User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Two swings, two misses with colchicine, Vascepa in COVID-19
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Deeper dive’ into opioid overdose deaths during COVID pandemic
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases top 200,000, vaccinations down
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.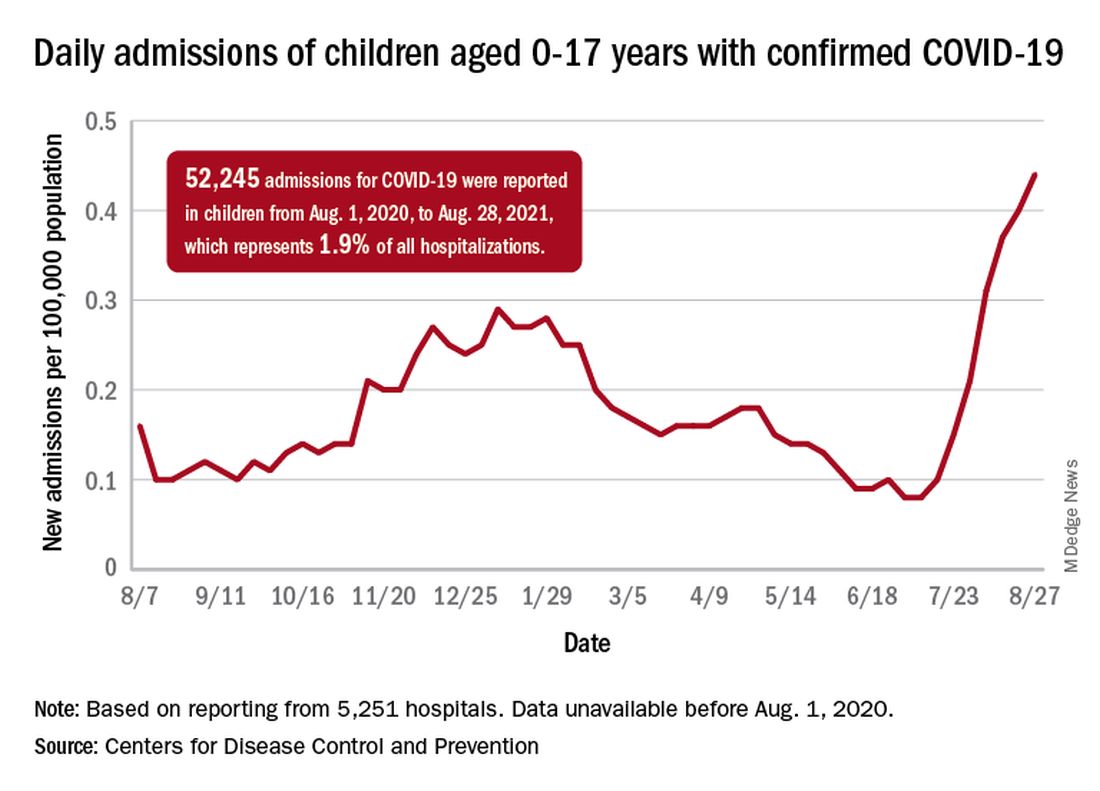
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
CDC panel unanimously backs Pfizer vax, fortifying FDA approval
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
Advocates seek to reframe masks as a disability accommodation
As governors and legislatures in states such as Texas, Florida, South Carolina, and Arkansas have banned schools and other entities from implementing mask mandates, disability rights advocates have pushed back. In federal civil rights lawsuits, they argue that bans on mask mandates violate antidiscrimination laws protecting people with disabilities.
For unvaccinated and immunosuppressed individuals, masks can provide crucial protection from SARS-CoV-2.
argues Mical Raz, MD, PhD, a professor at the University of Rochester (N.Y.) and a physician at Strong Memorial Hospital, also in Rochester, New York, in an article published in JAMA with coauthor Doron Dorfman, LLB, JSD.
This news organization talked with Dr. Raz about approaching mask requirements as disability accommodation during the COVID-19 pandemic. The following interview was lightly edited for length and clarity.
How did you come to think about mask requirements as a form of disability accommodation?
I saw a tweet from a professor at a university who said they couldn’t ask students about their vaccination status or to wear a mask. All agency was removed from the professor to take care of and protect themselves. I thought, well, that can’t be right. And ostensibly, that would be particularly dangerous for somebody with immunosuppression for whom the vaccine is not adequately protective. So, I called my friend, Doron Dorfman, and asked him to help me think through the legal part of this. We fleshed it out and wrote the article that same night.
How novel is it to view accommodations for people who are immunosuppressed through the lens of disability accommodation?
I think there has not been enough focus during the pandemic on individuals with disabilities or on how disability law can be mobilized during this pandemic to help supplement the public health law. This framework should be used a lot more because it’s good for everybody, not just for individuals with disabilities.
For example, take what’s called the “curb effect.” If you expand sidewalks, yes, it helps individuals who use a wheelchair. But it also helps me as a mom with a stroller. It helps somebody with a shopping cart, or a kid with a bike. If we adopt policies that are inclusive to those who are disadvantaged, it’s good for everybody. We should always strive to be an inclusive society, not just because it’s the right thing to do but because it really makes our society better.
How can mask requirements be used as a form of disability accommodation, as you argue in the JAMA article?
The ADA requires employers to provide reasonable accommodations for disability. In this case, the disability is your immunosuppressive status. We have an abundance of evidence showing individuals who are immunocompromised and vaccinated are still inadequately protected from the SARS-CoV-2 virus. So, there is absolute data to show individuals with immunosuppression have a disability that requires accommodation.
The ADA has a mandate requiring employers to adjust or modify policies in order to accommodate a disability. There are certain situations in which you cannot or do not need to accommodate a disability, when it would fundamentally alter the kind of employment you offer or if it’s an undue burden or hardship. But given that we’ve been wearing masks and working remotely for a year now, arguing that somehow these accommodations are no longer possible seems disingenuous.
In that way, allowing a person who’s immunocompromised to require those around them to mask is a form of modified protective policies. And in this case, those policies line up with a public health good, masking in the face of the highly contagious Delta variant ravaging our country right now.
In your view, can this argument be used in the mask debates happening right now across the country?
This argument can and should be useful for a couple of different lawsuits that are now underway in different states. I hope our article will provide further support for those suits. And I hope in school board hearings, when parents and teachers are talking about their concerns, this could be one way to argue for why we should allow mask mandates in classes. I’ve received emails from parents who said they’re going to bring this article to their school board hearing.
I also hope this could shift the narrative around the pandemic. Instead of focusing on individual responsibility – I got my vaccine shot so I’m fine – let’s focus on how we create an inclusive environment where we protect everybody, including those who cannot be vaccinated because of age or disability, or those who are vaccinated but inadequately protected because of their underlying conditions.
In the JAMA article, you talk about how our pandemic response has focused on individual health and how that individual focus can be ableist. Can you explain that point?
I think this idea that we just make our choices – like whether to get vaccinated or wear a mask, or not – and live with it really perpetuates a highly individualistic and ableist mindset. It doesn’t consider the people I admit to the hospital who are vaccinated but have a heart transplant and didn’t mount the sufficient immune response. Or even the people who chose not to be vaccinated because they were exposed to hours and hours of misinformation on TV.
We like to individualize everything, focusing on personal responsibility and choices, but a pandemic is one of those moments where everybody’s choices affect everybody else. Laying responsibility at the doorstep of each person, rather than thinking about what steps we as a society could be taking, is cheap and politically expedient. There is no public health rationale behind the bans on mask requirements in states like Texas, Iowa, and Florida. These choices are about politics. And the price is always borne by the most disadvantaged among us.
A version of this article first appeared on Medscape.com.
As governors and legislatures in states such as Texas, Florida, South Carolina, and Arkansas have banned schools and other entities from implementing mask mandates, disability rights advocates have pushed back. In federal civil rights lawsuits, they argue that bans on mask mandates violate antidiscrimination laws protecting people with disabilities.
For unvaccinated and immunosuppressed individuals, masks can provide crucial protection from SARS-CoV-2.
argues Mical Raz, MD, PhD, a professor at the University of Rochester (N.Y.) and a physician at Strong Memorial Hospital, also in Rochester, New York, in an article published in JAMA with coauthor Doron Dorfman, LLB, JSD.
This news organization talked with Dr. Raz about approaching mask requirements as disability accommodation during the COVID-19 pandemic. The following interview was lightly edited for length and clarity.
How did you come to think about mask requirements as a form of disability accommodation?
I saw a tweet from a professor at a university who said they couldn’t ask students about their vaccination status or to wear a mask. All agency was removed from the professor to take care of and protect themselves. I thought, well, that can’t be right. And ostensibly, that would be particularly dangerous for somebody with immunosuppression for whom the vaccine is not adequately protective. So, I called my friend, Doron Dorfman, and asked him to help me think through the legal part of this. We fleshed it out and wrote the article that same night.
How novel is it to view accommodations for people who are immunosuppressed through the lens of disability accommodation?
I think there has not been enough focus during the pandemic on individuals with disabilities or on how disability law can be mobilized during this pandemic to help supplement the public health law. This framework should be used a lot more because it’s good for everybody, not just for individuals with disabilities.
For example, take what’s called the “curb effect.” If you expand sidewalks, yes, it helps individuals who use a wheelchair. But it also helps me as a mom with a stroller. It helps somebody with a shopping cart, or a kid with a bike. If we adopt policies that are inclusive to those who are disadvantaged, it’s good for everybody. We should always strive to be an inclusive society, not just because it’s the right thing to do but because it really makes our society better.
How can mask requirements be used as a form of disability accommodation, as you argue in the JAMA article?
The ADA requires employers to provide reasonable accommodations for disability. In this case, the disability is your immunosuppressive status. We have an abundance of evidence showing individuals who are immunocompromised and vaccinated are still inadequately protected from the SARS-CoV-2 virus. So, there is absolute data to show individuals with immunosuppression have a disability that requires accommodation.
The ADA has a mandate requiring employers to adjust or modify policies in order to accommodate a disability. There are certain situations in which you cannot or do not need to accommodate a disability, when it would fundamentally alter the kind of employment you offer or if it’s an undue burden or hardship. But given that we’ve been wearing masks and working remotely for a year now, arguing that somehow these accommodations are no longer possible seems disingenuous.
In that way, allowing a person who’s immunocompromised to require those around them to mask is a form of modified protective policies. And in this case, those policies line up with a public health good, masking in the face of the highly contagious Delta variant ravaging our country right now.
In your view, can this argument be used in the mask debates happening right now across the country?
This argument can and should be useful for a couple of different lawsuits that are now underway in different states. I hope our article will provide further support for those suits. And I hope in school board hearings, when parents and teachers are talking about their concerns, this could be one way to argue for why we should allow mask mandates in classes. I’ve received emails from parents who said they’re going to bring this article to their school board hearing.
I also hope this could shift the narrative around the pandemic. Instead of focusing on individual responsibility – I got my vaccine shot so I’m fine – let’s focus on how we create an inclusive environment where we protect everybody, including those who cannot be vaccinated because of age or disability, or those who are vaccinated but inadequately protected because of their underlying conditions.
In the JAMA article, you talk about how our pandemic response has focused on individual health and how that individual focus can be ableist. Can you explain that point?
I think this idea that we just make our choices – like whether to get vaccinated or wear a mask, or not – and live with it really perpetuates a highly individualistic and ableist mindset. It doesn’t consider the people I admit to the hospital who are vaccinated but have a heart transplant and didn’t mount the sufficient immune response. Or even the people who chose not to be vaccinated because they were exposed to hours and hours of misinformation on TV.
We like to individualize everything, focusing on personal responsibility and choices, but a pandemic is one of those moments where everybody’s choices affect everybody else. Laying responsibility at the doorstep of each person, rather than thinking about what steps we as a society could be taking, is cheap and politically expedient. There is no public health rationale behind the bans on mask requirements in states like Texas, Iowa, and Florida. These choices are about politics. And the price is always borne by the most disadvantaged among us.
A version of this article first appeared on Medscape.com.
As governors and legislatures in states such as Texas, Florida, South Carolina, and Arkansas have banned schools and other entities from implementing mask mandates, disability rights advocates have pushed back. In federal civil rights lawsuits, they argue that bans on mask mandates violate antidiscrimination laws protecting people with disabilities.
For unvaccinated and immunosuppressed individuals, masks can provide crucial protection from SARS-CoV-2.
argues Mical Raz, MD, PhD, a professor at the University of Rochester (N.Y.) and a physician at Strong Memorial Hospital, also in Rochester, New York, in an article published in JAMA with coauthor Doron Dorfman, LLB, JSD.
This news organization talked with Dr. Raz about approaching mask requirements as disability accommodation during the COVID-19 pandemic. The following interview was lightly edited for length and clarity.
How did you come to think about mask requirements as a form of disability accommodation?
I saw a tweet from a professor at a university who said they couldn’t ask students about their vaccination status or to wear a mask. All agency was removed from the professor to take care of and protect themselves. I thought, well, that can’t be right. And ostensibly, that would be particularly dangerous for somebody with immunosuppression for whom the vaccine is not adequately protective. So, I called my friend, Doron Dorfman, and asked him to help me think through the legal part of this. We fleshed it out and wrote the article that same night.
How novel is it to view accommodations for people who are immunosuppressed through the lens of disability accommodation?
I think there has not been enough focus during the pandemic on individuals with disabilities or on how disability law can be mobilized during this pandemic to help supplement the public health law. This framework should be used a lot more because it’s good for everybody, not just for individuals with disabilities.
For example, take what’s called the “curb effect.” If you expand sidewalks, yes, it helps individuals who use a wheelchair. But it also helps me as a mom with a stroller. It helps somebody with a shopping cart, or a kid with a bike. If we adopt policies that are inclusive to those who are disadvantaged, it’s good for everybody. We should always strive to be an inclusive society, not just because it’s the right thing to do but because it really makes our society better.
How can mask requirements be used as a form of disability accommodation, as you argue in the JAMA article?
The ADA requires employers to provide reasonable accommodations for disability. In this case, the disability is your immunosuppressive status. We have an abundance of evidence showing individuals who are immunocompromised and vaccinated are still inadequately protected from the SARS-CoV-2 virus. So, there is absolute data to show individuals with immunosuppression have a disability that requires accommodation.
The ADA has a mandate requiring employers to adjust or modify policies in order to accommodate a disability. There are certain situations in which you cannot or do not need to accommodate a disability, when it would fundamentally alter the kind of employment you offer or if it’s an undue burden or hardship. But given that we’ve been wearing masks and working remotely for a year now, arguing that somehow these accommodations are no longer possible seems disingenuous.
In that way, allowing a person who’s immunocompromised to require those around them to mask is a form of modified protective policies. And in this case, those policies line up with a public health good, masking in the face of the highly contagious Delta variant ravaging our country right now.
In your view, can this argument be used in the mask debates happening right now across the country?
This argument can and should be useful for a couple of different lawsuits that are now underway in different states. I hope our article will provide further support for those suits. And I hope in school board hearings, when parents and teachers are talking about their concerns, this could be one way to argue for why we should allow mask mandates in classes. I’ve received emails from parents who said they’re going to bring this article to their school board hearing.
I also hope this could shift the narrative around the pandemic. Instead of focusing on individual responsibility – I got my vaccine shot so I’m fine – let’s focus on how we create an inclusive environment where we protect everybody, including those who cannot be vaccinated because of age or disability, or those who are vaccinated but inadequately protected because of their underlying conditions.
In the JAMA article, you talk about how our pandemic response has focused on individual health and how that individual focus can be ableist. Can you explain that point?
I think this idea that we just make our choices – like whether to get vaccinated or wear a mask, or not – and live with it really perpetuates a highly individualistic and ableist mindset. It doesn’t consider the people I admit to the hospital who are vaccinated but have a heart transplant and didn’t mount the sufficient immune response. Or even the people who chose not to be vaccinated because they were exposed to hours and hours of misinformation on TV.
We like to individualize everything, focusing on personal responsibility and choices, but a pandemic is one of those moments where everybody’s choices affect everybody else. Laying responsibility at the doorstep of each person, rather than thinking about what steps we as a society could be taking, is cheap and politically expedient. There is no public health rationale behind the bans on mask requirements in states like Texas, Iowa, and Florida. These choices are about politics. And the price is always borne by the most disadvantaged among us.
A version of this article first appeared on Medscape.com.
EAACI review urges reduction in antibiotic overuse with allergy
Urgent recommendations from a European Academy of Allergy and Clinical Immunology (EAACI) task force are aimed at reducing antibiotic overuse with allergic disease.
Top recommendations include limiting antibiotic therapy in pregnancy and early childhood to help reduce the allergy epidemic in children, and restricting antibiotic therapy in exacerbations and chronic treatment of allergic diseases, especially asthma and atopic dermatitis.
The review, by lead author Gerdien Tramper-Stranders, MD, PhD, department of pediatrics, Franciscus Gasthuis & Vlietland Hospital, Rotterdam, the Netherlands, and colleagues, was published online Aug. 13 in the journal Allergy.
Several studies have shown that use of antibiotics in childhood and during pregnancy is associated with disturbing the intestinal and respiratory microbiome, which in turn leads to dysbiosis and an increased risk of acquiring allergic diseases, the authors noted.
In addition, patients with allergic diseases such as asthma have a higher risk of being prescribed antibiotics for infections compared with the general population, despite lack of clear clinical benefit.
“In fact, there are no clear data supporting antibiotic prescriptions for acute exacerbations; and clinical and/or laboratory criteria are lacking,” the authors wrote.
Despite that lack of data, antibiotics are often prescribed for exacerbations along with oral corticosteroids, Dr. Tramper-Stranders said in an interview. Some patients may benefit from antibiotics in a flare-up, she said, but more research is needed to determine which ones.
Dr. Tramper-Stranders said Franciscus has begun a large study that includes patients with asthma exacerbations to find biomarkers that might predict the type or origin of exacerbation to personalize treatment.
Recommendations have global relevance
She said although the recommendations are coming from the EAACI group, they apply worldwide.
“Especially in countries outside Northern Europe, antibiotic use is tremendous, leading to high rates of antibiotic resistance; but also increasing the risk for developing allergic diseases when prescribed in infancy,” she said.
She pointed out that in the United States, as many as one in six children receive unnecessary antibiotics for an asthma exacerbation. Overtreatment in adults with flare-ups is also prevalent, at rates from 40%-50%.
Millie Kwan, MD, PhD, an allergy specialist at University of North Carolina in Chapel Hill, said in an interview that in the U.S. there’s been a culture change in the direction of antibiotic restraint – but there are still problems.
“It’s a lot easier for us to whip out our prescription pads and prescribe antibiotics for an asthma patient who’s having a flare-up or a patient who has atopic dermatitis before addressing the underlying mechanism directly,” Dr. Kwan said. She agreed that antibiotic overuse is prevalent in pregnancies in the U.S., and she said that starts with the high prevalence of cesarean births. Nearly one-third of all births in the U.S. are by C-section, twice the rate recommended by the World Health Organization.
“Just bypassing the birth canal actually changes what kind of microflora the infant is being exposed to,” Dr. Kwan said. “That’s the first huge problem.”
The second problem, she said, is the potential for overuse of antibiotics with the surgical procedure.
The researchers wrote that pre-, pro- or postbiotics might alter the course of allergic disease, but clear evidence is lacking.
Until now, Dr. Tramper-Stranders said, pre- or probiotic treatment in infancy, irrespective of previous antibiotic use, has not proved effective in preventing allergies.
Data describing the effect of pre- or probiotics after an antibiotic course are scarce, are limited to older children and adults, and are focused on short-term effects, such as diarrhea prevention, she explained.
Dr. Kwan says she agrees that current data are not strong enough to recommend one over another.
“We don’t even know what the normal amount of bacteria should be to constitute an environment where the immune system develops ‘normally,’ “ she said.
Antibiotics should be prescribed cautiously and by following current recommendations to use the narrowest spectrum available, the authors wrote. Future research in antibiotic stewardship should incorporate biomarker-guided therapy to determine which patients might benefit most from antibiotic therapy.
“Practicing antibiotic stewardship needs recurrent attention and we hope that with this initiative, we specifically reach allergy doctors who will rethink their next [antibiotic] prescription. Within our EAACI task force, we will next work on a guideline for rational antibiotic use in asthma,” Dr. Tramper-Stranders said.
The review’s authors and Dr. Kwan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Urgent recommendations from a European Academy of Allergy and Clinical Immunology (EAACI) task force are aimed at reducing antibiotic overuse with allergic disease.
Top recommendations include limiting antibiotic therapy in pregnancy and early childhood to help reduce the allergy epidemic in children, and restricting antibiotic therapy in exacerbations and chronic treatment of allergic diseases, especially asthma and atopic dermatitis.
The review, by lead author Gerdien Tramper-Stranders, MD, PhD, department of pediatrics, Franciscus Gasthuis & Vlietland Hospital, Rotterdam, the Netherlands, and colleagues, was published online Aug. 13 in the journal Allergy.
Several studies have shown that use of antibiotics in childhood and during pregnancy is associated with disturbing the intestinal and respiratory microbiome, which in turn leads to dysbiosis and an increased risk of acquiring allergic diseases, the authors noted.
In addition, patients with allergic diseases such as asthma have a higher risk of being prescribed antibiotics for infections compared with the general population, despite lack of clear clinical benefit.
“In fact, there are no clear data supporting antibiotic prescriptions for acute exacerbations; and clinical and/or laboratory criteria are lacking,” the authors wrote.
Despite that lack of data, antibiotics are often prescribed for exacerbations along with oral corticosteroids, Dr. Tramper-Stranders said in an interview. Some patients may benefit from antibiotics in a flare-up, she said, but more research is needed to determine which ones.
Dr. Tramper-Stranders said Franciscus has begun a large study that includes patients with asthma exacerbations to find biomarkers that might predict the type or origin of exacerbation to personalize treatment.
Recommendations have global relevance
She said although the recommendations are coming from the EAACI group, they apply worldwide.
“Especially in countries outside Northern Europe, antibiotic use is tremendous, leading to high rates of antibiotic resistance; but also increasing the risk for developing allergic diseases when prescribed in infancy,” she said.
She pointed out that in the United States, as many as one in six children receive unnecessary antibiotics for an asthma exacerbation. Overtreatment in adults with flare-ups is also prevalent, at rates from 40%-50%.
Millie Kwan, MD, PhD, an allergy specialist at University of North Carolina in Chapel Hill, said in an interview that in the U.S. there’s been a culture change in the direction of antibiotic restraint – but there are still problems.
“It’s a lot easier for us to whip out our prescription pads and prescribe antibiotics for an asthma patient who’s having a flare-up or a patient who has atopic dermatitis before addressing the underlying mechanism directly,” Dr. Kwan said. She agreed that antibiotic overuse is prevalent in pregnancies in the U.S., and she said that starts with the high prevalence of cesarean births. Nearly one-third of all births in the U.S. are by C-section, twice the rate recommended by the World Health Organization.
“Just bypassing the birth canal actually changes what kind of microflora the infant is being exposed to,” Dr. Kwan said. “That’s the first huge problem.”
The second problem, she said, is the potential for overuse of antibiotics with the surgical procedure.
The researchers wrote that pre-, pro- or postbiotics might alter the course of allergic disease, but clear evidence is lacking.
Until now, Dr. Tramper-Stranders said, pre- or probiotic treatment in infancy, irrespective of previous antibiotic use, has not proved effective in preventing allergies.
Data describing the effect of pre- or probiotics after an antibiotic course are scarce, are limited to older children and adults, and are focused on short-term effects, such as diarrhea prevention, she explained.
Dr. Kwan says she agrees that current data are not strong enough to recommend one over another.
“We don’t even know what the normal amount of bacteria should be to constitute an environment where the immune system develops ‘normally,’ “ she said.
Antibiotics should be prescribed cautiously and by following current recommendations to use the narrowest spectrum available, the authors wrote. Future research in antibiotic stewardship should incorporate biomarker-guided therapy to determine which patients might benefit most from antibiotic therapy.
“Practicing antibiotic stewardship needs recurrent attention and we hope that with this initiative, we specifically reach allergy doctors who will rethink their next [antibiotic] prescription. Within our EAACI task force, we will next work on a guideline for rational antibiotic use in asthma,” Dr. Tramper-Stranders said.
The review’s authors and Dr. Kwan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Urgent recommendations from a European Academy of Allergy and Clinical Immunology (EAACI) task force are aimed at reducing antibiotic overuse with allergic disease.
Top recommendations include limiting antibiotic therapy in pregnancy and early childhood to help reduce the allergy epidemic in children, and restricting antibiotic therapy in exacerbations and chronic treatment of allergic diseases, especially asthma and atopic dermatitis.
The review, by lead author Gerdien Tramper-Stranders, MD, PhD, department of pediatrics, Franciscus Gasthuis & Vlietland Hospital, Rotterdam, the Netherlands, and colleagues, was published online Aug. 13 in the journal Allergy.
Several studies have shown that use of antibiotics in childhood and during pregnancy is associated with disturbing the intestinal and respiratory microbiome, which in turn leads to dysbiosis and an increased risk of acquiring allergic diseases, the authors noted.
In addition, patients with allergic diseases such as asthma have a higher risk of being prescribed antibiotics for infections compared with the general population, despite lack of clear clinical benefit.
“In fact, there are no clear data supporting antibiotic prescriptions for acute exacerbations; and clinical and/or laboratory criteria are lacking,” the authors wrote.
Despite that lack of data, antibiotics are often prescribed for exacerbations along with oral corticosteroids, Dr. Tramper-Stranders said in an interview. Some patients may benefit from antibiotics in a flare-up, she said, but more research is needed to determine which ones.
Dr. Tramper-Stranders said Franciscus has begun a large study that includes patients with asthma exacerbations to find biomarkers that might predict the type or origin of exacerbation to personalize treatment.
Recommendations have global relevance
She said although the recommendations are coming from the EAACI group, they apply worldwide.
“Especially in countries outside Northern Europe, antibiotic use is tremendous, leading to high rates of antibiotic resistance; but also increasing the risk for developing allergic diseases when prescribed in infancy,” she said.
She pointed out that in the United States, as many as one in six children receive unnecessary antibiotics for an asthma exacerbation. Overtreatment in adults with flare-ups is also prevalent, at rates from 40%-50%.
Millie Kwan, MD, PhD, an allergy specialist at University of North Carolina in Chapel Hill, said in an interview that in the U.S. there’s been a culture change in the direction of antibiotic restraint – but there are still problems.
“It’s a lot easier for us to whip out our prescription pads and prescribe antibiotics for an asthma patient who’s having a flare-up or a patient who has atopic dermatitis before addressing the underlying mechanism directly,” Dr. Kwan said. She agreed that antibiotic overuse is prevalent in pregnancies in the U.S., and she said that starts with the high prevalence of cesarean births. Nearly one-third of all births in the U.S. are by C-section, twice the rate recommended by the World Health Organization.
“Just bypassing the birth canal actually changes what kind of microflora the infant is being exposed to,” Dr. Kwan said. “That’s the first huge problem.”
The second problem, she said, is the potential for overuse of antibiotics with the surgical procedure.
The researchers wrote that pre-, pro- or postbiotics might alter the course of allergic disease, but clear evidence is lacking.
Until now, Dr. Tramper-Stranders said, pre- or probiotic treatment in infancy, irrespective of previous antibiotic use, has not proved effective in preventing allergies.
Data describing the effect of pre- or probiotics after an antibiotic course are scarce, are limited to older children and adults, and are focused on short-term effects, such as diarrhea prevention, she explained.
Dr. Kwan says she agrees that current data are not strong enough to recommend one over another.
“We don’t even know what the normal amount of bacteria should be to constitute an environment where the immune system develops ‘normally,’ “ she said.
Antibiotics should be prescribed cautiously and by following current recommendations to use the narrowest spectrum available, the authors wrote. Future research in antibiotic stewardship should incorporate biomarker-guided therapy to determine which patients might benefit most from antibiotic therapy.
“Practicing antibiotic stewardship needs recurrent attention and we hope that with this initiative, we specifically reach allergy doctors who will rethink their next [antibiotic] prescription. Within our EAACI task force, we will next work on a guideline for rational antibiotic use in asthma,” Dr. Tramper-Stranders said.
The review’s authors and Dr. Kwan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One-third in U.S. had been infected by SARS-CoV-2 through 2020: Study
, according to a modeling study published Aug. 26 online in Nature.
Jeffrey Shaman, PhD, professor in the department of environmental health sciences and director of the climate and health program at the Columbia University Mailman School of Public Health, New York City, and colleagues developed a model to simulate how SARS-CoV-2 was transmitted within and between all 3,142 counties in the United States.
In their model, the researchers considered migration data between counties, the observed case numbers, and estimates of infections based on the number of people who test positive for SARS-CoV-2 antibodies.
The United States had the highest number of confirmed COVID-19 cases and deaths in the world during 2020. More than 19.6 million cases were reported by the end of the year.
But the authors point out that “69% of the population remained susceptible to viral infection.”
The researchers also studied the ascertainment rate, or the ratio of detected cases to the number of confirmed cases. Nationally, that value increased from 11.3% in March 2020 to 24.5% in December 2020.
That’s one of the biggest pandemic lessons from the data, Dr. Shaman said: “It is vitally important when there is an outbreak and you’re counting cases that there are many more people infected in your community who are contagious than reported cases. Each individual is infectious for multiple days, and there are many more unreported cases.”
That applies now with the Delta variant, he said.
“Vaccinated people who get infected with the Delta variant are part of the transmission chain,” he said.
Fatality rates dropped
Some of the data were very positive, Dr. Shaman told this news organization. The infection fatality rate fell from 0.77% in April to 0.31% in December. The authors suggest that that may be because of improvements in diagnosis and treatment, patient care, and reduced disease severity.
However, the fatality rate was still nearly four times as high as the estimated fatality rate for seasonal influenza (0.08%) and the 2009 influenza pandemic (0.0076%), the authors point out.
Joe K. Gerald, MD, PhD, associate professor and program director of public health policy and management at the University of Arizona, Tucson, told this news organization that this article helps confirm that COVID is much deadlier than the flu and that the intensity of the response has been appropriate.
“We should be willing to invest a lot more in mitigating COVID-19 than seasonal influenza because it has much greater consequences,” he said.
The numbers emphasize that testing must improve.
“We didn’t have enough tests available, and they weren’t easily accessible. For much of the year we were flying in the dark,” Dr. Gerald said.
The number of tests has increased this year, he acknowledged, but testing still lags. “We just can’t miss this many infections or diagnoses and hope to gain control,” he said.
The study also points out the huge variation by state and by county in infections and deaths, and that variation continues. Gerald noted that the varied numbers make it difficult for some regions to accept broader mandates, because the threat from COVID-19 appears very different where they are.
“We have to think about regions, how many people are susceptible, and what the testing capacity is,” he said. “States and even counties should have some leeway to make some important public health decisions, because local conditions are going to differ at different points in time.”
‘We have not turned the corner’
Jill Foster, MD, a pediatric infectious disease physician at the University of Minnesota Medical School, Minneapolis, said in an interview that the study adds evidence: “We have not turned the corner on COVID-19 and are nowhere near herd immunity – if it exists for SARS-CoV-2.”
She said the numbers presented are particularly concerning in regard to how many people were susceptible and were actively able to infect others: “Much higher than most people imagined and very much higher than their comparison, influenza.
“There are still more people susceptible than we had believed,” Dr. Foster added. “If the pattern continues where the Delta variant infects a significant portion of those vaccinated, the number of people susceptible rises even higher than was predicted.”
She said that it is reassuring that the analysis shows a decrease in case fatality and said the finding supports the common opinion that medicine is better able to fight the disease.
“However,” she said, “the optimism is tempered by acknowledging that in order to benefit from these advances, we must not overwhelm the facilities where patients are cared for so that optimal care can be delivered.”
Dr. Foster said these numbers represent a warning that COVID should be treated as a continuing threat.
“We need to acknowledge that there is COVID-19 infection simmering and periodically erupting throughout the country,” she said. “It is not monolithic and varies by geography and seasons in ways that are difficult to predict other than at any given time there is likely more infection present than we are identifying and more people susceptible to infection than we have calculated.”
The authors and Dr. Gerald have disclosed no relevant financial relationships. Dr. Foster has received clinical trials funding from Moderna.
A version of this article first appeared on Medscape.com.
, according to a modeling study published Aug. 26 online in Nature.
Jeffrey Shaman, PhD, professor in the department of environmental health sciences and director of the climate and health program at the Columbia University Mailman School of Public Health, New York City, and colleagues developed a model to simulate how SARS-CoV-2 was transmitted within and between all 3,142 counties in the United States.
In their model, the researchers considered migration data between counties, the observed case numbers, and estimates of infections based on the number of people who test positive for SARS-CoV-2 antibodies.
The United States had the highest number of confirmed COVID-19 cases and deaths in the world during 2020. More than 19.6 million cases were reported by the end of the year.
But the authors point out that “69% of the population remained susceptible to viral infection.”
The researchers also studied the ascertainment rate, or the ratio of detected cases to the number of confirmed cases. Nationally, that value increased from 11.3% in March 2020 to 24.5% in December 2020.
That’s one of the biggest pandemic lessons from the data, Dr. Shaman said: “It is vitally important when there is an outbreak and you’re counting cases that there are many more people infected in your community who are contagious than reported cases. Each individual is infectious for multiple days, and there are many more unreported cases.”
That applies now with the Delta variant, he said.
“Vaccinated people who get infected with the Delta variant are part of the transmission chain,” he said.
Fatality rates dropped
Some of the data were very positive, Dr. Shaman told this news organization. The infection fatality rate fell from 0.77% in April to 0.31% in December. The authors suggest that that may be because of improvements in diagnosis and treatment, patient care, and reduced disease severity.
However, the fatality rate was still nearly four times as high as the estimated fatality rate for seasonal influenza (0.08%) and the 2009 influenza pandemic (0.0076%), the authors point out.
Joe K. Gerald, MD, PhD, associate professor and program director of public health policy and management at the University of Arizona, Tucson, told this news organization that this article helps confirm that COVID is much deadlier than the flu and that the intensity of the response has been appropriate.
“We should be willing to invest a lot more in mitigating COVID-19 than seasonal influenza because it has much greater consequences,” he said.
The numbers emphasize that testing must improve.
“We didn’t have enough tests available, and they weren’t easily accessible. For much of the year we were flying in the dark,” Dr. Gerald said.
The number of tests has increased this year, he acknowledged, but testing still lags. “We just can’t miss this many infections or diagnoses and hope to gain control,” he said.
The study also points out the huge variation by state and by county in infections and deaths, and that variation continues. Gerald noted that the varied numbers make it difficult for some regions to accept broader mandates, because the threat from COVID-19 appears very different where they are.
“We have to think about regions, how many people are susceptible, and what the testing capacity is,” he said. “States and even counties should have some leeway to make some important public health decisions, because local conditions are going to differ at different points in time.”
‘We have not turned the corner’
Jill Foster, MD, a pediatric infectious disease physician at the University of Minnesota Medical School, Minneapolis, said in an interview that the study adds evidence: “We have not turned the corner on COVID-19 and are nowhere near herd immunity – if it exists for SARS-CoV-2.”
She said the numbers presented are particularly concerning in regard to how many people were susceptible and were actively able to infect others: “Much higher than most people imagined and very much higher than their comparison, influenza.
“There are still more people susceptible than we had believed,” Dr. Foster added. “If the pattern continues where the Delta variant infects a significant portion of those vaccinated, the number of people susceptible rises even higher than was predicted.”
She said that it is reassuring that the analysis shows a decrease in case fatality and said the finding supports the common opinion that medicine is better able to fight the disease.
“However,” she said, “the optimism is tempered by acknowledging that in order to benefit from these advances, we must not overwhelm the facilities where patients are cared for so that optimal care can be delivered.”
Dr. Foster said these numbers represent a warning that COVID should be treated as a continuing threat.
“We need to acknowledge that there is COVID-19 infection simmering and periodically erupting throughout the country,” she said. “It is not monolithic and varies by geography and seasons in ways that are difficult to predict other than at any given time there is likely more infection present than we are identifying and more people susceptible to infection than we have calculated.”
The authors and Dr. Gerald have disclosed no relevant financial relationships. Dr. Foster has received clinical trials funding from Moderna.
A version of this article first appeared on Medscape.com.
, according to a modeling study published Aug. 26 online in Nature.
Jeffrey Shaman, PhD, professor in the department of environmental health sciences and director of the climate and health program at the Columbia University Mailman School of Public Health, New York City, and colleagues developed a model to simulate how SARS-CoV-2 was transmitted within and between all 3,142 counties in the United States.
In their model, the researchers considered migration data between counties, the observed case numbers, and estimates of infections based on the number of people who test positive for SARS-CoV-2 antibodies.
The United States had the highest number of confirmed COVID-19 cases and deaths in the world during 2020. More than 19.6 million cases were reported by the end of the year.
But the authors point out that “69% of the population remained susceptible to viral infection.”
The researchers also studied the ascertainment rate, or the ratio of detected cases to the number of confirmed cases. Nationally, that value increased from 11.3% in March 2020 to 24.5% in December 2020.
That’s one of the biggest pandemic lessons from the data, Dr. Shaman said: “It is vitally important when there is an outbreak and you’re counting cases that there are many more people infected in your community who are contagious than reported cases. Each individual is infectious for multiple days, and there are many more unreported cases.”
That applies now with the Delta variant, he said.
“Vaccinated people who get infected with the Delta variant are part of the transmission chain,” he said.
Fatality rates dropped
Some of the data were very positive, Dr. Shaman told this news organization. The infection fatality rate fell from 0.77% in April to 0.31% in December. The authors suggest that that may be because of improvements in diagnosis and treatment, patient care, and reduced disease severity.
However, the fatality rate was still nearly four times as high as the estimated fatality rate for seasonal influenza (0.08%) and the 2009 influenza pandemic (0.0076%), the authors point out.
Joe K. Gerald, MD, PhD, associate professor and program director of public health policy and management at the University of Arizona, Tucson, told this news organization that this article helps confirm that COVID is much deadlier than the flu and that the intensity of the response has been appropriate.
“We should be willing to invest a lot more in mitigating COVID-19 than seasonal influenza because it has much greater consequences,” he said.
The numbers emphasize that testing must improve.
“We didn’t have enough tests available, and they weren’t easily accessible. For much of the year we were flying in the dark,” Dr. Gerald said.
The number of tests has increased this year, he acknowledged, but testing still lags. “We just can’t miss this many infections or diagnoses and hope to gain control,” he said.
The study also points out the huge variation by state and by county in infections and deaths, and that variation continues. Gerald noted that the varied numbers make it difficult for some regions to accept broader mandates, because the threat from COVID-19 appears very different where they are.
“We have to think about regions, how many people are susceptible, and what the testing capacity is,” he said. “States and even counties should have some leeway to make some important public health decisions, because local conditions are going to differ at different points in time.”
‘We have not turned the corner’
Jill Foster, MD, a pediatric infectious disease physician at the University of Minnesota Medical School, Minneapolis, said in an interview that the study adds evidence: “We have not turned the corner on COVID-19 and are nowhere near herd immunity – if it exists for SARS-CoV-2.”
She said the numbers presented are particularly concerning in regard to how many people were susceptible and were actively able to infect others: “Much higher than most people imagined and very much higher than their comparison, influenza.
“There are still more people susceptible than we had believed,” Dr. Foster added. “If the pattern continues where the Delta variant infects a significant portion of those vaccinated, the number of people susceptible rises even higher than was predicted.”
She said that it is reassuring that the analysis shows a decrease in case fatality and said the finding supports the common opinion that medicine is better able to fight the disease.
“However,” she said, “the optimism is tempered by acknowledging that in order to benefit from these advances, we must not overwhelm the facilities where patients are cared for so that optimal care can be delivered.”
Dr. Foster said these numbers represent a warning that COVID should be treated as a continuing threat.
“We need to acknowledge that there is COVID-19 infection simmering and periodically erupting throughout the country,” she said. “It is not monolithic and varies by geography and seasons in ways that are difficult to predict other than at any given time there is likely more infection present than we are identifying and more people susceptible to infection than we have calculated.”
The authors and Dr. Gerald have disclosed no relevant financial relationships. Dr. Foster has received clinical trials funding from Moderna.
A version of this article first appeared on Medscape.com.
AHA targets rising prevalence of obstructive sleep apnea in children
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Children and COVID: New cases soar to near-record level
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.
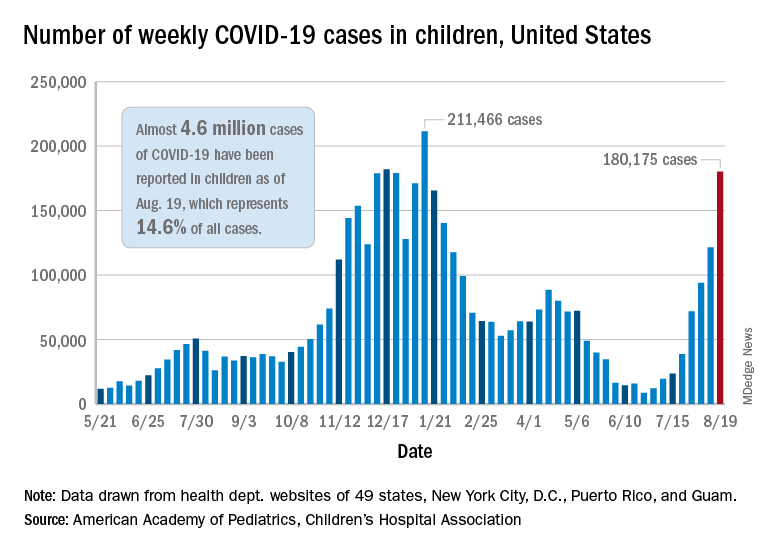
The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.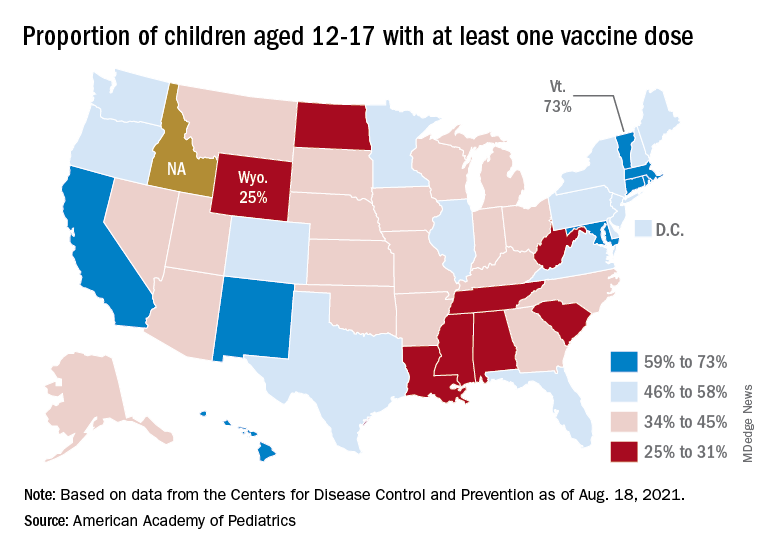
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Health care workers eager for COVID booster shots
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.









