User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Physician offices should have bigger role in vaccine rollout: MGMA
Physician offices, which have been deemphasized in the COVID-19 vaccine rollout, should have a more prominent role in the effort going forward, said the Medical Group Management Association in a letter sent to President Joe Biden on Jan. 26.
“Due to our members’ role as community providers, we ask that the Administration include medical group practices in COVID-19 vaccine distribution strategies moving forward,” Halee Fischer-Wright, MD, president and CEO of MGMA, stated in the letter.
“Current vaccine efforts are haphazard at best and appear to rely on a passive first come first served approach with the public rushing to sign up for vaccines when scant supply becomes available,” MGMA noted. “This favors patients who can advocate for themselves or have family members able to do the same. Yet medical group practices already have patient relationships and experience vaccinating patients for influenza and other conditions.”
Moreover, physician practices have data on patient demographics, preexisting conditions, and risk factors. This is valuable information not available to hospitals, pharmacies, and state health departments, MGMA said.
“Furthermore, in a time of uncertainty and misinformation, patients are looking to their own physicians as a trusted source for information on vaccine safety and efficacy,” the letter stated. “Physician group practices can and should play a significant role in vaccine education.”
Despite these advantages of vaccinating patients in doctors’ offices, MGMA pointed out that “states have largely not leveraged physician practices in vaccine rollout efforts.”
In an MGMA survey conducted last week, 85% of independent practices and 45% of hospital- or health system–owned practices that sought COVID-19 vaccine for their patients were unable to obtain any. Of the practices able to get vaccine supplies, the majority said they had received only enough to vaccinate 1% or less of their patients.
Susan R. Bailey, MD, president of the American Medical Association commented in an interview that, “once enough supplies are available, we encourage the administration to ensure physician practices have an adequate supply of COVID-19 vaccines to vaccinate their patients. Physician practices will be an integral part of the vaccine administration process. Physicians are a trusted source of information for patients and their direct conversations and recommendations for patients to get vaccinated will help address hesitancy and result in more people getting vaccinated.”
Many groups, MGMA said, had been approved by their states to distribute the vaccine but received little or no inventory. Practice phone lines have been “flooded” by patients wanting to know why their physicians can’t vaccinate them.
Programs vary by state
In an interview, Dr. Fischer-Wright said that most practices want to vaccinate their patients. But only some states have set up programs that allow them to apply for the COVID-19 vaccines. “Most of our practices that were eligible for vaccination have applied for it,” she added.
The New York State Health Department is taking a different approach, according to Dial Hewlett Jr., MD, medical director for disease control services with the Westchester County Department of Health in White Plains, N.Y.. The state health department has designated specific sites across New York as vaccination hubs; in Westchester County, the hub is the Westchester Medical Center. When the hospital receives a vaccine shipment, it distributes some of it to smaller sites such as the county health department, which includes a vaccination clinic.
“So far, they haven’t gotten to the point where they’re distributing to pharmacies or doctors’ offices,” Dr. Hewlett said in an interview.
Right now, he said, the chief limiting factor is vaccine supply. When that expands, he said, physician offices will likely get more vaccine doses.
Both Dr. Hewlett and Dr. Fischer-Wright pointed out that physician offices are limited because they aren’t able to store the Pfizer vaccine, which requires ultracold freezers. “But now that we have the Moderna vaccine, 50% of the 200 million doses that have been promised can be delivered in a physician office,” said Dr. Fischer-Wright.
So why haven’t practices received more vaccine? Besides the inadequate supply across the nation, Dr. Fischer-Wright said, there have been difficulties in getting the vaccine to physician offices. Some MGMA members, she added, did receive vaccine supplies immediately. “These were independent practices that had over 200 physicians.”
Dr. Hewlett noted that some smaller practices have complained to the county department that they couldn’t obtain vaccine because they lacked the clout to compete with larger groups. “They’re not ordering enough product to make it a priority for whoever is involved with the distribution.”
Another problem – evident in the results of MGMA’s recent poll – is that health care systems that have vaccine supplies are sharing them with their own practices before they make any available to community practices.
“If you’re working for Northwell Health, you probably won’t have the kinds of challenges that the small mom-and-pop practice would have,” Dr. Hewlett said.
Overcoming vaccine hesitancy
More than a quarter of the U.S. population has indicated they are hesitant to get the COVID-19 vaccine. This is an area where Dr. Fischer-Wright believes physicians can help immensely.
“The benefit of having that type of activity occur in the physician office is that it’s a place where physicians have already established trust with patients,” she said. “And one of the reasons why some people don’t want a shot is that they don’t trust the vaccine. Having a human being that you have a relationship with provide you with the pros and cons is very compelling to get people to make an alternative choice.”
Physicians and their staff will also need to be educated before they administer the vaccine, Dr. Hewlett noted. “There will have to be education on the handling of the vaccine, but I think that can easily be done. Many practices have physician assistants and nurse practitioners who have been doing a lot of vaccinations in the office setting.”
Complex logistics
Based on the experience of his department’s vaccination clinic, which has been giving COVID-19 shots since Jan. 5, Dr. Hewlett said private practices have a lot to consider before they launch their own vaccination efforts.
To begin with, he said, “it’s a tricky situation with these vaccines that require two doses.” Before his clinic makes an appointment to vaccinate a patient, the scheduler has to make sure that the patient can return in 21 or 28 days, depending on whether they’re getting the Pfizer or Moderna vaccine.
“It’s difficult if they can’t show up 28 days after that date because we expect the same number of people to show up 28 days later for their second dose,” he said. “This is quite different from a standard medical practice. There aren’t too many situations where a person has to come back to the office after 28 days or 21 days.”
While the Centers for Disease Control and Prevention recently said the immunization schedule can be more flexible, Dr. Hewlett added, his clinic prefers to get patients back on the recommended schedule to make sure the vaccine will be maximally effective.
The clinic also has to follow state regulations requiring that all vaccines it receives be administered within a week of receipt. Right now, the clinic is open 6 days a week, giving about 300-400 shots a day. Each morning, a clerk records how many doses were administered the previous day, along with the lot numbers – and all data must be reported to the state.
The operation is fairly labor intensive. The clinic has a staff of about 30 people, most of whom are now engaged full time in the COVID-19 vaccination effort.
“We have people who check patients in and who screen to make sure no one has COVID symptoms. Other people escort patients to the vaccination stations. We have about 15 nurse practitioners and public health nurses who give the shots, and we have to make sure they’re accounting for every dose that’s given. And we have to make sure everybody getting a dose meets the eligibility criteria for shots,” he said. “We also have an area where patients are watched for 15 minutes after they’re vaccinated. Then there’s a group of five data entry people who locate appointment slots 28 days from today.”
It’s all still “a work in progress,” Dr. Hewlett said, but the staff who give COVID-19 shots and the patients who receive them are gratified to be making a difference.
A version of this article first appeared on Medscape.com.
Physician offices, which have been deemphasized in the COVID-19 vaccine rollout, should have a more prominent role in the effort going forward, said the Medical Group Management Association in a letter sent to President Joe Biden on Jan. 26.
“Due to our members’ role as community providers, we ask that the Administration include medical group practices in COVID-19 vaccine distribution strategies moving forward,” Halee Fischer-Wright, MD, president and CEO of MGMA, stated in the letter.
“Current vaccine efforts are haphazard at best and appear to rely on a passive first come first served approach with the public rushing to sign up for vaccines when scant supply becomes available,” MGMA noted. “This favors patients who can advocate for themselves or have family members able to do the same. Yet medical group practices already have patient relationships and experience vaccinating patients for influenza and other conditions.”
Moreover, physician practices have data on patient demographics, preexisting conditions, and risk factors. This is valuable information not available to hospitals, pharmacies, and state health departments, MGMA said.
“Furthermore, in a time of uncertainty and misinformation, patients are looking to their own physicians as a trusted source for information on vaccine safety and efficacy,” the letter stated. “Physician group practices can and should play a significant role in vaccine education.”
Despite these advantages of vaccinating patients in doctors’ offices, MGMA pointed out that “states have largely not leveraged physician practices in vaccine rollout efforts.”
In an MGMA survey conducted last week, 85% of independent practices and 45% of hospital- or health system–owned practices that sought COVID-19 vaccine for their patients were unable to obtain any. Of the practices able to get vaccine supplies, the majority said they had received only enough to vaccinate 1% or less of their patients.
Susan R. Bailey, MD, president of the American Medical Association commented in an interview that, “once enough supplies are available, we encourage the administration to ensure physician practices have an adequate supply of COVID-19 vaccines to vaccinate their patients. Physician practices will be an integral part of the vaccine administration process. Physicians are a trusted source of information for patients and their direct conversations and recommendations for patients to get vaccinated will help address hesitancy and result in more people getting vaccinated.”
Many groups, MGMA said, had been approved by their states to distribute the vaccine but received little or no inventory. Practice phone lines have been “flooded” by patients wanting to know why their physicians can’t vaccinate them.
Programs vary by state
In an interview, Dr. Fischer-Wright said that most practices want to vaccinate their patients. But only some states have set up programs that allow them to apply for the COVID-19 vaccines. “Most of our practices that were eligible for vaccination have applied for it,” she added.
The New York State Health Department is taking a different approach, according to Dial Hewlett Jr., MD, medical director for disease control services with the Westchester County Department of Health in White Plains, N.Y.. The state health department has designated specific sites across New York as vaccination hubs; in Westchester County, the hub is the Westchester Medical Center. When the hospital receives a vaccine shipment, it distributes some of it to smaller sites such as the county health department, which includes a vaccination clinic.
“So far, they haven’t gotten to the point where they’re distributing to pharmacies or doctors’ offices,” Dr. Hewlett said in an interview.
Right now, he said, the chief limiting factor is vaccine supply. When that expands, he said, physician offices will likely get more vaccine doses.
Both Dr. Hewlett and Dr. Fischer-Wright pointed out that physician offices are limited because they aren’t able to store the Pfizer vaccine, which requires ultracold freezers. “But now that we have the Moderna vaccine, 50% of the 200 million doses that have been promised can be delivered in a physician office,” said Dr. Fischer-Wright.
So why haven’t practices received more vaccine? Besides the inadequate supply across the nation, Dr. Fischer-Wright said, there have been difficulties in getting the vaccine to physician offices. Some MGMA members, she added, did receive vaccine supplies immediately. “These were independent practices that had over 200 physicians.”
Dr. Hewlett noted that some smaller practices have complained to the county department that they couldn’t obtain vaccine because they lacked the clout to compete with larger groups. “They’re not ordering enough product to make it a priority for whoever is involved with the distribution.”
Another problem – evident in the results of MGMA’s recent poll – is that health care systems that have vaccine supplies are sharing them with their own practices before they make any available to community practices.
“If you’re working for Northwell Health, you probably won’t have the kinds of challenges that the small mom-and-pop practice would have,” Dr. Hewlett said.
Overcoming vaccine hesitancy
More than a quarter of the U.S. population has indicated they are hesitant to get the COVID-19 vaccine. This is an area where Dr. Fischer-Wright believes physicians can help immensely.
“The benefit of having that type of activity occur in the physician office is that it’s a place where physicians have already established trust with patients,” she said. “And one of the reasons why some people don’t want a shot is that they don’t trust the vaccine. Having a human being that you have a relationship with provide you with the pros and cons is very compelling to get people to make an alternative choice.”
Physicians and their staff will also need to be educated before they administer the vaccine, Dr. Hewlett noted. “There will have to be education on the handling of the vaccine, but I think that can easily be done. Many practices have physician assistants and nurse practitioners who have been doing a lot of vaccinations in the office setting.”
Complex logistics
Based on the experience of his department’s vaccination clinic, which has been giving COVID-19 shots since Jan. 5, Dr. Hewlett said private practices have a lot to consider before they launch their own vaccination efforts.
To begin with, he said, “it’s a tricky situation with these vaccines that require two doses.” Before his clinic makes an appointment to vaccinate a patient, the scheduler has to make sure that the patient can return in 21 or 28 days, depending on whether they’re getting the Pfizer or Moderna vaccine.
“It’s difficult if they can’t show up 28 days after that date because we expect the same number of people to show up 28 days later for their second dose,” he said. “This is quite different from a standard medical practice. There aren’t too many situations where a person has to come back to the office after 28 days or 21 days.”
While the Centers for Disease Control and Prevention recently said the immunization schedule can be more flexible, Dr. Hewlett added, his clinic prefers to get patients back on the recommended schedule to make sure the vaccine will be maximally effective.
The clinic also has to follow state regulations requiring that all vaccines it receives be administered within a week of receipt. Right now, the clinic is open 6 days a week, giving about 300-400 shots a day. Each morning, a clerk records how many doses were administered the previous day, along with the lot numbers – and all data must be reported to the state.
The operation is fairly labor intensive. The clinic has a staff of about 30 people, most of whom are now engaged full time in the COVID-19 vaccination effort.
“We have people who check patients in and who screen to make sure no one has COVID symptoms. Other people escort patients to the vaccination stations. We have about 15 nurse practitioners and public health nurses who give the shots, and we have to make sure they’re accounting for every dose that’s given. And we have to make sure everybody getting a dose meets the eligibility criteria for shots,” he said. “We also have an area where patients are watched for 15 minutes after they’re vaccinated. Then there’s a group of five data entry people who locate appointment slots 28 days from today.”
It’s all still “a work in progress,” Dr. Hewlett said, but the staff who give COVID-19 shots and the patients who receive them are gratified to be making a difference.
A version of this article first appeared on Medscape.com.
Physician offices, which have been deemphasized in the COVID-19 vaccine rollout, should have a more prominent role in the effort going forward, said the Medical Group Management Association in a letter sent to President Joe Biden on Jan. 26.
“Due to our members’ role as community providers, we ask that the Administration include medical group practices in COVID-19 vaccine distribution strategies moving forward,” Halee Fischer-Wright, MD, president and CEO of MGMA, stated in the letter.
“Current vaccine efforts are haphazard at best and appear to rely on a passive first come first served approach with the public rushing to sign up for vaccines when scant supply becomes available,” MGMA noted. “This favors patients who can advocate for themselves or have family members able to do the same. Yet medical group practices already have patient relationships and experience vaccinating patients for influenza and other conditions.”
Moreover, physician practices have data on patient demographics, preexisting conditions, and risk factors. This is valuable information not available to hospitals, pharmacies, and state health departments, MGMA said.
“Furthermore, in a time of uncertainty and misinformation, patients are looking to their own physicians as a trusted source for information on vaccine safety and efficacy,” the letter stated. “Physician group practices can and should play a significant role in vaccine education.”
Despite these advantages of vaccinating patients in doctors’ offices, MGMA pointed out that “states have largely not leveraged physician practices in vaccine rollout efforts.”
In an MGMA survey conducted last week, 85% of independent practices and 45% of hospital- or health system–owned practices that sought COVID-19 vaccine for their patients were unable to obtain any. Of the practices able to get vaccine supplies, the majority said they had received only enough to vaccinate 1% or less of their patients.
Susan R. Bailey, MD, president of the American Medical Association commented in an interview that, “once enough supplies are available, we encourage the administration to ensure physician practices have an adequate supply of COVID-19 vaccines to vaccinate their patients. Physician practices will be an integral part of the vaccine administration process. Physicians are a trusted source of information for patients and their direct conversations and recommendations for patients to get vaccinated will help address hesitancy and result in more people getting vaccinated.”
Many groups, MGMA said, had been approved by their states to distribute the vaccine but received little or no inventory. Practice phone lines have been “flooded” by patients wanting to know why their physicians can’t vaccinate them.
Programs vary by state
In an interview, Dr. Fischer-Wright said that most practices want to vaccinate their patients. But only some states have set up programs that allow them to apply for the COVID-19 vaccines. “Most of our practices that were eligible for vaccination have applied for it,” she added.
The New York State Health Department is taking a different approach, according to Dial Hewlett Jr., MD, medical director for disease control services with the Westchester County Department of Health in White Plains, N.Y.. The state health department has designated specific sites across New York as vaccination hubs; in Westchester County, the hub is the Westchester Medical Center. When the hospital receives a vaccine shipment, it distributes some of it to smaller sites such as the county health department, which includes a vaccination clinic.
“So far, they haven’t gotten to the point where they’re distributing to pharmacies or doctors’ offices,” Dr. Hewlett said in an interview.
Right now, he said, the chief limiting factor is vaccine supply. When that expands, he said, physician offices will likely get more vaccine doses.
Both Dr. Hewlett and Dr. Fischer-Wright pointed out that physician offices are limited because they aren’t able to store the Pfizer vaccine, which requires ultracold freezers. “But now that we have the Moderna vaccine, 50% of the 200 million doses that have been promised can be delivered in a physician office,” said Dr. Fischer-Wright.
So why haven’t practices received more vaccine? Besides the inadequate supply across the nation, Dr. Fischer-Wright said, there have been difficulties in getting the vaccine to physician offices. Some MGMA members, she added, did receive vaccine supplies immediately. “These were independent practices that had over 200 physicians.”
Dr. Hewlett noted that some smaller practices have complained to the county department that they couldn’t obtain vaccine because they lacked the clout to compete with larger groups. “They’re not ordering enough product to make it a priority for whoever is involved with the distribution.”
Another problem – evident in the results of MGMA’s recent poll – is that health care systems that have vaccine supplies are sharing them with their own practices before they make any available to community practices.
“If you’re working for Northwell Health, you probably won’t have the kinds of challenges that the small mom-and-pop practice would have,” Dr. Hewlett said.
Overcoming vaccine hesitancy
More than a quarter of the U.S. population has indicated they are hesitant to get the COVID-19 vaccine. This is an area where Dr. Fischer-Wright believes physicians can help immensely.
“The benefit of having that type of activity occur in the physician office is that it’s a place where physicians have already established trust with patients,” she said. “And one of the reasons why some people don’t want a shot is that they don’t trust the vaccine. Having a human being that you have a relationship with provide you with the pros and cons is very compelling to get people to make an alternative choice.”
Physicians and their staff will also need to be educated before they administer the vaccine, Dr. Hewlett noted. “There will have to be education on the handling of the vaccine, but I think that can easily be done. Many practices have physician assistants and nurse practitioners who have been doing a lot of vaccinations in the office setting.”
Complex logistics
Based on the experience of his department’s vaccination clinic, which has been giving COVID-19 shots since Jan. 5, Dr. Hewlett said private practices have a lot to consider before they launch their own vaccination efforts.
To begin with, he said, “it’s a tricky situation with these vaccines that require two doses.” Before his clinic makes an appointment to vaccinate a patient, the scheduler has to make sure that the patient can return in 21 or 28 days, depending on whether they’re getting the Pfizer or Moderna vaccine.
“It’s difficult if they can’t show up 28 days after that date because we expect the same number of people to show up 28 days later for their second dose,” he said. “This is quite different from a standard medical practice. There aren’t too many situations where a person has to come back to the office after 28 days or 21 days.”
While the Centers for Disease Control and Prevention recently said the immunization schedule can be more flexible, Dr. Hewlett added, his clinic prefers to get patients back on the recommended schedule to make sure the vaccine will be maximally effective.
The clinic also has to follow state regulations requiring that all vaccines it receives be administered within a week of receipt. Right now, the clinic is open 6 days a week, giving about 300-400 shots a day. Each morning, a clerk records how many doses were administered the previous day, along with the lot numbers – and all data must be reported to the state.
The operation is fairly labor intensive. The clinic has a staff of about 30 people, most of whom are now engaged full time in the COVID-19 vaccination effort.
“We have people who check patients in and who screen to make sure no one has COVID symptoms. Other people escort patients to the vaccination stations. We have about 15 nurse practitioners and public health nurses who give the shots, and we have to make sure they’re accounting for every dose that’s given. And we have to make sure everybody getting a dose meets the eligibility criteria for shots,” he said. “We also have an area where patients are watched for 15 minutes after they’re vaccinated. Then there’s a group of five data entry people who locate appointment slots 28 days from today.”
It’s all still “a work in progress,” Dr. Hewlett said, but the staff who give COVID-19 shots and the patients who receive them are gratified to be making a difference.
A version of this article first appeared on Medscape.com.
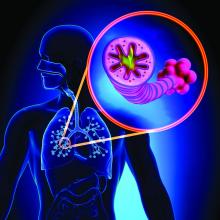
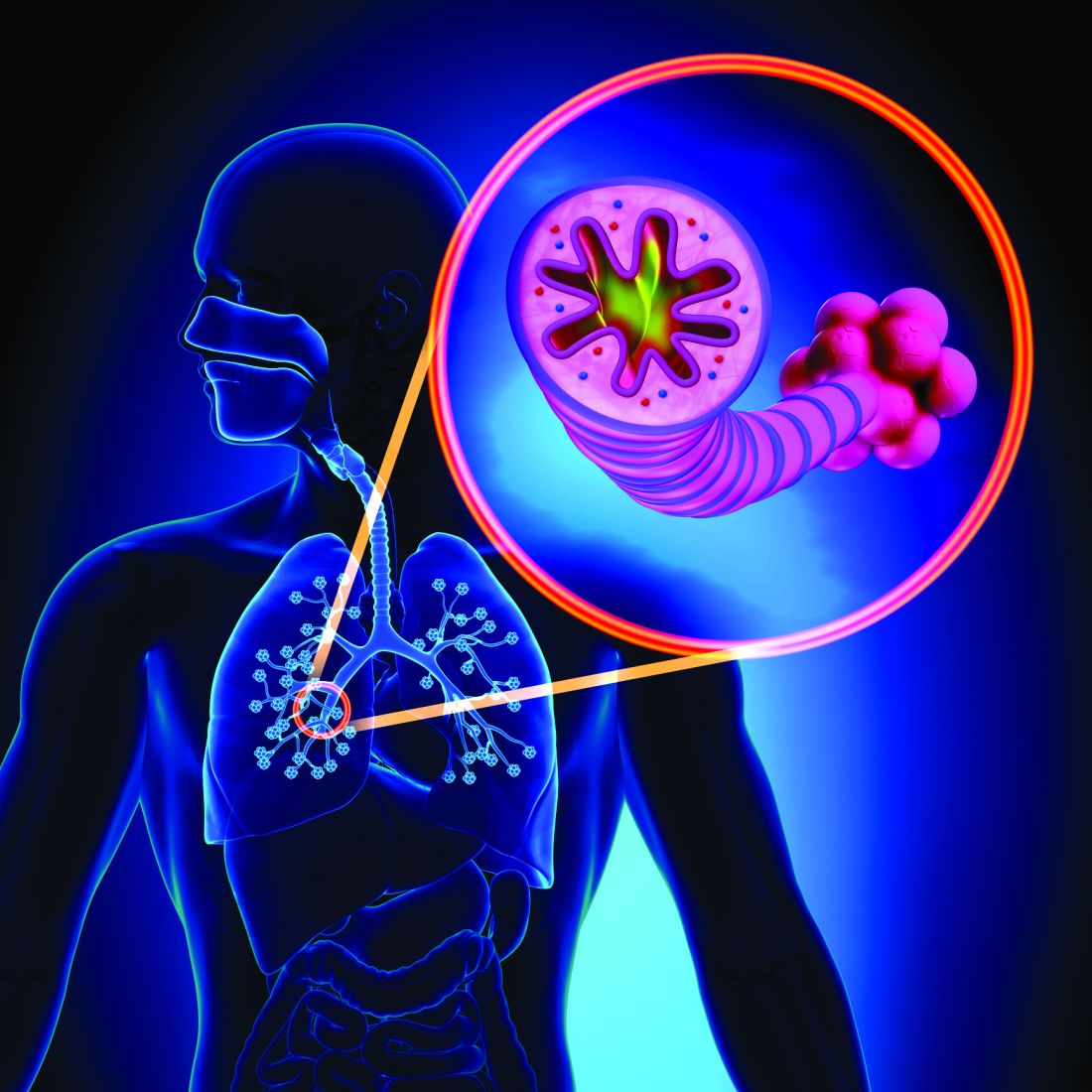
Doctors search for missing link between COVID-19 and ITP
Hospitalist Sarah Stone, MD, arrived for her day shift at Sharp Chula Vista one day in late December. The ICU and hospital wards were still overflowing with COVID-19 patients. But over the previous couple of months, she’d also seen more and more recovered patients presenting with a myriad of symptoms: pulmonary emboli, cardiomyopathy, a shocking case of aspergillosis, and those rare cases of “long COVID,” the patients who just can’t get better.
This morning it was a woman in her 30s. She felt fine, but 2 weeks after recovering from COVID-19, she had unexplained bruising on her arm, a petechiae rash on her legs, and her gums were bleeding. Once admitted to the emergency department, her platelet count of 5000/mm3 was a dead giveaway of immune thrombocytopenic purpura (ITP).
In Dr. Stone’s experience, new and otherwise unexplained symptoms so soon post COVID-19 can’t be written off as a coincidence without some additional consideration. But a quick preliminary search of the literature during her rounds came up almost empty. She found one report with three cases of post-COVID-19 ITP. But other online resources made no mention of it. Kenneth Johnson, MD, the hematologist/oncologist consulting on the new case, told Dr. Stone he’d seen one other case of post-COVID-19 ITP only earlier that month. Dr. Stone called a sister hospital. They’d seen one other case just weeks before.
“I was surprised to find just three cases in the literature when we had seen three among us in a matter of weeks,” Dr. Stone said in an interview. Something was missing.
A missing link
ITP is caused by an immune reaction against a patient’s own platelets.
“We know that infections like influenza can cause ITP, so in this light, [COVID-19-associated ITP] might not be surprising,” Gerard Jansen, MD, PhD, an internist and hematologist in Rotterdam, the Netherlands, said in an interview.
Dr. Jansen and colleagues recorded three cases of post-COVID-19 ITP in May 2020 – the report Dr. Stone had found during her shift. Two patients developed ITP several weeks after COVID-19 and responded to treatment with corticosteroids and intravenous immunoglobulin G (IVIG). The third patient, however, died of intracerebral bleeding while still battling COVID-19. He was retrospectively diagnosed with COVID-19-associated ITP.
A deeper dive into the literature uncovers additional case reports from India, France, the United Kingdom, Turkey, and one from China as early as January 2020. A September 2020 review of ITP secondary to COVID-19 included 23 papers and a total of 45 patients. The review authors noted that more than 70% of cases occurred in patients who were aged over 50 years and 75% had had moderate to severe COVID-19 infections. However, the sample size of 45 is too small to definitively describe what’s happening in the overall population.
ITP’s link to COVID-19 gained a media spotlight after the Miami obstetrician, Gregory Michael, MD, developed ITP days after getting the Pfizer COVID-19 vaccine. In early January, after 2 weeks in the ICU, Dr. Michael died of a hemorrhagic stroke caused by the low platelet count.
Pfizer said in a statement that the company is “actively investigating” the case, “but we don’t believe at this time that there is any direct connection to the vaccine.” Other experts have said the timing, particularly in a relatively young and healthy man, means a link to the vaccine is possible or even likely, but final results won›t be known until the Centers for Disease Control and Prevention finishes its investigation.
But “it is quite unusual to die from ITP,” San Diego hematologist Dr. Johnson said in an interview. In his more than 20 years of practice, he has never had a patient die from the condition.
For his part, Dr. Jansen, the hematologist in Rotterdam, said that at this point we just don’t know if there’s a link between the vaccine and ITP. Both infection and drugs are well established causes of ITP, so with that general mechanism or pathology in mind it makes sense that COVID-19 and the vaccine could instigate ITP. But it would be very difficult to prove in just one instance, he said. And considering the millions who have thus far received the vaccine without incident, and the known risks and dangers of COVID-19, “we still advise to vaccinate,” he said.
The number of cases is underestimated
Since his original case report in May, Dr. Jansen has seen five or so additional cases. But the causal link between the coronavirus and the hematologic symptoms is still undefined. “We don’t know much about platelet counts in COVID-19 at all,” he said. It could be that COVID-19 somehow inhibits platelet production or that it kills existing platelets. Whatever the exact relationship to the virus, Dr. Jansen expects that the true number of COVID-19-related ITP cases is higher than current estimates suggest.
One reason it isn’t coming up more often, Dr. Jansen said, may be that the cause of ITP in COVID-19 patients is hard to pin down. In the case report from May, Dr. Jansen and colleagues wrote: “And there are numerous other factors that can cause thrombocytopenia where COVID is concerned. For instance the coagulation activation by COVID‐19 infection leading to disseminated intravascular coagulation (DIC) and subsequent thrombocytopenia. Also, treatments for COVID‐19, including heparin, azithromycin and hydroxychloroquine, may lead to thrombocytopenia.”
Tracking and understanding COVID-19-associated ITP first requires the extensive process of elimination needed to diagnose it.
In addition, drugs used to treat COVID-19 could be masking COVID-19-related ITP. “Dexamethasone is a mainstay of COVID treatment. And it’s how we treat ITP,” Dr. Johnson said, which means physicians may be treating ITP without even registering it. And that’s one hypothesis for why Dr. Stone and Dr. Johnson didn’t see a case until 9 months into the pandemic.
Treating COVID-19-associated ITP also has its challenges, particularly in patients who develop it during an acute COVID-19 infection and are at risk for both internal bleeding and thrombosis. This was the case for the third patient in Dr. Jansen’s case report. The patient developed a pulmonary embolism and had a falling platelet count. He was given a platelet infusion and then an anticoagulant for the thrombosis. But a retrospective look at the case revealed the transfusion “did not increase numbers at all – which suggests ITP,” Dr. Jansen said. Intracerebral bleeding was the cause of death.
That’s why “it’s important to be aware of this phenomenon,” Dr. Jansen said of COVID-19-associated ITP. If a transfusion is unsuccessful, consider that the patient may have ITP and adjust. Dr. Johnson hasn’t had to treat a patient battling both complications simultaneously but says the ideal course of action would be to raise platelets with steroids and IVIG and then give the anticoagulant once the platelet count is higher. But reality is rarely ideal. Often these two treatments will have to be given concurrently since the patient faces two life-threatening risks, he said. “It’s a very challenging situation,” he said.
The good news is that standard treatments for ITP seem to work for COVID-19-associated ITP. The 30-year-old patient of Dr. Stone and Dr. Johnson responded so well to intravenous steroids that IVIG was unnecessary. She’s now on a slow prednisone taper and maintains platelet counts at 114,000/mm3 at her weekly follow-up appointments with Dr. Johnson.
Meanwhile, Dr. Jansen’s two other patients, now nearly a year out of treatment, require no additional medication. One of the patients is fully recovered and, though the other still has lower than normal platelet counts, she has no bleeding symptoms and her platelet counts remain stable. Still, Dr. Jansen is anxious for more data looking at the platelet counts in every COVID-19 patient and to combine findings from existing COVID-19-associated ITP patients.
For Dr. Stone, she says she’s added one COVID-19-associated complication to her belt. One less aftereffect will catch her off guard. And she wants others to have the same information.
“It’s just a little bit daunting. We don’t know how bad post-COVID will be,” she said. “There’s so many levels to this disease. Some people deal with it for so long and some people just get better and move on – we think ... so far.”
A version of this article first appeared on Medscape.com.
Hospitalist Sarah Stone, MD, arrived for her day shift at Sharp Chula Vista one day in late December. The ICU and hospital wards were still overflowing with COVID-19 patients. But over the previous couple of months, she’d also seen more and more recovered patients presenting with a myriad of symptoms: pulmonary emboli, cardiomyopathy, a shocking case of aspergillosis, and those rare cases of “long COVID,” the patients who just can’t get better.
This morning it was a woman in her 30s. She felt fine, but 2 weeks after recovering from COVID-19, she had unexplained bruising on her arm, a petechiae rash on her legs, and her gums were bleeding. Once admitted to the emergency department, her platelet count of 5000/mm3 was a dead giveaway of immune thrombocytopenic purpura (ITP).
In Dr. Stone’s experience, new and otherwise unexplained symptoms so soon post COVID-19 can’t be written off as a coincidence without some additional consideration. But a quick preliminary search of the literature during her rounds came up almost empty. She found one report with three cases of post-COVID-19 ITP. But other online resources made no mention of it. Kenneth Johnson, MD, the hematologist/oncologist consulting on the new case, told Dr. Stone he’d seen one other case of post-COVID-19 ITP only earlier that month. Dr. Stone called a sister hospital. They’d seen one other case just weeks before.
“I was surprised to find just three cases in the literature when we had seen three among us in a matter of weeks,” Dr. Stone said in an interview. Something was missing.
A missing link
ITP is caused by an immune reaction against a patient’s own platelets.
“We know that infections like influenza can cause ITP, so in this light, [COVID-19-associated ITP] might not be surprising,” Gerard Jansen, MD, PhD, an internist and hematologist in Rotterdam, the Netherlands, said in an interview.
Dr. Jansen and colleagues recorded three cases of post-COVID-19 ITP in May 2020 – the report Dr. Stone had found during her shift. Two patients developed ITP several weeks after COVID-19 and responded to treatment with corticosteroids and intravenous immunoglobulin G (IVIG). The third patient, however, died of intracerebral bleeding while still battling COVID-19. He was retrospectively diagnosed with COVID-19-associated ITP.
A deeper dive into the literature uncovers additional case reports from India, France, the United Kingdom, Turkey, and one from China as early as January 2020. A September 2020 review of ITP secondary to COVID-19 included 23 papers and a total of 45 patients. The review authors noted that more than 70% of cases occurred in patients who were aged over 50 years and 75% had had moderate to severe COVID-19 infections. However, the sample size of 45 is too small to definitively describe what’s happening in the overall population.
ITP’s link to COVID-19 gained a media spotlight after the Miami obstetrician, Gregory Michael, MD, developed ITP days after getting the Pfizer COVID-19 vaccine. In early January, after 2 weeks in the ICU, Dr. Michael died of a hemorrhagic stroke caused by the low platelet count.
Pfizer said in a statement that the company is “actively investigating” the case, “but we don’t believe at this time that there is any direct connection to the vaccine.” Other experts have said the timing, particularly in a relatively young and healthy man, means a link to the vaccine is possible or even likely, but final results won›t be known until the Centers for Disease Control and Prevention finishes its investigation.
But “it is quite unusual to die from ITP,” San Diego hematologist Dr. Johnson said in an interview. In his more than 20 years of practice, he has never had a patient die from the condition.
For his part, Dr. Jansen, the hematologist in Rotterdam, said that at this point we just don’t know if there’s a link between the vaccine and ITP. Both infection and drugs are well established causes of ITP, so with that general mechanism or pathology in mind it makes sense that COVID-19 and the vaccine could instigate ITP. But it would be very difficult to prove in just one instance, he said. And considering the millions who have thus far received the vaccine without incident, and the known risks and dangers of COVID-19, “we still advise to vaccinate,” he said.
The number of cases is underestimated
Since his original case report in May, Dr. Jansen has seen five or so additional cases. But the causal link between the coronavirus and the hematologic symptoms is still undefined. “We don’t know much about platelet counts in COVID-19 at all,” he said. It could be that COVID-19 somehow inhibits platelet production or that it kills existing platelets. Whatever the exact relationship to the virus, Dr. Jansen expects that the true number of COVID-19-related ITP cases is higher than current estimates suggest.
One reason it isn’t coming up more often, Dr. Jansen said, may be that the cause of ITP in COVID-19 patients is hard to pin down. In the case report from May, Dr. Jansen and colleagues wrote: “And there are numerous other factors that can cause thrombocytopenia where COVID is concerned. For instance the coagulation activation by COVID‐19 infection leading to disseminated intravascular coagulation (DIC) and subsequent thrombocytopenia. Also, treatments for COVID‐19, including heparin, azithromycin and hydroxychloroquine, may lead to thrombocytopenia.”
Tracking and understanding COVID-19-associated ITP first requires the extensive process of elimination needed to diagnose it.
In addition, drugs used to treat COVID-19 could be masking COVID-19-related ITP. “Dexamethasone is a mainstay of COVID treatment. And it’s how we treat ITP,” Dr. Johnson said, which means physicians may be treating ITP without even registering it. And that’s one hypothesis for why Dr. Stone and Dr. Johnson didn’t see a case until 9 months into the pandemic.
Treating COVID-19-associated ITP also has its challenges, particularly in patients who develop it during an acute COVID-19 infection and are at risk for both internal bleeding and thrombosis. This was the case for the third patient in Dr. Jansen’s case report. The patient developed a pulmonary embolism and had a falling platelet count. He was given a platelet infusion and then an anticoagulant for the thrombosis. But a retrospective look at the case revealed the transfusion “did not increase numbers at all – which suggests ITP,” Dr. Jansen said. Intracerebral bleeding was the cause of death.
That’s why “it’s important to be aware of this phenomenon,” Dr. Jansen said of COVID-19-associated ITP. If a transfusion is unsuccessful, consider that the patient may have ITP and adjust. Dr. Johnson hasn’t had to treat a patient battling both complications simultaneously but says the ideal course of action would be to raise platelets with steroids and IVIG and then give the anticoagulant once the platelet count is higher. But reality is rarely ideal. Often these two treatments will have to be given concurrently since the patient faces two life-threatening risks, he said. “It’s a very challenging situation,” he said.
The good news is that standard treatments for ITP seem to work for COVID-19-associated ITP. The 30-year-old patient of Dr. Stone and Dr. Johnson responded so well to intravenous steroids that IVIG was unnecessary. She’s now on a slow prednisone taper and maintains platelet counts at 114,000/mm3 at her weekly follow-up appointments with Dr. Johnson.
Meanwhile, Dr. Jansen’s two other patients, now nearly a year out of treatment, require no additional medication. One of the patients is fully recovered and, though the other still has lower than normal platelet counts, she has no bleeding symptoms and her platelet counts remain stable. Still, Dr. Jansen is anxious for more data looking at the platelet counts in every COVID-19 patient and to combine findings from existing COVID-19-associated ITP patients.
For Dr. Stone, she says she’s added one COVID-19-associated complication to her belt. One less aftereffect will catch her off guard. And she wants others to have the same information.
“It’s just a little bit daunting. We don’t know how bad post-COVID will be,” she said. “There’s so many levels to this disease. Some people deal with it for so long and some people just get better and move on – we think ... so far.”
A version of this article first appeared on Medscape.com.
Hospitalist Sarah Stone, MD, arrived for her day shift at Sharp Chula Vista one day in late December. The ICU and hospital wards were still overflowing with COVID-19 patients. But over the previous couple of months, she’d also seen more and more recovered patients presenting with a myriad of symptoms: pulmonary emboli, cardiomyopathy, a shocking case of aspergillosis, and those rare cases of “long COVID,” the patients who just can’t get better.
This morning it was a woman in her 30s. She felt fine, but 2 weeks after recovering from COVID-19, she had unexplained bruising on her arm, a petechiae rash on her legs, and her gums were bleeding. Once admitted to the emergency department, her platelet count of 5000/mm3 was a dead giveaway of immune thrombocytopenic purpura (ITP).
In Dr. Stone’s experience, new and otherwise unexplained symptoms so soon post COVID-19 can’t be written off as a coincidence without some additional consideration. But a quick preliminary search of the literature during her rounds came up almost empty. She found one report with three cases of post-COVID-19 ITP. But other online resources made no mention of it. Kenneth Johnson, MD, the hematologist/oncologist consulting on the new case, told Dr. Stone he’d seen one other case of post-COVID-19 ITP only earlier that month. Dr. Stone called a sister hospital. They’d seen one other case just weeks before.
“I was surprised to find just three cases in the literature when we had seen three among us in a matter of weeks,” Dr. Stone said in an interview. Something was missing.
A missing link
ITP is caused by an immune reaction against a patient’s own platelets.
“We know that infections like influenza can cause ITP, so in this light, [COVID-19-associated ITP] might not be surprising,” Gerard Jansen, MD, PhD, an internist and hematologist in Rotterdam, the Netherlands, said in an interview.
Dr. Jansen and colleagues recorded three cases of post-COVID-19 ITP in May 2020 – the report Dr. Stone had found during her shift. Two patients developed ITP several weeks after COVID-19 and responded to treatment with corticosteroids and intravenous immunoglobulin G (IVIG). The third patient, however, died of intracerebral bleeding while still battling COVID-19. He was retrospectively diagnosed with COVID-19-associated ITP.
A deeper dive into the literature uncovers additional case reports from India, France, the United Kingdom, Turkey, and one from China as early as January 2020. A September 2020 review of ITP secondary to COVID-19 included 23 papers and a total of 45 patients. The review authors noted that more than 70% of cases occurred in patients who were aged over 50 years and 75% had had moderate to severe COVID-19 infections. However, the sample size of 45 is too small to definitively describe what’s happening in the overall population.
ITP’s link to COVID-19 gained a media spotlight after the Miami obstetrician, Gregory Michael, MD, developed ITP days after getting the Pfizer COVID-19 vaccine. In early January, after 2 weeks in the ICU, Dr. Michael died of a hemorrhagic stroke caused by the low platelet count.
Pfizer said in a statement that the company is “actively investigating” the case, “but we don’t believe at this time that there is any direct connection to the vaccine.” Other experts have said the timing, particularly in a relatively young and healthy man, means a link to the vaccine is possible or even likely, but final results won›t be known until the Centers for Disease Control and Prevention finishes its investigation.
But “it is quite unusual to die from ITP,” San Diego hematologist Dr. Johnson said in an interview. In his more than 20 years of practice, he has never had a patient die from the condition.
For his part, Dr. Jansen, the hematologist in Rotterdam, said that at this point we just don’t know if there’s a link between the vaccine and ITP. Both infection and drugs are well established causes of ITP, so with that general mechanism or pathology in mind it makes sense that COVID-19 and the vaccine could instigate ITP. But it would be very difficult to prove in just one instance, he said. And considering the millions who have thus far received the vaccine without incident, and the known risks and dangers of COVID-19, “we still advise to vaccinate,” he said.
The number of cases is underestimated
Since his original case report in May, Dr. Jansen has seen five or so additional cases. But the causal link between the coronavirus and the hematologic symptoms is still undefined. “We don’t know much about platelet counts in COVID-19 at all,” he said. It could be that COVID-19 somehow inhibits platelet production or that it kills existing platelets. Whatever the exact relationship to the virus, Dr. Jansen expects that the true number of COVID-19-related ITP cases is higher than current estimates suggest.
One reason it isn’t coming up more often, Dr. Jansen said, may be that the cause of ITP in COVID-19 patients is hard to pin down. In the case report from May, Dr. Jansen and colleagues wrote: “And there are numerous other factors that can cause thrombocytopenia where COVID is concerned. For instance the coagulation activation by COVID‐19 infection leading to disseminated intravascular coagulation (DIC) and subsequent thrombocytopenia. Also, treatments for COVID‐19, including heparin, azithromycin and hydroxychloroquine, may lead to thrombocytopenia.”
Tracking and understanding COVID-19-associated ITP first requires the extensive process of elimination needed to diagnose it.
In addition, drugs used to treat COVID-19 could be masking COVID-19-related ITP. “Dexamethasone is a mainstay of COVID treatment. And it’s how we treat ITP,” Dr. Johnson said, which means physicians may be treating ITP without even registering it. And that’s one hypothesis for why Dr. Stone and Dr. Johnson didn’t see a case until 9 months into the pandemic.
Treating COVID-19-associated ITP also has its challenges, particularly in patients who develop it during an acute COVID-19 infection and are at risk for both internal bleeding and thrombosis. This was the case for the third patient in Dr. Jansen’s case report. The patient developed a pulmonary embolism and had a falling platelet count. He was given a platelet infusion and then an anticoagulant for the thrombosis. But a retrospective look at the case revealed the transfusion “did not increase numbers at all – which suggests ITP,” Dr. Jansen said. Intracerebral bleeding was the cause of death.
That’s why “it’s important to be aware of this phenomenon,” Dr. Jansen said of COVID-19-associated ITP. If a transfusion is unsuccessful, consider that the patient may have ITP and adjust. Dr. Johnson hasn’t had to treat a patient battling both complications simultaneously but says the ideal course of action would be to raise platelets with steroids and IVIG and then give the anticoagulant once the platelet count is higher. But reality is rarely ideal. Often these two treatments will have to be given concurrently since the patient faces two life-threatening risks, he said. “It’s a very challenging situation,” he said.
The good news is that standard treatments for ITP seem to work for COVID-19-associated ITP. The 30-year-old patient of Dr. Stone and Dr. Johnson responded so well to intravenous steroids that IVIG was unnecessary. She’s now on a slow prednisone taper and maintains platelet counts at 114,000/mm3 at her weekly follow-up appointments with Dr. Johnson.
Meanwhile, Dr. Jansen’s two other patients, now nearly a year out of treatment, require no additional medication. One of the patients is fully recovered and, though the other still has lower than normal platelet counts, she has no bleeding symptoms and her platelet counts remain stable. Still, Dr. Jansen is anxious for more data looking at the platelet counts in every COVID-19 patient and to combine findings from existing COVID-19-associated ITP patients.
For Dr. Stone, she says she’s added one COVID-19-associated complication to her belt. One less aftereffect will catch her off guard. And she wants others to have the same information.
“It’s just a little bit daunting. We don’t know how bad post-COVID will be,” she said. “There’s so many levels to this disease. Some people deal with it for so long and some people just get better and move on – we think ... so far.”
A version of this article first appeared on Medscape.com.
CDC panel: No COVID-19 vaccine safety surprises
The United States is nearly 6 weeks into its historic campaign to vaccinate Americans against the virus that causes COVID-19, and so far, the two vaccines in use look remarkably low risk, according to new data presented today at a meeting of vaccine experts that advise the Centers for Disease Control and Prevention.
With 23.5 million doses of the Pfizer and Moderna vaccines now given, there have been very few serious side effects. In addition, deaths reported after people got the vaccine do not seem to be related to it.
The most common symptoms reported after vaccination were pain where people got the shot, fatigue, headache, and muscle soreness. These were more common after the second dose. In addition, about one in four people reported fever and chills after the second shot.
“On the whole, I thought it was very reassuring,” said William Schaffner, MD, an infectious disease expert with Vanderbilt University, Nashville, Tenn., who listened to the presentations.
The CDC is collecting safety information through multiple channels. These include a new smartphone-based app called V-Safe, which collects daily information from people who’ve been vaccinated; the federal Vaccine Adverse Event Reporting System, which accepts reports from anyone; and the Vaccine Safety Datalink, which is a collaboration between the CDC and nine major hospital systems. There’s also the Clinical Immunization Safety Assessment Project, a collaboration between the CDC and vaccine safety experts.
After surveying these systems, experts heading the safety committee for the CDC’s Advisory Committee on Immunization Practices said there have been few serious side effects reported.
Very rarely, severe allergic reactions – called anaphylaxis – have occurred after vaccination. There have been 50 of these cases reported after the Pfizer vaccine and 21 cases reported after the Moderna vaccine to date. Nearly all of them – 94% of the anaphylaxis cases after Pfizer vaccines and 100% of those after Moderna’s vaccine – have been in women, though it’s not clear why.
That translates to a rate of about five cases of anaphylaxis for every million doses of the Pfizer vaccine and about three for every million doses of the Moderna vaccine. Most of these occur within 15 minutes after getting a vaccine dose, with one reported as long as 20 hours after the shot.
The CDC suspects these may be related to an ingredient called polyethylene glycol (PEG). PEG is a part of the particles that slip the vaccines’ mRNA into cells with instructions to make the spike protein of the virus. Cells then express these spikes on their surfaces so the immune system can learn to recognize them and make defenses against them. PEG is a common ingredient in many drugs and occasionally triggers anaphylaxis.
Reported deaths seem unrelated to vaccines
Through Jan. 18, 196 people have died after getting a vaccine.
Most of these deaths (129) were in patients in long term care facilities. These deaths are still being investigated, but when they were compared with the number of deaths that might be expected over the same period because of natural causes, they seemed to be coincidental and not caused by the vaccine, said Tom Shimabukuro, MD, deputy director of the Immunization Safety Office at the CDC, who studied the data.
In fact, death rates were lower among vaccinated nursing home residents, compared with those who had not been vaccinated.
“These findings suggest that short-term mortality rates appear unrelated to vaccination for COVID-19,” Dr. Shimabukuro said.
This also appeared to be true for younger adults who died after their shots.
There were 28 people aged under 65 years who died after being vaccinated. Most of these deaths were heart related, according to autopsy reports. When investigators compared the number of sudden cardiac deaths expected to occur in this population naturally, they found people who were vaccinated had a lower rate than would have been expected without vaccination. This suggests that these deaths were also unrelated to the vaccine.
More vaccines on the horizon
The panel also heard an update from drug company AstraZeneca on its vaccine. It’s being used in 18 countries but has not yet been authorized in the United States.
That vaccine is currently in phase 3 of its U.S. clinical trials, and more than 26,000 people who have volunteered to get the shot had received their second dose as of Jan. 21, the company said.
The Food and Drug Administration requires at least 2 months of follow-up before it will evaluate a vaccine for an emergency-use authorization, which means the company would be ready to submit by the end of March, with a possible approval by April.
The AstraZeneca vaccine uses a more traditional method to create immunity, slipping a key part of the virus that causes COVID-19 into the shell of an adenovirus – a virus that causes cold-like symptoms – that normally infects monkeys. When the immune system sees the virus, it generates protective defenses against it.
The two-dose vaccine can be stored in a regular refrigerator for up to 6 months, which makes it easier to handle than the mRNA vaccines, which require much colder storage. Another advantage appears to be that it’s less likely to trigger severe allergic reactions. So far, there have been no cases of anaphylaxis reported after this shot.
In total, four serious side effects have been reported with the AstraZeneca vaccine, including two cases of transverse myelitis, a serious condition that causes swelling of the spinal cord, leading to pain, muscle weakness, and paralysis. One of these was in the group that got the placebo. The reports paused the trial, but it was allowed to continue after a safety review.
This vaccine also appears to be less effective than the mRNA shots. Data presented to the panel show it appears to cut the risk of developing a COVID infection that has symptoms by 62%. That’s over the 50% threshold the FDA set for approval but less than seen with the mRNA vaccines, which are more than 90% effective at preventing infections.
“Is the average person going to want to take the AstraZeneca shot? What role is this going to play in our vaccination program?” Dr. Schaffner said.
Johnson & Johnson will have enough data from its clinical trials to submit it to the FDA within the next week, the company said in a call with shareholders on Tuesday. So far, its one-dose shots looks to be about as effective as both the Pfizer and Moderna vaccines.
“It could be that we wind up with four vaccines: Three that can run very fast, and one that’s not so fast,” Dr. Schaffner said.
A version of this article first appeared on Medscape.com.
The United States is nearly 6 weeks into its historic campaign to vaccinate Americans against the virus that causes COVID-19, and so far, the two vaccines in use look remarkably low risk, according to new data presented today at a meeting of vaccine experts that advise the Centers for Disease Control and Prevention.
With 23.5 million doses of the Pfizer and Moderna vaccines now given, there have been very few serious side effects. In addition, deaths reported after people got the vaccine do not seem to be related to it.
The most common symptoms reported after vaccination were pain where people got the shot, fatigue, headache, and muscle soreness. These were more common after the second dose. In addition, about one in four people reported fever and chills after the second shot.
“On the whole, I thought it was very reassuring,” said William Schaffner, MD, an infectious disease expert with Vanderbilt University, Nashville, Tenn., who listened to the presentations.
The CDC is collecting safety information through multiple channels. These include a new smartphone-based app called V-Safe, which collects daily information from people who’ve been vaccinated; the federal Vaccine Adverse Event Reporting System, which accepts reports from anyone; and the Vaccine Safety Datalink, which is a collaboration between the CDC and nine major hospital systems. There’s also the Clinical Immunization Safety Assessment Project, a collaboration between the CDC and vaccine safety experts.
After surveying these systems, experts heading the safety committee for the CDC’s Advisory Committee on Immunization Practices said there have been few serious side effects reported.
Very rarely, severe allergic reactions – called anaphylaxis – have occurred after vaccination. There have been 50 of these cases reported after the Pfizer vaccine and 21 cases reported after the Moderna vaccine to date. Nearly all of them – 94% of the anaphylaxis cases after Pfizer vaccines and 100% of those after Moderna’s vaccine – have been in women, though it’s not clear why.
That translates to a rate of about five cases of anaphylaxis for every million doses of the Pfizer vaccine and about three for every million doses of the Moderna vaccine. Most of these occur within 15 minutes after getting a vaccine dose, with one reported as long as 20 hours after the shot.
The CDC suspects these may be related to an ingredient called polyethylene glycol (PEG). PEG is a part of the particles that slip the vaccines’ mRNA into cells with instructions to make the spike protein of the virus. Cells then express these spikes on their surfaces so the immune system can learn to recognize them and make defenses against them. PEG is a common ingredient in many drugs and occasionally triggers anaphylaxis.
Reported deaths seem unrelated to vaccines
Through Jan. 18, 196 people have died after getting a vaccine.
Most of these deaths (129) were in patients in long term care facilities. These deaths are still being investigated, but when they were compared with the number of deaths that might be expected over the same period because of natural causes, they seemed to be coincidental and not caused by the vaccine, said Tom Shimabukuro, MD, deputy director of the Immunization Safety Office at the CDC, who studied the data.
In fact, death rates were lower among vaccinated nursing home residents, compared with those who had not been vaccinated.
“These findings suggest that short-term mortality rates appear unrelated to vaccination for COVID-19,” Dr. Shimabukuro said.
This also appeared to be true for younger adults who died after their shots.
There were 28 people aged under 65 years who died after being vaccinated. Most of these deaths were heart related, according to autopsy reports. When investigators compared the number of sudden cardiac deaths expected to occur in this population naturally, they found people who were vaccinated had a lower rate than would have been expected without vaccination. This suggests that these deaths were also unrelated to the vaccine.
More vaccines on the horizon
The panel also heard an update from drug company AstraZeneca on its vaccine. It’s being used in 18 countries but has not yet been authorized in the United States.
That vaccine is currently in phase 3 of its U.S. clinical trials, and more than 26,000 people who have volunteered to get the shot had received their second dose as of Jan. 21, the company said.
The Food and Drug Administration requires at least 2 months of follow-up before it will evaluate a vaccine for an emergency-use authorization, which means the company would be ready to submit by the end of March, with a possible approval by April.
The AstraZeneca vaccine uses a more traditional method to create immunity, slipping a key part of the virus that causes COVID-19 into the shell of an adenovirus – a virus that causes cold-like symptoms – that normally infects monkeys. When the immune system sees the virus, it generates protective defenses against it.
The two-dose vaccine can be stored in a regular refrigerator for up to 6 months, which makes it easier to handle than the mRNA vaccines, which require much colder storage. Another advantage appears to be that it’s less likely to trigger severe allergic reactions. So far, there have been no cases of anaphylaxis reported after this shot.
In total, four serious side effects have been reported with the AstraZeneca vaccine, including two cases of transverse myelitis, a serious condition that causes swelling of the spinal cord, leading to pain, muscle weakness, and paralysis. One of these was in the group that got the placebo. The reports paused the trial, but it was allowed to continue after a safety review.
This vaccine also appears to be less effective than the mRNA shots. Data presented to the panel show it appears to cut the risk of developing a COVID infection that has symptoms by 62%. That’s over the 50% threshold the FDA set for approval but less than seen with the mRNA vaccines, which are more than 90% effective at preventing infections.
“Is the average person going to want to take the AstraZeneca shot? What role is this going to play in our vaccination program?” Dr. Schaffner said.
Johnson & Johnson will have enough data from its clinical trials to submit it to the FDA within the next week, the company said in a call with shareholders on Tuesday. So far, its one-dose shots looks to be about as effective as both the Pfizer and Moderna vaccines.
“It could be that we wind up with four vaccines: Three that can run very fast, and one that’s not so fast,” Dr. Schaffner said.
A version of this article first appeared on Medscape.com.
The United States is nearly 6 weeks into its historic campaign to vaccinate Americans against the virus that causes COVID-19, and so far, the two vaccines in use look remarkably low risk, according to new data presented today at a meeting of vaccine experts that advise the Centers for Disease Control and Prevention.
With 23.5 million doses of the Pfizer and Moderna vaccines now given, there have been very few serious side effects. In addition, deaths reported after people got the vaccine do not seem to be related to it.
The most common symptoms reported after vaccination were pain where people got the shot, fatigue, headache, and muscle soreness. These were more common after the second dose. In addition, about one in four people reported fever and chills after the second shot.
“On the whole, I thought it was very reassuring,” said William Schaffner, MD, an infectious disease expert with Vanderbilt University, Nashville, Tenn., who listened to the presentations.
The CDC is collecting safety information through multiple channels. These include a new smartphone-based app called V-Safe, which collects daily information from people who’ve been vaccinated; the federal Vaccine Adverse Event Reporting System, which accepts reports from anyone; and the Vaccine Safety Datalink, which is a collaboration between the CDC and nine major hospital systems. There’s also the Clinical Immunization Safety Assessment Project, a collaboration between the CDC and vaccine safety experts.
After surveying these systems, experts heading the safety committee for the CDC’s Advisory Committee on Immunization Practices said there have been few serious side effects reported.
Very rarely, severe allergic reactions – called anaphylaxis – have occurred after vaccination. There have been 50 of these cases reported after the Pfizer vaccine and 21 cases reported after the Moderna vaccine to date. Nearly all of them – 94% of the anaphylaxis cases after Pfizer vaccines and 100% of those after Moderna’s vaccine – have been in women, though it’s not clear why.
That translates to a rate of about five cases of anaphylaxis for every million doses of the Pfizer vaccine and about three for every million doses of the Moderna vaccine. Most of these occur within 15 minutes after getting a vaccine dose, with one reported as long as 20 hours after the shot.
The CDC suspects these may be related to an ingredient called polyethylene glycol (PEG). PEG is a part of the particles that slip the vaccines’ mRNA into cells with instructions to make the spike protein of the virus. Cells then express these spikes on their surfaces so the immune system can learn to recognize them and make defenses against them. PEG is a common ingredient in many drugs and occasionally triggers anaphylaxis.
Reported deaths seem unrelated to vaccines
Through Jan. 18, 196 people have died after getting a vaccine.
Most of these deaths (129) were in patients in long term care facilities. These deaths are still being investigated, but when they were compared with the number of deaths that might be expected over the same period because of natural causes, they seemed to be coincidental and not caused by the vaccine, said Tom Shimabukuro, MD, deputy director of the Immunization Safety Office at the CDC, who studied the data.
In fact, death rates were lower among vaccinated nursing home residents, compared with those who had not been vaccinated.
“These findings suggest that short-term mortality rates appear unrelated to vaccination for COVID-19,” Dr. Shimabukuro said.
This also appeared to be true for younger adults who died after their shots.
There were 28 people aged under 65 years who died after being vaccinated. Most of these deaths were heart related, according to autopsy reports. When investigators compared the number of sudden cardiac deaths expected to occur in this population naturally, they found people who were vaccinated had a lower rate than would have been expected without vaccination. This suggests that these deaths were also unrelated to the vaccine.
More vaccines on the horizon
The panel also heard an update from drug company AstraZeneca on its vaccine. It’s being used in 18 countries but has not yet been authorized in the United States.
That vaccine is currently in phase 3 of its U.S. clinical trials, and more than 26,000 people who have volunteered to get the shot had received their second dose as of Jan. 21, the company said.
The Food and Drug Administration requires at least 2 months of follow-up before it will evaluate a vaccine for an emergency-use authorization, which means the company would be ready to submit by the end of March, with a possible approval by April.
The AstraZeneca vaccine uses a more traditional method to create immunity, slipping a key part of the virus that causes COVID-19 into the shell of an adenovirus – a virus that causes cold-like symptoms – that normally infects monkeys. When the immune system sees the virus, it generates protective defenses against it.
The two-dose vaccine can be stored in a regular refrigerator for up to 6 months, which makes it easier to handle than the mRNA vaccines, which require much colder storage. Another advantage appears to be that it’s less likely to trigger severe allergic reactions. So far, there have been no cases of anaphylaxis reported after this shot.
In total, four serious side effects have been reported with the AstraZeneca vaccine, including two cases of transverse myelitis, a serious condition that causes swelling of the spinal cord, leading to pain, muscle weakness, and paralysis. One of these was in the group that got the placebo. The reports paused the trial, but it was allowed to continue after a safety review.
This vaccine also appears to be less effective than the mRNA shots. Data presented to the panel show it appears to cut the risk of developing a COVID infection that has symptoms by 62%. That’s over the 50% threshold the FDA set for approval but less than seen with the mRNA vaccines, which are more than 90% effective at preventing infections.
“Is the average person going to want to take the AstraZeneca shot? What role is this going to play in our vaccination program?” Dr. Schaffner said.
Johnson & Johnson will have enough data from its clinical trials to submit it to the FDA within the next week, the company said in a call with shareholders on Tuesday. So far, its one-dose shots looks to be about as effective as both the Pfizer and Moderna vaccines.
“It could be that we wind up with four vaccines: Three that can run very fast, and one that’s not so fast,” Dr. Schaffner said.
A version of this article first appeared on Medscape.com.
Are there COVID-19–related ‘long-haul’ skin issues?
– as a result of infection with or exposure to the SARS-CoV-2 virus, but some dermatologists question if the skin signs and symptoms are truly related.
In their commentary in the Lancet Infectious Diseases, Esther P. Freeman, MD, PhD, and colleagues who lead and participate in the American Academy of Dermatology’s international registry said their analysis “revealed a previously unreported subset of patients who experience long-haul symptoms in dermatology-dominant COVID-19.”
Some of the data was presented at the 29th European Academy of Dermatology and Venereology in late October 2020, but has since been updated with more cases.
Dermatologists who spoke with this news organization said it has not been settled that some skin manifestations – such as pernio/chilblains rashes, seen primarily in nonhospitalized patients, and described in the registry – are definitively caused by COVID. They also noted that in some cases, patients who initially test negative for COVID-19 by polymerase chain reaction (PCR) sometimes do not ever develop antibodies, which could mean they were never actually exposed to SARS-CoV-2.
“I still question whether the perniosis is directly related to infection with SARS-CoV-2 or not,” said Anthony Fernandez, MD, PhD, director of medical and inpatient dermatology and assistant professor of dermatopathology at the Cleveland Clinic. His uncertainty is driven by the lack of seroconversion and that few cases were seen over the summer in the United States – suggesting that it may still be a result of cold temperatures.
“I’m not sure there is a definitive correct answer, definitely not that everyone would agree on,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn.
Dr. Freeman, however, believed that pernio and especially persistent lesions are caused by an immune response to COVID.
In an interview, she noted the multiple cases of patients in the registry who did seroconvert and that, while a registry is not a perfect means of getting an answer, it is good for generating questions. Taken collectively, the cases in the registry can “tell a story for further hypotheses,” said Dr. Freeman, who is director of global health dermatology at Massachusetts General Hospital and assistant professor of dermatology at Harvard University, both in Boston.
“We were noticing this signal across the world” that patients “developed these toe lesions and they never got better,” said Dr. Freeman. Generally, people who experience pernio, also described as COVID toes or “COVID fingers,” recover in 4-8 weeks. But in the registry, “we did have this subset of patients who really were experiencing these very longstanding symptoms,” she added.
Two patients with lab-confirmed COVID have had long-lasting pernio of 133 days and 150 days. “I’m caring for a cohort in Boston who have had long COVID of the skin and symptoms for over 10 months,” Dr. Freeman said.
Pernio dominates
The registry – a collaboration between the AAD and the International League of Dermatological Societies – was launched in April 2020. Any medical professional can enter case information. From April to October, 1,030 total cases and 331 laboratory-confirmed or suspected COVID-19 cases with dermatological manifestations were entered from 41 countries.
Most of the cases were just recorded at a single time point, which is an acknowledged limitation of the study.
Dr. Freeman and colleagues reached out to registry participants in June and August to get updates on COVID lab test results and sign and symptom duration. Overall, 234 total and 96 lab-confirmed COVID infections had more lengthy data about sign and symptom duration.
Pernio lasted a median of 15 days in patients with suspected disease and 12 days for those with lab-confirmed COVID, compared with a median of 7 days for morbilliform eruptions, 4 days for urticarial eruptions, and 20 days for papulosquamous eruptions – all in patients with lab-confirmed disease.
Of the 103 cases of pernio, 7 had symptoms lasting more than 60 days. Only two of those seven patients had lab-confirmed COVID. Initially, the one patient tested negative with nasopharyngeal PCR, and serum IgM and IgG. Six weeks after pernio onset, the patient – still experiencing fatigue and pernio – seroconverted to anti–SARS-CoV-2 IgM positivity.
The other long-haul patient, after a negative PCR, tested positive for SARS-CoV-2 serum IgG 1 month after pernio onset.
Robust immune response?
Dr. Freeman said these patients might have a very high interferon response initially to the virus, which makes for a mild to nonexistent disease, but could create inflammation elsewhere. “I almost view the toes as an innocent bystander of a robust immune response to SARS-CoV-2.”
Although he has not seen extended pernio or other skin manifestations in his patients, Dr. Fernandez said the interferon hypothesis is “fair,” and “the best that’s out there.” Dr. Fernandez is currently studying cutaneous manifestations of COVID-19 as a principal investigator of a trial sponsored by the Clinical and Translational Science Collaborative of Cleveland.
Dr. Ko said in an interview that she has not observed long-haul skin issues in her patients, but Yale colleagues have.
In a study, she and Yale colleagues published in September, SARS-CoV-2 spike protein was detected in perniotic lesions, but not nuclear protein or viral RNA. The test they used – immunohistochemistry – can be nonspecific, which muddied results.
She does not think there is replicating virus in the skin or the lesions. Instead, said Dr. Ko, “either there is viral spike protein that has somehow become disassociated from actively replicating virus that somehow got deposited in endothelial cells,” or the staining “was spurious,” or some other protein is cross-reacting. “And the people who are unlucky enough to have that protein in their endothelial cells can manifest this COVID-toe, COVID-finger phenomenon.”
To her, it’s an unsolved mystery. “The weird thing is, we’ve never before had this much perniosis,” Dr. Ko said.
Dr. Fernandez is not convinced yet, noting that, in Cleveland, more pernio cases were observed in March and April than in the summer. “If it is a manifestation of the infection then you also need the right environment, the cold weather for this manifestation to present,” he said. “Or, it really isn’t a direct manifestation of COVID-19 but may be more related to other factors,” such as lifestyle changes related to limitations implemented to help mitigate the spread of the disease.
“To me the jury is still out whether or not the perniotic lesions really can tell us something about a patient’s exposure and infection with SARS-CoV-2,” he said.
Dr. Freeman reported receiving a grant from the International League of Dermatological Societies and nonfinancial support from the AAD for the study. Dr. Ko reported no conflicts. Dr. Fernadnez had no disclosures.
– as a result of infection with or exposure to the SARS-CoV-2 virus, but some dermatologists question if the skin signs and symptoms are truly related.
In their commentary in the Lancet Infectious Diseases, Esther P. Freeman, MD, PhD, and colleagues who lead and participate in the American Academy of Dermatology’s international registry said their analysis “revealed a previously unreported subset of patients who experience long-haul symptoms in dermatology-dominant COVID-19.”
Some of the data was presented at the 29th European Academy of Dermatology and Venereology in late October 2020, but has since been updated with more cases.
Dermatologists who spoke with this news organization said it has not been settled that some skin manifestations – such as pernio/chilblains rashes, seen primarily in nonhospitalized patients, and described in the registry – are definitively caused by COVID. They also noted that in some cases, patients who initially test negative for COVID-19 by polymerase chain reaction (PCR) sometimes do not ever develop antibodies, which could mean they were never actually exposed to SARS-CoV-2.
“I still question whether the perniosis is directly related to infection with SARS-CoV-2 or not,” said Anthony Fernandez, MD, PhD, director of medical and inpatient dermatology and assistant professor of dermatopathology at the Cleveland Clinic. His uncertainty is driven by the lack of seroconversion and that few cases were seen over the summer in the United States – suggesting that it may still be a result of cold temperatures.
“I’m not sure there is a definitive correct answer, definitely not that everyone would agree on,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn.
Dr. Freeman, however, believed that pernio and especially persistent lesions are caused by an immune response to COVID.
In an interview, she noted the multiple cases of patients in the registry who did seroconvert and that, while a registry is not a perfect means of getting an answer, it is good for generating questions. Taken collectively, the cases in the registry can “tell a story for further hypotheses,” said Dr. Freeman, who is director of global health dermatology at Massachusetts General Hospital and assistant professor of dermatology at Harvard University, both in Boston.
“We were noticing this signal across the world” that patients “developed these toe lesions and they never got better,” said Dr. Freeman. Generally, people who experience pernio, also described as COVID toes or “COVID fingers,” recover in 4-8 weeks. But in the registry, “we did have this subset of patients who really were experiencing these very longstanding symptoms,” she added.
Two patients with lab-confirmed COVID have had long-lasting pernio of 133 days and 150 days. “I’m caring for a cohort in Boston who have had long COVID of the skin and symptoms for over 10 months,” Dr. Freeman said.
Pernio dominates
The registry – a collaboration between the AAD and the International League of Dermatological Societies – was launched in April 2020. Any medical professional can enter case information. From April to October, 1,030 total cases and 331 laboratory-confirmed or suspected COVID-19 cases with dermatological manifestations were entered from 41 countries.
Most of the cases were just recorded at a single time point, which is an acknowledged limitation of the study.
Dr. Freeman and colleagues reached out to registry participants in June and August to get updates on COVID lab test results and sign and symptom duration. Overall, 234 total and 96 lab-confirmed COVID infections had more lengthy data about sign and symptom duration.
Pernio lasted a median of 15 days in patients with suspected disease and 12 days for those with lab-confirmed COVID, compared with a median of 7 days for morbilliform eruptions, 4 days for urticarial eruptions, and 20 days for papulosquamous eruptions – all in patients with lab-confirmed disease.
Of the 103 cases of pernio, 7 had symptoms lasting more than 60 days. Only two of those seven patients had lab-confirmed COVID. Initially, the one patient tested negative with nasopharyngeal PCR, and serum IgM and IgG. Six weeks after pernio onset, the patient – still experiencing fatigue and pernio – seroconverted to anti–SARS-CoV-2 IgM positivity.
The other long-haul patient, after a negative PCR, tested positive for SARS-CoV-2 serum IgG 1 month after pernio onset.
Robust immune response?
Dr. Freeman said these patients might have a very high interferon response initially to the virus, which makes for a mild to nonexistent disease, but could create inflammation elsewhere. “I almost view the toes as an innocent bystander of a robust immune response to SARS-CoV-2.”
Although he has not seen extended pernio or other skin manifestations in his patients, Dr. Fernandez said the interferon hypothesis is “fair,” and “the best that’s out there.” Dr. Fernandez is currently studying cutaneous manifestations of COVID-19 as a principal investigator of a trial sponsored by the Clinical and Translational Science Collaborative of Cleveland.
Dr. Ko said in an interview that she has not observed long-haul skin issues in her patients, but Yale colleagues have.
In a study, she and Yale colleagues published in September, SARS-CoV-2 spike protein was detected in perniotic lesions, but not nuclear protein or viral RNA. The test they used – immunohistochemistry – can be nonspecific, which muddied results.
She does not think there is replicating virus in the skin or the lesions. Instead, said Dr. Ko, “either there is viral spike protein that has somehow become disassociated from actively replicating virus that somehow got deposited in endothelial cells,” or the staining “was spurious,” or some other protein is cross-reacting. “And the people who are unlucky enough to have that protein in their endothelial cells can manifest this COVID-toe, COVID-finger phenomenon.”
To her, it’s an unsolved mystery. “The weird thing is, we’ve never before had this much perniosis,” Dr. Ko said.
Dr. Fernandez is not convinced yet, noting that, in Cleveland, more pernio cases were observed in March and April than in the summer. “If it is a manifestation of the infection then you also need the right environment, the cold weather for this manifestation to present,” he said. “Or, it really isn’t a direct manifestation of COVID-19 but may be more related to other factors,” such as lifestyle changes related to limitations implemented to help mitigate the spread of the disease.
“To me the jury is still out whether or not the perniotic lesions really can tell us something about a patient’s exposure and infection with SARS-CoV-2,” he said.
Dr. Freeman reported receiving a grant from the International League of Dermatological Societies and nonfinancial support from the AAD for the study. Dr. Ko reported no conflicts. Dr. Fernadnez had no disclosures.
– as a result of infection with or exposure to the SARS-CoV-2 virus, but some dermatologists question if the skin signs and symptoms are truly related.
In their commentary in the Lancet Infectious Diseases, Esther P. Freeman, MD, PhD, and colleagues who lead and participate in the American Academy of Dermatology’s international registry said their analysis “revealed a previously unreported subset of patients who experience long-haul symptoms in dermatology-dominant COVID-19.”
Some of the data was presented at the 29th European Academy of Dermatology and Venereology in late October 2020, but has since been updated with more cases.
Dermatologists who spoke with this news organization said it has not been settled that some skin manifestations – such as pernio/chilblains rashes, seen primarily in nonhospitalized patients, and described in the registry – are definitively caused by COVID. They also noted that in some cases, patients who initially test negative for COVID-19 by polymerase chain reaction (PCR) sometimes do not ever develop antibodies, which could mean they were never actually exposed to SARS-CoV-2.
“I still question whether the perniosis is directly related to infection with SARS-CoV-2 or not,” said Anthony Fernandez, MD, PhD, director of medical and inpatient dermatology and assistant professor of dermatopathology at the Cleveland Clinic. His uncertainty is driven by the lack of seroconversion and that few cases were seen over the summer in the United States – suggesting that it may still be a result of cold temperatures.
“I’m not sure there is a definitive correct answer, definitely not that everyone would agree on,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn.
Dr. Freeman, however, believed that pernio and especially persistent lesions are caused by an immune response to COVID.
In an interview, she noted the multiple cases of patients in the registry who did seroconvert and that, while a registry is not a perfect means of getting an answer, it is good for generating questions. Taken collectively, the cases in the registry can “tell a story for further hypotheses,” said Dr. Freeman, who is director of global health dermatology at Massachusetts General Hospital and assistant professor of dermatology at Harvard University, both in Boston.
“We were noticing this signal across the world” that patients “developed these toe lesions and they never got better,” said Dr. Freeman. Generally, people who experience pernio, also described as COVID toes or “COVID fingers,” recover in 4-8 weeks. But in the registry, “we did have this subset of patients who really were experiencing these very longstanding symptoms,” she added.
Two patients with lab-confirmed COVID have had long-lasting pernio of 133 days and 150 days. “I’m caring for a cohort in Boston who have had long COVID of the skin and symptoms for over 10 months,” Dr. Freeman said.
Pernio dominates
The registry – a collaboration between the AAD and the International League of Dermatological Societies – was launched in April 2020. Any medical professional can enter case information. From April to October, 1,030 total cases and 331 laboratory-confirmed or suspected COVID-19 cases with dermatological manifestations were entered from 41 countries.
Most of the cases were just recorded at a single time point, which is an acknowledged limitation of the study.
Dr. Freeman and colleagues reached out to registry participants in June and August to get updates on COVID lab test results and sign and symptom duration. Overall, 234 total and 96 lab-confirmed COVID infections had more lengthy data about sign and symptom duration.
Pernio lasted a median of 15 days in patients with suspected disease and 12 days for those with lab-confirmed COVID, compared with a median of 7 days for morbilliform eruptions, 4 days for urticarial eruptions, and 20 days for papulosquamous eruptions – all in patients with lab-confirmed disease.
Of the 103 cases of pernio, 7 had symptoms lasting more than 60 days. Only two of those seven patients had lab-confirmed COVID. Initially, the one patient tested negative with nasopharyngeal PCR, and serum IgM and IgG. Six weeks after pernio onset, the patient – still experiencing fatigue and pernio – seroconverted to anti–SARS-CoV-2 IgM positivity.
The other long-haul patient, after a negative PCR, tested positive for SARS-CoV-2 serum IgG 1 month after pernio onset.
Robust immune response?
Dr. Freeman said these patients might have a very high interferon response initially to the virus, which makes for a mild to nonexistent disease, but could create inflammation elsewhere. “I almost view the toes as an innocent bystander of a robust immune response to SARS-CoV-2.”
Although he has not seen extended pernio or other skin manifestations in his patients, Dr. Fernandez said the interferon hypothesis is “fair,” and “the best that’s out there.” Dr. Fernandez is currently studying cutaneous manifestations of COVID-19 as a principal investigator of a trial sponsored by the Clinical and Translational Science Collaborative of Cleveland.
Dr. Ko said in an interview that she has not observed long-haul skin issues in her patients, but Yale colleagues have.
In a study, she and Yale colleagues published in September, SARS-CoV-2 spike protein was detected in perniotic lesions, but not nuclear protein or viral RNA. The test they used – immunohistochemistry – can be nonspecific, which muddied results.
She does not think there is replicating virus in the skin or the lesions. Instead, said Dr. Ko, “either there is viral spike protein that has somehow become disassociated from actively replicating virus that somehow got deposited in endothelial cells,” or the staining “was spurious,” or some other protein is cross-reacting. “And the people who are unlucky enough to have that protein in their endothelial cells can manifest this COVID-toe, COVID-finger phenomenon.”
To her, it’s an unsolved mystery. “The weird thing is, we’ve never before had this much perniosis,” Dr. Ko said.
Dr. Fernandez is not convinced yet, noting that, in Cleveland, more pernio cases were observed in March and April than in the summer. “If it is a manifestation of the infection then you also need the right environment, the cold weather for this manifestation to present,” he said. “Or, it really isn’t a direct manifestation of COVID-19 but may be more related to other factors,” such as lifestyle changes related to limitations implemented to help mitigate the spread of the disease.
“To me the jury is still out whether or not the perniotic lesions really can tell us something about a patient’s exposure and infection with SARS-CoV-2,” he said.
Dr. Freeman reported receiving a grant from the International League of Dermatological Societies and nonfinancial support from the AAD for the study. Dr. Ko reported no conflicts. Dr. Fernadnez had no disclosures.
FROM THE LANCET INFECTIOUS DISEASES
New COPD mortality risk model includes imaging-derived variables
All-cause mortality in patients with COPD over 10 years of follow-up was accurately predicted by a newly developed model based on a point system incorporating imaging-derived variables.
Identifying risk factors is important to develop treatments and preventive strategies, but the role of imaging variables in COPD mortality among smokers has not been well studied, wrote investigator Matthew Strand, PhD, of National Jewish Health in Denver, and colleagues.
An established risk model is the body mass index–airflow Obstruction-Dyspnea-Exercise capacity (BODE) index, developed to predict mortality in COPD patients over a 4-year period. The investigators noted that while models such as BODE provide useful information about predictors of mortality in COPD, they were developed using participants in the Global initiative for obstructive Lung Disease (GOLD) spirometry grades 1-4, and have been largely constructed without quantitative computed tomography (CT) imaging variables until recently.
“The BODE index was created as a simple point scoring system to predict risk of all-cause mortality within 4 years, and is based on FEV1 [forced expiratory volume at 1 second], [6-minute walk test], dyspnea and BMI, a subset of predictors we considered in our model,” the investigators noted. The new model includes data from pulmonary function tests and volumetric CT scans.
In a study published in Chronic Obstructive Pulmonary Diseases, the researchers identified 9,074 current and past smokers in the COPD Genetic Epidemiology study (COPDGene) for whom complete data were available. They developed a point system to determine mortality risk in current and former smokers after controlling for multiple risk factors. The average age of the study population was 60 years. All participants were current or former smokers with a smoking history of at least 10 pack-years.
Assessments of the study participants included a medical history, pre- and post-bronchodilator spirometry, a 6-minute walk distance test, and inspiratory and expiratory CT scans. The researchers analyzed mortality risk in the context of Global Initiative for Obstructive Lung Disease (GOLD) classifications of patients in the sample.
Overall, the average 10-year mortality risk was 18% for women and 25% for men. Performance on the 6-minute walk test (distances less than 500 feet), FEV1 (less than 20), and older age (80 years and older) were the strongest predictors of mortality.
The model showed strong predictive accuracy, with an area under the receiver operating characteristic curve averaging 0.797 that was validated in an external cohort, the researchers said.
The study findings were limited by the observational design that does not allow for estimating the causal effects of such modifiable factors as smoking cessation, that might impact the walking test and FEV1 values, the researchers noted. In addition, the model did not allow for testing the effects of smoking vs. not smoking.
However, the model developed in the study “will allow physicians and patients to better understand factors affecting risk of an adverse event, some of which may be modifiable,” the researchers said. “The risk estimates can be used to target groups of individuals for future clinical trials, including those not currently classified as having COPD based on GOLD criteria,” they said.
The study was supported by the National Heart, Lung, and Blood Institute and by the COPD Foundation through contributions to an industry advisory committee including AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
All-cause mortality in patients with COPD over 10 years of follow-up was accurately predicted by a newly developed model based on a point system incorporating imaging-derived variables.
Identifying risk factors is important to develop treatments and preventive strategies, but the role of imaging variables in COPD mortality among smokers has not been well studied, wrote investigator Matthew Strand, PhD, of National Jewish Health in Denver, and colleagues.
An established risk model is the body mass index–airflow Obstruction-Dyspnea-Exercise capacity (BODE) index, developed to predict mortality in COPD patients over a 4-year period. The investigators noted that while models such as BODE provide useful information about predictors of mortality in COPD, they were developed using participants in the Global initiative for obstructive Lung Disease (GOLD) spirometry grades 1-4, and have been largely constructed without quantitative computed tomography (CT) imaging variables until recently.
“The BODE index was created as a simple point scoring system to predict risk of all-cause mortality within 4 years, and is based on FEV1 [forced expiratory volume at 1 second], [6-minute walk test], dyspnea and BMI, a subset of predictors we considered in our model,” the investigators noted. The new model includes data from pulmonary function tests and volumetric CT scans.
In a study published in Chronic Obstructive Pulmonary Diseases, the researchers identified 9,074 current and past smokers in the COPD Genetic Epidemiology study (COPDGene) for whom complete data were available. They developed a point system to determine mortality risk in current and former smokers after controlling for multiple risk factors. The average age of the study population was 60 years. All participants were current or former smokers with a smoking history of at least 10 pack-years.
Assessments of the study participants included a medical history, pre- and post-bronchodilator spirometry, a 6-minute walk distance test, and inspiratory and expiratory CT scans. The researchers analyzed mortality risk in the context of Global Initiative for Obstructive Lung Disease (GOLD) classifications of patients in the sample.
Overall, the average 10-year mortality risk was 18% for women and 25% for men. Performance on the 6-minute walk test (distances less than 500 feet), FEV1 (less than 20), and older age (80 years and older) were the strongest predictors of mortality.
The model showed strong predictive accuracy, with an area under the receiver operating characteristic curve averaging 0.797 that was validated in an external cohort, the researchers said.
The study findings were limited by the observational design that does not allow for estimating the causal effects of such modifiable factors as smoking cessation, that might impact the walking test and FEV1 values, the researchers noted. In addition, the model did not allow for testing the effects of smoking vs. not smoking.
However, the model developed in the study “will allow physicians and patients to better understand factors affecting risk of an adverse event, some of which may be modifiable,” the researchers said. “The risk estimates can be used to target groups of individuals for future clinical trials, including those not currently classified as having COPD based on GOLD criteria,” they said.
The study was supported by the National Heart, Lung, and Blood Institute and by the COPD Foundation through contributions to an industry advisory committee including AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
All-cause mortality in patients with COPD over 10 years of follow-up was accurately predicted by a newly developed model based on a point system incorporating imaging-derived variables.
Identifying risk factors is important to develop treatments and preventive strategies, but the role of imaging variables in COPD mortality among smokers has not been well studied, wrote investigator Matthew Strand, PhD, of National Jewish Health in Denver, and colleagues.
An established risk model is the body mass index–airflow Obstruction-Dyspnea-Exercise capacity (BODE) index, developed to predict mortality in COPD patients over a 4-year period. The investigators noted that while models such as BODE provide useful information about predictors of mortality in COPD, they were developed using participants in the Global initiative for obstructive Lung Disease (GOLD) spirometry grades 1-4, and have been largely constructed without quantitative computed tomography (CT) imaging variables until recently.
“The BODE index was created as a simple point scoring system to predict risk of all-cause mortality within 4 years, and is based on FEV1 [forced expiratory volume at 1 second], [6-minute walk test], dyspnea and BMI, a subset of predictors we considered in our model,” the investigators noted. The new model includes data from pulmonary function tests and volumetric CT scans.
In a study published in Chronic Obstructive Pulmonary Diseases, the researchers identified 9,074 current and past smokers in the COPD Genetic Epidemiology study (COPDGene) for whom complete data were available. They developed a point system to determine mortality risk in current and former smokers after controlling for multiple risk factors. The average age of the study population was 60 years. All participants were current or former smokers with a smoking history of at least 10 pack-years.
Assessments of the study participants included a medical history, pre- and post-bronchodilator spirometry, a 6-minute walk distance test, and inspiratory and expiratory CT scans. The researchers analyzed mortality risk in the context of Global Initiative for Obstructive Lung Disease (GOLD) classifications of patients in the sample.
Overall, the average 10-year mortality risk was 18% for women and 25% for men. Performance on the 6-minute walk test (distances less than 500 feet), FEV1 (less than 20), and older age (80 years and older) were the strongest predictors of mortality.
The model showed strong predictive accuracy, with an area under the receiver operating characteristic curve averaging 0.797 that was validated in an external cohort, the researchers said.
The study findings were limited by the observational design that does not allow for estimating the causal effects of such modifiable factors as smoking cessation, that might impact the walking test and FEV1 values, the researchers noted. In addition, the model did not allow for testing the effects of smoking vs. not smoking.
However, the model developed in the study “will allow physicians and patients to better understand factors affecting risk of an adverse event, some of which may be modifiable,” the researchers said. “The risk estimates can be used to target groups of individuals for future clinical trials, including those not currently classified as having COPD based on GOLD criteria,” they said.
The study was supported by the National Heart, Lung, and Blood Institute and by the COPD Foundation through contributions to an industry advisory committee including AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
FROM CHRONIC OBSTRUCTIVE PULMONARY DISEASES
Myocarditis by CMR may be rare after COVID-19 in elite athletes
Two recent observational studies suggest that myocarditis, at least on cardiac magnetic resonance (CMR) imaging, might be far less common in elite-level athletes recovering from COVID-19 than suggested in influential earlier reports.
Both new studies documented a rate less than one-quarter as high as those previously reported from smaller cohorts, raising questions about the diagnostic yield of CMR in highly conditioned athletes with recent COVID-19 absent other evidence, such as from biomarker assays or electrocardiography (ECG).
That could have implications for some top-tier university athletics programs that mandate CMR imaging, biomarker assays, and other evaluations for myocarditis on all their players who test positive for SARS-CoV-2 before they can return to play.
The findings collectively point to CMR imaging features that might be a hallmark of an athlete’s heart, characterized by normal myocardial remodeling brought on by elite-level exercise training, which in athletes with recent COVID-19 could be misinterpreted as evidence of myocarditis. That may have thrown off prevalence estimates in the literature, the studies’ investigators speculated.
The two studies were retrospective takes on university athletes who underwent CMR imaging while recovering from COVID-19, who were either asymptomatic or with only mild to moderate symptoms and were generally without ECG or troponin evidence of myocarditis.
One of them showed a less than 2% incidence of myocarditis by CMR among 145 such cases, a low yield for imaging that is “raising doubt regarding its utility to evaluate athletes without a clinical presentation or abnormal ancillary tests to support the diagnosis of myocarditis,” argues a report published Jan. 14 in JAMA Cardiology, with lead author Jitka Starekova, MD, University of Wisconsin – Madison.
“Part of the problem is that occult myocarditis is, at least with other viruses, a risk factor for sudden death in competitive athletes. So you don’t want to let one slip through the cracks,” senior author Scott B. Reeder, MD, PhD, from the same institution, said in an interview.
Whether a policy of routine CMR imaging in elite athletes who test positive for the new coronavirus is better than more selective use driven by symptoms or other screening tests is unknown. But the more pressing issue, Dr. Reeder said, “is if they have a normal electrocardiogram and troponins, do they still need cardiac magnetic resonance imaging?”
The current study, he said, “certainly provides helpful evidence that maybe we don’t need as many.”
The other study, which featured two control groups, saw a similarly low incidence of myocarditis by CMR in athletes with recent COVID-19. One of the control groups included university athletes imaged prior to the advent of SARS-CoV-2 in the university’s region of the country. The other consisted of apparently healthy adult nonathletes.
Armed with two non-COVID-19 cohorts and two athlete cohorts, the researchers found comparable rates of myocarditis by CMR in both the COVID-19 athletes and the healthy athletes. And only 3% of the COVID-19 athletes had the tell-tale CMR signs, notes the report, published Dec. 17 in Circulation, with lead author Daniel E. Clark, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn.
Reassurance and concern
“The incidence is much lower than we feared, and so that’s reassuring,” Clark said in an interview. Still, the athletes with myocarditis by CMR “would have been completely missed by a protocol that did not include cardiac MR, and that’s concerning,” he said. “Both had active myocarditis.”
The study’s two non-COVID-19 control groups – elite athletes in one and nonathletes in the other – allowed them to tease out the potential contribution of athletic myocardial remodeling to CMR features that could be interpreted as scar tissue, which are characterized by late gadolinium enhancement (LGE).
As it turned out, focal regions of LGE located in the right ventricular (RV) septum on the scans were often seen in both athlete cohorts. “This kind of trivial nonischemic fibrosis in the mid RV septal insertion site was common among athletic control subjects. It was seen in 24% of them, which is almost identical to the percentage that we saw in the COVID-19 athletes, 22%,” Dr. Clark said.
The LGE finding, wrote Dr. Clark and coauthors, “may represent remodeling from athletic training, and should not be conflated with myocarditis.”
Of note, the other study saw a comparable incidence of the same or a very similar CMR feature in its athletes; 26% of the Wisconsin COVID-19 athlete cohort showed limited focal LGE in the inferior RV insertion site.
“And you get a little bit in the mid-septum, as well,” Dr. Reeder said. But the sign, in the absence of any corresponding T2 abnormalities, was not judged to represent myocarditis. “We interpreted all of these studies with this potential confounder in mind.”
Conceivably, Dr. Reeder proposed, the earlier studies may have “over-called” the prevalence of myocarditis in their cohorts. “I haven’t seen their images, but it’s possible there could be false-positives.”
It’s noteworthy that the Vanderbilt and Wisconsin reports saw closely similar incidences of the tell-tale CMR sign in all the athlete cohorts whether or not COVID-19 was involved, Aaron L. Baggish, MD, Massachusetts General Hospital, Boston, said in an interview.
“It looks very much like just an unrecognized part of athletic remodeling and isn’t in any way, shape, or form implicated as being a COVID-related issue,” said Dr. Baggish, who directs the cardiovascular performance program at his center and is unaffiliated with either study.
Still, that connection remains unproven given how little is yet known about the prevalence of clinically important myocarditis in milder cases of COVID-19, according to an accompanying editorial from Jonathan H. Kim, MD, MSc.
Although isolated LGE at the interventricular RV insertion site is “more commonly described among masters-level endurance athletes, the clinical significance and prevalence of this finding in youthful athletes is uncertain and should not be assumed to be a normal consequence of intense athletic training in young competitive athletes,” argued Dr. Kim, of Emory University, Atlanta.
There’s probably little about being a young competitive athlete that would render a person any more or less prone to COVID-19 cardiac involvement, Dr. Baggish said. Rather, “I think what we’re seeing, as the studies continue to come out, is that prevalence estimates are getting into the low single digits.”
The estimates are similar to those associated with influenza before the COVID-19 age; about 2% of patients showed cardiac involvement, Dr. Baggish said. “So the degree to which COVID is a special virus from this perspective, I think, is still a topic of some debate.”
The two current studies have limitations and neither is positioned to change practice, he said. “I would say that they are both kind of important, reassuring pieces of an unfinished jigsaw puzzle. But we still don’t know what the picture on the puzzle is.”
Routine CMR for positive cases
The University of Wisconsin group looked at all of the institution’s competitive athletes who underwent gadolinium-enhanced CMR imaging and other tests during recovery from COVID-19 from the beginning of the pandemic to the end of November 2020.
The imaging was performed on average about 2 weeks after a first positive SARS-CoV-2 assay result. About one-half and one-fourth of the cohort had experienced mild and moderate symptoms, respectively, and about 17% were asymptomatic; none had been hospitalized.
All CMR scans were reviewed by two experienced radiologists for, among other things, evidence of myocarditis according to modified Lake Louise criteria, the group wrote. Those criteria are based on CMR markers of fibrosis and other characteristics of scarring from myocarditis.
Such evidence was seen in only two members of the cohort, or 1.4%, one with elevated troponins but normal with respect to other biomarkers, and the other negative for all assays. Both were asymptomatic at the time of imaging, the report noted.
The Vanderbilt analysis from Dr. Clark and associates centered on 59 university athletes recently with COVID-19 who underwent CMR imaging along with other tests about 3 weeks after confirmation of SARS-CoV-2 infection. Symptoms had been mild in 78% of the group, and the remainder were asymptomatic.
They were compared with 60 retrospectively identified college athletes and elite-conditioned military personnel who had undergone CMR imaging prior to the advent of COVID-19, and to 27 apparently healthy nonathlete adults in whom CMR had been previously performed to define normal CMR imaging criteria at that center.
The only two post-COVID-19 athletes who met modified Lake Louise criteria for myocarditis showed no abnormalities on ECG or myocardial strain echocardiography, and had normal troponins, the group reported.
The COVID-19 athletes showed increased cardiac chamber volumes and myocardial mass “consistent with athletic remodeling,” compared with the healthy control subjects, the group wrote. But “most standard CMR parameters were similar” between the COVID-19 athletes and the control athletes, consistent with the 22% and 24% rates, respectively, for the finding of focal late LGE isolated to the inferoseptal RV insertion site.
At the end of the day, all published experiences on athletes with recent COVID-19 “are descriptive studies, without any hint of follow-up,” Dr. Baggish noted, so their clinical implications are unknown.
“We need time to sit and watch to see what happens to these individuals,” he said. “And if the answer is nothing, then that’s a very reassuring story. If the answer is that we start to see events, then that’s really important for us to take stock of.”
Dr. Starekova had no disclosures. Dr. Reeder reports that the University of Wisconsin receives research support from GE Healthcare and Bracco Diagnostics; and that he has ownership interests in Calimetrix, Reveal Pharmaceuticals, Cellectar Biosciences, Elucent Medical, and HeartVista; and has received grant support from Bayer Healthcare. Disclosures for the other coauthors are in the report. Dr. Clark and coauthors had no disclosures. Dr. Baggish reported no conflicts. Kim discloses receiving funding from the National Heart, Lung, and Blood Institute; compensation as team cardiologist for the Atlanta Falcons; and research stipends from the Atlanta Track Club.
A version of this article first appeared on Medscape.com.
Two recent observational studies suggest that myocarditis, at least on cardiac magnetic resonance (CMR) imaging, might be far less common in elite-level athletes recovering from COVID-19 than suggested in influential earlier reports.
Both new studies documented a rate less than one-quarter as high as those previously reported from smaller cohorts, raising questions about the diagnostic yield of CMR in highly conditioned athletes with recent COVID-19 absent other evidence, such as from biomarker assays or electrocardiography (ECG).
That could have implications for some top-tier university athletics programs that mandate CMR imaging, biomarker assays, and other evaluations for myocarditis on all their players who test positive for SARS-CoV-2 before they can return to play.
The findings collectively point to CMR imaging features that might be a hallmark of an athlete’s heart, characterized by normal myocardial remodeling brought on by elite-level exercise training, which in athletes with recent COVID-19 could be misinterpreted as evidence of myocarditis. That may have thrown off prevalence estimates in the literature, the studies’ investigators speculated.
The two studies were retrospective takes on university athletes who underwent CMR imaging while recovering from COVID-19, who were either asymptomatic or with only mild to moderate symptoms and were generally without ECG or troponin evidence of myocarditis.
One of them showed a less than 2% incidence of myocarditis by CMR among 145 such cases, a low yield for imaging that is “raising doubt regarding its utility to evaluate athletes without a clinical presentation or abnormal ancillary tests to support the diagnosis of myocarditis,” argues a report published Jan. 14 in JAMA Cardiology, with lead author Jitka Starekova, MD, University of Wisconsin – Madison.
“Part of the problem is that occult myocarditis is, at least with other viruses, a risk factor for sudden death in competitive athletes. So you don’t want to let one slip through the cracks,” senior author Scott B. Reeder, MD, PhD, from the same institution, said in an interview.
Whether a policy of routine CMR imaging in elite athletes who test positive for the new coronavirus is better than more selective use driven by symptoms or other screening tests is unknown. But the more pressing issue, Dr. Reeder said, “is if they have a normal electrocardiogram and troponins, do they still need cardiac magnetic resonance imaging?”
The current study, he said, “certainly provides helpful evidence that maybe we don’t need as many.”
The other study, which featured two control groups, saw a similarly low incidence of myocarditis by CMR in athletes with recent COVID-19. One of the control groups included university athletes imaged prior to the advent of SARS-CoV-2 in the university’s region of the country. The other consisted of apparently healthy adult nonathletes.
Armed with two non-COVID-19 cohorts and two athlete cohorts, the researchers found comparable rates of myocarditis by CMR in both the COVID-19 athletes and the healthy athletes. And only 3% of the COVID-19 athletes had the tell-tale CMR signs, notes the report, published Dec. 17 in Circulation, with lead author Daniel E. Clark, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn.
Reassurance and concern
“The incidence is much lower than we feared, and so that’s reassuring,” Clark said in an interview. Still, the athletes with myocarditis by CMR “would have been completely missed by a protocol that did not include cardiac MR, and that’s concerning,” he said. “Both had active myocarditis.”
The study’s two non-COVID-19 control groups – elite athletes in one and nonathletes in the other – allowed them to tease out the potential contribution of athletic myocardial remodeling to CMR features that could be interpreted as scar tissue, which are characterized by late gadolinium enhancement (LGE).
As it turned out, focal regions of LGE located in the right ventricular (RV) septum on the scans were often seen in both athlete cohorts. “This kind of trivial nonischemic fibrosis in the mid RV septal insertion site was common among athletic control subjects. It was seen in 24% of them, which is almost identical to the percentage that we saw in the COVID-19 athletes, 22%,” Dr. Clark said.
The LGE finding, wrote Dr. Clark and coauthors, “may represent remodeling from athletic training, and should not be conflated with myocarditis.”
Of note, the other study saw a comparable incidence of the same or a very similar CMR feature in its athletes; 26% of the Wisconsin COVID-19 athlete cohort showed limited focal LGE in the inferior RV insertion site.
“And you get a little bit in the mid-septum, as well,” Dr. Reeder said. But the sign, in the absence of any corresponding T2 abnormalities, was not judged to represent myocarditis. “We interpreted all of these studies with this potential confounder in mind.”
Conceivably, Dr. Reeder proposed, the earlier studies may have “over-called” the prevalence of myocarditis in their cohorts. “I haven’t seen their images, but it’s possible there could be false-positives.”
It’s noteworthy that the Vanderbilt and Wisconsin reports saw closely similar incidences of the tell-tale CMR sign in all the athlete cohorts whether or not COVID-19 was involved, Aaron L. Baggish, MD, Massachusetts General Hospital, Boston, said in an interview.
“It looks very much like just an unrecognized part of athletic remodeling and isn’t in any way, shape, or form implicated as being a COVID-related issue,” said Dr. Baggish, who directs the cardiovascular performance program at his center and is unaffiliated with either study.
Still, that connection remains unproven given how little is yet known about the prevalence of clinically important myocarditis in milder cases of COVID-19, according to an accompanying editorial from Jonathan H. Kim, MD, MSc.
Although isolated LGE at the interventricular RV insertion site is “more commonly described among masters-level endurance athletes, the clinical significance and prevalence of this finding in youthful athletes is uncertain and should not be assumed to be a normal consequence of intense athletic training in young competitive athletes,” argued Dr. Kim, of Emory University, Atlanta.
There’s probably little about being a young competitive athlete that would render a person any more or less prone to COVID-19 cardiac involvement, Dr. Baggish said. Rather, “I think what we’re seeing, as the studies continue to come out, is that prevalence estimates are getting into the low single digits.”
The estimates are similar to those associated with influenza before the COVID-19 age; about 2% of patients showed cardiac involvement, Dr. Baggish said. “So the degree to which COVID is a special virus from this perspective, I think, is still a topic of some debate.”
The two current studies have limitations and neither is positioned to change practice, he said. “I would say that they are both kind of important, reassuring pieces of an unfinished jigsaw puzzle. But we still don’t know what the picture on the puzzle is.”
Routine CMR for positive cases
The University of Wisconsin group looked at all of the institution’s competitive athletes who underwent gadolinium-enhanced CMR imaging and other tests during recovery from COVID-19 from the beginning of the pandemic to the end of November 2020.
The imaging was performed on average about 2 weeks after a first positive SARS-CoV-2 assay result. About one-half and one-fourth of the cohort had experienced mild and moderate symptoms, respectively, and about 17% were asymptomatic; none had been hospitalized.
All CMR scans were reviewed by two experienced radiologists for, among other things, evidence of myocarditis according to modified Lake Louise criteria, the group wrote. Those criteria are based on CMR markers of fibrosis and other characteristics of scarring from myocarditis.
Such evidence was seen in only two members of the cohort, or 1.4%, one with elevated troponins but normal with respect to other biomarkers, and the other negative for all assays. Both were asymptomatic at the time of imaging, the report noted.
The Vanderbilt analysis from Dr. Clark and associates centered on 59 university athletes recently with COVID-19 who underwent CMR imaging along with other tests about 3 weeks after confirmation of SARS-CoV-2 infection. Symptoms had been mild in 78% of the group, and the remainder were asymptomatic.
They were compared with 60 retrospectively identified college athletes and elite-conditioned military personnel who had undergone CMR imaging prior to the advent of COVID-19, and to 27 apparently healthy nonathlete adults in whom CMR had been previously performed to define normal CMR imaging criteria at that center.
The only two post-COVID-19 athletes who met modified Lake Louise criteria for myocarditis showed no abnormalities on ECG or myocardial strain echocardiography, and had normal troponins, the group reported.
The COVID-19 athletes showed increased cardiac chamber volumes and myocardial mass “consistent with athletic remodeling,” compared with the healthy control subjects, the group wrote. But “most standard CMR parameters were similar” between the COVID-19 athletes and the control athletes, consistent with the 22% and 24% rates, respectively, for the finding of focal late LGE isolated to the inferoseptal RV insertion site.
At the end of the day, all published experiences on athletes with recent COVID-19 “are descriptive studies, without any hint of follow-up,” Dr. Baggish noted, so their clinical implications are unknown.
“We need time to sit and watch to see what happens to these individuals,” he said. “And if the answer is nothing, then that’s a very reassuring story. If the answer is that we start to see events, then that’s really important for us to take stock of.”
Dr. Starekova had no disclosures. Dr. Reeder reports that the University of Wisconsin receives research support from GE Healthcare and Bracco Diagnostics; and that he has ownership interests in Calimetrix, Reveal Pharmaceuticals, Cellectar Biosciences, Elucent Medical, and HeartVista; and has received grant support from Bayer Healthcare. Disclosures for the other coauthors are in the report. Dr. Clark and coauthors had no disclosures. Dr. Baggish reported no conflicts. Kim discloses receiving funding from the National Heart, Lung, and Blood Institute; compensation as team cardiologist for the Atlanta Falcons; and research stipends from the Atlanta Track Club.
A version of this article first appeared on Medscape.com.
Two recent observational studies suggest that myocarditis, at least on cardiac magnetic resonance (CMR) imaging, might be far less common in elite-level athletes recovering from COVID-19 than suggested in influential earlier reports.
Both new studies documented a rate less than one-quarter as high as those previously reported from smaller cohorts, raising questions about the diagnostic yield of CMR in highly conditioned athletes with recent COVID-19 absent other evidence, such as from biomarker assays or electrocardiography (ECG).
That could have implications for some top-tier university athletics programs that mandate CMR imaging, biomarker assays, and other evaluations for myocarditis on all their players who test positive for SARS-CoV-2 before they can return to play.
The findings collectively point to CMR imaging features that might be a hallmark of an athlete’s heart, characterized by normal myocardial remodeling brought on by elite-level exercise training, which in athletes with recent COVID-19 could be misinterpreted as evidence of myocarditis. That may have thrown off prevalence estimates in the literature, the studies’ investigators speculated.
The two studies were retrospective takes on university athletes who underwent CMR imaging while recovering from COVID-19, who were either asymptomatic or with only mild to moderate symptoms and were generally without ECG or troponin evidence of myocarditis.
One of them showed a less than 2% incidence of myocarditis by CMR among 145 such cases, a low yield for imaging that is “raising doubt regarding its utility to evaluate athletes without a clinical presentation or abnormal ancillary tests to support the diagnosis of myocarditis,” argues a report published Jan. 14 in JAMA Cardiology, with lead author Jitka Starekova, MD, University of Wisconsin – Madison.
“Part of the problem is that occult myocarditis is, at least with other viruses, a risk factor for sudden death in competitive athletes. So you don’t want to let one slip through the cracks,” senior author Scott B. Reeder, MD, PhD, from the same institution, said in an interview.
Whether a policy of routine CMR imaging in elite athletes who test positive for the new coronavirus is better than more selective use driven by symptoms or other screening tests is unknown. But the more pressing issue, Dr. Reeder said, “is if they have a normal electrocardiogram and troponins, do they still need cardiac magnetic resonance imaging?”
The current study, he said, “certainly provides helpful evidence that maybe we don’t need as many.”
The other study, which featured two control groups, saw a similarly low incidence of myocarditis by CMR in athletes with recent COVID-19. One of the control groups included university athletes imaged prior to the advent of SARS-CoV-2 in the university’s region of the country. The other consisted of apparently healthy adult nonathletes.
Armed with two non-COVID-19 cohorts and two athlete cohorts, the researchers found comparable rates of myocarditis by CMR in both the COVID-19 athletes and the healthy athletes. And only 3% of the COVID-19 athletes had the tell-tale CMR signs, notes the report, published Dec. 17 in Circulation, with lead author Daniel E. Clark, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn.
Reassurance and concern
“The incidence is much lower than we feared, and so that’s reassuring,” Clark said in an interview. Still, the athletes with myocarditis by CMR “would have been completely missed by a protocol that did not include cardiac MR, and that’s concerning,” he said. “Both had active myocarditis.”
The study’s two non-COVID-19 control groups – elite athletes in one and nonathletes in the other – allowed them to tease out the potential contribution of athletic myocardial remodeling to CMR features that could be interpreted as scar tissue, which are characterized by late gadolinium enhancement (LGE).
As it turned out, focal regions of LGE located in the right ventricular (RV) septum on the scans were often seen in both athlete cohorts. “This kind of trivial nonischemic fibrosis in the mid RV septal insertion site was common among athletic control subjects. It was seen in 24% of them, which is almost identical to the percentage that we saw in the COVID-19 athletes, 22%,” Dr. Clark said.
The LGE finding, wrote Dr. Clark and coauthors, “may represent remodeling from athletic training, and should not be conflated with myocarditis.”
Of note, the other study saw a comparable incidence of the same or a very similar CMR feature in its athletes; 26% of the Wisconsin COVID-19 athlete cohort showed limited focal LGE in the inferior RV insertion site.
“And you get a little bit in the mid-septum, as well,” Dr. Reeder said. But the sign, in the absence of any corresponding T2 abnormalities, was not judged to represent myocarditis. “We interpreted all of these studies with this potential confounder in mind.”
Conceivably, Dr. Reeder proposed, the earlier studies may have “over-called” the prevalence of myocarditis in their cohorts. “I haven’t seen their images, but it’s possible there could be false-positives.”
It’s noteworthy that the Vanderbilt and Wisconsin reports saw closely similar incidences of the tell-tale CMR sign in all the athlete cohorts whether or not COVID-19 was involved, Aaron L. Baggish, MD, Massachusetts General Hospital, Boston, said in an interview.
“It looks very much like just an unrecognized part of athletic remodeling and isn’t in any way, shape, or form implicated as being a COVID-related issue,” said Dr. Baggish, who directs the cardiovascular performance program at his center and is unaffiliated with either study.
Still, that connection remains unproven given how little is yet known about the prevalence of clinically important myocarditis in milder cases of COVID-19, according to an accompanying editorial from Jonathan H. Kim, MD, MSc.
Although isolated LGE at the interventricular RV insertion site is “more commonly described among masters-level endurance athletes, the clinical significance and prevalence of this finding in youthful athletes is uncertain and should not be assumed to be a normal consequence of intense athletic training in young competitive athletes,” argued Dr. Kim, of Emory University, Atlanta.
There’s probably little about being a young competitive athlete that would render a person any more or less prone to COVID-19 cardiac involvement, Dr. Baggish said. Rather, “I think what we’re seeing, as the studies continue to come out, is that prevalence estimates are getting into the low single digits.”
The estimates are similar to those associated with influenza before the COVID-19 age; about 2% of patients showed cardiac involvement, Dr. Baggish said. “So the degree to which COVID is a special virus from this perspective, I think, is still a topic of some debate.”
The two current studies have limitations and neither is positioned to change practice, he said. “I would say that they are both kind of important, reassuring pieces of an unfinished jigsaw puzzle. But we still don’t know what the picture on the puzzle is.”
Routine CMR for positive cases
The University of Wisconsin group looked at all of the institution’s competitive athletes who underwent gadolinium-enhanced CMR imaging and other tests during recovery from COVID-19 from the beginning of the pandemic to the end of November 2020.
The imaging was performed on average about 2 weeks after a first positive SARS-CoV-2 assay result. About one-half and one-fourth of the cohort had experienced mild and moderate symptoms, respectively, and about 17% were asymptomatic; none had been hospitalized.
All CMR scans were reviewed by two experienced radiologists for, among other things, evidence of myocarditis according to modified Lake Louise criteria, the group wrote. Those criteria are based on CMR markers of fibrosis and other characteristics of scarring from myocarditis.
Such evidence was seen in only two members of the cohort, or 1.4%, one with elevated troponins but normal with respect to other biomarkers, and the other negative for all assays. Both were asymptomatic at the time of imaging, the report noted.
The Vanderbilt analysis from Dr. Clark and associates centered on 59 university athletes recently with COVID-19 who underwent CMR imaging along with other tests about 3 weeks after confirmation of SARS-CoV-2 infection. Symptoms had been mild in 78% of the group, and the remainder were asymptomatic.
They were compared with 60 retrospectively identified college athletes and elite-conditioned military personnel who had undergone CMR imaging prior to the advent of COVID-19, and to 27 apparently healthy nonathlete adults in whom CMR had been previously performed to define normal CMR imaging criteria at that center.
The only two post-COVID-19 athletes who met modified Lake Louise criteria for myocarditis showed no abnormalities on ECG or myocardial strain echocardiography, and had normal troponins, the group reported.
The COVID-19 athletes showed increased cardiac chamber volumes and myocardial mass “consistent with athletic remodeling,” compared with the healthy control subjects, the group wrote. But “most standard CMR parameters were similar” between the COVID-19 athletes and the control athletes, consistent with the 22% and 24% rates, respectively, for the finding of focal late LGE isolated to the inferoseptal RV insertion site.
At the end of the day, all published experiences on athletes with recent COVID-19 “are descriptive studies, without any hint of follow-up,” Dr. Baggish noted, so their clinical implications are unknown.
“We need time to sit and watch to see what happens to these individuals,” he said. “And if the answer is nothing, then that’s a very reassuring story. If the answer is that we start to see events, then that’s really important for us to take stock of.”
Dr. Starekova had no disclosures. Dr. Reeder reports that the University of Wisconsin receives research support from GE Healthcare and Bracco Diagnostics; and that he has ownership interests in Calimetrix, Reveal Pharmaceuticals, Cellectar Biosciences, Elucent Medical, and HeartVista; and has received grant support from Bayer Healthcare. Disclosures for the other coauthors are in the report. Dr. Clark and coauthors had no disclosures. Dr. Baggish reported no conflicts. Kim discloses receiving funding from the National Heart, Lung, and Blood Institute; compensation as team cardiologist for the Atlanta Falcons; and research stipends from the Atlanta Track Club.
A version of this article first appeared on Medscape.com.
Lung disease raises mortality risk in older RA patients
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
FROM RHEUMATOLOGY
U.K. variant spreading in the U.S. as COVID mutations raise stakes
The U.K.’s B117 variant is circulating in at least 24 states, according to new data from the Centers for Disease Control and Prevention COVID-19 variant surveillance. The CDC projects that the U.K. variant will become the dominant strain in the United States by March.
From any vantage point, the United Kingdom appears to be in the crosshairs of COVID-19: Weeks after a new, highly contagious variant emerged that fueled a surge in cases and fresh lockdowns, the United Kingdom was revealed to have the world’s highest coronavirus death rate.
But the United Kingdom also has a not-so-secret weapon of its own: A genomic sequencing program widely believed to be the most coordinated and advanced any nation has forged. In the vise grip of the virus, the Brits have gleaned key insights into the behavior and consequences of SARS-CoV-2.
But B117 is also notable for what it is missing: In this case, producing a negative result on certain polymerase chain reaction (PCR) tests in the spike protein, or S-gene.
One of the S-gene mutations specific to the variant deletes two amino acids, causing that portion of the PCR test to show up negative. The coincidental finding known as an S-gene target failure has become an integral proxy to help track where and when the variant is spreading in the United Kingdom, where about 5% of samples from COVID-19–infected patients are sequenced, said Sharon Peacock, PhD, executive director and chair of the COVID-19 Genomics U.K. Consortium.
That same tactic could prove valuable to clinicians similarly overwhelmed with cases and deaths but lacking high-level sequencing information on the virus, Dr. Peacock said in an interview. A British report released Friday stated that there is a “realistic possibility” that the variant has a higher death rate than other cases of SARS-CoV-2.
“In this particular variant, a deletion in the genome leads to one part of the diagnostic test failing,” Dr. Peacock explained. “Several targets are positive, but this is negative. In the U.K., this has been used as a surrogate marker.”
Targeting an invisible adversary
B117 is not the only variant that produces this result, Dr. Peacock cautioned, “but in screening for it, you can have this in mind.”
“Since the U.K. is sequencing about 5% of the cases they detect, this gives them really important clues about what’s happening there,” said Anderson Brito, PhD, a virologist and postdoctoral researcher at Yale University, New Haven, Conn., where investigators are creating custom PCR tests to detect the B117 variant.
Dr. Brito, who lived in the United Kingdom for 4 years while studying for his doctorate at Imperial College London, said a “major advantage” is the more unified process to collect and sequence samples. Crucial information – including the date and place of collection – comes with each sample, which fuels not only sequencing, but an epidemiologic perspective.
“They’re not in the dark at all,” Dr. Brito said in an interview. “I think no other country in the world knows better which virus lineages are circulating.”
The CDC launched the SPHERES consortium in May 2020 to coordinate the sequencing of SARS-CoV-2 genomes across the United States.
But American genomic efforts are “not as centralized,” said Dr. Brito, whose lab detected the first two cases of the U.K. variant in Connecticut on Jan. 6. “We struggle to get samples, because they’re decentralized to a level where there’s little coordination between hospitals and research centers. They’re not as connected as in the U.K. If we just get a sample and it has no date of collection and no origin information, for example, it’s basically useless.”
Global genomic collaborations include GISAID, an international database where researchers share new genomes from various coronaviruses. As of mid-January, the United States had submitted about 68,000 sequences to GISAID, adding about 3,000 new samples every week and expecting even more from commercial labs in coming days, according to the CDC.
“The U.K. is definitely much more on top of looking for variants as they pop up,” said Gigi Gronvall, PhD, an immunologist and senior scholar at Johns Hopkins Center for Health Security in Baltimore. “The U.S. has now turned that up.”
Warning from British scientists to the world
Despite these genomic accomplishments, some British scientists said they have regrets too, wishing they’d known just how rapidly SARS-CoV-2 was actually spreading a year ago, when it hit western Europe.
That information was crucial not only for preventive efforts, but because viruses inevitably mutate faster the more people who are infected, said Igor Rudan, MD, PhD, director of the Center for Global Health Research at University of Edinburgh.
“Italy showed us just how fast it was spreading and how deadly it is for the very old and people with multiple comorbidities,” said Dr. Rudan, who also editor in chief of the Journal of Global Health. “We wish we knew it was spreading so fast, and we wish we knew the threshold of cases we could allow to be infected before the virus would mutate.”
More mutations mean more new strains of SARS-CoV-2, Dr. Rudan said in an interview. “We’ve reached that threshold now and will see more of these mutations.”
Despite its current struggles, the United Kingdom is reaching beyond tracking its new variant’s spread and trying to identify new mutations that might change the way the virus behaves.
Three features of any emerging variant are particularly important, Dr. Peacock explained: Is it more transmissible? Is it more lethal? And does it cut the ability of natural- or vaccine-induced immunity to protect people from infection?
“We need to sequence people coming to the hospital who are sicker,” said Dr. Peacock, also a professor of public health and microbiology at the University of Cambridge (England). “Also, if anyone has the infection after they’ve already been sick or had the vaccine, we really want to know what that looks like” genomically.
SARS-CoV-2 has already logged more than 4,000 mutations, Dr. Peacock said. But “knowing that viruses mutate all the time is not sufficient reason not to look. We really want to know if mutations lead to changes in amino acids, and if that can lead to changes in functionality.”
For the moment, however, experts say they’re relieved that the U.K. strain doesn’t seem able to evade COVID-19 vaccines or render them less effective.
“Even though mutations are common, those able to change the viral coding are rare,” Dr. Brito explained. If necessary, vaccines could be tweaked to replace the spike gene sequence “within a matter of weeks. We already do this for flu vaccines. Every year, we have to monitor variants of the virus circulating to develop a vaccine that covers most of them. If we end up having to do it for SARS-CoV-2, I would not be surprised.”
But variant-fueled increases in infections will require more people to be vaccinated before herd immunity can be achieved, Dr. Rudan warned. “If it spreads faster, we’ll need to vaccinate probably 85% of people versus 70% to reach herd immunity.”
One lesson the COVID-19 pandemic has driven home “is to always be on your guard about what happens next,” Dr. Peacock said. Although confident about the genomic efforts in the United Kingdom to date, she and her colleagues feel they’re still reaching for a complete understanding of the evolutionary changes of the virus.
“We’re ahead of the curve right now, but we want to get in front of the curve,” Dr. Peacock said. “It’s essential to get ahead of what might be around the corner because we don’t know how the virus is going to evolve.”
A version of this article first appeared on Medscape.com.
The U.K.’s B117 variant is circulating in at least 24 states, according to new data from the Centers for Disease Control and Prevention COVID-19 variant surveillance. The CDC projects that the U.K. variant will become the dominant strain in the United States by March.
From any vantage point, the United Kingdom appears to be in the crosshairs of COVID-19: Weeks after a new, highly contagious variant emerged that fueled a surge in cases and fresh lockdowns, the United Kingdom was revealed to have the world’s highest coronavirus death rate.
But the United Kingdom also has a not-so-secret weapon of its own: A genomic sequencing program widely believed to be the most coordinated and advanced any nation has forged. In the vise grip of the virus, the Brits have gleaned key insights into the behavior and consequences of SARS-CoV-2.
But B117 is also notable for what it is missing: In this case, producing a negative result on certain polymerase chain reaction (PCR) tests in the spike protein, or S-gene.
One of the S-gene mutations specific to the variant deletes two amino acids, causing that portion of the PCR test to show up negative. The coincidental finding known as an S-gene target failure has become an integral proxy to help track where and when the variant is spreading in the United Kingdom, where about 5% of samples from COVID-19–infected patients are sequenced, said Sharon Peacock, PhD, executive director and chair of the COVID-19 Genomics U.K. Consortium.
That same tactic could prove valuable to clinicians similarly overwhelmed with cases and deaths but lacking high-level sequencing information on the virus, Dr. Peacock said in an interview. A British report released Friday stated that there is a “realistic possibility” that the variant has a higher death rate than other cases of SARS-CoV-2.
“In this particular variant, a deletion in the genome leads to one part of the diagnostic test failing,” Dr. Peacock explained. “Several targets are positive, but this is negative. In the U.K., this has been used as a surrogate marker.”
Targeting an invisible adversary
B117 is not the only variant that produces this result, Dr. Peacock cautioned, “but in screening for it, you can have this in mind.”
“Since the U.K. is sequencing about 5% of the cases they detect, this gives them really important clues about what’s happening there,” said Anderson Brito, PhD, a virologist and postdoctoral researcher at Yale University, New Haven, Conn., where investigators are creating custom PCR tests to detect the B117 variant.
Dr. Brito, who lived in the United Kingdom for 4 years while studying for his doctorate at Imperial College London, said a “major advantage” is the more unified process to collect and sequence samples. Crucial information – including the date and place of collection – comes with each sample, which fuels not only sequencing, but an epidemiologic perspective.
“They’re not in the dark at all,” Dr. Brito said in an interview. “I think no other country in the world knows better which virus lineages are circulating.”
The CDC launched the SPHERES consortium in May 2020 to coordinate the sequencing of SARS-CoV-2 genomes across the United States.
But American genomic efforts are “not as centralized,” said Dr. Brito, whose lab detected the first two cases of the U.K. variant in Connecticut on Jan. 6. “We struggle to get samples, because they’re decentralized to a level where there’s little coordination between hospitals and research centers. They’re not as connected as in the U.K. If we just get a sample and it has no date of collection and no origin information, for example, it’s basically useless.”
Global genomic collaborations include GISAID, an international database where researchers share new genomes from various coronaviruses. As of mid-January, the United States had submitted about 68,000 sequences to GISAID, adding about 3,000 new samples every week and expecting even more from commercial labs in coming days, according to the CDC.
“The U.K. is definitely much more on top of looking for variants as they pop up,” said Gigi Gronvall, PhD, an immunologist and senior scholar at Johns Hopkins Center for Health Security in Baltimore. “The U.S. has now turned that up.”
Warning from British scientists to the world
Despite these genomic accomplishments, some British scientists said they have regrets too, wishing they’d known just how rapidly SARS-CoV-2 was actually spreading a year ago, when it hit western Europe.
That information was crucial not only for preventive efforts, but because viruses inevitably mutate faster the more people who are infected, said Igor Rudan, MD, PhD, director of the Center for Global Health Research at University of Edinburgh.
“Italy showed us just how fast it was spreading and how deadly it is for the very old and people with multiple comorbidities,” said Dr. Rudan, who also editor in chief of the Journal of Global Health. “We wish we knew it was spreading so fast, and we wish we knew the threshold of cases we could allow to be infected before the virus would mutate.”
More mutations mean more new strains of SARS-CoV-2, Dr. Rudan said in an interview. “We’ve reached that threshold now and will see more of these mutations.”
Despite its current struggles, the United Kingdom is reaching beyond tracking its new variant’s spread and trying to identify new mutations that might change the way the virus behaves.
Three features of any emerging variant are particularly important, Dr. Peacock explained: Is it more transmissible? Is it more lethal? And does it cut the ability of natural- or vaccine-induced immunity to protect people from infection?
“We need to sequence people coming to the hospital who are sicker,” said Dr. Peacock, also a professor of public health and microbiology at the University of Cambridge (England). “Also, if anyone has the infection after they’ve already been sick or had the vaccine, we really want to know what that looks like” genomically.
SARS-CoV-2 has already logged more than 4,000 mutations, Dr. Peacock said. But “knowing that viruses mutate all the time is not sufficient reason not to look. We really want to know if mutations lead to changes in amino acids, and if that can lead to changes in functionality.”
For the moment, however, experts say they’re relieved that the U.K. strain doesn’t seem able to evade COVID-19 vaccines or render them less effective.
“Even though mutations are common, those able to change the viral coding are rare,” Dr. Brito explained. If necessary, vaccines could be tweaked to replace the spike gene sequence “within a matter of weeks. We already do this for flu vaccines. Every year, we have to monitor variants of the virus circulating to develop a vaccine that covers most of them. If we end up having to do it for SARS-CoV-2, I would not be surprised.”
But variant-fueled increases in infections will require more people to be vaccinated before herd immunity can be achieved, Dr. Rudan warned. “If it spreads faster, we’ll need to vaccinate probably 85% of people versus 70% to reach herd immunity.”
One lesson the COVID-19 pandemic has driven home “is to always be on your guard about what happens next,” Dr. Peacock said. Although confident about the genomic efforts in the United Kingdom to date, she and her colleagues feel they’re still reaching for a complete understanding of the evolutionary changes of the virus.
“We’re ahead of the curve right now, but we want to get in front of the curve,” Dr. Peacock said. “It’s essential to get ahead of what might be around the corner because we don’t know how the virus is going to evolve.”
A version of this article first appeared on Medscape.com.
The U.K.’s B117 variant is circulating in at least 24 states, according to new data from the Centers for Disease Control and Prevention COVID-19 variant surveillance. The CDC projects that the U.K. variant will become the dominant strain in the United States by March.
From any vantage point, the United Kingdom appears to be in the crosshairs of COVID-19: Weeks after a new, highly contagious variant emerged that fueled a surge in cases and fresh lockdowns, the United Kingdom was revealed to have the world’s highest coronavirus death rate.
But the United Kingdom also has a not-so-secret weapon of its own: A genomic sequencing program widely believed to be the most coordinated and advanced any nation has forged. In the vise grip of the virus, the Brits have gleaned key insights into the behavior and consequences of SARS-CoV-2.
But B117 is also notable for what it is missing: In this case, producing a negative result on certain polymerase chain reaction (PCR) tests in the spike protein, or S-gene.
One of the S-gene mutations specific to the variant deletes two amino acids, causing that portion of the PCR test to show up negative. The coincidental finding known as an S-gene target failure has become an integral proxy to help track where and when the variant is spreading in the United Kingdom, where about 5% of samples from COVID-19–infected patients are sequenced, said Sharon Peacock, PhD, executive director and chair of the COVID-19 Genomics U.K. Consortium.
That same tactic could prove valuable to clinicians similarly overwhelmed with cases and deaths but lacking high-level sequencing information on the virus, Dr. Peacock said in an interview. A British report released Friday stated that there is a “realistic possibility” that the variant has a higher death rate than other cases of SARS-CoV-2.
“In this particular variant, a deletion in the genome leads to one part of the diagnostic test failing,” Dr. Peacock explained. “Several targets are positive, but this is negative. In the U.K., this has been used as a surrogate marker.”
Targeting an invisible adversary
B117 is not the only variant that produces this result, Dr. Peacock cautioned, “but in screening for it, you can have this in mind.”
“Since the U.K. is sequencing about 5% of the cases they detect, this gives them really important clues about what’s happening there,” said Anderson Brito, PhD, a virologist and postdoctoral researcher at Yale University, New Haven, Conn., where investigators are creating custom PCR tests to detect the B117 variant.
Dr. Brito, who lived in the United Kingdom for 4 years while studying for his doctorate at Imperial College London, said a “major advantage” is the more unified process to collect and sequence samples. Crucial information – including the date and place of collection – comes with each sample, which fuels not only sequencing, but an epidemiologic perspective.
“They’re not in the dark at all,” Dr. Brito said in an interview. “I think no other country in the world knows better which virus lineages are circulating.”
The CDC launched the SPHERES consortium in May 2020 to coordinate the sequencing of SARS-CoV-2 genomes across the United States.
But American genomic efforts are “not as centralized,” said Dr. Brito, whose lab detected the first two cases of the U.K. variant in Connecticut on Jan. 6. “We struggle to get samples, because they’re decentralized to a level where there’s little coordination between hospitals and research centers. They’re not as connected as in the U.K. If we just get a sample and it has no date of collection and no origin information, for example, it’s basically useless.”
Global genomic collaborations include GISAID, an international database where researchers share new genomes from various coronaviruses. As of mid-January, the United States had submitted about 68,000 sequences to GISAID, adding about 3,000 new samples every week and expecting even more from commercial labs in coming days, according to the CDC.
“The U.K. is definitely much more on top of looking for variants as they pop up,” said Gigi Gronvall, PhD, an immunologist and senior scholar at Johns Hopkins Center for Health Security in Baltimore. “The U.S. has now turned that up.”
Warning from British scientists to the world
Despite these genomic accomplishments, some British scientists said they have regrets too, wishing they’d known just how rapidly SARS-CoV-2 was actually spreading a year ago, when it hit western Europe.
That information was crucial not only for preventive efforts, but because viruses inevitably mutate faster the more people who are infected, said Igor Rudan, MD, PhD, director of the Center for Global Health Research at University of Edinburgh.
“Italy showed us just how fast it was spreading and how deadly it is for the very old and people with multiple comorbidities,” said Dr. Rudan, who also editor in chief of the Journal of Global Health. “We wish we knew it was spreading so fast, and we wish we knew the threshold of cases we could allow to be infected before the virus would mutate.”
More mutations mean more new strains of SARS-CoV-2, Dr. Rudan said in an interview. “We’ve reached that threshold now and will see more of these mutations.”
Despite its current struggles, the United Kingdom is reaching beyond tracking its new variant’s spread and trying to identify new mutations that might change the way the virus behaves.
Three features of any emerging variant are particularly important, Dr. Peacock explained: Is it more transmissible? Is it more lethal? And does it cut the ability of natural- or vaccine-induced immunity to protect people from infection?
“We need to sequence people coming to the hospital who are sicker,” said Dr. Peacock, also a professor of public health and microbiology at the University of Cambridge (England). “Also, if anyone has the infection after they’ve already been sick or had the vaccine, we really want to know what that looks like” genomically.
SARS-CoV-2 has already logged more than 4,000 mutations, Dr. Peacock said. But “knowing that viruses mutate all the time is not sufficient reason not to look. We really want to know if mutations lead to changes in amino acids, and if that can lead to changes in functionality.”
For the moment, however, experts say they’re relieved that the U.K. strain doesn’t seem able to evade COVID-19 vaccines or render them less effective.
“Even though mutations are common, those able to change the viral coding are rare,” Dr. Brito explained. If necessary, vaccines could be tweaked to replace the spike gene sequence “within a matter of weeks. We already do this for flu vaccines. Every year, we have to monitor variants of the virus circulating to develop a vaccine that covers most of them. If we end up having to do it for SARS-CoV-2, I would not be surprised.”
But variant-fueled increases in infections will require more people to be vaccinated before herd immunity can be achieved, Dr. Rudan warned. “If it spreads faster, we’ll need to vaccinate probably 85% of people versus 70% to reach herd immunity.”
One lesson the COVID-19 pandemic has driven home “is to always be on your guard about what happens next,” Dr. Peacock said. Although confident about the genomic efforts in the United Kingdom to date, she and her colleagues feel they’re still reaching for a complete understanding of the evolutionary changes of the virus.
“We’re ahead of the curve right now, but we want to get in front of the curve,” Dr. Peacock said. “It’s essential to get ahead of what might be around the corner because we don’t know how the virus is going to evolve.”
A version of this article first appeared on Medscape.com.
Brazilian researchers tracking reinfection by new virus variant
Just as Brazil surpassed 200,000 deaths from COVID-19 on Jan. 7, news from Bahia added another layer of concern: A platform case report in a preprint detailed the first case of reinfection in that state, apparently caused by a new strain, one having the E484K mutation.
That variant, now called Brazil P.1, has migrated to the United States. The Minnesota Department of Health announced on Jan. 25 the nation’s first known COVID-19 case associated with it.
The mutation is located in the protein gene of the virus’ spike, which forms the crown structure of coronaviruses and is responsible for the virus’ binding to human cells. The E484K mutation is now the focus because it’s associated with mutations that escape the immune system’s neutralizing antibodies.
“This mutation is at the center of worldwide concern, and it is the first time that it has appeared in a reinfection,” the study’s first author, Bruno Solano de Freitas Souza, MD, a researcher at the Salvador regional unit of Instituto D’Or of Teaching and Research, based at Hospital São Rafael, Salvador, Brazil, explained in an interview.
“We will wait for the sample from Bahia to confirm the case from the perspective of the Ministry of Health’s surveillance network,” said Fernando Motta, PhD, deputy head of the Laboratory for Respiratory Virus and Measles at the Oswaldo Cruz Institute in Rio de Janeiro, which acts as a national reference center for respiratory viruses with the Brazilian Ministry of Health (MS) and as a reference for the World Health Organization.
A case of reinfection
The case patient that led to the alarm was a 45-year-old woman who is a health care executive. She had no comorbidities. The team had been following health care professionals and patients who had tested positive on reverse transcription–polymerase chain reaction (RT-PCR) testing more than once to understand whether they represented cases of prolonged viral persistence or new infections.
The woman had symptoms of viral infection on two occasions (May 26 and Oct. 26). On both occasions, results of RT-PCR testing for SARS-CoV-2 on nasopharyngeal samples were positive. In the first episode, the patient had diarrhea, myalgia, asthenia, and odynophagia for about 7 days. She returned to activities 21 days later. In the second episode, she had more severe symptoms that lasted longer, but she still did not require hospitalization.
“It was the first confirmed case of reinfection in Bahia, and in the second episode, we observed a mutation that could have an impact on the ability of antibodies to neutralize the virus,” Dr. Souza said. “The research continues with the investigation of cases in which the patient has a positive SARS-CoV-2 RT-PCR more than once in an interval greater than 45 days, to have a higher level of evidence.”
He stressed that “it is very important to reinforce measures to control the pandemic, social distance, use of masks, and speed up vaccination to be able to control the circulation of the virus, while monitoring the evolution of it.”
On alert for more cases
A person who twice tests positive for SARS-CoV-2 on real-time RT-PCR is suspected of having been reinfected, provided 90 or more days have elapsed between the two episodes, regardless of the condition observed. To confirm the suspected case, the samples must be sent to reference laboratories according to a plan established by the Ministry of Health in Brazil.
A health professional living in the Brazilian city of Natal represented the first confirmed case of reinfection by the new coronavirus in Brazil. That case was announced on Dec. 10, 2020.
“We communicated this case of reinfection to the MS in early December 2020. And the second sample already had the E484K mutation on the spike, as in the case of Bahia,” said Dr. Motta.
The first step in differentiating reinfection from persistence is to observe differences in the genotyping of the virus. For the technique to be successful, Dr. Souza said, researchers need a large amount of viral genetic material, which usually cannot be obtained.
“That is why there are many more suspected than confirmed cases,” Dr. Souza explained. He admitted that, although there are few cases, “it is increasingly clear that reinfection is a reality.”
Markers of mutations
What worried the researchers most was not only the possibility of reinfection but also the fact that preliminary analyses showed a specific mutation.
“The E484K mutation is present in a group of variants identified in South Africa that have been associated with increased infectivity and has been observed in a strain recently described in Brazil,” Dr. Souza said.
Mutations are expected, appear spontaneously, and in most cases have no effects on transmission or clinical outcome – they are simply used as markers and are useful for contact tracing or studying transmission routes. But some mutations can last because they provide an advantage for the pathogen, even if only momentary. In the case of SARS-CoV-2, mutations in the protein spike gene (S) are relevant because they may give clues to that advantage – as well as to changes in infectivity, transmission potential, antibodies, and response to vaccines.
A variant of the virus that has eight changes that affect the protein S gene – and several others in different genes – is behind the increase in the number of cases in London and southeastern England. Researchers from the University of São Paulo identified one of the factors that made this new variant – classified as B.1.1.7 – more infectious.
With bioinformatics tools, they found that the protein S gene in the new viral strain has a stronger molecular interaction with the ACE2 receptor, which is on the surface of human cells and to which the virus binds, making infection possible. The variant has already spread to the rest of the world, and the first two cases have been confirmed in Brazil by the Adolf Lutz Institute.
The alert for a new variant in Africa – similar to B.1.1.7 in the United Kingdom in that it carries nine changes in protein S at position 501 – was made by the Brazilian virologist Tulio de Oliveira, PhD.
“We found that this strain seems to be spreading much faster,” Dr. Oliveira, who is with the University of KwaZulu Natal, told the journal Science. His work first alerted British scientists to the importance of the position N501Y.
“The new variants just described in the United Kingdom and South Africa are slightly more transmissible and have already been identified in cases imported into Brazil,” Dr. Motta said. “Unfortunately, we believe it is only a matter of time before it becomes indigenous.”
The viral family grows
Viruses such as SARS-CoV-2 are classified into strains on the basis of small differences in their genetic material. Since Dec. 26, 2020, in addition to the British and South African variants, it appears the Carioca lineage also is a player.
In a preprint article, researchers analyzed the evolution of the epidemic in Rio de Janeiro from April 2020 until just before the new increase in incidence in December. They compared the complete sequences of the viral genome of 180 patients from different municipalities. The study, which is being jointly conducted by members of the Federal University of Rio de Janeiro and the National Laboratory for Scientific Computing, identified a new variant of SARS-CoV-2 that has five unique mutations (from one of the predominant strains). Concern arose because, in addition to those five genetic changes, many of the samples had a sixth – the well-known E484K mutation.
“The three lines – the U.K., South Africa, and Brazil – were almost synchronous publications, but there is no clear evidence that they have any kind of common ancestry,” Carolina M. Voloch, PhD, the article’s first author and a biologist and researcher at the Molecular Virology Laboratory and associate professor in the department of genetics at the Federal University of Rio de Janeiro, said in an interview.
Dr. Voloch’s research focuses on the use of bioinformatics tools to study the molecular, phylogenetic, and genomic evolution of viruses.
“The emergence of new strains is common for viruses,” she said. “It can be happening anywhere in the world at any time.”
She stressed that identifying when mutations emerge will help to define the new Brazilian lineage. Researchers are working to determine whether the neutralizing antibodies of patients who have been infected with other strains respond to this Rio de Janeiro strain.
“We hope to soon be sharing these results,” Dr. Voloch said.
The article’s authors estimated that the new strain likely appeared in early July. They say more analysis is needed to predict whether the changes have a major effect on viral infectivity, the host’s immune response, or the severity of the disease. Asked about the lineage that caused the reinfection in Bahia, Dr. Voloch said she hadn’t yet contacted the authors to conduct a joint analysis but added that the data disclosed in the preprint would not represent the same variant.
“There are only two of the five mutations that characterize the Rio de Janeiro lineage. However, it has the E484K mutation that is present in more than 94% of the samples of the new variant of Rio,” she said.
She added that there’s a possibility of reinfection by the lineage that’s circulating in Rio de Janeiro and in other states, as well as countries such as the United States, the United Kingdom, and Japan.
“The Carioca virus is being exported to the rest of the world,” Dr. Voloch said.
Virus’ diversity still unknown
Researchers now know that SARS-CoV-2 probably circulated silently in Brazil as early as February 2020 and reached all the nation’s regions before air travel was restricted. Since the first half of 2020, there have been two predominant strains.
“More than a dozen strains have been identified in Brazil, but more important than counting strains to identify the speed with which they arise – which is directly associated with the rate of infection, which is very high in the country,” said Dr. Motta.
The so-called variant of Rio de Janeiro, he said, has also been detected in other states in four regions of Brazil. The key to documenting variants is to get a more representative sample with genomes from other parts of the country.
As of Jan. 10, a total of 347,000 complete genome sequences had been shared globally through open databases since SARS-CoV-2 was first identified, but the contribution of countries is uneven. Although the cost and complexity of genetic sequencing has dropped significantly over time, effective sequencing programs still require substantial investments in personnel, equipment, reagents, and bioinformatics infrastructure.
According to Dr. Voloch, it will only be possible to combat the new coronavirus by knowing its diversity and understanding how it evolves. The Fiocruz Genomic Network has made an infographic available so researchers can track the strains circulating in Brazil. It›s the result of collaboration between researchers from Fiocruz and the GISAID Initiative, an international partnership that promotes rapid data sharing.
As of Jan. 5, researchers in Brazil had studied 1,897 genomes – not nearly enough.
“In Brazil, there is little testing and even less sequencing,” lamented Dr. Souza.
“In the U.K., 1 in 600 cases is sequenced. In Brazil it is less than 1 in 10 million cases,” Dr. Voloch added.
So far, no decisive factors for public health, such as greater virulence or greater transmissibility, have been identified in any of the strains established in Brazil. The million-dollar question is whether the emergence of new strains could have an impact on the effectiveness of vaccines being administered today.
“In one way or another, the vaccine is our best bet ever, even if in the future we identify escapist mutants and have to modify it,” Dr. Motta said. “It is what we do annually with influenza.”
Dr. Voloch, Dr. Motta, and Dr. Souza disclosed no relevant financial relationships.
A version of this article first appeared on the Portuguese edition of Medscape.com.
Just as Brazil surpassed 200,000 deaths from COVID-19 on Jan. 7, news from Bahia added another layer of concern: A platform case report in a preprint detailed the first case of reinfection in that state, apparently caused by a new strain, one having the E484K mutation.
That variant, now called Brazil P.1, has migrated to the United States. The Minnesota Department of Health announced on Jan. 25 the nation’s first known COVID-19 case associated with it.
The mutation is located in the protein gene of the virus’ spike, which forms the crown structure of coronaviruses and is responsible for the virus’ binding to human cells. The E484K mutation is now the focus because it’s associated with mutations that escape the immune system’s neutralizing antibodies.
“This mutation is at the center of worldwide concern, and it is the first time that it has appeared in a reinfection,” the study’s first author, Bruno Solano de Freitas Souza, MD, a researcher at the Salvador regional unit of Instituto D’Or of Teaching and Research, based at Hospital São Rafael, Salvador, Brazil, explained in an interview.
“We will wait for the sample from Bahia to confirm the case from the perspective of the Ministry of Health’s surveillance network,” said Fernando Motta, PhD, deputy head of the Laboratory for Respiratory Virus and Measles at the Oswaldo Cruz Institute in Rio de Janeiro, which acts as a national reference center for respiratory viruses with the Brazilian Ministry of Health (MS) and as a reference for the World Health Organization.
A case of reinfection
The case patient that led to the alarm was a 45-year-old woman who is a health care executive. She had no comorbidities. The team had been following health care professionals and patients who had tested positive on reverse transcription–polymerase chain reaction (RT-PCR) testing more than once to understand whether they represented cases of prolonged viral persistence or new infections.
The woman had symptoms of viral infection on two occasions (May 26 and Oct. 26). On both occasions, results of RT-PCR testing for SARS-CoV-2 on nasopharyngeal samples were positive. In the first episode, the patient had diarrhea, myalgia, asthenia, and odynophagia for about 7 days. She returned to activities 21 days later. In the second episode, she had more severe symptoms that lasted longer, but she still did not require hospitalization.
“It was the first confirmed case of reinfection in Bahia, and in the second episode, we observed a mutation that could have an impact on the ability of antibodies to neutralize the virus,” Dr. Souza said. “The research continues with the investigation of cases in which the patient has a positive SARS-CoV-2 RT-PCR more than once in an interval greater than 45 days, to have a higher level of evidence.”
He stressed that “it is very important to reinforce measures to control the pandemic, social distance, use of masks, and speed up vaccination to be able to control the circulation of the virus, while monitoring the evolution of it.”
On alert for more cases
A person who twice tests positive for SARS-CoV-2 on real-time RT-PCR is suspected of having been reinfected, provided 90 or more days have elapsed between the two episodes, regardless of the condition observed. To confirm the suspected case, the samples must be sent to reference laboratories according to a plan established by the Ministry of Health in Brazil.
A health professional living in the Brazilian city of Natal represented the first confirmed case of reinfection by the new coronavirus in Brazil. That case was announced on Dec. 10, 2020.
“We communicated this case of reinfection to the MS in early December 2020. And the second sample already had the E484K mutation on the spike, as in the case of Bahia,” said Dr. Motta.
The first step in differentiating reinfection from persistence is to observe differences in the genotyping of the virus. For the technique to be successful, Dr. Souza said, researchers need a large amount of viral genetic material, which usually cannot be obtained.
“That is why there are many more suspected than confirmed cases,” Dr. Souza explained. He admitted that, although there are few cases, “it is increasingly clear that reinfection is a reality.”
Markers of mutations
What worried the researchers most was not only the possibility of reinfection but also the fact that preliminary analyses showed a specific mutation.
“The E484K mutation is present in a group of variants identified in South Africa that have been associated with increased infectivity and has been observed in a strain recently described in Brazil,” Dr. Souza said.
Mutations are expected, appear spontaneously, and in most cases have no effects on transmission or clinical outcome – they are simply used as markers and are useful for contact tracing or studying transmission routes. But some mutations can last because they provide an advantage for the pathogen, even if only momentary. In the case of SARS-CoV-2, mutations in the protein spike gene (S) are relevant because they may give clues to that advantage – as well as to changes in infectivity, transmission potential, antibodies, and response to vaccines.
A variant of the virus that has eight changes that affect the protein S gene – and several others in different genes – is behind the increase in the number of cases in London and southeastern England. Researchers from the University of São Paulo identified one of the factors that made this new variant – classified as B.1.1.7 – more infectious.
With bioinformatics tools, they found that the protein S gene in the new viral strain has a stronger molecular interaction with the ACE2 receptor, which is on the surface of human cells and to which the virus binds, making infection possible. The variant has already spread to the rest of the world, and the first two cases have been confirmed in Brazil by the Adolf Lutz Institute.
The alert for a new variant in Africa – similar to B.1.1.7 in the United Kingdom in that it carries nine changes in protein S at position 501 – was made by the Brazilian virologist Tulio de Oliveira, PhD.
“We found that this strain seems to be spreading much faster,” Dr. Oliveira, who is with the University of KwaZulu Natal, told the journal Science. His work first alerted British scientists to the importance of the position N501Y.
“The new variants just described in the United Kingdom and South Africa are slightly more transmissible and have already been identified in cases imported into Brazil,” Dr. Motta said. “Unfortunately, we believe it is only a matter of time before it becomes indigenous.”
The viral family grows
Viruses such as SARS-CoV-2 are classified into strains on the basis of small differences in their genetic material. Since Dec. 26, 2020, in addition to the British and South African variants, it appears the Carioca lineage also is a player.
In a preprint article, researchers analyzed the evolution of the epidemic in Rio de Janeiro from April 2020 until just before the new increase in incidence in December. They compared the complete sequences of the viral genome of 180 patients from different municipalities. The study, which is being jointly conducted by members of the Federal University of Rio de Janeiro and the National Laboratory for Scientific Computing, identified a new variant of SARS-CoV-2 that has five unique mutations (from one of the predominant strains). Concern arose because, in addition to those five genetic changes, many of the samples had a sixth – the well-known E484K mutation.
“The three lines – the U.K., South Africa, and Brazil – were almost synchronous publications, but there is no clear evidence that they have any kind of common ancestry,” Carolina M. Voloch, PhD, the article’s first author and a biologist and researcher at the Molecular Virology Laboratory and associate professor in the department of genetics at the Federal University of Rio de Janeiro, said in an interview.
Dr. Voloch’s research focuses on the use of bioinformatics tools to study the molecular, phylogenetic, and genomic evolution of viruses.
“The emergence of new strains is common for viruses,” she said. “It can be happening anywhere in the world at any time.”
She stressed that identifying when mutations emerge will help to define the new Brazilian lineage. Researchers are working to determine whether the neutralizing antibodies of patients who have been infected with other strains respond to this Rio de Janeiro strain.
“We hope to soon be sharing these results,” Dr. Voloch said.
The article’s authors estimated that the new strain likely appeared in early July. They say more analysis is needed to predict whether the changes have a major effect on viral infectivity, the host’s immune response, or the severity of the disease. Asked about the lineage that caused the reinfection in Bahia, Dr. Voloch said she hadn’t yet contacted the authors to conduct a joint analysis but added that the data disclosed in the preprint would not represent the same variant.
“There are only two of the five mutations that characterize the Rio de Janeiro lineage. However, it has the E484K mutation that is present in more than 94% of the samples of the new variant of Rio,” she said.
She added that there’s a possibility of reinfection by the lineage that’s circulating in Rio de Janeiro and in other states, as well as countries such as the United States, the United Kingdom, and Japan.
“The Carioca virus is being exported to the rest of the world,” Dr. Voloch said.
Virus’ diversity still unknown
Researchers now know that SARS-CoV-2 probably circulated silently in Brazil as early as February 2020 and reached all the nation’s regions before air travel was restricted. Since the first half of 2020, there have been two predominant strains.
“More than a dozen strains have been identified in Brazil, but more important than counting strains to identify the speed with which they arise – which is directly associated with the rate of infection, which is very high in the country,” said Dr. Motta.
The so-called variant of Rio de Janeiro, he said, has also been detected in other states in four regions of Brazil. The key to documenting variants is to get a more representative sample with genomes from other parts of the country.
As of Jan. 10, a total of 347,000 complete genome sequences had been shared globally through open databases since SARS-CoV-2 was first identified, but the contribution of countries is uneven. Although the cost and complexity of genetic sequencing has dropped significantly over time, effective sequencing programs still require substantial investments in personnel, equipment, reagents, and bioinformatics infrastructure.
According to Dr. Voloch, it will only be possible to combat the new coronavirus by knowing its diversity and understanding how it evolves. The Fiocruz Genomic Network has made an infographic available so researchers can track the strains circulating in Brazil. It›s the result of collaboration between researchers from Fiocruz and the GISAID Initiative, an international partnership that promotes rapid data sharing.
As of Jan. 5, researchers in Brazil had studied 1,897 genomes – not nearly enough.
“In Brazil, there is little testing and even less sequencing,” lamented Dr. Souza.
“In the U.K., 1 in 600 cases is sequenced. In Brazil it is less than 1 in 10 million cases,” Dr. Voloch added.
So far, no decisive factors for public health, such as greater virulence or greater transmissibility, have been identified in any of the strains established in Brazil. The million-dollar question is whether the emergence of new strains could have an impact on the effectiveness of vaccines being administered today.
“In one way or another, the vaccine is our best bet ever, even if in the future we identify escapist mutants and have to modify it,” Dr. Motta said. “It is what we do annually with influenza.”
Dr. Voloch, Dr. Motta, and Dr. Souza disclosed no relevant financial relationships.
A version of this article first appeared on the Portuguese edition of Medscape.com.
Just as Brazil surpassed 200,000 deaths from COVID-19 on Jan. 7, news from Bahia added another layer of concern: A platform case report in a preprint detailed the first case of reinfection in that state, apparently caused by a new strain, one having the E484K mutation.
That variant, now called Brazil P.1, has migrated to the United States. The Minnesota Department of Health announced on Jan. 25 the nation’s first known COVID-19 case associated with it.
The mutation is located in the protein gene of the virus’ spike, which forms the crown structure of coronaviruses and is responsible for the virus’ binding to human cells. The E484K mutation is now the focus because it’s associated with mutations that escape the immune system’s neutralizing antibodies.
“This mutation is at the center of worldwide concern, and it is the first time that it has appeared in a reinfection,” the study’s first author, Bruno Solano de Freitas Souza, MD, a researcher at the Salvador regional unit of Instituto D’Or of Teaching and Research, based at Hospital São Rafael, Salvador, Brazil, explained in an interview.
“We will wait for the sample from Bahia to confirm the case from the perspective of the Ministry of Health’s surveillance network,” said Fernando Motta, PhD, deputy head of the Laboratory for Respiratory Virus and Measles at the Oswaldo Cruz Institute in Rio de Janeiro, which acts as a national reference center for respiratory viruses with the Brazilian Ministry of Health (MS) and as a reference for the World Health Organization.
A case of reinfection
The case patient that led to the alarm was a 45-year-old woman who is a health care executive. She had no comorbidities. The team had been following health care professionals and patients who had tested positive on reverse transcription–polymerase chain reaction (RT-PCR) testing more than once to understand whether they represented cases of prolonged viral persistence or new infections.
The woman had symptoms of viral infection on two occasions (May 26 and Oct. 26). On both occasions, results of RT-PCR testing for SARS-CoV-2 on nasopharyngeal samples were positive. In the first episode, the patient had diarrhea, myalgia, asthenia, and odynophagia for about 7 days. She returned to activities 21 days later. In the second episode, she had more severe symptoms that lasted longer, but she still did not require hospitalization.
“It was the first confirmed case of reinfection in Bahia, and in the second episode, we observed a mutation that could have an impact on the ability of antibodies to neutralize the virus,” Dr. Souza said. “The research continues with the investigation of cases in which the patient has a positive SARS-CoV-2 RT-PCR more than once in an interval greater than 45 days, to have a higher level of evidence.”
He stressed that “it is very important to reinforce measures to control the pandemic, social distance, use of masks, and speed up vaccination to be able to control the circulation of the virus, while monitoring the evolution of it.”
On alert for more cases
A person who twice tests positive for SARS-CoV-2 on real-time RT-PCR is suspected of having been reinfected, provided 90 or more days have elapsed between the two episodes, regardless of the condition observed. To confirm the suspected case, the samples must be sent to reference laboratories according to a plan established by the Ministry of Health in Brazil.
A health professional living in the Brazilian city of Natal represented the first confirmed case of reinfection by the new coronavirus in Brazil. That case was announced on Dec. 10, 2020.
“We communicated this case of reinfection to the MS in early December 2020. And the second sample already had the E484K mutation on the spike, as in the case of Bahia,” said Dr. Motta.
The first step in differentiating reinfection from persistence is to observe differences in the genotyping of the virus. For the technique to be successful, Dr. Souza said, researchers need a large amount of viral genetic material, which usually cannot be obtained.
“That is why there are many more suspected than confirmed cases,” Dr. Souza explained. He admitted that, although there are few cases, “it is increasingly clear that reinfection is a reality.”
Markers of mutations
What worried the researchers most was not only the possibility of reinfection but also the fact that preliminary analyses showed a specific mutation.
“The E484K mutation is present in a group of variants identified in South Africa that have been associated with increased infectivity and has been observed in a strain recently described in Brazil,” Dr. Souza said.
Mutations are expected, appear spontaneously, and in most cases have no effects on transmission or clinical outcome – they are simply used as markers and are useful for contact tracing or studying transmission routes. But some mutations can last because they provide an advantage for the pathogen, even if only momentary. In the case of SARS-CoV-2, mutations in the protein spike gene (S) are relevant because they may give clues to that advantage – as well as to changes in infectivity, transmission potential, antibodies, and response to vaccines.
A variant of the virus that has eight changes that affect the protein S gene – and several others in different genes – is behind the increase in the number of cases in London and southeastern England. Researchers from the University of São Paulo identified one of the factors that made this new variant – classified as B.1.1.7 – more infectious.
With bioinformatics tools, they found that the protein S gene in the new viral strain has a stronger molecular interaction with the ACE2 receptor, which is on the surface of human cells and to which the virus binds, making infection possible. The variant has already spread to the rest of the world, and the first two cases have been confirmed in Brazil by the Adolf Lutz Institute.
The alert for a new variant in Africa – similar to B.1.1.7 in the United Kingdom in that it carries nine changes in protein S at position 501 – was made by the Brazilian virologist Tulio de Oliveira, PhD.
“We found that this strain seems to be spreading much faster,” Dr. Oliveira, who is with the University of KwaZulu Natal, told the journal Science. His work first alerted British scientists to the importance of the position N501Y.
“The new variants just described in the United Kingdom and South Africa are slightly more transmissible and have already been identified in cases imported into Brazil,” Dr. Motta said. “Unfortunately, we believe it is only a matter of time before it becomes indigenous.”
The viral family grows
Viruses such as SARS-CoV-2 are classified into strains on the basis of small differences in their genetic material. Since Dec. 26, 2020, in addition to the British and South African variants, it appears the Carioca lineage also is a player.
In a preprint article, researchers analyzed the evolution of the epidemic in Rio de Janeiro from April 2020 until just before the new increase in incidence in December. They compared the complete sequences of the viral genome of 180 patients from different municipalities. The study, which is being jointly conducted by members of the Federal University of Rio de Janeiro and the National Laboratory for Scientific Computing, identified a new variant of SARS-CoV-2 that has five unique mutations (from one of the predominant strains). Concern arose because, in addition to those five genetic changes, many of the samples had a sixth – the well-known E484K mutation.
“The three lines – the U.K., South Africa, and Brazil – were almost synchronous publications, but there is no clear evidence that they have any kind of common ancestry,” Carolina M. Voloch, PhD, the article’s first author and a biologist and researcher at the Molecular Virology Laboratory and associate professor in the department of genetics at the Federal University of Rio de Janeiro, said in an interview.
Dr. Voloch’s research focuses on the use of bioinformatics tools to study the molecular, phylogenetic, and genomic evolution of viruses.
“The emergence of new strains is common for viruses,” she said. “It can be happening anywhere in the world at any time.”
She stressed that identifying when mutations emerge will help to define the new Brazilian lineage. Researchers are working to determine whether the neutralizing antibodies of patients who have been infected with other strains respond to this Rio de Janeiro strain.
“We hope to soon be sharing these results,” Dr. Voloch said.
The article’s authors estimated that the new strain likely appeared in early July. They say more analysis is needed to predict whether the changes have a major effect on viral infectivity, the host’s immune response, or the severity of the disease. Asked about the lineage that caused the reinfection in Bahia, Dr. Voloch said she hadn’t yet contacted the authors to conduct a joint analysis but added that the data disclosed in the preprint would not represent the same variant.
“There are only two of the five mutations that characterize the Rio de Janeiro lineage. However, it has the E484K mutation that is present in more than 94% of the samples of the new variant of Rio,” she said.
She added that there’s a possibility of reinfection by the lineage that’s circulating in Rio de Janeiro and in other states, as well as countries such as the United States, the United Kingdom, and Japan.
“The Carioca virus is being exported to the rest of the world,” Dr. Voloch said.
Virus’ diversity still unknown
Researchers now know that SARS-CoV-2 probably circulated silently in Brazil as early as February 2020 and reached all the nation’s regions before air travel was restricted. Since the first half of 2020, there have been two predominant strains.
“More than a dozen strains have been identified in Brazil, but more important than counting strains to identify the speed with which they arise – which is directly associated with the rate of infection, which is very high in the country,” said Dr. Motta.
The so-called variant of Rio de Janeiro, he said, has also been detected in other states in four regions of Brazil. The key to documenting variants is to get a more representative sample with genomes from other parts of the country.
As of Jan. 10, a total of 347,000 complete genome sequences had been shared globally through open databases since SARS-CoV-2 was first identified, but the contribution of countries is uneven. Although the cost and complexity of genetic sequencing has dropped significantly over time, effective sequencing programs still require substantial investments in personnel, equipment, reagents, and bioinformatics infrastructure.
According to Dr. Voloch, it will only be possible to combat the new coronavirus by knowing its diversity and understanding how it evolves. The Fiocruz Genomic Network has made an infographic available so researchers can track the strains circulating in Brazil. It›s the result of collaboration between researchers from Fiocruz and the GISAID Initiative, an international partnership that promotes rapid data sharing.
As of Jan. 5, researchers in Brazil had studied 1,897 genomes – not nearly enough.
“In Brazil, there is little testing and even less sequencing,” lamented Dr. Souza.
“In the U.K., 1 in 600 cases is sequenced. In Brazil it is less than 1 in 10 million cases,” Dr. Voloch added.
So far, no decisive factors for public health, such as greater virulence or greater transmissibility, have been identified in any of the strains established in Brazil. The million-dollar question is whether the emergence of new strains could have an impact on the effectiveness of vaccines being administered today.
“In one way or another, the vaccine is our best bet ever, even if in the future we identify escapist mutants and have to modify it,” Dr. Motta said. “It is what we do annually with influenza.”
Dr. Voloch, Dr. Motta, and Dr. Souza disclosed no relevant financial relationships.
A version of this article first appeared on the Portuguese edition of Medscape.com.
President Biden to up states’ vaccine supplies, targets more doses
Seven days into his presidency, Joe Biden announced that he is taking new steps to speed vaccines to Americans.
The president said he would increase the supply of vaccines to states from 8.6 million doses to 10 million doses per week, a 16% increase, for at least the next 3 weeks.
He said he was working to give states more advanced notice of their allotments so they could better plan their campaigns. He also said doses would be doled out based on population.
“We will both increase the supply and give our state and local partners more certainty about when doses will arrive,” he said Tuesday.
Finally, Mr. Biden announced that the United States would “soon be able to confirm” the purchase of 200 million more doses of the Pfizer and Moderna vaccines – 100 million of each – to effectively double the nation’s supply by “early summer.” That would increase the nation’s supply enough to fully vaccinate 300 million Americans by fall.
Mr. Biden said he was also working to shift the focus to getting more doses to economically disadvantaged communities and rural areas, which have fallen further behind as the vaccine rollout has faltered.
Even with these steps, Mr. Biden stressed that it would take months for vaccines to curb infections and deaths. He said, for the time being, masks, not vaccines, are the best way to save lives.
“The brutal truth is its going to take months before we get the majority of Americans vaccinated. Months,” he said, adding that wearing masks until at least April could save to save 50,000 lives.
“Let me be clear,” Mr. Biden said, “Things are going to get worse before they get better.
“We didn’t get into this mess overnight. It’s going to take months for us to turn things around. But let me be equally clear we’re going to get through this. We will defeat this pandemic,” he said.
A version of this article first appeared on WebMD.com.
Seven days into his presidency, Joe Biden announced that he is taking new steps to speed vaccines to Americans.
The president said he would increase the supply of vaccines to states from 8.6 million doses to 10 million doses per week, a 16% increase, for at least the next 3 weeks.
He said he was working to give states more advanced notice of their allotments so they could better plan their campaigns. He also said doses would be doled out based on population.
“We will both increase the supply and give our state and local partners more certainty about when doses will arrive,” he said Tuesday.
Finally, Mr. Biden announced that the United States would “soon be able to confirm” the purchase of 200 million more doses of the Pfizer and Moderna vaccines – 100 million of each – to effectively double the nation’s supply by “early summer.” That would increase the nation’s supply enough to fully vaccinate 300 million Americans by fall.
Mr. Biden said he was also working to shift the focus to getting more doses to economically disadvantaged communities and rural areas, which have fallen further behind as the vaccine rollout has faltered.
Even with these steps, Mr. Biden stressed that it would take months for vaccines to curb infections and deaths. He said, for the time being, masks, not vaccines, are the best way to save lives.
“The brutal truth is its going to take months before we get the majority of Americans vaccinated. Months,” he said, adding that wearing masks until at least April could save to save 50,000 lives.
“Let me be clear,” Mr. Biden said, “Things are going to get worse before they get better.
“We didn’t get into this mess overnight. It’s going to take months for us to turn things around. But let me be equally clear we’re going to get through this. We will defeat this pandemic,” he said.
A version of this article first appeared on WebMD.com.
Seven days into his presidency, Joe Biden announced that he is taking new steps to speed vaccines to Americans.
The president said he would increase the supply of vaccines to states from 8.6 million doses to 10 million doses per week, a 16% increase, for at least the next 3 weeks.
He said he was working to give states more advanced notice of their allotments so they could better plan their campaigns. He also said doses would be doled out based on population.
“We will both increase the supply and give our state and local partners more certainty about when doses will arrive,” he said Tuesday.
Finally, Mr. Biden announced that the United States would “soon be able to confirm” the purchase of 200 million more doses of the Pfizer and Moderna vaccines – 100 million of each – to effectively double the nation’s supply by “early summer.” That would increase the nation’s supply enough to fully vaccinate 300 million Americans by fall.
Mr. Biden said he was also working to shift the focus to getting more doses to economically disadvantaged communities and rural areas, which have fallen further behind as the vaccine rollout has faltered.
Even with these steps, Mr. Biden stressed that it would take months for vaccines to curb infections and deaths. He said, for the time being, masks, not vaccines, are the best way to save lives.
“The brutal truth is its going to take months before we get the majority of Americans vaccinated. Months,” he said, adding that wearing masks until at least April could save to save 50,000 lives.
“Let me be clear,” Mr. Biden said, “Things are going to get worse before they get better.
“We didn’t get into this mess overnight. It’s going to take months for us to turn things around. But let me be equally clear we’re going to get through this. We will defeat this pandemic,” he said.
A version of this article first appeared on WebMD.com.