User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Inside the flawed White House testing scheme that did not protect Trump
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Use of e-cigarettes may be linked to sleep deprivation
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.
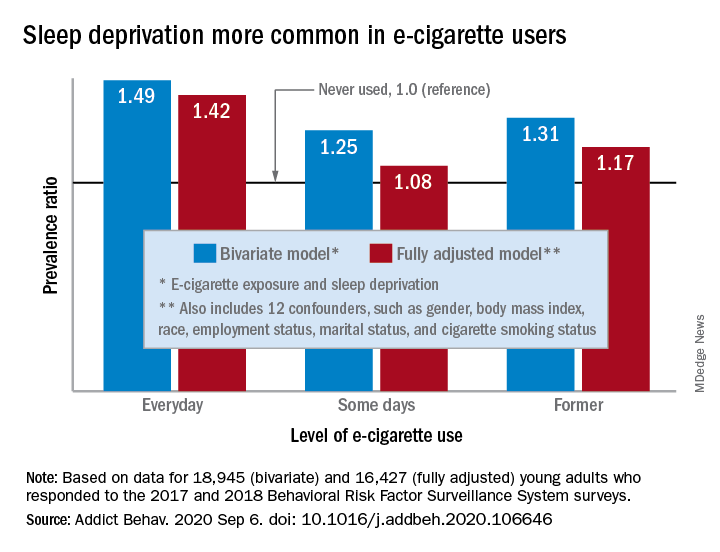
“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
FROM ADDICTIVE BEHAVIORS
Mental illness tied to increased mortality in COVID-19
A psychiatric diagnosis for patients hospitalized with COVID-19 is linked to a significantly increased risk for death, new research shows.
Investigators found that patients who were hospitalized with COVID-19 and who had been diagnosed with a psychiatric disorder had a 50% increased risk for a COVID-related death in comparison with COVID-19 patients who had not received a psychiatric diagnosis.
“Pay attention and potentially address/treat a prior psychiatric diagnosis if a patient is hospitalized for COVID-19, as this risk factor can impact the patient’s outcome – death – while in the hospital,” lead investigator Luming Li, MD, assistant professor of psychiatry and associate medical director of quality improvement, Yale New Haven Psychiatric Hospital, New Haven, Conn., said in an interview.
The study was published Sept. 30 in JAMA Network Open.
Negative impact
“We were interested to learn more about the impact of psychiatric diagnoses on COVID-19 mortality, as prior large cohort studies included neurological and other medical conditions but did not assess for a priori psychiatric diagnoses,” said Dr. Li.
“We know from the literature that prior psychiatric diagnoses can have a negative impact on the outcomes of medical conditions, and therefore we tested our hypothesis on a cohort of patients who were hospitalized with COVID-19,” she added.
To investigate, the researchers analyzed data on 1,685 patients hospitalized with COVID-19 between Feb. 15 and April 25, 2020, and whose cases were followed to May 27, 2020. The patients (mean age, 65.2 years; 52.6% men) were drawn from the Yale New Haven Health System.
The median follow-up period was 8 days (interquartile range, 4-16 days) .
Of these patients, 28% had received a psychiatric diagnosis prior to hospitalization. (i.e., cancer, cerebrovascular disease, heart failure, diabetes, kidney disease, liver disease, MI, and/or HIV).
Psychiatric diagnoses were defined in accordance with ICD codes that included mental and behavioral health, Alzheimer’s disease, and self-injury.
Vulnerability to stress
In the unadjusted model, the risk for COVID-19–related hospital death was greater for those who had received any psychiatric diagnosis, compared with those had not (hazard ratio, 2.3; 95% CI, 1.8-2.9; P < .001).
In the adjusted model that controlled for demographic characteristics, other medical comorbidities, and hospital location, the mortality risk somewhat decreased but still remained significantly higher (HR, 1.5; 95% CI, 1.1-1.9; P = .003).
Dr. Li noted a number of factors that might account for the higher mortality rate among psychiatric patients who had COVID-19 in comparison with COVD-19 patients who did not have a psychiatric disorder. These included “potential inflammatory and stress responses that the body experiences related to prior psychiatric conditions,” she said.
Having been previously diagnosed with a psychiatric disorder may also “reflect existing neurochemical differences, compared to those who do not have a prior psychiatric diagnosis, [and] these differences may make the population with the prior psychiatric diagnosis more vulnerable to respond to an acute stressor such as COVID-19,” she said.
Quality care
Harold Pincus, MD, professor and vice chair of the department of psychiatry at Columbia University, New York, said it “adds to the fairly well-known and well-established phenomenon that people with mental illnesses have a high risk of all sorts of morbidity and mortality for non–mental health conditions.”
The researchers “adjusted for various expected [mortality] risks that would be independent of the presence of COVID-19,” so “there was something else going on associated with mortality,” said Dr. Pincus, who is also codirector of the Irving Institute for Clinical and Translation Research. He was not involved with the study.
Beyond the possibility of “some basic immunologic process affected by the presence of a mental disorder,” it is possible that the vulnerability is “related to access to quality care for the comorbid general condition that is not being effectively treated,” he said.
“The take-home message is that people with mental disorders are at higher risk for death, and we need to make sure that, irrespective of COVID-19, they get adequate preventive and chronic-disease care, which would be the most effective way to intervene and protect the impact of a serious disease like COVID-19,” he noted. This would include being appropriately vaccinated and receiving preventive healthcare to reduce smoking and encourage weight loss.
No source of funding for the study was provided. Dr. Li reported receiving grants from a Health and Aging Policy Fellowship during the conduct of the study. Dr. Pincus reported no relevant financial relationships.
A psychiatric diagnosis for patients hospitalized with COVID-19 is linked to a significantly increased risk for death, new research shows.
Investigators found that patients who were hospitalized with COVID-19 and who had been diagnosed with a psychiatric disorder had a 50% increased risk for a COVID-related death in comparison with COVID-19 patients who had not received a psychiatric diagnosis.
“Pay attention and potentially address/treat a prior psychiatric diagnosis if a patient is hospitalized for COVID-19, as this risk factor can impact the patient’s outcome – death – while in the hospital,” lead investigator Luming Li, MD, assistant professor of psychiatry and associate medical director of quality improvement, Yale New Haven Psychiatric Hospital, New Haven, Conn., said in an interview.
The study was published Sept. 30 in JAMA Network Open.
Negative impact
“We were interested to learn more about the impact of psychiatric diagnoses on COVID-19 mortality, as prior large cohort studies included neurological and other medical conditions but did not assess for a priori psychiatric diagnoses,” said Dr. Li.
“We know from the literature that prior psychiatric diagnoses can have a negative impact on the outcomes of medical conditions, and therefore we tested our hypothesis on a cohort of patients who were hospitalized with COVID-19,” she added.
To investigate, the researchers analyzed data on 1,685 patients hospitalized with COVID-19 between Feb. 15 and April 25, 2020, and whose cases were followed to May 27, 2020. The patients (mean age, 65.2 years; 52.6% men) were drawn from the Yale New Haven Health System.
The median follow-up period was 8 days (interquartile range, 4-16 days) .
Of these patients, 28% had received a psychiatric diagnosis prior to hospitalization. (i.e., cancer, cerebrovascular disease, heart failure, diabetes, kidney disease, liver disease, MI, and/or HIV).
Psychiatric diagnoses were defined in accordance with ICD codes that included mental and behavioral health, Alzheimer’s disease, and self-injury.
Vulnerability to stress
In the unadjusted model, the risk for COVID-19–related hospital death was greater for those who had received any psychiatric diagnosis, compared with those had not (hazard ratio, 2.3; 95% CI, 1.8-2.9; P < .001).
In the adjusted model that controlled for demographic characteristics, other medical comorbidities, and hospital location, the mortality risk somewhat decreased but still remained significantly higher (HR, 1.5; 95% CI, 1.1-1.9; P = .003).
Dr. Li noted a number of factors that might account for the higher mortality rate among psychiatric patients who had COVID-19 in comparison with COVD-19 patients who did not have a psychiatric disorder. These included “potential inflammatory and stress responses that the body experiences related to prior psychiatric conditions,” she said.
Having been previously diagnosed with a psychiatric disorder may also “reflect existing neurochemical differences, compared to those who do not have a prior psychiatric diagnosis, [and] these differences may make the population with the prior psychiatric diagnosis more vulnerable to respond to an acute stressor such as COVID-19,” she said.
Quality care
Harold Pincus, MD, professor and vice chair of the department of psychiatry at Columbia University, New York, said it “adds to the fairly well-known and well-established phenomenon that people with mental illnesses have a high risk of all sorts of morbidity and mortality for non–mental health conditions.”
The researchers “adjusted for various expected [mortality] risks that would be independent of the presence of COVID-19,” so “there was something else going on associated with mortality,” said Dr. Pincus, who is also codirector of the Irving Institute for Clinical and Translation Research. He was not involved with the study.
Beyond the possibility of “some basic immunologic process affected by the presence of a mental disorder,” it is possible that the vulnerability is “related to access to quality care for the comorbid general condition that is not being effectively treated,” he said.
“The take-home message is that people with mental disorders are at higher risk for death, and we need to make sure that, irrespective of COVID-19, they get adequate preventive and chronic-disease care, which would be the most effective way to intervene and protect the impact of a serious disease like COVID-19,” he noted. This would include being appropriately vaccinated and receiving preventive healthcare to reduce smoking and encourage weight loss.
No source of funding for the study was provided. Dr. Li reported receiving grants from a Health and Aging Policy Fellowship during the conduct of the study. Dr. Pincus reported no relevant financial relationships.
A psychiatric diagnosis for patients hospitalized with COVID-19 is linked to a significantly increased risk for death, new research shows.
Investigators found that patients who were hospitalized with COVID-19 and who had been diagnosed with a psychiatric disorder had a 50% increased risk for a COVID-related death in comparison with COVID-19 patients who had not received a psychiatric diagnosis.
“Pay attention and potentially address/treat a prior psychiatric diagnosis if a patient is hospitalized for COVID-19, as this risk factor can impact the patient’s outcome – death – while in the hospital,” lead investigator Luming Li, MD, assistant professor of psychiatry and associate medical director of quality improvement, Yale New Haven Psychiatric Hospital, New Haven, Conn., said in an interview.
The study was published Sept. 30 in JAMA Network Open.
Negative impact
“We were interested to learn more about the impact of psychiatric diagnoses on COVID-19 mortality, as prior large cohort studies included neurological and other medical conditions but did not assess for a priori psychiatric diagnoses,” said Dr. Li.
“We know from the literature that prior psychiatric diagnoses can have a negative impact on the outcomes of medical conditions, and therefore we tested our hypothesis on a cohort of patients who were hospitalized with COVID-19,” she added.
To investigate, the researchers analyzed data on 1,685 patients hospitalized with COVID-19 between Feb. 15 and April 25, 2020, and whose cases were followed to May 27, 2020. The patients (mean age, 65.2 years; 52.6% men) were drawn from the Yale New Haven Health System.
The median follow-up period was 8 days (interquartile range, 4-16 days) .
Of these patients, 28% had received a psychiatric diagnosis prior to hospitalization. (i.e., cancer, cerebrovascular disease, heart failure, diabetes, kidney disease, liver disease, MI, and/or HIV).
Psychiatric diagnoses were defined in accordance with ICD codes that included mental and behavioral health, Alzheimer’s disease, and self-injury.
Vulnerability to stress
In the unadjusted model, the risk for COVID-19–related hospital death was greater for those who had received any psychiatric diagnosis, compared with those had not (hazard ratio, 2.3; 95% CI, 1.8-2.9; P < .001).
In the adjusted model that controlled for demographic characteristics, other medical comorbidities, and hospital location, the mortality risk somewhat decreased but still remained significantly higher (HR, 1.5; 95% CI, 1.1-1.9; P = .003).
Dr. Li noted a number of factors that might account for the higher mortality rate among psychiatric patients who had COVID-19 in comparison with COVD-19 patients who did not have a psychiatric disorder. These included “potential inflammatory and stress responses that the body experiences related to prior psychiatric conditions,” she said.
Having been previously diagnosed with a psychiatric disorder may also “reflect existing neurochemical differences, compared to those who do not have a prior psychiatric diagnosis, [and] these differences may make the population with the prior psychiatric diagnosis more vulnerable to respond to an acute stressor such as COVID-19,” she said.
Quality care
Harold Pincus, MD, professor and vice chair of the department of psychiatry at Columbia University, New York, said it “adds to the fairly well-known and well-established phenomenon that people with mental illnesses have a high risk of all sorts of morbidity and mortality for non–mental health conditions.”
The researchers “adjusted for various expected [mortality] risks that would be independent of the presence of COVID-19,” so “there was something else going on associated with mortality,” said Dr. Pincus, who is also codirector of the Irving Institute for Clinical and Translation Research. He was not involved with the study.
Beyond the possibility of “some basic immunologic process affected by the presence of a mental disorder,” it is possible that the vulnerability is “related to access to quality care for the comorbid general condition that is not being effectively treated,” he said.
“The take-home message is that people with mental disorders are at higher risk for death, and we need to make sure that, irrespective of COVID-19, they get adequate preventive and chronic-disease care, which would be the most effective way to intervene and protect the impact of a serious disease like COVID-19,” he noted. This would include being appropriately vaccinated and receiving preventive healthcare to reduce smoking and encourage weight loss.
No source of funding for the study was provided. Dr. Li reported receiving grants from a Health and Aging Policy Fellowship during the conduct of the study. Dr. Pincus reported no relevant financial relationships.
‘Celebration’ will be ‘short-lived’ if COVID vaccine rushed: Experts
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
Nerve damage linked to prone positioning in COVID-19
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF ANAESTHESIOLOGY
Trump signs Medicare loan relief bill delaying repayments
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
‘Overwhelming evidence’ FDA’s opioid approval process is shoddy
Despite the ongoing epidemic of misuse, overuse, and diversion of opioids, the Food and Drug Administration has set a low bar for approval of these medications over the past 20 years, new research suggests.
The study results also show that the FDA did not require manufacturers to collect safety data on tolerance, withdrawal, overdose, misuse, and diversion in any rigorous fashion.
In addition, during the study period, 17 of the 39 new drug applications (NDAs) (only one was an innovator product, known as a new molecular entity) for chronic pain were approved with an “enriched enrollment randomized withdrawal” (EERW) trial design. Such a design, in this case, allowed manufacturers to exclude 32%-43% of the initially enrolled patients from the double-blind treatment phase.
“The question for regulators, policy makers, and others is: How did we get to a point where these approvals took place based on trials that were by design unlikely to yield some of the most important information about safety and efficacy that patients and clinicians would care about?” study investigator G. Caleb Alexander, MD, Johns Hopkins University, Baltimore, said in an interview.
The study was published online Sept. 29 in the Annals of Internal Medicine.
‘Cooking the books’
Little is known about the evidence required by the FDA for new approvals of opioid analgesics.
To characterize the quality of safety and efficacy data in NDAs for opioid analgesics approved by the FDA between 1997 and 2018, the investigators conducted the cross-sectional analysis using data from ClinicalTrials.gov, FDA reviews, and peer-reviewed publications regarding phase 3 pivotal trials.
The investigators examined the key characteristics of each NDA, including the number, size, and duration of pivotal trials, trial control groups, use of EERW, and systematically measured safety outcomes.
Results showed that most of the 48 NDAs evaluated were for new dosage forms (52.1%) or new formulations (18.8%). Only one (2.1%) was for a new molecular entity.
Of 39 NDAs approved for the treatment of chronic pain, only 21 products were supported by at least one pivotal trial. The mean duration of these 28 trials was 84 days, and they enrolled a median of 299 patients.
Results showed that, for 17 of the 39 opioids approved for chronic pain, pivotal trials had an EERW design. For the latest period – 2012-2018 – trials of all eight of the approved opioids used the EERW method.
This EERW design allows the manufacturer to assess efficacy “among a subset of patients most likely to respond and least likely to have adverse effects, reducing generalizability to real-world settings,” the investigators noted.
They called on the FDA to stop relying on this type of trial to assess opioid efficacy.
In an August 2020 article, Andrew Kolodny, MD, pointed out the pitfalls of the EERW approach. In such a study, all participants are made physiologically dependent on the opioid in a 4- to 6-week open-label phase. Only those who tolerate the drug and find it helpful are included in the randomized study. Dr. Kolodny is codirector of opioid policy research at Brandeis University, Waltham, Mass.
“Critics of EERW have correctly described this methodology as ‘cooking the books,’ ” Dr. Kolodny writes.
He noted that the agency’s decision to rely on EERW trials for opioids was “based on discussions at private meetings between FDA officials and pharmaceutical company executives hosted by an organization called Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.” The 2013 meetings were reported in an article published in the Washington Post.
Little sign of change
Among NDAs for chronic pain, the investigators found that eight (20.5%) included pooled safety reviews that reported systematic assessment of diversion. Seven (17.9%) reported systematic measurement of nonmedical use, and 15 (38.5%) assessed incident tolerance.
The study revealed that eight of nine products that were approved for acute pain were supported by at least one pivotal trial. The median duration of these 19 trials was 1 day, and they enrolled a median of 329 patients.
The investigators noted that the findings “underscore the evidence gaps that have limited clinicians’ and patients’ understanding and appreciation of the inherent risks of prescription opioid analgesics.”
Dr. Alexander, who has been an FDA advisory committee chairman and currently serves as a consultant to plaintiffs who are suing opioid manufacturers in federal multidistrict litigation, said the study “is a story about missed opportunities to improve the safety and to improve the regulatory review of these products.”
Coinvestigator Peter Lurie, MD, who was an official at the FDA from 2009 to 2017, said that “there’s not a lot of signs that things are changing” at the agency.
The study shows that the FDA has “accepted what the companies have been presenting,” said Dr. Lurie, who is president of the Center for Science in the Public Interest.
The FDA “absolutely has the authority” to require manufacturers to undertake more rigorous trials, but agency culture keeps it from making such demands, especially if doing so means a new applicant might have to conduct trials that weren’t previously required, Dr. Lurie said in an interview.
“FDA is pretty rigorous about trying to establish a level playing field. That’s a virtuous thing, but it becomes problematic when that prevents change,” said Dr. Lurie.
The most recent FDA guidance to manufacturers, issued in 2019, does not provide advice on criteria for endpoints, study duration, or which populations are most likely to benefit from opioid treatment. The agency also does not require drug manufacturers to formally collect data on safety, tolerance, overdose symptoms, or constipation.
The guidance does suggest that the agency would likely take into account public health considerations when evaluating opioids, such as the risk to the overall population for overdose and diversion.
‘Overwhelming evidence’
Dr. Kolodny said that, as far as he is aware, “this is the first scientific publication in a peer-reviewed journal demonstrating clearly the problems with FDA’s opioid approval process.”
The article offers “overwhelming evidence that they are improperly approving the most dangerous medications – medications that killed more people than any other medication on the market,” added Dr. Kolodny, who is also president of Physicians for Responsible Opioid Prescribing.
Asked to respond to the study findings, FDA spokesperson Charles Kohler said the agency “does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
A version of this article originally appeared on Medscape.com.
Despite the ongoing epidemic of misuse, overuse, and diversion of opioids, the Food and Drug Administration has set a low bar for approval of these medications over the past 20 years, new research suggests.
The study results also show that the FDA did not require manufacturers to collect safety data on tolerance, withdrawal, overdose, misuse, and diversion in any rigorous fashion.
In addition, during the study period, 17 of the 39 new drug applications (NDAs) (only one was an innovator product, known as a new molecular entity) for chronic pain were approved with an “enriched enrollment randomized withdrawal” (EERW) trial design. Such a design, in this case, allowed manufacturers to exclude 32%-43% of the initially enrolled patients from the double-blind treatment phase.
“The question for regulators, policy makers, and others is: How did we get to a point where these approvals took place based on trials that were by design unlikely to yield some of the most important information about safety and efficacy that patients and clinicians would care about?” study investigator G. Caleb Alexander, MD, Johns Hopkins University, Baltimore, said in an interview.
The study was published online Sept. 29 in the Annals of Internal Medicine.
‘Cooking the books’
Little is known about the evidence required by the FDA for new approvals of opioid analgesics.
To characterize the quality of safety and efficacy data in NDAs for opioid analgesics approved by the FDA between 1997 and 2018, the investigators conducted the cross-sectional analysis using data from ClinicalTrials.gov, FDA reviews, and peer-reviewed publications regarding phase 3 pivotal trials.
The investigators examined the key characteristics of each NDA, including the number, size, and duration of pivotal trials, trial control groups, use of EERW, and systematically measured safety outcomes.
Results showed that most of the 48 NDAs evaluated were for new dosage forms (52.1%) or new formulations (18.8%). Only one (2.1%) was for a new molecular entity.
Of 39 NDAs approved for the treatment of chronic pain, only 21 products were supported by at least one pivotal trial. The mean duration of these 28 trials was 84 days, and they enrolled a median of 299 patients.
Results showed that, for 17 of the 39 opioids approved for chronic pain, pivotal trials had an EERW design. For the latest period – 2012-2018 – trials of all eight of the approved opioids used the EERW method.
This EERW design allows the manufacturer to assess efficacy “among a subset of patients most likely to respond and least likely to have adverse effects, reducing generalizability to real-world settings,” the investigators noted.
They called on the FDA to stop relying on this type of trial to assess opioid efficacy.
In an August 2020 article, Andrew Kolodny, MD, pointed out the pitfalls of the EERW approach. In such a study, all participants are made physiologically dependent on the opioid in a 4- to 6-week open-label phase. Only those who tolerate the drug and find it helpful are included in the randomized study. Dr. Kolodny is codirector of opioid policy research at Brandeis University, Waltham, Mass.
“Critics of EERW have correctly described this methodology as ‘cooking the books,’ ” Dr. Kolodny writes.
He noted that the agency’s decision to rely on EERW trials for opioids was “based on discussions at private meetings between FDA officials and pharmaceutical company executives hosted by an organization called Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.” The 2013 meetings were reported in an article published in the Washington Post.
Little sign of change
Among NDAs for chronic pain, the investigators found that eight (20.5%) included pooled safety reviews that reported systematic assessment of diversion. Seven (17.9%) reported systematic measurement of nonmedical use, and 15 (38.5%) assessed incident tolerance.
The study revealed that eight of nine products that were approved for acute pain were supported by at least one pivotal trial. The median duration of these 19 trials was 1 day, and they enrolled a median of 329 patients.
The investigators noted that the findings “underscore the evidence gaps that have limited clinicians’ and patients’ understanding and appreciation of the inherent risks of prescription opioid analgesics.”
Dr. Alexander, who has been an FDA advisory committee chairman and currently serves as a consultant to plaintiffs who are suing opioid manufacturers in federal multidistrict litigation, said the study “is a story about missed opportunities to improve the safety and to improve the regulatory review of these products.”
Coinvestigator Peter Lurie, MD, who was an official at the FDA from 2009 to 2017, said that “there’s not a lot of signs that things are changing” at the agency.
The study shows that the FDA has “accepted what the companies have been presenting,” said Dr. Lurie, who is president of the Center for Science in the Public Interest.
The FDA “absolutely has the authority” to require manufacturers to undertake more rigorous trials, but agency culture keeps it from making such demands, especially if doing so means a new applicant might have to conduct trials that weren’t previously required, Dr. Lurie said in an interview.
“FDA is pretty rigorous about trying to establish a level playing field. That’s a virtuous thing, but it becomes problematic when that prevents change,” said Dr. Lurie.
The most recent FDA guidance to manufacturers, issued in 2019, does not provide advice on criteria for endpoints, study duration, or which populations are most likely to benefit from opioid treatment. The agency also does not require drug manufacturers to formally collect data on safety, tolerance, overdose symptoms, or constipation.
The guidance does suggest that the agency would likely take into account public health considerations when evaluating opioids, such as the risk to the overall population for overdose and diversion.
‘Overwhelming evidence’
Dr. Kolodny said that, as far as he is aware, “this is the first scientific publication in a peer-reviewed journal demonstrating clearly the problems with FDA’s opioid approval process.”
The article offers “overwhelming evidence that they are improperly approving the most dangerous medications – medications that killed more people than any other medication on the market,” added Dr. Kolodny, who is also president of Physicians for Responsible Opioid Prescribing.
Asked to respond to the study findings, FDA spokesperson Charles Kohler said the agency “does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
A version of this article originally appeared on Medscape.com.
Despite the ongoing epidemic of misuse, overuse, and diversion of opioids, the Food and Drug Administration has set a low bar for approval of these medications over the past 20 years, new research suggests.
The study results also show that the FDA did not require manufacturers to collect safety data on tolerance, withdrawal, overdose, misuse, and diversion in any rigorous fashion.
In addition, during the study period, 17 of the 39 new drug applications (NDAs) (only one was an innovator product, known as a new molecular entity) for chronic pain were approved with an “enriched enrollment randomized withdrawal” (EERW) trial design. Such a design, in this case, allowed manufacturers to exclude 32%-43% of the initially enrolled patients from the double-blind treatment phase.
“The question for regulators, policy makers, and others is: How did we get to a point where these approvals took place based on trials that were by design unlikely to yield some of the most important information about safety and efficacy that patients and clinicians would care about?” study investigator G. Caleb Alexander, MD, Johns Hopkins University, Baltimore, said in an interview.
The study was published online Sept. 29 in the Annals of Internal Medicine.
‘Cooking the books’
Little is known about the evidence required by the FDA for new approvals of opioid analgesics.
To characterize the quality of safety and efficacy data in NDAs for opioid analgesics approved by the FDA between 1997 and 2018, the investigators conducted the cross-sectional analysis using data from ClinicalTrials.gov, FDA reviews, and peer-reviewed publications regarding phase 3 pivotal trials.
The investigators examined the key characteristics of each NDA, including the number, size, and duration of pivotal trials, trial control groups, use of EERW, and systematically measured safety outcomes.
Results showed that most of the 48 NDAs evaluated were for new dosage forms (52.1%) or new formulations (18.8%). Only one (2.1%) was for a new molecular entity.
Of 39 NDAs approved for the treatment of chronic pain, only 21 products were supported by at least one pivotal trial. The mean duration of these 28 trials was 84 days, and they enrolled a median of 299 patients.
Results showed that, for 17 of the 39 opioids approved for chronic pain, pivotal trials had an EERW design. For the latest period – 2012-2018 – trials of all eight of the approved opioids used the EERW method.
This EERW design allows the manufacturer to assess efficacy “among a subset of patients most likely to respond and least likely to have adverse effects, reducing generalizability to real-world settings,” the investigators noted.
They called on the FDA to stop relying on this type of trial to assess opioid efficacy.
In an August 2020 article, Andrew Kolodny, MD, pointed out the pitfalls of the EERW approach. In such a study, all participants are made physiologically dependent on the opioid in a 4- to 6-week open-label phase. Only those who tolerate the drug and find it helpful are included in the randomized study. Dr. Kolodny is codirector of opioid policy research at Brandeis University, Waltham, Mass.
“Critics of EERW have correctly described this methodology as ‘cooking the books,’ ” Dr. Kolodny writes.
He noted that the agency’s decision to rely on EERW trials for opioids was “based on discussions at private meetings between FDA officials and pharmaceutical company executives hosted by an organization called Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.” The 2013 meetings were reported in an article published in the Washington Post.
Little sign of change
Among NDAs for chronic pain, the investigators found that eight (20.5%) included pooled safety reviews that reported systematic assessment of diversion. Seven (17.9%) reported systematic measurement of nonmedical use, and 15 (38.5%) assessed incident tolerance.
The study revealed that eight of nine products that were approved for acute pain were supported by at least one pivotal trial. The median duration of these 19 trials was 1 day, and they enrolled a median of 329 patients.
The investigators noted that the findings “underscore the evidence gaps that have limited clinicians’ and patients’ understanding and appreciation of the inherent risks of prescription opioid analgesics.”
Dr. Alexander, who has been an FDA advisory committee chairman and currently serves as a consultant to plaintiffs who are suing opioid manufacturers in federal multidistrict litigation, said the study “is a story about missed opportunities to improve the safety and to improve the regulatory review of these products.”
Coinvestigator Peter Lurie, MD, who was an official at the FDA from 2009 to 2017, said that “there’s not a lot of signs that things are changing” at the agency.
The study shows that the FDA has “accepted what the companies have been presenting,” said Dr. Lurie, who is president of the Center for Science in the Public Interest.
The FDA “absolutely has the authority” to require manufacturers to undertake more rigorous trials, but agency culture keeps it from making such demands, especially if doing so means a new applicant might have to conduct trials that weren’t previously required, Dr. Lurie said in an interview.
“FDA is pretty rigorous about trying to establish a level playing field. That’s a virtuous thing, but it becomes problematic when that prevents change,” said Dr. Lurie.
The most recent FDA guidance to manufacturers, issued in 2019, does not provide advice on criteria for endpoints, study duration, or which populations are most likely to benefit from opioid treatment. The agency also does not require drug manufacturers to formally collect data on safety, tolerance, overdose symptoms, or constipation.
The guidance does suggest that the agency would likely take into account public health considerations when evaluating opioids, such as the risk to the overall population for overdose and diversion.
‘Overwhelming evidence’
Dr. Kolodny said that, as far as he is aware, “this is the first scientific publication in a peer-reviewed journal demonstrating clearly the problems with FDA’s opioid approval process.”
The article offers “overwhelming evidence that they are improperly approving the most dangerous medications – medications that killed more people than any other medication on the market,” added Dr. Kolodny, who is also president of Physicians for Responsible Opioid Prescribing.
Asked to respond to the study findings, FDA spokesperson Charles Kohler said the agency “does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
A version of this article originally appeared on Medscape.com.
COVID-19’s psychological impact gets a name
During normal times, the U.K.-based charity No Panic offers itself as an easily accessible service to those with anxiety disorders and phobias. Visitors to the website who can receive immediate, remote support from trained volunteers. But this spring was anything but normal, as the reality of COVID-19’s worldwide spread became terrifyingly clear.
COVID-19 cases peaked in the United Kingdom in early April. Nationwide lockdown efforts contributed to a gradual but ultimately substantial decline in cases, yet, despite the favorable trend lines, No Panic has remained busier than ever.
Beyond the physical symptoms associated with COVID-19, the psychological outcomes are vast and, it seems, prolonged. Researchers have now formalized a definition of the long-term mental maladies associated with the pandemic, collectively deeming them “coronaphobia.”
The term is a catch-all phrase for the fear and the emotional and social strain experienced by the general public in response to COVID-19. Obsessive behaviors, distress, avoidance reaction, panic, anxiety, hoarding, paranoia, and depression are some of the responses associated with coronaphobia. On the surface, these appear to be normal, somewhat fitting reactions to this surreal and frightening moment in time. However, for those experiencing coronaphobia, they are distinctly maladaptive and harmful.
“We had a serious rise in the use of our services, notably the helpline and email enquiries,” explained Sarah Floyd, No Panic’s volunteer advisor and social media coordinator. “It has been up and down all along, but more of an up since lockdown is easing.”
The group’s experience offers yet more evidence that the anxieties and fears caused by this global pandemic don’t flatten alongside the curve but instead linger as chronic problems requiring ongoing care.
“Every week in my clinic, I’m seeing people who are experiencing more anxiety and hopelessness and having an emotional response that is perhaps out of proportion to what one would expect, which is directly related to what is going on in the world right now with coronavirus,” said Gregory Scott Brown, MD, founder and director of the Center for Green Psychiatry in West Lake Hills, Tex. “Simply put, I think what we are looking at is adjustment disorder. That is probably how the DSM would define it.”
Adjustment disorder is one of the most frequently diagnosed mental health conditions, although it is also relatively understudied. It is really a set of disorders that follow in the wake of a significant stressor, which can vary from serious illness or the death of a loved one to relocating or experiencing work problems. The resulting dysfunction and distress that the person experiences are considered out of proportion in duration or scale with what would normally be expected. Diagnosing an adjustment disorder is made difficult by the lack of a valid and reliable screening measure.
Recent literature suggests that coronaphobia may be likely to occur in those who feel vulnerable to disease, are predisposed to anxiety, or are intolerant of uncertainty. Preexisting mental health conditions can also be exacerbated by periods of quarantine, self-isolation, and lockdown, which can lead to panic attacks, chronophobia (fear of passing time), and suicidality.
Although imperfect comparisons, findings from earlier 21st century disease outbreaks, such as severe acute respiratory syndrome and the Ebola virus, signal that containment efforts themselves play a role in deteriorating mental health. A recent rapid review found that, in studies comparing persons who had previously undergone quarantines and those who had not, the former were significantly more likely to experience acute stress disorder, posttraumatic stress symptoms, and depression. Quarantine was found to result in long-term behavioral changes, such as avoiding crowds, among the general public and health care practitioners.
That tremendous psychological morbidity should accompany a global pandemic of this scale is not surprising, according to Amit Anand, MD, vice chair for research for the Center for Behavioral Health and director of the Mood and Emotional Disorders Across the Life Span program at the Cleveland Clinic.
“The technical definition of anxiety is an impending sense of doom, and I think all of us are living with that,” Dr. Anand said. “The basic question then becomes, what is normal and when does it become abnormal?”
He added that most classifications of psychiatric disorders are set during periods of relative stability, which the current moment is most certainly not.
“This is such an unusual situation, so I think it will depend on case-by-case basis, keeping the whole context in mind as whether the patient is thinking or behaving with an abnormal amount of anxiety,” Dr. Anand said.
Investigators are currently trying to give clinicians the tools to better make that determination. In the first scientific study of this clinical condition, Sherman Lee, MD, reported that five symptoms – dizziness, sleep disturbances, tonic immobility, appetite loss, and nausea/abdominal distress – were strong factors for distinguishing coronaphobia from otherwise normal concerns about COVID-19 that did not result in functional impairment. Dr. Lee and colleagues have since published further evidence that coronaphobia “is a unique predictor of psychological distress during the COVID-19 crisis.” They are working on validating a self-reported mental health screener for this condition.
Having the tools to identify patients struggling with coronaphobia may go some ways toward addressing another area of declining health. At the outset of the COVID-19 pandemic, there was a question as to whether doctors would be beset by a surge of the “worried well” – persons mistakenly believing themselves to be infected. Now months into the pandemic, the converse phenomenon – a fear of contracting COVID-19 that is driving patients away from practitioners – appears to be the more valid concern.
In early spring, the pandemic’s first surge was accompanied by reports of approximately 40% and 60% drops in visits to EDs and ambulatory centers, respectively. Stories of acute stroke patients avoiding treatment began to appear in the press. Major U.S. cities saw noteworthy declines in 911 calls, indicating a hesitancy to be taken to a hospital. That COVID-19 has been accompanied by mass unemployment and subsequent loss of insurance complicates the notion that fear alone is keeping people from treatment. In other countries, it has been explicitly linked. Investigators in Singapore noted that coronaphobia played a role in reducing willingness to attend in-person visits among adolescents with eating disorders. Similarly, case reports in Israel suggest that coronaphobia has contributed to delays in diagnoses of common pediatric diseases.
There is also a concern, colloquially termed “reentry anxiety,” that mental health problems caused by the pandemic, the accompanying lockdown, self-isolation, and quarantine practices will prove alarmingly durable. Even after this challenging moment in history draws to a close, many people may face substantial stress in returning to the normal activities of life – social, professional, familial – once taken for granted.
“We are in the beginning phase of that now,” said Dr. Anand. “ I think the longer it goes on for, the more difficult it will be.”
In the United States, that day may seem far away. Nonetheless, it is important to begin laying the therapeutic groundwork now, according to Dr. Brown.
“I am recommending unconventional therapies like meet-up groups, online forums,” he said. “Everything has shifted online, and so there are a lot of support groups that patients can participate to learn coping skills and really hear what other people are going through.”
Before reaching that stage, Dr. Brown recommends that clinicians first simply discuss such anxieties with their patients in order to normalize them.
“Realize that everyone essentially is going through some degree of this right now. The coronavirus pandemic is literally impacting every person on the face of the planet. Sometimes just pointing that out to people can really help,” he said.
A version of this article originally appeared on Medscape.com.
During normal times, the U.K.-based charity No Panic offers itself as an easily accessible service to those with anxiety disorders and phobias. Visitors to the website who can receive immediate, remote support from trained volunteers. But this spring was anything but normal, as the reality of COVID-19’s worldwide spread became terrifyingly clear.
COVID-19 cases peaked in the United Kingdom in early April. Nationwide lockdown efforts contributed to a gradual but ultimately substantial decline in cases, yet, despite the favorable trend lines, No Panic has remained busier than ever.
Beyond the physical symptoms associated with COVID-19, the psychological outcomes are vast and, it seems, prolonged. Researchers have now formalized a definition of the long-term mental maladies associated with the pandemic, collectively deeming them “coronaphobia.”
The term is a catch-all phrase for the fear and the emotional and social strain experienced by the general public in response to COVID-19. Obsessive behaviors, distress, avoidance reaction, panic, anxiety, hoarding, paranoia, and depression are some of the responses associated with coronaphobia. On the surface, these appear to be normal, somewhat fitting reactions to this surreal and frightening moment in time. However, for those experiencing coronaphobia, they are distinctly maladaptive and harmful.
“We had a serious rise in the use of our services, notably the helpline and email enquiries,” explained Sarah Floyd, No Panic’s volunteer advisor and social media coordinator. “It has been up and down all along, but more of an up since lockdown is easing.”
The group’s experience offers yet more evidence that the anxieties and fears caused by this global pandemic don’t flatten alongside the curve but instead linger as chronic problems requiring ongoing care.
“Every week in my clinic, I’m seeing people who are experiencing more anxiety and hopelessness and having an emotional response that is perhaps out of proportion to what one would expect, which is directly related to what is going on in the world right now with coronavirus,” said Gregory Scott Brown, MD, founder and director of the Center for Green Psychiatry in West Lake Hills, Tex. “Simply put, I think what we are looking at is adjustment disorder. That is probably how the DSM would define it.”
Adjustment disorder is one of the most frequently diagnosed mental health conditions, although it is also relatively understudied. It is really a set of disorders that follow in the wake of a significant stressor, which can vary from serious illness or the death of a loved one to relocating or experiencing work problems. The resulting dysfunction and distress that the person experiences are considered out of proportion in duration or scale with what would normally be expected. Diagnosing an adjustment disorder is made difficult by the lack of a valid and reliable screening measure.
Recent literature suggests that coronaphobia may be likely to occur in those who feel vulnerable to disease, are predisposed to anxiety, or are intolerant of uncertainty. Preexisting mental health conditions can also be exacerbated by periods of quarantine, self-isolation, and lockdown, which can lead to panic attacks, chronophobia (fear of passing time), and suicidality.
Although imperfect comparisons, findings from earlier 21st century disease outbreaks, such as severe acute respiratory syndrome and the Ebola virus, signal that containment efforts themselves play a role in deteriorating mental health. A recent rapid review found that, in studies comparing persons who had previously undergone quarantines and those who had not, the former were significantly more likely to experience acute stress disorder, posttraumatic stress symptoms, and depression. Quarantine was found to result in long-term behavioral changes, such as avoiding crowds, among the general public and health care practitioners.
That tremendous psychological morbidity should accompany a global pandemic of this scale is not surprising, according to Amit Anand, MD, vice chair for research for the Center for Behavioral Health and director of the Mood and Emotional Disorders Across the Life Span program at the Cleveland Clinic.
“The technical definition of anxiety is an impending sense of doom, and I think all of us are living with that,” Dr. Anand said. “The basic question then becomes, what is normal and when does it become abnormal?”
He added that most classifications of psychiatric disorders are set during periods of relative stability, which the current moment is most certainly not.
“This is such an unusual situation, so I think it will depend on case-by-case basis, keeping the whole context in mind as whether the patient is thinking or behaving with an abnormal amount of anxiety,” Dr. Anand said.
Investigators are currently trying to give clinicians the tools to better make that determination. In the first scientific study of this clinical condition, Sherman Lee, MD, reported that five symptoms – dizziness, sleep disturbances, tonic immobility, appetite loss, and nausea/abdominal distress – were strong factors for distinguishing coronaphobia from otherwise normal concerns about COVID-19 that did not result in functional impairment. Dr. Lee and colleagues have since published further evidence that coronaphobia “is a unique predictor of psychological distress during the COVID-19 crisis.” They are working on validating a self-reported mental health screener for this condition.
Having the tools to identify patients struggling with coronaphobia may go some ways toward addressing another area of declining health. At the outset of the COVID-19 pandemic, there was a question as to whether doctors would be beset by a surge of the “worried well” – persons mistakenly believing themselves to be infected. Now months into the pandemic, the converse phenomenon – a fear of contracting COVID-19 that is driving patients away from practitioners – appears to be the more valid concern.
In early spring, the pandemic’s first surge was accompanied by reports of approximately 40% and 60% drops in visits to EDs and ambulatory centers, respectively. Stories of acute stroke patients avoiding treatment began to appear in the press. Major U.S. cities saw noteworthy declines in 911 calls, indicating a hesitancy to be taken to a hospital. That COVID-19 has been accompanied by mass unemployment and subsequent loss of insurance complicates the notion that fear alone is keeping people from treatment. In other countries, it has been explicitly linked. Investigators in Singapore noted that coronaphobia played a role in reducing willingness to attend in-person visits among adolescents with eating disorders. Similarly, case reports in Israel suggest that coronaphobia has contributed to delays in diagnoses of common pediatric diseases.
There is also a concern, colloquially termed “reentry anxiety,” that mental health problems caused by the pandemic, the accompanying lockdown, self-isolation, and quarantine practices will prove alarmingly durable. Even after this challenging moment in history draws to a close, many people may face substantial stress in returning to the normal activities of life – social, professional, familial – once taken for granted.
“We are in the beginning phase of that now,” said Dr. Anand. “ I think the longer it goes on for, the more difficult it will be.”
In the United States, that day may seem far away. Nonetheless, it is important to begin laying the therapeutic groundwork now, according to Dr. Brown.
“I am recommending unconventional therapies like meet-up groups, online forums,” he said. “Everything has shifted online, and so there are a lot of support groups that patients can participate to learn coping skills and really hear what other people are going through.”
Before reaching that stage, Dr. Brown recommends that clinicians first simply discuss such anxieties with their patients in order to normalize them.
“Realize that everyone essentially is going through some degree of this right now. The coronavirus pandemic is literally impacting every person on the face of the planet. Sometimes just pointing that out to people can really help,” he said.
A version of this article originally appeared on Medscape.com.
During normal times, the U.K.-based charity No Panic offers itself as an easily accessible service to those with anxiety disorders and phobias. Visitors to the website who can receive immediate, remote support from trained volunteers. But this spring was anything but normal, as the reality of COVID-19’s worldwide spread became terrifyingly clear.
COVID-19 cases peaked in the United Kingdom in early April. Nationwide lockdown efforts contributed to a gradual but ultimately substantial decline in cases, yet, despite the favorable trend lines, No Panic has remained busier than ever.
Beyond the physical symptoms associated with COVID-19, the psychological outcomes are vast and, it seems, prolonged. Researchers have now formalized a definition of the long-term mental maladies associated with the pandemic, collectively deeming them “coronaphobia.”
The term is a catch-all phrase for the fear and the emotional and social strain experienced by the general public in response to COVID-19. Obsessive behaviors, distress, avoidance reaction, panic, anxiety, hoarding, paranoia, and depression are some of the responses associated with coronaphobia. On the surface, these appear to be normal, somewhat fitting reactions to this surreal and frightening moment in time. However, for those experiencing coronaphobia, they are distinctly maladaptive and harmful.
“We had a serious rise in the use of our services, notably the helpline and email enquiries,” explained Sarah Floyd, No Panic’s volunteer advisor and social media coordinator. “It has been up and down all along, but more of an up since lockdown is easing.”
The group’s experience offers yet more evidence that the anxieties and fears caused by this global pandemic don’t flatten alongside the curve but instead linger as chronic problems requiring ongoing care.
“Every week in my clinic, I’m seeing people who are experiencing more anxiety and hopelessness and having an emotional response that is perhaps out of proportion to what one would expect, which is directly related to what is going on in the world right now with coronavirus,” said Gregory Scott Brown, MD, founder and director of the Center for Green Psychiatry in West Lake Hills, Tex. “Simply put, I think what we are looking at is adjustment disorder. That is probably how the DSM would define it.”
Adjustment disorder is one of the most frequently diagnosed mental health conditions, although it is also relatively understudied. It is really a set of disorders that follow in the wake of a significant stressor, which can vary from serious illness or the death of a loved one to relocating or experiencing work problems. The resulting dysfunction and distress that the person experiences are considered out of proportion in duration or scale with what would normally be expected. Diagnosing an adjustment disorder is made difficult by the lack of a valid and reliable screening measure.
Recent literature suggests that coronaphobia may be likely to occur in those who feel vulnerable to disease, are predisposed to anxiety, or are intolerant of uncertainty. Preexisting mental health conditions can also be exacerbated by periods of quarantine, self-isolation, and lockdown, which can lead to panic attacks, chronophobia (fear of passing time), and suicidality.
Although imperfect comparisons, findings from earlier 21st century disease outbreaks, such as severe acute respiratory syndrome and the Ebola virus, signal that containment efforts themselves play a role in deteriorating mental health. A recent rapid review found that, in studies comparing persons who had previously undergone quarantines and those who had not, the former were significantly more likely to experience acute stress disorder, posttraumatic stress symptoms, and depression. Quarantine was found to result in long-term behavioral changes, such as avoiding crowds, among the general public and health care practitioners.
That tremendous psychological morbidity should accompany a global pandemic of this scale is not surprising, according to Amit Anand, MD, vice chair for research for the Center for Behavioral Health and director of the Mood and Emotional Disorders Across the Life Span program at the Cleveland Clinic.
“The technical definition of anxiety is an impending sense of doom, and I think all of us are living with that,” Dr. Anand said. “The basic question then becomes, what is normal and when does it become abnormal?”
He added that most classifications of psychiatric disorders are set during periods of relative stability, which the current moment is most certainly not.
“This is such an unusual situation, so I think it will depend on case-by-case basis, keeping the whole context in mind as whether the patient is thinking or behaving with an abnormal amount of anxiety,” Dr. Anand said.
Investigators are currently trying to give clinicians the tools to better make that determination. In the first scientific study of this clinical condition, Sherman Lee, MD, reported that five symptoms – dizziness, sleep disturbances, tonic immobility, appetite loss, and nausea/abdominal distress – were strong factors for distinguishing coronaphobia from otherwise normal concerns about COVID-19 that did not result in functional impairment. Dr. Lee and colleagues have since published further evidence that coronaphobia “is a unique predictor of psychological distress during the COVID-19 crisis.” They are working on validating a self-reported mental health screener for this condition.
Having the tools to identify patients struggling with coronaphobia may go some ways toward addressing another area of declining health. At the outset of the COVID-19 pandemic, there was a question as to whether doctors would be beset by a surge of the “worried well” – persons mistakenly believing themselves to be infected. Now months into the pandemic, the converse phenomenon – a fear of contracting COVID-19 that is driving patients away from practitioners – appears to be the more valid concern.
In early spring, the pandemic’s first surge was accompanied by reports of approximately 40% and 60% drops in visits to EDs and ambulatory centers, respectively. Stories of acute stroke patients avoiding treatment began to appear in the press. Major U.S. cities saw noteworthy declines in 911 calls, indicating a hesitancy to be taken to a hospital. That COVID-19 has been accompanied by mass unemployment and subsequent loss of insurance complicates the notion that fear alone is keeping people from treatment. In other countries, it has been explicitly linked. Investigators in Singapore noted that coronaphobia played a role in reducing willingness to attend in-person visits among adolescents with eating disorders. Similarly, case reports in Israel suggest that coronaphobia has contributed to delays in diagnoses of common pediatric diseases.
There is also a concern, colloquially termed “reentry anxiety,” that mental health problems caused by the pandemic, the accompanying lockdown, self-isolation, and quarantine practices will prove alarmingly durable. Even after this challenging moment in history draws to a close, many people may face substantial stress in returning to the normal activities of life – social, professional, familial – once taken for granted.
“We are in the beginning phase of that now,” said Dr. Anand. “ I think the longer it goes on for, the more difficult it will be.”
In the United States, that day may seem far away. Nonetheless, it is important to begin laying the therapeutic groundwork now, according to Dr. Brown.
“I am recommending unconventional therapies like meet-up groups, online forums,” he said. “Everything has shifted online, and so there are a lot of support groups that patients can participate to learn coping skills and really hear what other people are going through.”
Before reaching that stage, Dr. Brown recommends that clinicians first simply discuss such anxieties with their patients in order to normalize them.
“Realize that everyone essentially is going through some degree of this right now. The coronavirus pandemic is literally impacting every person on the face of the planet. Sometimes just pointing that out to people can really help,” he said.
A version of this article originally appeared on Medscape.com.
Listening to Mozart helps tame epilepsy
Listening to Mozart’s piano music improves epilepsy, according to a meta-analysis presented at the virtual congress of the European College of Neuropsychopharmacology.
The results of the meta-analysis of 12 published studies of the so-called Mozart Effect that met rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines demonstrate that listening to Mozart results in significant reductions in both epileptic seizure frequency and interictal epileptiform discharges (IED), compared with baseline.
The benefits were apparent during and after even a single listening session, although the effect was greater with regular daily listening sessions, according to Gianluca Sesso, MD, a resident in child and adolescent psychiatry at the University of Pisa (Italy.)
“Obviously other music may have similar effects, but it may be that Mozart’s sonatas have distinctive rhythmic structures which are particularly suited to working on epilepsy,” he speculated, adding that the mechanism involved in the Mozart Effect on brain systems remains unclear.
“The highly consistent results of our meta-analysis strongly suggest that music-based neurostimulation may improve the clinical outcome in epilepsy by reducing seizures and IED, and thus deserves to be included in the set of nonpharmacologic complementary approaches for treating epilepsy,” Dr. Sesso added.
Four studies examined the effects of listening to Mozart’s Sonata for Two Pianos in D, K.448, the most-studied piece of music as a treatment for epilepsy. The data documented a 31% reduction in seizure frequency and 28% decrease in IED during a single listen, and a 79% reduction in IED after long-term Mozart music therapy. Similarly, studies demonstrated that listening to a set of Mozart’s compositions resulted in a 36% reduction in IED during and 38% decrease after a single listen, while regular listening in a prolonged treatment period resulted in a 66% reduction in seizure frequency from baseline.
Several studies compared the benefits of listening to K. 488 with those accrued through listening to Piano Sonata No. 16 in C major, K. 545. There was no significant difference between the two, according to Dr. Sesso.
He reported having no financial conflicts regarding his meta-analysis, carried out free of commercial support.
The full details of the meta-analysis were recently published in Clinical Neurophysiology.
Listening to Mozart’s piano music improves epilepsy, according to a meta-analysis presented at the virtual congress of the European College of Neuropsychopharmacology.
The results of the meta-analysis of 12 published studies of the so-called Mozart Effect that met rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines demonstrate that listening to Mozart results in significant reductions in both epileptic seizure frequency and interictal epileptiform discharges (IED), compared with baseline.
The benefits were apparent during and after even a single listening session, although the effect was greater with regular daily listening sessions, according to Gianluca Sesso, MD, a resident in child and adolescent psychiatry at the University of Pisa (Italy.)
“Obviously other music may have similar effects, but it may be that Mozart’s sonatas have distinctive rhythmic structures which are particularly suited to working on epilepsy,” he speculated, adding that the mechanism involved in the Mozart Effect on brain systems remains unclear.
“The highly consistent results of our meta-analysis strongly suggest that music-based neurostimulation may improve the clinical outcome in epilepsy by reducing seizures and IED, and thus deserves to be included in the set of nonpharmacologic complementary approaches for treating epilepsy,” Dr. Sesso added.
Four studies examined the effects of listening to Mozart’s Sonata for Two Pianos in D, K.448, the most-studied piece of music as a treatment for epilepsy. The data documented a 31% reduction in seizure frequency and 28% decrease in IED during a single listen, and a 79% reduction in IED after long-term Mozart music therapy. Similarly, studies demonstrated that listening to a set of Mozart’s compositions resulted in a 36% reduction in IED during and 38% decrease after a single listen, while regular listening in a prolonged treatment period resulted in a 66% reduction in seizure frequency from baseline.
Several studies compared the benefits of listening to K. 488 with those accrued through listening to Piano Sonata No. 16 in C major, K. 545. There was no significant difference between the two, according to Dr. Sesso.
He reported having no financial conflicts regarding his meta-analysis, carried out free of commercial support.
The full details of the meta-analysis were recently published in Clinical Neurophysiology.
Listening to Mozart’s piano music improves epilepsy, according to a meta-analysis presented at the virtual congress of the European College of Neuropsychopharmacology.
The results of the meta-analysis of 12 published studies of the so-called Mozart Effect that met rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines demonstrate that listening to Mozart results in significant reductions in both epileptic seizure frequency and interictal epileptiform discharges (IED), compared with baseline.
The benefits were apparent during and after even a single listening session, although the effect was greater with regular daily listening sessions, according to Gianluca Sesso, MD, a resident in child and adolescent psychiatry at the University of Pisa (Italy.)
“Obviously other music may have similar effects, but it may be that Mozart’s sonatas have distinctive rhythmic structures which are particularly suited to working on epilepsy,” he speculated, adding that the mechanism involved in the Mozart Effect on brain systems remains unclear.
“The highly consistent results of our meta-analysis strongly suggest that music-based neurostimulation may improve the clinical outcome in epilepsy by reducing seizures and IED, and thus deserves to be included in the set of nonpharmacologic complementary approaches for treating epilepsy,” Dr. Sesso added.
Four studies examined the effects of listening to Mozart’s Sonata for Two Pianos in D, K.448, the most-studied piece of music as a treatment for epilepsy. The data documented a 31% reduction in seizure frequency and 28% decrease in IED during a single listen, and a 79% reduction in IED after long-term Mozart music therapy. Similarly, studies demonstrated that listening to a set of Mozart’s compositions resulted in a 36% reduction in IED during and 38% decrease after a single listen, while regular listening in a prolonged treatment period resulted in a 66% reduction in seizure frequency from baseline.
Several studies compared the benefits of listening to K. 488 with those accrued through listening to Piano Sonata No. 16 in C major, K. 545. There was no significant difference between the two, according to Dr. Sesso.
He reported having no financial conflicts regarding his meta-analysis, carried out free of commercial support.
The full details of the meta-analysis were recently published in Clinical Neurophysiology.
FROM ECNP 2020
Suicide in America: The urban-rural divide
The gap in suicide rates between rural and urban areas has widened since 2000 for both males and females, according to a recent report from the National Center for Health Statistics.

After remaining stable from 2000 to 2007, the suicide rate for rural males rose 34% from 2007 to 2018, versus 17% among urban males over the same period. Suicide rates for females were significantly lower than those of men, but the changes were larger. For rural females, the rate increased 91% from 2000 to 2018, compared with 51% for urban females, Kristen Pettrone, MD, MPH, and Sally C. Curtin, MA, said in an NCHS Data Brief.
For 2018, the last year with available data, the age-adjusted rates look like this: 21.5 per 100,000 population for urban males, 30.7 for rural males, 5.9 per 100,000 for urban females, and 8.0 for rural females. The overall rate for the United States was 14.2 per 100,000, with combined male/female rates of 13.4 in urban areas and 19.4 in rural areas, the researchers said.
Methods of suicide also varied by sex and urban-rural status. Firearms were the leading method for males in both rural and urban areas, but females split between firearms in rural areas and suffocation (including hangings) in urban areas, said Dr. Pettrone of the Centers for Disease Control and Prevention and Ms. Curtin of the NCHS.
Suffocation, however, was the fastest-growing method from 2000 to 2018, regardless of sex or location. Suffocation-related suicide rates more than quadrupled for rural females, and more than doubled for urban females and rural males, while rates rose 85% among males in urban areas, based on data from the National Vital Statistics System.
“Suicide has remained the 10th leading cause of death in the United States since 2008,” they wrote, and
SOURCE: Pettrone K, Curtin SC. 2020 Aug. NCHS Data Brief, No 373.
The gap in suicide rates between rural and urban areas has widened since 2000 for both males and females, according to a recent report from the National Center for Health Statistics.

After remaining stable from 2000 to 2007, the suicide rate for rural males rose 34% from 2007 to 2018, versus 17% among urban males over the same period. Suicide rates for females were significantly lower than those of men, but the changes were larger. For rural females, the rate increased 91% from 2000 to 2018, compared with 51% for urban females, Kristen Pettrone, MD, MPH, and Sally C. Curtin, MA, said in an NCHS Data Brief.
For 2018, the last year with available data, the age-adjusted rates look like this: 21.5 per 100,000 population for urban males, 30.7 for rural males, 5.9 per 100,000 for urban females, and 8.0 for rural females. The overall rate for the United States was 14.2 per 100,000, with combined male/female rates of 13.4 in urban areas and 19.4 in rural areas, the researchers said.
Methods of suicide also varied by sex and urban-rural status. Firearms were the leading method for males in both rural and urban areas, but females split between firearms in rural areas and suffocation (including hangings) in urban areas, said Dr. Pettrone of the Centers for Disease Control and Prevention and Ms. Curtin of the NCHS.
Suffocation, however, was the fastest-growing method from 2000 to 2018, regardless of sex or location. Suffocation-related suicide rates more than quadrupled for rural females, and more than doubled for urban females and rural males, while rates rose 85% among males in urban areas, based on data from the National Vital Statistics System.
“Suicide has remained the 10th leading cause of death in the United States since 2008,” they wrote, and
SOURCE: Pettrone K, Curtin SC. 2020 Aug. NCHS Data Brief, No 373.
The gap in suicide rates between rural and urban areas has widened since 2000 for both males and females, according to a recent report from the National Center for Health Statistics.

After remaining stable from 2000 to 2007, the suicide rate for rural males rose 34% from 2007 to 2018, versus 17% among urban males over the same period. Suicide rates for females were significantly lower than those of men, but the changes were larger. For rural females, the rate increased 91% from 2000 to 2018, compared with 51% for urban females, Kristen Pettrone, MD, MPH, and Sally C. Curtin, MA, said in an NCHS Data Brief.
For 2018, the last year with available data, the age-adjusted rates look like this: 21.5 per 100,000 population for urban males, 30.7 for rural males, 5.9 per 100,000 for urban females, and 8.0 for rural females. The overall rate for the United States was 14.2 per 100,000, with combined male/female rates of 13.4 in urban areas and 19.4 in rural areas, the researchers said.
Methods of suicide also varied by sex and urban-rural status. Firearms were the leading method for males in both rural and urban areas, but females split between firearms in rural areas and suffocation (including hangings) in urban areas, said Dr. Pettrone of the Centers for Disease Control and Prevention and Ms. Curtin of the NCHS.
Suffocation, however, was the fastest-growing method from 2000 to 2018, regardless of sex or location. Suffocation-related suicide rates more than quadrupled for rural females, and more than doubled for urban females and rural males, while rates rose 85% among males in urban areas, based on data from the National Vital Statistics System.
“Suicide has remained the 10th leading cause of death in the United States since 2008,” they wrote, and
SOURCE: Pettrone K, Curtin SC. 2020 Aug. NCHS Data Brief, No 373.

