User login
Get triage plans in place before COVID-19 surge hits, critical care experts say
, according to authors of recent reports that offer advice on how to prepare for surges in demand.
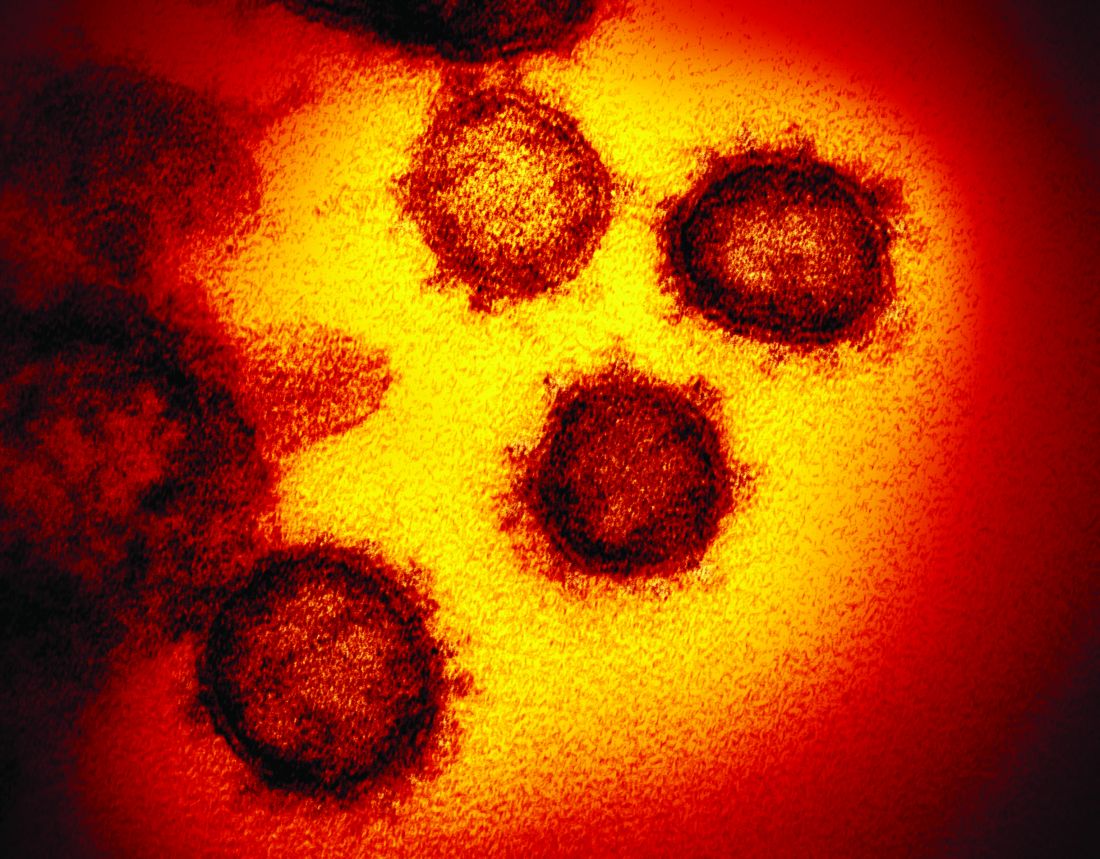
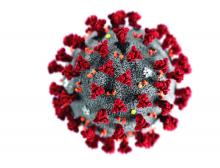
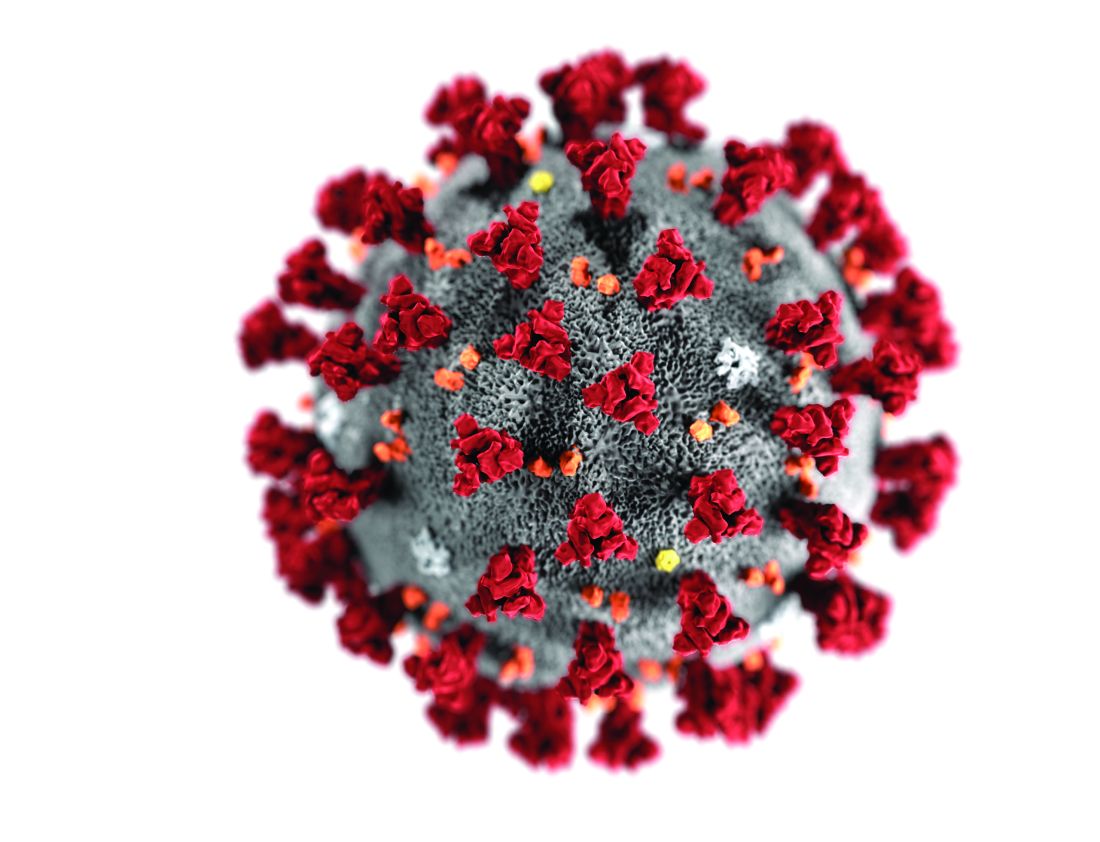
Even modest numbers of critically ill COVID-19 patients have already rapidly overwhelmed existing hospital capacity in hard-hit areas including Italy, Spain, and New York City, said authors of an expert panel report released in CHEST.
“The ethical burden this places on hospitals, health systems, and society is enormous,” said Ryan C. Maves, MD, FCCP, of the Naval Medical Center in San Diego, lead author of the expert panel report from the Task Force for Mass Critical Care and the American College of Chest Physicians (CHEST).
Triage decisions could be especially daunting for resource-intensive therapies such as extracorporeal membrane oxygenation (ECMO), as physicians may be forced to decide when and if to offer such support after demand outstrips a hospital’s ability to provide it.
“ECMO requires a lot of specialized capability to initiate on a patient, and then, it requires a lot of specialized capability to maintain and do safely,” said Steven P. Keller, MD, of the division of emergency critical care medicine in the department of emergency medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Those resource requirements can present a challenge to health care systems already overtaxed by COVID-19, according to Dr. Keller, coauthor of a guidance document in Annals of the American Thoracic Society. The guidance suggests a pandemic approach to ECMO response that’s tiered depending on the intensity of the surge over usual hospital volumes.
Mild surges call for a focus on increasing ECMO capacity, while a moderate surge may indicate a need to focus on allocating scarce resources, and a major surge may signal the need to limit or defer use of scarce resources, according to the guidance.
“If your health care system is stretched from a resource standpoint, at what point do you say, ‘we don’t even have the capability to even safely do ECMO, and so, perhaps we should not even be offering the support’?” Dr. Keller said in an interview. “That’s what we tried to get at in the paper – helping institutions think about how to prepare for that pandemic, and then when to make decisions on when it should and should not be offered.”
Critical care guidance for COVID-19
The guidance from the Task Force for Mass Critical Care and CHEST offers nine specific actions that authors suggest as part of a framework for communities to establish the infrastructure needed to triage critical care resources and “equitably” meet the needs of the largest number of COVID-19 patients.
“It is the goal of the task force to minimize the need for allocation of scarce resources as much as possible,” the authors stated.
The framework starts with surge planning that includes an inventory of intensive care unit resources such as ventilators, beds, supplies, and staff that could be marshaled to meet a surge in demand, followed by establishing “identification triggers” for triage initiation by a regional authority, should clinical demand reach a crisis stage.
The next step is preparing the triage system, which includes creating a committee at the regional level, identifying members of tertiary triage teams and the support structures they will need, and preparing and distributing training materials.
Agreeing on a triage protocol is important to ensure equitable targeting of resources, and how to allocate limited life-sustaining measures needs to be considered, according to the panel of experts. They also recommend adaptations to the standards of care such as modification of end-of-life care policies, support for health care workers, family, and the public, and consideration of pediatric issues including transport, concentration of care at specific centers, and potential increases in age thresholds to accommodate surges.
Barriers to triage?
When asked about potential barriers to rolling out a triage plan, Dr. Maves said the first is acknowledging the possible need for such a plan: “It is a difficult concept for most in critical care to accept – the idea that we may not be able to provide an individual patient with interventions that we consider routine,” he said.
Beyond acknowledging need, other potential barriers to successful implementation include the limited evidence base to support development of these protocols, as well as the need to address public trust.
“If a triage system is perceived as unjust or biased, or if people think that triage favors or excludes certain groups unfairly, it will undermine any system,” Dr. Maves said. “Making sure the public both understands and has input into system development is critical if we are going to be able to make this work.”
Dr. Maves and coauthors reported that some of the authors of their guidance are United States government employees or military service members, and that their opinions and assertions do not reflect the official views or position of those institutions. Dr. Keller reported no disclosures related to the ECMO guidance.
SOURCES: Maves RC et al. Chest. 2020 Apr 11. pii: S0012-3692(20)30691-7. doi: 10.1016/j.chest.2020.03.063; Seethara R and Keller SP. Ann Am Thorac Soc. 2020 Apr 15. doi: 10.1513/AnnalsATS.202003-233PS.
, according to authors of recent reports that offer advice on how to prepare for surges in demand.
Even modest numbers of critically ill COVID-19 patients have already rapidly overwhelmed existing hospital capacity in hard-hit areas including Italy, Spain, and New York City, said authors of an expert panel report released in CHEST.
“The ethical burden this places on hospitals, health systems, and society is enormous,” said Ryan C. Maves, MD, FCCP, of the Naval Medical Center in San Diego, lead author of the expert panel report from the Task Force for Mass Critical Care and the American College of Chest Physicians (CHEST).
Triage decisions could be especially daunting for resource-intensive therapies such as extracorporeal membrane oxygenation (ECMO), as physicians may be forced to decide when and if to offer such support after demand outstrips a hospital’s ability to provide it.
“ECMO requires a lot of specialized capability to initiate on a patient, and then, it requires a lot of specialized capability to maintain and do safely,” said Steven P. Keller, MD, of the division of emergency critical care medicine in the department of emergency medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Those resource requirements can present a challenge to health care systems already overtaxed by COVID-19, according to Dr. Keller, coauthor of a guidance document in Annals of the American Thoracic Society. The guidance suggests a pandemic approach to ECMO response that’s tiered depending on the intensity of the surge over usual hospital volumes.
Mild surges call for a focus on increasing ECMO capacity, while a moderate surge may indicate a need to focus on allocating scarce resources, and a major surge may signal the need to limit or defer use of scarce resources, according to the guidance.
“If your health care system is stretched from a resource standpoint, at what point do you say, ‘we don’t even have the capability to even safely do ECMO, and so, perhaps we should not even be offering the support’?” Dr. Keller said in an interview. “That’s what we tried to get at in the paper – helping institutions think about how to prepare for that pandemic, and then when to make decisions on when it should and should not be offered.”
Critical care guidance for COVID-19
The guidance from the Task Force for Mass Critical Care and CHEST offers nine specific actions that authors suggest as part of a framework for communities to establish the infrastructure needed to triage critical care resources and “equitably” meet the needs of the largest number of COVID-19 patients.
“It is the goal of the task force to minimize the need for allocation of scarce resources as much as possible,” the authors stated.
The framework starts with surge planning that includes an inventory of intensive care unit resources such as ventilators, beds, supplies, and staff that could be marshaled to meet a surge in demand, followed by establishing “identification triggers” for triage initiation by a regional authority, should clinical demand reach a crisis stage.
The next step is preparing the triage system, which includes creating a committee at the regional level, identifying members of tertiary triage teams and the support structures they will need, and preparing and distributing training materials.
Agreeing on a triage protocol is important to ensure equitable targeting of resources, and how to allocate limited life-sustaining measures needs to be considered, according to the panel of experts. They also recommend adaptations to the standards of care such as modification of end-of-life care policies, support for health care workers, family, and the public, and consideration of pediatric issues including transport, concentration of care at specific centers, and potential increases in age thresholds to accommodate surges.
Barriers to triage?
When asked about potential barriers to rolling out a triage plan, Dr. Maves said the first is acknowledging the possible need for such a plan: “It is a difficult concept for most in critical care to accept – the idea that we may not be able to provide an individual patient with interventions that we consider routine,” he said.
Beyond acknowledging need, other potential barriers to successful implementation include the limited evidence base to support development of these protocols, as well as the need to address public trust.
“If a triage system is perceived as unjust or biased, or if people think that triage favors or excludes certain groups unfairly, it will undermine any system,” Dr. Maves said. “Making sure the public both understands and has input into system development is critical if we are going to be able to make this work.”
Dr. Maves and coauthors reported that some of the authors of their guidance are United States government employees or military service members, and that their opinions and assertions do not reflect the official views or position of those institutions. Dr. Keller reported no disclosures related to the ECMO guidance.
SOURCES: Maves RC et al. Chest. 2020 Apr 11. pii: S0012-3692(20)30691-7. doi: 10.1016/j.chest.2020.03.063; Seethara R and Keller SP. Ann Am Thorac Soc. 2020 Apr 15. doi: 10.1513/AnnalsATS.202003-233PS.
, according to authors of recent reports that offer advice on how to prepare for surges in demand.
Even modest numbers of critically ill COVID-19 patients have already rapidly overwhelmed existing hospital capacity in hard-hit areas including Italy, Spain, and New York City, said authors of an expert panel report released in CHEST.
“The ethical burden this places on hospitals, health systems, and society is enormous,” said Ryan C. Maves, MD, FCCP, of the Naval Medical Center in San Diego, lead author of the expert panel report from the Task Force for Mass Critical Care and the American College of Chest Physicians (CHEST).
Triage decisions could be especially daunting for resource-intensive therapies such as extracorporeal membrane oxygenation (ECMO), as physicians may be forced to decide when and if to offer such support after demand outstrips a hospital’s ability to provide it.
“ECMO requires a lot of specialized capability to initiate on a patient, and then, it requires a lot of specialized capability to maintain and do safely,” said Steven P. Keller, MD, of the division of emergency critical care medicine in the department of emergency medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Those resource requirements can present a challenge to health care systems already overtaxed by COVID-19, according to Dr. Keller, coauthor of a guidance document in Annals of the American Thoracic Society. The guidance suggests a pandemic approach to ECMO response that’s tiered depending on the intensity of the surge over usual hospital volumes.
Mild surges call for a focus on increasing ECMO capacity, while a moderate surge may indicate a need to focus on allocating scarce resources, and a major surge may signal the need to limit or defer use of scarce resources, according to the guidance.
“If your health care system is stretched from a resource standpoint, at what point do you say, ‘we don’t even have the capability to even safely do ECMO, and so, perhaps we should not even be offering the support’?” Dr. Keller said in an interview. “That’s what we tried to get at in the paper – helping institutions think about how to prepare for that pandemic, and then when to make decisions on when it should and should not be offered.”
Critical care guidance for COVID-19
The guidance from the Task Force for Mass Critical Care and CHEST offers nine specific actions that authors suggest as part of a framework for communities to establish the infrastructure needed to triage critical care resources and “equitably” meet the needs of the largest number of COVID-19 patients.
“It is the goal of the task force to minimize the need for allocation of scarce resources as much as possible,” the authors stated.
The framework starts with surge planning that includes an inventory of intensive care unit resources such as ventilators, beds, supplies, and staff that could be marshaled to meet a surge in demand, followed by establishing “identification triggers” for triage initiation by a regional authority, should clinical demand reach a crisis stage.
The next step is preparing the triage system, which includes creating a committee at the regional level, identifying members of tertiary triage teams and the support structures they will need, and preparing and distributing training materials.
Agreeing on a triage protocol is important to ensure equitable targeting of resources, and how to allocate limited life-sustaining measures needs to be considered, according to the panel of experts. They also recommend adaptations to the standards of care such as modification of end-of-life care policies, support for health care workers, family, and the public, and consideration of pediatric issues including transport, concentration of care at specific centers, and potential increases in age thresholds to accommodate surges.
Barriers to triage?
When asked about potential barriers to rolling out a triage plan, Dr. Maves said the first is acknowledging the possible need for such a plan: “It is a difficult concept for most in critical care to accept – the idea that we may not be able to provide an individual patient with interventions that we consider routine,” he said.
Beyond acknowledging need, other potential barriers to successful implementation include the limited evidence base to support development of these protocols, as well as the need to address public trust.
“If a triage system is perceived as unjust or biased, or if people think that triage favors or excludes certain groups unfairly, it will undermine any system,” Dr. Maves said. “Making sure the public both understands and has input into system development is critical if we are going to be able to make this work.”
Dr. Maves and coauthors reported that some of the authors of their guidance are United States government employees or military service members, and that their opinions and assertions do not reflect the official views or position of those institutions. Dr. Keller reported no disclosures related to the ECMO guidance.
SOURCES: Maves RC et al. Chest. 2020 Apr 11. pii: S0012-3692(20)30691-7. doi: 10.1016/j.chest.2020.03.063; Seethara R and Keller SP. Ann Am Thorac Soc. 2020 Apr 15. doi: 10.1513/AnnalsATS.202003-233PS.
FROM CHEST AND ANNALS OF THE AMERICAN THORACIC SOCIETY
ACEI/ARBs linked with survival in hypertensive, Chinese COVID-19 patients
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
FROM CIRCULATION RESEARCH
Transitions: From editor to president
As I transition out of the role of medical editor for The Hospitalist, and into the role of president of the Society of Hospital Medicine, it is a bittersweet but exciting transition.
In the relatively short time I have served as editor, so much has changed in our hospitalist community! In the last 4 years alone, we have increased:
• Membership from 14,000 to 20,000
• Chapters from 46 to 68
• Special Interest Groups from 8 to 22
• Subscribers to The Hospitalist from 15,000 to 30,000.
This is all a testimony to the engagement of our membership. SHM is clearly no ordinary specialty society; it is full of incredibly intelligent, invested, and talented members, who actively participate in the society for the betterment of their local teams and patients. It is such a privilege to lead this amazing team.
As for The Hospitalist, I would like to warmly welcome Weijen Chang, MD, FACP, SFHM, as the incoming editor. Weijen served as the pediatrics editor for many years and has been extensively involved on The Hospitalist’s editorial advisory board for even longer. He also has a broad track record of experience as a hospitalist in many settings; that combined with an inquisitive mind and curious spirit makes him the ideal editor for The Hospitalist. He brings energy and enthusiasm and will serve us very well.
While I will miss being intimately involved with The Hospitalist, I am very much looking forward to serving in the role of SHM president starting in April. During this pivotal year, SHM will transition from our one-and-only CEO, Larry Wellikson, MD, MHM, to our newly minted CEO Eric Howell, MD, MHM, who will officially transition in July 2020.
This is a very exciting time in the history of SHM, as we refocus on our mission, vision, values, and core activities. As a membership organization, our primary focus has been, and will always be, serving our member’s needs! As a “Big Tent” organization, we have always supported a broad and diverse set of members, ranging far beyond physician hospitalists, to trainees, medical students, nurse practitioners, physician assistants, practice administrators, and other hospital-based specialists. Being in such a dynamic industry, our diverse members needs are constantly and rapidly changing along with the dramatic transformations in the landscape, including profound shifts in care and reimbursement models that could change the very definition of a hospitalist.
While we continuously scour the landscape and anticipate our members’ needs, we will never lose sight of our core mission, which is to promote exceptional care for hospitalized patients. We will continue to do this by supporting all of our members with tools and materials to help them be the very best they can, for all of our patients. As a humble and servant leader, I am prepared to meet the demands and challenges of the year ahead, with energy and focus, and fulfill the needs of our members, so that together, we can make health care better for those we serve.
Thank you in advance for allowing me the great pleasure of serving this amazing and innovative organization!
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is the outgoing medical editor of The Hospitalist, and president-elect of SHM.
As I transition out of the role of medical editor for The Hospitalist, and into the role of president of the Society of Hospital Medicine, it is a bittersweet but exciting transition.
In the relatively short time I have served as editor, so much has changed in our hospitalist community! In the last 4 years alone, we have increased:
• Membership from 14,000 to 20,000
• Chapters from 46 to 68
• Special Interest Groups from 8 to 22
• Subscribers to The Hospitalist from 15,000 to 30,000.
This is all a testimony to the engagement of our membership. SHM is clearly no ordinary specialty society; it is full of incredibly intelligent, invested, and talented members, who actively participate in the society for the betterment of their local teams and patients. It is such a privilege to lead this amazing team.
As for The Hospitalist, I would like to warmly welcome Weijen Chang, MD, FACP, SFHM, as the incoming editor. Weijen served as the pediatrics editor for many years and has been extensively involved on The Hospitalist’s editorial advisory board for even longer. He also has a broad track record of experience as a hospitalist in many settings; that combined with an inquisitive mind and curious spirit makes him the ideal editor for The Hospitalist. He brings energy and enthusiasm and will serve us very well.
While I will miss being intimately involved with The Hospitalist, I am very much looking forward to serving in the role of SHM president starting in April. During this pivotal year, SHM will transition from our one-and-only CEO, Larry Wellikson, MD, MHM, to our newly minted CEO Eric Howell, MD, MHM, who will officially transition in July 2020.
This is a very exciting time in the history of SHM, as we refocus on our mission, vision, values, and core activities. As a membership organization, our primary focus has been, and will always be, serving our member’s needs! As a “Big Tent” organization, we have always supported a broad and diverse set of members, ranging far beyond physician hospitalists, to trainees, medical students, nurse practitioners, physician assistants, practice administrators, and other hospital-based specialists. Being in such a dynamic industry, our diverse members needs are constantly and rapidly changing along with the dramatic transformations in the landscape, including profound shifts in care and reimbursement models that could change the very definition of a hospitalist.
While we continuously scour the landscape and anticipate our members’ needs, we will never lose sight of our core mission, which is to promote exceptional care for hospitalized patients. We will continue to do this by supporting all of our members with tools and materials to help them be the very best they can, for all of our patients. As a humble and servant leader, I am prepared to meet the demands and challenges of the year ahead, with energy and focus, and fulfill the needs of our members, so that together, we can make health care better for those we serve.
Thank you in advance for allowing me the great pleasure of serving this amazing and innovative organization!
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is the outgoing medical editor of The Hospitalist, and president-elect of SHM.
As I transition out of the role of medical editor for The Hospitalist, and into the role of president of the Society of Hospital Medicine, it is a bittersweet but exciting transition.
In the relatively short time I have served as editor, so much has changed in our hospitalist community! In the last 4 years alone, we have increased:
• Membership from 14,000 to 20,000
• Chapters from 46 to 68
• Special Interest Groups from 8 to 22
• Subscribers to The Hospitalist from 15,000 to 30,000.
This is all a testimony to the engagement of our membership. SHM is clearly no ordinary specialty society; it is full of incredibly intelligent, invested, and talented members, who actively participate in the society for the betterment of their local teams and patients. It is such a privilege to lead this amazing team.
As for The Hospitalist, I would like to warmly welcome Weijen Chang, MD, FACP, SFHM, as the incoming editor. Weijen served as the pediatrics editor for many years and has been extensively involved on The Hospitalist’s editorial advisory board for even longer. He also has a broad track record of experience as a hospitalist in many settings; that combined with an inquisitive mind and curious spirit makes him the ideal editor for The Hospitalist. He brings energy and enthusiasm and will serve us very well.
While I will miss being intimately involved with The Hospitalist, I am very much looking forward to serving in the role of SHM president starting in April. During this pivotal year, SHM will transition from our one-and-only CEO, Larry Wellikson, MD, MHM, to our newly minted CEO Eric Howell, MD, MHM, who will officially transition in July 2020.
This is a very exciting time in the history of SHM, as we refocus on our mission, vision, values, and core activities. As a membership organization, our primary focus has been, and will always be, serving our member’s needs! As a “Big Tent” organization, we have always supported a broad and diverse set of members, ranging far beyond physician hospitalists, to trainees, medical students, nurse practitioners, physician assistants, practice administrators, and other hospital-based specialists. Being in such a dynamic industry, our diverse members needs are constantly and rapidly changing along with the dramatic transformations in the landscape, including profound shifts in care and reimbursement models that could change the very definition of a hospitalist.
While we continuously scour the landscape and anticipate our members’ needs, we will never lose sight of our core mission, which is to promote exceptional care for hospitalized patients. We will continue to do this by supporting all of our members with tools and materials to help them be the very best they can, for all of our patients. As a humble and servant leader, I am prepared to meet the demands and challenges of the year ahead, with energy and focus, and fulfill the needs of our members, so that together, we can make health care better for those we serve.
Thank you in advance for allowing me the great pleasure of serving this amazing and innovative organization!
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is the outgoing medical editor of The Hospitalist, and president-elect of SHM.
COVID-19 strikes hard at state-run veterans nursing homes
In early March, 35 residents in the Life Care Center in Kirkland, Washington, died due to complications associated with COVID-19. And that facility thus became the first example of how extremely vulnerable nursing home residents are to COVID-19. Since then, around the US, thousands of nursing home residents have died from complications of the virus. US Department of Veterans Affairs (VA) nursing homes, while rated high in VA health inspection reports, have not been exempt.
As of April 21, the VA had confirmed > 5,500 coronavirus cases in 50 states, the District of Columbia, and Puerto Rico. More than 350 veterans have died of COVID-19, according to VA data. The VA calculates its rates by health care system or VA medical center and does not provide separate data for the community living centers (CLCs).
The VA initiated an isolation strategy on March 10 that suspended most new admissions and barred outsiders from all of its 134 nursing homes. The only exception to the rule was when a patient was expected to die soon. The VA has taken other precautions as well, including extra screening and directing patients to use telehealth where possible.
State-run long-term care facilities for veterans have been hard hit across the country. At the Soldiers’ Home in Holyoke, Massachusetts, which is run by the state of Massachusetts, 5 of 11 veterans who died recently tested positive for COVID-19. At the 4 state-run nursing homes in Alabama, as of April 14, 45 people were confirmed positive and 2 residents had died. The largest outbreak was in the Bill Nichols State Veterans Home in Alexander City. Alabama State Rep. Ed Oliver and Commissioner Kent Davis, of the Alabama Department of Veterans Affairs (ADVA), are looking into how the outbreak started and whether it could have been prevented. “We have reports of lack of hand sanitizers, and those are the things we’re looking at right now,” Rep. Oliver said. The ADVA says residents who test positive are isolated for treatment, and infected employees are prohibited from entering the homes.
States have deployed National Guard troops to facilities following large scale outbreaks and multiple deaths. Pennsylvania deployed 30 National Guard troops to its Southeastern Veterans Center facility in Spring City after at least 10 veterans had died and at least 19 health care workers had tested positive for the virus. The facility is 1 of 6 extended-care facilities run by the Pennsylvania Department of Military and Veterans Affairs. In New Jersey, 40 National Guard troops, 25 New Jersey Department of Health nurses, and 90 VA nurses were deployed to 2 of its veterans facilities amid worsening outbreaks. At the Paramus facility, 155 residents had tested positive and 39 had died, and at the home in Edison, 86 veterans had tested positive and 25 died; 6 more died at a third state facility.
However, reporting remains inconsistent across many states and facilities. Only on April 19 did the Centers for Medicare and Medicaid Services (CMS) order nursing home facilities to inform residents and families about COVID-19 cases inside. This followed similar orders in New Jersey, New York, California, Washington, and other states.
“Nursing homes have been ground zero for COVID-19,” said CMS Administrator Seema Verma in a written statement. “Nursing home reporting to the [Centers for Disease Control and Prevention] is a critical component of the go-forward national COVID-19 surveillance system and to efforts to reopen America.”
In early March, 35 residents in the Life Care Center in Kirkland, Washington, died due to complications associated with COVID-19. And that facility thus became the first example of how extremely vulnerable nursing home residents are to COVID-19. Since then, around the US, thousands of nursing home residents have died from complications of the virus. US Department of Veterans Affairs (VA) nursing homes, while rated high in VA health inspection reports, have not been exempt.
As of April 21, the VA had confirmed > 5,500 coronavirus cases in 50 states, the District of Columbia, and Puerto Rico. More than 350 veterans have died of COVID-19, according to VA data. The VA calculates its rates by health care system or VA medical center and does not provide separate data for the community living centers (CLCs).
The VA initiated an isolation strategy on March 10 that suspended most new admissions and barred outsiders from all of its 134 nursing homes. The only exception to the rule was when a patient was expected to die soon. The VA has taken other precautions as well, including extra screening and directing patients to use telehealth where possible.
State-run long-term care facilities for veterans have been hard hit across the country. At the Soldiers’ Home in Holyoke, Massachusetts, which is run by the state of Massachusetts, 5 of 11 veterans who died recently tested positive for COVID-19. At the 4 state-run nursing homes in Alabama, as of April 14, 45 people were confirmed positive and 2 residents had died. The largest outbreak was in the Bill Nichols State Veterans Home in Alexander City. Alabama State Rep. Ed Oliver and Commissioner Kent Davis, of the Alabama Department of Veterans Affairs (ADVA), are looking into how the outbreak started and whether it could have been prevented. “We have reports of lack of hand sanitizers, and those are the things we’re looking at right now,” Rep. Oliver said. The ADVA says residents who test positive are isolated for treatment, and infected employees are prohibited from entering the homes.
States have deployed National Guard troops to facilities following large scale outbreaks and multiple deaths. Pennsylvania deployed 30 National Guard troops to its Southeastern Veterans Center facility in Spring City after at least 10 veterans had died and at least 19 health care workers had tested positive for the virus. The facility is 1 of 6 extended-care facilities run by the Pennsylvania Department of Military and Veterans Affairs. In New Jersey, 40 National Guard troops, 25 New Jersey Department of Health nurses, and 90 VA nurses were deployed to 2 of its veterans facilities amid worsening outbreaks. At the Paramus facility, 155 residents had tested positive and 39 had died, and at the home in Edison, 86 veterans had tested positive and 25 died; 6 more died at a third state facility.
However, reporting remains inconsistent across many states and facilities. Only on April 19 did the Centers for Medicare and Medicaid Services (CMS) order nursing home facilities to inform residents and families about COVID-19 cases inside. This followed similar orders in New Jersey, New York, California, Washington, and other states.
“Nursing homes have been ground zero for COVID-19,” said CMS Administrator Seema Verma in a written statement. “Nursing home reporting to the [Centers for Disease Control and Prevention] is a critical component of the go-forward national COVID-19 surveillance system and to efforts to reopen America.”
In early March, 35 residents in the Life Care Center in Kirkland, Washington, died due to complications associated with COVID-19. And that facility thus became the first example of how extremely vulnerable nursing home residents are to COVID-19. Since then, around the US, thousands of nursing home residents have died from complications of the virus. US Department of Veterans Affairs (VA) nursing homes, while rated high in VA health inspection reports, have not been exempt.
As of April 21, the VA had confirmed > 5,500 coronavirus cases in 50 states, the District of Columbia, and Puerto Rico. More than 350 veterans have died of COVID-19, according to VA data. The VA calculates its rates by health care system or VA medical center and does not provide separate data for the community living centers (CLCs).
The VA initiated an isolation strategy on March 10 that suspended most new admissions and barred outsiders from all of its 134 nursing homes. The only exception to the rule was when a patient was expected to die soon. The VA has taken other precautions as well, including extra screening and directing patients to use telehealth where possible.
State-run long-term care facilities for veterans have been hard hit across the country. At the Soldiers’ Home in Holyoke, Massachusetts, which is run by the state of Massachusetts, 5 of 11 veterans who died recently tested positive for COVID-19. At the 4 state-run nursing homes in Alabama, as of April 14, 45 people were confirmed positive and 2 residents had died. The largest outbreak was in the Bill Nichols State Veterans Home in Alexander City. Alabama State Rep. Ed Oliver and Commissioner Kent Davis, of the Alabama Department of Veterans Affairs (ADVA), are looking into how the outbreak started and whether it could have been prevented. “We have reports of lack of hand sanitizers, and those are the things we’re looking at right now,” Rep. Oliver said. The ADVA says residents who test positive are isolated for treatment, and infected employees are prohibited from entering the homes.
States have deployed National Guard troops to facilities following large scale outbreaks and multiple deaths. Pennsylvania deployed 30 National Guard troops to its Southeastern Veterans Center facility in Spring City after at least 10 veterans had died and at least 19 health care workers had tested positive for the virus. The facility is 1 of 6 extended-care facilities run by the Pennsylvania Department of Military and Veterans Affairs. In New Jersey, 40 National Guard troops, 25 New Jersey Department of Health nurses, and 90 VA nurses were deployed to 2 of its veterans facilities amid worsening outbreaks. At the Paramus facility, 155 residents had tested positive and 39 had died, and at the home in Edison, 86 veterans had tested positive and 25 died; 6 more died at a third state facility.
However, reporting remains inconsistent across many states and facilities. Only on April 19 did the Centers for Medicare and Medicaid Services (CMS) order nursing home facilities to inform residents and families about COVID-19 cases inside. This followed similar orders in New Jersey, New York, California, Washington, and other states.
“Nursing homes have been ground zero for COVID-19,” said CMS Administrator Seema Verma in a written statement. “Nursing home reporting to the [Centers for Disease Control and Prevention] is a critical component of the go-forward national COVID-19 surveillance system and to efforts to reopen America.”
Mislabeled clopidogrel lot recalled, may contain simvastatin
International Laboratories has initiated a voluntary recall to the consumer level in the United States of a single lot of the antiplatelet clopidogrel because it is mislabeled and may contain simvastatin, a cholesterol-lowering drug, instead of clopidogrel.
The recalled product ― lot number 117099A of clopidogrel tablets (USP 75 mg) packaged in bottles of 30 tablets ― may contain clopidogrel 75 mg tablets or it could contain simvastatin tablets (USP 10 mg), according to a company announcement posted on the US Food and Drug Administration (FDA) website.
“Missed doses of clopidogrel increases the risk of heart attack and stroke which can be life threatening. Additionally, unintentional consumption of simvastatin could include the common side effects associated with its use and may cause fetal harm when administered to a pregnant woman,” the company cautions.
To date, the company has not received any reports of harm arising from the problem that prompted the recall.
The recalled product was distributed nationwide and was delivered to distribution centers in Arkansas, Georgia, Indiana, California, and Maryland and to retail stores in all US states.
International Laboratories is notifying distributors and customers by letter and is arranging for the return of all recalled products.
For questions regarding this recall, contact Inmar by phone 855-258-7280 (weekdays between 9:00 AM and 5:00 PM EST) or by email at [email protected].
Adverse reactions or quality problems experienced with the use of this product should be reported to the FDA’s MedWatch adverse event reporting program.
This article first appeared on Medscape.com.
International Laboratories has initiated a voluntary recall to the consumer level in the United States of a single lot of the antiplatelet clopidogrel because it is mislabeled and may contain simvastatin, a cholesterol-lowering drug, instead of clopidogrel.
The recalled product ― lot number 117099A of clopidogrel tablets (USP 75 mg) packaged in bottles of 30 tablets ― may contain clopidogrel 75 mg tablets or it could contain simvastatin tablets (USP 10 mg), according to a company announcement posted on the US Food and Drug Administration (FDA) website.
“Missed doses of clopidogrel increases the risk of heart attack and stroke which can be life threatening. Additionally, unintentional consumption of simvastatin could include the common side effects associated with its use and may cause fetal harm when administered to a pregnant woman,” the company cautions.
To date, the company has not received any reports of harm arising from the problem that prompted the recall.
The recalled product was distributed nationwide and was delivered to distribution centers in Arkansas, Georgia, Indiana, California, and Maryland and to retail stores in all US states.
International Laboratories is notifying distributors and customers by letter and is arranging for the return of all recalled products.
For questions regarding this recall, contact Inmar by phone 855-258-7280 (weekdays between 9:00 AM and 5:00 PM EST) or by email at [email protected].
Adverse reactions or quality problems experienced with the use of this product should be reported to the FDA’s MedWatch adverse event reporting program.
This article first appeared on Medscape.com.
International Laboratories has initiated a voluntary recall to the consumer level in the United States of a single lot of the antiplatelet clopidogrel because it is mislabeled and may contain simvastatin, a cholesterol-lowering drug, instead of clopidogrel.
The recalled product ― lot number 117099A of clopidogrel tablets (USP 75 mg) packaged in bottles of 30 tablets ― may contain clopidogrel 75 mg tablets or it could contain simvastatin tablets (USP 10 mg), according to a company announcement posted on the US Food and Drug Administration (FDA) website.
“Missed doses of clopidogrel increases the risk of heart attack and stroke which can be life threatening. Additionally, unintentional consumption of simvastatin could include the common side effects associated with its use and may cause fetal harm when administered to a pregnant woman,” the company cautions.
To date, the company has not received any reports of harm arising from the problem that prompted the recall.
The recalled product was distributed nationwide and was delivered to distribution centers in Arkansas, Georgia, Indiana, California, and Maryland and to retail stores in all US states.
International Laboratories is notifying distributors and customers by letter and is arranging for the return of all recalled products.
For questions regarding this recall, contact Inmar by phone 855-258-7280 (weekdays between 9:00 AM and 5:00 PM EST) or by email at [email protected].
Adverse reactions or quality problems experienced with the use of this product should be reported to the FDA’s MedWatch adverse event reporting program.
This article first appeared on Medscape.com.
Doctors push back on treating COVID-19 as HAPE
For Luanne Freer, MD, an expert in high-altitude pulmonary edema (HAPE) and founder and director of Everest ER, a nonprofit seasonal clinic at the Mt. Everest base camp in Nepal (elevation, 17,600 ft), a sudden flurry of messages and questions she received about a possible COVID-19/HAPE link was startling.
“That’s why it kind of poked me in the eye,” she said, referencing her extensive experience treating HAPE, which she described as a pressure-related phenomenon. “My goodness, they are so completely different.”
Dr. Freer, an emergency physician, reached out to several pulmonary intensivists with experience treating both HAPE and COVID-19 to gauge their reactions, and within 36 hours, they had drafted their response. In the commentary, published in High Altitude Medicine & Biology, the clinicians note that the comparison between HAPE and COVID-19 is potentially risky.
“As a group of physicians who have in some cases cared for patients with COVID-19 and in all cases cared for patients with HAPE and studied its pathophysiology and management, we feel it important to correct this misconception, as continued amplification of this message could have adverse effects on management of these patients,” they wrote.
The suggestion that COVID-19 lung injury sometimes looks more like HAPE than like acute respiratory distress syndrome (ARDS) appeared in a journal review article in late March and was put forth by medical professionals on social media where it gained traction in recent weeks and was amplified in multiple media outlets, including this one.
“With COVID, we don’t understand everything that’s going on, but we know for sure it’s an inflammatory process – not a pressure-related problem,” Dr. Freer said. “I thought ... this could be so dangerous to load the medicines that we use when we’re treating HAPE onto patients with COVID-19.”
The pathophysiological mechanisms in HAPE are different than those in other respiratory syndromes, including those associated with COVID-19, said Andrew M. Luks, MD, of the UW Medicine, Seattle, and the first author on the commentary.
“HAPE is a noncardiogenic form of pulmonary edema, as are ARDS due to bacteria or viral pneumonia, re-expansion pulmonary edema, immersion pulmonary edema, negative pressure pulmonary edema, and neurogenic pulmonary edema,” Dr. Luks, Dr. Freer, and colleagues wrote in the commentary, explaining that all of these entities cause varying degrees of hypoxemia and diffuse bilateral opacities on chest imaging. “Importantly, in all of these cases, edema accumulates in the interstitial and alveolar spaces of the lung as a result of imbalance in Starling forces.”
A difference between these entities, however, is “the mechanism by which that imbalance develops,” they noted.
The excessive and uneven hypoxic pulmonary vasoconstriction that leads to a marked increase in pulmonary artery pressure, subsequent lung overperfusion, increased pulmonary capillary hydrostatic pressure, and leakage of fluid from the vascular space into the alveolar space as seen in HAPE, is a “fundamentally different phenomenon than what is seen in COVID-19-related ARDS, which involves viral-mediated inflammatory responses as the primary pathophysiological mechanism,” they added.
The authors described several other differences between the conditions, ultimately noting that “understanding the distinction between the pathophysiological mechanisms of these entities is critical for patient management.”
In HAPE, supplemental oxygen alone may be sufficient; in COVID-19, it may improve hypoxemia but won’t resolve the underlying inflammation or injury, they explained, adding that “only good supportive care including mechanical ventilation, quite often for long periods of time, allows some patients to survive until their disease resolves.”
Further, HAPE can be prevented or treated with pulmonary vasodilators such a nifedipine or sildenafil, which decrease pulmonary artery pressure and, as a result lower pulmonary capillary hydrostatic pressure, they said.
Use of such medications for COVID-19 might decrease pulmonary artery pressure and improve right ventricular function in COVID-19, but “by releasing hypoxic pulmonary vasoconstriction and increasing perfusion to nonventilated regions of the lung, they could also worsen ventilation-perfusion mismatch” and thereby worsen hypoxemia, they explained, adding that the treatments can also cause or worsen hypotension.
Efforts to share observations and experience are important in medicine, but sometimes, as in this circumstance, “they get out there, spread around – like a brushfire almost – and get [unwarranted] face validity,” Dr. Luks said, noting that in response to information circulating about COVID-19 and HAPE, he has already heard medical professionals floating the idea of treating COVID-19 with treatments used for HAPE.
It’s true that some COVID-19 lung injury cases are behaving differently than typical ARDS, he said, adding that presentation can vary.
“But trying to equate HAPE and COVID-19 is just wrong,” he said. “HAPE and COVID-19 may share several features ...but those are features that are shared by a lot of different forms of respiratory failure.”
In a recent video interview, WebMD’s chief medical officer John Whyte, MD, spoke with a New York City physician trained in critical care and emergency medicine, Cameron Kyle-Sidell, MD, who raised the need to consider different respiratory protocols for COVID-19, noting that standard protocols were falling short in many cases.
“What we’re seeing ... is something unusual, it’s something that we are not used to,” Dr. Kyle-Sidell of Maimonides Medical Center said in that interview, stressing that the presentation differed from that seen in typical ARDS. “The patterns I was seeing did not make sense.”
Like others, he noted that COVID-19 patients were presenting with illness that clinically looked more like HAPE, but that the pathophysiology is not necessary similar to HAPE.
At around the same time, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues, published a letter to the editor in the American Journal of Respiratory and Critical Care Medicine stressing that the ARDS presentation in COVID-19 patients is atypical and requires a patient physiology–driven treatment approach, rather than a standard protocol–driven approach. Dr. Gattinoni and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation.
Dr. Luks agreed that “some patients with COVID-19 do not have the same physiologic derangements that we see in a lot of other people with ARDS.”
“[Dr. Gattinoni] is making the point that we need to treat these people differently ... and I think that’s a valid point, and honestly, that’s a point that applied even before COVID-19,” he said. “Most of the things that we see in clinical practice – there’s a lot of heterogeneity between patients, and you have to be prepared to tailor your therapy in light of the differences that you’re picking up from your observations at the bedside and other data that you’re getting on the patient.”
The main concern Dr. Luks and his coauthors wanted to convey, they said, is making sure that the anecdotal experiences and observations of clinicians struggling to find answers don’t spiral out of control without proper vetting, thereby leading to patient harm.
“In this challenging time, we must identify the best means to care for these critically ill patients. That approach should be grounded in sound pulmonary physiology, clinical experience and, when available, evidence from clinical studies,” they concluded.
Dr. Luks and Dr. Freer reported having no financial disclosures.
For Luanne Freer, MD, an expert in high-altitude pulmonary edema (HAPE) and founder and director of Everest ER, a nonprofit seasonal clinic at the Mt. Everest base camp in Nepal (elevation, 17,600 ft), a sudden flurry of messages and questions she received about a possible COVID-19/HAPE link was startling.
“That’s why it kind of poked me in the eye,” she said, referencing her extensive experience treating HAPE, which she described as a pressure-related phenomenon. “My goodness, they are so completely different.”
Dr. Freer, an emergency physician, reached out to several pulmonary intensivists with experience treating both HAPE and COVID-19 to gauge their reactions, and within 36 hours, they had drafted their response. In the commentary, published in High Altitude Medicine & Biology, the clinicians note that the comparison between HAPE and COVID-19 is potentially risky.
“As a group of physicians who have in some cases cared for patients with COVID-19 and in all cases cared for patients with HAPE and studied its pathophysiology and management, we feel it important to correct this misconception, as continued amplification of this message could have adverse effects on management of these patients,” they wrote.
The suggestion that COVID-19 lung injury sometimes looks more like HAPE than like acute respiratory distress syndrome (ARDS) appeared in a journal review article in late March and was put forth by medical professionals on social media where it gained traction in recent weeks and was amplified in multiple media outlets, including this one.
“With COVID, we don’t understand everything that’s going on, but we know for sure it’s an inflammatory process – not a pressure-related problem,” Dr. Freer said. “I thought ... this could be so dangerous to load the medicines that we use when we’re treating HAPE onto patients with COVID-19.”
The pathophysiological mechanisms in HAPE are different than those in other respiratory syndromes, including those associated with COVID-19, said Andrew M. Luks, MD, of the UW Medicine, Seattle, and the first author on the commentary.
“HAPE is a noncardiogenic form of pulmonary edema, as are ARDS due to bacteria or viral pneumonia, re-expansion pulmonary edema, immersion pulmonary edema, negative pressure pulmonary edema, and neurogenic pulmonary edema,” Dr. Luks, Dr. Freer, and colleagues wrote in the commentary, explaining that all of these entities cause varying degrees of hypoxemia and diffuse bilateral opacities on chest imaging. “Importantly, in all of these cases, edema accumulates in the interstitial and alveolar spaces of the lung as a result of imbalance in Starling forces.”
A difference between these entities, however, is “the mechanism by which that imbalance develops,” they noted.
The excessive and uneven hypoxic pulmonary vasoconstriction that leads to a marked increase in pulmonary artery pressure, subsequent lung overperfusion, increased pulmonary capillary hydrostatic pressure, and leakage of fluid from the vascular space into the alveolar space as seen in HAPE, is a “fundamentally different phenomenon than what is seen in COVID-19-related ARDS, which involves viral-mediated inflammatory responses as the primary pathophysiological mechanism,” they added.
The authors described several other differences between the conditions, ultimately noting that “understanding the distinction between the pathophysiological mechanisms of these entities is critical for patient management.”
In HAPE, supplemental oxygen alone may be sufficient; in COVID-19, it may improve hypoxemia but won’t resolve the underlying inflammation or injury, they explained, adding that “only good supportive care including mechanical ventilation, quite often for long periods of time, allows some patients to survive until their disease resolves.”
Further, HAPE can be prevented or treated with pulmonary vasodilators such a nifedipine or sildenafil, which decrease pulmonary artery pressure and, as a result lower pulmonary capillary hydrostatic pressure, they said.
Use of such medications for COVID-19 might decrease pulmonary artery pressure and improve right ventricular function in COVID-19, but “by releasing hypoxic pulmonary vasoconstriction and increasing perfusion to nonventilated regions of the lung, they could also worsen ventilation-perfusion mismatch” and thereby worsen hypoxemia, they explained, adding that the treatments can also cause or worsen hypotension.
Efforts to share observations and experience are important in medicine, but sometimes, as in this circumstance, “they get out there, spread around – like a brushfire almost – and get [unwarranted] face validity,” Dr. Luks said, noting that in response to information circulating about COVID-19 and HAPE, he has already heard medical professionals floating the idea of treating COVID-19 with treatments used for HAPE.
It’s true that some COVID-19 lung injury cases are behaving differently than typical ARDS, he said, adding that presentation can vary.
“But trying to equate HAPE and COVID-19 is just wrong,” he said. “HAPE and COVID-19 may share several features ...but those are features that are shared by a lot of different forms of respiratory failure.”
In a recent video interview, WebMD’s chief medical officer John Whyte, MD, spoke with a New York City physician trained in critical care and emergency medicine, Cameron Kyle-Sidell, MD, who raised the need to consider different respiratory protocols for COVID-19, noting that standard protocols were falling short in many cases.
“What we’re seeing ... is something unusual, it’s something that we are not used to,” Dr. Kyle-Sidell of Maimonides Medical Center said in that interview, stressing that the presentation differed from that seen in typical ARDS. “The patterns I was seeing did not make sense.”
Like others, he noted that COVID-19 patients were presenting with illness that clinically looked more like HAPE, but that the pathophysiology is not necessary similar to HAPE.
At around the same time, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues, published a letter to the editor in the American Journal of Respiratory and Critical Care Medicine stressing that the ARDS presentation in COVID-19 patients is atypical and requires a patient physiology–driven treatment approach, rather than a standard protocol–driven approach. Dr. Gattinoni and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation.
Dr. Luks agreed that “some patients with COVID-19 do not have the same physiologic derangements that we see in a lot of other people with ARDS.”
“[Dr. Gattinoni] is making the point that we need to treat these people differently ... and I think that’s a valid point, and honestly, that’s a point that applied even before COVID-19,” he said. “Most of the things that we see in clinical practice – there’s a lot of heterogeneity between patients, and you have to be prepared to tailor your therapy in light of the differences that you’re picking up from your observations at the bedside and other data that you’re getting on the patient.”
The main concern Dr. Luks and his coauthors wanted to convey, they said, is making sure that the anecdotal experiences and observations of clinicians struggling to find answers don’t spiral out of control without proper vetting, thereby leading to patient harm.
“In this challenging time, we must identify the best means to care for these critically ill patients. That approach should be grounded in sound pulmonary physiology, clinical experience and, when available, evidence from clinical studies,” they concluded.
Dr. Luks and Dr. Freer reported having no financial disclosures.
For Luanne Freer, MD, an expert in high-altitude pulmonary edema (HAPE) and founder and director of Everest ER, a nonprofit seasonal clinic at the Mt. Everest base camp in Nepal (elevation, 17,600 ft), a sudden flurry of messages and questions she received about a possible COVID-19/HAPE link was startling.
“That’s why it kind of poked me in the eye,” she said, referencing her extensive experience treating HAPE, which she described as a pressure-related phenomenon. “My goodness, they are so completely different.”
Dr. Freer, an emergency physician, reached out to several pulmonary intensivists with experience treating both HAPE and COVID-19 to gauge their reactions, and within 36 hours, they had drafted their response. In the commentary, published in High Altitude Medicine & Biology, the clinicians note that the comparison between HAPE and COVID-19 is potentially risky.
“As a group of physicians who have in some cases cared for patients with COVID-19 and in all cases cared for patients with HAPE and studied its pathophysiology and management, we feel it important to correct this misconception, as continued amplification of this message could have adverse effects on management of these patients,” they wrote.
The suggestion that COVID-19 lung injury sometimes looks more like HAPE than like acute respiratory distress syndrome (ARDS) appeared in a journal review article in late March and was put forth by medical professionals on social media where it gained traction in recent weeks and was amplified in multiple media outlets, including this one.
“With COVID, we don’t understand everything that’s going on, but we know for sure it’s an inflammatory process – not a pressure-related problem,” Dr. Freer said. “I thought ... this could be so dangerous to load the medicines that we use when we’re treating HAPE onto patients with COVID-19.”
The pathophysiological mechanisms in HAPE are different than those in other respiratory syndromes, including those associated with COVID-19, said Andrew M. Luks, MD, of the UW Medicine, Seattle, and the first author on the commentary.
“HAPE is a noncardiogenic form of pulmonary edema, as are ARDS due to bacteria or viral pneumonia, re-expansion pulmonary edema, immersion pulmonary edema, negative pressure pulmonary edema, and neurogenic pulmonary edema,” Dr. Luks, Dr. Freer, and colleagues wrote in the commentary, explaining that all of these entities cause varying degrees of hypoxemia and diffuse bilateral opacities on chest imaging. “Importantly, in all of these cases, edema accumulates in the interstitial and alveolar spaces of the lung as a result of imbalance in Starling forces.”
A difference between these entities, however, is “the mechanism by which that imbalance develops,” they noted.
The excessive and uneven hypoxic pulmonary vasoconstriction that leads to a marked increase in pulmonary artery pressure, subsequent lung overperfusion, increased pulmonary capillary hydrostatic pressure, and leakage of fluid from the vascular space into the alveolar space as seen in HAPE, is a “fundamentally different phenomenon than what is seen in COVID-19-related ARDS, which involves viral-mediated inflammatory responses as the primary pathophysiological mechanism,” they added.
The authors described several other differences between the conditions, ultimately noting that “understanding the distinction between the pathophysiological mechanisms of these entities is critical for patient management.”
In HAPE, supplemental oxygen alone may be sufficient; in COVID-19, it may improve hypoxemia but won’t resolve the underlying inflammation or injury, they explained, adding that “only good supportive care including mechanical ventilation, quite often for long periods of time, allows some patients to survive until their disease resolves.”
Further, HAPE can be prevented or treated with pulmonary vasodilators such a nifedipine or sildenafil, which decrease pulmonary artery pressure and, as a result lower pulmonary capillary hydrostatic pressure, they said.
Use of such medications for COVID-19 might decrease pulmonary artery pressure and improve right ventricular function in COVID-19, but “by releasing hypoxic pulmonary vasoconstriction and increasing perfusion to nonventilated regions of the lung, they could also worsen ventilation-perfusion mismatch” and thereby worsen hypoxemia, they explained, adding that the treatments can also cause or worsen hypotension.
Efforts to share observations and experience are important in medicine, but sometimes, as in this circumstance, “they get out there, spread around – like a brushfire almost – and get [unwarranted] face validity,” Dr. Luks said, noting that in response to information circulating about COVID-19 and HAPE, he has already heard medical professionals floating the idea of treating COVID-19 with treatments used for HAPE.
It’s true that some COVID-19 lung injury cases are behaving differently than typical ARDS, he said, adding that presentation can vary.
“But trying to equate HAPE and COVID-19 is just wrong,” he said. “HAPE and COVID-19 may share several features ...but those are features that are shared by a lot of different forms of respiratory failure.”
In a recent video interview, WebMD’s chief medical officer John Whyte, MD, spoke with a New York City physician trained in critical care and emergency medicine, Cameron Kyle-Sidell, MD, who raised the need to consider different respiratory protocols for COVID-19, noting that standard protocols were falling short in many cases.
“What we’re seeing ... is something unusual, it’s something that we are not used to,” Dr. Kyle-Sidell of Maimonides Medical Center said in that interview, stressing that the presentation differed from that seen in typical ARDS. “The patterns I was seeing did not make sense.”
Like others, he noted that COVID-19 patients were presenting with illness that clinically looked more like HAPE, but that the pathophysiology is not necessary similar to HAPE.
At around the same time, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues, published a letter to the editor in the American Journal of Respiratory and Critical Care Medicine stressing that the ARDS presentation in COVID-19 patients is atypical and requires a patient physiology–driven treatment approach, rather than a standard protocol–driven approach. Dr. Gattinoni and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation.
Dr. Luks agreed that “some patients with COVID-19 do not have the same physiologic derangements that we see in a lot of other people with ARDS.”
“[Dr. Gattinoni] is making the point that we need to treat these people differently ... and I think that’s a valid point, and honestly, that’s a point that applied even before COVID-19,” he said. “Most of the things that we see in clinical practice – there’s a lot of heterogeneity between patients, and you have to be prepared to tailor your therapy in light of the differences that you’re picking up from your observations at the bedside and other data that you’re getting on the patient.”
The main concern Dr. Luks and his coauthors wanted to convey, they said, is making sure that the anecdotal experiences and observations of clinicians struggling to find answers don’t spiral out of control without proper vetting, thereby leading to patient harm.
“In this challenging time, we must identify the best means to care for these critically ill patients. That approach should be grounded in sound pulmonary physiology, clinical experience and, when available, evidence from clinical studies,” they concluded.
Dr. Luks and Dr. Freer reported having no financial disclosures.
ASE issues echocardiography guidance amid COVID-19 pandemic
The American Society of Echocardiography (ASE) has issued a statement on protecting patients and echocardiography service providers during the COVID-19 pandemic.
Given the risk for cardiovascular complications associated with COVID-19, echocardiographic services will likely be needed for patients with suspected or confirmed COVID-19, meaning echo providers will be exposed to SARS-CoV-2, write the statement authors, led by James N. Kirkpatrick, MD, director of the echocardiography laboratory at University of Washington Medical Center in Seattle.
The statement was published online April 6 in the Journal of the American College of Cardiology.
The authors say the statement is intended to help guide the practice of echocardiography in this “challenging time.” It was developed with input from a variety of echocardiography providers and institutions who have experience with the COVID-19, or have been “actively and thoughtfully preparing for it.”
Who, When, Where, and How
The statement covers triaging and decision pathways for handling requests for echocardiography, as well as indications and recommended procedures, in cases of suspected or confirmed COVID-19.
Among the recommendations:
- Only perform transthoracic echocardiograms (TTE), stress echocardiograms, and transesophageal echocardiograms (TEE) if they are expected to provide clinical benefit. Appropriate-use criteria represent the first decision point as to whether an echocardiographic test should be performed.
- Determine which studies are “elective” and reschedule them, performing all others. Identify “nonelective” (urgent/emergent) indications and defer all others.
- Determine the clinical benefit of echocardiography for symptomatic patients whose SARS-CoV-2 status is unknown.
- Cautiously consider the benefit of a TEE examination weighed against the risk for exposure of healthcare personnel to aerosolization in a patient with suspected or confirmed COVID-19.
- Postpone or cancel TEEs if an alternative imaging modality can provide the necessary information.
- Note that treadmill or bicycle stress echo tests in patients with COVID-19 may lead to exposure because of deep breathing and/or coughing during exercise. These tests should generally be deferred or converted to a pharmacologic stress echo.
The ASE statement also provides advice on safe imaging protocol and adequate personal protection measures.
“In addition to limiting the number of echocardiography practitioners involved in scanning, consideration should be given to limiting the exposure of staff who may be particularly susceptible to severe complications of COVID-19,” the ASE advises.
Staff who are older than 60 years, who have chronic conditions, are immunocompromised or are pregnant may wish to avoid contact with patients suspected or confirmed to have COVID-19.
It’s also important to realize the risk for transmission in reading rooms. “Keyboards, monitors, mice, chairs, phones, desktops, and door knobs should be frequently cleaned, and ventilation provided wherever possible,” the ASE advises. When the echo lab reading room is located in a high-traffic area, remote review of images or via webinar might be advisable, they suggest.
Summing up, Kirkland and colleagues say providing echocardiographic service “remains crucial in this difficult time of the SARS-CoV-2 outbreak. Working together, we can continue to provide high-quality care while minimizing risk to ourselves, our patients, and the public at large. Carefully considering ‘Whom to Image’, ‘Where to Image’ and ‘How to Image’ has the potential to reduce the risks of transmission.”
The authors note that the statements and recommendations are primarily based on expert opinion rather than on scientifically verified data and are subject to change as the COVID-19 outbreak continues to evolve and new data emerges.
This article first appeared on Medscape.com.
The American Society of Echocardiography (ASE) has issued a statement on protecting patients and echocardiography service providers during the COVID-19 pandemic.
Given the risk for cardiovascular complications associated with COVID-19, echocardiographic services will likely be needed for patients with suspected or confirmed COVID-19, meaning echo providers will be exposed to SARS-CoV-2, write the statement authors, led by James N. Kirkpatrick, MD, director of the echocardiography laboratory at University of Washington Medical Center in Seattle.
The statement was published online April 6 in the Journal of the American College of Cardiology.
The authors say the statement is intended to help guide the practice of echocardiography in this “challenging time.” It was developed with input from a variety of echocardiography providers and institutions who have experience with the COVID-19, or have been “actively and thoughtfully preparing for it.”
Who, When, Where, and How
The statement covers triaging and decision pathways for handling requests for echocardiography, as well as indications and recommended procedures, in cases of suspected or confirmed COVID-19.
Among the recommendations:
- Only perform transthoracic echocardiograms (TTE), stress echocardiograms, and transesophageal echocardiograms (TEE) if they are expected to provide clinical benefit. Appropriate-use criteria represent the first decision point as to whether an echocardiographic test should be performed.
- Determine which studies are “elective” and reschedule them, performing all others. Identify “nonelective” (urgent/emergent) indications and defer all others.
- Determine the clinical benefit of echocardiography for symptomatic patients whose SARS-CoV-2 status is unknown.
- Cautiously consider the benefit of a TEE examination weighed against the risk for exposure of healthcare personnel to aerosolization in a patient with suspected or confirmed COVID-19.
- Postpone or cancel TEEs if an alternative imaging modality can provide the necessary information.
- Note that treadmill or bicycle stress echo tests in patients with COVID-19 may lead to exposure because of deep breathing and/or coughing during exercise. These tests should generally be deferred or converted to a pharmacologic stress echo.
The ASE statement also provides advice on safe imaging protocol and adequate personal protection measures.
“In addition to limiting the number of echocardiography practitioners involved in scanning, consideration should be given to limiting the exposure of staff who may be particularly susceptible to severe complications of COVID-19,” the ASE advises.
Staff who are older than 60 years, who have chronic conditions, are immunocompromised or are pregnant may wish to avoid contact with patients suspected or confirmed to have COVID-19.
It’s also important to realize the risk for transmission in reading rooms. “Keyboards, monitors, mice, chairs, phones, desktops, and door knobs should be frequently cleaned, and ventilation provided wherever possible,” the ASE advises. When the echo lab reading room is located in a high-traffic area, remote review of images or via webinar might be advisable, they suggest.
Summing up, Kirkland and colleagues say providing echocardiographic service “remains crucial in this difficult time of the SARS-CoV-2 outbreak. Working together, we can continue to provide high-quality care while minimizing risk to ourselves, our patients, and the public at large. Carefully considering ‘Whom to Image’, ‘Where to Image’ and ‘How to Image’ has the potential to reduce the risks of transmission.”
The authors note that the statements and recommendations are primarily based on expert opinion rather than on scientifically verified data and are subject to change as the COVID-19 outbreak continues to evolve and new data emerges.
This article first appeared on Medscape.com.
The American Society of Echocardiography (ASE) has issued a statement on protecting patients and echocardiography service providers during the COVID-19 pandemic.
Given the risk for cardiovascular complications associated with COVID-19, echocardiographic services will likely be needed for patients with suspected or confirmed COVID-19, meaning echo providers will be exposed to SARS-CoV-2, write the statement authors, led by James N. Kirkpatrick, MD, director of the echocardiography laboratory at University of Washington Medical Center in Seattle.
The statement was published online April 6 in the Journal of the American College of Cardiology.
The authors say the statement is intended to help guide the practice of echocardiography in this “challenging time.” It was developed with input from a variety of echocardiography providers and institutions who have experience with the COVID-19, or have been “actively and thoughtfully preparing for it.”
Who, When, Where, and How
The statement covers triaging and decision pathways for handling requests for echocardiography, as well as indications and recommended procedures, in cases of suspected or confirmed COVID-19.
Among the recommendations:
- Only perform transthoracic echocardiograms (TTE), stress echocardiograms, and transesophageal echocardiograms (TEE) if they are expected to provide clinical benefit. Appropriate-use criteria represent the first decision point as to whether an echocardiographic test should be performed.
- Determine which studies are “elective” and reschedule them, performing all others. Identify “nonelective” (urgent/emergent) indications and defer all others.
- Determine the clinical benefit of echocardiography for symptomatic patients whose SARS-CoV-2 status is unknown.
- Cautiously consider the benefit of a TEE examination weighed against the risk for exposure of healthcare personnel to aerosolization in a patient with suspected or confirmed COVID-19.
- Postpone or cancel TEEs if an alternative imaging modality can provide the necessary information.
- Note that treadmill or bicycle stress echo tests in patients with COVID-19 may lead to exposure because of deep breathing and/or coughing during exercise. These tests should generally be deferred or converted to a pharmacologic stress echo.
The ASE statement also provides advice on safe imaging protocol and adequate personal protection measures.
“In addition to limiting the number of echocardiography practitioners involved in scanning, consideration should be given to limiting the exposure of staff who may be particularly susceptible to severe complications of COVID-19,” the ASE advises.
Staff who are older than 60 years, who have chronic conditions, are immunocompromised or are pregnant may wish to avoid contact with patients suspected or confirmed to have COVID-19.
It’s also important to realize the risk for transmission in reading rooms. “Keyboards, monitors, mice, chairs, phones, desktops, and door knobs should be frequently cleaned, and ventilation provided wherever possible,” the ASE advises. When the echo lab reading room is located in a high-traffic area, remote review of images or via webinar might be advisable, they suggest.
Summing up, Kirkland and colleagues say providing echocardiographic service “remains crucial in this difficult time of the SARS-CoV-2 outbreak. Working together, we can continue to provide high-quality care while minimizing risk to ourselves, our patients, and the public at large. Carefully considering ‘Whom to Image’, ‘Where to Image’ and ‘How to Image’ has the potential to reduce the risks of transmission.”
The authors note that the statements and recommendations are primarily based on expert opinion rather than on scientifically verified data and are subject to change as the COVID-19 outbreak continues to evolve and new data emerges.
This article first appeared on Medscape.com.
Overcoming COVID-related stress
As a department chief managing during this crisis, everyone greets me sympathetically: “This must be so stressful for you! Are you doing OK?” “Um, I’m great,” I answer contritely. Yes, this is hard, yet I feel fine. But why? Shouldn’t I be fretting the damage done by the COVID cyclone? Our operations are smashed and our staff scrambled, my family and friends are out of work; these are difficult times. But a harmful effect on my health or yours is not inevitable. There are things we can do to inoculate ourselves.
No doubt, exercise (if you can find weights!), eating well, sleeping, and meditating help, but they are secondary. None of these protect much if you still believe stress is killing you. You must first reframe what is happening. Health psychologist Kelly McGonigal, PhD, from Stanford (Calif.) University, is a world expert on this topic. If you’ve not seen her TED talk about stress, then watch it now. She teaches how stress is indeed harmful to your health – but only if you believe it to be so. Many studies have borne this out. One showed that people who reported high stress in the previous year were 43% more likely to die than those who did not. But that risk held only when they believed stress was harmful to them. Those who did not think that stress was harmful not only fared better but also had the lowest likelihood of death, lower even than those who reported little stress! So it wasn’t the stress that mattered, it was the physiologic response to it. And that you can control.
Changing your beliefs is no easy feat. There is work to be done, Dr. McGonigal would argue. You must not only reframe our stress as healthful, but also act in ways to make this true. This is easier for us as physicians. First, we understand better than most that difficulty is a normal part of life. We have countless stories of hardship, tragedy, pain and suffering from the work we do. The pandemic may be extraordinary in breadth, but not in depth. We’ve seen worse happen to patients. Second, we have firsthand experience that suffering ends and often leads to strength and resilience. Even in our own lives, it was by traveling through the extraordinary stress of medical school and residency that we arrived here.
Cortisol increases when we are under duress. So does oxytocin. The former gets most of the press, the latter is more interesting. That oxytocin release during stress conferred survival benefits to us as a species: When a threat arrived, we not only ran, but also grabbed the kids, too! Oxytocin is the “tend and befriend” compliment to cortisol’s “fight or flight.” Focusing on this priming to strengthen social ties, listen, spend (Zoom) time together, and provide emotional support is key to our recovery. Even small acts of giving for our staff, friends, family, and strangers can significantly shift consequences of this stress from harmful to beneficial.
Last year, my uncle died in a tragic accident. My aunt, who is alone, is now also isolated. She’s lost her partner, her guardian, and she is afraid. Rather than succumb to the stress, she imagined something she could do to wrest some control. Last week, she filled her minivan with pink and yellow tulips bunched in bouquets and tied with handwritten notes of encouragement. She then drove up and down the streets in her North Attleboro, Mass., neighborhood and left the flowers on doorsteps until her van was empty. She did so to share with them the bit of joy that spring brings, she says, and to encourage people to stay inside!
This is a difficult time for us, and yet even more difficult for others. Perhaps the best we can do is to find ways to bring a bit of joy or comfort to others.
“In some ways suffering ceases to be suffering at the moment it finds a meaning, such as the meaning of a sacrifice.” – Viktor Frankl
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He had no relevant disclosures. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
As a department chief managing during this crisis, everyone greets me sympathetically: “This must be so stressful for you! Are you doing OK?” “Um, I’m great,” I answer contritely. Yes, this is hard, yet I feel fine. But why? Shouldn’t I be fretting the damage done by the COVID cyclone? Our operations are smashed and our staff scrambled, my family and friends are out of work; these are difficult times. But a harmful effect on my health or yours is not inevitable. There are things we can do to inoculate ourselves.
No doubt, exercise (if you can find weights!), eating well, sleeping, and meditating help, but they are secondary. None of these protect much if you still believe stress is killing you. You must first reframe what is happening. Health psychologist Kelly McGonigal, PhD, from Stanford (Calif.) University, is a world expert on this topic. If you’ve not seen her TED talk about stress, then watch it now. She teaches how stress is indeed harmful to your health – but only if you believe it to be so. Many studies have borne this out. One showed that people who reported high stress in the previous year were 43% more likely to die than those who did not. But that risk held only when they believed stress was harmful to them. Those who did not think that stress was harmful not only fared better but also had the lowest likelihood of death, lower even than those who reported little stress! So it wasn’t the stress that mattered, it was the physiologic response to it. And that you can control.
Changing your beliefs is no easy feat. There is work to be done, Dr. McGonigal would argue. You must not only reframe our stress as healthful, but also act in ways to make this true. This is easier for us as physicians. First, we understand better than most that difficulty is a normal part of life. We have countless stories of hardship, tragedy, pain and suffering from the work we do. The pandemic may be extraordinary in breadth, but not in depth. We’ve seen worse happen to patients. Second, we have firsthand experience that suffering ends and often leads to strength and resilience. Even in our own lives, it was by traveling through the extraordinary stress of medical school and residency that we arrived here.
Cortisol increases when we are under duress. So does oxytocin. The former gets most of the press, the latter is more interesting. That oxytocin release during stress conferred survival benefits to us as a species: When a threat arrived, we not only ran, but also grabbed the kids, too! Oxytocin is the “tend and befriend” compliment to cortisol’s “fight or flight.” Focusing on this priming to strengthen social ties, listen, spend (Zoom) time together, and provide emotional support is key to our recovery. Even small acts of giving for our staff, friends, family, and strangers can significantly shift consequences of this stress from harmful to beneficial.
Last year, my uncle died in a tragic accident. My aunt, who is alone, is now also isolated. She’s lost her partner, her guardian, and she is afraid. Rather than succumb to the stress, she imagined something she could do to wrest some control. Last week, she filled her minivan with pink and yellow tulips bunched in bouquets and tied with handwritten notes of encouragement. She then drove up and down the streets in her North Attleboro, Mass., neighborhood and left the flowers on doorsteps until her van was empty. She did so to share with them the bit of joy that spring brings, she says, and to encourage people to stay inside!
This is a difficult time for us, and yet even more difficult for others. Perhaps the best we can do is to find ways to bring a bit of joy or comfort to others.
“In some ways suffering ceases to be suffering at the moment it finds a meaning, such as the meaning of a sacrifice.” – Viktor Frankl
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He had no relevant disclosures. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
As a department chief managing during this crisis, everyone greets me sympathetically: “This must be so stressful for you! Are you doing OK?” “Um, I’m great,” I answer contritely. Yes, this is hard, yet I feel fine. But why? Shouldn’t I be fretting the damage done by the COVID cyclone? Our operations are smashed and our staff scrambled, my family and friends are out of work; these are difficult times. But a harmful effect on my health or yours is not inevitable. There are things we can do to inoculate ourselves.
No doubt, exercise (if you can find weights!), eating well, sleeping, and meditating help, but they are secondary. None of these protect much if you still believe stress is killing you. You must first reframe what is happening. Health psychologist Kelly McGonigal, PhD, from Stanford (Calif.) University, is a world expert on this topic. If you’ve not seen her TED talk about stress, then watch it now. She teaches how stress is indeed harmful to your health – but only if you believe it to be so. Many studies have borne this out. One showed that people who reported high stress in the previous year were 43% more likely to die than those who did not. But that risk held only when they believed stress was harmful to them. Those who did not think that stress was harmful not only fared better but also had the lowest likelihood of death, lower even than those who reported little stress! So it wasn’t the stress that mattered, it was the physiologic response to it. And that you can control.
Changing your beliefs is no easy feat. There is work to be done, Dr. McGonigal would argue. You must not only reframe our stress as healthful, but also act in ways to make this true. This is easier for us as physicians. First, we understand better than most that difficulty is a normal part of life. We have countless stories of hardship, tragedy, pain and suffering from the work we do. The pandemic may be extraordinary in breadth, but not in depth. We’ve seen worse happen to patients. Second, we have firsthand experience that suffering ends and often leads to strength and resilience. Even in our own lives, it was by traveling through the extraordinary stress of medical school and residency that we arrived here.
Cortisol increases when we are under duress. So does oxytocin. The former gets most of the press, the latter is more interesting. That oxytocin release during stress conferred survival benefits to us as a species: When a threat arrived, we not only ran, but also grabbed the kids, too! Oxytocin is the “tend and befriend” compliment to cortisol’s “fight or flight.” Focusing on this priming to strengthen social ties, listen, spend (Zoom) time together, and provide emotional support is key to our recovery. Even small acts of giving for our staff, friends, family, and strangers can significantly shift consequences of this stress from harmful to beneficial.
Last year, my uncle died in a tragic accident. My aunt, who is alone, is now also isolated. She’s lost her partner, her guardian, and she is afraid. Rather than succumb to the stress, she imagined something she could do to wrest some control. Last week, she filled her minivan with pink and yellow tulips bunched in bouquets and tied with handwritten notes of encouragement. She then drove up and down the streets in her North Attleboro, Mass., neighborhood and left the flowers on doorsteps until her van was empty. She did so to share with them the bit of joy that spring brings, she says, and to encourage people to stay inside!
This is a difficult time for us, and yet even more difficult for others. Perhaps the best we can do is to find ways to bring a bit of joy or comfort to others.
“In some ways suffering ceases to be suffering at the moment it finds a meaning, such as the meaning of a sacrifice.” – Viktor Frankl
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. He had no relevant disclosures. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
FDA authorizes first COVID-19 test kit with home collection option
a reissue of the emergency use authorization allowing for testing of samples self-collected by patients at home with the Pixel by LabCorp COVID-19 RT-PCR Test.
The reissued authorization allows for testing of a sample taken from the nose by way of a self-collection kit that contains nasal swabs and saline, according to the FDA press release. After self-swabbing, users should send the samples in an insulated package to a LabCorp laboratory for testing. LabCorp intends to make the Pixel test available to consumers in most states, accessible through doctors’ orders.
The Pixel test includes a specific Q-tip–style cotton swab for patients to use to collect their samples, the FDA noted. Because of concerns with sterility and cross-reactivity caused by inherent genetic material in cotton swabs, generic cotton swabs should not be used as a substitute. The FDA will work with test developers to determine if generic cotton swabs can be used safely and effectively with other tests.
“Throughout this pandemic we have been facilitating test development to ensure patients’ access to accurate diagnostics, which includes supporting the development of reliable and accurate at-home sample collection options. ... [The FDA] worked with LabCorp to ensure the data demonstrated from at-home patient sample collection is as safe and accurate as sample collection at a doctor’s office, hospital, or other testing site. With this action, there is now a convenient and reliable option for patient sample collection from the comfort and safety of their home,” FDA Commissioner Stephen M. Hahn, MD, said in the press release.
a reissue of the emergency use authorization allowing for testing of samples self-collected by patients at home with the Pixel by LabCorp COVID-19 RT-PCR Test.
The reissued authorization allows for testing of a sample taken from the nose by way of a self-collection kit that contains nasal swabs and saline, according to the FDA press release. After self-swabbing, users should send the samples in an insulated package to a LabCorp laboratory for testing. LabCorp intends to make the Pixel test available to consumers in most states, accessible through doctors’ orders.
The Pixel test includes a specific Q-tip–style cotton swab for patients to use to collect their samples, the FDA noted. Because of concerns with sterility and cross-reactivity caused by inherent genetic material in cotton swabs, generic cotton swabs should not be used as a substitute. The FDA will work with test developers to determine if generic cotton swabs can be used safely and effectively with other tests.
“Throughout this pandemic we have been facilitating test development to ensure patients’ access to accurate diagnostics, which includes supporting the development of reliable and accurate at-home sample collection options. ... [The FDA] worked with LabCorp to ensure the data demonstrated from at-home patient sample collection is as safe and accurate as sample collection at a doctor’s office, hospital, or other testing site. With this action, there is now a convenient and reliable option for patient sample collection from the comfort and safety of their home,” FDA Commissioner Stephen M. Hahn, MD, said in the press release.
a reissue of the emergency use authorization allowing for testing of samples self-collected by patients at home with the Pixel by LabCorp COVID-19 RT-PCR Test.
The reissued authorization allows for testing of a sample taken from the nose by way of a self-collection kit that contains nasal swabs and saline, according to the FDA press release. After self-swabbing, users should send the samples in an insulated package to a LabCorp laboratory for testing. LabCorp intends to make the Pixel test available to consumers in most states, accessible through doctors’ orders.
The Pixel test includes a specific Q-tip–style cotton swab for patients to use to collect their samples, the FDA noted. Because of concerns with sterility and cross-reactivity caused by inherent genetic material in cotton swabs, generic cotton swabs should not be used as a substitute. The FDA will work with test developers to determine if generic cotton swabs can be used safely and effectively with other tests.
“Throughout this pandemic we have been facilitating test development to ensure patients’ access to accurate diagnostics, which includes supporting the development of reliable and accurate at-home sample collection options. ... [The FDA] worked with LabCorp to ensure the data demonstrated from at-home patient sample collection is as safe and accurate as sample collection at a doctor’s office, hospital, or other testing site. With this action, there is now a convenient and reliable option for patient sample collection from the comfort and safety of their home,” FDA Commissioner Stephen M. Hahn, MD, said in the press release.
PCSK9 inhibitors unexpectedly link with lower VTE, aortic stenosis
Post hoc analyses of recent large, clinical outcomes studies of PCSK9 inhibitors have revealed two tantalizing and unexpected potential benefits from these drugs: an ability to substantially reduce the incidence or severity of venous thromboembolism and aortic stenosis.
The evidence also suggests that these effects are linked to the ability of these drugs to reduce blood levels of Lp(a) lipoprotein by roughly a quarter, currently the biggest known effect on Lp(a) levels of any approved medication.
One study ran post hoc analyses of venous thromboembolism (VTE) events in the FOURIER pivotal trial of evolocumab (Repatha), with more than 27,500 randomized patients (N Engl J Med. 2017 May 4; 376[18]:1713-22), and in the ODYSSEY OUTCOMES pivotal trial of alirocumab (Praluent), with nearly 19,000 randomized patients (N Engl J Med. 2018 Nov 29;379[22]:2097-2107). The analyses showed that, with evolocumab treatment, the incidence of VTE events fell by a statistically significant 29%, compared with patients on placebo, while in ODYSSEY OUTCOMES patients treated with alirocumab had a 33% cut in VTE events, compared with placebo-treated patients, a difference that just missed statistical significance (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046524) in analyses that were not prespecified before these trials started, Nicholas A. Marston, MD, said in a presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
A combined analysis of 46,488 patients from both studies showed a 31% cut in VTE events with PCSK9 inhibitor treatment, a highly significant finding using VTE endpoints that were not specifically tallied nor adjudicated but collected as part of the serious adverse event reporting in the two pivotal trials, said Dr. Marston, a cardiologist at Brigham and Women’s Hospital in Boston. This is the first report of a statistically significant link between treatment with PCSK9-inhibiting agents and a reduction in VTE, he added. Researchers from the ODYSSEY OUTCOMES trial had reported a VTE analysis in 2019, and while data from that trial on its own showed a nominal 33% lower VTE rate with alirocumab treatment, it just missed statistical significance.
The VTE effect took about a year on treatment to start to manifest. During the first 12 months of FOURIER, the rate of VTE events among patients in the two treatment arms was virtually identical. But starting during months 13-18 on treatment, the event curves in the two arms began to increasingly diverge, and overall during the period from month 13 to the end of the study treatment with evolocumab was linked with a statistically significant 46% reduction in VTE events, compared with patients who received placebo. The results Dr. Marston reported were also published online (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046397).
The suggestion that this association may be linked to the impact of PCSK9 inhibitors on Lp(a) came from an additional analysis that Dr. Marston presented, which looked at the link between evolocumab use and a change in VTE event rates, compared with placebo, depending on baseline lipoprotein levels. Evolocumab treatment was associated with a roughly similar, modest, and not statistically significant reduction in VTE events, compared with placebo regardless of whether patients had baseline levels of LDL cholesterol below the median or at or above the median. In contrast, when a similar analysis divided patients based on whether their Lp(a) level at baseline was below, or at or above, the median the results showed no discernible effect of evolocumab treatment, compared with on VTE events in patients with lower baseline Lp(a), but in those with higher levels treatment with evolocumab linked with a 48% cut in VTE events, compared with placebo, a statistically significant difference.
In FOURIER, treatment with evolocumab lowered baseline Lp(a) levels by a median of 27%, compared with placebo, among the 25,096 enrolled patients who had their baseline levels measured. As previously reported, prespecified analysis of FOURIER data also showed that the impact of evolocumab, compared with placebo, on the combined rate of coronary heart disease death, MI, or need for urgent coronary revascularization was enhanced among patients with elevated baseline Lp(a) and moderated in those who entered with lower levels. Among patients who entered FOURIER with Lp(a) levels at or below the median treatment with evolocumab cut the primary endpoint by 7%, compared with placebo, a difference that was not statistically significant. Among patients who began the study with Lp(a) levels above the median, evolocumab treatment cut the primary endpoint by 23%, compared with placebo, a statistically significant effect (Circulation. 2019 Mar 19;139[12]:1483-92).
The aortic stenosis connection
A second study reported in the online scientific sessions (Abstract 914-08) used only FOURIER data, and showed that patients treated with evolocumab had a roughly similar response pattern in their incidence of aortic stenosis (AS) events as they did for VTE events.
During the first year of the study, the incidence of AS events was virtually identical among patients treated with evolocumab and those who received placebo. But after the first 12 months and through the study’s end, patients on evolocumab showed a statistically significant 52% relative reduction in AS events, compared with control patients, said Brian A. Bergmark, MD. For the entire study duration, treatment with evolocumab linked with a 34% relative reduction in AS events, compared with placebo, a difference that did not reach statistical significance, added Dr. Bergmark, an interventional cardiologist also at Brigham and Women’s Hospital. The observed halving in total AS events that linked with evolocumab treatment after the first year of the study included a similar-magnitude reduction specifically in the incidence of aortic valve replacement procedures in the evolocumab-treated patients.
Further analysis of both total AS events and aortic valve replacements in FOURIER patients showed that they occurred at a significantly elevated rate in patients who entered the study with higher baseline Lp(a) levels in a multivariate analysis, but a similar analysis showed no significant association between the incidence of these AS-related events and baseline levels of LDL cholesterol, he said.
The AS analysis carried the same important limitations as the VTE analysis: It ran on a post hoc basis and focused on events that were relatively uncommon and not adjudicated, Dr. Bergmark cautioned. Nonetheless, other investigators saw important potential implications from both the VTE and AS observations, with the huge caveat that they need replication in prospective studies designed to specifically address the validity of these findings.
What it could mean
These observed associations between PCSK9 inhibitor treatment and apparent reductions in the rate of both VTE and AS events “represent a tremendous clinical breakthrough,” commented Michelle L. O’Donoghue, MD, a cardiologist at Brigham and Women’s Hospital who is a FOURIER coinvestigator and has led some of the Lp(a) analyses run from that study.
“To date, we have not identified any therapies that slow progression of AS. Other classes of lipid-lowering therapies, such as statins, have been tested and not demonstrated a significant effect,” Dr. O’Donoghue said in an interview.
“For AS, the results are very intriguing. If confirmed, it could be groundbreaking. AS is the most common valve disease in the developed world, and no medical therapy exists. The potential is immense,” commented George Thanassoulis, MD, director of preventive and genomic cardiology at McGill University, Montreal. “Having a medical treatment that could slow AS progression would completely change the disease. It’s conceivable to slow the disease enough that patients may never require valve replacement.” But an interview he cautioned that, “although the results are exciting, the analysis has many limitations. What we need is a dedicated, randomized trial for AS. I hope this stimulates that.”
“For VTE, it’s an interesting finding, but I don’t think it will have clinical utility because we have good treatment for VTE,” added Dr. Thanassoulis, but others saw more opportunity from what could be a new way to reduce VTE risk.
“Given that many patients have difficulty with the bleeding risk from anticoagulants, this option [a PCSK9 inhibitor] may be quite welcome for preventing VTE,” commented Gregory Piazza, MD, a cardiologist and VTE specialist at Brigham and Women’s Hospital who was not involved in any of the PCSK9 inhibitor studies.
“At this time we would not suggest that PCSK9 inhibitors replace an anticoagulant for patients with an established clot or at high risk for a recurrent clot, but if patients have an indication for a PCSK9 inhibitor, the further reduction in venous clot can be viewed as an additional benefit of this therapy,” said Dr. O’Donoghue.
How it might work
A possible mechanism underlying a VTE effect is unclear. Results from the JUPITER trial more than a decade ago had shown a significant association between treatment with 20 mg/day of rosuvastatin and a cut in VTE episodes, compared with placebo, in a prespecified, secondary analysis of the trial with nearly 18,000 patients selected for having a relatively high level of high-sensitivity C-reactive protein (N Engl J Med. 2009 Apr 30;360[18]:1851-61). But a meta-analysis of 29 controlled statin trials that used a variety of statin types and dosages (and included the JUPITER results) failed to confirm a statistically significant change in VTE rates from statins, though they produced a small, nominal reduction (PLoS Med. 2012 Sep 18. doi: 10.1371/journal.pmed.1001310).
Lp(a) “has long been linked to thrombosis, in particular arterial thrombosis,” so the link observed in the PCSK9 inhibitor trials “is not surprising,” said Dr. Piazza. Dr. O’Donoghue agreed that prior evidence had “suggested a prothrombotic role for Lp(a).”
Dr. Thanassoulis was more skeptical of a Lp(a) connection to VTE. “There has always been controversy regarding the prothrombotic effects of Lp(a) and whether it’s clinically relevant,” he said. “The genetic data, from Mendelian randomization studies, is not consistent” with a Lp(a) and VTE link.
The association of AS and Lp(a) may be stronger. “Our team showed that people with genetic variants that predispose to high Lp(a) have a much higher incidence of AS,” Dr. Thanassoulis noted. “We and others have also demonstrated that both Lp(a) and LDL are likely causal mediators of aortic valve calcification and stenosis.”
Dr. O’Donoghue also cited observational genetic data that linked elevated Lp(a) with AS. “Mendelian randomization studies have demonstrated that Lp(a) is a causal contributer to AS, and evolocumab reduced Lp(a) by 25%-30%, raising the possibility that Lp(a) lowering with these drugs may be the mechanism,” she said.
The future of Lp(a) lowering
This last point from Dr. O’Donoghue, that PCSK9 inhibitors cut Lp(a) levels by about 25%-30%, means that they are the most potent Lp(a)-lowering agents currently available, but it also leaves lots of room for other agents to do even better in cutting Lp(a).
“There are now drugs in development that block production of the Lp(a) protein and dramatically reduce its concentration, by about 80%,” Dr. O’Donoghue noted. “It will be of interest to study whether these novel therapies, now in phase 2 and phase 3 studies, have any effect on the risk for VTE and AS.”
“Several drugs in development, including antisense RNA and RNA-interfering molecules, are much more potent and lower Lp(a) by 80%-90%. Because of this potency they can completely normalize Lp(a) in most patients. For Lp(a) lowering, the future is in these new molecules. Randomized trials have started, and we will hopefully have some results in about 5 years,” said Dr. Thanassoulis.
Until then, the prospect of possibly soon documenting benefits from PCSK9 inhibitors beyond their impact on cutting LDL cholesterol raises some hope to get more bang for the considerable buck these drugs cost. But Dr. Thanassoulis was skeptical it would move the cost-benefit ratio much. “VTE and AS are relatively rare, compared with atherosclerotic cardiovascular events, and therefore the added value at the population level would be small,” he predicted. But if treatment with a drug could help patients avoid surgical or percutaneous valve interventions “that could be really interesting from a cost-benefit perspective.”
FOURIER was funded by Amgen, the company that markets evolocumab (Repatha). ODYSSEY OUTCOMES was funded by Sanofi and Regeneron, the companies that developed and market alirocumab (Praluent). Dr. Marston had no disclosures. Dr. Bergmark has been a consultant to Daiichi Sankyo, Janssen, Quark, and Servier and has received research funding from Abbott Vascular, AstraZeneca, and MedImmune. Dr. O’Donoghue has been a consultant to and has received research funding from Amgen; has been a consultant to Janssen and Novartis; and has received research funding from AstraZeneca, Eisai, GlaxoSmithKline, Janssen, Medimmune, Merck, and The Medicines Company. Dr. Thanassoulis has been an adviser to and speaker for Amgen; an adviser to Ionis and Sanofi/Regeneron; a speaker on behalf of Boehringer Ingelheim, Sanofi, and Servier; and has received research funding from Ionis and Servier. Dr. Piazza has been a consultant to Optum, Pfizer, and Thrombolex and he has received research funding from Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Ekos, Janssen, and Portola.
Post hoc analyses of recent large, clinical outcomes studies of PCSK9 inhibitors have revealed two tantalizing and unexpected potential benefits from these drugs: an ability to substantially reduce the incidence or severity of venous thromboembolism and aortic stenosis.
The evidence also suggests that these effects are linked to the ability of these drugs to reduce blood levels of Lp(a) lipoprotein by roughly a quarter, currently the biggest known effect on Lp(a) levels of any approved medication.
One study ran post hoc analyses of venous thromboembolism (VTE) events in the FOURIER pivotal trial of evolocumab (Repatha), with more than 27,500 randomized patients (N Engl J Med. 2017 May 4; 376[18]:1713-22), and in the ODYSSEY OUTCOMES pivotal trial of alirocumab (Praluent), with nearly 19,000 randomized patients (N Engl J Med. 2018 Nov 29;379[22]:2097-2107). The analyses showed that, with evolocumab treatment, the incidence of VTE events fell by a statistically significant 29%, compared with patients on placebo, while in ODYSSEY OUTCOMES patients treated with alirocumab had a 33% cut in VTE events, compared with placebo-treated patients, a difference that just missed statistical significance (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046524) in analyses that were not prespecified before these trials started, Nicholas A. Marston, MD, said in a presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
A combined analysis of 46,488 patients from both studies showed a 31% cut in VTE events with PCSK9 inhibitor treatment, a highly significant finding using VTE endpoints that were not specifically tallied nor adjudicated but collected as part of the serious adverse event reporting in the two pivotal trials, said Dr. Marston, a cardiologist at Brigham and Women’s Hospital in Boston. This is the first report of a statistically significant link between treatment with PCSK9-inhibiting agents and a reduction in VTE, he added. Researchers from the ODYSSEY OUTCOMES trial had reported a VTE analysis in 2019, and while data from that trial on its own showed a nominal 33% lower VTE rate with alirocumab treatment, it just missed statistical significance.
The VTE effect took about a year on treatment to start to manifest. During the first 12 months of FOURIER, the rate of VTE events among patients in the two treatment arms was virtually identical. But starting during months 13-18 on treatment, the event curves in the two arms began to increasingly diverge, and overall during the period from month 13 to the end of the study treatment with evolocumab was linked with a statistically significant 46% reduction in VTE events, compared with patients who received placebo. The results Dr. Marston reported were also published online (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046397).
The suggestion that this association may be linked to the impact of PCSK9 inhibitors on Lp(a) came from an additional analysis that Dr. Marston presented, which looked at the link between evolocumab use and a change in VTE event rates, compared with placebo, depending on baseline lipoprotein levels. Evolocumab treatment was associated with a roughly similar, modest, and not statistically significant reduction in VTE events, compared with placebo regardless of whether patients had baseline levels of LDL cholesterol below the median or at or above the median. In contrast, when a similar analysis divided patients based on whether their Lp(a) level at baseline was below, or at or above, the median the results showed no discernible effect of evolocumab treatment, compared with on VTE events in patients with lower baseline Lp(a), but in those with higher levels treatment with evolocumab linked with a 48% cut in VTE events, compared with placebo, a statistically significant difference.
In FOURIER, treatment with evolocumab lowered baseline Lp(a) levels by a median of 27%, compared with placebo, among the 25,096 enrolled patients who had their baseline levels measured. As previously reported, prespecified analysis of FOURIER data also showed that the impact of evolocumab, compared with placebo, on the combined rate of coronary heart disease death, MI, or need for urgent coronary revascularization was enhanced among patients with elevated baseline Lp(a) and moderated in those who entered with lower levels. Among patients who entered FOURIER with Lp(a) levels at or below the median treatment with evolocumab cut the primary endpoint by 7%, compared with placebo, a difference that was not statistically significant. Among patients who began the study with Lp(a) levels above the median, evolocumab treatment cut the primary endpoint by 23%, compared with placebo, a statistically significant effect (Circulation. 2019 Mar 19;139[12]:1483-92).
The aortic stenosis connection
A second study reported in the online scientific sessions (Abstract 914-08) used only FOURIER data, and showed that patients treated with evolocumab had a roughly similar response pattern in their incidence of aortic stenosis (AS) events as they did for VTE events.
During the first year of the study, the incidence of AS events was virtually identical among patients treated with evolocumab and those who received placebo. But after the first 12 months and through the study’s end, patients on evolocumab showed a statistically significant 52% relative reduction in AS events, compared with control patients, said Brian A. Bergmark, MD. For the entire study duration, treatment with evolocumab linked with a 34% relative reduction in AS events, compared with placebo, a difference that did not reach statistical significance, added Dr. Bergmark, an interventional cardiologist also at Brigham and Women’s Hospital. The observed halving in total AS events that linked with evolocumab treatment after the first year of the study included a similar-magnitude reduction specifically in the incidence of aortic valve replacement procedures in the evolocumab-treated patients.
Further analysis of both total AS events and aortic valve replacements in FOURIER patients showed that they occurred at a significantly elevated rate in patients who entered the study with higher baseline Lp(a) levels in a multivariate analysis, but a similar analysis showed no significant association between the incidence of these AS-related events and baseline levels of LDL cholesterol, he said.
The AS analysis carried the same important limitations as the VTE analysis: It ran on a post hoc basis and focused on events that were relatively uncommon and not adjudicated, Dr. Bergmark cautioned. Nonetheless, other investigators saw important potential implications from both the VTE and AS observations, with the huge caveat that they need replication in prospective studies designed to specifically address the validity of these findings.
What it could mean
These observed associations between PCSK9 inhibitor treatment and apparent reductions in the rate of both VTE and AS events “represent a tremendous clinical breakthrough,” commented Michelle L. O’Donoghue, MD, a cardiologist at Brigham and Women’s Hospital who is a FOURIER coinvestigator and has led some of the Lp(a) analyses run from that study.
“To date, we have not identified any therapies that slow progression of AS. Other classes of lipid-lowering therapies, such as statins, have been tested and not demonstrated a significant effect,” Dr. O’Donoghue said in an interview.
“For AS, the results are very intriguing. If confirmed, it could be groundbreaking. AS is the most common valve disease in the developed world, and no medical therapy exists. The potential is immense,” commented George Thanassoulis, MD, director of preventive and genomic cardiology at McGill University, Montreal. “Having a medical treatment that could slow AS progression would completely change the disease. It’s conceivable to slow the disease enough that patients may never require valve replacement.” But an interview he cautioned that, “although the results are exciting, the analysis has many limitations. What we need is a dedicated, randomized trial for AS. I hope this stimulates that.”
“For VTE, it’s an interesting finding, but I don’t think it will have clinical utility because we have good treatment for VTE,” added Dr. Thanassoulis, but others saw more opportunity from what could be a new way to reduce VTE risk.
“Given that many patients have difficulty with the bleeding risk from anticoagulants, this option [a PCSK9 inhibitor] may be quite welcome for preventing VTE,” commented Gregory Piazza, MD, a cardiologist and VTE specialist at Brigham and Women’s Hospital who was not involved in any of the PCSK9 inhibitor studies.
“At this time we would not suggest that PCSK9 inhibitors replace an anticoagulant for patients with an established clot or at high risk for a recurrent clot, but if patients have an indication for a PCSK9 inhibitor, the further reduction in venous clot can be viewed as an additional benefit of this therapy,” said Dr. O’Donoghue.
How it might work
A possible mechanism underlying a VTE effect is unclear. Results from the JUPITER trial more than a decade ago had shown a significant association between treatment with 20 mg/day of rosuvastatin and a cut in VTE episodes, compared with placebo, in a prespecified, secondary analysis of the trial with nearly 18,000 patients selected for having a relatively high level of high-sensitivity C-reactive protein (N Engl J Med. 2009 Apr 30;360[18]:1851-61). But a meta-analysis of 29 controlled statin trials that used a variety of statin types and dosages (and included the JUPITER results) failed to confirm a statistically significant change in VTE rates from statins, though they produced a small, nominal reduction (PLoS Med. 2012 Sep 18. doi: 10.1371/journal.pmed.1001310).
Lp(a) “has long been linked to thrombosis, in particular arterial thrombosis,” so the link observed in the PCSK9 inhibitor trials “is not surprising,” said Dr. Piazza. Dr. O’Donoghue agreed that prior evidence had “suggested a prothrombotic role for Lp(a).”
Dr. Thanassoulis was more skeptical of a Lp(a) connection to VTE. “There has always been controversy regarding the prothrombotic effects of Lp(a) and whether it’s clinically relevant,” he said. “The genetic data, from Mendelian randomization studies, is not consistent” with a Lp(a) and VTE link.
The association of AS and Lp(a) may be stronger. “Our team showed that people with genetic variants that predispose to high Lp(a) have a much higher incidence of AS,” Dr. Thanassoulis noted. “We and others have also demonstrated that both Lp(a) and LDL are likely causal mediators of aortic valve calcification and stenosis.”
Dr. O’Donoghue also cited observational genetic data that linked elevated Lp(a) with AS. “Mendelian randomization studies have demonstrated that Lp(a) is a causal contributer to AS, and evolocumab reduced Lp(a) by 25%-30%, raising the possibility that Lp(a) lowering with these drugs may be the mechanism,” she said.
The future of Lp(a) lowering
This last point from Dr. O’Donoghue, that PCSK9 inhibitors cut Lp(a) levels by about 25%-30%, means that they are the most potent Lp(a)-lowering agents currently available, but it also leaves lots of room for other agents to do even better in cutting Lp(a).
“There are now drugs in development that block production of the Lp(a) protein and dramatically reduce its concentration, by about 80%,” Dr. O’Donoghue noted. “It will be of interest to study whether these novel therapies, now in phase 2 and phase 3 studies, have any effect on the risk for VTE and AS.”
“Several drugs in development, including antisense RNA and RNA-interfering molecules, are much more potent and lower Lp(a) by 80%-90%. Because of this potency they can completely normalize Lp(a) in most patients. For Lp(a) lowering, the future is in these new molecules. Randomized trials have started, and we will hopefully have some results in about 5 years,” said Dr. Thanassoulis.
Until then, the prospect of possibly soon documenting benefits from PCSK9 inhibitors beyond their impact on cutting LDL cholesterol raises some hope to get more bang for the considerable buck these drugs cost. But Dr. Thanassoulis was skeptical it would move the cost-benefit ratio much. “VTE and AS are relatively rare, compared with atherosclerotic cardiovascular events, and therefore the added value at the population level would be small,” he predicted. But if treatment with a drug could help patients avoid surgical or percutaneous valve interventions “that could be really interesting from a cost-benefit perspective.”
FOURIER was funded by Amgen, the company that markets evolocumab (Repatha). ODYSSEY OUTCOMES was funded by Sanofi and Regeneron, the companies that developed and market alirocumab (Praluent). Dr. Marston had no disclosures. Dr. Bergmark has been a consultant to Daiichi Sankyo, Janssen, Quark, and Servier and has received research funding from Abbott Vascular, AstraZeneca, and MedImmune. Dr. O’Donoghue has been a consultant to and has received research funding from Amgen; has been a consultant to Janssen and Novartis; and has received research funding from AstraZeneca, Eisai, GlaxoSmithKline, Janssen, Medimmune, Merck, and The Medicines Company. Dr. Thanassoulis has been an adviser to and speaker for Amgen; an adviser to Ionis and Sanofi/Regeneron; a speaker on behalf of Boehringer Ingelheim, Sanofi, and Servier; and has received research funding from Ionis and Servier. Dr. Piazza has been a consultant to Optum, Pfizer, and Thrombolex and he has received research funding from Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Ekos, Janssen, and Portola.
Post hoc analyses of recent large, clinical outcomes studies of PCSK9 inhibitors have revealed two tantalizing and unexpected potential benefits from these drugs: an ability to substantially reduce the incidence or severity of venous thromboembolism and aortic stenosis.
The evidence also suggests that these effects are linked to the ability of these drugs to reduce blood levels of Lp(a) lipoprotein by roughly a quarter, currently the biggest known effect on Lp(a) levels of any approved medication.
One study ran post hoc analyses of venous thromboembolism (VTE) events in the FOURIER pivotal trial of evolocumab (Repatha), with more than 27,500 randomized patients (N Engl J Med. 2017 May 4; 376[18]:1713-22), and in the ODYSSEY OUTCOMES pivotal trial of alirocumab (Praluent), with nearly 19,000 randomized patients (N Engl J Med. 2018 Nov 29;379[22]:2097-2107). The analyses showed that, with evolocumab treatment, the incidence of VTE events fell by a statistically significant 29%, compared with patients on placebo, while in ODYSSEY OUTCOMES patients treated with alirocumab had a 33% cut in VTE events, compared with placebo-treated patients, a difference that just missed statistical significance (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046524) in analyses that were not prespecified before these trials started, Nicholas A. Marston, MD, said in a presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
A combined analysis of 46,488 patients from both studies showed a 31% cut in VTE events with PCSK9 inhibitor treatment, a highly significant finding using VTE endpoints that were not specifically tallied nor adjudicated but collected as part of the serious adverse event reporting in the two pivotal trials, said Dr. Marston, a cardiologist at Brigham and Women’s Hospital in Boston. This is the first report of a statistically significant link between treatment with PCSK9-inhibiting agents and a reduction in VTE, he added. Researchers from the ODYSSEY OUTCOMES trial had reported a VTE analysis in 2019, and while data from that trial on its own showed a nominal 33% lower VTE rate with alirocumab treatment, it just missed statistical significance.
The VTE effect took about a year on treatment to start to manifest. During the first 12 months of FOURIER, the rate of VTE events among patients in the two treatment arms was virtually identical. But starting during months 13-18 on treatment, the event curves in the two arms began to increasingly diverge, and overall during the period from month 13 to the end of the study treatment with evolocumab was linked with a statistically significant 46% reduction in VTE events, compared with patients who received placebo. The results Dr. Marston reported were also published online (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046397).
The suggestion that this association may be linked to the impact of PCSK9 inhibitors on Lp(a) came from an additional analysis that Dr. Marston presented, which looked at the link between evolocumab use and a change in VTE event rates, compared with placebo, depending on baseline lipoprotein levels. Evolocumab treatment was associated with a roughly similar, modest, and not statistically significant reduction in VTE events, compared with placebo regardless of whether patients had baseline levels of LDL cholesterol below the median or at or above the median. In contrast, when a similar analysis divided patients based on whether their Lp(a) level at baseline was below, or at or above, the median the results showed no discernible effect of evolocumab treatment, compared with on VTE events in patients with lower baseline Lp(a), but in those with higher levels treatment with evolocumab linked with a 48% cut in VTE events, compared with placebo, a statistically significant difference.
In FOURIER, treatment with evolocumab lowered baseline Lp(a) levels by a median of 27%, compared with placebo, among the 25,096 enrolled patients who had their baseline levels measured. As previously reported, prespecified analysis of FOURIER data also showed that the impact of evolocumab, compared with placebo, on the combined rate of coronary heart disease death, MI, or need for urgent coronary revascularization was enhanced among patients with elevated baseline Lp(a) and moderated in those who entered with lower levels. Among patients who entered FOURIER with Lp(a) levels at or below the median treatment with evolocumab cut the primary endpoint by 7%, compared with placebo, a difference that was not statistically significant. Among patients who began the study with Lp(a) levels above the median, evolocumab treatment cut the primary endpoint by 23%, compared with placebo, a statistically significant effect (Circulation. 2019 Mar 19;139[12]:1483-92).
The aortic stenosis connection
A second study reported in the online scientific sessions (Abstract 914-08) used only FOURIER data, and showed that patients treated with evolocumab had a roughly similar response pattern in their incidence of aortic stenosis (AS) events as they did for VTE events.
During the first year of the study, the incidence of AS events was virtually identical among patients treated with evolocumab and those who received placebo. But after the first 12 months and through the study’s end, patients on evolocumab showed a statistically significant 52% relative reduction in AS events, compared with control patients, said Brian A. Bergmark, MD. For the entire study duration, treatment with evolocumab linked with a 34% relative reduction in AS events, compared with placebo, a difference that did not reach statistical significance, added Dr. Bergmark, an interventional cardiologist also at Brigham and Women’s Hospital. The observed halving in total AS events that linked with evolocumab treatment after the first year of the study included a similar-magnitude reduction specifically in the incidence of aortic valve replacement procedures in the evolocumab-treated patients.
Further analysis of both total AS events and aortic valve replacements in FOURIER patients showed that they occurred at a significantly elevated rate in patients who entered the study with higher baseline Lp(a) levels in a multivariate analysis, but a similar analysis showed no significant association between the incidence of these AS-related events and baseline levels of LDL cholesterol, he said.
The AS analysis carried the same important limitations as the VTE analysis: It ran on a post hoc basis and focused on events that were relatively uncommon and not adjudicated, Dr. Bergmark cautioned. Nonetheless, other investigators saw important potential implications from both the VTE and AS observations, with the huge caveat that they need replication in prospective studies designed to specifically address the validity of these findings.
What it could mean
These observed associations between PCSK9 inhibitor treatment and apparent reductions in the rate of both VTE and AS events “represent a tremendous clinical breakthrough,” commented Michelle L. O’Donoghue, MD, a cardiologist at Brigham and Women’s Hospital who is a FOURIER coinvestigator and has led some of the Lp(a) analyses run from that study.
“To date, we have not identified any therapies that slow progression of AS. Other classes of lipid-lowering therapies, such as statins, have been tested and not demonstrated a significant effect,” Dr. O’Donoghue said in an interview.
“For AS, the results are very intriguing. If confirmed, it could be groundbreaking. AS is the most common valve disease in the developed world, and no medical therapy exists. The potential is immense,” commented George Thanassoulis, MD, director of preventive and genomic cardiology at McGill University, Montreal. “Having a medical treatment that could slow AS progression would completely change the disease. It’s conceivable to slow the disease enough that patients may never require valve replacement.” But an interview he cautioned that, “although the results are exciting, the analysis has many limitations. What we need is a dedicated, randomized trial for AS. I hope this stimulates that.”
“For VTE, it’s an interesting finding, but I don’t think it will have clinical utility because we have good treatment for VTE,” added Dr. Thanassoulis, but others saw more opportunity from what could be a new way to reduce VTE risk.
“Given that many patients have difficulty with the bleeding risk from anticoagulants, this option [a PCSK9 inhibitor] may be quite welcome for preventing VTE,” commented Gregory Piazza, MD, a cardiologist and VTE specialist at Brigham and Women’s Hospital who was not involved in any of the PCSK9 inhibitor studies.
“At this time we would not suggest that PCSK9 inhibitors replace an anticoagulant for patients with an established clot or at high risk for a recurrent clot, but if patients have an indication for a PCSK9 inhibitor, the further reduction in venous clot can be viewed as an additional benefit of this therapy,” said Dr. O’Donoghue.
How it might work
A possible mechanism underlying a VTE effect is unclear. Results from the JUPITER trial more than a decade ago had shown a significant association between treatment with 20 mg/day of rosuvastatin and a cut in VTE episodes, compared with placebo, in a prespecified, secondary analysis of the trial with nearly 18,000 patients selected for having a relatively high level of high-sensitivity C-reactive protein (N Engl J Med. 2009 Apr 30;360[18]:1851-61). But a meta-analysis of 29 controlled statin trials that used a variety of statin types and dosages (and included the JUPITER results) failed to confirm a statistically significant change in VTE rates from statins, though they produced a small, nominal reduction (PLoS Med. 2012 Sep 18. doi: 10.1371/journal.pmed.1001310).
Lp(a) “has long been linked to thrombosis, in particular arterial thrombosis,” so the link observed in the PCSK9 inhibitor trials “is not surprising,” said Dr. Piazza. Dr. O’Donoghue agreed that prior evidence had “suggested a prothrombotic role for Lp(a).”
Dr. Thanassoulis was more skeptical of a Lp(a) connection to VTE. “There has always been controversy regarding the prothrombotic effects of Lp(a) and whether it’s clinically relevant,” he said. “The genetic data, from Mendelian randomization studies, is not consistent” with a Lp(a) and VTE link.
The association of AS and Lp(a) may be stronger. “Our team showed that people with genetic variants that predispose to high Lp(a) have a much higher incidence of AS,” Dr. Thanassoulis noted. “We and others have also demonstrated that both Lp(a) and LDL are likely causal mediators of aortic valve calcification and stenosis.”
Dr. O’Donoghue also cited observational genetic data that linked elevated Lp(a) with AS. “Mendelian randomization studies have demonstrated that Lp(a) is a causal contributer to AS, and evolocumab reduced Lp(a) by 25%-30%, raising the possibility that Lp(a) lowering with these drugs may be the mechanism,” she said.
The future of Lp(a) lowering
This last point from Dr. O’Donoghue, that PCSK9 inhibitors cut Lp(a) levels by about 25%-30%, means that they are the most potent Lp(a)-lowering agents currently available, but it also leaves lots of room for other agents to do even better in cutting Lp(a).
“There are now drugs in development that block production of the Lp(a) protein and dramatically reduce its concentration, by about 80%,” Dr. O’Donoghue noted. “It will be of interest to study whether these novel therapies, now in phase 2 and phase 3 studies, have any effect on the risk for VTE and AS.”
“Several drugs in development, including antisense RNA and RNA-interfering molecules, are much more potent and lower Lp(a) by 80%-90%. Because of this potency they can completely normalize Lp(a) in most patients. For Lp(a) lowering, the future is in these new molecules. Randomized trials have started, and we will hopefully have some results in about 5 years,” said Dr. Thanassoulis.
Until then, the prospect of possibly soon documenting benefits from PCSK9 inhibitors beyond their impact on cutting LDL cholesterol raises some hope to get more bang for the considerable buck these drugs cost. But Dr. Thanassoulis was skeptical it would move the cost-benefit ratio much. “VTE and AS are relatively rare, compared with atherosclerotic cardiovascular events, and therefore the added value at the population level would be small,” he predicted. But if treatment with a drug could help patients avoid surgical or percutaneous valve interventions “that could be really interesting from a cost-benefit perspective.”
FOURIER was funded by Amgen, the company that markets evolocumab (Repatha). ODYSSEY OUTCOMES was funded by Sanofi and Regeneron, the companies that developed and market alirocumab (Praluent). Dr. Marston had no disclosures. Dr. Bergmark has been a consultant to Daiichi Sankyo, Janssen, Quark, and Servier and has received research funding from Abbott Vascular, AstraZeneca, and MedImmune. Dr. O’Donoghue has been a consultant to and has received research funding from Amgen; has been a consultant to Janssen and Novartis; and has received research funding from AstraZeneca, Eisai, GlaxoSmithKline, Janssen, Medimmune, Merck, and The Medicines Company. Dr. Thanassoulis has been an adviser to and speaker for Amgen; an adviser to Ionis and Sanofi/Regeneron; a speaker on behalf of Boehringer Ingelheim, Sanofi, and Servier; and has received research funding from Ionis and Servier. Dr. Piazza has been a consultant to Optum, Pfizer, and Thrombolex and he has received research funding from Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Ekos, Janssen, and Portola.
REPORTING FROM ACC 20