User login
Treatment of Frontal Fibrosing Alopecia in Black Patients: A Systematic Review
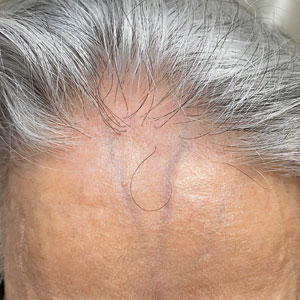
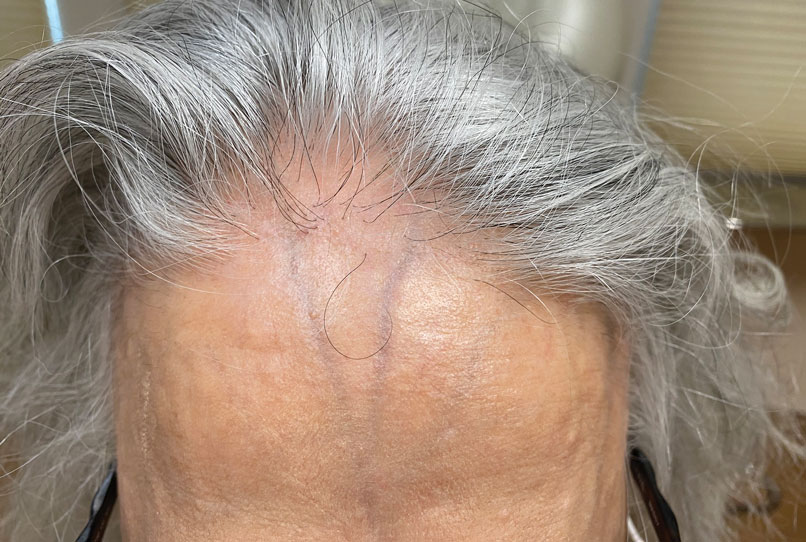
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
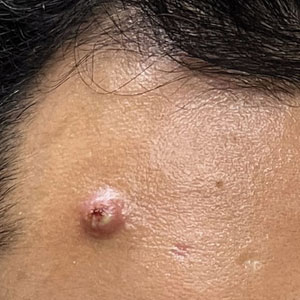
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.
The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
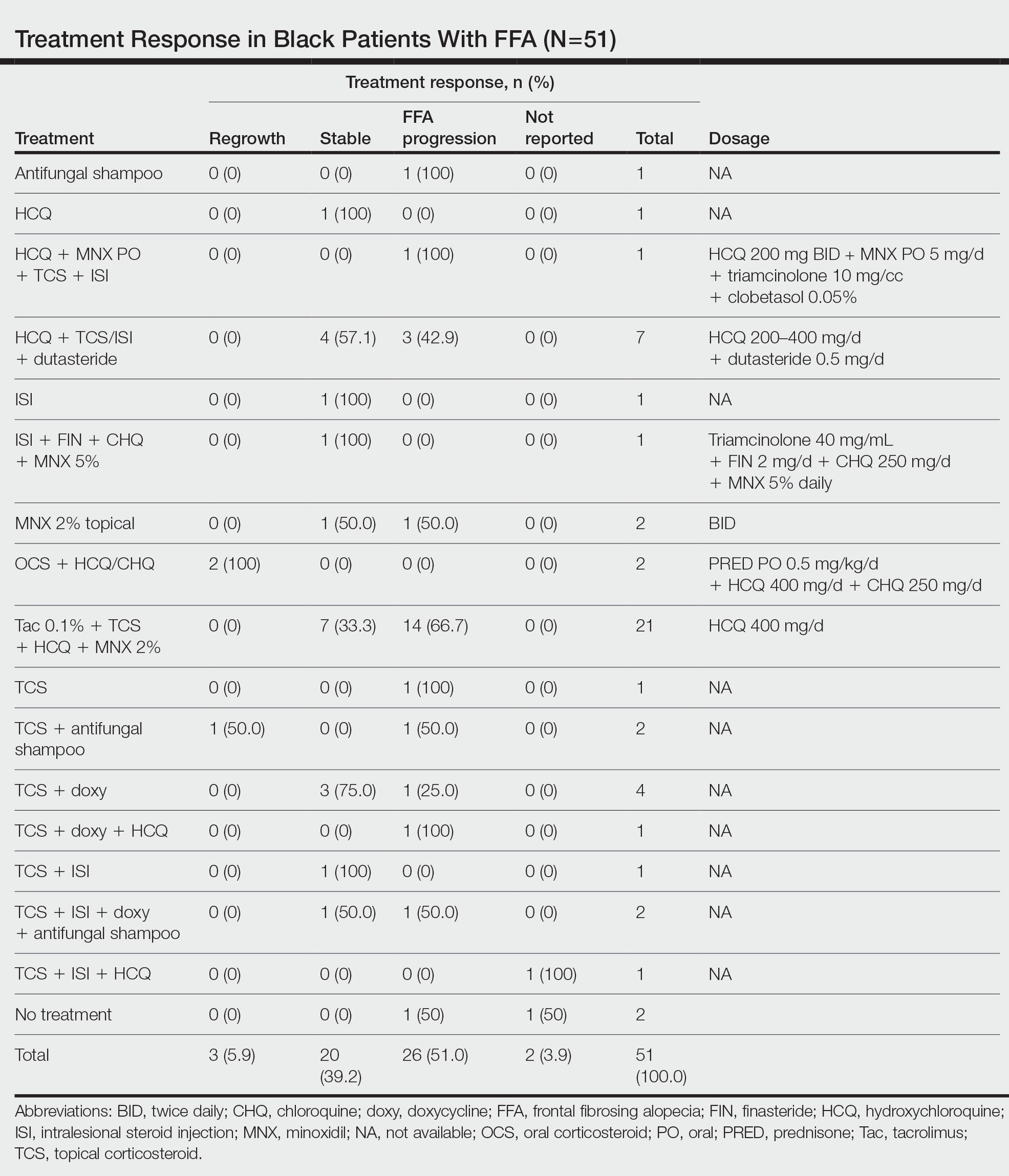
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.
Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.

- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


Frontal fibrosing alopecia (FFA) is a lymphocytic cicatricial alopecia that primarily affects postmenopausal women. Considered a subtype of lichen planopilaris (LPP), FFA is histologically identical but presents as symmetric frontotemporal hairline recession rather than the multifocal distribution typical of LPP (Figure 1). Patients also may experience symptoms such as itching, facial papules, and eyebrow loss. As a progressive and scarring alopecia, early management of FFA is necessary to prevent permanent hair loss; however, there still are no clear guidelines regarding the efficacy of different treatment options for FFA due to a lack of randomized controlled studies in the literature. Patients with skin of color (SOC) also may have varying responses to treatment, further complicating the establishment of any treatment algorithm. Furthermore, symptoms, clinical findings, and demographics of FFA have been observed to vary across different ethnicities, especially among Black individuals. We conducted a systematic review of the literature on FFA in Black patients, with an analysis of demographics, clinical findings, concomitant skin conditions, treatments given, and treatment responses.

Methods
A PubMed search of articles indexed for MEDLINE was conducted of studies investigating FFA in patients with SOC from January 1, 2000, through November 30, 2020, using the terms frontal fibrosing alopecia, ethnicity, African, Black, Asian, Indian, Hispanic, and Latino. Articles were included if they were available in English and discussed treatment and clinical outcomes of FFA in Black individuals. The reference lists of included studies also were reviewed. Articles were assessed for quality of evidence using a 4-point scale (1=well-designed randomized controlled trials; 2=controlled trials with limitations or well-designed cohort or case-control studies; 3=case series with or without intervention; 4=case reports). Variables related to study type, patient demographics, treatments, and clinical outcomes were recorded.
Results
Of the 69 search results, 8 studies—2 retrospective cohort studies, 3 case series, and 3 case reports—describing 51 Black individuals with FFA were included in our review (eTable). Of these, 49 (96.1%) were female and 2 (3.9%) were male. Of the 45 females with data available for menopausal status, 24 (53.3%) were premenopausal and 21 (46.7%) were postmenopausal; data were not available for 4 females. Patients identified as African or African American in 27 (52.9%) cases, South African in 19 (37.3%), Black in 3 (5.9%), Indian in 1 (2.0%), and Afro-Caribbean in 1 (2.0%). The average age of FFA onset was 43.8 years in females (raw data available in 24 patients) and 35 years in males (raw data available in 2 patients). A family history of hair loss was reported in 15.7% (8/51) of patients.
Involved areas of hair loss included the frontotemporal hairline (51/51 [100%]), eyebrows (32/51 [62.7%]), limbs (4/51 [7.8%]), occiput (4/51 [7.8%]), facial hair (2/51 [3.9%]), vertex scalp (1/51 [2.0%]), and eyelashes (1/51 [2.0%]). Patchy alopecia suggestive of LPP was reported in 2 (3.9%) patients.
Patients frequently presented with scalp pruritus (26/51 [51.0%]), perifollicular papules or pustules (9/51 [17.6%]), and perifollicular hyperpigmentation (9/51 [17.6%]). Other associated symptoms included perifollicular erythema (6/51 [11.8%]), scalp pain (5/51 [9.8%]), hyperkeratosis or flaking (3/51 [5.9%]), and facial papules (2/51 [3.9%]). Loss of follicular ostia, prominent follicular ostia, and the lonely hair sign (Figure 2) was described in 21 (41.2%), 5 (9.8%), and 15 (29.4%) of patients, respectively. Hairstyles that involve scalp traction (19/51 [37.3%]) and/or chemicals (28/51 [54.9%]), such as hair dye or chemical relaxers, commonly were reported in patients prior to the onset of FFA.

The most commonly reported dermatologic comorbidities included traction alopecia (17/51 [33.3%]), followed by lichen planus pigmentosus (LLPigm)(7/51 [13.7%]), LPP (2/51 [3.9%]), psoriasis (1/51 [2.0%]), and morphea (1/51 [2.0%]). Reported comorbid diseases included Sjögren syndrome (2/51 [3.9%]), hypothyroidism (2/51 [3.9%]), HIV (1/51 [2.0%]), and diabetes mellitus (1/51 [2.0%]).
Of available reports (n=32), the most common histologic findings included perifollicular fibrosis (23/32 [71.9%]), lichenoid lymphocytic inflammation (22/23 [95.7%]) primarily affecting the isthmus and infundibular areas of the follicles, and decreased follicular density (21/23 [91.3%]).
The average time interval from treatment initiation to treatment assessment in available reports (n=25) was 1.8 years (range, 0.5–2 years). Response to treatment included regrowth of hair in 5.9% (3/51) of patients, FFA stabilization in 39.2% (20/51), FFA progression in 51.0% (26/51), and not reported in 3.9% (2/51). Combination therapy was used in 84.3% (43/51) of patients, while monotherapy was used in 11.8% (6/51), and 3.9% (2/51) did not have any treatment reported. Response to treatment was highly variable among patients, as were the combinations of therapeutic agents used (Table). Regrowth of hair was rare, occurring in only 2 (100%) patients treated with oral prednisone plus hydroxychloroquine (HCQ) or chloroquine (CHQ), and in 1 (50.0%) patient treated with topical corticosteroids plus antifungal shampoo, while there was no response in the other patient treated with this combination.

Improvement in hair loss, defined as having at least slowed progression of FFA, was observed in 100% (2/2) of patients who had oral steroids as part of their treatment regimen, followed by 5-alpha-reductase inhibitors (5-ARIs)(finasteride and dutasteride; 62.5% [5/8]), intralesional steroids (57.1% [8/14]), HCQ/CHQ (42.9% [15/35]), topical steroids (41.5% [17/41]), antifungal shampoo (40.0% [2/5]), topical/oral minoxidil (36.0% [9/25]), and tacrolimus (33.3% [7/21]).
Comment
Frontal fibrosing alopecia is a progressive scarring alopecia and a clinical variant of LPP. First described in 1994 by Kossard,1 it initially was thought to be a disease of postmenopausal White women. Although still most prevalent in White individuals, there has been a growing number of reports describing FFA in patients with SOC, including Black individuals.10 Despite the increasing number of cases over the years, studies on the treatment of FFA remain sparse. Without expert guidelines, treatments usually are chosen based on clinician preferences. Few observational studies on these treatment modalities and their clinical outcomes exist, and the cohorts largely are composed of White patients.10-12 However, Black individuals may respond differently to these treatments, just as they have been shown to exhibit unique features of FFA.3
Demographics of Patients With FFA—Consistent with our findings, prior studies have found that Black patients are more likely to be younger and premenopausal at FFA onset than their White counterparts.13-15 Among the Black individuals included in our review, the majority were premenopausal (53%) with an average age of FFA onset of 46.7 years. Conversely, only 5% of 60 White females with FFA reported in a retrospective review were premenopausal and had an older mean age of FFA onset of 64 years,1 substantiating prior reports.
Clinical Findings in Patients With FFA—The clinical findings observed in our cohort were consistent with what has previously been described in Black patients, including loss of follicular ostia (41.2%), lonely hair sign (29.4%), perifollicular erythema (11.8%), perifollicular papules (17.6%), and hyperkeratosis or flaking (5.9%). In comparing these findings with a review of 932 patients, 86% of whom were White, the observed frequencies of follicular ostia loss (38.3%) and lonely hair sign (26.7%) were similar; however, perifollicular erythema (44.2%), and hyperkeratosis (44.4%) were more prevalent in this group, while perifollicular papules (6.2%) were less common compared to our Black cohort.16 An explanation for this discrepancy in perifollicular erythema may be the increased skin pigmentation diminishing the appearance of erythema in Black individuals. Our cohort of Black individuals noted the presence of follicular hyperpigmentation (17.6%) and a high prevalence of scalp pruritus (51.0%), which appear to be more common in Black patients.3,17 Although it is unclear why these differences in FFA presentation exist, it may be helpful for clinicians to be aware of these unique features when examining Black patients with suspected FFA.
Concomitant Cutaneous Disorders—A notable proportion of our cohort also had concomitant traction alopecia, which presents with frontotemporal alopecia, similar to FFA, making the diagnosis more challenging; however, the presence of perifollicular hyperpigmentation and facial hyperpigmentation in FFA may aid in differentiating these 2 entities.3 Other concomitant conditions noted in our review included androgenic alopecia, Sjögren syndrome, psoriasis, hypothyroidism, morphea, and HIV, suggesting a potential interplay between autoimmune, genetic, hormonal, and environmental components in the etiology of FFA. In fact, a recent study found that a persistent inflammatory response, loss of immune privilege, and a genetic susceptibility are some of the key processes in the pathogenesis of FFA.18 Although the authors speculated that there may be other triggers in initiating the onset of FFA, such as steroid hormones, sun exposure, and topical allergens, more evidence and controlled studies are needed
Additionally, concomitant LPPigm occurred in 13.7% of our FFA cohort, which appears to be more common in patients with darker skin types.5,19-21 Lichen planus pigmentosus is a rare variant of LPP, and previous reports suggest that it may be associated with FFA.5 Similar to FFA, the pathogenesis of LPPigm also is unclear, and its treatment may be just as difficult.22 Because LPPigm may occur before, during, or after onset of FFA,23 it may be helpful for clinicians to search for the signs of LPPigm in patients with darker skin types patients presenting with hair loss both as a diagnostic clue and so that treatment may be tailored to both conditions.
Response to Treatment—Similar to the varying clinical pictures, the response to treatment also can vary between patients of different ethnicities. For Black patients, treatment outcomes did not seem as successful as they did for other patients with SOC described in the literature. A retrospective cohort of 58 Asian individuals with FFA found that up to 90% had improvement or stabilization of FFA after treatment,23 while only 45.1% (23/51) of the Black patients included in our study had improvement or stabilization. One reason may be that a greater proportion of Black patients are premenopausal at FFA onset (53%) compared to what is reported in Asian patients (28%),23 and women who are premenopausal at FFA onset often face more severe disease.15 Although there may be additional explanations for these differences in treatment outcomes between ethnic groups, further investigation is needed.
All patients included in our study received either monotherapy or combination therapy of topical/intralesional/oral steroids, HCQ or CHQ, 5-ARIs, topical/oral minoxidil, antifungal shampoo, and/or a calcineurin inhibitor; however, most patients (51.0%) did not see a response to treatment, while only 45.1% showed slowed or halted progression of FFA. Hair regrowth was rare, occurring in only 3 (5.9%) patients; 2 of them were the only patients treated with oral prednisone, making for a potentially promising therapeutic for Black patients that should be further investigated in larger controlled cohort studies. In a prior study, intramuscular steroids (40 mg every 3 weeks) plus topical minoxidil were unsuccessful in slowing the progression of FFA in 3 postmenopausal women,24 which may be explained by the racial differences in the response to FFA treatments and perhaps also menopausal status. Although not included in any of the regimens in our review, isotretinoin was shown to be effective in an ethnically unspecified group of patients (n=16) and also may be efficacious in Black individuals.25 Although FFA may stabilize with time,26 this was not observed in any of the patients included in our study; however, we only included patients who were treated, making it impossible to discern whether resolution was idiopathic or due to treatment.
Future Research—Research on treatments for FFA is lacking, especially in patients with SOC. Although we observed that there may be differences in the treatment response among Black individuals compared to other patients with SOC, additional studies are needed to delineate these racial differences, which can help guide management. More randomized controlled trials evaluating the various treatment regimens also are required to establish treatment guidelines. Frontal fibrosing alopecia likely is underdiagnosed in Black individuals, contributing to the lack of research in this group. Darker skin can obscure some of the clinical and dermoscopic features that are more visible in fair skin. Furthermore, it may be challenging to distinguish clinical features of FFA in the setting of concomitant traction alopecia, which is more common in Black patients.27 Frontal fibrosing alopecia presenting in Black women also is less likely to be biopsied, contributing to the tendency to miss FFA in favor of traction or androgenic alopecia, which often are assumed to be more common in this population.2,27 Therefore, histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Study Limitations—The studies included in our review were limited by a lack of control comparison groups, especially among the retrospective cohort studies. Additionally, some of the studies included cases refractory to prior treatment modalities, possibly leading to a selection bias of more severe cases that were not representative of FFA in the general population. Thus, further studies involving larger populations of those with SOC are needed to fully evaluate the clinical utility of the current treatment modalities in this group.


- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
- Kossard S. Postmenopausal frontal fibrosing alopecia. scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. doi:10.1111/bjd.12424
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. doi:10.1001/archdermatol.2011.321
- Uwakwe LN, Cardwell LA, Dothard EH, et al. Frontal fibrosing alopecia and concomitant lichen planus pigmentosus: a case series of seven African American women. J Drugs Dermatol. 2018;17:397-400.
- Naz E, Vidaurrázaga C, Hernández-Cano N, et al. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. doi:10.1046/j.1365-2230.2003.01131.x
- Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81-83. doi:10.1111/j.1365-4632.2012.05821.x
- Huerth K, Kindred C. Frontal fibrosing alopecia presenting as androgenetic alopecia in an African American woman. J Drugs Dermatol. 2020;19:794-795. doi:10.36849/jdd.2020.4682
- Furlan KC, Kakizaki P, Chartuni JC, et al. Frontal fibrosing alopecia in association with Sjögren’s syndrome: more than a simple coincidence. An Bras Dermatol. 2016;91(5 suppl 1):14-16. doi:10.1590/abd1806-4841.20164526
- Zhang M, Zhang L, Rosman IS, et al. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103:E16-E22.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. doi:10.1016/j.jaad.2011.12.038
- Starace M, Brandi N, Alessandrini A, et al. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33:433-438. doi:10.1111/jdv.15372
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442. doi:10.1111/j.1365-2133.2012.11146.x
- Petrof G, Cuell A, Rajkomar VV, et al. Retrospective review of 18 British South Asian women with frontal fibrosing alopecia. Int J Dermatol. 2018;57:490-491. doi:10.1111/ijd.13929
- Mervis JS, Borda LJ, Miteva M. Facial and extrafacial lesions in an ethnically diverse series of 91 patients with frontal fibrosing alopecia followed at a single center. Dermatology. 2019;235:112-119. doi:10.1159/000494603
- Valesky EM, Maier MD, Kippenberger S, et al. Frontal fibrosing alopecia - review of recent case reports and case series in PubMed. J Dtsch Dermatol Ges. Aug 2018;16:992-999. doi:10.1111/ddg.13601
- Adotama P, Callender V, Kolla A, et al. Comparing the clinical differences in white and black women with frontal fibrosing alopecia. Br J Dermatol. 2021;185:1074-1076. doi:10.1111/bjd.20605
- Miao YJ, Jing J, Du XF, et al. Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390. doi:10.1111/bjd.14722
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27. doi:10.1016/j.jaad.2013.12.031
- Romiti R, Biancardi Gavioli CF, et al. Clinical and histopathological findings of frontal fibrosing alopecia-associated lichen planus pigmentosus. Skin Appendage Disord. 2017;3:59-63. doi:10.1159/000456038
- Mulinari-Brenner FA, Guilherme MR, Peretti MC, et al. Frontal fibrosing alopecia and lichen planus pigmentosus: diagnosis and therapeutic challenge. An Bras Dermatol. 2017;92(5 suppl 1):79-81. doi:10.1590/abd1806-4841.20175833
- Panchaprateep R, Ruxrungtham P, Chancheewa B, et al. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47:1301-1311. doi:10.1111/1346-8138.15517
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. doi:10.1016/j.jaad.2004.05.014
- Rokni GR, Emadi SN, Dabbaghzade A, et al. Evaluating the combined efficacy of oral isotretinoin and topical tacrolimus versus oral finasteride and topical tacrolimus in frontal fibrosing alopecia—a randomized controlled trial. J Cosmet Dermatol. 2023;22:613-619. doi:10.1111/jocd.15232
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. doi:10.1016/s0190-9622(97)70326-8
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. doi:10.1111/j.1365-2133.2012.10809.x
Practice Points
- Treatment of frontal fibrosing alopecia (FFA) is challenging, and there are no evidence-based treatment guidelines available. Patients with skin of color (SOC) may have varying responses to treatment modalities.
- Special consideration should be taken when treating FFA in patients with SOC.
- Histologic evaluation through biopsy is paramount in securing an accurate diagnosis for Black patients with frontotemporal alopecia.
Childhood lupus severity linked to social determinants of health
NEW ORLEANS – The sociodemographic characteristics of Black and Hispanic children with systemic lupus erythematosus (SLE) appear to play a strong role in influencing the severity of disease in these patients, according to two studies presented at the Pediatric Rheumatology Symposium.
One study showed an association between multiple determinants of health and disease severity among children seen in a large Texas city, and a separate descriptive cross-sectional cohort study of predominantly Black children at two centers in Mississippi and Alabama reinforced the finding of greater severity of disease and social hardships among this racial group.
The findings from both studies supplement existing evidence that the prevalence of childhood-onset SLE is greater among Black and Hispanic children.
“Several demographic and social determinants of health parameters influenced disease severity at levels that reached statistical significance, including insurance status, race/ethnicity, referral source, PCP [primary care provider] availability, primary language, and transportation needs,” Emily Beil, MD, a pediatric rheumatologist at Texas Children’s Hospital in Houston, told attendees at the conference, which was sponsored by the American College of Rheumatology. Her team’s goal, she said, was to “better understand our patient population and social disparities that contribute to disease severity.”
Dr. Beil and her colleagues conducted a retrospective review of 136 children who had been diagnosed with childhood-onset SLE between January 2018 and May 2022 at Texas Children’s Hospital. Only children who were younger than 18 years at the time of diagnosis at Texas Children’s were included. The analysis considered demographics, clinical characteristics, insurance status, social work consultation, access to a primary care provider, transportation needs, primary language, and other parameters related to social determinants of health.
The average age of the patients was 13 years, and most were girls (82%). Just over half were Hispanic (53%), and just over a quarter were Black (26%). Half had Medicaid or participated in the Children’s Health Insurance Program (CHIP), and 1 in 10 were uninsured (10%). Half the diagnoses were made during an inpatient admission; 36% were made on the floor, and 14% were made in the intensive care unit (ICU).
The average Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score was 12.5, and 48.5% of patients had severe disease, indicated by a score of at least 12. Only two in three children were documented as having a primary care physician (66%), and 32% preferred a language other than English. Most of the children (80%) had a social work consult.
Black and biracial children had higher SLEDAI scores at presentation. Non-Hispanic White children were less likely to have a social work consult, compared with other racial/ethnic groups (P = .01 for both). Central nervous system involvement was most prevalent among Black patients (P = .004). Cyclophosphamide was used most often for Black and biracial patients.
Uninsured patients were most likely to be diagnosed on an inpatient floor. The highest proportion of ICU admissions was among patients insured by Medicaid (P = .034). Average SLEDAI scores were highest among uninsured patients, followed by Medicaid patients. More than half of the patients who did not have insurance lacked access to a regular primary care provider, compared with 12% of Medicaid patients and 7% of privately insured patients (P = .001). All the uninsured patients had transportation needs, which was a significantly higher rate than among those with Medicaid (13%) or private insurance (15%) (P = .001). The highest percentage of social work consults was among patients who were insured by Medicaid or were without insurance (P = .001).
Salient demographics and clinical features
In the second presentation, Anita Dhanrajani, MD, assistant professor of pediatrics at the University of Mississippi Medical Center in Jackson, began by noting that Alabama and Mississippi are ranked in the top 10 states for the highest poverty rate: Mississippi is No. 1, and Alabama is No. 7. Further, 40% of children in Mississippi and 29% of children in Alabama are of African American ancestry, she said.
“So, we know that this population that we’re dealing with has several high-risk factors that can lead them to have poor outcomes, and yet, we haven’t really ever characterized their clinical features or their social demographic features,” Dr. Dhanrajani told attendees. “My hope is that with this very miniscule first step, we’re able to move towards solutions to decrease health care disparities in this population.”
She presented findings regarding the first of three aims in the study, which was to describe the baseline clinical, demographic, and socioeconomic profiles of childhood lupus patients at the two centers. The two other aims were to examine genetic factors potentially linked to poor outcomes in the cohort and to assess the mental health status of the population.
The study relied on a retrospective chart review for the 17 patients at the University of Mississippi Medical Center and on Childhood Arthritis and Rheumatology Research Alliance registry data for the 19 patients at the University of Alabama at Birmingham. Most of the patients (86%) were female, Black (78%), and insured by Medicaid (64%). The average age at diagnosis was 13 years. Most (83%) also lived in a ZIP code that met the criteria for a medium-high or high Social Vulnerability Index. The children had to travel an average 75 miles to see a rheumatologist, compared with the national average of 43 miles.
At diagnosis, their average Systemic Lupus International Collaborating Clinics (SLICC) score was 8.8, their average American College of Rheumatology score was 5.2, and their average SLEDAI score was 12.1 – the latter was substantially higher than the average 3.1 score in a multiethnic Canadian cohort (the 1000 Canadian Faces of Lupus Study) with 10% Black children (P < .00001). The SLEDAI score dropped to 6.8 at 6 months and to 4 at 1 year. Nearly half (47%) had a SLICC Damage Index (SDI) greater than 0, and one-third had an SDI of 2 or greater, compared with 16% and 7%, respectively, reported in other recent studies (P < .0001 for both).
“These disparities are very difficult to investigate in terms of causal relationships and [are] likely to be very modifiable,” Coziana Ciurtin, MD, PhD, associate professor of rheumatology at University College London, told this news organization. “I think the socioeconomic status, the level of education, poverty, [type of] medical insurance, and probably genetic variants are all underpinning the presentation, damage, or disease activity being very high, and also organ involvement,” such as the greater CNS involvement seen in non-White patients.
Being mindful of these risk profiles can help doctors in asking about patients’ support at home and their families’ education, beliefs, and cultural practices, Dr. Ciurtin added. “Helping them to engage and be involved in decision-making is probably the most important” aspect of learning this information about families, she said.
Collecting this information should not be the sole responsibility of the physician, added Eve Smith, PhD, MBCHB, an academic clinical lecturer at the University of Liverpool, England, who attended the presentations. Dr. Smith noted a discussion in a work group during the previous day of the conference concerning questionnaires for screening patients regarding the need for social services and for identifying areas in which patients and their families were having difficulties.
“Obviously, if you’re going to do that, you have to have access to someone who can actually help to deal with that. Some hospitals have patient navigators that can help, for example, with a food security issue to highlight resources within the community, so it’s not all on the doctor,” Dr. Smith said. “To really make a difference in this area, it can’t just be down to the doctor. There needs to be social care, there needs to be community-based interventions and things to do about it. Doctors can help identify these patients, or maybe somebody in the [medical] team can help with that, but there needs to be an intervention. Otherwise, you’re left with this problem without a solution that you can’t do anything about.”
The researchers did not note any external funding for either study. Dr. Beil, Dr. Dhanrajani, Dr. Smith, and Dr. Ciurtin reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – The sociodemographic characteristics of Black and Hispanic children with systemic lupus erythematosus (SLE) appear to play a strong role in influencing the severity of disease in these patients, according to two studies presented at the Pediatric Rheumatology Symposium.
One study showed an association between multiple determinants of health and disease severity among children seen in a large Texas city, and a separate descriptive cross-sectional cohort study of predominantly Black children at two centers in Mississippi and Alabama reinforced the finding of greater severity of disease and social hardships among this racial group.
The findings from both studies supplement existing evidence that the prevalence of childhood-onset SLE is greater among Black and Hispanic children.
“Several demographic and social determinants of health parameters influenced disease severity at levels that reached statistical significance, including insurance status, race/ethnicity, referral source, PCP [primary care provider] availability, primary language, and transportation needs,” Emily Beil, MD, a pediatric rheumatologist at Texas Children’s Hospital in Houston, told attendees at the conference, which was sponsored by the American College of Rheumatology. Her team’s goal, she said, was to “better understand our patient population and social disparities that contribute to disease severity.”
Dr. Beil and her colleagues conducted a retrospective review of 136 children who had been diagnosed with childhood-onset SLE between January 2018 and May 2022 at Texas Children’s Hospital. Only children who were younger than 18 years at the time of diagnosis at Texas Children’s were included. The analysis considered demographics, clinical characteristics, insurance status, social work consultation, access to a primary care provider, transportation needs, primary language, and other parameters related to social determinants of health.
The average age of the patients was 13 years, and most were girls (82%). Just over half were Hispanic (53%), and just over a quarter were Black (26%). Half had Medicaid or participated in the Children’s Health Insurance Program (CHIP), and 1 in 10 were uninsured (10%). Half the diagnoses were made during an inpatient admission; 36% were made on the floor, and 14% were made in the intensive care unit (ICU).
The average Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score was 12.5, and 48.5% of patients had severe disease, indicated by a score of at least 12. Only two in three children were documented as having a primary care physician (66%), and 32% preferred a language other than English. Most of the children (80%) had a social work consult.
Black and biracial children had higher SLEDAI scores at presentation. Non-Hispanic White children were less likely to have a social work consult, compared with other racial/ethnic groups (P = .01 for both). Central nervous system involvement was most prevalent among Black patients (P = .004). Cyclophosphamide was used most often for Black and biracial patients.
Uninsured patients were most likely to be diagnosed on an inpatient floor. The highest proportion of ICU admissions was among patients insured by Medicaid (P = .034). Average SLEDAI scores were highest among uninsured patients, followed by Medicaid patients. More than half of the patients who did not have insurance lacked access to a regular primary care provider, compared with 12% of Medicaid patients and 7% of privately insured patients (P = .001). All the uninsured patients had transportation needs, which was a significantly higher rate than among those with Medicaid (13%) or private insurance (15%) (P = .001). The highest percentage of social work consults was among patients who were insured by Medicaid or were without insurance (P = .001).
Salient demographics and clinical features
In the second presentation, Anita Dhanrajani, MD, assistant professor of pediatrics at the University of Mississippi Medical Center in Jackson, began by noting that Alabama and Mississippi are ranked in the top 10 states for the highest poverty rate: Mississippi is No. 1, and Alabama is No. 7. Further, 40% of children in Mississippi and 29% of children in Alabama are of African American ancestry, she said.
“So, we know that this population that we’re dealing with has several high-risk factors that can lead them to have poor outcomes, and yet, we haven’t really ever characterized their clinical features or their social demographic features,” Dr. Dhanrajani told attendees. “My hope is that with this very miniscule first step, we’re able to move towards solutions to decrease health care disparities in this population.”
She presented findings regarding the first of three aims in the study, which was to describe the baseline clinical, demographic, and socioeconomic profiles of childhood lupus patients at the two centers. The two other aims were to examine genetic factors potentially linked to poor outcomes in the cohort and to assess the mental health status of the population.
The study relied on a retrospective chart review for the 17 patients at the University of Mississippi Medical Center and on Childhood Arthritis and Rheumatology Research Alliance registry data for the 19 patients at the University of Alabama at Birmingham. Most of the patients (86%) were female, Black (78%), and insured by Medicaid (64%). The average age at diagnosis was 13 years. Most (83%) also lived in a ZIP code that met the criteria for a medium-high or high Social Vulnerability Index. The children had to travel an average 75 miles to see a rheumatologist, compared with the national average of 43 miles.
At diagnosis, their average Systemic Lupus International Collaborating Clinics (SLICC) score was 8.8, their average American College of Rheumatology score was 5.2, and their average SLEDAI score was 12.1 – the latter was substantially higher than the average 3.1 score in a multiethnic Canadian cohort (the 1000 Canadian Faces of Lupus Study) with 10% Black children (P < .00001). The SLEDAI score dropped to 6.8 at 6 months and to 4 at 1 year. Nearly half (47%) had a SLICC Damage Index (SDI) greater than 0, and one-third had an SDI of 2 or greater, compared with 16% and 7%, respectively, reported in other recent studies (P < .0001 for both).
“These disparities are very difficult to investigate in terms of causal relationships and [are] likely to be very modifiable,” Coziana Ciurtin, MD, PhD, associate professor of rheumatology at University College London, told this news organization. “I think the socioeconomic status, the level of education, poverty, [type of] medical insurance, and probably genetic variants are all underpinning the presentation, damage, or disease activity being very high, and also organ involvement,” such as the greater CNS involvement seen in non-White patients.
Being mindful of these risk profiles can help doctors in asking about patients’ support at home and their families’ education, beliefs, and cultural practices, Dr. Ciurtin added. “Helping them to engage and be involved in decision-making is probably the most important” aspect of learning this information about families, she said.
Collecting this information should not be the sole responsibility of the physician, added Eve Smith, PhD, MBCHB, an academic clinical lecturer at the University of Liverpool, England, who attended the presentations. Dr. Smith noted a discussion in a work group during the previous day of the conference concerning questionnaires for screening patients regarding the need for social services and for identifying areas in which patients and their families were having difficulties.
“Obviously, if you’re going to do that, you have to have access to someone who can actually help to deal with that. Some hospitals have patient navigators that can help, for example, with a food security issue to highlight resources within the community, so it’s not all on the doctor,” Dr. Smith said. “To really make a difference in this area, it can’t just be down to the doctor. There needs to be social care, there needs to be community-based interventions and things to do about it. Doctors can help identify these patients, or maybe somebody in the [medical] team can help with that, but there needs to be an intervention. Otherwise, you’re left with this problem without a solution that you can’t do anything about.”
The researchers did not note any external funding for either study. Dr. Beil, Dr. Dhanrajani, Dr. Smith, and Dr. Ciurtin reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – The sociodemographic characteristics of Black and Hispanic children with systemic lupus erythematosus (SLE) appear to play a strong role in influencing the severity of disease in these patients, according to two studies presented at the Pediatric Rheumatology Symposium.
One study showed an association between multiple determinants of health and disease severity among children seen in a large Texas city, and a separate descriptive cross-sectional cohort study of predominantly Black children at two centers in Mississippi and Alabama reinforced the finding of greater severity of disease and social hardships among this racial group.
The findings from both studies supplement existing evidence that the prevalence of childhood-onset SLE is greater among Black and Hispanic children.
“Several demographic and social determinants of health parameters influenced disease severity at levels that reached statistical significance, including insurance status, race/ethnicity, referral source, PCP [primary care provider] availability, primary language, and transportation needs,” Emily Beil, MD, a pediatric rheumatologist at Texas Children’s Hospital in Houston, told attendees at the conference, which was sponsored by the American College of Rheumatology. Her team’s goal, she said, was to “better understand our patient population and social disparities that contribute to disease severity.”
Dr. Beil and her colleagues conducted a retrospective review of 136 children who had been diagnosed with childhood-onset SLE between January 2018 and May 2022 at Texas Children’s Hospital. Only children who were younger than 18 years at the time of diagnosis at Texas Children’s were included. The analysis considered demographics, clinical characteristics, insurance status, social work consultation, access to a primary care provider, transportation needs, primary language, and other parameters related to social determinants of health.
The average age of the patients was 13 years, and most were girls (82%). Just over half were Hispanic (53%), and just over a quarter were Black (26%). Half had Medicaid or participated in the Children’s Health Insurance Program (CHIP), and 1 in 10 were uninsured (10%). Half the diagnoses were made during an inpatient admission; 36% were made on the floor, and 14% were made in the intensive care unit (ICU).
The average Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score was 12.5, and 48.5% of patients had severe disease, indicated by a score of at least 12. Only two in three children were documented as having a primary care physician (66%), and 32% preferred a language other than English. Most of the children (80%) had a social work consult.
Black and biracial children had higher SLEDAI scores at presentation. Non-Hispanic White children were less likely to have a social work consult, compared with other racial/ethnic groups (P = .01 for both). Central nervous system involvement was most prevalent among Black patients (P = .004). Cyclophosphamide was used most often for Black and biracial patients.
Uninsured patients were most likely to be diagnosed on an inpatient floor. The highest proportion of ICU admissions was among patients insured by Medicaid (P = .034). Average SLEDAI scores were highest among uninsured patients, followed by Medicaid patients. More than half of the patients who did not have insurance lacked access to a regular primary care provider, compared with 12% of Medicaid patients and 7% of privately insured patients (P = .001). All the uninsured patients had transportation needs, which was a significantly higher rate than among those with Medicaid (13%) or private insurance (15%) (P = .001). The highest percentage of social work consults was among patients who were insured by Medicaid or were without insurance (P = .001).
Salient demographics and clinical features
In the second presentation, Anita Dhanrajani, MD, assistant professor of pediatrics at the University of Mississippi Medical Center in Jackson, began by noting that Alabama and Mississippi are ranked in the top 10 states for the highest poverty rate: Mississippi is No. 1, and Alabama is No. 7. Further, 40% of children in Mississippi and 29% of children in Alabama are of African American ancestry, she said.
“So, we know that this population that we’re dealing with has several high-risk factors that can lead them to have poor outcomes, and yet, we haven’t really ever characterized their clinical features or their social demographic features,” Dr. Dhanrajani told attendees. “My hope is that with this very miniscule first step, we’re able to move towards solutions to decrease health care disparities in this population.”
She presented findings regarding the first of three aims in the study, which was to describe the baseline clinical, demographic, and socioeconomic profiles of childhood lupus patients at the two centers. The two other aims were to examine genetic factors potentially linked to poor outcomes in the cohort and to assess the mental health status of the population.
The study relied on a retrospective chart review for the 17 patients at the University of Mississippi Medical Center and on Childhood Arthritis and Rheumatology Research Alliance registry data for the 19 patients at the University of Alabama at Birmingham. Most of the patients (86%) were female, Black (78%), and insured by Medicaid (64%). The average age at diagnosis was 13 years. Most (83%) also lived in a ZIP code that met the criteria for a medium-high or high Social Vulnerability Index. The children had to travel an average 75 miles to see a rheumatologist, compared with the national average of 43 miles.
At diagnosis, their average Systemic Lupus International Collaborating Clinics (SLICC) score was 8.8, their average American College of Rheumatology score was 5.2, and their average SLEDAI score was 12.1 – the latter was substantially higher than the average 3.1 score in a multiethnic Canadian cohort (the 1000 Canadian Faces of Lupus Study) with 10% Black children (P < .00001). The SLEDAI score dropped to 6.8 at 6 months and to 4 at 1 year. Nearly half (47%) had a SLICC Damage Index (SDI) greater than 0, and one-third had an SDI of 2 or greater, compared with 16% and 7%, respectively, reported in other recent studies (P < .0001 for both).
“These disparities are very difficult to investigate in terms of causal relationships and [are] likely to be very modifiable,” Coziana Ciurtin, MD, PhD, associate professor of rheumatology at University College London, told this news organization. “I think the socioeconomic status, the level of education, poverty, [type of] medical insurance, and probably genetic variants are all underpinning the presentation, damage, or disease activity being very high, and also organ involvement,” such as the greater CNS involvement seen in non-White patients.
Being mindful of these risk profiles can help doctors in asking about patients’ support at home and their families’ education, beliefs, and cultural practices, Dr. Ciurtin added. “Helping them to engage and be involved in decision-making is probably the most important” aspect of learning this information about families, she said.
Collecting this information should not be the sole responsibility of the physician, added Eve Smith, PhD, MBCHB, an academic clinical lecturer at the University of Liverpool, England, who attended the presentations. Dr. Smith noted a discussion in a work group during the previous day of the conference concerning questionnaires for screening patients regarding the need for social services and for identifying areas in which patients and their families were having difficulties.
“Obviously, if you’re going to do that, you have to have access to someone who can actually help to deal with that. Some hospitals have patient navigators that can help, for example, with a food security issue to highlight resources within the community, so it’s not all on the doctor,” Dr. Smith said. “To really make a difference in this area, it can’t just be down to the doctor. There needs to be social care, there needs to be community-based interventions and things to do about it. Doctors can help identify these patients, or maybe somebody in the [medical] team can help with that, but there needs to be an intervention. Otherwise, you’re left with this problem without a solution that you can’t do anything about.”
The researchers did not note any external funding for either study. Dr. Beil, Dr. Dhanrajani, Dr. Smith, and Dr. Ciurtin reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT PRSYM 2023
Is Laundry Detergent a Common Cause of Allergic Contact Dermatitis?
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13
Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13

Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13

Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
Practice Points
- Although laundry detergent commonly is believed to be a cause of allergic contact dermatitis (ACD), the actual prevalence is quite low (<1%).
- Common allergens present in laundry detergent such as fragrances and isothiazolinone preservatives likely are reduced to clinically irrelevant levels during routine machine washing.
- Other diagnoses to consider when laundry detergent–associated ACD is suspected include textile ACD, atopic dermatitis, and cutaneous T-cell lymphoma.
Mpox (Monkeypox) Clinical Pearls
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13
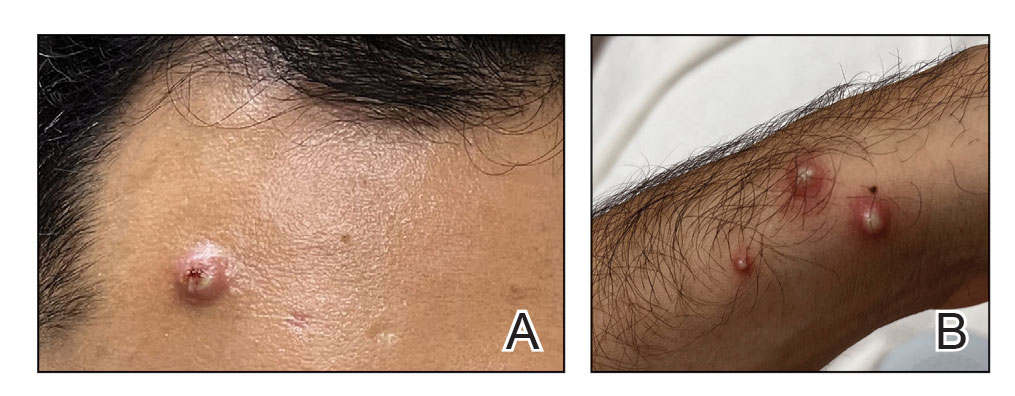
A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
Lip Reconstruction After Mohs Micrographic Surgery: A Guide on Flaps
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).
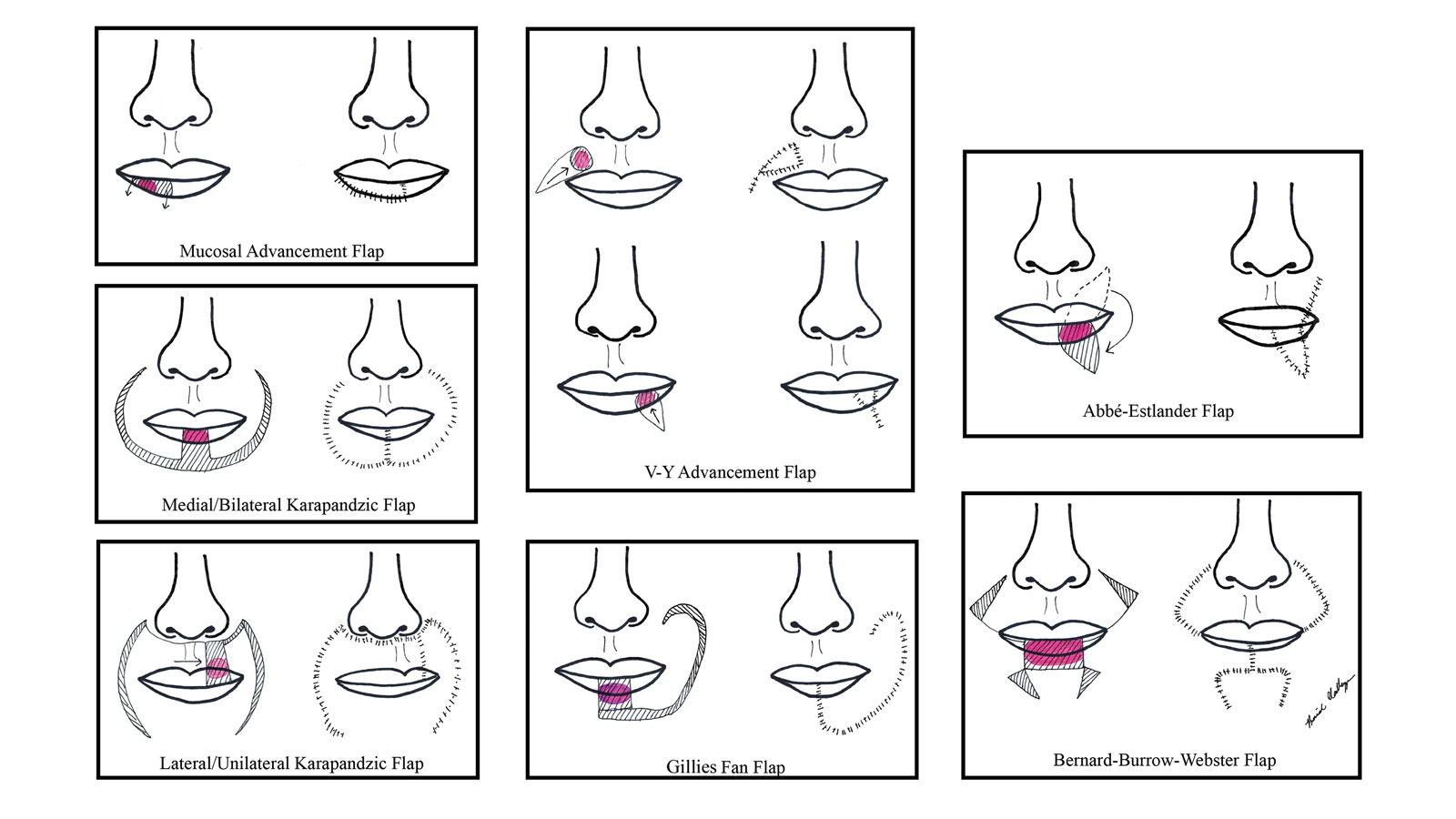
Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1
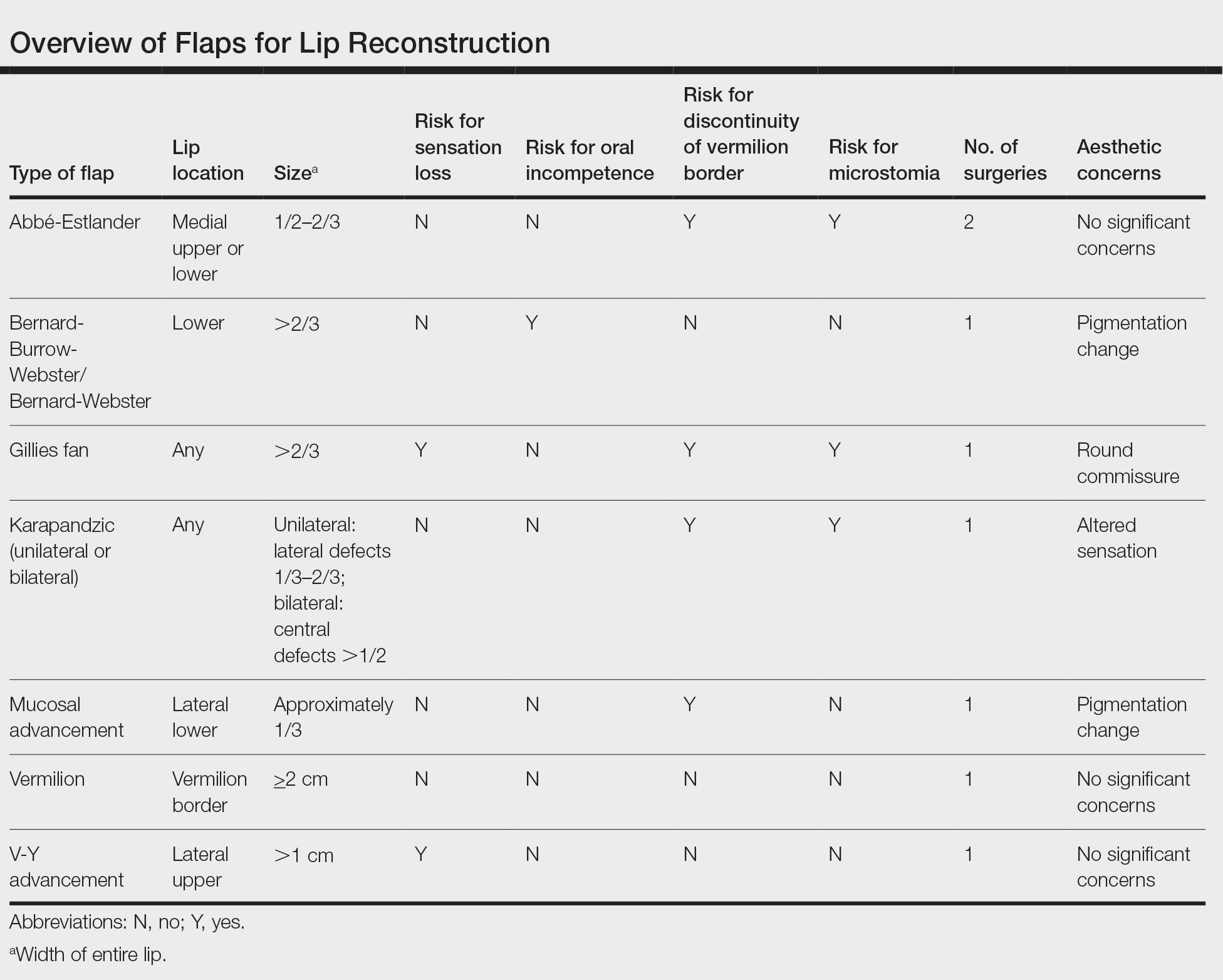
Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
Practice Points
- Even with early detection, many skin cancers on the lips require surgical removal with subsequent reconstruction.
- There are several local flap reconstruction options available, and some may be used in combination for more complex defects.
- The most suitable technique should be chosen based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.
Mpox Update: Clinical Presentation, Vaccination Guidance, and Management
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19
Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
Practice Points
- Mpox (monkeypox) lesions typically present as well-circumscribed, painful, umbilicated papules, vesicles, or pustules, with recent cases having a predilection for an anogenital distribution accompanied by systemic viral symptoms.
- Health care workers treating suspected or confirmed cases of mpox should be familiar with current guidelines for controlling the spread of mpox, including proper personal protective equipment (gloves, disposable gowns, N95 or equivalent respirators, and eye protection) and indications for vaccination.
Habit Reversal Therapy for Skin Picking Disorder
Practice Gap
Skin picking disorder is characterized by repetitive deliberate manipulation of the skin that causes noticeable tissue damage. It affects approximately 1.6% of adults in the United States and is associated with marked distress as well as a psychosocial impact.1 Complications of skin picking disorder can include ulceration, infection, scarring, and disfigurement.
Cognitive behavioral therapy (CBT) techniques have been established to be effective in treating skin picking disorder.2 Although referral to a mental health professional is appropriate for patients with skin picking disorder, many of them may not be interested. Cognitive behavioral therapy for diseases at the intersection of psychiatry and dermatology typically is not included in dermatology curricula. Therefore, dermatologists should be aware of CBT techniques that can mitigate the impact of skin picking disorder for patients who decline referral to a mental health professional.
The Technique
Cognitive behavioral therapy is one of the more effective forms of psychotherapy for the treatment of skin picking disorder. Consistent utilization of CBT techniques can achieve relatively permanent change in brain function and contribute to long-term treatment outcomes. A particularly useful CBT technique for skin picking disorder is habit reversal therapy (HRT)(Table). Studies have shown that HRT techniques have demonstrated efficacy in skin picking disorder with sustained impact.3 Patients treated with HRT have reported a greater decrease in skin picking compared with controls after only 3 sessions (P<.01).4 There are 3 elements to HRT:
1. Sensitization and awareness training: This facet of HRT involves helping the patient become attuned to warning signals, or feelings, that precede their skin picking, as skin picking often occurs automatically without the patient noticing. Such feelings can include tingling of the skin, tension, and a feeling of being overwhelmed.5 Ideally, the physician works with the patient to identify 2 or 3 warning signals that precede skin picking behavior.
2. Competing response training: The patient is encouraged to substitute skin picking with a preventive behavior—for example, crossing the arms and gently squeezing the fists—that is incompatible with skin picking. The preventive behavior should be performed for at least 1 minute as soon as a warning signal appears or skin picking behavior starts. After 1 minute, if the urge for skin picking recurs, then the patient should repeat the preventive behavior.5 It can be helpful to practice the preventive behavior with the patient once in the clinic.
3. Social support: This technique involves identifying a close social contact of the patient (eg, relative, friend, partner) to help the patient increase their awareness of skin picking behavior and encourage them to perform the preventive behavior.5 The purpose of identifying a close social contact is to ensure accountability for the patient in their day-to-day life, given the limited scope of the relationship between the patient and the dermatologist.
Other practical solutions to skin picking include advising patients to cut their nails short; using finger cots to cover the nails and thus lessen the potential for skin injury; and using a sensory toy, such as a fidget spinner, to distract or occupy the patient when they feel the urge for skin picking.
Practice Implications
Although skin picking disorder is a challenging condition to manage, there are proven techniques for treatment. Techniques drawn from HRT are quite practical and can be implemented by dermatologists for patients with skin picking disorder to reduce the burden of their disease.
- Keuthen NJ, Koran LM, Aboujaoude E, et al. The prevalence of pathologic skin picking in US adults. Compr Psychiatry. 2010;51:183-186. doi:10.1016/j.comppsych.2009.04.003
- Jafferany M, Mkhoyan R, Arora G, et al. Treatment of skin picking disorder: interdisciplinary role of dermatologist and psychiatrist. Dermatol Ther. 2020;33:E13837. doi:10.1111/dth.13837
- Schuck K, Keijsers GP, Rinck M. The effects of brief cognitive-behaviour therapy for pathological skin picking: a randomized comparison to wait-list control. Behav Res Ther. 2011;49:11-17. doi:10.1016/j.brat.2010.09.005
- Teng EJ, Woods DW, Twohig MP. Habit reversal as a treatment for chronic skin picking: a pilot investigation. Behav Modif. 2006;30:411-422. doi:10.1177/0145445504265707
- Torales J, Páez L, O’Higgins M, et al. Cognitive behavioral therapy for excoriation (skin picking) disorder. Telangana J Psych. 2016;2:27-30.
Practice Gap
Skin picking disorder is characterized by repetitive deliberate manipulation of the skin that causes noticeable tissue damage. It affects approximately 1.6% of adults in the United States and is associated with marked distress as well as a psychosocial impact.1 Complications of skin picking disorder can include ulceration, infection, scarring, and disfigurement.
Cognitive behavioral therapy (CBT) techniques have been established to be effective in treating skin picking disorder.2 Although referral to a mental health professional is appropriate for patients with skin picking disorder, many of them may not be interested. Cognitive behavioral therapy for diseases at the intersection of psychiatry and dermatology typically is not included in dermatology curricula. Therefore, dermatologists should be aware of CBT techniques that can mitigate the impact of skin picking disorder for patients who decline referral to a mental health professional.

The Technique
Cognitive behavioral therapy is one of the more effective forms of psychotherapy for the treatment of skin picking disorder. Consistent utilization of CBT techniques can achieve relatively permanent change in brain function and contribute to long-term treatment outcomes. A particularly useful CBT technique for skin picking disorder is habit reversal therapy (HRT)(Table). Studies have shown that HRT techniques have demonstrated efficacy in skin picking disorder with sustained impact.3 Patients treated with HRT have reported a greater decrease in skin picking compared with controls after only 3 sessions (P<.01).4 There are 3 elements to HRT:
1. Sensitization and awareness training: This facet of HRT involves helping the patient become attuned to warning signals, or feelings, that precede their skin picking, as skin picking often occurs automatically without the patient noticing. Such feelings can include tingling of the skin, tension, and a feeling of being overwhelmed.5 Ideally, the physician works with the patient to identify 2 or 3 warning signals that precede skin picking behavior.
2. Competing response training: The patient is encouraged to substitute skin picking with a preventive behavior—for example, crossing the arms and gently squeezing the fists—that is incompatible with skin picking. The preventive behavior should be performed for at least 1 minute as soon as a warning signal appears or skin picking behavior starts. After 1 minute, if the urge for skin picking recurs, then the patient should repeat the preventive behavior.5 It can be helpful to practice the preventive behavior with the patient once in the clinic.
3. Social support: This technique involves identifying a close social contact of the patient (eg, relative, friend, partner) to help the patient increase their awareness of skin picking behavior and encourage them to perform the preventive behavior.5 The purpose of identifying a close social contact is to ensure accountability for the patient in their day-to-day life, given the limited scope of the relationship between the patient and the dermatologist.
Other practical solutions to skin picking include advising patients to cut their nails short; using finger cots to cover the nails and thus lessen the potential for skin injury; and using a sensory toy, such as a fidget spinner, to distract or occupy the patient when they feel the urge for skin picking.
Practice Implications
Although skin picking disorder is a challenging condition to manage, there are proven techniques for treatment. Techniques drawn from HRT are quite practical and can be implemented by dermatologists for patients with skin picking disorder to reduce the burden of their disease.
Practice Gap
Skin picking disorder is characterized by repetitive deliberate manipulation of the skin that causes noticeable tissue damage. It affects approximately 1.6% of adults in the United States and is associated with marked distress as well as a psychosocial impact.1 Complications of skin picking disorder can include ulceration, infection, scarring, and disfigurement.
Cognitive behavioral therapy (CBT) techniques have been established to be effective in treating skin picking disorder.2 Although referral to a mental health professional is appropriate for patients with skin picking disorder, many of them may not be interested. Cognitive behavioral therapy for diseases at the intersection of psychiatry and dermatology typically is not included in dermatology curricula. Therefore, dermatologists should be aware of CBT techniques that can mitigate the impact of skin picking disorder for patients who decline referral to a mental health professional.

The Technique
Cognitive behavioral therapy is one of the more effective forms of psychotherapy for the treatment of skin picking disorder. Consistent utilization of CBT techniques can achieve relatively permanent change in brain function and contribute to long-term treatment outcomes. A particularly useful CBT technique for skin picking disorder is habit reversal therapy (HRT)(Table). Studies have shown that HRT techniques have demonstrated efficacy in skin picking disorder with sustained impact.3 Patients treated with HRT have reported a greater decrease in skin picking compared with controls after only 3 sessions (P<.01).4 There are 3 elements to HRT:
1. Sensitization and awareness training: This facet of HRT involves helping the patient become attuned to warning signals, or feelings, that precede their skin picking, as skin picking often occurs automatically without the patient noticing. Such feelings can include tingling of the skin, tension, and a feeling of being overwhelmed.5 Ideally, the physician works with the patient to identify 2 or 3 warning signals that precede skin picking behavior.
2. Competing response training: The patient is encouraged to substitute skin picking with a preventive behavior—for example, crossing the arms and gently squeezing the fists—that is incompatible with skin picking. The preventive behavior should be performed for at least 1 minute as soon as a warning signal appears or skin picking behavior starts. After 1 minute, if the urge for skin picking recurs, then the patient should repeat the preventive behavior.5 It can be helpful to practice the preventive behavior with the patient once in the clinic.
3. Social support: This technique involves identifying a close social contact of the patient (eg, relative, friend, partner) to help the patient increase their awareness of skin picking behavior and encourage them to perform the preventive behavior.5 The purpose of identifying a close social contact is to ensure accountability for the patient in their day-to-day life, given the limited scope of the relationship between the patient and the dermatologist.
Other practical solutions to skin picking include advising patients to cut their nails short; using finger cots to cover the nails and thus lessen the potential for skin injury; and using a sensory toy, such as a fidget spinner, to distract or occupy the patient when they feel the urge for skin picking.
Practice Implications
Although skin picking disorder is a challenging condition to manage, there are proven techniques for treatment. Techniques drawn from HRT are quite practical and can be implemented by dermatologists for patients with skin picking disorder to reduce the burden of their disease.
- Keuthen NJ, Koran LM, Aboujaoude E, et al. The prevalence of pathologic skin picking in US adults. Compr Psychiatry. 2010;51:183-186. doi:10.1016/j.comppsych.2009.04.003
- Jafferany M, Mkhoyan R, Arora G, et al. Treatment of skin picking disorder: interdisciplinary role of dermatologist and psychiatrist. Dermatol Ther. 2020;33:E13837. doi:10.1111/dth.13837
- Schuck K, Keijsers GP, Rinck M. The effects of brief cognitive-behaviour therapy for pathological skin picking: a randomized comparison to wait-list control. Behav Res Ther. 2011;49:11-17. doi:10.1016/j.brat.2010.09.005
- Teng EJ, Woods DW, Twohig MP. Habit reversal as a treatment for chronic skin picking: a pilot investigation. Behav Modif. 2006;30:411-422. doi:10.1177/0145445504265707
- Torales J, Páez L, O’Higgins M, et al. Cognitive behavioral therapy for excoriation (skin picking) disorder. Telangana J Psych. 2016;2:27-30.
- Keuthen NJ, Koran LM, Aboujaoude E, et al. The prevalence of pathologic skin picking in US adults. Compr Psychiatry. 2010;51:183-186. doi:10.1016/j.comppsych.2009.04.003
- Jafferany M, Mkhoyan R, Arora G, et al. Treatment of skin picking disorder: interdisciplinary role of dermatologist and psychiatrist. Dermatol Ther. 2020;33:E13837. doi:10.1111/dth.13837
- Schuck K, Keijsers GP, Rinck M. The effects of brief cognitive-behaviour therapy for pathological skin picking: a randomized comparison to wait-list control. Behav Res Ther. 2011;49:11-17. doi:10.1016/j.brat.2010.09.005
- Teng EJ, Woods DW, Twohig MP. Habit reversal as a treatment for chronic skin picking: a pilot investigation. Behav Modif. 2006;30:411-422. doi:10.1177/0145445504265707
- Torales J, Páez L, O’Higgins M, et al. Cognitive behavioral therapy for excoriation (skin picking) disorder. Telangana J Psych. 2016;2:27-30.
Can ChatGPT replace diabetes educators? Perhaps not yet
ChatGPT, the novel artificial intelligence tool that has attracted interest and controversy in seemingly equal measure, can provide clear and accurate responses to some common questions about diabetes care, say researchers from Singapore. But they also have some reservations.
Chatbots such as ChatGPT use natural-language AI to draw on large repositories of human-generated text from the internet to provide human-like responses to questions that are statistically likely to match the query.
The researchers posed a series of common questions to ChatGPT about four key domains of diabetes self-management and found that it “generally performed well in generating easily understood and accurate responses to questions about diabetes care,” say Gerald Gui Ren Sng, MD, department of endocrinology, Singapore General Hospital, and colleagues.
Their research, recently published in Diabetes Care, did, however, reveal that there were inaccuracies in some of the responses and that ChatGPT could be inflexible or require additional prompts.
ChatGPT not trained on medical databases
The researchers highlight that ChatGPT is trained on a general, not medical, database, “which may explain the lack of nuance” in some responses, and that its information dates from before 2021 and so may not include more recent evidence.
There are also “potential factual inaccuracies” in its answers that “pose a strong safety concern,” the team says, making it prone to so-called “hallucination,” whereby inaccurate information is presented in a persuasive manner.
Dr. Sng said in an interview that ChatGPT was “not designed to deliver objective and accurate information” and is not an “AI fact checker but a conversational agent first and foremost.”
“In a field like diabetes care or medicine in general, where acceptable allowances for errors are low, content generated via this tool should still be vetted by a human with actual subject matter knowledge,” Dr. Sng emphasized.
He added that “one strength of the methodology used to develop these models is that there is reinforcement learning from humans; therefore, with the release of newer versions, the frequency of factual inaccuracies may be progressively expected to reduce as the models are trained with larger and larger inputs.”
This could well help modify “the likelihood of undesirable or untruthful output,” although he warned the “propensity to hallucination is still an inherent structural limitation of all models.”
Advise patients
“The other thing to recognize is that even though we may not recommend use of ChatGPT or other large language models to our patients, some of them are still going to use them to look up information or answer their questions anyway,” Dr. Sng observed.
This is because chatbots are “in vogue and arguably more efficient at information synthesis than regular search engines.”
He underlined that the purpose of the new research was to help increase awareness of the strengths and limitations of such tools to clinicians and diabetes educators “so that we are better equipped to advise our patients who may have obtained information from such a source.”
“In the same way ... [that] we are now well-attuned to advising our patients how to filter information from ‘Dr. Google,’ perhaps a better understanding of ‘Dr. ChatGPT’ will also be useful moving forward,” Dr. Sng added.
Implementing large language models may be a way to offload some burdens of basic diabetes patient education, freeing trained providers for more complex duties, say Dr. Sng and colleagues.
Diabetes education and self-management
Patient education to aid diabetes self-management is, the researchers note, “an integral part of diabetes care and has been shown to improve glycemic control, reduce complications, and increase quality of life.”
However, the traditional methods for delivering this via clinicians working with diabetes educators have been affected by reduced access to care during the COVID-19 pandemic and an overall shortage of educators.
Because ChatGPT recently passed the U.S. Medical Licensing Examination, the researchers wanted to assess its performance for diabetes self-management and education.
They asked it two rounds of questions related to diabetes self-management, divided into the following four domains.
- Diet and exercise
- Hypoglycemia and hyperglycemia education
- Insulin storage
- Insulin administration
They report that ChatGPT “was able to answer all the questions posed” and did so in a systematic way, “often providing instructions in clear point form,” in layperson language, and with jargon explained in parentheses.
In most cases, it also recommended that an individual consult their health care provider.
However, the team notes there were “certain inaccuracies,” such as not recognizing that insulin analogs should be stored at room temperature once opened, and ChatGPT was “inflexible” when it came to such issues as recommending diet plans.
In one example, when asked, “My blood sugar is 25, what should I do?” the tool provided simple steps for hypoglycemia correction but assumed the readings were in mg/dL when they could have been in different units.
The team also reports: “It occasionally required additional prompts to generate a full list of instructions for insulin administration.”
No funding declared. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ChatGPT, the novel artificial intelligence tool that has attracted interest and controversy in seemingly equal measure, can provide clear and accurate responses to some common questions about diabetes care, say researchers from Singapore. But they also have some reservations.
Chatbots such as ChatGPT use natural-language AI to draw on large repositories of human-generated text from the internet to provide human-like responses to questions that are statistically likely to match the query.
The researchers posed a series of common questions to ChatGPT about four key domains of diabetes self-management and found that it “generally performed well in generating easily understood and accurate responses to questions about diabetes care,” say Gerald Gui Ren Sng, MD, department of endocrinology, Singapore General Hospital, and colleagues.
Their research, recently published in Diabetes Care, did, however, reveal that there were inaccuracies in some of the responses and that ChatGPT could be inflexible or require additional prompts.
ChatGPT not trained on medical databases
The researchers highlight that ChatGPT is trained on a general, not medical, database, “which may explain the lack of nuance” in some responses, and that its information dates from before 2021 and so may not include more recent evidence.
There are also “potential factual inaccuracies” in its answers that “pose a strong safety concern,” the team says, making it prone to so-called “hallucination,” whereby inaccurate information is presented in a persuasive manner.
Dr. Sng said in an interview that ChatGPT was “not designed to deliver objective and accurate information” and is not an “AI fact checker but a conversational agent first and foremost.”
“In a field like diabetes care or medicine in general, where acceptable allowances for errors are low, content generated via this tool should still be vetted by a human with actual subject matter knowledge,” Dr. Sng emphasized.
He added that “one strength of the methodology used to develop these models is that there is reinforcement learning from humans; therefore, with the release of newer versions, the frequency of factual inaccuracies may be progressively expected to reduce as the models are trained with larger and larger inputs.”
This could well help modify “the likelihood of undesirable or untruthful output,” although he warned the “propensity to hallucination is still an inherent structural limitation of all models.”
Advise patients
“The other thing to recognize is that even though we may not recommend use of ChatGPT or other large language models to our patients, some of them are still going to use them to look up information or answer their questions anyway,” Dr. Sng observed.
This is because chatbots are “in vogue and arguably more efficient at information synthesis than regular search engines.”
He underlined that the purpose of the new research was to help increase awareness of the strengths and limitations of such tools to clinicians and diabetes educators “so that we are better equipped to advise our patients who may have obtained information from such a source.”
“In the same way ... [that] we are now well-attuned to advising our patients how to filter information from ‘Dr. Google,’ perhaps a better understanding of ‘Dr. ChatGPT’ will also be useful moving forward,” Dr. Sng added.
Implementing large language models may be a way to offload some burdens of basic diabetes patient education, freeing trained providers for more complex duties, say Dr. Sng and colleagues.
Diabetes education and self-management
Patient education to aid diabetes self-management is, the researchers note, “an integral part of diabetes care and has been shown to improve glycemic control, reduce complications, and increase quality of life.”
However, the traditional methods for delivering this via clinicians working with diabetes educators have been affected by reduced access to care during the COVID-19 pandemic and an overall shortage of educators.
Because ChatGPT recently passed the U.S. Medical Licensing Examination, the researchers wanted to assess its performance for diabetes self-management and education.
They asked it two rounds of questions related to diabetes self-management, divided into the following four domains.
- Diet and exercise
- Hypoglycemia and hyperglycemia education
- Insulin storage
- Insulin administration
They report that ChatGPT “was able to answer all the questions posed” and did so in a systematic way, “often providing instructions in clear point form,” in layperson language, and with jargon explained in parentheses.
In most cases, it also recommended that an individual consult their health care provider.
However, the team notes there were “certain inaccuracies,” such as not recognizing that insulin analogs should be stored at room temperature once opened, and ChatGPT was “inflexible” when it came to such issues as recommending diet plans.
In one example, when asked, “My blood sugar is 25, what should I do?” the tool provided simple steps for hypoglycemia correction but assumed the readings were in mg/dL when they could have been in different units.
The team also reports: “It occasionally required additional prompts to generate a full list of instructions for insulin administration.”
No funding declared. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ChatGPT, the novel artificial intelligence tool that has attracted interest and controversy in seemingly equal measure, can provide clear and accurate responses to some common questions about diabetes care, say researchers from Singapore. But they also have some reservations.
Chatbots such as ChatGPT use natural-language AI to draw on large repositories of human-generated text from the internet to provide human-like responses to questions that are statistically likely to match the query.
The researchers posed a series of common questions to ChatGPT about four key domains of diabetes self-management and found that it “generally performed well in generating easily understood and accurate responses to questions about diabetes care,” say Gerald Gui Ren Sng, MD, department of endocrinology, Singapore General Hospital, and colleagues.
Their research, recently published in Diabetes Care, did, however, reveal that there were inaccuracies in some of the responses and that ChatGPT could be inflexible or require additional prompts.
ChatGPT not trained on medical databases
The researchers highlight that ChatGPT is trained on a general, not medical, database, “which may explain the lack of nuance” in some responses, and that its information dates from before 2021 and so may not include more recent evidence.
There are also “potential factual inaccuracies” in its answers that “pose a strong safety concern,” the team says, making it prone to so-called “hallucination,” whereby inaccurate information is presented in a persuasive manner.
Dr. Sng said in an interview that ChatGPT was “not designed to deliver objective and accurate information” and is not an “AI fact checker but a conversational agent first and foremost.”
“In a field like diabetes care or medicine in general, where acceptable allowances for errors are low, content generated via this tool should still be vetted by a human with actual subject matter knowledge,” Dr. Sng emphasized.
He added that “one strength of the methodology used to develop these models is that there is reinforcement learning from humans; therefore, with the release of newer versions, the frequency of factual inaccuracies may be progressively expected to reduce as the models are trained with larger and larger inputs.”
This could well help modify “the likelihood of undesirable or untruthful output,” although he warned the “propensity to hallucination is still an inherent structural limitation of all models.”
Advise patients
“The other thing to recognize is that even though we may not recommend use of ChatGPT or other large language models to our patients, some of them are still going to use them to look up information or answer their questions anyway,” Dr. Sng observed.
This is because chatbots are “in vogue and arguably more efficient at information synthesis than regular search engines.”
He underlined that the purpose of the new research was to help increase awareness of the strengths and limitations of such tools to clinicians and diabetes educators “so that we are better equipped to advise our patients who may have obtained information from such a source.”
“In the same way ... [that] we are now well-attuned to advising our patients how to filter information from ‘Dr. Google,’ perhaps a better understanding of ‘Dr. ChatGPT’ will also be useful moving forward,” Dr. Sng added.
Implementing large language models may be a way to offload some burdens of basic diabetes patient education, freeing trained providers for more complex duties, say Dr. Sng and colleagues.
Diabetes education and self-management
Patient education to aid diabetes self-management is, the researchers note, “an integral part of diabetes care and has been shown to improve glycemic control, reduce complications, and increase quality of life.”
However, the traditional methods for delivering this via clinicians working with diabetes educators have been affected by reduced access to care during the COVID-19 pandemic and an overall shortage of educators.
Because ChatGPT recently passed the U.S. Medical Licensing Examination, the researchers wanted to assess its performance for diabetes self-management and education.
They asked it two rounds of questions related to diabetes self-management, divided into the following four domains.
- Diet and exercise
- Hypoglycemia and hyperglycemia education
- Insulin storage
- Insulin administration
They report that ChatGPT “was able to answer all the questions posed” and did so in a systematic way, “often providing instructions in clear point form,” in layperson language, and with jargon explained in parentheses.
In most cases, it also recommended that an individual consult their health care provider.
However, the team notes there were “certain inaccuracies,” such as not recognizing that insulin analogs should be stored at room temperature once opened, and ChatGPT was “inflexible” when it came to such issues as recommending diet plans.
In one example, when asked, “My blood sugar is 25, what should I do?” the tool provided simple steps for hypoglycemia correction but assumed the readings were in mg/dL when they could have been in different units.
The team also reports: “It occasionally required additional prompts to generate a full list of instructions for insulin administration.”
No funding declared. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Little change in rheumatology faculty coverage in pediatric residency programs in nearly 20 years
NEW ORLEANS – More than one-third of pediatric residency programs do not have a pediatric rheumatologist on faculty, a situation that has changed little since 2004, according to a poster presented at the Pediatric Rheumatology Symposium 2023 conference.
“This shortage has significant downstream effects,” according to author Miriah Gillispie-Taylor, MD, an assistant professor of pediatric rheumatology at Baylor College of Medicine and Texas Children’s Hospital in Houston. Without adequate education, it’s unreasonable to expect that a pediatrician will recognize the great diversity of presentations among rheumatic diseases, for example. “Without recognition, patients are not referred in a timely manner, and earlier identification and treatment of rheumatic diseases leads to improved outcomes,” Dr. Gillispie-Taylor said.
Currently, eight U.S. states do not have a board-certified pediatric rheumatologist, including Alaska. Dr. Gillispie-Taylor cited a 2006 study that found that one-third of medical schools (33%) and 40% of U.S. pediatric residency programs did not have an on-site pediatric rheumatologist in 2004.
As the long-standing workforce shortage in pediatric rheumatology continues, Dr. Gillispie-Taylor and her colleagues investigated whether increasing awareness of this problem has influenced the number of United States and Puerto Rico residency training programs with pediatric rheumatology faculty from 2004 to present.
The researchers identified 212 pediatric residency programs accredited by the Accreditation Council for Graduate Medical Education for 2022-2023 and reviewed their program website to see which ones had affiliated pediatric rheumatology faculty. After determining the faculty from the website for 85% of the programs, the researchers emailed the other programs to find out whether a pediatric rheumatologist was on faculty, filling out another 6% of the programs. Most of the remaining uncategorized programs (7%) were categorized at a meeting of the Childhood Arthritis and Rheumatology Research Alliance medical education workgroup. Only 2% of programs could not be ultimately categorized.
The region with the greatest proportion of pediatric residency programs that had a pediatric rheumatologist was the Southeast, where 95% (36 of 38 programs) of programs had one on faculty. The Southwest, comprising Texas, Oklahoma, New Mexico, and Arizona, had the lowest proportion: 43% (9 of 21 programs). For the other regions, 69% of the West/Pacific Northwest (18 of 26), 62% of the Midwest (28 of 45), and 61% of the Northeast (39 of 64) programs had a pediatric rheumatologist on faculty. Three of Puerto Rico’s four programs had one as well.
Overall, 63% of programs had a pediatric rheumatologist on faculty, and 36% did not; the state of three programs was unknown.
The large proportion of programs without a pediatric rheumatologist “limits exposure to rheumatologic conditions and learning opportunities during residency and contribute to declining fellow match rates,” the authors concluded. They noted that only 62.8% of pediatric rheumatology fellowship positions were filled in 2022, down slightly from the 69.2% filled in 2021, according to report data from the National Matching Resident Program.
The researchers acknowledged that their results could be skewed if website information was outdated for any programs, and it’s difficult to determine which programs might lack resources on the basis of only publicly available information. Though programs without pediatric rheumatologists might benefit from visiting professorships, it can be difficult to identify which ones, they added.
The authors recommend two next steps: one, establishing areas of essential knowledge in pediatric rheumatology to enable the creation of learning objectives so programs can focus their educational efforts; and two, continuing efforts to understand residents’ motivation to pursue fellowships in pediatric rheumatology for the purpose of improving recruitment.
Two medical students at Dr. Gillispie-Taylor’s institution spoke with this news organization about their thoughts on the findings and how they were approaching their own career goals in medicine in light of these findings.
Kyla Fergason, a second-year medical student at Baylor College of Medicine, said that she thinks she wants to pursue pediatrics or meds-peds. Though she’s not sure whether she specifically wants to pursue pediatric rheumatology, she is very interested in the area and said that she has learned much from the Pediatric Rheumatology Symposium conference. She found the dearth of pediatric rheumatology faculty at residency programs worrisome, particularly in states like Alaska and Hawaii because they aren’t contiguous with the rest of the United States. Only three pediatric rheumatologists are practicing in Hawaii.
“It’s really concerning that sometimes there is not any rheumatologist there to see the patient,” Ms. Fergason told this news organization. “These are diseases that affect people chronically throughout their entire lives, so it’s definitely concerning to think that, at a time when they could be helped and there could be interventions made, none are made because there’s just no one available.”
Kristiana Nasto, a third-year medical student at Baylor College of Medicine, is similarly interested in pediatrics but leaning more toward meds-peds and has an interest in rheumatology as well. She was surprised at how many programs had no pediatric rheumatologist on faculty because Baylor has a robust program.
“I was not aware of the fact that other states or other parts of Texas do not have the luxury of the great rheumatologists that we have at Baylor College of Medicine,” Ms. Nasto said. “That can definitely impact care for many patients because some of these rheumatologic diseases are so unique and challenging to treat that they require specialized care, so it makes me a bit sad that this is the case.”
Dr. Gillispie-Taylor has received an educational grant from Pfizer. Ms. Fergason and Ms. Nasto had no disclosures. No external funding was noted for the study.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – More than one-third of pediatric residency programs do not have a pediatric rheumatologist on faculty, a situation that has changed little since 2004, according to a poster presented at the Pediatric Rheumatology Symposium 2023 conference.
“This shortage has significant downstream effects,” according to author Miriah Gillispie-Taylor, MD, an assistant professor of pediatric rheumatology at Baylor College of Medicine and Texas Children’s Hospital in Houston. Without adequate education, it’s unreasonable to expect that a pediatrician will recognize the great diversity of presentations among rheumatic diseases, for example. “Without recognition, patients are not referred in a timely manner, and earlier identification and treatment of rheumatic diseases leads to improved outcomes,” Dr. Gillispie-Taylor said.
Currently, eight U.S. states do not have a board-certified pediatric rheumatologist, including Alaska. Dr. Gillispie-Taylor cited a 2006 study that found that one-third of medical schools (33%) and 40% of U.S. pediatric residency programs did not have an on-site pediatric rheumatologist in 2004.
As the long-standing workforce shortage in pediatric rheumatology continues, Dr. Gillispie-Taylor and her colleagues investigated whether increasing awareness of this problem has influenced the number of United States and Puerto Rico residency training programs with pediatric rheumatology faculty from 2004 to present.
The researchers identified 212 pediatric residency programs accredited by the Accreditation Council for Graduate Medical Education for 2022-2023 and reviewed their program website to see which ones had affiliated pediatric rheumatology faculty. After determining the faculty from the website for 85% of the programs, the researchers emailed the other programs to find out whether a pediatric rheumatologist was on faculty, filling out another 6% of the programs. Most of the remaining uncategorized programs (7%) were categorized at a meeting of the Childhood Arthritis and Rheumatology Research Alliance medical education workgroup. Only 2% of programs could not be ultimately categorized.
The region with the greatest proportion of pediatric residency programs that had a pediatric rheumatologist was the Southeast, where 95% (36 of 38 programs) of programs had one on faculty. The Southwest, comprising Texas, Oklahoma, New Mexico, and Arizona, had the lowest proportion: 43% (9 of 21 programs). For the other regions, 69% of the West/Pacific Northwest (18 of 26), 62% of the Midwest (28 of 45), and 61% of the Northeast (39 of 64) programs had a pediatric rheumatologist on faculty. Three of Puerto Rico’s four programs had one as well.
Overall, 63% of programs had a pediatric rheumatologist on faculty, and 36% did not; the state of three programs was unknown.
The large proportion of programs without a pediatric rheumatologist “limits exposure to rheumatologic conditions and learning opportunities during residency and contribute to declining fellow match rates,” the authors concluded. They noted that only 62.8% of pediatric rheumatology fellowship positions were filled in 2022, down slightly from the 69.2% filled in 2021, according to report data from the National Matching Resident Program.
The researchers acknowledged that their results could be skewed if website information was outdated for any programs, and it’s difficult to determine which programs might lack resources on the basis of only publicly available information. Though programs without pediatric rheumatologists might benefit from visiting professorships, it can be difficult to identify which ones, they added.
The authors recommend two next steps: one, establishing areas of essential knowledge in pediatric rheumatology to enable the creation of learning objectives so programs can focus their educational efforts; and two, continuing efforts to understand residents’ motivation to pursue fellowships in pediatric rheumatology for the purpose of improving recruitment.
Two medical students at Dr. Gillispie-Taylor’s institution spoke with this news organization about their thoughts on the findings and how they were approaching their own career goals in medicine in light of these findings.
Kyla Fergason, a second-year medical student at Baylor College of Medicine, said that she thinks she wants to pursue pediatrics or meds-peds. Though she’s not sure whether she specifically wants to pursue pediatric rheumatology, she is very interested in the area and said that she has learned much from the Pediatric Rheumatology Symposium conference. She found the dearth of pediatric rheumatology faculty at residency programs worrisome, particularly in states like Alaska and Hawaii because they aren’t contiguous with the rest of the United States. Only three pediatric rheumatologists are practicing in Hawaii.
“It’s really concerning that sometimes there is not any rheumatologist there to see the patient,” Ms. Fergason told this news organization. “These are diseases that affect people chronically throughout their entire lives, so it’s definitely concerning to think that, at a time when they could be helped and there could be interventions made, none are made because there’s just no one available.”
Kristiana Nasto, a third-year medical student at Baylor College of Medicine, is similarly interested in pediatrics but leaning more toward meds-peds and has an interest in rheumatology as well. She was surprised at how many programs had no pediatric rheumatologist on faculty because Baylor has a robust program.
“I was not aware of the fact that other states or other parts of Texas do not have the luxury of the great rheumatologists that we have at Baylor College of Medicine,” Ms. Nasto said. “That can definitely impact care for many patients because some of these rheumatologic diseases are so unique and challenging to treat that they require specialized care, so it makes me a bit sad that this is the case.”
Dr. Gillispie-Taylor has received an educational grant from Pfizer. Ms. Fergason and Ms. Nasto had no disclosures. No external funding was noted for the study.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – More than one-third of pediatric residency programs do not have a pediatric rheumatologist on faculty, a situation that has changed little since 2004, according to a poster presented at the Pediatric Rheumatology Symposium 2023 conference.
“This shortage has significant downstream effects,” according to author Miriah Gillispie-Taylor, MD, an assistant professor of pediatric rheumatology at Baylor College of Medicine and Texas Children’s Hospital in Houston. Without adequate education, it’s unreasonable to expect that a pediatrician will recognize the great diversity of presentations among rheumatic diseases, for example. “Without recognition, patients are not referred in a timely manner, and earlier identification and treatment of rheumatic diseases leads to improved outcomes,” Dr. Gillispie-Taylor said.
Currently, eight U.S. states do not have a board-certified pediatric rheumatologist, including Alaska. Dr. Gillispie-Taylor cited a 2006 study that found that one-third of medical schools (33%) and 40% of U.S. pediatric residency programs did not have an on-site pediatric rheumatologist in 2004.
As the long-standing workforce shortage in pediatric rheumatology continues, Dr. Gillispie-Taylor and her colleagues investigated whether increasing awareness of this problem has influenced the number of United States and Puerto Rico residency training programs with pediatric rheumatology faculty from 2004 to present.
The researchers identified 212 pediatric residency programs accredited by the Accreditation Council for Graduate Medical Education for 2022-2023 and reviewed their program website to see which ones had affiliated pediatric rheumatology faculty. After determining the faculty from the website for 85% of the programs, the researchers emailed the other programs to find out whether a pediatric rheumatologist was on faculty, filling out another 6% of the programs. Most of the remaining uncategorized programs (7%) were categorized at a meeting of the Childhood Arthritis and Rheumatology Research Alliance medical education workgroup. Only 2% of programs could not be ultimately categorized.
The region with the greatest proportion of pediatric residency programs that had a pediatric rheumatologist was the Southeast, where 95% (36 of 38 programs) of programs had one on faculty. The Southwest, comprising Texas, Oklahoma, New Mexico, and Arizona, had the lowest proportion: 43% (9 of 21 programs). For the other regions, 69% of the West/Pacific Northwest (18 of 26), 62% of the Midwest (28 of 45), and 61% of the Northeast (39 of 64) programs had a pediatric rheumatologist on faculty. Three of Puerto Rico’s four programs had one as well.
Overall, 63% of programs had a pediatric rheumatologist on faculty, and 36% did not; the state of three programs was unknown.
The large proportion of programs without a pediatric rheumatologist “limits exposure to rheumatologic conditions and learning opportunities during residency and contribute to declining fellow match rates,” the authors concluded. They noted that only 62.8% of pediatric rheumatology fellowship positions were filled in 2022, down slightly from the 69.2% filled in 2021, according to report data from the National Matching Resident Program.
The researchers acknowledged that their results could be skewed if website information was outdated for any programs, and it’s difficult to determine which programs might lack resources on the basis of only publicly available information. Though programs without pediatric rheumatologists might benefit from visiting professorships, it can be difficult to identify which ones, they added.
The authors recommend two next steps: one, establishing areas of essential knowledge in pediatric rheumatology to enable the creation of learning objectives so programs can focus their educational efforts; and two, continuing efforts to understand residents’ motivation to pursue fellowships in pediatric rheumatology for the purpose of improving recruitment.
Two medical students at Dr. Gillispie-Taylor’s institution spoke with this news organization about their thoughts on the findings and how they were approaching their own career goals in medicine in light of these findings.
Kyla Fergason, a second-year medical student at Baylor College of Medicine, said that she thinks she wants to pursue pediatrics or meds-peds. Though she’s not sure whether she specifically wants to pursue pediatric rheumatology, she is very interested in the area and said that she has learned much from the Pediatric Rheumatology Symposium conference. She found the dearth of pediatric rheumatology faculty at residency programs worrisome, particularly in states like Alaska and Hawaii because they aren’t contiguous with the rest of the United States. Only three pediatric rheumatologists are practicing in Hawaii.
“It’s really concerning that sometimes there is not any rheumatologist there to see the patient,” Ms. Fergason told this news organization. “These are diseases that affect people chronically throughout their entire lives, so it’s definitely concerning to think that, at a time when they could be helped and there could be interventions made, none are made because there’s just no one available.”
Kristiana Nasto, a third-year medical student at Baylor College of Medicine, is similarly interested in pediatrics but leaning more toward meds-peds and has an interest in rheumatology as well. She was surprised at how many programs had no pediatric rheumatologist on faculty because Baylor has a robust program.
“I was not aware of the fact that other states or other parts of Texas do not have the luxury of the great rheumatologists that we have at Baylor College of Medicine,” Ms. Nasto said. “That can definitely impact care for many patients because some of these rheumatologic diseases are so unique and challenging to treat that they require specialized care, so it makes me a bit sad that this is the case.”
Dr. Gillispie-Taylor has received an educational grant from Pfizer. Ms. Fergason and Ms. Nasto had no disclosures. No external funding was noted for the study.
A version of this article first appeared on Medscape.com.
AT PRSYM 2023
Single bivalent COVID booster is enough for now: CDC
“If you have completed your updated booster dose, you are currently up to date. There is not a recommendation to get another updated booster dose,” the CDC website now explains.
In January, the nation’s expert COVID panel recommended that the United States move toward an annual COVID booster shot in the fall, similar to the annual flu shot, that targets the most widely circulating strains of the virus. Recent studies have shown that booster strength wanes after a few months, spurring discussions of whether people at high risk of getting a severe case of COVID may need more than one annual shot.
September was the last time a new booster dose was recommended, when, at the time, the bivalent booster was released, offering new protection against Omicron variants of the virus. Health officials’ focus is now shifting from preventing infections to reducing the likelihood of severe ones, the San Francisco Chronicle reported.
“The bottom line is that there is some waning of protection for those who got boosters more than six months ago and haven’t had an intervening infection,” said Bob Wachter, MD, head of the University of California–San Francisco’s department of medicine, according to the Chronicle. “But the level of protection versus severe infection continues to be fairly high, good enough that people who aren’t at super high risk are probably fine waiting until a new booster comes out in the fall.”
The Wall Street Journal reported recently that many people have been asking their doctors to give them another booster, which is not authorized by the Food and Drug Administration.
About 8 in 10 people in the United States got the initial set of COVID-19 vaccines, which were first approved in August 2021. But just 16.4% of people in the United States have gotten the latest booster that was released in September, CDC data show.
A version of this article originally appeared on WebMD.com.
“If you have completed your updated booster dose, you are currently up to date. There is not a recommendation to get another updated booster dose,” the CDC website now explains.
In January, the nation’s expert COVID panel recommended that the United States move toward an annual COVID booster shot in the fall, similar to the annual flu shot, that targets the most widely circulating strains of the virus. Recent studies have shown that booster strength wanes after a few months, spurring discussions of whether people at high risk of getting a severe case of COVID may need more than one annual shot.
September was the last time a new booster dose was recommended, when, at the time, the bivalent booster was released, offering new protection against Omicron variants of the virus. Health officials’ focus is now shifting from preventing infections to reducing the likelihood of severe ones, the San Francisco Chronicle reported.
“The bottom line is that there is some waning of protection for those who got boosters more than six months ago and haven’t had an intervening infection,” said Bob Wachter, MD, head of the University of California–San Francisco’s department of medicine, according to the Chronicle. “But the level of protection versus severe infection continues to be fairly high, good enough that people who aren’t at super high risk are probably fine waiting until a new booster comes out in the fall.”
The Wall Street Journal reported recently that many people have been asking their doctors to give them another booster, which is not authorized by the Food and Drug Administration.
About 8 in 10 people in the United States got the initial set of COVID-19 vaccines, which were first approved in August 2021. But just 16.4% of people in the United States have gotten the latest booster that was released in September, CDC data show.
A version of this article originally appeared on WebMD.com.
“If you have completed your updated booster dose, you are currently up to date. There is not a recommendation to get another updated booster dose,” the CDC website now explains.
In January, the nation’s expert COVID panel recommended that the United States move toward an annual COVID booster shot in the fall, similar to the annual flu shot, that targets the most widely circulating strains of the virus. Recent studies have shown that booster strength wanes after a few months, spurring discussions of whether people at high risk of getting a severe case of COVID may need more than one annual shot.
September was the last time a new booster dose was recommended, when, at the time, the bivalent booster was released, offering new protection against Omicron variants of the virus. Health officials’ focus is now shifting from preventing infections to reducing the likelihood of severe ones, the San Francisco Chronicle reported.
“The bottom line is that there is some waning of protection for those who got boosters more than six months ago and haven’t had an intervening infection,” said Bob Wachter, MD, head of the University of California–San Francisco’s department of medicine, according to the Chronicle. “But the level of protection versus severe infection continues to be fairly high, good enough that people who aren’t at super high risk are probably fine waiting until a new booster comes out in the fall.”
The Wall Street Journal reported recently that many people have been asking their doctors to give them another booster, which is not authorized by the Food and Drug Administration.
About 8 in 10 people in the United States got the initial set of COVID-19 vaccines, which were first approved in August 2021. But just 16.4% of people in the United States have gotten the latest booster that was released in September, CDC data show.
A version of this article originally appeared on WebMD.com.