User login
CDC chief overrules panel, OKs boosters for health care workers
The CDC’s Advisory Committee on Immunization Practices earlier Thursday voted to allow several groups of Americans to get a booster shot, but voted not to recommend it for adults age 18 to 64 who live or work in a place where the risk of COVID-19 is high. That would have included health care workers and other frontline employees.
But CDC Director Rochelle Walensky, MD, decided to reverse that recommendation and include the 18-to-64-year-olds in her final decision.
“As CDC Director, it is my job to recognize where our actions can have the greatest impact,” Dr. Walensky said in a statement late Thursday night, according to published reports. “At CDC, we are tasked with analyzing complex, often imperfect data to make concrete recommendations that optimize health. In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good.”
Dr. Walensky agreed with the rest of the advisory committee's decisions, which included recommendations that the following groups also be eligible for a booster shot:
- Adults ages 65 and up and residents of long-term care facilities
- Adults ages 50 to 64 who have an underlying medical condition that may increase their risk from a COVID infection
- Adults ages 18 to 49 who may be at increased risk from a COVID-19 infection because of an underlying medical condition, if a person feels like they need one based on a consideration of their individual benefit and risks.
About 26 million Americans are at least 6 months past the last dose of the Pfizer vaccines, making them eligible to receive a third dose. About 13.6 million of them are over the age of 65. Another 5.3 million are ages 50 to 64.
In making the recommendations, the committee left out healthcare workers. This was a departure from the Food and Drug Administration’s authorization which included boosters for those 65 and over, and for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers – such as those in healthcare -- whose jobs increase their risk for infection.
This is the group Dr. Walensky added to the eligible list on her own.
Committee members “did not buy the need in occupational or institutional settings,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville. Dr. Schaffner sits on the ACIP workgroup that considered the evidence behind boosters. He said that he would have voted yes to offer boosters to healthcare and other essential workers.
“There was a real split in the committee,” he said.
The vote on boosters for healthcare and other high-risk workers was rejected 9 to 6.
“I think that there is ample evidence that people such as healthcare workers do not have repeated exposure in the workplace,” said Beth Bell, MD, a clinical professor at the University of Washington. “They’re using PPE as they should and they’re following the other policies within the healthcare setting. There’s lots of evidence that suggest that health care workers who become infected become infected because of exposures in the community.”
She was not alone in feeling cautious.
“I think this is an extremely slippery slope,” said Sarah Long, MD, a pediatric infectious disease specialist at Drexel University in Philadelphia, before her vote to reject boosters for healthcare and other high-risk workers.
“We might as well just say, ‘Give it to everybody 18 and over.’ We have an extremely effective vaccine. It’s like saying it’s not working, and it is working.”
The committee saw data showing that all of the vaccines remain highly protective against hospitalization and death for all age groups, though protection against getting sick with COVID has waned slightly over time and with the dominance of the more contagious Delta variant. Those at highest risk for a severe breakthrough infection — those that cause hospitalization or death — are older adults.
How much will the U.S. benefit from boosters?
Some felt squeamish about broadly recommending boosters at all.
“We have too much hope on the line with these boosters,” said James Loehr, MD, who is a family physician in Ithaca, N.Y. Dr. Loehr said he felt the goal of giving boosters in the United States should be to decrease hospitalizations, and he felt they would, but that the impact would likely be smaller than appreciated.
Based on his calculations of the benefits of boosters for each age group, Dr. Loehr said if boosters were given to all 13 million seniors previously vaccinated with the Pfizer vaccine, we might prevent 200 hospitalizations a day, “which would be a lot,” he noted. But, he said, “considering that we have 10,000 hospitalizations a day now, it’s probably not that much.”
Others agreed.
“I really think this is a solution looking for a problem,” said Jason Goldman, MD, an associate professor at Florida Atlantic University who was representing the American College of Physicians. “You know, I don’t think it’s going to address the issue of the pandemic. I really think it’s just going to create more confusion on the provider from the position of implementation, and I really think it’s going really far afield of the data.”
ACIP Chair Grace Lee, MD, a pediatric infectious disease specialist at Stanford, said she had cared for children who had died of COVID.
“I can tell you that their family members really wished they had extra protection for their kids, because they weren’t symptomatic. Nobody else was sick at home,” she said.
Dr. Lee said for her, access was paramount, and she was in favor of expanding access to boosters for as many people as possible.
Next steps
People who were initially vaccinated with either Moderna or Johnson & Johnson vaccines are excluded from booster recommendations, something many on the committee were uncomfortable with.
The FDA is still considering Moderna’s application to market booster doses. Johnson & Johnson hasn’t yet applied to the FDA for permission to offer second doses in the United States.
While the ACIP’s recommendations are important, in this case, they may not have a huge practical effect, said Schaffner. The CDC has already approved third shots for people who are immunocompromised, and no proof of a medical condition is required to get one.
More than 2 million people have already gotten a third dose, he noted, and not all of them are immunocompromised.
“They have heard the president say that, you know, everybody should get a booster, and they’ve taken that at face value,” he said.
A version of this article first appeared on WebMD.com.
The CDC’s Advisory Committee on Immunization Practices earlier Thursday voted to allow several groups of Americans to get a booster shot, but voted not to recommend it for adults age 18 to 64 who live or work in a place where the risk of COVID-19 is high. That would have included health care workers and other frontline employees.
But CDC Director Rochelle Walensky, MD, decided to reverse that recommendation and include the 18-to-64-year-olds in her final decision.
“As CDC Director, it is my job to recognize where our actions can have the greatest impact,” Dr. Walensky said in a statement late Thursday night, according to published reports. “At CDC, we are tasked with analyzing complex, often imperfect data to make concrete recommendations that optimize health. In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good.”
Dr. Walensky agreed with the rest of the advisory committee's decisions, which included recommendations that the following groups also be eligible for a booster shot:
- Adults ages 65 and up and residents of long-term care facilities
- Adults ages 50 to 64 who have an underlying medical condition that may increase their risk from a COVID infection
- Adults ages 18 to 49 who may be at increased risk from a COVID-19 infection because of an underlying medical condition, if a person feels like they need one based on a consideration of their individual benefit and risks.
About 26 million Americans are at least 6 months past the last dose of the Pfizer vaccines, making them eligible to receive a third dose. About 13.6 million of them are over the age of 65. Another 5.3 million are ages 50 to 64.
In making the recommendations, the committee left out healthcare workers. This was a departure from the Food and Drug Administration’s authorization which included boosters for those 65 and over, and for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers – such as those in healthcare -- whose jobs increase their risk for infection.
This is the group Dr. Walensky added to the eligible list on her own.
Committee members “did not buy the need in occupational or institutional settings,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville. Dr. Schaffner sits on the ACIP workgroup that considered the evidence behind boosters. He said that he would have voted yes to offer boosters to healthcare and other essential workers.
“There was a real split in the committee,” he said.
The vote on boosters for healthcare and other high-risk workers was rejected 9 to 6.
“I think that there is ample evidence that people such as healthcare workers do not have repeated exposure in the workplace,” said Beth Bell, MD, a clinical professor at the University of Washington. “They’re using PPE as they should and they’re following the other policies within the healthcare setting. There’s lots of evidence that suggest that health care workers who become infected become infected because of exposures in the community.”
She was not alone in feeling cautious.
“I think this is an extremely slippery slope,” said Sarah Long, MD, a pediatric infectious disease specialist at Drexel University in Philadelphia, before her vote to reject boosters for healthcare and other high-risk workers.
“We might as well just say, ‘Give it to everybody 18 and over.’ We have an extremely effective vaccine. It’s like saying it’s not working, and it is working.”
The committee saw data showing that all of the vaccines remain highly protective against hospitalization and death for all age groups, though protection against getting sick with COVID has waned slightly over time and with the dominance of the more contagious Delta variant. Those at highest risk for a severe breakthrough infection — those that cause hospitalization or death — are older adults.
How much will the U.S. benefit from boosters?
Some felt squeamish about broadly recommending boosters at all.
“We have too much hope on the line with these boosters,” said James Loehr, MD, who is a family physician in Ithaca, N.Y. Dr. Loehr said he felt the goal of giving boosters in the United States should be to decrease hospitalizations, and he felt they would, but that the impact would likely be smaller than appreciated.
Based on his calculations of the benefits of boosters for each age group, Dr. Loehr said if boosters were given to all 13 million seniors previously vaccinated with the Pfizer vaccine, we might prevent 200 hospitalizations a day, “which would be a lot,” he noted. But, he said, “considering that we have 10,000 hospitalizations a day now, it’s probably not that much.”
Others agreed.
“I really think this is a solution looking for a problem,” said Jason Goldman, MD, an associate professor at Florida Atlantic University who was representing the American College of Physicians. “You know, I don’t think it’s going to address the issue of the pandemic. I really think it’s just going to create more confusion on the provider from the position of implementation, and I really think it’s going really far afield of the data.”
ACIP Chair Grace Lee, MD, a pediatric infectious disease specialist at Stanford, said she had cared for children who had died of COVID.
“I can tell you that their family members really wished they had extra protection for their kids, because they weren’t symptomatic. Nobody else was sick at home,” she said.
Dr. Lee said for her, access was paramount, and she was in favor of expanding access to boosters for as many people as possible.
Next steps
People who were initially vaccinated with either Moderna or Johnson & Johnson vaccines are excluded from booster recommendations, something many on the committee were uncomfortable with.
The FDA is still considering Moderna’s application to market booster doses. Johnson & Johnson hasn’t yet applied to the FDA for permission to offer second doses in the United States.
While the ACIP’s recommendations are important, in this case, they may not have a huge practical effect, said Schaffner. The CDC has already approved third shots for people who are immunocompromised, and no proof of a medical condition is required to get one.
More than 2 million people have already gotten a third dose, he noted, and not all of them are immunocompromised.
“They have heard the president say that, you know, everybody should get a booster, and they’ve taken that at face value,” he said.
A version of this article first appeared on WebMD.com.
The CDC’s Advisory Committee on Immunization Practices earlier Thursday voted to allow several groups of Americans to get a booster shot, but voted not to recommend it for adults age 18 to 64 who live or work in a place where the risk of COVID-19 is high. That would have included health care workers and other frontline employees.
But CDC Director Rochelle Walensky, MD, decided to reverse that recommendation and include the 18-to-64-year-olds in her final decision.
“As CDC Director, it is my job to recognize where our actions can have the greatest impact,” Dr. Walensky said in a statement late Thursday night, according to published reports. “At CDC, we are tasked with analyzing complex, often imperfect data to make concrete recommendations that optimize health. In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good.”
Dr. Walensky agreed with the rest of the advisory committee's decisions, which included recommendations that the following groups also be eligible for a booster shot:
- Adults ages 65 and up and residents of long-term care facilities
- Adults ages 50 to 64 who have an underlying medical condition that may increase their risk from a COVID infection
- Adults ages 18 to 49 who may be at increased risk from a COVID-19 infection because of an underlying medical condition, if a person feels like they need one based on a consideration of their individual benefit and risks.
About 26 million Americans are at least 6 months past the last dose of the Pfizer vaccines, making them eligible to receive a third dose. About 13.6 million of them are over the age of 65. Another 5.3 million are ages 50 to 64.
In making the recommendations, the committee left out healthcare workers. This was a departure from the Food and Drug Administration’s authorization which included boosters for those 65 and over, and for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers – such as those in healthcare -- whose jobs increase their risk for infection.
This is the group Dr. Walensky added to the eligible list on her own.
Committee members “did not buy the need in occupational or institutional settings,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville. Dr. Schaffner sits on the ACIP workgroup that considered the evidence behind boosters. He said that he would have voted yes to offer boosters to healthcare and other essential workers.
“There was a real split in the committee,” he said.
The vote on boosters for healthcare and other high-risk workers was rejected 9 to 6.
“I think that there is ample evidence that people such as healthcare workers do not have repeated exposure in the workplace,” said Beth Bell, MD, a clinical professor at the University of Washington. “They’re using PPE as they should and they’re following the other policies within the healthcare setting. There’s lots of evidence that suggest that health care workers who become infected become infected because of exposures in the community.”
She was not alone in feeling cautious.
“I think this is an extremely slippery slope,” said Sarah Long, MD, a pediatric infectious disease specialist at Drexel University in Philadelphia, before her vote to reject boosters for healthcare and other high-risk workers.
“We might as well just say, ‘Give it to everybody 18 and over.’ We have an extremely effective vaccine. It’s like saying it’s not working, and it is working.”
The committee saw data showing that all of the vaccines remain highly protective against hospitalization and death for all age groups, though protection against getting sick with COVID has waned slightly over time and with the dominance of the more contagious Delta variant. Those at highest risk for a severe breakthrough infection — those that cause hospitalization or death — are older adults.
How much will the U.S. benefit from boosters?
Some felt squeamish about broadly recommending boosters at all.
“We have too much hope on the line with these boosters,” said James Loehr, MD, who is a family physician in Ithaca, N.Y. Dr. Loehr said he felt the goal of giving boosters in the United States should be to decrease hospitalizations, and he felt they would, but that the impact would likely be smaller than appreciated.
Based on his calculations of the benefits of boosters for each age group, Dr. Loehr said if boosters were given to all 13 million seniors previously vaccinated with the Pfizer vaccine, we might prevent 200 hospitalizations a day, “which would be a lot,” he noted. But, he said, “considering that we have 10,000 hospitalizations a day now, it’s probably not that much.”
Others agreed.
“I really think this is a solution looking for a problem,” said Jason Goldman, MD, an associate professor at Florida Atlantic University who was representing the American College of Physicians. “You know, I don’t think it’s going to address the issue of the pandemic. I really think it’s just going to create more confusion on the provider from the position of implementation, and I really think it’s going really far afield of the data.”
ACIP Chair Grace Lee, MD, a pediatric infectious disease specialist at Stanford, said she had cared for children who had died of COVID.
“I can tell you that their family members really wished they had extra protection for their kids, because they weren’t symptomatic. Nobody else was sick at home,” she said.
Dr. Lee said for her, access was paramount, and she was in favor of expanding access to boosters for as many people as possible.
Next steps
People who were initially vaccinated with either Moderna or Johnson & Johnson vaccines are excluded from booster recommendations, something many on the committee were uncomfortable with.
The FDA is still considering Moderna’s application to market booster doses. Johnson & Johnson hasn’t yet applied to the FDA for permission to offer second doses in the United States.
While the ACIP’s recommendations are important, in this case, they may not have a huge practical effect, said Schaffner. The CDC has already approved third shots for people who are immunocompromised, and no proof of a medical condition is required to get one.
More than 2 million people have already gotten a third dose, he noted, and not all of them are immunocompromised.
“They have heard the president say that, you know, everybody should get a booster, and they’ve taken that at face value,” he said.
A version of this article first appeared on WebMD.com.
Remdesivir sharply cuts COVID hospitalization risk, Gilead says
Remdesivir (Veklury, Gilead) was found to reduce some COVID-19 patients’ risk of hospitalization by 87% in a phase 3 trial, the drug’s manufacturer announced Sept. 22 in a press release.
The randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of a 3-day course of intravenous remdesivir in an analysis of 562 nonhospitalized patients at high risk for disease progression.
Remdesivir demonstrated a statistically significant 87% reduction in risk for COVID-19–related hospitalization or all-cause death by Day 28 (0.7% [2/279]) compared with placebo (5.3% [15/283]) P = .008. Participants were assigned 1:1 to remdesivir or the placebo group.
Researchers also found an 81% reduction in risk for the composite secondary endpoint – medical visits due to COVID-19 or all-cause death by Day 28. Only 1.6% had COVID-19 medical visits ([4/246]) compared with those in the placebo group (8.3% [21/252]) P = .002. No deaths were observed in either arm by Day 28.
“These latest data show remdesivir’s potential to help high-risk patients recover before they get sicker and stay out of the hospital altogether,” coauthor Robert L. Gottlieb, MD, PhD, from Baylor University Medical Center, Houston, said in the press release.
Remdesivir is the only drug approved by the U.S. Food and Drug Administration for hospitalized COVID-19 patients at least 12 years old. Its treatment of nonhospitalized patients with 3 days of dosing is investigational, and the safety and efficacy for this use and dosing duration have not been established or approved by any regulatory agency, the Gilead press release notes.
The patients in this study were considered high-risk for disease progression based on comorbidities – commonly obesity, hypertension, and diabetes – and age, but had not recently been hospitalized due to COVID-19.
A third of the participants were at least 60 years old. Participants in the study must have received a positive diagnosis within 4 days of starting treatment and experienced symptoms for 7 days or less.
Use of remdesivir controversial
Results from the Adaptive COVID-19 Treatment Trial (ACTT-1) showed remdesivir was superior to placebo in shortening time to recovery in adults hospitalized with COVID-19 with evidence of lower respiratory tract infection.
However, a large trial of more than 11,000 people in 30 countries, sponsored by the World Health Organization, did not show any benefit for the drug in reducing COVID deaths.
The WHO has conditionally recommended against using remdesivir in hospitalized patients, regardless of disease severity, “as there is currently no evidence that remdesivir improves survival and other outcomes in these patients.”
The drug also is given intravenously, and this study tested three infusions over 3 days, a difficult treatment for nonhospitalized patients.
The study results were released ahead of IDWeek, where the late-breaking abstract will be presented at the virtual conference in full at the end of next week.
A version of this article first appeared on Medscape.com.
Remdesivir (Veklury, Gilead) was found to reduce some COVID-19 patients’ risk of hospitalization by 87% in a phase 3 trial, the drug’s manufacturer announced Sept. 22 in a press release.
The randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of a 3-day course of intravenous remdesivir in an analysis of 562 nonhospitalized patients at high risk for disease progression.
Remdesivir demonstrated a statistically significant 87% reduction in risk for COVID-19–related hospitalization or all-cause death by Day 28 (0.7% [2/279]) compared with placebo (5.3% [15/283]) P = .008. Participants were assigned 1:1 to remdesivir or the placebo group.
Researchers also found an 81% reduction in risk for the composite secondary endpoint – medical visits due to COVID-19 or all-cause death by Day 28. Only 1.6% had COVID-19 medical visits ([4/246]) compared with those in the placebo group (8.3% [21/252]) P = .002. No deaths were observed in either arm by Day 28.
“These latest data show remdesivir’s potential to help high-risk patients recover before they get sicker and stay out of the hospital altogether,” coauthor Robert L. Gottlieb, MD, PhD, from Baylor University Medical Center, Houston, said in the press release.
Remdesivir is the only drug approved by the U.S. Food and Drug Administration for hospitalized COVID-19 patients at least 12 years old. Its treatment of nonhospitalized patients with 3 days of dosing is investigational, and the safety and efficacy for this use and dosing duration have not been established or approved by any regulatory agency, the Gilead press release notes.
The patients in this study were considered high-risk for disease progression based on comorbidities – commonly obesity, hypertension, and diabetes – and age, but had not recently been hospitalized due to COVID-19.
A third of the participants were at least 60 years old. Participants in the study must have received a positive diagnosis within 4 days of starting treatment and experienced symptoms for 7 days or less.
Use of remdesivir controversial
Results from the Adaptive COVID-19 Treatment Trial (ACTT-1) showed remdesivir was superior to placebo in shortening time to recovery in adults hospitalized with COVID-19 with evidence of lower respiratory tract infection.
However, a large trial of more than 11,000 people in 30 countries, sponsored by the World Health Organization, did not show any benefit for the drug in reducing COVID deaths.
The WHO has conditionally recommended against using remdesivir in hospitalized patients, regardless of disease severity, “as there is currently no evidence that remdesivir improves survival and other outcomes in these patients.”
The drug also is given intravenously, and this study tested three infusions over 3 days, a difficult treatment for nonhospitalized patients.
The study results were released ahead of IDWeek, where the late-breaking abstract will be presented at the virtual conference in full at the end of next week.
A version of this article first appeared on Medscape.com.
Remdesivir (Veklury, Gilead) was found to reduce some COVID-19 patients’ risk of hospitalization by 87% in a phase 3 trial, the drug’s manufacturer announced Sept. 22 in a press release.
The randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of a 3-day course of intravenous remdesivir in an analysis of 562 nonhospitalized patients at high risk for disease progression.
Remdesivir demonstrated a statistically significant 87% reduction in risk for COVID-19–related hospitalization or all-cause death by Day 28 (0.7% [2/279]) compared with placebo (5.3% [15/283]) P = .008. Participants were assigned 1:1 to remdesivir or the placebo group.
Researchers also found an 81% reduction in risk for the composite secondary endpoint – medical visits due to COVID-19 or all-cause death by Day 28. Only 1.6% had COVID-19 medical visits ([4/246]) compared with those in the placebo group (8.3% [21/252]) P = .002. No deaths were observed in either arm by Day 28.
“These latest data show remdesivir’s potential to help high-risk patients recover before they get sicker and stay out of the hospital altogether,” coauthor Robert L. Gottlieb, MD, PhD, from Baylor University Medical Center, Houston, said in the press release.
Remdesivir is the only drug approved by the U.S. Food and Drug Administration for hospitalized COVID-19 patients at least 12 years old. Its treatment of nonhospitalized patients with 3 days of dosing is investigational, and the safety and efficacy for this use and dosing duration have not been established or approved by any regulatory agency, the Gilead press release notes.
The patients in this study were considered high-risk for disease progression based on comorbidities – commonly obesity, hypertension, and diabetes – and age, but had not recently been hospitalized due to COVID-19.
A third of the participants were at least 60 years old. Participants in the study must have received a positive diagnosis within 4 days of starting treatment and experienced symptoms for 7 days or less.
Use of remdesivir controversial
Results from the Adaptive COVID-19 Treatment Trial (ACTT-1) showed remdesivir was superior to placebo in shortening time to recovery in adults hospitalized with COVID-19 with evidence of lower respiratory tract infection.
However, a large trial of more than 11,000 people in 30 countries, sponsored by the World Health Organization, did not show any benefit for the drug in reducing COVID deaths.
The WHO has conditionally recommended against using remdesivir in hospitalized patients, regardless of disease severity, “as there is currently no evidence that remdesivir improves survival and other outcomes in these patients.”
The drug also is given intravenously, and this study tested three infusions over 3 days, a difficult treatment for nonhospitalized patients.
The study results were released ahead of IDWeek, where the late-breaking abstract will be presented at the virtual conference in full at the end of next week.
A version of this article first appeared on Medscape.com.
New COVID-19 strain has reached the U.S.
Deadline, citing a Centers for Disease Control and Prevention report, said 26 residents and 20 workers tested positive for COVID-19 at a skilled care nursing home. The facility has 83 residents and 116 employees.
On March 1, 28 specimens that had been subjected to whole genome sequencing were found to have “mutations aligning with the R.1 lineage,” Deadline said.
About 90% of the facility’s residents and 52% of the staff had received two COVID vaccine doses, the CDC said. Because of the high vaccination rate, the finding raises concerns about “reduced protective immunity” in relation to the R.1 variant, the CDC said.
However, the nursing home case appears to show that the vaccine keeps most people from getting extremely sick, the CDC said. The vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective for employees.
“Compared with unvaccinated persons, vaccinated persons had reduced risk for SARS-CoV-2 infection and symptomatic COVID-19,” the CDC said. The vaccination of nursing home residents and health care workers “is essential to reduce the risk for symptomatic COVID-19, as is continued focus on infection prevention and control practices,” the CDC said.
Since being reported in Kentucky, R.1 has been detected more than 10,000 times in the United States, Forbes reported, basing that number on entries in the GISAID SARS-CoV-2 database.
Overall, more than 42 million cases of COVID have been reported since the start of the pandemic.
Deadline reported that the R.1 strain was first detected in Japan in January among three members of one family. The family members had no history of traveling abroad, Deadline said, citing an National Institutes of Health report.
The CDC has not classified R.1 as a variant of concern yet but noted it has “several mutations of importance” and “demonstrates evidence of increasing virus transmissibility.”
A version of this article first appeared on WebMD.com.
Deadline, citing a Centers for Disease Control and Prevention report, said 26 residents and 20 workers tested positive for COVID-19 at a skilled care nursing home. The facility has 83 residents and 116 employees.
On March 1, 28 specimens that had been subjected to whole genome sequencing were found to have “mutations aligning with the R.1 lineage,” Deadline said.
About 90% of the facility’s residents and 52% of the staff had received two COVID vaccine doses, the CDC said. Because of the high vaccination rate, the finding raises concerns about “reduced protective immunity” in relation to the R.1 variant, the CDC said.
However, the nursing home case appears to show that the vaccine keeps most people from getting extremely sick, the CDC said. The vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective for employees.
“Compared with unvaccinated persons, vaccinated persons had reduced risk for SARS-CoV-2 infection and symptomatic COVID-19,” the CDC said. The vaccination of nursing home residents and health care workers “is essential to reduce the risk for symptomatic COVID-19, as is continued focus on infection prevention and control practices,” the CDC said.
Since being reported in Kentucky, R.1 has been detected more than 10,000 times in the United States, Forbes reported, basing that number on entries in the GISAID SARS-CoV-2 database.
Overall, more than 42 million cases of COVID have been reported since the start of the pandemic.
Deadline reported that the R.1 strain was first detected in Japan in January among three members of one family. The family members had no history of traveling abroad, Deadline said, citing an National Institutes of Health report.
The CDC has not classified R.1 as a variant of concern yet but noted it has “several mutations of importance” and “demonstrates evidence of increasing virus transmissibility.”
A version of this article first appeared on WebMD.com.
Deadline, citing a Centers for Disease Control and Prevention report, said 26 residents and 20 workers tested positive for COVID-19 at a skilled care nursing home. The facility has 83 residents and 116 employees.
On March 1, 28 specimens that had been subjected to whole genome sequencing were found to have “mutations aligning with the R.1 lineage,” Deadline said.
About 90% of the facility’s residents and 52% of the staff had received two COVID vaccine doses, the CDC said. Because of the high vaccination rate, the finding raises concerns about “reduced protective immunity” in relation to the R.1 variant, the CDC said.
However, the nursing home case appears to show that the vaccine keeps most people from getting extremely sick, the CDC said. The vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective for employees.
“Compared with unvaccinated persons, vaccinated persons had reduced risk for SARS-CoV-2 infection and symptomatic COVID-19,” the CDC said. The vaccination of nursing home residents and health care workers “is essential to reduce the risk for symptomatic COVID-19, as is continued focus on infection prevention and control practices,” the CDC said.
Since being reported in Kentucky, R.1 has been detected more than 10,000 times in the United States, Forbes reported, basing that number on entries in the GISAID SARS-CoV-2 database.
Overall, more than 42 million cases of COVID have been reported since the start of the pandemic.
Deadline reported that the R.1 strain was first detected in Japan in January among three members of one family. The family members had no history of traveling abroad, Deadline said, citing an National Institutes of Health report.
The CDC has not classified R.1 as a variant of concern yet but noted it has “several mutations of importance” and “demonstrates evidence of increasing virus transmissibility.”
A version of this article first appeared on WebMD.com.
Mean leadership
The differences between the mean and median of leadership data
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.
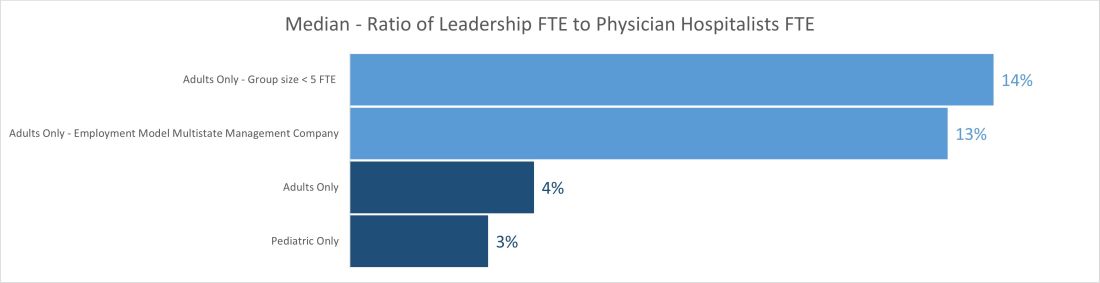
The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.
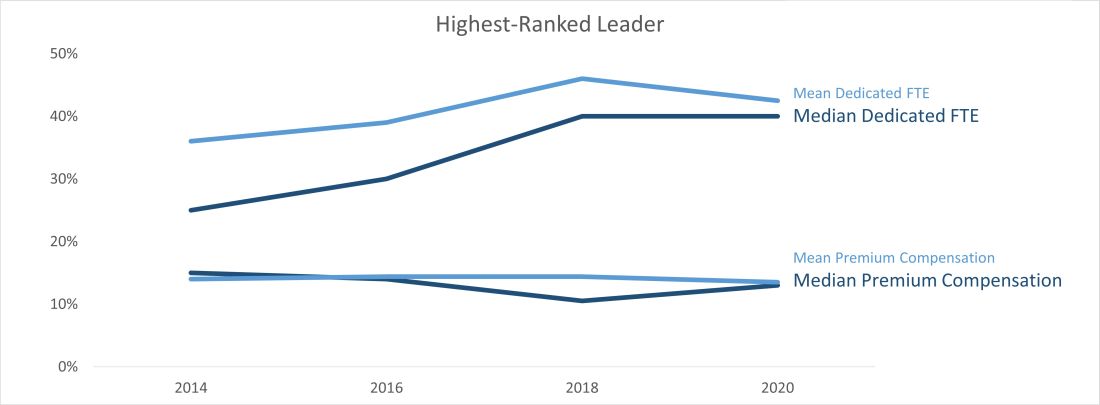
At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
The differences between the mean and median of leadership data
The differences between the mean and median of leadership data
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.

The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.

At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.

The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.

At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
Cardiogenic shock teams again tied to lower mortality
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
FDA OKs Pfizer COVID booster for 65 and over, those at high risk
The agency’s move comes as a Centers for Disease Control and Prevention (CDC) panel ended the first day of a 2-day meeting. That panel, the Advisory Committee on Immunization Practices (ACIP), is expected to vote Sept. 23 to instruct doctors on how to administer the boosters.
The FDA officially authorized the vaccine not only for individuals 65 and older, but also for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection.
“After considering the totality of the available scientific evidence and the deliberations of our advisory committee of independent, external experts, the FDA amended the EUA for the Pfizer-BioNTech COVID-19 vaccine to allow for a booster dose in certain populations such as health care workers, teachers and daycare staff, grocery workers and those in homeless shelters or prisons, among others,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release.
The recommendations align with those from an FDA advisory panel Sept. 17.
The agency determined that the benefits of a booster dose outweigh the risks for people now authorized to receive it, according to the news release.
Other questions remain
So, how will this work? That was the main question weighing on the minds of the CDC’s ACIP during their first day of a 2-day meeting where they are expected to make recommendations on booster doses for Americans.
The panel discussed situations the FDA will still need to consider, such as what should be done for Americans who were originally vaccinated with a Moderna or Johnson and Johnson vaccine, but are not covered under the revised EUA, which is only for those people who received Pfizer’s two-dose vaccine regimen.
“That’s going to leave half of the people immunized in this age group having received the vaccine and being told that they’re at risk now for waning immunity and hospitalization unable to get a booster dose,” said committee member Sarah S. Long, MD, a professor of pediatrics at Drexel University College of Medicine in Philadelphia. “So that’s a big public health panic that we would like to avoid.”
Johnson and Johnson recently reported that second doses of its vaccine boosted its efficacy to almost 94% against COVID-19. A new study, published ahead of peer review, suggests that the efficacy of the single-dose Johnson and Johnson shot has fallen to about 78% against symptomatic infection during the Delta surge.
Moderna has applied for permission to market third doses of its vaccine in the United States, but the FDA has given no timeline on when it might make a decision.
Doran Fink, MD, PhD, deputy director of the FDA’s Division of Vaccines and Related Products Applications, a representative advising the committee Sept. 22, said the agency was working as rapidly as possible on Moderna’s submission.
Regarding the question of whether it was OK to mix vaccines, rather than match them, Dr. Fink said there are currently not enough data available to inform that decision.
Those answers are coming, though. John Beigel, MD, associate director of clinical research at the National Institute of Allergy and Infectious Diseases, revealed that the federal government has a study underway to see what happens when the vaccines are mixed with each other.
He said that data from the study would be available later this fall, and would certainly help physicians and other healthcare providers know whether it’s effective or safe to use them interchangeably.
Correlates of immunity
The ACIP left much of its schedule open Sept. 23 to discuss extra Pfizer doses and vote on how they should be used.
Pfizer had originally applied to the FDA for an amendment to its FDA approval, which would have given doctors a freer hand to prescribe third doses as they saw fit, in patients as young as 16.
But the FDA’s Vaccines and Related Biological Products Advisory Committee voted Sept. 17 against granting the amendment. The committee was particularly concerned about the lack of data in teens ages 16 and 17, who have the highest risk for a rare side effect that causes heart inflammation that requires hospital care.
Instead, they recommended — and the FDA agreed per their decision Sept. 22 — that third doses should be given to people at higher risk for severe breakthrough infections because of advanced age or because they work in an occupation that puts them at high risk for exposure.
The CDC panel heard important presentations on new science that is helping to identify the correlates of immunity.
The correlates of immunity are biomarkers that can be measured in blood that help doctors understand how protected a person may be against COVID-19. These markers of immunity are not yet known for the COVID-19 vaccines.
Emerging evidence shows that booster doses of the Pfizer vaccine cause front-line immune defenders — called binding antibodies — to roughly triple soon after a person gets the third shot.
Neutralizing antibodies also jump soon after two vaccine doses, but they fall over time, which is natural. The body doesn’t need these foot soldiers to be on guard all the time, so they go away.
The body retains its memory of how to make them, however, so they can quickly be marshaled again, if needed.
Early studies suggest that antibodies account for about two thirds of a person’s protection against COVID, while the longer-lasting T-cells and B-cells account for about one third.
After the antibody levels fall, it may take a few days to recreate this army. In the meantime, the virus can try to break in. This can cause symptoms, which can make a person feel terrible, but for the most part, vaccinated individuals don’t need hospital care and are nearly always protected from dying — even against the Delta variant.
Those most likely to be at risk for a breakthrough infection are older, because immune function wanes with age.
Essential workers
Essential workers, such as those who work in healthcare, may also benefit from high antibody levels, which can minimize symptoms and help them get back to work more quickly.
Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, said that in her area staffing levels are critical right now.
“I’m actually sitting in one of the deepest red [states] with high rates of COVID. We don’t have enough health care workers currently to take care of the unvaccinated,” she said.
“When we have beds, we are often missing staff, and so the idea of vaccinating health care workers is to be a little bit different than our idea of using vaccines in the general population,” Dr. Talbot said.
Oliver Brooks, MD, chief medical officer of the Watts Healthcare Corporation in Los Angeles, said he was in favor of making a public statement about the temporary nature of the potential recommendations Sept. 23, because they probably won’t cover all who might need a third shot.
“We may want to go on record stating what it is that would allow us to broaden our recommendation or restrict our recommendation,” Dr. Brooks said.
The considerations of who should get an extra dose are not always straightforward.
New modeling by the Harvard TH Chan School of Public Health and the CDC to assist the government’s decisions on boosters had a surprise finding: in nursing homes, it’s more effective to vaccinate healthcare workers than it is to give booster doses to these residents. Nursing homes are at the mercy of community transmission.
In regions with high transmission, it’s easy for a caregiver to bring the virus into a facility — so the models found that the transmission from these workers is a more effective strategy than giving third doses to the already highly vaccinated group of seniors who live in them.
A version of this article first appeared on Medscape.com.
The agency’s move comes as a Centers for Disease Control and Prevention (CDC) panel ended the first day of a 2-day meeting. That panel, the Advisory Committee on Immunization Practices (ACIP), is expected to vote Sept. 23 to instruct doctors on how to administer the boosters.
The FDA officially authorized the vaccine not only for individuals 65 and older, but also for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection.
“After considering the totality of the available scientific evidence and the deliberations of our advisory committee of independent, external experts, the FDA amended the EUA for the Pfizer-BioNTech COVID-19 vaccine to allow for a booster dose in certain populations such as health care workers, teachers and daycare staff, grocery workers and those in homeless shelters or prisons, among others,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release.
The recommendations align with those from an FDA advisory panel Sept. 17.
The agency determined that the benefits of a booster dose outweigh the risks for people now authorized to receive it, according to the news release.
Other questions remain
So, how will this work? That was the main question weighing on the minds of the CDC’s ACIP during their first day of a 2-day meeting where they are expected to make recommendations on booster doses for Americans.
The panel discussed situations the FDA will still need to consider, such as what should be done for Americans who were originally vaccinated with a Moderna or Johnson and Johnson vaccine, but are not covered under the revised EUA, which is only for those people who received Pfizer’s two-dose vaccine regimen.
“That’s going to leave half of the people immunized in this age group having received the vaccine and being told that they’re at risk now for waning immunity and hospitalization unable to get a booster dose,” said committee member Sarah S. Long, MD, a professor of pediatrics at Drexel University College of Medicine in Philadelphia. “So that’s a big public health panic that we would like to avoid.”
Johnson and Johnson recently reported that second doses of its vaccine boosted its efficacy to almost 94% against COVID-19. A new study, published ahead of peer review, suggests that the efficacy of the single-dose Johnson and Johnson shot has fallen to about 78% against symptomatic infection during the Delta surge.
Moderna has applied for permission to market third doses of its vaccine in the United States, but the FDA has given no timeline on when it might make a decision.
Doran Fink, MD, PhD, deputy director of the FDA’s Division of Vaccines and Related Products Applications, a representative advising the committee Sept. 22, said the agency was working as rapidly as possible on Moderna’s submission.
Regarding the question of whether it was OK to mix vaccines, rather than match them, Dr. Fink said there are currently not enough data available to inform that decision.
Those answers are coming, though. John Beigel, MD, associate director of clinical research at the National Institute of Allergy and Infectious Diseases, revealed that the federal government has a study underway to see what happens when the vaccines are mixed with each other.
He said that data from the study would be available later this fall, and would certainly help physicians and other healthcare providers know whether it’s effective or safe to use them interchangeably.
Correlates of immunity
The ACIP left much of its schedule open Sept. 23 to discuss extra Pfizer doses and vote on how they should be used.
Pfizer had originally applied to the FDA for an amendment to its FDA approval, which would have given doctors a freer hand to prescribe third doses as they saw fit, in patients as young as 16.
But the FDA’s Vaccines and Related Biological Products Advisory Committee voted Sept. 17 against granting the amendment. The committee was particularly concerned about the lack of data in teens ages 16 and 17, who have the highest risk for a rare side effect that causes heart inflammation that requires hospital care.
Instead, they recommended — and the FDA agreed per their decision Sept. 22 — that third doses should be given to people at higher risk for severe breakthrough infections because of advanced age or because they work in an occupation that puts them at high risk for exposure.
The CDC panel heard important presentations on new science that is helping to identify the correlates of immunity.
The correlates of immunity are biomarkers that can be measured in blood that help doctors understand how protected a person may be against COVID-19. These markers of immunity are not yet known for the COVID-19 vaccines.
Emerging evidence shows that booster doses of the Pfizer vaccine cause front-line immune defenders — called binding antibodies — to roughly triple soon after a person gets the third shot.
Neutralizing antibodies also jump soon after two vaccine doses, but they fall over time, which is natural. The body doesn’t need these foot soldiers to be on guard all the time, so they go away.
The body retains its memory of how to make them, however, so they can quickly be marshaled again, if needed.
Early studies suggest that antibodies account for about two thirds of a person’s protection against COVID, while the longer-lasting T-cells and B-cells account for about one third.
After the antibody levels fall, it may take a few days to recreate this army. In the meantime, the virus can try to break in. This can cause symptoms, which can make a person feel terrible, but for the most part, vaccinated individuals don’t need hospital care and are nearly always protected from dying — even against the Delta variant.
Those most likely to be at risk for a breakthrough infection are older, because immune function wanes with age.
Essential workers
Essential workers, such as those who work in healthcare, may also benefit from high antibody levels, which can minimize symptoms and help them get back to work more quickly.
Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, said that in her area staffing levels are critical right now.
“I’m actually sitting in one of the deepest red [states] with high rates of COVID. We don’t have enough health care workers currently to take care of the unvaccinated,” she said.
“When we have beds, we are often missing staff, and so the idea of vaccinating health care workers is to be a little bit different than our idea of using vaccines in the general population,” Dr. Talbot said.
Oliver Brooks, MD, chief medical officer of the Watts Healthcare Corporation in Los Angeles, said he was in favor of making a public statement about the temporary nature of the potential recommendations Sept. 23, because they probably won’t cover all who might need a third shot.
“We may want to go on record stating what it is that would allow us to broaden our recommendation or restrict our recommendation,” Dr. Brooks said.
The considerations of who should get an extra dose are not always straightforward.
New modeling by the Harvard TH Chan School of Public Health and the CDC to assist the government’s decisions on boosters had a surprise finding: in nursing homes, it’s more effective to vaccinate healthcare workers than it is to give booster doses to these residents. Nursing homes are at the mercy of community transmission.
In regions with high transmission, it’s easy for a caregiver to bring the virus into a facility — so the models found that the transmission from these workers is a more effective strategy than giving third doses to the already highly vaccinated group of seniors who live in them.
A version of this article first appeared on Medscape.com.
The agency’s move comes as a Centers for Disease Control and Prevention (CDC) panel ended the first day of a 2-day meeting. That panel, the Advisory Committee on Immunization Practices (ACIP), is expected to vote Sept. 23 to instruct doctors on how to administer the boosters.
The FDA officially authorized the vaccine not only for individuals 65 and older, but also for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection.
“After considering the totality of the available scientific evidence and the deliberations of our advisory committee of independent, external experts, the FDA amended the EUA for the Pfizer-BioNTech COVID-19 vaccine to allow for a booster dose in certain populations such as health care workers, teachers and daycare staff, grocery workers and those in homeless shelters or prisons, among others,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release.
The recommendations align with those from an FDA advisory panel Sept. 17.
The agency determined that the benefits of a booster dose outweigh the risks for people now authorized to receive it, according to the news release.
Other questions remain
So, how will this work? That was the main question weighing on the minds of the CDC’s ACIP during their first day of a 2-day meeting where they are expected to make recommendations on booster doses for Americans.
The panel discussed situations the FDA will still need to consider, such as what should be done for Americans who were originally vaccinated with a Moderna or Johnson and Johnson vaccine, but are not covered under the revised EUA, which is only for those people who received Pfizer’s two-dose vaccine regimen.
“That’s going to leave half of the people immunized in this age group having received the vaccine and being told that they’re at risk now for waning immunity and hospitalization unable to get a booster dose,” said committee member Sarah S. Long, MD, a professor of pediatrics at Drexel University College of Medicine in Philadelphia. “So that’s a big public health panic that we would like to avoid.”
Johnson and Johnson recently reported that second doses of its vaccine boosted its efficacy to almost 94% against COVID-19. A new study, published ahead of peer review, suggests that the efficacy of the single-dose Johnson and Johnson shot has fallen to about 78% against symptomatic infection during the Delta surge.
Moderna has applied for permission to market third doses of its vaccine in the United States, but the FDA has given no timeline on when it might make a decision.
Doran Fink, MD, PhD, deputy director of the FDA’s Division of Vaccines and Related Products Applications, a representative advising the committee Sept. 22, said the agency was working as rapidly as possible on Moderna’s submission.
Regarding the question of whether it was OK to mix vaccines, rather than match them, Dr. Fink said there are currently not enough data available to inform that decision.
Those answers are coming, though. John Beigel, MD, associate director of clinical research at the National Institute of Allergy and Infectious Diseases, revealed that the federal government has a study underway to see what happens when the vaccines are mixed with each other.
He said that data from the study would be available later this fall, and would certainly help physicians and other healthcare providers know whether it’s effective or safe to use them interchangeably.
Correlates of immunity
The ACIP left much of its schedule open Sept. 23 to discuss extra Pfizer doses and vote on how they should be used.
Pfizer had originally applied to the FDA for an amendment to its FDA approval, which would have given doctors a freer hand to prescribe third doses as they saw fit, in patients as young as 16.
But the FDA’s Vaccines and Related Biological Products Advisory Committee voted Sept. 17 against granting the amendment. The committee was particularly concerned about the lack of data in teens ages 16 and 17, who have the highest risk for a rare side effect that causes heart inflammation that requires hospital care.
Instead, they recommended — and the FDA agreed per their decision Sept. 22 — that third doses should be given to people at higher risk for severe breakthrough infections because of advanced age or because they work in an occupation that puts them at high risk for exposure.
The CDC panel heard important presentations on new science that is helping to identify the correlates of immunity.
The correlates of immunity are biomarkers that can be measured in blood that help doctors understand how protected a person may be against COVID-19. These markers of immunity are not yet known for the COVID-19 vaccines.
Emerging evidence shows that booster doses of the Pfizer vaccine cause front-line immune defenders — called binding antibodies — to roughly triple soon after a person gets the third shot.
Neutralizing antibodies also jump soon after two vaccine doses, but they fall over time, which is natural. The body doesn’t need these foot soldiers to be on guard all the time, so they go away.
The body retains its memory of how to make them, however, so they can quickly be marshaled again, if needed.
Early studies suggest that antibodies account for about two thirds of a person’s protection against COVID, while the longer-lasting T-cells and B-cells account for about one third.
After the antibody levels fall, it may take a few days to recreate this army. In the meantime, the virus can try to break in. This can cause symptoms, which can make a person feel terrible, but for the most part, vaccinated individuals don’t need hospital care and are nearly always protected from dying — even against the Delta variant.
Those most likely to be at risk for a breakthrough infection are older, because immune function wanes with age.
Essential workers
Essential workers, such as those who work in healthcare, may also benefit from high antibody levels, which can minimize symptoms and help them get back to work more quickly.
Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, said that in her area staffing levels are critical right now.
“I’m actually sitting in one of the deepest red [states] with high rates of COVID. We don’t have enough health care workers currently to take care of the unvaccinated,” she said.
“When we have beds, we are often missing staff, and so the idea of vaccinating health care workers is to be a little bit different than our idea of using vaccines in the general population,” Dr. Talbot said.
Oliver Brooks, MD, chief medical officer of the Watts Healthcare Corporation in Los Angeles, said he was in favor of making a public statement about the temporary nature of the potential recommendations Sept. 23, because they probably won’t cover all who might need a third shot.
“We may want to go on record stating what it is that would allow us to broaden our recommendation or restrict our recommendation,” Dr. Brooks said.
The considerations of who should get an extra dose are not always straightforward.
New modeling by the Harvard TH Chan School of Public Health and the CDC to assist the government’s decisions on boosters had a surprise finding: in nursing homes, it’s more effective to vaccinate healthcare workers than it is to give booster doses to these residents. Nursing homes are at the mercy of community transmission.
In regions with high transmission, it’s easy for a caregiver to bring the virus into a facility — so the models found that the transmission from these workers is a more effective strategy than giving third doses to the already highly vaccinated group of seniors who live in them.
A version of this article first appeared on Medscape.com.
Should hospitalists use albumin to treat non-SBP infections in patients with cirrhosis?
Caution is advised in patients at risk of pulmonary edema
Case
A 56 year-old male with hypertension, alcohol use disorder, stage II chronic kidney disease, and biopsy-proven cirrhosis presents with fever and chills, pyuria, flank pain, and an acute kidney injury concerning for pyelonephritis. Is there a benefit in treating with albumin in addition to guideline-based antibiotics?
Brief overview of the issue
Albumin is a negatively charged human protein produced by the liver. Albumin comprises 50% of plasma protein and over 75% of plasma oncotic pressure.1 It was first used at Walter Reed Hospital in 1940 and subsequently for burn injuries after the attack on Pearl Harbor in 1941.2
Albumin serves several important physiologic functions including maintaining oncotic pressure, endothelial support, antioxidation, nitrogen oxide scavenging, and buffering and transport of solutes and drugs, including antibiotics. In cirrhosis, albumin is diluted due to sodium and water retention. There is increased redistribution, decreased synthesis by the liver, and impaired albumin molecule binding.3
For patients with liver disease, per the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD), albumin should be administered to prevent post paracentesis circulatory dysfunction after large volume paracentesis, to prevent renal failure and mortality in the setting of spontaneous bacterial peritonitis (SBP), and in the diagnosis and treatment of hepatorenal syndrome (HRS) type I to potentially improve mortality.4,5 Beyond these three guideline-based indications, other uses for albumin for patients with liver disease have been proposed, including treatment of hyponatremia, posttransplant fluid resuscitation, diuretic unresponsive ascites, and long-term management of cirrhosis. There has yet to be strong evidence supporting these additional indications. However, given the known benefits of albumin in patients with SBP, there has been recent research into treatment of non-SBP infections, including urinary tract infections.
Overview of the data
There have been three randomized controlled trials (RCTs) regarding albumin administration for the treatment of non-SBP infections for hospitalized patients with cirrhosis. All three trials randomized patients to a treatment arm of albumin and antibiotics versus a control group of antibiotics alone. The treatment protocol prescribed 20% albumin with 1.5 g/kg on day 1 and 1.0 g/kg on day 3. The most common infections studied were pneumonia and urinary tract infection. These RCTs found that albumin administration was associated with improved renal and/or circulatory function, but not with a reduction in mortality.

First, there was a single center RCT by Guevara et al. in 2012 of 110 patients with cirrhosis and infection based on SIRS criteria.6 The primary outcome was 90-day survival with secondary outcomes of renal failure development, renal function at days 3,7 and 14, and circulatory function measured by plasma renin, aldosterone, and norepinephrine. Renal function and circulatory function improved in the albumin group, but not mortality. In a multivariable regression analysis, albumin was statistically predictive of survival (hazard ratio of 0.294).
Second, there was a multicenter RCT by Thévenot et al. in 2015 of 193 patients.7 The primary outcome was 90-day renal failure and the secondary outcome was 90-day survival. Renal failure was chosen as the primary endpoint because of its association with survival in this patient population. The treatment group had delayed onset of renal failure, but no difference in the development of 90-day renal failure or 90-day mortality rate. Notably, eight patients (8.3%) in the albumin group developed pulmonary edema with two deaths. This led the oversight committee to prematurely terminate the study.
Third and most recently, there was a multicenter RCT by Fernández et al. in 2019 of 118 patients.8 The primary outcome was in-hospital mortality, with secondary outcomes of circulatory dysfunction measured by plasma renin concentration, systemic inflammation measured by plasma IL-6 and biomarkers, complications including acute-on-chronic liver failure (ACLF) and nosocomial bacterial infections, and 90-day mortality. Between the albumin and control group, there were no differences in in-hospital mortality (13.1% vs. 10.5%, P > .66), inflammation, circulatory dysfunction, or liver severity. However, a significantly higher proportion of patients in the albumin group had resolution of their ACLF (82.3% vs. 33.3%, P = .003) and a lower proportion developed nosocomial infections (6.6% vs. 24.6%, P = .007). A major weakness of this study was that patients in the albumin group had a higher combined rate of ACLF and kidney dysfunction (44.3% vs. 24.6%, P = .02).
Beyond these three randomized controlled trials, there was a study on the long-term administration of albumin for patients with cirrhosis and ascites. Patients who received twice weekly albumin infusions had a lower 2-year mortality rate and a reduction in the incidence of both SBP and non-SBP infections.9 Another long-term study of albumin administration found similar results with greater 18-month survival and fewer non-SBP infections.10 A trial looking at inflammation in patients without bacterial infections and in biobanked samples from cirrhotic patients with bacterial infections found that treatment with albumin reduced systemic inflammation.11
In summary, the three RCTs looked at comparable patients with cirrhosis and a non-SBP infection and all underwent similar treatment protocols with 20% albumin dosed at 1.5 g/kg on day 1 and 1.0 g/kg on day 3. All studies evaluated mortality in either the primary or secondary outcome, and none found significant differences in mortality between treatment and control groups. Each study also evaluated and found improvement in renal and/or circulatory function. Fernández et al. also found increased resolution of ACLF, fewer nosocomial infections, and reduction in some inflammatory markers. However, all studies had relatively small sample sizes that were underpowered to detect mortality differences. Furthermore, randomization did not lead to well-matched groups, with the treatment group patients in the Fernández study having higher rates of ACLF and kidney dysfunction.
The data suggest that albumin may be beneficial in improving renal and circulatory function. In select patients with ACLF and elevated serum creatinine, albumin treatment may be considered. There has been discussion about the use of albumin preferentially in patients with subdiaphragmatic bacterial infections, most related to increased risk of renal failure such as biliary and urinary tract infections.12 The authors of these studies also note that albumin may be more beneficial in patients with higher baseline creatinine. Caution is warranted for patients with impaired cardiac function or poor respiratory status given the possibility of pulmonary edema. Finally, the high cost of albumin in many medical centers is a major limitation of this treatment approach.
Application of data to our patient
Our patient has cirrhosis and is acutely presenting with pyelonephritis and acute kidney injury. He has no baseline pulmonary disease or oxygen requirement. His recent transthoracic echocardiogram is reviewed and he has no evidence of cardiac disease.
Because he has an elevated creatinine, an infectious process associated with progressive renal failure, and is not at an elevated baseline risk of developing pulmonary edema, albumin would be reasonable to administer at 1.5 g/kg on day 1 and 1.0 g/kg on day 3 of hospitalization.
Bottom line
In certain patients with cirrhosis and a non-SBP infection, the use of albumin to help improve renal and circulatory function is reasonable. There is no evidence that albumin will improve mortality and caution is warranted for patients at risk for pulmonary edema.
Dr. Rambachan is an academic hospital medicine fellow at the University of California, San Francisco.
References
1. Caironi P and Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus. 2009;7(4):259-67. doi: 10.2450/2009.0002-09.
2. Paine CH et al. Albumin in cirrhosis: More than a colloid. Curr Treat Options Gastroenterol. 2019;17(2):231-43. doi: 10.1007/s11938-019-00227-4.
3. Walayat S et al. Role of albumin in cirrhosis: from a hospitalist’s perspective. J Community Hosp Intern Med Perspect. 2017;7(1):8-14. 2017 Mar 31. doi: 10.1080/20009666.2017.1302704.
4. Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline [for the] management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013 Apr;57(4):1651-3. doi: 10.1002/hep.26359.
5. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis [published correction appears in J Hepatol. 2018 Nov;69(5):1207]. J Hepatol. 2018 Aug;69(2):406-60. doi: 10.1016/j.jhep.2018.03.024.
6. Guevara M et al. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol. 2012 Oct;57(4):759-65. doi: 10.1016/j.jhep.2012.06.013.
7. Thévenot T et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol. 2015 Apr;62(4):822-30. doi: 10.1016/j.jhep.2014.11.017.
8. Fernández J et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2020 Apr;18(4):963-73.e14. doi: 10.1016/j.c gh.2019.07.055.
9. Di Pascoli M et al. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019 Jan;39(1):98-105. doi: 10.1111/liv.13968.
10. Caraceni P et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial [published correction appears in Lancet. 2018 Aug 4;392(10145):386]. Lancet. 2018 June;391(10138):2417-29. doi: 10.1016/S0140-6736(18)30840-7.
11. Fernández J et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019 July;157(1):149-62. doi: 10.1053/j.gastro.2019.03.021.
12. Fasolato S et al. Renal failure and bacterial infections in patients with cirrhosis: Epidemiology and clinical features. Hepatology. 2007;45(1):223-9. doi: 10.1002/hep.21443.
Key points
- In patients with spontaneous bacterial peritonitis, hepatorenal syndrome, and for large volume paracentesis, albumin improves outcomes and is recommended by guidelines.
- In patients with cirrhosis and a non-SBP infection, there is some evidence that albumin may improve renal and circulatory function.
- Clinicians should be cautious about albumin use in patients at an elevated risk for development of pulmonary edema.
Quiz
Which of the following is not a guideline-recommended use of albumin for patients with cirrhosis?
A. Treatment of type 1 hepatorenal syndrome
B. Treatment of spontaneous bacterial peritonitis
C. To correct plasma albumin < 2.5 g/dL in nontransplant patients
D. Post large-volume paracentesis
The answer is C. Per the EASL and AASLD, A,B, and D are recommended. There is not strong evidence to support administering albumin to correct low plasma albumin.
Additional reading
- Bernardi M et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. 2020 June;69(6):1127-38. doi: 10.1136/gutjnl-2019-318843.
- Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline [for the] management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013 Apr;57(4):1651-3. doi: 10.1002/hep.26359.
- Paine CH et al. Albumin in cirrhosis: More than a colloid. Curr Treat Options Gastroenterol. 2019 June;17(2):231-43. doi: 10.1007/s11938-019-00227-4.
Caution is advised in patients at risk of pulmonary edema
Caution is advised in patients at risk of pulmonary edema
Case
A 56 year-old male with hypertension, alcohol use disorder, stage II chronic kidney disease, and biopsy-proven cirrhosis presents with fever and chills, pyuria, flank pain, and an acute kidney injury concerning for pyelonephritis. Is there a benefit in treating with albumin in addition to guideline-based antibiotics?
Brief overview of the issue
Albumin is a negatively charged human protein produced by the liver. Albumin comprises 50% of plasma protein and over 75% of plasma oncotic pressure.1 It was first used at Walter Reed Hospital in 1940 and subsequently for burn injuries after the attack on Pearl Harbor in 1941.2
Albumin serves several important physiologic functions including maintaining oncotic pressure, endothelial support, antioxidation, nitrogen oxide scavenging, and buffering and transport of solutes and drugs, including antibiotics. In cirrhosis, albumin is diluted due to sodium and water retention. There is increased redistribution, decreased synthesis by the liver, and impaired albumin molecule binding.3
For patients with liver disease, per the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD), albumin should be administered to prevent post paracentesis circulatory dysfunction after large volume paracentesis, to prevent renal failure and mortality in the setting of spontaneous bacterial peritonitis (SBP), and in the diagnosis and treatment of hepatorenal syndrome (HRS) type I to potentially improve mortality.4,5 Beyond these three guideline-based indications, other uses for albumin for patients with liver disease have been proposed, including treatment of hyponatremia, posttransplant fluid resuscitation, diuretic unresponsive ascites, and long-term management of cirrhosis. There has yet to be strong evidence supporting these additional indications. However, given the known benefits of albumin in patients with SBP, there has been recent research into treatment of non-SBP infections, including urinary tract infections.
Overview of the data
There have been three randomized controlled trials (RCTs) regarding albumin administration for the treatment of non-SBP infections for hospitalized patients with cirrhosis. All three trials randomized patients to a treatment arm of albumin and antibiotics versus a control group of antibiotics alone. The treatment protocol prescribed 20% albumin with 1.5 g/kg on day 1 and 1.0 g/kg on day 3. The most common infections studied were pneumonia and urinary tract infection. These RCTs found that albumin administration was associated with improved renal and/or circulatory function, but not with a reduction in mortality.

First, there was a single center RCT by Guevara et al. in 2012 of 110 patients with cirrhosis and infection based on SIRS criteria.6 The primary outcome was 90-day survival with secondary outcomes of renal failure development, renal function at days 3,7 and 14, and circulatory function measured by plasma renin, aldosterone, and norepinephrine. Renal function and circulatory function improved in the albumin group, but not mortality. In a multivariable regression analysis, albumin was statistically predictive of survival (hazard ratio of 0.294).
Second, there was a multicenter RCT by Thévenot et al. in 2015 of 193 patients.7 The primary outcome was 90-day renal failure and the secondary outcome was 90-day survival. Renal failure was chosen as the primary endpoint because of its association with survival in this patient population. The treatment group had delayed onset of renal failure, but no difference in the development of 90-day renal failure or 90-day mortality rate. Notably, eight patients (8.3%) in the albumin group developed pulmonary edema with two deaths. This led the oversight committee to prematurely terminate the study.
Third and most recently, there was a multicenter RCT by Fernández et al. in 2019 of 118 patients.8 The primary outcome was in-hospital mortality, with secondary outcomes of circulatory dysfunction measured by plasma renin concentration, systemic inflammation measured by plasma IL-6 and biomarkers, complications including acute-on-chronic liver failure (ACLF) and nosocomial bacterial infections, and 90-day mortality. Between the albumin and control group, there were no differences in in-hospital mortality (13.1% vs. 10.5%, P > .66), inflammation, circulatory dysfunction, or liver severity. However, a significantly higher proportion of patients in the albumin group had resolution of their ACLF (82.3% vs. 33.3%, P = .003) and a lower proportion developed nosocomial infections (6.6% vs. 24.6%, P = .007). A major weakness of this study was that patients in the albumin group had a higher combined rate of ACLF and kidney dysfunction (44.3% vs. 24.6%, P = .02).
Beyond these three randomized controlled trials, there was a study on the long-term administration of albumin for patients with cirrhosis and ascites. Patients who received twice weekly albumin infusions had a lower 2-year mortality rate and a reduction in the incidence of both SBP and non-SBP infections.9 Another long-term study of albumin administration found similar results with greater 18-month survival and fewer non-SBP infections.10 A trial looking at inflammation in patients without bacterial infections and in biobanked samples from cirrhotic patients with bacterial infections found that treatment with albumin reduced systemic inflammation.11
In summary, the three RCTs looked at comparable patients with cirrhosis and a non-SBP infection and all underwent similar treatment protocols with 20% albumin dosed at 1.5 g/kg on day 1 and 1.0 g/kg on day 3. All studies evaluated mortality in either the primary or secondary outcome, and none found significant differences in mortality between treatment and control groups. Each study also evaluated and found improvement in renal and/or circulatory function. Fernández et al. also found increased resolution of ACLF, fewer nosocomial infections, and reduction in some inflammatory markers. However, all studies had relatively small sample sizes that were underpowered to detect mortality differences. Furthermore, randomization did not lead to well-matched groups, with the treatment group patients in the Fernández study having higher rates of ACLF and kidney dysfunction.
The data suggest that albumin may be beneficial in improving renal and circulatory function. In select patients with ACLF and elevated serum creatinine, albumin treatment may be considered. There has been discussion about the use of albumin preferentially in patients with subdiaphragmatic bacterial infections, most related to increased risk of renal failure such as biliary and urinary tract infections.12 The authors of these studies also note that albumin may be more beneficial in patients with higher baseline creatinine. Caution is warranted for patients with impaired cardiac function or poor respiratory status given the possibility of pulmonary edema. Finally, the high cost of albumin in many medical centers is a major limitation of this treatment approach.
Application of data to our patient
Our patient has cirrhosis and is acutely presenting with pyelonephritis and acute kidney injury. He has no baseline pulmonary disease or oxygen requirement. His recent transthoracic echocardiogram is reviewed and he has no evidence of cardiac disease.
Because he has an elevated creatinine, an infectious process associated with progressive renal failure, and is not at an elevated baseline risk of developing pulmonary edema, albumin would be reasonable to administer at 1.5 g/kg on day 1 and 1.0 g/kg on day 3 of hospitalization.
Bottom line
In certain patients with cirrhosis and a non-SBP infection, the use of albumin to help improve renal and circulatory function is reasonable. There is no evidence that albumin will improve mortality and caution is warranted for patients at risk for pulmonary edema.
Dr. Rambachan is an academic hospital medicine fellow at the University of California, San Francisco.
References
1. Caironi P and Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus. 2009;7(4):259-67. doi: 10.2450/2009.0002-09.
2. Paine CH et al. Albumin in cirrhosis: More than a colloid. Curr Treat Options Gastroenterol. 2019;17(2):231-43. doi: 10.1007/s11938-019-00227-4.
3. Walayat S et al. Role of albumin in cirrhosis: from a hospitalist’s perspective. J Community Hosp Intern Med Perspect. 2017;7(1):8-14. 2017 Mar 31. doi: 10.1080/20009666.2017.1302704.
4. Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline [for the] management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013 Apr;57(4):1651-3. doi: 10.1002/hep.26359.
5. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis [published correction appears in J Hepatol. 2018 Nov;69(5):1207]. J Hepatol. 2018 Aug;69(2):406-60. doi: 10.1016/j.jhep.2018.03.024.
6. Guevara M et al. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol. 2012 Oct;57(4):759-65. doi: 10.1016/j.jhep.2012.06.013.
7. Thévenot T et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol. 2015 Apr;62(4):822-30. doi: 10.1016/j.jhep.2014.11.017.
8. Fernández J et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2020 Apr;18(4):963-73.e14. doi: 10.1016/j.c gh.2019.07.055.
9. Di Pascoli M et al. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019 Jan;39(1):98-105. doi: 10.1111/liv.13968.
10. Caraceni P et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial [published correction appears in Lancet. 2018 Aug 4;392(10145):386]. Lancet. 2018 June;391(10138):2417-29. doi: 10.1016/S0140-6736(18)30840-7.
11. Fernández J et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019 July;157(1):149-62. doi: 10.1053/j.gastro.2019.03.021.
12. Fasolato S et al. Renal failure and bacterial infections in patients with cirrhosis: Epidemiology and clinical features. Hepatology. 2007;45(1):223-9. doi: 10.1002/hep.21443.
Key points
- In patients with spontaneous bacterial peritonitis, hepatorenal syndrome, and for large volume paracentesis, albumin improves outcomes and is recommended by guidelines.
- In patients with cirrhosis and a non-SBP infection, there is some evidence that albumin may improve renal and circulatory function.
- Clinicians should be cautious about albumin use in patients at an elevated risk for development of pulmonary edema.
Quiz
Which of the following is not a guideline-recommended use of albumin for patients with cirrhosis?
A. Treatment of type 1 hepatorenal syndrome
B. Treatment of spontaneous bacterial peritonitis
C. To correct plasma albumin < 2.5 g/dL in nontransplant patients
D. Post large-volume paracentesis
The answer is C. Per the EASL and AASLD, A,B, and D are recommended. There is not strong evidence to support administering albumin to correct low plasma albumin.
Additional reading
- Bernardi M et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. 2020 June;69(6):1127-38. doi: 10.1136/gutjnl-2019-318843.
- Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline [for the] management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013 Apr;57(4):1651-3. doi: 10.1002/hep.26359.
- Paine CH et al. Albumin in cirrhosis: More than a colloid. Curr Treat Options Gastroenterol. 2019 June;17(2):231-43. doi: 10.1007/s11938-019-00227-4.
Case
A 56 year-old male with hypertension, alcohol use disorder, stage II chronic kidney disease, and biopsy-proven cirrhosis presents with fever and chills, pyuria, flank pain, and an acute kidney injury concerning for pyelonephritis. Is there a benefit in treating with albumin in addition to guideline-based antibiotics?
Brief overview of the issue
Albumin is a negatively charged human protein produced by the liver. Albumin comprises 50% of plasma protein and over 75% of plasma oncotic pressure.1 It was first used at Walter Reed Hospital in 1940 and subsequently for burn injuries after the attack on Pearl Harbor in 1941.2
Albumin serves several important physiologic functions including maintaining oncotic pressure, endothelial support, antioxidation, nitrogen oxide scavenging, and buffering and transport of solutes and drugs, including antibiotics. In cirrhosis, albumin is diluted due to sodium and water retention. There is increased redistribution, decreased synthesis by the liver, and impaired albumin molecule binding.3
For patients with liver disease, per the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD), albumin should be administered to prevent post paracentesis circulatory dysfunction after large volume paracentesis, to prevent renal failure and mortality in the setting of spontaneous bacterial peritonitis (SBP), and in the diagnosis and treatment of hepatorenal syndrome (HRS) type I to potentially improve mortality.4,5 Beyond these three guideline-based indications, other uses for albumin for patients with liver disease have been proposed, including treatment of hyponatremia, posttransplant fluid resuscitation, diuretic unresponsive ascites, and long-term management of cirrhosis. There has yet to be strong evidence supporting these additional indications. However, given the known benefits of albumin in patients with SBP, there has been recent research into treatment of non-SBP infections, including urinary tract infections.
Overview of the data
There have been three randomized controlled trials (RCTs) regarding albumin administration for the treatment of non-SBP infections for hospitalized patients with cirrhosis. All three trials randomized patients to a treatment arm of albumin and antibiotics versus a control group of antibiotics alone. The treatment protocol prescribed 20% albumin with 1.5 g/kg on day 1 and 1.0 g/kg on day 3. The most common infections studied were pneumonia and urinary tract infection. These RCTs found that albumin administration was associated with improved renal and/or circulatory function, but not with a reduction in mortality.

First, there was a single center RCT by Guevara et al. in 2012 of 110 patients with cirrhosis and infection based on SIRS criteria.6 The primary outcome was 90-day survival with secondary outcomes of renal failure development, renal function at days 3,7 and 14, and circulatory function measured by plasma renin, aldosterone, and norepinephrine. Renal function and circulatory function improved in the albumin group, but not mortality. In a multivariable regression analysis, albumin was statistically predictive of survival (hazard ratio of 0.294).
Second, there was a multicenter RCT by Thévenot et al. in 2015 of 193 patients.7 The primary outcome was 90-day renal failure and the secondary outcome was 90-day survival. Renal failure was chosen as the primary endpoint because of its association with survival in this patient population. The treatment group had delayed onset of renal failure, but no difference in the development of 90-day renal failure or 90-day mortality rate. Notably, eight patients (8.3%) in the albumin group developed pulmonary edema with two deaths. This led the oversight committee to prematurely terminate the study.
Third and most recently, there was a multicenter RCT by Fernández et al. in 2019 of 118 patients.8 The primary outcome was in-hospital mortality, with secondary outcomes of circulatory dysfunction measured by plasma renin concentration, systemic inflammation measured by plasma IL-6 and biomarkers, complications including acute-on-chronic liver failure (ACLF) and nosocomial bacterial infections, and 90-day mortality. Between the albumin and control group, there were no differences in in-hospital mortality (13.1% vs. 10.5%, P > .66), inflammation, circulatory dysfunction, or liver severity. However, a significantly higher proportion of patients in the albumin group had resolution of their ACLF (82.3% vs. 33.3%, P = .003) and a lower proportion developed nosocomial infections (6.6% vs. 24.6%, P = .007). A major weakness of this study was that patients in the albumin group had a higher combined rate of ACLF and kidney dysfunction (44.3% vs. 24.6%, P = .02).
Beyond these three randomized controlled trials, there was a study on the long-term administration of albumin for patients with cirrhosis and ascites. Patients who received twice weekly albumin infusions had a lower 2-year mortality rate and a reduction in the incidence of both SBP and non-SBP infections.9 Another long-term study of albumin administration found similar results with greater 18-month survival and fewer non-SBP infections.10 A trial looking at inflammation in patients without bacterial infections and in biobanked samples from cirrhotic patients with bacterial infections found that treatment with albumin reduced systemic inflammation.11
In summary, the three RCTs looked at comparable patients with cirrhosis and a non-SBP infection and all underwent similar treatment protocols with 20% albumin dosed at 1.5 g/kg on day 1 and 1.0 g/kg on day 3. All studies evaluated mortality in either the primary or secondary outcome, and none found significant differences in mortality between treatment and control groups. Each study also evaluated and found improvement in renal and/or circulatory function. Fernández et al. also found increased resolution of ACLF, fewer nosocomial infections, and reduction in some inflammatory markers. However, all studies had relatively small sample sizes that were underpowered to detect mortality differences. Furthermore, randomization did not lead to well-matched groups, with the treatment group patients in the Fernández study having higher rates of ACLF and kidney dysfunction.
The data suggest that albumin may be beneficial in improving renal and circulatory function. In select patients with ACLF and elevated serum creatinine, albumin treatment may be considered. There has been discussion about the use of albumin preferentially in patients with subdiaphragmatic bacterial infections, most related to increased risk of renal failure such as biliary and urinary tract infections.12 The authors of these studies also note that albumin may be more beneficial in patients with higher baseline creatinine. Caution is warranted for patients with impaired cardiac function or poor respiratory status given the possibility of pulmonary edema. Finally, the high cost of albumin in many medical centers is a major limitation of this treatment approach.
Application of data to our patient
Our patient has cirrhosis and is acutely presenting with pyelonephritis and acute kidney injury. He has no baseline pulmonary disease or oxygen requirement. His recent transthoracic echocardiogram is reviewed and he has no evidence of cardiac disease.
Because he has an elevated creatinine, an infectious process associated with progressive renal failure, and is not at an elevated baseline risk of developing pulmonary edema, albumin would be reasonable to administer at 1.5 g/kg on day 1 and 1.0 g/kg on day 3 of hospitalization.
Bottom line
In certain patients with cirrhosis and a non-SBP infection, the use of albumin to help improve renal and circulatory function is reasonable. There is no evidence that albumin will improve mortality and caution is warranted for patients at risk for pulmonary edema.
Dr. Rambachan is an academic hospital medicine fellow at the University of California, San Francisco.
References
1. Caironi P and Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus. 2009;7(4):259-67. doi: 10.2450/2009.0002-09.
2. Paine CH et al. Albumin in cirrhosis: More than a colloid. Curr Treat Options Gastroenterol. 2019;17(2):231-43. doi: 10.1007/s11938-019-00227-4.
3. Walayat S et al. Role of albumin in cirrhosis: from a hospitalist’s perspective. J Community Hosp Intern Med Perspect. 2017;7(1):8-14. 2017 Mar 31. doi: 10.1080/20009666.2017.1302704.
4. Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline [for the] management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013 Apr;57(4):1651-3. doi: 10.1002/hep.26359.
5. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis [published correction appears in J Hepatol. 2018 Nov;69(5):1207]. J Hepatol. 2018 Aug;69(2):406-60. doi: 10.1016/j.jhep.2018.03.024.
6. Guevara M et al. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol. 2012 Oct;57(4):759-65. doi: 10.1016/j.jhep.2012.06.013.
7. Thévenot T et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol. 2015 Apr;62(4):822-30. doi: 10.1016/j.jhep.2014.11.017.
8. Fernández J et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2020 Apr;18(4):963-73.e14. doi: 10.1016/j.c gh.2019.07.055.
9. Di Pascoli M et al. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019 Jan;39(1):98-105. doi: 10.1111/liv.13968.
10. Caraceni P et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial [published correction appears in Lancet. 2018 Aug 4;392(10145):386]. Lancet. 2018 June;391(10138):2417-29. doi: 10.1016/S0140-6736(18)30840-7.
11. Fernández J et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019 July;157(1):149-62. doi: 10.1053/j.gastro.2019.03.021.
12. Fasolato S et al. Renal failure and bacterial infections in patients with cirrhosis: Epidemiology and clinical features. Hepatology. 2007;45(1):223-9. doi: 10.1002/hep.21443.
Key points
- In patients with spontaneous bacterial peritonitis, hepatorenal syndrome, and for large volume paracentesis, albumin improves outcomes and is recommended by guidelines.
- In patients with cirrhosis and a non-SBP infection, there is some evidence that albumin may improve renal and circulatory function.
- Clinicians should be cautious about albumin use in patients at an elevated risk for development of pulmonary edema.
Quiz
Which of the following is not a guideline-recommended use of albumin for patients with cirrhosis?
A. Treatment of type 1 hepatorenal syndrome
B. Treatment of spontaneous bacterial peritonitis
C. To correct plasma albumin < 2.5 g/dL in nontransplant patients
D. Post large-volume paracentesis
The answer is C. Per the EASL and AASLD, A,B, and D are recommended. There is not strong evidence to support administering albumin to correct low plasma albumin.
Additional reading
- Bernardi M et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. 2020 June;69(6):1127-38. doi: 10.1136/gutjnl-2019-318843.
- Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline [for the] management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013 Apr;57(4):1651-3. doi: 10.1002/hep.26359.
- Paine CH et al. Albumin in cirrhosis: More than a colloid. Curr Treat Options Gastroenterol. 2019 June;17(2):231-43. doi: 10.1007/s11938-019-00227-4.
Decline in child COVID may signal end of latest surge
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
Nurses ‘at the breaking point,’ consider quitting due to COVID issues: Survey
As hospitals have been flooded with critically ill patients, nurses have been overwhelmed.
“What we’re hearing from our nurses is really shocking,” Amanda Bettencourt, PhD, APRN, CCRN-K, president-elect of the American Association of Critical-Care Nurses (AACN), said in an interview. “They’re saying they’re at the breaking point.”
Between Aug. 26 and Aug. 30, the AACN surveyed more than 6,000 critical care nurses, zeroing in on four key questions regarding the pandemic and its impact on nursing. The results were alarming – not only with regard to individual nurses but also for the nursing profession and the future of health care. A full 66% of those surveyed said their experiences during the pandemic have caused them to consider leaving nursing. The respondents’ take on their colleagues was even more concerning. Ninety-two percent agreed with the following two statements: “I believe the pandemic has depleted nurses at my hospital. Their careers will be shorter than they intended.”
“This puts the entire health care system at risk,” says Dr. Bettencourt, assistant professor in the department of family and community health at the University of Pennsylvania School of Nursing, Philadelphia. Intensive care unit (ICU) nurses are highly trained and are skilled in caring for critically ill patients with complex medical needs. “It’s not easy to replace a critical care nurse when one leaves,” she said.
And when nurses leave, patients suffer, said Beth Wathen, MSN, RN, CCRN-K, president of the ACCN and frontline nurse at Children’s Hospital Colorado, in Aurora. “Hospitals can have all the beds and all the rooms and all the equipment they want, but without nurses and others at the front lines to provide that essential care, none of it really matters, whether we’re talking about caring for COVID patients or caring for patients with other health ailments.”
Heartbreak of the unvaccinated
The problem is not just overwork because of the flood of COVID-19 patients. The emotional strain is enormous as well. “What’s demoralizing for us is not that patients are sick and that it’s physically exhausting to take care of sick patients. We’re used to that,” said Dr. Bettencourt.
But few nurses have experienced the sheer magnitude of patients caused by this pandemic. “The past 18 months have been grueling,” says Ms. Wathen. “The burden on frontline caregivers and our nurses at the front line has been immense.”
The situation is made worse by how unnecessary much of the suffering is at this point. Seventy-six percent of the survey’s respondents agreed with the following statement: “People who hold out on getting vaccinated undermine nurses’ physical and mental well-being.” That comment doesn’t convey the nature or extent of the effect on caregivers’ well-being. “That 9 out of 10 of the people we’re seeing in ICU right now are unvaccinated just adds to the sense of heartbreak and frustration,” says Ms. Wathen. “These deaths don’t have to be happening right now. And that’s hard to bear witness to.”
The politicization of public health has also taken a toll. “That’s been the hard part of this entire pandemic,” says Ms. Wathen. “This really isn’t at all about politics. This is about your health; this is about my health. This is about our collective health as a community and as a country.”
Like the rest of the world, nurses are also concerned about their own loved ones. The survey statement, “I fear taking care of patients with COVID puts my family’s health at risk,” garnered 67% agreement. Ms. Wathen points out that nurses take the appropriate precautions but still worry about taking infection home to their families. “This disease is a tricky one,” she says. She points out that until this pandemic is over, in addition to being vaccinated, nurses and the public still need to be vigilant about wearing masks, social distancing, and taking other precautions to ensure the safety of us all. “Our individual decisions don’t just affect ourselves. They affect our family, the people in our circle, and the people in our community,” she said.
Avoiding a professional exodus
It’s too early yet to have reliable national data on how many nurses have already left their jobs because of COVID-19, but it is clear that there are too few nurses of all kinds. The American Nurses Association sent a letter to the U.S. Secretary of Health and Human Services urging the agency to declare the nursing shortage a crisis and to take immediate steps to find solutions.
The nursing shortage predates the pandemic, and COVID-19 has brought a simmering problem to the boil. Nurses are calling on the public and the health care system for help. From inside the industry, the needs are pretty much what they were before the pandemic. Dr. Bettencourt and Ms. Wathen point to the need for supportive leadership, healthy work environments, sufficient staffing to meet patients’ needs, and a voice in decisions, such as decisions about staffing, that affect nurses and their patients. Nurses want to be heard and appreciated. “It’s not that these are new things,” said Dr. Bettencourt. “We just need them even more now because we’re stressed even more than we were before.”
Critical care nurses have a different request of the public. They’re asking – pleading, actually – with the public to get vaccinated, wear masks in public, practice social distancing, and bring this pandemic to an end.
“COVID kills, and it’s a really difficult, tragic, and lonely death,” said Ms. Wathen. “We’ve witnessed hundreds of thousands of those deaths. But now we have a way to stop it. If many more people get vaccinated, we can stop this pandemic. And hopefully that will stop this current trend of nurses leaving.”
A version of this article first appeared on Medscape.com.
As hospitals have been flooded with critically ill patients, nurses have been overwhelmed.
“What we’re hearing from our nurses is really shocking,” Amanda Bettencourt, PhD, APRN, CCRN-K, president-elect of the American Association of Critical-Care Nurses (AACN), said in an interview. “They’re saying they’re at the breaking point.”
Between Aug. 26 and Aug. 30, the AACN surveyed more than 6,000 critical care nurses, zeroing in on four key questions regarding the pandemic and its impact on nursing. The results were alarming – not only with regard to individual nurses but also for the nursing profession and the future of health care. A full 66% of those surveyed said their experiences during the pandemic have caused them to consider leaving nursing. The respondents’ take on their colleagues was even more concerning. Ninety-two percent agreed with the following two statements: “I believe the pandemic has depleted nurses at my hospital. Their careers will be shorter than they intended.”
“This puts the entire health care system at risk,” says Dr. Bettencourt, assistant professor in the department of family and community health at the University of Pennsylvania School of Nursing, Philadelphia. Intensive care unit (ICU) nurses are highly trained and are skilled in caring for critically ill patients with complex medical needs. “It’s not easy to replace a critical care nurse when one leaves,” she said.
And when nurses leave, patients suffer, said Beth Wathen, MSN, RN, CCRN-K, president of the ACCN and frontline nurse at Children’s Hospital Colorado, in Aurora. “Hospitals can have all the beds and all the rooms and all the equipment they want, but without nurses and others at the front lines to provide that essential care, none of it really matters, whether we’re talking about caring for COVID patients or caring for patients with other health ailments.”
Heartbreak of the unvaccinated
The problem is not just overwork because of the flood of COVID-19 patients. The emotional strain is enormous as well. “What’s demoralizing for us is not that patients are sick and that it’s physically exhausting to take care of sick patients. We’re used to that,” said Dr. Bettencourt.
But few nurses have experienced the sheer magnitude of patients caused by this pandemic. “The past 18 months have been grueling,” says Ms. Wathen. “The burden on frontline caregivers and our nurses at the front line has been immense.”
The situation is made worse by how unnecessary much of the suffering is at this point. Seventy-six percent of the survey’s respondents agreed with the following statement: “People who hold out on getting vaccinated undermine nurses’ physical and mental well-being.” That comment doesn’t convey the nature or extent of the effect on caregivers’ well-being. “That 9 out of 10 of the people we’re seeing in ICU right now are unvaccinated just adds to the sense of heartbreak and frustration,” says Ms. Wathen. “These deaths don’t have to be happening right now. And that’s hard to bear witness to.”
The politicization of public health has also taken a toll. “That’s been the hard part of this entire pandemic,” says Ms. Wathen. “This really isn’t at all about politics. This is about your health; this is about my health. This is about our collective health as a community and as a country.”
Like the rest of the world, nurses are also concerned about their own loved ones. The survey statement, “I fear taking care of patients with COVID puts my family’s health at risk,” garnered 67% agreement. Ms. Wathen points out that nurses take the appropriate precautions but still worry about taking infection home to their families. “This disease is a tricky one,” she says. She points out that until this pandemic is over, in addition to being vaccinated, nurses and the public still need to be vigilant about wearing masks, social distancing, and taking other precautions to ensure the safety of us all. “Our individual decisions don’t just affect ourselves. They affect our family, the people in our circle, and the people in our community,” she said.
Avoiding a professional exodus
It’s too early yet to have reliable national data on how many nurses have already left their jobs because of COVID-19, but it is clear that there are too few nurses of all kinds. The American Nurses Association sent a letter to the U.S. Secretary of Health and Human Services urging the agency to declare the nursing shortage a crisis and to take immediate steps to find solutions.
The nursing shortage predates the pandemic, and COVID-19 has brought a simmering problem to the boil. Nurses are calling on the public and the health care system for help. From inside the industry, the needs are pretty much what they were before the pandemic. Dr. Bettencourt and Ms. Wathen point to the need for supportive leadership, healthy work environments, sufficient staffing to meet patients’ needs, and a voice in decisions, such as decisions about staffing, that affect nurses and their patients. Nurses want to be heard and appreciated. “It’s not that these are new things,” said Dr. Bettencourt. “We just need them even more now because we’re stressed even more than we were before.”
Critical care nurses have a different request of the public. They’re asking – pleading, actually – with the public to get vaccinated, wear masks in public, practice social distancing, and bring this pandemic to an end.
“COVID kills, and it’s a really difficult, tragic, and lonely death,” said Ms. Wathen. “We’ve witnessed hundreds of thousands of those deaths. But now we have a way to stop it. If many more people get vaccinated, we can stop this pandemic. And hopefully that will stop this current trend of nurses leaving.”
A version of this article first appeared on Medscape.com.
As hospitals have been flooded with critically ill patients, nurses have been overwhelmed.
“What we’re hearing from our nurses is really shocking,” Amanda Bettencourt, PhD, APRN, CCRN-K, president-elect of the American Association of Critical-Care Nurses (AACN), said in an interview. “They’re saying they’re at the breaking point.”
Between Aug. 26 and Aug. 30, the AACN surveyed more than 6,000 critical care nurses, zeroing in on four key questions regarding the pandemic and its impact on nursing. The results were alarming – not only with regard to individual nurses but also for the nursing profession and the future of health care. A full 66% of those surveyed said their experiences during the pandemic have caused them to consider leaving nursing. The respondents’ take on their colleagues was even more concerning. Ninety-two percent agreed with the following two statements: “I believe the pandemic has depleted nurses at my hospital. Their careers will be shorter than they intended.”
“This puts the entire health care system at risk,” says Dr. Bettencourt, assistant professor in the department of family and community health at the University of Pennsylvania School of Nursing, Philadelphia. Intensive care unit (ICU) nurses are highly trained and are skilled in caring for critically ill patients with complex medical needs. “It’s not easy to replace a critical care nurse when one leaves,” she said.
And when nurses leave, patients suffer, said Beth Wathen, MSN, RN, CCRN-K, president of the ACCN and frontline nurse at Children’s Hospital Colorado, in Aurora. “Hospitals can have all the beds and all the rooms and all the equipment they want, but without nurses and others at the front lines to provide that essential care, none of it really matters, whether we’re talking about caring for COVID patients or caring for patients with other health ailments.”
Heartbreak of the unvaccinated
The problem is not just overwork because of the flood of COVID-19 patients. The emotional strain is enormous as well. “What’s demoralizing for us is not that patients are sick and that it’s physically exhausting to take care of sick patients. We’re used to that,” said Dr. Bettencourt.
But few nurses have experienced the sheer magnitude of patients caused by this pandemic. “The past 18 months have been grueling,” says Ms. Wathen. “The burden on frontline caregivers and our nurses at the front line has been immense.”
The situation is made worse by how unnecessary much of the suffering is at this point. Seventy-six percent of the survey’s respondents agreed with the following statement: “People who hold out on getting vaccinated undermine nurses’ physical and mental well-being.” That comment doesn’t convey the nature or extent of the effect on caregivers’ well-being. “That 9 out of 10 of the people we’re seeing in ICU right now are unvaccinated just adds to the sense of heartbreak and frustration,” says Ms. Wathen. “These deaths don’t have to be happening right now. And that’s hard to bear witness to.”
The politicization of public health has also taken a toll. “That’s been the hard part of this entire pandemic,” says Ms. Wathen. “This really isn’t at all about politics. This is about your health; this is about my health. This is about our collective health as a community and as a country.”
Like the rest of the world, nurses are also concerned about their own loved ones. The survey statement, “I fear taking care of patients with COVID puts my family’s health at risk,” garnered 67% agreement. Ms. Wathen points out that nurses take the appropriate precautions but still worry about taking infection home to their families. “This disease is a tricky one,” she says. She points out that until this pandemic is over, in addition to being vaccinated, nurses and the public still need to be vigilant about wearing masks, social distancing, and taking other precautions to ensure the safety of us all. “Our individual decisions don’t just affect ourselves. They affect our family, the people in our circle, and the people in our community,” she said.
Avoiding a professional exodus
It’s too early yet to have reliable national data on how many nurses have already left their jobs because of COVID-19, but it is clear that there are too few nurses of all kinds. The American Nurses Association sent a letter to the U.S. Secretary of Health and Human Services urging the agency to declare the nursing shortage a crisis and to take immediate steps to find solutions.
The nursing shortage predates the pandemic, and COVID-19 has brought a simmering problem to the boil. Nurses are calling on the public and the health care system for help. From inside the industry, the needs are pretty much what they were before the pandemic. Dr. Bettencourt and Ms. Wathen point to the need for supportive leadership, healthy work environments, sufficient staffing to meet patients’ needs, and a voice in decisions, such as decisions about staffing, that affect nurses and their patients. Nurses want to be heard and appreciated. “It’s not that these are new things,” said Dr. Bettencourt. “We just need them even more now because we’re stressed even more than we were before.”
Critical care nurses have a different request of the public. They’re asking – pleading, actually – with the public to get vaccinated, wear masks in public, practice social distancing, and bring this pandemic to an end.
“COVID kills, and it’s a really difficult, tragic, and lonely death,” said Ms. Wathen. “We’ve witnessed hundreds of thousands of those deaths. But now we have a way to stop it. If many more people get vaccinated, we can stop this pandemic. And hopefully that will stop this current trend of nurses leaving.”
A version of this article first appeared on Medscape.com.
Embedding diversity, equity, inclusion, and justice in hospital medicine
A road map for success
The language of equality in America’s founding was never truly embraced, resulting in a painful legacy of slavery, racial injustice, and gender inequality inherited by all generations. However, for as long as America has fallen short of this unfulfilled promise, individuals have dedicated their lives to the tireless work of correcting injustice. Although the process has been painstakingly slow, our nation has incrementally inched toward the promised vision of equality, and these efforts continue today. With increased attention to social justice movements such as #MeToo and Black Lives Matter, our collective social consciousness may be finally waking up to the systemic injustices embedded into our fundamental institutions.
Medicine is not immune to these injustices. Persistent underrepresentation of women and minorities remains in medical school faculty and the broader physician workforce, and the same inequities exist in hospital medicine.1-6 The report by the Association of American Medical Colleges (AAMC) on diversity in medicine highlights the impact widespread implicit and explicit bias has on creating exclusionary environments, exemplified by research demonstrating lower promotion rates in non-White faculty.7-8 The report calls us, as physicians, to a broader mission: “Focusing solely on increasing compositional diversity along the academic continuum is insufficient. To effectively enact institutional change at academic medical centers ... leaders must focus their efforts on developing inclusive, equity-minded environments.”7
We have a clear moral imperative to correct these shortcomings for our profession and our patients. It is incumbent on our institutions and hospital medicine groups (HMGs) to embark on the necessary process of systemic institutional change to address inequality and justice within our field.
A road map for DEI and justice in hospital medicine
The policies and biases allowing these inequities to persist have existed for decades, and superficial efforts will not bring sufficient change. Our institutions require new building blocks from which the foundation of a wholly inclusive and equal system of practice can be constructed. Encouragingly, some institutions and HMGs have taken steps to modernize their practices. We offer examples and suggestions of concrete practices to begin this journey, organizing these efforts into three broad categories:
1. Recruitment and retention
2. Scholarship, mentorship, and sponsorship
3. Community engagement and partnership.
Recruitment and retention
Improving equity and inclusion begins with recruitment. Search and hiring committees should be assembled intentionally, with gender balance, and ideally with diversity or equity experts invited to join. All members should receive unconscious bias training. For example, the University of Colorado utilizes a toolkit to ensure appropriate steps are followed in the recruitment process, including predetermined candidate selection criteria that are ranked in advance.
Job descriptions should be reviewed by a diversity expert, ensuring unbiased and ungendered language within written text. Advertisements should be wide-reaching, and the committee should consider asking applicants for a diversity statement. Interviews should include a variety of interviewers and interview types (e.g., 1:1, group, etc.). Letters of recommendation deserve special scrutiny; letters for women and minorities may be at risk of being shorter and less record focused, and may be subject to less professional respect, such as use of first names over honorifics or titles.
Once candidates are hired, institutions and HMGs should prioritize developing strategies to improve retention of a diverse workforce. This includes special attention to workplace culture, and thoughtfully striving for cultural intelligence within the group. Some examples may include developing affinity groups, such as underrepresented in medicine (UIM), women in medicine (WIM), or LGBTQ+ groups. Affinity groups provide a safe space for members and allies to support and uplift each other. Institutional and HMG leaders must educate themselves and their members on the importance of language (see table), and the more insidious forms of bias and discrimination that adversely affect workplace culture. Microinsults and microinvalidations, for example, can hurt and result in failure to recruit or turnover. 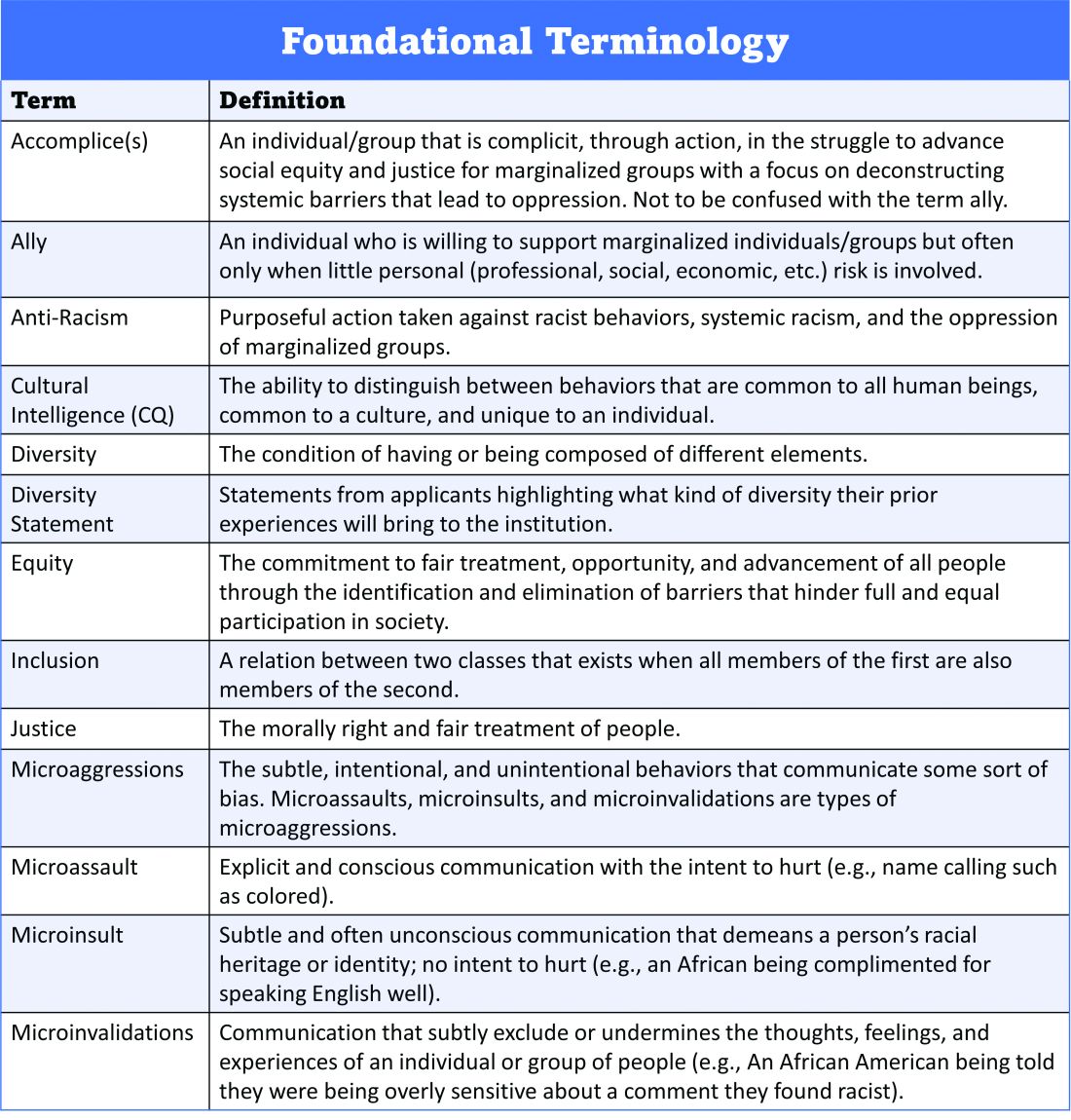
Conducting exit interviews when any hospitalist leaves is important to learn how to improve, but holding ‘stay’ interviews is mission critical. Stay interviews are an opportunity for HMG leaders to proactively understand why hospitalists stay, and what can be done to create more inclusive and equitable environments to retain them. This process creates psychological safety that brings challenges to the fore to be addressed, and spotlights best practices to be maintained and scaled.
Scholarship, mentorship, and sponsorship
Women and minorities are known to be over-mentored and under-sponsored. Sponsorship is defined by Ayyala et al. as “active support by someone appropriately placed in the organization who has significant influence on decision making processes or structures and who is advocating for the career advancement of an individual and recommends them for leadership roles, awards, or high-profile speaking opportunities.”9 While the goal of mentorship is professional development, sponsorship emphasizes professional advancement. Deliberate steps to both mentor and then sponsor diverse hospitalists and future hospitalists (including trainees) are important to ensure equity.
More inclusive HMGs can be bolstered by prioritizing peer education on the professional imperative that we have a diverse workforce and equitable, just workplaces. Academic institutions may use existing structures such as grand rounds to provide education on these crucial topics, and all HMGs can host journal clubs and professional development sessions on leadership competencies that foster inclusion and equity. Sessions coordinated by women and minorities are also a form of justice, by helping overcome barriers to career advancement. Diverse faculty presenting in educational venues will result in content that is relevant to more audience members and will exemplify that leaders and experts are of all races, ethnicities, genders, ages, and abilities.
Groups should prioritize mentoring trainees and early-career hospitalists on scholarly projects that examine equity in opportunities of care, which signals that this science is valued as much as basic research. When used to demonstrate areas needing improvement, these projects can drive meaningful change. Even projects as straightforward as studying diversity in conference presenters, disparities in adherence to guidelines, or QI projects on how race is portrayed in the medical record can be powerful tools in advancing equity.
A key part of mentoring is training hospitalists and future hospitalists in how to be an upstander, as in how to intervene when a peer or patient is affected by bias, harassment, or discrimination. Receiving such training can prepare hospitalists for these nearly inevitable experiences and receiving training during usual work hours communicates that this is a valuable and necessary professional competency.
Community engagement and partnership
Institutions and HMGs should deliberately work to promote community engagement and partnership within their groups. Beyond promoting health equity, community engagement also fosters inclusivity by allowing community members to share their ideas and give recommendations to the institutions that serve them.
There is a growing body of literature that demonstrates how disadvantages by individual and neighborhood-level socioeconomic status (SES) contribute to disparities in specific disease conditions.10-11 Strategies to narrow the gap in SES disadvantages may help reduce race-related health disparities. Institutions that engage the community and develop programs to promote health equity can do so through bidirectional exchange of knowledge and mutual benefit.
An institution-specific example is Medicine for the Greater Good at Johns Hopkins. The founders of this program wrote, “health is not synonymous with medicine. To truly care for our patients and their communities, health care professionals must understand how to deliver equitable health care that meets the needs of the diverse populations we care for. The mission of Medicine for the Greater Good is to promote health and wellness beyond the confines of the hospital through an interactive and engaging partnership with the community ...” Community engagement also provides an opportunity for growing the cultural intelligence of institutions and HMGs.
Tools for advancing comprehensive change – Repurposing PDSA cycles
Whether institutions and HMGs are at the beginning of their journey or further along in the work of reducing disparities, having a systematic approach for implementing and refining policies and procedures can cultivate more inclusive and equitable environments. Thankfully, hospitalists are already equipped with the fundamental tools needed to advance change across their institutions – QI processes in the form of Plan-Do-Study-Act (PDSA) cycles.
They allow a continuous cycle of successful incremental change based on direct evidence and experience. Any efforts to deconstruct systematic bias within our organizations must also be a continual process. Our female colleagues and colleagues of color need our institutions to engage unceasingly to bring about the equality they deserve. To that end, PDSA cycles are an apt tool to utilize in this work as they can naturally function in a never-ending process of improvement.
With PDSA as a model, we envision a cycle with steps that are intentionally purposed to fit the needs of equitable institutional change: Target-Engage-Assess-Modify. As highlighted (see graphic), these modifications ensure that stakeholders (i.e., those that unequal practices and policies affect the most) are engaged early and remain involved throughout the cycle. 
As hospitalists, we have significant work ahead to ensure that we develop and maintain a diverse, equitable and inclusive workforce. This work to bring change will not be easy and will require a considerable investment of time and resources. However, with the strategies and tools that we have outlined, our institutions and HMGs can start the change needed in our profession for our patients and the workforce. In doing so, we can all be accomplices in the fight to achieve racial and gender equity, and social justice.
Dr. Delapenha and Dr. Kisuule are based in the department of internal medicine, division of hospital medicine, at the Johns Hopkins University, Baltimore. Dr. Martin is based in the department of medicine, section of hospital medicine at the University of Chicago. Dr. Barrett is a hospitalist in the department of internal medicine, University of New Mexico, Albuquerque.
References
1. Diversity in Medicine: Facts and Figures 2019: Figure 19. Percentage of physicians by sex, 2018. AAMC website.
2. Diversity in Medicine: Facts and Figures 2019. Figure 16. Percentage of full-time U.S. medical school faculty by sex and race/ethnicity, 2018. AAMC website.
3. Diversity in Medicine: Facts and Figures 2019. Figure 15. Percentage of full-time U.S. medical school faculty by race/ethnicity, 2018. AAMC website.
4. Diversity in Medicine: Facts and Figures 2019. Figure 6. Percentage of acceptees to U.S. medical schools by race/ethnicity (alone), academic year 2018-2019. AAMC website.
5. Diversity in Medicine: Facts and Figures 2019 Figure 18. Percentage of all active physicians by race/ethnicity, 2018. AAMC website.
6. Herzke C et al. Gender issues in academic hospital medicine: A national survey of hospitalist leaders. J Gen Intern Med. 2020;35(6):1641-6.
7. Diversity in Medicine: Facts and Figures 2019. Fostering diversity and inclusion. AAMC website.
8. Diversity in Medicine: Facts and Figures 2019. Executive summary. AAMC website.
9. Ayyala MS et al. Mentorship is not enough: Exploring sponsorship and its role in career advancement in academic medicine. Acad Med. 2019;94(1):94-100.
10. Ejike OC et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021 Apr 15;203(8):987-97.
11. Galiatsatos P et al. The effect of community socioeconomic status on sepsis-attributable mortality. J Crit Care. 2018 Aug;46:129-33.
A road map for success
A road map for success
The language of equality in America’s founding was never truly embraced, resulting in a painful legacy of slavery, racial injustice, and gender inequality inherited by all generations. However, for as long as America has fallen short of this unfulfilled promise, individuals have dedicated their lives to the tireless work of correcting injustice. Although the process has been painstakingly slow, our nation has incrementally inched toward the promised vision of equality, and these efforts continue today. With increased attention to social justice movements such as #MeToo and Black Lives Matter, our collective social consciousness may be finally waking up to the systemic injustices embedded into our fundamental institutions.
Medicine is not immune to these injustices. Persistent underrepresentation of women and minorities remains in medical school faculty and the broader physician workforce, and the same inequities exist in hospital medicine.1-6 The report by the Association of American Medical Colleges (AAMC) on diversity in medicine highlights the impact widespread implicit and explicit bias has on creating exclusionary environments, exemplified by research demonstrating lower promotion rates in non-White faculty.7-8 The report calls us, as physicians, to a broader mission: “Focusing solely on increasing compositional diversity along the academic continuum is insufficient. To effectively enact institutional change at academic medical centers ... leaders must focus their efforts on developing inclusive, equity-minded environments.”7
We have a clear moral imperative to correct these shortcomings for our profession and our patients. It is incumbent on our institutions and hospital medicine groups (HMGs) to embark on the necessary process of systemic institutional change to address inequality and justice within our field.
A road map for DEI and justice in hospital medicine
The policies and biases allowing these inequities to persist have existed for decades, and superficial efforts will not bring sufficient change. Our institutions require new building blocks from which the foundation of a wholly inclusive and equal system of practice can be constructed. Encouragingly, some institutions and HMGs have taken steps to modernize their practices. We offer examples and suggestions of concrete practices to begin this journey, organizing these efforts into three broad categories:
1. Recruitment and retention
2. Scholarship, mentorship, and sponsorship
3. Community engagement and partnership.
Recruitment and retention
Improving equity and inclusion begins with recruitment. Search and hiring committees should be assembled intentionally, with gender balance, and ideally with diversity or equity experts invited to join. All members should receive unconscious bias training. For example, the University of Colorado utilizes a toolkit to ensure appropriate steps are followed in the recruitment process, including predetermined candidate selection criteria that are ranked in advance.
Job descriptions should be reviewed by a diversity expert, ensuring unbiased and ungendered language within written text. Advertisements should be wide-reaching, and the committee should consider asking applicants for a diversity statement. Interviews should include a variety of interviewers and interview types (e.g., 1:1, group, etc.). Letters of recommendation deserve special scrutiny; letters for women and minorities may be at risk of being shorter and less record focused, and may be subject to less professional respect, such as use of first names over honorifics or titles.
Once candidates are hired, institutions and HMGs should prioritize developing strategies to improve retention of a diverse workforce. This includes special attention to workplace culture, and thoughtfully striving for cultural intelligence within the group. Some examples may include developing affinity groups, such as underrepresented in medicine (UIM), women in medicine (WIM), or LGBTQ+ groups. Affinity groups provide a safe space for members and allies to support and uplift each other. Institutional and HMG leaders must educate themselves and their members on the importance of language (see table), and the more insidious forms of bias and discrimination that adversely affect workplace culture. Microinsults and microinvalidations, for example, can hurt and result in failure to recruit or turnover. 
Conducting exit interviews when any hospitalist leaves is important to learn how to improve, but holding ‘stay’ interviews is mission critical. Stay interviews are an opportunity for HMG leaders to proactively understand why hospitalists stay, and what can be done to create more inclusive and equitable environments to retain them. This process creates psychological safety that brings challenges to the fore to be addressed, and spotlights best practices to be maintained and scaled.
Scholarship, mentorship, and sponsorship
Women and minorities are known to be over-mentored and under-sponsored. Sponsorship is defined by Ayyala et al. as “active support by someone appropriately placed in the organization who has significant influence on decision making processes or structures and who is advocating for the career advancement of an individual and recommends them for leadership roles, awards, or high-profile speaking opportunities.”9 While the goal of mentorship is professional development, sponsorship emphasizes professional advancement. Deliberate steps to both mentor and then sponsor diverse hospitalists and future hospitalists (including trainees) are important to ensure equity.
More inclusive HMGs can be bolstered by prioritizing peer education on the professional imperative that we have a diverse workforce and equitable, just workplaces. Academic institutions may use existing structures such as grand rounds to provide education on these crucial topics, and all HMGs can host journal clubs and professional development sessions on leadership competencies that foster inclusion and equity. Sessions coordinated by women and minorities are also a form of justice, by helping overcome barriers to career advancement. Diverse faculty presenting in educational venues will result in content that is relevant to more audience members and will exemplify that leaders and experts are of all races, ethnicities, genders, ages, and abilities.
Groups should prioritize mentoring trainees and early-career hospitalists on scholarly projects that examine equity in opportunities of care, which signals that this science is valued as much as basic research. When used to demonstrate areas needing improvement, these projects can drive meaningful change. Even projects as straightforward as studying diversity in conference presenters, disparities in adherence to guidelines, or QI projects on how race is portrayed in the medical record can be powerful tools in advancing equity.
A key part of mentoring is training hospitalists and future hospitalists in how to be an upstander, as in how to intervene when a peer or patient is affected by bias, harassment, or discrimination. Receiving such training can prepare hospitalists for these nearly inevitable experiences and receiving training during usual work hours communicates that this is a valuable and necessary professional competency.
Community engagement and partnership
Institutions and HMGs should deliberately work to promote community engagement and partnership within their groups. Beyond promoting health equity, community engagement also fosters inclusivity by allowing community members to share their ideas and give recommendations to the institutions that serve them.
There is a growing body of literature that demonstrates how disadvantages by individual and neighborhood-level socioeconomic status (SES) contribute to disparities in specific disease conditions.10-11 Strategies to narrow the gap in SES disadvantages may help reduce race-related health disparities. Institutions that engage the community and develop programs to promote health equity can do so through bidirectional exchange of knowledge and mutual benefit.
An institution-specific example is Medicine for the Greater Good at Johns Hopkins. The founders of this program wrote, “health is not synonymous with medicine. To truly care for our patients and their communities, health care professionals must understand how to deliver equitable health care that meets the needs of the diverse populations we care for. The mission of Medicine for the Greater Good is to promote health and wellness beyond the confines of the hospital through an interactive and engaging partnership with the community ...” Community engagement also provides an opportunity for growing the cultural intelligence of institutions and HMGs.
Tools for advancing comprehensive change – Repurposing PDSA cycles
Whether institutions and HMGs are at the beginning of their journey or further along in the work of reducing disparities, having a systematic approach for implementing and refining policies and procedures can cultivate more inclusive and equitable environments. Thankfully, hospitalists are already equipped with the fundamental tools needed to advance change across their institutions – QI processes in the form of Plan-Do-Study-Act (PDSA) cycles.
They allow a continuous cycle of successful incremental change based on direct evidence and experience. Any efforts to deconstruct systematic bias within our organizations must also be a continual process. Our female colleagues and colleagues of color need our institutions to engage unceasingly to bring about the equality they deserve. To that end, PDSA cycles are an apt tool to utilize in this work as they can naturally function in a never-ending process of improvement.
With PDSA as a model, we envision a cycle with steps that are intentionally purposed to fit the needs of equitable institutional change: Target-Engage-Assess-Modify. As highlighted (see graphic), these modifications ensure that stakeholders (i.e., those that unequal practices and policies affect the most) are engaged early and remain involved throughout the cycle. 
As hospitalists, we have significant work ahead to ensure that we develop and maintain a diverse, equitable and inclusive workforce. This work to bring change will not be easy and will require a considerable investment of time and resources. However, with the strategies and tools that we have outlined, our institutions and HMGs can start the change needed in our profession for our patients and the workforce. In doing so, we can all be accomplices in the fight to achieve racial and gender equity, and social justice.
Dr. Delapenha and Dr. Kisuule are based in the department of internal medicine, division of hospital medicine, at the Johns Hopkins University, Baltimore. Dr. Martin is based in the department of medicine, section of hospital medicine at the University of Chicago. Dr. Barrett is a hospitalist in the department of internal medicine, University of New Mexico, Albuquerque.
References
1. Diversity in Medicine: Facts and Figures 2019: Figure 19. Percentage of physicians by sex, 2018. AAMC website.
2. Diversity in Medicine: Facts and Figures 2019. Figure 16. Percentage of full-time U.S. medical school faculty by sex and race/ethnicity, 2018. AAMC website.
3. Diversity in Medicine: Facts and Figures 2019. Figure 15. Percentage of full-time U.S. medical school faculty by race/ethnicity, 2018. AAMC website.
4. Diversity in Medicine: Facts and Figures 2019. Figure 6. Percentage of acceptees to U.S. medical schools by race/ethnicity (alone), academic year 2018-2019. AAMC website.
5. Diversity in Medicine: Facts and Figures 2019 Figure 18. Percentage of all active physicians by race/ethnicity, 2018. AAMC website.
6. Herzke C et al. Gender issues in academic hospital medicine: A national survey of hospitalist leaders. J Gen Intern Med. 2020;35(6):1641-6.
7. Diversity in Medicine: Facts and Figures 2019. Fostering diversity and inclusion. AAMC website.
8. Diversity in Medicine: Facts and Figures 2019. Executive summary. AAMC website.
9. Ayyala MS et al. Mentorship is not enough: Exploring sponsorship and its role in career advancement in academic medicine. Acad Med. 2019;94(1):94-100.
10. Ejike OC et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021 Apr 15;203(8):987-97.
11. Galiatsatos P et al. The effect of community socioeconomic status on sepsis-attributable mortality. J Crit Care. 2018 Aug;46:129-33.
The language of equality in America’s founding was never truly embraced, resulting in a painful legacy of slavery, racial injustice, and gender inequality inherited by all generations. However, for as long as America has fallen short of this unfulfilled promise, individuals have dedicated their lives to the tireless work of correcting injustice. Although the process has been painstakingly slow, our nation has incrementally inched toward the promised vision of equality, and these efforts continue today. With increased attention to social justice movements such as #MeToo and Black Lives Matter, our collective social consciousness may be finally waking up to the systemic injustices embedded into our fundamental institutions.
Medicine is not immune to these injustices. Persistent underrepresentation of women and minorities remains in medical school faculty and the broader physician workforce, and the same inequities exist in hospital medicine.1-6 The report by the Association of American Medical Colleges (AAMC) on diversity in medicine highlights the impact widespread implicit and explicit bias has on creating exclusionary environments, exemplified by research demonstrating lower promotion rates in non-White faculty.7-8 The report calls us, as physicians, to a broader mission: “Focusing solely on increasing compositional diversity along the academic continuum is insufficient. To effectively enact institutional change at academic medical centers ... leaders must focus their efforts on developing inclusive, equity-minded environments.”7
We have a clear moral imperative to correct these shortcomings for our profession and our patients. It is incumbent on our institutions and hospital medicine groups (HMGs) to embark on the necessary process of systemic institutional change to address inequality and justice within our field.
A road map for DEI and justice in hospital medicine
The policies and biases allowing these inequities to persist have existed for decades, and superficial efforts will not bring sufficient change. Our institutions require new building blocks from which the foundation of a wholly inclusive and equal system of practice can be constructed. Encouragingly, some institutions and HMGs have taken steps to modernize their practices. We offer examples and suggestions of concrete practices to begin this journey, organizing these efforts into three broad categories:
1. Recruitment and retention
2. Scholarship, mentorship, and sponsorship
3. Community engagement and partnership.
Recruitment and retention
Improving equity and inclusion begins with recruitment. Search and hiring committees should be assembled intentionally, with gender balance, and ideally with diversity or equity experts invited to join. All members should receive unconscious bias training. For example, the University of Colorado utilizes a toolkit to ensure appropriate steps are followed in the recruitment process, including predetermined candidate selection criteria that are ranked in advance.
Job descriptions should be reviewed by a diversity expert, ensuring unbiased and ungendered language within written text. Advertisements should be wide-reaching, and the committee should consider asking applicants for a diversity statement. Interviews should include a variety of interviewers and interview types (e.g., 1:1, group, etc.). Letters of recommendation deserve special scrutiny; letters for women and minorities may be at risk of being shorter and less record focused, and may be subject to less professional respect, such as use of first names over honorifics or titles.
Once candidates are hired, institutions and HMGs should prioritize developing strategies to improve retention of a diverse workforce. This includes special attention to workplace culture, and thoughtfully striving for cultural intelligence within the group. Some examples may include developing affinity groups, such as underrepresented in medicine (UIM), women in medicine (WIM), or LGBTQ+ groups. Affinity groups provide a safe space for members and allies to support and uplift each other. Institutional and HMG leaders must educate themselves and their members on the importance of language (see table), and the more insidious forms of bias and discrimination that adversely affect workplace culture. Microinsults and microinvalidations, for example, can hurt and result in failure to recruit or turnover. 
Conducting exit interviews when any hospitalist leaves is important to learn how to improve, but holding ‘stay’ interviews is mission critical. Stay interviews are an opportunity for HMG leaders to proactively understand why hospitalists stay, and what can be done to create more inclusive and equitable environments to retain them. This process creates psychological safety that brings challenges to the fore to be addressed, and spotlights best practices to be maintained and scaled.
Scholarship, mentorship, and sponsorship
Women and minorities are known to be over-mentored and under-sponsored. Sponsorship is defined by Ayyala et al. as “active support by someone appropriately placed in the organization who has significant influence on decision making processes or structures and who is advocating for the career advancement of an individual and recommends them for leadership roles, awards, or high-profile speaking opportunities.”9 While the goal of mentorship is professional development, sponsorship emphasizes professional advancement. Deliberate steps to both mentor and then sponsor diverse hospitalists and future hospitalists (including trainees) are important to ensure equity.
More inclusive HMGs can be bolstered by prioritizing peer education on the professional imperative that we have a diverse workforce and equitable, just workplaces. Academic institutions may use existing structures such as grand rounds to provide education on these crucial topics, and all HMGs can host journal clubs and professional development sessions on leadership competencies that foster inclusion and equity. Sessions coordinated by women and minorities are also a form of justice, by helping overcome barriers to career advancement. Diverse faculty presenting in educational venues will result in content that is relevant to more audience members and will exemplify that leaders and experts are of all races, ethnicities, genders, ages, and abilities.
Groups should prioritize mentoring trainees and early-career hospitalists on scholarly projects that examine equity in opportunities of care, which signals that this science is valued as much as basic research. When used to demonstrate areas needing improvement, these projects can drive meaningful change. Even projects as straightforward as studying diversity in conference presenters, disparities in adherence to guidelines, or QI projects on how race is portrayed in the medical record can be powerful tools in advancing equity.
A key part of mentoring is training hospitalists and future hospitalists in how to be an upstander, as in how to intervene when a peer or patient is affected by bias, harassment, or discrimination. Receiving such training can prepare hospitalists for these nearly inevitable experiences and receiving training during usual work hours communicates that this is a valuable and necessary professional competency.
Community engagement and partnership
Institutions and HMGs should deliberately work to promote community engagement and partnership within their groups. Beyond promoting health equity, community engagement also fosters inclusivity by allowing community members to share their ideas and give recommendations to the institutions that serve them.
There is a growing body of literature that demonstrates how disadvantages by individual and neighborhood-level socioeconomic status (SES) contribute to disparities in specific disease conditions.10-11 Strategies to narrow the gap in SES disadvantages may help reduce race-related health disparities. Institutions that engage the community and develop programs to promote health equity can do so through bidirectional exchange of knowledge and mutual benefit.
An institution-specific example is Medicine for the Greater Good at Johns Hopkins. The founders of this program wrote, “health is not synonymous with medicine. To truly care for our patients and their communities, health care professionals must understand how to deliver equitable health care that meets the needs of the diverse populations we care for. The mission of Medicine for the Greater Good is to promote health and wellness beyond the confines of the hospital through an interactive and engaging partnership with the community ...” Community engagement also provides an opportunity for growing the cultural intelligence of institutions and HMGs.
Tools for advancing comprehensive change – Repurposing PDSA cycles
Whether institutions and HMGs are at the beginning of their journey or further along in the work of reducing disparities, having a systematic approach for implementing and refining policies and procedures can cultivate more inclusive and equitable environments. Thankfully, hospitalists are already equipped with the fundamental tools needed to advance change across their institutions – QI processes in the form of Plan-Do-Study-Act (PDSA) cycles.
They allow a continuous cycle of successful incremental change based on direct evidence and experience. Any efforts to deconstruct systematic bias within our organizations must also be a continual process. Our female colleagues and colleagues of color need our institutions to engage unceasingly to bring about the equality they deserve. To that end, PDSA cycles are an apt tool to utilize in this work as they can naturally function in a never-ending process of improvement.
With PDSA as a model, we envision a cycle with steps that are intentionally purposed to fit the needs of equitable institutional change: Target-Engage-Assess-Modify. As highlighted (see graphic), these modifications ensure that stakeholders (i.e., those that unequal practices and policies affect the most) are engaged early and remain involved throughout the cycle. 
As hospitalists, we have significant work ahead to ensure that we develop and maintain a diverse, equitable and inclusive workforce. This work to bring change will not be easy and will require a considerable investment of time and resources. However, with the strategies and tools that we have outlined, our institutions and HMGs can start the change needed in our profession for our patients and the workforce. In doing so, we can all be accomplices in the fight to achieve racial and gender equity, and social justice.
Dr. Delapenha and Dr. Kisuule are based in the department of internal medicine, division of hospital medicine, at the Johns Hopkins University, Baltimore. Dr. Martin is based in the department of medicine, section of hospital medicine at the University of Chicago. Dr. Barrett is a hospitalist in the department of internal medicine, University of New Mexico, Albuquerque.
References
1. Diversity in Medicine: Facts and Figures 2019: Figure 19. Percentage of physicians by sex, 2018. AAMC website.
2. Diversity in Medicine: Facts and Figures 2019. Figure 16. Percentage of full-time U.S. medical school faculty by sex and race/ethnicity, 2018. AAMC website.
3. Diversity in Medicine: Facts and Figures 2019. Figure 15. Percentage of full-time U.S. medical school faculty by race/ethnicity, 2018. AAMC website.
4. Diversity in Medicine: Facts and Figures 2019. Figure 6. Percentage of acceptees to U.S. medical schools by race/ethnicity (alone), academic year 2018-2019. AAMC website.
5. Diversity in Medicine: Facts and Figures 2019 Figure 18. Percentage of all active physicians by race/ethnicity, 2018. AAMC website.
6. Herzke C et al. Gender issues in academic hospital medicine: A national survey of hospitalist leaders. J Gen Intern Med. 2020;35(6):1641-6.
7. Diversity in Medicine: Facts and Figures 2019. Fostering diversity and inclusion. AAMC website.
8. Diversity in Medicine: Facts and Figures 2019. Executive summary. AAMC website.
9. Ayyala MS et al. Mentorship is not enough: Exploring sponsorship and its role in career advancement in academic medicine. Acad Med. 2019;94(1):94-100.
10. Ejike OC et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021 Apr 15;203(8):987-97.
11. Galiatsatos P et al. The effect of community socioeconomic status on sepsis-attributable mortality. J Crit Care. 2018 Aug;46:129-33.