User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Ozempic Curbs Hunger – And Not Just for Food
This transcript has been edited for clarity.
If you’ve been paying attention only to the headlines, when you think of “Ozempic” you’ll think of a few things: a blockbuster weight loss drug or the tip of the spear of a completely new industry — why not? A drug so popular that the people it was invented for (those with diabetes) can’t even get it.
Ozempic and other GLP-1 receptor agonists are undeniable game changers. Insofar as obesity is the number-one public health risk in the United States, antiobesity drugs hold immense promise even if all they do is reduce obesity.
In 2023, an article in Scientific Reports presented data suggesting that people on Ozempic might be reducing their alcohol intake, not just their total calories.
A 2024 article in Molecular Psychiatry found that the drug might positively impact cannabis use disorder. An article from Brain Sciences suggests that the drug reduces compulsive shopping.
A picture is starting to form, a picture that suggests these drugs curb hunger both literally and figuratively. That GLP-1 receptor agonists like Ozempic and Mounjaro are fundamentally anticonsumption drugs. In a society that — some would argue — is plagued by overconsumption, these drugs might be just what the doctor ordered.
If only they could stop people from smoking.
Oh, wait — they can.
At least it seems they can, based on a new study appearing in Annals of Internal Medicine.
Before we get too excited, this is not a randomized trial. There actually was a small randomized trial of exenatide (Byetta), which is in the same class as Ozempic but probably a bit less potent, with promising results for smoking cessation. 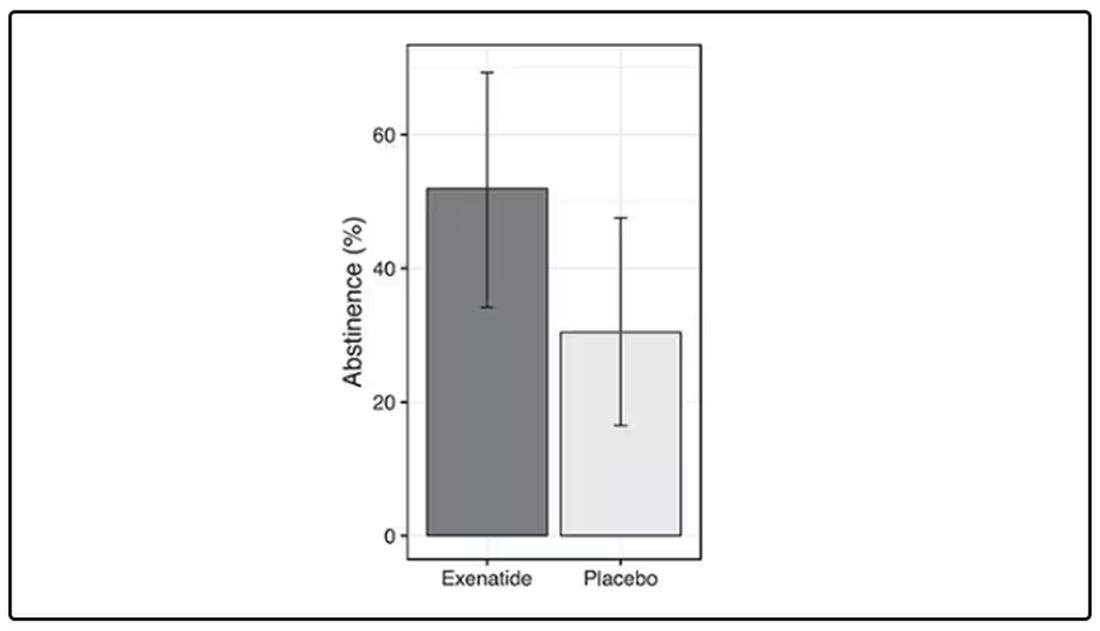
But Byetta is the weaker drug in this class; the market leader is Ozempic. So how can you figure out whether Ozempic can reduce smoking without doing a huge and expensive randomized trial? You can do what Nora Volkow and colleagues from the National Institute on Drug Abuse did: a target trial emulation study.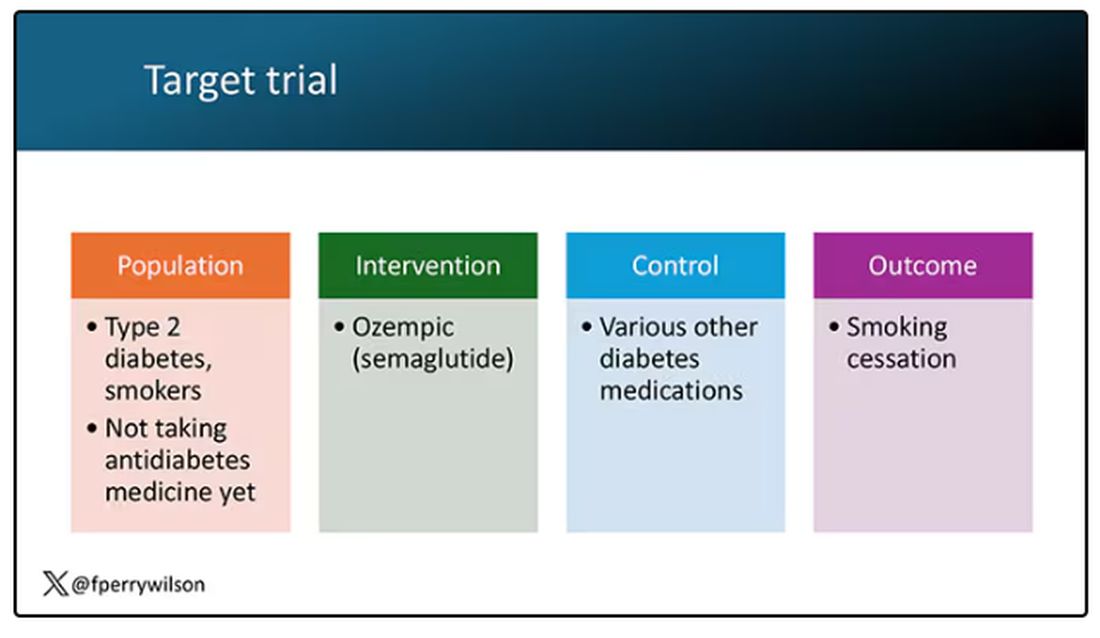
A target trial emulation study is more or less what it sounds like. First, you decide what your dream randomized controlled trial would be and you plan it all out in great detail. You define the population you would recruit, with all the relevant inclusion and exclusion criteria. You define the intervention and the control, and you define the outcome.
But you don’t actually do the trial. You could if someone would lend you $10-$50 million, but assuming you don’t have that lying around, you do the next best thing, which is to dig into a medical record database to find all the people who would be eligible for your imaginary trial. And you analyze them.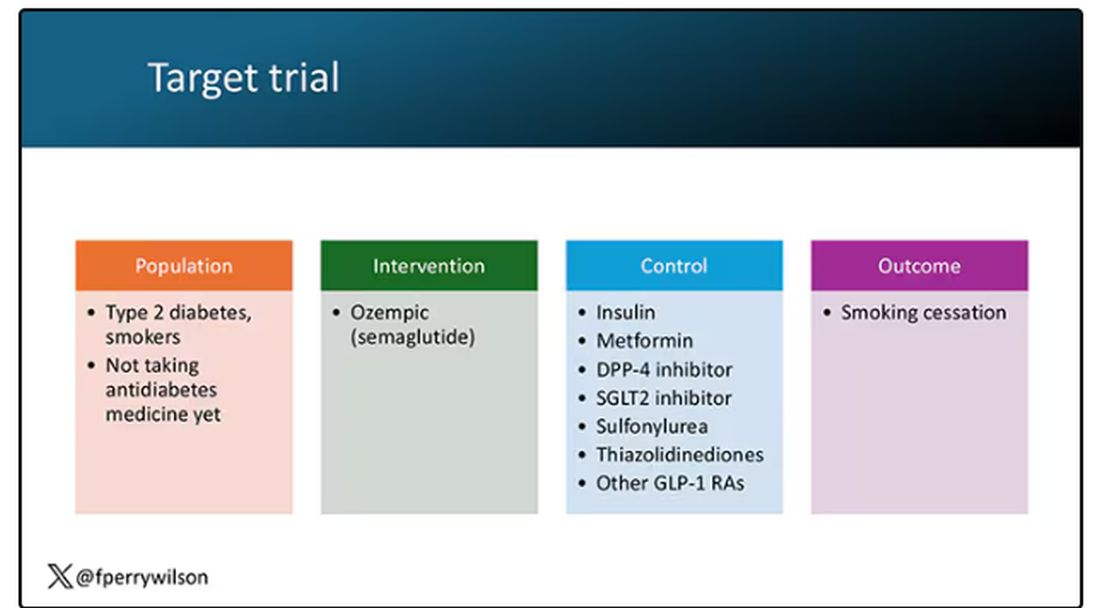
The authors wanted to study the effect of Ozempic on smoking among people with diabetes; that’s why all the comparator agents are antidiabetes drugs. They figured out whether these folks were smoking on the basis of a medical record diagnosis of tobacco use disorder before they started one of the drugs of interest. This code is fairly specific: If a patient has it, you can be pretty sure they are smoking. But it’s not very sensitive; not every smoker has this diagnostic code. This is an age-old limitation of using EHR data instead of asking patients, but it’s part of the tradeoff for not having to spend $50 million.
After applying all those inclusion and exclusion criteria, they have a defined population who could be in their dream trial. And, as luck would have it, some of those people really were treated with Ozempic and some really were treated with those other agents. Although decisions about what to prescribe were not randomized, the authors account for this confounding-by-indication using propensity-score matching. You can find a little explainer on propensity-score matching in an earlier column here. 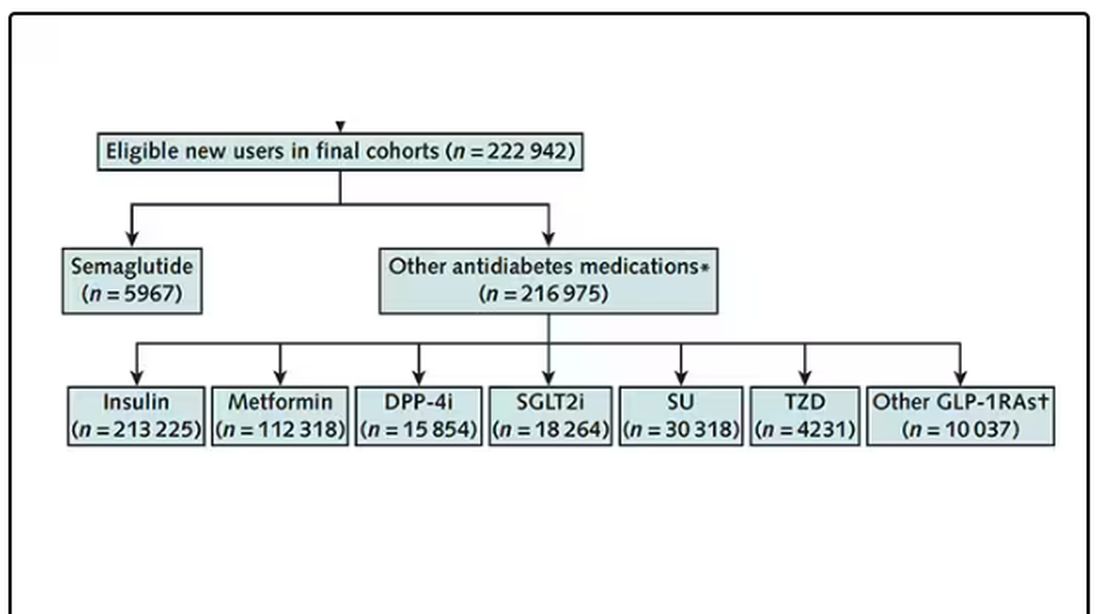
It’s easy enough, using the EHR, to figure out who has diabetes and who got which drug. But how do you know who quit smoking? Remember, everyone had a diagnosis code for tobacco use disorder prior to starting Ozempic or a comparator drug. The authors decided that if the patient had a medical visit where someone again coded tobacco-use disorder, they were still smoking. If someone prescribed smoking cessation meds like a nicotine patch or varenicline, they were obviously still smoking. If someone billed for tobacco-cessation counseling, the patient is still smoking. We’ll get back to the implications of this outcome definition in a minute.
Let’s talk about the results, which are pretty intriguing. 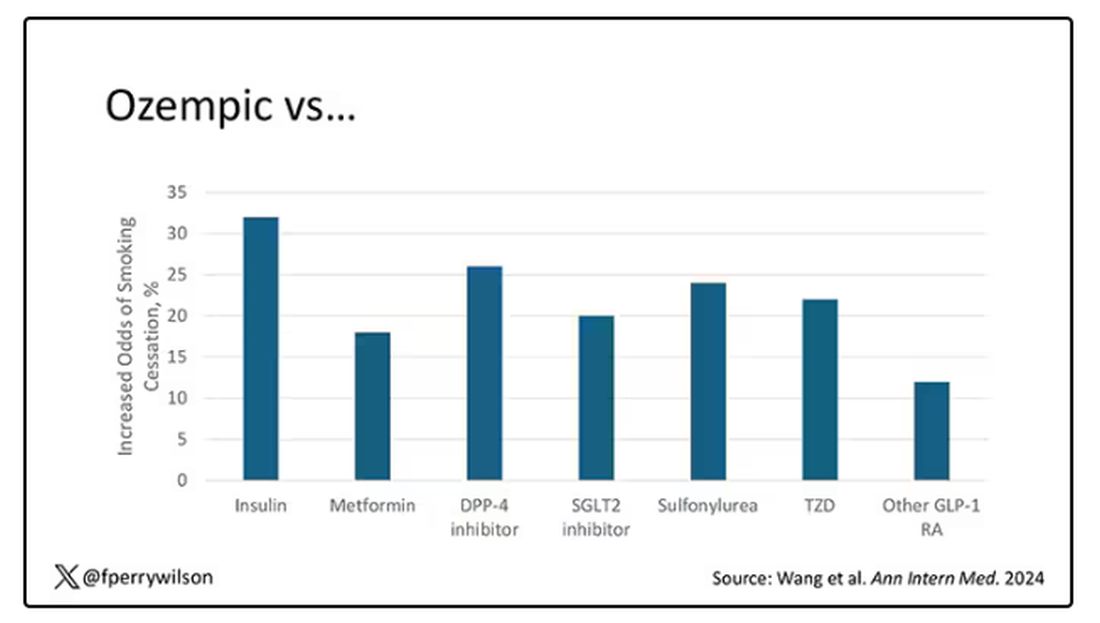
When Ozempic is compared with insulin among smokers with diabetes, those on Ozempic were about 30% more likely to quit smoking. They were about 18% more likely to quit smoking than those who took metformin. They were even slightly more likely to quit smoking than those on other GLP-1 receptor antagonists, though I should note that Mounjaro, which is probably the more potent GLP-1 drug in terms of weight loss, was not among the comparators.
This is pretty impressive for a drug that was not designed to be a smoking cessation drug. It speaks to this emerging idea that these drugs do more than curb appetite by slowing down gastric emptying or something. They work in the brain, modulating some of the reward circuitry that keeps us locked into our bad habits.
There are, of course, some caveats. As I pointed out, this study captured the idea of “still smoking” through the use of administrative codes in the EHR and prescription of smoking cessation aids. You could see similar results if taking Ozempic makes people less likely to address their smoking at all; maybe they shut down the doctor before they even talk about it, or there is too much to discuss during these visits to even get to the subject of smoking. You could also see results like this if people taking Ozempic had fewer visits overall, but the authors showed that that, at least, was not the case.
I’m inclined to believe that this effect is real, simply because we keep seeing signals from multiple sources. If that turns out to be the case, these new “weight loss” drugs may prove to be much more than that; they may turn out to be the drugs that can finally save us from ourselves.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
If you’ve been paying attention only to the headlines, when you think of “Ozempic” you’ll think of a few things: a blockbuster weight loss drug or the tip of the spear of a completely new industry — why not? A drug so popular that the people it was invented for (those with diabetes) can’t even get it.
Ozempic and other GLP-1 receptor agonists are undeniable game changers. Insofar as obesity is the number-one public health risk in the United States, antiobesity drugs hold immense promise even if all they do is reduce obesity.
In 2023, an article in Scientific Reports presented data suggesting that people on Ozempic might be reducing their alcohol intake, not just their total calories.
A 2024 article in Molecular Psychiatry found that the drug might positively impact cannabis use disorder. An article from Brain Sciences suggests that the drug reduces compulsive shopping.
A picture is starting to form, a picture that suggests these drugs curb hunger both literally and figuratively. That GLP-1 receptor agonists like Ozempic and Mounjaro are fundamentally anticonsumption drugs. In a society that — some would argue — is plagued by overconsumption, these drugs might be just what the doctor ordered.
If only they could stop people from smoking.
Oh, wait — they can.
At least it seems they can, based on a new study appearing in Annals of Internal Medicine.
Before we get too excited, this is not a randomized trial. There actually was a small randomized trial of exenatide (Byetta), which is in the same class as Ozempic but probably a bit less potent, with promising results for smoking cessation. 
But Byetta is the weaker drug in this class; the market leader is Ozempic. So how can you figure out whether Ozempic can reduce smoking without doing a huge and expensive randomized trial? You can do what Nora Volkow and colleagues from the National Institute on Drug Abuse did: a target trial emulation study.
A target trial emulation study is more or less what it sounds like. First, you decide what your dream randomized controlled trial would be and you plan it all out in great detail. You define the population you would recruit, with all the relevant inclusion and exclusion criteria. You define the intervention and the control, and you define the outcome.
But you don’t actually do the trial. You could if someone would lend you $10-$50 million, but assuming you don’t have that lying around, you do the next best thing, which is to dig into a medical record database to find all the people who would be eligible for your imaginary trial. And you analyze them.
The authors wanted to study the effect of Ozempic on smoking among people with diabetes; that’s why all the comparator agents are antidiabetes drugs. They figured out whether these folks were smoking on the basis of a medical record diagnosis of tobacco use disorder before they started one of the drugs of interest. This code is fairly specific: If a patient has it, you can be pretty sure they are smoking. But it’s not very sensitive; not every smoker has this diagnostic code. This is an age-old limitation of using EHR data instead of asking patients, but it’s part of the tradeoff for not having to spend $50 million.
After applying all those inclusion and exclusion criteria, they have a defined population who could be in their dream trial. And, as luck would have it, some of those people really were treated with Ozempic and some really were treated with those other agents. Although decisions about what to prescribe were not randomized, the authors account for this confounding-by-indication using propensity-score matching. You can find a little explainer on propensity-score matching in an earlier column here. 
It’s easy enough, using the EHR, to figure out who has diabetes and who got which drug. But how do you know who quit smoking? Remember, everyone had a diagnosis code for tobacco use disorder prior to starting Ozempic or a comparator drug. The authors decided that if the patient had a medical visit where someone again coded tobacco-use disorder, they were still smoking. If someone prescribed smoking cessation meds like a nicotine patch or varenicline, they were obviously still smoking. If someone billed for tobacco-cessation counseling, the patient is still smoking. We’ll get back to the implications of this outcome definition in a minute.
Let’s talk about the results, which are pretty intriguing. 
When Ozempic is compared with insulin among smokers with diabetes, those on Ozempic were about 30% more likely to quit smoking. They were about 18% more likely to quit smoking than those who took metformin. They were even slightly more likely to quit smoking than those on other GLP-1 receptor antagonists, though I should note that Mounjaro, which is probably the more potent GLP-1 drug in terms of weight loss, was not among the comparators.
This is pretty impressive for a drug that was not designed to be a smoking cessation drug. It speaks to this emerging idea that these drugs do more than curb appetite by slowing down gastric emptying or something. They work in the brain, modulating some of the reward circuitry that keeps us locked into our bad habits.
There are, of course, some caveats. As I pointed out, this study captured the idea of “still smoking” through the use of administrative codes in the EHR and prescription of smoking cessation aids. You could see similar results if taking Ozempic makes people less likely to address their smoking at all; maybe they shut down the doctor before they even talk about it, or there is too much to discuss during these visits to even get to the subject of smoking. You could also see results like this if people taking Ozempic had fewer visits overall, but the authors showed that that, at least, was not the case.
I’m inclined to believe that this effect is real, simply because we keep seeing signals from multiple sources. If that turns out to be the case, these new “weight loss” drugs may prove to be much more than that; they may turn out to be the drugs that can finally save us from ourselves.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
If you’ve been paying attention only to the headlines, when you think of “Ozempic” you’ll think of a few things: a blockbuster weight loss drug or the tip of the spear of a completely new industry — why not? A drug so popular that the people it was invented for (those with diabetes) can’t even get it.
Ozempic and other GLP-1 receptor agonists are undeniable game changers. Insofar as obesity is the number-one public health risk in the United States, antiobesity drugs hold immense promise even if all they do is reduce obesity.
In 2023, an article in Scientific Reports presented data suggesting that people on Ozempic might be reducing their alcohol intake, not just their total calories.
A 2024 article in Molecular Psychiatry found that the drug might positively impact cannabis use disorder. An article from Brain Sciences suggests that the drug reduces compulsive shopping.
A picture is starting to form, a picture that suggests these drugs curb hunger both literally and figuratively. That GLP-1 receptor agonists like Ozempic and Mounjaro are fundamentally anticonsumption drugs. In a society that — some would argue — is plagued by overconsumption, these drugs might be just what the doctor ordered.
If only they could stop people from smoking.
Oh, wait — they can.
At least it seems they can, based on a new study appearing in Annals of Internal Medicine.
Before we get too excited, this is not a randomized trial. There actually was a small randomized trial of exenatide (Byetta), which is in the same class as Ozempic but probably a bit less potent, with promising results for smoking cessation. 
But Byetta is the weaker drug in this class; the market leader is Ozempic. So how can you figure out whether Ozempic can reduce smoking without doing a huge and expensive randomized trial? You can do what Nora Volkow and colleagues from the National Institute on Drug Abuse did: a target trial emulation study.
A target trial emulation study is more or less what it sounds like. First, you decide what your dream randomized controlled trial would be and you plan it all out in great detail. You define the population you would recruit, with all the relevant inclusion and exclusion criteria. You define the intervention and the control, and you define the outcome.
But you don’t actually do the trial. You could if someone would lend you $10-$50 million, but assuming you don’t have that lying around, you do the next best thing, which is to dig into a medical record database to find all the people who would be eligible for your imaginary trial. And you analyze them.
The authors wanted to study the effect of Ozempic on smoking among people with diabetes; that’s why all the comparator agents are antidiabetes drugs. They figured out whether these folks were smoking on the basis of a medical record diagnosis of tobacco use disorder before they started one of the drugs of interest. This code is fairly specific: If a patient has it, you can be pretty sure they are smoking. But it’s not very sensitive; not every smoker has this diagnostic code. This is an age-old limitation of using EHR data instead of asking patients, but it’s part of the tradeoff for not having to spend $50 million.
After applying all those inclusion and exclusion criteria, they have a defined population who could be in their dream trial. And, as luck would have it, some of those people really were treated with Ozempic and some really were treated with those other agents. Although decisions about what to prescribe were not randomized, the authors account for this confounding-by-indication using propensity-score matching. You can find a little explainer on propensity-score matching in an earlier column here. 
It’s easy enough, using the EHR, to figure out who has diabetes and who got which drug. But how do you know who quit smoking? Remember, everyone had a diagnosis code for tobacco use disorder prior to starting Ozempic or a comparator drug. The authors decided that if the patient had a medical visit where someone again coded tobacco-use disorder, they were still smoking. If someone prescribed smoking cessation meds like a nicotine patch or varenicline, they were obviously still smoking. If someone billed for tobacco-cessation counseling, the patient is still smoking. We’ll get back to the implications of this outcome definition in a minute.
Let’s talk about the results, which are pretty intriguing. 
When Ozempic is compared with insulin among smokers with diabetes, those on Ozempic were about 30% more likely to quit smoking. They were about 18% more likely to quit smoking than those who took metformin. They were even slightly more likely to quit smoking than those on other GLP-1 receptor antagonists, though I should note that Mounjaro, which is probably the more potent GLP-1 drug in terms of weight loss, was not among the comparators.
This is pretty impressive for a drug that was not designed to be a smoking cessation drug. It speaks to this emerging idea that these drugs do more than curb appetite by slowing down gastric emptying or something. They work in the brain, modulating some of the reward circuitry that keeps us locked into our bad habits.
There are, of course, some caveats. As I pointed out, this study captured the idea of “still smoking” through the use of administrative codes in the EHR and prescription of smoking cessation aids. You could see similar results if taking Ozempic makes people less likely to address their smoking at all; maybe they shut down the doctor before they even talk about it, or there is too much to discuss during these visits to even get to the subject of smoking. You could also see results like this if people taking Ozempic had fewer visits overall, but the authors showed that that, at least, was not the case.
I’m inclined to believe that this effect is real, simply because we keep seeing signals from multiple sources. If that turns out to be the case, these new “weight loss” drugs may prove to be much more than that; they may turn out to be the drugs that can finally save us from ourselves.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Too Much Coffee Linked to Accelerated Cognitive Decline
PHILADELPHIA – results from a large study suggest.
Investigators examined the impact of different amounts of coffee and tea on fluid intelligence — a measure of cognitive functions including abstract reasoning, pattern recognition, and logical thinking.
“It’s the old adage that too much of anything isn’t good. It’s all about balance, so moderate coffee consumption is okay but too much is probably not recommended,” said study investigator Kelsey R. Sewell, PhD, Advent Health Research Institute, Orlando, Florida.
The findings of the study were presented at the 2024 Alzheimer’s Association International Conference (AAIC).
One of the World’s Most Widely Consumed Beverages
Coffee is one of the most widely consumed beverages around the world. The beans contain a range of bioactive compounds, including caffeine, chlorogenic acid, and small amounts of vitamins and minerals.
Consistent evidence from observational and epidemiologic studies indicates that intake of both coffee and tea has beneficial effects on stroke, heart failure, cancers, diabetes, and Parkinson’s disease.
Several studies also suggest that coffee may reduce the risk for Alzheimer’s disease, said Dr. Sewell. However, there are limited longitudinal data on associations between coffee and tea intake and cognitive decline, particularly in distinct cognitive domains.
Dr. Sewell’s group previously published a study of cognitively unimpaired older adults that found greater coffee consumption was associated with slower cognitive decline and slower accumulation of brain beta-amyloid.
Their current study extends some of the prior findings and investigates the relationship between both coffee and tea intake and cognitive decline over time in a larger sample of older adults.
This new study included 8451 mostly female (60%) and White (97%) cognitively unimpaired adults older than 60 (mean age, 67.8 years) in the UK Biobank, a large-scale research resource containing in-depth, deidentified genetic and health information from half a million UK participants. Study subjects had a mean body mass index (BMI) of 26, and about 26% were apolipoprotein epsilon 4 (APOE e4) gene carriers.
Researchers divided coffee and tea consumption into tertiles: high, moderate, and no consumption.
For daily coffee consumption, 18% reported drinking four or more cups (high consumption), 58% reported drinking one to three cups (moderate consumption), and 25% reported that they never drink coffee. For daily tea consumption, 47% reported drinking four or more cups (high consumption), 38% reported drinking one to three cups (moderate consumption), and 15% reported that they never drink tea.
The study assessed cognitive function at baseline and at least two additional patient visits.
Researchers used linear mixed models to assess the relationships between coffee and tea intake and cognitive outcomes. The models adjusted for age, sex, Townsend deprivation index (reflecting socioeconomic status), ethnicity, APOE e4 status, and BMI.
Steeper Decline
Compared with high coffee consumption (four or more cups daily), people who never consumed coffee (beta, 0.06; standard error [SE], 0.02; P = .005) and those with moderate consumption (beta, 0.07; SE, 0.02; P = < .001) had slower decline in fluid intelligence after an average of 8.83 years of follow-up.
“We can see that those with high coffee consumption showed the steepest decline in fluid intelligence across the follow up, compared to those with moderate coffee consumption and those never consuming coffee,” said Dr. Sewell, referring to illustrative graphs.
At the same time, “our data suggest that across this time period, moderate coffee consumption can serve as some kind of protective factor against cognitive decline,” she added.
For tea, there was a somewhat different pattern. People who never drank tea had a greater decline in fluid intelligence, compared with those who had moderate consumption (beta, 0.06; SE, 0.02; P = .0090) or high consumption (beta, 0.06; SE, 0.02; P = .003).
Because this is an observational study, “we still need randomized controlled trials to better understand the neuroprotective mechanism of coffee and tea compounds,” said Dr. Sewell.
Responding later to a query from a meeting delegate about how moderate coffee drinking could be protective, Dr. Sewell said there are probably “different levels of mechanisms,” including at the molecular level (possibly involving amyloid toxicity) and the behavioral level (possibly involving sleep patterns).
Dr. Sewell said that she hopes this line of investigation will lead to new avenues of research in preventive strategies for Alzheimer’s disease.
“We hope that coffee and tea intake could contribute to the development of a safe and inexpensive strategy for delaying the onset and reducing the incidence for Alzheimer’s disease.”
A limitation of the study is possible recall bias, because coffee and tea consumption were self-reported. However, this may not be much of an issue because coffee and tea consumption “is usually quite a habitual behavior,” said Dr. Sewell.
The study also had no data on midlife coffee or tea consumption and did not compare the effect of different preparation methods or types of coffee and tea — for example, green tea versus black tea.
When asked if the study controlled for smoking, Dr. Sewell said it didn’t but added that it would be interesting to explore its impact on cognition.
Dr. Sewell reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – results from a large study suggest.
Investigators examined the impact of different amounts of coffee and tea on fluid intelligence — a measure of cognitive functions including abstract reasoning, pattern recognition, and logical thinking.
“It’s the old adage that too much of anything isn’t good. It’s all about balance, so moderate coffee consumption is okay but too much is probably not recommended,” said study investigator Kelsey R. Sewell, PhD, Advent Health Research Institute, Orlando, Florida.
The findings of the study were presented at the 2024 Alzheimer’s Association International Conference (AAIC).
One of the World’s Most Widely Consumed Beverages
Coffee is one of the most widely consumed beverages around the world. The beans contain a range of bioactive compounds, including caffeine, chlorogenic acid, and small amounts of vitamins and minerals.
Consistent evidence from observational and epidemiologic studies indicates that intake of both coffee and tea has beneficial effects on stroke, heart failure, cancers, diabetes, and Parkinson’s disease.
Several studies also suggest that coffee may reduce the risk for Alzheimer’s disease, said Dr. Sewell. However, there are limited longitudinal data on associations between coffee and tea intake and cognitive decline, particularly in distinct cognitive domains.
Dr. Sewell’s group previously published a study of cognitively unimpaired older adults that found greater coffee consumption was associated with slower cognitive decline and slower accumulation of brain beta-amyloid.
Their current study extends some of the prior findings and investigates the relationship between both coffee and tea intake and cognitive decline over time in a larger sample of older adults.
This new study included 8451 mostly female (60%) and White (97%) cognitively unimpaired adults older than 60 (mean age, 67.8 years) in the UK Biobank, a large-scale research resource containing in-depth, deidentified genetic and health information from half a million UK participants. Study subjects had a mean body mass index (BMI) of 26, and about 26% were apolipoprotein epsilon 4 (APOE e4) gene carriers.
Researchers divided coffee and tea consumption into tertiles: high, moderate, and no consumption.
For daily coffee consumption, 18% reported drinking four or more cups (high consumption), 58% reported drinking one to three cups (moderate consumption), and 25% reported that they never drink coffee. For daily tea consumption, 47% reported drinking four or more cups (high consumption), 38% reported drinking one to three cups (moderate consumption), and 15% reported that they never drink tea.
The study assessed cognitive function at baseline and at least two additional patient visits.
Researchers used linear mixed models to assess the relationships between coffee and tea intake and cognitive outcomes. The models adjusted for age, sex, Townsend deprivation index (reflecting socioeconomic status), ethnicity, APOE e4 status, and BMI.
Steeper Decline
Compared with high coffee consumption (four or more cups daily), people who never consumed coffee (beta, 0.06; standard error [SE], 0.02; P = .005) and those with moderate consumption (beta, 0.07; SE, 0.02; P = < .001) had slower decline in fluid intelligence after an average of 8.83 years of follow-up.
“We can see that those with high coffee consumption showed the steepest decline in fluid intelligence across the follow up, compared to those with moderate coffee consumption and those never consuming coffee,” said Dr. Sewell, referring to illustrative graphs.
At the same time, “our data suggest that across this time period, moderate coffee consumption can serve as some kind of protective factor against cognitive decline,” she added.
For tea, there was a somewhat different pattern. People who never drank tea had a greater decline in fluid intelligence, compared with those who had moderate consumption (beta, 0.06; SE, 0.02; P = .0090) or high consumption (beta, 0.06; SE, 0.02; P = .003).
Because this is an observational study, “we still need randomized controlled trials to better understand the neuroprotective mechanism of coffee and tea compounds,” said Dr. Sewell.
Responding later to a query from a meeting delegate about how moderate coffee drinking could be protective, Dr. Sewell said there are probably “different levels of mechanisms,” including at the molecular level (possibly involving amyloid toxicity) and the behavioral level (possibly involving sleep patterns).
Dr. Sewell said that she hopes this line of investigation will lead to new avenues of research in preventive strategies for Alzheimer’s disease.
“We hope that coffee and tea intake could contribute to the development of a safe and inexpensive strategy for delaying the onset and reducing the incidence for Alzheimer’s disease.”
A limitation of the study is possible recall bias, because coffee and tea consumption were self-reported. However, this may not be much of an issue because coffee and tea consumption “is usually quite a habitual behavior,” said Dr. Sewell.
The study also had no data on midlife coffee or tea consumption and did not compare the effect of different preparation methods or types of coffee and tea — for example, green tea versus black tea.
When asked if the study controlled for smoking, Dr. Sewell said it didn’t but added that it would be interesting to explore its impact on cognition.
Dr. Sewell reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – results from a large study suggest.
Investigators examined the impact of different amounts of coffee and tea on fluid intelligence — a measure of cognitive functions including abstract reasoning, pattern recognition, and logical thinking.
“It’s the old adage that too much of anything isn’t good. It’s all about balance, so moderate coffee consumption is okay but too much is probably not recommended,” said study investigator Kelsey R. Sewell, PhD, Advent Health Research Institute, Orlando, Florida.
The findings of the study were presented at the 2024 Alzheimer’s Association International Conference (AAIC).
One of the World’s Most Widely Consumed Beverages
Coffee is one of the most widely consumed beverages around the world. The beans contain a range of bioactive compounds, including caffeine, chlorogenic acid, and small amounts of vitamins and minerals.
Consistent evidence from observational and epidemiologic studies indicates that intake of both coffee and tea has beneficial effects on stroke, heart failure, cancers, diabetes, and Parkinson’s disease.
Several studies also suggest that coffee may reduce the risk for Alzheimer’s disease, said Dr. Sewell. However, there are limited longitudinal data on associations between coffee and tea intake and cognitive decline, particularly in distinct cognitive domains.
Dr. Sewell’s group previously published a study of cognitively unimpaired older adults that found greater coffee consumption was associated with slower cognitive decline and slower accumulation of brain beta-amyloid.
Their current study extends some of the prior findings and investigates the relationship between both coffee and tea intake and cognitive decline over time in a larger sample of older adults.
This new study included 8451 mostly female (60%) and White (97%) cognitively unimpaired adults older than 60 (mean age, 67.8 years) in the UK Biobank, a large-scale research resource containing in-depth, deidentified genetic and health information from half a million UK participants. Study subjects had a mean body mass index (BMI) of 26, and about 26% were apolipoprotein epsilon 4 (APOE e4) gene carriers.
Researchers divided coffee and tea consumption into tertiles: high, moderate, and no consumption.
For daily coffee consumption, 18% reported drinking four or more cups (high consumption), 58% reported drinking one to three cups (moderate consumption), and 25% reported that they never drink coffee. For daily tea consumption, 47% reported drinking four or more cups (high consumption), 38% reported drinking one to three cups (moderate consumption), and 15% reported that they never drink tea.
The study assessed cognitive function at baseline and at least two additional patient visits.
Researchers used linear mixed models to assess the relationships between coffee and tea intake and cognitive outcomes. The models adjusted for age, sex, Townsend deprivation index (reflecting socioeconomic status), ethnicity, APOE e4 status, and BMI.
Steeper Decline
Compared with high coffee consumption (four or more cups daily), people who never consumed coffee (beta, 0.06; standard error [SE], 0.02; P = .005) and those with moderate consumption (beta, 0.07; SE, 0.02; P = < .001) had slower decline in fluid intelligence after an average of 8.83 years of follow-up.
“We can see that those with high coffee consumption showed the steepest decline in fluid intelligence across the follow up, compared to those with moderate coffee consumption and those never consuming coffee,” said Dr. Sewell, referring to illustrative graphs.
At the same time, “our data suggest that across this time period, moderate coffee consumption can serve as some kind of protective factor against cognitive decline,” she added.
For tea, there was a somewhat different pattern. People who never drank tea had a greater decline in fluid intelligence, compared with those who had moderate consumption (beta, 0.06; SE, 0.02; P = .0090) or high consumption (beta, 0.06; SE, 0.02; P = .003).
Because this is an observational study, “we still need randomized controlled trials to better understand the neuroprotective mechanism of coffee and tea compounds,” said Dr. Sewell.
Responding later to a query from a meeting delegate about how moderate coffee drinking could be protective, Dr. Sewell said there are probably “different levels of mechanisms,” including at the molecular level (possibly involving amyloid toxicity) and the behavioral level (possibly involving sleep patterns).
Dr. Sewell said that she hopes this line of investigation will lead to new avenues of research in preventive strategies for Alzheimer’s disease.
“We hope that coffee and tea intake could contribute to the development of a safe and inexpensive strategy for delaying the onset and reducing the incidence for Alzheimer’s disease.”
A limitation of the study is possible recall bias, because coffee and tea consumption were self-reported. However, this may not be much of an issue because coffee and tea consumption “is usually quite a habitual behavior,” said Dr. Sewell.
The study also had no data on midlife coffee or tea consumption and did not compare the effect of different preparation methods or types of coffee and tea — for example, green tea versus black tea.
When asked if the study controlled for smoking, Dr. Sewell said it didn’t but added that it would be interesting to explore its impact on cognition.
Dr. Sewell reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM AAIC 2024
Insurers’ Rules and AI for Preauthorization: ‘Ethically Nuts,’ Says Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
Almost 50% of Global Dementia Cases May Be Preventable
PHILADELPHIA – a report from the Lancet Commission on dementia prevention, intervention, and care.
The report adds two new modifiable risk factors for dementia — high cholesterol and vision loss — to the 12 risk factors identified in the 2020 Lancet Commission report, which were linked to about 40% of all dementia cases.
The original Lancet Commission report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one third of dementia cases.
“Our new report reveals that there is much more that can and should be done to reduce the risk of dementia. It’s never too early or too late to act, with opportunities to make an impact at any stage of life,” lead author Gill Livingston, MD, from University College London in England, said in a statement.
The 57-page report was published online in The Lancet Neurology (to coincide with its presentation at the 2024 Alzheimer’s Association International Conference (AAIC).
‘Compelling’ New Evidence
The 12 risk factors cited in the 2020 report are lower levels of education, hearing loss, hypertension, smoking, obesity, depression, physical inactivity, diabetes, excessive alcohol consumption, traumatic brain injury (TBI), air pollution, and social isolation.
According to the authors of the current report, there is “new compelling evidence” that untreated vision loss and elevated low-density lipoprotein (LDL) cholesterol are also risk factors for dementia.
These two added risk factors are associated with 9% of all dementia cases — with an estimated 7% of cases caused by high LDL cholesterol from about age 40 years, and 2% of cases caused by untreated vision loss in later life, the authors said.
Out of all 14 risk factors, those tied to the greatest proportion of dementia in the global population are hearing impairment and high LDL cholesterol (7% each), along with less education in early life, and social isolation in later life (5% each), the report estimates.
The new report also outlines 13 recommendations aimed at individuals and governments to help guard against dementia. They include preventing and treating hearing loss, vision loss, and depression; being cognitively active throughout life; using head protection in contact sports; reducing vascular risk factors (high cholesterol, diabetes, obesity, hypertension); improving air quality; and providing supportive community environments to increase social contact.
Tara Spires-Jones, PhD, president of the British Neuroscience Association, emphasized that, while this research doesn’t directly link specific factors to dementia, it supports evidence that a healthy lifestyle — encompassing education, social activities, exercise, cognitive engagement, and avoiding head injuries and harmful factors for heart and lung health — can enhance brain resilience and prevent dementia.
In an interview, Heather M. Snyder, PhD, senior vice president of medical and scientific relations, Alzheimer’s Association, said: “Our brains are complex and what happens throughout our lives may increase or decrease our risk for dementia as we age. Protecting brain health as we age requires a comprehensive approach that includes discussions on diet, exercise, heart health, hearing, and vision.”
Also weighing in on the new report, Shaheen Lakhan, MD, PhD, neurologist and researcher based in Miami, Florida, said the addition of high cholesterol is “particularly noteworthy as it reinforces the intricate connection between vascular health and brain health — a link we’ve long suspected but can now target more effectively.”
As for vision loss, “it’s not just a matter of seeing clearly; it’s a matter of thinking clearly. Untreated vision loss can lead to social isolation, reduced physical activity, and cognitive decline,” said Dr. Lakhan.
Dementia Is Not Inevitable
In his view, “the potential to prevent or delay nearly half of dementia cases by addressing these risk factors is nothing short of revolutionary. It shifts our perspective from viewing dementia as an inevitable part of aging to seeing it as a condition we can actively work to prevent,” Dr. Lakhan added.
He said the report’s emphasis on health equity is also important.
“Dementia risk factors disproportionately affect socioeconomically disadvantaged groups and low- and middle-income countries. Addressing these disparities isn’t just a matter of fairness in the fight against dementia, equality in prevention is as important as equality in treatment,” Dr. Lakhan commented.
While the report offers hope, it also presents a challenge, he said.
Implementing the recommended preventive measures requires a “coordinated effort from individuals, healthcare systems, and policymakers. The potential benefits, both in terms of quality of life and economic savings, make this effort not just worthwhile but imperative. Preventing dementia is not just a medical imperative — it’s an economic and humanitarian one,” Dr. Lakhan said.
Masud Husain, PhD, with the University of Oxford in England, agreed.
The conclusions in this report are “very important for all of us, but particularly for health policy makers and government,” he said.
“If we did simple things well such as screening for some of the factors identified in this report, with adequate resources to perform this, we have the potential to prevent dementia on a national scale. This would be far more cost effective than developing high-tech treatments, which so far have been disappointing in their impacts on people with established dementia,” Dr. Husain said.
The Lancet Commission was funded by University College London, Alzheimer’s Society, Alzheimer’s Research UK, and the Economic and Social Research Council. A complete list of author disclosures is available with the original article. Dr. Snyder, Dr. Lakhan, Dr. Husain and Dr. Spires-Jones have no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA – a report from the Lancet Commission on dementia prevention, intervention, and care.
The report adds two new modifiable risk factors for dementia — high cholesterol and vision loss — to the 12 risk factors identified in the 2020 Lancet Commission report, which were linked to about 40% of all dementia cases.
The original Lancet Commission report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one third of dementia cases.
“Our new report reveals that there is much more that can and should be done to reduce the risk of dementia. It’s never too early or too late to act, with opportunities to make an impact at any stage of life,” lead author Gill Livingston, MD, from University College London in England, said in a statement.
The 57-page report was published online in The Lancet Neurology (to coincide with its presentation at the 2024 Alzheimer’s Association International Conference (AAIC).
‘Compelling’ New Evidence
The 12 risk factors cited in the 2020 report are lower levels of education, hearing loss, hypertension, smoking, obesity, depression, physical inactivity, diabetes, excessive alcohol consumption, traumatic brain injury (TBI), air pollution, and social isolation.
According to the authors of the current report, there is “new compelling evidence” that untreated vision loss and elevated low-density lipoprotein (LDL) cholesterol are also risk factors for dementia.
These two added risk factors are associated with 9% of all dementia cases — with an estimated 7% of cases caused by high LDL cholesterol from about age 40 years, and 2% of cases caused by untreated vision loss in later life, the authors said.
Out of all 14 risk factors, those tied to the greatest proportion of dementia in the global population are hearing impairment and high LDL cholesterol (7% each), along with less education in early life, and social isolation in later life (5% each), the report estimates.
The new report also outlines 13 recommendations aimed at individuals and governments to help guard against dementia. They include preventing and treating hearing loss, vision loss, and depression; being cognitively active throughout life; using head protection in contact sports; reducing vascular risk factors (high cholesterol, diabetes, obesity, hypertension); improving air quality; and providing supportive community environments to increase social contact.
Tara Spires-Jones, PhD, president of the British Neuroscience Association, emphasized that, while this research doesn’t directly link specific factors to dementia, it supports evidence that a healthy lifestyle — encompassing education, social activities, exercise, cognitive engagement, and avoiding head injuries and harmful factors for heart and lung health — can enhance brain resilience and prevent dementia.
In an interview, Heather M. Snyder, PhD, senior vice president of medical and scientific relations, Alzheimer’s Association, said: “Our brains are complex and what happens throughout our lives may increase or decrease our risk for dementia as we age. Protecting brain health as we age requires a comprehensive approach that includes discussions on diet, exercise, heart health, hearing, and vision.”
Also weighing in on the new report, Shaheen Lakhan, MD, PhD, neurologist and researcher based in Miami, Florida, said the addition of high cholesterol is “particularly noteworthy as it reinforces the intricate connection between vascular health and brain health — a link we’ve long suspected but can now target more effectively.”
As for vision loss, “it’s not just a matter of seeing clearly; it’s a matter of thinking clearly. Untreated vision loss can lead to social isolation, reduced physical activity, and cognitive decline,” said Dr. Lakhan.
Dementia Is Not Inevitable
In his view, “the potential to prevent or delay nearly half of dementia cases by addressing these risk factors is nothing short of revolutionary. It shifts our perspective from viewing dementia as an inevitable part of aging to seeing it as a condition we can actively work to prevent,” Dr. Lakhan added.
He said the report’s emphasis on health equity is also important.
“Dementia risk factors disproportionately affect socioeconomically disadvantaged groups and low- and middle-income countries. Addressing these disparities isn’t just a matter of fairness in the fight against dementia, equality in prevention is as important as equality in treatment,” Dr. Lakhan commented.
While the report offers hope, it also presents a challenge, he said.
Implementing the recommended preventive measures requires a “coordinated effort from individuals, healthcare systems, and policymakers. The potential benefits, both in terms of quality of life and economic savings, make this effort not just worthwhile but imperative. Preventing dementia is not just a medical imperative — it’s an economic and humanitarian one,” Dr. Lakhan said.
Masud Husain, PhD, with the University of Oxford in England, agreed.
The conclusions in this report are “very important for all of us, but particularly for health policy makers and government,” he said.
“If we did simple things well such as screening for some of the factors identified in this report, with adequate resources to perform this, we have the potential to prevent dementia on a national scale. This would be far more cost effective than developing high-tech treatments, which so far have been disappointing in their impacts on people with established dementia,” Dr. Husain said.
The Lancet Commission was funded by University College London, Alzheimer’s Society, Alzheimer’s Research UK, and the Economic and Social Research Council. A complete list of author disclosures is available with the original article. Dr. Snyder, Dr. Lakhan, Dr. Husain and Dr. Spires-Jones have no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA – a report from the Lancet Commission on dementia prevention, intervention, and care.
The report adds two new modifiable risk factors for dementia — high cholesterol and vision loss — to the 12 risk factors identified in the 2020 Lancet Commission report, which were linked to about 40% of all dementia cases.
The original Lancet Commission report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one third of dementia cases.
“Our new report reveals that there is much more that can and should be done to reduce the risk of dementia. It’s never too early or too late to act, with opportunities to make an impact at any stage of life,” lead author Gill Livingston, MD, from University College London in England, said in a statement.
The 57-page report was published online in The Lancet Neurology (to coincide with its presentation at the 2024 Alzheimer’s Association International Conference (AAIC).
‘Compelling’ New Evidence
The 12 risk factors cited in the 2020 report are lower levels of education, hearing loss, hypertension, smoking, obesity, depression, physical inactivity, diabetes, excessive alcohol consumption, traumatic brain injury (TBI), air pollution, and social isolation.
According to the authors of the current report, there is “new compelling evidence” that untreated vision loss and elevated low-density lipoprotein (LDL) cholesterol are also risk factors for dementia.
These two added risk factors are associated with 9% of all dementia cases — with an estimated 7% of cases caused by high LDL cholesterol from about age 40 years, and 2% of cases caused by untreated vision loss in later life, the authors said.
Out of all 14 risk factors, those tied to the greatest proportion of dementia in the global population are hearing impairment and high LDL cholesterol (7% each), along with less education in early life, and social isolation in later life (5% each), the report estimates.
The new report also outlines 13 recommendations aimed at individuals and governments to help guard against dementia. They include preventing and treating hearing loss, vision loss, and depression; being cognitively active throughout life; using head protection in contact sports; reducing vascular risk factors (high cholesterol, diabetes, obesity, hypertension); improving air quality; and providing supportive community environments to increase social contact.
Tara Spires-Jones, PhD, president of the British Neuroscience Association, emphasized that, while this research doesn’t directly link specific factors to dementia, it supports evidence that a healthy lifestyle — encompassing education, social activities, exercise, cognitive engagement, and avoiding head injuries and harmful factors for heart and lung health — can enhance brain resilience and prevent dementia.
In an interview, Heather M. Snyder, PhD, senior vice president of medical and scientific relations, Alzheimer’s Association, said: “Our brains are complex and what happens throughout our lives may increase or decrease our risk for dementia as we age. Protecting brain health as we age requires a comprehensive approach that includes discussions on diet, exercise, heart health, hearing, and vision.”
Also weighing in on the new report, Shaheen Lakhan, MD, PhD, neurologist and researcher based in Miami, Florida, said the addition of high cholesterol is “particularly noteworthy as it reinforces the intricate connection between vascular health and brain health — a link we’ve long suspected but can now target more effectively.”
As for vision loss, “it’s not just a matter of seeing clearly; it’s a matter of thinking clearly. Untreated vision loss can lead to social isolation, reduced physical activity, and cognitive decline,” said Dr. Lakhan.
Dementia Is Not Inevitable
In his view, “the potential to prevent or delay nearly half of dementia cases by addressing these risk factors is nothing short of revolutionary. It shifts our perspective from viewing dementia as an inevitable part of aging to seeing it as a condition we can actively work to prevent,” Dr. Lakhan added.
He said the report’s emphasis on health equity is also important.
“Dementia risk factors disproportionately affect socioeconomically disadvantaged groups and low- and middle-income countries. Addressing these disparities isn’t just a matter of fairness in the fight against dementia, equality in prevention is as important as equality in treatment,” Dr. Lakhan commented.
While the report offers hope, it also presents a challenge, he said.
Implementing the recommended preventive measures requires a “coordinated effort from individuals, healthcare systems, and policymakers. The potential benefits, both in terms of quality of life and economic savings, make this effort not just worthwhile but imperative. Preventing dementia is not just a medical imperative — it’s an economic and humanitarian one,” Dr. Lakhan said.
Masud Husain, PhD, with the University of Oxford in England, agreed.
The conclusions in this report are “very important for all of us, but particularly for health policy makers and government,” he said.
“If we did simple things well such as screening for some of the factors identified in this report, with adequate resources to perform this, we have the potential to prevent dementia on a national scale. This would be far more cost effective than developing high-tech treatments, which so far have been disappointing in their impacts on people with established dementia,” Dr. Husain said.
The Lancet Commission was funded by University College London, Alzheimer’s Society, Alzheimer’s Research UK, and the Economic and Social Research Council. A complete list of author disclosures is available with the original article. Dr. Snyder, Dr. Lakhan, Dr. Husain and Dr. Spires-Jones have no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAIC 2024
‘Psychological Weight’ Crucial in Patients With Obesity
Increasingly recognized as a multifactorial disease, obesity demands an approach that involves multiple healthcare professionals. For psychologist Andréa Levy, coordinator and founder of the nongovernmental organization Obesity Brazil, addressing the patient’s “psychological weight” is crucial.
In an interview with this news organization, Ms. Levy, who was one of the speakers at the International Congress on Obesity in 2024, emphasized the importance of integrating emotional and behavioral aspects into treatment, because these factors often influence eating habits and weight gain.
She also highlighted the essential collaboration between endocrinologists, nutritionists, psychiatrists, and psychologists, who must work together to provide comprehensive and effective care to patients.
How do psychological factors affect the treatment of obesity?
Psychological factors are important triggers for weight gain. As the degree of obesity increases, so does the predisposition to mental health problems such as anxiety, mood disorders, personality disorders, and eating disorders. Understanding these factors is important because accurate psychodiagnosis is essential for effective disease treatment.
Without a proper diagnosis, the treatment may be incomplete and omit relevant factors. For example, a person with undiagnosed depression who is starting treatment for weight loss may feel discouraged and low on energy. He or she may wrongly attribute these symptoms to the diet or surgery. Similarly, someone undergoing bariatric surgery may confuse malnutrition symptoms with depression, resulting in inadequate treatment with antidepressants and possible iatrogenic complications.
Furthermore, psychotherapy and psychological follow-up are essential to help the individual organize better and understand the treatment and the disease itself. This is especially important in stigmatized diseases and those subject to prejudice such as obesity, where understanding and acceptance are often challenging, which affects treatment adherence.
Is the collaboration between psychologist and psychiatrist always necessary?
Often, it is necessary to have the support of both a psychologist and a psychiatrist. The process generally begins with a good psychodiagnosis. Initially, there may not be a case that requires treatment, but it is important to perform this evaluation to rule out any issues.
The follow-up, unlike weekly psychotherapy, can be monthly or at an interval agreed on with the patient. It is crucial to help him or her navigate the various stages of obesity treatment. For example, the patient may be going through a period of mourning or separation, or a happier moment, such as the beginning of a relationship or the birth of a child in the family. These moments affect eating habits and need to be well managed.
Depending on the degree of the pathology, such as depression, severe binge-eating disorder, or personality disorders, the psychologist works in conjunction with the psychiatrist. When we talk about obesity, we are possibly also talking about a psychiatric population because it is a disease that, besides being highly recurrent, involves many other factors, such as the gaze of others, difficulty with dressing, body pains, mobility, and relationships. Therefore, having this disease alone is already a trigger for disorders such as depression.
What is the main evidence regarding the psychological follow-up of patients with obesity?
Several studies have investigated the relationship between obesity and mental health. Research indicates that the greater the obesity, the higher the likelihood of a positive diagnosis for a psychiatric disorder. Additionally, there is evidence of the benefits of psychological treatment for patients with obesity.
A study published in the Journal of Clinical Endocrinology and Metabolism addressed the impact of cognitive-behavioral therapy (CBT), which helps patients manage goals and treat maladaptive behaviors such as binge-eating disorders. CBT has a modest effect on weight loss, but its integration as part of a lifestyle modification amplifies the results of this loss.
Recent research also shows that weight loss through bariatric surgery offers significant psychological benefits. In the past, it was believed that this procedure could cause depression and other severe psychiatric disorders, but it is now more than proven that weight loss, when done properly and without misconduct or malnutrition, improves psychological and psychiatric issues.
How does psychological follow-up affect the use of medication during obesity treatment?
Many people who take medications, such as corticosteroids for chronic pain or psychiatric medications, may experience weight gain. It is essential to discuss these issues with the psychiatrist because if the patient already has a predisposition to weight gain, medication X should be chosen instead of medication Y, or the dosage should be adjusted. The psychiatrist needs to understand obesity to medicate correctly. Other types of medication, such as chemotherapeutics, may also cause weight gain, often resulting in more abdominal obesity.
There is also lipedema, a hormone-dependent disease that is different from obesity. In this disease, the person gains weight mainly in the legs and arms. In this case, bariatric surgery may result in weight loss only in specific areas, causing disproportionality and difficulty in understanding for the patient. Therefore, when treating obesity, it is important to analyze the patient from all angles: psychological, physiologic, and physical, considering the diversity of the body, its functioning, and hormonal reactions.
Although psychologists do not prescribe medications, they often explain their functioning to the patient. For example, if a patient is taking a glucagon-like peptide 1 analog and experiences initial nausea, he or she may stop using the treatment because the wrong dose had been started. In this case, the psychologist can explain how the medication works and encourage the patient to discuss adjustments with the doctor, avoiding premature discontinuation.
How has the mental health follow-up of patients with obesity evolved over the years?
I started working with people with obesity 25 years ago, when I myself underwent bariatric surgery. At that time, surgeons were used to “solving” the problem and sending the person home. Often, the patient did not even return for surgical follow-up because, in theory, the problem was solved.
Over time, I believe that surgeons learned to talk to the patient, understanding that there is a whole process that even involves creating a bond with the individual who underwent the surgical procedure. Within this process, the importance of the mental health of patients was recognized, and how common it is to confuse a degree of malnutrition with a mental disorder.
Even though I am not a nutritionist, I need to know the difference between a case of malnutrition and depression. So, it is a whole set of factors that needs to be worked on like an orchestra. It is not necessary for this work to be done in the same physical space, but dialogue is important.
Of course, there are things that the patient will only share with the psychologist or with the surgeon, but there are also pieces of information that need to be shared for positive management. I have had patients who were afraid to go back to the nutritionist because they did not lose weight. If they are afraid, it is because the professional is guiding them incorrectly.
What tips would you give to clinicians regarding the psychological approach to people with obesity?
Accessibility is crucial. When someone tells me they are dealing with obesity and depression, I usually ask, “Did you know you have two chronic diseases?” It is essential to explain these concepts because the patient may often think they are free after a successful diet and weight loss, which is not true because of the high relapse associated with obesity. Depression and anxiety follow similar patterns. If the same person wears prescription glasses, I interact by saying, “Did you know you have three chronic diseases?” This question often causes surprise. “I hadn’t thought of that.”
It is essential to use accessible language for the patient to understand the functioning of the disease. More important than choosing a treatment approach is understanding the pathophysiology of obesity and its psychological impact. This avoids a one-size-fits-all approach for all patients.
For example, the impact on someone who developed obesity in childhood after suffering physical, moral, or sexual abuse will probably be deeper than on someone in a healthy family who gained weight after becoming sedentary. Each life story requires a personalized approach.
Sometimes, a patient with mild obesity (grade 1) may not seem to need specific interventions at first glance, but it is crucial to listen to his or her story. Similarly, patients with severe obesity (grades 3 or 4) who resist surgery are entitled to other treatment options, and this is perfectly valid. Therefore, it is always important to ask, “Who is this person? What does obesity represent in their story?” Then propose the most appropriate treatment.
Ms. Levy reported having no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Increasingly recognized as a multifactorial disease, obesity demands an approach that involves multiple healthcare professionals. For psychologist Andréa Levy, coordinator and founder of the nongovernmental organization Obesity Brazil, addressing the patient’s “psychological weight” is crucial.
In an interview with this news organization, Ms. Levy, who was one of the speakers at the International Congress on Obesity in 2024, emphasized the importance of integrating emotional and behavioral aspects into treatment, because these factors often influence eating habits and weight gain.
She also highlighted the essential collaboration between endocrinologists, nutritionists, psychiatrists, and psychologists, who must work together to provide comprehensive and effective care to patients.
How do psychological factors affect the treatment of obesity?
Psychological factors are important triggers for weight gain. As the degree of obesity increases, so does the predisposition to mental health problems such as anxiety, mood disorders, personality disorders, and eating disorders. Understanding these factors is important because accurate psychodiagnosis is essential for effective disease treatment.
Without a proper diagnosis, the treatment may be incomplete and omit relevant factors. For example, a person with undiagnosed depression who is starting treatment for weight loss may feel discouraged and low on energy. He or she may wrongly attribute these symptoms to the diet or surgery. Similarly, someone undergoing bariatric surgery may confuse malnutrition symptoms with depression, resulting in inadequate treatment with antidepressants and possible iatrogenic complications.
Furthermore, psychotherapy and psychological follow-up are essential to help the individual organize better and understand the treatment and the disease itself. This is especially important in stigmatized diseases and those subject to prejudice such as obesity, where understanding and acceptance are often challenging, which affects treatment adherence.
Is the collaboration between psychologist and psychiatrist always necessary?
Often, it is necessary to have the support of both a psychologist and a psychiatrist. The process generally begins with a good psychodiagnosis. Initially, there may not be a case that requires treatment, but it is important to perform this evaluation to rule out any issues.
The follow-up, unlike weekly psychotherapy, can be monthly or at an interval agreed on with the patient. It is crucial to help him or her navigate the various stages of obesity treatment. For example, the patient may be going through a period of mourning or separation, or a happier moment, such as the beginning of a relationship or the birth of a child in the family. These moments affect eating habits and need to be well managed.
Depending on the degree of the pathology, such as depression, severe binge-eating disorder, or personality disorders, the psychologist works in conjunction with the psychiatrist. When we talk about obesity, we are possibly also talking about a psychiatric population because it is a disease that, besides being highly recurrent, involves many other factors, such as the gaze of others, difficulty with dressing, body pains, mobility, and relationships. Therefore, having this disease alone is already a trigger for disorders such as depression.
What is the main evidence regarding the psychological follow-up of patients with obesity?
Several studies have investigated the relationship between obesity and mental health. Research indicates that the greater the obesity, the higher the likelihood of a positive diagnosis for a psychiatric disorder. Additionally, there is evidence of the benefits of psychological treatment for patients with obesity.
A study published in the Journal of Clinical Endocrinology and Metabolism addressed the impact of cognitive-behavioral therapy (CBT), which helps patients manage goals and treat maladaptive behaviors such as binge-eating disorders. CBT has a modest effect on weight loss, but its integration as part of a lifestyle modification amplifies the results of this loss.
Recent research also shows that weight loss through bariatric surgery offers significant psychological benefits. In the past, it was believed that this procedure could cause depression and other severe psychiatric disorders, but it is now more than proven that weight loss, when done properly and without misconduct or malnutrition, improves psychological and psychiatric issues.
How does psychological follow-up affect the use of medication during obesity treatment?
Many people who take medications, such as corticosteroids for chronic pain or psychiatric medications, may experience weight gain. It is essential to discuss these issues with the psychiatrist because if the patient already has a predisposition to weight gain, medication X should be chosen instead of medication Y, or the dosage should be adjusted. The psychiatrist needs to understand obesity to medicate correctly. Other types of medication, such as chemotherapeutics, may also cause weight gain, often resulting in more abdominal obesity.
There is also lipedema, a hormone-dependent disease that is different from obesity. In this disease, the person gains weight mainly in the legs and arms. In this case, bariatric surgery may result in weight loss only in specific areas, causing disproportionality and difficulty in understanding for the patient. Therefore, when treating obesity, it is important to analyze the patient from all angles: psychological, physiologic, and physical, considering the diversity of the body, its functioning, and hormonal reactions.
Although psychologists do not prescribe medications, they often explain their functioning to the patient. For example, if a patient is taking a glucagon-like peptide 1 analog and experiences initial nausea, he or she may stop using the treatment because the wrong dose had been started. In this case, the psychologist can explain how the medication works and encourage the patient to discuss adjustments with the doctor, avoiding premature discontinuation.
How has the mental health follow-up of patients with obesity evolved over the years?
I started working with people with obesity 25 years ago, when I myself underwent bariatric surgery. At that time, surgeons were used to “solving” the problem and sending the person home. Often, the patient did not even return for surgical follow-up because, in theory, the problem was solved.
Over time, I believe that surgeons learned to talk to the patient, understanding that there is a whole process that even involves creating a bond with the individual who underwent the surgical procedure. Within this process, the importance of the mental health of patients was recognized, and how common it is to confuse a degree of malnutrition with a mental disorder.
Even though I am not a nutritionist, I need to know the difference between a case of malnutrition and depression. So, it is a whole set of factors that needs to be worked on like an orchestra. It is not necessary for this work to be done in the same physical space, but dialogue is important.
Of course, there are things that the patient will only share with the psychologist or with the surgeon, but there are also pieces of information that need to be shared for positive management. I have had patients who were afraid to go back to the nutritionist because they did not lose weight. If they are afraid, it is because the professional is guiding them incorrectly.
What tips would you give to clinicians regarding the psychological approach to people with obesity?
Accessibility is crucial. When someone tells me they are dealing with obesity and depression, I usually ask, “Did you know you have two chronic diseases?” It is essential to explain these concepts because the patient may often think they are free after a successful diet and weight loss, which is not true because of the high relapse associated with obesity. Depression and anxiety follow similar patterns. If the same person wears prescription glasses, I interact by saying, “Did you know you have three chronic diseases?” This question often causes surprise. “I hadn’t thought of that.”
It is essential to use accessible language for the patient to understand the functioning of the disease. More important than choosing a treatment approach is understanding the pathophysiology of obesity and its psychological impact. This avoids a one-size-fits-all approach for all patients.
For example, the impact on someone who developed obesity in childhood after suffering physical, moral, or sexual abuse will probably be deeper than on someone in a healthy family who gained weight after becoming sedentary. Each life story requires a personalized approach.
Sometimes, a patient with mild obesity (grade 1) may not seem to need specific interventions at first glance, but it is crucial to listen to his or her story. Similarly, patients with severe obesity (grades 3 or 4) who resist surgery are entitled to other treatment options, and this is perfectly valid. Therefore, it is always important to ask, “Who is this person? What does obesity represent in their story?” Then propose the most appropriate treatment.
Ms. Levy reported having no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Increasingly recognized as a multifactorial disease, obesity demands an approach that involves multiple healthcare professionals. For psychologist Andréa Levy, coordinator and founder of the nongovernmental organization Obesity Brazil, addressing the patient’s “psychological weight” is crucial.
In an interview with this news organization, Ms. Levy, who was one of the speakers at the International Congress on Obesity in 2024, emphasized the importance of integrating emotional and behavioral aspects into treatment, because these factors often influence eating habits and weight gain.
She also highlighted the essential collaboration between endocrinologists, nutritionists, psychiatrists, and psychologists, who must work together to provide comprehensive and effective care to patients.
How do psychological factors affect the treatment of obesity?
Psychological factors are important triggers for weight gain. As the degree of obesity increases, so does the predisposition to mental health problems such as anxiety, mood disorders, personality disorders, and eating disorders. Understanding these factors is important because accurate psychodiagnosis is essential for effective disease treatment.
Without a proper diagnosis, the treatment may be incomplete and omit relevant factors. For example, a person with undiagnosed depression who is starting treatment for weight loss may feel discouraged and low on energy. He or she may wrongly attribute these symptoms to the diet or surgery. Similarly, someone undergoing bariatric surgery may confuse malnutrition symptoms with depression, resulting in inadequate treatment with antidepressants and possible iatrogenic complications.
Furthermore, psychotherapy and psychological follow-up are essential to help the individual organize better and understand the treatment and the disease itself. This is especially important in stigmatized diseases and those subject to prejudice such as obesity, where understanding and acceptance are often challenging, which affects treatment adherence.
Is the collaboration between psychologist and psychiatrist always necessary?
Often, it is necessary to have the support of both a psychologist and a psychiatrist. The process generally begins with a good psychodiagnosis. Initially, there may not be a case that requires treatment, but it is important to perform this evaluation to rule out any issues.
The follow-up, unlike weekly psychotherapy, can be monthly or at an interval agreed on with the patient. It is crucial to help him or her navigate the various stages of obesity treatment. For example, the patient may be going through a period of mourning or separation, or a happier moment, such as the beginning of a relationship or the birth of a child in the family. These moments affect eating habits and need to be well managed.
Depending on the degree of the pathology, such as depression, severe binge-eating disorder, or personality disorders, the psychologist works in conjunction with the psychiatrist. When we talk about obesity, we are possibly also talking about a psychiatric population because it is a disease that, besides being highly recurrent, involves many other factors, such as the gaze of others, difficulty with dressing, body pains, mobility, and relationships. Therefore, having this disease alone is already a trigger for disorders such as depression.
What is the main evidence regarding the psychological follow-up of patients with obesity?
Several studies have investigated the relationship between obesity and mental health. Research indicates that the greater the obesity, the higher the likelihood of a positive diagnosis for a psychiatric disorder. Additionally, there is evidence of the benefits of psychological treatment for patients with obesity.
A study published in the Journal of Clinical Endocrinology and Metabolism addressed the impact of cognitive-behavioral therapy (CBT), which helps patients manage goals and treat maladaptive behaviors such as binge-eating disorders. CBT has a modest effect on weight loss, but its integration as part of a lifestyle modification amplifies the results of this loss.
Recent research also shows that weight loss through bariatric surgery offers significant psychological benefits. In the past, it was believed that this procedure could cause depression and other severe psychiatric disorders, but it is now more than proven that weight loss, when done properly and without misconduct or malnutrition, improves psychological and psychiatric issues.
How does psychological follow-up affect the use of medication during obesity treatment?
Many people who take medications, such as corticosteroids for chronic pain or psychiatric medications, may experience weight gain. It is essential to discuss these issues with the psychiatrist because if the patient already has a predisposition to weight gain, medication X should be chosen instead of medication Y, or the dosage should be adjusted. The psychiatrist needs to understand obesity to medicate correctly. Other types of medication, such as chemotherapeutics, may also cause weight gain, often resulting in more abdominal obesity.
There is also lipedema, a hormone-dependent disease that is different from obesity. In this disease, the person gains weight mainly in the legs and arms. In this case, bariatric surgery may result in weight loss only in specific areas, causing disproportionality and difficulty in understanding for the patient. Therefore, when treating obesity, it is important to analyze the patient from all angles: psychological, physiologic, and physical, considering the diversity of the body, its functioning, and hormonal reactions.
Although psychologists do not prescribe medications, they often explain their functioning to the patient. For example, if a patient is taking a glucagon-like peptide 1 analog and experiences initial nausea, he or she may stop using the treatment because the wrong dose had been started. In this case, the psychologist can explain how the medication works and encourage the patient to discuss adjustments with the doctor, avoiding premature discontinuation.
How has the mental health follow-up of patients with obesity evolved over the years?
I started working with people with obesity 25 years ago, when I myself underwent bariatric surgery. At that time, surgeons were used to “solving” the problem and sending the person home. Often, the patient did not even return for surgical follow-up because, in theory, the problem was solved.
Over time, I believe that surgeons learned to talk to the patient, understanding that there is a whole process that even involves creating a bond with the individual who underwent the surgical procedure. Within this process, the importance of the mental health of patients was recognized, and how common it is to confuse a degree of malnutrition with a mental disorder.
Even though I am not a nutritionist, I need to know the difference between a case of malnutrition and depression. So, it is a whole set of factors that needs to be worked on like an orchestra. It is not necessary for this work to be done in the same physical space, but dialogue is important.
Of course, there are things that the patient will only share with the psychologist or with the surgeon, but there are also pieces of information that need to be shared for positive management. I have had patients who were afraid to go back to the nutritionist because they did not lose weight. If they are afraid, it is because the professional is guiding them incorrectly.
What tips would you give to clinicians regarding the psychological approach to people with obesity?
Accessibility is crucial. When someone tells me they are dealing with obesity and depression, I usually ask, “Did you know you have two chronic diseases?” It is essential to explain these concepts because the patient may often think they are free after a successful diet and weight loss, which is not true because of the high relapse associated with obesity. Depression and anxiety follow similar patterns. If the same person wears prescription glasses, I interact by saying, “Did you know you have three chronic diseases?” This question often causes surprise. “I hadn’t thought of that.”
It is essential to use accessible language for the patient to understand the functioning of the disease. More important than choosing a treatment approach is understanding the pathophysiology of obesity and its psychological impact. This avoids a one-size-fits-all approach for all patients.
For example, the impact on someone who developed obesity in childhood after suffering physical, moral, or sexual abuse will probably be deeper than on someone in a healthy family who gained weight after becoming sedentary. Each life story requires a personalized approach.
Sometimes, a patient with mild obesity (grade 1) may not seem to need specific interventions at first glance, but it is crucial to listen to his or her story. Similarly, patients with severe obesity (grades 3 or 4) who resist surgery are entitled to other treatment options, and this is perfectly valid. Therefore, it is always important to ask, “Who is this person? What does obesity represent in their story?” Then propose the most appropriate treatment.
Ms. Levy reported having no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Promising New Data Support GLP-1s for Dementia Prevention
PHILADELPHIA –
In the phase 2b ELAD clinical trial, adults with early-stage Alzheimer’s disease taking the GLP-1 receptor agonist liraglutide exhibited slower decline in memory and thinking and experienced less brain atrophy over 12 months, compared with placebo.
“The slower loss of brain volume suggests liraglutide protects the brain, much like statins protect the heart,” study chief Paul Edison, MD, PhD, with Imperial College London, London, England, said in a statement.
“While further research is needed, liraglutide may work through various mechanisms, such as reducing inflammation in the brain, lowering insulin resistance and the toxic effects of Alzheimer’s biomarkers amyloid beta and tau, and improving how the brain’s nerve cells communicate,” Dr. Edison said.
He presented the study results at the 2024 Alzheimer’s Association International Conference (AAIC).
Brain Benefits
Liraglutide has previously demonstrated promising neuroprotective effects in animal models of Alzheimer’s disease and epidemiologic studies.
In ELAD, 204 patients with mild to moderate Alzheimer’s disease were randomly allocated (1:1) to a daily subcutaneous injection of up to 1.8 mg of liraglutide or placebo for 12 months; 80 patients in the liraglutide group and 89 in the placebo group completed the study.
Brain MRI was performed at baseline and at 12 months, along with neuropsychometric evaluation and 18F-fludeoxyglucose PET.
The study’s primary endpoint — change in the cerebral glucose metabolic rate in the cortical regions of the brain (hippocampus, medial temporal lobe, and posterior cingulate) — was not met.
However, patients taking liraglutide experienced a significant slowing of cognitive decline, compared with placebo group (P = .01), which was a key secondary outcome, calculated as a composite score of 18 different tests of memory, comprehension, language, and spatial orientation.
Although the study was not powered to assess cognitive changes, adults taking liraglutide had an 18% slower decline in cognitive function over 12 months, compared with those on placebo, Dr. Edison reported.
In addition, patients treated with liraglutide had nearly 50% less volume loss in several areas of the brain involved in memory, language, and decision-making, including frontal, temporal, parietal, and total gray matter, as measured by MRI.
Liraglutide daily subcutaneous injections were safe and well tolerated in patients with Alzheimer’s disease, Dr. Edison reported. There were 25 serious side effects — 18 in the placebo group and 7 in the liraglutide group — and most were considered unlikely to be related to the study treatment. There were no deaths.
Promising, Preliminary
This study shows a positive effect of liraglutide on the brain in terms of “slowing down of brain atrophy and slowing down the rate of cognitive decline,” said Howard Fillit, MD, founding executive director of the Alzheimer’s Drug Discovery Foundation, who wasn’t involved in the study.
Heather Snyder, PhD, vice-president of medical and scientific relations at the Alzheimer’s Association, said it’s “interesting” to see slowing of brain volume loss and some cognitive benefit “especially as the study was not powered necessarily to see some of those changes. The fact that they did see these changes in this small study provides a window into what may happen, but we certainly need larger phase 3 studies.”
In a statement from the UK nonprofit Science Media Centre, Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, called the data “promising.”
“There are clear links from strong data in the field between vascular risk factors including diabetes and obesity being associated with increased risk of dementia. The GLP-1 drug should help reduce these risk factors as well as potentially directly protecting brain cells,” Dr. Spires-Jones said.
However, she said “more research in bigger trials is needed to confirm whether this type of treatment will be effective in people with Alzheimer’s disease.”
Stephen Evans, MSc, emeritus professor, London School of Hygiene and Tropical Medicine, noted that the repurposing of drugs is “an important avenue of research but there is a lot of uncertainty here.”
He cautioned that the “50% brain volume change may not translate to important cognitive effects, and reporting only on those who completed the full 52 weeks of treatment could bring bias into the results. It sounds like it is worth pursuing a larger trial, but these results cannot demonstrate that liraglutide can protect against dementia.”
The ongoing phase 3 EVOKE trial is investigating the effects of the GLP-1 receptor agonist semaglutide in early Alzheimer’s disease.
Funding for the study was provided by Alzheimer’s Society UK, Alzheimer’s Drug Discovery Foundation, Novo Nordisk, John and Lucille Van Geest Foundation, and the National Institute for Health and Care Research Biomedical Research Centre. Dr. Edison, Dr. Fillit, Dr. Snyder, Mr. Evans, and Dr. Spires-Jones had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
PHILADELPHIA –
In the phase 2b ELAD clinical trial, adults with early-stage Alzheimer’s disease taking the GLP-1 receptor agonist liraglutide exhibited slower decline in memory and thinking and experienced less brain atrophy over 12 months, compared with placebo.
“The slower loss of brain volume suggests liraglutide protects the brain, much like statins protect the heart,” study chief Paul Edison, MD, PhD, with Imperial College London, London, England, said in a statement.
“While further research is needed, liraglutide may work through various mechanisms, such as reducing inflammation in the brain, lowering insulin resistance and the toxic effects of Alzheimer’s biomarkers amyloid beta and tau, and improving how the brain’s nerve cells communicate,” Dr. Edison said.
He presented the study results at the 2024 Alzheimer’s Association International Conference (AAIC).
Brain Benefits
Liraglutide has previously demonstrated promising neuroprotective effects in animal models of Alzheimer’s disease and epidemiologic studies.
In ELAD, 204 patients with mild to moderate Alzheimer’s disease were randomly allocated (1:1) to a daily subcutaneous injection of up to 1.8 mg of liraglutide or placebo for 12 months; 80 patients in the liraglutide group and 89 in the placebo group completed the study.
Brain MRI was performed at baseline and at 12 months, along with neuropsychometric evaluation and 18F-fludeoxyglucose PET.
The study’s primary endpoint — change in the cerebral glucose metabolic rate in the cortical regions of the brain (hippocampus, medial temporal lobe, and posterior cingulate) — was not met.
However, patients taking liraglutide experienced a significant slowing of cognitive decline, compared with placebo group (P = .01), which was a key secondary outcome, calculated as a composite score of 18 different tests of memory, comprehension, language, and spatial orientation.
Although the study was not powered to assess cognitive changes, adults taking liraglutide had an 18% slower decline in cognitive function over 12 months, compared with those on placebo, Dr. Edison reported.
In addition, patients treated with liraglutide had nearly 50% less volume loss in several areas of the brain involved in memory, language, and decision-making, including frontal, temporal, parietal, and total gray matter, as measured by MRI.
Liraglutide daily subcutaneous injections were safe and well tolerated in patients with Alzheimer’s disease, Dr. Edison reported. There were 25 serious side effects — 18 in the placebo group and 7 in the liraglutide group — and most were considered unlikely to be related to the study treatment. There were no deaths.
Promising, Preliminary
This study shows a positive effect of liraglutide on the brain in terms of “slowing down of brain atrophy and slowing down the rate of cognitive decline,” said Howard Fillit, MD, founding executive director of the Alzheimer’s Drug Discovery Foundation, who wasn’t involved in the study.
Heather Snyder, PhD, vice-president of medical and scientific relations at the Alzheimer’s Association, said it’s “interesting” to see slowing of brain volume loss and some cognitive benefit “especially as the study was not powered necessarily to see some of those changes. The fact that they did see these changes in this small study provides a window into what may happen, but we certainly need larger phase 3 studies.”
In a statement from the UK nonprofit Science Media Centre, Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, called the data “promising.”
“There are clear links from strong data in the field between vascular risk factors including diabetes and obesity being associated with increased risk of dementia. The GLP-1 drug should help reduce these risk factors as well as potentially directly protecting brain cells,” Dr. Spires-Jones said.
However, she said “more research in bigger trials is needed to confirm whether this type of treatment will be effective in people with Alzheimer’s disease.”
Stephen Evans, MSc, emeritus professor, London School of Hygiene and Tropical Medicine, noted that the repurposing of drugs is “an important avenue of research but there is a lot of uncertainty here.”
He cautioned that the “50% brain volume change may not translate to important cognitive effects, and reporting only on those who completed the full 52 weeks of treatment could bring bias into the results. It sounds like it is worth pursuing a larger trial, but these results cannot demonstrate that liraglutide can protect against dementia.”
The ongoing phase 3 EVOKE trial is investigating the effects of the GLP-1 receptor agonist semaglutide in early Alzheimer’s disease.
Funding for the study was provided by Alzheimer’s Society UK, Alzheimer’s Drug Discovery Foundation, Novo Nordisk, John and Lucille Van Geest Foundation, and the National Institute for Health and Care Research Biomedical Research Centre. Dr. Edison, Dr. Fillit, Dr. Snyder, Mr. Evans, and Dr. Spires-Jones had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
PHILADELPHIA –
In the phase 2b ELAD clinical trial, adults with early-stage Alzheimer’s disease taking the GLP-1 receptor agonist liraglutide exhibited slower decline in memory and thinking and experienced less brain atrophy over 12 months, compared with placebo.
“The slower loss of brain volume suggests liraglutide protects the brain, much like statins protect the heart,” study chief Paul Edison, MD, PhD, with Imperial College London, London, England, said in a statement.
“While further research is needed, liraglutide may work through various mechanisms, such as reducing inflammation in the brain, lowering insulin resistance and the toxic effects of Alzheimer’s biomarkers amyloid beta and tau, and improving how the brain’s nerve cells communicate,” Dr. Edison said.
He presented the study results at the 2024 Alzheimer’s Association International Conference (AAIC).
Brain Benefits
Liraglutide has previously demonstrated promising neuroprotective effects in animal models of Alzheimer’s disease and epidemiologic studies.
In ELAD, 204 patients with mild to moderate Alzheimer’s disease were randomly allocated (1:1) to a daily subcutaneous injection of up to 1.8 mg of liraglutide or placebo for 12 months; 80 patients in the liraglutide group and 89 in the placebo group completed the study.
Brain MRI was performed at baseline and at 12 months, along with neuropsychometric evaluation and 18F-fludeoxyglucose PET.
The study’s primary endpoint — change in the cerebral glucose metabolic rate in the cortical regions of the brain (hippocampus, medial temporal lobe, and posterior cingulate) — was not met.
However, patients taking liraglutide experienced a significant slowing of cognitive decline, compared with placebo group (P = .01), which was a key secondary outcome, calculated as a composite score of 18 different tests of memory, comprehension, language, and spatial orientation.
Although the study was not powered to assess cognitive changes, adults taking liraglutide had an 18% slower decline in cognitive function over 12 months, compared with those on placebo, Dr. Edison reported.
In addition, patients treated with liraglutide had nearly 50% less volume loss in several areas of the brain involved in memory, language, and decision-making, including frontal, temporal, parietal, and total gray matter, as measured by MRI.
Liraglutide daily subcutaneous injections were safe and well tolerated in patients with Alzheimer’s disease, Dr. Edison reported. There were 25 serious side effects — 18 in the placebo group and 7 in the liraglutide group — and most were considered unlikely to be related to the study treatment. There were no deaths.
Promising, Preliminary
This study shows a positive effect of liraglutide on the brain in terms of “slowing down of brain atrophy and slowing down the rate of cognitive decline,” said Howard Fillit, MD, founding executive director of the Alzheimer’s Drug Discovery Foundation, who wasn’t involved in the study.
Heather Snyder, PhD, vice-president of medical and scientific relations at the Alzheimer’s Association, said it’s “interesting” to see slowing of brain volume loss and some cognitive benefit “especially as the study was not powered necessarily to see some of those changes. The fact that they did see these changes in this small study provides a window into what may happen, but we certainly need larger phase 3 studies.”
In a statement from the UK nonprofit Science Media Centre, Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, called the data “promising.”
“There are clear links from strong data in the field between vascular risk factors including diabetes and obesity being associated with increased risk of dementia. The GLP-1 drug should help reduce these risk factors as well as potentially directly protecting brain cells,” Dr. Spires-Jones said.
However, she said “more research in bigger trials is needed to confirm whether this type of treatment will be effective in people with Alzheimer’s disease.”
Stephen Evans, MSc, emeritus professor, London School of Hygiene and Tropical Medicine, noted that the repurposing of drugs is “an important avenue of research but there is a lot of uncertainty here.”
He cautioned that the “50% brain volume change may not translate to important cognitive effects, and reporting only on those who completed the full 52 weeks of treatment could bring bias into the results. It sounds like it is worth pursuing a larger trial, but these results cannot demonstrate that liraglutide can protect against dementia.”
The ongoing phase 3 EVOKE trial is investigating the effects of the GLP-1 receptor agonist semaglutide in early Alzheimer’s disease.
Funding for the study was provided by Alzheimer’s Society UK, Alzheimer’s Drug Discovery Foundation, Novo Nordisk, John and Lucille Van Geest Foundation, and the National Institute for Health and Care Research Biomedical Research Centre. Dr. Edison, Dr. Fillit, Dr. Snyder, Mr. Evans, and Dr. Spires-Jones had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM AAIC 2024
Red Meat Tied to Increased Dementia Risk
PHILADELPHIA – preliminary research shows.
Study participants who consumed 0.25 or more servings of processed meat per day, or roughly two servings per week, had a 15% higher risk for dementia, compared with those who consumed less than 0.10 serving per day, which is about three servings per month.
“Our study found a higher intake of red meat — particularly processed red meat — was associated with a higher risk of developing dementia, as well as worse cognition,” said study author Yuhan Li, MHS, research assistant, Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
However, the study also showed that replacing processed red meat with nuts and legumes could potentially lower this increased risk.
The findings were presented on at the 2024 Alzheimer’s Association International Conference (AAIC).
Inconsistent Research
Previous studies have shown an inconsistent association between red meat intake and cognitive health.
To assess the relationship between diet and dementia, the researchers used data from the Nurses’ Health Study, which began recruiting female registered nurses aged 30-55 years in 1976, and the Health Professionals Follow-Up Study, which began recruiting male health professionals aged 40-75 in 1986.
They assessed processed red meat intake by validated semi-quantitative food frequency questionnaires administered every 2-4 years. Participants were asked how often they consumed a serving of processed red meat.
Investigators also assessed intake of unprocessed red meat, including beef, pork, or lamb as a main dish, in a sandwich or hamburger, or in a mixed dish.
The investigators also looked at participants’ intake of nuts and legumes.
Dementia outcome was a composite endpoint of self-reported dementia and dementia-related death. “Specifically, participants reported a physician diagnosis of Alzheimer’s disease or other forms of dementia by questionnaire. Deaths were identified through state vital statistics records, the National Death Index, family reports, and the postal system,” said Ms. Li.
Three Cognitive Outcomes
Researchers examined three outcomes: dementia, subjective cognitive decline, and objective cognitive function. For dementia, they ascertained incident cases in 87,424 individuals in the UK’s National Health Service database without Parkinson’s disease or baseline dementia, stroke, or cancer.
They longitudinally collected information on subjective cognitive decline from 33,908 Nurses’ Health Study participants and 10,058 participants in the Health Professionals Follow-Up Study.
Cognitive function was assessed using the Telephone Interview for Cognitive Status (1995-2008) in a subset of 17,458 Nurses’ Health Study participants.
Over a follow-up of 38 years (1980-2018), there were 6856 dementia cases in the Nurses’ Health Study. Participants with processed red meat intake of 0.25 or more serving/day, compared with less than 0.10 serving/day, had 15% higher risk for dementia (hazard ratio [HR], 1.15; 95% CI, 1.08-1.23; P < .001).
In addition to an increased risk for dementia, intake of processed red meat was associated with accelerated cognitive aging in global cognition (1.61 years per 1–serving/day increment; 95% CI, 0.20, 3.03) and verbal memory (1.69 years per 1–serving/day increment; 95% CI, 0.13, 3.25; both P = .03).
Participants with processed red meat intake of 0.25 or more serving/day had a 14% higher likelihood of subjective cognitive decline, compared with those with intake less than 0.10 serving/day (odds ratio [OR], 1.14; 95% CI, 1.04-1.24; P = .004).
For unprocessed red meat, consuming 1.00 or more serving/day versus less than 0.50 serving/day was associated with a 16% higher likelihood of subjective cognitive decline (OR, 1.16; 95% CI, 1.04-1.30; P = .02).
Substitution Analysis
Researchers modeled the effects of replacing 1 serving/day of processed red meat with 1 serving/day of nuts and legumes on cognitive outcomes. They did this by treating food intakes as continuous variables and calculating the differences in coefficients of the two food items.
They found that substituting legumes and nuts was associated with a 23% lower risk for dementia (HR, 0.77; 95% CI, 0.69-0.86), 1.37 fewer years of cognitive aging (95% CI, –2.49 to –0.25), and 20% lower odds of subjective cognitive decline (OR, 0.80, 95% CI, 0.69-0.92).
The research cannot determine whether it’s the processing method itself or the type of red meat that affects cognition, Ms. Li cautioned.
“Our study is an epidemiologic study, not a biological mechanism study, but based on our findings, red meat may be related to worse cognition, and processed red meat may add additional risk,” she said.
She also noted that because the study focused solely on red meats, the study cannot determine the potential on the impact of other processed meats on cognition.
Although the study doesn’t address a possible mechanism linking processed red meat with cognition, Ms. Li said it’s possible such meats have high levels of relatively harmful substances, such as nitrites, N-nitroso compounds, and sodium, and that “these carry the additional risk to brain health.”
There are currently no specific guidelines regarding the “safe” amount of processed meat consumption specifically related to cognition, she said.
The study is important because of its large sample size, long follow-up period, and inclusion of repeated measurements of diet, the investigators noted. In addition, researchers assessed both processed and unprocessed red meat and evaluated multiple cognitive outcomes.
The investigators plan to assess the association between other modifiable factors and cognitive health.
Experts Weigh In
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, agreed past studies on the topic have been “mixed,” with only some studies reporting links between cognition or dementia and processed red meat.
Another unique aspect of the study, said Dr. Sexton, was the replacement analysis showing the brain benefits of eating nuts and legumes in place of processed red meat. “So, it’s not just suggesting to people what not to do, but also what they can be doing instead.”
That’s why this large study with more than 130,000 adults that tracked individuals for close to 40 years in some cases “is so valuable,” she added.
In a release from the Science Media Centre in the United Kingdom, several other experts commented on the study. Among them, Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England, said that “it’s pretty well impossible to get a clear message from the information that is available so far about this research. It is a conference paper, and all we have seen so far is a press release, a brief summary of the research, and a diagram. There isn’t a detailed, peer-reviewed research report, not yet anyway. Putting out limited information like this isn’t the right way to report science.”
Dr. McConway also noted that the observational study recorded participants’ diets and dementia diagnoses over several years without assigning specific diets. Those who ate more red processed meat had higher rates of dementia and cognitive decline. However, it’s unclear if these differences are caused by red meat consumption or other factors, such as diet, age, ethnicity, or location.
Researchers typically adjust for these factors, but the available information doesn’t specify what adjustments were made or their impact, he noted, and without detailed data, it’s impossible to evaluate the study’s quality. Although eating more red processed meat might increase dementia risk, more research is needed to confirm this, Dr. McConway added.
Also commenting, Sebastian Walsh, a National Institute for Health and Care Research doctoral fellow who researches population-level approaches to dementia risk reduction at University of Cambridge, Cambridge, England, said that without seeing the full paper, it’s difficult to know exactly what to make of the study’s findings.
“On the surface, this is a large and long study. But it isn’t clear how the analysis was done — specifically what other factors were taken into account when looking at this apparent relationship between red meat and dementia.
“Despite a lot of research looking at specific foods and different diseases, the basic public health advice that eating a healthy, balanced diet is good for health is essentially unchanged. Most people know and accept this. What is most important is to find ways of supporting people, particularly those from poorer backgrounds, to follow this advice and address the obesity epidemic,” said Mr. Walsh.
The study was funded by a National Institutes of Health research grant. Ms. Li reports no relevant conflicts of interest. Dr. Sexton, Dr. McConway, and Mr. Walsh report no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – preliminary research shows.
Study participants who consumed 0.25 or more servings of processed meat per day, or roughly two servings per week, had a 15% higher risk for dementia, compared with those who consumed less than 0.10 serving per day, which is about three servings per month.
“Our study found a higher intake of red meat — particularly processed red meat — was associated with a higher risk of developing dementia, as well as worse cognition,” said study author Yuhan Li, MHS, research assistant, Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
However, the study also showed that replacing processed red meat with nuts and legumes could potentially lower this increased risk.
The findings were presented on at the 2024 Alzheimer’s Association International Conference (AAIC).
Inconsistent Research
Previous studies have shown an inconsistent association between red meat intake and cognitive health.
To assess the relationship between diet and dementia, the researchers used data from the Nurses’ Health Study, which began recruiting female registered nurses aged 30-55 years in 1976, and the Health Professionals Follow-Up Study, which began recruiting male health professionals aged 40-75 in 1986.
They assessed processed red meat intake by validated semi-quantitative food frequency questionnaires administered every 2-4 years. Participants were asked how often they consumed a serving of processed red meat.
Investigators also assessed intake of unprocessed red meat, including beef, pork, or lamb as a main dish, in a sandwich or hamburger, or in a mixed dish.
The investigators also looked at participants’ intake of nuts and legumes.
Dementia outcome was a composite endpoint of self-reported dementia and dementia-related death. “Specifically, participants reported a physician diagnosis of Alzheimer’s disease or other forms of dementia by questionnaire. Deaths were identified through state vital statistics records, the National Death Index, family reports, and the postal system,” said Ms. Li.
Three Cognitive Outcomes
Researchers examined three outcomes: dementia, subjective cognitive decline, and objective cognitive function. For dementia, they ascertained incident cases in 87,424 individuals in the UK’s National Health Service database without Parkinson’s disease or baseline dementia, stroke, or cancer.
They longitudinally collected information on subjective cognitive decline from 33,908 Nurses’ Health Study participants and 10,058 participants in the Health Professionals Follow-Up Study.
Cognitive function was assessed using the Telephone Interview for Cognitive Status (1995-2008) in a subset of 17,458 Nurses’ Health Study participants.
Over a follow-up of 38 years (1980-2018), there were 6856 dementia cases in the Nurses’ Health Study. Participants with processed red meat intake of 0.25 or more serving/day, compared with less than 0.10 serving/day, had 15% higher risk for dementia (hazard ratio [HR], 1.15; 95% CI, 1.08-1.23; P < .001).
In addition to an increased risk for dementia, intake of processed red meat was associated with accelerated cognitive aging in global cognition (1.61 years per 1–serving/day increment; 95% CI, 0.20, 3.03) and verbal memory (1.69 years per 1–serving/day increment; 95% CI, 0.13, 3.25; both P = .03).
Participants with processed red meat intake of 0.25 or more serving/day had a 14% higher likelihood of subjective cognitive decline, compared with those with intake less than 0.10 serving/day (odds ratio [OR], 1.14; 95% CI, 1.04-1.24; P = .004).
For unprocessed red meat, consuming 1.00 or more serving/day versus less than 0.50 serving/day was associated with a 16% higher likelihood of subjective cognitive decline (OR, 1.16; 95% CI, 1.04-1.30; P = .02).
Substitution Analysis
Researchers modeled the effects of replacing 1 serving/day of processed red meat with 1 serving/day of nuts and legumes on cognitive outcomes. They did this by treating food intakes as continuous variables and calculating the differences in coefficients of the two food items.
They found that substituting legumes and nuts was associated with a 23% lower risk for dementia (HR, 0.77; 95% CI, 0.69-0.86), 1.37 fewer years of cognitive aging (95% CI, –2.49 to –0.25), and 20% lower odds of subjective cognitive decline (OR, 0.80, 95% CI, 0.69-0.92).
The research cannot determine whether it’s the processing method itself or the type of red meat that affects cognition, Ms. Li cautioned.
“Our study is an epidemiologic study, not a biological mechanism study, but based on our findings, red meat may be related to worse cognition, and processed red meat may add additional risk,” she said.
She also noted that because the study focused solely on red meats, the study cannot determine the potential on the impact of other processed meats on cognition.
Although the study doesn’t address a possible mechanism linking processed red meat with cognition, Ms. Li said it’s possible such meats have high levels of relatively harmful substances, such as nitrites, N-nitroso compounds, and sodium, and that “these carry the additional risk to brain health.”
There are currently no specific guidelines regarding the “safe” amount of processed meat consumption specifically related to cognition, she said.
The study is important because of its large sample size, long follow-up period, and inclusion of repeated measurements of diet, the investigators noted. In addition, researchers assessed both processed and unprocessed red meat and evaluated multiple cognitive outcomes.
The investigators plan to assess the association between other modifiable factors and cognitive health.
Experts Weigh In
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, agreed past studies on the topic have been “mixed,” with only some studies reporting links between cognition or dementia and processed red meat.
Another unique aspect of the study, said Dr. Sexton, was the replacement analysis showing the brain benefits of eating nuts and legumes in place of processed red meat. “So, it’s not just suggesting to people what not to do, but also what they can be doing instead.”
That’s why this large study with more than 130,000 adults that tracked individuals for close to 40 years in some cases “is so valuable,” she added.
In a release from the Science Media Centre in the United Kingdom, several other experts commented on the study. Among them, Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England, said that “it’s pretty well impossible to get a clear message from the information that is available so far about this research. It is a conference paper, and all we have seen so far is a press release, a brief summary of the research, and a diagram. There isn’t a detailed, peer-reviewed research report, not yet anyway. Putting out limited information like this isn’t the right way to report science.”
Dr. McConway also noted that the observational study recorded participants’ diets and dementia diagnoses over several years without assigning specific diets. Those who ate more red processed meat had higher rates of dementia and cognitive decline. However, it’s unclear if these differences are caused by red meat consumption or other factors, such as diet, age, ethnicity, or location.
Researchers typically adjust for these factors, but the available information doesn’t specify what adjustments were made or their impact, he noted, and without detailed data, it’s impossible to evaluate the study’s quality. Although eating more red processed meat might increase dementia risk, more research is needed to confirm this, Dr. McConway added.
Also commenting, Sebastian Walsh, a National Institute for Health and Care Research doctoral fellow who researches population-level approaches to dementia risk reduction at University of Cambridge, Cambridge, England, said that without seeing the full paper, it’s difficult to know exactly what to make of the study’s findings.
“On the surface, this is a large and long study. But it isn’t clear how the analysis was done — specifically what other factors were taken into account when looking at this apparent relationship between red meat and dementia.
“Despite a lot of research looking at specific foods and different diseases, the basic public health advice that eating a healthy, balanced diet is good for health is essentially unchanged. Most people know and accept this. What is most important is to find ways of supporting people, particularly those from poorer backgrounds, to follow this advice and address the obesity epidemic,” said Mr. Walsh.
The study was funded by a National Institutes of Health research grant. Ms. Li reports no relevant conflicts of interest. Dr. Sexton, Dr. McConway, and Mr. Walsh report no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – preliminary research shows.
Study participants who consumed 0.25 or more servings of processed meat per day, or roughly two servings per week, had a 15% higher risk for dementia, compared with those who consumed less than 0.10 serving per day, which is about three servings per month.
“Our study found a higher intake of red meat — particularly processed red meat — was associated with a higher risk of developing dementia, as well as worse cognition,” said study author Yuhan Li, MHS, research assistant, Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
However, the study also showed that replacing processed red meat with nuts and legumes could potentially lower this increased risk.
The findings were presented on at the 2024 Alzheimer’s Association International Conference (AAIC).
Inconsistent Research
Previous studies have shown an inconsistent association between red meat intake and cognitive health.
To assess the relationship between diet and dementia, the researchers used data from the Nurses’ Health Study, which began recruiting female registered nurses aged 30-55 years in 1976, and the Health Professionals Follow-Up Study, which began recruiting male health professionals aged 40-75 in 1986.
They assessed processed red meat intake by validated semi-quantitative food frequency questionnaires administered every 2-4 years. Participants were asked how often they consumed a serving of processed red meat.
Investigators also assessed intake of unprocessed red meat, including beef, pork, or lamb as a main dish, in a sandwich or hamburger, or in a mixed dish.
The investigators also looked at participants’ intake of nuts and legumes.
Dementia outcome was a composite endpoint of self-reported dementia and dementia-related death. “Specifically, participants reported a physician diagnosis of Alzheimer’s disease or other forms of dementia by questionnaire. Deaths were identified through state vital statistics records, the National Death Index, family reports, and the postal system,” said Ms. Li.
Three Cognitive Outcomes
Researchers examined three outcomes: dementia, subjective cognitive decline, and objective cognitive function. For dementia, they ascertained incident cases in 87,424 individuals in the UK’s National Health Service database without Parkinson’s disease or baseline dementia, stroke, or cancer.
They longitudinally collected information on subjective cognitive decline from 33,908 Nurses’ Health Study participants and 10,058 participants in the Health Professionals Follow-Up Study.
Cognitive function was assessed using the Telephone Interview for Cognitive Status (1995-2008) in a subset of 17,458 Nurses’ Health Study participants.
Over a follow-up of 38 years (1980-2018), there were 6856 dementia cases in the Nurses’ Health Study. Participants with processed red meat intake of 0.25 or more serving/day, compared with less than 0.10 serving/day, had 15% higher risk for dementia (hazard ratio [HR], 1.15; 95% CI, 1.08-1.23; P < .001).
In addition to an increased risk for dementia, intake of processed red meat was associated with accelerated cognitive aging in global cognition (1.61 years per 1–serving/day increment; 95% CI, 0.20, 3.03) and verbal memory (1.69 years per 1–serving/day increment; 95% CI, 0.13, 3.25; both P = .03).
Participants with processed red meat intake of 0.25 or more serving/day had a 14% higher likelihood of subjective cognitive decline, compared with those with intake less than 0.10 serving/day (odds ratio [OR], 1.14; 95% CI, 1.04-1.24; P = .004).
For unprocessed red meat, consuming 1.00 or more serving/day versus less than 0.50 serving/day was associated with a 16% higher likelihood of subjective cognitive decline (OR, 1.16; 95% CI, 1.04-1.30; P = .02).
Substitution Analysis
Researchers modeled the effects of replacing 1 serving/day of processed red meat with 1 serving/day of nuts and legumes on cognitive outcomes. They did this by treating food intakes as continuous variables and calculating the differences in coefficients of the two food items.
They found that substituting legumes and nuts was associated with a 23% lower risk for dementia (HR, 0.77; 95% CI, 0.69-0.86), 1.37 fewer years of cognitive aging (95% CI, –2.49 to –0.25), and 20% lower odds of subjective cognitive decline (OR, 0.80, 95% CI, 0.69-0.92).
The research cannot determine whether it’s the processing method itself or the type of red meat that affects cognition, Ms. Li cautioned.
“Our study is an epidemiologic study, not a biological mechanism study, but based on our findings, red meat may be related to worse cognition, and processed red meat may add additional risk,” she said.
She also noted that because the study focused solely on red meats, the study cannot determine the potential on the impact of other processed meats on cognition.
Although the study doesn’t address a possible mechanism linking processed red meat with cognition, Ms. Li said it’s possible such meats have high levels of relatively harmful substances, such as nitrites, N-nitroso compounds, and sodium, and that “these carry the additional risk to brain health.”
There are currently no specific guidelines regarding the “safe” amount of processed meat consumption specifically related to cognition, she said.
The study is important because of its large sample size, long follow-up period, and inclusion of repeated measurements of diet, the investigators noted. In addition, researchers assessed both processed and unprocessed red meat and evaluated multiple cognitive outcomes.
The investigators plan to assess the association between other modifiable factors and cognitive health.
Experts Weigh In
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, agreed past studies on the topic have been “mixed,” with only some studies reporting links between cognition or dementia and processed red meat.
Another unique aspect of the study, said Dr. Sexton, was the replacement analysis showing the brain benefits of eating nuts and legumes in place of processed red meat. “So, it’s not just suggesting to people what not to do, but also what they can be doing instead.”
That’s why this large study with more than 130,000 adults that tracked individuals for close to 40 years in some cases “is so valuable,” she added.
In a release from the Science Media Centre in the United Kingdom, several other experts commented on the study. Among them, Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England, said that “it’s pretty well impossible to get a clear message from the information that is available so far about this research. It is a conference paper, and all we have seen so far is a press release, a brief summary of the research, and a diagram. There isn’t a detailed, peer-reviewed research report, not yet anyway. Putting out limited information like this isn’t the right way to report science.”
Dr. McConway also noted that the observational study recorded participants’ diets and dementia diagnoses over several years without assigning specific diets. Those who ate more red processed meat had higher rates of dementia and cognitive decline. However, it’s unclear if these differences are caused by red meat consumption or other factors, such as diet, age, ethnicity, or location.
Researchers typically adjust for these factors, but the available information doesn’t specify what adjustments were made or their impact, he noted, and without detailed data, it’s impossible to evaluate the study’s quality. Although eating more red processed meat might increase dementia risk, more research is needed to confirm this, Dr. McConway added.
Also commenting, Sebastian Walsh, a National Institute for Health and Care Research doctoral fellow who researches population-level approaches to dementia risk reduction at University of Cambridge, Cambridge, England, said that without seeing the full paper, it’s difficult to know exactly what to make of the study’s findings.
“On the surface, this is a large and long study. But it isn’t clear how the analysis was done — specifically what other factors were taken into account when looking at this apparent relationship between red meat and dementia.
“Despite a lot of research looking at specific foods and different diseases, the basic public health advice that eating a healthy, balanced diet is good for health is essentially unchanged. Most people know and accept this. What is most important is to find ways of supporting people, particularly those from poorer backgrounds, to follow this advice and address the obesity epidemic,” said Mr. Walsh.
The study was funded by a National Institutes of Health research grant. Ms. Li reports no relevant conflicts of interest. Dr. Sexton, Dr. McConway, and Mr. Walsh report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM AAIC 2024
Tau Blood Test Flags Preclinical Alzheimer’s Disease
Recruiting preclinical Alzheimer’s disease participants for clinical research is challenging, owing to a lack of symptoms and the high cost and invasiveness of cerebrospinal fluid (CSF) tests and brain amyloid PET imaging.
Plasma p-tau217 has consistently shown high performance in detecting Alzheimer’s disease pathology in patients with mild cognitive impairment and dementia, but there has been concern that it may have lower accuracy in cognitively unimpaired adults, said lead investigator Gemma Salvadó, PhD, with the Clinical Memory Research Unit, Lund University, Lund, Sweden.
However, “our study shows that plasma p-tau217, alone or in combination with invasive tests, can be used accurately to assess amyloid positivity in cognitively unimpaired participants, to streamline the inclusion of these participants in preventive clinical trials,” she said.
The findings were presented at the 2024 Alzheimer’s Association International Conference (AAIC).
Correlation to CSF, PET Amyloid Status
The investigators assessed the clinical accuracy of plasma p-tau217 as a prescreening method in 2917 cognitively unimpaired adults (mean age, 67 years; 57% women) across 12 independent cohorts who had available plasma p-tau217 and amyloid beta PET imaging or CSF samples.
They found that plasma p-tau217 levels correlated with amyloid beta CSF status and PET load.
As a standalone test, plasma p-tau217 identified amyloid beta PET–positive cognitively normal adults with a positive predictive value of 80% or greater.
The positive predictive value increased to 95% or greater when amyloid beta CSF or PET was used to confirm a positive plasma p-tau217 result.
As a first step, plasma p-tau217 could significantly reduce the number of invasive tests performed because only individuals with a positive p-tau217 test would go on to PET imaging or CSF sampling, Dr. Salvadó told conference attendees. This may reduce trial recruitment costs and get more patients enrolled.
Although the study had a large sample size, “these results should be replicated in independent studies, [in] more heterogeneous participants, and coming from the clinical setting instead of observational studies to avoid possible bias,” Dr. Salvadó added.
A New Diagnostic Era
Commenting on the research, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said what’s particularly interesting about this study is that the researchers examined multiple cohorts of cognitively unimpaired individuals and “consistently” found that plasma p-tau217 could identify individuals with amyloid-positive PET and CSF with high accuracy.
“This may reduce the need for more expensive and more invasive scans or lumbar punctures to confirm if an individual has the biology,” Dr. Snyder said.
“Blood tests are revolutionizing Alzheimer’s detection, diagnosis and ultimately treatment,” added Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation.
He predicted that blood tests will “soon replace more invasive and costly PET scans as the standard of care and serve as the first line of defense in diagnosing the disease.”
“After many years of research, the field is in a place where we have novel biomarkers and diagnostics to support a diagnosis,” the way cholesterol is used to help detect heart disease, said Dr. Fillit.
“The diagnostic framework for Alzheimer’s — an incredibly complex disease — is constantly evolving. As we usher in the new era of care, we are moving closer to the day when blood tests will be complemented by digital tools to provide precise and timely diagnoses and risk assessments backed by numerous data points, complementing existing cognitive tests,” he added.
Funding for the study was provided by the Alzheimer’s Association, the European Union’s Horizon 2020 Research and Innovation Program, Alzheimerfonden, and Strategic Research Area MultiPark. Dr. Salvadó, Dr. Snyder, and Dr. Fillit have no relevant disclosures.
A version of this article appeared on Medscape.com.
Recruiting preclinical Alzheimer’s disease participants for clinical research is challenging, owing to a lack of symptoms and the high cost and invasiveness of cerebrospinal fluid (CSF) tests and brain amyloid PET imaging.
Plasma p-tau217 has consistently shown high performance in detecting Alzheimer’s disease pathology in patients with mild cognitive impairment and dementia, but there has been concern that it may have lower accuracy in cognitively unimpaired adults, said lead investigator Gemma Salvadó, PhD, with the Clinical Memory Research Unit, Lund University, Lund, Sweden.
However, “our study shows that plasma p-tau217, alone or in combination with invasive tests, can be used accurately to assess amyloid positivity in cognitively unimpaired participants, to streamline the inclusion of these participants in preventive clinical trials,” she said.
The findings were presented at the 2024 Alzheimer’s Association International Conference (AAIC).
Correlation to CSF, PET Amyloid Status
The investigators assessed the clinical accuracy of plasma p-tau217 as a prescreening method in 2917 cognitively unimpaired adults (mean age, 67 years; 57% women) across 12 independent cohorts who had available plasma p-tau217 and amyloid beta PET imaging or CSF samples.
They found that plasma p-tau217 levels correlated with amyloid beta CSF status and PET load.
As a standalone test, plasma p-tau217 identified amyloid beta PET–positive cognitively normal adults with a positive predictive value of 80% or greater.
The positive predictive value increased to 95% or greater when amyloid beta CSF or PET was used to confirm a positive plasma p-tau217 result.
As a first step, plasma p-tau217 could significantly reduce the number of invasive tests performed because only individuals with a positive p-tau217 test would go on to PET imaging or CSF sampling, Dr. Salvadó told conference attendees. This may reduce trial recruitment costs and get more patients enrolled.
Although the study had a large sample size, “these results should be replicated in independent studies, [in] more heterogeneous participants, and coming from the clinical setting instead of observational studies to avoid possible bias,” Dr. Salvadó added.
A New Diagnostic Era
Commenting on the research, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said what’s particularly interesting about this study is that the researchers examined multiple cohorts of cognitively unimpaired individuals and “consistently” found that plasma p-tau217 could identify individuals with amyloid-positive PET and CSF with high accuracy.
“This may reduce the need for more expensive and more invasive scans or lumbar punctures to confirm if an individual has the biology,” Dr. Snyder said.
“Blood tests are revolutionizing Alzheimer’s detection, diagnosis and ultimately treatment,” added Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation.
He predicted that blood tests will “soon replace more invasive and costly PET scans as the standard of care and serve as the first line of defense in diagnosing the disease.”
“After many years of research, the field is in a place where we have novel biomarkers and diagnostics to support a diagnosis,” the way cholesterol is used to help detect heart disease, said Dr. Fillit.
“The diagnostic framework for Alzheimer’s — an incredibly complex disease — is constantly evolving. As we usher in the new era of care, we are moving closer to the day when blood tests will be complemented by digital tools to provide precise and timely diagnoses and risk assessments backed by numerous data points, complementing existing cognitive tests,” he added.
Funding for the study was provided by the Alzheimer’s Association, the European Union’s Horizon 2020 Research and Innovation Program, Alzheimerfonden, and Strategic Research Area MultiPark. Dr. Salvadó, Dr. Snyder, and Dr. Fillit have no relevant disclosures.
A version of this article appeared on Medscape.com.
Recruiting preclinical Alzheimer’s disease participants for clinical research is challenging, owing to a lack of symptoms and the high cost and invasiveness of cerebrospinal fluid (CSF) tests and brain amyloid PET imaging.
Plasma p-tau217 has consistently shown high performance in detecting Alzheimer’s disease pathology in patients with mild cognitive impairment and dementia, but there has been concern that it may have lower accuracy in cognitively unimpaired adults, said lead investigator Gemma Salvadó, PhD, with the Clinical Memory Research Unit, Lund University, Lund, Sweden.
However, “our study shows that plasma p-tau217, alone or in combination with invasive tests, can be used accurately to assess amyloid positivity in cognitively unimpaired participants, to streamline the inclusion of these participants in preventive clinical trials,” she said.
The findings were presented at the 2024 Alzheimer’s Association International Conference (AAIC).
Correlation to CSF, PET Amyloid Status
The investigators assessed the clinical accuracy of plasma p-tau217 as a prescreening method in 2917 cognitively unimpaired adults (mean age, 67 years; 57% women) across 12 independent cohorts who had available plasma p-tau217 and amyloid beta PET imaging or CSF samples.
They found that plasma p-tau217 levels correlated with amyloid beta CSF status and PET load.
As a standalone test, plasma p-tau217 identified amyloid beta PET–positive cognitively normal adults with a positive predictive value of 80% or greater.
The positive predictive value increased to 95% or greater when amyloid beta CSF or PET was used to confirm a positive plasma p-tau217 result.
As a first step, plasma p-tau217 could significantly reduce the number of invasive tests performed because only individuals with a positive p-tau217 test would go on to PET imaging or CSF sampling, Dr. Salvadó told conference attendees. This may reduce trial recruitment costs and get more patients enrolled.
Although the study had a large sample size, “these results should be replicated in independent studies, [in] more heterogeneous participants, and coming from the clinical setting instead of observational studies to avoid possible bias,” Dr. Salvadó added.
A New Diagnostic Era
Commenting on the research, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said what’s particularly interesting about this study is that the researchers examined multiple cohorts of cognitively unimpaired individuals and “consistently” found that plasma p-tau217 could identify individuals with amyloid-positive PET and CSF with high accuracy.
“This may reduce the need for more expensive and more invasive scans or lumbar punctures to confirm if an individual has the biology,” Dr. Snyder said.
“Blood tests are revolutionizing Alzheimer’s detection, diagnosis and ultimately treatment,” added Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation.
He predicted that blood tests will “soon replace more invasive and costly PET scans as the standard of care and serve as the first line of defense in diagnosing the disease.”
“After many years of research, the field is in a place where we have novel biomarkers and diagnostics to support a diagnosis,” the way cholesterol is used to help detect heart disease, said Dr. Fillit.
“The diagnostic framework for Alzheimer’s — an incredibly complex disease — is constantly evolving. As we usher in the new era of care, we are moving closer to the day when blood tests will be complemented by digital tools to provide precise and timely diagnoses and risk assessments backed by numerous data points, complementing existing cognitive tests,” he added.
Funding for the study was provided by the Alzheimer’s Association, the European Union’s Horizon 2020 Research and Innovation Program, Alzheimerfonden, and Strategic Research Area MultiPark. Dr. Salvadó, Dr. Snyder, and Dr. Fillit have no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAIC 2024
Blood Biomarkers Are Highly Accurate in Diagnosing Alzheimer’s Disease
PHILADELPHIA — new research showed.
Accurate early diagnosis of Alzheimer’s disease is important because two monoclonal antibodies donanemab (Kisunla) and lecanemab (Leqembi) are now approved by the Food and Drug Administration (FDA) for early-stage Alzheimer’s disease. However, the use of these agents requires amyloid confirmation.
A key finding of the study was that primary care physicians had a diagnostic accuracy of 61%, and dementia specialists had an accuracy of 73%, after completing standard clinical evaluations and before seeing results of the blood test or other Alzheimer’s disease biomarkers, while the blood test used in the study had an accuracy of 91% for correctly classifying clinical, biomarker-verified Alzheimer’s disease.
“This underscores the potential improvement in diagnostic accuracy, especially in primary care, when implementing such a blood test,” said study investigator Sebastian Palmqvist, MD, PhD, associate professor of neurology at Lund University, Lund, and a consultant at Skåne University Hospital, Malmö, Sweden. “It also highlights the challenges in accurately identifying Alzheimer’s disease based solely on clinical evaluation and cognitive testing, even for specialists.”
The findings were presented at the 2024 Alzheimer’s Association International Conference (AAIC) and simultaneously published online in JAMA.
The study included two cohorts from primary and secondary care clinics in Sweden. Researchers analyzed plasma samples together at one time point in a single batch.
It also included two cohorts from Swedish primary and secondary care clinics where the plasma samples were analyzed prospectively (biweekly) in batches throughout the enrollment period, which more closely resembles clinical practice.
Primary care physicians and dementia specialists documented whether they believed their patients had Alzheimer’s disease pathology, basing the diagnoses on the standard evaluation that includes clinical examination, cognitive testing, and a CT scan prior to seeing any Alzheimer’s disease biomarker results.
They reported their certainty of the presence of Alzheimer’s disease pathology on a scale from 0 (not at all certain) to 10 (completely certain).
Plasma analyses were performed by personnel blinded to all clinical or biomarker data. Mass spectrometry assays were used to analyze Abeta42, Abeta40, phosphorylated tau 217 (p-tau217), and non–p-tau217.
Biomarkers used in the study included the percentage of plasma p-tau217, which is the ratio of p-tau217 relative to non–p-tau217, and the Abeta42 to Abeta40 ratio (the amyloid probability score 2 [APS2]). Researchers determined p-tau217 alone and when combined with the APS2.
The study included 1213 patients with cognitive symptoms — mean age 74.2 years and 48% women. Researchers applied biomarker cutoff values to the primary care cohort (n = 307) and the secondary care cohort (n = 300) and then evaluated the blood test prospectively in the primary care cohort (n = 208) and the secondary care cohort (n = 398).
The blood biomarker cutoff value was set at 90% specificity for Alzheimer’s disease pathology (the 1 cutoff-value approach). A 2 cutoff-value approach (using 1 upper and 1 lower cutoff value) was also used with values corresponding to 95% sensitivity and 95% specificity.
The primary outcome was presence of Alzheimer’s disease pathology. A positive finding of the Abeta biomarker was defined according to the FDA-approved cutoff value (≤ 0.072). A positive finding of the tau biomarker was defined as a p-tau217 level > 11.42 pg/mL in cerebrospinal fluid.
Researchers calculated the positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy, as well as area under the curve (AUC) values.
Accuracy in Specialty Versus Primary Care
When the plasma samples were analyzed in a single batch in the primary care cohort, the AUC was 0.97 when the APS2 was used. In the secondary care cohort, the AUC was 0.96 when the APS2 was used.
When plasma samples were analyzed prospectively (biweekly) in the primary care cohort, the AUC was 0.96 when the APS2 was used. In the secondary care cohort, the AUC was 0.97 when the APS2 was used.
The 2 cutoff-value approach achieved PPVs of 97%-99% in patients with cognitive impairment, which is the target population of currently available antiamyloid treatments.
Although NPVs were slightly lower in these patients (87%-92% using the APS2), “we argue that a very high positive predictive value is probably more important in diagnosing patients as having Alzheimer’s disease, especially before initiating costly and burdensome antiamyloid treatment,” the investigators noted.
The PPVs were less than optimal for accurate identification of Alzheimer’s disease pathology in patients with subjective cognitive decline regardless of the cutoff-value approach used. The researchers pointed out that this could be a disadvantage for clinical trials that include patients with presymptomatic Alzheimer’s disease but not in clinical practice because there are no clinical criteria for diagnosing Alzheimer’s disease at the subjective cognitive decline stage.
The NPVs were higher in patients with subjective cognitive decline (91%-94% for the APS2 or percentage of p-tau217 alone). This indicates the blood test would be more useful for ruling out underlying Alzheimer’s disease when only subtle symptoms are present, the researchers noted.
As for doctors identifying clinical Alzheimer’s disease, primary care physicians had a diagnostic accuracy of 61% (95% CI, 53%-69%) versus 91% (95% CI, 86%-96%) using the APS2. Dementia specialists had a diagnostic accuracy of 73% (95% CI, 68%-79%) versus 91% (95% CI, 86%-95%) using the APS2.
In the overall population, the diagnostic accuracy using the APS2 (90%; 95% CI, 88%-92%) was not different from that using the percentage of p-tau217 alone (90%; 95% CI, 88%-91%).
Very little was known about how a blood test would perform in a primary care setting, said Dr. Palmqvist. “Seeing that the test was just as accurate in primary care (about 90%) as it was in secondary care is really encouraging, especially since primary care is the first, and often final, point of entry into the healthcare system for cognitive evaluations.”
He said he was surprised the biomarkers performed so well in prospective, biweekly analyses throughout the study. “Previous studies have only demonstrated their effectiveness when all collected samples are analyzed at a single time point, which does not reflect how a blood test is used in clinical practice.”
He added that he was surprised that the tests were just as accurate in primary care as in a memory clinic setting with referred patients. This, despite older age and higher prevalence of comorbidities in primary care, such as chronic kidney disease (present in 26% of the primary care cohort), can be a confounding factor causing increased concentrations of p-tau217.
Next Steps
The diagnostic accuracy of the blood tests is on par with FDA-cleared cerebrospinal fluid biomarkers, noted the investigators, led by senior author Oskar Hansson, MD, PhD, Clinical Memory Research Unit, Department of Clinical Sciences Malm
As blood tests are “more time effective, cost effective, and convenient” for patients, “they could also potentially replace cerebrospinal fluid tests and PET,” they added.
Dr. Palmqvist emphasized that these tests should not be used as stand-alone diagnostic tools for Alzheimer’s disease but should complement the standard clinical evaluation that includes cognitive testing and a thorough interview with the patient and a spouse or relative.
“This is crucial because Alzheimer’s disease pathology can be asymptomatic for many years, and cognitive symptoms in some patients with Alzheimer’s disease pathology may primarily result from other conditions. Misinterpreting a positive Alzheimer’s disease blood test could lead to underdiagnosis of common non–Alzheimer’s disease conditions.”
With new antiamyloid treatments possibly slowing disease progression by 30%-40% when initiated early on, a blood test for Alzheimer’s disease could lead to more people receiving an accurate and earlier diagnosis, said Dr. Palmqvist. “This could potentially result in a better response to treatment. Results from drug trials clearly indicate that the earlier treatment begins, the more effectively it can slow disease progression.”
The test used in the study is already available in the United States, the investigators said, and a similar test will be accessible in Sweden within a few months. “However, the rollout will probably be gradual and will depend on how international and national guidelines recommend their use, so developing these guidelines will be a crucial next step for widespread implementation, particularly in primary care,” said Dr. Palmqvist.
He also underlined the importance of replicating the findings in more diverse populations. “This will help ensure the tests’ reliability and effectiveness across various demographic and clinical contexts.”
An important next research step is to examine how implementing a blood test for Alzheimer’s disease affects patient care. “This includes looking at changes in management, such as referrals, other examinations, and the initiation of appropriate treatments,” said Dr. Palmqvist.
Another study presented at the meeting showed that a highly accurate blood test could significantly reduce diagnostic wait times.
Convincing Research
In an accompanying editorial, Stephen Salloway, MD, Departments of Psychiatry and Neurology, Warren Alpert Medical School, Brown University, Providence, Rhode Island, and colleagues said the study “makes the case convincingly that highly sensitive blood measures of Alzheimer’s disease can be integrated into the clinical decision-making process, including in the primary care setting.”
These tests, they wrote, “can be used to enhance the ability of clinicians to accurately identify individuals with cognitive impairment and dementia due to Alzheimer’s disease.
“Current practice should focus on using these blood biomarkers in individuals with cognitive impairment rather than in those with normal cognition or subjective cognitive decline until further research demonstrates effective interventions for individuals considered cognitively normal with elevated levels of amyloid.”
A key limitation of the study was the lack of diversity in the study sample. This makes it difficult to generalize the results across other ethnic and racial groups, the editorialists noted. Plasma assays for Alzheimer’s disease in the United States will require approval from the FDA and coverage by the Centers for Medicare & Medicaid Services to be widely adopted.
The editorialists also pointed out that advances in the diagnosis and treatment of Alzheimer’s disease will require important changes to healthcare models, including providing additional resources and staffing.
The study was supported by the Alzheimer’s Association, National Institute on Aging, European Research Council, Swedish Research Council, the GHR Foundation, and other groups. The study was conducted as an academic collaboration between Lund University and C2N Diagnostics in the United States. Lund University or its affiliated researchers received no funding or compensation from C2N Diagnostics. C2N Diagnostics performed the plasma analyses blinded to any biomarker or clinical data and had no role in the statistical analysis or results. Dr. Palmqvist reported receiving institutional research support from ki:elements, Alzheimer’s Drug Discovery Foundation, and Avid Radiopharmaceuticals and consultancy or speaker fees from BioArctic, Biogen, Esai, Eli Lilly, and Roche. Dr. Hansson reported receiving personal fees from AC Immune, ALZpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Roche, Bristol-Myers Squibb, Merck, Novartis, Novo Nordisk, Roche, Sanofi, and Siemens and institutional research support from ADX, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. Dr. Salloway reported receiving grants from Biogen, Roche, Lilly, Genentech, Eisai, and Novartis; personal fees from Biogen, Roche, Lilly, Genentech, Eisai, Novo Nordisk, Prothena, AbbVie, Acumen, and Kisbee; and nonfinancial support (travel expenses for conference attendance) from Biogen, Roche, Lilly, and Acumen.
A version of this article appeared on Medscape.com.
PHILADELPHIA — new research showed.
Accurate early diagnosis of Alzheimer’s disease is important because two monoclonal antibodies donanemab (Kisunla) and lecanemab (Leqembi) are now approved by the Food and Drug Administration (FDA) for early-stage Alzheimer’s disease. However, the use of these agents requires amyloid confirmation.
A key finding of the study was that primary care physicians had a diagnostic accuracy of 61%, and dementia specialists had an accuracy of 73%, after completing standard clinical evaluations and before seeing results of the blood test or other Alzheimer’s disease biomarkers, while the blood test used in the study had an accuracy of 91% for correctly classifying clinical, biomarker-verified Alzheimer’s disease.
“This underscores the potential improvement in diagnostic accuracy, especially in primary care, when implementing such a blood test,” said study investigator Sebastian Palmqvist, MD, PhD, associate professor of neurology at Lund University, Lund, and a consultant at Skåne University Hospital, Malmö, Sweden. “It also highlights the challenges in accurately identifying Alzheimer’s disease based solely on clinical evaluation and cognitive testing, even for specialists.”
The findings were presented at the 2024 Alzheimer’s Association International Conference (AAIC) and simultaneously published online in JAMA.
The study included two cohorts from primary and secondary care clinics in Sweden. Researchers analyzed plasma samples together at one time point in a single batch.
It also included two cohorts from Swedish primary and secondary care clinics where the plasma samples were analyzed prospectively (biweekly) in batches throughout the enrollment period, which more closely resembles clinical practice.
Primary care physicians and dementia specialists documented whether they believed their patients had Alzheimer’s disease pathology, basing the diagnoses on the standard evaluation that includes clinical examination, cognitive testing, and a CT scan prior to seeing any Alzheimer’s disease biomarker results.
They reported their certainty of the presence of Alzheimer’s disease pathology on a scale from 0 (not at all certain) to 10 (completely certain).
Plasma analyses were performed by personnel blinded to all clinical or biomarker data. Mass spectrometry assays were used to analyze Abeta42, Abeta40, phosphorylated tau 217 (p-tau217), and non–p-tau217.
Biomarkers used in the study included the percentage of plasma p-tau217, which is the ratio of p-tau217 relative to non–p-tau217, and the Abeta42 to Abeta40 ratio (the amyloid probability score 2 [APS2]). Researchers determined p-tau217 alone and when combined with the APS2.
The study included 1213 patients with cognitive symptoms — mean age 74.2 years and 48% women. Researchers applied biomarker cutoff values to the primary care cohort (n = 307) and the secondary care cohort (n = 300) and then evaluated the blood test prospectively in the primary care cohort (n = 208) and the secondary care cohort (n = 398).
The blood biomarker cutoff value was set at 90% specificity for Alzheimer’s disease pathology (the 1 cutoff-value approach). A 2 cutoff-value approach (using 1 upper and 1 lower cutoff value) was also used with values corresponding to 95% sensitivity and 95% specificity.
The primary outcome was presence of Alzheimer’s disease pathology. A positive finding of the Abeta biomarker was defined according to the FDA-approved cutoff value (≤ 0.072). A positive finding of the tau biomarker was defined as a p-tau217 level > 11.42 pg/mL in cerebrospinal fluid.
Researchers calculated the positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy, as well as area under the curve (AUC) values.
Accuracy in Specialty Versus Primary Care
When the plasma samples were analyzed in a single batch in the primary care cohort, the AUC was 0.97 when the APS2 was used. In the secondary care cohort, the AUC was 0.96 when the APS2 was used.
When plasma samples were analyzed prospectively (biweekly) in the primary care cohort, the AUC was 0.96 when the APS2 was used. In the secondary care cohort, the AUC was 0.97 when the APS2 was used.
The 2 cutoff-value approach achieved PPVs of 97%-99% in patients with cognitive impairment, which is the target population of currently available antiamyloid treatments.
Although NPVs were slightly lower in these patients (87%-92% using the APS2), “we argue that a very high positive predictive value is probably more important in diagnosing patients as having Alzheimer’s disease, especially before initiating costly and burdensome antiamyloid treatment,” the investigators noted.
The PPVs were less than optimal for accurate identification of Alzheimer’s disease pathology in patients with subjective cognitive decline regardless of the cutoff-value approach used. The researchers pointed out that this could be a disadvantage for clinical trials that include patients with presymptomatic Alzheimer’s disease but not in clinical practice because there are no clinical criteria for diagnosing Alzheimer’s disease at the subjective cognitive decline stage.
The NPVs were higher in patients with subjective cognitive decline (91%-94% for the APS2 or percentage of p-tau217 alone). This indicates the blood test would be more useful for ruling out underlying Alzheimer’s disease when only subtle symptoms are present, the researchers noted.
As for doctors identifying clinical Alzheimer’s disease, primary care physicians had a diagnostic accuracy of 61% (95% CI, 53%-69%) versus 91% (95% CI, 86%-96%) using the APS2. Dementia specialists had a diagnostic accuracy of 73% (95% CI, 68%-79%) versus 91% (95% CI, 86%-95%) using the APS2.
In the overall population, the diagnostic accuracy using the APS2 (90%; 95% CI, 88%-92%) was not different from that using the percentage of p-tau217 alone (90%; 95% CI, 88%-91%).
Very little was known about how a blood test would perform in a primary care setting, said Dr. Palmqvist. “Seeing that the test was just as accurate in primary care (about 90%) as it was in secondary care is really encouraging, especially since primary care is the first, and often final, point of entry into the healthcare system for cognitive evaluations.”
He said he was surprised the biomarkers performed so well in prospective, biweekly analyses throughout the study. “Previous studies have only demonstrated their effectiveness when all collected samples are analyzed at a single time point, which does not reflect how a blood test is used in clinical practice.”
He added that he was surprised that the tests were just as accurate in primary care as in a memory clinic setting with referred patients. This, despite older age and higher prevalence of comorbidities in primary care, such as chronic kidney disease (present in 26% of the primary care cohort), can be a confounding factor causing increased concentrations of p-tau217.
Next Steps
The diagnostic accuracy of the blood tests is on par with FDA-cleared cerebrospinal fluid biomarkers, noted the investigators, led by senior author Oskar Hansson, MD, PhD, Clinical Memory Research Unit, Department of Clinical Sciences Malm
As blood tests are “more time effective, cost effective, and convenient” for patients, “they could also potentially replace cerebrospinal fluid tests and PET,” they added.
Dr. Palmqvist emphasized that these tests should not be used as stand-alone diagnostic tools for Alzheimer’s disease but should complement the standard clinical evaluation that includes cognitive testing and a thorough interview with the patient and a spouse or relative.
“This is crucial because Alzheimer’s disease pathology can be asymptomatic for many years, and cognitive symptoms in some patients with Alzheimer’s disease pathology may primarily result from other conditions. Misinterpreting a positive Alzheimer’s disease blood test could lead to underdiagnosis of common non–Alzheimer’s disease conditions.”
With new antiamyloid treatments possibly slowing disease progression by 30%-40% when initiated early on, a blood test for Alzheimer’s disease could lead to more people receiving an accurate and earlier diagnosis, said Dr. Palmqvist. “This could potentially result in a better response to treatment. Results from drug trials clearly indicate that the earlier treatment begins, the more effectively it can slow disease progression.”
The test used in the study is already available in the United States, the investigators said, and a similar test will be accessible in Sweden within a few months. “However, the rollout will probably be gradual and will depend on how international and national guidelines recommend their use, so developing these guidelines will be a crucial next step for widespread implementation, particularly in primary care,” said Dr. Palmqvist.
He also underlined the importance of replicating the findings in more diverse populations. “This will help ensure the tests’ reliability and effectiveness across various demographic and clinical contexts.”
An important next research step is to examine how implementing a blood test for Alzheimer’s disease affects patient care. “This includes looking at changes in management, such as referrals, other examinations, and the initiation of appropriate treatments,” said Dr. Palmqvist.
Another study presented at the meeting showed that a highly accurate blood test could significantly reduce diagnostic wait times.
Convincing Research
In an accompanying editorial, Stephen Salloway, MD, Departments of Psychiatry and Neurology, Warren Alpert Medical School, Brown University, Providence, Rhode Island, and colleagues said the study “makes the case convincingly that highly sensitive blood measures of Alzheimer’s disease can be integrated into the clinical decision-making process, including in the primary care setting.”
These tests, they wrote, “can be used to enhance the ability of clinicians to accurately identify individuals with cognitive impairment and dementia due to Alzheimer’s disease.
“Current practice should focus on using these blood biomarkers in individuals with cognitive impairment rather than in those with normal cognition or subjective cognitive decline until further research demonstrates effective interventions for individuals considered cognitively normal with elevated levels of amyloid.”
A key limitation of the study was the lack of diversity in the study sample. This makes it difficult to generalize the results across other ethnic and racial groups, the editorialists noted. Plasma assays for Alzheimer’s disease in the United States will require approval from the FDA and coverage by the Centers for Medicare & Medicaid Services to be widely adopted.
The editorialists also pointed out that advances in the diagnosis and treatment of Alzheimer’s disease will require important changes to healthcare models, including providing additional resources and staffing.
The study was supported by the Alzheimer’s Association, National Institute on Aging, European Research Council, Swedish Research Council, the GHR Foundation, and other groups. The study was conducted as an academic collaboration between Lund University and C2N Diagnostics in the United States. Lund University or its affiliated researchers received no funding or compensation from C2N Diagnostics. C2N Diagnostics performed the plasma analyses blinded to any biomarker or clinical data and had no role in the statistical analysis or results. Dr. Palmqvist reported receiving institutional research support from ki:elements, Alzheimer’s Drug Discovery Foundation, and Avid Radiopharmaceuticals and consultancy or speaker fees from BioArctic, Biogen, Esai, Eli Lilly, and Roche. Dr. Hansson reported receiving personal fees from AC Immune, ALZpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Roche, Bristol-Myers Squibb, Merck, Novartis, Novo Nordisk, Roche, Sanofi, and Siemens and institutional research support from ADX, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. Dr. Salloway reported receiving grants from Biogen, Roche, Lilly, Genentech, Eisai, and Novartis; personal fees from Biogen, Roche, Lilly, Genentech, Eisai, Novo Nordisk, Prothena, AbbVie, Acumen, and Kisbee; and nonfinancial support (travel expenses for conference attendance) from Biogen, Roche, Lilly, and Acumen.
A version of this article appeared on Medscape.com.
PHILADELPHIA — new research showed.
Accurate early diagnosis of Alzheimer’s disease is important because two monoclonal antibodies donanemab (Kisunla) and lecanemab (Leqembi) are now approved by the Food and Drug Administration (FDA) for early-stage Alzheimer’s disease. However, the use of these agents requires amyloid confirmation.
A key finding of the study was that primary care physicians had a diagnostic accuracy of 61%, and dementia specialists had an accuracy of 73%, after completing standard clinical evaluations and before seeing results of the blood test or other Alzheimer’s disease biomarkers, while the blood test used in the study had an accuracy of 91% for correctly classifying clinical, biomarker-verified Alzheimer’s disease.
“This underscores the potential improvement in diagnostic accuracy, especially in primary care, when implementing such a blood test,” said study investigator Sebastian Palmqvist, MD, PhD, associate professor of neurology at Lund University, Lund, and a consultant at Skåne University Hospital, Malmö, Sweden. “It also highlights the challenges in accurately identifying Alzheimer’s disease based solely on clinical evaluation and cognitive testing, even for specialists.”
The findings were presented at the 2024 Alzheimer’s Association International Conference (AAIC) and simultaneously published online in JAMA.
The study included two cohorts from primary and secondary care clinics in Sweden. Researchers analyzed plasma samples together at one time point in a single batch.
It also included two cohorts from Swedish primary and secondary care clinics where the plasma samples were analyzed prospectively (biweekly) in batches throughout the enrollment period, which more closely resembles clinical practice.
Primary care physicians and dementia specialists documented whether they believed their patients had Alzheimer’s disease pathology, basing the diagnoses on the standard evaluation that includes clinical examination, cognitive testing, and a CT scan prior to seeing any Alzheimer’s disease biomarker results.
They reported their certainty of the presence of Alzheimer’s disease pathology on a scale from 0 (not at all certain) to 10 (completely certain).
Plasma analyses were performed by personnel blinded to all clinical or biomarker data. Mass spectrometry assays were used to analyze Abeta42, Abeta40, phosphorylated tau 217 (p-tau217), and non–p-tau217.
Biomarkers used in the study included the percentage of plasma p-tau217, which is the ratio of p-tau217 relative to non–p-tau217, and the Abeta42 to Abeta40 ratio (the amyloid probability score 2 [APS2]). Researchers determined p-tau217 alone and when combined with the APS2.
The study included 1213 patients with cognitive symptoms — mean age 74.2 years and 48% women. Researchers applied biomarker cutoff values to the primary care cohort (n = 307) and the secondary care cohort (n = 300) and then evaluated the blood test prospectively in the primary care cohort (n = 208) and the secondary care cohort (n = 398).
The blood biomarker cutoff value was set at 90% specificity for Alzheimer’s disease pathology (the 1 cutoff-value approach). A 2 cutoff-value approach (using 1 upper and 1 lower cutoff value) was also used with values corresponding to 95% sensitivity and 95% specificity.
The primary outcome was presence of Alzheimer’s disease pathology. A positive finding of the Abeta biomarker was defined according to the FDA-approved cutoff value (≤ 0.072). A positive finding of the tau biomarker was defined as a p-tau217 level > 11.42 pg/mL in cerebrospinal fluid.
Researchers calculated the positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy, as well as area under the curve (AUC) values.
Accuracy in Specialty Versus Primary Care
When the plasma samples were analyzed in a single batch in the primary care cohort, the AUC was 0.97 when the APS2 was used. In the secondary care cohort, the AUC was 0.96 when the APS2 was used.
When plasma samples were analyzed prospectively (biweekly) in the primary care cohort, the AUC was 0.96 when the APS2 was used. In the secondary care cohort, the AUC was 0.97 when the APS2 was used.
The 2 cutoff-value approach achieved PPVs of 97%-99% in patients with cognitive impairment, which is the target population of currently available antiamyloid treatments.
Although NPVs were slightly lower in these patients (87%-92% using the APS2), “we argue that a very high positive predictive value is probably more important in diagnosing patients as having Alzheimer’s disease, especially before initiating costly and burdensome antiamyloid treatment,” the investigators noted.
The PPVs were less than optimal for accurate identification of Alzheimer’s disease pathology in patients with subjective cognitive decline regardless of the cutoff-value approach used. The researchers pointed out that this could be a disadvantage for clinical trials that include patients with presymptomatic Alzheimer’s disease but not in clinical practice because there are no clinical criteria for diagnosing Alzheimer’s disease at the subjective cognitive decline stage.
The NPVs were higher in patients with subjective cognitive decline (91%-94% for the APS2 or percentage of p-tau217 alone). This indicates the blood test would be more useful for ruling out underlying Alzheimer’s disease when only subtle symptoms are present, the researchers noted.
As for doctors identifying clinical Alzheimer’s disease, primary care physicians had a diagnostic accuracy of 61% (95% CI, 53%-69%) versus 91% (95% CI, 86%-96%) using the APS2. Dementia specialists had a diagnostic accuracy of 73% (95% CI, 68%-79%) versus 91% (95% CI, 86%-95%) using the APS2.
In the overall population, the diagnostic accuracy using the APS2 (90%; 95% CI, 88%-92%) was not different from that using the percentage of p-tau217 alone (90%; 95% CI, 88%-91%).
Very little was known about how a blood test would perform in a primary care setting, said Dr. Palmqvist. “Seeing that the test was just as accurate in primary care (about 90%) as it was in secondary care is really encouraging, especially since primary care is the first, and often final, point of entry into the healthcare system for cognitive evaluations.”
He said he was surprised the biomarkers performed so well in prospective, biweekly analyses throughout the study. “Previous studies have only demonstrated their effectiveness when all collected samples are analyzed at a single time point, which does not reflect how a blood test is used in clinical practice.”
He added that he was surprised that the tests were just as accurate in primary care as in a memory clinic setting with referred patients. This, despite older age and higher prevalence of comorbidities in primary care, such as chronic kidney disease (present in 26% of the primary care cohort), can be a confounding factor causing increased concentrations of p-tau217.
Next Steps
The diagnostic accuracy of the blood tests is on par with FDA-cleared cerebrospinal fluid biomarkers, noted the investigators, led by senior author Oskar Hansson, MD, PhD, Clinical Memory Research Unit, Department of Clinical Sciences Malm
As blood tests are “more time effective, cost effective, and convenient” for patients, “they could also potentially replace cerebrospinal fluid tests and PET,” they added.
Dr. Palmqvist emphasized that these tests should not be used as stand-alone diagnostic tools for Alzheimer’s disease but should complement the standard clinical evaluation that includes cognitive testing and a thorough interview with the patient and a spouse or relative.
“This is crucial because Alzheimer’s disease pathology can be asymptomatic for many years, and cognitive symptoms in some patients with Alzheimer’s disease pathology may primarily result from other conditions. Misinterpreting a positive Alzheimer’s disease blood test could lead to underdiagnosis of common non–Alzheimer’s disease conditions.”
With new antiamyloid treatments possibly slowing disease progression by 30%-40% when initiated early on, a blood test for Alzheimer’s disease could lead to more people receiving an accurate and earlier diagnosis, said Dr. Palmqvist. “This could potentially result in a better response to treatment. Results from drug trials clearly indicate that the earlier treatment begins, the more effectively it can slow disease progression.”
The test used in the study is already available in the United States, the investigators said, and a similar test will be accessible in Sweden within a few months. “However, the rollout will probably be gradual and will depend on how international and national guidelines recommend their use, so developing these guidelines will be a crucial next step for widespread implementation, particularly in primary care,” said Dr. Palmqvist.
He also underlined the importance of replicating the findings in more diverse populations. “This will help ensure the tests’ reliability and effectiveness across various demographic and clinical contexts.”
An important next research step is to examine how implementing a blood test for Alzheimer’s disease affects patient care. “This includes looking at changes in management, such as referrals, other examinations, and the initiation of appropriate treatments,” said Dr. Palmqvist.
Another study presented at the meeting showed that a highly accurate blood test could significantly reduce diagnostic wait times.
Convincing Research
In an accompanying editorial, Stephen Salloway, MD, Departments of Psychiatry and Neurology, Warren Alpert Medical School, Brown University, Providence, Rhode Island, and colleagues said the study “makes the case convincingly that highly sensitive blood measures of Alzheimer’s disease can be integrated into the clinical decision-making process, including in the primary care setting.”
These tests, they wrote, “can be used to enhance the ability of clinicians to accurately identify individuals with cognitive impairment and dementia due to Alzheimer’s disease.
“Current practice should focus on using these blood biomarkers in individuals with cognitive impairment rather than in those with normal cognition or subjective cognitive decline until further research demonstrates effective interventions for individuals considered cognitively normal with elevated levels of amyloid.”
A key limitation of the study was the lack of diversity in the study sample. This makes it difficult to generalize the results across other ethnic and racial groups, the editorialists noted. Plasma assays for Alzheimer’s disease in the United States will require approval from the FDA and coverage by the Centers for Medicare & Medicaid Services to be widely adopted.
The editorialists also pointed out that advances in the diagnosis and treatment of Alzheimer’s disease will require important changes to healthcare models, including providing additional resources and staffing.
The study was supported by the Alzheimer’s Association, National Institute on Aging, European Research Council, Swedish Research Council, the GHR Foundation, and other groups. The study was conducted as an academic collaboration between Lund University and C2N Diagnostics in the United States. Lund University or its affiliated researchers received no funding or compensation from C2N Diagnostics. C2N Diagnostics performed the plasma analyses blinded to any biomarker or clinical data and had no role in the statistical analysis or results. Dr. Palmqvist reported receiving institutional research support from ki:elements, Alzheimer’s Drug Discovery Foundation, and Avid Radiopharmaceuticals and consultancy or speaker fees from BioArctic, Biogen, Esai, Eli Lilly, and Roche. Dr. Hansson reported receiving personal fees from AC Immune, ALZpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Roche, Bristol-Myers Squibb, Merck, Novartis, Novo Nordisk, Roche, Sanofi, and Siemens and institutional research support from ADX, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. Dr. Salloway reported receiving grants from Biogen, Roche, Lilly, Genentech, Eisai, and Novartis; personal fees from Biogen, Roche, Lilly, Genentech, Eisai, Novo Nordisk, Prothena, AbbVie, Acumen, and Kisbee; and nonfinancial support (travel expenses for conference attendance) from Biogen, Roche, Lilly, and Acumen.
A version of this article appeared on Medscape.com.
FROM AAIC 2024
Alzheimer’s Blood Test in Primary Care Could Slash Diagnostic, Treatment Wait Times
As disease-modifying treatments for Alzheimer’s disease (AD) become available, . Currently, the patient diagnostic journey is often prolonged owing to the limited number of AD specialists, causing concern among healthcare providers and patients alike. Now, a new study suggests that use of high-performing blood tests in primary care could identify potential patients with AD much earlier, possibly reducing wait times for specialist care and receipt of treatment.
“We need to triage in primary care and send preferentially the ones that actually could be eligible for treatment, and not those who are just worried because their grandmother reported that she has Alzheimer’s,” lead researcher Soeren Mattke, MD, DSc, told this news organization.
“By combining a brief cognitive test with an accurate blood test of Alzheimer’s pathology in primary care, we can reduce unnecessary referrals, and shorten appointment wait times,” said Dr. Mattke, director of the Brain Health Observatory at the University of Southern California in Los Angeles.
The findings were presented at the Alzheimer’s Association International Conference (AAIC) 2024.
Projected Wait Times 100 Months by 2033
The investigators used a Markov model to estimate wait times for patients eligible for AD treatment, taking into account constrained capacity for specialist visits.
The model included the projected US population of people aged 55 years or older from 2023 to 2032. It assumed that individuals would undergo a brief cognitive assessment in primary care and, if suggestive of early-stage cognitive impairment, be referred to a AD specialist under three scenarios: no blood test, blood test to rule out AD pathology, and blood test to confirm AD pathology.
According to the model, without an accurate blood test for AD pathology, projected wait times to see a specialist are about 12 months in 2024 and will increase to more than 100 months in 2033, largely owing to a lack of specialist appointments.
In contrast, with the availability of an accurate blood test to rule out AD, average wait times would be just 3 months in 2024 and increase to only about 13 months in 2033, because far fewer patients would need to see a specialist.
Availability of a blood test to rule in AD pathology in primary care would have a limited effect on wait times because 50% of patients would still undergo confirmatory testing based on expert assumptions, the model suggests.
Prioritizing Resources
“Millions of people have mild memory complaints, and if they all start coming to neurologists, it could completely flood the system and create long wait times for everybody,” Dr. Mattke told this news organization.
The problem, he said, is that brief cognitive tests performed in primary care are not particularly specific for mild cognitive impairment.
“They work pretty well for manifest advanced dementia but for mild cognitive impairment, which is a very subtle, symptomatic disease, they are only about 75% accurate. One quarter are false-positives. That’s a lot of people,” Dr. Mattke said.
He also noted that although earlier blood tests were about 75% accurate, they are now about 90% accurate, “so we are getting to a level where we can pretty much say with confidence that this is likely Alzheimer’s,” Dr. Mattke said.
Commenting on this research for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said it is clear that blood tests, “once confirmed, could have a significant impact on the wait times” for dementia assessment.
“After an initial blood test, we might be able to rule out or rule in individuals who should go to a specialist for further follow-up and testing. This allows us to really ensure that we’re prioritizing resources accordingly,” said Dr. Snyder, who was not involved in the study.
This project was supported by a research contract from C2N Diagnostics LLC to USC. Dr. Mattke serves on the board of directors of Senscio Systems Inc. and the scientific advisory board of ALZPath and Boston Millennia Partners and has received consulting fees from Biogen, C2N, Eisai, Eli Lilly, Novartis, and Roche/Genentech. Dr. Snyder has no relevant disclosures.
A version of this article first appeared on Medscape.com.
As disease-modifying treatments for Alzheimer’s disease (AD) become available, . Currently, the patient diagnostic journey is often prolonged owing to the limited number of AD specialists, causing concern among healthcare providers and patients alike. Now, a new study suggests that use of high-performing blood tests in primary care could identify potential patients with AD much earlier, possibly reducing wait times for specialist care and receipt of treatment.
“We need to triage in primary care and send preferentially the ones that actually could be eligible for treatment, and not those who are just worried because their grandmother reported that she has Alzheimer’s,” lead researcher Soeren Mattke, MD, DSc, told this news organization.
“By combining a brief cognitive test with an accurate blood test of Alzheimer’s pathology in primary care, we can reduce unnecessary referrals, and shorten appointment wait times,” said Dr. Mattke, director of the Brain Health Observatory at the University of Southern California in Los Angeles.
The findings were presented at the Alzheimer’s Association International Conference (AAIC) 2024.
Projected Wait Times 100 Months by 2033
The investigators used a Markov model to estimate wait times for patients eligible for AD treatment, taking into account constrained capacity for specialist visits.
The model included the projected US population of people aged 55 years or older from 2023 to 2032. It assumed that individuals would undergo a brief cognitive assessment in primary care and, if suggestive of early-stage cognitive impairment, be referred to a AD specialist under three scenarios: no blood test, blood test to rule out AD pathology, and blood test to confirm AD pathology.
According to the model, without an accurate blood test for AD pathology, projected wait times to see a specialist are about 12 months in 2024 and will increase to more than 100 months in 2033, largely owing to a lack of specialist appointments.
In contrast, with the availability of an accurate blood test to rule out AD, average wait times would be just 3 months in 2024 and increase to only about 13 months in 2033, because far fewer patients would need to see a specialist.
Availability of a blood test to rule in AD pathology in primary care would have a limited effect on wait times because 50% of patients would still undergo confirmatory testing based on expert assumptions, the model suggests.
Prioritizing Resources
“Millions of people have mild memory complaints, and if they all start coming to neurologists, it could completely flood the system and create long wait times for everybody,” Dr. Mattke told this news organization.
The problem, he said, is that brief cognitive tests performed in primary care are not particularly specific for mild cognitive impairment.
“They work pretty well for manifest advanced dementia but for mild cognitive impairment, which is a very subtle, symptomatic disease, they are only about 75% accurate. One quarter are false-positives. That’s a lot of people,” Dr. Mattke said.
He also noted that although earlier blood tests were about 75% accurate, they are now about 90% accurate, “so we are getting to a level where we can pretty much say with confidence that this is likely Alzheimer’s,” Dr. Mattke said.
Commenting on this research for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said it is clear that blood tests, “once confirmed, could have a significant impact on the wait times” for dementia assessment.
“After an initial blood test, we might be able to rule out or rule in individuals who should go to a specialist for further follow-up and testing. This allows us to really ensure that we’re prioritizing resources accordingly,” said Dr. Snyder, who was not involved in the study.
This project was supported by a research contract from C2N Diagnostics LLC to USC. Dr. Mattke serves on the board of directors of Senscio Systems Inc. and the scientific advisory board of ALZPath and Boston Millennia Partners and has received consulting fees from Biogen, C2N, Eisai, Eli Lilly, Novartis, and Roche/Genentech. Dr. Snyder has no relevant disclosures.
A version of this article first appeared on Medscape.com.
As disease-modifying treatments for Alzheimer’s disease (AD) become available, . Currently, the patient diagnostic journey is often prolonged owing to the limited number of AD specialists, causing concern among healthcare providers and patients alike. Now, a new study suggests that use of high-performing blood tests in primary care could identify potential patients with AD much earlier, possibly reducing wait times for specialist care and receipt of treatment.
“We need to triage in primary care and send preferentially the ones that actually could be eligible for treatment, and not those who are just worried because their grandmother reported that she has Alzheimer’s,” lead researcher Soeren Mattke, MD, DSc, told this news organization.
“By combining a brief cognitive test with an accurate blood test of Alzheimer’s pathology in primary care, we can reduce unnecessary referrals, and shorten appointment wait times,” said Dr. Mattke, director of the Brain Health Observatory at the University of Southern California in Los Angeles.
The findings were presented at the Alzheimer’s Association International Conference (AAIC) 2024.
Projected Wait Times 100 Months by 2033
The investigators used a Markov model to estimate wait times for patients eligible for AD treatment, taking into account constrained capacity for specialist visits.
The model included the projected US population of people aged 55 years or older from 2023 to 2032. It assumed that individuals would undergo a brief cognitive assessment in primary care and, if suggestive of early-stage cognitive impairment, be referred to a AD specialist under three scenarios: no blood test, blood test to rule out AD pathology, and blood test to confirm AD pathology.
According to the model, without an accurate blood test for AD pathology, projected wait times to see a specialist are about 12 months in 2024 and will increase to more than 100 months in 2033, largely owing to a lack of specialist appointments.
In contrast, with the availability of an accurate blood test to rule out AD, average wait times would be just 3 months in 2024 and increase to only about 13 months in 2033, because far fewer patients would need to see a specialist.
Availability of a blood test to rule in AD pathology in primary care would have a limited effect on wait times because 50% of patients would still undergo confirmatory testing based on expert assumptions, the model suggests.
Prioritizing Resources
“Millions of people have mild memory complaints, and if they all start coming to neurologists, it could completely flood the system and create long wait times for everybody,” Dr. Mattke told this news organization.
The problem, he said, is that brief cognitive tests performed in primary care are not particularly specific for mild cognitive impairment.
“They work pretty well for manifest advanced dementia but for mild cognitive impairment, which is a very subtle, symptomatic disease, they are only about 75% accurate. One quarter are false-positives. That’s a lot of people,” Dr. Mattke said.
He also noted that although earlier blood tests were about 75% accurate, they are now about 90% accurate, “so we are getting to a level where we can pretty much say with confidence that this is likely Alzheimer’s,” Dr. Mattke said.
Commenting on this research for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said it is clear that blood tests, “once confirmed, could have a significant impact on the wait times” for dementia assessment.
“After an initial blood test, we might be able to rule out or rule in individuals who should go to a specialist for further follow-up and testing. This allows us to really ensure that we’re prioritizing resources accordingly,” said Dr. Snyder, who was not involved in the study.
This project was supported by a research contract from C2N Diagnostics LLC to USC. Dr. Mattke serves on the board of directors of Senscio Systems Inc. and the scientific advisory board of ALZPath and Boston Millennia Partners and has received consulting fees from Biogen, C2N, Eisai, Eli Lilly, Novartis, and Roche/Genentech. Dr. Snyder has no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM AAIC 2024