User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Post–COVID-19 cardiac involvement in college athletes much rarer than thought
In a multicenter study conducted during September-December 2020, only 0.7% of 3,018 collegiate athletes who tested positive for SARS-CoV-2 infection were found to have definite, probable, or possible infection-related cardiac involvement.
None experienced an adverse cardiac event and only five (0.2%) required hospitalization for noncardiac complications of COVID-19.
“The take-home message is that cardiac involvement does not happen as much as we had initially feared. It’s in the range of 0.5% to 3%, depending on how you define cardiac involvement, which is not nothing, but it’s not the 30% or 50% that some early studies hinted at,” said Kimberly G. Harmon, MD, of the University of Washington, Seattle.
Dr. Harmon, along with Jeffrey A. Drezner, MD, also from UW, and Aaron L. Baggish, MD, of Massachusetts General Hospital, Boston, were co–primary investigators of the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) study. The group’s findings were published April 17 in Circulation.
Nearly 20,000 athletes tested
The researchers prospectively tested 19,378 athletes for SARS-CoV-2 infection from 42 U.S. colleges and universities during the study period. A total of 3,018 (16%; mean age, 20 years; 32% female) tested positive and underwent cardiac evaluation.
“We didn’t prescribe what the schools had to do in terms of cardiac evaluation, but most of these colleges are well resourced, and about 74% of athletes were evaluated using the triad testing strategy of 12-lead electrocardiography, cardiac troponin, and transthoracic echocardiography [TEE], with cardiac magnetic resonance [CMR ]when indicated,” explained Dr. Harmon. Only 198 athletes underwent primary screening with CMR.
Athletes were often tested multiple times for SARS-CoV-2 infection by participating institutions and were included in this study if they had any positive test and underwent postinfection cardiac screening.
The cohort includes athletes representing 26 distinct sporting disciplines, including American-style football (36%), basketball (9%), and cross country/track and field (8%). Most were asymptomatic or had only mild COVID-19 symptoms (33% and 29%, respectively).
‘Exercise appears to be protective’
Abnormal findings suggestive of SARS-CoV-2 cardiac involvement were detected by ECG in 0.7% of athletes (21 of 2,999), cardiac troponin elevation in 0.9% (24/2,719), and abnormal TTE findings in 0.9% (24/2,556).
The odds of having cardiac involvement was 3.1 times higher in athletes with cardiopulmonary symptoms.
“One thing we’ve seen in the literature and in this cohort, is that exercise appears to be protective to some extent from COVID-19. We had a lot of cases, but in the whole cohort, only five athletes were hospitalized with COVID and those were for noncardiac reasons,” said Dr. Harmon.
During a median clinical surveillance of 113 days, there was one (0.03%) adverse cardiac event likely unrelated to SARS-CoV-2 infection.
The diagnostic yield for probable or definite cardiac involvement was 6.7 times higher for a CMR obtained for clinical reasons (10.1%) versus a primary screening CMR (1.5%).
“This is data we desperately needed. Small, single-center studies early in the pandemic had indicated a higher prevalence of cardiac involvement, which led us to be very conservative about return-to-play in the early days,” said Jeffrey Lander, MD, who was not involved in the study.
The study is complementary, he noted, to one published in March that looked at professional athletes post–COVID-19 and also found cardiac pathology in fewer than 1%. The mean age in that study was 25 years.
“They saw a similarly low rate of cardiac involvement in professional athletes, and together with this study, it gives us new information that is also reassuring,” added Dr. Lander, codirector of sports cardiology at Saint Barnabas Medical Center in Livingston, N.J., an RWJBarnabas Health facility, and team cardiologist for Seton Hall University in South Orange, N.J.
Limit CMR to symptomatic athletes
“I think this data can be extended beyond the college athlete. And it’s fair to say to high school athletes and young recreational athletes who have had asymptomatic or mild infection, you probably don’t need further workup if you’re feeling fine,” suggested Dr. Harmon.
“For those with moderate or severe illness, then the triple screen protocol is a good idea, particularly if they are having any symptoms,” she added.
Dr. Lander agrees that athletes should be screened by appropriate providers before returning to sports, but that CMR should not be used routinely for return-to-play screening.
“We’ve never taken a group of, say, 1,000 college athletes who just recovered from the flu and done cardiac MRIs on them, so it’s a bit like opening Pandora’s box when it’s used too liberally. It’s difficult to assess if the findings are secondary to COVID infection or from something entirely unrelated,” he noted.
ORCCA is a collaboration of the American Heart Association and the American Medical Society for Sports Medicine to track COVID-19 cases among National Collegiate Athletic Association (NCAA) athletes. The current study was supported by a grant from the American Medical Society for Sports Medicine.
In a multicenter study conducted during September-December 2020, only 0.7% of 3,018 collegiate athletes who tested positive for SARS-CoV-2 infection were found to have definite, probable, or possible infection-related cardiac involvement.
None experienced an adverse cardiac event and only five (0.2%) required hospitalization for noncardiac complications of COVID-19.
“The take-home message is that cardiac involvement does not happen as much as we had initially feared. It’s in the range of 0.5% to 3%, depending on how you define cardiac involvement, which is not nothing, but it’s not the 30% or 50% that some early studies hinted at,” said Kimberly G. Harmon, MD, of the University of Washington, Seattle.
Dr. Harmon, along with Jeffrey A. Drezner, MD, also from UW, and Aaron L. Baggish, MD, of Massachusetts General Hospital, Boston, were co–primary investigators of the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) study. The group’s findings were published April 17 in Circulation.
Nearly 20,000 athletes tested
The researchers prospectively tested 19,378 athletes for SARS-CoV-2 infection from 42 U.S. colleges and universities during the study period. A total of 3,018 (16%; mean age, 20 years; 32% female) tested positive and underwent cardiac evaluation.
“We didn’t prescribe what the schools had to do in terms of cardiac evaluation, but most of these colleges are well resourced, and about 74% of athletes were evaluated using the triad testing strategy of 12-lead electrocardiography, cardiac troponin, and transthoracic echocardiography [TEE], with cardiac magnetic resonance [CMR ]when indicated,” explained Dr. Harmon. Only 198 athletes underwent primary screening with CMR.
Athletes were often tested multiple times for SARS-CoV-2 infection by participating institutions and were included in this study if they had any positive test and underwent postinfection cardiac screening.
The cohort includes athletes representing 26 distinct sporting disciplines, including American-style football (36%), basketball (9%), and cross country/track and field (8%). Most were asymptomatic or had only mild COVID-19 symptoms (33% and 29%, respectively).
‘Exercise appears to be protective’
Abnormal findings suggestive of SARS-CoV-2 cardiac involvement were detected by ECG in 0.7% of athletes (21 of 2,999), cardiac troponin elevation in 0.9% (24/2,719), and abnormal TTE findings in 0.9% (24/2,556).
The odds of having cardiac involvement was 3.1 times higher in athletes with cardiopulmonary symptoms.
“One thing we’ve seen in the literature and in this cohort, is that exercise appears to be protective to some extent from COVID-19. We had a lot of cases, but in the whole cohort, only five athletes were hospitalized with COVID and those were for noncardiac reasons,” said Dr. Harmon.
During a median clinical surveillance of 113 days, there was one (0.03%) adverse cardiac event likely unrelated to SARS-CoV-2 infection.
The diagnostic yield for probable or definite cardiac involvement was 6.7 times higher for a CMR obtained for clinical reasons (10.1%) versus a primary screening CMR (1.5%).
“This is data we desperately needed. Small, single-center studies early in the pandemic had indicated a higher prevalence of cardiac involvement, which led us to be very conservative about return-to-play in the early days,” said Jeffrey Lander, MD, who was not involved in the study.
The study is complementary, he noted, to one published in March that looked at professional athletes post–COVID-19 and also found cardiac pathology in fewer than 1%. The mean age in that study was 25 years.
“They saw a similarly low rate of cardiac involvement in professional athletes, and together with this study, it gives us new information that is also reassuring,” added Dr. Lander, codirector of sports cardiology at Saint Barnabas Medical Center in Livingston, N.J., an RWJBarnabas Health facility, and team cardiologist for Seton Hall University in South Orange, N.J.
Limit CMR to symptomatic athletes
“I think this data can be extended beyond the college athlete. And it’s fair to say to high school athletes and young recreational athletes who have had asymptomatic or mild infection, you probably don’t need further workup if you’re feeling fine,” suggested Dr. Harmon.
“For those with moderate or severe illness, then the triple screen protocol is a good idea, particularly if they are having any symptoms,” she added.
Dr. Lander agrees that athletes should be screened by appropriate providers before returning to sports, but that CMR should not be used routinely for return-to-play screening.
“We’ve never taken a group of, say, 1,000 college athletes who just recovered from the flu and done cardiac MRIs on them, so it’s a bit like opening Pandora’s box when it’s used too liberally. It’s difficult to assess if the findings are secondary to COVID infection or from something entirely unrelated,” he noted.
ORCCA is a collaboration of the American Heart Association and the American Medical Society for Sports Medicine to track COVID-19 cases among National Collegiate Athletic Association (NCAA) athletes. The current study was supported by a grant from the American Medical Society for Sports Medicine.
In a multicenter study conducted during September-December 2020, only 0.7% of 3,018 collegiate athletes who tested positive for SARS-CoV-2 infection were found to have definite, probable, or possible infection-related cardiac involvement.
None experienced an adverse cardiac event and only five (0.2%) required hospitalization for noncardiac complications of COVID-19.
“The take-home message is that cardiac involvement does not happen as much as we had initially feared. It’s in the range of 0.5% to 3%, depending on how you define cardiac involvement, which is not nothing, but it’s not the 30% or 50% that some early studies hinted at,” said Kimberly G. Harmon, MD, of the University of Washington, Seattle.
Dr. Harmon, along with Jeffrey A. Drezner, MD, also from UW, and Aaron L. Baggish, MD, of Massachusetts General Hospital, Boston, were co–primary investigators of the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) study. The group’s findings were published April 17 in Circulation.
Nearly 20,000 athletes tested
The researchers prospectively tested 19,378 athletes for SARS-CoV-2 infection from 42 U.S. colleges and universities during the study period. A total of 3,018 (16%; mean age, 20 years; 32% female) tested positive and underwent cardiac evaluation.
“We didn’t prescribe what the schools had to do in terms of cardiac evaluation, but most of these colleges are well resourced, and about 74% of athletes were evaluated using the triad testing strategy of 12-lead electrocardiography, cardiac troponin, and transthoracic echocardiography [TEE], with cardiac magnetic resonance [CMR ]when indicated,” explained Dr. Harmon. Only 198 athletes underwent primary screening with CMR.
Athletes were often tested multiple times for SARS-CoV-2 infection by participating institutions and were included in this study if they had any positive test and underwent postinfection cardiac screening.
The cohort includes athletes representing 26 distinct sporting disciplines, including American-style football (36%), basketball (9%), and cross country/track and field (8%). Most were asymptomatic or had only mild COVID-19 symptoms (33% and 29%, respectively).
‘Exercise appears to be protective’
Abnormal findings suggestive of SARS-CoV-2 cardiac involvement were detected by ECG in 0.7% of athletes (21 of 2,999), cardiac troponin elevation in 0.9% (24/2,719), and abnormal TTE findings in 0.9% (24/2,556).
The odds of having cardiac involvement was 3.1 times higher in athletes with cardiopulmonary symptoms.
“One thing we’ve seen in the literature and in this cohort, is that exercise appears to be protective to some extent from COVID-19. We had a lot of cases, but in the whole cohort, only five athletes were hospitalized with COVID and those were for noncardiac reasons,” said Dr. Harmon.
During a median clinical surveillance of 113 days, there was one (0.03%) adverse cardiac event likely unrelated to SARS-CoV-2 infection.
The diagnostic yield for probable or definite cardiac involvement was 6.7 times higher for a CMR obtained for clinical reasons (10.1%) versus a primary screening CMR (1.5%).
“This is data we desperately needed. Small, single-center studies early in the pandemic had indicated a higher prevalence of cardiac involvement, which led us to be very conservative about return-to-play in the early days,” said Jeffrey Lander, MD, who was not involved in the study.
The study is complementary, he noted, to one published in March that looked at professional athletes post–COVID-19 and also found cardiac pathology in fewer than 1%. The mean age in that study was 25 years.
“They saw a similarly low rate of cardiac involvement in professional athletes, and together with this study, it gives us new information that is also reassuring,” added Dr. Lander, codirector of sports cardiology at Saint Barnabas Medical Center in Livingston, N.J., an RWJBarnabas Health facility, and team cardiologist for Seton Hall University in South Orange, N.J.
Limit CMR to symptomatic athletes
“I think this data can be extended beyond the college athlete. And it’s fair to say to high school athletes and young recreational athletes who have had asymptomatic or mild infection, you probably don’t need further workup if you’re feeling fine,” suggested Dr. Harmon.
“For those with moderate or severe illness, then the triple screen protocol is a good idea, particularly if they are having any symptoms,” she added.
Dr. Lander agrees that athletes should be screened by appropriate providers before returning to sports, but that CMR should not be used routinely for return-to-play screening.
“We’ve never taken a group of, say, 1,000 college athletes who just recovered from the flu and done cardiac MRIs on them, so it’s a bit like opening Pandora’s box when it’s used too liberally. It’s difficult to assess if the findings are secondary to COVID infection or from something entirely unrelated,” he noted.
ORCCA is a collaboration of the American Heart Association and the American Medical Society for Sports Medicine to track COVID-19 cases among National Collegiate Athletic Association (NCAA) athletes. The current study was supported by a grant from the American Medical Society for Sports Medicine.
FROM CIRCULATION
Can we get to ‘COVID zero’? Experts predict the next 8 months
COVID-19 is likely to follow a seasonal pattern – similar to some other respiratory viruses – with fewer cases come summer 2021 followed by a jump next winter, experts predicted in a Thursday briefing.
If that pattern holds, it could mean a need to reinforce the mask-wearing message as the weather gets colder and people once again congregate indoors.
“Right now, we are projecting the United States all the way to Aug. 1 [will have] 619,000 deaths from COVID-19, with 4.7 million globally,” said Ali H. Mokdad, PhD, professor of health metrics sciences at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle, during today’s media briefing sponsored by the Infectious Diseases Society of America and IHME.
The encouraging news is the vaccines appear to be working, and more Americans are getting them. “If you look at the data for these vaccines, they are extremely safe, they are extremely efficacious, and they make you basically impervious – for the most part – to getting serious disease, hospitalization, or death,” said Amesh Adalja, MD, senior scholar at Johns Hopkins University Center for Health Security in Baltimore.
“These vaccines do what they were meant to do: defang this virus,” said Dr. Adalja, who is an IDSA Fellow and adjunct assistant professor at Johns Hopkins Bloomberg School of Public Health. Emerging data out of Israel and other countries suggest a vaccinated person is less likely to transmit the virus as well, he added.
Still aiming for herd immunity
Furthermore, the U.S. Food and Drug Administration is likely to approve emergency use authorization (EUA) among teenagers 12-15 years old “imminently,” thereby expanding the pool of people potentially protected by vaccines.
Such authorization could help with overall public health efforts. “That’s simply a mathematical formula,” Dr. Adalja said. “The more people that are vaccinated, including children, the quicker we’ll get to herd immunity.”
In addition, with lower case numbers expected this summer, herd immunity might become more achievable, said Dr. Mokdad, who is also chief strategy officer for population health at the University of Washington.
As important as herd immunity is, so-called decoupling is “more important to me,” Dr. Adalja said. Decoupling refers to separating infections from the more severe outcomes, so people who get COVID-19 are less likely to need hospitalization or die from it.
Vaccines get the credit here, he added, including with the variants. “Even if you get a breakthrough infection with a variant, it’s not likely to land you in the hospital or cause serious disease or death,” Dr. Adalja said.
Masks and the uncommon cold
Wearing a mask until we reach herd immunity is important because it’s not possible to tell who is vaccinated and who isn’t, Dr. Mokdad said. “Remember, as many people are waiting to get a vaccine, all of us have access to a mask,” he said.
Dr. Adalja agreed, adding that public health guidance on masks will likely stay in place until we cross that herd immunity threshold and community circulation of the virus goes down.
“People are probably going to want to continue wearing masks, at least some proportion, because they see the benefit for other respiratory viruses,” Dr. Adalja said. “How many of you had a common cold this year?”
Variants: Some good news?
Experts are monitoring the spread of variants of concern in the United States and abroad. On a positive note, the B.1.1.7 variant first identified in the United Kingdom appears to be dominant in the United States at this time, which is potentially good for two reasons. One is that the available COVID-19 vaccines show sufficient efficacy against the strain, Dr. Mokdad said.
Second, a predominance of B.1.1.7 makes it more difficult for other emerging variants of concern like P1 [Brazil] or B.1.351 [South Africa] to gain control, Dr. Adalja said.
“B.1.1.7 is such an efficient transmitter,” he said. “That’s kind of an advantage … because the more B.1.1.7, you have the less opportunity B.1.351 and P1 have to set up shop.”
Hesitancy from misinformation
Vaccine hesitancy remains a concern, particularly at a time when some predict a drop in the number of Americans seeking vaccination. Although needle phobia plays a role in dissuading some from vaccination, the bigger issue is vaccine misinformation, Dr. Adalja said.
“Some people are just terrified when they see the needle. That’s a small part of the proportion of people who don’t want to get vaccinated,” Dr. Adalja said. In contrast, he attributed most hesitancy to misinformation about the vaccine, including reports that the vaccines are fake.
Even celebrities are getting drawn into the misinformation.
“I just had to answer something about Mariah Carey’s vaccination,” he said. Someone believed “that it was done with a retractable needle that didn’t really go into her arm.”
Vaccine hesitancy is more about people not understanding the risk-benefit analysis, taking side effects out of out of context if there are side effects, or being influenced by “arbitrary statements about microchips, infertility, or whatever it might be,” Dr. Adalja said.
The future is subject to change
“We’re expecting another rise in cases and more mortality in our winter season here in the United States,” Dr. Mokdad said, adding that the efficacy of the vaccines is likely to attenuate the mortality rate in particular.
However, as the epidemiology of the pandemic evolves, so too will the long-term predictions. Factors that could influence future numbers include the expansion of vaccination to teens 12-15 years old and (eventually) younger children, a need for booster vaccines, emerging variants, and the changing proportion of the population who are fully vaccinated or were previously infected.
Again, getting people to adhere to mask wearing come winter could be challenging if the scenario over the summer is “close to normal with less than 200 deaths a day in the United States,” he added. Asking people to wear masks again will be like “swimming upstream.”
“I think it’s a mistake to think that we’re going to get to ‘COVID zero,’ ” Dr. Adalja said. “This is not an eradicable disease. There’s only been one human infectious disease eradicated from the planet, and that’s smallpox, and it had very different characteristics.”
A version of this article first appeared on Medscape.com.
COVID-19 is likely to follow a seasonal pattern – similar to some other respiratory viruses – with fewer cases come summer 2021 followed by a jump next winter, experts predicted in a Thursday briefing.
If that pattern holds, it could mean a need to reinforce the mask-wearing message as the weather gets colder and people once again congregate indoors.
“Right now, we are projecting the United States all the way to Aug. 1 [will have] 619,000 deaths from COVID-19, with 4.7 million globally,” said Ali H. Mokdad, PhD, professor of health metrics sciences at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle, during today’s media briefing sponsored by the Infectious Diseases Society of America and IHME.
The encouraging news is the vaccines appear to be working, and more Americans are getting them. “If you look at the data for these vaccines, they are extremely safe, they are extremely efficacious, and they make you basically impervious – for the most part – to getting serious disease, hospitalization, or death,” said Amesh Adalja, MD, senior scholar at Johns Hopkins University Center for Health Security in Baltimore.
“These vaccines do what they were meant to do: defang this virus,” said Dr. Adalja, who is an IDSA Fellow and adjunct assistant professor at Johns Hopkins Bloomberg School of Public Health. Emerging data out of Israel and other countries suggest a vaccinated person is less likely to transmit the virus as well, he added.
Still aiming for herd immunity
Furthermore, the U.S. Food and Drug Administration is likely to approve emergency use authorization (EUA) among teenagers 12-15 years old “imminently,” thereby expanding the pool of people potentially protected by vaccines.
Such authorization could help with overall public health efforts. “That’s simply a mathematical formula,” Dr. Adalja said. “The more people that are vaccinated, including children, the quicker we’ll get to herd immunity.”
In addition, with lower case numbers expected this summer, herd immunity might become more achievable, said Dr. Mokdad, who is also chief strategy officer for population health at the University of Washington.
As important as herd immunity is, so-called decoupling is “more important to me,” Dr. Adalja said. Decoupling refers to separating infections from the more severe outcomes, so people who get COVID-19 are less likely to need hospitalization or die from it.
Vaccines get the credit here, he added, including with the variants. “Even if you get a breakthrough infection with a variant, it’s not likely to land you in the hospital or cause serious disease or death,” Dr. Adalja said.
Masks and the uncommon cold
Wearing a mask until we reach herd immunity is important because it’s not possible to tell who is vaccinated and who isn’t, Dr. Mokdad said. “Remember, as many people are waiting to get a vaccine, all of us have access to a mask,” he said.
Dr. Adalja agreed, adding that public health guidance on masks will likely stay in place until we cross that herd immunity threshold and community circulation of the virus goes down.
“People are probably going to want to continue wearing masks, at least some proportion, because they see the benefit for other respiratory viruses,” Dr. Adalja said. “How many of you had a common cold this year?”
Variants: Some good news?
Experts are monitoring the spread of variants of concern in the United States and abroad. On a positive note, the B.1.1.7 variant first identified in the United Kingdom appears to be dominant in the United States at this time, which is potentially good for two reasons. One is that the available COVID-19 vaccines show sufficient efficacy against the strain, Dr. Mokdad said.
Second, a predominance of B.1.1.7 makes it more difficult for other emerging variants of concern like P1 [Brazil] or B.1.351 [South Africa] to gain control, Dr. Adalja said.
“B.1.1.7 is such an efficient transmitter,” he said. “That’s kind of an advantage … because the more B.1.1.7, you have the less opportunity B.1.351 and P1 have to set up shop.”
Hesitancy from misinformation
Vaccine hesitancy remains a concern, particularly at a time when some predict a drop in the number of Americans seeking vaccination. Although needle phobia plays a role in dissuading some from vaccination, the bigger issue is vaccine misinformation, Dr. Adalja said.
“Some people are just terrified when they see the needle. That’s a small part of the proportion of people who don’t want to get vaccinated,” Dr. Adalja said. In contrast, he attributed most hesitancy to misinformation about the vaccine, including reports that the vaccines are fake.
Even celebrities are getting drawn into the misinformation.
“I just had to answer something about Mariah Carey’s vaccination,” he said. Someone believed “that it was done with a retractable needle that didn’t really go into her arm.”
Vaccine hesitancy is more about people not understanding the risk-benefit analysis, taking side effects out of out of context if there are side effects, or being influenced by “arbitrary statements about microchips, infertility, or whatever it might be,” Dr. Adalja said.
The future is subject to change
“We’re expecting another rise in cases and more mortality in our winter season here in the United States,” Dr. Mokdad said, adding that the efficacy of the vaccines is likely to attenuate the mortality rate in particular.
However, as the epidemiology of the pandemic evolves, so too will the long-term predictions. Factors that could influence future numbers include the expansion of vaccination to teens 12-15 years old and (eventually) younger children, a need for booster vaccines, emerging variants, and the changing proportion of the population who are fully vaccinated or were previously infected.
Again, getting people to adhere to mask wearing come winter could be challenging if the scenario over the summer is “close to normal with less than 200 deaths a day in the United States,” he added. Asking people to wear masks again will be like “swimming upstream.”
“I think it’s a mistake to think that we’re going to get to ‘COVID zero,’ ” Dr. Adalja said. “This is not an eradicable disease. There’s only been one human infectious disease eradicated from the planet, and that’s smallpox, and it had very different characteristics.”
A version of this article first appeared on Medscape.com.
COVID-19 is likely to follow a seasonal pattern – similar to some other respiratory viruses – with fewer cases come summer 2021 followed by a jump next winter, experts predicted in a Thursday briefing.
If that pattern holds, it could mean a need to reinforce the mask-wearing message as the weather gets colder and people once again congregate indoors.
“Right now, we are projecting the United States all the way to Aug. 1 [will have] 619,000 deaths from COVID-19, with 4.7 million globally,” said Ali H. Mokdad, PhD, professor of health metrics sciences at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle, during today’s media briefing sponsored by the Infectious Diseases Society of America and IHME.
The encouraging news is the vaccines appear to be working, and more Americans are getting them. “If you look at the data for these vaccines, they are extremely safe, they are extremely efficacious, and they make you basically impervious – for the most part – to getting serious disease, hospitalization, or death,” said Amesh Adalja, MD, senior scholar at Johns Hopkins University Center for Health Security in Baltimore.
“These vaccines do what they were meant to do: defang this virus,” said Dr. Adalja, who is an IDSA Fellow and adjunct assistant professor at Johns Hopkins Bloomberg School of Public Health. Emerging data out of Israel and other countries suggest a vaccinated person is less likely to transmit the virus as well, he added.
Still aiming for herd immunity
Furthermore, the U.S. Food and Drug Administration is likely to approve emergency use authorization (EUA) among teenagers 12-15 years old “imminently,” thereby expanding the pool of people potentially protected by vaccines.
Such authorization could help with overall public health efforts. “That’s simply a mathematical formula,” Dr. Adalja said. “The more people that are vaccinated, including children, the quicker we’ll get to herd immunity.”
In addition, with lower case numbers expected this summer, herd immunity might become more achievable, said Dr. Mokdad, who is also chief strategy officer for population health at the University of Washington.
As important as herd immunity is, so-called decoupling is “more important to me,” Dr. Adalja said. Decoupling refers to separating infections from the more severe outcomes, so people who get COVID-19 are less likely to need hospitalization or die from it.
Vaccines get the credit here, he added, including with the variants. “Even if you get a breakthrough infection with a variant, it’s not likely to land you in the hospital or cause serious disease or death,” Dr. Adalja said.
Masks and the uncommon cold
Wearing a mask until we reach herd immunity is important because it’s not possible to tell who is vaccinated and who isn’t, Dr. Mokdad said. “Remember, as many people are waiting to get a vaccine, all of us have access to a mask,” he said.
Dr. Adalja agreed, adding that public health guidance on masks will likely stay in place until we cross that herd immunity threshold and community circulation of the virus goes down.
“People are probably going to want to continue wearing masks, at least some proportion, because they see the benefit for other respiratory viruses,” Dr. Adalja said. “How many of you had a common cold this year?”
Variants: Some good news?
Experts are monitoring the spread of variants of concern in the United States and abroad. On a positive note, the B.1.1.7 variant first identified in the United Kingdom appears to be dominant in the United States at this time, which is potentially good for two reasons. One is that the available COVID-19 vaccines show sufficient efficacy against the strain, Dr. Mokdad said.
Second, a predominance of B.1.1.7 makes it more difficult for other emerging variants of concern like P1 [Brazil] or B.1.351 [South Africa] to gain control, Dr. Adalja said.
“B.1.1.7 is such an efficient transmitter,” he said. “That’s kind of an advantage … because the more B.1.1.7, you have the less opportunity B.1.351 and P1 have to set up shop.”
Hesitancy from misinformation
Vaccine hesitancy remains a concern, particularly at a time when some predict a drop in the number of Americans seeking vaccination. Although needle phobia plays a role in dissuading some from vaccination, the bigger issue is vaccine misinformation, Dr. Adalja said.
“Some people are just terrified when they see the needle. That’s a small part of the proportion of people who don’t want to get vaccinated,” Dr. Adalja said. In contrast, he attributed most hesitancy to misinformation about the vaccine, including reports that the vaccines are fake.
Even celebrities are getting drawn into the misinformation.
“I just had to answer something about Mariah Carey’s vaccination,” he said. Someone believed “that it was done with a retractable needle that didn’t really go into her arm.”
Vaccine hesitancy is more about people not understanding the risk-benefit analysis, taking side effects out of out of context if there are side effects, or being influenced by “arbitrary statements about microchips, infertility, or whatever it might be,” Dr. Adalja said.
The future is subject to change
“We’re expecting another rise in cases and more mortality in our winter season here in the United States,” Dr. Mokdad said, adding that the efficacy of the vaccines is likely to attenuate the mortality rate in particular.
However, as the epidemiology of the pandemic evolves, so too will the long-term predictions. Factors that could influence future numbers include the expansion of vaccination to teens 12-15 years old and (eventually) younger children, a need for booster vaccines, emerging variants, and the changing proportion of the population who are fully vaccinated or were previously infected.
Again, getting people to adhere to mask wearing come winter could be challenging if the scenario over the summer is “close to normal with less than 200 deaths a day in the United States,” he added. Asking people to wear masks again will be like “swimming upstream.”
“I think it’s a mistake to think that we’re going to get to ‘COVID zero,’ ” Dr. Adalja said. “This is not an eradicable disease. There’s only been one human infectious disease eradicated from the planet, and that’s smallpox, and it had very different characteristics.”
A version of this article first appeared on Medscape.com.
FDA expands use of SLIT pollen allergy treatment to children
The Food and Drug Administration has approved a new indication for ALK’s under-the-tongue immunotherapy tablet Ragwitek (Ambrosia artemisiifolia) to treat ragweed pollen–induced hay fever in children aged 5-17 years.
Ragwitek received FDA approval in 2014 to treat short ragweed pollen–induced hay fever, with or without allergic rhinoconjunctivitis, in adults aged 18-65 years.
The approval for Ragwitek comes with a boxed warning regarding a risk for life-threatening allergic reactions associated with the immunotherapy treatment, including anaphylaxis and severe laryngopharyngeal restriction. The package insert specifies that physicians should prescribe autoinjectable epinephrine with the drug.
“Ragwitek tablets provide a new immunotherapy treatment option for children and adolescents with seasonal ragweed allergies which often causes uncomfortable nasal symptoms and red, itchy eyes during the late summer and early fall,” David I. Bernstein, MD, University of Cincinnati, Bernstein Clinical Research, said in a company press release.
Short ragweed pollen is one of the most common weed allergies. Allergic rhinitis, or hay fever, affects 10%-30% of the population worldwide, according to the American Academy of Allergy Asthma & Immunology. In the United States, approximately 7.7% of adults and 7.2% of children were diagnosed with it annually, according to the Centers for Disease Control and Prevention.
The new indication was based partly on data from a phase 3 clinical trial in children with short ragweed–induced allergic rhinitis, or hay fever, published in the Journal of Allergy and Clinical Immunology. In the study, researchers evaluated the efficacy and safety of the treatment in 1,022 participants aged 5-17 years with a history of ragweed-induced rhinoconjunctivitis and sensitivity to ragweed over a 20- to 28-week treatment period.
Researchers found that Ragwitek improved symptoms in children and adolescents and decreased their use of symptom-relieving medication, compared with placebo.
Among children and adolescents aged 5-17 years, the most common adverse reactions reported were throat irritation/tickle (48.3% in the Ragwitek group vs. 17.7% in the placebo group), itching in the mouth (47.8% vs. 11.2%), itching in the ear (33.9% vs. 6.3%), mouth pain (18.9% vs. 4.5%), swelling of the lips (13.8% vs. 1.2%), nausea (11.5% vs. 3.3%), swelling of the tongue (11.3% vs. 0.8%), throat swelling (10.7% vs. 1.6%), and stomach pain (10.1% vs. 4.5%).
The FDA also recommends that Ragwitek not be prescribed to people with severe, unstable, or uncontrolled asthma, those with a history of severe systemic allergic reactions, and those with a history of eosinophilic esophagitis. The immunotherapy treatment also may not be suitable for people who are unresponsive to epinephrine or inhaled bronchodilators.
In addition, the treatment is not approved for the immediate relief of allergic symptoms in children or adults. The once-daily treatment, which contains an extract from short ragweed pollen, should begin 12 weeks before the start of ragweed pollen season and continue throughout the season, according to the FDA.
Dr. Bernstein said that the under-the-tongue immunotherapy works by targeting the specific allergy trigger and reducing allergy symptoms by “stimulating the immune system.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new indication for ALK’s under-the-tongue immunotherapy tablet Ragwitek (Ambrosia artemisiifolia) to treat ragweed pollen–induced hay fever in children aged 5-17 years.
Ragwitek received FDA approval in 2014 to treat short ragweed pollen–induced hay fever, with or without allergic rhinoconjunctivitis, in adults aged 18-65 years.
The approval for Ragwitek comes with a boxed warning regarding a risk for life-threatening allergic reactions associated with the immunotherapy treatment, including anaphylaxis and severe laryngopharyngeal restriction. The package insert specifies that physicians should prescribe autoinjectable epinephrine with the drug.
“Ragwitek tablets provide a new immunotherapy treatment option for children and adolescents with seasonal ragweed allergies which often causes uncomfortable nasal symptoms and red, itchy eyes during the late summer and early fall,” David I. Bernstein, MD, University of Cincinnati, Bernstein Clinical Research, said in a company press release.
Short ragweed pollen is one of the most common weed allergies. Allergic rhinitis, or hay fever, affects 10%-30% of the population worldwide, according to the American Academy of Allergy Asthma & Immunology. In the United States, approximately 7.7% of adults and 7.2% of children were diagnosed with it annually, according to the Centers for Disease Control and Prevention.
The new indication was based partly on data from a phase 3 clinical trial in children with short ragweed–induced allergic rhinitis, or hay fever, published in the Journal of Allergy and Clinical Immunology. In the study, researchers evaluated the efficacy and safety of the treatment in 1,022 participants aged 5-17 years with a history of ragweed-induced rhinoconjunctivitis and sensitivity to ragweed over a 20- to 28-week treatment period.
Researchers found that Ragwitek improved symptoms in children and adolescents and decreased their use of symptom-relieving medication, compared with placebo.
Among children and adolescents aged 5-17 years, the most common adverse reactions reported were throat irritation/tickle (48.3% in the Ragwitek group vs. 17.7% in the placebo group), itching in the mouth (47.8% vs. 11.2%), itching in the ear (33.9% vs. 6.3%), mouth pain (18.9% vs. 4.5%), swelling of the lips (13.8% vs. 1.2%), nausea (11.5% vs. 3.3%), swelling of the tongue (11.3% vs. 0.8%), throat swelling (10.7% vs. 1.6%), and stomach pain (10.1% vs. 4.5%).
The FDA also recommends that Ragwitek not be prescribed to people with severe, unstable, or uncontrolled asthma, those with a history of severe systemic allergic reactions, and those with a history of eosinophilic esophagitis. The immunotherapy treatment also may not be suitable for people who are unresponsive to epinephrine or inhaled bronchodilators.
In addition, the treatment is not approved for the immediate relief of allergic symptoms in children or adults. The once-daily treatment, which contains an extract from short ragweed pollen, should begin 12 weeks before the start of ragweed pollen season and continue throughout the season, according to the FDA.
Dr. Bernstein said that the under-the-tongue immunotherapy works by targeting the specific allergy trigger and reducing allergy symptoms by “stimulating the immune system.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new indication for ALK’s under-the-tongue immunotherapy tablet Ragwitek (Ambrosia artemisiifolia) to treat ragweed pollen–induced hay fever in children aged 5-17 years.
Ragwitek received FDA approval in 2014 to treat short ragweed pollen–induced hay fever, with or without allergic rhinoconjunctivitis, in adults aged 18-65 years.
The approval for Ragwitek comes with a boxed warning regarding a risk for life-threatening allergic reactions associated with the immunotherapy treatment, including anaphylaxis and severe laryngopharyngeal restriction. The package insert specifies that physicians should prescribe autoinjectable epinephrine with the drug.
“Ragwitek tablets provide a new immunotherapy treatment option for children and adolescents with seasonal ragweed allergies which often causes uncomfortable nasal symptoms and red, itchy eyes during the late summer and early fall,” David I. Bernstein, MD, University of Cincinnati, Bernstein Clinical Research, said in a company press release.
Short ragweed pollen is one of the most common weed allergies. Allergic rhinitis, or hay fever, affects 10%-30% of the population worldwide, according to the American Academy of Allergy Asthma & Immunology. In the United States, approximately 7.7% of adults and 7.2% of children were diagnosed with it annually, according to the Centers for Disease Control and Prevention.
The new indication was based partly on data from a phase 3 clinical trial in children with short ragweed–induced allergic rhinitis, or hay fever, published in the Journal of Allergy and Clinical Immunology. In the study, researchers evaluated the efficacy and safety of the treatment in 1,022 participants aged 5-17 years with a history of ragweed-induced rhinoconjunctivitis and sensitivity to ragweed over a 20- to 28-week treatment period.
Researchers found that Ragwitek improved symptoms in children and adolescents and decreased their use of symptom-relieving medication, compared with placebo.
Among children and adolescents aged 5-17 years, the most common adverse reactions reported were throat irritation/tickle (48.3% in the Ragwitek group vs. 17.7% in the placebo group), itching in the mouth (47.8% vs. 11.2%), itching in the ear (33.9% vs. 6.3%), mouth pain (18.9% vs. 4.5%), swelling of the lips (13.8% vs. 1.2%), nausea (11.5% vs. 3.3%), swelling of the tongue (11.3% vs. 0.8%), throat swelling (10.7% vs. 1.6%), and stomach pain (10.1% vs. 4.5%).
The FDA also recommends that Ragwitek not be prescribed to people with severe, unstable, or uncontrolled asthma, those with a history of severe systemic allergic reactions, and those with a history of eosinophilic esophagitis. The immunotherapy treatment also may not be suitable for people who are unresponsive to epinephrine or inhaled bronchodilators.
In addition, the treatment is not approved for the immediate relief of allergic symptoms in children or adults. The once-daily treatment, which contains an extract from short ragweed pollen, should begin 12 weeks before the start of ragweed pollen season and continue throughout the season, according to the FDA.
Dr. Bernstein said that the under-the-tongue immunotherapy works by targeting the specific allergy trigger and reducing allergy symptoms by “stimulating the immune system.”
A version of this article first appeared on Medscape.com.
COVID-19 infection conveys imperfect immunity in young adults
Do your patients think that getting COVID-19 is fully protective against subsequent reinfection? Tell it to the Marines.
A study of U.S. Marine recruits on their way to boot camp at Parris Island, S.C., showed that those who were seropositive at baseline, indicating prior exposure to SARS-CoV-2, remained at some risk for reinfection. They had about one-fifth the risk of subsequent infection, compared with seronegative recruits during basic training, but reinfections did occur.
The study, by Stuart C. Sealfon, MD, of Icahn School of Medicine at Mount Sinai in New York, and colleagues, was published in The Lancet Respiratory Medicine.
“Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralization activity or immunity against subsequent infection,” they wrote.
An infectious disease specialist who was not involved in the study said that the findings provide further evidence about the level of immunity acquired after an infection.
“It’s quite clear that reinfections do occur, they are of public health importance, and they’re something we need to be mindful of in terms of advising patients about whether a prior infection protects them from reinfection,” Mark Siedner, MD, MPH, a clinician and researcher in the division of infectious diseases at Massachusetts General Hospital, Boston, said in an interview.
The study results reinforce that “not all antibodies are the same,” said Sachin Gupta, MD, an attending physician in pulmonary and critical care medicine at Alameda Health System in Oakland, Calif. “We’re seeing still that 10% of folks who have antibodies can get infected again,” he said in an interview.
CHARM initiative
Dr. Sealfon and colleagues presented an analysis of data from the ironically named CHARM (COVID-19 Health Action Response for Marines) prospective study.
CHARM included U.S. Marine recruits, most of them male, aged 18-20 years, who were instructed to follow a 2-week unsupervised quarantine at home, after which they reported to a Marine-supervised facility for an additional 2-week quarantine.
At baseline, participants were tested for SARS-CoV-2 immunoglobulin G (IgG) seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein enzyme-linked immunosorbent assay (ELISA).
The recruits filled out questionnaires asking them to report any of 14 specific COVID-19–related symptoms or any other unspecified symptom, as well as demographic information, risk factors, and a brief medical history.
Investigators tested recruits for SARS-CoV-2 infection by polymerase chain reaction (PCR) assay at weeks 0, 1, and 2 of quarantine, and any who had positive PCR results during quarantine were excluded.
Participants who had three negative swab PCR results during quarantine and a baseline serology test at the beginning of the supervised quarantine period – either seronegative or seropositive – then went on to enjoy their basic training at the Marine Corps Recruit Depot, Parris Island, S.C.
The participants were followed prospectively with PCR tests at weeks 2, 4, and 6 in both the seropositive and seronegative groups, and sera were obtained at the same time.
Holes in immunologic armor
Full data were available for a total of 189 participants who were seropositive and 2,247 who were seronegative at enrollment.
In all, 19 of 189 seropositive recruits (10%) had at least one PCR test positive for SARS-CoV-2 infection during the 6-week follow-up period. This translated into an incidence of 1.1 cases per person-year.
Of the 2,247 participants seronegative at baseline, 1,079 tested positive (6.2 cases per person-year; incidence rate ratio 0.18).
It appeared that antibodies provided some protection for seropositive recruits, as evidenced by a higher likelihood of infection among those with lower baseline full-length spike protein IgG titers than in those with higher baseline titers (hazard ratio 0.4, P < .001).
Among the seropositive participants who did acquire a second SARS-CoV-2 infection, viral loads in mid-turbinate nasal swabs were about 10-fold lower than in seronegative recruits who acquired infections during follow-up.
“This finding suggests that some reinfected individuals could have a similar capacity to transmit infection as those who are infected for the first time. The rate at which reinfection occurs after vaccines and natural immunity is important for estimating the proportion of the population that needs to be vaccinated to suppress the pandemic,” the investigators wrote.
Baseline neutralizing antibody titers were detected in 45 of the first 54 seropositive recruits who remained PCR negative throughout follow-up, but also in 6 of 19 seropositive participants who became infected during the 6 weeks of observation.
Lessons
Both Dr. Siedner and Dr. Gupta agreed with the authors that the risks for reinfection that were observed in young, physically fit people may differ for other populations, such as women (only 10% of seropositive recruits and 8% of seronegative recruits were female), older patients, or those who are immunocompromised.
Given that the adjusted odds ratio for reinfection in this study was nearly identical to that of a recent British study comparing infection rates between seropositive and seronegative health care workers, the risk of reinfection for other young adults and for the general population may be similar, Dr. Sealfon and colleagues wrote.
Adding to the challenge of reaching herd immunity is the observation that some patients who have recovered from COVID-19 are skeptical about the need for further protection.
“There are patients who feel like vaccination is of low benefit to them, and I think these are the same people who would be hesitant to get the vaccine anyway,” Dr. Gupta said.
Although no vaccine is perfect – the vaccine failure rate from the mRNA-based vaccines from Moderna and Pfizer/Biontech is about 5% – the protections they afford are unmistakable, Dr. Siedner said.
“I think it’s important to make the distinction that most postvaccination infections by and large have been very mild,” he said. “In people with normal immune systems, we have not seen an onslaught of postvaccination infections requiring hospitalization. Even if people do get infected after vaccination, the vaccines protect people from severe infection, and that’s what we want them to do.”
The investigators stated, “Young adults, of whom a high proportion are asymptomatically infected and become seropositive in the absence of known infection, can be an important source of transmission to more vulnerable populations. Evaluating the protection against subsequent SARS-CoV-2 infection conferred by seropositivity in young adults is important for determining the need for vaccinating previously infected individuals in this age group.”
The study was funded by the Defense Health Agency and Defense Advanced Research Projects Agency. Dr. Sealfon, Dr. Siedner, and Dr. Gupta have no conflicts of interest to report. Dr. Gupta is a member of the editorial advisory board for this publication.
Do your patients think that getting COVID-19 is fully protective against subsequent reinfection? Tell it to the Marines.
A study of U.S. Marine recruits on their way to boot camp at Parris Island, S.C., showed that those who were seropositive at baseline, indicating prior exposure to SARS-CoV-2, remained at some risk for reinfection. They had about one-fifth the risk of subsequent infection, compared with seronegative recruits during basic training, but reinfections did occur.
The study, by Stuart C. Sealfon, MD, of Icahn School of Medicine at Mount Sinai in New York, and colleagues, was published in The Lancet Respiratory Medicine.
“Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralization activity or immunity against subsequent infection,” they wrote.
An infectious disease specialist who was not involved in the study said that the findings provide further evidence about the level of immunity acquired after an infection.
“It’s quite clear that reinfections do occur, they are of public health importance, and they’re something we need to be mindful of in terms of advising patients about whether a prior infection protects them from reinfection,” Mark Siedner, MD, MPH, a clinician and researcher in the division of infectious diseases at Massachusetts General Hospital, Boston, said in an interview.
The study results reinforce that “not all antibodies are the same,” said Sachin Gupta, MD, an attending physician in pulmonary and critical care medicine at Alameda Health System in Oakland, Calif. “We’re seeing still that 10% of folks who have antibodies can get infected again,” he said in an interview.
CHARM initiative
Dr. Sealfon and colleagues presented an analysis of data from the ironically named CHARM (COVID-19 Health Action Response for Marines) prospective study.
CHARM included U.S. Marine recruits, most of them male, aged 18-20 years, who were instructed to follow a 2-week unsupervised quarantine at home, after which they reported to a Marine-supervised facility for an additional 2-week quarantine.
At baseline, participants were tested for SARS-CoV-2 immunoglobulin G (IgG) seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein enzyme-linked immunosorbent assay (ELISA).
The recruits filled out questionnaires asking them to report any of 14 specific COVID-19–related symptoms or any other unspecified symptom, as well as demographic information, risk factors, and a brief medical history.
Investigators tested recruits for SARS-CoV-2 infection by polymerase chain reaction (PCR) assay at weeks 0, 1, and 2 of quarantine, and any who had positive PCR results during quarantine were excluded.
Participants who had three negative swab PCR results during quarantine and a baseline serology test at the beginning of the supervised quarantine period – either seronegative or seropositive – then went on to enjoy their basic training at the Marine Corps Recruit Depot, Parris Island, S.C.
The participants were followed prospectively with PCR tests at weeks 2, 4, and 6 in both the seropositive and seronegative groups, and sera were obtained at the same time.
Holes in immunologic armor
Full data were available for a total of 189 participants who were seropositive and 2,247 who were seronegative at enrollment.
In all, 19 of 189 seropositive recruits (10%) had at least one PCR test positive for SARS-CoV-2 infection during the 6-week follow-up period. This translated into an incidence of 1.1 cases per person-year.
Of the 2,247 participants seronegative at baseline, 1,079 tested positive (6.2 cases per person-year; incidence rate ratio 0.18).
It appeared that antibodies provided some protection for seropositive recruits, as evidenced by a higher likelihood of infection among those with lower baseline full-length spike protein IgG titers than in those with higher baseline titers (hazard ratio 0.4, P < .001).
Among the seropositive participants who did acquire a second SARS-CoV-2 infection, viral loads in mid-turbinate nasal swabs were about 10-fold lower than in seronegative recruits who acquired infections during follow-up.
“This finding suggests that some reinfected individuals could have a similar capacity to transmit infection as those who are infected for the first time. The rate at which reinfection occurs after vaccines and natural immunity is important for estimating the proportion of the population that needs to be vaccinated to suppress the pandemic,” the investigators wrote.
Baseline neutralizing antibody titers were detected in 45 of the first 54 seropositive recruits who remained PCR negative throughout follow-up, but also in 6 of 19 seropositive participants who became infected during the 6 weeks of observation.
Lessons
Both Dr. Siedner and Dr. Gupta agreed with the authors that the risks for reinfection that were observed in young, physically fit people may differ for other populations, such as women (only 10% of seropositive recruits and 8% of seronegative recruits were female), older patients, or those who are immunocompromised.
Given that the adjusted odds ratio for reinfection in this study was nearly identical to that of a recent British study comparing infection rates between seropositive and seronegative health care workers, the risk of reinfection for other young adults and for the general population may be similar, Dr. Sealfon and colleagues wrote.
Adding to the challenge of reaching herd immunity is the observation that some patients who have recovered from COVID-19 are skeptical about the need for further protection.
“There are patients who feel like vaccination is of low benefit to them, and I think these are the same people who would be hesitant to get the vaccine anyway,” Dr. Gupta said.
Although no vaccine is perfect – the vaccine failure rate from the mRNA-based vaccines from Moderna and Pfizer/Biontech is about 5% – the protections they afford are unmistakable, Dr. Siedner said.
“I think it’s important to make the distinction that most postvaccination infections by and large have been very mild,” he said. “In people with normal immune systems, we have not seen an onslaught of postvaccination infections requiring hospitalization. Even if people do get infected after vaccination, the vaccines protect people from severe infection, and that’s what we want them to do.”
The investigators stated, “Young adults, of whom a high proportion are asymptomatically infected and become seropositive in the absence of known infection, can be an important source of transmission to more vulnerable populations. Evaluating the protection against subsequent SARS-CoV-2 infection conferred by seropositivity in young adults is important for determining the need for vaccinating previously infected individuals in this age group.”
The study was funded by the Defense Health Agency and Defense Advanced Research Projects Agency. Dr. Sealfon, Dr. Siedner, and Dr. Gupta have no conflicts of interest to report. Dr. Gupta is a member of the editorial advisory board for this publication.
Do your patients think that getting COVID-19 is fully protective against subsequent reinfection? Tell it to the Marines.
A study of U.S. Marine recruits on their way to boot camp at Parris Island, S.C., showed that those who were seropositive at baseline, indicating prior exposure to SARS-CoV-2, remained at some risk for reinfection. They had about one-fifth the risk of subsequent infection, compared with seronegative recruits during basic training, but reinfections did occur.
The study, by Stuart C. Sealfon, MD, of Icahn School of Medicine at Mount Sinai in New York, and colleagues, was published in The Lancet Respiratory Medicine.
“Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralization activity or immunity against subsequent infection,” they wrote.
An infectious disease specialist who was not involved in the study said that the findings provide further evidence about the level of immunity acquired after an infection.
“It’s quite clear that reinfections do occur, they are of public health importance, and they’re something we need to be mindful of in terms of advising patients about whether a prior infection protects them from reinfection,” Mark Siedner, MD, MPH, a clinician and researcher in the division of infectious diseases at Massachusetts General Hospital, Boston, said in an interview.
The study results reinforce that “not all antibodies are the same,” said Sachin Gupta, MD, an attending physician in pulmonary and critical care medicine at Alameda Health System in Oakland, Calif. “We’re seeing still that 10% of folks who have antibodies can get infected again,” he said in an interview.
CHARM initiative
Dr. Sealfon and colleagues presented an analysis of data from the ironically named CHARM (COVID-19 Health Action Response for Marines) prospective study.
CHARM included U.S. Marine recruits, most of them male, aged 18-20 years, who were instructed to follow a 2-week unsupervised quarantine at home, after which they reported to a Marine-supervised facility for an additional 2-week quarantine.
At baseline, participants were tested for SARS-CoV-2 immunoglobulin G (IgG) seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein enzyme-linked immunosorbent assay (ELISA).
The recruits filled out questionnaires asking them to report any of 14 specific COVID-19–related symptoms or any other unspecified symptom, as well as demographic information, risk factors, and a brief medical history.
Investigators tested recruits for SARS-CoV-2 infection by polymerase chain reaction (PCR) assay at weeks 0, 1, and 2 of quarantine, and any who had positive PCR results during quarantine were excluded.
Participants who had three negative swab PCR results during quarantine and a baseline serology test at the beginning of the supervised quarantine period – either seronegative or seropositive – then went on to enjoy their basic training at the Marine Corps Recruit Depot, Parris Island, S.C.
The participants were followed prospectively with PCR tests at weeks 2, 4, and 6 in both the seropositive and seronegative groups, and sera were obtained at the same time.
Holes in immunologic armor
Full data were available for a total of 189 participants who were seropositive and 2,247 who were seronegative at enrollment.
In all, 19 of 189 seropositive recruits (10%) had at least one PCR test positive for SARS-CoV-2 infection during the 6-week follow-up period. This translated into an incidence of 1.1 cases per person-year.
Of the 2,247 participants seronegative at baseline, 1,079 tested positive (6.2 cases per person-year; incidence rate ratio 0.18).
It appeared that antibodies provided some protection for seropositive recruits, as evidenced by a higher likelihood of infection among those with lower baseline full-length spike protein IgG titers than in those with higher baseline titers (hazard ratio 0.4, P < .001).
Among the seropositive participants who did acquire a second SARS-CoV-2 infection, viral loads in mid-turbinate nasal swabs were about 10-fold lower than in seronegative recruits who acquired infections during follow-up.
“This finding suggests that some reinfected individuals could have a similar capacity to transmit infection as those who are infected for the first time. The rate at which reinfection occurs after vaccines and natural immunity is important for estimating the proportion of the population that needs to be vaccinated to suppress the pandemic,” the investigators wrote.
Baseline neutralizing antibody titers were detected in 45 of the first 54 seropositive recruits who remained PCR negative throughout follow-up, but also in 6 of 19 seropositive participants who became infected during the 6 weeks of observation.
Lessons
Both Dr. Siedner and Dr. Gupta agreed with the authors that the risks for reinfection that were observed in young, physically fit people may differ for other populations, such as women (only 10% of seropositive recruits and 8% of seronegative recruits were female), older patients, or those who are immunocompromised.
Given that the adjusted odds ratio for reinfection in this study was nearly identical to that of a recent British study comparing infection rates between seropositive and seronegative health care workers, the risk of reinfection for other young adults and for the general population may be similar, Dr. Sealfon and colleagues wrote.
Adding to the challenge of reaching herd immunity is the observation that some patients who have recovered from COVID-19 are skeptical about the need for further protection.
“There are patients who feel like vaccination is of low benefit to them, and I think these are the same people who would be hesitant to get the vaccine anyway,” Dr. Gupta said.
Although no vaccine is perfect – the vaccine failure rate from the mRNA-based vaccines from Moderna and Pfizer/Biontech is about 5% – the protections they afford are unmistakable, Dr. Siedner said.
“I think it’s important to make the distinction that most postvaccination infections by and large have been very mild,” he said. “In people with normal immune systems, we have not seen an onslaught of postvaccination infections requiring hospitalization. Even if people do get infected after vaccination, the vaccines protect people from severe infection, and that’s what we want them to do.”
The investigators stated, “Young adults, of whom a high proportion are asymptomatically infected and become seropositive in the absence of known infection, can be an important source of transmission to more vulnerable populations. Evaluating the protection against subsequent SARS-CoV-2 infection conferred by seropositivity in young adults is important for determining the need for vaccinating previously infected individuals in this age group.”
The study was funded by the Defense Health Agency and Defense Advanced Research Projects Agency. Dr. Sealfon, Dr. Siedner, and Dr. Gupta have no conflicts of interest to report. Dr. Gupta is a member of the editorial advisory board for this publication.
FROM THE LANCET RESPIRATORY MEDICINE
COVID plus MI confers poor prognosis; 1 in 3 die in hospital
COVID-19 patients with ST-segment elevation MI (STEMI) represent a population with unique demographic and clinical features resulting in a high risk for mortality, according to initial findings from the North American Cardiovascular COVID-19 Myocardial Infarction (NACMI) Registry.

“This is the largest registry of COVID-positive patients presenting with STEMI [and] the results clearly illustrate the challenges and uniqueness of this patient population that deserves prompt and special attention,” study cochair Timothy Henry, MD, president-elect of the Society for Cardiovascular Angiography & Interventions, said in a news release.
The NACMI registry is a collaborative effort between the SCAI, the American College of Cardiology Interventional Council, and the Canadian Association of Interventional Cardiology.
“The rapid development of this ongoing, critically important prospective registry reflects the strong and unique collaboration of all three societies. It was gratifying to be part of this process and hopefully the results will improve the care of our patients and stimulate further research,” Dr. Henry said in the news release.
The registry has enrolled 1,185 patients presenting with STEMI at 64 sites across the United States and Canada. Participants include 230 COVID-positive STEMI patients; 495 STEMI patients suspected but ultimately confirmed not to have COVID-19; and 460 age-and sex-matched control STEMI patients treated prior to the pandemic who are part of the Midwest STEMI Consortium.
The initial findings from the registry were published online in the Journal of the American College of Cardiology.
Atypical symptoms may explain high death rate
The primary outcome – a composite of in-hospital death, stroke, recurrent MI, or repeat unplanned revascularization – occurred in 36% of COVID-positive patients, compared with 13% of COVID-negative patients and 5% of control patients (P < .001 relative to controls).
This difference was driven largely by a “very high” in-hospital death rate in COVID-positive patients, lead author Santiago Garcia, MD, Minneapolis Heart Institute Foundation, said in an interview.
The in-hospital death rate was 33% in COVID-positive patients, compared with 11% in COVID-negative patients and 4% in controls. Stroke also occurred more often in COVID-positive patients at 3% versus 2% in COVID-negative and 0% in controls.
These initial findings suggest that the combination of STEMI and COVID-19 infection “confers a poor prognosis, with one in three patients succumbing to the disease, even among patients selected for invasive angiography (28% mortality),” the investigators wrote.
The data also show that STEMI in COVID-positive patients disproportionately affects ethnic minorities (23% Hispanic and 24% Black) with diabetes, which was present in 46% of COVID-positive patients.
COVID-positive patients with STEMI are more likely to present with atypical symptoms such as dyspnea (54%), pulmonary infiltrates on chest x-ray (46%), and high-risk conditions such as cardiogenic shock (18%), “which may explain the high fatality rate,” Dr. Garcia said.
Despite these high-risk features, COVID-positive patients are less apt to undergo invasive angiography when compared with COVID-negative and control STEMI patients (78% vs. 96% vs. 100%).
The majority of patients (71%) who did under angiography received primary percutaneous coronary intervention (PPCI) with very small treatment delays (at 15 minutes) during the pandemic.
Another notable finding is that “many patients (23%) have ‘no culprit’ vessel and may represent different etiologies of ST-segment elevation including microemboli, myocarditis, Takotsubo cardiomyopathy,” Dr. Garcia said in an interview.
“In line with current guidelines, patients with suspected STEMI should be managed with PPCI, without delay while the safety of health care providers is ensured,” Ran Kornowski, MD, and Katia Orvin, MD, both with Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, wrote in a linked editorial.
“In this case, PPCI should be performed routinely, even if the patient is presumed to have COVID-19, because PPCI should not be postponed. Confirmation of SARS-CoV-2 infection should not delay urgent decision management concerning reperfusion strategy,” they advised.
Looking ahead, Garcia said plans for the registry include determining predictors of in-hospital mortality and studying demographic and treatment trends as the pandemic continues with more virulent strains of the virus.
Various subgroup analyses are also planned as well as an independent angiographic and electrocardiographic core lab analysis. A comparative analysis of data from the US and Canada is also planned.
This work was supported by an ACC Accreditation Grant, Saskatchewan Health Research Foundation, and grants from Medtronic and Abbott Vascular to SCAI. Dr. Garcia has received institutional research grants from Edwards Lifesciences, BSCI, Medtronic, and Abbott Vascular; has served as a consultant for Medtronic and BSCI; and has served as a proctor for Edwards Lifesciences. Dr. Kornowski and Dr. Orvin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients with ST-segment elevation MI (STEMI) represent a population with unique demographic and clinical features resulting in a high risk for mortality, according to initial findings from the North American Cardiovascular COVID-19 Myocardial Infarction (NACMI) Registry.

“This is the largest registry of COVID-positive patients presenting with STEMI [and] the results clearly illustrate the challenges and uniqueness of this patient population that deserves prompt and special attention,” study cochair Timothy Henry, MD, president-elect of the Society for Cardiovascular Angiography & Interventions, said in a news release.
The NACMI registry is a collaborative effort between the SCAI, the American College of Cardiology Interventional Council, and the Canadian Association of Interventional Cardiology.
“The rapid development of this ongoing, critically important prospective registry reflects the strong and unique collaboration of all three societies. It was gratifying to be part of this process and hopefully the results will improve the care of our patients and stimulate further research,” Dr. Henry said in the news release.
The registry has enrolled 1,185 patients presenting with STEMI at 64 sites across the United States and Canada. Participants include 230 COVID-positive STEMI patients; 495 STEMI patients suspected but ultimately confirmed not to have COVID-19; and 460 age-and sex-matched control STEMI patients treated prior to the pandemic who are part of the Midwest STEMI Consortium.
The initial findings from the registry were published online in the Journal of the American College of Cardiology.
Atypical symptoms may explain high death rate
The primary outcome – a composite of in-hospital death, stroke, recurrent MI, or repeat unplanned revascularization – occurred in 36% of COVID-positive patients, compared with 13% of COVID-negative patients and 5% of control patients (P < .001 relative to controls).
This difference was driven largely by a “very high” in-hospital death rate in COVID-positive patients, lead author Santiago Garcia, MD, Minneapolis Heart Institute Foundation, said in an interview.
The in-hospital death rate was 33% in COVID-positive patients, compared with 11% in COVID-negative patients and 4% in controls. Stroke also occurred more often in COVID-positive patients at 3% versus 2% in COVID-negative and 0% in controls.
These initial findings suggest that the combination of STEMI and COVID-19 infection “confers a poor prognosis, with one in three patients succumbing to the disease, even among patients selected for invasive angiography (28% mortality),” the investigators wrote.
The data also show that STEMI in COVID-positive patients disproportionately affects ethnic minorities (23% Hispanic and 24% Black) with diabetes, which was present in 46% of COVID-positive patients.
COVID-positive patients with STEMI are more likely to present with atypical symptoms such as dyspnea (54%), pulmonary infiltrates on chest x-ray (46%), and high-risk conditions such as cardiogenic shock (18%), “which may explain the high fatality rate,” Dr. Garcia said.
Despite these high-risk features, COVID-positive patients are less apt to undergo invasive angiography when compared with COVID-negative and control STEMI patients (78% vs. 96% vs. 100%).
The majority of patients (71%) who did under angiography received primary percutaneous coronary intervention (PPCI) with very small treatment delays (at 15 minutes) during the pandemic.
Another notable finding is that “many patients (23%) have ‘no culprit’ vessel and may represent different etiologies of ST-segment elevation including microemboli, myocarditis, Takotsubo cardiomyopathy,” Dr. Garcia said in an interview.
“In line with current guidelines, patients with suspected STEMI should be managed with PPCI, without delay while the safety of health care providers is ensured,” Ran Kornowski, MD, and Katia Orvin, MD, both with Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, wrote in a linked editorial.
“In this case, PPCI should be performed routinely, even if the patient is presumed to have COVID-19, because PPCI should not be postponed. Confirmation of SARS-CoV-2 infection should not delay urgent decision management concerning reperfusion strategy,” they advised.
Looking ahead, Garcia said plans for the registry include determining predictors of in-hospital mortality and studying demographic and treatment trends as the pandemic continues with more virulent strains of the virus.
Various subgroup analyses are also planned as well as an independent angiographic and electrocardiographic core lab analysis. A comparative analysis of data from the US and Canada is also planned.
This work was supported by an ACC Accreditation Grant, Saskatchewan Health Research Foundation, and grants from Medtronic and Abbott Vascular to SCAI. Dr. Garcia has received institutional research grants from Edwards Lifesciences, BSCI, Medtronic, and Abbott Vascular; has served as a consultant for Medtronic and BSCI; and has served as a proctor for Edwards Lifesciences. Dr. Kornowski and Dr. Orvin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients with ST-segment elevation MI (STEMI) represent a population with unique demographic and clinical features resulting in a high risk for mortality, according to initial findings from the North American Cardiovascular COVID-19 Myocardial Infarction (NACMI) Registry.

“This is the largest registry of COVID-positive patients presenting with STEMI [and] the results clearly illustrate the challenges and uniqueness of this patient population that deserves prompt and special attention,” study cochair Timothy Henry, MD, president-elect of the Society for Cardiovascular Angiography & Interventions, said in a news release.
The NACMI registry is a collaborative effort between the SCAI, the American College of Cardiology Interventional Council, and the Canadian Association of Interventional Cardiology.
“The rapid development of this ongoing, critically important prospective registry reflects the strong and unique collaboration of all three societies. It was gratifying to be part of this process and hopefully the results will improve the care of our patients and stimulate further research,” Dr. Henry said in the news release.
The registry has enrolled 1,185 patients presenting with STEMI at 64 sites across the United States and Canada. Participants include 230 COVID-positive STEMI patients; 495 STEMI patients suspected but ultimately confirmed not to have COVID-19; and 460 age-and sex-matched control STEMI patients treated prior to the pandemic who are part of the Midwest STEMI Consortium.
The initial findings from the registry were published online in the Journal of the American College of Cardiology.
Atypical symptoms may explain high death rate
The primary outcome – a composite of in-hospital death, stroke, recurrent MI, or repeat unplanned revascularization – occurred in 36% of COVID-positive patients, compared with 13% of COVID-negative patients and 5% of control patients (P < .001 relative to controls).
This difference was driven largely by a “very high” in-hospital death rate in COVID-positive patients, lead author Santiago Garcia, MD, Minneapolis Heart Institute Foundation, said in an interview.
The in-hospital death rate was 33% in COVID-positive patients, compared with 11% in COVID-negative patients and 4% in controls. Stroke also occurred more often in COVID-positive patients at 3% versus 2% in COVID-negative and 0% in controls.
These initial findings suggest that the combination of STEMI and COVID-19 infection “confers a poor prognosis, with one in three patients succumbing to the disease, even among patients selected for invasive angiography (28% mortality),” the investigators wrote.
The data also show that STEMI in COVID-positive patients disproportionately affects ethnic minorities (23% Hispanic and 24% Black) with diabetes, which was present in 46% of COVID-positive patients.
COVID-positive patients with STEMI are more likely to present with atypical symptoms such as dyspnea (54%), pulmonary infiltrates on chest x-ray (46%), and high-risk conditions such as cardiogenic shock (18%), “which may explain the high fatality rate,” Dr. Garcia said.
Despite these high-risk features, COVID-positive patients are less apt to undergo invasive angiography when compared with COVID-negative and control STEMI patients (78% vs. 96% vs. 100%).
The majority of patients (71%) who did under angiography received primary percutaneous coronary intervention (PPCI) with very small treatment delays (at 15 minutes) during the pandemic.
Another notable finding is that “many patients (23%) have ‘no culprit’ vessel and may represent different etiologies of ST-segment elevation including microemboli, myocarditis, Takotsubo cardiomyopathy,” Dr. Garcia said in an interview.
“In line with current guidelines, patients with suspected STEMI should be managed with PPCI, without delay while the safety of health care providers is ensured,” Ran Kornowski, MD, and Katia Orvin, MD, both with Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, wrote in a linked editorial.
“In this case, PPCI should be performed routinely, even if the patient is presumed to have COVID-19, because PPCI should not be postponed. Confirmation of SARS-CoV-2 infection should not delay urgent decision management concerning reperfusion strategy,” they advised.
Looking ahead, Garcia said plans for the registry include determining predictors of in-hospital mortality and studying demographic and treatment trends as the pandemic continues with more virulent strains of the virus.
Various subgroup analyses are also planned as well as an independent angiographic and electrocardiographic core lab analysis. A comparative analysis of data from the US and Canada is also planned.
This work was supported by an ACC Accreditation Grant, Saskatchewan Health Research Foundation, and grants from Medtronic and Abbott Vascular to SCAI. Dr. Garcia has received institutional research grants from Edwards Lifesciences, BSCI, Medtronic, and Abbott Vascular; has served as a consultant for Medtronic and BSCI; and has served as a proctor for Edwards Lifesciences. Dr. Kornowski and Dr. Orvin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ten reasons airborne transmission of SARS-CoV-2 appears airtight
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
CDC panel: Pause of J&J COVID-19 vaccine to remain for now
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
How to counsel worried patients about the J&J vaccine news
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
Data about COVID-19-related skin manifestations in children continue to emerge
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
COVID-19 in children: New cases on the rise again
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
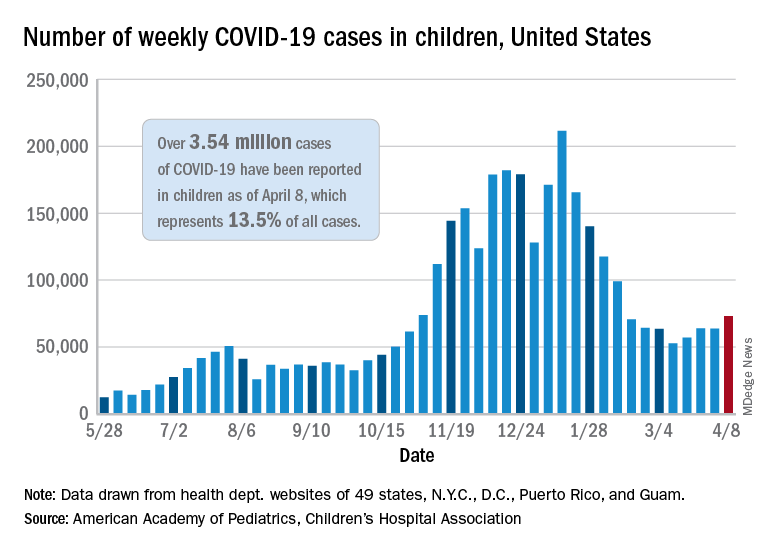
Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.











