User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Next winter may be rough: Models predict ‘considerable surge’ of COVID
It’s likely the United States will see another surge of COVID-19 this winter, warned Christopher Murray, MD, director of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
Speaking at the national conference of State of Reform on April 8, Dr. Murray cited the seasonality of the SARS-CoV-2 virus, which wanes in the summer and waxes in the winter. The “optimistic forecast” of IHME, which has modeled the course of the pandemic for the past 13 months, is that daily deaths will rise a bit in the next month, then decline from May through August, he said.
“Summer should be fairly quiet in terms of COVID, if vaccinations rise and people don’t stop wearing masks,” Dr. Murray said.
But he added that “a considerable surge will occur over next winter,” because the new variants are more transmissible, and people will likely relax social distancing and mask wearing. The IHME predicts that the percentage of Americans who usually don masks will decline from 73% today to 21% by Aug. 1.
With a rapid decline in mask use and a rise in mobility, there will still be more than 1,000 deaths each day by July 1, Dr. Murray said. In a forecast released the day after Dr. Murray spoke, the IHME predicted that by Aug. 1, there will be a total of 618,523 U.S. deaths from COVID-19. Deaths could be as high as 696,651 if mobility among the vaccinated returns to prepandemic levels, the institute forecasts.
Based on cell phone data, Dr. Murray said, the amount of mobility in the United States has already risen to the level of March 2020, when the pandemic was just getting underway.
Decreased infections
If there’s one piece of good news in the latest IHME report, it’s that the estimated number of people infected (including those not tested) will drop from 111,581 today to a projected 17,502 on Aug. 1. But in a worst-case scenario, with sharply higher mobility among vaccinated people, the case count on that date would only fall to 73,842.
The SARS-CoV-2 variants are another factor of concern. Dr. Murray distinguished between variants like the one first identified in the U.K. (B.1.1.7) and other “escape variants.”
B.1.1.7, which is now the dominant strain in the United States, increases transmission but doesn’t necessarily escape the immune system or vaccines, he explained.
In contrast, if someone is infected with a variant such as the South African or the Brazilian mutations, he said, a previous COVID-19 infection might not protect the person, and vaccines are less effective against those variants.
Cross-variant immunity may range from 0% to 60% for escape variants, based on the slim amount of data now available, Dr. Murray said. In his view, these variants will be the long-term driver of the pandemic in the United States, while the United Kingdom variant is the short-term driver.
The latest data, he said, show that the Pfizer/BioNTech and Moderna vaccines are 75% effective against the escape variants, with lower efficacy for other vaccines. But booster shots may still be required to protect people against some variants.
Human factors
Human behavior will also help determine the course of the pandemic, he noted. Vaccine hesitancy, for example, is still high in the United States.
By the end of May, he predicted, about 180 million people will have received about two doses of vaccine. After that, he said, “vaccination will flatline due to lack of demand.” The two unknowns are how much campaigns to promote vaccination will increase vaccine confidence, and when children will be vaccinated.
In the United States, he said, 69% of adults have been vaccinated or want to get a shot. But that percentage has dropped 5 points since February, and vaccine confidence varies by state.
Dr. Murray emphasized that the winter surge he predicts can be blocked if people change their behaviors. These include a rise in vaccine confidence to 80% and continued mask wearing by most people.
However, if vaccine confidence and mask wearing decline, state governments continue to drop social distancing rules, and the uptake of boosters is low, the winter surge could be more serious, he said.
Double surge
Murray also raised the possibility of a double surge of COVID-19 and influenza this winter. Widely expected last winter, this double surge never materialized here or elsewhere, partly because of mask wearing. But Dr. Murray said it could happen this year: History shows that the flu tends to be stronger in years after weak outbreaks.
He advised hospitals to prepare now for whatever might come later this year. Public health authorities, he said, should speed up vaccination, monitor variants closely with additional sequencing, and try to modify behavior in high-risk groups.
Asked to explain the recent surge of COVID-19 cases in Michigan, Dr. Murray attributed it partly to the spread of the B.1.1.7 (U.K.) variant. But he noted that the U.K. variant has expanded even more widely in some other states that haven’t had an explosive surge like Michigan’s.
Moreover, he noted, Michigan doesn’t have low mask use or high mobility. So the upward spiral of COVID-19 infections there is very concerning, he said.
In regard to the role of children as reservoirs of the virus, Dr. Murray pointed out that views on this have changed around the world. For a while, people thought kids didn’t spread COVID-19 very much. That view shifted when U.K. data showed that child transmission of the B.1.1.7 variant increased by half to 9% of contacts in comparison with the original virus strain.
Dutch data, similarly, showed schools contributing to the latest outbreaks, and some European nations have closed schools. In the United States, the trend is to open them.
A version of this article first appeared on Medscape.com.
It’s likely the United States will see another surge of COVID-19 this winter, warned Christopher Murray, MD, director of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
Speaking at the national conference of State of Reform on April 8, Dr. Murray cited the seasonality of the SARS-CoV-2 virus, which wanes in the summer and waxes in the winter. The “optimistic forecast” of IHME, which has modeled the course of the pandemic for the past 13 months, is that daily deaths will rise a bit in the next month, then decline from May through August, he said.
“Summer should be fairly quiet in terms of COVID, if vaccinations rise and people don’t stop wearing masks,” Dr. Murray said.
But he added that “a considerable surge will occur over next winter,” because the new variants are more transmissible, and people will likely relax social distancing and mask wearing. The IHME predicts that the percentage of Americans who usually don masks will decline from 73% today to 21% by Aug. 1.
With a rapid decline in mask use and a rise in mobility, there will still be more than 1,000 deaths each day by July 1, Dr. Murray said. In a forecast released the day after Dr. Murray spoke, the IHME predicted that by Aug. 1, there will be a total of 618,523 U.S. deaths from COVID-19. Deaths could be as high as 696,651 if mobility among the vaccinated returns to prepandemic levels, the institute forecasts.
Based on cell phone data, Dr. Murray said, the amount of mobility in the United States has already risen to the level of March 2020, when the pandemic was just getting underway.
Decreased infections
If there’s one piece of good news in the latest IHME report, it’s that the estimated number of people infected (including those not tested) will drop from 111,581 today to a projected 17,502 on Aug. 1. But in a worst-case scenario, with sharply higher mobility among vaccinated people, the case count on that date would only fall to 73,842.
The SARS-CoV-2 variants are another factor of concern. Dr. Murray distinguished between variants like the one first identified in the U.K. (B.1.1.7) and other “escape variants.”
B.1.1.7, which is now the dominant strain in the United States, increases transmission but doesn’t necessarily escape the immune system or vaccines, he explained.
In contrast, if someone is infected with a variant such as the South African or the Brazilian mutations, he said, a previous COVID-19 infection might not protect the person, and vaccines are less effective against those variants.
Cross-variant immunity may range from 0% to 60% for escape variants, based on the slim amount of data now available, Dr. Murray said. In his view, these variants will be the long-term driver of the pandemic in the United States, while the United Kingdom variant is the short-term driver.
The latest data, he said, show that the Pfizer/BioNTech and Moderna vaccines are 75% effective against the escape variants, with lower efficacy for other vaccines. But booster shots may still be required to protect people against some variants.
Human factors
Human behavior will also help determine the course of the pandemic, he noted. Vaccine hesitancy, for example, is still high in the United States.
By the end of May, he predicted, about 180 million people will have received about two doses of vaccine. After that, he said, “vaccination will flatline due to lack of demand.” The two unknowns are how much campaigns to promote vaccination will increase vaccine confidence, and when children will be vaccinated.
In the United States, he said, 69% of adults have been vaccinated or want to get a shot. But that percentage has dropped 5 points since February, and vaccine confidence varies by state.
Dr. Murray emphasized that the winter surge he predicts can be blocked if people change their behaviors. These include a rise in vaccine confidence to 80% and continued mask wearing by most people.
However, if vaccine confidence and mask wearing decline, state governments continue to drop social distancing rules, and the uptake of boosters is low, the winter surge could be more serious, he said.
Double surge
Murray also raised the possibility of a double surge of COVID-19 and influenza this winter. Widely expected last winter, this double surge never materialized here or elsewhere, partly because of mask wearing. But Dr. Murray said it could happen this year: History shows that the flu tends to be stronger in years after weak outbreaks.
He advised hospitals to prepare now for whatever might come later this year. Public health authorities, he said, should speed up vaccination, monitor variants closely with additional sequencing, and try to modify behavior in high-risk groups.
Asked to explain the recent surge of COVID-19 cases in Michigan, Dr. Murray attributed it partly to the spread of the B.1.1.7 (U.K.) variant. But he noted that the U.K. variant has expanded even more widely in some other states that haven’t had an explosive surge like Michigan’s.
Moreover, he noted, Michigan doesn’t have low mask use or high mobility. So the upward spiral of COVID-19 infections there is very concerning, he said.
In regard to the role of children as reservoirs of the virus, Dr. Murray pointed out that views on this have changed around the world. For a while, people thought kids didn’t spread COVID-19 very much. That view shifted when U.K. data showed that child transmission of the B.1.1.7 variant increased by half to 9% of contacts in comparison with the original virus strain.
Dutch data, similarly, showed schools contributing to the latest outbreaks, and some European nations have closed schools. In the United States, the trend is to open them.
A version of this article first appeared on Medscape.com.
It’s likely the United States will see another surge of COVID-19 this winter, warned Christopher Murray, MD, director of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
Speaking at the national conference of State of Reform on April 8, Dr. Murray cited the seasonality of the SARS-CoV-2 virus, which wanes in the summer and waxes in the winter. The “optimistic forecast” of IHME, which has modeled the course of the pandemic for the past 13 months, is that daily deaths will rise a bit in the next month, then decline from May through August, he said.
“Summer should be fairly quiet in terms of COVID, if vaccinations rise and people don’t stop wearing masks,” Dr. Murray said.
But he added that “a considerable surge will occur over next winter,” because the new variants are more transmissible, and people will likely relax social distancing and mask wearing. The IHME predicts that the percentage of Americans who usually don masks will decline from 73% today to 21% by Aug. 1.
With a rapid decline in mask use and a rise in mobility, there will still be more than 1,000 deaths each day by July 1, Dr. Murray said. In a forecast released the day after Dr. Murray spoke, the IHME predicted that by Aug. 1, there will be a total of 618,523 U.S. deaths from COVID-19. Deaths could be as high as 696,651 if mobility among the vaccinated returns to prepandemic levels, the institute forecasts.
Based on cell phone data, Dr. Murray said, the amount of mobility in the United States has already risen to the level of March 2020, when the pandemic was just getting underway.
Decreased infections
If there’s one piece of good news in the latest IHME report, it’s that the estimated number of people infected (including those not tested) will drop from 111,581 today to a projected 17,502 on Aug. 1. But in a worst-case scenario, with sharply higher mobility among vaccinated people, the case count on that date would only fall to 73,842.
The SARS-CoV-2 variants are another factor of concern. Dr. Murray distinguished between variants like the one first identified in the U.K. (B.1.1.7) and other “escape variants.”
B.1.1.7, which is now the dominant strain in the United States, increases transmission but doesn’t necessarily escape the immune system or vaccines, he explained.
In contrast, if someone is infected with a variant such as the South African or the Brazilian mutations, he said, a previous COVID-19 infection might not protect the person, and vaccines are less effective against those variants.
Cross-variant immunity may range from 0% to 60% for escape variants, based on the slim amount of data now available, Dr. Murray said. In his view, these variants will be the long-term driver of the pandemic in the United States, while the United Kingdom variant is the short-term driver.
The latest data, he said, show that the Pfizer/BioNTech and Moderna vaccines are 75% effective against the escape variants, with lower efficacy for other vaccines. But booster shots may still be required to protect people against some variants.
Human factors
Human behavior will also help determine the course of the pandemic, he noted. Vaccine hesitancy, for example, is still high in the United States.
By the end of May, he predicted, about 180 million people will have received about two doses of vaccine. After that, he said, “vaccination will flatline due to lack of demand.” The two unknowns are how much campaigns to promote vaccination will increase vaccine confidence, and when children will be vaccinated.
In the United States, he said, 69% of adults have been vaccinated or want to get a shot. But that percentage has dropped 5 points since February, and vaccine confidence varies by state.
Dr. Murray emphasized that the winter surge he predicts can be blocked if people change their behaviors. These include a rise in vaccine confidence to 80% and continued mask wearing by most people.
However, if vaccine confidence and mask wearing decline, state governments continue to drop social distancing rules, and the uptake of boosters is low, the winter surge could be more serious, he said.
Double surge
Murray also raised the possibility of a double surge of COVID-19 and influenza this winter. Widely expected last winter, this double surge never materialized here or elsewhere, partly because of mask wearing. But Dr. Murray said it could happen this year: History shows that the flu tends to be stronger in years after weak outbreaks.
He advised hospitals to prepare now for whatever might come later this year. Public health authorities, he said, should speed up vaccination, monitor variants closely with additional sequencing, and try to modify behavior in high-risk groups.
Asked to explain the recent surge of COVID-19 cases in Michigan, Dr. Murray attributed it partly to the spread of the B.1.1.7 (U.K.) variant. But he noted that the U.K. variant has expanded even more widely in some other states that haven’t had an explosive surge like Michigan’s.
Moreover, he noted, Michigan doesn’t have low mask use or high mobility. So the upward spiral of COVID-19 infections there is very concerning, he said.
In regard to the role of children as reservoirs of the virus, Dr. Murray pointed out that views on this have changed around the world. For a while, people thought kids didn’t spread COVID-19 very much. That view shifted when U.K. data showed that child transmission of the B.1.1.7 variant increased by half to 9% of contacts in comparison with the original virus strain.
Dutch data, similarly, showed schools contributing to the latest outbreaks, and some European nations have closed schools. In the United States, the trend is to open them.
A version of this article first appeared on Medscape.com.
FDA, CDC urge pause of J&J COVID vaccine
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
Sarcoidosis: An FP’s primer on an enigmatic disease
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
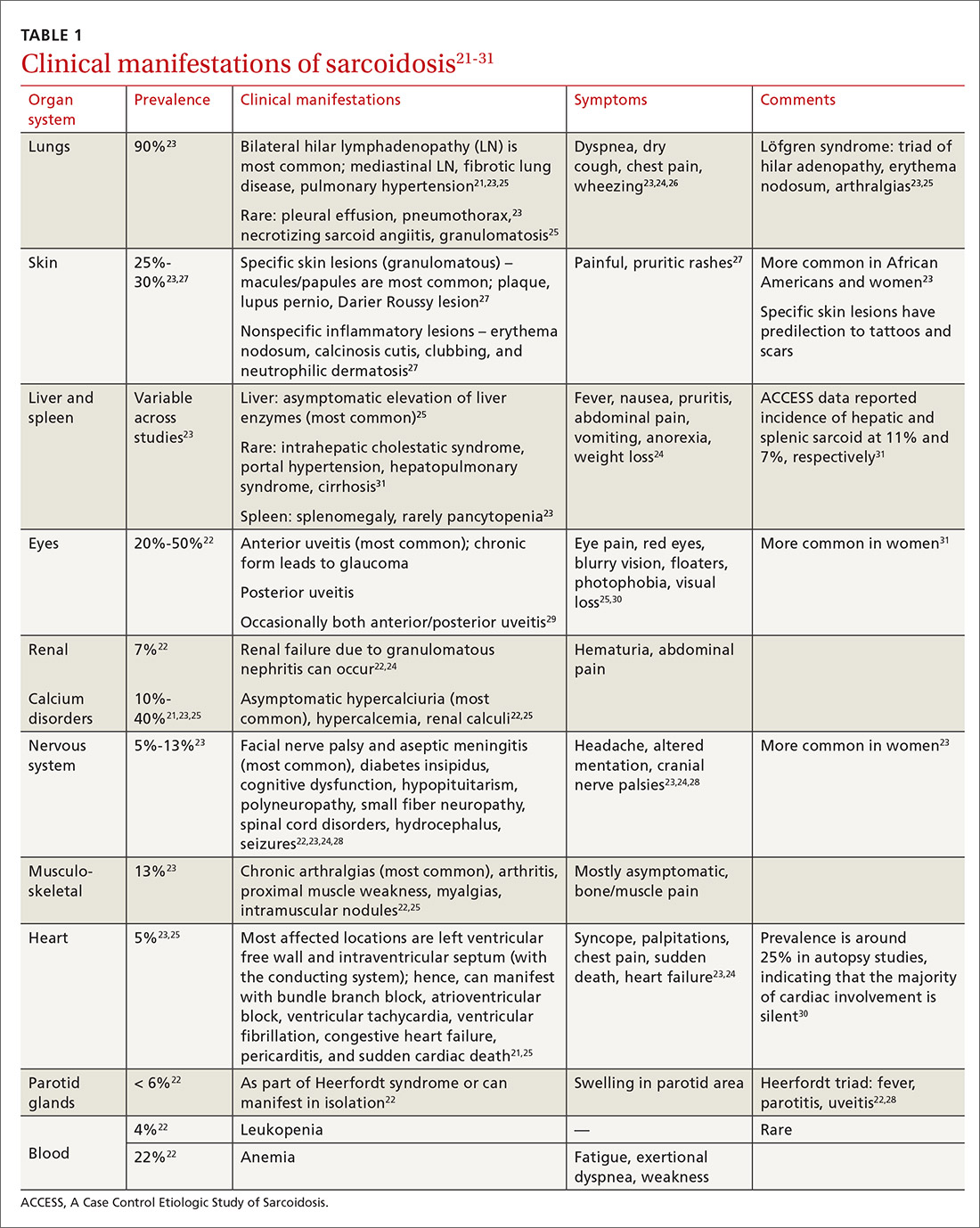
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
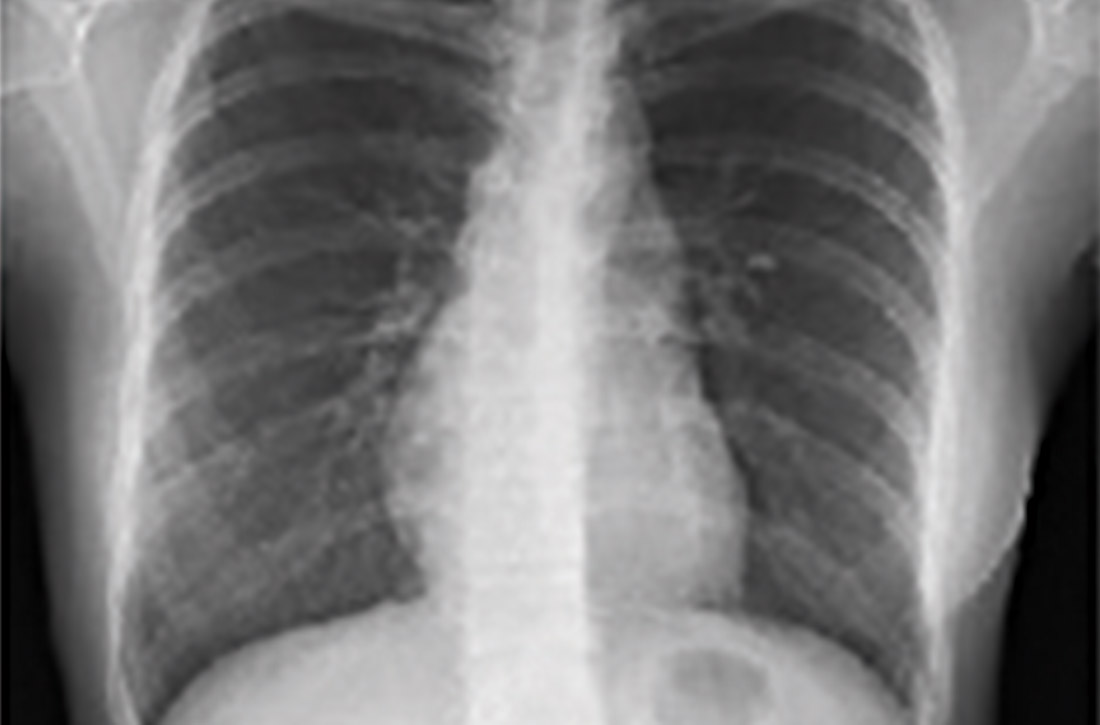
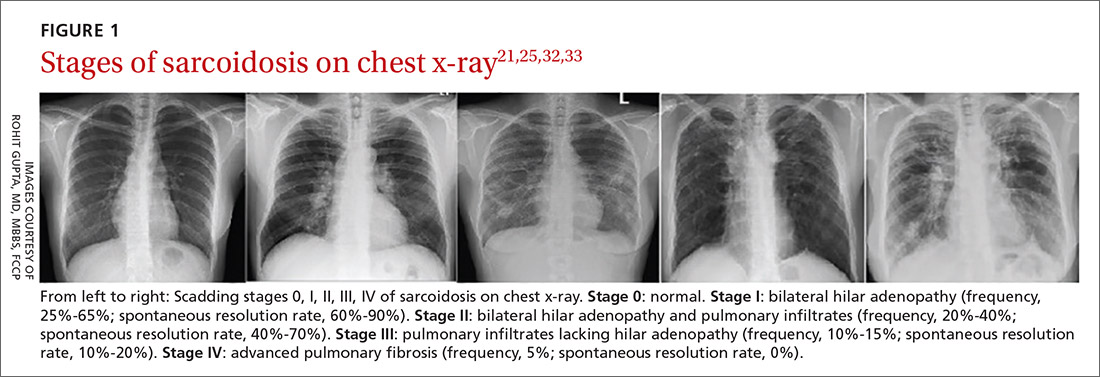
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
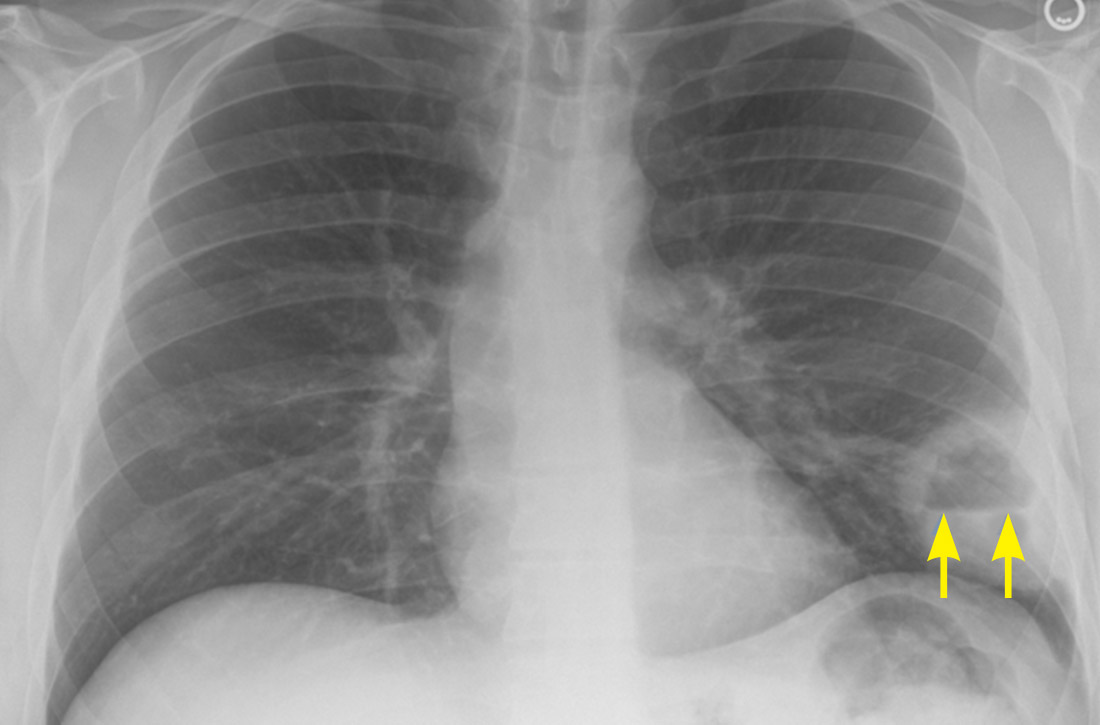
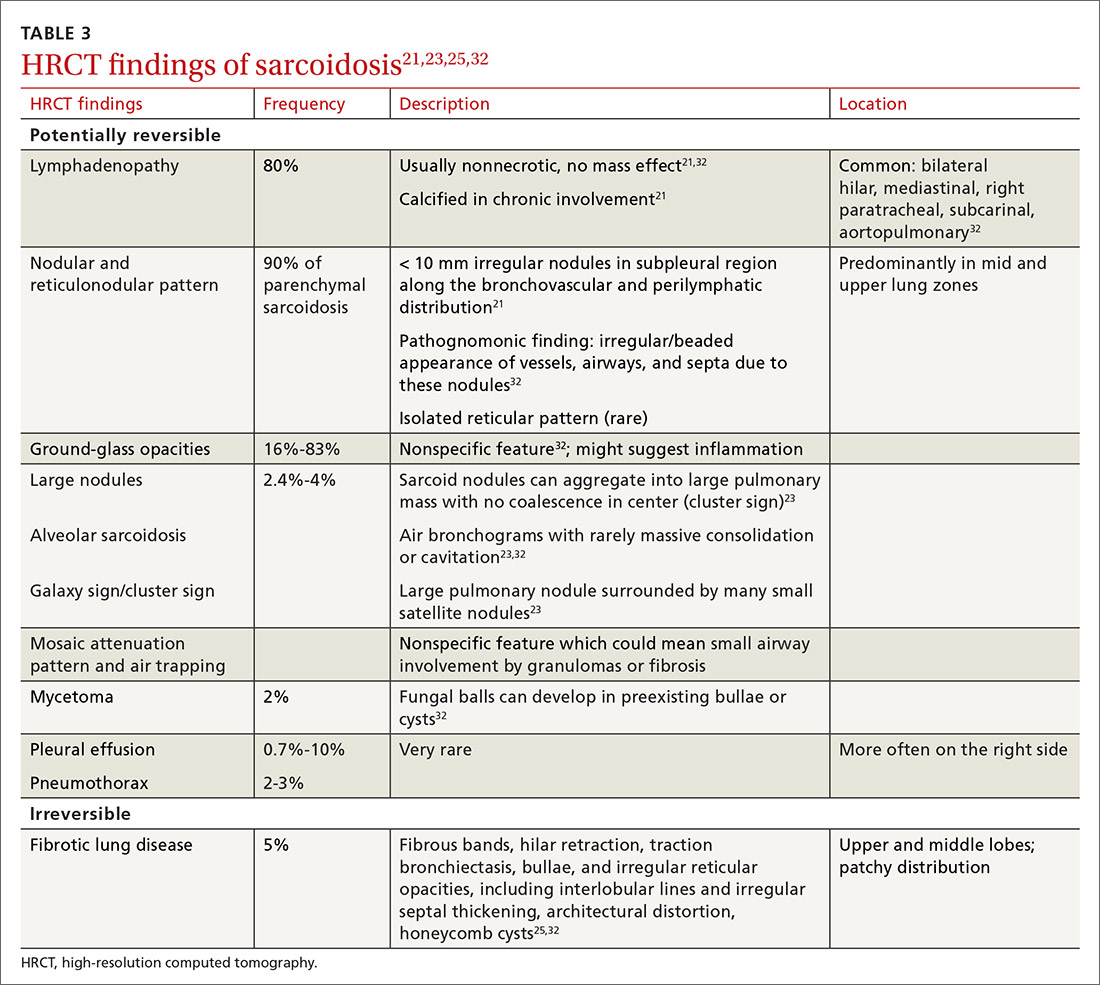
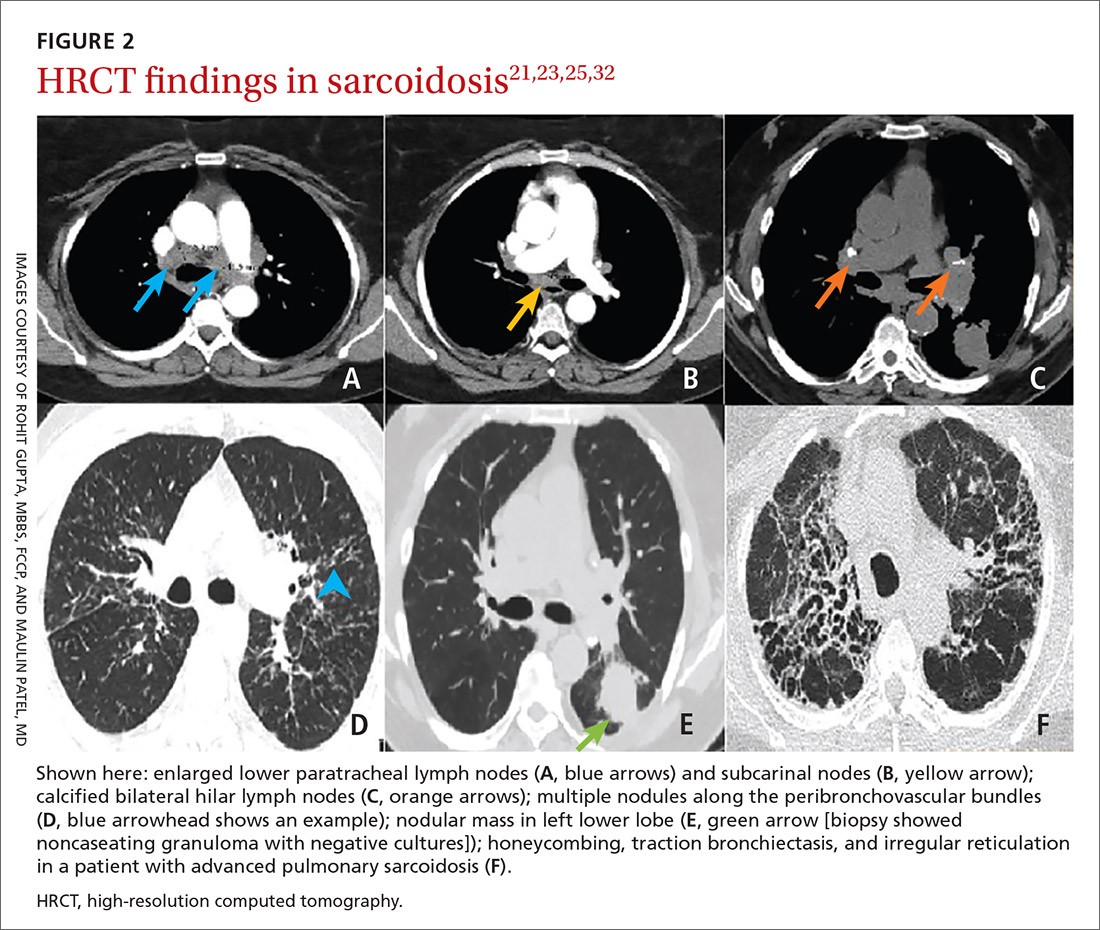
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
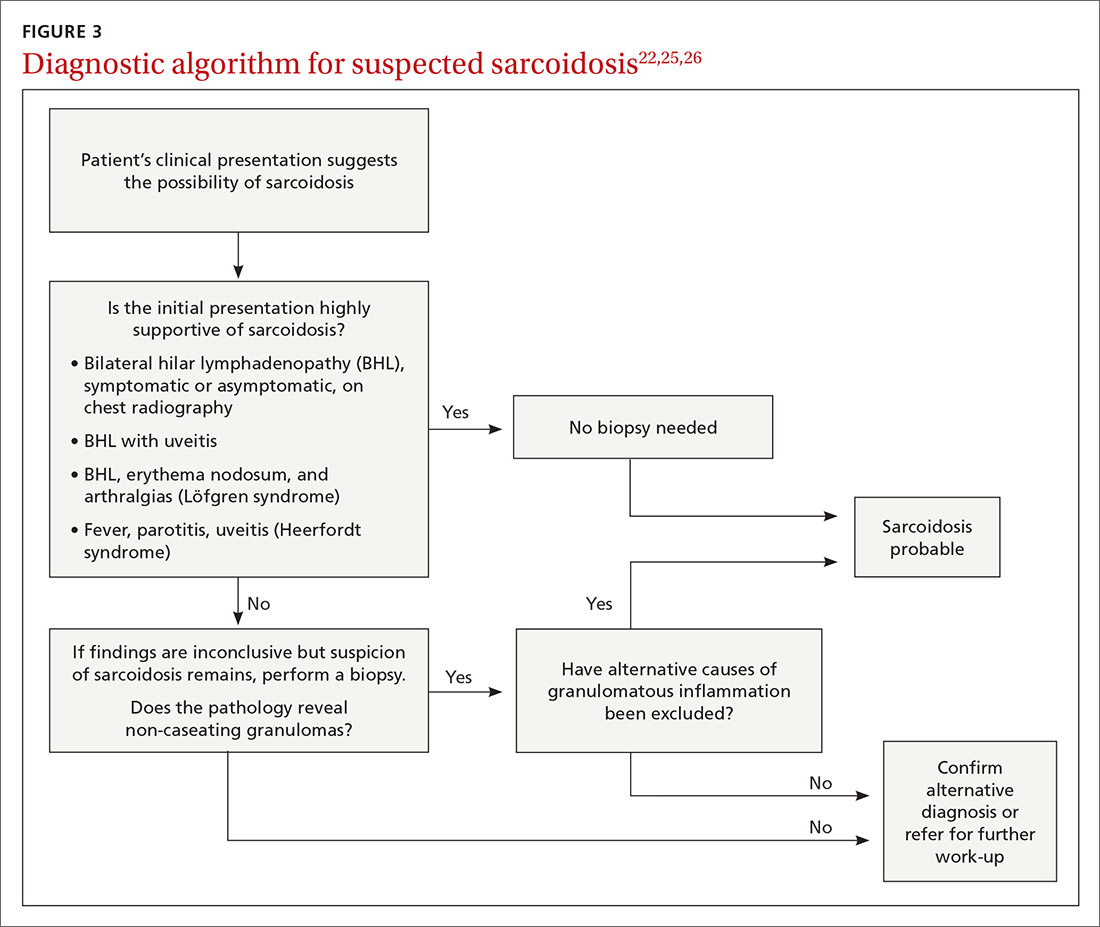
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
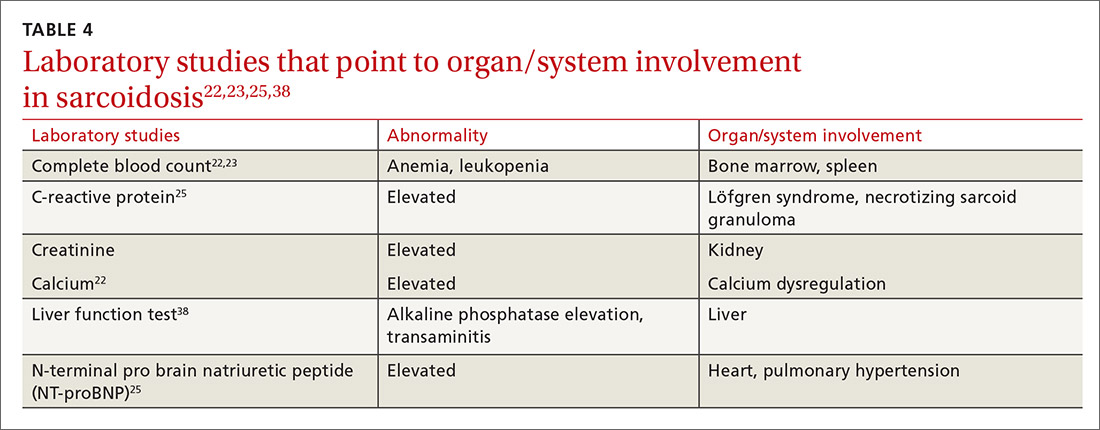
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
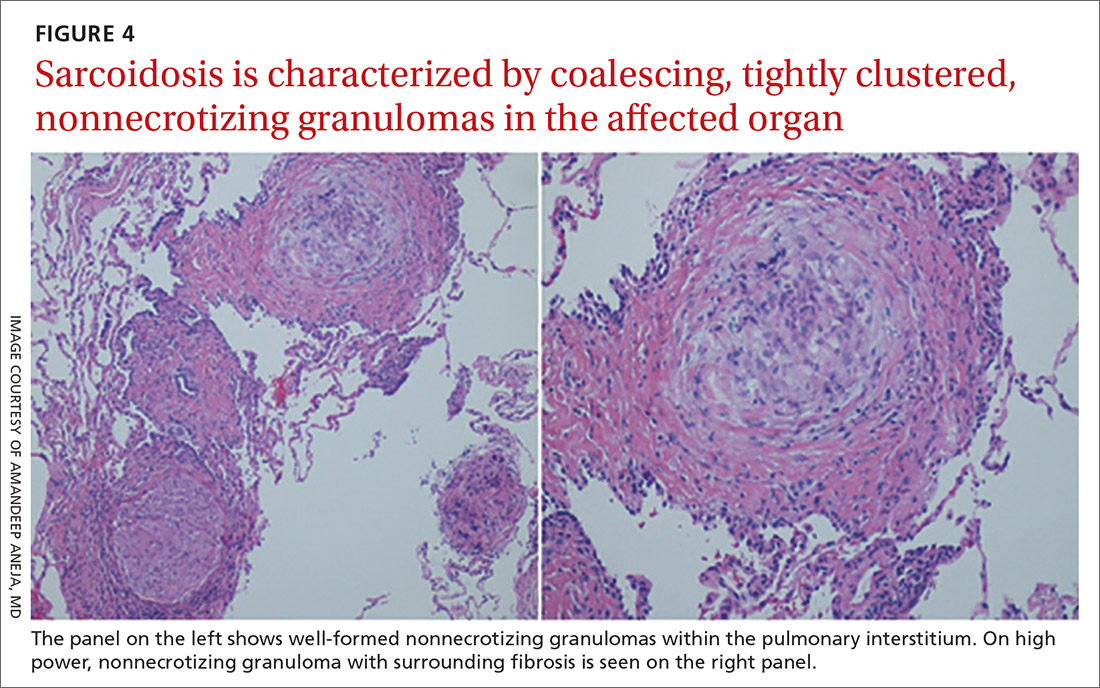
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
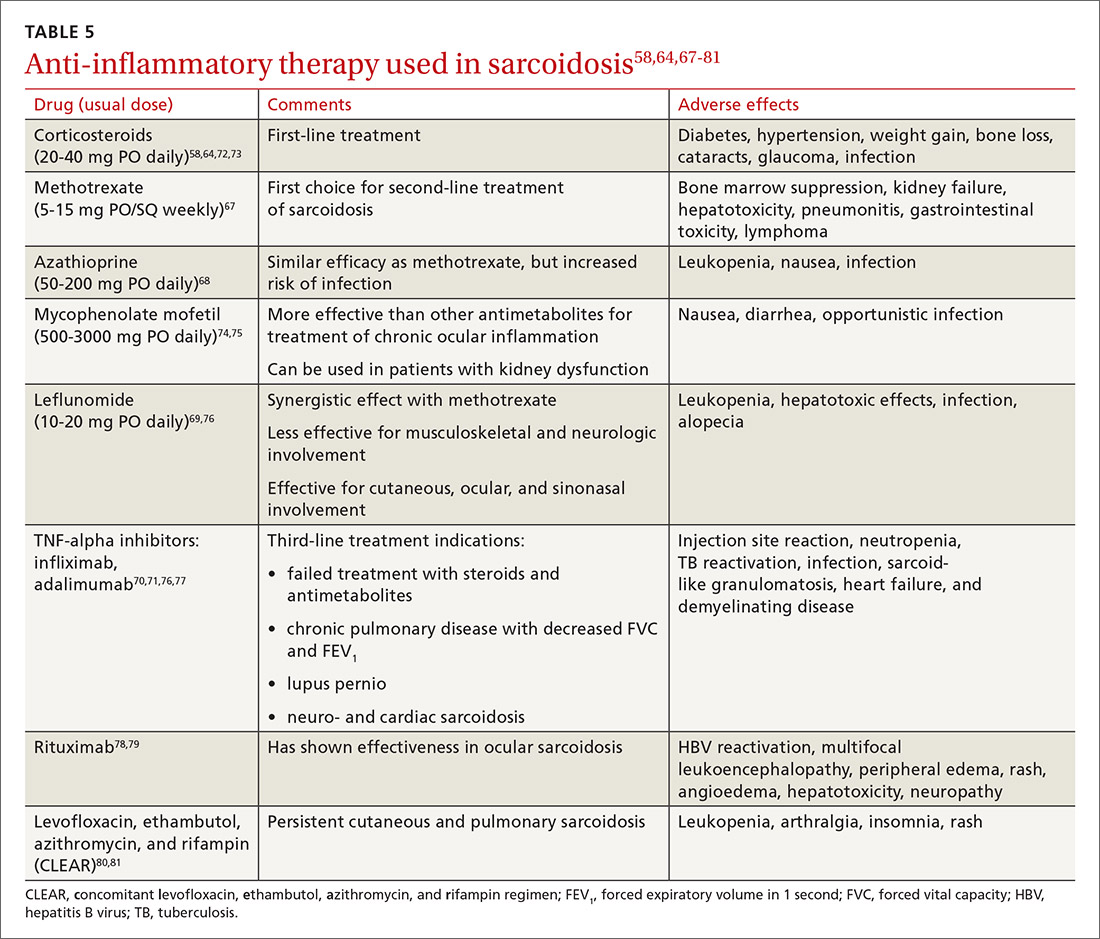
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22

Diagnostic work-up

Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.

Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.

Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.

Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26

Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21

Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.

In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81

The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22

Diagnostic work-up

Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.

Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.

Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.

Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26

Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21

Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.

In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81

The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
PRACTICE RECOMMENDATIONS
› Consider biopsy to aid in diagnosing sarcoidosis; it may be avoided with a high clinical suspicion for sarcoidosis (eg, Löfgren syndrome, lupus pernio, or Heerfordt syndrome). C
› Rule out alternative diagnoses such as infection, malignancy, collagen vascular disease, and vasculitis. C
› Identify extra-pulmonary organ involvement, as clinically indicated, by screening with a baseline eye examination; complete blood count; creatinine, alkaline phosphatase, and calcium levels; electrocardiogram, and other organ-specific studies. C
› Make a patient-centered decision whether to begin antiinflammatory treatment based on symptomatology and risk of organ failure or death. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Study IDs most common lingering symptoms 8 months after mild COVID
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
U.S. finally hits its stride with COVID-19 vaccination rollouts
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
37-year-old man • cough • increasing shortness of breath • pleuritic chest pain • Dx?
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.
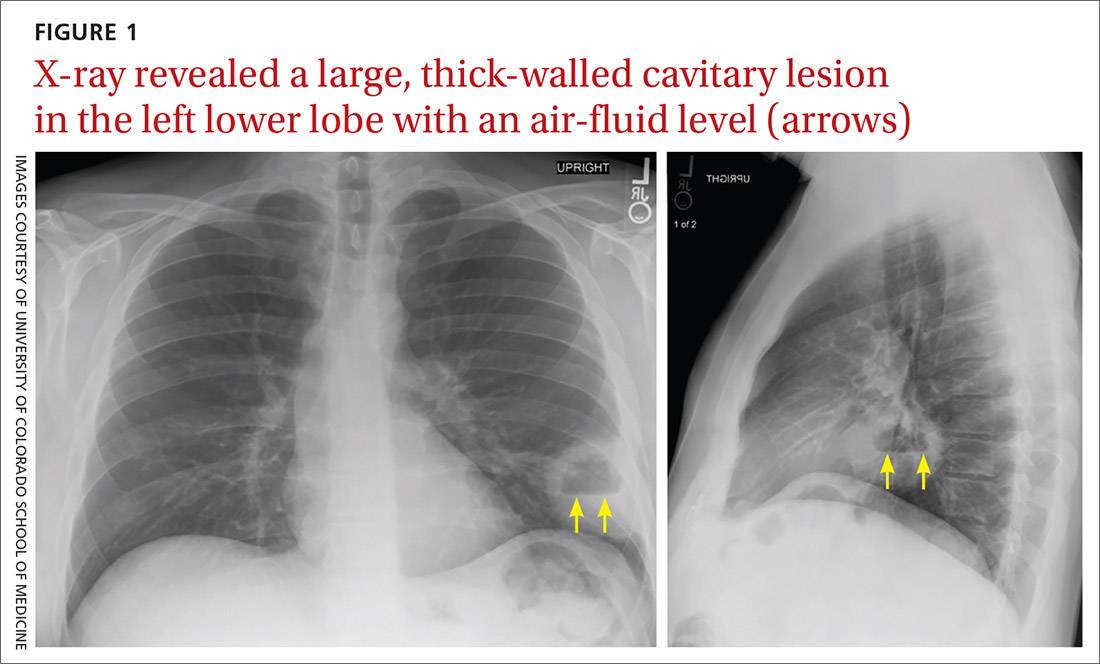
A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.
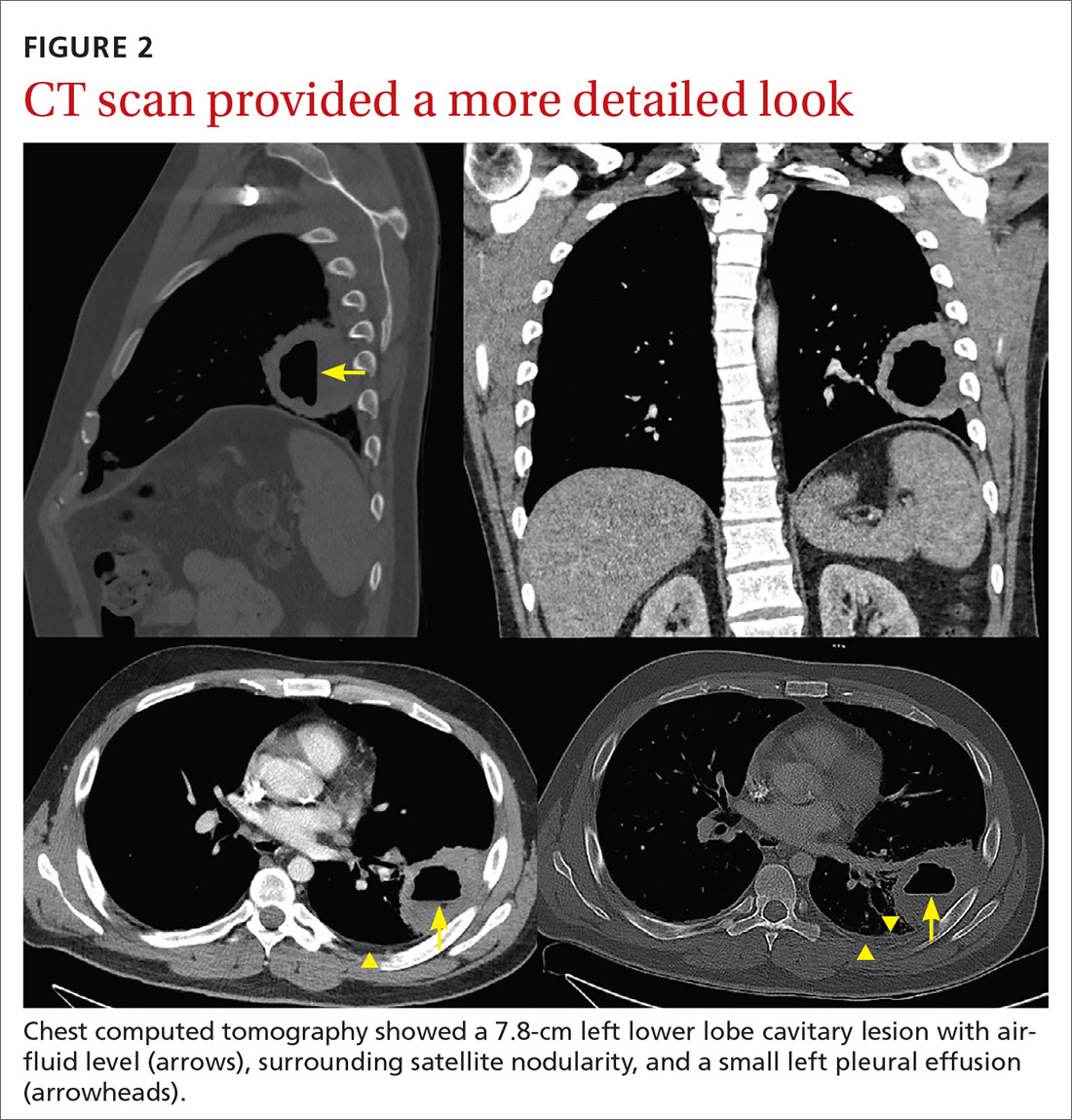
THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4
Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).
THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.

A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.

THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4

Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).

THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.

A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.

THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4

Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).

THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
‘Beyond a reasonable doubt’: COVID-19 brain health fallout is real, severe
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
List of COVID-19 high-risk comorbidities expanded
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
Risk for erectile dysfunction sixfold higher in men with COVID-19
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study suggests no added risk of blood clots in COVID-19 outpatients
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.