User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Getting COVID shots in same arm may be more effective, study says
Scientists in Germany looked at health data for 303 people who got the mRNA vaccine and then a booster shot. Their antibody levels were measured two weeks after the second shot. None of the people had had COVID before the vaccinations.
Scientists found that the number of protective “killer T cells” was higher in the 147 study participants who got both shots in the same arm, said the study published in EBioMedicine.
The killer cells were found in 67% of cases in which both shots went into the same arm, compared with 43% of cases with different arms.
“That may suggest that that ipsilateral vaccination (in the same arm) is more likely to provide better protection should the vaccinated person become infected with the SARS-CoV-2 virus,” Laura Ziegler, a doctoral student at Saarland University, Germany, said in a news release.
William Schaffner, MD, a professor in the Division of Infectious Diseases at Vanderbilt University Medical Center, Nashville, Tenn., told CBS News that same-arm vaccinations may work better because the cells that provide the immune response are in local lymph nodes.
There’s greater immunological response if the immune cells in the lymph nodes are restimulated in the same place, said Dr. Schaffner, who was not involved in the German study.
The scientists from Saarland University said more research is needed before they can be certain that having vaccinations in the same arm is actually more effective for COVID shots and sequential vaccinations against diseases such as the flu.
A version of this article first appeared on Medscape.com.
Scientists in Germany looked at health data for 303 people who got the mRNA vaccine and then a booster shot. Their antibody levels were measured two weeks after the second shot. None of the people had had COVID before the vaccinations.
Scientists found that the number of protective “killer T cells” was higher in the 147 study participants who got both shots in the same arm, said the study published in EBioMedicine.
The killer cells were found in 67% of cases in which both shots went into the same arm, compared with 43% of cases with different arms.
“That may suggest that that ipsilateral vaccination (in the same arm) is more likely to provide better protection should the vaccinated person become infected with the SARS-CoV-2 virus,” Laura Ziegler, a doctoral student at Saarland University, Germany, said in a news release.
William Schaffner, MD, a professor in the Division of Infectious Diseases at Vanderbilt University Medical Center, Nashville, Tenn., told CBS News that same-arm vaccinations may work better because the cells that provide the immune response are in local lymph nodes.
There’s greater immunological response if the immune cells in the lymph nodes are restimulated in the same place, said Dr. Schaffner, who was not involved in the German study.
The scientists from Saarland University said more research is needed before they can be certain that having vaccinations in the same arm is actually more effective for COVID shots and sequential vaccinations against diseases such as the flu.
A version of this article first appeared on Medscape.com.
Scientists in Germany looked at health data for 303 people who got the mRNA vaccine and then a booster shot. Their antibody levels were measured two weeks after the second shot. None of the people had had COVID before the vaccinations.
Scientists found that the number of protective “killer T cells” was higher in the 147 study participants who got both shots in the same arm, said the study published in EBioMedicine.
The killer cells were found in 67% of cases in which both shots went into the same arm, compared with 43% of cases with different arms.
“That may suggest that that ipsilateral vaccination (in the same arm) is more likely to provide better protection should the vaccinated person become infected with the SARS-CoV-2 virus,” Laura Ziegler, a doctoral student at Saarland University, Germany, said in a news release.
William Schaffner, MD, a professor in the Division of Infectious Diseases at Vanderbilt University Medical Center, Nashville, Tenn., told CBS News that same-arm vaccinations may work better because the cells that provide the immune response are in local lymph nodes.
There’s greater immunological response if the immune cells in the lymph nodes are restimulated in the same place, said Dr. Schaffner, who was not involved in the German study.
The scientists from Saarland University said more research is needed before they can be certain that having vaccinations in the same arm is actually more effective for COVID shots and sequential vaccinations against diseases such as the flu.
A version of this article first appeared on Medscape.com.
FROM EBIOMEDICINE
Parental bias about a doctor can’t trump a patient’s health
This transcript has been edited for clarity.
I’d like to present you today with a case that raised a large amount of discussion and debate. I got involved as an ethics consultant on the case. I think you’ll find it very interesting and I also think there are going to be some differences of opinion about how to manage the case. I’ll be looking forward to getting comments and feedback on this.
The case involved a 14-year-old boy who had been brought into the hospital by his parents, suffering from severe bouts of anxiety that were just almost overwhelming to him. When he was brought in, he was assigned a health care provider who had a West African last name. Prior to meeting the patient, I have to say that the father of this kid told the intake department nurse that he requested someone else. He saw the name – he hadn’t even met the provider – and he said he wanted someone who might be Catholic.
The parents are both from the Dominican Republic. They identified as White, but they appeared to be non-White Latinx to the nurse who was doing some of the initial intake. They got reassigned to a different provider in the department who identified as African American.
The first month of treatment for the young boy went very well, and he seemed to be getting along extremely well with his provider. He was reporting relief to both parents of some of his anxiety, and the provider felt very connected to the child. A good doctor-patient alliance had been formed.
Nevertheless, at the end of the first month, the father connected back to one of the administrators at the hospital and complained, saying he still wanted a different provider. When asked why, he said, “Well, I don’t really want to answer that,” but getting pressed, he basically said he wasn’t comfortable with having an African American doctor take care of his child. He eventually went back to the argument that what he wanted was someone with a Catholic background, although I don’t know that he knew whether this particular provider was religious – Catholic or anything else.
Some people felt that, as the father in charge of the child’s care, if we could accommodate what he wanted in terms of the parents being comfortable, then that’s something we should do. I absolutely did not agree.
My view is that in a situation where a strong provider-patient relationship has been established, where trust is going both ways, where there are no issues coming up between this 14-year-old and the provider, and when a serious mental health issue is being adequately addressed, the patient’s interest must come first.
Once that therapeutic alliance had been established and both the patient and the provider felt satisfied, I don’t think the father’s wishes made any sense. He may have been acting more out of bigotry or just discomfort about difference in terms of who the provider was. I don’t think that’s something that any health system should have to accommodate unless it is getting in the way of patient care.
I hope that we treat all physicians as properly trained to deal with all kinds of patients, regardless of their religion, ethnicity, or skin color. They should have the skills to manage and do well with any patient. There may be situations where it just doesn’t work or where people don’t get along. Yes, I think we then should try, perhaps, to shift the doctor, get a different nurse, or have a different person do an exam. That’s because of the inability to get the patient’s health interests addressed.
Listening to this dad about what he preferred in terms of religion or ethnicity seemed to me to be interfering with medical success. Could I stop him from moving this patient out entirely from the care setting? Probably not, but I think the way to manage this is to try to talk to him – and, by the way, to talk to the mother.
When we did bring the mom into the situation, she was very happy with the health care provider. She didn’t agree with the dad and wanted to have a meeting with the social worker, the dad, and her to get him to get over the worries, concerns, and maybe even biases he was bringing in about the kind of provider he wanted. That’s exactly what we did.
I know that there are many instances where patients may say, “I don’t want a particular doctor or a particular type.” My view is that we shouldn’t accommodate that. We should say that our doctors are trained to help and care for all manner of people. Unless we can think of some reason that there might be a gap or a problem in the actual delivery of the quality of care, we are not going to accommodate racism, bigotry, or bias.
We certainly shouldn’t be accommodating that once a successful therapeutic relationship is established. Even when it’s a child, I would argue that the patient’s best interest has to trump parental desires, parental worries, and parental concerns about the background, ethnicity, and religion of the provider.
Dr. Caplan is director of the division of medical ethics at NYU Langone Medical Center, New York. He disclosed a conflict of interest with Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to present you today with a case that raised a large amount of discussion and debate. I got involved as an ethics consultant on the case. I think you’ll find it very interesting and I also think there are going to be some differences of opinion about how to manage the case. I’ll be looking forward to getting comments and feedback on this.
The case involved a 14-year-old boy who had been brought into the hospital by his parents, suffering from severe bouts of anxiety that were just almost overwhelming to him. When he was brought in, he was assigned a health care provider who had a West African last name. Prior to meeting the patient, I have to say that the father of this kid told the intake department nurse that he requested someone else. He saw the name – he hadn’t even met the provider – and he said he wanted someone who might be Catholic.
The parents are both from the Dominican Republic. They identified as White, but they appeared to be non-White Latinx to the nurse who was doing some of the initial intake. They got reassigned to a different provider in the department who identified as African American.
The first month of treatment for the young boy went very well, and he seemed to be getting along extremely well with his provider. He was reporting relief to both parents of some of his anxiety, and the provider felt very connected to the child. A good doctor-patient alliance had been formed.
Nevertheless, at the end of the first month, the father connected back to one of the administrators at the hospital and complained, saying he still wanted a different provider. When asked why, he said, “Well, I don’t really want to answer that,” but getting pressed, he basically said he wasn’t comfortable with having an African American doctor take care of his child. He eventually went back to the argument that what he wanted was someone with a Catholic background, although I don’t know that he knew whether this particular provider was religious – Catholic or anything else.
Some people felt that, as the father in charge of the child’s care, if we could accommodate what he wanted in terms of the parents being comfortable, then that’s something we should do. I absolutely did not agree.
My view is that in a situation where a strong provider-patient relationship has been established, where trust is going both ways, where there are no issues coming up between this 14-year-old and the provider, and when a serious mental health issue is being adequately addressed, the patient’s interest must come first.
Once that therapeutic alliance had been established and both the patient and the provider felt satisfied, I don’t think the father’s wishes made any sense. He may have been acting more out of bigotry or just discomfort about difference in terms of who the provider was. I don’t think that’s something that any health system should have to accommodate unless it is getting in the way of patient care.
I hope that we treat all physicians as properly trained to deal with all kinds of patients, regardless of their religion, ethnicity, or skin color. They should have the skills to manage and do well with any patient. There may be situations where it just doesn’t work or where people don’t get along. Yes, I think we then should try, perhaps, to shift the doctor, get a different nurse, or have a different person do an exam. That’s because of the inability to get the patient’s health interests addressed.
Listening to this dad about what he preferred in terms of religion or ethnicity seemed to me to be interfering with medical success. Could I stop him from moving this patient out entirely from the care setting? Probably not, but I think the way to manage this is to try to talk to him – and, by the way, to talk to the mother.
When we did bring the mom into the situation, she was very happy with the health care provider. She didn’t agree with the dad and wanted to have a meeting with the social worker, the dad, and her to get him to get over the worries, concerns, and maybe even biases he was bringing in about the kind of provider he wanted. That’s exactly what we did.
I know that there are many instances where patients may say, “I don’t want a particular doctor or a particular type.” My view is that we shouldn’t accommodate that. We should say that our doctors are trained to help and care for all manner of people. Unless we can think of some reason that there might be a gap or a problem in the actual delivery of the quality of care, we are not going to accommodate racism, bigotry, or bias.
We certainly shouldn’t be accommodating that once a successful therapeutic relationship is established. Even when it’s a child, I would argue that the patient’s best interest has to trump parental desires, parental worries, and parental concerns about the background, ethnicity, and religion of the provider.
Dr. Caplan is director of the division of medical ethics at NYU Langone Medical Center, New York. He disclosed a conflict of interest with Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to present you today with a case that raised a large amount of discussion and debate. I got involved as an ethics consultant on the case. I think you’ll find it very interesting and I also think there are going to be some differences of opinion about how to manage the case. I’ll be looking forward to getting comments and feedback on this.
The case involved a 14-year-old boy who had been brought into the hospital by his parents, suffering from severe bouts of anxiety that were just almost overwhelming to him. When he was brought in, he was assigned a health care provider who had a West African last name. Prior to meeting the patient, I have to say that the father of this kid told the intake department nurse that he requested someone else. He saw the name – he hadn’t even met the provider – and he said he wanted someone who might be Catholic.
The parents are both from the Dominican Republic. They identified as White, but they appeared to be non-White Latinx to the nurse who was doing some of the initial intake. They got reassigned to a different provider in the department who identified as African American.
The first month of treatment for the young boy went very well, and he seemed to be getting along extremely well with his provider. He was reporting relief to both parents of some of his anxiety, and the provider felt very connected to the child. A good doctor-patient alliance had been formed.
Nevertheless, at the end of the first month, the father connected back to one of the administrators at the hospital and complained, saying he still wanted a different provider. When asked why, he said, “Well, I don’t really want to answer that,” but getting pressed, he basically said he wasn’t comfortable with having an African American doctor take care of his child. He eventually went back to the argument that what he wanted was someone with a Catholic background, although I don’t know that he knew whether this particular provider was religious – Catholic or anything else.
Some people felt that, as the father in charge of the child’s care, if we could accommodate what he wanted in terms of the parents being comfortable, then that’s something we should do. I absolutely did not agree.
My view is that in a situation where a strong provider-patient relationship has been established, where trust is going both ways, where there are no issues coming up between this 14-year-old and the provider, and when a serious mental health issue is being adequately addressed, the patient’s interest must come first.
Once that therapeutic alliance had been established and both the patient and the provider felt satisfied, I don’t think the father’s wishes made any sense. He may have been acting more out of bigotry or just discomfort about difference in terms of who the provider was. I don’t think that’s something that any health system should have to accommodate unless it is getting in the way of patient care.
I hope that we treat all physicians as properly trained to deal with all kinds of patients, regardless of their religion, ethnicity, or skin color. They should have the skills to manage and do well with any patient. There may be situations where it just doesn’t work or where people don’t get along. Yes, I think we then should try, perhaps, to shift the doctor, get a different nurse, or have a different person do an exam. That’s because of the inability to get the patient’s health interests addressed.
Listening to this dad about what he preferred in terms of religion or ethnicity seemed to me to be interfering with medical success. Could I stop him from moving this patient out entirely from the care setting? Probably not, but I think the way to manage this is to try to talk to him – and, by the way, to talk to the mother.
When we did bring the mom into the situation, she was very happy with the health care provider. She didn’t agree with the dad and wanted to have a meeting with the social worker, the dad, and her to get him to get over the worries, concerns, and maybe even biases he was bringing in about the kind of provider he wanted. That’s exactly what we did.
I know that there are many instances where patients may say, “I don’t want a particular doctor or a particular type.” My view is that we shouldn’t accommodate that. We should say that our doctors are trained to help and care for all manner of people. Unless we can think of some reason that there might be a gap or a problem in the actual delivery of the quality of care, we are not going to accommodate racism, bigotry, or bias.
We certainly shouldn’t be accommodating that once a successful therapeutic relationship is established. Even when it’s a child, I would argue that the patient’s best interest has to trump parental desires, parental worries, and parental concerns about the background, ethnicity, and religion of the provider.
Dr. Caplan is director of the division of medical ethics at NYU Langone Medical Center, New York. He disclosed a conflict of interest with Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Delayed introduction of allergens increases allergy risk
These findings were published in Allergy.
Launched in April 2011, the French ELFE study aims to monitor children from birth to adulthood to better understand the factors from the intrauterine period to adolescence that affect their development, health, social skills, and school career. Thanks to this cohort, a team of scientists has reviewed the relationship between complementary feeding practices and allergies in French children.
The study focused on 6,662 children who had no signs of an allergic reaction before 2 months of age. Data on feeding practices were collected monthly from ages 3 months to 10 months. Their age at complementary feeding introduction was calculated, and a food diversity score was determined at 8 and 10 months. The number of major allergenic foods (out of eggs, fish, wheat, and dairy products) not introduced at 8 and 10 months was also determined. Allergic diseases (food allergy, eczema, asthma, and rhinoconjunctivitis) were reported by parents at 2 months and at 1, 2, 3.5, and 5.5 years.
Initially, scientists determined that just 62% of children began complementary feeding in the recommended age window, which is between ages 4 months and 6 months. They then closely studied the link between delayed introduction of major allergenic foods and the risk of food allergies. They saw that for 1 in 10 children, at least two major allergens, from eggs, fish, wheat, and dairy products, had still not been introduced into the diet of infants by the age of 10 months. Now, these children have a risk of developing a food allergy before the age of 5.5 years that is two times greater than that of children in whom the four major allergens were introduced before the age of 10 months.
These findings therefore confirm the importance of not delaying the introduction of major food allergens to prevent the occurrence of childhood allergic diseases. They provide convincing arguments in support of new recommendations made by the French pediatric and allergy societies as well as those issued by Public Health France.
ELFE: The first cohort to follow children from birth to adulthood
ELFE is the first longitudinal nationwide French study dedicated to monitoring children from birth to adulthood. More than 18,000 children born in metropolitan France in 2011 were included in this study, which represents 1 in 50 children born in 2011. From the time that researchers first met the families in the maternity ward, the parents who agreed to participate in this great scientific adventure have been questioned at regular intervals to better understand how environment, family members, and living conditions affect the development, health, and socialization of children.
This article was translated from the Medscape French Edition. A version of this article first appeared on Medscape.com.
These findings were published in Allergy.
Launched in April 2011, the French ELFE study aims to monitor children from birth to adulthood to better understand the factors from the intrauterine period to adolescence that affect their development, health, social skills, and school career. Thanks to this cohort, a team of scientists has reviewed the relationship between complementary feeding practices and allergies in French children.
The study focused on 6,662 children who had no signs of an allergic reaction before 2 months of age. Data on feeding practices were collected monthly from ages 3 months to 10 months. Their age at complementary feeding introduction was calculated, and a food diversity score was determined at 8 and 10 months. The number of major allergenic foods (out of eggs, fish, wheat, and dairy products) not introduced at 8 and 10 months was also determined. Allergic diseases (food allergy, eczema, asthma, and rhinoconjunctivitis) were reported by parents at 2 months and at 1, 2, 3.5, and 5.5 years.
Initially, scientists determined that just 62% of children began complementary feeding in the recommended age window, which is between ages 4 months and 6 months. They then closely studied the link between delayed introduction of major allergenic foods and the risk of food allergies. They saw that for 1 in 10 children, at least two major allergens, from eggs, fish, wheat, and dairy products, had still not been introduced into the diet of infants by the age of 10 months. Now, these children have a risk of developing a food allergy before the age of 5.5 years that is two times greater than that of children in whom the four major allergens were introduced before the age of 10 months.
These findings therefore confirm the importance of not delaying the introduction of major food allergens to prevent the occurrence of childhood allergic diseases. They provide convincing arguments in support of new recommendations made by the French pediatric and allergy societies as well as those issued by Public Health France.
ELFE: The first cohort to follow children from birth to adulthood
ELFE is the first longitudinal nationwide French study dedicated to monitoring children from birth to adulthood. More than 18,000 children born in metropolitan France in 2011 were included in this study, which represents 1 in 50 children born in 2011. From the time that researchers first met the families in the maternity ward, the parents who agreed to participate in this great scientific adventure have been questioned at regular intervals to better understand how environment, family members, and living conditions affect the development, health, and socialization of children.
This article was translated from the Medscape French Edition. A version of this article first appeared on Medscape.com.
These findings were published in Allergy.
Launched in April 2011, the French ELFE study aims to monitor children from birth to adulthood to better understand the factors from the intrauterine period to adolescence that affect their development, health, social skills, and school career. Thanks to this cohort, a team of scientists has reviewed the relationship between complementary feeding practices and allergies in French children.
The study focused on 6,662 children who had no signs of an allergic reaction before 2 months of age. Data on feeding practices were collected monthly from ages 3 months to 10 months. Their age at complementary feeding introduction was calculated, and a food diversity score was determined at 8 and 10 months. The number of major allergenic foods (out of eggs, fish, wheat, and dairy products) not introduced at 8 and 10 months was also determined. Allergic diseases (food allergy, eczema, asthma, and rhinoconjunctivitis) were reported by parents at 2 months and at 1, 2, 3.5, and 5.5 years.
Initially, scientists determined that just 62% of children began complementary feeding in the recommended age window, which is between ages 4 months and 6 months. They then closely studied the link between delayed introduction of major allergenic foods and the risk of food allergies. They saw that for 1 in 10 children, at least two major allergens, from eggs, fish, wheat, and dairy products, had still not been introduced into the diet of infants by the age of 10 months. Now, these children have a risk of developing a food allergy before the age of 5.5 years that is two times greater than that of children in whom the four major allergens were introduced before the age of 10 months.
These findings therefore confirm the importance of not delaying the introduction of major food allergens to prevent the occurrence of childhood allergic diseases. They provide convincing arguments in support of new recommendations made by the French pediatric and allergy societies as well as those issued by Public Health France.
ELFE: The first cohort to follow children from birth to adulthood
ELFE is the first longitudinal nationwide French study dedicated to monitoring children from birth to adulthood. More than 18,000 children born in metropolitan France in 2011 were included in this study, which represents 1 in 50 children born in 2011. From the time that researchers first met the families in the maternity ward, the parents who agreed to participate in this great scientific adventure have been questioned at regular intervals to better understand how environment, family members, and living conditions affect the development, health, and socialization of children.
This article was translated from the Medscape French Edition. A version of this article first appeared on Medscape.com.
FROM ALLERGY
FDA approves first RSV vaccine for pregnancy
The vaccine, known as Abrysvo, can be given between weeks 32 and 36 of pregnancy and is designed to protect infants from the virus from birth to 6 months of age.
Administered as a single-dose, intramuscular injection, the FDA approved Abrysvo at the end of May for the prevention of lower respiratory tract illness caused by RSV in people aged 60 years and older.
However, “RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, pointed out in a news release. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Most children are infected with the contagious virus at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis, and in clinical trials, the new vaccine reduced that risk by up to 82%.
Before the vaccine became available, up to 3% of infants infected with RSV needed to be hospitalized, according to the Centers for Disease Control and Prevention. In the hospital, treatment typically includes oxygen, intravenous fluids, and mechanical ventilation.
RSV often causes common cold symptoms, but the virus poses the risk of severe complications that can lead to death among young children and older people. The CDC estimates 100-300 deaths of children younger than 5 years and 6,000-10,000 deaths of people aged 65 years and older are linked to RSV annually.
This is also the first year that an antibody shot is available to be given after birth to prevent severe RSV in infants younger than 1 year.
In its approval announcement, the FDA pointed out that preeclampsia occurred in 1.8% of pregnancies after Abrysvo, compared with 1.4% of those who received placebo. The FDA also reported that, in infants, low birth weight and jaundice occurred at a higher rate among the pregnant Abrysvo recipients, compared with the placebo group.
Studies have also shown that pregnant vaccine recipients experienced preterm birth at a rate of 5.7%, compared with a rate of 4.7% among those who received placebo. The FDA called the difference “a numerical imbalance” but said in the approval announcement that a “causal relationship” could not be established.
The FDA also noted that people already at high risk of preterm birth were excluded from clinical trials and that Pfizer must conduct ongoing studies to monitor the risk of preeclampsia as well as preterm birth.
A version of this article first appeared on Medscape.com.
The vaccine, known as Abrysvo, can be given between weeks 32 and 36 of pregnancy and is designed to protect infants from the virus from birth to 6 months of age.
Administered as a single-dose, intramuscular injection, the FDA approved Abrysvo at the end of May for the prevention of lower respiratory tract illness caused by RSV in people aged 60 years and older.
However, “RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, pointed out in a news release. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Most children are infected with the contagious virus at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis, and in clinical trials, the new vaccine reduced that risk by up to 82%.
Before the vaccine became available, up to 3% of infants infected with RSV needed to be hospitalized, according to the Centers for Disease Control and Prevention. In the hospital, treatment typically includes oxygen, intravenous fluids, and mechanical ventilation.
RSV often causes common cold symptoms, but the virus poses the risk of severe complications that can lead to death among young children and older people. The CDC estimates 100-300 deaths of children younger than 5 years and 6,000-10,000 deaths of people aged 65 years and older are linked to RSV annually.
This is also the first year that an antibody shot is available to be given after birth to prevent severe RSV in infants younger than 1 year.
In its approval announcement, the FDA pointed out that preeclampsia occurred in 1.8% of pregnancies after Abrysvo, compared with 1.4% of those who received placebo. The FDA also reported that, in infants, low birth weight and jaundice occurred at a higher rate among the pregnant Abrysvo recipients, compared with the placebo group.
Studies have also shown that pregnant vaccine recipients experienced preterm birth at a rate of 5.7%, compared with a rate of 4.7% among those who received placebo. The FDA called the difference “a numerical imbalance” but said in the approval announcement that a “causal relationship” could not be established.
The FDA also noted that people already at high risk of preterm birth were excluded from clinical trials and that Pfizer must conduct ongoing studies to monitor the risk of preeclampsia as well as preterm birth.
A version of this article first appeared on Medscape.com.
The vaccine, known as Abrysvo, can be given between weeks 32 and 36 of pregnancy and is designed to protect infants from the virus from birth to 6 months of age.
Administered as a single-dose, intramuscular injection, the FDA approved Abrysvo at the end of May for the prevention of lower respiratory tract illness caused by RSV in people aged 60 years and older.
However, “RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, pointed out in a news release. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Most children are infected with the contagious virus at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis, and in clinical trials, the new vaccine reduced that risk by up to 82%.
Before the vaccine became available, up to 3% of infants infected with RSV needed to be hospitalized, according to the Centers for Disease Control and Prevention. In the hospital, treatment typically includes oxygen, intravenous fluids, and mechanical ventilation.
RSV often causes common cold symptoms, but the virus poses the risk of severe complications that can lead to death among young children and older people. The CDC estimates 100-300 deaths of children younger than 5 years and 6,000-10,000 deaths of people aged 65 years and older are linked to RSV annually.
This is also the first year that an antibody shot is available to be given after birth to prevent severe RSV in infants younger than 1 year.
In its approval announcement, the FDA pointed out that preeclampsia occurred in 1.8% of pregnancies after Abrysvo, compared with 1.4% of those who received placebo. The FDA also reported that, in infants, low birth weight and jaundice occurred at a higher rate among the pregnant Abrysvo recipients, compared with the placebo group.
Studies have also shown that pregnant vaccine recipients experienced preterm birth at a rate of 5.7%, compared with a rate of 4.7% among those who received placebo. The FDA called the difference “a numerical imbalance” but said in the approval announcement that a “causal relationship” could not be established.
The FDA also noted that people already at high risk of preterm birth were excluded from clinical trials and that Pfizer must conduct ongoing studies to monitor the risk of preeclampsia as well as preterm birth.
A version of this article first appeared on Medscape.com.
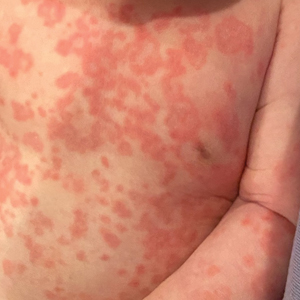
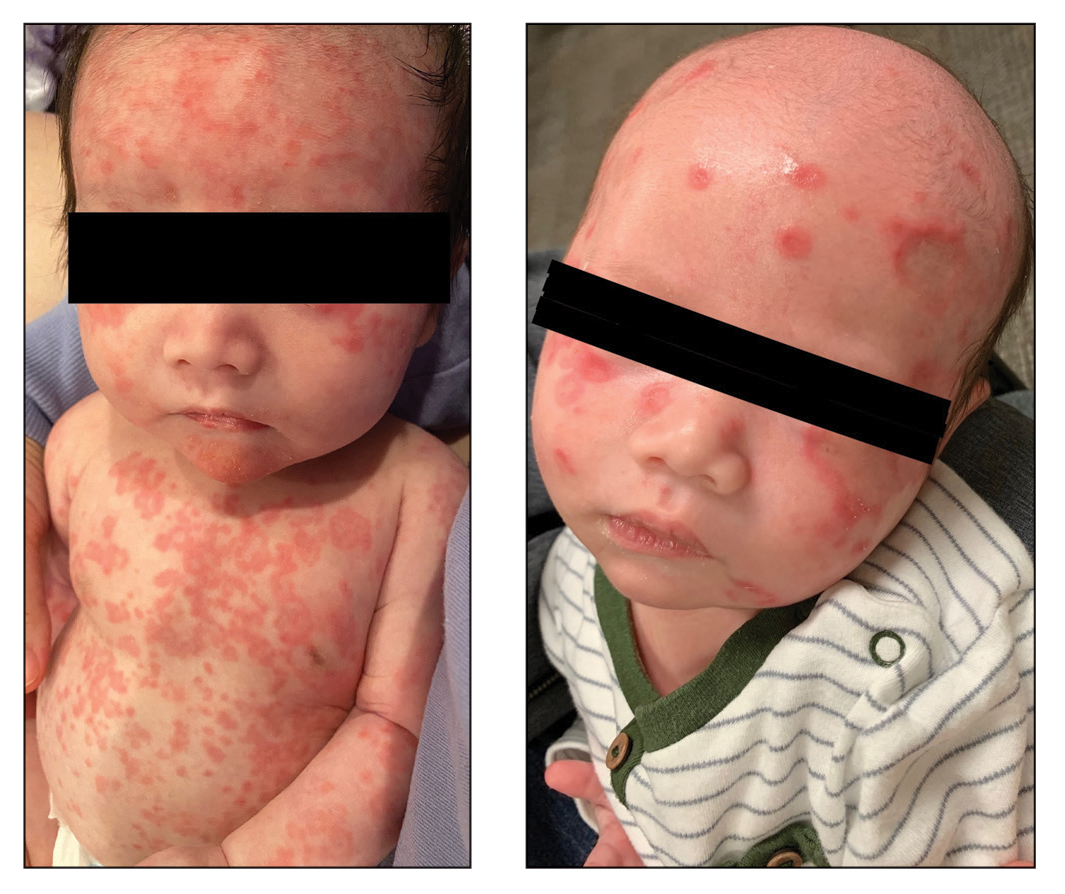
Diffuse Annular Plaques in an Infant
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
A 5-week-old infant boy presented with a rash at birth (left). The pregnancy was full term without complications, and he was otherwise healthy. A family history revealed that his older brother developed a similar rash 2 weeks after birth (right). Physical examination revealed polycyclic annular patches with an erythematous border and central clearing diffusely located on the trunk, extremities, scalp, and face with periorbital edema.
Could a malpractice insurer drop you when you need it most?
You’ve practiced medicine for years without issues, but now you are facing a medical malpractice case. No worries – you’ve had professional liability insurance all this time, so surely there’s nothing to be concerned about. Undoubtedly, your medical malpractice insurer will cover the costs of defending you. Or will they? One case casts questions on just this issue.
Professional liability insurance
According to the American Medical Association, almost one in three physicians (31%) have had a medical malpractice lawsuit filed against them at some point in their careers. These numbers only increase the longer a physician practices; almost half of doctors 55 and over have been sued, compared with less than 10% of physicians under 40.
And while the majority of cases are dropped or dismissed, and the small minority of cases that do go to trial are mostly won by the defense, the cost of defending these cases can be extremely high. Physicians have medical malpractice insurance to defray these costs.
Malpractice insurance generally covers the costs of attorney fees, court costs, arbitration, compensatory damages, and settlements related to patient injury or death. Insurance sometimes, but not always, pays for the costs of malpractice lawsuits arising out of Health Insurance Portability and Accountability Act (HIPAA) violations.
But it is what the policies don’t pay for that should be of most interest to practitioners.
Exclusions to medical malpractice insurance
All professional liability insurance policies contain exclusions, and it is essential that you know what they are. While the exclusions may vary by policy, most malpractice insurance policies exclude claims stemming from:
- Reckless or intentional acts.
- Illegal/criminal activities, including theft.
- Misrepresentation, including dishonesty, fraudulent activity, falsification, and misrepresentation on forms.
- Practicing under the influence of alcohol or drugs.
- Altering patient or hospital records.
- Sexual misconduct.
- Cyber security issues, which typically require a separate cyber liability policy to protect against cyber attacks and data breaches affecting patient medical records.
It’s essential to know what your specific policy’s exclusions are, or you may be surprised to find that your malpractice liability insurance doesn’t cover you when you expected that it would. Such was the situation in a recently decided case.
Also essential is knowing what type of coverage your policy provides – claims-made or occurrence-based. Occurrence policies offer lifetime coverage for incidents that occurred during the policy period, no matter when the claim is made. Claims-made policies cover only incidents that occur and are reported within the policy’s time period (unless a “tail” policy is purchased to extend the reporting period).
The case
Dr. P was a neurologist specializing in pain management. He had a professional liability insurance policy with an insurance company. In 2012, Dr. P’s insurance agent saw a television news story about the physician being accused by the state medical board for overprescribing opioids, resulting in the deaths of 17 patients. The next day, the agent obtained copies of documents from the state medical board, including a summary suspension order and a notice of contemplated action.
The notice of contemplated action specified that Dr. P had deviated from the standard of care through injudicious prescribing, leading to approximately 17 patient deaths due to drug toxicity. Because the agent realized that lawsuits could be filed against Dr. P for the deaths, she sent the insurance company the paperwork from the medical board so the insurer would be aware of the potential claims.
However, when the insurer received the information, it did not investigate or seek more information as it was required to do. The insurer failed to get medical records, or specific patient names, and none of the 17 deaths were recorded in the insurance company’s claims system (a failure to follow company procedure). Instead, the insurance company decided to cancel Dr. P’s policy effective the following month.
The company sent Dr. P a cancellation letter advising him that his policy was being terminated due to “license suspension, nature of allegations, and practice profile,” and offered him a tail policy to purchase.
The insurance company did not advise Dr. P that he should ensure all potential claims were reported, including the 17 deaths, before his policy expired. The company also did not advise him that he had a claims-made policy and what that meant regarding future lawsuits that might be filed after his policy period expired.
A year later, Dr. P was sued in two wrongful death lawsuits by the families of two of the 17 opioid-related deaths. When he was served with the papers, he promptly notified the insurance company. The insurance company issued a denial letter, incorrectly asserting that the 17 drug-toxicity deaths that they were aware of did not qualify as claims under Dr. P’s policy.
After his insurance company failed to represent him, Dr. P divorced his wife of 35 years and filed for bankruptcy. The only creditors with claims were the two families who had sued him. The bankruptcy trustee filed a lawsuit against the insurance company on behalf of Dr. P for the insurer’s failure to defend and indemnify Dr. P against the wrongful death lawsuits. In 2017, the bankruptcy trustee settled the two wrongful death cases by paying the families a certain amount of cash and assigning the insurance bad faith lawsuit to them.
Court and jury decide
In 2020, the case against the insurance company ended up in court. By 2022, the court had decided some of the issues and left some for the jury to determine.
The court found that the insurance company had breached its obligation to defend and indemnify Dr. P, committed unfair insurance claims practices, and committed bad faith in failing to defend the physician. The court limited the compensation to the amount of cash that had been paid to settle the two cases, and any fees and costs that Dr. P had incurred defending himself.
However, this still left the jury to decide whether the insurance company had committed bad faith in failing to indemnify (secure a person against legal liability for his/her actions) Dr. P, whether it had violated the state’s Unfair Insurance Practices Act, and whether punitive damages should be levied against the insurer.
The jury trial ended in a stunning $52 million verdict against the insurance company after less than 2 hours of deliberation. The jury found that the insurance company had acted in bad faith and willfully violated the Unfair Insurances Practices Act.
While the jury ultimately decided against the insurance company and sent it a strong message with a large verdict, Dr. P’s career was still over. He had stopped practicing medicine, was bankrupt, and his personal life was in shambles. The litigation had taken about a decade. Sometimes a win isn’t a victory.
Protecting yourself
The best way to protect yourself from a situation in which your insurer will not defend you is to really know and understand your insurance policy. Is it occurrence-based or claims-made insurance? What exactly does it cover? How are claims supposed to be made? Your professional liability insurance can be extremely important if you get sued, so it is equally important to choose it carefully and to really understand what is being covered.
Other ways to protect yourself:
- Know your agent. Your agent is key to explaining your policy as well as helping in the event that you need to make a claim. Dr. P’s agent saw a news story about him on television, which is why she submitted the information to the insurance company. Dr. P would have been far better off calling the agent directly when he was being investigated by the state medical board to explain the situation and seek advice.
- Be aware of exclusions to your policy. Many – such as criminal acts, reckless or intentional acts, or practicing under the influence – were mentioned earlier in this article. Some may be unexpected, so it is extremely important that you understand the specific exclusions to your particular policy.
- Be aware of your state law, and how changes might affect you. For example, in states that have outlawed or criminalized abortion, an insurance company would probably not have to represent a policy holder who was sued for malpractice involving an abortion. On the other hand, be aware that not treating a patient who needs life-saving care because you are afraid of running afoul of the law can also get you in trouble if the patient is harmed by not being treated. (For example, the Centers for Medicare & Medicaid Services is currently investigating two hospitals that failed to provide necessary stabilizing abortion care to a patient with an emergency medication condition resulting from a miscarriage.)
- Know how your policy defines ‘intentional’ acts (which are typically excluded from coverage). This is important. In some jurisdictions, the insured clinician has to merely intend to commit the acts in order for the claim to be excluded. In other jurisdictions, the insured doctor has to intend to cause the resulting damage. This can result in a very different outcome.
- The best thing doctors can do is to really understand what the policy covers and be prepared to make some noise if the company is not covering something that it should. Don’t be afraid to ask questions if you think your insurer is doing something wrong, and if the answers don’t satisfy you, consult an attorney.
The future
In the fall of 2022, at least partially in response to the Dobbs v. Jackson Women’s Health Organization decision regarding abortion, one professional liability company (Physician’s Insurance) launched criminal defense reimbursement coverage for physicians and hospitals to pay for defense costs incurred in responding to criminal allegations arising directly from patient care.
The add-on Criminal Defense Reimbursement Endorsement was made available in Washington State in January 2023, and will be offered in other states pending regulatory approval. It reimburses defense costs up to $250,000 when criminal actions have arisen from direct patient care.
In a press release announcing the new coverage, Physician’s Insurance CEO Bill Cotter explained the company’s reasoning in providing it: “The already challenging environment for physicians and hospitals has been made even more difficult as they now navigate the legal ramifications of increased criminal medical negligence claims as seen in the case of the Nashville nurse at the Vanderbilt University Medical Center, the potential for criminal state claims arising out of the U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization, and the subsequent state criminalization of healthcare practices that have long been the professionally accepted standard of care.”
Expect to see more insurance companies offering new coverage options for physicians in the future as they recognize that physicians may be facing more than just medical malpractice lawsuits arising out of patient care.
A version of this article first appeared on Medscape.com.
You’ve practiced medicine for years without issues, but now you are facing a medical malpractice case. No worries – you’ve had professional liability insurance all this time, so surely there’s nothing to be concerned about. Undoubtedly, your medical malpractice insurer will cover the costs of defending you. Or will they? One case casts questions on just this issue.
Professional liability insurance
According to the American Medical Association, almost one in three physicians (31%) have had a medical malpractice lawsuit filed against them at some point in their careers. These numbers only increase the longer a physician practices; almost half of doctors 55 and over have been sued, compared with less than 10% of physicians under 40.
And while the majority of cases are dropped or dismissed, and the small minority of cases that do go to trial are mostly won by the defense, the cost of defending these cases can be extremely high. Physicians have medical malpractice insurance to defray these costs.
Malpractice insurance generally covers the costs of attorney fees, court costs, arbitration, compensatory damages, and settlements related to patient injury or death. Insurance sometimes, but not always, pays for the costs of malpractice lawsuits arising out of Health Insurance Portability and Accountability Act (HIPAA) violations.
But it is what the policies don’t pay for that should be of most interest to practitioners.
Exclusions to medical malpractice insurance
All professional liability insurance policies contain exclusions, and it is essential that you know what they are. While the exclusions may vary by policy, most malpractice insurance policies exclude claims stemming from:
- Reckless or intentional acts.
- Illegal/criminal activities, including theft.
- Misrepresentation, including dishonesty, fraudulent activity, falsification, and misrepresentation on forms.
- Practicing under the influence of alcohol or drugs.
- Altering patient or hospital records.
- Sexual misconduct.
- Cyber security issues, which typically require a separate cyber liability policy to protect against cyber attacks and data breaches affecting patient medical records.
It’s essential to know what your specific policy’s exclusions are, or you may be surprised to find that your malpractice liability insurance doesn’t cover you when you expected that it would. Such was the situation in a recently decided case.
Also essential is knowing what type of coverage your policy provides – claims-made or occurrence-based. Occurrence policies offer lifetime coverage for incidents that occurred during the policy period, no matter when the claim is made. Claims-made policies cover only incidents that occur and are reported within the policy’s time period (unless a “tail” policy is purchased to extend the reporting period).
The case
Dr. P was a neurologist specializing in pain management. He had a professional liability insurance policy with an insurance company. In 2012, Dr. P’s insurance agent saw a television news story about the physician being accused by the state medical board for overprescribing opioids, resulting in the deaths of 17 patients. The next day, the agent obtained copies of documents from the state medical board, including a summary suspension order and a notice of contemplated action.
The notice of contemplated action specified that Dr. P had deviated from the standard of care through injudicious prescribing, leading to approximately 17 patient deaths due to drug toxicity. Because the agent realized that lawsuits could be filed against Dr. P for the deaths, she sent the insurance company the paperwork from the medical board so the insurer would be aware of the potential claims.
However, when the insurer received the information, it did not investigate or seek more information as it was required to do. The insurer failed to get medical records, or specific patient names, and none of the 17 deaths were recorded in the insurance company’s claims system (a failure to follow company procedure). Instead, the insurance company decided to cancel Dr. P’s policy effective the following month.
The company sent Dr. P a cancellation letter advising him that his policy was being terminated due to “license suspension, nature of allegations, and practice profile,” and offered him a tail policy to purchase.
The insurance company did not advise Dr. P that he should ensure all potential claims were reported, including the 17 deaths, before his policy expired. The company also did not advise him that he had a claims-made policy and what that meant regarding future lawsuits that might be filed after his policy period expired.
A year later, Dr. P was sued in two wrongful death lawsuits by the families of two of the 17 opioid-related deaths. When he was served with the papers, he promptly notified the insurance company. The insurance company issued a denial letter, incorrectly asserting that the 17 drug-toxicity deaths that they were aware of did not qualify as claims under Dr. P’s policy.
After his insurance company failed to represent him, Dr. P divorced his wife of 35 years and filed for bankruptcy. The only creditors with claims were the two families who had sued him. The bankruptcy trustee filed a lawsuit against the insurance company on behalf of Dr. P for the insurer’s failure to defend and indemnify Dr. P against the wrongful death lawsuits. In 2017, the bankruptcy trustee settled the two wrongful death cases by paying the families a certain amount of cash and assigning the insurance bad faith lawsuit to them.
Court and jury decide
In 2020, the case against the insurance company ended up in court. By 2022, the court had decided some of the issues and left some for the jury to determine.
The court found that the insurance company had breached its obligation to defend and indemnify Dr. P, committed unfair insurance claims practices, and committed bad faith in failing to defend the physician. The court limited the compensation to the amount of cash that had been paid to settle the two cases, and any fees and costs that Dr. P had incurred defending himself.
However, this still left the jury to decide whether the insurance company had committed bad faith in failing to indemnify (secure a person against legal liability for his/her actions) Dr. P, whether it had violated the state’s Unfair Insurance Practices Act, and whether punitive damages should be levied against the insurer.
The jury trial ended in a stunning $52 million verdict against the insurance company after less than 2 hours of deliberation. The jury found that the insurance company had acted in bad faith and willfully violated the Unfair Insurances Practices Act.
While the jury ultimately decided against the insurance company and sent it a strong message with a large verdict, Dr. P’s career was still over. He had stopped practicing medicine, was bankrupt, and his personal life was in shambles. The litigation had taken about a decade. Sometimes a win isn’t a victory.
Protecting yourself
The best way to protect yourself from a situation in which your insurer will not defend you is to really know and understand your insurance policy. Is it occurrence-based or claims-made insurance? What exactly does it cover? How are claims supposed to be made? Your professional liability insurance can be extremely important if you get sued, so it is equally important to choose it carefully and to really understand what is being covered.
Other ways to protect yourself:
- Know your agent. Your agent is key to explaining your policy as well as helping in the event that you need to make a claim. Dr. P’s agent saw a news story about him on television, which is why she submitted the information to the insurance company. Dr. P would have been far better off calling the agent directly when he was being investigated by the state medical board to explain the situation and seek advice.
- Be aware of exclusions to your policy. Many – such as criminal acts, reckless or intentional acts, or practicing under the influence – were mentioned earlier in this article. Some may be unexpected, so it is extremely important that you understand the specific exclusions to your particular policy.
- Be aware of your state law, and how changes might affect you. For example, in states that have outlawed or criminalized abortion, an insurance company would probably not have to represent a policy holder who was sued for malpractice involving an abortion. On the other hand, be aware that not treating a patient who needs life-saving care because you are afraid of running afoul of the law can also get you in trouble if the patient is harmed by not being treated. (For example, the Centers for Medicare & Medicaid Services is currently investigating two hospitals that failed to provide necessary stabilizing abortion care to a patient with an emergency medication condition resulting from a miscarriage.)
- Know how your policy defines ‘intentional’ acts (which are typically excluded from coverage). This is important. In some jurisdictions, the insured clinician has to merely intend to commit the acts in order for the claim to be excluded. In other jurisdictions, the insured doctor has to intend to cause the resulting damage. This can result in a very different outcome.
- The best thing doctors can do is to really understand what the policy covers and be prepared to make some noise if the company is not covering something that it should. Don’t be afraid to ask questions if you think your insurer is doing something wrong, and if the answers don’t satisfy you, consult an attorney.
The future
In the fall of 2022, at least partially in response to the Dobbs v. Jackson Women’s Health Organization decision regarding abortion, one professional liability company (Physician’s Insurance) launched criminal defense reimbursement coverage for physicians and hospitals to pay for defense costs incurred in responding to criminal allegations arising directly from patient care.
The add-on Criminal Defense Reimbursement Endorsement was made available in Washington State in January 2023, and will be offered in other states pending regulatory approval. It reimburses defense costs up to $250,000 when criminal actions have arisen from direct patient care.
In a press release announcing the new coverage, Physician’s Insurance CEO Bill Cotter explained the company’s reasoning in providing it: “The already challenging environment for physicians and hospitals has been made even more difficult as they now navigate the legal ramifications of increased criminal medical negligence claims as seen in the case of the Nashville nurse at the Vanderbilt University Medical Center, the potential for criminal state claims arising out of the U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization, and the subsequent state criminalization of healthcare practices that have long been the professionally accepted standard of care.”
Expect to see more insurance companies offering new coverage options for physicians in the future as they recognize that physicians may be facing more than just medical malpractice lawsuits arising out of patient care.
A version of this article first appeared on Medscape.com.
You’ve practiced medicine for years without issues, but now you are facing a medical malpractice case. No worries – you’ve had professional liability insurance all this time, so surely there’s nothing to be concerned about. Undoubtedly, your medical malpractice insurer will cover the costs of defending you. Or will they? One case casts questions on just this issue.
Professional liability insurance
According to the American Medical Association, almost one in three physicians (31%) have had a medical malpractice lawsuit filed against them at some point in their careers. These numbers only increase the longer a physician practices; almost half of doctors 55 and over have been sued, compared with less than 10% of physicians under 40.
And while the majority of cases are dropped or dismissed, and the small minority of cases that do go to trial are mostly won by the defense, the cost of defending these cases can be extremely high. Physicians have medical malpractice insurance to defray these costs.
Malpractice insurance generally covers the costs of attorney fees, court costs, arbitration, compensatory damages, and settlements related to patient injury or death. Insurance sometimes, but not always, pays for the costs of malpractice lawsuits arising out of Health Insurance Portability and Accountability Act (HIPAA) violations.
But it is what the policies don’t pay for that should be of most interest to practitioners.
Exclusions to medical malpractice insurance
All professional liability insurance policies contain exclusions, and it is essential that you know what they are. While the exclusions may vary by policy, most malpractice insurance policies exclude claims stemming from:
- Reckless or intentional acts.
- Illegal/criminal activities, including theft.
- Misrepresentation, including dishonesty, fraudulent activity, falsification, and misrepresentation on forms.
- Practicing under the influence of alcohol or drugs.
- Altering patient or hospital records.
- Sexual misconduct.
- Cyber security issues, which typically require a separate cyber liability policy to protect against cyber attacks and data breaches affecting patient medical records.
It’s essential to know what your specific policy’s exclusions are, or you may be surprised to find that your malpractice liability insurance doesn’t cover you when you expected that it would. Such was the situation in a recently decided case.
Also essential is knowing what type of coverage your policy provides – claims-made or occurrence-based. Occurrence policies offer lifetime coverage for incidents that occurred during the policy period, no matter when the claim is made. Claims-made policies cover only incidents that occur and are reported within the policy’s time period (unless a “tail” policy is purchased to extend the reporting period).
The case
Dr. P was a neurologist specializing in pain management. He had a professional liability insurance policy with an insurance company. In 2012, Dr. P’s insurance agent saw a television news story about the physician being accused by the state medical board for overprescribing opioids, resulting in the deaths of 17 patients. The next day, the agent obtained copies of documents from the state medical board, including a summary suspension order and a notice of contemplated action.
The notice of contemplated action specified that Dr. P had deviated from the standard of care through injudicious prescribing, leading to approximately 17 patient deaths due to drug toxicity. Because the agent realized that lawsuits could be filed against Dr. P for the deaths, she sent the insurance company the paperwork from the medical board so the insurer would be aware of the potential claims.
However, when the insurer received the information, it did not investigate or seek more information as it was required to do. The insurer failed to get medical records, or specific patient names, and none of the 17 deaths were recorded in the insurance company’s claims system (a failure to follow company procedure). Instead, the insurance company decided to cancel Dr. P’s policy effective the following month.
The company sent Dr. P a cancellation letter advising him that his policy was being terminated due to “license suspension, nature of allegations, and practice profile,” and offered him a tail policy to purchase.
The insurance company did not advise Dr. P that he should ensure all potential claims were reported, including the 17 deaths, before his policy expired. The company also did not advise him that he had a claims-made policy and what that meant regarding future lawsuits that might be filed after his policy period expired.
A year later, Dr. P was sued in two wrongful death lawsuits by the families of two of the 17 opioid-related deaths. When he was served with the papers, he promptly notified the insurance company. The insurance company issued a denial letter, incorrectly asserting that the 17 drug-toxicity deaths that they were aware of did not qualify as claims under Dr. P’s policy.
After his insurance company failed to represent him, Dr. P divorced his wife of 35 years and filed for bankruptcy. The only creditors with claims were the two families who had sued him. The bankruptcy trustee filed a lawsuit against the insurance company on behalf of Dr. P for the insurer’s failure to defend and indemnify Dr. P against the wrongful death lawsuits. In 2017, the bankruptcy trustee settled the two wrongful death cases by paying the families a certain amount of cash and assigning the insurance bad faith lawsuit to them.
Court and jury decide
In 2020, the case against the insurance company ended up in court. By 2022, the court had decided some of the issues and left some for the jury to determine.
The court found that the insurance company had breached its obligation to defend and indemnify Dr. P, committed unfair insurance claims practices, and committed bad faith in failing to defend the physician. The court limited the compensation to the amount of cash that had been paid to settle the two cases, and any fees and costs that Dr. P had incurred defending himself.
However, this still left the jury to decide whether the insurance company had committed bad faith in failing to indemnify (secure a person against legal liability for his/her actions) Dr. P, whether it had violated the state’s Unfair Insurance Practices Act, and whether punitive damages should be levied against the insurer.
The jury trial ended in a stunning $52 million verdict against the insurance company after less than 2 hours of deliberation. The jury found that the insurance company had acted in bad faith and willfully violated the Unfair Insurances Practices Act.
While the jury ultimately decided against the insurance company and sent it a strong message with a large verdict, Dr. P’s career was still over. He had stopped practicing medicine, was bankrupt, and his personal life was in shambles. The litigation had taken about a decade. Sometimes a win isn’t a victory.
Protecting yourself
The best way to protect yourself from a situation in which your insurer will not defend you is to really know and understand your insurance policy. Is it occurrence-based or claims-made insurance? What exactly does it cover? How are claims supposed to be made? Your professional liability insurance can be extremely important if you get sued, so it is equally important to choose it carefully and to really understand what is being covered.
Other ways to protect yourself:
- Know your agent. Your agent is key to explaining your policy as well as helping in the event that you need to make a claim. Dr. P’s agent saw a news story about him on television, which is why she submitted the information to the insurance company. Dr. P would have been far better off calling the agent directly when he was being investigated by the state medical board to explain the situation and seek advice.
- Be aware of exclusions to your policy. Many – such as criminal acts, reckless or intentional acts, or practicing under the influence – were mentioned earlier in this article. Some may be unexpected, so it is extremely important that you understand the specific exclusions to your particular policy.
- Be aware of your state law, and how changes might affect you. For example, in states that have outlawed or criminalized abortion, an insurance company would probably not have to represent a policy holder who was sued for malpractice involving an abortion. On the other hand, be aware that not treating a patient who needs life-saving care because you are afraid of running afoul of the law can also get you in trouble if the patient is harmed by not being treated. (For example, the Centers for Medicare & Medicaid Services is currently investigating two hospitals that failed to provide necessary stabilizing abortion care to a patient with an emergency medication condition resulting from a miscarriage.)
- Know how your policy defines ‘intentional’ acts (which are typically excluded from coverage). This is important. In some jurisdictions, the insured clinician has to merely intend to commit the acts in order for the claim to be excluded. In other jurisdictions, the insured doctor has to intend to cause the resulting damage. This can result in a very different outcome.
- The best thing doctors can do is to really understand what the policy covers and be prepared to make some noise if the company is not covering something that it should. Don’t be afraid to ask questions if you think your insurer is doing something wrong, and if the answers don’t satisfy you, consult an attorney.
The future
In the fall of 2022, at least partially in response to the Dobbs v. Jackson Women’s Health Organization decision regarding abortion, one professional liability company (Physician’s Insurance) launched criminal defense reimbursement coverage for physicians and hospitals to pay for defense costs incurred in responding to criminal allegations arising directly from patient care.
The add-on Criminal Defense Reimbursement Endorsement was made available in Washington State in January 2023, and will be offered in other states pending regulatory approval. It reimburses defense costs up to $250,000 when criminal actions have arisen from direct patient care.
In a press release announcing the new coverage, Physician’s Insurance CEO Bill Cotter explained the company’s reasoning in providing it: “The already challenging environment for physicians and hospitals has been made even more difficult as they now navigate the legal ramifications of increased criminal medical negligence claims as seen in the case of the Nashville nurse at the Vanderbilt University Medical Center, the potential for criminal state claims arising out of the U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization, and the subsequent state criminalization of healthcare practices that have long been the professionally accepted standard of care.”
Expect to see more insurance companies offering new coverage options for physicians in the future as they recognize that physicians may be facing more than just medical malpractice lawsuits arising out of patient care.
A version of this article first appeared on Medscape.com.
Docs using AI? Some love it, most remain wary
When OpenAI released ChatGPT-3 publicly last November, some doctors decided to try out the free AI tool that learns language and writes human-like text. Some physicians found the chatbot made mistakes and stopped using it, while others were happy with the results and plan to use it more often.
“We’ve played around with it. It was very early on in AI and we noticed it gave us incorrect information with regards to clinical guidance,” said Monalisa Tailor, MD, an internal medicine physician at Norton Health Care in Louisville, Ky. “We decided not to pursue it further,” she said.
Orthopedic spine surgeon Daniel Choi, MD, who owns a small medical/surgical practice in Long Island, New York, tested the chatbot’s performance with a few administrative tasks, including writing a job listing for an administrator and prior authorization letters.
He was enthusiastic. “A well-polished job posting that would usually take me 2-3 hours to write was done in 5 minutes,” Dr. Choi said. “I was blown away by the writing – it was much better than anything I could write.”
The chatbot can also automate administrative tasks in doctors’ practices from appointment scheduling and billing to clinical documentation, saving doctors time and money, experts say.
Most physicians are proceeding cautiously. About 10% of more than 500 medical group leaders, responding to a March poll by the Medical Group Management Association, said their practices regularly use AI tools.
More than half of the respondents not using AI said they first want more evidence that the technology works as intended.
“None of them work as advertised,” said one respondent.
MGMA practice management consultant Dawn Plested acknowledges that many of the physician practices she’s worked with are still wary. “I have yet to encounter a practice that is using any AI tool, even something as low-risk as appointment scheduling,” she said.
Physician groups may be concerned about the costs and logistics of integrating ChatGPT with their electronic health record systems (EHRs) and how that would work, said Ms. Plested.
Doctors may also be skeptical of AI based on their experience with EHRs, she said.
“They were promoted as a panacea to many problems; they were supposed to automate business practice, reduce staff and clinician’s work, and improve billing/coding/documentation. Unfortunately, they have become a major source of frustration for doctors,” said Ms. Plested.
Drawing the line at patient care
Patients are worried about their doctors relying on AI for their care, according to a Pew Research Center poll released in February. About 60% of U.S. adults say they would feel uncomfortable if their own health care professional relied on artificial intelligence to do things like diagnose disease and recommend treatments; about 40% say they would feel comfortable with this.
“We have not yet gone into using ChatGPT for clinical purposes and will be very cautious with these types of applications due to concerns about inaccuracies,” Dr. Choi said.
Practice leaders reported in the MGMA poll that the most common uses of AI were nonclinical, such as:
- Patient communications, including call center answering service to help triage calls, to sort/distribute incoming fax messages, and outreach such as appointment reminders and marketing materials.
- Capturing clinical documentation, often with natural language processing or speech recognition platforms to help virtually scribe.
- Improving billing operations and predictive analytics.
Some doctors told The New York Times that ChatGPT helped them communicate with patients in a more compassionate way.
They used chatbots “to find words to break bad news and express concerns about a patient’s suffering, or to just more clearly explain medical recommendations,” the story noted.
Is regulation needed?
Some legal scholars and medical groups say that AI should be regulated to protect patients and doctors from risks, including medical errors, that could harm patients.
“It’s very important to evaluate the accuracy, safety, and privacy of language learning models (LLMs) before integrating them into the medical system. The same should be true of any new medical tool,” said Mason Marks, MD, JD, a health law professor at the Florida State University College of Law in Tallahassee.
In mid-June, the American Medical Association approved two resolutions calling for greater government oversight of AI. The AMA will develop proposed state and federal regulations and work with the federal government and other organizations to protect patients from false or misleading AI-generated medical advice.
Dr. Marks pointed to existing federal rules that apply to AI. “The Federal Trade Commission already has regulation that can potentially be used to combat unfair or deceptive trade practices associated with chatbots,” he said.
In addition, “the U.S. Food and Drug Administration can also regulate these tools, but it needs to update how it approaches risk when it comes to AI. The FDA has an outdated view of risk as physical harm, for instance, from traditional medical devices. That view of risk needs to be updated and expanded to encompass the unique harms of AI,” Dr. Marks said.
There should also be more transparency about how LLM software is used in medicine, he said. “That could be a norm implemented by the LLM developers and it could also be enforced by federal agencies. For instance, the FDA could require developers to be more transparent regarding training data and methods, and the FTC could require greater transparency regarding how consumer data might be used and opportunities to opt out of certain uses,” said Dr. Marks.
What should doctors do?
Dr. Marks advised doctors to be cautious when using ChatGPT and other LLMs, especially for medical advice. “The same would apply to any new medical tool, but we know that the current generation of LLMs [is] particularly prone to making things up, which could lead to medical errors if relied on in clinical settings,” he said.
There is also potential for breaches of patient confidentiality if doctors input clinical information. ChatGPT and OpenAI-enabled tools may not be compliant with the Health Insurance Portability and Accountability Act, which set national standards to protect individuals’ medical records and individually identifiable health information.
“The best approach is to use chatbots cautiously and with skepticism. Don’t input patient information, confirm the accuracy of information produced, and don’t use them as replacements for professional judgment,” Dr. Marks recommended.
Ms. Plested suggested that doctors who want to experiment with AI start with a low-risk tool such as appointment reminders that could save staff time and money. “I never recommend they start with something as high-stakes as coding/billing,” she said.
A version of this article appeared on Medscape.com.
When OpenAI released ChatGPT-3 publicly last November, some doctors decided to try out the free AI tool that learns language and writes human-like text. Some physicians found the chatbot made mistakes and stopped using it, while others were happy with the results and plan to use it more often.
“We’ve played around with it. It was very early on in AI and we noticed it gave us incorrect information with regards to clinical guidance,” said Monalisa Tailor, MD, an internal medicine physician at Norton Health Care in Louisville, Ky. “We decided not to pursue it further,” she said.
Orthopedic spine surgeon Daniel Choi, MD, who owns a small medical/surgical practice in Long Island, New York, tested the chatbot’s performance with a few administrative tasks, including writing a job listing for an administrator and prior authorization letters.
He was enthusiastic. “A well-polished job posting that would usually take me 2-3 hours to write was done in 5 minutes,” Dr. Choi said. “I was blown away by the writing – it was much better than anything I could write.”
The chatbot can also automate administrative tasks in doctors’ practices from appointment scheduling and billing to clinical documentation, saving doctors time and money, experts say.
Most physicians are proceeding cautiously. About 10% of more than 500 medical group leaders, responding to a March poll by the Medical Group Management Association, said their practices regularly use AI tools.
More than half of the respondents not using AI said they first want more evidence that the technology works as intended.
“None of them work as advertised,” said one respondent.
MGMA practice management consultant Dawn Plested acknowledges that many of the physician practices she’s worked with are still wary. “I have yet to encounter a practice that is using any AI tool, even something as low-risk as appointment scheduling,” she said.
Physician groups may be concerned about the costs and logistics of integrating ChatGPT with their electronic health record systems (EHRs) and how that would work, said Ms. Plested.
Doctors may also be skeptical of AI based on their experience with EHRs, she said.
“They were promoted as a panacea to many problems; they were supposed to automate business practice, reduce staff and clinician’s work, and improve billing/coding/documentation. Unfortunately, they have become a major source of frustration for doctors,” said Ms. Plested.
Drawing the line at patient care
Patients are worried about their doctors relying on AI for their care, according to a Pew Research Center poll released in February. About 60% of U.S. adults say they would feel uncomfortable if their own health care professional relied on artificial intelligence to do things like diagnose disease and recommend treatments; about 40% say they would feel comfortable with this.
“We have not yet gone into using ChatGPT for clinical purposes and will be very cautious with these types of applications due to concerns about inaccuracies,” Dr. Choi said.
Practice leaders reported in the MGMA poll that the most common uses of AI were nonclinical, such as:
- Patient communications, including call center answering service to help triage calls, to sort/distribute incoming fax messages, and outreach such as appointment reminders and marketing materials.
- Capturing clinical documentation, often with natural language processing or speech recognition platforms to help virtually scribe.
- Improving billing operations and predictive analytics.
Some doctors told The New York Times that ChatGPT helped them communicate with patients in a more compassionate way.
They used chatbots “to find words to break bad news and express concerns about a patient’s suffering, or to just more clearly explain medical recommendations,” the story noted.
Is regulation needed?
Some legal scholars and medical groups say that AI should be regulated to protect patients and doctors from risks, including medical errors, that could harm patients.
“It’s very important to evaluate the accuracy, safety, and privacy of language learning models (LLMs) before integrating them into the medical system. The same should be true of any new medical tool,” said Mason Marks, MD, JD, a health law professor at the Florida State University College of Law in Tallahassee.
In mid-June, the American Medical Association approved two resolutions calling for greater government oversight of AI. The AMA will develop proposed state and federal regulations and work with the federal government and other organizations to protect patients from false or misleading AI-generated medical advice.
Dr. Marks pointed to existing federal rules that apply to AI. “The Federal Trade Commission already has regulation that can potentially be used to combat unfair or deceptive trade practices associated with chatbots,” he said.
In addition, “the U.S. Food and Drug Administration can also regulate these tools, but it needs to update how it approaches risk when it comes to AI. The FDA has an outdated view of risk as physical harm, for instance, from traditional medical devices. That view of risk needs to be updated and expanded to encompass the unique harms of AI,” Dr. Marks said.
There should also be more transparency about how LLM software is used in medicine, he said. “That could be a norm implemented by the LLM developers and it could also be enforced by federal agencies. For instance, the FDA could require developers to be more transparent regarding training data and methods, and the FTC could require greater transparency regarding how consumer data might be used and opportunities to opt out of certain uses,” said Dr. Marks.
What should doctors do?
Dr. Marks advised doctors to be cautious when using ChatGPT and other LLMs, especially for medical advice. “The same would apply to any new medical tool, but we know that the current generation of LLMs [is] particularly prone to making things up, which could lead to medical errors if relied on in clinical settings,” he said.
There is also potential for breaches of patient confidentiality if doctors input clinical information. ChatGPT and OpenAI-enabled tools may not be compliant with the Health Insurance Portability and Accountability Act, which set national standards to protect individuals’ medical records and individually identifiable health information.
“The best approach is to use chatbots cautiously and with skepticism. Don’t input patient information, confirm the accuracy of information produced, and don’t use them as replacements for professional judgment,” Dr. Marks recommended.
Ms. Plested suggested that doctors who want to experiment with AI start with a low-risk tool such as appointment reminders that could save staff time and money. “I never recommend they start with something as high-stakes as coding/billing,” she said.
A version of this article appeared on Medscape.com.
When OpenAI released ChatGPT-3 publicly last November, some doctors decided to try out the free AI tool that learns language and writes human-like text. Some physicians found the chatbot made mistakes and stopped using it, while others were happy with the results and plan to use it more often.
“We’ve played around with it. It was very early on in AI and we noticed it gave us incorrect information with regards to clinical guidance,” said Monalisa Tailor, MD, an internal medicine physician at Norton Health Care in Louisville, Ky. “We decided not to pursue it further,” she said.
Orthopedic spine surgeon Daniel Choi, MD, who owns a small medical/surgical practice in Long Island, New York, tested the chatbot’s performance with a few administrative tasks, including writing a job listing for an administrator and prior authorization letters.
He was enthusiastic. “A well-polished job posting that would usually take me 2-3 hours to write was done in 5 minutes,” Dr. Choi said. “I was blown away by the writing – it was much better than anything I could write.”
The chatbot can also automate administrative tasks in doctors’ practices from appointment scheduling and billing to clinical documentation, saving doctors time and money, experts say.
Most physicians are proceeding cautiously. About 10% of more than 500 medical group leaders, responding to a March poll by the Medical Group Management Association, said their practices regularly use AI tools.
More than half of the respondents not using AI said they first want more evidence that the technology works as intended.
“None of them work as advertised,” said one respondent.
MGMA practice management consultant Dawn Plested acknowledges that many of the physician practices she’s worked with are still wary. “I have yet to encounter a practice that is using any AI tool, even something as low-risk as appointment scheduling,” she said.
Physician groups may be concerned about the costs and logistics of integrating ChatGPT with their electronic health record systems (EHRs) and how that would work, said Ms. Plested.
Doctors may also be skeptical of AI based on their experience with EHRs, she said.
“They were promoted as a panacea to many problems; they were supposed to automate business practice, reduce staff and clinician’s work, and improve billing/coding/documentation. Unfortunately, they have become a major source of frustration for doctors,” said Ms. Plested.
Drawing the line at patient care
Patients are worried about their doctors relying on AI for their care, according to a Pew Research Center poll released in February. About 60% of U.S. adults say they would feel uncomfortable if their own health care professional relied on artificial intelligence to do things like diagnose disease and recommend treatments; about 40% say they would feel comfortable with this.
“We have not yet gone into using ChatGPT for clinical purposes and will be very cautious with these types of applications due to concerns about inaccuracies,” Dr. Choi said.
Practice leaders reported in the MGMA poll that the most common uses of AI were nonclinical, such as:
- Patient communications, including call center answering service to help triage calls, to sort/distribute incoming fax messages, and outreach such as appointment reminders and marketing materials.
- Capturing clinical documentation, often with natural language processing or speech recognition platforms to help virtually scribe.
- Improving billing operations and predictive analytics.
Some doctors told The New York Times that ChatGPT helped them communicate with patients in a more compassionate way.
They used chatbots “to find words to break bad news and express concerns about a patient’s suffering, or to just more clearly explain medical recommendations,” the story noted.
Is regulation needed?
Some legal scholars and medical groups say that AI should be regulated to protect patients and doctors from risks, including medical errors, that could harm patients.
“It’s very important to evaluate the accuracy, safety, and privacy of language learning models (LLMs) before integrating them into the medical system. The same should be true of any new medical tool,” said Mason Marks, MD, JD, a health law professor at the Florida State University College of Law in Tallahassee.
In mid-June, the American Medical Association approved two resolutions calling for greater government oversight of AI. The AMA will develop proposed state and federal regulations and work with the federal government and other organizations to protect patients from false or misleading AI-generated medical advice.
Dr. Marks pointed to existing federal rules that apply to AI. “The Federal Trade Commission already has regulation that can potentially be used to combat unfair or deceptive trade practices associated with chatbots,” he said.
In addition, “the U.S. Food and Drug Administration can also regulate these tools, but it needs to update how it approaches risk when it comes to AI. The FDA has an outdated view of risk as physical harm, for instance, from traditional medical devices. That view of risk needs to be updated and expanded to encompass the unique harms of AI,” Dr. Marks said.
There should also be more transparency about how LLM software is used in medicine, he said. “That could be a norm implemented by the LLM developers and it could also be enforced by federal agencies. For instance, the FDA could require developers to be more transparent regarding training data and methods, and the FTC could require greater transparency regarding how consumer data might be used and opportunities to opt out of certain uses,” said Dr. Marks.
What should doctors do?
Dr. Marks advised doctors to be cautious when using ChatGPT and other LLMs, especially for medical advice. “The same would apply to any new medical tool, but we know that the current generation of LLMs [is] particularly prone to making things up, which could lead to medical errors if relied on in clinical settings,” he said.
There is also potential for breaches of patient confidentiality if doctors input clinical information. ChatGPT and OpenAI-enabled tools may not be compliant with the Health Insurance Portability and Accountability Act, which set national standards to protect individuals’ medical records and individually identifiable health information.
“The best approach is to use chatbots cautiously and with skepticism. Don’t input patient information, confirm the accuracy of information produced, and don’t use them as replacements for professional judgment,” Dr. Marks recommended.
Ms. Plested suggested that doctors who want to experiment with AI start with a low-risk tool such as appointment reminders that could save staff time and money. “I never recommend they start with something as high-stakes as coding/billing,” she said.
A version of this article appeared on Medscape.com.
Your practice was bought out by private equity: Now what?
After her emergency medicine group was acquired by a staffing firm backed by a large private equity (PE) firm, Michelle Wiener, MD, said the workflow changes came swiftly.
“Our staffing has been greatly reduced,” the Detroit physician said. “At this point, we have no say in anything. We have no say in the scheduling. We aren’t allowed to see what is billed under our name. The morale has really gone down.”
Dr. Wiener, who practices at Ascension St. John Hospital, said she and fellow physicians have repeatedly brought their concerns to TeamHealth, which in 2015 took over St. John Emergency Services PC. TeamHealth is owned by PE giant Blackstone.
“It’s very frustrating,” Dr. Wiener said. “We’re taking it from all sides.”
Blackstone and Ascension St. John did not respond to this news organization’s request for comment.
TeamHealth would not respond directly to questions about the Ascension St. John Hospital physicians or their concerns.
Spokesman Josh Hopson provided only a general statement: “TeamHealth is committed to making sure that clinicians have the resources and support needed to provide first-class care to patients, particularly with regard to staffing and compensation. TeamHealth has and will always put patient care first, and that is not impacted by its ownership model.”
Acquisitions of medical practices and hospitals by PE firms are rapidly growing, with more than 1,400 PE deals in health care in 2021 totaling upwards of $208 billion, according to PitchBook Data Inc., a Seattle-based firm that tracks mergers and acquisitions.
Some physicians praise the partnerships as an opportunity to improve technology and efficiency, whereas others decry them as raising patient costs and lowering the quality of care. A recent UC Berkeley study found that PE ownership of medical practices was linked to consumer price increases for 8 of 10 specialties, most notably oncology and gastroenterology.
What should you expect after PE acquisition?
Since his practice partnered with a PE firm in 2020, Milwaukee-based otolaryngologist Madan Kandula, MD, said he has found the changes positive. The practice has grown and improved operations in finance, accounting, compliance, and information technology, said Dr. Kandula, founder and CEO of Advent, an ENT practice with 15 clinics in four Midwestern states.
Dr. Kandula said his group already had a sound business practice, and that the goal of partnering with a PE firm wasn’t to change day-to-day operations but to propel the organization forward.
“From patient load to visit time to how we staff our clinics, there has been no change,” he said. “My private equity firm does not, [and] cannot, impose their will on our clinical decisions.”
Experts say the impact of PE acquisitions on individual physicians often depends on where a doctor ranks in the organization, what stage they are in their career, and how much control they had over the deal.
“It’s the older physicians who are usually selling the practice and getting the big payout,” said Anjali Dooley, a St. Louis–based health law attorney who counsels physicians about PE deals. “The younger doctors are usually not part of the deal, as they may still be employees. They don’t have any negotiating power. Hopefully, there is some transparency, but sometimes there is not, and they are blindsided by the deal.”
When it comes to workload, most PE-owned groups are put on a production-based model, such as a wRVU-based model, said Roger Strode, a Chicago-based health law attorney who focuses on health care mergers and acquisitions. Most already operate under such a model, but there might be some changes after a buyout.
Staffing may also change, added Ms. Dooley. The PE firms may want to add partners or companies already in their portfolios to create efficiencies, causing training or workflow changes.
In a hospital buyout, changes may depend on whether a department is a significant revenue generator for the hospital, Ms. Dooley noted.
PE firms frequently favor higher revenue–generating specialties, such as neurosurgery, cardiology, orthopedics, gastroenterology, and plastic surgery. They closely scrutinize departments said that make less money, such as the emergency department or primary care, Ms. Dooley said. Physicians or teams that don’t fit the firm’s cost-efficiency plans may be terminated or replaced.
On the other hand, Mr. Strode said physicians may see improved electronic health records and collections.
“Some of your overall overhead costs may be reduced, because they’re better at it,” Mr. Strode said. “When you’ve got more scale, the cost per patient, the cost per hour, the cost per procedure, goes down, and the cost that’s applied against your production will go down. As [practices grow], they have more bargaining power with payers and you can potentially get better rates. At least, that’s the promise.”
Analysts note that PE health care acquisitions show no signs of slowing and that it pays for physicians to know what to expect and how to cope if their practice or hospital is acquired. Whether physicians have some control over a buyout or are blindsided by the transition, it’s critical to know what to consider, how workloads might change, and your options for settling in or settling up.
The PE industry has about $2 trillion lined up for potential investments in 2023, said Ms. Dooley.
“PE firms are looking at health care to expend some of this dry powder,” Ms. Dooley continued. “If done correctly, PE firms that are aware of health care regulations, compliance, and patient care issues can ... remove redundant services and improve ... efficiencies, but the bad is when that doesn’t happen, and the quality of care goes down or there are patient safety risks.”
How to prepare for and cope with PE partnerships
If your practice is considering a PE partnership, it’s important to explore the terms and conditions and carefully weigh the pros and cons, said Gary Herschman, a Newark, N.J.–based attorney who advises PE-owned physician groups.
“My recommendation is that physicians at a minimum conduct due diligence on all potential strategic options for their groups, and then make an informed decision regarding whether a partnership transaction is right for their group, as it’s not right for every group,” he said.
When Texas cardiologist Rick Snyder, MD, was considering PE partnerships, he spoke with physicians who made similar deals to determine whether they were satisfied years later, he said. In April, Snyder’s practice, HeartPlace, the largest physician-owned cardiology practice in Texas, was acquired by US Heart & Vascular, a practice management platform backed by PE firm Ares Management.
“I called every group that I knew that had done private equity for any meaningful amount of time,” Dr. Snyder said. “For the first year or two, everybody is in the honeymoon period. If the model is going to succeed or break down, it’s not going to be in the first year or two. So I wanted to talk to groups that had done this for a longer amount of time and find out what their pitfalls were. What would they have done differently? Has it been a productive relationship? Did they grow?”
Dr. Snyder, president of the Texas Medical Association, said his practice met with seven or eight firms before choosing one that best met their needs. His group wanted a platform that preserved their clinical autonomy, governance, and culture, he said. They also wanted to ensure they were not entering into a “buy and flip” scenario, but rather a “buy and build” plan.
“Thus, financial capital was not sufficient, they also had to have intellectual capital and relationship capital on their bench,” he said. “When we found the partner that embraced all of these factors as well as a history of buying and long-term building, we pulled the trigger and partnered with Ares and US Heart & Vascular Management. The partner we chose did not offer us the most money. We put a premium on these other criteria.”
“I always tell docs, know the culture of your group and your vision,” he said. “Before you go down that route, ask yourself what you want to accomplish and if it makes sense having a private equity partner to accomplish that vision with.”
For younger physicians or those with little control over buyouts, experts recommend they review their contracts and consider consulting with an attorney to better understand how the deal may affect their earnings and career prospects.
Those who have a much longer career runway need to weigh whether they want to work for a PE-linked practice, Mr. Strode said. For some, it’s time to check when their noncompete agreements end and find a position elsewhere.
Also, physicians should know their rights and the laws in their states regarding the corporate practice of medicine. Statutes vary by state, and knowing the provisions in your state helps doctors recognize their legal rights, learn possible exceptions to the requirements, and know the penalties for violations.
In Michigan, a group of physicians and other health professionals at Ascension St. John has voted to unionize. Doctors hope that the union, which includes advanced practice clinicians, nurse practitioners, and physician assistants, will help improve patient care and protect working conditions for staff, Dr. Wiener said.
She advises physicians who are unhappy after acquisitions to speak up and stick together.
“That’s the biggest thing I think physicians should start doing,” she said. “Support each other and stand up. You are stronger together.”
Why is PE so attracted to health care?
PE firms typically buy practices or hospitals, work to make the entities more profitable, and then sell them, with the goal of doubling or tripling their investment over a short period. In general, PE firms aim for annual returns exceeding 20% after 3-7 years.
These firms know that health care is relatively recession-proof, that providers have third-party payers, and that the industry is fragmented and requires more efficiency, Ms. Dooley said.
When PE practice acquisitions started gaining momentum about 12 years ago, traditional hospital-based specialties such as anesthesiology and radiology were prime targets, said Mr. Strode.
At the same time, increasing challenges in private practice, such as declining compensation from payers, pressure to participate in value-based care programs, and rising regional competitors have fueled more physician groups to partner with PE firms, Mr. Herschman noted.
Physicians who partner with PE firms often benefit by having new access to capital to grow their practices, cost savings through group purchasing, and the ability to compete with larger health groups, Mr. Herschman said.
Questions remain, however, about how PE involvement affects health care use and spending. An April 2023 JAMA Viewpoint article called out the lack of oversight and regulation in the health care/PE space, suggesting that a stronger framework for regulation and transparency is needed.
A 2022 study in JAMA Health Forum that examined changes in prices and utilization associated with the PE acquisitions of 578 dermatology, gastroenterology, and ophthalmology physician practices from 2016 to 2020 found that prices increased by an average of 11%, and volume rose by 16%, after acquisition.
“We found that acquisitions were associated with increases in health care spending and utilization, as well as some other patterns of care like potential upcoding,” said Jane M. Zhu, MD, an author of the study and assistant professor at Oregon Health & Science University in Portland.
Another recent study that Dr. Zhu coauthored, published in Health Affairs, found that physician practices acquired by PE firms experience greater staff turnover and rely more heavily on advanced practice professionals than doctors.
“To the extent that that turnover indicates physicians are dissatisfied after private equity comes in, that’s really important to investigate further,” Dr. Zhu said.
PE firms owned 4% of U.S. hospitals in 2021 and 11% of nursing homes, according to a Medicare Payment Advisory Commission (MedPAC) report. The report does not include 2021 data on medical practices but notes that from 2013 to 2016, PE firms acquired at least 2% of physician practices. Estimates of PE deals are probably lower than actual numbers because of the lack of comprehensive information sources, according to the MedPAC report.
A version of this article appeared on Medscape.com.
After her emergency medicine group was acquired by a staffing firm backed by a large private equity (PE) firm, Michelle Wiener, MD, said the workflow changes came swiftly.
“Our staffing has been greatly reduced,” the Detroit physician said. “At this point, we have no say in anything. We have no say in the scheduling. We aren’t allowed to see what is billed under our name. The morale has really gone down.”
Dr. Wiener, who practices at Ascension St. John Hospital, said she and fellow physicians have repeatedly brought their concerns to TeamHealth, which in 2015 took over St. John Emergency Services PC. TeamHealth is owned by PE giant Blackstone.
“It’s very frustrating,” Dr. Wiener said. “We’re taking it from all sides.”
Blackstone and Ascension St. John did not respond to this news organization’s request for comment.
TeamHealth would not respond directly to questions about the Ascension St. John Hospital physicians or their concerns.
Spokesman Josh Hopson provided only a general statement: “TeamHealth is committed to making sure that clinicians have the resources and support needed to provide first-class care to patients, particularly with regard to staffing and compensation. TeamHealth has and will always put patient care first, and that is not impacted by its ownership model.”
Acquisitions of medical practices and hospitals by PE firms are rapidly growing, with more than 1,400 PE deals in health care in 2021 totaling upwards of $208 billion, according to PitchBook Data Inc., a Seattle-based firm that tracks mergers and acquisitions.
Some physicians praise the partnerships as an opportunity to improve technology and efficiency, whereas others decry them as raising patient costs and lowering the quality of care. A recent UC Berkeley study found that PE ownership of medical practices was linked to consumer price increases for 8 of 10 specialties, most notably oncology and gastroenterology.
What should you expect after PE acquisition?
Since his practice partnered with a PE firm in 2020, Milwaukee-based otolaryngologist Madan Kandula, MD, said he has found the changes positive. The practice has grown and improved operations in finance, accounting, compliance, and information technology, said Dr. Kandula, founder and CEO of Advent, an ENT practice with 15 clinics in four Midwestern states.
Dr. Kandula said his group already had a sound business practice, and that the goal of partnering with a PE firm wasn’t to change day-to-day operations but to propel the organization forward.
“From patient load to visit time to how we staff our clinics, there has been no change,” he said. “My private equity firm does not, [and] cannot, impose their will on our clinical decisions.”
Experts say the impact of PE acquisitions on individual physicians often depends on where a doctor ranks in the organization, what stage they are in their career, and how much control they had over the deal.
“It’s the older physicians who are usually selling the practice and getting the big payout,” said Anjali Dooley, a St. Louis–based health law attorney who counsels physicians about PE deals. “The younger doctors are usually not part of the deal, as they may still be employees. They don’t have any negotiating power. Hopefully, there is some transparency, but sometimes there is not, and they are blindsided by the deal.”
When it comes to workload, most PE-owned groups are put on a production-based model, such as a wRVU-based model, said Roger Strode, a Chicago-based health law attorney who focuses on health care mergers and acquisitions. Most already operate under such a model, but there might be some changes after a buyout.
Staffing may also change, added Ms. Dooley. The PE firms may want to add partners or companies already in their portfolios to create efficiencies, causing training or workflow changes.
In a hospital buyout, changes may depend on whether a department is a significant revenue generator for the hospital, Ms. Dooley noted.
PE firms frequently favor higher revenue–generating specialties, such as neurosurgery, cardiology, orthopedics, gastroenterology, and plastic surgery. They closely scrutinize departments said that make less money, such as the emergency department or primary care, Ms. Dooley said. Physicians or teams that don’t fit the firm’s cost-efficiency plans may be terminated or replaced.
On the other hand, Mr. Strode said physicians may see improved electronic health records and collections.
“Some of your overall overhead costs may be reduced, because they’re better at it,” Mr. Strode said. “When you’ve got more scale, the cost per patient, the cost per hour, the cost per procedure, goes down, and the cost that’s applied against your production will go down. As [practices grow], they have more bargaining power with payers and you can potentially get better rates. At least, that’s the promise.”
Analysts note that PE health care acquisitions show no signs of slowing and that it pays for physicians to know what to expect and how to cope if their practice or hospital is acquired. Whether physicians have some control over a buyout or are blindsided by the transition, it’s critical to know what to consider, how workloads might change, and your options for settling in or settling up.
The PE industry has about $2 trillion lined up for potential investments in 2023, said Ms. Dooley.
“PE firms are looking at health care to expend some of this dry powder,” Ms. Dooley continued. “If done correctly, PE firms that are aware of health care regulations, compliance, and patient care issues can ... remove redundant services and improve ... efficiencies, but the bad is when that doesn’t happen, and the quality of care goes down or there are patient safety risks.”
How to prepare for and cope with PE partnerships
If your practice is considering a PE partnership, it’s important to explore the terms and conditions and carefully weigh the pros and cons, said Gary Herschman, a Newark, N.J.–based attorney who advises PE-owned physician groups.
“My recommendation is that physicians at a minimum conduct due diligence on all potential strategic options for their groups, and then make an informed decision regarding whether a partnership transaction is right for their group, as it’s not right for every group,” he said.
When Texas cardiologist Rick Snyder, MD, was considering PE partnerships, he spoke with physicians who made similar deals to determine whether they were satisfied years later, he said. In April, Snyder’s practice, HeartPlace, the largest physician-owned cardiology practice in Texas, was acquired by US Heart & Vascular, a practice management platform backed by PE firm Ares Management.
“I called every group that I knew that had done private equity for any meaningful amount of time,” Dr. Snyder said. “For the first year or two, everybody is in the honeymoon period. If the model is going to succeed or break down, it’s not going to be in the first year or two. So I wanted to talk to groups that had done this for a longer amount of time and find out what their pitfalls were. What would they have done differently? Has it been a productive relationship? Did they grow?”
Dr. Snyder, president of the Texas Medical Association, said his practice met with seven or eight firms before choosing one that best met their needs. His group wanted a platform that preserved their clinical autonomy, governance, and culture, he said. They also wanted to ensure they were not entering into a “buy and flip” scenario, but rather a “buy and build” plan.
“Thus, financial capital was not sufficient, they also had to have intellectual capital and relationship capital on their bench,” he said. “When we found the partner that embraced all of these factors as well as a history of buying and long-term building, we pulled the trigger and partnered with Ares and US Heart & Vascular Management. The partner we chose did not offer us the most money. We put a premium on these other criteria.”
“I always tell docs, know the culture of your group and your vision,” he said. “Before you go down that route, ask yourself what you want to accomplish and if it makes sense having a private equity partner to accomplish that vision with.”
For younger physicians or those with little control over buyouts, experts recommend they review their contracts and consider consulting with an attorney to better understand how the deal may affect their earnings and career prospects.
Those who have a much longer career runway need to weigh whether they want to work for a PE-linked practice, Mr. Strode said. For some, it’s time to check when their noncompete agreements end and find a position elsewhere.
Also, physicians should know their rights and the laws in their states regarding the corporate practice of medicine. Statutes vary by state, and knowing the provisions in your state helps doctors recognize their legal rights, learn possible exceptions to the requirements, and know the penalties for violations.
In Michigan, a group of physicians and other health professionals at Ascension St. John has voted to unionize. Doctors hope that the union, which includes advanced practice clinicians, nurse practitioners, and physician assistants, will help improve patient care and protect working conditions for staff, Dr. Wiener said.
She advises physicians who are unhappy after acquisitions to speak up and stick together.
“That’s the biggest thing I think physicians should start doing,” she said. “Support each other and stand up. You are stronger together.”
Why is PE so attracted to health care?
PE firms typically buy practices or hospitals, work to make the entities more profitable, and then sell them, with the goal of doubling or tripling their investment over a short period. In general, PE firms aim for annual returns exceeding 20% after 3-7 years.
These firms know that health care is relatively recession-proof, that providers have third-party payers, and that the industry is fragmented and requires more efficiency, Ms. Dooley said.
When PE practice acquisitions started gaining momentum about 12 years ago, traditional hospital-based specialties such as anesthesiology and radiology were prime targets, said Mr. Strode.
At the same time, increasing challenges in private practice, such as declining compensation from payers, pressure to participate in value-based care programs, and rising regional competitors have fueled more physician groups to partner with PE firms, Mr. Herschman noted.
Physicians who partner with PE firms often benefit by having new access to capital to grow their practices, cost savings through group purchasing, and the ability to compete with larger health groups, Mr. Herschman said.
Questions remain, however, about how PE involvement affects health care use and spending. An April 2023 JAMA Viewpoint article called out the lack of oversight and regulation in the health care/PE space, suggesting that a stronger framework for regulation and transparency is needed.
A 2022 study in JAMA Health Forum that examined changes in prices and utilization associated with the PE acquisitions of 578 dermatology, gastroenterology, and ophthalmology physician practices from 2016 to 2020 found that prices increased by an average of 11%, and volume rose by 16%, after acquisition.
“We found that acquisitions were associated with increases in health care spending and utilization, as well as some other patterns of care like potential upcoding,” said Jane M. Zhu, MD, an author of the study and assistant professor at Oregon Health & Science University in Portland.
Another recent study that Dr. Zhu coauthored, published in Health Affairs, found that physician practices acquired by PE firms experience greater staff turnover and rely more heavily on advanced practice professionals than doctors.
“To the extent that that turnover indicates physicians are dissatisfied after private equity comes in, that’s really important to investigate further,” Dr. Zhu said.
PE firms owned 4% of U.S. hospitals in 2021 and 11% of nursing homes, according to a Medicare Payment Advisory Commission (MedPAC) report. The report does not include 2021 data on medical practices but notes that from 2013 to 2016, PE firms acquired at least 2% of physician practices. Estimates of PE deals are probably lower than actual numbers because of the lack of comprehensive information sources, according to the MedPAC report.
A version of this article appeared on Medscape.com.
After her emergency medicine group was acquired by a staffing firm backed by a large private equity (PE) firm, Michelle Wiener, MD, said the workflow changes came swiftly.
“Our staffing has been greatly reduced,” the Detroit physician said. “At this point, we have no say in anything. We have no say in the scheduling. We aren’t allowed to see what is billed under our name. The morale has really gone down.”
Dr. Wiener, who practices at Ascension St. John Hospital, said she and fellow physicians have repeatedly brought their concerns to TeamHealth, which in 2015 took over St. John Emergency Services PC. TeamHealth is owned by PE giant Blackstone.
“It’s very frustrating,” Dr. Wiener said. “We’re taking it from all sides.”
Blackstone and Ascension St. John did not respond to this news organization’s request for comment.
TeamHealth would not respond directly to questions about the Ascension St. John Hospital physicians or their concerns.
Spokesman Josh Hopson provided only a general statement: “TeamHealth is committed to making sure that clinicians have the resources and support needed to provide first-class care to patients, particularly with regard to staffing and compensation. TeamHealth has and will always put patient care first, and that is not impacted by its ownership model.”
Acquisitions of medical practices and hospitals by PE firms are rapidly growing, with more than 1,400 PE deals in health care in 2021 totaling upwards of $208 billion, according to PitchBook Data Inc., a Seattle-based firm that tracks mergers and acquisitions.
Some physicians praise the partnerships as an opportunity to improve technology and efficiency, whereas others decry them as raising patient costs and lowering the quality of care. A recent UC Berkeley study found that PE ownership of medical practices was linked to consumer price increases for 8 of 10 specialties, most notably oncology and gastroenterology.
What should you expect after PE acquisition?
Since his practice partnered with a PE firm in 2020, Milwaukee-based otolaryngologist Madan Kandula, MD, said he has found the changes positive. The practice has grown and improved operations in finance, accounting, compliance, and information technology, said Dr. Kandula, founder and CEO of Advent, an ENT practice with 15 clinics in four Midwestern states.
Dr. Kandula said his group already had a sound business practice, and that the goal of partnering with a PE firm wasn’t to change day-to-day operations but to propel the organization forward.
“From patient load to visit time to how we staff our clinics, there has been no change,” he said. “My private equity firm does not, [and] cannot, impose their will on our clinical decisions.”
Experts say the impact of PE acquisitions on individual physicians often depends on where a doctor ranks in the organization, what stage they are in their career, and how much control they had over the deal.
“It’s the older physicians who are usually selling the practice and getting the big payout,” said Anjali Dooley, a St. Louis–based health law attorney who counsels physicians about PE deals. “The younger doctors are usually not part of the deal, as they may still be employees. They don’t have any negotiating power. Hopefully, there is some transparency, but sometimes there is not, and they are blindsided by the deal.”
When it comes to workload, most PE-owned groups are put on a production-based model, such as a wRVU-based model, said Roger Strode, a Chicago-based health law attorney who focuses on health care mergers and acquisitions. Most already operate under such a model, but there might be some changes after a buyout.
Staffing may also change, added Ms. Dooley. The PE firms may want to add partners or companies already in their portfolios to create efficiencies, causing training or workflow changes.
In a hospital buyout, changes may depend on whether a department is a significant revenue generator for the hospital, Ms. Dooley noted.
PE firms frequently favor higher revenue–generating specialties, such as neurosurgery, cardiology, orthopedics, gastroenterology, and plastic surgery. They closely scrutinize departments said that make less money, such as the emergency department or primary care, Ms. Dooley said. Physicians or teams that don’t fit the firm’s cost-efficiency plans may be terminated or replaced.
On the other hand, Mr. Strode said physicians may see improved electronic health records and collections.
“Some of your overall overhead costs may be reduced, because they’re better at it,” Mr. Strode said. “When you’ve got more scale, the cost per patient, the cost per hour, the cost per procedure, goes down, and the cost that’s applied against your production will go down. As [practices grow], they have more bargaining power with payers and you can potentially get better rates. At least, that’s the promise.”
Analysts note that PE health care acquisitions show no signs of slowing and that it pays for physicians to know what to expect and how to cope if their practice or hospital is acquired. Whether physicians have some control over a buyout or are blindsided by the transition, it’s critical to know what to consider, how workloads might change, and your options for settling in or settling up.
The PE industry has about $2 trillion lined up for potential investments in 2023, said Ms. Dooley.
“PE firms are looking at health care to expend some of this dry powder,” Ms. Dooley continued. “If done correctly, PE firms that are aware of health care regulations, compliance, and patient care issues can ... remove redundant services and improve ... efficiencies, but the bad is when that doesn’t happen, and the quality of care goes down or there are patient safety risks.”
How to prepare for and cope with PE partnerships
If your practice is considering a PE partnership, it’s important to explore the terms and conditions and carefully weigh the pros and cons, said Gary Herschman, a Newark, N.J.–based attorney who advises PE-owned physician groups.
“My recommendation is that physicians at a minimum conduct due diligence on all potential strategic options for their groups, and then make an informed decision regarding whether a partnership transaction is right for their group, as it’s not right for every group,” he said.
When Texas cardiologist Rick Snyder, MD, was considering PE partnerships, he spoke with physicians who made similar deals to determine whether they were satisfied years later, he said. In April, Snyder’s practice, HeartPlace, the largest physician-owned cardiology practice in Texas, was acquired by US Heart & Vascular, a practice management platform backed by PE firm Ares Management.
“I called every group that I knew that had done private equity for any meaningful amount of time,” Dr. Snyder said. “For the first year or two, everybody is in the honeymoon period. If the model is going to succeed or break down, it’s not going to be in the first year or two. So I wanted to talk to groups that had done this for a longer amount of time and find out what their pitfalls were. What would they have done differently? Has it been a productive relationship? Did they grow?”
Dr. Snyder, president of the Texas Medical Association, said his practice met with seven or eight firms before choosing one that best met their needs. His group wanted a platform that preserved their clinical autonomy, governance, and culture, he said. They also wanted to ensure they were not entering into a “buy and flip” scenario, but rather a “buy and build” plan.
“Thus, financial capital was not sufficient, they also had to have intellectual capital and relationship capital on their bench,” he said. “When we found the partner that embraced all of these factors as well as a history of buying and long-term building, we pulled the trigger and partnered with Ares and US Heart & Vascular Management. The partner we chose did not offer us the most money. We put a premium on these other criteria.”
“I always tell docs, know the culture of your group and your vision,” he said. “Before you go down that route, ask yourself what you want to accomplish and if it makes sense having a private equity partner to accomplish that vision with.”
For younger physicians or those with little control over buyouts, experts recommend they review their contracts and consider consulting with an attorney to better understand how the deal may affect their earnings and career prospects.
Those who have a much longer career runway need to weigh whether they want to work for a PE-linked practice, Mr. Strode said. For some, it’s time to check when their noncompete agreements end and find a position elsewhere.
Also, physicians should know their rights and the laws in their states regarding the corporate practice of medicine. Statutes vary by state, and knowing the provisions in your state helps doctors recognize their legal rights, learn possible exceptions to the requirements, and know the penalties for violations.
In Michigan, a group of physicians and other health professionals at Ascension St. John has voted to unionize. Doctors hope that the union, which includes advanced practice clinicians, nurse practitioners, and physician assistants, will help improve patient care and protect working conditions for staff, Dr. Wiener said.
She advises physicians who are unhappy after acquisitions to speak up and stick together.
“That’s the biggest thing I think physicians should start doing,” she said. “Support each other and stand up. You are stronger together.”
Why is PE so attracted to health care?
PE firms typically buy practices or hospitals, work to make the entities more profitable, and then sell them, with the goal of doubling or tripling their investment over a short period. In general, PE firms aim for annual returns exceeding 20% after 3-7 years.
These firms know that health care is relatively recession-proof, that providers have third-party payers, and that the industry is fragmented and requires more efficiency, Ms. Dooley said.
When PE practice acquisitions started gaining momentum about 12 years ago, traditional hospital-based specialties such as anesthesiology and radiology were prime targets, said Mr. Strode.
At the same time, increasing challenges in private practice, such as declining compensation from payers, pressure to participate in value-based care programs, and rising regional competitors have fueled more physician groups to partner with PE firms, Mr. Herschman noted.
Physicians who partner with PE firms often benefit by having new access to capital to grow their practices, cost savings through group purchasing, and the ability to compete with larger health groups, Mr. Herschman said.
Questions remain, however, about how PE involvement affects health care use and spending. An April 2023 JAMA Viewpoint article called out the lack of oversight and regulation in the health care/PE space, suggesting that a stronger framework for regulation and transparency is needed.
A 2022 study in JAMA Health Forum that examined changes in prices and utilization associated with the PE acquisitions of 578 dermatology, gastroenterology, and ophthalmology physician practices from 2016 to 2020 found that prices increased by an average of 11%, and volume rose by 16%, after acquisition.
“We found that acquisitions were associated with increases in health care spending and utilization, as well as some other patterns of care like potential upcoding,” said Jane M. Zhu, MD, an author of the study and assistant professor at Oregon Health & Science University in Portland.
Another recent study that Dr. Zhu coauthored, published in Health Affairs, found that physician practices acquired by PE firms experience greater staff turnover and rely more heavily on advanced practice professionals than doctors.
“To the extent that that turnover indicates physicians are dissatisfied after private equity comes in, that’s really important to investigate further,” Dr. Zhu said.
PE firms owned 4% of U.S. hospitals in 2021 and 11% of nursing homes, according to a Medicare Payment Advisory Commission (MedPAC) report. The report does not include 2021 data on medical practices but notes that from 2013 to 2016, PE firms acquired at least 2% of physician practices. Estimates of PE deals are probably lower than actual numbers because of the lack of comprehensive information sources, according to the MedPAC report.
A version of this article appeared on Medscape.com.
Battling pediatric cancer outcome disparities, new interventions aim to close gaps
Pediatric oncologist Lena Winestone, MD, recalls treating a 2-year-old leukemia patient who underwent a bone marrow transplant as her only chance for a cure.
The girl’s family, who spoke only Spanish and struggled with literacy, could not pay their rent or afford the girl’s weekly transportation to the hospital for after-transplant care. The family had three other children and lived more than 2 hours from the transplant center, remembers Dr. Winestone, an assistant professor of pediatrics in the division of malignancies and bone & marrow transplant at the University of California, San Francisco.
The hospital’s social worker was able to secure grant support for the family’s housing and worked with the patient’s insurance to arrange for transportation. However, the departure times were rigid, Dr. Winestone said, and the family sometimes had to leave the hospital before the child’s graft vs. host disease (GvHD) treatment was complete for the day.
“If we had not finished her treatment, we had to disconnect her from the machine early,” Dr. Winestone said. “Her mother also had to load her oxygen tanks [three of them], her BiPAP machine, and her tube feeds into the transportation every week in order to make sure she could be safely transported. She was treated for GvHD for almost 2 years, but unfortunately, her GvHD started to affect her lungs and ultimately, she passed away.”
Dr. Winestone says it’s difficult to know whether the girl’s death was directly related to her socioeconomic status, but that it certainly made all aspects of the child’s care more complicated and forced health care providers to adapt her cancer care to accommodate the family’s circumstances.
This story is one of countless cases where socioeconomic status impacted a young patient’s cancer care and likely contributed to a worse outcome.
A 2022 study for example, found that children from marginalized racial/ethnic groups and those living in poverty were more likely to have inferior 5-year overall survival, compared with other children, even when assigned to receive the same initial treatment. Of 696 children with high-risk neuroblastoma, 47% of Hispanic children had a 5-year overall survival (OS), compared with 50% for other non-Hispanic children, and 61% for white non-Hispanic patients. Children on public health insurance (a proxy for household poverty) had a 53% 5-year OS, compared with 63% for children unexposed to household poverty. Pediatric patients exposed to neighborhood poverty had a 54% 5-year OS, compared with 62% for unexposed children.
In another study, children with acute lymphoblastic leukemia who lived in high-poverty areas were more likely to experience early relapse than other patients, despite having the same treatment. Of the 575 children studied, 92% of children from high-poverty areas who relapsed, experienced early relapse, defined as less than 36 months after remission. By comparison, only 48% of other children who relapsed experienced early relapse.
Reasons behind the relapse and survival disparities are multifold, which has led to challenges in addressing the gaps and improving cancer outcomes for poverty-stricken children. A research infrastructure that is largely based on biological, rather than social determinants of health, acts as another barrier, oncologists say.
Historically, interventions to address disparities in pediatric oncology have never been evaluated, said Kira Bona, MD, MPH, a pediatric oncologist at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. This is in large part because the body of literature illustrating the disparities is relatively new, said Dr. Bona, whose research focuses on poverty-associated outcome disparities in childhood cancer.
However, new efforts aim to change this landscape by using the growing data to develop and analyze possible interventions. A set of three novel interventions led by Dr. Bona and her research team are in the works, some of which have shown promise in early studies.
“Now is the time to begin to actively intervene on disparities in childhood cancer,” Dr. Bona said. “We’re really good at studying genetic mutations in cancer cells that might lead to a risk of relapse, and when we identify those mutations, what we do is intervene. We try new chemotherapy agents, new ways of delivering therapy. We are now at the point where we have identified that social determinants of health may be equally ‘risky’ but we haven’t taken the next step to begin intervening in the same way.”
What is causing disparities in pediatric cancer outcomes?
Lack of access to the health care system is a top contributor to the disparities, although there is no single root cause, said Sharon Castellino, MD, director of the Leukemia and Lymphoma Program at the Aflac Cancer & Blood Disorders Center of Children’s Healthcare of Atlanta, and a professor in the department of pediatrics at Emory University, Atlanta.
Even before cancer diagnosis, Dr. Castellino notes that many children of color and/or of lower socioeconomic status are not receiving regular health care, leading to sicker children and more advanced-stage cancer by the time they are diagnosed.
Lack of insurance is a primary barrier to this access, adds Xu Ji, PhD, MSPH, an assistant professor in the department of pediatrics at Emory University and a member of the Cancer Prevention and Control Research Program at the university’s Winship Cancer Institute.
Studies have long shown that uninsured children are more likely to go without needed care, compared with those with private insurance. Patients of color are at much higher risk of being uninsured than White patients, with the uninsured rates for Hispanic, American Indian, and Alaska Native patients being more than 2.5 times higher than that of White patients.
“We all know that insurance is a strong predictor of health outcomes,” said Dr. Ji, whose research focuses on insurance disparities and gains among cancer patients. “Lack of insurance coverage and therefore lack of access to care along the pediatric cancer continuum from early detection to early diagnosis to timely initiation of treatment to receipt of high-quality treatment to access to recommended survivorship care and even access to palliative and end-of-life care are all very important constructs in the pathway from poverty to ultimate cancer outcomes for children.”
Unstable housing, employment difficulties, and lack of family support can also come into play. Dr. Castellino remembers the case of a 12-year-old cancer patient who entered treatment with advanced-stage Hodgkin Lymphoma. The girl came from a low-income, single-parent household without stable housing. Dr. Castellino said when the child was granted a wish from the Make-a-Wish Foundation, she asked for her own bed.
“We had been working with her every week for 6 months when that request came up,” she recalled. “We said, ‘You don’t have to wait for your make-a-wish, we can get you a bed now.’ We don’t even know the extent of what happens at home for many of these children.”
The impact of toxic stress on child cancer patients is an emerging area of research, said Dr. Winestone, whose research explores racial, ethnic, and socioeconomic disparities in access to care and outcomes of leukemia and lymphoma treatment. For example, Dr. Winestone’s research includes understanding how exposure to poverty or adverse experiences in childhood may influence a patient’s biological response to chemotherapy.
Other contributors to disparities include transportation issues, lack of childcare for other children, literacy, and language barriers. A 2016 study suggests that language barriers negatively impact the quality of informed decision-making and the care experience for Spanish-speaking parents of pediatric cancer patients with limited English proficiency.
Such access issues are also compounded by systemic factors, including a shortage of physicians of color who may be able to forge better trust relationships with families of similar race and ethnicity, Dr. Castellino adds. Lower enrollment of pediatric cancer patients with higher social vulnerabilities in clinical trials is another problem.
“In childhood cancer, I believe our improvements have been built on the backs of prior generations of families and children who have enrolled in trials. We learn things, and the next generation of therapy improves,” Dr. Castellino said. “If you have a whole group of the population not represented in trials, you don’t know what’s driving the fact they may or may not improve.”
Working toward solutions
With such a diverse set of factors fueling outcome gaps, a similarly diverse approach is needed to help bridge the divide, say disparity researchers.
To this end, Dr. Bona and her research team are currently building the first portfolio of health equity interventions, each designed to address a different adverse social determinant of health differently.
The Pediatric Cancer Resource Equity (PediCARE) intervention is a centrally delivered, household material hardship (HMH)–targeted intervention that provides transportation and groceries to low-income pediatric oncology families. The intervention was recently studied in a pilot, randomized, controlled trial at Dana-Farber Cancer Institute and the University of Alabama between May 2019 and August 2021.
Families were first screened for HMH and randomized into receiving either the intervention or usual care for 6 months. The intervention group received groceries via Instacart and transportation to and from the hospital coordinated through the Ride Health platform using Uber or Lyft. For families with their own cars, gas cards were provided. Of the families offered the chance to participate, 100% agreed to participate in the program, and there was 0% attrition in either arm of the program during the 6 months, according to the study findings, which were presented at the 2023 American Society of Clinical Oncology annual meeting in June.
Among families who received the PediCARE intervention, 100% successfully received grocery and transportation resources, 100% reported that it was “easier to buy food for my family,” 85% reported it was easier to get to and from the hospital, and 95% reported they would be “very likely to recommend the intervention to other families,” according to the results.
“The key takeaway is that we had excellent feasibility outcomes,” said Haley Newman, MD, lead author of the study and an attending physician in the division of oncology at The Children’s Hospital of Philadelphia. “From this study, we learned that PediCARE is accessible and feasible in very diverse settings. From this, what we really took away is that PediCARE could be successfully rolled out in a phase 3 randomized trial, which would be the best way to examine efficacy.”
Another initiative in its early stages is Pediatric RISE, a guaranteed income intervention being developed with support from the Children’s Cancer Research Fund, the American Cancer Society, and other donors. The intervention will provide unrestricted cash transfers to low-income families during the early months of chemotherapy, Dr. Bona said. Families are currently being enrolled in a pilot study with a goal of refining the intervention before it’s tested for feasibility and efficacy.
“The goal here is ultimately to evaluate the question: If we are able to successfully provide income support to low-income families going through childhood cancer treatment, might we be able to ameliorate some of the disparities associated with living in poverty that we have already described in childhood cancer,” Dr. Bona said.
Pediatric Assist, a developing intervention centering on benefits, is a third initiative that will soon be evaluated. The intervention will provide newly diagnosed families with systemic access to a centralized benefits counselor who can help them determine which existing government benefits they might be eligible for and assist them in navigating the application process.
“The idea here is that we know many lower-income families in the U.S. are eligible for existing supports, but may not be accessing them because of how incredibly difficult the system is to navigate,” Dr. Bona said. “For example, we know that low-income families may be eligible for SNAP benefits, but figuring out if you are eligible and then applying for SNAP involves multiple, complicated steps that are often infeasible for a parent when their child is admitted to the hospital with a newly diagnosed, life-threatening illness.”
Pilot refinement of the intervention is expected in the fall of 2023.
Overcoming barriers, addressing challenges
Investigators are also making headway in proving that collecting social determinants of health (SDoH) data during existing clinical trials is easily achievable.
Past Children’s Oncology Group trials have collected only race, ethnicity, insurance, and zip code data as proxies for exposure to adverse SDoH. Dr. Winestone and her colleagues recently investigated the feasibility and acceptability of the first COG trial to prospectively embed SDoH data collection.
Of eligible participants, 360 of 413 opted-in to the embedded SDOH aim across 101 COG sites (87.2% consent rate). Among participants, 316 surveys (87.8%) were completed a median of 11 days post enrollment, according to the findings, which were presented at the ASCO annual meeting.
“We’ve come to realize the importance of the social determinants of health [as it pertains] to outcomes, but it has been a process to learn how to effectively collect that data in a large collaborative environment,” said Dr. Winestone. “This abstract demonstrates that patients are very willing to provide this data, and they’re able to do it in an efficient way. People think of these questions as very sensitive and that families may not want to share the answers, but this study demonstrates those presumptions are false.”
The authors hope the findings fuel incorporation of SDoH data collection in future National Clinical Trials Network trials to inform impactful health equity research.
While such research and intervention efforts are gaining momentum, challenges to do the work remain. A lack of research funding and support are among the obstacles, Dr. Winestone said.
To date, much of pediatric cancer work has focused on developing new therapeutic approaches to reach a cure for more patients, she explained.
“While that’s incredibly essential, if we’re creating these approaches that only work for a subset of patients that have resources, we’re contributing to the inequities in the system,” Dr. Winestone said. “Really, [we need] dedicated support to studying how to make sure the interventions we know are effective are reaching all populations, and that the patients are poised to benefit from those interventions by setting them up for success.”
A strong research infrastructure exists to evaluate and support clinical drug trials in pediatric oncology, but the same does not exist for health equity interventions, Dr. Bona adds. A significant question that needs to be addressed is how best to integrate health equity evaluation into existing infrastructure or whether to build a parallel infrastructure.
Despite the challenges, Dr. Bona believes now is exactly the right time to investigate and intervene in poverty as a risk factor for childhood cancer relapse and outcomes. What has led to success in childhood cancer is how pediatric oncology has collaborated across the country to operate clinical drug trials at various centers, all in the same way, to identify which treatments work best, she said.
“We have an opportunity now in pediatrics to take advantage of this highly successful clinical trials research infrastructure to integrate interventions to address disparities in a way that has not been done previously,” she said. “The opportunity to significantly improve survival in childhood cancer by reducing disparities exists if we take this head on from a research and funding perspective and approach social risk factors just as we already know how to approach tumor genomic risk factors.”
Pediatric oncologist Lena Winestone, MD, recalls treating a 2-year-old leukemia patient who underwent a bone marrow transplant as her only chance for a cure.
The girl’s family, who spoke only Spanish and struggled with literacy, could not pay their rent or afford the girl’s weekly transportation to the hospital for after-transplant care. The family had three other children and lived more than 2 hours from the transplant center, remembers Dr. Winestone, an assistant professor of pediatrics in the division of malignancies and bone & marrow transplant at the University of California, San Francisco.
The hospital’s social worker was able to secure grant support for the family’s housing and worked with the patient’s insurance to arrange for transportation. However, the departure times were rigid, Dr. Winestone said, and the family sometimes had to leave the hospital before the child’s graft vs. host disease (GvHD) treatment was complete for the day.
“If we had not finished her treatment, we had to disconnect her from the machine early,” Dr. Winestone said. “Her mother also had to load her oxygen tanks [three of them], her BiPAP machine, and her tube feeds into the transportation every week in order to make sure she could be safely transported. She was treated for GvHD for almost 2 years, but unfortunately, her GvHD started to affect her lungs and ultimately, she passed away.”
Dr. Winestone says it’s difficult to know whether the girl’s death was directly related to her socioeconomic status, but that it certainly made all aspects of the child’s care more complicated and forced health care providers to adapt her cancer care to accommodate the family’s circumstances.
This story is one of countless cases where socioeconomic status impacted a young patient’s cancer care and likely contributed to a worse outcome.
A 2022 study for example, found that children from marginalized racial/ethnic groups and those living in poverty were more likely to have inferior 5-year overall survival, compared with other children, even when assigned to receive the same initial treatment. Of 696 children with high-risk neuroblastoma, 47% of Hispanic children had a 5-year overall survival (OS), compared with 50% for other non-Hispanic children, and 61% for white non-Hispanic patients. Children on public health insurance (a proxy for household poverty) had a 53% 5-year OS, compared with 63% for children unexposed to household poverty. Pediatric patients exposed to neighborhood poverty had a 54% 5-year OS, compared with 62% for unexposed children.
In another study, children with acute lymphoblastic leukemia who lived in high-poverty areas were more likely to experience early relapse than other patients, despite having the same treatment. Of the 575 children studied, 92% of children from high-poverty areas who relapsed, experienced early relapse, defined as less than 36 months after remission. By comparison, only 48% of other children who relapsed experienced early relapse.
Reasons behind the relapse and survival disparities are multifold, which has led to challenges in addressing the gaps and improving cancer outcomes for poverty-stricken children. A research infrastructure that is largely based on biological, rather than social determinants of health, acts as another barrier, oncologists say.
Historically, interventions to address disparities in pediatric oncology have never been evaluated, said Kira Bona, MD, MPH, a pediatric oncologist at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. This is in large part because the body of literature illustrating the disparities is relatively new, said Dr. Bona, whose research focuses on poverty-associated outcome disparities in childhood cancer.
However, new efforts aim to change this landscape by using the growing data to develop and analyze possible interventions. A set of three novel interventions led by Dr. Bona and her research team are in the works, some of which have shown promise in early studies.
“Now is the time to begin to actively intervene on disparities in childhood cancer,” Dr. Bona said. “We’re really good at studying genetic mutations in cancer cells that might lead to a risk of relapse, and when we identify those mutations, what we do is intervene. We try new chemotherapy agents, new ways of delivering therapy. We are now at the point where we have identified that social determinants of health may be equally ‘risky’ but we haven’t taken the next step to begin intervening in the same way.”
What is causing disparities in pediatric cancer outcomes?
Lack of access to the health care system is a top contributor to the disparities, although there is no single root cause, said Sharon Castellino, MD, director of the Leukemia and Lymphoma Program at the Aflac Cancer & Blood Disorders Center of Children’s Healthcare of Atlanta, and a professor in the department of pediatrics at Emory University, Atlanta.
Even before cancer diagnosis, Dr. Castellino notes that many children of color and/or of lower socioeconomic status are not receiving regular health care, leading to sicker children and more advanced-stage cancer by the time they are diagnosed.
Lack of insurance is a primary barrier to this access, adds Xu Ji, PhD, MSPH, an assistant professor in the department of pediatrics at Emory University and a member of the Cancer Prevention and Control Research Program at the university’s Winship Cancer Institute.
Studies have long shown that uninsured children are more likely to go without needed care, compared with those with private insurance. Patients of color are at much higher risk of being uninsured than White patients, with the uninsured rates for Hispanic, American Indian, and Alaska Native patients being more than 2.5 times higher than that of White patients.
“We all know that insurance is a strong predictor of health outcomes,” said Dr. Ji, whose research focuses on insurance disparities and gains among cancer patients. “Lack of insurance coverage and therefore lack of access to care along the pediatric cancer continuum from early detection to early diagnosis to timely initiation of treatment to receipt of high-quality treatment to access to recommended survivorship care and even access to palliative and end-of-life care are all very important constructs in the pathway from poverty to ultimate cancer outcomes for children.”
Unstable housing, employment difficulties, and lack of family support can also come into play. Dr. Castellino remembers the case of a 12-year-old cancer patient who entered treatment with advanced-stage Hodgkin Lymphoma. The girl came from a low-income, single-parent household without stable housing. Dr. Castellino said when the child was granted a wish from the Make-a-Wish Foundation, she asked for her own bed.
“We had been working with her every week for 6 months when that request came up,” she recalled. “We said, ‘You don’t have to wait for your make-a-wish, we can get you a bed now.’ We don’t even know the extent of what happens at home for many of these children.”
The impact of toxic stress on child cancer patients is an emerging area of research, said Dr. Winestone, whose research explores racial, ethnic, and socioeconomic disparities in access to care and outcomes of leukemia and lymphoma treatment. For example, Dr. Winestone’s research includes understanding how exposure to poverty or adverse experiences in childhood may influence a patient’s biological response to chemotherapy.
Other contributors to disparities include transportation issues, lack of childcare for other children, literacy, and language barriers. A 2016 study suggests that language barriers negatively impact the quality of informed decision-making and the care experience for Spanish-speaking parents of pediatric cancer patients with limited English proficiency.
Such access issues are also compounded by systemic factors, including a shortage of physicians of color who may be able to forge better trust relationships with families of similar race and ethnicity, Dr. Castellino adds. Lower enrollment of pediatric cancer patients with higher social vulnerabilities in clinical trials is another problem.
“In childhood cancer, I believe our improvements have been built on the backs of prior generations of families and children who have enrolled in trials. We learn things, and the next generation of therapy improves,” Dr. Castellino said. “If you have a whole group of the population not represented in trials, you don’t know what’s driving the fact they may or may not improve.”
Working toward solutions
With such a diverse set of factors fueling outcome gaps, a similarly diverse approach is needed to help bridge the divide, say disparity researchers.
To this end, Dr. Bona and her research team are currently building the first portfolio of health equity interventions, each designed to address a different adverse social determinant of health differently.
The Pediatric Cancer Resource Equity (PediCARE) intervention is a centrally delivered, household material hardship (HMH)–targeted intervention that provides transportation and groceries to low-income pediatric oncology families. The intervention was recently studied in a pilot, randomized, controlled trial at Dana-Farber Cancer Institute and the University of Alabama between May 2019 and August 2021.
Families were first screened for HMH and randomized into receiving either the intervention or usual care for 6 months. The intervention group received groceries via Instacart and transportation to and from the hospital coordinated through the Ride Health platform using Uber or Lyft. For families with their own cars, gas cards were provided. Of the families offered the chance to participate, 100% agreed to participate in the program, and there was 0% attrition in either arm of the program during the 6 months, according to the study findings, which were presented at the 2023 American Society of Clinical Oncology annual meeting in June.
Among families who received the PediCARE intervention, 100% successfully received grocery and transportation resources, 100% reported that it was “easier to buy food for my family,” 85% reported it was easier to get to and from the hospital, and 95% reported they would be “very likely to recommend the intervention to other families,” according to the results.
“The key takeaway is that we had excellent feasibility outcomes,” said Haley Newman, MD, lead author of the study and an attending physician in the division of oncology at The Children’s Hospital of Philadelphia. “From this study, we learned that PediCARE is accessible and feasible in very diverse settings. From this, what we really took away is that PediCARE could be successfully rolled out in a phase 3 randomized trial, which would be the best way to examine efficacy.”
Another initiative in its early stages is Pediatric RISE, a guaranteed income intervention being developed with support from the Children’s Cancer Research Fund, the American Cancer Society, and other donors. The intervention will provide unrestricted cash transfers to low-income families during the early months of chemotherapy, Dr. Bona said. Families are currently being enrolled in a pilot study with a goal of refining the intervention before it’s tested for feasibility and efficacy.
“The goal here is ultimately to evaluate the question: If we are able to successfully provide income support to low-income families going through childhood cancer treatment, might we be able to ameliorate some of the disparities associated with living in poverty that we have already described in childhood cancer,” Dr. Bona said.
Pediatric Assist, a developing intervention centering on benefits, is a third initiative that will soon be evaluated. The intervention will provide newly diagnosed families with systemic access to a centralized benefits counselor who can help them determine which existing government benefits they might be eligible for and assist them in navigating the application process.
“The idea here is that we know many lower-income families in the U.S. are eligible for existing supports, but may not be accessing them because of how incredibly difficult the system is to navigate,” Dr. Bona said. “For example, we know that low-income families may be eligible for SNAP benefits, but figuring out if you are eligible and then applying for SNAP involves multiple, complicated steps that are often infeasible for a parent when their child is admitted to the hospital with a newly diagnosed, life-threatening illness.”
Pilot refinement of the intervention is expected in the fall of 2023.
Overcoming barriers, addressing challenges
Investigators are also making headway in proving that collecting social determinants of health (SDoH) data during existing clinical trials is easily achievable.
Past Children’s Oncology Group trials have collected only race, ethnicity, insurance, and zip code data as proxies for exposure to adverse SDoH. Dr. Winestone and her colleagues recently investigated the feasibility and acceptability of the first COG trial to prospectively embed SDoH data collection.
Of eligible participants, 360 of 413 opted-in to the embedded SDOH aim across 101 COG sites (87.2% consent rate). Among participants, 316 surveys (87.8%) were completed a median of 11 days post enrollment, according to the findings, which were presented at the ASCO annual meeting.
“We’ve come to realize the importance of the social determinants of health [as it pertains] to outcomes, but it has been a process to learn how to effectively collect that data in a large collaborative environment,” said Dr. Winestone. “This abstract demonstrates that patients are very willing to provide this data, and they’re able to do it in an efficient way. People think of these questions as very sensitive and that families may not want to share the answers, but this study demonstrates those presumptions are false.”
The authors hope the findings fuel incorporation of SDoH data collection in future National Clinical Trials Network trials to inform impactful health equity research.
While such research and intervention efforts are gaining momentum, challenges to do the work remain. A lack of research funding and support are among the obstacles, Dr. Winestone said.
To date, much of pediatric cancer work has focused on developing new therapeutic approaches to reach a cure for more patients, she explained.
“While that’s incredibly essential, if we’re creating these approaches that only work for a subset of patients that have resources, we’re contributing to the inequities in the system,” Dr. Winestone said. “Really, [we need] dedicated support to studying how to make sure the interventions we know are effective are reaching all populations, and that the patients are poised to benefit from those interventions by setting them up for success.”
A strong research infrastructure exists to evaluate and support clinical drug trials in pediatric oncology, but the same does not exist for health equity interventions, Dr. Bona adds. A significant question that needs to be addressed is how best to integrate health equity evaluation into existing infrastructure or whether to build a parallel infrastructure.
Despite the challenges, Dr. Bona believes now is exactly the right time to investigate and intervene in poverty as a risk factor for childhood cancer relapse and outcomes. What has led to success in childhood cancer is how pediatric oncology has collaborated across the country to operate clinical drug trials at various centers, all in the same way, to identify which treatments work best, she said.
“We have an opportunity now in pediatrics to take advantage of this highly successful clinical trials research infrastructure to integrate interventions to address disparities in a way that has not been done previously,” she said. “The opportunity to significantly improve survival in childhood cancer by reducing disparities exists if we take this head on from a research and funding perspective and approach social risk factors just as we already know how to approach tumor genomic risk factors.”
Pediatric oncologist Lena Winestone, MD, recalls treating a 2-year-old leukemia patient who underwent a bone marrow transplant as her only chance for a cure.
The girl’s family, who spoke only Spanish and struggled with literacy, could not pay their rent or afford the girl’s weekly transportation to the hospital for after-transplant care. The family had three other children and lived more than 2 hours from the transplant center, remembers Dr. Winestone, an assistant professor of pediatrics in the division of malignancies and bone & marrow transplant at the University of California, San Francisco.
The hospital’s social worker was able to secure grant support for the family’s housing and worked with the patient’s insurance to arrange for transportation. However, the departure times were rigid, Dr. Winestone said, and the family sometimes had to leave the hospital before the child’s graft vs. host disease (GvHD) treatment was complete for the day.
“If we had not finished her treatment, we had to disconnect her from the machine early,” Dr. Winestone said. “Her mother also had to load her oxygen tanks [three of them], her BiPAP machine, and her tube feeds into the transportation every week in order to make sure she could be safely transported. She was treated for GvHD for almost 2 years, but unfortunately, her GvHD started to affect her lungs and ultimately, she passed away.”
Dr. Winestone says it’s difficult to know whether the girl’s death was directly related to her socioeconomic status, but that it certainly made all aspects of the child’s care more complicated and forced health care providers to adapt her cancer care to accommodate the family’s circumstances.
This story is one of countless cases where socioeconomic status impacted a young patient’s cancer care and likely contributed to a worse outcome.
A 2022 study for example, found that children from marginalized racial/ethnic groups and those living in poverty were more likely to have inferior 5-year overall survival, compared with other children, even when assigned to receive the same initial treatment. Of 696 children with high-risk neuroblastoma, 47% of Hispanic children had a 5-year overall survival (OS), compared with 50% for other non-Hispanic children, and 61% for white non-Hispanic patients. Children on public health insurance (a proxy for household poverty) had a 53% 5-year OS, compared with 63% for children unexposed to household poverty. Pediatric patients exposed to neighborhood poverty had a 54% 5-year OS, compared with 62% for unexposed children.
In another study, children with acute lymphoblastic leukemia who lived in high-poverty areas were more likely to experience early relapse than other patients, despite having the same treatment. Of the 575 children studied, 92% of children from high-poverty areas who relapsed, experienced early relapse, defined as less than 36 months after remission. By comparison, only 48% of other children who relapsed experienced early relapse.
Reasons behind the relapse and survival disparities are multifold, which has led to challenges in addressing the gaps and improving cancer outcomes for poverty-stricken children. A research infrastructure that is largely based on biological, rather than social determinants of health, acts as another barrier, oncologists say.
Historically, interventions to address disparities in pediatric oncology have never been evaluated, said Kira Bona, MD, MPH, a pediatric oncologist at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. This is in large part because the body of literature illustrating the disparities is relatively new, said Dr. Bona, whose research focuses on poverty-associated outcome disparities in childhood cancer.
However, new efforts aim to change this landscape by using the growing data to develop and analyze possible interventions. A set of three novel interventions led by Dr. Bona and her research team are in the works, some of which have shown promise in early studies.
“Now is the time to begin to actively intervene on disparities in childhood cancer,” Dr. Bona said. “We’re really good at studying genetic mutations in cancer cells that might lead to a risk of relapse, and when we identify those mutations, what we do is intervene. We try new chemotherapy agents, new ways of delivering therapy. We are now at the point where we have identified that social determinants of health may be equally ‘risky’ but we haven’t taken the next step to begin intervening in the same way.”
What is causing disparities in pediatric cancer outcomes?
Lack of access to the health care system is a top contributor to the disparities, although there is no single root cause, said Sharon Castellino, MD, director of the Leukemia and Lymphoma Program at the Aflac Cancer & Blood Disorders Center of Children’s Healthcare of Atlanta, and a professor in the department of pediatrics at Emory University, Atlanta.
Even before cancer diagnosis, Dr. Castellino notes that many children of color and/or of lower socioeconomic status are not receiving regular health care, leading to sicker children and more advanced-stage cancer by the time they are diagnosed.
Lack of insurance is a primary barrier to this access, adds Xu Ji, PhD, MSPH, an assistant professor in the department of pediatrics at Emory University and a member of the Cancer Prevention and Control Research Program at the university’s Winship Cancer Institute.
Studies have long shown that uninsured children are more likely to go without needed care, compared with those with private insurance. Patients of color are at much higher risk of being uninsured than White patients, with the uninsured rates for Hispanic, American Indian, and Alaska Native patients being more than 2.5 times higher than that of White patients.
“We all know that insurance is a strong predictor of health outcomes,” said Dr. Ji, whose research focuses on insurance disparities and gains among cancer patients. “Lack of insurance coverage and therefore lack of access to care along the pediatric cancer continuum from early detection to early diagnosis to timely initiation of treatment to receipt of high-quality treatment to access to recommended survivorship care and even access to palliative and end-of-life care are all very important constructs in the pathway from poverty to ultimate cancer outcomes for children.”
Unstable housing, employment difficulties, and lack of family support can also come into play. Dr. Castellino remembers the case of a 12-year-old cancer patient who entered treatment with advanced-stage Hodgkin Lymphoma. The girl came from a low-income, single-parent household without stable housing. Dr. Castellino said when the child was granted a wish from the Make-a-Wish Foundation, she asked for her own bed.
“We had been working with her every week for 6 months when that request came up,” she recalled. “We said, ‘You don’t have to wait for your make-a-wish, we can get you a bed now.’ We don’t even know the extent of what happens at home for many of these children.”
The impact of toxic stress on child cancer patients is an emerging area of research, said Dr. Winestone, whose research explores racial, ethnic, and socioeconomic disparities in access to care and outcomes of leukemia and lymphoma treatment. For example, Dr. Winestone’s research includes understanding how exposure to poverty or adverse experiences in childhood may influence a patient’s biological response to chemotherapy.
Other contributors to disparities include transportation issues, lack of childcare for other children, literacy, and language barriers. A 2016 study suggests that language barriers negatively impact the quality of informed decision-making and the care experience for Spanish-speaking parents of pediatric cancer patients with limited English proficiency.
Such access issues are also compounded by systemic factors, including a shortage of physicians of color who may be able to forge better trust relationships with families of similar race and ethnicity, Dr. Castellino adds. Lower enrollment of pediatric cancer patients with higher social vulnerabilities in clinical trials is another problem.
“In childhood cancer, I believe our improvements have been built on the backs of prior generations of families and children who have enrolled in trials. We learn things, and the next generation of therapy improves,” Dr. Castellino said. “If you have a whole group of the population not represented in trials, you don’t know what’s driving the fact they may or may not improve.”
Working toward solutions
With such a diverse set of factors fueling outcome gaps, a similarly diverse approach is needed to help bridge the divide, say disparity researchers.
To this end, Dr. Bona and her research team are currently building the first portfolio of health equity interventions, each designed to address a different adverse social determinant of health differently.
The Pediatric Cancer Resource Equity (PediCARE) intervention is a centrally delivered, household material hardship (HMH)–targeted intervention that provides transportation and groceries to low-income pediatric oncology families. The intervention was recently studied in a pilot, randomized, controlled trial at Dana-Farber Cancer Institute and the University of Alabama between May 2019 and August 2021.
Families were first screened for HMH and randomized into receiving either the intervention or usual care for 6 months. The intervention group received groceries via Instacart and transportation to and from the hospital coordinated through the Ride Health platform using Uber or Lyft. For families with their own cars, gas cards were provided. Of the families offered the chance to participate, 100% agreed to participate in the program, and there was 0% attrition in either arm of the program during the 6 months, according to the study findings, which were presented at the 2023 American Society of Clinical Oncology annual meeting in June.
Among families who received the PediCARE intervention, 100% successfully received grocery and transportation resources, 100% reported that it was “easier to buy food for my family,” 85% reported it was easier to get to and from the hospital, and 95% reported they would be “very likely to recommend the intervention to other families,” according to the results.
“The key takeaway is that we had excellent feasibility outcomes,” said Haley Newman, MD, lead author of the study and an attending physician in the division of oncology at The Children’s Hospital of Philadelphia. “From this study, we learned that PediCARE is accessible and feasible in very diverse settings. From this, what we really took away is that PediCARE could be successfully rolled out in a phase 3 randomized trial, which would be the best way to examine efficacy.”
Another initiative in its early stages is Pediatric RISE, a guaranteed income intervention being developed with support from the Children’s Cancer Research Fund, the American Cancer Society, and other donors. The intervention will provide unrestricted cash transfers to low-income families during the early months of chemotherapy, Dr. Bona said. Families are currently being enrolled in a pilot study with a goal of refining the intervention before it’s tested for feasibility and efficacy.
“The goal here is ultimately to evaluate the question: If we are able to successfully provide income support to low-income families going through childhood cancer treatment, might we be able to ameliorate some of the disparities associated with living in poverty that we have already described in childhood cancer,” Dr. Bona said.
Pediatric Assist, a developing intervention centering on benefits, is a third initiative that will soon be evaluated. The intervention will provide newly diagnosed families with systemic access to a centralized benefits counselor who can help them determine which existing government benefits they might be eligible for and assist them in navigating the application process.
“The idea here is that we know many lower-income families in the U.S. are eligible for existing supports, but may not be accessing them because of how incredibly difficult the system is to navigate,” Dr. Bona said. “For example, we know that low-income families may be eligible for SNAP benefits, but figuring out if you are eligible and then applying for SNAP involves multiple, complicated steps that are often infeasible for a parent when their child is admitted to the hospital with a newly diagnosed, life-threatening illness.”
Pilot refinement of the intervention is expected in the fall of 2023.
Overcoming barriers, addressing challenges
Investigators are also making headway in proving that collecting social determinants of health (SDoH) data during existing clinical trials is easily achievable.
Past Children’s Oncology Group trials have collected only race, ethnicity, insurance, and zip code data as proxies for exposure to adverse SDoH. Dr. Winestone and her colleagues recently investigated the feasibility and acceptability of the first COG trial to prospectively embed SDoH data collection.
Of eligible participants, 360 of 413 opted-in to the embedded SDOH aim across 101 COG sites (87.2% consent rate). Among participants, 316 surveys (87.8%) were completed a median of 11 days post enrollment, according to the findings, which were presented at the ASCO annual meeting.
“We’ve come to realize the importance of the social determinants of health [as it pertains] to outcomes, but it has been a process to learn how to effectively collect that data in a large collaborative environment,” said Dr. Winestone. “This abstract demonstrates that patients are very willing to provide this data, and they’re able to do it in an efficient way. People think of these questions as very sensitive and that families may not want to share the answers, but this study demonstrates those presumptions are false.”
The authors hope the findings fuel incorporation of SDoH data collection in future National Clinical Trials Network trials to inform impactful health equity research.
While such research and intervention efforts are gaining momentum, challenges to do the work remain. A lack of research funding and support are among the obstacles, Dr. Winestone said.
To date, much of pediatric cancer work has focused on developing new therapeutic approaches to reach a cure for more patients, she explained.
“While that’s incredibly essential, if we’re creating these approaches that only work for a subset of patients that have resources, we’re contributing to the inequities in the system,” Dr. Winestone said. “Really, [we need] dedicated support to studying how to make sure the interventions we know are effective are reaching all populations, and that the patients are poised to benefit from those interventions by setting them up for success.”
A strong research infrastructure exists to evaluate and support clinical drug trials in pediatric oncology, but the same does not exist for health equity interventions, Dr. Bona adds. A significant question that needs to be addressed is how best to integrate health equity evaluation into existing infrastructure or whether to build a parallel infrastructure.
Despite the challenges, Dr. Bona believes now is exactly the right time to investigate and intervene in poverty as a risk factor for childhood cancer relapse and outcomes. What has led to success in childhood cancer is how pediatric oncology has collaborated across the country to operate clinical drug trials at various centers, all in the same way, to identify which treatments work best, she said.
“We have an opportunity now in pediatrics to take advantage of this highly successful clinical trials research infrastructure to integrate interventions to address disparities in a way that has not been done previously,” she said. “The opportunity to significantly improve survival in childhood cancer by reducing disparities exists if we take this head on from a research and funding perspective and approach social risk factors just as we already know how to approach tumor genomic risk factors.”
CDC tracking new COVID strain
On Aug. 17, the agency posted on X, formerly known as Twitter, that the lineage has been detected in the United States, Denmark, and Israel.
“As we learn more about BA.2.86, CDC’s advice on protecting yourself from COVID-19 remains the same,” the CDC said on X.
A case of BA.2.86 was detected at a laboratory at the University of Michigan, CBS News reported. It’s not clear how the university obtained the sample that was sequenced. A case was also detected in the United Kingdom, the news outlet said.
The World Health Organization is also tracking BA.2.86 and has classified it as a “variant under monitoring.”
“More data are needed to understand this COVID-19 variant and the extent of its spread, but the number of mutations warrants attention. WHO will update countries and the public as we learn more,” the WHO said on X.
The strain is so new that scientists don’t know if BA.2.86 is more easily spread, causes more severe symptoms than existing strains, or will be more resistant to vaccines and natural immunity developed over the last few years.
Early research indicates BA.2.86 “will have equal or greater escape than XBB.1.5 from antibodies elicited by pre-Omicron and first-generation Omicron variants,” Jesse Bloom, PhD, a virologist at the Fred Hutchinson Cancer Center, said in a slide deck published Aug. 17. (XBB.1.5 is the Omicron subvariant that is targeted in the updated COVID booster shot to be released soon.)
Still, Dr. Bloom noted that “even if a highly mutated new variant like BA.2.86 starts to spread, we will be in a far better place than we were in 2020 and 2021, since most people have some immunity to SARS-CoV-2 now.”
A version of this article first appeared on WebMD.com.
On Aug. 17, the agency posted on X, formerly known as Twitter, that the lineage has been detected in the United States, Denmark, and Israel.
“As we learn more about BA.2.86, CDC’s advice on protecting yourself from COVID-19 remains the same,” the CDC said on X.
A case of BA.2.86 was detected at a laboratory at the University of Michigan, CBS News reported. It’s not clear how the university obtained the sample that was sequenced. A case was also detected in the United Kingdom, the news outlet said.
The World Health Organization is also tracking BA.2.86 and has classified it as a “variant under monitoring.”
“More data are needed to understand this COVID-19 variant and the extent of its spread, but the number of mutations warrants attention. WHO will update countries and the public as we learn more,” the WHO said on X.
The strain is so new that scientists don’t know if BA.2.86 is more easily spread, causes more severe symptoms than existing strains, or will be more resistant to vaccines and natural immunity developed over the last few years.
Early research indicates BA.2.86 “will have equal or greater escape than XBB.1.5 from antibodies elicited by pre-Omicron and first-generation Omicron variants,” Jesse Bloom, PhD, a virologist at the Fred Hutchinson Cancer Center, said in a slide deck published Aug. 17. (XBB.1.5 is the Omicron subvariant that is targeted in the updated COVID booster shot to be released soon.)
Still, Dr. Bloom noted that “even if a highly mutated new variant like BA.2.86 starts to spread, we will be in a far better place than we were in 2020 and 2021, since most people have some immunity to SARS-CoV-2 now.”
A version of this article first appeared on WebMD.com.
On Aug. 17, the agency posted on X, formerly known as Twitter, that the lineage has been detected in the United States, Denmark, and Israel.
“As we learn more about BA.2.86, CDC’s advice on protecting yourself from COVID-19 remains the same,” the CDC said on X.
A case of BA.2.86 was detected at a laboratory at the University of Michigan, CBS News reported. It’s not clear how the university obtained the sample that was sequenced. A case was also detected in the United Kingdom, the news outlet said.
The World Health Organization is also tracking BA.2.86 and has classified it as a “variant under monitoring.”
“More data are needed to understand this COVID-19 variant and the extent of its spread, but the number of mutations warrants attention. WHO will update countries and the public as we learn more,” the WHO said on X.
The strain is so new that scientists don’t know if BA.2.86 is more easily spread, causes more severe symptoms than existing strains, or will be more resistant to vaccines and natural immunity developed over the last few years.
Early research indicates BA.2.86 “will have equal or greater escape than XBB.1.5 from antibodies elicited by pre-Omicron and first-generation Omicron variants,” Jesse Bloom, PhD, a virologist at the Fred Hutchinson Cancer Center, said in a slide deck published Aug. 17. (XBB.1.5 is the Omicron subvariant that is targeted in the updated COVID booster shot to be released soon.)
Still, Dr. Bloom noted that “even if a highly mutated new variant like BA.2.86 starts to spread, we will be in a far better place than we were in 2020 and 2021, since most people have some immunity to SARS-CoV-2 now.”
A version of this article first appeared on WebMD.com.