User login
Limiting antibiotic therapy after surgical drainage for native joint bacterial arthritis
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
The state of inpatient COVID-19 care
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.
At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
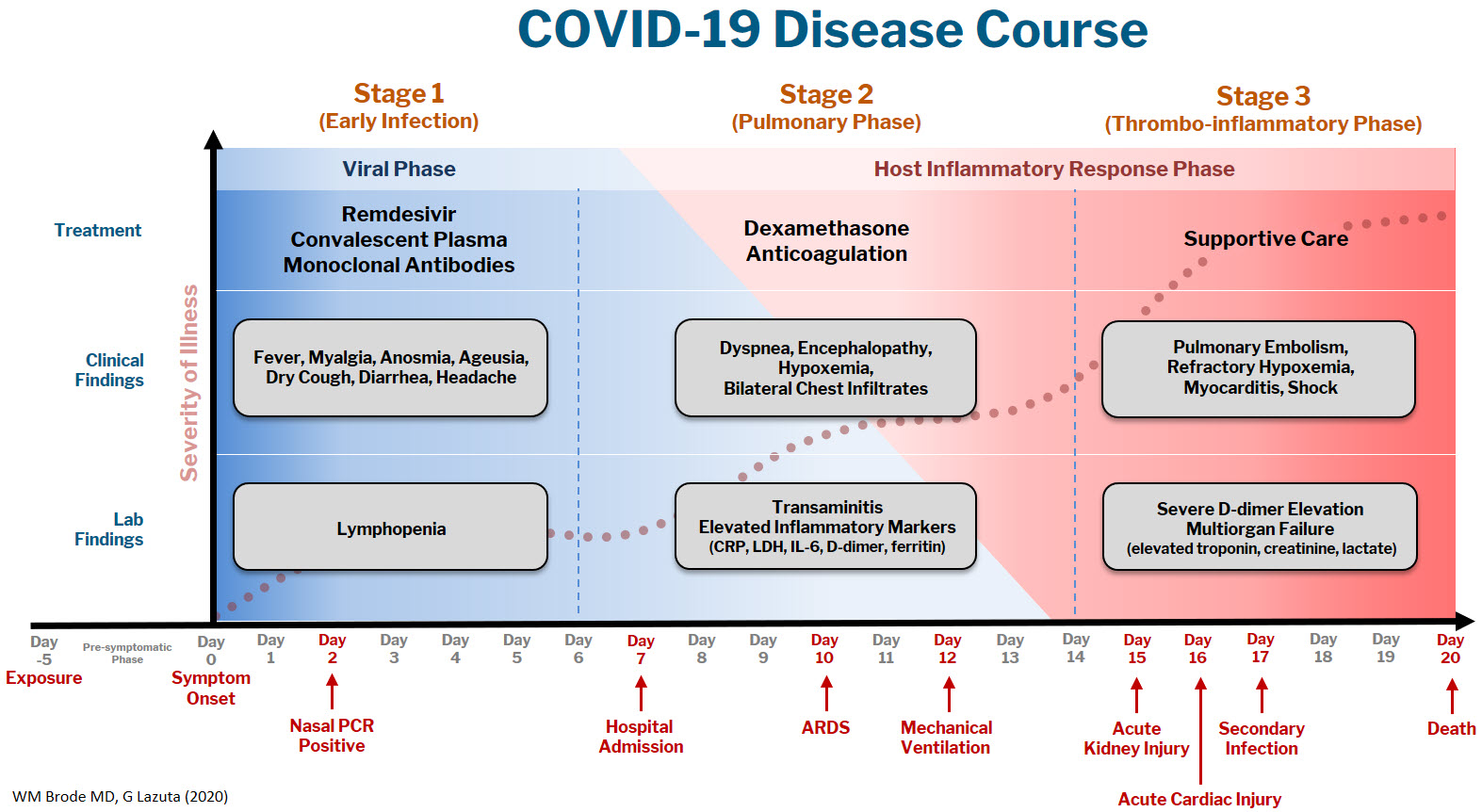
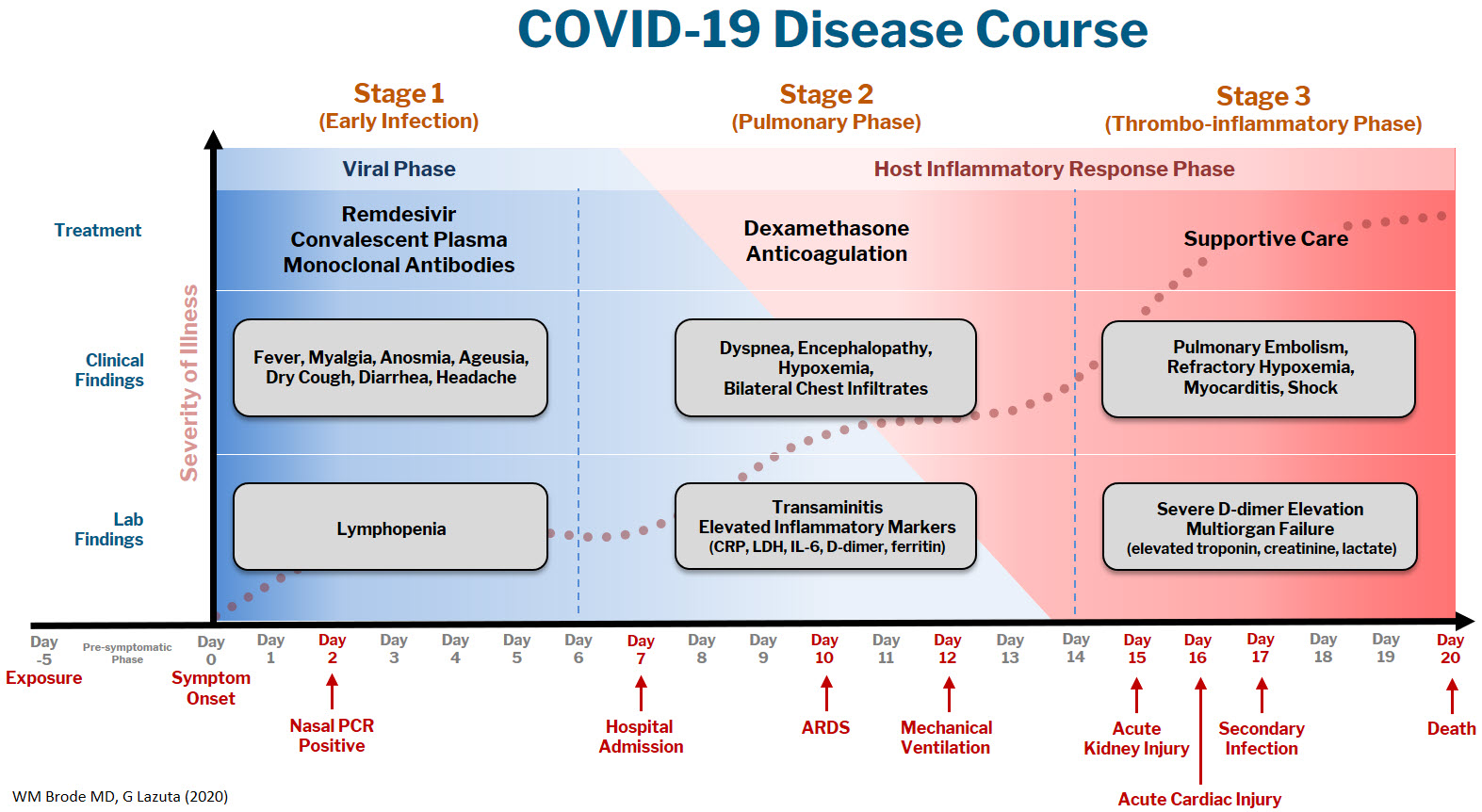
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
A brief evidence-based review of everything we have learned
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
President Biden kicks off health agenda with COVID actions, WHO outreach
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
COVID-19 may damage blood vessels in the brain
Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Dr. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Dr. Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung‑Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Dr. Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Dr. Cervantes has no relevant financial disclosures.
Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Dr. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Dr. Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung‑Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Dr. Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Dr. Cervantes has no relevant financial disclosures.
Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Dr. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Dr. Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung‑Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Dr. Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Dr. Cervantes has no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Think twice before intensifying BP regimen in older hospitalized patients
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Further warning on SGLT2 inhibitor use and DKA risk in COVID-19
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
COVID-19 in children: Latest weekly increase is largest yet
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
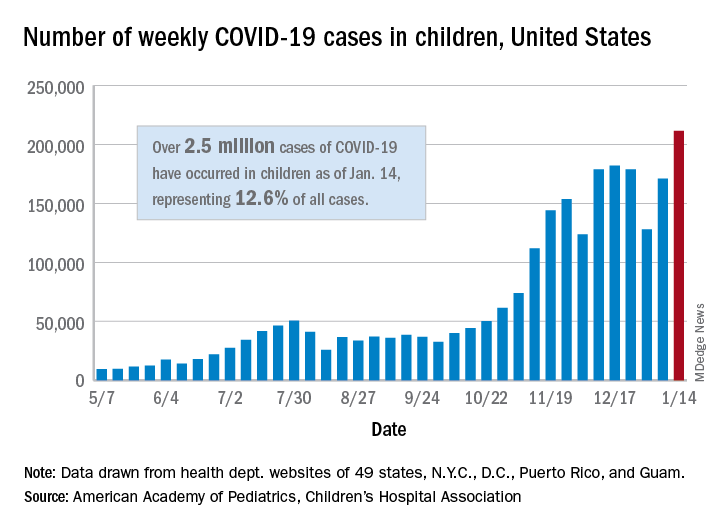
There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
Biomarker HF risk score envisioned as SGLT2 inhibitor lodestar in diabetes
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
HHS will drop buprenorphine waiver rule for most physicians
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Covert stroke after noncardiac surgery linked with cognitive decline
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.