User login
Tips for self-care during the COVID-19 crisis
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
AMA president calls for greater reliance on science in COVID-19 fight
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
Nearly 24 tests for the novel coronavirus are available
according to the Infectious Diseases Society of America (IDSA).
“Based on what we know about influenza, it’s unlikely that all of these tests are going to perform exactly the same way,” said Angela M. Caliendo, MD, executive vice chair of the department of medicine at Brown University in Providence, R.I., at a press briefing. Although these tests are good, no test is perfect, she added.
The development and availability of testing has improved over time, but clinical laboratories still face challenges, said Kimberly E. Hanson, MD, associate professor of internal medicine at University of Utah, Salt Lake City. These challenges include shortages of devices for specimen collection, media, test tubes, and reagents. Although the goal is to test all symptomatic patients, these shortages require laboratories to prioritize health care workers and the sickest patients.
Tests are being approved through an abbreviated process
Two types of test, rapid tests and serology tests, are in use. Rapid tests use polymerase chain reactions to detect the virus in a clinical specimen. This type of testing is used to diagnose infection. Serology tests measure antibodies to the virus and are more appropriate for indicating whether a patient has been exposed to the virus.
The declaration of a national emergency enabled the FDA to activate its EUA policy, which allows for quicker approval of tests. Normally, a test must be assessed in the laboratory (such as with a mock specimen or an inactivated virus) and in a clinical study of patients. Under the EUA, clinical assessment is not required for the approval of a test. Consequently, the clinical performance of a test approved under EUA is unknown.
Collecting a specimen of good quality is critical to the quality of the test result, said Dr. Caliendo, the secretary of IDSA’s board of directors. Clinicians and investigators have used nasopharyngeal swabs, sputum, and specimens collected from deep within the lung. “We’re still collecting data to determine which is the best specimen type.” As coronavirus testing expands, particularly to drive-through testing sites, “we may be using people who are not as experienced, and so you might not get as high a quality specimen in that situation,” Dr. Caliendo added.
The timing of the test influences the quality of the result, as well, because the amount of virus is lower at the onset of symptoms than it is later. Another factor that affects the quality of the results is the test’s sensitivity.
The time to obtain results varies
The value of having several tests available is that it enables many patients to be tested simultaneously, said Dr. Hanson, a member of IDSA’s board of directors. It also helps to reduce potential problems with the supply of test kits. A test manufacturer, however, may supply parts of the test kit but not the whole kit. This requires the hospital or laboratory to obtain the remaining parts from other suppliers. Furthermore, test manufacturers may need to prioritize areas with high rates of infection or transmission when they ship their tests, which limits testing in other areas.
One reason for the lack of a national plan for testing is that the virus has affected different regions at different times, said Dr. Caliendo. Some tests are more difficult to perform than others, and not all laboratories are equally sophisticated, which can limit testing. It is necessary to test not only symptomatic patients who have been hospitalized, but also symptomatic patients in the community, said Dr. Caliendo. “Ideally, we’re going to need to couple acute diagnostics [testing while people are sick] with serologic testing. Serologic testing is going to be important for us to see who has been infected. That will give us an idea of who is left in our community who is at risk for developing infection.”
How quickly test results are available depends on the type of test and where it is administered. Recently established drive-through clinics can provide results in about 30 minutes. Tests performed in hospitals may take between 1 and 6 hours to yield results. “The issue is, do we have reagents that day?” said Dr. Caliendo. “We have to be careful whom we choose to test, and we screen that in the hospital so that we have enough tests to run as we need them.” But many locations have backlogs. “When you have a backlog of testing, you’re going to wait days, unfortunately, to get a result,” said Dr. Caliendo.
Dr. Caliendo and Dr. Hanson did not report disclosures for this briefing.
according to the Infectious Diseases Society of America (IDSA).
“Based on what we know about influenza, it’s unlikely that all of these tests are going to perform exactly the same way,” said Angela M. Caliendo, MD, executive vice chair of the department of medicine at Brown University in Providence, R.I., at a press briefing. Although these tests are good, no test is perfect, she added.
The development and availability of testing has improved over time, but clinical laboratories still face challenges, said Kimberly E. Hanson, MD, associate professor of internal medicine at University of Utah, Salt Lake City. These challenges include shortages of devices for specimen collection, media, test tubes, and reagents. Although the goal is to test all symptomatic patients, these shortages require laboratories to prioritize health care workers and the sickest patients.
Tests are being approved through an abbreviated process
Two types of test, rapid tests and serology tests, are in use. Rapid tests use polymerase chain reactions to detect the virus in a clinical specimen. This type of testing is used to diagnose infection. Serology tests measure antibodies to the virus and are more appropriate for indicating whether a patient has been exposed to the virus.
The declaration of a national emergency enabled the FDA to activate its EUA policy, which allows for quicker approval of tests. Normally, a test must be assessed in the laboratory (such as with a mock specimen or an inactivated virus) and in a clinical study of patients. Under the EUA, clinical assessment is not required for the approval of a test. Consequently, the clinical performance of a test approved under EUA is unknown.
Collecting a specimen of good quality is critical to the quality of the test result, said Dr. Caliendo, the secretary of IDSA’s board of directors. Clinicians and investigators have used nasopharyngeal swabs, sputum, and specimens collected from deep within the lung. “We’re still collecting data to determine which is the best specimen type.” As coronavirus testing expands, particularly to drive-through testing sites, “we may be using people who are not as experienced, and so you might not get as high a quality specimen in that situation,” Dr. Caliendo added.
The timing of the test influences the quality of the result, as well, because the amount of virus is lower at the onset of symptoms than it is later. Another factor that affects the quality of the results is the test’s sensitivity.
The time to obtain results varies
The value of having several tests available is that it enables many patients to be tested simultaneously, said Dr. Hanson, a member of IDSA’s board of directors. It also helps to reduce potential problems with the supply of test kits. A test manufacturer, however, may supply parts of the test kit but not the whole kit. This requires the hospital or laboratory to obtain the remaining parts from other suppliers. Furthermore, test manufacturers may need to prioritize areas with high rates of infection or transmission when they ship their tests, which limits testing in other areas.
One reason for the lack of a national plan for testing is that the virus has affected different regions at different times, said Dr. Caliendo. Some tests are more difficult to perform than others, and not all laboratories are equally sophisticated, which can limit testing. It is necessary to test not only symptomatic patients who have been hospitalized, but also symptomatic patients in the community, said Dr. Caliendo. “Ideally, we’re going to need to couple acute diagnostics [testing while people are sick] with serologic testing. Serologic testing is going to be important for us to see who has been infected. That will give us an idea of who is left in our community who is at risk for developing infection.”
How quickly test results are available depends on the type of test and where it is administered. Recently established drive-through clinics can provide results in about 30 minutes. Tests performed in hospitals may take between 1 and 6 hours to yield results. “The issue is, do we have reagents that day?” said Dr. Caliendo. “We have to be careful whom we choose to test, and we screen that in the hospital so that we have enough tests to run as we need them.” But many locations have backlogs. “When you have a backlog of testing, you’re going to wait days, unfortunately, to get a result,” said Dr. Caliendo.
Dr. Caliendo and Dr. Hanson did not report disclosures for this briefing.
according to the Infectious Diseases Society of America (IDSA).
“Based on what we know about influenza, it’s unlikely that all of these tests are going to perform exactly the same way,” said Angela M. Caliendo, MD, executive vice chair of the department of medicine at Brown University in Providence, R.I., at a press briefing. Although these tests are good, no test is perfect, she added.
The development and availability of testing has improved over time, but clinical laboratories still face challenges, said Kimberly E. Hanson, MD, associate professor of internal medicine at University of Utah, Salt Lake City. These challenges include shortages of devices for specimen collection, media, test tubes, and reagents. Although the goal is to test all symptomatic patients, these shortages require laboratories to prioritize health care workers and the sickest patients.
Tests are being approved through an abbreviated process
Two types of test, rapid tests and serology tests, are in use. Rapid tests use polymerase chain reactions to detect the virus in a clinical specimen. This type of testing is used to diagnose infection. Serology tests measure antibodies to the virus and are more appropriate for indicating whether a patient has been exposed to the virus.
The declaration of a national emergency enabled the FDA to activate its EUA policy, which allows for quicker approval of tests. Normally, a test must be assessed in the laboratory (such as with a mock specimen or an inactivated virus) and in a clinical study of patients. Under the EUA, clinical assessment is not required for the approval of a test. Consequently, the clinical performance of a test approved under EUA is unknown.
Collecting a specimen of good quality is critical to the quality of the test result, said Dr. Caliendo, the secretary of IDSA’s board of directors. Clinicians and investigators have used nasopharyngeal swabs, sputum, and specimens collected from deep within the lung. “We’re still collecting data to determine which is the best specimen type.” As coronavirus testing expands, particularly to drive-through testing sites, “we may be using people who are not as experienced, and so you might not get as high a quality specimen in that situation,” Dr. Caliendo added.
The timing of the test influences the quality of the result, as well, because the amount of virus is lower at the onset of symptoms than it is later. Another factor that affects the quality of the results is the test’s sensitivity.
The time to obtain results varies
The value of having several tests available is that it enables many patients to be tested simultaneously, said Dr. Hanson, a member of IDSA’s board of directors. It also helps to reduce potential problems with the supply of test kits. A test manufacturer, however, may supply parts of the test kit but not the whole kit. This requires the hospital or laboratory to obtain the remaining parts from other suppliers. Furthermore, test manufacturers may need to prioritize areas with high rates of infection or transmission when they ship their tests, which limits testing in other areas.
One reason for the lack of a national plan for testing is that the virus has affected different regions at different times, said Dr. Caliendo. Some tests are more difficult to perform than others, and not all laboratories are equally sophisticated, which can limit testing. It is necessary to test not only symptomatic patients who have been hospitalized, but also symptomatic patients in the community, said Dr. Caliendo. “Ideally, we’re going to need to couple acute diagnostics [testing while people are sick] with serologic testing. Serologic testing is going to be important for us to see who has been infected. That will give us an idea of who is left in our community who is at risk for developing infection.”
How quickly test results are available depends on the type of test and where it is administered. Recently established drive-through clinics can provide results in about 30 minutes. Tests performed in hospitals may take between 1 and 6 hours to yield results. “The issue is, do we have reagents that day?” said Dr. Caliendo. “We have to be careful whom we choose to test, and we screen that in the hospital so that we have enough tests to run as we need them.” But many locations have backlogs. “When you have a backlog of testing, you’re going to wait days, unfortunately, to get a result,” said Dr. Caliendo.
Dr. Caliendo and Dr. Hanson did not report disclosures for this briefing.
Cytokine release syndrome in severe COVID-19: Is tocilizumab effective?
A large amount of data suggest that mild or severe cytokine storms, accompanied by high expression of interleukin-6 (IL-6), occur in patients with severe coronavirus disease and can be an important cause of death. Blocking the signal transduction pathway of IL-6 is expected to become a new method for the treatment of patients with severe COVID-19, with the IL-6 inhibitor, tocilizumab (Actemra), poised to become an effective drug for these patients, according to the authors of a review published online in the International Journal of Antimicrobial Agents.
The reviewers from China detailed the metabolic pathways and regulation of cytokine release syndrome, especially with respect to what is known about severe COVID-19, and discussed the results of recent trials with tocilizumab, which is currently used for treatment of CRS in a variety of cancers and other metabolic disorders.
Tocilizumab is a recombinant humanized monoclonal antibody against human IL-6 receptor of immunoglobulin IgG1 subtype and has been approved for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis. The antibody specifically binds soluble- and membrane-bound IL-6 receptors (sIL-6R and mIL-6R) and inhibits sIL-6R– and mIL-6R–mediated signal transduction. It has been shown to be effective in the treatment of severe CRS patients. In 2017, the U.S. Food and Drug Administration approved tocilizumab for the treatment of CRS caused by CAR-T (chimeric antigen receptor T-cell immunotherapy) therapy.
A small clinical trial in China examined the effectiveness of tocilizumab in 21 patients who met the criteria for severe or critical COVID-19, including respiratory failure requiring mechanical ventilation, shock, or admission to the ICU with other organ failure. After a few days of tocilizumab treatment, the body temperatures returned to normal (initially, all 21 patients had fevers), and all other symptoms were significantly improved, according to the authors. A total of 75% (15/20) of the patients reduced their oxygen intake, and 1 patient did not need oxygen. CT scanning showed that 90.5% (19/21) of the patients had absorption of pulmonary lesions, and lab tests showed that the proportion of peripheral blood lymphocytes and C-reactive protein in the patients returned to normal.
The main deficiency of the study was that only the level of IL-6 in peripheral blood before treatment with tocilizumab was reported (mean value, 132.38 ± 278.54 pg/mL), but the level of IL-6 following treatment was not given, according to the reviewers. Serum levels of IL-6 in normal patients are undetectable or very low.
Based upon their analysis of COVID-19’s possible mechanism and the small samples of clinical data available, tocilizumab appeared effective, and “we suggest that it should be used in critically ill COVID-19 patients with significantly elevated IL-6,” the authors stated.
“CRS occurs in a large number of patients with severe COVID-19, which is also an important cause of death. IL-6 is the key molecule of CRS, so IL-6R antagonist tocilizumab may be an important drug to save patients’ lives,” the researchers concluded.
This study was supported by China Mega-Project for Infectious Diseases and the China Mega-Project for Innovative Drugs. The authors reported that they had no conflicts.
SOURCE: Zhang C et al. Int J Antimicrobial Agents. 2020. doi. org/10.1016/j.ijantimicag.2020.105954.
A large amount of data suggest that mild or severe cytokine storms, accompanied by high expression of interleukin-6 (IL-6), occur in patients with severe coronavirus disease and can be an important cause of death. Blocking the signal transduction pathway of IL-6 is expected to become a new method for the treatment of patients with severe COVID-19, with the IL-6 inhibitor, tocilizumab (Actemra), poised to become an effective drug for these patients, according to the authors of a review published online in the International Journal of Antimicrobial Agents.
The reviewers from China detailed the metabolic pathways and regulation of cytokine release syndrome, especially with respect to what is known about severe COVID-19, and discussed the results of recent trials with tocilizumab, which is currently used for treatment of CRS in a variety of cancers and other metabolic disorders.
Tocilizumab is a recombinant humanized monoclonal antibody against human IL-6 receptor of immunoglobulin IgG1 subtype and has been approved for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis. The antibody specifically binds soluble- and membrane-bound IL-6 receptors (sIL-6R and mIL-6R) and inhibits sIL-6R– and mIL-6R–mediated signal transduction. It has been shown to be effective in the treatment of severe CRS patients. In 2017, the U.S. Food and Drug Administration approved tocilizumab for the treatment of CRS caused by CAR-T (chimeric antigen receptor T-cell immunotherapy) therapy.
A small clinical trial in China examined the effectiveness of tocilizumab in 21 patients who met the criteria for severe or critical COVID-19, including respiratory failure requiring mechanical ventilation, shock, or admission to the ICU with other organ failure. After a few days of tocilizumab treatment, the body temperatures returned to normal (initially, all 21 patients had fevers), and all other symptoms were significantly improved, according to the authors. A total of 75% (15/20) of the patients reduced their oxygen intake, and 1 patient did not need oxygen. CT scanning showed that 90.5% (19/21) of the patients had absorption of pulmonary lesions, and lab tests showed that the proportion of peripheral blood lymphocytes and C-reactive protein in the patients returned to normal.
The main deficiency of the study was that only the level of IL-6 in peripheral blood before treatment with tocilizumab was reported (mean value, 132.38 ± 278.54 pg/mL), but the level of IL-6 following treatment was not given, according to the reviewers. Serum levels of IL-6 in normal patients are undetectable or very low.
Based upon their analysis of COVID-19’s possible mechanism and the small samples of clinical data available, tocilizumab appeared effective, and “we suggest that it should be used in critically ill COVID-19 patients with significantly elevated IL-6,” the authors stated.
“CRS occurs in a large number of patients with severe COVID-19, which is also an important cause of death. IL-6 is the key molecule of CRS, so IL-6R antagonist tocilizumab may be an important drug to save patients’ lives,” the researchers concluded.
This study was supported by China Mega-Project for Infectious Diseases and the China Mega-Project for Innovative Drugs. The authors reported that they had no conflicts.
SOURCE: Zhang C et al. Int J Antimicrobial Agents. 2020. doi. org/10.1016/j.ijantimicag.2020.105954.
A large amount of data suggest that mild or severe cytokine storms, accompanied by high expression of interleukin-6 (IL-6), occur in patients with severe coronavirus disease and can be an important cause of death. Blocking the signal transduction pathway of IL-6 is expected to become a new method for the treatment of patients with severe COVID-19, with the IL-6 inhibitor, tocilizumab (Actemra), poised to become an effective drug for these patients, according to the authors of a review published online in the International Journal of Antimicrobial Agents.
The reviewers from China detailed the metabolic pathways and regulation of cytokine release syndrome, especially with respect to what is known about severe COVID-19, and discussed the results of recent trials with tocilizumab, which is currently used for treatment of CRS in a variety of cancers and other metabolic disorders.
Tocilizumab is a recombinant humanized monoclonal antibody against human IL-6 receptor of immunoglobulin IgG1 subtype and has been approved for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis. The antibody specifically binds soluble- and membrane-bound IL-6 receptors (sIL-6R and mIL-6R) and inhibits sIL-6R– and mIL-6R–mediated signal transduction. It has been shown to be effective in the treatment of severe CRS patients. In 2017, the U.S. Food and Drug Administration approved tocilizumab for the treatment of CRS caused by CAR-T (chimeric antigen receptor T-cell immunotherapy) therapy.
A small clinical trial in China examined the effectiveness of tocilizumab in 21 patients who met the criteria for severe or critical COVID-19, including respiratory failure requiring mechanical ventilation, shock, or admission to the ICU with other organ failure. After a few days of tocilizumab treatment, the body temperatures returned to normal (initially, all 21 patients had fevers), and all other symptoms were significantly improved, according to the authors. A total of 75% (15/20) of the patients reduced their oxygen intake, and 1 patient did not need oxygen. CT scanning showed that 90.5% (19/21) of the patients had absorption of pulmonary lesions, and lab tests showed that the proportion of peripheral blood lymphocytes and C-reactive protein in the patients returned to normal.
The main deficiency of the study was that only the level of IL-6 in peripheral blood before treatment with tocilizumab was reported (mean value, 132.38 ± 278.54 pg/mL), but the level of IL-6 following treatment was not given, according to the reviewers. Serum levels of IL-6 in normal patients are undetectable or very low.
Based upon their analysis of COVID-19’s possible mechanism and the small samples of clinical data available, tocilizumab appeared effective, and “we suggest that it should be used in critically ill COVID-19 patients with significantly elevated IL-6,” the authors stated.
“CRS occurs in a large number of patients with severe COVID-19, which is also an important cause of death. IL-6 is the key molecule of CRS, so IL-6R antagonist tocilizumab may be an important drug to save patients’ lives,” the researchers concluded.
This study was supported by China Mega-Project for Infectious Diseases and the China Mega-Project for Innovative Drugs. The authors reported that they had no conflicts.
SOURCE: Zhang C et al. Int J Antimicrobial Agents. 2020. doi. org/10.1016/j.ijantimicag.2020.105954.
FROM THE INTERNATIONAL JOURNAL OF ANTIMICROBIAL AGENTS
U.S. hospitals facing severe challenges from COVID-19, HHS report says
Hospitals across the country encountered severe challenges as the first wave of the COVID-19 pandemic swept over them, and they anticipated much worse to come, according to a new report from the Office of Inspector General of the Department of Health and Human Services (HHS).
From March 23 to 27, the OIG interviewed 323 hospitals of several types in 46 states, the District of Columbia, and Puerto Rico. The report it pulled together from these interviews is intended to help HHS manage the crisis, rather than to review its response to the pandemic, the OIG said.
The most significant hospital challenges, the report states, were testing and caring for patients with known or suspected COVID-19 and protecting staff members. In addition, the hospitals faced challenges in maintaining or expanding their capacities to treat COVID-19 patients and ensuring the adequacy of basic supplies.
The critical shortages of ventilators, personal protective equipment (PPE), and test kits in hospitals have been widely reported by the media. But the OIG report also focused on some areas that have received less press attention.
To begin with, the shortage of tests has not only slowed the national response to the pandemic, but has had a major impact on inpatient care, according to the report’s authors. The limited number of test kits means that only symptomatic staff members and patients can be tested; in some hospitals, there aren’t even enough tests for that, and some facilities subdivided the test kits they had, the report states.
Moreover, the test results often took 7 days or more to come back from commercial or government labs, the report states. In the meantime, symptomatic patients were presumed to have the coronavirus. While awaiting the results, they had to stay in the hospital, using beds and requiring staff who could otherwise have been assigned to other patients.
The doctors and nurse who cared for these presumptive COVID-19 patients also had to take time suiting up in PPE before seeing them; much of that scarce PPE was wasted on those who were later found not to have the illness.
As one administrator explained to OIG, “Sitting with 60 patients with presumed positives in our hospital isn’t healthy for anybody.”
Delayed test results also reduced hospitals’ ability to provide care by sidelining clinicians who reported COVID-19 symptoms. In one hospital, 20% to 25% of staff were determined to be presumptively positive for COVID-19. As a result of their tests not being analyzed promptly, these doctors and nurses were prevented from providing clinical services for longer than necessary.
Supply Shortages
The report also described some factors contributing to mask shortages. Because of the fear factor, for example, all staff members in one hospital were wearing masks, instead of just those in designated areas. An administrator said the hospital was using 2,000 masks a day, 10 times the number before the COVID-19 crisis.
Another hospital received 2,300 N95 masks from a state reserve, but they were unusable because the elastic bands had dry-rotted.
Meanwhile, some vendors were profiteering. Masks that used to cost 50 cents now sold for $6 each, one administrator said.
To combat the supply chain disruptions, some facilities were buying PPE from nontraditional sources such as online retailers, home supply stores, paint stores, autobody supply shops, and beauty salons. Other hospitals were using non–medical-grade PPE such as construction masks and handmade masks and gowns.
Other hospitals reported they were conserving and reusing PPE to stretch their supplies. In some cases, they had even changed policies to reduce the extent and frequency of patient interactions with clinicians so the latter would have to change their gear less often.
Shortages of other critical supplies and materials were also reported. Hospitals were running out of supplies that supported patient rooms, such as IV poles, medical gas, linens, toilet paper, and food.
Hospitals across the country were also expecting or experiencing a shortage of ventilators, although none said any patients had been denied access to them. Some institutions were adapting anesthesia machines and single-use emergency transport ventilators.
Also concerning to hospitals was the shortage of intensive-care specialists and nurses to operate the ventilators and care for critically ill patients. Some facilities were training anesthesiologists, hospitalists, and other nonintensivists on how to use the lifesaving equipment.
Meanwhile, patients with COVID-19 symptoms were continuing to show up in droves at emergency departments. Hospitals were concerned about potential shortages of ICU beds, negative-pressure rooms, and isolation units. Given limited bed availability, some administrators said, it was getting hard to separate COVID-19 from non–COVID-19 patients.
What Hospitals Want
As the COVID-19 crisis continues to mount, many hospitals are facing financial emergencies as well, the report noted.
“Hospitals described increasing costs and decreasing revenues as a threat to their financial viability. Hospitals reported that ceasing elective procedures and other services decreased revenues at the same time that their costs have increased as they prepare for a potential surge of patients. Many hospitals reported that their cash reserves were quickly depleting, which could disrupt ongoing hospital operations,” the authors write.
This report was conducted a few days before the passage of the CURES Act, which earmarked $100 billion for hospitals on the frontline of the crisis. As a recent analysis of financial hospital data revealed, however, even with the 20% bump in Medicare payments for COVID-19 care that this cash infusion represents, many hospitals will face a cash-flow crunch within 60 to 90 days, as reported by Medscape Medical News.
Besides higher Medicare payments, the OIG report said, hospitals wanted the government to drop the 14-day waiting period for reimbursement and to offer them loans and grants.
Hospitals also want federal and state governments to relax regulations on professional licensing of, and business relationships with, doctors and other clinicians. They’d like the government to:
- Let them reassign licensed professionals within their hospitals and across healthcare networks
- Provide flexibility with respect to licensed professionals practicing across state lines
- Provide relief from regulations that may restrict using contracted staff or physicians based on business relationships
This article first appeared on Medscape.com.
Hospitals across the country encountered severe challenges as the first wave of the COVID-19 pandemic swept over them, and they anticipated much worse to come, according to a new report from the Office of Inspector General of the Department of Health and Human Services (HHS).
From March 23 to 27, the OIG interviewed 323 hospitals of several types in 46 states, the District of Columbia, and Puerto Rico. The report it pulled together from these interviews is intended to help HHS manage the crisis, rather than to review its response to the pandemic, the OIG said.
The most significant hospital challenges, the report states, were testing and caring for patients with known or suspected COVID-19 and protecting staff members. In addition, the hospitals faced challenges in maintaining or expanding their capacities to treat COVID-19 patients and ensuring the adequacy of basic supplies.
The critical shortages of ventilators, personal protective equipment (PPE), and test kits in hospitals have been widely reported by the media. But the OIG report also focused on some areas that have received less press attention.
To begin with, the shortage of tests has not only slowed the national response to the pandemic, but has had a major impact on inpatient care, according to the report’s authors. The limited number of test kits means that only symptomatic staff members and patients can be tested; in some hospitals, there aren’t even enough tests for that, and some facilities subdivided the test kits they had, the report states.
Moreover, the test results often took 7 days or more to come back from commercial or government labs, the report states. In the meantime, symptomatic patients were presumed to have the coronavirus. While awaiting the results, they had to stay in the hospital, using beds and requiring staff who could otherwise have been assigned to other patients.
The doctors and nurse who cared for these presumptive COVID-19 patients also had to take time suiting up in PPE before seeing them; much of that scarce PPE was wasted on those who were later found not to have the illness.
As one administrator explained to OIG, “Sitting with 60 patients with presumed positives in our hospital isn’t healthy for anybody.”
Delayed test results also reduced hospitals’ ability to provide care by sidelining clinicians who reported COVID-19 symptoms. In one hospital, 20% to 25% of staff were determined to be presumptively positive for COVID-19. As a result of their tests not being analyzed promptly, these doctors and nurses were prevented from providing clinical services for longer than necessary.
Supply Shortages
The report also described some factors contributing to mask shortages. Because of the fear factor, for example, all staff members in one hospital were wearing masks, instead of just those in designated areas. An administrator said the hospital was using 2,000 masks a day, 10 times the number before the COVID-19 crisis.
Another hospital received 2,300 N95 masks from a state reserve, but they were unusable because the elastic bands had dry-rotted.
Meanwhile, some vendors were profiteering. Masks that used to cost 50 cents now sold for $6 each, one administrator said.
To combat the supply chain disruptions, some facilities were buying PPE from nontraditional sources such as online retailers, home supply stores, paint stores, autobody supply shops, and beauty salons. Other hospitals were using non–medical-grade PPE such as construction masks and handmade masks and gowns.
Other hospitals reported they were conserving and reusing PPE to stretch their supplies. In some cases, they had even changed policies to reduce the extent and frequency of patient interactions with clinicians so the latter would have to change their gear less often.
Shortages of other critical supplies and materials were also reported. Hospitals were running out of supplies that supported patient rooms, such as IV poles, medical gas, linens, toilet paper, and food.
Hospitals across the country were also expecting or experiencing a shortage of ventilators, although none said any patients had been denied access to them. Some institutions were adapting anesthesia machines and single-use emergency transport ventilators.
Also concerning to hospitals was the shortage of intensive-care specialists and nurses to operate the ventilators and care for critically ill patients. Some facilities were training anesthesiologists, hospitalists, and other nonintensivists on how to use the lifesaving equipment.
Meanwhile, patients with COVID-19 symptoms were continuing to show up in droves at emergency departments. Hospitals were concerned about potential shortages of ICU beds, negative-pressure rooms, and isolation units. Given limited bed availability, some administrators said, it was getting hard to separate COVID-19 from non–COVID-19 patients.
What Hospitals Want
As the COVID-19 crisis continues to mount, many hospitals are facing financial emergencies as well, the report noted.
“Hospitals described increasing costs and decreasing revenues as a threat to their financial viability. Hospitals reported that ceasing elective procedures and other services decreased revenues at the same time that their costs have increased as they prepare for a potential surge of patients. Many hospitals reported that their cash reserves were quickly depleting, which could disrupt ongoing hospital operations,” the authors write.
This report was conducted a few days before the passage of the CURES Act, which earmarked $100 billion for hospitals on the frontline of the crisis. As a recent analysis of financial hospital data revealed, however, even with the 20% bump in Medicare payments for COVID-19 care that this cash infusion represents, many hospitals will face a cash-flow crunch within 60 to 90 days, as reported by Medscape Medical News.
Besides higher Medicare payments, the OIG report said, hospitals wanted the government to drop the 14-day waiting period for reimbursement and to offer them loans and grants.
Hospitals also want federal and state governments to relax regulations on professional licensing of, and business relationships with, doctors and other clinicians. They’d like the government to:
- Let them reassign licensed professionals within their hospitals and across healthcare networks
- Provide flexibility with respect to licensed professionals practicing across state lines
- Provide relief from regulations that may restrict using contracted staff or physicians based on business relationships
This article first appeared on Medscape.com.
Hospitals across the country encountered severe challenges as the first wave of the COVID-19 pandemic swept over them, and they anticipated much worse to come, according to a new report from the Office of Inspector General of the Department of Health and Human Services (HHS).
From March 23 to 27, the OIG interviewed 323 hospitals of several types in 46 states, the District of Columbia, and Puerto Rico. The report it pulled together from these interviews is intended to help HHS manage the crisis, rather than to review its response to the pandemic, the OIG said.
The most significant hospital challenges, the report states, were testing and caring for patients with known or suspected COVID-19 and protecting staff members. In addition, the hospitals faced challenges in maintaining or expanding their capacities to treat COVID-19 patients and ensuring the adequacy of basic supplies.
The critical shortages of ventilators, personal protective equipment (PPE), and test kits in hospitals have been widely reported by the media. But the OIG report also focused on some areas that have received less press attention.
To begin with, the shortage of tests has not only slowed the national response to the pandemic, but has had a major impact on inpatient care, according to the report’s authors. The limited number of test kits means that only symptomatic staff members and patients can be tested; in some hospitals, there aren’t even enough tests for that, and some facilities subdivided the test kits they had, the report states.
Moreover, the test results often took 7 days or more to come back from commercial or government labs, the report states. In the meantime, symptomatic patients were presumed to have the coronavirus. While awaiting the results, they had to stay in the hospital, using beds and requiring staff who could otherwise have been assigned to other patients.
The doctors and nurse who cared for these presumptive COVID-19 patients also had to take time suiting up in PPE before seeing them; much of that scarce PPE was wasted on those who were later found not to have the illness.
As one administrator explained to OIG, “Sitting with 60 patients with presumed positives in our hospital isn’t healthy for anybody.”
Delayed test results also reduced hospitals’ ability to provide care by sidelining clinicians who reported COVID-19 symptoms. In one hospital, 20% to 25% of staff were determined to be presumptively positive for COVID-19. As a result of their tests not being analyzed promptly, these doctors and nurses were prevented from providing clinical services for longer than necessary.
Supply Shortages
The report also described some factors contributing to mask shortages. Because of the fear factor, for example, all staff members in one hospital were wearing masks, instead of just those in designated areas. An administrator said the hospital was using 2,000 masks a day, 10 times the number before the COVID-19 crisis.
Another hospital received 2,300 N95 masks from a state reserve, but they were unusable because the elastic bands had dry-rotted.
Meanwhile, some vendors were profiteering. Masks that used to cost 50 cents now sold for $6 each, one administrator said.
To combat the supply chain disruptions, some facilities were buying PPE from nontraditional sources such as online retailers, home supply stores, paint stores, autobody supply shops, and beauty salons. Other hospitals were using non–medical-grade PPE such as construction masks and handmade masks and gowns.
Other hospitals reported they were conserving and reusing PPE to stretch their supplies. In some cases, they had even changed policies to reduce the extent and frequency of patient interactions with clinicians so the latter would have to change their gear less often.
Shortages of other critical supplies and materials were also reported. Hospitals were running out of supplies that supported patient rooms, such as IV poles, medical gas, linens, toilet paper, and food.
Hospitals across the country were also expecting or experiencing a shortage of ventilators, although none said any patients had been denied access to them. Some institutions were adapting anesthesia machines and single-use emergency transport ventilators.
Also concerning to hospitals was the shortage of intensive-care specialists and nurses to operate the ventilators and care for critically ill patients. Some facilities were training anesthesiologists, hospitalists, and other nonintensivists on how to use the lifesaving equipment.
Meanwhile, patients with COVID-19 symptoms were continuing to show up in droves at emergency departments. Hospitals were concerned about potential shortages of ICU beds, negative-pressure rooms, and isolation units. Given limited bed availability, some administrators said, it was getting hard to separate COVID-19 from non–COVID-19 patients.
What Hospitals Want
As the COVID-19 crisis continues to mount, many hospitals are facing financial emergencies as well, the report noted.
“Hospitals described increasing costs and decreasing revenues as a threat to their financial viability. Hospitals reported that ceasing elective procedures and other services decreased revenues at the same time that their costs have increased as they prepare for a potential surge of patients. Many hospitals reported that their cash reserves were quickly depleting, which could disrupt ongoing hospital operations,” the authors write.
This report was conducted a few days before the passage of the CURES Act, which earmarked $100 billion for hospitals on the frontline of the crisis. As a recent analysis of financial hospital data revealed, however, even with the 20% bump in Medicare payments for COVID-19 care that this cash infusion represents, many hospitals will face a cash-flow crunch within 60 to 90 days, as reported by Medscape Medical News.
Besides higher Medicare payments, the OIG report said, hospitals wanted the government to drop the 14-day waiting period for reimbursement and to offer them loans and grants.
Hospitals also want federal and state governments to relax regulations on professional licensing of, and business relationships with, doctors and other clinicians. They’d like the government to:
- Let them reassign licensed professionals within their hospitals and across healthcare networks
- Provide flexibility with respect to licensed professionals practicing across state lines
- Provide relief from regulations that may restrict using contracted staff or physicians based on business relationships
This article first appeared on Medscape.com.
The future of hospital medicine
Assured? Or a definite maybe?
When I started at SHM in 2000, there were fewer than 1,000 hospitalists in the US, and now there are more than 60,000. SHM (back then, we were the National Association of Inpatient Physicians) had about 300 members; now, we have more than 20,000.
Today, hospitalists are part of the medical staff at virtually every hospital in the country, and hospital medicine is recognized as a unique medical specialty with our own knowledge base, textbooks, competencies, meetings, and medical professional society. In a health care environment swirling with change, we are one of the few specialties forged with the ability to adapt and, at times, lead this change. Yet there is so much disruption and instability that there are still many twists and turns in the road that will affect hospitalists’ ability to carve out an even brighter future.
Consolidation has come to health care on a large scale. Hospitals are merging. Health insurers are combining, and even large hospital medicine companies like TeamHealth, Sound, Envision, and others are merging, growing, and acquiring.
At the same time, outside forces from industries not usually associated with health care or inpatient care are swarming into our world: CVS acquires Aetna and aims to reshape primary care; Amazon dominates health care supply chains and moves into pharmacy benefits, and even gets into health care delivery via their partnership with Berkshire Hathaway and JP Morgan; Walmart merges with Humana to create one of the biggest players in Medicare; and Apple expands their inroads into wearables and chronic disease management.
Employment of clinicians has grown logarithmically, especially with inpatient physicians, reshaping the medical staff compensation and accountability. At the same time, payers, both government and private, are evolving into population health with an emphasis not so much on transactions (visits and procedures), but more aligned with outcomes, effectiveness, and efficiency.
All of this leads to a new paradigm of what is important and a new set of values that seems at times more like corporate America where the loyalty of employees can be torn between their employer and the patient. This is especially troublesome in a field traditionally based on the primacy of the doctor-patient relationship. This can put the hospitalist right in the middle at the time when the patient can be most vulnerable.
This has led to new ways to deliver the care that hospitalists provide. First as a pilot and now moving more mainstream, patients with several diagnoses (e.g., heart failure, dehydration, or pneumonia) are now managed not in bricks and mortar hospitals, but in “hospitals at home.” The last few days of a typical hospitalization now take place outside the hospital in a skilled nursing facility (SNF). Fear of uncompensated and unnecessary readmissions leads hospitals to engage hospitalists to handle the first few post-discharge outpatient visits.
This is just a small part of the expanding scope for hospitalists. In addition to managing SNFs and the discharge clinic, hospitalists are now the major providers of perioperative care and play a growing role in palliative care, especially for inpatients. As other specialties that abut hospital medicine have increasing demands and yet fewer new specialists, hospitalists are taking on more critical care and geriatrics, providing procedures, and occupy an evolving role in the emergency room.
There is a lot of work coming towards hospital medicine, and to expand our workforce, hospital medicine groups have incorporated advanced practice providers, including nurse practitioners and physician assistants. But building a true team of health professionals is not seamless or easy with each constituency having a unique scope of practice, limits on their licensure, their own culture, and a distinct training background.
But wait. There will be more new players on the hospital medicine team going forward – some we cannot even anticipate at the present time. In the future, the hospitalist may not even touch the electronic health record (EHR). Clinicians have never excelled at data entry or analysis, and it is time to use a combination of artificial intelligence (AI), voice-activated gathering of history into the record, and staff trained to manage the EHR on both the input and the output sides.
While there may be cheering for this new approach to the EHR – especially because it is a major factor in hospitalist burnout – this will refocus the role and work of the hospitalist to be more of a reviewer and integrator of data, and a strategist and decision-maker overseeing 30 or more patients. As Amazon, CVS, and Walmart move into health care, they will look for the best way to utilize the $300-400/hour hospitalist to the top of our skill level.
In the end, this all comes back to how hospitalists add value, how we can create a career that is rewarding, and how we can help hospitalists be resilient and avoid burnout.
The good news is that hospitalists will not be replaced by AI, nor should we expect to have our incomes cut as less well-trained alternatives replace highly compensated physicians in other specialties. This is a real prospect for many other specialties like dermatology, radiology, pathology, anesthesiology, and even cardiology. But hospitalists will need to adapt to changes in what is valued (i.e., how you can be the most effective and efficient) and to a new job description (i.e., overseeing more patients and managing a team that does more of the H&P, data collecting, and bedside work).
After 20 years of coming out of nowhere to being in the middle of everything in health care, I am confident that hospitalists, with the help of SHM, can continue to forge a path where we can be key difference makers and where we can create a rewarding and sustainable career. It won’t “just happen.” It is not inevitable. But if the past 20 years is any example, we are well-positioned to make the adaptation to succeed in the next 20 years. It is up to all of us to make it happen.
Dr. Wellikson is the CEO of SHM and is retiring from his role in 2020. This article is the second in a series celebrating Dr. Wellikson’s tenure as CEO.
Assured? Or a definite maybe?
Assured? Or a definite maybe?
When I started at SHM in 2000, there were fewer than 1,000 hospitalists in the US, and now there are more than 60,000. SHM (back then, we were the National Association of Inpatient Physicians) had about 300 members; now, we have more than 20,000.
Today, hospitalists are part of the medical staff at virtually every hospital in the country, and hospital medicine is recognized as a unique medical specialty with our own knowledge base, textbooks, competencies, meetings, and medical professional society. In a health care environment swirling with change, we are one of the few specialties forged with the ability to adapt and, at times, lead this change. Yet there is so much disruption and instability that there are still many twists and turns in the road that will affect hospitalists’ ability to carve out an even brighter future.
Consolidation has come to health care on a large scale. Hospitals are merging. Health insurers are combining, and even large hospital medicine companies like TeamHealth, Sound, Envision, and others are merging, growing, and acquiring.
At the same time, outside forces from industries not usually associated with health care or inpatient care are swarming into our world: CVS acquires Aetna and aims to reshape primary care; Amazon dominates health care supply chains and moves into pharmacy benefits, and even gets into health care delivery via their partnership with Berkshire Hathaway and JP Morgan; Walmart merges with Humana to create one of the biggest players in Medicare; and Apple expands their inroads into wearables and chronic disease management.
Employment of clinicians has grown logarithmically, especially with inpatient physicians, reshaping the medical staff compensation and accountability. At the same time, payers, both government and private, are evolving into population health with an emphasis not so much on transactions (visits and procedures), but more aligned with outcomes, effectiveness, and efficiency.
All of this leads to a new paradigm of what is important and a new set of values that seems at times more like corporate America where the loyalty of employees can be torn between their employer and the patient. This is especially troublesome in a field traditionally based on the primacy of the doctor-patient relationship. This can put the hospitalist right in the middle at the time when the patient can be most vulnerable.
This has led to new ways to deliver the care that hospitalists provide. First as a pilot and now moving more mainstream, patients with several diagnoses (e.g., heart failure, dehydration, or pneumonia) are now managed not in bricks and mortar hospitals, but in “hospitals at home.” The last few days of a typical hospitalization now take place outside the hospital in a skilled nursing facility (SNF). Fear of uncompensated and unnecessary readmissions leads hospitals to engage hospitalists to handle the first few post-discharge outpatient visits.
This is just a small part of the expanding scope for hospitalists. In addition to managing SNFs and the discharge clinic, hospitalists are now the major providers of perioperative care and play a growing role in palliative care, especially for inpatients. As other specialties that abut hospital medicine have increasing demands and yet fewer new specialists, hospitalists are taking on more critical care and geriatrics, providing procedures, and occupy an evolving role in the emergency room.
There is a lot of work coming towards hospital medicine, and to expand our workforce, hospital medicine groups have incorporated advanced practice providers, including nurse practitioners and physician assistants. But building a true team of health professionals is not seamless or easy with each constituency having a unique scope of practice, limits on their licensure, their own culture, and a distinct training background.
But wait. There will be more new players on the hospital medicine team going forward – some we cannot even anticipate at the present time. In the future, the hospitalist may not even touch the electronic health record (EHR). Clinicians have never excelled at data entry or analysis, and it is time to use a combination of artificial intelligence (AI), voice-activated gathering of history into the record, and staff trained to manage the EHR on both the input and the output sides.
While there may be cheering for this new approach to the EHR – especially because it is a major factor in hospitalist burnout – this will refocus the role and work of the hospitalist to be more of a reviewer and integrator of data, and a strategist and decision-maker overseeing 30 or more patients. As Amazon, CVS, and Walmart move into health care, they will look for the best way to utilize the $300-400/hour hospitalist to the top of our skill level.
In the end, this all comes back to how hospitalists add value, how we can create a career that is rewarding, and how we can help hospitalists be resilient and avoid burnout.
The good news is that hospitalists will not be replaced by AI, nor should we expect to have our incomes cut as less well-trained alternatives replace highly compensated physicians in other specialties. This is a real prospect for many other specialties like dermatology, radiology, pathology, anesthesiology, and even cardiology. But hospitalists will need to adapt to changes in what is valued (i.e., how you can be the most effective and efficient) and to a new job description (i.e., overseeing more patients and managing a team that does more of the H&P, data collecting, and bedside work).
After 20 years of coming out of nowhere to being in the middle of everything in health care, I am confident that hospitalists, with the help of SHM, can continue to forge a path where we can be key difference makers and where we can create a rewarding and sustainable career. It won’t “just happen.” It is not inevitable. But if the past 20 years is any example, we are well-positioned to make the adaptation to succeed in the next 20 years. It is up to all of us to make it happen.
Dr. Wellikson is the CEO of SHM and is retiring from his role in 2020. This article is the second in a series celebrating Dr. Wellikson’s tenure as CEO.
When I started at SHM in 2000, there were fewer than 1,000 hospitalists in the US, and now there are more than 60,000. SHM (back then, we were the National Association of Inpatient Physicians) had about 300 members; now, we have more than 20,000.
Today, hospitalists are part of the medical staff at virtually every hospital in the country, and hospital medicine is recognized as a unique medical specialty with our own knowledge base, textbooks, competencies, meetings, and medical professional society. In a health care environment swirling with change, we are one of the few specialties forged with the ability to adapt and, at times, lead this change. Yet there is so much disruption and instability that there are still many twists and turns in the road that will affect hospitalists’ ability to carve out an even brighter future.
Consolidation has come to health care on a large scale. Hospitals are merging. Health insurers are combining, and even large hospital medicine companies like TeamHealth, Sound, Envision, and others are merging, growing, and acquiring.
At the same time, outside forces from industries not usually associated with health care or inpatient care are swarming into our world: CVS acquires Aetna and aims to reshape primary care; Amazon dominates health care supply chains and moves into pharmacy benefits, and even gets into health care delivery via their partnership with Berkshire Hathaway and JP Morgan; Walmart merges with Humana to create one of the biggest players in Medicare; and Apple expands their inroads into wearables and chronic disease management.
Employment of clinicians has grown logarithmically, especially with inpatient physicians, reshaping the medical staff compensation and accountability. At the same time, payers, both government and private, are evolving into population health with an emphasis not so much on transactions (visits and procedures), but more aligned with outcomes, effectiveness, and efficiency.
All of this leads to a new paradigm of what is important and a new set of values that seems at times more like corporate America where the loyalty of employees can be torn between their employer and the patient. This is especially troublesome in a field traditionally based on the primacy of the doctor-patient relationship. This can put the hospitalist right in the middle at the time when the patient can be most vulnerable.
This has led to new ways to deliver the care that hospitalists provide. First as a pilot and now moving more mainstream, patients with several diagnoses (e.g., heart failure, dehydration, or pneumonia) are now managed not in bricks and mortar hospitals, but in “hospitals at home.” The last few days of a typical hospitalization now take place outside the hospital in a skilled nursing facility (SNF). Fear of uncompensated and unnecessary readmissions leads hospitals to engage hospitalists to handle the first few post-discharge outpatient visits.
This is just a small part of the expanding scope for hospitalists. In addition to managing SNFs and the discharge clinic, hospitalists are now the major providers of perioperative care and play a growing role in palliative care, especially for inpatients. As other specialties that abut hospital medicine have increasing demands and yet fewer new specialists, hospitalists are taking on more critical care and geriatrics, providing procedures, and occupy an evolving role in the emergency room.
There is a lot of work coming towards hospital medicine, and to expand our workforce, hospital medicine groups have incorporated advanced practice providers, including nurse practitioners and physician assistants. But building a true team of health professionals is not seamless or easy with each constituency having a unique scope of practice, limits on their licensure, their own culture, and a distinct training background.
But wait. There will be more new players on the hospital medicine team going forward – some we cannot even anticipate at the present time. In the future, the hospitalist may not even touch the electronic health record (EHR). Clinicians have never excelled at data entry or analysis, and it is time to use a combination of artificial intelligence (AI), voice-activated gathering of history into the record, and staff trained to manage the EHR on both the input and the output sides.
While there may be cheering for this new approach to the EHR – especially because it is a major factor in hospitalist burnout – this will refocus the role and work of the hospitalist to be more of a reviewer and integrator of data, and a strategist and decision-maker overseeing 30 or more patients. As Amazon, CVS, and Walmart move into health care, they will look for the best way to utilize the $300-400/hour hospitalist to the top of our skill level.
In the end, this all comes back to how hospitalists add value, how we can create a career that is rewarding, and how we can help hospitalists be resilient and avoid burnout.
The good news is that hospitalists will not be replaced by AI, nor should we expect to have our incomes cut as less well-trained alternatives replace highly compensated physicians in other specialties. This is a real prospect for many other specialties like dermatology, radiology, pathology, anesthesiology, and even cardiology. But hospitalists will need to adapt to changes in what is valued (i.e., how you can be the most effective and efficient) and to a new job description (i.e., overseeing more patients and managing a team that does more of the H&P, data collecting, and bedside work).
After 20 years of coming out of nowhere to being in the middle of everything in health care, I am confident that hospitalists, with the help of SHM, can continue to forge a path where we can be key difference makers and where we can create a rewarding and sustainable career. It won’t “just happen.” It is not inevitable. But if the past 20 years is any example, we are well-positioned to make the adaptation to succeed in the next 20 years. It is up to all of us to make it happen.
Dr. Wellikson is the CEO of SHM and is retiring from his role in 2020. This article is the second in a series celebrating Dr. Wellikson’s tenure as CEO.
Many children with COVID-19 don’t have cough or fever
according to the Centers for Disease and Prevention Control.
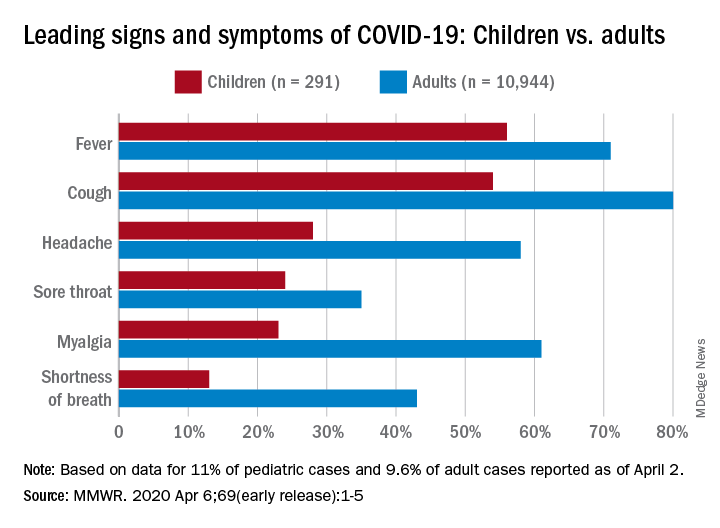
Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
according to the Centers for Disease and Prevention Control.

Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
according to the Centers for Disease and Prevention Control.

Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
FROM MMWR
AAP issues guidance on managing infants born to mothers with COVID-19
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
Is protocol-driven COVID-19 respiratory therapy doing more harm than good?
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
[email protected]
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
[email protected]
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
[email protected]
A decade of telemedicine policy has advanced in just 2 weeks
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.















