User login
Bimekizumab approved in Europe for psoriasis treatment
, according to a statement from the manufacturer.
Bimekizumab (Bimzelx), a humanized IgG1 monoclonal antibody, is the first approved treatment for moderate to severe plaque psoriasis that selectively inhibits interleukin (IL)–17A and IL-17F, the statement from UCB said.
In the United States, the Food and Drug Administration is expected to make a decision on approval of bimekizumab for treating psoriasis on Oct. 15.
Approval in the EU was based on data from three phase 3 trials including a total of 1,480 adult patients with moderate to severe psoriasis, which found that those treated with bimekizumab experienced significantly greater skin clearance, compared with placebo, ustekinumab, and adalimumab, with a favorable safety profile, according to the company.
In all three studies (BE VIVID, BE READY, and BE SURE), more than 80% of patients treated with bimekizumab showed improved skin clearance after 16 weeks, significantly more than those treated with ustekinumab, placebo, or adalimumab, based on an improvement of at least 90% in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). In all three studies, these clinical responses persisted after 1 year.
The recommended dose of bimekizumab is 320 mg, given in two subcutaneous injections every 4 weeks to week 16, then every 8 weeks. However, for “some patients” weighing 120 kg or more who have not achieved complete skin clearance at 16 weeks, 320 mg every 4 weeks after that time may improve response to treatment, according to the company statement.
The most common treatment-related adverse events in the studies were upper respiratory tract infections (a majority of which were nasopharyngitis), reported by 14.5% of patients, followed by oral candidiasis, reported by 7.3%.
Results of BE READY and BE VIVID were published in The Lancet. Results of the BE SURE study were published in The New England Journal of Medicine.
Bimekizumab is contraindicated for individuals with clinically important active infections such as tuberculosis, and for individuals with any hypersensitivity to the active substance. More details on bimekizumab are available on the website of the European Medicines Agency.
, according to a statement from the manufacturer.
Bimekizumab (Bimzelx), a humanized IgG1 monoclonal antibody, is the first approved treatment for moderate to severe plaque psoriasis that selectively inhibits interleukin (IL)–17A and IL-17F, the statement from UCB said.
In the United States, the Food and Drug Administration is expected to make a decision on approval of bimekizumab for treating psoriasis on Oct. 15.
Approval in the EU was based on data from three phase 3 trials including a total of 1,480 adult patients with moderate to severe psoriasis, which found that those treated with bimekizumab experienced significantly greater skin clearance, compared with placebo, ustekinumab, and adalimumab, with a favorable safety profile, according to the company.
In all three studies (BE VIVID, BE READY, and BE SURE), more than 80% of patients treated with bimekizumab showed improved skin clearance after 16 weeks, significantly more than those treated with ustekinumab, placebo, or adalimumab, based on an improvement of at least 90% in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). In all three studies, these clinical responses persisted after 1 year.
The recommended dose of bimekizumab is 320 mg, given in two subcutaneous injections every 4 weeks to week 16, then every 8 weeks. However, for “some patients” weighing 120 kg or more who have not achieved complete skin clearance at 16 weeks, 320 mg every 4 weeks after that time may improve response to treatment, according to the company statement.
The most common treatment-related adverse events in the studies were upper respiratory tract infections (a majority of which were nasopharyngitis), reported by 14.5% of patients, followed by oral candidiasis, reported by 7.3%.
Results of BE READY and BE VIVID were published in The Lancet. Results of the BE SURE study were published in The New England Journal of Medicine.
Bimekizumab is contraindicated for individuals with clinically important active infections such as tuberculosis, and for individuals with any hypersensitivity to the active substance. More details on bimekizumab are available on the website of the European Medicines Agency.
, according to a statement from the manufacturer.
Bimekizumab (Bimzelx), a humanized IgG1 monoclonal antibody, is the first approved treatment for moderate to severe plaque psoriasis that selectively inhibits interleukin (IL)–17A and IL-17F, the statement from UCB said.
In the United States, the Food and Drug Administration is expected to make a decision on approval of bimekizumab for treating psoriasis on Oct. 15.
Approval in the EU was based on data from three phase 3 trials including a total of 1,480 adult patients with moderate to severe psoriasis, which found that those treated with bimekizumab experienced significantly greater skin clearance, compared with placebo, ustekinumab, and adalimumab, with a favorable safety profile, according to the company.
In all three studies (BE VIVID, BE READY, and BE SURE), more than 80% of patients treated with bimekizumab showed improved skin clearance after 16 weeks, significantly more than those treated with ustekinumab, placebo, or adalimumab, based on an improvement of at least 90% in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). In all three studies, these clinical responses persisted after 1 year.
The recommended dose of bimekizumab is 320 mg, given in two subcutaneous injections every 4 weeks to week 16, then every 8 weeks. However, for “some patients” weighing 120 kg or more who have not achieved complete skin clearance at 16 weeks, 320 mg every 4 weeks after that time may improve response to treatment, according to the company statement.
The most common treatment-related adverse events in the studies were upper respiratory tract infections (a majority of which were nasopharyngitis), reported by 14.5% of patients, followed by oral candidiasis, reported by 7.3%.
Results of BE READY and BE VIVID were published in The Lancet. Results of the BE SURE study were published in The New England Journal of Medicine.
Bimekizumab is contraindicated for individuals with clinically important active infections such as tuberculosis, and for individuals with any hypersensitivity to the active substance. More details on bimekizumab are available on the website of the European Medicines Agency.
Psoriatic arthritis health care costs continue to rise over time
Annual health care costs for patients with psoriatic arthritis rose over recent 5-year periods across all categories of resource use to a significantly greater extent than among patients with psoriasis only or those without any psoriatic disease diagnoses, according to commercial insurance claims data.
Using an IBM MarketScan Commercial Database, researchers examined claims data for 208,434 patients with psoriasis, 47,274 with PsA, and 255,708 controls who had neither psoriasis nor PsA. Controls were matched for age and sex. Those with RA, ankylosing spondylitis, Crohn’s disease, or ulcerative colitis were excluded.
The investigators examined data for 2009-2020, following patients for 5 years within that period. They looked at hospitalizations, outpatient and pharmacy services, lab services, and office visits, Steven Peterson, director of market access for rheumatology at Janssen Pharmaceuticals, said in his presentation of the data at the Pan American League of Associations for Rheumatology 2021 annual meeting, held recently as a virtual event.
The research was also published online May 2, 2021, in Clinical Rheumatology.
Big differences between the groups were seen in the first year, when the average health care costs for the PsA group were $28,322, about half of which was outpatient drug costs. That compared with $12,039 for the psoriasis group and $6,672 for the control group.
The differences tended to widen over time. By the fifth year, average costs for the PsA group were $34,290, nearly 60% of which were drug costs. That compared with $12,877 for the psoriasis group and $8,569 for the control group. In each year examined, outpatient drug costs accounted for less than half of the expenses for the psoriasis group and about a quarter for the control group.
Researchers found that the PsA group needed 28.7 prescriptions per person per year, compared with 17.0 and 12.7 in the psoriasis and control groups, respectively, Mr. Peterson said. He also noted that patients with PsA and psoriasis tended to have higher rates of hypertension, depression, and anxiety.
“The cost and resource utilization disparity between these patient groups demonstrates the high remaining unmet medical need for patients with psoriasis and psoriatic arthritis,” Mr. Peterson said during the virtual proceedings.
Do findings reflect treatment advances?
Elaine Husni, MD, MPH, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic, where she studies health outcomes in PsA, said the findings are helpful in pointing to a trend across a large sample. But she added it’s important to remember that the increasing costs could reflect recent advances in PsA treatment, which include costly biologic drugs.
“There’s a ton more treatments for psoriasis and psoriatic arthritis than there were even just 5 years ago,” she said in an interview. She was not involved in the research.
Dr. Husni would like to see a more detailed look at the costs, from the categories of expenses to the patients who are incurring the highest costs.
“Is it just a couple of percent of really sick patients that are driving the psoriatic arthritis group?” she wondered.
She also pointed out that PsA is going to be more expensive by its very nature. PsA tends to develop 3-10 years after psoriasis, adding to the costs for someone who already has psoriasis and at a time when they are older and likely have higher health care costs because of comorbidities that develop with age.
Dr. Husni said she does think about treatment costs, in that a less expensive first-line drug might be more appropriate than going straight to a more expensive biologic, especially because they also tend to be safer. She said it’s not just a simple question of curbing costs.
“Is there a way that we can personalize medicine?” she asked. “Is there a way that we can be more accurate about which people may need the more expensive drugs, and which patients may need the less expensive drugs? Are we getting better at monitoring so we can avoid high-cost events?”
Mr. Peterson is an employee of Janssen Pharmaceuticals. Dr. Husni reported serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, UCB, Novartis, Lilly, and Pfizer.
* Update, 9/28/21: The headline and parts of this story were updated to better reflect the study on which it reports.
A version of this article first appeared on Medscape.com.
Annual health care costs for patients with psoriatic arthritis rose over recent 5-year periods across all categories of resource use to a significantly greater extent than among patients with psoriasis only or those without any psoriatic disease diagnoses, according to commercial insurance claims data.
Using an IBM MarketScan Commercial Database, researchers examined claims data for 208,434 patients with psoriasis, 47,274 with PsA, and 255,708 controls who had neither psoriasis nor PsA. Controls were matched for age and sex. Those with RA, ankylosing spondylitis, Crohn’s disease, or ulcerative colitis were excluded.
The investigators examined data for 2009-2020, following patients for 5 years within that period. They looked at hospitalizations, outpatient and pharmacy services, lab services, and office visits, Steven Peterson, director of market access for rheumatology at Janssen Pharmaceuticals, said in his presentation of the data at the Pan American League of Associations for Rheumatology 2021 annual meeting, held recently as a virtual event.
The research was also published online May 2, 2021, in Clinical Rheumatology.
Big differences between the groups were seen in the first year, when the average health care costs for the PsA group were $28,322, about half of which was outpatient drug costs. That compared with $12,039 for the psoriasis group and $6,672 for the control group.
The differences tended to widen over time. By the fifth year, average costs for the PsA group were $34,290, nearly 60% of which were drug costs. That compared with $12,877 for the psoriasis group and $8,569 for the control group. In each year examined, outpatient drug costs accounted for less than half of the expenses for the psoriasis group and about a quarter for the control group.
Researchers found that the PsA group needed 28.7 prescriptions per person per year, compared with 17.0 and 12.7 in the psoriasis and control groups, respectively, Mr. Peterson said. He also noted that patients with PsA and psoriasis tended to have higher rates of hypertension, depression, and anxiety.
“The cost and resource utilization disparity between these patient groups demonstrates the high remaining unmet medical need for patients with psoriasis and psoriatic arthritis,” Mr. Peterson said during the virtual proceedings.
Do findings reflect treatment advances?
Elaine Husni, MD, MPH, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic, where she studies health outcomes in PsA, said the findings are helpful in pointing to a trend across a large sample. But she added it’s important to remember that the increasing costs could reflect recent advances in PsA treatment, which include costly biologic drugs.
“There’s a ton more treatments for psoriasis and psoriatic arthritis than there were even just 5 years ago,” she said in an interview. She was not involved in the research.
Dr. Husni would like to see a more detailed look at the costs, from the categories of expenses to the patients who are incurring the highest costs.
“Is it just a couple of percent of really sick patients that are driving the psoriatic arthritis group?” she wondered.
She also pointed out that PsA is going to be more expensive by its very nature. PsA tends to develop 3-10 years after psoriasis, adding to the costs for someone who already has psoriasis and at a time when they are older and likely have higher health care costs because of comorbidities that develop with age.
Dr. Husni said she does think about treatment costs, in that a less expensive first-line drug might be more appropriate than going straight to a more expensive biologic, especially because they also tend to be safer. She said it’s not just a simple question of curbing costs.
“Is there a way that we can personalize medicine?” she asked. “Is there a way that we can be more accurate about which people may need the more expensive drugs, and which patients may need the less expensive drugs? Are we getting better at monitoring so we can avoid high-cost events?”
Mr. Peterson is an employee of Janssen Pharmaceuticals. Dr. Husni reported serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, UCB, Novartis, Lilly, and Pfizer.
* Update, 9/28/21: The headline and parts of this story were updated to better reflect the study on which it reports.
A version of this article first appeared on Medscape.com.
Annual health care costs for patients with psoriatic arthritis rose over recent 5-year periods across all categories of resource use to a significantly greater extent than among patients with psoriasis only or those without any psoriatic disease diagnoses, according to commercial insurance claims data.
Using an IBM MarketScan Commercial Database, researchers examined claims data for 208,434 patients with psoriasis, 47,274 with PsA, and 255,708 controls who had neither psoriasis nor PsA. Controls were matched for age and sex. Those with RA, ankylosing spondylitis, Crohn’s disease, or ulcerative colitis were excluded.
The investigators examined data for 2009-2020, following patients for 5 years within that period. They looked at hospitalizations, outpatient and pharmacy services, lab services, and office visits, Steven Peterson, director of market access for rheumatology at Janssen Pharmaceuticals, said in his presentation of the data at the Pan American League of Associations for Rheumatology 2021 annual meeting, held recently as a virtual event.
The research was also published online May 2, 2021, in Clinical Rheumatology.
Big differences between the groups were seen in the first year, when the average health care costs for the PsA group were $28,322, about half of which was outpatient drug costs. That compared with $12,039 for the psoriasis group and $6,672 for the control group.
The differences tended to widen over time. By the fifth year, average costs for the PsA group were $34,290, nearly 60% of which were drug costs. That compared with $12,877 for the psoriasis group and $8,569 for the control group. In each year examined, outpatient drug costs accounted for less than half of the expenses for the psoriasis group and about a quarter for the control group.
Researchers found that the PsA group needed 28.7 prescriptions per person per year, compared with 17.0 and 12.7 in the psoriasis and control groups, respectively, Mr. Peterson said. He also noted that patients with PsA and psoriasis tended to have higher rates of hypertension, depression, and anxiety.
“The cost and resource utilization disparity between these patient groups demonstrates the high remaining unmet medical need for patients with psoriasis and psoriatic arthritis,” Mr. Peterson said during the virtual proceedings.
Do findings reflect treatment advances?
Elaine Husni, MD, MPH, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic, where she studies health outcomes in PsA, said the findings are helpful in pointing to a trend across a large sample. But she added it’s important to remember that the increasing costs could reflect recent advances in PsA treatment, which include costly biologic drugs.
“There’s a ton more treatments for psoriasis and psoriatic arthritis than there were even just 5 years ago,” she said in an interview. She was not involved in the research.
Dr. Husni would like to see a more detailed look at the costs, from the categories of expenses to the patients who are incurring the highest costs.
“Is it just a couple of percent of really sick patients that are driving the psoriatic arthritis group?” she wondered.
She also pointed out that PsA is going to be more expensive by its very nature. PsA tends to develop 3-10 years after psoriasis, adding to the costs for someone who already has psoriasis and at a time when they are older and likely have higher health care costs because of comorbidities that develop with age.
Dr. Husni said she does think about treatment costs, in that a less expensive first-line drug might be more appropriate than going straight to a more expensive biologic, especially because they also tend to be safer. She said it’s not just a simple question of curbing costs.
“Is there a way that we can personalize medicine?” she asked. “Is there a way that we can be more accurate about which people may need the more expensive drugs, and which patients may need the less expensive drugs? Are we getting better at monitoring so we can avoid high-cost events?”
Mr. Peterson is an employee of Janssen Pharmaceuticals. Dr. Husni reported serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, UCB, Novartis, Lilly, and Pfizer.
* Update, 9/28/21: The headline and parts of this story were updated to better reflect the study on which it reports.
A version of this article first appeared on Medscape.com.
Translating the 2019 AAD-NPF Guidelines of Care for Psoriasis With Attention to Comorbidities
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.

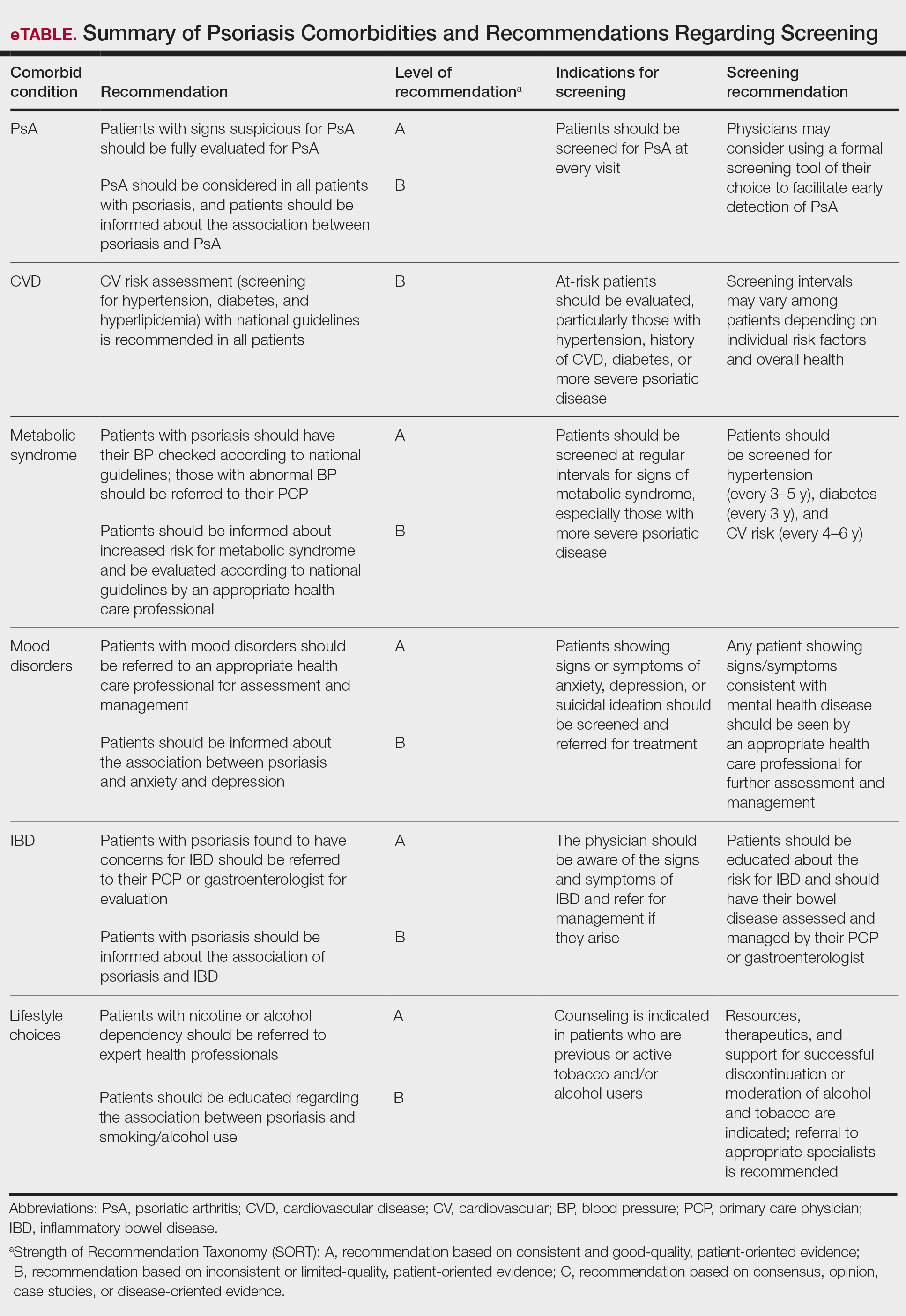
Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Practice Points
- Educating patients about psoriasis and its extracutaneous manifestations, available treatment options, and the impact of lifestyle choices is advised to maximize their patient’s disease awareness and to promote a collaborative physician-patient partnership.
- Physicians are strongly recommended to screen patients with psoriasis for the presence of disease comorbidities to ensure comprehensive management of their disease.
- Managing psoriasis as a multisystem inflammatory disorder requires the combined effort of dermatologists and other specialists to prevent and treat disease comorbidities and enhance patients’ quality of life.
Psoriatic Arthritis Diagnosis and Management in the Era of Telehealth
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
Western diet promoted skin, joint inflammation in preclinical study
A short-term Western diet facilitated the development of interleukin (IL)-23-mediated psoriasis-like skin and joint inflammation and caused shifts in the intestinal microbiota in a murine model – , say the investigators and other experts who reviewed the findings.
The mice did not become obese during the short duration of the multilayered study, which suggests that a Western diet (high sugar, moderate fat) can be impactful independent of obesity, Samuel T. Hwang, MD, PhD, professor and chair of dermatology at the University of California, Davis, and senior author of the study, said in an interview. The study was published in the Journal of Investigative Dermatology.
In an accompanying commentary, Renuka R. Nayak, MD, PhD, of the department of rheumatology at the University of California, San Francisco, wrote that the findings “add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and [psoriatic arthritis] and raise the possibility that the microbiome may contribute to disease severity”.
Mice were fed a Western diet (WD) or conventional chow diet for 6 weeks and then injected with IL-23 minicircle (MC) DNA to induce systemic IL-23 overexpression – or a control minicircle DNA injection – and continued on these diets for another 4 weeks.
The mice in the WD/IL-23 MC DNA group developed erythema and scaling and increased epidermal thickness in the ears; such changes were “remarkably milder” or nonexistent in the other groups. Skin and joint immune cell populations, such as gamma delta T cells, neutrophils, and T helper type 17 cytokines were elevated in WD-fed mice, as were other markers of IL-23-mediated joint inflammation.
Recent research has suggested that the gut microbiota is dysbiotic in patients with psoriasis, and this new study found that WD-fed mice had less microbial diversity than that of mice fed a conventional diet. After IL-23 MC delivery, WD-fed reduced microbial diversity and pronounced dysbiosis.
“When we combined the Western diet and IL-23, we saw some very different microbes in abundance. The whole landscape changed,” Dr. Hwang said in the interview.
The data “suggest that WD and overexpression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner,” he and his coinvestigators wrote.
Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in the WD-fed mice – and moderately affected skin inflammation in conventionally-fed mice as well – which affirmed the role of dysbiosis.
And “notably,” in another layer of the study, mice that switched diets from a WD to a conventional diet had reduced skin and joint inflammation and increased diversity of gut microbiota. (Mice that were fed a WD for 6 weeks and given the IL-23 MC DNA were randomized to continue this diet for another 4 weeks or switch to a conventional diet.)
Commenting on the new research, Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, said it “provides evidence” that diet can affect not only psoriasis, but psoriatic arthritis (PsA) as well, “through altering the ratio of good to bad bacteria in the gut.”
Going forward, better understanding “which specific gut bacteria and bacterial products lead to increased psoriatic inflammation, and the immunologic mechanism by which this occurs” will be important and could lead to novel treatments for psoriasis and PsA, said Dr. Liao, director of the UCSF Psoriasis and Skin Treatment Center.
Next on his research agenda, Dr. Hwang said, is the question of “how microbiota in the gut are actually able to influence inflammation at very distant sites in the joints and the skin.
“We want to understand the metabolic mechanisms,” he said, noting that “we invariably talk about cytokines, but there are other substances, like certain bile acids that are metabolized through the gut microbiome,” which may play a role.
The findings also offer a basis for treatment experiments in humans – of diet, probiotic therapy, or selective antibiotic modulation, for instance, Dr. Hwang said.
And in the meantime, the findings should encourage patients who are interested in making dietary changes, such as reducing sugar intake. “There’s wide interest – patients will ask, is there something I can change to make this better?” Dr. Hwang said. “Before, we could say it might be logical, but now we have some evidence. The message now is [high-sugar, moderate-fat] diets, apart from their ability to stimulate obesity, probably have some effects.”
Dietary change may not replace the need for other psoriasis treatments, he said, “but I think there’s good reason to believe that if you do change your diet, your treatment will be better than it would be without that dietary change,” he said.
In their discussion, Dr. Hwang and coauthors note that WD with IL-23 overexpression also decreased the mRNA expression of barrier-forming tight junction proteins, thus increasing intestinal permeability. This finding may be relevant, they wrote, because “leaky gut has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis, and enhanced immune response,” and has been observed in a number of autoimmune diseases, including psoriasis.
Dr. Hwang, lead author Zhenrui Shi, MD, PhD, and coauthors reported no conflicts of interest. Their study was supported by the National Psoriasis Foundation, as well as the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Cancer Institute.
A short-term Western diet facilitated the development of interleukin (IL)-23-mediated psoriasis-like skin and joint inflammation and caused shifts in the intestinal microbiota in a murine model – , say the investigators and other experts who reviewed the findings.
The mice did not become obese during the short duration of the multilayered study, which suggests that a Western diet (high sugar, moderate fat) can be impactful independent of obesity, Samuel T. Hwang, MD, PhD, professor and chair of dermatology at the University of California, Davis, and senior author of the study, said in an interview. The study was published in the Journal of Investigative Dermatology.
In an accompanying commentary, Renuka R. Nayak, MD, PhD, of the department of rheumatology at the University of California, San Francisco, wrote that the findings “add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and [psoriatic arthritis] and raise the possibility that the microbiome may contribute to disease severity”.
Mice were fed a Western diet (WD) or conventional chow diet for 6 weeks and then injected with IL-23 minicircle (MC) DNA to induce systemic IL-23 overexpression – or a control minicircle DNA injection – and continued on these diets for another 4 weeks.
The mice in the WD/IL-23 MC DNA group developed erythema and scaling and increased epidermal thickness in the ears; such changes were “remarkably milder” or nonexistent in the other groups. Skin and joint immune cell populations, such as gamma delta T cells, neutrophils, and T helper type 17 cytokines were elevated in WD-fed mice, as were other markers of IL-23-mediated joint inflammation.
Recent research has suggested that the gut microbiota is dysbiotic in patients with psoriasis, and this new study found that WD-fed mice had less microbial diversity than that of mice fed a conventional diet. After IL-23 MC delivery, WD-fed reduced microbial diversity and pronounced dysbiosis.
“When we combined the Western diet and IL-23, we saw some very different microbes in abundance. The whole landscape changed,” Dr. Hwang said in the interview.
The data “suggest that WD and overexpression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner,” he and his coinvestigators wrote.
Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in the WD-fed mice – and moderately affected skin inflammation in conventionally-fed mice as well – which affirmed the role of dysbiosis.
And “notably,” in another layer of the study, mice that switched diets from a WD to a conventional diet had reduced skin and joint inflammation and increased diversity of gut microbiota. (Mice that were fed a WD for 6 weeks and given the IL-23 MC DNA were randomized to continue this diet for another 4 weeks or switch to a conventional diet.)
Commenting on the new research, Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, said it “provides evidence” that diet can affect not only psoriasis, but psoriatic arthritis (PsA) as well, “through altering the ratio of good to bad bacteria in the gut.”
Going forward, better understanding “which specific gut bacteria and bacterial products lead to increased psoriatic inflammation, and the immunologic mechanism by which this occurs” will be important and could lead to novel treatments for psoriasis and PsA, said Dr. Liao, director of the UCSF Psoriasis and Skin Treatment Center.
Next on his research agenda, Dr. Hwang said, is the question of “how microbiota in the gut are actually able to influence inflammation at very distant sites in the joints and the skin.
“We want to understand the metabolic mechanisms,” he said, noting that “we invariably talk about cytokines, but there are other substances, like certain bile acids that are metabolized through the gut microbiome,” which may play a role.
The findings also offer a basis for treatment experiments in humans – of diet, probiotic therapy, or selective antibiotic modulation, for instance, Dr. Hwang said.
And in the meantime, the findings should encourage patients who are interested in making dietary changes, such as reducing sugar intake. “There’s wide interest – patients will ask, is there something I can change to make this better?” Dr. Hwang said. “Before, we could say it might be logical, but now we have some evidence. The message now is [high-sugar, moderate-fat] diets, apart from their ability to stimulate obesity, probably have some effects.”
Dietary change may not replace the need for other psoriasis treatments, he said, “but I think there’s good reason to believe that if you do change your diet, your treatment will be better than it would be without that dietary change,” he said.
In their discussion, Dr. Hwang and coauthors note that WD with IL-23 overexpression also decreased the mRNA expression of barrier-forming tight junction proteins, thus increasing intestinal permeability. This finding may be relevant, they wrote, because “leaky gut has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis, and enhanced immune response,” and has been observed in a number of autoimmune diseases, including psoriasis.
Dr. Hwang, lead author Zhenrui Shi, MD, PhD, and coauthors reported no conflicts of interest. Their study was supported by the National Psoriasis Foundation, as well as the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Cancer Institute.
A short-term Western diet facilitated the development of interleukin (IL)-23-mediated psoriasis-like skin and joint inflammation and caused shifts in the intestinal microbiota in a murine model – , say the investigators and other experts who reviewed the findings.
The mice did not become obese during the short duration of the multilayered study, which suggests that a Western diet (high sugar, moderate fat) can be impactful independent of obesity, Samuel T. Hwang, MD, PhD, professor and chair of dermatology at the University of California, Davis, and senior author of the study, said in an interview. The study was published in the Journal of Investigative Dermatology.
In an accompanying commentary, Renuka R. Nayak, MD, PhD, of the department of rheumatology at the University of California, San Francisco, wrote that the findings “add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and [psoriatic arthritis] and raise the possibility that the microbiome may contribute to disease severity”.
Mice were fed a Western diet (WD) or conventional chow diet for 6 weeks and then injected with IL-23 minicircle (MC) DNA to induce systemic IL-23 overexpression – or a control minicircle DNA injection – and continued on these diets for another 4 weeks.
The mice in the WD/IL-23 MC DNA group developed erythema and scaling and increased epidermal thickness in the ears; such changes were “remarkably milder” or nonexistent in the other groups. Skin and joint immune cell populations, such as gamma delta T cells, neutrophils, and T helper type 17 cytokines were elevated in WD-fed mice, as were other markers of IL-23-mediated joint inflammation.
Recent research has suggested that the gut microbiota is dysbiotic in patients with psoriasis, and this new study found that WD-fed mice had less microbial diversity than that of mice fed a conventional diet. After IL-23 MC delivery, WD-fed reduced microbial diversity and pronounced dysbiosis.
“When we combined the Western diet and IL-23, we saw some very different microbes in abundance. The whole landscape changed,” Dr. Hwang said in the interview.
The data “suggest that WD and overexpression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner,” he and his coinvestigators wrote.
Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in the WD-fed mice – and moderately affected skin inflammation in conventionally-fed mice as well – which affirmed the role of dysbiosis.
And “notably,” in another layer of the study, mice that switched diets from a WD to a conventional diet had reduced skin and joint inflammation and increased diversity of gut microbiota. (Mice that were fed a WD for 6 weeks and given the IL-23 MC DNA were randomized to continue this diet for another 4 weeks or switch to a conventional diet.)
Commenting on the new research, Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, said it “provides evidence” that diet can affect not only psoriasis, but psoriatic arthritis (PsA) as well, “through altering the ratio of good to bad bacteria in the gut.”
Going forward, better understanding “which specific gut bacteria and bacterial products lead to increased psoriatic inflammation, and the immunologic mechanism by which this occurs” will be important and could lead to novel treatments for psoriasis and PsA, said Dr. Liao, director of the UCSF Psoriasis and Skin Treatment Center.
Next on his research agenda, Dr. Hwang said, is the question of “how microbiota in the gut are actually able to influence inflammation at very distant sites in the joints and the skin.
“We want to understand the metabolic mechanisms,” he said, noting that “we invariably talk about cytokines, but there are other substances, like certain bile acids that are metabolized through the gut microbiome,” which may play a role.
The findings also offer a basis for treatment experiments in humans – of diet, probiotic therapy, or selective antibiotic modulation, for instance, Dr. Hwang said.
And in the meantime, the findings should encourage patients who are interested in making dietary changes, such as reducing sugar intake. “There’s wide interest – patients will ask, is there something I can change to make this better?” Dr. Hwang said. “Before, we could say it might be logical, but now we have some evidence. The message now is [high-sugar, moderate-fat] diets, apart from their ability to stimulate obesity, probably have some effects.”
Dietary change may not replace the need for other psoriasis treatments, he said, “but I think there’s good reason to believe that if you do change your diet, your treatment will be better than it would be without that dietary change,” he said.
In their discussion, Dr. Hwang and coauthors note that WD with IL-23 overexpression also decreased the mRNA expression of barrier-forming tight junction proteins, thus increasing intestinal permeability. This finding may be relevant, they wrote, because “leaky gut has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis, and enhanced immune response,” and has been observed in a number of autoimmune diseases, including psoriasis.
Dr. Hwang, lead author Zhenrui Shi, MD, PhD, and coauthors reported no conflicts of interest. Their study was supported by the National Psoriasis Foundation, as well as the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Cancer Institute.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
HBV screening often incomplete or forgone when starting tocilizumab, tofacitinib
People beginning treatment with the immunosuppressive drugs tocilizumab (Actemra) or tofacitinib (Xeljanz) are infrequently screened for hepatitis B virus (HBV) infection, according to a new study of patients with rheumatic diseases who are starting one of the two treatments.
“Perhaps not unexpectedly, these screening patterns conform more with recommendations from the American College of Rheumatology, which do not explicitly stipulate universal HBV screening,” wrote lead author Amir M. Mohareb, MD, of Massachusetts General Hospital in Boston. The study was published in The Journal of Rheumatology.
To determine the frequency of HBV screening among this specific population, the researchers conducted a retrospective, cross-sectional study of patients 18 years or older within the Mass General Brigham health system in the Boston area who initiated either of the two drugs before Dec. 31, 2018. Tocilizumab was approved by the Food and Drug Administration on Jan. 11, 2010, and tofacitinib was approved on Nov. 6, 2012.
The final study population included 678 patients on tocilizumab and 391 patients on tofacitinib. The mean age of the patients in each group was 61 years for tocilizumab and 60 years for tofacitinib. A large majority were female (78% of the tocilizumab group, 88% of the tofacitinib group) and 84% of patients in both groups were white. Their primary diagnosis was rheumatoid arthritis (53% of the tocilizumab group, 77% of the tofacitinib group), and most of them – 57% of patients on tocilizumab and 72% of patients on tofacitinib – had a history of being on both conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs).
HBV screening patterns were classified into three categories: complete (all three of the HBV surface antigen [HBsAg], total core antibody [anti-HBcAb], and surface antibody [HBsAb] tests); partial (any one to two tests); and none. Of the 678 patients on tocilizumab, 194 (29%) underwent complete screening, 307 (45%) underwent partial screening, and 177 (26%) had no screening. Of the 391 patients on tofacitinib, 94 (24%) underwent complete screening, 195 (50%) underwent partial screening, and 102 (26%) had none.
Inappropriate testing – defined as either HBV e-antigen (HBeAg), anti-HBcAb IgM, or HBV DNA without a positive HBsAg or total anti-HBcAb – occurred in 22% of patients on tocilizumab and 23% of patients on tofacitinib. After multivariable analysis, the authors found that Whites were less likely to undergo complete screening (odds ratio, 0.74; 95% confidence interval, 0.57-0.95) compared to non-Whites. Previous use of immunosuppressive agents such as conventional synthetic DMARDs (OR, 1.05; 95% CI, 0.72-1.55) and biologic DMARDs with or without prior csDMARDs (OR, 0.73; 95% CI, 0.48-1.12) was not associated with a likelihood of complete appropriate screening.
“These data add to the evidence indicating that clinicians are not completing pretreatment screening for latent infections prior to patients starting high-risk immunosuppressant drugs,” Gabriela Schmajuk, MD, of the University of California, San Francisco, said in an interview. “It can be dangerous, since a fraction of these patients may reactivate latent infections with HBV that can result in liver failure or death.
“On the bright side,” she added, “we have antivirals that can be given as prophylaxis against reactivation of latent HBV if patients do test positive.”
Dr. Schmajuk was previously the senior author of a similar study from the 2019 American College of Rheumatology annual meeting that found only a small percentage of patients who were new users of biologics or new synthetic DMARDs were screened for HBV or hepatitis C virus.
When asked if anything in the study stood out, she acknowledged being “somewhat surprised that patients with prior immunosuppression did not have higher rates of screening. One might expect that since those patients had more opportunities for screening – since they started new medications more times – they would have higher rates, but this did not appear to be the case.”
As a message to rheumatologists who may be starting their patients on any biologic or new synthetic DMARD, she reinforced that “we need universal HBV screening for patients starting these medications. Many clinicians are used to ordering a hepatitis B surface antigen test, but one key message is that we also need to be ordering hepatitis B core antibody tests. Patients with a positive core antibody are still at risk for reactivation.”
The authors noted their study’s limitations, including the data being retrospectively collected and some of the subjects potentially being screened in laboratories outside of the Mass General Brigham health system. In addition, they stated that their findings “may not be generalizable to nonrheumatologic settings or other immunomodulators,” although they added that studies of other patient populations have also uncovered “similarly low HBV screening frequencies.”
Several of the authors reported being supported by institutes within the National Institutes of Health. Beyond that, they declared no potential conflicts of interest.
People beginning treatment with the immunosuppressive drugs tocilizumab (Actemra) or tofacitinib (Xeljanz) are infrequently screened for hepatitis B virus (HBV) infection, according to a new study of patients with rheumatic diseases who are starting one of the two treatments.
“Perhaps not unexpectedly, these screening patterns conform more with recommendations from the American College of Rheumatology, which do not explicitly stipulate universal HBV screening,” wrote lead author Amir M. Mohareb, MD, of Massachusetts General Hospital in Boston. The study was published in The Journal of Rheumatology.
To determine the frequency of HBV screening among this specific population, the researchers conducted a retrospective, cross-sectional study of patients 18 years or older within the Mass General Brigham health system in the Boston area who initiated either of the two drugs before Dec. 31, 2018. Tocilizumab was approved by the Food and Drug Administration on Jan. 11, 2010, and tofacitinib was approved on Nov. 6, 2012.
The final study population included 678 patients on tocilizumab and 391 patients on tofacitinib. The mean age of the patients in each group was 61 years for tocilizumab and 60 years for tofacitinib. A large majority were female (78% of the tocilizumab group, 88% of the tofacitinib group) and 84% of patients in both groups were white. Their primary diagnosis was rheumatoid arthritis (53% of the tocilizumab group, 77% of the tofacitinib group), and most of them – 57% of patients on tocilizumab and 72% of patients on tofacitinib – had a history of being on both conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs).
HBV screening patterns were classified into three categories: complete (all three of the HBV surface antigen [HBsAg], total core antibody [anti-HBcAb], and surface antibody [HBsAb] tests); partial (any one to two tests); and none. Of the 678 patients on tocilizumab, 194 (29%) underwent complete screening, 307 (45%) underwent partial screening, and 177 (26%) had no screening. Of the 391 patients on tofacitinib, 94 (24%) underwent complete screening, 195 (50%) underwent partial screening, and 102 (26%) had none.
Inappropriate testing – defined as either HBV e-antigen (HBeAg), anti-HBcAb IgM, or HBV DNA without a positive HBsAg or total anti-HBcAb – occurred in 22% of patients on tocilizumab and 23% of patients on tofacitinib. After multivariable analysis, the authors found that Whites were less likely to undergo complete screening (odds ratio, 0.74; 95% confidence interval, 0.57-0.95) compared to non-Whites. Previous use of immunosuppressive agents such as conventional synthetic DMARDs (OR, 1.05; 95% CI, 0.72-1.55) and biologic DMARDs with or without prior csDMARDs (OR, 0.73; 95% CI, 0.48-1.12) was not associated with a likelihood of complete appropriate screening.
“These data add to the evidence indicating that clinicians are not completing pretreatment screening for latent infections prior to patients starting high-risk immunosuppressant drugs,” Gabriela Schmajuk, MD, of the University of California, San Francisco, said in an interview. “It can be dangerous, since a fraction of these patients may reactivate latent infections with HBV that can result in liver failure or death.
“On the bright side,” she added, “we have antivirals that can be given as prophylaxis against reactivation of latent HBV if patients do test positive.”
Dr. Schmajuk was previously the senior author of a similar study from the 2019 American College of Rheumatology annual meeting that found only a small percentage of patients who were new users of biologics or new synthetic DMARDs were screened for HBV or hepatitis C virus.
When asked if anything in the study stood out, she acknowledged being “somewhat surprised that patients with prior immunosuppression did not have higher rates of screening. One might expect that since those patients had more opportunities for screening – since they started new medications more times – they would have higher rates, but this did not appear to be the case.”
As a message to rheumatologists who may be starting their patients on any biologic or new synthetic DMARD, she reinforced that “we need universal HBV screening for patients starting these medications. Many clinicians are used to ordering a hepatitis B surface antigen test, but one key message is that we also need to be ordering hepatitis B core antibody tests. Patients with a positive core antibody are still at risk for reactivation.”
The authors noted their study’s limitations, including the data being retrospectively collected and some of the subjects potentially being screened in laboratories outside of the Mass General Brigham health system. In addition, they stated that their findings “may not be generalizable to nonrheumatologic settings or other immunomodulators,” although they added that studies of other patient populations have also uncovered “similarly low HBV screening frequencies.”
Several of the authors reported being supported by institutes within the National Institutes of Health. Beyond that, they declared no potential conflicts of interest.
People beginning treatment with the immunosuppressive drugs tocilizumab (Actemra) or tofacitinib (Xeljanz) are infrequently screened for hepatitis B virus (HBV) infection, according to a new study of patients with rheumatic diseases who are starting one of the two treatments.
“Perhaps not unexpectedly, these screening patterns conform more with recommendations from the American College of Rheumatology, which do not explicitly stipulate universal HBV screening,” wrote lead author Amir M. Mohareb, MD, of Massachusetts General Hospital in Boston. The study was published in The Journal of Rheumatology.
To determine the frequency of HBV screening among this specific population, the researchers conducted a retrospective, cross-sectional study of patients 18 years or older within the Mass General Brigham health system in the Boston area who initiated either of the two drugs before Dec. 31, 2018. Tocilizumab was approved by the Food and Drug Administration on Jan. 11, 2010, and tofacitinib was approved on Nov. 6, 2012.
The final study population included 678 patients on tocilizumab and 391 patients on tofacitinib. The mean age of the patients in each group was 61 years for tocilizumab and 60 years for tofacitinib. A large majority were female (78% of the tocilizumab group, 88% of the tofacitinib group) and 84% of patients in both groups were white. Their primary diagnosis was rheumatoid arthritis (53% of the tocilizumab group, 77% of the tofacitinib group), and most of them – 57% of patients on tocilizumab and 72% of patients on tofacitinib – had a history of being on both conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs).
HBV screening patterns were classified into three categories: complete (all three of the HBV surface antigen [HBsAg], total core antibody [anti-HBcAb], and surface antibody [HBsAb] tests); partial (any one to two tests); and none. Of the 678 patients on tocilizumab, 194 (29%) underwent complete screening, 307 (45%) underwent partial screening, and 177 (26%) had no screening. Of the 391 patients on tofacitinib, 94 (24%) underwent complete screening, 195 (50%) underwent partial screening, and 102 (26%) had none.
Inappropriate testing – defined as either HBV e-antigen (HBeAg), anti-HBcAb IgM, or HBV DNA without a positive HBsAg or total anti-HBcAb – occurred in 22% of patients on tocilizumab and 23% of patients on tofacitinib. After multivariable analysis, the authors found that Whites were less likely to undergo complete screening (odds ratio, 0.74; 95% confidence interval, 0.57-0.95) compared to non-Whites. Previous use of immunosuppressive agents such as conventional synthetic DMARDs (OR, 1.05; 95% CI, 0.72-1.55) and biologic DMARDs with or without prior csDMARDs (OR, 0.73; 95% CI, 0.48-1.12) was not associated with a likelihood of complete appropriate screening.
“These data add to the evidence indicating that clinicians are not completing pretreatment screening for latent infections prior to patients starting high-risk immunosuppressant drugs,” Gabriela Schmajuk, MD, of the University of California, San Francisco, said in an interview. “It can be dangerous, since a fraction of these patients may reactivate latent infections with HBV that can result in liver failure or death.
“On the bright side,” she added, “we have antivirals that can be given as prophylaxis against reactivation of latent HBV if patients do test positive.”
Dr. Schmajuk was previously the senior author of a similar study from the 2019 American College of Rheumatology annual meeting that found only a small percentage of patients who were new users of biologics or new synthetic DMARDs were screened for HBV or hepatitis C virus.
When asked if anything in the study stood out, she acknowledged being “somewhat surprised that patients with prior immunosuppression did not have higher rates of screening. One might expect that since those patients had more opportunities for screening – since they started new medications more times – they would have higher rates, but this did not appear to be the case.”
As a message to rheumatologists who may be starting their patients on any biologic or new synthetic DMARD, she reinforced that “we need universal HBV screening for patients starting these medications. Many clinicians are used to ordering a hepatitis B surface antigen test, but one key message is that we also need to be ordering hepatitis B core antibody tests. Patients with a positive core antibody are still at risk for reactivation.”
The authors noted their study’s limitations, including the data being retrospectively collected and some of the subjects potentially being screened in laboratories outside of the Mass General Brigham health system. In addition, they stated that their findings “may not be generalizable to nonrheumatologic settings or other immunomodulators,” although they added that studies of other patient populations have also uncovered “similarly low HBV screening frequencies.”
Several of the authors reported being supported by institutes within the National Institutes of Health. Beyond that, they declared no potential conflicts of interest.
FROM THE JOURNAL OF RHEUMATOLOGY
Physicians question the future of TNF inhibitors for psoriasis, PsA
Tumor necrosis factor inhibitors have long been the go-to treatment of choice for patients with psoriasis and psoriatic arthritis (PsA). They’ve served patients well since etanercept was first approved for PsA in 2002, but today, with the availability of more attractive interleukin-17 and IL-23 inhibitors, dermatologists and rheumatologists are asking whether it’s time to reconsider the use of TNF inhibitors as first-line therapy in psoriasis and PsA.
“TNF inhibitors have served psoriasis patients well for many years. The question is, ‘Is it time to move on from them as first-line agents for psoriasis?’ ” said April W. Armstrong, MD, MPH, a dermatologist and associate dean for clinical research at the University of Southern California, Los Angeles. Dr. Armstrong participated in a point/counterpoint debate about the merits of IL-17 and IL-23 inhibitors over TNF inhibitors at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. “For the majority of our patients, IL-17 and IL-23 inhibitors are probably rationally better than TNF inhibitors as first-line agents for moderate to severe plaque psoriasis,” she said.
In this debate, dermatologists and rheumatologists cited studies showing the safety and efficacy of IL-17 and IL-23 inhibitors over TNF inhibitors. TNF inhibitors include etanercept (Enbrel and biosimilars), infliximab (Remicade and biosimilars), adalimumab (Humira and biosimilars), certolizumab pegol (Cimzia), and golimumab (Simponi). IL-12/23 inhibitors are limited to ustekinumab (Stelara). IL-17 inhibitors include secukinumab (Cosentyx), ixekizumab (Taltz), and brodalumab (Siliq). IL-23 inhibitors include guselkumab (Tremfya), tildrakizumab (Ilumya), and risankizumab (Skyrizi).
TNF inhibitors are recommended by the American College of Rheumatology as first-line therapy for treatment-naive patients with active PsA, and they, along with IL-12/23, IL-17, and IL-23 inhibitors are all recommended by the American Academy of Dermatology as monotherapy treatment options in adult patients with moderate to severe plaque psoriasis. However, some studies have shown that non–TNF-inhibitor biologics have a higher efficacy than TNF inhibitors in some cases for some patients, such as those with moderate to severe psoriasis alone or for musculoskeletal efficacy in patients with PsA who have peripheral arthritis, enthesitis, dactylitis, or axial manifestations.
Favorable characteristics of non–TNF-inhibitor biologics
Dr. Armstrong cited a number of head-to-head trials to support her view that IL-17 and IL-23 inhibitors are better than TNF inhibitors as first-line agents for patients with moderate to severe plaque psoriasis. In the first head-to-head study of its kind in patients with moderate to severe psoriasis, ustekinumab proved superior to etanercept. Guselkumab was shown to be superior to adalimumab for patients with moderate to severe psoriasis. Tildrakizumab also proved superior to etanercept for patients with psoriasis. Risankizumab bested adalimumab in patients with moderate to severe psoriasis. Ixekizumab proved superior to etanercept in two pivotal studies of patients with widespread moderate-to-severe psoriasis.
IL-23 and IL-17 inhibitors tend to have less frequent maintenance dosing, with IL-17 inhibitors being once every 2 or 4 weeks and IL-23 inhibitors once every 8 or 12 weeks, compared with frequencies ranging from every week to every 8 weeks with TNF inhibitors, Dr. Armstrong said.
IL-17 and IL-23 inhibitors also appear to have fewer safety concerns than TNF inhibitors, although there is less long-term data for them overall and there are some notable exceptions in certain patient populations. TNF inhibitors should be avoided in patients with a history of demyelinating disease or hepatitis B virus infection, and they are not preferred in patients who have a history of latent tuberculosis or advanced heart failure. IL-17 inhibitors should not be used in patients with a history of inflammatory bowel disease, and their use is associated with a higher rate of oral candidiasis. IL-23 inhibitors have a good safety profile overall, she said.
“The IL-17/23 axis is very important to psoriatic arthritis and should be the focus of our treatments” for PsA, said Deepak Jadon, MBBCh, MRCP, PhD, a rheumatologist and director of the rheumatology research unit at Addenbrooke’s Hospital, Cambridge (England) University Hospitals NHS Foundation Trust. In his presentation, he proposed that IL-17 inhibitors and IL-23 inhibitors be used as first-line therapies in PsA ahead of TNF inhibitors.
One reason to go with IL-17 and IL-23 inhibitors may be to ”get it right immunologically the first time,” Dr. Jadon said. He cited evidence showing substantially better response to guselkumab when given to biologic-naive patients with PsA versus those who had a inadequate response to TNF inhibitors, as well as data indicating better response with secukinumab regardless of previous TNF inhibitor use.
IL-17 inhibitors target more domains of psoriatic disease than do TNF inhibitors, he said, noting that “they have excellent musculoskeletal efficacy in patients with moderate skin psoriasis, not just those with severe psoriasis.” Ixekizumab proved superior to adalimumab in biologic-naive patients with PsA. The results of this study also indicated that IL-17 inhibitors should not be reserved only for patients with severe psoriasis since a higher percentage of patients with moderate psoriasis who were taking ixekizumab achieved very low PsA activity. Secukinumab also beat adalimumab in a head-to-head comparison and showed a greater impact on some measures of health-related quality of life.
IL-17 inhibitors also do not require concomitant methotrexate, he said, “which is a major bonus for our patients. All of my patients wish to stop methotrexate even if tolerated. Not having to cope with prescribed methotrexate improves risk of adverse events and frequency of blood test monitoring.”
IL-17 and IL-23 inhibitors appear to have good efficacy against axial disease in patients with PsA. Randomized trial results for secukinumab versus placebo show high percentages of patients improving either 20% or 40% in Assessment in Spondyloarthritis International Society response criteria and reduced inflammatory MRI lesions in the spine and sacroiliac joints. Analyses of trial results in guselkumab-treated patients with axial manifestations of PsA have shown the IL-23 inhibitor’s efficacy versus placebo across different measures of disease activity.
Dr. Jadon also cited real-world data showing that patients stay longer on IL-17 and IL-12/23 inhibitors versus TNF inhibitors. A 2016 study of patients with psoriasis in the PSOLAR registry showed that patients persisted on treatment longer with ustekinumab than with adalimumab, etanercept, or infliximab. Similarly, a 2020 study of patients with psoriasis from the British Association of Dermatologists Biologics and Immunomodulators Register found that both ustekinumab and secukinumab had better sustained drug survival than did adalimumab.
Accessibility weighs heavily in using TNF inhibitor first
Clinical trials data show that IL-17 inhibitors outperform TNF inhibitors for psoriasis, but in clinical practice, TNF inhibitors still perform very well in individual patients and are well tolerated, said Amit Garg, MD, founding chair of the department of dermatology at Hofstra University, Hempstead, N.Y.
He argued in favor of TNF inhibitors as first-line therapy over IL-17 inhibitors for psoriasis. In this case, treatment decisions often come down to accessibility, Dr. Garg said. Not all insurance companies cover the cost of the newer IL-23 inhibitors. Plus, access to TNF inhibitors is widespread and costs are generally lower.
“As a physician, I don’t have complete autonomy in prescribing what I want. The reality is whether it be because of cross indication or discount pricing, [TNF inhibitors] – in particular adalimumab – is widely available on all plans and is usually the preferred treatment plan, at least in our area,” he said. “I’m not a big fan of plans that allow drugs at low or no cost for a year or 2, and then abandon the patients at that point thereafter. I like to use something that insurance will cover sustainably, and, quite frankly, TNFs have served well in that regard.”
However, TNF inhibitors are associated with more safety signals, plus they carry a greater risk of infection, leading to tolerability and persistence issues with patients.
“Psoriasis is a lifelong disease. I wish I could tell you that every drug is going to work well forever for individual patients, but I don’t think we know that yet. From my perspective, for efficacy, general well tolerance, convenience, and access, TNFs are still an important part of our ability to treat psoriasis effectively. I have no problem starting there and transitioning as needed for individual patients.
“In my experience, I think patients on TNFs generally do well. We don’t always get the patients clear and certainly there’s drop off of efficacy over time, but I’m not sure that’s a rationale for [changing treatment],” Dr. Garg said.
Ying Ying (Katy) Leung, MD, a rheumatologist with Singapore General Hospital, and a member of the GRAPPA peripheral arthritis working group, argued against the use of IL-17 and IL-23 inhibitors as first-line treatment for PsA over TNF inhibitors. She reasoned that TNF blockers are more accessible, have more long-term safety data (including data indicating safety during pregnancy), and have better cardiovascular protection. She also noted that GRAPPA treatment recommendations strongly advise using TNF blockers (or IL-17 inhibitors) for treatment-naive patients with PsA.
“Accessibility is very important as I learned along the way of leading the peripheral arthritis [GRAPPA] working group. Accessibility [issues] can be coming from a lot of sources, but if you don’t take good care of accessibility, you might be developing a guideline that is way out of reality and nobody is going to use it,” she said.
In her native Singapore, Dr. Leung said that patients pay for biologics out of pocket, so cost is a key factor for her patients. She stated that adalimumab is available as a biosimilar at about $200 monthly for patients with PsA in Singapore, while the average monthly costs are $1,400 for originator infliximab and $1,500 for originator etanercept. By comparison, secukinumab sells for about $750 monthly, ixekizumab $540 monthly, and guselkumab $2,000 monthly.
Treatment choices should be aligned with the disease manifestations of PsA, Dr. Leung said, keeping in mind that accessibility and individual patient needs and preferences should be considered as well. She conducted an informal comparison that found TNF inhibitors are most effective for patients with uveitis or inflammatory bowel disease. Evidence from head-to-head studies indicates that TNF inhibitors and IL-17 inhibitors have similar efficacy for peripheral arthritis, enthesitis, and dactylitis. But caution is warranted, she suggested, for determining the best biologics for axial disease because no head-to-head comparison trials have been conducted for IL-17 or IL-23 inhibitors versus TNF inhibitors.
Dr. Armstrong has been a consultant to AbbVie, Bristol-Myers Squibb, Dermira, Genzyme, Incyte, Janssen, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB. Dr. Jadon has been a consultant to, has been on speakers bureaus for, and has received grant/research support from AbbVie, Amgen, Celgene, Celltrion, Gilead, Janssen, Eli Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, and UCB. Dr. Garg has consulted for AbbVie, Boehringer Ingelheim, Janssen, and UCB. Dr. Leung has been a consultant to AbbVie, Boehringer Ingelheim, Janssen, Eli Lilly, Novartis, and Pfizer. She has been on speakers bureaus for AbbVie, Janssen Eli Lilly, and Novartis. She has received grant/research support from Pfizer and conference support from AbbVie,
Tumor necrosis factor inhibitors have long been the go-to treatment of choice for patients with psoriasis and psoriatic arthritis (PsA). They’ve served patients well since etanercept was first approved for PsA in 2002, but today, with the availability of more attractive interleukin-17 and IL-23 inhibitors, dermatologists and rheumatologists are asking whether it’s time to reconsider the use of TNF inhibitors as first-line therapy in psoriasis and PsA.
“TNF inhibitors have served psoriasis patients well for many years. The question is, ‘Is it time to move on from them as first-line agents for psoriasis?’ ” said April W. Armstrong, MD, MPH, a dermatologist and associate dean for clinical research at the University of Southern California, Los Angeles. Dr. Armstrong participated in a point/counterpoint debate about the merits of IL-17 and IL-23 inhibitors over TNF inhibitors at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. “For the majority of our patients, IL-17 and IL-23 inhibitors are probably rationally better than TNF inhibitors as first-line agents for moderate to severe plaque psoriasis,” she said.
In this debate, dermatologists and rheumatologists cited studies showing the safety and efficacy of IL-17 and IL-23 inhibitors over TNF inhibitors. TNF inhibitors include etanercept (Enbrel and biosimilars), infliximab (Remicade and biosimilars), adalimumab (Humira and biosimilars), certolizumab pegol (Cimzia), and golimumab (Simponi). IL-12/23 inhibitors are limited to ustekinumab (Stelara). IL-17 inhibitors include secukinumab (Cosentyx), ixekizumab (Taltz), and brodalumab (Siliq). IL-23 inhibitors include guselkumab (Tremfya), tildrakizumab (Ilumya), and risankizumab (Skyrizi).
TNF inhibitors are recommended by the American College of Rheumatology as first-line therapy for treatment-naive patients with active PsA, and they, along with IL-12/23, IL-17, and IL-23 inhibitors are all recommended by the American Academy of Dermatology as monotherapy treatment options in adult patients with moderate to severe plaque psoriasis. However, some studies have shown that non–TNF-inhibitor biologics have a higher efficacy than TNF inhibitors in some cases for some patients, such as those with moderate to severe psoriasis alone or for musculoskeletal efficacy in patients with PsA who have peripheral arthritis, enthesitis, dactylitis, or axial manifestations.
Favorable characteristics of non–TNF-inhibitor biologics
Dr. Armstrong cited a number of head-to-head trials to support her view that IL-17 and IL-23 inhibitors are better than TNF inhibitors as first-line agents for patients with moderate to severe plaque psoriasis. In the first head-to-head study of its kind in patients with moderate to severe psoriasis, ustekinumab proved superior to etanercept. Guselkumab was shown to be superior to adalimumab for patients with moderate to severe psoriasis. Tildrakizumab also proved superior to etanercept for patients with psoriasis. Risankizumab bested adalimumab in patients with moderate to severe psoriasis. Ixekizumab proved superior to etanercept in two pivotal studies of patients with widespread moderate-to-severe psoriasis.
IL-23 and IL-17 inhibitors tend to have less frequent maintenance dosing, with IL-17 inhibitors being once every 2 or 4 weeks and IL-23 inhibitors once every 8 or 12 weeks, compared with frequencies ranging from every week to every 8 weeks with TNF inhibitors, Dr. Armstrong said.
IL-17 and IL-23 inhibitors also appear to have fewer safety concerns than TNF inhibitors, although there is less long-term data for them overall and there are some notable exceptions in certain patient populations. TNF inhibitors should be avoided in patients with a history of demyelinating disease or hepatitis B virus infection, and they are not preferred in patients who have a history of latent tuberculosis or advanced heart failure. IL-17 inhibitors should not be used in patients with a history of inflammatory bowel disease, and their use is associated with a higher rate of oral candidiasis. IL-23 inhibitors have a good safety profile overall, she said.
“The IL-17/23 axis is very important to psoriatic arthritis and should be the focus of our treatments” for PsA, said Deepak Jadon, MBBCh, MRCP, PhD, a rheumatologist and director of the rheumatology research unit at Addenbrooke’s Hospital, Cambridge (England) University Hospitals NHS Foundation Trust. In his presentation, he proposed that IL-17 inhibitors and IL-23 inhibitors be used as first-line therapies in PsA ahead of TNF inhibitors.
One reason to go with IL-17 and IL-23 inhibitors may be to ”get it right immunologically the first time,” Dr. Jadon said. He cited evidence showing substantially better response to guselkumab when given to biologic-naive patients with PsA versus those who had a inadequate response to TNF inhibitors, as well as data indicating better response with secukinumab regardless of previous TNF inhibitor use.
IL-17 inhibitors target more domains of psoriatic disease than do TNF inhibitors, he said, noting that “they have excellent musculoskeletal efficacy in patients with moderate skin psoriasis, not just those with severe psoriasis.” Ixekizumab proved superior to adalimumab in biologic-naive patients with PsA. The results of this study also indicated that IL-17 inhibitors should not be reserved only for patients with severe psoriasis since a higher percentage of patients with moderate psoriasis who were taking ixekizumab achieved very low PsA activity. Secukinumab also beat adalimumab in a head-to-head comparison and showed a greater impact on some measures of health-related quality of life.
IL-17 inhibitors also do not require concomitant methotrexate, he said, “which is a major bonus for our patients. All of my patients wish to stop methotrexate even if tolerated. Not having to cope with prescribed methotrexate improves risk of adverse events and frequency of blood test monitoring.”
IL-17 and IL-23 inhibitors appear to have good efficacy against axial disease in patients with PsA. Randomized trial results for secukinumab versus placebo show high percentages of patients improving either 20% or 40% in Assessment in Spondyloarthritis International Society response criteria and reduced inflammatory MRI lesions in the spine and sacroiliac joints. Analyses of trial results in guselkumab-treated patients with axial manifestations of PsA have shown the IL-23 inhibitor’s efficacy versus placebo across different measures of disease activity.
Dr. Jadon also cited real-world data showing that patients stay longer on IL-17 and IL-12/23 inhibitors versus TNF inhibitors. A 2016 study of patients with psoriasis in the PSOLAR registry showed that patients persisted on treatment longer with ustekinumab than with adalimumab, etanercept, or infliximab. Similarly, a 2020 study of patients with psoriasis from the British Association of Dermatologists Biologics and Immunomodulators Register found that both ustekinumab and secukinumab had better sustained drug survival than did adalimumab.
Accessibility weighs heavily in using TNF inhibitor first
Clinical trials data show that IL-17 inhibitors outperform TNF inhibitors for psoriasis, but in clinical practice, TNF inhibitors still perform very well in individual patients and are well tolerated, said Amit Garg, MD, founding chair of the department of dermatology at Hofstra University, Hempstead, N.Y.
He argued in favor of TNF inhibitors as first-line therapy over IL-17 inhibitors for psoriasis. In this case, treatment decisions often come down to accessibility, Dr. Garg said. Not all insurance companies cover the cost of the newer IL-23 inhibitors. Plus, access to TNF inhibitors is widespread and costs are generally lower.
“As a physician, I don’t have complete autonomy in prescribing what I want. The reality is whether it be because of cross indication or discount pricing, [TNF inhibitors] – in particular adalimumab – is widely available on all plans and is usually the preferred treatment plan, at least in our area,” he said. “I’m not a big fan of plans that allow drugs at low or no cost for a year or 2, and then abandon the patients at that point thereafter. I like to use something that insurance will cover sustainably, and, quite frankly, TNFs have served well in that regard.”
However, TNF inhibitors are associated with more safety signals, plus they carry a greater risk of infection, leading to tolerability and persistence issues with patients.
“Psoriasis is a lifelong disease. I wish I could tell you that every drug is going to work well forever for individual patients, but I don’t think we know that yet. From my perspective, for efficacy, general well tolerance, convenience, and access, TNFs are still an important part of our ability to treat psoriasis effectively. I have no problem starting there and transitioning as needed for individual patients.
“In my experience, I think patients on TNFs generally do well. We don’t always get the patients clear and certainly there’s drop off of efficacy over time, but I’m not sure that’s a rationale for [changing treatment],” Dr. Garg said.
Ying Ying (Katy) Leung, MD, a rheumatologist with Singapore General Hospital, and a member of the GRAPPA peripheral arthritis working group, argued against the use of IL-17 and IL-23 inhibitors as first-line treatment for PsA over TNF inhibitors. She reasoned that TNF blockers are more accessible, have more long-term safety data (including data indicating safety during pregnancy), and have better cardiovascular protection. She also noted that GRAPPA treatment recommendations strongly advise using TNF blockers (or IL-17 inhibitors) for treatment-naive patients with PsA.
“Accessibility is very important as I learned along the way of leading the peripheral arthritis [GRAPPA] working group. Accessibility [issues] can be coming from a lot of sources, but if you don’t take good care of accessibility, you might be developing a guideline that is way out of reality and nobody is going to use it,” she said.
In her native Singapore, Dr. Leung said that patients pay for biologics out of pocket, so cost is a key factor for her patients. She stated that adalimumab is available as a biosimilar at about $200 monthly for patients with PsA in Singapore, while the average monthly costs are $1,400 for originator infliximab and $1,500 for originator etanercept. By comparison, secukinumab sells for about $750 monthly, ixekizumab $540 monthly, and guselkumab $2,000 monthly.
Treatment choices should be aligned with the disease manifestations of PsA, Dr. Leung said, keeping in mind that accessibility and individual patient needs and preferences should be considered as well. She conducted an informal comparison that found TNF inhibitors are most effective for patients with uveitis or inflammatory bowel disease. Evidence from head-to-head studies indicates that TNF inhibitors and IL-17 inhibitors have similar efficacy for peripheral arthritis, enthesitis, and dactylitis. But caution is warranted, she suggested, for determining the best biologics for axial disease because no head-to-head comparison trials have been conducted for IL-17 or IL-23 inhibitors versus TNF inhibitors.
Dr. Armstrong has been a consultant to AbbVie, Bristol-Myers Squibb, Dermira, Genzyme, Incyte, Janssen, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB. Dr. Jadon has been a consultant to, has been on speakers bureaus for, and has received grant/research support from AbbVie, Amgen, Celgene, Celltrion, Gilead, Janssen, Eli Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, and UCB. Dr. Garg has consulted for AbbVie, Boehringer Ingelheim, Janssen, and UCB. Dr. Leung has been a consultant to AbbVie, Boehringer Ingelheim, Janssen, Eli Lilly, Novartis, and Pfizer. She has been on speakers bureaus for AbbVie, Janssen Eli Lilly, and Novartis. She has received grant/research support from Pfizer and conference support from AbbVie,
Tumor necrosis factor inhibitors have long been the go-to treatment of choice for patients with psoriasis and psoriatic arthritis (PsA). They’ve served patients well since etanercept was first approved for PsA in 2002, but today, with the availability of more attractive interleukin-17 and IL-23 inhibitors, dermatologists and rheumatologists are asking whether it’s time to reconsider the use of TNF inhibitors as first-line therapy in psoriasis and PsA.
“TNF inhibitors have served psoriasis patients well for many years. The question is, ‘Is it time to move on from them as first-line agents for psoriasis?’ ” said April W. Armstrong, MD, MPH, a dermatologist and associate dean for clinical research at the University of Southern California, Los Angeles. Dr. Armstrong participated in a point/counterpoint debate about the merits of IL-17 and IL-23 inhibitors over TNF inhibitors at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. “For the majority of our patients, IL-17 and IL-23 inhibitors are probably rationally better than TNF inhibitors as first-line agents for moderate to severe plaque psoriasis,” she said.
In this debate, dermatologists and rheumatologists cited studies showing the safety and efficacy of IL-17 and IL-23 inhibitors over TNF inhibitors. TNF inhibitors include etanercept (Enbrel and biosimilars), infliximab (Remicade and biosimilars), adalimumab (Humira and biosimilars), certolizumab pegol (Cimzia), and golimumab (Simponi). IL-12/23 inhibitors are limited to ustekinumab (Stelara). IL-17 inhibitors include secukinumab (Cosentyx), ixekizumab (Taltz), and brodalumab (Siliq). IL-23 inhibitors include guselkumab (Tremfya), tildrakizumab (Ilumya), and risankizumab (Skyrizi).
TNF inhibitors are recommended by the American College of Rheumatology as first-line therapy for treatment-naive patients with active PsA, and they, along with IL-12/23, IL-17, and IL-23 inhibitors are all recommended by the American Academy of Dermatology as monotherapy treatment options in adult patients with moderate to severe plaque psoriasis. However, some studies have shown that non–TNF-inhibitor biologics have a higher efficacy than TNF inhibitors in some cases for some patients, such as those with moderate to severe psoriasis alone or for musculoskeletal efficacy in patients with PsA who have peripheral arthritis, enthesitis, dactylitis, or axial manifestations.
Favorable characteristics of non–TNF-inhibitor biologics
Dr. Armstrong cited a number of head-to-head trials to support her view that IL-17 and IL-23 inhibitors are better than TNF inhibitors as first-line agents for patients with moderate to severe plaque psoriasis. In the first head-to-head study of its kind in patients with moderate to severe psoriasis, ustekinumab proved superior to etanercept. Guselkumab was shown to be superior to adalimumab for patients with moderate to severe psoriasis. Tildrakizumab also proved superior to etanercept for patients with psoriasis. Risankizumab bested adalimumab in patients with moderate to severe psoriasis. Ixekizumab proved superior to etanercept in two pivotal studies of patients with widespread moderate-to-severe psoriasis.
IL-23 and IL-17 inhibitors tend to have less frequent maintenance dosing, with IL-17 inhibitors being once every 2 or 4 weeks and IL-23 inhibitors once every 8 or 12 weeks, compared with frequencies ranging from every week to every 8 weeks with TNF inhibitors, Dr. Armstrong said.
IL-17 and IL-23 inhibitors also appear to have fewer safety concerns than TNF inhibitors, although there is less long-term data for them overall and there are some notable exceptions in certain patient populations. TNF inhibitors should be avoided in patients with a history of demyelinating disease or hepatitis B virus infection, and they are not preferred in patients who have a history of latent tuberculosis or advanced heart failure. IL-17 inhibitors should not be used in patients with a history of inflammatory bowel disease, and their use is associated with a higher rate of oral candidiasis. IL-23 inhibitors have a good safety profile overall, she said.
“The IL-17/23 axis is very important to psoriatic arthritis and should be the focus of our treatments” for PsA, said Deepak Jadon, MBBCh, MRCP, PhD, a rheumatologist and director of the rheumatology research unit at Addenbrooke’s Hospital, Cambridge (England) University Hospitals NHS Foundation Trust. In his presentation, he proposed that IL-17 inhibitors and IL-23 inhibitors be used as first-line therapies in PsA ahead of TNF inhibitors.
One reason to go with IL-17 and IL-23 inhibitors may be to ”get it right immunologically the first time,” Dr. Jadon said. He cited evidence showing substantially better response to guselkumab when given to biologic-naive patients with PsA versus those who had a inadequate response to TNF inhibitors, as well as data indicating better response with secukinumab regardless of previous TNF inhibitor use.
IL-17 inhibitors target more domains of psoriatic disease than do TNF inhibitors, he said, noting that “they have excellent musculoskeletal efficacy in patients with moderate skin psoriasis, not just those with severe psoriasis.” Ixekizumab proved superior to adalimumab in biologic-naive patients with PsA. The results of this study also indicated that IL-17 inhibitors should not be reserved only for patients with severe psoriasis since a higher percentage of patients with moderate psoriasis who were taking ixekizumab achieved very low PsA activity. Secukinumab also beat adalimumab in a head-to-head comparison and showed a greater impact on some measures of health-related quality of life.
IL-17 inhibitors also do not require concomitant methotrexate, he said, “which is a major bonus for our patients. All of my patients wish to stop methotrexate even if tolerated. Not having to cope with prescribed methotrexate improves risk of adverse events and frequency of blood test monitoring.”
IL-17 and IL-23 inhibitors appear to have good efficacy against axial disease in patients with PsA. Randomized trial results for secukinumab versus placebo show high percentages of patients improving either 20% or 40% in Assessment in Spondyloarthritis International Society response criteria and reduced inflammatory MRI lesions in the spine and sacroiliac joints. Analyses of trial results in guselkumab-treated patients with axial manifestations of PsA have shown the IL-23 inhibitor’s efficacy versus placebo across different measures of disease activity.
Dr. Jadon also cited real-world data showing that patients stay longer on IL-17 and IL-12/23 inhibitors versus TNF inhibitors. A 2016 study of patients with psoriasis in the PSOLAR registry showed that patients persisted on treatment longer with ustekinumab than with adalimumab, etanercept, or infliximab. Similarly, a 2020 study of patients with psoriasis from the British Association of Dermatologists Biologics and Immunomodulators Register found that both ustekinumab and secukinumab had better sustained drug survival than did adalimumab.
Accessibility weighs heavily in using TNF inhibitor first
Clinical trials data show that IL-17 inhibitors outperform TNF inhibitors for psoriasis, but in clinical practice, TNF inhibitors still perform very well in individual patients and are well tolerated, said Amit Garg, MD, founding chair of the department of dermatology at Hofstra University, Hempstead, N.Y.
He argued in favor of TNF inhibitors as first-line therapy over IL-17 inhibitors for psoriasis. In this case, treatment decisions often come down to accessibility, Dr. Garg said. Not all insurance companies cover the cost of the newer IL-23 inhibitors. Plus, access to TNF inhibitors is widespread and costs are generally lower.
“As a physician, I don’t have complete autonomy in prescribing what I want. The reality is whether it be because of cross indication or discount pricing, [TNF inhibitors] – in particular adalimumab – is widely available on all plans and is usually the preferred treatment plan, at least in our area,” he said. “I’m not a big fan of plans that allow drugs at low or no cost for a year or 2, and then abandon the patients at that point thereafter. I like to use something that insurance will cover sustainably, and, quite frankly, TNFs have served well in that regard.”
However, TNF inhibitors are associated with more safety signals, plus they carry a greater risk of infection, leading to tolerability and persistence issues with patients.
“Psoriasis is a lifelong disease. I wish I could tell you that every drug is going to work well forever for individual patients, but I don’t think we know that yet. From my perspective, for efficacy, general well tolerance, convenience, and access, TNFs are still an important part of our ability to treat psoriasis effectively. I have no problem starting there and transitioning as needed for individual patients.
“In my experience, I think patients on TNFs generally do well. We don’t always get the patients clear and certainly there’s drop off of efficacy over time, but I’m not sure that’s a rationale for [changing treatment],” Dr. Garg said.
Ying Ying (Katy) Leung, MD, a rheumatologist with Singapore General Hospital, and a member of the GRAPPA peripheral arthritis working group, argued against the use of IL-17 and IL-23 inhibitors as first-line treatment for PsA over TNF inhibitors. She reasoned that TNF blockers are more accessible, have more long-term safety data (including data indicating safety during pregnancy), and have better cardiovascular protection. She also noted that GRAPPA treatment recommendations strongly advise using TNF blockers (or IL-17 inhibitors) for treatment-naive patients with PsA.
“Accessibility is very important as I learned along the way of leading the peripheral arthritis [GRAPPA] working group. Accessibility [issues] can be coming from a lot of sources, but if you don’t take good care of accessibility, you might be developing a guideline that is way out of reality and nobody is going to use it,” she said.
In her native Singapore, Dr. Leung said that patients pay for biologics out of pocket, so cost is a key factor for her patients. She stated that adalimumab is available as a biosimilar at about $200 monthly for patients with PsA in Singapore, while the average monthly costs are $1,400 for originator infliximab and $1,500 for originator etanercept. By comparison, secukinumab sells for about $750 monthly, ixekizumab $540 monthly, and guselkumab $2,000 monthly.
Treatment choices should be aligned with the disease manifestations of PsA, Dr. Leung said, keeping in mind that accessibility and individual patient needs and preferences should be considered as well. She conducted an informal comparison that found TNF inhibitors are most effective for patients with uveitis or inflammatory bowel disease. Evidence from head-to-head studies indicates that TNF inhibitors and IL-17 inhibitors have similar efficacy for peripheral arthritis, enthesitis, and dactylitis. But caution is warranted, she suggested, for determining the best biologics for axial disease because no head-to-head comparison trials have been conducted for IL-17 or IL-23 inhibitors versus TNF inhibitors.
Dr. Armstrong has been a consultant to AbbVie, Bristol-Myers Squibb, Dermira, Genzyme, Incyte, Janssen, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB. Dr. Jadon has been a consultant to, has been on speakers bureaus for, and has received grant/research support from AbbVie, Amgen, Celgene, Celltrion, Gilead, Janssen, Eli Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, and UCB. Dr. Garg has consulted for AbbVie, Boehringer Ingelheim, Janssen, and UCB. Dr. Leung has been a consultant to AbbVie, Boehringer Ingelheim, Janssen, Eli Lilly, Novartis, and Pfizer. She has been on speakers bureaus for AbbVie, Janssen Eli Lilly, and Novartis. She has received grant/research support from Pfizer and conference support from AbbVie,
FROM THE GRAPPA 2021 ANNUAL MEETING
Clinical Edge Journal Scan Commentary: PsA August 2021
In most patients with psoriatic arthritis (PsA), the musculoskeletal manifestations occur after the onset of cutaneous manifestations. The mechanisms underlying the triggering of joint disease is still not well understood, one burning question is whether early and effective treatment of cutaneous psoriasis will reduce the incidence of PsA. In a retrospective non-randomised study, Gisondi et al compared the incidence rates of PsA in patients with chronic plaque psoriasis receiving either continuous treatment with a biologic disease modifying anti-rheumatic drugs (bDMARD- infliximab, etanercept, adalimumab, ustekinumab and secukinumab) for at least 5 years (n=234, 1584 person-year of follow-up) or at least three courses of narrow band ultraviolet B (nb-UVB) phototherapy (n=230, 1478 person-year of follow-up). bDMARDs treatment was associated with a lower risk of incident PsA (adjusted hazard ratio 0.27, 95% Confidence Interval 0.11–0.66). However, analysis after propensity score matching found no significant difference between treatment with bDMARDs and nb-UVB phototherapy and the risk of incident PsA. Prospective studies are required to answer this important question. Interestingly, nail psoriasis was associated with higher risk of PsA, confirming previous observations.
Due to lack of disease activity biomarkers clinical assessment of disease activity in PsA patients with concomitant fibromyalgia can be challenging. Ultrasound (US) may however be useful in providing objective assessment of disease activity. Polachek et al compared 42 patients with PsA and coexisting fibromyalgia syndrome (FMS) (satisfying CASPAR criteria and the 2016 fibromyalgia classification criteria) to 114 PsA patients without FMS (satisfying CASPAR criteria alone). All patients underwent detailed US evaluation including 52 joints, 40 tendons and 14 entheses, and a scores for synovitis, tenosynovitis and enthesitis were summed to obtain a final US disease activity score for each patient. Those with FMS had higher scores of composite clinical disease activity indices. However, the total US score and its subcategories were similar for those with and without FMS. The total US score significantly correlated with composite indices in PsA patients without FMS but not in PsA patients with FMS. Thus, US is a tool that can be employed to determine PsA disease activity in patients with concomitant FMS. However, the scanning protocol as described is time consuming. A shortened protocol as well as training of rheumatologists and radiologists for reliably assessing synovitis, tenosynovitis and enthesitis is required before US can be feasibly and reliably used in clinical practice.
IL-23 inhibitors were not found to be efficacious in the treatment of axial spondyloarthritis. It is not clear whether these inhibitors improve axial disease in PsA patients, and if indeed axial PsA is distinct from primary axial spondyloarthritis. In a post-hoc analyses of the DISCOVER 1 and DISCOVER 2 studies that included 312 patients with PsA with imaging-confirmed sacroiliitis randomly assigned to either placebo (n=118), guselkumab Q4 (n=103), or Q8 (n=91), Mease et al demonstrated that at week 24, guselkumab Q4 and Q8 groups vs placebo showed higher least-squares mean changes in BASDAI (−2.7 and −2.7 vs −1.3; P less than .0001) and ASDAS (−1.4 and −1.4 vs −0.7; P less than .0001) scores, which were maintained until week 52. Thus, Guselkumab may improve axial PsA. However, axial PsA has not yet been formally defined, and BASDAI and ASDAS are not specific for axial PsA. Once axial PsA is defined, prospective randomised clinical trials with associated MRI studies will be required to determine if IL-23 inhibitors improve symptoms of axial PsA.
In most patients with psoriatic arthritis (PsA), the musculoskeletal manifestations occur after the onset of cutaneous manifestations. The mechanisms underlying the triggering of joint disease is still not well understood, one burning question is whether early and effective treatment of cutaneous psoriasis will reduce the incidence of PsA. In a retrospective non-randomised study, Gisondi et al compared the incidence rates of PsA in patients with chronic plaque psoriasis receiving either continuous treatment with a biologic disease modifying anti-rheumatic drugs (bDMARD- infliximab, etanercept, adalimumab, ustekinumab and secukinumab) for at least 5 years (n=234, 1584 person-year of follow-up) or at least three courses of narrow band ultraviolet B (nb-UVB) phototherapy (n=230, 1478 person-year of follow-up). bDMARDs treatment was associated with a lower risk of incident PsA (adjusted hazard ratio 0.27, 95% Confidence Interval 0.11–0.66). However, analysis after propensity score matching found no significant difference between treatment with bDMARDs and nb-UVB phototherapy and the risk of incident PsA. Prospective studies are required to answer this important question. Interestingly, nail psoriasis was associated with higher risk of PsA, confirming previous observations.
Due to lack of disease activity biomarkers clinical assessment of disease activity in PsA patients with concomitant fibromyalgia can be challenging. Ultrasound (US) may however be useful in providing objective assessment of disease activity. Polachek et al compared 42 patients with PsA and coexisting fibromyalgia syndrome (FMS) (satisfying CASPAR criteria and the 2016 fibromyalgia classification criteria) to 114 PsA patients without FMS (satisfying CASPAR criteria alone). All patients underwent detailed US evaluation including 52 joints, 40 tendons and 14 entheses, and a scores for synovitis, tenosynovitis and enthesitis were summed to obtain a final US disease activity score for each patient. Those with FMS had higher scores of composite clinical disease activity indices. However, the total US score and its subcategories were similar for those with and without FMS. The total US score significantly correlated with composite indices in PsA patients without FMS but not in PsA patients with FMS. Thus, US is a tool that can be employed to determine PsA disease activity in patients with concomitant FMS. However, the scanning protocol as described is time consuming. A shortened protocol as well as training of rheumatologists and radiologists for reliably assessing synovitis, tenosynovitis and enthesitis is required before US can be feasibly and reliably used in clinical practice.
IL-23 inhibitors were not found to be efficacious in the treatment of axial spondyloarthritis. It is not clear whether these inhibitors improve axial disease in PsA patients, and if indeed axial PsA is distinct from primary axial spondyloarthritis. In a post-hoc analyses of the DISCOVER 1 and DISCOVER 2 studies that included 312 patients with PsA with imaging-confirmed sacroiliitis randomly assigned to either placebo (n=118), guselkumab Q4 (n=103), or Q8 (n=91), Mease et al demonstrated that at week 24, guselkumab Q4 and Q8 groups vs placebo showed higher least-squares mean changes in BASDAI (−2.7 and −2.7 vs −1.3; P less than .0001) and ASDAS (−1.4 and −1.4 vs −0.7; P less than .0001) scores, which were maintained until week 52. Thus, Guselkumab may improve axial PsA. However, axial PsA has not yet been formally defined, and BASDAI and ASDAS are not specific for axial PsA. Once axial PsA is defined, prospective randomised clinical trials with associated MRI studies will be required to determine if IL-23 inhibitors improve symptoms of axial PsA.
In most patients with psoriatic arthritis (PsA), the musculoskeletal manifestations occur after the onset of cutaneous manifestations. The mechanisms underlying the triggering of joint disease is still not well understood, one burning question is whether early and effective treatment of cutaneous psoriasis will reduce the incidence of PsA. In a retrospective non-randomised study, Gisondi et al compared the incidence rates of PsA in patients with chronic plaque psoriasis receiving either continuous treatment with a biologic disease modifying anti-rheumatic drugs (bDMARD- infliximab, etanercept, adalimumab, ustekinumab and secukinumab) for at least 5 years (n=234, 1584 person-year of follow-up) or at least three courses of narrow band ultraviolet B (nb-UVB) phototherapy (n=230, 1478 person-year of follow-up). bDMARDs treatment was associated with a lower risk of incident PsA (adjusted hazard ratio 0.27, 95% Confidence Interval 0.11–0.66). However, analysis after propensity score matching found no significant difference between treatment with bDMARDs and nb-UVB phototherapy and the risk of incident PsA. Prospective studies are required to answer this important question. Interestingly, nail psoriasis was associated with higher risk of PsA, confirming previous observations.
Due to lack of disease activity biomarkers clinical assessment of disease activity in PsA patients with concomitant fibromyalgia can be challenging. Ultrasound (US) may however be useful in providing objective assessment of disease activity. Polachek et al compared 42 patients with PsA and coexisting fibromyalgia syndrome (FMS) (satisfying CASPAR criteria and the 2016 fibromyalgia classification criteria) to 114 PsA patients without FMS (satisfying CASPAR criteria alone). All patients underwent detailed US evaluation including 52 joints, 40 tendons and 14 entheses, and a scores for synovitis, tenosynovitis and enthesitis were summed to obtain a final US disease activity score for each patient. Those with FMS had higher scores of composite clinical disease activity indices. However, the total US score and its subcategories were similar for those with and without FMS. The total US score significantly correlated with composite indices in PsA patients without FMS but not in PsA patients with FMS. Thus, US is a tool that can be employed to determine PsA disease activity in patients with concomitant FMS. However, the scanning protocol as described is time consuming. A shortened protocol as well as training of rheumatologists and radiologists for reliably assessing synovitis, tenosynovitis and enthesitis is required before US can be feasibly and reliably used in clinical practice.
IL-23 inhibitors were not found to be efficacious in the treatment of axial spondyloarthritis. It is not clear whether these inhibitors improve axial disease in PsA patients, and if indeed axial PsA is distinct from primary axial spondyloarthritis. In a post-hoc analyses of the DISCOVER 1 and DISCOVER 2 studies that included 312 patients with PsA with imaging-confirmed sacroiliitis randomly assigned to either placebo (n=118), guselkumab Q4 (n=103), or Q8 (n=91), Mease et al demonstrated that at week 24, guselkumab Q4 and Q8 groups vs placebo showed higher least-squares mean changes in BASDAI (−2.7 and −2.7 vs −1.3; P less than .0001) and ASDAS (−1.4 and −1.4 vs −0.7; P less than .0001) scores, which were maintained until week 52. Thus, Guselkumab may improve axial PsA. However, axial PsA has not yet been formally defined, and BASDAI and ASDAS are not specific for axial PsA. Once axial PsA is defined, prospective randomised clinical trials with associated MRI studies will be required to determine if IL-23 inhibitors improve symptoms of axial PsA.
Risk for MACEs higher in new users of IL12/23 and IL17 inhibitors vs TNF inhibitors in PsA
Key clinical point: Among patients with psoriatic arthritis (PsA), risk for major adverse cardiovascular events (MACEs) was higher in those who initiated interleukin (IL)12/23 and IL17 inhibitors vs tumor necrosis factor (TNF) inhibitors. However, risk for MACEs was not different among new-users of TNF inhibitors or apremilast.
Major finding: Overall, 51 MACEs (crude incidence rate, 3.4/1,000 patient-years) were observed. The risk of MACEs was higher (P less than .0001) with IL12/23 (weighted hazard ratio [wHR], 2.0; 95% confidence interval [CI], 1.3-3.0) and IL17 (wHR, 1.9; 95% CI, 1.2-3.0) inhibitors but not with apremilast (wHR, 1.3; 95% CI, 0.8-2.2) vs TNF inhibitors.
Study details: Findings are from a nationwide PsA cohort without a history of cardiovascular diseases involving 9,510 new users of biological disease-modifying anti-rheumatic drugs (TNF inhibitor, n=7289; IL12/23 inhibitor, n=1058; IL17 inhibitor, n=1,163) and 1,885 new users of apremilast.
Disclosures: The study did not receive any funding. P Claudepierre reported receiving consulting fees from and being an investigator for various pharmaceutical companies. Other authors declared no conflicts of interest.
Source: Vegas LP et al. Rheumatology. 2021 Jul 9. doi: 10.1093/rheumatology/keab522.
Key clinical point: Among patients with psoriatic arthritis (PsA), risk for major adverse cardiovascular events (MACEs) was higher in those who initiated interleukin (IL)12/23 and IL17 inhibitors vs tumor necrosis factor (TNF) inhibitors. However, risk for MACEs was not different among new-users of TNF inhibitors or apremilast.
Major finding: Overall, 51 MACEs (crude incidence rate, 3.4/1,000 patient-years) were observed. The risk of MACEs was higher (P less than .0001) with IL12/23 (weighted hazard ratio [wHR], 2.0; 95% confidence interval [CI], 1.3-3.0) and IL17 (wHR, 1.9; 95% CI, 1.2-3.0) inhibitors but not with apremilast (wHR, 1.3; 95% CI, 0.8-2.2) vs TNF inhibitors.
Study details: Findings are from a nationwide PsA cohort without a history of cardiovascular diseases involving 9,510 new users of biological disease-modifying anti-rheumatic drugs (TNF inhibitor, n=7289; IL12/23 inhibitor, n=1058; IL17 inhibitor, n=1,163) and 1,885 new users of apremilast.
Disclosures: The study did not receive any funding. P Claudepierre reported receiving consulting fees from and being an investigator for various pharmaceutical companies. Other authors declared no conflicts of interest.
Source: Vegas LP et al. Rheumatology. 2021 Jul 9. doi: 10.1093/rheumatology/keab522.
Key clinical point: Among patients with psoriatic arthritis (PsA), risk for major adverse cardiovascular events (MACEs) was higher in those who initiated interleukin (IL)12/23 and IL17 inhibitors vs tumor necrosis factor (TNF) inhibitors. However, risk for MACEs was not different among new-users of TNF inhibitors or apremilast.
Major finding: Overall, 51 MACEs (crude incidence rate, 3.4/1,000 patient-years) were observed. The risk of MACEs was higher (P less than .0001) with IL12/23 (weighted hazard ratio [wHR], 2.0; 95% confidence interval [CI], 1.3-3.0) and IL17 (wHR, 1.9; 95% CI, 1.2-3.0) inhibitors but not with apremilast (wHR, 1.3; 95% CI, 0.8-2.2) vs TNF inhibitors.
Study details: Findings are from a nationwide PsA cohort without a history of cardiovascular diseases involving 9,510 new users of biological disease-modifying anti-rheumatic drugs (TNF inhibitor, n=7289; IL12/23 inhibitor, n=1058; IL17 inhibitor, n=1,163) and 1,885 new users of apremilast.
Disclosures: The study did not receive any funding. P Claudepierre reported receiving consulting fees from and being an investigator for various pharmaceutical companies. Other authors declared no conflicts of interest.
Source: Vegas LP et al. Rheumatology. 2021 Jul 9. doi: 10.1093/rheumatology/keab522.
First administered bDMARDs show good drug survival rates in PsA
Key clinical point: The drug survival rate was good for the first biological disease-modifying antirheumatic drug (bDMARD) in patients with psoriatic arthritis (PsA). Moreover, female sex may be a predisposing risk factor for flares and therapeutic switches.
Major finding: Overall, 44.32% of patients switched to another bDMARD. The mean time to first bDMARD discontinuation was 72 months. Overall, the drug survival rate in patients treated with antitumor necrosis factor-α and anti-interleukin (IL)-12/23 or anti-IL17 was 75% at 2 years and 60% at 5 years without a significant difference between the biological agents (P = .66). Female sex was associated with a higher risk for first bDMARD discontinuation (hazard ratio, 2.39; 95% confidence interval, 1.50-3.81).
Study details: The data come from a 15-year, monocentric, real-life study involving 264 patients with PsA who received biologics treatment.
Disclosures: The study reported no external funding. R Ramonda and A Doria reported receiving honoraria and speaker fees from Novartis, AbbVie, Pfizer, MSD, and Janssen. All the other authors declared no conflicts of interest.
Source: Lorenzin M et al. Clin Rheumatol. 2021 Jun 16. doi: 10.1007/s10067-021-05799-0.
Key clinical point: The drug survival rate was good for the first biological disease-modifying antirheumatic drug (bDMARD) in patients with psoriatic arthritis (PsA). Moreover, female sex may be a predisposing risk factor for flares and therapeutic switches.
Major finding: Overall, 44.32% of patients switched to another bDMARD. The mean time to first bDMARD discontinuation was 72 months. Overall, the drug survival rate in patients treated with antitumor necrosis factor-α and anti-interleukin (IL)-12/23 or anti-IL17 was 75% at 2 years and 60% at 5 years without a significant difference between the biological agents (P = .66). Female sex was associated with a higher risk for first bDMARD discontinuation (hazard ratio, 2.39; 95% confidence interval, 1.50-3.81).
Study details: The data come from a 15-year, monocentric, real-life study involving 264 patients with PsA who received biologics treatment.
Disclosures: The study reported no external funding. R Ramonda and A Doria reported receiving honoraria and speaker fees from Novartis, AbbVie, Pfizer, MSD, and Janssen. All the other authors declared no conflicts of interest.
Source: Lorenzin M et al. Clin Rheumatol. 2021 Jun 16. doi: 10.1007/s10067-021-05799-0.
Key clinical point: The drug survival rate was good for the first biological disease-modifying antirheumatic drug (bDMARD) in patients with psoriatic arthritis (PsA). Moreover, female sex may be a predisposing risk factor for flares and therapeutic switches.
Major finding: Overall, 44.32% of patients switched to another bDMARD. The mean time to first bDMARD discontinuation was 72 months. Overall, the drug survival rate in patients treated with antitumor necrosis factor-α and anti-interleukin (IL)-12/23 or anti-IL17 was 75% at 2 years and 60% at 5 years without a significant difference between the biological agents (P = .66). Female sex was associated with a higher risk for first bDMARD discontinuation (hazard ratio, 2.39; 95% confidence interval, 1.50-3.81).
Study details: The data come from a 15-year, monocentric, real-life study involving 264 patients with PsA who received biologics treatment.
Disclosures: The study reported no external funding. R Ramonda and A Doria reported receiving honoraria and speaker fees from Novartis, AbbVie, Pfizer, MSD, and Janssen. All the other authors declared no conflicts of interest.
Source: Lorenzin M et al. Clin Rheumatol. 2021 Jun 16. doi: 10.1007/s10067-021-05799-0.











