User login
Children and COVID: New cases soar to near-record level
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.
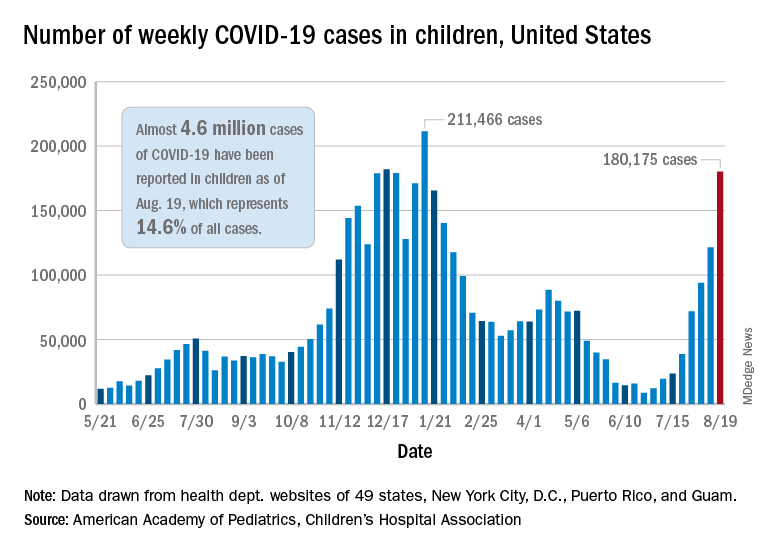
The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.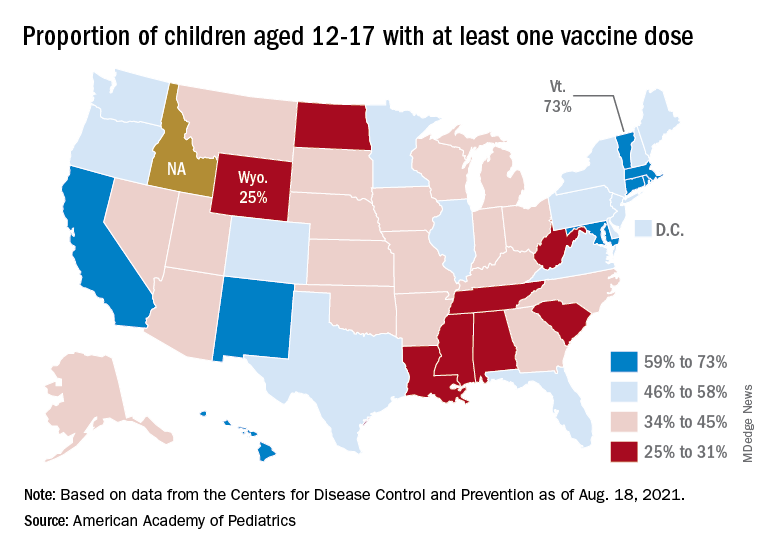
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
U.S. kidney transplants grow in number and success
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
CRP as a biomarker for community-acquired pneumonia
Background: In the United States, CAP was responsible for nearly 50,000 deaths in 2017. Prompt and accurate diagnosis promotes early treatment and avoids unnecessary antibiotic treatment for nonpneumonia lower respiratory tract infection patients. Diagnosis is based on signs and symptoms, as well as available imaging. Inflammatory markers such as CRP, white blood cell count, and procalcitonin are readily available in the ED and outpatient settings.
Study design: Bivariate meta-analysis.
Setting: A systematic review of literature was done via PubMed search to identify prospective studies evaluating the accuracy of biomarkers in patients with cough or suspected CAP.
Synopsis: Fourteen studies met the criteria to be included in the meta-analysis. Summary receiver operating characteristic (ROC) curves generated reported area under the curve of 0.802 for CRP (95% confidence interval, 0.78-0.85), 0.777 for leukocytosis (95% CI, 0.74-0.81), and 0.771 for procalcitonin (95% CI, 0.74-0.81). The combination of CRP greater than 49.5 mg/L and procalcitonin greater than 0.1 mcg/L had a positive likelihood ratio of 2.24 and a negative likelihood ratio of 0.44.
The study had a some of limitations. The blinding of the person performing the index test to the reference standard and vice versa was not clear. Further, it was unclear if the person interpreting the reference standard was blinded to the index test in five studies and absent in one. Other limitations were inconsistent reporting of abnormal post hoc cutoffs and only two biomarkers being reported in a single study.
Combining a biomarker with signs and symptoms has the potential to improve diagnostic accuracy in the outpatient setting further. CRP was found to be most accurate regardless of the cutoff used; however, further studies without threshold effect will prove beneficial.
Bottom line: CRP is a more accurate and useful biomarker for outpatient CAP diagnosis than procalcitonin or leukocytosis.
Citation: Ebell MH et al. Accuracy of biomarkers for the diagnosis of adult community-acquired pneumonia: A meta-analysis. Acad Emerg Med. 2020;27(3):195-206.
Dr. Castellanos is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: In the United States, CAP was responsible for nearly 50,000 deaths in 2017. Prompt and accurate diagnosis promotes early treatment and avoids unnecessary antibiotic treatment for nonpneumonia lower respiratory tract infection patients. Diagnosis is based on signs and symptoms, as well as available imaging. Inflammatory markers such as CRP, white blood cell count, and procalcitonin are readily available in the ED and outpatient settings.
Study design: Bivariate meta-analysis.
Setting: A systematic review of literature was done via PubMed search to identify prospective studies evaluating the accuracy of biomarkers in patients with cough or suspected CAP.
Synopsis: Fourteen studies met the criteria to be included in the meta-analysis. Summary receiver operating characteristic (ROC) curves generated reported area under the curve of 0.802 for CRP (95% confidence interval, 0.78-0.85), 0.777 for leukocytosis (95% CI, 0.74-0.81), and 0.771 for procalcitonin (95% CI, 0.74-0.81). The combination of CRP greater than 49.5 mg/L and procalcitonin greater than 0.1 mcg/L had a positive likelihood ratio of 2.24 and a negative likelihood ratio of 0.44.
The study had a some of limitations. The blinding of the person performing the index test to the reference standard and vice versa was not clear. Further, it was unclear if the person interpreting the reference standard was blinded to the index test in five studies and absent in one. Other limitations were inconsistent reporting of abnormal post hoc cutoffs and only two biomarkers being reported in a single study.
Combining a biomarker with signs and symptoms has the potential to improve diagnostic accuracy in the outpatient setting further. CRP was found to be most accurate regardless of the cutoff used; however, further studies without threshold effect will prove beneficial.
Bottom line: CRP is a more accurate and useful biomarker for outpatient CAP diagnosis than procalcitonin or leukocytosis.
Citation: Ebell MH et al. Accuracy of biomarkers for the diagnosis of adult community-acquired pneumonia: A meta-analysis. Acad Emerg Med. 2020;27(3):195-206.
Dr. Castellanos is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: In the United States, CAP was responsible for nearly 50,000 deaths in 2017. Prompt and accurate diagnosis promotes early treatment and avoids unnecessary antibiotic treatment for nonpneumonia lower respiratory tract infection patients. Diagnosis is based on signs and symptoms, as well as available imaging. Inflammatory markers such as CRP, white blood cell count, and procalcitonin are readily available in the ED and outpatient settings.
Study design: Bivariate meta-analysis.
Setting: A systematic review of literature was done via PubMed search to identify prospective studies evaluating the accuracy of biomarkers in patients with cough or suspected CAP.
Synopsis: Fourteen studies met the criteria to be included in the meta-analysis. Summary receiver operating characteristic (ROC) curves generated reported area under the curve of 0.802 for CRP (95% confidence interval, 0.78-0.85), 0.777 for leukocytosis (95% CI, 0.74-0.81), and 0.771 for procalcitonin (95% CI, 0.74-0.81). The combination of CRP greater than 49.5 mg/L and procalcitonin greater than 0.1 mcg/L had a positive likelihood ratio of 2.24 and a negative likelihood ratio of 0.44.
The study had a some of limitations. The blinding of the person performing the index test to the reference standard and vice versa was not clear. Further, it was unclear if the person interpreting the reference standard was blinded to the index test in five studies and absent in one. Other limitations were inconsistent reporting of abnormal post hoc cutoffs and only two biomarkers being reported in a single study.
Combining a biomarker with signs and symptoms has the potential to improve diagnostic accuracy in the outpatient setting further. CRP was found to be most accurate regardless of the cutoff used; however, further studies without threshold effect will prove beneficial.
Bottom line: CRP is a more accurate and useful biomarker for outpatient CAP diagnosis than procalcitonin or leukocytosis.
Citation: Ebell MH et al. Accuracy of biomarkers for the diagnosis of adult community-acquired pneumonia: A meta-analysis. Acad Emerg Med. 2020;27(3):195-206.
Dr. Castellanos is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Health care workers eager for COVID booster shots
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.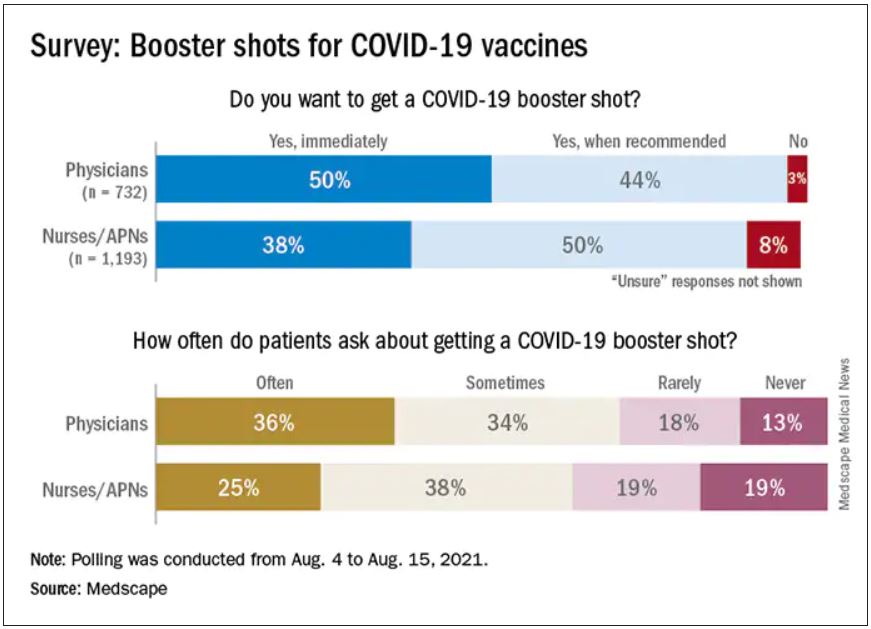
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
SGLT2 inhibitor use rising in patients with DKD
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
FROM DIABETES CARE
Apixaban more effective, safer than rivaroxaban for Afib patients
Background: Direct oral anticoagulants have proven to be more efficacious, safe, and easy to use, compared with warfarin, in patients with atrial fibrillation (Afib). An indirect comparison showed apixaban to be more effective and safer than rivaroxaban. But randomized controlled trials and head-to-head comparison data regarding the same have been lacking until now.
Study design: Retrospective cohort study.
Setting: A U.S. nationwide commercial health care claims database was searched for persons older than 18 years, with a new diagnosis of atrial fibrillation or flutter who were started on apixaban or rivaroxaban from Dec. 28, 2012, to Jan. 1, 2019.
Synopsis: Optum Clinformatics was used to identify a total of 99,878 patients who were eligible for the analysis. Of these patients, 39,531 newly prescribed apixaban patients were propensity score matched to 39,351 newly prescribed rivaroxaban patients. After propensity score matching, the study found ischemic stroke or systemic embolism rate for new apixaban users to be 6.6 events per 1,000 person-years versus 8.0 events per 1,000 person-years for new rivaroxaban users (hazard ratio, 0.82; 95% confidence interval, 0.68-0.98). The rate of major bleeding after propensity score matching was 12.9 per 1,000 person-years for new apixaban users versus 21.9 per 1,000 person-years for new rivaroxaban users (HR, 0.58; 95% CI, 0.52-0.66).
This observational study has several limitations including an inability to balance unmeasured confounding factors, both ICD-9 and ICD-10 codes being used for defined outcomes, an inability to account for time-varying confounders for stroke or bleeding, an inability to capture patients from locations other than primary internist and cardiologists, and a shorter follow-up period, compared with that of clinical trials.
Bottom line: In routine practice, apixaban is more effective and safer than rivaroxaban with a lower rate of strokes, systemic embolism, and major bleeding.
Citation: Fralick M et al. Effectiveness and safety of apixaban compared with rivaroxaban for patients with atrial fibrillation in routine practice: a cohort study. Ann Intern Med. 2020 Apr 7. doi: 10.7326/M19-2522.
Dr. Almagdub is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Direct oral anticoagulants have proven to be more efficacious, safe, and easy to use, compared with warfarin, in patients with atrial fibrillation (Afib). An indirect comparison showed apixaban to be more effective and safer than rivaroxaban. But randomized controlled trials and head-to-head comparison data regarding the same have been lacking until now.
Study design: Retrospective cohort study.
Setting: A U.S. nationwide commercial health care claims database was searched for persons older than 18 years, with a new diagnosis of atrial fibrillation or flutter who were started on apixaban or rivaroxaban from Dec. 28, 2012, to Jan. 1, 2019.
Synopsis: Optum Clinformatics was used to identify a total of 99,878 patients who were eligible for the analysis. Of these patients, 39,531 newly prescribed apixaban patients were propensity score matched to 39,351 newly prescribed rivaroxaban patients. After propensity score matching, the study found ischemic stroke or systemic embolism rate for new apixaban users to be 6.6 events per 1,000 person-years versus 8.0 events per 1,000 person-years for new rivaroxaban users (hazard ratio, 0.82; 95% confidence interval, 0.68-0.98). The rate of major bleeding after propensity score matching was 12.9 per 1,000 person-years for new apixaban users versus 21.9 per 1,000 person-years for new rivaroxaban users (HR, 0.58; 95% CI, 0.52-0.66).
This observational study has several limitations including an inability to balance unmeasured confounding factors, both ICD-9 and ICD-10 codes being used for defined outcomes, an inability to account for time-varying confounders for stroke or bleeding, an inability to capture patients from locations other than primary internist and cardiologists, and a shorter follow-up period, compared with that of clinical trials.
Bottom line: In routine practice, apixaban is more effective and safer than rivaroxaban with a lower rate of strokes, systemic embolism, and major bleeding.
Citation: Fralick M et al. Effectiveness and safety of apixaban compared with rivaroxaban for patients with atrial fibrillation in routine practice: a cohort study. Ann Intern Med. 2020 Apr 7. doi: 10.7326/M19-2522.
Dr. Almagdub is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Direct oral anticoagulants have proven to be more efficacious, safe, and easy to use, compared with warfarin, in patients with atrial fibrillation (Afib). An indirect comparison showed apixaban to be more effective and safer than rivaroxaban. But randomized controlled trials and head-to-head comparison data regarding the same have been lacking until now.
Study design: Retrospective cohort study.
Setting: A U.S. nationwide commercial health care claims database was searched for persons older than 18 years, with a new diagnosis of atrial fibrillation or flutter who were started on apixaban or rivaroxaban from Dec. 28, 2012, to Jan. 1, 2019.
Synopsis: Optum Clinformatics was used to identify a total of 99,878 patients who were eligible for the analysis. Of these patients, 39,531 newly prescribed apixaban patients were propensity score matched to 39,351 newly prescribed rivaroxaban patients. After propensity score matching, the study found ischemic stroke or systemic embolism rate for new apixaban users to be 6.6 events per 1,000 person-years versus 8.0 events per 1,000 person-years for new rivaroxaban users (hazard ratio, 0.82; 95% confidence interval, 0.68-0.98). The rate of major bleeding after propensity score matching was 12.9 per 1,000 person-years for new apixaban users versus 21.9 per 1,000 person-years for new rivaroxaban users (HR, 0.58; 95% CI, 0.52-0.66).
This observational study has several limitations including an inability to balance unmeasured confounding factors, both ICD-9 and ICD-10 codes being used for defined outcomes, an inability to account for time-varying confounders for stroke or bleeding, an inability to capture patients from locations other than primary internist and cardiologists, and a shorter follow-up period, compared with that of clinical trials.
Bottom line: In routine practice, apixaban is more effective and safer than rivaroxaban with a lower rate of strokes, systemic embolism, and major bleeding.
Citation: Fralick M et al. Effectiveness and safety of apixaban compared with rivaroxaban for patients with atrial fibrillation in routine practice: a cohort study. Ann Intern Med. 2020 Apr 7. doi: 10.7326/M19-2522.
Dr. Almagdub is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
FDA fully approves Pfizer COVID-19 vaccine
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
Weathering this storm and the next
Perspectives on disaster preparedness amid COVID-19
The COVID-19 pandemic has tested disaster preparedness in hospitals across the nation. The pandemic brought many unique disaster planning challenges not commonly seen with other emergencies disasters. These included an uncertain and prolonged time frame, the implementation of physical distancing, and the challenges of preserving the health care work force.
But how do we prepare for the next disaster when the health care system and staff are already stretched thin? Here, we discuss the concept of maintaining a state of preparedness through and beyond COVID-19, using a disaster preparedness cycle – including continuous assessments of vulnerabilities, dynamic staffing adjustments to support patient and hospital needs, and broadening of the pandemic response to incorporate planning for the next disaster.
Disaster preparedness and assessing ongoing needs
Disaster preparedness cycle and Hazard Vulnerability Assessment
The disaster preparedness cycle illustrates that disaster preparedness is continuous. Disaster preparedness is achieved with the non-stop cycle of planning, coordinating, and recognizing vulnerable areas.1-5 Hazard vulnerability analysis (HVA) can play a critical role in recognizing areas in which a hospital system has strengths and weaknesses for different disaster scenarios. There are several tools available, but the overarching goal is to provide an objective and systematic approach to evaluate the potential damage and impact a disaster could have on the health care system and surrounding community.
The HVA can also be utilized to reassess system or personnel vulnerabilities that may have been exposed or highlighted during the pandemic.6,7 These vulnerabilities must be addressed during preparations for the next disaster while concurrently “assuming the incident happens at the worst possible time.”7
Disaster preparedness staffing considerations
Management, communication, and staffing issues are critical to disaster response. Key leadership responses during COVID-19 included providing frequent and transparent communication, down-staffing for physical distancing during low census, and prioritizing staff well-being. These measures serve as a strong foundation moving into preparations for the next disaster.8
To ensure adequate staffing during an unexpected natural disaster, we recommend creating “ride-out” and “relief teams” as part of disaster staffing preparedness.9,10 The ride-out team provides the initial care and these providers are expected to stay in the hospital during the primary impact of the event. Once the initial threat of disaster is over and it is deemed safe to travel, the relief team is activated and offers reprieve to the ride-out team. Leaders and backup leaders within these teams should be identified in the event teams are activated. These assignments should be made at the start of the year and updated yearly or more frequently, if needed.
While the COVID-19 pandemic did not significantly affect children, our ride-out and relief teams would have played a significant role in case a surge of pediatric cases had occurred.
Other considerations for disaster staffing include expanding backup coverage and for multisite groups, identifying site leads to help field specific questions or concerns. Lastly, understanding the staffing needs of the hospital during a disaster is vital – bidirectional communication between physicians and hospital leadership optimizes preparedness plans. These measures will help staff feel supported before, during, and after a disaster.
Dynamic disaster response
Supporting patient and hospital needs
The next step in the disaster preparedness cycle is adjusting to changing needs during the disaster. The pediatric inpatient population was less affected initially by COVID-19, allowing hospitalists to support the unpredicted needs of the pandemic. A dynamic and flexible physician response is important to disaster preparedness.
As there has been a continued shift to telehealth during the pandemic, our group has engaged in telehealth calls related to COVID-19. Seizing these new opportunities not only provided additional services to our patients, but also strengthened community support, physician worth, and the hospital’s financial state. This is also an opportunity for higher-risk clinicians or quarantined faculty to offer patient care during the pandemic.
Cram et al. describe the importance of “unspecializing” during the COVID-19 pandemic.11 Starting discussions early with adult and pediatric critical care colleagues is vital. Hospitalists take care of a broad patient population, and therefore, can adapt to where the clinical need may be. Optimizing and expanding our skill sets can bring value to the hospital system during uncertain times.
Hospitalists are also instrumental for patient flow during the pandemic. To address this, our group partnered with hospital leadership from many different areas including administration, nursing, emergency medicine, critical care, and ancillary services. By collaborating as one cohesive hospital unit, we were able to efficiently develop, implement, and update best clinical care guidelines and algorithms for COVID-19–related topics such as testing indications, admission criteria, infection control, and proper personal protective equipment use. Lastly, working with specialists to consolidate teams during a pandemic presents an opportunity for hospitalists to highlight expertise while bringing value to the hospital.
Unique staffing situations related to COVID-19
Different from other disasters, the COVID-19 pandemic affected older or immunocompromised staff in a unique way. Beauhaus et al. note that 20% of the physician workforce in the United Sates is between 55 and 64 years of age, and 9% are 65 years and older.12 Hospitalist groups should focus on how to optimize and preserve their workforce, specifically those that are higher risk due to age or other health conditions.
We used a tiered guide to safely accommodate our physicians that were determined to be at higher-risk for complications of COVID-19; these recommendations included limiting exposure to patients with acute respiratory illnesses and shifting some providers to a different clinical environments with a lower exposure risk, such as telemedicine visits.
Other COVID-19 preparedness considerations that affected our group in particular include the changes in learner staffing. Similar to attending down-staffing to encourage physical distancing during low census, learners (residents, medical students, and physician assistant students) also experienced decreased hours or suspension of rotations. To maintain optimal patient care, adjusting to changing disaster needs may include assessing attendings’ capacity to assume responsibilities typically supported by learners.
Due to the ongoing nature of the pandemic, we have had to maintain a dynamic response while adjusting to changing and ongoing needs during recovery. Creating a measured and staggered approach helps facilitate a smooth transition back to nonemergent activities. The education of learners, academic and scholarly work, and administrative duties will resume, but likely in a different steady state. Also, awareness of physician burnout and fatigue is critical as an institution enters a phase of recovery.
Preparing for the next disaster during the pandemic
This brings us back to the beginning of the disaster preparedness cycle and the need to plan for the next disaster. Current disaster preparedness plans among physician groups and hospitals are likely focused on an individual disaster scenario, but adjusting current disaster plans to account for the uncertain time frame of an event like the COVID-19 pandemic is critical. Several articles in the national news posed similar questions, although these publications focused mainly on the Federal Emergency Management Agency and the governmental response to prepare for the next disaster when resources are already stretched.13-15
How do we adequately plan, maintain a dynamic response, and continue to efficiently move through the disaster staffing cycle during an event like the COVID-19 pandemic? Being aware of current vulnerabilities and addressing gaps at the department and hospital level are vital to disaster preparedness. For example, we reassessed disaster (ride-out/relief) teams and the minimum number of staff needed to maintain safe and quality care, and what in-house arrangements would be needed (food, supplies, sleeping arrangements) while having to maintain physical distance.
Newman et al. explain “in disaster planning, having as many physicians as possible on hand may seem like an advantage, but being overstaffed in tight quarters was almost as bad as being understaffed.”9 This has been particularly true during the COVID-19 pandemic. It is crucial to have backup plans for faculty that are unable to serve ride-out duties from unexpected issues – such as availability, illnesses/quarantines, childcare/dependents. Also, it is important to be aware that some supply chains are already strained because of the pandemic and how this may play a role in the availability of certain supplies. Being aware and proactive about specific constraints allows for a better level of preparedness. Continued collaboration and communication with other services to provide care should be ongoing throughout the disaster preparedness cycle.
Conclusion
Providing and maintaining optimal and safe patient care should be the overarching goal throughout disaster preparedness. Being aware of group and institutional vulnerabilities, collaboration with hospital leadership, and remaining flexible as hospitalists are critical components for successful preparedness amid disasters. A dynamic and responsive disaster plan has been vital amid COVID-19, and for the next disasters we will certainly encounter.
Dr. Hadvani is assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Uremovich is assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Quinonez is associate professor of pediatrics and chief of pediatric hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Lopez is assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Mothner is associate professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital and is the pediatric hospital medicine medical director for the main campus.
References
1. Malilay J et al. The role of applied epidemiology methods in the disaster management cycle. Am J Public Health. 2014;104(11):2092-102. doi: 10.2105/AJPH.2014.302010.
2. Federal Emergency Management Agency. Developing and maintaining emergency operations plans. 2010 Nov.
3. Federal Emergency Management Agency. National preparedness system. 2020 Jul 31.
4. Federal Emergency Management Agency. National preparedness goal. 2011 Sep.
5. Environmental health in emergencies and disasters: A practical guide. World Health Organization, Geneva. 2002:9-24. Edited by B. Wisner and J. Adams.
6. U.S. Department of Health and Human Services. Topic collection: Hazard vulnerability/risk assessment.
7. Hospital Association of Southern California. Hazard and vulnerability analysis.
8. Meier K et al. Pediatric hospital medicine management, staffing, and well-being in the face of COVID-19. J Hosp Med. 2020 May;15(5):308-10. doi: 10.12788/jhm.3435.
9. Newman B and Gallion C. Hurricane Harvey: Firsthand preparedness in graduate medical education. Acad Med. 2019 Sep;94(9):1267-69. doi: 10.1097/ACM.0000000000002696.
10. Brevard S et al. Analysis of disaster response plans and the aftermath of Hurricane Katrina: Lessons learned from a level I trauma center. J Trauma. 2008 Nov;65(5):1126-32. doi: 10.1097/TA.0b013e318188d6e5.
11. Cram P et al. All hands on deck learning to “un-specialize” in the COVID-19 pandemic. J Hosp Med. 2020 May;15(5):314-5. doi: 10.12788/jhm.3426.
12. Buerhaus P et al. Older clinicians and the surge in novel coronavirus disease 2019 (COVID-19). JAMA. 2020 May 12;323(18):1777-8. doi: 10.1001/jama.2020.4978.
13. VOX Media. Imagine Hurricane Katrina during a pandemic. The US needs to prepare for that – now. 2020 May 27.
14. The Hill. Democratic lawmakers ask how FEMA is planning to balance natural disasters, COVID-19 response. 2020 Apr 20.
15. The Atlantic. What happens if a ‘big one’ strikes during the pandemic? 2020 May 9.
Perspectives on disaster preparedness amid COVID-19
Perspectives on disaster preparedness amid COVID-19
The COVID-19 pandemic has tested disaster preparedness in hospitals across the nation. The pandemic brought many unique disaster planning challenges not commonly seen with other emergencies disasters. These included an uncertain and prolonged time frame, the implementation of physical distancing, and the challenges of preserving the health care work force.
But how do we prepare for the next disaster when the health care system and staff are already stretched thin? Here, we discuss the concept of maintaining a state of preparedness through and beyond COVID-19, using a disaster preparedness cycle – including continuous assessments of vulnerabilities, dynamic staffing adjustments to support patient and hospital needs, and broadening of the pandemic response to incorporate planning for the next disaster.
Disaster preparedness and assessing ongoing needs
Disaster preparedness cycle and Hazard Vulnerability Assessment
The disaster preparedness cycle illustrates that disaster preparedness is continuous. Disaster preparedness is achieved with the non-stop cycle of planning, coordinating, and recognizing vulnerable areas.1-5 Hazard vulnerability analysis (HVA) can play a critical role in recognizing areas in which a hospital system has strengths and weaknesses for different disaster scenarios. There are several tools available, but the overarching goal is to provide an objective and systematic approach to evaluate the potential damage and impact a disaster could have on the health care system and surrounding community.
The HVA can also be utilized to reassess system or personnel vulnerabilities that may have been exposed or highlighted during the pandemic.6,7 These vulnerabilities must be addressed during preparations for the next disaster while concurrently “assuming the incident happens at the worst possible time.”7
Disaster preparedness staffing considerations
Management, communication, and staffing issues are critical to disaster response. Key leadership responses during COVID-19 included providing frequent and transparent communication, down-staffing for physical distancing during low census, and prioritizing staff well-being. These measures serve as a strong foundation moving into preparations for the next disaster.8
To ensure adequate staffing during an unexpected natural disaster, we recommend creating “ride-out” and “relief teams” as part of disaster staffing preparedness.9,10 The ride-out team provides the initial care and these providers are expected to stay in the hospital during the primary impact of the event. Once the initial threat of disaster is over and it is deemed safe to travel, the relief team is activated and offers reprieve to the ride-out team. Leaders and backup leaders within these teams should be identified in the event teams are activated. These assignments should be made at the start of the year and updated yearly or more frequently, if needed.
While the COVID-19 pandemic did not significantly affect children, our ride-out and relief teams would have played a significant role in case a surge of pediatric cases had occurred.
Other considerations for disaster staffing include expanding backup coverage and for multisite groups, identifying site leads to help field specific questions or concerns. Lastly, understanding the staffing needs of the hospital during a disaster is vital – bidirectional communication between physicians and hospital leadership optimizes preparedness plans. These measures will help staff feel supported before, during, and after a disaster.
Dynamic disaster response
Supporting patient and hospital needs
The next step in the disaster preparedness cycle is adjusting to changing needs during the disaster. The pediatric inpatient population was less affected initially by COVID-19, allowing hospitalists to support the unpredicted needs of the pandemic. A dynamic and flexible physician response is important to disaster preparedness.
As there has been a continued shift to telehealth during the pandemic, our group has engaged in telehealth calls related to COVID-19. Seizing these new opportunities not only provided additional services to our patients, but also strengthened community support, physician worth, and the hospital’s financial state. This is also an opportunity for higher-risk clinicians or quarantined faculty to offer patient care during the pandemic.
Cram et al. describe the importance of “unspecializing” during the COVID-19 pandemic.11 Starting discussions early with adult and pediatric critical care colleagues is vital. Hospitalists take care of a broad patient population, and therefore, can adapt to where the clinical need may be. Optimizing and expanding our skill sets can bring value to the hospital system during uncertain times.
Hospitalists are also instrumental for patient flow during the pandemic. To address this, our group partnered with hospital leadership from many different areas including administration, nursing, emergency medicine, critical care, and ancillary services. By collaborating as one cohesive hospital unit, we were able to efficiently develop, implement, and update best clinical care guidelines and algorithms for COVID-19–related topics such as testing indications, admission criteria, infection control, and proper personal protective equipment use. Lastly, working with specialists to consolidate teams during a pandemic presents an opportunity for hospitalists to highlight expertise while bringing value to the hospital.
Unique staffing situations related to COVID-19
Different from other disasters, the COVID-19 pandemic affected older or immunocompromised staff in a unique way. Beauhaus et al. note that 20% of the physician workforce in the United Sates is between 55 and 64 years of age, and 9% are 65 years and older.12 Hospitalist groups should focus on how to optimize and preserve their workforce, specifically those that are higher risk due to age or other health conditions.
We used a tiered guide to safely accommodate our physicians that were determined to be at higher-risk for complications of COVID-19; these recommendations included limiting exposure to patients with acute respiratory illnesses and shifting some providers to a different clinical environments with a lower exposure risk, such as telemedicine visits.
Other COVID-19 preparedness considerations that affected our group in particular include the changes in learner staffing. Similar to attending down-staffing to encourage physical distancing during low census, learners (residents, medical students, and physician assistant students) also experienced decreased hours or suspension of rotations. To maintain optimal patient care, adjusting to changing disaster needs may include assessing attendings’ capacity to assume responsibilities typically supported by learners.
Due to the ongoing nature of the pandemic, we have had to maintain a dynamic response while adjusting to changing and ongoing needs during recovery. Creating a measured and staggered approach helps facilitate a smooth transition back to nonemergent activities. The education of learners, academic and scholarly work, and administrative duties will resume, but likely in a different steady state. Also, awareness of physician burnout and fatigue is critical as an institution enters a phase of recovery.
Preparing for the next disaster during the pandemic
This brings us back to the beginning of the disaster preparedness cycle and the need to plan for the next disaster. Current disaster preparedness plans among physician groups and hospitals are likely focused on an individual disaster scenario, but adjusting current disaster plans to account for the uncertain time frame of an event like the COVID-19 pandemic is critical. Several articles in the national news posed similar questions, although these publications focused mainly on the Federal Emergency Management Agency and the governmental response to prepare for the next disaster when resources are already stretched.13-15
How do we adequately plan, maintain a dynamic response, and continue to efficiently move through the disaster staffing cycle during an event like the COVID-19 pandemic? Being aware of current vulnerabilities and addressing gaps at the department and hospital level are vital to disaster preparedness. For example, we reassessed disaster (ride-out/relief) teams and the minimum number of staff needed to maintain safe and quality care, and what in-house arrangements would be needed (food, supplies, sleeping arrangements) while having to maintain physical distance.
Newman et al. explain “in disaster planning, having as many physicians as possible on hand may seem like an advantage, but being overstaffed in tight quarters was almost as bad as being understaffed.”9 This has been particularly true during the COVID-19 pandemic. It is crucial to have backup plans for faculty that are unable to serve ride-out duties from unexpected issues – such as availability, illnesses/quarantines, childcare/dependents. Also, it is important to be aware that some supply chains are already strained because of the pandemic and how this may play a role in the availability of certain supplies. Being aware and proactive about specific constraints allows for a better level of preparedness. Continued collaboration and communication with other services to provide care should be ongoing throughout the disaster preparedness cycle.
Conclusion
Providing and maintaining optimal and safe patient care should be the overarching goal throughout disaster preparedness. Being aware of group and institutional vulnerabilities, collaboration with hospital leadership, and remaining flexible as hospitalists are critical components for successful preparedness amid disasters. A dynamic and responsive disaster plan has been vital amid COVID-19, and for the next disasters we will certainly encounter.
Dr. Hadvani is assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Uremovich is assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Quinonez is associate professor of pediatrics and chief of pediatric hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Lopez is assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Mothner is associate professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital and is the pediatric hospital medicine medical director for the main campus.
References
1. Malilay J et al. The role of applied epidemiology methods in the disaster management cycle. Am J Public Health. 2014;104(11):2092-102. doi: 10.2105/AJPH.2014.302010.
2. Federal Emergency Management Agency. Developing and maintaining emergency operations plans. 2010 Nov.
3. Federal Emergency Management Agency. National preparedness system. 2020 Jul 31.
4. Federal Emergency Management Agency. National preparedness goal. 2011 Sep.
5. Environmental health in emergencies and disasters: A practical guide. World Health Organization, Geneva. 2002:9-24. Edited by B. Wisner and J. Adams.
6. U.S. Department of Health and Human Services. Topic collection: Hazard vulnerability/risk assessment.
7. Hospital Association of Southern California. Hazard and vulnerability analysis.
8. Meier K et al. Pediatric hospital medicine management, staffing, and well-being in the face of COVID-19. J Hosp Med. 2020 May;15(5):308-10. doi: 10.12788/jhm.3435.
9. Newman B and Gallion C. Hurricane Harvey: Firsthand preparedness in graduate medical education. Acad Med. 2019 Sep;94(9):1267-69. doi: 10.1097/ACM.0000000000002696.
10. Brevard S et al. Analysis of disaster response plans and the aftermath of Hurricane Katrina: Lessons learned from a level I trauma center. J Trauma. 2008 Nov;65(5):1126-32. doi: 10.1097/TA.0b013e318188d6e5.
11. Cram P et al. All hands on deck learning to “un-specialize” in the COVID-19 pandemic. J Hosp Med. 2020 May;15(5):314-5. doi: 10.12788/jhm.3426.
12. Buerhaus P et al. Older clinicians and the surge in novel coronavirus disease 2019 (COVID-19). JAMA. 2020 May 12;323(18):1777-8. doi: 10.1001/jama.2020.4978.
13. VOX Media. Imagine Hurricane Katrina during a pandemic. The US needs to prepare for that – now. 2020 May 27.
14. The Hill. Democratic lawmakers ask how FEMA is planning to balance natural disasters, COVID-19 response. 2020 Apr 20.
15. The Atlantic. What happens if a ‘big one’ strikes during the pandemic? 2020 May 9.
The COVID-19 pandemic has tested disaster preparedness in hospitals across the nation. The pandemic brought many unique disaster planning challenges not commonly seen with other emergencies disasters. These included an uncertain and prolonged time frame, the implementation of physical distancing, and the challenges of preserving the health care work force.
But how do we prepare for the next disaster when the health care system and staff are already stretched thin? Here, we discuss the concept of maintaining a state of preparedness through and beyond COVID-19, using a disaster preparedness cycle – including continuous assessments of vulnerabilities, dynamic staffing adjustments to support patient and hospital needs, and broadening of the pandemic response to incorporate planning for the next disaster.
Disaster preparedness and assessing ongoing needs
Disaster preparedness cycle and Hazard Vulnerability Assessment
The disaster preparedness cycle illustrates that disaster preparedness is continuous. Disaster preparedness is achieved with the non-stop cycle of planning, coordinating, and recognizing vulnerable areas.1-5 Hazard vulnerability analysis (HVA) can play a critical role in recognizing areas in which a hospital system has strengths and weaknesses for different disaster scenarios. There are several tools available, but the overarching goal is to provide an objective and systematic approach to evaluate the potential damage and impact a disaster could have on the health care system and surrounding community.
The HVA can also be utilized to reassess system or personnel vulnerabilities that may have been exposed or highlighted during the pandemic.6,7 These vulnerabilities must be addressed during preparations for the next disaster while concurrently “assuming the incident happens at the worst possible time.”7
Disaster preparedness staffing considerations
Management, communication, and staffing issues are critical to disaster response. Key leadership responses during COVID-19 included providing frequent and transparent communication, down-staffing for physical distancing during low census, and prioritizing staff well-being. These measures serve as a strong foundation moving into preparations for the next disaster.8
To ensure adequate staffing during an unexpected natural disaster, we recommend creating “ride-out” and “relief teams” as part of disaster staffing preparedness.9,10 The ride-out team provides the initial care and these providers are expected to stay in the hospital during the primary impact of the event. Once the initial threat of disaster is over and it is deemed safe to travel, the relief team is activated and offers reprieve to the ride-out team. Leaders and backup leaders within these teams should be identified in the event teams are activated. These assignments should be made at the start of the year and updated yearly or more frequently, if needed.
While the COVID-19 pandemic did not significantly affect children, our ride-out and relief teams would have played a significant role in case a surge of pediatric cases had occurred.
Other considerations for disaster staffing include expanding backup coverage and for multisite groups, identifying site leads to help field specific questions or concerns. Lastly, understanding the staffing needs of the hospital during a disaster is vital – bidirectional communication between physicians and hospital leadership optimizes preparedness plans. These measures will help staff feel supported before, during, and after a disaster.
Dynamic disaster response
Supporting patient and hospital needs
The next step in the disaster preparedness cycle is adjusting to changing needs during the disaster. The pediatric inpatient population was less affected initially by COVID-19, allowing hospitalists to support the unpredicted needs of the pandemic. A dynamic and flexible physician response is important to disaster preparedness.
As there has been a continued shift to telehealth during the pandemic, our group has engaged in telehealth calls related to COVID-19. Seizing these new opportunities not only provided additional services to our patients, but also strengthened community support, physician worth, and the hospital’s financial state. This is also an opportunity for higher-risk clinicians or quarantined faculty to offer patient care during the pandemic.
Cram et al. describe the importance of “unspecializing” during the COVID-19 pandemic.11 Starting discussions early with adult and pediatric critical care colleagues is vital. Hospitalists take care of a broad patient population, and therefore, can adapt to where the clinical need may be. Optimizing and expanding our skill sets can bring value to the hospital system during uncertain times.
Hospitalists are also instrumental for patient flow during the pandemic. To address this, our group partnered with hospital leadership from many different areas including administration, nursing, emergency medicine, critical care, and ancillary services. By collaborating as one cohesive hospital unit, we were able to efficiently develop, implement, and update best clinical care guidelines and algorithms for COVID-19–related topics such as testing indications, admission criteria, infection control, and proper personal protective equipment use. Lastly, working with specialists to consolidate teams during a pandemic presents an opportunity for hospitalists to highlight expertise while bringing value to the hospital.
Unique staffing situations related to COVID-19
Different from other disasters, the COVID-19 pandemic affected older or immunocompromised staff in a unique way. Beauhaus et al. note that 20% of the physician workforce in the United Sates is between 55 and 64 years of age, and 9% are 65 years and older.12 Hospitalist groups should focus on how to optimize and preserve their workforce, specifically those that are higher risk due to age or other health conditions.
We used a tiered guide to safely accommodate our physicians that were determined to be at higher-risk for complications of COVID-19; these recommendations included limiting exposure to patients with acute respiratory illnesses and shifting some providers to a different clinical environments with a lower exposure risk, such as telemedicine visits.
Other COVID-19 preparedness considerations that affected our group in particular include the changes in learner staffing. Similar to attending down-staffing to encourage physical distancing during low census, learners (residents, medical students, and physician assistant students) also experienced decreased hours or suspension of rotations. To maintain optimal patient care, adjusting to changing disaster needs may include assessing attendings’ capacity to assume responsibilities typically supported by learners.
Due to the ongoing nature of the pandemic, we have had to maintain a dynamic response while adjusting to changing and ongoing needs during recovery. Creating a measured and staggered approach helps facilitate a smooth transition back to nonemergent activities. The education of learners, academic and scholarly work, and administrative duties will resume, but likely in a different steady state. Also, awareness of physician burnout and fatigue is critical as an institution enters a phase of recovery.
Preparing for the next disaster during the pandemic
This brings us back to the beginning of the disaster preparedness cycle and the need to plan for the next disaster. Current disaster preparedness plans among physician groups and hospitals are likely focused on an individual disaster scenario, but adjusting current disaster plans to account for the uncertain time frame of an event like the COVID-19 pandemic is critical. Several articles in the national news posed similar questions, although these publications focused mainly on the Federal Emergency Management Agency and the governmental response to prepare for the next disaster when resources are already stretched.13-15
How do we adequately plan, maintain a dynamic response, and continue to efficiently move through the disaster staffing cycle during an event like the COVID-19 pandemic? Being aware of current vulnerabilities and addressing gaps at the department and hospital level are vital to disaster preparedness. For example, we reassessed disaster (ride-out/relief) teams and the minimum number of staff needed to maintain safe and quality care, and what in-house arrangements would be needed (food, supplies, sleeping arrangements) while having to maintain physical distance.
Newman et al. explain “in disaster planning, having as many physicians as possible on hand may seem like an advantage, but being overstaffed in tight quarters was almost as bad as being understaffed.”9 This has been particularly true during the COVID-19 pandemic. It is crucial to have backup plans for faculty that are unable to serve ride-out duties from unexpected issues – such as availability, illnesses/quarantines, childcare/dependents. Also, it is important to be aware that some supply chains are already strained because of the pandemic and how this may play a role in the availability of certain supplies. Being aware and proactive about specific constraints allows for a better level of preparedness. Continued collaboration and communication with other services to provide care should be ongoing throughout the disaster preparedness cycle.
Conclusion
Providing and maintaining optimal and safe patient care should be the overarching goal throughout disaster preparedness. Being aware of group and institutional vulnerabilities, collaboration with hospital leadership, and remaining flexible as hospitalists are critical components for successful preparedness amid disasters. A dynamic and responsive disaster plan has been vital amid COVID-19, and for the next disasters we will certainly encounter.
Dr. Hadvani is assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Uremovich is assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Quinonez is associate professor of pediatrics and chief of pediatric hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Lopez is assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital. Dr. Mothner is associate professor of pediatrics in the section of hospital medicine at Baylor College of Medicine, Texas Children’s Hospital and is the pediatric hospital medicine medical director for the main campus.
References
1. Malilay J et al. The role of applied epidemiology methods in the disaster management cycle. Am J Public Health. 2014;104(11):2092-102. doi: 10.2105/AJPH.2014.302010.
2. Federal Emergency Management Agency. Developing and maintaining emergency operations plans. 2010 Nov.
3. Federal Emergency Management Agency. National preparedness system. 2020 Jul 31.
4. Federal Emergency Management Agency. National preparedness goal. 2011 Sep.
5. Environmental health in emergencies and disasters: A practical guide. World Health Organization, Geneva. 2002:9-24. Edited by B. Wisner and J. Adams.
6. U.S. Department of Health and Human Services. Topic collection: Hazard vulnerability/risk assessment.
7. Hospital Association of Southern California. Hazard and vulnerability analysis.
8. Meier K et al. Pediatric hospital medicine management, staffing, and well-being in the face of COVID-19. J Hosp Med. 2020 May;15(5):308-10. doi: 10.12788/jhm.3435.
9. Newman B and Gallion C. Hurricane Harvey: Firsthand preparedness in graduate medical education. Acad Med. 2019 Sep;94(9):1267-69. doi: 10.1097/ACM.0000000000002696.
10. Brevard S et al. Analysis of disaster response plans and the aftermath of Hurricane Katrina: Lessons learned from a level I trauma center. J Trauma. 2008 Nov;65(5):1126-32. doi: 10.1097/TA.0b013e318188d6e5.
11. Cram P et al. All hands on deck learning to “un-specialize” in the COVID-19 pandemic. J Hosp Med. 2020 May;15(5):314-5. doi: 10.12788/jhm.3426.
12. Buerhaus P et al. Older clinicians and the surge in novel coronavirus disease 2019 (COVID-19). JAMA. 2020 May 12;323(18):1777-8. doi: 10.1001/jama.2020.4978.
13. VOX Media. Imagine Hurricane Katrina during a pandemic. The US needs to prepare for that – now. 2020 May 27.
14. The Hill. Democratic lawmakers ask how FEMA is planning to balance natural disasters, COVID-19 response. 2020 Apr 20.
15. The Atlantic. What happens if a ‘big one’ strikes during the pandemic? 2020 May 9.
Tocilizumab shortage continues as pandemic wears on
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at [email protected].
A version of this article first appeared on Medscape.com.
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at [email protected].
A version of this article first appeared on Medscape.com.
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at [email protected].
A version of this article first appeared on Medscape.com.
Empagliflozin gets HFrEF approval from FDA
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.