User login
Intermittent pain and stiffness
The history and findings in this case are consistent with a diagnosis of psoriatic spondylitis.
Psoriatic spondylitis is a form of psoriatic arthritis (PsA) that affects the spine and the joints in the pelvis (axial involvement). PsA is a chronic, heterogeneous condition that affects approximately 25%-30% of patients with psoriasis, particularly those with severe psoriasis or nail or scalp involvement. It is characterized by musculoskeletal inflammation (arthritis, enthesitis, spondylitis, and dactylitis). PsA is a spondyloarthritis that can be found either in the peripheral or axial skeleton. If not treated, it may result in permanent joint damage and loss of function.
Patients with PsA may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Common symptoms of axial involvement in PsA include morning back/neck stiffness that lasts longer than 30 minutes, neck or back pain that improves with activity and worsens after prolonged inactivity, and diminished mobility. PsA affects men and women equally, and typically develops when patients are between 30 and 50 years of age. As with psoriasis, PsA is associated with numerous comorbidities, such as cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
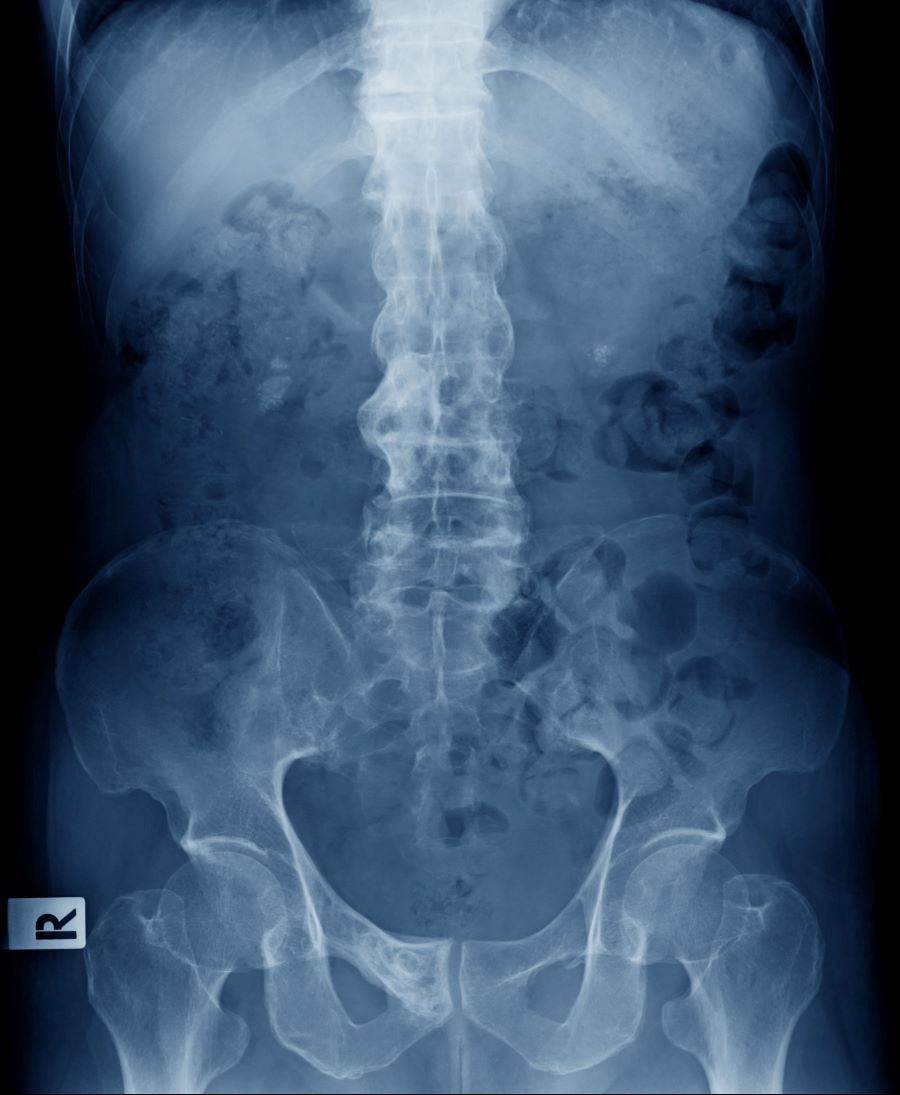
The diagnosis of psoriatic spondylitis is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosus). Useful imaging tools for evaluating patients with PsA include plain radiography, CT, ultrasound, and MRI. Although MRI and ultrasound may be more sensitive than plain radiography for detecting early joint inflammation and damage and axial changes, including sacroiliitis, they are not mandatory for a diagnosis of PsA to be made.
International guidelines have been developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), the European Alliance of Associations for Rheumatology (EULAR), and the Assessment of Spondyloarthritis International Society to guide the treatment of axial PsA. The goals of treatment include minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; improving functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Treatment options for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or have erosive disease or other indications of high disease activity, guidelines recommend initiation of a tumor necrosis factor (TNF) inhibitor (eg, adalimumab, etanercept, infliximab, golimumab, certolizumab pegol). Disease-modifying antirheumatic drugs (eg, methotrexate) are not routinely prescribed for patients with axial disease because they have not been shown to be effective. In patients with significant skin involvement, treatment with interleukin-17A inhibitors may be preferred to TNF inhibitors.
If patients have an inadequate response to a first trial of a TNF inhibitor, guidelines recommend trying a second TNF inhibitor before switching to a different class of biologic. For patients who do not respond to TNF inhibitors, a Janus kinase inhibitor (tofacitinib) may be considered. Additionally, nonpharmacologic therapies (eg, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are consistent with a diagnosis of psoriatic spondylitis.
Psoriatic spondylitis is a form of psoriatic arthritis (PsA) that affects the spine and the joints in the pelvis (axial involvement). PsA is a chronic, heterogeneous condition that affects approximately 25%-30% of patients with psoriasis, particularly those with severe psoriasis or nail or scalp involvement. It is characterized by musculoskeletal inflammation (arthritis, enthesitis, spondylitis, and dactylitis). PsA is a spondyloarthritis that can be found either in the peripheral or axial skeleton. If not treated, it may result in permanent joint damage and loss of function.
Patients with PsA may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Common symptoms of axial involvement in PsA include morning back/neck stiffness that lasts longer than 30 minutes, neck or back pain that improves with activity and worsens after prolonged inactivity, and diminished mobility. PsA affects men and women equally, and typically develops when patients are between 30 and 50 years of age. As with psoriasis, PsA is associated with numerous comorbidities, such as cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
The diagnosis of psoriatic spondylitis is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosus). Useful imaging tools for evaluating patients with PsA include plain radiography, CT, ultrasound, and MRI. Although MRI and ultrasound may be more sensitive than plain radiography for detecting early joint inflammation and damage and axial changes, including sacroiliitis, they are not mandatory for a diagnosis of PsA to be made.
International guidelines have been developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), the European Alliance of Associations for Rheumatology (EULAR), and the Assessment of Spondyloarthritis International Society to guide the treatment of axial PsA. The goals of treatment include minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; improving functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Treatment options for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or have erosive disease or other indications of high disease activity, guidelines recommend initiation of a tumor necrosis factor (TNF) inhibitor (eg, adalimumab, etanercept, infliximab, golimumab, certolizumab pegol). Disease-modifying antirheumatic drugs (eg, methotrexate) are not routinely prescribed for patients with axial disease because they have not been shown to be effective. In patients with significant skin involvement, treatment with interleukin-17A inhibitors may be preferred to TNF inhibitors.
If patients have an inadequate response to a first trial of a TNF inhibitor, guidelines recommend trying a second TNF inhibitor before switching to a different class of biologic. For patients who do not respond to TNF inhibitors, a Janus kinase inhibitor (tofacitinib) may be considered. Additionally, nonpharmacologic therapies (eg, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are consistent with a diagnosis of psoriatic spondylitis.
Psoriatic spondylitis is a form of psoriatic arthritis (PsA) that affects the spine and the joints in the pelvis (axial involvement). PsA is a chronic, heterogeneous condition that affects approximately 25%-30% of patients with psoriasis, particularly those with severe psoriasis or nail or scalp involvement. It is characterized by musculoskeletal inflammation (arthritis, enthesitis, spondylitis, and dactylitis). PsA is a spondyloarthritis that can be found either in the peripheral or axial skeleton. If not treated, it may result in permanent joint damage and loss of function.
Patients with PsA may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Common symptoms of axial involvement in PsA include morning back/neck stiffness that lasts longer than 30 minutes, neck or back pain that improves with activity and worsens after prolonged inactivity, and diminished mobility. PsA affects men and women equally, and typically develops when patients are between 30 and 50 years of age. As with psoriasis, PsA is associated with numerous comorbidities, such as cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
The diagnosis of psoriatic spondylitis is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosus). Useful imaging tools for evaluating patients with PsA include plain radiography, CT, ultrasound, and MRI. Although MRI and ultrasound may be more sensitive than plain radiography for detecting early joint inflammation and damage and axial changes, including sacroiliitis, they are not mandatory for a diagnosis of PsA to be made.
International guidelines have been developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), the European Alliance of Associations for Rheumatology (EULAR), and the Assessment of Spondyloarthritis International Society to guide the treatment of axial PsA. The goals of treatment include minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; improving functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Treatment options for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or have erosive disease or other indications of high disease activity, guidelines recommend initiation of a tumor necrosis factor (TNF) inhibitor (eg, adalimumab, etanercept, infliximab, golimumab, certolizumab pegol). Disease-modifying antirheumatic drugs (eg, methotrexate) are not routinely prescribed for patients with axial disease because they have not been shown to be effective. In patients with significant skin involvement, treatment with interleukin-17A inhibitors may be preferred to TNF inhibitors.
If patients have an inadequate response to a first trial of a TNF inhibitor, guidelines recommend trying a second TNF inhibitor before switching to a different class of biologic. For patients who do not respond to TNF inhibitors, a Janus kinase inhibitor (tofacitinib) may be considered. Additionally, nonpharmacologic therapies (eg, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 41-year-old man with a 5-year history of moderate to severe scalp psoriasis presents with complaints of intermittent pain and stiffness in his left hip and lower back of approximately 6 months' duration. The patient states that his back pain has been severe enough to wake him up on several occasions. Treatment with over-the-counter ibuprofen is moderately effective at relieving his pain. He also reports morning back stiffness that improves with motion, usually within an hour of awakening. The patient reports no fever, pain, swelling, or worsening of his scalp psoriasis. He is not aware of any injury or other triggering factor for his back pain. He takes an over-the-counter multivitamin daily and treats his scalp psoriasis with fluocinolone acetonide 0.01% oil. The patient is 5 ft 9 in and weighs 176 lb (BMI 26).
Physical examination reveals tenderness in the lumbar spine and associated decreased range of motion, as well as psoriatic plaques on the scalp. Vital signs are within normal ranges. Pertinent laboratory findings include erythrocyte sedimentation rate of 19 mm/h and C-reactive protein of 10 mg/L. Rheumatoid factor, antinuclear antibody, and anti-cyclic citrullinated peptide antibody were negative. Radiographic findings include sacroiliitis and bulky nonmarginal syndesmophytes.
Self-talk overhaul may help patients achieve weight loss
It’s common knowledge that the recommended first-line treatment for obesity is behavioral or “lifestyle” intervention, with the goal of losing a modest amount of weight to gain significant health benefits. Unfortunately, when pursuing weight loss, patients often think they need to beat themselves up to stay motivated. I’ve heard patients call themselves “weak,” saying they need to “stop being lazy” and gain some self-control in order to be less of a “failure.” They label their bodies as “disgusting” and themselves as “worthless,” all because of their weight.
Some patients may worry that if they are kind to themselves or “too accepting” of their bodies, they’ll lose motivation to stick with their health behavior goals. That’s a question that my colleagues and I have explored in recent research that attempts to reduce weight stigma as part of standard weight-related care.
Misguided societal view drives blame game
This tendency for people to blame and disparage themselves for their weight is largely driven by the misguided societal view of body weight as an issue of personal responsibility. We’re constantly exposed to messages telling us that there’s a narrow range of acceptable body weights and sizes, and that if we have enough willpower and discipline to eat healthily and exercise, then we should be able to control our weight. These messages are prevalent in the news and in social media, but often they are communicated in health care settings too. Narratives of this kind usually ignore the complex environmental and biological factors that contribute to body size and shape, instead attributing high body weight to laziness and moral failings.
Such messages exemplify weight bias and stigma, or the negative attitudes toward and mistreatment of individuals with a high body weight. Given society’s harsh judgment of people with larger bodies, it’s no surprise that many individuals internalize these beliefs and stigmatize themselves for their weight. This internalized or self-directed stigma is known to be harmful to mental and physical health.
Contrary to beliefs that negative self-talk and self-blame can be motivators to improve health, we know that high levels of weight self-stigma are linked to unhealthy eating behaviors and less engagement in physical activity, among other poor health outcomes. Thus, ironically, internalizing weight stigma actually undermines efforts to lose weight and maintain weight loss, rather than motivating healthy behavior change.
Combating internalized weight stigma
How do we combat these negative weight messages in our culture and reduce, or ideally prevent, internalization of judgment and blame? Fundamental changes in policies, health care practices, and public attitudes are needed to eradicate weight stigma. While such initiatives are underway, there are many individuals who have already experienced and internalized weight stigma and need support now. Interventions such as peer support and psychological counseling may be helpful for challenging negative, internalized beliefs about weight; learning to cope with exposure to weight stigma without internalizing it; increasing self-acceptance and self-compassion; and feeling empowered to fight back against weight bias and stigma.
In our latest study, my colleagues and I tested the long-term effects of including a group intervention to address weight stigma in a standard behavioral weight management program. More than 100 adults with obesity who had experienced and internalized weight stigma were recruited for this clinical trial, which randomly assigned participants to receive either the Weight Bias Internalization and Stigma (Weight BIAS) program combined with standard behavioral weight loss treatment, or standard weight loss treatment alone.
The Weight BIAS program adapted evidence-based psychotherapy techniques to target weight self-stigma, while also providing peer support in a group treatment format. Specific topics included challenging myths and stereotypes about weight; identifying and changing negative thought patterns related to weight and how they affect emotions and behaviors; and responding to experiences of weight stigma.
For example, to challenge negative thoughts (for example, that they were a “failure” because of their weight), patients worked together to examine all of the evidence that proved these beliefs were not true, and came up with ideas for how to revise these thoughts to be less judgmental and more fair and accurate.
Other topics focused on building confidence, increasing body- and self-acceptance, and advocating for themselves and others who are mistreated because of their weight. Many patients shared examples of stigmatizing experiences in health care settings and discussed what they could say or do when facing judgment or discrimination from health care providers, as well as the importance of finding health care providers who treated them with respect. Group discussions also tied in information relevant to health behavior goals, such as overcoming self-consciousness about weight to enjoy physical activity.
Participants were offered weekly group meetings for 20 weeks, followed by a year of less frequent meetings. At the study’s end, participants in the group that received weight loss treatment with the Weight BIAS program on average lost about 7% of their starting weight, compared with an average weight loss of about 5% in the group that received weight loss treatment alone. Weight losses of these magnitudes are known to have meaningful health benefits. Results from our study showed comparable improvements in most outcomes across groups, with some added benefit of the Weight BIAS program for certain psychological and behavioral outcomes. These findings challenge the notion that reducing weight stigma and promoting body acceptance will undermine motivation to engage in healthy behaviors and lose weight. We found no such effect.
What did participants say?
When asked questions such as how much they liked the program, what they learned, and how they used the new skills and changed their self-perceptions, participants who received the Weight BIAS program gave higher ratings than those who received only the weight loss treatment. Positive feedback from free-response questions indicated that many participants identified social support as their favorite aspect of the program. Others highlighted how the program helped them to gain “the ability to think differently about myself and other people” and “an understanding that weight really is separate from the person.” They also described how they brought together the goals of weight loss and body and self-acceptance, saying, “I am more accepting of me and at the same time more dedicated to obtaining a healthier weight,” and “It’s okay to be happy the way I am and still want to change.”
Participants who didn’t receive the Weight BIAS program also shared positive feedback, writing that their favorite part of the program was “being part of such a supportive group of people who can relate to the things that I think and feel” and that they learned “how not to be so hard on myself.” This might suggest that even without an intervention specifically for weight stigma, providing respectful, compassionate care and peer support may help patients to feel less alone and to be kinder to themselves.
Our study results suggest that reducing negative self-talk and internalized beliefs about weight certainly won’t undermine treatment outcomes and may have some benefits beyond standard weight loss treatment. At the same time, we also all need to do our part to change how society views and treats people with larger bodies and prevent the harms of experiencing and internalizing weight stigma.
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL140176. The content is solely the responsibility of the author and does not necessarily reflect the official views of the National Institutes of Health.
Dr. Pearl is assistant professor, clinical and health psychology, University of Florida, Gainesville. She has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
It’s common knowledge that the recommended first-line treatment for obesity is behavioral or “lifestyle” intervention, with the goal of losing a modest amount of weight to gain significant health benefits. Unfortunately, when pursuing weight loss, patients often think they need to beat themselves up to stay motivated. I’ve heard patients call themselves “weak,” saying they need to “stop being lazy” and gain some self-control in order to be less of a “failure.” They label their bodies as “disgusting” and themselves as “worthless,” all because of their weight.
Some patients may worry that if they are kind to themselves or “too accepting” of their bodies, they’ll lose motivation to stick with their health behavior goals. That’s a question that my colleagues and I have explored in recent research that attempts to reduce weight stigma as part of standard weight-related care.
Misguided societal view drives blame game
This tendency for people to blame and disparage themselves for their weight is largely driven by the misguided societal view of body weight as an issue of personal responsibility. We’re constantly exposed to messages telling us that there’s a narrow range of acceptable body weights and sizes, and that if we have enough willpower and discipline to eat healthily and exercise, then we should be able to control our weight. These messages are prevalent in the news and in social media, but often they are communicated in health care settings too. Narratives of this kind usually ignore the complex environmental and biological factors that contribute to body size and shape, instead attributing high body weight to laziness and moral failings.
Such messages exemplify weight bias and stigma, or the negative attitudes toward and mistreatment of individuals with a high body weight. Given society’s harsh judgment of people with larger bodies, it’s no surprise that many individuals internalize these beliefs and stigmatize themselves for their weight. This internalized or self-directed stigma is known to be harmful to mental and physical health.
Contrary to beliefs that negative self-talk and self-blame can be motivators to improve health, we know that high levels of weight self-stigma are linked to unhealthy eating behaviors and less engagement in physical activity, among other poor health outcomes. Thus, ironically, internalizing weight stigma actually undermines efforts to lose weight and maintain weight loss, rather than motivating healthy behavior change.
Combating internalized weight stigma
How do we combat these negative weight messages in our culture and reduce, or ideally prevent, internalization of judgment and blame? Fundamental changes in policies, health care practices, and public attitudes are needed to eradicate weight stigma. While such initiatives are underway, there are many individuals who have already experienced and internalized weight stigma and need support now. Interventions such as peer support and psychological counseling may be helpful for challenging negative, internalized beliefs about weight; learning to cope with exposure to weight stigma without internalizing it; increasing self-acceptance and self-compassion; and feeling empowered to fight back against weight bias and stigma.
In our latest study, my colleagues and I tested the long-term effects of including a group intervention to address weight stigma in a standard behavioral weight management program. More than 100 adults with obesity who had experienced and internalized weight stigma were recruited for this clinical trial, which randomly assigned participants to receive either the Weight Bias Internalization and Stigma (Weight BIAS) program combined with standard behavioral weight loss treatment, or standard weight loss treatment alone.
The Weight BIAS program adapted evidence-based psychotherapy techniques to target weight self-stigma, while also providing peer support in a group treatment format. Specific topics included challenging myths and stereotypes about weight; identifying and changing negative thought patterns related to weight and how they affect emotions and behaviors; and responding to experiences of weight stigma.
For example, to challenge negative thoughts (for example, that they were a “failure” because of their weight), patients worked together to examine all of the evidence that proved these beliefs were not true, and came up with ideas for how to revise these thoughts to be less judgmental and more fair and accurate.
Other topics focused on building confidence, increasing body- and self-acceptance, and advocating for themselves and others who are mistreated because of their weight. Many patients shared examples of stigmatizing experiences in health care settings and discussed what they could say or do when facing judgment or discrimination from health care providers, as well as the importance of finding health care providers who treated them with respect. Group discussions also tied in information relevant to health behavior goals, such as overcoming self-consciousness about weight to enjoy physical activity.
Participants were offered weekly group meetings for 20 weeks, followed by a year of less frequent meetings. At the study’s end, participants in the group that received weight loss treatment with the Weight BIAS program on average lost about 7% of their starting weight, compared with an average weight loss of about 5% in the group that received weight loss treatment alone. Weight losses of these magnitudes are known to have meaningful health benefits. Results from our study showed comparable improvements in most outcomes across groups, with some added benefit of the Weight BIAS program for certain psychological and behavioral outcomes. These findings challenge the notion that reducing weight stigma and promoting body acceptance will undermine motivation to engage in healthy behaviors and lose weight. We found no such effect.
What did participants say?
When asked questions such as how much they liked the program, what they learned, and how they used the new skills and changed their self-perceptions, participants who received the Weight BIAS program gave higher ratings than those who received only the weight loss treatment. Positive feedback from free-response questions indicated that many participants identified social support as their favorite aspect of the program. Others highlighted how the program helped them to gain “the ability to think differently about myself and other people” and “an understanding that weight really is separate from the person.” They also described how they brought together the goals of weight loss and body and self-acceptance, saying, “I am more accepting of me and at the same time more dedicated to obtaining a healthier weight,” and “It’s okay to be happy the way I am and still want to change.”
Participants who didn’t receive the Weight BIAS program also shared positive feedback, writing that their favorite part of the program was “being part of such a supportive group of people who can relate to the things that I think and feel” and that they learned “how not to be so hard on myself.” This might suggest that even without an intervention specifically for weight stigma, providing respectful, compassionate care and peer support may help patients to feel less alone and to be kinder to themselves.
Our study results suggest that reducing negative self-talk and internalized beliefs about weight certainly won’t undermine treatment outcomes and may have some benefits beyond standard weight loss treatment. At the same time, we also all need to do our part to change how society views and treats people with larger bodies and prevent the harms of experiencing and internalizing weight stigma.
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL140176. The content is solely the responsibility of the author and does not necessarily reflect the official views of the National Institutes of Health.
Dr. Pearl is assistant professor, clinical and health psychology, University of Florida, Gainesville. She has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
It’s common knowledge that the recommended first-line treatment for obesity is behavioral or “lifestyle” intervention, with the goal of losing a modest amount of weight to gain significant health benefits. Unfortunately, when pursuing weight loss, patients often think they need to beat themselves up to stay motivated. I’ve heard patients call themselves “weak,” saying they need to “stop being lazy” and gain some self-control in order to be less of a “failure.” They label their bodies as “disgusting” and themselves as “worthless,” all because of their weight.
Some patients may worry that if they are kind to themselves or “too accepting” of their bodies, they’ll lose motivation to stick with their health behavior goals. That’s a question that my colleagues and I have explored in recent research that attempts to reduce weight stigma as part of standard weight-related care.
Misguided societal view drives blame game
This tendency for people to blame and disparage themselves for their weight is largely driven by the misguided societal view of body weight as an issue of personal responsibility. We’re constantly exposed to messages telling us that there’s a narrow range of acceptable body weights and sizes, and that if we have enough willpower and discipline to eat healthily and exercise, then we should be able to control our weight. These messages are prevalent in the news and in social media, but often they are communicated in health care settings too. Narratives of this kind usually ignore the complex environmental and biological factors that contribute to body size and shape, instead attributing high body weight to laziness and moral failings.
Such messages exemplify weight bias and stigma, or the negative attitudes toward and mistreatment of individuals with a high body weight. Given society’s harsh judgment of people with larger bodies, it’s no surprise that many individuals internalize these beliefs and stigmatize themselves for their weight. This internalized or self-directed stigma is known to be harmful to mental and physical health.
Contrary to beliefs that negative self-talk and self-blame can be motivators to improve health, we know that high levels of weight self-stigma are linked to unhealthy eating behaviors and less engagement in physical activity, among other poor health outcomes. Thus, ironically, internalizing weight stigma actually undermines efforts to lose weight and maintain weight loss, rather than motivating healthy behavior change.
Combating internalized weight stigma
How do we combat these negative weight messages in our culture and reduce, or ideally prevent, internalization of judgment and blame? Fundamental changes in policies, health care practices, and public attitudes are needed to eradicate weight stigma. While such initiatives are underway, there are many individuals who have already experienced and internalized weight stigma and need support now. Interventions such as peer support and psychological counseling may be helpful for challenging negative, internalized beliefs about weight; learning to cope with exposure to weight stigma without internalizing it; increasing self-acceptance and self-compassion; and feeling empowered to fight back against weight bias and stigma.
In our latest study, my colleagues and I tested the long-term effects of including a group intervention to address weight stigma in a standard behavioral weight management program. More than 100 adults with obesity who had experienced and internalized weight stigma were recruited for this clinical trial, which randomly assigned participants to receive either the Weight Bias Internalization and Stigma (Weight BIAS) program combined with standard behavioral weight loss treatment, or standard weight loss treatment alone.
The Weight BIAS program adapted evidence-based psychotherapy techniques to target weight self-stigma, while also providing peer support in a group treatment format. Specific topics included challenging myths and stereotypes about weight; identifying and changing negative thought patterns related to weight and how they affect emotions and behaviors; and responding to experiences of weight stigma.
For example, to challenge negative thoughts (for example, that they were a “failure” because of their weight), patients worked together to examine all of the evidence that proved these beliefs were not true, and came up with ideas for how to revise these thoughts to be less judgmental and more fair and accurate.
Other topics focused on building confidence, increasing body- and self-acceptance, and advocating for themselves and others who are mistreated because of their weight. Many patients shared examples of stigmatizing experiences in health care settings and discussed what they could say or do when facing judgment or discrimination from health care providers, as well as the importance of finding health care providers who treated them with respect. Group discussions also tied in information relevant to health behavior goals, such as overcoming self-consciousness about weight to enjoy physical activity.
Participants were offered weekly group meetings for 20 weeks, followed by a year of less frequent meetings. At the study’s end, participants in the group that received weight loss treatment with the Weight BIAS program on average lost about 7% of their starting weight, compared with an average weight loss of about 5% in the group that received weight loss treatment alone. Weight losses of these magnitudes are known to have meaningful health benefits. Results from our study showed comparable improvements in most outcomes across groups, with some added benefit of the Weight BIAS program for certain psychological and behavioral outcomes. These findings challenge the notion that reducing weight stigma and promoting body acceptance will undermine motivation to engage in healthy behaviors and lose weight. We found no such effect.
What did participants say?
When asked questions such as how much they liked the program, what they learned, and how they used the new skills and changed their self-perceptions, participants who received the Weight BIAS program gave higher ratings than those who received only the weight loss treatment. Positive feedback from free-response questions indicated that many participants identified social support as their favorite aspect of the program. Others highlighted how the program helped them to gain “the ability to think differently about myself and other people” and “an understanding that weight really is separate from the person.” They also described how they brought together the goals of weight loss and body and self-acceptance, saying, “I am more accepting of me and at the same time more dedicated to obtaining a healthier weight,” and “It’s okay to be happy the way I am and still want to change.”
Participants who didn’t receive the Weight BIAS program also shared positive feedback, writing that their favorite part of the program was “being part of such a supportive group of people who can relate to the things that I think and feel” and that they learned “how not to be so hard on myself.” This might suggest that even without an intervention specifically for weight stigma, providing respectful, compassionate care and peer support may help patients to feel less alone and to be kinder to themselves.
Our study results suggest that reducing negative self-talk and internalized beliefs about weight certainly won’t undermine treatment outcomes and may have some benefits beyond standard weight loss treatment. At the same time, we also all need to do our part to change how society views and treats people with larger bodies and prevent the harms of experiencing and internalizing weight stigma.
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL140176. The content is solely the responsibility of the author and does not necessarily reflect the official views of the National Institutes of Health.
Dr. Pearl is assistant professor, clinical and health psychology, University of Florida, Gainesville. She has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Multiple sclerosis has a misdiagnosis problem
DENVER – that potentially puts patients at prolonged and unnecessary risk. Experts warn that false-negative diagnoses cause treatment delays, while false-positive diagnoses run the risk for potential harm from needless treatment.
“MS has a misdiagnosis problem,” said Patricia Coyle, MD, professor of neurology and vice chair (academic affairs), department of neurology, Stony Brook (N.Y.) University, in presenting on the issue at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“We currently lack a diagnostic biomarker test, yet diagnosis is key. If you get it wrong – that really can be a problem,” Dr. Coyle said. Recent research indicates that MS misdiagnosis is a widespread problem, she added.
For instance, one research paper reported that nearly 20% of patients were misdiagnosed with MS and that more than 50% carried the misdiagnosis for at least 3 years, while 5% were misdiagnosed for 20 years or more.
The misdiagnosis problem is also reflected at large MS referral centers, where 30%-67% of patients turn out not to have the disease, Dr. Coyle noted.
A study from Argentina further highlights some of the key characteristics of misdiagnosis. In this study, examination of a cohort of 572 patients diagnosed with MS revealed that 16% were incorrectly diagnosed with MS and that women were at an 83% greater risk for misdiagnosis than men. Furthermore, the study showed that MS misdiagnosis increased by 8% per year of older age. The most frequent confirmed diagnoses among those who had been initially misdiagnosed as having MS were cerebrovascular disease, radiologically isolated syndrome, and headache.
The majority (83%) of patients incorrectly diagnosed with MS had an atypical presentation that did not indicate demyelination, 70% had an atypical brain magnetic resonance imaging, and 61% received a prescription for a disease-modifying treatment (DMT), despite not having confirmed MS.
The dangers of misdiagnosis
Misdiagnosis and incorrect treatment can be particularly dangerous if patients are diagnosed with MS when, in fact, they have neuromyelitis optica spectrum disorder (NMOSD), commonly mistaken for MS, Dr. Coyle noted.
“Several MS DMTs make NMOSD worse. You are also basically giving an unnecessary and inappropriate drug with potential side effects to the misdiagnosed patient,” she said.
There have been some advances in MS diagnosis on MRI. However, there are many caveats, Dr. Coyle noted.
For instance, leptomeningeal enhancement has been considered as an MS diagnostic indicator, but it is not unique to MS, Dr. Coyle noted. In addition, subpial demyelination is MS specific, but it is hard to see and is often missed, she added.
Central vein sign has received significant attention as an important MRI marker for MS, but, Dr. Coyle said, it is “not ready for prime time. It’s somewhat tedious and you need to use special protocols to identify it,” she said.
In the future, artificial intelligence and deep learning may be key to improving some of these technologies, Dr. Coyle noted.
Best hope for an accurate diagnosis
In the meantime, Dr. Coyle said she believes spinal fluid evaluation offers the best chance for a reliable MS diagnosis and is her preference. “I personally find spinal fluid to be extremely helpful to support MS diagnosis. Spinal fluid oligoclonal bands are positive in the vast majority of people with MS, and it is an independent finding from MRI to support an MS diagnosis. Added to MRI, it makes you much more comfortable,” she said.
Dr. Coyle said that a comprehensive workup should include:
- A thorough neurologic history and exam.
- MRI of the brain and spinal cord ensuring use of the MS protocol, and personally reading the studies with a neuroradiologist.
- Adding spinal fluid evaluation, especially in any atypical cases.
- Ruling out myelin oligodendrocyte glycoprotein antibody disease and NMOSD, diseases that mimic relapsing MS, via blood IgG to aquaporin 4.
“You want to be as certain as possible. Everything starts with a thorough workup,” Dr. Coyle said.
Dr. Coyle’s disclosures include consulting, nonbranded speaker fees, and/or research support with Actelion, Alkermes, Accordant, Biogen, Bristol Myers Squibb, Celgene, CorEvitas LLC, GlaxoSmithKline, Genentech/Roche, Horizon Therapeutics, Janssen, MedDay, Labcorp, Eli Lilly, Mylan, NINDS, Novartis, Sanofi Genzyme, and TG Therapeutics.
A version of this article originally appeared on Medscape.com.
DENVER – that potentially puts patients at prolonged and unnecessary risk. Experts warn that false-negative diagnoses cause treatment delays, while false-positive diagnoses run the risk for potential harm from needless treatment.
“MS has a misdiagnosis problem,” said Patricia Coyle, MD, professor of neurology and vice chair (academic affairs), department of neurology, Stony Brook (N.Y.) University, in presenting on the issue at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“We currently lack a diagnostic biomarker test, yet diagnosis is key. If you get it wrong – that really can be a problem,” Dr. Coyle said. Recent research indicates that MS misdiagnosis is a widespread problem, she added.
For instance, one research paper reported that nearly 20% of patients were misdiagnosed with MS and that more than 50% carried the misdiagnosis for at least 3 years, while 5% were misdiagnosed for 20 years or more.
The misdiagnosis problem is also reflected at large MS referral centers, where 30%-67% of patients turn out not to have the disease, Dr. Coyle noted.
A study from Argentina further highlights some of the key characteristics of misdiagnosis. In this study, examination of a cohort of 572 patients diagnosed with MS revealed that 16% were incorrectly diagnosed with MS and that women were at an 83% greater risk for misdiagnosis than men. Furthermore, the study showed that MS misdiagnosis increased by 8% per year of older age. The most frequent confirmed diagnoses among those who had been initially misdiagnosed as having MS were cerebrovascular disease, radiologically isolated syndrome, and headache.
The majority (83%) of patients incorrectly diagnosed with MS had an atypical presentation that did not indicate demyelination, 70% had an atypical brain magnetic resonance imaging, and 61% received a prescription for a disease-modifying treatment (DMT), despite not having confirmed MS.
The dangers of misdiagnosis
Misdiagnosis and incorrect treatment can be particularly dangerous if patients are diagnosed with MS when, in fact, they have neuromyelitis optica spectrum disorder (NMOSD), commonly mistaken for MS, Dr. Coyle noted.
“Several MS DMTs make NMOSD worse. You are also basically giving an unnecessary and inappropriate drug with potential side effects to the misdiagnosed patient,” she said.
There have been some advances in MS diagnosis on MRI. However, there are many caveats, Dr. Coyle noted.
For instance, leptomeningeal enhancement has been considered as an MS diagnostic indicator, but it is not unique to MS, Dr. Coyle noted. In addition, subpial demyelination is MS specific, but it is hard to see and is often missed, she added.
Central vein sign has received significant attention as an important MRI marker for MS, but, Dr. Coyle said, it is “not ready for prime time. It’s somewhat tedious and you need to use special protocols to identify it,” she said.
In the future, artificial intelligence and deep learning may be key to improving some of these technologies, Dr. Coyle noted.
Best hope for an accurate diagnosis
In the meantime, Dr. Coyle said she believes spinal fluid evaluation offers the best chance for a reliable MS diagnosis and is her preference. “I personally find spinal fluid to be extremely helpful to support MS diagnosis. Spinal fluid oligoclonal bands are positive in the vast majority of people with MS, and it is an independent finding from MRI to support an MS diagnosis. Added to MRI, it makes you much more comfortable,” she said.
Dr. Coyle said that a comprehensive workup should include:
- A thorough neurologic history and exam.
- MRI of the brain and spinal cord ensuring use of the MS protocol, and personally reading the studies with a neuroradiologist.
- Adding spinal fluid evaluation, especially in any atypical cases.
- Ruling out myelin oligodendrocyte glycoprotein antibody disease and NMOSD, diseases that mimic relapsing MS, via blood IgG to aquaporin 4.
“You want to be as certain as possible. Everything starts with a thorough workup,” Dr. Coyle said.
Dr. Coyle’s disclosures include consulting, nonbranded speaker fees, and/or research support with Actelion, Alkermes, Accordant, Biogen, Bristol Myers Squibb, Celgene, CorEvitas LLC, GlaxoSmithKline, Genentech/Roche, Horizon Therapeutics, Janssen, MedDay, Labcorp, Eli Lilly, Mylan, NINDS, Novartis, Sanofi Genzyme, and TG Therapeutics.
A version of this article originally appeared on Medscape.com.
DENVER – that potentially puts patients at prolonged and unnecessary risk. Experts warn that false-negative diagnoses cause treatment delays, while false-positive diagnoses run the risk for potential harm from needless treatment.
“MS has a misdiagnosis problem,” said Patricia Coyle, MD, professor of neurology and vice chair (academic affairs), department of neurology, Stony Brook (N.Y.) University, in presenting on the issue at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“We currently lack a diagnostic biomarker test, yet diagnosis is key. If you get it wrong – that really can be a problem,” Dr. Coyle said. Recent research indicates that MS misdiagnosis is a widespread problem, she added.
For instance, one research paper reported that nearly 20% of patients were misdiagnosed with MS and that more than 50% carried the misdiagnosis for at least 3 years, while 5% were misdiagnosed for 20 years or more.
The misdiagnosis problem is also reflected at large MS referral centers, where 30%-67% of patients turn out not to have the disease, Dr. Coyle noted.
A study from Argentina further highlights some of the key characteristics of misdiagnosis. In this study, examination of a cohort of 572 patients diagnosed with MS revealed that 16% were incorrectly diagnosed with MS and that women were at an 83% greater risk for misdiagnosis than men. Furthermore, the study showed that MS misdiagnosis increased by 8% per year of older age. The most frequent confirmed diagnoses among those who had been initially misdiagnosed as having MS were cerebrovascular disease, radiologically isolated syndrome, and headache.
The majority (83%) of patients incorrectly diagnosed with MS had an atypical presentation that did not indicate demyelination, 70% had an atypical brain magnetic resonance imaging, and 61% received a prescription for a disease-modifying treatment (DMT), despite not having confirmed MS.
The dangers of misdiagnosis
Misdiagnosis and incorrect treatment can be particularly dangerous if patients are diagnosed with MS when, in fact, they have neuromyelitis optica spectrum disorder (NMOSD), commonly mistaken for MS, Dr. Coyle noted.
“Several MS DMTs make NMOSD worse. You are also basically giving an unnecessary and inappropriate drug with potential side effects to the misdiagnosed patient,” she said.
There have been some advances in MS diagnosis on MRI. However, there are many caveats, Dr. Coyle noted.
For instance, leptomeningeal enhancement has been considered as an MS diagnostic indicator, but it is not unique to MS, Dr. Coyle noted. In addition, subpial demyelination is MS specific, but it is hard to see and is often missed, she added.
Central vein sign has received significant attention as an important MRI marker for MS, but, Dr. Coyle said, it is “not ready for prime time. It’s somewhat tedious and you need to use special protocols to identify it,” she said.
In the future, artificial intelligence and deep learning may be key to improving some of these technologies, Dr. Coyle noted.
Best hope for an accurate diagnosis
In the meantime, Dr. Coyle said she believes spinal fluid evaluation offers the best chance for a reliable MS diagnosis and is her preference. “I personally find spinal fluid to be extremely helpful to support MS diagnosis. Spinal fluid oligoclonal bands are positive in the vast majority of people with MS, and it is an independent finding from MRI to support an MS diagnosis. Added to MRI, it makes you much more comfortable,” she said.
Dr. Coyle said that a comprehensive workup should include:
- A thorough neurologic history and exam.
- MRI of the brain and spinal cord ensuring use of the MS protocol, and personally reading the studies with a neuroradiologist.
- Adding spinal fluid evaluation, especially in any atypical cases.
- Ruling out myelin oligodendrocyte glycoprotein antibody disease and NMOSD, diseases that mimic relapsing MS, via blood IgG to aquaporin 4.
“You want to be as certain as possible. Everything starts with a thorough workup,” Dr. Coyle said.
Dr. Coyle’s disclosures include consulting, nonbranded speaker fees, and/or research support with Actelion, Alkermes, Accordant, Biogen, Bristol Myers Squibb, Celgene, CorEvitas LLC, GlaxoSmithKline, Genentech/Roche, Horizon Therapeutics, Janssen, MedDay, Labcorp, Eli Lilly, Mylan, NINDS, Novartis, Sanofi Genzyme, and TG Therapeutics.
A version of this article originally appeared on Medscape.com.
AT CMSC 2023
Latest data: COVID vaccine safety, protection, and breakthrough infections in inflammatory, autoimmune diseases
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
AT EULAR 2023
ACS officer provides ASCO highlights: Targeting hidden cancer, AI in oncology
And it didn’t just sparkle because of the sequined Taylor Swift fans clogging the nearby streets during the meeting.
Arif Kamal, MD, MBA, MHS, who is also an oncologist at Duke University, Durham, N.C., said he was impressed by a pair of landmark studies released at the meeting that show hidden cancer can be targeted with “really remarkable outcomes.” He also highlighted sessions that examined the role of artificial intelligence (AI) in oncology, during an interview.
Below are lightly edited excerpts from a conversation with Dr. Kamal:
Question: What are some of most groundbreaking studies released at ASCO?
Answer: One is an interim analysis of the NATALEE trial, which involved patients with early-stage hormone receptor-positive, HER2-negative (HR+/HER2–) breast tumors. This phase 3 randomized trial compared maintenance therapy with the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib (Kisqali) plus endocrine therapy with an aromatase inhibitor to endocrine therapy alone in patients with node-positive or node-negative and stage II or III HR+/HER– breast cancer.
For a long time, the standard care in these patients has been to use endocrine therapy alone. This is the first big trial to show that upstream usage of additional therapy in early stages is also beneficial for disease-free survival. The 3-year invasive disease-free survival rate was 90.4% in the rebociclib-endocrine therapy group vs. 87.1% for patients who received only endocrine therapy (P = .0014).
Q: How do these findings add to current knowledge?
A: Typically, we let people get metastatic disease before we use CDK4/6 inhibitors. These findings show that systemic treatment beyond endocrine therapy will be helpful in cases where you’ve got smaller disease that has not spread yet.
Even in patients with node-negative breast cancer, micrometastatic disease is clearly there, because the medication killed the negative lymph nodes.
Q: What else struck you as especially important research?
A: The NATALEE findings match what we saw in another study – the ADAURA trial, which looked at adjuvant osimertinib in non–small-cell lung cancer patients with EGFR-mutated, stage IB to IIIA disease – cancer that has not spread to the lymph nodes.
This is another example where you have a treatment being used in earlier-stage disease that’s showing really remarkable outcomes. The study found that 5-year overall survival was 88% in an osimertinib group vs. 78% in a placebo group (P < .001). This is a disease where, in stage IB, we wouldn’t even necessarily give these patients treatment at all, other than surgical resection of the tumor and maybe give them a little bit of chemotherapy.
Even in these smaller, early tumors, osimertinib makes a difference.
Q: As a whole, what are these studies telling us about cancer cells that can’t be easily detected?
A: To find a disease-free survival benefit with adding ribociclib in a stage II, stage III setting, particularly in node-negative disease, is remarkable because it says that the cells in hiding are bad actors, and they are going to cause trouble. The study shows that medications can find these cells and reverse that risk of bad outcomes.
If you think about the paradigm of cancer, that’s pretty remarkable because the ADAURA trial does the same thing: You do surgery for [early-stage] lung cancers that have not spread to the lymph nodes and you figure, “Well, I’ve got it all, right? The margins are real big, healthy, clean.” And yet, people still have recurrences, and you ask the same question: “Can any medicine find those few cells, the hundreds of cells that are still left somewhere in hiding?” And the answer is again, yes. It’s changing the paradigm of our understanding of minimal residual disease.
That’s why there’s so much interest in liquid biopsies. Let’s say that after treatment we don’t see any cancer radiologically, but there’s a signal from a liquid biopsy [detecting residual cancer]. These two trials demonstrate that there’s something we can do about it.
Q: There were quite a few studies about artificial intelligence released at ASCO. Where do we stand on that front?
A: We’re just at the beginning of people thinking about the use of generative AI for clinical decision support, clinical trial matching, and pathology review. But AI, at least for now, still has the issue of making up things that aren’t true. That’s not something patients are going to be okay with.
Q: How can AI be helpful to medical providers considering its limitations?
A: AI is going to be very good at the data-to-information transition. You’ll start seeing people use AI to start clinical notes for them and to match patients to the best clinical trials for them. But fundamentally, the clinician’s role will continue to be to check facts and offer wisdom.
Q: Will AI threaten the careers of oncologists?
A: The body of knowledge about oncology is growing exponentially, and no one can actually keep up. There’s so much data that’s out there that needs to be turned into usable information amid a shortage of oncologists. At the same time, the prevalence of cancer is going up, even though mortality is going down.
Synthesis of data is what oncologists are waiting for from AI. They’ll welcome it as opposed to being worried. That’s the sentiment I heard from my colleagues.
Dr. Kamal has no disclosures.
And it didn’t just sparkle because of the sequined Taylor Swift fans clogging the nearby streets during the meeting.
Arif Kamal, MD, MBA, MHS, who is also an oncologist at Duke University, Durham, N.C., said he was impressed by a pair of landmark studies released at the meeting that show hidden cancer can be targeted with “really remarkable outcomes.” He also highlighted sessions that examined the role of artificial intelligence (AI) in oncology, during an interview.
Below are lightly edited excerpts from a conversation with Dr. Kamal:
Question: What are some of most groundbreaking studies released at ASCO?
Answer: One is an interim analysis of the NATALEE trial, which involved patients with early-stage hormone receptor-positive, HER2-negative (HR+/HER2–) breast tumors. This phase 3 randomized trial compared maintenance therapy with the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib (Kisqali) plus endocrine therapy with an aromatase inhibitor to endocrine therapy alone in patients with node-positive or node-negative and stage II or III HR+/HER– breast cancer.
For a long time, the standard care in these patients has been to use endocrine therapy alone. This is the first big trial to show that upstream usage of additional therapy in early stages is also beneficial for disease-free survival. The 3-year invasive disease-free survival rate was 90.4% in the rebociclib-endocrine therapy group vs. 87.1% for patients who received only endocrine therapy (P = .0014).
Q: How do these findings add to current knowledge?
A: Typically, we let people get metastatic disease before we use CDK4/6 inhibitors. These findings show that systemic treatment beyond endocrine therapy will be helpful in cases where you’ve got smaller disease that has not spread yet.
Even in patients with node-negative breast cancer, micrometastatic disease is clearly there, because the medication killed the negative lymph nodes.
Q: What else struck you as especially important research?
A: The NATALEE findings match what we saw in another study – the ADAURA trial, which looked at adjuvant osimertinib in non–small-cell lung cancer patients with EGFR-mutated, stage IB to IIIA disease – cancer that has not spread to the lymph nodes.
This is another example where you have a treatment being used in earlier-stage disease that’s showing really remarkable outcomes. The study found that 5-year overall survival was 88% in an osimertinib group vs. 78% in a placebo group (P < .001). This is a disease where, in stage IB, we wouldn’t even necessarily give these patients treatment at all, other than surgical resection of the tumor and maybe give them a little bit of chemotherapy.
Even in these smaller, early tumors, osimertinib makes a difference.
Q: As a whole, what are these studies telling us about cancer cells that can’t be easily detected?
A: To find a disease-free survival benefit with adding ribociclib in a stage II, stage III setting, particularly in node-negative disease, is remarkable because it says that the cells in hiding are bad actors, and they are going to cause trouble. The study shows that medications can find these cells and reverse that risk of bad outcomes.
If you think about the paradigm of cancer, that’s pretty remarkable because the ADAURA trial does the same thing: You do surgery for [early-stage] lung cancers that have not spread to the lymph nodes and you figure, “Well, I’ve got it all, right? The margins are real big, healthy, clean.” And yet, people still have recurrences, and you ask the same question: “Can any medicine find those few cells, the hundreds of cells that are still left somewhere in hiding?” And the answer is again, yes. It’s changing the paradigm of our understanding of minimal residual disease.
That’s why there’s so much interest in liquid biopsies. Let’s say that after treatment we don’t see any cancer radiologically, but there’s a signal from a liquid biopsy [detecting residual cancer]. These two trials demonstrate that there’s something we can do about it.
Q: There were quite a few studies about artificial intelligence released at ASCO. Where do we stand on that front?
A: We’re just at the beginning of people thinking about the use of generative AI for clinical decision support, clinical trial matching, and pathology review. But AI, at least for now, still has the issue of making up things that aren’t true. That’s not something patients are going to be okay with.
Q: How can AI be helpful to medical providers considering its limitations?
A: AI is going to be very good at the data-to-information transition. You’ll start seeing people use AI to start clinical notes for them and to match patients to the best clinical trials for them. But fundamentally, the clinician’s role will continue to be to check facts and offer wisdom.
Q: Will AI threaten the careers of oncologists?
A: The body of knowledge about oncology is growing exponentially, and no one can actually keep up. There’s so much data that’s out there that needs to be turned into usable information amid a shortage of oncologists. At the same time, the prevalence of cancer is going up, even though mortality is going down.
Synthesis of data is what oncologists are waiting for from AI. They’ll welcome it as opposed to being worried. That’s the sentiment I heard from my colleagues.
Dr. Kamal has no disclosures.
And it didn’t just sparkle because of the sequined Taylor Swift fans clogging the nearby streets during the meeting.
Arif Kamal, MD, MBA, MHS, who is also an oncologist at Duke University, Durham, N.C., said he was impressed by a pair of landmark studies released at the meeting that show hidden cancer can be targeted with “really remarkable outcomes.” He also highlighted sessions that examined the role of artificial intelligence (AI) in oncology, during an interview.
Below are lightly edited excerpts from a conversation with Dr. Kamal:
Question: What are some of most groundbreaking studies released at ASCO?
Answer: One is an interim analysis of the NATALEE trial, which involved patients with early-stage hormone receptor-positive, HER2-negative (HR+/HER2–) breast tumors. This phase 3 randomized trial compared maintenance therapy with the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib (Kisqali) plus endocrine therapy with an aromatase inhibitor to endocrine therapy alone in patients with node-positive or node-negative and stage II or III HR+/HER– breast cancer.
For a long time, the standard care in these patients has been to use endocrine therapy alone. This is the first big trial to show that upstream usage of additional therapy in early stages is also beneficial for disease-free survival. The 3-year invasive disease-free survival rate was 90.4% in the rebociclib-endocrine therapy group vs. 87.1% for patients who received only endocrine therapy (P = .0014).
Q: How do these findings add to current knowledge?
A: Typically, we let people get metastatic disease before we use CDK4/6 inhibitors. These findings show that systemic treatment beyond endocrine therapy will be helpful in cases where you’ve got smaller disease that has not spread yet.
Even in patients with node-negative breast cancer, micrometastatic disease is clearly there, because the medication killed the negative lymph nodes.
Q: What else struck you as especially important research?
A: The NATALEE findings match what we saw in another study – the ADAURA trial, which looked at adjuvant osimertinib in non–small-cell lung cancer patients with EGFR-mutated, stage IB to IIIA disease – cancer that has not spread to the lymph nodes.
This is another example where you have a treatment being used in earlier-stage disease that’s showing really remarkable outcomes. The study found that 5-year overall survival was 88% in an osimertinib group vs. 78% in a placebo group (P < .001). This is a disease where, in stage IB, we wouldn’t even necessarily give these patients treatment at all, other than surgical resection of the tumor and maybe give them a little bit of chemotherapy.
Even in these smaller, early tumors, osimertinib makes a difference.
Q: As a whole, what are these studies telling us about cancer cells that can’t be easily detected?
A: To find a disease-free survival benefit with adding ribociclib in a stage II, stage III setting, particularly in node-negative disease, is remarkable because it says that the cells in hiding are bad actors, and they are going to cause trouble. The study shows that medications can find these cells and reverse that risk of bad outcomes.
If you think about the paradigm of cancer, that’s pretty remarkable because the ADAURA trial does the same thing: You do surgery for [early-stage] lung cancers that have not spread to the lymph nodes and you figure, “Well, I’ve got it all, right? The margins are real big, healthy, clean.” And yet, people still have recurrences, and you ask the same question: “Can any medicine find those few cells, the hundreds of cells that are still left somewhere in hiding?” And the answer is again, yes. It’s changing the paradigm of our understanding of minimal residual disease.
That’s why there’s so much interest in liquid biopsies. Let’s say that after treatment we don’t see any cancer radiologically, but there’s a signal from a liquid biopsy [detecting residual cancer]. These two trials demonstrate that there’s something we can do about it.
Q: There were quite a few studies about artificial intelligence released at ASCO. Where do we stand on that front?
A: We’re just at the beginning of people thinking about the use of generative AI for clinical decision support, clinical trial matching, and pathology review. But AI, at least for now, still has the issue of making up things that aren’t true. That’s not something patients are going to be okay with.
Q: How can AI be helpful to medical providers considering its limitations?
A: AI is going to be very good at the data-to-information transition. You’ll start seeing people use AI to start clinical notes for them and to match patients to the best clinical trials for them. But fundamentally, the clinician’s role will continue to be to check facts and offer wisdom.
Q: Will AI threaten the careers of oncologists?
A: The body of knowledge about oncology is growing exponentially, and no one can actually keep up. There’s so much data that’s out there that needs to be turned into usable information amid a shortage of oncologists. At the same time, the prevalence of cancer is going up, even though mortality is going down.
Synthesis of data is what oncologists are waiting for from AI. They’ll welcome it as opposed to being worried. That’s the sentiment I heard from my colleagues.
Dr. Kamal has no disclosures.
AT ASCO 2023
Vitamin D deficiency: Can we improve diagnosis?
CHICAGO – , new research suggests.
The study supports previous data suggesting that a ratio cut-off of greater than 100 is associated with the development of secondary hyperparathyroidism and the need for correction with supplementation, while a level greater than 50 suggests mild to moderate deficiency, Zhinous Shahidzadeh Yazdi, MD, noted in a poster presented at the annual meeting of the Endocrine Society
Current Endocrine Society guidelines published in 2011 advise measurement of plasma circulating 25(OH)D levels to evaluate vitamin D status in patients at risk for deficiency, defined as < 20 ng/mL (50 nmol/L). Revised guidelines are due out in early 2024.
“We don’t think measuring 25 hydroxy D is optimal because of the impact of vitamin D binding protein,” Dr. Yazdi said in an interview.
“Over 99% of all metabolites are bound to vitamin D binding protein, but only the free fraction is biologically active. By measuring total plasma 25(OH)D – as we do right now in clinic – we cannot account for the impact of vitamin D binding proteins, which vary by threefold across the population,” she added.
Thus, the total 25(OH)D deficiency cut-off of < 20 mg/mL currently recommended by the Endocrine Society may signal clinically significant vitamin D deficiency in one person but not another, noted Dr. Yazdi, a postdoctoral fellow at the University of Maryland, Baltimore.
Directly measuring binding protein or the free fraction would be ideal, but “there aren’t good commercial assays for those, and it’s more difficult to do. So, as an alternative, the vitamin D metabolite ratios implicitly adjust for individual differences in vitamin D binding protein,” she explained.
The ratio that Dr. Yazdi and colleagues propose to measure is that of the vitamin D metabolites 1,25(OH)2D/24,25 (OH)2D (shortened to 1,25D/24,25D), which they say reflect the body’s homeostatic response to vitamin D levels, and which rises in the setting of deficiency. It is a measurement > 100 in this ratio that they believe means the patient should receive vitamin D supplementation.
Controversial topic, ratio proposal is “very early in the game”
The issue of vitamin D deficiency has long generated controversy, particularly since publication of findings from the VITAL study in 2022, which showed vitamin D supplements did not significantly reduce the risk of fracture among adults in midlife and older compared with placebo.
According to the senior author of the new study, Simeon I. Taylor, MD, professor of medicine at the University of Maryland, what still remains controversial after VITAL is the question: “How can you identify people who have sufficiently bad vitamin D deficiency that it’s adversely impacting their bones?”
He added that there is a suggestion that small subpopulations in VITAL really did benefit from vitamin D supplementation, but the study “wasn’t designed to look at that.”
Indeed, the authors of an editorial accompanying the publication of the VITAL study said the findings mean there is no justification for measuring 25(OH)D in the general population or for treating to a target level.
Asked to comment on Dr. Yazdi and colleagues’ ratio proposal for diagnosing vitamin D deficiency, the coauthor of the VITAL study editorial, Clifford J. Rosen, MD, said in an interview: “I do think it’s important to point out that changes in the vitamin D binding protein can have a significant impact on the level of 25 [OH] D ... People should recognize that.”
And, Dr. Rosen noted, “I like the idea that the ... [ratio] is a measure of what’s happening in the body in response to vitamin D stores. So, when you supplement, it comes back up ... In certain individuals at high risk for fractures, for example, you might want to consider a more extensive workup like they’re suggesting.”
However, Dr. Rosen, of the Rosen Musculoskeletal Laboratory at Maine Medical Center Research Institute, Scarborough, added: “If the 25[OH]D level is below 20 [ng/mL] you’re going to treat regardless. When we think about sensitivity, a 25[OH]D level less than 20 [ng/mL] is a good screen ... Those individuals need to be treated, especially if they have low bone mass or fractures.”
To validate the ratio for clinical use, Dr. Rosen said, larger numbers of individuals would need to be evaluated. Moreover, “you’d need to run a standard of vitamin D binding protein by mass spectrometry versus their assumed method using ratios. Ratios are always a little tricky to interpret. So, I think this is very early in the game.”
And measuring the ratio of 1,25D/24,25D “is quite expensive,” he added.
He also pointed out that “calcium intake is really critical. You can have a [25(OH)D] level of 18 ng/mL and not have any of those secondary changes because [you’re] taking adequate calcium ... So, that always is a consideration that has to be worked into the evaluation.”
Same 25(OH)D, different risk level
In their poster, Dr. Yazdi and colleagues explain that to assess vitamin D status “one needs to understand regulation of vitamin D metabolism.” 25(OH)D undergoes two alternative fates: 1α-hydroxylation in the kidney, generating 1,25D (the biologically active form) or 24-hydroxylation leading to 24,25D (a biologically inactive metabolite).
For their study, they analyzed pilot data from 11 otherwise healthy individuals who had total baseline plasma 25(OH)D levels < 20 ng/mL, and compared 25(OH)D, 1,25D, 24,25D, and parathyroid hormone before versus after treating them with vitamin D3 supplementation of 50,000 IU per week for 4-6 weeks, aiming for a total 25D level above 30 ng/mL.
They then modeled how the body maintains 1,25D in a normal range and calculated/compared two vitamin D metabolite ratios in vitamin D deficient versus sufficient states: 25(OH)D/1,25D and 1,25D/24,25D. They then evaluated the applicability of these ratios for assessment of vitamin D status.
They explained that suppression of 24-hydroxylase is the first line of defense to maintain 1,25D levels. Secondary hyperparathyroidism is the second line of defense and occurs in severe vitamin D deficiency when the first line is maximally deployed.
Overall, there was poor correlation between 25[OH]D and 1,25D, “consistent with previous evidence that in mild to moderate vitamin D deficiency, 1,25D is maintained in the normal range, and therefore not a useful index for assessing vitamin D status,” the researchers said in their poster.
Hence, they said, the need to add the ratio of 1,25D/24,25D.
They presented a comparison of two study participants: one with a baseline 25[OH]D of 12.3 ng/mL, the other of 11.7 ng/mL. Although both would therefore be classified as deficient according to current guidelines, their 1,25D/24,25D ratios were 20 and 110, respectively.
In the first participant, the parathyroid hormone response to vitamin D supplementation was negligible, at +5%, compared with a dramatic 34% drop in the second participant.
“We think only the one with very high 1,25D/24,25D [ratio of 110] and a significant drop in parathyroid hormone after vitamin D supplementation [-34%] was vitamin D deficient,” the researchers said.
However, Dr. Taylor noted: “The diagnostic cut-offs we describe should be viewed as tentative for the time being. Additional research will be required to fully validate the optimal diagnostic criteria.”
Dr. Yazdi and Dr. Rosen have reported no relevant financial relationships. Dr. Taylor has reported being a consultant for Ionis Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
CHICAGO – , new research suggests.
The study supports previous data suggesting that a ratio cut-off of greater than 100 is associated with the development of secondary hyperparathyroidism and the need for correction with supplementation, while a level greater than 50 suggests mild to moderate deficiency, Zhinous Shahidzadeh Yazdi, MD, noted in a poster presented at the annual meeting of the Endocrine Society
Current Endocrine Society guidelines published in 2011 advise measurement of plasma circulating 25(OH)D levels to evaluate vitamin D status in patients at risk for deficiency, defined as < 20 ng/mL (50 nmol/L). Revised guidelines are due out in early 2024.
“We don’t think measuring 25 hydroxy D is optimal because of the impact of vitamin D binding protein,” Dr. Yazdi said in an interview.
“Over 99% of all metabolites are bound to vitamin D binding protein, but only the free fraction is biologically active. By measuring total plasma 25(OH)D – as we do right now in clinic – we cannot account for the impact of vitamin D binding proteins, which vary by threefold across the population,” she added.
Thus, the total 25(OH)D deficiency cut-off of < 20 mg/mL currently recommended by the Endocrine Society may signal clinically significant vitamin D deficiency in one person but not another, noted Dr. Yazdi, a postdoctoral fellow at the University of Maryland, Baltimore.
Directly measuring binding protein or the free fraction would be ideal, but “there aren’t good commercial assays for those, and it’s more difficult to do. So, as an alternative, the vitamin D metabolite ratios implicitly adjust for individual differences in vitamin D binding protein,” she explained.
The ratio that Dr. Yazdi and colleagues propose to measure is that of the vitamin D metabolites 1,25(OH)2D/24,25 (OH)2D (shortened to 1,25D/24,25D), which they say reflect the body’s homeostatic response to vitamin D levels, and which rises in the setting of deficiency. It is a measurement > 100 in this ratio that they believe means the patient should receive vitamin D supplementation.
Controversial topic, ratio proposal is “very early in the game”
The issue of vitamin D deficiency has long generated controversy, particularly since publication of findings from the VITAL study in 2022, which showed vitamin D supplements did not significantly reduce the risk of fracture among adults in midlife and older compared with placebo.
According to the senior author of the new study, Simeon I. Taylor, MD, professor of medicine at the University of Maryland, what still remains controversial after VITAL is the question: “How can you identify people who have sufficiently bad vitamin D deficiency that it’s adversely impacting their bones?”
He added that there is a suggestion that small subpopulations in VITAL really did benefit from vitamin D supplementation, but the study “wasn’t designed to look at that.”
Indeed, the authors of an editorial accompanying the publication of the VITAL study said the findings mean there is no justification for measuring 25(OH)D in the general population or for treating to a target level.
Asked to comment on Dr. Yazdi and colleagues’ ratio proposal for diagnosing vitamin D deficiency, the coauthor of the VITAL study editorial, Clifford J. Rosen, MD, said in an interview: “I do think it’s important to point out that changes in the vitamin D binding protein can have a significant impact on the level of 25 [OH] D ... People should recognize that.”
And, Dr. Rosen noted, “I like the idea that the ... [ratio] is a measure of what’s happening in the body in response to vitamin D stores. So, when you supplement, it comes back up ... In certain individuals at high risk for fractures, for example, you might want to consider a more extensive workup like they’re suggesting.”
However, Dr. Rosen, of the Rosen Musculoskeletal Laboratory at Maine Medical Center Research Institute, Scarborough, added: “If the 25[OH]D level is below 20 [ng/mL] you’re going to treat regardless. When we think about sensitivity, a 25[OH]D level less than 20 [ng/mL] is a good screen ... Those individuals need to be treated, especially if they have low bone mass or fractures.”
To validate the ratio for clinical use, Dr. Rosen said, larger numbers of individuals would need to be evaluated. Moreover, “you’d need to run a standard of vitamin D binding protein by mass spectrometry versus their assumed method using ratios. Ratios are always a little tricky to interpret. So, I think this is very early in the game.”
And measuring the ratio of 1,25D/24,25D “is quite expensive,” he added.
He also pointed out that “calcium intake is really critical. You can have a [25(OH)D] level of 18 ng/mL and not have any of those secondary changes because [you’re] taking adequate calcium ... So, that always is a consideration that has to be worked into the evaluation.”
Same 25(OH)D, different risk level
In their poster, Dr. Yazdi and colleagues explain that to assess vitamin D status “one needs to understand regulation of vitamin D metabolism.” 25(OH)D undergoes two alternative fates: 1α-hydroxylation in the kidney, generating 1,25D (the biologically active form) or 24-hydroxylation leading to 24,25D (a biologically inactive metabolite).
For their study, they analyzed pilot data from 11 otherwise healthy individuals who had total baseline plasma 25(OH)D levels < 20 ng/mL, and compared 25(OH)D, 1,25D, 24,25D, and parathyroid hormone before versus after treating them with vitamin D3 supplementation of 50,000 IU per week for 4-6 weeks, aiming for a total 25D level above 30 ng/mL.
They then modeled how the body maintains 1,25D in a normal range and calculated/compared two vitamin D metabolite ratios in vitamin D deficient versus sufficient states: 25(OH)D/1,25D and 1,25D/24,25D. They then evaluated the applicability of these ratios for assessment of vitamin D status.
They explained that suppression of 24-hydroxylase is the first line of defense to maintain 1,25D levels. Secondary hyperparathyroidism is the second line of defense and occurs in severe vitamin D deficiency when the first line is maximally deployed.
Overall, there was poor correlation between 25[OH]D and 1,25D, “consistent with previous evidence that in mild to moderate vitamin D deficiency, 1,25D is maintained in the normal range, and therefore not a useful index for assessing vitamin D status,” the researchers said in their poster.
Hence, they said, the need to add the ratio of 1,25D/24,25D.
They presented a comparison of two study participants: one with a baseline 25[OH]D of 12.3 ng/mL, the other of 11.7 ng/mL. Although both would therefore be classified as deficient according to current guidelines, their 1,25D/24,25D ratios were 20 and 110, respectively.
In the first participant, the parathyroid hormone response to vitamin D supplementation was negligible, at +5%, compared with a dramatic 34% drop in the second participant.
“We think only the one with very high 1,25D/24,25D [ratio of 110] and a significant drop in parathyroid hormone after vitamin D supplementation [-34%] was vitamin D deficient,” the researchers said.
However, Dr. Taylor noted: “The diagnostic cut-offs we describe should be viewed as tentative for the time being. Additional research will be required to fully validate the optimal diagnostic criteria.”
Dr. Yazdi and Dr. Rosen have reported no relevant financial relationships. Dr. Taylor has reported being a consultant for Ionis Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
CHICAGO – , new research suggests.
The study supports previous data suggesting that a ratio cut-off of greater than 100 is associated with the development of secondary hyperparathyroidism and the need for correction with supplementation, while a level greater than 50 suggests mild to moderate deficiency, Zhinous Shahidzadeh Yazdi, MD, noted in a poster presented at the annual meeting of the Endocrine Society
Current Endocrine Society guidelines published in 2011 advise measurement of plasma circulating 25(OH)D levels to evaluate vitamin D status in patients at risk for deficiency, defined as < 20 ng/mL (50 nmol/L). Revised guidelines are due out in early 2024.
“We don’t think measuring 25 hydroxy D is optimal because of the impact of vitamin D binding protein,” Dr. Yazdi said in an interview.
“Over 99% of all metabolites are bound to vitamin D binding protein, but only the free fraction is biologically active. By measuring total plasma 25(OH)D – as we do right now in clinic – we cannot account for the impact of vitamin D binding proteins, which vary by threefold across the population,” she added.
Thus, the total 25(OH)D deficiency cut-off of < 20 mg/mL currently recommended by the Endocrine Society may signal clinically significant vitamin D deficiency in one person but not another, noted Dr. Yazdi, a postdoctoral fellow at the University of Maryland, Baltimore.
Directly measuring binding protein or the free fraction would be ideal, but “there aren’t good commercial assays for those, and it’s more difficult to do. So, as an alternative, the vitamin D metabolite ratios implicitly adjust for individual differences in vitamin D binding protein,” she explained.
The ratio that Dr. Yazdi and colleagues propose to measure is that of the vitamin D metabolites 1,25(OH)2D/24,25 (OH)2D (shortened to 1,25D/24,25D), which they say reflect the body’s homeostatic response to vitamin D levels, and which rises in the setting of deficiency. It is a measurement > 100 in this ratio that they believe means the patient should receive vitamin D supplementation.
Controversial topic, ratio proposal is “very early in the game”
The issue of vitamin D deficiency has long generated controversy, particularly since publication of findings from the VITAL study in 2022, which showed vitamin D supplements did not significantly reduce the risk of fracture among adults in midlife and older compared with placebo.
According to the senior author of the new study, Simeon I. Taylor, MD, professor of medicine at the University of Maryland, what still remains controversial after VITAL is the question: “How can you identify people who have sufficiently bad vitamin D deficiency that it’s adversely impacting their bones?”
He added that there is a suggestion that small subpopulations in VITAL really did benefit from vitamin D supplementation, but the study “wasn’t designed to look at that.”
Indeed, the authors of an editorial accompanying the publication of the VITAL study said the findings mean there is no justification for measuring 25(OH)D in the general population or for treating to a target level.
Asked to comment on Dr. Yazdi and colleagues’ ratio proposal for diagnosing vitamin D deficiency, the coauthor of the VITAL study editorial, Clifford J. Rosen, MD, said in an interview: “I do think it’s important to point out that changes in the vitamin D binding protein can have a significant impact on the level of 25 [OH] D ... People should recognize that.”
And, Dr. Rosen noted, “I like the idea that the ... [ratio] is a measure of what’s happening in the body in response to vitamin D stores. So, when you supplement, it comes back up ... In certain individuals at high risk for fractures, for example, you might want to consider a more extensive workup like they’re suggesting.”
However, Dr. Rosen, of the Rosen Musculoskeletal Laboratory at Maine Medical Center Research Institute, Scarborough, added: “If the 25[OH]D level is below 20 [ng/mL] you’re going to treat regardless. When we think about sensitivity, a 25[OH]D level less than 20 [ng/mL] is a good screen ... Those individuals need to be treated, especially if they have low bone mass or fractures.”
To validate the ratio for clinical use, Dr. Rosen said, larger numbers of individuals would need to be evaluated. Moreover, “you’d need to run a standard of vitamin D binding protein by mass spectrometry versus their assumed method using ratios. Ratios are always a little tricky to interpret. So, I think this is very early in the game.”
And measuring the ratio of 1,25D/24,25D “is quite expensive,” he added.
He also pointed out that “calcium intake is really critical. You can have a [25(OH)D] level of 18 ng/mL and not have any of those secondary changes because [you’re] taking adequate calcium ... So, that always is a consideration that has to be worked into the evaluation.”
Same 25(OH)D, different risk level
In their poster, Dr. Yazdi and colleagues explain that to assess vitamin D status “one needs to understand regulation of vitamin D metabolism.” 25(OH)D undergoes two alternative fates: 1α-hydroxylation in the kidney, generating 1,25D (the biologically active form) or 24-hydroxylation leading to 24,25D (a biologically inactive metabolite).
For their study, they analyzed pilot data from 11 otherwise healthy individuals who had total baseline plasma 25(OH)D levels < 20 ng/mL, and compared 25(OH)D, 1,25D, 24,25D, and parathyroid hormone before versus after treating them with vitamin D3 supplementation of 50,000 IU per week for 4-6 weeks, aiming for a total 25D level above 30 ng/mL.
They then modeled how the body maintains 1,25D in a normal range and calculated/compared two vitamin D metabolite ratios in vitamin D deficient versus sufficient states: 25(OH)D/1,25D and 1,25D/24,25D. They then evaluated the applicability of these ratios for assessment of vitamin D status.
They explained that suppression of 24-hydroxylase is the first line of defense to maintain 1,25D levels. Secondary hyperparathyroidism is the second line of defense and occurs in severe vitamin D deficiency when the first line is maximally deployed.
Overall, there was poor correlation between 25[OH]D and 1,25D, “consistent with previous evidence that in mild to moderate vitamin D deficiency, 1,25D is maintained in the normal range, and therefore not a useful index for assessing vitamin D status,” the researchers said in their poster.
Hence, they said, the need to add the ratio of 1,25D/24,25D.
They presented a comparison of two study participants: one with a baseline 25[OH]D of 12.3 ng/mL, the other of 11.7 ng/mL. Although both would therefore be classified as deficient according to current guidelines, their 1,25D/24,25D ratios were 20 and 110, respectively.
In the first participant, the parathyroid hormone response to vitamin D supplementation was negligible, at +5%, compared with a dramatic 34% drop in the second participant.
“We think only the one with very high 1,25D/24,25D [ratio of 110] and a significant drop in parathyroid hormone after vitamin D supplementation [-34%] was vitamin D deficient,” the researchers said.
However, Dr. Taylor noted: “The diagnostic cut-offs we describe should be viewed as tentative for the time being. Additional research will be required to fully validate the optimal diagnostic criteria.”
Dr. Yazdi and Dr. Rosen have reported no relevant financial relationships. Dr. Taylor has reported being a consultant for Ionis Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
AT ENDO 2023
‘New standard of care’ for capecitabine hand-foot syndrome
researchers reported in a study that has been hailed by experts as “practice changing.”
Hand-foot syndrome causes painful, bleeding blisters and ulcers on the palms and soles. It often leads to dose reductions and sometimes even discontinuations, both of which limit the effectiveness of capecitabine, a standard oral chemotherapy drug widely used for colorectal and breast cancers.
In a new study presented at the annual meeting of the American Society of Clinical Oncology, Indian researchers reported that a cheap, safe, and widely available over-the-counter nonsteroidal anti-inflammatory gel containing 1% diclofenac reduced the incidence of hand-foot syndrome by 75% among patients with cancer being treated with capecitabine.
Up until now, the oral anti-inflammatory celecoxib (Celebrex) was the only agent proven to prevent the problem, but it’s rarely used because of the risk for strokes, gastric bleeding, and other issues, none of which are a concern with topical diclofenac, which osteoarthritis patients have used safely for years.
The Indian trial, dubbed D-Torch, establishes “1% topical diclofenac gel as the new standard of care to prevent capecitabine-associated hand-foot syndrome,” said investigator and study presenter Atul Batra, MD, a medical oncologist at the All India Institute of Medical Sciences, New Delhi.
Dr. Batra told ASCO Daily News that there is no need for a second trial. “We don’t feel there’s a need to replicate these results” in a larger study “because this was adequately powered, and the results speak for themselves. There’s no confusion about these results. Diclofenac is clearly effective.”
Dr. Batra also commented that his clinic now uses topical diclofenac routinely during capecitabine treatment and that he hopes oncology practices elsewhere will do the same.
Diclofenac gel is sold under the brand name Voltaren and is also available as a generic; in the United States, a 150-gram tube costs about $18 at Walmart.
‘The most practice-changing study’ at ASCO 2023
Audience members at ASCO’s annual meeting immediately saw the importance of the study.
Tarah Ballinger, MD, a breast cancer specialist at Indiana University, Indianapolis, said on Twitter that “this might be the most practice changing study I heard at ASCO23.” Topical diclofenac is “widely available, affordable, [and] addresses [a] major” quality of life issue.
The study discussant at the meeting, gastrointestinal cancer specialist Pallavi Kumar, MD, of the University of Pennsylvania, Philadelphia, concurred: “For me as a GI oncologist, topical diclofenac for prevention of HFS for patients on capecitabine is practice changing,” she said.
The takeaway is “that topical diclofenac significantly reduces the incidence of grade 2 or higher HFS in patients receiving capecitabine.” The results are “very impressive,” Dr. Kumar said.
Study details
The idea for the new study came after Batra and colleagues realized that celecoxib, a COX-2 enzyme inhibitor, helps prevent capecitabine hand-foot syndrome (HFS) by blocking a key process that leads to it, the up-regulation of COX-2 and subsequent release of proinflammatory prostaglandins.
They turned to diclofenac gel hoping to get the same effect but more safely; diclofenac is also a COX-2 blocker, and its topical formulation has a strong safety record.
To test the approach, the team randomly assigned 130 patients to topical diclofenac and 133 to placebo – the gel vehicle without the medication – while they were being treated with capecitabine for 12 weeks; 56% were being treated for breast cancer and the rest for gastrointestinal cancers.
Subjects rubbed one fingertip’s worth of gel – about half a gram – on each palm and the back of each hand twice a day. The dose was about 4 grams/day, which is well below maximal dosages for osteoarthritis (up to 32 g/day over all affected joints). Adherence to treatment was about 95% in both arms.
By the end of 12 weeks, the incidence of grade 2 or higher HFS was 3.8% in the diclofenac arm (5 patients) versus 15% (n = 20) with placebo (P = .003), a 75% risk reduction.
The incidence of any grade HFS was 6.1% in the treatment group versus 18.1% with placebo (P = .003).
Hand-foot syndrome led to dose reductions of capecitabine in 13.5% of placebo but only 3.8% of those in the diclofenac group (P = .002).
The findings held regardless of whether patients were being treated for breast or GI cancer or if they were men or women.
Other capecitabine-induced adverse events, including diarrhea, mucositis, and myelosuppression, were not significantly different between the groups.
The treatment arms were well balanced, with a median age of 47 years in both groups and women making up about 70% of each. About 40% of subjects in each group were on capecitabine monotherapy with the rest on combination treatments. The mean dose of capecitabine was just over 1,880 mg/m2 in both groups.
At the meeting, Dr. Batra was asked if topical diclofenac would also work for another common problem in oncology: hand-food syndrome occurring as a side-effect with VEGF–tyrosine kinase inhibitors. He didn’t think so because it probably has a different cause than capecitabine HFS, one not strongly related to COX-2 up-regulation.
The study was partly funded by the Indian Supportive Care of Cancer Association. The investigators reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers reported in a study that has been hailed by experts as “practice changing.”
Hand-foot syndrome causes painful, bleeding blisters and ulcers on the palms and soles. It often leads to dose reductions and sometimes even discontinuations, both of which limit the effectiveness of capecitabine, a standard oral chemotherapy drug widely used for colorectal and breast cancers.
In a new study presented at the annual meeting of the American Society of Clinical Oncology, Indian researchers reported that a cheap, safe, and widely available over-the-counter nonsteroidal anti-inflammatory gel containing 1% diclofenac reduced the incidence of hand-foot syndrome by 75% among patients with cancer being treated with capecitabine.
Up until now, the oral anti-inflammatory celecoxib (Celebrex) was the only agent proven to prevent the problem, but it’s rarely used because of the risk for strokes, gastric bleeding, and other issues, none of which are a concern with topical diclofenac, which osteoarthritis patients have used safely for years.
The Indian trial, dubbed D-Torch, establishes “1% topical diclofenac gel as the new standard of care to prevent capecitabine-associated hand-foot syndrome,” said investigator and study presenter Atul Batra, MD, a medical oncologist at the All India Institute of Medical Sciences, New Delhi.
Dr. Batra told ASCO Daily News that there is no need for a second trial. “We don’t feel there’s a need to replicate these results” in a larger study “because this was adequately powered, and the results speak for themselves. There’s no confusion about these results. Diclofenac is clearly effective.”
Dr. Batra also commented that his clinic now uses topical diclofenac routinely during capecitabine treatment and that he hopes oncology practices elsewhere will do the same.
Diclofenac gel is sold under the brand name Voltaren and is also available as a generic; in the United States, a 150-gram tube costs about $18 at Walmart.
‘The most practice-changing study’ at ASCO 2023
Audience members at ASCO’s annual meeting immediately saw the importance of the study.
Tarah Ballinger, MD, a breast cancer specialist at Indiana University, Indianapolis, said on Twitter that “this might be the most practice changing study I heard at ASCO23.” Topical diclofenac is “widely available, affordable, [and] addresses [a] major” quality of life issue.
The study discussant at the meeting, gastrointestinal cancer specialist Pallavi Kumar, MD, of the University of Pennsylvania, Philadelphia, concurred: “For me as a GI oncologist, topical diclofenac for prevention of HFS for patients on capecitabine is practice changing,” she said.
The takeaway is “that topical diclofenac significantly reduces the incidence of grade 2 or higher HFS in patients receiving capecitabine.” The results are “very impressive,” Dr. Kumar said.
Study details
The idea for the new study came after Batra and colleagues realized that celecoxib, a COX-2 enzyme inhibitor, helps prevent capecitabine hand-foot syndrome (HFS) by blocking a key process that leads to it, the up-regulation of COX-2 and subsequent release of proinflammatory prostaglandins.
They turned to diclofenac gel hoping to get the same effect but more safely; diclofenac is also a COX-2 blocker, and its topical formulation has a strong safety record.
To test the approach, the team randomly assigned 130 patients to topical diclofenac and 133 to placebo – the gel vehicle without the medication – while they were being treated with capecitabine for 12 weeks; 56% were being treated for breast cancer and the rest for gastrointestinal cancers.
Subjects rubbed one fingertip’s worth of gel – about half a gram – on each palm and the back of each hand twice a day. The dose was about 4 grams/day, which is well below maximal dosages for osteoarthritis (up to 32 g/day over all affected joints). Adherence to treatment was about 95% in both arms.
By the end of 12 weeks, the incidence of grade 2 or higher HFS was 3.8% in the diclofenac arm (5 patients) versus 15% (n = 20) with placebo (P = .003), a 75% risk reduction.
The incidence of any grade HFS was 6.1% in the treatment group versus 18.1% with placebo (P = .003).
Hand-foot syndrome led to dose reductions of capecitabine in 13.5% of placebo but only 3.8% of those in the diclofenac group (P = .002).
The findings held regardless of whether patients were being treated for breast or GI cancer or if they were men or women.
Other capecitabine-induced adverse events, including diarrhea, mucositis, and myelosuppression, were not significantly different between the groups.
The treatment arms were well balanced, with a median age of 47 years in both groups and women making up about 70% of each. About 40% of subjects in each group were on capecitabine monotherapy with the rest on combination treatments. The mean dose of capecitabine was just over 1,880 mg/m2 in both groups.
At the meeting, Dr. Batra was asked if topical diclofenac would also work for another common problem in oncology: hand-food syndrome occurring as a side-effect with VEGF–tyrosine kinase inhibitors. He didn’t think so because it probably has a different cause than capecitabine HFS, one not strongly related to COX-2 up-regulation.
The study was partly funded by the Indian Supportive Care of Cancer Association. The investigators reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers reported in a study that has been hailed by experts as “practice changing.”
Hand-foot syndrome causes painful, bleeding blisters and ulcers on the palms and soles. It often leads to dose reductions and sometimes even discontinuations, both of which limit the effectiveness of capecitabine, a standard oral chemotherapy drug widely used for colorectal and breast cancers.
In a new study presented at the annual meeting of the American Society of Clinical Oncology, Indian researchers reported that a cheap, safe, and widely available over-the-counter nonsteroidal anti-inflammatory gel containing 1% diclofenac reduced the incidence of hand-foot syndrome by 75% among patients with cancer being treated with capecitabine.
Up until now, the oral anti-inflammatory celecoxib (Celebrex) was the only agent proven to prevent the problem, but it’s rarely used because of the risk for strokes, gastric bleeding, and other issues, none of which are a concern with topical diclofenac, which osteoarthritis patients have used safely for years.
The Indian trial, dubbed D-Torch, establishes “1% topical diclofenac gel as the new standard of care to prevent capecitabine-associated hand-foot syndrome,” said investigator and study presenter Atul Batra, MD, a medical oncologist at the All India Institute of Medical Sciences, New Delhi.
Dr. Batra told ASCO Daily News that there is no need for a second trial. “We don’t feel there’s a need to replicate these results” in a larger study “because this was adequately powered, and the results speak for themselves. There’s no confusion about these results. Diclofenac is clearly effective.”
Dr. Batra also commented that his clinic now uses topical diclofenac routinely during capecitabine treatment and that he hopes oncology practices elsewhere will do the same.
Diclofenac gel is sold under the brand name Voltaren and is also available as a generic; in the United States, a 150-gram tube costs about $18 at Walmart.
‘The most practice-changing study’ at ASCO 2023
Audience members at ASCO’s annual meeting immediately saw the importance of the study.
Tarah Ballinger, MD, a breast cancer specialist at Indiana University, Indianapolis, said on Twitter that “this might be the most practice changing study I heard at ASCO23.” Topical diclofenac is “widely available, affordable, [and] addresses [a] major” quality of life issue.
The study discussant at the meeting, gastrointestinal cancer specialist Pallavi Kumar, MD, of the University of Pennsylvania, Philadelphia, concurred: “For me as a GI oncologist, topical diclofenac for prevention of HFS for patients on capecitabine is practice changing,” she said.
The takeaway is “that topical diclofenac significantly reduces the incidence of grade 2 or higher HFS in patients receiving capecitabine.” The results are “very impressive,” Dr. Kumar said.
Study details
The idea for the new study came after Batra and colleagues realized that celecoxib, a COX-2 enzyme inhibitor, helps prevent capecitabine hand-foot syndrome (HFS) by blocking a key process that leads to it, the up-regulation of COX-2 and subsequent release of proinflammatory prostaglandins.
They turned to diclofenac gel hoping to get the same effect but more safely; diclofenac is also a COX-2 blocker, and its topical formulation has a strong safety record.
To test the approach, the team randomly assigned 130 patients to topical diclofenac and 133 to placebo – the gel vehicle without the medication – while they were being treated with capecitabine for 12 weeks; 56% were being treated for breast cancer and the rest for gastrointestinal cancers.
Subjects rubbed one fingertip’s worth of gel – about half a gram – on each palm and the back of each hand twice a day. The dose was about 4 grams/day, which is well below maximal dosages for osteoarthritis (up to 32 g/day over all affected joints). Adherence to treatment was about 95% in both arms.
By the end of 12 weeks, the incidence of grade 2 or higher HFS was 3.8% in the diclofenac arm (5 patients) versus 15% (n = 20) with placebo (P = .003), a 75% risk reduction.
The incidence of any grade HFS was 6.1% in the treatment group versus 18.1% with placebo (P = .003).
Hand-foot syndrome led to dose reductions of capecitabine in 13.5% of placebo but only 3.8% of those in the diclofenac group (P = .002).
The findings held regardless of whether patients were being treated for breast or GI cancer or if they were men or women.
Other capecitabine-induced adverse events, including diarrhea, mucositis, and myelosuppression, were not significantly different between the groups.
The treatment arms were well balanced, with a median age of 47 years in both groups and women making up about 70% of each. About 40% of subjects in each group were on capecitabine monotherapy with the rest on combination treatments. The mean dose of capecitabine was just over 1,880 mg/m2 in both groups.
At the meeting, Dr. Batra was asked if topical diclofenac would also work for another common problem in oncology: hand-food syndrome occurring as a side-effect with VEGF–tyrosine kinase inhibitors. He didn’t think so because it probably has a different cause than capecitabine HFS, one not strongly related to COX-2 up-regulation.
The study was partly funded by the Indian Supportive Care of Cancer Association. The investigators reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ASCO 2023
Millions who had COVID-19 still don’t have sense of smell, taste
Almost 36 million people were diagnosed in 2021, and 60% of them reported accompanying losses in smell or taste, according to the study by Mass Eye and Ear, which is affiliated with Harvard Medical School, Boston. The study was published in The Laryngoscope.
Most people fully regained the senses, but about 24% didn’t get smell back completely, and more than 3% had no recovery, the researchers reported. The numbers were similar among those who lost the sense of taste, they added.
“Many people never fully recovered,” Neil Bhattacharyya, MD, professor of otolaryngology and one of the study’s authors, told Fortune, estimating that up to 6 million people still have lingering symptoms. “If you lost your sense of smell, did you get it back? There’s about a one in four chance you didn’t. That’s terrible.”
Researchers looked at the records of 30,000 adults who had COVID-19 in 2021. They reported that patients who suffered more severe cases were less likely to regain some or all their senses.
Some patients said they lost appetite because they couldn’t smell food. There’s concern, too, about losing the ability to smell gas and smoke, spoiled food, and dirty diapers.
People with symptoms should see their doctors, Dr. Bhattacharyya said. The symptoms might be caused by something other than lingering COVID-19 effects and might be treatable.
A version of this article first appeared on WebMD.com.
Almost 36 million people were diagnosed in 2021, and 60% of them reported accompanying losses in smell or taste, according to the study by Mass Eye and Ear, which is affiliated with Harvard Medical School, Boston. The study was published in The Laryngoscope.
Most people fully regained the senses, but about 24% didn’t get smell back completely, and more than 3% had no recovery, the researchers reported. The numbers were similar among those who lost the sense of taste, they added.
“Many people never fully recovered,” Neil Bhattacharyya, MD, professor of otolaryngology and one of the study’s authors, told Fortune, estimating that up to 6 million people still have lingering symptoms. “If you lost your sense of smell, did you get it back? There’s about a one in four chance you didn’t. That’s terrible.”
Researchers looked at the records of 30,000 adults who had COVID-19 in 2021. They reported that patients who suffered more severe cases were less likely to regain some or all their senses.
Some patients said they lost appetite because they couldn’t smell food. There’s concern, too, about losing the ability to smell gas and smoke, spoiled food, and dirty diapers.
People with symptoms should see their doctors, Dr. Bhattacharyya said. The symptoms might be caused by something other than lingering COVID-19 effects and might be treatable.
A version of this article first appeared on WebMD.com.
Almost 36 million people were diagnosed in 2021, and 60% of them reported accompanying losses in smell or taste, according to the study by Mass Eye and Ear, which is affiliated with Harvard Medical School, Boston. The study was published in The Laryngoscope.
Most people fully regained the senses, but about 24% didn’t get smell back completely, and more than 3% had no recovery, the researchers reported. The numbers were similar among those who lost the sense of taste, they added.
“Many people never fully recovered,” Neil Bhattacharyya, MD, professor of otolaryngology and one of the study’s authors, told Fortune, estimating that up to 6 million people still have lingering symptoms. “If you lost your sense of smell, did you get it back? There’s about a one in four chance you didn’t. That’s terrible.”
Researchers looked at the records of 30,000 adults who had COVID-19 in 2021. They reported that patients who suffered more severe cases were less likely to regain some or all their senses.
Some patients said they lost appetite because they couldn’t smell food. There’s concern, too, about losing the ability to smell gas and smoke, spoiled food, and dirty diapers.
People with symptoms should see their doctors, Dr. Bhattacharyya said. The symptoms might be caused by something other than lingering COVID-19 effects and might be treatable.
A version of this article first appeared on WebMD.com.
FROM THE LARYNGOSCOPE
Hospital patient catches on fire, highlighting need for prevention
On Thanksgiving Day 2022, Kathy Stark watched as her husband of 35 years, Bobby Ray Stark, caught fire at a Nashville hospital. According to Clint Kelly, Kathy Stark’s attorney, the hospital staff was performing cardioversion to restore Bobby Ray’s heart rhythm when a spark ignited the oxygen and set the patient aflame.
Mr. Stark, 64, died of “a combination of cardiovascular disease and thermal burns,” according to a local news report. In May, Kathy Stark filed a malpractice lawsuit in U.S. District Court. Mr. Kelly hopes that the lawsuit will help improve patient safety. Meanwhile, Kathy Stark “goes to bed at night and sees her husband on fire,” Mr. Kelly says. A similar incident occurred last December in the operating room at Oregon Health & Science University, resulting in minor injuries to a patient.
Underreported, but likely dropping
Reliable data on the incidence of surgical fires is lacking because incidents may go unreported over litigation fears, says Jeffrey Feldman, MD, MSE, anesthesiologist at Children’s Hospital of Philadelphia and chair of the Anesthesia Patient Safety Foundation’s Committee on Technology.
The Pennsylvania Patient Safety Authority has been tracking surgical fires for decades, however, and experts have used the agency’s data to extrapolate how often they occur in the United States.
In 2005, nationwide incidence was estimated to be somewhere in the neighborhood of 550-600 fires annually, says Barbara G. Malanga, acting director of health care incident investigation and technology consulting at ECRI (formerly the Emergency Care Research Institute). By 2011, that number appeared to have dropped to 200-240 incidents per year.
A similar analysis in 2018 found the incidence may now be as low as 88-105 a year. The drop is likely a result of increased awareness because of educational efforts on the part of the ECRI and the APSF, including a widely disseminated video on fire safety.
The decline of surgical fires “sounds great,” says Dr. Feldman, “except that it’s a 100% preventable complication, and they’re still happening.”
Accidents waiting to happen
How do these fires happen? It comes down to the ‘fire triangle’ often taught in grade school. Fire requires three things: an ignition source, fuel, and oxygen or an oxidizing agent. Ignition sources are plentiful in a surgical suite, including any of a variety of electrical devices commonly used in surgical procedures, including defibrillators. Gowns, gauze, drapes, sponges, oxygen masks, nasal cannulae, a patient’s hair or their clothing – all provide the necessary fuel.
But the key factor for surgical fire risk is the presence of high concentrations of oxygen.
Safety protocols
The best and most obvious way to mitigate risk is to reduce the amount of supplemental oxygen, explains Dr. Feldman.
“Many patients do not require a high concentration of oxygen during sedation,” he says.
When a patient does require a higher concentration for their safety, the APSF and ECRI recommend placing an endotracheal tube or supraglottic airway rather than using an oxygen mask or a nasal cannula. “You want to deliver the oxygen in such a way that high concentration doesn’t exist in the surgical field,” Dr. Feldman says. In cases where supplemental oxygen is necessary, ECRI and APSF recommend reducing the oxygen concentration to less than 30%.
In addition, safety protocols include giving flammable prep solutions time to dry before applying towels or drapes and beginning the procedure. These precautions to ensure the safety of patients take just a moment, says Chester H. Lake Jr, MD, MS, of the department of anesthesiology at the University of Mississippi Medical Center, Jackson.
Making fire safety part of the preop routine
These safety protocols are straightforward but not always observed, experts say. Part of the reason is a matter of culture. Both anesthesiologists and surgeons have absorbed the attitude that placing an airway escalates the procedure beyond what the patient needs, says Dr. Feldman. And indeed, according to a 2013 analysis of the American Society of Anesthesiologists closed claims database, 85% of surgical fires occur in outpatient settings where airways are less likely to be placed, and 81% of those claims were for procedures that used monitored anesthesia care.
In an article on prevention of surgical fires, Dr. Lake and colleagues recommend in-house education on preventing and responding to fires at least once a year. But it shouldn’t stop there. Because these fires – horrific as they are – are fairly rare, it’s important to maintain awareness. Making fire safety a regular part of the surgical “time-out” can help further reduce incidents, he says. ECRI and the APSF have teamed up to create a poster that can help surgical teams make fire safety a regular part of their routines.
Although the national decline in surgical fires is encouraging, the problem remains serious. “You can classify these incidents as low, but it’s not low if it happens to you or a family member,” says Dr. Lake. “One is too many.”
ECRI’s Ms. Malanga agrees. “I do like to emphasize that it’s rare,” she says. “But I’d like to see us reduce this until it’s zero.”
A version of this article originally appeared on Medscape.com.
On Thanksgiving Day 2022, Kathy Stark watched as her husband of 35 years, Bobby Ray Stark, caught fire at a Nashville hospital. According to Clint Kelly, Kathy Stark’s attorney, the hospital staff was performing cardioversion to restore Bobby Ray’s heart rhythm when a spark ignited the oxygen and set the patient aflame.
Mr. Stark, 64, died of “a combination of cardiovascular disease and thermal burns,” according to a local news report. In May, Kathy Stark filed a malpractice lawsuit in U.S. District Court. Mr. Kelly hopes that the lawsuit will help improve patient safety. Meanwhile, Kathy Stark “goes to bed at night and sees her husband on fire,” Mr. Kelly says. A similar incident occurred last December in the operating room at Oregon Health & Science University, resulting in minor injuries to a patient.
Underreported, but likely dropping
Reliable data on the incidence of surgical fires is lacking because incidents may go unreported over litigation fears, says Jeffrey Feldman, MD, MSE, anesthesiologist at Children’s Hospital of Philadelphia and chair of the Anesthesia Patient Safety Foundation’s Committee on Technology.
The Pennsylvania Patient Safety Authority has been tracking surgical fires for decades, however, and experts have used the agency’s data to extrapolate how often they occur in the United States.
In 2005, nationwide incidence was estimated to be somewhere in the neighborhood of 550-600 fires annually, says Barbara G. Malanga, acting director of health care incident investigation and technology consulting at ECRI (formerly the Emergency Care Research Institute). By 2011, that number appeared to have dropped to 200-240 incidents per year.
A similar analysis in 2018 found the incidence may now be as low as 88-105 a year. The drop is likely a result of increased awareness because of educational efforts on the part of the ECRI and the APSF, including a widely disseminated video on fire safety.
The decline of surgical fires “sounds great,” says Dr. Feldman, “except that it’s a 100% preventable complication, and they’re still happening.”
Accidents waiting to happen
How do these fires happen? It comes down to the ‘fire triangle’ often taught in grade school. Fire requires three things: an ignition source, fuel, and oxygen or an oxidizing agent. Ignition sources are plentiful in a surgical suite, including any of a variety of electrical devices commonly used in surgical procedures, including defibrillators. Gowns, gauze, drapes, sponges, oxygen masks, nasal cannulae, a patient’s hair or their clothing – all provide the necessary fuel.
But the key factor for surgical fire risk is the presence of high concentrations of oxygen.
Safety protocols
The best and most obvious way to mitigate risk is to reduce the amount of supplemental oxygen, explains Dr. Feldman.
“Many patients do not require a high concentration of oxygen during sedation,” he says.
When a patient does require a higher concentration for their safety, the APSF and ECRI recommend placing an endotracheal tube or supraglottic airway rather than using an oxygen mask or a nasal cannula. “You want to deliver the oxygen in such a way that high concentration doesn’t exist in the surgical field,” Dr. Feldman says. In cases where supplemental oxygen is necessary, ECRI and APSF recommend reducing the oxygen concentration to less than 30%.
In addition, safety protocols include giving flammable prep solutions time to dry before applying towels or drapes and beginning the procedure. These precautions to ensure the safety of patients take just a moment, says Chester H. Lake Jr, MD, MS, of the department of anesthesiology at the University of Mississippi Medical Center, Jackson.
Making fire safety part of the preop routine
These safety protocols are straightforward but not always observed, experts say. Part of the reason is a matter of culture. Both anesthesiologists and surgeons have absorbed the attitude that placing an airway escalates the procedure beyond what the patient needs, says Dr. Feldman. And indeed, according to a 2013 analysis of the American Society of Anesthesiologists closed claims database, 85% of surgical fires occur in outpatient settings where airways are less likely to be placed, and 81% of those claims were for procedures that used monitored anesthesia care.
In an article on prevention of surgical fires, Dr. Lake and colleagues recommend in-house education on preventing and responding to fires at least once a year. But it shouldn’t stop there. Because these fires – horrific as they are – are fairly rare, it’s important to maintain awareness. Making fire safety a regular part of the surgical “time-out” can help further reduce incidents, he says. ECRI and the APSF have teamed up to create a poster that can help surgical teams make fire safety a regular part of their routines.
Although the national decline in surgical fires is encouraging, the problem remains serious. “You can classify these incidents as low, but it’s not low if it happens to you or a family member,” says Dr. Lake. “One is too many.”
ECRI’s Ms. Malanga agrees. “I do like to emphasize that it’s rare,” she says. “But I’d like to see us reduce this until it’s zero.”
A version of this article originally appeared on Medscape.com.
On Thanksgiving Day 2022, Kathy Stark watched as her husband of 35 years, Bobby Ray Stark, caught fire at a Nashville hospital. According to Clint Kelly, Kathy Stark’s attorney, the hospital staff was performing cardioversion to restore Bobby Ray’s heart rhythm when a spark ignited the oxygen and set the patient aflame.
Mr. Stark, 64, died of “a combination of cardiovascular disease and thermal burns,” according to a local news report. In May, Kathy Stark filed a malpractice lawsuit in U.S. District Court. Mr. Kelly hopes that the lawsuit will help improve patient safety. Meanwhile, Kathy Stark “goes to bed at night and sees her husband on fire,” Mr. Kelly says. A similar incident occurred last December in the operating room at Oregon Health & Science University, resulting in minor injuries to a patient.
Underreported, but likely dropping
Reliable data on the incidence of surgical fires is lacking because incidents may go unreported over litigation fears, says Jeffrey Feldman, MD, MSE, anesthesiologist at Children’s Hospital of Philadelphia and chair of the Anesthesia Patient Safety Foundation’s Committee on Technology.
The Pennsylvania Patient Safety Authority has been tracking surgical fires for decades, however, and experts have used the agency’s data to extrapolate how often they occur in the United States.
In 2005, nationwide incidence was estimated to be somewhere in the neighborhood of 550-600 fires annually, says Barbara G. Malanga, acting director of health care incident investigation and technology consulting at ECRI (formerly the Emergency Care Research Institute). By 2011, that number appeared to have dropped to 200-240 incidents per year.
A similar analysis in 2018 found the incidence may now be as low as 88-105 a year. The drop is likely a result of increased awareness because of educational efforts on the part of the ECRI and the APSF, including a widely disseminated video on fire safety.
The decline of surgical fires “sounds great,” says Dr. Feldman, “except that it’s a 100% preventable complication, and they’re still happening.”
Accidents waiting to happen
How do these fires happen? It comes down to the ‘fire triangle’ often taught in grade school. Fire requires three things: an ignition source, fuel, and oxygen or an oxidizing agent. Ignition sources are plentiful in a surgical suite, including any of a variety of electrical devices commonly used in surgical procedures, including defibrillators. Gowns, gauze, drapes, sponges, oxygen masks, nasal cannulae, a patient’s hair or their clothing – all provide the necessary fuel.
But the key factor for surgical fire risk is the presence of high concentrations of oxygen.
Safety protocols
The best and most obvious way to mitigate risk is to reduce the amount of supplemental oxygen, explains Dr. Feldman.
“Many patients do not require a high concentration of oxygen during sedation,” he says.
When a patient does require a higher concentration for their safety, the APSF and ECRI recommend placing an endotracheal tube or supraglottic airway rather than using an oxygen mask or a nasal cannula. “You want to deliver the oxygen in such a way that high concentration doesn’t exist in the surgical field,” Dr. Feldman says. In cases where supplemental oxygen is necessary, ECRI and APSF recommend reducing the oxygen concentration to less than 30%.
In addition, safety protocols include giving flammable prep solutions time to dry before applying towels or drapes and beginning the procedure. These precautions to ensure the safety of patients take just a moment, says Chester H. Lake Jr, MD, MS, of the department of anesthesiology at the University of Mississippi Medical Center, Jackson.
Making fire safety part of the preop routine
These safety protocols are straightforward but not always observed, experts say. Part of the reason is a matter of culture. Both anesthesiologists and surgeons have absorbed the attitude that placing an airway escalates the procedure beyond what the patient needs, says Dr. Feldman. And indeed, according to a 2013 analysis of the American Society of Anesthesiologists closed claims database, 85% of surgical fires occur in outpatient settings where airways are less likely to be placed, and 81% of those claims were for procedures that used monitored anesthesia care.
In an article on prevention of surgical fires, Dr. Lake and colleagues recommend in-house education on preventing and responding to fires at least once a year. But it shouldn’t stop there. Because these fires – horrific as they are – are fairly rare, it’s important to maintain awareness. Making fire safety a regular part of the surgical “time-out” can help further reduce incidents, he says. ECRI and the APSF have teamed up to create a poster that can help surgical teams make fire safety a regular part of their routines.
Although the national decline in surgical fires is encouraging, the problem remains serious. “You can classify these incidents as low, but it’s not low if it happens to you or a family member,” says Dr. Lake. “One is too many.”
ECRI’s Ms. Malanga agrees. “I do like to emphasize that it’s rare,” she says. “But I’d like to see us reduce this until it’s zero.”
A version of this article originally appeared on Medscape.com.
‘Impressive’ results for intranasal ketamine in chronic, refractory migraine
Half of the study participants who used IN ketamine for chronic, treatment-refractory migraine in a new retrospective cohort study reported it as “very effective” and over one-third said it boosted their quality of life.
“In our study, we showed that with even a few uses per day, intranasal ketamine can still improve patients’ quality of life,” lead investigator Hsiangkuo Yuan, MD, PhD, said in an interview. Dr. Yuan is associate professor of neurology at Thomas Jefferson University, Philadelphia, and director of clinical research at the Jefferson Headache Center.
He added that “multiple medications failed these patients, and the majority of patients were having daily headaches. So, if anything works, even partially and shortly, it may still give patients some relief to get through the day.”
The findings were published online in Regional Anesthesia & Pain Medicine.
Daily migraine, failed medications
Use of IN ketamine has not been studied for the treatment of chronic, treatment-refractory migraine – although it has been studied in patients with cluster headache and migraine, the investigators note.
Ketamine is not yet approved by the Food and Drug Administration to treat migraine.
To further explore ketamine’s effect in those with chronic, treatment-refractory migraine, the investigators retrospectively analyzed electronic health records of patients at the Jefferson Headache Center who had received IN ketamine for the treatment of migraine between January 2019 and February 2020.
Of 242 patients who had received IN ketamine, Dr. Yuan’s team followed up with 169 who agreed to be part of the study.
The majority (67%) had daily migraine, and 85% had tried more than three classes of preventive medications for migraine. They currently used a median of two medications, the most common of which was a CGRP monoclonal antibody.
On average, patients used six sprays per day for a median 10 days per month. Median pain relief onset was 52 minutes after dosage.
Almost three-quarters of patients reported at least one side effect from the ketamine, most commonly fatigue (22%), double/blurred vision (21%), and confusion/dissociation (21%). These effects were mostly temporary, the researchers report.
The most common reasons for initiating IN ketamine included an incomplete response to prior acute medications (59%), incomplete response to prior preventive medications (31%), and prior benefit from IV ketamine (23%).
Study investigators noted that ketamine has the potential to become addictive and indicated that “clinicians should only consider the use of a potentially addictive medication such as ketamine for significantly disabled patients with migraine.”
About half of the participants who used IN ketamine found it “very effective,” and 40% found it “somewhat effective.” Within the same group, 36% and 43% found the overall impact of IN ketamine on their quality of life was much better and somewhat better, respectively.
Among those still using ketamine during study follow-up, 82% reported that ketamine was very effective.
Compared with other acute headache medications, IN ketamine was considered much better (43%) or somewhat better (30%).
Nearly 75% of participants reported using fewer pain relievers when using IN ketamine.
Dr. Yuan said that future research might focus on finding predictors for IN ketamine response or determining the optimal effective and safe dose for the drug in those with chronic, treatment-refractory migraine.
“We still need a prospective, randomized controlled trial to assess the efficacy and tolerability of intranasal ketamine,” he added.
‘Impressive result’
Commenting on the findings for this article, Richard Lipton, MD, professor of neurology, psychiatry and behavioral sciences and director of the Montefiore Headache Center at Albert Einstein College of Medicine, New York, said that “in this refractory population with multiple treatment failures, this is a very impressive, open-label result.”
“This real-world data suggests that ketamine is an effective option for people with medically intractable chronic migraine,” said Dr. Lipton, who was not part of the study. “In these very difficult to treat patients, 65% of those who started on ketamine persisted. Of those who remained on ketamine, 82% found it very effective.”
“This study makes me more confident that intranasal ketamine is a helpful treatment option, and I plan to use it more often in the future,” he added.
Like Dr. Yuan, Dr. Lipton highlighted the need for “well-designed placebo-controlled trials” and “rigorous comparative effectiveness studies.”
The study was funded by Miles for Migraine. Dr. Yuan has received institutional support for serving as an investigator from Teva and AbbVie, and royalties from Cambridge University Press and MedLink. Dr. Lipton has received compensation for consultation from Alder/Lumbeck, Axsome, Supernus, Theranica, Upsher-Smith, and Satsuma. He has participated in speaker bureaus for Eli Lilly and Amgen/Novartis and has received institutional support for serving as principal investigator from Teva, GammaCore, and Allergan/AbbVie. He has received payments for authorship or royalties from Demos Medical, Cambridge University Press, and MedLink.
A version of this article originally appeared on Medscape.com.
Half of the study participants who used IN ketamine for chronic, treatment-refractory migraine in a new retrospective cohort study reported it as “very effective” and over one-third said it boosted their quality of life.
“In our study, we showed that with even a few uses per day, intranasal ketamine can still improve patients’ quality of life,” lead investigator Hsiangkuo Yuan, MD, PhD, said in an interview. Dr. Yuan is associate professor of neurology at Thomas Jefferson University, Philadelphia, and director of clinical research at the Jefferson Headache Center.
He added that “multiple medications failed these patients, and the majority of patients were having daily headaches. So, if anything works, even partially and shortly, it may still give patients some relief to get through the day.”
The findings were published online in Regional Anesthesia & Pain Medicine.
Daily migraine, failed medications
Use of IN ketamine has not been studied for the treatment of chronic, treatment-refractory migraine – although it has been studied in patients with cluster headache and migraine, the investigators note.
Ketamine is not yet approved by the Food and Drug Administration to treat migraine.
To further explore ketamine’s effect in those with chronic, treatment-refractory migraine, the investigators retrospectively analyzed electronic health records of patients at the Jefferson Headache Center who had received IN ketamine for the treatment of migraine between January 2019 and February 2020.
Of 242 patients who had received IN ketamine, Dr. Yuan’s team followed up with 169 who agreed to be part of the study.
The majority (67%) had daily migraine, and 85% had tried more than three classes of preventive medications for migraine. They currently used a median of two medications, the most common of which was a CGRP monoclonal antibody.
On average, patients used six sprays per day for a median 10 days per month. Median pain relief onset was 52 minutes after dosage.
Almost three-quarters of patients reported at least one side effect from the ketamine, most commonly fatigue (22%), double/blurred vision (21%), and confusion/dissociation (21%). These effects were mostly temporary, the researchers report.
The most common reasons for initiating IN ketamine included an incomplete response to prior acute medications (59%), incomplete response to prior preventive medications (31%), and prior benefit from IV ketamine (23%).
Study investigators noted that ketamine has the potential to become addictive and indicated that “clinicians should only consider the use of a potentially addictive medication such as ketamine for significantly disabled patients with migraine.”
About half of the participants who used IN ketamine found it “very effective,” and 40% found it “somewhat effective.” Within the same group, 36% and 43% found the overall impact of IN ketamine on their quality of life was much better and somewhat better, respectively.
Among those still using ketamine during study follow-up, 82% reported that ketamine was very effective.
Compared with other acute headache medications, IN ketamine was considered much better (43%) or somewhat better (30%).
Nearly 75% of participants reported using fewer pain relievers when using IN ketamine.
Dr. Yuan said that future research might focus on finding predictors for IN ketamine response or determining the optimal effective and safe dose for the drug in those with chronic, treatment-refractory migraine.
“We still need a prospective, randomized controlled trial to assess the efficacy and tolerability of intranasal ketamine,” he added.
‘Impressive result’
Commenting on the findings for this article, Richard Lipton, MD, professor of neurology, psychiatry and behavioral sciences and director of the Montefiore Headache Center at Albert Einstein College of Medicine, New York, said that “in this refractory population with multiple treatment failures, this is a very impressive, open-label result.”
“This real-world data suggests that ketamine is an effective option for people with medically intractable chronic migraine,” said Dr. Lipton, who was not part of the study. “In these very difficult to treat patients, 65% of those who started on ketamine persisted. Of those who remained on ketamine, 82% found it very effective.”
“This study makes me more confident that intranasal ketamine is a helpful treatment option, and I plan to use it more often in the future,” he added.
Like Dr. Yuan, Dr. Lipton highlighted the need for “well-designed placebo-controlled trials” and “rigorous comparative effectiveness studies.”
The study was funded by Miles for Migraine. Dr. Yuan has received institutional support for serving as an investigator from Teva and AbbVie, and royalties from Cambridge University Press and MedLink. Dr. Lipton has received compensation for consultation from Alder/Lumbeck, Axsome, Supernus, Theranica, Upsher-Smith, and Satsuma. He has participated in speaker bureaus for Eli Lilly and Amgen/Novartis and has received institutional support for serving as principal investigator from Teva, GammaCore, and Allergan/AbbVie. He has received payments for authorship or royalties from Demos Medical, Cambridge University Press, and MedLink.
A version of this article originally appeared on Medscape.com.
Half of the study participants who used IN ketamine for chronic, treatment-refractory migraine in a new retrospective cohort study reported it as “very effective” and over one-third said it boosted their quality of life.
“In our study, we showed that with even a few uses per day, intranasal ketamine can still improve patients’ quality of life,” lead investigator Hsiangkuo Yuan, MD, PhD, said in an interview. Dr. Yuan is associate professor of neurology at Thomas Jefferson University, Philadelphia, and director of clinical research at the Jefferson Headache Center.
He added that “multiple medications failed these patients, and the majority of patients were having daily headaches. So, if anything works, even partially and shortly, it may still give patients some relief to get through the day.”
The findings were published online in Regional Anesthesia & Pain Medicine.
Daily migraine, failed medications
Use of IN ketamine has not been studied for the treatment of chronic, treatment-refractory migraine – although it has been studied in patients with cluster headache and migraine, the investigators note.
Ketamine is not yet approved by the Food and Drug Administration to treat migraine.
To further explore ketamine’s effect in those with chronic, treatment-refractory migraine, the investigators retrospectively analyzed electronic health records of patients at the Jefferson Headache Center who had received IN ketamine for the treatment of migraine between January 2019 and February 2020.
Of 242 patients who had received IN ketamine, Dr. Yuan’s team followed up with 169 who agreed to be part of the study.
The majority (67%) had daily migraine, and 85% had tried more than three classes of preventive medications for migraine. They currently used a median of two medications, the most common of which was a CGRP monoclonal antibody.
On average, patients used six sprays per day for a median 10 days per month. Median pain relief onset was 52 minutes after dosage.
Almost three-quarters of patients reported at least one side effect from the ketamine, most commonly fatigue (22%), double/blurred vision (21%), and confusion/dissociation (21%). These effects were mostly temporary, the researchers report.
The most common reasons for initiating IN ketamine included an incomplete response to prior acute medications (59%), incomplete response to prior preventive medications (31%), and prior benefit from IV ketamine (23%).
Study investigators noted that ketamine has the potential to become addictive and indicated that “clinicians should only consider the use of a potentially addictive medication such as ketamine for significantly disabled patients with migraine.”
About half of the participants who used IN ketamine found it “very effective,” and 40% found it “somewhat effective.” Within the same group, 36% and 43% found the overall impact of IN ketamine on their quality of life was much better and somewhat better, respectively.
Among those still using ketamine during study follow-up, 82% reported that ketamine was very effective.
Compared with other acute headache medications, IN ketamine was considered much better (43%) or somewhat better (30%).
Nearly 75% of participants reported using fewer pain relievers when using IN ketamine.
Dr. Yuan said that future research might focus on finding predictors for IN ketamine response or determining the optimal effective and safe dose for the drug in those with chronic, treatment-refractory migraine.
“We still need a prospective, randomized controlled trial to assess the efficacy and tolerability of intranasal ketamine,” he added.
‘Impressive result’
Commenting on the findings for this article, Richard Lipton, MD, professor of neurology, psychiatry and behavioral sciences and director of the Montefiore Headache Center at Albert Einstein College of Medicine, New York, said that “in this refractory population with multiple treatment failures, this is a very impressive, open-label result.”
“This real-world data suggests that ketamine is an effective option for people with medically intractable chronic migraine,” said Dr. Lipton, who was not part of the study. “In these very difficult to treat patients, 65% of those who started on ketamine persisted. Of those who remained on ketamine, 82% found it very effective.”
“This study makes me more confident that intranasal ketamine is a helpful treatment option, and I plan to use it more often in the future,” he added.
Like Dr. Yuan, Dr. Lipton highlighted the need for “well-designed placebo-controlled trials” and “rigorous comparative effectiveness studies.”
The study was funded by Miles for Migraine. Dr. Yuan has received institutional support for serving as an investigator from Teva and AbbVie, and royalties from Cambridge University Press and MedLink. Dr. Lipton has received compensation for consultation from Alder/Lumbeck, Axsome, Supernus, Theranica, Upsher-Smith, and Satsuma. He has participated in speaker bureaus for Eli Lilly and Amgen/Novartis and has received institutional support for serving as principal investigator from Teva, GammaCore, and Allergan/AbbVie. He has received payments for authorship or royalties from Demos Medical, Cambridge University Press, and MedLink.
A version of this article originally appeared on Medscape.com.
FROM REGIONAL ANESTHESIA & PAIN MEDICINE