User login
Pandemic took a cut of cosmetic procedures in 2020
pandemic, according to the American Society of Plastic Surgeons.
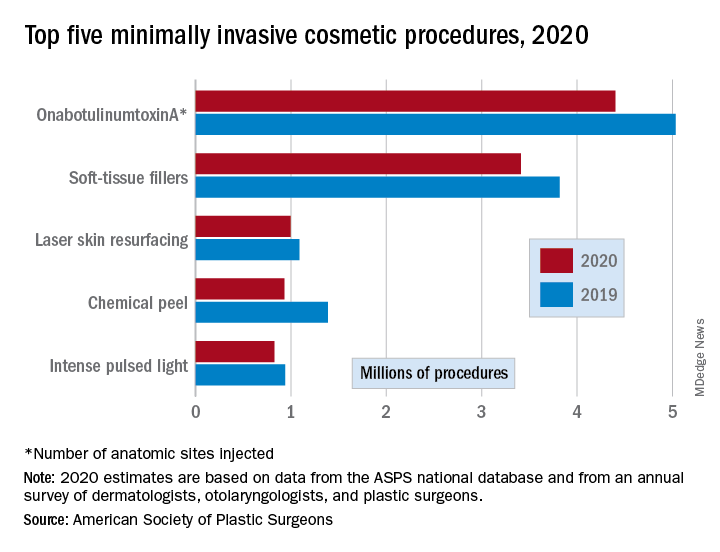
There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
Goodbye, OTC hydroquinone
In 1972, an over-the-counter drug review process was established by the Food and Drug Administration to regulate the safety and efficacy of over-the-counter (OTC) drugs. This created a book or “monograph” for each medication category that describes the active ingredients, indications, doses, route of administration, testing, and labeling. If a drug meets the criteria in its therapeutic category, it does not have to undergo an FDA review before being marketed to consumers.
As part of this process, drugs are classified into one of three categories: category I: generally recognized as safe and effective (GRASE) and not misbranded; category II: not GRASE; category III: lacking sufficient data on safety and efficacy to permit classification. This methodology was outdated and made it difficult under the old guidelines to make changes to medications in the evolving world of drug development. Some categories of OTC drugs, including hand sanitizers, hydroquinone, and sunscreens, have been marketed for years without a final monograph.
The signing of the “Coronavirus Aid, Relief, and Economic Security” (CARES) Act in March 2020 included reforms in the FDA monograph process for OTC medications. Under this proceeding, a final monograph determination was made for all OTC categories. While drugs in category I and some in category III may remain on the market, if certain specifications are met, category II drugs had to be removed within 180 days of the enactment of the CARES Act.
Hydroquinone was one of those that fell victim to the ban. This ban is similar to hydroquinone bans in other places, including Europe. However, for manufacturers, this issue was under the radar and packaged in a seemingly irrelevant piece of legislation.
Among dermatologists, there is no consensus as to whether 2% hydroquinone is safe or not. However, the unmonitored use and overuse that is common for this type of medication has led to heightened safety concerns. Common side effects of hydroquinone include irritant and allergic contact dermatitis; the most difficult to treat side effect with long-term use is ochronosis. But there are no reported cancer data in humans with the use of topical hydroquinone as previously thought. Hydroquinone used short term is a very safe and effective treatment for hard to treat hyperpigmentation and is often necessary when other topicals are ineffective, particularly in our patients with skin of color.
The bigger problem however is the legislative process involved, as exemplified by this ban, which only came to light because of the CARES act.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
In 1972, an over-the-counter drug review process was established by the Food and Drug Administration to regulate the safety and efficacy of over-the-counter (OTC) drugs. This created a book or “monograph” for each medication category that describes the active ingredients, indications, doses, route of administration, testing, and labeling. If a drug meets the criteria in its therapeutic category, it does not have to undergo an FDA review before being marketed to consumers.
As part of this process, drugs are classified into one of three categories: category I: generally recognized as safe and effective (GRASE) and not misbranded; category II: not GRASE; category III: lacking sufficient data on safety and efficacy to permit classification. This methodology was outdated and made it difficult under the old guidelines to make changes to medications in the evolving world of drug development. Some categories of OTC drugs, including hand sanitizers, hydroquinone, and sunscreens, have been marketed for years without a final monograph.
The signing of the “Coronavirus Aid, Relief, and Economic Security” (CARES) Act in March 2020 included reforms in the FDA monograph process for OTC medications. Under this proceeding, a final monograph determination was made for all OTC categories. While drugs in category I and some in category III may remain on the market, if certain specifications are met, category II drugs had to be removed within 180 days of the enactment of the CARES Act.
Hydroquinone was one of those that fell victim to the ban. This ban is similar to hydroquinone bans in other places, including Europe. However, for manufacturers, this issue was under the radar and packaged in a seemingly irrelevant piece of legislation.
Among dermatologists, there is no consensus as to whether 2% hydroquinone is safe or not. However, the unmonitored use and overuse that is common for this type of medication has led to heightened safety concerns. Common side effects of hydroquinone include irritant and allergic contact dermatitis; the most difficult to treat side effect with long-term use is ochronosis. But there are no reported cancer data in humans with the use of topical hydroquinone as previously thought. Hydroquinone used short term is a very safe and effective treatment for hard to treat hyperpigmentation and is often necessary when other topicals are ineffective, particularly in our patients with skin of color.
The bigger problem however is the legislative process involved, as exemplified by this ban, which only came to light because of the CARES act.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
In 1972, an over-the-counter drug review process was established by the Food and Drug Administration to regulate the safety and efficacy of over-the-counter (OTC) drugs. This created a book or “monograph” for each medication category that describes the active ingredients, indications, doses, route of administration, testing, and labeling. If a drug meets the criteria in its therapeutic category, it does not have to undergo an FDA review before being marketed to consumers.
As part of this process, drugs are classified into one of three categories: category I: generally recognized as safe and effective (GRASE) and not misbranded; category II: not GRASE; category III: lacking sufficient data on safety and efficacy to permit classification. This methodology was outdated and made it difficult under the old guidelines to make changes to medications in the evolving world of drug development. Some categories of OTC drugs, including hand sanitizers, hydroquinone, and sunscreens, have been marketed for years without a final monograph.
The signing of the “Coronavirus Aid, Relief, and Economic Security” (CARES) Act in March 2020 included reforms in the FDA monograph process for OTC medications. Under this proceeding, a final monograph determination was made for all OTC categories. While drugs in category I and some in category III may remain on the market, if certain specifications are met, category II drugs had to be removed within 180 days of the enactment of the CARES Act.
Hydroquinone was one of those that fell victim to the ban. This ban is similar to hydroquinone bans in other places, including Europe. However, for manufacturers, this issue was under the radar and packaged in a seemingly irrelevant piece of legislation.
Among dermatologists, there is no consensus as to whether 2% hydroquinone is safe or not. However, the unmonitored use and overuse that is common for this type of medication has led to heightened safety concerns. Common side effects of hydroquinone include irritant and allergic contact dermatitis; the most difficult to treat side effect with long-term use is ochronosis. But there are no reported cancer data in humans with the use of topical hydroquinone as previously thought. Hydroquinone used short term is a very safe and effective treatment for hard to treat hyperpigmentation and is often necessary when other topicals are ineffective, particularly in our patients with skin of color.
The bigger problem however is the legislative process involved, as exemplified by this ban, which only came to light because of the CARES act.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Chart review for cosmetic procedures: 2019 was a good year
Cosmetic procedures continued to increase in popularity in 2019, and body sculpting and laser/light/energy-based procedures led the way, according to a survey by the American Society of Dermatologic Surgery.

the largest proportional rise among the major categories of cosmetic procedures, the ASDS reported in its annual member survey.
Use of laser/light/energy-based devices was up by a much lower 18%, but the increase in the actual number of procedures – nearly 637,000 more than 2018 – was larger than any other category, the ASDS said.
Procedures categorized as “other rejuvenation” – microneedling, platelet-rich plasma, hair rejuvenation, and thread lifts – were another bright spot in the cosmetic lineup in 2019, rising 38% over their 2018 volume. Injectable neuromodulator treatments, which were second in overall volume with almost 2.4 million procedures, were up by 12.5% in 2019, compared with 2018, the ASDS said.
The injectable soft-tissue fillers, however, did not produce any noteworthy gain in the volume of procedures for the second consecutive year. Meanwhile, the number of chemical peel procedures dropped by almost 16% in 2019 after rising for the last 2 years, the ASDS reported.
A closer look at the body-sculpting sector also shows some declines in 2019, despite the overall success: Cryolipolysis procedures were down by 10.3% and deoxycholic acid procedures slipped 13.7%. The biggest addition – over 157,000 procedures – came from muscle-toning devices, which were new to the survey last year, the ASDS noted.
The survey was conducted among the society’s members from May 7 to July 31, 2020, and the 514 responses were generalized to the entire ASDS membership of over 6,400 physicians.
Cosmetic procedures continued to increase in popularity in 2019, and body sculpting and laser/light/energy-based procedures led the way, according to a survey by the American Society of Dermatologic Surgery.

the largest proportional rise among the major categories of cosmetic procedures, the ASDS reported in its annual member survey.
Use of laser/light/energy-based devices was up by a much lower 18%, but the increase in the actual number of procedures – nearly 637,000 more than 2018 – was larger than any other category, the ASDS said.
Procedures categorized as “other rejuvenation” – microneedling, platelet-rich plasma, hair rejuvenation, and thread lifts – were another bright spot in the cosmetic lineup in 2019, rising 38% over their 2018 volume. Injectable neuromodulator treatments, which were second in overall volume with almost 2.4 million procedures, were up by 12.5% in 2019, compared with 2018, the ASDS said.
The injectable soft-tissue fillers, however, did not produce any noteworthy gain in the volume of procedures for the second consecutive year. Meanwhile, the number of chemical peel procedures dropped by almost 16% in 2019 after rising for the last 2 years, the ASDS reported.
A closer look at the body-sculpting sector also shows some declines in 2019, despite the overall success: Cryolipolysis procedures were down by 10.3% and deoxycholic acid procedures slipped 13.7%. The biggest addition – over 157,000 procedures – came from muscle-toning devices, which were new to the survey last year, the ASDS noted.
The survey was conducted among the society’s members from May 7 to July 31, 2020, and the 514 responses were generalized to the entire ASDS membership of over 6,400 physicians.
Cosmetic procedures continued to increase in popularity in 2019, and body sculpting and laser/light/energy-based procedures led the way, according to a survey by the American Society of Dermatologic Surgery.

the largest proportional rise among the major categories of cosmetic procedures, the ASDS reported in its annual member survey.
Use of laser/light/energy-based devices was up by a much lower 18%, but the increase in the actual number of procedures – nearly 637,000 more than 2018 – was larger than any other category, the ASDS said.
Procedures categorized as “other rejuvenation” – microneedling, platelet-rich plasma, hair rejuvenation, and thread lifts – were another bright spot in the cosmetic lineup in 2019, rising 38% over their 2018 volume. Injectable neuromodulator treatments, which were second in overall volume with almost 2.4 million procedures, were up by 12.5% in 2019, compared with 2018, the ASDS said.
The injectable soft-tissue fillers, however, did not produce any noteworthy gain in the volume of procedures for the second consecutive year. Meanwhile, the number of chemical peel procedures dropped by almost 16% in 2019 after rising for the last 2 years, the ASDS reported.
A closer look at the body-sculpting sector also shows some declines in 2019, despite the overall success: Cryolipolysis procedures were down by 10.3% and deoxycholic acid procedures slipped 13.7%. The biggest addition – over 157,000 procedures – came from muscle-toning devices, which were new to the survey last year, the ASDS noted.
The survey was conducted among the society’s members from May 7 to July 31, 2020, and the 514 responses were generalized to the entire ASDS membership of over 6,400 physicians.
Expert spotlights three emerging technologies for dermatology practice
New technologies being developed at the Wellman Center for Photomedicine, Boston, that .
During a virtual course on laser and aesthetic skin therapy, Lilit Garibyan, MD, PhD, discussed findings from a swine study published online in January 2020 that used an injectable physiologic ice slurry for the nonsurgical removal of fat, a technology that could give CoolSculpting a run for its money. “It does lead to more efficient and effective cryolipolysis,” said Dr. Garibyan, the lead study author who is an assistant professor of dermatology at Harvard University, and director of The Magic Wand Initiative at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The treatment of fat tissue with ice slurry injection can be done in less than 1 minute, as opposed to an hour of cooling with CoolSculpting. In addition, because cooling is delivered directly into target tissue, it is more effective.”
For the study, she and her colleagues at the Wellman Center injected the slurry – a mix of ice, saline, and glycol – into the flanks of swine and followed them for up to 8 weeks. They used ultrasound imaging to show the location of the fat loss and to quantify it. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% fat gain in swine who served as controls. “This is because the pig is growing and gaining weight, so the fat is increasing,” she explained.
Gross histologic images also showed fat loss in the subcutaneous fat tissue of treated swine, but not in controls. “When we quantified this loss, there was about a 60% loss of fat after a single injection of ice slurry in the subcutaneous fat,” Dr. Garibyan said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “On histology there was loss of fat in the subcutaneous area and it was replaced by new collagen. No damage to surrounding skin or muscle tissue was seen.”
She characterized the approach as “a minimally invasive and novel method of adipose tissue removal. It’s very simple, because it’s just a simple injection, and it’s very efficient and effective in fat removal. Most importantly, it can target any anatomic site accessible with a needle.”
Human studies are currently underway.
Another emerging technology Dr. Garibyan discussed is a novel controlled skin cooling device for the treatment of benign pigmented lesions. The approach, known as Cryomodulation, was invented by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry HL Chan, MD, at Massachusetts General Hospital, Boston, and is being commercialized by R2 Technologies. It delivers precise controlled and titratable freezing of benign pigmented lesions without damage to the epidermal barrier. It has been cleared by the Food and Drug Administration, and R2 Technologies plans to launch its first commercial product in the United States in December 2020.
The handpiece of the device, which is placed on top of the skin, provides localized and controlled freezing to targeted benign pigmented lesions. “The cold, or the freeze, is delivered to where the melanocytes reside,” Dr. Garibyan said. “The ice nucleation essentially pauses melanin production. As cell turnover occurs, cells that are melanin-free migrate upward and renew freshly healthy skin. So, melanocyte function is still preserved but there is no destruction to the epidermal barrier. This technology is totally color blind, and there is no persistent inflammatory response.”
After this treatment, histology reveals a reduction of epidermal melanin without destruction of melanocytes. The treatment impairs melanocyte transfer, but not the melanocytes. “Clinically, that is seen as lightening of the skin,” she said. More than 550 patients have been treated with Cryomodulation to demonstrate its safety and effectiveness, described in a study published in 2019, and an ASLMS e-poster.
The final technology Dr. Garibyan discussed is a novel device for removing dermal pigment with a highly focused laser beam. “The problem with current lasers is that the maximum absorption of energy happens at the dermal/epidermal junction,” she said. “This not only increases the risk of epidermal injury, especially in skin of color, but it also leaves very little energy to reach the pigmented target tissue or cells. In addition, there is scattering in the skin, which also reduces the amount of fluence or energy that can reach the target depth, therefore reducing the efficacy of treatment with currently available laser.”
The investigative focused laser beam with high-speed scanning creates a large differential between the fluence at the surface and the fluence at the target, which improves safety. “It’s able to deliver enhanced energy to the target,” she said. “Therefore it’s more effective than destroying the target pigmented cells. There is no injury outside of the focal point, so it offers improved safety, efficacy, and spatial selectivity. The end result on histology is a selective destruction of the pigmented cells, which are typically melanophages.”
Dr. Garibyan predicted that this device will be an ideal therapy for postinflammatory hyperpigmentation and for melasma, “as no effective therapies are available for those conditions.”
She disclosed that she has received royalties/inventorship assigned to MGH. She holds equity in, is a consultant to, and is a member of the scientific advisory board of Brixton Biosciences. She is a consultant to Vyome Therapeutics, Blossom Innovations, Aegle Therapeutics, and ClearifiRx.
New technologies being developed at the Wellman Center for Photomedicine, Boston, that .
During a virtual course on laser and aesthetic skin therapy, Lilit Garibyan, MD, PhD, discussed findings from a swine study published online in January 2020 that used an injectable physiologic ice slurry for the nonsurgical removal of fat, a technology that could give CoolSculpting a run for its money. “It does lead to more efficient and effective cryolipolysis,” said Dr. Garibyan, the lead study author who is an assistant professor of dermatology at Harvard University, and director of The Magic Wand Initiative at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The treatment of fat tissue with ice slurry injection can be done in less than 1 minute, as opposed to an hour of cooling with CoolSculpting. In addition, because cooling is delivered directly into target tissue, it is more effective.”
For the study, she and her colleagues at the Wellman Center injected the slurry – a mix of ice, saline, and glycol – into the flanks of swine and followed them for up to 8 weeks. They used ultrasound imaging to show the location of the fat loss and to quantify it. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% fat gain in swine who served as controls. “This is because the pig is growing and gaining weight, so the fat is increasing,” she explained.
Gross histologic images also showed fat loss in the subcutaneous fat tissue of treated swine, but not in controls. “When we quantified this loss, there was about a 60% loss of fat after a single injection of ice slurry in the subcutaneous fat,” Dr. Garibyan said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “On histology there was loss of fat in the subcutaneous area and it was replaced by new collagen. No damage to surrounding skin or muscle tissue was seen.”
She characterized the approach as “a minimally invasive and novel method of adipose tissue removal. It’s very simple, because it’s just a simple injection, and it’s very efficient and effective in fat removal. Most importantly, it can target any anatomic site accessible with a needle.”
Human studies are currently underway.
Another emerging technology Dr. Garibyan discussed is a novel controlled skin cooling device for the treatment of benign pigmented lesions. The approach, known as Cryomodulation, was invented by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry HL Chan, MD, at Massachusetts General Hospital, Boston, and is being commercialized by R2 Technologies. It delivers precise controlled and titratable freezing of benign pigmented lesions without damage to the epidermal barrier. It has been cleared by the Food and Drug Administration, and R2 Technologies plans to launch its first commercial product in the United States in December 2020.
The handpiece of the device, which is placed on top of the skin, provides localized and controlled freezing to targeted benign pigmented lesions. “The cold, or the freeze, is delivered to where the melanocytes reside,” Dr. Garibyan said. “The ice nucleation essentially pauses melanin production. As cell turnover occurs, cells that are melanin-free migrate upward and renew freshly healthy skin. So, melanocyte function is still preserved but there is no destruction to the epidermal barrier. This technology is totally color blind, and there is no persistent inflammatory response.”
After this treatment, histology reveals a reduction of epidermal melanin without destruction of melanocytes. The treatment impairs melanocyte transfer, but not the melanocytes. “Clinically, that is seen as lightening of the skin,” she said. More than 550 patients have been treated with Cryomodulation to demonstrate its safety and effectiveness, described in a study published in 2019, and an ASLMS e-poster.
The final technology Dr. Garibyan discussed is a novel device for removing dermal pigment with a highly focused laser beam. “The problem with current lasers is that the maximum absorption of energy happens at the dermal/epidermal junction,” she said. “This not only increases the risk of epidermal injury, especially in skin of color, but it also leaves very little energy to reach the pigmented target tissue or cells. In addition, there is scattering in the skin, which also reduces the amount of fluence or energy that can reach the target depth, therefore reducing the efficacy of treatment with currently available laser.”
The investigative focused laser beam with high-speed scanning creates a large differential between the fluence at the surface and the fluence at the target, which improves safety. “It’s able to deliver enhanced energy to the target,” she said. “Therefore it’s more effective than destroying the target pigmented cells. There is no injury outside of the focal point, so it offers improved safety, efficacy, and spatial selectivity. The end result on histology is a selective destruction of the pigmented cells, which are typically melanophages.”
Dr. Garibyan predicted that this device will be an ideal therapy for postinflammatory hyperpigmentation and for melasma, “as no effective therapies are available for those conditions.”
She disclosed that she has received royalties/inventorship assigned to MGH. She holds equity in, is a consultant to, and is a member of the scientific advisory board of Brixton Biosciences. She is a consultant to Vyome Therapeutics, Blossom Innovations, Aegle Therapeutics, and ClearifiRx.
New technologies being developed at the Wellman Center for Photomedicine, Boston, that .
During a virtual course on laser and aesthetic skin therapy, Lilit Garibyan, MD, PhD, discussed findings from a swine study published online in January 2020 that used an injectable physiologic ice slurry for the nonsurgical removal of fat, a technology that could give CoolSculpting a run for its money. “It does lead to more efficient and effective cryolipolysis,” said Dr. Garibyan, the lead study author who is an assistant professor of dermatology at Harvard University, and director of The Magic Wand Initiative at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The treatment of fat tissue with ice slurry injection can be done in less than 1 minute, as opposed to an hour of cooling with CoolSculpting. In addition, because cooling is delivered directly into target tissue, it is more effective.”
For the study, she and her colleagues at the Wellman Center injected the slurry – a mix of ice, saline, and glycol – into the flanks of swine and followed them for up to 8 weeks. They used ultrasound imaging to show the location of the fat loss and to quantify it. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% fat gain in swine who served as controls. “This is because the pig is growing and gaining weight, so the fat is increasing,” she explained.
Gross histologic images also showed fat loss in the subcutaneous fat tissue of treated swine, but not in controls. “When we quantified this loss, there was about a 60% loss of fat after a single injection of ice slurry in the subcutaneous fat,” Dr. Garibyan said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “On histology there was loss of fat in the subcutaneous area and it was replaced by new collagen. No damage to surrounding skin or muscle tissue was seen.”
She characterized the approach as “a minimally invasive and novel method of adipose tissue removal. It’s very simple, because it’s just a simple injection, and it’s very efficient and effective in fat removal. Most importantly, it can target any anatomic site accessible with a needle.”
Human studies are currently underway.
Another emerging technology Dr. Garibyan discussed is a novel controlled skin cooling device for the treatment of benign pigmented lesions. The approach, known as Cryomodulation, was invented by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry HL Chan, MD, at Massachusetts General Hospital, Boston, and is being commercialized by R2 Technologies. It delivers precise controlled and titratable freezing of benign pigmented lesions without damage to the epidermal barrier. It has been cleared by the Food and Drug Administration, and R2 Technologies plans to launch its first commercial product in the United States in December 2020.
The handpiece of the device, which is placed on top of the skin, provides localized and controlled freezing to targeted benign pigmented lesions. “The cold, or the freeze, is delivered to where the melanocytes reside,” Dr. Garibyan said. “The ice nucleation essentially pauses melanin production. As cell turnover occurs, cells that are melanin-free migrate upward and renew freshly healthy skin. So, melanocyte function is still preserved but there is no destruction to the epidermal barrier. This technology is totally color blind, and there is no persistent inflammatory response.”
After this treatment, histology reveals a reduction of epidermal melanin without destruction of melanocytes. The treatment impairs melanocyte transfer, but not the melanocytes. “Clinically, that is seen as lightening of the skin,” she said. More than 550 patients have been treated with Cryomodulation to demonstrate its safety and effectiveness, described in a study published in 2019, and an ASLMS e-poster.
The final technology Dr. Garibyan discussed is a novel device for removing dermal pigment with a highly focused laser beam. “The problem with current lasers is that the maximum absorption of energy happens at the dermal/epidermal junction,” she said. “This not only increases the risk of epidermal injury, especially in skin of color, but it also leaves very little energy to reach the pigmented target tissue or cells. In addition, there is scattering in the skin, which also reduces the amount of fluence or energy that can reach the target depth, therefore reducing the efficacy of treatment with currently available laser.”
The investigative focused laser beam with high-speed scanning creates a large differential between the fluence at the surface and the fluence at the target, which improves safety. “It’s able to deliver enhanced energy to the target,” she said. “Therefore it’s more effective than destroying the target pigmented cells. There is no injury outside of the focal point, so it offers improved safety, efficacy, and spatial selectivity. The end result on histology is a selective destruction of the pigmented cells, which are typically melanophages.”
Dr. Garibyan predicted that this device will be an ideal therapy for postinflammatory hyperpigmentation and for melasma, “as no effective therapies are available for those conditions.”
She disclosed that she has received royalties/inventorship assigned to MGH. She holds equity in, is a consultant to, and is a member of the scientific advisory board of Brixton Biosciences. She is a consultant to Vyome Therapeutics, Blossom Innovations, Aegle Therapeutics, and ClearifiRx.
EXPERT ANALYSIS FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Counterfeits: An ugly truth in aesthetic medicine
according to the results of two recent surveys of such providers.

“Counterfeit medical devices and injectables may be more prevalent in aesthetic medicine than most practitioners might estimate,” Jordan V. Wang, MD, of Sidney Kimmel Medical College, Philadelphia, and associates wrote in Dermatologic Surgery, even though “the vast majority [believe] that they are inferior and even potentially harmful.”
In one of the online surveys, conducted among members of the American Society of Dermatologic Surgery, 41.1% of the 616 respondents said they had encountered counterfeit injectables, more than half (56.5%) of whom were solicited to buy such products. Just over 10% had purchased counterfeit injectables, although nearly 80% did so unknowingly, the investigators said.
In the second survey, 37.4% of the 765 respondents (members of the ASDS as well as the American Society for Laser Medicine and Surgery) said that they had encountered counterfeit medical devices, and nearly half had been approached to purchase such devices. Of those who were approached, 4.6% had actually purchased a counterfeit, but only 6.1% did so unknowingly, Dr. Wang and associates reported.
In the medical device survey, almost a quarter (24.2%) acknowledged that they know of other providers using them, while 29.3% of those surveyed about injectables know of others who use counterfeits, they said.
Over 90% of practitioners in each survey agreed that counterfeits are worse in terms of safety, reliability, and effectiveness, but the proportions were smaller when they were asked if counterfeits were either very or extremely endangering to patient safety: 70.5% for injectables and 59.2% for devices, the investigators said.
Experience with adverse events from counterfeits in patients was reported by 39.7% of respondents to the injectables survey and by 20.1% of those in the device survey, they added.
Majorities in both surveys – 73.7% for injectables and 68.9% for devices – also said that they were either not familiar or only somewhat familiar with the Food and Drug Administration’s regulations on counterfeits. “This is especially problematic considering the potentially severe adverse events and steep punishments,” Dr. Wang and associates wrote.
The authors disclosed that they had no significant interest with commercial supporters. Dr. Wang is now a fellow at the Laser & Skin Surgery Center of New York.
SOURCE: Wang JV et al. Dermatol. Surg. 2020 Oct;46(10):1323-6.
according to the results of two recent surveys of such providers.

“Counterfeit medical devices and injectables may be more prevalent in aesthetic medicine than most practitioners might estimate,” Jordan V. Wang, MD, of Sidney Kimmel Medical College, Philadelphia, and associates wrote in Dermatologic Surgery, even though “the vast majority [believe] that they are inferior and even potentially harmful.”
In one of the online surveys, conducted among members of the American Society of Dermatologic Surgery, 41.1% of the 616 respondents said they had encountered counterfeit injectables, more than half (56.5%) of whom were solicited to buy such products. Just over 10% had purchased counterfeit injectables, although nearly 80% did so unknowingly, the investigators said.
In the second survey, 37.4% of the 765 respondents (members of the ASDS as well as the American Society for Laser Medicine and Surgery) said that they had encountered counterfeit medical devices, and nearly half had been approached to purchase such devices. Of those who were approached, 4.6% had actually purchased a counterfeit, but only 6.1% did so unknowingly, Dr. Wang and associates reported.
In the medical device survey, almost a quarter (24.2%) acknowledged that they know of other providers using them, while 29.3% of those surveyed about injectables know of others who use counterfeits, they said.
Over 90% of practitioners in each survey agreed that counterfeits are worse in terms of safety, reliability, and effectiveness, but the proportions were smaller when they were asked if counterfeits were either very or extremely endangering to patient safety: 70.5% for injectables and 59.2% for devices, the investigators said.
Experience with adverse events from counterfeits in patients was reported by 39.7% of respondents to the injectables survey and by 20.1% of those in the device survey, they added.
Majorities in both surveys – 73.7% for injectables and 68.9% for devices – also said that they were either not familiar or only somewhat familiar with the Food and Drug Administration’s regulations on counterfeits. “This is especially problematic considering the potentially severe adverse events and steep punishments,” Dr. Wang and associates wrote.
The authors disclosed that they had no significant interest with commercial supporters. Dr. Wang is now a fellow at the Laser & Skin Surgery Center of New York.
SOURCE: Wang JV et al. Dermatol. Surg. 2020 Oct;46(10):1323-6.
according to the results of two recent surveys of such providers.

“Counterfeit medical devices and injectables may be more prevalent in aesthetic medicine than most practitioners might estimate,” Jordan V. Wang, MD, of Sidney Kimmel Medical College, Philadelphia, and associates wrote in Dermatologic Surgery, even though “the vast majority [believe] that they are inferior and even potentially harmful.”
In one of the online surveys, conducted among members of the American Society of Dermatologic Surgery, 41.1% of the 616 respondents said they had encountered counterfeit injectables, more than half (56.5%) of whom were solicited to buy such products. Just over 10% had purchased counterfeit injectables, although nearly 80% did so unknowingly, the investigators said.
In the second survey, 37.4% of the 765 respondents (members of the ASDS as well as the American Society for Laser Medicine and Surgery) said that they had encountered counterfeit medical devices, and nearly half had been approached to purchase such devices. Of those who were approached, 4.6% had actually purchased a counterfeit, but only 6.1% did so unknowingly, Dr. Wang and associates reported.
In the medical device survey, almost a quarter (24.2%) acknowledged that they know of other providers using them, while 29.3% of those surveyed about injectables know of others who use counterfeits, they said.
Over 90% of practitioners in each survey agreed that counterfeits are worse in terms of safety, reliability, and effectiveness, but the proportions were smaller when they were asked if counterfeits were either very or extremely endangering to patient safety: 70.5% for injectables and 59.2% for devices, the investigators said.
Experience with adverse events from counterfeits in patients was reported by 39.7% of respondents to the injectables survey and by 20.1% of those in the device survey, they added.
Majorities in both surveys – 73.7% for injectables and 68.9% for devices – also said that they were either not familiar or only somewhat familiar with the Food and Drug Administration’s regulations on counterfeits. “This is especially problematic considering the potentially severe adverse events and steep punishments,” Dr. Wang and associates wrote.
The authors disclosed that they had no significant interest with commercial supporters. Dr. Wang is now a fellow at the Laser & Skin Surgery Center of New York.
SOURCE: Wang JV et al. Dermatol. Surg. 2020 Oct;46(10):1323-6.
FROM DERMATOLOGIC SURGERY
Authors of picosecond laser review predict more widespread use of the technology
Ever since the first picosecond laser hit the market in 2012 as an option for treating unwanted tattoos and pigmented lesions, clinicians have used the technology to safely and effectively treat an expanding range of dermatologic conditions, from Nevus of Ota and melasma to rejuvenation.
. They called for further development of the technology and predicted that application of the devices will become more widespread.
“Future directions may include the development of even shorter pulse durations, improvements in fractionation method and delivery, and exploration of the utility of pulsing other laser wavelengths in the picosecond (or shorter) domain,” first author Douglas C. Wu, MD, PhD, of Cosmetic Laser Dermatology and colleagues wrote in the review. “The introduction of newer devices along with continued improvements in clinical technique and experience will drive the refinement and expansion of this technology.”
The authors evaluated medical literature on the topic published up to March 2020 and classified 78 studies into one of the following categories: discrete pigmented lesions, other nonmelasma pigmented conditions, rejuvenation, melasma, scar revision, and tattoo removal. They assessed the level of evidence for each indication according to modified criteria published by the Oxford Centre of Evidence-Based Medicine and proposed recommendations based on the medical literature in combination with the authors’ collective clinical experience with picosecond laser.
In the category of discrete pigmented lesions, the authors assigned level of evidence 1a to Nevus of Ota and Hori’s macules, level of evidence 2b to solar lentigines and freckles, level of evidence 3c to café au lait macules, and level of evidence 4 to all other benign pigmentary conditions. “Comparative studies utilizing clinical, histological, and microscopic endpoints further suggest that picosecond laser may be safer and more effective than nanosecond laser in some situations, with potentially reduced risk of inducing postinflammatory hyperpigmentation,” the authors wrote. “This increased safety level may be due to the reduction of non-specific photothermal damage of the melanocyte and dermal-epidermal junction,” they noted. They called for more robust clinical comparative data with a focus on shorter pulse durations and refined clinical endpoints “to further distinguish the differences between picosecond and nanosecond laser for the treatment of some benign pigmented lesions.”
Based on seven prospective open-label trials and three split-face comparison trials involving the use of picosecond lasers for photorejuvenation, the authors assigned a level of evidence 2a to this category. “The studies show a high level of safety associated with a moderate level of efficacy,” they wrote. “Indeed, when compared with traditional non-ablative fractional laser, fractionated picosecond laser may have an improved side effect profile without sacrificing treatment efficacy. This could be due to the unique mechanism of action of fractionated picosecond laser, which results in greater confinement of tissue injury to focal and precise points within the epidermis and papillary dermis.”
Clinical data on using picosecond lasers to treat melasma remains “mixed and unclear,” but it may have a role as an adjunctive treatment combined with rigorous photoprotection, topical melanin inhibitors, “and potentially other laser or systemic therapies as dictated by clinical circumstance,” the authors said. They do not recommend the picosecond laser as a monotherapy for melasma, and they assigned a level of evidence 2a to this category.
Although the fractionated picosecond laser is cleared by the Food and Drug Administration for the treatment of acne scars, Dr. Wu and his colleagues noted that rigorous clinical data on using the technology for this indication is limited. “Encouragingly, reports thus far seem to suggest that the risk of post-inflammatory pigmentary alteration is low when using fractionated picosecond laser, which has added significance due to the high prevalence of acne scarring in skin of color,” they wrote. They assigned a level of evidence 2b to this category. Meanwhile, clinical data on the use of picosecond lasers for non-acne scars are limited to cases series and retrospective reviews, reaching evidence level 3c. “Although the level of evidence is weak, there is likely an effective role for fractionated picosecond laser for the improvement of hyperpigmented scars given its more robust track record for the treatment of hyperpigmentation due to other causes such as benign pigmentary conditions and photodamage,” the authors wrote.
The manuscript concludes with a discussion of the picosecond laser’s role in tattoo removal, which represents the oldest and most established dermatologic indication for the technology. “The accumulated scientific and clinical evidence to date concludes that the shorter pulse duration confers a distinct advantage when other laser parameters remain equal,” the authors wrote. “The evidence also suggests that the shorter the pulse gets (within currently commercially available and tested devices), the greater becomes the efficacy for tattoo removal. There is no evidence to suggest that larger tattoo particles are more optimally targeted by longer nanosecond pulses.” They assigned a level of evidence 1a to this category and described using the picosecond laser for tattoo removal of almost any color as “the gold standard.”
In an interview, Arisa Ortiz, MD, described the manuscript as a thorough review of the clinical indications for picosecond lasers. “Overall, the review shows evidence for slightly better improvement of efficacy with picosecond lasers compared to nanosecond lasers,” said Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, who was not involved with the review. “They also show a slightly improved side effect profile with picosecond lasers [and] notably, less risk of postinflammatory hyperpigmentation in darker skin types compared to nanosecond lasers. One issue that was not addressed was the cost of picosecond lasers. The cost of a picosecond lasers remains substantially higher than the cost of a nanosecond laser. I am not sure that this extra cost justifies a slightly improved efficacy or slightly improved side effect profile.”
According to Eric F. Bernstein, MD, director of the Main Line Center for Laser Surgery in Ardmore, Penn., the versatility of picosecond lasers offers an advantage to dermatologists. “Most of them have three wavelengths at least,” said Dr. Bernstein, who was not involved with the systematic review. “That means you can treat skin types I-VI. I was never able to offer much for my patients with skin types V and VI for fractionated rejuvenation and treatment of acne scarring. But now, with these lasers, I have an option for them. That’s a huge advantage.”
He credited laser engineers as “the real heroes” in the success of picosecond lasers in dermatology. “They’re passionate, they’re brilliant, and they’re creative,” Dr. Bernstein said. “They’re the ones that build and produce these devices for multiple manufacturers. In our space, the innovation really comes from industry.”
The review authors and Dr. Ortiz reported having no relevant disclosures. Dr. Bernstein disclosed that he is head of Candela’s medical advisory board.
SOURCE: Wu DC et al. Lasers Surg Med. 2020. doi: 10.1002/lsm.23244.
Ever since the first picosecond laser hit the market in 2012 as an option for treating unwanted tattoos and pigmented lesions, clinicians have used the technology to safely and effectively treat an expanding range of dermatologic conditions, from Nevus of Ota and melasma to rejuvenation.
. They called for further development of the technology and predicted that application of the devices will become more widespread.
“Future directions may include the development of even shorter pulse durations, improvements in fractionation method and delivery, and exploration of the utility of pulsing other laser wavelengths in the picosecond (or shorter) domain,” first author Douglas C. Wu, MD, PhD, of Cosmetic Laser Dermatology and colleagues wrote in the review. “The introduction of newer devices along with continued improvements in clinical technique and experience will drive the refinement and expansion of this technology.”
The authors evaluated medical literature on the topic published up to March 2020 and classified 78 studies into one of the following categories: discrete pigmented lesions, other nonmelasma pigmented conditions, rejuvenation, melasma, scar revision, and tattoo removal. They assessed the level of evidence for each indication according to modified criteria published by the Oxford Centre of Evidence-Based Medicine and proposed recommendations based on the medical literature in combination with the authors’ collective clinical experience with picosecond laser.
In the category of discrete pigmented lesions, the authors assigned level of evidence 1a to Nevus of Ota and Hori’s macules, level of evidence 2b to solar lentigines and freckles, level of evidence 3c to café au lait macules, and level of evidence 4 to all other benign pigmentary conditions. “Comparative studies utilizing clinical, histological, and microscopic endpoints further suggest that picosecond laser may be safer and more effective than nanosecond laser in some situations, with potentially reduced risk of inducing postinflammatory hyperpigmentation,” the authors wrote. “This increased safety level may be due to the reduction of non-specific photothermal damage of the melanocyte and dermal-epidermal junction,” they noted. They called for more robust clinical comparative data with a focus on shorter pulse durations and refined clinical endpoints “to further distinguish the differences between picosecond and nanosecond laser for the treatment of some benign pigmented lesions.”
Based on seven prospective open-label trials and three split-face comparison trials involving the use of picosecond lasers for photorejuvenation, the authors assigned a level of evidence 2a to this category. “The studies show a high level of safety associated with a moderate level of efficacy,” they wrote. “Indeed, when compared with traditional non-ablative fractional laser, fractionated picosecond laser may have an improved side effect profile without sacrificing treatment efficacy. This could be due to the unique mechanism of action of fractionated picosecond laser, which results in greater confinement of tissue injury to focal and precise points within the epidermis and papillary dermis.”
Clinical data on using picosecond lasers to treat melasma remains “mixed and unclear,” but it may have a role as an adjunctive treatment combined with rigorous photoprotection, topical melanin inhibitors, “and potentially other laser or systemic therapies as dictated by clinical circumstance,” the authors said. They do not recommend the picosecond laser as a monotherapy for melasma, and they assigned a level of evidence 2a to this category.
Although the fractionated picosecond laser is cleared by the Food and Drug Administration for the treatment of acne scars, Dr. Wu and his colleagues noted that rigorous clinical data on using the technology for this indication is limited. “Encouragingly, reports thus far seem to suggest that the risk of post-inflammatory pigmentary alteration is low when using fractionated picosecond laser, which has added significance due to the high prevalence of acne scarring in skin of color,” they wrote. They assigned a level of evidence 2b to this category. Meanwhile, clinical data on the use of picosecond lasers for non-acne scars are limited to cases series and retrospective reviews, reaching evidence level 3c. “Although the level of evidence is weak, there is likely an effective role for fractionated picosecond laser for the improvement of hyperpigmented scars given its more robust track record for the treatment of hyperpigmentation due to other causes such as benign pigmentary conditions and photodamage,” the authors wrote.
The manuscript concludes with a discussion of the picosecond laser’s role in tattoo removal, which represents the oldest and most established dermatologic indication for the technology. “The accumulated scientific and clinical evidence to date concludes that the shorter pulse duration confers a distinct advantage when other laser parameters remain equal,” the authors wrote. “The evidence also suggests that the shorter the pulse gets (within currently commercially available and tested devices), the greater becomes the efficacy for tattoo removal. There is no evidence to suggest that larger tattoo particles are more optimally targeted by longer nanosecond pulses.” They assigned a level of evidence 1a to this category and described using the picosecond laser for tattoo removal of almost any color as “the gold standard.”
In an interview, Arisa Ortiz, MD, described the manuscript as a thorough review of the clinical indications for picosecond lasers. “Overall, the review shows evidence for slightly better improvement of efficacy with picosecond lasers compared to nanosecond lasers,” said Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, who was not involved with the review. “They also show a slightly improved side effect profile with picosecond lasers [and] notably, less risk of postinflammatory hyperpigmentation in darker skin types compared to nanosecond lasers. One issue that was not addressed was the cost of picosecond lasers. The cost of a picosecond lasers remains substantially higher than the cost of a nanosecond laser. I am not sure that this extra cost justifies a slightly improved efficacy or slightly improved side effect profile.”
According to Eric F. Bernstein, MD, director of the Main Line Center for Laser Surgery in Ardmore, Penn., the versatility of picosecond lasers offers an advantage to dermatologists. “Most of them have three wavelengths at least,” said Dr. Bernstein, who was not involved with the systematic review. “That means you can treat skin types I-VI. I was never able to offer much for my patients with skin types V and VI for fractionated rejuvenation and treatment of acne scarring. But now, with these lasers, I have an option for them. That’s a huge advantage.”
He credited laser engineers as “the real heroes” in the success of picosecond lasers in dermatology. “They’re passionate, they’re brilliant, and they’re creative,” Dr. Bernstein said. “They’re the ones that build and produce these devices for multiple manufacturers. In our space, the innovation really comes from industry.”
The review authors and Dr. Ortiz reported having no relevant disclosures. Dr. Bernstein disclosed that he is head of Candela’s medical advisory board.
SOURCE: Wu DC et al. Lasers Surg Med. 2020. doi: 10.1002/lsm.23244.
Ever since the first picosecond laser hit the market in 2012 as an option for treating unwanted tattoos and pigmented lesions, clinicians have used the technology to safely and effectively treat an expanding range of dermatologic conditions, from Nevus of Ota and melasma to rejuvenation.
. They called for further development of the technology and predicted that application of the devices will become more widespread.
“Future directions may include the development of even shorter pulse durations, improvements in fractionation method and delivery, and exploration of the utility of pulsing other laser wavelengths in the picosecond (or shorter) domain,” first author Douglas C. Wu, MD, PhD, of Cosmetic Laser Dermatology and colleagues wrote in the review. “The introduction of newer devices along with continued improvements in clinical technique and experience will drive the refinement and expansion of this technology.”
The authors evaluated medical literature on the topic published up to March 2020 and classified 78 studies into one of the following categories: discrete pigmented lesions, other nonmelasma pigmented conditions, rejuvenation, melasma, scar revision, and tattoo removal. They assessed the level of evidence for each indication according to modified criteria published by the Oxford Centre of Evidence-Based Medicine and proposed recommendations based on the medical literature in combination with the authors’ collective clinical experience with picosecond laser.
In the category of discrete pigmented lesions, the authors assigned level of evidence 1a to Nevus of Ota and Hori’s macules, level of evidence 2b to solar lentigines and freckles, level of evidence 3c to café au lait macules, and level of evidence 4 to all other benign pigmentary conditions. “Comparative studies utilizing clinical, histological, and microscopic endpoints further suggest that picosecond laser may be safer and more effective than nanosecond laser in some situations, with potentially reduced risk of inducing postinflammatory hyperpigmentation,” the authors wrote. “This increased safety level may be due to the reduction of non-specific photothermal damage of the melanocyte and dermal-epidermal junction,” they noted. They called for more robust clinical comparative data with a focus on shorter pulse durations and refined clinical endpoints “to further distinguish the differences between picosecond and nanosecond laser for the treatment of some benign pigmented lesions.”
Based on seven prospective open-label trials and three split-face comparison trials involving the use of picosecond lasers for photorejuvenation, the authors assigned a level of evidence 2a to this category. “The studies show a high level of safety associated with a moderate level of efficacy,” they wrote. “Indeed, when compared with traditional non-ablative fractional laser, fractionated picosecond laser may have an improved side effect profile without sacrificing treatment efficacy. This could be due to the unique mechanism of action of fractionated picosecond laser, which results in greater confinement of tissue injury to focal and precise points within the epidermis and papillary dermis.”
Clinical data on using picosecond lasers to treat melasma remains “mixed and unclear,” but it may have a role as an adjunctive treatment combined with rigorous photoprotection, topical melanin inhibitors, “and potentially other laser or systemic therapies as dictated by clinical circumstance,” the authors said. They do not recommend the picosecond laser as a monotherapy for melasma, and they assigned a level of evidence 2a to this category.
Although the fractionated picosecond laser is cleared by the Food and Drug Administration for the treatment of acne scars, Dr. Wu and his colleagues noted that rigorous clinical data on using the technology for this indication is limited. “Encouragingly, reports thus far seem to suggest that the risk of post-inflammatory pigmentary alteration is low when using fractionated picosecond laser, which has added significance due to the high prevalence of acne scarring in skin of color,” they wrote. They assigned a level of evidence 2b to this category. Meanwhile, clinical data on the use of picosecond lasers for non-acne scars are limited to cases series and retrospective reviews, reaching evidence level 3c. “Although the level of evidence is weak, there is likely an effective role for fractionated picosecond laser for the improvement of hyperpigmented scars given its more robust track record for the treatment of hyperpigmentation due to other causes such as benign pigmentary conditions and photodamage,” the authors wrote.
The manuscript concludes with a discussion of the picosecond laser’s role in tattoo removal, which represents the oldest and most established dermatologic indication for the technology. “The accumulated scientific and clinical evidence to date concludes that the shorter pulse duration confers a distinct advantage when other laser parameters remain equal,” the authors wrote. “The evidence also suggests that the shorter the pulse gets (within currently commercially available and tested devices), the greater becomes the efficacy for tattoo removal. There is no evidence to suggest that larger tattoo particles are more optimally targeted by longer nanosecond pulses.” They assigned a level of evidence 1a to this category and described using the picosecond laser for tattoo removal of almost any color as “the gold standard.”
In an interview, Arisa Ortiz, MD, described the manuscript as a thorough review of the clinical indications for picosecond lasers. “Overall, the review shows evidence for slightly better improvement of efficacy with picosecond lasers compared to nanosecond lasers,” said Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, who was not involved with the review. “They also show a slightly improved side effect profile with picosecond lasers [and] notably, less risk of postinflammatory hyperpigmentation in darker skin types compared to nanosecond lasers. One issue that was not addressed was the cost of picosecond lasers. The cost of a picosecond lasers remains substantially higher than the cost of a nanosecond laser. I am not sure that this extra cost justifies a slightly improved efficacy or slightly improved side effect profile.”
According to Eric F. Bernstein, MD, director of the Main Line Center for Laser Surgery in Ardmore, Penn., the versatility of picosecond lasers offers an advantage to dermatologists. “Most of them have three wavelengths at least,” said Dr. Bernstein, who was not involved with the systematic review. “That means you can treat skin types I-VI. I was never able to offer much for my patients with skin types V and VI for fractionated rejuvenation and treatment of acne scarring. But now, with these lasers, I have an option for them. That’s a huge advantage.”
He credited laser engineers as “the real heroes” in the success of picosecond lasers in dermatology. “They’re passionate, they’re brilliant, and they’re creative,” Dr. Bernstein said. “They’re the ones that build and produce these devices for multiple manufacturers. In our space, the innovation really comes from industry.”
The review authors and Dr. Ortiz reported having no relevant disclosures. Dr. Bernstein disclosed that he is head of Candela’s medical advisory board.
SOURCE: Wu DC et al. Lasers Surg Med. 2020. doi: 10.1002/lsm.23244.
FROM LASERS IN SURGERY AND MEDICINE
Experts recommend slow, steady approach to reopening laser and cosmetic surgery practices
“People talk about reinventing the wheel,” Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., said during an hour-long webinar on May 5 sponsored by the American Society for Laser Medicine and Surgery. “In this case, we’re inventing the wheel; no one’s ever done this before – not in our lifetimes. The last pandemic was over 100 years ago, when there wasn’t aesthetic medicine.”
Dr. Dover joined a panel of four other experts from around the country to discuss how to reopen practices safely and effectively. Paul M. Friedman, MD, director of the Houston Cosmetic Dermatology & Laser Center, moderated the event.
In Florida, which reopened certain businesses on May 4, 2020, Jill S. Waibel, MD, plans to start at 25% capacity at Miami Dermatology and Laser Institute, and build from there. “We’re trying to take care of skin cancer patients first,” said Dr. Waibel, a dermatologist who owns the practice. “Then we’re going to start doing less aggressive cosmetic procedures like injectables, nonablative procedures. We’ll move into the more aggressive procedures as we ease back into it. We really want to see what’s going to happen 2-3 weeks down the line now that things are starting to open up.”
In Maryland, where state officials announced on May 6 that guidelines would be issued to allow for nonmedical procedures, Elizabeth L. Tanzi, MD, founder and director of Capital Laser & Skin Care in Chevy Chase, expects things to “look very different” once her practice reopens. “We are taking it very slowly,” she said. “Teledermatology for acne and other follow-ups is not something we did before, but it is certainly something that we’ll continue.”
The way she sees it, having the proper personal protective equipment is a key part of any reopening discussion. “I am not going near anyone’s face without an N95 mask that fits well, and without a face shield,” she said. “If you’re delegating these procedures to people that you don’t trust to be wearing the PPE correctly, then you shouldn’t be delegating them, because a key is the PPE. You have to assume that everyone has the virus at every time.”
In Ardmore, Pa., the Main Line Center for Laser Surgery remains closed because of current state regulations. When practice director Eric F. Bernstein, MD, gets the green light to reopen, patients will undergo a consultation by phone or videoconference and pay their bill before they set foot in the office. “We’re on the second floor, so patients can take a stairwell and avoid the elevator,” Dr. Bernstein said. “They’ll come in, not check in at the desk; go right to the room. There will be one treater and one assistant. If the patient doesn’t come in with a mask, we’ll supply one. It’s going to be a very different process. People are setting their hours longer because they’re going to be seeing fewer people. There will be no sitting in the waiting room.”
In the COVID-19 epicenter, Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, has been performing Mohs procedures and treating children with vascular malformations, but everything else is on hold. “Once the governor [Andrew Cuomo] lifts the stay-at-home restrictions, we’ll ease into things,” he said. “The issue of performing more invasive procedures – like ablative fractional resurfacing – is something that we are concerned about. I’m concerned about any laser that has environmental plume. For example, with our tattoo-removal procedures, I intend to treat every patient through a gel for the short term, and perhaps even for the long term. One can do that safely, and that eliminates the plume altogether.”
At the center, Dr. Geronemus added, “we do a fair amount of ablative fractional resurfacing and some fully ablative resurfacing. I intend to use large facial shields with these patients. We do use vacuum in each room as it stands right now, not only with electrosurgery, but we’ll be adding that to laser procedures as well. That will be helpful.”
In Chestnut Hill, Mass., Dr. Dover and his colleagues plan to practice what he termed “universal COVID precaution” by wearing a face mask, goggles, or a face shield, gloves, and protective clothing when necessary. “We are not going to do any ablative procedures, no procedures with plume, and we’re going to try and eliminate risk as much as we can,” he said. “We will have no waiting room; the patients will walk right to an exam room. They’ll be prescreened on the phone. The only thing they’ll have done when they first come in is to have their temperature taken, and they’ll be checked in and out with the doctor and the nurse in the room, and that’s it. There will be no other extraneous people to help to eliminate risk. We’re cutting our schedules down by 75% so that we can socially distance within our practice,” Dr. Dover said.
Dr. Dover served as lead author on “A path to resume aesthetic care: Executive summary of Project AesCert guidance supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” which was published online in Facial Plastic Surgery & Aesthetic Medicine (2002 May 5. doi: 10.1089/fpsam.2020.0239). His coauthors included a facial plastic surgeon and three infectious disease experts.
Dr. Dover said, “We took the advice of these experts in infectious diseases, who said, ‘we don’t know all the right answers [to resuming aesthetic care]. We can mitigate risk, but we cannot eliminate risk. You have to treat every patient in your office as if they’re COVID-19 positive. If you do that, you’ll have a safe office. It’ll be the safest place in your world, safer than a grocery store, where you have no idea who you’re standing beside.’ ”
“The problem with this virus, compared to, say, SARS-CoV-1, is that these patients are positive and shedding virus 2-3 days before they get a fever,” he added. “With SARS-CoV-1, they had a fever first and then they shed virus. What I learned was, treat everybody with universal precautions.” The document includes tips for communicating with patient about expectations for office visits, clinic schedule management, cleaning procedures, PPE, treatment room set-up, and employee health screening and training.
During the webinar, an ASLMS member posed a question to the panelists about their comfort level in performing mechanical microneedling and radiofrequency (RF) microneedling procedures as aesthetic practices begin to reopen. “Generally, there’s no plume with microneedling with or without RF,” Dr. Geronemus said. “Depending upon the procedure that you’re doing, some of the microneedling procedures are very bloody; that may carry a risk unto itself. Other procedures where you’re using a thermal component have less bleeding. I’m more inclined to proceed with an RF with microneedling procedure and less inclined to proceed with a bloody, more aggressive microneedling procedure.”
Dr. Waibel emphasized the importance of disinfecting the microneedling device between uses. “If you have disposable needle cartridges, I think it’s a lot safer than if you have to clean [them],” she said. “We know that COVID-19 can live up to 3 hours, at least in a lab scenario, so you don’t want to transmit it from patient to patient. If someone has COVID-19 on their nose, and you microneedle over it, and that’s not completely disinfected, you could spread it to the next patient. We have really amped up our cleaning in between rooms. We have a whole crew that cleans every surface with [disinfectant wipes] and 90% alcohol.”
With reported shortages of N95 in many health care settings, some panelists said that they plan to reuse masks until the supply chain improves. Dr. Dover said that one option is to “use a mask, label it, number it, drop it in into a paper bag or into a [sealed plastic food container] upside down without touching the front of it,” he said. “If it sits for a week and you see patients 5 days a week, that mask will be dried out and highly effective a week later. That’s what we’re going to do until there is a big supply of them.”
The pandemic has also thrown a monkey wrench into aesthetic and medical dermatology clinical research efforts. According to Dr. Dover, many aesthetic studies have been shut down, “and most companies are giving us little guidance,” he said. “As they figure things out, they ask us to do things over and over again. So, I hope that clinical research will improve because of COVID-19 in the long term, but in the short term, it’s been a bit of a nightmare.”
Dr. Geronemus added that, in order to fulfill criteria for most studies, clinicians are required to see patients in a certain number of days. “We’re out of protocol in many different studies, so we’re requesting that protocols be amended and that the FDA [Food and Drug Administration] and the sponsors will consider opportunities to make those changes,” he said. “We’ll do as much as we can virtually, but if you’re studying an acne scar, you really need to see the patient [in person].”
Strict social distancing measures are also disrupting agreements that dermatologists may have had with trainees and fellows before the pandemic hit. “We’ve had to send letters and e-mails to people who were planning visits and preceptorships,” Dr. Dover said. “Even with our fellows, we’re going to have to figure out a way to practice so as not to complicate the issue in the room. The more people in the room, the more risk there is for transmitting disease. It’s really an issue.”
Dr. Tanzi limits everyone in the room during procedures. “We’re screening patients beforehand and telling them no family members, unless there’s a disability; no kids, unless it’s a kid coming in for acne treatment and they have to bring their parent; no drivers – they can wait outside,” she said.
Another ASLMS member asked the panelists if they plan to incorporate an informed consent form for COVID-19 risk into their practices, similar to the one developed by the American Society of Plastic Surgeons. “That’s a tough one,” Dr. Waibel said. “Before patients enter our practice, we take their temperature and ask them several COVID-related symptoms and contact questions – which they validate as true.”
Dr. Geronemus said that he will consider the idea. “The downside is logistical,” he said. “Patients sign so many forms already; they’re complaining that it takes so long to get into see me, and my hand is tired from signing so many forms.’”
Dr. Dover said that he and his colleagues are planning to use a COVID-19 risk consent form. “I’d err on the side of yes rather than on the side of no, because you’re better off overdoing it than underdoing it,” he said. “This is not the time for shortcuts.”
[email protected]

“People talk about reinventing the wheel,” Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., said during an hour-long webinar on May 5 sponsored by the American Society for Laser Medicine and Surgery. “In this case, we’re inventing the wheel; no one’s ever done this before – not in our lifetimes. The last pandemic was over 100 years ago, when there wasn’t aesthetic medicine.”
Dr. Dover joined a panel of four other experts from around the country to discuss how to reopen practices safely and effectively. Paul M. Friedman, MD, director of the Houston Cosmetic Dermatology & Laser Center, moderated the event.
In Florida, which reopened certain businesses on May 4, 2020, Jill S. Waibel, MD, plans to start at 25% capacity at Miami Dermatology and Laser Institute, and build from there. “We’re trying to take care of skin cancer patients first,” said Dr. Waibel, a dermatologist who owns the practice. “Then we’re going to start doing less aggressive cosmetic procedures like injectables, nonablative procedures. We’ll move into the more aggressive procedures as we ease back into it. We really want to see what’s going to happen 2-3 weeks down the line now that things are starting to open up.”
In Maryland, where state officials announced on May 6 that guidelines would be issued to allow for nonmedical procedures, Elizabeth L. Tanzi, MD, founder and director of Capital Laser & Skin Care in Chevy Chase, expects things to “look very different” once her practice reopens. “We are taking it very slowly,” she said. “Teledermatology for acne and other follow-ups is not something we did before, but it is certainly something that we’ll continue.”
The way she sees it, having the proper personal protective equipment is a key part of any reopening discussion. “I am not going near anyone’s face without an N95 mask that fits well, and without a face shield,” she said. “If you’re delegating these procedures to people that you don’t trust to be wearing the PPE correctly, then you shouldn’t be delegating them, because a key is the PPE. You have to assume that everyone has the virus at every time.”
In Ardmore, Pa., the Main Line Center for Laser Surgery remains closed because of current state regulations. When practice director Eric F. Bernstein, MD, gets the green light to reopen, patients will undergo a consultation by phone or videoconference and pay their bill before they set foot in the office. “We’re on the second floor, so patients can take a stairwell and avoid the elevator,” Dr. Bernstein said. “They’ll come in, not check in at the desk; go right to the room. There will be one treater and one assistant. If the patient doesn’t come in with a mask, we’ll supply one. It’s going to be a very different process. People are setting their hours longer because they’re going to be seeing fewer people. There will be no sitting in the waiting room.”
In the COVID-19 epicenter, Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, has been performing Mohs procedures and treating children with vascular malformations, but everything else is on hold. “Once the governor [Andrew Cuomo] lifts the stay-at-home restrictions, we’ll ease into things,” he said. “The issue of performing more invasive procedures – like ablative fractional resurfacing – is something that we are concerned about. I’m concerned about any laser that has environmental plume. For example, with our tattoo-removal procedures, I intend to treat every patient through a gel for the short term, and perhaps even for the long term. One can do that safely, and that eliminates the plume altogether.”
At the center, Dr. Geronemus added, “we do a fair amount of ablative fractional resurfacing and some fully ablative resurfacing. I intend to use large facial shields with these patients. We do use vacuum in each room as it stands right now, not only with electrosurgery, but we’ll be adding that to laser procedures as well. That will be helpful.”
In Chestnut Hill, Mass., Dr. Dover and his colleagues plan to practice what he termed “universal COVID precaution” by wearing a face mask, goggles, or a face shield, gloves, and protective clothing when necessary. “We are not going to do any ablative procedures, no procedures with plume, and we’re going to try and eliminate risk as much as we can,” he said. “We will have no waiting room; the patients will walk right to an exam room. They’ll be prescreened on the phone. The only thing they’ll have done when they first come in is to have their temperature taken, and they’ll be checked in and out with the doctor and the nurse in the room, and that’s it. There will be no other extraneous people to help to eliminate risk. We’re cutting our schedules down by 75% so that we can socially distance within our practice,” Dr. Dover said.
Dr. Dover served as lead author on “A path to resume aesthetic care: Executive summary of Project AesCert guidance supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” which was published online in Facial Plastic Surgery & Aesthetic Medicine (2002 May 5. doi: 10.1089/fpsam.2020.0239). His coauthors included a facial plastic surgeon and three infectious disease experts.
Dr. Dover said, “We took the advice of these experts in infectious diseases, who said, ‘we don’t know all the right answers [to resuming aesthetic care]. We can mitigate risk, but we cannot eliminate risk. You have to treat every patient in your office as if they’re COVID-19 positive. If you do that, you’ll have a safe office. It’ll be the safest place in your world, safer than a grocery store, where you have no idea who you’re standing beside.’ ”
“The problem with this virus, compared to, say, SARS-CoV-1, is that these patients are positive and shedding virus 2-3 days before they get a fever,” he added. “With SARS-CoV-1, they had a fever first and then they shed virus. What I learned was, treat everybody with universal precautions.” The document includes tips for communicating with patient about expectations for office visits, clinic schedule management, cleaning procedures, PPE, treatment room set-up, and employee health screening and training.
During the webinar, an ASLMS member posed a question to the panelists about their comfort level in performing mechanical microneedling and radiofrequency (RF) microneedling procedures as aesthetic practices begin to reopen. “Generally, there’s no plume with microneedling with or without RF,” Dr. Geronemus said. “Depending upon the procedure that you’re doing, some of the microneedling procedures are very bloody; that may carry a risk unto itself. Other procedures where you’re using a thermal component have less bleeding. I’m more inclined to proceed with an RF with microneedling procedure and less inclined to proceed with a bloody, more aggressive microneedling procedure.”
Dr. Waibel emphasized the importance of disinfecting the microneedling device between uses. “If you have disposable needle cartridges, I think it’s a lot safer than if you have to clean [them],” she said. “We know that COVID-19 can live up to 3 hours, at least in a lab scenario, so you don’t want to transmit it from patient to patient. If someone has COVID-19 on their nose, and you microneedle over it, and that’s not completely disinfected, you could spread it to the next patient. We have really amped up our cleaning in between rooms. We have a whole crew that cleans every surface with [disinfectant wipes] and 90% alcohol.”
With reported shortages of N95 in many health care settings, some panelists said that they plan to reuse masks until the supply chain improves. Dr. Dover said that one option is to “use a mask, label it, number it, drop it in into a paper bag or into a [sealed plastic food container] upside down without touching the front of it,” he said. “If it sits for a week and you see patients 5 days a week, that mask will be dried out and highly effective a week later. That’s what we’re going to do until there is a big supply of them.”
The pandemic has also thrown a monkey wrench into aesthetic and medical dermatology clinical research efforts. According to Dr. Dover, many aesthetic studies have been shut down, “and most companies are giving us little guidance,” he said. “As they figure things out, they ask us to do things over and over again. So, I hope that clinical research will improve because of COVID-19 in the long term, but in the short term, it’s been a bit of a nightmare.”
Dr. Geronemus added that, in order to fulfill criteria for most studies, clinicians are required to see patients in a certain number of days. “We’re out of protocol in many different studies, so we’re requesting that protocols be amended and that the FDA [Food and Drug Administration] and the sponsors will consider opportunities to make those changes,” he said. “We’ll do as much as we can virtually, but if you’re studying an acne scar, you really need to see the patient [in person].”
Strict social distancing measures are also disrupting agreements that dermatologists may have had with trainees and fellows before the pandemic hit. “We’ve had to send letters and e-mails to people who were planning visits and preceptorships,” Dr. Dover said. “Even with our fellows, we’re going to have to figure out a way to practice so as not to complicate the issue in the room. The more people in the room, the more risk there is for transmitting disease. It’s really an issue.”
Dr. Tanzi limits everyone in the room during procedures. “We’re screening patients beforehand and telling them no family members, unless there’s a disability; no kids, unless it’s a kid coming in for acne treatment and they have to bring their parent; no drivers – they can wait outside,” she said.
Another ASLMS member asked the panelists if they plan to incorporate an informed consent form for COVID-19 risk into their practices, similar to the one developed by the American Society of Plastic Surgeons. “That’s a tough one,” Dr. Waibel said. “Before patients enter our practice, we take their temperature and ask them several COVID-related symptoms and contact questions – which they validate as true.”
Dr. Geronemus said that he will consider the idea. “The downside is logistical,” he said. “Patients sign so many forms already; they’re complaining that it takes so long to get into see me, and my hand is tired from signing so many forms.’”
Dr. Dover said that he and his colleagues are planning to use a COVID-19 risk consent form. “I’d err on the side of yes rather than on the side of no, because you’re better off overdoing it than underdoing it,” he said. “This is not the time for shortcuts.”
[email protected]

“People talk about reinventing the wheel,” Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., said during an hour-long webinar on May 5 sponsored by the American Society for Laser Medicine and Surgery. “In this case, we’re inventing the wheel; no one’s ever done this before – not in our lifetimes. The last pandemic was over 100 years ago, when there wasn’t aesthetic medicine.”
Dr. Dover joined a panel of four other experts from around the country to discuss how to reopen practices safely and effectively. Paul M. Friedman, MD, director of the Houston Cosmetic Dermatology & Laser Center, moderated the event.
In Florida, which reopened certain businesses on May 4, 2020, Jill S. Waibel, MD, plans to start at 25% capacity at Miami Dermatology and Laser Institute, and build from there. “We’re trying to take care of skin cancer patients first,” said Dr. Waibel, a dermatologist who owns the practice. “Then we’re going to start doing less aggressive cosmetic procedures like injectables, nonablative procedures. We’ll move into the more aggressive procedures as we ease back into it. We really want to see what’s going to happen 2-3 weeks down the line now that things are starting to open up.”
In Maryland, where state officials announced on May 6 that guidelines would be issued to allow for nonmedical procedures, Elizabeth L. Tanzi, MD, founder and director of Capital Laser & Skin Care in Chevy Chase, expects things to “look very different” once her practice reopens. “We are taking it very slowly,” she said. “Teledermatology for acne and other follow-ups is not something we did before, but it is certainly something that we’ll continue.”
The way she sees it, having the proper personal protective equipment is a key part of any reopening discussion. “I am not going near anyone’s face without an N95 mask that fits well, and without a face shield,” she said. “If you’re delegating these procedures to people that you don’t trust to be wearing the PPE correctly, then you shouldn’t be delegating them, because a key is the PPE. You have to assume that everyone has the virus at every time.”
In Ardmore, Pa., the Main Line Center for Laser Surgery remains closed because of current state regulations. When practice director Eric F. Bernstein, MD, gets the green light to reopen, patients will undergo a consultation by phone or videoconference and pay their bill before they set foot in the office. “We’re on the second floor, so patients can take a stairwell and avoid the elevator,” Dr. Bernstein said. “They’ll come in, not check in at the desk; go right to the room. There will be one treater and one assistant. If the patient doesn’t come in with a mask, we’ll supply one. It’s going to be a very different process. People are setting their hours longer because they’re going to be seeing fewer people. There will be no sitting in the waiting room.”
In the COVID-19 epicenter, Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, has been performing Mohs procedures and treating children with vascular malformations, but everything else is on hold. “Once the governor [Andrew Cuomo] lifts the stay-at-home restrictions, we’ll ease into things,” he said. “The issue of performing more invasive procedures – like ablative fractional resurfacing – is something that we are concerned about. I’m concerned about any laser that has environmental plume. For example, with our tattoo-removal procedures, I intend to treat every patient through a gel for the short term, and perhaps even for the long term. One can do that safely, and that eliminates the plume altogether.”
At the center, Dr. Geronemus added, “we do a fair amount of ablative fractional resurfacing and some fully ablative resurfacing. I intend to use large facial shields with these patients. We do use vacuum in each room as it stands right now, not only with electrosurgery, but we’ll be adding that to laser procedures as well. That will be helpful.”
In Chestnut Hill, Mass., Dr. Dover and his colleagues plan to practice what he termed “universal COVID precaution” by wearing a face mask, goggles, or a face shield, gloves, and protective clothing when necessary. “We are not going to do any ablative procedures, no procedures with plume, and we’re going to try and eliminate risk as much as we can,” he said. “We will have no waiting room; the patients will walk right to an exam room. They’ll be prescreened on the phone. The only thing they’ll have done when they first come in is to have their temperature taken, and they’ll be checked in and out with the doctor and the nurse in the room, and that’s it. There will be no other extraneous people to help to eliminate risk. We’re cutting our schedules down by 75% so that we can socially distance within our practice,” Dr. Dover said.
Dr. Dover served as lead author on “A path to resume aesthetic care: Executive summary of Project AesCert guidance supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” which was published online in Facial Plastic Surgery & Aesthetic Medicine (2002 May 5. doi: 10.1089/fpsam.2020.0239). His coauthors included a facial plastic surgeon and three infectious disease experts.
Dr. Dover said, “We took the advice of these experts in infectious diseases, who said, ‘we don’t know all the right answers [to resuming aesthetic care]. We can mitigate risk, but we cannot eliminate risk. You have to treat every patient in your office as if they’re COVID-19 positive. If you do that, you’ll have a safe office. It’ll be the safest place in your world, safer than a grocery store, where you have no idea who you’re standing beside.’ ”
“The problem with this virus, compared to, say, SARS-CoV-1, is that these patients are positive and shedding virus 2-3 days before they get a fever,” he added. “With SARS-CoV-1, they had a fever first and then they shed virus. What I learned was, treat everybody with universal precautions.” The document includes tips for communicating with patient about expectations for office visits, clinic schedule management, cleaning procedures, PPE, treatment room set-up, and employee health screening and training.
During the webinar, an ASLMS member posed a question to the panelists about their comfort level in performing mechanical microneedling and radiofrequency (RF) microneedling procedures as aesthetic practices begin to reopen. “Generally, there’s no plume with microneedling with or without RF,” Dr. Geronemus said. “Depending upon the procedure that you’re doing, some of the microneedling procedures are very bloody; that may carry a risk unto itself. Other procedures where you’re using a thermal component have less bleeding. I’m more inclined to proceed with an RF with microneedling procedure and less inclined to proceed with a bloody, more aggressive microneedling procedure.”
Dr. Waibel emphasized the importance of disinfecting the microneedling device between uses. “If you have disposable needle cartridges, I think it’s a lot safer than if you have to clean [them],” she said. “We know that COVID-19 can live up to 3 hours, at least in a lab scenario, so you don’t want to transmit it from patient to patient. If someone has COVID-19 on their nose, and you microneedle over it, and that’s not completely disinfected, you could spread it to the next patient. We have really amped up our cleaning in between rooms. We have a whole crew that cleans every surface with [disinfectant wipes] and 90% alcohol.”
With reported shortages of N95 in many health care settings, some panelists said that they plan to reuse masks until the supply chain improves. Dr. Dover said that one option is to “use a mask, label it, number it, drop it in into a paper bag or into a [sealed plastic food container] upside down without touching the front of it,” he said. “If it sits for a week and you see patients 5 days a week, that mask will be dried out and highly effective a week later. That’s what we’re going to do until there is a big supply of them.”
The pandemic has also thrown a monkey wrench into aesthetic and medical dermatology clinical research efforts. According to Dr. Dover, many aesthetic studies have been shut down, “and most companies are giving us little guidance,” he said. “As they figure things out, they ask us to do things over and over again. So, I hope that clinical research will improve because of COVID-19 in the long term, but in the short term, it’s been a bit of a nightmare.”
Dr. Geronemus added that, in order to fulfill criteria for most studies, clinicians are required to see patients in a certain number of days. “We’re out of protocol in many different studies, so we’re requesting that protocols be amended and that the FDA [Food and Drug Administration] and the sponsors will consider opportunities to make those changes,” he said. “We’ll do as much as we can virtually, but if you’re studying an acne scar, you really need to see the patient [in person].”
Strict social distancing measures are also disrupting agreements that dermatologists may have had with trainees and fellows before the pandemic hit. “We’ve had to send letters and e-mails to people who were planning visits and preceptorships,” Dr. Dover said. “Even with our fellows, we’re going to have to figure out a way to practice so as not to complicate the issue in the room. The more people in the room, the more risk there is for transmitting disease. It’s really an issue.”
Dr. Tanzi limits everyone in the room during procedures. “We’re screening patients beforehand and telling them no family members, unless there’s a disability; no kids, unless it’s a kid coming in for acne treatment and they have to bring their parent; no drivers – they can wait outside,” she said.
Another ASLMS member asked the panelists if they plan to incorporate an informed consent form for COVID-19 risk into their practices, similar to the one developed by the American Society of Plastic Surgeons. “That’s a tough one,” Dr. Waibel said. “Before patients enter our practice, we take their temperature and ask them several COVID-related symptoms and contact questions – which they validate as true.”
Dr. Geronemus said that he will consider the idea. “The downside is logistical,” he said. “Patients sign so many forms already; they’re complaining that it takes so long to get into see me, and my hand is tired from signing so many forms.’”
Dr. Dover said that he and his colleagues are planning to use a COVID-19 risk consent form. “I’d err on the side of yes rather than on the side of no, because you’re better off overdoing it than underdoing it,” he said. “This is not the time for shortcuts.”
[email protected]
Social media may negatively influence acne treatment
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
FROM PEDIATRIC DERMATOLOGY
Online resources influencing cosmetic treatment choices
Online resources are affecting most consumers’ selections of cosmetic providers, and social media are now a top-three influence on cosmetic procedure choices and skin care purchases, according to a new survey from the American Society for Dermatologic Surgery.
Almost 70% of respondents said that their use of rate and review websites had an impact on the choice of provider for cosmetic procedures: WebMD was the site most often visited, followed by Facebook, physician websites, and Yelp, the ASDS said based on its annual consumer survey.
For 43% of consumers, the decision to schedule an appointment was influenced by a provider’s social media presence, and 41% of patients said that they follow their current or potential provider on social media, the ASDS said.
“Online resources and social media platforms are clearly influencing consumers’ behavior and perception of skin health,” ASDS President Murad Alam, MD, MBA, chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, Chicago, said in a written statement.
Dermatologists, however, remain the leading influence on the decision to have a cosmetic procedure – named as a resource by 34% of respondents, who could select more than one possibility from a list of 15 – but social media moved ahead of primary care physicians into third place (24%), just behind friends (30%), the survey showed. Dermatologists, on the other hand, had polled at 50%-55% for the previous 5 years.
The dermatologists’ lead remained stronger as the top influencer for skin care purchases, selected by 45% of respondents, compared with 32% for friends and 28% for social media. In this category there were 14 factors from which respondents could choose. As for the cost of those skin care products, 48% of consumers spent $1-$50 a month, 31% said that they spent $51-$100 a month, and 12% reported spending $101-$150 a month, the ASDS said.
The society received 3,645 responses to the 2019 Consumer Survey on Cosmetic Dermatologic Procedures, which was conducted online from July 30 to Aug. 27 by Survata.
Online resources are affecting most consumers’ selections of cosmetic providers, and social media are now a top-three influence on cosmetic procedure choices and skin care purchases, according to a new survey from the American Society for Dermatologic Surgery.

Almost 70% of respondents said that their use of rate and review websites had an impact on the choice of provider for cosmetic procedures: WebMD was the site most often visited, followed by Facebook, physician websites, and Yelp, the ASDS said based on its annual consumer survey.
For 43% of consumers, the decision to schedule an appointment was influenced by a provider’s social media presence, and 41% of patients said that they follow their current or potential provider on social media, the ASDS said.
“Online resources and social media platforms are clearly influencing consumers’ behavior and perception of skin health,” ASDS President Murad Alam, MD, MBA, chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, Chicago, said in a written statement.
Dermatologists, however, remain the leading influence on the decision to have a cosmetic procedure – named as a resource by 34% of respondents, who could select more than one possibility from a list of 15 – but social media moved ahead of primary care physicians into third place (24%), just behind friends (30%), the survey showed. Dermatologists, on the other hand, had polled at 50%-55% for the previous 5 years.
The dermatologists’ lead remained stronger as the top influencer for skin care purchases, selected by 45% of respondents, compared with 32% for friends and 28% for social media. In this category there were 14 factors from which respondents could choose. As for the cost of those skin care products, 48% of consumers spent $1-$50 a month, 31% said that they spent $51-$100 a month, and 12% reported spending $101-$150 a month, the ASDS said.
The society received 3,645 responses to the 2019 Consumer Survey on Cosmetic Dermatologic Procedures, which was conducted online from July 30 to Aug. 27 by Survata.
Online resources are affecting most consumers’ selections of cosmetic providers, and social media are now a top-three influence on cosmetic procedure choices and skin care purchases, according to a new survey from the American Society for Dermatologic Surgery.

Almost 70% of respondents said that their use of rate and review websites had an impact on the choice of provider for cosmetic procedures: WebMD was the site most often visited, followed by Facebook, physician websites, and Yelp, the ASDS said based on its annual consumer survey.
For 43% of consumers, the decision to schedule an appointment was influenced by a provider’s social media presence, and 41% of patients said that they follow their current or potential provider on social media, the ASDS said.
“Online resources and social media platforms are clearly influencing consumers’ behavior and perception of skin health,” ASDS President Murad Alam, MD, MBA, chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, Chicago, said in a written statement.
Dermatologists, however, remain the leading influence on the decision to have a cosmetic procedure – named as a resource by 34% of respondents, who could select more than one possibility from a list of 15 – but social media moved ahead of primary care physicians into third place (24%), just behind friends (30%), the survey showed. Dermatologists, on the other hand, had polled at 50%-55% for the previous 5 years.
The dermatologists’ lead remained stronger as the top influencer for skin care purchases, selected by 45% of respondents, compared with 32% for friends and 28% for social media. In this category there were 14 factors from which respondents could choose. As for the cost of those skin care products, 48% of consumers spent $1-$50 a month, 31% said that they spent $51-$100 a month, and 12% reported spending $101-$150 a month, the ASDS said.
The society received 3,645 responses to the 2019 Consumer Survey on Cosmetic Dermatologic Procedures, which was conducted online from July 30 to Aug. 27 by Survata.
Combined treatments provide control of pseudofolliculitis barbae in women
NEW YORK – For has been found highly effective, Wendy Roberts, MD, reported at the Skin of Color Update 2019.
“We didn’t have great treatments for this problem in the past, but the technology has evolved, and you can now get most women clear,” Dr. Roberts, a dermatologist who practices in Rancho Mirage, Calif., said at the meeting.
This approach is appropriate in all women, but Dr. Roberts focused on her experience with black patients, for whom an antioxidant cream is added to address the inflammatory-associated hyperpigmentation that often accompanies pseudofolliculitis barbae, a chronic inflammatory skin condition typically characterized by small, painful papules and pustules.
Start with microdermabrasion to treat the hypertrophic hair follicles and address keratin plugs, Dr. Roberts said. The microdermabrasion smooths the skin and increases penetration of subsequent creams and topics, she said.
“In the same session, I treat with Nd-YAG 1064 nm laser using short pulses,” she noted. For black women, she makes four passes with the laser at a level of moderate intensity. For those with lighter skin, she might perform as many as six passes with the laser set higher.
The microdermabrasion is repeated monthly for three or four treatments, but can be extended for those with persistent symptoms, Dr. Roberts pointed out. She presented a case of a patient who required seven treatments to achieve a satisfactory response.
Patients are instructed to avoid hair plucking and over the course of treatment nightly topical tretinoin is recommended for maintenance. Regular use of emollients is also recommended. For black women who have developed hyperpigmentation as a complication of pseudofolliculitis barbae, Dr. Roberts prescribes a lightening cream.
“I have pretty much moved away from hydroquinone,” said Dr. Roberts, explaining that she has achieved better results with topical cysteamine, a product that she has been using for about 3 years.
In outlining her treatment strategy, she employed case studies of two black women, both of whom achieved resolution of the problem and were satisfied with the results. She said that the same approach is suitable for women of other racial and ethnic groups.
Most commonly seen in black men, pseudofolliculitis barbae – also known as razor bumps – can occur as a complication of shaving in men or women from any racial and ethnic group. However, because of their embarrassment, women often fail to volunteer that they are struggling with this problem. Some women have been afflicted for years and have developed a regular routine of shaving or plucking hairs and then applying makeup for camouflage, Dr. Roberts said.
“This is a patient who rarely presents the problem to the dermatologist. Yet, she is in every one of our practices,” she added. Due to the frequency with which she has identified pseudofolliculitis barbae in patients who are being seen for a different complaint, she now routinely asks patients about this issue when taking a history. Early detection is useful because pseudofolliculitis barbae is more easily resolved in younger women than in older women.
When the problem is resolved, patient satisfaction is very high. For this reason, Dr. Roberts called diagnosis and treatment of pseudofolliculitis barbae “a practice builder.” Based on her approach, “you can really get these ladies clear.”
Dr. Roberts reports financial relationships with an extensive list of companies that market dermatologic and cosmetic products.
NEW YORK – For has been found highly effective, Wendy Roberts, MD, reported at the Skin of Color Update 2019.
“We didn’t have great treatments for this problem in the past, but the technology has evolved, and you can now get most women clear,” Dr. Roberts, a dermatologist who practices in Rancho Mirage, Calif., said at the meeting.
This approach is appropriate in all women, but Dr. Roberts focused on her experience with black patients, for whom an antioxidant cream is added to address the inflammatory-associated hyperpigmentation that often accompanies pseudofolliculitis barbae, a chronic inflammatory skin condition typically characterized by small, painful papules and pustules.
Start with microdermabrasion to treat the hypertrophic hair follicles and address keratin plugs, Dr. Roberts said. The microdermabrasion smooths the skin and increases penetration of subsequent creams and topics, she said.
“In the same session, I treat with Nd-YAG 1064 nm laser using short pulses,” she noted. For black women, she makes four passes with the laser at a level of moderate intensity. For those with lighter skin, she might perform as many as six passes with the laser set higher.
The microdermabrasion is repeated monthly for three or four treatments, but can be extended for those with persistent symptoms, Dr. Roberts pointed out. She presented a case of a patient who required seven treatments to achieve a satisfactory response.
Patients are instructed to avoid hair plucking and over the course of treatment nightly topical tretinoin is recommended for maintenance. Regular use of emollients is also recommended. For black women who have developed hyperpigmentation as a complication of pseudofolliculitis barbae, Dr. Roberts prescribes a lightening cream.
“I have pretty much moved away from hydroquinone,” said Dr. Roberts, explaining that she has achieved better results with topical cysteamine, a product that she has been using for about 3 years.
In outlining her treatment strategy, she employed case studies of two black women, both of whom achieved resolution of the problem and were satisfied with the results. She said that the same approach is suitable for women of other racial and ethnic groups.
Most commonly seen in black men, pseudofolliculitis barbae – also known as razor bumps – can occur as a complication of shaving in men or women from any racial and ethnic group. However, because of their embarrassment, women often fail to volunteer that they are struggling with this problem. Some women have been afflicted for years and have developed a regular routine of shaving or plucking hairs and then applying makeup for camouflage, Dr. Roberts said.
“This is a patient who rarely presents the problem to the dermatologist. Yet, she is in every one of our practices,” she added. Due to the frequency with which she has identified pseudofolliculitis barbae in patients who are being seen for a different complaint, she now routinely asks patients about this issue when taking a history. Early detection is useful because pseudofolliculitis barbae is more easily resolved in younger women than in older women.
When the problem is resolved, patient satisfaction is very high. For this reason, Dr. Roberts called diagnosis and treatment of pseudofolliculitis barbae “a practice builder.” Based on her approach, “you can really get these ladies clear.”
Dr. Roberts reports financial relationships with an extensive list of companies that market dermatologic and cosmetic products.
NEW YORK – For has been found highly effective, Wendy Roberts, MD, reported at the Skin of Color Update 2019.
“We didn’t have great treatments for this problem in the past, but the technology has evolved, and you can now get most women clear,” Dr. Roberts, a dermatologist who practices in Rancho Mirage, Calif., said at the meeting.
This approach is appropriate in all women, but Dr. Roberts focused on her experience with black patients, for whom an antioxidant cream is added to address the inflammatory-associated hyperpigmentation that often accompanies pseudofolliculitis barbae, a chronic inflammatory skin condition typically characterized by small, painful papules and pustules.
Start with microdermabrasion to treat the hypertrophic hair follicles and address keratin plugs, Dr. Roberts said. The microdermabrasion smooths the skin and increases penetration of subsequent creams and topics, she said.
“In the same session, I treat with Nd-YAG 1064 nm laser using short pulses,” she noted. For black women, she makes four passes with the laser at a level of moderate intensity. For those with lighter skin, she might perform as many as six passes with the laser set higher.
The microdermabrasion is repeated monthly for three or four treatments, but can be extended for those with persistent symptoms, Dr. Roberts pointed out. She presented a case of a patient who required seven treatments to achieve a satisfactory response.
Patients are instructed to avoid hair plucking and over the course of treatment nightly topical tretinoin is recommended for maintenance. Regular use of emollients is also recommended. For black women who have developed hyperpigmentation as a complication of pseudofolliculitis barbae, Dr. Roberts prescribes a lightening cream.
“I have pretty much moved away from hydroquinone,” said Dr. Roberts, explaining that she has achieved better results with topical cysteamine, a product that she has been using for about 3 years.
In outlining her treatment strategy, she employed case studies of two black women, both of whom achieved resolution of the problem and were satisfied with the results. She said that the same approach is suitable for women of other racial and ethnic groups.
Most commonly seen in black men, pseudofolliculitis barbae – also known as razor bumps – can occur as a complication of shaving in men or women from any racial and ethnic group. However, because of their embarrassment, women often fail to volunteer that they are struggling with this problem. Some women have been afflicted for years and have developed a regular routine of shaving or plucking hairs and then applying makeup for camouflage, Dr. Roberts said.
“This is a patient who rarely presents the problem to the dermatologist. Yet, she is in every one of our practices,” she added. Due to the frequency with which she has identified pseudofolliculitis barbae in patients who are being seen for a different complaint, she now routinely asks patients about this issue when taking a history. Early detection is useful because pseudofolliculitis barbae is more easily resolved in younger women than in older women.
When the problem is resolved, patient satisfaction is very high. For this reason, Dr. Roberts called diagnosis and treatment of pseudofolliculitis barbae “a practice builder.” Based on her approach, “you can really get these ladies clear.”
Dr. Roberts reports financial relationships with an extensive list of companies that market dermatologic and cosmetic products.
REPORTING FROM SOC 2019















