart block. Therefore, complete heart block in a young patient should raise the possibility of this diagnosis. 12
Ventricular tachycardia (sustained or nonsustained) and ventricular premature beats are the second most common presentation. Up to 22% of patients with sarcoidosis have electrocardiographic evidence of ventricular arrythmias. 13 The cause is believed to be myocardial scar tissue resulting from the sarcoid granulomas, leading to electrical reentry.14 Sudden death due to ventricular tachyarrhythmias or conduction blocks accounts for 25% to 65% of deaths from cardiac sarcoidosis.9,15,16
Heart failure may result from sarcoidosis when there is extensive granulomatous disease in the myocardium. Depending on the location of the granulomas, both systolic and diastolic dysfunction can occur. In severe cases, extensive granulomas can cause left ventricular aneurysms.
The diagnosis of cardiac sarcoidosis as the cause of heart failure can be difficult to establish, especially in patients without evidence of sarcoidosis elsewhere. Such patients are often given a diagnosis of idiopathic dilated cardiomyopathy. However, compared with patients with idiopathic dilated cardiomyopathy, those with cardiac sarcoidosis have a greater incidence of advanced atrioventricular block, abnormal wall thickness, focal wall motion abnormalities, and perfusion defects of the anteroseptal and apical regions.17
Progressive heart failure is the second most frequent cause of death (after sudden death) and accounts for 25% to 75% of sarcoid-related cardiac deaths.9,18,19
DIAGNOSING CARDIAC SARCOIDOSIS
4. How is cardiac sarcoidosis diagnosed?
- Electrocardiography
- Echocardiography
- Computed tomography
- Endomyocardial biopsy
- There are no guidelines for diagnosis
Given the variable extent and location of granulomas in sarcoidosis, the diagnosis of cardiac sarcoidosis is often challenging.
To make the diagnosis of sarcoidosis in general, the American Thoracic Society2 says that the clinical picture should be compatible with this diagnosis, noncaseating granulomas should be histologically confirmed, and other diseases capable of producing a similar clinical or histologic picture should be excluded.
A newer diagnostic tool, the Sarcoidosis Three-Dimensional Assessment Instrument,20 incorporates two earlier tools.20,21 It assesses three axes: organ involvement, sarcoidosis severity, and sarcoidosis activity and categorizes the diagnosis of sarcoidosis as “definite,” “probable,” or “possible.”20
In Japan, where sarcoidosis is more common, the Ministry of Health and Welfare22 says that cardiac sarcoidosis can be diagnosed histologically if operative or endomyocardial biopsy specimens contain noncaseating granuloma. In addition, the diagnosis can be suspected in patients who have a histologic diagnosis of extracardiac sarcoidosis if the first item in the list below and one or more of the rest are present:
- Complete right bundle branch block, left axis deviation, atrioventricular block, ventricular tachycardia, premature ventricular contractions (> grade 2 of the Lown classification), or Q or ST-T wave abnormalities
- Abnormal wall motion, regional wall thinning, or dilation of the left ventricle on echocardiography
- Perfusion defects on thallium 201 myocardial scintigraphy or abnormal accumulation of gallium citrate Ga 67 or technetium 99m on myocardial scintigraphy
- Abnormal intracardiac pressure, low cardiac output, or abnormal wall motion or depressed left ventricular ejection fraction on cardiac catheterization
- Nonspecific interstitial fibrosis or cellular infiltration on myocardial biopsy.
The current diagnostic guidelines from the American Thoracic Society2 and the Japanese Ministry of Health and Welfare22 and the Sarcoidosis Three-Dimensional Assessment Instrument,20 however, do not incorporate newer imaging studies as part of their criteria.
A DEFINITIVE DIAGNOSIS
5. Endomyocardial biopsy often provides the definitive diagnosis of cardiac sarcoidosis.
- True
- False
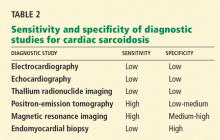
False. Endomyocardial biopsy often fails to reveal noncaseating granulomas, which have a patchy distribution.13 Table 2 summarizes the accuracy of tests for cardiac sarcoidosis.
Electrocardiography is abnormal in up to 50% of patients with sarcoidosis,23 reflecting the conduction disease or arrhythmias commonly seen in cardiac involvement.
Chest radiography classically shows hilar lymphadenopathy and interstitial disease, and may show cardiomegaly, pericardial effusion, or left ventricular aneurysm.
Echocardiography is nonspecific for sarcoid disease, but granulomatous involvement and scar tissue of cardiac tissue may appear hyperechogenic, particularly in the ventricular septum or left ventricular free wall.24
Angiography. Primary sarcoidosis rarely involves the coronary arteries,25 so angiography is most useful in excluding the diagnosis of atherosclerotic coronary artery disease.
Radionuclide imaging with thallium 201 in patients with suspected cardiac sarcoidosis may be useful to suggest myocardial involvement and to exclude cardiac dysfunction secondary to coronary artery disease. Segmental areas with defective thallium 201 uptake correspond to fibrogranulomatous tissue. In resting images, the pattern may be similar to that seen in coronary artery disease. However, during exercise, perfusion defects increase in patients who have ischemia but actually decrease in those with cardiac sarcoidosis.26
Nevertheless, some conclude that thallium scanning is too nonspecific for it to be used as a diagnostic or screening test.27,28 The combined use of thallium 201 and gallium 67 may better detect cardiac sarcoidosis, as gallium is taken up in areas of active inflammation.
Positron-emission tomography (PET) with fluorodeoxyglucose F 18 (FDG), with the patient fasting, appears to be useful in detecting the early inflammation of cardiac sarcoidosis29–34 and monitoring disease activity.30,31 FDG is a glucose analogue that is taken up by granulomatous tissue in the myocardium.34 The uptake in cardiac sarcoidosis is in a focal distribution.30,31,34 The abnormal FDG uptake resolves with steroid treatment.31,32
MRI has promise for diagnosing cardiac sarcoidosis. With gadolinium contrast, MRI has superior image resolution and can detect cardiac involvement early in its course.27,29,35–44
Inflammation of the myocardium due to sarcoid involvement appears as focal zones of increased signal intensity on both T2-weighted and early gadolinium T1-weighted images. Late myocardial enhancement after gadolinium infusion is the most typical finding of cardiac sarcoidosis on MRI, and likely represents fibrogranulomatous tissue.27 Delayed gadolinium enhancement is also seen in myocardial infarction but differs in its distribution.1,35,45 Cardiac sarcoidosis most commonly affects the basal and lateral segments. In one study, the finding of delayed enhancement had a sensitivity of 100% and a specificity of 78%,1,27 though it may not sufficiently differentiate active inflammation from scar.30
Like FDG-PET, MRI has also been shown to be useful for monitoring treatment.33,46 However, PET is more useful for follow-up in patients who receive a pacemaker or implantable cardioverter-defibrillator, in whom MRI is contraindicated. One case report29 described using both delayed-enhancement MRI and FDG-PET to diagnose cardiac sarcoidosis.
TREATMENT
6. How is cardiac sarcoidosis currently treated?
- Implantable cardioverter-defibrillator
- Corticosteroids
- Heart transplantation
- All of the above
- None of the above
Corticosteroids
Corticosteroids are the mainstay of treatment of cardiac sarcoidosis, as they attenuate the characteristic inflammation and fibrosis of sarcoid granulomas. The goal is to prevent compromise of cardiac structure or function.47 Although most of the supporting data are anecdotal, steroids have been shown to improve ventricular contractility,48 arrhythmias,49 and conduction abnormalities.50 MRI and FDG-PET studies have shown cardiac lesions resolving after steroids were started.31,45,46
The optimal dosage remains unknown. Initial doses of 30 to 60 mg daily, gradually tapered over 6 to 12 months to maintenance doses of 5 to 10 mg daily, have been effective.45,51
Relapses are common and require vigilant monitoring.
Alternative agents such as cyclophosphamide (Cytoxan),52 methotrexate (Rheumatrex), 53 and cyclosporine (Sandimmune)54 can be given to patients whose disease does not respond to corticosteroids or who cannot tolerate their side effects.
Implantable cardioverter-defibrillator
Sudden death due to ventricular tachyarrhythmias or conduction block accounts for 30% to 65% of deaths in patients with cardiac sarcoidosis.10 The rates of recurrent ventricular tachycardia and sudden death are high, even with antiarrhythmic drug therapy.55
Although experience with implantable cardiac defibrillators is limited in patients with cardiac sarcoidosis,55–58 some have argued that they be strongly considered to prevent sudden cardiac death in this high-risk group.57,58
Heart transplantation
The largest body of data on transplantation comes from the United Network for Organ Sharing database. In the 65 patients with cardiac sarcoidosis who underwent cardiac transplantation in the 18 years from October 1987 to September 2005, the 1-year post-transplant survival rate was 88%, which was better than in patients with all other diagnoses (85%). The 5-year survival rate was 80%.59,60
Recurrence of sarcoidosis within the cardiac allograft and transmission of sarcoidosis from donor to recipient have both been documented after heart transplantation.61,62
CAUSES OF DEATH
7. What is the most common cause of death in patients with cardiac sarcoidosis?
- Respiratory failure
- Conduction disturbances
- Progressive heart failure
- Ventricular tachyarrhythmias
- None of the above
The prognosis of symptomatic cardiac sarcoidosis is not well defined, owing to the variable extent and severity of the disease. The mortality rate in sarcoidosis without cardiac involvement is about 1% to 5% per year.63,64 Cardiac involvement portends a worse prognosis, with a 55% survival rate at 5 years and 44% at 10 years.17,65 Most patients in the reported series ultimately died of cardiac complications of sarcoidosis, including ventricular tachyarrhythmias, conduction disturbances, and progressive cardiomyopathy.10,17
Since corticosteroids, pacemakers, and implantable cardioverter-defibrillators have begun to be used, the cause of death has shifted from sudden death to progressive heart failure.66
CASE CONTINUED
Electrophysiologic testing revealed inducible monomorphic sustained ventricular tachycardia. The patient subsequently had a biventricular cardioverter-defibrillator implanted. He was started on an angiotensin-converting enzyme inhibitor and a beta-blocker for his heart failure. Further imaging of his chest and abdomen revealed lesions in his thyroid and liver. As of this writing, he is undergoing further workup. Because of active infection with Clostridium difficile, steroid therapy was deferred.
- Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997; 145:234–241.
- Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann NY Acad Sci 1976; 278:455–469.
- Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004; 83:315–334.
- Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994; 11:26–31.
- Thomsen TK, Eriksson T. Myocardial sarcoidosis in forensic medicine. Am J Forensic Med Pathol 1999; 20:52–56.
- Silverman KJ, Hutchins GM, Buckley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978; 58:1204–1211.
- Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977; 63:86–108.
- Bargout R, Kelly R. Sarcoid heart disease: clinical course and treatment. Int J Cardiol 2004; 97:173–182.
- Abeler V. Sarcoidosis of the cardiac conducting system. Am Heart J 1979; 97:701–707.
- Fleming HA, Bailey SM. Sarcoid heart disease. J R Coll Physicians Lond 1981; 15:245–253.
- Sekiguchi M, Numao Y, Imai M, Furuie T, Mikami R. Clinical and histological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn Circ J 1980; 44:249–263.
- Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol 2004; 27:217–222.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Reuhl J, Schneider M, Sievert H, Lutz FU, Zieger G. Myocardial sarcoidosis as a rare cause of sudden cardiac death. Forensic Sci Int 1997; 89:145–153.
- Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol 1998; 82:537–540.
- Fleming H. Cardiac sarcoidosis. In:James DG, editor. Sarcoidosis and Other Granulomatous Disorders. New York, NY: Dekker 1994; 73:323–334.
- Padilla M. Cardiac sarcoidosis. In:Baughman R, editor. Lung Biology in Health and Disease (Sarcoidosis), vol 210. New York, NY: Taylor & Francis Group; 2006:515–552.
- Judson MA. A proposed solution to the clinical assessment of sarcoidosis: the sarcoidosis three-dimensional assessment instrument (STAI). Med Hypotheses 2007; 68:1080–1087.
- Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16:75–86.
- Hiraga H, Yuwai K, Hiroe M, et al. Guideline for diagnosis of cardiac sarcoidosis. Study report of diffuse pulmonary diseases. Tokyo, Japan: The Japanese Ministry of Health and Welfare, 1993:23–24 (in Japanese).
- Stein E, Jackler I, Stimmel B, Stein W, Siltzbach LE. Asymptomatic electrocardiographic alterations in sarcoidosis. Am Heart J 1973; 86:474–477.
- Fahy GJ, Marwick T, McCreery CJ, Quigley PJ, Maurer BJ. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 1996; 109:62–66.
- Butany J, Bahl NE, Morales K, et al. The intricacies of cardiac sarcoidosis: a case report involving the coronary arteries and a review of the literature. Cardiovasc Pathol 2006; 15:222–227.
- Haywood LJ, Sharma OP, Siegel ME, et al. Detection of myocardial sarcoidosis by thallium-201 imaging. J Natl Med Assoc 1982; 74:959–964.
- Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol 2005; 185:110–115.
- Kinney EL, Caldwell JW. Do thallium myocardial perfusion scan abnormalities predict survival in sarcoid patients without cardiac symptoms? Angiology 1990; 41:573–576.
- Pandya C, Brunken RC, Tchou P, Schoenhagen P, Culver DA. Detecting cardiac involvement in sarcoidosis: a call for prospective studies of newer imaging techniques. Eur Respir J 2007; 29:418–422.
- Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008; 35:933–941.
- Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with 13N-NH3/18F-FDG PET. J Nucl Med 2003; 44:1030–1036.
- Takeda N, Yokoyama I, Hiroi Y, et al. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation 2002; 105:1144–1145.
- Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005; 26:1538–1543.
- Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004; 45:1989–1998.
- Schulz-Menger J, Wassmuth R, Abdel-Aty H, et al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006; 92:399–400.
- Kiuchi S, Teraoka K, Koizumi K, Takazawa K, Yamashina A. Usefulness of late gadolinium enhancement combined with MRI and 67-Ga scintigraphy in the diagnosis of cardiac sarcoidosis and disease activity evaluation. Int J Cardiovasc Imaging 2007; 23:237–241.
- Matsuki M, Matsuo M. MR findings of myocardial sarcoidosis. Clin Radiol 2000; 55:323–325.
- Inoue S, Shimada T, Murakami Y. Clinical significance of gadolinium-DTPA-enhanced MRI for detection of myocardial lesions in a patient with sarcoidosis. Clin Radiol 1999; 54:70–72.
- Vignaux O, Dhote R, Dudoc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrastenhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr 2002; 26:762–767.
- Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol 2005; 184:249–254.
- Smedema JP, Snoep G, Van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Doherty MJ, Kumar SK, Nicholson AA, McGivern DV. Cardiac sarcoidosis: the value of magnetic resonance imagine in diagnosis and assessment of response to treatment. Respir Med 1998; 92:697–699.
- Smedema JP, Truter R, de Klerk PA, Zaaiman L, White L, Doubell AF. Cardiac sarcoidosis evaluated with gadolinium-enhanced magnetic resonance and contrast-enhanced 64-slice computed tomography. Int J Cardiol 2006; 112:261–263.
- Kanao S, Tadamura E, Yamamuro M, et al. Demonstration of cardiac involvement of sarcoidosis by contrast-enhanced multislice computed tomography and delayed-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2005; 29:745–748.
- Vignaux O, Dhote R, Duboc D, et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest 2002; 122:1895–1901.
- Shimada T, Shimada K, Sakane T, et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med 2001; 110:520–527.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of longterm survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Ishikawa T, Kondoh H, Nakagawa S, Koiwaya Y, Tanaka K. Steroid therapy in cardiac sarcoidosis. Increased left ventricular contractility concomitant with electrocardiographic improvement after prednisolone. Chest 1984; 85:445–447.
- Walsh MJ. Systemic sarcoidosis with refractory ventricular tachycardia and heart failure. Br Heart J 1978; 40:931–933.
- Lash R, Coker J, Wong BY. Treatment of heart block due to sarcoid heart disease. J Electrocardiol 1979; 12:325–329.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and implications. Ann N Y Acad Sci 1986; 465:702–712.
- Demeter SL. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest 1988; 94:202–203.
- Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995; 155:846–851.
- York EL, Kovithavongs T, Man SF, Rebuck AS, Sproule BJ. Cyclosporine and chronic sarcoidosis. Chest 1990; 98:1026–1029.
- Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 1991; 18:937–943.
- Bajaj AK, Kopelman HA, Echt DS. Cardiac sarcoidosis with sudden death: treatment with automatic implantable cardioverter defibrillator. Am Heart J 1988; 116:557–560.
- Paz HL, McCormick DJ, Kutalek SP, Patchefsky A. The automated implantable cardiac defibrillator. Prophylaxis in cardiac sarcoidosis. Chest 1994; 106:1603–1607.
- Becker D, Berger E, Chmielewski C. Cardiac sarcoidosis: a report of four cases with ventricular tachycardia. J Cardiovasc Electrophysiol 1990; 1:214–219.
- Zaidi AR, Zaidi A, Vaitkus PT. Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant 2007; 26:714–717.
- Valantine HA, Tazelaar HD, Macoviak J, et al. Cardiac sarcoidosis: response to steroids and transplantation. J Heart Transplant 1987; 6:244–250.
- Oni AA, Hershberger RE, Norman DJ, et al. Recurrence of sarcoidosis in a cardiac allograft: control with augmented corticosteroids. J Heart Lung Transplant 1992; 11:367–369.
- Burke WM, Keogh A, Maloney PJ, Delprado W, Bryant DH, Spratt P. Transmission of sarcoidosis via cardiac transplantation. Lancet 1990; 336:1579.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and complications. Ann N Y Acad Sci 1986; 465:702–712.
- Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med 1996; 100:423–427.
- Fleming HA, Bailey SM. The prognosis of sarcoid heart disease in the United Kingdom. Ann N Y Acad Sci 1986; 465:543–550.
- Takada K, Ina Y, Yamamoto M, Satoh T, Morishita M. Prognosis after pacemaker implantation in cardiac sarcoidosis in Japan. Clinical evaluation of corticosteroid therapy. Sarcoidosis 1994; 11:113–117.
A 61-year-old white man presents with progressive fatigue, which began several months ago and has accelerated in severity over the past week. He says he has had no shortness of breath, chest pain, or symptoms of heart failure, but he has noticed a decrease in exertional capacity and now has trouble completing his daily 5-mile walk.
He saw his primary physician, who obtained an electrocardiogram that showed a new left bundle branch block. Transthoracic echocardiography indicated that his left ventricular ejection fraction, which was 60% a year earlier, was now 35%.
He has hypertension, dyslipidemia, type 2 diabetes, and chronic kidney disease. Although he was previously morbidly obese, he has lost more than 100 pounds with diet and exercise over the past 10 years. He also used to smoke; in fact, he has a 30-pack-year history, but he quit in 1987. He has a family history of premature coronary artery disease.
Physical examination. His heart rate is 75 beats per minute, blood pressure 142/85 mm Hg, and blood oxygen saturation 96% while breathing room air. He weighs 169 pounds (76.6 kg) and he is 6 feet tall (182.9 cm), so his body mass index is 22.9 kg/m2.
Electrocardiography reveals sinus rhythm with a left bundle branch block and left axis deviation (Figure 1), which were not present 1 year ago.
Chest roentgenography is normal.
A WORRISOME PICTURE
1. Which of the following is associated with left bundle branch block?
- Myocardial injury
- Hypertension
- Aortic stenosis
- Intrinsic conduction system disease
- All of the above
All of the above are true. For left bundle branch block to be diagnosed, the rhythm must be supraventricular and the QRS duration must be 120 ms or more. There should be a QS or RS complex in V1 and a monophasic R wave in I and V6. Also, the T wave should be deflected opposite the terminal deflection of the QRS complex. This is known as appropriate T-wave discordance with bundle branch block. A concordant T wave is nonspecific but suggests ischemia or myocardial infarction.
Potential causes of a new left bundle branch block include hypertension, acute myocardial infarction, aortic stenosis, and conduction system disease. A new left bundle branch block with a concomitant decrease in ejection fraction, especially in a patient with cardiac risk factors, is very worrisome, raising the possibility of ischemic heart disease.
MORE CARDIAC TESTING
The patient undergoes more cardiac testing.
Transthoracic echocardiography is done again. The left ventricle is normal in size, but the ejection fraction is 35%. In addition, stage 1 diastolic dysfunction (abnormal relaxation) and evidence of mechanical dyssynchrony (disruption in the normal sequence of activation and contraction of segments of the left ventricular wall) are seen. The right ventricle is normal in size and function. There is trivial mitral regurgitation and mild tricuspid regurgitation with normal right-sided pressures.
A gated rubidium-82 dipyridamole stress test yields no evidence of a fixed or reversible perfusion defect.
Left heart catheterization reveals angiographically normal coronary arteries.
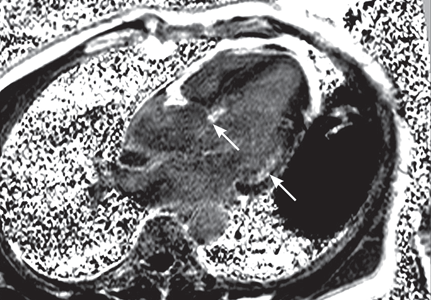
Magnetic resonance imaging (MRI) shows a moderately hypertrophied left ventricle with moderately to severely depressed systolic function (left ventricular ejection fraction 27%). The left ventricle appears dyssynchronous. Delayed-enhancement imaging reveals patchy delayed enhancement in the basal septum and the basal inferolateral walls. These findings suggest cardiac sarcoidosis, with a sensitivity of nearly 100% and a specificity of approximately 78%.1
SARCOIDOSIS IS A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem disease characterized by noncaseating granulomas. Almost any organ can be affected, but it most commonly involves the respiratory and lymphatic systems.2 Although infectious, environmental, and genetic factors have been implicated, the cause remains unknown. The prevalence is approximately 20 per 100,000, being higher in black3 and Japanese 4 populations.
CARDIAC SARCOIDOSIS
2. What percentage of patients with sarcoidosis have cardiac involvement?
- 10%–20%
- 20%–30%
- 50%
- 80%
Cardiac involvement is seen in 20% to 30% of patients with sarcoidosis.5–7 However, most cases are subclinical, and symptomatic cardiac involvement is present in only about 5% of patients with systemic sarcoidosis.8 Isolated cardiac sarcoidosis has been described in case reports but is rare.9
The clinical manifestations of cardiac sarcoidosis depend on the location and extent of granulomatous inflammation. In a necropsy study of 113 patients with cardiac sarcoidosis, the left ventricular free wall was the most common location, followed by the interventricular septum.10
3. How does cardiac sarcoidosis most commonly present?
- Conduction abnormalities
- Ventricular tachycardia
- Cardiomyopathy
- Sudden death
- None of the above
Common presentations of cardiac sarcoidosis include conduction system disease and arrhythmias (which can sometimes result in sudden death), and heart failure.
Conduction abnormalities due to granulomas (in the active phase of sarcoidosis) and fibrosis (in the fibrotic phase) in the atrioventricular node or bundle of His are the most common presentation of cardiac sarcoidosis.9 These lesions may result in relatively benign first-degree heart block or may be as potentially devastating as complete heart block.
In almost all patients with conduction abnormalities, the basal interventricular septum is involved.11 Patients who develop complete heart block from sarcoidosis tend to be younger than those with idiopathic heart block. Therefore, complete heart block in a young patient should raise the possibility of this diagnosis. 12
Ventricular tachycardia (sustained or nonsustained) and ventricular premature beats are the second most common presentation. Up to 22% of patients with sarcoidosis have electrocardiographic evidence of ventricular arrythmias. 13 The cause is believed to be myocardial scar tissue resulting from the sarcoid granulomas, leading to electrical reentry.14 Sudden death due to ventricular tachyarrhythmias or conduction blocks accounts for 25% to 65% of deaths from cardiac sarcoidosis.9,15,16
Heart failure may result from sarcoidosis when there is extensive granulomatous disease in the myocardium. Depending on the location of the granulomas, both systolic and diastolic dysfunction can occur. In severe cases, extensive granulomas can cause left ventricular aneurysms.
The diagnosis of cardiac sarcoidosis as the cause of heart failure can be difficult to establish, especially in patients without evidence of sarcoidosis elsewhere. Such patients are often given a diagnosis of idiopathic dilated cardiomyopathy. However, compared with patients with idiopathic dilated cardiomyopathy, those with cardiac sarcoidosis have a greater incidence of advanced atrioventricular block, abnormal wall thickness, focal wall motion abnormalities, and perfusion defects of the anteroseptal and apical regions.17
Progressive heart failure is the second most frequent cause of death (after sudden death) and accounts for 25% to 75% of sarcoid-related cardiac deaths.9,18,19
DIAGNOSING CARDIAC SARCOIDOSIS
4. How is cardiac sarcoidosis diagnosed?
- Electrocardiography
- Echocardiography
- Computed tomography
- Endomyocardial biopsy
- There are no guidelines for diagnosis
Given the variable extent and location of granulomas in sarcoidosis, the diagnosis of cardiac sarcoidosis is often challenging.
To make the diagnosis of sarcoidosis in general, the American Thoracic Society2 says that the clinical picture should be compatible with this diagnosis, noncaseating granulomas should be histologically confirmed, and other diseases capable of producing a similar clinical or histologic picture should be excluded.
A newer diagnostic tool, the Sarcoidosis Three-Dimensional Assessment Instrument,20 incorporates two earlier tools.20,21 It assesses three axes: organ involvement, sarcoidosis severity, and sarcoidosis activity and categorizes the diagnosis of sarcoidosis as “definite,” “probable,” or “possible.”20
In Japan, where sarcoidosis is more common, the Ministry of Health and Welfare22 says that cardiac sarcoidosis can be diagnosed histologically if operative or endomyocardial biopsy specimens contain noncaseating granuloma. In addition, the diagnosis can be suspected in patients who have a histologic diagnosis of extracardiac sarcoidosis if the first item in the list below and one or more of the rest are present:
- Complete right bundle branch block, left axis deviation, atrioventricular block, ventricular tachycardia, premature ventricular contractions (> grade 2 of the Lown classification), or Q or ST-T wave abnormalities
- Abnormal wall motion, regional wall thinning, or dilation of the left ventricle on echocardiography
- Perfusion defects on thallium 201 myocardial scintigraphy or abnormal accumulation of gallium citrate Ga 67 or technetium 99m on myocardial scintigraphy
- Abnormal intracardiac pressure, low cardiac output, or abnormal wall motion or depressed left ventricular ejection fraction on cardiac catheterization
- Nonspecific interstitial fibrosis or cellular infiltration on myocardial biopsy.
The current diagnostic guidelines from the American Thoracic Society2 and the Japanese Ministry of Health and Welfare22 and the Sarcoidosis Three-Dimensional Assessment Instrument,20 however, do not incorporate newer imaging studies as part of their criteria.
A DEFINITIVE DIAGNOSIS
5. Endomyocardial biopsy often provides the definitive diagnosis of cardiac sarcoidosis.
- True
- False
False. Endomyocardial biopsy often fails to reveal noncaseating granulomas, which have a patchy distribution.13 Table 2 summarizes the accuracy of tests for cardiac sarcoidosis.
Electrocardiography is abnormal in up to 50% of patients with sarcoidosis,23 reflecting the conduction disease or arrhythmias commonly seen in cardiac involvement.
Chest radiography classically shows hilar lymphadenopathy and interstitial disease, and may show cardiomegaly, pericardial effusion, or left ventricular aneurysm.
Echocardiography is nonspecific for sarcoid disease, but granulomatous involvement and scar tissue of cardiac tissue may appear hyperechogenic, particularly in the ventricular septum or left ventricular free wall.24
Angiography. Primary sarcoidosis rarely involves the coronary arteries,25 so angiography is most useful in excluding the diagnosis of atherosclerotic coronary artery disease.
Radionuclide imaging with thallium 201 in patients with suspected cardiac sarcoidosis may be useful to suggest myocardial involvement and to exclude cardiac dysfunction secondary to coronary artery disease. Segmental areas with defective thallium 201 uptake correspond to fibrogranulomatous tissue. In resting images, the pattern may be similar to that seen in coronary artery disease. However, during exercise, perfusion defects increase in patients who have ischemia but actually decrease in those with cardiac sarcoidosis.26
Nevertheless, some conclude that thallium scanning is too nonspecific for it to be used as a diagnostic or screening test.27,28 The combined use of thallium 201 and gallium 67 may better detect cardiac sarcoidosis, as gallium is taken up in areas of active inflammation.
Positron-emission tomography (PET) with fluorodeoxyglucose F 18 (FDG), with the patient fasting, appears to be useful in detecting the early inflammation of cardiac sarcoidosis29–34 and monitoring disease activity.30,31 FDG is a glucose analogue that is taken up by granulomatous tissue in the myocardium.34 The uptake in cardiac sarcoidosis is in a focal distribution.30,31,34 The abnormal FDG uptake resolves with steroid treatment.31,32
MRI has promise for diagnosing cardiac sarcoidosis. With gadolinium contrast, MRI has superior image resolution and can detect cardiac involvement early in its course.27,29,35–44
Inflammation of the myocardium due to sarcoid involvement appears as focal zones of increased signal intensity on both T2-weighted and early gadolinium T1-weighted images. Late myocardial enhancement after gadolinium infusion is the most typical finding of cardiac sarcoidosis on MRI, and likely represents fibrogranulomatous tissue.27 Delayed gadolinium enhancement is also seen in myocardial infarction but differs in its distribution.1,35,45 Cardiac sarcoidosis most commonly affects the basal and lateral segments. In one study, the finding of delayed enhancement had a sensitivity of 100% and a specificity of 78%,1,27 though it may not sufficiently differentiate active inflammation from scar.30
Like FDG-PET, MRI has also been shown to be useful for monitoring treatment.33,46 However, PET is more useful for follow-up in patients who receive a pacemaker or implantable cardioverter-defibrillator, in whom MRI is contraindicated. One case report29 described using both delayed-enhancement MRI and FDG-PET to diagnose cardiac sarcoidosis.
TREATMENT
6. How is cardiac sarcoidosis currently treated?
- Implantable cardioverter-defibrillator
- Corticosteroids
- Heart transplantation
- All of the above
- None of the above
Corticosteroids
Corticosteroids are the mainstay of treatment of cardiac sarcoidosis, as they attenuate the characteristic inflammation and fibrosis of sarcoid granulomas. The goal is to prevent compromise of cardiac structure or function.47 Although most of the supporting data are anecdotal, steroids have been shown to improve ventricular contractility,48 arrhythmias,49 and conduction abnormalities.50 MRI and FDG-PET studies have shown cardiac lesions resolving after steroids were started.31,45,46
The optimal dosage remains unknown. Initial doses of 30 to 60 mg daily, gradually tapered over 6 to 12 months to maintenance doses of 5 to 10 mg daily, have been effective.45,51
Relapses are common and require vigilant monitoring.
Alternative agents such as cyclophosphamide (Cytoxan),52 methotrexate (Rheumatrex), 53 and cyclosporine (Sandimmune)54 can be given to patients whose disease does not respond to corticosteroids or who cannot tolerate their side effects.
Implantable cardioverter-defibrillator
Sudden death due to ventricular tachyarrhythmias or conduction block accounts for 30% to 65% of deaths in patients with cardiac sarcoidosis.10 The rates of recurrent ventricular tachycardia and sudden death are high, even with antiarrhythmic drug therapy.55
Although experience with implantable cardiac defibrillators is limited in patients with cardiac sarcoidosis,55–58 some have argued that they be strongly considered to prevent sudden cardiac death in this high-risk group.57,58
Heart transplantation
The largest body of data on transplantation comes from the United Network for Organ Sharing database. In the 65 patients with cardiac sarcoidosis who underwent cardiac transplantation in the 18 years from October 1987 to September 2005, the 1-year post-transplant survival rate was 88%, which was better than in patients with all other diagnoses (85%). The 5-year survival rate was 80%.59,60
Recurrence of sarcoidosis within the cardiac allograft and transmission of sarcoidosis from donor to recipient have both been documented after heart transplantation.61,62
CAUSES OF DEATH
7. What is the most common cause of death in patients with cardiac sarcoidosis?
- Respiratory failure
- Conduction disturbances
- Progressive heart failure
- Ventricular tachyarrhythmias
- None of the above
The prognosis of symptomatic cardiac sarcoidosis is not well defined, owing to the variable extent and severity of the disease. The mortality rate in sarcoidosis without cardiac involvement is about 1% to 5% per year.63,64 Cardiac involvement portends a worse prognosis, with a 55% survival rate at 5 years and 44% at 10 years.17,65 Most patients in the reported series ultimately died of cardiac complications of sarcoidosis, including ventricular tachyarrhythmias, conduction disturbances, and progressive cardiomyopathy.10,17
Since corticosteroids, pacemakers, and implantable cardioverter-defibrillators have begun to be used, the cause of death has shifted from sudden death to progressive heart failure.66
CASE CONTINUED
Electrophysiologic testing revealed inducible monomorphic sustained ventricular tachycardia. The patient subsequently had a biventricular cardioverter-defibrillator implanted. He was started on an angiotensin-converting enzyme inhibitor and a beta-blocker for his heart failure. Further imaging of his chest and abdomen revealed lesions in his thyroid and liver. As of this writing, he is undergoing further workup. Because of active infection with Clostridium difficile, steroid therapy was deferred.
A 61-year-old white man presents with progressive fatigue, which began several months ago and has accelerated in severity over the past week. He says he has had no shortness of breath, chest pain, or symptoms of heart failure, but he has noticed a decrease in exertional capacity and now has trouble completing his daily 5-mile walk.
He saw his primary physician, who obtained an electrocardiogram that showed a new left bundle branch block. Transthoracic echocardiography indicated that his left ventricular ejection fraction, which was 60% a year earlier, was now 35%.
He has hypertension, dyslipidemia, type 2 diabetes, and chronic kidney disease. Although he was previously morbidly obese, he has lost more than 100 pounds with diet and exercise over the past 10 years. He also used to smoke; in fact, he has a 30-pack-year history, but he quit in 1987. He has a family history of premature coronary artery disease.
Physical examination. His heart rate is 75 beats per minute, blood pressure 142/85 mm Hg, and blood oxygen saturation 96% while breathing room air. He weighs 169 pounds (76.6 kg) and he is 6 feet tall (182.9 cm), so his body mass index is 22.9 kg/m2.
Electrocardiography reveals sinus rhythm with a left bundle branch block and left axis deviation (Figure 1), which were not present 1 year ago.
Chest roentgenography is normal.
A WORRISOME PICTURE
1. Which of the following is associated with left bundle branch block?
- Myocardial injury
- Hypertension
- Aortic stenosis
- Intrinsic conduction system disease
- All of the above
All of the above are true. For left bundle branch block to be diagnosed, the rhythm must be supraventricular and the QRS duration must be 120 ms or more. There should be a QS or RS complex in V1 and a monophasic R wave in I and V6. Also, the T wave should be deflected opposite the terminal deflection of the QRS complex. This is known as appropriate T-wave discordance with bundle branch block. A concordant T wave is nonspecific but suggests ischemia or myocardial infarction.
Potential causes of a new left bundle branch block include hypertension, acute myocardial infarction, aortic stenosis, and conduction system disease. A new left bundle branch block with a concomitant decrease in ejection fraction, especially in a patient with cardiac risk factors, is very worrisome, raising the possibility of ischemic heart disease.
MORE CARDIAC TESTING
The patient undergoes more cardiac testing.
Transthoracic echocardiography is done again. The left ventricle is normal in size, but the ejection fraction is 35%. In addition, stage 1 diastolic dysfunction (abnormal relaxation) and evidence of mechanical dyssynchrony (disruption in the normal sequence of activation and contraction of segments of the left ventricular wall) are seen. The right ventricle is normal in size and function. There is trivial mitral regurgitation and mild tricuspid regurgitation with normal right-sided pressures.
A gated rubidium-82 dipyridamole stress test yields no evidence of a fixed or reversible perfusion defect.
Left heart catheterization reveals angiographically normal coronary arteries.
Magnetic resonance imaging (MRI) shows a moderately hypertrophied left ventricle with moderately to severely depressed systolic function (left ventricular ejection fraction 27%). The left ventricle appears dyssynchronous. Delayed-enhancement imaging reveals patchy delayed enhancement in the basal septum and the basal inferolateral walls. These findings suggest cardiac sarcoidosis, with a sensitivity of nearly 100% and a specificity of approximately 78%.1
SARCOIDOSIS IS A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem disease characterized by noncaseating granulomas. Almost any organ can be affected, but it most commonly involves the respiratory and lymphatic systems.2 Although infectious, environmental, and genetic factors have been implicated, the cause remains unknown. The prevalence is approximately 20 per 100,000, being higher in black3 and Japanese 4 populations.
CARDIAC SARCOIDOSIS
2. What percentage of patients with sarcoidosis have cardiac involvement?
- 10%–20%
- 20%–30%
- 50%
- 80%
Cardiac involvement is seen in 20% to 30% of patients with sarcoidosis.5–7 However, most cases are subclinical, and symptomatic cardiac involvement is present in only about 5% of patients with systemic sarcoidosis.8 Isolated cardiac sarcoidosis has been described in case reports but is rare.9
The clinical manifestations of cardiac sarcoidosis depend on the location and extent of granulomatous inflammation. In a necropsy study of 113 patients with cardiac sarcoidosis, the left ventricular free wall was the most common location, followed by the interventricular septum.10
3. How does cardiac sarcoidosis most commonly present?
- Conduction abnormalities
- Ventricular tachycardia
- Cardiomyopathy
- Sudden death
- None of the above
Common presentations of cardiac sarcoidosis include conduction system disease and arrhythmias (which can sometimes result in sudden death), and heart failure.
Conduction abnormalities due to granulomas (in the active phase of sarcoidosis) and fibrosis (in the fibrotic phase) in the atrioventricular node or bundle of His are the most common presentation of cardiac sarcoidosis.9 These lesions may result in relatively benign first-degree heart block or may be as potentially devastating as complete heart block.
In almost all patients with conduction abnormalities, the basal interventricular septum is involved.11 Patients who develop complete heart block from sarcoidosis tend to be younger than those with idiopathic heart block. Therefore, complete heart block in a young patient should raise the possibility of this diagnosis. 12
Ventricular tachycardia (sustained or nonsustained) and ventricular premature beats are the second most common presentation. Up to 22% of patients with sarcoidosis have electrocardiographic evidence of ventricular arrythmias. 13 The cause is believed to be myocardial scar tissue resulting from the sarcoid granulomas, leading to electrical reentry.14 Sudden death due to ventricular tachyarrhythmias or conduction blocks accounts for 25% to 65% of deaths from cardiac sarcoidosis.9,15,16
Heart failure may result from sarcoidosis when there is extensive granulomatous disease in the myocardium. Depending on the location of the granulomas, both systolic and diastolic dysfunction can occur. In severe cases, extensive granulomas can cause left ventricular aneurysms.
The diagnosis of cardiac sarcoidosis as the cause of heart failure can be difficult to establish, especially in patients without evidence of sarcoidosis elsewhere. Such patients are often given a diagnosis of idiopathic dilated cardiomyopathy. However, compared with patients with idiopathic dilated cardiomyopathy, those with cardiac sarcoidosis have a greater incidence of advanced atrioventricular block, abnormal wall thickness, focal wall motion abnormalities, and perfusion defects of the anteroseptal and apical regions.17
Progressive heart failure is the second most frequent cause of death (after sudden death) and accounts for 25% to 75% of sarcoid-related cardiac deaths.9,18,19
DIAGNOSING CARDIAC SARCOIDOSIS
4. How is cardiac sarcoidosis diagnosed?
- Electrocardiography
- Echocardiography
- Computed tomography
- Endomyocardial biopsy
- There are no guidelines for diagnosis
Given the variable extent and location of granulomas in sarcoidosis, the diagnosis of cardiac sarcoidosis is often challenging.
To make the diagnosis of sarcoidosis in general, the American Thoracic Society2 says that the clinical picture should be compatible with this diagnosis, noncaseating granulomas should be histologically confirmed, and other diseases capable of producing a similar clinical or histologic picture should be excluded.
A newer diagnostic tool, the Sarcoidosis Three-Dimensional Assessment Instrument,20 incorporates two earlier tools.20,21 It assesses three axes: organ involvement, sarcoidosis severity, and sarcoidosis activity and categorizes the diagnosis of sarcoidosis as “definite,” “probable,” or “possible.”20
In Japan, where sarcoidosis is more common, the Ministry of Health and Welfare22 says that cardiac sarcoidosis can be diagnosed histologically if operative or endomyocardial biopsy specimens contain noncaseating granuloma. In addition, the diagnosis can be suspected in patients who have a histologic diagnosis of extracardiac sarcoidosis if the first item in the list below and one or more of the rest are present:
- Complete right bundle branch block, left axis deviation, atrioventricular block, ventricular tachycardia, premature ventricular contractions (> grade 2 of the Lown classification), or Q or ST-T wave abnormalities
- Abnormal wall motion, regional wall thinning, or dilation of the left ventricle on echocardiography
- Perfusion defects on thallium 201 myocardial scintigraphy or abnormal accumulation of gallium citrate Ga 67 or technetium 99m on myocardial scintigraphy
- Abnormal intracardiac pressure, low cardiac output, or abnormal wall motion or depressed left ventricular ejection fraction on cardiac catheterization
- Nonspecific interstitial fibrosis or cellular infiltration on myocardial biopsy.
The current diagnostic guidelines from the American Thoracic Society2 and the Japanese Ministry of Health and Welfare22 and the Sarcoidosis Three-Dimensional Assessment Instrument,20 however, do not incorporate newer imaging studies as part of their criteria.
A DEFINITIVE DIAGNOSIS
5. Endomyocardial biopsy often provides the definitive diagnosis of cardiac sarcoidosis.
- True
- False
False. Endomyocardial biopsy often fails to reveal noncaseating granulomas, which have a patchy distribution.13 Table 2 summarizes the accuracy of tests for cardiac sarcoidosis.
Electrocardiography is abnormal in up to 50% of patients with sarcoidosis,23 reflecting the conduction disease or arrhythmias commonly seen in cardiac involvement.
Chest radiography classically shows hilar lymphadenopathy and interstitial disease, and may show cardiomegaly, pericardial effusion, or left ventricular aneurysm.
Echocardiography is nonspecific for sarcoid disease, but granulomatous involvement and scar tissue of cardiac tissue may appear hyperechogenic, particularly in the ventricular septum or left ventricular free wall.24
Angiography. Primary sarcoidosis rarely involves the coronary arteries,25 so angiography is most useful in excluding the diagnosis of atherosclerotic coronary artery disease.
Radionuclide imaging with thallium 201 in patients with suspected cardiac sarcoidosis may be useful to suggest myocardial involvement and to exclude cardiac dysfunction secondary to coronary artery disease. Segmental areas with defective thallium 201 uptake correspond to fibrogranulomatous tissue. In resting images, the pattern may be similar to that seen in coronary artery disease. However, during exercise, perfusion defects increase in patients who have ischemia but actually decrease in those with cardiac sarcoidosis.26
Nevertheless, some conclude that thallium scanning is too nonspecific for it to be used as a diagnostic or screening test.27,28 The combined use of thallium 201 and gallium 67 may better detect cardiac sarcoidosis, as gallium is taken up in areas of active inflammation.
Positron-emission tomography (PET) with fluorodeoxyglucose F 18 (FDG), with the patient fasting, appears to be useful in detecting the early inflammation of cardiac sarcoidosis29–34 and monitoring disease activity.30,31 FDG is a glucose analogue that is taken up by granulomatous tissue in the myocardium.34 The uptake in cardiac sarcoidosis is in a focal distribution.30,31,34 The abnormal FDG uptake resolves with steroid treatment.31,32
MRI has promise for diagnosing cardiac sarcoidosis. With gadolinium contrast, MRI has superior image resolution and can detect cardiac involvement early in its course.27,29,35–44
Inflammation of the myocardium due to sarcoid involvement appears as focal zones of increased signal intensity on both T2-weighted and early gadolinium T1-weighted images. Late myocardial enhancement after gadolinium infusion is the most typical finding of cardiac sarcoidosis on MRI, and likely represents fibrogranulomatous tissue.27 Delayed gadolinium enhancement is also seen in myocardial infarction but differs in its distribution.1,35,45 Cardiac sarcoidosis most commonly affects the basal and lateral segments. In one study, the finding of delayed enhancement had a sensitivity of 100% and a specificity of 78%,1,27 though it may not sufficiently differentiate active inflammation from scar.30
Like FDG-PET, MRI has also been shown to be useful for monitoring treatment.33,46 However, PET is more useful for follow-up in patients who receive a pacemaker or implantable cardioverter-defibrillator, in whom MRI is contraindicated. One case report29 described using both delayed-enhancement MRI and FDG-PET to diagnose cardiac sarcoidosis.
TREATMENT
6. How is cardiac sarcoidosis currently treated?
- Implantable cardioverter-defibrillator
- Corticosteroids
- Heart transplantation
- All of the above
- None of the above
Corticosteroids
Corticosteroids are the mainstay of treatment of cardiac sarcoidosis, as they attenuate the characteristic inflammation and fibrosis of sarcoid granulomas. The goal is to prevent compromise of cardiac structure or function.47 Although most of the supporting data are anecdotal, steroids have been shown to improve ventricular contractility,48 arrhythmias,49 and conduction abnormalities.50 MRI and FDG-PET studies have shown cardiac lesions resolving after steroids were started.31,45,46
The optimal dosage remains unknown. Initial doses of 30 to 60 mg daily, gradually tapered over 6 to 12 months to maintenance doses of 5 to 10 mg daily, have been effective.45,51
Relapses are common and require vigilant monitoring.
Alternative agents such as cyclophosphamide (Cytoxan),52 methotrexate (Rheumatrex), 53 and cyclosporine (Sandimmune)54 can be given to patients whose disease does not respond to corticosteroids or who cannot tolerate their side effects.
Implantable cardioverter-defibrillator
Sudden death due to ventricular tachyarrhythmias or conduction block accounts for 30% to 65% of deaths in patients with cardiac sarcoidosis.10 The rates of recurrent ventricular tachycardia and sudden death are high, even with antiarrhythmic drug therapy.55
Although experience with implantable cardiac defibrillators is limited in patients with cardiac sarcoidosis,55–58 some have argued that they be strongly considered to prevent sudden cardiac death in this high-risk group.57,58
Heart transplantation
The largest body of data on transplantation comes from the United Network for Organ Sharing database. In the 65 patients with cardiac sarcoidosis who underwent cardiac transplantation in the 18 years from October 1987 to September 2005, the 1-year post-transplant survival rate was 88%, which was better than in patients with all other diagnoses (85%). The 5-year survival rate was 80%.59,60
Recurrence of sarcoidosis within the cardiac allograft and transmission of sarcoidosis from donor to recipient have both been documented after heart transplantation.61,62
CAUSES OF DEATH
7. What is the most common cause of death in patients with cardiac sarcoidosis?
- Respiratory failure
- Conduction disturbances
- Progressive heart failure
- Ventricular tachyarrhythmias
- None of the above
The prognosis of symptomatic cardiac sarcoidosis is not well defined, owing to the variable extent and severity of the disease. The mortality rate in sarcoidosis without cardiac involvement is about 1% to 5% per year.63,64 Cardiac involvement portends a worse prognosis, with a 55% survival rate at 5 years and 44% at 10 years.17,65 Most patients in the reported series ultimately died of cardiac complications of sarcoidosis, including ventricular tachyarrhythmias, conduction disturbances, and progressive cardiomyopathy.10,17
Since corticosteroids, pacemakers, and implantable cardioverter-defibrillators have begun to be used, the cause of death has shifted from sudden death to progressive heart failure.66
CASE CONTINUED
Electrophysiologic testing revealed inducible monomorphic sustained ventricular tachycardia. The patient subsequently had a biventricular cardioverter-defibrillator implanted. He was started on an angiotensin-converting enzyme inhibitor and a beta-blocker for his heart failure. Further imaging of his chest and abdomen revealed lesions in his thyroid and liver. As of this writing, he is undergoing further workup. Because of active infection with Clostridium difficile, steroid therapy was deferred.
- Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997; 145:234–241.
- Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann NY Acad Sci 1976; 278:455–469.
- Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004; 83:315–334.
- Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994; 11:26–31.
- Thomsen TK, Eriksson T. Myocardial sarcoidosis in forensic medicine. Am J Forensic Med Pathol 1999; 20:52–56.
- Silverman KJ, Hutchins GM, Buckley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978; 58:1204–1211.
- Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977; 63:86–108.
- Bargout R, Kelly R. Sarcoid heart disease: clinical course and treatment. Int J Cardiol 2004; 97:173–182.
- Abeler V. Sarcoidosis of the cardiac conducting system. Am Heart J 1979; 97:701–707.
- Fleming HA, Bailey SM. Sarcoid heart disease. J R Coll Physicians Lond 1981; 15:245–253.
- Sekiguchi M, Numao Y, Imai M, Furuie T, Mikami R. Clinical and histological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn Circ J 1980; 44:249–263.
- Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol 2004; 27:217–222.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Reuhl J, Schneider M, Sievert H, Lutz FU, Zieger G. Myocardial sarcoidosis as a rare cause of sudden cardiac death. Forensic Sci Int 1997; 89:145–153.
- Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol 1998; 82:537–540.
- Fleming H. Cardiac sarcoidosis. In:James DG, editor. Sarcoidosis and Other Granulomatous Disorders. New York, NY: Dekker 1994; 73:323–334.
- Padilla M. Cardiac sarcoidosis. In:Baughman R, editor. Lung Biology in Health and Disease (Sarcoidosis), vol 210. New York, NY: Taylor & Francis Group; 2006:515–552.
- Judson MA. A proposed solution to the clinical assessment of sarcoidosis: the sarcoidosis three-dimensional assessment instrument (STAI). Med Hypotheses 2007; 68:1080–1087.
- Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16:75–86.
- Hiraga H, Yuwai K, Hiroe M, et al. Guideline for diagnosis of cardiac sarcoidosis. Study report of diffuse pulmonary diseases. Tokyo, Japan: The Japanese Ministry of Health and Welfare, 1993:23–24 (in Japanese).
- Stein E, Jackler I, Stimmel B, Stein W, Siltzbach LE. Asymptomatic electrocardiographic alterations in sarcoidosis. Am Heart J 1973; 86:474–477.
- Fahy GJ, Marwick T, McCreery CJ, Quigley PJ, Maurer BJ. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 1996; 109:62–66.
- Butany J, Bahl NE, Morales K, et al. The intricacies of cardiac sarcoidosis: a case report involving the coronary arteries and a review of the literature. Cardiovasc Pathol 2006; 15:222–227.
- Haywood LJ, Sharma OP, Siegel ME, et al. Detection of myocardial sarcoidosis by thallium-201 imaging. J Natl Med Assoc 1982; 74:959–964.
- Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol 2005; 185:110–115.
- Kinney EL, Caldwell JW. Do thallium myocardial perfusion scan abnormalities predict survival in sarcoid patients without cardiac symptoms? Angiology 1990; 41:573–576.
- Pandya C, Brunken RC, Tchou P, Schoenhagen P, Culver DA. Detecting cardiac involvement in sarcoidosis: a call for prospective studies of newer imaging techniques. Eur Respir J 2007; 29:418–422.
- Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008; 35:933–941.
- Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with 13N-NH3/18F-FDG PET. J Nucl Med 2003; 44:1030–1036.
- Takeda N, Yokoyama I, Hiroi Y, et al. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation 2002; 105:1144–1145.
- Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005; 26:1538–1543.
- Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004; 45:1989–1998.
- Schulz-Menger J, Wassmuth R, Abdel-Aty H, et al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006; 92:399–400.
- Kiuchi S, Teraoka K, Koizumi K, Takazawa K, Yamashina A. Usefulness of late gadolinium enhancement combined with MRI and 67-Ga scintigraphy in the diagnosis of cardiac sarcoidosis and disease activity evaluation. Int J Cardiovasc Imaging 2007; 23:237–241.
- Matsuki M, Matsuo M. MR findings of myocardial sarcoidosis. Clin Radiol 2000; 55:323–325.
- Inoue S, Shimada T, Murakami Y. Clinical significance of gadolinium-DTPA-enhanced MRI for detection of myocardial lesions in a patient with sarcoidosis. Clin Radiol 1999; 54:70–72.
- Vignaux O, Dhote R, Dudoc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrastenhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr 2002; 26:762–767.
- Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol 2005; 184:249–254.
- Smedema JP, Snoep G, Van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Doherty MJ, Kumar SK, Nicholson AA, McGivern DV. Cardiac sarcoidosis: the value of magnetic resonance imagine in diagnosis and assessment of response to treatment. Respir Med 1998; 92:697–699.
- Smedema JP, Truter R, de Klerk PA, Zaaiman L, White L, Doubell AF. Cardiac sarcoidosis evaluated with gadolinium-enhanced magnetic resonance and contrast-enhanced 64-slice computed tomography. Int J Cardiol 2006; 112:261–263.
- Kanao S, Tadamura E, Yamamuro M, et al. Demonstration of cardiac involvement of sarcoidosis by contrast-enhanced multislice computed tomography and delayed-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2005; 29:745–748.
- Vignaux O, Dhote R, Duboc D, et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest 2002; 122:1895–1901.
- Shimada T, Shimada K, Sakane T, et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med 2001; 110:520–527.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of longterm survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Ishikawa T, Kondoh H, Nakagawa S, Koiwaya Y, Tanaka K. Steroid therapy in cardiac sarcoidosis. Increased left ventricular contractility concomitant with electrocardiographic improvement after prednisolone. Chest 1984; 85:445–447.
- Walsh MJ. Systemic sarcoidosis with refractory ventricular tachycardia and heart failure. Br Heart J 1978; 40:931–933.
- Lash R, Coker J, Wong BY. Treatment of heart block due to sarcoid heart disease. J Electrocardiol 1979; 12:325–329.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and implications. Ann N Y Acad Sci 1986; 465:702–712.
- Demeter SL. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest 1988; 94:202–203.
- Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995; 155:846–851.
- York EL, Kovithavongs T, Man SF, Rebuck AS, Sproule BJ. Cyclosporine and chronic sarcoidosis. Chest 1990; 98:1026–1029.
- Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 1991; 18:937–943.
- Bajaj AK, Kopelman HA, Echt DS. Cardiac sarcoidosis with sudden death: treatment with automatic implantable cardioverter defibrillator. Am Heart J 1988; 116:557–560.
- Paz HL, McCormick DJ, Kutalek SP, Patchefsky A. The automated implantable cardiac defibrillator. Prophylaxis in cardiac sarcoidosis. Chest 1994; 106:1603–1607.
- Becker D, Berger E, Chmielewski C. Cardiac sarcoidosis: a report of four cases with ventricular tachycardia. J Cardiovasc Electrophysiol 1990; 1:214–219.
- Zaidi AR, Zaidi A, Vaitkus PT. Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant 2007; 26:714–717.
- Valantine HA, Tazelaar HD, Macoviak J, et al. Cardiac sarcoidosis: response to steroids and transplantation. J Heart Transplant 1987; 6:244–250.
- Oni AA, Hershberger RE, Norman DJ, et al. Recurrence of sarcoidosis in a cardiac allograft: control with augmented corticosteroids. J Heart Lung Transplant 1992; 11:367–369.
- Burke WM, Keogh A, Maloney PJ, Delprado W, Bryant DH, Spratt P. Transmission of sarcoidosis via cardiac transplantation. Lancet 1990; 336:1579.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and complications. Ann N Y Acad Sci 1986; 465:702–712.
- Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med 1996; 100:423–427.
- Fleming HA, Bailey SM. The prognosis of sarcoid heart disease in the United Kingdom. Ann N Y Acad Sci 1986; 465:543–550.
- Takada K, Ina Y, Yamamoto M, Satoh T, Morishita M. Prognosis after pacemaker implantation in cardiac sarcoidosis in Japan. Clinical evaluation of corticosteroid therapy. Sarcoidosis 1994; 11:113–117.
- Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997; 145:234–241.
- Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann NY Acad Sci 1976; 278:455–469.
- Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004; 83:315–334.
- Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994; 11:26–31.
- Thomsen TK, Eriksson T. Myocardial sarcoidosis in forensic medicine. Am J Forensic Med Pathol 1999; 20:52–56.
- Silverman KJ, Hutchins GM, Buckley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978; 58:1204–1211.
- Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977; 63:86–108.
- Bargout R, Kelly R. Sarcoid heart disease: clinical course and treatment. Int J Cardiol 2004; 97:173–182.
- Abeler V. Sarcoidosis of the cardiac conducting system. Am Heart J 1979; 97:701–707.
- Fleming HA, Bailey SM. Sarcoid heart disease. J R Coll Physicians Lond 1981; 15:245–253.
- Sekiguchi M, Numao Y, Imai M, Furuie T, Mikami R. Clinical and histological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn Circ J 1980; 44:249–263.
- Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol 2004; 27:217–222.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Reuhl J, Schneider M, Sievert H, Lutz FU, Zieger G. Myocardial sarcoidosis as a rare cause of sudden cardiac death. Forensic Sci Int 1997; 89:145–153.
- Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol 1998; 82:537–540.
- Fleming H. Cardiac sarcoidosis. In:James DG, editor. Sarcoidosis and Other Granulomatous Disorders. New York, NY: Dekker 1994; 73:323–334.
- Padilla M. Cardiac sarcoidosis. In:Baughman R, editor. Lung Biology in Health and Disease (Sarcoidosis), vol 210. New York, NY: Taylor & Francis Group; 2006:515–552.
- Judson MA. A proposed solution to the clinical assessment of sarcoidosis: the sarcoidosis three-dimensional assessment instrument (STAI). Med Hypotheses 2007; 68:1080–1087.
- Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16:75–86.
- Hiraga H, Yuwai K, Hiroe M, et al. Guideline for diagnosis of cardiac sarcoidosis. Study report of diffuse pulmonary diseases. Tokyo, Japan: The Japanese Ministry of Health and Welfare, 1993:23–24 (in Japanese).
- Stein E, Jackler I, Stimmel B, Stein W, Siltzbach LE. Asymptomatic electrocardiographic alterations in sarcoidosis. Am Heart J 1973; 86:474–477.
- Fahy GJ, Marwick T, McCreery CJ, Quigley PJ, Maurer BJ. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 1996; 109:62–66.
- Butany J, Bahl NE, Morales K, et al. The intricacies of cardiac sarcoidosis: a case report involving the coronary arteries and a review of the literature. Cardiovasc Pathol 2006; 15:222–227.
- Haywood LJ, Sharma OP, Siegel ME, et al. Detection of myocardial sarcoidosis by thallium-201 imaging. J Natl Med Assoc 1982; 74:959–964.
- Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol 2005; 185:110–115.
- Kinney EL, Caldwell JW. Do thallium myocardial perfusion scan abnormalities predict survival in sarcoid patients without cardiac symptoms? Angiology 1990; 41:573–576.
- Pandya C, Brunken RC, Tchou P, Schoenhagen P, Culver DA. Detecting cardiac involvement in sarcoidosis: a call for prospective studies of newer imaging techniques. Eur Respir J 2007; 29:418–422.
- Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008; 35:933–941.
- Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with 13N-NH3/18F-FDG PET. J Nucl Med 2003; 44:1030–1036.
- Takeda N, Yokoyama I, Hiroi Y, et al. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation 2002; 105:1144–1145.
- Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005; 26:1538–1543.
- Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004; 45:1989–1998.
- Schulz-Menger J, Wassmuth R, Abdel-Aty H, et al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006; 92:399–400.
- Kiuchi S, Teraoka K, Koizumi K, Takazawa K, Yamashina A. Usefulness of late gadolinium enhancement combined with MRI and 67-Ga scintigraphy in the diagnosis of cardiac sarcoidosis and disease activity evaluation. Int J Cardiovasc Imaging 2007; 23:237–241.
- Matsuki M, Matsuo M. MR findings of myocardial sarcoidosis. Clin Radiol 2000; 55:323–325.
- Inoue S, Shimada T, Murakami Y. Clinical significance of gadolinium-DTPA-enhanced MRI for detection of myocardial lesions in a patient with sarcoidosis. Clin Radiol 1999; 54:70–72.
- Vignaux O, Dhote R, Dudoc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrastenhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr 2002; 26:762–767.
- Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol 2005; 184:249–254.
- Smedema JP, Snoep G, Van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Doherty MJ, Kumar SK, Nicholson AA, McGivern DV. Cardiac sarcoidosis: the value of magnetic resonance imagine in diagnosis and assessment of response to treatment. Respir Med 1998; 92:697–699.
- Smedema JP, Truter R, de Klerk PA, Zaaiman L, White L, Doubell AF. Cardiac sarcoidosis evaluated with gadolinium-enhanced magnetic resonance and contrast-enhanced 64-slice computed tomography. Int J Cardiol 2006; 112:261–263.
- Kanao S, Tadamura E, Yamamuro M, et al. Demonstration of cardiac involvement of sarcoidosis by contrast-enhanced multislice computed tomography and delayed-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2005; 29:745–748.
- Vignaux O, Dhote R, Duboc D, et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest 2002; 122:1895–1901.
- Shimada T, Shimada K, Sakane T, et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med 2001; 110:520–527.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of longterm survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Ishikawa T, Kondoh H, Nakagawa S, Koiwaya Y, Tanaka K. Steroid therapy in cardiac sarcoidosis. Increased left ventricular contractility concomitant with electrocardiographic improvement after prednisolone. Chest 1984; 85:445–447.
- Walsh MJ. Systemic sarcoidosis with refractory ventricular tachycardia and heart failure. Br Heart J 1978; 40:931–933.
- Lash R, Coker J, Wong BY. Treatment of heart block due to sarcoid heart disease. J Electrocardiol 1979; 12:325–329.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and implications. Ann N Y Acad Sci 1986; 465:702–712.
- Demeter SL. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest 1988; 94:202–203.
- Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995; 155:846–851.
- York EL, Kovithavongs T, Man SF, Rebuck AS, Sproule BJ. Cyclosporine and chronic sarcoidosis. Chest 1990; 98:1026–1029.
- Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 1991; 18:937–943.
- Bajaj AK, Kopelman HA, Echt DS. Cardiac sarcoidosis with sudden death: treatment with automatic implantable cardioverter defibrillator. Am Heart J 1988; 116:557–560.
- Paz HL, McCormick DJ, Kutalek SP, Patchefsky A. The automated implantable cardiac defibrillator. Prophylaxis in cardiac sarcoidosis. Chest 1994; 106:1603–1607.
- Becker D, Berger E, Chmielewski C. Cardiac sarcoidosis: a report of four cases with ventricular tachycardia. J Cardiovasc Electrophysiol 1990; 1:214–219.
- Zaidi AR, Zaidi A, Vaitkus PT. Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant 2007; 26:714–717.
- Valantine HA, Tazelaar HD, Macoviak J, et al. Cardiac sarcoidosis: response to steroids and transplantation. J Heart Transplant 1987; 6:244–250.
- Oni AA, Hershberger RE, Norman DJ, et al. Recurrence of sarcoidosis in a cardiac allograft: control with augmented corticosteroids. J Heart Lung Transplant 1992; 11:367–369.
- Burke WM, Keogh A, Maloney PJ, Delprado W, Bryant DH, Spratt P. Transmission of sarcoidosis via cardiac transplantation. Lancet 1990; 336:1579.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and complications. Ann N Y Acad Sci 1986; 465:702–712.
- Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med 1996; 100:423–427.
- Fleming HA, Bailey SM. The prognosis of sarcoid heart disease in the United Kingdom. Ann N Y Acad Sci 1986; 465:543–550.
- Takada K, Ina Y, Yamamoto M, Satoh T, Morishita M. Prognosis after pacemaker implantation in cardiac sarcoidosis in Japan. Clinical evaluation of corticosteroid therapy. Sarcoidosis 1994; 11:113–117.
Update on 2009 pandemic influenza A (H1N1) virus
A 69-year-old ohio man with leukemia was treated in another state in late June. During the car trip back to Ohio, he developed a sore throat, fever, cough, and nasal congestion. He was admitted to Cleveland Clinic with a presumed diagnosis of neutropenic fever; his absolute neutrophil count was 0.4 × 109/L (reference range 1.8–7.7). His chest radiograph was normal. He was treated with empiric broad-spectrum antimicrobials. On his second day in the hospital, he was tested for influenza by a polymerase chain reaction (PCR) test, which was positive for influenza A. He was moved to a private room and started on oseltamivir (Tamiflu) and rimantadine (Flumadine). The patient’s previous roommate subsequently tested positive for influenza A, as did two health care workers working on the ward. All patients on the floor received prophylactic oseltamivir.
The patient’s condition worsened, and he subsequently went into respiratory distress with diffuse pulmonary infiltrates. He was transferred to the intensive care unit, where he was intubated. Influenza A was isolated from a bronchoscopic specimen. He subsequently recovered after a prolonged course and was discharged on hospital day 50. Testing by the Ohio Department of Health confirmed that this was the 2009 pandemic influenza A (H1N1) virus.
THE CHALLENGES WE FACE
We are now in the midst of an influenza pandemic of the 2009 influenza A (H1N1) virus, with pandemic defined as “worldwide sustained community transmission.” The circulation of seasonal and 2009 pandemic influenza A (H1N1) strains will make this flu season both interesting and challenging.
The approaches to vaccination, prophylaxis, and treatment will be more complex. As of this writing (mid-September 2009), it is clear that we will be giving two influenza vaccines this season: a trivalent vaccine for seasonal influenza, and a monovalent vaccine for pandemic H1N1. It appears the monovalent vaccine may require only one dose to provide protective immunity.1 Fortunately, the vast majority of cases of pandemic H1N1 are relatively mild and uncomplicated. Still, some people are at higher risk of complications, including young patients, pregnant women, and people with immune deficiency or concomitant health conditions that put them at higher risk of flu-associated complications. Thus, clinicians will need to be educated about whom to test, who needs prophylaxis, and who should not be treated.
As our case demonstrates, unsuspected cases of influenza in hospitalized patients or health care workers working with influenza pose the greatest threat for transmission of influenza within the hospital. Adults hospitalized with influenza tend to present late (more than 48 hours after the onset of symptoms) and tend to have prolonged illness.2 Ambulatory adults shed virus for 3 to 6 days; virus shedding is more prolonged for hospitalized patients. Antiviral agents started within 4 days of illness enhance viral clearance and are associated with a shorter stay.3 Therefore, we should have a low threshold for testing for influenza and for isolating all suspected cases.
This is also creating a paradigm shift for health care workers, who are notorious for working through an illness. If you are sick, stay home! This applies whether you have pandemic H1N1 or something else.
EPIDEMIOLOGY OF PANDEMIC 2009 INFLUENZA A (H1N1) VIRUS
The location of cases can now be found on Google Maps; the US Centers for Disease Control and Prevention (CDC) provides weekly influenza reports at www.cdc.gov/flu/weekly/fluactivity.htm.
Pandemic H1N1 appeared in the spring of 2009, and cases continued to mount all summer in the United States (when influenza is normally absent) and around the world. In Mexico in March and April 2009, 2,155 cases of pneumonia, 821 hospitalizations, and 100 deaths were reported.4
In contrast with seasonal influenza, children and younger adults were hit the hardest in Mexico. The age group 5 through 59 years accounted for 87% of the deaths (usually, they account for about 17%) and 71% of the cases of severe pneumonia (usually, they account for 32%). These observations may be explained in part by the possibility that people who were alive during the 1957 pandemic (which was an H1N1 strain) have some immunity to the new virus. However, the case-fatality rate was highest in people age 65 and older.4
As of July 2009, there were more than 43,000 confirmed cases of pandemic H1N1 in the United States, and actual cases probably exceed 1 million, with more than 400 deaths. An underlying risk factor was identified in more than half of the fatal cases.5 Ten percent of the women who died were pregnant.
Pandemic H1N1 has several distinctive epidemiologic features:
- The distribution of cases is similar across multiple geographic areas.
- The distribution of cases by age group is markedly different than that of seasonal influenza, with more cases in school children and fewer cases in older adults.
- Fewer cases have been reported in older adults, but this group has the highest case-fatality rate.
2009 PANDEMIC H1N1 IS A MONGREL
There are three types of influenza viruses, designated A, B, and C. Type A undergoes antigenic shift (rapid changes) and antigenic drift (gradual changes) from year to year, and so it is the type associated with pandemics. In contrast, type B undergoes antigenic drift only, and type C is relatively stable.
Influenza virus is subtyped on the basis of surface glycoproteins: 16 hemagglutinins and nine neuraminidases. The circulating subtypes change every year; the current circulating human subtypes are a seasonal subtype of H1N1 that is different than the pandemic H1N1 subtype, and H3N2.
The 2009 pandemic H1N1 is a new virus never seen before in North America.6 Genetically, it is a mongrel, coming from three recognized sources (pigs, birds, and humans) which were combined in pigs.7 It is similar to subtypes that circulated in the 1920s through the 1940s.
Most influenza in the Western world comes from Asia every fall, and its arrival is probably facilitated by air travel. The spread is usually unidirectional and is unlikely to contribute to long-term viral evolution.8 It appears that 2009 H1N1 virus is the predominant strain circulating in the current influenza season in the Southern Hemisphere. Virologic studies indicate that the H1N1 virus strain has remained antigenically stable since it appeared in April 2009. Thus, it appears likely that the strain selected by the United States for vaccine manufacturing will match the currently circulating seasonal and pandemic H1N1 strains.
VACCINATION IS THE FIRST LINE OF DEFENSE
In addition to the trivalent vaccine against seasonal influenza, a monovalent vaccine for pandemic H1N1 virus is being produced. The CDC has indicated that 45 million doses of pandemic influenza vaccine are expected in October 2009, with an average of 20 million doses each week thereafter. It is anticipated that half of these will be in multidose vials, that 20% will be in prefilled syringes for children over 5 years old and for pregnant women, and that 20% will be in the form of live-attenuated influenza vaccine (nasal spray). The inhaled vaccine should not be given to children under 2 years old, to children under 5 years old who have recurrent wheezing, or to anyone with severe asthma. Neither vaccine should be given to people allergic to hen eggs, from which the vaccine is produced.
An ample supply of the seasonal trivalent vaccine should be available. Once the CDC has more information about specific product availability of the pandemic H1N1 vaccine, that vaccine will be distributed. It can be given concurrently with seasonal influenza vaccine.
Several definitions should be kept in mind when discussing vaccination strategies. Supply is the number of vaccine doses available for distribution. Availability is the ability of a person recommended to be vaccinated to do so in a local venue. Prioritization is the recommendation to vaccination venues to selectively use vaccine for certain population groups first. Targeting is the recommendation that immunization programs encourage and promote vaccination for certain population groups.
The Advisory Committee on Immunization Practices and the CDC recommend both seasonal and H1N1 vaccinations for anyone 6 months of age or older who is at risk of becoming ill or of transmitting the viruses to others. Based on a review of epidemiologic data, the recommendation is for targeting the following five groups for H1N1 vaccination: children and young adults aged 6 months through 24 years; pregnant women; health care workers and emergency medical service workers; people ages 25 through 64 years who have certain health conditions (eg, diabetes, heart disease, lung disease); and people who live with or care for children younger than 6 months of age. This represents approximately 159 million people in the United States.
If the estimates for the vaccine supply are met, and if pandemic H1N1 vaccine requires only a single injection, there should be no need for prioritization of vaccine. If the supply of pandemic H1N1 vaccine is inadequate, then those groups who are targeted would also receive the first doses of the pandemic H1N1 vaccine. It should be used only with caution after consideration of potential benefits and risks in people who have had Guillain-Barré syndrome during the previous 6 weeks, in people with altered immunocompetence, or in people with medical conditions predisposing to influenza complications.
A mass vaccination campaign involving two separate flu vaccines can pose challenges in execution and messaging for public health officials and politicians. In 1976, an aggressive vaccination program turned into a disaster, as there was no pandemic and the vaccine was associated with adverse effects such as Guillain-Barré syndrome. The government and the medical profession need to prepare for a vaccine controversy and to communicate and continue to explain the plan to the public. As pointed out in a recent op-ed piece,9 we would hope that all expectant women in the fall flu season will get the flu vaccines. We also know that, normally, one in seven pregnancies would be expected to miscarry. The challenge for public health officials and physicians will be to explain to these patients that there may be an association rather than a causal relationship.
In health care workers, the average vaccination rate is only 37%. We should be doing much better. Cleveland Clinic previously increased the rate of vaccination among its employees via a program in which all workers must either be vaccinated or formally declare (on an internal Web site) that they decline to be vaccinated.10 This season, even more resources are being directed at decreasing the barriers to flu vaccinations for our health care workers with the support from hospital leadership.
INFECTION CONTROL IN THE HOSPITAL AND IN THE COMMUNITY
Influenza is very contagious and is spread in droplets via sneezing and coughing (within a 3-foot radius), or via unwashed hands—thus the infection-control campaigns urging you to cover your cough and wash your hands.
As noted, for patients being admitted or transferred to the hospital, we need to have a low threshold for testing for influenza and for isolating patients suspected of having influenza. For patients with suspected or proven seasonal influenza, transmission precautions are those recommended by the CDC for droplet precautions (www.cdc.gov/ncidod/dhqp/gl_isolation_droplet.html). A face mask is deemed adequate to protect transmission when coming within 3 feet of an infected person. CDC guidelines for pandemic H1N1 recommends airborne-transmission-based precautions for health care workers who are in close contact with patients with proven or possible H1N1 (www.cdc.gov/ncidod/dhqp/gl_isolation_airborne.html). This recommendation implies the use of fit-tested N95 respirators and negative air pressure rooms (if available).
The recent Institute of Medicine report, Respiratory Protection for Healthcare Workers in the Workplace Against Novel H1N1 Influenza A (www.iom.edu/CMS/3740/71769/72967/72970.aspx) endorses the current CDC guidelines and recommends following these guidelines until we have evidence that other forms of protection or guidelines are equally or more effective.
Personally, I am against this requirement because it creates a terrible administrative burden with no proven benefit. Requiring a respirator means requiring fit-testing, and this will negatively affect our ability to deliver patient care. Recent studies have shown that surgical masks may not be as effective11 but are probably sufficient. Lim et al12 reported that 79 (37%) of 212 workers who responded to a survey experienced headaches while wearing N95 masks. This remains a controversial issue.
Besides getting the flu shot, what can one do to avoid getting influenza or transmitting to others?
- Cover your cough (cough etiquette) and sneeze.
- Practice good hand hygiene.
- Avoid close contact with people who are sick.
- Do not go to school or work if sick.
A recent study of influenza in households suggested that having the person with flu and household contacts wear face masks and practice hand hygiene within the first 36 hours decreased transmission of flu within the household.13
The United States does have a national influenza pandemic plan that outlines specific roles in the event of a pandemic, and I urge you to peruse it at www.hhs.gov/pandemicflu/plan/.
RECOGNIZING AND DIAGNOSING INFLUENZA
The familiar signs and symptoms of influenza—fever, cough, muscle aches, and headache—are nonspecific. Call et al14 analyzed the diagnostic accuracy of symptoms and signs of influenza and found that fever and cough during an epidemic suggest but do not confirm influenza, and that sneezing in those over age 60 argues against influenza. They concluded that signs and symptoms can tell us whether a patient has an influenza-like illness, but do not confirm or exclude the diagnosis of influenza: “Clinicians need to consider whether influenza is circulating in their communities, and then either treat patients with influenza-like illness empirically or obtain a rapid influenza test.”14
The signs and symptoms of pandemic 2009 H1N1 are the same as for seasonal flu, except that about 25% of patients with pandemic flu develop gastrointestinal symptoms. It has not been more virulent than seasonal influenza to date.
Should you order a test for influenza?
Most people with influenza are neither tested nor treated. Before ordering a test for influenza, ask, “Does this patient actually have influenza?” Patients diagnosed with “influenza” may have a range of infectious and noninfectious causes, such as vasculitis, endocarditis, or any other condition that can cause a fever and cough.
If I truly suspect influenza, I would still only order a test if the results would change how I manage the patient—for example, a patient being admitted to the hospital where isolation would be required.
Pandemic H1N1 will be detected only as influenza A in our current PCR screen for human influenza. The test does not differentiate between seasonal strains of influenza A (which is resistant to oseltamivir) and pandemic H1N1 (which is susceptible to oseltamivir). This means if you intend to treat, you will have to address further complexity.
Testing for influenza
The clinician should be familiar with the types of tests available. Each test has advantages and disadvantages15:
Rapid antigen assay is a point-of-care test that can give results in 15 minutes but unfortunately is only 20% to 30% sensitive, so a negative result does not exclude the diagnosis. The positive predictive value is high, meaning a positive test means the patient does have the flu.
Direct fluorescent antibody testing takes about 2.5 hours to complete and requires special training for technicians. It has a sensitivity of 47%, a positive predictive value of 95%, and a negative predictive value of 92%.
PCR testing takes about 6 hours and has a sensitivity of 98%, a positive predictive value of 100%, and a negative predictive value of 98%. This is probably the best test, in view of its all-around performance, but it is not a point-of-care test.
Culture takes 2 to 3 days, has a sensitivity of 89%, a positive predictive value of 100%, and a negative predictive value of 88%.
These tests can determine that the patient has influenza A, but a confirmatory test is always required to confirm pandemic H1N1. This confirmatory testing can be done by the CDC, by state public health laboratories, and by commercial reference laboratories.
ANTIVIRAL TREATMENT
Since influenza test results do not specify whether the patient has seasonal or pandemic influenza, treatment decisions are a sticky wicket. Most patients with pandemic H1N1 do not need to be tested or treated.
Several drugs are approved for treating influenza and shorten the duration of symptoms by about 1 day. The earlier the treatment is started, the better: the time of antiviral initiation affects influenza viral load and the duration of viral shedding.3
The neuraminidase inhibitors oseltamivir and zanamivir (Relenza) block release of virus from the cell. Resistance to oseltamivir is emerging in seasonal influenza A, while most pandemic H1N1 strains are susceptible.
Oseltamivir resistance in pandemic H1N1
A total of 11 cases of oseltamivir-resistant pandemic H1N1 have been confirmed worldwide, including 3 in the United States (2 in immunosuppressed patients in Seattle, WA). Ten of the 11 cases occurred with oseltamivir exposure. All involved a histidine-to-tyrosine substitution at position 275 (H275Y) of the neuraminidase gene. Most were susceptible to zanamivir.
Supplies of oseltamivir and zanamivir are limited, so they should be used only in those who will benefit the most, ie, those at higher risk of influenza complications. These include children under 5 years old, adults age 65 and older, children and adolescents on long-term aspirin therapy, pregnant women, patients who have chronic conditions or who are immunosuppressed, and residents of long-term care facilities.
- Greenberg MA, Lai MH, Hartel GF. Response after one dose of a monovalent influenza A (H1N1) 2009 vaccine—preliminary report. N Engl J Med 2009;361doi:10.1056/NEJMoa0907413 [published online ahead of print].
- Ison M. Influenza in hospitalized adults: gaining insight into a significant problem. J Infect Dis 2009; 200:485–488.
- Lee N, Chan PKS, Hui DSC, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200:492–500.
- Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679.
- Vaillant L, La Ruche G, Tarantola A, Barboza P; for the Epidemic Intelligence Team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009; 14(33):1–6. Available online at www.eurosurveillance.org/ViewArticle.aspx?ArticleID=19309.
- Zimmer SM, Burke DS. Historical perspective—emergence of influenza A (H1N1) viruses. N Engl J Med 2009; 361:279–285.
- Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201.
- Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340–346.
- Allen A. Prepare for a vaccine controversy. New York Times. 9/1/2009.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis 2009; 49:275–277.
- Lim EC, Seet RC, Lee KH, Wilder-Smith EP, Chuah BY, Ong BK. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol Scand 2006; 113:199–202.
- Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a randomized trial. Ann Intern Med 2009; 151(6 Oct) [published online ahead of print].
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005; 293:987–997.
- Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol 2009; 45:191–195.
- US Centers for Disease Control and Prevention. Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR 2009; 58:893–896.
A 69-year-old ohio man with leukemia was treated in another state in late June. During the car trip back to Ohio, he developed a sore throat, fever, cough, and nasal congestion. He was admitted to Cleveland Clinic with a presumed diagnosis of neutropenic fever; his absolute neutrophil count was 0.4 × 109/L (reference range 1.8–7.7). His chest radiograph was normal. He was treated with empiric broad-spectrum antimicrobials. On his second day in the hospital, he was tested for influenza by a polymerase chain reaction (PCR) test, which was positive for influenza A. He was moved to a private room and started on oseltamivir (Tamiflu) and rimantadine (Flumadine). The patient’s previous roommate subsequently tested positive for influenza A, as did two health care workers working on the ward. All patients on the floor received prophylactic oseltamivir.
The patient’s condition worsened, and he subsequently went into respiratory distress with diffuse pulmonary infiltrates. He was transferred to the intensive care unit, where he was intubated. Influenza A was isolated from a bronchoscopic specimen. He subsequently recovered after a prolonged course and was discharged on hospital day 50. Testing by the Ohio Department of Health confirmed that this was the 2009 pandemic influenza A (H1N1) virus.
THE CHALLENGES WE FACE
We are now in the midst of an influenza pandemic of the 2009 influenza A (H1N1) virus, with pandemic defined as “worldwide sustained community transmission.” The circulation of seasonal and 2009 pandemic influenza A (H1N1) strains will make this flu season both interesting and challenging.
The approaches to vaccination, prophylaxis, and treatment will be more complex. As of this writing (mid-September 2009), it is clear that we will be giving two influenza vaccines this season: a trivalent vaccine for seasonal influenza, and a monovalent vaccine for pandemic H1N1. It appears the monovalent vaccine may require only one dose to provide protective immunity.1 Fortunately, the vast majority of cases of pandemic H1N1 are relatively mild and uncomplicated. Still, some people are at higher risk of complications, including young patients, pregnant women, and people with immune deficiency or concomitant health conditions that put them at higher risk of flu-associated complications. Thus, clinicians will need to be educated about whom to test, who needs prophylaxis, and who should not be treated.
As our case demonstrates, unsuspected cases of influenza in hospitalized patients or health care workers working with influenza pose the greatest threat for transmission of influenza within the hospital. Adults hospitalized with influenza tend to present late (more than 48 hours after the onset of symptoms) and tend to have prolonged illness.2 Ambulatory adults shed virus for 3 to 6 days; virus shedding is more prolonged for hospitalized patients. Antiviral agents started within 4 days of illness enhance viral clearance and are associated with a shorter stay.3 Therefore, we should have a low threshold for testing for influenza and for isolating all suspected cases.
This is also creating a paradigm shift for health care workers, who are notorious for working through an illness. If you are sick, stay home! This applies whether you have pandemic H1N1 or something else.
EPIDEMIOLOGY OF PANDEMIC 2009 INFLUENZA A (H1N1) VIRUS
The location of cases can now be found on Google Maps; the US Centers for Disease Control and Prevention (CDC) provides weekly influenza reports at www.cdc.gov/flu/weekly/fluactivity.htm.
Pandemic H1N1 appeared in the spring of 2009, and cases continued to mount all summer in the United States (when influenza is normally absent) and around the world. In Mexico in March and April 2009, 2,155 cases of pneumonia, 821 hospitalizations, and 100 deaths were reported.4
In contrast with seasonal influenza, children and younger adults were hit the hardest in Mexico. The age group 5 through 59 years accounted for 87% of the deaths (usually, they account for about 17%) and 71% of the cases of severe pneumonia (usually, they account for 32%). These observations may be explained in part by the possibility that people who were alive during the 1957 pandemic (which was an H1N1 strain) have some immunity to the new virus. However, the case-fatality rate was highest in people age 65 and older.4
As of July 2009, there were more than 43,000 confirmed cases of pandemic H1N1 in the United States, and actual cases probably exceed 1 million, with more than 400 deaths. An underlying risk factor was identified in more than half of the fatal cases.5 Ten percent of the women who died were pregnant.
Pandemic H1N1 has several distinctive epidemiologic features:
- The distribution of cases is similar across multiple geographic areas.
- The distribution of cases by age group is markedly different than that of seasonal influenza, with more cases in school children and fewer cases in older adults.
- Fewer cases have been reported in older adults, but this group has the highest case-fatality rate.
2009 PANDEMIC H1N1 IS A MONGREL
There are three types of influenza viruses, designated A, B, and C. Type A undergoes antigenic shift (rapid changes) and antigenic drift (gradual changes) from year to year, and so it is the type associated with pandemics. In contrast, type B undergoes antigenic drift only, and type C is relatively stable.
Influenza virus is subtyped on the basis of surface glycoproteins: 16 hemagglutinins and nine neuraminidases. The circulating subtypes change every year; the current circulating human subtypes are a seasonal subtype of H1N1 that is different than the pandemic H1N1 subtype, and H3N2.
The 2009 pandemic H1N1 is a new virus never seen before in North America.6 Genetically, it is a mongrel, coming from three recognized sources (pigs, birds, and humans) which were combined in pigs.7 It is similar to subtypes that circulated in the 1920s through the 1940s.
Most influenza in the Western world comes from Asia every fall, and its arrival is probably facilitated by air travel. The spread is usually unidirectional and is unlikely to contribute to long-term viral evolution.8 It appears that 2009 H1N1 virus is the predominant strain circulating in the current influenza season in the Southern Hemisphere. Virologic studies indicate that the H1N1 virus strain has remained antigenically stable since it appeared in April 2009. Thus, it appears likely that the strain selected by the United States for vaccine manufacturing will match the currently circulating seasonal and pandemic H1N1 strains.
VACCINATION IS THE FIRST LINE OF DEFENSE
In addition to the trivalent vaccine against seasonal influenza, a monovalent vaccine for pandemic H1N1 virus is being produced. The CDC has indicated that 45 million doses of pandemic influenza vaccine are expected in October 2009, with an average of 20 million doses each week thereafter. It is anticipated that half of these will be in multidose vials, that 20% will be in prefilled syringes for children over 5 years old and for pregnant women, and that 20% will be in the form of live-attenuated influenza vaccine (nasal spray). The inhaled vaccine should not be given to children under 2 years old, to children under 5 years old who have recurrent wheezing, or to anyone with severe asthma. Neither vaccine should be given to people allergic to hen eggs, from which the vaccine is produced.
An ample supply of the seasonal trivalent vaccine should be available. Once the CDC has more information about specific product availability of the pandemic H1N1 vaccine, that vaccine will be distributed. It can be given concurrently with seasonal influenza vaccine.
Several definitions should be kept in mind when discussing vaccination strategies. Supply is the number of vaccine doses available for distribution. Availability is the ability of a person recommended to be vaccinated to do so in a local venue. Prioritization is the recommendation to vaccination venues to selectively use vaccine for certain population groups first. Targeting is the recommendation that immunization programs encourage and promote vaccination for certain population groups.
The Advisory Committee on Immunization Practices and the CDC recommend both seasonal and H1N1 vaccinations for anyone 6 months of age or older who is at risk of becoming ill or of transmitting the viruses to others. Based on a review of epidemiologic data, the recommendation is for targeting the following five groups for H1N1 vaccination: children and young adults aged 6 months through 24 years; pregnant women; health care workers and emergency medical service workers; people ages 25 through 64 years who have certain health conditions (eg, diabetes, heart disease, lung disease); and people who live with or care for children younger than 6 months of age. This represents approximately 159 million people in the United States.
If the estimates for the vaccine supply are met, and if pandemic H1N1 vaccine requires only a single injection, there should be no need for prioritization of vaccine. If the supply of pandemic H1N1 vaccine is inadequate, then those groups who are targeted would also receive the first doses of the pandemic H1N1 vaccine. It should be used only with caution after consideration of potential benefits and risks in people who have had Guillain-Barré syndrome during the previous 6 weeks, in people with altered immunocompetence, or in people with medical conditions predisposing to influenza complications.
A mass vaccination campaign involving two separate flu vaccines can pose challenges in execution and messaging for public health officials and politicians. In 1976, an aggressive vaccination program turned into a disaster, as there was no pandemic and the vaccine was associated with adverse effects such as Guillain-Barré syndrome. The government and the medical profession need to prepare for a vaccine controversy and to communicate and continue to explain the plan to the public. As pointed out in a recent op-ed piece,9 we would hope that all expectant women in the fall flu season will get the flu vaccines. We also know that, normally, one in seven pregnancies would be expected to miscarry. The challenge for public health officials and physicians will be to explain to these patients that there may be an association rather than a causal relationship.
In health care workers, the average vaccination rate is only 37%. We should be doing much better. Cleveland Clinic previously increased the rate of vaccination among its employees via a program in which all workers must either be vaccinated or formally declare (on an internal Web site) that they decline to be vaccinated.10 This season, even more resources are being directed at decreasing the barriers to flu vaccinations for our health care workers with the support from hospital leadership.
INFECTION CONTROL IN THE HOSPITAL AND IN THE COMMUNITY
Influenza is very contagious and is spread in droplets via sneezing and coughing (within a 3-foot radius), or via unwashed hands—thus the infection-control campaigns urging you to cover your cough and wash your hands.
As noted, for patients being admitted or transferred to the hospital, we need to have a low threshold for testing for influenza and for isolating patients suspected of having influenza. For patients with suspected or proven seasonal influenza, transmission precautions are those recommended by the CDC for droplet precautions (www.cdc.gov/ncidod/dhqp/gl_isolation_droplet.html). A face mask is deemed adequate to protect transmission when coming within 3 feet of an infected person. CDC guidelines for pandemic H1N1 recommends airborne-transmission-based precautions for health care workers who are in close contact with patients with proven or possible H1N1 (www.cdc.gov/ncidod/dhqp/gl_isolation_airborne.html). This recommendation implies the use of fit-tested N95 respirators and negative air pressure rooms (if available).
The recent Institute of Medicine report, Respiratory Protection for Healthcare Workers in the Workplace Against Novel H1N1 Influenza A (www.iom.edu/CMS/3740/71769/72967/72970.aspx) endorses the current CDC guidelines and recommends following these guidelines until we have evidence that other forms of protection or guidelines are equally or more effective.
Personally, I am against this requirement because it creates a terrible administrative burden with no proven benefit. Requiring a respirator means requiring fit-testing, and this will negatively affect our ability to deliver patient care. Recent studies have shown that surgical masks may not be as effective11 but are probably sufficient. Lim et al12 reported that 79 (37%) of 212 workers who responded to a survey experienced headaches while wearing N95 masks. This remains a controversial issue.
Besides getting the flu shot, what can one do to avoid getting influenza or transmitting to others?
- Cover your cough (cough etiquette) and sneeze.
- Practice good hand hygiene.
- Avoid close contact with people who are sick.
- Do not go to school or work if sick.
A recent study of influenza in households suggested that having the person with flu and household contacts wear face masks and practice hand hygiene within the first 36 hours decreased transmission of flu within the household.13
The United States does have a national influenza pandemic plan that outlines specific roles in the event of a pandemic, and I urge you to peruse it at www.hhs.gov/pandemicflu/plan/.
RECOGNIZING AND DIAGNOSING INFLUENZA
The familiar signs and symptoms of influenza—fever, cough, muscle aches, and headache—are nonspecific. Call et al14 analyzed the diagnostic accuracy of symptoms and signs of influenza and found that fever and cough during an epidemic suggest but do not confirm influenza, and that sneezing in those over age 60 argues against influenza. They concluded that signs and symptoms can tell us whether a patient has an influenza-like illness, but do not confirm or exclude the diagnosis of influenza: “Clinicians need to consider whether influenza is circulating in their communities, and then either treat patients with influenza-like illness empirically or obtain a rapid influenza test.”14
The signs and symptoms of pandemic 2009 H1N1 are the same as for seasonal flu, except that about 25% of patients with pandemic flu develop gastrointestinal symptoms. It has not been more virulent than seasonal influenza to date.
Should you order a test for influenza?
Most people with influenza are neither tested nor treated. Before ordering a test for influenza, ask, “Does this patient actually have influenza?” Patients diagnosed with “influenza” may have a range of infectious and noninfectious causes, such as vasculitis, endocarditis, or any other condition that can cause a fever and cough.
If I truly suspect influenza, I would still only order a test if the results would change how I manage the patient—for example, a patient being admitted to the hospital where isolation would be required.
Pandemic H1N1 will be detected only as influenza A in our current PCR screen for human influenza. The test does not differentiate between seasonal strains of influenza A (which is resistant to oseltamivir) and pandemic H1N1 (which is susceptible to oseltamivir). This means if you intend to treat, you will have to address further complexity.
Testing for influenza
The clinician should be familiar with the types of tests available. Each test has advantages and disadvantages15:
Rapid antigen assay is a point-of-care test that can give results in 15 minutes but unfortunately is only 20% to 30% sensitive, so a negative result does not exclude the diagnosis. The positive predictive value is high, meaning a positive test means the patient does have the flu.
Direct fluorescent antibody testing takes about 2.5 hours to complete and requires special training for technicians. It has a sensitivity of 47%, a positive predictive value of 95%, and a negative predictive value of 92%.
PCR testing takes about 6 hours and has a sensitivity of 98%, a positive predictive value of 100%, and a negative predictive value of 98%. This is probably the best test, in view of its all-around performance, but it is not a point-of-care test.
Culture takes 2 to 3 days, has a sensitivity of 89%, a positive predictive value of 100%, and a negative predictive value of 88%.
These tests can determine that the patient has influenza A, but a confirmatory test is always required to confirm pandemic H1N1. This confirmatory testing can be done by the CDC, by state public health laboratories, and by commercial reference laboratories.
ANTIVIRAL TREATMENT
Since influenza test results do not specify whether the patient has seasonal or pandemic influenza, treatment decisions are a sticky wicket. Most patients with pandemic H1N1 do not need to be tested or treated.
Several drugs are approved for treating influenza and shorten the duration of symptoms by about 1 day. The earlier the treatment is started, the better: the time of antiviral initiation affects influenza viral load and the duration of viral shedding.3
The neuraminidase inhibitors oseltamivir and zanamivir (Relenza) block release of virus from the cell. Resistance to oseltamivir is emerging in seasonal influenza A, while most pandemic H1N1 strains are susceptible.
Oseltamivir resistance in pandemic H1N1
A total of 11 cases of oseltamivir-resistant pandemic H1N1 have been confirmed worldwide, including 3 in the United States (2 in immunosuppressed patients in Seattle, WA). Ten of the 11 cases occurred with oseltamivir exposure. All involved a histidine-to-tyrosine substitution at position 275 (H275Y) of the neuraminidase gene. Most were susceptible to zanamivir.
Supplies of oseltamivir and zanamivir are limited, so they should be used only in those who will benefit the most, ie, those at higher risk of influenza complications. These include children under 5 years old, adults age 65 and older, children and adolescents on long-term aspirin therapy, pregnant women, patients who have chronic conditions or who are immunosuppressed, and residents of long-term care facilities.
A 69-year-old ohio man with leukemia was treated in another state in late June. During the car trip back to Ohio, he developed a sore throat, fever, cough, and nasal congestion. He was admitted to Cleveland Clinic with a presumed diagnosis of neutropenic fever; his absolute neutrophil count was 0.4 × 109/L (reference range 1.8–7.7). His chest radiograph was normal. He was treated with empiric broad-spectrum antimicrobials. On his second day in the hospital, he was tested for influenza by a polymerase chain reaction (PCR) test, which was positive for influenza A. He was moved to a private room and started on oseltamivir (Tamiflu) and rimantadine (Flumadine). The patient’s previous roommate subsequently tested positive for influenza A, as did two health care workers working on the ward. All patients on the floor received prophylactic oseltamivir.
The patient’s condition worsened, and he subsequently went into respiratory distress with diffuse pulmonary infiltrates. He was transferred to the intensive care unit, where he was intubated. Influenza A was isolated from a bronchoscopic specimen. He subsequently recovered after a prolonged course and was discharged on hospital day 50. Testing by the Ohio Department of Health confirmed that this was the 2009 pandemic influenza A (H1N1) virus.
THE CHALLENGES WE FACE
We are now in the midst of an influenza pandemic of the 2009 influenza A (H1N1) virus, with pandemic defined as “worldwide sustained community transmission.” The circulation of seasonal and 2009 pandemic influenza A (H1N1) strains will make this flu season both interesting and challenging.
The approaches to vaccination, prophylaxis, and treatment will be more complex. As of this writing (mid-September 2009), it is clear that we will be giving two influenza vaccines this season: a trivalent vaccine for seasonal influenza, and a monovalent vaccine for pandemic H1N1. It appears the monovalent vaccine may require only one dose to provide protective immunity.1 Fortunately, the vast majority of cases of pandemic H1N1 are relatively mild and uncomplicated. Still, some people are at higher risk of complications, including young patients, pregnant women, and people with immune deficiency or concomitant health conditions that put them at higher risk of flu-associated complications. Thus, clinicians will need to be educated about whom to test, who needs prophylaxis, and who should not be treated.
As our case demonstrates, unsuspected cases of influenza in hospitalized patients or health care workers working with influenza pose the greatest threat for transmission of influenza within the hospital. Adults hospitalized with influenza tend to present late (more than 48 hours after the onset of symptoms) and tend to have prolonged illness.2 Ambulatory adults shed virus for 3 to 6 days; virus shedding is more prolonged for hospitalized patients. Antiviral agents started within 4 days of illness enhance viral clearance and are associated with a shorter stay.3 Therefore, we should have a low threshold for testing for influenza and for isolating all suspected cases.
This is also creating a paradigm shift for health care workers, who are notorious for working through an illness. If you are sick, stay home! This applies whether you have pandemic H1N1 or something else.
EPIDEMIOLOGY OF PANDEMIC 2009 INFLUENZA A (H1N1) VIRUS
The location of cases can now be found on Google Maps; the US Centers for Disease Control and Prevention (CDC) provides weekly influenza reports at www.cdc.gov/flu/weekly/fluactivity.htm.
Pandemic H1N1 appeared in the spring of 2009, and cases continued to mount all summer in the United States (when influenza is normally absent) and around the world. In Mexico in March and April 2009, 2,155 cases of pneumonia, 821 hospitalizations, and 100 deaths were reported.4
In contrast with seasonal influenza, children and younger adults were hit the hardest in Mexico. The age group 5 through 59 years accounted for 87% of the deaths (usually, they account for about 17%) and 71% of the cases of severe pneumonia (usually, they account for 32%). These observations may be explained in part by the possibility that people who were alive during the 1957 pandemic (which was an H1N1 strain) have some immunity to the new virus. However, the case-fatality rate was highest in people age 65 and older.4
As of July 2009, there were more than 43,000 confirmed cases of pandemic H1N1 in the United States, and actual cases probably exceed 1 million, with more than 400 deaths. An underlying risk factor was identified in more than half of the fatal cases.5 Ten percent of the women who died were pregnant.
Pandemic H1N1 has several distinctive epidemiologic features:
- The distribution of cases is similar across multiple geographic areas.
- The distribution of cases by age group is markedly different than that of seasonal influenza, with more cases in school children and fewer cases in older adults.
- Fewer cases have been reported in older adults, but this group has the highest case-fatality rate.
2009 PANDEMIC H1N1 IS A MONGREL
There are three types of influenza viruses, designated A, B, and C. Type A undergoes antigenic shift (rapid changes) and antigenic drift (gradual changes) from year to year, and so it is the type associated with pandemics. In contrast, type B undergoes antigenic drift only, and type C is relatively stable.
Influenza virus is subtyped on the basis of surface glycoproteins: 16 hemagglutinins and nine neuraminidases. The circulating subtypes change every year; the current circulating human subtypes are a seasonal subtype of H1N1 that is different than the pandemic H1N1 subtype, and H3N2.
The 2009 pandemic H1N1 is a new virus never seen before in North America.6 Genetically, it is a mongrel, coming from three recognized sources (pigs, birds, and humans) which were combined in pigs.7 It is similar to subtypes that circulated in the 1920s through the 1940s.
Most influenza in the Western world comes from Asia every fall, and its arrival is probably facilitated by air travel. The spread is usually unidirectional and is unlikely to contribute to long-term viral evolution.8 It appears that 2009 H1N1 virus is the predominant strain circulating in the current influenza season in the Southern Hemisphere. Virologic studies indicate that the H1N1 virus strain has remained antigenically stable since it appeared in April 2009. Thus, it appears likely that the strain selected by the United States for vaccine manufacturing will match the currently circulating seasonal and pandemic H1N1 strains.
VACCINATION IS THE FIRST LINE OF DEFENSE
In addition to the trivalent vaccine against seasonal influenza, a monovalent vaccine for pandemic H1N1 virus is being produced. The CDC has indicated that 45 million doses of pandemic influenza vaccine are expected in October 2009, with an average of 20 million doses each week thereafter. It is anticipated that half of these will be in multidose vials, that 20% will be in prefilled syringes for children over 5 years old and for pregnant women, and that 20% will be in the form of live-attenuated influenza vaccine (nasal spray). The inhaled vaccine should not be given to children under 2 years old, to children under 5 years old who have recurrent wheezing, or to anyone with severe asthma. Neither vaccine should be given to people allergic to hen eggs, from which the vaccine is produced.
An ample supply of the seasonal trivalent vaccine should be available. Once the CDC has more information about specific product availability of the pandemic H1N1 vaccine, that vaccine will be distributed. It can be given concurrently with seasonal influenza vaccine.
Several definitions should be kept in mind when discussing vaccination strategies. Supply is the number of vaccine doses available for distribution. Availability is the ability of a person recommended to be vaccinated to do so in a local venue. Prioritization is the recommendation to vaccination venues to selectively use vaccine for certain population groups first. Targeting is the recommendation that immunization programs encourage and promote vaccination for certain population groups.
The Advisory Committee on Immunization Practices and the CDC recommend both seasonal and H1N1 vaccinations for anyone 6 months of age or older who is at risk of becoming ill or of transmitting the viruses to others. Based on a review of epidemiologic data, the recommendation is for targeting the following five groups for H1N1 vaccination: children and young adults aged 6 months through 24 years; pregnant women; health care workers and emergency medical service workers; people ages 25 through 64 years who have certain health conditions (eg, diabetes, heart disease, lung disease); and people who live with or care for children younger than 6 months of age. This represents approximately 159 million people in the United States.
If the estimates for the vaccine supply are met, and if pandemic H1N1 vaccine requires only a single injection, there should be no need for prioritization of vaccine. If the supply of pandemic H1N1 vaccine is inadequate, then those groups who are targeted would also receive the first doses of the pandemic H1N1 vaccine. It should be used only with caution after consideration of potential benefits and risks in people who have had Guillain-Barré syndrome during the previous 6 weeks, in people with altered immunocompetence, or in people with medical conditions predisposing to influenza complications.
A mass vaccination campaign involving two separate flu vaccines can pose challenges in execution and messaging for public health officials and politicians. In 1976, an aggressive vaccination program turned into a disaster, as there was no pandemic and the vaccine was associated with adverse effects such as Guillain-Barré syndrome. The government and the medical profession need to prepare for a vaccine controversy and to communicate and continue to explain the plan to the public. As pointed out in a recent op-ed piece,9 we would hope that all expectant women in the fall flu season will get the flu vaccines. We also know that, normally, one in seven pregnancies would be expected to miscarry. The challenge for public health officials and physicians will be to explain to these patients that there may be an association rather than a causal relationship.
In health care workers, the average vaccination rate is only 37%. We should be doing much better. Cleveland Clinic previously increased the rate of vaccination among its employees via a program in which all workers must either be vaccinated or formally declare (on an internal Web site) that they decline to be vaccinated.10 This season, even more resources are being directed at decreasing the barriers to flu vaccinations for our health care workers with the support from hospital leadership.
INFECTION CONTROL IN THE HOSPITAL AND IN THE COMMUNITY
Influenza is very contagious and is spread in droplets via sneezing and coughing (within a 3-foot radius), or via unwashed hands—thus the infection-control campaigns urging you to cover your cough and wash your hands.
As noted, for patients being admitted or transferred to the hospital, we need to have a low threshold for testing for influenza and for isolating patients suspected of having influenza. For patients with suspected or proven seasonal influenza, transmission precautions are those recommended by the CDC for droplet precautions (www.cdc.gov/ncidod/dhqp/gl_isolation_droplet.html). A face mask is deemed adequate to protect transmission when coming within 3 feet of an infected person. CDC guidelines for pandemic H1N1 recommends airborne-transmission-based precautions for health care workers who are in close contact with patients with proven or possible H1N1 (www.cdc.gov/ncidod/dhqp/gl_isolation_airborne.html). This recommendation implies the use of fit-tested N95 respirators and negative air pressure rooms (if available).
The recent Institute of Medicine report, Respiratory Protection for Healthcare Workers in the Workplace Against Novel H1N1 Influenza A (www.iom.edu/CMS/3740/71769/72967/72970.aspx) endorses the current CDC guidelines and recommends following these guidelines until we have evidence that other forms of protection or guidelines are equally or more effective.
Personally, I am against this requirement because it creates a terrible administrative burden with no proven benefit. Requiring a respirator means requiring fit-testing, and this will negatively affect our ability to deliver patient care. Recent studies have shown that surgical masks may not be as effective11 but are probably sufficient. Lim et al12 reported that 79 (37%) of 212 workers who responded to a survey experienced headaches while wearing N95 masks. This remains a controversial issue.
Besides getting the flu shot, what can one do to avoid getting influenza or transmitting to others?
- Cover your cough (cough etiquette) and sneeze.
- Practice good hand hygiene.
- Avoid close contact with people who are sick.
- Do not go to school or work if sick.
A recent study of influenza in households suggested that having the person with flu and household contacts wear face masks and practice hand hygiene within the first 36 hours decreased transmission of flu within the household.13
The United States does have a national influenza pandemic plan that outlines specific roles in the event of a pandemic, and I urge you to peruse it at www.hhs.gov/pandemicflu/plan/.
RECOGNIZING AND DIAGNOSING INFLUENZA
The familiar signs and symptoms of influenza—fever, cough, muscle aches, and headache—are nonspecific. Call et al14 analyzed the diagnostic accuracy of symptoms and signs of influenza and found that fever and cough during an epidemic suggest but do not confirm influenza, and that sneezing in those over age 60 argues against influenza. They concluded that signs and symptoms can tell us whether a patient has an influenza-like illness, but do not confirm or exclude the diagnosis of influenza: “Clinicians need to consider whether influenza is circulating in their communities, and then either treat patients with influenza-like illness empirically or obtain a rapid influenza test.”14
The signs and symptoms of pandemic 2009 H1N1 are the same as for seasonal flu, except that about 25% of patients with pandemic flu develop gastrointestinal symptoms. It has not been more virulent than seasonal influenza to date.
Should you order a test for influenza?
Most people with influenza are neither tested nor treated. Before ordering a test for influenza, ask, “Does this patient actually have influenza?” Patients diagnosed with “influenza” may have a range of infectious and noninfectious causes, such as vasculitis, endocarditis, or any other condition that can cause a fever and cough.
If I truly suspect influenza, I would still only order a test if the results would change how I manage the patient—for example, a patient being admitted to the hospital where isolation would be required.
Pandemic H1N1 will be detected only as influenza A in our current PCR screen for human influenza. The test does not differentiate between seasonal strains of influenza A (which is resistant to oseltamivir) and pandemic H1N1 (which is susceptible to oseltamivir). This means if you intend to treat, you will have to address further complexity.
Testing for influenza
The clinician should be familiar with the types of tests available. Each test has advantages and disadvantages15:
Rapid antigen assay is a point-of-care test that can give results in 15 minutes but unfortunately is only 20% to 30% sensitive, so a negative result does not exclude the diagnosis. The positive predictive value is high, meaning a positive test means the patient does have the flu.
Direct fluorescent antibody testing takes about 2.5 hours to complete and requires special training for technicians. It has a sensitivity of 47%, a positive predictive value of 95%, and a negative predictive value of 92%.
PCR testing takes about 6 hours and has a sensitivity of 98%, a positive predictive value of 100%, and a negative predictive value of 98%. This is probably the best test, in view of its all-around performance, but it is not a point-of-care test.
Culture takes 2 to 3 days, has a sensitivity of 89%, a positive predictive value of 100%, and a negative predictive value of 88%.
These tests can determine that the patient has influenza A, but a confirmatory test is always required to confirm pandemic H1N1. This confirmatory testing can be done by the CDC, by state public health laboratories, and by commercial reference laboratories.
ANTIVIRAL TREATMENT
Since influenza test results do not specify whether the patient has seasonal or pandemic influenza, treatment decisions are a sticky wicket. Most patients with pandemic H1N1 do not need to be tested or treated.
Several drugs are approved for treating influenza and shorten the duration of symptoms by about 1 day. The earlier the treatment is started, the better: the time of antiviral initiation affects influenza viral load and the duration of viral shedding.3
The neuraminidase inhibitors oseltamivir and zanamivir (Relenza) block release of virus from the cell. Resistance to oseltamivir is emerging in seasonal influenza A, while most pandemic H1N1 strains are susceptible.
Oseltamivir resistance in pandemic H1N1
A total of 11 cases of oseltamivir-resistant pandemic H1N1 have been confirmed worldwide, including 3 in the United States (2 in immunosuppressed patients in Seattle, WA). Ten of the 11 cases occurred with oseltamivir exposure. All involved a histidine-to-tyrosine substitution at position 275 (H275Y) of the neuraminidase gene. Most were susceptible to zanamivir.
Supplies of oseltamivir and zanamivir are limited, so they should be used only in those who will benefit the most, ie, those at higher risk of influenza complications. These include children under 5 years old, adults age 65 and older, children and adolescents on long-term aspirin therapy, pregnant women, patients who have chronic conditions or who are immunosuppressed, and residents of long-term care facilities.
- Greenberg MA, Lai MH, Hartel GF. Response after one dose of a monovalent influenza A (H1N1) 2009 vaccine—preliminary report. N Engl J Med 2009;361doi:10.1056/NEJMoa0907413 [published online ahead of print].
- Ison M. Influenza in hospitalized adults: gaining insight into a significant problem. J Infect Dis 2009; 200:485–488.
- Lee N, Chan PKS, Hui DSC, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200:492–500.
- Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679.
- Vaillant L, La Ruche G, Tarantola A, Barboza P; for the Epidemic Intelligence Team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009; 14(33):1–6. Available online at www.eurosurveillance.org/ViewArticle.aspx?ArticleID=19309.
- Zimmer SM, Burke DS. Historical perspective—emergence of influenza A (H1N1) viruses. N Engl J Med 2009; 361:279–285.
- Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201.
- Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340–346.
- Allen A. Prepare for a vaccine controversy. New York Times. 9/1/2009.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis 2009; 49:275–277.
- Lim EC, Seet RC, Lee KH, Wilder-Smith EP, Chuah BY, Ong BK. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol Scand 2006; 113:199–202.
- Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a randomized trial. Ann Intern Med 2009; 151(6 Oct) [published online ahead of print].
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005; 293:987–997.
- Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol 2009; 45:191–195.
- US Centers for Disease Control and Prevention. Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR 2009; 58:893–896.
- Greenberg MA, Lai MH, Hartel GF. Response after one dose of a monovalent influenza A (H1N1) 2009 vaccine—preliminary report. N Engl J Med 2009;361doi:10.1056/NEJMoa0907413 [published online ahead of print].
- Ison M. Influenza in hospitalized adults: gaining insight into a significant problem. J Infect Dis 2009; 200:485–488.
- Lee N, Chan PKS, Hui DSC, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200:492–500.
- Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679.
- Vaillant L, La Ruche G, Tarantola A, Barboza P; for the Epidemic Intelligence Team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009; 14(33):1–6. Available online at www.eurosurveillance.org/ViewArticle.aspx?ArticleID=19309.
- Zimmer SM, Burke DS. Historical perspective—emergence of influenza A (H1N1) viruses. N Engl J Med 2009; 361:279–285.
- Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201.
- Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340–346.
- Allen A. Prepare for a vaccine controversy. New York Times. 9/1/2009.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis 2009; 49:275–277.
- Lim EC, Seet RC, Lee KH, Wilder-Smith EP, Chuah BY, Ong BK. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol Scand 2006; 113:199–202.
- Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a randomized trial. Ann Intern Med 2009; 151(6 Oct) [published online ahead of print].
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005; 293:987–997.
- Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol 2009; 45:191–195.
- US Centers for Disease Control and Prevention. Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR 2009; 58:893–896.
KEY POINTS
- Vaccination this season will require two vaccines: a trivalent vaccine for seasonal influenza and a monovalent vaccine for 2009 pandemic influenza A (H1N1).
- Recent studies indicate that the monovalent vaccine for 2009 pandemic influenza A (H1N1) may require only one injection.
- To date, 2009 pandemic influenza A (H1N1) virus has not been exceptionally virulent and differs from conventional influenza in that it seems to disproportionately affect children and young adults. Pregnant women are at a higher risk of complications.
- Most people with 2009 pandemic influenza A (H1N1) do not need to be tested, treated, or seen by a clinician.
- Antiviral drugs should be reserved only for those at high risk of influenza complications.
VHA Facilities Improve Colonoscope Reprocessing Compliance
Grand Rounds: Woman, 39, With Leg Weakness After Exercise Class
A 39-year-old woman presented to the emergency department (ED) with a chief complaint of muscle aches and pain. She stated that three days earlier, she had begun exercising in a 45-minute “spinning” class (ie, riding a stationary bicycle with a weighted front wheel). The patient had not engaged in any aerobic exercise for at least six months before the spinning class. She mentioned that much older participants in the class were outperforming her, but she did not feel the need to keep up with them.
After dismounting, the woman said, she experienced weakness in her legs and had great difficulty ambulating. She went home, took 400 mg of ibuprofen, and went to bed. She awoke with pain and swelling in both thighs and continued to take ibuprofen, in addition to applying a topical mentholated preparation to her thighs. She took an Epsom salts bath two days later.
On the morning of the third day after the spinning class, she voided black urine and presented to the ED.
The patient had no significant medical history. Surgical history was limited to removal of a ganglion cyst on her wrist. She denied any history of seizure disorder, thyroid disease, hepatitis, heart disease, or hyperlipidemia.
The patient had been taking ibuprofen as needed since the spinning class. She was taking no other medications. She denied any allergies to drugs or food.
The patient admitted to smoking one pack of cigarettes per week and to occasional alcohol consumption but denied use of illicit drugs. She was employed as an executive officer for a large business association.
On physical examination, the patient’s vital signs were blood pressure, 134/73 mm/Hg; pulse, 86 beats/min; and respirations, 16 breaths/min. She was afebrile, alert, and oriented. Her sclera were nonicteric. Her neck was supple with no anterior cervical lymphadenopathy. There was no thyroid enlargement, her lungs were clear to auscultation, and her heart sounds were regular. There was no peripheral edema, and dorsalis pedis pulses were present bilaterally. Her thighs appeared swollen but were not tender to palpation.
The patient’s history, combined with an extremely high level of serum creatine phosphokinase (CPK; ie, 123,800 U/L [reference range, 45 to 260 U/L1]), confirmed a diagnosis of rhabdomyolysis. She was admitted for close observation. The patient’s urinalysis revealed 2 to 5 red blood cells and 6 to 10 white blood cells per high-power field. Moderate occult blood was detected, with no casts or protein noted. A urine myoglobin test was not performed.
The patient underwent IV hydration with dextrose 5% in water and three ampules of sodium bicarbonate after being given a 2.0-L saline bolus. Ibuprofen was discontinued. IV hydration with bicarbonate solution was continued until the patient’s CPK level declined significantly. She underwent daily laboratory testing (see Table 1). Her renal function remained stable, and she was discharged on hospital day 7.
DISCUSSION
Rhabdomyolysis is a clinical condition defined as muscle necrosis resulting from the release of intracellular skeletal muscle components (including myoglobin, CPK, potassium, phosphorus, and aldolase) into the extracellular compartment.1-3 The condition was first described during the bombing of London in World War II, with high incidence of crush injuries, shock, and associated kidney damage.4 The preponderance of such injuries during a 1988 earthquake in Armenia led the International Society of Nephrology to form its Renal Disaster Relief Task Force, which has provided support at numerous other disaster scenes since then.5
Rhabdomyolysis has been identified with a variety of pathologic events: those that cause muscle trauma, those associated with muscle use or overuse, and other etiologies involving genetic, metabolic, infectious, or pharmaceutical factors.1 Many of the reported causes of rhabdomyolysis are listed in Table 2.1,2
For patients with muscle trauma, the etiology of rhabdomyolysis is clear, but for those with other disease states, diagnosis may be more elusive. Patients who present with rhabdomyolysis after excessive exercise, for example, may have underlying metabolic disorders that predispose them to exertional rhabdomyolysis, such as chronic hypokalemia resulting from primary hyperaldosteronism.1 Others may have a muscle enzyme deficiency, as in McArdle’s syndrome or carnitine deficiency.6
Alterations in blood chemistries can also contribute to development of rhabdomyolysis, even when more obvious etiologies for muscle necrosis are evident. Hypokalemia interferes with the vasodilation that normally occurs during exercise to increase muscle blood flow.1,7,8 Continued exercise can lead to muscle necrosis, raising a concern for athletes who take diuretics.1 Hypophosphatemia leads to a state of muscle necrosis; this is of particular concern for alcoholic patients who receive hyperalimentation without repletion of phosphates.9
Diagnosis
Patients with rhabdomyolysis usually present with myalgias, darkened urine (red, brown, or black), and a clinical scenario that corroborates the diagnosis (ie, history of trauma, excessive exercise, use of an offending medication).1 Some patients may have minimal to absent symptoms or symptoms that occur only after exercise.3
A careful history is key. While traumatic causes are obvious, it is important to ask a patient with rhabdomyolysis after exertion about previous history of excessive weakness during or immediately after exercise, excessive cramping, or discoloration of urine after exercise. The family history may point to a genetic abnormality. A thorough understanding of the patient’s use of medications, including OTC agents, is also important. Rhabdomyolysis has been reported in patients who use herbal remedies, including those taken to facilitate weight loss or to improve lipid profiles.10,11
For patients suspected of having rhabdomyolysis, a serum CPK level should be obtained; results exceeding normal values by five times confirm the diagnosis.3 Measurements for potassium, phosphorus, and calcium are also important to determine, as is renal function. A high level of serum aldolase (an enzyme that breaks down glucose in muscle tissue) can also support a diagnosis of rhabdomyolysis.1,12 Urinalysis and urine myoglobin testing are also warranted, although a negative urine myoglobin test result does not rule out rhabdomyolysis in the presence of an elevated CPK level. Myoglobin is cleared rapidly by the kidneys, whereas serum CPK levels change slowly.1
Any patient who presents with acute rhabdomyolysis and low to normal values for potassium or phosphate should be evaluated further for hypokalemia and hypophosphatemia as contributing or etiologic factors. Hypocalcemia may occur in the early course of rhabdomyolysis as calcium salt is deposited in muscle tissue. Patients recovering from rhabdomyolysis may experience rebound hypercalcemia as the damaged muscle releases the deposited calcium.7,13
In most cases of rhabdomyolysis, only laboratory values are needed to make the diagnosis and follow the course of the episode.1 However, when the etiology appears to involve metabolic deficiencies or genetic etiologies, it may become necessary to order additional diagnostic tests. These may include tests for thyroid function, a carnitine level to screen for glycogen storage diseases, and toxin screening (eg, for illicit drugs, such as cocaine).2,6
Treatment and Management
Effective treatment of rhabdomyolysis relies on recognizing the underlying disorder.1 For patients with muscle trauma (eg, crush injury) or muscle overuse, the mainstay of treatment is aggressive fluid resuscitation and prevention of acute injury to the kidneys.13 As for patients with an injury induced by a pharmaceutical agent or a toxin, removal of the offending agent is required, followed by hydration and prevention of renal damage. Supportive care during an infectious illness is also essential.14
Additionally, treatment must address the complications inherent with rhabdomyolysis.1 In addition to CPK, potassium, phosphorus, and myoglobin are also released from skeletal muscle tissue. Hyperkalemia can be fatal, and potassium levels must be monitored closely to avert this condition.7,8,13 During an episode of rhabdomyolysis, normal levels of both potassium and phosphorus should raise the clinician’s suspicion for underlying hypokalemia and hypophosphatemia—conditions that may have contributed to the episode of rhabdomyolysis. Hypocalcemia may also develop.13
Released myoglobin may cause acute kidney injury, as is the case in 33% to 50% of patients with rhabdomyolysis.3 In early studies, it was determined that alkalinizing the urine with IV isotonic bicarbonate might thwart onset of acute kidney injury.1,2,15 Time is critical, and even on the battlefield or at the scene of a recent disaster, most attempts at resuscitation are begun immediately. IV access may be problematic, but administration of oral bicarbonate solutions has also proven effective.15 Close follow-up of the serum urea and creatinine levels and measurement of the urine pH during alkalinization is warranted throughout the course of the episode.
Unfortunately, some patients respond poorly to these conservative measures, and the released myoglobin can cause renal tubular blockage and necrosis, resulting in acute kidney injury.1 Renal replacement therapy may be required.16 However, most episodes of dialysis-dependent acute renal injury do subside with time.
For patients with less elusive causes of rhabdomyolysis, treatment will hinge on a workup of the possible etiologies and follow-up treatment to target the apparent cause. For example, carnitine may be administered to patients with carnitine deficiency, and hypokalemic patients may be given potassium.1,6,7 These patients will also need counseling before they consider engaging in an exercise program.
Patient’s Outcome
The case patient presented with exertional rhabdomyolysis; improper hydration, severe deconditioning, and a relatively low serum potassium level may all have contributed to the muscle necrosis she experienced. She was given IV alkaline solutions and did not develop acute kidney injury. She was discharged from the hospital and at the time of this writing was awaiting outpatient follow-up.
It should be interesting to see whether the case patient experiences any further episodes of severe weakness after engaging in exercise. Her low-normal potassium level (reference range, 3.5 to 5.3 mmol/L17) warrants further follow-up, as does her mildly elevated thyroid-stimulating hormone level (reference range, 0.5 to 4.7 mcIU/mL17).
CONCLUSION
Patients with rhabdomyolysis may present with muscle aches, darkened urine, and/or weakness; an elevated CPK level confirms the diagnosis. Management is mainly conservative, with IV hydration accompanied by alkalinizing the urine and correcting any metabolic abnormalities, such as potassium deficiencies. For the few patients who experience severe acute kidney injury, renal replacement therapy may be necessary.
While most causes of rhabdomyolysis have obvious clinical scenarios, such as a crush injury, a search for muscle enzyme deficiencies, disorders of potassium homeostasis, and thyroid abnormalities is also warranted in patients who present with exertional rhabdomyolysis.
1. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
2. Warren JD, Blumbergs PC, Thompson PD. Rhabdomyolysis: a review. Muscle Nerve. 2002;25(3):332-347.
3. Lima RSA, da Silva GB Jr, Liborio AB, Daher ED. Acute kidney injury due to rhabdomyolysis. Saudi J Kidney Dis Transpl. 2008;19(5):721-729.
4. Bywaters EG, Beall D. Crush injuries with impairment of renal function [reprinted from BMJ, 1941]. J Am Soc Nephrol. 1998;9(2):322-332.
5. Vanholder R, Van Biesen W, Lameire N, Sever MS; International Society of Nephrology/Renal Disaster Relief Task Force. The role of the International Society of Nephrology/Renal Disaster Relief Task Force in the rescue of renal disaster victims. Contrib Nephrol. 2007;156:325-332.
6. Toledo R, López V, Martín G, et al. Rhabdomyolysis due to enzyme deficiency in muscles. Nefrología. 2009;29(1):77-80.
7. Agrawal S, Agrawal V, Taneja A. Hypokalemia causing rhabdomyolysis resulting in life-threatening hyperkalemia. Pediatr Nephrol. 2006;221(2): 289-291.
8. Knochel JP, Schlein EM. On the mechanism of rhabdomyolysis in potassium depletion. J Clin Invest. 1972:51(7):1750-1758.
9. Knochel JP. Hypophosphatemia and rhabdomyolysis. Am J Med. 1992;92(5):455-457.
10. Mansi IA, Huang J. Rhabdomyolysis in response to weight-loss herbal medicine. Am J Med Sci. 2004; 327(6): 356-357.
11. Heber D, Yip I, Ashley JM, et al. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr. 1999;69(2): 231-236.
12. Hooda AK, Narula AS. Exertional rhabdomyolysis causing acute renal failure. Med J Armed Forces India. 2005;61(4):395-396.
13. Chatzizisis YS, Misirli G, Hatzitolios AI, Giannoglou GD. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19(8): 568-574.
14. Blanco JR, Zabalza M, Salcedo J, et al. Rhabdomyolysis of infectious and noninfectious causes. South Med J. 2002;95(5):542-544.
15. Ron D, Taitelman U, Michaelson M, et al. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch Intern Med. 1984;144(2):277-280.
16. Soni SS, Nagarik AP, Adikey GK, Raman A. Using continuous renal replacement therapy to manage patients of shock and acute renal failure. J Emerg Trauma Shock. 2009;2(1):19-22.
17. Normal laboratory values. In: Beers MH, Berkow R, eds. The Merck Manual of Diagnosis and Therapy. 17th ed. Whitehouse Station, NJ: Merck Research Laboratories; 1999:2526-2546.
A 39-year-old woman presented to the emergency department (ED) with a chief complaint of muscle aches and pain. She stated that three days earlier, she had begun exercising in a 45-minute “spinning” class (ie, riding a stationary bicycle with a weighted front wheel). The patient had not engaged in any aerobic exercise for at least six months before the spinning class. She mentioned that much older participants in the class were outperforming her, but she did not feel the need to keep up with them.
After dismounting, the woman said, she experienced weakness in her legs and had great difficulty ambulating. She went home, took 400 mg of ibuprofen, and went to bed. She awoke with pain and swelling in both thighs and continued to take ibuprofen, in addition to applying a topical mentholated preparation to her thighs. She took an Epsom salts bath two days later.
On the morning of the third day after the spinning class, she voided black urine and presented to the ED.
The patient had no significant medical history. Surgical history was limited to removal of a ganglion cyst on her wrist. She denied any history of seizure disorder, thyroid disease, hepatitis, heart disease, or hyperlipidemia.
The patient had been taking ibuprofen as needed since the spinning class. She was taking no other medications. She denied any allergies to drugs or food.
The patient admitted to smoking one pack of cigarettes per week and to occasional alcohol consumption but denied use of illicit drugs. She was employed as an executive officer for a large business association.
On physical examination, the patient’s vital signs were blood pressure, 134/73 mm/Hg; pulse, 86 beats/min; and respirations, 16 breaths/min. She was afebrile, alert, and oriented. Her sclera were nonicteric. Her neck was supple with no anterior cervical lymphadenopathy. There was no thyroid enlargement, her lungs were clear to auscultation, and her heart sounds were regular. There was no peripheral edema, and dorsalis pedis pulses were present bilaterally. Her thighs appeared swollen but were not tender to palpation.
The patient’s history, combined with an extremely high level of serum creatine phosphokinase (CPK; ie, 123,800 U/L [reference range, 45 to 260 U/L1]), confirmed a diagnosis of rhabdomyolysis. She was admitted for close observation. The patient’s urinalysis revealed 2 to 5 red blood cells and 6 to 10 white blood cells per high-power field. Moderate occult blood was detected, with no casts or protein noted. A urine myoglobin test was not performed.
The patient underwent IV hydration with dextrose 5% in water and three ampules of sodium bicarbonate after being given a 2.0-L saline bolus. Ibuprofen was discontinued. IV hydration with bicarbonate solution was continued until the patient’s CPK level declined significantly. She underwent daily laboratory testing (see Table 1). Her renal function remained stable, and she was discharged on hospital day 7.
DISCUSSION
Rhabdomyolysis is a clinical condition defined as muscle necrosis resulting from the release of intracellular skeletal muscle components (including myoglobin, CPK, potassium, phosphorus, and aldolase) into the extracellular compartment.1-3 The condition was first described during the bombing of London in World War II, with high incidence of crush injuries, shock, and associated kidney damage.4 The preponderance of such injuries during a 1988 earthquake in Armenia led the International Society of Nephrology to form its Renal Disaster Relief Task Force, which has provided support at numerous other disaster scenes since then.5
Rhabdomyolysis has been identified with a variety of pathologic events: those that cause muscle trauma, those associated with muscle use or overuse, and other etiologies involving genetic, metabolic, infectious, or pharmaceutical factors.1 Many of the reported causes of rhabdomyolysis are listed in Table 2.1,2
For patients with muscle trauma, the etiology of rhabdomyolysis is clear, but for those with other disease states, diagnosis may be more elusive. Patients who present with rhabdomyolysis after excessive exercise, for example, may have underlying metabolic disorders that predispose them to exertional rhabdomyolysis, such as chronic hypokalemia resulting from primary hyperaldosteronism.1 Others may have a muscle enzyme deficiency, as in McArdle’s syndrome or carnitine deficiency.6
Alterations in blood chemistries can also contribute to development of rhabdomyolysis, even when more obvious etiologies for muscle necrosis are evident. Hypokalemia interferes with the vasodilation that normally occurs during exercise to increase muscle blood flow.1,7,8 Continued exercise can lead to muscle necrosis, raising a concern for athletes who take diuretics.1 Hypophosphatemia leads to a state of muscle necrosis; this is of particular concern for alcoholic patients who receive hyperalimentation without repletion of phosphates.9
Diagnosis
Patients with rhabdomyolysis usually present with myalgias, darkened urine (red, brown, or black), and a clinical scenario that corroborates the diagnosis (ie, history of trauma, excessive exercise, use of an offending medication).1 Some patients may have minimal to absent symptoms or symptoms that occur only after exercise.3
A careful history is key. While traumatic causes are obvious, it is important to ask a patient with rhabdomyolysis after exertion about previous history of excessive weakness during or immediately after exercise, excessive cramping, or discoloration of urine after exercise. The family history may point to a genetic abnormality. A thorough understanding of the patient’s use of medications, including OTC agents, is also important. Rhabdomyolysis has been reported in patients who use herbal remedies, including those taken to facilitate weight loss or to improve lipid profiles.10,11
For patients suspected of having rhabdomyolysis, a serum CPK level should be obtained; results exceeding normal values by five times confirm the diagnosis.3 Measurements for potassium, phosphorus, and calcium are also important to determine, as is renal function. A high level of serum aldolase (an enzyme that breaks down glucose in muscle tissue) can also support a diagnosis of rhabdomyolysis.1,12 Urinalysis and urine myoglobin testing are also warranted, although a negative urine myoglobin test result does not rule out rhabdomyolysis in the presence of an elevated CPK level. Myoglobin is cleared rapidly by the kidneys, whereas serum CPK levels change slowly.1
Any patient who presents with acute rhabdomyolysis and low to normal values for potassium or phosphate should be evaluated further for hypokalemia and hypophosphatemia as contributing or etiologic factors. Hypocalcemia may occur in the early course of rhabdomyolysis as calcium salt is deposited in muscle tissue. Patients recovering from rhabdomyolysis may experience rebound hypercalcemia as the damaged muscle releases the deposited calcium.7,13
In most cases of rhabdomyolysis, only laboratory values are needed to make the diagnosis and follow the course of the episode.1 However, when the etiology appears to involve metabolic deficiencies or genetic etiologies, it may become necessary to order additional diagnostic tests. These may include tests for thyroid function, a carnitine level to screen for glycogen storage diseases, and toxin screening (eg, for illicit drugs, such as cocaine).2,6
Treatment and Management
Effective treatment of rhabdomyolysis relies on recognizing the underlying disorder.1 For patients with muscle trauma (eg, crush injury) or muscle overuse, the mainstay of treatment is aggressive fluid resuscitation and prevention of acute injury to the kidneys.13 As for patients with an injury induced by a pharmaceutical agent or a toxin, removal of the offending agent is required, followed by hydration and prevention of renal damage. Supportive care during an infectious illness is also essential.14
Additionally, treatment must address the complications inherent with rhabdomyolysis.1 In addition to CPK, potassium, phosphorus, and myoglobin are also released from skeletal muscle tissue. Hyperkalemia can be fatal, and potassium levels must be monitored closely to avert this condition.7,8,13 During an episode of rhabdomyolysis, normal levels of both potassium and phosphorus should raise the clinician’s suspicion for underlying hypokalemia and hypophosphatemia—conditions that may have contributed to the episode of rhabdomyolysis. Hypocalcemia may also develop.13
Released myoglobin may cause acute kidney injury, as is the case in 33% to 50% of patients with rhabdomyolysis.3 In early studies, it was determined that alkalinizing the urine with IV isotonic bicarbonate might thwart onset of acute kidney injury.1,2,15 Time is critical, and even on the battlefield or at the scene of a recent disaster, most attempts at resuscitation are begun immediately. IV access may be problematic, but administration of oral bicarbonate solutions has also proven effective.15 Close follow-up of the serum urea and creatinine levels and measurement of the urine pH during alkalinization is warranted throughout the course of the episode.
Unfortunately, some patients respond poorly to these conservative measures, and the released myoglobin can cause renal tubular blockage and necrosis, resulting in acute kidney injury.1 Renal replacement therapy may be required.16 However, most episodes of dialysis-dependent acute renal injury do subside with time.
For patients with less elusive causes of rhabdomyolysis, treatment will hinge on a workup of the possible etiologies and follow-up treatment to target the apparent cause. For example, carnitine may be administered to patients with carnitine deficiency, and hypokalemic patients may be given potassium.1,6,7 These patients will also need counseling before they consider engaging in an exercise program.
Patient’s Outcome
The case patient presented with exertional rhabdomyolysis; improper hydration, severe deconditioning, and a relatively low serum potassium level may all have contributed to the muscle necrosis she experienced. She was given IV alkaline solutions and did not develop acute kidney injury. She was discharged from the hospital and at the time of this writing was awaiting outpatient follow-up.
It should be interesting to see whether the case patient experiences any further episodes of severe weakness after engaging in exercise. Her low-normal potassium level (reference range, 3.5 to 5.3 mmol/L17) warrants further follow-up, as does her mildly elevated thyroid-stimulating hormone level (reference range, 0.5 to 4.7 mcIU/mL17).
CONCLUSION
Patients with rhabdomyolysis may present with muscle aches, darkened urine, and/or weakness; an elevated CPK level confirms the diagnosis. Management is mainly conservative, with IV hydration accompanied by alkalinizing the urine and correcting any metabolic abnormalities, such as potassium deficiencies. For the few patients who experience severe acute kidney injury, renal replacement therapy may be necessary.
While most causes of rhabdomyolysis have obvious clinical scenarios, such as a crush injury, a search for muscle enzyme deficiencies, disorders of potassium homeostasis, and thyroid abnormalities is also warranted in patients who present with exertional rhabdomyolysis.
A 39-year-old woman presented to the emergency department (ED) with a chief complaint of muscle aches and pain. She stated that three days earlier, she had begun exercising in a 45-minute “spinning” class (ie, riding a stationary bicycle with a weighted front wheel). The patient had not engaged in any aerobic exercise for at least six months before the spinning class. She mentioned that much older participants in the class were outperforming her, but she did not feel the need to keep up with them.
After dismounting, the woman said, she experienced weakness in her legs and had great difficulty ambulating. She went home, took 400 mg of ibuprofen, and went to bed. She awoke with pain and swelling in both thighs and continued to take ibuprofen, in addition to applying a topical mentholated preparation to her thighs. She took an Epsom salts bath two days later.
On the morning of the third day after the spinning class, she voided black urine and presented to the ED.
The patient had no significant medical history. Surgical history was limited to removal of a ganglion cyst on her wrist. She denied any history of seizure disorder, thyroid disease, hepatitis, heart disease, or hyperlipidemia.
The patient had been taking ibuprofen as needed since the spinning class. She was taking no other medications. She denied any allergies to drugs or food.
The patient admitted to smoking one pack of cigarettes per week and to occasional alcohol consumption but denied use of illicit drugs. She was employed as an executive officer for a large business association.
On physical examination, the patient’s vital signs were blood pressure, 134/73 mm/Hg; pulse, 86 beats/min; and respirations, 16 breaths/min. She was afebrile, alert, and oriented. Her sclera were nonicteric. Her neck was supple with no anterior cervical lymphadenopathy. There was no thyroid enlargement, her lungs were clear to auscultation, and her heart sounds were regular. There was no peripheral edema, and dorsalis pedis pulses were present bilaterally. Her thighs appeared swollen but were not tender to palpation.
The patient’s history, combined with an extremely high level of serum creatine phosphokinase (CPK; ie, 123,800 U/L [reference range, 45 to 260 U/L1]), confirmed a diagnosis of rhabdomyolysis. She was admitted for close observation. The patient’s urinalysis revealed 2 to 5 red blood cells and 6 to 10 white blood cells per high-power field. Moderate occult blood was detected, with no casts or protein noted. A urine myoglobin test was not performed.
The patient underwent IV hydration with dextrose 5% in water and three ampules of sodium bicarbonate after being given a 2.0-L saline bolus. Ibuprofen was discontinued. IV hydration with bicarbonate solution was continued until the patient’s CPK level declined significantly. She underwent daily laboratory testing (see Table 1). Her renal function remained stable, and she was discharged on hospital day 7.
DISCUSSION
Rhabdomyolysis is a clinical condition defined as muscle necrosis resulting from the release of intracellular skeletal muscle components (including myoglobin, CPK, potassium, phosphorus, and aldolase) into the extracellular compartment.1-3 The condition was first described during the bombing of London in World War II, with high incidence of crush injuries, shock, and associated kidney damage.4 The preponderance of such injuries during a 1988 earthquake in Armenia led the International Society of Nephrology to form its Renal Disaster Relief Task Force, which has provided support at numerous other disaster scenes since then.5
Rhabdomyolysis has been identified with a variety of pathologic events: those that cause muscle trauma, those associated with muscle use or overuse, and other etiologies involving genetic, metabolic, infectious, or pharmaceutical factors.1 Many of the reported causes of rhabdomyolysis are listed in Table 2.1,2
For patients with muscle trauma, the etiology of rhabdomyolysis is clear, but for those with other disease states, diagnosis may be more elusive. Patients who present with rhabdomyolysis after excessive exercise, for example, may have underlying metabolic disorders that predispose them to exertional rhabdomyolysis, such as chronic hypokalemia resulting from primary hyperaldosteronism.1 Others may have a muscle enzyme deficiency, as in McArdle’s syndrome or carnitine deficiency.6
Alterations in blood chemistries can also contribute to development of rhabdomyolysis, even when more obvious etiologies for muscle necrosis are evident. Hypokalemia interferes with the vasodilation that normally occurs during exercise to increase muscle blood flow.1,7,8 Continued exercise can lead to muscle necrosis, raising a concern for athletes who take diuretics.1 Hypophosphatemia leads to a state of muscle necrosis; this is of particular concern for alcoholic patients who receive hyperalimentation without repletion of phosphates.9
Diagnosis
Patients with rhabdomyolysis usually present with myalgias, darkened urine (red, brown, or black), and a clinical scenario that corroborates the diagnosis (ie, history of trauma, excessive exercise, use of an offending medication).1 Some patients may have minimal to absent symptoms or symptoms that occur only after exercise.3
A careful history is key. While traumatic causes are obvious, it is important to ask a patient with rhabdomyolysis after exertion about previous history of excessive weakness during or immediately after exercise, excessive cramping, or discoloration of urine after exercise. The family history may point to a genetic abnormality. A thorough understanding of the patient’s use of medications, including OTC agents, is also important. Rhabdomyolysis has been reported in patients who use herbal remedies, including those taken to facilitate weight loss or to improve lipid profiles.10,11
For patients suspected of having rhabdomyolysis, a serum CPK level should be obtained; results exceeding normal values by five times confirm the diagnosis.3 Measurements for potassium, phosphorus, and calcium are also important to determine, as is renal function. A high level of serum aldolase (an enzyme that breaks down glucose in muscle tissue) can also support a diagnosis of rhabdomyolysis.1,12 Urinalysis and urine myoglobin testing are also warranted, although a negative urine myoglobin test result does not rule out rhabdomyolysis in the presence of an elevated CPK level. Myoglobin is cleared rapidly by the kidneys, whereas serum CPK levels change slowly.1
Any patient who presents with acute rhabdomyolysis and low to normal values for potassium or phosphate should be evaluated further for hypokalemia and hypophosphatemia as contributing or etiologic factors. Hypocalcemia may occur in the early course of rhabdomyolysis as calcium salt is deposited in muscle tissue. Patients recovering from rhabdomyolysis may experience rebound hypercalcemia as the damaged muscle releases the deposited calcium.7,13
In most cases of rhabdomyolysis, only laboratory values are needed to make the diagnosis and follow the course of the episode.1 However, when the etiology appears to involve metabolic deficiencies or genetic etiologies, it may become necessary to order additional diagnostic tests. These may include tests for thyroid function, a carnitine level to screen for glycogen storage diseases, and toxin screening (eg, for illicit drugs, such as cocaine).2,6
Treatment and Management
Effective treatment of rhabdomyolysis relies on recognizing the underlying disorder.1 For patients with muscle trauma (eg, crush injury) or muscle overuse, the mainstay of treatment is aggressive fluid resuscitation and prevention of acute injury to the kidneys.13 As for patients with an injury induced by a pharmaceutical agent or a toxin, removal of the offending agent is required, followed by hydration and prevention of renal damage. Supportive care during an infectious illness is also essential.14
Additionally, treatment must address the complications inherent with rhabdomyolysis.1 In addition to CPK, potassium, phosphorus, and myoglobin are also released from skeletal muscle tissue. Hyperkalemia can be fatal, and potassium levels must be monitored closely to avert this condition.7,8,13 During an episode of rhabdomyolysis, normal levels of both potassium and phosphorus should raise the clinician’s suspicion for underlying hypokalemia and hypophosphatemia—conditions that may have contributed to the episode of rhabdomyolysis. Hypocalcemia may also develop.13
Released myoglobin may cause acute kidney injury, as is the case in 33% to 50% of patients with rhabdomyolysis.3 In early studies, it was determined that alkalinizing the urine with IV isotonic bicarbonate might thwart onset of acute kidney injury.1,2,15 Time is critical, and even on the battlefield or at the scene of a recent disaster, most attempts at resuscitation are begun immediately. IV access may be problematic, but administration of oral bicarbonate solutions has also proven effective.15 Close follow-up of the serum urea and creatinine levels and measurement of the urine pH during alkalinization is warranted throughout the course of the episode.
Unfortunately, some patients respond poorly to these conservative measures, and the released myoglobin can cause renal tubular blockage and necrosis, resulting in acute kidney injury.1 Renal replacement therapy may be required.16 However, most episodes of dialysis-dependent acute renal injury do subside with time.
For patients with less elusive causes of rhabdomyolysis, treatment will hinge on a workup of the possible etiologies and follow-up treatment to target the apparent cause. For example, carnitine may be administered to patients with carnitine deficiency, and hypokalemic patients may be given potassium.1,6,7 These patients will also need counseling before they consider engaging in an exercise program.
Patient’s Outcome
The case patient presented with exertional rhabdomyolysis; improper hydration, severe deconditioning, and a relatively low serum potassium level may all have contributed to the muscle necrosis she experienced. She was given IV alkaline solutions and did not develop acute kidney injury. She was discharged from the hospital and at the time of this writing was awaiting outpatient follow-up.
It should be interesting to see whether the case patient experiences any further episodes of severe weakness after engaging in exercise. Her low-normal potassium level (reference range, 3.5 to 5.3 mmol/L17) warrants further follow-up, as does her mildly elevated thyroid-stimulating hormone level (reference range, 0.5 to 4.7 mcIU/mL17).
CONCLUSION
Patients with rhabdomyolysis may present with muscle aches, darkened urine, and/or weakness; an elevated CPK level confirms the diagnosis. Management is mainly conservative, with IV hydration accompanied by alkalinizing the urine and correcting any metabolic abnormalities, such as potassium deficiencies. For the few patients who experience severe acute kidney injury, renal replacement therapy may be necessary.
While most causes of rhabdomyolysis have obvious clinical scenarios, such as a crush injury, a search for muscle enzyme deficiencies, disorders of potassium homeostasis, and thyroid abnormalities is also warranted in patients who present with exertional rhabdomyolysis.
1. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
2. Warren JD, Blumbergs PC, Thompson PD. Rhabdomyolysis: a review. Muscle Nerve. 2002;25(3):332-347.
3. Lima RSA, da Silva GB Jr, Liborio AB, Daher ED. Acute kidney injury due to rhabdomyolysis. Saudi J Kidney Dis Transpl. 2008;19(5):721-729.
4. Bywaters EG, Beall D. Crush injuries with impairment of renal function [reprinted from BMJ, 1941]. J Am Soc Nephrol. 1998;9(2):322-332.
5. Vanholder R, Van Biesen W, Lameire N, Sever MS; International Society of Nephrology/Renal Disaster Relief Task Force. The role of the International Society of Nephrology/Renal Disaster Relief Task Force in the rescue of renal disaster victims. Contrib Nephrol. 2007;156:325-332.
6. Toledo R, López V, Martín G, et al. Rhabdomyolysis due to enzyme deficiency in muscles. Nefrología. 2009;29(1):77-80.
7. Agrawal S, Agrawal V, Taneja A. Hypokalemia causing rhabdomyolysis resulting in life-threatening hyperkalemia. Pediatr Nephrol. 2006;221(2): 289-291.
8. Knochel JP, Schlein EM. On the mechanism of rhabdomyolysis in potassium depletion. J Clin Invest. 1972:51(7):1750-1758.
9. Knochel JP. Hypophosphatemia and rhabdomyolysis. Am J Med. 1992;92(5):455-457.
10. Mansi IA, Huang J. Rhabdomyolysis in response to weight-loss herbal medicine. Am J Med Sci. 2004; 327(6): 356-357.
11. Heber D, Yip I, Ashley JM, et al. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr. 1999;69(2): 231-236.
12. Hooda AK, Narula AS. Exertional rhabdomyolysis causing acute renal failure. Med J Armed Forces India. 2005;61(4):395-396.
13. Chatzizisis YS, Misirli G, Hatzitolios AI, Giannoglou GD. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19(8): 568-574.
14. Blanco JR, Zabalza M, Salcedo J, et al. Rhabdomyolysis of infectious and noninfectious causes. South Med J. 2002;95(5):542-544.
15. Ron D, Taitelman U, Michaelson M, et al. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch Intern Med. 1984;144(2):277-280.
16. Soni SS, Nagarik AP, Adikey GK, Raman A. Using continuous renal replacement therapy to manage patients of shock and acute renal failure. J Emerg Trauma Shock. 2009;2(1):19-22.
17. Normal laboratory values. In: Beers MH, Berkow R, eds. The Merck Manual of Diagnosis and Therapy. 17th ed. Whitehouse Station, NJ: Merck Research Laboratories; 1999:2526-2546.
1. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
2. Warren JD, Blumbergs PC, Thompson PD. Rhabdomyolysis: a review. Muscle Nerve. 2002;25(3):332-347.
3. Lima RSA, da Silva GB Jr, Liborio AB, Daher ED. Acute kidney injury due to rhabdomyolysis. Saudi J Kidney Dis Transpl. 2008;19(5):721-729.
4. Bywaters EG, Beall D. Crush injuries with impairment of renal function [reprinted from BMJ, 1941]. J Am Soc Nephrol. 1998;9(2):322-332.
5. Vanholder R, Van Biesen W, Lameire N, Sever MS; International Society of Nephrology/Renal Disaster Relief Task Force. The role of the International Society of Nephrology/Renal Disaster Relief Task Force in the rescue of renal disaster victims. Contrib Nephrol. 2007;156:325-332.
6. Toledo R, López V, Martín G, et al. Rhabdomyolysis due to enzyme deficiency in muscles. Nefrología. 2009;29(1):77-80.
7. Agrawal S, Agrawal V, Taneja A. Hypokalemia causing rhabdomyolysis resulting in life-threatening hyperkalemia. Pediatr Nephrol. 2006;221(2): 289-291.
8. Knochel JP, Schlein EM. On the mechanism of rhabdomyolysis in potassium depletion. J Clin Invest. 1972:51(7):1750-1758.
9. Knochel JP. Hypophosphatemia and rhabdomyolysis. Am J Med. 1992;92(5):455-457.
10. Mansi IA, Huang J. Rhabdomyolysis in response to weight-loss herbal medicine. Am J Med Sci. 2004; 327(6): 356-357.
11. Heber D, Yip I, Ashley JM, et al. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr. 1999;69(2): 231-236.
12. Hooda AK, Narula AS. Exertional rhabdomyolysis causing acute renal failure. Med J Armed Forces India. 2005;61(4):395-396.
13. Chatzizisis YS, Misirli G, Hatzitolios AI, Giannoglou GD. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19(8): 568-574.
14. Blanco JR, Zabalza M, Salcedo J, et al. Rhabdomyolysis of infectious and noninfectious causes. South Med J. 2002;95(5):542-544.
15. Ron D, Taitelman U, Michaelson M, et al. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch Intern Med. 1984;144(2):277-280.
16. Soni SS, Nagarik AP, Adikey GK, Raman A. Using continuous renal replacement therapy to manage patients of shock and acute renal failure. J Emerg Trauma Shock. 2009;2(1):19-22.
17. Normal laboratory values. In: Beers MH, Berkow R, eds. The Merck Manual of Diagnosis and Therapy. 17th ed. Whitehouse Station, NJ: Merck Research Laboratories; 1999:2526-2546.
Nickel-Induced Facial Dermatitis: Adolescents Beware of the Cell Phone
Pediatric Malignant Melanoma: An Update on Epidemiology, Detection, and Prevention
Botanical Briefs: Garden Angelica (Angelica archangelica)
Buckle fractures in children: Is urgent treatment necessary?
PURPOSE To determine whether the clinical outcome of buckle fractures in children differs between those treated acutely on the same day of trauma and those treated subacutely, and whether a change in practice patterns based on these data would result in cost savings.
METHODS In this retrospective cohort study—approved by the institutional review board—we reviewed the cases of 341 consecutive patients <18 years of age seen by the pediatric orthopedic clinic for treatment of isolated extremity buckle fractures between July 1, 2004 and August 31, 2007. Time from injury to treatment was used to divide patients into 2 groups: acute (≤1 day; n=155) and subacute treatment (>1 day; n=186). Clinical outcome at final orthopedic follow-up was recorded for each patient. We defined adverse outcome as fractures requiring manipulation, clinically apparent deformity, or functional impairment. Charge analysis compared differences in management costs for patients with buckle fractures presenting initially to the emergency department (ED) and those seen solely in the orthopedic clinic.
RESULTS No adverse outcomes were identified in either acute or subacute treatment groups. Total clinical visits did not vary (acute, 3.2 vs subacute, 3.1; P=.051). Presence of mild angulation of fractures on radiographs did not differ significantly between acute and subacute management groups at initial presentation (6.5% vs 8.6%; P=.541) or at final follow-up (12.2% vs 12.4%; P=1.0). A cost savings of approximately $3000 could have been realized for each patient referred to the ED who might otherwise have been seen subacutely in the orthopedic clinic.
CONCLUSIONS No adverse clinical outcomes resulted from subacute treatment of stable buckle fractures. Cost and time savings may be realized with subacute management of buckle fractures without affecting clinical outcome.
Next time a child in your care has a suspected fracture as a result of a fall and x-ray films reveal a buckle fracture, consider telling parents there’s no need for an urgent visit to the ED. As long as the pain is manageable, treating the injury within a day or so will likely be more convenient for the family, will cost less, and will not result in any complications for the child.
Buckle (or torus) fractures—the most common type of fracture occurring in the pediatric population and accounting for a large number of visits to primary care physicians (PCPs), EDs, and orthopedic clinics each year1 —involve impaction of bone along only 1 cortex and are therefore inherently stable.2 Even with only minimal immobilization, the overwhelming majority of buckle fractures heal without complication.3 Although many patients present directly to the ED for management of these fractures, many others present initially to their PCP, given the relatively minor nature of their symptoms and mechanism of injury.
At our institution, the radiology department and referring physician jointly triage out-patients when radiographs requested by the referring physician show evidence of a fracture. Stable and unstable fractures are referred for immediate care—in the pediatric orthopedic clinic if the clinic is open and appointments are available; otherwise in the ED for initial splinting, with follow-up in the orthopedic clinic as soon as possible.
Referral of patients with buckle fractures for same-day care in the ED may bring about unnecessary costs and inconvenience for patients and families. However, policy at our institution dictates that all fractures, including stable buckle fractures, be referred for treatment immediately, once identified.
To determine whether patients with buckle fractures can be safely counseled on the possibility of nonurgent management, we compared the clinical outcomes of pediatric buckle fractures treated acutely or subacutely. The results of our study have practical implications for the timing of treatment or referrals, and for the management of buckle fractures by appropriately trained PCPs, especially in settings where orthopedic consultation may not be readily available.
Methods
Patient selection
The Vanderbilt Children’s Hospital institutional review board approved this retrospective cohort study, with waiver of patient consent. We reviewed 1923 consecutive charts of patients who were seen in the hospital’s pediatric orthopedic clinic for stable fractures between July, 1, 2004 and August 31, 2007. We identified patients for our study population by current procedural terminology (CPT) codes for fracture care that were compatible with buckle fracture or other stable fracture management without manipulation. Applicable CPT codes included the following fracture sites: radial head/neck (24650), ulnar shaft (25530), distal radius with or without ulnar styloid (25600), metacarpal (26600), phalanx of hand or foot (26720, 28510), distal fibula (27786), and metatarsal (28470).
Inclusion and exclusion criteria. Inclusion criteria among this screened population were an isolated buckle fracture mentioned in the official radiology report or pediatric orthopedic clinical note, and age <18 years at the time of injury. We excluded patients for the following reasons: uncertain date of injury (n=67), lack of final clinical follow-up (n=59), acute manipulation of the fracture (n=10), multiple concurrent injuries (n=11), or known metabolic bone disease (n=3) or coagulopathy (n=1).
After initial patient selection, a CAQ-certified pediatric radiologist (with additional fellowship training in pediatric musculoskeletal radiology) and a board-certified orthopedic surgeon (with pediatric orthopedic fellowship training) examined available radiographic images to confirm the diagnosis of a buckle fracture. We further excluded patients whose radiographic findings did not meet criteria for isolated buckle fractures.
Study populations
The final study population consisted of 341 children with confirmed isolated buckle fractures. We assigned patients to acute or subacute treatment groups based on the length of time between injury and presentation for care. Patients were assigned to acute treatment (n=155) if they presented for care on the same day as the injury. All others first seen >1 day after documented time of injury were assigned to subacute treatment (n=186).
We determined length of time between injury and presentation based on data available in the electronic medical record. If the injury was first documented in the orthopedic clinic, we reviewed notes to determine when the patient had initially sought care, and whether from our institution, a PCP, or an outside ED. If documentation showed that initial contact with any health care professional occurred within 1 day of the injury, we assigned the patient to acute management.
Data analysis
Data collection from computerized medical records included date of injury, date of initial care, anatomic location of fracture, mechanism of injury, referring physician, whether the patient was seen initially in the ED, date of last orthopedic follow-up, number of clinical visits, and clinical outcome. Clinical outcome was judged as “good” or “poor” at the last orthopedic follow-up visit, approximately 3 to 4 weeks after injury. A poor clinical outcome could indicate a clinically apparent deformity or functional impairment, need for subacute manipulation, or refracture. We deemed 1 patient’s outcome uncertain due to an ambiguous final clinical note, and we had this case reviewed by a pediatric orthopedist. Any visit to a PCP, ED, or orthopedic clinic was included in the total number of a patient’s clinical visits.
Consulting radiologists also noted the presence and degree of fracture angulation for each patient on initial and follow-up films. Degree of angulation was rated as mild (<10°), moderate (11°-20°), or severe (>20°). Follow-up films were not available for 14 patients (5.2% of acutely treated patients; 3.2% of subacutely treated patients), as final clinical follow-up occasionally occurred outside our institution. In these cases, we relied on the clinical note to determine degree of angulation, if present.
We obtained total charges (technical and professional) for buckle fracture treatment for patients treated initially in the ED and for patients seen initially in the orthopedic clinic.
Statistical analysis
We used an independent samples t-test to compare mean patient ages, times from initial treatment to final treatment, and the numbers of clinical encounters for patients in the acute and subacute treatment groups. For the acute treatment group, time from injury to initial care, by definition, was considered “0.” For the subacute treatment group, we constructed 99.9% confidence intervals around the mean time from injury to initial care to determine whether or not they included “0.”
We used Fisher’s exact test to gauge differences in the proportions of absent or mild initial angulation, absent or mild final angulation, and the point of initial care between the acute and subacute treatment groups. We used Pearson’s chi-squared test to assess between-group differences in the distribution of fracture sites (forearm, hand/foot, or leg), mechanism of injury (fall, direct blow, other), change in angulation (none, improved, worsened), and point of entry into the health care system (PCP, ED, orthopedic clinic). We performed statistical analyses with the statistical package SPSS v15 (SPSS, Inc., Chicago, Ill).
Results
Patient characteristics
Of the 1923 pediatric patients with stable fractures seen in the orthopedic clinic at our institution during the study period, 588 had isolated buckle fractures. Of these, we excluded 151 based on predefined criteria (see Methods). After consensus review of radiographs by a pediatric orthopedist and pediatric radiologist, we excluded an additional 96 patients with inconclusive radiographs. The final study group numbered 341 pediatric patients with confirmed isolated buckle fractures.
The forearm was most commonly affected, with isolated distal radius fractures accounting for 67.7% (231/341) of all fractures, and combined radius/ulna fractures accounting for 14.7% (50/341). The most common mechanism of injury was a fall (85.9%; 293/341), usually a direct fall, with a higher percentage of patients with direct falls in the acute management group ( TABLE 1 ). Mean age and sex were not significantly different for the 2 treatment groups.
TABLE 1
Baseline characteristics of study populations
| Acute (n=155) | Subacute (n=186) | P value | |
|---|---|---|---|
| Age, y ± SD (range) | 7.9 ± 4.0 (0.9-17.8) | 7.9 ± 3.8 (1.0-16.4) | .901 |
| Male, n (%) | 79 (51.0) | 86 (46.2) | .384 |
| Site of fracture, n (%) | .022 | ||
| Forearm | 142 (91.6) | 151 (81.2) | |
| Hand or foot | 12 (7.7) | 33 (17.7) | |
| Leg | 1 (0.6) | 2 (1.1) | |
| Mechanism of injury, n (%) | .045 | ||
| Fall | 141 (90.9) | 152 (81.7) | |
| Direct blow | 5 (3.2) | 15 (8.1) | |
| Other/unknown | 9 (5.8) | 19 (10.2) | |
| SD, standard deviation. | |||
Acute vs subacute management outcomes
Of the 341 patients included in the study, 155 patients were treated acutely and 186 patients were treated subacutely. For the subacute management group, mean time between injury and treatment was 2.5±2.6 days ( TABLE 2 ). We observed no poor clinical outcomes in either acute or subacute management groups. All patients, regardless of time elapsed from injury to initial splinting, recovered without complication. The difference in number of clinical visits between the acute and subacute management groups was not significant (acute 3.2±0.5; subacute 3.1±0.5). The mean length of clinical follow-up from initial splinting to discharge from orthopedic care was higher in the acute management group (acute 32.9±17.1 days; subacute 28.9±13.4 days).
Most patients presented with non-angulated fractures, regardless of time from injury to initial presentation ( TABLE 2 ). The degree of angulation worsened in a small proportion of fractures during convalescence. The difference in initial angulation, final angulation, or change in angulation between acute and subacute management groups was not significant.
A higher proportion of patients in the acute treatment group presented directly to the ED for care, whereas a higher proportion of patients in the subacute treatment group presented to their PCP during routine working hours and were referred to the orthopedic clinic ( TABLE 2 ). For both acute and subacute management groups, we compared outcomes for patients seen initially in the ED or orthopedic clinic. No adverse outcomes occurred among any of the studied patients.
TABLE 2
Clinical outcomes did not differ between acute and subacute management groups
| Acute (n=155) | Subacute (n=186) | P value | |
|---|---|---|---|
| Time from injury to initial care, d ± SD (range) | 0 | 2.5 ± 2.6 (1-14) | <.001 |
| Good outcome, n (%) | 155 (100) | 186 (100) | 1.0 |
| Time from initial treatment to final follow-up, d ± SD (range) | 32.9 ± 17.1 (8-169) | 28.9 ± 13.4 (9-164) | .016 |
| Number of clinical encounters (primary care physician, ED, or orthopedic clinic), n ± SD (range) | 3.2 ± 0.5 (2-5) | 3.1 ± 0.5 (2-5) | .051 |
| Initial angulation, n (%) | .541 | ||
| None | 145 (93.5) | 170 (91.4) | |
| Mild (<10°) | 10 (6.5) | 16 (8.6) | |
| Final angulation, n (%) | 1.0 | ||
| None | 136 (87.7) | 163 (87.6) | |
| Mild (<10°) | 19 (12.2) | 23 (12.4) | |
| Change in angulation, n (%) | .907 | ||
| No change | 144 (92.9) | 175 (94.1) | |
| Worse | 10 (6.4) | 10 (5.4) | |
| Improved | 1 (0.6) | 1 (0.5) | |
| Point of entry to health care system, n (%) | <.001 | ||
| Primary care physician | 44 (28.4) | 115 (61.8) | |
| ED | 108 (69.7) | 54 (29.0) | |
| Orthopedic clinic | 3 (1.9) | 17 (9.1) | |
| Location of initial management, n (%) | <.001 | ||
| ED | 132 (85.2) | 81 (43.5) | |
| Orthopedic clinic | 23 (14.8) | 105 (56.4) | |
| ED, emergency department; SD, standard deviation. | |||
Charge analysis
We compared total charges (professional and technical) for managing buckle fractures initially in the ED with those initially seen in the orthopedic clinic. Total charge per patient in the ED, including subsequent follow-up in the orthopedic clinic, was $4397 ($2516, professional; $1881, technical). Total charge per patient for treatment only in the orthopedic clinic was $1426 ($918, professional; $508, technical). Total charge per patient was $2971 more for patients treated initially in the ED.
Between July 1, 2004, and August 31, 2007, 159 patients (46.6%) with buckle fractures entered the health care system through their primary care physician. Of these, 44 patients were seen acutely by the physician; 115 patients were seen on a subacute basis. Of the 44 patients seen acutely, 24 (54%) were referred directly to the ED; 20 (45%) were referred to the orthopedic clinic. Of the 115 patients seen subacutely, 27 (23%) were referred directly to the ED, and the remaining 88 (76%) were referred to the orthopedic clinic. In sum, 51 patients (32%) were seen initially by a PCP, who referred them to the ED. The cost savings with each patient seen subacutely in the orthopedic clinic was $2971, and avoiding ED treatment for all patients could have yielded a total gross savings of approximately $150,000.
Discussion
Buckle fractures are inherently stable and almost universally heal without complication.4,5 Perhaps because of the high likelihood of good outcome, there is a relative paucity of articles in the recent literature addressing the management of this common pediatric fracture. Older studies have addressed casting vs splinting and the need for follow-up, but no study has yet examined whether immediate treatment is necessary.6,7 Although some studies have noted incidentally that many children have delayed presentation for care,5 none has specifically examined the clinical or economic impact of a delay in care or the effect of subacute treatment on outcomes.
Delayed treatment does not adversely affect clinical outcome. Our study objective was to compare clinical outcomes of buckle fractures treated acutely on the same day of injury with outcomes of those treated subacutely. The 2 groups did not differ in extent or angulation of fracture at presentation. We found no difference in outcomes between the groups; all fractures healed without complication. We observed no difference in final angulation of fracture on follow-up imaging. Though our institution routinely obtains follow-up films, it is worth mentioning that the utility of repeat films in pediatric buckle fractures with minimal initial angulation has been debated.5 These data suggest that subacute treatment of a buckle fracture is a safe and reasonable option.
Non-ED treatment substantially reduces cost. One goal of efficient health care delivery is to decrease the cost and burden of care without increasing long-term morbidity and disability. Evidence suggests that families may prefer less acute management options that allow greater convenience and flexibility, provided that clinical outcomes are not compromised.8 In the case of pediatric buckle fractures, higher costs (for both the patient and the hospital) and longer wait times related to ED care may be avoided by counseling patients on the option of subacute care. Our study found that referring patients directly to the orthopedic clinic, even if this results in a delay in definitive management, leads to a reduction in health care burden without a change in clinical outcome.
Children with buckle fractures are frequently (46.6%) taken to their PCP for initial care. Many pediatricians and family physicians—especially the increasing number of physicians who have completed additional fellowship training in sports medicine—may prefer to manage buckle fractures within their practices. Many other PCPs may be practicing in communities lacking local orthopedic expertise. The results of this study provide reassurance regarding the positive outcome of buckle fractures. Furthermore, managing buckle fractures in the primary care setting may be even more cost effective than referring patients to a specialty orthopedic clinic—but additional research on this point is needed.
We do not advocate delayed imaging or treatment of suspected fractures. However, once a diagnosis of buckle fracture is confirmed radiographically, our data show that subacute treatment yields significant cost and time savings without affecting final clinical outcome.
Study limitations
This study is limited by its retrospective data collection in 1 pediatric tertiary care hospital. As current clinical practice is to treat all buckle fractures once identified, very few patients with known injury were specifically treated in a subacute fashion. We defined the subacute care group as patients who were treated >1 day from the time of injury. Because initial splinting did not occur in this group, we expect that the observed results would be similar, and no worse, compared with buckle fracture care directed by a subacute treatment algorithm.
This study examined only patients with a diagnosis of isolated buckle fracture. Non-buckle stable fractures were excluded a priori from our patient population. Although it is possible that most stable fractures (eg, nondisplaced transverse fractures, Salter-Harris I injuries) could be managed subacutely, we addressed only isolated buckle fractures.
Because of the universally positive outcomes in these cases, most of our patients had no orthopedic follow-up beyond 1 month. We are not able to comment on whether any longer-term abnormalities in function occurred. This question could be addressed through a prospective trial requiring reevaluation of each patient at a set endpoint of the study.
Although buckle fractures are inherently stable and do not present a significant risk of displacement with delayed treatment, they are nevertheless painful fractures that can be a cause of considerable anxiety for both patient and family. The goal of the physician, beyond ensuring the best medical outcome, extends to provide emotional support to the patient and family. Pain control and reassurance are therefore central to the discussion of fracture management, and are most likely the driving factor for a patient to seek urgent care. A key limitation of this study was the inability to determine differences in pain control between acute and subacute treatment. As mentioned above, a prospective study would enable the issue of pain control to be better addressed.
CORRESPONDENCE
Debbie Lee Bennett, MD, Massachusetts General Hospital, 55 Fruit Street, FND-216, Boston, MA 02114; [email protected]
(At the time this study was accepted for publication, Dr. Bennett was at Vanderbilt Children’s Hospital.)
1. Plint AC, Clifford T, Perry J, et al. Wrist buckle fractures: a survey of current practice patterns and attitudes toward immobilization. Can J Emerg Med. 2003;5:95-100.
2. England SP, Sundberg S. Management of common pediatric fractures. In: England SP, ed. Common Orthopedic Problems II. Philadelphia, Pa: WB Saunders; 1996:991–1012.
3. Van Bosse HJP, Patel RJ, Thacker M, et al. Minimalistic approach to treating wrist torus fractures. J Pediatr Orthop. 2005;25:495-500.
4. Davidson JS, Brown DJ, Barnes SN, et al. Simple treatment for torus fractures of the distal radius. J Bone Joint Surg Br. 2001;83-B:1173-1175.
5. Plint AC, Perry JJ, Tsang JLY. Pediatric wrist buckle fractures: should we just splint and go? Can J Emerg Med. 2004;6:397-401.
6. Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33:503-505.
7. Plint AC, Perry JJ, Correll R, et al. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117:691-697.
8. Symons S, Rowsell M, Bhowal B, et al. Hospital versus home management of children with buckle fractures of the distal radius. A prospective, randomised trial. J Bone Joint Surg Br. 2001;83:556-560.
PURPOSE To determine whether the clinical outcome of buckle fractures in children differs between those treated acutely on the same day of trauma and those treated subacutely, and whether a change in practice patterns based on these data would result in cost savings.
METHODS In this retrospective cohort study—approved by the institutional review board—we reviewed the cases of 341 consecutive patients <18 years of age seen by the pediatric orthopedic clinic for treatment of isolated extremity buckle fractures between July 1, 2004 and August 31, 2007. Time from injury to treatment was used to divide patients into 2 groups: acute (≤1 day; n=155) and subacute treatment (>1 day; n=186). Clinical outcome at final orthopedic follow-up was recorded for each patient. We defined adverse outcome as fractures requiring manipulation, clinically apparent deformity, or functional impairment. Charge analysis compared differences in management costs for patients with buckle fractures presenting initially to the emergency department (ED) and those seen solely in the orthopedic clinic.
RESULTS No adverse outcomes were identified in either acute or subacute treatment groups. Total clinical visits did not vary (acute, 3.2 vs subacute, 3.1; P=.051). Presence of mild angulation of fractures on radiographs did not differ significantly between acute and subacute management groups at initial presentation (6.5% vs 8.6%; P=.541) or at final follow-up (12.2% vs 12.4%; P=1.0). A cost savings of approximately $3000 could have been realized for each patient referred to the ED who might otherwise have been seen subacutely in the orthopedic clinic.
CONCLUSIONS No adverse clinical outcomes resulted from subacute treatment of stable buckle fractures. Cost and time savings may be realized with subacute management of buckle fractures without affecting clinical outcome.
Next time a child in your care has a suspected fracture as a result of a fall and x-ray films reveal a buckle fracture, consider telling parents there’s no need for an urgent visit to the ED. As long as the pain is manageable, treating the injury within a day or so will likely be more convenient for the family, will cost less, and will not result in any complications for the child.
Buckle (or torus) fractures—the most common type of fracture occurring in the pediatric population and accounting for a large number of visits to primary care physicians (PCPs), EDs, and orthopedic clinics each year1 —involve impaction of bone along only 1 cortex and are therefore inherently stable.2 Even with only minimal immobilization, the overwhelming majority of buckle fractures heal without complication.3 Although many patients present directly to the ED for management of these fractures, many others present initially to their PCP, given the relatively minor nature of their symptoms and mechanism of injury.
At our institution, the radiology department and referring physician jointly triage out-patients when radiographs requested by the referring physician show evidence of a fracture. Stable and unstable fractures are referred for immediate care—in the pediatric orthopedic clinic if the clinic is open and appointments are available; otherwise in the ED for initial splinting, with follow-up in the orthopedic clinic as soon as possible.
Referral of patients with buckle fractures for same-day care in the ED may bring about unnecessary costs and inconvenience for patients and families. However, policy at our institution dictates that all fractures, including stable buckle fractures, be referred for treatment immediately, once identified.
To determine whether patients with buckle fractures can be safely counseled on the possibility of nonurgent management, we compared the clinical outcomes of pediatric buckle fractures treated acutely or subacutely. The results of our study have practical implications for the timing of treatment or referrals, and for the management of buckle fractures by appropriately trained PCPs, especially in settings where orthopedic consultation may not be readily available.
Methods
Patient selection
The Vanderbilt Children’s Hospital institutional review board approved this retrospective cohort study, with waiver of patient consent. We reviewed 1923 consecutive charts of patients who were seen in the hospital’s pediatric orthopedic clinic for stable fractures between July, 1, 2004 and August 31, 2007. We identified patients for our study population by current procedural terminology (CPT) codes for fracture care that were compatible with buckle fracture or other stable fracture management without manipulation. Applicable CPT codes included the following fracture sites: radial head/neck (24650), ulnar shaft (25530), distal radius with or without ulnar styloid (25600), metacarpal (26600), phalanx of hand or foot (26720, 28510), distal fibula (27786), and metatarsal (28470).
Inclusion and exclusion criteria. Inclusion criteria among this screened population were an isolated buckle fracture mentioned in the official radiology report or pediatric orthopedic clinical note, and age <18 years at the time of injury. We excluded patients for the following reasons: uncertain date of injury (n=67), lack of final clinical follow-up (n=59), acute manipulation of the fracture (n=10), multiple concurrent injuries (n=11), or known metabolic bone disease (n=3) or coagulopathy (n=1).
After initial patient selection, a CAQ-certified pediatric radiologist (with additional fellowship training in pediatric musculoskeletal radiology) and a board-certified orthopedic surgeon (with pediatric orthopedic fellowship training) examined available radiographic images to confirm the diagnosis of a buckle fracture. We further excluded patients whose radiographic findings did not meet criteria for isolated buckle fractures.
Study populations
The final study population consisted of 341 children with confirmed isolated buckle fractures. We assigned patients to acute or subacute treatment groups based on the length of time between injury and presentation for care. Patients were assigned to acute treatment (n=155) if they presented for care on the same day as the injury. All others first seen >1 day after documented time of injury were assigned to subacute treatment (n=186).
We determined length of time between injury and presentation based on data available in the electronic medical record. If the injury was first documented in the orthopedic clinic, we reviewed notes to determine when the patient had initially sought care, and whether from our institution, a PCP, or an outside ED. If documentation showed that initial contact with any health care professional occurred within 1 day of the injury, we assigned the patient to acute management.
Data analysis
Data collection from computerized medical records included date of injury, date of initial care, anatomic location of fracture, mechanism of injury, referring physician, whether the patient was seen initially in the ED, date of last orthopedic follow-up, number of clinical visits, and clinical outcome. Clinical outcome was judged as “good” or “poor” at the last orthopedic follow-up visit, approximately 3 to 4 weeks after injury. A poor clinical outcome could indicate a clinically apparent deformity or functional impairment, need for subacute manipulation, or refracture. We deemed 1 patient’s outcome uncertain due to an ambiguous final clinical note, and we had this case reviewed by a pediatric orthopedist. Any visit to a PCP, ED, or orthopedic clinic was included in the total number of a patient’s clinical visits.
Consulting radiologists also noted the presence and degree of fracture angulation for each patient on initial and follow-up films. Degree of angulation was rated as mild (<10°), moderate (11°-20°), or severe (>20°). Follow-up films were not available for 14 patients (5.2% of acutely treated patients; 3.2% of subacutely treated patients), as final clinical follow-up occasionally occurred outside our institution. In these cases, we relied on the clinical note to determine degree of angulation, if present.
We obtained total charges (technical and professional) for buckle fracture treatment for patients treated initially in the ED and for patients seen initially in the orthopedic clinic.
Statistical analysis
We used an independent samples t-test to compare mean patient ages, times from initial treatment to final treatment, and the numbers of clinical encounters for patients in the acute and subacute treatment groups. For the acute treatment group, time from injury to initial care, by definition, was considered “0.” For the subacute treatment group, we constructed 99.9% confidence intervals around the mean time from injury to initial care to determine whether or not they included “0.”
We used Fisher’s exact test to gauge differences in the proportions of absent or mild initial angulation, absent or mild final angulation, and the point of initial care between the acute and subacute treatment groups. We used Pearson’s chi-squared test to assess between-group differences in the distribution of fracture sites (forearm, hand/foot, or leg), mechanism of injury (fall, direct blow, other), change in angulation (none, improved, worsened), and point of entry into the health care system (PCP, ED, orthopedic clinic). We performed statistical analyses with the statistical package SPSS v15 (SPSS, Inc., Chicago, Ill).
Results
Patient characteristics
Of the 1923 pediatric patients with stable fractures seen in the orthopedic clinic at our institution during the study period, 588 had isolated buckle fractures. Of these, we excluded 151 based on predefined criteria (see Methods). After consensus review of radiographs by a pediatric orthopedist and pediatric radiologist, we excluded an additional 96 patients with inconclusive radiographs. The final study group numbered 341 pediatric patients with confirmed isolated buckle fractures.
The forearm was most commonly affected, with isolated distal radius fractures accounting for 67.7% (231/341) of all fractures, and combined radius/ulna fractures accounting for 14.7% (50/341). The most common mechanism of injury was a fall (85.9%; 293/341), usually a direct fall, with a higher percentage of patients with direct falls in the acute management group ( TABLE 1 ). Mean age and sex were not significantly different for the 2 treatment groups.
TABLE 1
Baseline characteristics of study populations
| Acute (n=155) | Subacute (n=186) | P value | |
|---|---|---|---|
| Age, y ± SD (range) | 7.9 ± 4.0 (0.9-17.8) | 7.9 ± 3.8 (1.0-16.4) | .901 |
| Male, n (%) | 79 (51.0) | 86 (46.2) | .384 |
| Site of fracture, n (%) | .022 | ||
| Forearm | 142 (91.6) | 151 (81.2) | |
| Hand or foot | 12 (7.7) | 33 (17.7) | |
| Leg | 1 (0.6) | 2 (1.1) | |
| Mechanism of injury, n (%) | .045 | ||
| Fall | 141 (90.9) | 152 (81.7) | |
| Direct blow | 5 (3.2) | 15 (8.1) | |
| Other/unknown | 9 (5.8) | 19 (10.2) | |
| SD, standard deviation. | |||
Acute vs subacute management outcomes
Of the 341 patients included in the study, 155 patients were treated acutely and 186 patients were treated subacutely. For the subacute management group, mean time between injury and treatment was 2.5±2.6 days ( TABLE 2 ). We observed no poor clinical outcomes in either acute or subacute management groups. All patients, regardless of time elapsed from injury to initial splinting, recovered without complication. The difference in number of clinical visits between the acute and subacute management groups was not significant (acute 3.2±0.5; subacute 3.1±0.5). The mean length of clinical follow-up from initial splinting to discharge from orthopedic care was higher in the acute management group (acute 32.9±17.1 days; subacute 28.9±13.4 days).
Most patients presented with non-angulated fractures, regardless of time from injury to initial presentation ( TABLE 2 ). The degree of angulation worsened in a small proportion of fractures during convalescence. The difference in initial angulation, final angulation, or change in angulation between acute and subacute management groups was not significant.
A higher proportion of patients in the acute treatment group presented directly to the ED for care, whereas a higher proportion of patients in the subacute treatment group presented to their PCP during routine working hours and were referred to the orthopedic clinic ( TABLE 2 ). For both acute and subacute management groups, we compared outcomes for patients seen initially in the ED or orthopedic clinic. No adverse outcomes occurred among any of the studied patients.
TABLE 2
Clinical outcomes did not differ between acute and subacute management groups
| Acute (n=155) | Subacute (n=186) | P value | |
|---|---|---|---|
| Time from injury to initial care, d ± SD (range) | 0 | 2.5 ± 2.6 (1-14) | <.001 |
| Good outcome, n (%) | 155 (100) | 186 (100) | 1.0 |
| Time from initial treatment to final follow-up, d ± SD (range) | 32.9 ± 17.1 (8-169) | 28.9 ± 13.4 (9-164) | .016 |
| Number of clinical encounters (primary care physician, ED, or orthopedic clinic), n ± SD (range) | 3.2 ± 0.5 (2-5) | 3.1 ± 0.5 (2-5) | .051 |
| Initial angulation, n (%) | .541 | ||
| None | 145 (93.5) | 170 (91.4) | |
| Mild (<10°) | 10 (6.5) | 16 (8.6) | |
| Final angulation, n (%) | 1.0 | ||
| None | 136 (87.7) | 163 (87.6) | |
| Mild (<10°) | 19 (12.2) | 23 (12.4) | |
| Change in angulation, n (%) | .907 | ||
| No change | 144 (92.9) | 175 (94.1) | |
| Worse | 10 (6.4) | 10 (5.4) | |
| Improved | 1 (0.6) | 1 (0.5) | |
| Point of entry to health care system, n (%) | <.001 | ||
| Primary care physician | 44 (28.4) | 115 (61.8) | |
| ED | 108 (69.7) | 54 (29.0) | |
| Orthopedic clinic | 3 (1.9) | 17 (9.1) | |
| Location of initial management, n (%) | <.001 | ||
| ED | 132 (85.2) | 81 (43.5) | |
| Orthopedic clinic | 23 (14.8) | 105 (56.4) | |
| ED, emergency department; SD, standard deviation. | |||
Charge analysis
We compared total charges (professional and technical) for managing buckle fractures initially in the ED with those initially seen in the orthopedic clinic. Total charge per patient in the ED, including subsequent follow-up in the orthopedic clinic, was $4397 ($2516, professional; $1881, technical). Total charge per patient for treatment only in the orthopedic clinic was $1426 ($918, professional; $508, technical). Total charge per patient was $2971 more for patients treated initially in the ED.
Between July 1, 2004, and August 31, 2007, 159 patients (46.6%) with buckle fractures entered the health care system through their primary care physician. Of these, 44 patients were seen acutely by the physician; 115 patients were seen on a subacute basis. Of the 44 patients seen acutely, 24 (54%) were referred directly to the ED; 20 (45%) were referred to the orthopedic clinic. Of the 115 patients seen subacutely, 27 (23%) were referred directly to the ED, and the remaining 88 (76%) were referred to the orthopedic clinic. In sum, 51 patients (32%) were seen initially by a PCP, who referred them to the ED. The cost savings with each patient seen subacutely in the orthopedic clinic was $2971, and avoiding ED treatment for all patients could have yielded a total gross savings of approximately $150,000.
Discussion
Buckle fractures are inherently stable and almost universally heal without complication.4,5 Perhaps because of the high likelihood of good outcome, there is a relative paucity of articles in the recent literature addressing the management of this common pediatric fracture. Older studies have addressed casting vs splinting and the need for follow-up, but no study has yet examined whether immediate treatment is necessary.6,7 Although some studies have noted incidentally that many children have delayed presentation for care,5 none has specifically examined the clinical or economic impact of a delay in care or the effect of subacute treatment on outcomes.
Delayed treatment does not adversely affect clinical outcome. Our study objective was to compare clinical outcomes of buckle fractures treated acutely on the same day of injury with outcomes of those treated subacutely. The 2 groups did not differ in extent or angulation of fracture at presentation. We found no difference in outcomes between the groups; all fractures healed without complication. We observed no difference in final angulation of fracture on follow-up imaging. Though our institution routinely obtains follow-up films, it is worth mentioning that the utility of repeat films in pediatric buckle fractures with minimal initial angulation has been debated.5 These data suggest that subacute treatment of a buckle fracture is a safe and reasonable option.
Non-ED treatment substantially reduces cost. One goal of efficient health care delivery is to decrease the cost and burden of care without increasing long-term morbidity and disability. Evidence suggests that families may prefer less acute management options that allow greater convenience and flexibility, provided that clinical outcomes are not compromised.8 In the case of pediatric buckle fractures, higher costs (for both the patient and the hospital) and longer wait times related to ED care may be avoided by counseling patients on the option of subacute care. Our study found that referring patients directly to the orthopedic clinic, even if this results in a delay in definitive management, leads to a reduction in health care burden without a change in clinical outcome.
Children with buckle fractures are frequently (46.6%) taken to their PCP for initial care. Many pediatricians and family physicians—especially the increasing number of physicians who have completed additional fellowship training in sports medicine—may prefer to manage buckle fractures within their practices. Many other PCPs may be practicing in communities lacking local orthopedic expertise. The results of this study provide reassurance regarding the positive outcome of buckle fractures. Furthermore, managing buckle fractures in the primary care setting may be even more cost effective than referring patients to a specialty orthopedic clinic—but additional research on this point is needed.
We do not advocate delayed imaging or treatment of suspected fractures. However, once a diagnosis of buckle fracture is confirmed radiographically, our data show that subacute treatment yields significant cost and time savings without affecting final clinical outcome.
Study limitations
This study is limited by its retrospective data collection in 1 pediatric tertiary care hospital. As current clinical practice is to treat all buckle fractures once identified, very few patients with known injury were specifically treated in a subacute fashion. We defined the subacute care group as patients who were treated >1 day from the time of injury. Because initial splinting did not occur in this group, we expect that the observed results would be similar, and no worse, compared with buckle fracture care directed by a subacute treatment algorithm.
This study examined only patients with a diagnosis of isolated buckle fracture. Non-buckle stable fractures were excluded a priori from our patient population. Although it is possible that most stable fractures (eg, nondisplaced transverse fractures, Salter-Harris I injuries) could be managed subacutely, we addressed only isolated buckle fractures.
Because of the universally positive outcomes in these cases, most of our patients had no orthopedic follow-up beyond 1 month. We are not able to comment on whether any longer-term abnormalities in function occurred. This question could be addressed through a prospective trial requiring reevaluation of each patient at a set endpoint of the study.
Although buckle fractures are inherently stable and do not present a significant risk of displacement with delayed treatment, they are nevertheless painful fractures that can be a cause of considerable anxiety for both patient and family. The goal of the physician, beyond ensuring the best medical outcome, extends to provide emotional support to the patient and family. Pain control and reassurance are therefore central to the discussion of fracture management, and are most likely the driving factor for a patient to seek urgent care. A key limitation of this study was the inability to determine differences in pain control between acute and subacute treatment. As mentioned above, a prospective study would enable the issue of pain control to be better addressed.
CORRESPONDENCE
Debbie Lee Bennett, MD, Massachusetts General Hospital, 55 Fruit Street, FND-216, Boston, MA 02114; [email protected]
(At the time this study was accepted for publication, Dr. Bennett was at Vanderbilt Children’s Hospital.)
PURPOSE To determine whether the clinical outcome of buckle fractures in children differs between those treated acutely on the same day of trauma and those treated subacutely, and whether a change in practice patterns based on these data would result in cost savings.
METHODS In this retrospective cohort study—approved by the institutional review board—we reviewed the cases of 341 consecutive patients <18 years of age seen by the pediatric orthopedic clinic for treatment of isolated extremity buckle fractures between July 1, 2004 and August 31, 2007. Time from injury to treatment was used to divide patients into 2 groups: acute (≤1 day; n=155) and subacute treatment (>1 day; n=186). Clinical outcome at final orthopedic follow-up was recorded for each patient. We defined adverse outcome as fractures requiring manipulation, clinically apparent deformity, or functional impairment. Charge analysis compared differences in management costs for patients with buckle fractures presenting initially to the emergency department (ED) and those seen solely in the orthopedic clinic.
RESULTS No adverse outcomes were identified in either acute or subacute treatment groups. Total clinical visits did not vary (acute, 3.2 vs subacute, 3.1; P=.051). Presence of mild angulation of fractures on radiographs did not differ significantly between acute and subacute management groups at initial presentation (6.5% vs 8.6%; P=.541) or at final follow-up (12.2% vs 12.4%; P=1.0). A cost savings of approximately $3000 could have been realized for each patient referred to the ED who might otherwise have been seen subacutely in the orthopedic clinic.
CONCLUSIONS No adverse clinical outcomes resulted from subacute treatment of stable buckle fractures. Cost and time savings may be realized with subacute management of buckle fractures without affecting clinical outcome.
Next time a child in your care has a suspected fracture as a result of a fall and x-ray films reveal a buckle fracture, consider telling parents there’s no need for an urgent visit to the ED. As long as the pain is manageable, treating the injury within a day or so will likely be more convenient for the family, will cost less, and will not result in any complications for the child.
Buckle (or torus) fractures—the most common type of fracture occurring in the pediatric population and accounting for a large number of visits to primary care physicians (PCPs), EDs, and orthopedic clinics each year1 —involve impaction of bone along only 1 cortex and are therefore inherently stable.2 Even with only minimal immobilization, the overwhelming majority of buckle fractures heal without complication.3 Although many patients present directly to the ED for management of these fractures, many others present initially to their PCP, given the relatively minor nature of their symptoms and mechanism of injury.
At our institution, the radiology department and referring physician jointly triage out-patients when radiographs requested by the referring physician show evidence of a fracture. Stable and unstable fractures are referred for immediate care—in the pediatric orthopedic clinic if the clinic is open and appointments are available; otherwise in the ED for initial splinting, with follow-up in the orthopedic clinic as soon as possible.
Referral of patients with buckle fractures for same-day care in the ED may bring about unnecessary costs and inconvenience for patients and families. However, policy at our institution dictates that all fractures, including stable buckle fractures, be referred for treatment immediately, once identified.
To determine whether patients with buckle fractures can be safely counseled on the possibility of nonurgent management, we compared the clinical outcomes of pediatric buckle fractures treated acutely or subacutely. The results of our study have practical implications for the timing of treatment or referrals, and for the management of buckle fractures by appropriately trained PCPs, especially in settings where orthopedic consultation may not be readily available.
Methods
Patient selection
The Vanderbilt Children’s Hospital institutional review board approved this retrospective cohort study, with waiver of patient consent. We reviewed 1923 consecutive charts of patients who were seen in the hospital’s pediatric orthopedic clinic for stable fractures between July, 1, 2004 and August 31, 2007. We identified patients for our study population by current procedural terminology (CPT) codes for fracture care that were compatible with buckle fracture or other stable fracture management without manipulation. Applicable CPT codes included the following fracture sites: radial head/neck (24650), ulnar shaft (25530), distal radius with or without ulnar styloid (25600), metacarpal (26600), phalanx of hand or foot (26720, 28510), distal fibula (27786), and metatarsal (28470).
Inclusion and exclusion criteria. Inclusion criteria among this screened population were an isolated buckle fracture mentioned in the official radiology report or pediatric orthopedic clinical note, and age <18 years at the time of injury. We excluded patients for the following reasons: uncertain date of injury (n=67), lack of final clinical follow-up (n=59), acute manipulation of the fracture (n=10), multiple concurrent injuries (n=11), or known metabolic bone disease (n=3) or coagulopathy (n=1).
After initial patient selection, a CAQ-certified pediatric radiologist (with additional fellowship training in pediatric musculoskeletal radiology) and a board-certified orthopedic surgeon (with pediatric orthopedic fellowship training) examined available radiographic images to confirm the diagnosis of a buckle fracture. We further excluded patients whose radiographic findings did not meet criteria for isolated buckle fractures.
Study populations
The final study population consisted of 341 children with confirmed isolated buckle fractures. We assigned patients to acute or subacute treatment groups based on the length of time between injury and presentation for care. Patients were assigned to acute treatment (n=155) if they presented for care on the same day as the injury. All others first seen >1 day after documented time of injury were assigned to subacute treatment (n=186).
We determined length of time between injury and presentation based on data available in the electronic medical record. If the injury was first documented in the orthopedic clinic, we reviewed notes to determine when the patient had initially sought care, and whether from our institution, a PCP, or an outside ED. If documentation showed that initial contact with any health care professional occurred within 1 day of the injury, we assigned the patient to acute management.
Data analysis
Data collection from computerized medical records included date of injury, date of initial care, anatomic location of fracture, mechanism of injury, referring physician, whether the patient was seen initially in the ED, date of last orthopedic follow-up, number of clinical visits, and clinical outcome. Clinical outcome was judged as “good” or “poor” at the last orthopedic follow-up visit, approximately 3 to 4 weeks after injury. A poor clinical outcome could indicate a clinically apparent deformity or functional impairment, need for subacute manipulation, or refracture. We deemed 1 patient’s outcome uncertain due to an ambiguous final clinical note, and we had this case reviewed by a pediatric orthopedist. Any visit to a PCP, ED, or orthopedic clinic was included in the total number of a patient’s clinical visits.
Consulting radiologists also noted the presence and degree of fracture angulation for each patient on initial and follow-up films. Degree of angulation was rated as mild (<10°), moderate (11°-20°), or severe (>20°). Follow-up films were not available for 14 patients (5.2% of acutely treated patients; 3.2% of subacutely treated patients), as final clinical follow-up occasionally occurred outside our institution. In these cases, we relied on the clinical note to determine degree of angulation, if present.
We obtained total charges (technical and professional) for buckle fracture treatment for patients treated initially in the ED and for patients seen initially in the orthopedic clinic.
Statistical analysis
We used an independent samples t-test to compare mean patient ages, times from initial treatment to final treatment, and the numbers of clinical encounters for patients in the acute and subacute treatment groups. For the acute treatment group, time from injury to initial care, by definition, was considered “0.” For the subacute treatment group, we constructed 99.9% confidence intervals around the mean time from injury to initial care to determine whether or not they included “0.”
We used Fisher’s exact test to gauge differences in the proportions of absent or mild initial angulation, absent or mild final angulation, and the point of initial care between the acute and subacute treatment groups. We used Pearson’s chi-squared test to assess between-group differences in the distribution of fracture sites (forearm, hand/foot, or leg), mechanism of injury (fall, direct blow, other), change in angulation (none, improved, worsened), and point of entry into the health care system (PCP, ED, orthopedic clinic). We performed statistical analyses with the statistical package SPSS v15 (SPSS, Inc., Chicago, Ill).
Results
Patient characteristics
Of the 1923 pediatric patients with stable fractures seen in the orthopedic clinic at our institution during the study period, 588 had isolated buckle fractures. Of these, we excluded 151 based on predefined criteria (see Methods). After consensus review of radiographs by a pediatric orthopedist and pediatric radiologist, we excluded an additional 96 patients with inconclusive radiographs. The final study group numbered 341 pediatric patients with confirmed isolated buckle fractures.
The forearm was most commonly affected, with isolated distal radius fractures accounting for 67.7% (231/341) of all fractures, and combined radius/ulna fractures accounting for 14.7% (50/341). The most common mechanism of injury was a fall (85.9%; 293/341), usually a direct fall, with a higher percentage of patients with direct falls in the acute management group ( TABLE 1 ). Mean age and sex were not significantly different for the 2 treatment groups.
TABLE 1
Baseline characteristics of study populations
| Acute (n=155) | Subacute (n=186) | P value | |
|---|---|---|---|
| Age, y ± SD (range) | 7.9 ± 4.0 (0.9-17.8) | 7.9 ± 3.8 (1.0-16.4) | .901 |
| Male, n (%) | 79 (51.0) | 86 (46.2) | .384 |
| Site of fracture, n (%) | .022 | ||
| Forearm | 142 (91.6) | 151 (81.2) | |
| Hand or foot | 12 (7.7) | 33 (17.7) | |
| Leg | 1 (0.6) | 2 (1.1) | |
| Mechanism of injury, n (%) | .045 | ||
| Fall | 141 (90.9) | 152 (81.7) | |
| Direct blow | 5 (3.2) | 15 (8.1) | |
| Other/unknown | 9 (5.8) | 19 (10.2) | |
| SD, standard deviation. | |||
Acute vs subacute management outcomes
Of the 341 patients included in the study, 155 patients were treated acutely and 186 patients were treated subacutely. For the subacute management group, mean time between injury and treatment was 2.5±2.6 days ( TABLE 2 ). We observed no poor clinical outcomes in either acute or subacute management groups. All patients, regardless of time elapsed from injury to initial splinting, recovered without complication. The difference in number of clinical visits between the acute and subacute management groups was not significant (acute 3.2±0.5; subacute 3.1±0.5). The mean length of clinical follow-up from initial splinting to discharge from orthopedic care was higher in the acute management group (acute 32.9±17.1 days; subacute 28.9±13.4 days).
Most patients presented with non-angulated fractures, regardless of time from injury to initial presentation ( TABLE 2 ). The degree of angulation worsened in a small proportion of fractures during convalescence. The difference in initial angulation, final angulation, or change in angulation between acute and subacute management groups was not significant.
A higher proportion of patients in the acute treatment group presented directly to the ED for care, whereas a higher proportion of patients in the subacute treatment group presented to their PCP during routine working hours and were referred to the orthopedic clinic ( TABLE 2 ). For both acute and subacute management groups, we compared outcomes for patients seen initially in the ED or orthopedic clinic. No adverse outcomes occurred among any of the studied patients.
TABLE 2
Clinical outcomes did not differ between acute and subacute management groups
| Acute (n=155) | Subacute (n=186) | P value | |
|---|---|---|---|
| Time from injury to initial care, d ± SD (range) | 0 | 2.5 ± 2.6 (1-14) | <.001 |
| Good outcome, n (%) | 155 (100) | 186 (100) | 1.0 |
| Time from initial treatment to final follow-up, d ± SD (range) | 32.9 ± 17.1 (8-169) | 28.9 ± 13.4 (9-164) | .016 |
| Number of clinical encounters (primary care physician, ED, or orthopedic clinic), n ± SD (range) | 3.2 ± 0.5 (2-5) | 3.1 ± 0.5 (2-5) | .051 |
| Initial angulation, n (%) | .541 | ||
| None | 145 (93.5) | 170 (91.4) | |
| Mild (<10°) | 10 (6.5) | 16 (8.6) | |
| Final angulation, n (%) | 1.0 | ||
| None | 136 (87.7) | 163 (87.6) | |
| Mild (<10°) | 19 (12.2) | 23 (12.4) | |
| Change in angulation, n (%) | .907 | ||
| No change | 144 (92.9) | 175 (94.1) | |
| Worse | 10 (6.4) | 10 (5.4) | |
| Improved | 1 (0.6) | 1 (0.5) | |
| Point of entry to health care system, n (%) | <.001 | ||
| Primary care physician | 44 (28.4) | 115 (61.8) | |
| ED | 108 (69.7) | 54 (29.0) | |
| Orthopedic clinic | 3 (1.9) | 17 (9.1) | |
| Location of initial management, n (%) | <.001 | ||
| ED | 132 (85.2) | 81 (43.5) | |
| Orthopedic clinic | 23 (14.8) | 105 (56.4) | |
| ED, emergency department; SD, standard deviation. | |||
Charge analysis
We compared total charges (professional and technical) for managing buckle fractures initially in the ED with those initially seen in the orthopedic clinic. Total charge per patient in the ED, including subsequent follow-up in the orthopedic clinic, was $4397 ($2516, professional; $1881, technical). Total charge per patient for treatment only in the orthopedic clinic was $1426 ($918, professional; $508, technical). Total charge per patient was $2971 more for patients treated initially in the ED.
Between July 1, 2004, and August 31, 2007, 159 patients (46.6%) with buckle fractures entered the health care system through their primary care physician. Of these, 44 patients were seen acutely by the physician; 115 patients were seen on a subacute basis. Of the 44 patients seen acutely, 24 (54%) were referred directly to the ED; 20 (45%) were referred to the orthopedic clinic. Of the 115 patients seen subacutely, 27 (23%) were referred directly to the ED, and the remaining 88 (76%) were referred to the orthopedic clinic. In sum, 51 patients (32%) were seen initially by a PCP, who referred them to the ED. The cost savings with each patient seen subacutely in the orthopedic clinic was $2971, and avoiding ED treatment for all patients could have yielded a total gross savings of approximately $150,000.
Discussion
Buckle fractures are inherently stable and almost universally heal without complication.4,5 Perhaps because of the high likelihood of good outcome, there is a relative paucity of articles in the recent literature addressing the management of this common pediatric fracture. Older studies have addressed casting vs splinting and the need for follow-up, but no study has yet examined whether immediate treatment is necessary.6,7 Although some studies have noted incidentally that many children have delayed presentation for care,5 none has specifically examined the clinical or economic impact of a delay in care or the effect of subacute treatment on outcomes.
Delayed treatment does not adversely affect clinical outcome. Our study objective was to compare clinical outcomes of buckle fractures treated acutely on the same day of injury with outcomes of those treated subacutely. The 2 groups did not differ in extent or angulation of fracture at presentation. We found no difference in outcomes between the groups; all fractures healed without complication. We observed no difference in final angulation of fracture on follow-up imaging. Though our institution routinely obtains follow-up films, it is worth mentioning that the utility of repeat films in pediatric buckle fractures with minimal initial angulation has been debated.5 These data suggest that subacute treatment of a buckle fracture is a safe and reasonable option.
Non-ED treatment substantially reduces cost. One goal of efficient health care delivery is to decrease the cost and burden of care without increasing long-term morbidity and disability. Evidence suggests that families may prefer less acute management options that allow greater convenience and flexibility, provided that clinical outcomes are not compromised.8 In the case of pediatric buckle fractures, higher costs (for both the patient and the hospital) and longer wait times related to ED care may be avoided by counseling patients on the option of subacute care. Our study found that referring patients directly to the orthopedic clinic, even if this results in a delay in definitive management, leads to a reduction in health care burden without a change in clinical outcome.
Children with buckle fractures are frequently (46.6%) taken to their PCP for initial care. Many pediatricians and family physicians—especially the increasing number of physicians who have completed additional fellowship training in sports medicine—may prefer to manage buckle fractures within their practices. Many other PCPs may be practicing in communities lacking local orthopedic expertise. The results of this study provide reassurance regarding the positive outcome of buckle fractures. Furthermore, managing buckle fractures in the primary care setting may be even more cost effective than referring patients to a specialty orthopedic clinic—but additional research on this point is needed.
We do not advocate delayed imaging or treatment of suspected fractures. However, once a diagnosis of buckle fracture is confirmed radiographically, our data show that subacute treatment yields significant cost and time savings without affecting final clinical outcome.
Study limitations
This study is limited by its retrospective data collection in 1 pediatric tertiary care hospital. As current clinical practice is to treat all buckle fractures once identified, very few patients with known injury were specifically treated in a subacute fashion. We defined the subacute care group as patients who were treated >1 day from the time of injury. Because initial splinting did not occur in this group, we expect that the observed results would be similar, and no worse, compared with buckle fracture care directed by a subacute treatment algorithm.
This study examined only patients with a diagnosis of isolated buckle fracture. Non-buckle stable fractures were excluded a priori from our patient population. Although it is possible that most stable fractures (eg, nondisplaced transverse fractures, Salter-Harris I injuries) could be managed subacutely, we addressed only isolated buckle fractures.
Because of the universally positive outcomes in these cases, most of our patients had no orthopedic follow-up beyond 1 month. We are not able to comment on whether any longer-term abnormalities in function occurred. This question could be addressed through a prospective trial requiring reevaluation of each patient at a set endpoint of the study.
Although buckle fractures are inherently stable and do not present a significant risk of displacement with delayed treatment, they are nevertheless painful fractures that can be a cause of considerable anxiety for both patient and family. The goal of the physician, beyond ensuring the best medical outcome, extends to provide emotional support to the patient and family. Pain control and reassurance are therefore central to the discussion of fracture management, and are most likely the driving factor for a patient to seek urgent care. A key limitation of this study was the inability to determine differences in pain control between acute and subacute treatment. As mentioned above, a prospective study would enable the issue of pain control to be better addressed.
CORRESPONDENCE
Debbie Lee Bennett, MD, Massachusetts General Hospital, 55 Fruit Street, FND-216, Boston, MA 02114; [email protected]
(At the time this study was accepted for publication, Dr. Bennett was at Vanderbilt Children’s Hospital.)
1. Plint AC, Clifford T, Perry J, et al. Wrist buckle fractures: a survey of current practice patterns and attitudes toward immobilization. Can J Emerg Med. 2003;5:95-100.
2. England SP, Sundberg S. Management of common pediatric fractures. In: England SP, ed. Common Orthopedic Problems II. Philadelphia, Pa: WB Saunders; 1996:991–1012.
3. Van Bosse HJP, Patel RJ, Thacker M, et al. Minimalistic approach to treating wrist torus fractures. J Pediatr Orthop. 2005;25:495-500.
4. Davidson JS, Brown DJ, Barnes SN, et al. Simple treatment for torus fractures of the distal radius. J Bone Joint Surg Br. 2001;83-B:1173-1175.
5. Plint AC, Perry JJ, Tsang JLY. Pediatric wrist buckle fractures: should we just splint and go? Can J Emerg Med. 2004;6:397-401.
6. Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33:503-505.
7. Plint AC, Perry JJ, Correll R, et al. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117:691-697.
8. Symons S, Rowsell M, Bhowal B, et al. Hospital versus home management of children with buckle fractures of the distal radius. A prospective, randomised trial. J Bone Joint Surg Br. 2001;83:556-560.
1. Plint AC, Clifford T, Perry J, et al. Wrist buckle fractures: a survey of current practice patterns and attitudes toward immobilization. Can J Emerg Med. 2003;5:95-100.
2. England SP, Sundberg S. Management of common pediatric fractures. In: England SP, ed. Common Orthopedic Problems II. Philadelphia, Pa: WB Saunders; 1996:991–1012.
3. Van Bosse HJP, Patel RJ, Thacker M, et al. Minimalistic approach to treating wrist torus fractures. J Pediatr Orthop. 2005;25:495-500.
4. Davidson JS, Brown DJ, Barnes SN, et al. Simple treatment for torus fractures of the distal radius. J Bone Joint Surg Br. 2001;83-B:1173-1175.
5. Plint AC, Perry JJ, Tsang JLY. Pediatric wrist buckle fractures: should we just splint and go? Can J Emerg Med. 2004;6:397-401.
6. Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33:503-505.
7. Plint AC, Perry JJ, Correll R, et al. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117:691-697.
8. Symons S, Rowsell M, Bhowal B, et al. Hospital versus home management of children with buckle fractures of the distal radius. A prospective, randomised trial. J Bone Joint Surg Br. 2001;83:556-560.
PELVIC FLOOR DYSFUNCTION
The authors report no financial relationships relevant to this article.
Overactive bladder (OAB)—urinary urgency, with or without incontinence, usually with frequency and nocturia1—is a common problem among women who seek care from an ObGyn. In fact, the condition is estimated to carry a health-care cost in excess of $12 billion annually in the United States.2
A recent community-based survey in Norway estimated the prevalence of urinary incontinence there to be 27% in women between the ages of 65 and 69 years and 35% to 40% in those 80 years or older.3 A population-based study in the United States suggested an even higher rate of urinary incontinence here: greater than 50% in women 60 years or older, with 1) urge urinary incontinence (UUI) predominating4 and 2) the prevalence particularly high among older women who are homebound or who live in a long-term care facility.5
OAB can undermine quality of life in several ways: social isolation, anxiety, poor sleep, higher risk of fracture after a fall,6 reduced ability to function, and poor self-perception. Despite these harmful effects, many women delay seeking care for OAB because they are embarrassed to talk about it with their physician.
Treatment by generalists is feasible—but there is a catch
It’s possible to treat most patients with OAB without referral to a specialist. Two common concerns, however, may set up a roadblock to successful management: the adverse effects associated with some agents and suboptimal control of symptoms.
In this Update, we review recent findings about 1) the potential that anticholinergic therapy has for impairing cognitive function in the older population of women and 2) the important role that concomitant behavioral therapy plays in the long-term success of, and patients’ satisfaction with, treatment of OAB.
Behavioral therapy for OAB: Is it worth all the effort?
Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370–374.
The authors of this article followed a randomized clinical trial of older women that compared behavioral and drug therapy for OAB. In the trial, biofeedback-assisted behavioral training (comprising anorectal biofeedback, urge strategies, pelvic muscle biofeedback, and practitioner-directed review with optimization) was compared with treatment with oxybutynin, between 2.5 and 15 mg/day. Both biofeedback-assisted behavioral therapy and the drug regimen were found effective, although neither treatment provided an entirely satisfactory result for all patients. (For a brief description of what constitutes behavioral treatment, see “6 tenets of behavioral therapy for urge urinary incontinence.”)
Second phase of the trial. To determine if treatment satisfaction could be enhanced, the investigators performed a modified crossover study to determine whether combination therapy—biofeedback-assisted behavioral training plus oxybutynin—added any benefit over treatment with behavioral therapy or drug therapy alone. Eligibility was determined by age (55 years or older), demonstrated UUI for at least 3 months, and incomplete dryness or incomplete satisfaction with the outcome of 8 weeks of single-intervention treatment (with either treatment) during the initial phase of the trial.
This subgroup was offered an additional 8 weeks of combination therapy. The primary outcome measure was a reduction in the frequency of episodes of incontinence episodes as recorded by subjects in a bladder diary.
Of 197 women who participated in the original randomized clinical trial, 35—27 who completed drug therapy and 8 who completed behavioral treatment—elected to receive combination therapy. Those 35 subjects did not differ in any of the multiple baseline variables; mean age was 69.3 years (standard deviation [SD], ±7.9 years).
Among subjects originally assigned to behavioral therapy alone, overall reduction in incontinence increased from a mean of 57.5% to a mean of 88.5% after combined therapy (P=.034). Subjects originally assigned to drug therapy alone demonstrated an improvement from 72.7% reduction in incontinence to a mean 84.3% overall reduction with combined therapy (P=.001).
These data suggest that combined therapy can be more effective than behavioral therapy or drug therapy alone. The impact of this study is limited, however, by the relatively low percentage (12.7%) of patients who had received behavioral therapy and chose to add drug therapy, compared with the 41.5% who moved from drug therapy alone to add behavioral therapy.
Furthermore, subjects were self-selected: They chose to continue with an additional 8 weeks of therapy after their initial suboptimal outcome. It is possible that some subjects who were neither totally continent nor completely satisfied with initial therapy chose not to continue with the crossover segment of the trial because it posed too great a burden or because they were discouraged with the initial degree of improvement.
Generalizing these results to all older women with UUI is difficult. The authors point out, however, that, in practice, patients may be more likely than not to choose combination therapy in the hope of shortening the duration of medical therapy. Although it isn’t known whether providing combination therapy from the outset would have yielded better outcomes than either single therapy did, the authors hypothesize that initial combination therapy may result in greater improvement because patients have a high level of motivation and expectation of improvement at the beginning of treatment.
Importance of this article. The investigators demonstrated that a combination of behavioral and drug therapies can provide increased effectiveness in patients for whom each treatment alone led to suboptimal satisfaction. Furthermore, by targeting women older than 55 years, the investigators were able to demonstrate this effectiveness in a group for whom pelvic-floor training may be more difficult than it is for younger women.
It will be interesting to see if future research will 1) validate these findings and 2) determine whether combined therapy can reduce the duration of drug therapy in this older population through behavioral modification and pelvic floor reeducation.
Fluid management
This first-step therapy can involve providing a handout to the patient that details techniques she can use to monitor and control her fluid intake in a manner that addresses her problem. Among such steps:
- avoiding caffeine and artificial sweeteners
- tracking her diet to identify any other bladder irritants
- limiting fluids before times she is more likely to be incontinent—during a long drive, for example, or, in the case of nocturia, after the evening meal.
Scheduled voiding
With scheduled, or prompted, voiding, the patient empties her bladder at a set interval—usually, every 1.5 to 2 hours. If nocturia, or the more severe enuresis, is a problem, the patient can be prompted by an alarm clock or (if she is institutionalized) by nursing staff. Combining scheduled voiding with fluid management principles helps the patient avoid reaching a bladder volume at which an episode of incontinence becomes more likely.
Bladder training
This is a modification of scheduled voiding that attempts to establish a normal voiding interval in patients who have significant frequency but a small voided volume. It imposes a regimented voiding schedule that gradually (over 7 to 10 days) extends the duration between voids.
Pelvic floor-muscle exercises
The focus here is on using pelvic-floor muscles to prevent incontinence. The muscles are strengthened by having the patient perform Kegel exercises (named for Arnold H. Kegel, MD, who, in 1948, recognized the role of pelvic floor-muscle rehabilitation in the treatment of incontinence). The exercises involve simultaneous 1) contraction of the pelvic and periurethral musculature and 2) relaxation of other muscles, including abdominal muscles, which can increase pressure on the bladder.
Once the patient learns to perform Kegel exercises, she can use them to suppress urgency: Instead of hurrying to the bathroom when urgency arises, she is encouraged to sit down, relax, and contract the pelvic-floor muscles repeatedly until the urge to void diminishes. Once it does, the patient proceeds to the toilet to void normally.
Pelvic exam
By self-exam, the patient can identify and familiarize herself with her purposeful contractions of the pelvic-floor musculature and thereby strengthen those muscles with effective exercise.
Biofeedback
Direct feedback about contractions of the pelvic-floor muscles—by a display of data on a gauge or computer monitor, gathered using an intravaginal or anorectal sensor or probe—allows a patient who is exercising those muscles to better target her efforts and maximize their effectiveness.
Combining behavioral therapy and an anticholinergic medication for urge urinary incontinence may yield a superior result after either modality alone has been disappointing by the patient’s account of success.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
Does oxybutynin for UUI further erode cognition in elderly women who are cognitively impaired?
Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56:862–870.
Although anticholinergic therapy is modestly effective against UUI in nursing home residents, past studies have suggested that such treatment can impair, or further impair, cognition in this population—a concern that may lead to underuse. This double-blinded, randomized, placebo-controlled trial compared short-term oral extended-release oxybutynin with placebo.
Consequently, the authors sought to determine the cognitive effect, safety, and tolerability of 5 mg/day oral extended-release oxybutynin (the most commonly prescribed dosage) in cognitively impaired older nursing home residents who have UUI.
Subjects were eligible if they:
- were 65 years or older
- had UUI
- lived in a nursing home longer than 3 months
- had cognitive impairment.
Women already being treated for urinary incontinence, those who had an indwelling Foley catheter or urinary retention, and those who were bed-bound or incommunicative were excluded.
Fifty women, mean age 88.6 years (SD, ±6.2), from 12 nursing home facilities, agreed to participate. They were further stratified based on the score of a Mini-Mental State Exam (MMSE): 13 had severe cognitive impairment (MMSE score, 5–10) and 37 had mild or moderate impairment (score, 11–23).
Subjects were randomized to 4 weeks’ treatment with either 5 mg/day oral extended-release oxybutynin or one placebo tablet daily. A nurse practitioner who was blinded to randomization collected all data. The Confusion Assessment Method (CAM) algorithm, MMSE, and Severe Impairment Battery (SIB) were used to assess cognitive decline. The Brief Agitation Rating Scale (BARS) assessed agitation.
No baseline differences were noted with regard to: age; demographic, functional, and neuropsychiatric characteristics; clinical factors predisposing to delirium; and serum anticholinergic activity. Adherence was similar in the treatment (97%) and placebo (97.4%) groups.
Finding: Cognitive impairment. Treatment and placebo groups in the baseline mild-or-moderate stratum (by MMSE) showed equivalent mean changes in CAM scores at all time points. Because of the small sample size, however, CAM score equivalence could not be definitively determined for the groups in the severe impairment stratum. Evaluation of mean MMSE and BARS scores showed no significant changes between groups.
Finding: Tolerability. Excellent tolerability was noted in the treatment group: 96% of subjects completed the trial (compared with 92% of the placebo group). No difference in the rate of adverse events was noted between treatment and placebo groups; of adverse events recorded, 90% were judged “mild” by the investigators. Constipation and dry mouth were most common.
Finding: Falls. More than half—54%—of subjects in both groups experienced at least one fall during the trial or during the preceding or following 3 months. Despite this, no difference in the rate of falls between the treatment and placebo groups was noted. Furthermore, regression analysis revealed no treatment or period effect on falls per month across the time of observation.
Conclusions. Treatment with 5 mg/day oral extended-release oxybutynin in older patients with some cognitive impairment is well tolerated, the study’s findings suggest, with minimal risk of further cognitive decline or delirium over the short term. The potential that long-term therapy has to harm cognitive function remains, however; data on long-term treatment are needed to illuminate that area.
The authors also address the importance of dosing, especially over time, and discuss the lower potential of newer-generation anticholinergics to produce cognitive impairment.
A limited number of articles in the medical literature address anticholinergics in an older population, specifically, and only a few of those evaluated the effects of the drugs on cognitive function. By investigating patients who had an existing cognitive impairment, the authors of this article were able to target a cohort at risk of further cognitive impairment from medication use—thereby giving further weight to their findings of no significant effect.
Main strengths and limitations of the study. The investigators used validated, standardized cognitive tests that were administered by a uniform blinded evaluator in a randomized, controlled trial. The study was limited, however, because patients were evaluated only over a relatively short period (1 month) and because the efficacy of therapy was not addressed.
Further studies of anticholinergic medications, using the same rigorous scientific approach that these investigators applied, are needed to address 1) the long-term efficacy of oxybutynin and similar agents and 2) the cognitive effects of long-term treatment in this older population.
Further impairment is unlikely over the short term when a cognitively impaired nursing home patient who has urge urinary incontinence is treated with 5 mg/day oral extended-release oxybutynin.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116-126.
2. Hu TW, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123-1128.
3. Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150-1157.
4. Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537-542.
5. Fantl JA, Newman DK, Colling J, et al. Managing Acute and Chronic Urinary Incontinence. Clinical Practice Guideline. Quick Reference Guide for Clinicians, No. 2, 1996 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. AHCPR Pub. No. 96-0686. January 1996. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat6.chapter.32554. Accessed September 11, 2009.
6. Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721-725.
The authors report no financial relationships relevant to this article.
Overactive bladder (OAB)—urinary urgency, with or without incontinence, usually with frequency and nocturia1—is a common problem among women who seek care from an ObGyn. In fact, the condition is estimated to carry a health-care cost in excess of $12 billion annually in the United States.2
A recent community-based survey in Norway estimated the prevalence of urinary incontinence there to be 27% in women between the ages of 65 and 69 years and 35% to 40% in those 80 years or older.3 A population-based study in the United States suggested an even higher rate of urinary incontinence here: greater than 50% in women 60 years or older, with 1) urge urinary incontinence (UUI) predominating4 and 2) the prevalence particularly high among older women who are homebound or who live in a long-term care facility.5
OAB can undermine quality of life in several ways: social isolation, anxiety, poor sleep, higher risk of fracture after a fall,6 reduced ability to function, and poor self-perception. Despite these harmful effects, many women delay seeking care for OAB because they are embarrassed to talk about it with their physician.
Treatment by generalists is feasible—but there is a catch
It’s possible to treat most patients with OAB without referral to a specialist. Two common concerns, however, may set up a roadblock to successful management: the adverse effects associated with some agents and suboptimal control of symptoms.
In this Update, we review recent findings about 1) the potential that anticholinergic therapy has for impairing cognitive function in the older population of women and 2) the important role that concomitant behavioral therapy plays in the long-term success of, and patients’ satisfaction with, treatment of OAB.
Behavioral therapy for OAB: Is it worth all the effort?
Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370–374.
The authors of this article followed a randomized clinical trial of older women that compared behavioral and drug therapy for OAB. In the trial, biofeedback-assisted behavioral training (comprising anorectal biofeedback, urge strategies, pelvic muscle biofeedback, and practitioner-directed review with optimization) was compared with treatment with oxybutynin, between 2.5 and 15 mg/day. Both biofeedback-assisted behavioral therapy and the drug regimen were found effective, although neither treatment provided an entirely satisfactory result for all patients. (For a brief description of what constitutes behavioral treatment, see “6 tenets of behavioral therapy for urge urinary incontinence.”)
Second phase of the trial. To determine if treatment satisfaction could be enhanced, the investigators performed a modified crossover study to determine whether combination therapy—biofeedback-assisted behavioral training plus oxybutynin—added any benefit over treatment with behavioral therapy or drug therapy alone. Eligibility was determined by age (55 years or older), demonstrated UUI for at least 3 months, and incomplete dryness or incomplete satisfaction with the outcome of 8 weeks of single-intervention treatment (with either treatment) during the initial phase of the trial.
This subgroup was offered an additional 8 weeks of combination therapy. The primary outcome measure was a reduction in the frequency of episodes of incontinence episodes as recorded by subjects in a bladder diary.
Of 197 women who participated in the original randomized clinical trial, 35—27 who completed drug therapy and 8 who completed behavioral treatment—elected to receive combination therapy. Those 35 subjects did not differ in any of the multiple baseline variables; mean age was 69.3 years (standard deviation [SD], ±7.9 years).
Among subjects originally assigned to behavioral therapy alone, overall reduction in incontinence increased from a mean of 57.5% to a mean of 88.5% after combined therapy (P=.034). Subjects originally assigned to drug therapy alone demonstrated an improvement from 72.7% reduction in incontinence to a mean 84.3% overall reduction with combined therapy (P=.001).
These data suggest that combined therapy can be more effective than behavioral therapy or drug therapy alone. The impact of this study is limited, however, by the relatively low percentage (12.7%) of patients who had received behavioral therapy and chose to add drug therapy, compared with the 41.5% who moved from drug therapy alone to add behavioral therapy.
Furthermore, subjects were self-selected: They chose to continue with an additional 8 weeks of therapy after their initial suboptimal outcome. It is possible that some subjects who were neither totally continent nor completely satisfied with initial therapy chose not to continue with the crossover segment of the trial because it posed too great a burden or because they were discouraged with the initial degree of improvement.
Generalizing these results to all older women with UUI is difficult. The authors point out, however, that, in practice, patients may be more likely than not to choose combination therapy in the hope of shortening the duration of medical therapy. Although it isn’t known whether providing combination therapy from the outset would have yielded better outcomes than either single therapy did, the authors hypothesize that initial combination therapy may result in greater improvement because patients have a high level of motivation and expectation of improvement at the beginning of treatment.
Importance of this article. The investigators demonstrated that a combination of behavioral and drug therapies can provide increased effectiveness in patients for whom each treatment alone led to suboptimal satisfaction. Furthermore, by targeting women older than 55 years, the investigators were able to demonstrate this effectiveness in a group for whom pelvic-floor training may be more difficult than it is for younger women.
It will be interesting to see if future research will 1) validate these findings and 2) determine whether combined therapy can reduce the duration of drug therapy in this older population through behavioral modification and pelvic floor reeducation.
Fluid management
This first-step therapy can involve providing a handout to the patient that details techniques she can use to monitor and control her fluid intake in a manner that addresses her problem. Among such steps:
- avoiding caffeine and artificial sweeteners
- tracking her diet to identify any other bladder irritants
- limiting fluids before times she is more likely to be incontinent—during a long drive, for example, or, in the case of nocturia, after the evening meal.
Scheduled voiding
With scheduled, or prompted, voiding, the patient empties her bladder at a set interval—usually, every 1.5 to 2 hours. If nocturia, or the more severe enuresis, is a problem, the patient can be prompted by an alarm clock or (if she is institutionalized) by nursing staff. Combining scheduled voiding with fluid management principles helps the patient avoid reaching a bladder volume at which an episode of incontinence becomes more likely.
Bladder training
This is a modification of scheduled voiding that attempts to establish a normal voiding interval in patients who have significant frequency but a small voided volume. It imposes a regimented voiding schedule that gradually (over 7 to 10 days) extends the duration between voids.
Pelvic floor-muscle exercises
The focus here is on using pelvic-floor muscles to prevent incontinence. The muscles are strengthened by having the patient perform Kegel exercises (named for Arnold H. Kegel, MD, who, in 1948, recognized the role of pelvic floor-muscle rehabilitation in the treatment of incontinence). The exercises involve simultaneous 1) contraction of the pelvic and periurethral musculature and 2) relaxation of other muscles, including abdominal muscles, which can increase pressure on the bladder.
Once the patient learns to perform Kegel exercises, she can use them to suppress urgency: Instead of hurrying to the bathroom when urgency arises, she is encouraged to sit down, relax, and contract the pelvic-floor muscles repeatedly until the urge to void diminishes. Once it does, the patient proceeds to the toilet to void normally.
Pelvic exam
By self-exam, the patient can identify and familiarize herself with her purposeful contractions of the pelvic-floor musculature and thereby strengthen those muscles with effective exercise.
Biofeedback
Direct feedback about contractions of the pelvic-floor muscles—by a display of data on a gauge or computer monitor, gathered using an intravaginal or anorectal sensor or probe—allows a patient who is exercising those muscles to better target her efforts and maximize their effectiveness.
Combining behavioral therapy and an anticholinergic medication for urge urinary incontinence may yield a superior result after either modality alone has been disappointing by the patient’s account of success.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
Does oxybutynin for UUI further erode cognition in elderly women who are cognitively impaired?
Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56:862–870.
Although anticholinergic therapy is modestly effective against UUI in nursing home residents, past studies have suggested that such treatment can impair, or further impair, cognition in this population—a concern that may lead to underuse. This double-blinded, randomized, placebo-controlled trial compared short-term oral extended-release oxybutynin with placebo.
Consequently, the authors sought to determine the cognitive effect, safety, and tolerability of 5 mg/day oral extended-release oxybutynin (the most commonly prescribed dosage) in cognitively impaired older nursing home residents who have UUI.
Subjects were eligible if they:
- were 65 years or older
- had UUI
- lived in a nursing home longer than 3 months
- had cognitive impairment.
Women already being treated for urinary incontinence, those who had an indwelling Foley catheter or urinary retention, and those who were bed-bound or incommunicative were excluded.
Fifty women, mean age 88.6 years (SD, ±6.2), from 12 nursing home facilities, agreed to participate. They were further stratified based on the score of a Mini-Mental State Exam (MMSE): 13 had severe cognitive impairment (MMSE score, 5–10) and 37 had mild or moderate impairment (score, 11–23).
Subjects were randomized to 4 weeks’ treatment with either 5 mg/day oral extended-release oxybutynin or one placebo tablet daily. A nurse practitioner who was blinded to randomization collected all data. The Confusion Assessment Method (CAM) algorithm, MMSE, and Severe Impairment Battery (SIB) were used to assess cognitive decline. The Brief Agitation Rating Scale (BARS) assessed agitation.
No baseline differences were noted with regard to: age; demographic, functional, and neuropsychiatric characteristics; clinical factors predisposing to delirium; and serum anticholinergic activity. Adherence was similar in the treatment (97%) and placebo (97.4%) groups.
Finding: Cognitive impairment. Treatment and placebo groups in the baseline mild-or-moderate stratum (by MMSE) showed equivalent mean changes in CAM scores at all time points. Because of the small sample size, however, CAM score equivalence could not be definitively determined for the groups in the severe impairment stratum. Evaluation of mean MMSE and BARS scores showed no significant changes between groups.
Finding: Tolerability. Excellent tolerability was noted in the treatment group: 96% of subjects completed the trial (compared with 92% of the placebo group). No difference in the rate of adverse events was noted between treatment and placebo groups; of adverse events recorded, 90% were judged “mild” by the investigators. Constipation and dry mouth were most common.
Finding: Falls. More than half—54%—of subjects in both groups experienced at least one fall during the trial or during the preceding or following 3 months. Despite this, no difference in the rate of falls between the treatment and placebo groups was noted. Furthermore, regression analysis revealed no treatment or period effect on falls per month across the time of observation.
Conclusions. Treatment with 5 mg/day oral extended-release oxybutynin in older patients with some cognitive impairment is well tolerated, the study’s findings suggest, with minimal risk of further cognitive decline or delirium over the short term. The potential that long-term therapy has to harm cognitive function remains, however; data on long-term treatment are needed to illuminate that area.
The authors also address the importance of dosing, especially over time, and discuss the lower potential of newer-generation anticholinergics to produce cognitive impairment.
A limited number of articles in the medical literature address anticholinergics in an older population, specifically, and only a few of those evaluated the effects of the drugs on cognitive function. By investigating patients who had an existing cognitive impairment, the authors of this article were able to target a cohort at risk of further cognitive impairment from medication use—thereby giving further weight to their findings of no significant effect.
Main strengths and limitations of the study. The investigators used validated, standardized cognitive tests that were administered by a uniform blinded evaluator in a randomized, controlled trial. The study was limited, however, because patients were evaluated only over a relatively short period (1 month) and because the efficacy of therapy was not addressed.
Further studies of anticholinergic medications, using the same rigorous scientific approach that these investigators applied, are needed to address 1) the long-term efficacy of oxybutynin and similar agents and 2) the cognitive effects of long-term treatment in this older population.
Further impairment is unlikely over the short term when a cognitively impaired nursing home patient who has urge urinary incontinence is treated with 5 mg/day oral extended-release oxybutynin.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
The authors report no financial relationships relevant to this article.
Overactive bladder (OAB)—urinary urgency, with or without incontinence, usually with frequency and nocturia1—is a common problem among women who seek care from an ObGyn. In fact, the condition is estimated to carry a health-care cost in excess of $12 billion annually in the United States.2
A recent community-based survey in Norway estimated the prevalence of urinary incontinence there to be 27% in women between the ages of 65 and 69 years and 35% to 40% in those 80 years or older.3 A population-based study in the United States suggested an even higher rate of urinary incontinence here: greater than 50% in women 60 years or older, with 1) urge urinary incontinence (UUI) predominating4 and 2) the prevalence particularly high among older women who are homebound or who live in a long-term care facility.5
OAB can undermine quality of life in several ways: social isolation, anxiety, poor sleep, higher risk of fracture after a fall,6 reduced ability to function, and poor self-perception. Despite these harmful effects, many women delay seeking care for OAB because they are embarrassed to talk about it with their physician.
Treatment by generalists is feasible—but there is a catch
It’s possible to treat most patients with OAB without referral to a specialist. Two common concerns, however, may set up a roadblock to successful management: the adverse effects associated with some agents and suboptimal control of symptoms.
In this Update, we review recent findings about 1) the potential that anticholinergic therapy has for impairing cognitive function in the older population of women and 2) the important role that concomitant behavioral therapy plays in the long-term success of, and patients’ satisfaction with, treatment of OAB.
Behavioral therapy for OAB: Is it worth all the effort?
Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370–374.
The authors of this article followed a randomized clinical trial of older women that compared behavioral and drug therapy for OAB. In the trial, biofeedback-assisted behavioral training (comprising anorectal biofeedback, urge strategies, pelvic muscle biofeedback, and practitioner-directed review with optimization) was compared with treatment with oxybutynin, between 2.5 and 15 mg/day. Both biofeedback-assisted behavioral therapy and the drug regimen were found effective, although neither treatment provided an entirely satisfactory result for all patients. (For a brief description of what constitutes behavioral treatment, see “6 tenets of behavioral therapy for urge urinary incontinence.”)
Second phase of the trial. To determine if treatment satisfaction could be enhanced, the investigators performed a modified crossover study to determine whether combination therapy—biofeedback-assisted behavioral training plus oxybutynin—added any benefit over treatment with behavioral therapy or drug therapy alone. Eligibility was determined by age (55 years or older), demonstrated UUI for at least 3 months, and incomplete dryness or incomplete satisfaction with the outcome of 8 weeks of single-intervention treatment (with either treatment) during the initial phase of the trial.
This subgroup was offered an additional 8 weeks of combination therapy. The primary outcome measure was a reduction in the frequency of episodes of incontinence episodes as recorded by subjects in a bladder diary.
Of 197 women who participated in the original randomized clinical trial, 35—27 who completed drug therapy and 8 who completed behavioral treatment—elected to receive combination therapy. Those 35 subjects did not differ in any of the multiple baseline variables; mean age was 69.3 years (standard deviation [SD], ±7.9 years).
Among subjects originally assigned to behavioral therapy alone, overall reduction in incontinence increased from a mean of 57.5% to a mean of 88.5% after combined therapy (P=.034). Subjects originally assigned to drug therapy alone demonstrated an improvement from 72.7% reduction in incontinence to a mean 84.3% overall reduction with combined therapy (P=.001).
These data suggest that combined therapy can be more effective than behavioral therapy or drug therapy alone. The impact of this study is limited, however, by the relatively low percentage (12.7%) of patients who had received behavioral therapy and chose to add drug therapy, compared with the 41.5% who moved from drug therapy alone to add behavioral therapy.
Furthermore, subjects were self-selected: They chose to continue with an additional 8 weeks of therapy after their initial suboptimal outcome. It is possible that some subjects who were neither totally continent nor completely satisfied with initial therapy chose not to continue with the crossover segment of the trial because it posed too great a burden or because they were discouraged with the initial degree of improvement.
Generalizing these results to all older women with UUI is difficult. The authors point out, however, that, in practice, patients may be more likely than not to choose combination therapy in the hope of shortening the duration of medical therapy. Although it isn’t known whether providing combination therapy from the outset would have yielded better outcomes than either single therapy did, the authors hypothesize that initial combination therapy may result in greater improvement because patients have a high level of motivation and expectation of improvement at the beginning of treatment.
Importance of this article. The investigators demonstrated that a combination of behavioral and drug therapies can provide increased effectiveness in patients for whom each treatment alone led to suboptimal satisfaction. Furthermore, by targeting women older than 55 years, the investigators were able to demonstrate this effectiveness in a group for whom pelvic-floor training may be more difficult than it is for younger women.
It will be interesting to see if future research will 1) validate these findings and 2) determine whether combined therapy can reduce the duration of drug therapy in this older population through behavioral modification and pelvic floor reeducation.
Fluid management
This first-step therapy can involve providing a handout to the patient that details techniques she can use to monitor and control her fluid intake in a manner that addresses her problem. Among such steps:
- avoiding caffeine and artificial sweeteners
- tracking her diet to identify any other bladder irritants
- limiting fluids before times she is more likely to be incontinent—during a long drive, for example, or, in the case of nocturia, after the evening meal.
Scheduled voiding
With scheduled, or prompted, voiding, the patient empties her bladder at a set interval—usually, every 1.5 to 2 hours. If nocturia, or the more severe enuresis, is a problem, the patient can be prompted by an alarm clock or (if she is institutionalized) by nursing staff. Combining scheduled voiding with fluid management principles helps the patient avoid reaching a bladder volume at which an episode of incontinence becomes more likely.
Bladder training
This is a modification of scheduled voiding that attempts to establish a normal voiding interval in patients who have significant frequency but a small voided volume. It imposes a regimented voiding schedule that gradually (over 7 to 10 days) extends the duration between voids.
Pelvic floor-muscle exercises
The focus here is on using pelvic-floor muscles to prevent incontinence. The muscles are strengthened by having the patient perform Kegel exercises (named for Arnold H. Kegel, MD, who, in 1948, recognized the role of pelvic floor-muscle rehabilitation in the treatment of incontinence). The exercises involve simultaneous 1) contraction of the pelvic and periurethral musculature and 2) relaxation of other muscles, including abdominal muscles, which can increase pressure on the bladder.
Once the patient learns to perform Kegel exercises, she can use them to suppress urgency: Instead of hurrying to the bathroom when urgency arises, she is encouraged to sit down, relax, and contract the pelvic-floor muscles repeatedly until the urge to void diminishes. Once it does, the patient proceeds to the toilet to void normally.
Pelvic exam
By self-exam, the patient can identify and familiarize herself with her purposeful contractions of the pelvic-floor musculature and thereby strengthen those muscles with effective exercise.
Biofeedback
Direct feedback about contractions of the pelvic-floor muscles—by a display of data on a gauge or computer monitor, gathered using an intravaginal or anorectal sensor or probe—allows a patient who is exercising those muscles to better target her efforts and maximize their effectiveness.
Combining behavioral therapy and an anticholinergic medication for urge urinary incontinence may yield a superior result after either modality alone has been disappointing by the patient’s account of success.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
Does oxybutynin for UUI further erode cognition in elderly women who are cognitively impaired?
Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56:862–870.
Although anticholinergic therapy is modestly effective against UUI in nursing home residents, past studies have suggested that such treatment can impair, or further impair, cognition in this population—a concern that may lead to underuse. This double-blinded, randomized, placebo-controlled trial compared short-term oral extended-release oxybutynin with placebo.
Consequently, the authors sought to determine the cognitive effect, safety, and tolerability of 5 mg/day oral extended-release oxybutynin (the most commonly prescribed dosage) in cognitively impaired older nursing home residents who have UUI.
Subjects were eligible if they:
- were 65 years or older
- had UUI
- lived in a nursing home longer than 3 months
- had cognitive impairment.
Women already being treated for urinary incontinence, those who had an indwelling Foley catheter or urinary retention, and those who were bed-bound or incommunicative were excluded.
Fifty women, mean age 88.6 years (SD, ±6.2), from 12 nursing home facilities, agreed to participate. They were further stratified based on the score of a Mini-Mental State Exam (MMSE): 13 had severe cognitive impairment (MMSE score, 5–10) and 37 had mild or moderate impairment (score, 11–23).
Subjects were randomized to 4 weeks’ treatment with either 5 mg/day oral extended-release oxybutynin or one placebo tablet daily. A nurse practitioner who was blinded to randomization collected all data. The Confusion Assessment Method (CAM) algorithm, MMSE, and Severe Impairment Battery (SIB) were used to assess cognitive decline. The Brief Agitation Rating Scale (BARS) assessed agitation.
No baseline differences were noted with regard to: age; demographic, functional, and neuropsychiatric characteristics; clinical factors predisposing to delirium; and serum anticholinergic activity. Adherence was similar in the treatment (97%) and placebo (97.4%) groups.
Finding: Cognitive impairment. Treatment and placebo groups in the baseline mild-or-moderate stratum (by MMSE) showed equivalent mean changes in CAM scores at all time points. Because of the small sample size, however, CAM score equivalence could not be definitively determined for the groups in the severe impairment stratum. Evaluation of mean MMSE and BARS scores showed no significant changes between groups.
Finding: Tolerability. Excellent tolerability was noted in the treatment group: 96% of subjects completed the trial (compared with 92% of the placebo group). No difference in the rate of adverse events was noted between treatment and placebo groups; of adverse events recorded, 90% were judged “mild” by the investigators. Constipation and dry mouth were most common.
Finding: Falls. More than half—54%—of subjects in both groups experienced at least one fall during the trial or during the preceding or following 3 months. Despite this, no difference in the rate of falls between the treatment and placebo groups was noted. Furthermore, regression analysis revealed no treatment or period effect on falls per month across the time of observation.
Conclusions. Treatment with 5 mg/day oral extended-release oxybutynin in older patients with some cognitive impairment is well tolerated, the study’s findings suggest, with minimal risk of further cognitive decline or delirium over the short term. The potential that long-term therapy has to harm cognitive function remains, however; data on long-term treatment are needed to illuminate that area.
The authors also address the importance of dosing, especially over time, and discuss the lower potential of newer-generation anticholinergics to produce cognitive impairment.
A limited number of articles in the medical literature address anticholinergics in an older population, specifically, and only a few of those evaluated the effects of the drugs on cognitive function. By investigating patients who had an existing cognitive impairment, the authors of this article were able to target a cohort at risk of further cognitive impairment from medication use—thereby giving further weight to their findings of no significant effect.
Main strengths and limitations of the study. The investigators used validated, standardized cognitive tests that were administered by a uniform blinded evaluator in a randomized, controlled trial. The study was limited, however, because patients were evaluated only over a relatively short period (1 month) and because the efficacy of therapy was not addressed.
Further studies of anticholinergic medications, using the same rigorous scientific approach that these investigators applied, are needed to address 1) the long-term efficacy of oxybutynin and similar agents and 2) the cognitive effects of long-term treatment in this older population.
Further impairment is unlikely over the short term when a cognitively impaired nursing home patient who has urge urinary incontinence is treated with 5 mg/day oral extended-release oxybutynin.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116-126.
2. Hu TW, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123-1128.
3. Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150-1157.
4. Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537-542.
5. Fantl JA, Newman DK, Colling J, et al. Managing Acute and Chronic Urinary Incontinence. Clinical Practice Guideline. Quick Reference Guide for Clinicians, No. 2, 1996 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. AHCPR Pub. No. 96-0686. January 1996. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat6.chapter.32554. Accessed September 11, 2009.
6. Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721-725.
1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116-126.
2. Hu TW, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123-1128.
3. Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150-1157.
4. Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537-542.
5. Fantl JA, Newman DK, Colling J, et al. Managing Acute and Chronic Urinary Incontinence. Clinical Practice Guideline. Quick Reference Guide for Clinicians, No. 2, 1996 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. AHCPR Pub. No. 96-0686. January 1996. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat6.chapter.32554. Accessed September 11, 2009.
6. Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721-725.
Policy & Practice : Can't get enough Policy & Practice? Check out our new podcast each Monday. egmnblog.wordpress.com
Flavored Cigarettes Snubbed Out
The Food and Drug Administration has banned fruit- and candy-flavored cigarettes as part of its effort to prevent children from starting to smoke. The agency said it will act against any company that continues to make, ship, or sell such products in the United States. The tobacco control legislation approved by Congress last spring authorized the FDA to target flavored cigarettes, and the agency said it is also examining options for regulating menthol cigarettes and flavored tobacco products other than cigarettes. Almost 90% of adult smokers start the habit as teenagers, and studies have shown that 17-year-old smokers are three times as likely to use flavored cigarettes as are smokers over 25, the FDA said. “Candy and fruit flavorings have unfortunately been some of the most egregious examples of marketing tobacco products to children, and the academy supported the inclusion of this ban in the legislation,” American Academy of Pediatrics President David Tayloe Jr. said in a statement.
FDA Makes Device Grants
In an effort to have more medical devices available for children, the FDA has awarded a total of $2 million in grants to three nonprofit device consortiums. A panel of experts reviewed 16 applications for the grants, which were mandated by Congress in 2007 and will be administered by the FDA's Office of Orphan Products Development. The grants, to groups based in California, Massachusetts, and Michigan, are to encourage connections between innovators and potential manufacturers of pediatric medical devices. Each of the grant recipients will coordinate efforts of the FDA, device companies, and the National Institutes of Health to bring pediatric medical devices to market sooner. Development of medical devices for children is a challenge because of differences in size, growth, and body chemistry between age groups. As a result, availability of pediatric devices lags up to a decade behind similar devices intended for adults, according to the FDA.
Review Raps Medicaid Services
Preventive care for children and adults is lagging in Medicaid, the Government Accountability Office (GAO) found. It reported that many children covered by Medicaid are not receiving well-child checkups and that providers may not be aware that obesity-related services are covered for youngsters in the program. Most states told GAO that they have set goals for and monitored children's utilization of preventive services available and that they have taken steps to increase the number of children who received those services through Medicaid. However, the GAO study found that only 58% of children who were eligible under the program to receive a periodic screening, diagnostic, or treatment service in 2007 actually received one.
HHS Supports Health Centers
The Department of Health and Human Services has granted $25.7 million to increase and improve health and support services at public health centers, which are treating many more children than they did before the economic downturn. The federal health center system, overseen by the Health Resources and Services Administration, served more than 17 million medically needy people in 2008, up from 10 million patients in 2001, according to HHS. Since the economic downturn began, the health center patient population has grown by another million people, one-third of them children.
Obesity Counseling Found Ineffective
Primary care obesity screening followed by a series of counseling sessions failed to improve body mass index, physical activity, or nutrition in overweight or mildly obese children, a study in the British Medical Journal found. A total of 139 overweight and mildly obese children aged 5-10 years underwent four brief consultations with their physicians in Melbourne over 12 weeks. The objective was to change the children's behavior. But when compared with that of a control group after a year, the intervention group's BMI had not fallen significantly, the study found. Money might be better spent on obesity-prevention activities at the community and population levels, rather than on individual counseling by primary care physicians, the authors concluded.
HHS Awards Adoption Incentives
The Department of Health and Human Services announced the distribution of $35 million to 38 states and Puerto Rico to increase adoptions among children in foster care. Congress created the Adoptions Incentive program in 1997 as part of the Adoption and Safe Families Act, particularly to move older children and those with special needs into permanent homes. As part of the program, states can earn $4,000 for each additional adopted foster child above a baseline rate established in 2007. They receive additional payments for the adoption of foster children older than age 8 and those with special needs. States use the incentive payments to improve their programs for abused and neglected children, according to HHS.
Flavored Cigarettes Snubbed Out
The Food and Drug Administration has banned fruit- and candy-flavored cigarettes as part of its effort to prevent children from starting to smoke. The agency said it will act against any company that continues to make, ship, or sell such products in the United States. The tobacco control legislation approved by Congress last spring authorized the FDA to target flavored cigarettes, and the agency said it is also examining options for regulating menthol cigarettes and flavored tobacco products other than cigarettes. Almost 90% of adult smokers start the habit as teenagers, and studies have shown that 17-year-old smokers are three times as likely to use flavored cigarettes as are smokers over 25, the FDA said. “Candy and fruit flavorings have unfortunately been some of the most egregious examples of marketing tobacco products to children, and the academy supported the inclusion of this ban in the legislation,” American Academy of Pediatrics President David Tayloe Jr. said in a statement.
FDA Makes Device Grants
In an effort to have more medical devices available for children, the FDA has awarded a total of $2 million in grants to three nonprofit device consortiums. A panel of experts reviewed 16 applications for the grants, which were mandated by Congress in 2007 and will be administered by the FDA's Office of Orphan Products Development. The grants, to groups based in California, Massachusetts, and Michigan, are to encourage connections between innovators and potential manufacturers of pediatric medical devices. Each of the grant recipients will coordinate efforts of the FDA, device companies, and the National Institutes of Health to bring pediatric medical devices to market sooner. Development of medical devices for children is a challenge because of differences in size, growth, and body chemistry between age groups. As a result, availability of pediatric devices lags up to a decade behind similar devices intended for adults, according to the FDA.
Review Raps Medicaid Services
Preventive care for children and adults is lagging in Medicaid, the Government Accountability Office (GAO) found. It reported that many children covered by Medicaid are not receiving well-child checkups and that providers may not be aware that obesity-related services are covered for youngsters in the program. Most states told GAO that they have set goals for and monitored children's utilization of preventive services available and that they have taken steps to increase the number of children who received those services through Medicaid. However, the GAO study found that only 58% of children who were eligible under the program to receive a periodic screening, diagnostic, or treatment service in 2007 actually received one.
HHS Supports Health Centers
The Department of Health and Human Services has granted $25.7 million to increase and improve health and support services at public health centers, which are treating many more children than they did before the economic downturn. The federal health center system, overseen by the Health Resources and Services Administration, served more than 17 million medically needy people in 2008, up from 10 million patients in 2001, according to HHS. Since the economic downturn began, the health center patient population has grown by another million people, one-third of them children.
Obesity Counseling Found Ineffective
Primary care obesity screening followed by a series of counseling sessions failed to improve body mass index, physical activity, or nutrition in overweight or mildly obese children, a study in the British Medical Journal found. A total of 139 overweight and mildly obese children aged 5-10 years underwent four brief consultations with their physicians in Melbourne over 12 weeks. The objective was to change the children's behavior. But when compared with that of a control group after a year, the intervention group's BMI had not fallen significantly, the study found. Money might be better spent on obesity-prevention activities at the community and population levels, rather than on individual counseling by primary care physicians, the authors concluded.
HHS Awards Adoption Incentives
The Department of Health and Human Services announced the distribution of $35 million to 38 states and Puerto Rico to increase adoptions among children in foster care. Congress created the Adoptions Incentive program in 1997 as part of the Adoption and Safe Families Act, particularly to move older children and those with special needs into permanent homes. As part of the program, states can earn $4,000 for each additional adopted foster child above a baseline rate established in 2007. They receive additional payments for the adoption of foster children older than age 8 and those with special needs. States use the incentive payments to improve their programs for abused and neglected children, according to HHS.
Flavored Cigarettes Snubbed Out
The Food and Drug Administration has banned fruit- and candy-flavored cigarettes as part of its effort to prevent children from starting to smoke. The agency said it will act against any company that continues to make, ship, or sell such products in the United States. The tobacco control legislation approved by Congress last spring authorized the FDA to target flavored cigarettes, and the agency said it is also examining options for regulating menthol cigarettes and flavored tobacco products other than cigarettes. Almost 90% of adult smokers start the habit as teenagers, and studies have shown that 17-year-old smokers are three times as likely to use flavored cigarettes as are smokers over 25, the FDA said. “Candy and fruit flavorings have unfortunately been some of the most egregious examples of marketing tobacco products to children, and the academy supported the inclusion of this ban in the legislation,” American Academy of Pediatrics President David Tayloe Jr. said in a statement.
FDA Makes Device Grants
In an effort to have more medical devices available for children, the FDA has awarded a total of $2 million in grants to three nonprofit device consortiums. A panel of experts reviewed 16 applications for the grants, which were mandated by Congress in 2007 and will be administered by the FDA's Office of Orphan Products Development. The grants, to groups based in California, Massachusetts, and Michigan, are to encourage connections between innovators and potential manufacturers of pediatric medical devices. Each of the grant recipients will coordinate efforts of the FDA, device companies, and the National Institutes of Health to bring pediatric medical devices to market sooner. Development of medical devices for children is a challenge because of differences in size, growth, and body chemistry between age groups. As a result, availability of pediatric devices lags up to a decade behind similar devices intended for adults, according to the FDA.
Review Raps Medicaid Services
Preventive care for children and adults is lagging in Medicaid, the Government Accountability Office (GAO) found. It reported that many children covered by Medicaid are not receiving well-child checkups and that providers may not be aware that obesity-related services are covered for youngsters in the program. Most states told GAO that they have set goals for and monitored children's utilization of preventive services available and that they have taken steps to increase the number of children who received those services through Medicaid. However, the GAO study found that only 58% of children who were eligible under the program to receive a periodic screening, diagnostic, or treatment service in 2007 actually received one.
HHS Supports Health Centers
The Department of Health and Human Services has granted $25.7 million to increase and improve health and support services at public health centers, which are treating many more children than they did before the economic downturn. The federal health center system, overseen by the Health Resources and Services Administration, served more than 17 million medically needy people in 2008, up from 10 million patients in 2001, according to HHS. Since the economic downturn began, the health center patient population has grown by another million people, one-third of them children.
Obesity Counseling Found Ineffective
Primary care obesity screening followed by a series of counseling sessions failed to improve body mass index, physical activity, or nutrition in overweight or mildly obese children, a study in the British Medical Journal found. A total of 139 overweight and mildly obese children aged 5-10 years underwent four brief consultations with their physicians in Melbourne over 12 weeks. The objective was to change the children's behavior. But when compared with that of a control group after a year, the intervention group's BMI had not fallen significantly, the study found. Money might be better spent on obesity-prevention activities at the community and population levels, rather than on individual counseling by primary care physicians, the authors concluded.
HHS Awards Adoption Incentives
The Department of Health and Human Services announced the distribution of $35 million to 38 states and Puerto Rico to increase adoptions among children in foster care. Congress created the Adoptions Incentive program in 1997 as part of the Adoption and Safe Families Act, particularly to move older children and those with special needs into permanent homes. As part of the program, states can earn $4,000 for each additional adopted foster child above a baseline rate established in 2007. They receive additional payments for the adoption of foster children older than age 8 and those with special needs. States use the incentive payments to improve their programs for abused and neglected children, according to HHS.