User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Pfizer files for FDA emergency use authorization of COVID vaccine
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Tips for physicians, patients to make the most of the holidays amid COVID
“We must accept finite disappointment, but never lose infinite hope,” Martin Luther King, Jr.
This holiday season will be like no other. We will remember it for the rest of our lives, and we will look back to see how we faced the holidays during a pandemic.
Like the rest of 2020, the holidays will need to be reimagined. Years and even decades of tradition will be broken as we place health above merriment.
Here are a few tips to help all of us and our patients make the most of this holiday season.
- Reprioritize: This holiday season will be about depth not breadth, quality not quantity, and less not more. Trips are canceled and gatherings have shrunk. We are not running from store to store or party to party. Instead, you will find yourself surrounded by fewer friends and family. Some will be alone to optimally protect their health and the health of others. Do your best to focus on the half-full portion.
- Embrace change: Don’t compare or try to make this year like previous years. Be creative and try to find ways to make a new format fun. Meeting during the day and limiting alcohol intake can assist in making sure everyone stays safe. It has been interesting to see how many of my patients have decreased their alcohol use during quarantine. I hope this pattern will continue over the next weeks and months.
- Practice self-care: As health care professionals, we must remember the old adage “physician, heal thyself.” This year has been so difficult for almost all of us. It was filled with unprecedented levels of personal and professional stress. Holidays are often about what we can do for others, but this year we may need to place self-care first. Do what brings you happiness.
Even though you aren’t traveling, you can still disconnect from work. Set up a schedule and stick to it making sure you take plenty of time to rest and enjoy. Many of us have been working extremely long hours and a break is so needed. Take it if you possibly can. Detox from your screen! Limit the news. Creativity and productivity will be enhanced in 2021 if we can come in recharged.
For those remaining on the front lines, be patient; the end is nearing. Take care of yourself when you are not working. We are all so grateful to those in our field who have sacrificed so much to care for others. Eat, drink, and rest well to keep your immune system strong.
- Acknowledge your negative emotions: As we all know, if you try to deny negative emotions, they continue to pop up. If we give them time and space to be felt, we will find they diminish in intensity. Long work hours may have prevented us from feeling our emotions, so don’t be surprised if they surface when we take a break.
Let yourself feel the sadness for what you have experienced this year. Be open about missing those who can’t be with you because of travel or other restrictions. Let yourself feel the disappointment about your holiday travel plans that you can’t embark upon.
You may elect to share these emotions with someone close to you or with a professional. To paraphrase Carl Jung, “what we resist, persists,” so don’t try to hide from your negative emotions. Most of us had lots of them in 2020, so don’t be shy about admitting it.
- Focus on growth: What have we learned from 2020 and how can we be better equipped in 2021 and beyond?
Trauma can bring growth not just disorder. This year has returned well-deserved prestige to our fields. We are being lauded as heroes as we have scarified our health and the health of our loved ones to serve others. Can we choose to celebrate our accomplishments?
We have become more resilient and learned to continue on in the face of great hardship. Many of us have gained confidence as we confronted this historic challenge. As we have been reminded of death daily, we learn to appreciate life more fully and not take any day for granted.
I am proud to be a physician during this pandemic, and I hope you are, too!
- Find joy: Often times, we find real happiness in smaller moments and experiences. For many, this time of year is filled with so much stress that it can be hard to carve out moments of joy. As we may be less busy socially this holiday season, might we find even more joy?
Joy can only be experienced in the present moment. Tap into all your senses. Eat slowly making sure to smell and taste every bite. Cherish those who can still gather at your table. If you find yourself alone, embrace that experience. Safety must continue to come first, and we can’t let down our guard now.
- Reflect: New Year’s Eve is always a time for reflection and hope for the future. Most of us will be glad to see 2020 in the rearview mirror. With multiple and very promising vaccines on the horizon, we can anticipate a brighter future. We must continue to work hard; remain patient; and be creative, resilient, and optimistic. Let’s try to fill our days with hope and purpose and work together to achieve a brighter future for all.
“Learn from yesterday, live for today, hope for tomorrow,” Albert Einstein
Wishing you health and happiness in this holiday season and beyond.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She also is founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world.
“We must accept finite disappointment, but never lose infinite hope,” Martin Luther King, Jr.
This holiday season will be like no other. We will remember it for the rest of our lives, and we will look back to see how we faced the holidays during a pandemic.
Like the rest of 2020, the holidays will need to be reimagined. Years and even decades of tradition will be broken as we place health above merriment.
Here are a few tips to help all of us and our patients make the most of this holiday season.
- Reprioritize: This holiday season will be about depth not breadth, quality not quantity, and less not more. Trips are canceled and gatherings have shrunk. We are not running from store to store or party to party. Instead, you will find yourself surrounded by fewer friends and family. Some will be alone to optimally protect their health and the health of others. Do your best to focus on the half-full portion.
- Embrace change: Don’t compare or try to make this year like previous years. Be creative and try to find ways to make a new format fun. Meeting during the day and limiting alcohol intake can assist in making sure everyone stays safe. It has been interesting to see how many of my patients have decreased their alcohol use during quarantine. I hope this pattern will continue over the next weeks and months.
- Practice self-care: As health care professionals, we must remember the old adage “physician, heal thyself.” This year has been so difficult for almost all of us. It was filled with unprecedented levels of personal and professional stress. Holidays are often about what we can do for others, but this year we may need to place self-care first. Do what brings you happiness.
Even though you aren’t traveling, you can still disconnect from work. Set up a schedule and stick to it making sure you take plenty of time to rest and enjoy. Many of us have been working extremely long hours and a break is so needed. Take it if you possibly can. Detox from your screen! Limit the news. Creativity and productivity will be enhanced in 2021 if we can come in recharged.
For those remaining on the front lines, be patient; the end is nearing. Take care of yourself when you are not working. We are all so grateful to those in our field who have sacrificed so much to care for others. Eat, drink, and rest well to keep your immune system strong.
- Acknowledge your negative emotions: As we all know, if you try to deny negative emotions, they continue to pop up. If we give them time and space to be felt, we will find they diminish in intensity. Long work hours may have prevented us from feeling our emotions, so don’t be surprised if they surface when we take a break.
Let yourself feel the sadness for what you have experienced this year. Be open about missing those who can’t be with you because of travel or other restrictions. Let yourself feel the disappointment about your holiday travel plans that you can’t embark upon.
You may elect to share these emotions with someone close to you or with a professional. To paraphrase Carl Jung, “what we resist, persists,” so don’t try to hide from your negative emotions. Most of us had lots of them in 2020, so don’t be shy about admitting it.
- Focus on growth: What have we learned from 2020 and how can we be better equipped in 2021 and beyond?
Trauma can bring growth not just disorder. This year has returned well-deserved prestige to our fields. We are being lauded as heroes as we have scarified our health and the health of our loved ones to serve others. Can we choose to celebrate our accomplishments?
We have become more resilient and learned to continue on in the face of great hardship. Many of us have gained confidence as we confronted this historic challenge. As we have been reminded of death daily, we learn to appreciate life more fully and not take any day for granted.
I am proud to be a physician during this pandemic, and I hope you are, too!
- Find joy: Often times, we find real happiness in smaller moments and experiences. For many, this time of year is filled with so much stress that it can be hard to carve out moments of joy. As we may be less busy socially this holiday season, might we find even more joy?
Joy can only be experienced in the present moment. Tap into all your senses. Eat slowly making sure to smell and taste every bite. Cherish those who can still gather at your table. If you find yourself alone, embrace that experience. Safety must continue to come first, and we can’t let down our guard now.
- Reflect: New Year’s Eve is always a time for reflection and hope for the future. Most of us will be glad to see 2020 in the rearview mirror. With multiple and very promising vaccines on the horizon, we can anticipate a brighter future. We must continue to work hard; remain patient; and be creative, resilient, and optimistic. Let’s try to fill our days with hope and purpose and work together to achieve a brighter future for all.
“Learn from yesterday, live for today, hope for tomorrow,” Albert Einstein
Wishing you health and happiness in this holiday season and beyond.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She also is founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world.
“We must accept finite disappointment, but never lose infinite hope,” Martin Luther King, Jr.
This holiday season will be like no other. We will remember it for the rest of our lives, and we will look back to see how we faced the holidays during a pandemic.
Like the rest of 2020, the holidays will need to be reimagined. Years and even decades of tradition will be broken as we place health above merriment.
Here are a few tips to help all of us and our patients make the most of this holiday season.
- Reprioritize: This holiday season will be about depth not breadth, quality not quantity, and less not more. Trips are canceled and gatherings have shrunk. We are not running from store to store or party to party. Instead, you will find yourself surrounded by fewer friends and family. Some will be alone to optimally protect their health and the health of others. Do your best to focus on the half-full portion.
- Embrace change: Don’t compare or try to make this year like previous years. Be creative and try to find ways to make a new format fun. Meeting during the day and limiting alcohol intake can assist in making sure everyone stays safe. It has been interesting to see how many of my patients have decreased their alcohol use during quarantine. I hope this pattern will continue over the next weeks and months.
- Practice self-care: As health care professionals, we must remember the old adage “physician, heal thyself.” This year has been so difficult for almost all of us. It was filled with unprecedented levels of personal and professional stress. Holidays are often about what we can do for others, but this year we may need to place self-care first. Do what brings you happiness.
Even though you aren’t traveling, you can still disconnect from work. Set up a schedule and stick to it making sure you take plenty of time to rest and enjoy. Many of us have been working extremely long hours and a break is so needed. Take it if you possibly can. Detox from your screen! Limit the news. Creativity and productivity will be enhanced in 2021 if we can come in recharged.
For those remaining on the front lines, be patient; the end is nearing. Take care of yourself when you are not working. We are all so grateful to those in our field who have sacrificed so much to care for others. Eat, drink, and rest well to keep your immune system strong.
- Acknowledge your negative emotions: As we all know, if you try to deny negative emotions, they continue to pop up. If we give them time and space to be felt, we will find they diminish in intensity. Long work hours may have prevented us from feeling our emotions, so don’t be surprised if they surface when we take a break.
Let yourself feel the sadness for what you have experienced this year. Be open about missing those who can’t be with you because of travel or other restrictions. Let yourself feel the disappointment about your holiday travel plans that you can’t embark upon.
You may elect to share these emotions with someone close to you or with a professional. To paraphrase Carl Jung, “what we resist, persists,” so don’t try to hide from your negative emotions. Most of us had lots of them in 2020, so don’t be shy about admitting it.
- Focus on growth: What have we learned from 2020 and how can we be better equipped in 2021 and beyond?
Trauma can bring growth not just disorder. This year has returned well-deserved prestige to our fields. We are being lauded as heroes as we have scarified our health and the health of our loved ones to serve others. Can we choose to celebrate our accomplishments?
We have become more resilient and learned to continue on in the face of great hardship. Many of us have gained confidence as we confronted this historic challenge. As we have been reminded of death daily, we learn to appreciate life more fully and not take any day for granted.
I am proud to be a physician during this pandemic, and I hope you are, too!
- Find joy: Often times, we find real happiness in smaller moments and experiences. For many, this time of year is filled with so much stress that it can be hard to carve out moments of joy. As we may be less busy socially this holiday season, might we find even more joy?
Joy can only be experienced in the present moment. Tap into all your senses. Eat slowly making sure to smell and taste every bite. Cherish those who can still gather at your table. If you find yourself alone, embrace that experience. Safety must continue to come first, and we can’t let down our guard now.
- Reflect: New Year’s Eve is always a time for reflection and hope for the future. Most of us will be glad to see 2020 in the rearview mirror. With multiple and very promising vaccines on the horizon, we can anticipate a brighter future. We must continue to work hard; remain patient; and be creative, resilient, and optimistic. Let’s try to fill our days with hope and purpose and work together to achieve a brighter future for all.
“Learn from yesterday, live for today, hope for tomorrow,” Albert Einstein
Wishing you health and happiness in this holiday season and beyond.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She also is founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world.
FDA authorizes baricitinib combo for COVID-19
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
FDA approves first at-home COVID-19 test kit
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
Inattention to heightened CV risk common theme in clozapine deaths teaser
Death while on clozapine for schizophrenia is often associated with substandard treatment of cardiometabolic risk factors, Sharon Taub, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Inadequate treatment for metabolic syndrome was found to be a mortality predictor while on clozapine therapy. Patients who died were less likely to receive appropriate treatment for hyperlipidemia and type 2 diabetes, despite having been diagnosed with those conditions,” she said in presenting the results of her retrospective cohort study.
“Better preventive care, with special attention to those conditions, might prevent morbidity and improve life expectancy in this population,” concluded Dr. Taub of Geha Mental Health Center in Petah Tikva, Israel, and Tel Aviv University.
She reported on all 1,817 patients on clozapine for schizophrenia included in a large Israeli health care electronic medical records database, of whom 112, or 6.2%, died during 2 years of follow-up. Mortality while on the atypical antipsychotic was associated with a higher prevalence of hyperlipidemia, type 2 diabetes, hypertension, known ischemic heart disease, and a history of acute MI, compared with survivors.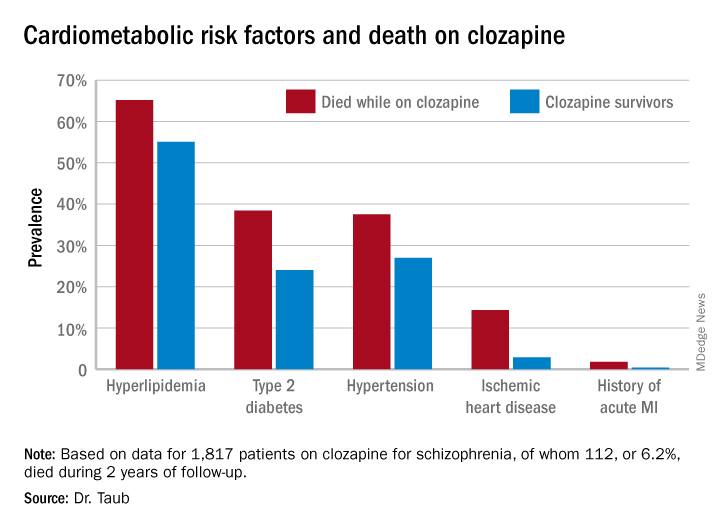
Similarly, only 16.3% of those known to have type 2 diabetes who died while on clozapine were on hypoglycemic agents, compared with 67.1% of diabetic survivors. The between-group difference in the use of antihypertensive drug therapy for patients diagnosed with hypertension – 28.6% in nonsurvivors on clozapine, 40.1% in survivors – did not achieve statistical significance.
In a multivariate analysis adjusted for age, sex, and socioeconomic status, schizophrenia patients with type 2 diabetes who weren’t on hypoglycemic medication were at 695% increased risk of mortality, compared with those who were. Similarly, hyperlipidemic patients on clozapine who weren’t on a statin had a 579% increase in mortality risk.
Patients who died while on clozapine had no increased risk of use of medical services while living in the community.
This evidence of a pattern of inadequate care with regard to management of cardiometabolic risk factors in patients on clozapine is disturbing for several reasons. For one, clozapine is known to be associated with increased risk of serious side effects, including development or worsening of metabolic syndrome. Also, clozapine is an important drug in psychiatric practice: “Clozapine is the only antipsychotic indicated for refractory schizophrenia. It is highly effective in treatment-resistant disease, present in 25%-30% of individuals with schizophrenia,” Dr. Taub noted. “Clozapine is underused, mostly because of severe side effects. Its administration is often postponed.”
She reported having no financial conflicts regarding her study, which was voted by conference attendees one of the top presentations at ECNP 2020.
Death while on clozapine for schizophrenia is often associated with substandard treatment of cardiometabolic risk factors, Sharon Taub, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Inadequate treatment for metabolic syndrome was found to be a mortality predictor while on clozapine therapy. Patients who died were less likely to receive appropriate treatment for hyperlipidemia and type 2 diabetes, despite having been diagnosed with those conditions,” she said in presenting the results of her retrospective cohort study.
“Better preventive care, with special attention to those conditions, might prevent morbidity and improve life expectancy in this population,” concluded Dr. Taub of Geha Mental Health Center in Petah Tikva, Israel, and Tel Aviv University.
She reported on all 1,817 patients on clozapine for schizophrenia included in a large Israeli health care electronic medical records database, of whom 112, or 6.2%, died during 2 years of follow-up. Mortality while on the atypical antipsychotic was associated with a higher prevalence of hyperlipidemia, type 2 diabetes, hypertension, known ischemic heart disease, and a history of acute MI, compared with survivors.
Similarly, only 16.3% of those known to have type 2 diabetes who died while on clozapine were on hypoglycemic agents, compared with 67.1% of diabetic survivors. The between-group difference in the use of antihypertensive drug therapy for patients diagnosed with hypertension – 28.6% in nonsurvivors on clozapine, 40.1% in survivors – did not achieve statistical significance.
In a multivariate analysis adjusted for age, sex, and socioeconomic status, schizophrenia patients with type 2 diabetes who weren’t on hypoglycemic medication were at 695% increased risk of mortality, compared with those who were. Similarly, hyperlipidemic patients on clozapine who weren’t on a statin had a 579% increase in mortality risk.
Patients who died while on clozapine had no increased risk of use of medical services while living in the community.
This evidence of a pattern of inadequate care with regard to management of cardiometabolic risk factors in patients on clozapine is disturbing for several reasons. For one, clozapine is known to be associated with increased risk of serious side effects, including development or worsening of metabolic syndrome. Also, clozapine is an important drug in psychiatric practice: “Clozapine is the only antipsychotic indicated for refractory schizophrenia. It is highly effective in treatment-resistant disease, present in 25%-30% of individuals with schizophrenia,” Dr. Taub noted. “Clozapine is underused, mostly because of severe side effects. Its administration is often postponed.”
She reported having no financial conflicts regarding her study, which was voted by conference attendees one of the top presentations at ECNP 2020.
Death while on clozapine for schizophrenia is often associated with substandard treatment of cardiometabolic risk factors, Sharon Taub, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Inadequate treatment for metabolic syndrome was found to be a mortality predictor while on clozapine therapy. Patients who died were less likely to receive appropriate treatment for hyperlipidemia and type 2 diabetes, despite having been diagnosed with those conditions,” she said in presenting the results of her retrospective cohort study.
“Better preventive care, with special attention to those conditions, might prevent morbidity and improve life expectancy in this population,” concluded Dr. Taub of Geha Mental Health Center in Petah Tikva, Israel, and Tel Aviv University.
She reported on all 1,817 patients on clozapine for schizophrenia included in a large Israeli health care electronic medical records database, of whom 112, or 6.2%, died during 2 years of follow-up. Mortality while on the atypical antipsychotic was associated with a higher prevalence of hyperlipidemia, type 2 diabetes, hypertension, known ischemic heart disease, and a history of acute MI, compared with survivors.
Similarly, only 16.3% of those known to have type 2 diabetes who died while on clozapine were on hypoglycemic agents, compared with 67.1% of diabetic survivors. The between-group difference in the use of antihypertensive drug therapy for patients diagnosed with hypertension – 28.6% in nonsurvivors on clozapine, 40.1% in survivors – did not achieve statistical significance.
In a multivariate analysis adjusted for age, sex, and socioeconomic status, schizophrenia patients with type 2 diabetes who weren’t on hypoglycemic medication were at 695% increased risk of mortality, compared with those who were. Similarly, hyperlipidemic patients on clozapine who weren’t on a statin had a 579% increase in mortality risk.
Patients who died while on clozapine had no increased risk of use of medical services while living in the community.
This evidence of a pattern of inadequate care with regard to management of cardiometabolic risk factors in patients on clozapine is disturbing for several reasons. For one, clozapine is known to be associated with increased risk of serious side effects, including development or worsening of metabolic syndrome. Also, clozapine is an important drug in psychiatric practice: “Clozapine is the only antipsychotic indicated for refractory schizophrenia. It is highly effective in treatment-resistant disease, present in 25%-30% of individuals with schizophrenia,” Dr. Taub noted. “Clozapine is underused, mostly because of severe side effects. Its administration is often postponed.”
She reported having no financial conflicts regarding her study, which was voted by conference attendees one of the top presentations at ECNP 2020.
FROM ECNP 2020
Can a probiotic prevent COVID-19?
On the Nov. 12 episode of the Blood & Cancer podcast, Anthony D. Sung, MD, of Duke University, Durham, N.C., joined host David H. Henry, MD, of Penn Medicine in Philadelphia, to discuss the trial of LGG as well as other research. The following transcript of that discussion has been edited for length and clarity.
David Henry, MD: Here we are in COVID. We’re recording this the first week in November. Sadly, cases are spiking in the country. And I understand you’ve got some information that you might share about how manipulating ... the microbiome that we all exist with inside our gut might somehow play into doing better or worse with COVID.
Anthony Sung, MD: Absolutely. So, as associate director of the Duke Microbiome Center, I was approached by one of my colleagues, Paul Wischmeyer, who is a professor of anesthesiology and critical care medicine at Duke. Paul had previously done some very nice murine studies with the probiotic Lactobacillus rhamnosus GG, or LGG.
He showed, in a murine model of pseudomonas pneumonia, that giving LGG to mice would help modulate their microbiome and, in turn, their immune system, leading to decreased inflammation, decreased TNF-alpha, IL [interleukin]-2, and IL-6, [and] increased Treg cells [Clin Nutr. 2017;36[6]:1549-57]. This also helped prevent lung injury, and it actually significantly improved survival in mice receiving LGG [Shock. 2013;40[6]:496-503].
In addition, there has been a randomized clinical trial of LGG showing that its administration would help prevent ventilator-associated pneumonia, or VAP [Am J Respir Crit Care Med. 2010 Oct 15;182[8]:1058-64].
And a few years ago, there was another RCT [randomized, controlled trial], published in Nature, showing that another Lactobacillus product significantly decreased the combined endpoint of sepsis and mortality, primarily by reducing lower respiratory tract infection [Nature. 2017 Aug 24;548[7668]:407-12].
Dr. Henry: And how is that working? What is the bacillus doing to help us?
Dr. Sung: We think it’s through modulating the immune system. As mentioned in Paul’s studies, we saw significantly decreased amounts of TNF-alpha, IL-2, and IL-6, which are the same cytokines that have been implicated in COVID-19 and associated with increased lung injury in patients during this pandemic.
And we believe that by giving individuals this probiotic, LGG, we may help modulate the immune system, decrease lung injury and symptoms, and maybe even prevent COVID-19.
So with support from the Duke Microbiome Center, as well as private donations and philanthropy, we are conducting a randomized clinical trial of LGG to prevent COVID-19 in household contacts who’ve been exposed to the disease. In other words, if someone in the house gets COVID-19, we want to try to prophylax everybody else living in that house and prevent them from coming down with the same infection.
Dr. Henry: And this is an oral administration?
Dr. Sung: Correct. This is an oral pill, two pills once a day.
Dr. Henry: And it’s an ongoing study, of course, in COVID right now?
Dr. Sung: Correct. So we have an IND [investigational new drug application] from the FDA [Food and Drug Administration], and we are actively recruiting subjects both at Duke University, but also due to the unique study design, we can enroll patients anywhere across the country. Because of the importance of social distancing, everything is done remotely.
So a household contact can hear about us, either through your podcast or one of our Facebook ads or through other media. They can reach out to our study website, which is https://sites.duke.edu/protectehc, or reach out to us at our study email, [email protected].
And we can go ahead and screen them for eligibility in our trial. And if they are eligible and they consent to participate, we will mail them a package basically overnight, FedEx, containing either LGG or placebo, as well as kits so that they can self-collect their stool and nasal swabs so we can test it for SARS-CoV-2 by PCR [polymerase chain reaction] and look at the microbiome.
Dr. Sung and Dr. Henry have no relevant disclosures. Funding for the trial is provided by the Duke Microbiome Center and philanthropic giving. The LGG and placebo used in the trial are provided by DSM.
On the Nov. 12 episode of the Blood & Cancer podcast, Anthony D. Sung, MD, of Duke University, Durham, N.C., joined host David H. Henry, MD, of Penn Medicine in Philadelphia, to discuss the trial of LGG as well as other research. The following transcript of that discussion has been edited for length and clarity.
David Henry, MD: Here we are in COVID. We’re recording this the first week in November. Sadly, cases are spiking in the country. And I understand you’ve got some information that you might share about how manipulating ... the microbiome that we all exist with inside our gut might somehow play into doing better or worse with COVID.
Anthony Sung, MD: Absolutely. So, as associate director of the Duke Microbiome Center, I was approached by one of my colleagues, Paul Wischmeyer, who is a professor of anesthesiology and critical care medicine at Duke. Paul had previously done some very nice murine studies with the probiotic Lactobacillus rhamnosus GG, or LGG.
He showed, in a murine model of pseudomonas pneumonia, that giving LGG to mice would help modulate their microbiome and, in turn, their immune system, leading to decreased inflammation, decreased TNF-alpha, IL [interleukin]-2, and IL-6, [and] increased Treg cells [Clin Nutr. 2017;36[6]:1549-57]. This also helped prevent lung injury, and it actually significantly improved survival in mice receiving LGG [Shock. 2013;40[6]:496-503].
In addition, there has been a randomized clinical trial of LGG showing that its administration would help prevent ventilator-associated pneumonia, or VAP [Am J Respir Crit Care Med. 2010 Oct 15;182[8]:1058-64].
And a few years ago, there was another RCT [randomized, controlled trial], published in Nature, showing that another Lactobacillus product significantly decreased the combined endpoint of sepsis and mortality, primarily by reducing lower respiratory tract infection [Nature. 2017 Aug 24;548[7668]:407-12].
Dr. Henry: And how is that working? What is the bacillus doing to help us?
Dr. Sung: We think it’s through modulating the immune system. As mentioned in Paul’s studies, we saw significantly decreased amounts of TNF-alpha, IL-2, and IL-6, which are the same cytokines that have been implicated in COVID-19 and associated with increased lung injury in patients during this pandemic.
And we believe that by giving individuals this probiotic, LGG, we may help modulate the immune system, decrease lung injury and symptoms, and maybe even prevent COVID-19.
So with support from the Duke Microbiome Center, as well as private donations and philanthropy, we are conducting a randomized clinical trial of LGG to prevent COVID-19 in household contacts who’ve been exposed to the disease. In other words, if someone in the house gets COVID-19, we want to try to prophylax everybody else living in that house and prevent them from coming down with the same infection.
Dr. Henry: And this is an oral administration?
Dr. Sung: Correct. This is an oral pill, two pills once a day.
Dr. Henry: And it’s an ongoing study, of course, in COVID right now?
Dr. Sung: Correct. So we have an IND [investigational new drug application] from the FDA [Food and Drug Administration], and we are actively recruiting subjects both at Duke University, but also due to the unique study design, we can enroll patients anywhere across the country. Because of the importance of social distancing, everything is done remotely.
So a household contact can hear about us, either through your podcast or one of our Facebook ads or through other media. They can reach out to our study website, which is https://sites.duke.edu/protectehc, or reach out to us at our study email, [email protected].
And we can go ahead and screen them for eligibility in our trial. And if they are eligible and they consent to participate, we will mail them a package basically overnight, FedEx, containing either LGG or placebo, as well as kits so that they can self-collect their stool and nasal swabs so we can test it for SARS-CoV-2 by PCR [polymerase chain reaction] and look at the microbiome.
Dr. Sung and Dr. Henry have no relevant disclosures. Funding for the trial is provided by the Duke Microbiome Center and philanthropic giving. The LGG and placebo used in the trial are provided by DSM.
On the Nov. 12 episode of the Blood & Cancer podcast, Anthony D. Sung, MD, of Duke University, Durham, N.C., joined host David H. Henry, MD, of Penn Medicine in Philadelphia, to discuss the trial of LGG as well as other research. The following transcript of that discussion has been edited for length and clarity.
David Henry, MD: Here we are in COVID. We’re recording this the first week in November. Sadly, cases are spiking in the country. And I understand you’ve got some information that you might share about how manipulating ... the microbiome that we all exist with inside our gut might somehow play into doing better or worse with COVID.
Anthony Sung, MD: Absolutely. So, as associate director of the Duke Microbiome Center, I was approached by one of my colleagues, Paul Wischmeyer, who is a professor of anesthesiology and critical care medicine at Duke. Paul had previously done some very nice murine studies with the probiotic Lactobacillus rhamnosus GG, or LGG.
He showed, in a murine model of pseudomonas pneumonia, that giving LGG to mice would help modulate their microbiome and, in turn, their immune system, leading to decreased inflammation, decreased TNF-alpha, IL [interleukin]-2, and IL-6, [and] increased Treg cells [Clin Nutr. 2017;36[6]:1549-57]. This also helped prevent lung injury, and it actually significantly improved survival in mice receiving LGG [Shock. 2013;40[6]:496-503].
In addition, there has been a randomized clinical trial of LGG showing that its administration would help prevent ventilator-associated pneumonia, or VAP [Am J Respir Crit Care Med. 2010 Oct 15;182[8]:1058-64].
And a few years ago, there was another RCT [randomized, controlled trial], published in Nature, showing that another Lactobacillus product significantly decreased the combined endpoint of sepsis and mortality, primarily by reducing lower respiratory tract infection [Nature. 2017 Aug 24;548[7668]:407-12].
Dr. Henry: And how is that working? What is the bacillus doing to help us?
Dr. Sung: We think it’s through modulating the immune system. As mentioned in Paul’s studies, we saw significantly decreased amounts of TNF-alpha, IL-2, and IL-6, which are the same cytokines that have been implicated in COVID-19 and associated with increased lung injury in patients during this pandemic.
And we believe that by giving individuals this probiotic, LGG, we may help modulate the immune system, decrease lung injury and symptoms, and maybe even prevent COVID-19.
So with support from the Duke Microbiome Center, as well as private donations and philanthropy, we are conducting a randomized clinical trial of LGG to prevent COVID-19 in household contacts who’ve been exposed to the disease. In other words, if someone in the house gets COVID-19, we want to try to prophylax everybody else living in that house and prevent them from coming down with the same infection.
Dr. Henry: And this is an oral administration?
Dr. Sung: Correct. This is an oral pill, two pills once a day.
Dr. Henry: And it’s an ongoing study, of course, in COVID right now?
Dr. Sung: Correct. So we have an IND [investigational new drug application] from the FDA [Food and Drug Administration], and we are actively recruiting subjects both at Duke University, but also due to the unique study design, we can enroll patients anywhere across the country. Because of the importance of social distancing, everything is done remotely.
So a household contact can hear about us, either through your podcast or one of our Facebook ads or through other media. They can reach out to our study website, which is https://sites.duke.edu/protectehc, or reach out to us at our study email, [email protected].
And we can go ahead and screen them for eligibility in our trial. And if they are eligible and they consent to participate, we will mail them a package basically overnight, FedEx, containing either LGG or placebo, as well as kits so that they can self-collect their stool and nasal swabs so we can test it for SARS-CoV-2 by PCR [polymerase chain reaction] and look at the microbiome.
Dr. Sung and Dr. Henry have no relevant disclosures. Funding for the trial is provided by the Duke Microbiome Center and philanthropic giving. The LGG and placebo used in the trial are provided by DSM.
Pfizer’s COVID-19 vaccine 95% effective in final phase 3 results
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
‘Hospital at home’ increases COVID capacity in large study
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
Common newborn hearing test promising for early detection of autism
new research shows.
Results from one of the largest studies of its kind show the auditory brainstem response (ABR) test, which is carried out on most newborns, represents “a huge untapped potential” to detect autism, lead author Oren Miron, research associate, department of biomedical informatics, Harvard Medical School, Boston, and a PhD candidate at Ben Gurion University in Beersheba, Israel, said in an interview.
“The findings further reinforce our understanding that autism, in many cases, has a sensorial and auditory aspect to it,” said Mr. Miron, adding that an adverse response to sound is one of the earliest behavioral signs of autism.
The research was published online Oct. 31 in Autism Research.
Early intervention critical
Autism spectrum disorder (ASD), which involves problems in social communication and interaction, affects an estimated 1 in 59 children. Early identification and intervention are critical for improving outcomes and decreasing the economic burden associated with ASD.
The ABR test, which is used for Universal Newborn Hearing Screening (UNHS), uses surface electrodes to measure auditory nerve and brainstem responses to sound.
Previous studies identified abnormal ABR amplitude in children with ASD. However, it’s unclear whether healthy newborns who later develop autism also show ABR differences vs those who don’t develop the disorder.
Researchers used UNHS data, which allowed them to examine a larger, younger, and healthier sample compared with previous studies. The study included 321 newborns later diagnosed with ASD and 138,844 controls without a subsequent ASD diagnosis.
The mean ABR testing age was 1.76 days for newborns later diagnosed with ASD and 1.86 for those in the non-ASD group.
The ASD group was 77% male and the non-ASD group was 51% male. The rate of neonatal intensive care unit admission was 8% in the ASD group and 10% in the non-ASD group.
The hearing test involves placing an earpiece in the baby’s ear and delivering a click sound at 35 dB above normal hearing level (nHL) at a rate of 77 clicks per second in the right ear and 79 clicks per second in the left ear.
Brainstem abnormalities?
The clicks create electrical activity, which is recorded by a surface electrode and used to extract the ABR waveform. When a sound reaches the brain stem, it creates five consecutive waveforms – waves I, II, III, IV, and V.
Previous studies focused on wave V, which is easiest to detect. The current study used low intensity sound that resulted in a weaker signal.
To overcome this low intensity issue, researchers focused on the negative drop (latency) after the wave V (Vn), which is easier to detect, and on the ABR phase, or entire waveform. They illustrated the differences between the ASD and non-ASD groups in a series of graphs.
Results showed that the ABR phase in the right ear was significantly prolonged in the ASD vs non-ASD group (P < .001). ABR phase in the left ear was also significantly prolonged in the ASD group (P = .021)
Vn latency in the right ear was significantly prolonged in the ASD group compared with the non-ASD group (P = .048); however, this was not the case in the left ear.
The prolongation could mean that the V-negative wave might appear after 8 ms in normally developing children compared with 8.5 or 9 ms in children with autism, said Mr. Miron.
The new study is the first to show V-negative and phase abnormalities are associated with ASD, the authors note. The brainstem prolongation could be due to anatomical abnormalities in the brainstem in individuals with ASD, the researchers added.
Present before birth?
The presence of ABR biomarkers of ASD in the first weeks after birth suggests the disorder is likely present before birth in a large group of these individuals, the researchers note.
It’s possible the ABR test could be modified to use lower intensities not only to detect hearing impairment but autism risk, said Mr. Miron. “The test has been optimized to detect hearing impairment, and it does so brilliantly and helps thousands of children. We want to do the same kind of optimization for autism.”
This could lead to earlier behavioral diagnoses, which, in turn, could lead to earlier treatment and better outcomes for children with ASD, said Mr. Miron.
At this time, the level of prolongation to detect ASD is unclear. “I would think a lot of people would want to make it one standard deviation, but it depends on a lot of factors, including for example, whether a baby is preterm,” said Mr. Miron.
More research and better accuracy and specificity are needed before the newborn hearing test is clinically useful.
He noted that the hearing test is only one marker of autism and that it could potentially be combined with other behavioral signs and genetic markers to facilitate earlier diagnosis and treatment and improve outcomes for patients with ASD.
Future research by his group will investigate whether the degree of auditory prolongation relates to autism severity. They also plan to research ASD subgroups including children with comorbid epilepsy.
Terrific, clever research
Jeremy Veenstra-VanderWeele, MD, professor, child and adolescent psychiatry, Columbia University, New York, said in an interview that the study is “terrific” and a “clever use” of an existing dataset.
“They showed a difference between a large group of kids with autism and a large group of kids without.”
However, he added, more research is needed before the test can be used as an autism screening tool.
“In order for this to be a screening test that could be broadly applied you would need to identify a cutoff where you’d think a child was at risk for autism, and if you look at the graphs in the article, there are no clear cutoffs,” said Dr. Veenstra-VanderWeele.
To turn this into a useful test, “you would have to establish sensitivity and specificity, you would have to look not just at the comparison of kids with autism and kids without but apply it in a predictive way in a second population.”
The study authors and Dr. Veenstra-VanderWeele have reported no relevant financial relationships. Veenstra-VanderWeele is an associate editor at Autism Research, which published the article, but he did not handle or view it before being interviewed.
A version of this article originally appeared on Medscape.com.
new research shows.
Results from one of the largest studies of its kind show the auditory brainstem response (ABR) test, which is carried out on most newborns, represents “a huge untapped potential” to detect autism, lead author Oren Miron, research associate, department of biomedical informatics, Harvard Medical School, Boston, and a PhD candidate at Ben Gurion University in Beersheba, Israel, said in an interview.
“The findings further reinforce our understanding that autism, in many cases, has a sensorial and auditory aspect to it,” said Mr. Miron, adding that an adverse response to sound is one of the earliest behavioral signs of autism.
The research was published online Oct. 31 in Autism Research.
Early intervention critical
Autism spectrum disorder (ASD), which involves problems in social communication and interaction, affects an estimated 1 in 59 children. Early identification and intervention are critical for improving outcomes and decreasing the economic burden associated with ASD.
The ABR test, which is used for Universal Newborn Hearing Screening (UNHS), uses surface electrodes to measure auditory nerve and brainstem responses to sound.
Previous studies identified abnormal ABR amplitude in children with ASD. However, it’s unclear whether healthy newborns who later develop autism also show ABR differences vs those who don’t develop the disorder.
Researchers used UNHS data, which allowed them to examine a larger, younger, and healthier sample compared with previous studies. The study included 321 newborns later diagnosed with ASD and 138,844 controls without a subsequent ASD diagnosis.
The mean ABR testing age was 1.76 days for newborns later diagnosed with ASD and 1.86 for those in the non-ASD group.
The ASD group was 77% male and the non-ASD group was 51% male. The rate of neonatal intensive care unit admission was 8% in the ASD group and 10% in the non-ASD group.
The hearing test involves placing an earpiece in the baby’s ear and delivering a click sound at 35 dB above normal hearing level (nHL) at a rate of 77 clicks per second in the right ear and 79 clicks per second in the left ear.
Brainstem abnormalities?
The clicks create electrical activity, which is recorded by a surface electrode and used to extract the ABR waveform. When a sound reaches the brain stem, it creates five consecutive waveforms – waves I, II, III, IV, and V.
Previous studies focused on wave V, which is easiest to detect. The current study used low intensity sound that resulted in a weaker signal.
To overcome this low intensity issue, researchers focused on the negative drop (latency) after the wave V (Vn), which is easier to detect, and on the ABR phase, or entire waveform. They illustrated the differences between the ASD and non-ASD groups in a series of graphs.
Results showed that the ABR phase in the right ear was significantly prolonged in the ASD vs non-ASD group (P < .001). ABR phase in the left ear was also significantly prolonged in the ASD group (P = .021)
Vn latency in the right ear was significantly prolonged in the ASD group compared with the non-ASD group (P = .048); however, this was not the case in the left ear.
The prolongation could mean that the V-negative wave might appear after 8 ms in normally developing children compared with 8.5 or 9 ms in children with autism, said Mr. Miron.
The new study is the first to show V-negative and phase abnormalities are associated with ASD, the authors note. The brainstem prolongation could be due to anatomical abnormalities in the brainstem in individuals with ASD, the researchers added.
Present before birth?
The presence of ABR biomarkers of ASD in the first weeks after birth suggests the disorder is likely present before birth in a large group of these individuals, the researchers note.
It’s possible the ABR test could be modified to use lower intensities not only to detect hearing impairment but autism risk, said Mr. Miron. “The test has been optimized to detect hearing impairment, and it does so brilliantly and helps thousands of children. We want to do the same kind of optimization for autism.”
This could lead to earlier behavioral diagnoses, which, in turn, could lead to earlier treatment and better outcomes for children with ASD, said Mr. Miron.
At this time, the level of prolongation to detect ASD is unclear. “I would think a lot of people would want to make it one standard deviation, but it depends on a lot of factors, including for example, whether a baby is preterm,” said Mr. Miron.
More research and better accuracy and specificity are needed before the newborn hearing test is clinically useful.
He noted that the hearing test is only one marker of autism and that it could potentially be combined with other behavioral signs and genetic markers to facilitate earlier diagnosis and treatment and improve outcomes for patients with ASD.
Future research by his group will investigate whether the degree of auditory prolongation relates to autism severity. They also plan to research ASD subgroups including children with comorbid epilepsy.
Terrific, clever research
Jeremy Veenstra-VanderWeele, MD, professor, child and adolescent psychiatry, Columbia University, New York, said in an interview that the study is “terrific” and a “clever use” of an existing dataset.
“They showed a difference between a large group of kids with autism and a large group of kids without.”
However, he added, more research is needed before the test can be used as an autism screening tool.
“In order for this to be a screening test that could be broadly applied you would need to identify a cutoff where you’d think a child was at risk for autism, and if you look at the graphs in the article, there are no clear cutoffs,” said Dr. Veenstra-VanderWeele.
To turn this into a useful test, “you would have to establish sensitivity and specificity, you would have to look not just at the comparison of kids with autism and kids without but apply it in a predictive way in a second population.”
The study authors and Dr. Veenstra-VanderWeele have reported no relevant financial relationships. Veenstra-VanderWeele is an associate editor at Autism Research, which published the article, but he did not handle or view it before being interviewed.
A version of this article originally appeared on Medscape.com.
new research shows.
Results from one of the largest studies of its kind show the auditory brainstem response (ABR) test, which is carried out on most newborns, represents “a huge untapped potential” to detect autism, lead author Oren Miron, research associate, department of biomedical informatics, Harvard Medical School, Boston, and a PhD candidate at Ben Gurion University in Beersheba, Israel, said in an interview.
“The findings further reinforce our understanding that autism, in many cases, has a sensorial and auditory aspect to it,” said Mr. Miron, adding that an adverse response to sound is one of the earliest behavioral signs of autism.
The research was published online Oct. 31 in Autism Research.
Early intervention critical
Autism spectrum disorder (ASD), which involves problems in social communication and interaction, affects an estimated 1 in 59 children. Early identification and intervention are critical for improving outcomes and decreasing the economic burden associated with ASD.
The ABR test, which is used for Universal Newborn Hearing Screening (UNHS), uses surface electrodes to measure auditory nerve and brainstem responses to sound.
Previous studies identified abnormal ABR amplitude in children with ASD. However, it’s unclear whether healthy newborns who later develop autism also show ABR differences vs those who don’t develop the disorder.
Researchers used UNHS data, which allowed them to examine a larger, younger, and healthier sample compared with previous studies. The study included 321 newborns later diagnosed with ASD and 138,844 controls without a subsequent ASD diagnosis.
The mean ABR testing age was 1.76 days for newborns later diagnosed with ASD and 1.86 for those in the non-ASD group.
The ASD group was 77% male and the non-ASD group was 51% male. The rate of neonatal intensive care unit admission was 8% in the ASD group and 10% in the non-ASD group.
The hearing test involves placing an earpiece in the baby’s ear and delivering a click sound at 35 dB above normal hearing level (nHL) at a rate of 77 clicks per second in the right ear and 79 clicks per second in the left ear.
Brainstem abnormalities?
The clicks create electrical activity, which is recorded by a surface electrode and used to extract the ABR waveform. When a sound reaches the brain stem, it creates five consecutive waveforms – waves I, II, III, IV, and V.
Previous studies focused on wave V, which is easiest to detect. The current study used low intensity sound that resulted in a weaker signal.
To overcome this low intensity issue, researchers focused on the negative drop (latency) after the wave V (Vn), which is easier to detect, and on the ABR phase, or entire waveform. They illustrated the differences between the ASD and non-ASD groups in a series of graphs.
Results showed that the ABR phase in the right ear was significantly prolonged in the ASD vs non-ASD group (P < .001). ABR phase in the left ear was also significantly prolonged in the ASD group (P = .021)
Vn latency in the right ear was significantly prolonged in the ASD group compared with the non-ASD group (P = .048); however, this was not the case in the left ear.
The prolongation could mean that the V-negative wave might appear after 8 ms in normally developing children compared with 8.5 or 9 ms in children with autism, said Mr. Miron.
The new study is the first to show V-negative and phase abnormalities are associated with ASD, the authors note. The brainstem prolongation could be due to anatomical abnormalities in the brainstem in individuals with ASD, the researchers added.
Present before birth?
The presence of ABR biomarkers of ASD in the first weeks after birth suggests the disorder is likely present before birth in a large group of these individuals, the researchers note.
It’s possible the ABR test could be modified to use lower intensities not only to detect hearing impairment but autism risk, said Mr. Miron. “The test has been optimized to detect hearing impairment, and it does so brilliantly and helps thousands of children. We want to do the same kind of optimization for autism.”
This could lead to earlier behavioral diagnoses, which, in turn, could lead to earlier treatment and better outcomes for children with ASD, said Mr. Miron.
At this time, the level of prolongation to detect ASD is unclear. “I would think a lot of people would want to make it one standard deviation, but it depends on a lot of factors, including for example, whether a baby is preterm,” said Mr. Miron.
More research and better accuracy and specificity are needed before the newborn hearing test is clinically useful.
He noted that the hearing test is only one marker of autism and that it could potentially be combined with other behavioral signs and genetic markers to facilitate earlier diagnosis and treatment and improve outcomes for patients with ASD.
Future research by his group will investigate whether the degree of auditory prolongation relates to autism severity. They also plan to research ASD subgroups including children with comorbid epilepsy.
Terrific, clever research
Jeremy Veenstra-VanderWeele, MD, professor, child and adolescent psychiatry, Columbia University, New York, said in an interview that the study is “terrific” and a “clever use” of an existing dataset.
“They showed a difference between a large group of kids with autism and a large group of kids without.”
However, he added, more research is needed before the test can be used as an autism screening tool.
“In order for this to be a screening test that could be broadly applied you would need to identify a cutoff where you’d think a child was at risk for autism, and if you look at the graphs in the article, there are no clear cutoffs,” said Dr. Veenstra-VanderWeele.
To turn this into a useful test, “you would have to establish sensitivity and specificity, you would have to look not just at the comparison of kids with autism and kids without but apply it in a predictive way in a second population.”
The study authors and Dr. Veenstra-VanderWeele have reported no relevant financial relationships. Veenstra-VanderWeele is an associate editor at Autism Research, which published the article, but he did not handle or view it before being interviewed.
A version of this article originally appeared on Medscape.com.
Painful ethical choices in 2020 vs. 2010: How has thinking changed?
Much has changed in the 10 years since Medscape’s first survey on what physicians would do when faced with painful choices in patient care.
A new report, Ethics 2020: Life, Death, and Painful Dilemmas, shows that physicians’ value judgments have shifted in many respects, sometimes as a result of increased regulations and fears of litigation.
End-of-life decisions
Several of the questions in the survey revolved around end-of-life decisions, and in some cases, the differences seen in just a decade were striking. One example concerned life support decisions in the context of a family’s choices.
Age also seemed to play a role in the 2020 answers to that question: Physicians younger than 45 were more likely (28%) to answer “yes” (that they would withdraw life support in that instance) than were those 45 and older (16%).
A critical care physician said, “If the family appears to have an underlying motivation that may not be in the patient’s best interest, I might be inclined to pursue a legal decision prior to withdrawing support.”
A cardiologist had a more pointed response to the question: “To me, that would be murder.”
Another example of how perspectives have changed over the past 10 years concerns whether physician-aided dying should be legal for terminally ill patients. The practice is now mandated by law in eight states and the District of Columbia, and it is mandated by court ruling in two additional states.
In 2010, 41% said “no.” That number dropped to 28% in 2020.
On legalization, a psychiatrist said, “Yes, when there is truly no hope and the quality of remaining life is too poor. We show more compassion to our sick animals than we do to our human population.”
Conversely, a neurologist answered, “No, I see younger physicians already becoming comfortable with the idea of deciding ASAP whether there is a reasonable chance of survival and then pressing for the right code status. This change would make things worse.”
Assisted death and incurable suffering
Far fewer physicians supported physician-assisted death for those who had years to live but faced incurable suffering: Thirty-seven percent said “yes,” 34% said “no,” and 29% said “it depends.”
However, support was significantly higher than it was just 2 years ago, in 2018, when only 27% supported the concept, the report authors noted.
“The shift reflects movements by many states to legalize assisted dying for the terminally ill,” Arthur Caplan, PhD, director of the division of medical ethics, New York University, said in the report. “Legalization has not been abused, so some doctors are more willing to press further beyond terminal illness as a trigger to suffering.”
Conversely, many more physicians (44% vs. 24% a decade ago) said they would provide life-sustaining therapy if the family requested it, even if the physician thought it was futile.
“Concerns over a malpractice lawsuit and potential negative patient/family online reviews are factors that play into this change,” the survey authors wrote.
Shared decision making also increased in the past decade.
Would you undertreat pain?
Primary care physicians fear the consequences of what they consider adequate pain management more than specialists do (24% vs. 17%), the survey authors noted.
Ten years ago, Medscape asked physicians whether they would undertreat a patient’s pain because of fear of repercussions or the patient’s becoming addicted: Eighty-four percent said “no,” and 6% said “yes.” The rest said “it depends.”
In 2020, the question was asked slightly differently: “Would you undertreat a patient’s pain for fear of addiction or Drug Enforcement Administration or medical board scrutiny?” This year, three times as many said “yes” (18%); 63% said “no.”
“Respondents this year talked about investigations and reprimands by medical boards, and how much they wanted to avoid that,” the survey authors wrote.
Should physicians be required to treat COVID-19 patients?
Some questions were new this year, including one on whether physicians should be required to treat COVID-19 patients. Fewer than half (47%) answered “yes,” 24% said “no,” and 29% answered “it depends.”
Doctors’ answers to this question differed slightly by gender: Fifty percent of men and 43% of women said “yes.” In their responses, many physicians said consideration should be given to risk factors, such as age, underlying conditions, risk of family members, and availability of personal protective equipment (PPE).
Another pandemic-related question asked whether physicians felt they should correct physicians who post misinformation about the pandemic on social media. Half (50%) said “yes,” 19% said “no,” and 31% said “it depends.”
Speaking out against the workplace
This year, many physicians have felt betrayed when they didn’t have adequate PPE during the pandemic.
Asked, “Is it right to speak out against your hospital or workplace when they don’t give you what you need?” 53% of physicians said “yes,” 8% said “no,” and 40% said “it depends.”
A cardiologist made the value judgment this way: “Speaking out just because you had an argument with your boss is inappropriate. Bringing to the public repeated failures to correct situations that have been brought through the proper channels is necessary to incite change.”
Random drug testing for physicians?
Another question in the survey asked whether physicians should be subjected to random drug testing for alcohol and drug abuse. About one-third (34%) said yes, 43% said no, and 23% said “it depends.” A study found that between 10% and 15% of physicians have abused a substance at some point in their careers.
The subject continues to hit a nerve in medicine.
A family physician wrote, “This should not be done unless a particular physician had a problem with drug or alcohol abuse and shows signs of impairment.”
An internist took a different view, saying, “Military service men and women, police, firefighters, airline pilots, and other professions that have responsibilities affecting people’s lives are subject to testing; why not physicians?”
A version of this article originally appeared on Medscape.com.
Much has changed in the 10 years since Medscape’s first survey on what physicians would do when faced with painful choices in patient care.
A new report, Ethics 2020: Life, Death, and Painful Dilemmas, shows that physicians’ value judgments have shifted in many respects, sometimes as a result of increased regulations and fears of litigation.
End-of-life decisions
Several of the questions in the survey revolved around end-of-life decisions, and in some cases, the differences seen in just a decade were striking. One example concerned life support decisions in the context of a family’s choices.
Age also seemed to play a role in the 2020 answers to that question: Physicians younger than 45 were more likely (28%) to answer “yes” (that they would withdraw life support in that instance) than were those 45 and older (16%).
A critical care physician said, “If the family appears to have an underlying motivation that may not be in the patient’s best interest, I might be inclined to pursue a legal decision prior to withdrawing support.”
A cardiologist had a more pointed response to the question: “To me, that would be murder.”
Another example of how perspectives have changed over the past 10 years concerns whether physician-aided dying should be legal for terminally ill patients. The practice is now mandated by law in eight states and the District of Columbia, and it is mandated by court ruling in two additional states.
In 2010, 41% said “no.” That number dropped to 28% in 2020.
On legalization, a psychiatrist said, “Yes, when there is truly no hope and the quality of remaining life is too poor. We show more compassion to our sick animals than we do to our human population.”
Conversely, a neurologist answered, “No, I see younger physicians already becoming comfortable with the idea of deciding ASAP whether there is a reasonable chance of survival and then pressing for the right code status. This change would make things worse.”
Assisted death and incurable suffering
Far fewer physicians supported physician-assisted death for those who had years to live but faced incurable suffering: Thirty-seven percent said “yes,” 34% said “no,” and 29% said “it depends.”
However, support was significantly higher than it was just 2 years ago, in 2018, when only 27% supported the concept, the report authors noted.
“The shift reflects movements by many states to legalize assisted dying for the terminally ill,” Arthur Caplan, PhD, director of the division of medical ethics, New York University, said in the report. “Legalization has not been abused, so some doctors are more willing to press further beyond terminal illness as a trigger to suffering.”
Conversely, many more physicians (44% vs. 24% a decade ago) said they would provide life-sustaining therapy if the family requested it, even if the physician thought it was futile.
“Concerns over a malpractice lawsuit and potential negative patient/family online reviews are factors that play into this change,” the survey authors wrote.
Shared decision making also increased in the past decade.
Would you undertreat pain?
Primary care physicians fear the consequences of what they consider adequate pain management more than specialists do (24% vs. 17%), the survey authors noted.
Ten years ago, Medscape asked physicians whether they would undertreat a patient’s pain because of fear of repercussions or the patient’s becoming addicted: Eighty-four percent said “no,” and 6% said “yes.” The rest said “it depends.”
In 2020, the question was asked slightly differently: “Would you undertreat a patient’s pain for fear of addiction or Drug Enforcement Administration or medical board scrutiny?” This year, three times as many said “yes” (18%); 63% said “no.”
“Respondents this year talked about investigations and reprimands by medical boards, and how much they wanted to avoid that,” the survey authors wrote.
Should physicians be required to treat COVID-19 patients?
Some questions were new this year, including one on whether physicians should be required to treat COVID-19 patients. Fewer than half (47%) answered “yes,” 24% said “no,” and 29% answered “it depends.”
Doctors’ answers to this question differed slightly by gender: Fifty percent of men and 43% of women said “yes.” In their responses, many physicians said consideration should be given to risk factors, such as age, underlying conditions, risk of family members, and availability of personal protective equipment (PPE).
Another pandemic-related question asked whether physicians felt they should correct physicians who post misinformation about the pandemic on social media. Half (50%) said “yes,” 19% said “no,” and 31% said “it depends.”
Speaking out against the workplace
This year, many physicians have felt betrayed when they didn’t have adequate PPE during the pandemic.
Asked, “Is it right to speak out against your hospital or workplace when they don’t give you what you need?” 53% of physicians said “yes,” 8% said “no,” and 40% said “it depends.”
A cardiologist made the value judgment this way: “Speaking out just because you had an argument with your boss is inappropriate. Bringing to the public repeated failures to correct situations that have been brought through the proper channels is necessary to incite change.”
Random drug testing for physicians?
Another question in the survey asked whether physicians should be subjected to random drug testing for alcohol and drug abuse. About one-third (34%) said yes, 43% said no, and 23% said “it depends.” A study found that between 10% and 15% of physicians have abused a substance at some point in their careers.
The subject continues to hit a nerve in medicine.
A family physician wrote, “This should not be done unless a particular physician had a problem with drug or alcohol abuse and shows signs of impairment.”
An internist took a different view, saying, “Military service men and women, police, firefighters, airline pilots, and other professions that have responsibilities affecting people’s lives are subject to testing; why not physicians?”
A version of this article originally appeared on Medscape.com.
Much has changed in the 10 years since Medscape’s first survey on what physicians would do when faced with painful choices in patient care.
A new report, Ethics 2020: Life, Death, and Painful Dilemmas, shows that physicians’ value judgments have shifted in many respects, sometimes as a result of increased regulations and fears of litigation.
End-of-life decisions
Several of the questions in the survey revolved around end-of-life decisions, and in some cases, the differences seen in just a decade were striking. One example concerned life support decisions in the context of a family’s choices.
Age also seemed to play a role in the 2020 answers to that question: Physicians younger than 45 were more likely (28%) to answer “yes” (that they would withdraw life support in that instance) than were those 45 and older (16%).
A critical care physician said, “If the family appears to have an underlying motivation that may not be in the patient’s best interest, I might be inclined to pursue a legal decision prior to withdrawing support.”
A cardiologist had a more pointed response to the question: “To me, that would be murder.”
Another example of how perspectives have changed over the past 10 years concerns whether physician-aided dying should be legal for terminally ill patients. The practice is now mandated by law in eight states and the District of Columbia, and it is mandated by court ruling in two additional states.
In 2010, 41% said “no.” That number dropped to 28% in 2020.
On legalization, a psychiatrist said, “Yes, when there is truly no hope and the quality of remaining life is too poor. We show more compassion to our sick animals than we do to our human population.”
Conversely, a neurologist answered, “No, I see younger physicians already becoming comfortable with the idea of deciding ASAP whether there is a reasonable chance of survival and then pressing for the right code status. This change would make things worse.”
Assisted death and incurable suffering
Far fewer physicians supported physician-assisted death for those who had years to live but faced incurable suffering: Thirty-seven percent said “yes,” 34% said “no,” and 29% said “it depends.”
However, support was significantly higher than it was just 2 years ago, in 2018, when only 27% supported the concept, the report authors noted.
“The shift reflects movements by many states to legalize assisted dying for the terminally ill,” Arthur Caplan, PhD, director of the division of medical ethics, New York University, said in the report. “Legalization has not been abused, so some doctors are more willing to press further beyond terminal illness as a trigger to suffering.”
Conversely, many more physicians (44% vs. 24% a decade ago) said they would provide life-sustaining therapy if the family requested it, even if the physician thought it was futile.
“Concerns over a malpractice lawsuit and potential negative patient/family online reviews are factors that play into this change,” the survey authors wrote.
Shared decision making also increased in the past decade.
Would you undertreat pain?
Primary care physicians fear the consequences of what they consider adequate pain management more than specialists do (24% vs. 17%), the survey authors noted.
Ten years ago, Medscape asked physicians whether they would undertreat a patient’s pain because of fear of repercussions or the patient’s becoming addicted: Eighty-four percent said “no,” and 6% said “yes.” The rest said “it depends.”
In 2020, the question was asked slightly differently: “Would you undertreat a patient’s pain for fear of addiction or Drug Enforcement Administration or medical board scrutiny?” This year, three times as many said “yes” (18%); 63% said “no.”
“Respondents this year talked about investigations and reprimands by medical boards, and how much they wanted to avoid that,” the survey authors wrote.
Should physicians be required to treat COVID-19 patients?
Some questions were new this year, including one on whether physicians should be required to treat COVID-19 patients. Fewer than half (47%) answered “yes,” 24% said “no,” and 29% answered “it depends.”
Doctors’ answers to this question differed slightly by gender: Fifty percent of men and 43% of women said “yes.” In their responses, many physicians said consideration should be given to risk factors, such as age, underlying conditions, risk of family members, and availability of personal protective equipment (PPE).
Another pandemic-related question asked whether physicians felt they should correct physicians who post misinformation about the pandemic on social media. Half (50%) said “yes,” 19% said “no,” and 31% said “it depends.”
Speaking out against the workplace
This year, many physicians have felt betrayed when they didn’t have adequate PPE during the pandemic.
Asked, “Is it right to speak out against your hospital or workplace when they don’t give you what you need?” 53% of physicians said “yes,” 8% said “no,” and 40% said “it depends.”
A cardiologist made the value judgment this way: “Speaking out just because you had an argument with your boss is inappropriate. Bringing to the public repeated failures to correct situations that have been brought through the proper channels is necessary to incite change.”
Random drug testing for physicians?
Another question in the survey asked whether physicians should be subjected to random drug testing for alcohol and drug abuse. About one-third (34%) said yes, 43% said no, and 23% said “it depends.” A study found that between 10% and 15% of physicians have abused a substance at some point in their careers.
The subject continues to hit a nerve in medicine.
A family physician wrote, “This should not be done unless a particular physician had a problem with drug or alcohol abuse and shows signs of impairment.”
An internist took a different view, saying, “Military service men and women, police, firefighters, airline pilots, and other professions that have responsibilities affecting people’s lives are subject to testing; why not physicians?”
A version of this article originally appeared on Medscape.com.




