User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
One-third of critical illness survivors emerge from ICU with functional deterioration
More patients are surviving critical illnesses requiring ICU care but many emerge with physical debility that may or may not eventually resolve.
Over the past decade, functional status deterioration after critical illness has become more common and of greater magnitude, despite concurrent efforts to reduce post–intensive care syndrome, based on a retrospective analysis of more than 100,000 patients.
Almost one-third of patients who survived nonsurgical ICU admission had evidence of functional status decline, reported lead author Nicholas E. Ingraham, MD, of the University of Minnesota, Minneapolis, and colleagues.
“Increasing capacity and decreasing mortality have created an evolving and diverse population of ICU survivors,” the investigators wrote in Critical Care Medicine. “Today’s survivors of critical illness are increasingly burdened by extensive physical and psychological comorbidities, often resulting in reduced quality of life.”
To determine trends in post–intensive care syndrome from 2008 to 2016, Dr. Ingraham and colleagues analyzed data from the Cerner Acute Physiology and Chronic Health Evaluation outcomes database, a national prospective cohort. Out of 202,786 adult patients admitted to the ICU, 129,917 were eligible for the study. Patients were excluded because of surgical admission, death, lack of functional status documentation, or inadequate hospital size or duration of participation. The final dataset had a median age of 63 years, with a slight predominance of male patients (54.0%). Most patients (80.9%) were White.
The primary outcome was defined as presence or absence of functional status deterioration, based on functional status at admission versus time of discharge. The secondary outcome was magnitude of deterioration over time.
The analysis, which controlled for age and severity of illness, revealed concerning trends for both outcomes.
Across the entire cohort 38,116 patients (29.3%) had functional status deterioration, with a 15% increase in prevalence over the course of the decade that spanned all disease categories (prevalence rate ratio, 1.15; 95% confidence interval, 1.13-1.17; P < .001). The magnitude of functional status decline also increased by 4% (odds ratio, 1.04; P < .001), with all but nonsurgical trauma patients showing greater deterioration over time.
“However, despite the decreasing magnitude of functional status deterioration in nonsurgical trauma, many admission diagnoses in this category remain in the top quartile of higher risk for functional status deterioration,” the investigators noted.
Functional status decline was most common among patients with head and polytrauma (OR, 3.39), followed closely by chest and spine trauma (OR, 3.38), and spine trauma (OR, 3.19). The top quartile of categories for prevalence of deterioration included nonsurgical trauma, neurologic, pulmonary, and gastrointestinal diseases.
Functional status decline was least common among patients diagnosed with diabetic ketoacidosis (OR, 0.27) or asthma (OR, 0.35).
“We believe our study provides important information that can be used in beginning to identify patients at high risk of functional status decline,” the investigators concluded. “Improving the identification of these patients and targeting appropriate interventions to mitigate this decline will be important directions for future studies in this area.”
According to David L. Bowton, MD, FCCP, professor emeritus, section on critical care, Wake Forest Baptist Health, Winston-Salem, N.C., the findings show just how common functional decline is after critical illness, and may actually underestimate prevalence.
“Because the authors employed a course evaluation tool employing only three categories of ability/disability and abstracted the level of disability from the medical record, they likely underestimated the frequency of clinically important, though not detected, disability at the time of hospital discharge,” Dr. Bowton said. “The study did not address cognitive impairment which can be detected in half of patients at 3 months following critical illness, and which significantly affects patients’ quality of life (Am J Respir Crit Care Med. 2020;202[2]:193-201).”
Dr. Bowton suggested that evidence-based methods of preventing post–intensive care syndrome are limited.
“Current efforts to improve post-ICU functional and cognitive outcomes suffer from the lack of proven effective interventions (Crit Care Med. 2019;47[11]:1607-18),” he said. “Observational data indicates that compliance with the ABCDEF bundle decreases the duration and incidence of delirium, ICU length of stay, duration of mechanical ventilation, and mortality (Crit Care Med. 2019;47[1]:3-14). However, the implications of these improvements on postdischarge functional outcomes are unknown as area the relative importance of individual elements of the bundle. Early mobility and patient and family diaries appear to improve functional status at discharge and postdischarge anxiety and depression, though the evidence supporting this is thin.”
Appropriate intervention may be especially challenging during the COVID-19 pandemic, he added.
“The impact of COVID on ICU staffing adequacy and stress is significant and the impact on quality bundle compliance and the availability of support services is currently not clear, but likely to be detrimental, especially to support services such as physical therapy that are already commonly understaffed,” Dr. Bowton said.
The study was supported by grants from the University of Minnesota’s Critical Care Research and Programmatic Development Program; the National Heart, Lung, and Blood Institute; and the University of Minnesota Clinical and Translational Science via the National Center for Advancing Translational Sciences. The investigators reported financial relationships with no other relevant organizations. Dr. Bowton reported no conflicts of interest.
SOURCE: Ingraham NE et al. Crit Care Med. 2020 Nov. doi: 10.1097/CCM.0000000000004524.
More patients are surviving critical illnesses requiring ICU care but many emerge with physical debility that may or may not eventually resolve.
Over the past decade, functional status deterioration after critical illness has become more common and of greater magnitude, despite concurrent efforts to reduce post–intensive care syndrome, based on a retrospective analysis of more than 100,000 patients.
Almost one-third of patients who survived nonsurgical ICU admission had evidence of functional status decline, reported lead author Nicholas E. Ingraham, MD, of the University of Minnesota, Minneapolis, and colleagues.
“Increasing capacity and decreasing mortality have created an evolving and diverse population of ICU survivors,” the investigators wrote in Critical Care Medicine. “Today’s survivors of critical illness are increasingly burdened by extensive physical and psychological comorbidities, often resulting in reduced quality of life.”
To determine trends in post–intensive care syndrome from 2008 to 2016, Dr. Ingraham and colleagues analyzed data from the Cerner Acute Physiology and Chronic Health Evaluation outcomes database, a national prospective cohort. Out of 202,786 adult patients admitted to the ICU, 129,917 were eligible for the study. Patients were excluded because of surgical admission, death, lack of functional status documentation, or inadequate hospital size or duration of participation. The final dataset had a median age of 63 years, with a slight predominance of male patients (54.0%). Most patients (80.9%) were White.
The primary outcome was defined as presence or absence of functional status deterioration, based on functional status at admission versus time of discharge. The secondary outcome was magnitude of deterioration over time.
The analysis, which controlled for age and severity of illness, revealed concerning trends for both outcomes.
Across the entire cohort 38,116 patients (29.3%) had functional status deterioration, with a 15% increase in prevalence over the course of the decade that spanned all disease categories (prevalence rate ratio, 1.15; 95% confidence interval, 1.13-1.17; P < .001). The magnitude of functional status decline also increased by 4% (odds ratio, 1.04; P < .001), with all but nonsurgical trauma patients showing greater deterioration over time.
“However, despite the decreasing magnitude of functional status deterioration in nonsurgical trauma, many admission diagnoses in this category remain in the top quartile of higher risk for functional status deterioration,” the investigators noted.
Functional status decline was most common among patients with head and polytrauma (OR, 3.39), followed closely by chest and spine trauma (OR, 3.38), and spine trauma (OR, 3.19). The top quartile of categories for prevalence of deterioration included nonsurgical trauma, neurologic, pulmonary, and gastrointestinal diseases.
Functional status decline was least common among patients diagnosed with diabetic ketoacidosis (OR, 0.27) or asthma (OR, 0.35).
“We believe our study provides important information that can be used in beginning to identify patients at high risk of functional status decline,” the investigators concluded. “Improving the identification of these patients and targeting appropriate interventions to mitigate this decline will be important directions for future studies in this area.”
According to David L. Bowton, MD, FCCP, professor emeritus, section on critical care, Wake Forest Baptist Health, Winston-Salem, N.C., the findings show just how common functional decline is after critical illness, and may actually underestimate prevalence.
“Because the authors employed a course evaluation tool employing only three categories of ability/disability and abstracted the level of disability from the medical record, they likely underestimated the frequency of clinically important, though not detected, disability at the time of hospital discharge,” Dr. Bowton said. “The study did not address cognitive impairment which can be detected in half of patients at 3 months following critical illness, and which significantly affects patients’ quality of life (Am J Respir Crit Care Med. 2020;202[2]:193-201).”
Dr. Bowton suggested that evidence-based methods of preventing post–intensive care syndrome are limited.
“Current efforts to improve post-ICU functional and cognitive outcomes suffer from the lack of proven effective interventions (Crit Care Med. 2019;47[11]:1607-18),” he said. “Observational data indicates that compliance with the ABCDEF bundle decreases the duration and incidence of delirium, ICU length of stay, duration of mechanical ventilation, and mortality (Crit Care Med. 2019;47[1]:3-14). However, the implications of these improvements on postdischarge functional outcomes are unknown as area the relative importance of individual elements of the bundle. Early mobility and patient and family diaries appear to improve functional status at discharge and postdischarge anxiety and depression, though the evidence supporting this is thin.”
Appropriate intervention may be especially challenging during the COVID-19 pandemic, he added.
“The impact of COVID on ICU staffing adequacy and stress is significant and the impact on quality bundle compliance and the availability of support services is currently not clear, but likely to be detrimental, especially to support services such as physical therapy that are already commonly understaffed,” Dr. Bowton said.
The study was supported by grants from the University of Minnesota’s Critical Care Research and Programmatic Development Program; the National Heart, Lung, and Blood Institute; and the University of Minnesota Clinical and Translational Science via the National Center for Advancing Translational Sciences. The investigators reported financial relationships with no other relevant organizations. Dr. Bowton reported no conflicts of interest.
SOURCE: Ingraham NE et al. Crit Care Med. 2020 Nov. doi: 10.1097/CCM.0000000000004524.
More patients are surviving critical illnesses requiring ICU care but many emerge with physical debility that may or may not eventually resolve.
Over the past decade, functional status deterioration after critical illness has become more common and of greater magnitude, despite concurrent efforts to reduce post–intensive care syndrome, based on a retrospective analysis of more than 100,000 patients.
Almost one-third of patients who survived nonsurgical ICU admission had evidence of functional status decline, reported lead author Nicholas E. Ingraham, MD, of the University of Minnesota, Minneapolis, and colleagues.
“Increasing capacity and decreasing mortality have created an evolving and diverse population of ICU survivors,” the investigators wrote in Critical Care Medicine. “Today’s survivors of critical illness are increasingly burdened by extensive physical and psychological comorbidities, often resulting in reduced quality of life.”
To determine trends in post–intensive care syndrome from 2008 to 2016, Dr. Ingraham and colleagues analyzed data from the Cerner Acute Physiology and Chronic Health Evaluation outcomes database, a national prospective cohort. Out of 202,786 adult patients admitted to the ICU, 129,917 were eligible for the study. Patients were excluded because of surgical admission, death, lack of functional status documentation, or inadequate hospital size or duration of participation. The final dataset had a median age of 63 years, with a slight predominance of male patients (54.0%). Most patients (80.9%) were White.
The primary outcome was defined as presence or absence of functional status deterioration, based on functional status at admission versus time of discharge. The secondary outcome was magnitude of deterioration over time.
The analysis, which controlled for age and severity of illness, revealed concerning trends for both outcomes.
Across the entire cohort 38,116 patients (29.3%) had functional status deterioration, with a 15% increase in prevalence over the course of the decade that spanned all disease categories (prevalence rate ratio, 1.15; 95% confidence interval, 1.13-1.17; P < .001). The magnitude of functional status decline also increased by 4% (odds ratio, 1.04; P < .001), with all but nonsurgical trauma patients showing greater deterioration over time.
“However, despite the decreasing magnitude of functional status deterioration in nonsurgical trauma, many admission diagnoses in this category remain in the top quartile of higher risk for functional status deterioration,” the investigators noted.
Functional status decline was most common among patients with head and polytrauma (OR, 3.39), followed closely by chest and spine trauma (OR, 3.38), and spine trauma (OR, 3.19). The top quartile of categories for prevalence of deterioration included nonsurgical trauma, neurologic, pulmonary, and gastrointestinal diseases.
Functional status decline was least common among patients diagnosed with diabetic ketoacidosis (OR, 0.27) or asthma (OR, 0.35).
“We believe our study provides important information that can be used in beginning to identify patients at high risk of functional status decline,” the investigators concluded. “Improving the identification of these patients and targeting appropriate interventions to mitigate this decline will be important directions for future studies in this area.”
According to David L. Bowton, MD, FCCP, professor emeritus, section on critical care, Wake Forest Baptist Health, Winston-Salem, N.C., the findings show just how common functional decline is after critical illness, and may actually underestimate prevalence.
“Because the authors employed a course evaluation tool employing only three categories of ability/disability and abstracted the level of disability from the medical record, they likely underestimated the frequency of clinically important, though not detected, disability at the time of hospital discharge,” Dr. Bowton said. “The study did not address cognitive impairment which can be detected in half of patients at 3 months following critical illness, and which significantly affects patients’ quality of life (Am J Respir Crit Care Med. 2020;202[2]:193-201).”
Dr. Bowton suggested that evidence-based methods of preventing post–intensive care syndrome are limited.
“Current efforts to improve post-ICU functional and cognitive outcomes suffer from the lack of proven effective interventions (Crit Care Med. 2019;47[11]:1607-18),” he said. “Observational data indicates that compliance with the ABCDEF bundle decreases the duration and incidence of delirium, ICU length of stay, duration of mechanical ventilation, and mortality (Crit Care Med. 2019;47[1]:3-14). However, the implications of these improvements on postdischarge functional outcomes are unknown as area the relative importance of individual elements of the bundle. Early mobility and patient and family diaries appear to improve functional status at discharge and postdischarge anxiety and depression, though the evidence supporting this is thin.”
Appropriate intervention may be especially challenging during the COVID-19 pandemic, he added.
“The impact of COVID on ICU staffing adequacy and stress is significant and the impact on quality bundle compliance and the availability of support services is currently not clear, but likely to be detrimental, especially to support services such as physical therapy that are already commonly understaffed,” Dr. Bowton said.
The study was supported by grants from the University of Minnesota’s Critical Care Research and Programmatic Development Program; the National Heart, Lung, and Blood Institute; and the University of Minnesota Clinical and Translational Science via the National Center for Advancing Translational Sciences. The investigators reported financial relationships with no other relevant organizations. Dr. Bowton reported no conflicts of interest.
SOURCE: Ingraham NE et al. Crit Care Med. 2020 Nov. doi: 10.1097/CCM.0000000000004524.
FROM CRITICAL CARE MEDICINE
COVID-19 burdens follow patients after discharge
COVID-19 patients who survive their hospitalization don’t leave the disease behind upon discharge, as a significant percentage died within 60 days of discharge, with an ICU admission heightening the risk, according to an observational study of 38 Michigan hospitals. What’s more, many of them were burdened with health and emotional challenges ranging from hospital readmission to job loss and financial problems.
“These data confirm that the toll of COVID-19 extends well beyond hospitalization, a finding consistent with long-term sequelae from sepsis and other severe respiratory viral illnesses,” wrote lead author Vineet Chopra, MBBS, of the University of Michigan, Ann Arbor, and colleagues (Ann Intern Med. 2020 Nov 11: doi: 10.7326/M20-5661)
The researchers found that 29.2% of all patients hospitalized for COVID-19 from March 16 to July 1 died. The observational cohort study included 1,648 COVID-19 patients hospitalized at 38 Michigan hospitals participating in a statewide collaborative.
The bulk of those deaths occurred during hospitalization: 24.2% of patients (n = 398). Of the 1,250 patients discharged, 78% (n = 975) went home and 12.6% (n = 158) went to a skilled nursing facility, with the remainder unaccounted for. Within 60 days of discharge, 6.7% (n = 84) of hospitalized survivors had died and 15.2% (n = 189) were readmitted. The researchers gathered 60-day postdischarge data via a telephone survey, contacting 41.8% (n = 488) of discharged patients.
Outcomes were even worse for discharged patients who spent time in the ICU. The death rate among this group was 10.4% (17 of 165) after discharge. That resulted in an overall study death rate of 63.5% (n = 257) for the 405 patients who were in the ICU.
While the study data were in the first wave of the novel coronavirus, the findings have relevance today, said Mary Jo Farmer, MD, PhD, FCCP, directory of pulmonary hypertension services at Baystate Health in Springfield, Mass.
“This is the best information we have to date,” she said. “We have to continue to have an open mind and expect that this information may change as the virus possibly mutates as it spreads, and we should continue doing these types of outcomes studies at 90 days, 120 days, etc.”
The median age of study patients was 62, with a range of 50-72. The three leading comorbidities among discharged patients were hypertension (n = 800, 64%), diabetes (34.9%, n = 436), and cardiovascular disease (24.1%, n = 301).
Poor postdischarge outcomes weren’t limited to mortality and readmission. Almost 19% (n = 92) reported new or worsening cardiopulmonary symptoms such as cough and dyspnea, 13.3% had a persistent loss of taste or smell, and 12% (n = 58) reported more difficulty with daily living tasks.
The after-effects were not only physical. Nearly half of discharged patients (48.7%, n = 238) reported emotional effects and almost 6% (n = 28) sought mental health care. Among the 40% (n = 195) employed before they were hospitalized, 36% (n = 78) couldn’t return to work because of health issues or layoffs. Sixty percent (n = 117) of the pre-employed discharged patients did return to work, but 25% (n = 30) did so with reduced hours or modified job duties because of health problems.
Financial problems were also a burden. More than a third, 36.7% (n = 179), reported some financial impact from their hospitalization. About 10% (n = 47) said they used most or all of their savings, and 7% (n = 35) said they resorted to rationing necessities such as food or medications.
The researchers noted that one in five patients had no primary care follow-up at 2 months post discharge. “Collectively, these findings suggest that better models to support COVID-19 survivors are necessary,” said Dr. Chopra and colleagues.
The postdischarge course for patients involves two humps, said Sachin Gupta, MD, FCCP a pulmonary and critical care specialist at Alameda Health System in Oakland, Calif.: Getting over the hospitalization itself and the recovery phase. “As you look at the median age of the survivors, elderly patients who survive a hospital stay are still going to have a period of recovery, and like any viral illness that leads to someone being hospitalized, when you have an elderly patient with comorbidities, not all of them can make it over that final hump.”
He echoed the study authors’ call for better postdischarge support for COVID-19 patients. “There’s typically, although not at every hospital, a one-size-fits-all discharge planning process,” Dr. Gupta said. “For older patients, particularly with comorbid conditions, close follow-up after discharge is important.”
Dr. Farmer noted that one challenge in discharge support may be a matter of personnel. “The providers of this care might be fearful of patients who have had COVID-19 – Do the patients remain contagious? What if symptoms of COVID-19 return such as dry cough, fever? – and of contracting the disease themselves,” she said.
The findings regarding the emotional status of discharged patients should factor into discharge planning, she added. “Providers of posthospital care need to be educated in the emotional impact of this disease (e.g., the patients may feel ostracized or that no one wants to be around them) to assist in their recovery.”
Dr. Chopra and Dr. Farmer have no financial relationships to disclose. Dr. Gupta is an employee and shareholder of Genentech.
SOURCE: Chopra V et al. Ann Intern Med. 2020 Nov 11. doi: 10.7326/M20-5661.
COVID-19 patients who survive their hospitalization don’t leave the disease behind upon discharge, as a significant percentage died within 60 days of discharge, with an ICU admission heightening the risk, according to an observational study of 38 Michigan hospitals. What’s more, many of them were burdened with health and emotional challenges ranging from hospital readmission to job loss and financial problems.
“These data confirm that the toll of COVID-19 extends well beyond hospitalization, a finding consistent with long-term sequelae from sepsis and other severe respiratory viral illnesses,” wrote lead author Vineet Chopra, MBBS, of the University of Michigan, Ann Arbor, and colleagues (Ann Intern Med. 2020 Nov 11: doi: 10.7326/M20-5661)
The researchers found that 29.2% of all patients hospitalized for COVID-19 from March 16 to July 1 died. The observational cohort study included 1,648 COVID-19 patients hospitalized at 38 Michigan hospitals participating in a statewide collaborative.
The bulk of those deaths occurred during hospitalization: 24.2% of patients (n = 398). Of the 1,250 patients discharged, 78% (n = 975) went home and 12.6% (n = 158) went to a skilled nursing facility, with the remainder unaccounted for. Within 60 days of discharge, 6.7% (n = 84) of hospitalized survivors had died and 15.2% (n = 189) were readmitted. The researchers gathered 60-day postdischarge data via a telephone survey, contacting 41.8% (n = 488) of discharged patients.
Outcomes were even worse for discharged patients who spent time in the ICU. The death rate among this group was 10.4% (17 of 165) after discharge. That resulted in an overall study death rate of 63.5% (n = 257) for the 405 patients who were in the ICU.
While the study data were in the first wave of the novel coronavirus, the findings have relevance today, said Mary Jo Farmer, MD, PhD, FCCP, directory of pulmonary hypertension services at Baystate Health in Springfield, Mass.
“This is the best information we have to date,” she said. “We have to continue to have an open mind and expect that this information may change as the virus possibly mutates as it spreads, and we should continue doing these types of outcomes studies at 90 days, 120 days, etc.”
The median age of study patients was 62, with a range of 50-72. The three leading comorbidities among discharged patients were hypertension (n = 800, 64%), diabetes (34.9%, n = 436), and cardiovascular disease (24.1%, n = 301).
Poor postdischarge outcomes weren’t limited to mortality and readmission. Almost 19% (n = 92) reported new or worsening cardiopulmonary symptoms such as cough and dyspnea, 13.3% had a persistent loss of taste or smell, and 12% (n = 58) reported more difficulty with daily living tasks.
The after-effects were not only physical. Nearly half of discharged patients (48.7%, n = 238) reported emotional effects and almost 6% (n = 28) sought mental health care. Among the 40% (n = 195) employed before they were hospitalized, 36% (n = 78) couldn’t return to work because of health issues or layoffs. Sixty percent (n = 117) of the pre-employed discharged patients did return to work, but 25% (n = 30) did so with reduced hours or modified job duties because of health problems.
Financial problems were also a burden. More than a third, 36.7% (n = 179), reported some financial impact from their hospitalization. About 10% (n = 47) said they used most or all of their savings, and 7% (n = 35) said they resorted to rationing necessities such as food or medications.
The researchers noted that one in five patients had no primary care follow-up at 2 months post discharge. “Collectively, these findings suggest that better models to support COVID-19 survivors are necessary,” said Dr. Chopra and colleagues.
The postdischarge course for patients involves two humps, said Sachin Gupta, MD, FCCP a pulmonary and critical care specialist at Alameda Health System in Oakland, Calif.: Getting over the hospitalization itself and the recovery phase. “As you look at the median age of the survivors, elderly patients who survive a hospital stay are still going to have a period of recovery, and like any viral illness that leads to someone being hospitalized, when you have an elderly patient with comorbidities, not all of them can make it over that final hump.”
He echoed the study authors’ call for better postdischarge support for COVID-19 patients. “There’s typically, although not at every hospital, a one-size-fits-all discharge planning process,” Dr. Gupta said. “For older patients, particularly with comorbid conditions, close follow-up after discharge is important.”
Dr. Farmer noted that one challenge in discharge support may be a matter of personnel. “The providers of this care might be fearful of patients who have had COVID-19 – Do the patients remain contagious? What if symptoms of COVID-19 return such as dry cough, fever? – and of contracting the disease themselves,” she said.
The findings regarding the emotional status of discharged patients should factor into discharge planning, she added. “Providers of posthospital care need to be educated in the emotional impact of this disease (e.g., the patients may feel ostracized or that no one wants to be around them) to assist in their recovery.”
Dr. Chopra and Dr. Farmer have no financial relationships to disclose. Dr. Gupta is an employee and shareholder of Genentech.
SOURCE: Chopra V et al. Ann Intern Med. 2020 Nov 11. doi: 10.7326/M20-5661.
COVID-19 patients who survive their hospitalization don’t leave the disease behind upon discharge, as a significant percentage died within 60 days of discharge, with an ICU admission heightening the risk, according to an observational study of 38 Michigan hospitals. What’s more, many of them were burdened with health and emotional challenges ranging from hospital readmission to job loss and financial problems.
“These data confirm that the toll of COVID-19 extends well beyond hospitalization, a finding consistent with long-term sequelae from sepsis and other severe respiratory viral illnesses,” wrote lead author Vineet Chopra, MBBS, of the University of Michigan, Ann Arbor, and colleagues (Ann Intern Med. 2020 Nov 11: doi: 10.7326/M20-5661)
The researchers found that 29.2% of all patients hospitalized for COVID-19 from March 16 to July 1 died. The observational cohort study included 1,648 COVID-19 patients hospitalized at 38 Michigan hospitals participating in a statewide collaborative.
The bulk of those deaths occurred during hospitalization: 24.2% of patients (n = 398). Of the 1,250 patients discharged, 78% (n = 975) went home and 12.6% (n = 158) went to a skilled nursing facility, with the remainder unaccounted for. Within 60 days of discharge, 6.7% (n = 84) of hospitalized survivors had died and 15.2% (n = 189) were readmitted. The researchers gathered 60-day postdischarge data via a telephone survey, contacting 41.8% (n = 488) of discharged patients.
Outcomes were even worse for discharged patients who spent time in the ICU. The death rate among this group was 10.4% (17 of 165) after discharge. That resulted in an overall study death rate of 63.5% (n = 257) for the 405 patients who were in the ICU.
While the study data were in the first wave of the novel coronavirus, the findings have relevance today, said Mary Jo Farmer, MD, PhD, FCCP, directory of pulmonary hypertension services at Baystate Health in Springfield, Mass.
“This is the best information we have to date,” she said. “We have to continue to have an open mind and expect that this information may change as the virus possibly mutates as it spreads, and we should continue doing these types of outcomes studies at 90 days, 120 days, etc.”
The median age of study patients was 62, with a range of 50-72. The three leading comorbidities among discharged patients were hypertension (n = 800, 64%), diabetes (34.9%, n = 436), and cardiovascular disease (24.1%, n = 301).
Poor postdischarge outcomes weren’t limited to mortality and readmission. Almost 19% (n = 92) reported new or worsening cardiopulmonary symptoms such as cough and dyspnea, 13.3% had a persistent loss of taste or smell, and 12% (n = 58) reported more difficulty with daily living tasks.
The after-effects were not only physical. Nearly half of discharged patients (48.7%, n = 238) reported emotional effects and almost 6% (n = 28) sought mental health care. Among the 40% (n = 195) employed before they were hospitalized, 36% (n = 78) couldn’t return to work because of health issues or layoffs. Sixty percent (n = 117) of the pre-employed discharged patients did return to work, but 25% (n = 30) did so with reduced hours or modified job duties because of health problems.
Financial problems were also a burden. More than a third, 36.7% (n = 179), reported some financial impact from their hospitalization. About 10% (n = 47) said they used most or all of their savings, and 7% (n = 35) said they resorted to rationing necessities such as food or medications.
The researchers noted that one in five patients had no primary care follow-up at 2 months post discharge. “Collectively, these findings suggest that better models to support COVID-19 survivors are necessary,” said Dr. Chopra and colleagues.
The postdischarge course for patients involves two humps, said Sachin Gupta, MD, FCCP a pulmonary and critical care specialist at Alameda Health System in Oakland, Calif.: Getting over the hospitalization itself and the recovery phase. “As you look at the median age of the survivors, elderly patients who survive a hospital stay are still going to have a period of recovery, and like any viral illness that leads to someone being hospitalized, when you have an elderly patient with comorbidities, not all of them can make it over that final hump.”
He echoed the study authors’ call for better postdischarge support for COVID-19 patients. “There’s typically, although not at every hospital, a one-size-fits-all discharge planning process,” Dr. Gupta said. “For older patients, particularly with comorbid conditions, close follow-up after discharge is important.”
Dr. Farmer noted that one challenge in discharge support may be a matter of personnel. “The providers of this care might be fearful of patients who have had COVID-19 – Do the patients remain contagious? What if symptoms of COVID-19 return such as dry cough, fever? – and of contracting the disease themselves,” she said.
The findings regarding the emotional status of discharged patients should factor into discharge planning, she added. “Providers of posthospital care need to be educated in the emotional impact of this disease (e.g., the patients may feel ostracized or that no one wants to be around them) to assist in their recovery.”
Dr. Chopra and Dr. Farmer have no financial relationships to disclose. Dr. Gupta is an employee and shareholder of Genentech.
SOURCE: Chopra V et al. Ann Intern Med. 2020 Nov 11. doi: 10.7326/M20-5661.
FROM ANNALS OF INTERNAL MEDICINE
Siblings of patients with bipolar disorder at increased risk
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
FROM ECNP 2020
Open enrollment 2021: A big start for HealthCare.gov
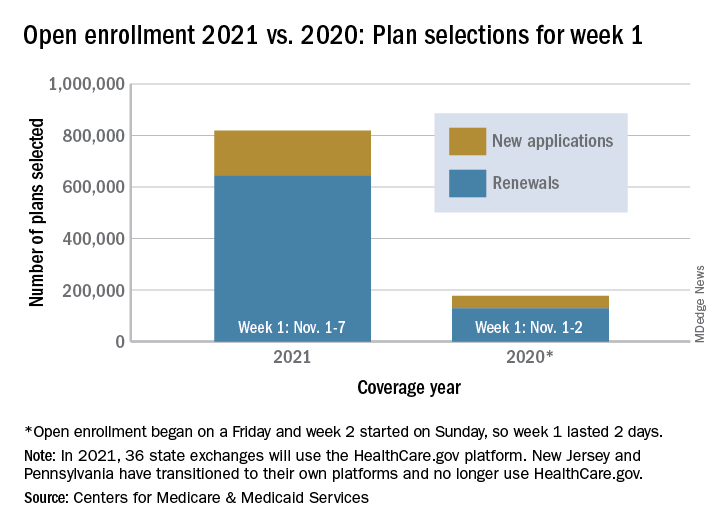
Over 818,000 plans were selected for the 2021 coverage year during the first week, Nov.1-7, of this year’s open enrollment on the federal health insurance exchange, according to the Centers for Medicare & Medicaid Services.
The bulk of those plans, nearly 79%, were renewals by consumers who had coverage through the federal exchange this year. The balance covers new plans selected by individuals who were not covered through HealthCare.gov this year, the CMS noted in a written statement.
The total enrollment for week 1 marks a considerable increase over last year’s first week of open enrollment, which saw approximately 177,000 plans selected, but Nov. 1 fell on a Friday in 2019, so that total represents only 2 days since weeks are tracked as running from Sunday to Saturday, the CMS explained.
For the 2021 benefit year, the HealthCare.gov platform covers 36 states, down from 38 for the 2020 benefit year, because New Jersey and Pennsylvania have “transitioned to their own state-based exchange platforms,” the CMS noted, adding that the two accounted for 7% of all plans selected last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS said, and its weekly snapshot “does not report the number of consumers who have paid premiums to effectuate their enrollment.”
This year’s open-enrollment period on HealthCare.gov is scheduled to conclude Dec. 15.
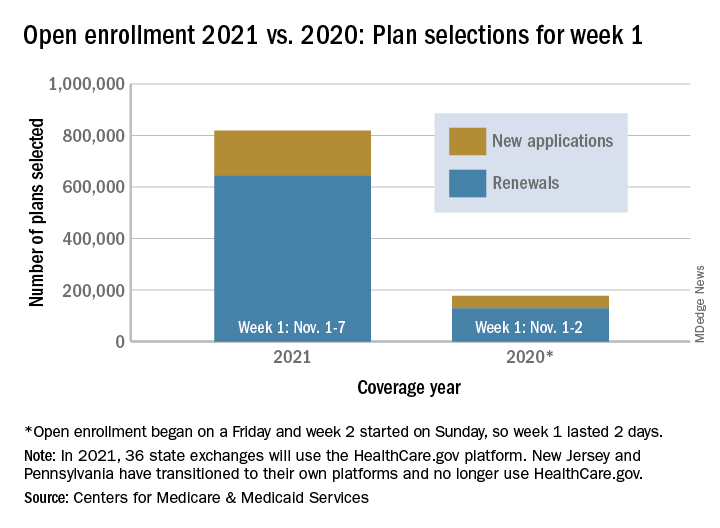
Over 818,000 plans were selected for the 2021 coverage year during the first week, Nov.1-7, of this year’s open enrollment on the federal health insurance exchange, according to the Centers for Medicare & Medicaid Services.

The bulk of those plans, nearly 79%, were renewals by consumers who had coverage through the federal exchange this year. The balance covers new plans selected by individuals who were not covered through HealthCare.gov this year, the CMS noted in a written statement.
The total enrollment for week 1 marks a considerable increase over last year’s first week of open enrollment, which saw approximately 177,000 plans selected, but Nov. 1 fell on a Friday in 2019, so that total represents only 2 days since weeks are tracked as running from Sunday to Saturday, the CMS explained.
For the 2021 benefit year, the HealthCare.gov platform covers 36 states, down from 38 for the 2020 benefit year, because New Jersey and Pennsylvania have “transitioned to their own state-based exchange platforms,” the CMS noted, adding that the two accounted for 7% of all plans selected last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS said, and its weekly snapshot “does not report the number of consumers who have paid premiums to effectuate their enrollment.”
This year’s open-enrollment period on HealthCare.gov is scheduled to conclude Dec. 15.
Over 818,000 plans were selected for the 2021 coverage year during the first week, Nov.1-7, of this year’s open enrollment on the federal health insurance exchange, according to the Centers for Medicare & Medicaid Services.

The bulk of those plans, nearly 79%, were renewals by consumers who had coverage through the federal exchange this year. The balance covers new plans selected by individuals who were not covered through HealthCare.gov this year, the CMS noted in a written statement.
The total enrollment for week 1 marks a considerable increase over last year’s first week of open enrollment, which saw approximately 177,000 plans selected, but Nov. 1 fell on a Friday in 2019, so that total represents only 2 days since weeks are tracked as running from Sunday to Saturday, the CMS explained.
For the 2021 benefit year, the HealthCare.gov platform covers 36 states, down from 38 for the 2020 benefit year, because New Jersey and Pennsylvania have “transitioned to their own state-based exchange platforms,” the CMS noted, adding that the two accounted for 7% of all plans selected last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS said, and its weekly snapshot “does not report the number of consumers who have paid premiums to effectuate their enrollment.”
This year’s open-enrollment period on HealthCare.gov is scheduled to conclude Dec. 15.
Practicing medicine without judgment
“What do you think of all this election stuff?” I froze. Sitting on the exam table was a 50-something-year-old woman. Her hair was long, but not gray. She was wearing a mask without distinctive markings, such as Trump lips or #BLM to identify the political leanings of the owner. She had a subtle New York accent, perhaps dating back to the Giuliani years. It was hard to know her intention. “It’s a trap!” I could hear Admiral Ackbar’s voice in my head. “Don’t engage.” We all know nothing erodes trust faster than showing your blue or red colors before you know which your patient identifies.
Instead, I replied that indeed it has been a stressful year for us all. Then I paused. She shifted a bit and tugged at the gown sleeves and admitted this was the most stress she felt in years. She was seeing me for lichen sclerosus et atrophicus, a terribly itchy, sometimes-disfiguring eruption that can occur in the vulva. She was dealing with COVID-19, kids, divorce, a new partner, working from home, parents, and now the election drama.
At this point in the visit, I knew I could help her. First, the treatment for lichen sclerosus is straightforward and mostly effective. Second, I knew I’d have 7 minutes to spare to just listen. It was a lucky break, as often no such gift of time presents itself while seeing patients in a busy clinic. We take vitals, history (typing), do an exam, make a diagnosis (more typing), and maybe a procedure (yet more typing). All of this is necessary, but sometimes not what our patient needs. Some really need just to connect and share their burden with someone who isn’t a friend or family. As physicians, we have a unique opportunity to see and hear people without judgment.
This reminds me of a recent episode from Sam Harris’s podcast, “Making Sense.” Mr. Harris, a philosopher (and “blue” all the way through) revealed his insight into Presidents Trump’s appeal. Leaving policy aside, Mr. Harris notes that people are drawn to the President because he never judges you. He is incapable of being sanctimonious, Mr. Harris argues, and therefore creates a safe space for people to continue their lives, however flawed, without expectation that they improve.
I’m unsure just how much of this theory explains the devotion of his supporters, but it resonated with me. We doctors are sanctimonious by nature. The better part of my day is spent prodding people to be better: Wear more sunscreen, exercise more, stop believing in conspiracy theories, get your flu shot, and above all, stop scratching! In doing so, I’m in a way judging them. Finger wagging: You’re lazy or poor or dumb or stubborn. “You aren’t as good as me,” is what they might feel after 15 minutes of my pep talk.
But what if that’s wrong? What if they are just fine exactly the way they are? Perhaps what my lichen sclerosis patient needs more than anything is unconditional attention? She, like most of our patients, is well aware of how her shortcomings might contribute to her own anxiety or difficulties. And now she has this rash and that’s probably somehow her fault too, she thinks.
How can I best help her? Betamethasone dipropionate b.i.d. for 2 weeks and spend the last 7 minutes just sitting and listening without judgment or advice. I don’t know who she wanted to win the election. It didn’t matter, she was exactly right to believe what she believed, either way.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“What do you think of all this election stuff?” I froze. Sitting on the exam table was a 50-something-year-old woman. Her hair was long, but not gray. She was wearing a mask without distinctive markings, such as Trump lips or #BLM to identify the political leanings of the owner. She had a subtle New York accent, perhaps dating back to the Giuliani years. It was hard to know her intention. “It’s a trap!” I could hear Admiral Ackbar’s voice in my head. “Don’t engage.” We all know nothing erodes trust faster than showing your blue or red colors before you know which your patient identifies.
Instead, I replied that indeed it has been a stressful year for us all. Then I paused. She shifted a bit and tugged at the gown sleeves and admitted this was the most stress she felt in years. She was seeing me for lichen sclerosus et atrophicus, a terribly itchy, sometimes-disfiguring eruption that can occur in the vulva. She was dealing with COVID-19, kids, divorce, a new partner, working from home, parents, and now the election drama.
At this point in the visit, I knew I could help her. First, the treatment for lichen sclerosus is straightforward and mostly effective. Second, I knew I’d have 7 minutes to spare to just listen. It was a lucky break, as often no such gift of time presents itself while seeing patients in a busy clinic. We take vitals, history (typing), do an exam, make a diagnosis (more typing), and maybe a procedure (yet more typing). All of this is necessary, but sometimes not what our patient needs. Some really need just to connect and share their burden with someone who isn’t a friend or family. As physicians, we have a unique opportunity to see and hear people without judgment.
This reminds me of a recent episode from Sam Harris’s podcast, “Making Sense.” Mr. Harris, a philosopher (and “blue” all the way through) revealed his insight into Presidents Trump’s appeal. Leaving policy aside, Mr. Harris notes that people are drawn to the President because he never judges you. He is incapable of being sanctimonious, Mr. Harris argues, and therefore creates a safe space for people to continue their lives, however flawed, without expectation that they improve.
I’m unsure just how much of this theory explains the devotion of his supporters, but it resonated with me. We doctors are sanctimonious by nature. The better part of my day is spent prodding people to be better: Wear more sunscreen, exercise more, stop believing in conspiracy theories, get your flu shot, and above all, stop scratching! In doing so, I’m in a way judging them. Finger wagging: You’re lazy or poor or dumb or stubborn. “You aren’t as good as me,” is what they might feel after 15 minutes of my pep talk.
But what if that’s wrong? What if they are just fine exactly the way they are? Perhaps what my lichen sclerosis patient needs more than anything is unconditional attention? She, like most of our patients, is well aware of how her shortcomings might contribute to her own anxiety or difficulties. And now she has this rash and that’s probably somehow her fault too, she thinks.
How can I best help her? Betamethasone dipropionate b.i.d. for 2 weeks and spend the last 7 minutes just sitting and listening without judgment or advice. I don’t know who she wanted to win the election. It didn’t matter, she was exactly right to believe what she believed, either way.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“What do you think of all this election stuff?” I froze. Sitting on the exam table was a 50-something-year-old woman. Her hair was long, but not gray. She was wearing a mask without distinctive markings, such as Trump lips or #BLM to identify the political leanings of the owner. She had a subtle New York accent, perhaps dating back to the Giuliani years. It was hard to know her intention. “It’s a trap!” I could hear Admiral Ackbar’s voice in my head. “Don’t engage.” We all know nothing erodes trust faster than showing your blue or red colors before you know which your patient identifies.
Instead, I replied that indeed it has been a stressful year for us all. Then I paused. She shifted a bit and tugged at the gown sleeves and admitted this was the most stress she felt in years. She was seeing me for lichen sclerosus et atrophicus, a terribly itchy, sometimes-disfiguring eruption that can occur in the vulva. She was dealing with COVID-19, kids, divorce, a new partner, working from home, parents, and now the election drama.
At this point in the visit, I knew I could help her. First, the treatment for lichen sclerosus is straightforward and mostly effective. Second, I knew I’d have 7 minutes to spare to just listen. It was a lucky break, as often no such gift of time presents itself while seeing patients in a busy clinic. We take vitals, history (typing), do an exam, make a diagnosis (more typing), and maybe a procedure (yet more typing). All of this is necessary, but sometimes not what our patient needs. Some really need just to connect and share their burden with someone who isn’t a friend or family. As physicians, we have a unique opportunity to see and hear people without judgment.
This reminds me of a recent episode from Sam Harris’s podcast, “Making Sense.” Mr. Harris, a philosopher (and “blue” all the way through) revealed his insight into Presidents Trump’s appeal. Leaving policy aside, Mr. Harris notes that people are drawn to the President because he never judges you. He is incapable of being sanctimonious, Mr. Harris argues, and therefore creates a safe space for people to continue their lives, however flawed, without expectation that they improve.
I’m unsure just how much of this theory explains the devotion of his supporters, but it resonated with me. We doctors are sanctimonious by nature. The better part of my day is spent prodding people to be better: Wear more sunscreen, exercise more, stop believing in conspiracy theories, get your flu shot, and above all, stop scratching! In doing so, I’m in a way judging them. Finger wagging: You’re lazy or poor or dumb or stubborn. “You aren’t as good as me,” is what they might feel after 15 minutes of my pep talk.
But what if that’s wrong? What if they are just fine exactly the way they are? Perhaps what my lichen sclerosis patient needs more than anything is unconditional attention? She, like most of our patients, is well aware of how her shortcomings might contribute to her own anxiety or difficulties. And now she has this rash and that’s probably somehow her fault too, she thinks.
How can I best help her? Betamethasone dipropionate b.i.d. for 2 weeks and spend the last 7 minutes just sitting and listening without judgment or advice. I don’t know who she wanted to win the election. It didn’t matter, she was exactly right to believe what she believed, either way.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Moderna: Interim data show 94.5% efficacy for COVID-19 vaccine, will seek FDA EUA
The Moderna mRNA-1273 vaccine, in development to prevent COVID-19, yielded 94.5% efficacy in early results and is generally well tolerated, the company announced early Monday. The product can be stored at refrigeration temperatures common to many physician offices, pharmacies, and hospitals.
The first interim results of the phase 3 COVE trial included 95 participants with confirmed COVID-19. An independent data safety monitoring board, which was appointed by the National Institutes of Health, informed Moderna that 90 of the patients who were positive for COVID-19 were in a placebo group and that 5 patients were in the mRNA-1273 vaccine group, resulting in a vaccine efficacy of 94.5% (P < .0001).
Interim data included 11 patients with severe COVID-19, all of whom were in the placebo group.
“This positive interim analysis from our phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease,” said Stéphane Bancel, CEO of Moderna, said in a statement.
The vaccine met its primary study endpoint, which was based on adjudicated data that were collected starting 2 weeks after the second dose of mRNA-1273. The interim study population included people who could be at higher risk for COVID-19, including 15 adults aged 65 years and older and 20 participants from diverse communities.
Safety data
The DSMB also reviewed safety data for the COVE study interim results. The vaccine was generally safe and well tolerated, as determined on the basis of solicited adverse events. Most adverse events were mild to moderate and were generally short-lived, according to a company news release.
Injection-site pain was reported in 2.7% of participants after the first dose. After the second dose, 9.7% of participants reported fatigue, 8.9% reported myalgia, 5.2% reported arthralgia, 4.5% reported headache, 4.1% reported pain, and 2.0% reported erythema or redness at the injection site.
Moderna plans to request emergency-use authorization (EUA) from the Food and Drug Administration in the coming weeks. The company expects that the EUA will be based on more data from the COVE study, including a final analysis of 151 patients with a median follow-up of more than 2 months. Moderna also plans to seek authorizations from global regulatory agencies.
The company expects to have approximately 20 million doses of mRNA-1273 ready to ship in the United States by the end of the year. In addition, the company says it remains on track to manufacture between 500 million and 1 billion doses globally in 2021.
Moderna is developing distribution plans in conjunction with the Centers for Disease Control and Prevention, the federal government’s Operation Warp Speed, and McKesson, a COVID-19 vaccine distributor contracted by the U.S. government.
Refrigeration requirements
The mRNA-1273 vaccine can be shipped and stored for up to 6 months at –20° C (about –4° F), a temperature maintained in most home or medical freezers, according to Moderna. The company expects that, after the product thaws, it will remain stable at standard refrigerator temperatures of 2°-8° C (36°-46° F) for up to 30 days within the 6-month shelf life.
Because the mRNA-1273 vaccine is stable at these refrigerator temperatures, it can be stored at most physicians’ offices, pharmacies, and hospitals, the company noted. In contrast, the similar Pfizer BTN162b2 vaccine – early results for which showed a 90% efficacy rate – requires shipment and storage at “deep-freeze” conditions of –70° C or –80° C, which is more challenging from a logistic point of view.
Moderna’s mRNA-1273 can be kept at room temperature for up to 12 hours after removal from a refrigerator for patient administration. The vaccine will not require dilution prior to use.
More than 30,000 people aged older than 18 years in the United States are enrolled in the COVE study. The research is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases and the Biomedical Advanced Research and Development Authority, part of the Office of the Assistant Secretary for Preparedness and Response at the Department of Health & Human Services.
A version of this article originally appeared on Medscape.com.
The Moderna mRNA-1273 vaccine, in development to prevent COVID-19, yielded 94.5% efficacy in early results and is generally well tolerated, the company announced early Monday. The product can be stored at refrigeration temperatures common to many physician offices, pharmacies, and hospitals.
The first interim results of the phase 3 COVE trial included 95 participants with confirmed COVID-19. An independent data safety monitoring board, which was appointed by the National Institutes of Health, informed Moderna that 90 of the patients who were positive for COVID-19 were in a placebo group and that 5 patients were in the mRNA-1273 vaccine group, resulting in a vaccine efficacy of 94.5% (P < .0001).
Interim data included 11 patients with severe COVID-19, all of whom were in the placebo group.
“This positive interim analysis from our phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease,” said Stéphane Bancel, CEO of Moderna, said in a statement.
The vaccine met its primary study endpoint, which was based on adjudicated data that were collected starting 2 weeks after the second dose of mRNA-1273. The interim study population included people who could be at higher risk for COVID-19, including 15 adults aged 65 years and older and 20 participants from diverse communities.
Safety data
The DSMB also reviewed safety data for the COVE study interim results. The vaccine was generally safe and well tolerated, as determined on the basis of solicited adverse events. Most adverse events were mild to moderate and were generally short-lived, according to a company news release.
Injection-site pain was reported in 2.7% of participants after the first dose. After the second dose, 9.7% of participants reported fatigue, 8.9% reported myalgia, 5.2% reported arthralgia, 4.5% reported headache, 4.1% reported pain, and 2.0% reported erythema or redness at the injection site.
Moderna plans to request emergency-use authorization (EUA) from the Food and Drug Administration in the coming weeks. The company expects that the EUA will be based on more data from the COVE study, including a final analysis of 151 patients with a median follow-up of more than 2 months. Moderna also plans to seek authorizations from global regulatory agencies.
The company expects to have approximately 20 million doses of mRNA-1273 ready to ship in the United States by the end of the year. In addition, the company says it remains on track to manufacture between 500 million and 1 billion doses globally in 2021.
Moderna is developing distribution plans in conjunction with the Centers for Disease Control and Prevention, the federal government’s Operation Warp Speed, and McKesson, a COVID-19 vaccine distributor contracted by the U.S. government.
Refrigeration requirements
The mRNA-1273 vaccine can be shipped and stored for up to 6 months at –20° C (about –4° F), a temperature maintained in most home or medical freezers, according to Moderna. The company expects that, after the product thaws, it will remain stable at standard refrigerator temperatures of 2°-8° C (36°-46° F) for up to 30 days within the 6-month shelf life.
Because the mRNA-1273 vaccine is stable at these refrigerator temperatures, it can be stored at most physicians’ offices, pharmacies, and hospitals, the company noted. In contrast, the similar Pfizer BTN162b2 vaccine – early results for which showed a 90% efficacy rate – requires shipment and storage at “deep-freeze” conditions of –70° C or –80° C, which is more challenging from a logistic point of view.
Moderna’s mRNA-1273 can be kept at room temperature for up to 12 hours after removal from a refrigerator for patient administration. The vaccine will not require dilution prior to use.
More than 30,000 people aged older than 18 years in the United States are enrolled in the COVE study. The research is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases and the Biomedical Advanced Research and Development Authority, part of the Office of the Assistant Secretary for Preparedness and Response at the Department of Health & Human Services.
A version of this article originally appeared on Medscape.com.
The Moderna mRNA-1273 vaccine, in development to prevent COVID-19, yielded 94.5% efficacy in early results and is generally well tolerated, the company announced early Monday. The product can be stored at refrigeration temperatures common to many physician offices, pharmacies, and hospitals.
The first interim results of the phase 3 COVE trial included 95 participants with confirmed COVID-19. An independent data safety monitoring board, which was appointed by the National Institutes of Health, informed Moderna that 90 of the patients who were positive for COVID-19 were in a placebo group and that 5 patients were in the mRNA-1273 vaccine group, resulting in a vaccine efficacy of 94.5% (P < .0001).
Interim data included 11 patients with severe COVID-19, all of whom were in the placebo group.
“This positive interim analysis from our phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease,” said Stéphane Bancel, CEO of Moderna, said in a statement.
The vaccine met its primary study endpoint, which was based on adjudicated data that were collected starting 2 weeks after the second dose of mRNA-1273. The interim study population included people who could be at higher risk for COVID-19, including 15 adults aged 65 years and older and 20 participants from diverse communities.
Safety data
The DSMB also reviewed safety data for the COVE study interim results. The vaccine was generally safe and well tolerated, as determined on the basis of solicited adverse events. Most adverse events were mild to moderate and were generally short-lived, according to a company news release.
Injection-site pain was reported in 2.7% of participants after the first dose. After the second dose, 9.7% of participants reported fatigue, 8.9% reported myalgia, 5.2% reported arthralgia, 4.5% reported headache, 4.1% reported pain, and 2.0% reported erythema or redness at the injection site.
Moderna plans to request emergency-use authorization (EUA) from the Food and Drug Administration in the coming weeks. The company expects that the EUA will be based on more data from the COVE study, including a final analysis of 151 patients with a median follow-up of more than 2 months. Moderna also plans to seek authorizations from global regulatory agencies.
The company expects to have approximately 20 million doses of mRNA-1273 ready to ship in the United States by the end of the year. In addition, the company says it remains on track to manufacture between 500 million and 1 billion doses globally in 2021.
Moderna is developing distribution plans in conjunction with the Centers for Disease Control and Prevention, the federal government’s Operation Warp Speed, and McKesson, a COVID-19 vaccine distributor contracted by the U.S. government.
Refrigeration requirements
The mRNA-1273 vaccine can be shipped and stored for up to 6 months at –20° C (about –4° F), a temperature maintained in most home or medical freezers, according to Moderna. The company expects that, after the product thaws, it will remain stable at standard refrigerator temperatures of 2°-8° C (36°-46° F) for up to 30 days within the 6-month shelf life.
Because the mRNA-1273 vaccine is stable at these refrigerator temperatures, it can be stored at most physicians’ offices, pharmacies, and hospitals, the company noted. In contrast, the similar Pfizer BTN162b2 vaccine – early results for which showed a 90% efficacy rate – requires shipment and storage at “deep-freeze” conditions of –70° C or –80° C, which is more challenging from a logistic point of view.
Moderna’s mRNA-1273 can be kept at room temperature for up to 12 hours after removal from a refrigerator for patient administration. The vaccine will not require dilution prior to use.
More than 30,000 people aged older than 18 years in the United States are enrolled in the COVE study. The research is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases and the Biomedical Advanced Research and Development Authority, part of the Office of the Assistant Secretary for Preparedness and Response at the Department of Health & Human Services.
A version of this article originally appeared on Medscape.com.
Escalate HIV adherence strategies amid COVID-19
"The writing is on the wall” that virtual care is not meeting the needs of people with HIV who struggled with viral suppression even before the COVID-19 pandemic, said Jason Farley, PhD, ANP-BC, AACRN, associate professor of nursing at Johns Hopkins University, Baltimore. So it’s time for HIV care teams, especially clinics in the Ryan White HIV/AIDS Program, to get creative in bringing wraparound services to patients.
That may mean reallocating the workforce so that one person serves as a community health worker. Or it could mean increasing texts and video calls; helping patients find online support groups to address problems with alcohol or drug use; and conducting an overall assessment of patients’ needs as the pandemic continues.
“The virtual patient-centered medical home may be the new normal after COVID-19, and we have to be thinking about how we use this model with patients for whom it works, but supplement this model in patients that it does not,” Farley said at the virtual Association of Nurses in AIDS Care (ANAC) 2020 Annual Meeting. That work “is essential to our being able to facilitate the best patient outcomes possible.”
Early data, tiered interventions
Farley referred to an article published in September in the Journal AIDS that confirmed unpublished data mentioned at the International AIDS Conference 2020. The article reported that viral suppression rates among people with HIV who attended San Francisco’s Ward 86 HIV clinic dropped by 31% from pre-COVID levels.
Of the 1766 people who attended the clinic, about 1 in 5 had detectable HIV viral loads at any point in 2019. But that rate was 31% higher after shelter-in-place orders were issued. And although patients participated in telemedicine visits at more or less the same rate before and after the pandemic (31% vs. 30% no-shows), viral suppression rates dropped. The impact was especially acute for homeless individuals.
“This destabilization occurred despite our population attending telemedicine visits at a higher rate than expected, given the 60% drop in ambulatory care visit volume nationwide,” the authors stated in their article. “Telehealth visits, while offering greater patient convenience, may lead to less access to clinic-based social support services essential to achieving viral suppression among vulnerable groups.”
That’s the challenge HIV clinics now face, Farley said at the ANAC meeting.
He suggested a differentiated care approach in which there are four tiers of care, starting with the standard level of outreach, which may include email, electronic health record blasts, and robo-calls to remind people of their appointments and to refill their medications. Those with sustained viral suppression may only need 90-day automatic refills of their medications. Those who are vulnerable to nonadherence may need to be contacted weekly or more often by the clinic. Such contact could be made by a social worker, a community health worker, or through some form of virtual support.
Patients at tier 4, who have labile viral suppression, need far more than that. These are the 15% of patients with HIV who struggled with viral suppression before the pandemic. They are the patients that Farley’s team focuses on at Baltimore’s John G. Bartlett Specialty Clinic for Infectious Disease.
“We’ve completely deconstructed the patient-centered medical home,” he said of the early move to virtual care. He suggested that clinicians assess their services and ask themselves some questions:
- Has someone on the team reached out to every patient and checked in to see what their biggest needs are, medical or not, during the pandemic? Have they assessed the patient’s ability to receive video calls or text messages?
- How have group-support programs that address stigma or the social determinants of health fared in the transition to virtual medicine?
- Are patients who are in recovery being supported in order that they may engage with recovery programs online?
- How well have counseling services done in engaging people in virtual care? Currently, given the overall increase in mental health challenges during the pandemic, one would expect that the use of mental health counseling is increasing. “If they’re stagnant or going down, someone needs to be reflecting on that issue internally in the clinic,” he said.
- Are patients being contacted regarding the effects that isolation is having on their lives? “The things that would normally allow us to self-mitigate and self-manage these conditions, like going to the gym, meeting with friends, religious services – all of those are being cut,” he said.
- Is there an early alert from an in-person pharmacy to trigger outreach via a community health worker for patients who haven’t picked up their medications in a week or more?
Farley pointed to a 2015 model for an enhanced e-health approach to chronic care management that called for e-support from the community and that was enhanced through virtual communities.
These are some of the approaches Farley has taken at his clinic. He leads a team that focuses specifically on patients who struggled with engagement before the pandemic. Through a grant from the US Department of Health & Human Services’ Health Resources and Services Administration – even before the pandemic – that team has been funding community health workers who have multiple contacts with patients online and virtually and are able to offer what he calls “unapologetically enabling” support for patients so that they are able to focus on their health.
He gave the following example. Before the pandemic, a community health worker on the team had been working with a patient who showed up at every scheduled visit and swore that she was taking her medications, although clearly she was not. A community health worker, who was made available through the grant, was able to recognize that the patient’s biggest challenge in her life was providing childcare for her special-needs child. The community health worker worked with the patient for months to find stable childcare for the child, paid 2 months of rent for the patient so that she would not become homeless, and helped her find transitional housing. When the pandemic hit, the community health worker was already texting and conducting video calls with the patient regularly.
For the past 9 months, that patient has had an undetectable viral load, Farley said.
“Nine months during a pandemic,” Farley reiterated, “and the community health worker keeps working with her, keeps meeting with her.”
Stigma on stigma
The need for this level of support from the clinic may be even more important for people with HIV who acquire COVID-19, said Orlando Harris, PhD, assistant professor of community health systems at the University of California, San Francisco, (UCSF) School of Nursing. HIV-related stigma is a well-known deterrent to care for people living with the virus. During the presentation, Harris asked Farley about the impact of COVID-19 stigma on people with both HIV and COVID-19.
Farley said that patients at his clinic have told him that they have “ostracized” friends who have tested positive for COVID-19. Harris remembered a person with HIV who participated in one of his trials telling the researchers that despite all his precautions – wearing a mask, staying socially distant – he still acquired COVID-19. There was nothing he could have done, Harris said, other than just not go to the grocery store.
The fear of contracting another disease that is associated with stigma, as well as the need to disclose it, can inflame memories of the trauma of being diagnosed with HIV, Harris said. And with patient-centered medical homes struggling to reconstitute their wraparound services via telehealth, he said he wonders whether clinicians should be doing more.
“I worry about people who have survived being diagnosed with HIV in the ‘80s and the ‘90s before antiretroviral therapy showed up on the scene,” he told Medscape Medical News. “I worry that the folks that survived one pandemic [may] be feeling fearful or living in that fear that this new pandemic might take them out. That’s why I’m stressing the need for us to really consider, as clinicians and also as researchers the support systems, the coping mechanisms, the counseling, or what have you to support those living with HIV and vulnerable to COVID-19.”
During telehealth visits, that can be achieved simply by asking people how they are really doing and what their coping mechanisms are.
For their part, the clinicians at San Francisco’s Ward 86 are not trying to provide that support through telehealth on the same level as they were at the beginning of the pandemic, said Matthew Spinelli, MD, assistant professor of medicine, and Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases and Global Medicine, who are both at UCSF and are coauthors of the study.
They still offer telemedicine appointments to patients who request them, said Spinelli. He said about one-third of his patients still prefer to receive their care virtually. The rest have gone back to face-to-face support.
“The analysis led us to promptly open up care as much as possible to our patients, with the idea that telehealth is not cutting it for vulnerable patients with HIV,” Gandhi told Medscape Medical News via email. “We don’t think it’s right for a population who relies on social support from the clinic.”
This article first appeared on Medscape.com.
"The writing is on the wall” that virtual care is not meeting the needs of people with HIV who struggled with viral suppression even before the COVID-19 pandemic, said Jason Farley, PhD, ANP-BC, AACRN, associate professor of nursing at Johns Hopkins University, Baltimore. So it’s time for HIV care teams, especially clinics in the Ryan White HIV/AIDS Program, to get creative in bringing wraparound services to patients.
That may mean reallocating the workforce so that one person serves as a community health worker. Or it could mean increasing texts and video calls; helping patients find online support groups to address problems with alcohol or drug use; and conducting an overall assessment of patients’ needs as the pandemic continues.
“The virtual patient-centered medical home may be the new normal after COVID-19, and we have to be thinking about how we use this model with patients for whom it works, but supplement this model in patients that it does not,” Farley said at the virtual Association of Nurses in AIDS Care (ANAC) 2020 Annual Meeting. That work “is essential to our being able to facilitate the best patient outcomes possible.”
Early data, tiered interventions
Farley referred to an article published in September in the Journal AIDS that confirmed unpublished data mentioned at the International AIDS Conference 2020. The article reported that viral suppression rates among people with HIV who attended San Francisco’s Ward 86 HIV clinic dropped by 31% from pre-COVID levels.
Of the 1766 people who attended the clinic, about 1 in 5 had detectable HIV viral loads at any point in 2019. But that rate was 31% higher after shelter-in-place orders were issued. And although patients participated in telemedicine visits at more or less the same rate before and after the pandemic (31% vs. 30% no-shows), viral suppression rates dropped. The impact was especially acute for homeless individuals.
“This destabilization occurred despite our population attending telemedicine visits at a higher rate than expected, given the 60% drop in ambulatory care visit volume nationwide,” the authors stated in their article. “Telehealth visits, while offering greater patient convenience, may lead to less access to clinic-based social support services essential to achieving viral suppression among vulnerable groups.”
That’s the challenge HIV clinics now face, Farley said at the ANAC meeting.
He suggested a differentiated care approach in which there are four tiers of care, starting with the standard level of outreach, which may include email, electronic health record blasts, and robo-calls to remind people of their appointments and to refill their medications. Those with sustained viral suppression may only need 90-day automatic refills of their medications. Those who are vulnerable to nonadherence may need to be contacted weekly or more often by the clinic. Such contact could be made by a social worker, a community health worker, or through some form of virtual support.
Patients at tier 4, who have labile viral suppression, need far more than that. These are the 15% of patients with HIV who struggled with viral suppression before the pandemic. They are the patients that Farley’s team focuses on at Baltimore’s John G. Bartlett Specialty Clinic for Infectious Disease.
“We’ve completely deconstructed the patient-centered medical home,” he said of the early move to virtual care. He suggested that clinicians assess their services and ask themselves some questions:
- Has someone on the team reached out to every patient and checked in to see what their biggest needs are, medical or not, during the pandemic? Have they assessed the patient’s ability to receive video calls or text messages?
- How have group-support programs that address stigma or the social determinants of health fared in the transition to virtual medicine?
- Are patients who are in recovery being supported in order that they may engage with recovery programs online?
- How well have counseling services done in engaging people in virtual care? Currently, given the overall increase in mental health challenges during the pandemic, one would expect that the use of mental health counseling is increasing. “If they’re stagnant or going down, someone needs to be reflecting on that issue internally in the clinic,” he said.
- Are patients being contacted regarding the effects that isolation is having on their lives? “The things that would normally allow us to self-mitigate and self-manage these conditions, like going to the gym, meeting with friends, religious services – all of those are being cut,” he said.
- Is there an early alert from an in-person pharmacy to trigger outreach via a community health worker for patients who haven’t picked up their medications in a week or more?
Farley pointed to a 2015 model for an enhanced e-health approach to chronic care management that called for e-support from the community and that was enhanced through virtual communities.
These are some of the approaches Farley has taken at his clinic. He leads a team that focuses specifically on patients who struggled with engagement before the pandemic. Through a grant from the US Department of Health & Human Services’ Health Resources and Services Administration – even before the pandemic – that team has been funding community health workers who have multiple contacts with patients online and virtually and are able to offer what he calls “unapologetically enabling” support for patients so that they are able to focus on their health.
He gave the following example. Before the pandemic, a community health worker on the team had been working with a patient who showed up at every scheduled visit and swore that she was taking her medications, although clearly she was not. A community health worker, who was made available through the grant, was able to recognize that the patient’s biggest challenge in her life was providing childcare for her special-needs child. The community health worker worked with the patient for months to find stable childcare for the child, paid 2 months of rent for the patient so that she would not become homeless, and helped her find transitional housing. When the pandemic hit, the community health worker was already texting and conducting video calls with the patient regularly.
For the past 9 months, that patient has had an undetectable viral load, Farley said.
“Nine months during a pandemic,” Farley reiterated, “and the community health worker keeps working with her, keeps meeting with her.”
Stigma on stigma
The need for this level of support from the clinic may be even more important for people with HIV who acquire COVID-19, said Orlando Harris, PhD, assistant professor of community health systems at the University of California, San Francisco, (UCSF) School of Nursing. HIV-related stigma is a well-known deterrent to care for people living with the virus. During the presentation, Harris asked Farley about the impact of COVID-19 stigma on people with both HIV and COVID-19.
Farley said that patients at his clinic have told him that they have “ostracized” friends who have tested positive for COVID-19. Harris remembered a person with HIV who participated in one of his trials telling the researchers that despite all his precautions – wearing a mask, staying socially distant – he still acquired COVID-19. There was nothing he could have done, Harris said, other than just not go to the grocery store.
The fear of contracting another disease that is associated with stigma, as well as the need to disclose it, can inflame memories of the trauma of being diagnosed with HIV, Harris said. And with patient-centered medical homes struggling to reconstitute their wraparound services via telehealth, he said he wonders whether clinicians should be doing more.
“I worry about people who have survived being diagnosed with HIV in the ‘80s and the ‘90s before antiretroviral therapy showed up on the scene,” he told Medscape Medical News. “I worry that the folks that survived one pandemic [may] be feeling fearful or living in that fear that this new pandemic might take them out. That’s why I’m stressing the need for us to really consider, as clinicians and also as researchers the support systems, the coping mechanisms, the counseling, or what have you to support those living with HIV and vulnerable to COVID-19.”
During telehealth visits, that can be achieved simply by asking people how they are really doing and what their coping mechanisms are.
For their part, the clinicians at San Francisco’s Ward 86 are not trying to provide that support through telehealth on the same level as they were at the beginning of the pandemic, said Matthew Spinelli, MD, assistant professor of medicine, and Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases and Global Medicine, who are both at UCSF and are coauthors of the study.
They still offer telemedicine appointments to patients who request them, said Spinelli. He said about one-third of his patients still prefer to receive their care virtually. The rest have gone back to face-to-face support.
“The analysis led us to promptly open up care as much as possible to our patients, with the idea that telehealth is not cutting it for vulnerable patients with HIV,” Gandhi told Medscape Medical News via email. “We don’t think it’s right for a population who relies on social support from the clinic.”
This article first appeared on Medscape.com.
"The writing is on the wall” that virtual care is not meeting the needs of people with HIV who struggled with viral suppression even before the COVID-19 pandemic, said Jason Farley, PhD, ANP-BC, AACRN, associate professor of nursing at Johns Hopkins University, Baltimore. So it’s time for HIV care teams, especially clinics in the Ryan White HIV/AIDS Program, to get creative in bringing wraparound services to patients.
That may mean reallocating the workforce so that one person serves as a community health worker. Or it could mean increasing texts and video calls; helping patients find online support groups to address problems with alcohol or drug use; and conducting an overall assessment of patients’ needs as the pandemic continues.
“The virtual patient-centered medical home may be the new normal after COVID-19, and we have to be thinking about how we use this model with patients for whom it works, but supplement this model in patients that it does not,” Farley said at the virtual Association of Nurses in AIDS Care (ANAC) 2020 Annual Meeting. That work “is essential to our being able to facilitate the best patient outcomes possible.”
Early data, tiered interventions
Farley referred to an article published in September in the Journal AIDS that confirmed unpublished data mentioned at the International AIDS Conference 2020. The article reported that viral suppression rates among people with HIV who attended San Francisco’s Ward 86 HIV clinic dropped by 31% from pre-COVID levels.
Of the 1766 people who attended the clinic, about 1 in 5 had detectable HIV viral loads at any point in 2019. But that rate was 31% higher after shelter-in-place orders were issued. And although patients participated in telemedicine visits at more or less the same rate before and after the pandemic (31% vs. 30% no-shows), viral suppression rates dropped. The impact was especially acute for homeless individuals.
“This destabilization occurred despite our population attending telemedicine visits at a higher rate than expected, given the 60% drop in ambulatory care visit volume nationwide,” the authors stated in their article. “Telehealth visits, while offering greater patient convenience, may lead to less access to clinic-based social support services essential to achieving viral suppression among vulnerable groups.”
That’s the challenge HIV clinics now face, Farley said at the ANAC meeting.
He suggested a differentiated care approach in which there are four tiers of care, starting with the standard level of outreach, which may include email, electronic health record blasts, and robo-calls to remind people of their appointments and to refill their medications. Those with sustained viral suppression may only need 90-day automatic refills of their medications. Those who are vulnerable to nonadherence may need to be contacted weekly or more often by the clinic. Such contact could be made by a social worker, a community health worker, or through some form of virtual support.
Patients at tier 4, who have labile viral suppression, need far more than that. These are the 15% of patients with HIV who struggled with viral suppression before the pandemic. They are the patients that Farley’s team focuses on at Baltimore’s John G. Bartlett Specialty Clinic for Infectious Disease.
“We’ve completely deconstructed the patient-centered medical home,” he said of the early move to virtual care. He suggested that clinicians assess their services and ask themselves some questions:
- Has someone on the team reached out to every patient and checked in to see what their biggest needs are, medical or not, during the pandemic? Have they assessed the patient’s ability to receive video calls or text messages?
- How have group-support programs that address stigma or the social determinants of health fared in the transition to virtual medicine?
- Are patients who are in recovery being supported in order that they may engage with recovery programs online?
- How well have counseling services done in engaging people in virtual care? Currently, given the overall increase in mental health challenges during the pandemic, one would expect that the use of mental health counseling is increasing. “If they’re stagnant or going down, someone needs to be reflecting on that issue internally in the clinic,” he said.
- Are patients being contacted regarding the effects that isolation is having on their lives? “The things that would normally allow us to self-mitigate and self-manage these conditions, like going to the gym, meeting with friends, religious services – all of those are being cut,” he said.
- Is there an early alert from an in-person pharmacy to trigger outreach via a community health worker for patients who haven’t picked up their medications in a week or more?
Farley pointed to a 2015 model for an enhanced e-health approach to chronic care management that called for e-support from the community and that was enhanced through virtual communities.
These are some of the approaches Farley has taken at his clinic. He leads a team that focuses specifically on patients who struggled with engagement before the pandemic. Through a grant from the US Department of Health & Human Services’ Health Resources and Services Administration – even before the pandemic – that team has been funding community health workers who have multiple contacts with patients online and virtually and are able to offer what he calls “unapologetically enabling” support for patients so that they are able to focus on their health.
He gave the following example. Before the pandemic, a community health worker on the team had been working with a patient who showed up at every scheduled visit and swore that she was taking her medications, although clearly she was not. A community health worker, who was made available through the grant, was able to recognize that the patient’s biggest challenge in her life was providing childcare for her special-needs child. The community health worker worked with the patient for months to find stable childcare for the child, paid 2 months of rent for the patient so that she would not become homeless, and helped her find transitional housing. When the pandemic hit, the community health worker was already texting and conducting video calls with the patient regularly.
For the past 9 months, that patient has had an undetectable viral load, Farley said.
“Nine months during a pandemic,” Farley reiterated, “and the community health worker keeps working with her, keeps meeting with her.”
Stigma on stigma
The need for this level of support from the clinic may be even more important for people with HIV who acquire COVID-19, said Orlando Harris, PhD, assistant professor of community health systems at the University of California, San Francisco, (UCSF) School of Nursing. HIV-related stigma is a well-known deterrent to care for people living with the virus. During the presentation, Harris asked Farley about the impact of COVID-19 stigma on people with both HIV and COVID-19.
Farley said that patients at his clinic have told him that they have “ostracized” friends who have tested positive for COVID-19. Harris remembered a person with HIV who participated in one of his trials telling the researchers that despite all his precautions – wearing a mask, staying socially distant – he still acquired COVID-19. There was nothing he could have done, Harris said, other than just not go to the grocery store.
The fear of contracting another disease that is associated with stigma, as well as the need to disclose it, can inflame memories of the trauma of being diagnosed with HIV, Harris said. And with patient-centered medical homes struggling to reconstitute their wraparound services via telehealth, he said he wonders whether clinicians should be doing more.
“I worry about people who have survived being diagnosed with HIV in the ‘80s and the ‘90s before antiretroviral therapy showed up on the scene,” he told Medscape Medical News. “I worry that the folks that survived one pandemic [may] be feeling fearful or living in that fear that this new pandemic might take them out. That’s why I’m stressing the need for us to really consider, as clinicians and also as researchers the support systems, the coping mechanisms, the counseling, or what have you to support those living with HIV and vulnerable to COVID-19.”
During telehealth visits, that can be achieved simply by asking people how they are really doing and what their coping mechanisms are.
For their part, the clinicians at San Francisco’s Ward 86 are not trying to provide that support through telehealth on the same level as they were at the beginning of the pandemic, said Matthew Spinelli, MD, assistant professor of medicine, and Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases and Global Medicine, who are both at UCSF and are coauthors of the study.
They still offer telemedicine appointments to patients who request them, said Spinelli. He said about one-third of his patients still prefer to receive their care virtually. The rest have gone back to face-to-face support.
“The analysis led us to promptly open up care as much as possible to our patients, with the idea that telehealth is not cutting it for vulnerable patients with HIV,” Gandhi told Medscape Medical News via email. “We don’t think it’s right for a population who relies on social support from the clinic.”
This article first appeared on Medscape.com.
Situation ‘dire’ as COVID spike in West, Midwest worsens, experts say
Coronavirus infections are expected to continue to climb in the upper Midwest and intermountain West of the United States, which will strain an already-maxed-out system as increased hospitalizations and deaths follow, say infectious diseases specialists.
“I think the situation in 2 to 4 weeks is going to be grim,” said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah School of Medicine in Salt Lake City, on a call yesterday with reporters, sponsored by the Infectious Diseases Society of America (IDSA).
Cases began rising in Utah in mid-September and have gone up steeply since, increasing from 450 cases per day to 2,650 reported on Nov. 8, according to the Johns Hopkins Coronavirus Resource Center. The New York Times reports that the 7-day rolling average for hospitalizations have gone up 34% and deaths have risen 93%, with 11 deaths this past Tuesday.
Other states in the west – Montana, Idaho, and Wyoming, which reported 1,232 cases on Tuesday and have been averaging 660 cases a day in the last week, according to the Times – are being equally hard hit. The same is true for states in the upper Midwest, including North Dakota, South Dakota, Minnesota, Wisconsin, and Iowa.
Most of the states being hit now have large swaths of rural countryside, which means health resources are limited and spread out, said Pavia.
“The situation really has to be described as dire,” said Pavia, noting that intensive care units in Utah are full, including contingency units that were purpose-built for the pandemic. Physicians and nurses are burned out and in short supply, he said. Instead of a 1:1 or 1:2 nurse-to-ICU patient ratio, the ratio is now 1:4, said Pavia. “Throughout the region, people are facing a crisis in staffing.”
The University of Utah hospital normally takes referrals from Idaho, Wyoming, and northern Arizona, but is prioritizing Utah residents for ICU admission, said Pavia.
Both Pavia and Daniel P. McQuillen, MD, president-elect of IDSA, also noted the shortage of infectious diseases specialists, which began at least a decade ago. McQuillen, senior infectious diseases physician at Beth Israel Lahey Health in Boston, said he and colleagues had done some research earlier this year anticipating the pandemic’s spread, and found that some 80% of counties – including the rural counties in the states now being hit – have one or zero infectious disease specialists.
Those specialists can help improve patient outcomes, explained McQuillen.
Colleges likely driving spike
Pavia said the reasons for sharp increases in the region vary, but there are several areas of commonality. Most of the states didn’t have many cases early in the pandemic, “so perhaps there was less fear of the virus.” There were fewer actions by government officials, driven perhaps by the reluctance to take on individuals who are distrustful of government, he said.
Cases started going up after some events – such as the August motorcycle rally in Sturgis, South Dakota – but the acceleration in September was likely driven by the reopening of colleges across the region, said Pavia.
“Most of the states have kept in-person schooling, and probably more importantly, they’ve kept extracurricular activities in sports,” he said, adding that in many of the areas the weather has turned cooler, driving people indoors.
McQuillen said it has been shown that a significant amount of transmission occurs within homes – and college students may be bringing the virus home and fueling spread, in addition to people not wearing masks while at small family gatherings.
Both he and Pavia said more emphasis needs to be placed on mitigation measures such as mask-wearing as well as on testing. IDSA is starting #MaskUpAmerica, a public service campaign aimed at getting people to wear masks in all community settings, including at work, in churches, at social gatherings, in gyms, and on public transportation.
Pavia said in some places people are refusing to be tested because they don’t want to be quarantined.
Utah Gov. Gary Herbert (R) issued a statewide mask mandate this past weekend and announced some other restrictions, including a 2-week pause on most, but not all, athletic events, according to CBS News. But local pushback could weaken those measures, said Pavia.
Many people are looking to vaccines to usher in a return to normal. But, said Pavia, “vaccines aren’t going to help us out much this winter,” noting that initial doses will be given mostly to first responders and healthcare workers.
“The only way we’re going to get out of this this winter is by doing the things that we’ve been talking about for months – wearing a mask, watching your social distance, and avoiding large gatherings,” he said.
There is an end in sight, said Pavia, but it won’t be in early 2021. “That end is next summer or fall,” he said. “And that’s a hard message to give but it’s really critical.”
McQuillen agreed: “Wearing masks and distancing are exactly all we have probably until middle of next year.”
This article first appeared on Medscape.com.
Coronavirus infections are expected to continue to climb in the upper Midwest and intermountain West of the United States, which will strain an already-maxed-out system as increased hospitalizations and deaths follow, say infectious diseases specialists.
“I think the situation in 2 to 4 weeks is going to be grim,” said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah School of Medicine in Salt Lake City, on a call yesterday with reporters, sponsored by the Infectious Diseases Society of America (IDSA).
Cases began rising in Utah in mid-September and have gone up steeply since, increasing from 450 cases per day to 2,650 reported on Nov. 8, according to the Johns Hopkins Coronavirus Resource Center. The New York Times reports that the 7-day rolling average for hospitalizations have gone up 34% and deaths have risen 93%, with 11 deaths this past Tuesday.
Other states in the west – Montana, Idaho, and Wyoming, which reported 1,232 cases on Tuesday and have been averaging 660 cases a day in the last week, according to the Times – are being equally hard hit. The same is true for states in the upper Midwest, including North Dakota, South Dakota, Minnesota, Wisconsin, and Iowa.
Most of the states being hit now have large swaths of rural countryside, which means health resources are limited and spread out, said Pavia.
“The situation really has to be described as dire,” said Pavia, noting that intensive care units in Utah are full, including contingency units that were purpose-built for the pandemic. Physicians and nurses are burned out and in short supply, he said. Instead of a 1:1 or 1:2 nurse-to-ICU patient ratio, the ratio is now 1:4, said Pavia. “Throughout the region, people are facing a crisis in staffing.”
The University of Utah hospital normally takes referrals from Idaho, Wyoming, and northern Arizona, but is prioritizing Utah residents for ICU admission, said Pavia.
Both Pavia and Daniel P. McQuillen, MD, president-elect of IDSA, also noted the shortage of infectious diseases specialists, which began at least a decade ago. McQuillen, senior infectious diseases physician at Beth Israel Lahey Health in Boston, said he and colleagues had done some research earlier this year anticipating the pandemic’s spread, and found that some 80% of counties – including the rural counties in the states now being hit – have one or zero infectious disease specialists.
Those specialists can help improve patient outcomes, explained McQuillen.
Colleges likely driving spike
Pavia said the reasons for sharp increases in the region vary, but there are several areas of commonality. Most of the states didn’t have many cases early in the pandemic, “so perhaps there was less fear of the virus.” There were fewer actions by government officials, driven perhaps by the reluctance to take on individuals who are distrustful of government, he said.
Cases started going up after some events – such as the August motorcycle rally in Sturgis, South Dakota – but the acceleration in September was likely driven by the reopening of colleges across the region, said Pavia.
“Most of the states have kept in-person schooling, and probably more importantly, they’ve kept extracurricular activities in sports,” he said, adding that in many of the areas the weather has turned cooler, driving people indoors.
McQuillen said it has been shown that a significant amount of transmission occurs within homes – and college students may be bringing the virus home and fueling spread, in addition to people not wearing masks while at small family gatherings.
Both he and Pavia said more emphasis needs to be placed on mitigation measures such as mask-wearing as well as on testing. IDSA is starting #MaskUpAmerica, a public service campaign aimed at getting people to wear masks in all community settings, including at work, in churches, at social gatherings, in gyms, and on public transportation.
Pavia said in some places people are refusing to be tested because they don’t want to be quarantined.
Utah Gov. Gary Herbert (R) issued a statewide mask mandate this past weekend and announced some other restrictions, including a 2-week pause on most, but not all, athletic events, according to CBS News. But local pushback could weaken those measures, said Pavia.
Many people are looking to vaccines to usher in a return to normal. But, said Pavia, “vaccines aren’t going to help us out much this winter,” noting that initial doses will be given mostly to first responders and healthcare workers.
“The only way we’re going to get out of this this winter is by doing the things that we’ve been talking about for months – wearing a mask, watching your social distance, and avoiding large gatherings,” he said.
There is an end in sight, said Pavia, but it won’t be in early 2021. “That end is next summer or fall,” he said. “And that’s a hard message to give but it’s really critical.”
McQuillen agreed: “Wearing masks and distancing are exactly all we have probably until middle of next year.”
This article first appeared on Medscape.com.
Coronavirus infections are expected to continue to climb in the upper Midwest and intermountain West of the United States, which will strain an already-maxed-out system as increased hospitalizations and deaths follow, say infectious diseases specialists.
“I think the situation in 2 to 4 weeks is going to be grim,” said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah School of Medicine in Salt Lake City, on a call yesterday with reporters, sponsored by the Infectious Diseases Society of America (IDSA).
Cases began rising in Utah in mid-September and have gone up steeply since, increasing from 450 cases per day to 2,650 reported on Nov. 8, according to the Johns Hopkins Coronavirus Resource Center. The New York Times reports that the 7-day rolling average for hospitalizations have gone up 34% and deaths have risen 93%, with 11 deaths this past Tuesday.
Other states in the west – Montana, Idaho, and Wyoming, which reported 1,232 cases on Tuesday and have been averaging 660 cases a day in the last week, according to the Times – are being equally hard hit. The same is true for states in the upper Midwest, including North Dakota, South Dakota, Minnesota, Wisconsin, and Iowa.
Most of the states being hit now have large swaths of rural countryside, which means health resources are limited and spread out, said Pavia.
“The situation really has to be described as dire,” said Pavia, noting that intensive care units in Utah are full, including contingency units that were purpose-built for the pandemic. Physicians and nurses are burned out and in short supply, he said. Instead of a 1:1 or 1:2 nurse-to-ICU patient ratio, the ratio is now 1:4, said Pavia. “Throughout the region, people are facing a crisis in staffing.”
The University of Utah hospital normally takes referrals from Idaho, Wyoming, and northern Arizona, but is prioritizing Utah residents for ICU admission, said Pavia.
Both Pavia and Daniel P. McQuillen, MD, president-elect of IDSA, also noted the shortage of infectious diseases specialists, which began at least a decade ago. McQuillen, senior infectious diseases physician at Beth Israel Lahey Health in Boston, said he and colleagues had done some research earlier this year anticipating the pandemic’s spread, and found that some 80% of counties – including the rural counties in the states now being hit – have one or zero infectious disease specialists.
Those specialists can help improve patient outcomes, explained McQuillen.
Colleges likely driving spike
Pavia said the reasons for sharp increases in the region vary, but there are several areas of commonality. Most of the states didn’t have many cases early in the pandemic, “so perhaps there was less fear of the virus.” There were fewer actions by government officials, driven perhaps by the reluctance to take on individuals who are distrustful of government, he said.
Cases started going up after some events – such as the August motorcycle rally in Sturgis, South Dakota – but the acceleration in September was likely driven by the reopening of colleges across the region, said Pavia.
“Most of the states have kept in-person schooling, and probably more importantly, they’ve kept extracurricular activities in sports,” he said, adding that in many of the areas the weather has turned cooler, driving people indoors.
McQuillen said it has been shown that a significant amount of transmission occurs within homes – and college students may be bringing the virus home and fueling spread, in addition to people not wearing masks while at small family gatherings.
Both he and Pavia said more emphasis needs to be placed on mitigation measures such as mask-wearing as well as on testing. IDSA is starting #MaskUpAmerica, a public service campaign aimed at getting people to wear masks in all community settings, including at work, in churches, at social gatherings, in gyms, and on public transportation.
Pavia said in some places people are refusing to be tested because they don’t want to be quarantined.
Utah Gov. Gary Herbert (R) issued a statewide mask mandate this past weekend and announced some other restrictions, including a 2-week pause on most, but not all, athletic events, according to CBS News. But local pushback could weaken those measures, said Pavia.
Many people are looking to vaccines to usher in a return to normal. But, said Pavia, “vaccines aren’t going to help us out much this winter,” noting that initial doses will be given mostly to first responders and healthcare workers.
“The only way we’re going to get out of this this winter is by doing the things that we’ve been talking about for months – wearing a mask, watching your social distance, and avoiding large gatherings,” he said.
There is an end in sight, said Pavia, but it won’t be in early 2021. “That end is next summer or fall,” he said. “And that’s a hard message to give but it’s really critical.”
McQuillen agreed: “Wearing masks and distancing are exactly all we have probably until middle of next year.”
This article first appeared on Medscape.com.