User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Laser, Radiofrequency Therapies Offer Little Benefit for Genitourinary Syndrome of Menopause
CHICAGO — Use of CO2 lasers and similar “energy-based” treatments result in little to no benefit for genitourinary syndrome of menopause (GSM) symptoms, according to research presented at the The Menopause Society 2024 Annual Meeting in Chicago on September 12.
“There was a concern that menopausal women are being targeted for treatments that may not have a lot of benefit and might have significant harms,” Elisheva Danan, MD, MPH, a physician at the Minneapolis VA Health Care System and an assistant professor of medicine at the University of Minnesota Medical School in Minneapolis, told this news organization. While she was not surprised to find little evidence of benefit, “we were a little bit surprised that we also didn’t find significant evidence of harms.”
The study was unable to evaluate the potential for financial harms, but Dr. Danan noted that these therapies are often expensive and not typically covered by insurance. The treatments appear to be used primarily in private practice, she said, while “most academic clinicians were not familiar with these and do not use these lasers.”
The American Urological Association had requested the review, Dr. Danan said, “to inform clinical guidelines that they could put out for practitioners about treating genital urinary syndrome from menopause.” Yet the evidence available remains slim. “There’s a lot of outcomes that were not looked at by most of these [trials], or they were looked at in a way that we couldn’t separate out,” she said.
Kamalini Das, MD, a professor of ob.gyn. at the University of Minnesota who was not involved in the research, was surprised by the findings because studies to date have been variable, “but since this looks at multiple studies and they find no benefits, I would take these results as more significant than any of the small studies,” she told this news organization.
Dr. Das said she has patients who ask about using these therapies and have had them done. “So far, I’ve told them the jury is out on whether it will help or not, that there are some studies that say they’re beneficial and some studies that they’re not,” Dr. Das said.
But this new review changes what she will tell patients going forward, she said. “This is a good study because it consolidates lots of little studies, so I think I would use this to say, looking at all the studies together, this treatment is not beneficial.”
GSM occurs due to the body’s reduced production of estrogen and affects anywhere from 27% to 84% of postmenopausal women. It can involve a constellation of symptoms ranging from vaginal discomfort and irritation to painful urination or intercourse. Typical recommended treatments for GSM include systemic hormone therapy, localized hormonal treatments such as vaginal estrogen or dehydroepiandrosterone, nonhormonal creams and moisturizers, and the prescription drug ospemifene.
Most of these have been found effective, according to a recent systematic review Dr. Danan published in the Annals of Internal Medicine that this news organization covered. But recent years have also seen a rapid increase in interest and the availability of energy-based treatments for GSM, such as CO2 laser and radiofrequency interventions, particularly for those who cannot or do not want to use hormonal treatments. The idea behind these newer therapies is that they “heat tissue to cause a denaturation of collagen fibers and induce a wound-healing response,” with the aim of “enhancement of vaginal elasticity, restoration of premenopausal epithelial function, and symptom improvement,” the authors wrote.
Evidence has been scant and uneven for the safety and effectiveness of these treatments, and they have not been evaluated by the US Food and Drug Administration. The agency issued a warning in 2018 with remarks from then Commissioner Scott Gottlieb that the “products have serious risks and don’t have adequate evidence to support their use for these purposes.”
Much of the evidence has focused on CO2 lasers instead of other energy-based treatments, however, and a raft of new studies have been published on these interventions in the past 2 years. Dr. Danan and colleagues, therefore, assessed the most current state of the research with a systematic review of randomized controlled trials (RCTs) and prospective observational studies with control groups published through December 11, 2023.
Included studies needed to evaluate an energy-based treatment for at least 8 weeks in a minimum of 40 postmenopausal women (20 in each group) who had one or more GSM symptoms. The authors also included nonrandomized and uncontrolled studies with a follow-up of a year or more to assess possible adverse events. The studies also needed to assess at least one of eight core outcomes: Dyspareunia; vulvovaginal dryness; vulvovaginal discomfort/irritation; dysuria; change in most bothersome symptom; treatment satisfaction; adverse events; and distress, bother, or interference associated with genitourinary symptoms.
The authors identified 32 studies, including 16 RCTs, one quasi-RCT, and 15 nonrandomized studies. The researchers extracted and analyzed data from the 10 RCTs and one quasi-RCT that were rated as having low to moderate risk for bias.
Most of these studies assessed CO2 lasers alone, while three assessed erbium:yttrium-aluminum-garnet (Er:YAG) laser, and one looked at CO2 lasers vs radiofrequency treatments.
The average age of participants ranged from 56 to 64 years, and most trials were in the United States. Results showed that CO2 lasers led to little or no difference in dysuria, dyspareunia, or quality of life when compared with sham lasers. The CO2 laser therapy also showed little to no difference compared with vaginal estrogen creams for dyspareunia, dryness, discomfort/irritation, dysuria, or quality of life.
Most CO2 laser studies reported on most outcomes, but the Er:YAG studies tended to report only on quality of life and/or one or two other outcomes. The radiofrequency study only assessed dyspareunia and quality of life.
“Treatment effects on other outcomes and effects of Er:YAG laser or radiofrequency on any outcomes are very uncertain,” the authors reported. Few adverse events and no serious adverse events were reported based on 15 studies, including the additional non-RCTs that had follow-up for at least a year.
“There are case reports and other types of studies that have shown some bad outcomes using laser therapies, and we really wanted to be expansive and include anything, especially because this is such a new treatment and all these trials were in the last couple of years,” Dr. Danan said.
The review was limited by inconsistent or nonvalidated outcome reporting in the studies as well as small populations and short follow-up, typically less than 3 months.
The research was funded by the Agency for Healthcare Research and Quality and Patient-Centered Outcomes Research Institute. Dr. Danan and Dr. Das had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO — Use of CO2 lasers and similar “energy-based” treatments result in little to no benefit for genitourinary syndrome of menopause (GSM) symptoms, according to research presented at the The Menopause Society 2024 Annual Meeting in Chicago on September 12.
“There was a concern that menopausal women are being targeted for treatments that may not have a lot of benefit and might have significant harms,” Elisheva Danan, MD, MPH, a physician at the Minneapolis VA Health Care System and an assistant professor of medicine at the University of Minnesota Medical School in Minneapolis, told this news organization. While she was not surprised to find little evidence of benefit, “we were a little bit surprised that we also didn’t find significant evidence of harms.”
The study was unable to evaluate the potential for financial harms, but Dr. Danan noted that these therapies are often expensive and not typically covered by insurance. The treatments appear to be used primarily in private practice, she said, while “most academic clinicians were not familiar with these and do not use these lasers.”
The American Urological Association had requested the review, Dr. Danan said, “to inform clinical guidelines that they could put out for practitioners about treating genital urinary syndrome from menopause.” Yet the evidence available remains slim. “There’s a lot of outcomes that were not looked at by most of these [trials], or they were looked at in a way that we couldn’t separate out,” she said.
Kamalini Das, MD, a professor of ob.gyn. at the University of Minnesota who was not involved in the research, was surprised by the findings because studies to date have been variable, “but since this looks at multiple studies and they find no benefits, I would take these results as more significant than any of the small studies,” she told this news organization.
Dr. Das said she has patients who ask about using these therapies and have had them done. “So far, I’ve told them the jury is out on whether it will help or not, that there are some studies that say they’re beneficial and some studies that they’re not,” Dr. Das said.
But this new review changes what she will tell patients going forward, she said. “This is a good study because it consolidates lots of little studies, so I think I would use this to say, looking at all the studies together, this treatment is not beneficial.”
GSM occurs due to the body’s reduced production of estrogen and affects anywhere from 27% to 84% of postmenopausal women. It can involve a constellation of symptoms ranging from vaginal discomfort and irritation to painful urination or intercourse. Typical recommended treatments for GSM include systemic hormone therapy, localized hormonal treatments such as vaginal estrogen or dehydroepiandrosterone, nonhormonal creams and moisturizers, and the prescription drug ospemifene.
Most of these have been found effective, according to a recent systematic review Dr. Danan published in the Annals of Internal Medicine that this news organization covered. But recent years have also seen a rapid increase in interest and the availability of energy-based treatments for GSM, such as CO2 laser and radiofrequency interventions, particularly for those who cannot or do not want to use hormonal treatments. The idea behind these newer therapies is that they “heat tissue to cause a denaturation of collagen fibers and induce a wound-healing response,” with the aim of “enhancement of vaginal elasticity, restoration of premenopausal epithelial function, and symptom improvement,” the authors wrote.
Evidence has been scant and uneven for the safety and effectiveness of these treatments, and they have not been evaluated by the US Food and Drug Administration. The agency issued a warning in 2018 with remarks from then Commissioner Scott Gottlieb that the “products have serious risks and don’t have adequate evidence to support their use for these purposes.”
Much of the evidence has focused on CO2 lasers instead of other energy-based treatments, however, and a raft of new studies have been published on these interventions in the past 2 years. Dr. Danan and colleagues, therefore, assessed the most current state of the research with a systematic review of randomized controlled trials (RCTs) and prospective observational studies with control groups published through December 11, 2023.
Included studies needed to evaluate an energy-based treatment for at least 8 weeks in a minimum of 40 postmenopausal women (20 in each group) who had one or more GSM symptoms. The authors also included nonrandomized and uncontrolled studies with a follow-up of a year or more to assess possible adverse events. The studies also needed to assess at least one of eight core outcomes: Dyspareunia; vulvovaginal dryness; vulvovaginal discomfort/irritation; dysuria; change in most bothersome symptom; treatment satisfaction; adverse events; and distress, bother, or interference associated with genitourinary symptoms.
The authors identified 32 studies, including 16 RCTs, one quasi-RCT, and 15 nonrandomized studies. The researchers extracted and analyzed data from the 10 RCTs and one quasi-RCT that were rated as having low to moderate risk for bias.
Most of these studies assessed CO2 lasers alone, while three assessed erbium:yttrium-aluminum-garnet (Er:YAG) laser, and one looked at CO2 lasers vs radiofrequency treatments.
The average age of participants ranged from 56 to 64 years, and most trials were in the United States. Results showed that CO2 lasers led to little or no difference in dysuria, dyspareunia, or quality of life when compared with sham lasers. The CO2 laser therapy also showed little to no difference compared with vaginal estrogen creams for dyspareunia, dryness, discomfort/irritation, dysuria, or quality of life.
Most CO2 laser studies reported on most outcomes, but the Er:YAG studies tended to report only on quality of life and/or one or two other outcomes. The radiofrequency study only assessed dyspareunia and quality of life.
“Treatment effects on other outcomes and effects of Er:YAG laser or radiofrequency on any outcomes are very uncertain,” the authors reported. Few adverse events and no serious adverse events were reported based on 15 studies, including the additional non-RCTs that had follow-up for at least a year.
“There are case reports and other types of studies that have shown some bad outcomes using laser therapies, and we really wanted to be expansive and include anything, especially because this is such a new treatment and all these trials were in the last couple of years,” Dr. Danan said.
The review was limited by inconsistent or nonvalidated outcome reporting in the studies as well as small populations and short follow-up, typically less than 3 months.
The research was funded by the Agency for Healthcare Research and Quality and Patient-Centered Outcomes Research Institute. Dr. Danan and Dr. Das had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO — Use of CO2 lasers and similar “energy-based” treatments result in little to no benefit for genitourinary syndrome of menopause (GSM) symptoms, according to research presented at the The Menopause Society 2024 Annual Meeting in Chicago on September 12.
“There was a concern that menopausal women are being targeted for treatments that may not have a lot of benefit and might have significant harms,” Elisheva Danan, MD, MPH, a physician at the Minneapolis VA Health Care System and an assistant professor of medicine at the University of Minnesota Medical School in Minneapolis, told this news organization. While she was not surprised to find little evidence of benefit, “we were a little bit surprised that we also didn’t find significant evidence of harms.”
The study was unable to evaluate the potential for financial harms, but Dr. Danan noted that these therapies are often expensive and not typically covered by insurance. The treatments appear to be used primarily in private practice, she said, while “most academic clinicians were not familiar with these and do not use these lasers.”
The American Urological Association had requested the review, Dr. Danan said, “to inform clinical guidelines that they could put out for practitioners about treating genital urinary syndrome from menopause.” Yet the evidence available remains slim. “There’s a lot of outcomes that were not looked at by most of these [trials], or they were looked at in a way that we couldn’t separate out,” she said.
Kamalini Das, MD, a professor of ob.gyn. at the University of Minnesota who was not involved in the research, was surprised by the findings because studies to date have been variable, “but since this looks at multiple studies and they find no benefits, I would take these results as more significant than any of the small studies,” she told this news organization.
Dr. Das said she has patients who ask about using these therapies and have had them done. “So far, I’ve told them the jury is out on whether it will help or not, that there are some studies that say they’re beneficial and some studies that they’re not,” Dr. Das said.
But this new review changes what she will tell patients going forward, she said. “This is a good study because it consolidates lots of little studies, so I think I would use this to say, looking at all the studies together, this treatment is not beneficial.”
GSM occurs due to the body’s reduced production of estrogen and affects anywhere from 27% to 84% of postmenopausal women. It can involve a constellation of symptoms ranging from vaginal discomfort and irritation to painful urination or intercourse. Typical recommended treatments for GSM include systemic hormone therapy, localized hormonal treatments such as vaginal estrogen or dehydroepiandrosterone, nonhormonal creams and moisturizers, and the prescription drug ospemifene.
Most of these have been found effective, according to a recent systematic review Dr. Danan published in the Annals of Internal Medicine that this news organization covered. But recent years have also seen a rapid increase in interest and the availability of energy-based treatments for GSM, such as CO2 laser and radiofrequency interventions, particularly for those who cannot or do not want to use hormonal treatments. The idea behind these newer therapies is that they “heat tissue to cause a denaturation of collagen fibers and induce a wound-healing response,” with the aim of “enhancement of vaginal elasticity, restoration of premenopausal epithelial function, and symptom improvement,” the authors wrote.
Evidence has been scant and uneven for the safety and effectiveness of these treatments, and they have not been evaluated by the US Food and Drug Administration. The agency issued a warning in 2018 with remarks from then Commissioner Scott Gottlieb that the “products have serious risks and don’t have adequate evidence to support their use for these purposes.”
Much of the evidence has focused on CO2 lasers instead of other energy-based treatments, however, and a raft of new studies have been published on these interventions in the past 2 years. Dr. Danan and colleagues, therefore, assessed the most current state of the research with a systematic review of randomized controlled trials (RCTs) and prospective observational studies with control groups published through December 11, 2023.
Included studies needed to evaluate an energy-based treatment for at least 8 weeks in a minimum of 40 postmenopausal women (20 in each group) who had one or more GSM symptoms. The authors also included nonrandomized and uncontrolled studies with a follow-up of a year or more to assess possible adverse events. The studies also needed to assess at least one of eight core outcomes: Dyspareunia; vulvovaginal dryness; vulvovaginal discomfort/irritation; dysuria; change in most bothersome symptom; treatment satisfaction; adverse events; and distress, bother, or interference associated with genitourinary symptoms.
The authors identified 32 studies, including 16 RCTs, one quasi-RCT, and 15 nonrandomized studies. The researchers extracted and analyzed data from the 10 RCTs and one quasi-RCT that were rated as having low to moderate risk for bias.
Most of these studies assessed CO2 lasers alone, while three assessed erbium:yttrium-aluminum-garnet (Er:YAG) laser, and one looked at CO2 lasers vs radiofrequency treatments.
The average age of participants ranged from 56 to 64 years, and most trials were in the United States. Results showed that CO2 lasers led to little or no difference in dysuria, dyspareunia, or quality of life when compared with sham lasers. The CO2 laser therapy also showed little to no difference compared with vaginal estrogen creams for dyspareunia, dryness, discomfort/irritation, dysuria, or quality of life.
Most CO2 laser studies reported on most outcomes, but the Er:YAG studies tended to report only on quality of life and/or one or two other outcomes. The radiofrequency study only assessed dyspareunia and quality of life.
“Treatment effects on other outcomes and effects of Er:YAG laser or radiofrequency on any outcomes are very uncertain,” the authors reported. Few adverse events and no serious adverse events were reported based on 15 studies, including the additional non-RCTs that had follow-up for at least a year.
“There are case reports and other types of studies that have shown some bad outcomes using laser therapies, and we really wanted to be expansive and include anything, especially because this is such a new treatment and all these trials were in the last couple of years,” Dr. Danan said.
The review was limited by inconsistent or nonvalidated outcome reporting in the studies as well as small populations and short follow-up, typically less than 3 months.
The research was funded by the Agency for Healthcare Research and Quality and Patient-Centered Outcomes Research Institute. Dr. Danan and Dr. Das had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY 2024
When You and Your Malpractice Insurer Disagree on Your Case
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
Hot Flashes: Do They Predict CVD and Dementia?
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
Is Minimal Access Nipple-Sparing Mastectomy a Safer Option?
TOPLINE:
Given that both procedures appear safe overall, the choice may be guided by patients’ preference.
METHODOLOGY:
- Compared with a conventional mastectomy, a nipple-sparing mastectomy offers superior esthetic outcomes in patients with breast cancer. However, even the typical nipple-sparing approach often results in visible scarring and a high risk for nipple or areola necrosis. A minimal access approach, using endoscopic or robotic techniques, offers the potential to minimize scarring and better outcomes, but the complication risks are not well understood.
- Researchers performed a retrospective study that included 1583 patients with breast cancer who underwent conventional nipple-sparing mastectomy (n = 1356) or minimal access nipple-sparing mastectomy (n = 227) between 2018 and 2020 across 21 institutions in the Republic of Korea.
- Postoperative complications, categorized as short term (< 30 days) and long term (< 90 days), were compared between the two groups.
- The minimal access group had a higher percentage of premenopausal patients (73.57% vs 66.67%) and women with firm breasts (66.08% vs 31.27%).
TAKEAWAY:
- In total, 72 individuals (5.31%) in the conventional nipple-sparing mastectomy group and 7 (3.08%) in the minimal access nipple-sparing mastectomy group developed postoperative complications of grade IIIb or higher.
- The rate of complications between the conventional and minimal access nipple-sparing mastectomy groups in the short term (34.29% for conventional vs 32.16% for minimal access; P = .53) and long term (38.72% vs 32.16%, respectively; P = .06) was not significantly different.
- The conventional group experienced significantly fewer wound infections — both in the short term (1.62% vs 7.49%) and long term (4.28% vs 7.93%) — but a significantly higher rate of seroma (14.23% vs 9.25%), likely because of the variations in surgical instruments used during the procedures.
- Necrosis of the nipple or areola occurred more often in the minimal access group in the short term (8.81% vs 3.91%) but occurred more frequently in the conventional group in the long term (6.71% vs 2.20%).
IN PRACTICE:
“The similar complication rates suggest that both C-NSM [conventional nipple-sparing mastectomy] and M-NSM [minimal access nipple-sparing mastectomy] may be equally safe options,” the authors wrote. “Therefore, the choice of surgical approach should be tailored to patient preferences and individual needs.”
SOURCE:
The study, led by Joo Heung Kim, MD, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, South Korea, was published online on August 14, 2024, in JAMA Surgery.
LIMITATIONS:
The retrospective design comes with inherent biases. The nonrandom assignment of the participants to the two groups and the relatively small number of cases of minimal access nipple-sparing mastectomy may have affected the findings. The involvement of different surgeons and use of early robotic surgery techniques may have introduced bias as well.
DISCLOSURES:
This study was supported by Yonsei University College of Medicine and the National Research Foundation of Korea. Two authors reported receiving grants and consulting fees outside of this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Given that both procedures appear safe overall, the choice may be guided by patients’ preference.
METHODOLOGY:
- Compared with a conventional mastectomy, a nipple-sparing mastectomy offers superior esthetic outcomes in patients with breast cancer. However, even the typical nipple-sparing approach often results in visible scarring and a high risk for nipple or areola necrosis. A minimal access approach, using endoscopic or robotic techniques, offers the potential to minimize scarring and better outcomes, but the complication risks are not well understood.
- Researchers performed a retrospective study that included 1583 patients with breast cancer who underwent conventional nipple-sparing mastectomy (n = 1356) or minimal access nipple-sparing mastectomy (n = 227) between 2018 and 2020 across 21 institutions in the Republic of Korea.
- Postoperative complications, categorized as short term (< 30 days) and long term (< 90 days), were compared between the two groups.
- The minimal access group had a higher percentage of premenopausal patients (73.57% vs 66.67%) and women with firm breasts (66.08% vs 31.27%).
TAKEAWAY:
- In total, 72 individuals (5.31%) in the conventional nipple-sparing mastectomy group and 7 (3.08%) in the minimal access nipple-sparing mastectomy group developed postoperative complications of grade IIIb or higher.
- The rate of complications between the conventional and minimal access nipple-sparing mastectomy groups in the short term (34.29% for conventional vs 32.16% for minimal access; P = .53) and long term (38.72% vs 32.16%, respectively; P = .06) was not significantly different.
- The conventional group experienced significantly fewer wound infections — both in the short term (1.62% vs 7.49%) and long term (4.28% vs 7.93%) — but a significantly higher rate of seroma (14.23% vs 9.25%), likely because of the variations in surgical instruments used during the procedures.
- Necrosis of the nipple or areola occurred more often in the minimal access group in the short term (8.81% vs 3.91%) but occurred more frequently in the conventional group in the long term (6.71% vs 2.20%).
IN PRACTICE:
“The similar complication rates suggest that both C-NSM [conventional nipple-sparing mastectomy] and M-NSM [minimal access nipple-sparing mastectomy] may be equally safe options,” the authors wrote. “Therefore, the choice of surgical approach should be tailored to patient preferences and individual needs.”
SOURCE:
The study, led by Joo Heung Kim, MD, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, South Korea, was published online on August 14, 2024, in JAMA Surgery.
LIMITATIONS:
The retrospective design comes with inherent biases. The nonrandom assignment of the participants to the two groups and the relatively small number of cases of minimal access nipple-sparing mastectomy may have affected the findings. The involvement of different surgeons and use of early robotic surgery techniques may have introduced bias as well.
DISCLOSURES:
This study was supported by Yonsei University College of Medicine and the National Research Foundation of Korea. Two authors reported receiving grants and consulting fees outside of this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Given that both procedures appear safe overall, the choice may be guided by patients’ preference.
METHODOLOGY:
- Compared with a conventional mastectomy, a nipple-sparing mastectomy offers superior esthetic outcomes in patients with breast cancer. However, even the typical nipple-sparing approach often results in visible scarring and a high risk for nipple or areola necrosis. A minimal access approach, using endoscopic or robotic techniques, offers the potential to minimize scarring and better outcomes, but the complication risks are not well understood.
- Researchers performed a retrospective study that included 1583 patients with breast cancer who underwent conventional nipple-sparing mastectomy (n = 1356) or minimal access nipple-sparing mastectomy (n = 227) between 2018 and 2020 across 21 institutions in the Republic of Korea.
- Postoperative complications, categorized as short term (< 30 days) and long term (< 90 days), were compared between the two groups.
- The minimal access group had a higher percentage of premenopausal patients (73.57% vs 66.67%) and women with firm breasts (66.08% vs 31.27%).
TAKEAWAY:
- In total, 72 individuals (5.31%) in the conventional nipple-sparing mastectomy group and 7 (3.08%) in the minimal access nipple-sparing mastectomy group developed postoperative complications of grade IIIb or higher.
- The rate of complications between the conventional and minimal access nipple-sparing mastectomy groups in the short term (34.29% for conventional vs 32.16% for minimal access; P = .53) and long term (38.72% vs 32.16%, respectively; P = .06) was not significantly different.
- The conventional group experienced significantly fewer wound infections — both in the short term (1.62% vs 7.49%) and long term (4.28% vs 7.93%) — but a significantly higher rate of seroma (14.23% vs 9.25%), likely because of the variations in surgical instruments used during the procedures.
- Necrosis of the nipple or areola occurred more often in the minimal access group in the short term (8.81% vs 3.91%) but occurred more frequently in the conventional group in the long term (6.71% vs 2.20%).
IN PRACTICE:
“The similar complication rates suggest that both C-NSM [conventional nipple-sparing mastectomy] and M-NSM [minimal access nipple-sparing mastectomy] may be equally safe options,” the authors wrote. “Therefore, the choice of surgical approach should be tailored to patient preferences and individual needs.”
SOURCE:
The study, led by Joo Heung Kim, MD, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, South Korea, was published online on August 14, 2024, in JAMA Surgery.
LIMITATIONS:
The retrospective design comes with inherent biases. The nonrandom assignment of the participants to the two groups and the relatively small number of cases of minimal access nipple-sparing mastectomy may have affected the findings. The involvement of different surgeons and use of early robotic surgery techniques may have introduced bias as well.
DISCLOSURES:
This study was supported by Yonsei University College of Medicine and the National Research Foundation of Korea. Two authors reported receiving grants and consulting fees outside of this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Should Genetic Testing Be Routine for Breast Cancer?
TOPLINE:
METHODOLOGY:
- Traditional risk-based criteria, including family history and ancestry, are used to guide genetic testing decisions in women with breast cancer. However, these criteria may overlook patients with actionable genetic variants, particularly those outside the typical risk profile.
- To assess the efficacy of universal genetic testing, researchers conducted a cross-sectional study that included 729 women (median age at diagnosis, 53 years; 65.4% White women) newly diagnosed with invasive breast cancer between September 2019 and April 2022 at three Canadian institutions.
- All patients received genetic counseling followed by testing for the presence of germline pathogenic variants in 17 breast cancer susceptibility genes. The primary gene panel included screening for BRCA1, BRCA2, and PALB2, and the optional secondary panel included 14 additional breast cancer susceptibility genes.
- Of the participants, 659 (90.4%) were tested for both primary and secondary gene panels, whereas 70 (9.6%) underwent testing for only the primary panel. The majority of the cohort (66.8) were diagnosed with estrogen receptor–positive breast cancer, while 15.4% had triple-negative breast cancer.
TAKEAWAY:
- The prevalence of germline pathogenic variants was 7.3% (53 patients) — 5.3% for the primary gene panel and 2.1% for the secondary panel.
- Younger age (< 40 years; odds ratio [OR], 6.83), family history of ovarian cancer (OR, 9.75), high-grade disease (OR, 1.68), and triple-negative breast cancer (OR, 3.19) were independently associated with the presence of pathogenic genetic variants in BRCA1, BRCA2, or PALB2.
- Overall, 34.3% of patients with germline pathogenic variants in BRCA1, BRCA2, or PALB2, and 85.7% of carriers of secondary panel variants would not have qualified for traditional genetic testing according to the current risk factors.
- A total of 13 patients with BRCA1, BRCA2, or PALB2 variants had confirmed pathogenic mutations and were eligible for poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
IN PRACTICE:
These findings have “informed our clinical practice, and we now offer mainstream, oncology-led genetic testing to all women diagnosed with incident invasive breast cancer younger than 50 years of age, those with triple-negative breast cancer and/or bilateral breast cancer, those potentially eligible for PARP inhibitors,” as well as to men with breast cancer, the authors wrote.
SOURCE:
The study was led by Zoulikha Rezoug, MSc, Lady Davis Institute of the Jewish General Hospital, McGill University in Montreal, Québec, Canada. It was published online on September 3, 2024, in JAMA Network Open.
LIMITATIONS:
The COVID-19 pandemic resulted in a 6-month recruitment pause. Adjustments in recruitment criteria, focus on younger patients and those with triple-negative breast cancer could have overestimated prevalence of genetic pathogenic variants among women aged ≥ 70 years.
DISCLOSURES:
The study was supported by grants from the Jewish General Hospital Foundation and the Québec Breast Cancer Foundation, as well as an award from the Fonds de Recherche du Québec - Santé. Two authors reported receiving grants or personal fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Traditional risk-based criteria, including family history and ancestry, are used to guide genetic testing decisions in women with breast cancer. However, these criteria may overlook patients with actionable genetic variants, particularly those outside the typical risk profile.
- To assess the efficacy of universal genetic testing, researchers conducted a cross-sectional study that included 729 women (median age at diagnosis, 53 years; 65.4% White women) newly diagnosed with invasive breast cancer between September 2019 and April 2022 at three Canadian institutions.
- All patients received genetic counseling followed by testing for the presence of germline pathogenic variants in 17 breast cancer susceptibility genes. The primary gene panel included screening for BRCA1, BRCA2, and PALB2, and the optional secondary panel included 14 additional breast cancer susceptibility genes.
- Of the participants, 659 (90.4%) were tested for both primary and secondary gene panels, whereas 70 (9.6%) underwent testing for only the primary panel. The majority of the cohort (66.8) were diagnosed with estrogen receptor–positive breast cancer, while 15.4% had triple-negative breast cancer.
TAKEAWAY:
- The prevalence of germline pathogenic variants was 7.3% (53 patients) — 5.3% for the primary gene panel and 2.1% for the secondary panel.
- Younger age (< 40 years; odds ratio [OR], 6.83), family history of ovarian cancer (OR, 9.75), high-grade disease (OR, 1.68), and triple-negative breast cancer (OR, 3.19) were independently associated with the presence of pathogenic genetic variants in BRCA1, BRCA2, or PALB2.
- Overall, 34.3% of patients with germline pathogenic variants in BRCA1, BRCA2, or PALB2, and 85.7% of carriers of secondary panel variants would not have qualified for traditional genetic testing according to the current risk factors.
- A total of 13 patients with BRCA1, BRCA2, or PALB2 variants had confirmed pathogenic mutations and were eligible for poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
IN PRACTICE:
These findings have “informed our clinical practice, and we now offer mainstream, oncology-led genetic testing to all women diagnosed with incident invasive breast cancer younger than 50 years of age, those with triple-negative breast cancer and/or bilateral breast cancer, those potentially eligible for PARP inhibitors,” as well as to men with breast cancer, the authors wrote.
SOURCE:
The study was led by Zoulikha Rezoug, MSc, Lady Davis Institute of the Jewish General Hospital, McGill University in Montreal, Québec, Canada. It was published online on September 3, 2024, in JAMA Network Open.
LIMITATIONS:
The COVID-19 pandemic resulted in a 6-month recruitment pause. Adjustments in recruitment criteria, focus on younger patients and those with triple-negative breast cancer could have overestimated prevalence of genetic pathogenic variants among women aged ≥ 70 years.
DISCLOSURES:
The study was supported by grants from the Jewish General Hospital Foundation and the Québec Breast Cancer Foundation, as well as an award from the Fonds de Recherche du Québec - Santé. Two authors reported receiving grants or personal fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Traditional risk-based criteria, including family history and ancestry, are used to guide genetic testing decisions in women with breast cancer. However, these criteria may overlook patients with actionable genetic variants, particularly those outside the typical risk profile.
- To assess the efficacy of universal genetic testing, researchers conducted a cross-sectional study that included 729 women (median age at diagnosis, 53 years; 65.4% White women) newly diagnosed with invasive breast cancer between September 2019 and April 2022 at three Canadian institutions.
- All patients received genetic counseling followed by testing for the presence of germline pathogenic variants in 17 breast cancer susceptibility genes. The primary gene panel included screening for BRCA1, BRCA2, and PALB2, and the optional secondary panel included 14 additional breast cancer susceptibility genes.
- Of the participants, 659 (90.4%) were tested for both primary and secondary gene panels, whereas 70 (9.6%) underwent testing for only the primary panel. The majority of the cohort (66.8) were diagnosed with estrogen receptor–positive breast cancer, while 15.4% had triple-negative breast cancer.
TAKEAWAY:
- The prevalence of germline pathogenic variants was 7.3% (53 patients) — 5.3% for the primary gene panel and 2.1% for the secondary panel.
- Younger age (< 40 years; odds ratio [OR], 6.83), family history of ovarian cancer (OR, 9.75), high-grade disease (OR, 1.68), and triple-negative breast cancer (OR, 3.19) were independently associated with the presence of pathogenic genetic variants in BRCA1, BRCA2, or PALB2.
- Overall, 34.3% of patients with germline pathogenic variants in BRCA1, BRCA2, or PALB2, and 85.7% of carriers of secondary panel variants would not have qualified for traditional genetic testing according to the current risk factors.
- A total of 13 patients with BRCA1, BRCA2, or PALB2 variants had confirmed pathogenic mutations and were eligible for poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
IN PRACTICE:
These findings have “informed our clinical practice, and we now offer mainstream, oncology-led genetic testing to all women diagnosed with incident invasive breast cancer younger than 50 years of age, those with triple-negative breast cancer and/or bilateral breast cancer, those potentially eligible for PARP inhibitors,” as well as to men with breast cancer, the authors wrote.
SOURCE:
The study was led by Zoulikha Rezoug, MSc, Lady Davis Institute of the Jewish General Hospital, McGill University in Montreal, Québec, Canada. It was published online on September 3, 2024, in JAMA Network Open.
LIMITATIONS:
The COVID-19 pandemic resulted in a 6-month recruitment pause. Adjustments in recruitment criteria, focus on younger patients and those with triple-negative breast cancer could have overestimated prevalence of genetic pathogenic variants among women aged ≥ 70 years.
DISCLOSURES:
The study was supported by grants from the Jewish General Hospital Foundation and the Québec Breast Cancer Foundation, as well as an award from the Fonds de Recherche du Québec - Santé. Two authors reported receiving grants or personal fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
‘Reform School’ for Pharmacy Benefit Managers: How Might Legislation Help Patients?
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).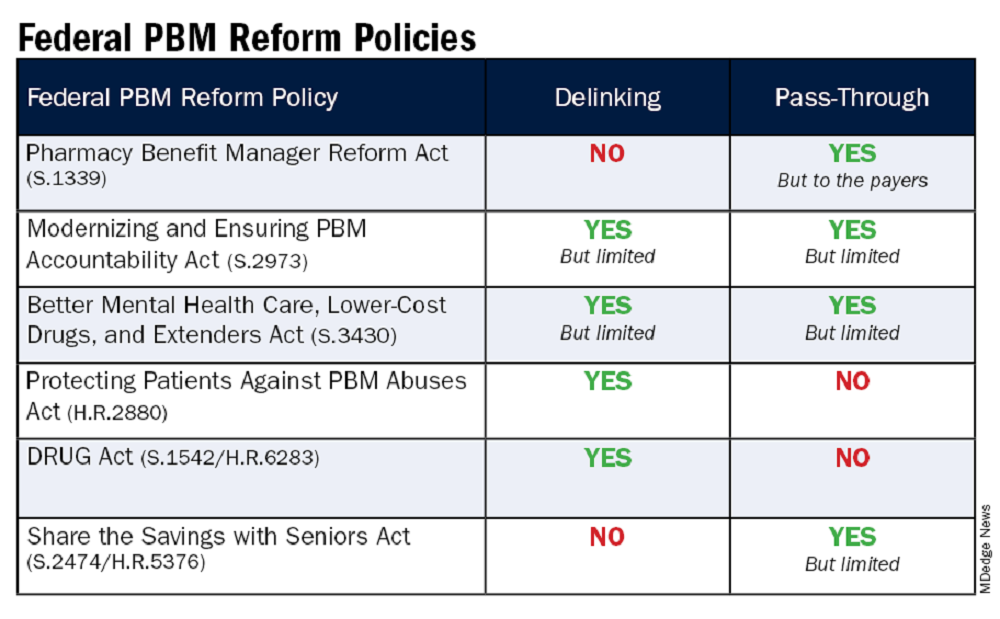
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
Stones, Bones, Groans, and Moans: Could This Be Primary Hyperparathyroidism?
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams.
Paul, we’re going to talk about our primary hyperparathyroidism podcast with Dr. Lindsay Kuo. It’s a topic that I feel much more clear on now.
Now, Paul, in primary care, you see a lot of calcium that is just slightly high. Can we just blame that on thiazide diuretics?
Paul N. Williams, MD: It’s a place to start. As you’re starting to think about the possible etiologies, primary hyperparathyroidism and malignancy are the two that roll right off the tongue, but it is worth going back to the patient’s medication list and making sure you’re not missing something.
Thiazides famously cause hypercalcemia, but in some of the reading I did for this episode, they may just uncover it a little bit early. Patients who are on thiazides who become hypercalcemic seem to go on to develop primary hyperthyroidism anyway. So I don’t think you can solely blame the thiazide.
Another medication that can be causative is lithium. So a good place to look first after you’ve repeated the labs and confirmed hypercalcemia is the patient’s medication list.
Dr. Watto: We’ve talked before about the basic workup for hypercalcemia, and determining whether it’s PTH dependent or PTH independent. On the podcast, we talk more about the full workup, but I wanted to talk about the classic symptoms. Our expert made the point that we don’t see them as much anymore, although we do see kidney stones. People used to present very late in the disease because they weren’t having labs done routinely.
The classic symptoms include osteoporosis and bone tumors. People can get nephrocalcinosis and kidney stones. I hadn’t really thought of it this way because we’re used to diagnosing it early now. Do you feel the same?
Dr. Williams: As labs have started routinely reporting calcium levels, this is more and more often how it’s picked up. The other aspect is that as we are screening for and finding osteoporosis, part of the workup almost always involves getting a parathyroid hormone and a calcium level. We’re seeing these lab abnormalities before we’re seeing symptoms, which is good.
But it also makes things more diagnostically thorny.
Dr. Watto: Dr. Lindsay Kuo made the point that when she sees patients before and after surgery, she’s aware of these nonclassic symptoms — the stones, bones, groans, and the psychiatric overtones that can be anything from fatigue or irritability to dysphoria.
Some people have a generalized weakness that’s very nonspecific. Dr. Kuo said that sometimes these symptoms will disappear after surgery. The patients may just have gotten used to them, or they thought these symptoms were caused by something else, but after surgery they went away.
There are these nonclassic symptoms that are harder to pin down. I was surprised by that.
Dr. Williams: She mentioned polydipsia and polyuria, which have been reported in other studies. It seems like it can be anything. You have to take a good history, but none of those things in and of themselves is an indication for operating unless the patient has the classic renal or bone manifestations.
Dr. Watto: The other thing we talked about is a normal calcium level in a patient with primary hyperparathyroidism, or the finding of a PTH level in the normal range but with a high calcium level that is inappropriate. Can you talk a little bit about those two situations?
Dr. Williams: They’re hard to say but kind of easy to manage because you treat them the same way as someone who has elevated calcium and PTH levels.
The normocalcemic patient is something we might stumble across with osteoporosis screening. Initially the calcium level is elevated, so you repeat it and it’s normal but with an elevated PTH level. You’re like, shoot. Now what?
It turns out that most endocrine surgeons say that the indications for surgery for the classic form of primary hyperparathyroidism apply to these patients as well, and it probably helps with the bone outcomes, which is one of the things they follow most closely. If you have hypercalcemia, you should have a suppressed PTH level, the so-called normohormonal hyperparathyroidism, which is not normal at all. So even if the PTH is in the normal range, it’s still relatively elevated compared with what it should be. That situation is treated in the same way as the classic elevated PTH and elevated calcium levels.
Dr. Watto: If the calcium is abnormal and the PTH is not quite what you’d expect it to be, you can always ask your friendly neighborhood endocrinologist to help you figure out whether the patient really has one of these conditions. You have to make sure that they don’t have a simple secondary cause like a low vitamin D level. In that case, you fix the vitamin D and then recheck the numbers to see if they’ve normalized. But I have found a bunch of these edge cases in which it has been helpful to confer with an endocrinologist, especially before you send someone to a surgeon to take out their parathyroid gland.
This was a really fantastic conversation. If you want to hear the full podcast episode, click here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, served as a director, officer, partner, employee, adviser, consultant, or trustee for The Curbsiders, and has received income in an amount equal to or greater than $250 from The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams.
Paul, we’re going to talk about our primary hyperparathyroidism podcast with Dr. Lindsay Kuo. It’s a topic that I feel much more clear on now.
Now, Paul, in primary care, you see a lot of calcium that is just slightly high. Can we just blame that on thiazide diuretics?
Paul N. Williams, MD: It’s a place to start. As you’re starting to think about the possible etiologies, primary hyperparathyroidism and malignancy are the two that roll right off the tongue, but it is worth going back to the patient’s medication list and making sure you’re not missing something.
Thiazides famously cause hypercalcemia, but in some of the reading I did for this episode, they may just uncover it a little bit early. Patients who are on thiazides who become hypercalcemic seem to go on to develop primary hyperthyroidism anyway. So I don’t think you can solely blame the thiazide.
Another medication that can be causative is lithium. So a good place to look first after you’ve repeated the labs and confirmed hypercalcemia is the patient’s medication list.
Dr. Watto: We’ve talked before about the basic workup for hypercalcemia, and determining whether it’s PTH dependent or PTH independent. On the podcast, we talk more about the full workup, but I wanted to talk about the classic symptoms. Our expert made the point that we don’t see them as much anymore, although we do see kidney stones. People used to present very late in the disease because they weren’t having labs done routinely.
The classic symptoms include osteoporosis and bone tumors. People can get nephrocalcinosis and kidney stones. I hadn’t really thought of it this way because we’re used to diagnosing it early now. Do you feel the same?
Dr. Williams: As labs have started routinely reporting calcium levels, this is more and more often how it’s picked up. The other aspect is that as we are screening for and finding osteoporosis, part of the workup almost always involves getting a parathyroid hormone and a calcium level. We’re seeing these lab abnormalities before we’re seeing symptoms, which is good.
But it also makes things more diagnostically thorny.
Dr. Watto: Dr. Lindsay Kuo made the point that when she sees patients before and after surgery, she’s aware of these nonclassic symptoms — the stones, bones, groans, and the psychiatric overtones that can be anything from fatigue or irritability to dysphoria.
Some people have a generalized weakness that’s very nonspecific. Dr. Kuo said that sometimes these symptoms will disappear after surgery. The patients may just have gotten used to them, or they thought these symptoms were caused by something else, but after surgery they went away.
There are these nonclassic symptoms that are harder to pin down. I was surprised by that.
Dr. Williams: She mentioned polydipsia and polyuria, which have been reported in other studies. It seems like it can be anything. You have to take a good history, but none of those things in and of themselves is an indication for operating unless the patient has the classic renal or bone manifestations.
Dr. Watto: The other thing we talked about is a normal calcium level in a patient with primary hyperparathyroidism, or the finding of a PTH level in the normal range but with a high calcium level that is inappropriate. Can you talk a little bit about those two situations?
Dr. Williams: They’re hard to say but kind of easy to manage because you treat them the same way as someone who has elevated calcium and PTH levels.
The normocalcemic patient is something we might stumble across with osteoporosis screening. Initially the calcium level is elevated, so you repeat it and it’s normal but with an elevated PTH level. You’re like, shoot. Now what?
It turns out that most endocrine surgeons say that the indications for surgery for the classic form of primary hyperparathyroidism apply to these patients as well, and it probably helps with the bone outcomes, which is one of the things they follow most closely. If you have hypercalcemia, you should have a suppressed PTH level, the so-called normohormonal hyperparathyroidism, which is not normal at all. So even if the PTH is in the normal range, it’s still relatively elevated compared with what it should be. That situation is treated in the same way as the classic elevated PTH and elevated calcium levels.
Dr. Watto: If the calcium is abnormal and the PTH is not quite what you’d expect it to be, you can always ask your friendly neighborhood endocrinologist to help you figure out whether the patient really has one of these conditions. You have to make sure that they don’t have a simple secondary cause like a low vitamin D level. In that case, you fix the vitamin D and then recheck the numbers to see if they’ve normalized. But I have found a bunch of these edge cases in which it has been helpful to confer with an endocrinologist, especially before you send someone to a surgeon to take out their parathyroid gland.
This was a really fantastic conversation. If you want to hear the full podcast episode, click here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, served as a director, officer, partner, employee, adviser, consultant, or trustee for The Curbsiders, and has received income in an amount equal to or greater than $250 from The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams.
Paul, we’re going to talk about our primary hyperparathyroidism podcast with Dr. Lindsay Kuo. It’s a topic that I feel much more clear on now.
Now, Paul, in primary care, you see a lot of calcium that is just slightly high. Can we just blame that on thiazide diuretics?
Paul N. Williams, MD: It’s a place to start. As you’re starting to think about the possible etiologies, primary hyperparathyroidism and malignancy are the two that roll right off the tongue, but it is worth going back to the patient’s medication list and making sure you’re not missing something.
Thiazides famously cause hypercalcemia, but in some of the reading I did for this episode, they may just uncover it a little bit early. Patients who are on thiazides who become hypercalcemic seem to go on to develop primary hyperthyroidism anyway. So I don’t think you can solely blame the thiazide.
Another medication that can be causative is lithium. So a good place to look first after you’ve repeated the labs and confirmed hypercalcemia is the patient’s medication list.
Dr. Watto: We’ve talked before about the basic workup for hypercalcemia, and determining whether it’s PTH dependent or PTH independent. On the podcast, we talk more about the full workup, but I wanted to talk about the classic symptoms. Our expert made the point that we don’t see them as much anymore, although we do see kidney stones. People used to present very late in the disease because they weren’t having labs done routinely.
The classic symptoms include osteoporosis and bone tumors. People can get nephrocalcinosis and kidney stones. I hadn’t really thought of it this way because we’re used to diagnosing it early now. Do you feel the same?
Dr. Williams: As labs have started routinely reporting calcium levels, this is more and more often how it’s picked up. The other aspect is that as we are screening for and finding osteoporosis, part of the workup almost always involves getting a parathyroid hormone and a calcium level. We’re seeing these lab abnormalities before we’re seeing symptoms, which is good.
But it also makes things more diagnostically thorny.
Dr. Watto: Dr. Lindsay Kuo made the point that when she sees patients before and after surgery, she’s aware of these nonclassic symptoms — the stones, bones, groans, and the psychiatric overtones that can be anything from fatigue or irritability to dysphoria.
Some people have a generalized weakness that’s very nonspecific. Dr. Kuo said that sometimes these symptoms will disappear after surgery. The patients may just have gotten used to them, or they thought these symptoms were caused by something else, but after surgery they went away.
There are these nonclassic symptoms that are harder to pin down. I was surprised by that.
Dr. Williams: She mentioned polydipsia and polyuria, which have been reported in other studies. It seems like it can be anything. You have to take a good history, but none of those things in and of themselves is an indication for operating unless the patient has the classic renal or bone manifestations.
Dr. Watto: The other thing we talked about is a normal calcium level in a patient with primary hyperparathyroidism, or the finding of a PTH level in the normal range but with a high calcium level that is inappropriate. Can you talk a little bit about those two situations?
Dr. Williams: They’re hard to say but kind of easy to manage because you treat them the same way as someone who has elevated calcium and PTH levels.
The normocalcemic patient is something we might stumble across with osteoporosis screening. Initially the calcium level is elevated, so you repeat it and it’s normal but with an elevated PTH level. You’re like, shoot. Now what?
It turns out that most endocrine surgeons say that the indications for surgery for the classic form of primary hyperparathyroidism apply to these patients as well, and it probably helps with the bone outcomes, which is one of the things they follow most closely. If you have hypercalcemia, you should have a suppressed PTH level, the so-called normohormonal hyperparathyroidism, which is not normal at all. So even if the PTH is in the normal range, it’s still relatively elevated compared with what it should be. That situation is treated in the same way as the classic elevated PTH and elevated calcium levels.
Dr. Watto: If the calcium is abnormal and the PTH is not quite what you’d expect it to be, you can always ask your friendly neighborhood endocrinologist to help you figure out whether the patient really has one of these conditions. You have to make sure that they don’t have a simple secondary cause like a low vitamin D level. In that case, you fix the vitamin D and then recheck the numbers to see if they’ve normalized. But I have found a bunch of these edge cases in which it has been helpful to confer with an endocrinologist, especially before you send someone to a surgeon to take out their parathyroid gland.
This was a really fantastic conversation. If you want to hear the full podcast episode, click here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, served as a director, officer, partner, employee, adviser, consultant, or trustee for The Curbsiders, and has received income in an amount equal to or greater than $250 from The Curbsiders.
A version of this article first appeared on Medscape.com.
AI-Powered Clinical Documentation Tool Reduces EHR Time for Clinicians
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
NSAIDs Offer No Relief for Pain From IUD Placement
Research on pain management during placement of intrauterine devices (IUD) is lacking, but most studies so far indicate that nonsteroidal anti-inflammatory drugs (NSAIDs) are not effective, according to a poster presented at Pain Week 2024 in Las Vegas.
Roughly 79% of the 14 studies included in the systematic review found NSAIDs — one of the most common drugs clinicians advise patients to take before placement — did not diminish discomfort.
“We’re challenging the current practice of using just NSAIDs as a first-line of treatment,” said Kevin Rowland, PhD, professor and chair of biomedical sciences at Tilman J. Fertitta Family College of Medicine in Houston, who helped conduct the meta-analysis. “We need additional measures.”
Some studies found the drugs offered virtually no improvement for patients, while the biggest drop in pain shown in one study was about 40%. The range of pain levels women reported while using NSAIDs was between 1.8 and 7.3 on the visual analog scale (VAS), with an average score of 4.25.
The review included 10 types of NSAIDs and dosages administered to patients before the procedure. One intramuscular NSAID was included while the remaining were oral. All studies were peer-reviewed, used the VAS pain scale, and were not limited to any specific population.
The findings highlight a longstanding but unresolved problem in reproductive health: An overall lack of effective pain management strategies for gynecologic procedures.
“We went into this having a pretty good idea of what we were going to find because [the lack of NSAID efficacy] has been shown before, it’s been talked about before, and we’re just not listening as a medical community,” said Isabella D. Martingano, an MD candidate at Tilman J. Fertitta Family College of Medicine, who led the review.
The research also points to a lack of robust studies on pain during IUD placement, said Emma Lakey, a coauthor and medical student at Tilman J. Fertitta Family College of Medicine.
“We were only able to review 14 studies, which was enough to go off of, but considering we were looking for trials about pain control for a procedure that helps prevent pregnancy, that’s just not enough research,” Ms. Lakey said.
Discomfort associated with IUD placement ranges from mild to severe, can last for over a week, and includes cramping, bleeding, lightheadedness, nausea, and fainting. Some research suggests that providers may underestimate the level of pain the procedures cause.
“Unfortunately, the pain associated with IUD insertion and removal has been underplayed for a long time and many practitioners in the field likely haven’t counseled patients fully on what the procedure will feel like,” said Jennifer Chin, MD, an ob.gyn. and assistant professor of obstetrics and gynecology at the University of Washington in Seattle.
NSAIDs are not mentioned in the recently expanded guidelines on IUD placement from the US Centers for Disease Control and Prevention (CDC). The CDC recommends lidocaine paracervical blocks, gels, sprays, and creams, plus counseling women about pain ahead of the procedures.
IUDs are one of the most effective forms of birth control, with a failure rate below 1%.
Yet hearing about painful placement keeps many women from seeking out an IUD or replacing an existing device, Dr. Rowland said. The review adds to the body of evidence that current strategies are not working and that more research is needed, he said.
According to Dr. Chin, making IUDs more accessible means taking a more personalized approach to pain management while understanding that what may be a painless procedure for one patient may be excruciating for another.
Dr. Chin offers a range of options for her patients, including NSAIDs, lorazepam for anxiety, paracervical blocks, lidocaine jelly and spray, intravenous sedation, and general anesthesia. She also talks to her patients through the procedure and provides guided imagery and meditation.
“We should always make sure we’re prioritizing the patients and providing evidence-based, compassionate, and individualized care,” said Dr. Chin. “Each patient comes to us in a particular context and with a specific set of experiences and history that will make a difference in how we’re best able to take care of them.”
The authors reported no disclosures and no sources of funding. Dr. Chin reported no disclosures.
A version of this article first appeared on Medscape.com.
Research on pain management during placement of intrauterine devices (IUD) is lacking, but most studies so far indicate that nonsteroidal anti-inflammatory drugs (NSAIDs) are not effective, according to a poster presented at Pain Week 2024 in Las Vegas.
Roughly 79% of the 14 studies included in the systematic review found NSAIDs — one of the most common drugs clinicians advise patients to take before placement — did not diminish discomfort.
“We’re challenging the current practice of using just NSAIDs as a first-line of treatment,” said Kevin Rowland, PhD, professor and chair of biomedical sciences at Tilman J. Fertitta Family College of Medicine in Houston, who helped conduct the meta-analysis. “We need additional measures.”
Some studies found the drugs offered virtually no improvement for patients, while the biggest drop in pain shown in one study was about 40%. The range of pain levels women reported while using NSAIDs was between 1.8 and 7.3 on the visual analog scale (VAS), with an average score of 4.25.
The review included 10 types of NSAIDs and dosages administered to patients before the procedure. One intramuscular NSAID was included while the remaining were oral. All studies were peer-reviewed, used the VAS pain scale, and were not limited to any specific population.
The findings highlight a longstanding but unresolved problem in reproductive health: An overall lack of effective pain management strategies for gynecologic procedures.
“We went into this having a pretty good idea of what we were going to find because [the lack of NSAID efficacy] has been shown before, it’s been talked about before, and we’re just not listening as a medical community,” said Isabella D. Martingano, an MD candidate at Tilman J. Fertitta Family College of Medicine, who led the review.
The research also points to a lack of robust studies on pain during IUD placement, said Emma Lakey, a coauthor and medical student at Tilman J. Fertitta Family College of Medicine.
“We were only able to review 14 studies, which was enough to go off of, but considering we were looking for trials about pain control for a procedure that helps prevent pregnancy, that’s just not enough research,” Ms. Lakey said.
Discomfort associated with IUD placement ranges from mild to severe, can last for over a week, and includes cramping, bleeding, lightheadedness, nausea, and fainting. Some research suggests that providers may underestimate the level of pain the procedures cause.
“Unfortunately, the pain associated with IUD insertion and removal has been underplayed for a long time and many practitioners in the field likely haven’t counseled patients fully on what the procedure will feel like,” said Jennifer Chin, MD, an ob.gyn. and assistant professor of obstetrics and gynecology at the University of Washington in Seattle.
NSAIDs are not mentioned in the recently expanded guidelines on IUD placement from the US Centers for Disease Control and Prevention (CDC). The CDC recommends lidocaine paracervical blocks, gels, sprays, and creams, plus counseling women about pain ahead of the procedures.
IUDs are one of the most effective forms of birth control, with a failure rate below 1%.
Yet hearing about painful placement keeps many women from seeking out an IUD or replacing an existing device, Dr. Rowland said. The review adds to the body of evidence that current strategies are not working and that more research is needed, he said.
According to Dr. Chin, making IUDs more accessible means taking a more personalized approach to pain management while understanding that what may be a painless procedure for one patient may be excruciating for another.
Dr. Chin offers a range of options for her patients, including NSAIDs, lorazepam for anxiety, paracervical blocks, lidocaine jelly and spray, intravenous sedation, and general anesthesia. She also talks to her patients through the procedure and provides guided imagery and meditation.
“We should always make sure we’re prioritizing the patients and providing evidence-based, compassionate, and individualized care,” said Dr. Chin. “Each patient comes to us in a particular context and with a specific set of experiences and history that will make a difference in how we’re best able to take care of them.”
The authors reported no disclosures and no sources of funding. Dr. Chin reported no disclosures.
A version of this article first appeared on Medscape.com.
Research on pain management during placement of intrauterine devices (IUD) is lacking, but most studies so far indicate that nonsteroidal anti-inflammatory drugs (NSAIDs) are not effective, according to a poster presented at Pain Week 2024 in Las Vegas.
Roughly 79% of the 14 studies included in the systematic review found NSAIDs — one of the most common drugs clinicians advise patients to take before placement — did not diminish discomfort.
“We’re challenging the current practice of using just NSAIDs as a first-line of treatment,” said Kevin Rowland, PhD, professor and chair of biomedical sciences at Tilman J. Fertitta Family College of Medicine in Houston, who helped conduct the meta-analysis. “We need additional measures.”
Some studies found the drugs offered virtually no improvement for patients, while the biggest drop in pain shown in one study was about 40%. The range of pain levels women reported while using NSAIDs was between 1.8 and 7.3 on the visual analog scale (VAS), with an average score of 4.25.
The review included 10 types of NSAIDs and dosages administered to patients before the procedure. One intramuscular NSAID was included while the remaining were oral. All studies were peer-reviewed, used the VAS pain scale, and were not limited to any specific population.
The findings highlight a longstanding but unresolved problem in reproductive health: An overall lack of effective pain management strategies for gynecologic procedures.
“We went into this having a pretty good idea of what we were going to find because [the lack of NSAID efficacy] has been shown before, it’s been talked about before, and we’re just not listening as a medical community,” said Isabella D. Martingano, an MD candidate at Tilman J. Fertitta Family College of Medicine, who led the review.
The research also points to a lack of robust studies on pain during IUD placement, said Emma Lakey, a coauthor and medical student at Tilman J. Fertitta Family College of Medicine.
“We were only able to review 14 studies, which was enough to go off of, but considering we were looking for trials about pain control for a procedure that helps prevent pregnancy, that’s just not enough research,” Ms. Lakey said.
Discomfort associated with IUD placement ranges from mild to severe, can last for over a week, and includes cramping, bleeding, lightheadedness, nausea, and fainting. Some research suggests that providers may underestimate the level of pain the procedures cause.
“Unfortunately, the pain associated with IUD insertion and removal has been underplayed for a long time and many practitioners in the field likely haven’t counseled patients fully on what the procedure will feel like,” said Jennifer Chin, MD, an ob.gyn. and assistant professor of obstetrics and gynecology at the University of Washington in Seattle.
NSAIDs are not mentioned in the recently expanded guidelines on IUD placement from the US Centers for Disease Control and Prevention (CDC). The CDC recommends lidocaine paracervical blocks, gels, sprays, and creams, plus counseling women about pain ahead of the procedures.
IUDs are one of the most effective forms of birth control, with a failure rate below 1%.
Yet hearing about painful placement keeps many women from seeking out an IUD or replacing an existing device, Dr. Rowland said. The review adds to the body of evidence that current strategies are not working and that more research is needed, he said.
According to Dr. Chin, making IUDs more accessible means taking a more personalized approach to pain management while understanding that what may be a painless procedure for one patient may be excruciating for another.
Dr. Chin offers a range of options for her patients, including NSAIDs, lorazepam for anxiety, paracervical blocks, lidocaine jelly and spray, intravenous sedation, and general anesthesia. She also talks to her patients through the procedure and provides guided imagery and meditation.
“We should always make sure we’re prioritizing the patients and providing evidence-based, compassionate, and individualized care,” said Dr. Chin. “Each patient comes to us in a particular context and with a specific set of experiences and history that will make a difference in how we’re best able to take care of them.”
The authors reported no disclosures and no sources of funding. Dr. Chin reported no disclosures.
A version of this article first appeared on Medscape.com.
Long-Term Exposure to Road Traffic Noise and Air Pollution Linked to Infertility
TOPLINE:
Long-term exposure to particulate matter < 2.5 μm in diameter (PM2.5) is linked to a higher risk for infertility in men. Exposure to road traffic noise is associated with a higher risk for infertility in women aged > 35 years and possibly in men aged > 37 years.
METHODOLOGY:
- This nationwide prospective cohort study evaluated the association between long-term exposure to road traffic noise and PM2.5 and infertility in 526,056 men (mean age, 33.6 years) and 377,850 women (mean age, 32.7 years) who were cohabiting or married, had fewer than two children, and lived in Denmark between 2000 and 2017.
- Residential exposure to road traffic noise (most exposed facade of the home) and PM2.5 was estimated using validated models and linked to data from national registers.
- Diagnoses of infertility were identified in men and women from the Danish National Patient Register over a mean follow-up of 4.3 years and 4.2 years, respectively.
TAKEAWAY:
- Each 2.9 µg/m3 increase in the 5-year average exposure to PM2.5 was associated with a 24% increase in the risk for infertility in men aged 30-45 years (adjusted hazard ratio [aHR], 1.24).
- No significant association was found between exposure to PM2.5 and infertility in women.
- Each 10.2 dB increase in the 5-year average exposure to road traffic noise was associated with a 14% increase in infertility (aHR, 1.14; 95% CI, 1.10-1.18) in women aged 35-45 years.
- Exposure to noise was associated with a reduced risk for infertility in men aged 30.0-36.9 years (aHR, 0.93; 95% CI, 0.91-0.96) and an increased risk in those aged 37-45 years (aHR, 1.06; 95% CI, 1.02-1.11).
IN PRACTICE:
“As many Western countries are facing declining birth rates and increasing maternal age at the birth of a first child, knowledge on environmental pollutants affecting fertility is crucial,” the authors of the study wrote. “It suggests that political implementation of air pollution and noise mitigations may be important tools for improving birth rates in the Western world,” they added.
SOURCE:
The study, led by Mette Sorensen, of the Danish Cancer Institute in Copenhagen, Denmark, was published online in The BMJ.
LIMITATIONS:
The study’s reliance on register data meant information on lifestyle factors such as alcohol use, body mass index, and smoking was unavailable. The lack of data on exposure to noise and PM2.5 at work and during leisure activities may affect the size and statistical precision of risk estimates.
DISCLOSURES:
The study did not receive any external funding. The authors declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Long-term exposure to particulate matter < 2.5 μm in diameter (PM2.5) is linked to a higher risk for infertility in men. Exposure to road traffic noise is associated with a higher risk for infertility in women aged > 35 years and possibly in men aged > 37 years.
METHODOLOGY:
- This nationwide prospective cohort study evaluated the association between long-term exposure to road traffic noise and PM2.5 and infertility in 526,056 men (mean age, 33.6 years) and 377,850 women (mean age, 32.7 years) who were cohabiting or married, had fewer than two children, and lived in Denmark between 2000 and 2017.
- Residential exposure to road traffic noise (most exposed facade of the home) and PM2.5 was estimated using validated models and linked to data from national registers.
- Diagnoses of infertility were identified in men and women from the Danish National Patient Register over a mean follow-up of 4.3 years and 4.2 years, respectively.
TAKEAWAY:
- Each 2.9 µg/m3 increase in the 5-year average exposure to PM2.5 was associated with a 24% increase in the risk for infertility in men aged 30-45 years (adjusted hazard ratio [aHR], 1.24).
- No significant association was found between exposure to PM2.5 and infertility in women.
- Each 10.2 dB increase in the 5-year average exposure to road traffic noise was associated with a 14% increase in infertility (aHR, 1.14; 95% CI, 1.10-1.18) in women aged 35-45 years.
- Exposure to noise was associated with a reduced risk for infertility in men aged 30.0-36.9 years (aHR, 0.93; 95% CI, 0.91-0.96) and an increased risk in those aged 37-45 years (aHR, 1.06; 95% CI, 1.02-1.11).
IN PRACTICE:
“As many Western countries are facing declining birth rates and increasing maternal age at the birth of a first child, knowledge on environmental pollutants affecting fertility is crucial,” the authors of the study wrote. “It suggests that political implementation of air pollution and noise mitigations may be important tools for improving birth rates in the Western world,” they added.
SOURCE:
The study, led by Mette Sorensen, of the Danish Cancer Institute in Copenhagen, Denmark, was published online in The BMJ.
LIMITATIONS:
The study’s reliance on register data meant information on lifestyle factors such as alcohol use, body mass index, and smoking was unavailable. The lack of data on exposure to noise and PM2.5 at work and during leisure activities may affect the size and statistical precision of risk estimates.
DISCLOSURES:
The study did not receive any external funding. The authors declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Long-term exposure to particulate matter < 2.5 μm in diameter (PM2.5) is linked to a higher risk for infertility in men. Exposure to road traffic noise is associated with a higher risk for infertility in women aged > 35 years and possibly in men aged > 37 years.
METHODOLOGY:
- This nationwide prospective cohort study evaluated the association between long-term exposure to road traffic noise and PM2.5 and infertility in 526,056 men (mean age, 33.6 years) and 377,850 women (mean age, 32.7 years) who were cohabiting or married, had fewer than two children, and lived in Denmark between 2000 and 2017.
- Residential exposure to road traffic noise (most exposed facade of the home) and PM2.5 was estimated using validated models and linked to data from national registers.
- Diagnoses of infertility were identified in men and women from the Danish National Patient Register over a mean follow-up of 4.3 years and 4.2 years, respectively.
TAKEAWAY:
- Each 2.9 µg/m3 increase in the 5-year average exposure to PM2.5 was associated with a 24% increase in the risk for infertility in men aged 30-45 years (adjusted hazard ratio [aHR], 1.24).
- No significant association was found between exposure to PM2.5 and infertility in women.
- Each 10.2 dB increase in the 5-year average exposure to road traffic noise was associated with a 14% increase in infertility (aHR, 1.14; 95% CI, 1.10-1.18) in women aged 35-45 years.
- Exposure to noise was associated with a reduced risk for infertility in men aged 30.0-36.9 years (aHR, 0.93; 95% CI, 0.91-0.96) and an increased risk in those aged 37-45 years (aHR, 1.06; 95% CI, 1.02-1.11).
IN PRACTICE:
“As many Western countries are facing declining birth rates and increasing maternal age at the birth of a first child, knowledge on environmental pollutants affecting fertility is crucial,” the authors of the study wrote. “It suggests that political implementation of air pollution and noise mitigations may be important tools for improving birth rates in the Western world,” they added.
SOURCE:
The study, led by Mette Sorensen, of the Danish Cancer Institute in Copenhagen, Denmark, was published online in The BMJ.
LIMITATIONS:
The study’s reliance on register data meant information on lifestyle factors such as alcohol use, body mass index, and smoking was unavailable. The lack of data on exposure to noise and PM2.5 at work and during leisure activities may affect the size and statistical precision of risk estimates.
DISCLOSURES:
The study did not receive any external funding. The authors declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.

