User login
How lovers, limes, and drug samples can plague your patients
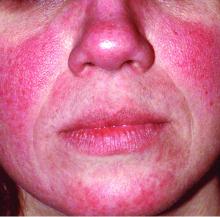
MONTEREY, CALIF. – “Consort dermatitis” – when a patient is allergic to his or her partner. “Lime dermatitis” – when gin and tonics are the culprit. And “sample dermatitis” – when an unprescribed drug sample turns out to be the cause of a mysterious reaction.
Dermatologist Vincent DeLeo, MD, of the University of Southern California, Los Angeles, has seen them all. He provided insight about how to diagnose these unusual conditions at the Coastal Dermatology Symposium.
The following are a few unusual causes of dermatitis that he discussed:
- Romantic partners. A patient’s partner can be the cause of a reaction, as in the case of a 25-year-old woman who turned out to be allergic to her boyfriend’s cologne. In another case, a 50-year-old man had a 3-year history of recurrent dermatitis on his left arm and the left side of his chest. The cause was a mystery until it became clear that it was caused by exposure to hair dye, but not his. “He didn’t color his hair, but his wife did, and she always slept on that side of him,” Dr. DeLeo recalled. “When she stopped coloring her hair, his disease cleared.”
- Black henna. The dye known as “black henna,” or just “henna,” can cause reactions in adults (who use it as a hair dye or to decorate the skin) and children (who can be exposed to it with temporary tattoos). “Because henna typically produces a brown, orange-brown, or reddish-brown tint, other ingredients must be added to produce other colors, such as those marketed as ‘black henna’ and ‘blue henna,’ ” according to a Food and Drug Administration statement. “Even brown shades of products marketed as henna may contain other ingredients intended to make them darker or make the stain last longer on the skin. The problem? “The extra ingredient used to blacken henna is often a coal-tar hair dye containing p-Phenylenediamine, an ingredient that can cause dangerous skin reactions in some people,” the statement says. Dr. DeLeo said that one good rule of thumb is to consider a reaction to black henna if a patient acknowledges using a henna dye and their hair is any color but red. That’s a sign, he said, that they’re actually using black henna.
- Makeup applicators. Dr. DeLeo has seen two cases of patients with facial dermatitis who turned out to be allergic to thiuram, a component of rubber. Their skin was reacting to the rubber in some sponges used to apply makeup.
- Lime and sun exposure. Patients are impressed when Dr. DeLeo correctly guesses what they were drinking the previous weekend, because of their telltale blisters indicating a lime allergy. Noninflammatory blisters on the fingers or hyperpigmentation can be caused by touching the skin of a lime and then having subsequent exposure to ultraviolet light. It may take days for the blisters to appear, he noted. A weekend after mixing gin and tonics with lime, for example, a patient “may show up on Tuesday of the following week. The patient doesn’t always think of what they did over the weekend.”
- Liquid detergents. As a general rule, laundry detergents do not cause dermatitis, Dr. DeLeo said. “By the time that clothing is rinsed in your washer, there’s not enough left of anything on the clothing to cause a problem.” But there’s an exception: When people hand wash clothing with liquid detergents, such as Woolite. “It’s not the fragrance,” he said. “It’s the preservative in the detergent.”
- Unexpected nickel. Skin allergy to nickel is common, and the metal can lurk in unexpected places, as he discovered when he treated a Columbia University student who was “allergic to his tuba.” The tuba was made of brass, not nickel. But “the little things connecting the tubes to each other are alloy metals,” he said, including nickel.
- Drug samples. Dr. DeLeo recalled the case of a dermatology office administrator with a recurrent neck rash. Dermatologist after dermatologist failed to find the cause. Patch and photopatch testing turned up nothing. Then Dr. DeLeo asked her to bring in every skin product she was using. She returned with a large bag full of dermatologic samples, including Drithocreme (anthralin), which can be an irritant. None of the drugs were prescribed. “This is case of sample dermatitis,” which may occur among employees and family members of dermatologists, he said. “Always think of having patients bring in what they’re using,” he added, “because you can be surprised.”
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. DeLeo disclosed consulting work for Estée Lauder.
MONTEREY, CALIF. – “Consort dermatitis” – when a patient is allergic to his or her partner. “Lime dermatitis” – when gin and tonics are the culprit. And “sample dermatitis” – when an unprescribed drug sample turns out to be the cause of a mysterious reaction.
Dermatologist Vincent DeLeo, MD, of the University of Southern California, Los Angeles, has seen them all. He provided insight about how to diagnose these unusual conditions at the Coastal Dermatology Symposium.
The following are a few unusual causes of dermatitis that he discussed:
- Romantic partners. A patient’s partner can be the cause of a reaction, as in the case of a 25-year-old woman who turned out to be allergic to her boyfriend’s cologne. In another case, a 50-year-old man had a 3-year history of recurrent dermatitis on his left arm and the left side of his chest. The cause was a mystery until it became clear that it was caused by exposure to hair dye, but not his. “He didn’t color his hair, but his wife did, and she always slept on that side of him,” Dr. DeLeo recalled. “When she stopped coloring her hair, his disease cleared.”
- Black henna. The dye known as “black henna,” or just “henna,” can cause reactions in adults (who use it as a hair dye or to decorate the skin) and children (who can be exposed to it with temporary tattoos). “Because henna typically produces a brown, orange-brown, or reddish-brown tint, other ingredients must be added to produce other colors, such as those marketed as ‘black henna’ and ‘blue henna,’ ” according to a Food and Drug Administration statement. “Even brown shades of products marketed as henna may contain other ingredients intended to make them darker or make the stain last longer on the skin. The problem? “The extra ingredient used to blacken henna is often a coal-tar hair dye containing p-Phenylenediamine, an ingredient that can cause dangerous skin reactions in some people,” the statement says. Dr. DeLeo said that one good rule of thumb is to consider a reaction to black henna if a patient acknowledges using a henna dye and their hair is any color but red. That’s a sign, he said, that they’re actually using black henna.
- Makeup applicators. Dr. DeLeo has seen two cases of patients with facial dermatitis who turned out to be allergic to thiuram, a component of rubber. Their skin was reacting to the rubber in some sponges used to apply makeup.
- Lime and sun exposure. Patients are impressed when Dr. DeLeo correctly guesses what they were drinking the previous weekend, because of their telltale blisters indicating a lime allergy. Noninflammatory blisters on the fingers or hyperpigmentation can be caused by touching the skin of a lime and then having subsequent exposure to ultraviolet light. It may take days for the blisters to appear, he noted. A weekend after mixing gin and tonics with lime, for example, a patient “may show up on Tuesday of the following week. The patient doesn’t always think of what they did over the weekend.”
- Liquid detergents. As a general rule, laundry detergents do not cause dermatitis, Dr. DeLeo said. “By the time that clothing is rinsed in your washer, there’s not enough left of anything on the clothing to cause a problem.” But there’s an exception: When people hand wash clothing with liquid detergents, such as Woolite. “It’s not the fragrance,” he said. “It’s the preservative in the detergent.”
- Unexpected nickel. Skin allergy to nickel is common, and the metal can lurk in unexpected places, as he discovered when he treated a Columbia University student who was “allergic to his tuba.” The tuba was made of brass, not nickel. But “the little things connecting the tubes to each other are alloy metals,” he said, including nickel.
- Drug samples. Dr. DeLeo recalled the case of a dermatology office administrator with a recurrent neck rash. Dermatologist after dermatologist failed to find the cause. Patch and photopatch testing turned up nothing. Then Dr. DeLeo asked her to bring in every skin product she was using. She returned with a large bag full of dermatologic samples, including Drithocreme (anthralin), which can be an irritant. None of the drugs were prescribed. “This is case of sample dermatitis,” which may occur among employees and family members of dermatologists, he said. “Always think of having patients bring in what they’re using,” he added, “because you can be surprised.”
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. DeLeo disclosed consulting work for Estée Lauder.
MONTEREY, CALIF. – “Consort dermatitis” – when a patient is allergic to his or her partner. “Lime dermatitis” – when gin and tonics are the culprit. And “sample dermatitis” – when an unprescribed drug sample turns out to be the cause of a mysterious reaction.
Dermatologist Vincent DeLeo, MD, of the University of Southern California, Los Angeles, has seen them all. He provided insight about how to diagnose these unusual conditions at the Coastal Dermatology Symposium.
The following are a few unusual causes of dermatitis that he discussed:
- Romantic partners. A patient’s partner can be the cause of a reaction, as in the case of a 25-year-old woman who turned out to be allergic to her boyfriend’s cologne. In another case, a 50-year-old man had a 3-year history of recurrent dermatitis on his left arm and the left side of his chest. The cause was a mystery until it became clear that it was caused by exposure to hair dye, but not his. “He didn’t color his hair, but his wife did, and she always slept on that side of him,” Dr. DeLeo recalled. “When she stopped coloring her hair, his disease cleared.”
- Black henna. The dye known as “black henna,” or just “henna,” can cause reactions in adults (who use it as a hair dye or to decorate the skin) and children (who can be exposed to it with temporary tattoos). “Because henna typically produces a brown, orange-brown, or reddish-brown tint, other ingredients must be added to produce other colors, such as those marketed as ‘black henna’ and ‘blue henna,’ ” according to a Food and Drug Administration statement. “Even brown shades of products marketed as henna may contain other ingredients intended to make them darker or make the stain last longer on the skin. The problem? “The extra ingredient used to blacken henna is often a coal-tar hair dye containing p-Phenylenediamine, an ingredient that can cause dangerous skin reactions in some people,” the statement says. Dr. DeLeo said that one good rule of thumb is to consider a reaction to black henna if a patient acknowledges using a henna dye and their hair is any color but red. That’s a sign, he said, that they’re actually using black henna.
- Makeup applicators. Dr. DeLeo has seen two cases of patients with facial dermatitis who turned out to be allergic to thiuram, a component of rubber. Their skin was reacting to the rubber in some sponges used to apply makeup.
- Lime and sun exposure. Patients are impressed when Dr. DeLeo correctly guesses what they were drinking the previous weekend, because of their telltale blisters indicating a lime allergy. Noninflammatory blisters on the fingers or hyperpigmentation can be caused by touching the skin of a lime and then having subsequent exposure to ultraviolet light. It may take days for the blisters to appear, he noted. A weekend after mixing gin and tonics with lime, for example, a patient “may show up on Tuesday of the following week. The patient doesn’t always think of what they did over the weekend.”
- Liquid detergents. As a general rule, laundry detergents do not cause dermatitis, Dr. DeLeo said. “By the time that clothing is rinsed in your washer, there’s not enough left of anything on the clothing to cause a problem.” But there’s an exception: When people hand wash clothing with liquid detergents, such as Woolite. “It’s not the fragrance,” he said. “It’s the preservative in the detergent.”
- Unexpected nickel. Skin allergy to nickel is common, and the metal can lurk in unexpected places, as he discovered when he treated a Columbia University student who was “allergic to his tuba.” The tuba was made of brass, not nickel. But “the little things connecting the tubes to each other are alloy metals,” he said, including nickel.
- Drug samples. Dr. DeLeo recalled the case of a dermatology office administrator with a recurrent neck rash. Dermatologist after dermatologist failed to find the cause. Patch and photopatch testing turned up nothing. Then Dr. DeLeo asked her to bring in every skin product she was using. She returned with a large bag full of dermatologic samples, including Drithocreme (anthralin), which can be an irritant. None of the drugs were prescribed. “This is case of sample dermatitis,” which may occur among employees and family members of dermatologists, he said. “Always think of having patients bring in what they’re using,” he added, “because you can be surprised.”
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. DeLeo disclosed consulting work for Estée Lauder.
REPORTING FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Q and A with Dr. Julie Harper: Treating acne and rosacea
MONTEREY, CALIF. – Julie C. Harper, MD, likes to warn her patients with acne about an unexpected possible side effect of treatment with isotretinoin. “You may become a dermatologist.”
After all, that’s exactly how Dr. Harper herself was inspired to pursue a career in dermatology. As a teenager, she had acne and was treated with isotretinoin three times. The experience was so influential that she went into dermatology with a specific goal of treating acne.
“I love all of it, and in my practice I treat everything,” said Dr. Harper, “but I have a special interest in helping people with acne be as clear as they can be.” Indeed, she helped found the American Acne and Rosacea Society, which she now serves as president.
Dr. Harper, who practices in Birmingham, Ala., spoke about her approach to acne and rosacea in an interview following one of her presentations at the annual Coastal Dermatology Symposium.
DERMATOLOGY NEWS: What drew you to focus on rosacea in addition to acne?
Dr. Harper: But they’re very distinct diagnoses, and their pathogenesis is completely different. My interest in treating rosacea was secondary to acne, but I love to treat them both.
DN: Are they both equally challenging to treat?
Dr. Harper: In some ways, rosacea is more challenging to treat.
With acne, we have a pretty good algorithm for how we treat it. We can end with isotretinoin, which for many people is a cure. But we really don’t have that last step in rosacea.
DN: What are you doing differently with rosacea than you might not have done a few years ago?
Dr. Harper: More combination therapy. The trend is more toward a comprehensive combination approach to treat everything we see in rosacea: Hit this as hard as you can. Hit everything you see. Part of that is because we have some newer drugs like the alpha-adrenergic agonists that work differently than anything we’ve had before.
We have a couple of good combination studies. One study examined ivermectin plus brimonidine (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Those two worked better together if you did not delay the brimonidine for 4 weeks and only used it with the ivermectin for part of the study.
There are also the newer studies that look at doxycycline plus ivermectin and compare it with ivermectin plus placebo. The combination works better, and it works faster (Adv Ther. 2016;33[9]:1481-1501; unpublished clinical trial data on file with Galderma, NCT03075891).
On top of those treatments, we may need to add laser for background redness, or an oral beta-blocker for flushing if the patient still complains of the symptoms.
DN: What’s most challenging to treat in rosacea?
Dr. Harper: The redness and phymatous changes are the hardest. Once you get phymatous changes, you have to do a physical modality.
Most of us think that if we treat rosacea aggressively up front, maybe we can prevent the phymatous changes. Prevention is key, just like prevention of acne scarring is easier than getting rid of scars once you have it.
Other than phyma, it’s the redness. Even the Food and Drug Administration–approved products we have for redness don’t work for flushing. Patients stand up to give up a presentation and “Oh no, here comes a red face.” That’s the hardest part to manage.
DN: How do beta-blockers fare at treating flushing?
Dr. Harper: They can help, but I don’t know that they can knock it out completely.
And we should remember that there are no FDA-approved beta-blockers to treat this. Most of the data we have are small case reports or case series. We don’t have a lot of data.
DN: Is there anything that’s used too much in rosacea?
Dr. Harper: Probably metronidazole. I understand why it’s used. It’s not a bad drug. But we have better drugs now.
I think we use metronidazole whenever things aren’t covered by insurance. And we use it to do too many things. Don’t try to make metronidazole do everything.
Metronidazole is FDA-approved for papules and pustules. It wasn’t ever intended to help with flushing and background erythema, and you’ll need to use something else with it.
DN: What’s coming down the line for rosacea?
Dr. Harper: We’ve got a couple new antibiotics: a new topical antibiotic and another oral antibiotic.
DN: Let’s talk about acne. Do you think isotretinoin is underused?
Dr. Harper: We should be using more of it. Why do we hold this drug hostage from our patients? In many people, it will cure their acne if they take it for just 5-6 months.
Are we worried about inflammatory bowel disease? The most recent studies say that’s not really an association. Are we worried about depression? We’ve had a meta-analysis that suggests if you take all that data, depression – if anything – gets better in people who take isotretinoin (Am J Gastroenterol. 2014 Apr;109[4]:563-9; J Am Acad Dermatol. 2017 Jun;76[6]:1068-76.e9).
We need to take [the risk with pregnancy] seriously. But we need to be putting more people on the drug and giving them the opportunity to be clear.
DN: What should be used less in acne?
Dr. Harper: We should use less antibiotics and more of everything else – more hormonal treatments, more isotretinoin, more topical retinoids.
That doesn’t mean no antibiotics. But instead of doing three repetitive courses of antibiotics, do one. If acne recurs, go to isotretinoin. Go to an alternative.
DN: What about spironolactone in acne?
Dr. Harper: It’s a blood pressure medicine, but it’s got an antiandrogenic qualities. It blocks the androgen receptor so it’s like getting the benefits of the birth control pill without the estrogen. It can be very beneficial for acne in women.
Its use increased from 2004 to 2013, and people are getting the hang of it. But when you compare it with the number of antibiotics prescribed, antibiotics are written a whole lot more (J Am Acad Dermatol. 2017 Sep;77[3],456-63.e4).
DN: Is there anything that is especially helpful in treating men?
Dr. Harper: Part of the way that birth control and spironolactone work is by decreasing sebum, and we don’t have anything like that for men. But potentially, there may be a topical antiandrogen product that decreases sebum.
DN: How do you deal with patients who are in a lot of distress because of acne or rosacea?
Dr. Harper: You listen to them and tell them you hear what they’re saying. “I understand that you want to be clear, and I’ll help you do that.”
Listen to why they’re not doing well and why they’re frustrated with what they’ve used. They might say, “I don’t use what you gave me because I don’t like the way it feels.” Or, “the drug that you prescribed is too expensive.”
If they’re really doing everything you said, and they’re not doing well, in both of those conditions it may be time for isotretinoin.
In acne, I’ve never seen it fail. It doesn’t work as predictably in rosacea, but it does pretty well if you do low-dose, intermittent isotretinoin.
DN: Do you ever try treatments that are unexpected for acne and rosacea?
Dr. Harper: If you use the right combination of what we have, and try to target pathogenesis, I don’t think we have to go off the reservation very often. We can get good results with what we have available.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications. Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, La Roche–Posay, and Ortho Pharmaceutical, and has served as an investigator for Bayer.
MONTEREY, CALIF. – Julie C. Harper, MD, likes to warn her patients with acne about an unexpected possible side effect of treatment with isotretinoin. “You may become a dermatologist.”
After all, that’s exactly how Dr. Harper herself was inspired to pursue a career in dermatology. As a teenager, she had acne and was treated with isotretinoin three times. The experience was so influential that she went into dermatology with a specific goal of treating acne.
“I love all of it, and in my practice I treat everything,” said Dr. Harper, “but I have a special interest in helping people with acne be as clear as they can be.” Indeed, she helped found the American Acne and Rosacea Society, which she now serves as president.
Dr. Harper, who practices in Birmingham, Ala., spoke about her approach to acne and rosacea in an interview following one of her presentations at the annual Coastal Dermatology Symposium.
DERMATOLOGY NEWS: What drew you to focus on rosacea in addition to acne?
Dr. Harper: But they’re very distinct diagnoses, and their pathogenesis is completely different. My interest in treating rosacea was secondary to acne, but I love to treat them both.
DN: Are they both equally challenging to treat?
Dr. Harper: In some ways, rosacea is more challenging to treat.
With acne, we have a pretty good algorithm for how we treat it. We can end with isotretinoin, which for many people is a cure. But we really don’t have that last step in rosacea.
DN: What are you doing differently with rosacea than you might not have done a few years ago?
Dr. Harper: More combination therapy. The trend is more toward a comprehensive combination approach to treat everything we see in rosacea: Hit this as hard as you can. Hit everything you see. Part of that is because we have some newer drugs like the alpha-adrenergic agonists that work differently than anything we’ve had before.
We have a couple of good combination studies. One study examined ivermectin plus brimonidine (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Those two worked better together if you did not delay the brimonidine for 4 weeks and only used it with the ivermectin for part of the study.
There are also the newer studies that look at doxycycline plus ivermectin and compare it with ivermectin plus placebo. The combination works better, and it works faster (Adv Ther. 2016;33[9]:1481-1501; unpublished clinical trial data on file with Galderma, NCT03075891).
On top of those treatments, we may need to add laser for background redness, or an oral beta-blocker for flushing if the patient still complains of the symptoms.
DN: What’s most challenging to treat in rosacea?
Dr. Harper: The redness and phymatous changes are the hardest. Once you get phymatous changes, you have to do a physical modality.
Most of us think that if we treat rosacea aggressively up front, maybe we can prevent the phymatous changes. Prevention is key, just like prevention of acne scarring is easier than getting rid of scars once you have it.
Other than phyma, it’s the redness. Even the Food and Drug Administration–approved products we have for redness don’t work for flushing. Patients stand up to give up a presentation and “Oh no, here comes a red face.” That’s the hardest part to manage.
DN: How do beta-blockers fare at treating flushing?
Dr. Harper: They can help, but I don’t know that they can knock it out completely.
And we should remember that there are no FDA-approved beta-blockers to treat this. Most of the data we have are small case reports or case series. We don’t have a lot of data.
DN: Is there anything that’s used too much in rosacea?
Dr. Harper: Probably metronidazole. I understand why it’s used. It’s not a bad drug. But we have better drugs now.
I think we use metronidazole whenever things aren’t covered by insurance. And we use it to do too many things. Don’t try to make metronidazole do everything.
Metronidazole is FDA-approved for papules and pustules. It wasn’t ever intended to help with flushing and background erythema, and you’ll need to use something else with it.
DN: What’s coming down the line for rosacea?
Dr. Harper: We’ve got a couple new antibiotics: a new topical antibiotic and another oral antibiotic.
DN: Let’s talk about acne. Do you think isotretinoin is underused?
Dr. Harper: We should be using more of it. Why do we hold this drug hostage from our patients? In many people, it will cure their acne if they take it for just 5-6 months.
Are we worried about inflammatory bowel disease? The most recent studies say that’s not really an association. Are we worried about depression? We’ve had a meta-analysis that suggests if you take all that data, depression – if anything – gets better in people who take isotretinoin (Am J Gastroenterol. 2014 Apr;109[4]:563-9; J Am Acad Dermatol. 2017 Jun;76[6]:1068-76.e9).
We need to take [the risk with pregnancy] seriously. But we need to be putting more people on the drug and giving them the opportunity to be clear.
DN: What should be used less in acne?
Dr. Harper: We should use less antibiotics and more of everything else – more hormonal treatments, more isotretinoin, more topical retinoids.
That doesn’t mean no antibiotics. But instead of doing three repetitive courses of antibiotics, do one. If acne recurs, go to isotretinoin. Go to an alternative.
DN: What about spironolactone in acne?
Dr. Harper: It’s a blood pressure medicine, but it’s got an antiandrogenic qualities. It blocks the androgen receptor so it’s like getting the benefits of the birth control pill without the estrogen. It can be very beneficial for acne in women.
Its use increased from 2004 to 2013, and people are getting the hang of it. But when you compare it with the number of antibiotics prescribed, antibiotics are written a whole lot more (J Am Acad Dermatol. 2017 Sep;77[3],456-63.e4).
DN: Is there anything that is especially helpful in treating men?
Dr. Harper: Part of the way that birth control and spironolactone work is by decreasing sebum, and we don’t have anything like that for men. But potentially, there may be a topical antiandrogen product that decreases sebum.
DN: How do you deal with patients who are in a lot of distress because of acne or rosacea?
Dr. Harper: You listen to them and tell them you hear what they’re saying. “I understand that you want to be clear, and I’ll help you do that.”
Listen to why they’re not doing well and why they’re frustrated with what they’ve used. They might say, “I don’t use what you gave me because I don’t like the way it feels.” Or, “the drug that you prescribed is too expensive.”
If they’re really doing everything you said, and they’re not doing well, in both of those conditions it may be time for isotretinoin.
In acne, I’ve never seen it fail. It doesn’t work as predictably in rosacea, but it does pretty well if you do low-dose, intermittent isotretinoin.
DN: Do you ever try treatments that are unexpected for acne and rosacea?
Dr. Harper: If you use the right combination of what we have, and try to target pathogenesis, I don’t think we have to go off the reservation very often. We can get good results with what we have available.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications. Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, La Roche–Posay, and Ortho Pharmaceutical, and has served as an investigator for Bayer.
MONTEREY, CALIF. – Julie C. Harper, MD, likes to warn her patients with acne about an unexpected possible side effect of treatment with isotretinoin. “You may become a dermatologist.”
After all, that’s exactly how Dr. Harper herself was inspired to pursue a career in dermatology. As a teenager, she had acne and was treated with isotretinoin three times. The experience was so influential that she went into dermatology with a specific goal of treating acne.
“I love all of it, and in my practice I treat everything,” said Dr. Harper, “but I have a special interest in helping people with acne be as clear as they can be.” Indeed, she helped found the American Acne and Rosacea Society, which she now serves as president.
Dr. Harper, who practices in Birmingham, Ala., spoke about her approach to acne and rosacea in an interview following one of her presentations at the annual Coastal Dermatology Symposium.
DERMATOLOGY NEWS: What drew you to focus on rosacea in addition to acne?
Dr. Harper: But they’re very distinct diagnoses, and their pathogenesis is completely different. My interest in treating rosacea was secondary to acne, but I love to treat them both.
DN: Are they both equally challenging to treat?
Dr. Harper: In some ways, rosacea is more challenging to treat.
With acne, we have a pretty good algorithm for how we treat it. We can end with isotretinoin, which for many people is a cure. But we really don’t have that last step in rosacea.
DN: What are you doing differently with rosacea than you might not have done a few years ago?
Dr. Harper: More combination therapy. The trend is more toward a comprehensive combination approach to treat everything we see in rosacea: Hit this as hard as you can. Hit everything you see. Part of that is because we have some newer drugs like the alpha-adrenergic agonists that work differently than anything we’ve had before.
We have a couple of good combination studies. One study examined ivermectin plus brimonidine (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Those two worked better together if you did not delay the brimonidine for 4 weeks and only used it with the ivermectin for part of the study.
There are also the newer studies that look at doxycycline plus ivermectin and compare it with ivermectin plus placebo. The combination works better, and it works faster (Adv Ther. 2016;33[9]:1481-1501; unpublished clinical trial data on file with Galderma, NCT03075891).
On top of those treatments, we may need to add laser for background redness, or an oral beta-blocker for flushing if the patient still complains of the symptoms.
DN: What’s most challenging to treat in rosacea?
Dr. Harper: The redness and phymatous changes are the hardest. Once you get phymatous changes, you have to do a physical modality.
Most of us think that if we treat rosacea aggressively up front, maybe we can prevent the phymatous changes. Prevention is key, just like prevention of acne scarring is easier than getting rid of scars once you have it.
Other than phyma, it’s the redness. Even the Food and Drug Administration–approved products we have for redness don’t work for flushing. Patients stand up to give up a presentation and “Oh no, here comes a red face.” That’s the hardest part to manage.
DN: How do beta-blockers fare at treating flushing?
Dr. Harper: They can help, but I don’t know that they can knock it out completely.
And we should remember that there are no FDA-approved beta-blockers to treat this. Most of the data we have are small case reports or case series. We don’t have a lot of data.
DN: Is there anything that’s used too much in rosacea?
Dr. Harper: Probably metronidazole. I understand why it’s used. It’s not a bad drug. But we have better drugs now.
I think we use metronidazole whenever things aren’t covered by insurance. And we use it to do too many things. Don’t try to make metronidazole do everything.
Metronidazole is FDA-approved for papules and pustules. It wasn’t ever intended to help with flushing and background erythema, and you’ll need to use something else with it.
DN: What’s coming down the line for rosacea?
Dr. Harper: We’ve got a couple new antibiotics: a new topical antibiotic and another oral antibiotic.
DN: Let’s talk about acne. Do you think isotretinoin is underused?
Dr. Harper: We should be using more of it. Why do we hold this drug hostage from our patients? In many people, it will cure their acne if they take it for just 5-6 months.
Are we worried about inflammatory bowel disease? The most recent studies say that’s not really an association. Are we worried about depression? We’ve had a meta-analysis that suggests if you take all that data, depression – if anything – gets better in people who take isotretinoin (Am J Gastroenterol. 2014 Apr;109[4]:563-9; J Am Acad Dermatol. 2017 Jun;76[6]:1068-76.e9).
We need to take [the risk with pregnancy] seriously. But we need to be putting more people on the drug and giving them the opportunity to be clear.
DN: What should be used less in acne?
Dr. Harper: We should use less antibiotics and more of everything else – more hormonal treatments, more isotretinoin, more topical retinoids.
That doesn’t mean no antibiotics. But instead of doing three repetitive courses of antibiotics, do one. If acne recurs, go to isotretinoin. Go to an alternative.
DN: What about spironolactone in acne?
Dr. Harper: It’s a blood pressure medicine, but it’s got an antiandrogenic qualities. It blocks the androgen receptor so it’s like getting the benefits of the birth control pill without the estrogen. It can be very beneficial for acne in women.
Its use increased from 2004 to 2013, and people are getting the hang of it. But when you compare it with the number of antibiotics prescribed, antibiotics are written a whole lot more (J Am Acad Dermatol. 2017 Sep;77[3],456-63.e4).
DN: Is there anything that is especially helpful in treating men?
Dr. Harper: Part of the way that birth control and spironolactone work is by decreasing sebum, and we don’t have anything like that for men. But potentially, there may be a topical antiandrogen product that decreases sebum.
DN: How do you deal with patients who are in a lot of distress because of acne or rosacea?
Dr. Harper: You listen to them and tell them you hear what they’re saying. “I understand that you want to be clear, and I’ll help you do that.”
Listen to why they’re not doing well and why they’re frustrated with what they’ve used. They might say, “I don’t use what you gave me because I don’t like the way it feels.” Or, “the drug that you prescribed is too expensive.”
If they’re really doing everything you said, and they’re not doing well, in both of those conditions it may be time for isotretinoin.
In acne, I’ve never seen it fail. It doesn’t work as predictably in rosacea, but it does pretty well if you do low-dose, intermittent isotretinoin.
DN: Do you ever try treatments that are unexpected for acne and rosacea?
Dr. Harper: If you use the right combination of what we have, and try to target pathogenesis, I don’t think we have to go off the reservation very often. We can get good results with what we have available.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications. Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, La Roche–Posay, and Ortho Pharmaceutical, and has served as an investigator for Bayer.
EXPERT ANALYSIS FROM COASTAL DERMATOLOGY SYMPOSIUM
Stubborn derm condition? Don’t just rely on emails and data
MONTEREY, CALIF. – Looking for a better treatment for a stubborn dermatologic condition? Pick up the telephone, work closely with nondermatologists, and find a dermatology specialty pharmacy. Seek out clinical trials that fit your patient’s needs. And be aware that data may not provide the best dosage protocols.
These tips and more came from Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego, who spoke about best practices in drug therapy at the annual Coastal Dermatology Symposium. Dr. Bhatia urged colleagues not to give up on conditions such as vitiligo, alopecia areata, severe atopic dermatitis (AD), granulomatous disorders, recalcitrant urticaria, and itching, even when there is resistance from insurance companies. Instead, he said, rely on persistence and the power of a united front with other specialists.
Rheumatologists, oncologists, and allergists may be helpful allies in certain cases, he said, as can dermatologic specialty pharmacies. And if you’re dealing with a medical director of an insurance company, he said, make sure to call. Don’t write a letter or send a fax.
Because some treatments never get evaluated in clinical trials because of cost or lack of interest, he also recommended that dermatologists keep an eye on anecdotal protocols, which can provide helpful “real-world options,” he said in an interview. “Searching for conclusions from case reports and small independent studies can be just as informative and beneficial to patient care as pivotal data from large late-phase studies. Practical information, pearls on management, and other important tips can be found in anecdotes that were either too small or short in duration to be conducted as a validated trial.”
One option is to check ClinicalTrials.gov for a trial; another is to pursue an investigator-initiated study. “Some companies will offer the option to fund a small study for one or several patients to get them treatment and drugs without a high expense for the study or funding for the site’s costs,” Dr. Bhatia said.
In his presentation, Dr. Bhatia referred to a variety of dermatologic medications are showing promising results in trials, and some relatively new options.
Topical hypochlorous acid (Sebuderm gel) for dermatoses, such as seborrheic dermatitis. This prescription nonsteroidal gel is now available in the United States and was cleared by the Food and Drug Administration for seborrhea and seborrheic dermatitis in 2015. Dr. Bhatia referred to a study presented in a poster at a meeting in 2017 that showed improvement in 20 of 24 patients with mild to moderate seborrheic dermatitis treated with this product after 28 days. None of the patients worsened, and overall disease activity fell by more than half.
Loyon lotion (Cetiol oil and dimethicone), a nonmedicated descaling treatment for scaly patches in infants with cradle cap (seborrheic dermatitis). A 2014 pilot study found that it improved scaling in 80% of 20 infants and children aged 3-36 months over 8 days, and it reached “treatment success” in 50% (Dermatol Ther [Heidelb]. 2014 Dec;4[2]:221-32).
(As for cost, GoodRx.com states that various pharmacies sell one 8-ounce [227-gram] bottle of Sebuderm gel for about $300 with a coupon. Loyon is also expensive, with a GoodRx.com listing its price at about $300, with coupon, for one 50-ml spray bottle.)
Dr. Bhatia also reviewed drugs in the research pipeline. Several drugs for AD are in early phases of research, while some phase 3 trials involving Janus kinase (JAK) inhibitors for AD are starting – or are close to starting.
He pointed to other research that’s underway on treatments for acne and rosacea. Three acne drugs that he said are “almost here” are minocycline gel and cortexolone 17 alpha-propionate 1% cream. He also referred to sarecycline, a tetracycline-derived antibiotic taken orally, which was approved by the Food and Drug Administration on October 2 for moderate to severe acne vulgaris in patients aged 9 years and older.
This symposium was jointly presented by the University of Louisville (Ky.) and the Global Academy for Medical Education. This publication and the Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Bhatia reported affiliations with multiple drugmakers: Abbvie, Aclaris, Almirall, Bayer, Biofrontera, BioPharmX, Dermira, Encore, EPI Health, Ferndale, Foamix, Galderma, IntraDerm, ISDIN, La Roche-Posay, Leo, Mayne, Menlo, Novartis, Ortho, Pfizer, Pierre Fabre, Promius, Regeneron, Sanofi, Skinfix, Soligenix, Sun, and Vidac.
MONTEREY, CALIF. – Looking for a better treatment for a stubborn dermatologic condition? Pick up the telephone, work closely with nondermatologists, and find a dermatology specialty pharmacy. Seek out clinical trials that fit your patient’s needs. And be aware that data may not provide the best dosage protocols.
These tips and more came from Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego, who spoke about best practices in drug therapy at the annual Coastal Dermatology Symposium. Dr. Bhatia urged colleagues not to give up on conditions such as vitiligo, alopecia areata, severe atopic dermatitis (AD), granulomatous disorders, recalcitrant urticaria, and itching, even when there is resistance from insurance companies. Instead, he said, rely on persistence and the power of a united front with other specialists.
Rheumatologists, oncologists, and allergists may be helpful allies in certain cases, he said, as can dermatologic specialty pharmacies. And if you’re dealing with a medical director of an insurance company, he said, make sure to call. Don’t write a letter or send a fax.
Because some treatments never get evaluated in clinical trials because of cost or lack of interest, he also recommended that dermatologists keep an eye on anecdotal protocols, which can provide helpful “real-world options,” he said in an interview. “Searching for conclusions from case reports and small independent studies can be just as informative and beneficial to patient care as pivotal data from large late-phase studies. Practical information, pearls on management, and other important tips can be found in anecdotes that were either too small or short in duration to be conducted as a validated trial.”
One option is to check ClinicalTrials.gov for a trial; another is to pursue an investigator-initiated study. “Some companies will offer the option to fund a small study for one or several patients to get them treatment and drugs without a high expense for the study or funding for the site’s costs,” Dr. Bhatia said.
In his presentation, Dr. Bhatia referred to a variety of dermatologic medications are showing promising results in trials, and some relatively new options.
Topical hypochlorous acid (Sebuderm gel) for dermatoses, such as seborrheic dermatitis. This prescription nonsteroidal gel is now available in the United States and was cleared by the Food and Drug Administration for seborrhea and seborrheic dermatitis in 2015. Dr. Bhatia referred to a study presented in a poster at a meeting in 2017 that showed improvement in 20 of 24 patients with mild to moderate seborrheic dermatitis treated with this product after 28 days. None of the patients worsened, and overall disease activity fell by more than half.
Loyon lotion (Cetiol oil and dimethicone), a nonmedicated descaling treatment for scaly patches in infants with cradle cap (seborrheic dermatitis). A 2014 pilot study found that it improved scaling in 80% of 20 infants and children aged 3-36 months over 8 days, and it reached “treatment success” in 50% (Dermatol Ther [Heidelb]. 2014 Dec;4[2]:221-32).
(As for cost, GoodRx.com states that various pharmacies sell one 8-ounce [227-gram] bottle of Sebuderm gel for about $300 with a coupon. Loyon is also expensive, with a GoodRx.com listing its price at about $300, with coupon, for one 50-ml spray bottle.)
Dr. Bhatia also reviewed drugs in the research pipeline. Several drugs for AD are in early phases of research, while some phase 3 trials involving Janus kinase (JAK) inhibitors for AD are starting – or are close to starting.
He pointed to other research that’s underway on treatments for acne and rosacea. Three acne drugs that he said are “almost here” are minocycline gel and cortexolone 17 alpha-propionate 1% cream. He also referred to sarecycline, a tetracycline-derived antibiotic taken orally, which was approved by the Food and Drug Administration on October 2 for moderate to severe acne vulgaris in patients aged 9 years and older.
This symposium was jointly presented by the University of Louisville (Ky.) and the Global Academy for Medical Education. This publication and the Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Bhatia reported affiliations with multiple drugmakers: Abbvie, Aclaris, Almirall, Bayer, Biofrontera, BioPharmX, Dermira, Encore, EPI Health, Ferndale, Foamix, Galderma, IntraDerm, ISDIN, La Roche-Posay, Leo, Mayne, Menlo, Novartis, Ortho, Pfizer, Pierre Fabre, Promius, Regeneron, Sanofi, Skinfix, Soligenix, Sun, and Vidac.
MONTEREY, CALIF. – Looking for a better treatment for a stubborn dermatologic condition? Pick up the telephone, work closely with nondermatologists, and find a dermatology specialty pharmacy. Seek out clinical trials that fit your patient’s needs. And be aware that data may not provide the best dosage protocols.
These tips and more came from Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego, who spoke about best practices in drug therapy at the annual Coastal Dermatology Symposium. Dr. Bhatia urged colleagues not to give up on conditions such as vitiligo, alopecia areata, severe atopic dermatitis (AD), granulomatous disorders, recalcitrant urticaria, and itching, even when there is resistance from insurance companies. Instead, he said, rely on persistence and the power of a united front with other specialists.
Rheumatologists, oncologists, and allergists may be helpful allies in certain cases, he said, as can dermatologic specialty pharmacies. And if you’re dealing with a medical director of an insurance company, he said, make sure to call. Don’t write a letter or send a fax.
Because some treatments never get evaluated in clinical trials because of cost or lack of interest, he also recommended that dermatologists keep an eye on anecdotal protocols, which can provide helpful “real-world options,” he said in an interview. “Searching for conclusions from case reports and small independent studies can be just as informative and beneficial to patient care as pivotal data from large late-phase studies. Practical information, pearls on management, and other important tips can be found in anecdotes that were either too small or short in duration to be conducted as a validated trial.”
One option is to check ClinicalTrials.gov for a trial; another is to pursue an investigator-initiated study. “Some companies will offer the option to fund a small study for one or several patients to get them treatment and drugs without a high expense for the study or funding for the site’s costs,” Dr. Bhatia said.
In his presentation, Dr. Bhatia referred to a variety of dermatologic medications are showing promising results in trials, and some relatively new options.
Topical hypochlorous acid (Sebuderm gel) for dermatoses, such as seborrheic dermatitis. This prescription nonsteroidal gel is now available in the United States and was cleared by the Food and Drug Administration for seborrhea and seborrheic dermatitis in 2015. Dr. Bhatia referred to a study presented in a poster at a meeting in 2017 that showed improvement in 20 of 24 patients with mild to moderate seborrheic dermatitis treated with this product after 28 days. None of the patients worsened, and overall disease activity fell by more than half.
Loyon lotion (Cetiol oil and dimethicone), a nonmedicated descaling treatment for scaly patches in infants with cradle cap (seborrheic dermatitis). A 2014 pilot study found that it improved scaling in 80% of 20 infants and children aged 3-36 months over 8 days, and it reached “treatment success” in 50% (Dermatol Ther [Heidelb]. 2014 Dec;4[2]:221-32).
(As for cost, GoodRx.com states that various pharmacies sell one 8-ounce [227-gram] bottle of Sebuderm gel for about $300 with a coupon. Loyon is also expensive, with a GoodRx.com listing its price at about $300, with coupon, for one 50-ml spray bottle.)
Dr. Bhatia also reviewed drugs in the research pipeline. Several drugs for AD are in early phases of research, while some phase 3 trials involving Janus kinase (JAK) inhibitors for AD are starting – or are close to starting.
He pointed to other research that’s underway on treatments for acne and rosacea. Three acne drugs that he said are “almost here” are minocycline gel and cortexolone 17 alpha-propionate 1% cream. He also referred to sarecycline, a tetracycline-derived antibiotic taken orally, which was approved by the Food and Drug Administration on October 2 for moderate to severe acne vulgaris in patients aged 9 years and older.
This symposium was jointly presented by the University of Louisville (Ky.) and the Global Academy for Medical Education. This publication and the Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Bhatia reported affiliations with multiple drugmakers: Abbvie, Aclaris, Almirall, Bayer, Biofrontera, BioPharmX, Dermira, Encore, EPI Health, Ferndale, Foamix, Galderma, IntraDerm, ISDIN, La Roche-Posay, Leo, Mayne, Menlo, Novartis, Ortho, Pfizer, Pierre Fabre, Promius, Regeneron, Sanofi, Skinfix, Soligenix, Sun, and Vidac.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
In rosacea, a single treatment may not be enough
MONTEREY, CALIF. – , a dermatologist said at the annual Coastal Dermatology Symposium. Don’t assume you can just prescribe one drug like you might with acne, she advised.
“Treat everything that you see,” said dermatologist Julie C. Harper, MD, of Birmingham, Ala. “That may mean a laser or something you’re using off-label. Different lesions and signs of rosacea will require multiple modes of treatment.”
Dr. Harper offered these other pearls to consider when treating rosacea:
- Don’t get hung up on subtypes.
The four subtypes of rosacea should be used to classify lesions, not people, she said. That’s because patients can fall into more than one of the four categories – erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea, she noted.
“Document the redness you see and ask them what’s bothering them the most,” she said. And ask yourself, she added, “Do I have them on everything that I should have them on?”
- Talk to patients about triggers.
For the first visit, “we have to talk to patients about skin care and triggers,” Dr. Harper noted. According to the American Academy of Dermatology, common rosacea triggers include sunlight, hairspray, heat, stress, alcohol, and spicy foods.
- Consider an ivermectin-brimonidine combination.
“Targeting inflammation in papules and pustules doesn’t necessarily translate to less background erythema,” Dr. Harper said. What to do? She pointed to a 2017 study that examined a combination treatment of ivermectin 1% topical cream (Soolantra) and brimonidine 0.33% topical gel (Mirvaso) for patients with rosacea with moderate to severe persistent erythema and inflammatory lesions. Ivermectin is indicated for inflammatory lesions, while brimonidine treats persistent erythema.
At week 12, the proportion of patients who achieved investigator global assessment of clear or almost clear was 55.8% in the combination group, versus 36.8% of those in the vehicle group (P = .007), according to the study (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Dr. Harper highlighted the effect of brimonidine when added to ivermectin. “In a period of 3 hours,” she said, “we had twice as many people fall into clear or almost clear.”
- Consider adding botulinum toxin to your toolbox.
This “really does work,” Dr. Harper said. She pointed to a 2015 report of botulinum toxin use in two cases of refractory flushing and erythema and a 2012 report of 13 cases in patients with the same symptoms (Dermatology. 2015;230:299-301; J Drugs Dermatol. 2012 Dec;11[12]:e76-9). Dr. Harper said that she usually uses the full 50-unit dose of Botox.
- Consider a beta-blocker.
According to a 2018 report, the beta-blocker carvedilol (Coreg) showed benefit when added to other treatments in five patients with facial flushing and persistent erythema.
- Keep isotretinoin in mind.
A 2016 report suggested low-dose isotretinoin had value for difficult-to-treat papulopustular rosacea. As Dr. Harper noted, 57% of those who took isotretinoin reached the primary endpoint, versus 10% of those taking the placebo. However, relapses over 4 months were common, which is a sign that it may be wise to prescribe low doses over the long term, but not in females of child-bearing potential, she said.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, LaRoche Posay, and Ortho and has served as investigator for Bayer.
MONTEREY, CALIF. – , a dermatologist said at the annual Coastal Dermatology Symposium. Don’t assume you can just prescribe one drug like you might with acne, she advised.
“Treat everything that you see,” said dermatologist Julie C. Harper, MD, of Birmingham, Ala. “That may mean a laser or something you’re using off-label. Different lesions and signs of rosacea will require multiple modes of treatment.”
Dr. Harper offered these other pearls to consider when treating rosacea:
- Don’t get hung up on subtypes.
The four subtypes of rosacea should be used to classify lesions, not people, she said. That’s because patients can fall into more than one of the four categories – erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea, she noted.
“Document the redness you see and ask them what’s bothering them the most,” she said. And ask yourself, she added, “Do I have them on everything that I should have them on?”
- Talk to patients about triggers.
For the first visit, “we have to talk to patients about skin care and triggers,” Dr. Harper noted. According to the American Academy of Dermatology, common rosacea triggers include sunlight, hairspray, heat, stress, alcohol, and spicy foods.
- Consider an ivermectin-brimonidine combination.
“Targeting inflammation in papules and pustules doesn’t necessarily translate to less background erythema,” Dr. Harper said. What to do? She pointed to a 2017 study that examined a combination treatment of ivermectin 1% topical cream (Soolantra) and brimonidine 0.33% topical gel (Mirvaso) for patients with rosacea with moderate to severe persistent erythema and inflammatory lesions. Ivermectin is indicated for inflammatory lesions, while brimonidine treats persistent erythema.
At week 12, the proportion of patients who achieved investigator global assessment of clear or almost clear was 55.8% in the combination group, versus 36.8% of those in the vehicle group (P = .007), according to the study (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Dr. Harper highlighted the effect of brimonidine when added to ivermectin. “In a period of 3 hours,” she said, “we had twice as many people fall into clear or almost clear.”
- Consider adding botulinum toxin to your toolbox.
This “really does work,” Dr. Harper said. She pointed to a 2015 report of botulinum toxin use in two cases of refractory flushing and erythema and a 2012 report of 13 cases in patients with the same symptoms (Dermatology. 2015;230:299-301; J Drugs Dermatol. 2012 Dec;11[12]:e76-9). Dr. Harper said that she usually uses the full 50-unit dose of Botox.
- Consider a beta-blocker.
According to a 2018 report, the beta-blocker carvedilol (Coreg) showed benefit when added to other treatments in five patients with facial flushing and persistent erythema.
- Keep isotretinoin in mind.
A 2016 report suggested low-dose isotretinoin had value for difficult-to-treat papulopustular rosacea. As Dr. Harper noted, 57% of those who took isotretinoin reached the primary endpoint, versus 10% of those taking the placebo. However, relapses over 4 months were common, which is a sign that it may be wise to prescribe low doses over the long term, but not in females of child-bearing potential, she said.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, LaRoche Posay, and Ortho and has served as investigator for Bayer.
MONTEREY, CALIF. – , a dermatologist said at the annual Coastal Dermatology Symposium. Don’t assume you can just prescribe one drug like you might with acne, she advised.
“Treat everything that you see,” said dermatologist Julie C. Harper, MD, of Birmingham, Ala. “That may mean a laser or something you’re using off-label. Different lesions and signs of rosacea will require multiple modes of treatment.”
Dr. Harper offered these other pearls to consider when treating rosacea:
- Don’t get hung up on subtypes.
The four subtypes of rosacea should be used to classify lesions, not people, she said. That’s because patients can fall into more than one of the four categories – erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea, she noted.
“Document the redness you see and ask them what’s bothering them the most,” she said. And ask yourself, she added, “Do I have them on everything that I should have them on?”
- Talk to patients about triggers.
For the first visit, “we have to talk to patients about skin care and triggers,” Dr. Harper noted. According to the American Academy of Dermatology, common rosacea triggers include sunlight, hairspray, heat, stress, alcohol, and spicy foods.
- Consider an ivermectin-brimonidine combination.
“Targeting inflammation in papules and pustules doesn’t necessarily translate to less background erythema,” Dr. Harper said. What to do? She pointed to a 2017 study that examined a combination treatment of ivermectin 1% topical cream (Soolantra) and brimonidine 0.33% topical gel (Mirvaso) for patients with rosacea with moderate to severe persistent erythema and inflammatory lesions. Ivermectin is indicated for inflammatory lesions, while brimonidine treats persistent erythema.
At week 12, the proportion of patients who achieved investigator global assessment of clear or almost clear was 55.8% in the combination group, versus 36.8% of those in the vehicle group (P = .007), according to the study (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Dr. Harper highlighted the effect of brimonidine when added to ivermectin. “In a period of 3 hours,” she said, “we had twice as many people fall into clear or almost clear.”
- Consider adding botulinum toxin to your toolbox.
This “really does work,” Dr. Harper said. She pointed to a 2015 report of botulinum toxin use in two cases of refractory flushing and erythema and a 2012 report of 13 cases in patients with the same symptoms (Dermatology. 2015;230:299-301; J Drugs Dermatol. 2012 Dec;11[12]:e76-9). Dr. Harper said that she usually uses the full 50-unit dose of Botox.
- Consider a beta-blocker.
According to a 2018 report, the beta-blocker carvedilol (Coreg) showed benefit when added to other treatments in five patients with facial flushing and persistent erythema.
- Keep isotretinoin in mind.
A 2016 report suggested low-dose isotretinoin had value for difficult-to-treat papulopustular rosacea. As Dr. Harper noted, 57% of those who took isotretinoin reached the primary endpoint, versus 10% of those taking the placebo. However, relapses over 4 months were common, which is a sign that it may be wise to prescribe low doses over the long term, but not in females of child-bearing potential, she said.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, LaRoche Posay, and Ortho and has served as investigator for Bayer.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Beware drug reactions from methotrexate, voriconazole, and BRAF inhibitors
MONTEREY, CALIF. – Cutaneous necrosis. Porphyria cutanea tarda, accelerated photoaging, and actinic keratosis (AK). Cutaneous keratinocytic neoplasias. Two drugs – and a class of drugs commonly used in oncologic dermatology – can produce these skin conditions, a dermatologist cautioned his colleagues.
J. Mark Jackson, MD, of the University of Louisville (Ky.), highlighted these drug reactions in a presentation at the annual Coastal Dermatology Symposium. The
Dr. Jackson referred to reports of cutaneous necrosis associated with methotrexate and highlighted a 2017 case series that compared 24 patients who developed the condition with a control population of patients taking methotrexate who did not develop it. The patients with this reaction were more likely to be older, had a higher starting dose, and had signs of kidney problems. They were also less likely to be taking folic acid supplements (J Am Acad Dermatol. 2017 Aug;77[2]:247-55.e2).
“It’s pretty alarming,” he said. “They look like Stevens-Johnson syndrome/TEN [toxic epidermal necrolysis], but the pathology was differentiated,” he pointed out.
He cautioned, though, that this is not “a typical reaction.”
The oral antifungal drug voriconazole is often used in immunosuppressed patients, such as transplant patients, either as prophylaxis or therapy. It is highly photosensitizing and has been linked to porphyria cutanea tarda, accelerated photoaging, development of AKs, and aggressive cutaneous squamous cell carcinoma (Am J Transplant 2008 Apr;8[4]:877-80; AIDS. 2008 Apr 23;22[7]:905-6; J Am Acad Dermatol. 2010 Jan;62[1]:31-7; Dermatol Surg. 2010 Nov;36[11]:1752-5).
The risk of nonmelanoma skin cancer may be quadrupled in patients who take this medication, Dr. Jackson said.
There also are reports of patients on voriconazole developing tense bullae that are suggestive of porphyria cutanea tarda but with normal porphyrin levels, he said. This resolves over time, once therapy has ceased.
The BRAF inhibitor chemotherapy drugs – vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) – are used to treat metastatic melanoma. They’ve been linked to rash and cutaneous keratinocytic neoplasias. Patients on these agents should be “closely monitored” for these conditions (Chem Immunol Allergy. 2012;97:191-202). Dr. Jackson emphasized the importance of photoprotection with these patients and noted that it’s crucial to see these patients every month because neoplasias can develop quickly, even within 4 weeks of starting the medication.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Jackson reported relationships with AbbVie, Accuitis, Aclaris, Galderma, Janssen, Lilly, Medimetriks, Novartis, Promius, Ralexar, and TopMD.
MONTEREY, CALIF. – Cutaneous necrosis. Porphyria cutanea tarda, accelerated photoaging, and actinic keratosis (AK). Cutaneous keratinocytic neoplasias. Two drugs – and a class of drugs commonly used in oncologic dermatology – can produce these skin conditions, a dermatologist cautioned his colleagues.
J. Mark Jackson, MD, of the University of Louisville (Ky.), highlighted these drug reactions in a presentation at the annual Coastal Dermatology Symposium. The
Dr. Jackson referred to reports of cutaneous necrosis associated with methotrexate and highlighted a 2017 case series that compared 24 patients who developed the condition with a control population of patients taking methotrexate who did not develop it. The patients with this reaction were more likely to be older, had a higher starting dose, and had signs of kidney problems. They were also less likely to be taking folic acid supplements (J Am Acad Dermatol. 2017 Aug;77[2]:247-55.e2).
“It’s pretty alarming,” he said. “They look like Stevens-Johnson syndrome/TEN [toxic epidermal necrolysis], but the pathology was differentiated,” he pointed out.
He cautioned, though, that this is not “a typical reaction.”
The oral antifungal drug voriconazole is often used in immunosuppressed patients, such as transplant patients, either as prophylaxis or therapy. It is highly photosensitizing and has been linked to porphyria cutanea tarda, accelerated photoaging, development of AKs, and aggressive cutaneous squamous cell carcinoma (Am J Transplant 2008 Apr;8[4]:877-80; AIDS. 2008 Apr 23;22[7]:905-6; J Am Acad Dermatol. 2010 Jan;62[1]:31-7; Dermatol Surg. 2010 Nov;36[11]:1752-5).
The risk of nonmelanoma skin cancer may be quadrupled in patients who take this medication, Dr. Jackson said.
There also are reports of patients on voriconazole developing tense bullae that are suggestive of porphyria cutanea tarda but with normal porphyrin levels, he said. This resolves over time, once therapy has ceased.
The BRAF inhibitor chemotherapy drugs – vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) – are used to treat metastatic melanoma. They’ve been linked to rash and cutaneous keratinocytic neoplasias. Patients on these agents should be “closely monitored” for these conditions (Chem Immunol Allergy. 2012;97:191-202). Dr. Jackson emphasized the importance of photoprotection with these patients and noted that it’s crucial to see these patients every month because neoplasias can develop quickly, even within 4 weeks of starting the medication.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Jackson reported relationships with AbbVie, Accuitis, Aclaris, Galderma, Janssen, Lilly, Medimetriks, Novartis, Promius, Ralexar, and TopMD.
MONTEREY, CALIF. – Cutaneous necrosis. Porphyria cutanea tarda, accelerated photoaging, and actinic keratosis (AK). Cutaneous keratinocytic neoplasias. Two drugs – and a class of drugs commonly used in oncologic dermatology – can produce these skin conditions, a dermatologist cautioned his colleagues.
J. Mark Jackson, MD, of the University of Louisville (Ky.), highlighted these drug reactions in a presentation at the annual Coastal Dermatology Symposium. The
Dr. Jackson referred to reports of cutaneous necrosis associated with methotrexate and highlighted a 2017 case series that compared 24 patients who developed the condition with a control population of patients taking methotrexate who did not develop it. The patients with this reaction were more likely to be older, had a higher starting dose, and had signs of kidney problems. They were also less likely to be taking folic acid supplements (J Am Acad Dermatol. 2017 Aug;77[2]:247-55.e2).
“It’s pretty alarming,” he said. “They look like Stevens-Johnson syndrome/TEN [toxic epidermal necrolysis], but the pathology was differentiated,” he pointed out.
He cautioned, though, that this is not “a typical reaction.”
The oral antifungal drug voriconazole is often used in immunosuppressed patients, such as transplant patients, either as prophylaxis or therapy. It is highly photosensitizing and has been linked to porphyria cutanea tarda, accelerated photoaging, development of AKs, and aggressive cutaneous squamous cell carcinoma (Am J Transplant 2008 Apr;8[4]:877-80; AIDS. 2008 Apr 23;22[7]:905-6; J Am Acad Dermatol. 2010 Jan;62[1]:31-7; Dermatol Surg. 2010 Nov;36[11]:1752-5).
The risk of nonmelanoma skin cancer may be quadrupled in patients who take this medication, Dr. Jackson said.
There also are reports of patients on voriconazole developing tense bullae that are suggestive of porphyria cutanea tarda but with normal porphyrin levels, he said. This resolves over time, once therapy has ceased.
The BRAF inhibitor chemotherapy drugs – vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) – are used to treat metastatic melanoma. They’ve been linked to rash and cutaneous keratinocytic neoplasias. Patients on these agents should be “closely monitored” for these conditions (Chem Immunol Allergy. 2012;97:191-202). Dr. Jackson emphasized the importance of photoprotection with these patients and noted that it’s crucial to see these patients every month because neoplasias can develop quickly, even within 4 weeks of starting the medication.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Jackson reported relationships with AbbVie, Accuitis, Aclaris, Galderma, Janssen, Lilly, Medimetriks, Novartis, Promius, Ralexar, and TopMD.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
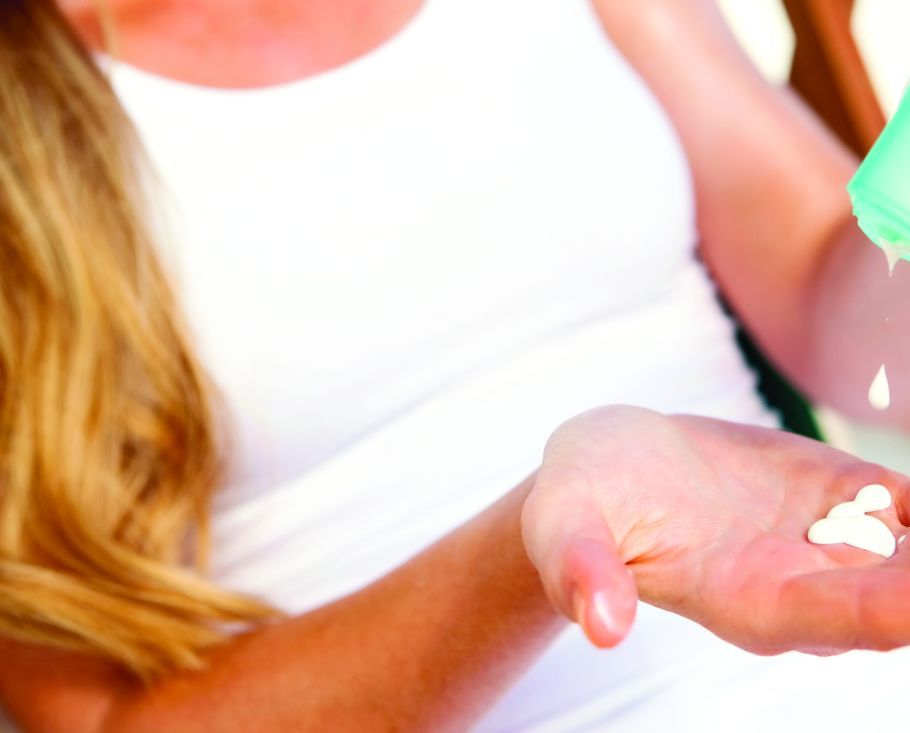
Sunscreens: Misleading labels, poor performance, and hype about their risks
MONTEREY, CALIF. – Heads up! “Natural” mineral-based sunscreens don’t provide the protection of their rivals. Patients may get burned by scary hype about the supposed dangers of sunscreen. And sunscreen spray is great for the scalp of people whose hair is thinning.
In a presentation on sunscreens at the annual Coastal Dermatology Symposium, Vincent DeLeo, MD, of the University of Southern California, Los Angeles, offered the following tips on sunscreen and more.
Here’s a roundup of his pearls:
Sunscreens are getting better and are faring poorly, too.
Dr. DeLeo said. A 2013 comparison of sunscreens in 1997 and 2009 found that, among available sunscreens, the percentage of those with low SPF (under 15) fell from 27% to 6% during that time. (The Food and Drug Administration declared in 2011 that manufacturers must tell consumers that low SPF and/or non–broad spectrum sunscreens protect only against sunburn, not against skin cancer or skin aging.) The study also found that the percentage of sunscreens with UVA-1 (such as avobenzone or zinc oxide) filters grew from 5% to 70% (Photochem Photobiol Sci. 2013 Jan;12[1]:197-202).
But the label of sunscreens may not always be accurate. Earlier this year, Consumer Reports wrote that 36 of 73 sunscreens tested failed to correctly list their SPF protection level; 23 sunscreens missed their listed SPF levels by more than half. “Natural” or “mineral-only” sunscreens, which rely on such blockers as zinc oxide or titanium dioxide, performed the worst. Some patients prefer to use these sunscreens because they aren’t chemical based, and “may want to have a more natural sunscreen,” Dr. DeLeo said. “But they should be aware the sunscreens don’t always live up to the SPF level on the label.”
Beware of warnings about sunscreens.
Reports have warned Americans about supposed risks of sunscreen use such as low vitamin D levels from the lack of sun exposure, the exposure to titanium dioxide and zinc oxide nanoparticles, and the exposure to retinyl palmitate in sunscreen. Hawaiian officials, meanwhile, are banning some types of sunscreen chemicals in order to protect coral reefs.
Typical use of sunscreen will not dangerously lower vitamin D levels, Dr. DeLeo said, but people who use it every day may want to be cautious. He dismissed the concerns about nanoparticles and retinyl palmitate.
Dr. DeLeo said two sunscreen risks are real; sunscreens can trigger irritation, at a rate as high as 20%, and, rarely, allergic reactions, as well.
American sunscreens don’t stack up worldwide.
Simplicity often is a virtue. But, Dr. DeLeo said, it’s not helpful when it comes to the components of American sunscreens.
U.S. regulations only allow 16 ingredients in sunscreen while several more are allowed in Europe, he said. According to him, this helps explain why European sunscreens do a better job. European sunscreens “are much more absorbent, much better at absorbing radiation than the U.S. sunscreens,” he said. “It’s because we don’t have the same products as they have in Europe.”
The good news, he said, is that the FDA is considering expanding the number of ingredients allowed in sunscreen. The Sunscreen Innovation Act of 2014, a law passed by Congress, allows the FDA to use efficacy and safety data from Europe without requiring manufacturers to launch new, multimillion dollar tests, he said.
That’s good news for companies that want to improve U.S. sunscreens by selling a wider variety of types. “Sooner or later,” he said, “we will probably get these.”
Sunscreen sprays are tops at scalp protection.
Sunscreen sprays shouldn’t be applied to the face in children, Dr. DeLeo said, but they’re great for solo people because they facilitate protecting the back when there isn’t someone around to help them apply topical sunscreen.
How much spray should people use? A lot, he said. He added that sunscreen sprays are especially useful for the scalps of people with thinning hair.
Dr. DeLeo disclosed consulting work for Estée Lauder.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – Heads up! “Natural” mineral-based sunscreens don’t provide the protection of their rivals. Patients may get burned by scary hype about the supposed dangers of sunscreen. And sunscreen spray is great for the scalp of people whose hair is thinning.
In a presentation on sunscreens at the annual Coastal Dermatology Symposium, Vincent DeLeo, MD, of the University of Southern California, Los Angeles, offered the following tips on sunscreen and more.
Here’s a roundup of his pearls:
Sunscreens are getting better and are faring poorly, too.
Dr. DeLeo said. A 2013 comparison of sunscreens in 1997 and 2009 found that, among available sunscreens, the percentage of those with low SPF (under 15) fell from 27% to 6% during that time. (The Food and Drug Administration declared in 2011 that manufacturers must tell consumers that low SPF and/or non–broad spectrum sunscreens protect only against sunburn, not against skin cancer or skin aging.) The study also found that the percentage of sunscreens with UVA-1 (such as avobenzone or zinc oxide) filters grew from 5% to 70% (Photochem Photobiol Sci. 2013 Jan;12[1]:197-202).
But the label of sunscreens may not always be accurate. Earlier this year, Consumer Reports wrote that 36 of 73 sunscreens tested failed to correctly list their SPF protection level; 23 sunscreens missed their listed SPF levels by more than half. “Natural” or “mineral-only” sunscreens, which rely on such blockers as zinc oxide or titanium dioxide, performed the worst. Some patients prefer to use these sunscreens because they aren’t chemical based, and “may want to have a more natural sunscreen,” Dr. DeLeo said. “But they should be aware the sunscreens don’t always live up to the SPF level on the label.”
Beware of warnings about sunscreens.
Reports have warned Americans about supposed risks of sunscreen use such as low vitamin D levels from the lack of sun exposure, the exposure to titanium dioxide and zinc oxide nanoparticles, and the exposure to retinyl palmitate in sunscreen. Hawaiian officials, meanwhile, are banning some types of sunscreen chemicals in order to protect coral reefs.
Typical use of sunscreen will not dangerously lower vitamin D levels, Dr. DeLeo said, but people who use it every day may want to be cautious. He dismissed the concerns about nanoparticles and retinyl palmitate.
Dr. DeLeo said two sunscreen risks are real; sunscreens can trigger irritation, at a rate as high as 20%, and, rarely, allergic reactions, as well.
American sunscreens don’t stack up worldwide.
Simplicity often is a virtue. But, Dr. DeLeo said, it’s not helpful when it comes to the components of American sunscreens.
U.S. regulations only allow 16 ingredients in sunscreen while several more are allowed in Europe, he said. According to him, this helps explain why European sunscreens do a better job. European sunscreens “are much more absorbent, much better at absorbing radiation than the U.S. sunscreens,” he said. “It’s because we don’t have the same products as they have in Europe.”
The good news, he said, is that the FDA is considering expanding the number of ingredients allowed in sunscreen. The Sunscreen Innovation Act of 2014, a law passed by Congress, allows the FDA to use efficacy and safety data from Europe without requiring manufacturers to launch new, multimillion dollar tests, he said.
That’s good news for companies that want to improve U.S. sunscreens by selling a wider variety of types. “Sooner or later,” he said, “we will probably get these.”
Sunscreen sprays are tops at scalp protection.
Sunscreen sprays shouldn’t be applied to the face in children, Dr. DeLeo said, but they’re great for solo people because they facilitate protecting the back when there isn’t someone around to help them apply topical sunscreen.
How much spray should people use? A lot, he said. He added that sunscreen sprays are especially useful for the scalps of people with thinning hair.
Dr. DeLeo disclosed consulting work for Estée Lauder.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – Heads up! “Natural” mineral-based sunscreens don’t provide the protection of their rivals. Patients may get burned by scary hype about the supposed dangers of sunscreen. And sunscreen spray is great for the scalp of people whose hair is thinning.
In a presentation on sunscreens at the annual Coastal Dermatology Symposium, Vincent DeLeo, MD, of the University of Southern California, Los Angeles, offered the following tips on sunscreen and more.
Here’s a roundup of his pearls:
Sunscreens are getting better and are faring poorly, too.
Dr. DeLeo said. A 2013 comparison of sunscreens in 1997 and 2009 found that, among available sunscreens, the percentage of those with low SPF (under 15) fell from 27% to 6% during that time. (The Food and Drug Administration declared in 2011 that manufacturers must tell consumers that low SPF and/or non–broad spectrum sunscreens protect only against sunburn, not against skin cancer or skin aging.) The study also found that the percentage of sunscreens with UVA-1 (such as avobenzone or zinc oxide) filters grew from 5% to 70% (Photochem Photobiol Sci. 2013 Jan;12[1]:197-202).
But the label of sunscreens may not always be accurate. Earlier this year, Consumer Reports wrote that 36 of 73 sunscreens tested failed to correctly list their SPF protection level; 23 sunscreens missed their listed SPF levels by more than half. “Natural” or “mineral-only” sunscreens, which rely on such blockers as zinc oxide or titanium dioxide, performed the worst. Some patients prefer to use these sunscreens because they aren’t chemical based, and “may want to have a more natural sunscreen,” Dr. DeLeo said. “But they should be aware the sunscreens don’t always live up to the SPF level on the label.”
Beware of warnings about sunscreens.
Reports have warned Americans about supposed risks of sunscreen use such as low vitamin D levels from the lack of sun exposure, the exposure to titanium dioxide and zinc oxide nanoparticles, and the exposure to retinyl palmitate in sunscreen. Hawaiian officials, meanwhile, are banning some types of sunscreen chemicals in order to protect coral reefs.
Typical use of sunscreen will not dangerously lower vitamin D levels, Dr. DeLeo said, but people who use it every day may want to be cautious. He dismissed the concerns about nanoparticles and retinyl palmitate.
Dr. DeLeo said two sunscreen risks are real; sunscreens can trigger irritation, at a rate as high as 20%, and, rarely, allergic reactions, as well.
American sunscreens don’t stack up worldwide.
Simplicity often is a virtue. But, Dr. DeLeo said, it’s not helpful when it comes to the components of American sunscreens.
U.S. regulations only allow 16 ingredients in sunscreen while several more are allowed in Europe, he said. According to him, this helps explain why European sunscreens do a better job. European sunscreens “are much more absorbent, much better at absorbing radiation than the U.S. sunscreens,” he said. “It’s because we don’t have the same products as they have in Europe.”
The good news, he said, is that the FDA is considering expanding the number of ingredients allowed in sunscreen. The Sunscreen Innovation Act of 2014, a law passed by Congress, allows the FDA to use efficacy and safety data from Europe without requiring manufacturers to launch new, multimillion dollar tests, he said.
That’s good news for companies that want to improve U.S. sunscreens by selling a wider variety of types. “Sooner or later,” he said, “we will probably get these.”
Sunscreen sprays are tops at scalp protection.
Sunscreen sprays shouldn’t be applied to the face in children, Dr. DeLeo said, but they’re great for solo people because they facilitate protecting the back when there isn’t someone around to help them apply topical sunscreen.
How much spray should people use? A lot, he said. He added that sunscreen sprays are especially useful for the scalps of people with thinning hair.
Dr. DeLeo disclosed consulting work for Estée Lauder.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
REPORTING FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Allergen of the year may be nearer than you think
MONTEREY, CALIF. – It’s only found in 2%-3% of allergy cases. So Because, a dermatologist told colleagues, it’s so common.
“If you’re allergic to it, it’s tough to stay away from it,” said Joseph F. Fowler Jr., MD, clinical professor of dermatology at the University of Louisville (Ky.) in a presentation about contact dermatitis at the annual Coastal Dermatology Symposium.
Indeed, the synthetic compound PG is found in skin care products and cosmetics, coated pills, topical medications such as corticosteroids, foods (including bread, food coloring, and such flavorings as vanilla extracts). “It’s in every topical acne product I know of,” and is even in brake fluid and so-called nontoxic antifreeze, he said. (Propylene glycol shouldn’t be confused with the poisonous toxin ethylene glycol, which also is found in antifreeze.)
Patients can be tested for allergy to PG, Dr. Fowler pointed out, but it’s important to understand that it can trigger an irritation reaction that can be mistaken for an allergic reaction.
Dr. Fowler offered the following tips related to contact dermatitis and allergens. Be aware that metals, topical antibiotics, fragrances, and preservatives are most likely to cause allergic contact dermatitis. According to 2016 figures on allergen prevalence from the North American Contact Dermatitis Group (NACDG), allergy to the metal nickel is the most common (16%); followed by neomycin (9%); fragrance mix I, a mixture of fragrances used in allergen testing (9%); bacitracin (8%); and myroxylon, also known as balsam of Peru, which is used for a variety of purposes in food, medicines, and fragrances (7%).
These are followed by the metal cobalt (6%); the preservatives quaternium 15 and formaldehyde (both 6%); para-phenylenediamine, also known as PPD, which is used in hair dye (5%); and the fragrance mix II (5%), another mix of fragrances used in allergen testing.
Dr. Fowler cautioned that nickel can trigger an intense body-wide allergic reaction in children with atopic dermatitis. “In this situation, it’s really good to be compulsive and tell parents to absolutely keep that person away from nickel as much as humanly possible,” he said.
Keep an eye out for allergens that aren’t on the NACDG list, which includes 70 items. According to Dr. Fowler, more than 20% of his patients were positive to allergens not on the NACDG list.
Contact dermatitis is as common in children as in adults and can even be more common in children. An Italian study published in 2012 found that 70% of children aged 1-15 years tested via patch test were allergic to at least one allergen, a number that’s similar in adults (Dermatitis. 2012 Nov-Dec;23[6]:275-80). There are wide disparities in reported levels of children who are allergic to nickel, cobalt, and myroxylon, Dr. Fowler said.
The T.R.U.E. Test patch test system has value, compared with standard patch tests, but beware of its limitations, he advised. T.R.U.E. is easy to use and requires no prep time, he said, but the number of allergens is limited. By contrast, his clinic mostly uses the Finn Chambers on Scanpor tape system, which can test for many more allergens and is cheaper if used at least 5-10 times a month.
He cautioned that T.R.U.E. could miss the cause of contact dermatitis as often as 39% of the time, as demonstrated in one study of children undergoing patch testing (Arch Dermatol. 2008 Oct;144[10]:1329-36). However, he said, the T.R.U.E test has value in detecting allergies to nickel, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), and neomycin (J Am Acad Dermatol. 2001 Dec;45[6]:836-9).
Consider patch testing in a child with eczema if the eczema is not in normal atopic areas, it spreads beyond normal areas, it doesn’t respond to usual treatments, or it begins later than 5 years of age.
And, Dr. Fowler added, it’s fine to perform patch testing on patients who are taking antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.
Dr. Fowler disclosed consulting for IntraDerm, serving on speakers bureaus for SmartPractice and Regeneron/Sanofi, and serving as an investigator for companies that include AbbVie, Allergan, Bayer, Dow, Galderma, Johnson & Johnson, Eli Lilly, Merck, Regeneron, SmartPractice, and Valeant (now Bausch).
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – It’s only found in 2%-3% of allergy cases. So Because, a dermatologist told colleagues, it’s so common.
“If you’re allergic to it, it’s tough to stay away from it,” said Joseph F. Fowler Jr., MD, clinical professor of dermatology at the University of Louisville (Ky.) in a presentation about contact dermatitis at the annual Coastal Dermatology Symposium.
Indeed, the synthetic compound PG is found in skin care products and cosmetics, coated pills, topical medications such as corticosteroids, foods (including bread, food coloring, and such flavorings as vanilla extracts). “It’s in every topical acne product I know of,” and is even in brake fluid and so-called nontoxic antifreeze, he said. (Propylene glycol shouldn’t be confused with the poisonous toxin ethylene glycol, which also is found in antifreeze.)
Patients can be tested for allergy to PG, Dr. Fowler pointed out, but it’s important to understand that it can trigger an irritation reaction that can be mistaken for an allergic reaction.
Dr. Fowler offered the following tips related to contact dermatitis and allergens. Be aware that metals, topical antibiotics, fragrances, and preservatives are most likely to cause allergic contact dermatitis. According to 2016 figures on allergen prevalence from the North American Contact Dermatitis Group (NACDG), allergy to the metal nickel is the most common (16%); followed by neomycin (9%); fragrance mix I, a mixture of fragrances used in allergen testing (9%); bacitracin (8%); and myroxylon, also known as balsam of Peru, which is used for a variety of purposes in food, medicines, and fragrances (7%).
These are followed by the metal cobalt (6%); the preservatives quaternium 15 and formaldehyde (both 6%); para-phenylenediamine, also known as PPD, which is used in hair dye (5%); and the fragrance mix II (5%), another mix of fragrances used in allergen testing.
Dr. Fowler cautioned that nickel can trigger an intense body-wide allergic reaction in children with atopic dermatitis. “In this situation, it’s really good to be compulsive and tell parents to absolutely keep that person away from nickel as much as humanly possible,” he said.
Keep an eye out for allergens that aren’t on the NACDG list, which includes 70 items. According to Dr. Fowler, more than 20% of his patients were positive to allergens not on the NACDG list.
Contact dermatitis is as common in children as in adults and can even be more common in children. An Italian study published in 2012 found that 70% of children aged 1-15 years tested via patch test were allergic to at least one allergen, a number that’s similar in adults (Dermatitis. 2012 Nov-Dec;23[6]:275-80). There are wide disparities in reported levels of children who are allergic to nickel, cobalt, and myroxylon, Dr. Fowler said.
The T.R.U.E. Test patch test system has value, compared with standard patch tests, but beware of its limitations, he advised. T.R.U.E. is easy to use and requires no prep time, he said, but the number of allergens is limited. By contrast, his clinic mostly uses the Finn Chambers on Scanpor tape system, which can test for many more allergens and is cheaper if used at least 5-10 times a month.
He cautioned that T.R.U.E. could miss the cause of contact dermatitis as often as 39% of the time, as demonstrated in one study of children undergoing patch testing (Arch Dermatol. 2008 Oct;144[10]:1329-36). However, he said, the T.R.U.E test has value in detecting allergies to nickel, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), and neomycin (J Am Acad Dermatol. 2001 Dec;45[6]:836-9).
Consider patch testing in a child with eczema if the eczema is not in normal atopic areas, it spreads beyond normal areas, it doesn’t respond to usual treatments, or it begins later than 5 years of age.
And, Dr. Fowler added, it’s fine to perform patch testing on patients who are taking antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.
Dr. Fowler disclosed consulting for IntraDerm, serving on speakers bureaus for SmartPractice and Regeneron/Sanofi, and serving as an investigator for companies that include AbbVie, Allergan, Bayer, Dow, Galderma, Johnson & Johnson, Eli Lilly, Merck, Regeneron, SmartPractice, and Valeant (now Bausch).
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – It’s only found in 2%-3% of allergy cases. So Because, a dermatologist told colleagues, it’s so common.
“If you’re allergic to it, it’s tough to stay away from it,” said Joseph F. Fowler Jr., MD, clinical professor of dermatology at the University of Louisville (Ky.) in a presentation about contact dermatitis at the annual Coastal Dermatology Symposium.
Indeed, the synthetic compound PG is found in skin care products and cosmetics, coated pills, topical medications such as corticosteroids, foods (including bread, food coloring, and such flavorings as vanilla extracts). “It’s in every topical acne product I know of,” and is even in brake fluid and so-called nontoxic antifreeze, he said. (Propylene glycol shouldn’t be confused with the poisonous toxin ethylene glycol, which also is found in antifreeze.)
Patients can be tested for allergy to PG, Dr. Fowler pointed out, but it’s important to understand that it can trigger an irritation reaction that can be mistaken for an allergic reaction.
Dr. Fowler offered the following tips related to contact dermatitis and allergens. Be aware that metals, topical antibiotics, fragrances, and preservatives are most likely to cause allergic contact dermatitis. According to 2016 figures on allergen prevalence from the North American Contact Dermatitis Group (NACDG), allergy to the metal nickel is the most common (16%); followed by neomycin (9%); fragrance mix I, a mixture of fragrances used in allergen testing (9%); bacitracin (8%); and myroxylon, also known as balsam of Peru, which is used for a variety of purposes in food, medicines, and fragrances (7%).
These are followed by the metal cobalt (6%); the preservatives quaternium 15 and formaldehyde (both 6%); para-phenylenediamine, also known as PPD, which is used in hair dye (5%); and the fragrance mix II (5%), another mix of fragrances used in allergen testing.
Dr. Fowler cautioned that nickel can trigger an intense body-wide allergic reaction in children with atopic dermatitis. “In this situation, it’s really good to be compulsive and tell parents to absolutely keep that person away from nickel as much as humanly possible,” he said.
Keep an eye out for allergens that aren’t on the NACDG list, which includes 70 items. According to Dr. Fowler, more than 20% of his patients were positive to allergens not on the NACDG list.
Contact dermatitis is as common in children as in adults and can even be more common in children. An Italian study published in 2012 found that 70% of children aged 1-15 years tested via patch test were allergic to at least one allergen, a number that’s similar in adults (Dermatitis. 2012 Nov-Dec;23[6]:275-80). There are wide disparities in reported levels of children who are allergic to nickel, cobalt, and myroxylon, Dr. Fowler said.
The T.R.U.E. Test patch test system has value, compared with standard patch tests, but beware of its limitations, he advised. T.R.U.E. is easy to use and requires no prep time, he said, but the number of allergens is limited. By contrast, his clinic mostly uses the Finn Chambers on Scanpor tape system, which can test for many more allergens and is cheaper if used at least 5-10 times a month.
He cautioned that T.R.U.E. could miss the cause of contact dermatitis as often as 39% of the time, as demonstrated in one study of children undergoing patch testing (Arch Dermatol. 2008 Oct;144[10]:1329-36). However, he said, the T.R.U.E test has value in detecting allergies to nickel, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), and neomycin (J Am Acad Dermatol. 2001 Dec;45[6]:836-9).
Consider patch testing in a child with eczema if the eczema is not in normal atopic areas, it spreads beyond normal areas, it doesn’t respond to usual treatments, or it begins later than 5 years of age.
And, Dr. Fowler added, it’s fine to perform patch testing on patients who are taking antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.
Dr. Fowler disclosed consulting for IntraDerm, serving on speakers bureaus for SmartPractice and Regeneron/Sanofi, and serving as an investigator for companies that include AbbVie, Allergan, Bayer, Dow, Galderma, Johnson & Johnson, Eli Lilly, Merck, Regeneron, SmartPractice, and Valeant (now Bausch).
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
IL inhibitor options move psoriasis treatment forward
Psoriasis patients have many options, and more are on the way, according to J. Mark Jackson, MD, of the University of Louisville, Ky.
“Know the information regarding each [treatment] to best care for your patients,” Dr. Jackson said in a presentation at the annual Coastal Dermatology Symposium.
Dr. Jackson particularly addressed the interleukin (IL)-17 inhibitors (brodalumab, ixekizumab, and secukinumab) and the IL-23 inhibitors (guselkumab, risankizumab, and tildrakizumab).
Complete clearance rates can reach 50% and higher over the long term when treating patients with IL-17 inhibitors, but patients must maintain regular dosing to maintain a response, he said.
Overall, comparisons of IL-17 inhibitors with etanercept, adalimumab, and ustekinumab “demonstrate better efficacy with no evidence of compromising safety,” he noted.
For example, secukinumab demonstrated significantly superior results when compared with ustekinumab in a randomized trial (J Am Acad Dermatol. 2015;73: 400-9). After 16 weeks of treatment, 79% of secukinumab patients achieved a 90% reduction in Psoriasis Area and Severity Index score (PASI 90) versus 58% of ustekinumab patients, he said, and the drug safety profile was consistent with the pivotal phase 3 studies of secukinumab.
Concerns persist about increased risk of inflammatory bowel disease, Crohn’s disease, and ulcerative colitis in patients taking secukinumab and other IL-17 inhibitors, but data indicate that rates are low. The risk is low “and may be related to psoriasis and not the therapy,” he explained.
Ixekizumab has been associated with more injection site reactions than secukinumab, but these tend to be mild, Dr. Jackson said. Advantages of ixekizumab are that it works quickly and has demonstrated effectiveness against genital, palmoplantar, scalp, and nail psoriasis, he added.
Brodalumab also works quickly, but it has the unique inclusion of a Risk Evaluation and Mitigation Strategies (REMS) program because of suicidal ideation and behavior in clinical trials, he noted, adding that there are more data showing rates are low and the REMS program is easier to deal with than the isotretinoin REMS. The increased risk of superficial Staphylococcus and Candida infections are noted on IL-17 inhibitor labels, but this has not been a significant issue in trials or clinical practice, he said.
What is also exciting about the IL-17 inhibitors are the approvals of ixekizumab and secukinumab for patients with psoriatic arthritis (PsA), with both agents demonstrating the ability to inhibit the structural progression of joint damage over time, Dr. Jackson commented. These data seem to be on par with that of the TNF-inhibitors, although time will tell how this bears out clinically, he noted.
IL-23 inhibitors guselkumab, tildrakizumab, and risankizumab (not yet approved) have shown similar effectiveness and are well tolerated by patients, with few injection site reactions or adverse events reported, Dr. Jackson said. The dosing regimens of each of these drugs, administered subcutaneously, are easy to follow: Treatment starts with an initial dose of either 100 mg (guselkumab and tildrakizumab) or 150 mg (risankizumab), which is followed by doses at 4 weeks and then doses every 8 weeks (guselkumab) or 12 weeks (tildrakizumab and risankizumab).
For example, in a comparison study of risankizumab with a dosage of 150 mg subcutaneously at week 0, 4, then every 12 weeks, 75% of risankizumab patients achieved PASI 90 at 16 weeks and 82% at 52 weeks, compared with 42% and 44%, respectively, for adalimumab patients.
In addition, the IL-23 inhibitors have demonstrated some benefits for PsA patients in clinical trials, but they are not currently indicated for PsA, he said.
Dr. Jackson disclosed having received research, honoraria, consulting, and/or other support from AbbVie, Accuitis, Aclaris, Celgene, Dr. Reddy’s, Galderma, Janssen, Lilly, Medimetriks, Novartis, Pfizer, Promius, Ralexar, Sienna, and TopMD.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Psoriasis patients have many options, and more are on the way, according to J. Mark Jackson, MD, of the University of Louisville, Ky.
“Know the information regarding each [treatment] to best care for your patients,” Dr. Jackson said in a presentation at the annual Coastal Dermatology Symposium.
Dr. Jackson particularly addressed the interleukin (IL)-17 inhibitors (brodalumab, ixekizumab, and secukinumab) and the IL-23 inhibitors (guselkumab, risankizumab, and tildrakizumab).
Complete clearance rates can reach 50% and higher over the long term when treating patients with IL-17 inhibitors, but patients must maintain regular dosing to maintain a response, he said.
Overall, comparisons of IL-17 inhibitors with etanercept, adalimumab, and ustekinumab “demonstrate better efficacy with no evidence of compromising safety,” he noted.
For example, secukinumab demonstrated significantly superior results when compared with ustekinumab in a randomized trial (J Am Acad Dermatol. 2015;73: 400-9). After 16 weeks of treatment, 79% of secukinumab patients achieved a 90% reduction in Psoriasis Area and Severity Index score (PASI 90) versus 58% of ustekinumab patients, he said, and the drug safety profile was consistent with the pivotal phase 3 studies of secukinumab.
Concerns persist about increased risk of inflammatory bowel disease, Crohn’s disease, and ulcerative colitis in patients taking secukinumab and other IL-17 inhibitors, but data indicate that rates are low. The risk is low “and may be related to psoriasis and not the therapy,” he explained.
Ixekizumab has been associated with more injection site reactions than secukinumab, but these tend to be mild, Dr. Jackson said. Advantages of ixekizumab are that it works quickly and has demonstrated effectiveness against genital, palmoplantar, scalp, and nail psoriasis, he added.
Brodalumab also works quickly, but it has the unique inclusion of a Risk Evaluation and Mitigation Strategies (REMS) program because of suicidal ideation and behavior in clinical trials, he noted, adding that there are more data showing rates are low and the REMS program is easier to deal with than the isotretinoin REMS. The increased risk of superficial Staphylococcus and Candida infections are noted on IL-17 inhibitor labels, but this has not been a significant issue in trials or clinical practice, he said.
What is also exciting about the IL-17 inhibitors are the approvals of ixekizumab and secukinumab for patients with psoriatic arthritis (PsA), with both agents demonstrating the ability to inhibit the structural progression of joint damage over time, Dr. Jackson commented. These data seem to be on par with that of the TNF-inhibitors, although time will tell how this bears out clinically, he noted.
IL-23 inhibitors guselkumab, tildrakizumab, and risankizumab (not yet approved) have shown similar effectiveness and are well tolerated by patients, with few injection site reactions or adverse events reported, Dr. Jackson said. The dosing regimens of each of these drugs, administered subcutaneously, are easy to follow: Treatment starts with an initial dose of either 100 mg (guselkumab and tildrakizumab) or 150 mg (risankizumab), which is followed by doses at 4 weeks and then doses every 8 weeks (guselkumab) or 12 weeks (tildrakizumab and risankizumab).
For example, in a comparison study of risankizumab with a dosage of 150 mg subcutaneously at week 0, 4, then every 12 weeks, 75% of risankizumab patients achieved PASI 90 at 16 weeks and 82% at 52 weeks, compared with 42% and 44%, respectively, for adalimumab patients.
In addition, the IL-23 inhibitors have demonstrated some benefits for PsA patients in clinical trials, but they are not currently indicated for PsA, he said.
Dr. Jackson disclosed having received research, honoraria, consulting, and/or other support from AbbVie, Accuitis, Aclaris, Celgene, Dr. Reddy’s, Galderma, Janssen, Lilly, Medimetriks, Novartis, Pfizer, Promius, Ralexar, Sienna, and TopMD.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Psoriasis patients have many options, and more are on the way, according to J. Mark Jackson, MD, of the University of Louisville, Ky.
“Know the information regarding each [treatment] to best care for your patients,” Dr. Jackson said in a presentation at the annual Coastal Dermatology Symposium.
Dr. Jackson particularly addressed the interleukin (IL)-17 inhibitors (brodalumab, ixekizumab, and secukinumab) and the IL-23 inhibitors (guselkumab, risankizumab, and tildrakizumab).
Complete clearance rates can reach 50% and higher over the long term when treating patients with IL-17 inhibitors, but patients must maintain regular dosing to maintain a response, he said.
Overall, comparisons of IL-17 inhibitors with etanercept, adalimumab, and ustekinumab “demonstrate better efficacy with no evidence of compromising safety,” he noted.
For example, secukinumab demonstrated significantly superior results when compared with ustekinumab in a randomized trial (J Am Acad Dermatol. 2015;73: 400-9). After 16 weeks of treatment, 79% of secukinumab patients achieved a 90% reduction in Psoriasis Area and Severity Index score (PASI 90) versus 58% of ustekinumab patients, he said, and the drug safety profile was consistent with the pivotal phase 3 studies of secukinumab.
Concerns persist about increased risk of inflammatory bowel disease, Crohn’s disease, and ulcerative colitis in patients taking secukinumab and other IL-17 inhibitors, but data indicate that rates are low. The risk is low “and may be related to psoriasis and not the therapy,” he explained.
Ixekizumab has been associated with more injection site reactions than secukinumab, but these tend to be mild, Dr. Jackson said. Advantages of ixekizumab are that it works quickly and has demonstrated effectiveness against genital, palmoplantar, scalp, and nail psoriasis, he added.
Brodalumab also works quickly, but it has the unique inclusion of a Risk Evaluation and Mitigation Strategies (REMS) program because of suicidal ideation and behavior in clinical trials, he noted, adding that there are more data showing rates are low and the REMS program is easier to deal with than the isotretinoin REMS. The increased risk of superficial Staphylococcus and Candida infections are noted on IL-17 inhibitor labels, but this has not been a significant issue in trials or clinical practice, he said.
What is also exciting about the IL-17 inhibitors are the approvals of ixekizumab and secukinumab for patients with psoriatic arthritis (PsA), with both agents demonstrating the ability to inhibit the structural progression of joint damage over time, Dr. Jackson commented. These data seem to be on par with that of the TNF-inhibitors, although time will tell how this bears out clinically, he noted.
IL-23 inhibitors guselkumab, tildrakizumab, and risankizumab (not yet approved) have shown similar effectiveness and are well tolerated by patients, with few injection site reactions or adverse events reported, Dr. Jackson said. The dosing regimens of each of these drugs, administered subcutaneously, are easy to follow: Treatment starts with an initial dose of either 100 mg (guselkumab and tildrakizumab) or 150 mg (risankizumab), which is followed by doses at 4 weeks and then doses every 8 weeks (guselkumab) or 12 weeks (tildrakizumab and risankizumab).
For example, in a comparison study of risankizumab with a dosage of 150 mg subcutaneously at week 0, 4, then every 12 weeks, 75% of risankizumab patients achieved PASI 90 at 16 weeks and 82% at 52 weeks, compared with 42% and 44%, respectively, for adalimumab patients.
In addition, the IL-23 inhibitors have demonstrated some benefits for PsA patients in clinical trials, but they are not currently indicated for PsA, he said.
Dr. Jackson disclosed having received research, honoraria, consulting, and/or other support from AbbVie, Accuitis, Aclaris, Celgene, Dr. Reddy’s, Galderma, Janssen, Lilly, Medimetriks, Novartis, Pfizer, Promius, Ralexar, Sienna, and TopMD.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
FROM THE COASTAL DERMATOLOGY SYMPOSIUM