User login
Flu activity declines again but remains high
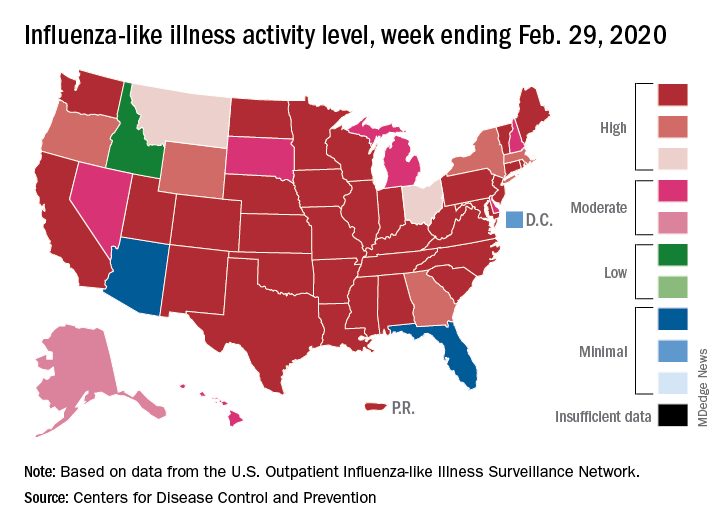
Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.

Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.

Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.
Novel coronavirus may cause environmental contamination through fecal shedding
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
FROM JAMA
Telehealth seen as a key tool to help fight COVID-19
Telehealth is increasingly being viewed as a key way to help fight the COVID-19 outbreak in the United States. Recognizing the potential of this technology to slow the spread of the disease, the House of Representatives included a provision in an $8.3 billion emergency response bill it approved today that would temporarily lift restrictions on Medicare telehealth coverage to assist in the efforts to contain the virus.
Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC), said that hospitals should be prepared to use telehealth as one of their tools in fighting the outbreak, according to a recent news release from the American Hospital Association (AHA).
Congress is responding to that need by including the service in the new coronavirus legislation now headed to the Senate, after the funding bill was approved in a 415-2 vote by the House.
The bill empowers the Secretary of Health and Human Services (HHS) to “waive or modify application of certain Medicare requirements with respect to telehealth services furnished during certain emergency periods.”
While the measure adds telehealth to the waiver authority that the HHS secretary currently has during national emergencies, it’s only for the coronavirus crisis in this case, Krista Drobac, executive director of the Alliance for Connected Care, told Medscape Medical News.
The waiver would apply to originating sites of telehealth visits, she noted. Thus Medicare coverage of telemedicine would be expanded beyond rural areas.
In addition, the waiver would allow coverage of virtual visits conducted on smartphones with audio and video capabilities. A “qualified provider,” as defined by the legislation, would be a practitioner who has an established relationship with the patient or who is in the same practice as the provider who has that relationship.
An advantage of telehealth, proponents say, is that it can enable people who believe they have COVID-19 to be seen at home rather than visit offices or emergency departments (EDs) where they might spread the disease or be in proximity to others who have it.
In an editorial published March 2 in Modern Healthcare, medical directors from Stanford Medicine, MedStar Health, and Intermountain Healthcare also noted that telehealth can give patients 24/7 access to care, allow surveillance of patients at risk while keeping them at home, ensure that treatment in hospitals is reserved for high-need patients, and enable providers to triage and screen more patients than can be handled in brick-and-mortar care settings.
However, telehealth screening would allow physicians only to judge whether a patient’s symptoms might be indicative of COVID-19, the Alliance for Connected Care, a telehealth advocacy group, noted in a letter to Congressional leaders. Patients would still have to be seen in person to be tested for the disease.
The group, which represents technology companies, health insurers, pharmacies, and other healthcare players, has been lobbying Congress to include telehealth in federal funds to combat the outbreak.
The American Telemedicine Association (ATA) also supports this goal, ATA President Joseph Kvedar, MD, told Medscape Medical News. And the authors of the Modern Healthcare editorial also advocated for this legislative solution. Because the fatality rate for COVID-19 is significantly higher for older people than for other age groups, they noted, telehealth should be an economically viable option for all seniors.
The Centers for Medicare and Medicaid Services (CMS) long covered telemedicine only in rural areas and only when initiated in healthcare settings. Recently, however, CMS loosened its approach to some extent. Virtual “check-in visits” can now be initiated from any location, including home, to determine whether a Medicare patient needs to be seen in the office. In addition, CMS allows Medicare Advantage plans to offer telemedicine as a core benefit.
Are healthcare systems prepared?
Some large healthcare systems such as Stanford, MedStar, and Intermountain are already using telehealth to diagnose and treat patients who have traditional influenza. Telehealth providers at Stanford estimate that almost 50% of these patients are being prescribed the antiviral drug Tamiflu.
It’s unclear whether other healthcare systems are this well prepared to offer telehealth on a large scale. But, according to an AHA survey, Kvedar noted, three quarters of AHA members are engaged in some form of telehealth.
Drobac said “it wouldn’t require too much effort” to ramp up a wide-scale telehealth program that could help reduce the impact of the outbreak. “The technology is there,” she noted. “You need a HIPAA-compliant telehealth platform, but there are so many out there.”
Kvedar agreed. To begin with, he said, hospitals might sequester patients who visit the ED with COVID-19 symptoms in a video-equipped “isolation room.” Staff members could then do the patient intake from a different location in the hospital.
He admitted that this approach would be infeasible if a lot of patients arrived in EDs with coronavirus symptoms. However, Kvedar noted, “All the tools are in place to go well beyond that. American Well, Teladoc, and others are all offering ways to get out in front of this. There are plenty of vendors out there, and most people have a connected cell phone that you can do a video call on.”
Hospital leaders would have to decide whether to embrace telehealth, which would mean less use of services in their institutions, he said. “But it would be for the greater good of the public.”
Kvedar recalled that there was some use of telehealth in the New York area after 9/11. Telehealth was also used in the aftermath of Hurricane Katrina in 2005. But the ATA president, who is also vice president of connected health at Partners HealthCare in Boston, noted that the COVID-19 outbreak is the first public health emergency to occur in the era of Skype and smartphones.
If Congress does ultimately authorize CMS to cover telehealth across the board during this emergency, might that lead to a permanent change in Medicare coverage policy? Kvedar wouldn’t venture an opinion. “However, the current CMS leadership has been incredibly telehealth friendly,” he said. “So it’s possible they would [embrace a lifting of restrictions]. As patients get a sense of this modality of care and how convenient it is for them, they’ll start asking for more.”
Meanwhile, he said, the telehealth opportunity goes beyond video visits with doctors to mitigate the outbreak. Telehealth data could also be used to track disease spread, similar to how researchers have studied Google searches to predict the spread of the flu, he noted.
Teladoc, a major telehealth vendor, recently told stock analysts it’s already working with the CDC on disease surveillance, according to a report in FierceHealthcare.
This article first appeared on Medscape.com.
Telehealth is increasingly being viewed as a key way to help fight the COVID-19 outbreak in the United States. Recognizing the potential of this technology to slow the spread of the disease, the House of Representatives included a provision in an $8.3 billion emergency response bill it approved today that would temporarily lift restrictions on Medicare telehealth coverage to assist in the efforts to contain the virus.
Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC), said that hospitals should be prepared to use telehealth as one of their tools in fighting the outbreak, according to a recent news release from the American Hospital Association (AHA).
Congress is responding to that need by including the service in the new coronavirus legislation now headed to the Senate, after the funding bill was approved in a 415-2 vote by the House.
The bill empowers the Secretary of Health and Human Services (HHS) to “waive or modify application of certain Medicare requirements with respect to telehealth services furnished during certain emergency periods.”
While the measure adds telehealth to the waiver authority that the HHS secretary currently has during national emergencies, it’s only for the coronavirus crisis in this case, Krista Drobac, executive director of the Alliance for Connected Care, told Medscape Medical News.
The waiver would apply to originating sites of telehealth visits, she noted. Thus Medicare coverage of telemedicine would be expanded beyond rural areas.
In addition, the waiver would allow coverage of virtual visits conducted on smartphones with audio and video capabilities. A “qualified provider,” as defined by the legislation, would be a practitioner who has an established relationship with the patient or who is in the same practice as the provider who has that relationship.
An advantage of telehealth, proponents say, is that it can enable people who believe they have COVID-19 to be seen at home rather than visit offices or emergency departments (EDs) where they might spread the disease or be in proximity to others who have it.
In an editorial published March 2 in Modern Healthcare, medical directors from Stanford Medicine, MedStar Health, and Intermountain Healthcare also noted that telehealth can give patients 24/7 access to care, allow surveillance of patients at risk while keeping them at home, ensure that treatment in hospitals is reserved for high-need patients, and enable providers to triage and screen more patients than can be handled in brick-and-mortar care settings.
However, telehealth screening would allow physicians only to judge whether a patient’s symptoms might be indicative of COVID-19, the Alliance for Connected Care, a telehealth advocacy group, noted in a letter to Congressional leaders. Patients would still have to be seen in person to be tested for the disease.
The group, which represents technology companies, health insurers, pharmacies, and other healthcare players, has been lobbying Congress to include telehealth in federal funds to combat the outbreak.
The American Telemedicine Association (ATA) also supports this goal, ATA President Joseph Kvedar, MD, told Medscape Medical News. And the authors of the Modern Healthcare editorial also advocated for this legislative solution. Because the fatality rate for COVID-19 is significantly higher for older people than for other age groups, they noted, telehealth should be an economically viable option for all seniors.
The Centers for Medicare and Medicaid Services (CMS) long covered telemedicine only in rural areas and only when initiated in healthcare settings. Recently, however, CMS loosened its approach to some extent. Virtual “check-in visits” can now be initiated from any location, including home, to determine whether a Medicare patient needs to be seen in the office. In addition, CMS allows Medicare Advantage plans to offer telemedicine as a core benefit.
Are healthcare systems prepared?
Some large healthcare systems such as Stanford, MedStar, and Intermountain are already using telehealth to diagnose and treat patients who have traditional influenza. Telehealth providers at Stanford estimate that almost 50% of these patients are being prescribed the antiviral drug Tamiflu.
It’s unclear whether other healthcare systems are this well prepared to offer telehealth on a large scale. But, according to an AHA survey, Kvedar noted, three quarters of AHA members are engaged in some form of telehealth.
Drobac said “it wouldn’t require too much effort” to ramp up a wide-scale telehealth program that could help reduce the impact of the outbreak. “The technology is there,” she noted. “You need a HIPAA-compliant telehealth platform, but there are so many out there.”
Kvedar agreed. To begin with, he said, hospitals might sequester patients who visit the ED with COVID-19 symptoms in a video-equipped “isolation room.” Staff members could then do the patient intake from a different location in the hospital.
He admitted that this approach would be infeasible if a lot of patients arrived in EDs with coronavirus symptoms. However, Kvedar noted, “All the tools are in place to go well beyond that. American Well, Teladoc, and others are all offering ways to get out in front of this. There are plenty of vendors out there, and most people have a connected cell phone that you can do a video call on.”
Hospital leaders would have to decide whether to embrace telehealth, which would mean less use of services in their institutions, he said. “But it would be for the greater good of the public.”
Kvedar recalled that there was some use of telehealth in the New York area after 9/11. Telehealth was also used in the aftermath of Hurricane Katrina in 2005. But the ATA president, who is also vice president of connected health at Partners HealthCare in Boston, noted that the COVID-19 outbreak is the first public health emergency to occur in the era of Skype and smartphones.
If Congress does ultimately authorize CMS to cover telehealth across the board during this emergency, might that lead to a permanent change in Medicare coverage policy? Kvedar wouldn’t venture an opinion. “However, the current CMS leadership has been incredibly telehealth friendly,” he said. “So it’s possible they would [embrace a lifting of restrictions]. As patients get a sense of this modality of care and how convenient it is for them, they’ll start asking for more.”
Meanwhile, he said, the telehealth opportunity goes beyond video visits with doctors to mitigate the outbreak. Telehealth data could also be used to track disease spread, similar to how researchers have studied Google searches to predict the spread of the flu, he noted.
Teladoc, a major telehealth vendor, recently told stock analysts it’s already working with the CDC on disease surveillance, according to a report in FierceHealthcare.
This article first appeared on Medscape.com.
Telehealth is increasingly being viewed as a key way to help fight the COVID-19 outbreak in the United States. Recognizing the potential of this technology to slow the spread of the disease, the House of Representatives included a provision in an $8.3 billion emergency response bill it approved today that would temporarily lift restrictions on Medicare telehealth coverage to assist in the efforts to contain the virus.
Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC), said that hospitals should be prepared to use telehealth as one of their tools in fighting the outbreak, according to a recent news release from the American Hospital Association (AHA).
Congress is responding to that need by including the service in the new coronavirus legislation now headed to the Senate, after the funding bill was approved in a 415-2 vote by the House.
The bill empowers the Secretary of Health and Human Services (HHS) to “waive or modify application of certain Medicare requirements with respect to telehealth services furnished during certain emergency periods.”
While the measure adds telehealth to the waiver authority that the HHS secretary currently has during national emergencies, it’s only for the coronavirus crisis in this case, Krista Drobac, executive director of the Alliance for Connected Care, told Medscape Medical News.
The waiver would apply to originating sites of telehealth visits, she noted. Thus Medicare coverage of telemedicine would be expanded beyond rural areas.
In addition, the waiver would allow coverage of virtual visits conducted on smartphones with audio and video capabilities. A “qualified provider,” as defined by the legislation, would be a practitioner who has an established relationship with the patient or who is in the same practice as the provider who has that relationship.
An advantage of telehealth, proponents say, is that it can enable people who believe they have COVID-19 to be seen at home rather than visit offices or emergency departments (EDs) where they might spread the disease or be in proximity to others who have it.
In an editorial published March 2 in Modern Healthcare, medical directors from Stanford Medicine, MedStar Health, and Intermountain Healthcare also noted that telehealth can give patients 24/7 access to care, allow surveillance of patients at risk while keeping them at home, ensure that treatment in hospitals is reserved for high-need patients, and enable providers to triage and screen more patients than can be handled in brick-and-mortar care settings.
However, telehealth screening would allow physicians only to judge whether a patient’s symptoms might be indicative of COVID-19, the Alliance for Connected Care, a telehealth advocacy group, noted in a letter to Congressional leaders. Patients would still have to be seen in person to be tested for the disease.
The group, which represents technology companies, health insurers, pharmacies, and other healthcare players, has been lobbying Congress to include telehealth in federal funds to combat the outbreak.
The American Telemedicine Association (ATA) also supports this goal, ATA President Joseph Kvedar, MD, told Medscape Medical News. And the authors of the Modern Healthcare editorial also advocated for this legislative solution. Because the fatality rate for COVID-19 is significantly higher for older people than for other age groups, they noted, telehealth should be an economically viable option for all seniors.
The Centers for Medicare and Medicaid Services (CMS) long covered telemedicine only in rural areas and only when initiated in healthcare settings. Recently, however, CMS loosened its approach to some extent. Virtual “check-in visits” can now be initiated from any location, including home, to determine whether a Medicare patient needs to be seen in the office. In addition, CMS allows Medicare Advantage plans to offer telemedicine as a core benefit.
Are healthcare systems prepared?
Some large healthcare systems such as Stanford, MedStar, and Intermountain are already using telehealth to diagnose and treat patients who have traditional influenza. Telehealth providers at Stanford estimate that almost 50% of these patients are being prescribed the antiviral drug Tamiflu.
It’s unclear whether other healthcare systems are this well prepared to offer telehealth on a large scale. But, according to an AHA survey, Kvedar noted, three quarters of AHA members are engaged in some form of telehealth.
Drobac said “it wouldn’t require too much effort” to ramp up a wide-scale telehealth program that could help reduce the impact of the outbreak. “The technology is there,” she noted. “You need a HIPAA-compliant telehealth platform, but there are so many out there.”
Kvedar agreed. To begin with, he said, hospitals might sequester patients who visit the ED with COVID-19 symptoms in a video-equipped “isolation room.” Staff members could then do the patient intake from a different location in the hospital.
He admitted that this approach would be infeasible if a lot of patients arrived in EDs with coronavirus symptoms. However, Kvedar noted, “All the tools are in place to go well beyond that. American Well, Teladoc, and others are all offering ways to get out in front of this. There are plenty of vendors out there, and most people have a connected cell phone that you can do a video call on.”
Hospital leaders would have to decide whether to embrace telehealth, which would mean less use of services in their institutions, he said. “But it would be for the greater good of the public.”
Kvedar recalled that there was some use of telehealth in the New York area after 9/11. Telehealth was also used in the aftermath of Hurricane Katrina in 2005. But the ATA president, who is also vice president of connected health at Partners HealthCare in Boston, noted that the COVID-19 outbreak is the first public health emergency to occur in the era of Skype and smartphones.
If Congress does ultimately authorize CMS to cover telehealth across the board during this emergency, might that lead to a permanent change in Medicare coverage policy? Kvedar wouldn’t venture an opinion. “However, the current CMS leadership has been incredibly telehealth friendly,” he said. “So it’s possible they would [embrace a lifting of restrictions]. As patients get a sense of this modality of care and how convenient it is for them, they’ll start asking for more.”
Meanwhile, he said, the telehealth opportunity goes beyond video visits with doctors to mitigate the outbreak. Telehealth data could also be used to track disease spread, similar to how researchers have studied Google searches to predict the spread of the flu, he noted.
Teladoc, a major telehealth vendor, recently told stock analysts it’s already working with the CDC on disease surveillance, according to a report in FierceHealthcare.
This article first appeared on Medscape.com.
COVID-19 and public health preparedness in the United States
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Hospitalist profile: Charu Puri, MD
Charu Puri, MD, FHM, is a hospitalist and medical informaticist at Sutter East Bay Medical Group in Oakland, Calif. She also serves as medical director for onboarding, mentoring, and physician development.
Dr. Puri has been a member of the Society of Hospital Medicine since 2009, and attended the Society’s Leadership Academy, where she was inspired to create a mentorship program at her own institution. She is a member of the San Francisco Bay chapter of SHM and serves on the Performance Measurement and Reporting Committee.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
It was early on in my residency that it became clear to me that I wanted to pursue the hospitalist track. It was a natural fit, and I gravitated toward the hospitalist side of medicine. What appealed to me most was that we had the opportunity and privilege to provide care to patients in their most vulnerable state and experience the effects of that care in real time. I found that very gratifying.
There is also a sense of community and camaraderie that comes with working in a hospital setting. Everyone is working together, trying to help patients. The collegiality and the relationships that develop are very rewarding. I have been fortunate enough to have built strong friendships with the hospitalists in my group as well as colleagues from other disciplines in medicine that work in the hospital.
What is your current role at Sutter Health?
Alta Bates Summit Medical Center is part of the larger Sutter Health system. I have an administrative role with my medical group in addition to the clinical work I do at the medical center, although first and foremost I identify myself as a hospitalist. About 5 years ago I took on a role in clinical informatics, when our hospital implemented an EHR. Since then I have been working as an inpatient physician informaticist. Most recently I took on a new role as medical director for onboarding, mentoring, and physician development in my medical group.
How do you balance the different duties of your various roles?
I am full time in my administration role, between my informatics role and my onboarding role. I technically don’t have to do clinical shifts if I don’t want to, but it’s important to me to continue clinical practice and maintain my skills and connection to the hospital and colleagues. I do about four clinical shifts a month, and plan to continue doing that. In our group you must do 14 shifts a month to be considered full time, so what I do could be considered about one-third of that.
What are your favorite areas of clinical practice and/or research?
I haven’t had a lot of research experience. My residency program was a community-based program, and my current setting is a community hospital. I haven’t been involved much in the academic side of hospital medicine. As far as clinical practices goes, I think it’s the diversity of hospital medicine that appeals to me. You really get to be a jack of all trades, and experience all the different disciplines of medicine. I like the variety.
Both my informatics and onboarding roles came out of a need that I identified, and just began doing the work before there was an official role. When we implemented our EHR, it was essential to get our doctors organized to make sure they were ready to take care of patients that first day of go live. By the time our hospital went live on the EHR, I had a good understanding of how it worked, and so I was able to create a miniature curriculum for our physicians – templates, order sets, workflows, etc. – to help ensure everything went smoothly. A few months after we implemented the EHR, I was officially offered a physician informaticist role.
The onboarding role came about in an interesting way. I was participating in the leadership course offered by SHM and was lucky enough to be in the pilot for the Capstone course. That leadership course is focused around mentoring and sponsorship, and one of the faculty members was Nancy Spector, MD, the associate dean of faculty development at Drexel University, Philadelphia. She talked a lot about mentoring, and I was inspired to set up a mentoring program for our hospitalists. Dr. Spector graciously agreed to mentor me as I worked on my Capstone project, which was to create a mentoring program in a community-based hospitalist group. As I continued to work on the project, coincidentally our medical group decided to redesign our new physician onboarding process. Because I was already involved in the onboarding and training related to our EHR, I became very involved with our medical group's onboarding redesign.
My group's CEO decided to create a new directorship role for onboarding and mentoring, which I recently interviewed for and was offered about two months ago.
I think setting up systems to support our doctors is the common threat between the informatics and the onboarding roles. I want to implement systems that support our doctors, help them succeed, and hopefully make their jobs a little easier.
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
We practice in a very urban environment, with many low-income patients who have limited resources and access to health care. That can be very challenging. You always wonder if these patients have all the support they need after leaving the hospital. Sometimes I feel that I am just putting a band-aid on the medical problem, so to speak, but not solving the underlying issue. But it can be very rewarding during those times when the hospital and the broader community can bring our resources together to create interventions to help at-risk patients. It doesn’t happen as frequently as we would like, but when it does happen it feels good.
Another challenging aspect is related to perception. There are a lot of consultants in the hospital who view hospitalists as "house staff." That can be very frustrating, and it’s important to steer the conversations away from that perspective, and really try to establish ourselves as colleagues and peers.
How will hospital medicine change in the next decade or 2?
It’s a relatively young field, and we’re still figuring it out. I really don’t know how hospital medicine is going to change, but I do know that the field will continue to evolve, given the way U.S. health care is rapidly changing.
Do you have any advice for students and residents interested in hospital medicine?
It’s a fun way to practice medicine and I would encourage students to go into hospital medicine. It’s great for work/life balance. The advice I would give is that it is very important to get involved early in your career. Get involved in medical group or hospital committees. Stay away from the “shift mentality” – that I’m going to work my shifts and leave. That can lead to early burnout, which is a real concern in our field now. Early engagement is essential, so you can help lead these conversations at your hospital.
Charu Puri, MD, FHM, is a hospitalist and medical informaticist at Sutter East Bay Medical Group in Oakland, Calif. She also serves as medical director for onboarding, mentoring, and physician development.
Dr. Puri has been a member of the Society of Hospital Medicine since 2009, and attended the Society’s Leadership Academy, where she was inspired to create a mentorship program at her own institution. She is a member of the San Francisco Bay chapter of SHM and serves on the Performance Measurement and Reporting Committee.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
It was early on in my residency that it became clear to me that I wanted to pursue the hospitalist track. It was a natural fit, and I gravitated toward the hospitalist side of medicine. What appealed to me most was that we had the opportunity and privilege to provide care to patients in their most vulnerable state and experience the effects of that care in real time. I found that very gratifying.
There is also a sense of community and camaraderie that comes with working in a hospital setting. Everyone is working together, trying to help patients. The collegiality and the relationships that develop are very rewarding. I have been fortunate enough to have built strong friendships with the hospitalists in my group as well as colleagues from other disciplines in medicine that work in the hospital.
What is your current role at Sutter Health?
Alta Bates Summit Medical Center is part of the larger Sutter Health system. I have an administrative role with my medical group in addition to the clinical work I do at the medical center, although first and foremost I identify myself as a hospitalist. About 5 years ago I took on a role in clinical informatics, when our hospital implemented an EHR. Since then I have been working as an inpatient physician informaticist. Most recently I took on a new role as medical director for onboarding, mentoring, and physician development in my medical group.
How do you balance the different duties of your various roles?
I am full time in my administration role, between my informatics role and my onboarding role. I technically don’t have to do clinical shifts if I don’t want to, but it’s important to me to continue clinical practice and maintain my skills and connection to the hospital and colleagues. I do about four clinical shifts a month, and plan to continue doing that. In our group you must do 14 shifts a month to be considered full time, so what I do could be considered about one-third of that.
What are your favorite areas of clinical practice and/or research?
I haven’t had a lot of research experience. My residency program was a community-based program, and my current setting is a community hospital. I haven’t been involved much in the academic side of hospital medicine. As far as clinical practices goes, I think it’s the diversity of hospital medicine that appeals to me. You really get to be a jack of all trades, and experience all the different disciplines of medicine. I like the variety.
Both my informatics and onboarding roles came out of a need that I identified, and just began doing the work before there was an official role. When we implemented our EHR, it was essential to get our doctors organized to make sure they were ready to take care of patients that first day of go live. By the time our hospital went live on the EHR, I had a good understanding of how it worked, and so I was able to create a miniature curriculum for our physicians – templates, order sets, workflows, etc. – to help ensure everything went smoothly. A few months after we implemented the EHR, I was officially offered a physician informaticist role.
The onboarding role came about in an interesting way. I was participating in the leadership course offered by SHM and was lucky enough to be in the pilot for the Capstone course. That leadership course is focused around mentoring and sponsorship, and one of the faculty members was Nancy Spector, MD, the associate dean of faculty development at Drexel University, Philadelphia. She talked a lot about mentoring, and I was inspired to set up a mentoring program for our hospitalists. Dr. Spector graciously agreed to mentor me as I worked on my Capstone project, which was to create a mentoring program in a community-based hospitalist group. As I continued to work on the project, coincidentally our medical group decided to redesign our new physician onboarding process. Because I was already involved in the onboarding and training related to our EHR, I became very involved with our medical group's onboarding redesign.
My group's CEO decided to create a new directorship role for onboarding and mentoring, which I recently interviewed for and was offered about two months ago.
I think setting up systems to support our doctors is the common threat between the informatics and the onboarding roles. I want to implement systems that support our doctors, help them succeed, and hopefully make their jobs a little easier.
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
We practice in a very urban environment, with many low-income patients who have limited resources and access to health care. That can be very challenging. You always wonder if these patients have all the support they need after leaving the hospital. Sometimes I feel that I am just putting a band-aid on the medical problem, so to speak, but not solving the underlying issue. But it can be very rewarding during those times when the hospital and the broader community can bring our resources together to create interventions to help at-risk patients. It doesn’t happen as frequently as we would like, but when it does happen it feels good.
Another challenging aspect is related to perception. There are a lot of consultants in the hospital who view hospitalists as "house staff." That can be very frustrating, and it’s important to steer the conversations away from that perspective, and really try to establish ourselves as colleagues and peers.
How will hospital medicine change in the next decade or 2?
It’s a relatively young field, and we’re still figuring it out. I really don’t know how hospital medicine is going to change, but I do know that the field will continue to evolve, given the way U.S. health care is rapidly changing.
Do you have any advice for students and residents interested in hospital medicine?
It’s a fun way to practice medicine and I would encourage students to go into hospital medicine. It’s great for work/life balance. The advice I would give is that it is very important to get involved early in your career. Get involved in medical group or hospital committees. Stay away from the “shift mentality” – that I’m going to work my shifts and leave. That can lead to early burnout, which is a real concern in our field now. Early engagement is essential, so you can help lead these conversations at your hospital.
Charu Puri, MD, FHM, is a hospitalist and medical informaticist at Sutter East Bay Medical Group in Oakland, Calif. She also serves as medical director for onboarding, mentoring, and physician development.
Dr. Puri has been a member of the Society of Hospital Medicine since 2009, and attended the Society’s Leadership Academy, where she was inspired to create a mentorship program at her own institution. She is a member of the San Francisco Bay chapter of SHM and serves on the Performance Measurement and Reporting Committee.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
It was early on in my residency that it became clear to me that I wanted to pursue the hospitalist track. It was a natural fit, and I gravitated toward the hospitalist side of medicine. What appealed to me most was that we had the opportunity and privilege to provide care to patients in their most vulnerable state and experience the effects of that care in real time. I found that very gratifying.
There is also a sense of community and camaraderie that comes with working in a hospital setting. Everyone is working together, trying to help patients. The collegiality and the relationships that develop are very rewarding. I have been fortunate enough to have built strong friendships with the hospitalists in my group as well as colleagues from other disciplines in medicine that work in the hospital.
What is your current role at Sutter Health?
Alta Bates Summit Medical Center is part of the larger Sutter Health system. I have an administrative role with my medical group in addition to the clinical work I do at the medical center, although first and foremost I identify myself as a hospitalist. About 5 years ago I took on a role in clinical informatics, when our hospital implemented an EHR. Since then I have been working as an inpatient physician informaticist. Most recently I took on a new role as medical director for onboarding, mentoring, and physician development in my medical group.
How do you balance the different duties of your various roles?
I am full time in my administration role, between my informatics role and my onboarding role. I technically don’t have to do clinical shifts if I don’t want to, but it’s important to me to continue clinical practice and maintain my skills and connection to the hospital and colleagues. I do about four clinical shifts a month, and plan to continue doing that. In our group you must do 14 shifts a month to be considered full time, so what I do could be considered about one-third of that.
What are your favorite areas of clinical practice and/or research?
I haven’t had a lot of research experience. My residency program was a community-based program, and my current setting is a community hospital. I haven’t been involved much in the academic side of hospital medicine. As far as clinical practices goes, I think it’s the diversity of hospital medicine that appeals to me. You really get to be a jack of all trades, and experience all the different disciplines of medicine. I like the variety.
Both my informatics and onboarding roles came out of a need that I identified, and just began doing the work before there was an official role. When we implemented our EHR, it was essential to get our doctors organized to make sure they were ready to take care of patients that first day of go live. By the time our hospital went live on the EHR, I had a good understanding of how it worked, and so I was able to create a miniature curriculum for our physicians – templates, order sets, workflows, etc. – to help ensure everything went smoothly. A few months after we implemented the EHR, I was officially offered a physician informaticist role.
The onboarding role came about in an interesting way. I was participating in the leadership course offered by SHM and was lucky enough to be in the pilot for the Capstone course. That leadership course is focused around mentoring and sponsorship, and one of the faculty members was Nancy Spector, MD, the associate dean of faculty development at Drexel University, Philadelphia. She talked a lot about mentoring, and I was inspired to set up a mentoring program for our hospitalists. Dr. Spector graciously agreed to mentor me as I worked on my Capstone project, which was to create a mentoring program in a community-based hospitalist group. As I continued to work on the project, coincidentally our medical group decided to redesign our new physician onboarding process. Because I was already involved in the onboarding and training related to our EHR, I became very involved with our medical group's onboarding redesign.
My group's CEO decided to create a new directorship role for onboarding and mentoring, which I recently interviewed for and was offered about two months ago.
I think setting up systems to support our doctors is the common threat between the informatics and the onboarding roles. I want to implement systems that support our doctors, help them succeed, and hopefully make their jobs a little easier.
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
We practice in a very urban environment, with many low-income patients who have limited resources and access to health care. That can be very challenging. You always wonder if these patients have all the support they need after leaving the hospital. Sometimes I feel that I am just putting a band-aid on the medical problem, so to speak, but not solving the underlying issue. But it can be very rewarding during those times when the hospital and the broader community can bring our resources together to create interventions to help at-risk patients. It doesn’t happen as frequently as we would like, but when it does happen it feels good.
Another challenging aspect is related to perception. There are a lot of consultants in the hospital who view hospitalists as "house staff." That can be very frustrating, and it’s important to steer the conversations away from that perspective, and really try to establish ourselves as colleagues and peers.
How will hospital medicine change in the next decade or 2?
It’s a relatively young field, and we’re still figuring it out. I really don’t know how hospital medicine is going to change, but I do know that the field will continue to evolve, given the way U.S. health care is rapidly changing.
Do you have any advice for students and residents interested in hospital medicine?
It’s a fun way to practice medicine and I would encourage students to go into hospital medicine. It’s great for work/life balance. The advice I would give is that it is very important to get involved early in your career. Get involved in medical group or hospital committees. Stay away from the “shift mentality” – that I’m going to work my shifts and leave. That can lead to early burnout, which is a real concern in our field now. Early engagement is essential, so you can help lead these conversations at your hospital.
Infection control protects hospital staff from COVID-19, study shows
Hospital-related infections have been widely reported during the ongoing coronavirus outbreak, with healthcare professionals bearing a disproportionate risk. However, a proactive response in Hong Kong’s public hospital system appears to have bucked this trend and successfully protected both patients and staff from SARS-CoV-2, according to a study published online today in Infection Control & Hospital Epidemiology.
During the first 42 days of the outbreak, the 43 hospitals in the network tested 1275 suspected cases and treated 42 patients with confirmed COVID-19, the disease caused by SARS-CoV-2 infection. Yet, there were no nosocomial infections or infections among healthcare personnel, report Vincent C.C. Cheng, MD, FRCPath, the hospital’s infection control officer, and colleagues.
Cheng and colleagues note that 11 out of 413 healthcare workers who treat patients with confirmed infections had unprotected exposure and were in quarantine for 14 days, but none became ill.
In comparison, they note, the 2003 SARS outbreak saw almost 60% of nosocomial cases occurring in healthcare workers.
Proactive bundle
The Hong Kong success story may be due to a stepped-up proactive bundle of measures that included enhanced laboratory surveillance, early airborne infection isolation, and rapid-turnaround molecular diagnostics. Other strategies included staff forums and one-on-one discussions about infection control, employee training in protective equipment use, hand-hygiene compliance enforcement, and contact tracing for workers with unprotected exposure.
In addition, surgical masks were provided for all healthcare workers, patients, and visitors to clinical areas, a practice previously associated with reduced in-hospital transmission during influenza outbreaks, the authors note.
Hospitals also mandated use of personal protective equipment (PPE) for aerosol-generating procedures (AGPs), such as endotracheal intubation, open suctioning, and high-flow oxygen use, as AGPs had been linked to nosocomial transmission to healthcare workers during the 2003 SARS outbreak.
The infection control measures, which were part of a preparedness plan developed after the SARS outbreak, were initiated on December 31, when the first reports of a cluster of infections came from Wuhan, China.
As the outbreak evolved, the Hong Kong hospitals quickly widened the epidemiologic criteria for screening, from initially including only those who had been to a wet market in Wuhan within 14 days of symptom onset, to eventually including anyone who had been to Hubei province, been in a medical facility in mainland China, or in contact with a known case.
All suspected cases were sent to an airborne-infection isolation room (AIIR) or a ward with at least a meter of space between patients.
“Appropriate hospital infection control measures could prevent nosocomial transmission of SARS-CoV-2,” the authors write. “Vigilance in hand hygiene practice, wearing of surgical mask in the hospital, and appropriate use of PPE in patient care, especially [when] performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2 even before the availability of effective antiviral agents and vaccine.”
Asked for his perspective on the report, Aaron E. Glatt, MD, chairman of the department of medicine and chief of infectious diseases at Mount Sinai South Nassau in Oceanside, New York, said that apart from the widespread issuing of surgical masks to workers, patients, and visitors, the measures taken in Hong Kong are not different from standard infection-control practices in American hospitals. Glatt, who is also a hospital epidemiologist, said it was unclear how much impact the masks would have.
“Although the infection control was impressive, I don’t see any evidence of a difference in care,” he told Medscape Medical News.
Could zero infection transmission be achieved in the more far-flung and variable settings of hospitals across the United States? “The ability to get zero transmission is only possible if people adhere to the strictest infection-control guidelines,” Glatt said. “That is clearly the goal, and it will take time to see if our existing strict guidelines are sufficient to maintain zero or close to zero contamination and transmission rates in our hospitals.”
Rather than looking to change US practices, he stressed adherence to widely established tenets of care. “It’s critically important to keep paying close attention to the basics, to the simple blocking and tackling, and to identify which patients are at risk, and therefore, when workers need protective equipment,” he said.
“Follow the recommended standards,” continued Glatt, who is also a spokesperson for the Infectious Diseases Society of America and did not participate in this study.
In a finding from an ancillary pilot experiment, the Hong Kong researchers found exhaled air from a patient with a moderate coronavirus load showed no evidence of the virus, whether the patient was breathing normally or heavily, speaking, or coughing. And spot tests around the room detected the virus in just one location.
“We may not be able to make a definite conclusion based on the analysis of a single patient,” the authors write. “However, it may help to reassure our staff that the exhaled air may be rapidly diluted inside the AIIR with 12 air changes per hour, or probably the SARS-CoV-2 may not be predominantly transmitted by [the] airborne route.”
However, a recent Singapore study showed widespread environmental contamination by SARS-CoV-2 through respiratory droplets and fecal shedding, underlining the need for strict adherence to environmental and hand hygiene. Post-cleaning samples tested negative, suggesting that standard decontamination practices are effective.
This work was partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Education of China. The authors and Glatt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hospital-related infections have been widely reported during the ongoing coronavirus outbreak, with healthcare professionals bearing a disproportionate risk. However, a proactive response in Hong Kong’s public hospital system appears to have bucked this trend and successfully protected both patients and staff from SARS-CoV-2, according to a study published online today in Infection Control & Hospital Epidemiology.
During the first 42 days of the outbreak, the 43 hospitals in the network tested 1275 suspected cases and treated 42 patients with confirmed COVID-19, the disease caused by SARS-CoV-2 infection. Yet, there were no nosocomial infections or infections among healthcare personnel, report Vincent C.C. Cheng, MD, FRCPath, the hospital’s infection control officer, and colleagues.
Cheng and colleagues note that 11 out of 413 healthcare workers who treat patients with confirmed infections had unprotected exposure and were in quarantine for 14 days, but none became ill.
In comparison, they note, the 2003 SARS outbreak saw almost 60% of nosocomial cases occurring in healthcare workers.
Proactive bundle
The Hong Kong success story may be due to a stepped-up proactive bundle of measures that included enhanced laboratory surveillance, early airborne infection isolation, and rapid-turnaround molecular diagnostics. Other strategies included staff forums and one-on-one discussions about infection control, employee training in protective equipment use, hand-hygiene compliance enforcement, and contact tracing for workers with unprotected exposure.
In addition, surgical masks were provided for all healthcare workers, patients, and visitors to clinical areas, a practice previously associated with reduced in-hospital transmission during influenza outbreaks, the authors note.
Hospitals also mandated use of personal protective equipment (PPE) for aerosol-generating procedures (AGPs), such as endotracheal intubation, open suctioning, and high-flow oxygen use, as AGPs had been linked to nosocomial transmission to healthcare workers during the 2003 SARS outbreak.
The infection control measures, which were part of a preparedness plan developed after the SARS outbreak, were initiated on December 31, when the first reports of a cluster of infections came from Wuhan, China.
As the outbreak evolved, the Hong Kong hospitals quickly widened the epidemiologic criteria for screening, from initially including only those who had been to a wet market in Wuhan within 14 days of symptom onset, to eventually including anyone who had been to Hubei province, been in a medical facility in mainland China, or in contact with a known case.
All suspected cases were sent to an airborne-infection isolation room (AIIR) or a ward with at least a meter of space between patients.
“Appropriate hospital infection control measures could prevent nosocomial transmission of SARS-CoV-2,” the authors write. “Vigilance in hand hygiene practice, wearing of surgical mask in the hospital, and appropriate use of PPE in patient care, especially [when] performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2 even before the availability of effective antiviral agents and vaccine.”
Asked for his perspective on the report, Aaron E. Glatt, MD, chairman of the department of medicine and chief of infectious diseases at Mount Sinai South Nassau in Oceanside, New York, said that apart from the widespread issuing of surgical masks to workers, patients, and visitors, the measures taken in Hong Kong are not different from standard infection-control practices in American hospitals. Glatt, who is also a hospital epidemiologist, said it was unclear how much impact the masks would have.
“Although the infection control was impressive, I don’t see any evidence of a difference in care,” he told Medscape Medical News.
Could zero infection transmission be achieved in the more far-flung and variable settings of hospitals across the United States? “The ability to get zero transmission is only possible if people adhere to the strictest infection-control guidelines,” Glatt said. “That is clearly the goal, and it will take time to see if our existing strict guidelines are sufficient to maintain zero or close to zero contamination and transmission rates in our hospitals.”
Rather than looking to change US practices, he stressed adherence to widely established tenets of care. “It’s critically important to keep paying close attention to the basics, to the simple blocking and tackling, and to identify which patients are at risk, and therefore, when workers need protective equipment,” he said.
“Follow the recommended standards,” continued Glatt, who is also a spokesperson for the Infectious Diseases Society of America and did not participate in this study.
In a finding from an ancillary pilot experiment, the Hong Kong researchers found exhaled air from a patient with a moderate coronavirus load showed no evidence of the virus, whether the patient was breathing normally or heavily, speaking, or coughing. And spot tests around the room detected the virus in just one location.
“We may not be able to make a definite conclusion based on the analysis of a single patient,” the authors write. “However, it may help to reassure our staff that the exhaled air may be rapidly diluted inside the AIIR with 12 air changes per hour, or probably the SARS-CoV-2 may not be predominantly transmitted by [the] airborne route.”
However, a recent Singapore study showed widespread environmental contamination by SARS-CoV-2 through respiratory droplets and fecal shedding, underlining the need for strict adherence to environmental and hand hygiene. Post-cleaning samples tested negative, suggesting that standard decontamination practices are effective.
This work was partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Education of China. The authors and Glatt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hospital-related infections have been widely reported during the ongoing coronavirus outbreak, with healthcare professionals bearing a disproportionate risk. However, a proactive response in Hong Kong’s public hospital system appears to have bucked this trend and successfully protected both patients and staff from SARS-CoV-2, according to a study published online today in Infection Control & Hospital Epidemiology.
During the first 42 days of the outbreak, the 43 hospitals in the network tested 1275 suspected cases and treated 42 patients with confirmed COVID-19, the disease caused by SARS-CoV-2 infection. Yet, there were no nosocomial infections or infections among healthcare personnel, report Vincent C.C. Cheng, MD, FRCPath, the hospital’s infection control officer, and colleagues.
Cheng and colleagues note that 11 out of 413 healthcare workers who treat patients with confirmed infections had unprotected exposure and were in quarantine for 14 days, but none became ill.
In comparison, they note, the 2003 SARS outbreak saw almost 60% of nosocomial cases occurring in healthcare workers.
Proactive bundle
The Hong Kong success story may be due to a stepped-up proactive bundle of measures that included enhanced laboratory surveillance, early airborne infection isolation, and rapid-turnaround molecular diagnostics. Other strategies included staff forums and one-on-one discussions about infection control, employee training in protective equipment use, hand-hygiene compliance enforcement, and contact tracing for workers with unprotected exposure.
In addition, surgical masks were provided for all healthcare workers, patients, and visitors to clinical areas, a practice previously associated with reduced in-hospital transmission during influenza outbreaks, the authors note.
Hospitals also mandated use of personal protective equipment (PPE) for aerosol-generating procedures (AGPs), such as endotracheal intubation, open suctioning, and high-flow oxygen use, as AGPs had been linked to nosocomial transmission to healthcare workers during the 2003 SARS outbreak.
The infection control measures, which were part of a preparedness plan developed after the SARS outbreak, were initiated on December 31, when the first reports of a cluster of infections came from Wuhan, China.
As the outbreak evolved, the Hong Kong hospitals quickly widened the epidemiologic criteria for screening, from initially including only those who had been to a wet market in Wuhan within 14 days of symptom onset, to eventually including anyone who had been to Hubei province, been in a medical facility in mainland China, or in contact with a known case.
All suspected cases were sent to an airborne-infection isolation room (AIIR) or a ward with at least a meter of space between patients.
“Appropriate hospital infection control measures could prevent nosocomial transmission of SARS-CoV-2,” the authors write. “Vigilance in hand hygiene practice, wearing of surgical mask in the hospital, and appropriate use of PPE in patient care, especially [when] performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2 even before the availability of effective antiviral agents and vaccine.”
Asked for his perspective on the report, Aaron E. Glatt, MD, chairman of the department of medicine and chief of infectious diseases at Mount Sinai South Nassau in Oceanside, New York, said that apart from the widespread issuing of surgical masks to workers, patients, and visitors, the measures taken in Hong Kong are not different from standard infection-control practices in American hospitals. Glatt, who is also a hospital epidemiologist, said it was unclear how much impact the masks would have.
“Although the infection control was impressive, I don’t see any evidence of a difference in care,” he told Medscape Medical News.
Could zero infection transmission be achieved in the more far-flung and variable settings of hospitals across the United States? “The ability to get zero transmission is only possible if people adhere to the strictest infection-control guidelines,” Glatt said. “That is clearly the goal, and it will take time to see if our existing strict guidelines are sufficient to maintain zero or close to zero contamination and transmission rates in our hospitals.”
Rather than looking to change US practices, he stressed adherence to widely established tenets of care. “It’s critically important to keep paying close attention to the basics, to the simple blocking and tackling, and to identify which patients are at risk, and therefore, when workers need protective equipment,” he said.
“Follow the recommended standards,” continued Glatt, who is also a spokesperson for the Infectious Diseases Society of America and did not participate in this study.
In a finding from an ancillary pilot experiment, the Hong Kong researchers found exhaled air from a patient with a moderate coronavirus load showed no evidence of the virus, whether the patient was breathing normally or heavily, speaking, or coughing. And spot tests around the room detected the virus in just one location.
“We may not be able to make a definite conclusion based on the analysis of a single patient,” the authors write. “However, it may help to reassure our staff that the exhaled air may be rapidly diluted inside the AIIR with 12 air changes per hour, or probably the SARS-CoV-2 may not be predominantly transmitted by [the] airborne route.”
However, a recent Singapore study showed widespread environmental contamination by SARS-CoV-2 through respiratory droplets and fecal shedding, underlining the need for strict adherence to environmental and hand hygiene. Post-cleaning samples tested negative, suggesting that standard decontamination practices are effective.
This work was partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Education of China. The authors and Glatt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
CMS issues guidance on containing spread of coronavirus
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
AFib patients do best on a DOAC started 7-10 days post stroke
LOS ANGELES – When a patient with atrial fibrillation (AFib) has a cardioembolic stroke, the best blood thinner to start may be a direct-acting oral anticoagulant (DOAC), possibly beginning 7-10 days after the index stroke, according to an analysis of 90-day, observational outcomes data from nearly 1,300 patients.
The analysis also suggested that the use of “bridging” anticoagulant treatment by injection before a patient with atrial fibrillation (AFib) starts a daily oral anticoagulant regimen following a cardioembolic stroke is not a good idea. Patients who received bridging anticoagulation had a nearly threefold higher rate of symptomatic intracranial hemorrhage than did patients who did not, and their bridging treatment failed to protect them from recurrent ischemic events, Shadi Yaghi, MD, said at the International Stroke Conference, sponsored by the American Heart Association. The bridging regimens delivered either heparin or low-molecular-weight heparin.
Based on the findings, “it seems reasonable to avoid bridging unless absolutely necessary, to initiate a DOAC unless it’s contraindicated, and to start the DOAC on day 7-10 following the stroke in most patients,” said Dr. Yaghi, a vascular neurologist and director of stroke research at NYU Langone Health in New York.
“It’s been hard to develop a broad guideline on when to start oral anticoagulation” after a cardioembolic stroke in AFib patients. The best time “depends on a lot of variables and how the patient responded to acute treatment,” commented Alexis Simpkins, MD, a vascular and stroke neurologist at the University of Florida in Gainesville. “You want to start treatment before the patient has another stroke, but not so soon that the treatment causes symptomatic hemorrhagic transformation.”
Dr. Yaghi’s suggestion, based on his findings, to start treatment for most patients with a DOAC 7-10 days after their index stroke “shows consistency” with the prevailing guideline recommendation from the AHA/American Stroke Association to start oral anticoagulation in this patient population 4-14 days after the index stroke (Stroke. 2018 March;49[3]:e46-e99), she noted.
A recent article reviewed the uncertainty about the best time to start oral anticoagulation in AFib patients after a cardioembolic stroke and the subtle differences that distinguish various international medical groups that, like the ASA, have made recommendations (Lancet Neurol. 2019 Jan 1;18[1]:117-26). According to this review, a major limitation of these various recommendations has been the lack of actual evidence collected from AFib patients who began receiving a DOAC shortly after a cardioembolic stroke, although the article added that several studies in progress are collecting these data.
The study reported by Dr. Yaghi pooled data collected from 2,084 recent AFib patients with a cardioembolic stroke treated at any of eight comprehensive U.S. stroke centers. They excluded patients who died from causes unrelated to the primary endpoint, those who did not receive an anticoagulant or had incomplete data, and patients lost to follow-up, leaving 1,289 evaluable patients. During their 90-day follow-up, 10% of the patients had an ischemic event, a symptomatic intracranial hemorrhage, or an extracranial hemorrhage.
The study’s primary analysis showed no statistically significant difference in the incidence of recurrent ischemic events, symptomatic intracranial hemorrhage, or both based on when oral anticoagulant treatment began: 0-3 days, 4-14 days, or more than 14 days after the index stroke.
The investigators then subdivided patients into the subgroup that started treatment with a DOAC and the subgroup that started treatment with warfarin and also further subdivided the 4-14 day time window for starting treatment. Results of this analysis showed that patients who received a DOAC and began this treatment 7-10 days after their stroke had a 50% cut in their 90-day events compared with other patients, a difference that fell just short of statistical significance at P = .07. All the other combinations of oral anticoagulant and time of treatment initiation analyzed showed neutral effects that never came near statistical significance.
Secondary data analyses also showed that both patients with a history of a stroke prior to their index stroke and patients with ipsilateral atherosclerosis came close to having a statistically significant increased rate of a subsequent ischemic event during 90-day follow-up. Furthermore, women, patients with a history of hyperlipidemia, and patients who developed hemorrhagic transformation of their index stroke all had significantly increased rates of developing a symptomatic intracranial hemorrhage during 90-day follow-up. When the endpoint was limited to recurrent ischemic events only, patients who received a DOAC were 50% less likely to have an event than were patients treated with warfarin, a statistically significant difference.
Although starting a DOAC 7-10 days after the index stroke seems reasonable based on this analysis, the question needs a prospective, randomized study to create an appropriate evidence base, Dr. Yaghi said.
Dr. Yaghi disclosed a financial relationship with Medtronic. Dr. Simpkins had no disclosures.
SOURCE: Yaghi S et al. Stroke. 2020 Feb;51(suppl 1):A119.
LOS ANGELES – When a patient with atrial fibrillation (AFib) has a cardioembolic stroke, the best blood thinner to start may be a direct-acting oral anticoagulant (DOAC), possibly beginning 7-10 days after the index stroke, according to an analysis of 90-day, observational outcomes data from nearly 1,300 patients.
The analysis also suggested that the use of “bridging” anticoagulant treatment by injection before a patient with atrial fibrillation (AFib) starts a daily oral anticoagulant regimen following a cardioembolic stroke is not a good idea. Patients who received bridging anticoagulation had a nearly threefold higher rate of symptomatic intracranial hemorrhage than did patients who did not, and their bridging treatment failed to protect them from recurrent ischemic events, Shadi Yaghi, MD, said at the International Stroke Conference, sponsored by the American Heart Association. The bridging regimens delivered either heparin or low-molecular-weight heparin.
Based on the findings, “it seems reasonable to avoid bridging unless absolutely necessary, to initiate a DOAC unless it’s contraindicated, and to start the DOAC on day 7-10 following the stroke in most patients,” said Dr. Yaghi, a vascular neurologist and director of stroke research at NYU Langone Health in New York.
“It’s been hard to develop a broad guideline on when to start oral anticoagulation” after a cardioembolic stroke in AFib patients. The best time “depends on a lot of variables and how the patient responded to acute treatment,” commented Alexis Simpkins, MD, a vascular and stroke neurologist at the University of Florida in Gainesville. “You want to start treatment before the patient has another stroke, but not so soon that the treatment causes symptomatic hemorrhagic transformation.”
Dr. Yaghi’s suggestion, based on his findings, to start treatment for most patients with a DOAC 7-10 days after their index stroke “shows consistency” with the prevailing guideline recommendation from the AHA/American Stroke Association to start oral anticoagulation in this patient population 4-14 days after the index stroke (Stroke. 2018 March;49[3]:e46-e99), she noted.
A recent article reviewed the uncertainty about the best time to start oral anticoagulation in AFib patients after a cardioembolic stroke and the subtle differences that distinguish various international medical groups that, like the ASA, have made recommendations (Lancet Neurol. 2019 Jan 1;18[1]:117-26). According to this review, a major limitation of these various recommendations has been the lack of actual evidence collected from AFib patients who began receiving a DOAC shortly after a cardioembolic stroke, although the article added that several studies in progress are collecting these data.
The study reported by Dr. Yaghi pooled data collected from 2,084 recent AFib patients with a cardioembolic stroke treated at any of eight comprehensive U.S. stroke centers. They excluded patients who died from causes unrelated to the primary endpoint, those who did not receive an anticoagulant or had incomplete data, and patients lost to follow-up, leaving 1,289 evaluable patients. During their 90-day follow-up, 10% of the patients had an ischemic event, a symptomatic intracranial hemorrhage, or an extracranial hemorrhage.
The study’s primary analysis showed no statistically significant difference in the incidence of recurrent ischemic events, symptomatic intracranial hemorrhage, or both based on when oral anticoagulant treatment began: 0-3 days, 4-14 days, or more than 14 days after the index stroke.
The investigators then subdivided patients into the subgroup that started treatment with a DOAC and the subgroup that started treatment with warfarin and also further subdivided the 4-14 day time window for starting treatment. Results of this analysis showed that patients who received a DOAC and began this treatment 7-10 days after their stroke had a 50% cut in their 90-day events compared with other patients, a difference that fell just short of statistical significance at P = .07. All the other combinations of oral anticoagulant and time of treatment initiation analyzed showed neutral effects that never came near statistical significance.
Secondary data analyses also showed that both patients with a history of a stroke prior to their index stroke and patients with ipsilateral atherosclerosis came close to having a statistically significant increased rate of a subsequent ischemic event during 90-day follow-up. Furthermore, women, patients with a history of hyperlipidemia, and patients who developed hemorrhagic transformation of their index stroke all had significantly increased rates of developing a symptomatic intracranial hemorrhage during 90-day follow-up. When the endpoint was limited to recurrent ischemic events only, patients who received a DOAC were 50% less likely to have an event than were patients treated with warfarin, a statistically significant difference.
Although starting a DOAC 7-10 days after the index stroke seems reasonable based on this analysis, the question needs a prospective, randomized study to create an appropriate evidence base, Dr. Yaghi said.
Dr. Yaghi disclosed a financial relationship with Medtronic. Dr. Simpkins had no disclosures.
SOURCE: Yaghi S et al. Stroke. 2020 Feb;51(suppl 1):A119.
LOS ANGELES – When a patient with atrial fibrillation (AFib) has a cardioembolic stroke, the best blood thinner to start may be a direct-acting oral anticoagulant (DOAC), possibly beginning 7-10 days after the index stroke, according to an analysis of 90-day, observational outcomes data from nearly 1,300 patients.
The analysis also suggested that the use of “bridging” anticoagulant treatment by injection before a patient with atrial fibrillation (AFib) starts a daily oral anticoagulant regimen following a cardioembolic stroke is not a good idea. Patients who received bridging anticoagulation had a nearly threefold higher rate of symptomatic intracranial hemorrhage than did patients who did not, and their bridging treatment failed to protect them from recurrent ischemic events, Shadi Yaghi, MD, said at the International Stroke Conference, sponsored by the American Heart Association. The bridging regimens delivered either heparin or low-molecular-weight heparin.
Based on the findings, “it seems reasonable to avoid bridging unless absolutely necessary, to initiate a DOAC unless it’s contraindicated, and to start the DOAC on day 7-10 following the stroke in most patients,” said Dr. Yaghi, a vascular neurologist and director of stroke research at NYU Langone Health in New York.
“It’s been hard to develop a broad guideline on when to start oral anticoagulation” after a cardioembolic stroke in AFib patients. The best time “depends on a lot of variables and how the patient responded to acute treatment,” commented Alexis Simpkins, MD, a vascular and stroke neurologist at the University of Florida in Gainesville. “You want to start treatment before the patient has another stroke, but not so soon that the treatment causes symptomatic hemorrhagic transformation.”
Dr. Yaghi’s suggestion, based on his findings, to start treatment for most patients with a DOAC 7-10 days after their index stroke “shows consistency” with the prevailing guideline recommendation from the AHA/American Stroke Association to start oral anticoagulation in this patient population 4-14 days after the index stroke (Stroke. 2018 March;49[3]:e46-e99), she noted.
A recent article reviewed the uncertainty about the best time to start oral anticoagulation in AFib patients after a cardioembolic stroke and the subtle differences that distinguish various international medical groups that, like the ASA, have made recommendations (Lancet Neurol. 2019 Jan 1;18[1]:117-26). According to this review, a major limitation of these various recommendations has been the lack of actual evidence collected from AFib patients who began receiving a DOAC shortly after a cardioembolic stroke, although the article added that several studies in progress are collecting these data.
The study reported by Dr. Yaghi pooled data collected from 2,084 recent AFib patients with a cardioembolic stroke treated at any of eight comprehensive U.S. stroke centers. They excluded patients who died from causes unrelated to the primary endpoint, those who did not receive an anticoagulant or had incomplete data, and patients lost to follow-up, leaving 1,289 evaluable patients. During their 90-day follow-up, 10% of the patients had an ischemic event, a symptomatic intracranial hemorrhage, or an extracranial hemorrhage.
The study’s primary analysis showed no statistically significant difference in the incidence of recurrent ischemic events, symptomatic intracranial hemorrhage, or both based on when oral anticoagulant treatment began: 0-3 days, 4-14 days, or more than 14 days after the index stroke.
The investigators then subdivided patients into the subgroup that started treatment with a DOAC and the subgroup that started treatment with warfarin and also further subdivided the 4-14 day time window for starting treatment. Results of this analysis showed that patients who received a DOAC and began this treatment 7-10 days after their stroke had a 50% cut in their 90-day events compared with other patients, a difference that fell just short of statistical significance at P = .07. All the other combinations of oral anticoagulant and time of treatment initiation analyzed showed neutral effects that never came near statistical significance.
Secondary data analyses also showed that both patients with a history of a stroke prior to their index stroke and patients with ipsilateral atherosclerosis came close to having a statistically significant increased rate of a subsequent ischemic event during 90-day follow-up. Furthermore, women, patients with a history of hyperlipidemia, and patients who developed hemorrhagic transformation of their index stroke all had significantly increased rates of developing a symptomatic intracranial hemorrhage during 90-day follow-up. When the endpoint was limited to recurrent ischemic events only, patients who received a DOAC were 50% less likely to have an event than were patients treated with warfarin, a statistically significant difference.
Although starting a DOAC 7-10 days after the index stroke seems reasonable based on this analysis, the question needs a prospective, randomized study to create an appropriate evidence base, Dr. Yaghi said.
Dr. Yaghi disclosed a financial relationship with Medtronic. Dr. Simpkins had no disclosures.
SOURCE: Yaghi S et al. Stroke. 2020 Feb;51(suppl 1):A119.
REPORTING FROM ISC 2020
MACE benefits with dapagliflozin improve with disease duration
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
FROM DIABETES, OBESITY AND METABOLISM
HM20 course director influenced by POCUS, global health
Dr. Benji Mathews praises mentors for his SHM roles
Benji K. Mathews, MD, SFHM, CLHM, is chief of hospital medicine at Regions Hospital in St. Paul, Minn., and director of point of care ultrasound (POCUS) for hospital medicine at HealthPartners. He is also the course director for the Society of Hospital Medicine’s 2020 Annual Conference (HM20), to be held April 16-18 in San Diego.
Dr. Mathews, an associate professor of medicine at the University of Minnesota, Minneapolis, is recognized by fellow hospitalists as a pioneer in the use of bedside ultrasound. In fact, his Certificate of Leadership in Hospital Medicine (CLHM) was completed with a focus on ultrasound in hospital medicine, and he is a Fellow in Diagnostic Safety through the Society to Improve Diagnosis in Medicine. “While a resident, I took an interest in the field of improving diagnosis and combined it with the 21st-century innovative tool of bedside ultrasound,” he said. “Now, I continue to teach clinicians, educators, and learners.”
In addition to his interest in POCUS and medical education, Dr. Mathews also has a passion for global health, rooted in a commitment to reducing health care disparities both locally and globally. He has worked with medical missions, nongovernmental organizations, and orphanages in Nepal, India, Bolivia, Honduras, and Costa Rica. This led him to complete the global health course at the University of Minnesota.
Dr. Mathews spent a few minutes with The Hospitalist to discuss his background and his new role of course director of the HM20 Annual Conference.
Can you describe your journey to becoming a hospitalist?
I’ve been a hospitalist for most of the last decade. I was fortunate to be a part of a great residency program at the University of Minnesota Medical School, which started a hospital medicine pathway that had several nationally recognized hospital medicine leaders as mentors. I was lucky to work with several of them through the HealthPartners organization in Saint Paul, and that developed in me a further desire to practice hospital medicine. The group and mentors provided opportunities to develop further niches in my practice, like bedside ultrasound.
How did you first get involved with SHM?
I entered SHM through the influence of mentors at HealthPartners, especially Burke Kealey, MD, SFHM, senior medical director for hospital specialties at HealthPartners Medical Group in Bloomington, Minn. and a past president of the Society, who encouraged me to participate on SHM committees. I eventually applied for the Annual Conference Committee, and somehow was accepted.
At that time, I was a community hospitalist among a lot of academic hospitalists. I thought that my voice could probably diversify the conversation, and bring the perspective of an early-career hospitalist to the discussion around educational offerings at the Annual Conference. I benefited from good mentorship on that committee, and with that experience I started getting involved with our local chapter in Minnesota. That was very important. I became our local chapter president and was able to combine my efforts with SHM nationally with our regional initiatives.
You have a particular interest in point-of-care ultrasound for hospitalists. How did that make its way into your involvement with SHM?
Point-of-care ultrasound and diagnostic error work really took off when I was a resident. My interest in that funneled naturally into the base curriculum of the Annual Conference, where once a year I could come together with 18 of my best hospitalist friends from across the nation to discuss curriculum. We talk about what content is applicable for frontline clinicians, what is right for early learners, and what innovations are coming in the future. Toward that last point, I was always involved as a judge or volunteer for the Research, Innovations and Clinical Vignettes – or RIV – competition at the Annual Conference. That’s the scientific abstract and poster competition at the conference. My interest grew to a point at which I decided to apply for one of the leadership roles in the RIV. I had the opportunity to serve as an Innovations Lead at RIV one year, and then chaired the overall RIV competition. Those opportunities helped me better understand the cutting-edge research that hospitalists should be aware of and which researchers and clinicians we should be in conversation with.
All these roles together have led me to my service as HM20 course director. I see myself as a lucky guy who has benefited from great mentorship, and I want to take advantage of my opportunities to serve.
We’ve been told that your elementary school–age children have learned to use ultrasound!
Well, they’ve learned how to use handheld ultrasound devices on each other. They’re able to find their siblings’ kidneys and hearts. I often show an image of this to encourage hospitalists that, if children can pick it up, highly educated providers can do the same and more.
To register for the Society of Hospital Medicine’s 2020 Annual Conference, please visit the HM20 Registration page.
Dr. Benji Mathews praises mentors for his SHM roles
Dr. Benji Mathews praises mentors for his SHM roles
Benji K. Mathews, MD, SFHM, CLHM, is chief of hospital medicine at Regions Hospital in St. Paul, Minn., and director of point of care ultrasound (POCUS) for hospital medicine at HealthPartners. He is also the course director for the Society of Hospital Medicine’s 2020 Annual Conference (HM20), to be held April 16-18 in San Diego.
Dr. Mathews, an associate professor of medicine at the University of Minnesota, Minneapolis, is recognized by fellow hospitalists as a pioneer in the use of bedside ultrasound. In fact, his Certificate of Leadership in Hospital Medicine (CLHM) was completed with a focus on ultrasound in hospital medicine, and he is a Fellow in Diagnostic Safety through the Society to Improve Diagnosis in Medicine. “While a resident, I took an interest in the field of improving diagnosis and combined it with the 21st-century innovative tool of bedside ultrasound,” he said. “Now, I continue to teach clinicians, educators, and learners.”
In addition to his interest in POCUS and medical education, Dr. Mathews also has a passion for global health, rooted in a commitment to reducing health care disparities both locally and globally. He has worked with medical missions, nongovernmental organizations, and orphanages in Nepal, India, Bolivia, Honduras, and Costa Rica. This led him to complete the global health course at the University of Minnesota.
Dr. Mathews spent a few minutes with The Hospitalist to discuss his background and his new role of course director of the HM20 Annual Conference.
Can you describe your journey to becoming a hospitalist?
I’ve been a hospitalist for most of the last decade. I was fortunate to be a part of a great residency program at the University of Minnesota Medical School, which started a hospital medicine pathway that had several nationally recognized hospital medicine leaders as mentors. I was lucky to work with several of them through the HealthPartners organization in Saint Paul, and that developed in me a further desire to practice hospital medicine. The group and mentors provided opportunities to develop further niches in my practice, like bedside ultrasound.
How did you first get involved with SHM?
I entered SHM through the influence of mentors at HealthPartners, especially Burke Kealey, MD, SFHM, senior medical director for hospital specialties at HealthPartners Medical Group in Bloomington, Minn. and a past president of the Society, who encouraged me to participate on SHM committees. I eventually applied for the Annual Conference Committee, and somehow was accepted.
At that time, I was a community hospitalist among a lot of academic hospitalists. I thought that my voice could probably diversify the conversation, and bring the perspective of an early-career hospitalist to the discussion around educational offerings at the Annual Conference. I benefited from good mentorship on that committee, and with that experience I started getting involved with our local chapter in Minnesota. That was very important. I became our local chapter president and was able to combine my efforts with SHM nationally with our regional initiatives.
You have a particular interest in point-of-care ultrasound for hospitalists. How did that make its way into your involvement with SHM?
Point-of-care ultrasound and diagnostic error work really took off when I was a resident. My interest in that funneled naturally into the base curriculum of the Annual Conference, where once a year I could come together with 18 of my best hospitalist friends from across the nation to discuss curriculum. We talk about what content is applicable for frontline clinicians, what is right for early learners, and what innovations are coming in the future. Toward that last point, I was always involved as a judge or volunteer for the Research, Innovations and Clinical Vignettes – or RIV – competition at the Annual Conference. That’s the scientific abstract and poster competition at the conference. My interest grew to a point at which I decided to apply for one of the leadership roles in the RIV. I had the opportunity to serve as an Innovations Lead at RIV one year, and then chaired the overall RIV competition. Those opportunities helped me better understand the cutting-edge research that hospitalists should be aware of and which researchers and clinicians we should be in conversation with.
All these roles together have led me to my service as HM20 course director. I see myself as a lucky guy who has benefited from great mentorship, and I want to take advantage of my opportunities to serve.
We’ve been told that your elementary school–age children have learned to use ultrasound!
Well, they’ve learned how to use handheld ultrasound devices on each other. They’re able to find their siblings’ kidneys and hearts. I often show an image of this to encourage hospitalists that, if children can pick it up, highly educated providers can do the same and more.
To register for the Society of Hospital Medicine’s 2020 Annual Conference, please visit the HM20 Registration page.
Benji K. Mathews, MD, SFHM, CLHM, is chief of hospital medicine at Regions Hospital in St. Paul, Minn., and director of point of care ultrasound (POCUS) for hospital medicine at HealthPartners. He is also the course director for the Society of Hospital Medicine’s 2020 Annual Conference (HM20), to be held April 16-18 in San Diego.
Dr. Mathews, an associate professor of medicine at the University of Minnesota, Minneapolis, is recognized by fellow hospitalists as a pioneer in the use of bedside ultrasound. In fact, his Certificate of Leadership in Hospital Medicine (CLHM) was completed with a focus on ultrasound in hospital medicine, and he is a Fellow in Diagnostic Safety through the Society to Improve Diagnosis in Medicine. “While a resident, I took an interest in the field of improving diagnosis and combined it with the 21st-century innovative tool of bedside ultrasound,” he said. “Now, I continue to teach clinicians, educators, and learners.”
In addition to his interest in POCUS and medical education, Dr. Mathews also has a passion for global health, rooted in a commitment to reducing health care disparities both locally and globally. He has worked with medical missions, nongovernmental organizations, and orphanages in Nepal, India, Bolivia, Honduras, and Costa Rica. This led him to complete the global health course at the University of Minnesota.
Dr. Mathews spent a few minutes with The Hospitalist to discuss his background and his new role of course director of the HM20 Annual Conference.
Can you describe your journey to becoming a hospitalist?
I’ve been a hospitalist for most of the last decade. I was fortunate to be a part of a great residency program at the University of Minnesota Medical School, which started a hospital medicine pathway that had several nationally recognized hospital medicine leaders as mentors. I was lucky to work with several of them through the HealthPartners organization in Saint Paul, and that developed in me a further desire to practice hospital medicine. The group and mentors provided opportunities to develop further niches in my practice, like bedside ultrasound.
How did you first get involved with SHM?
I entered SHM through the influence of mentors at HealthPartners, especially Burke Kealey, MD, SFHM, senior medical director for hospital specialties at HealthPartners Medical Group in Bloomington, Minn. and a past president of the Society, who encouraged me to participate on SHM committees. I eventually applied for the Annual Conference Committee, and somehow was accepted.
At that time, I was a community hospitalist among a lot of academic hospitalists. I thought that my voice could probably diversify the conversation, and bring the perspective of an early-career hospitalist to the discussion around educational offerings at the Annual Conference. I benefited from good mentorship on that committee, and with that experience I started getting involved with our local chapter in Minnesota. That was very important. I became our local chapter president and was able to combine my efforts with SHM nationally with our regional initiatives.
You have a particular interest in point-of-care ultrasound for hospitalists. How did that make its way into your involvement with SHM?
Point-of-care ultrasound and diagnostic error work really took off when I was a resident. My interest in that funneled naturally into the base curriculum of the Annual Conference, where once a year I could come together with 18 of my best hospitalist friends from across the nation to discuss curriculum. We talk about what content is applicable for frontline clinicians, what is right for early learners, and what innovations are coming in the future. Toward that last point, I was always involved as a judge or volunteer for the Research, Innovations and Clinical Vignettes – or RIV – competition at the Annual Conference. That’s the scientific abstract and poster competition at the conference. My interest grew to a point at which I decided to apply for one of the leadership roles in the RIV. I had the opportunity to serve as an Innovations Lead at RIV one year, and then chaired the overall RIV competition. Those opportunities helped me better understand the cutting-edge research that hospitalists should be aware of and which researchers and clinicians we should be in conversation with.
All these roles together have led me to my service as HM20 course director. I see myself as a lucky guy who has benefited from great mentorship, and I want to take advantage of my opportunities to serve.
We’ve been told that your elementary school–age children have learned to use ultrasound!
Well, they’ve learned how to use handheld ultrasound devices on each other. They’re able to find their siblings’ kidneys and hearts. I often show an image of this to encourage hospitalists that, if children can pick it up, highly educated providers can do the same and more.
To register for the Society of Hospital Medicine’s 2020 Annual Conference, please visit the HM20 Registration page.
















