User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
What's the diagnosis?
At the week follow-up, the lesions were unchanged and the swelling on the left lateral eyebrow was worsening. A biopsy of the yellow lesion on the back and one of the scaly papules on the abdomen was performed. A fungal and bacterial cultures were also ordered.
He was referred to ophthalmology for evaluation of the eyelid swelling and an ultrasound was requested.
The skin biopsy showed a clonal proliferation of reniform histiocytes with eosinophils within the dermis. The cells were positive for S100, CD207 (langerin), and CD1a and negative for pancytokeratin and Melan-A, supportive of the diagnosis of Langerhans cell histiocytosis (LCH).
Diagnosis
The patient was admitted to the hospital, where a skeletal survey was performed, which showed an asymmetric lucency involving the left frontal calvarium extending to the superior lateral orbital rim. The brain MRI demonstrated a destructive avidly enhancing soft-tissue process which involved the superior left orbital rim likely with some degree of intracranial extension. This lesion exerts mass effect upon surrounding structures to the left ocular globe. With the skin and skeletal findings, the patient was diagnosed with LCH. His blood count was significant for thrombocytopenia. His liver and kidney function were normal. His electrolytes were also with in normal range. He was started on chemotherapy with vinblastine and systemic corticosteroids with resolution of the rash and decrease on the size of the lesion on the orbit within a few weeks.
Infantile LCH is a rare neoplastic disorder of hematopoietic myeloid precursor cells caused by activating mutations in the mitogen-activated protein kinase (MAPK) pathway, particularly BRAF-V600E mutation. White male children are mostly affected, with a peak incidence of 1-3 years of age. Nine out of 10 children with cutaneous involvement also have multisystemic disease, such as the case of our patient. LCH is classified as single or multisystem organ disease. Two-thirds of the cases present with single system involvement. Organs most commonly affected include the bone (the skull being the most commonly affected), skin, and high-risk organs like the liver, spleen, and bone marrow, and less commonly the lungs, lymph nodes, and central nervous system. Some patients can present with fever, lethargy, and weight loss. None were noted in our patient.
Skin findings of LCH can have multiple morphologies and presentations and often described as a big mimicker. In young infants like our patient, the seborrheic dermatitis–mimicking type is often seen. In other cases, the skin lesions can appear eczematous, petechial, with scabbing, crusting, or purpura. Xanthoma-like lesions, like that one our patient had in the back, have also been described. Resistant diaper dermatitis and cradle cap should prompt the clinician to think about LCH. Lesions can be so varied that can present with hypopigmentation (vitiligo like), hyperpigmentation, varicella-like papulo-pustules, and red blue nodules within others. Oral mucosa and nail involvement can also occur.
Bone involvement can present as soft-tissue mass with swelling and pain as it occur in our patient.
Endocrinopathies have been described in patients with LCH including diabetes insipidus, growth hormone deficiency, and less likely thyroid disease.
Multidisciplinary care
The diagnosis of LCH in infants necessitates a combination of clinical, radiological, and histopathologic findings. In infants, cutaneous involvement is a frequent initial presentation, with characteristic lesions that are often misdiagnosed as other dermatologic conditions. Timely recognition of these lesions and appropriate skin biopsies for histological examination are essential steps in achieving an accurate diagnosis.
Radiological imaging, including x-rays, CT, and MRI, plays a crucial role in assessing the extent of involvement.
The management of LCH in infants requires a well-coordinated multidisciplinary approach involving pediatric oncologists, dermatologists, radiologists, orthopedic surgeons, and other relevant specialists. Treatment strategies vary depending on the extent of disease involvement and the presence of risk factors. In localized cases, observation with close monitoring may be considered, as some cases of LCH in infants may undergo spontaneous regression. However, cases with severe symptoms, extensive organ involvement, or high-risk features may require systemic therapies.
Chemotherapy agents, including vinblastine and prednisone have been utilized in the treatment of infantile LCH with varying success. The selection of treatment regimens should be tailored to each individual case, considering disease severity, potential toxicities, and long-term effects. In cases of bone lesions causing significant deformities or functional impairment, surgical intervention may be necessary. Skin only disease can be treated with topical corticosteroids.
Prognosis
Survival rates in patients with single-organ involvement without risk-organ involvement is close to 100% and with risk-organ involvement of 98% at 5 years.
Long-term follow-up is essential for infants diagnosed with LCH, as recurrence and late effects can occur even after successful treatment. Continued monitoring allows for the timely detection of relapses or the development of secondary complications.
Infants thought to have common skin conditions like eczema, seborrheic dermatitis, or diaper dermatitis not responding to treatment should be referred to pediatric dermatology for evaluation to rule out the possibility of LCH.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Krooks J et al. J Am Acad Dermatol. 2018 Jun;78(6):1035-44.
Krooks J et al. J Am Acad Dermatol. 2018 Jun;78(6):1047-56.
Leung AKC et al. World J Pediatr. 2019 Dec;15(6):536-45.
At the week follow-up, the lesions were unchanged and the swelling on the left lateral eyebrow was worsening. A biopsy of the yellow lesion on the back and one of the scaly papules on the abdomen was performed. A fungal and bacterial cultures were also ordered.
He was referred to ophthalmology for evaluation of the eyelid swelling and an ultrasound was requested.
The skin biopsy showed a clonal proliferation of reniform histiocytes with eosinophils within the dermis. The cells were positive for S100, CD207 (langerin), and CD1a and negative for pancytokeratin and Melan-A, supportive of the diagnosis of Langerhans cell histiocytosis (LCH).
Diagnosis
The patient was admitted to the hospital, where a skeletal survey was performed, which showed an asymmetric lucency involving the left frontal calvarium extending to the superior lateral orbital rim. The brain MRI demonstrated a destructive avidly enhancing soft-tissue process which involved the superior left orbital rim likely with some degree of intracranial extension. This lesion exerts mass effect upon surrounding structures to the left ocular globe. With the skin and skeletal findings, the patient was diagnosed with LCH. His blood count was significant for thrombocytopenia. His liver and kidney function were normal. His electrolytes were also with in normal range. He was started on chemotherapy with vinblastine and systemic corticosteroids with resolution of the rash and decrease on the size of the lesion on the orbit within a few weeks.
Infantile LCH is a rare neoplastic disorder of hematopoietic myeloid precursor cells caused by activating mutations in the mitogen-activated protein kinase (MAPK) pathway, particularly BRAF-V600E mutation. White male children are mostly affected, with a peak incidence of 1-3 years of age. Nine out of 10 children with cutaneous involvement also have multisystemic disease, such as the case of our patient. LCH is classified as single or multisystem organ disease. Two-thirds of the cases present with single system involvement. Organs most commonly affected include the bone (the skull being the most commonly affected), skin, and high-risk organs like the liver, spleen, and bone marrow, and less commonly the lungs, lymph nodes, and central nervous system. Some patients can present with fever, lethargy, and weight loss. None were noted in our patient.
Skin findings of LCH can have multiple morphologies and presentations and often described as a big mimicker. In young infants like our patient, the seborrheic dermatitis–mimicking type is often seen. In other cases, the skin lesions can appear eczematous, petechial, with scabbing, crusting, or purpura. Xanthoma-like lesions, like that one our patient had in the back, have also been described. Resistant diaper dermatitis and cradle cap should prompt the clinician to think about LCH. Lesions can be so varied that can present with hypopigmentation (vitiligo like), hyperpigmentation, varicella-like papulo-pustules, and red blue nodules within others. Oral mucosa and nail involvement can also occur.
Bone involvement can present as soft-tissue mass with swelling and pain as it occur in our patient.
Endocrinopathies have been described in patients with LCH including diabetes insipidus, growth hormone deficiency, and less likely thyroid disease.
Multidisciplinary care
The diagnosis of LCH in infants necessitates a combination of clinical, radiological, and histopathologic findings. In infants, cutaneous involvement is a frequent initial presentation, with characteristic lesions that are often misdiagnosed as other dermatologic conditions. Timely recognition of these lesions and appropriate skin biopsies for histological examination are essential steps in achieving an accurate diagnosis.
Radiological imaging, including x-rays, CT, and MRI, plays a crucial role in assessing the extent of involvement.
The management of LCH in infants requires a well-coordinated multidisciplinary approach involving pediatric oncologists, dermatologists, radiologists, orthopedic surgeons, and other relevant specialists. Treatment strategies vary depending on the extent of disease involvement and the presence of risk factors. In localized cases, observation with close monitoring may be considered, as some cases of LCH in infants may undergo spontaneous regression. However, cases with severe symptoms, extensive organ involvement, or high-risk features may require systemic therapies.
Chemotherapy agents, including vinblastine and prednisone have been utilized in the treatment of infantile LCH with varying success. The selection of treatment regimens should be tailored to each individual case, considering disease severity, potential toxicities, and long-term effects. In cases of bone lesions causing significant deformities or functional impairment, surgical intervention may be necessary. Skin only disease can be treated with topical corticosteroids.
Prognosis
Survival rates in patients with single-organ involvement without risk-organ involvement is close to 100% and with risk-organ involvement of 98% at 5 years.
Long-term follow-up is essential for infants diagnosed with LCH, as recurrence and late effects can occur even after successful treatment. Continued monitoring allows for the timely detection of relapses or the development of secondary complications.
Infants thought to have common skin conditions like eczema, seborrheic dermatitis, or diaper dermatitis not responding to treatment should be referred to pediatric dermatology for evaluation to rule out the possibility of LCH.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Krooks J et al. J Am Acad Dermatol. 2018 Jun;78(6):1035-44.
Krooks J et al. J Am Acad Dermatol. 2018 Jun;78(6):1047-56.
Leung AKC et al. World J Pediatr. 2019 Dec;15(6):536-45.
At the week follow-up, the lesions were unchanged and the swelling on the left lateral eyebrow was worsening. A biopsy of the yellow lesion on the back and one of the scaly papules on the abdomen was performed. A fungal and bacterial cultures were also ordered.
He was referred to ophthalmology for evaluation of the eyelid swelling and an ultrasound was requested.
The skin biopsy showed a clonal proliferation of reniform histiocytes with eosinophils within the dermis. The cells were positive for S100, CD207 (langerin), and CD1a and negative for pancytokeratin and Melan-A, supportive of the diagnosis of Langerhans cell histiocytosis (LCH).
Diagnosis
The patient was admitted to the hospital, where a skeletal survey was performed, which showed an asymmetric lucency involving the left frontal calvarium extending to the superior lateral orbital rim. The brain MRI demonstrated a destructive avidly enhancing soft-tissue process which involved the superior left orbital rim likely with some degree of intracranial extension. This lesion exerts mass effect upon surrounding structures to the left ocular globe. With the skin and skeletal findings, the patient was diagnosed with LCH. His blood count was significant for thrombocytopenia. His liver and kidney function were normal. His electrolytes were also with in normal range. He was started on chemotherapy with vinblastine and systemic corticosteroids with resolution of the rash and decrease on the size of the lesion on the orbit within a few weeks.
Infantile LCH is a rare neoplastic disorder of hematopoietic myeloid precursor cells caused by activating mutations in the mitogen-activated protein kinase (MAPK) pathway, particularly BRAF-V600E mutation. White male children are mostly affected, with a peak incidence of 1-3 years of age. Nine out of 10 children with cutaneous involvement also have multisystemic disease, such as the case of our patient. LCH is classified as single or multisystem organ disease. Two-thirds of the cases present with single system involvement. Organs most commonly affected include the bone (the skull being the most commonly affected), skin, and high-risk organs like the liver, spleen, and bone marrow, and less commonly the lungs, lymph nodes, and central nervous system. Some patients can present with fever, lethargy, and weight loss. None were noted in our patient.
Skin findings of LCH can have multiple morphologies and presentations and often described as a big mimicker. In young infants like our patient, the seborrheic dermatitis–mimicking type is often seen. In other cases, the skin lesions can appear eczematous, petechial, with scabbing, crusting, or purpura. Xanthoma-like lesions, like that one our patient had in the back, have also been described. Resistant diaper dermatitis and cradle cap should prompt the clinician to think about LCH. Lesions can be so varied that can present with hypopigmentation (vitiligo like), hyperpigmentation, varicella-like papulo-pustules, and red blue nodules within others. Oral mucosa and nail involvement can also occur.
Bone involvement can present as soft-tissue mass with swelling and pain as it occur in our patient.
Endocrinopathies have been described in patients with LCH including diabetes insipidus, growth hormone deficiency, and less likely thyroid disease.
Multidisciplinary care
The diagnosis of LCH in infants necessitates a combination of clinical, radiological, and histopathologic findings. In infants, cutaneous involvement is a frequent initial presentation, with characteristic lesions that are often misdiagnosed as other dermatologic conditions. Timely recognition of these lesions and appropriate skin biopsies for histological examination are essential steps in achieving an accurate diagnosis.
Radiological imaging, including x-rays, CT, and MRI, plays a crucial role in assessing the extent of involvement.
The management of LCH in infants requires a well-coordinated multidisciplinary approach involving pediatric oncologists, dermatologists, radiologists, orthopedic surgeons, and other relevant specialists. Treatment strategies vary depending on the extent of disease involvement and the presence of risk factors. In localized cases, observation with close monitoring may be considered, as some cases of LCH in infants may undergo spontaneous regression. However, cases with severe symptoms, extensive organ involvement, or high-risk features may require systemic therapies.
Chemotherapy agents, including vinblastine and prednisone have been utilized in the treatment of infantile LCH with varying success. The selection of treatment regimens should be tailored to each individual case, considering disease severity, potential toxicities, and long-term effects. In cases of bone lesions causing significant deformities or functional impairment, surgical intervention may be necessary. Skin only disease can be treated with topical corticosteroids.
Prognosis
Survival rates in patients with single-organ involvement without risk-organ involvement is close to 100% and with risk-organ involvement of 98% at 5 years.
Long-term follow-up is essential for infants diagnosed with LCH, as recurrence and late effects can occur even after successful treatment. Continued monitoring allows for the timely detection of relapses or the development of secondary complications.
Infants thought to have common skin conditions like eczema, seborrheic dermatitis, or diaper dermatitis not responding to treatment should be referred to pediatric dermatology for evaluation to rule out the possibility of LCH.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Krooks J et al. J Am Acad Dermatol. 2018 Jun;78(6):1035-44.
Krooks J et al. J Am Acad Dermatol. 2018 Jun;78(6):1047-56.
Leung AKC et al. World J Pediatr. 2019 Dec;15(6):536-45.
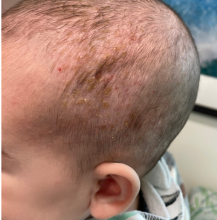
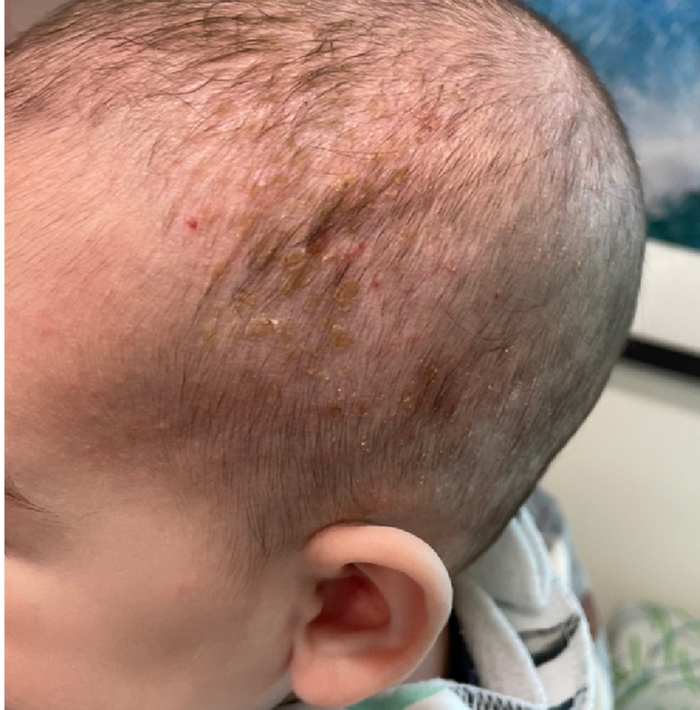
A 4-month male was referred to the pediatric dermatology clinic for a rash on the scalp, torso, and the diaper area since he was 2 months of age. He has been treated with nystatin, clotrimazole, and zinc oxide paste with partial improvement. After 2 months of partial improvement the rash worsened again, and he was referred to pediatric dermatology. The mother also reported asymptomatic left upper lateral eyebrow swelling noted a few weeks prior.
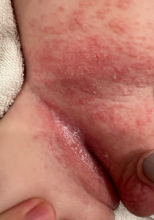
On the torso and diaper area, he had multiple scaly pink papules. On the groin he had eroded pink scaly plaques (Picture 2).
On his back he had a 3-mm yellow papule (Picture 3).
COVID hospitalizations climb for fourth straight week
Weekly new hospitalizations for COVID-19 have climbed for the fourth straight week.
according to newly updated Centers for Disease Control and Prevention figures. Hospitalizations reached an all-time low of about 6,300 per week in July.
The CDC stopped tracking the number of people infected by the virus earlier in 2023, and now relies on hospitalization data to gauge the current impact of COVID-19.
“We have to remember that we’re still dealing with numbers that are far less than what we’ve seen for the pandemic,” John Brownstein, PhD, a professor of biomedical informatics at Harvard Medical School, Boston, told ABC News. “We have to zoom out to look at our experience for the entire pandemic, to understand that what we’re dealing with now is far from any crisis that we’ve experienced with previous waves.”
The current predominant strain remains EG.5, and experts believe it is not more severe or more contagious than other recent variants.
Dr. Brownstein told ABC News that one reason for the concern about rising COVID metrics, despite their overall low levels, is that a surge occurred in the summer of 2021 with the dangerous Delta variant.
“But each new variant so far that has come through has subsequently had less of a population impact,” he said. “Now, is it possible we may see one in the future that is worthy, a real concern? Absolutely. But overall, we’ve seen a dampening of effect over the last several variants that have come through.”
A version of this article appeared on WebMD.com.
Weekly new hospitalizations for COVID-19 have climbed for the fourth straight week.
according to newly updated Centers for Disease Control and Prevention figures. Hospitalizations reached an all-time low of about 6,300 per week in July.
The CDC stopped tracking the number of people infected by the virus earlier in 2023, and now relies on hospitalization data to gauge the current impact of COVID-19.
“We have to remember that we’re still dealing with numbers that are far less than what we’ve seen for the pandemic,” John Brownstein, PhD, a professor of biomedical informatics at Harvard Medical School, Boston, told ABC News. “We have to zoom out to look at our experience for the entire pandemic, to understand that what we’re dealing with now is far from any crisis that we’ve experienced with previous waves.”
The current predominant strain remains EG.5, and experts believe it is not more severe or more contagious than other recent variants.
Dr. Brownstein told ABC News that one reason for the concern about rising COVID metrics, despite their overall low levels, is that a surge occurred in the summer of 2021 with the dangerous Delta variant.
“But each new variant so far that has come through has subsequently had less of a population impact,” he said. “Now, is it possible we may see one in the future that is worthy, a real concern? Absolutely. But overall, we’ve seen a dampening of effect over the last several variants that have come through.”
A version of this article appeared on WebMD.com.
Weekly new hospitalizations for COVID-19 have climbed for the fourth straight week.
according to newly updated Centers for Disease Control and Prevention figures. Hospitalizations reached an all-time low of about 6,300 per week in July.
The CDC stopped tracking the number of people infected by the virus earlier in 2023, and now relies on hospitalization data to gauge the current impact of COVID-19.
“We have to remember that we’re still dealing with numbers that are far less than what we’ve seen for the pandemic,” John Brownstein, PhD, a professor of biomedical informatics at Harvard Medical School, Boston, told ABC News. “We have to zoom out to look at our experience for the entire pandemic, to understand that what we’re dealing with now is far from any crisis that we’ve experienced with previous waves.”
The current predominant strain remains EG.5, and experts believe it is not more severe or more contagious than other recent variants.
Dr. Brownstein told ABC News that one reason for the concern about rising COVID metrics, despite their overall low levels, is that a surge occurred in the summer of 2021 with the dangerous Delta variant.
“But each new variant so far that has come through has subsequently had less of a population impact,” he said. “Now, is it possible we may see one in the future that is worthy, a real concern? Absolutely. But overall, we’ve seen a dampening of effect over the last several variants that have come through.”
A version of this article appeared on WebMD.com.
Child assault tied to triple the risk for mental illness within 1 year
The greatest risk was found in the first year following the assault, increasing to three times the risk of being diagnosed with mental illness, compared with children not assaulted. Mood and anxiety disorders were the most common diagnoses.
“From a clinical and policy perspective, our study highlights that there is a critical opportunity for health care clinicians to support children in the first year following physical assault,” Natasha Saunders, MD, MSc, of the Hospital for Sick Children, Toronto, and colleagues wrote. “There is a need to develop and implement targeted mental illness prevention, screening, and treatment programs for assaulted children.”
The findings were published online in JAMA Network Open.
While it has been well established that children exposed to assault have an increased risk for subsequent mental illness, Dr. Saunders and coinvestigators noted that using an age-matched, population-based cohort study would enable them to obtain detailed information on the patterns and timing of subsequent psychiatric diagnoses.
To that end, the researchers used several medical databases in Ontario to find 5,487 children (infants to age 13 years) who required an ED visit or hospitalization for a physical assault in Ontario between 2006 and 2014.
These children were matched on a 1:4 basis with 21,948 children not exposed to physical assault. The children were followed until their 18th birthday or until the study ended in March 2019.
The researchers found that more than a third of the children (39%) who were exposed to assault received a mental health diagnosis, according to health records, compared with 23% of unexposed children.
Mood and anxiety disorders were the most common diagnoses among children exposed to assault (16.2% vs. 10.6%, respectively); followed by select childhood behavior disorders, such as ADHD, oppositional defiant disorder, or conduct disorder (9.9% vs. 5.2%); and substance use disorders (2.4% vs. 0.4%).
Triple risk of mental illness in first year
The researchers found that the children exposed to assault were nearly twice as likely to be diagnosed with a mental illness over a median follow-up of 7 years, compared with those not exposed to assault (adjusted hazard ratio, 1.96; 95% confidence interval, 1.85,2.08).
In the year following the assault, children exposed to assault bore three times the risk of being diagnosed with a mental illness, compared with unexposed children (aHR, 3.08; 95% CI, 2.68,3.54).
In addition, the children who had been assaulted were more likely to be diagnosed in an acute care setting than those who were not assaulted (14% vs. 2.8%).
The children who had been assaulted were an average age of 7 years and were more often boys (55% vs. 45%). Children who were assaulted were also more likely to have mothers with mental illness (35% vs. 19%).
The investigators noted that the study likely underestimated the number of children exposed to assault, as many do not end up in the ED.
In addition to highlighting the need for medical personnel to support children in the first year following assault, the investigators wrote that “our results also advocate for accessible mental health care outside of the acute setting and for care that addresses the social and health needs of mothers, who themselves have high social and health risks.”
This study received funding from the National Foundation to End Child Abuse and Neglect and the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Saunders reported receiving personal fees from The BMJ Group, Archives of Diseases in Childhood outside the submitted work.
A version of this article first appeared on Medscape.com.
The greatest risk was found in the first year following the assault, increasing to three times the risk of being diagnosed with mental illness, compared with children not assaulted. Mood and anxiety disorders were the most common diagnoses.
“From a clinical and policy perspective, our study highlights that there is a critical opportunity for health care clinicians to support children in the first year following physical assault,” Natasha Saunders, MD, MSc, of the Hospital for Sick Children, Toronto, and colleagues wrote. “There is a need to develop and implement targeted mental illness prevention, screening, and treatment programs for assaulted children.”
The findings were published online in JAMA Network Open.
While it has been well established that children exposed to assault have an increased risk for subsequent mental illness, Dr. Saunders and coinvestigators noted that using an age-matched, population-based cohort study would enable them to obtain detailed information on the patterns and timing of subsequent psychiatric diagnoses.
To that end, the researchers used several medical databases in Ontario to find 5,487 children (infants to age 13 years) who required an ED visit or hospitalization for a physical assault in Ontario between 2006 and 2014.
These children were matched on a 1:4 basis with 21,948 children not exposed to physical assault. The children were followed until their 18th birthday or until the study ended in March 2019.
The researchers found that more than a third of the children (39%) who were exposed to assault received a mental health diagnosis, according to health records, compared with 23% of unexposed children.
Mood and anxiety disorders were the most common diagnoses among children exposed to assault (16.2% vs. 10.6%, respectively); followed by select childhood behavior disorders, such as ADHD, oppositional defiant disorder, or conduct disorder (9.9% vs. 5.2%); and substance use disorders (2.4% vs. 0.4%).
Triple risk of mental illness in first year
The researchers found that the children exposed to assault were nearly twice as likely to be diagnosed with a mental illness over a median follow-up of 7 years, compared with those not exposed to assault (adjusted hazard ratio, 1.96; 95% confidence interval, 1.85,2.08).
In the year following the assault, children exposed to assault bore three times the risk of being diagnosed with a mental illness, compared with unexposed children (aHR, 3.08; 95% CI, 2.68,3.54).
In addition, the children who had been assaulted were more likely to be diagnosed in an acute care setting than those who were not assaulted (14% vs. 2.8%).
The children who had been assaulted were an average age of 7 years and were more often boys (55% vs. 45%). Children who were assaulted were also more likely to have mothers with mental illness (35% vs. 19%).
The investigators noted that the study likely underestimated the number of children exposed to assault, as many do not end up in the ED.
In addition to highlighting the need for medical personnel to support children in the first year following assault, the investigators wrote that “our results also advocate for accessible mental health care outside of the acute setting and for care that addresses the social and health needs of mothers, who themselves have high social and health risks.”
This study received funding from the National Foundation to End Child Abuse and Neglect and the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Saunders reported receiving personal fees from The BMJ Group, Archives of Diseases in Childhood outside the submitted work.
A version of this article first appeared on Medscape.com.
The greatest risk was found in the first year following the assault, increasing to three times the risk of being diagnosed with mental illness, compared with children not assaulted. Mood and anxiety disorders were the most common diagnoses.
“From a clinical and policy perspective, our study highlights that there is a critical opportunity for health care clinicians to support children in the first year following physical assault,” Natasha Saunders, MD, MSc, of the Hospital for Sick Children, Toronto, and colleagues wrote. “There is a need to develop and implement targeted mental illness prevention, screening, and treatment programs for assaulted children.”
The findings were published online in JAMA Network Open.
While it has been well established that children exposed to assault have an increased risk for subsequent mental illness, Dr. Saunders and coinvestigators noted that using an age-matched, population-based cohort study would enable them to obtain detailed information on the patterns and timing of subsequent psychiatric diagnoses.
To that end, the researchers used several medical databases in Ontario to find 5,487 children (infants to age 13 years) who required an ED visit or hospitalization for a physical assault in Ontario between 2006 and 2014.
These children were matched on a 1:4 basis with 21,948 children not exposed to physical assault. The children were followed until their 18th birthday or until the study ended in March 2019.
The researchers found that more than a third of the children (39%) who were exposed to assault received a mental health diagnosis, according to health records, compared with 23% of unexposed children.
Mood and anxiety disorders were the most common diagnoses among children exposed to assault (16.2% vs. 10.6%, respectively); followed by select childhood behavior disorders, such as ADHD, oppositional defiant disorder, or conduct disorder (9.9% vs. 5.2%); and substance use disorders (2.4% vs. 0.4%).
Triple risk of mental illness in first year
The researchers found that the children exposed to assault were nearly twice as likely to be diagnosed with a mental illness over a median follow-up of 7 years, compared with those not exposed to assault (adjusted hazard ratio, 1.96; 95% confidence interval, 1.85,2.08).
In the year following the assault, children exposed to assault bore three times the risk of being diagnosed with a mental illness, compared with unexposed children (aHR, 3.08; 95% CI, 2.68,3.54).
In addition, the children who had been assaulted were more likely to be diagnosed in an acute care setting than those who were not assaulted (14% vs. 2.8%).
The children who had been assaulted were an average age of 7 years and were more often boys (55% vs. 45%). Children who were assaulted were also more likely to have mothers with mental illness (35% vs. 19%).
The investigators noted that the study likely underestimated the number of children exposed to assault, as many do not end up in the ED.
In addition to highlighting the need for medical personnel to support children in the first year following assault, the investigators wrote that “our results also advocate for accessible mental health care outside of the acute setting and for care that addresses the social and health needs of mothers, who themselves have high social and health risks.”
This study received funding from the National Foundation to End Child Abuse and Neglect and the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Saunders reported receiving personal fees from The BMJ Group, Archives of Diseases in Childhood outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Healthy babies can still get very sick from RSV
Any parent might naturally assume that their newborn is at little risk from respiratory syncytial virus (RSV), which in healthy infants has been thought to cause mild symptoms similar to having a cold. But a new study challenges the assumption that only infirm children are at risk for the worst outcomes from RSV, finding that
The researchers, who published their study in JAMA Network Open, said the results reinforce the importance of a new preventive injection that can lower the risk for severe RSV infection in babies.
“RSV is the number one cause of hospitalizations in young infants,” said Natasha Halasa, MD, MPH, an infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn., and the lead author of the new study. But “the vast majority of kids didn’t have underlying medical conditions” when they got sick.
Every infant in the study was in an intensive care unit for at least 24 hours, Dr. Halasa said, and most babies gave no prior indication that RSV would affect them so profoundly.
“Two to three of every 100 babies in the United States will be hospitalized for RSV in their first year of life,” added study author Angela Campbell, MD, MPH, of the Coronavirus and Other Respiratory Viruses Division of the Centers for Disease Control and Prevention in Atlanta.
Until recently, only one treatment was available for children up to age 2 at high risk for RSV, the monoclonal antibody palivizumab (Synagis). Palivizumab is reserved for children who are born prematurely, are immunocompromised, or have chronic heart or lung disease. The injection is given monthly during the 5-month peak of RSV season, from fall to spring.
In July, the Food and Drug Administration approved, and the CDC has since recommended, a new monoclonal antibody called nirsevimab (Beyfortus) to prevent the worst effects of RSV. Nirsevimab is intended for all newborns under age 8 months who were born during the RSV season, or babies who will be entering that season before reaching 8 months. The injection is given only once and can act for 150 days. The FDA and CDC actions came following a clinical trial showing that nirsevimab lowers the risk for hospitalization from RSV among infants by more than 75%.
“We’re very excited that this product exists now,” Dr. Campbell said.
Chart reviews during the ‘tripledemic’
In fall 2022 the United States experienced a “tripledemic” of elevated hospitalizations for COVID-19, influenza, and RSV. For the new study, Dr. Halasa and her colleagues examined the medical records of 600 infants (under age 1; average age, 2.6 months) admitted to U.S. ICUs for lower respiratory tract infections caused by RSV from October to December 2022, during the height of the tripledemic.
More than 60% of admissions, 361, were boys; 44% were White, 23% were Hispanic, 16% were Black, 10% were unknown race, 5% were multiple race, and 2% were Asian.
Of the 600 infants, 572 (95.3%) required oxygen at the hospital and 487 (81.2%) had no underlying medical conditions linked to higher risk from RSV. The other infants had at least one ailment, such as a cardiac or lung condition, that could result in more severe RSV outcomes.
The 169 preemies in the study population were more likely to be intubated in the ICU than were those born at term. But 90 of the 143 total recorded intubations happened among full-term infants. Two children in the study group died.
Christopher Horvat, MD, MHA, who works in the pediatric ICU at the University of Pittsburgh Medical Center, called the new study “important,” adding that it shows “the RSV burden is substantial for children who are otherwise healthy.” Dr. Horvat, who was not involved in the work, said the new data highlight the value of preventive measures to prevent any repeat of the tripledemic.
On the same day the new study was published, the American Academy of Pediatrics (AAP) released a statement calling for widespread access to nirsevimab.
“The American Academy of Pediatrics recommends that all infants – and especially those at high risk – receive the new preventive antibody, nirsevimab, to protect against severe disease caused by respiratory syncytial virus (RSV), which is common, highly contagious, and sometimes deadly,” the organization said in a statement.
The AAP called for the CDC and the Centers for Medicaid & Medicare Services to work together to ensure that any parent in America can obtain nirsevimab for their children if needed. Anyone who cannot access nirsevimab this year, the AAP said, should rely on the older treatment palivizumab instead.
The sources in this story reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any parent might naturally assume that their newborn is at little risk from respiratory syncytial virus (RSV), which in healthy infants has been thought to cause mild symptoms similar to having a cold. But a new study challenges the assumption that only infirm children are at risk for the worst outcomes from RSV, finding that
The researchers, who published their study in JAMA Network Open, said the results reinforce the importance of a new preventive injection that can lower the risk for severe RSV infection in babies.
“RSV is the number one cause of hospitalizations in young infants,” said Natasha Halasa, MD, MPH, an infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn., and the lead author of the new study. But “the vast majority of kids didn’t have underlying medical conditions” when they got sick.
Every infant in the study was in an intensive care unit for at least 24 hours, Dr. Halasa said, and most babies gave no prior indication that RSV would affect them so profoundly.
“Two to three of every 100 babies in the United States will be hospitalized for RSV in their first year of life,” added study author Angela Campbell, MD, MPH, of the Coronavirus and Other Respiratory Viruses Division of the Centers for Disease Control and Prevention in Atlanta.
Until recently, only one treatment was available for children up to age 2 at high risk for RSV, the monoclonal antibody palivizumab (Synagis). Palivizumab is reserved for children who are born prematurely, are immunocompromised, or have chronic heart or lung disease. The injection is given monthly during the 5-month peak of RSV season, from fall to spring.
In July, the Food and Drug Administration approved, and the CDC has since recommended, a new monoclonal antibody called nirsevimab (Beyfortus) to prevent the worst effects of RSV. Nirsevimab is intended for all newborns under age 8 months who were born during the RSV season, or babies who will be entering that season before reaching 8 months. The injection is given only once and can act for 150 days. The FDA and CDC actions came following a clinical trial showing that nirsevimab lowers the risk for hospitalization from RSV among infants by more than 75%.
“We’re very excited that this product exists now,” Dr. Campbell said.
Chart reviews during the ‘tripledemic’
In fall 2022 the United States experienced a “tripledemic” of elevated hospitalizations for COVID-19, influenza, and RSV. For the new study, Dr. Halasa and her colleagues examined the medical records of 600 infants (under age 1; average age, 2.6 months) admitted to U.S. ICUs for lower respiratory tract infections caused by RSV from October to December 2022, during the height of the tripledemic.
More than 60% of admissions, 361, were boys; 44% were White, 23% were Hispanic, 16% were Black, 10% were unknown race, 5% were multiple race, and 2% were Asian.
Of the 600 infants, 572 (95.3%) required oxygen at the hospital and 487 (81.2%) had no underlying medical conditions linked to higher risk from RSV. The other infants had at least one ailment, such as a cardiac or lung condition, that could result in more severe RSV outcomes.
The 169 preemies in the study population were more likely to be intubated in the ICU than were those born at term. But 90 of the 143 total recorded intubations happened among full-term infants. Two children in the study group died.
Christopher Horvat, MD, MHA, who works in the pediatric ICU at the University of Pittsburgh Medical Center, called the new study “important,” adding that it shows “the RSV burden is substantial for children who are otherwise healthy.” Dr. Horvat, who was not involved in the work, said the new data highlight the value of preventive measures to prevent any repeat of the tripledemic.
On the same day the new study was published, the American Academy of Pediatrics (AAP) released a statement calling for widespread access to nirsevimab.
“The American Academy of Pediatrics recommends that all infants – and especially those at high risk – receive the new preventive antibody, nirsevimab, to protect against severe disease caused by respiratory syncytial virus (RSV), which is common, highly contagious, and sometimes deadly,” the organization said in a statement.
The AAP called for the CDC and the Centers for Medicaid & Medicare Services to work together to ensure that any parent in America can obtain nirsevimab for their children if needed. Anyone who cannot access nirsevimab this year, the AAP said, should rely on the older treatment palivizumab instead.
The sources in this story reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any parent might naturally assume that their newborn is at little risk from respiratory syncytial virus (RSV), which in healthy infants has been thought to cause mild symptoms similar to having a cold. But a new study challenges the assumption that only infirm children are at risk for the worst outcomes from RSV, finding that
The researchers, who published their study in JAMA Network Open, said the results reinforce the importance of a new preventive injection that can lower the risk for severe RSV infection in babies.
“RSV is the number one cause of hospitalizations in young infants,” said Natasha Halasa, MD, MPH, an infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn., and the lead author of the new study. But “the vast majority of kids didn’t have underlying medical conditions” when they got sick.
Every infant in the study was in an intensive care unit for at least 24 hours, Dr. Halasa said, and most babies gave no prior indication that RSV would affect them so profoundly.
“Two to three of every 100 babies in the United States will be hospitalized for RSV in their first year of life,” added study author Angela Campbell, MD, MPH, of the Coronavirus and Other Respiratory Viruses Division of the Centers for Disease Control and Prevention in Atlanta.
Until recently, only one treatment was available for children up to age 2 at high risk for RSV, the monoclonal antibody palivizumab (Synagis). Palivizumab is reserved for children who are born prematurely, are immunocompromised, or have chronic heart or lung disease. The injection is given monthly during the 5-month peak of RSV season, from fall to spring.
In July, the Food and Drug Administration approved, and the CDC has since recommended, a new monoclonal antibody called nirsevimab (Beyfortus) to prevent the worst effects of RSV. Nirsevimab is intended for all newborns under age 8 months who were born during the RSV season, or babies who will be entering that season before reaching 8 months. The injection is given only once and can act for 150 days. The FDA and CDC actions came following a clinical trial showing that nirsevimab lowers the risk for hospitalization from RSV among infants by more than 75%.
“We’re very excited that this product exists now,” Dr. Campbell said.
Chart reviews during the ‘tripledemic’
In fall 2022 the United States experienced a “tripledemic” of elevated hospitalizations for COVID-19, influenza, and RSV. For the new study, Dr. Halasa and her colleagues examined the medical records of 600 infants (under age 1; average age, 2.6 months) admitted to U.S. ICUs for lower respiratory tract infections caused by RSV from October to December 2022, during the height of the tripledemic.
More than 60% of admissions, 361, were boys; 44% were White, 23% were Hispanic, 16% were Black, 10% were unknown race, 5% were multiple race, and 2% were Asian.
Of the 600 infants, 572 (95.3%) required oxygen at the hospital and 487 (81.2%) had no underlying medical conditions linked to higher risk from RSV. The other infants had at least one ailment, such as a cardiac or lung condition, that could result in more severe RSV outcomes.
The 169 preemies in the study population were more likely to be intubated in the ICU than were those born at term. But 90 of the 143 total recorded intubations happened among full-term infants. Two children in the study group died.
Christopher Horvat, MD, MHA, who works in the pediatric ICU at the University of Pittsburgh Medical Center, called the new study “important,” adding that it shows “the RSV burden is substantial for children who are otherwise healthy.” Dr. Horvat, who was not involved in the work, said the new data highlight the value of preventive measures to prevent any repeat of the tripledemic.
On the same day the new study was published, the American Academy of Pediatrics (AAP) released a statement calling for widespread access to nirsevimab.
“The American Academy of Pediatrics recommends that all infants – and especially those at high risk – receive the new preventive antibody, nirsevimab, to protect against severe disease caused by respiratory syncytial virus (RSV), which is common, highly contagious, and sometimes deadly,” the organization said in a statement.
The AAP called for the CDC and the Centers for Medicaid & Medicare Services to work together to ensure that any parent in America can obtain nirsevimab for their children if needed. Anyone who cannot access nirsevimab this year, the AAP said, should rely on the older treatment palivizumab instead.
The sources in this story reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Playing football linked to higher Parkinson’s risk
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Analysis reveals recent acne prescribing trends
While.
Notably, isotretinoin prescribing among men and women decreased slightly during the study period, “which may reflect ongoing administrative burdens associated with iPLEDGE,” study author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, told this news organization.
For the cross-sectional study, which was published online as a research letter in JAMA Dermatology, Dr. Barbieri drew from the Truven Health MarketScan Commercial Claims Database from Jan. 1, 2017, to Dec. 31, 2020, to identify individuals with an encounter for acne, prescriptions for oral tetracycline antibiotics (doxycycline, minocycline), other commonly prescribed oral antibiotics (trimethoprim-sulfamethoxazole, amoxicillin, cephalexin), spironolactone, and isotretinoin. Only drug courses greater than 28 days were included in the analysis, and Dr. Barbieri stratified them according to clinician type (dermatologist, nondermatology physician, and nurse-practitioner or physician assistant). To normalize prescribing rates (to address possible changes in the number of patients treated for acne over time), the number of treatment courses prescribed each year was standardized to the number of encounters for acne with that clinician type during the same calendar year.
The study period included a mean of 1.9 million acne encounters per year.
Dr. Barbieri found that dermatologists prescribed more oral antibiotics per clinician for acne than any other major medical specialty and that oral antibiotics remained frequently prescribed for treating acne by both dermatologists and nondermatologists. “Among oral antibiotics, minocycline and trimethoprim-sulfamethoxazole remain relatively commonly prescribed, despite potential safety concerns and a lack of evidence that they are any more effective than doxycycline,” he said in an interview.
“Patient outcomes could likely be improved by reducing use of minocycline and particularly trimethoprim-sulfamethoxazole given its high risk of serious side effects such as SJS/TEN [Stevens-Johnson syndrome/toxic epidermal necrolysis] and acute respiratory failure,” he added.
Dr. Barbieri noted that there are likely opportunities to consider nonantibiotic alternatives such as hormonal therapy (spironolactone, combined oral contraceptives) and isotretinoin. “There is also a need for continued research to identify nonantibiotic treatment options for patients with acne,” he said.
The analysis revealed that for women with acne prescriptions for spironolactone increased about three- to fourfold during the study period among all clinician types. In 2017, oral antibiotics were prescribed about two- to threefold more often than spironolactone, but by 2020 they were being prescribed at about the same frequency. “Given spironolactone may have similar effectiveness to oral antibiotics in the treatment of acne, this shift in practice has the potential to improve outcomes for patients by reducing the risk of antibiotic-associated complications,” Dr. Barbieri wrote. Still, in 2020, oral antibiotics were still slightly more commonly prescribed than spironolactone by nondermatology physicians and NP or PAs.
In other findings, isotretinoin prescribing decreased slightly among male and female patients during the study period. Among antibiotic prescriptions, prescribing for doxycycline increased at a higher rate than prescribing for minocycline, especially among dermatologists and NPs or PAs.
In the interview, Dr. Barbieri acknowledged certain limitations of the study, including the fact that the dataset “does not allow for evaluation of severity of acne and it is not possible to directly link prescriptions to diagnoses, so some prescriptions might not be for acne and others that are for acne might not have been included.”
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the results, said that, while a course of antibiotic therapy was tied to an office visit in the analysis, the duration of each course of therapy was unclear. It would be interesting to see if antibiotic courses became shorter during the time period analyzed, such as 1-3 months versus 4 or more months, he added, “as this should reduce risks associated with long-term use of oral antibiotics.”
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
While.
Notably, isotretinoin prescribing among men and women decreased slightly during the study period, “which may reflect ongoing administrative burdens associated with iPLEDGE,” study author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, told this news organization.
For the cross-sectional study, which was published online as a research letter in JAMA Dermatology, Dr. Barbieri drew from the Truven Health MarketScan Commercial Claims Database from Jan. 1, 2017, to Dec. 31, 2020, to identify individuals with an encounter for acne, prescriptions for oral tetracycline antibiotics (doxycycline, minocycline), other commonly prescribed oral antibiotics (trimethoprim-sulfamethoxazole, amoxicillin, cephalexin), spironolactone, and isotretinoin. Only drug courses greater than 28 days were included in the analysis, and Dr. Barbieri stratified them according to clinician type (dermatologist, nondermatology physician, and nurse-practitioner or physician assistant). To normalize prescribing rates (to address possible changes in the number of patients treated for acne over time), the number of treatment courses prescribed each year was standardized to the number of encounters for acne with that clinician type during the same calendar year.
The study period included a mean of 1.9 million acne encounters per year.
Dr. Barbieri found that dermatologists prescribed more oral antibiotics per clinician for acne than any other major medical specialty and that oral antibiotics remained frequently prescribed for treating acne by both dermatologists and nondermatologists. “Among oral antibiotics, minocycline and trimethoprim-sulfamethoxazole remain relatively commonly prescribed, despite potential safety concerns and a lack of evidence that they are any more effective than doxycycline,” he said in an interview.
“Patient outcomes could likely be improved by reducing use of minocycline and particularly trimethoprim-sulfamethoxazole given its high risk of serious side effects such as SJS/TEN [Stevens-Johnson syndrome/toxic epidermal necrolysis] and acute respiratory failure,” he added.
Dr. Barbieri noted that there are likely opportunities to consider nonantibiotic alternatives such as hormonal therapy (spironolactone, combined oral contraceptives) and isotretinoin. “There is also a need for continued research to identify nonantibiotic treatment options for patients with acne,” he said.
The analysis revealed that for women with acne prescriptions for spironolactone increased about three- to fourfold during the study period among all clinician types. In 2017, oral antibiotics were prescribed about two- to threefold more often than spironolactone, but by 2020 they were being prescribed at about the same frequency. “Given spironolactone may have similar effectiveness to oral antibiotics in the treatment of acne, this shift in practice has the potential to improve outcomes for patients by reducing the risk of antibiotic-associated complications,” Dr. Barbieri wrote. Still, in 2020, oral antibiotics were still slightly more commonly prescribed than spironolactone by nondermatology physicians and NP or PAs.
In other findings, isotretinoin prescribing decreased slightly among male and female patients during the study period. Among antibiotic prescriptions, prescribing for doxycycline increased at a higher rate than prescribing for minocycline, especially among dermatologists and NPs or PAs.
In the interview, Dr. Barbieri acknowledged certain limitations of the study, including the fact that the dataset “does not allow for evaluation of severity of acne and it is not possible to directly link prescriptions to diagnoses, so some prescriptions might not be for acne and others that are for acne might not have been included.”
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the results, said that, while a course of antibiotic therapy was tied to an office visit in the analysis, the duration of each course of therapy was unclear. It would be interesting to see if antibiotic courses became shorter during the time period analyzed, such as 1-3 months versus 4 or more months, he added, “as this should reduce risks associated with long-term use of oral antibiotics.”
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
While.
Notably, isotretinoin prescribing among men and women decreased slightly during the study period, “which may reflect ongoing administrative burdens associated with iPLEDGE,” study author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, told this news organization.
For the cross-sectional study, which was published online as a research letter in JAMA Dermatology, Dr. Barbieri drew from the Truven Health MarketScan Commercial Claims Database from Jan. 1, 2017, to Dec. 31, 2020, to identify individuals with an encounter for acne, prescriptions for oral tetracycline antibiotics (doxycycline, minocycline), other commonly prescribed oral antibiotics (trimethoprim-sulfamethoxazole, amoxicillin, cephalexin), spironolactone, and isotretinoin. Only drug courses greater than 28 days were included in the analysis, and Dr. Barbieri stratified them according to clinician type (dermatologist, nondermatology physician, and nurse-practitioner or physician assistant). To normalize prescribing rates (to address possible changes in the number of patients treated for acne over time), the number of treatment courses prescribed each year was standardized to the number of encounters for acne with that clinician type during the same calendar year.
The study period included a mean of 1.9 million acne encounters per year.
Dr. Barbieri found that dermatologists prescribed more oral antibiotics per clinician for acne than any other major medical specialty and that oral antibiotics remained frequently prescribed for treating acne by both dermatologists and nondermatologists. “Among oral antibiotics, minocycline and trimethoprim-sulfamethoxazole remain relatively commonly prescribed, despite potential safety concerns and a lack of evidence that they are any more effective than doxycycline,” he said in an interview.
“Patient outcomes could likely be improved by reducing use of minocycline and particularly trimethoprim-sulfamethoxazole given its high risk of serious side effects such as SJS/TEN [Stevens-Johnson syndrome/toxic epidermal necrolysis] and acute respiratory failure,” he added.
Dr. Barbieri noted that there are likely opportunities to consider nonantibiotic alternatives such as hormonal therapy (spironolactone, combined oral contraceptives) and isotretinoin. “There is also a need for continued research to identify nonantibiotic treatment options for patients with acne,” he said.
The analysis revealed that for women with acne prescriptions for spironolactone increased about three- to fourfold during the study period among all clinician types. In 2017, oral antibiotics were prescribed about two- to threefold more often than spironolactone, but by 2020 they were being prescribed at about the same frequency. “Given spironolactone may have similar effectiveness to oral antibiotics in the treatment of acne, this shift in practice has the potential to improve outcomes for patients by reducing the risk of antibiotic-associated complications,” Dr. Barbieri wrote. Still, in 2020, oral antibiotics were still slightly more commonly prescribed than spironolactone by nondermatology physicians and NP or PAs.
In other findings, isotretinoin prescribing decreased slightly among male and female patients during the study period. Among antibiotic prescriptions, prescribing for doxycycline increased at a higher rate than prescribing for minocycline, especially among dermatologists and NPs or PAs.
In the interview, Dr. Barbieri acknowledged certain limitations of the study, including the fact that the dataset “does not allow for evaluation of severity of acne and it is not possible to directly link prescriptions to diagnoses, so some prescriptions might not be for acne and others that are for acne might not have been included.”
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the results, said that, while a course of antibiotic therapy was tied to an office visit in the analysis, the duration of each course of therapy was unclear. It would be interesting to see if antibiotic courses became shorter during the time period analyzed, such as 1-3 months versus 4 or more months, he added, “as this should reduce risks associated with long-term use of oral antibiotics.”
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM JAMA DERMATOLOGY
Gender-affirming care: The role of the pediatrician in a changing landscape
As the political targeting of transgender youth and families continues to play out on the national stage, it is more important than ever for pediatricians and other primary care providers to support this vulnerable population by defending the recommendations and guidelines of reputable medical organizations based in science and to show grace and humility in caring for their patients.
Guidelines and resources
All leading medical groups in the United States with statements or policies related to gender-affirming care (including the American Medical Association, the American Academy of Pediatrics, the Society for Adolescent Health and Medicine, the Pediatric Endocrine Society, the American Academy of Child and Adolescent Psychiatry, and many more) recognize that this care is medically necessary and that exclusions for gender-related services are harmful to patients and their families. As pediatricians, families and youth rely on our expertise and guidance related to childhood and adolescent development, including the development of gender identity and ways to create safe and supportive environments needed for youth to reach their full potential.1
While pediatricians are experts in youth development, some may have had limited access to training specific to LGBTQ+ identity development and interventions related to gender-affirming care. There are, however, readily accessible resources to help guide pediatricians in providing support and recommendations to families with concerns around gender or sexuality (See Resources). The American Academy of Pediatrics and Bright Futures recommend discussing the differences between assigned sex at birth and gender identity development with parents of those younger than 12 months of age as well as beginning to discuss and explore gender identity with all youth beginning at 4-5 years of age. Beginning at 8 years of age, pediatricians are also recommended to assess for a patient’s understanding and feelings toward emerging puberty to identify any potential concerns for gender dysphoria.2 If concerns or questions emerge from screening, the family can then be referred to a gender-affirming care specialist for more support.
Gender dysphoria and gender-affirming care
Gender dysphoria may present in different ways and at different times for each patient. Some patients may present early in childhood with gender-diverse behaviors or the assertion of a gender identity different than their assigned sex at birth. However, the most commonly seen presentation is just prior to or during puberty, when one’s physical body starts to change in ways that are not consistent with their gender identity. Many patients report distress around gender before this time, but the distress that comes with the physical changes of puberty often prompts patients to reach out to parents, friends, and/or medical providers for help. Other than youth specifically disclosing their gender identity, as with any life stressors, gender dysphoria may initially present with a decline in school or social functioning, increased mood irritability, depression, or anxiety.3
The goal of support prior to puberty is for youth to grow and thrive as any other child and to not have gender dysphoria get in the way of normal development and social functioning.4 Some families will pursue social transition, the process of making changes within different areas of social interaction (such as name, pronouns, clothing, hairstyle, etc.) to decrease distress around gender. The decision of whether to pursue social transition is unique to each patient and family. The goal of this process is to allow youth to explore these changes in an effort to decrease the distress they experience in social interactions. Youth should be centered in this process and be the leader of any potential changes with parents and schools providing safe and supportive environments.5 Social transition has been shown to decrease rates of depression in gender-diverse youth to the same level as that of their cisgender peers.6
It is important to note that there are no recommended medical interventions for gender-affirming care before the time of puberty and, once a patient reaches Sexual Maturity Rating II (early puberty), the first potential treatment option is the reversible suppression of puberty using gonadotropin-releasing hormone (GnRH) analogues. The goal of this type of medication is to allow youth more time to explore their gender and avoid the permanent physical changes that occur during their endogenous puberty that can have a significant negative impact on their gender dysphoria and psychosocial functioning. Youth and their families can then later decide to discontinue the medication and go through their endogenous puberty or to proceed with gender-affirming hormone treatment.7
With the growing number of states who have or are attempting to ban gender-affirming care for youth, more patients and families will be left with no options for accessing this potentially life-saving care and support within their home state. Some families have already been forced to relocate to more supportive environments or to travel significant distances to receive medically necessary care.8 This summer, the American Academy of Pediatrics reaffirmed their current policy stating, “The AAP opposes any laws or regulations that discriminate against transgender and gender-diverse individuals, or that interfere in the doctor-patient relationship,” and they “support giving transgender adolescents access to the health care they need.”9
Pediatricians should continue to utilize existing resources for recommended routine screening and subsequent referral for patients or families with concerns around gender identity. When possible, connect patients and families in need of more supportive services around gender-affirming care to appropriate specialty providers. If providers are uncertain about the current legal climate in their state, it is recommended to consult with legal counsel if needed. As pediatricians, we must strive to uphold the tenets of medicine, follow expert recommendations and guidelines based on the best available evidence to provide comprehensive care to all patients, and continue to advocate for our patients and families.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is assistant professor of pediatrics at University of Southern California, Los Angeles.
Resources
Bright Futures – Promoting healthy development of sexuality and gender identity (Implementation tip sheet).
Rafferty J. AAP Committee on Psychosocial Aspects of Child and Family Health, AAP Committee on Adolescence, AAP Section on Lesbian, Gay, Bisexual, and Transgender Health and Wellness. Ensuring comprehensive care and support for transgender and gender-diverse children and adolescents.
References
1. Rafferty J. AAP Committee on Psychosocial Aspects of Child and Family Health, AAP Committee on Adolescence, AAP Section on Lesbian, Gay, Bisexual, and Transgender Health and Wellness. Ensuring comprehensive care and support for transgender and gender-diverse children and adolescents.
2. Bright Futures – Promoting healthy development of sexuality and gender identity (Implementation tip sheet).
3. Shumer DE et al. Advances in the care of transgender children and adolescents.
4. Vance SR et al. Psychological and medical care of gender nonconforming youth.
5. Ehrensaft D et al. Prepubertal social gender transitions: What we know; what we can learn – A view from a gender affirmative lens.
6. Olson KR et al. Mental health of transgender children who are supported in their identities.
7. Olson J et al. Management of the transgender adolescent.
8. Rodgers A and Goldberg M. New State laws force families with trans kids to seek gender-affirming care elsewhere.
9. Wyckoff AS, ed. AAP reaffirms gender-affirming care policy, authorizes systematic review of evidence to guide update.
As the political targeting of transgender youth and families continues to play out on the national stage, it is more important than ever for pediatricians and other primary care providers to support this vulnerable population by defending the recommendations and guidelines of reputable medical organizations based in science and to show grace and humility in caring for their patients.
Guidelines and resources
All leading medical groups in the United States with statements or policies related to gender-affirming care (including the American Medical Association, the American Academy of Pediatrics, the Society for Adolescent Health and Medicine, the Pediatric Endocrine Society, the American Academy of Child and Adolescent Psychiatry, and many more) recognize that this care is medically necessary and that exclusions for gender-related services are harmful to patients and their families. As pediatricians, families and youth rely on our expertise and guidance related to childhood and adolescent development, including the development of gender identity and ways to create safe and supportive environments needed for youth to reach their full potential.1
While pediatricians are experts in youth development, some may have had limited access to training specific to LGBTQ+ identity development and interventions related to gender-affirming care. There are, however, readily accessible resources to help guide pediatricians in providing support and recommendations to families with concerns around gender or sexuality (See Resources). The American Academy of Pediatrics and Bright Futures recommend discussing the differences between assigned sex at birth and gender identity development with parents of those younger than 12 months of age as well as beginning to discuss and explore gender identity with all youth beginning at 4-5 years of age. Beginning at 8 years of age, pediatricians are also recommended to assess for a patient’s understanding and feelings toward emerging puberty to identify any potential concerns for gender dysphoria.2 If concerns or questions emerge from screening, the family can then be referred to a gender-affirming care specialist for more support.
Gender dysphoria and gender-affirming care
Gender dysphoria may present in different ways and at different times for each patient. Some patients may present early in childhood with gender-diverse behaviors or the assertion of a gender identity different than their assigned sex at birth. However, the most commonly seen presentation is just prior to or during puberty, when one’s physical body starts to change in ways that are not consistent with their gender identity. Many patients report distress around gender before this time, but the distress that comes with the physical changes of puberty often prompts patients to reach out to parents, friends, and/or medical providers for help. Other than youth specifically disclosing their gender identity, as with any life stressors, gender dysphoria may initially present with a decline in school or social functioning, increased mood irritability, depression, or anxiety.3
The goal of support prior to puberty is for youth to grow and thrive as any other child and to not have gender dysphoria get in the way of normal development and social functioning.4 Some families will pursue social transition, the process of making changes within different areas of social interaction (such as name, pronouns, clothing, hairstyle, etc.) to decrease distress around gender. The decision of whether to pursue social transition is unique to each patient and family. The goal of this process is to allow youth to explore these changes in an effort to decrease the distress they experience in social interactions. Youth should be centered in this process and be the leader of any potential changes with parents and schools providing safe and supportive environments.5 Social transition has been shown to decrease rates of depression in gender-diverse youth to the same level as that of their cisgender peers.6
It is important to note that there are no recommended medical interventions for gender-affirming care before the time of puberty and, once a patient reaches Sexual Maturity Rating II (early puberty), the first potential treatment option is the reversible suppression of puberty using gonadotropin-releasing hormone (GnRH) analogues. The goal of this type of medication is to allow youth more time to explore their gender and avoid the permanent physical changes that occur during their endogenous puberty that can have a significant negative impact on their gender dysphoria and psychosocial functioning. Youth and their families can then later decide to discontinue the medication and go through their endogenous puberty or to proceed with gender-affirming hormone treatment.7
With the growing number of states who have or are attempting to ban gender-affirming care for youth, more patients and families will be left with no options for accessing this potentially life-saving care and support within their home state. Some families have already been forced to relocate to more supportive environments or to travel significant distances to receive medically necessary care.8 This summer, the American Academy of Pediatrics reaffirmed their current policy stating, “The AAP opposes any laws or regulations that discriminate against transgender and gender-diverse individuals, or that interfere in the doctor-patient relationship,” and they “support giving transgender adolescents access to the health care they need.”9
Pediatricians should continue to utilize existing resources for recommended routine screening and subsequent referral for patients or families with concerns around gender identity. When possible, connect patients and families in need of more supportive services around gender-affirming care to appropriate specialty providers. If providers are uncertain about the current legal climate in their state, it is recommended to consult with legal counsel if needed. As pediatricians, we must strive to uphold the tenets of medicine, follow expert recommendations and guidelines based on the best available evidence to provide comprehensive care to all patients, and continue to advocate for our patients and families.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is assistant professor of pediatrics at University of Southern California, Los Angeles.
Resources
Bright Futures – Promoting healthy development of sexuality and gender identity (Implementation tip sheet).
Rafferty J. AAP Committee on Psychosocial Aspects of Child and Family Health, AAP Committee on Adolescence, AAP Section on Lesbian, Gay, Bisexual, and Transgender Health and Wellness. Ensuring comprehensive care and support for transgender and gender-diverse children and adolescents.
References
1. Rafferty J. AAP Committee on Psychosocial Aspects of Child and Family Health, AAP Committee on Adolescence, AAP Section on Lesbian, Gay, Bisexual, and Transgender Health and Wellness. Ensuring comprehensive care and support for transgender and gender-diverse children and adolescents.
2. Bright Futures – Promoting healthy development of sexuality and gender identity (Implementation tip sheet).
3. Shumer DE et al. Advances in the care of transgender children and adolescents.
4. Vance SR et al. Psychological and medical care of gender nonconforming youth.
5. Ehrensaft D et al. Prepubertal social gender transitions: What we know; what we can learn – A view from a gender affirmative lens.
6. Olson KR et al. Mental health of transgender children who are supported in their identities.
7. Olson J et al. Management of the transgender adolescent.
8. Rodgers A and Goldberg M. New State laws force families with trans kids to seek gender-affirming care elsewhere.
9. Wyckoff AS, ed. AAP reaffirms gender-affirming care policy, authorizes systematic review of evidence to guide update.
As the political targeting of transgender youth and families continues to play out on the national stage, it is more important than ever for pediatricians and other primary care providers to support this vulnerable population by defending the recommendations and guidelines of reputable medical organizations based in science and to show grace and humility in caring for their patients.
Guidelines and resources
All leading medical groups in the United States with statements or policies related to gender-affirming care (including the American Medical Association, the American Academy of Pediatrics, the Society for Adolescent Health and Medicine, the Pediatric Endocrine Society, the American Academy of Child and Adolescent Psychiatry, and many more) recognize that this care is medically necessary and that exclusions for gender-related services are harmful to patients and their families. As pediatricians, families and youth rely on our expertise and guidance related to childhood and adolescent development, including the development of gender identity and ways to create safe and supportive environments needed for youth to reach their full potential.1
While pediatricians are experts in youth development, some may have had limited access to training specific to LGBTQ+ identity development and interventions related to gender-affirming care. There are, however, readily accessible resources to help guide pediatricians in providing support and recommendations to families with concerns around gender or sexuality (See Resources). The American Academy of Pediatrics and Bright Futures recommend discussing the differences between assigned sex at birth and gender identity development with parents of those younger than 12 months of age as well as beginning to discuss and explore gender identity with all youth beginning at 4-5 years of age. Beginning at 8 years of age, pediatricians are also recommended to assess for a patient’s understanding and feelings toward emerging puberty to identify any potential concerns for gender dysphoria.2 If concerns or questions emerge from screening, the family can then be referred to a gender-affirming care specialist for more support.
Gender dysphoria and gender-affirming care
Gender dysphoria may present in different ways and at different times for each patient. Some patients may present early in childhood with gender-diverse behaviors or the assertion of a gender identity different than their assigned sex at birth. However, the most commonly seen presentation is just prior to or during puberty, when one’s physical body starts to change in ways that are not consistent with their gender identity. Many patients report distress around gender before this time, but the distress that comes with the physical changes of puberty often prompts patients to reach out to parents, friends, and/or medical providers for help. Other than youth specifically disclosing their gender identity, as with any life stressors, gender dysphoria may initially present with a decline in school or social functioning, increased mood irritability, depression, or anxiety.3
The goal of support prior to puberty is for youth to grow and thrive as any other child and to not have gender dysphoria get in the way of normal development and social functioning.4 Some families will pursue social transition, the process of making changes within different areas of social interaction (such as name, pronouns, clothing, hairstyle, etc.) to decrease distress around gender. The decision of whether to pursue social transition is unique to each patient and family. The goal of this process is to allow youth to explore these changes in an effort to decrease the distress they experience in social interactions. Youth should be centered in this process and be the leader of any potential changes with parents and schools providing safe and supportive environments.5 Social transition has been shown to decrease rates of depression in gender-diverse youth to the same level as that of their cisgender peers.6
It is important to note that there are no recommended medical interventions for gender-affirming care before the time of puberty and, once a patient reaches Sexual Maturity Rating II (early puberty), the first potential treatment option is the reversible suppression of puberty using gonadotropin-releasing hormone (GnRH) analogues. The goal of this type of medication is to allow youth more time to explore their gender and avoid the permanent physical changes that occur during their endogenous puberty that can have a significant negative impact on their gender dysphoria and psychosocial functioning. Youth and their families can then later decide to discontinue the medication and go through their endogenous puberty or to proceed with gender-affirming hormone treatment.7
With the growing number of states who have or are attempting to ban gender-affirming care for youth, more patients and families will be left with no options for accessing this potentially life-saving care and support within their home state. Some families have already been forced to relocate to more supportive environments or to travel significant distances to receive medically necessary care.8 This summer, the American Academy of Pediatrics reaffirmed their current policy stating, “The AAP opposes any laws or regulations that discriminate against transgender and gender-diverse individuals, or that interfere in the doctor-patient relationship,” and they “support giving transgender adolescents access to the health care they need.”9
Pediatricians should continue to utilize existing resources for recommended routine screening and subsequent referral for patients or families with concerns around gender identity. When possible, connect patients and families in need of more supportive services around gender-affirming care to appropriate specialty providers. If providers are uncertain about the current legal climate in their state, it is recommended to consult with legal counsel if needed. As pediatricians, we must strive to uphold the tenets of medicine, follow expert recommendations and guidelines based on the best available evidence to provide comprehensive care to all patients, and continue to advocate for our patients and families.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is assistant professor of pediatrics at University of Southern California, Los Angeles.
Resources
Bright Futures – Promoting healthy development of sexuality and gender identity (Implementation tip sheet).
Rafferty J. AAP Committee on Psychosocial Aspects of Child and Family Health, AAP Committee on Adolescence, AAP Section on Lesbian, Gay, Bisexual, and Transgender Health and Wellness. Ensuring comprehensive care and support for transgender and gender-diverse children and adolescents.
References
1. Rafferty J. AAP Committee on Psychosocial Aspects of Child and Family Health, AAP Committee on Adolescence, AAP Section on Lesbian, Gay, Bisexual, and Transgender Health and Wellness. Ensuring comprehensive care and support for transgender and gender-diverse children and adolescents.
2. Bright Futures – Promoting healthy development of sexuality and gender identity (Implementation tip sheet).
3. Shumer DE et al. Advances in the care of transgender children and adolescents.
4. Vance SR et al. Psychological and medical care of gender nonconforming youth.
5. Ehrensaft D et al. Prepubertal social gender transitions: What we know; what we can learn – A view from a gender affirmative lens.
6. Olson KR et al. Mental health of transgender children who are supported in their identities.
7. Olson J et al. Management of the transgender adolescent.
8. Rodgers A and Goldberg M. New State laws force families with trans kids to seek gender-affirming care elsewhere.
9. Wyckoff AS, ed. AAP reaffirms gender-affirming care policy, authorizes systematic review of evidence to guide update.
New evidence suggests genetic risk factors in hidradenitis suppurativa
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with lesions that include deep-seated nodules and abscesses, draining tracts, and fibrotic scars, and has been reported to be highly heritable.
A genomewide association study (GWAS) that involved a meta-analysis of data from three large biobanks (UK Biobank, FinnGen, and BioVU) identified one significant locus and two suggestive loci. The researchers were able to replicate the association with HS for two loci near the SOX9 and KLF5 genes in BioVU.
In addition, they also looked at genetic correlations between HS and autoimmune and inflammatory diseases, and results suggested a positive association with inflammatory bowel disease, psoriasis, and type 2 diabetes.
However, while plausible, the variant associations near candidate genes do not prove a causal effect of these variants or genes on disease risk, and further study is needed.
“It’s possible that these genes aren’t the ones affected by the variations we describe, but both are very strong candidates,” said study author Christopher J. Sayed, MD, associate professor of dermatology, University of North Carolina at Chapel Hill. “This improves our understanding of HS as a disease that is likely related to genetic changes that result in dysregulated hair follicle maintenance, inflammation, and wound healing.”
In turn, this will allow clinicians to educate patients about the underlying genetic influences on HS, he explained, as opposed to stigmatizing misconceptions focusing only on weight, smoking, and hygiene.
“Larger studies are underway and will be needed to find variants that may predict things like disease severity and response to treatment, but this is a big first step in the right direction,” Dr. Sayed added.
The study was published online in JAMA Dermatology.
Loci identified
GWASs have been limited in HS, and previous research has not identified significant risk loci. In the current study, Dr. Sayed and colleagues sought to identify underlying genes and genetic mechanisms that may underlie pathogenesis in HS.
They performed a GWAS in a cohort of 720 patients who were part of the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the UNC department of dermatology, and controls from the National Longitudinal Study of Adolescent to Adult Health (Add Health) study, a U.S.-based study following adolescents through adulthood.
The UK Biobank (UKB) is a prospective biobank with about 500,000 individuals aged 40-69 years, and FinnGen collects and analyzes genome and health data from Finnish biobank participants. To increase power to detect associations, a GWAS was performed using participants from the UKB (247 HS cases, 453,048 controls). The HS ProCARE GWAS results were meta-analyzed with UKB, along with data from FinnGen (673 HS cases, 297,544 controls). This three-way meta-analysis revealed one genomewide significant locus and two suggestive loci.
The authors found that the most strongly associated variant, rs10512572 located on chromosome 17, showed the strongest association in FinnGen; at the second locus, the most strongly associated variant was rs17090189 located on chromosome 13 and also showed the strongest association in FinnGen; and at the third locus, the most strongly associated variant, rs5792315, located on chromosome 11, showed the strongest association in HS ProCARE.
Next, they tested the 10 most strongly associated variants at the three loci in the BioVU biobank, which has 290 HS cases, including 189 individuals of European ancestry and 101 individuals of African ancestry, with 64,234 and 12,105 controls, respectively. The locus on chromosome 17 was replicated in BioVU in the same direction of effect, while one variant at the chromosome 13 locus showed nominal evidence of association in the same direction.
In a four-way meta-analysis of BioVU combined with the other three studies, the chromosome locus became more significant and the chromosome 13 locus exceeded the genomewide significance threshold. In contrast, the chromosome 11 locus was not replicated in BioVU (P = .27).
The authors pointed out that variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, which play a role in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
Finally, they looked to see if there were shared genetic components between HS and autoimmune and inflammatory diseases. A nominally significant genetic correlation was observed between HS and inflammatory bowel disease (P = .04), psoriasis (P = .03), and type 2 diabetes (P = .04), although none reached significance.
Different manifestation with genetic variant
In a related study, researchers assessed the prevalence of the NCSTN:c.671_682del variant among individuals with HS in Malta, the island country in the Mediterranean, which has a high prevalence of HS and its associated risk factors, particularly obesity.
Led by Dillon Mintoff, MD, of the department of pathology at the University of Malta, Msida, they conducted a cross-sectional study of 113 adults with HS. In this group, 14 (12.39%) were found to be heterozygous for the NCSTN:c.671_682del variant. Individuals who had this variant were more likely to develop symptoms earlier and to manifest them in atypical skin sites, including the scalp, neck, torso, and antecubital fossae. Additionally, even though their symptoms weren’t more severe than those without the variant, patients with the variant were more likely to require treatment with biologic agents.
Studies move genetics in HS forward
Writing in an accompanying editorial, Atlas Khan, PhD, and Lynn Petukhova, PhD, from Columbia University, New York, noted that both of these HS genetic studies “set a solid foundation for future studies aimed at understanding the biological and clinical relevance of new HS genetic evidence.”
“Each study suggests a series of experiments that will allow us to gain new knowledge about HS,” they wrote, including coming closer to providing patients with a genetic diagnosis.
In addition, evidence from the GWAS paper suggests that with “larger HS GWASs we will be able to better prioritize drug-repurposing studies,” concluded the editorialists. “Both of these goals will help to improve health outcomes for patients with HS and their family members.”
Dr. Sayed reported grants and/or personal fees from AbbVie, Novartis, UCB, Incyte, InflaRx, Alumis, and ChemoCentryx outside the submitted work; and serving as a volunteer member of the board of the HS Foundation and member of the European HS Foundation. The study in Malta was funded by the Government of Malta’s Tertiary Education Scholarship Scheme and Institutional Funds from the University of Malta. Dr. Mintoff reported grants from the Government of Malta Ministry for Education, Sport, Youth, Research and Innovation during the conduct of the study. Dr. Khan reported receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases. Dr. Petukhova reported receiving grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Columbia University’s Precision Medicine Initiative, Herbert Irving Comprehensive Cancer Center, and Data Science Institute.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with lesions that include deep-seated nodules and abscesses, draining tracts, and fibrotic scars, and has been reported to be highly heritable.
A genomewide association study (GWAS) that involved a meta-analysis of data from three large biobanks (UK Biobank, FinnGen, and BioVU) identified one significant locus and two suggestive loci. The researchers were able to replicate the association with HS for two loci near the SOX9 and KLF5 genes in BioVU.
In addition, they also looked at genetic correlations between HS and autoimmune and inflammatory diseases, and results suggested a positive association with inflammatory bowel disease, psoriasis, and type 2 diabetes.
However, while plausible, the variant associations near candidate genes do not prove a causal effect of these variants or genes on disease risk, and further study is needed.
“It’s possible that these genes aren’t the ones affected by the variations we describe, but both are very strong candidates,” said study author Christopher J. Sayed, MD, associate professor of dermatology, University of North Carolina at Chapel Hill. “This improves our understanding of HS as a disease that is likely related to genetic changes that result in dysregulated hair follicle maintenance, inflammation, and wound healing.”
In turn, this will allow clinicians to educate patients about the underlying genetic influences on HS, he explained, as opposed to stigmatizing misconceptions focusing only on weight, smoking, and hygiene.
“Larger studies are underway and will be needed to find variants that may predict things like disease severity and response to treatment, but this is a big first step in the right direction,” Dr. Sayed added.
The study was published online in JAMA Dermatology.
Loci identified
GWASs have been limited in HS, and previous research has not identified significant risk loci. In the current study, Dr. Sayed and colleagues sought to identify underlying genes and genetic mechanisms that may underlie pathogenesis in HS.
They performed a GWAS in a cohort of 720 patients who were part of the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the UNC department of dermatology, and controls from the National Longitudinal Study of Adolescent to Adult Health (Add Health) study, a U.S.-based study following adolescents through adulthood.
The UK Biobank (UKB) is a prospective biobank with about 500,000 individuals aged 40-69 years, and FinnGen collects and analyzes genome and health data from Finnish biobank participants. To increase power to detect associations, a GWAS was performed using participants from the UKB (247 HS cases, 453,048 controls). The HS ProCARE GWAS results were meta-analyzed with UKB, along with data from FinnGen (673 HS cases, 297,544 controls). This three-way meta-analysis revealed one genomewide significant locus and two suggestive loci.
The authors found that the most strongly associated variant, rs10512572 located on chromosome 17, showed the strongest association in FinnGen; at the second locus, the most strongly associated variant was rs17090189 located on chromosome 13 and also showed the strongest association in FinnGen; and at the third locus, the most strongly associated variant, rs5792315, located on chromosome 11, showed the strongest association in HS ProCARE.
Next, they tested the 10 most strongly associated variants at the three loci in the BioVU biobank, which has 290 HS cases, including 189 individuals of European ancestry and 101 individuals of African ancestry, with 64,234 and 12,105 controls, respectively. The locus on chromosome 17 was replicated in BioVU in the same direction of effect, while one variant at the chromosome 13 locus showed nominal evidence of association in the same direction.
In a four-way meta-analysis of BioVU combined with the other three studies, the chromosome locus became more significant and the chromosome 13 locus exceeded the genomewide significance threshold. In contrast, the chromosome 11 locus was not replicated in BioVU (P = .27).
The authors pointed out that variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, which play a role in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
Finally, they looked to see if there were shared genetic components between HS and autoimmune and inflammatory diseases. A nominally significant genetic correlation was observed between HS and inflammatory bowel disease (P = .04), psoriasis (P = .03), and type 2 diabetes (P = .04), although none reached significance.
Different manifestation with genetic variant
In a related study, researchers assessed the prevalence of the NCSTN:c.671_682del variant among individuals with HS in Malta, the island country in the Mediterranean, which has a high prevalence of HS and its associated risk factors, particularly obesity.
Led by Dillon Mintoff, MD, of the department of pathology at the University of Malta, Msida, they conducted a cross-sectional study of 113 adults with HS. In this group, 14 (12.39%) were found to be heterozygous for the NCSTN:c.671_682del variant. Individuals who had this variant were more likely to develop symptoms earlier and to manifest them in atypical skin sites, including the scalp, neck, torso, and antecubital fossae. Additionally, even though their symptoms weren’t more severe than those without the variant, patients with the variant were more likely to require treatment with biologic agents.
Studies move genetics in HS forward
Writing in an accompanying editorial, Atlas Khan, PhD, and Lynn Petukhova, PhD, from Columbia University, New York, noted that both of these HS genetic studies “set a solid foundation for future studies aimed at understanding the biological and clinical relevance of new HS genetic evidence.”
“Each study suggests a series of experiments that will allow us to gain new knowledge about HS,” they wrote, including coming closer to providing patients with a genetic diagnosis.
In addition, evidence from the GWAS paper suggests that with “larger HS GWASs we will be able to better prioritize drug-repurposing studies,” concluded the editorialists. “Both of these goals will help to improve health outcomes for patients with HS and their family members.”
Dr. Sayed reported grants and/or personal fees from AbbVie, Novartis, UCB, Incyte, InflaRx, Alumis, and ChemoCentryx outside the submitted work; and serving as a volunteer member of the board of the HS Foundation and member of the European HS Foundation. The study in Malta was funded by the Government of Malta’s Tertiary Education Scholarship Scheme and Institutional Funds from the University of Malta. Dr. Mintoff reported grants from the Government of Malta Ministry for Education, Sport, Youth, Research and Innovation during the conduct of the study. Dr. Khan reported receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases. Dr. Petukhova reported receiving grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Columbia University’s Precision Medicine Initiative, Herbert Irving Comprehensive Cancer Center, and Data Science Institute.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with lesions that include deep-seated nodules and abscesses, draining tracts, and fibrotic scars, and has been reported to be highly heritable.
A genomewide association study (GWAS) that involved a meta-analysis of data from three large biobanks (UK Biobank, FinnGen, and BioVU) identified one significant locus and two suggestive loci. The researchers were able to replicate the association with HS for two loci near the SOX9 and KLF5 genes in BioVU.
In addition, they also looked at genetic correlations between HS and autoimmune and inflammatory diseases, and results suggested a positive association with inflammatory bowel disease, psoriasis, and type 2 diabetes.
However, while plausible, the variant associations near candidate genes do not prove a causal effect of these variants or genes on disease risk, and further study is needed.
“It’s possible that these genes aren’t the ones affected by the variations we describe, but both are very strong candidates,” said study author Christopher J. Sayed, MD, associate professor of dermatology, University of North Carolina at Chapel Hill. “This improves our understanding of HS as a disease that is likely related to genetic changes that result in dysregulated hair follicle maintenance, inflammation, and wound healing.”
In turn, this will allow clinicians to educate patients about the underlying genetic influences on HS, he explained, as opposed to stigmatizing misconceptions focusing only on weight, smoking, and hygiene.
“Larger studies are underway and will be needed to find variants that may predict things like disease severity and response to treatment, but this is a big first step in the right direction,” Dr. Sayed added.
The study was published online in JAMA Dermatology.
Loci identified
GWASs have been limited in HS, and previous research has not identified significant risk loci. In the current study, Dr. Sayed and colleagues sought to identify underlying genes and genetic mechanisms that may underlie pathogenesis in HS.
They performed a GWAS in a cohort of 720 patients who were part of the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the UNC department of dermatology, and controls from the National Longitudinal Study of Adolescent to Adult Health (Add Health) study, a U.S.-based study following adolescents through adulthood.
The UK Biobank (UKB) is a prospective biobank with about 500,000 individuals aged 40-69 years, and FinnGen collects and analyzes genome and health data from Finnish biobank participants. To increase power to detect associations, a GWAS was performed using participants from the UKB (247 HS cases, 453,048 controls). The HS ProCARE GWAS results were meta-analyzed with UKB, along with data from FinnGen (673 HS cases, 297,544 controls). This three-way meta-analysis revealed one genomewide significant locus and two suggestive loci.
The authors found that the most strongly associated variant, rs10512572 located on chromosome 17, showed the strongest association in FinnGen; at the second locus, the most strongly associated variant was rs17090189 located on chromosome 13 and also showed the strongest association in FinnGen; and at the third locus, the most strongly associated variant, rs5792315, located on chromosome 11, showed the strongest association in HS ProCARE.
Next, they tested the 10 most strongly associated variants at the three loci in the BioVU biobank, which has 290 HS cases, including 189 individuals of European ancestry and 101 individuals of African ancestry, with 64,234 and 12,105 controls, respectively. The locus on chromosome 17 was replicated in BioVU in the same direction of effect, while one variant at the chromosome 13 locus showed nominal evidence of association in the same direction.
In a four-way meta-analysis of BioVU combined with the other three studies, the chromosome locus became more significant and the chromosome 13 locus exceeded the genomewide significance threshold. In contrast, the chromosome 11 locus was not replicated in BioVU (P = .27).
The authors pointed out that variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, which play a role in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
Finally, they looked to see if there were shared genetic components between HS and autoimmune and inflammatory diseases. A nominally significant genetic correlation was observed between HS and inflammatory bowel disease (P = .04), psoriasis (P = .03), and type 2 diabetes (P = .04), although none reached significance.
Different manifestation with genetic variant
In a related study, researchers assessed the prevalence of the NCSTN:c.671_682del variant among individuals with HS in Malta, the island country in the Mediterranean, which has a high prevalence of HS and its associated risk factors, particularly obesity.
Led by Dillon Mintoff, MD, of the department of pathology at the University of Malta, Msida, they conducted a cross-sectional study of 113 adults with HS. In this group, 14 (12.39%) were found to be heterozygous for the NCSTN:c.671_682del variant. Individuals who had this variant were more likely to develop symptoms earlier and to manifest them in atypical skin sites, including the scalp, neck, torso, and antecubital fossae. Additionally, even though their symptoms weren’t more severe than those without the variant, patients with the variant were more likely to require treatment with biologic agents.
Studies move genetics in HS forward
Writing in an accompanying editorial, Atlas Khan, PhD, and Lynn Petukhova, PhD, from Columbia University, New York, noted that both of these HS genetic studies “set a solid foundation for future studies aimed at understanding the biological and clinical relevance of new HS genetic evidence.”
“Each study suggests a series of experiments that will allow us to gain new knowledge about HS,” they wrote, including coming closer to providing patients with a genetic diagnosis.
In addition, evidence from the GWAS paper suggests that with “larger HS GWASs we will be able to better prioritize drug-repurposing studies,” concluded the editorialists. “Both of these goals will help to improve health outcomes for patients with HS and their family members.”
Dr. Sayed reported grants and/or personal fees from AbbVie, Novartis, UCB, Incyte, InflaRx, Alumis, and ChemoCentryx outside the submitted work; and serving as a volunteer member of the board of the HS Foundation and member of the European HS Foundation. The study in Malta was funded by the Government of Malta’s Tertiary Education Scholarship Scheme and Institutional Funds from the University of Malta. Dr. Mintoff reported grants from the Government of Malta Ministry for Education, Sport, Youth, Research and Innovation during the conduct of the study. Dr. Khan reported receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases. Dr. Petukhova reported receiving grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Columbia University’s Precision Medicine Initiative, Herbert Irving Comprehensive Cancer Center, and Data Science Institute.
FROM JAMA DERMATOLOGY
Primary care vision testing rates in children low
In data from the 2018-2020 National Survey of Children’s Health, an annual, nationally representative cross-sectional study, fewer than half of children aged 3-5 with private insurance received vision testing, with rates even lower in those lacking private insurance. The findings point to unmet eye care needs, especially in young children for whom early testing to ward off vision loss is highly recommended.
The report was published online in JAMA Ophthalmology by Olivia J. Killeen MD, MS, an ophthalmologist during the study in the department of ophthalmology and visual sciences at the University of Michigan, Ann Arbor, and colleagues.
Future work should focus on improving primary care provider vision screening rates, especially for the 3- to 5-year age group, the authors wrote.
“Because children often are not aware they have an eye problem, routine vision testing in the primary care setting can help identify those with potential eye disease,” Dr. Killeen said. “We have an opportunity to improve the early detection and treatment of eye disease by improving primary care vision testing.”
Childhood vision testing, which falls into the vital signs section of the wellness check, is critical because undiagnosed problems can cause amblyopia or “lazy eye.” The American Academy of Pediatrics recommends vision testing at well-child visits starting at age 3. Regional studies,however, have suggested low rates of PCP vision testing.
Study findings
In a sample of 89,936 participants, with a mean age of 10 years (51.1% male), an estimated 30.7% overall received vision testing in primary care. Adjusted odds of vision testing in primary care decreased by 41% (odds ratio, 0.59; 95% confidence interval [CI], 0.49-0.72) for uninsured and by 24% (OR, 0.76; 95% CI, 0.70-0.82) for publicly insured participants vs. those with private insurance.
Adjusted estimated probability of vision testing was 22% (95% CI, 18.8%-25.2%) for uninsured participants, 26.6% (95% CI, 25.3%-27.9%) for those publicly insured, and 32.3% (95% CI, 31.4%-33.%) for those privately insured.
In children aged 3-5, estimated probability was 29.7% (95% CI, 25.6%-33.7%) for those uninsured, 35.2% (95% CI, 33.1%-37.3%) for those publicly insured, and 41.6% (95% CI, 39.8%-43.5%) for those privately insured – all well under 50%. The children least likely to be tested, regardless of insurance status, were adolescents aged 12-17.
Commenting on the study but not involved in it, Natalie J. Choi, MD, assistant professor of family medicine at Northwestern Medicine in Chicago, noted that the authors did not address other screening venues such as schools. She also pointed out that it is challenging for primary care physicians to address all the recommended prevention measures in addition to acute issues during an office visit.
“Oftentimes parents are the first to notice a concern about vision, and parental concerns are routinely addressed in primary care,” she said.
According to Dr. Killeen, vision testing should always be worked into the exam flow as a routine part of well-child visits. “There are devices called photoscreeners which provide automated vision tests for young children and can save a lot of time and make vision testing less burdensome for physicians and their staff,” she said. Not all primary care offices, however, offer these testing modalities.
Dr. Choi said low vision screening rates are part of the larger problem of preventive care. “In the study, the percentage of children attending a preventive health visit within the last 12 months varied by insurance coverage and was definitely not 100%,” she said. “Investing in preventive medicine helps us identify things before they become a problem, so I would love to see the number of well-child checks increase.” Identifying this gap in vision care is one of many steps, she said.
This work was supported by the University of Michigan National Clinician Scholars Program and by an unrestricted grant to the University of Michigan Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness. Study coauthor Brian C. Stagg, MD, reported a grant from the National Eye Institute during the conduct of the study. Dr. Choi disclosed no conflicts of interest relevant to her comments.
In data from the 2018-2020 National Survey of Children’s Health, an annual, nationally representative cross-sectional study, fewer than half of children aged 3-5 with private insurance received vision testing, with rates even lower in those lacking private insurance. The findings point to unmet eye care needs, especially in young children for whom early testing to ward off vision loss is highly recommended.
The report was published online in JAMA Ophthalmology by Olivia J. Killeen MD, MS, an ophthalmologist during the study in the department of ophthalmology and visual sciences at the University of Michigan, Ann Arbor, and colleagues.
Future work should focus on improving primary care provider vision screening rates, especially for the 3- to 5-year age group, the authors wrote.
“Because children often are not aware they have an eye problem, routine vision testing in the primary care setting can help identify those with potential eye disease,” Dr. Killeen said. “We have an opportunity to improve the early detection and treatment of eye disease by improving primary care vision testing.”
Childhood vision testing, which falls into the vital signs section of the wellness check, is critical because undiagnosed problems can cause amblyopia or “lazy eye.” The American Academy of Pediatrics recommends vision testing at well-child visits starting at age 3. Regional studies,however, have suggested low rates of PCP vision testing.
Study findings
In a sample of 89,936 participants, with a mean age of 10 years (51.1% male), an estimated 30.7% overall received vision testing in primary care. Adjusted odds of vision testing in primary care decreased by 41% (odds ratio, 0.59; 95% confidence interval [CI], 0.49-0.72) for uninsured and by 24% (OR, 0.76; 95% CI, 0.70-0.82) for publicly insured participants vs. those with private insurance.
Adjusted estimated probability of vision testing was 22% (95% CI, 18.8%-25.2%) for uninsured participants, 26.6% (95% CI, 25.3%-27.9%) for those publicly insured, and 32.3% (95% CI, 31.4%-33.%) for those privately insured.
In children aged 3-5, estimated probability was 29.7% (95% CI, 25.6%-33.7%) for those uninsured, 35.2% (95% CI, 33.1%-37.3%) for those publicly insured, and 41.6% (95% CI, 39.8%-43.5%) for those privately insured – all well under 50%. The children least likely to be tested, regardless of insurance status, were adolescents aged 12-17.
Commenting on the study but not involved in it, Natalie J. Choi, MD, assistant professor of family medicine at Northwestern Medicine in Chicago, noted that the authors did not address other screening venues such as schools. She also pointed out that it is challenging for primary care physicians to address all the recommended prevention measures in addition to acute issues during an office visit.
“Oftentimes parents are the first to notice a concern about vision, and parental concerns are routinely addressed in primary care,” she said.
According to Dr. Killeen, vision testing should always be worked into the exam flow as a routine part of well-child visits. “There are devices called photoscreeners which provide automated vision tests for young children and can save a lot of time and make vision testing less burdensome for physicians and their staff,” she said. Not all primary care offices, however, offer these testing modalities.
Dr. Choi said low vision screening rates are part of the larger problem of preventive care. “In the study, the percentage of children attending a preventive health visit within the last 12 months varied by insurance coverage and was definitely not 100%,” she said. “Investing in preventive medicine helps us identify things before they become a problem, so I would love to see the number of well-child checks increase.” Identifying this gap in vision care is one of many steps, she said.
This work was supported by the University of Michigan National Clinician Scholars Program and by an unrestricted grant to the University of Michigan Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness. Study coauthor Brian C. Stagg, MD, reported a grant from the National Eye Institute during the conduct of the study. Dr. Choi disclosed no conflicts of interest relevant to her comments.
In data from the 2018-2020 National Survey of Children’s Health, an annual, nationally representative cross-sectional study, fewer than half of children aged 3-5 with private insurance received vision testing, with rates even lower in those lacking private insurance. The findings point to unmet eye care needs, especially in young children for whom early testing to ward off vision loss is highly recommended.
The report was published online in JAMA Ophthalmology by Olivia J. Killeen MD, MS, an ophthalmologist during the study in the department of ophthalmology and visual sciences at the University of Michigan, Ann Arbor, and colleagues.
Future work should focus on improving primary care provider vision screening rates, especially for the 3- to 5-year age group, the authors wrote.
“Because children often are not aware they have an eye problem, routine vision testing in the primary care setting can help identify those with potential eye disease,” Dr. Killeen said. “We have an opportunity to improve the early detection and treatment of eye disease by improving primary care vision testing.”
Childhood vision testing, which falls into the vital signs section of the wellness check, is critical because undiagnosed problems can cause amblyopia or “lazy eye.” The American Academy of Pediatrics recommends vision testing at well-child visits starting at age 3. Regional studies,however, have suggested low rates of PCP vision testing.
Study findings
In a sample of 89,936 participants, with a mean age of 10 years (51.1% male), an estimated 30.7% overall received vision testing in primary care. Adjusted odds of vision testing in primary care decreased by 41% (odds ratio, 0.59; 95% confidence interval [CI], 0.49-0.72) for uninsured and by 24% (OR, 0.76; 95% CI, 0.70-0.82) for publicly insured participants vs. those with private insurance.
Adjusted estimated probability of vision testing was 22% (95% CI, 18.8%-25.2%) for uninsured participants, 26.6% (95% CI, 25.3%-27.9%) for those publicly insured, and 32.3% (95% CI, 31.4%-33.%) for those privately insured.
In children aged 3-5, estimated probability was 29.7% (95% CI, 25.6%-33.7%) for those uninsured, 35.2% (95% CI, 33.1%-37.3%) for those publicly insured, and 41.6% (95% CI, 39.8%-43.5%) for those privately insured – all well under 50%. The children least likely to be tested, regardless of insurance status, were adolescents aged 12-17.
Commenting on the study but not involved in it, Natalie J. Choi, MD, assistant professor of family medicine at Northwestern Medicine in Chicago, noted that the authors did not address other screening venues such as schools. She also pointed out that it is challenging for primary care physicians to address all the recommended prevention measures in addition to acute issues during an office visit.
“Oftentimes parents are the first to notice a concern about vision, and parental concerns are routinely addressed in primary care,” she said.
According to Dr. Killeen, vision testing should always be worked into the exam flow as a routine part of well-child visits. “There are devices called photoscreeners which provide automated vision tests for young children and can save a lot of time and make vision testing less burdensome for physicians and their staff,” she said. Not all primary care offices, however, offer these testing modalities.
Dr. Choi said low vision screening rates are part of the larger problem of preventive care. “In the study, the percentage of children attending a preventive health visit within the last 12 months varied by insurance coverage and was definitely not 100%,” she said. “Investing in preventive medicine helps us identify things before they become a problem, so I would love to see the number of well-child checks increase.” Identifying this gap in vision care is one of many steps, she said.
This work was supported by the University of Michigan National Clinician Scholars Program and by an unrestricted grant to the University of Michigan Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness. Study coauthor Brian C. Stagg, MD, reported a grant from the National Eye Institute during the conduct of the study. Dr. Choi disclosed no conflicts of interest relevant to her comments.
FROM JAMA OPHTHALMOLOGY
Telehealth visit helps reconnect adolescents lost to follow-up
A telehealth primary care visit more than doubled the well-visit show rate for a cohort of hard-to-reach adolescents, results of a small pilot study show.
Brian P. Jenssen, MD, MSHP, department of pediatrics, University of Pennsylvania, Philadelphia, led the pilot study and the project team, which included physicians, researchers, and experts in innovation, quality improvement, and data analytics.
Findings were published online in Annals of Family Medicine.
Keeping adolescents in consistent primary care can be challenging for many reasons. The study authors note, “Only 50% of adolescents have had a health supervision visit in the past year, missing a critical opportunity for clinicians to influence health, development, screening, and counseling.”
Interest high in hard-to-reach group
This study included a particularly hard-to-reach group of 18-year-old patients at an urban primary care clinic who were lost to follow-up and had Medicaid insurance. They had not completed a well visit in more than 2 years and had a history of no-show visits.
Interest in the pilot program was high. The authors write: “We contacted patients (or their caregivers) to gauge interest in a virtual well visit with a goal to fill five telehealth slots in one evening block with one clinician. Due to high patient interest and demand, we expanded to 15 slots over three evenings, filling the slots after contacting just 24 patients.”
Professional organizations have recommended a telehealth/in-person hybrid care model to meet hard-to-reach adolescents “wherever they are,” the authors note, but the concept has not been well studied.
Under the hybrid model, the first visit is through telehealth and in-person follow-up is scheduled as needed.
Navigators contacted patients to remind them of the appointment, and helped activate the patient portal and complete previsit screening questions for depression and other health risks.
Telehealth visits were billed as preventive visits and in-person follow-up visits as no-charge nurse visits, and these payments were supported by Medicaid.
Sharp increase in show rate
In the pilot study, of the 15 patients scheduled for the telehealth visit, 11 connected virtually (73% show rate). Of those, nine needed in-person follow-up, and five completed the follow-up.
Before the intervention, the average well-visit show rate for this patient group was 33%.
Clinicians counseled all the patients about substance use and safe sex. One patient screened positive for depression and was then connected to services. Two patients were started on birth control.
During the in-person follow-up, all patients received vaccinations (influenza, meningococcal, and/or COVID-19) and were screened for sexually transmitted infection. Eight patients completed the satisfaction survey and all said they liked the convenience of the telehealth visit.
Telehealth may reduce barriers for teens
Anthony Cheng, MD, a family medicine physician at Oregon Health & Science University in Portland, who was not part of the study, said he found the hybrid model promising.
One reason is that telehealth eliminates the need for transportation to medical appointments, which can be a barrier for adolescents.
Among the top causes of death for young people are mental health issues and addressing those, Dr. Cheng noted, is well-suited to a telehealth visit.
“There’s so much we can do if we can establish a relationship and maintain a relationship with our patients as young adults,” he said. “People do better when they have a regular source of care.”
He added that adolescents also have grown up communicating via screens so it’s often more comfortable for them to communicate with health care providers this way.
Dr. Cheng said adopting such a model may be difficult for providers reluctant to switch from the practice model with which they are most comfortable.
“We prefer to do things we have the most confidence in,” he said. “It does take an investment to train staff and build your own clinical comfort. If that experience wasn’t good over the past 3 years, you may be anxious to get back to your normal way of doing business.”
The authors and Dr. Cheng have no relevant financial relationships to disclose.
A telehealth primary care visit more than doubled the well-visit show rate for a cohort of hard-to-reach adolescents, results of a small pilot study show.
Brian P. Jenssen, MD, MSHP, department of pediatrics, University of Pennsylvania, Philadelphia, led the pilot study and the project team, which included physicians, researchers, and experts in innovation, quality improvement, and data analytics.
Findings were published online in Annals of Family Medicine.
Keeping adolescents in consistent primary care can be challenging for many reasons. The study authors note, “Only 50% of adolescents have had a health supervision visit in the past year, missing a critical opportunity for clinicians to influence health, development, screening, and counseling.”
Interest high in hard-to-reach group
This study included a particularly hard-to-reach group of 18-year-old patients at an urban primary care clinic who were lost to follow-up and had Medicaid insurance. They had not completed a well visit in more than 2 years and had a history of no-show visits.
Interest in the pilot program was high. The authors write: “We contacted patients (or their caregivers) to gauge interest in a virtual well visit with a goal to fill five telehealth slots in one evening block with one clinician. Due to high patient interest and demand, we expanded to 15 slots over three evenings, filling the slots after contacting just 24 patients.”
Professional organizations have recommended a telehealth/in-person hybrid care model to meet hard-to-reach adolescents “wherever they are,” the authors note, but the concept has not been well studied.
Under the hybrid model, the first visit is through telehealth and in-person follow-up is scheduled as needed.
Navigators contacted patients to remind them of the appointment, and helped activate the patient portal and complete previsit screening questions for depression and other health risks.
Telehealth visits were billed as preventive visits and in-person follow-up visits as no-charge nurse visits, and these payments were supported by Medicaid.
Sharp increase in show rate
In the pilot study, of the 15 patients scheduled for the telehealth visit, 11 connected virtually (73% show rate). Of those, nine needed in-person follow-up, and five completed the follow-up.
Before the intervention, the average well-visit show rate for this patient group was 33%.
Clinicians counseled all the patients about substance use and safe sex. One patient screened positive for depression and was then connected to services. Two patients were started on birth control.
During the in-person follow-up, all patients received vaccinations (influenza, meningococcal, and/or COVID-19) and were screened for sexually transmitted infection. Eight patients completed the satisfaction survey and all said they liked the convenience of the telehealth visit.
Telehealth may reduce barriers for teens
Anthony Cheng, MD, a family medicine physician at Oregon Health & Science University in Portland, who was not part of the study, said he found the hybrid model promising.
One reason is that telehealth eliminates the need for transportation to medical appointments, which can be a barrier for adolescents.
Among the top causes of death for young people are mental health issues and addressing those, Dr. Cheng noted, is well-suited to a telehealth visit.
“There’s so much we can do if we can establish a relationship and maintain a relationship with our patients as young adults,” he said. “People do better when they have a regular source of care.”
He added that adolescents also have grown up communicating via screens so it’s often more comfortable for them to communicate with health care providers this way.
Dr. Cheng said adopting such a model may be difficult for providers reluctant to switch from the practice model with which they are most comfortable.
“We prefer to do things we have the most confidence in,” he said. “It does take an investment to train staff and build your own clinical comfort. If that experience wasn’t good over the past 3 years, you may be anxious to get back to your normal way of doing business.”
The authors and Dr. Cheng have no relevant financial relationships to disclose.
A telehealth primary care visit more than doubled the well-visit show rate for a cohort of hard-to-reach adolescents, results of a small pilot study show.
Brian P. Jenssen, MD, MSHP, department of pediatrics, University of Pennsylvania, Philadelphia, led the pilot study and the project team, which included physicians, researchers, and experts in innovation, quality improvement, and data analytics.
Findings were published online in Annals of Family Medicine.
Keeping adolescents in consistent primary care can be challenging for many reasons. The study authors note, “Only 50% of adolescents have had a health supervision visit in the past year, missing a critical opportunity for clinicians to influence health, development, screening, and counseling.”
Interest high in hard-to-reach group
This study included a particularly hard-to-reach group of 18-year-old patients at an urban primary care clinic who were lost to follow-up and had Medicaid insurance. They had not completed a well visit in more than 2 years and had a history of no-show visits.
Interest in the pilot program was high. The authors write: “We contacted patients (or their caregivers) to gauge interest in a virtual well visit with a goal to fill five telehealth slots in one evening block with one clinician. Due to high patient interest and demand, we expanded to 15 slots over three evenings, filling the slots after contacting just 24 patients.”
Professional organizations have recommended a telehealth/in-person hybrid care model to meet hard-to-reach adolescents “wherever they are,” the authors note, but the concept has not been well studied.
Under the hybrid model, the first visit is through telehealth and in-person follow-up is scheduled as needed.
Navigators contacted patients to remind them of the appointment, and helped activate the patient portal and complete previsit screening questions for depression and other health risks.
Telehealth visits were billed as preventive visits and in-person follow-up visits as no-charge nurse visits, and these payments were supported by Medicaid.
Sharp increase in show rate
In the pilot study, of the 15 patients scheduled for the telehealth visit, 11 connected virtually (73% show rate). Of those, nine needed in-person follow-up, and five completed the follow-up.
Before the intervention, the average well-visit show rate for this patient group was 33%.
Clinicians counseled all the patients about substance use and safe sex. One patient screened positive for depression and was then connected to services. Two patients were started on birth control.
During the in-person follow-up, all patients received vaccinations (influenza, meningococcal, and/or COVID-19) and were screened for sexually transmitted infection. Eight patients completed the satisfaction survey and all said they liked the convenience of the telehealth visit.
Telehealth may reduce barriers for teens
Anthony Cheng, MD, a family medicine physician at Oregon Health & Science University in Portland, who was not part of the study, said he found the hybrid model promising.
One reason is that telehealth eliminates the need for transportation to medical appointments, which can be a barrier for adolescents.
Among the top causes of death for young people are mental health issues and addressing those, Dr. Cheng noted, is well-suited to a telehealth visit.
“There’s so much we can do if we can establish a relationship and maintain a relationship with our patients as young adults,” he said. “People do better when they have a regular source of care.”
He added that adolescents also have grown up communicating via screens so it’s often more comfortable for them to communicate with health care providers this way.
Dr. Cheng said adopting such a model may be difficult for providers reluctant to switch from the practice model with which they are most comfortable.
“We prefer to do things we have the most confidence in,” he said. “It does take an investment to train staff and build your own clinical comfort. If that experience wasn’t good over the past 3 years, you may be anxious to get back to your normal way of doing business.”
The authors and Dr. Cheng have no relevant financial relationships to disclose.
FROM ANNALS OF INTERNAL MEDICINE