User login
Many people becoming reinfected as BA.5 dominates new COVID-19 cases
When the COVID-19 pandemic first began, the general thought was that once people were infected, they were then protected from the virus.
It’s hard to say how many. The ABC News analysis found at least 1.6 million reinfections in 24 states, but the actual number is probably a lot higher.
“These are not the real numbers because many people are not reporting cases,” Ali Mokdad, MD, an epidemiologist with the University of Washington, Seattle, told ABC.
The latest variant, BA.5, has become the dominant strain in the United States, making up more than 65% of all COVID-19 cases as of July 13, according to data from the CDC.
Prior infections and vaccines aren’t providing as much protection against the newly dominant BA.5 strain as they did against earlier variants.
But evidence doesn’t show this subvariant of Omicron to be more harmful than earlier, less transmissible versions.
Several factors are contributing to rising reinfections, experts say. For example, fewer people are wearing masks than in the first year or so of the pandemic. Dr. Mokdad said just 18% of Americans reported always wearing a mask in public at the end of May, down from 44% the year before.
The emergence of the Omicron variant, of which BA.5 is a subvariant, is indicating that less protection is being offered by prior infections.
A version of this article first appeared on WebMD.com.
When the COVID-19 pandemic first began, the general thought was that once people were infected, they were then protected from the virus.
It’s hard to say how many. The ABC News analysis found at least 1.6 million reinfections in 24 states, but the actual number is probably a lot higher.
“These are not the real numbers because many people are not reporting cases,” Ali Mokdad, MD, an epidemiologist with the University of Washington, Seattle, told ABC.
The latest variant, BA.5, has become the dominant strain in the United States, making up more than 65% of all COVID-19 cases as of July 13, according to data from the CDC.
Prior infections and vaccines aren’t providing as much protection against the newly dominant BA.5 strain as they did against earlier variants.
But evidence doesn’t show this subvariant of Omicron to be more harmful than earlier, less transmissible versions.
Several factors are contributing to rising reinfections, experts say. For example, fewer people are wearing masks than in the first year or so of the pandemic. Dr. Mokdad said just 18% of Americans reported always wearing a mask in public at the end of May, down from 44% the year before.
The emergence of the Omicron variant, of which BA.5 is a subvariant, is indicating that less protection is being offered by prior infections.
A version of this article first appeared on WebMD.com.
When the COVID-19 pandemic first began, the general thought was that once people were infected, they were then protected from the virus.
It’s hard to say how many. The ABC News analysis found at least 1.6 million reinfections in 24 states, but the actual number is probably a lot higher.
“These are not the real numbers because many people are not reporting cases,” Ali Mokdad, MD, an epidemiologist with the University of Washington, Seattle, told ABC.
The latest variant, BA.5, has become the dominant strain in the United States, making up more than 65% of all COVID-19 cases as of July 13, according to data from the CDC.
Prior infections and vaccines aren’t providing as much protection against the newly dominant BA.5 strain as they did against earlier variants.
But evidence doesn’t show this subvariant of Omicron to be more harmful than earlier, less transmissible versions.
Several factors are contributing to rising reinfections, experts say. For example, fewer people are wearing masks than in the first year or so of the pandemic. Dr. Mokdad said just 18% of Americans reported always wearing a mask in public at the end of May, down from 44% the year before.
The emergence of the Omicron variant, of which BA.5 is a subvariant, is indicating that less protection is being offered by prior infections.
A version of this article first appeared on WebMD.com.
Some have heavier periods after COVID vaccine
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
FROM SCIENCE ADVANCES
Cancer drug significantly cuts risk for COVID-19 death
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
FROM NEJM EVIDENCE
FDA grants emergency authorization for Novavax COVID vaccine
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
Children and COVID: Vaccination a harder sell in the summer
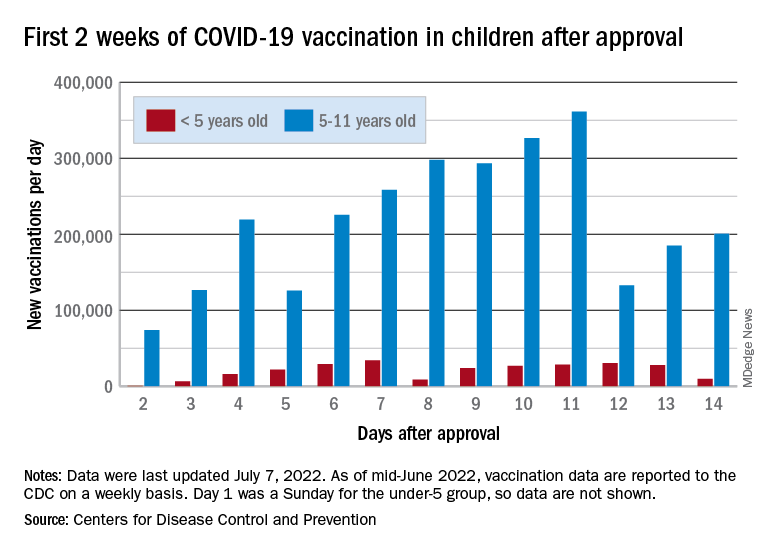
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.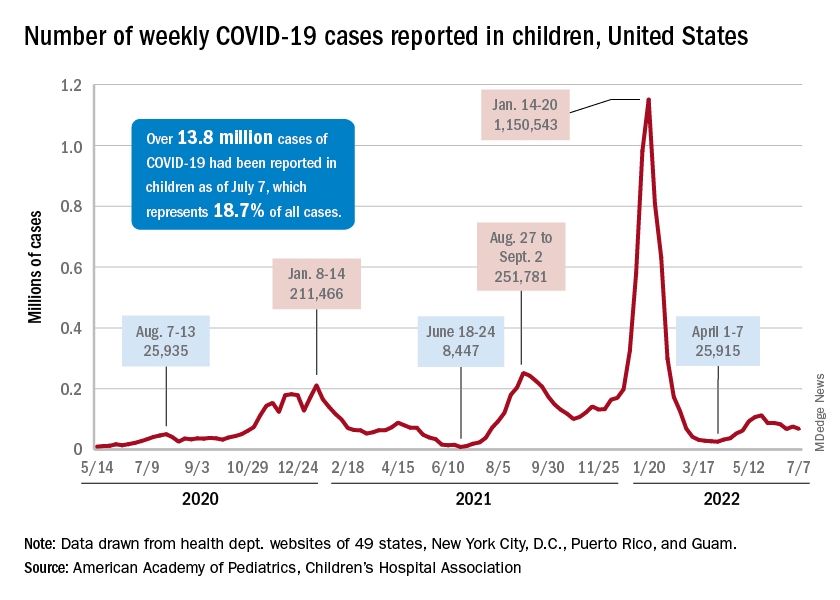
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
BA.4 and BA.5 subvariants are more evasive of antibodies, but not of cellular immunity
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
Obesity links to faster fading of COVID vaccine protection
Researchers published the study covered in this summary on medRxiv.org as a preprint that has not yet been peer reviewed.
Key takeaways
- The study results suggest that
- The findings documented evidence of reduced neutralizing antibody capacity 6 months after primary vaccination in people with severe obesity.
- This was a large study involving about more than 3.5 million people who had received at least two doses of COVID-19 vaccine, including more than 650,000 with obesity.
Why this matters
- Obesity is associated with comorbidities that independently increase the risk for severe COVID-19, including type 2 diabetes, chronic kidney disease, and heart failure.
- The authors concluded that additional or more frequent booster doses are likely to be required to maintain protection among people with obesity against COVID-19.
Study design
- Prospective longitudinal study of the incidence and severity of COVID-19 infections and immune responses in a cohort of more than 3.5 million adults from a Scottish healthcare database who received two or three doses of COVID-19 vaccine. The data came from the study, centered at the University of Edinburgh.
- About 16% had obesity with a body mass index of 30-39.9 kg/m2, and an additional 3% had severe obesity with a BMI of 40 or greater.
- Although not specified in this preprint, another said that the vaccines administered in Scotland have been the Pfizer-BioNTech and Oxford-AstraZeneca formulations.
Key results
- Between Sept. 14, 2020, and March 19, 2022, 10,983 people (0.3% of the total cohort; 6.0 events per 1,000 person-years) had severe COVID-19, consisting of 9,733 who were hospitalized and 2,207 who died (957 of those hospitalized also died).
- People with obesity or severe obesity were at higher risk of hospitalization or death from COVID-19 after both a second and third (booster) dose of vaccine.
- Compared with those with normal weight, those with severe obesity (BMI higher than 40) were at significantly increased risk for severe COVID-19 after a second vaccine dose, with an adjusted rate ratio 1.76, whereas those with standard obesity (BMI, 30-40) were at a modestly but significantly increased risk with an adjusted rate ratio of 1.11.
- Breakthrough infections after the second dose for those with severe obesity, obesity, and normal weight occurred on average at 10 weeks, 15 weeks, and 20 weeks, respectively.
- Interaction testing showed that vaccine effectiveness significantly diminished over time across BMI groups, and protection waned more rapidly as BMI increased.
- Results from immunophenotyping studies run in a subgroup of several dozen subjects with severe obesity or normal weight showed significant decrements in the robustness of antibody responses in those with severe obesity 6 months after a second or third vaccine dose.
Limitations
- The authors did not specify any limitations.
Disclosures
- The study received no commercial funding.
- One author received funding from Wellcome.
This is a summary of a preprint research study , “Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity,” published by researchers primarily at the University of Cambridge (England), on medRxiv. This study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on medRxiv.org as a preprint that has not yet been peer reviewed.
Key takeaways
- The study results suggest that
- The findings documented evidence of reduced neutralizing antibody capacity 6 months after primary vaccination in people with severe obesity.
- This was a large study involving about more than 3.5 million people who had received at least two doses of COVID-19 vaccine, including more than 650,000 with obesity.
Why this matters
- Obesity is associated with comorbidities that independently increase the risk for severe COVID-19, including type 2 diabetes, chronic kidney disease, and heart failure.
- The authors concluded that additional or more frequent booster doses are likely to be required to maintain protection among people with obesity against COVID-19.
Study design
- Prospective longitudinal study of the incidence and severity of COVID-19 infections and immune responses in a cohort of more than 3.5 million adults from a Scottish healthcare database who received two or three doses of COVID-19 vaccine. The data came from the study, centered at the University of Edinburgh.
- About 16% had obesity with a body mass index of 30-39.9 kg/m2, and an additional 3% had severe obesity with a BMI of 40 or greater.
- Although not specified in this preprint, another said that the vaccines administered in Scotland have been the Pfizer-BioNTech and Oxford-AstraZeneca formulations.
Key results
- Between Sept. 14, 2020, and March 19, 2022, 10,983 people (0.3% of the total cohort; 6.0 events per 1,000 person-years) had severe COVID-19, consisting of 9,733 who were hospitalized and 2,207 who died (957 of those hospitalized also died).
- People with obesity or severe obesity were at higher risk of hospitalization or death from COVID-19 after both a second and third (booster) dose of vaccine.
- Compared with those with normal weight, those with severe obesity (BMI higher than 40) were at significantly increased risk for severe COVID-19 after a second vaccine dose, with an adjusted rate ratio 1.76, whereas those with standard obesity (BMI, 30-40) were at a modestly but significantly increased risk with an adjusted rate ratio of 1.11.
- Breakthrough infections after the second dose for those with severe obesity, obesity, and normal weight occurred on average at 10 weeks, 15 weeks, and 20 weeks, respectively.
- Interaction testing showed that vaccine effectiveness significantly diminished over time across BMI groups, and protection waned more rapidly as BMI increased.
- Results from immunophenotyping studies run in a subgroup of several dozen subjects with severe obesity or normal weight showed significant decrements in the robustness of antibody responses in those with severe obesity 6 months after a second or third vaccine dose.
Limitations
- The authors did not specify any limitations.
Disclosures
- The study received no commercial funding.
- One author received funding from Wellcome.
This is a summary of a preprint research study , “Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity,” published by researchers primarily at the University of Cambridge (England), on medRxiv. This study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on medRxiv.org as a preprint that has not yet been peer reviewed.
Key takeaways
- The study results suggest that
- The findings documented evidence of reduced neutralizing antibody capacity 6 months after primary vaccination in people with severe obesity.
- This was a large study involving about more than 3.5 million people who had received at least two doses of COVID-19 vaccine, including more than 650,000 with obesity.
Why this matters
- Obesity is associated with comorbidities that independently increase the risk for severe COVID-19, including type 2 diabetes, chronic kidney disease, and heart failure.
- The authors concluded that additional or more frequent booster doses are likely to be required to maintain protection among people with obesity against COVID-19.
Study design
- Prospective longitudinal study of the incidence and severity of COVID-19 infections and immune responses in a cohort of more than 3.5 million adults from a Scottish healthcare database who received two or three doses of COVID-19 vaccine. The data came from the study, centered at the University of Edinburgh.
- About 16% had obesity with a body mass index of 30-39.9 kg/m2, and an additional 3% had severe obesity with a BMI of 40 or greater.
- Although not specified in this preprint, another said that the vaccines administered in Scotland have been the Pfizer-BioNTech and Oxford-AstraZeneca formulations.
Key results
- Between Sept. 14, 2020, and March 19, 2022, 10,983 people (0.3% of the total cohort; 6.0 events per 1,000 person-years) had severe COVID-19, consisting of 9,733 who were hospitalized and 2,207 who died (957 of those hospitalized also died).
- People with obesity or severe obesity were at higher risk of hospitalization or death from COVID-19 after both a second and third (booster) dose of vaccine.
- Compared with those with normal weight, those with severe obesity (BMI higher than 40) were at significantly increased risk for severe COVID-19 after a second vaccine dose, with an adjusted rate ratio 1.76, whereas those with standard obesity (BMI, 30-40) were at a modestly but significantly increased risk with an adjusted rate ratio of 1.11.
- Breakthrough infections after the second dose for those with severe obesity, obesity, and normal weight occurred on average at 10 weeks, 15 weeks, and 20 weeks, respectively.
- Interaction testing showed that vaccine effectiveness significantly diminished over time across BMI groups, and protection waned more rapidly as BMI increased.
- Results from immunophenotyping studies run in a subgroup of several dozen subjects with severe obesity or normal weight showed significant decrements in the robustness of antibody responses in those with severe obesity 6 months after a second or third vaccine dose.
Limitations
- The authors did not specify any limitations.
Disclosures
- The study received no commercial funding.
- One author received funding from Wellcome.
This is a summary of a preprint research study , “Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity,” published by researchers primarily at the University of Cambridge (England), on medRxiv. This study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Long COVID-19 in children and adolescents: What do we know?
Among scientists, the existence of long COVID-19 in children and adolescents has been the subject of debate.
Published by a Mexican multidisciplinary group in Scientific Reports, the first study is a systematic review and meta-analysis. It identified mood symptoms as the most prevalent clinical manifestations of long COVID-19 in children and adolescents. These symptoms included sadness, tension, anger, depression, and anxiety (16.50%); fatigue (9.66%); and sleep disorders (8.42%).
The second study, LongCOVIDKidsDK, was conducted in Denmark. It compared 11,000 children younger than 14 years who had tested positive for COVID-19 with 33,000 children who had no history of COVID-19. The study was published in The Lancet Child and Adolescent Health.
Definitions are changing
In their meta-analysis, the researchers estimated the prevalence and counted signs and symptoms of long COVID-19, as defined by the United Kingdom’s National Institute for Health and Care Excellence. Long COVID-19 was defined as the presence of one or more symptoms more than 4 weeks after SARS-CoV-2 infection. For search terms, the researchers used “COVID-19,” “COVID,” “SARSCOV-2,” “coronavirus,” “long COVID,” “postCOVID,” “PASC,” “long-haulers,” “prolonged,” “post-acute,” “persistent,” “convalescent,” “sequelae,” and “postviral.”
Of the 8,373 citations returned by the search as of Feb. 10, 2022, 21 prospective studies, 2 of them on preprint servers, met the authors’ selection criteria. Those studies included a total of 80,071 children and adolescents younger than 18 years.
In the meta-analysis, the prevalence of long COVID-19 among children and adolescents was reported to be 25.24% (95% confidence interval, 18.17-33.02; I2, 99.61%), regardless of whether the case had been asymptomatic, mild, moderate, severe, or serious. For patients who had been hospitalized, the prevalence was 29.19% (95% CI, 17.83-41.98; I2, 80.84%).
These numbers, while striking, are not the focus of the study, according to first author Sandra Lopez-Leon, MD, PhD, associate professor of pharmacoepidemiology at Rutgers University, New Brunswick, N.J. “It’s important that we don’t focus on that 25%,” she said in an interview. “It’s a disease that we’re learning about, we’re at a time when the definitions are still changing, and, depending on when it is measured, a different number will be given. The message we want to give is that long COVID-19 exists, it’s happening in children and adolescents, and patients need this recognition. And also to show that it can affect the whole body.”
The study showed that the children and adolescents who presented with SARS-CoV-2 infection were at higher risk of subsequent long dyspnea, anosmia/ageusia, or fever, compared with control persons.
In total, in the studies that were included, more than 40 long-term clinical manifestations associated with COVID-19 in the pediatric population were identified.
The most common symptoms among children aged 0-3 years were mood swings, skin rashes, and stomachaches. In 4- to 11-year-olds, the most common symptoms were mood swings, trouble remembering or concentrating, and skin rashes. In 12- to 14-year-olds, they were fatigue, mood swings, and trouble remembering or concentrating. These data are based on parent responses.
The list of signs and symptoms also includes headache, respiratory symptoms, cognitive symptoms (such as decreased concentration, learning difficulties, confusion, and memory loss), loss of appetite, and smell disorder (hyposmia, anosmia, hyperosmia, parosmia, and phantom smell).
In the studies, the prevalence of the following symptoms was less than 5%: hyperhidrosis, chest pain, dizziness, cough, myalgia/arthralgia, changes in body weight, taste disorder, otalgia (tinnitus, ear pain, vertigo), ophthalmologic symptoms (conjunctivitis, dry eye, blurred vision, photophobia, pain), dermatologic symptoms (dry skin, itchy skin, rashes, hives, hair loss), urinary symptoms, abdominal pain, throat pain, chest tightness, variations in heart rate, palpitations, constipation, dysphonia, fever, diarrhea, vomiting/nausea, menstrual changes, neurological abnormalities, speech disorders, and dysphagia.
The authors made it clear that the frequency and severity of these symptoms can fluctuate from one patient to another.
“The meta-analysis is important because it brings together 21 studies selected from more than 8,000 articles – and in them, a large number of children – to study the most common manifestations of long COVID-19,” Gabriela Ensinck, MD, head of the infectious diseases department at the Víctor J. Vilela Children’s Hospital in Rosario, Argentina, told this news organization. Dr. Ensinck did not participate in the study. “The important thing here is that long COVID-19 exists in pediatrics. And that it is a prolongation of signs or symptoms over time, a time for which there is no single definition.”
“It’s a snapshot of all the symptoms that can remain after COVID-19,” Dr. Lopez-Leon explained. “The meta-analysis seeks to see if there’s an association between having had COVID-19 and having the symptoms, but at no time does it speak of causality.”
The prevalence of symptoms largely depends on the time since the onset of acute COVID-19. Most symptoms improve over time. In the studies that were included in the meta-analysis, the follow-up time varied between 1 and 13 months. It is important to understand what symptoms are associated with each period after the onset of infection, the authors said.
Danish parent survey
The Danish study LongCOVIDKidsDK followed the World Health Organization criteria for long COVID-19 and included children and adolescents aged 0-14 years who received a diagnosis of COVID-19 and who experienced symptoms that lasted at least 2 months.
Between July 20, 2021, and Sept. 15, 2021, a questionnaire was sent to 38,152 case patients and 147,212 control persons. Of this group, 10,997 (28.8%) case patients and 33,016 (22.4%) control persons answered the survey.
Children who had been diagnosed with SARS-CoV-2 infection were more likely to experience long-lasting symptoms than children who had never been diagnosed. Approximately one-third of children with a positive SARS-CoV-2 test experienced symptoms that were not present before infection. Children who experienced long-lasting symptoms included 40% of children diagnosed with COVID-19 and 27% of control persons aged 0-3 years, 38% of case patients and 34% of control persons aged 4-11 years, and 46% of case patients and 41% of control persons aged 12-14 years.
Interestingly, those diagnosed with COVID-19 reported fewer psychological and social problems than those in the control group. Among the oldest (aged 12-14 years), quality of life scores were higher and anxiety scores were lower for those who had tested positive for SARS-CoV-2.
More information needed
Given the diversity of symptoms in the meta-analysis and the LongCOVIDKidsDK study, a multidisciplinary approach is imperative. Dr. Lopez-Leon suggests that there is a need to raise awareness among parents, clinicians, researchers, and the health system about the conditions that can occur after COVID-19. Clinicians must better understand the sequelae to provide targeted care and treatment. The authors of the Danish study recommend establishing clinics for long COVID-19 with multispecialty care.
Maren J. Heilskov Rytter, PhD, associate professor of clinical medicine at the University of Copenhagen, wrote an editorial in The Lancet Child and Adolescent Health about the Danish study. Until it is clarified whether SARS-CoV-2 does indeed cause persistent symptoms, she wrote, “it seems excessive and premature to establish specific multidisciplinary clinics for children with long COVID-19.”
Dr. Rytter highlighted the difficulty of interpreting LongCOVIDKidsDK data, owing to recall bias, the failure to exclude other causes of symptoms in the cases analyzed, and the number of symptoms in the control persons. In addition, the data analyzed in Denmark are of limited clinical relevance, she said, given a greater presence of mild symptoms and, paradoxically, a higher quality of life.
She concluded, “In the majority of children with nonspecific symptoms after COVID-19, the symptoms presented are more likely to have been caused by something other than COVID-19, and if they are related to COVID-19, they are likely to go away over time.”
Dr. Ensinck, who is coauthor of the Argentine Ministry of Health’s guide for long COVID-19 monitoring for children and adolescents and who represented the Infectious Diseases Committee of the Argentine Society of Pediatrics, highlighted another aspect of the problem. “What should be taken into account in these data is to see how much the confinement contributed. Children are the ones who suffered the most in the period in which schools were closed; they could not meet their peers, they had sick relatives, they felt fear. … all this must be taken into account.”
There is as yet no agreement on how to define and diagnose long COVID-19 in adults, a population that has been studied more closely. Part of the problem is that long COVID-19 has been linked to more than 200 symptoms, which can range in severity from inconvenient to debilitating, can last for months or years, and can recur, sometimes months after apparent recovery. Thus, there are still disparate answers to basic questions about the syndrome’s frequency and its effects on vaccination, reinfection, and the latest variant of SARS-CoV-2.
This article has been translated from the Medscape Spanish edition. A version appeared on Medscape.com.
Among scientists, the existence of long COVID-19 in children and adolescents has been the subject of debate.
Published by a Mexican multidisciplinary group in Scientific Reports, the first study is a systematic review and meta-analysis. It identified mood symptoms as the most prevalent clinical manifestations of long COVID-19 in children and adolescents. These symptoms included sadness, tension, anger, depression, and anxiety (16.50%); fatigue (9.66%); and sleep disorders (8.42%).
The second study, LongCOVIDKidsDK, was conducted in Denmark. It compared 11,000 children younger than 14 years who had tested positive for COVID-19 with 33,000 children who had no history of COVID-19. The study was published in The Lancet Child and Adolescent Health.
Definitions are changing
In their meta-analysis, the researchers estimated the prevalence and counted signs and symptoms of long COVID-19, as defined by the United Kingdom’s National Institute for Health and Care Excellence. Long COVID-19 was defined as the presence of one or more symptoms more than 4 weeks after SARS-CoV-2 infection. For search terms, the researchers used “COVID-19,” “COVID,” “SARSCOV-2,” “coronavirus,” “long COVID,” “postCOVID,” “PASC,” “long-haulers,” “prolonged,” “post-acute,” “persistent,” “convalescent,” “sequelae,” and “postviral.”
Of the 8,373 citations returned by the search as of Feb. 10, 2022, 21 prospective studies, 2 of them on preprint servers, met the authors’ selection criteria. Those studies included a total of 80,071 children and adolescents younger than 18 years.
In the meta-analysis, the prevalence of long COVID-19 among children and adolescents was reported to be 25.24% (95% confidence interval, 18.17-33.02; I2, 99.61%), regardless of whether the case had been asymptomatic, mild, moderate, severe, or serious. For patients who had been hospitalized, the prevalence was 29.19% (95% CI, 17.83-41.98; I2, 80.84%).
These numbers, while striking, are not the focus of the study, according to first author Sandra Lopez-Leon, MD, PhD, associate professor of pharmacoepidemiology at Rutgers University, New Brunswick, N.J. “It’s important that we don’t focus on that 25%,” she said in an interview. “It’s a disease that we’re learning about, we’re at a time when the definitions are still changing, and, depending on when it is measured, a different number will be given. The message we want to give is that long COVID-19 exists, it’s happening in children and adolescents, and patients need this recognition. And also to show that it can affect the whole body.”
The study showed that the children and adolescents who presented with SARS-CoV-2 infection were at higher risk of subsequent long dyspnea, anosmia/ageusia, or fever, compared with control persons.
In total, in the studies that were included, more than 40 long-term clinical manifestations associated with COVID-19 in the pediatric population were identified.
The most common symptoms among children aged 0-3 years were mood swings, skin rashes, and stomachaches. In 4- to 11-year-olds, the most common symptoms were mood swings, trouble remembering or concentrating, and skin rashes. In 12- to 14-year-olds, they were fatigue, mood swings, and trouble remembering or concentrating. These data are based on parent responses.
The list of signs and symptoms also includes headache, respiratory symptoms, cognitive symptoms (such as decreased concentration, learning difficulties, confusion, and memory loss), loss of appetite, and smell disorder (hyposmia, anosmia, hyperosmia, parosmia, and phantom smell).
In the studies, the prevalence of the following symptoms was less than 5%: hyperhidrosis, chest pain, dizziness, cough, myalgia/arthralgia, changes in body weight, taste disorder, otalgia (tinnitus, ear pain, vertigo), ophthalmologic symptoms (conjunctivitis, dry eye, blurred vision, photophobia, pain), dermatologic symptoms (dry skin, itchy skin, rashes, hives, hair loss), urinary symptoms, abdominal pain, throat pain, chest tightness, variations in heart rate, palpitations, constipation, dysphonia, fever, diarrhea, vomiting/nausea, menstrual changes, neurological abnormalities, speech disorders, and dysphagia.
The authors made it clear that the frequency and severity of these symptoms can fluctuate from one patient to another.
“The meta-analysis is important because it brings together 21 studies selected from more than 8,000 articles – and in them, a large number of children – to study the most common manifestations of long COVID-19,” Gabriela Ensinck, MD, head of the infectious diseases department at the Víctor J. Vilela Children’s Hospital in Rosario, Argentina, told this news organization. Dr. Ensinck did not participate in the study. “The important thing here is that long COVID-19 exists in pediatrics. And that it is a prolongation of signs or symptoms over time, a time for which there is no single definition.”
“It’s a snapshot of all the symptoms that can remain after COVID-19,” Dr. Lopez-Leon explained. “The meta-analysis seeks to see if there’s an association between having had COVID-19 and having the symptoms, but at no time does it speak of causality.”
The prevalence of symptoms largely depends on the time since the onset of acute COVID-19. Most symptoms improve over time. In the studies that were included in the meta-analysis, the follow-up time varied between 1 and 13 months. It is important to understand what symptoms are associated with each period after the onset of infection, the authors said.
Danish parent survey
The Danish study LongCOVIDKidsDK followed the World Health Organization criteria for long COVID-19 and included children and adolescents aged 0-14 years who received a diagnosis of COVID-19 and who experienced symptoms that lasted at least 2 months.
Between July 20, 2021, and Sept. 15, 2021, a questionnaire was sent to 38,152 case patients and 147,212 control persons. Of this group, 10,997 (28.8%) case patients and 33,016 (22.4%) control persons answered the survey.
Children who had been diagnosed with SARS-CoV-2 infection were more likely to experience long-lasting symptoms than children who had never been diagnosed. Approximately one-third of children with a positive SARS-CoV-2 test experienced symptoms that were not present before infection. Children who experienced long-lasting symptoms included 40% of children diagnosed with COVID-19 and 27% of control persons aged 0-3 years, 38% of case patients and 34% of control persons aged 4-11 years, and 46% of case patients and 41% of control persons aged 12-14 years.
Interestingly, those diagnosed with COVID-19 reported fewer psychological and social problems than those in the control group. Among the oldest (aged 12-14 years), quality of life scores were higher and anxiety scores were lower for those who had tested positive for SARS-CoV-2.
More information needed
Given the diversity of symptoms in the meta-analysis and the LongCOVIDKidsDK study, a multidisciplinary approach is imperative. Dr. Lopez-Leon suggests that there is a need to raise awareness among parents, clinicians, researchers, and the health system about the conditions that can occur after COVID-19. Clinicians must better understand the sequelae to provide targeted care and treatment. The authors of the Danish study recommend establishing clinics for long COVID-19 with multispecialty care.
Maren J. Heilskov Rytter, PhD, associate professor of clinical medicine at the University of Copenhagen, wrote an editorial in The Lancet Child and Adolescent Health about the Danish study. Until it is clarified whether SARS-CoV-2 does indeed cause persistent symptoms, she wrote, “it seems excessive and premature to establish specific multidisciplinary clinics for children with long COVID-19.”
Dr. Rytter highlighted the difficulty of interpreting LongCOVIDKidsDK data, owing to recall bias, the failure to exclude other causes of symptoms in the cases analyzed, and the number of symptoms in the control persons. In addition, the data analyzed in Denmark are of limited clinical relevance, she said, given a greater presence of mild symptoms and, paradoxically, a higher quality of life.
She concluded, “In the majority of children with nonspecific symptoms after COVID-19, the symptoms presented are more likely to have been caused by something other than COVID-19, and if they are related to COVID-19, they are likely to go away over time.”
Dr. Ensinck, who is coauthor of the Argentine Ministry of Health’s guide for long COVID-19 monitoring for children and adolescents and who represented the Infectious Diseases Committee of the Argentine Society of Pediatrics, highlighted another aspect of the problem. “What should be taken into account in these data is to see how much the confinement contributed. Children are the ones who suffered the most in the period in which schools were closed; they could not meet their peers, they had sick relatives, they felt fear. … all this must be taken into account.”
There is as yet no agreement on how to define and diagnose long COVID-19 in adults, a population that has been studied more closely. Part of the problem is that long COVID-19 has been linked to more than 200 symptoms, which can range in severity from inconvenient to debilitating, can last for months or years, and can recur, sometimes months after apparent recovery. Thus, there are still disparate answers to basic questions about the syndrome’s frequency and its effects on vaccination, reinfection, and the latest variant of SARS-CoV-2.
This article has been translated from the Medscape Spanish edition. A version appeared on Medscape.com.
Among scientists, the existence of long COVID-19 in children and adolescents has been the subject of debate.
Published by a Mexican multidisciplinary group in Scientific Reports, the first study is a systematic review and meta-analysis. It identified mood symptoms as the most prevalent clinical manifestations of long COVID-19 in children and adolescents. These symptoms included sadness, tension, anger, depression, and anxiety (16.50%); fatigue (9.66%); and sleep disorders (8.42%).
The second study, LongCOVIDKidsDK, was conducted in Denmark. It compared 11,000 children younger than 14 years who had tested positive for COVID-19 with 33,000 children who had no history of COVID-19. The study was published in The Lancet Child and Adolescent Health.
Definitions are changing
In their meta-analysis, the researchers estimated the prevalence and counted signs and symptoms of long COVID-19, as defined by the United Kingdom’s National Institute for Health and Care Excellence. Long COVID-19 was defined as the presence of one or more symptoms more than 4 weeks after SARS-CoV-2 infection. For search terms, the researchers used “COVID-19,” “COVID,” “SARSCOV-2,” “coronavirus,” “long COVID,” “postCOVID,” “PASC,” “long-haulers,” “prolonged,” “post-acute,” “persistent,” “convalescent,” “sequelae,” and “postviral.”
Of the 8,373 citations returned by the search as of Feb. 10, 2022, 21 prospective studies, 2 of them on preprint servers, met the authors’ selection criteria. Those studies included a total of 80,071 children and adolescents younger than 18 years.
In the meta-analysis, the prevalence of long COVID-19 among children and adolescents was reported to be 25.24% (95% confidence interval, 18.17-33.02; I2, 99.61%), regardless of whether the case had been asymptomatic, mild, moderate, severe, or serious. For patients who had been hospitalized, the prevalence was 29.19% (95% CI, 17.83-41.98; I2, 80.84%).
These numbers, while striking, are not the focus of the study, according to first author Sandra Lopez-Leon, MD, PhD, associate professor of pharmacoepidemiology at Rutgers University, New Brunswick, N.J. “It’s important that we don’t focus on that 25%,” she said in an interview. “It’s a disease that we’re learning about, we’re at a time when the definitions are still changing, and, depending on when it is measured, a different number will be given. The message we want to give is that long COVID-19 exists, it’s happening in children and adolescents, and patients need this recognition. And also to show that it can affect the whole body.”
The study showed that the children and adolescents who presented with SARS-CoV-2 infection were at higher risk of subsequent long dyspnea, anosmia/ageusia, or fever, compared with control persons.
In total, in the studies that were included, more than 40 long-term clinical manifestations associated with COVID-19 in the pediatric population were identified.
The most common symptoms among children aged 0-3 years were mood swings, skin rashes, and stomachaches. In 4- to 11-year-olds, the most common symptoms were mood swings, trouble remembering or concentrating, and skin rashes. In 12- to 14-year-olds, they were fatigue, mood swings, and trouble remembering or concentrating. These data are based on parent responses.
The list of signs and symptoms also includes headache, respiratory symptoms, cognitive symptoms (such as decreased concentration, learning difficulties, confusion, and memory loss), loss of appetite, and smell disorder (hyposmia, anosmia, hyperosmia, parosmia, and phantom smell).
In the studies, the prevalence of the following symptoms was less than 5%: hyperhidrosis, chest pain, dizziness, cough, myalgia/arthralgia, changes in body weight, taste disorder, otalgia (tinnitus, ear pain, vertigo), ophthalmologic symptoms (conjunctivitis, dry eye, blurred vision, photophobia, pain), dermatologic symptoms (dry skin, itchy skin, rashes, hives, hair loss), urinary symptoms, abdominal pain, throat pain, chest tightness, variations in heart rate, palpitations, constipation, dysphonia, fever, diarrhea, vomiting/nausea, menstrual changes, neurological abnormalities, speech disorders, and dysphagia.
The authors made it clear that the frequency and severity of these symptoms can fluctuate from one patient to another.
“The meta-analysis is important because it brings together 21 studies selected from more than 8,000 articles – and in them, a large number of children – to study the most common manifestations of long COVID-19,” Gabriela Ensinck, MD, head of the infectious diseases department at the Víctor J. Vilela Children’s Hospital in Rosario, Argentina, told this news organization. Dr. Ensinck did not participate in the study. “The important thing here is that long COVID-19 exists in pediatrics. And that it is a prolongation of signs or symptoms over time, a time for which there is no single definition.”
“It’s a snapshot of all the symptoms that can remain after COVID-19,” Dr. Lopez-Leon explained. “The meta-analysis seeks to see if there’s an association between having had COVID-19 and having the symptoms, but at no time does it speak of causality.”
The prevalence of symptoms largely depends on the time since the onset of acute COVID-19. Most symptoms improve over time. In the studies that were included in the meta-analysis, the follow-up time varied between 1 and 13 months. It is important to understand what symptoms are associated with each period after the onset of infection, the authors said.
Danish parent survey
The Danish study LongCOVIDKidsDK followed the World Health Organization criteria for long COVID-19 and included children and adolescents aged 0-14 years who received a diagnosis of COVID-19 and who experienced symptoms that lasted at least 2 months.
Between July 20, 2021, and Sept. 15, 2021, a questionnaire was sent to 38,152 case patients and 147,212 control persons. Of this group, 10,997 (28.8%) case patients and 33,016 (22.4%) control persons answered the survey.
Children who had been diagnosed with SARS-CoV-2 infection were more likely to experience long-lasting symptoms than children who had never been diagnosed. Approximately one-third of children with a positive SARS-CoV-2 test experienced symptoms that were not present before infection. Children who experienced long-lasting symptoms included 40% of children diagnosed with COVID-19 and 27% of control persons aged 0-3 years, 38% of case patients and 34% of control persons aged 4-11 years, and 46% of case patients and 41% of control persons aged 12-14 years.
Interestingly, those diagnosed with COVID-19 reported fewer psychological and social problems than those in the control group. Among the oldest (aged 12-14 years), quality of life scores were higher and anxiety scores were lower for those who had tested positive for SARS-CoV-2.
More information needed
Given the diversity of symptoms in the meta-analysis and the LongCOVIDKidsDK study, a multidisciplinary approach is imperative. Dr. Lopez-Leon suggests that there is a need to raise awareness among parents, clinicians, researchers, and the health system about the conditions that can occur after COVID-19. Clinicians must better understand the sequelae to provide targeted care and treatment. The authors of the Danish study recommend establishing clinics for long COVID-19 with multispecialty care.
Maren J. Heilskov Rytter, PhD, associate professor of clinical medicine at the University of Copenhagen, wrote an editorial in The Lancet Child and Adolescent Health about the Danish study. Until it is clarified whether SARS-CoV-2 does indeed cause persistent symptoms, she wrote, “it seems excessive and premature to establish specific multidisciplinary clinics for children with long COVID-19.”
Dr. Rytter highlighted the difficulty of interpreting LongCOVIDKidsDK data, owing to recall bias, the failure to exclude other causes of symptoms in the cases analyzed, and the number of symptoms in the control persons. In addition, the data analyzed in Denmark are of limited clinical relevance, she said, given a greater presence of mild symptoms and, paradoxically, a higher quality of life.
She concluded, “In the majority of children with nonspecific symptoms after COVID-19, the symptoms presented are more likely to have been caused by something other than COVID-19, and if they are related to COVID-19, they are likely to go away over time.”
Dr. Ensinck, who is coauthor of the Argentine Ministry of Health’s guide for long COVID-19 monitoring for children and adolescents and who represented the Infectious Diseases Committee of the Argentine Society of Pediatrics, highlighted another aspect of the problem. “What should be taken into account in these data is to see how much the confinement contributed. Children are the ones who suffered the most in the period in which schools were closed; they could not meet their peers, they had sick relatives, they felt fear. … all this must be taken into account.”
There is as yet no agreement on how to define and diagnose long COVID-19 in adults, a population that has been studied more closely. Part of the problem is that long COVID-19 has been linked to more than 200 symptoms, which can range in severity from inconvenient to debilitating, can last for months or years, and can recur, sometimes months after apparent recovery. Thus, there are still disparate answers to basic questions about the syndrome’s frequency and its effects on vaccination, reinfection, and the latest variant of SARS-CoV-2.
This article has been translated from the Medscape Spanish edition. A version appeared on Medscape.com.
FROM SCIENTIFIC REPORTS AND THE LANCET CHILD AND ADOLESCENT HEALTH
IBD study hints at cause of postacute COVID
A new study among patients with inflammatory bowel disease (IBD) suggests that viral antigen persistence in the gut may contribute to post-acute COVID-19 syndrome.
Postacute COVID-19 syndrome is now understood to be a multiorgan condition with symptoms that may include fatigue, cognitive dysfunction, and pain. Poor baseline health and severe acute infection are risk factors for the condition, but nonhospitalized illness can also lead to persistent symptoms.
Researchers found that nearly two-thirds of IBD patients had persistence of the antigen in infected tissues up to 8 months after a mild (nonhospitalized) acute COVID-19 infection. The study is the first to tie gut antigen persistence to post-acute COVID symptoms, and the results imply that the antigen may lead to immune perturbation and ongoing symptoms.
The study was published online in Gastroenterology.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses the membrane-bound angiotensin-converting enzyme 2 to gain entry into cells, which is expressed in the brush border enterocytes, as well as elsewhere in the body.
Previous research using intestinal epithelial organoids confirmed that SARS-CoV-2 is capable of infecting the human epithelium and that the virus can be detected in anal swabs long after it is cleared from nasal passages.
One potential explanation is viral immune perturbation or inflammatory tissue injury. Supporting evidence includes neural accumulation of memory T cells in patients with neuropsychiatric symptoms such as malaise and depression, and similar changes are seen with age-related immune senescence and tissue injury. Hyperactivated B and T cells, as well as other innate immune cells, have also been linked to postacute COVID-19, as has heightened expression of proinflammatory cytokines.
To explore the potential role of persistent viral antigens, the researchers gathered biopsies during upper- and lower-gastrointestinal endoscopy in 46 patients with IBD whose prior COVID-19 infection (mean, 7.3 months previous) had been confirmed by polymerase chain reaction and who were seen at the IBD outpatient unit of the investigators’ institution. In all, 43.5% of patients were female, and the average age was 44.67 years. Overall, 67.4% had been diagnosed with Crohn’s disease, 28.3% with ulcerative colitis, and 4.3% had unclassified IBD; 23.9% had a history of exposure to anti–tumor necrosis factor therapy. Among patients in the study, 32 of the patients tested positive for mucosal SARS-CoV-2 RNA, and there was no association between the presence of viral RNA and IBD type.
The researchers found that 52%-70% of patients had antigen persistence in any gut segment, as measured by nucleocapsid immunofluorescence or expression of one of four viral transcripts. They detected persistence of the nucleocapsid in epithelial cells and CD8+ T cells. Viral antigens persisted in patients with and without exposure to immunosuppressive therapy, and there was no association with antigen persistence and severity of acute COVID-19 infection or the presence of inflammation at the time of the endoscopy.
The researchers believed that the persistent viral antigen reflects incomplete clearance from the original infection rather than a latent or persistent infection because they could not replicate the virus in biopsy samples. Most biopsies within a patient produced some, but not all, of the viral transcripts tested. The authors suggest that immunosuppressive therapy may lead to incomplete viral clearance. Some patients lacked humoral nucleocapsid IgG antibodies, especially among those with gut antigen persistence.
In fact, only patients with gut viral RNA persistence had symptoms of postacute COVID. “This observation strongly argues for a role of viral antigen persistence in postacute COVID-19 and it appears plausible that SARS-CoV-2 antigen persistence, possibly in infected tissues beyond the gut, could impact host immune responses underlying the postacute COVID-19 syndrome,” the researchers wrote.
There is precedent for such a phenomenon in influenza. Mouse models have shown that ineffective clearance can influence adaptive immune responses and memory T-cell formation in lymph nodes of the lung. Another report found that COVID-19 pneumonia survivors have persistent changes to pulmonary CD8+ T cells.
The study is limited by its small sample size and a lack of a replication cohort. The study was also conducted in IBD patients because the researchers believed they were at higher risk of COVID-19 infection, although the researchers note that viral antigen persistence has been observed 2 months after recovery from COVID-19 in patients without IBD or exposure to immunosuppressants.
The researchers call for studies in patients without IBD to determine whether viral antigen persistence is a key mechanism in postacute COVID-19.
The researchers have no relevant financial disclosures.
Understanding the cause and risk factors for the postacute COVID-19 condition is an urgent research priority. The study by Zollner et al. found new clues about the cause of the post–COVID-19 condition in intestinal tissues of patients with IBD. The first important finding was that most adult patients with IBD have persistent viral antigen in their intestine months after even mild acute COVID-19. Importantly, researchers could not recover replicating virus from these tissues, indicating there was unlikely persistent active infection or viral transmissibility. The second major finding was that the presence of persistent viral antigen in intestinal tissue was strongly associated with postacute COVID-19 symptoms. This suggests that persistence of SARS‑CoV‑2 antigen after acute infection could perpetuate an ongoing inflammatory response that causes the postacute COVID-19 condition.
Zollner et al. used the intestine as a window onto how this virus may lead to long-lasting symptoms in IBD patients. However, it does not change our understanding that corticosteroids, poorly controlled IBD, and comorbidities, and not biologic or immunomodulator therapy, increase the risk of severe illness and mortality related to acute COVID-19 in IBD patients.
Michael J. Rosen, MD, MSCI, is Endowed Professor for Pediatric IBD & Celiac Disease and director for the Center for Pediatric IBD & Celiac Disease at Stanford (Calif.) University. Dr. Rosen served on an advisory board for Pfizer.
Understanding the cause and risk factors for the postacute COVID-19 condition is an urgent research priority. The study by Zollner et al. found new clues about the cause of the post–COVID-19 condition in intestinal tissues of patients with IBD. The first important finding was that most adult patients with IBD have persistent viral antigen in their intestine months after even mild acute COVID-19. Importantly, researchers could not recover replicating virus from these tissues, indicating there was unlikely persistent active infection or viral transmissibility. The second major finding was that the presence of persistent viral antigen in intestinal tissue was strongly associated with postacute COVID-19 symptoms. This suggests that persistence of SARS‑CoV‑2 antigen after acute infection could perpetuate an ongoing inflammatory response that causes the postacute COVID-19 condition.
Zollner et al. used the intestine as a window onto how this virus may lead to long-lasting symptoms in IBD patients. However, it does not change our understanding that corticosteroids, poorly controlled IBD, and comorbidities, and not biologic or immunomodulator therapy, increase the risk of severe illness and mortality related to acute COVID-19 in IBD patients.
Michael J. Rosen, MD, MSCI, is Endowed Professor for Pediatric IBD & Celiac Disease and director for the Center for Pediatric IBD & Celiac Disease at Stanford (Calif.) University. Dr. Rosen served on an advisory board for Pfizer.
Understanding the cause and risk factors for the postacute COVID-19 condition is an urgent research priority. The study by Zollner et al. found new clues about the cause of the post–COVID-19 condition in intestinal tissues of patients with IBD. The first important finding was that most adult patients with IBD have persistent viral antigen in their intestine months after even mild acute COVID-19. Importantly, researchers could not recover replicating virus from these tissues, indicating there was unlikely persistent active infection or viral transmissibility. The second major finding was that the presence of persistent viral antigen in intestinal tissue was strongly associated with postacute COVID-19 symptoms. This suggests that persistence of SARS‑CoV‑2 antigen after acute infection could perpetuate an ongoing inflammatory response that causes the postacute COVID-19 condition.
Zollner et al. used the intestine as a window onto how this virus may lead to long-lasting symptoms in IBD patients. However, it does not change our understanding that corticosteroids, poorly controlled IBD, and comorbidities, and not biologic or immunomodulator therapy, increase the risk of severe illness and mortality related to acute COVID-19 in IBD patients.
Michael J. Rosen, MD, MSCI, is Endowed Professor for Pediatric IBD & Celiac Disease and director for the Center for Pediatric IBD & Celiac Disease at Stanford (Calif.) University. Dr. Rosen served on an advisory board for Pfizer.
A new study among patients with inflammatory bowel disease (IBD) suggests that viral antigen persistence in the gut may contribute to post-acute COVID-19 syndrome.
Postacute COVID-19 syndrome is now understood to be a multiorgan condition with symptoms that may include fatigue, cognitive dysfunction, and pain. Poor baseline health and severe acute infection are risk factors for the condition, but nonhospitalized illness can also lead to persistent symptoms.
Researchers found that nearly two-thirds of IBD patients had persistence of the antigen in infected tissues up to 8 months after a mild (nonhospitalized) acute COVID-19 infection. The study is the first to tie gut antigen persistence to post-acute COVID symptoms, and the results imply that the antigen may lead to immune perturbation and ongoing symptoms.
The study was published online in Gastroenterology.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses the membrane-bound angiotensin-converting enzyme 2 to gain entry into cells, which is expressed in the brush border enterocytes, as well as elsewhere in the body.
Previous research using intestinal epithelial organoids confirmed that SARS-CoV-2 is capable of infecting the human epithelium and that the virus can be detected in anal swabs long after it is cleared from nasal passages.
One potential explanation is viral immune perturbation or inflammatory tissue injury. Supporting evidence includes neural accumulation of memory T cells in patients with neuropsychiatric symptoms such as malaise and depression, and similar changes are seen with age-related immune senescence and tissue injury. Hyperactivated B and T cells, as well as other innate immune cells, have also been linked to postacute COVID-19, as has heightened expression of proinflammatory cytokines.
To explore the potential role of persistent viral antigens, the researchers gathered biopsies during upper- and lower-gastrointestinal endoscopy in 46 patients with IBD whose prior COVID-19 infection (mean, 7.3 months previous) had been confirmed by polymerase chain reaction and who were seen at the IBD outpatient unit of the investigators’ institution. In all, 43.5% of patients were female, and the average age was 44.67 years. Overall, 67.4% had been diagnosed with Crohn’s disease, 28.3% with ulcerative colitis, and 4.3% had unclassified IBD; 23.9% had a history of exposure to anti–tumor necrosis factor therapy. Among patients in the study, 32 of the patients tested positive for mucosal SARS-CoV-2 RNA, and there was no association between the presence of viral RNA and IBD type.
The researchers found that 52%-70% of patients had antigen persistence in any gut segment, as measured by nucleocapsid immunofluorescence or expression of one of four viral transcripts. They detected persistence of the nucleocapsid in epithelial cells and CD8+ T cells. Viral antigens persisted in patients with and without exposure to immunosuppressive therapy, and there was no association with antigen persistence and severity of acute COVID-19 infection or the presence of inflammation at the time of the endoscopy.
The researchers believed that the persistent viral antigen reflects incomplete clearance from the original infection rather than a latent or persistent infection because they could not replicate the virus in biopsy samples. Most biopsies within a patient produced some, but not all, of the viral transcripts tested. The authors suggest that immunosuppressive therapy may lead to incomplete viral clearance. Some patients lacked humoral nucleocapsid IgG antibodies, especially among those with gut antigen persistence.
In fact, only patients with gut viral RNA persistence had symptoms of postacute COVID. “This observation strongly argues for a role of viral antigen persistence in postacute COVID-19 and it appears plausible that SARS-CoV-2 antigen persistence, possibly in infected tissues beyond the gut, could impact host immune responses underlying the postacute COVID-19 syndrome,” the researchers wrote.
There is precedent for such a phenomenon in influenza. Mouse models have shown that ineffective clearance can influence adaptive immune responses and memory T-cell formation in lymph nodes of the lung. Another report found that COVID-19 pneumonia survivors have persistent changes to pulmonary CD8+ T cells.
The study is limited by its small sample size and a lack of a replication cohort. The study was also conducted in IBD patients because the researchers believed they were at higher risk of COVID-19 infection, although the researchers note that viral antigen persistence has been observed 2 months after recovery from COVID-19 in patients without IBD or exposure to immunosuppressants.
The researchers call for studies in patients without IBD to determine whether viral antigen persistence is a key mechanism in postacute COVID-19.
The researchers have no relevant financial disclosures.
A new study among patients with inflammatory bowel disease (IBD) suggests that viral antigen persistence in the gut may contribute to post-acute COVID-19 syndrome.
Postacute COVID-19 syndrome is now understood to be a multiorgan condition with symptoms that may include fatigue, cognitive dysfunction, and pain. Poor baseline health and severe acute infection are risk factors for the condition, but nonhospitalized illness can also lead to persistent symptoms.
Researchers found that nearly two-thirds of IBD patients had persistence of the antigen in infected tissues up to 8 months after a mild (nonhospitalized) acute COVID-19 infection. The study is the first to tie gut antigen persistence to post-acute COVID symptoms, and the results imply that the antigen may lead to immune perturbation and ongoing symptoms.
The study was published online in Gastroenterology.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses the membrane-bound angiotensin-converting enzyme 2 to gain entry into cells, which is expressed in the brush border enterocytes, as well as elsewhere in the body.
Previous research using intestinal epithelial organoids confirmed that SARS-CoV-2 is capable of infecting the human epithelium and that the virus can be detected in anal swabs long after it is cleared from nasal passages.
One potential explanation is viral immune perturbation or inflammatory tissue injury. Supporting evidence includes neural accumulation of memory T cells in patients with neuropsychiatric symptoms such as malaise and depression, and similar changes are seen with age-related immune senescence and tissue injury. Hyperactivated B and T cells, as well as other innate immune cells, have also been linked to postacute COVID-19, as has heightened expression of proinflammatory cytokines.
To explore the potential role of persistent viral antigens, the researchers gathered biopsies during upper- and lower-gastrointestinal endoscopy in 46 patients with IBD whose prior COVID-19 infection (mean, 7.3 months previous) had been confirmed by polymerase chain reaction and who were seen at the IBD outpatient unit of the investigators’ institution. In all, 43.5% of patients were female, and the average age was 44.67 years. Overall, 67.4% had been diagnosed with Crohn’s disease, 28.3% with ulcerative colitis, and 4.3% had unclassified IBD; 23.9% had a history of exposure to anti–tumor necrosis factor therapy. Among patients in the study, 32 of the patients tested positive for mucosal SARS-CoV-2 RNA, and there was no association between the presence of viral RNA and IBD type.
The researchers found that 52%-70% of patients had antigen persistence in any gut segment, as measured by nucleocapsid immunofluorescence or expression of one of four viral transcripts. They detected persistence of the nucleocapsid in epithelial cells and CD8+ T cells. Viral antigens persisted in patients with and without exposure to immunosuppressive therapy, and there was no association with antigen persistence and severity of acute COVID-19 infection or the presence of inflammation at the time of the endoscopy.
The researchers believed that the persistent viral antigen reflects incomplete clearance from the original infection rather than a latent or persistent infection because they could not replicate the virus in biopsy samples. Most biopsies within a patient produced some, but not all, of the viral transcripts tested. The authors suggest that immunosuppressive therapy may lead to incomplete viral clearance. Some patients lacked humoral nucleocapsid IgG antibodies, especially among those with gut antigen persistence.
In fact, only patients with gut viral RNA persistence had symptoms of postacute COVID. “This observation strongly argues for a role of viral antigen persistence in postacute COVID-19 and it appears plausible that SARS-CoV-2 antigen persistence, possibly in infected tissues beyond the gut, could impact host immune responses underlying the postacute COVID-19 syndrome,” the researchers wrote.
There is precedent for such a phenomenon in influenza. Mouse models have shown that ineffective clearance can influence adaptive immune responses and memory T-cell formation in lymph nodes of the lung. Another report found that COVID-19 pneumonia survivors have persistent changes to pulmonary CD8+ T cells.
The study is limited by its small sample size and a lack of a replication cohort. The study was also conducted in IBD patients because the researchers believed they were at higher risk of COVID-19 infection, although the researchers note that viral antigen persistence has been observed 2 months after recovery from COVID-19 in patients without IBD or exposure to immunosuppressants.
The researchers call for studies in patients without IBD to determine whether viral antigen persistence is a key mechanism in postacute COVID-19.
The researchers have no relevant financial disclosures.
FROM GASTROENTEROLOGY
‘Myriad’ dermatologic reactions after COVID-19 vaccination
GLASGOW – Individuals given COVID-19 vaccination may experience a wide range of dermatologic reactions, some of which may be life-threatening, reveals a prospective Indian study that suggests histopathological assessment is key to understanding the cause.
Studying more than 130 patients who presented with vaccine-related dermatologic reactions, the researchers found that the most common acute adverse events were acute urticaria, generalized pruritus, and maculopapular rash.
Dermal hypersensitivity reactions occurred within 3 days of vaccination, which suggests the culprit is an immediate type 1 hypersensitivity reaction, said study presenter Alpana Mohta, MD, department of dermatology, Sardar Patel Medical College, Bikaner, Rajasthan, India. Most of the patients had received the AstraZeneca vaccine, she said.
, which occurred within 3-4 weeks of vaccination and could be a result of delayed hypersensitivity or a T cell–mediated skin reaction caused by “molecular mimicry with a viral epitope,” Dr. Mohta said.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 5.
Dr. Mohta said that, given the “surge” in the number of people who have been vaccinated, it is “imperative as dermatologists” to maintain a “very high index of suspicion to differentiate reactions caused by vaccination” from other causes, and a proper assessment should be performed in “every patient” who presents with a possible reaction.
She also emphasized that “since so many clinical [COVID-19] variants are being encountered,” histopathological assessment could “help in better understanding the underlying pathophysiology” of every reaction.
Dr. Mohta began her presentation by explaining that India is running one of the “world’s largest vaccination drives” for COVID-19, with almost 90% of adults fully vaccinated.
She added that studies have indicated that the incidence of cutaneous adverse reactions following COVID-19 vaccination ranges from 1.0% to 1.9% and that dermatologists have encountered a “plethora” of related reactions.
Dr. Mohta emphasized that the “myriad presentations” of these reactions means that correlating clinical and pathological findings is “key” to understanding the underlying pathophysiology.
She and her colleagues therefore conducted a prospective, hospital-based study of patients who self-reported mucocutaneous adverse reactions from April to December 2021, within 4 weeks of receiving a COVID-19 vaccine.
They gathered information on the patients’ signs and symptoms, as well as the date of vaccine administration and the type of vaccine given, alongside a detailed medical history, including previous allergies, prior COVID-19 infection, and any comorbidities.
The patients also underwent a clinical examination and laboratory investigations, and their cases were assessed by two senior dermatologists to determine whether the association between the adverse event and COVID-19 vaccination was likely causal.
Dr. Mohta said that 132 adult patients, with an average age of 38.2 years, were identified as having vaccine-related reactions.
This included 84 (63.6%) patients with a mild reaction, defined as resolving with symptomatic treatment; 43 (32.6%) patients with a moderate reaction, defined as extensive and lasting for more than 4 weeks; and five (3.8%) patients with severe reactions, defined as systemic and potentially life-threatening.
The mild group included 21 patients with acute urticaria, with a mean onset of 1.2 days following vaccination, as well as 20 cases of maculopapular rash, with a mean onset of 2.4 days; 18 cases of pityriasis rosea, with a mean onset of 17.4 days; and nine cases of eruptive pseudoangioma, with a mean onset of 3.5 days.
There were 16 cases of lichen planus in the moderate group, with a mean onset of 22.7 days after COVID-19 vaccination; nine cases of herpes zoster, with a mean onset of 15.3 days; and one case of pityriasis lichenoides et varioliformis acuta (PLEVA), among others.
The severe group included two cases of erythroderma, with a mean onset of 9 days after vaccination; one case of drug rash with eosinophilia and systemic symptoms (DRESS), with a mean onset of 20 days; and one case each of subacute cutaneous lupus erythematosus (SCLE) and bullous pemphigoid, with mean onsets of 15 days and 14 days, respectively.
Turning to the histopathological results, Dr. Mohta explained that only 57 patients from their cohort agreed to have a skin biopsy.
Results of those skin biopsies showed that 21 (36.8%) patients had vaccine-related eruption of papules and plaques, predominantly spongiotic dermatitis. This correlated with the clinical diagnoses of pityriasis rosea, maculopapular and papulosquamous rash, and DRESS.
Lichenoid and interface dermatitis were seen in 13 (22.8%) patients, which correlated with the clinical diagnoses of lichen planus, PLEVA, and SCLE. Eleven (19.3%) patients had a dermal hypersensitivity reaction, equated to the clinical diagnoses of urticaria, and eruptive pseudoangioma.
Dr. Mohta acknowledged that the study was limited by the inability to calculate the “true prevalence of vaccine-associated reactions,” and because immunohistochemistry was not performed.
Session chair Saleem Taibjee, MD, department of dermatology, Dorset County Hospital NHS Foundation Trust, Dorchester, United Kingdom, congratulated Dr. Mohta on her “very interesting” presentation, highlighting their “extensive experience in such a large cohort of patients.”
He asked what type of COVID-19 vaccines the patients had received, and whether Dr. Mohta could provide any “insights into which patients you can safely give the vaccine again to, and those [to whom] you may avoid giving further doses.”
Dr. Mohta said that the majority of the patients in the study received the AstraZeneca COVID-19 vaccine, as that was the one most commonly used in India at the time, with around 30 patients receiving the Indian Covishield version of the AstraZeneca vaccine. (The two-dose AstraZeneca vaccine, which is cheaper to manufacture and easier to store at typical refrigerated temperatures than mRNA-based vaccines, has been authorized by the World Health Organization, the European Medicines Agency, and over 50 countries but has not been authorized in the United States.)
She added that none of the patients in the study with mild-to-moderate skin reactions were advised against receiving further doses” but that those with severe reactions “were advised not to take any further doses.”
Consequently, in the case of mild reactions, “further doses are not a contraindication,” Dr. Mohta said, but patients with more severe reactions should be considered on a “case by case basis.”
A version of this article first appeared on Medscape.com.
GLASGOW – Individuals given COVID-19 vaccination may experience a wide range of dermatologic reactions, some of which may be life-threatening, reveals a prospective Indian study that suggests histopathological assessment is key to understanding the cause.
Studying more than 130 patients who presented with vaccine-related dermatologic reactions, the researchers found that the most common acute adverse events were acute urticaria, generalized pruritus, and maculopapular rash.
Dermal hypersensitivity reactions occurred within 3 days of vaccination, which suggests the culprit is an immediate type 1 hypersensitivity reaction, said study presenter Alpana Mohta, MD, department of dermatology, Sardar Patel Medical College, Bikaner, Rajasthan, India. Most of the patients had received the AstraZeneca vaccine, she said.
, which occurred within 3-4 weeks of vaccination and could be a result of delayed hypersensitivity or a T cell–mediated skin reaction caused by “molecular mimicry with a viral epitope,” Dr. Mohta said.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 5.
Dr. Mohta said that, given the “surge” in the number of people who have been vaccinated, it is “imperative as dermatologists” to maintain a “very high index of suspicion to differentiate reactions caused by vaccination” from other causes, and a proper assessment should be performed in “every patient” who presents with a possible reaction.
She also emphasized that “since so many clinical [COVID-19] variants are being encountered,” histopathological assessment could “help in better understanding the underlying pathophysiology” of every reaction.
Dr. Mohta began her presentation by explaining that India is running one of the “world’s largest vaccination drives” for COVID-19, with almost 90% of adults fully vaccinated.
She added that studies have indicated that the incidence of cutaneous adverse reactions following COVID-19 vaccination ranges from 1.0% to 1.9% and that dermatologists have encountered a “plethora” of related reactions.
Dr. Mohta emphasized that the “myriad presentations” of these reactions means that correlating clinical and pathological findings is “key” to understanding the underlying pathophysiology.
She and her colleagues therefore conducted a prospective, hospital-based study of patients who self-reported mucocutaneous adverse reactions from April to December 2021, within 4 weeks of receiving a COVID-19 vaccine.
They gathered information on the patients’ signs and symptoms, as well as the date of vaccine administration and the type of vaccine given, alongside a detailed medical history, including previous allergies, prior COVID-19 infection, and any comorbidities.
The patients also underwent a clinical examination and laboratory investigations, and their cases were assessed by two senior dermatologists to determine whether the association between the adverse event and COVID-19 vaccination was likely causal.
Dr. Mohta said that 132 adult patients, with an average age of 38.2 years, were identified as having vaccine-related reactions.
This included 84 (63.6%) patients with a mild reaction, defined as resolving with symptomatic treatment; 43 (32.6%) patients with a moderate reaction, defined as extensive and lasting for more than 4 weeks; and five (3.8%) patients with severe reactions, defined as systemic and potentially life-threatening.
The mild group included 21 patients with acute urticaria, with a mean onset of 1.2 days following vaccination, as well as 20 cases of maculopapular rash, with a mean onset of 2.4 days; 18 cases of pityriasis rosea, with a mean onset of 17.4 days; and nine cases of eruptive pseudoangioma, with a mean onset of 3.5 days.
There were 16 cases of lichen planus in the moderate group, with a mean onset of 22.7 days after COVID-19 vaccination; nine cases of herpes zoster, with a mean onset of 15.3 days; and one case of pityriasis lichenoides et varioliformis acuta (PLEVA), among others.
The severe group included two cases of erythroderma, with a mean onset of 9 days after vaccination; one case of drug rash with eosinophilia and systemic symptoms (DRESS), with a mean onset of 20 days; and one case each of subacute cutaneous lupus erythematosus (SCLE) and bullous pemphigoid, with mean onsets of 15 days and 14 days, respectively.
Turning to the histopathological results, Dr. Mohta explained that only 57 patients from their cohort agreed to have a skin biopsy.
Results of those skin biopsies showed that 21 (36.8%) patients had vaccine-related eruption of papules and plaques, predominantly spongiotic dermatitis. This correlated with the clinical diagnoses of pityriasis rosea, maculopapular and papulosquamous rash, and DRESS.
Lichenoid and interface dermatitis were seen in 13 (22.8%) patients, which correlated with the clinical diagnoses of lichen planus, PLEVA, and SCLE. Eleven (19.3%) patients had a dermal hypersensitivity reaction, equated to the clinical diagnoses of urticaria, and eruptive pseudoangioma.
Dr. Mohta acknowledged that the study was limited by the inability to calculate the “true prevalence of vaccine-associated reactions,” and because immunohistochemistry was not performed.
Session chair Saleem Taibjee, MD, department of dermatology, Dorset County Hospital NHS Foundation Trust, Dorchester, United Kingdom, congratulated Dr. Mohta on her “very interesting” presentation, highlighting their “extensive experience in such a large cohort of patients.”
He asked what type of COVID-19 vaccines the patients had received, and whether Dr. Mohta could provide any “insights into which patients you can safely give the vaccine again to, and those [to whom] you may avoid giving further doses.”
Dr. Mohta said that the majority of the patients in the study received the AstraZeneca COVID-19 vaccine, as that was the one most commonly used in India at the time, with around 30 patients receiving the Indian Covishield version of the AstraZeneca vaccine. (The two-dose AstraZeneca vaccine, which is cheaper to manufacture and easier to store at typical refrigerated temperatures than mRNA-based vaccines, has been authorized by the World Health Organization, the European Medicines Agency, and over 50 countries but has not been authorized in the United States.)
She added that none of the patients in the study with mild-to-moderate skin reactions were advised against receiving further doses” but that those with severe reactions “were advised not to take any further doses.”
Consequently, in the case of mild reactions, “further doses are not a contraindication,” Dr. Mohta said, but patients with more severe reactions should be considered on a “case by case basis.”
A version of this article first appeared on Medscape.com.
GLASGOW – Individuals given COVID-19 vaccination may experience a wide range of dermatologic reactions, some of which may be life-threatening, reveals a prospective Indian study that suggests histopathological assessment is key to understanding the cause.
Studying more than 130 patients who presented with vaccine-related dermatologic reactions, the researchers found that the most common acute adverse events were acute urticaria, generalized pruritus, and maculopapular rash.
Dermal hypersensitivity reactions occurred within 3 days of vaccination, which suggests the culprit is an immediate type 1 hypersensitivity reaction, said study presenter Alpana Mohta, MD, department of dermatology, Sardar Patel Medical College, Bikaner, Rajasthan, India. Most of the patients had received the AstraZeneca vaccine, she said.
, which occurred within 3-4 weeks of vaccination and could be a result of delayed hypersensitivity or a T cell–mediated skin reaction caused by “molecular mimicry with a viral epitope,” Dr. Mohta said.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 5.
Dr. Mohta said that, given the “surge” in the number of people who have been vaccinated, it is “imperative as dermatologists” to maintain a “very high index of suspicion to differentiate reactions caused by vaccination” from other causes, and a proper assessment should be performed in “every patient” who presents with a possible reaction.
She also emphasized that “since so many clinical [COVID-19] variants are being encountered,” histopathological assessment could “help in better understanding the underlying pathophysiology” of every reaction.
Dr. Mohta began her presentation by explaining that India is running one of the “world’s largest vaccination drives” for COVID-19, with almost 90% of adults fully vaccinated.
She added that studies have indicated that the incidence of cutaneous adverse reactions following COVID-19 vaccination ranges from 1.0% to 1.9% and that dermatologists have encountered a “plethora” of related reactions.
Dr. Mohta emphasized that the “myriad presentations” of these reactions means that correlating clinical and pathological findings is “key” to understanding the underlying pathophysiology.
She and her colleagues therefore conducted a prospective, hospital-based study of patients who self-reported mucocutaneous adverse reactions from April to December 2021, within 4 weeks of receiving a COVID-19 vaccine.
They gathered information on the patients’ signs and symptoms, as well as the date of vaccine administration and the type of vaccine given, alongside a detailed medical history, including previous allergies, prior COVID-19 infection, and any comorbidities.
The patients also underwent a clinical examination and laboratory investigations, and their cases were assessed by two senior dermatologists to determine whether the association between the adverse event and COVID-19 vaccination was likely causal.
Dr. Mohta said that 132 adult patients, with an average age of 38.2 years, were identified as having vaccine-related reactions.
This included 84 (63.6%) patients with a mild reaction, defined as resolving with symptomatic treatment; 43 (32.6%) patients with a moderate reaction, defined as extensive and lasting for more than 4 weeks; and five (3.8%) patients with severe reactions, defined as systemic and potentially life-threatening.
The mild group included 21 patients with acute urticaria, with a mean onset of 1.2 days following vaccination, as well as 20 cases of maculopapular rash, with a mean onset of 2.4 days; 18 cases of pityriasis rosea, with a mean onset of 17.4 days; and nine cases of eruptive pseudoangioma, with a mean onset of 3.5 days.
There were 16 cases of lichen planus in the moderate group, with a mean onset of 22.7 days after COVID-19 vaccination; nine cases of herpes zoster, with a mean onset of 15.3 days; and one case of pityriasis lichenoides et varioliformis acuta (PLEVA), among others.
The severe group included two cases of erythroderma, with a mean onset of 9 days after vaccination; one case of drug rash with eosinophilia and systemic symptoms (DRESS), with a mean onset of 20 days; and one case each of subacute cutaneous lupus erythematosus (SCLE) and bullous pemphigoid, with mean onsets of 15 days and 14 days, respectively.
Turning to the histopathological results, Dr. Mohta explained that only 57 patients from their cohort agreed to have a skin biopsy.
Results of those skin biopsies showed that 21 (36.8%) patients had vaccine-related eruption of papules and plaques, predominantly spongiotic dermatitis. This correlated with the clinical diagnoses of pityriasis rosea, maculopapular and papulosquamous rash, and DRESS.
Lichenoid and interface dermatitis were seen in 13 (22.8%) patients, which correlated with the clinical diagnoses of lichen planus, PLEVA, and SCLE. Eleven (19.3%) patients had a dermal hypersensitivity reaction, equated to the clinical diagnoses of urticaria, and eruptive pseudoangioma.
Dr. Mohta acknowledged that the study was limited by the inability to calculate the “true prevalence of vaccine-associated reactions,” and because immunohistochemistry was not performed.
Session chair Saleem Taibjee, MD, department of dermatology, Dorset County Hospital NHS Foundation Trust, Dorchester, United Kingdom, congratulated Dr. Mohta on her “very interesting” presentation, highlighting their “extensive experience in such a large cohort of patients.”
He asked what type of COVID-19 vaccines the patients had received, and whether Dr. Mohta could provide any “insights into which patients you can safely give the vaccine again to, and those [to whom] you may avoid giving further doses.”
Dr. Mohta said that the majority of the patients in the study received the AstraZeneca COVID-19 vaccine, as that was the one most commonly used in India at the time, with around 30 patients receiving the Indian Covishield version of the AstraZeneca vaccine. (The two-dose AstraZeneca vaccine, which is cheaper to manufacture and easier to store at typical refrigerated temperatures than mRNA-based vaccines, has been authorized by the World Health Organization, the European Medicines Agency, and over 50 countries but has not been authorized in the United States.)
She added that none of the patients in the study with mild-to-moderate skin reactions were advised against receiving further doses” but that those with severe reactions “were advised not to take any further doses.”
Consequently, in the case of mild reactions, “further doses are not a contraindication,” Dr. Mohta said, but patients with more severe reactions should be considered on a “case by case basis.”
A version of this article first appeared on Medscape.com.

