User login
FDA approves adalimumab biosimilar Hyrimoz
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
compared with patients with hypertension alone.
“The results suggested that hypertensive patients with concurrent psoriasis experienced an earlier and more aggressive disease progression of hypertension, compared with general hypertensive patients,” Hsien-Yi Chiu, MD, PhD, from the department of dermatology at the National Taiwan University Hospital in Hsinchu, Taiwan, and his colleagues wrote in the Journal of Dermatology. “Thus, patients with hypertension and psoriasis should be considered for more aggressive strategies for prevention of primary [cardiovascular disease] and more intense assessments for cardiovascular interventions needed to improve [cardiovascular disease] outcome in these patients.”
They performed a nationwide cohort study of patients in the Taiwan National Health Insurance Research Database with new onset hypertension from 2005 to 2006. Those with psoriasis (4,039 patients) were matched by age and sex to patients in the database who were diagnosed with hypertension but not psoriasis; the mean follow-up was 5.62 years. Their mean age was 58 years and about 31% of the psoriasis cohort were female. They were divided into groups based on psoriasis severity (mild and severe psoriasis) and type (psoriasis with and without arthritis). Researchers noted patients with both psoriasis and hypertension also had higher rates of cerebrovascular disease, coronary heart disease, hyperlipidemia, and diabetes mellitus during the year prior to the study.
The outcome measured was having a cardiovascular procedure (percutaneous coronary intervention with/without stenting or percutaneous transluminal coronary angioplasty and transcatheter radiofrequency ablation for arrhythmia) and cardiovascular surgery (coronary artery bypass grafting and other surgery for heart valves, arrhythmia, cerebrovascular disease, peripheral vessels, and the aorta).
Patients with both psoriasis and hypertension were at an increased risk for having a cardiovascular procedure and surgery (adjusted hazard ratio, 1.28; 95% confidence interval, 1.07-1.53), compared with patients with only hypertension. The risk of this outcome was also increased among patients with severe psoriasis or psoriatic arthritis, compared with patients who had mild psoriasis (aHR, 1.22; 95% CI, 0.98-1.51) and with patients with psoriasis but not arthritis (aHR, 1.15; 95% CI, 0.84-1.58); however, the results did not reach statistical significance after adjustment, which the researchers attributed to the small subgroup size.
“Another possible explanation was that the observed increased requirement for cardiovascular procedure and surgery in patients with severe psoriasis was mediated by a complex interplay among inflammation, traditional risk factors for [cardiovascular disease], and antirheumatic drugs, which probably attenuate the effects conferred by psoriasis,” the authors wrote.
Limitations in the study included reliance on administrative claims data for psoriasis diagnosis, unavailability of some details of the cardiovascular procedures and surgery, lack of blood pressure data to identify hypertension severity, as well as unmeasured factors and confounders. Further, “comparative occurrence of a requirement for cardiovascular procedure and surgery in the two groups may be influenced by a competing risk for death,” the researchers noted.
This study was supported in part through grants by the National Taiwan University Hospital, Asia-Pacific La Roche–Posay Foundation 2014, and the Ministry of Science and Technology. Dr. Chiu is on the speaker’s bureau for AbbVie, Janssen Pharmaceuticals, Novartis, Eli Lilly and Pfizer. Another author has conducted clinical trials for or received fees for being a consultant or speaker for companies that include Abbvie, Boehringer Ingelheim, and Celgene. The remaining authors reported no relevant conflicts of interest.
SOURCE: Chiu H-Y et al. J Dermatol. 2018 Oct 16. doi: 10.1111/1346-8138.14654.
compared with patients with hypertension alone.
“The results suggested that hypertensive patients with concurrent psoriasis experienced an earlier and more aggressive disease progression of hypertension, compared with general hypertensive patients,” Hsien-Yi Chiu, MD, PhD, from the department of dermatology at the National Taiwan University Hospital in Hsinchu, Taiwan, and his colleagues wrote in the Journal of Dermatology. “Thus, patients with hypertension and psoriasis should be considered for more aggressive strategies for prevention of primary [cardiovascular disease] and more intense assessments for cardiovascular interventions needed to improve [cardiovascular disease] outcome in these patients.”
They performed a nationwide cohort study of patients in the Taiwan National Health Insurance Research Database with new onset hypertension from 2005 to 2006. Those with psoriasis (4,039 patients) were matched by age and sex to patients in the database who were diagnosed with hypertension but not psoriasis; the mean follow-up was 5.62 years. Their mean age was 58 years and about 31% of the psoriasis cohort were female. They were divided into groups based on psoriasis severity (mild and severe psoriasis) and type (psoriasis with and without arthritis). Researchers noted patients with both psoriasis and hypertension also had higher rates of cerebrovascular disease, coronary heart disease, hyperlipidemia, and diabetes mellitus during the year prior to the study.
The outcome measured was having a cardiovascular procedure (percutaneous coronary intervention with/without stenting or percutaneous transluminal coronary angioplasty and transcatheter radiofrequency ablation for arrhythmia) and cardiovascular surgery (coronary artery bypass grafting and other surgery for heart valves, arrhythmia, cerebrovascular disease, peripheral vessels, and the aorta).
Patients with both psoriasis and hypertension were at an increased risk for having a cardiovascular procedure and surgery (adjusted hazard ratio, 1.28; 95% confidence interval, 1.07-1.53), compared with patients with only hypertension. The risk of this outcome was also increased among patients with severe psoriasis or psoriatic arthritis, compared with patients who had mild psoriasis (aHR, 1.22; 95% CI, 0.98-1.51) and with patients with psoriasis but not arthritis (aHR, 1.15; 95% CI, 0.84-1.58); however, the results did not reach statistical significance after adjustment, which the researchers attributed to the small subgroup size.
“Another possible explanation was that the observed increased requirement for cardiovascular procedure and surgery in patients with severe psoriasis was mediated by a complex interplay among inflammation, traditional risk factors for [cardiovascular disease], and antirheumatic drugs, which probably attenuate the effects conferred by psoriasis,” the authors wrote.
Limitations in the study included reliance on administrative claims data for psoriasis diagnosis, unavailability of some details of the cardiovascular procedures and surgery, lack of blood pressure data to identify hypertension severity, as well as unmeasured factors and confounders. Further, “comparative occurrence of a requirement for cardiovascular procedure and surgery in the two groups may be influenced by a competing risk for death,” the researchers noted.
This study was supported in part through grants by the National Taiwan University Hospital, Asia-Pacific La Roche–Posay Foundation 2014, and the Ministry of Science and Technology. Dr. Chiu is on the speaker’s bureau for AbbVie, Janssen Pharmaceuticals, Novartis, Eli Lilly and Pfizer. Another author has conducted clinical trials for or received fees for being a consultant or speaker for companies that include Abbvie, Boehringer Ingelheim, and Celgene. The remaining authors reported no relevant conflicts of interest.
SOURCE: Chiu H-Y et al. J Dermatol. 2018 Oct 16. doi: 10.1111/1346-8138.14654.
compared with patients with hypertension alone.
“The results suggested that hypertensive patients with concurrent psoriasis experienced an earlier and more aggressive disease progression of hypertension, compared with general hypertensive patients,” Hsien-Yi Chiu, MD, PhD, from the department of dermatology at the National Taiwan University Hospital in Hsinchu, Taiwan, and his colleagues wrote in the Journal of Dermatology. “Thus, patients with hypertension and psoriasis should be considered for more aggressive strategies for prevention of primary [cardiovascular disease] and more intense assessments for cardiovascular interventions needed to improve [cardiovascular disease] outcome in these patients.”
They performed a nationwide cohort study of patients in the Taiwan National Health Insurance Research Database with new onset hypertension from 2005 to 2006. Those with psoriasis (4,039 patients) were matched by age and sex to patients in the database who were diagnosed with hypertension but not psoriasis; the mean follow-up was 5.62 years. Their mean age was 58 years and about 31% of the psoriasis cohort were female. They were divided into groups based on psoriasis severity (mild and severe psoriasis) and type (psoriasis with and without arthritis). Researchers noted patients with both psoriasis and hypertension also had higher rates of cerebrovascular disease, coronary heart disease, hyperlipidemia, and diabetes mellitus during the year prior to the study.
The outcome measured was having a cardiovascular procedure (percutaneous coronary intervention with/without stenting or percutaneous transluminal coronary angioplasty and transcatheter radiofrequency ablation for arrhythmia) and cardiovascular surgery (coronary artery bypass grafting and other surgery for heart valves, arrhythmia, cerebrovascular disease, peripheral vessels, and the aorta).
Patients with both psoriasis and hypertension were at an increased risk for having a cardiovascular procedure and surgery (adjusted hazard ratio, 1.28; 95% confidence interval, 1.07-1.53), compared with patients with only hypertension. The risk of this outcome was also increased among patients with severe psoriasis or psoriatic arthritis, compared with patients who had mild psoriasis (aHR, 1.22; 95% CI, 0.98-1.51) and with patients with psoriasis but not arthritis (aHR, 1.15; 95% CI, 0.84-1.58); however, the results did not reach statistical significance after adjustment, which the researchers attributed to the small subgroup size.
“Another possible explanation was that the observed increased requirement for cardiovascular procedure and surgery in patients with severe psoriasis was mediated by a complex interplay among inflammation, traditional risk factors for [cardiovascular disease], and antirheumatic drugs, which probably attenuate the effects conferred by psoriasis,” the authors wrote.
Limitations in the study included reliance on administrative claims data for psoriasis diagnosis, unavailability of some details of the cardiovascular procedures and surgery, lack of blood pressure data to identify hypertension severity, as well as unmeasured factors and confounders. Further, “comparative occurrence of a requirement for cardiovascular procedure and surgery in the two groups may be influenced by a competing risk for death,” the researchers noted.
This study was supported in part through grants by the National Taiwan University Hospital, Asia-Pacific La Roche–Posay Foundation 2014, and the Ministry of Science and Technology. Dr. Chiu is on the speaker’s bureau for AbbVie, Janssen Pharmaceuticals, Novartis, Eli Lilly and Pfizer. Another author has conducted clinical trials for or received fees for being a consultant or speaker for companies that include Abbvie, Boehringer Ingelheim, and Celgene. The remaining authors reported no relevant conflicts of interest.
SOURCE: Chiu H-Y et al. J Dermatol. 2018 Oct 16. doi: 10.1111/1346-8138.14654.
FROM THE JOURNAL OF DERMATOLOGY
Key clinical point: More aggressive cardiovascular disease preventive strategies should be considered in patients with hypertension who also have psoriasis.
Major finding: Patients with both psoriasis and hypertension were at an increased risk for requiring a cardiovascular procedure and surgery (adjusted hazard ratio, 1.28), compared with patients with hypertension alone.
Study details: A retrospective cohort study evaluated risk of this outcome in 4,039 patients with psoriasis and hypertension, compared with patients who had hypertension, matched for age and sex.
Disclosures: This study was supported in part through grants by the National Taiwan University Hospital Hsin-Chu Branch, Asia-Pacific La Roche–Posay Foundation 2014, and the Ministry of Science and Technology. Dr. Chiu is on the speaker’s bureau for companies including AbbVie, Novartis, and Eli Lilly. Another author has conducted clinical trials for or received fees for being a consultant or speaker for Abbvie, Boehringer Ingelheim, Celgene, Janssen Pharmaceuticals, Eli Lilly, Galderma, Novartis, and Pfizer. The other authors reported no relevant conflicts of interest.
Source: Chiu H-Y et al. J Dermatol. 2018 Oct 16. doi:10.1111/1346-8138.14654.
ACR readies first-ever guidelines on managing reproductive health in rheumatology
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM THE ACR ANNUAL MEETING
Flu vaccination lags among patients with psoriasis
Psoriasis patients are more vulnerable to systemic infections, including influenza-related pneumonia, but a new study shows that they are less likely to receive the influenza vaccine than patients with RA.
Vaccination rates were higher in psoriasis patients aged over 50 years, those who were female, and those with other chronic medical conditions, however.
Megan H. Noe, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and her coauthors referred to recent evidence suggesting that psoriasis involves systemic inflammation that increase the risk of comorbidities and that hospitalization rates for serious infections, including lower respiratory tract infections and pneumonia, are higher among adults with psoriasis than those who do not have psoriasis.
drawing from administrative and commercial claims data from OptumInsight Clinformatics Data Mart. They examined all adult patients with psoriasis, RA, or chronic hypertension who required oral antihypertensive medication. The study population included individuals tracked during the 2010-2011 flu season and 24 months prior (September 2008 to March 2011). This year was chosen because it was labeled as a “typical” season by the Centers for Disease Control and Prevention.
The primary outcome was a claim for an influenza vaccine, and covariates included age, length of residency, gender, and a clinical history of a range of conditions known to be associated with greater risk of influenza complications.
The population included 17,078 patients with psoriasis, 21,832 with RA, and 496,972 with chronic hypertension. After controlling for sex and age, the probability of getting a flu vaccine was similar between psoriasis and hypertension patients, but RA patients were more likely to be vaccinated than patients with psoriasis (odds ratio, 1.08; 95% confidence interval, 1.03-1.13). But the likelihood varied with age: 30-year-old patients with RA were more likely than a 30-year-old psoriasis patient to get a flu shot (OR, 1.30; 95% CI, 1.18-1.45), while a 70-year-old patient with RA was about as likely to get the flu vaccine as a 70-year-old patient with psoriasis.
Female psoriasis patients were more likely to get a flu shot than males (OR, 1.29; 95% CI, 1.20-1.38). Among the psoriasis patients, having some medical comorbidities were linked to a greater likelihood of being vaccinated, including asthma (OR, 1.58; 95% CI, 1.40-1.77), chronic liver disease (OR, 1.23; 95%, 1.03-1.47), diabetes (OR, 1.48; 95% CI, 1.36-1.63), HIV (OR, 3.68; 95% CI, 2.06-6.57), history of malignancy (OR, 1.21; 95% CI, 1.09-1.34), and psoriatic arthritis (OR, 1.40; 95% CI, 1.25-1.58).
There was no association between the use of an oral systemic therapy or biologic treatment and vaccination rates.
The authors suggested that psoriasis patients, especially younger ones, may not get adequate counseling on the value of the flu vaccine from their physicians. Studies have shown that, among the American public, health care providers are the most influential source of information about the flu vaccine. Among younger patients, the dermatologist may be a psoriasis patient’s primary health care provider, so it is important for dermatologists to counsel patients about the recommended vaccines, the authors wrote.
“Further research understanding why adults with psoriasis do not receive recommended vaccinations will help to create targeted interventions to improve vaccination rates and decrease hospitalizations in adults with psoriasis,” they concluded.
The study relied on administrative claims, so the results may not be generalizable to patients with insurance types other than those in the database or who are uninsured, the authors noted.
This study was funded by the National Psoriasis Foundation, the Dermatology Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Noe and three other authors did not report any disclosures, the fifth author reported multiple disclosures related to various pharmaceutical companies.
SOURCE: Noe MH et al. J Invest Dermatol. 2018 Oct 10. doi: 10.1016/j.jid.2018.09.012.
Psoriasis patients are more vulnerable to systemic infections, including influenza-related pneumonia, but a new study shows that they are less likely to receive the influenza vaccine than patients with RA.
Vaccination rates were higher in psoriasis patients aged over 50 years, those who were female, and those with other chronic medical conditions, however.
Megan H. Noe, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and her coauthors referred to recent evidence suggesting that psoriasis involves systemic inflammation that increase the risk of comorbidities and that hospitalization rates for serious infections, including lower respiratory tract infections and pneumonia, are higher among adults with psoriasis than those who do not have psoriasis.
drawing from administrative and commercial claims data from OptumInsight Clinformatics Data Mart. They examined all adult patients with psoriasis, RA, or chronic hypertension who required oral antihypertensive medication. The study population included individuals tracked during the 2010-2011 flu season and 24 months prior (September 2008 to March 2011). This year was chosen because it was labeled as a “typical” season by the Centers for Disease Control and Prevention.
The primary outcome was a claim for an influenza vaccine, and covariates included age, length of residency, gender, and a clinical history of a range of conditions known to be associated with greater risk of influenza complications.
The population included 17,078 patients with psoriasis, 21,832 with RA, and 496,972 with chronic hypertension. After controlling for sex and age, the probability of getting a flu vaccine was similar between psoriasis and hypertension patients, but RA patients were more likely to be vaccinated than patients with psoriasis (odds ratio, 1.08; 95% confidence interval, 1.03-1.13). But the likelihood varied with age: 30-year-old patients with RA were more likely than a 30-year-old psoriasis patient to get a flu shot (OR, 1.30; 95% CI, 1.18-1.45), while a 70-year-old patient with RA was about as likely to get the flu vaccine as a 70-year-old patient with psoriasis.
Female psoriasis patients were more likely to get a flu shot than males (OR, 1.29; 95% CI, 1.20-1.38). Among the psoriasis patients, having some medical comorbidities were linked to a greater likelihood of being vaccinated, including asthma (OR, 1.58; 95% CI, 1.40-1.77), chronic liver disease (OR, 1.23; 95%, 1.03-1.47), diabetes (OR, 1.48; 95% CI, 1.36-1.63), HIV (OR, 3.68; 95% CI, 2.06-6.57), history of malignancy (OR, 1.21; 95% CI, 1.09-1.34), and psoriatic arthritis (OR, 1.40; 95% CI, 1.25-1.58).
There was no association between the use of an oral systemic therapy or biologic treatment and vaccination rates.
The authors suggested that psoriasis patients, especially younger ones, may not get adequate counseling on the value of the flu vaccine from their physicians. Studies have shown that, among the American public, health care providers are the most influential source of information about the flu vaccine. Among younger patients, the dermatologist may be a psoriasis patient’s primary health care provider, so it is important for dermatologists to counsel patients about the recommended vaccines, the authors wrote.
“Further research understanding why adults with psoriasis do not receive recommended vaccinations will help to create targeted interventions to improve vaccination rates and decrease hospitalizations in adults with psoriasis,” they concluded.
The study relied on administrative claims, so the results may not be generalizable to patients with insurance types other than those in the database or who are uninsured, the authors noted.
This study was funded by the National Psoriasis Foundation, the Dermatology Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Noe and three other authors did not report any disclosures, the fifth author reported multiple disclosures related to various pharmaceutical companies.
SOURCE: Noe MH et al. J Invest Dermatol. 2018 Oct 10. doi: 10.1016/j.jid.2018.09.012.
Psoriasis patients are more vulnerable to systemic infections, including influenza-related pneumonia, but a new study shows that they are less likely to receive the influenza vaccine than patients with RA.
Vaccination rates were higher in psoriasis patients aged over 50 years, those who were female, and those with other chronic medical conditions, however.
Megan H. Noe, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and her coauthors referred to recent evidence suggesting that psoriasis involves systemic inflammation that increase the risk of comorbidities and that hospitalization rates for serious infections, including lower respiratory tract infections and pneumonia, are higher among adults with psoriasis than those who do not have psoriasis.
drawing from administrative and commercial claims data from OptumInsight Clinformatics Data Mart. They examined all adult patients with psoriasis, RA, or chronic hypertension who required oral antihypertensive medication. The study population included individuals tracked during the 2010-2011 flu season and 24 months prior (September 2008 to March 2011). This year was chosen because it was labeled as a “typical” season by the Centers for Disease Control and Prevention.
The primary outcome was a claim for an influenza vaccine, and covariates included age, length of residency, gender, and a clinical history of a range of conditions known to be associated with greater risk of influenza complications.
The population included 17,078 patients with psoriasis, 21,832 with RA, and 496,972 with chronic hypertension. After controlling for sex and age, the probability of getting a flu vaccine was similar between psoriasis and hypertension patients, but RA patients were more likely to be vaccinated than patients with psoriasis (odds ratio, 1.08; 95% confidence interval, 1.03-1.13). But the likelihood varied with age: 30-year-old patients with RA were more likely than a 30-year-old psoriasis patient to get a flu shot (OR, 1.30; 95% CI, 1.18-1.45), while a 70-year-old patient with RA was about as likely to get the flu vaccine as a 70-year-old patient with psoriasis.
Female psoriasis patients were more likely to get a flu shot than males (OR, 1.29; 95% CI, 1.20-1.38). Among the psoriasis patients, having some medical comorbidities were linked to a greater likelihood of being vaccinated, including asthma (OR, 1.58; 95% CI, 1.40-1.77), chronic liver disease (OR, 1.23; 95%, 1.03-1.47), diabetes (OR, 1.48; 95% CI, 1.36-1.63), HIV (OR, 3.68; 95% CI, 2.06-6.57), history of malignancy (OR, 1.21; 95% CI, 1.09-1.34), and psoriatic arthritis (OR, 1.40; 95% CI, 1.25-1.58).
There was no association between the use of an oral systemic therapy or biologic treatment and vaccination rates.
The authors suggested that psoriasis patients, especially younger ones, may not get adequate counseling on the value of the flu vaccine from their physicians. Studies have shown that, among the American public, health care providers are the most influential source of information about the flu vaccine. Among younger patients, the dermatologist may be a psoriasis patient’s primary health care provider, so it is important for dermatologists to counsel patients about the recommended vaccines, the authors wrote.
“Further research understanding why adults with psoriasis do not receive recommended vaccinations will help to create targeted interventions to improve vaccination rates and decrease hospitalizations in adults with psoriasis,” they concluded.
The study relied on administrative claims, so the results may not be generalizable to patients with insurance types other than those in the database or who are uninsured, the authors noted.
This study was funded by the National Psoriasis Foundation, the Dermatology Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Noe and three other authors did not report any disclosures, the fifth author reported multiple disclosures related to various pharmaceutical companies.
SOURCE: Noe MH et al. J Invest Dermatol. 2018 Oct 10. doi: 10.1016/j.jid.2018.09.012.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Despite vulnerability to complications, fewer psoriasis patients received the vaccine, compared with RA patients.
Major finding: Patients with RA were 8% more likely to receive a flu vaccine than patients with psoriasis.
Study details: A retrospective cohort study of 535,882 subjects with psoriasis, RA, or hypertension.
Disclosures: This study was funded by the National Psoriasis Foundation, the Dermatology Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Four authors did not report any disclosures; the fifth author reported multiple disclosures related to various pharmaceutical companies.
Source: Noe MH et al. J Invest Dermatol. 2018 Oct 10. doi: 10.1016/j.jid.2018.09.012.
IL inhibitor options move psoriasis treatment forward
Psoriasis patients have many options, and more are on the way, according to J. Mark Jackson, MD, of the University of Louisville, Ky.
“Know the information regarding each [treatment] to best care for your patients,” Dr. Jackson said in a presentation at the annual Coastal Dermatology Symposium.
Dr. Jackson particularly addressed the interleukin (IL)-17 inhibitors (brodalumab, ixekizumab, and secukinumab) and the IL-23 inhibitors (guselkumab, risankizumab, and tildrakizumab).
Complete clearance rates can reach 50% and higher over the long term when treating patients with IL-17 inhibitors, but patients must maintain regular dosing to maintain a response, he said.
Overall, comparisons of IL-17 inhibitors with etanercept, adalimumab, and ustekinumab “demonstrate better efficacy with no evidence of compromising safety,” he noted.
For example, secukinumab demonstrated significantly superior results when compared with ustekinumab in a randomized trial (J Am Acad Dermatol. 2015;73: 400-9). After 16 weeks of treatment, 79% of secukinumab patients achieved a 90% reduction in Psoriasis Area and Severity Index score (PASI 90) versus 58% of ustekinumab patients, he said, and the drug safety profile was consistent with the pivotal phase 3 studies of secukinumab.
Concerns persist about increased risk of inflammatory bowel disease, Crohn’s disease, and ulcerative colitis in patients taking secukinumab and other IL-17 inhibitors, but data indicate that rates are low. The risk is low “and may be related to psoriasis and not the therapy,” he explained.
Ixekizumab has been associated with more injection site reactions than secukinumab, but these tend to be mild, Dr. Jackson said. Advantages of ixekizumab are that it works quickly and has demonstrated effectiveness against genital, palmoplantar, scalp, and nail psoriasis, he added.
Brodalumab also works quickly, but it has the unique inclusion of a Risk Evaluation and Mitigation Strategies (REMS) program because of suicidal ideation and behavior in clinical trials, he noted, adding that there are more data showing rates are low and the REMS program is easier to deal with than the isotretinoin REMS. The increased risk of superficial Staphylococcus and Candida infections are noted on IL-17 inhibitor labels, but this has not been a significant issue in trials or clinical practice, he said.
What is also exciting about the IL-17 inhibitors are the approvals of ixekizumab and secukinumab for patients with psoriatic arthritis (PsA), with both agents demonstrating the ability to inhibit the structural progression of joint damage over time, Dr. Jackson commented. These data seem to be on par with that of the TNF-inhibitors, although time will tell how this bears out clinically, he noted.
IL-23 inhibitors guselkumab, tildrakizumab, and risankizumab (not yet approved) have shown similar effectiveness and are well tolerated by patients, with few injection site reactions or adverse events reported, Dr. Jackson said. The dosing regimens of each of these drugs, administered subcutaneously, are easy to follow: Treatment starts with an initial dose of either 100 mg (guselkumab and tildrakizumab) or 150 mg (risankizumab), which is followed by doses at 4 weeks and then doses every 8 weeks (guselkumab) or 12 weeks (tildrakizumab and risankizumab).
For example, in a comparison study of risankizumab with a dosage of 150 mg subcutaneously at week 0, 4, then every 12 weeks, 75% of risankizumab patients achieved PASI 90 at 16 weeks and 82% at 52 weeks, compared with 42% and 44%, respectively, for adalimumab patients.
In addition, the IL-23 inhibitors have demonstrated some benefits for PsA patients in clinical trials, but they are not currently indicated for PsA, he said.
Dr. Jackson disclosed having received research, honoraria, consulting, and/or other support from AbbVie, Accuitis, Aclaris, Celgene, Dr. Reddy’s, Galderma, Janssen, Lilly, Medimetriks, Novartis, Pfizer, Promius, Ralexar, Sienna, and TopMD.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Psoriasis patients have many options, and more are on the way, according to J. Mark Jackson, MD, of the University of Louisville, Ky.
“Know the information regarding each [treatment] to best care for your patients,” Dr. Jackson said in a presentation at the annual Coastal Dermatology Symposium.
Dr. Jackson particularly addressed the interleukin (IL)-17 inhibitors (brodalumab, ixekizumab, and secukinumab) and the IL-23 inhibitors (guselkumab, risankizumab, and tildrakizumab).
Complete clearance rates can reach 50% and higher over the long term when treating patients with IL-17 inhibitors, but patients must maintain regular dosing to maintain a response, he said.
Overall, comparisons of IL-17 inhibitors with etanercept, adalimumab, and ustekinumab “demonstrate better efficacy with no evidence of compromising safety,” he noted.
For example, secukinumab demonstrated significantly superior results when compared with ustekinumab in a randomized trial (J Am Acad Dermatol. 2015;73: 400-9). After 16 weeks of treatment, 79% of secukinumab patients achieved a 90% reduction in Psoriasis Area and Severity Index score (PASI 90) versus 58% of ustekinumab patients, he said, and the drug safety profile was consistent with the pivotal phase 3 studies of secukinumab.
Concerns persist about increased risk of inflammatory bowel disease, Crohn’s disease, and ulcerative colitis in patients taking secukinumab and other IL-17 inhibitors, but data indicate that rates are low. The risk is low “and may be related to psoriasis and not the therapy,” he explained.
Ixekizumab has been associated with more injection site reactions than secukinumab, but these tend to be mild, Dr. Jackson said. Advantages of ixekizumab are that it works quickly and has demonstrated effectiveness against genital, palmoplantar, scalp, and nail psoriasis, he added.
Brodalumab also works quickly, but it has the unique inclusion of a Risk Evaluation and Mitigation Strategies (REMS) program because of suicidal ideation and behavior in clinical trials, he noted, adding that there are more data showing rates are low and the REMS program is easier to deal with than the isotretinoin REMS. The increased risk of superficial Staphylococcus and Candida infections are noted on IL-17 inhibitor labels, but this has not been a significant issue in trials or clinical practice, he said.
What is also exciting about the IL-17 inhibitors are the approvals of ixekizumab and secukinumab for patients with psoriatic arthritis (PsA), with both agents demonstrating the ability to inhibit the structural progression of joint damage over time, Dr. Jackson commented. These data seem to be on par with that of the TNF-inhibitors, although time will tell how this bears out clinically, he noted.
IL-23 inhibitors guselkumab, tildrakizumab, and risankizumab (not yet approved) have shown similar effectiveness and are well tolerated by patients, with few injection site reactions or adverse events reported, Dr. Jackson said. The dosing regimens of each of these drugs, administered subcutaneously, are easy to follow: Treatment starts with an initial dose of either 100 mg (guselkumab and tildrakizumab) or 150 mg (risankizumab), which is followed by doses at 4 weeks and then doses every 8 weeks (guselkumab) or 12 weeks (tildrakizumab and risankizumab).
For example, in a comparison study of risankizumab with a dosage of 150 mg subcutaneously at week 0, 4, then every 12 weeks, 75% of risankizumab patients achieved PASI 90 at 16 weeks and 82% at 52 weeks, compared with 42% and 44%, respectively, for adalimumab patients.
In addition, the IL-23 inhibitors have demonstrated some benefits for PsA patients in clinical trials, but they are not currently indicated for PsA, he said.
Dr. Jackson disclosed having received research, honoraria, consulting, and/or other support from AbbVie, Accuitis, Aclaris, Celgene, Dr. Reddy’s, Galderma, Janssen, Lilly, Medimetriks, Novartis, Pfizer, Promius, Ralexar, Sienna, and TopMD.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Psoriasis patients have many options, and more are on the way, according to J. Mark Jackson, MD, of the University of Louisville, Ky.
“Know the information regarding each [treatment] to best care for your patients,” Dr. Jackson said in a presentation at the annual Coastal Dermatology Symposium.
Dr. Jackson particularly addressed the interleukin (IL)-17 inhibitors (brodalumab, ixekizumab, and secukinumab) and the IL-23 inhibitors (guselkumab, risankizumab, and tildrakizumab).
Complete clearance rates can reach 50% and higher over the long term when treating patients with IL-17 inhibitors, but patients must maintain regular dosing to maintain a response, he said.
Overall, comparisons of IL-17 inhibitors with etanercept, adalimumab, and ustekinumab “demonstrate better efficacy with no evidence of compromising safety,” he noted.
For example, secukinumab demonstrated significantly superior results when compared with ustekinumab in a randomized trial (J Am Acad Dermatol. 2015;73: 400-9). After 16 weeks of treatment, 79% of secukinumab patients achieved a 90% reduction in Psoriasis Area and Severity Index score (PASI 90) versus 58% of ustekinumab patients, he said, and the drug safety profile was consistent with the pivotal phase 3 studies of secukinumab.
Concerns persist about increased risk of inflammatory bowel disease, Crohn’s disease, and ulcerative colitis in patients taking secukinumab and other IL-17 inhibitors, but data indicate that rates are low. The risk is low “and may be related to psoriasis and not the therapy,” he explained.
Ixekizumab has been associated with more injection site reactions than secukinumab, but these tend to be mild, Dr. Jackson said. Advantages of ixekizumab are that it works quickly and has demonstrated effectiveness against genital, palmoplantar, scalp, and nail psoriasis, he added.
Brodalumab also works quickly, but it has the unique inclusion of a Risk Evaluation and Mitigation Strategies (REMS) program because of suicidal ideation and behavior in clinical trials, he noted, adding that there are more data showing rates are low and the REMS program is easier to deal with than the isotretinoin REMS. The increased risk of superficial Staphylococcus and Candida infections are noted on IL-17 inhibitor labels, but this has not been a significant issue in trials or clinical practice, he said.
What is also exciting about the IL-17 inhibitors are the approvals of ixekizumab and secukinumab for patients with psoriatic arthritis (PsA), with both agents demonstrating the ability to inhibit the structural progression of joint damage over time, Dr. Jackson commented. These data seem to be on par with that of the TNF-inhibitors, although time will tell how this bears out clinically, he noted.
IL-23 inhibitors guselkumab, tildrakizumab, and risankizumab (not yet approved) have shown similar effectiveness and are well tolerated by patients, with few injection site reactions or adverse events reported, Dr. Jackson said. The dosing regimens of each of these drugs, administered subcutaneously, are easy to follow: Treatment starts with an initial dose of either 100 mg (guselkumab and tildrakizumab) or 150 mg (risankizumab), which is followed by doses at 4 weeks and then doses every 8 weeks (guselkumab) or 12 weeks (tildrakizumab and risankizumab).
For example, in a comparison study of risankizumab with a dosage of 150 mg subcutaneously at week 0, 4, then every 12 weeks, 75% of risankizumab patients achieved PASI 90 at 16 weeks and 82% at 52 weeks, compared with 42% and 44%, respectively, for adalimumab patients.
In addition, the IL-23 inhibitors have demonstrated some benefits for PsA patients in clinical trials, but they are not currently indicated for PsA, he said.
Dr. Jackson disclosed having received research, honoraria, consulting, and/or other support from AbbVie, Accuitis, Aclaris, Celgene, Dr. Reddy’s, Galderma, Janssen, Lilly, Medimetriks, Novartis, Pfizer, Promius, Ralexar, Sienna, and TopMD.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Adalimumab safety update finds no new signals
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
No evidence of subclinical axial involvement seen in skin psoriasis
A study of individuals with longstanding skin psoriasis but no clinical arthritis or spondylitis has found no evidence of subclinical involvement of the sacroiliac joint or spine.
The prevalence of sacroiliac lesions on blinded MRI assessment was similar in 20 patients who had skin psoriasis for a median of 23 years and in 22 healthy controls, and no sacroiliac ankylosis was seen in either group. Similarly, there was no significant difference between the two groups in spinal lesions on MRI, nor in any of the five levels of lesion frequency, Vlad A. Bratu, MD, of the department of radiology at University Hospital Basel (Switzerland) and his coauthors reported in Arthritis Care & Research.
On blinded MRI assessment, five (25%) patients with skin psoriasis and two (9.1%) controls were classified as having inflammation of the sacroiliac joint. Three of these patients in the psoriasis group and one in the control group were older than 50, and the three with psoriasis had had the condition for 26-35 years.
Dr. Bratu and his colleagues said that subclinical peripheral joint inflammation on MRI had previously been a common finding in patients who had skin psoriasis but no clinical signs of psoriatic arthritis. But given the limited evidence of concomitant subclinical axial or spinal inflammation in their study, the authors argued there was no support for routine screening for potential subclinical axial inflammation in patients with longstanding skin psoriasis.
They noted that bone marrow edema lesions in at least two sacroiliac joint quadrants were seen in 35% of patients with psoriasis and 23% of healthy controls, a finding that reflected those seen in other studies in healthy individuals.
“If a specificity threshold for a given MRI lesion of at least 0.9 is applied for axial MRI to discriminate between axial SpA [spondyloarthritis] and background variation in healthy controls or in differential diagnostic conditions, no more than 10% of healthy controls in our study should meet this criterion by an individual level data analysis,” they wrote.
The authors also pointed out the impact of age on lesion frequency, which was more evident in spinal lesions.
“This observation supports the hypothesis that some spinal alterations in higher age may reflect degenerative rather than inflammatory changes,” they wrote. “However, there is a gap in knowledge with virtually no evidence about presence and pattern of degenerative versus inflammatory spinal lesions in subjects beyond 50 years of age.”
The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
SOURCE: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
A study of individuals with longstanding skin psoriasis but no clinical arthritis or spondylitis has found no evidence of subclinical involvement of the sacroiliac joint or spine.
The prevalence of sacroiliac lesions on blinded MRI assessment was similar in 20 patients who had skin psoriasis for a median of 23 years and in 22 healthy controls, and no sacroiliac ankylosis was seen in either group. Similarly, there was no significant difference between the two groups in spinal lesions on MRI, nor in any of the five levels of lesion frequency, Vlad A. Bratu, MD, of the department of radiology at University Hospital Basel (Switzerland) and his coauthors reported in Arthritis Care & Research.
On blinded MRI assessment, five (25%) patients with skin psoriasis and two (9.1%) controls were classified as having inflammation of the sacroiliac joint. Three of these patients in the psoriasis group and one in the control group were older than 50, and the three with psoriasis had had the condition for 26-35 years.
Dr. Bratu and his colleagues said that subclinical peripheral joint inflammation on MRI had previously been a common finding in patients who had skin psoriasis but no clinical signs of psoriatic arthritis. But given the limited evidence of concomitant subclinical axial or spinal inflammation in their study, the authors argued there was no support for routine screening for potential subclinical axial inflammation in patients with longstanding skin psoriasis.
They noted that bone marrow edema lesions in at least two sacroiliac joint quadrants were seen in 35% of patients with psoriasis and 23% of healthy controls, a finding that reflected those seen in other studies in healthy individuals.
“If a specificity threshold for a given MRI lesion of at least 0.9 is applied for axial MRI to discriminate between axial SpA [spondyloarthritis] and background variation in healthy controls or in differential diagnostic conditions, no more than 10% of healthy controls in our study should meet this criterion by an individual level data analysis,” they wrote.
The authors also pointed out the impact of age on lesion frequency, which was more evident in spinal lesions.
“This observation supports the hypothesis that some spinal alterations in higher age may reflect degenerative rather than inflammatory changes,” they wrote. “However, there is a gap in knowledge with virtually no evidence about presence and pattern of degenerative versus inflammatory spinal lesions in subjects beyond 50 years of age.”
The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
SOURCE: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
A study of individuals with longstanding skin psoriasis but no clinical arthritis or spondylitis has found no evidence of subclinical involvement of the sacroiliac joint or spine.
The prevalence of sacroiliac lesions on blinded MRI assessment was similar in 20 patients who had skin psoriasis for a median of 23 years and in 22 healthy controls, and no sacroiliac ankylosis was seen in either group. Similarly, there was no significant difference between the two groups in spinal lesions on MRI, nor in any of the five levels of lesion frequency, Vlad A. Bratu, MD, of the department of radiology at University Hospital Basel (Switzerland) and his coauthors reported in Arthritis Care & Research.
On blinded MRI assessment, five (25%) patients with skin psoriasis and two (9.1%) controls were classified as having inflammation of the sacroiliac joint. Three of these patients in the psoriasis group and one in the control group were older than 50, and the three with psoriasis had had the condition for 26-35 years.
Dr. Bratu and his colleagues said that subclinical peripheral joint inflammation on MRI had previously been a common finding in patients who had skin psoriasis but no clinical signs of psoriatic arthritis. But given the limited evidence of concomitant subclinical axial or spinal inflammation in their study, the authors argued there was no support for routine screening for potential subclinical axial inflammation in patients with longstanding skin psoriasis.
They noted that bone marrow edema lesions in at least two sacroiliac joint quadrants were seen in 35% of patients with psoriasis and 23% of healthy controls, a finding that reflected those seen in other studies in healthy individuals.
“If a specificity threshold for a given MRI lesion of at least 0.9 is applied for axial MRI to discriminate between axial SpA [spondyloarthritis] and background variation in healthy controls or in differential diagnostic conditions, no more than 10% of healthy controls in our study should meet this criterion by an individual level data analysis,” they wrote.
The authors also pointed out the impact of age on lesion frequency, which was more evident in spinal lesions.
“This observation supports the hypothesis that some spinal alterations in higher age may reflect degenerative rather than inflammatory changes,” they wrote. “However, there is a gap in knowledge with virtually no evidence about presence and pattern of degenerative versus inflammatory spinal lesions in subjects beyond 50 years of age.”
The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
SOURCE: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: The prevalence of sacroiliac bone marrow lesions was similar between patients with skin psoriasis and healthy controls.
Study details: Case-control study in 20 patients with skin psoriasis and 22 healthy controls.
Disclosures: The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
Source: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
Novel oral agent shows unprecedented efficacy in psoriasis
PARIS – A novel in a phase 2 clinical trial including 267 adults with moderate to severe disease, James G. Krueger, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I would say the clinical response here is almost dead-on as a copy for ustekinumab, which is an [injectable interleukin] IL-23/IL-12 blocker. And we’re only at 12 weeks here; some of the curves look like they’re on a trajectory to go up further in terms of improvement. So I’m getting a performance with an oral drug that is just so much better than the approved alternatives that we have,” said Dr. Krueger, head of the laboratory of investigative dermatology and professor in clinical investigation at Rockefeller University in New York.
Oral apremilast (Otezla), for example, can’t touch those PASI 75 response rates in patients with moderate to severe psoriasis. Indeed, many psoriasis experts favor reserving apremilast for patients with moderate disease.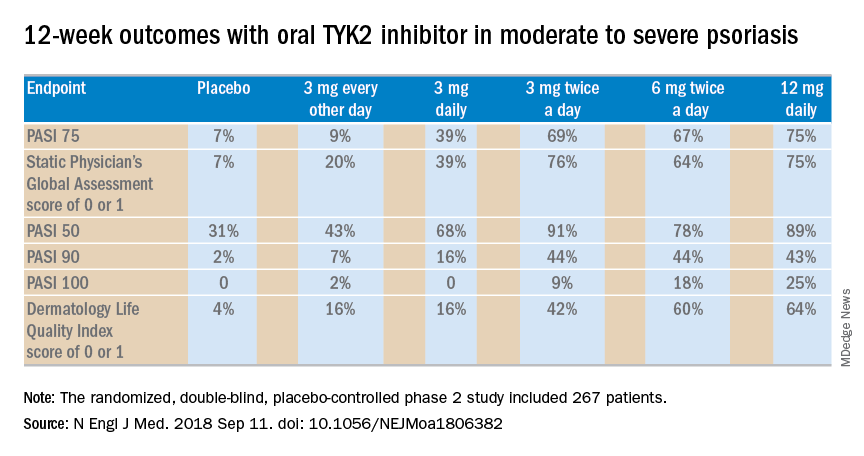
The 12-week, double-blind, placebo-controlled study was conducted at 82 sites in the United States and seven other countries. In this dose-ranging study, participants were randomized to the oral selective tyrosine kinase 2 (TYK2) inhibitor, known for the time being as BMS-986165, at 3 mg every other day, 3 mg daily, 3 mg twice a day, 6 mg twice a day, 12 mg daily, or to placebo.
The primary outcome was a 75% or greater reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) at week 12. The TYK2 inhibitor outperformed placebo in dose-dependent fashion starting at the 3 mg/day dose. The PASI 75 rate was 7% with placebo, 9% with 3 mg of BMS-986165 every other day, 39% with 3 mg daily, 69% with 3 mg BID, 67% with 6 mg BID, and 75% with 12 mg/day. All secondary endpoints followed suit.
A striking finding in the phase 2 study was that when the drug was stopped for a month at the end of the 12-week treatment period, for the most part, the PASI 75 response and other clinical benefits were retained.
“I would contrast this to experiments that I have personally done with cyclosporine, where I have cleared people with cyclosporine, stopped it, and a month later every single patient has rip-roaring disease back. So I think this TYK2 inhibitor has some different performance features than just blocking a downstream T-cell transduction molecule,” observed the dermatologist, who is credited as the discoverer of the importance of the T cell in psoriasis pathogenesis.
The strong multidimensional evidence of clinical efficacy in the phase 2 study was supported mechanistically by analysis of skin biopsies obtained on study days 1, 15, and 85. The laboratory studies showed that the oral drug improved molecular, cellular, and clinical biomarkers associated with treatment efficacy. For example, at doses of 3 mg twice a day or higher, the TYK2 inhibitor reduced expression of IL-19 and IL-36A, which are key drivers of keratinocyte activation and epidermal hyperplasia. The drug also markedly decreased expression of genes in the Th17 pathway and essentially normalized expression of the proinflammatory genes beta defensin and S100A9.
In contrast to the Janus kinase (JAK) 1/3 and JAK 2 inhibitors in development for treatment of psoriasis, which paint with a much broader brush, the TYK2 inhibitor is highly selective for IL-23, IL-12, and interferon alpha.
“Previous studies have shown pan-JAK inhibition can be very effective in remitting psoriasis. The problem is that if one inhibits JAK1 and JAK3, one blocks the transduction of effector cytokines that are essentially there for protective immunity. That could lead to undesirable levels of immunosuppression,” Dr. Krueger explained.
The most important cytokine in the pathogenesis of psoriasis is clearly IL-23, he continued. In cell-based assays, the TYK2 inhibitor has been shown to be 100 times more selective in inhibiting IL-23 , IL-12, and interferon-alpha than JAK 1/3 inhibitors and 3,000 times more selective than JAK 2 inhibitors. This high degree of selectivity makes for fewer off-target effects and for a favorable safety profile.
“There were no major safety signals that would lead you to be concerned,” Dr. Krueger said. Indeed, based upon the encouraging safety and efficacy demonstrated this phase 2 study, a phase 3 program known as POETYK-PSO is underway (POETYK-PSO-1 and POETYK-PSO-2).
The phase 2 clinical trial results were published online in conjunction with the EADV congress.
The TYK2 inhibitor is being developed by Bristol-Myers Squibb. Dr. Krueger reported receiving personal fees as well as research grants paid directly to Rockefeller University from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
PARIS – A novel in a phase 2 clinical trial including 267 adults with moderate to severe disease, James G. Krueger, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I would say the clinical response here is almost dead-on as a copy for ustekinumab, which is an [injectable interleukin] IL-23/IL-12 blocker. And we’re only at 12 weeks here; some of the curves look like they’re on a trajectory to go up further in terms of improvement. So I’m getting a performance with an oral drug that is just so much better than the approved alternatives that we have,” said Dr. Krueger, head of the laboratory of investigative dermatology and professor in clinical investigation at Rockefeller University in New York.
Oral apremilast (Otezla), for example, can’t touch those PASI 75 response rates in patients with moderate to severe psoriasis. Indeed, many psoriasis experts favor reserving apremilast for patients with moderate disease.
The 12-week, double-blind, placebo-controlled study was conducted at 82 sites in the United States and seven other countries. In this dose-ranging study, participants were randomized to the oral selective tyrosine kinase 2 (TYK2) inhibitor, known for the time being as BMS-986165, at 3 mg every other day, 3 mg daily, 3 mg twice a day, 6 mg twice a day, 12 mg daily, or to placebo.
The primary outcome was a 75% or greater reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) at week 12. The TYK2 inhibitor outperformed placebo in dose-dependent fashion starting at the 3 mg/day dose. The PASI 75 rate was 7% with placebo, 9% with 3 mg of BMS-986165 every other day, 39% with 3 mg daily, 69% with 3 mg BID, 67% with 6 mg BID, and 75% with 12 mg/day. All secondary endpoints followed suit.
A striking finding in the phase 2 study was that when the drug was stopped for a month at the end of the 12-week treatment period, for the most part, the PASI 75 response and other clinical benefits were retained.
“I would contrast this to experiments that I have personally done with cyclosporine, where I have cleared people with cyclosporine, stopped it, and a month later every single patient has rip-roaring disease back. So I think this TYK2 inhibitor has some different performance features than just blocking a downstream T-cell transduction molecule,” observed the dermatologist, who is credited as the discoverer of the importance of the T cell in psoriasis pathogenesis.
The strong multidimensional evidence of clinical efficacy in the phase 2 study was supported mechanistically by analysis of skin biopsies obtained on study days 1, 15, and 85. The laboratory studies showed that the oral drug improved molecular, cellular, and clinical biomarkers associated with treatment efficacy. For example, at doses of 3 mg twice a day or higher, the TYK2 inhibitor reduced expression of IL-19 and IL-36A, which are key drivers of keratinocyte activation and epidermal hyperplasia. The drug also markedly decreased expression of genes in the Th17 pathway and essentially normalized expression of the proinflammatory genes beta defensin and S100A9.
In contrast to the Janus kinase (JAK) 1/3 and JAK 2 inhibitors in development for treatment of psoriasis, which paint with a much broader brush, the TYK2 inhibitor is highly selective for IL-23, IL-12, and interferon alpha.
“Previous studies have shown pan-JAK inhibition can be very effective in remitting psoriasis. The problem is that if one inhibits JAK1 and JAK3, one blocks the transduction of effector cytokines that are essentially there for protective immunity. That could lead to undesirable levels of immunosuppression,” Dr. Krueger explained.
The most important cytokine in the pathogenesis of psoriasis is clearly IL-23, he continued. In cell-based assays, the TYK2 inhibitor has been shown to be 100 times more selective in inhibiting IL-23 , IL-12, and interferon-alpha than JAK 1/3 inhibitors and 3,000 times more selective than JAK 2 inhibitors. This high degree of selectivity makes for fewer off-target effects and for a favorable safety profile.
“There were no major safety signals that would lead you to be concerned,” Dr. Krueger said. Indeed, based upon the encouraging safety and efficacy demonstrated this phase 2 study, a phase 3 program known as POETYK-PSO is underway (POETYK-PSO-1 and POETYK-PSO-2).
The phase 2 clinical trial results were published online in conjunction with the EADV congress.
The TYK2 inhibitor is being developed by Bristol-Myers Squibb. Dr. Krueger reported receiving personal fees as well as research grants paid directly to Rockefeller University from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
PARIS – A novel in a phase 2 clinical trial including 267 adults with moderate to severe disease, James G. Krueger, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I would say the clinical response here is almost dead-on as a copy for ustekinumab, which is an [injectable interleukin] IL-23/IL-12 blocker. And we’re only at 12 weeks here; some of the curves look like they’re on a trajectory to go up further in terms of improvement. So I’m getting a performance with an oral drug that is just so much better than the approved alternatives that we have,” said Dr. Krueger, head of the laboratory of investigative dermatology and professor in clinical investigation at Rockefeller University in New York.
Oral apremilast (Otezla), for example, can’t touch those PASI 75 response rates in patients with moderate to severe psoriasis. Indeed, many psoriasis experts favor reserving apremilast for patients with moderate disease.
The 12-week, double-blind, placebo-controlled study was conducted at 82 sites in the United States and seven other countries. In this dose-ranging study, participants were randomized to the oral selective tyrosine kinase 2 (TYK2) inhibitor, known for the time being as BMS-986165, at 3 mg every other day, 3 mg daily, 3 mg twice a day, 6 mg twice a day, 12 mg daily, or to placebo.
The primary outcome was a 75% or greater reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) at week 12. The TYK2 inhibitor outperformed placebo in dose-dependent fashion starting at the 3 mg/day dose. The PASI 75 rate was 7% with placebo, 9% with 3 mg of BMS-986165 every other day, 39% with 3 mg daily, 69% with 3 mg BID, 67% with 6 mg BID, and 75% with 12 mg/day. All secondary endpoints followed suit.
A striking finding in the phase 2 study was that when the drug was stopped for a month at the end of the 12-week treatment period, for the most part, the PASI 75 response and other clinical benefits were retained.
“I would contrast this to experiments that I have personally done with cyclosporine, where I have cleared people with cyclosporine, stopped it, and a month later every single patient has rip-roaring disease back. So I think this TYK2 inhibitor has some different performance features than just blocking a downstream T-cell transduction molecule,” observed the dermatologist, who is credited as the discoverer of the importance of the T cell in psoriasis pathogenesis.
The strong multidimensional evidence of clinical efficacy in the phase 2 study was supported mechanistically by analysis of skin biopsies obtained on study days 1, 15, and 85. The laboratory studies showed that the oral drug improved molecular, cellular, and clinical biomarkers associated with treatment efficacy. For example, at doses of 3 mg twice a day or higher, the TYK2 inhibitor reduced expression of IL-19 and IL-36A, which are key drivers of keratinocyte activation and epidermal hyperplasia. The drug also markedly decreased expression of genes in the Th17 pathway and essentially normalized expression of the proinflammatory genes beta defensin and S100A9.
In contrast to the Janus kinase (JAK) 1/3 and JAK 2 inhibitors in development for treatment of psoriasis, which paint with a much broader brush, the TYK2 inhibitor is highly selective for IL-23, IL-12, and interferon alpha.
“Previous studies have shown pan-JAK inhibition can be very effective in remitting psoriasis. The problem is that if one inhibits JAK1 and JAK3, one blocks the transduction of effector cytokines that are essentially there for protective immunity. That could lead to undesirable levels of immunosuppression,” Dr. Krueger explained.
The most important cytokine in the pathogenesis of psoriasis is clearly IL-23, he continued. In cell-based assays, the TYK2 inhibitor has been shown to be 100 times more selective in inhibiting IL-23 , IL-12, and interferon-alpha than JAK 1/3 inhibitors and 3,000 times more selective than JAK 2 inhibitors. This high degree of selectivity makes for fewer off-target effects and for a favorable safety profile.
“There were no major safety signals that would lead you to be concerned,” Dr. Krueger said. Indeed, based upon the encouraging safety and efficacy demonstrated this phase 2 study, a phase 3 program known as POETYK-PSO is underway (POETYK-PSO-1 and POETYK-PSO-2).
The phase 2 clinical trial results were published online in conjunction with the EADV congress.
The TYK2 inhibitor is being developed by Bristol-Myers Squibb. Dr. Krueger reported receiving personal fees as well as research grants paid directly to Rockefeller University from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
REPORTING FROM THE EADV CONGRESS
Key clinical point: A novel selective tyrosine kinase 2 inhibitor achieves response rates previously unheard of in oral therapy for moderate to severe psoriasis.
Major finding: At the top dose of oral BMS-986165 studied to date, the PASI 75 rate at 12 weeks was 75%.
Study details: This eight-country, randomized, double-blind, placebo-controlled phase 2 study included 267 patients with moderate to severe psoriasis.
Disclosures: The study was sponsored by Bristol-Myers Squibb. The presenter reported receiving personal fees and institutional research grants from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
Elevated type 2 diabetes risk seen in PsA patients
Patients with incident psoriatic arthritis are at a significantly increased risk of developing type 2 diabetes when compared against patients with psoriasis alone and with the general population, according to recent research published in Rheumatology.
Rachel Charlton, PhD, of the department of pharmacy and pharmacology at the University of Bath (England), and her colleagues performed an analysis of 6,783 incident cases of psoriatic arthritis (PsA) from the U.K. Clinical Practice Research Datalink who were diagnosed during 1998-2014. Patients were between 18 years and 89 years old with a median age of 49 years at PsA diagnosis.
In the study, the researchers randomly matched PsA cases at a 1:4 ratio to either a cohort of general population patients with no PsA, psoriasis, or inflammatory arthritis or a cohort of patients with psoriasis but no PsA or inflammatory arthritis. Patients were followed from match to the point where they either no longer met inclusion criteria for the cohort or received a diagnosis of type 2 diabetes, cerebrovascular disease (CVD), ischemic heart disease (IHD), or peripheral vascular disease (PVD) with a mean follow-up duration of approximately 5.5 years across all patient groups.
Patients in the PsA group had a significantly higher incidence of type 2 diabetes, compared with the general population (adjusted relative risk, 1.40; 95% confidence interval, 1.15-1.70; P = .0007) and psoriasis groups (adjusted RR, 1.53; 95% CI, 1.19-1.97; P = .0009). In the PsA group, risk of CVD (adjusted RR, 1.24; 95% CI, 0.99-1.56; P = .06), IHD (adjusted RR, 1.27; 95% CI, 1.05-1.54; P = .02), and PVD (adjusted RR, 1.40; 95% CI, 1.02-1.92; P = .04) were significantly higher than in the general population but not when compared with the psoriasis group. The overall risk of cardiovascular disease (including CVD, IHD, and PVD) for the PsA group was significantly higher (adjusted RR, 1.29; 95% CI, 1.12-1.48; P = .0005), compared with the general population.
“These results support the proposal in existing clinical guidelines that, in order to reduce cardiovascular risk in patients with PsA, it is important to treat inflammatory disease as well as to screen and treat traditional risk factors early in the disease course,” Ms. Charlton and her colleagues wrote in their study.
This study was funded by a grant from the National Institute for Health Research in the United Kingdom. The authors reported no relevant conflicts of interest.
SOURCE: Charlton RA et al. Rheumatology. 2018 Sep 6. doi: 10.1093/rheumatology/key286.
Patients with incident psoriatic arthritis are at a significantly increased risk of developing type 2 diabetes when compared against patients with psoriasis alone and with the general population, according to recent research published in Rheumatology.
Rachel Charlton, PhD, of the department of pharmacy and pharmacology at the University of Bath (England), and her colleagues performed an analysis of 6,783 incident cases of psoriatic arthritis (PsA) from the U.K. Clinical Practice Research Datalink who were diagnosed during 1998-2014. Patients were between 18 years and 89 years old with a median age of 49 years at PsA diagnosis.
In the study, the researchers randomly matched PsA cases at a 1:4 ratio to either a cohort of general population patients with no PsA, psoriasis, or inflammatory arthritis or a cohort of patients with psoriasis but no PsA or inflammatory arthritis. Patients were followed from match to the point where they either no longer met inclusion criteria for the cohort or received a diagnosis of type 2 diabetes, cerebrovascular disease (CVD), ischemic heart disease (IHD), or peripheral vascular disease (PVD) with a mean follow-up duration of approximately 5.5 years across all patient groups.
Patients in the PsA group had a significantly higher incidence of type 2 diabetes, compared with the general population (adjusted relative risk, 1.40; 95% confidence interval, 1.15-1.70; P = .0007) and psoriasis groups (adjusted RR, 1.53; 95% CI, 1.19-1.97; P = .0009). In the PsA group, risk of CVD (adjusted RR, 1.24; 95% CI, 0.99-1.56; P = .06), IHD (adjusted RR, 1.27; 95% CI, 1.05-1.54; P = .02), and PVD (adjusted RR, 1.40; 95% CI, 1.02-1.92; P = .04) were significantly higher than in the general population but not when compared with the psoriasis group. The overall risk of cardiovascular disease (including CVD, IHD, and PVD) for the PsA group was significantly higher (adjusted RR, 1.29; 95% CI, 1.12-1.48; P = .0005), compared with the general population.
“These results support the proposal in existing clinical guidelines that, in order to reduce cardiovascular risk in patients with PsA, it is important to treat inflammatory disease as well as to screen and treat traditional risk factors early in the disease course,” Ms. Charlton and her colleagues wrote in their study.
This study was funded by a grant from the National Institute for Health Research in the United Kingdom. The authors reported no relevant conflicts of interest.
SOURCE: Charlton RA et al. Rheumatology. 2018 Sep 6. doi: 10.1093/rheumatology/key286.
Patients with incident psoriatic arthritis are at a significantly increased risk of developing type 2 diabetes when compared against patients with psoriasis alone and with the general population, according to recent research published in Rheumatology.
Rachel Charlton, PhD, of the department of pharmacy and pharmacology at the University of Bath (England), and her colleagues performed an analysis of 6,783 incident cases of psoriatic arthritis (PsA) from the U.K. Clinical Practice Research Datalink who were diagnosed during 1998-2014. Patients were between 18 years and 89 years old with a median age of 49 years at PsA diagnosis.
In the study, the researchers randomly matched PsA cases at a 1:4 ratio to either a cohort of general population patients with no PsA, psoriasis, or inflammatory arthritis or a cohort of patients with psoriasis but no PsA or inflammatory arthritis. Patients were followed from match to the point where they either no longer met inclusion criteria for the cohort or received a diagnosis of type 2 diabetes, cerebrovascular disease (CVD), ischemic heart disease (IHD), or peripheral vascular disease (PVD) with a mean follow-up duration of approximately 5.5 years across all patient groups.
Patients in the PsA group had a significantly higher incidence of type 2 diabetes, compared with the general population (adjusted relative risk, 1.40; 95% confidence interval, 1.15-1.70; P = .0007) and psoriasis groups (adjusted RR, 1.53; 95% CI, 1.19-1.97; P = .0009). In the PsA group, risk of CVD (adjusted RR, 1.24; 95% CI, 0.99-1.56; P = .06), IHD (adjusted RR, 1.27; 95% CI, 1.05-1.54; P = .02), and PVD (adjusted RR, 1.40; 95% CI, 1.02-1.92; P = .04) were significantly higher than in the general population but not when compared with the psoriasis group. The overall risk of cardiovascular disease (including CVD, IHD, and PVD) for the PsA group was significantly higher (adjusted RR, 1.29; 95% CI, 1.12-1.48; P = .0005), compared with the general population.
“These results support the proposal in existing clinical guidelines that, in order to reduce cardiovascular risk in patients with PsA, it is important to treat inflammatory disease as well as to screen and treat traditional risk factors early in the disease course,” Ms. Charlton and her colleagues wrote in their study.
This study was funded by a grant from the National Institute for Health Research in the United Kingdom. The authors reported no relevant conflicts of interest.
SOURCE: Charlton RA et al. Rheumatology. 2018 Sep 6. doi: 10.1093/rheumatology/key286.
FROM RHEUMATOLOGY
Key clinical point: It is important to treat inflammatory disease as well as to screen and treat traditional cardiovascular risk factors early in the course of PsA.
Major finding: (adjusted RR = 1.53) and a general population control group (adjusted RR = 1.40).
Study details: An analysis of 6,783 patients with psoriatic arthritis in the U.K. Clinical Practice Research Datalink who were diagnosed between 1998 and 2014.
Disclosures: This study was funded by a grant from the National Institute for Health Research in the United Kingdom. The authors reported no relevant conflicts of interest.
Source: Charlton RA et al. Rheumatology. 2018 Sep 6. doi: 10.1093/rheumatology/key286.
Psoriatic arthritis activity spikes briefly postpartum
Disease activity for women with psoriatic arthritis seeking pregnancy was relatively stable through 1 year after delivery, but there was a significant jump at 6 months postpartum follow-up, based on data from a prospective study of more than 100 patients.
Previous research has described rheumatoid arthritis remission during pregnancy, but the symptoms of psoriatic arthritis (PsA) before, during, and after pregnancy have not been well studied, wrote Kristin Ursin, MD, of Trondheim (Norway) University Hospital, and her colleagues.
In a study published in Arthritis Care & Research, the investigators reviewed data from 108 pregnancies in 103 women with PsA who were diagnosed between January 2006 and October 2017.
The participants were enrolled in a Norwegian nationwide registry that followed women with inflammatory diseases from preconception through 1 year after delivery. Disease activity was measured using the DAS28-CRP (28-Joint Disease Activity Score with C-reactive Protein). Participants were assessed at seven time points: before pregnancy, during each trimester, and at 6 weeks, 6 months, and 12 months after delivery.
Although approximately 75% of the women had stable disease activity throughout the study period, activity spiked at 6 months after delivery; DAS28-CRP scores at 6 months post partum were significantly higher than scores at 6 weeks post partum (2.71 vs. 2.45, respectively; P = .016).
The researchers conducted an additional analysis of the potential role of tumor necrosis factor inhibitor use and found that women taking a TNFi had significantly lower disease activity during pregnancy, compared with women not taking a TNFi; mean DAS28-CRP scores at 6 months post partum for these groups were 2.22 and 2.72, respectively (P = .043).
The study was limited by the use of DAS28-CRP as the main measure of disease activity; the index does not include potentially affected distal interphalangeal joints. In addition, not all the participants were assessed at each of the seven time points. However, the results suggest that most pregnant women with PsA experience low levels of disease activity, the researchers said. “Future research on pregnancy in women with PsA should include extended joint count (66/68 joints), and assessment of dactylitis, entheses, axial skeleton, and psoriasis,” they added.
The researchers had no financial conflicts to disclose. The study was funded by the department of rheumatology at Trondheim University Hospital and the Research Fund of the Norwegian organization for people with rheumatic disease.
SOURCE: Ursin K et al. Arthritis Care Res. 2018 Sep 7. doi: 10.1002/acr.23747.
Disease activity for women with psoriatic arthritis seeking pregnancy was relatively stable through 1 year after delivery, but there was a significant jump at 6 months postpartum follow-up, based on data from a prospective study of more than 100 patients.
Previous research has described rheumatoid arthritis remission during pregnancy, but the symptoms of psoriatic arthritis (PsA) before, during, and after pregnancy have not been well studied, wrote Kristin Ursin, MD, of Trondheim (Norway) University Hospital, and her colleagues.
In a study published in Arthritis Care & Research, the investigators reviewed data from 108 pregnancies in 103 women with PsA who were diagnosed between January 2006 and October 2017.
The participants were enrolled in a Norwegian nationwide registry that followed women with inflammatory diseases from preconception through 1 year after delivery. Disease activity was measured using the DAS28-CRP (28-Joint Disease Activity Score with C-reactive Protein). Participants were assessed at seven time points: before pregnancy, during each trimester, and at 6 weeks, 6 months, and 12 months after delivery.
Although approximately 75% of the women had stable disease activity throughout the study period, activity spiked at 6 months after delivery; DAS28-CRP scores at 6 months post partum were significantly higher than scores at 6 weeks post partum (2.71 vs. 2.45, respectively; P = .016).
The researchers conducted an additional analysis of the potential role of tumor necrosis factor inhibitor use and found that women taking a TNFi had significantly lower disease activity during pregnancy, compared with women not taking a TNFi; mean DAS28-CRP scores at 6 months post partum for these groups were 2.22 and 2.72, respectively (P = .043).
The study was limited by the use of DAS28-CRP as the main measure of disease activity; the index does not include potentially affected distal interphalangeal joints. In addition, not all the participants were assessed at each of the seven time points. However, the results suggest that most pregnant women with PsA experience low levels of disease activity, the researchers said. “Future research on pregnancy in women with PsA should include extended joint count (66/68 joints), and assessment of dactylitis, entheses, axial skeleton, and psoriasis,” they added.
The researchers had no financial conflicts to disclose. The study was funded by the department of rheumatology at Trondheim University Hospital and the Research Fund of the Norwegian organization for people with rheumatic disease.
SOURCE: Ursin K et al. Arthritis Care Res. 2018 Sep 7. doi: 10.1002/acr.23747.
Disease activity for women with psoriatic arthritis seeking pregnancy was relatively stable through 1 year after delivery, but there was a significant jump at 6 months postpartum follow-up, based on data from a prospective study of more than 100 patients.
Previous research has described rheumatoid arthritis remission during pregnancy, but the symptoms of psoriatic arthritis (PsA) before, during, and after pregnancy have not been well studied, wrote Kristin Ursin, MD, of Trondheim (Norway) University Hospital, and her colleagues.
In a study published in Arthritis Care & Research, the investigators reviewed data from 108 pregnancies in 103 women with PsA who were diagnosed between January 2006 and October 2017.
The participants were enrolled in a Norwegian nationwide registry that followed women with inflammatory diseases from preconception through 1 year after delivery. Disease activity was measured using the DAS28-CRP (28-Joint Disease Activity Score with C-reactive Protein). Participants were assessed at seven time points: before pregnancy, during each trimester, and at 6 weeks, 6 months, and 12 months after delivery.
Although approximately 75% of the women had stable disease activity throughout the study period, activity spiked at 6 months after delivery; DAS28-CRP scores at 6 months post partum were significantly higher than scores at 6 weeks post partum (2.71 vs. 2.45, respectively; P = .016).
The researchers conducted an additional analysis of the potential role of tumor necrosis factor inhibitor use and found that women taking a TNFi had significantly lower disease activity during pregnancy, compared with women not taking a TNFi; mean DAS28-CRP scores at 6 months post partum for these groups were 2.22 and 2.72, respectively (P = .043).
The study was limited by the use of DAS28-CRP as the main measure of disease activity; the index does not include potentially affected distal interphalangeal joints. In addition, not all the participants were assessed at each of the seven time points. However, the results suggest that most pregnant women with PsA experience low levels of disease activity, the researchers said. “Future research on pregnancy in women with PsA should include extended joint count (66/68 joints), and assessment of dactylitis, entheses, axial skeleton, and psoriasis,” they added.
The researchers had no financial conflicts to disclose. The study was funded by the department of rheumatology at Trondheim University Hospital and the Research Fund of the Norwegian organization for people with rheumatic disease.
SOURCE: Ursin K et al. Arthritis Care Res. 2018 Sep 7. doi: 10.1002/acr.23747.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Disease activity for pregnant women with PsA decreased during pregnancy, increased significantly by 6 months post partum, and returned to baseline by 12 months post partum.
Major finding: The average DAS28-CRP was 2.71 at 6 months vs. 2.45 at 6 weeks (P = .016).
Study details: The data come from 108 women with 103 pregnancies who were part of a national registry in Norway.
Disclosures: The researchers had no financial conflicts to disclose. The study was funded by the department of rheumatology at Trondheim University Hospital and the Research Fund of the Norwegian organization for people with rheumatic disease.
Source: Ursin K et al. Arthritis Care Res. 2018 Sep 7. doi: 10.1002/acr.23747.