User login
FDA approves first-ever OTC erectile dysfunction gel
The gel, which can help users get an erection within 10 minutes, is already available without a prescription in Europe.
The Food and Drug Administration has approved the drug, called Eroxon, noting that it is a first-of-its-kind treatment. Eroxon is made by the British pharmaceutical company Futura Medical, which specializes in drugs that are given through the skin.
According to the product’s leaflet, Eroxon “stimulates blood flow in the penis through a unique physical cooling then warming effect, helping you get and keep an erection hard enough for sex.” The company said on the product’s website that 65% of people who used the drug were able to have sex.
A company spokesperson told CNN that the price of the product has not been set in the United States, but it costs the equivalent of about $31 in the United Kingdom. Futura Medical has not announced when it will be available in the United States.
Harvard Health reports that 30 million people in the United States have erectile dysfunction, which means a person cannot get an erection at all or one firm enough to have sex. The disorder is often linked to other physical or mental health problems, such as heart problems or clogged arteries.
Erectile dysfunction affects 1% of men in their 40s, 17% of men in their 60s, and nearly 50% of men who are age 75 or older, according to Harvard Health.
A version of this article originally appeared on WebMD.com.
The gel, which can help users get an erection within 10 minutes, is already available without a prescription in Europe.
The Food and Drug Administration has approved the drug, called Eroxon, noting that it is a first-of-its-kind treatment. Eroxon is made by the British pharmaceutical company Futura Medical, which specializes in drugs that are given through the skin.
According to the product’s leaflet, Eroxon “stimulates blood flow in the penis through a unique physical cooling then warming effect, helping you get and keep an erection hard enough for sex.” The company said on the product’s website that 65% of people who used the drug were able to have sex.
A company spokesperson told CNN that the price of the product has not been set in the United States, but it costs the equivalent of about $31 in the United Kingdom. Futura Medical has not announced when it will be available in the United States.
Harvard Health reports that 30 million people in the United States have erectile dysfunction, which means a person cannot get an erection at all or one firm enough to have sex. The disorder is often linked to other physical or mental health problems, such as heart problems or clogged arteries.
Erectile dysfunction affects 1% of men in their 40s, 17% of men in their 60s, and nearly 50% of men who are age 75 or older, according to Harvard Health.
A version of this article originally appeared on WebMD.com.
The gel, which can help users get an erection within 10 minutes, is already available without a prescription in Europe.
The Food and Drug Administration has approved the drug, called Eroxon, noting that it is a first-of-its-kind treatment. Eroxon is made by the British pharmaceutical company Futura Medical, which specializes in drugs that are given through the skin.
According to the product’s leaflet, Eroxon “stimulates blood flow in the penis through a unique physical cooling then warming effect, helping you get and keep an erection hard enough for sex.” The company said on the product’s website that 65% of people who used the drug were able to have sex.
A company spokesperson told CNN that the price of the product has not been set in the United States, but it costs the equivalent of about $31 in the United Kingdom. Futura Medical has not announced when it will be available in the United States.
Harvard Health reports that 30 million people in the United States have erectile dysfunction, which means a person cannot get an erection at all or one firm enough to have sex. The disorder is often linked to other physical or mental health problems, such as heart problems or clogged arteries.
Erectile dysfunction affects 1% of men in their 40s, 17% of men in their 60s, and nearly 50% of men who are age 75 or older, according to Harvard Health.
A version of this article originally appeared on WebMD.com.
Report eyes complications from microwave energy devices for hyperhidrosis
database showed.
While microwave energy devices (MEDs) are used to treat hyperhidrosis, the largest MED clinical trial included only 101 patients, Samantha Jo Albucker and Shari Lipner, MD, PhD, wrote in a research letter reporting the results.
For the study, published online in the Journal of the American Academy of Dermatology, Ms. Albucker, a student at Tulane University, New Orleans, and Dr. Lipner, associate professor of clinical dermatology at Weill Cornell Medicine, New York, searched the MAUDE database between Feb. 28, 2013, and Dec. 29, 2022, for adverse events (AEs) involving MEDs for hyperhidrosis treatment. Of the 502 medical device reports identified over the study period, the axilla was the most frequent injury site in 50.4% of cases. The three most common complications were infections (45.4%); neurological symptoms including neuropathy, nerve damage, and numbness (21.7%); and burns/ulcerations/erosions (19.1%).
In other findings, 2.4% of patients required hospitalization, most often because of infection (83.3%), followed by burn and coma (8.3% each). The average symptom onset was 2 months postprocedure, and the most common treatment was antibiotics in 62.2% of cases, followed by incision and drainage/aspiration in 21.7% of cases.
A codiagnosis of hidradenitis suppurativa (HS) was reported in 5.4% of all medical device reports. The researchers noted that in a published randomized clinical trial of eight HS patients undergoing MED treatment to assess the effect on HS symptoms, the treatment showed no clinical advantage. In addition, they referred to two case reports describing new-onset HS after MED treatment for hyperhidrosis.
“Therefore, we recommend questioning patients about HS history and examining for HS clinical findings before performing MED for hyperhidrosis,” they wrote, adding that the data, “taken together, suggests that avoidance of MED treatment of hyperhidrosis in HS patients is prudent and alternative treatments may be prescribed.”
The researchers acknowledged certain limitations of their analysis, including uncompleted medical device reports, patient reporting, and unverified causes of adverse events. “Large multicenter studies are needed to corroborate our results,” they concluded.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that primary idiopathic hyperhidrosis is a common medical condition that is often overlooked as a legitimate concern, and causes a quality-of-life burden. “Even with the striking numbers in the millions, there are limited treatment options available for axillary let alone other forms of primary hyperhidrosis,” said Dr. Friedman, who was not involved with the study.
“Therefore, for the short treatment list we have, it is important to have some predictive power with respect to clinical impact to provide realistic expectations as well as potential adverse events to ensure best practices and meaningful patient guidance. In this research letter, our colleagues highlight complications that can ensue from microwave therapy for hyperhidrosis and the frequency of said adverse events. Knowing these data is half the battle, and I for one would not have assumed infection was number one on the list of adverse events.”
Ms. Albucker had no relevant conflicts of interest to disclose. Dr. Lipner disclosed that she has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, BelleTorus Corporation, and Moberg Pharmaceuticals.
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
database showed.
While microwave energy devices (MEDs) are used to treat hyperhidrosis, the largest MED clinical trial included only 101 patients, Samantha Jo Albucker and Shari Lipner, MD, PhD, wrote in a research letter reporting the results.
For the study, published online in the Journal of the American Academy of Dermatology, Ms. Albucker, a student at Tulane University, New Orleans, and Dr. Lipner, associate professor of clinical dermatology at Weill Cornell Medicine, New York, searched the MAUDE database between Feb. 28, 2013, and Dec. 29, 2022, for adverse events (AEs) involving MEDs for hyperhidrosis treatment. Of the 502 medical device reports identified over the study period, the axilla was the most frequent injury site in 50.4% of cases. The three most common complications were infections (45.4%); neurological symptoms including neuropathy, nerve damage, and numbness (21.7%); and burns/ulcerations/erosions (19.1%).
In other findings, 2.4% of patients required hospitalization, most often because of infection (83.3%), followed by burn and coma (8.3% each). The average symptom onset was 2 months postprocedure, and the most common treatment was antibiotics in 62.2% of cases, followed by incision and drainage/aspiration in 21.7% of cases.
A codiagnosis of hidradenitis suppurativa (HS) was reported in 5.4% of all medical device reports. The researchers noted that in a published randomized clinical trial of eight HS patients undergoing MED treatment to assess the effect on HS symptoms, the treatment showed no clinical advantage. In addition, they referred to two case reports describing new-onset HS after MED treatment for hyperhidrosis.
“Therefore, we recommend questioning patients about HS history and examining for HS clinical findings before performing MED for hyperhidrosis,” they wrote, adding that the data, “taken together, suggests that avoidance of MED treatment of hyperhidrosis in HS patients is prudent and alternative treatments may be prescribed.”
The researchers acknowledged certain limitations of their analysis, including uncompleted medical device reports, patient reporting, and unverified causes of adverse events. “Large multicenter studies are needed to corroborate our results,” they concluded.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that primary idiopathic hyperhidrosis is a common medical condition that is often overlooked as a legitimate concern, and causes a quality-of-life burden. “Even with the striking numbers in the millions, there are limited treatment options available for axillary let alone other forms of primary hyperhidrosis,” said Dr. Friedman, who was not involved with the study.
“Therefore, for the short treatment list we have, it is important to have some predictive power with respect to clinical impact to provide realistic expectations as well as potential adverse events to ensure best practices and meaningful patient guidance. In this research letter, our colleagues highlight complications that can ensue from microwave therapy for hyperhidrosis and the frequency of said adverse events. Knowing these data is half the battle, and I for one would not have assumed infection was number one on the list of adverse events.”
Ms. Albucker had no relevant conflicts of interest to disclose. Dr. Lipner disclosed that she has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, BelleTorus Corporation, and Moberg Pharmaceuticals.
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
database showed.
While microwave energy devices (MEDs) are used to treat hyperhidrosis, the largest MED clinical trial included only 101 patients, Samantha Jo Albucker and Shari Lipner, MD, PhD, wrote in a research letter reporting the results.
For the study, published online in the Journal of the American Academy of Dermatology, Ms. Albucker, a student at Tulane University, New Orleans, and Dr. Lipner, associate professor of clinical dermatology at Weill Cornell Medicine, New York, searched the MAUDE database between Feb. 28, 2013, and Dec. 29, 2022, for adverse events (AEs) involving MEDs for hyperhidrosis treatment. Of the 502 medical device reports identified over the study period, the axilla was the most frequent injury site in 50.4% of cases. The three most common complications were infections (45.4%); neurological symptoms including neuropathy, nerve damage, and numbness (21.7%); and burns/ulcerations/erosions (19.1%).
In other findings, 2.4% of patients required hospitalization, most often because of infection (83.3%), followed by burn and coma (8.3% each). The average symptom onset was 2 months postprocedure, and the most common treatment was antibiotics in 62.2% of cases, followed by incision and drainage/aspiration in 21.7% of cases.
A codiagnosis of hidradenitis suppurativa (HS) was reported in 5.4% of all medical device reports. The researchers noted that in a published randomized clinical trial of eight HS patients undergoing MED treatment to assess the effect on HS symptoms, the treatment showed no clinical advantage. In addition, they referred to two case reports describing new-onset HS after MED treatment for hyperhidrosis.
“Therefore, we recommend questioning patients about HS history and examining for HS clinical findings before performing MED for hyperhidrosis,” they wrote, adding that the data, “taken together, suggests that avoidance of MED treatment of hyperhidrosis in HS patients is prudent and alternative treatments may be prescribed.”
The researchers acknowledged certain limitations of their analysis, including uncompleted medical device reports, patient reporting, and unverified causes of adverse events. “Large multicenter studies are needed to corroborate our results,” they concluded.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that primary idiopathic hyperhidrosis is a common medical condition that is often overlooked as a legitimate concern, and causes a quality-of-life burden. “Even with the striking numbers in the millions, there are limited treatment options available for axillary let alone other forms of primary hyperhidrosis,” said Dr. Friedman, who was not involved with the study.
“Therefore, for the short treatment list we have, it is important to have some predictive power with respect to clinical impact to provide realistic expectations as well as potential adverse events to ensure best practices and meaningful patient guidance. In this research letter, our colleagues highlight complications that can ensue from microwave therapy for hyperhidrosis and the frequency of said adverse events. Knowing these data is half the battle, and I for one would not have assumed infection was number one on the list of adverse events.”
Ms. Albucker had no relevant conflicts of interest to disclose. Dr. Lipner disclosed that she has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, BelleTorus Corporation, and Moberg Pharmaceuticals.
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Cuffless blood pressure monitors: Still a numbers game
Medscape’s Editor-in-Chief Eric Topol, MD, referred to continual noninvasive, cuffless, accurate blood pressure devices as “a holy grail in sensor technology.”
He personally tested a cuff-calibrated, over-the-counter device available in Europe that claims to monitor daily blood pressure changes and produce data that can help physicians titrate medications.
Dr. Topol does not believe that it is ready for prime time. Yes, cuffless devices are easy to use, and generate lots of data. But are those data accurate?
Many experts say not yet, even as the market continues to grow and more devices are introduced and highlighted at high-profile consumer events.
Burned before
Limitations of cuffed devices are well known, including errors related to cuff size, patient positioning, patient habits or behaviors (for example, caffeine/nicotine use, acute meal digestion, full bladder, very recent physical activity) and clinicians’ failure to take accurate measurements.
Like many clinicians, Timothy B. Plante, MD, MHS, assistant professor at the University of Vermont Medical Center thrombosis & hemostasis program in Burlington, is very excited about cuffless technology. However, “we’ve been burned by it before,” he said in an interview.
Dr. Plante’s 2016 validation study of an instant blood pressure smartphone app found that its measurements were “highly inaccurate,” with such low sensitivity that more than three-quarters of individuals with hypertensive blood levels would be falsely reassured that their blood pressure was in the normal range.
His team’s 2023 review of the current landscape, which includes more sophisticated devices, concluded that accuracy remains an issue: “Unfortunately, the pace of regulation of these devices has failed to match the speed of innovation and direct availability to patient consumers. There is an urgent need to develop a consensus on standards by which cuffless BP devices can be tested for accuracy.”
Devices, indications differ
Cuffless devices estimate blood pressure indirectly. Most operate based on pulse wave analysis and pulse arrival time (PWA-PAT), explained Ramakrishna Mukkamala, PhD, in a commentary. Dr. Mukkamala is a professor in the departments of bioengineering and anesthesiology and perioperative medicine at the University of Pittsburgh.
PWA involves measuring a peripheral arterial waveform using an optical sensor such as the green lights on the back of a wrist-worn device, or a ‘force sensor’ such as a finger cuff or pressing on a smartphone. Certain features are extracted from the waveform using machine learning and calibrated to blood pressure values.
PAT techniques work together with PWA; they record the ECG and extract features from that signal as well as the arterial waveform for calibration to blood pressure values.
The algorithm used to generate the BP numbers comprises a proprietary baseline model that may include demographics and other patient characteristics. A cuff measurement is often part of the baseline model because most cuffless devices require periodic (typically weekly or monthly) calibration using a cuffed device.
Cuffless devices that require cuff calibration compare the estimate they get to the cuff-calibrated number. In this scenario, the cuffless device may come up with the same blood pressure numbers simply because the baseline model – which is made up of thousands of data points relevant to the patient – has not changed.
This has led some experts to question whether PWA-PAT cuffless device readings actually add anything to the baseline model.
They don’t, according to Microsoft Research in what Dr. Mukkamala and coauthors referred to (in a review published in Hypertension) as “a complex article describing perhaps the most important and highest resource project to date (Aurora Project) on assessing the accuracy of PWA and PWA devices.”
The Microsoft article was written for bioengineers. The review in Hypertension explains the project for clinicians, and concludes that, “Cuffless BP devices based on PWA and PWA-PAT, which are similar to some regulatory-cleared devices, were of no additional value in measuring auscultatory or 24-hour ambulatory cuff BP when compared with a baseline model in which BP was predicted without an actual measurement.”
IEEE and FDA validation
Despite these concerns, several cuffless devices using PWA and PAT have been cleared by the Food and Drug Administration.
Validating cuffless devices is no simple matter. The Institute of Electrical and Electronics Engineers published a validation protocol for cuffless blood pressure devices in 2014 that was amended in 2019 to include a requirement to evaluate performance in different positions and in the presence of motion with varying degrees of noise artifact.
However, Daichi Shimbo, MD, codirector of the Columbia Hypertension Center in New York and vice chair of the American Heart Association Statement on blood pressure monitoring, and colleagues point out limitations, even in the updated standard. These include not requiring evaluation for drift over time; lack of specific dynamic testing protocols for stressors such as exercise or environmental temperatures; and an unsuitable reference standard (oscillometric cuff-based devices) during movement.
Dr. Shimbo said in an interview that, although he is excited about them, “these cuffless devices are not aligned with regulatory bodies. If a device gives someone a wrong blood pressure, they might be diagnosed with hypertension when they don’t have it or might miss the fact that they’re hypertensive because they get a normal blood pressure reading. If there’s no yardstick by which you say these devices are good, what are we really doing – helping, or causing a problem?”
“The specifics of how a device estimates blood pressure can determine what testing is needed to ensure that it is providing accurate performance in the intended conditions of use,” Jeremy Kahn, an FDA press officer, said in an interview. “For example, for cuffless devices that are calibrated initially with a cuff-based blood pressure device, the cuffless device needs to specify the period over which it can provide accurate readings and have testing to demonstrate that it provides accurate results over that period of use.”
The FDA said its testing is different from what the Microsoft Aurora Project used in their study.
“The intent of that testing, as the agency understands it, is to evaluate whether the device is providing useful input based on the current physiology of the patient rather than relying on predetermined values based on calibration or patient attributes. We evaluate this clinically in two separate tests: an induced change in blood pressure test and tracking of natural blood pressure changes with longer term device use,” Mr. Kahn explained.
Analyzing a device’s performance on individuals who have had natural changes in blood pressure as compared to a calibration value or initial reading “can also help discern if the device is using physiological data from the patient to determine their blood pressure accurately,” he said.
Experts interviewed for this article who remain skeptical about cuffless BP monitoring question whether the numbers that appear during the induced blood pressure change, and with the natural blood pressure changes that may occur over time, accurately reflect a patient’s blood pressure.
“The FDA doesn’t approve these devices; they clear them,” Dr. Shimbo pointed out. “Clearing them means they can be sold to the general public in the U.S. It’s not a strong statement that they’re accurate.”
Moving toward validation, standards
Ultimately, cuffless BP monitors may require more than one validation protocol and standard, depending on their technology, how and where they will be used, and by whom.
And as Dr. Plante and colleagues write, “Importantly, validation should be performed in diverse and special populations, including pregnant women and individuals across a range of heart rates, skin tones, wrist sizes, common arrhythmias, and beta-blocker use.”
Organizations that might be expected to help move validation and standards forward have mostly remained silent. The American Medical Association’s US Blood Pressure Validated Device Listing website includes only cuffed devices, as does the website of the international scientific nonprofit STRIDE BP.
The European Society of Hypertension 2022 consensus statement on cuffless devices concluded that, until there is an internationally accepted accuracy standard and the devices have been tested in healthy people and those with suspected or diagnosed hypertension, “cuffless BP devices should not be used for the evaluation or management of hypertension in clinical practice.”
This month, ESH published recommendations for “specific, clinically meaningful, and pragmatic validation procedures for different types of intermittent cuffless devices” that will be presented at their upcoming annual meeting June 26.
Updated protocols from IEEE “are coming out soon,” according to Dr. Shimbo. The FDA says currently cleared devices won’t need to revalidate according to new standards unless the sponsor makes significant modifications in software algorithms, device hardware, or targeted patient populations.
Device makers take the initiative
In the face of conflicting reports on accuracy and lack of a robust standard, some device makers are publishing their own tests or encouraging validation by potential customers.
For example, institutions that are considering using the Biobeat cuffless blood pressure monitor watch “usually start with small pilots with our devices to do internal validation,” Lior Ben Shettrit, the company’s vice president of business development, said in an interview. “Only after they complete the internal validation are they willing to move forward to full implementation.”
Cardiologist Dean Nachman, MD, is leading validation studies of the Biobeat device at the Hadassah Ein Kerem Medical Center in Jerusalem. For the first validation, the team recruited 1,057 volunteers who did a single blood pressure measurement with the cuffless device and with a cuffed device.
“We found 96.3% agreement in identifying hypertension and an interclass correlation coefficient of 0.99 and 0.97 for systolic and diastolic measurements, respectively,” he said. “Then we took it to the next level and compared the device to ambulatory 24-hour blood pressure monitoring and found comparable measurements.”
The investigators are not done yet. “We need data from thousands of patients, with subgroups, to not have any concerns,” he says. “Right now, we are using the device as a general monitor – as an EKG plus heart rate plus oxygen saturation level monitor – and as a blood pressure monitor for 24-hour blood pressure monitoring.”
The developers of the Aktiia device, which is the one Dr. Topol tested, take a different perspective. “When somebody introduces a new technology that is disrupting something that has been in place for over 100 years, there will always be some grumblings, ruffling of feathers, people saying it’s not ready, it’s not ready, it’s not ready,” Aktiia’s chief medical officer Jay Shah, MD, noted.
“But a lot of those comments are coming from the isolation of an ivory tower,” he said.
Aktiia cofounder and chief technology officer Josep Solà said that “no device is probably as accurate as if you have an invasive catheter,” adding that “we engage patients to look at their blood pressure day by day. … If each individual measurement of each of those patient is slightly less accurate than a cuff, who cares? We have 40 measurements per day on each patient. The accuracy and precision of each of those is good.”
Researchers from the George Institute for Global Health recently compared the Aktiia device to conventional ambulatory monitoring in 41 patients and found that “it did not accurately track night-time BP decline and results suggested it was unable to track medication-induced BP changes.”
“In the context of 24/7 monitoring of hypertensive patients,” Mr. Solà said, “whatever you do, if it’s better than a sham device or a baseline model and you track the blood pressure changes, it’s a hundred times much better than doing nothing.”
Dr. Nachman and Dr. Plante reported no relevant financial relationships. Dr. Shimbo reported that he received funding from NIH and has consulted for Abbott Vascular, Edward Lifesciences, Medtronic, and Tryton Medical.
A version of this article first appeared on Medscape.com.
Medscape’s Editor-in-Chief Eric Topol, MD, referred to continual noninvasive, cuffless, accurate blood pressure devices as “a holy grail in sensor technology.”
He personally tested a cuff-calibrated, over-the-counter device available in Europe that claims to monitor daily blood pressure changes and produce data that can help physicians titrate medications.
Dr. Topol does not believe that it is ready for prime time. Yes, cuffless devices are easy to use, and generate lots of data. But are those data accurate?
Many experts say not yet, even as the market continues to grow and more devices are introduced and highlighted at high-profile consumer events.
Burned before
Limitations of cuffed devices are well known, including errors related to cuff size, patient positioning, patient habits or behaviors (for example, caffeine/nicotine use, acute meal digestion, full bladder, very recent physical activity) and clinicians’ failure to take accurate measurements.
Like many clinicians, Timothy B. Plante, MD, MHS, assistant professor at the University of Vermont Medical Center thrombosis & hemostasis program in Burlington, is very excited about cuffless technology. However, “we’ve been burned by it before,” he said in an interview.
Dr. Plante’s 2016 validation study of an instant blood pressure smartphone app found that its measurements were “highly inaccurate,” with such low sensitivity that more than three-quarters of individuals with hypertensive blood levels would be falsely reassured that their blood pressure was in the normal range.
His team’s 2023 review of the current landscape, which includes more sophisticated devices, concluded that accuracy remains an issue: “Unfortunately, the pace of regulation of these devices has failed to match the speed of innovation and direct availability to patient consumers. There is an urgent need to develop a consensus on standards by which cuffless BP devices can be tested for accuracy.”
Devices, indications differ
Cuffless devices estimate blood pressure indirectly. Most operate based on pulse wave analysis and pulse arrival time (PWA-PAT), explained Ramakrishna Mukkamala, PhD, in a commentary. Dr. Mukkamala is a professor in the departments of bioengineering and anesthesiology and perioperative medicine at the University of Pittsburgh.
PWA involves measuring a peripheral arterial waveform using an optical sensor such as the green lights on the back of a wrist-worn device, or a ‘force sensor’ such as a finger cuff or pressing on a smartphone. Certain features are extracted from the waveform using machine learning and calibrated to blood pressure values.
PAT techniques work together with PWA; they record the ECG and extract features from that signal as well as the arterial waveform for calibration to blood pressure values.
The algorithm used to generate the BP numbers comprises a proprietary baseline model that may include demographics and other patient characteristics. A cuff measurement is often part of the baseline model because most cuffless devices require periodic (typically weekly or monthly) calibration using a cuffed device.
Cuffless devices that require cuff calibration compare the estimate they get to the cuff-calibrated number. In this scenario, the cuffless device may come up with the same blood pressure numbers simply because the baseline model – which is made up of thousands of data points relevant to the patient – has not changed.
This has led some experts to question whether PWA-PAT cuffless device readings actually add anything to the baseline model.
They don’t, according to Microsoft Research in what Dr. Mukkamala and coauthors referred to (in a review published in Hypertension) as “a complex article describing perhaps the most important and highest resource project to date (Aurora Project) on assessing the accuracy of PWA and PWA devices.”
The Microsoft article was written for bioengineers. The review in Hypertension explains the project for clinicians, and concludes that, “Cuffless BP devices based on PWA and PWA-PAT, which are similar to some regulatory-cleared devices, were of no additional value in measuring auscultatory or 24-hour ambulatory cuff BP when compared with a baseline model in which BP was predicted without an actual measurement.”
IEEE and FDA validation
Despite these concerns, several cuffless devices using PWA and PAT have been cleared by the Food and Drug Administration.
Validating cuffless devices is no simple matter. The Institute of Electrical and Electronics Engineers published a validation protocol for cuffless blood pressure devices in 2014 that was amended in 2019 to include a requirement to evaluate performance in different positions and in the presence of motion with varying degrees of noise artifact.
However, Daichi Shimbo, MD, codirector of the Columbia Hypertension Center in New York and vice chair of the American Heart Association Statement on blood pressure monitoring, and colleagues point out limitations, even in the updated standard. These include not requiring evaluation for drift over time; lack of specific dynamic testing protocols for stressors such as exercise or environmental temperatures; and an unsuitable reference standard (oscillometric cuff-based devices) during movement.
Dr. Shimbo said in an interview that, although he is excited about them, “these cuffless devices are not aligned with regulatory bodies. If a device gives someone a wrong blood pressure, they might be diagnosed with hypertension when they don’t have it or might miss the fact that they’re hypertensive because they get a normal blood pressure reading. If there’s no yardstick by which you say these devices are good, what are we really doing – helping, or causing a problem?”
“The specifics of how a device estimates blood pressure can determine what testing is needed to ensure that it is providing accurate performance in the intended conditions of use,” Jeremy Kahn, an FDA press officer, said in an interview. “For example, for cuffless devices that are calibrated initially with a cuff-based blood pressure device, the cuffless device needs to specify the period over which it can provide accurate readings and have testing to demonstrate that it provides accurate results over that period of use.”
The FDA said its testing is different from what the Microsoft Aurora Project used in their study.
“The intent of that testing, as the agency understands it, is to evaluate whether the device is providing useful input based on the current physiology of the patient rather than relying on predetermined values based on calibration or patient attributes. We evaluate this clinically in two separate tests: an induced change in blood pressure test and tracking of natural blood pressure changes with longer term device use,” Mr. Kahn explained.
Analyzing a device’s performance on individuals who have had natural changes in blood pressure as compared to a calibration value or initial reading “can also help discern if the device is using physiological data from the patient to determine their blood pressure accurately,” he said.
Experts interviewed for this article who remain skeptical about cuffless BP monitoring question whether the numbers that appear during the induced blood pressure change, and with the natural blood pressure changes that may occur over time, accurately reflect a patient’s blood pressure.
“The FDA doesn’t approve these devices; they clear them,” Dr. Shimbo pointed out. “Clearing them means they can be sold to the general public in the U.S. It’s not a strong statement that they’re accurate.”
Moving toward validation, standards
Ultimately, cuffless BP monitors may require more than one validation protocol and standard, depending on their technology, how and where they will be used, and by whom.
And as Dr. Plante and colleagues write, “Importantly, validation should be performed in diverse and special populations, including pregnant women and individuals across a range of heart rates, skin tones, wrist sizes, common arrhythmias, and beta-blocker use.”
Organizations that might be expected to help move validation and standards forward have mostly remained silent. The American Medical Association’s US Blood Pressure Validated Device Listing website includes only cuffed devices, as does the website of the international scientific nonprofit STRIDE BP.
The European Society of Hypertension 2022 consensus statement on cuffless devices concluded that, until there is an internationally accepted accuracy standard and the devices have been tested in healthy people and those with suspected or diagnosed hypertension, “cuffless BP devices should not be used for the evaluation or management of hypertension in clinical practice.”
This month, ESH published recommendations for “specific, clinically meaningful, and pragmatic validation procedures for different types of intermittent cuffless devices” that will be presented at their upcoming annual meeting June 26.
Updated protocols from IEEE “are coming out soon,” according to Dr. Shimbo. The FDA says currently cleared devices won’t need to revalidate according to new standards unless the sponsor makes significant modifications in software algorithms, device hardware, or targeted patient populations.
Device makers take the initiative
In the face of conflicting reports on accuracy and lack of a robust standard, some device makers are publishing their own tests or encouraging validation by potential customers.
For example, institutions that are considering using the Biobeat cuffless blood pressure monitor watch “usually start with small pilots with our devices to do internal validation,” Lior Ben Shettrit, the company’s vice president of business development, said in an interview. “Only after they complete the internal validation are they willing to move forward to full implementation.”
Cardiologist Dean Nachman, MD, is leading validation studies of the Biobeat device at the Hadassah Ein Kerem Medical Center in Jerusalem. For the first validation, the team recruited 1,057 volunteers who did a single blood pressure measurement with the cuffless device and with a cuffed device.
“We found 96.3% agreement in identifying hypertension and an interclass correlation coefficient of 0.99 and 0.97 for systolic and diastolic measurements, respectively,” he said. “Then we took it to the next level and compared the device to ambulatory 24-hour blood pressure monitoring and found comparable measurements.”
The investigators are not done yet. “We need data from thousands of patients, with subgroups, to not have any concerns,” he says. “Right now, we are using the device as a general monitor – as an EKG plus heart rate plus oxygen saturation level monitor – and as a blood pressure monitor for 24-hour blood pressure monitoring.”
The developers of the Aktiia device, which is the one Dr. Topol tested, take a different perspective. “When somebody introduces a new technology that is disrupting something that has been in place for over 100 years, there will always be some grumblings, ruffling of feathers, people saying it’s not ready, it’s not ready, it’s not ready,” Aktiia’s chief medical officer Jay Shah, MD, noted.
“But a lot of those comments are coming from the isolation of an ivory tower,” he said.
Aktiia cofounder and chief technology officer Josep Solà said that “no device is probably as accurate as if you have an invasive catheter,” adding that “we engage patients to look at their blood pressure day by day. … If each individual measurement of each of those patient is slightly less accurate than a cuff, who cares? We have 40 measurements per day on each patient. The accuracy and precision of each of those is good.”
Researchers from the George Institute for Global Health recently compared the Aktiia device to conventional ambulatory monitoring in 41 patients and found that “it did not accurately track night-time BP decline and results suggested it was unable to track medication-induced BP changes.”
“In the context of 24/7 monitoring of hypertensive patients,” Mr. Solà said, “whatever you do, if it’s better than a sham device or a baseline model and you track the blood pressure changes, it’s a hundred times much better than doing nothing.”
Dr. Nachman and Dr. Plante reported no relevant financial relationships. Dr. Shimbo reported that he received funding from NIH and has consulted for Abbott Vascular, Edward Lifesciences, Medtronic, and Tryton Medical.
A version of this article first appeared on Medscape.com.
Medscape’s Editor-in-Chief Eric Topol, MD, referred to continual noninvasive, cuffless, accurate blood pressure devices as “a holy grail in sensor technology.”
He personally tested a cuff-calibrated, over-the-counter device available in Europe that claims to monitor daily blood pressure changes and produce data that can help physicians titrate medications.
Dr. Topol does not believe that it is ready for prime time. Yes, cuffless devices are easy to use, and generate lots of data. But are those data accurate?
Many experts say not yet, even as the market continues to grow and more devices are introduced and highlighted at high-profile consumer events.
Burned before
Limitations of cuffed devices are well known, including errors related to cuff size, patient positioning, patient habits or behaviors (for example, caffeine/nicotine use, acute meal digestion, full bladder, very recent physical activity) and clinicians’ failure to take accurate measurements.
Like many clinicians, Timothy B. Plante, MD, MHS, assistant professor at the University of Vermont Medical Center thrombosis & hemostasis program in Burlington, is very excited about cuffless technology. However, “we’ve been burned by it before,” he said in an interview.
Dr. Plante’s 2016 validation study of an instant blood pressure smartphone app found that its measurements were “highly inaccurate,” with such low sensitivity that more than three-quarters of individuals with hypertensive blood levels would be falsely reassured that their blood pressure was in the normal range.
His team’s 2023 review of the current landscape, which includes more sophisticated devices, concluded that accuracy remains an issue: “Unfortunately, the pace of regulation of these devices has failed to match the speed of innovation and direct availability to patient consumers. There is an urgent need to develop a consensus on standards by which cuffless BP devices can be tested for accuracy.”
Devices, indications differ
Cuffless devices estimate blood pressure indirectly. Most operate based on pulse wave analysis and pulse arrival time (PWA-PAT), explained Ramakrishna Mukkamala, PhD, in a commentary. Dr. Mukkamala is a professor in the departments of bioengineering and anesthesiology and perioperative medicine at the University of Pittsburgh.
PWA involves measuring a peripheral arterial waveform using an optical sensor such as the green lights on the back of a wrist-worn device, or a ‘force sensor’ such as a finger cuff or pressing on a smartphone. Certain features are extracted from the waveform using machine learning and calibrated to blood pressure values.
PAT techniques work together with PWA; they record the ECG and extract features from that signal as well as the arterial waveform for calibration to blood pressure values.
The algorithm used to generate the BP numbers comprises a proprietary baseline model that may include demographics and other patient characteristics. A cuff measurement is often part of the baseline model because most cuffless devices require periodic (typically weekly or monthly) calibration using a cuffed device.
Cuffless devices that require cuff calibration compare the estimate they get to the cuff-calibrated number. In this scenario, the cuffless device may come up with the same blood pressure numbers simply because the baseline model – which is made up of thousands of data points relevant to the patient – has not changed.
This has led some experts to question whether PWA-PAT cuffless device readings actually add anything to the baseline model.
They don’t, according to Microsoft Research in what Dr. Mukkamala and coauthors referred to (in a review published in Hypertension) as “a complex article describing perhaps the most important and highest resource project to date (Aurora Project) on assessing the accuracy of PWA and PWA devices.”
The Microsoft article was written for bioengineers. The review in Hypertension explains the project for clinicians, and concludes that, “Cuffless BP devices based on PWA and PWA-PAT, which are similar to some regulatory-cleared devices, were of no additional value in measuring auscultatory or 24-hour ambulatory cuff BP when compared with a baseline model in which BP was predicted without an actual measurement.”
IEEE and FDA validation
Despite these concerns, several cuffless devices using PWA and PAT have been cleared by the Food and Drug Administration.
Validating cuffless devices is no simple matter. The Institute of Electrical and Electronics Engineers published a validation protocol for cuffless blood pressure devices in 2014 that was amended in 2019 to include a requirement to evaluate performance in different positions and in the presence of motion with varying degrees of noise artifact.
However, Daichi Shimbo, MD, codirector of the Columbia Hypertension Center in New York and vice chair of the American Heart Association Statement on blood pressure monitoring, and colleagues point out limitations, even in the updated standard. These include not requiring evaluation for drift over time; lack of specific dynamic testing protocols for stressors such as exercise or environmental temperatures; and an unsuitable reference standard (oscillometric cuff-based devices) during movement.
Dr. Shimbo said in an interview that, although he is excited about them, “these cuffless devices are not aligned with regulatory bodies. If a device gives someone a wrong blood pressure, they might be diagnosed with hypertension when they don’t have it or might miss the fact that they’re hypertensive because they get a normal blood pressure reading. If there’s no yardstick by which you say these devices are good, what are we really doing – helping, or causing a problem?”
“The specifics of how a device estimates blood pressure can determine what testing is needed to ensure that it is providing accurate performance in the intended conditions of use,” Jeremy Kahn, an FDA press officer, said in an interview. “For example, for cuffless devices that are calibrated initially with a cuff-based blood pressure device, the cuffless device needs to specify the period over which it can provide accurate readings and have testing to demonstrate that it provides accurate results over that period of use.”
The FDA said its testing is different from what the Microsoft Aurora Project used in their study.
“The intent of that testing, as the agency understands it, is to evaluate whether the device is providing useful input based on the current physiology of the patient rather than relying on predetermined values based on calibration or patient attributes. We evaluate this clinically in two separate tests: an induced change in blood pressure test and tracking of natural blood pressure changes with longer term device use,” Mr. Kahn explained.
Analyzing a device’s performance on individuals who have had natural changes in blood pressure as compared to a calibration value or initial reading “can also help discern if the device is using physiological data from the patient to determine their blood pressure accurately,” he said.
Experts interviewed for this article who remain skeptical about cuffless BP monitoring question whether the numbers that appear during the induced blood pressure change, and with the natural blood pressure changes that may occur over time, accurately reflect a patient’s blood pressure.
“The FDA doesn’t approve these devices; they clear them,” Dr. Shimbo pointed out. “Clearing them means they can be sold to the general public in the U.S. It’s not a strong statement that they’re accurate.”
Moving toward validation, standards
Ultimately, cuffless BP monitors may require more than one validation protocol and standard, depending on their technology, how and where they will be used, and by whom.
And as Dr. Plante and colleagues write, “Importantly, validation should be performed in diverse and special populations, including pregnant women and individuals across a range of heart rates, skin tones, wrist sizes, common arrhythmias, and beta-blocker use.”
Organizations that might be expected to help move validation and standards forward have mostly remained silent. The American Medical Association’s US Blood Pressure Validated Device Listing website includes only cuffed devices, as does the website of the international scientific nonprofit STRIDE BP.
The European Society of Hypertension 2022 consensus statement on cuffless devices concluded that, until there is an internationally accepted accuracy standard and the devices have been tested in healthy people and those with suspected or diagnosed hypertension, “cuffless BP devices should not be used for the evaluation or management of hypertension in clinical practice.”
This month, ESH published recommendations for “specific, clinically meaningful, and pragmatic validation procedures for different types of intermittent cuffless devices” that will be presented at their upcoming annual meeting June 26.
Updated protocols from IEEE “are coming out soon,” according to Dr. Shimbo. The FDA says currently cleared devices won’t need to revalidate according to new standards unless the sponsor makes significant modifications in software algorithms, device hardware, or targeted patient populations.
Device makers take the initiative
In the face of conflicting reports on accuracy and lack of a robust standard, some device makers are publishing their own tests or encouraging validation by potential customers.
For example, institutions that are considering using the Biobeat cuffless blood pressure monitor watch “usually start with small pilots with our devices to do internal validation,” Lior Ben Shettrit, the company’s vice president of business development, said in an interview. “Only after they complete the internal validation are they willing to move forward to full implementation.”
Cardiologist Dean Nachman, MD, is leading validation studies of the Biobeat device at the Hadassah Ein Kerem Medical Center in Jerusalem. For the first validation, the team recruited 1,057 volunteers who did a single blood pressure measurement with the cuffless device and with a cuffed device.
“We found 96.3% agreement in identifying hypertension and an interclass correlation coefficient of 0.99 and 0.97 for systolic and diastolic measurements, respectively,” he said. “Then we took it to the next level and compared the device to ambulatory 24-hour blood pressure monitoring and found comparable measurements.”
The investigators are not done yet. “We need data from thousands of patients, with subgroups, to not have any concerns,” he says. “Right now, we are using the device as a general monitor – as an EKG plus heart rate plus oxygen saturation level monitor – and as a blood pressure monitor for 24-hour blood pressure monitoring.”
The developers of the Aktiia device, which is the one Dr. Topol tested, take a different perspective. “When somebody introduces a new technology that is disrupting something that has been in place for over 100 years, there will always be some grumblings, ruffling of feathers, people saying it’s not ready, it’s not ready, it’s not ready,” Aktiia’s chief medical officer Jay Shah, MD, noted.
“But a lot of those comments are coming from the isolation of an ivory tower,” he said.
Aktiia cofounder and chief technology officer Josep Solà said that “no device is probably as accurate as if you have an invasive catheter,” adding that “we engage patients to look at their blood pressure day by day. … If each individual measurement of each of those patient is slightly less accurate than a cuff, who cares? We have 40 measurements per day on each patient. The accuracy and precision of each of those is good.”
Researchers from the George Institute for Global Health recently compared the Aktiia device to conventional ambulatory monitoring in 41 patients and found that “it did not accurately track night-time BP decline and results suggested it was unable to track medication-induced BP changes.”
“In the context of 24/7 monitoring of hypertensive patients,” Mr. Solà said, “whatever you do, if it’s better than a sham device or a baseline model and you track the blood pressure changes, it’s a hundred times much better than doing nothing.”
Dr. Nachman and Dr. Plante reported no relevant financial relationships. Dr. Shimbo reported that he received funding from NIH and has consulted for Abbott Vascular, Edward Lifesciences, Medtronic, and Tryton Medical.
A version of this article first appeared on Medscape.com.
Increase in message volume begs the question: ‘Should we be compensated for our time?’
The American Gastroenterological Association and other gastrointestinal-specific organizations have excellent resources available to members that focus on optimizing reimbursement in your clinical and endoscopic practice.
During the COVID-19 pandemic and public health emergency (PHE), many previously noncovered services were now covered under rules of the Centers for Medicare & Medicaid Services. During the pandemic, patient portal messages increased by 157%, meaning more work for health care teams, negatively impacting physician satisfaction, and increasing burnout.1 Medical burnout has been associated with increased time spent on electronic health records, with some subspeciality gastroenterology (GI) groups having a high EHR burden, according to a recently published article in the American Journal of Gastroenterology.2
This topic is a timely discussion as several large health systems have implemented processes to bill for non–face-to-face services (termed “asynchronous care”), some of which have not been well received in the lay media. It is important to note that despite these implementations, studies have shown only 1% of all incoming portal messages would meet criteria to be submitted for reimbursement. This impact might be slightly higher in chronic care management practices.
Providers and practices have several options when considering billing for non–face-to-face encounters, which we outline in Table 1.3
The focus of this article will be to review the more common non–face-to-face evaluation and management services, such as telephone E/M (patient phone call) and e-visits (patient portal messages) as these have recently generated the most interest and discussion amongst health care providers.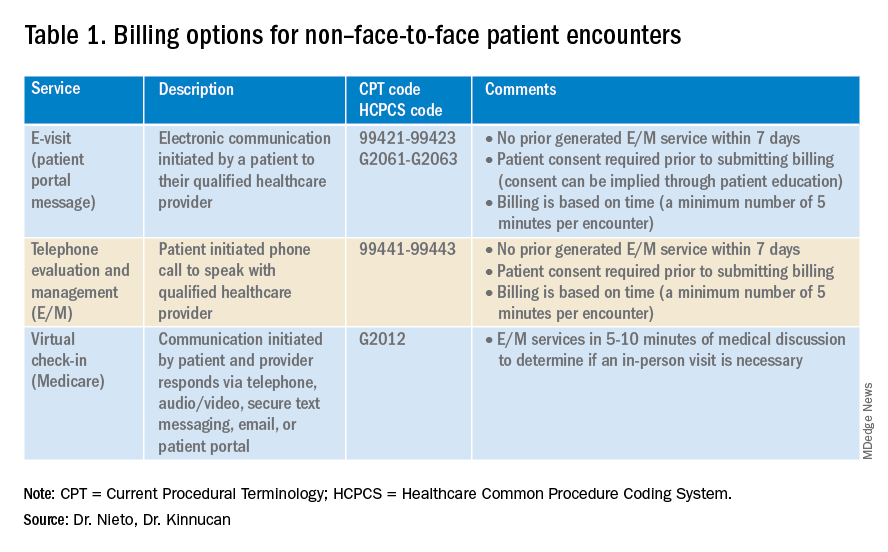
Telemedicine after COVID-19 pandemic
During the beginning of the pandemic, a web-based survey study found that almost all providers in GI practices implemented some form of telemedicine to continue to provide care for patients, compared to 32% prior to the pandemic.4,5 The high demand and essential requirement for telehealth evaluation facilitated its reimbursement, eliminating the primary barrier to previous use.6
One of the new covered benefits by CMS was asynchronous telehealth care.7 The PHE ended in May 2023, and since then a qualified health care provider (QHCP) does not have the full flexibility to deliver telemedicine services across state lines. The U.S. Department of Health and Human Services has considered some telehealth policy changes after the COVID-19 PHE and many of those will be extended, at least through 2024.8 As during the pandemic, where the U.S. national payer network (CMS, state Medicaid, and private payers) and state health agencies assisted to ensure patients get the care they need by authorizing providers to be compensated for non–face-to-face services, we believe this service will continue to be part of our clinical practice.
We recommend you stay informed about local and federal laws, regulations, and alternatives for reimbursement as they may be modified at the beginning of a new calendar year. Remember, you can always talk with your revenue cycle team to clarify any query.
Telephone evaluation and management services
The patient requests to speak with you.
Telephone evaluation and management services became more widely used after the pandemic and were recognized by CMS as a covered medical service under PHE. As outlined in Table 1, there are associated codes with this service and it can only apply to an established patient in your practice. The cumulative time spent over a 7-day period without generating an immediate follow-up visit could qualify for this CPT code. However, for a patient with a high-complexity diagnosis and/or decisions being made about care, it might be better to consider a virtual office visit as this would value the complex care at a higher level than the time spent during the telephone E/M encounter.
A common question comes up: Can my nurse or support team bill for telephone care? No, only QHCP can, which means physicians and advanced practice providers can bill for this E/M service, and it does not include time spent by other members of clinical staff in patient care. However, there are CPT codes for chronic care management, which is not covered in this article.
Virtual evaluation and management services
You respond to a patient-initiated portal message.
Patient portal messages increased exponentially during the pandemic with 2.5 more minutes spent per message, resulting in more EHR work by practitioners, compared with prior to the pandemic. One study showed an immediate postpandemic increase in EHR patient-initiated messages with no return to prepandemic baseline.1
Although studies evaluating postpandemic telemedicine services are needed, we believe that this trend will continue, and for this reason, it is important to create sustainable workflows to continue to provide this patient driven avenue of care.9
E-visits are asynchronous patient or guardian portal messages that require a minimum of 5 minutes to provide medical decision-making without prior E/M services in the last 7 days. To obtain reimbursement for this service, it cannot be initiated by the provider, and patient consent must be obtained. Documentation should include this information and the time spent in the encounter. The associated CPT codes with this e-service are outlined in Table 1.
A common question is, “Are there additional codes I should use if a portal message E/M visit lasts more than 30 minutes?” No. If an e-visit lasts more than 30 minutes, the QHCP should bill the CPT code 99423. However, we would advise that, if this care requires more than 30 minutes, then either virtual or face-to-face E/M be considered for the optimal reimbursement for provider time spent. Another common question is around consent for services, and we advise providers to review this requirement with their compliance colleagues as each institution has different policies.
Virtual check-in
Medicare also covers brief communication technology–based services also known as virtual check-ins, where patients can communicate with their provider after having established care. During this brief conversation that can be via telephone, audio/video, secure text messaging, email, or patient portal, providers will determine if an in-person visit is necessary. CMS has designed G codes for these virtual check-ins that are from the Healthcare Common Procedure Coding System (HCPCS). Two codes are available for this E/M service: G2012, which is outlined in Table 1, and G2010, which covers the evaluation of images and/or recorded videos. In order to be reimbursed for a G2010 code, providers need at least a 5-minute response to make a clinical determination or give the patient a medical impression.
Patient satisfaction, physician well-being and quality of care outcomes
Large health care systems like Kaiser Permanente implemented secure message patient-physician communication (the patient portal) even before the pandemic, showing promising results in 2010 with reduction in office visits, improvement in measurable quality outcomes, and high level of patient satisfaction.10 Post pandemic, several large health care centers opted to announce the billing implementation for patient-initiated portal messages.11 A focus was placed on educating their patients about when a message will and will not be billed. Using this type of strategy can help to improve patient awareness about potential billing without affecting patient satisfaction and care outcomes. Studies have shown the EHR has contributed to physician burnout and some physicians reducing their clinical time or leaving medicine; a reduction in messaging might have a positive impact on physician well-being.
The challenge is that medical billing is not routinely included as a curriculum topic in many residency and fellowship programs; however, trainees are part of E/M services and have limited knowledge of billing processes. Unfortunately, at this time, trainees cannot submit for reimbursement for asynchronous care as described above. We hope that this brief article will help junior gastroenterologists optimize their outpatient billing practices.
Dr. Nieto is an internal medicine chief resident with WellStar Cobb Medical Center, Austell, Ga. Dr. Kinnucan is a gastroenterologist with Mayo Clinic, Jacksonville, Fla. The authors have no conflicts of interest to disclose for this article. The authors certify that no financial and grant support has been received for this article.
References
1. Holmgren AJ et al. J Am Med Inform Assoc. 2021 Dec 9. doi: 10.1093/jamia/ocab268.
2. Bali AS et al. Am J Gastroenterol. 2023 Apr 24. doi: 10.14309/ajg.0000000000002254.
3. AAFP. Family Physician. “Coding Scenario: Coding for Virtual-Digital Visits”
4. Keihanian T. et al. Telehealth Utilization in Gastroenterology Clinics Amid the COVID-19 Pandemic: Impact on Clinical Practice and Gastroenterology Training. Gastroenterology. 2020 Jun 20. doi: 10.1053/j.gastro.2020.06.040.
5. Lewin S et al. J Crohns Colitis. 2020 Oct 21. doi: 10.1093/ecco-jcc/jjaa140.
6. Perisetti A and H Goyal. Dig Dis Sci. 2021 Mar 3. doi: 10.1007/s10620-021-06874-x.
7. Telehealth.HHS.gov. Medicaid and Medicare billing for asynchronous telehealth. Updated: 2022 May 4.
8. Telehealth.HHS.gov. Telehealth policy changes after the COVID-19 public health emergency. Last updated: 2023 Jan 23.
9. Fox B and Sizemore JO. Telehealth: Fad or the future. Epic Health Research Network. 2020 Aug 18.
10. Baer D. Patient-physician e-mail communication: the kaiser permanente experience. J Oncol Pract. 2011 Jul. doi: 10.1200/JOP.2011.000323.
11. Myclevelandclinic.org. MyChart Messaging.
12. Sinsky CA et al. J Gen Intern Med. 2022 Aug 29. doi: 10.1007/s11606-022-07766-0.
The American Gastroenterological Association and other gastrointestinal-specific organizations have excellent resources available to members that focus on optimizing reimbursement in your clinical and endoscopic practice.
During the COVID-19 pandemic and public health emergency (PHE), many previously noncovered services were now covered under rules of the Centers for Medicare & Medicaid Services. During the pandemic, patient portal messages increased by 157%, meaning more work for health care teams, negatively impacting physician satisfaction, and increasing burnout.1 Medical burnout has been associated with increased time spent on electronic health records, with some subspeciality gastroenterology (GI) groups having a high EHR burden, according to a recently published article in the American Journal of Gastroenterology.2
This topic is a timely discussion as several large health systems have implemented processes to bill for non–face-to-face services (termed “asynchronous care”), some of which have not been well received in the lay media. It is important to note that despite these implementations, studies have shown only 1% of all incoming portal messages would meet criteria to be submitted for reimbursement. This impact might be slightly higher in chronic care management practices.
Providers and practices have several options when considering billing for non–face-to-face encounters, which we outline in Table 1.3
The focus of this article will be to review the more common non–face-to-face evaluation and management services, such as telephone E/M (patient phone call) and e-visits (patient portal messages) as these have recently generated the most interest and discussion amongst health care providers.
Telemedicine after COVID-19 pandemic
During the beginning of the pandemic, a web-based survey study found that almost all providers in GI practices implemented some form of telemedicine to continue to provide care for patients, compared to 32% prior to the pandemic.4,5 The high demand and essential requirement for telehealth evaluation facilitated its reimbursement, eliminating the primary barrier to previous use.6
One of the new covered benefits by CMS was asynchronous telehealth care.7 The PHE ended in May 2023, and since then a qualified health care provider (QHCP) does not have the full flexibility to deliver telemedicine services across state lines. The U.S. Department of Health and Human Services has considered some telehealth policy changes after the COVID-19 PHE and many of those will be extended, at least through 2024.8 As during the pandemic, where the U.S. national payer network (CMS, state Medicaid, and private payers) and state health agencies assisted to ensure patients get the care they need by authorizing providers to be compensated for non–face-to-face services, we believe this service will continue to be part of our clinical practice.
We recommend you stay informed about local and federal laws, regulations, and alternatives for reimbursement as they may be modified at the beginning of a new calendar year. Remember, you can always talk with your revenue cycle team to clarify any query.
Telephone evaluation and management services
The patient requests to speak with you.
Telephone evaluation and management services became more widely used after the pandemic and were recognized by CMS as a covered medical service under PHE. As outlined in Table 1, there are associated codes with this service and it can only apply to an established patient in your practice. The cumulative time spent over a 7-day period without generating an immediate follow-up visit could qualify for this CPT code. However, for a patient with a high-complexity diagnosis and/or decisions being made about care, it might be better to consider a virtual office visit as this would value the complex care at a higher level than the time spent during the telephone E/M encounter.
A common question comes up: Can my nurse or support team bill for telephone care? No, only QHCP can, which means physicians and advanced practice providers can bill for this E/M service, and it does not include time spent by other members of clinical staff in patient care. However, there are CPT codes for chronic care management, which is not covered in this article.
Virtual evaluation and management services
You respond to a patient-initiated portal message.
Patient portal messages increased exponentially during the pandemic with 2.5 more minutes spent per message, resulting in more EHR work by practitioners, compared with prior to the pandemic. One study showed an immediate postpandemic increase in EHR patient-initiated messages with no return to prepandemic baseline.1
Although studies evaluating postpandemic telemedicine services are needed, we believe that this trend will continue, and for this reason, it is important to create sustainable workflows to continue to provide this patient driven avenue of care.9
E-visits are asynchronous patient or guardian portal messages that require a minimum of 5 minutes to provide medical decision-making without prior E/M services in the last 7 days. To obtain reimbursement for this service, it cannot be initiated by the provider, and patient consent must be obtained. Documentation should include this information and the time spent in the encounter. The associated CPT codes with this e-service are outlined in Table 1.
A common question is, “Are there additional codes I should use if a portal message E/M visit lasts more than 30 minutes?” No. If an e-visit lasts more than 30 minutes, the QHCP should bill the CPT code 99423. However, we would advise that, if this care requires more than 30 minutes, then either virtual or face-to-face E/M be considered for the optimal reimbursement for provider time spent. Another common question is around consent for services, and we advise providers to review this requirement with their compliance colleagues as each institution has different policies.
Virtual check-in
Medicare also covers brief communication technology–based services also known as virtual check-ins, where patients can communicate with their provider after having established care. During this brief conversation that can be via telephone, audio/video, secure text messaging, email, or patient portal, providers will determine if an in-person visit is necessary. CMS has designed G codes for these virtual check-ins that are from the Healthcare Common Procedure Coding System (HCPCS). Two codes are available for this E/M service: G2012, which is outlined in Table 1, and G2010, which covers the evaluation of images and/or recorded videos. In order to be reimbursed for a G2010 code, providers need at least a 5-minute response to make a clinical determination or give the patient a medical impression.
Patient satisfaction, physician well-being and quality of care outcomes
Large health care systems like Kaiser Permanente implemented secure message patient-physician communication (the patient portal) even before the pandemic, showing promising results in 2010 with reduction in office visits, improvement in measurable quality outcomes, and high level of patient satisfaction.10 Post pandemic, several large health care centers opted to announce the billing implementation for patient-initiated portal messages.11 A focus was placed on educating their patients about when a message will and will not be billed. Using this type of strategy can help to improve patient awareness about potential billing without affecting patient satisfaction and care outcomes. Studies have shown the EHR has contributed to physician burnout and some physicians reducing their clinical time or leaving medicine; a reduction in messaging might have a positive impact on physician well-being.
The challenge is that medical billing is not routinely included as a curriculum topic in many residency and fellowship programs; however, trainees are part of E/M services and have limited knowledge of billing processes. Unfortunately, at this time, trainees cannot submit for reimbursement for asynchronous care as described above. We hope that this brief article will help junior gastroenterologists optimize their outpatient billing practices.
Dr. Nieto is an internal medicine chief resident with WellStar Cobb Medical Center, Austell, Ga. Dr. Kinnucan is a gastroenterologist with Mayo Clinic, Jacksonville, Fla. The authors have no conflicts of interest to disclose for this article. The authors certify that no financial and grant support has been received for this article.
References
1. Holmgren AJ et al. J Am Med Inform Assoc. 2021 Dec 9. doi: 10.1093/jamia/ocab268.
2. Bali AS et al. Am J Gastroenterol. 2023 Apr 24. doi: 10.14309/ajg.0000000000002254.
3. AAFP. Family Physician. “Coding Scenario: Coding for Virtual-Digital Visits”
4. Keihanian T. et al. Telehealth Utilization in Gastroenterology Clinics Amid the COVID-19 Pandemic: Impact on Clinical Practice and Gastroenterology Training. Gastroenterology. 2020 Jun 20. doi: 10.1053/j.gastro.2020.06.040.
5. Lewin S et al. J Crohns Colitis. 2020 Oct 21. doi: 10.1093/ecco-jcc/jjaa140.
6. Perisetti A and H Goyal. Dig Dis Sci. 2021 Mar 3. doi: 10.1007/s10620-021-06874-x.
7. Telehealth.HHS.gov. Medicaid and Medicare billing for asynchronous telehealth. Updated: 2022 May 4.
8. Telehealth.HHS.gov. Telehealth policy changes after the COVID-19 public health emergency. Last updated: 2023 Jan 23.
9. Fox B and Sizemore JO. Telehealth: Fad or the future. Epic Health Research Network. 2020 Aug 18.
10. Baer D. Patient-physician e-mail communication: the kaiser permanente experience. J Oncol Pract. 2011 Jul. doi: 10.1200/JOP.2011.000323.
11. Myclevelandclinic.org. MyChart Messaging.
12. Sinsky CA et al. J Gen Intern Med. 2022 Aug 29. doi: 10.1007/s11606-022-07766-0.
The American Gastroenterological Association and other gastrointestinal-specific organizations have excellent resources available to members that focus on optimizing reimbursement in your clinical and endoscopic practice.
During the COVID-19 pandemic and public health emergency (PHE), many previously noncovered services were now covered under rules of the Centers for Medicare & Medicaid Services. During the pandemic, patient portal messages increased by 157%, meaning more work for health care teams, negatively impacting physician satisfaction, and increasing burnout.1 Medical burnout has been associated with increased time spent on electronic health records, with some subspeciality gastroenterology (GI) groups having a high EHR burden, according to a recently published article in the American Journal of Gastroenterology.2
This topic is a timely discussion as several large health systems have implemented processes to bill for non–face-to-face services (termed “asynchronous care”), some of which have not been well received in the lay media. It is important to note that despite these implementations, studies have shown only 1% of all incoming portal messages would meet criteria to be submitted for reimbursement. This impact might be slightly higher in chronic care management practices.
Providers and practices have several options when considering billing for non–face-to-face encounters, which we outline in Table 1.3
The focus of this article will be to review the more common non–face-to-face evaluation and management services, such as telephone E/M (patient phone call) and e-visits (patient portal messages) as these have recently generated the most interest and discussion amongst health care providers.
Telemedicine after COVID-19 pandemic
During the beginning of the pandemic, a web-based survey study found that almost all providers in GI practices implemented some form of telemedicine to continue to provide care for patients, compared to 32% prior to the pandemic.4,5 The high demand and essential requirement for telehealth evaluation facilitated its reimbursement, eliminating the primary barrier to previous use.6
One of the new covered benefits by CMS was asynchronous telehealth care.7 The PHE ended in May 2023, and since then a qualified health care provider (QHCP) does not have the full flexibility to deliver telemedicine services across state lines. The U.S. Department of Health and Human Services has considered some telehealth policy changes after the COVID-19 PHE and many of those will be extended, at least through 2024.8 As during the pandemic, where the U.S. national payer network (CMS, state Medicaid, and private payers) and state health agencies assisted to ensure patients get the care they need by authorizing providers to be compensated for non–face-to-face services, we believe this service will continue to be part of our clinical practice.
We recommend you stay informed about local and federal laws, regulations, and alternatives for reimbursement as they may be modified at the beginning of a new calendar year. Remember, you can always talk with your revenue cycle team to clarify any query.
Telephone evaluation and management services
The patient requests to speak with you.
Telephone evaluation and management services became more widely used after the pandemic and were recognized by CMS as a covered medical service under PHE. As outlined in Table 1, there are associated codes with this service and it can only apply to an established patient in your practice. The cumulative time spent over a 7-day period without generating an immediate follow-up visit could qualify for this CPT code. However, for a patient with a high-complexity diagnosis and/or decisions being made about care, it might be better to consider a virtual office visit as this would value the complex care at a higher level than the time spent during the telephone E/M encounter.
A common question comes up: Can my nurse or support team bill for telephone care? No, only QHCP can, which means physicians and advanced practice providers can bill for this E/M service, and it does not include time spent by other members of clinical staff in patient care. However, there are CPT codes for chronic care management, which is not covered in this article.
Virtual evaluation and management services
You respond to a patient-initiated portal message.
Patient portal messages increased exponentially during the pandemic with 2.5 more minutes spent per message, resulting in more EHR work by practitioners, compared with prior to the pandemic. One study showed an immediate postpandemic increase in EHR patient-initiated messages with no return to prepandemic baseline.1
Although studies evaluating postpandemic telemedicine services are needed, we believe that this trend will continue, and for this reason, it is important to create sustainable workflows to continue to provide this patient driven avenue of care.9
E-visits are asynchronous patient or guardian portal messages that require a minimum of 5 minutes to provide medical decision-making without prior E/M services in the last 7 days. To obtain reimbursement for this service, it cannot be initiated by the provider, and patient consent must be obtained. Documentation should include this information and the time spent in the encounter. The associated CPT codes with this e-service are outlined in Table 1.
A common question is, “Are there additional codes I should use if a portal message E/M visit lasts more than 30 minutes?” No. If an e-visit lasts more than 30 minutes, the QHCP should bill the CPT code 99423. However, we would advise that, if this care requires more than 30 minutes, then either virtual or face-to-face E/M be considered for the optimal reimbursement for provider time spent. Another common question is around consent for services, and we advise providers to review this requirement with their compliance colleagues as each institution has different policies.
Virtual check-in
Medicare also covers brief communication technology–based services also known as virtual check-ins, where patients can communicate with their provider after having established care. During this brief conversation that can be via telephone, audio/video, secure text messaging, email, or patient portal, providers will determine if an in-person visit is necessary. CMS has designed G codes for these virtual check-ins that are from the Healthcare Common Procedure Coding System (HCPCS). Two codes are available for this E/M service: G2012, which is outlined in Table 1, and G2010, which covers the evaluation of images and/or recorded videos. In order to be reimbursed for a G2010 code, providers need at least a 5-minute response to make a clinical determination or give the patient a medical impression.
Patient satisfaction, physician well-being and quality of care outcomes
Large health care systems like Kaiser Permanente implemented secure message patient-physician communication (the patient portal) even before the pandemic, showing promising results in 2010 with reduction in office visits, improvement in measurable quality outcomes, and high level of patient satisfaction.10 Post pandemic, several large health care centers opted to announce the billing implementation for patient-initiated portal messages.11 A focus was placed on educating their patients about when a message will and will not be billed. Using this type of strategy can help to improve patient awareness about potential billing without affecting patient satisfaction and care outcomes. Studies have shown the EHR has contributed to physician burnout and some physicians reducing their clinical time or leaving medicine; a reduction in messaging might have a positive impact on physician well-being.
The challenge is that medical billing is not routinely included as a curriculum topic in many residency and fellowship programs; however, trainees are part of E/M services and have limited knowledge of billing processes. Unfortunately, at this time, trainees cannot submit for reimbursement for asynchronous care as described above. We hope that this brief article will help junior gastroenterologists optimize their outpatient billing practices.
Dr. Nieto is an internal medicine chief resident with WellStar Cobb Medical Center, Austell, Ga. Dr. Kinnucan is a gastroenterologist with Mayo Clinic, Jacksonville, Fla. The authors have no conflicts of interest to disclose for this article. The authors certify that no financial and grant support has been received for this article.
References
1. Holmgren AJ et al. J Am Med Inform Assoc. 2021 Dec 9. doi: 10.1093/jamia/ocab268.
2. Bali AS et al. Am J Gastroenterol. 2023 Apr 24. doi: 10.14309/ajg.0000000000002254.
3. AAFP. Family Physician. “Coding Scenario: Coding for Virtual-Digital Visits”
4. Keihanian T. et al. Telehealth Utilization in Gastroenterology Clinics Amid the COVID-19 Pandemic: Impact on Clinical Practice and Gastroenterology Training. Gastroenterology. 2020 Jun 20. doi: 10.1053/j.gastro.2020.06.040.
5. Lewin S et al. J Crohns Colitis. 2020 Oct 21. doi: 10.1093/ecco-jcc/jjaa140.
6. Perisetti A and H Goyal. Dig Dis Sci. 2021 Mar 3. doi: 10.1007/s10620-021-06874-x.
7. Telehealth.HHS.gov. Medicaid and Medicare billing for asynchronous telehealth. Updated: 2022 May 4.
8. Telehealth.HHS.gov. Telehealth policy changes after the COVID-19 public health emergency. Last updated: 2023 Jan 23.
9. Fox B and Sizemore JO. Telehealth: Fad or the future. Epic Health Research Network. 2020 Aug 18.
10. Baer D. Patient-physician e-mail communication: the kaiser permanente experience. J Oncol Pract. 2011 Jul. doi: 10.1200/JOP.2011.000323.
11. Myclevelandclinic.org. MyChart Messaging.
12. Sinsky CA et al. J Gen Intern Med. 2022 Aug 29. doi: 10.1007/s11606-022-07766-0.
Crusted scalp rash

Dermoscopy showed not only the erythema, inflammation, and crusting visible during the initial examination, but it also revealed that each lesion had a hair growing through it. This pointed to a diagnosis of superficial folliculitis of the scalp.
The physician ruled out tinea capitis, acne keloidalis nuchae, and scarring alopecia based on the dermoscopic exam. There were no broken hairs that one would expect with tinea capitis. Also, there was no polytrichia (multiple hairs pushed into a single follicular opening due to scarring of the skin) that would be expected with acne keloidalis nuchae and scarring alopecias.
There are multiple types of scalp folliculitis. This patient had superficial folliculitis, in which pustules develop at the ostium of the hair follicles. Deep folliculitis is more severe and includes furuncles and carbuncles.1
Folliculitis is usually caused by a bacterial infection and, less commonly, fungal infection. In addition to superficial and deep folliculitis, inflammation with scarring of the follicles occurs with folliculitis decalvans, which is one of the scarring alopecias.1
Mild cases of superficial bacterial folliculitis are treated with topical antibiotics (eg, topical clindamycin 1% applied twice daily). Depending on the severity, oral antibiotics including doxycycline 100 mg twice daily for 7 days or trimethoprim sulfamethoxazole 160 mg/800 mg (double strength) twice daily for 7 days may be used. There is also a chronic nonscarring form of scalp folliculitis that often responds initially to antibiotics but then recurs. This has been treated with longer courses of oral antibiotics and, if the lesions don’t respond or continue to recur, with low-dose isotretinoin.2
Due to the amount of scalp involvement, crusting, and inflammation seen on this patient’s scalp, he was treated with trimethoprim sulfamethoxazole 160 mg/800 mg twice daily for 7 days. After 1 week, he reported that he was doing much better and that the lesions had nearly resolved. He was told to return for reevaluation if the lesions did not completely resolve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Lugović-Mihić L, Barisić F, Bulat V, et al. Differential diagnosis of the scalp hair folliculitis. Acta Clin Croat. 2011;50:395-402.
2. Romero-Maté A, Arias-Palomo D, Hernández-Núñez A, et al. Chronic nonscarring scalp folliculitis: retrospective case series study of 34 cases. J Am Acad Dermatol. 2019;81:1023-1024. doi: 10.1016/j.jaad.2019.02.065

Dermoscopy showed not only the erythema, inflammation, and crusting visible during the initial examination, but it also revealed that each lesion had a hair growing through it. This pointed to a diagnosis of superficial folliculitis of the scalp.
The physician ruled out tinea capitis, acne keloidalis nuchae, and scarring alopecia based on the dermoscopic exam. There were no broken hairs that one would expect with tinea capitis. Also, there was no polytrichia (multiple hairs pushed into a single follicular opening due to scarring of the skin) that would be expected with acne keloidalis nuchae and scarring alopecias.
There are multiple types of scalp folliculitis. This patient had superficial folliculitis, in which pustules develop at the ostium of the hair follicles. Deep folliculitis is more severe and includes furuncles and carbuncles.1
Folliculitis is usually caused by a bacterial infection and, less commonly, fungal infection. In addition to superficial and deep folliculitis, inflammation with scarring of the follicles occurs with folliculitis decalvans, which is one of the scarring alopecias.1
Mild cases of superficial bacterial folliculitis are treated with topical antibiotics (eg, topical clindamycin 1% applied twice daily). Depending on the severity, oral antibiotics including doxycycline 100 mg twice daily for 7 days or trimethoprim sulfamethoxazole 160 mg/800 mg (double strength) twice daily for 7 days may be used. There is also a chronic nonscarring form of scalp folliculitis that often responds initially to antibiotics but then recurs. This has been treated with longer courses of oral antibiotics and, if the lesions don’t respond or continue to recur, with low-dose isotretinoin.2
Due to the amount of scalp involvement, crusting, and inflammation seen on this patient’s scalp, he was treated with trimethoprim sulfamethoxazole 160 mg/800 mg twice daily for 7 days. After 1 week, he reported that he was doing much better and that the lesions had nearly resolved. He was told to return for reevaluation if the lesions did not completely resolve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Dermoscopy showed not only the erythema, inflammation, and crusting visible during the initial examination, but it also revealed that each lesion had a hair growing through it. This pointed to a diagnosis of superficial folliculitis of the scalp.
The physician ruled out tinea capitis, acne keloidalis nuchae, and scarring alopecia based on the dermoscopic exam. There were no broken hairs that one would expect with tinea capitis. Also, there was no polytrichia (multiple hairs pushed into a single follicular opening due to scarring of the skin) that would be expected with acne keloidalis nuchae and scarring alopecias.
There are multiple types of scalp folliculitis. This patient had superficial folliculitis, in which pustules develop at the ostium of the hair follicles. Deep folliculitis is more severe and includes furuncles and carbuncles.1
Folliculitis is usually caused by a bacterial infection and, less commonly, fungal infection. In addition to superficial and deep folliculitis, inflammation with scarring of the follicles occurs with folliculitis decalvans, which is one of the scarring alopecias.1
Mild cases of superficial bacterial folliculitis are treated with topical antibiotics (eg, topical clindamycin 1% applied twice daily). Depending on the severity, oral antibiotics including doxycycline 100 mg twice daily for 7 days or trimethoprim sulfamethoxazole 160 mg/800 mg (double strength) twice daily for 7 days may be used. There is also a chronic nonscarring form of scalp folliculitis that often responds initially to antibiotics but then recurs. This has been treated with longer courses of oral antibiotics and, if the lesions don’t respond or continue to recur, with low-dose isotretinoin.2
Due to the amount of scalp involvement, crusting, and inflammation seen on this patient’s scalp, he was treated with trimethoprim sulfamethoxazole 160 mg/800 mg twice daily for 7 days. After 1 week, he reported that he was doing much better and that the lesions had nearly resolved. He was told to return for reevaluation if the lesions did not completely resolve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Lugović-Mihić L, Barisić F, Bulat V, et al. Differential diagnosis of the scalp hair folliculitis. Acta Clin Croat. 2011;50:395-402.
2. Romero-Maté A, Arias-Palomo D, Hernández-Núñez A, et al. Chronic nonscarring scalp folliculitis: retrospective case series study of 34 cases. J Am Acad Dermatol. 2019;81:1023-1024. doi: 10.1016/j.jaad.2019.02.065
1. Lugović-Mihić L, Barisić F, Bulat V, et al. Differential diagnosis of the scalp hair folliculitis. Acta Clin Croat. 2011;50:395-402.
2. Romero-Maté A, Arias-Palomo D, Hernández-Núñez A, et al. Chronic nonscarring scalp folliculitis: retrospective case series study of 34 cases. J Am Acad Dermatol. 2019;81:1023-1024. doi: 10.1016/j.jaad.2019.02.065
Persistent scaling rash
The clinical pattern of a scaly herald patch predating the eruption of multiple scaly macules is the hallmark of pityriasis rosea (PR). This patient’s severe itching is also classic for PR.
PR’s etiology is believed to be a reactivation of infection from human herpes viruses 6 and 7.1 Prodromal viral symptoms of malaise, sore throat, myalgias, and fever are common.2 Along with the prodromal symptoms, there is often a several-centimeter herald patch that occurs on the trunk. It is often confused with eczema or tinea due to its erythema and scale. (Secondary syphilis is also in the differential.) Sometimes PR can be differentiated by the scale pattern being a collarette instead of diffuse. The diagnosis becomes clearer 1 to 2 weeks later with the onset of multiple small scaly macules across the trunk following the Langer’s skin lines. The course is self-limited but takes several weeks to months to resolve.
If severe, PR may be treated with acyclovir 800 mg orally 5 times daily for 5 days; this is the same regimen for treating varicella zoster (shingles).1,2 Estimated recurrence rates are 4% to 24%.1,3
At age 49 years, this woman was older than the average patient with PR, as the usual age range is 10 to 35 years.1 Her physician advised her that the outbreak might recur. She was also given a prescription for oral hydroxyzine 25 mg to be taken at bedtime if the itching was interfering with her sleep. Her physician told her to return for reevaluation if the rash did not resolve in 3 months. She did not return for reevaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Drago F, Ciccarese G, Parodi A. Commentary on: "pityriasis rosea recurrence is much higher than previously known: a prospective study." Acta Derm Venereol. 2019;99:1053-1054. doi: 10.2340/00015555-3265
2. Villalon-Gomez JM. Pityriasis rosea: diagnosis and treatment. Am Fam Physician. 2018;97:38-44.
3. Yüksel M. Pityriasis rosea recurrence is much higher than previously known: a prospective study. Acta Derm Venereol. 2019;99:664-667. doi: 10.2340/00015555-3169

The clinical pattern of a scaly herald patch predating the eruption of multiple scaly macules is the hallmark of pityriasis rosea (PR). This patient’s severe itching is also classic for PR.
PR’s etiology is believed to be a reactivation of infection from human herpes viruses 6 and 7.1 Prodromal viral symptoms of malaise, sore throat, myalgias, and fever are common.2 Along with the prodromal symptoms, there is often a several-centimeter herald patch that occurs on the trunk. It is often confused with eczema or tinea due to its erythema and scale. (Secondary syphilis is also in the differential.) Sometimes PR can be differentiated by the scale pattern being a collarette instead of diffuse. The diagnosis becomes clearer 1 to 2 weeks later with the onset of multiple small scaly macules across the trunk following the Langer’s skin lines. The course is self-limited but takes several weeks to months to resolve.
If severe, PR may be treated with acyclovir 800 mg orally 5 times daily for 5 days; this is the same regimen for treating varicella zoster (shingles).1,2 Estimated recurrence rates are 4% to 24%.1,3
At age 49 years, this woman was older than the average patient with PR, as the usual age range is 10 to 35 years.1 Her physician advised her that the outbreak might recur. She was also given a prescription for oral hydroxyzine 25 mg to be taken at bedtime if the itching was interfering with her sleep. Her physician told her to return for reevaluation if the rash did not resolve in 3 months. She did not return for reevaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The clinical pattern of a scaly herald patch predating the eruption of multiple scaly macules is the hallmark of pityriasis rosea (PR). This patient’s severe itching is also classic for PR.
PR’s etiology is believed to be a reactivation of infection from human herpes viruses 6 and 7.1 Prodromal viral symptoms of malaise, sore throat, myalgias, and fever are common.2 Along with the prodromal symptoms, there is often a several-centimeter herald patch that occurs on the trunk. It is often confused with eczema or tinea due to its erythema and scale. (Secondary syphilis is also in the differential.) Sometimes PR can be differentiated by the scale pattern being a collarette instead of diffuse. The diagnosis becomes clearer 1 to 2 weeks later with the onset of multiple small scaly macules across the trunk following the Langer’s skin lines. The course is self-limited but takes several weeks to months to resolve.
If severe, PR may be treated with acyclovir 800 mg orally 5 times daily for 5 days; this is the same regimen for treating varicella zoster (shingles).1,2 Estimated recurrence rates are 4% to 24%.1,3
At age 49 years, this woman was older than the average patient with PR, as the usual age range is 10 to 35 years.1 Her physician advised her that the outbreak might recur. She was also given a prescription for oral hydroxyzine 25 mg to be taken at bedtime if the itching was interfering with her sleep. Her physician told her to return for reevaluation if the rash did not resolve in 3 months. She did not return for reevaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Drago F, Ciccarese G, Parodi A. Commentary on: "pityriasis rosea recurrence is much higher than previously known: a prospective study." Acta Derm Venereol. 2019;99:1053-1054. doi: 10.2340/00015555-3265
2. Villalon-Gomez JM. Pityriasis rosea: diagnosis and treatment. Am Fam Physician. 2018;97:38-44.
3. Yüksel M. Pityriasis rosea recurrence is much higher than previously known: a prospective study. Acta Derm Venereol. 2019;99:664-667. doi: 10.2340/00015555-3169
1. Drago F, Ciccarese G, Parodi A. Commentary on: "pityriasis rosea recurrence is much higher than previously known: a prospective study." Acta Derm Venereol. 2019;99:1053-1054. doi: 10.2340/00015555-3265
2. Villalon-Gomez JM. Pityriasis rosea: diagnosis and treatment. Am Fam Physician. 2018;97:38-44.
3. Yüksel M. Pityriasis rosea recurrence is much higher than previously known: a prospective study. Acta Derm Venereol. 2019;99:664-667. doi: 10.2340/00015555-3169
Concomitant med use may explain poor antidepressant response
Investigators studied over 800 patients who were taking antidepressants for major depressive disorder (MDD) and found that close to two-thirds were taking at least one nonpsychiatric medication with potential depressive symptom side effects (PDSS), more than 30% were taking two or more such medications, and 20% at least three such medications.
These medications, which included antihypertensive medications and corticosteroids, among others, were associated with higher odds of moderate-to-severe depressive symptoms, compared with medications without PDSS.
“When evaluating the reasons for inadequate response to treatment for depression, clinicians should consider whether their patient is also receiving a nonpsychiatric medication with a potential for depressive symptom side effects,” study investigator Mark Olfson, MD, MPH, Elizabeth K. Dollard professor of psychiatry, medicine, and law and professor of epidemiology, Columbia University Irving Medical Center, New York, said in an interview.
The study was published online in the Journal of Clinical Psychiatry.
Previous research limited
“In earlier research, we found that people who were taking medications with a potential to cause depressive symptom side effects were at increased risk of depression, especially those adults who were taking more than one of these medications,” said Dr. Olfson.
This finding led Dr. Olfson and his team to “wonder whether the risks of depressive symptoms associated with these medications extended to people who were being actively treated with antidepressants for depression.”
To investigate, they turned to the National Health and Nutrition Examination Survey (NHANES) – a nationally representative cross-sectional survey of the United States general population.
The study was based on the 2013-2014, 2015-2016, and 2017-2018 waves and included 885 adults who reported using antidepressant medications for greater than or equal to 6 weeks for depression and whose depression could be ascertained.
Prescription medications with PDSS were identified through Micromedex, whose accuracy is “established” and primarily based on the U.S. Food and Drug Administration’s labeled side effects.
Nonantidepressant psychiatric medications and medications for Alzheimer’s disease or substance use disorders were not included in the analysis.
Antidepressant-treated MDD was defined as taking an antidepressant for MDD for greater than or equal to 6 weeks. Depressive symptoms were ascertained using the Patient Health Questionnaire-9 (PHQ-9) with a score of less than 5 representing no/minimal depressive symptoms and a score of greater than or equal to 10 indicating moderate/severe symptoms.
Other variables included self-reported sex, age, race/ethnicity, income, education, health insurance, and common chronic medical conditions such as hypertension, arthritis, lung disease, diabetes mellitus, thyroid disease, cancer, heart disease, liver disease, stroke, and congestive heart failure.
Recovery interrupted
Of the patients in the study treated with antidepressants, most were female, greater than or equal to 50 years, non-Hispanic White, and with a college education (70.55, 62.0%, 81.7%, and 69.4%, respectively).
Selective serotonin reuptake inhibitors were used by 67.9% of participants with MDD. Most had been on the same antidepressant medication for a “long time,” the authors report, with 79.2% and 67.8% taking them for greater than 1 year and greater than 2 years, respectively.
Despite the large number of patients on antidepressants, only 43.0% scored in the no/minimal symptoms range, based on the PHQ-9, while 28.4% scored in the moderate/severe range.
Most patients (85%) took at least one medication for medical conditions, with the majority medications with PDSS: 66.7% took at least one medication with PDSS, 37.3% took at least two, 21.6% took at least three, 10.7% took at least four, and 4.9% took at least five.
Almost 75% were using greater than or equal to 1 medication without PDSS, and about 50% were using greater than 1.
The number of medications with PDSS was significantly associated with lower odds of no/minimal depressive symptoms (AOR, 0.75 [95% CI, 0.64-0.87]; P < .001) and higher odds of moderate/severe symptoms (AOR, 1.14 [1.004-1.29]; P = .044).
“The predicted probability of no/minimal symptoms in those taking 5 medications with PDSS was less than half the predicted probability in those taking no medications with PDSS (0.23 vs. 0.52),” the authors report.
Conversely, the predicted probability of moderate/severe symptoms was ~50% higher in individuals taking 5 versus 0 medications with PDSS (0.36 vs. 0.24).
No corresponding associations were found for medications without PDSS.
The results were even stronger when the researchers repeated their adjusted regression analyses to focus on the 10 individual medications most associated with the severity of depressive symptoms. These were omeprazole, gabapentin, meloxicam, tramadol, ranitidine, baclofen, oxycodone, tizanidine, propranolol, and morphine, with an AOR of 0.42 [0.30-0.60] for no/minimal symptoms and 1.68 [1.24-2.27] for moderate/severe symptoms.
“Many widely prescribed medications, from antihypertensives, such as atenolol and metoprolol to corticosteroids, such as dexamethasone and triamcinolone, are associated with depression side effects,” said Dr. Olfson.
“These medications could interfere with recovery from depression. When available, consideration should be given to selecting a substitute with lower risk for depressive symptoms,” he said.
Role in treatment-resistant depression
In a comment, Dima Qato, PharmD, MPH, PhD, Hygeia Centennial chair and associate professor, University of Southern California School of Pharmacy, Los Angeles, said the study “is an important reminder that the use of medications with depressive symptoms side effects is increasingly common and may contribute to delays in responsiveness or worsen depressive symptoms among individuals being treated for depression.”
Dr. Qato, who is also the director of the Program on Medicines and Public Health, USC School of Pharmacy, and was not involved with the study, recommended that clinicians “consider the role of medications with depression side effects when evaluating patients with treatment-resistant depression.”
The study was not supported by any funding agency. Dr. Olfson and coauthors have disclosed no relevant financial relationships. Dr. Qato is a consultant for the Public Citizen Health Research Group.
A version of this article first appeared on Medscape.com.
Investigators studied over 800 patients who were taking antidepressants for major depressive disorder (MDD) and found that close to two-thirds were taking at least one nonpsychiatric medication with potential depressive symptom side effects (PDSS), more than 30% were taking two or more such medications, and 20% at least three such medications.
These medications, which included antihypertensive medications and corticosteroids, among others, were associated with higher odds of moderate-to-severe depressive symptoms, compared with medications without PDSS.
“When evaluating the reasons for inadequate response to treatment for depression, clinicians should consider whether their patient is also receiving a nonpsychiatric medication with a potential for depressive symptom side effects,” study investigator Mark Olfson, MD, MPH, Elizabeth K. Dollard professor of psychiatry, medicine, and law and professor of epidemiology, Columbia University Irving Medical Center, New York, said in an interview.
The study was published online in the Journal of Clinical Psychiatry.
Previous research limited
“In earlier research, we found that people who were taking medications with a potential to cause depressive symptom side effects were at increased risk of depression, especially those adults who were taking more than one of these medications,” said Dr. Olfson.
This finding led Dr. Olfson and his team to “wonder whether the risks of depressive symptoms associated with these medications extended to people who were being actively treated with antidepressants for depression.”
To investigate, they turned to the National Health and Nutrition Examination Survey (NHANES) – a nationally representative cross-sectional survey of the United States general population.
The study was based on the 2013-2014, 2015-2016, and 2017-2018 waves and included 885 adults who reported using antidepressant medications for greater than or equal to 6 weeks for depression and whose depression could be ascertained.
Prescription medications with PDSS were identified through Micromedex, whose accuracy is “established” and primarily based on the U.S. Food and Drug Administration’s labeled side effects.
Nonantidepressant psychiatric medications and medications for Alzheimer’s disease or substance use disorders were not included in the analysis.
Antidepressant-treated MDD was defined as taking an antidepressant for MDD for greater than or equal to 6 weeks. Depressive symptoms were ascertained using the Patient Health Questionnaire-9 (PHQ-9) with a score of less than 5 representing no/minimal depressive symptoms and a score of greater than or equal to 10 indicating moderate/severe symptoms.
Other variables included self-reported sex, age, race/ethnicity, income, education, health insurance, and common chronic medical conditions such as hypertension, arthritis, lung disease, diabetes mellitus, thyroid disease, cancer, heart disease, liver disease, stroke, and congestive heart failure.
Recovery interrupted
Of the patients in the study treated with antidepressants, most were female, greater than or equal to 50 years, non-Hispanic White, and with a college education (70.55, 62.0%, 81.7%, and 69.4%, respectively).
Selective serotonin reuptake inhibitors were used by 67.9% of participants with MDD. Most had been on the same antidepressant medication for a “long time,” the authors report, with 79.2% and 67.8% taking them for greater than 1 year and greater than 2 years, respectively.
Despite the large number of patients on antidepressants, only 43.0% scored in the no/minimal symptoms range, based on the PHQ-9, while 28.4% scored in the moderate/severe range.
Most patients (85%) took at least one medication for medical conditions, with the majority medications with PDSS: 66.7% took at least one medication with PDSS, 37.3% took at least two, 21.6% took at least three, 10.7% took at least four, and 4.9% took at least five.
Almost 75% were using greater than or equal to 1 medication without PDSS, and about 50% were using greater than 1.
The number of medications with PDSS was significantly associated with lower odds of no/minimal depressive symptoms (AOR, 0.75 [95% CI, 0.64-0.87]; P < .001) and higher odds of moderate/severe symptoms (AOR, 1.14 [1.004-1.29]; P = .044).
“The predicted probability of no/minimal symptoms in those taking 5 medications with PDSS was less than half the predicted probability in those taking no medications with PDSS (0.23 vs. 0.52),” the authors report.
Conversely, the predicted probability of moderate/severe symptoms was ~50% higher in individuals taking 5 versus 0 medications with PDSS (0.36 vs. 0.24).
No corresponding associations were found for medications without PDSS.
The results were even stronger when the researchers repeated their adjusted regression analyses to focus on the 10 individual medications most associated with the severity of depressive symptoms. These were omeprazole, gabapentin, meloxicam, tramadol, ranitidine, baclofen, oxycodone, tizanidine, propranolol, and morphine, with an AOR of 0.42 [0.30-0.60] for no/minimal symptoms and 1.68 [1.24-2.27] for moderate/severe symptoms.
“Many widely prescribed medications, from antihypertensives, such as atenolol and metoprolol to corticosteroids, such as dexamethasone and triamcinolone, are associated with depression side effects,” said Dr. Olfson.
“These medications could interfere with recovery from depression. When available, consideration should be given to selecting a substitute with lower risk for depressive symptoms,” he said.
Role in treatment-resistant depression
In a comment, Dima Qato, PharmD, MPH, PhD, Hygeia Centennial chair and associate professor, University of Southern California School of Pharmacy, Los Angeles, said the study “is an important reminder that the use of medications with depressive symptoms side effects is increasingly common and may contribute to delays in responsiveness or worsen depressive symptoms among individuals being treated for depression.”
Dr. Qato, who is also the director of the Program on Medicines and Public Health, USC School of Pharmacy, and was not involved with the study, recommended that clinicians “consider the role of medications with depression side effects when evaluating patients with treatment-resistant depression.”
The study was not supported by any funding agency. Dr. Olfson and coauthors have disclosed no relevant financial relationships. Dr. Qato is a consultant for the Public Citizen Health Research Group.
A version of this article first appeared on Medscape.com.
Investigators studied over 800 patients who were taking antidepressants for major depressive disorder (MDD) and found that close to two-thirds were taking at least one nonpsychiatric medication with potential depressive symptom side effects (PDSS), more than 30% were taking two or more such medications, and 20% at least three such medications.
These medications, which included antihypertensive medications and corticosteroids, among others, were associated with higher odds of moderate-to-severe depressive symptoms, compared with medications without PDSS.
“When evaluating the reasons for inadequate response to treatment for depression, clinicians should consider whether their patient is also receiving a nonpsychiatric medication with a potential for depressive symptom side effects,” study investigator Mark Olfson, MD, MPH, Elizabeth K. Dollard professor of psychiatry, medicine, and law and professor of epidemiology, Columbia University Irving Medical Center, New York, said in an interview.
The study was published online in the Journal of Clinical Psychiatry.
Previous research limited
“In earlier research, we found that people who were taking medications with a potential to cause depressive symptom side effects were at increased risk of depression, especially those adults who were taking more than one of these medications,” said Dr. Olfson.
This finding led Dr. Olfson and his team to “wonder whether the risks of depressive symptoms associated with these medications extended to people who were being actively treated with antidepressants for depression.”
To investigate, they turned to the National Health and Nutrition Examination Survey (NHANES) – a nationally representative cross-sectional survey of the United States general population.
The study was based on the 2013-2014, 2015-2016, and 2017-2018 waves and included 885 adults who reported using antidepressant medications for greater than or equal to 6 weeks for depression and whose depression could be ascertained.
Prescription medications with PDSS were identified through Micromedex, whose accuracy is “established” and primarily based on the U.S. Food and Drug Administration’s labeled side effects.
Nonantidepressant psychiatric medications and medications for Alzheimer’s disease or substance use disorders were not included in the analysis.
Antidepressant-treated MDD was defined as taking an antidepressant for MDD for greater than or equal to 6 weeks. Depressive symptoms were ascertained using the Patient Health Questionnaire-9 (PHQ-9) with a score of less than 5 representing no/minimal depressive symptoms and a score of greater than or equal to 10 indicating moderate/severe symptoms.
Other variables included self-reported sex, age, race/ethnicity, income, education, health insurance, and common chronic medical conditions such as hypertension, arthritis, lung disease, diabetes mellitus, thyroid disease, cancer, heart disease, liver disease, stroke, and congestive heart failure.
Recovery interrupted
Of the patients in the study treated with antidepressants, most were female, greater than or equal to 50 years, non-Hispanic White, and with a college education (70.55, 62.0%, 81.7%, and 69.4%, respectively).
Selective serotonin reuptake inhibitors were used by 67.9% of participants with MDD. Most had been on the same antidepressant medication for a “long time,” the authors report, with 79.2% and 67.8% taking them for greater than 1 year and greater than 2 years, respectively.
Despite the large number of patients on antidepressants, only 43.0% scored in the no/minimal symptoms range, based on the PHQ-9, while 28.4% scored in the moderate/severe range.
Most patients (85%) took at least one medication for medical conditions, with the majority medications with PDSS: 66.7% took at least one medication with PDSS, 37.3% took at least two, 21.6% took at least three, 10.7% took at least four, and 4.9% took at least five.
Almost 75% were using greater than or equal to 1 medication without PDSS, and about 50% were using greater than 1.
The number of medications with PDSS was significantly associated with lower odds of no/minimal depressive symptoms (AOR, 0.75 [95% CI, 0.64-0.87]; P < .001) and higher odds of moderate/severe symptoms (AOR, 1.14 [1.004-1.29]; P = .044).
“The predicted probability of no/minimal symptoms in those taking 5 medications with PDSS was less than half the predicted probability in those taking no medications with PDSS (0.23 vs. 0.52),” the authors report.
Conversely, the predicted probability of moderate/severe symptoms was ~50% higher in individuals taking 5 versus 0 medications with PDSS (0.36 vs. 0.24).
No corresponding associations were found for medications without PDSS.
The results were even stronger when the researchers repeated their adjusted regression analyses to focus on the 10 individual medications most associated with the severity of depressive symptoms. These were omeprazole, gabapentin, meloxicam, tramadol, ranitidine, baclofen, oxycodone, tizanidine, propranolol, and morphine, with an AOR of 0.42 [0.30-0.60] for no/minimal symptoms and 1.68 [1.24-2.27] for moderate/severe symptoms.
“Many widely prescribed medications, from antihypertensives, such as atenolol and metoprolol to corticosteroids, such as dexamethasone and triamcinolone, are associated with depression side effects,” said Dr. Olfson.
“These medications could interfere with recovery from depression. When available, consideration should be given to selecting a substitute with lower risk for depressive symptoms,” he said.
Role in treatment-resistant depression
In a comment, Dima Qato, PharmD, MPH, PhD, Hygeia Centennial chair and associate professor, University of Southern California School of Pharmacy, Los Angeles, said the study “is an important reminder that the use of medications with depressive symptoms side effects is increasingly common and may contribute to delays in responsiveness or worsen depressive symptoms among individuals being treated for depression.”
Dr. Qato, who is also the director of the Program on Medicines and Public Health, USC School of Pharmacy, and was not involved with the study, recommended that clinicians “consider the role of medications with depression side effects when evaluating patients with treatment-resistant depression.”
The study was not supported by any funding agency. Dr. Olfson and coauthors have disclosed no relevant financial relationships. Dr. Qato is a consultant for the Public Citizen Health Research Group.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Novel agent promising for major depression: Phase 3 data
TOPLINE
Patients who received zuranolone 50 mg/d demonstrated significantly greater improvement in depressive symptoms than those who received placebo, with a rapid onset of effect.
METHODOLOGY
The Food and Drug Administration has accepted filing of a new drug application for zuranolone, a neuroactive steroid that targets g-aminobutyric acid type A receptors (GABAAR), for the treatment of major depressive disorder (MDD) and postpartum depression.
The study included 543 mostly White female patients with MDD. The mean age of the patients was 40 years. Participants were randomly assigned to receive oral zuranolone 50 mg or placebo once daily for 14 days.
About 30% of patients were taking an antidepressant.
The primary endpoint was change in Hamilton Depression Rating Scale (HAM-D) score at day 15.
TAKEAWAY
The zuranolone group showed significantly greater improvement in depressive symptoms at 15 days compared with the placebo group (least square mean [LSM] change on HAM-D, –14.1, vs. –12.3; P = .01; Cohen’s d = 0.23).
Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Results favored zuranolone regardless of the use of antidepressant therapies.
Patients with anxiety who received the active drug experienced improvement in anxiety symptoms compared to the patients who received placebo.
The drug was well tolerated, and there were no new safety findings. The most common treatment-emergent adverse events were somnolence and headache. There was no weight gain, sexual dysfunction, withdrawal symptoms, or increased suicidal ideation or behavior.
IN PRACTICE
The study adds to evidence suggesting zuranolone is a promising novel therapy for treating MDD, the authors noted.
STUDY DETAILS
The study was conducted by Anita H. Clayton, MD, department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville, and colleagues. It was published online May 3 in The American Journal of Psychiatry.
LIMITATIONS
The study was short term, and the patient population was severely depressed at study entry, which may limit application to those with mild or moderate symptoms. There was a robust placebo response, possibly partly due to the COVID-19 pandemic, when there was an increase in depressive symptoms in the U.S. population, and so frequent in-person visits may have led to an improvement in symptoms even if the patient was receiving placebo.
DISCLOSURES
The study was funded by Sage Therapeutics and Biogen.
A version of this article first appeared on Medscape.com.
TOPLINE
Patients who received zuranolone 50 mg/d demonstrated significantly greater improvement in depressive symptoms than those who received placebo, with a rapid onset of effect.
METHODOLOGY
The Food and Drug Administration has accepted filing of a new drug application for zuranolone, a neuroactive steroid that targets g-aminobutyric acid type A receptors (GABAAR), for the treatment of major depressive disorder (MDD) and postpartum depression.
The study included 543 mostly White female patients with MDD. The mean age of the patients was 40 years. Participants were randomly assigned to receive oral zuranolone 50 mg or placebo once daily for 14 days.
About 30% of patients were taking an antidepressant.
The primary endpoint was change in Hamilton Depression Rating Scale (HAM-D) score at day 15.
TAKEAWAY
The zuranolone group showed significantly greater improvement in depressive symptoms at 15 days compared with the placebo group (least square mean [LSM] change on HAM-D, –14.1, vs. –12.3; P = .01; Cohen’s d = 0.23).
Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Results favored zuranolone regardless of the use of antidepressant therapies.
Patients with anxiety who received the active drug experienced improvement in anxiety symptoms compared to the patients who received placebo.
The drug was well tolerated, and there were no new safety findings. The most common treatment-emergent adverse events were somnolence and headache. There was no weight gain, sexual dysfunction, withdrawal symptoms, or increased suicidal ideation or behavior.
IN PRACTICE
The study adds to evidence suggesting zuranolone is a promising novel therapy for treating MDD, the authors noted.
STUDY DETAILS
The study was conducted by Anita H. Clayton, MD, department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville, and colleagues. It was published online May 3 in The American Journal of Psychiatry.
LIMITATIONS
The study was short term, and the patient population was severely depressed at study entry, which may limit application to those with mild or moderate symptoms. There was a robust placebo response, possibly partly due to the COVID-19 pandemic, when there was an increase in depressive symptoms in the U.S. population, and so frequent in-person visits may have led to an improvement in symptoms even if the patient was receiving placebo.
DISCLOSURES
The study was funded by Sage Therapeutics and Biogen.
A version of this article first appeared on Medscape.com.
TOPLINE
Patients who received zuranolone 50 mg/d demonstrated significantly greater improvement in depressive symptoms than those who received placebo, with a rapid onset of effect.
METHODOLOGY
The Food and Drug Administration has accepted filing of a new drug application for zuranolone, a neuroactive steroid that targets g-aminobutyric acid type A receptors (GABAAR), for the treatment of major depressive disorder (MDD) and postpartum depression.
The study included 543 mostly White female patients with MDD. The mean age of the patients was 40 years. Participants were randomly assigned to receive oral zuranolone 50 mg or placebo once daily for 14 days.
About 30% of patients were taking an antidepressant.
The primary endpoint was change in Hamilton Depression Rating Scale (HAM-D) score at day 15.
TAKEAWAY
The zuranolone group showed significantly greater improvement in depressive symptoms at 15 days compared with the placebo group (least square mean [LSM] change on HAM-D, –14.1, vs. –12.3; P = .01; Cohen’s d = 0.23).
Improvements were observed on day 3, the earliest assessment, and were sustained at all subsequent visits during the treatment and follow-up period (through day 42).
Results favored zuranolone regardless of the use of antidepressant therapies.
Patients with anxiety who received the active drug experienced improvement in anxiety symptoms compared to the patients who received placebo.
The drug was well tolerated, and there were no new safety findings. The most common treatment-emergent adverse events were somnolence and headache. There was no weight gain, sexual dysfunction, withdrawal symptoms, or increased suicidal ideation or behavior.
IN PRACTICE
The study adds to evidence suggesting zuranolone is a promising novel therapy for treating MDD, the authors noted.
STUDY DETAILS
The study was conducted by Anita H. Clayton, MD, department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville, and colleagues. It was published online May 3 in The American Journal of Psychiatry.
LIMITATIONS
The study was short term, and the patient population was severely depressed at study entry, which may limit application to those with mild or moderate symptoms. There was a robust placebo response, possibly partly due to the COVID-19 pandemic, when there was an increase in depressive symptoms in the U.S. population, and so frequent in-person visits may have led to an improvement in symptoms even if the patient was receiving placebo.
DISCLOSURES
The study was funded by Sage Therapeutics and Biogen.
A version of this article first appeared on Medscape.com.
Strategies for complete B-cell depletion evolve for patients with lupus nephritis
SEOUL, SOUTH KOREA – B cell–depleting therapies in patients with lupus nephritis have a higher likelihood of complete response if B cells are almost completely depleted, and strategies for achieving more complete B-cell depletion continue to be tested, according to evidence presented by Richard A. Furie, MD, at an international congress on systemic lupus erythematosus (SLE).
“If you go back about 20 years ago or so, when we designed the LUNAR and EXPLORER trials, we were scared to death of rituximab [Rituxan and biosimilars], about what would happen when you deplete B cells,” said Dr. Furie, chief of the division of rheumatology at Northwell Health in New York.
The LUNAR trial, which compared rituximab with placebo in patients with lupus nephritis, did not show a statistically significant difference in renal outcomes at 1 year. However, a post hoc analysis done several years later told a different story. It looked at patients who achieved complete peripheral depletion of B cells, defined as zero cells per microliter in peripheral blood. “You can see about a fourfold increase in complete response rates in those who were complete B-cell depleters at 1 year,” Dr. Furie told the conference.
It therefore raises the question of how to achieve greater B-cell depletion rates in patients. Dr. Furie said one strategy might be to first mobilize memory B cells and neutralize B cell–activating factor using belimumab (Benlysta), and then treat with rituximab to eliminate B cells. This strategy of sequential belimumab-rituximab treatment has been taken in several clinical trials.
More potent B-cell depletion with obinutuzumab
Another approach is to choose more potent B cell–depleting therapies, such as obinutuzumab (Gazyva), which is an anti-CD20 monoclonal antibody that was approved in 2013 for the treatment of chronic lymphocytic leukemia.
The NOBILITY trial compared obinutuzumab with placebo in 125 patients with lupus nephritis who were on background treatment with mycophenolate and corticosteroids. At 1 year, significantly more patients achieved B-cell thresholds either below 5 cells per microliter or even zero cells per microliter than had been seen previously with rituximab.
That also translated into clinical benefit, Dr. Furie said. By week 76, half the patients who had sustained depletion of B cells below 0.4 cells per microliter had a complete response, compared with 35% of those who still had detectable B cells and 18% of the placebo group. Treatment with obinutuzumab did not show any link to higher rates of serious adverse events, serious infections, or deaths.
“I think we’re all pretty much convinced more is better, without introducing safety issues,” Dr. Furie said in an interview.
Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said the data did suggest that renal outcomes were better with more complete depletion, but raised the question of whether this might increase the risk of infections or infectious severity.
Dr. Furie noted that complete response not only required improvement in proteinuria, complement levels, and anti–double-stranded DNA antibodies, but also in serum creatinine, “because maintenance of eGFR [estimated glomerular filtration rate] is the name of the game with lupus nephritis.”
However, he also pointed out that there may be a ceiling for response rates in patients with lupus nephritis when using stricter endpoints for serum creatinine. The NOBILITY trial required patients to achieve a serum creatinine that did not increase by more than 15% from baseline. But when researchers did an analysis that instead only required patients to achieve a reduction in proteinuria and maintain normal creatinine, the complete response rate in complete B-cell depleters increased to 72%, compared with 50% in partial depleters and 37% in the placebo group.
Newer strategies for greater B-cell depletion
A third strategy for achieving greater B-cell depletion is bispecific T-cell engagers, or BiTEs. “I called it a ‘frenemy,’ where it’s taking the activated T cell and introducing it to the B cell, and it can kill it via direct T-cell killing,” Dr. Furie said in an interview. Mosunetuzumab (Lunsumio) is one example, and is currently in a phase 1 clinical trial of patients with SLE.
And the fourth strategy, which has proved so successful in lymphoma, is chimeric antigen receptor T-cell therapy (CAR T). Dr. Furie cited the recent publication of data from a CAR T clinical trial in five patients with refractory SLE. He said the data were impressive but the question for this treatment approach will be which patients are most likely to benefit and whether CAR T will experience the same ceiling effect because of pre-existing kidney damage.
“We won’t be seeing 100% response rates,” he said. “What we’ll be seeing, as a maximum, might be about 70%.” The big question for B-cell depletion in lupus was therefore how best to achieve it. “Is the future a potent monoclonal antibody, or is it in fact CAR T?”
Dr. Merrill said the analyses from B-cell depletion trials, showing greater response rates among more complete depleters, highlighted the importance of a personalized approach to treating lupus.
“One size fits all is never optimal in any disease, but it will prove a nonstarter in lupus, where we ought to be trying to find the optimal treatment regimen for each patient guided by biomarkers,” she said in an interview.
Dr. Furie reported having financial relationships with Genentech/Roche, which manufactures obinutuzumab and rituximab, as well as GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda. Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and AstraZeneca.
SEOUL, SOUTH KOREA – B cell–depleting therapies in patients with lupus nephritis have a higher likelihood of complete response if B cells are almost completely depleted, and strategies for achieving more complete B-cell depletion continue to be tested, according to evidence presented by Richard A. Furie, MD, at an international congress on systemic lupus erythematosus (SLE).
“If you go back about 20 years ago or so, when we designed the LUNAR and EXPLORER trials, we were scared to death of rituximab [Rituxan and biosimilars], about what would happen when you deplete B cells,” said Dr. Furie, chief of the division of rheumatology at Northwell Health in New York.
The LUNAR trial, which compared rituximab with placebo in patients with lupus nephritis, did not show a statistically significant difference in renal outcomes at 1 year. However, a post hoc analysis done several years later told a different story. It looked at patients who achieved complete peripheral depletion of B cells, defined as zero cells per microliter in peripheral blood. “You can see about a fourfold increase in complete response rates in those who were complete B-cell depleters at 1 year,” Dr. Furie told the conference.
It therefore raises the question of how to achieve greater B-cell depletion rates in patients. Dr. Furie said one strategy might be to first mobilize memory B cells and neutralize B cell–activating factor using belimumab (Benlysta), and then treat with rituximab to eliminate B cells. This strategy of sequential belimumab-rituximab treatment has been taken in several clinical trials.
More potent B-cell depletion with obinutuzumab
Another approach is to choose more potent B cell–depleting therapies, such as obinutuzumab (Gazyva), which is an anti-CD20 monoclonal antibody that was approved in 2013 for the treatment of chronic lymphocytic leukemia.
The NOBILITY trial compared obinutuzumab with placebo in 125 patients with lupus nephritis who were on background treatment with mycophenolate and corticosteroids. At 1 year, significantly more patients achieved B-cell thresholds either below 5 cells per microliter or even zero cells per microliter than had been seen previously with rituximab.
That also translated into clinical benefit, Dr. Furie said. By week 76, half the patients who had sustained depletion of B cells below 0.4 cells per microliter had a complete response, compared with 35% of those who still had detectable B cells and 18% of the placebo group. Treatment with obinutuzumab did not show any link to higher rates of serious adverse events, serious infections, or deaths.
“I think we’re all pretty much convinced more is better, without introducing safety issues,” Dr. Furie said in an interview.
Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said the data did suggest that renal outcomes were better with more complete depletion, but raised the question of whether this might increase the risk of infections or infectious severity.
Dr. Furie noted that complete response not only required improvement in proteinuria, complement levels, and anti–double-stranded DNA antibodies, but also in serum creatinine, “because maintenance of eGFR [estimated glomerular filtration rate] is the name of the game with lupus nephritis.”
However, he also pointed out that there may be a ceiling for response rates in patients with lupus nephritis when using stricter endpoints for serum creatinine. The NOBILITY trial required patients to achieve a serum creatinine that did not increase by more than 15% from baseline. But when researchers did an analysis that instead only required patients to achieve a reduction in proteinuria and maintain normal creatinine, the complete response rate in complete B-cell depleters increased to 72%, compared with 50% in partial depleters and 37% in the placebo group.
Newer strategies for greater B-cell depletion
A third strategy for achieving greater B-cell depletion is bispecific T-cell engagers, or BiTEs. “I called it a ‘frenemy,’ where it’s taking the activated T cell and introducing it to the B cell, and it can kill it via direct T-cell killing,” Dr. Furie said in an interview. Mosunetuzumab (Lunsumio) is one example, and is currently in a phase 1 clinical trial of patients with SLE.
And the fourth strategy, which has proved so successful in lymphoma, is chimeric antigen receptor T-cell therapy (CAR T). Dr. Furie cited the recent publication of data from a CAR T clinical trial in five patients with refractory SLE. He said the data were impressive but the question for this treatment approach will be which patients are most likely to benefit and whether CAR T will experience the same ceiling effect because of pre-existing kidney damage.
“We won’t be seeing 100% response rates,” he said. “What we’ll be seeing, as a maximum, might be about 70%.” The big question for B-cell depletion in lupus was therefore how best to achieve it. “Is the future a potent monoclonal antibody, or is it in fact CAR T?”
Dr. Merrill said the analyses from B-cell depletion trials, showing greater response rates among more complete depleters, highlighted the importance of a personalized approach to treating lupus.
“One size fits all is never optimal in any disease, but it will prove a nonstarter in lupus, where we ought to be trying to find the optimal treatment regimen for each patient guided by biomarkers,” she said in an interview.
Dr. Furie reported having financial relationships with Genentech/Roche, which manufactures obinutuzumab and rituximab, as well as GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda. Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and AstraZeneca.
SEOUL, SOUTH KOREA – B cell–depleting therapies in patients with lupus nephritis have a higher likelihood of complete response if B cells are almost completely depleted, and strategies for achieving more complete B-cell depletion continue to be tested, according to evidence presented by Richard A. Furie, MD, at an international congress on systemic lupus erythematosus (SLE).
“If you go back about 20 years ago or so, when we designed the LUNAR and EXPLORER trials, we were scared to death of rituximab [Rituxan and biosimilars], about what would happen when you deplete B cells,” said Dr. Furie, chief of the division of rheumatology at Northwell Health in New York.
The LUNAR trial, which compared rituximab with placebo in patients with lupus nephritis, did not show a statistically significant difference in renal outcomes at 1 year. However, a post hoc analysis done several years later told a different story. It looked at patients who achieved complete peripheral depletion of B cells, defined as zero cells per microliter in peripheral blood. “You can see about a fourfold increase in complete response rates in those who were complete B-cell depleters at 1 year,” Dr. Furie told the conference.
It therefore raises the question of how to achieve greater B-cell depletion rates in patients. Dr. Furie said one strategy might be to first mobilize memory B cells and neutralize B cell–activating factor using belimumab (Benlysta), and then treat with rituximab to eliminate B cells. This strategy of sequential belimumab-rituximab treatment has been taken in several clinical trials.
More potent B-cell depletion with obinutuzumab
Another approach is to choose more potent B cell–depleting therapies, such as obinutuzumab (Gazyva), which is an anti-CD20 monoclonal antibody that was approved in 2013 for the treatment of chronic lymphocytic leukemia.
The NOBILITY trial compared obinutuzumab with placebo in 125 patients with lupus nephritis who were on background treatment with mycophenolate and corticosteroids. At 1 year, significantly more patients achieved B-cell thresholds either below 5 cells per microliter or even zero cells per microliter than had been seen previously with rituximab.
That also translated into clinical benefit, Dr. Furie said. By week 76, half the patients who had sustained depletion of B cells below 0.4 cells per microliter had a complete response, compared with 35% of those who still had detectable B cells and 18% of the placebo group. Treatment with obinutuzumab did not show any link to higher rates of serious adverse events, serious infections, or deaths.
“I think we’re all pretty much convinced more is better, without introducing safety issues,” Dr. Furie said in an interview.
Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said the data did suggest that renal outcomes were better with more complete depletion, but raised the question of whether this might increase the risk of infections or infectious severity.
Dr. Furie noted that complete response not only required improvement in proteinuria, complement levels, and anti–double-stranded DNA antibodies, but also in serum creatinine, “because maintenance of eGFR [estimated glomerular filtration rate] is the name of the game with lupus nephritis.”
However, he also pointed out that there may be a ceiling for response rates in patients with lupus nephritis when using stricter endpoints for serum creatinine. The NOBILITY trial required patients to achieve a serum creatinine that did not increase by more than 15% from baseline. But when researchers did an analysis that instead only required patients to achieve a reduction in proteinuria and maintain normal creatinine, the complete response rate in complete B-cell depleters increased to 72%, compared with 50% in partial depleters and 37% in the placebo group.
Newer strategies for greater B-cell depletion
A third strategy for achieving greater B-cell depletion is bispecific T-cell engagers, or BiTEs. “I called it a ‘frenemy,’ where it’s taking the activated T cell and introducing it to the B cell, and it can kill it via direct T-cell killing,” Dr. Furie said in an interview. Mosunetuzumab (Lunsumio) is one example, and is currently in a phase 1 clinical trial of patients with SLE.
And the fourth strategy, which has proved so successful in lymphoma, is chimeric antigen receptor T-cell therapy (CAR T). Dr. Furie cited the recent publication of data from a CAR T clinical trial in five patients with refractory SLE. He said the data were impressive but the question for this treatment approach will be which patients are most likely to benefit and whether CAR T will experience the same ceiling effect because of pre-existing kidney damage.
“We won’t be seeing 100% response rates,” he said. “What we’ll be seeing, as a maximum, might be about 70%.” The big question for B-cell depletion in lupus was therefore how best to achieve it. “Is the future a potent monoclonal antibody, or is it in fact CAR T?”
Dr. Merrill said the analyses from B-cell depletion trials, showing greater response rates among more complete depleters, highlighted the importance of a personalized approach to treating lupus.
“One size fits all is never optimal in any disease, but it will prove a nonstarter in lupus, where we ought to be trying to find the optimal treatment regimen for each patient guided by biomarkers,” she said in an interview.
Dr. Furie reported having financial relationships with Genentech/Roche, which manufactures obinutuzumab and rituximab, as well as GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda. Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and AstraZeneca.
AT LUPUS 2023
In TNBC, repeated biopsies may reveal emergent HER2-low expression
Triple-negative breast cancer (TNBC) is characterized by the absence of hormonal receptors and human epidermal growth factor receptor 2 (HER2) expression. were found to be ineffective in patients with TNBC and known HER2-zero status.
These HER2-low results were of great clinical significance for this patient population, said Yael Bar, MD, PhD, during her presentation of the research, at the annual meeting of the American Society of Clinical Oncology (ASCO).
Previously, the DESTINY-Breast04 trial demonstrated that the antibody-drug conjugate (ADC) trastuzumab deruxtecan (T-DXd) improved progression-free survival (PFS) and overall survival (OS) for patients with HER2-low metastatic breast cancer. “As a result [of the DESTINY-Breast04 findings], T-DXd is now approved for HER2-low but not HER2-zero triple-negative metastatic breast cancer."
“While HER2-low is detected in about 30%-50% of patients with triple-negative breast cancer, several studies have shown that HER2 status is heterogeneous and also dynamic over time, said Dr. Bar, who is an international research fellow in the breast cancer group at Mass General Cancer Center, Boston.
In the new study, Dr. Bar and her co-authors retrospectively identified 512 TNBC patients from 2000 to 2022 from an institutional database. They included core, surgical, or metastatic biopsies. Participants had a mean age of 52 years, with 54% over age 50. They were 83% White, 7% African American, 5% Asian, 3% Hispanic, and 2% other. Stage II was most common at diagnosis at 48%, followed by stage 1 (28%), stage 3 (14%), and stage IV (8%).
Most patients had undergone one (38%) or two (45%) biopsies, while 9% underwent three biopsies, 6% underwent four biopsies, and 2% underwent five or more.
Among all 512 patients in the study, 60% had a HER2-low result on their first biopsy. As of the second biopsy, 73% had at least one HER2-low result, with 13% of the first HER2-low results occurring at the second biopsy. As of the third biopsy, 81% had a HER2-low result, with 9% occurring for the first time. At the fourth biopsy, 86% had a positive result, with 8% occurring for the first time. All patients with five or more biopsies had at least one HER2-low result and none were first-time results.
At the second biopsy, a HER2-low result was detected for 32% of patients for the first time. At the third biopsy, a new HER2-low result was detected in 33%, and at the fourth biopsy, a new HER-2 result was detected in 38%.
The researchers matched early and metastatic biopsies in 71 patients, and 44% had changed status: 68% of those with a status change went HER2-low to HER2-zero, 26% from HER2-zero to HER2-low, and 6% from HER2-low to HER2-positive. Among 50 patients with matched metastatic biopsies, 33% had a change in status, with 63% going from HER2-zero to HER2-low, 31% from HER2-low to HER2-zero, and 6% from HER2-low to HER2-positive.
“We showed here that repeat biopsies can identify new HER2-low results for patients who were previously ineligible for T-DXd; and therefore, we think that a repeat biopsy could be considered if feasible and safe. Also, if a repeat biopsy is performed for any reason, but mainly upon metastatic recurrence, receptors should be retested,” said Dr. Bar.
After Dr. Bar’s presentation, Barbara Pistilli, MD served as a discussant. She noted the increased HER2-low results over successive biopsies. “However, here the question is, are these results related to the changes in the analytical methods over the past 20 years or the changes in the guidelines in terms of definition of HER2 status, or are they more related to a true evolution of HER2 status with the evolution of the disease?” she said during her presentation. Dr. Pistilli is chair of the breast disease committee at Gustave Roussy in Villejuif, France.
She also said that HER2 expression can vary even between different parts of the same tumor and called for alternative methods to following HER2 expression. “I don’t think that we can follow our patients with multiple biopsies over the disease evolution, so we have to find other tools, such as target-positive [circulating tumor cells], or antibody-radiolabeled PET scan in order to better follow the intermetastasis target heterogeneity over time, and finally define what is the optimal ADC sequential strategy for each patient,” said Dr. Pistilli.
Comoderator Michael Danso, MD, also weighed in when asked for comment.
“It was an important trial to show that serial biopsies potentially allow more patients to receive trastuzumab deruxtecan,” said Dr. Danso, who is the research director at Virginia Oncology Associates, Norfolk. However, he pointed out the concerns of a statistician who had spoken up during the question-and-answer session who said that the positive results could simply be the consequence of repeated testing. “If you do a test often enough, statistically you’re going to get a difference in outcome. That was an important point made. Also, if you’re going to get 100% of patients who are eventually going to [develop HER2-low status], the question is, can you just treat everybody with trastuzumab deruxtecan and not do these sequential biopsies? Obviously that is subject to cost; it’s subject to toxicity as well, so you probably want documentation that there is a HER2-low result,” said Dr. Danso.
Dr. Bar has no relevant financial disclosures. Dr. Pistilli has consulted for or advised AstraZeneca, Daiichi Sankyo/UCB Japan, Myriad Genetics, Novartis, PIERRE FABRE, and Puma Biotechnology. She has received research funding through her institution from AstraZeneca, Daiichi Sankyo, Gilead Sciences, Merus, Pfizer, and Puma Biotechnology. She has received travel or accommodation expenses from AstraZeneca, Daiichi Sankyo Europe, MSD Oncology, Novartis, Pfizer, and Pierre Fabre. Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen.
*This story was updated on 6/13/2023.
Triple-negative breast cancer (TNBC) is characterized by the absence of hormonal receptors and human epidermal growth factor receptor 2 (HER2) expression. were found to be ineffective in patients with TNBC and known HER2-zero status.
These HER2-low results were of great clinical significance for this patient population, said Yael Bar, MD, PhD, during her presentation of the research, at the annual meeting of the American Society of Clinical Oncology (ASCO).
Previously, the DESTINY-Breast04 trial demonstrated that the antibody-drug conjugate (ADC) trastuzumab deruxtecan (T-DXd) improved progression-free survival (PFS) and overall survival (OS) for patients with HER2-low metastatic breast cancer. “As a result [of the DESTINY-Breast04 findings], T-DXd is now approved for HER2-low but not HER2-zero triple-negative metastatic breast cancer."
“While HER2-low is detected in about 30%-50% of patients with triple-negative breast cancer, several studies have shown that HER2 status is heterogeneous and also dynamic over time, said Dr. Bar, who is an international research fellow in the breast cancer group at Mass General Cancer Center, Boston.
In the new study, Dr. Bar and her co-authors retrospectively identified 512 TNBC patients from 2000 to 2022 from an institutional database. They included core, surgical, or metastatic biopsies. Participants had a mean age of 52 years, with 54% over age 50. They were 83% White, 7% African American, 5% Asian, 3% Hispanic, and 2% other. Stage II was most common at diagnosis at 48%, followed by stage 1 (28%), stage 3 (14%), and stage IV (8%).
Most patients had undergone one (38%) or two (45%) biopsies, while 9% underwent three biopsies, 6% underwent four biopsies, and 2% underwent five or more.
Among all 512 patients in the study, 60% had a HER2-low result on their first biopsy. As of the second biopsy, 73% had at least one HER2-low result, with 13% of the first HER2-low results occurring at the second biopsy. As of the third biopsy, 81% had a HER2-low result, with 9% occurring for the first time. At the fourth biopsy, 86% had a positive result, with 8% occurring for the first time. All patients with five or more biopsies had at least one HER2-low result and none were first-time results.
At the second biopsy, a HER2-low result was detected for 32% of patients for the first time. At the third biopsy, a new HER2-low result was detected in 33%, and at the fourth biopsy, a new HER-2 result was detected in 38%.
The researchers matched early and metastatic biopsies in 71 patients, and 44% had changed status: 68% of those with a status change went HER2-low to HER2-zero, 26% from HER2-zero to HER2-low, and 6% from HER2-low to HER2-positive. Among 50 patients with matched metastatic biopsies, 33% had a change in status, with 63% going from HER2-zero to HER2-low, 31% from HER2-low to HER2-zero, and 6% from HER2-low to HER2-positive.
“We showed here that repeat biopsies can identify new HER2-low results for patients who were previously ineligible for T-DXd; and therefore, we think that a repeat biopsy could be considered if feasible and safe. Also, if a repeat biopsy is performed for any reason, but mainly upon metastatic recurrence, receptors should be retested,” said Dr. Bar.
After Dr. Bar’s presentation, Barbara Pistilli, MD served as a discussant. She noted the increased HER2-low results over successive biopsies. “However, here the question is, are these results related to the changes in the analytical methods over the past 20 years or the changes in the guidelines in terms of definition of HER2 status, or are they more related to a true evolution of HER2 status with the evolution of the disease?” she said during her presentation. Dr. Pistilli is chair of the breast disease committee at Gustave Roussy in Villejuif, France.
She also said that HER2 expression can vary even between different parts of the same tumor and called for alternative methods to following HER2 expression. “I don’t think that we can follow our patients with multiple biopsies over the disease evolution, so we have to find other tools, such as target-positive [circulating tumor cells], or antibody-radiolabeled PET scan in order to better follow the intermetastasis target heterogeneity over time, and finally define what is the optimal ADC sequential strategy for each patient,” said Dr. Pistilli.
Comoderator Michael Danso, MD, also weighed in when asked for comment.
“It was an important trial to show that serial biopsies potentially allow more patients to receive trastuzumab deruxtecan,” said Dr. Danso, who is the research director at Virginia Oncology Associates, Norfolk. However, he pointed out the concerns of a statistician who had spoken up during the question-and-answer session who said that the positive results could simply be the consequence of repeated testing. “If you do a test often enough, statistically you’re going to get a difference in outcome. That was an important point made. Also, if you’re going to get 100% of patients who are eventually going to [develop HER2-low status], the question is, can you just treat everybody with trastuzumab deruxtecan and not do these sequential biopsies? Obviously that is subject to cost; it’s subject to toxicity as well, so you probably want documentation that there is a HER2-low result,” said Dr. Danso.
Dr. Bar has no relevant financial disclosures. Dr. Pistilli has consulted for or advised AstraZeneca, Daiichi Sankyo/UCB Japan, Myriad Genetics, Novartis, PIERRE FABRE, and Puma Biotechnology. She has received research funding through her institution from AstraZeneca, Daiichi Sankyo, Gilead Sciences, Merus, Pfizer, and Puma Biotechnology. She has received travel or accommodation expenses from AstraZeneca, Daiichi Sankyo Europe, MSD Oncology, Novartis, Pfizer, and Pierre Fabre. Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen.
*This story was updated on 6/13/2023.
Triple-negative breast cancer (TNBC) is characterized by the absence of hormonal receptors and human epidermal growth factor receptor 2 (HER2) expression. were found to be ineffective in patients with TNBC and known HER2-zero status.
These HER2-low results were of great clinical significance for this patient population, said Yael Bar, MD, PhD, during her presentation of the research, at the annual meeting of the American Society of Clinical Oncology (ASCO).
Previously, the DESTINY-Breast04 trial demonstrated that the antibody-drug conjugate (ADC) trastuzumab deruxtecan (T-DXd) improved progression-free survival (PFS) and overall survival (OS) for patients with HER2-low metastatic breast cancer. “As a result [of the DESTINY-Breast04 findings], T-DXd is now approved for HER2-low but not HER2-zero triple-negative metastatic breast cancer."
“While HER2-low is detected in about 30%-50% of patients with triple-negative breast cancer, several studies have shown that HER2 status is heterogeneous and also dynamic over time, said Dr. Bar, who is an international research fellow in the breast cancer group at Mass General Cancer Center, Boston.
In the new study, Dr. Bar and her co-authors retrospectively identified 512 TNBC patients from 2000 to 2022 from an institutional database. They included core, surgical, or metastatic biopsies. Participants had a mean age of 52 years, with 54% over age 50. They were 83% White, 7% African American, 5% Asian, 3% Hispanic, and 2% other. Stage II was most common at diagnosis at 48%, followed by stage 1 (28%), stage 3 (14%), and stage IV (8%).
Most patients had undergone one (38%) or two (45%) biopsies, while 9% underwent three biopsies, 6% underwent four biopsies, and 2% underwent five or more.
Among all 512 patients in the study, 60% had a HER2-low result on their first biopsy. As of the second biopsy, 73% had at least one HER2-low result, with 13% of the first HER2-low results occurring at the second biopsy. As of the third biopsy, 81% had a HER2-low result, with 9% occurring for the first time. At the fourth biopsy, 86% had a positive result, with 8% occurring for the first time. All patients with five or more biopsies had at least one HER2-low result and none were first-time results.
At the second biopsy, a HER2-low result was detected for 32% of patients for the first time. At the third biopsy, a new HER2-low result was detected in 33%, and at the fourth biopsy, a new HER-2 result was detected in 38%.
The researchers matched early and metastatic biopsies in 71 patients, and 44% had changed status: 68% of those with a status change went HER2-low to HER2-zero, 26% from HER2-zero to HER2-low, and 6% from HER2-low to HER2-positive. Among 50 patients with matched metastatic biopsies, 33% had a change in status, with 63% going from HER2-zero to HER2-low, 31% from HER2-low to HER2-zero, and 6% from HER2-low to HER2-positive.
“We showed here that repeat biopsies can identify new HER2-low results for patients who were previously ineligible for T-DXd; and therefore, we think that a repeat biopsy could be considered if feasible and safe. Also, if a repeat biopsy is performed for any reason, but mainly upon metastatic recurrence, receptors should be retested,” said Dr. Bar.
After Dr. Bar’s presentation, Barbara Pistilli, MD served as a discussant. She noted the increased HER2-low results over successive biopsies. “However, here the question is, are these results related to the changes in the analytical methods over the past 20 years or the changes in the guidelines in terms of definition of HER2 status, or are they more related to a true evolution of HER2 status with the evolution of the disease?” she said during her presentation. Dr. Pistilli is chair of the breast disease committee at Gustave Roussy in Villejuif, France.
She also said that HER2 expression can vary even between different parts of the same tumor and called for alternative methods to following HER2 expression. “I don’t think that we can follow our patients with multiple biopsies over the disease evolution, so we have to find other tools, such as target-positive [circulating tumor cells], or antibody-radiolabeled PET scan in order to better follow the intermetastasis target heterogeneity over time, and finally define what is the optimal ADC sequential strategy for each patient,” said Dr. Pistilli.
Comoderator Michael Danso, MD, also weighed in when asked for comment.
“It was an important trial to show that serial biopsies potentially allow more patients to receive trastuzumab deruxtecan,” said Dr. Danso, who is the research director at Virginia Oncology Associates, Norfolk. However, he pointed out the concerns of a statistician who had spoken up during the question-and-answer session who said that the positive results could simply be the consequence of repeated testing. “If you do a test often enough, statistically you’re going to get a difference in outcome. That was an important point made. Also, if you’re going to get 100% of patients who are eventually going to [develop HER2-low status], the question is, can you just treat everybody with trastuzumab deruxtecan and not do these sequential biopsies? Obviously that is subject to cost; it’s subject to toxicity as well, so you probably want documentation that there is a HER2-low result,” said Dr. Danso.
Dr. Bar has no relevant financial disclosures. Dr. Pistilli has consulted for or advised AstraZeneca, Daiichi Sankyo/UCB Japan, Myriad Genetics, Novartis, PIERRE FABRE, and Puma Biotechnology. She has received research funding through her institution from AstraZeneca, Daiichi Sankyo, Gilead Sciences, Merus, Pfizer, and Puma Biotechnology. She has received travel or accommodation expenses from AstraZeneca, Daiichi Sankyo Europe, MSD Oncology, Novartis, Pfizer, and Pierre Fabre. Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen.
*This story was updated on 6/13/2023.
FROM ASCO 2023