User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Issues with the Maintenance of Certification program; Overcoming a ‘quadruple threat’
Issues with the MOC
In Dr. Nasrallah’s editorial “Revamp the MOC” (From the Editor,
I was not so fortunate to have been grandfathered with lifetime certification, so I have been forced to recertify twice now. I will be 70 years old when I will need to decide whether to recertify once again. It is my belief that the MOC process is cumbersome and nonsensical, having little, if any, relevance in assessing one’s competency. Again, the ABPN’s purpose is not to “protect the public” and ensure safe and competent care, but to generate tremendous revenue for the Board. How can any rational individual believe that this exam is a legitimate test of one’s knowledge and competency when the pass rates are so stratospherically high year after year? I do not know of a single individual who has failed the recertification exam, so it would appear that if you pay the fees and sit for the exam, you will pass. It saddens me that the Board can perpetrate such a hoax on the public, leading them to believe that the MOC actually means something.
The cost to recertify is not inexpensive. Apparently, in a desire to add to its coffers, the ABPN has recently implemented the Physician Folios portal, whereby psychiatrists are forced to pay an annual fee. Its purpose, according to the Board, is to provide“a dynamic conduit for important data exchange such as making updates to personal contact information, updating medical license information, and applying and paying for an examination.”1 Give me a break!
It is my hope that a better, less expensive, more appropriate system is developed, allowing the psychiatrist to focus his/her efforts on treating patients.
Terrence Boyadjis, MD
Private psychiatric practice
West Chester, Pennsylvania
Reference
1. American Board of Psychiatry and Neurology. ABPN Physician Folios. https://application.abpn.com/webclient/landing_page.asp. Accessed October 20, 2020.
Dr. Nasrallah’s editorial about the MOC process is another addition to his collection of many of the best editorials I’ve ever read. I related fondly to his experiences taking the oral exam, which I took in 1972. I also became an examiner during the mid-1970s. Dr. Nasrallah continues to be a source of down-to-earth wisdom for our beloved profession.
Richard W. Worst, MD
Twin Falls, Idaho
Continue to: Overcoming a ‘quadruple threat’
Overcoming a ‘quadruple threat’
Dr. Nasrallah’s editorial “Enduring the ordeal of a quadruple threat is especially arduous for psychiatric patients” (From the Editor,
Robert W. Pollack, MD
Founder/COO
Psychiatric Associates of Southwest Florida
Fort Myers, Florida
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Issues with the MOC
In Dr. Nasrallah’s editorial “Revamp the MOC” (From the Editor,
I was not so fortunate to have been grandfathered with lifetime certification, so I have been forced to recertify twice now. I will be 70 years old when I will need to decide whether to recertify once again. It is my belief that the MOC process is cumbersome and nonsensical, having little, if any, relevance in assessing one’s competency. Again, the ABPN’s purpose is not to “protect the public” and ensure safe and competent care, but to generate tremendous revenue for the Board. How can any rational individual believe that this exam is a legitimate test of one’s knowledge and competency when the pass rates are so stratospherically high year after year? I do not know of a single individual who has failed the recertification exam, so it would appear that if you pay the fees and sit for the exam, you will pass. It saddens me that the Board can perpetrate such a hoax on the public, leading them to believe that the MOC actually means something.
The cost to recertify is not inexpensive. Apparently, in a desire to add to its coffers, the ABPN has recently implemented the Physician Folios portal, whereby psychiatrists are forced to pay an annual fee. Its purpose, according to the Board, is to provide“a dynamic conduit for important data exchange such as making updates to personal contact information, updating medical license information, and applying and paying for an examination.”1 Give me a break!
It is my hope that a better, less expensive, more appropriate system is developed, allowing the psychiatrist to focus his/her efforts on treating patients.
Terrence Boyadjis, MD
Private psychiatric practice
West Chester, Pennsylvania
Reference
1. American Board of Psychiatry and Neurology. ABPN Physician Folios. https://application.abpn.com/webclient/landing_page.asp. Accessed October 20, 2020.
Dr. Nasrallah’s editorial about the MOC process is another addition to his collection of many of the best editorials I’ve ever read. I related fondly to his experiences taking the oral exam, which I took in 1972. I also became an examiner during the mid-1970s. Dr. Nasrallah continues to be a source of down-to-earth wisdom for our beloved profession.
Richard W. Worst, MD
Twin Falls, Idaho
Continue to: Overcoming a ‘quadruple threat’
Overcoming a ‘quadruple threat’
Dr. Nasrallah’s editorial “Enduring the ordeal of a quadruple threat is especially arduous for psychiatric patients” (From the Editor,
Robert W. Pollack, MD
Founder/COO
Psychiatric Associates of Southwest Florida
Fort Myers, Florida
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Issues with the MOC
In Dr. Nasrallah’s editorial “Revamp the MOC” (From the Editor,
I was not so fortunate to have been grandfathered with lifetime certification, so I have been forced to recertify twice now. I will be 70 years old when I will need to decide whether to recertify once again. It is my belief that the MOC process is cumbersome and nonsensical, having little, if any, relevance in assessing one’s competency. Again, the ABPN’s purpose is not to “protect the public” and ensure safe and competent care, but to generate tremendous revenue for the Board. How can any rational individual believe that this exam is a legitimate test of one’s knowledge and competency when the pass rates are so stratospherically high year after year? I do not know of a single individual who has failed the recertification exam, so it would appear that if you pay the fees and sit for the exam, you will pass. It saddens me that the Board can perpetrate such a hoax on the public, leading them to believe that the MOC actually means something.
The cost to recertify is not inexpensive. Apparently, in a desire to add to its coffers, the ABPN has recently implemented the Physician Folios portal, whereby psychiatrists are forced to pay an annual fee. Its purpose, according to the Board, is to provide“a dynamic conduit for important data exchange such as making updates to personal contact information, updating medical license information, and applying and paying for an examination.”1 Give me a break!
It is my hope that a better, less expensive, more appropriate system is developed, allowing the psychiatrist to focus his/her efforts on treating patients.
Terrence Boyadjis, MD
Private psychiatric practice
West Chester, Pennsylvania
Reference
1. American Board of Psychiatry and Neurology. ABPN Physician Folios. https://application.abpn.com/webclient/landing_page.asp. Accessed October 20, 2020.
Dr. Nasrallah’s editorial about the MOC process is another addition to his collection of many of the best editorials I’ve ever read. I related fondly to his experiences taking the oral exam, which I took in 1972. I also became an examiner during the mid-1970s. Dr. Nasrallah continues to be a source of down-to-earth wisdom for our beloved profession.
Richard W. Worst, MD
Twin Falls, Idaho
Continue to: Overcoming a ‘quadruple threat’
Overcoming a ‘quadruple threat’
Dr. Nasrallah’s editorial “Enduring the ordeal of a quadruple threat is especially arduous for psychiatric patients” (From the Editor,
Robert W. Pollack, MD
Founder/COO
Psychiatric Associates of Southwest Florida
Fort Myers, Florida
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Unmet needs in the pharmacotherapy of psychiatric brain syndromes
Let’s face it: The greatest unmet need in psychiatry is discovering a treatment for the infamous syndrome of toxic political extremism. Its ugly symptoms include blind hatred, visceral malice, bigotry, vandalism, hypocrisy, racism, hubris, intransigence, narcissism, demagoguery, mutual contempt, and intense schadenfreude.
This corrosive affliction has engulfed and polluted our society, and compromised our well-being and quality of life. Treating this malignant syndrome is beyond the reach of psychopharmacology!
Thus, we psychiatrists should focus on the mood, psychotic, anxiety, and addiction syndromes that we encounter daily in our hospitals, clinics, and private offices. They affect tens of millions of patients. We currently have many psychotropic medications for these conditions. When combined with psychotherapy, the resulting synergy can be magical and immensely gratifying. However, some of those agents have limited efficacy due to the extensive heterogeneity of syndromes such as schizophrenia or depression, which are often confounded with comorbidities. A perfect balance between efficacy, tolerability, and safety are often hard to come by in pharmacotherapy.
The most glaring psychopharmacologic unmet need is that 80% of DSM disorders still do not have a single FDA-approved (evidence-based) medication.1 It will take decades, hundreds of billions of dollars, and the motivation of the often-maligned pharmaceutical industry (indispensable, because they are the only entity with the large R&D infrastructure to develop medications for psychiatry). Both academic and clinical psychiatrists must advise pharmaceutical companies about the unmet needs in our field and urge them to develop novel pharmacotherapies to address the gaps in the clinical care of psychiatric patients.
An inventory of unmet needs
With that in mind, here is a list of unmet needs I have been thinking about lately, and hoping that they will be resolved to help our patients achieve better clinical and functional outcomes.
Rapid-onset antipsychotics. The discovery that ketamine can rapidly convert refractory patients who are chronically depressed or suicidal to normal mood within a few hours shattered the dogma that weeks and months are needed for severe depression to improve, let alone achieve full remission. There is a similar dogma about psychosis requiring a protracted duration of antipsychotic treatment to attain significant impact. A rapid-acting antipsychotic agent would represent a major advance in psychiatry and its pharmaco-economic benefits would be substantial, given the high cost of inpatient hospitalization. Just as neurobiologic research guided the discovery of ketamine as a dramatic paradigm shift in treating depression, targeted research, especially focusing on glutamate pathways, may help identify a rapid-onset agent, whether oral, intranasal, IV, or even (why not) intrathecal. Research is known to enhance serendipity, which has been kind to psychiatry and has led to the discovery of several pharmacologic therapies in psychiatry, such as chlorpromazine, monoamine oxidase inhibitor antidepressants, and lithium.
Long-acting antidepressants and anxiolytics. This can be regarded as low-hanging fruit. Several technologies have been developed for long-acting formulations, yet they have been exploited mainly for antipsychotic medications. Some of these technologies can be employed to convert commonly used antidepressants (such as selective serotonin reuptake inhibitors) into long-acting antidepressants that can also reduce anxiety. Nonadherence among patients with depression is quite common, and relapses may lead to suicide attempts. The use of injectable, long-acting antidepressants can also reduce the incidence of overdoses because the patient will not have possession of potentially fatal pills.
Continue to: Long-acting mood stabilizers
Long-acting mood stabilizers. The rationale for long-acting mood stabilizers is the same as for long-acting antidepressants. Patients with bipolar disorder are known to stop taking their medications because they miss their “highs.” Some long-acting antipsychotics are approved for bipolar disorder, but these are often associated with adverse effects, such as metabolic dysregulation, extrapyramidal symptoms, and tardive dyskinesia. Mood stabilizers are essential for the bipolar spectrum.
A “real” treatment for alcohol use disorders that eliminates craving for alcohol. Alcoholism is associated with more than 100 medical complications and is one of the leading causes of disability in the world. It is frustrating that very few drug companies have focused on this widely prevalent brain disorder, which is also a common comorbid condition in many psychiatric syndromes.
Treatment-resistance pharmacotherapy solutions. All psychiatric syndromes are heterogeneous and contain ≥1 subgroups (biotypes) that fail to respond to what is considered the “standard” psychopharmacologic treatment (such as antipsychotics, antidepressants, mood stabilizers, or anti-obsessive medications). Technically, those so-called treatment-resistant subtypes need medications with a different mechanism of action. For example, clozapine for treatment-resistant schizophrenia and ketamine for treatment-resistant depression provide proof that treatment resistance is treatable but by a mechanism of action that is completely different from that of standard therapies, such as N-methyl-
Negative symptoms of schizophrenia cause significant functional disability and are well known to be a major unmet need. Some promising data are emerging on agents such as pimavanserin, cariprazine, and roluperidone, which is encouraging, but nothing is approved yet.
Cognitive deficits of schizophrenia, both neurocognition and social cognition, are another major unmet need that impair function in many patients. Many attempts to develop a pharmacologic treatment for these serious cognitive impairments have been made, but several candidates that initially appeared promising have bitten the dust. A focus on modulating the glutamate NMDA receptor may eventually lead to a breakthrough, and that may also help patients with bipolar disorder and major depressive disorder, both of whom also have cognitive deficits in several domains, albeit less severe than those experienced by patients with schizophrenia.
Continue to: Personality disorders
Personality disorders, especially borderline personality disorder, are very challenging to treat pharmacologically despite their prevalence and serious disruption to people’s lives. Hardly any FDA clinical trials have been conducted on any personality disorder. It is an unmet need that all psychiatrists would love to see addressed. But the mythical notion that personality disorders are untreatable may be an impediment in the pursuit of novel pharmacotherapy for borderline, narcissistic, antisocial, or schizotypal personality disorders, and other disorders. Heart attacks and religious conversion often change the baseline personality dramatically.
Childhood disorders. Apart from attention-deficit/hyperactivity disorder (ADHD), very few childhood psychiatric disorders have an FDA-approved medication. Why do drug companies avoid conducting controlled clinical trials in children age <10 who have autism, spectrum disorders, conduct disorder, oppositional defiant disorder, and other disorders? Effective pharmacotherapy for these children can be regarded as a desirable early intervention that may short-circuit their progression to serious adult psychopathology.
Parsimonious psychopharmacology for the treatment of trans-diagnostic psychiatric disorders. Recent research strongly suggests there is a strong overlap among psychiatric conditions, genetically, clinically, and biologically.2,3 For example, bipolar disorder is frequently accompanied by anxiety or substance use, patients with schizophrenia often experience anxiety, depression, or substance use, and ADHD has been found to share genes with autism.4,5
Eating disorders. There are no truly efficacious pharmacologic treatments for anorexia or bulimia nervosa. Research in this area is thin, and needs to be beefed up.
Sexual disorders. A huge unmet need exists for the pharmacotherapy of many sexual disorders that can have serious legal consequences (paraphilias) or quality-of-life repercussions (low sexual desire and orgasm disorders).
Continue to: A coordinated effort
A coordinated effort
It will take a massive collaboration among multiple stakeholders to launch the herculean process of addressing the unmet needs of all the above psychiatric disorders. This includes:
- the pharmaceutical industry (to provide the massive financial investment and R&D expertise)
- the federal government (to provide incentives)
- the FDA (to allow novel clinical trial designs)
- academic psychiatrists (to conduct research to discover the pathophysiology of psychiatric diseases)
- clinical psychiatrists (to provide consultations and advise about the clinical gaps in current psychopharmacological treatments)
- psychiatric patients (who are needed to volunteer for large-scale clinical trials).
This will be a veritable “psychiatric Manhattan Project” to advance the treatment of numerous psychiatric illnesses. The greatest benefit of discovering cures for disabling mental disorders is the evaporation of the virulent stigma that continues to plague our patients.
As for the political extremism that has corroded our society, it may be beyond pharmacologic redemption. An antidote to the “kool aid” has not yet been invented…
1. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
2. Nasrallah HA. Is there only 1 neurobiologic disorder, with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
3. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
4. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
5. Marshall M. Roots of mental illness. Nature. 2020;581:19-21.
Let’s face it: The greatest unmet need in psychiatry is discovering a treatment for the infamous syndrome of toxic political extremism. Its ugly symptoms include blind hatred, visceral malice, bigotry, vandalism, hypocrisy, racism, hubris, intransigence, narcissism, demagoguery, mutual contempt, and intense schadenfreude.
This corrosive affliction has engulfed and polluted our society, and compromised our well-being and quality of life. Treating this malignant syndrome is beyond the reach of psychopharmacology!
Thus, we psychiatrists should focus on the mood, psychotic, anxiety, and addiction syndromes that we encounter daily in our hospitals, clinics, and private offices. They affect tens of millions of patients. We currently have many psychotropic medications for these conditions. When combined with psychotherapy, the resulting synergy can be magical and immensely gratifying. However, some of those agents have limited efficacy due to the extensive heterogeneity of syndromes such as schizophrenia or depression, which are often confounded with comorbidities. A perfect balance between efficacy, tolerability, and safety are often hard to come by in pharmacotherapy.
The most glaring psychopharmacologic unmet need is that 80% of DSM disorders still do not have a single FDA-approved (evidence-based) medication.1 It will take decades, hundreds of billions of dollars, and the motivation of the often-maligned pharmaceutical industry (indispensable, because they are the only entity with the large R&D infrastructure to develop medications for psychiatry). Both academic and clinical psychiatrists must advise pharmaceutical companies about the unmet needs in our field and urge them to develop novel pharmacotherapies to address the gaps in the clinical care of psychiatric patients.
An inventory of unmet needs
With that in mind, here is a list of unmet needs I have been thinking about lately, and hoping that they will be resolved to help our patients achieve better clinical and functional outcomes.
Rapid-onset antipsychotics. The discovery that ketamine can rapidly convert refractory patients who are chronically depressed or suicidal to normal mood within a few hours shattered the dogma that weeks and months are needed for severe depression to improve, let alone achieve full remission. There is a similar dogma about psychosis requiring a protracted duration of antipsychotic treatment to attain significant impact. A rapid-acting antipsychotic agent would represent a major advance in psychiatry and its pharmaco-economic benefits would be substantial, given the high cost of inpatient hospitalization. Just as neurobiologic research guided the discovery of ketamine as a dramatic paradigm shift in treating depression, targeted research, especially focusing on glutamate pathways, may help identify a rapid-onset agent, whether oral, intranasal, IV, or even (why not) intrathecal. Research is known to enhance serendipity, which has been kind to psychiatry and has led to the discovery of several pharmacologic therapies in psychiatry, such as chlorpromazine, monoamine oxidase inhibitor antidepressants, and lithium.
Long-acting antidepressants and anxiolytics. This can be regarded as low-hanging fruit. Several technologies have been developed for long-acting formulations, yet they have been exploited mainly for antipsychotic medications. Some of these technologies can be employed to convert commonly used antidepressants (such as selective serotonin reuptake inhibitors) into long-acting antidepressants that can also reduce anxiety. Nonadherence among patients with depression is quite common, and relapses may lead to suicide attempts. The use of injectable, long-acting antidepressants can also reduce the incidence of overdoses because the patient will not have possession of potentially fatal pills.
Continue to: Long-acting mood stabilizers
Long-acting mood stabilizers. The rationale for long-acting mood stabilizers is the same as for long-acting antidepressants. Patients with bipolar disorder are known to stop taking their medications because they miss their “highs.” Some long-acting antipsychotics are approved for bipolar disorder, but these are often associated with adverse effects, such as metabolic dysregulation, extrapyramidal symptoms, and tardive dyskinesia. Mood stabilizers are essential for the bipolar spectrum.
A “real” treatment for alcohol use disorders that eliminates craving for alcohol. Alcoholism is associated with more than 100 medical complications and is one of the leading causes of disability in the world. It is frustrating that very few drug companies have focused on this widely prevalent brain disorder, which is also a common comorbid condition in many psychiatric syndromes.
Treatment-resistance pharmacotherapy solutions. All psychiatric syndromes are heterogeneous and contain ≥1 subgroups (biotypes) that fail to respond to what is considered the “standard” psychopharmacologic treatment (such as antipsychotics, antidepressants, mood stabilizers, or anti-obsessive medications). Technically, those so-called treatment-resistant subtypes need medications with a different mechanism of action. For example, clozapine for treatment-resistant schizophrenia and ketamine for treatment-resistant depression provide proof that treatment resistance is treatable but by a mechanism of action that is completely different from that of standard therapies, such as N-methyl-
Negative symptoms of schizophrenia cause significant functional disability and are well known to be a major unmet need. Some promising data are emerging on agents such as pimavanserin, cariprazine, and roluperidone, which is encouraging, but nothing is approved yet.
Cognitive deficits of schizophrenia, both neurocognition and social cognition, are another major unmet need that impair function in many patients. Many attempts to develop a pharmacologic treatment for these serious cognitive impairments have been made, but several candidates that initially appeared promising have bitten the dust. A focus on modulating the glutamate NMDA receptor may eventually lead to a breakthrough, and that may also help patients with bipolar disorder and major depressive disorder, both of whom also have cognitive deficits in several domains, albeit less severe than those experienced by patients with schizophrenia.
Continue to: Personality disorders
Personality disorders, especially borderline personality disorder, are very challenging to treat pharmacologically despite their prevalence and serious disruption to people’s lives. Hardly any FDA clinical trials have been conducted on any personality disorder. It is an unmet need that all psychiatrists would love to see addressed. But the mythical notion that personality disorders are untreatable may be an impediment in the pursuit of novel pharmacotherapy for borderline, narcissistic, antisocial, or schizotypal personality disorders, and other disorders. Heart attacks and religious conversion often change the baseline personality dramatically.
Childhood disorders. Apart from attention-deficit/hyperactivity disorder (ADHD), very few childhood psychiatric disorders have an FDA-approved medication. Why do drug companies avoid conducting controlled clinical trials in children age <10 who have autism, spectrum disorders, conduct disorder, oppositional defiant disorder, and other disorders? Effective pharmacotherapy for these children can be regarded as a desirable early intervention that may short-circuit their progression to serious adult psychopathology.
Parsimonious psychopharmacology for the treatment of trans-diagnostic psychiatric disorders. Recent research strongly suggests there is a strong overlap among psychiatric conditions, genetically, clinically, and biologically.2,3 For example, bipolar disorder is frequently accompanied by anxiety or substance use, patients with schizophrenia often experience anxiety, depression, or substance use, and ADHD has been found to share genes with autism.4,5
Eating disorders. There are no truly efficacious pharmacologic treatments for anorexia or bulimia nervosa. Research in this area is thin, and needs to be beefed up.
Sexual disorders. A huge unmet need exists for the pharmacotherapy of many sexual disorders that can have serious legal consequences (paraphilias) or quality-of-life repercussions (low sexual desire and orgasm disorders).
Continue to: A coordinated effort
A coordinated effort
It will take a massive collaboration among multiple stakeholders to launch the herculean process of addressing the unmet needs of all the above psychiatric disorders. This includes:
- the pharmaceutical industry (to provide the massive financial investment and R&D expertise)
- the federal government (to provide incentives)
- the FDA (to allow novel clinical trial designs)
- academic psychiatrists (to conduct research to discover the pathophysiology of psychiatric diseases)
- clinical psychiatrists (to provide consultations and advise about the clinical gaps in current psychopharmacological treatments)
- psychiatric patients (who are needed to volunteer for large-scale clinical trials).
This will be a veritable “psychiatric Manhattan Project” to advance the treatment of numerous psychiatric illnesses. The greatest benefit of discovering cures for disabling mental disorders is the evaporation of the virulent stigma that continues to plague our patients.
As for the political extremism that has corroded our society, it may be beyond pharmacologic redemption. An antidote to the “kool aid” has not yet been invented…
Let’s face it: The greatest unmet need in psychiatry is discovering a treatment for the infamous syndrome of toxic political extremism. Its ugly symptoms include blind hatred, visceral malice, bigotry, vandalism, hypocrisy, racism, hubris, intransigence, narcissism, demagoguery, mutual contempt, and intense schadenfreude.
This corrosive affliction has engulfed and polluted our society, and compromised our well-being and quality of life. Treating this malignant syndrome is beyond the reach of psychopharmacology!
Thus, we psychiatrists should focus on the mood, psychotic, anxiety, and addiction syndromes that we encounter daily in our hospitals, clinics, and private offices. They affect tens of millions of patients. We currently have many psychotropic medications for these conditions. When combined with psychotherapy, the resulting synergy can be magical and immensely gratifying. However, some of those agents have limited efficacy due to the extensive heterogeneity of syndromes such as schizophrenia or depression, which are often confounded with comorbidities. A perfect balance between efficacy, tolerability, and safety are often hard to come by in pharmacotherapy.
The most glaring psychopharmacologic unmet need is that 80% of DSM disorders still do not have a single FDA-approved (evidence-based) medication.1 It will take decades, hundreds of billions of dollars, and the motivation of the often-maligned pharmaceutical industry (indispensable, because they are the only entity with the large R&D infrastructure to develop medications for psychiatry). Both academic and clinical psychiatrists must advise pharmaceutical companies about the unmet needs in our field and urge them to develop novel pharmacotherapies to address the gaps in the clinical care of psychiatric patients.
An inventory of unmet needs
With that in mind, here is a list of unmet needs I have been thinking about lately, and hoping that they will be resolved to help our patients achieve better clinical and functional outcomes.
Rapid-onset antipsychotics. The discovery that ketamine can rapidly convert refractory patients who are chronically depressed or suicidal to normal mood within a few hours shattered the dogma that weeks and months are needed for severe depression to improve, let alone achieve full remission. There is a similar dogma about psychosis requiring a protracted duration of antipsychotic treatment to attain significant impact. A rapid-acting antipsychotic agent would represent a major advance in psychiatry and its pharmaco-economic benefits would be substantial, given the high cost of inpatient hospitalization. Just as neurobiologic research guided the discovery of ketamine as a dramatic paradigm shift in treating depression, targeted research, especially focusing on glutamate pathways, may help identify a rapid-onset agent, whether oral, intranasal, IV, or even (why not) intrathecal. Research is known to enhance serendipity, which has been kind to psychiatry and has led to the discovery of several pharmacologic therapies in psychiatry, such as chlorpromazine, monoamine oxidase inhibitor antidepressants, and lithium.
Long-acting antidepressants and anxiolytics. This can be regarded as low-hanging fruit. Several technologies have been developed for long-acting formulations, yet they have been exploited mainly for antipsychotic medications. Some of these technologies can be employed to convert commonly used antidepressants (such as selective serotonin reuptake inhibitors) into long-acting antidepressants that can also reduce anxiety. Nonadherence among patients with depression is quite common, and relapses may lead to suicide attempts. The use of injectable, long-acting antidepressants can also reduce the incidence of overdoses because the patient will not have possession of potentially fatal pills.
Continue to: Long-acting mood stabilizers
Long-acting mood stabilizers. The rationale for long-acting mood stabilizers is the same as for long-acting antidepressants. Patients with bipolar disorder are known to stop taking their medications because they miss their “highs.” Some long-acting antipsychotics are approved for bipolar disorder, but these are often associated with adverse effects, such as metabolic dysregulation, extrapyramidal symptoms, and tardive dyskinesia. Mood stabilizers are essential for the bipolar spectrum.
A “real” treatment for alcohol use disorders that eliminates craving for alcohol. Alcoholism is associated with more than 100 medical complications and is one of the leading causes of disability in the world. It is frustrating that very few drug companies have focused on this widely prevalent brain disorder, which is also a common comorbid condition in many psychiatric syndromes.
Treatment-resistance pharmacotherapy solutions. All psychiatric syndromes are heterogeneous and contain ≥1 subgroups (biotypes) that fail to respond to what is considered the “standard” psychopharmacologic treatment (such as antipsychotics, antidepressants, mood stabilizers, or anti-obsessive medications). Technically, those so-called treatment-resistant subtypes need medications with a different mechanism of action. For example, clozapine for treatment-resistant schizophrenia and ketamine for treatment-resistant depression provide proof that treatment resistance is treatable but by a mechanism of action that is completely different from that of standard therapies, such as N-methyl-
Negative symptoms of schizophrenia cause significant functional disability and are well known to be a major unmet need. Some promising data are emerging on agents such as pimavanserin, cariprazine, and roluperidone, which is encouraging, but nothing is approved yet.
Cognitive deficits of schizophrenia, both neurocognition and social cognition, are another major unmet need that impair function in many patients. Many attempts to develop a pharmacologic treatment for these serious cognitive impairments have been made, but several candidates that initially appeared promising have bitten the dust. A focus on modulating the glutamate NMDA receptor may eventually lead to a breakthrough, and that may also help patients with bipolar disorder and major depressive disorder, both of whom also have cognitive deficits in several domains, albeit less severe than those experienced by patients with schizophrenia.
Continue to: Personality disorders
Personality disorders, especially borderline personality disorder, are very challenging to treat pharmacologically despite their prevalence and serious disruption to people’s lives. Hardly any FDA clinical trials have been conducted on any personality disorder. It is an unmet need that all psychiatrists would love to see addressed. But the mythical notion that personality disorders are untreatable may be an impediment in the pursuit of novel pharmacotherapy for borderline, narcissistic, antisocial, or schizotypal personality disorders, and other disorders. Heart attacks and religious conversion often change the baseline personality dramatically.
Childhood disorders. Apart from attention-deficit/hyperactivity disorder (ADHD), very few childhood psychiatric disorders have an FDA-approved medication. Why do drug companies avoid conducting controlled clinical trials in children age <10 who have autism, spectrum disorders, conduct disorder, oppositional defiant disorder, and other disorders? Effective pharmacotherapy for these children can be regarded as a desirable early intervention that may short-circuit their progression to serious adult psychopathology.
Parsimonious psychopharmacology for the treatment of trans-diagnostic psychiatric disorders. Recent research strongly suggests there is a strong overlap among psychiatric conditions, genetically, clinically, and biologically.2,3 For example, bipolar disorder is frequently accompanied by anxiety or substance use, patients with schizophrenia often experience anxiety, depression, or substance use, and ADHD has been found to share genes with autism.4,5
Eating disorders. There are no truly efficacious pharmacologic treatments for anorexia or bulimia nervosa. Research in this area is thin, and needs to be beefed up.
Sexual disorders. A huge unmet need exists for the pharmacotherapy of many sexual disorders that can have serious legal consequences (paraphilias) or quality-of-life repercussions (low sexual desire and orgasm disorders).
Continue to: A coordinated effort
A coordinated effort
It will take a massive collaboration among multiple stakeholders to launch the herculean process of addressing the unmet needs of all the above psychiatric disorders. This includes:
- the pharmaceutical industry (to provide the massive financial investment and R&D expertise)
- the federal government (to provide incentives)
- the FDA (to allow novel clinical trial designs)
- academic psychiatrists (to conduct research to discover the pathophysiology of psychiatric diseases)
- clinical psychiatrists (to provide consultations and advise about the clinical gaps in current psychopharmacological treatments)
- psychiatric patients (who are needed to volunteer for large-scale clinical trials).
This will be a veritable “psychiatric Manhattan Project” to advance the treatment of numerous psychiatric illnesses. The greatest benefit of discovering cures for disabling mental disorders is the evaporation of the virulent stigma that continues to plague our patients.
As for the political extremism that has corroded our society, it may be beyond pharmacologic redemption. An antidote to the “kool aid” has not yet been invented…
1. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
2. Nasrallah HA. Is there only 1 neurobiologic disorder, with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
3. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
4. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
5. Marshall M. Roots of mental illness. Nature. 2020;581:19-21.
1. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
2. Nasrallah HA. Is there only 1 neurobiologic disorder, with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
3. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
4. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
5. Marshall M. Roots of mental illness. Nature. 2020;581:19-21.
Infected with COVID-19: One psychiatrist’s story
Emil: Coronavirus disease 2019 (COVID-19) wasn’t really on my mind until the first weekend in March, specifically Sunday, March 8, 2020. That weekend had us traveling from Chicago to Berwyn, Pennsylvania to attend the funeral of one of my older cousins. Though we were the only ones from his side at the graveside, his funeral had drawn numerous relatives, none of whom were “socially distanced.”
On our way home, I received an e-mail from a colleague in Brazil who had invited me to speak at a conference in São Paulo. He told me that several of my American colleagues had contacted him and informed him that their universities had banned travel because of COVID. “I’m coming,” I replied. “I don’t think COVID’s going to be a big deal here.” He said COVID wasn’t a “big deal” in Brazil, either. Famous last words.
The next weekend, I left early on Saturday morning to start my call duty at the hospital. After finishing rounds at one hospital and going to the next, I got a text from my wife, Anne, asking “What’s wrong with your people over there? What kind of doctors would take a 65-year-old colleague with a history of asthma, and history of an ICU stay with 10 days on a respirator with acute respiratory distress syndrome 10 years ago, and have him exposed to this lethal virus? Are they trying to kill you?”
It stopped me in my tracks. She was right. A lot had changed in a week. In that single week, it had become clear that COVID was a real threat, and I was vulnerable. I finished my call duty but made it clear to the “powers that be” I was going to stay home and isolate for the next few weeks, until we knew more. I was ahead of the curve, but not by much: within days, Chicago shut down with a “stay-at-home” order.
Anne: When the threat of COVID first became known, I said to family and friends, “If Emil gets this, it’s going to be very, very bad.” After that, we made certain to wear masks and gloves when we went out, which wasn’t often.
Emil: We stayed in for the next 3 months until we moved to Columbus, Ohio for my new position as Vice Chair for Research in the Department of Psychiatry and Behavioral Health at The Ohio State University Wexner Medical Center (OSUMC).
The day after arriving, I went to the emergency dental clinic because of a severe toothache. While they couldn’t save my tooth, I got something in return: COVID. The clinic took more than appropriate precautions, but I was in a very large room, not a private office, with many patients having their teeth drilled and whatever it is dentists do (actually, I do know; my father was a dentist).
Continue to: All was fine until 2 days later...
All was fine until 2 days later, when I began to feel a bit “unwell” on late Friday afternoon. I went out to do some chores the next morning, but soon returned home exhausted. The rest of the weekend was more of the same, and I was surprised at how I just couldn’t get anything done. On Monday, I felt a chill and thought I might have COVID.
The next morning, I went to OSUMC for a COVID test, but by then I already knew the result. The night before, Anne started complaining of a dry cough that would not stop.
Anne: When I realized Emil had COVID, I wrote to a friend, “If he gets bad and has to go to the hospital, or worse … he goes on a ventilator, I may need to be admitted to a psych ward!” I was still upset from the memory of sitting by Emil’s bedside when he was sick, and on a ventilator, 10 years ago, with his doctors talking with me about when, not if, he died.
Emil: My test came back within 8 hours on Tuesday. It was positive, as was the one for Anne the next day. The doctor I spoke to that evening thought I was only having a mild case and that I should just stay isolated. We immediately got a thermometer and a pulse oximeter to follow our symptoms. Anne’s oxygen saturation levels were always above 95%, but mine were lower, and by Friday, 3 days later and 1 week after my first symptoms, they were down to 92% or less. At that point, we both went to the ER at OSUMC.
Anne: We went to different places in the ER to be evaluated. As Emil was being wheeled away in the ER for his evaluation, I ran over for a kiss—with our masks on.
Continue to: As my ER evaluation...
As my ER evaluation was concluding, my doctor said, “I want someone, preferably the same person, to check in on you every day.” I replied I had a friend who is a critical care nurse. He smiled and said, “Excellent.” My friend called every day, and when she didn’t like how I sounded, on some days, she found an excuse to call again.
Emil: I barely recall my ER evaluation, except that I was to be admitted for observation and supplemental oxygen. I accepted this with aplomb, knowing I was in good hands and hoping I’d be home soon.
Anne: Because we were in the same ER, I thought I’d be able to see Emil once they decided to admit him. No. They wouldn’t even let me go to him to get his wallet for safekeeping. Instead, it was brought to me in a hazmat bag. Thus began our forced separation for the next 5 weeks.
Emil: I had to wait hours for a bed and was wheeled up late in the evening to a double room with one other patient, also with COVID, I supposed. While I had an oxygen mask on, we were only separated by a curtain. I had no idea I wouldn’t see Anne for weeks.
Anne: I returned “home” to a house I had spent less than 5 days in. We had barely moved in and it only had a bed, a couch, a TV, and a kitchen chair. I didn’t even know my neighbors to wave at, and … I was in quarantine. No one could come to me. Our eldest daughter was alone near Burlington, Vermont (where she had escaped to from New York City when it was the national epicenter for COVID back in March). Our youngest daughter was alone in Los Angeles, and our son, a newly minted First Lieutenant in the Army, was stationed in Afghanistan. “Good for him,” I thought. He could safely interact with his army buddies. It was so ironic; the one in the war zone was the only one of us who was safe from COVID.
Continue to: I reached out to family and friends...
I reached out to family and friends and asked for prayers. Emil was prayed for by all of our Catholic, Methodist, Jewish, Muslim, and Buddhist friends. As I told him later, he was prayed for from Afghanistan to Alaska. My extended family activated a text chain so all I had to do was reply and everyone on the chain would have the same information. I also received many notes and cards of support from friends and Emil’s family. Many told me how strong I was and how I would be fine. Later, I realized how many of these were from widows who were telling me I would survive bereavement, should that be the outcome.
Emil: The next day, the doctors started me on a 5-day course of the newly “approved” antiviral remdesivir, and the day after that, I received 2 units of convalescent plasma on “compassionate use” from the Mayo Clinic. It didn’t matter. I kept getting worse.
Anne: I received twice-daily updates from the nurses. When the updates were late in coming, I crawled the walls, waiting at least 2 hours before reaching out. One day, the nurse who answered said she couldn’t talk because his nurse was dealing with an emergency with him. I didn’t take a deep breath until his nurse called back to say he was stable. Regardless, he just kept getting sicker and sicker, and I began to fear he would not make it.
Emil: By Day 5, my X-ray showed clear evidence of a bilateral pneumonia (it had appeared “normal” on admission) and I was transferred to a “step-up unit.” The next day, I was transferred to the ICU and placed on a ventilator, in the prone position, for 16 hours a day.
Anne: The day Emil was transferred to the ICU, he told me he was worried about his fate. He called and asked me to stay on the phone with him while waiting to go to the ICU. We were both so weak we couldn’t do more than say “I love you” and listen to the other’s labored breathing. That was our last phone call until he was off the ventilator 10 days later.
Continue to: Emil's reply
Emil: At this point I had no idea what was going on. I was on a ventilator and I was “out.”
Anne: In the meantime, my family made sure I knew they were thinking of us. Every day I woke up with a text from one cousin asking how the night was while my sister checked in every afternoon. They sent flowers and baskets of goodies. Knowing how difficult it was waiting for updates, they sent me a jigsaw puzzle with a thousand pieces. I was surprised at how important that was for binding my anxiety. A friend sent books from my favorite writers.
Despite all this, I was absolutely beside myself the night Emil was placed on the ventilator. I cleaned and scrubbed the house; not that it needed it, I needed it. In the bedroom I saw a bottle under the bed. I retrieved it but couldn’t get up off the floor. I was weak and had tremendous muscle pain each time I moved. I had my phone, so knew I wouldn’t be stranded, but … I didn’t relish the idea of calling 911 and have them break down the front door in their hazmat suits. After more than 30 minutes, and much effort, I was able to get myself up; soon after, I put a house key outside.
When a friend who was taking care of our 2 dogs in Chicago heard that Emil was on the ventilator, she drove through the night to bring them to me so I would have them for solace. She couldn’t even come in the house. She stayed at a nearby hotel and visited with me from outside with masks on waiting for the updates.
Emil: Being an elder lawyer married to a physician, Anne knows a thing or 2 about medicine (because she’s seen a thing or 2 about medicine). She’s even been known to give her elderly clients Mini-Mental State Exams. In addition to talking with members of her support system, Anne was also talking with friends and relatives who are physicians. One exclaimed, “He’s having a cytokine storm!” and said I needed steroids. Another said, yes, that and serious “anti-inflammatory” drugs. At that moment, data supporting the use of steroids or “anti-inflammatories” in COVID hadn’t yet become public. The data on steroids came out early the next week in the Lancet and the data on “anti-inflammatories” was still in process until a few weeks later.
Continue to: Anne was ahead of the curve...
Anne was ahead of the curve and advocated hard for both treatments. At the same time, my OSUMC physicians were considering other options for me. They were checking on my inflammatory status by following my levels of C-reactive protein (CRP) and interleukin-6 (IL-6). On Days 2 and 3, my CRP level was 64 mg/L and my IL-6 level was 32 pg/mL (neither should be higher than 1).
While I don’t recall much before being on the ventilator, I do recall my alarm at seeing my CRP/IL-6 levels go up in real time on alerts from “My Chart” (my CRP/IL-6 levels were 149/123 within 4 days of admission, and reached a high of about 250/190 as I entered the ICU). I knew what those numbers meant. It was surreal; like watching myself die off in the distance, emotionally disconnected from the whole scene.
The decision to give steroids was relatively easy, and I was started on dexamethasone, a very inexpensive steroid, on Day 7 (ICU Day 2). The decision of which “antiinflammatory” to give was more difficult, as OSUMC had over 40 treatment protocols for COVID. Anne suggested 2 drugs based on recommendations from our physician friends—tocilizumab and acalabrutinib— both were on the market for other conditions and very expensive. The first is an IL-6 antagonist, while the second shuts down cytokine production in B cells, an effect also observed in lung tissue. While tocilizumab was not included in any of the OSUMC COVID protocols, acalabrutinib was, and I started on that medication on Day 8 (ICU Day 3).
Anne: My experience being the advocate was different than the first time 10 years before. That time, Emil had a community-acquired pneumonia, with which our doctors had much experience. This time, I was more active because no one had much information about how to deal with COVID and, thus, there was no standard of care. In fact, Emil was only the second patient to receive acalabrutinib at OSUMC; later, we found out that that patient did well.
Emil: The “anti-inflammatory” strategy worked. Within 5 days of starting the 2 drugs, my CRP and IL-6 levels were down to 10 and 5, respectively; a reduction of >95%. As these levels dropped, so did my oxygen requirements.
Continue to: Anne's reply
Anne: Emil was finally on the upswing. I woke up the next morning and, surprisingly, found that my first emotion wasn’t one of terror. His ICU doctor, a real booster for Emil, made it her mission to get him off the ventilator before the end of her ICU service week. She succeeded.
Emil: Five days after coming off the ventilator, I went to a rehab unit for reconditioning and to begin the long process of recovering my strength and stamina.
Most people say to me, “How awful for you! How terrible!” I smile and say, “Yeah, well, I missed all the excitement. It was really much worse for Anne.” I told them that, although you don’t recall anything while on the ventilator, you get retrograde amnesia for the several days prior to artificial ventilation. I have texts on my cell phone, written by me in those first few days, I don’t recall writing. Anne says we had conversations all the way up to my admission to the ICU; I recall none of those. Frankly, that’s for the best.
One thing to highlight is that your brain doesn’t stop working while you’re “out.” I had numerous vivid dreams, or whatever they were, while on the ventilator and after. Many were “bizarre and dark,” others were “dark and bizarre.” A few were amusing— in the end. I recall watching a TV news program segment describing how we donated our 2 little dogs to the Queen of England, who then gave them to her youngest son, Edward. I swear, I actually “saw” this TV program and watched the Queen and her son (and his wife) playing with our dogs. I was so convinced, I asked Anne where our dogs were; with her, of course. No, she assured me, we hadn’t given them to Queen Elizabeth II. Another conversation I swore I had with Anne was one in which she was telling me she was starting the vetting process to be a VP candidate for Joe Biden (Anne had been involved in Chicago politics so … not totally “crazy”). Nevertheless, I was quickly disabused of this one by my eldest daughter, also a lawyer.
Anne: This time, like the last time he was on a ventilator, Emil took a few more days to clear all the drugs keeping him sedated. Last time, his medical center sent his colleague, the Chair of Neurology, to check on him because there was a concern that he wasn’t “clearing” fast enough. This time, I was the one reassuring the doctors and nurses to be “patient.” At the same time, I was disabusing him of his far-fetched idea that he was head of all research at OSUMC and head of the ICU. He told me, “I don’t understand it. Don’t these people know they work for me?” “No,” I told him. “You are a patient there, and you need to behave.” Aside from that, Emil was fairly lucid. As one of his nurses said, “He’s oriented, he’s just wrong!”
Continue to: Emil's reply
Emil: Some people have asked me if this experience has changed my perspective. It could have, but I went through something worse 10 years ago when I was first brought back from the “mostly dead.” After that, I realized the most important things in life are the people you love and the people who love you; the good stuff is “gravy” and everything else isn’t worth spending much time or energy on. The first thing I said to Anne when we were face-to-face, as I entered the rehab facility (with masks on, of course), was “I can’t do this to you again.”
Anne: One of the most inhumane aspects of COVID is that you can’t be with your loved one while they are sick. Last time I spent 10 to 12 hours a day at the bedside. This time I couldn’t be there at all. It was especially hard because I knew from the last time how much my presence meant to him. If I left, he would get agitated. His heart rate would come down by 10 beats when I sat next to him.
When we had our first post-ventilator conversation on Father’s Day, he was surprised I was so excited to talk to him. Somehow, he thought I had abandoned him. What he didn’t know was that I was thinking about getting a job in Housekeeping at the hospital just so I could go see him!
Emil: In the end, I’m now back to baseline and grateful I’m alive. I still have things I want to do professionally and personally, and am appreciative I’ll have more time for those. However, I am appalled at how a serious public health issue has been turned into a political weapon by “science deniers” and that this is continuing to kill our citizens. That’s not a nightmare from when I was ill. It’s the “day-mare” we are living now.
Emil: Coronavirus disease 2019 (COVID-19) wasn’t really on my mind until the first weekend in March, specifically Sunday, March 8, 2020. That weekend had us traveling from Chicago to Berwyn, Pennsylvania to attend the funeral of one of my older cousins. Though we were the only ones from his side at the graveside, his funeral had drawn numerous relatives, none of whom were “socially distanced.”
On our way home, I received an e-mail from a colleague in Brazil who had invited me to speak at a conference in São Paulo. He told me that several of my American colleagues had contacted him and informed him that their universities had banned travel because of COVID. “I’m coming,” I replied. “I don’t think COVID’s going to be a big deal here.” He said COVID wasn’t a “big deal” in Brazil, either. Famous last words.
The next weekend, I left early on Saturday morning to start my call duty at the hospital. After finishing rounds at one hospital and going to the next, I got a text from my wife, Anne, asking “What’s wrong with your people over there? What kind of doctors would take a 65-year-old colleague with a history of asthma, and history of an ICU stay with 10 days on a respirator with acute respiratory distress syndrome 10 years ago, and have him exposed to this lethal virus? Are they trying to kill you?”
It stopped me in my tracks. She was right. A lot had changed in a week. In that single week, it had become clear that COVID was a real threat, and I was vulnerable. I finished my call duty but made it clear to the “powers that be” I was going to stay home and isolate for the next few weeks, until we knew more. I was ahead of the curve, but not by much: within days, Chicago shut down with a “stay-at-home” order.
Anne: When the threat of COVID first became known, I said to family and friends, “If Emil gets this, it’s going to be very, very bad.” After that, we made certain to wear masks and gloves when we went out, which wasn’t often.
Emil: We stayed in for the next 3 months until we moved to Columbus, Ohio for my new position as Vice Chair for Research in the Department of Psychiatry and Behavioral Health at The Ohio State University Wexner Medical Center (OSUMC).
The day after arriving, I went to the emergency dental clinic because of a severe toothache. While they couldn’t save my tooth, I got something in return: COVID. The clinic took more than appropriate precautions, but I was in a very large room, not a private office, with many patients having their teeth drilled and whatever it is dentists do (actually, I do know; my father was a dentist).
Continue to: All was fine until 2 days later...
All was fine until 2 days later, when I began to feel a bit “unwell” on late Friday afternoon. I went out to do some chores the next morning, but soon returned home exhausted. The rest of the weekend was more of the same, and I was surprised at how I just couldn’t get anything done. On Monday, I felt a chill and thought I might have COVID.
The next morning, I went to OSUMC for a COVID test, but by then I already knew the result. The night before, Anne started complaining of a dry cough that would not stop.
Anne: When I realized Emil had COVID, I wrote to a friend, “If he gets bad and has to go to the hospital, or worse … he goes on a ventilator, I may need to be admitted to a psych ward!” I was still upset from the memory of sitting by Emil’s bedside when he was sick, and on a ventilator, 10 years ago, with his doctors talking with me about when, not if, he died.
Emil: My test came back within 8 hours on Tuesday. It was positive, as was the one for Anne the next day. The doctor I spoke to that evening thought I was only having a mild case and that I should just stay isolated. We immediately got a thermometer and a pulse oximeter to follow our symptoms. Anne’s oxygen saturation levels were always above 95%, but mine were lower, and by Friday, 3 days later and 1 week after my first symptoms, they were down to 92% or less. At that point, we both went to the ER at OSUMC.
Anne: We went to different places in the ER to be evaluated. As Emil was being wheeled away in the ER for his evaluation, I ran over for a kiss—with our masks on.
Continue to: As my ER evaluation...
As my ER evaluation was concluding, my doctor said, “I want someone, preferably the same person, to check in on you every day.” I replied I had a friend who is a critical care nurse. He smiled and said, “Excellent.” My friend called every day, and when she didn’t like how I sounded, on some days, she found an excuse to call again.
Emil: I barely recall my ER evaluation, except that I was to be admitted for observation and supplemental oxygen. I accepted this with aplomb, knowing I was in good hands and hoping I’d be home soon.
Anne: Because we were in the same ER, I thought I’d be able to see Emil once they decided to admit him. No. They wouldn’t even let me go to him to get his wallet for safekeeping. Instead, it was brought to me in a hazmat bag. Thus began our forced separation for the next 5 weeks.
Emil: I had to wait hours for a bed and was wheeled up late in the evening to a double room with one other patient, also with COVID, I supposed. While I had an oxygen mask on, we were only separated by a curtain. I had no idea I wouldn’t see Anne for weeks.
Anne: I returned “home” to a house I had spent less than 5 days in. We had barely moved in and it only had a bed, a couch, a TV, and a kitchen chair. I didn’t even know my neighbors to wave at, and … I was in quarantine. No one could come to me. Our eldest daughter was alone near Burlington, Vermont (where she had escaped to from New York City when it was the national epicenter for COVID back in March). Our youngest daughter was alone in Los Angeles, and our son, a newly minted First Lieutenant in the Army, was stationed in Afghanistan. “Good for him,” I thought. He could safely interact with his army buddies. It was so ironic; the one in the war zone was the only one of us who was safe from COVID.
Continue to: I reached out to family and friends...
I reached out to family and friends and asked for prayers. Emil was prayed for by all of our Catholic, Methodist, Jewish, Muslim, and Buddhist friends. As I told him later, he was prayed for from Afghanistan to Alaska. My extended family activated a text chain so all I had to do was reply and everyone on the chain would have the same information. I also received many notes and cards of support from friends and Emil’s family. Many told me how strong I was and how I would be fine. Later, I realized how many of these were from widows who were telling me I would survive bereavement, should that be the outcome.
Emil: The next day, the doctors started me on a 5-day course of the newly “approved” antiviral remdesivir, and the day after that, I received 2 units of convalescent plasma on “compassionate use” from the Mayo Clinic. It didn’t matter. I kept getting worse.
Anne: I received twice-daily updates from the nurses. When the updates were late in coming, I crawled the walls, waiting at least 2 hours before reaching out. One day, the nurse who answered said she couldn’t talk because his nurse was dealing with an emergency with him. I didn’t take a deep breath until his nurse called back to say he was stable. Regardless, he just kept getting sicker and sicker, and I began to fear he would not make it.
Emil: By Day 5, my X-ray showed clear evidence of a bilateral pneumonia (it had appeared “normal” on admission) and I was transferred to a “step-up unit.” The next day, I was transferred to the ICU and placed on a ventilator, in the prone position, for 16 hours a day.
Anne: The day Emil was transferred to the ICU, he told me he was worried about his fate. He called and asked me to stay on the phone with him while waiting to go to the ICU. We were both so weak we couldn’t do more than say “I love you” and listen to the other’s labored breathing. That was our last phone call until he was off the ventilator 10 days later.
Continue to: Emil's reply
Emil: At this point I had no idea what was going on. I was on a ventilator and I was “out.”
Anne: In the meantime, my family made sure I knew they were thinking of us. Every day I woke up with a text from one cousin asking how the night was while my sister checked in every afternoon. They sent flowers and baskets of goodies. Knowing how difficult it was waiting for updates, they sent me a jigsaw puzzle with a thousand pieces. I was surprised at how important that was for binding my anxiety. A friend sent books from my favorite writers.
Despite all this, I was absolutely beside myself the night Emil was placed on the ventilator. I cleaned and scrubbed the house; not that it needed it, I needed it. In the bedroom I saw a bottle under the bed. I retrieved it but couldn’t get up off the floor. I was weak and had tremendous muscle pain each time I moved. I had my phone, so knew I wouldn’t be stranded, but … I didn’t relish the idea of calling 911 and have them break down the front door in their hazmat suits. After more than 30 minutes, and much effort, I was able to get myself up; soon after, I put a house key outside.
When a friend who was taking care of our 2 dogs in Chicago heard that Emil was on the ventilator, she drove through the night to bring them to me so I would have them for solace. She couldn’t even come in the house. She stayed at a nearby hotel and visited with me from outside with masks on waiting for the updates.
Emil: Being an elder lawyer married to a physician, Anne knows a thing or 2 about medicine (because she’s seen a thing or 2 about medicine). She’s even been known to give her elderly clients Mini-Mental State Exams. In addition to talking with members of her support system, Anne was also talking with friends and relatives who are physicians. One exclaimed, “He’s having a cytokine storm!” and said I needed steroids. Another said, yes, that and serious “anti-inflammatory” drugs. At that moment, data supporting the use of steroids or “anti-inflammatories” in COVID hadn’t yet become public. The data on steroids came out early the next week in the Lancet and the data on “anti-inflammatories” was still in process until a few weeks later.
Continue to: Anne was ahead of the curve...
Anne was ahead of the curve and advocated hard for both treatments. At the same time, my OSUMC physicians were considering other options for me. They were checking on my inflammatory status by following my levels of C-reactive protein (CRP) and interleukin-6 (IL-6). On Days 2 and 3, my CRP level was 64 mg/L and my IL-6 level was 32 pg/mL (neither should be higher than 1).
While I don’t recall much before being on the ventilator, I do recall my alarm at seeing my CRP/IL-6 levels go up in real time on alerts from “My Chart” (my CRP/IL-6 levels were 149/123 within 4 days of admission, and reached a high of about 250/190 as I entered the ICU). I knew what those numbers meant. It was surreal; like watching myself die off in the distance, emotionally disconnected from the whole scene.
The decision to give steroids was relatively easy, and I was started on dexamethasone, a very inexpensive steroid, on Day 7 (ICU Day 2). The decision of which “antiinflammatory” to give was more difficult, as OSUMC had over 40 treatment protocols for COVID. Anne suggested 2 drugs based on recommendations from our physician friends—tocilizumab and acalabrutinib— both were on the market for other conditions and very expensive. The first is an IL-6 antagonist, while the second shuts down cytokine production in B cells, an effect also observed in lung tissue. While tocilizumab was not included in any of the OSUMC COVID protocols, acalabrutinib was, and I started on that medication on Day 8 (ICU Day 3).
Anne: My experience being the advocate was different than the first time 10 years before. That time, Emil had a community-acquired pneumonia, with which our doctors had much experience. This time, I was more active because no one had much information about how to deal with COVID and, thus, there was no standard of care. In fact, Emil was only the second patient to receive acalabrutinib at OSUMC; later, we found out that that patient did well.
Emil: The “anti-inflammatory” strategy worked. Within 5 days of starting the 2 drugs, my CRP and IL-6 levels were down to 10 and 5, respectively; a reduction of >95%. As these levels dropped, so did my oxygen requirements.
Continue to: Anne's reply
Anne: Emil was finally on the upswing. I woke up the next morning and, surprisingly, found that my first emotion wasn’t one of terror. His ICU doctor, a real booster for Emil, made it her mission to get him off the ventilator before the end of her ICU service week. She succeeded.
Emil: Five days after coming off the ventilator, I went to a rehab unit for reconditioning and to begin the long process of recovering my strength and stamina.
Most people say to me, “How awful for you! How terrible!” I smile and say, “Yeah, well, I missed all the excitement. It was really much worse for Anne.” I told them that, although you don’t recall anything while on the ventilator, you get retrograde amnesia for the several days prior to artificial ventilation. I have texts on my cell phone, written by me in those first few days, I don’t recall writing. Anne says we had conversations all the way up to my admission to the ICU; I recall none of those. Frankly, that’s for the best.
One thing to highlight is that your brain doesn’t stop working while you’re “out.” I had numerous vivid dreams, or whatever they were, while on the ventilator and after. Many were “bizarre and dark,” others were “dark and bizarre.” A few were amusing— in the end. I recall watching a TV news program segment describing how we donated our 2 little dogs to the Queen of England, who then gave them to her youngest son, Edward. I swear, I actually “saw” this TV program and watched the Queen and her son (and his wife) playing with our dogs. I was so convinced, I asked Anne where our dogs were; with her, of course. No, she assured me, we hadn’t given them to Queen Elizabeth II. Another conversation I swore I had with Anne was one in which she was telling me she was starting the vetting process to be a VP candidate for Joe Biden (Anne had been involved in Chicago politics so … not totally “crazy”). Nevertheless, I was quickly disabused of this one by my eldest daughter, also a lawyer.
Anne: This time, like the last time he was on a ventilator, Emil took a few more days to clear all the drugs keeping him sedated. Last time, his medical center sent his colleague, the Chair of Neurology, to check on him because there was a concern that he wasn’t “clearing” fast enough. This time, I was the one reassuring the doctors and nurses to be “patient.” At the same time, I was disabusing him of his far-fetched idea that he was head of all research at OSUMC and head of the ICU. He told me, “I don’t understand it. Don’t these people know they work for me?” “No,” I told him. “You are a patient there, and you need to behave.” Aside from that, Emil was fairly lucid. As one of his nurses said, “He’s oriented, he’s just wrong!”
Continue to: Emil's reply
Emil: Some people have asked me if this experience has changed my perspective. It could have, but I went through something worse 10 years ago when I was first brought back from the “mostly dead.” After that, I realized the most important things in life are the people you love and the people who love you; the good stuff is “gravy” and everything else isn’t worth spending much time or energy on. The first thing I said to Anne when we were face-to-face, as I entered the rehab facility (with masks on, of course), was “I can’t do this to you again.”
Anne: One of the most inhumane aspects of COVID is that you can’t be with your loved one while they are sick. Last time I spent 10 to 12 hours a day at the bedside. This time I couldn’t be there at all. It was especially hard because I knew from the last time how much my presence meant to him. If I left, he would get agitated. His heart rate would come down by 10 beats when I sat next to him.
When we had our first post-ventilator conversation on Father’s Day, he was surprised I was so excited to talk to him. Somehow, he thought I had abandoned him. What he didn’t know was that I was thinking about getting a job in Housekeeping at the hospital just so I could go see him!
Emil: In the end, I’m now back to baseline and grateful I’m alive. I still have things I want to do professionally and personally, and am appreciative I’ll have more time for those. However, I am appalled at how a serious public health issue has been turned into a political weapon by “science deniers” and that this is continuing to kill our citizens. That’s not a nightmare from when I was ill. It’s the “day-mare” we are living now.
Emil: Coronavirus disease 2019 (COVID-19) wasn’t really on my mind until the first weekend in March, specifically Sunday, March 8, 2020. That weekend had us traveling from Chicago to Berwyn, Pennsylvania to attend the funeral of one of my older cousins. Though we were the only ones from his side at the graveside, his funeral had drawn numerous relatives, none of whom were “socially distanced.”
On our way home, I received an e-mail from a colleague in Brazil who had invited me to speak at a conference in São Paulo. He told me that several of my American colleagues had contacted him and informed him that their universities had banned travel because of COVID. “I’m coming,” I replied. “I don’t think COVID’s going to be a big deal here.” He said COVID wasn’t a “big deal” in Brazil, either. Famous last words.
The next weekend, I left early on Saturday morning to start my call duty at the hospital. After finishing rounds at one hospital and going to the next, I got a text from my wife, Anne, asking “What’s wrong with your people over there? What kind of doctors would take a 65-year-old colleague with a history of asthma, and history of an ICU stay with 10 days on a respirator with acute respiratory distress syndrome 10 years ago, and have him exposed to this lethal virus? Are they trying to kill you?”
It stopped me in my tracks. She was right. A lot had changed in a week. In that single week, it had become clear that COVID was a real threat, and I was vulnerable. I finished my call duty but made it clear to the “powers that be” I was going to stay home and isolate for the next few weeks, until we knew more. I was ahead of the curve, but not by much: within days, Chicago shut down with a “stay-at-home” order.
Anne: When the threat of COVID first became known, I said to family and friends, “If Emil gets this, it’s going to be very, very bad.” After that, we made certain to wear masks and gloves when we went out, which wasn’t often.
Emil: We stayed in for the next 3 months until we moved to Columbus, Ohio for my new position as Vice Chair for Research in the Department of Psychiatry and Behavioral Health at The Ohio State University Wexner Medical Center (OSUMC).
The day after arriving, I went to the emergency dental clinic because of a severe toothache. While they couldn’t save my tooth, I got something in return: COVID. The clinic took more than appropriate precautions, but I was in a very large room, not a private office, with many patients having their teeth drilled and whatever it is dentists do (actually, I do know; my father was a dentist).
Continue to: All was fine until 2 days later...
All was fine until 2 days later, when I began to feel a bit “unwell” on late Friday afternoon. I went out to do some chores the next morning, but soon returned home exhausted. The rest of the weekend was more of the same, and I was surprised at how I just couldn’t get anything done. On Monday, I felt a chill and thought I might have COVID.
The next morning, I went to OSUMC for a COVID test, but by then I already knew the result. The night before, Anne started complaining of a dry cough that would not stop.
Anne: When I realized Emil had COVID, I wrote to a friend, “If he gets bad and has to go to the hospital, or worse … he goes on a ventilator, I may need to be admitted to a psych ward!” I was still upset from the memory of sitting by Emil’s bedside when he was sick, and on a ventilator, 10 years ago, with his doctors talking with me about when, not if, he died.
Emil: My test came back within 8 hours on Tuesday. It was positive, as was the one for Anne the next day. The doctor I spoke to that evening thought I was only having a mild case and that I should just stay isolated. We immediately got a thermometer and a pulse oximeter to follow our symptoms. Anne’s oxygen saturation levels were always above 95%, but mine were lower, and by Friday, 3 days later and 1 week after my first symptoms, they were down to 92% or less. At that point, we both went to the ER at OSUMC.
Anne: We went to different places in the ER to be evaluated. As Emil was being wheeled away in the ER for his evaluation, I ran over for a kiss—with our masks on.
Continue to: As my ER evaluation...
As my ER evaluation was concluding, my doctor said, “I want someone, preferably the same person, to check in on you every day.” I replied I had a friend who is a critical care nurse. He smiled and said, “Excellent.” My friend called every day, and when she didn’t like how I sounded, on some days, she found an excuse to call again.
Emil: I barely recall my ER evaluation, except that I was to be admitted for observation and supplemental oxygen. I accepted this with aplomb, knowing I was in good hands and hoping I’d be home soon.
Anne: Because we were in the same ER, I thought I’d be able to see Emil once they decided to admit him. No. They wouldn’t even let me go to him to get his wallet for safekeeping. Instead, it was brought to me in a hazmat bag. Thus began our forced separation for the next 5 weeks.
Emil: I had to wait hours for a bed and was wheeled up late in the evening to a double room with one other patient, also with COVID, I supposed. While I had an oxygen mask on, we were only separated by a curtain. I had no idea I wouldn’t see Anne for weeks.
Anne: I returned “home” to a house I had spent less than 5 days in. We had barely moved in and it only had a bed, a couch, a TV, and a kitchen chair. I didn’t even know my neighbors to wave at, and … I was in quarantine. No one could come to me. Our eldest daughter was alone near Burlington, Vermont (where she had escaped to from New York City when it was the national epicenter for COVID back in March). Our youngest daughter was alone in Los Angeles, and our son, a newly minted First Lieutenant in the Army, was stationed in Afghanistan. “Good for him,” I thought. He could safely interact with his army buddies. It was so ironic; the one in the war zone was the only one of us who was safe from COVID.
Continue to: I reached out to family and friends...
I reached out to family and friends and asked for prayers. Emil was prayed for by all of our Catholic, Methodist, Jewish, Muslim, and Buddhist friends. As I told him later, he was prayed for from Afghanistan to Alaska. My extended family activated a text chain so all I had to do was reply and everyone on the chain would have the same information. I also received many notes and cards of support from friends and Emil’s family. Many told me how strong I was and how I would be fine. Later, I realized how many of these were from widows who were telling me I would survive bereavement, should that be the outcome.
Emil: The next day, the doctors started me on a 5-day course of the newly “approved” antiviral remdesivir, and the day after that, I received 2 units of convalescent plasma on “compassionate use” from the Mayo Clinic. It didn’t matter. I kept getting worse.
Anne: I received twice-daily updates from the nurses. When the updates were late in coming, I crawled the walls, waiting at least 2 hours before reaching out. One day, the nurse who answered said she couldn’t talk because his nurse was dealing with an emergency with him. I didn’t take a deep breath until his nurse called back to say he was stable. Regardless, he just kept getting sicker and sicker, and I began to fear he would not make it.
Emil: By Day 5, my X-ray showed clear evidence of a bilateral pneumonia (it had appeared “normal” on admission) and I was transferred to a “step-up unit.” The next day, I was transferred to the ICU and placed on a ventilator, in the prone position, for 16 hours a day.
Anne: The day Emil was transferred to the ICU, he told me he was worried about his fate. He called and asked me to stay on the phone with him while waiting to go to the ICU. We were both so weak we couldn’t do more than say “I love you” and listen to the other’s labored breathing. That was our last phone call until he was off the ventilator 10 days later.
Continue to: Emil's reply
Emil: At this point I had no idea what was going on. I was on a ventilator and I was “out.”
Anne: In the meantime, my family made sure I knew they were thinking of us. Every day I woke up with a text from one cousin asking how the night was while my sister checked in every afternoon. They sent flowers and baskets of goodies. Knowing how difficult it was waiting for updates, they sent me a jigsaw puzzle with a thousand pieces. I was surprised at how important that was for binding my anxiety. A friend sent books from my favorite writers.
Despite all this, I was absolutely beside myself the night Emil was placed on the ventilator. I cleaned and scrubbed the house; not that it needed it, I needed it. In the bedroom I saw a bottle under the bed. I retrieved it but couldn’t get up off the floor. I was weak and had tremendous muscle pain each time I moved. I had my phone, so knew I wouldn’t be stranded, but … I didn’t relish the idea of calling 911 and have them break down the front door in their hazmat suits. After more than 30 minutes, and much effort, I was able to get myself up; soon after, I put a house key outside.
When a friend who was taking care of our 2 dogs in Chicago heard that Emil was on the ventilator, she drove through the night to bring them to me so I would have them for solace. She couldn’t even come in the house. She stayed at a nearby hotel and visited with me from outside with masks on waiting for the updates.
Emil: Being an elder lawyer married to a physician, Anne knows a thing or 2 about medicine (because she’s seen a thing or 2 about medicine). She’s even been known to give her elderly clients Mini-Mental State Exams. In addition to talking with members of her support system, Anne was also talking with friends and relatives who are physicians. One exclaimed, “He’s having a cytokine storm!” and said I needed steroids. Another said, yes, that and serious “anti-inflammatory” drugs. At that moment, data supporting the use of steroids or “anti-inflammatories” in COVID hadn’t yet become public. The data on steroids came out early the next week in the Lancet and the data on “anti-inflammatories” was still in process until a few weeks later.
Continue to: Anne was ahead of the curve...
Anne was ahead of the curve and advocated hard for both treatments. At the same time, my OSUMC physicians were considering other options for me. They were checking on my inflammatory status by following my levels of C-reactive protein (CRP) and interleukin-6 (IL-6). On Days 2 and 3, my CRP level was 64 mg/L and my IL-6 level was 32 pg/mL (neither should be higher than 1).
While I don’t recall much before being on the ventilator, I do recall my alarm at seeing my CRP/IL-6 levels go up in real time on alerts from “My Chart” (my CRP/IL-6 levels were 149/123 within 4 days of admission, and reached a high of about 250/190 as I entered the ICU). I knew what those numbers meant. It was surreal; like watching myself die off in the distance, emotionally disconnected from the whole scene.
The decision to give steroids was relatively easy, and I was started on dexamethasone, a very inexpensive steroid, on Day 7 (ICU Day 2). The decision of which “antiinflammatory” to give was more difficult, as OSUMC had over 40 treatment protocols for COVID. Anne suggested 2 drugs based on recommendations from our physician friends—tocilizumab and acalabrutinib— both were on the market for other conditions and very expensive. The first is an IL-6 antagonist, while the second shuts down cytokine production in B cells, an effect also observed in lung tissue. While tocilizumab was not included in any of the OSUMC COVID protocols, acalabrutinib was, and I started on that medication on Day 8 (ICU Day 3).
Anne: My experience being the advocate was different than the first time 10 years before. That time, Emil had a community-acquired pneumonia, with which our doctors had much experience. This time, I was more active because no one had much information about how to deal with COVID and, thus, there was no standard of care. In fact, Emil was only the second patient to receive acalabrutinib at OSUMC; later, we found out that that patient did well.
Emil: The “anti-inflammatory” strategy worked. Within 5 days of starting the 2 drugs, my CRP and IL-6 levels were down to 10 and 5, respectively; a reduction of >95%. As these levels dropped, so did my oxygen requirements.
Continue to: Anne's reply
Anne: Emil was finally on the upswing. I woke up the next morning and, surprisingly, found that my first emotion wasn’t one of terror. His ICU doctor, a real booster for Emil, made it her mission to get him off the ventilator before the end of her ICU service week. She succeeded.
Emil: Five days after coming off the ventilator, I went to a rehab unit for reconditioning and to begin the long process of recovering my strength and stamina.
Most people say to me, “How awful for you! How terrible!” I smile and say, “Yeah, well, I missed all the excitement. It was really much worse for Anne.” I told them that, although you don’t recall anything while on the ventilator, you get retrograde amnesia for the several days prior to artificial ventilation. I have texts on my cell phone, written by me in those first few days, I don’t recall writing. Anne says we had conversations all the way up to my admission to the ICU; I recall none of those. Frankly, that’s for the best.
One thing to highlight is that your brain doesn’t stop working while you’re “out.” I had numerous vivid dreams, or whatever they were, while on the ventilator and after. Many were “bizarre and dark,” others were “dark and bizarre.” A few were amusing— in the end. I recall watching a TV news program segment describing how we donated our 2 little dogs to the Queen of England, who then gave them to her youngest son, Edward. I swear, I actually “saw” this TV program and watched the Queen and her son (and his wife) playing with our dogs. I was so convinced, I asked Anne where our dogs were; with her, of course. No, she assured me, we hadn’t given them to Queen Elizabeth II. Another conversation I swore I had with Anne was one in which she was telling me she was starting the vetting process to be a VP candidate for Joe Biden (Anne had been involved in Chicago politics so … not totally “crazy”). Nevertheless, I was quickly disabused of this one by my eldest daughter, also a lawyer.
Anne: This time, like the last time he was on a ventilator, Emil took a few more days to clear all the drugs keeping him sedated. Last time, his medical center sent his colleague, the Chair of Neurology, to check on him because there was a concern that he wasn’t “clearing” fast enough. This time, I was the one reassuring the doctors and nurses to be “patient.” At the same time, I was disabusing him of his far-fetched idea that he was head of all research at OSUMC and head of the ICU. He told me, “I don’t understand it. Don’t these people know they work for me?” “No,” I told him. “You are a patient there, and you need to behave.” Aside from that, Emil was fairly lucid. As one of his nurses said, “He’s oriented, he’s just wrong!”
Continue to: Emil's reply
Emil: Some people have asked me if this experience has changed my perspective. It could have, but I went through something worse 10 years ago when I was first brought back from the “mostly dead.” After that, I realized the most important things in life are the people you love and the people who love you; the good stuff is “gravy” and everything else isn’t worth spending much time or energy on. The first thing I said to Anne when we were face-to-face, as I entered the rehab facility (with masks on, of course), was “I can’t do this to you again.”
Anne: One of the most inhumane aspects of COVID is that you can’t be with your loved one while they are sick. Last time I spent 10 to 12 hours a day at the bedside. This time I couldn’t be there at all. It was especially hard because I knew from the last time how much my presence meant to him. If I left, he would get agitated. His heart rate would come down by 10 beats when I sat next to him.
When we had our first post-ventilator conversation on Father’s Day, he was surprised I was so excited to talk to him. Somehow, he thought I had abandoned him. What he didn’t know was that I was thinking about getting a job in Housekeeping at the hospital just so I could go see him!
Emil: In the end, I’m now back to baseline and grateful I’m alive. I still have things I want to do professionally and personally, and am appreciative I’ll have more time for those. However, I am appalled at how a serious public health issue has been turned into a political weapon by “science deniers” and that this is continuing to kill our citizens. That’s not a nightmare from when I was ill. It’s the “day-mare” we are living now.
Pharmacogenetic testing: 5 Questions
When selecting a psychotropic medication for a patient with a challenging illness, you may want to consider ordering pharmacogenetic testing. By characterizing how a patient’s genetic profile affects their medication metabolism, pharmacogenetic testing can potentially help improve medication adherence, reduce “trial-and-error” prescribing, and target an effective treatment. Here we address 5 important questions about using pharmacogenetic testing.
1. What can pharmacogenetic testing tell you? Pharmacogenetic testing looks for variants in genes that can affect how a patient metabolizes specific medications. While the results will not inform you about a specific medication’s effectiveness, they can describe the patient’s tolerability of that medication based on his/her metabolism. Most psychotropic medications are biotransformed in the liver by cytochrome P450 (CYP) through pathway enzymes such as 2D6, 2C19, and 3A4. For example, fluoxetine and paroxetine exert their inhibition on CYP2D6, while other psychotropic medications, such as lurasidone, are metabolized at CYP3A4 and are contraindicated with potent CYP3A4 inhibitors (eg, grapefruit juice).1
In addition to CYP450 enzymes, pharmacogenetic testing can assess for the serotonin transporter gene, SLC6A4, and its sequence promoter variant, 5-HTTLPR. This sequence variation influences response to selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tertiary amine tricyclic antidepressants.2 Pharmacogenetic testing also looks for genes related to Stevens-Johnson syndrome, such as HLA-B*1502, which is associated with adverse effects of carbamazepine and lamotrigine.
2. When should you order pharmacogenetic testing? Not all patients require pharmacogenetic testing. Anxious patients who have had multiple unsuccessful medication trials may be good candidates for testing. Consider testing for patients with a history of sensitivity to medications, or whose family members have experienced unusual drug responses.2
3. What steps should you take before ordering pharmacogenetic testing? First, obtain your patient’s informed consent, because clinical testing reveals personal genetic information. Make sure your patient understands that such testing is voluntary and that he/she can opt out. Also, explain that the information obtained from pharmacogenetic testing is confidential and will become part of the patient’s medical record.
Second, choose the best test for your patient’s needs. Pharmacogenetic tests can assess for single genes encoded for selected CYP450 enzymes based on pharmacokinetics (metabolism), or for multiple genes based on pharmacodynamic (mechanism of action) factors.5 In a recent randomized controlled trial, Bradley et al6 found that testing for multiple genes improved response and remission rates in patients with depression and/or anxiety.
Third, confirm that your patient’s insurance covers pharmacogenetic testing, because this testing can be expensive, although some genetic testing companies may offer patients financial assistance.
Continue to: How are samples taken?
4. How are samples taken? Several methods are used for obtaining samples, including saliva, buccal swab, and peripheral blood. Your patient should not smoke, eat, or drink for at least 30 minutes before providing a saliva sample. For a buccal swab, a cotton swab is rubbed in a circular motion along the oral lining inside each of the patient’s cheeks. The most invasive method is peripheral blood obtained via venipuncture. The sample is sent through expedited mail to an accredited genetic processing laboratory for analysis.
5. How do you interpret the results? Pharmacogenetic testing results are provided in a confidential report. A single gene report allows you to choose psychotropic agents based on pharmacokinetics.5 Some laboratories assess multiple genes in a single report, and create categories of medications (such as “use as directed” or “use with caution”) based on the pharmacodynamic factors of each agent.5,6 Certain laboratories offer dosing guidelines for types of medications that you should use with caution.1,5,6
When interpreting such testing results, it is critical to use your clinical judgment, because pharmacogenetic testing alone does not assess whether a medication will help improve the patient’s symptoms. It is of utmost importance that you have an understanding of pharmacodynamics, knowledge of the patient’s diet and age, and a caring doctor–patient relationship.
1. Madhusoodanan S, Velama U, Parmar J, et al. A current review of cytochrome P450 interactions of psychotropic drugs. Ann Clin Psychiatry. 2014;26(2):120-138.
2. Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69-76.
3. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166-184.
4. Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. doi: 10.4088/PCC.13m01520.
5. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded randomized, controlled study. J Psychiatr Res. 2019;111:59-67.
6. Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psych Res. 2018;96:100-107.
When selecting a psychotropic medication for a patient with a challenging illness, you may want to consider ordering pharmacogenetic testing. By characterizing how a patient’s genetic profile affects their medication metabolism, pharmacogenetic testing can potentially help improve medication adherence, reduce “trial-and-error” prescribing, and target an effective treatment. Here we address 5 important questions about using pharmacogenetic testing.
1. What can pharmacogenetic testing tell you? Pharmacogenetic testing looks for variants in genes that can affect how a patient metabolizes specific medications. While the results will not inform you about a specific medication’s effectiveness, they can describe the patient’s tolerability of that medication based on his/her metabolism. Most psychotropic medications are biotransformed in the liver by cytochrome P450 (CYP) through pathway enzymes such as 2D6, 2C19, and 3A4. For example, fluoxetine and paroxetine exert their inhibition on CYP2D6, while other psychotropic medications, such as lurasidone, are metabolized at CYP3A4 and are contraindicated with potent CYP3A4 inhibitors (eg, grapefruit juice).1
In addition to CYP450 enzymes, pharmacogenetic testing can assess for the serotonin transporter gene, SLC6A4, and its sequence promoter variant, 5-HTTLPR. This sequence variation influences response to selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tertiary amine tricyclic antidepressants.2 Pharmacogenetic testing also looks for genes related to Stevens-Johnson syndrome, such as HLA-B*1502, which is associated with adverse effects of carbamazepine and lamotrigine.
2. When should you order pharmacogenetic testing? Not all patients require pharmacogenetic testing. Anxious patients who have had multiple unsuccessful medication trials may be good candidates for testing. Consider testing for patients with a history of sensitivity to medications, or whose family members have experienced unusual drug responses.2
3. What steps should you take before ordering pharmacogenetic testing? First, obtain your patient’s informed consent, because clinical testing reveals personal genetic information. Make sure your patient understands that such testing is voluntary and that he/she can opt out. Also, explain that the information obtained from pharmacogenetic testing is confidential and will become part of the patient’s medical record.
Second, choose the best test for your patient’s needs. Pharmacogenetic tests can assess for single genes encoded for selected CYP450 enzymes based on pharmacokinetics (metabolism), or for multiple genes based on pharmacodynamic (mechanism of action) factors.5 In a recent randomized controlled trial, Bradley et al6 found that testing for multiple genes improved response and remission rates in patients with depression and/or anxiety.
Third, confirm that your patient’s insurance covers pharmacogenetic testing, because this testing can be expensive, although some genetic testing companies may offer patients financial assistance.
Continue to: How are samples taken?
4. How are samples taken? Several methods are used for obtaining samples, including saliva, buccal swab, and peripheral blood. Your patient should not smoke, eat, or drink for at least 30 minutes before providing a saliva sample. For a buccal swab, a cotton swab is rubbed in a circular motion along the oral lining inside each of the patient’s cheeks. The most invasive method is peripheral blood obtained via venipuncture. The sample is sent through expedited mail to an accredited genetic processing laboratory for analysis.
5. How do you interpret the results? Pharmacogenetic testing results are provided in a confidential report. A single gene report allows you to choose psychotropic agents based on pharmacokinetics.5 Some laboratories assess multiple genes in a single report, and create categories of medications (such as “use as directed” or “use with caution”) based on the pharmacodynamic factors of each agent.5,6 Certain laboratories offer dosing guidelines for types of medications that you should use with caution.1,5,6
When interpreting such testing results, it is critical to use your clinical judgment, because pharmacogenetic testing alone does not assess whether a medication will help improve the patient’s symptoms. It is of utmost importance that you have an understanding of pharmacodynamics, knowledge of the patient’s diet and age, and a caring doctor–patient relationship.
When selecting a psychotropic medication for a patient with a challenging illness, you may want to consider ordering pharmacogenetic testing. By characterizing how a patient’s genetic profile affects their medication metabolism, pharmacogenetic testing can potentially help improve medication adherence, reduce “trial-and-error” prescribing, and target an effective treatment. Here we address 5 important questions about using pharmacogenetic testing.
1. What can pharmacogenetic testing tell you? Pharmacogenetic testing looks for variants in genes that can affect how a patient metabolizes specific medications. While the results will not inform you about a specific medication’s effectiveness, they can describe the patient’s tolerability of that medication based on his/her metabolism. Most psychotropic medications are biotransformed in the liver by cytochrome P450 (CYP) through pathway enzymes such as 2D6, 2C19, and 3A4. For example, fluoxetine and paroxetine exert their inhibition on CYP2D6, while other psychotropic medications, such as lurasidone, are metabolized at CYP3A4 and are contraindicated with potent CYP3A4 inhibitors (eg, grapefruit juice).1
In addition to CYP450 enzymes, pharmacogenetic testing can assess for the serotonin transporter gene, SLC6A4, and its sequence promoter variant, 5-HTTLPR. This sequence variation influences response to selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tertiary amine tricyclic antidepressants.2 Pharmacogenetic testing also looks for genes related to Stevens-Johnson syndrome, such as HLA-B*1502, which is associated with adverse effects of carbamazepine and lamotrigine.
2. When should you order pharmacogenetic testing? Not all patients require pharmacogenetic testing. Anxious patients who have had multiple unsuccessful medication trials may be good candidates for testing. Consider testing for patients with a history of sensitivity to medications, or whose family members have experienced unusual drug responses.2
3. What steps should you take before ordering pharmacogenetic testing? First, obtain your patient’s informed consent, because clinical testing reveals personal genetic information. Make sure your patient understands that such testing is voluntary and that he/she can opt out. Also, explain that the information obtained from pharmacogenetic testing is confidential and will become part of the patient’s medical record.
Second, choose the best test for your patient’s needs. Pharmacogenetic tests can assess for single genes encoded for selected CYP450 enzymes based on pharmacokinetics (metabolism), or for multiple genes based on pharmacodynamic (mechanism of action) factors.5 In a recent randomized controlled trial, Bradley et al6 found that testing for multiple genes improved response and remission rates in patients with depression and/or anxiety.
Third, confirm that your patient’s insurance covers pharmacogenetic testing, because this testing can be expensive, although some genetic testing companies may offer patients financial assistance.
Continue to: How are samples taken?
4. How are samples taken? Several methods are used for obtaining samples, including saliva, buccal swab, and peripheral blood. Your patient should not smoke, eat, or drink for at least 30 minutes before providing a saliva sample. For a buccal swab, a cotton swab is rubbed in a circular motion along the oral lining inside each of the patient’s cheeks. The most invasive method is peripheral blood obtained via venipuncture. The sample is sent through expedited mail to an accredited genetic processing laboratory for analysis.
5. How do you interpret the results? Pharmacogenetic testing results are provided in a confidential report. A single gene report allows you to choose psychotropic agents based on pharmacokinetics.5 Some laboratories assess multiple genes in a single report, and create categories of medications (such as “use as directed” or “use with caution”) based on the pharmacodynamic factors of each agent.5,6 Certain laboratories offer dosing guidelines for types of medications that you should use with caution.1,5,6
When interpreting such testing results, it is critical to use your clinical judgment, because pharmacogenetic testing alone does not assess whether a medication will help improve the patient’s symptoms. It is of utmost importance that you have an understanding of pharmacodynamics, knowledge of the patient’s diet and age, and a caring doctor–patient relationship.
1. Madhusoodanan S, Velama U, Parmar J, et al. A current review of cytochrome P450 interactions of psychotropic drugs. Ann Clin Psychiatry. 2014;26(2):120-138.
2. Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69-76.
3. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166-184.
4. Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. doi: 10.4088/PCC.13m01520.
5. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded randomized, controlled study. J Psychiatr Res. 2019;111:59-67.
6. Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psych Res. 2018;96:100-107.
1. Madhusoodanan S, Velama U, Parmar J, et al. A current review of cytochrome P450 interactions of psychotropic drugs. Ann Clin Psychiatry. 2014;26(2):120-138.
2. Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69-76.
3. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166-184.
4. Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. doi: 10.4088/PCC.13m01520.
5. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded randomized, controlled study. J Psychiatr Res. 2019;111:59-67.
6. Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psych Res. 2018;96:100-107.
Lemborexant for insomnia
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
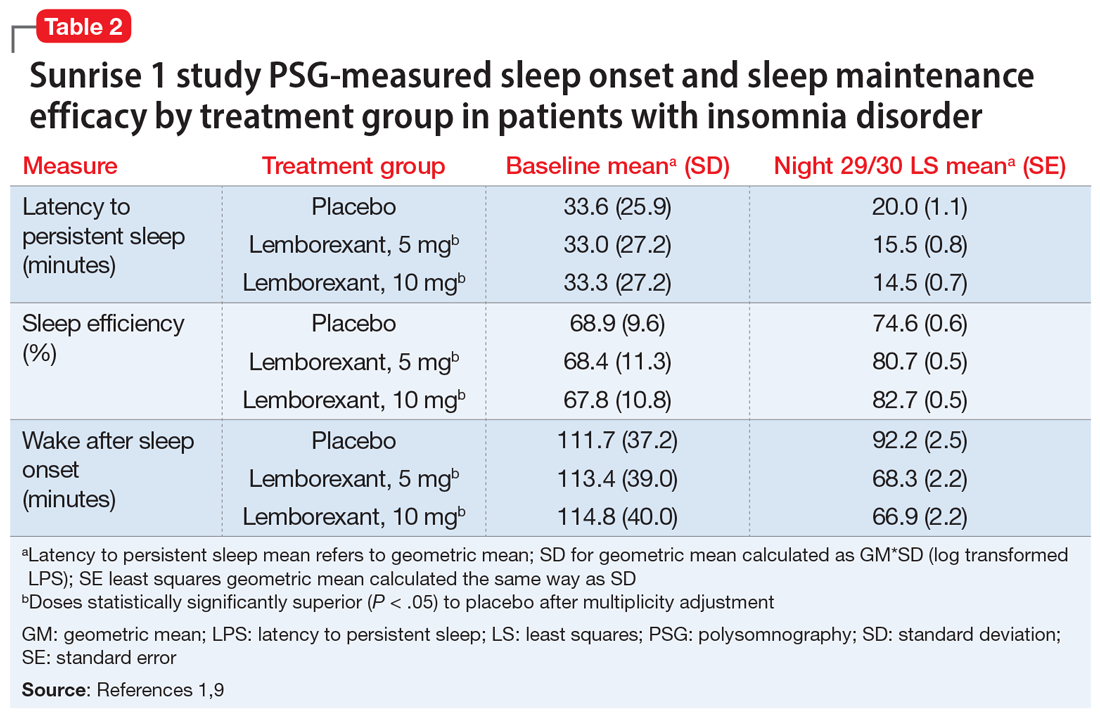
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
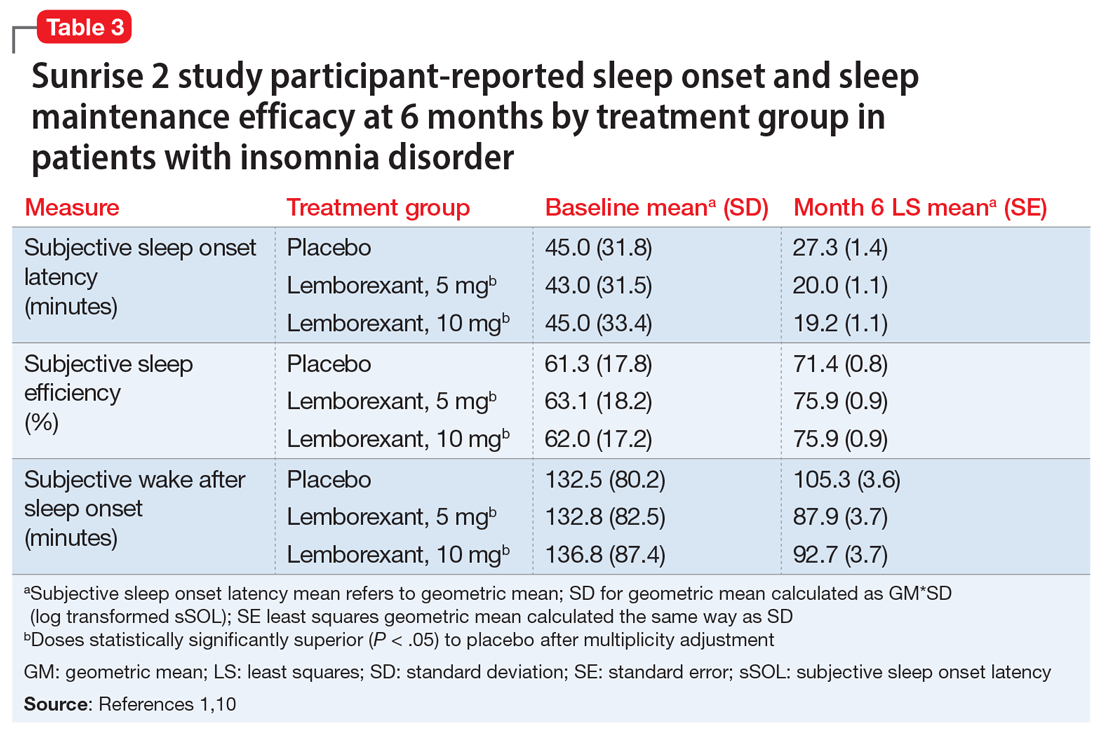
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.

In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.

Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.

In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.

Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Painful erections while being treated for OCD
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
Add-on atypicals for depression carry ‘substantial’ death risk
Adding a second-generation antipsychotic to an antidepressant to treat depression carries an increased mortality risk for middle-aged adults, results of a large, observational study show.
“Our study suggests physicians should consider prescribing antipsychotics to adults with depression carefully, as the potential health risks are substantial and the benefits are quite modest and controversially debated,” lead investigator Tobias Gerhard, PhD, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, N.J., said in a news release.
The results, he added, “emphasize the importance of considering newer antipsychotics only after nonresponse to less risky, evidence-based treatment options has been established.”
The study was published online September 30 in PLOS ONE.
A last resort
Previous research has demonstrated an increased mortality risk for elderly patients with dementia who take an atypical antipsychotic, but it’s unclear whether this risk occurs among nonelderly adults who use newer antipsychotics as augmentation treatment for depression.
To investigate, Gerhard and colleagues analyzed national healthcare claims from the Medicaid program from 2001 to 2010 for 39,582 Medicaid beneficiaries (mean age, 44.5 years; 78.5% women) who had been diagnosed with depression. Patients with alternative indications for antipsychotic therapy, such as schizophrenia, psychotic depression, or bipolar disorder, were excluded.
After at least 3 months of treatment with a single antidepressant, for more than half of the patients (56.6%), treatment was augmented with an atypical antipsychotic (quetiapine, risperidone, aripiprazole or olanzapine). For the remainder (43.4%), a second antidepressant was added.
The average chlorpromazine equivalent starting dose for all atypical antipsychotics was 68 mg/d. The dose was increased to 100 mg/d during follow-up.
A total of 153 patients died during 13,328 person-years of follow-up, including 105 for whom treatment was augmented with an atypical antipsychotic and 48 for whom treatment was augmented with a second antidepressant.
(adjusted hazard ratio, 1.45; 95% CI, 1.02 – 2.06).
This equates to an absolute risk difference of 37.7 deaths per 10,000 person-years of treatment (0.38% per year) and a number needed to harm of roughly 265 per year. For higher-risk subgroups, the number needed to harm decreased substantially, the authors note. The results were robust across several sensitivity analyses.
“We don’t know the mechanisms of the increased mortality risk, but cardiac and infectious causes are leading candidates,” said Gerhard.
“Our study in nonelderly adults with depression did not identify a single predominant cause of death. However, this may be a result of both the relatively small number of deaths in our study as well as of the well-recognized concerns regarding the accuracy of cause-of-death attribution in death certificates,” Gerhard said.
“As with the potential causes of death, the pathophysiological pathways involved are not well understood but could, among others, involve adverse metabolic effects, including weight gain, diabetes, dyslipidemia, QT prolongation, sedation, and falls – all of which have been associated with at least some of the newer antipsychotics,” he added.
The researchers state that atypical antipsychotics should be considered only “after non-response to evidence-based treatment options that are less risky.”
Another red flag
Commenting for Medscape Medical News, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Northwell Health’s Staten Island University Hospital in New York, said this is a “valid contribution” and represents the second large study that “raises the same concern.”
“We’ve been probably underestimating the risk in administering them, and that’s something people really need to know, because if you’re prescribing it for someone with mild to moderate depression, it may be helpful, but is it really worth the risk if you’re significantly increasing their risk of death?” said Sullivan, who wasn’t involved in the study.
Clearly, he said, this “raises a flag that we have to look at this a little more carefully and be a little clearer with patients about the risk. One could argue that we should not be so quick to add these drugs, even though they could be helpful, before we exhaust other less potentially risky options.”
Sullivan’s advice: “Do the three trials of antidepressants, look at antidepressant combinations, don’t be quick to jump to this particular option, because of the concerns. Certainly there are situations like psychotic depression where the risk of use is outweighed by the benefits, given the clinical syndrome, but for less severe forms, we probably should reformulate some of our algorithms.”
The study was supported by the National Institute of Mental Health (NIMH). Gerhard received grants from the NIMH and the National Institute on Aging during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; and personal fees from Eisai, Merck, Pfizer, Lilly, and IntraCellular Therapies outside the submitted work. Sullivan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adding a second-generation antipsychotic to an antidepressant to treat depression carries an increased mortality risk for middle-aged adults, results of a large, observational study show.
“Our study suggests physicians should consider prescribing antipsychotics to adults with depression carefully, as the potential health risks are substantial and the benefits are quite modest and controversially debated,” lead investigator Tobias Gerhard, PhD, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, N.J., said in a news release.
The results, he added, “emphasize the importance of considering newer antipsychotics only after nonresponse to less risky, evidence-based treatment options has been established.”
The study was published online September 30 in PLOS ONE.
A last resort
Previous research has demonstrated an increased mortality risk for elderly patients with dementia who take an atypical antipsychotic, but it’s unclear whether this risk occurs among nonelderly adults who use newer antipsychotics as augmentation treatment for depression.
To investigate, Gerhard and colleagues analyzed national healthcare claims from the Medicaid program from 2001 to 2010 for 39,582 Medicaid beneficiaries (mean age, 44.5 years; 78.5% women) who had been diagnosed with depression. Patients with alternative indications for antipsychotic therapy, such as schizophrenia, psychotic depression, or bipolar disorder, were excluded.
After at least 3 months of treatment with a single antidepressant, for more than half of the patients (56.6%), treatment was augmented with an atypical antipsychotic (quetiapine, risperidone, aripiprazole or olanzapine). For the remainder (43.4%), a second antidepressant was added.
The average chlorpromazine equivalent starting dose for all atypical antipsychotics was 68 mg/d. The dose was increased to 100 mg/d during follow-up.
A total of 153 patients died during 13,328 person-years of follow-up, including 105 for whom treatment was augmented with an atypical antipsychotic and 48 for whom treatment was augmented with a second antidepressant.
(adjusted hazard ratio, 1.45; 95% CI, 1.02 – 2.06).
This equates to an absolute risk difference of 37.7 deaths per 10,000 person-years of treatment (0.38% per year) and a number needed to harm of roughly 265 per year. For higher-risk subgroups, the number needed to harm decreased substantially, the authors note. The results were robust across several sensitivity analyses.
“We don’t know the mechanisms of the increased mortality risk, but cardiac and infectious causes are leading candidates,” said Gerhard.
“Our study in nonelderly adults with depression did not identify a single predominant cause of death. However, this may be a result of both the relatively small number of deaths in our study as well as of the well-recognized concerns regarding the accuracy of cause-of-death attribution in death certificates,” Gerhard said.
“As with the potential causes of death, the pathophysiological pathways involved are not well understood but could, among others, involve adverse metabolic effects, including weight gain, diabetes, dyslipidemia, QT prolongation, sedation, and falls – all of which have been associated with at least some of the newer antipsychotics,” he added.
The researchers state that atypical antipsychotics should be considered only “after non-response to evidence-based treatment options that are less risky.”
Another red flag
Commenting for Medscape Medical News, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Northwell Health’s Staten Island University Hospital in New York, said this is a “valid contribution” and represents the second large study that “raises the same concern.”
“We’ve been probably underestimating the risk in administering them, and that’s something people really need to know, because if you’re prescribing it for someone with mild to moderate depression, it may be helpful, but is it really worth the risk if you’re significantly increasing their risk of death?” said Sullivan, who wasn’t involved in the study.
Clearly, he said, this “raises a flag that we have to look at this a little more carefully and be a little clearer with patients about the risk. One could argue that we should not be so quick to add these drugs, even though they could be helpful, before we exhaust other less potentially risky options.”
Sullivan’s advice: “Do the three trials of antidepressants, look at antidepressant combinations, don’t be quick to jump to this particular option, because of the concerns. Certainly there are situations like psychotic depression where the risk of use is outweighed by the benefits, given the clinical syndrome, but for less severe forms, we probably should reformulate some of our algorithms.”
The study was supported by the National Institute of Mental Health (NIMH). Gerhard received grants from the NIMH and the National Institute on Aging during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; and personal fees from Eisai, Merck, Pfizer, Lilly, and IntraCellular Therapies outside the submitted work. Sullivan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adding a second-generation antipsychotic to an antidepressant to treat depression carries an increased mortality risk for middle-aged adults, results of a large, observational study show.
“Our study suggests physicians should consider prescribing antipsychotics to adults with depression carefully, as the potential health risks are substantial and the benefits are quite modest and controversially debated,” lead investigator Tobias Gerhard, PhD, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, N.J., said in a news release.
The results, he added, “emphasize the importance of considering newer antipsychotics only after nonresponse to less risky, evidence-based treatment options has been established.”
The study was published online September 30 in PLOS ONE.
A last resort
Previous research has demonstrated an increased mortality risk for elderly patients with dementia who take an atypical antipsychotic, but it’s unclear whether this risk occurs among nonelderly adults who use newer antipsychotics as augmentation treatment for depression.
To investigate, Gerhard and colleagues analyzed national healthcare claims from the Medicaid program from 2001 to 2010 for 39,582 Medicaid beneficiaries (mean age, 44.5 years; 78.5% women) who had been diagnosed with depression. Patients with alternative indications for antipsychotic therapy, such as schizophrenia, psychotic depression, or bipolar disorder, were excluded.
After at least 3 months of treatment with a single antidepressant, for more than half of the patients (56.6%), treatment was augmented with an atypical antipsychotic (quetiapine, risperidone, aripiprazole or olanzapine). For the remainder (43.4%), a second antidepressant was added.
The average chlorpromazine equivalent starting dose for all atypical antipsychotics was 68 mg/d. The dose was increased to 100 mg/d during follow-up.
A total of 153 patients died during 13,328 person-years of follow-up, including 105 for whom treatment was augmented with an atypical antipsychotic and 48 for whom treatment was augmented with a second antidepressant.
(adjusted hazard ratio, 1.45; 95% CI, 1.02 – 2.06).
This equates to an absolute risk difference of 37.7 deaths per 10,000 person-years of treatment (0.38% per year) and a number needed to harm of roughly 265 per year. For higher-risk subgroups, the number needed to harm decreased substantially, the authors note. The results were robust across several sensitivity analyses.
“We don’t know the mechanisms of the increased mortality risk, but cardiac and infectious causes are leading candidates,” said Gerhard.
“Our study in nonelderly adults with depression did not identify a single predominant cause of death. However, this may be a result of both the relatively small number of deaths in our study as well as of the well-recognized concerns regarding the accuracy of cause-of-death attribution in death certificates,” Gerhard said.
“As with the potential causes of death, the pathophysiological pathways involved are not well understood but could, among others, involve adverse metabolic effects, including weight gain, diabetes, dyslipidemia, QT prolongation, sedation, and falls – all of which have been associated with at least some of the newer antipsychotics,” he added.
The researchers state that atypical antipsychotics should be considered only “after non-response to evidence-based treatment options that are less risky.”
Another red flag
Commenting for Medscape Medical News, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Northwell Health’s Staten Island University Hospital in New York, said this is a “valid contribution” and represents the second large study that “raises the same concern.”
“We’ve been probably underestimating the risk in administering them, and that’s something people really need to know, because if you’re prescribing it for someone with mild to moderate depression, it may be helpful, but is it really worth the risk if you’re significantly increasing their risk of death?” said Sullivan, who wasn’t involved in the study.
Clearly, he said, this “raises a flag that we have to look at this a little more carefully and be a little clearer with patients about the risk. One could argue that we should not be so quick to add these drugs, even though they could be helpful, before we exhaust other less potentially risky options.”
Sullivan’s advice: “Do the three trials of antidepressants, look at antidepressant combinations, don’t be quick to jump to this particular option, because of the concerns. Certainly there are situations like psychotic depression where the risk of use is outweighed by the benefits, given the clinical syndrome, but for less severe forms, we probably should reformulate some of our algorithms.”
The study was supported by the National Institute of Mental Health (NIMH). Gerhard received grants from the NIMH and the National Institute on Aging during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; and personal fees from Eisai, Merck, Pfizer, Lilly, and IntraCellular Therapies outside the submitted work. Sullivan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hospitalists are natural leaders in the COVID-19 battle
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
COVID and med ed cost: Are future docs paying more for less?
Like most medical students, Kaitlyn Thomas’s education was abruptly interrupted by the pandemic. Her school, an osteopathic medicine institution in the Midwest, followed guidelines issued by the American Association of Medical Colleges in March, shifting lectures online and suspending activities in which students interacted with patients. But even as Ms. Thomas’s learning opportunities dwindled for the sake of safety, the costs kept piling up.
Instead of going home to live with her family, she stayed in her apartment near school – and kept paying rent – so she could be nearby for the two licensing exams she was scheduled to take 3 months later. Both tests were canceled 9 days before she was scheduled to take them, one without any notification. This meant she had to travel to two different testing sites in two different states. All told, she said, the whole thing cost her around $2,000.
Ms. Thomas’s experience isn’t rare. Across the country, medical students find themselves paying substantial costs for a medical education now greatly altered by the pandemic. Despite restrictions on time spent in hospitals, hands-on learning, social events, and access to libraries, gyms, study spaces, and instructors, the price of tuition hasn’t dropped but has remained the same or has even risen.
In response, students have become vocal about the return on their pricey investment. “Am I just going to end up doing most of my year online, and what does that look like for my future patients?” Ms. Thomas asked. “It really doesn’t feel like a time to be limiting education.”
Medical schools and administrators are scrambling to find creative solutions for safely educating students. No matter what those solutions may be, experts say, the pandemic has drawn fresh attention to enduring questions about how the cost of medical education compares to its value. Although many are frustrated, some see the potential for COVID to open new opportunities for lasting innovation. At the very least, the pandemic has sparked conversations about what matters most in terms of producing qualified physicians.
“While this is a challenging time, we will get through it, and we will continue to educate doctors, and we will get them through to practice,” says Robert Cain, president and CEO of the American Association of Colleges of Osteopathic Medicine. Many in the midst of training still have one lingering question: Is the price future doctors are now paying still worth it?
COVID’s “hidden costs” for students
Tom is a third-year student at an allopathic medicine institution in the Caribbean. He asked not to be fully identified here, owing to concern about possible backlash. In March, Tom was doing clinical rotations in New York City when his training was put on hold. He returned home to Connecticut and resumed working 60-80 hours a week as a paramedic. As much as 75% of that income went to pay for the New York City apartment he was no longer living in – an apartment that cost more than $2,000 a month – and for student loans that suddenly came due when his enrollment status changed.
Tom has been able to take some online courses through his school. But he still doesn’t know whether state licensing boards will accept them, how residency programs will view them, or whether he will eventually have to retake those online classes in person. At the end of September, he was allowed to return to the hospital but was relocated to Chicago and was forced to move on short notice.
Like many students, Tom has worried that the pandemic may prevent him from acquiring crucial elements for his residency applications, things like letters of recommendation or key experiences. That could delay his next stage of training, which would mean lost future income, increasing student loan interest, and lost work experience. “This could also mean the difference between getting a residency and being able to practice medicine and not being able to practice my intended specialty,” he said. “This is the real hidden cost we may have to deal with.”
International medical students hoping to practice in the United States face additional costs. Michelle Warncke earned her bachelor’s degree in America but went to the United Kingdom for her master’s and her medical degree, which she completed in 2019. She then moved to North Carolina with her husband and saved money to take the exams she needed for residency in the states. But her scheduled Step 2 CS exam was canceled because of the pandemic. Now, like hundreds or even thousands of other students, she said she is unable to apply for residency, even as her student loans collect interest. An active Facebook group of international medical graduates includes about 1,500 people with comparable dilemmas.
The path to becoming a physician carries a well-known price tag, one that is already quite high. Now, for many, that price is substantially increasing. “The only way I can actually keep my medical credentials up to date and passable, to be able to ever get a shot at a residency in the following years,” she said, “is to move to another country and work for less pay, pay for a visa, pay for my exams, pay for my language test, and wait and hope that I might be able to as an older graduate then be able to apply for residency.”
Scaling back the price of med school?
Questions about the economics of medical education aren’t new, says David Asch, MD, MBA, an internal medicine physician and executive director of the Center for Health Care Innovation at the University of Pennsylvania, Philadelphia. But the changes forced by COVID could lead to innovations that may finally better balance the financial scales.
Such innovations are necessary, many say, given how medical education costs have skyrocketed over the past half century. In the 1960s, 4 years of medical school cost about $40,000 in today’s dollars, Dr. Asch and colleagues wrote in a 2020 analysis, which they conducted before the pandemic began. By 2018, the price of a medical education in the United States had ballooned to about $300,000. About 75% of students were taking out loans. Upon graduating, the average debt was $200,000.
Medical school is expensive for many tangible reasons, Dr. Asch said. Schools must pay for curriculum, faculty, technology, textbooks, lab materials, facilities, administrators, and more. But policy changes could decrease those costs.
He says one idea would be for medical schools to join forces and give students access to the same basic lectures in the early years, delivered online by top-notch instructors. Students could then participate in on-campus programs that might only require 3 years to complete instead of 4. By demonstrating what can be done via online platforms, he said, the pandemic might pave the way to permanent changes that could reduce costs.
“I’m not trying to pick on biochemistry professors and medical schools, but how many do we need in the country?” Dr. Asch asked. “We’re all watching the same episode of Seinfeld. Why can’t we all watch the same episode of the Krebs cycle?” If all 190 or so medical schools in the United States shared such preclinical courses, he says, each would require a fraction of the current cost to produce. “We could save 99.5% of the cost. So why don’t we do that?”
Pandemic as opportunity
Although the price of medical education has yet to decrease, schools are working to leverage the pandemic to provide increased educational value.
This generation of physicians will not only have to cope with the fallout of this pandemic, they will be the ones responsible for confronting the next pandemic as well, says Donald Brady, MD, senior associate dean for health sciences education at Vanderbilt University, Nashville, Tenn. “They will be the leaders in the future who will better be able to know how to handle it [a pandemic] because they were able to watch it and be part of it safely in the current circumstance.”
As much as possible, Vanderbilt is using the pandemic as an opportunity. As soon as it became clear that students couldn’t be involved in certain hands-on training, instructors developed a course about pandemics that included lectures on ethics, global health, systemic racism, and other topics. It also included experiential components of pandemic management, such as opportunities to work with patients through telehealth.
Students say they feel that they are getting less for their money and that they are paying for experiences that are no longer available, such as hands-on patient contact and community events. However, Dr. Brady said, schools have had to account for new expenses, including various now-required technologies and transitioning to courses online.
Some challenges can’t be solved with money alone. Medical schools across the country are working together to ensure that they are still adequately preparing students. Vanderbilt participates in an AAMC group that meets regularly and is also one of 37 institutions involved in an American Medical Association Consortium (AACOM). These groups discuss challenges, strategies, and opportunities for optimizing medical education during the pandemic.
Some institutions have come up with creative solutions. Ohio University’s Heritage College of Osteopathic Medicine, in Athens, Ohio, in collaboration with the Ohio Department of Health, launched a 4-week rotation for third-year students that focuses on public health. Harvard Medical School, Boston, was one of several schools that allowed students to graduate early in the spring. “We’re constantly talking to our colleagues and friends,” Dr. Brady said. “We learn from each other. There’s a lot of sharing going on.”
Other organizations are also working to make sure students ultimately get what they are paying for: a high-quality education. As soon as the pandemic began, the AACOM organized four working groups to address how schools could better use technology to deliver curricula and how students could participate in public health efforts, among other topics. “For the students, the part they don’t see and can’t really be aware of is all the things that happen behind the scenes,” Mr. Cain said. “People were working really hard to make sure that their education was still delivered, and delivered in a way that was going to assure a good product at the end.”
Ultimately, that product will be held to a rigid standard, said Geoffrey Young, the AAMC’s senior director for student affairs and programs. Medical schools must still meet standards of competency set by the liaison committee on medical education. Mr. Young says that even now those standards remain rigorous enough to ensure that medical students are learning what they need to know. “The core elements for competency may be slightly altered to address the realities that we’re experiencing because of COVID, but the core tenants of competencies will not change,” he said.
Even as conversations continue about what a medical education is worth, the pandemic is drawing new attention to the profession. No signs suggest that the value of tuition or a shift to more virtual offerings are scaring students away. Applications for medical schools were up 17% for the fall of 2021.
Brady expects the surge in interest to continue. “The increased focus and emphasis on public health, the increased focus and emphasis on health equity, the increased focus on the need for a more diverse physician workforce, the interest in basic science research around viruses, the interest in COVID itself – there are a lot of different elements that are setting us up for a potential boom in applications to medical school,” he said.
Beyond increasing interest, the pandemic may also finally force a reckoning on the disconnection between how schools think about costs and how students think about value, Dr. Asch said. “When students say: ‘I’m not getting as much from this,’ they’re saying, ‘you should price this according to its lower value.’ And when the medical schools are saying: ‘Oh, but it’s costing us so much more,’ they’re talking about pricing according to the cost. It’s like one group is speaking Latin and the other group is speaking Greek.” Perhaps, he said, COVID-related changes will finally get them speaking the same language.
This article first appeared on Medscape.com.
Like most medical students, Kaitlyn Thomas’s education was abruptly interrupted by the pandemic. Her school, an osteopathic medicine institution in the Midwest, followed guidelines issued by the American Association of Medical Colleges in March, shifting lectures online and suspending activities in which students interacted with patients. But even as Ms. Thomas’s learning opportunities dwindled for the sake of safety, the costs kept piling up.
Instead of going home to live with her family, she stayed in her apartment near school – and kept paying rent – so she could be nearby for the two licensing exams she was scheduled to take 3 months later. Both tests were canceled 9 days before she was scheduled to take them, one without any notification. This meant she had to travel to two different testing sites in two different states. All told, she said, the whole thing cost her around $2,000.
Ms. Thomas’s experience isn’t rare. Across the country, medical students find themselves paying substantial costs for a medical education now greatly altered by the pandemic. Despite restrictions on time spent in hospitals, hands-on learning, social events, and access to libraries, gyms, study spaces, and instructors, the price of tuition hasn’t dropped but has remained the same or has even risen.
In response, students have become vocal about the return on their pricey investment. “Am I just going to end up doing most of my year online, and what does that look like for my future patients?” Ms. Thomas asked. “It really doesn’t feel like a time to be limiting education.”
Medical schools and administrators are scrambling to find creative solutions for safely educating students. No matter what those solutions may be, experts say, the pandemic has drawn fresh attention to enduring questions about how the cost of medical education compares to its value. Although many are frustrated, some see the potential for COVID to open new opportunities for lasting innovation. At the very least, the pandemic has sparked conversations about what matters most in terms of producing qualified physicians.
“While this is a challenging time, we will get through it, and we will continue to educate doctors, and we will get them through to practice,” says Robert Cain, president and CEO of the American Association of Colleges of Osteopathic Medicine. Many in the midst of training still have one lingering question: Is the price future doctors are now paying still worth it?
COVID’s “hidden costs” for students
Tom is a third-year student at an allopathic medicine institution in the Caribbean. He asked not to be fully identified here, owing to concern about possible backlash. In March, Tom was doing clinical rotations in New York City when his training was put on hold. He returned home to Connecticut and resumed working 60-80 hours a week as a paramedic. As much as 75% of that income went to pay for the New York City apartment he was no longer living in – an apartment that cost more than $2,000 a month – and for student loans that suddenly came due when his enrollment status changed.
Tom has been able to take some online courses through his school. But he still doesn’t know whether state licensing boards will accept them, how residency programs will view them, or whether he will eventually have to retake those online classes in person. At the end of September, he was allowed to return to the hospital but was relocated to Chicago and was forced to move on short notice.
Like many students, Tom has worried that the pandemic may prevent him from acquiring crucial elements for his residency applications, things like letters of recommendation or key experiences. That could delay his next stage of training, which would mean lost future income, increasing student loan interest, and lost work experience. “This could also mean the difference between getting a residency and being able to practice medicine and not being able to practice my intended specialty,” he said. “This is the real hidden cost we may have to deal with.”
International medical students hoping to practice in the United States face additional costs. Michelle Warncke earned her bachelor’s degree in America but went to the United Kingdom for her master’s and her medical degree, which she completed in 2019. She then moved to North Carolina with her husband and saved money to take the exams she needed for residency in the states. But her scheduled Step 2 CS exam was canceled because of the pandemic. Now, like hundreds or even thousands of other students, she said she is unable to apply for residency, even as her student loans collect interest. An active Facebook group of international medical graduates includes about 1,500 people with comparable dilemmas.
The path to becoming a physician carries a well-known price tag, one that is already quite high. Now, for many, that price is substantially increasing. “The only way I can actually keep my medical credentials up to date and passable, to be able to ever get a shot at a residency in the following years,” she said, “is to move to another country and work for less pay, pay for a visa, pay for my exams, pay for my language test, and wait and hope that I might be able to as an older graduate then be able to apply for residency.”
Scaling back the price of med school?
Questions about the economics of medical education aren’t new, says David Asch, MD, MBA, an internal medicine physician and executive director of the Center for Health Care Innovation at the University of Pennsylvania, Philadelphia. But the changes forced by COVID could lead to innovations that may finally better balance the financial scales.
Such innovations are necessary, many say, given how medical education costs have skyrocketed over the past half century. In the 1960s, 4 years of medical school cost about $40,000 in today’s dollars, Dr. Asch and colleagues wrote in a 2020 analysis, which they conducted before the pandemic began. By 2018, the price of a medical education in the United States had ballooned to about $300,000. About 75% of students were taking out loans. Upon graduating, the average debt was $200,000.
Medical school is expensive for many tangible reasons, Dr. Asch said. Schools must pay for curriculum, faculty, technology, textbooks, lab materials, facilities, administrators, and more. But policy changes could decrease those costs.
He says one idea would be for medical schools to join forces and give students access to the same basic lectures in the early years, delivered online by top-notch instructors. Students could then participate in on-campus programs that might only require 3 years to complete instead of 4. By demonstrating what can be done via online platforms, he said, the pandemic might pave the way to permanent changes that could reduce costs.
“I’m not trying to pick on biochemistry professors and medical schools, but how many do we need in the country?” Dr. Asch asked. “We’re all watching the same episode of Seinfeld. Why can’t we all watch the same episode of the Krebs cycle?” If all 190 or so medical schools in the United States shared such preclinical courses, he says, each would require a fraction of the current cost to produce. “We could save 99.5% of the cost. So why don’t we do that?”
Pandemic as opportunity
Although the price of medical education has yet to decrease, schools are working to leverage the pandemic to provide increased educational value.
This generation of physicians will not only have to cope with the fallout of this pandemic, they will be the ones responsible for confronting the next pandemic as well, says Donald Brady, MD, senior associate dean for health sciences education at Vanderbilt University, Nashville, Tenn. “They will be the leaders in the future who will better be able to know how to handle it [a pandemic] because they were able to watch it and be part of it safely in the current circumstance.”
As much as possible, Vanderbilt is using the pandemic as an opportunity. As soon as it became clear that students couldn’t be involved in certain hands-on training, instructors developed a course about pandemics that included lectures on ethics, global health, systemic racism, and other topics. It also included experiential components of pandemic management, such as opportunities to work with patients through telehealth.
Students say they feel that they are getting less for their money and that they are paying for experiences that are no longer available, such as hands-on patient contact and community events. However, Dr. Brady said, schools have had to account for new expenses, including various now-required technologies and transitioning to courses online.
Some challenges can’t be solved with money alone. Medical schools across the country are working together to ensure that they are still adequately preparing students. Vanderbilt participates in an AAMC group that meets regularly and is also one of 37 institutions involved in an American Medical Association Consortium (AACOM). These groups discuss challenges, strategies, and opportunities for optimizing medical education during the pandemic.
Some institutions have come up with creative solutions. Ohio University’s Heritage College of Osteopathic Medicine, in Athens, Ohio, in collaboration with the Ohio Department of Health, launched a 4-week rotation for third-year students that focuses on public health. Harvard Medical School, Boston, was one of several schools that allowed students to graduate early in the spring. “We’re constantly talking to our colleagues and friends,” Dr. Brady said. “We learn from each other. There’s a lot of sharing going on.”
Other organizations are also working to make sure students ultimately get what they are paying for: a high-quality education. As soon as the pandemic began, the AACOM organized four working groups to address how schools could better use technology to deliver curricula and how students could participate in public health efforts, among other topics. “For the students, the part they don’t see and can’t really be aware of is all the things that happen behind the scenes,” Mr. Cain said. “People were working really hard to make sure that their education was still delivered, and delivered in a way that was going to assure a good product at the end.”
Ultimately, that product will be held to a rigid standard, said Geoffrey Young, the AAMC’s senior director for student affairs and programs. Medical schools must still meet standards of competency set by the liaison committee on medical education. Mr. Young says that even now those standards remain rigorous enough to ensure that medical students are learning what they need to know. “The core elements for competency may be slightly altered to address the realities that we’re experiencing because of COVID, but the core tenants of competencies will not change,” he said.
Even as conversations continue about what a medical education is worth, the pandemic is drawing new attention to the profession. No signs suggest that the value of tuition or a shift to more virtual offerings are scaring students away. Applications for medical schools were up 17% for the fall of 2021.
Brady expects the surge in interest to continue. “The increased focus and emphasis on public health, the increased focus and emphasis on health equity, the increased focus on the need for a more diverse physician workforce, the interest in basic science research around viruses, the interest in COVID itself – there are a lot of different elements that are setting us up for a potential boom in applications to medical school,” he said.
Beyond increasing interest, the pandemic may also finally force a reckoning on the disconnection between how schools think about costs and how students think about value, Dr. Asch said. “When students say: ‘I’m not getting as much from this,’ they’re saying, ‘you should price this according to its lower value.’ And when the medical schools are saying: ‘Oh, but it’s costing us so much more,’ they’re talking about pricing according to the cost. It’s like one group is speaking Latin and the other group is speaking Greek.” Perhaps, he said, COVID-related changes will finally get them speaking the same language.
This article first appeared on Medscape.com.
Like most medical students, Kaitlyn Thomas’s education was abruptly interrupted by the pandemic. Her school, an osteopathic medicine institution in the Midwest, followed guidelines issued by the American Association of Medical Colleges in March, shifting lectures online and suspending activities in which students interacted with patients. But even as Ms. Thomas’s learning opportunities dwindled for the sake of safety, the costs kept piling up.
Instead of going home to live with her family, she stayed in her apartment near school – and kept paying rent – so she could be nearby for the two licensing exams she was scheduled to take 3 months later. Both tests were canceled 9 days before she was scheduled to take them, one without any notification. This meant she had to travel to two different testing sites in two different states. All told, she said, the whole thing cost her around $2,000.
Ms. Thomas’s experience isn’t rare. Across the country, medical students find themselves paying substantial costs for a medical education now greatly altered by the pandemic. Despite restrictions on time spent in hospitals, hands-on learning, social events, and access to libraries, gyms, study spaces, and instructors, the price of tuition hasn’t dropped but has remained the same or has even risen.
In response, students have become vocal about the return on their pricey investment. “Am I just going to end up doing most of my year online, and what does that look like for my future patients?” Ms. Thomas asked. “It really doesn’t feel like a time to be limiting education.”
Medical schools and administrators are scrambling to find creative solutions for safely educating students. No matter what those solutions may be, experts say, the pandemic has drawn fresh attention to enduring questions about how the cost of medical education compares to its value. Although many are frustrated, some see the potential for COVID to open new opportunities for lasting innovation. At the very least, the pandemic has sparked conversations about what matters most in terms of producing qualified physicians.
“While this is a challenging time, we will get through it, and we will continue to educate doctors, and we will get them through to practice,” says Robert Cain, president and CEO of the American Association of Colleges of Osteopathic Medicine. Many in the midst of training still have one lingering question: Is the price future doctors are now paying still worth it?
COVID’s “hidden costs” for students
Tom is a third-year student at an allopathic medicine institution in the Caribbean. He asked not to be fully identified here, owing to concern about possible backlash. In March, Tom was doing clinical rotations in New York City when his training was put on hold. He returned home to Connecticut and resumed working 60-80 hours a week as a paramedic. As much as 75% of that income went to pay for the New York City apartment he was no longer living in – an apartment that cost more than $2,000 a month – and for student loans that suddenly came due when his enrollment status changed.
Tom has been able to take some online courses through his school. But he still doesn’t know whether state licensing boards will accept them, how residency programs will view them, or whether he will eventually have to retake those online classes in person. At the end of September, he was allowed to return to the hospital but was relocated to Chicago and was forced to move on short notice.
Like many students, Tom has worried that the pandemic may prevent him from acquiring crucial elements for his residency applications, things like letters of recommendation or key experiences. That could delay his next stage of training, which would mean lost future income, increasing student loan interest, and lost work experience. “This could also mean the difference between getting a residency and being able to practice medicine and not being able to practice my intended specialty,” he said. “This is the real hidden cost we may have to deal with.”
International medical students hoping to practice in the United States face additional costs. Michelle Warncke earned her bachelor’s degree in America but went to the United Kingdom for her master’s and her medical degree, which she completed in 2019. She then moved to North Carolina with her husband and saved money to take the exams she needed for residency in the states. But her scheduled Step 2 CS exam was canceled because of the pandemic. Now, like hundreds or even thousands of other students, she said she is unable to apply for residency, even as her student loans collect interest. An active Facebook group of international medical graduates includes about 1,500 people with comparable dilemmas.
The path to becoming a physician carries a well-known price tag, one that is already quite high. Now, for many, that price is substantially increasing. “The only way I can actually keep my medical credentials up to date and passable, to be able to ever get a shot at a residency in the following years,” she said, “is to move to another country and work for less pay, pay for a visa, pay for my exams, pay for my language test, and wait and hope that I might be able to as an older graduate then be able to apply for residency.”
Scaling back the price of med school?
Questions about the economics of medical education aren’t new, says David Asch, MD, MBA, an internal medicine physician and executive director of the Center for Health Care Innovation at the University of Pennsylvania, Philadelphia. But the changes forced by COVID could lead to innovations that may finally better balance the financial scales.
Such innovations are necessary, many say, given how medical education costs have skyrocketed over the past half century. In the 1960s, 4 years of medical school cost about $40,000 in today’s dollars, Dr. Asch and colleagues wrote in a 2020 analysis, which they conducted before the pandemic began. By 2018, the price of a medical education in the United States had ballooned to about $300,000. About 75% of students were taking out loans. Upon graduating, the average debt was $200,000.
Medical school is expensive for many tangible reasons, Dr. Asch said. Schools must pay for curriculum, faculty, technology, textbooks, lab materials, facilities, administrators, and more. But policy changes could decrease those costs.
He says one idea would be for medical schools to join forces and give students access to the same basic lectures in the early years, delivered online by top-notch instructors. Students could then participate in on-campus programs that might only require 3 years to complete instead of 4. By demonstrating what can be done via online platforms, he said, the pandemic might pave the way to permanent changes that could reduce costs.
“I’m not trying to pick on biochemistry professors and medical schools, but how many do we need in the country?” Dr. Asch asked. “We’re all watching the same episode of Seinfeld. Why can’t we all watch the same episode of the Krebs cycle?” If all 190 or so medical schools in the United States shared such preclinical courses, he says, each would require a fraction of the current cost to produce. “We could save 99.5% of the cost. So why don’t we do that?”
Pandemic as opportunity
Although the price of medical education has yet to decrease, schools are working to leverage the pandemic to provide increased educational value.
This generation of physicians will not only have to cope with the fallout of this pandemic, they will be the ones responsible for confronting the next pandemic as well, says Donald Brady, MD, senior associate dean for health sciences education at Vanderbilt University, Nashville, Tenn. “They will be the leaders in the future who will better be able to know how to handle it [a pandemic] because they were able to watch it and be part of it safely in the current circumstance.”
As much as possible, Vanderbilt is using the pandemic as an opportunity. As soon as it became clear that students couldn’t be involved in certain hands-on training, instructors developed a course about pandemics that included lectures on ethics, global health, systemic racism, and other topics. It also included experiential components of pandemic management, such as opportunities to work with patients through telehealth.
Students say they feel that they are getting less for their money and that they are paying for experiences that are no longer available, such as hands-on patient contact and community events. However, Dr. Brady said, schools have had to account for new expenses, including various now-required technologies and transitioning to courses online.
Some challenges can’t be solved with money alone. Medical schools across the country are working together to ensure that they are still adequately preparing students. Vanderbilt participates in an AAMC group that meets regularly and is also one of 37 institutions involved in an American Medical Association Consortium (AACOM). These groups discuss challenges, strategies, and opportunities for optimizing medical education during the pandemic.
Some institutions have come up with creative solutions. Ohio University’s Heritage College of Osteopathic Medicine, in Athens, Ohio, in collaboration with the Ohio Department of Health, launched a 4-week rotation for third-year students that focuses on public health. Harvard Medical School, Boston, was one of several schools that allowed students to graduate early in the spring. “We’re constantly talking to our colleagues and friends,” Dr. Brady said. “We learn from each other. There’s a lot of sharing going on.”
Other organizations are also working to make sure students ultimately get what they are paying for: a high-quality education. As soon as the pandemic began, the AACOM organized four working groups to address how schools could better use technology to deliver curricula and how students could participate in public health efforts, among other topics. “For the students, the part they don’t see and can’t really be aware of is all the things that happen behind the scenes,” Mr. Cain said. “People were working really hard to make sure that their education was still delivered, and delivered in a way that was going to assure a good product at the end.”
Ultimately, that product will be held to a rigid standard, said Geoffrey Young, the AAMC’s senior director for student affairs and programs. Medical schools must still meet standards of competency set by the liaison committee on medical education. Mr. Young says that even now those standards remain rigorous enough to ensure that medical students are learning what they need to know. “The core elements for competency may be slightly altered to address the realities that we’re experiencing because of COVID, but the core tenants of competencies will not change,” he said.
Even as conversations continue about what a medical education is worth, the pandemic is drawing new attention to the profession. No signs suggest that the value of tuition or a shift to more virtual offerings are scaring students away. Applications for medical schools were up 17% for the fall of 2021.
Brady expects the surge in interest to continue. “The increased focus and emphasis on public health, the increased focus and emphasis on health equity, the increased focus on the need for a more diverse physician workforce, the interest in basic science research around viruses, the interest in COVID itself – there are a lot of different elements that are setting us up for a potential boom in applications to medical school,” he said.
Beyond increasing interest, the pandemic may also finally force a reckoning on the disconnection between how schools think about costs and how students think about value, Dr. Asch said. “When students say: ‘I’m not getting as much from this,’ they’re saying, ‘you should price this according to its lower value.’ And when the medical schools are saying: ‘Oh, but it’s costing us so much more,’ they’re talking about pricing according to the cost. It’s like one group is speaking Latin and the other group is speaking Greek.” Perhaps, he said, COVID-related changes will finally get them speaking the same language.
This article first appeared on Medscape.com.
Two COVID-19 outpatient antibody drugs show encouraging results
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.