User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Six big changes coming for office-visit coding
Betsy Nicoletti, MS, a nationally recognized coding expert, will take your coding questions via email and provide guidance on how to code properly to maximize reimbursement. Have a question about coding? Send it to [email protected].
thanks to the American Medical Association.
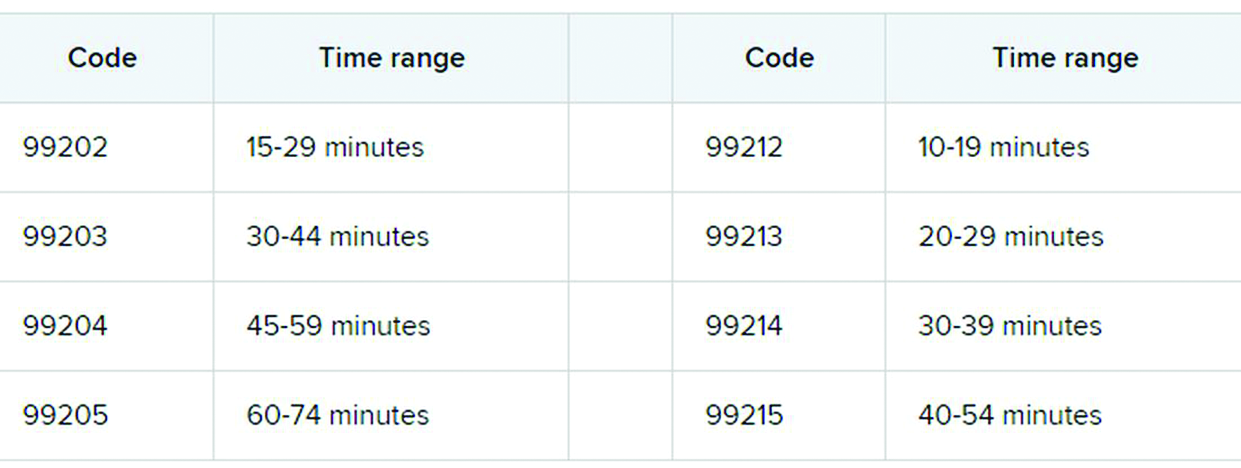
The first major changes to the definitions for E/M services will be in effect as of Jan. 1, 2021, with all payers expected to adopt these new guidelines. In particular, the AMA has revised the definitions for E/M codes 99202-99215 in the Current Procedural Terminology (CPT) 2021 codebook. The existing guidelines were developed in 1995 and 1997 and remain in effect for all other E/M services determined by history, exam, and medical decision-making (MDM).
What do the new changes mean to you? In 2021, for new and established office and other outpatient services reported with codes 99202-99215, a clinician may select the code on the basis of time or MDM.
There are three elements in MDM, and two of three are required. These elements are the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
Make sure you familiarize yourself with these six big changes. It may take a bit of time to integrate these new processes into your daily routine, but wrapping your head around them as soon as possible can help boost your bottom line:
1. History and exam don’t count toward level of service
Physicians, advanced practice registered nurses, and physician assistants won’t use history or exam to select what level of code to bill for office visits 99202-99215, as they did in the past. They need only document a medically appropriate history and exam. The history may be obtained by staff members and reviewed by the billing practitioner.
While specific history and exam requirements disappear for office visit codes, they remain for all other types of visits, selected on the basis of history, exam, and MDM, such as hospital services, nursing facility services, and home and domiciliary care. So, say goodbye to “all other systems reviewed and negative” in office notes, but keep it handy for those other E/M codes.
2. All time spent caring for the patient on a particular day counts
This includes all time spent on the day of service, including preparing to see the patient, seeing the patient, phone calls or other work done after the visit (if not billed with a care management or other CPT code), and documenting in the medical record. The AMA developed new guidelines for using time for office and other outpatient services. For codes 99202-99215, count all of the face-to-face and non–face-to-face time spent by the billing clinician on the day of the visit. Counseling does not need to be more than 50% of the total time.
Do not include any staff time or time spent on any days before or after the visit. This allows clinicians to capture the work when a significant amount of it takes place before or after the visit with the patient, and to bill for it on the day of the visit.
According to the 2021 CPT codebook, physician or other qualified health care professional time includes the following activities:
- Preparing to see the patient (e.g., review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).
3. Soon to be gone: ‘new to the examiner’ and ‘workup planned’
The current guidelines don’t differentiate between a new problem to the clinician or an established problem to the clinician. So it doesn’t matter whether you’re hearing about a particular problem for the first time or the fifth time. The new office and outpatient services guidelines define problems only as they relate to the patient. For example, when selecting a level of service, a chronic problem with a mild exacerbation is the same level whether it’s the primary care physician seeing the patient for the 10th time to help manage her diabetes or the endocrinologist seeing the patient for the first time.
In the current guidelines (1995 and 1997), additional weight is given in selecting the level of MDM for a problem that’s new to the examiner with a workup planned, yet when the diagnostic test couldn’t be completed at the visit. This concept is gone from element of number and complexity of new problems. Ordering diagnostic tests is part of the second element, the amount and/or complexity of data to be reviewed.
4. Different guidelines if you need a history from a parent or other source
The new guidelines recognize the additional work required by the clinician when the patient is unable to give a history or when the practitioner doesn’t find the history to be reliable.
For example, in the case of a baby or child who is unable to give a history, the parent counts as an “independent historian,” according to the new guidelines. Likewise, for a patient with dementia, the caregiver counts as a historian. Note, however, that the criteria is not met simply because the patient is accompanied by another person. The additional weight in selecting the level of service is based on the patient being unable to give a reliable history.
Bottom line: In cases where patients are unable to communicate clearly, physicians or other providers should document the necessity of getting a complete history and who provided it.
5. A new spin on social determinants of health (SDoH)
In the risk of morbidity and/or mortality element, conditions described as “social determinants of health” are considered moderate complexity. SDoH are social and environmental factors that affect a patient’s health and medical outcomes. These include homelessness, inability to afford medications, food insecurity, and occupational exposure to risk factors. These circumstances are reported with codes in categories Z55-Z65.
In the past, physicians often documented this information in their office notes but rarely added a diagnosis code that described the patient’s situation. The ICD-10-CM code set includes codes that describe these factors. Using them allows the practice to track patients who have increased needs, and it communicates to payers the complexity of caring for these patients.
6. Risks related to surgery are defined
The current guidelines assign different levels of risk to minor and major surgery. They also include differentiation for “minor surgery with no identified risk factors,” “minor surgery with identified risk factors,” “elective major surgery with no identified risk factors,” and “elective major surgery with identified risk factors.” The old guidelines didn’t state whether the risk factors pertained to the patient – such as smoking, heart disease, or high body mass index – or to the procedure itself.
The new guidelines specifically say that it’s both. In the risk column, “decision regarding minor surgery with identified patient or procedure risk factors” and “decision regarding elective major surgery without patient or procedure risk factors” are both considered moderate. “Decision regarding elective major surgery with identified patient or procedure risk factors” and “decision regarding emergency major surgery” are in the high complexity column for risk.
Keep in mind that two of three elements are required: the number and complexity of problems, amount of data, and morbidity/mortality risk. Risk of morbidity/mortality alone doesn’t count as the basis for selecting the code. Of course, when surgeons see this, they ask, “What major procedures don’t have identified risk factors?”
Note, too, that these new CPT guidelines do not define the terms “minor” and “major” surgery. For payment reasons related to the postop period, the Centers for Medicare & Medicaid Services defines minor surgery as a procedure with 0-10 global days and a major surgery as a procedure with 90 global days. However, there are many procedures with 0 global days (endoscopy, cardiac catheterization) that are not minor procedures. Hopefully, the AMA will clarify this in 2021.
What’s the take-away for clinicians?
There are sure to be shifts in coding patterns based on these new guidelines. Some specialties will find that not being able to select a service based on history and exam alone will lower the level of service for which they can bill. Some practices, on the other hand, will be able to code for more high-level visits, without the need for a complete review of systems or a comprehensive exam.
The biggest challenge will be for practices that provide services both in the hospital and in the office, because they’ll have to use both sets of guidelines, depending on which type of service they’re performing.
For more details on what’s coming your way beginning on New Year’s Day, you may want to read the 16-page AMA document .
A version of this article first appeared on Medscape.com.
Betsy Nicoletti, MS, a nationally recognized coding expert, will take your coding questions via email and provide guidance on how to code properly to maximize reimbursement. Have a question about coding? Send it to [email protected].
thanks to the American Medical Association.
The first major changes to the definitions for E/M services will be in effect as of Jan. 1, 2021, with all payers expected to adopt these new guidelines. In particular, the AMA has revised the definitions for E/M codes 99202-99215 in the Current Procedural Terminology (CPT) 2021 codebook. The existing guidelines were developed in 1995 and 1997 and remain in effect for all other E/M services determined by history, exam, and medical decision-making (MDM).
What do the new changes mean to you? In 2021, for new and established office and other outpatient services reported with codes 99202-99215, a clinician may select the code on the basis of time or MDM.
There are three elements in MDM, and two of three are required. These elements are the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
Make sure you familiarize yourself with these six big changes. It may take a bit of time to integrate these new processes into your daily routine, but wrapping your head around them as soon as possible can help boost your bottom line:
1. History and exam don’t count toward level of service
Physicians, advanced practice registered nurses, and physician assistants won’t use history or exam to select what level of code to bill for office visits 99202-99215, as they did in the past. They need only document a medically appropriate history and exam. The history may be obtained by staff members and reviewed by the billing practitioner.
While specific history and exam requirements disappear for office visit codes, they remain for all other types of visits, selected on the basis of history, exam, and MDM, such as hospital services, nursing facility services, and home and domiciliary care. So, say goodbye to “all other systems reviewed and negative” in office notes, but keep it handy for those other E/M codes.
2. All time spent caring for the patient on a particular day counts
This includes all time spent on the day of service, including preparing to see the patient, seeing the patient, phone calls or other work done after the visit (if not billed with a care management or other CPT code), and documenting in the medical record. The AMA developed new guidelines for using time for office and other outpatient services. For codes 99202-99215, count all of the face-to-face and non–face-to-face time spent by the billing clinician on the day of the visit. Counseling does not need to be more than 50% of the total time.
Do not include any staff time or time spent on any days before or after the visit. This allows clinicians to capture the work when a significant amount of it takes place before or after the visit with the patient, and to bill for it on the day of the visit.
According to the 2021 CPT codebook, physician or other qualified health care professional time includes the following activities:
- Preparing to see the patient (e.g., review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).

3. Soon to be gone: ‘new to the examiner’ and ‘workup planned’
The current guidelines don’t differentiate between a new problem to the clinician or an established problem to the clinician. So it doesn’t matter whether you’re hearing about a particular problem for the first time or the fifth time. The new office and outpatient services guidelines define problems only as they relate to the patient. For example, when selecting a level of service, a chronic problem with a mild exacerbation is the same level whether it’s the primary care physician seeing the patient for the 10th time to help manage her diabetes or the endocrinologist seeing the patient for the first time.
In the current guidelines (1995 and 1997), additional weight is given in selecting the level of MDM for a problem that’s new to the examiner with a workup planned, yet when the diagnostic test couldn’t be completed at the visit. This concept is gone from element of number and complexity of new problems. Ordering diagnostic tests is part of the second element, the amount and/or complexity of data to be reviewed.
4. Different guidelines if you need a history from a parent or other source
The new guidelines recognize the additional work required by the clinician when the patient is unable to give a history or when the practitioner doesn’t find the history to be reliable.
For example, in the case of a baby or child who is unable to give a history, the parent counts as an “independent historian,” according to the new guidelines. Likewise, for a patient with dementia, the caregiver counts as a historian. Note, however, that the criteria is not met simply because the patient is accompanied by another person. The additional weight in selecting the level of service is based on the patient being unable to give a reliable history.
Bottom line: In cases where patients are unable to communicate clearly, physicians or other providers should document the necessity of getting a complete history and who provided it.
5. A new spin on social determinants of health (SDoH)
In the risk of morbidity and/or mortality element, conditions described as “social determinants of health” are considered moderate complexity. SDoH are social and environmental factors that affect a patient’s health and medical outcomes. These include homelessness, inability to afford medications, food insecurity, and occupational exposure to risk factors. These circumstances are reported with codes in categories Z55-Z65.
In the past, physicians often documented this information in their office notes but rarely added a diagnosis code that described the patient’s situation. The ICD-10-CM code set includes codes that describe these factors. Using them allows the practice to track patients who have increased needs, and it communicates to payers the complexity of caring for these patients.
6. Risks related to surgery are defined
The current guidelines assign different levels of risk to minor and major surgery. They also include differentiation for “minor surgery with no identified risk factors,” “minor surgery with identified risk factors,” “elective major surgery with no identified risk factors,” and “elective major surgery with identified risk factors.” The old guidelines didn’t state whether the risk factors pertained to the patient – such as smoking, heart disease, or high body mass index – or to the procedure itself.
The new guidelines specifically say that it’s both. In the risk column, “decision regarding minor surgery with identified patient or procedure risk factors” and “decision regarding elective major surgery without patient or procedure risk factors” are both considered moderate. “Decision regarding elective major surgery with identified patient or procedure risk factors” and “decision regarding emergency major surgery” are in the high complexity column for risk.
Keep in mind that two of three elements are required: the number and complexity of problems, amount of data, and morbidity/mortality risk. Risk of morbidity/mortality alone doesn’t count as the basis for selecting the code. Of course, when surgeons see this, they ask, “What major procedures don’t have identified risk factors?”
Note, too, that these new CPT guidelines do not define the terms “minor” and “major” surgery. For payment reasons related to the postop period, the Centers for Medicare & Medicaid Services defines minor surgery as a procedure with 0-10 global days and a major surgery as a procedure with 90 global days. However, there are many procedures with 0 global days (endoscopy, cardiac catheterization) that are not minor procedures. Hopefully, the AMA will clarify this in 2021.
What’s the take-away for clinicians?
There are sure to be shifts in coding patterns based on these new guidelines. Some specialties will find that not being able to select a service based on history and exam alone will lower the level of service for which they can bill. Some practices, on the other hand, will be able to code for more high-level visits, without the need for a complete review of systems or a comprehensive exam.
The biggest challenge will be for practices that provide services both in the hospital and in the office, because they’ll have to use both sets of guidelines, depending on which type of service they’re performing.
For more details on what’s coming your way beginning on New Year’s Day, you may want to read the 16-page AMA document .
A version of this article first appeared on Medscape.com.
Betsy Nicoletti, MS, a nationally recognized coding expert, will take your coding questions via email and provide guidance on how to code properly to maximize reimbursement. Have a question about coding? Send it to [email protected].
thanks to the American Medical Association.
The first major changes to the definitions for E/M services will be in effect as of Jan. 1, 2021, with all payers expected to adopt these new guidelines. In particular, the AMA has revised the definitions for E/M codes 99202-99215 in the Current Procedural Terminology (CPT) 2021 codebook. The existing guidelines were developed in 1995 and 1997 and remain in effect for all other E/M services determined by history, exam, and medical decision-making (MDM).
What do the new changes mean to you? In 2021, for new and established office and other outpatient services reported with codes 99202-99215, a clinician may select the code on the basis of time or MDM.
There are three elements in MDM, and two of three are required. These elements are the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
Make sure you familiarize yourself with these six big changes. It may take a bit of time to integrate these new processes into your daily routine, but wrapping your head around them as soon as possible can help boost your bottom line:
1. History and exam don’t count toward level of service
Physicians, advanced practice registered nurses, and physician assistants won’t use history or exam to select what level of code to bill for office visits 99202-99215, as they did in the past. They need only document a medically appropriate history and exam. The history may be obtained by staff members and reviewed by the billing practitioner.
While specific history and exam requirements disappear for office visit codes, they remain for all other types of visits, selected on the basis of history, exam, and MDM, such as hospital services, nursing facility services, and home and domiciliary care. So, say goodbye to “all other systems reviewed and negative” in office notes, but keep it handy for those other E/M codes.
2. All time spent caring for the patient on a particular day counts
This includes all time spent on the day of service, including preparing to see the patient, seeing the patient, phone calls or other work done after the visit (if not billed with a care management or other CPT code), and documenting in the medical record. The AMA developed new guidelines for using time for office and other outpatient services. For codes 99202-99215, count all of the face-to-face and non–face-to-face time spent by the billing clinician on the day of the visit. Counseling does not need to be more than 50% of the total time.
Do not include any staff time or time spent on any days before or after the visit. This allows clinicians to capture the work when a significant amount of it takes place before or after the visit with the patient, and to bill for it on the day of the visit.
According to the 2021 CPT codebook, physician or other qualified health care professional time includes the following activities:
- Preparing to see the patient (e.g., review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).

3. Soon to be gone: ‘new to the examiner’ and ‘workup planned’
The current guidelines don’t differentiate between a new problem to the clinician or an established problem to the clinician. So it doesn’t matter whether you’re hearing about a particular problem for the first time or the fifth time. The new office and outpatient services guidelines define problems only as they relate to the patient. For example, when selecting a level of service, a chronic problem with a mild exacerbation is the same level whether it’s the primary care physician seeing the patient for the 10th time to help manage her diabetes or the endocrinologist seeing the patient for the first time.
In the current guidelines (1995 and 1997), additional weight is given in selecting the level of MDM for a problem that’s new to the examiner with a workup planned, yet when the diagnostic test couldn’t be completed at the visit. This concept is gone from element of number and complexity of new problems. Ordering diagnostic tests is part of the second element, the amount and/or complexity of data to be reviewed.
4. Different guidelines if you need a history from a parent or other source
The new guidelines recognize the additional work required by the clinician when the patient is unable to give a history or when the practitioner doesn’t find the history to be reliable.
For example, in the case of a baby or child who is unable to give a history, the parent counts as an “independent historian,” according to the new guidelines. Likewise, for a patient with dementia, the caregiver counts as a historian. Note, however, that the criteria is not met simply because the patient is accompanied by another person. The additional weight in selecting the level of service is based on the patient being unable to give a reliable history.
Bottom line: In cases where patients are unable to communicate clearly, physicians or other providers should document the necessity of getting a complete history and who provided it.
5. A new spin on social determinants of health (SDoH)
In the risk of morbidity and/or mortality element, conditions described as “social determinants of health” are considered moderate complexity. SDoH are social and environmental factors that affect a patient’s health and medical outcomes. These include homelessness, inability to afford medications, food insecurity, and occupational exposure to risk factors. These circumstances are reported with codes in categories Z55-Z65.
In the past, physicians often documented this information in their office notes but rarely added a diagnosis code that described the patient’s situation. The ICD-10-CM code set includes codes that describe these factors. Using them allows the practice to track patients who have increased needs, and it communicates to payers the complexity of caring for these patients.
6. Risks related to surgery are defined
The current guidelines assign different levels of risk to minor and major surgery. They also include differentiation for “minor surgery with no identified risk factors,” “minor surgery with identified risk factors,” “elective major surgery with no identified risk factors,” and “elective major surgery with identified risk factors.” The old guidelines didn’t state whether the risk factors pertained to the patient – such as smoking, heart disease, or high body mass index – or to the procedure itself.
The new guidelines specifically say that it’s both. In the risk column, “decision regarding minor surgery with identified patient or procedure risk factors” and “decision regarding elective major surgery without patient or procedure risk factors” are both considered moderate. “Decision regarding elective major surgery with identified patient or procedure risk factors” and “decision regarding emergency major surgery” are in the high complexity column for risk.
Keep in mind that two of three elements are required: the number and complexity of problems, amount of data, and morbidity/mortality risk. Risk of morbidity/mortality alone doesn’t count as the basis for selecting the code. Of course, when surgeons see this, they ask, “What major procedures don’t have identified risk factors?”
Note, too, that these new CPT guidelines do not define the terms “minor” and “major” surgery. For payment reasons related to the postop period, the Centers for Medicare & Medicaid Services defines minor surgery as a procedure with 0-10 global days and a major surgery as a procedure with 90 global days. However, there are many procedures with 0 global days (endoscopy, cardiac catheterization) that are not minor procedures. Hopefully, the AMA will clarify this in 2021.
What’s the take-away for clinicians?
There are sure to be shifts in coding patterns based on these new guidelines. Some specialties will find that not being able to select a service based on history and exam alone will lower the level of service for which they can bill. Some practices, on the other hand, will be able to code for more high-level visits, without the need for a complete review of systems or a comprehensive exam.
The biggest challenge will be for practices that provide services both in the hospital and in the office, because they’ll have to use both sets of guidelines, depending on which type of service they’re performing.
For more details on what’s coming your way beginning on New Year’s Day, you may want to read the 16-page AMA document .
A version of this article first appeared on Medscape.com.
To vape or not to vape: Is that really a question?
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at [email protected].
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at [email protected].
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at [email protected].
COVID-related harm to HCWs must be tracked more rigorously: NAS panel
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
PTSD, depression combo tied to high risk for early death in women
Middle-aged women with PTSD and comorbid depression have a nearly fourfold increased risk for early death from a variety of causes in comparison with their peers who do not have those conditions, new research shows.
“Women with more severe symptoms of depression and PTSD were more at risk, compared with those with fewer symptoms or women with symptoms of only PTSD or only depression,” lead investigator Andrea Roberts, PhD, Harvard School of Public Health, Boston, said in an interview.
Health care providers “should be aware that mental health is a critical component of overall health and is tightly entwined with physical health. Identifying and treating mental health issues should be a foundational part of general health practice,” said Dr. Roberts.
The study was published online Dec. 4 in JAMA Network Open.
Mental health fundamental to survival
The researchers studied more than 51,000 mostly White women from the Nurses Health Study II who were followed for 9 years (2008-2017). At baseline in 2008, the women were aged between 43 and 64 years (mean age, 53.3 years).
Women with high levels of PTSD (six or seven symptoms) and probable depression were nearly four times more likely to die during follow-up than their peers who did not have these conditions (hazard ratio, 3.8; 95% confidence interval, 2.65-5.45; P < .001).
With adjustment for health factors such as smoking and body mass index, women with a high level of PTSD and depression remained at increased risk for early death (HR, 3.11; 95% CI, 2.16-4.47; P < .001).
The risk for early death was also elevated among women with moderate PTSD (four or five symptoms) and depression (HR, 2.03; 95% CI, 1.35-3.03; P < .001) and women with subclinical PTSD and depression (HR, 2.85; 95% CI, 1.99-4.07; P < .001) compared with those who did not have PTSD or depression.
Among women with PTSD symptoms and depression, the incidence of death from nearly all major causes was increased, including death from cardiovascular disease, respiratory disease, type 2 diabetes, unintentional injury, suicide, and other causes.
“These findings provide further evidence that mental health is fundamental to physical health – and to our very survival. We ignore our emotional well-being at our peril,” senior author Karestan Koenen, PhD, said in a news release.
New knowledge
Commenting on the findings, Jennifer Sumner, PhD, said that it’s “critical to appreciate the physical health consequences of psychopathology in individuals who have experienced trauma. This study adds to a growing literature demonstrating that the impact extends far beyond emotional health.
“Furthermore, these results highlight the potential value of promoting healthy lifestyle changes in order to reduce the elevated mortality risk in trauma-exposed individuals with co-occurring PTSD and depression,” said Dr. Sumner, who is with the department of psychology, University of California, Los Angeles.
She noted that this study builds on other work that links PTSD to mortality in men.
“Most work on posttraumatic psychopathology and physical health has actually been conducted in predominantly male samples of veterans, so said Dr. Sumner.
“It’s also important to note that PTSD and depression are more prevalent in women than in men, so demonstrating these associations in women is particularly relevant,” she added.
Funding for the study was provided by the National Institutes of Heath. The authors disclosed no relevant financial relationships. Dr. Sumner has collaborated with the study investigators on prior studies.
A version of this article originally appeared on Medscape.com.
Middle-aged women with PTSD and comorbid depression have a nearly fourfold increased risk for early death from a variety of causes in comparison with their peers who do not have those conditions, new research shows.
“Women with more severe symptoms of depression and PTSD were more at risk, compared with those with fewer symptoms or women with symptoms of only PTSD or only depression,” lead investigator Andrea Roberts, PhD, Harvard School of Public Health, Boston, said in an interview.
Health care providers “should be aware that mental health is a critical component of overall health and is tightly entwined with physical health. Identifying and treating mental health issues should be a foundational part of general health practice,” said Dr. Roberts.
The study was published online Dec. 4 in JAMA Network Open.
Mental health fundamental to survival
The researchers studied more than 51,000 mostly White women from the Nurses Health Study II who were followed for 9 years (2008-2017). At baseline in 2008, the women were aged between 43 and 64 years (mean age, 53.3 years).
Women with high levels of PTSD (six or seven symptoms) and probable depression were nearly four times more likely to die during follow-up than their peers who did not have these conditions (hazard ratio, 3.8; 95% confidence interval, 2.65-5.45; P < .001).
With adjustment for health factors such as smoking and body mass index, women with a high level of PTSD and depression remained at increased risk for early death (HR, 3.11; 95% CI, 2.16-4.47; P < .001).
The risk for early death was also elevated among women with moderate PTSD (four or five symptoms) and depression (HR, 2.03; 95% CI, 1.35-3.03; P < .001) and women with subclinical PTSD and depression (HR, 2.85; 95% CI, 1.99-4.07; P < .001) compared with those who did not have PTSD or depression.
Among women with PTSD symptoms and depression, the incidence of death from nearly all major causes was increased, including death from cardiovascular disease, respiratory disease, type 2 diabetes, unintentional injury, suicide, and other causes.
“These findings provide further evidence that mental health is fundamental to physical health – and to our very survival. We ignore our emotional well-being at our peril,” senior author Karestan Koenen, PhD, said in a news release.
New knowledge
Commenting on the findings, Jennifer Sumner, PhD, said that it’s “critical to appreciate the physical health consequences of psychopathology in individuals who have experienced trauma. This study adds to a growing literature demonstrating that the impact extends far beyond emotional health.
“Furthermore, these results highlight the potential value of promoting healthy lifestyle changes in order to reduce the elevated mortality risk in trauma-exposed individuals with co-occurring PTSD and depression,” said Dr. Sumner, who is with the department of psychology, University of California, Los Angeles.
She noted that this study builds on other work that links PTSD to mortality in men.
“Most work on posttraumatic psychopathology and physical health has actually been conducted in predominantly male samples of veterans, so said Dr. Sumner.
“It’s also important to note that PTSD and depression are more prevalent in women than in men, so demonstrating these associations in women is particularly relevant,” she added.
Funding for the study was provided by the National Institutes of Heath. The authors disclosed no relevant financial relationships. Dr. Sumner has collaborated with the study investigators on prior studies.
A version of this article originally appeared on Medscape.com.
Middle-aged women with PTSD and comorbid depression have a nearly fourfold increased risk for early death from a variety of causes in comparison with their peers who do not have those conditions, new research shows.
“Women with more severe symptoms of depression and PTSD were more at risk, compared with those with fewer symptoms or women with symptoms of only PTSD or only depression,” lead investigator Andrea Roberts, PhD, Harvard School of Public Health, Boston, said in an interview.
Health care providers “should be aware that mental health is a critical component of overall health and is tightly entwined with physical health. Identifying and treating mental health issues should be a foundational part of general health practice,” said Dr. Roberts.
The study was published online Dec. 4 in JAMA Network Open.
Mental health fundamental to survival
The researchers studied more than 51,000 mostly White women from the Nurses Health Study II who were followed for 9 years (2008-2017). At baseline in 2008, the women were aged between 43 and 64 years (mean age, 53.3 years).
Women with high levels of PTSD (six or seven symptoms) and probable depression were nearly four times more likely to die during follow-up than their peers who did not have these conditions (hazard ratio, 3.8; 95% confidence interval, 2.65-5.45; P < .001).
With adjustment for health factors such as smoking and body mass index, women with a high level of PTSD and depression remained at increased risk for early death (HR, 3.11; 95% CI, 2.16-4.47; P < .001).
The risk for early death was also elevated among women with moderate PTSD (four or five symptoms) and depression (HR, 2.03; 95% CI, 1.35-3.03; P < .001) and women with subclinical PTSD and depression (HR, 2.85; 95% CI, 1.99-4.07; P < .001) compared with those who did not have PTSD or depression.
Among women with PTSD symptoms and depression, the incidence of death from nearly all major causes was increased, including death from cardiovascular disease, respiratory disease, type 2 diabetes, unintentional injury, suicide, and other causes.
“These findings provide further evidence that mental health is fundamental to physical health – and to our very survival. We ignore our emotional well-being at our peril,” senior author Karestan Koenen, PhD, said in a news release.
New knowledge
Commenting on the findings, Jennifer Sumner, PhD, said that it’s “critical to appreciate the physical health consequences of psychopathology in individuals who have experienced trauma. This study adds to a growing literature demonstrating that the impact extends far beyond emotional health.
“Furthermore, these results highlight the potential value of promoting healthy lifestyle changes in order to reduce the elevated mortality risk in trauma-exposed individuals with co-occurring PTSD and depression,” said Dr. Sumner, who is with the department of psychology, University of California, Los Angeles.
She noted that this study builds on other work that links PTSD to mortality in men.
“Most work on posttraumatic psychopathology and physical health has actually been conducted in predominantly male samples of veterans, so said Dr. Sumner.
“It’s also important to note that PTSD and depression are more prevalent in women than in men, so demonstrating these associations in women is particularly relevant,” she added.
Funding for the study was provided by the National Institutes of Heath. The authors disclosed no relevant financial relationships. Dr. Sumner has collaborated with the study investigators on prior studies.
A version of this article originally appeared on Medscape.com.
Proposed HIPAA overhaul to ease access to patient health info
The Department of Health & Human Services is proposing an overhaul of HIPAA that will make it easier to access patients’ personal health information, including the health records of patients with mental illness. The proposal would also do away with the requirement that all patients sign a notice of privacy practices.
The changes are contained in a 357-page proposed rule, which was unveiled by federal officials Dec. 10. Roger Severino, director of HHS’ Office for Civil Rights, said in a briefing that the sweeping proposal would empower patients, reduce the administrative burden for health care providers, and pave the way to better-coordinated care.
HHS estimated that the rule could save $3.2 billion over 5 years, but it’s not clear how much of that would accrue to clinical practices.
The most obvious cost-saving aspect for medical and dental practices is the proposal that practitioners would no longer have to provide and collect signed notifications of privacy practices.
“This has been a tremendous waste of time and effort and has caused massive confusion,” said Mr. Severino. He said some patients thought they were waiving privacy rights and that, in some cases, physicians refused to administer care unless patients signed the notices. “That was never the intent.”
Requiring that patients sign the form and that practices keep copies for 6 years is an “unnecessary burden,” said Mr. Severino. “We’ve lost whole forests from this regulation.”
Under the new proposal, health care providers would merely have to let patients know where to find their privacy policies.
Sharing mental health info
The rule would also ease the standard for sharing information about a patient who is in a mental health crisis, such as an exacerbation of a serious mental illness or a crisis related to a substance use disorder, including an overdose.
Currently, clinicians can choose to disclose protected health information – to a family member, a caregiver, a law enforcement official, a doctor, or an insurer – if they believe that doing so is advisable in their “professional judgment.” The rule proposes to ease that to a “good faith” belief that a disclosure would be in the best interest of the patient. In both instances, the patient can still object and block the disclosure.
As an example, HHS said that, in the case of a young adult who had experienced an overdose of opioids, a licensed health care professional could make the determination to “disclose relevant information to a parent who is involved in the patient’s treatment and who the young adult would expect, based on their relationship, to participate in or be involved with the patient’s recovery from the overdose.”
HHS is also proposing to let clinicians disclose information in cases in which an individual might be a threat to himself or others, provided the harm is “serious and reasonably foreseeable.”
Currently, information can only be disclosed if it appears there is a “serious and imminent” threat to health or safety. If an individual experienced suicidal ideation, for instance, a health care professional could notify family that the individual is at risk.
Fast, no-cost access
The rule also aims to make it easier for patients to get access to their own health care information quickly – within 15 days of a request – instead of the 30 days currently allowed, and sometimes at no cost.
The 30-day time frame is “a relic of a pre-Internet age that should be dispensed with,” said Mr. Severino.
Patients can also request that a treating physician get his or her records from a clinician who had previously treated the individual. The request would be fulfilled within 15 days, although extensions might be possible.
“That takes away the burden of coordination from the patient and puts it on those parties that are responsible for the actual provision of care and that are better positioned to do that coordination,” Mr. Severino said.
Health care professionals will also have to share with patients a fee schedule for records requests. However, if records are shared through a patient portal with view, download, and transmit capabilities, the provider can’t charge the patient for the time it took to upload the information into the system.
“We do not believe a patient’s personal medical record should be profit centers for providers,” Mr. Severino said.
Patients will be allowed to take photos with a smartphone of personal health information – such as an x-ray or sonogram – while receiving care.
The rule is open for public comment until mid-February. After that, it will become final in 180 days. The agency said it would not begin enforcement until 240 days after the final rule was published.
A version of this article originally appeared on Medscape.com.
The Department of Health & Human Services is proposing an overhaul of HIPAA that will make it easier to access patients’ personal health information, including the health records of patients with mental illness. The proposal would also do away with the requirement that all patients sign a notice of privacy practices.
The changes are contained in a 357-page proposed rule, which was unveiled by federal officials Dec. 10. Roger Severino, director of HHS’ Office for Civil Rights, said in a briefing that the sweeping proposal would empower patients, reduce the administrative burden for health care providers, and pave the way to better-coordinated care.
HHS estimated that the rule could save $3.2 billion over 5 years, but it’s not clear how much of that would accrue to clinical practices.
The most obvious cost-saving aspect for medical and dental practices is the proposal that practitioners would no longer have to provide and collect signed notifications of privacy practices.
“This has been a tremendous waste of time and effort and has caused massive confusion,” said Mr. Severino. He said some patients thought they were waiving privacy rights and that, in some cases, physicians refused to administer care unless patients signed the notices. “That was never the intent.”
Requiring that patients sign the form and that practices keep copies for 6 years is an “unnecessary burden,” said Mr. Severino. “We’ve lost whole forests from this regulation.”
Under the new proposal, health care providers would merely have to let patients know where to find their privacy policies.
Sharing mental health info
The rule would also ease the standard for sharing information about a patient who is in a mental health crisis, such as an exacerbation of a serious mental illness or a crisis related to a substance use disorder, including an overdose.
Currently, clinicians can choose to disclose protected health information – to a family member, a caregiver, a law enforcement official, a doctor, or an insurer – if they believe that doing so is advisable in their “professional judgment.” The rule proposes to ease that to a “good faith” belief that a disclosure would be in the best interest of the patient. In both instances, the patient can still object and block the disclosure.
As an example, HHS said that, in the case of a young adult who had experienced an overdose of opioids, a licensed health care professional could make the determination to “disclose relevant information to a parent who is involved in the patient’s treatment and who the young adult would expect, based on their relationship, to participate in or be involved with the patient’s recovery from the overdose.”
HHS is also proposing to let clinicians disclose information in cases in which an individual might be a threat to himself or others, provided the harm is “serious and reasonably foreseeable.”
Currently, information can only be disclosed if it appears there is a “serious and imminent” threat to health or safety. If an individual experienced suicidal ideation, for instance, a health care professional could notify family that the individual is at risk.
Fast, no-cost access
The rule also aims to make it easier for patients to get access to their own health care information quickly – within 15 days of a request – instead of the 30 days currently allowed, and sometimes at no cost.
The 30-day time frame is “a relic of a pre-Internet age that should be dispensed with,” said Mr. Severino.
Patients can also request that a treating physician get his or her records from a clinician who had previously treated the individual. The request would be fulfilled within 15 days, although extensions might be possible.
“That takes away the burden of coordination from the patient and puts it on those parties that are responsible for the actual provision of care and that are better positioned to do that coordination,” Mr. Severino said.
Health care professionals will also have to share with patients a fee schedule for records requests. However, if records are shared through a patient portal with view, download, and transmit capabilities, the provider can’t charge the patient for the time it took to upload the information into the system.
“We do not believe a patient’s personal medical record should be profit centers for providers,” Mr. Severino said.
Patients will be allowed to take photos with a smartphone of personal health information – such as an x-ray or sonogram – while receiving care.
The rule is open for public comment until mid-February. After that, it will become final in 180 days. The agency said it would not begin enforcement until 240 days after the final rule was published.
A version of this article originally appeared on Medscape.com.
The Department of Health & Human Services is proposing an overhaul of HIPAA that will make it easier to access patients’ personal health information, including the health records of patients with mental illness. The proposal would also do away with the requirement that all patients sign a notice of privacy practices.
The changes are contained in a 357-page proposed rule, which was unveiled by federal officials Dec. 10. Roger Severino, director of HHS’ Office for Civil Rights, said in a briefing that the sweeping proposal would empower patients, reduce the administrative burden for health care providers, and pave the way to better-coordinated care.
HHS estimated that the rule could save $3.2 billion over 5 years, but it’s not clear how much of that would accrue to clinical practices.
The most obvious cost-saving aspect for medical and dental practices is the proposal that practitioners would no longer have to provide and collect signed notifications of privacy practices.
“This has been a tremendous waste of time and effort and has caused massive confusion,” said Mr. Severino. He said some patients thought they were waiving privacy rights and that, in some cases, physicians refused to administer care unless patients signed the notices. “That was never the intent.”
Requiring that patients sign the form and that practices keep copies for 6 years is an “unnecessary burden,” said Mr. Severino. “We’ve lost whole forests from this regulation.”
Under the new proposal, health care providers would merely have to let patients know where to find their privacy policies.
Sharing mental health info
The rule would also ease the standard for sharing information about a patient who is in a mental health crisis, such as an exacerbation of a serious mental illness or a crisis related to a substance use disorder, including an overdose.
Currently, clinicians can choose to disclose protected health information – to a family member, a caregiver, a law enforcement official, a doctor, or an insurer – if they believe that doing so is advisable in their “professional judgment.” The rule proposes to ease that to a “good faith” belief that a disclosure would be in the best interest of the patient. In both instances, the patient can still object and block the disclosure.
As an example, HHS said that, in the case of a young adult who had experienced an overdose of opioids, a licensed health care professional could make the determination to “disclose relevant information to a parent who is involved in the patient’s treatment and who the young adult would expect, based on their relationship, to participate in or be involved with the patient’s recovery from the overdose.”
HHS is also proposing to let clinicians disclose information in cases in which an individual might be a threat to himself or others, provided the harm is “serious and reasonably foreseeable.”
Currently, information can only be disclosed if it appears there is a “serious and imminent” threat to health or safety. If an individual experienced suicidal ideation, for instance, a health care professional could notify family that the individual is at risk.
Fast, no-cost access
The rule also aims to make it easier for patients to get access to their own health care information quickly – within 15 days of a request – instead of the 30 days currently allowed, and sometimes at no cost.
The 30-day time frame is “a relic of a pre-Internet age that should be dispensed with,” said Mr. Severino.
Patients can also request that a treating physician get his or her records from a clinician who had previously treated the individual. The request would be fulfilled within 15 days, although extensions might be possible.
“That takes away the burden of coordination from the patient and puts it on those parties that are responsible for the actual provision of care and that are better positioned to do that coordination,” Mr. Severino said.
Health care professionals will also have to share with patients a fee schedule for records requests. However, if records are shared through a patient portal with view, download, and transmit capabilities, the provider can’t charge the patient for the time it took to upload the information into the system.
“We do not believe a patient’s personal medical record should be profit centers for providers,” Mr. Severino said.
Patients will be allowed to take photos with a smartphone of personal health information – such as an x-ray or sonogram – while receiving care.
The rule is open for public comment until mid-February. After that, it will become final in 180 days. The agency said it would not begin enforcement until 240 days after the final rule was published.
A version of this article originally appeared on Medscape.com.
COVID-19 drives innovation in addiction treatment
With the onset of the COVID-19 pandemic, there has been a rapid uptick in virtual recovery programs and telemedicine counseling sessions for patients with substance use disorders (SUDs). New research shows that these programs are acceptable and effective alternatives to in-person sessions.
Study results from three research teams at the University of South Carolina School of Medicine Greenville (USCSM-G) show that SUD counselors in the state were satisfied with their experience with telehealth and virtual recovery meetings.
In one of the studies, five counselors who utilized a virtual meeting platform after the COVID-19 pandemic made in-person visits unsafe were surveyed. The respondents said they much preferred in-person meetings. However, they could also see that virtual meetings were filling an important need for their patients.
Two other studies echoed the results from the first. Clinicians who were leery of the new technology at first became more enthusiastic after they gained experience using it.
“We have lived in a society where there has been one right way, which has always been in-person meetings for recovery, such as Alcoholics Anonymous. It is a very structured process,” lead author Haley Fulton, a fourth-year medical student at USCSM-G, said in an interview.
“The onset of COVID really upended a lot of things, but ... now there may not be just one right way for recovery. There are alternatives to offer,” Ms. Fulton said.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry, which was held online this year because of the pandemic.
Huge need
“Virtual meetings may not be ideal, but some version of recovery is better than none. If we can make these meetings accessible to more people, this could promote recovery from substance use disorder,” Ms. Fulton said.
There is a huge need for counseling, and past research has shown that failure to attend meetings can precipitate relapse in many individuals.
In Ms. Fulton’s study, counselors were asked to describe how they perceived the efficacy of virtual recovery meetings, compared with that of in-person meetings.
The investigators analyzed how often certain words, phrases, or issues came up during seven in-person recovery meetings held before the COVID-19 pandemic as well as observational data from seven virtual recovery-support meetings held during the pandemic.
On the pro side, the respondents cited convenience, comfort at home, and increased accessibility to counseling for patients.
In addition, because there was no need to travel, virtual meetings were cost effective. Such meetings could expand the recovery world, inasmuch as individuals could attend recovery meetings in other parts of the country.
Perceived disadvantages included challenges involving technology, because learning new apps such as Zoom could be a problem for some patients. Distractions at home and lack of privacy were also cited, but for many, the most important drawback to virtual meetings was the lessening of emotional connection with others.
Impact on SUD treatment
In a second study, another team from USCSM-G reported similar findings when it explored the impact of telehealth on counselors as well as on patients who were undergoing SUD treatment during the pandemic.
Led by fourth-year medical students Elizabeth Whiteside and Kyleigh Connolly, the researchers assessed data from a focus group of six behavioral health counselors representing rural and city agencies throughout South Carolina.
Themes that emerged included concerns about mental health – counselors and patients were experiencing increased stress, depression, and anxiety.
“People had to now home school, there were job layoffs, increased responsibilities at home. Also, Narcan [naloxone] distribution was decreased, and this contributed to rising overdose rates,” Ms. Whiteside said in an interview.
The focus group concluded that the advantages of telehealth included greater ability to accept new patients, an increase in scheduling flexibility, and cost-effectiveness because it obviated the need for child care or transportation.
Disadvantages included problems involving privacy, because for many patients who were undergoing SUD recovery, it was impossible to be alone in a room or a designated area of their own.
“Before COVID happened, [health care] barriers included transport to the actual center and finding care for children,” Ms. Connolly said in an interview.
“That’s where telehealth really bridged the gap for these people, and it actually became a lot easier for them to get in contact with their counselors, get into group meetings, and access other services,” she said.
Many of the study participants were not very optimistic about telehealth at first, Ms. Connolly noted. “They felt a little odd going on telehealth at first, but by the end, everybody said that they loved having it.”
“One of the things that came out often was that patients felt they could be more open and honest because they weren’t looking their counselor right in the face. They didn’t feel so horrible sharing,” Ms. Whiteside added.
Some counselors reported that some clients shared more details with them and that there was an ease of connecting. If a patient was a few minutes late to an appointment, telehealth would put in a call to find out where that patient was.
The counselors also had the ability to determine which of their patients would be good candidates for telehealth counseling and which patients would not do well with telehealth and would instead need in-patient care.
“This is something that really helped the experience go better for the counselors. They were able to determine which patient fit the mold for telehealth working for them. Obviously, patients who have more acute periods of mental health problems would do better with in-person care,” Ms. Whiteside said.
Here to stay?
In the third study from USCSM-G, investigators evaluated data from a focus group of four providers of medications for opioid use disorder (MOUD) who practiced in urban and rural areas throughout the state.
The respondents reflected on their experiences in using telemedicine for prescribing MOUD.
As in the previous studies, the providers had positive experiences with telemedicine. It increased patient access, participation, and satisfaction with treatment, and the benefits of telemedicine outweighed its potential limitations.
Still, technology was cited as a barrier to care, especially in rural areas.
“We found that there was a lack of good internet in certain rural parts of South Carolina, and that lack of the proper electronic devices ... could also make it difficult to access telemedicine,” lead author Kellie Shell said in an interview.
As noted in the other studies, the providers expressed a desire that telemedicine incorporate safeguards that would enable clinicians to identify a particular patient’s location in order that authorities could be dispatched if an emergency were to arise.
The clinicians also said that monitoring for diversion and performing pill counts were more difficult to do via telemedicine.
“We definitely have to improve infrastructure, especially in rural areas, so that all people have access to telemedicine,” Ms. Shell said.
“Overall, the providers were won over with telemedicine, and some predicted telehealth and virtual visits were here to stay, even after COVID,” she added.
The three posters provide useful insight into the potential advantages and disadvantages of telehealth in SUD settings, experts said.
Telehealth data ‘very limited’
Commenting on the research, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, noted that “there is such limited information” about the use of telehealth for patients with SUD.
“These insights are helpful for us to start understanding the things that need to be considered, including clinician attitudes and perceptions,” said Dr. Lin, who was not involved with the studies.
“It will be key to have data as use of telemedicine increases during COVID-19 to help us see exactly how it should be used and to better understand the actual impacts and whether or not it is increasing accessibility, and for which patients,” she added.
David Kan, MD, chief medical officer at Bright Heart Health, San Ramon, Calif., has had experience with telehealth for SUD and has found that conducting pill counts with his patients has not been a problem.
“The Shell poster covers telemedicine well,” Dr. Kan said in an interview.
However, “I disagree with their point that diversion prevention is harder via telemedicine. In my experience, it is easier, as you can do pill or wrapper counts almost on demand. You can also do daily observed dosing with pill counts if diversion is suspected,” he said.
Dr. Kan also suggested ways to cope with problems involving privacy. “Privacy concerns are always an issue but can be mitigated with headphones and a scan of the room with the telehealth technology if a privacy concern arises.”
He acknowledged that in-person meetings, especially through well-established programs, such as Alcoholics Anonymous (AA), will always be important. But he pointed out that people are finding ways to meet safely and have in-person connections.
“The AA has been providing virtual recovery meetings long before COVID. The common complaint is the loss of fellowship associated with recovery groups. I don’t know of a way to get around this short of vaccines,” Dr. Kan said. However, “people have adapted impressively with masked outdoor meetings and other forms of safe gathering.”
The investigators, Dr. Lin, and Dr. Kan reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
With the onset of the COVID-19 pandemic, there has been a rapid uptick in virtual recovery programs and telemedicine counseling sessions for patients with substance use disorders (SUDs). New research shows that these programs are acceptable and effective alternatives to in-person sessions.
Study results from three research teams at the University of South Carolina School of Medicine Greenville (USCSM-G) show that SUD counselors in the state were satisfied with their experience with telehealth and virtual recovery meetings.
In one of the studies, five counselors who utilized a virtual meeting platform after the COVID-19 pandemic made in-person visits unsafe were surveyed. The respondents said they much preferred in-person meetings. However, they could also see that virtual meetings were filling an important need for their patients.
Two other studies echoed the results from the first. Clinicians who were leery of the new technology at first became more enthusiastic after they gained experience using it.
“We have lived in a society where there has been one right way, which has always been in-person meetings for recovery, such as Alcoholics Anonymous. It is a very structured process,” lead author Haley Fulton, a fourth-year medical student at USCSM-G, said in an interview.
“The onset of COVID really upended a lot of things, but ... now there may not be just one right way for recovery. There are alternatives to offer,” Ms. Fulton said.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry, which was held online this year because of the pandemic.
Huge need
“Virtual meetings may not be ideal, but some version of recovery is better than none. If we can make these meetings accessible to more people, this could promote recovery from substance use disorder,” Ms. Fulton said.
There is a huge need for counseling, and past research has shown that failure to attend meetings can precipitate relapse in many individuals.
In Ms. Fulton’s study, counselors were asked to describe how they perceived the efficacy of virtual recovery meetings, compared with that of in-person meetings.
The investigators analyzed how often certain words, phrases, or issues came up during seven in-person recovery meetings held before the COVID-19 pandemic as well as observational data from seven virtual recovery-support meetings held during the pandemic.
On the pro side, the respondents cited convenience, comfort at home, and increased accessibility to counseling for patients.
In addition, because there was no need to travel, virtual meetings were cost effective. Such meetings could expand the recovery world, inasmuch as individuals could attend recovery meetings in other parts of the country.
Perceived disadvantages included challenges involving technology, because learning new apps such as Zoom could be a problem for some patients. Distractions at home and lack of privacy were also cited, but for many, the most important drawback to virtual meetings was the lessening of emotional connection with others.
Impact on SUD treatment
In a second study, another team from USCSM-G reported similar findings when it explored the impact of telehealth on counselors as well as on patients who were undergoing SUD treatment during the pandemic.
Led by fourth-year medical students Elizabeth Whiteside and Kyleigh Connolly, the researchers assessed data from a focus group of six behavioral health counselors representing rural and city agencies throughout South Carolina.
Themes that emerged included concerns about mental health – counselors and patients were experiencing increased stress, depression, and anxiety.
“People had to now home school, there were job layoffs, increased responsibilities at home. Also, Narcan [naloxone] distribution was decreased, and this contributed to rising overdose rates,” Ms. Whiteside said in an interview.
The focus group concluded that the advantages of telehealth included greater ability to accept new patients, an increase in scheduling flexibility, and cost-effectiveness because it obviated the need for child care or transportation.
Disadvantages included problems involving privacy, because for many patients who were undergoing SUD recovery, it was impossible to be alone in a room or a designated area of their own.
“Before COVID happened, [health care] barriers included transport to the actual center and finding care for children,” Ms. Connolly said in an interview.
“That’s where telehealth really bridged the gap for these people, and it actually became a lot easier for them to get in contact with their counselors, get into group meetings, and access other services,” she said.
Many of the study participants were not very optimistic about telehealth at first, Ms. Connolly noted. “They felt a little odd going on telehealth at first, but by the end, everybody said that they loved having it.”
“One of the things that came out often was that patients felt they could be more open and honest because they weren’t looking their counselor right in the face. They didn’t feel so horrible sharing,” Ms. Whiteside added.
Some counselors reported that some clients shared more details with them and that there was an ease of connecting. If a patient was a few minutes late to an appointment, telehealth would put in a call to find out where that patient was.
The counselors also had the ability to determine which of their patients would be good candidates for telehealth counseling and which patients would not do well with telehealth and would instead need in-patient care.
“This is something that really helped the experience go better for the counselors. They were able to determine which patient fit the mold for telehealth working for them. Obviously, patients who have more acute periods of mental health problems would do better with in-person care,” Ms. Whiteside said.
Here to stay?
In the third study from USCSM-G, investigators evaluated data from a focus group of four providers of medications for opioid use disorder (MOUD) who practiced in urban and rural areas throughout the state.
The respondents reflected on their experiences in using telemedicine for prescribing MOUD.
As in the previous studies, the providers had positive experiences with telemedicine. It increased patient access, participation, and satisfaction with treatment, and the benefits of telemedicine outweighed its potential limitations.
Still, technology was cited as a barrier to care, especially in rural areas.
“We found that there was a lack of good internet in certain rural parts of South Carolina, and that lack of the proper electronic devices ... could also make it difficult to access telemedicine,” lead author Kellie Shell said in an interview.
As noted in the other studies, the providers expressed a desire that telemedicine incorporate safeguards that would enable clinicians to identify a particular patient’s location in order that authorities could be dispatched if an emergency were to arise.
The clinicians also said that monitoring for diversion and performing pill counts were more difficult to do via telemedicine.
“We definitely have to improve infrastructure, especially in rural areas, so that all people have access to telemedicine,” Ms. Shell said.
“Overall, the providers were won over with telemedicine, and some predicted telehealth and virtual visits were here to stay, even after COVID,” she added.
The three posters provide useful insight into the potential advantages and disadvantages of telehealth in SUD settings, experts said.
Telehealth data ‘very limited’
Commenting on the research, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, noted that “there is such limited information” about the use of telehealth for patients with SUD.
“These insights are helpful for us to start understanding the things that need to be considered, including clinician attitudes and perceptions,” said Dr. Lin, who was not involved with the studies.
“It will be key to have data as use of telemedicine increases during COVID-19 to help us see exactly how it should be used and to better understand the actual impacts and whether or not it is increasing accessibility, and for which patients,” she added.
David Kan, MD, chief medical officer at Bright Heart Health, San Ramon, Calif., has had experience with telehealth for SUD and has found that conducting pill counts with his patients has not been a problem.
“The Shell poster covers telemedicine well,” Dr. Kan said in an interview.
However, “I disagree with their point that diversion prevention is harder via telemedicine. In my experience, it is easier, as you can do pill or wrapper counts almost on demand. You can also do daily observed dosing with pill counts if diversion is suspected,” he said.
Dr. Kan also suggested ways to cope with problems involving privacy. “Privacy concerns are always an issue but can be mitigated with headphones and a scan of the room with the telehealth technology if a privacy concern arises.”
He acknowledged that in-person meetings, especially through well-established programs, such as Alcoholics Anonymous (AA), will always be important. But he pointed out that people are finding ways to meet safely and have in-person connections.
“The AA has been providing virtual recovery meetings long before COVID. The common complaint is the loss of fellowship associated with recovery groups. I don’t know of a way to get around this short of vaccines,” Dr. Kan said. However, “people have adapted impressively with masked outdoor meetings and other forms of safe gathering.”
The investigators, Dr. Lin, and Dr. Kan reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
With the onset of the COVID-19 pandemic, there has been a rapid uptick in virtual recovery programs and telemedicine counseling sessions for patients with substance use disorders (SUDs). New research shows that these programs are acceptable and effective alternatives to in-person sessions.
Study results from three research teams at the University of South Carolina School of Medicine Greenville (USCSM-G) show that SUD counselors in the state were satisfied with their experience with telehealth and virtual recovery meetings.
In one of the studies, five counselors who utilized a virtual meeting platform after the COVID-19 pandemic made in-person visits unsafe were surveyed. The respondents said they much preferred in-person meetings. However, they could also see that virtual meetings were filling an important need for their patients.
Two other studies echoed the results from the first. Clinicians who were leery of the new technology at first became more enthusiastic after they gained experience using it.
“We have lived in a society where there has been one right way, which has always been in-person meetings for recovery, such as Alcoholics Anonymous. It is a very structured process,” lead author Haley Fulton, a fourth-year medical student at USCSM-G, said in an interview.
“The onset of COVID really upended a lot of things, but ... now there may not be just one right way for recovery. There are alternatives to offer,” Ms. Fulton said.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry, which was held online this year because of the pandemic.
Huge need
“Virtual meetings may not be ideal, but some version of recovery is better than none. If we can make these meetings accessible to more people, this could promote recovery from substance use disorder,” Ms. Fulton said.
There is a huge need for counseling, and past research has shown that failure to attend meetings can precipitate relapse in many individuals.
In Ms. Fulton’s study, counselors were asked to describe how they perceived the efficacy of virtual recovery meetings, compared with that of in-person meetings.
The investigators analyzed how often certain words, phrases, or issues came up during seven in-person recovery meetings held before the COVID-19 pandemic as well as observational data from seven virtual recovery-support meetings held during the pandemic.
On the pro side, the respondents cited convenience, comfort at home, and increased accessibility to counseling for patients.
In addition, because there was no need to travel, virtual meetings were cost effective. Such meetings could expand the recovery world, inasmuch as individuals could attend recovery meetings in other parts of the country.
Perceived disadvantages included challenges involving technology, because learning new apps such as Zoom could be a problem for some patients. Distractions at home and lack of privacy were also cited, but for many, the most important drawback to virtual meetings was the lessening of emotional connection with others.
Impact on SUD treatment
In a second study, another team from USCSM-G reported similar findings when it explored the impact of telehealth on counselors as well as on patients who were undergoing SUD treatment during the pandemic.
Led by fourth-year medical students Elizabeth Whiteside and Kyleigh Connolly, the researchers assessed data from a focus group of six behavioral health counselors representing rural and city agencies throughout South Carolina.
Themes that emerged included concerns about mental health – counselors and patients were experiencing increased stress, depression, and anxiety.
“People had to now home school, there were job layoffs, increased responsibilities at home. Also, Narcan [naloxone] distribution was decreased, and this contributed to rising overdose rates,” Ms. Whiteside said in an interview.
The focus group concluded that the advantages of telehealth included greater ability to accept new patients, an increase in scheduling flexibility, and cost-effectiveness because it obviated the need for child care or transportation.
Disadvantages included problems involving privacy, because for many patients who were undergoing SUD recovery, it was impossible to be alone in a room or a designated area of their own.
“Before COVID happened, [health care] barriers included transport to the actual center and finding care for children,” Ms. Connolly said in an interview.
“That’s where telehealth really bridged the gap for these people, and it actually became a lot easier for them to get in contact with their counselors, get into group meetings, and access other services,” she said.
Many of the study participants were not very optimistic about telehealth at first, Ms. Connolly noted. “They felt a little odd going on telehealth at first, but by the end, everybody said that they loved having it.”
“One of the things that came out often was that patients felt they could be more open and honest because they weren’t looking their counselor right in the face. They didn’t feel so horrible sharing,” Ms. Whiteside added.
Some counselors reported that some clients shared more details with them and that there was an ease of connecting. If a patient was a few minutes late to an appointment, telehealth would put in a call to find out where that patient was.
The counselors also had the ability to determine which of their patients would be good candidates for telehealth counseling and which patients would not do well with telehealth and would instead need in-patient care.
“This is something that really helped the experience go better for the counselors. They were able to determine which patient fit the mold for telehealth working for them. Obviously, patients who have more acute periods of mental health problems would do better with in-person care,” Ms. Whiteside said.
Here to stay?
In the third study from USCSM-G, investigators evaluated data from a focus group of four providers of medications for opioid use disorder (MOUD) who practiced in urban and rural areas throughout the state.
The respondents reflected on their experiences in using telemedicine for prescribing MOUD.
As in the previous studies, the providers had positive experiences with telemedicine. It increased patient access, participation, and satisfaction with treatment, and the benefits of telemedicine outweighed its potential limitations.
Still, technology was cited as a barrier to care, especially in rural areas.
“We found that there was a lack of good internet in certain rural parts of South Carolina, and that lack of the proper electronic devices ... could also make it difficult to access telemedicine,” lead author Kellie Shell said in an interview.
As noted in the other studies, the providers expressed a desire that telemedicine incorporate safeguards that would enable clinicians to identify a particular patient’s location in order that authorities could be dispatched if an emergency were to arise.
The clinicians also said that monitoring for diversion and performing pill counts were more difficult to do via telemedicine.
“We definitely have to improve infrastructure, especially in rural areas, so that all people have access to telemedicine,” Ms. Shell said.
“Overall, the providers were won over with telemedicine, and some predicted telehealth and virtual visits were here to stay, even after COVID,” she added.
The three posters provide useful insight into the potential advantages and disadvantages of telehealth in SUD settings, experts said.
Telehealth data ‘very limited’
Commenting on the research, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, noted that “there is such limited information” about the use of telehealth for patients with SUD.
“These insights are helpful for us to start understanding the things that need to be considered, including clinician attitudes and perceptions,” said Dr. Lin, who was not involved with the studies.
“It will be key to have data as use of telemedicine increases during COVID-19 to help us see exactly how it should be used and to better understand the actual impacts and whether or not it is increasing accessibility, and for which patients,” she added.
David Kan, MD, chief medical officer at Bright Heart Health, San Ramon, Calif., has had experience with telehealth for SUD and has found that conducting pill counts with his patients has not been a problem.
“The Shell poster covers telemedicine well,” Dr. Kan said in an interview.
However, “I disagree with their point that diversion prevention is harder via telemedicine. In my experience, it is easier, as you can do pill or wrapper counts almost on demand. You can also do daily observed dosing with pill counts if diversion is suspected,” he said.
Dr. Kan also suggested ways to cope with problems involving privacy. “Privacy concerns are always an issue but can be mitigated with headphones and a scan of the room with the telehealth technology if a privacy concern arises.”
He acknowledged that in-person meetings, especially through well-established programs, such as Alcoholics Anonymous (AA), will always be important. But he pointed out that people are finding ways to meet safely and have in-person connections.
“The AA has been providing virtual recovery meetings long before COVID. The common complaint is the loss of fellowship associated with recovery groups. I don’t know of a way to get around this short of vaccines,” Dr. Kan said. However, “people have adapted impressively with masked outdoor meetings and other forms of safe gathering.”
The investigators, Dr. Lin, and Dr. Kan reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Kennedy, NIMH demand urgent action on COVID-19 mental health toll
A public-private partnership, led by mental health advocate Patrick Kennedy and the head of the National Institute of Mental Health, Joshua Gordon, MD, PhD, want urgent action to address the wave of mental illness and suicide caused by COVID-19.
“Our country is in serious denial about the full impact of mental health in this country and certainly as part of this pandemic,” said former congressman Mr. Kennedy, cochair of the Action Alliance’s Mental Health & Suicide Prevention National Response to COVID-19, at a briefing unveiling the group’s new six-priority Action Plan.
“That’s reinforced when all we hear from is Dr. Fauci,” and only about the physical effects of the disease, said Mr. Kennedy, the founder of the Kennedy Forum, a nonprofit dedicated to changing the health system’s approach to mental health and substance use disorders.
“ he said. Mr. Kennedy noted the huge effort to speed therapeutics and vaccines to the American public. “We need to bring that same sense of urgency to these deaths of despair hiding in plain sight.”
Dr. Gordon, NIMH’s director and a cochair of the National Response group, was also at the briefing.
“We know many people report experiencing symptoms of distress, including anxiety, sleep problems, depression, substance use, and suicidal thoughts at rates two to three times higher than we might expect in times before the pandemic. Just as the country has come together to mitigate the physical impacts of pandemic, we also have to identify how to mitigate the mental health impacts,” said Dr. Gordon.
Plan of action
Mr. Kennedy emphasized that it is crucial that federal lawmakers and regulators find a way to increase parity between mental and physical health.
Paramount in that effort would be ensuring stronger enforcement of the Mental Health Parity and Addiction Equity Act, he said.
That 1996 law requires health plans to ensure that benefits for physical and mental health were equivalent, but it has frequently been ignored. In 2019, a U.S. federal court found that one of the nation’s largest behavioral health insurers, United Behavioral Health, had been violating the law. Mr. Kennedy said he expects this decision to continue to have a positive impact on achieving parity.
In November, United was ordered by a federal judge to reprocess 67,000 claims that it illegally denied.
The Alliance’s Action Plan has six priorities:
- Change the national conversation about mental health and suicide.
- Increase access to evidence-based treatments for substance use and mental health disorders in specialty and primary care, and include better reimbursement for services and make permanent reimbursement for telehealth services.
- Increase the use of nonpunitive and supportive crisis intervention services, including keeping people out of the criminal justice system.
- Establish near real-time data collection systems to promptly identify changes in rates of suicide, overdose, and other key events, and of clusters or spikes.
- Ensure the equitable delivery of comprehensive and effective suicide prevention and mental health services for Black Americans, Latin Americans, American Indian/Alaskan Natives, LGBTQ individuals, and others disproportionately impacted by the pandemic.
- Invest in prevention and early intervention approaches that treat the root causes of suicide and mental health problems.
Uptick in distress
Dr. Gordon noted that recent data indicate that, although ED visits for children are still down in 2020, compared with previous years, mental health ED visits are back to prepandemic levels.
A September survey showed an increase in suicidal thoughts and attempts, anxiety, and depression pandemic in youth because of the pandemic. Almost one-quarter of those surveyed said they knew a peer who developed suicidal thoughts since the start of the pandemic and 5% reported making a suicide attempt themselves.
In early December, research reported in JAMA Psychiatry showed the overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends in 2018 and 2019, and that all overdose-related incidents were about 17% above baseline in 2020.
COVID-19 also appears to be striking individuals who are living in behavioral health facilities, and some of those facilities are reducing inpatient care and other programs because they don’t have enough personal protective equipment, testing supplies, or staff to cope with the disease.
The facilities are not required to report infections to the federal government. Sen. Elizabeth Warren (D-Mass.), Rep. Carolyn Maloney (D-N.Y.), and Rep. Katie Porter (D-Calif.) issued a report based on their own offices’ survey of 10 large behavioral health program operators.
Eight of those operators – covering 376 facilities and more than 100,000 patients in 40 states and Puerto Rico – provided substantive responses.
More than half had at least one COVID case and 14% had large outbreaks of 10 or more cases. The infection rate for patients was in line with that of the general public.
A version of this article originally appeared on Medscape.com.
A public-private partnership, led by mental health advocate Patrick Kennedy and the head of the National Institute of Mental Health, Joshua Gordon, MD, PhD, want urgent action to address the wave of mental illness and suicide caused by COVID-19.
“Our country is in serious denial about the full impact of mental health in this country and certainly as part of this pandemic,” said former congressman Mr. Kennedy, cochair of the Action Alliance’s Mental Health & Suicide Prevention National Response to COVID-19, at a briefing unveiling the group’s new six-priority Action Plan.
“That’s reinforced when all we hear from is Dr. Fauci,” and only about the physical effects of the disease, said Mr. Kennedy, the founder of the Kennedy Forum, a nonprofit dedicated to changing the health system’s approach to mental health and substance use disorders.
“ he said. Mr. Kennedy noted the huge effort to speed therapeutics and vaccines to the American public. “We need to bring that same sense of urgency to these deaths of despair hiding in plain sight.”
Dr. Gordon, NIMH’s director and a cochair of the National Response group, was also at the briefing.
“We know many people report experiencing symptoms of distress, including anxiety, sleep problems, depression, substance use, and suicidal thoughts at rates two to three times higher than we might expect in times before the pandemic. Just as the country has come together to mitigate the physical impacts of pandemic, we also have to identify how to mitigate the mental health impacts,” said Dr. Gordon.
Plan of action
Mr. Kennedy emphasized that it is crucial that federal lawmakers and regulators find a way to increase parity between mental and physical health.
Paramount in that effort would be ensuring stronger enforcement of the Mental Health Parity and Addiction Equity Act, he said.
That 1996 law requires health plans to ensure that benefits for physical and mental health were equivalent, but it has frequently been ignored. In 2019, a U.S. federal court found that one of the nation’s largest behavioral health insurers, United Behavioral Health, had been violating the law. Mr. Kennedy said he expects this decision to continue to have a positive impact on achieving parity.
In November, United was ordered by a federal judge to reprocess 67,000 claims that it illegally denied.
The Alliance’s Action Plan has six priorities:
- Change the national conversation about mental health and suicide.
- Increase access to evidence-based treatments for substance use and mental health disorders in specialty and primary care, and include better reimbursement for services and make permanent reimbursement for telehealth services.
- Increase the use of nonpunitive and supportive crisis intervention services, including keeping people out of the criminal justice system.
- Establish near real-time data collection systems to promptly identify changes in rates of suicide, overdose, and other key events, and of clusters or spikes.
- Ensure the equitable delivery of comprehensive and effective suicide prevention and mental health services for Black Americans, Latin Americans, American Indian/Alaskan Natives, LGBTQ individuals, and others disproportionately impacted by the pandemic.
- Invest in prevention and early intervention approaches that treat the root causes of suicide and mental health problems.
Uptick in distress
Dr. Gordon noted that recent data indicate that, although ED visits for children are still down in 2020, compared with previous years, mental health ED visits are back to prepandemic levels.
A September survey showed an increase in suicidal thoughts and attempts, anxiety, and depression pandemic in youth because of the pandemic. Almost one-quarter of those surveyed said they knew a peer who developed suicidal thoughts since the start of the pandemic and 5% reported making a suicide attempt themselves.
In early December, research reported in JAMA Psychiatry showed the overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends in 2018 and 2019, and that all overdose-related incidents were about 17% above baseline in 2020.
COVID-19 also appears to be striking individuals who are living in behavioral health facilities, and some of those facilities are reducing inpatient care and other programs because they don’t have enough personal protective equipment, testing supplies, or staff to cope with the disease.
The facilities are not required to report infections to the federal government. Sen. Elizabeth Warren (D-Mass.), Rep. Carolyn Maloney (D-N.Y.), and Rep. Katie Porter (D-Calif.) issued a report based on their own offices’ survey of 10 large behavioral health program operators.
Eight of those operators – covering 376 facilities and more than 100,000 patients in 40 states and Puerto Rico – provided substantive responses.
More than half had at least one COVID case and 14% had large outbreaks of 10 or more cases. The infection rate for patients was in line with that of the general public.
A version of this article originally appeared on Medscape.com.
A public-private partnership, led by mental health advocate Patrick Kennedy and the head of the National Institute of Mental Health, Joshua Gordon, MD, PhD, want urgent action to address the wave of mental illness and suicide caused by COVID-19.
“Our country is in serious denial about the full impact of mental health in this country and certainly as part of this pandemic,” said former congressman Mr. Kennedy, cochair of the Action Alliance’s Mental Health & Suicide Prevention National Response to COVID-19, at a briefing unveiling the group’s new six-priority Action Plan.
“That’s reinforced when all we hear from is Dr. Fauci,” and only about the physical effects of the disease, said Mr. Kennedy, the founder of the Kennedy Forum, a nonprofit dedicated to changing the health system’s approach to mental health and substance use disorders.
“ he said. Mr. Kennedy noted the huge effort to speed therapeutics and vaccines to the American public. “We need to bring that same sense of urgency to these deaths of despair hiding in plain sight.”
Dr. Gordon, NIMH’s director and a cochair of the National Response group, was also at the briefing.
“We know many people report experiencing symptoms of distress, including anxiety, sleep problems, depression, substance use, and suicidal thoughts at rates two to three times higher than we might expect in times before the pandemic. Just as the country has come together to mitigate the physical impacts of pandemic, we also have to identify how to mitigate the mental health impacts,” said Dr. Gordon.
Plan of action
Mr. Kennedy emphasized that it is crucial that federal lawmakers and regulators find a way to increase parity between mental and physical health.
Paramount in that effort would be ensuring stronger enforcement of the Mental Health Parity and Addiction Equity Act, he said.
That 1996 law requires health plans to ensure that benefits for physical and mental health were equivalent, but it has frequently been ignored. In 2019, a U.S. federal court found that one of the nation’s largest behavioral health insurers, United Behavioral Health, had been violating the law. Mr. Kennedy said he expects this decision to continue to have a positive impact on achieving parity.
In November, United was ordered by a federal judge to reprocess 67,000 claims that it illegally denied.
The Alliance’s Action Plan has six priorities:
- Change the national conversation about mental health and suicide.
- Increase access to evidence-based treatments for substance use and mental health disorders in specialty and primary care, and include better reimbursement for services and make permanent reimbursement for telehealth services.
- Increase the use of nonpunitive and supportive crisis intervention services, including keeping people out of the criminal justice system.
- Establish near real-time data collection systems to promptly identify changes in rates of suicide, overdose, and other key events, and of clusters or spikes.
- Ensure the equitable delivery of comprehensive and effective suicide prevention and mental health services for Black Americans, Latin Americans, American Indian/Alaskan Natives, LGBTQ individuals, and others disproportionately impacted by the pandemic.
- Invest in prevention and early intervention approaches that treat the root causes of suicide and mental health problems.
Uptick in distress
Dr. Gordon noted that recent data indicate that, although ED visits for children are still down in 2020, compared with previous years, mental health ED visits are back to prepandemic levels.
A September survey showed an increase in suicidal thoughts and attempts, anxiety, and depression pandemic in youth because of the pandemic. Almost one-quarter of those surveyed said they knew a peer who developed suicidal thoughts since the start of the pandemic and 5% reported making a suicide attempt themselves.
In early December, research reported in JAMA Psychiatry showed the overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends in 2018 and 2019, and that all overdose-related incidents were about 17% above baseline in 2020.
COVID-19 also appears to be striking individuals who are living in behavioral health facilities, and some of those facilities are reducing inpatient care and other programs because they don’t have enough personal protective equipment, testing supplies, or staff to cope with the disease.
The facilities are not required to report infections to the federal government. Sen. Elizabeth Warren (D-Mass.), Rep. Carolyn Maloney (D-N.Y.), and Rep. Katie Porter (D-Calif.) issued a report based on their own offices’ survey of 10 large behavioral health program operators.
Eight of those operators – covering 376 facilities and more than 100,000 patients in 40 states and Puerto Rico – provided substantive responses.
More than half had at least one COVID case and 14% had large outbreaks of 10 or more cases. The infection rate for patients was in line with that of the general public.
A version of this article originally appeared on Medscape.com.
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Raising psychiatry up ‘from depths of the asylums’
New biography captures Dr. Anthony Clare’s complexity
In “Psychiatrist in the Chair,” authors Brendan Kelly and Muiris Houston tell the story of a fellow Irishman, Anthony Clare, MD, who brought intelligence and eloquence to psychiatry. They tell a well-measured, well-referenced story of Anthony Clare’s personal and professional life. They capture his eloquence, wit, charm, and success in psychiatry as well as alluding to Dr. Clare’s self-reported “some kind of Irish darkness.”
In 1983, I was a young Scottish psychiatrist entering a fusty profession. Suddenly, there was Dr. Anthony Clare on the BBC! In “In the Psychiatrist’s Chair,” Dr. Clare interviewed celebrities. In addition to describing his Irish darkness, Brendan Kelly, MD, PhD and Muiris Houston, MD, FRCGP, both of whom are affiliated with Trinity College Dublin, note that Dr. Clare said: “I’m better at destroying systems than I am at putting them together – I do rather look for people to interview who will not live up to the prediction; there’s an element of destructiveness that’s still in me.”
I still listen to his talks on YouTube. His delicate probing questioning of B.F. Skinner, PhD, is one of my favorites, as he expertly and in an ever-so-friendly manner, teases out Dr. Skinner’s views of his upbringing and tags them to his behavorialism. It is this skill as an interviewer that captured us; can psychiatrists really be this clever? Yes, we can. All of the young and hopeful psychiatrists could see a future.
Dr. Clare raised psychiatry up from the depths of the asylums. He showed that a psychiatrist can be kind, charming, and sophisticated – handsome and helpful, not the ghouls of old movies. He did what needed to be done to psychiatry at that time: He set us on a footing that was not scary to the public. His vision for psychiatry was to improve services to those in need, reduce stigma, and show the public that there is a continuum between health and illness. He also took on the push for diagnoses, which he felt separated the normal from the abnormal, us from them.
He wrote his seminal work 10 years after graduating from the University College of Dublin. “Psychiatry in Dissent: Controversial Issues in Thought and Practice” was published in 1976, and is still considered one of the most influential texts in psychiatry. Dr. Clare “legitimized psychiatry not only in the eyes of the public but in the eyes of psychiatrists too,” the authors wrote. He did not support psychoanalysis and eschewed the rigor attached to the learning of new psychotherapies. He took renowned experts to task, but in ever such an elegant way. He successfully took on Hans Eysenck, PhD, I think because Dr. Eysenck insulted the intelligence of the Irish. He had a measured response to the anti-psychiatrists Thomas Szasz, MD, and R.D. Laing, MD, incorporating their ideas into his view of psychiatry. Dr. Clare was a social psychiatrist who highlighted the role of poverty and lack of access to mental health services. He stated that psychiatry was a “shambles, a mess and at a very primitive level.”
I enjoyed learning about his fight to make the membership exam for entrance into the Royal College of Psychiatry worthy of its name. Dr. Clare helped found the Association of Psychiatrists in Training (APIT) and wrote eloquently about the difference between training and indoctrination, which he described as having people fit a predetermined paradigm of how psychiatry should be constructed and practiced, versus education, which he defined as forming the mind. He highlighted the lack of good training facilities, and teaching staff in many parts of the United Kingdom. When Dr. Clare studied candidates in Edinburgh, he found that 70% had no child, forensic, or intellectual disability training. By the time I did my training there, I was able to get experience in all three subspecialties. He opposed the granting of automatic membership to current consultants, many of whom he considered to be “dunderheads.” . I can attest to that!
In the later phase of his life, Dr. Clare likened his self-punishing regime at the height of his hyperproductive fame to an addiction – a fix, with its risk/reward, pain/pleasure kick. He identified fear as being an unacknowledged presence in most of his life. There are vague hints from Dr. Clare’s friends and colleagues that something drove him back to Ireland from a successful life in London. Although his wife was described as being fully supportive of him, her words on his tombstone indicate something: What they indicate you can decide. She called him “a loving husband, father and grandfather, orator, physician, writer and broadcaster.” No mention was made of his being one of the greatest psychiatrists of his generation.
In 2000, he wrote “On Men: Masculinity in Crisis,” about men and the patriarchy, and highlighted the concept of “performance-based self-worth” in men. He stated: “What is the point of an awful lot of what I do. I’m in my 50s. I think one should be spending a good deal of your time doing things you want to do ... and what is that? I want to see much more of my family and friends. I want to continue making a contribution, but how can I best do that? ... I am contaminated by patriarchy; there is no man who isn’t. There is hope for men only if they ‘acknowledge the end of patriarchal power and participate in the discussion of how the post-patriarchal age is to be negotiated”. He opined whether it is still the case that, if men do not reevaluate their roles, they will soon be entirely irrelevant as social beings. The value of men is less in income generation but more in cultivating involvement, awareness, consistency, and caring, he stated.
As always with famous and talented people, we are interested not only in their professional gifts to us but in their personal journeys, and the authors, Dr. Kelly and Dr. Houston have given us this rich profile of one of my lifelong heroes, Anthony Clare. Anthony Clare makes you feel good about being a psychiatrist, and that is such an important gift.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest.
New biography captures Dr. Anthony Clare’s complexity
New biography captures Dr. Anthony Clare’s complexity
In “Psychiatrist in the Chair,” authors Brendan Kelly and Muiris Houston tell the story of a fellow Irishman, Anthony Clare, MD, who brought intelligence and eloquence to psychiatry. They tell a well-measured, well-referenced story of Anthony Clare’s personal and professional life. They capture his eloquence, wit, charm, and success in psychiatry as well as alluding to Dr. Clare’s self-reported “some kind of Irish darkness.”
In 1983, I was a young Scottish psychiatrist entering a fusty profession. Suddenly, there was Dr. Anthony Clare on the BBC! In “In the Psychiatrist’s Chair,” Dr. Clare interviewed celebrities. In addition to describing his Irish darkness, Brendan Kelly, MD, PhD and Muiris Houston, MD, FRCGP, both of whom are affiliated with Trinity College Dublin, note that Dr. Clare said: “I’m better at destroying systems than I am at putting them together – I do rather look for people to interview who will not live up to the prediction; there’s an element of destructiveness that’s still in me.”
I still listen to his talks on YouTube. His delicate probing questioning of B.F. Skinner, PhD, is one of my favorites, as he expertly and in an ever-so-friendly manner, teases out Dr. Skinner’s views of his upbringing and tags them to his behavorialism. It is this skill as an interviewer that captured us; can psychiatrists really be this clever? Yes, we can. All of the young and hopeful psychiatrists could see a future.
Dr. Clare raised psychiatry up from the depths of the asylums. He showed that a psychiatrist can be kind, charming, and sophisticated – handsome and helpful, not the ghouls of old movies. He did what needed to be done to psychiatry at that time: He set us on a footing that was not scary to the public. His vision for psychiatry was to improve services to those in need, reduce stigma, and show the public that there is a continuum between health and illness. He also took on the push for diagnoses, which he felt separated the normal from the abnormal, us from them.
He wrote his seminal work 10 years after graduating from the University College of Dublin. “Psychiatry in Dissent: Controversial Issues in Thought and Practice” was published in 1976, and is still considered one of the most influential texts in psychiatry. Dr. Clare “legitimized psychiatry not only in the eyes of the public but in the eyes of psychiatrists too,” the authors wrote. He did not support psychoanalysis and eschewed the rigor attached to the learning of new psychotherapies. He took renowned experts to task, but in ever such an elegant way. He successfully took on Hans Eysenck, PhD, I think because Dr. Eysenck insulted the intelligence of the Irish. He had a measured response to the anti-psychiatrists Thomas Szasz, MD, and R.D. Laing, MD, incorporating their ideas into his view of psychiatry. Dr. Clare was a social psychiatrist who highlighted the role of poverty and lack of access to mental health services. He stated that psychiatry was a “shambles, a mess and at a very primitive level.”
I enjoyed learning about his fight to make the membership exam for entrance into the Royal College of Psychiatry worthy of its name. Dr. Clare helped found the Association of Psychiatrists in Training (APIT) and wrote eloquently about the difference between training and indoctrination, which he described as having people fit a predetermined paradigm of how psychiatry should be constructed and practiced, versus education, which he defined as forming the mind. He highlighted the lack of good training facilities, and teaching staff in many parts of the United Kingdom. When Dr. Clare studied candidates in Edinburgh, he found that 70% had no child, forensic, or intellectual disability training. By the time I did my training there, I was able to get experience in all three subspecialties. He opposed the granting of automatic membership to current consultants, many of whom he considered to be “dunderheads.” . I can attest to that!
In the later phase of his life, Dr. Clare likened his self-punishing regime at the height of his hyperproductive fame to an addiction – a fix, with its risk/reward, pain/pleasure kick. He identified fear as being an unacknowledged presence in most of his life. There are vague hints from Dr. Clare’s friends and colleagues that something drove him back to Ireland from a successful life in London. Although his wife was described as being fully supportive of him, her words on his tombstone indicate something: What they indicate you can decide. She called him “a loving husband, father and grandfather, orator, physician, writer and broadcaster.” No mention was made of his being one of the greatest psychiatrists of his generation.
In 2000, he wrote “On Men: Masculinity in Crisis,” about men and the patriarchy, and highlighted the concept of “performance-based self-worth” in men. He stated: “What is the point of an awful lot of what I do. I’m in my 50s. I think one should be spending a good deal of your time doing things you want to do ... and what is that? I want to see much more of my family and friends. I want to continue making a contribution, but how can I best do that? ... I am contaminated by patriarchy; there is no man who isn’t. There is hope for men only if they ‘acknowledge the end of patriarchal power and participate in the discussion of how the post-patriarchal age is to be negotiated”. He opined whether it is still the case that, if men do not reevaluate their roles, they will soon be entirely irrelevant as social beings. The value of men is less in income generation but more in cultivating involvement, awareness, consistency, and caring, he stated.
As always with famous and talented people, we are interested not only in their professional gifts to us but in their personal journeys, and the authors, Dr. Kelly and Dr. Houston have given us this rich profile of one of my lifelong heroes, Anthony Clare. Anthony Clare makes you feel good about being a psychiatrist, and that is such an important gift.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest.
In “Psychiatrist in the Chair,” authors Brendan Kelly and Muiris Houston tell the story of a fellow Irishman, Anthony Clare, MD, who brought intelligence and eloquence to psychiatry. They tell a well-measured, well-referenced story of Anthony Clare’s personal and professional life. They capture his eloquence, wit, charm, and success in psychiatry as well as alluding to Dr. Clare’s self-reported “some kind of Irish darkness.”
In 1983, I was a young Scottish psychiatrist entering a fusty profession. Suddenly, there was Dr. Anthony Clare on the BBC! In “In the Psychiatrist’s Chair,” Dr. Clare interviewed celebrities. In addition to describing his Irish darkness, Brendan Kelly, MD, PhD and Muiris Houston, MD, FRCGP, both of whom are affiliated with Trinity College Dublin, note that Dr. Clare said: “I’m better at destroying systems than I am at putting them together – I do rather look for people to interview who will not live up to the prediction; there’s an element of destructiveness that’s still in me.”
I still listen to his talks on YouTube. His delicate probing questioning of B.F. Skinner, PhD, is one of my favorites, as he expertly and in an ever-so-friendly manner, teases out Dr. Skinner’s views of his upbringing and tags them to his behavorialism. It is this skill as an interviewer that captured us; can psychiatrists really be this clever? Yes, we can. All of the young and hopeful psychiatrists could see a future.
Dr. Clare raised psychiatry up from the depths of the asylums. He showed that a psychiatrist can be kind, charming, and sophisticated – handsome and helpful, not the ghouls of old movies. He did what needed to be done to psychiatry at that time: He set us on a footing that was not scary to the public. His vision for psychiatry was to improve services to those in need, reduce stigma, and show the public that there is a continuum between health and illness. He also took on the push for diagnoses, which he felt separated the normal from the abnormal, us from them.
He wrote his seminal work 10 years after graduating from the University College of Dublin. “Psychiatry in Dissent: Controversial Issues in Thought and Practice” was published in 1976, and is still considered one of the most influential texts in psychiatry. Dr. Clare “legitimized psychiatry not only in the eyes of the public but in the eyes of psychiatrists too,” the authors wrote. He did not support psychoanalysis and eschewed the rigor attached to the learning of new psychotherapies. He took renowned experts to task, but in ever such an elegant way. He successfully took on Hans Eysenck, PhD, I think because Dr. Eysenck insulted the intelligence of the Irish. He had a measured response to the anti-psychiatrists Thomas Szasz, MD, and R.D. Laing, MD, incorporating their ideas into his view of psychiatry. Dr. Clare was a social psychiatrist who highlighted the role of poverty and lack of access to mental health services. He stated that psychiatry was a “shambles, a mess and at a very primitive level.”
I enjoyed learning about his fight to make the membership exam for entrance into the Royal College of Psychiatry worthy of its name. Dr. Clare helped found the Association of Psychiatrists in Training (APIT) and wrote eloquently about the difference between training and indoctrination, which he described as having people fit a predetermined paradigm of how psychiatry should be constructed and practiced, versus education, which he defined as forming the mind. He highlighted the lack of good training facilities, and teaching staff in many parts of the United Kingdom. When Dr. Clare studied candidates in Edinburgh, he found that 70% had no child, forensic, or intellectual disability training. By the time I did my training there, I was able to get experience in all three subspecialties. He opposed the granting of automatic membership to current consultants, many of whom he considered to be “dunderheads.” . I can attest to that!
In the later phase of his life, Dr. Clare likened his self-punishing regime at the height of his hyperproductive fame to an addiction – a fix, with its risk/reward, pain/pleasure kick. He identified fear as being an unacknowledged presence in most of his life. There are vague hints from Dr. Clare’s friends and colleagues that something drove him back to Ireland from a successful life in London. Although his wife was described as being fully supportive of him, her words on his tombstone indicate something: What they indicate you can decide. She called him “a loving husband, father and grandfather, orator, physician, writer and broadcaster.” No mention was made of his being one of the greatest psychiatrists of his generation.
In 2000, he wrote “On Men: Masculinity in Crisis,” about men and the patriarchy, and highlighted the concept of “performance-based self-worth” in men. He stated: “What is the point of an awful lot of what I do. I’m in my 50s. I think one should be spending a good deal of your time doing things you want to do ... and what is that? I want to see much more of my family and friends. I want to continue making a contribution, but how can I best do that? ... I am contaminated by patriarchy; there is no man who isn’t. There is hope for men only if they ‘acknowledge the end of patriarchal power and participate in the discussion of how the post-patriarchal age is to be negotiated”. He opined whether it is still the case that, if men do not reevaluate their roles, they will soon be entirely irrelevant as social beings. The value of men is less in income generation but more in cultivating involvement, awareness, consistency, and caring, he stated.
As always with famous and talented people, we are interested not only in their professional gifts to us but in their personal journeys, and the authors, Dr. Kelly and Dr. Houston have given us this rich profile of one of my lifelong heroes, Anthony Clare. Anthony Clare makes you feel good about being a psychiatrist, and that is such an important gift.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest.
CDC panel recommends Pfizer’s COVID-19 vaccine for people 16 and over
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.








