User login
Pediatric HM highlights from the 2020 State of Hospital Medicine Report
To improve the pediatric data in the State of Hospital Medicine (SoHM) Report, the Practice Analysis Committee (PAC) developed a pediatric task force to recommend content specific to pediatric practice and garner support for survey participation. The pediatric hospital medicine (PHM) community responded with its usual enthusiasm, resulting in a threefold increase in PHM participation (99 groups), making the data from 2020 SoHM Report the most meaningful ever for pediatric practices.
However, data collection for the 2020 SoHM Report concluded in February, just before the face of medical practice and hospital care changed dramatically. A recent report at the virtual Pediatric Hospital Medicine meeting stated that pre–COVID-19 hospital operating margins had already taken a significant decline (from 5% to 2%-3%), putting pressure on pediatric programs in community settings that typically do not generate much revenue. After COVID-19, hospital revenues took an even greater downturn, affecting many hospital-based pediatric programs. While the future direction of many PHM programs remains unclear, the robust nature of the pediatric data in the 2020 SoHM Report defines where we were and where we once again hope to be. In addition, the PAC conducted a supplemental survey designed to assess the impact of COVID-19 on the practice of hospital medicine. Here’s a quick review of PHM highlights from the 2020 SoHM Report, with preliminary findings from the supplemental survey.
Diversity of service and scope of practice: pediatric hospitalist programs continue to provide a wide variety of services beyond care on inpatient wards, with the most common being procedure performance (56.6%), care of healthy newborns (51.5%), and rapid response team (38.4%) coverage. In addition, most PHM programs have a role in comanagement of a wide variety of patient populations, with the greatest presence among the surgical specialties. Approximately 90% of programs report some role in the care of patients admitted to general surgery, orthopedic surgery, and other surgical subspecialties. The role for comanagement with medical specialties remains diverse, with PHM programs routinely having some role in caring for patients hospitalized for neurologic, gastroenterological, cardiac concerns, and others. With the recent decline in hospital revenues affecting PHM practices, one way to ensure program value is to continue to diversify. Based on data from the 2020 SoHM report, broadening of clinical coverage will not require a significant change in practice for most PHM programs.
PHM board certification: With the first certifying exam for PHM taking place just months before SoHM data collection, the survey sought to establish a baseline percentage of providers board certified in PHM. With 98 groups responding, an average of 26.4% of PHM practitioners per group were reported to be board certified. While no difference was seen based on academic status, practitioners in PHM programs employed by a hospital, health system, or integrated delivery system were much more likely to be board certified than those employed by a university or medical school (31% vs. 20%). Regional differences were noted as well, with the East region reporting a much higher median proportion of PHM-certified physicians. It will be interesting to watch the trend in board certification status evolve over the upcoming years.
Anticipated change of budgeted full-time equivalents in the next year/post–COVID-19 analysis: Of the PHM programs responding to the SoHM Survey, 46.5% predicted an increase in budgeted full-time equivalents in the next year, while only 5.1% anticipated a decrease. Expecting this to change in response to COVID-19, the supplemental survey sought to update this information. Of the 30 PHM respondents to the supplemental survey, 41% instituted a temporary hiring freeze because of COVID-19, while 8.3% instituted a hiring freeze felt likely to be permanent. As PHM programs gear up for the next viral season, we wait to see whether the impact of COVID-19 will continue to be reflected in the volume and variety of patients admitted. It is clear that PHM programs will need to remain nimble to stay ahead of the changing landscape of practice in the days ahead. View all data by obtaining access to the 2020 SoHM Report at hospitalmedicine.org/sohm.
Many thanks to pediatric task force members Jack Percelay, MD; Vivien Kon-Ea Sun, MD; Marcos Mestre, MD; Ann Allen, MD; Dimple Khona, MD; Jeff Grill, MD; and Michelle Marks, MD.
Dr. Gage is director of faculty development, pediatric hospital medicine, at Phoenix Children’s Hospital, and associate professor of pediatrics at the University of Arizona, Phoenix.
To improve the pediatric data in the State of Hospital Medicine (SoHM) Report, the Practice Analysis Committee (PAC) developed a pediatric task force to recommend content specific to pediatric practice and garner support for survey participation. The pediatric hospital medicine (PHM) community responded with its usual enthusiasm, resulting in a threefold increase in PHM participation (99 groups), making the data from 2020 SoHM Report the most meaningful ever for pediatric practices.
However, data collection for the 2020 SoHM Report concluded in February, just before the face of medical practice and hospital care changed dramatically. A recent report at the virtual Pediatric Hospital Medicine meeting stated that pre–COVID-19 hospital operating margins had already taken a significant decline (from 5% to 2%-3%), putting pressure on pediatric programs in community settings that typically do not generate much revenue. After COVID-19, hospital revenues took an even greater downturn, affecting many hospital-based pediatric programs. While the future direction of many PHM programs remains unclear, the robust nature of the pediatric data in the 2020 SoHM Report defines where we were and where we once again hope to be. In addition, the PAC conducted a supplemental survey designed to assess the impact of COVID-19 on the practice of hospital medicine. Here’s a quick review of PHM highlights from the 2020 SoHM Report, with preliminary findings from the supplemental survey.
Diversity of service and scope of practice: pediatric hospitalist programs continue to provide a wide variety of services beyond care on inpatient wards, with the most common being procedure performance (56.6%), care of healthy newborns (51.5%), and rapid response team (38.4%) coverage. In addition, most PHM programs have a role in comanagement of a wide variety of patient populations, with the greatest presence among the surgical specialties. Approximately 90% of programs report some role in the care of patients admitted to general surgery, orthopedic surgery, and other surgical subspecialties. The role for comanagement with medical specialties remains diverse, with PHM programs routinely having some role in caring for patients hospitalized for neurologic, gastroenterological, cardiac concerns, and others. With the recent decline in hospital revenues affecting PHM practices, one way to ensure program value is to continue to diversify. Based on data from the 2020 SoHM report, broadening of clinical coverage will not require a significant change in practice for most PHM programs.
PHM board certification: With the first certifying exam for PHM taking place just months before SoHM data collection, the survey sought to establish a baseline percentage of providers board certified in PHM. With 98 groups responding, an average of 26.4% of PHM practitioners per group were reported to be board certified. While no difference was seen based on academic status, practitioners in PHM programs employed by a hospital, health system, or integrated delivery system were much more likely to be board certified than those employed by a university or medical school (31% vs. 20%). Regional differences were noted as well, with the East region reporting a much higher median proportion of PHM-certified physicians. It will be interesting to watch the trend in board certification status evolve over the upcoming years.
Anticipated change of budgeted full-time equivalents in the next year/post–COVID-19 analysis: Of the PHM programs responding to the SoHM Survey, 46.5% predicted an increase in budgeted full-time equivalents in the next year, while only 5.1% anticipated a decrease. Expecting this to change in response to COVID-19, the supplemental survey sought to update this information. Of the 30 PHM respondents to the supplemental survey, 41% instituted a temporary hiring freeze because of COVID-19, while 8.3% instituted a hiring freeze felt likely to be permanent. As PHM programs gear up for the next viral season, we wait to see whether the impact of COVID-19 will continue to be reflected in the volume and variety of patients admitted. It is clear that PHM programs will need to remain nimble to stay ahead of the changing landscape of practice in the days ahead. View all data by obtaining access to the 2020 SoHM Report at hospitalmedicine.org/sohm.
Many thanks to pediatric task force members Jack Percelay, MD; Vivien Kon-Ea Sun, MD; Marcos Mestre, MD; Ann Allen, MD; Dimple Khona, MD; Jeff Grill, MD; and Michelle Marks, MD.
Dr. Gage is director of faculty development, pediatric hospital medicine, at Phoenix Children’s Hospital, and associate professor of pediatrics at the University of Arizona, Phoenix.
To improve the pediatric data in the State of Hospital Medicine (SoHM) Report, the Practice Analysis Committee (PAC) developed a pediatric task force to recommend content specific to pediatric practice and garner support for survey participation. The pediatric hospital medicine (PHM) community responded with its usual enthusiasm, resulting in a threefold increase in PHM participation (99 groups), making the data from 2020 SoHM Report the most meaningful ever for pediatric practices.
However, data collection for the 2020 SoHM Report concluded in February, just before the face of medical practice and hospital care changed dramatically. A recent report at the virtual Pediatric Hospital Medicine meeting stated that pre–COVID-19 hospital operating margins had already taken a significant decline (from 5% to 2%-3%), putting pressure on pediatric programs in community settings that typically do not generate much revenue. After COVID-19, hospital revenues took an even greater downturn, affecting many hospital-based pediatric programs. While the future direction of many PHM programs remains unclear, the robust nature of the pediatric data in the 2020 SoHM Report defines where we were and where we once again hope to be. In addition, the PAC conducted a supplemental survey designed to assess the impact of COVID-19 on the practice of hospital medicine. Here’s a quick review of PHM highlights from the 2020 SoHM Report, with preliminary findings from the supplemental survey.
Diversity of service and scope of practice: pediatric hospitalist programs continue to provide a wide variety of services beyond care on inpatient wards, with the most common being procedure performance (56.6%), care of healthy newborns (51.5%), and rapid response team (38.4%) coverage. In addition, most PHM programs have a role in comanagement of a wide variety of patient populations, with the greatest presence among the surgical specialties. Approximately 90% of programs report some role in the care of patients admitted to general surgery, orthopedic surgery, and other surgical subspecialties. The role for comanagement with medical specialties remains diverse, with PHM programs routinely having some role in caring for patients hospitalized for neurologic, gastroenterological, cardiac concerns, and others. With the recent decline in hospital revenues affecting PHM practices, one way to ensure program value is to continue to diversify. Based on data from the 2020 SoHM report, broadening of clinical coverage will not require a significant change in practice for most PHM programs.
PHM board certification: With the first certifying exam for PHM taking place just months before SoHM data collection, the survey sought to establish a baseline percentage of providers board certified in PHM. With 98 groups responding, an average of 26.4% of PHM practitioners per group were reported to be board certified. While no difference was seen based on academic status, practitioners in PHM programs employed by a hospital, health system, or integrated delivery system were much more likely to be board certified than those employed by a university or medical school (31% vs. 20%). Regional differences were noted as well, with the East region reporting a much higher median proportion of PHM-certified physicians. It will be interesting to watch the trend in board certification status evolve over the upcoming years.
Anticipated change of budgeted full-time equivalents in the next year/post–COVID-19 analysis: Of the PHM programs responding to the SoHM Survey, 46.5% predicted an increase in budgeted full-time equivalents in the next year, while only 5.1% anticipated a decrease. Expecting this to change in response to COVID-19, the supplemental survey sought to update this information. Of the 30 PHM respondents to the supplemental survey, 41% instituted a temporary hiring freeze because of COVID-19, while 8.3% instituted a hiring freeze felt likely to be permanent. As PHM programs gear up for the next viral season, we wait to see whether the impact of COVID-19 will continue to be reflected in the volume and variety of patients admitted. It is clear that PHM programs will need to remain nimble to stay ahead of the changing landscape of practice in the days ahead. View all data by obtaining access to the 2020 SoHM Report at hospitalmedicine.org/sohm.
Many thanks to pediatric task force members Jack Percelay, MD; Vivien Kon-Ea Sun, MD; Marcos Mestre, MD; Ann Allen, MD; Dimple Khona, MD; Jeff Grill, MD; and Michelle Marks, MD.
Dr. Gage is director of faculty development, pediatric hospital medicine, at Phoenix Children’s Hospital, and associate professor of pediatrics at the University of Arizona, Phoenix.
Income inequality plus race drive COVID incidence, death rates in U.S.
according to an analysis of U.S. county-level data.
The study, published in JAMA Network Open (2021 Jan 20. doi: 10.1001/jamanetworkopen.2020.34578), was led by Tim F. Liao, PhD, of the University of Illinois at Urbana-Champaign, and Fernando de Maio, of DePaul University, Chicago. They wrote: “This analysis confirms the association between racial/ethnic composition and COVID-19 incidence and mortality. A higher level of Black or Hispanic composition in a county is associated with a higher COVID-19 incidence and mortality; a higher level of economic inequality is also associated with a higher level of incidence and mortality.”
The analysis, which examined data from the first 200 days of the pandemic from January to August 2020, examined the joint associations between income inequality and racial and ethnic composition. Researchers mined data from the Centers for Disease Control and Prevention, the Census Bureau, the Kaiser Family Foundation, and other sources for 3,142 U.S. counties.
Income inequality was measured with the Gini index, on a 0-100 scale, with zero meaning perfect income equality (everyone has the same income) and 100 meaning perfect inequality (only one person or group has all of the income). The average Gini score across all the counties was 44.5, with a range of 25.6-66.5.
Researchers found that, for every 1.0% increase in a county’s Black population, there was a 1.9% increase in COVID-19 incidence (risk ratio, 1.019; 95% confidence interval, 1.016-1.022) and a 2.6% increase in COVID-19 mortality (RR, 1.026; 95% CI, 1.020-1.033). For every 1.0% increase in a county’s Hispanic population, there was a 2.4% increase in incidence (RR, 1.024; 95% CI, 1.012-1.025) and a 1.9% increase in mortality (RR, 1.019; 95% CI, 1.012-1.025).
Income inequality had an even greater effect on COVID-19 incidence and mortality. For each 1.0% rise in a county’s income inequality, there was a 2.0% rise in incidence (RR, 1.020; 95% CI, 1.012-1.027), and a 3.0% rise in mortality (RR, 1.030; 95% CI, 1.012-1.047).
In counties with lower percentages of Black and Hispanic population – up to about 50% for blacks and about 20%-30% for Hispanics – greater income inequality was correlated with higher COVID-19 incidence and mortality. But as the proportion of the Black and Hispanic population increased, race and ethnic population became the much more dominant predictive factor. In other words, the researchers said, income inequality seems to become less of a factor in COVID-related health as the minority population number grows in a given county.
“This finding implies that counties with relatively low proportions of Black or Hispanic residents may experience health effects of income inequality associated with the neomaterial pathway, which connects income inequality to population health through the breakdown of public infrastructure,” such as education, transportation and health care, the researchers said.
The study also examined the interaction between these factors and political attributes of a county, such as whether a governor faced a term limit, was Republican, or was male, and these were found to have no effect on COVID-19 incidence and mortality. Counties in states participating in Medicaid expansion under the Affordable Care Act had a 32% lower COVID-19 incidence rate, researchers found, but there was no correlation with mortality rates.
“This analysis found racial/ethnic composition, while important, does not reveal the full complexity of the story,” the researchers wrote. “Income inequality – a measure not typically included in public health county-level surveillance – also needs to be considered as a driver of the disproportionate burden borne by minoritized communities across the United States.”
The findings, they said, support using composite variables that “measure both income inequality and racial/ethnic composition simultaneously.”
The investigators had no disclosures.
according to an analysis of U.S. county-level data.
The study, published in JAMA Network Open (2021 Jan 20. doi: 10.1001/jamanetworkopen.2020.34578), was led by Tim F. Liao, PhD, of the University of Illinois at Urbana-Champaign, and Fernando de Maio, of DePaul University, Chicago. They wrote: “This analysis confirms the association between racial/ethnic composition and COVID-19 incidence and mortality. A higher level of Black or Hispanic composition in a county is associated with a higher COVID-19 incidence and mortality; a higher level of economic inequality is also associated with a higher level of incidence and mortality.”
The analysis, which examined data from the first 200 days of the pandemic from January to August 2020, examined the joint associations between income inequality and racial and ethnic composition. Researchers mined data from the Centers for Disease Control and Prevention, the Census Bureau, the Kaiser Family Foundation, and other sources for 3,142 U.S. counties.
Income inequality was measured with the Gini index, on a 0-100 scale, with zero meaning perfect income equality (everyone has the same income) and 100 meaning perfect inequality (only one person or group has all of the income). The average Gini score across all the counties was 44.5, with a range of 25.6-66.5.
Researchers found that, for every 1.0% increase in a county’s Black population, there was a 1.9% increase in COVID-19 incidence (risk ratio, 1.019; 95% confidence interval, 1.016-1.022) and a 2.6% increase in COVID-19 mortality (RR, 1.026; 95% CI, 1.020-1.033). For every 1.0% increase in a county’s Hispanic population, there was a 2.4% increase in incidence (RR, 1.024; 95% CI, 1.012-1.025) and a 1.9% increase in mortality (RR, 1.019; 95% CI, 1.012-1.025).
Income inequality had an even greater effect on COVID-19 incidence and mortality. For each 1.0% rise in a county’s income inequality, there was a 2.0% rise in incidence (RR, 1.020; 95% CI, 1.012-1.027), and a 3.0% rise in mortality (RR, 1.030; 95% CI, 1.012-1.047).
In counties with lower percentages of Black and Hispanic population – up to about 50% for blacks and about 20%-30% for Hispanics – greater income inequality was correlated with higher COVID-19 incidence and mortality. But as the proportion of the Black and Hispanic population increased, race and ethnic population became the much more dominant predictive factor. In other words, the researchers said, income inequality seems to become less of a factor in COVID-related health as the minority population number grows in a given county.
“This finding implies that counties with relatively low proportions of Black or Hispanic residents may experience health effects of income inequality associated with the neomaterial pathway, which connects income inequality to population health through the breakdown of public infrastructure,” such as education, transportation and health care, the researchers said.
The study also examined the interaction between these factors and political attributes of a county, such as whether a governor faced a term limit, was Republican, or was male, and these were found to have no effect on COVID-19 incidence and mortality. Counties in states participating in Medicaid expansion under the Affordable Care Act had a 32% lower COVID-19 incidence rate, researchers found, but there was no correlation with mortality rates.
“This analysis found racial/ethnic composition, while important, does not reveal the full complexity of the story,” the researchers wrote. “Income inequality – a measure not typically included in public health county-level surveillance – also needs to be considered as a driver of the disproportionate burden borne by minoritized communities across the United States.”
The findings, they said, support using composite variables that “measure both income inequality and racial/ethnic composition simultaneously.”
The investigators had no disclosures.
according to an analysis of U.S. county-level data.
The study, published in JAMA Network Open (2021 Jan 20. doi: 10.1001/jamanetworkopen.2020.34578), was led by Tim F. Liao, PhD, of the University of Illinois at Urbana-Champaign, and Fernando de Maio, of DePaul University, Chicago. They wrote: “This analysis confirms the association between racial/ethnic composition and COVID-19 incidence and mortality. A higher level of Black or Hispanic composition in a county is associated with a higher COVID-19 incidence and mortality; a higher level of economic inequality is also associated with a higher level of incidence and mortality.”
The analysis, which examined data from the first 200 days of the pandemic from January to August 2020, examined the joint associations between income inequality and racial and ethnic composition. Researchers mined data from the Centers for Disease Control and Prevention, the Census Bureau, the Kaiser Family Foundation, and other sources for 3,142 U.S. counties.
Income inequality was measured with the Gini index, on a 0-100 scale, with zero meaning perfect income equality (everyone has the same income) and 100 meaning perfect inequality (only one person or group has all of the income). The average Gini score across all the counties was 44.5, with a range of 25.6-66.5.
Researchers found that, for every 1.0% increase in a county’s Black population, there was a 1.9% increase in COVID-19 incidence (risk ratio, 1.019; 95% confidence interval, 1.016-1.022) and a 2.6% increase in COVID-19 mortality (RR, 1.026; 95% CI, 1.020-1.033). For every 1.0% increase in a county’s Hispanic population, there was a 2.4% increase in incidence (RR, 1.024; 95% CI, 1.012-1.025) and a 1.9% increase in mortality (RR, 1.019; 95% CI, 1.012-1.025).
Income inequality had an even greater effect on COVID-19 incidence and mortality. For each 1.0% rise in a county’s income inequality, there was a 2.0% rise in incidence (RR, 1.020; 95% CI, 1.012-1.027), and a 3.0% rise in mortality (RR, 1.030; 95% CI, 1.012-1.047).
In counties with lower percentages of Black and Hispanic population – up to about 50% for blacks and about 20%-30% for Hispanics – greater income inequality was correlated with higher COVID-19 incidence and mortality. But as the proportion of the Black and Hispanic population increased, race and ethnic population became the much more dominant predictive factor. In other words, the researchers said, income inequality seems to become less of a factor in COVID-related health as the minority population number grows in a given county.
“This finding implies that counties with relatively low proportions of Black or Hispanic residents may experience health effects of income inequality associated with the neomaterial pathway, which connects income inequality to population health through the breakdown of public infrastructure,” such as education, transportation and health care, the researchers said.
The study also examined the interaction between these factors and political attributes of a county, such as whether a governor faced a term limit, was Republican, or was male, and these were found to have no effect on COVID-19 incidence and mortality. Counties in states participating in Medicaid expansion under the Affordable Care Act had a 32% lower COVID-19 incidence rate, researchers found, but there was no correlation with mortality rates.
“This analysis found racial/ethnic composition, while important, does not reveal the full complexity of the story,” the researchers wrote. “Income inequality – a measure not typically included in public health county-level surveillance – also needs to be considered as a driver of the disproportionate burden borne by minoritized communities across the United States.”
The findings, they said, support using composite variables that “measure both income inequality and racial/ethnic composition simultaneously.”
The investigators had no disclosures.
FROM JAMA NETWORK OPEN
What we know and don’t know about virus variants and vaccines
About 20 states across the country have detected the more transmissible B.1.1.7 SARS-CoV-2 variant to date. Given the unknowns of the emerging situation, experts with the Infectious Diseases Society of America addressed vaccine effectiveness, how well equipped the United States is to track new mutations, and shared their impressions of President Joe Biden’s COVID-19 executive orders.
One of the major concerns remains the ability of COVID-19 vaccines to work on new strains. “All of our vaccines target the spike protein and try to elicit neutralizing antibodies that bind to that protein,” Mirella Salvatore, MD, assistant professor of medicine and population health sciences at Weill Cornell Medicine, New York, said during an IDSA press briefing on Thursday.
The B.1.1.7 mutation occurs in the “very important” spike protein, a component of the SARS-CoV-2 virus necessary for binding, which allows the virus to enter cells, added Dr. Salvatore, an IDSA fellow.
The evidence suggests that SARS-CoV-2 should be capable of producing one or two mutations per month. However, the B.1.1.7 variant surprised investigators in the United Kingdom when they first discovered the strain had 17 mutations, Dr. Salvatore said.
It’s still unknown why this particular strain is more transmissible, but Dr. Salvatore speculated that the mutation gives the virus an advantage and increases binding, allowing it to enter cells more easily. She added that the mutations might have arisen among immunocompromised people infected with SARS-CoV-2, but “that is just a hypothesis.”
On a positive note, Kathryn M. Edwards, MD, another IDSA fellow, explained at the briefing that the existing vaccines target more than one location on the virus’ spike protein. Therefore, “if there is a mutation that changes one structure of the spike protein, there will be other areas where the binding can occur.”
This polyclonal response “is why the vaccine can still be effective against this virus,” added Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program and professor of pediatrics at Vanderbilt University, Nashville, Tenn.
Dr. Salvatore emphasized that, although the new variant is more transmissible, it doesn’t appear to be more lethal. “This might affect overall mortality but not for the individual who gets the infection.”
Staying one step ahead
When asked for assurance that COVID-19 vaccines will work against emerging variants, Dr. Edwards said, “It may be we will have to change the vaccine so it is more responsive to new variants, but at this point that does not seem to be the case.”
Should the vaccines require an update, the messenger RNA vaccines have an advantage – researchers can rapidly revise them. “All you need to do is put all the little nucleotides together,” Dr. Edwards said.
“A number of us are looking at how this will work, and we look to influenza,” she added. Dr. Edwards drew an analogy to choosing – and sometimes updating – the influenza strains each year for the annual flu vaccine. With appropriate funding, the same system could be replicated to address any evolving changes to SARS-CoV-2.
On funding, Dr. Salvatore said more money would be required to optimize the surveillance system for emerging strains in the United States.
“We actually have this system – there is a wonderful network that sequences the influenza strains,” she said. “The structure exists, we just need the funding.”
“The CDC is getting the system tooled up to get more viruses to be sequenced,” Dr. Edwards said.
Both experts praised the CDC for its website with up-to-date surveillance information on emerging strains of SARS-CoV-2.
President Biden’s backing of science
A reporter asked each infectious disease expert to share their impression of President Biden’s newly signed COVID-19 executive orders.
“The biggest takeaway is the role of science and the lessons we’ve learned from masks, handwashing, and distancing,” Dr. Edwards said. “We need to heed the advice ... [especially] with a variant that is more contagious.
“It is encouraging that science will be listened to – that is the overall message,” she added.
Dr. Salvatore agreed, saying that the orders give “the feeling that we can now act by following science.”
“We have plenty of papers that show the effectiveness of masking,” for example, she said. Dr. Salvatore acknowledged that there are “a lot of contrasting ideas about masking” across the United States but stressed their importance.
“We should follow measures that we know work,” she said.
Both experts said more research is needed to stay ahead of this evolving scenario. “We still need a lot of basic science showing how this virus replicates in the cell,” Dr. Salvatore said. “We need to really characterize all these mutations and their functions.”
“We need to be concerned, do follow-up studies,” she added, “but we don’t need to panic.”
This article was based on an Infectious Diseases Society of America Media Briefing on Jan. 21, 2021. Dr. Salvatore disclosed that she is a site principal investigator on a study from Verily Life Sciences/Brin Foundation on Predictors of Severe COVID-19 Outcomes and principal investigator for an investigator-initiated study sponsored by Genentech on combination therapy in influenza. Dr. Edwards disclosed National Institutes of Health and Centers for Disease Control and Prevention grants; consulting for Bionet and IBM; and being a member of data safety and monitoring committees for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, and Merck.
A version of this article first appeared on Medscape.com.
About 20 states across the country have detected the more transmissible B.1.1.7 SARS-CoV-2 variant to date. Given the unknowns of the emerging situation, experts with the Infectious Diseases Society of America addressed vaccine effectiveness, how well equipped the United States is to track new mutations, and shared their impressions of President Joe Biden’s COVID-19 executive orders.
One of the major concerns remains the ability of COVID-19 vaccines to work on new strains. “All of our vaccines target the spike protein and try to elicit neutralizing antibodies that bind to that protein,” Mirella Salvatore, MD, assistant professor of medicine and population health sciences at Weill Cornell Medicine, New York, said during an IDSA press briefing on Thursday.
The B.1.1.7 mutation occurs in the “very important” spike protein, a component of the SARS-CoV-2 virus necessary for binding, which allows the virus to enter cells, added Dr. Salvatore, an IDSA fellow.
The evidence suggests that SARS-CoV-2 should be capable of producing one or two mutations per month. However, the B.1.1.7 variant surprised investigators in the United Kingdom when they first discovered the strain had 17 mutations, Dr. Salvatore said.
It’s still unknown why this particular strain is more transmissible, but Dr. Salvatore speculated that the mutation gives the virus an advantage and increases binding, allowing it to enter cells more easily. She added that the mutations might have arisen among immunocompromised people infected with SARS-CoV-2, but “that is just a hypothesis.”
On a positive note, Kathryn M. Edwards, MD, another IDSA fellow, explained at the briefing that the existing vaccines target more than one location on the virus’ spike protein. Therefore, “if there is a mutation that changes one structure of the spike protein, there will be other areas where the binding can occur.”
This polyclonal response “is why the vaccine can still be effective against this virus,” added Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program and professor of pediatrics at Vanderbilt University, Nashville, Tenn.
Dr. Salvatore emphasized that, although the new variant is more transmissible, it doesn’t appear to be more lethal. “This might affect overall mortality but not for the individual who gets the infection.”
Staying one step ahead
When asked for assurance that COVID-19 vaccines will work against emerging variants, Dr. Edwards said, “It may be we will have to change the vaccine so it is more responsive to new variants, but at this point that does not seem to be the case.”
Should the vaccines require an update, the messenger RNA vaccines have an advantage – researchers can rapidly revise them. “All you need to do is put all the little nucleotides together,” Dr. Edwards said.
“A number of us are looking at how this will work, and we look to influenza,” she added. Dr. Edwards drew an analogy to choosing – and sometimes updating – the influenza strains each year for the annual flu vaccine. With appropriate funding, the same system could be replicated to address any evolving changes to SARS-CoV-2.
On funding, Dr. Salvatore said more money would be required to optimize the surveillance system for emerging strains in the United States.
“We actually have this system – there is a wonderful network that sequences the influenza strains,” she said. “The structure exists, we just need the funding.”
“The CDC is getting the system tooled up to get more viruses to be sequenced,” Dr. Edwards said.
Both experts praised the CDC for its website with up-to-date surveillance information on emerging strains of SARS-CoV-2.
President Biden’s backing of science
A reporter asked each infectious disease expert to share their impression of President Biden’s newly signed COVID-19 executive orders.
“The biggest takeaway is the role of science and the lessons we’ve learned from masks, handwashing, and distancing,” Dr. Edwards said. “We need to heed the advice ... [especially] with a variant that is more contagious.
“It is encouraging that science will be listened to – that is the overall message,” she added.
Dr. Salvatore agreed, saying that the orders give “the feeling that we can now act by following science.”
“We have plenty of papers that show the effectiveness of masking,” for example, she said. Dr. Salvatore acknowledged that there are “a lot of contrasting ideas about masking” across the United States but stressed their importance.
“We should follow measures that we know work,” she said.
Both experts said more research is needed to stay ahead of this evolving scenario. “We still need a lot of basic science showing how this virus replicates in the cell,” Dr. Salvatore said. “We need to really characterize all these mutations and their functions.”
“We need to be concerned, do follow-up studies,” she added, “but we don’t need to panic.”
This article was based on an Infectious Diseases Society of America Media Briefing on Jan. 21, 2021. Dr. Salvatore disclosed that she is a site principal investigator on a study from Verily Life Sciences/Brin Foundation on Predictors of Severe COVID-19 Outcomes and principal investigator for an investigator-initiated study sponsored by Genentech on combination therapy in influenza. Dr. Edwards disclosed National Institutes of Health and Centers for Disease Control and Prevention grants; consulting for Bionet and IBM; and being a member of data safety and monitoring committees for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, and Merck.
A version of this article first appeared on Medscape.com.
About 20 states across the country have detected the more transmissible B.1.1.7 SARS-CoV-2 variant to date. Given the unknowns of the emerging situation, experts with the Infectious Diseases Society of America addressed vaccine effectiveness, how well equipped the United States is to track new mutations, and shared their impressions of President Joe Biden’s COVID-19 executive orders.
One of the major concerns remains the ability of COVID-19 vaccines to work on new strains. “All of our vaccines target the spike protein and try to elicit neutralizing antibodies that bind to that protein,” Mirella Salvatore, MD, assistant professor of medicine and population health sciences at Weill Cornell Medicine, New York, said during an IDSA press briefing on Thursday.
The B.1.1.7 mutation occurs in the “very important” spike protein, a component of the SARS-CoV-2 virus necessary for binding, which allows the virus to enter cells, added Dr. Salvatore, an IDSA fellow.
The evidence suggests that SARS-CoV-2 should be capable of producing one or two mutations per month. However, the B.1.1.7 variant surprised investigators in the United Kingdom when they first discovered the strain had 17 mutations, Dr. Salvatore said.
It’s still unknown why this particular strain is more transmissible, but Dr. Salvatore speculated that the mutation gives the virus an advantage and increases binding, allowing it to enter cells more easily. She added that the mutations might have arisen among immunocompromised people infected with SARS-CoV-2, but “that is just a hypothesis.”
On a positive note, Kathryn M. Edwards, MD, another IDSA fellow, explained at the briefing that the existing vaccines target more than one location on the virus’ spike protein. Therefore, “if there is a mutation that changes one structure of the spike protein, there will be other areas where the binding can occur.”
This polyclonal response “is why the vaccine can still be effective against this virus,” added Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program and professor of pediatrics at Vanderbilt University, Nashville, Tenn.
Dr. Salvatore emphasized that, although the new variant is more transmissible, it doesn’t appear to be more lethal. “This might affect overall mortality but not for the individual who gets the infection.”
Staying one step ahead
When asked for assurance that COVID-19 vaccines will work against emerging variants, Dr. Edwards said, “It may be we will have to change the vaccine so it is more responsive to new variants, but at this point that does not seem to be the case.”
Should the vaccines require an update, the messenger RNA vaccines have an advantage – researchers can rapidly revise them. “All you need to do is put all the little nucleotides together,” Dr. Edwards said.
“A number of us are looking at how this will work, and we look to influenza,” she added. Dr. Edwards drew an analogy to choosing – and sometimes updating – the influenza strains each year for the annual flu vaccine. With appropriate funding, the same system could be replicated to address any evolving changes to SARS-CoV-2.
On funding, Dr. Salvatore said more money would be required to optimize the surveillance system for emerging strains in the United States.
“We actually have this system – there is a wonderful network that sequences the influenza strains,” she said. “The structure exists, we just need the funding.”
“The CDC is getting the system tooled up to get more viruses to be sequenced,” Dr. Edwards said.
Both experts praised the CDC for its website with up-to-date surveillance information on emerging strains of SARS-CoV-2.
President Biden’s backing of science
A reporter asked each infectious disease expert to share their impression of President Biden’s newly signed COVID-19 executive orders.
“The biggest takeaway is the role of science and the lessons we’ve learned from masks, handwashing, and distancing,” Dr. Edwards said. “We need to heed the advice ... [especially] with a variant that is more contagious.
“It is encouraging that science will be listened to – that is the overall message,” she added.
Dr. Salvatore agreed, saying that the orders give “the feeling that we can now act by following science.”
“We have plenty of papers that show the effectiveness of masking,” for example, she said. Dr. Salvatore acknowledged that there are “a lot of contrasting ideas about masking” across the United States but stressed their importance.
“We should follow measures that we know work,” she said.
Both experts said more research is needed to stay ahead of this evolving scenario. “We still need a lot of basic science showing how this virus replicates in the cell,” Dr. Salvatore said. “We need to really characterize all these mutations and their functions.”
“We need to be concerned, do follow-up studies,” she added, “but we don’t need to panic.”
This article was based on an Infectious Diseases Society of America Media Briefing on Jan. 21, 2021. Dr. Salvatore disclosed that she is a site principal investigator on a study from Verily Life Sciences/Brin Foundation on Predictors of Severe COVID-19 Outcomes and principal investigator for an investigator-initiated study sponsored by Genentech on combination therapy in influenza. Dr. Edwards disclosed National Institutes of Health and Centers for Disease Control and Prevention grants; consulting for Bionet and IBM; and being a member of data safety and monitoring committees for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, and Merck.
A version of this article first appeared on Medscape.com.
Controversy flares over ivermectin for COVID-19
The National Institutes of Health has dropped its recommendation against the inexpensive antiparasitic drug ivermectin for treatment of COVID-19, and the agency now advises it can’t recommend for or against its use, leaving the decision to physicians and their patients.
“Results from adequately powered, well-designed, and well-conducted clinical trials are needed to provide more specific, evidence-based guidance on the role of ivermectin for the treatment of COVID-19,” according to new NIH guidance released last week.
Passionate arguments have been waged for and against the drug’s use.
The NIH update disappointed members of the Front Line COVID-19 Critical Care Alliance (FLCCC), which outlined its case for endorsing ivermectin in a public statement Jan. 18. Point by point, the group of 10 physicians argued against each limitation that drove the NIH’s ruling.
The group’s members said that, although grateful the recommendation against the drug was dropped, a neutral approach is not acceptable as total U.S. deaths surpassed 400,000 since last spring – and currently approach 4,000 a day. Results from research are enough to support its use, and the drug will immediately save lives, they say.
“Patients do not have time to wait,” they write, “and we as health care providers in society do not have that time either.”
NIH, which in August had recommended against ivermectin’s use, invited the group to present evidence to its treatment guidance panel on Jan. 6 to detail the emerging science surrounding ivermectin. The group cited rapidly growing evidence of the drug’s effectiveness.
Pierre Kory, MD, president/cofounder of FLCCC and a pulmonary and critical care specialist at Aurora St. Luke’s Medical Center in Milwaukee, also spoke before a Senate panel on Dec. 8 in a widely shared impassioned video, touting ivermectin as a COVID-19 “miracle” drug, a term he said he doesn’t use lightly.
Dr. Kory pleaded with the NIH to consider the emerging data. “Please, I’m just asking that they review our manuscript,” he told the senators.
“We have immense amounts of data to show that ivermectin must be implemented and implemented now,” he said.
Some draw parallels to hydroxychloroquine
Critics have said there’s not enough data to institute a protocol, and some draw parallels to another repurposed drug – hydroxychloroquine (HCQ) – which was once considered a promising treatment for COVID-19, based on flawed and incomplete evidence, and now is not recommended.
Paul Sax, MD, a professor of medicine at Harvard and clinical director of the HIV program and division of infectious diseases at Brigham and Women’s Hospital in Boston, wrote in a blog post earlier this month in the New England Journal of Medicine Journal Watch that ivermectin has more robust evidence for it than HCQ ever did.
“But we’re not quite yet at the ‘practice changing’ level,” he writes. “Results from at least five randomized clinical trials are expected soon that might further inform the decision.”
He said the best argument for the drug is seen in this explanation of a meta-analysis of studies of between 100 and 500 patients by Andrew Hill, MD, with the department of pharmacology, University of Liverpool (England).
Dr. Sax advises against two biases in considering ivermectin. One is assuming that because HCQ failed, other antiparasitic drugs will too.
The second bias to avoid, he says, is discounting studies done in low- and middle-income countries because “they weren’t done in the right places.”
“That’s not just bias,” he says. “It’s also snobbery.”
Ivermectin has been approved by the U.S. Food and Drug Administration for treatment of onchocerciasis (river blindness) and strongyloidiasis, but is not FDA-approved for the treatment of any viral infection. It also is sometimes used to treat animals.
In dropping the recommendation against ivermectin, the NIH gave it the same neutral declaration as monoclonal antibodies and convalescent plasma.
Some physicians say they won’t prescribe it
Some physicians say they won’t be recommending it to their COVID-19 patients.
Amesh Adalja, MD, an infectious disease expert and senior scholar at the Johns Hopkins University Center for Health Security in Baltimore,said in an interview that the NIH update hasn’t changed his mind and he isn’t prescribing it for his patients.
He said although “there’s enough of a signal” that he would like to see more data, “we haven’t seen anything in terms of a really robust study.”
He noted that the Infectious Diseases Society of America has 15 recommendations for COVID-19 treatment “and not one of them has to do with ivermectin.”
He added, “It’s not enough to see if it works, but we need to see who it works in and when it works in them.”
He also acknowledged that “some prominent physicians” are recommending it.
Among them is Paul Marik, MD, endowed professor of medicine and chief of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk. A cofounder of FLCCC, Dr. Marik has championed ivermectin and developed a protocol for its use to prevent and treat COVID-19.
The data surrounding ivermectin have met with hope, criticism, and warnings.
Australian researchers published a study ahead of print in Antiviral Research that found ivermectin inhibited the replication of SARS-CoV-2 in a laboratory setting.
The study concluded that the drug resulted post infection in a 5,000-fold reduction in viral RNA at 48 hours. After that study, however, the FDA in April warned consumers not to self-medicate with ivermectin products intended for animals.
The NIH acknowledged that several randomized trials and retrospective studies of ivermectin use in patients with COVID-19 have now been published in peer-reviewed journals or on preprint servers.
“Some clinical studies showed no benefits or worsening of disease after ivermectin use, whereas others reported shorter time to resolution of disease manifestations attributed to COVID-19, greater reduction in inflammatory markers, shorter time to viral clearance, or lower mortality rates in patients who received ivermectin than in patients who received comparator drugs or placebo,” the NIH guidance reads.
The NIH acknowledges limitations: the studies have been small; doses of ivermectin have varied; some patients were taking other medications at the same time (including doxycycline, hydroxychloroquine, azithromycin, zinc, and corticosteroids, which may be potential confounders); and patients’ severity of COVID was not always clearly described in the studies.
Nasia Safdar, MD, medical director of infection prevention at the University of Wisconsin Hospital in Madison, told this news organization she agrees more research is needed before ivermectin is recommended by regulatory bodies for COVID-19.
That said, Dr. Safdar added, “in individual circumstances if a physician is confronted with a patient in dire straits and you’re not sure what to do, might you consider it? I think after a discussion with the patient, perhaps, but the level of evidence certainly doesn’t rise to the level of a policy.”
A downside of recommending a treatment without conclusive data, even if harm isn’t the primary concern, she said, is that supplies could dwindle for its intended use in other diseases. Also, premature approval can limit the robust research needed to see not only whether it works better for prevention or treatment, but also if it’s effective depending on patient populations and the severity of COVID-19.
Dr. Adalja and Dr. Safdar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The National Institutes of Health has dropped its recommendation against the inexpensive antiparasitic drug ivermectin for treatment of COVID-19, and the agency now advises it can’t recommend for or against its use, leaving the decision to physicians and their patients.
“Results from adequately powered, well-designed, and well-conducted clinical trials are needed to provide more specific, evidence-based guidance on the role of ivermectin for the treatment of COVID-19,” according to new NIH guidance released last week.
Passionate arguments have been waged for and against the drug’s use.
The NIH update disappointed members of the Front Line COVID-19 Critical Care Alliance (FLCCC), which outlined its case for endorsing ivermectin in a public statement Jan. 18. Point by point, the group of 10 physicians argued against each limitation that drove the NIH’s ruling.
The group’s members said that, although grateful the recommendation against the drug was dropped, a neutral approach is not acceptable as total U.S. deaths surpassed 400,000 since last spring – and currently approach 4,000 a day. Results from research are enough to support its use, and the drug will immediately save lives, they say.
“Patients do not have time to wait,” they write, “and we as health care providers in society do not have that time either.”
NIH, which in August had recommended against ivermectin’s use, invited the group to present evidence to its treatment guidance panel on Jan. 6 to detail the emerging science surrounding ivermectin. The group cited rapidly growing evidence of the drug’s effectiveness.
Pierre Kory, MD, president/cofounder of FLCCC and a pulmonary and critical care specialist at Aurora St. Luke’s Medical Center in Milwaukee, also spoke before a Senate panel on Dec. 8 in a widely shared impassioned video, touting ivermectin as a COVID-19 “miracle” drug, a term he said he doesn’t use lightly.
Dr. Kory pleaded with the NIH to consider the emerging data. “Please, I’m just asking that they review our manuscript,” he told the senators.
“We have immense amounts of data to show that ivermectin must be implemented and implemented now,” he said.
Some draw parallels to hydroxychloroquine
Critics have said there’s not enough data to institute a protocol, and some draw parallels to another repurposed drug – hydroxychloroquine (HCQ) – which was once considered a promising treatment for COVID-19, based on flawed and incomplete evidence, and now is not recommended.
Paul Sax, MD, a professor of medicine at Harvard and clinical director of the HIV program and division of infectious diseases at Brigham and Women’s Hospital in Boston, wrote in a blog post earlier this month in the New England Journal of Medicine Journal Watch that ivermectin has more robust evidence for it than HCQ ever did.
“But we’re not quite yet at the ‘practice changing’ level,” he writes. “Results from at least five randomized clinical trials are expected soon that might further inform the decision.”
He said the best argument for the drug is seen in this explanation of a meta-analysis of studies of between 100 and 500 patients by Andrew Hill, MD, with the department of pharmacology, University of Liverpool (England).
Dr. Sax advises against two biases in considering ivermectin. One is assuming that because HCQ failed, other antiparasitic drugs will too.
The second bias to avoid, he says, is discounting studies done in low- and middle-income countries because “they weren’t done in the right places.”
“That’s not just bias,” he says. “It’s also snobbery.”
Ivermectin has been approved by the U.S. Food and Drug Administration for treatment of onchocerciasis (river blindness) and strongyloidiasis, but is not FDA-approved for the treatment of any viral infection. It also is sometimes used to treat animals.
In dropping the recommendation against ivermectin, the NIH gave it the same neutral declaration as monoclonal antibodies and convalescent plasma.
Some physicians say they won’t prescribe it
Some physicians say they won’t be recommending it to their COVID-19 patients.
Amesh Adalja, MD, an infectious disease expert and senior scholar at the Johns Hopkins University Center for Health Security in Baltimore,said in an interview that the NIH update hasn’t changed his mind and he isn’t prescribing it for his patients.
He said although “there’s enough of a signal” that he would like to see more data, “we haven’t seen anything in terms of a really robust study.”
He noted that the Infectious Diseases Society of America has 15 recommendations for COVID-19 treatment “and not one of them has to do with ivermectin.”
He added, “It’s not enough to see if it works, but we need to see who it works in and when it works in them.”
He also acknowledged that “some prominent physicians” are recommending it.
Among them is Paul Marik, MD, endowed professor of medicine and chief of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk. A cofounder of FLCCC, Dr. Marik has championed ivermectin and developed a protocol for its use to prevent and treat COVID-19.
The data surrounding ivermectin have met with hope, criticism, and warnings.
Australian researchers published a study ahead of print in Antiviral Research that found ivermectin inhibited the replication of SARS-CoV-2 in a laboratory setting.
The study concluded that the drug resulted post infection in a 5,000-fold reduction in viral RNA at 48 hours. After that study, however, the FDA in April warned consumers not to self-medicate with ivermectin products intended for animals.
The NIH acknowledged that several randomized trials and retrospective studies of ivermectin use in patients with COVID-19 have now been published in peer-reviewed journals or on preprint servers.
“Some clinical studies showed no benefits or worsening of disease after ivermectin use, whereas others reported shorter time to resolution of disease manifestations attributed to COVID-19, greater reduction in inflammatory markers, shorter time to viral clearance, or lower mortality rates in patients who received ivermectin than in patients who received comparator drugs or placebo,” the NIH guidance reads.
The NIH acknowledges limitations: the studies have been small; doses of ivermectin have varied; some patients were taking other medications at the same time (including doxycycline, hydroxychloroquine, azithromycin, zinc, and corticosteroids, which may be potential confounders); and patients’ severity of COVID was not always clearly described in the studies.
Nasia Safdar, MD, medical director of infection prevention at the University of Wisconsin Hospital in Madison, told this news organization she agrees more research is needed before ivermectin is recommended by regulatory bodies for COVID-19.
That said, Dr. Safdar added, “in individual circumstances if a physician is confronted with a patient in dire straits and you’re not sure what to do, might you consider it? I think after a discussion with the patient, perhaps, but the level of evidence certainly doesn’t rise to the level of a policy.”
A downside of recommending a treatment without conclusive data, even if harm isn’t the primary concern, she said, is that supplies could dwindle for its intended use in other diseases. Also, premature approval can limit the robust research needed to see not only whether it works better for prevention or treatment, but also if it’s effective depending on patient populations and the severity of COVID-19.
Dr. Adalja and Dr. Safdar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The National Institutes of Health has dropped its recommendation against the inexpensive antiparasitic drug ivermectin for treatment of COVID-19, and the agency now advises it can’t recommend for or against its use, leaving the decision to physicians and their patients.
“Results from adequately powered, well-designed, and well-conducted clinical trials are needed to provide more specific, evidence-based guidance on the role of ivermectin for the treatment of COVID-19,” according to new NIH guidance released last week.
Passionate arguments have been waged for and against the drug’s use.
The NIH update disappointed members of the Front Line COVID-19 Critical Care Alliance (FLCCC), which outlined its case for endorsing ivermectin in a public statement Jan. 18. Point by point, the group of 10 physicians argued against each limitation that drove the NIH’s ruling.
The group’s members said that, although grateful the recommendation against the drug was dropped, a neutral approach is not acceptable as total U.S. deaths surpassed 400,000 since last spring – and currently approach 4,000 a day. Results from research are enough to support its use, and the drug will immediately save lives, they say.
“Patients do not have time to wait,” they write, “and we as health care providers in society do not have that time either.”
NIH, which in August had recommended against ivermectin’s use, invited the group to present evidence to its treatment guidance panel on Jan. 6 to detail the emerging science surrounding ivermectin. The group cited rapidly growing evidence of the drug’s effectiveness.
Pierre Kory, MD, president/cofounder of FLCCC and a pulmonary and critical care specialist at Aurora St. Luke’s Medical Center in Milwaukee, also spoke before a Senate panel on Dec. 8 in a widely shared impassioned video, touting ivermectin as a COVID-19 “miracle” drug, a term he said he doesn’t use lightly.
Dr. Kory pleaded with the NIH to consider the emerging data. “Please, I’m just asking that they review our manuscript,” he told the senators.
“We have immense amounts of data to show that ivermectin must be implemented and implemented now,” he said.
Some draw parallels to hydroxychloroquine
Critics have said there’s not enough data to institute a protocol, and some draw parallels to another repurposed drug – hydroxychloroquine (HCQ) – which was once considered a promising treatment for COVID-19, based on flawed and incomplete evidence, and now is not recommended.
Paul Sax, MD, a professor of medicine at Harvard and clinical director of the HIV program and division of infectious diseases at Brigham and Women’s Hospital in Boston, wrote in a blog post earlier this month in the New England Journal of Medicine Journal Watch that ivermectin has more robust evidence for it than HCQ ever did.
“But we’re not quite yet at the ‘practice changing’ level,” he writes. “Results from at least five randomized clinical trials are expected soon that might further inform the decision.”
He said the best argument for the drug is seen in this explanation of a meta-analysis of studies of between 100 and 500 patients by Andrew Hill, MD, with the department of pharmacology, University of Liverpool (England).
Dr. Sax advises against two biases in considering ivermectin. One is assuming that because HCQ failed, other antiparasitic drugs will too.
The second bias to avoid, he says, is discounting studies done in low- and middle-income countries because “they weren’t done in the right places.”
“That’s not just bias,” he says. “It’s also snobbery.”
Ivermectin has been approved by the U.S. Food and Drug Administration for treatment of onchocerciasis (river blindness) and strongyloidiasis, but is not FDA-approved for the treatment of any viral infection. It also is sometimes used to treat animals.
In dropping the recommendation against ivermectin, the NIH gave it the same neutral declaration as monoclonal antibodies and convalescent plasma.
Some physicians say they won’t prescribe it
Some physicians say they won’t be recommending it to their COVID-19 patients.
Amesh Adalja, MD, an infectious disease expert and senior scholar at the Johns Hopkins University Center for Health Security in Baltimore,said in an interview that the NIH update hasn’t changed his mind and he isn’t prescribing it for his patients.
He said although “there’s enough of a signal” that he would like to see more data, “we haven’t seen anything in terms of a really robust study.”
He noted that the Infectious Diseases Society of America has 15 recommendations for COVID-19 treatment “and not one of them has to do with ivermectin.”
He added, “It’s not enough to see if it works, but we need to see who it works in and when it works in them.”
He also acknowledged that “some prominent physicians” are recommending it.
Among them is Paul Marik, MD, endowed professor of medicine and chief of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk. A cofounder of FLCCC, Dr. Marik has championed ivermectin and developed a protocol for its use to prevent and treat COVID-19.
The data surrounding ivermectin have met with hope, criticism, and warnings.
Australian researchers published a study ahead of print in Antiviral Research that found ivermectin inhibited the replication of SARS-CoV-2 in a laboratory setting.
The study concluded that the drug resulted post infection in a 5,000-fold reduction in viral RNA at 48 hours. After that study, however, the FDA in April warned consumers not to self-medicate with ivermectin products intended for animals.
The NIH acknowledged that several randomized trials and retrospective studies of ivermectin use in patients with COVID-19 have now been published in peer-reviewed journals or on preprint servers.
“Some clinical studies showed no benefits or worsening of disease after ivermectin use, whereas others reported shorter time to resolution of disease manifestations attributed to COVID-19, greater reduction in inflammatory markers, shorter time to viral clearance, or lower mortality rates in patients who received ivermectin than in patients who received comparator drugs or placebo,” the NIH guidance reads.
The NIH acknowledges limitations: the studies have been small; doses of ivermectin have varied; some patients were taking other medications at the same time (including doxycycline, hydroxychloroquine, azithromycin, zinc, and corticosteroids, which may be potential confounders); and patients’ severity of COVID was not always clearly described in the studies.
Nasia Safdar, MD, medical director of infection prevention at the University of Wisconsin Hospital in Madison, told this news organization she agrees more research is needed before ivermectin is recommended by regulatory bodies for COVID-19.
That said, Dr. Safdar added, “in individual circumstances if a physician is confronted with a patient in dire straits and you’re not sure what to do, might you consider it? I think after a discussion with the patient, perhaps, but the level of evidence certainly doesn’t rise to the level of a policy.”
A downside of recommending a treatment without conclusive data, even if harm isn’t the primary concern, she said, is that supplies could dwindle for its intended use in other diseases. Also, premature approval can limit the robust research needed to see not only whether it works better for prevention or treatment, but also if it’s effective depending on patient populations and the severity of COVID-19.
Dr. Adalja and Dr. Safdar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monoclonal antibody combo treatment reduces viral load in mild to moderate COVID-19
A combination treatment of neutralizing monoclonal antibodies bamlanivimab and etesevimab was associated with a statistically significant reduction in SARS-CoV-2 at day 11 compared with placebo among nonhospitalized patients who had mild to moderate COVID-19, new data indicate.
However, bamlanivimab alone in three different single-infusion doses showed no significant reduction in viral load, compared with placebo, according to the phase 2/3 study by Robert L. Gottlieb, MD, PhD, of the Baylor University Medical Center and the Baylor Scott & White Research Institute, both in Dallas, and colleagues.
Findings from the Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) study were published online Jan. 21 in JAMA. The results represent findings through Oct. 6, 2020.
BLAZE-1 was funded by Eli Lilly, which makes both of the antispike neutralizing antibodies. The trial was conducted at 49 U.S. centers and included 613 outpatients who tested positive for SARS-CoV-2 and had one or more mild to moderate symptoms.
Patients were randomized to one of five groups (four treatment groups and a placebo control), and researchers analyzed between-group differences.
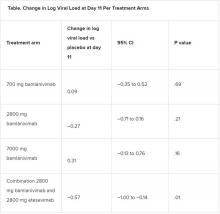
All four treatment arms suggest a trend toward reduction in viral load, which was the primary endpoint of the trial, but only the combination showed a statistically significant reduction.
The average age of patients was 44.7 years, 54.6% were female, 42.5% were Hispanic, and 67.1% had at least one risk factor for severe COVID-19 (aged ≥55 years, body mass index of at least 30, or relevant comorbidity such as hypertension).
Among secondary outcomes, there were no consistent differences between the monotherapy groups or the combination group versus placebo for the other measures of viral load or clinical symptom scores.
The proportion of patients who had COVID-19–related hospitalizations or ED visits was 5.8% (nine events) for placebo; 1.0% (one event) for the 700-mg group; 1.9% (two events) for 2,800 mg; 2.0% (two events) for 7,000 mg; and 0.9% (one event) for combination treatment.
“Combining these two neutralizing monoclonal antibodies in clinical use may enhance viral load reduction and decrease treatment-emergent resistant variants,” the authors concluded.
Safety profile comparison
As for adverse events, immediate hypersensitivity reactions were reported in nine patients (six bamlanivimab, two combination treatment, and one placebo). No deaths occurred during the study.
Serious adverse events unrelated to SARS-CoV-2 infection or considered related to the study drug occurred in 0% (0/309) of patients in the bamlanivimab monotherapy groups; in 0.9% (1/112) of patients in the combination group; and in 0.6% (1/156) of patients in the placebo group.
The serious adverse event in the combination group was a urinary tract infection deemed unrelated to the study drug, the authors wrote.
The two most frequently reported side effects were nausea (3.0% for the 700-mg group; 3.7% for the 2,800-mg group; 5.0% for the 7,000-mg group; 3.6% for the combination group; and 3.8% for the placebo group) and diarrhea (1.0%, 1.9%, 5.9%, 0.9%, and 4.5%, respectively).
The authors included in the study’s limitations that the primary endpoint at day 11 may have been too late to best detect treatment effects.
“All patients, including those who received placebo, demonstrated substantial viral reduction by day 11,” they noted. “An earlier time point like day 3 or day 7 could possibly have been more appropriate to measure viral load.”
Currently, only remdesivir has been approved by the Food and Drug Administration for treating COVID-19, but convalescent plasma and neutralizing monoclonal antibodies have been granted emergency-use authorization.
In an accompanying editor’s note, Preeti N. Malani, MD, with the division of infectious diseases at the University of Michigan, Ann Arbor, and associate editor of JAMA, and Robert M. Golub, MD, deputy editor of JAMA, pointed out that these results differ from an earlier interim analysis of BLAZE-1 data.
A previous publication by Peter Chen, MD, with the department of medicine at Cedars Sinai Medical Center, Los Angeles, compared the three monotherapy groups (no combination group) with placebo, and in that study the 2,800-mg dose of bamlanivimab versus placebo achieved statistical significance for reduction in viral load from baseline at day 11, whereas the other two doses did not.
The editors explain that, in the study by Dr. Chen, “Follow-up for the placebo group was incomplete at the time of the database lock on Sept. 5, 2020. In the final analysis reported in the current article, the database was locked on Oct. 6, 2020, and the longer follow-up for the placebo group, which is now complete, resulted in changes in the primary outcome among that group.”
They concluded: “The comparison of the monotherapy groups against the final results for the placebo group led to changes in the effect sizes,” and the statistical significance of the 2,800-mg group was erased.
The editors pointed out that monoclonal antibodies are likely to benefit certain patients but definitive answers regarding which patients will benefit and under what circumstances will likely take more time than clinicians have to make decisions on treatment.
Meanwhile, as this news organization reported, the United States has spent $375 million on bamlanivimab and $450 million on Regeneron’s monoclonal antibody cocktail of casirivimab plus imdevimab, with the promise to spend billions more.
However, 80% of the 660,000 doses delivered by the two companies are still sitting on shelves, federal officials said in a press briefing last week, because of doubts about efficacy, lack of resources for infusion centers, and questions on reimbursement.
“While the world waits for widespread administration of effective vaccines and additional data on treatments, local efforts should work to improve testing access and turnaround time and reduce logistical barriers to ensure that monoclonal therapies can be provided to patients who are most likely to benefit,” Dr. Malani and Dr. Golub wrote.
This trial was sponsored and funded by Eli Lilly. Dr. Gottlieb disclosed personal fees and nonfinancial support (medication for another trial) from Gilead Sciences and serving on an advisory board for Sentinel. Several coauthors have financial ties to Eli Lilly. Dr. Malani reported serving on the National Institute of Allergy and Infectious Diseases COVID-19 Preventive Monoclonal Antibody data and safety monitoring board but was not compensated. Dr. Golub disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A combination treatment of neutralizing monoclonal antibodies bamlanivimab and etesevimab was associated with a statistically significant reduction in SARS-CoV-2 at day 11 compared with placebo among nonhospitalized patients who had mild to moderate COVID-19, new data indicate.
However, bamlanivimab alone in three different single-infusion doses showed no significant reduction in viral load, compared with placebo, according to the phase 2/3 study by Robert L. Gottlieb, MD, PhD, of the Baylor University Medical Center and the Baylor Scott & White Research Institute, both in Dallas, and colleagues.
Findings from the Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) study were published online Jan. 21 in JAMA. The results represent findings through Oct. 6, 2020.
BLAZE-1 was funded by Eli Lilly, which makes both of the antispike neutralizing antibodies. The trial was conducted at 49 U.S. centers and included 613 outpatients who tested positive for SARS-CoV-2 and had one or more mild to moderate symptoms.
Patients were randomized to one of five groups (four treatment groups and a placebo control), and researchers analyzed between-group differences.
All four treatment arms suggest a trend toward reduction in viral load, which was the primary endpoint of the trial, but only the combination showed a statistically significant reduction.
The average age of patients was 44.7 years, 54.6% were female, 42.5% were Hispanic, and 67.1% had at least one risk factor for severe COVID-19 (aged ≥55 years, body mass index of at least 30, or relevant comorbidity such as hypertension).
Among secondary outcomes, there were no consistent differences between the monotherapy groups or the combination group versus placebo for the other measures of viral load or clinical symptom scores.
The proportion of patients who had COVID-19–related hospitalizations or ED visits was 5.8% (nine events) for placebo; 1.0% (one event) for the 700-mg group; 1.9% (two events) for 2,800 mg; 2.0% (two events) for 7,000 mg; and 0.9% (one event) for combination treatment.
“Combining these two neutralizing monoclonal antibodies in clinical use may enhance viral load reduction and decrease treatment-emergent resistant variants,” the authors concluded.
Safety profile comparison
As for adverse events, immediate hypersensitivity reactions were reported in nine patients (six bamlanivimab, two combination treatment, and one placebo). No deaths occurred during the study.
Serious adverse events unrelated to SARS-CoV-2 infection or considered related to the study drug occurred in 0% (0/309) of patients in the bamlanivimab monotherapy groups; in 0.9% (1/112) of patients in the combination group; and in 0.6% (1/156) of patients in the placebo group.
The serious adverse event in the combination group was a urinary tract infection deemed unrelated to the study drug, the authors wrote.
The two most frequently reported side effects were nausea (3.0% for the 700-mg group; 3.7% for the 2,800-mg group; 5.0% for the 7,000-mg group; 3.6% for the combination group; and 3.8% for the placebo group) and diarrhea (1.0%, 1.9%, 5.9%, 0.9%, and 4.5%, respectively).
The authors included in the study’s limitations that the primary endpoint at day 11 may have been too late to best detect treatment effects.
“All patients, including those who received placebo, demonstrated substantial viral reduction by day 11,” they noted. “An earlier time point like day 3 or day 7 could possibly have been more appropriate to measure viral load.”
Currently, only remdesivir has been approved by the Food and Drug Administration for treating COVID-19, but convalescent plasma and neutralizing monoclonal antibodies have been granted emergency-use authorization.
In an accompanying editor’s note, Preeti N. Malani, MD, with the division of infectious diseases at the University of Michigan, Ann Arbor, and associate editor of JAMA, and Robert M. Golub, MD, deputy editor of JAMA, pointed out that these results differ from an earlier interim analysis of BLAZE-1 data.
A previous publication by Peter Chen, MD, with the department of medicine at Cedars Sinai Medical Center, Los Angeles, compared the three monotherapy groups (no combination group) with placebo, and in that study the 2,800-mg dose of bamlanivimab versus placebo achieved statistical significance for reduction in viral load from baseline at day 11, whereas the other two doses did not.
The editors explain that, in the study by Dr. Chen, “Follow-up for the placebo group was incomplete at the time of the database lock on Sept. 5, 2020. In the final analysis reported in the current article, the database was locked on Oct. 6, 2020, and the longer follow-up for the placebo group, which is now complete, resulted in changes in the primary outcome among that group.”
They concluded: “The comparison of the monotherapy groups against the final results for the placebo group led to changes in the effect sizes,” and the statistical significance of the 2,800-mg group was erased.
The editors pointed out that monoclonal antibodies are likely to benefit certain patients but definitive answers regarding which patients will benefit and under what circumstances will likely take more time than clinicians have to make decisions on treatment.
Meanwhile, as this news organization reported, the United States has spent $375 million on bamlanivimab and $450 million on Regeneron’s monoclonal antibody cocktail of casirivimab plus imdevimab, with the promise to spend billions more.
However, 80% of the 660,000 doses delivered by the two companies are still sitting on shelves, federal officials said in a press briefing last week, because of doubts about efficacy, lack of resources for infusion centers, and questions on reimbursement.
“While the world waits for widespread administration of effective vaccines and additional data on treatments, local efforts should work to improve testing access and turnaround time and reduce logistical barriers to ensure that monoclonal therapies can be provided to patients who are most likely to benefit,” Dr. Malani and Dr. Golub wrote.
This trial was sponsored and funded by Eli Lilly. Dr. Gottlieb disclosed personal fees and nonfinancial support (medication for another trial) from Gilead Sciences and serving on an advisory board for Sentinel. Several coauthors have financial ties to Eli Lilly. Dr. Malani reported serving on the National Institute of Allergy and Infectious Diseases COVID-19 Preventive Monoclonal Antibody data and safety monitoring board but was not compensated. Dr. Golub disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A combination treatment of neutralizing monoclonal antibodies bamlanivimab and etesevimab was associated with a statistically significant reduction in SARS-CoV-2 at day 11 compared with placebo among nonhospitalized patients who had mild to moderate COVID-19, new data indicate.
However, bamlanivimab alone in three different single-infusion doses showed no significant reduction in viral load, compared with placebo, according to the phase 2/3 study by Robert L. Gottlieb, MD, PhD, of the Baylor University Medical Center and the Baylor Scott & White Research Institute, both in Dallas, and colleagues.
Findings from the Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) study were published online Jan. 21 in JAMA. The results represent findings through Oct. 6, 2020.
BLAZE-1 was funded by Eli Lilly, which makes both of the antispike neutralizing antibodies. The trial was conducted at 49 U.S. centers and included 613 outpatients who tested positive for SARS-CoV-2 and had one or more mild to moderate symptoms.
Patients were randomized to one of five groups (four treatment groups and a placebo control), and researchers analyzed between-group differences.
All four treatment arms suggest a trend toward reduction in viral load, which was the primary endpoint of the trial, but only the combination showed a statistically significant reduction.
The average age of patients was 44.7 years, 54.6% were female, 42.5% were Hispanic, and 67.1% had at least one risk factor for severe COVID-19 (aged ≥55 years, body mass index of at least 30, or relevant comorbidity such as hypertension).
Among secondary outcomes, there were no consistent differences between the monotherapy groups or the combination group versus placebo for the other measures of viral load or clinical symptom scores.
The proportion of patients who had COVID-19–related hospitalizations or ED visits was 5.8% (nine events) for placebo; 1.0% (one event) for the 700-mg group; 1.9% (two events) for 2,800 mg; 2.0% (two events) for 7,000 mg; and 0.9% (one event) for combination treatment.
“Combining these two neutralizing monoclonal antibodies in clinical use may enhance viral load reduction and decrease treatment-emergent resistant variants,” the authors concluded.
Safety profile comparison
As for adverse events, immediate hypersensitivity reactions were reported in nine patients (six bamlanivimab, two combination treatment, and one placebo). No deaths occurred during the study.
Serious adverse events unrelated to SARS-CoV-2 infection or considered related to the study drug occurred in 0% (0/309) of patients in the bamlanivimab monotherapy groups; in 0.9% (1/112) of patients in the combination group; and in 0.6% (1/156) of patients in the placebo group.
The serious adverse event in the combination group was a urinary tract infection deemed unrelated to the study drug, the authors wrote.
The two most frequently reported side effects were nausea (3.0% for the 700-mg group; 3.7% for the 2,800-mg group; 5.0% for the 7,000-mg group; 3.6% for the combination group; and 3.8% for the placebo group) and diarrhea (1.0%, 1.9%, 5.9%, 0.9%, and 4.5%, respectively).
The authors included in the study’s limitations that the primary endpoint at day 11 may have been too late to best detect treatment effects.
“All patients, including those who received placebo, demonstrated substantial viral reduction by day 11,” they noted. “An earlier time point like day 3 or day 7 could possibly have been more appropriate to measure viral load.”
Currently, only remdesivir has been approved by the Food and Drug Administration for treating COVID-19, but convalescent plasma and neutralizing monoclonal antibodies have been granted emergency-use authorization.
In an accompanying editor’s note, Preeti N. Malani, MD, with the division of infectious diseases at the University of Michigan, Ann Arbor, and associate editor of JAMA, and Robert M. Golub, MD, deputy editor of JAMA, pointed out that these results differ from an earlier interim analysis of BLAZE-1 data.
A previous publication by Peter Chen, MD, with the department of medicine at Cedars Sinai Medical Center, Los Angeles, compared the three monotherapy groups (no combination group) with placebo, and in that study the 2,800-mg dose of bamlanivimab versus placebo achieved statistical significance for reduction in viral load from baseline at day 11, whereas the other two doses did not.
The editors explain that, in the study by Dr. Chen, “Follow-up for the placebo group was incomplete at the time of the database lock on Sept. 5, 2020. In the final analysis reported in the current article, the database was locked on Oct. 6, 2020, and the longer follow-up for the placebo group, which is now complete, resulted in changes in the primary outcome among that group.”
They concluded: “The comparison of the monotherapy groups against the final results for the placebo group led to changes in the effect sizes,” and the statistical significance of the 2,800-mg group was erased.
The editors pointed out that monoclonal antibodies are likely to benefit certain patients but definitive answers regarding which patients will benefit and under what circumstances will likely take more time than clinicians have to make decisions on treatment.
Meanwhile, as this news organization reported, the United States has spent $375 million on bamlanivimab and $450 million on Regeneron’s monoclonal antibody cocktail of casirivimab plus imdevimab, with the promise to spend billions more.
However, 80% of the 660,000 doses delivered by the two companies are still sitting on shelves, federal officials said in a press briefing last week, because of doubts about efficacy, lack of resources for infusion centers, and questions on reimbursement.
“While the world waits for widespread administration of effective vaccines and additional data on treatments, local efforts should work to improve testing access and turnaround time and reduce logistical barriers to ensure that monoclonal therapies can be provided to patients who are most likely to benefit,” Dr. Malani and Dr. Golub wrote.
This trial was sponsored and funded by Eli Lilly. Dr. Gottlieb disclosed personal fees and nonfinancial support (medication for another trial) from Gilead Sciences and serving on an advisory board for Sentinel. Several coauthors have financial ties to Eli Lilly. Dr. Malani reported serving on the National Institute of Allergy and Infectious Diseases COVID-19 Preventive Monoclonal Antibody data and safety monitoring board but was not compensated. Dr. Golub disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ACEIs, ARBs safe to continue in COVID-19: Trial published
The BRACE-CORONA trial, the first randomized trial to address the question of whether patients with COVID-19 should continue to take ACE inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) – has now been published.
The study, which was conducted in patients hospitalized with COVID-19 who were taking ACEIs or ARBs before hospitalization, showed no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus those assigned to continue these medications.
There were, however, hints that continuing to take ACEIs or ARBs may be beneficial for patients with more severe COVID-19.
The study was first presented at last year’s European Society of Cardiology Congress and was reported by this news organization at that time. The study was published online in JAMA on Jan. 19, 2021.
“These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment,” the authors concluded.
Led by Renato D. Lopes, MD, Duke Clinical Research Institute, Durham, N.C., the researchers explained that there has been conflicting speculation about the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on the course of COVID-19.
On the one hand, observations from animal models suggest that ACEIs and ARBs up-regulate the expression of ACE2, a receptor involved in SARS-CoV-2 infection of host target cells. This led to suggestions that these medications may enhance viral binding and cell entry. Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.
The BRACE-CORONA trial included 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization. The median age of the patients was 55 years. Of these patients, 57.1% were considered to have mild cases at hospital admission, and 42.9% were considered to have moderate cases.
Results showed no significant difference in the number of days alive and out of the hospital for patients in the discontinuation group (mean, 21.9 days) in comparison with patients in the continuation group (mean, 22.9 days). The mean ratio was 0.95 (95% confidence interval, 0.90-1.01).
There also was no statistically significant difference in deaths (2.7% of the discontinuation group vs. 2.8% for the continuation group); cardiovascular death (0.6% vs. 0.3%), or COVID-19 progression (38.3% vs. 32.3%).
The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs. 7.7% in the continuation group), shock requiring vasopressors (8.4% vs. 7.1%), acute MI (7.5% vs. 4.6%), new or worsening heart failure (4.2% vs. 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs. 2.8%).
The authors note that hypertension is an important comorbidity in patients with COVID-19. Recent data suggest that immune dysfunction may contribute to poor outcomes among patients who have COVID-19 and hypertension.
It has been shown that, when use of long-term medications is discontinued during hospitalization, the use of those medications is often not resumed, owing to clinical inertia. Long-term outcomes worsen as a result, the authors reported. In the current study, all patients had hypertension, and more than 50% were obese; both of these comorbidities increase the risk for poor outcomes with COVID-19.
The investigators pointed out that a sensitivity analysis in which site was regarded as a random effect showed a statistically significant finding in favor of the group that continued ACEIs or ARBs. This finding was similar to that of the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission. For these patients, continuing ACEIs or ARBs may be beneficial.
“The primary analyses with the null results but wide 95% confidence intervals suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs,” they said.
Dr. Lopes has received grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
A version of this article first appeared on Medscape.com.
The BRACE-CORONA trial, the first randomized trial to address the question of whether patients with COVID-19 should continue to take ACE inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) – has now been published.
The study, which was conducted in patients hospitalized with COVID-19 who were taking ACEIs or ARBs before hospitalization, showed no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus those assigned to continue these medications.
There were, however, hints that continuing to take ACEIs or ARBs may be beneficial for patients with more severe COVID-19.
The study was first presented at last year’s European Society of Cardiology Congress and was reported by this news organization at that time. The study was published online in JAMA on Jan. 19, 2021.
“These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment,” the authors concluded.
Led by Renato D. Lopes, MD, Duke Clinical Research Institute, Durham, N.C., the researchers explained that there has been conflicting speculation about the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on the course of COVID-19.
On the one hand, observations from animal models suggest that ACEIs and ARBs up-regulate the expression of ACE2, a receptor involved in SARS-CoV-2 infection of host target cells. This led to suggestions that these medications may enhance viral binding and cell entry. Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.
The BRACE-CORONA trial included 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization. The median age of the patients was 55 years. Of these patients, 57.1% were considered to have mild cases at hospital admission, and 42.9% were considered to have moderate cases.
Results showed no significant difference in the number of days alive and out of the hospital for patients in the discontinuation group (mean, 21.9 days) in comparison with patients in the continuation group (mean, 22.9 days). The mean ratio was 0.95 (95% confidence interval, 0.90-1.01).
There also was no statistically significant difference in deaths (2.7% of the discontinuation group vs. 2.8% for the continuation group); cardiovascular death (0.6% vs. 0.3%), or COVID-19 progression (38.3% vs. 32.3%).
The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs. 7.7% in the continuation group), shock requiring vasopressors (8.4% vs. 7.1%), acute MI (7.5% vs. 4.6%), new or worsening heart failure (4.2% vs. 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs. 2.8%).
The authors note that hypertension is an important comorbidity in patients with COVID-19. Recent data suggest that immune dysfunction may contribute to poor outcomes among patients who have COVID-19 and hypertension.
It has been shown that, when use of long-term medications is discontinued during hospitalization, the use of those medications is often not resumed, owing to clinical inertia. Long-term outcomes worsen as a result, the authors reported. In the current study, all patients had hypertension, and more than 50% were obese; both of these comorbidities increase the risk for poor outcomes with COVID-19.
The investigators pointed out that a sensitivity analysis in which site was regarded as a random effect showed a statistically significant finding in favor of the group that continued ACEIs or ARBs. This finding was similar to that of the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission. For these patients, continuing ACEIs or ARBs may be beneficial.
“The primary analyses with the null results but wide 95% confidence intervals suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs,” they said.
Dr. Lopes has received grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
A version of this article first appeared on Medscape.com.
The BRACE-CORONA trial, the first randomized trial to address the question of whether patients with COVID-19 should continue to take ACE inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) – has now been published.
The study, which was conducted in patients hospitalized with COVID-19 who were taking ACEIs or ARBs before hospitalization, showed no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus those assigned to continue these medications.
There were, however, hints that continuing to take ACEIs or ARBs may be beneficial for patients with more severe COVID-19.
The study was first presented at last year’s European Society of Cardiology Congress and was reported by this news organization at that time. The study was published online in JAMA on Jan. 19, 2021.
“These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment,” the authors concluded.
Led by Renato D. Lopes, MD, Duke Clinical Research Institute, Durham, N.C., the researchers explained that there has been conflicting speculation about the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on the course of COVID-19.
On the one hand, observations from animal models suggest that ACEIs and ARBs up-regulate the expression of ACE2, a receptor involved in SARS-CoV-2 infection of host target cells. This led to suggestions that these medications may enhance viral binding and cell entry. Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.
The BRACE-CORONA trial included 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization. The median age of the patients was 55 years. Of these patients, 57.1% were considered to have mild cases at hospital admission, and 42.9% were considered to have moderate cases.
Results showed no significant difference in the number of days alive and out of the hospital for patients in the discontinuation group (mean, 21.9 days) in comparison with patients in the continuation group (mean, 22.9 days). The mean ratio was 0.95 (95% confidence interval, 0.90-1.01).
There also was no statistically significant difference in deaths (2.7% of the discontinuation group vs. 2.8% for the continuation group); cardiovascular death (0.6% vs. 0.3%), or COVID-19 progression (38.3% vs. 32.3%).
The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs. 7.7% in the continuation group), shock requiring vasopressors (8.4% vs. 7.1%), acute MI (7.5% vs. 4.6%), new or worsening heart failure (4.2% vs. 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs. 2.8%).
The authors note that hypertension is an important comorbidity in patients with COVID-19. Recent data suggest that immune dysfunction may contribute to poor outcomes among patients who have COVID-19 and hypertension.
It has been shown that, when use of long-term medications is discontinued during hospitalization, the use of those medications is often not resumed, owing to clinical inertia. Long-term outcomes worsen as a result, the authors reported. In the current study, all patients had hypertension, and more than 50% were obese; both of these comorbidities increase the risk for poor outcomes with COVID-19.
The investigators pointed out that a sensitivity analysis in which site was regarded as a random effect showed a statistically significant finding in favor of the group that continued ACEIs or ARBs. This finding was similar to that of the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission. For these patients, continuing ACEIs or ARBs may be beneficial.
“The primary analyses with the null results but wide 95% confidence intervals suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs,” they said.
Dr. Lopes has received grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
A version of this article first appeared on Medscape.com.
President Biden signs 10 new orders to help fight COVID-19

“For the past year, we couldn’t rely on the federal government to act with the urgency and focus and coordination we needed, and we have seen the tragic cost of that failure,” Mr. Biden said in remarks from the White House, unveiling his 198-page National Strategy for the COVID-19 Response and Pandemic Preparedness.
He said as many as 500,000 Americans will have died by February. “It’s going to take months for us to turn things around,” he said.
“Our national strategy is comprehensive – it’s based on science, not politics; it’s based on truth, not denial,” Mr. Biden said. He also promised to restore public trust, in part by having scientists and public health experts speak to the public. “That’s why you’ll be hearing a lot more from Dr. Fauci again, not from the president,” he said, adding that the experts will be “free from political interference.”
While the president’s executive orders can help accomplish some of the plan’s proposals, the majority will require new funding from Congress and will be included in the $1.9 trillion American Rescue package that Mr. Biden hopes legislators will approve.
Ten new orders
The 10 new pandemic-related orders Biden signed on Jan. 21 follow two he signed on his first day in office.
One establishes a COVID-19 Response Office responsible for coordinating the pandemic response across all federal departments and agencies and also reestablishes the White House Directorate on Global Health Security and Biodefense, which was disabled by the Trump administration.
The other order requires masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
Among the new orders will be directives that:
- Require individuals to also wear masks in airports and planes, and when using other modes of public transportation including trains, boats, and intercity buses, and also require international travelers to produce proof of a recent negative COVID-19 test prior to entry and to quarantine after entry.
- Federal agencies use all powers, including the Defense Production Act, to accelerate manufacturing and delivery of supplies such as N95 masks, gowns, gloves, swabs, reagents, pipette tips, rapid test kits, and nitrocellulose material for rapid antigen tests, and all equipment and material needed to accelerate manufacture, delivery, and administration of COVID-19 vaccine.
- Create a Pandemic Testing Board to expand supply and access, to promote more surge capacity, and to ensure equitable access to tests.
- Facilitate discovery, development, and trials of potential COVID-19 treatments, as well as expand access to programs that can meet the long-term health needs of those recovering from the disease.
- Facilitate more and better data sharing that will allow businesses, schools, hospitals, and individuals to make real-time decisions based on spread in their community.
- Direct the Education and Health & Human Services departments to provide schools and child-care operations guidance on how to reopen and operate safely.
- Direct the Occupational Safety and Health Administration (OSHA) to immediately release clear guidance for employers to help keep workers safe and to enforce health and safety requirements.
The plan also sets goals for vaccination – including 100 million shots in the administration’s first 100 days. President Biden had already previewed his goals for vaccination, including setting up mass vaccination sites and mobile vaccination sites. During his remarks, Mr. Biden said that he had already directed the Federal Emergency Management Agency (FEMA) to begin setting up the vaccination centers.
The administration is also going to look into improving reimbursement for giving vaccines. As a start, the HHS will ask the Centers for Medicare & Medicaid Services to consider if a higher rate “may more accurately compensate providers,” according to the Biden plan.
“But the brutal truth is it will take months before we can get the majority of Americans vaccinated,” said Mr. Biden.
As part of the goal of ensuring an equitable pandemic response, the president will sign an order that establishes a COVID-19 Health Equity Task Force. The task force is charged with providing recommendations for allocating resources and funding in communities with inequities in COVID-19 outcomes by race, ethnicity, geography, disability, and other considerations.
Finally, the administration has committed to being more transparent and sharing more information. The national plan calls for the federal government to conduct regular, expert-led, science-based public briefings and to release regular reports on the pandemic. The administration said it will launch massive science-based public information campaigns – in multiple languages – to educate Americans on masks, testing, and vaccines, and also work to counter misinformation and disinformation.
The American Academy of Family Physicians (AAFP) applauded Mr. Biden’s initiative. “If enacted, this bold legislative agenda will provide much-needed support to American families struggling during the pandemic – especially communities of color and those hardest hit by the virus,” Ada D. Stewart, MD, AAFP president, said in a statement.
Dr. Stewart also noted that family physicians “are uniquely positioned in their communities to educate patients, prioritize access, and coordinate administration of the COVID-19 vaccines,” and urged the administration to ensure that family physicians and staff be vaccinated as soon as possible, to help them “more safely provide care to their communities.”
A version of this article first appeared on Medscape.com.

“For the past year, we couldn’t rely on the federal government to act with the urgency and focus and coordination we needed, and we have seen the tragic cost of that failure,” Mr. Biden said in remarks from the White House, unveiling his 198-page National Strategy for the COVID-19 Response and Pandemic Preparedness.
He said as many as 500,000 Americans will have died by February. “It’s going to take months for us to turn things around,” he said.
“Our national strategy is comprehensive – it’s based on science, not politics; it’s based on truth, not denial,” Mr. Biden said. He also promised to restore public trust, in part by having scientists and public health experts speak to the public. “That’s why you’ll be hearing a lot more from Dr. Fauci again, not from the president,” he said, adding that the experts will be “free from political interference.”
While the president’s executive orders can help accomplish some of the plan’s proposals, the majority will require new funding from Congress and will be included in the $1.9 trillion American Rescue package that Mr. Biden hopes legislators will approve.
Ten new orders
The 10 new pandemic-related orders Biden signed on Jan. 21 follow two he signed on his first day in office.
One establishes a COVID-19 Response Office responsible for coordinating the pandemic response across all federal departments and agencies and also reestablishes the White House Directorate on Global Health Security and Biodefense, which was disabled by the Trump administration.
The other order requires masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
Among the new orders will be directives that:
- Require individuals to also wear masks in airports and planes, and when using other modes of public transportation including trains, boats, and intercity buses, and also require international travelers to produce proof of a recent negative COVID-19 test prior to entry and to quarantine after entry.
- Federal agencies use all powers, including the Defense Production Act, to accelerate manufacturing and delivery of supplies such as N95 masks, gowns, gloves, swabs, reagents, pipette tips, rapid test kits, and nitrocellulose material for rapid antigen tests, and all equipment and material needed to accelerate manufacture, delivery, and administration of COVID-19 vaccine.
- Create a Pandemic Testing Board to expand supply and access, to promote more surge capacity, and to ensure equitable access to tests.
- Facilitate discovery, development, and trials of potential COVID-19 treatments, as well as expand access to programs that can meet the long-term health needs of those recovering from the disease.
- Facilitate more and better data sharing that will allow businesses, schools, hospitals, and individuals to make real-time decisions based on spread in their community.
- Direct the Education and Health & Human Services departments to provide schools and child-care operations guidance on how to reopen and operate safely.
- Direct the Occupational Safety and Health Administration (OSHA) to immediately release clear guidance for employers to help keep workers safe and to enforce health and safety requirements.
The plan also sets goals for vaccination – including 100 million shots in the administration’s first 100 days. President Biden had already previewed his goals for vaccination, including setting up mass vaccination sites and mobile vaccination sites. During his remarks, Mr. Biden said that he had already directed the Federal Emergency Management Agency (FEMA) to begin setting up the vaccination centers.
The administration is also going to look into improving reimbursement for giving vaccines. As a start, the HHS will ask the Centers for Medicare & Medicaid Services to consider if a higher rate “may more accurately compensate providers,” according to the Biden plan.
“But the brutal truth is it will take months before we can get the majority of Americans vaccinated,” said Mr. Biden.
As part of the goal of ensuring an equitable pandemic response, the president will sign an order that establishes a COVID-19 Health Equity Task Force. The task force is charged with providing recommendations for allocating resources and funding in communities with inequities in COVID-19 outcomes by race, ethnicity, geography, disability, and other considerations.
Finally, the administration has committed to being more transparent and sharing more information. The national plan calls for the federal government to conduct regular, expert-led, science-based public briefings and to release regular reports on the pandemic. The administration said it will launch massive science-based public information campaigns – in multiple languages – to educate Americans on masks, testing, and vaccines, and also work to counter misinformation and disinformation.
The American Academy of Family Physicians (AAFP) applauded Mr. Biden’s initiative. “If enacted, this bold legislative agenda will provide much-needed support to American families struggling during the pandemic – especially communities of color and those hardest hit by the virus,” Ada D. Stewart, MD, AAFP president, said in a statement.
Dr. Stewart also noted that family physicians “are uniquely positioned in their communities to educate patients, prioritize access, and coordinate administration of the COVID-19 vaccines,” and urged the administration to ensure that family physicians and staff be vaccinated as soon as possible, to help them “more safely provide care to their communities.”
A version of this article first appeared on Medscape.com.

“For the past year, we couldn’t rely on the federal government to act with the urgency and focus and coordination we needed, and we have seen the tragic cost of that failure,” Mr. Biden said in remarks from the White House, unveiling his 198-page National Strategy for the COVID-19 Response and Pandemic Preparedness.
He said as many as 500,000 Americans will have died by February. “It’s going to take months for us to turn things around,” he said.
“Our national strategy is comprehensive – it’s based on science, not politics; it’s based on truth, not denial,” Mr. Biden said. He also promised to restore public trust, in part by having scientists and public health experts speak to the public. “That’s why you’ll be hearing a lot more from Dr. Fauci again, not from the president,” he said, adding that the experts will be “free from political interference.”
While the president’s executive orders can help accomplish some of the plan’s proposals, the majority will require new funding from Congress and will be included in the $1.9 trillion American Rescue package that Mr. Biden hopes legislators will approve.
Ten new orders
The 10 new pandemic-related orders Biden signed on Jan. 21 follow two he signed on his first day in office.
One establishes a COVID-19 Response Office responsible for coordinating the pandemic response across all federal departments and agencies and also reestablishes the White House Directorate on Global Health Security and Biodefense, which was disabled by the Trump administration.
The other order requires masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
Among the new orders will be directives that:
- Require individuals to also wear masks in airports and planes, and when using other modes of public transportation including trains, boats, and intercity buses, and also require international travelers to produce proof of a recent negative COVID-19 test prior to entry and to quarantine after entry.
- Federal agencies use all powers, including the Defense Production Act, to accelerate manufacturing and delivery of supplies such as N95 masks, gowns, gloves, swabs, reagents, pipette tips, rapid test kits, and nitrocellulose material for rapid antigen tests, and all equipment and material needed to accelerate manufacture, delivery, and administration of COVID-19 vaccine.
- Create a Pandemic Testing Board to expand supply and access, to promote more surge capacity, and to ensure equitable access to tests.
- Facilitate discovery, development, and trials of potential COVID-19 treatments, as well as expand access to programs that can meet the long-term health needs of those recovering from the disease.
- Facilitate more and better data sharing that will allow businesses, schools, hospitals, and individuals to make real-time decisions based on spread in their community.
- Direct the Education and Health & Human Services departments to provide schools and child-care operations guidance on how to reopen and operate safely.
- Direct the Occupational Safety and Health Administration (OSHA) to immediately release clear guidance for employers to help keep workers safe and to enforce health and safety requirements.
The plan also sets goals for vaccination – including 100 million shots in the administration’s first 100 days. President Biden had already previewed his goals for vaccination, including setting up mass vaccination sites and mobile vaccination sites. During his remarks, Mr. Biden said that he had already directed the Federal Emergency Management Agency (FEMA) to begin setting up the vaccination centers.
The administration is also going to look into improving reimbursement for giving vaccines. As a start, the HHS will ask the Centers for Medicare & Medicaid Services to consider if a higher rate “may more accurately compensate providers,” according to the Biden plan.
“But the brutal truth is it will take months before we can get the majority of Americans vaccinated,” said Mr. Biden.
As part of the goal of ensuring an equitable pandemic response, the president will sign an order that establishes a COVID-19 Health Equity Task Force. The task force is charged with providing recommendations for allocating resources and funding in communities with inequities in COVID-19 outcomes by race, ethnicity, geography, disability, and other considerations.
Finally, the administration has committed to being more transparent and sharing more information. The national plan calls for the federal government to conduct regular, expert-led, science-based public briefings and to release regular reports on the pandemic. The administration said it will launch massive science-based public information campaigns – in multiple languages – to educate Americans on masks, testing, and vaccines, and also work to counter misinformation and disinformation.
The American Academy of Family Physicians (AAFP) applauded Mr. Biden’s initiative. “If enacted, this bold legislative agenda will provide much-needed support to American families struggling during the pandemic – especially communities of color and those hardest hit by the virus,” Ada D. Stewart, MD, AAFP president, said in a statement.
Dr. Stewart also noted that family physicians “are uniquely positioned in their communities to educate patients, prioritize access, and coordinate administration of the COVID-19 vaccines,” and urged the administration to ensure that family physicians and staff be vaccinated as soon as possible, to help them “more safely provide care to their communities.”
A version of this article first appeared on Medscape.com.
Metformin treatment again linked to fewer deaths from COVID-19
People with type 2 diabetes who develop COVID-19 show a substantially reduced risk of dying if they are taking metformin, shows a study that adds to prior research indicating the drug might somehow play a role in reducing the severity of infection.
“Unlike several previous analyses, this was a study in a racially diverse population with a high proportion of Blacks/African Americans and [it] revealed that metformin treatment of diabetes prior to diagnosis with COVID-19 was associated with a dramatic threefold reduced mortality in subjects with type 2 diabetes, even after correcting for multiple covariates,” first author Anath Shalev, MD, of the Comprehensive Diabetes Center at the University of Alabama at Birmingham, said in an interview.
But Anne Peters, MD, a professor of clinical medicine at the University of Southern California, Los Angeles, said caution is needed when interpreting these findings.
“It’s hard to tease out the true effects because, for instance, those treated with insulin may be a sicker subset of patients with diabetes than those on metformin, or those with comorbidities such as renal insufficiency may not be treated with metformin” she said in an interview.
“In general, though, treatment obviously matters and people who are better treated tend to do better, so while I think this study raises the question of what role metformin plays in the risk of mortality and COVID-19, I don’t think it necessarily proves the association,” Dr. Peters asserted.
Diverse population
The new study, published this month in Frontiers of Endocrinology, included 25,326 individuals who were tested for COVID-19 at the University of Alabama at Birmingham Hospital between February and June 2020.
Overall, 2.4% tested positive for COVID-19 (n = 604), which the authors note is likely a low figure because screening included asymptomatic hospital staff and patients having elective procedures.
Black/African American patients had a significantly higher risk of COVID-19 positivity, compared with White patients (odds ratio, 2.6; P < .0001). Rates were also higher among those with hypertension (OR, 2.46), diabetes (OR, 2.11), and obesity (OR, 1.93), compared with those without each condition (all P < .0001).
The overall mortality rate in COVID-19-positive patients was 11%. Diabetes was associated with a dramatically increased risk of death (OR, 3.62; P < .0001), and remained an independent risk factor even after adjusting for age, race, sex, obesity, and hypertension.
Notably, the reduction in mortality among those with diabetes taking metformin prior to COVID-19 diagnosis was significant: 11% of those patients died, compared with 23% of those with diabetes not taking metformin (OR, 0.33; P = .021).
Similar findings reported across varied populations
The study adds to mounting research suggesting metformin could have a protective effect on COVID-19 mortality, including an early report from Wuhan, China, findings from the French CORONADO study, and a U.S. study linking treatment with decreased mortality among women with COVID-19.
Of note, the effects of metformin on mortality in the current study were observed in men and women alike, as well as in high-risk subgroups including African Americans.
“The fact that such similar results were obtained in different populations from around the world suggests that the observed reduction in mortality risk, associated with metformin use in subjects with type 2 diabetes and COVID-19, might be generalizable,” the authors wrote.
“Furthermore, these findings underline the importance of following general diabetes treatment and prevention guidelines and not delaying or discontinuing any metformin treatment,” they add.
Speculation of mechanisms includes anti-inflammatory effects
While the mechanisms behind metformin’s potential role in reducing mortality risk in COVID-19 are unknown, the authors note that the most obvious assumption – that improved glycemic control may be a key factor – is disputed by the study’s finding that blood glucose levels and hemoglobin A1c were not significantly different among COVID-19 survivors taking versus not taking metformin.
They point instead to metformin’s known anti-inflammatory and antithrombotic properties.
“We therefore hypothesize that, by exerting some of these effects, metformin might improve outcomes and we are now in the process of investigating this possibility further,” Dr. Shalev said.
Dr. Peters noted that anti-inflammatory properties, themselves, are not necessarily unique to metformin in the treatment of diabetes.
“Many other agents, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors can reduce inflammation, so I don’t know if that would explain it, but it certainly could help,” she said. “[Reducing inflammation] is a hypothesis you see commonly with diabetes drugs, but I think there are also a lot of metabolic benefits from metformin.”
“It was fascinating that they had the A1c data and that survival with metformin didn’t appear to be as related to A1c levels as one might think,” she added.
Notably, a key advantage, should the effects and mechanisms be validated, is metformin’s high accessibility, Dr. Peters added.
“This doesn’t necessarily tell us what we can do to reduce the health care disparities surrounding COVID-19, but the fact that metformin is low cost and easily available is very important, so maybe it will help as we try to grapple with other risk factors.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with type 2 diabetes who develop COVID-19 show a substantially reduced risk of dying if they are taking metformin, shows a study that adds to prior research indicating the drug might somehow play a role in reducing the severity of infection.
“Unlike several previous analyses, this was a study in a racially diverse population with a high proportion of Blacks/African Americans and [it] revealed that metformin treatment of diabetes prior to diagnosis with COVID-19 was associated with a dramatic threefold reduced mortality in subjects with type 2 diabetes, even after correcting for multiple covariates,” first author Anath Shalev, MD, of the Comprehensive Diabetes Center at the University of Alabama at Birmingham, said in an interview.
But Anne Peters, MD, a professor of clinical medicine at the University of Southern California, Los Angeles, said caution is needed when interpreting these findings.
“It’s hard to tease out the true effects because, for instance, those treated with insulin may be a sicker subset of patients with diabetes than those on metformin, or those with comorbidities such as renal insufficiency may not be treated with metformin” she said in an interview.
“In general, though, treatment obviously matters and people who are better treated tend to do better, so while I think this study raises the question of what role metformin plays in the risk of mortality and COVID-19, I don’t think it necessarily proves the association,” Dr. Peters asserted.
Diverse population
The new study, published this month in Frontiers of Endocrinology, included 25,326 individuals who were tested for COVID-19 at the University of Alabama at Birmingham Hospital between February and June 2020.
Overall, 2.4% tested positive for COVID-19 (n = 604), which the authors note is likely a low figure because screening included asymptomatic hospital staff and patients having elective procedures.
Black/African American patients had a significantly higher risk of COVID-19 positivity, compared with White patients (odds ratio, 2.6; P < .0001). Rates were also higher among those with hypertension (OR, 2.46), diabetes (OR, 2.11), and obesity (OR, 1.93), compared with those without each condition (all P < .0001).
The overall mortality rate in COVID-19-positive patients was 11%. Diabetes was associated with a dramatically increased risk of death (OR, 3.62; P < .0001), and remained an independent risk factor even after adjusting for age, race, sex, obesity, and hypertension.
Notably, the reduction in mortality among those with diabetes taking metformin prior to COVID-19 diagnosis was significant: 11% of those patients died, compared with 23% of those with diabetes not taking metformin (OR, 0.33; P = .021).
Similar findings reported across varied populations
The study adds to mounting research suggesting metformin could have a protective effect on COVID-19 mortality, including an early report from Wuhan, China, findings from the French CORONADO study, and a U.S. study linking treatment with decreased mortality among women with COVID-19.
Of note, the effects of metformin on mortality in the current study were observed in men and women alike, as well as in high-risk subgroups including African Americans.
“The fact that such similar results were obtained in different populations from around the world suggests that the observed reduction in mortality risk, associated with metformin use in subjects with type 2 diabetes and COVID-19, might be generalizable,” the authors wrote.
“Furthermore, these findings underline the importance of following general diabetes treatment and prevention guidelines and not delaying or discontinuing any metformin treatment,” they add.
Speculation of mechanisms includes anti-inflammatory effects
While the mechanisms behind metformin’s potential role in reducing mortality risk in COVID-19 are unknown, the authors note that the most obvious assumption – that improved glycemic control may be a key factor – is disputed by the study’s finding that blood glucose levels and hemoglobin A1c were not significantly different among COVID-19 survivors taking versus not taking metformin.
They point instead to metformin’s known anti-inflammatory and antithrombotic properties.
“We therefore hypothesize that, by exerting some of these effects, metformin might improve outcomes and we are now in the process of investigating this possibility further,” Dr. Shalev said.
Dr. Peters noted that anti-inflammatory properties, themselves, are not necessarily unique to metformin in the treatment of diabetes.
“Many other agents, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors can reduce inflammation, so I don’t know if that would explain it, but it certainly could help,” she said. “[Reducing inflammation] is a hypothesis you see commonly with diabetes drugs, but I think there are also a lot of metabolic benefits from metformin.”
“It was fascinating that they had the A1c data and that survival with metformin didn’t appear to be as related to A1c levels as one might think,” she added.
Notably, a key advantage, should the effects and mechanisms be validated, is metformin’s high accessibility, Dr. Peters added.
“This doesn’t necessarily tell us what we can do to reduce the health care disparities surrounding COVID-19, but the fact that metformin is low cost and easily available is very important, so maybe it will help as we try to grapple with other risk factors.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with type 2 diabetes who develop COVID-19 show a substantially reduced risk of dying if they are taking metformin, shows a study that adds to prior research indicating the drug might somehow play a role in reducing the severity of infection.
“Unlike several previous analyses, this was a study in a racially diverse population with a high proportion of Blacks/African Americans and [it] revealed that metformin treatment of diabetes prior to diagnosis with COVID-19 was associated with a dramatic threefold reduced mortality in subjects with type 2 diabetes, even after correcting for multiple covariates,” first author Anath Shalev, MD, of the Comprehensive Diabetes Center at the University of Alabama at Birmingham, said in an interview.
But Anne Peters, MD, a professor of clinical medicine at the University of Southern California, Los Angeles, said caution is needed when interpreting these findings.
“It’s hard to tease out the true effects because, for instance, those treated with insulin may be a sicker subset of patients with diabetes than those on metformin, or those with comorbidities such as renal insufficiency may not be treated with metformin” she said in an interview.
“In general, though, treatment obviously matters and people who are better treated tend to do better, so while I think this study raises the question of what role metformin plays in the risk of mortality and COVID-19, I don’t think it necessarily proves the association,” Dr. Peters asserted.
Diverse population
The new study, published this month in Frontiers of Endocrinology, included 25,326 individuals who were tested for COVID-19 at the University of Alabama at Birmingham Hospital between February and June 2020.
Overall, 2.4% tested positive for COVID-19 (n = 604), which the authors note is likely a low figure because screening included asymptomatic hospital staff and patients having elective procedures.
Black/African American patients had a significantly higher risk of COVID-19 positivity, compared with White patients (odds ratio, 2.6; P < .0001). Rates were also higher among those with hypertension (OR, 2.46), diabetes (OR, 2.11), and obesity (OR, 1.93), compared with those without each condition (all P < .0001).
The overall mortality rate in COVID-19-positive patients was 11%. Diabetes was associated with a dramatically increased risk of death (OR, 3.62; P < .0001), and remained an independent risk factor even after adjusting for age, race, sex, obesity, and hypertension.
Notably, the reduction in mortality among those with diabetes taking metformin prior to COVID-19 diagnosis was significant: 11% of those patients died, compared with 23% of those with diabetes not taking metformin (OR, 0.33; P = .021).
Similar findings reported across varied populations
The study adds to mounting research suggesting metformin could have a protective effect on COVID-19 mortality, including an early report from Wuhan, China, findings from the French CORONADO study, and a U.S. study linking treatment with decreased mortality among women with COVID-19.
Of note, the effects of metformin on mortality in the current study were observed in men and women alike, as well as in high-risk subgroups including African Americans.
“The fact that such similar results were obtained in different populations from around the world suggests that the observed reduction in mortality risk, associated with metformin use in subjects with type 2 diabetes and COVID-19, might be generalizable,” the authors wrote.
“Furthermore, these findings underline the importance of following general diabetes treatment and prevention guidelines and not delaying or discontinuing any metformin treatment,” they add.
Speculation of mechanisms includes anti-inflammatory effects
While the mechanisms behind metformin’s potential role in reducing mortality risk in COVID-19 are unknown, the authors note that the most obvious assumption – that improved glycemic control may be a key factor – is disputed by the study’s finding that blood glucose levels and hemoglobin A1c were not significantly different among COVID-19 survivors taking versus not taking metformin.
They point instead to metformin’s known anti-inflammatory and antithrombotic properties.
“We therefore hypothesize that, by exerting some of these effects, metformin might improve outcomes and we are now in the process of investigating this possibility further,” Dr. Shalev said.
Dr. Peters noted that anti-inflammatory properties, themselves, are not necessarily unique to metformin in the treatment of diabetes.
“Many other agents, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors can reduce inflammation, so I don’t know if that would explain it, but it certainly could help,” she said. “[Reducing inflammation] is a hypothesis you see commonly with diabetes drugs, but I think there are also a lot of metabolic benefits from metformin.”
“It was fascinating that they had the A1c data and that survival with metformin didn’t appear to be as related to A1c levels as one might think,” she added.
Notably, a key advantage, should the effects and mechanisms be validated, is metformin’s high accessibility, Dr. Peters added.
“This doesn’t necessarily tell us what we can do to reduce the health care disparities surrounding COVID-19, but the fact that metformin is low cost and easily available is very important, so maybe it will help as we try to grapple with other risk factors.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seven ways President Biden could now change health care
President Joe Biden has come into office after an unexpected shift in Congress. On Jan. 5, Democrats scored an upset by winning two U.S. Senate seats in runoff elections in Georgia, giving them control of the Senate.
Now the Democrats have control of all three levers of power – the Senate, the House, and the presidency – for the first time since the early years of the Obama administration.
How will President Biden use this new concentration of power to shape health care policy?
Democrats’ small majorities in both houses of Congress suggest that moderation and bipartisanship will be necessary to get things done. Moreover, Mr. Biden himself is calling for bipartisanship. “On this January day,” he said in his inauguration speech, “my whole soul is in this: Bringing America together, uniting our people, uniting our nation.”
Key health care actions that Mr. Biden could pursue include the following.
1. Passing a new COVID-19 relief bill
Above all, Mr. Biden is focused on overcoming the COVID-19 pandemic, which has been registering record deaths recently, and getting newly released vaccines to Americans.
“Dealing with the coronavirus pandemic is one of the most important battles our administration will face, and I will be informed by science and by experts,” the president said.
“There is no question that the pandemic is the highest priority for the Biden administration,” said Larry Levitt, executive vice president for health policy at the Henry J. Kaiser Family Foundation. “COVID will dominate the early weeks and months of this administration. His success rests, in particular, on improving the rollout of vaccines.”
Five days before his inauguration, the president-elect unveiled the American Rescue Plan, a massive, $1.9 trillion legislative package intended to hasten rollout of COVID-19 vaccines, improve COVID-19 testing, and provide financial help to businesses and individuals, among many other things.
The bill would add $1,400 to the recently passed $600 government relief payments for each American, amounting to a $2,000 check. It would also enact many non-COVID-19 measures, such as a $15-an-hour minimum wage and measures to bolster the Affordable Care Act (ACA).
If Democrats cannot reach a deal with the Republicans, they might turn the proposal into a reconciliation bill, which could then be passed with a simple majority. However, drafting a reconciliation bill is a long, complicated process that would require removing provisions that don’t meet the requirements of reconciliation, said Hazen Marshall, a Washington lobbyist and former staffer for Sen. Mitch McConnell.
Most importantly, Mr. Marshall said, reconciliation bills bring out diehard partisanship. “They involve a sledgehammer mentality,” he says. “You’re telling the other side that their views aren’t going to matter.” The final version of the ACA, for example, was passed as a reconciliation bill, with not one Republican vote.
In the Trump years, “the last four reconciliation bills did not get any votes from the minority,” added Rodney Whitlock, PhD, a political consultant at McDermott+Consulting, who worked 21 years for Republicans in the House. “When the majority chooses to use reconciliation, it is an admission that it has no interest in working with the minority.”
Hammering out a compromise will be tough, but Robert Pearl MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University, said that if anyone can do it, it would be President Biden. Having served in the Senate for 36 years, “Biden knows Congress better than any president since Lyndon Johnson,” he said. “He can reach across the aisle and get legislation passed as much as anyone could these days.”
2. Restoring Obamacare
Mr. Biden has vowed to undo a gradual dismantling of the ACA that went on during the Trump administration through executive orders, rule-making, and new laws. “Reinvigorating the ACA was a central part of Biden’s platform as a candidate,” Mr. Levitt said.
Each Trump action against the ACA must be undone in the same way. Presidential orders must be met with presidential orders, regulations with regulations, and legislation with legislation.
The ACA is also being challenged in the Supreme Court. Republicans under Trump passed a law that reduced the penalty for not buying health insurance under the ACA to zero. Then a group of 20 states, led by Texas, filed a lawsuit asserting that this change makes the ACA unconstitutional.
The lawsuit was heard by the Supreme Court in November. From remarks made by the justices then, it appears that the court might well uphold the law when a verdict comes down in June.
But just in case, Mr. Biden wants Congress to enact a small penalty for not buying health insurance, which would remove the basis of the lawsuit.
Mr. Biden’s choice for secretary of Health and Human Services shows his level of commitment to protecting the ACA. His HHS nominee is California Attorney General Xavier Becerra, who led a group of 17 states defending the ACA in the current lawsuit.
In addition to undoing Trump’s changes, Mr. Biden plans to expand the ACA beyond the original legislation. The new COVID-19 bill contains provisions that would expand subsidies to buy insurance on the exchanges and would lower the maximum percentage of income that anyone has to pay for health insurance to 8.5%.
Dealing with Medicaid is also related to the ACA. In 2012, the Supreme Court struck down a mandate that states expand their Medicaid programs, with substantial funding from the federal government.
To date, 12 states still do not participate in the Medicaid expansion. To lure them into the expansion, the Democrat-controlled House last session passed a bill that would offer to pay the entire bill for the first 3 years of Medicaid expansion if they chose to enact an expansion.
3. Undoing other Trump actions in health care
In addition to changes in the ACA, Trump also enacted a number of other changes in health care that President Biden could undo. For example, Mr. Biden says he will reenter the World Health Organization (WHO) so that the United States could better coordinate a COVID-19 response with other nations. Trump exited the WHO with the stroke of a pen, and Mr. Biden can do the same in reverse.
Under Trump, the Centers for Medicare & Medicaid Services used waivers to weaken the ACA and allow states to alter their Medicaid programs. One waiver allows Georgia to leave the ACA exchanges and put brokers in charge of buying coverage. Other waivers allow states to transform federal Medicaid payments into block grants, which several states are planning to do.
The Trump CMS has allowed several states to use Medicaid waivers to add work requirements for Medicaid recipients. The courts have blocked the work rules so far, and the Biden CMS may decide to reverse these waivers or modify them.
“Undoing waivers is normally a fairly simple thing,” Mr. Levitt said. In January, however, the Trump CMS asked some waiver states to sign new contracts in which the CMS pledges not to end a waiver without 9 months’ notice. It’s unclear how many states signed such contracts and what obligation the Biden CMS has to enforce them.
The Trump CMS also stopped reimbursing insurers for waiving deductibles and copayments for low-income customers, as directed by the ACA. Without federal reimbursement, some insurers raised premiums by as much as 20% to cover the costs. It is unclear how the Biden CMS would tackle this change.
4. Negotiating lower drug prices
Allowing Medicare to negotiate drug prices, a major plank in Mr. Biden’s campaign, would seem like a slam dunk for the Democrats. This approach is backed by 89% of Americans, including 84% of Republicans, according to a Kaiser Family Foundation survey in December.
“With that level of support, it’s hard to go wrong politically on this issue,” Mr. Levitt said.
Many Republicans, however, do not favor negotiating drug prices, and the two parties continue to be far apart on how to control drug prices. Trump signed an action that allows Americans to buy cheaper drugs abroad, an approach that Mr. Biden also supports, but it is now tied up in the courts.
“A drug pricing bill has always been difficult to pass,” Dr. Whitlock said. “The issue is popular with the public, but change does not come easily. The drug lobby is one the strongest in Washington, and now it may be even stronger, since it was the drug companies that gave us the COVID vaccines.”
Dr. Whitlock said Republicans will want Democrats to compromise on drug pricing, but he doubts they will do so. The House passed a bill to negotiate drug prices last year, which never was voted on in the Senate. “It is difficult to imagine that the Democrats will be able to move rightward from that House bill,” Dr. Whitlock said. “Democrats are likely to stand pat on drug pricing.”
5. Introducing a public option
President Biden’s campaign proposal for a public option – health insurance offered by the federal government – and to lower the age for Medicare eligibility from 65 years to 60 years, resulted from a compromise between two factions of the Democratic party on how to expand coverage.
Although Mr. Biden and other moderates wanted to focus on fixing the ACA, Democrats led by Sen. Bernie Sanders of Vermont called for a single-payer system, dubbed “Medicare for all.” A public option was seen as the middle ground between the two camps.
“A public option would be a very controversial,” Dr. Whitlock said. Critics say it would pay at Medicare rates, which would reduce doctors’ reimbursements, and save very little money compared with a single-payer system.
Dr. Pearl sees similar problems with lowering the Medicare age. “This would be an expensive change that the federal government could not afford, particularly with all the spending on the pandemic,” he said. “And it would be tough on doctors and hospitals, because Medicare pays less than the private insurance payment they are now getting.”
“The public option is likely to get serious discussion within the Democratic caucus and get onto the Senate floor,” Mr. Levitt said. “The party won’t ignore it.” He notes that in the new Senate, Sen. Sanders chairs the budget committee, and from that position he is likely to push for expanding access to care.
Mr. Levitt says the Biden CMS might allow states to experiment with a statewide public option or even a single-payer model, but he concedes that states, with their budgets ravaged by COVID-19, do not currently have the money to launch such programs.
6. Reviving the CMS
Under President Obama, the CMS was the engine that implemented the ACA and shepherded wider use of value-based reimbursements, which reward providers for quality and outcomes rather than volume.
Under the Trump administration, CMS leadership continued to uphold value-based reimbursement, Dr. Pearl observed. “CMS leadership championed value-based payments, but they encountered a lot of pushback from doctors and hospitals and had to scale back their goals,” he said.
On the other hand, the Trump CMS took a 180-degree turn on the ACA and worked to take it apart. This took a toll on staff morale, according to Donald M. Berwick, MD, who ran the CMS under President Obama. “Many people in CMS did not feel supported during the Trump administration, and some of them left,” Dr. Berwick said.
The CMS needs experienced staff on board to write comprehensible rules and regulations that can overcome court challenges.
Having a fully functioning CMS also requires consistent leadership, which was a problem for Obama. When Mr. Obama nominated Dr. Berwick, 60 Senate votes were needed to confirm him, and Republicans would not vote for him. Mr. Obama eventually brought Dr. Berwick in as a recess appointment, but it meant he could serve for only 17 months.
Since then, Senate confirmation rules have changed so that only a simple majority is needed to confirm appointments. This is important for Biden’s nominees, Dr. Berwick said. “For a president, having your team in place means you are able to execute the policies you want,” he said. “You need to have consistent leadership.”
7. Potentially changing health care without Congress
Even with their newly won control of the Senate, the Democrats’ thin majorities in both houses of Congress may not be enough to pass much legislation if Republicans are solidly opposed.
Democrats in the House also have a narrow path this session in which to pass legislation. The Democratic leadership has an 11-vote majority, but it must contend with 15 moderate representatives in purple districts (where Democrats and Republicans have about equal support).
A bigger problem looms before the Democrats. In 2022, the party may well lose its majorities in both houses. Mr. Whitlock notes that the party of an incoming president normally loses seats in the first midterm election. “The last incoming president to keep both houses of Congress in his first midterm was Jimmy Carter,” he said.
If this happens, President Biden would have to govern without the support of Congress, which is what Barack Obama had to do through most of his presidency. As Mr. Obama’s vice president, Mr. Biden is well aware how that goes. Governing without Congress means relying on presidential orders and decrees.
In health care, Mr. Biden has a powerful policy-making tool, the Center for Medicare & Medicaid Innovation (CMMI). The CMMI was empowered by the ACA to initiate pilot programs for new payment models.
So far, the CMMI’s work has been mainly limited to accountable care organizations, bundled payments, and patient-centered medical homes, but it could also be used to enact new federal policies that would normally require Congressional action, Mr. Levitt said.
Conclusion
Expectations have been very high for what President Joe Biden can do in health care. He needs to unite a very divided political system to defeat a deadly pandemic, restore Obamacare, and sign landmark legislation, such as a drug-pricing bill.
But shepherding bills through Congress will be a challenge. “You need to have accountability, unity, and civility, which is a Herculean task,” Mr. Whitlock said. “You have to keep policies off the table that could blow up the bipartisanship.”
A version of this article first appeared on Medscape.com.
President Joe Biden has come into office after an unexpected shift in Congress. On Jan. 5, Democrats scored an upset by winning two U.S. Senate seats in runoff elections in Georgia, giving them control of the Senate.
Now the Democrats have control of all three levers of power – the Senate, the House, and the presidency – for the first time since the early years of the Obama administration.
How will President Biden use this new concentration of power to shape health care policy?
Democrats’ small majorities in both houses of Congress suggest that moderation and bipartisanship will be necessary to get things done. Moreover, Mr. Biden himself is calling for bipartisanship. “On this January day,” he said in his inauguration speech, “my whole soul is in this: Bringing America together, uniting our people, uniting our nation.”
Key health care actions that Mr. Biden could pursue include the following.
1. Passing a new COVID-19 relief bill
Above all, Mr. Biden is focused on overcoming the COVID-19 pandemic, which has been registering record deaths recently, and getting newly released vaccines to Americans.
“Dealing with the coronavirus pandemic is one of the most important battles our administration will face, and I will be informed by science and by experts,” the president said.
“There is no question that the pandemic is the highest priority for the Biden administration,” said Larry Levitt, executive vice president for health policy at the Henry J. Kaiser Family Foundation. “COVID will dominate the early weeks and months of this administration. His success rests, in particular, on improving the rollout of vaccines.”
Five days before his inauguration, the president-elect unveiled the American Rescue Plan, a massive, $1.9 trillion legislative package intended to hasten rollout of COVID-19 vaccines, improve COVID-19 testing, and provide financial help to businesses and individuals, among many other things.
The bill would add $1,400 to the recently passed $600 government relief payments for each American, amounting to a $2,000 check. It would also enact many non-COVID-19 measures, such as a $15-an-hour minimum wage and measures to bolster the Affordable Care Act (ACA).
If Democrats cannot reach a deal with the Republicans, they might turn the proposal into a reconciliation bill, which could then be passed with a simple majority. However, drafting a reconciliation bill is a long, complicated process that would require removing provisions that don’t meet the requirements of reconciliation, said Hazen Marshall, a Washington lobbyist and former staffer for Sen. Mitch McConnell.
Most importantly, Mr. Marshall said, reconciliation bills bring out diehard partisanship. “They involve a sledgehammer mentality,” he says. “You’re telling the other side that their views aren’t going to matter.” The final version of the ACA, for example, was passed as a reconciliation bill, with not one Republican vote.
In the Trump years, “the last four reconciliation bills did not get any votes from the minority,” added Rodney Whitlock, PhD, a political consultant at McDermott+Consulting, who worked 21 years for Republicans in the House. “When the majority chooses to use reconciliation, it is an admission that it has no interest in working with the minority.”
Hammering out a compromise will be tough, but Robert Pearl MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University, said that if anyone can do it, it would be President Biden. Having served in the Senate for 36 years, “Biden knows Congress better than any president since Lyndon Johnson,” he said. “He can reach across the aisle and get legislation passed as much as anyone could these days.”
2. Restoring Obamacare
Mr. Biden has vowed to undo a gradual dismantling of the ACA that went on during the Trump administration through executive orders, rule-making, and new laws. “Reinvigorating the ACA was a central part of Biden’s platform as a candidate,” Mr. Levitt said.
Each Trump action against the ACA must be undone in the same way. Presidential orders must be met with presidential orders, regulations with regulations, and legislation with legislation.
The ACA is also being challenged in the Supreme Court. Republicans under Trump passed a law that reduced the penalty for not buying health insurance under the ACA to zero. Then a group of 20 states, led by Texas, filed a lawsuit asserting that this change makes the ACA unconstitutional.
The lawsuit was heard by the Supreme Court in November. From remarks made by the justices then, it appears that the court might well uphold the law when a verdict comes down in June.
But just in case, Mr. Biden wants Congress to enact a small penalty for not buying health insurance, which would remove the basis of the lawsuit.
Mr. Biden’s choice for secretary of Health and Human Services shows his level of commitment to protecting the ACA. His HHS nominee is California Attorney General Xavier Becerra, who led a group of 17 states defending the ACA in the current lawsuit.
In addition to undoing Trump’s changes, Mr. Biden plans to expand the ACA beyond the original legislation. The new COVID-19 bill contains provisions that would expand subsidies to buy insurance on the exchanges and would lower the maximum percentage of income that anyone has to pay for health insurance to 8.5%.
Dealing with Medicaid is also related to the ACA. In 2012, the Supreme Court struck down a mandate that states expand their Medicaid programs, with substantial funding from the federal government.
To date, 12 states still do not participate in the Medicaid expansion. To lure them into the expansion, the Democrat-controlled House last session passed a bill that would offer to pay the entire bill for the first 3 years of Medicaid expansion if they chose to enact an expansion.
3. Undoing other Trump actions in health care
In addition to changes in the ACA, Trump also enacted a number of other changes in health care that President Biden could undo. For example, Mr. Biden says he will reenter the World Health Organization (WHO) so that the United States could better coordinate a COVID-19 response with other nations. Trump exited the WHO with the stroke of a pen, and Mr. Biden can do the same in reverse.
Under Trump, the Centers for Medicare & Medicaid Services used waivers to weaken the ACA and allow states to alter their Medicaid programs. One waiver allows Georgia to leave the ACA exchanges and put brokers in charge of buying coverage. Other waivers allow states to transform federal Medicaid payments into block grants, which several states are planning to do.
The Trump CMS has allowed several states to use Medicaid waivers to add work requirements for Medicaid recipients. The courts have blocked the work rules so far, and the Biden CMS may decide to reverse these waivers or modify them.
“Undoing waivers is normally a fairly simple thing,” Mr. Levitt said. In January, however, the Trump CMS asked some waiver states to sign new contracts in which the CMS pledges not to end a waiver without 9 months’ notice. It’s unclear how many states signed such contracts and what obligation the Biden CMS has to enforce them.
The Trump CMS also stopped reimbursing insurers for waiving deductibles and copayments for low-income customers, as directed by the ACA. Without federal reimbursement, some insurers raised premiums by as much as 20% to cover the costs. It is unclear how the Biden CMS would tackle this change.
4. Negotiating lower drug prices
Allowing Medicare to negotiate drug prices, a major plank in Mr. Biden’s campaign, would seem like a slam dunk for the Democrats. This approach is backed by 89% of Americans, including 84% of Republicans, according to a Kaiser Family Foundation survey in December.
“With that level of support, it’s hard to go wrong politically on this issue,” Mr. Levitt said.
Many Republicans, however, do not favor negotiating drug prices, and the two parties continue to be far apart on how to control drug prices. Trump signed an action that allows Americans to buy cheaper drugs abroad, an approach that Mr. Biden also supports, but it is now tied up in the courts.
“A drug pricing bill has always been difficult to pass,” Dr. Whitlock said. “The issue is popular with the public, but change does not come easily. The drug lobby is one the strongest in Washington, and now it may be even stronger, since it was the drug companies that gave us the COVID vaccines.”
Dr. Whitlock said Republicans will want Democrats to compromise on drug pricing, but he doubts they will do so. The House passed a bill to negotiate drug prices last year, which never was voted on in the Senate. “It is difficult to imagine that the Democrats will be able to move rightward from that House bill,” Dr. Whitlock said. “Democrats are likely to stand pat on drug pricing.”
5. Introducing a public option
President Biden’s campaign proposal for a public option – health insurance offered by the federal government – and to lower the age for Medicare eligibility from 65 years to 60 years, resulted from a compromise between two factions of the Democratic party on how to expand coverage.
Although Mr. Biden and other moderates wanted to focus on fixing the ACA, Democrats led by Sen. Bernie Sanders of Vermont called for a single-payer system, dubbed “Medicare for all.” A public option was seen as the middle ground between the two camps.
“A public option would be a very controversial,” Dr. Whitlock said. Critics say it would pay at Medicare rates, which would reduce doctors’ reimbursements, and save very little money compared with a single-payer system.
Dr. Pearl sees similar problems with lowering the Medicare age. “This would be an expensive change that the federal government could not afford, particularly with all the spending on the pandemic,” he said. “And it would be tough on doctors and hospitals, because Medicare pays less than the private insurance payment they are now getting.”
“The public option is likely to get serious discussion within the Democratic caucus and get onto the Senate floor,” Mr. Levitt said. “The party won’t ignore it.” He notes that in the new Senate, Sen. Sanders chairs the budget committee, and from that position he is likely to push for expanding access to care.
Mr. Levitt says the Biden CMS might allow states to experiment with a statewide public option or even a single-payer model, but he concedes that states, with their budgets ravaged by COVID-19, do not currently have the money to launch such programs.
6. Reviving the CMS
Under President Obama, the CMS was the engine that implemented the ACA and shepherded wider use of value-based reimbursements, which reward providers for quality and outcomes rather than volume.
Under the Trump administration, CMS leadership continued to uphold value-based reimbursement, Dr. Pearl observed. “CMS leadership championed value-based payments, but they encountered a lot of pushback from doctors and hospitals and had to scale back their goals,” he said.
On the other hand, the Trump CMS took a 180-degree turn on the ACA and worked to take it apart. This took a toll on staff morale, according to Donald M. Berwick, MD, who ran the CMS under President Obama. “Many people in CMS did not feel supported during the Trump administration, and some of them left,” Dr. Berwick said.
The CMS needs experienced staff on board to write comprehensible rules and regulations that can overcome court challenges.
Having a fully functioning CMS also requires consistent leadership, which was a problem for Obama. When Mr. Obama nominated Dr. Berwick, 60 Senate votes were needed to confirm him, and Republicans would not vote for him. Mr. Obama eventually brought Dr. Berwick in as a recess appointment, but it meant he could serve for only 17 months.
Since then, Senate confirmation rules have changed so that only a simple majority is needed to confirm appointments. This is important for Biden’s nominees, Dr. Berwick said. “For a president, having your team in place means you are able to execute the policies you want,” he said. “You need to have consistent leadership.”
7. Potentially changing health care without Congress
Even with their newly won control of the Senate, the Democrats’ thin majorities in both houses of Congress may not be enough to pass much legislation if Republicans are solidly opposed.
Democrats in the House also have a narrow path this session in which to pass legislation. The Democratic leadership has an 11-vote majority, but it must contend with 15 moderate representatives in purple districts (where Democrats and Republicans have about equal support).
A bigger problem looms before the Democrats. In 2022, the party may well lose its majorities in both houses. Mr. Whitlock notes that the party of an incoming president normally loses seats in the first midterm election. “The last incoming president to keep both houses of Congress in his first midterm was Jimmy Carter,” he said.
If this happens, President Biden would have to govern without the support of Congress, which is what Barack Obama had to do through most of his presidency. As Mr. Obama’s vice president, Mr. Biden is well aware how that goes. Governing without Congress means relying on presidential orders and decrees.
In health care, Mr. Biden has a powerful policy-making tool, the Center for Medicare & Medicaid Innovation (CMMI). The CMMI was empowered by the ACA to initiate pilot programs for new payment models.
So far, the CMMI’s work has been mainly limited to accountable care organizations, bundled payments, and patient-centered medical homes, but it could also be used to enact new federal policies that would normally require Congressional action, Mr. Levitt said.
Conclusion
Expectations have been very high for what President Joe Biden can do in health care. He needs to unite a very divided political system to defeat a deadly pandemic, restore Obamacare, and sign landmark legislation, such as a drug-pricing bill.
But shepherding bills through Congress will be a challenge. “You need to have accountability, unity, and civility, which is a Herculean task,” Mr. Whitlock said. “You have to keep policies off the table that could blow up the bipartisanship.”
A version of this article first appeared on Medscape.com.
President Joe Biden has come into office after an unexpected shift in Congress. On Jan. 5, Democrats scored an upset by winning two U.S. Senate seats in runoff elections in Georgia, giving them control of the Senate.
Now the Democrats have control of all three levers of power – the Senate, the House, and the presidency – for the first time since the early years of the Obama administration.
How will President Biden use this new concentration of power to shape health care policy?
Democrats’ small majorities in both houses of Congress suggest that moderation and bipartisanship will be necessary to get things done. Moreover, Mr. Biden himself is calling for bipartisanship. “On this January day,” he said in his inauguration speech, “my whole soul is in this: Bringing America together, uniting our people, uniting our nation.”
Key health care actions that Mr. Biden could pursue include the following.
1. Passing a new COVID-19 relief bill
Above all, Mr. Biden is focused on overcoming the COVID-19 pandemic, which has been registering record deaths recently, and getting newly released vaccines to Americans.
“Dealing with the coronavirus pandemic is one of the most important battles our administration will face, and I will be informed by science and by experts,” the president said.
“There is no question that the pandemic is the highest priority for the Biden administration,” said Larry Levitt, executive vice president for health policy at the Henry J. Kaiser Family Foundation. “COVID will dominate the early weeks and months of this administration. His success rests, in particular, on improving the rollout of vaccines.”
Five days before his inauguration, the president-elect unveiled the American Rescue Plan, a massive, $1.9 trillion legislative package intended to hasten rollout of COVID-19 vaccines, improve COVID-19 testing, and provide financial help to businesses and individuals, among many other things.
The bill would add $1,400 to the recently passed $600 government relief payments for each American, amounting to a $2,000 check. It would also enact many non-COVID-19 measures, such as a $15-an-hour minimum wage and measures to bolster the Affordable Care Act (ACA).
If Democrats cannot reach a deal with the Republicans, they might turn the proposal into a reconciliation bill, which could then be passed with a simple majority. However, drafting a reconciliation bill is a long, complicated process that would require removing provisions that don’t meet the requirements of reconciliation, said Hazen Marshall, a Washington lobbyist and former staffer for Sen. Mitch McConnell.
Most importantly, Mr. Marshall said, reconciliation bills bring out diehard partisanship. “They involve a sledgehammer mentality,” he says. “You’re telling the other side that their views aren’t going to matter.” The final version of the ACA, for example, was passed as a reconciliation bill, with not one Republican vote.
In the Trump years, “the last four reconciliation bills did not get any votes from the minority,” added Rodney Whitlock, PhD, a political consultant at McDermott+Consulting, who worked 21 years for Republicans in the House. “When the majority chooses to use reconciliation, it is an admission that it has no interest in working with the minority.”
Hammering out a compromise will be tough, but Robert Pearl MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University, said that if anyone can do it, it would be President Biden. Having served in the Senate for 36 years, “Biden knows Congress better than any president since Lyndon Johnson,” he said. “He can reach across the aisle and get legislation passed as much as anyone could these days.”
2. Restoring Obamacare
Mr. Biden has vowed to undo a gradual dismantling of the ACA that went on during the Trump administration through executive orders, rule-making, and new laws. “Reinvigorating the ACA was a central part of Biden’s platform as a candidate,” Mr. Levitt said.
Each Trump action against the ACA must be undone in the same way. Presidential orders must be met with presidential orders, regulations with regulations, and legislation with legislation.
The ACA is also being challenged in the Supreme Court. Republicans under Trump passed a law that reduced the penalty for not buying health insurance under the ACA to zero. Then a group of 20 states, led by Texas, filed a lawsuit asserting that this change makes the ACA unconstitutional.
The lawsuit was heard by the Supreme Court in November. From remarks made by the justices then, it appears that the court might well uphold the law when a verdict comes down in June.
But just in case, Mr. Biden wants Congress to enact a small penalty for not buying health insurance, which would remove the basis of the lawsuit.
Mr. Biden’s choice for secretary of Health and Human Services shows his level of commitment to protecting the ACA. His HHS nominee is California Attorney General Xavier Becerra, who led a group of 17 states defending the ACA in the current lawsuit.
In addition to undoing Trump’s changes, Mr. Biden plans to expand the ACA beyond the original legislation. The new COVID-19 bill contains provisions that would expand subsidies to buy insurance on the exchanges and would lower the maximum percentage of income that anyone has to pay for health insurance to 8.5%.
Dealing with Medicaid is also related to the ACA. In 2012, the Supreme Court struck down a mandate that states expand their Medicaid programs, with substantial funding from the federal government.
To date, 12 states still do not participate in the Medicaid expansion. To lure them into the expansion, the Democrat-controlled House last session passed a bill that would offer to pay the entire bill for the first 3 years of Medicaid expansion if they chose to enact an expansion.
3. Undoing other Trump actions in health care
In addition to changes in the ACA, Trump also enacted a number of other changes in health care that President Biden could undo. For example, Mr. Biden says he will reenter the World Health Organization (WHO) so that the United States could better coordinate a COVID-19 response with other nations. Trump exited the WHO with the stroke of a pen, and Mr. Biden can do the same in reverse.
Under Trump, the Centers for Medicare & Medicaid Services used waivers to weaken the ACA and allow states to alter their Medicaid programs. One waiver allows Georgia to leave the ACA exchanges and put brokers in charge of buying coverage. Other waivers allow states to transform federal Medicaid payments into block grants, which several states are planning to do.
The Trump CMS has allowed several states to use Medicaid waivers to add work requirements for Medicaid recipients. The courts have blocked the work rules so far, and the Biden CMS may decide to reverse these waivers or modify them.
“Undoing waivers is normally a fairly simple thing,” Mr. Levitt said. In January, however, the Trump CMS asked some waiver states to sign new contracts in which the CMS pledges not to end a waiver without 9 months’ notice. It’s unclear how many states signed such contracts and what obligation the Biden CMS has to enforce them.
The Trump CMS also stopped reimbursing insurers for waiving deductibles and copayments for low-income customers, as directed by the ACA. Without federal reimbursement, some insurers raised premiums by as much as 20% to cover the costs. It is unclear how the Biden CMS would tackle this change.
4. Negotiating lower drug prices
Allowing Medicare to negotiate drug prices, a major plank in Mr. Biden’s campaign, would seem like a slam dunk for the Democrats. This approach is backed by 89% of Americans, including 84% of Republicans, according to a Kaiser Family Foundation survey in December.
“With that level of support, it’s hard to go wrong politically on this issue,” Mr. Levitt said.
Many Republicans, however, do not favor negotiating drug prices, and the two parties continue to be far apart on how to control drug prices. Trump signed an action that allows Americans to buy cheaper drugs abroad, an approach that Mr. Biden also supports, but it is now tied up in the courts.
“A drug pricing bill has always been difficult to pass,” Dr. Whitlock said. “The issue is popular with the public, but change does not come easily. The drug lobby is one the strongest in Washington, and now it may be even stronger, since it was the drug companies that gave us the COVID vaccines.”
Dr. Whitlock said Republicans will want Democrats to compromise on drug pricing, but he doubts they will do so. The House passed a bill to negotiate drug prices last year, which never was voted on in the Senate. “It is difficult to imagine that the Democrats will be able to move rightward from that House bill,” Dr. Whitlock said. “Democrats are likely to stand pat on drug pricing.”
5. Introducing a public option
President Biden’s campaign proposal for a public option – health insurance offered by the federal government – and to lower the age for Medicare eligibility from 65 years to 60 years, resulted from a compromise between two factions of the Democratic party on how to expand coverage.
Although Mr. Biden and other moderates wanted to focus on fixing the ACA, Democrats led by Sen. Bernie Sanders of Vermont called for a single-payer system, dubbed “Medicare for all.” A public option was seen as the middle ground between the two camps.
“A public option would be a very controversial,” Dr. Whitlock said. Critics say it would pay at Medicare rates, which would reduce doctors’ reimbursements, and save very little money compared with a single-payer system.
Dr. Pearl sees similar problems with lowering the Medicare age. “This would be an expensive change that the federal government could not afford, particularly with all the spending on the pandemic,” he said. “And it would be tough on doctors and hospitals, because Medicare pays less than the private insurance payment they are now getting.”
“The public option is likely to get serious discussion within the Democratic caucus and get onto the Senate floor,” Mr. Levitt said. “The party won’t ignore it.” He notes that in the new Senate, Sen. Sanders chairs the budget committee, and from that position he is likely to push for expanding access to care.
Mr. Levitt says the Biden CMS might allow states to experiment with a statewide public option or even a single-payer model, but he concedes that states, with their budgets ravaged by COVID-19, do not currently have the money to launch such programs.
6. Reviving the CMS
Under President Obama, the CMS was the engine that implemented the ACA and shepherded wider use of value-based reimbursements, which reward providers for quality and outcomes rather than volume.
Under the Trump administration, CMS leadership continued to uphold value-based reimbursement, Dr. Pearl observed. “CMS leadership championed value-based payments, but they encountered a lot of pushback from doctors and hospitals and had to scale back their goals,” he said.
On the other hand, the Trump CMS took a 180-degree turn on the ACA and worked to take it apart. This took a toll on staff morale, according to Donald M. Berwick, MD, who ran the CMS under President Obama. “Many people in CMS did not feel supported during the Trump administration, and some of them left,” Dr. Berwick said.
The CMS needs experienced staff on board to write comprehensible rules and regulations that can overcome court challenges.
Having a fully functioning CMS also requires consistent leadership, which was a problem for Obama. When Mr. Obama nominated Dr. Berwick, 60 Senate votes were needed to confirm him, and Republicans would not vote for him. Mr. Obama eventually brought Dr. Berwick in as a recess appointment, but it meant he could serve for only 17 months.
Since then, Senate confirmation rules have changed so that only a simple majority is needed to confirm appointments. This is important for Biden’s nominees, Dr. Berwick said. “For a president, having your team in place means you are able to execute the policies you want,” he said. “You need to have consistent leadership.”
7. Potentially changing health care without Congress
Even with their newly won control of the Senate, the Democrats’ thin majorities in both houses of Congress may not be enough to pass much legislation if Republicans are solidly opposed.
Democrats in the House also have a narrow path this session in which to pass legislation. The Democratic leadership has an 11-vote majority, but it must contend with 15 moderate representatives in purple districts (where Democrats and Republicans have about equal support).
A bigger problem looms before the Democrats. In 2022, the party may well lose its majorities in both houses. Mr. Whitlock notes that the party of an incoming president normally loses seats in the first midterm election. “The last incoming president to keep both houses of Congress in his first midterm was Jimmy Carter,” he said.
If this happens, President Biden would have to govern without the support of Congress, which is what Barack Obama had to do through most of his presidency. As Mr. Obama’s vice president, Mr. Biden is well aware how that goes. Governing without Congress means relying on presidential orders and decrees.
In health care, Mr. Biden has a powerful policy-making tool, the Center for Medicare & Medicaid Innovation (CMMI). The CMMI was empowered by the ACA to initiate pilot programs for new payment models.
So far, the CMMI’s work has been mainly limited to accountable care organizations, bundled payments, and patient-centered medical homes, but it could also be used to enact new federal policies that would normally require Congressional action, Mr. Levitt said.
Conclusion
Expectations have been very high for what President Joe Biden can do in health care. He needs to unite a very divided political system to defeat a deadly pandemic, restore Obamacare, and sign landmark legislation, such as a drug-pricing bill.
But shepherding bills through Congress will be a challenge. “You need to have accountability, unity, and civility, which is a Herculean task,” Mr. Whitlock said. “You have to keep policies off the table that could blow up the bipartisanship.”
A version of this article first appeared on Medscape.com.
On receiving the COVID-19 vaccine
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.