User login
Individualizing immunization for international travelers
International travel, whether for business, pleasure, child adoption, medical tourism, or adventure, continues to grow. In 2015, more than 70 million US citizens traveled internationally.1 Many individuals contact family physicians first about their plans for travel and questions about travel-related health advice. This article provides an overview of the vaccines recommended for travelers headed to international destinations. Because country-specific vaccination recommendations and requirements for entry and departure change over time, check the Centers for Disease Control and Prevention (CDC) Web site for up-to-date requirements and recommendations (www.cdc.gov/travel).
Vaccine schedules vary according to destination and individual risks
There is no single vaccination schedule that applies to all travelers. Each schedule should be individualized based on the traveler’s destination, risk assessment, previous immunizations, health status, and time available before departure.2,3 Pregnant or immunocompromised travelers should seek advice from an experienced travel medicine consultant on the immunization recommendations specifically meant for them.4,5
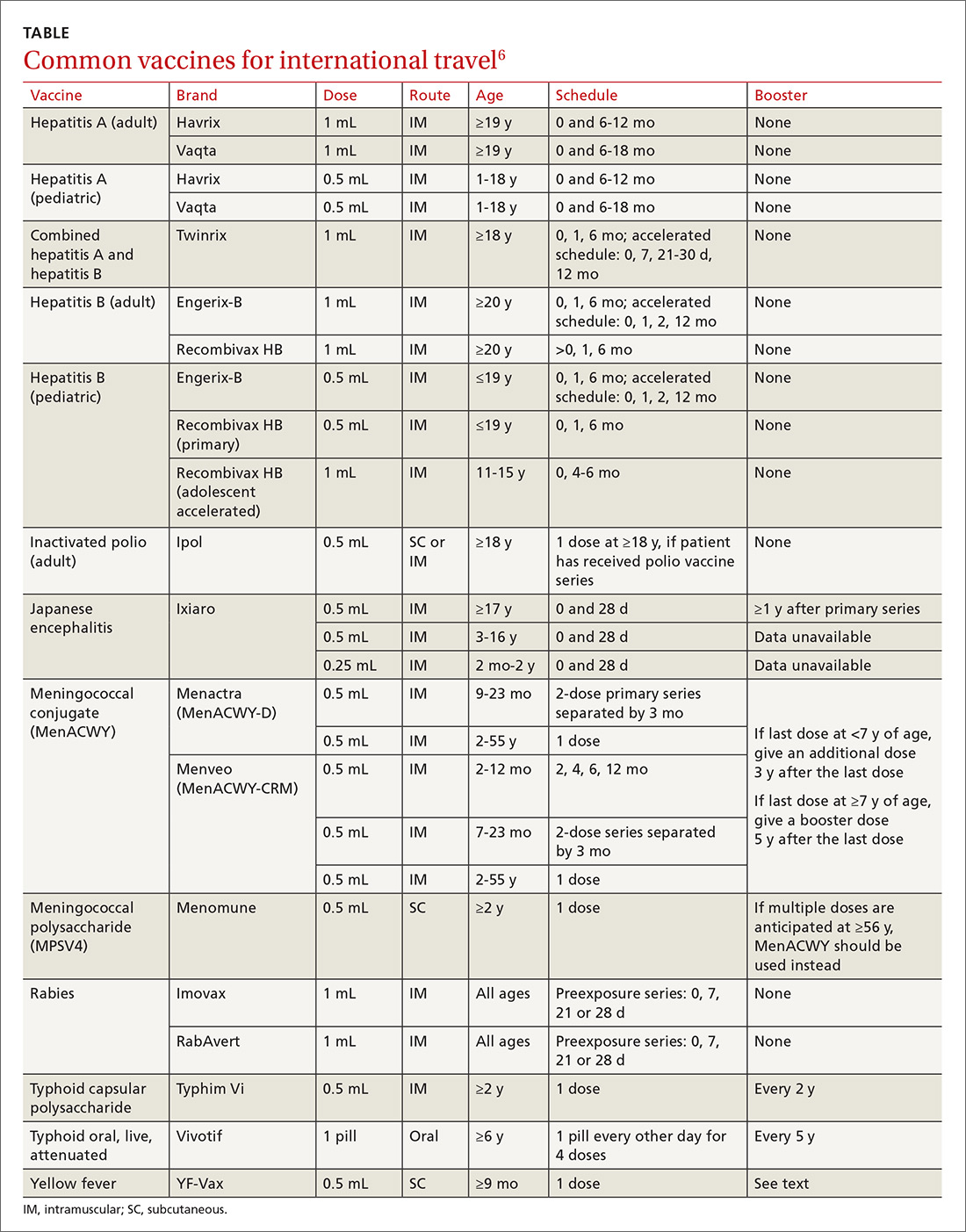
Travel vaccines (TABLE6) are generally categorized as routine, required, or recommended.
- Routine vaccines are the standard child and adult immunizations recommended by the Advisory Committee on Immunization Practices (ACIP). These include such vaccines as diphtheria-tetanus toxoids-acellular pertussis (DTaP), inactivated polio vaccine (IPV), Haemophilus influenzae type b (Hib), hepatitis B, rotavirus and pneumococcal vaccines, and human papillomavirus (HPV).
- Required vaccines—eg, yellow fever and meningococcal vaccines—must be documented on the International Certificate of Vaccination before entry into certain countries.
- Recommended vaccines are advised based on the travel destination and anticipated activities. These would include vaccines for typhoid, rabies, Japanese encephalitis, and polio (adult booster).
Routine vaccinations may need to be accelerated
Pre-travel patient encounters are an opportunity to update routine vaccinations.7,8 Immunization against childhood diseases remains suboptimal in developing countries, where vaccine-preventable illnesses occur more frequently.9
Routine vaccines may be administered on an accelerated basis depending on geographic destination, seasonal disease variations, anticipated exposures, and known outbreaks at the time of travel.
MMR vaccine. Measles is still common in many parts of the world, and unvaccinated or incompletely vaccinated travelers are at risk of acquiring the disease and importing it to the United States (see “Measles: Why it’s still a threat,” 2017;66:446-449.) In 2015, a large, widespread measles outbreak occurred in the United States, linked to an amusement park in California, likely originating with an infected traveler who visited the park.10
All children older than 12 months should receive 2 doses of measles-mumps-rubella (MMR) vaccine separated by at least 28 days before departure (regardless of their destination). Infants between 6 and 11 months are at risk for high morbidity and may therefore receive a single dose of MMR earlier than the routinely recommended age of 12 to 15 months. Adolescents and adults without evidence of immunity against measles should get 2 doses of MMR separated by at least 28 days.11 Acceptable presumptive evidence of immunity against measles includes written documentation of adequate vaccination, laboratory evidence of immunity, laboratory confirmation of measles, or birth before 1957.
Varicella vaccine. Children, adolescents, and young adults who have received only one dose of varicella should get a second dose prior to departure. For children 7 to 12 years, the recommended minimum interval between doses is 3 months. For individuals 13 years or older, the minimum interval is 4 weeks.7,8
Influenza vaccine is routinely recommended for all travelers 6 months of age or older, as flu season varies geographically. Flu season in the Northern Hemisphere may begin as early as October and can extend until May. In the Southern Hemisphere, it may begin in April and last through September. Travelers should be vaccinated at least 2 weeks before travel in order to develop adequate immunity.12,13
Required vaccinations: Proof is needed before traveling
Yellow fever (YF) is a mosquito-borne viral illness characterized by fever, chills, headache, myalgia, and vomiting. The disease can progress to coagulopathy, shock, and multisystem organ failure.14 YF vaccine is recommended for individuals 9 months or older who are traveling to or living in areas of South America or Africa where YF virus transmission is common (map: http://www.cdc.gov/yellowfever/maps/).
YF vaccine is a live-attenuated virus formulation and, therefore, should not be given to individuals with primary immunodeficiencies, transplant recipients or patients on immunosuppressive and immunomodulatory therapies, or patients with human immunodeficiency virus (HIV) whose CD4 count is below 200/mL. Other contraindications to YF vaccine are age younger than 6 months, allergy to a vaccine component, and thymic disorders. Serious adverse reactions to the vaccine are rare, but include 2 syndromes: YF-associated neurotropic disease and YF vaccine-associated viscerotropic disease.15
In many YF-endemic countries, vaccination is legally required for entry, and proof of vaccination must be documented on an International Certificate of Vaccination or Prophylaxis (ICVP). Additionally, some countries may require proof of vaccination before allowing travel through an endemic region, to prevent introduction of the disease elsewhere. Travelers with a specific contraindication to YF vaccine should obtain a waiver from a physician before traveling to a country requiring vaccination.16
The vaccination certificate is valid beginning 10 days after administration of YF vaccine. Immunity after a single dose is long lasting and may provide lifetime protection. Previously, re-vaccination was required every 10 years; however, in February 2015, ACIP approved a new recommendation stating a single dose of YF vaccine is adequate for most travelers.1
Although ACIP no longer recommends booster doses of YF vaccine for most travelers, clinicians and travelers should review the entry requirements for destination countries because changes to the International Health Regulations have not yet been fully implemented. Once this change is instituted, a completed ICVP will be valid for the lifetime of the vaccine.18,19 Country-specific requirements for YF can be found at http://www.cdc.gov/yellowfever/maps/. (Click on the link below the appropriate map.) In the United States, the YF vaccine is distributed only through approved vaccination centers. These designated clinics are listed in a registry on the CDC travel Web site at https://wwwnc.cdc.gov/travel/yellow-fever-vaccination-clinics/search.
Meningococcal disease. ACIP recommends routine vaccination against meningococcal disease for people 11 to 18 years of age and for individuals with persistent complement component deficiency, functional or anatomic asplenia, and HIV. Vaccination is recommended for travelers who visit or reside in areas where meningococcal disease is hyperendemic or epidemic, such as the meningitis belt of sub-Saharan Africa during the dry season of December to June (map: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/meningococcal-disease). Travelers to Saudi Arabia during the annual Hajj and Umrah pilgrimages are required to have a certificate of vaccination with quadrivalent (serogroups A, C, Y, W-135) meningococcal vaccine issued within 3 years (and not less than 10 days) before entry.
Several meningococcal vaccines are available in the United States. The quadrivalent vaccines are Menactra (MenACWY-D, Sanofi Pasteur) and Menveo (MenACWY-CRM, GSK). A bivalent (serogroups C and Y) conjugate vaccine MenHibrix (Hib-MenCY-TT, GSK) is also licensed for use in the United States, but infants traveling to areas with high endemic rates of meningococcal disease who received this vaccine are not protected against serogroups A and W and should receive quadrivalent meningococcal conjugate vaccine. Serogroup B vaccination is not routinely recommended for travelers. Approximately 7 to 10 days are required after vaccination for the development of protective antibody levels.7,8,20,21
Polio. Although polio has been nearly eradicated, as of the time this article was written, the disease has not been eliminated in Afghanistan, Guinea, Laos, Nigeria, or Pakistan. Other countries, such as Cameroon, Chad, and Ukraine remain vulnerable to international transmission.22 The CDC recommends that adults who are traveling to areas where wild polio virus (WPV) has circulated in the last 12 months and who are unvaccinated, incompletely vaccinated, or whose vaccination status is unknown should receive a series of 3 doses of IPV to prevent ongoing spread.23 Adults who completed the polio vaccine series as children and are traveling to areas where WPV has circulated in the last 12 months should receive a one-time booster dose of IPV.23
Infants and children in the United States should be vaccinated against polio as part of a routine age-appropriate series. If a child cannot complete the routine series before departure and is traveling to an area where WPV has circulated in the last 12 months, an accelerated schedule is recommended. Vaccination should be documented on the ICVP, as countries with active spread of poliovirus may require proof of polio vaccination upon exit. A list of the countries where the polio virus is currently circulating is available at http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/.
Both routine and accelerated vaccination schedules for children and adults are published annually by the CDC and are available at http://www.cdc.gov/vaccines/schedules/hcp/index.html.
Recommended vaccines
Japanese encephalitis (JE) is endemic throughout most of Asia and parts of the Western Pacific region (map: http://www.cdc.gov/japaneseencephalitis/maps/). JE vaccine is recommended for travelers who plan to spend more than a month in endemic areas during the JE virus transmission season. (In temperate areas of Asia, JE virus transmission is seasonal and usually peaks in the summer and fall. In the subtropics and tropics, transmission can occur year-round, often with a peak during the rainy season.)
This recommendation includes recurrent travelers or expatriates who are likely to visit endemic rural or agricultural areas during a high-risk period of JE virus transmission. Risk is low for travelers who spend less than a month in endemic areas and for those who confine their travel to urban centers. Nevertheless, vaccination should be considered if travel is planned for outside an urban area and includes such activities as camping, hiking, trekking, biking, fishing, hunting, or farming. Inactivated Vero cell culture-derived vaccine (Ixiaro) is the only JE vaccine licensed and available in the United States. Ixiaro is given as a 2-dose series, with the doses spaced 28 days apart. The last dose should be given at least one week before travel.24
Typhoid fever. Vaccination against typhoid fever is recommended for travelers to highly endemic areas such as the Indian subcontinent, Africa, and Central and South America. Two typhoid vaccines are available: Vi capsular polysaccharide vaccine (ViCPS) administered intramuscularly (IM), and oral live attenuated vaccine (Ty21a). Ty21a is a live vaccine and should not be given to immunocompromised people or those taking antibiotics, as it may reduce immunogenicity. Ty21a must be kept refrigerated at 35.6° F to 46.4° F (2° C - 8° C) and administered with cool liquid no warmer than 98.6° F (37° C). Both vaccines are only 50% to 80% efficacious, making access to clean food and water essential.3,5,25
Hepatitis A vaccine should be given to all children older than one year traveling to areas where there is an intermediate or high risk of the disease. Children younger than one year who are traveling to high-risk areas can receive a single dose of immunoglobulin (IG) 0.02 mL/kg IM, which provides protection for up to 3 months. One 0.06 mL/kg-dose IM provides protection for 3 to 5 months.
If travel continues, children should receive a second dose after 5 months. IG does not interfere with the response to YF vaccine, but can interfere with the response to other live injected vaccines (such as MMR and varicella).26
Hepatitis B vaccination should be administered to all unvaccinated travelers who plan to visit an area with intermediate to high prevalence of chronic hepatitis B (HBV surface antigen prevalence ≥2%). Unvaccinated travelers who may engage in high-risk sexual activity or injection drug use should receive hepatitis B vaccine regardless of destination. Additionally, travelers who access medical care for injury or illness while abroad may also be at risk of acquiring hepatitis B via contaminated blood products or medical equipment.27
Serologic testing and booster vaccination are not recommended before travel for immunocompetent adults who have been previously vaccinated. The combined hepatitis A and B vaccine provides effective and convenient dual protection for travelers and can be administered with an accelerated 0-, 7-, and 21-day schedule for last-minute travelers.7,8
Rabies remains endemic in developing countries of Africa and Asia, where appropriate post-exposure prophylaxis is limited or non-existent.28 Consider pre-exposure rabies prophylaxis for traveling patients based on the availability of rabies vaccine and immunoglobulin in their destination area, planned duration of stay, and the likelihood of animal exposure (eg, veterinarians, animal handlers, cavers, missionaries). Advise travelers who decline vaccination to avoid or minimize animal contact during travel. In the event the traveler sustains an animal bite or scratch, immediate cleansing of the wound substantially reduces the risk of infection, especially when followed by timely administration of post-exposure prophylaxis.
Post-exposure prophylaxis for unvaccinated individuals consists of local infiltration of rabies immunoglobulin at the site of the bite and a series of 4 injections of rabies vaccine over 14 days, or 5 doses over one month for immunosuppressed patients. The first dose of the 4-dose course should be administered as soon as possible after exposure. Two vaccines are licensed for use in the United States: human diploid cell vaccine (HDCV, Imovax Rabies, Sanofi Pasteur) and purified chick embryo cell vaccine (PCECV, RabAvert, Novartis Vaccines and Diagnostics). The vaccine should never be administered in the gluteal area, as this may result in lower antibody titers.29
Additionally, promising new vaccines against malaria and dengue fever are under clinical development and may be available in the near future.
CORRESPONDENCE
Vini Vijayan, MD, Division of Infectious Diseases, Arkansas Children's Hospital, 1 Children's Way, Slot 512-11, Little Rock, AR 72202; [email protected].
1. U.S. Department of Commerce, International Trade Administration, National Travel and Tourism Office (NTTO). 2015. Available at: http://travel.trade.gov/view/m-2015-O-001/index.html. Accessed July 12, 2017.
2. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499-1539.
3. Centers for Disease Control and Prevention. The pre-travel consultation. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/the-pre-travel-consultation. Accessed June 20, 2017.
4. Hochberg NS, Barnett ED, Chen LH, et al. International travel by persons with medical comorbidities: understanding risks and providing advice. Mayo Clin Proc. 2013;88:1231-1240.
5. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44-e100.
6. Centers for Disease Control and Prevention. Yellow Book table of contents: Chapter 3. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/table-of-contents. Accessed July 21, 2017.
7. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65;86-87.
8. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:88-90.
9. Boggild AK, Castelli F, Gautret P, et al. Vaccine preventable diseases in returned international travelers: results from the GeoSentinel Surveillance Network. Vaccine. 2010;28:7389-7395.
10. Sotir MJ, Esposito DH, Barnett ED, et al. Measles in the 21st century, a continuing preventable risk to travelers: data from the GeoSentinel Global Network. Clin Infect Dis. 2016;62:210-212.
11. Measles. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015:535-546.
12. Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
13. Marti F, Steffen R, Mutsch M. Influenza vaccine: a travelers’ vaccine? Expert Rev Vaccines. 2008;7:679-687.
14. Monath T, Gershman MD, Staples JE, et al. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 6th ed. London, England: W.B. Saunders; 2013:870-968.
15. Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-27.
16. World Health Organization. International Health Regulations. 2nd ed. Geneva, Switzerland: World Health Organization; 2005. Available at: http://whqlibdoc.who.int/publications/2008/9789241580410_eng.pdf. Accessed June 20, 2017.
17. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices: summary report. February 26, 2015. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2015-02.pdf. Accessed July 20, 2017.
18. Staples JE, Bocchini JA Jr, Rubin L, et al. Yellow fever vaccine booster doses: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:647-650.
19. World Health Organization. International travel and health: World–yellow fever vaccination booster. Geneva, Switzerland: World Health Organization; 2014. Available at: http://www.who.int/ith/updates/20140605/en. Accessed June 20, 2017.
20. Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1-28.
21. Memish ZA, Stephens GM, Steffen R, et al. Emergence of medicine for mass gatherings: lessons from the Hajj. Lancet Infect Dis. 2012;12:56-65.
22. World Health Organization. Twelfth meeting of the Emergency Committee under the International Health Regulations (2015) regarding the international spread of poliovirus. Available at: http://www.who.int/mediacentre/news/statements/2017/poliovirus-twelfth-ec/en/. Accessed June 21, 2017.
23. Centers for Disease Control and Prevention. Interim CDC Guidance for Travel to and from Countries Affected by the New Polio Vaccine Requirements. Available at http://wwwnc.cdc.gov/travel/news-announcements/polio-guidance-new-requirements. Accessed August 1, 2017.
24. Centers for Disease Control and Prevention. Use of Japanese encephalitis vaccine in children: recommendations of the advisory committee on immunization practices, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:898-900.
25. Mahon BE, Newton AE, Mintz ED. Effectiveness of typhoid vaccination in US travelers. Vaccine. 2014;32:3577-3579.
26. Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55:1-23.
27. Vivancos R, Abubakar I, Hunter PR. Foreign travel, casual sex, and sexually transmitted infections: systematic review and meta-analysis. Int J Infect Dis. 2010;14:e842-e851.
28. Gautret P, Harvey K, Pandey P, et al for the GeoSentinel Surveillance Network. Animal-associated exposure to rabies virus among travelers, 1997-2012. Emerg Infect Dis. 2015;21:569-577.
29. Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2010;59:1-9.
International travel, whether for business, pleasure, child adoption, medical tourism, or adventure, continues to grow. In 2015, more than 70 million US citizens traveled internationally.1 Many individuals contact family physicians first about their plans for travel and questions about travel-related health advice. This article provides an overview of the vaccines recommended for travelers headed to international destinations. Because country-specific vaccination recommendations and requirements for entry and departure change over time, check the Centers for Disease Control and Prevention (CDC) Web site for up-to-date requirements and recommendations (www.cdc.gov/travel).
Vaccine schedules vary according to destination and individual risks
There is no single vaccination schedule that applies to all travelers. Each schedule should be individualized based on the traveler’s destination, risk assessment, previous immunizations, health status, and time available before departure.2,3 Pregnant or immunocompromised travelers should seek advice from an experienced travel medicine consultant on the immunization recommendations specifically meant for them.4,5
Travel vaccines (TABLE6) are generally categorized as routine, required, or recommended.
- Routine vaccines are the standard child and adult immunizations recommended by the Advisory Committee on Immunization Practices (ACIP). These include such vaccines as diphtheria-tetanus toxoids-acellular pertussis (DTaP), inactivated polio vaccine (IPV), Haemophilus influenzae type b (Hib), hepatitis B, rotavirus and pneumococcal vaccines, and human papillomavirus (HPV).
- Required vaccines—eg, yellow fever and meningococcal vaccines—must be documented on the International Certificate of Vaccination before entry into certain countries.
- Recommended vaccines are advised based on the travel destination and anticipated activities. These would include vaccines for typhoid, rabies, Japanese encephalitis, and polio (adult booster).

Routine vaccinations may need to be accelerated
Pre-travel patient encounters are an opportunity to update routine vaccinations.7,8 Immunization against childhood diseases remains suboptimal in developing countries, where vaccine-preventable illnesses occur more frequently.9
Routine vaccines may be administered on an accelerated basis depending on geographic destination, seasonal disease variations, anticipated exposures, and known outbreaks at the time of travel.
MMR vaccine. Measles is still common in many parts of the world, and unvaccinated or incompletely vaccinated travelers are at risk of acquiring the disease and importing it to the United States (see “Measles: Why it’s still a threat,” 2017;66:446-449.) In 2015, a large, widespread measles outbreak occurred in the United States, linked to an amusement park in California, likely originating with an infected traveler who visited the park.10
All children older than 12 months should receive 2 doses of measles-mumps-rubella (MMR) vaccine separated by at least 28 days before departure (regardless of their destination). Infants between 6 and 11 months are at risk for high morbidity and may therefore receive a single dose of MMR earlier than the routinely recommended age of 12 to 15 months. Adolescents and adults without evidence of immunity against measles should get 2 doses of MMR separated by at least 28 days.11 Acceptable presumptive evidence of immunity against measles includes written documentation of adequate vaccination, laboratory evidence of immunity, laboratory confirmation of measles, or birth before 1957.
Varicella vaccine. Children, adolescents, and young adults who have received only one dose of varicella should get a second dose prior to departure. For children 7 to 12 years, the recommended minimum interval between doses is 3 months. For individuals 13 years or older, the minimum interval is 4 weeks.7,8
Influenza vaccine is routinely recommended for all travelers 6 months of age or older, as flu season varies geographically. Flu season in the Northern Hemisphere may begin as early as October and can extend until May. In the Southern Hemisphere, it may begin in April and last through September. Travelers should be vaccinated at least 2 weeks before travel in order to develop adequate immunity.12,13
Required vaccinations: Proof is needed before traveling
Yellow fever (YF) is a mosquito-borne viral illness characterized by fever, chills, headache, myalgia, and vomiting. The disease can progress to coagulopathy, shock, and multisystem organ failure.14 YF vaccine is recommended for individuals 9 months or older who are traveling to or living in areas of South America or Africa where YF virus transmission is common (map: http://www.cdc.gov/yellowfever/maps/).
YF vaccine is a live-attenuated virus formulation and, therefore, should not be given to individuals with primary immunodeficiencies, transplant recipients or patients on immunosuppressive and immunomodulatory therapies, or patients with human immunodeficiency virus (HIV) whose CD4 count is below 200/mL. Other contraindications to YF vaccine are age younger than 6 months, allergy to a vaccine component, and thymic disorders. Serious adverse reactions to the vaccine are rare, but include 2 syndromes: YF-associated neurotropic disease and YF vaccine-associated viscerotropic disease.15
In many YF-endemic countries, vaccination is legally required for entry, and proof of vaccination must be documented on an International Certificate of Vaccination or Prophylaxis (ICVP). Additionally, some countries may require proof of vaccination before allowing travel through an endemic region, to prevent introduction of the disease elsewhere. Travelers with a specific contraindication to YF vaccine should obtain a waiver from a physician before traveling to a country requiring vaccination.16
The vaccination certificate is valid beginning 10 days after administration of YF vaccine. Immunity after a single dose is long lasting and may provide lifetime protection. Previously, re-vaccination was required every 10 years; however, in February 2015, ACIP approved a new recommendation stating a single dose of YF vaccine is adequate for most travelers.1
Although ACIP no longer recommends booster doses of YF vaccine for most travelers, clinicians and travelers should review the entry requirements for destination countries because changes to the International Health Regulations have not yet been fully implemented. Once this change is instituted, a completed ICVP will be valid for the lifetime of the vaccine.18,19 Country-specific requirements for YF can be found at http://www.cdc.gov/yellowfever/maps/. (Click on the link below the appropriate map.) In the United States, the YF vaccine is distributed only through approved vaccination centers. These designated clinics are listed in a registry on the CDC travel Web site at https://wwwnc.cdc.gov/travel/yellow-fever-vaccination-clinics/search.
Meningococcal disease. ACIP recommends routine vaccination against meningococcal disease for people 11 to 18 years of age and for individuals with persistent complement component deficiency, functional or anatomic asplenia, and HIV. Vaccination is recommended for travelers who visit or reside in areas where meningococcal disease is hyperendemic or epidemic, such as the meningitis belt of sub-Saharan Africa during the dry season of December to June (map: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/meningococcal-disease). Travelers to Saudi Arabia during the annual Hajj and Umrah pilgrimages are required to have a certificate of vaccination with quadrivalent (serogroups A, C, Y, W-135) meningococcal vaccine issued within 3 years (and not less than 10 days) before entry.
Several meningococcal vaccines are available in the United States. The quadrivalent vaccines are Menactra (MenACWY-D, Sanofi Pasteur) and Menveo (MenACWY-CRM, GSK). A bivalent (serogroups C and Y) conjugate vaccine MenHibrix (Hib-MenCY-TT, GSK) is also licensed for use in the United States, but infants traveling to areas with high endemic rates of meningococcal disease who received this vaccine are not protected against serogroups A and W and should receive quadrivalent meningococcal conjugate vaccine. Serogroup B vaccination is not routinely recommended for travelers. Approximately 7 to 10 days are required after vaccination for the development of protective antibody levels.7,8,20,21
Polio. Although polio has been nearly eradicated, as of the time this article was written, the disease has not been eliminated in Afghanistan, Guinea, Laos, Nigeria, or Pakistan. Other countries, such as Cameroon, Chad, and Ukraine remain vulnerable to international transmission.22 The CDC recommends that adults who are traveling to areas where wild polio virus (WPV) has circulated in the last 12 months and who are unvaccinated, incompletely vaccinated, or whose vaccination status is unknown should receive a series of 3 doses of IPV to prevent ongoing spread.23 Adults who completed the polio vaccine series as children and are traveling to areas where WPV has circulated in the last 12 months should receive a one-time booster dose of IPV.23
Infants and children in the United States should be vaccinated against polio as part of a routine age-appropriate series. If a child cannot complete the routine series before departure and is traveling to an area where WPV has circulated in the last 12 months, an accelerated schedule is recommended. Vaccination should be documented on the ICVP, as countries with active spread of poliovirus may require proof of polio vaccination upon exit. A list of the countries where the polio virus is currently circulating is available at http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/.
Both routine and accelerated vaccination schedules for children and adults are published annually by the CDC and are available at http://www.cdc.gov/vaccines/schedules/hcp/index.html.
Recommended vaccines
Japanese encephalitis (JE) is endemic throughout most of Asia and parts of the Western Pacific region (map: http://www.cdc.gov/japaneseencephalitis/maps/). JE vaccine is recommended for travelers who plan to spend more than a month in endemic areas during the JE virus transmission season. (In temperate areas of Asia, JE virus transmission is seasonal and usually peaks in the summer and fall. In the subtropics and tropics, transmission can occur year-round, often with a peak during the rainy season.)
This recommendation includes recurrent travelers or expatriates who are likely to visit endemic rural or agricultural areas during a high-risk period of JE virus transmission. Risk is low for travelers who spend less than a month in endemic areas and for those who confine their travel to urban centers. Nevertheless, vaccination should be considered if travel is planned for outside an urban area and includes such activities as camping, hiking, trekking, biking, fishing, hunting, or farming. Inactivated Vero cell culture-derived vaccine (Ixiaro) is the only JE vaccine licensed and available in the United States. Ixiaro is given as a 2-dose series, with the doses spaced 28 days apart. The last dose should be given at least one week before travel.24
Typhoid fever. Vaccination against typhoid fever is recommended for travelers to highly endemic areas such as the Indian subcontinent, Africa, and Central and South America. Two typhoid vaccines are available: Vi capsular polysaccharide vaccine (ViCPS) administered intramuscularly (IM), and oral live attenuated vaccine (Ty21a). Ty21a is a live vaccine and should not be given to immunocompromised people or those taking antibiotics, as it may reduce immunogenicity. Ty21a must be kept refrigerated at 35.6° F to 46.4° F (2° C - 8° C) and administered with cool liquid no warmer than 98.6° F (37° C). Both vaccines are only 50% to 80% efficacious, making access to clean food and water essential.3,5,25
Hepatitis A vaccine should be given to all children older than one year traveling to areas where there is an intermediate or high risk of the disease. Children younger than one year who are traveling to high-risk areas can receive a single dose of immunoglobulin (IG) 0.02 mL/kg IM, which provides protection for up to 3 months. One 0.06 mL/kg-dose IM provides protection for 3 to 5 months.
If travel continues, children should receive a second dose after 5 months. IG does not interfere with the response to YF vaccine, but can interfere with the response to other live injected vaccines (such as MMR and varicella).26
Hepatitis B vaccination should be administered to all unvaccinated travelers who plan to visit an area with intermediate to high prevalence of chronic hepatitis B (HBV surface antigen prevalence ≥2%). Unvaccinated travelers who may engage in high-risk sexual activity or injection drug use should receive hepatitis B vaccine regardless of destination. Additionally, travelers who access medical care for injury or illness while abroad may also be at risk of acquiring hepatitis B via contaminated blood products or medical equipment.27
Serologic testing and booster vaccination are not recommended before travel for immunocompetent adults who have been previously vaccinated. The combined hepatitis A and B vaccine provides effective and convenient dual protection for travelers and can be administered with an accelerated 0-, 7-, and 21-day schedule for last-minute travelers.7,8
Rabies remains endemic in developing countries of Africa and Asia, where appropriate post-exposure prophylaxis is limited or non-existent.28 Consider pre-exposure rabies prophylaxis for traveling patients based on the availability of rabies vaccine and immunoglobulin in their destination area, planned duration of stay, and the likelihood of animal exposure (eg, veterinarians, animal handlers, cavers, missionaries). Advise travelers who decline vaccination to avoid or minimize animal contact during travel. In the event the traveler sustains an animal bite or scratch, immediate cleansing of the wound substantially reduces the risk of infection, especially when followed by timely administration of post-exposure prophylaxis.
Post-exposure prophylaxis for unvaccinated individuals consists of local infiltration of rabies immunoglobulin at the site of the bite and a series of 4 injections of rabies vaccine over 14 days, or 5 doses over one month for immunosuppressed patients. The first dose of the 4-dose course should be administered as soon as possible after exposure. Two vaccines are licensed for use in the United States: human diploid cell vaccine (HDCV, Imovax Rabies, Sanofi Pasteur) and purified chick embryo cell vaccine (PCECV, RabAvert, Novartis Vaccines and Diagnostics). The vaccine should never be administered in the gluteal area, as this may result in lower antibody titers.29
Additionally, promising new vaccines against malaria and dengue fever are under clinical development and may be available in the near future.
CORRESPONDENCE
Vini Vijayan, MD, Division of Infectious Diseases, Arkansas Children's Hospital, 1 Children's Way, Slot 512-11, Little Rock, AR 72202; [email protected].
International travel, whether for business, pleasure, child adoption, medical tourism, or adventure, continues to grow. In 2015, more than 70 million US citizens traveled internationally.1 Many individuals contact family physicians first about their plans for travel and questions about travel-related health advice. This article provides an overview of the vaccines recommended for travelers headed to international destinations. Because country-specific vaccination recommendations and requirements for entry and departure change over time, check the Centers for Disease Control and Prevention (CDC) Web site for up-to-date requirements and recommendations (www.cdc.gov/travel).
Vaccine schedules vary according to destination and individual risks
There is no single vaccination schedule that applies to all travelers. Each schedule should be individualized based on the traveler’s destination, risk assessment, previous immunizations, health status, and time available before departure.2,3 Pregnant or immunocompromised travelers should seek advice from an experienced travel medicine consultant on the immunization recommendations specifically meant for them.4,5
Travel vaccines (TABLE6) are generally categorized as routine, required, or recommended.
- Routine vaccines are the standard child and adult immunizations recommended by the Advisory Committee on Immunization Practices (ACIP). These include such vaccines as diphtheria-tetanus toxoids-acellular pertussis (DTaP), inactivated polio vaccine (IPV), Haemophilus influenzae type b (Hib), hepatitis B, rotavirus and pneumococcal vaccines, and human papillomavirus (HPV).
- Required vaccines—eg, yellow fever and meningococcal vaccines—must be documented on the International Certificate of Vaccination before entry into certain countries.
- Recommended vaccines are advised based on the travel destination and anticipated activities. These would include vaccines for typhoid, rabies, Japanese encephalitis, and polio (adult booster).

Routine vaccinations may need to be accelerated
Pre-travel patient encounters are an opportunity to update routine vaccinations.7,8 Immunization against childhood diseases remains suboptimal in developing countries, where vaccine-preventable illnesses occur more frequently.9
Routine vaccines may be administered on an accelerated basis depending on geographic destination, seasonal disease variations, anticipated exposures, and known outbreaks at the time of travel.
MMR vaccine. Measles is still common in many parts of the world, and unvaccinated or incompletely vaccinated travelers are at risk of acquiring the disease and importing it to the United States (see “Measles: Why it’s still a threat,” 2017;66:446-449.) In 2015, a large, widespread measles outbreak occurred in the United States, linked to an amusement park in California, likely originating with an infected traveler who visited the park.10
All children older than 12 months should receive 2 doses of measles-mumps-rubella (MMR) vaccine separated by at least 28 days before departure (regardless of their destination). Infants between 6 and 11 months are at risk for high morbidity and may therefore receive a single dose of MMR earlier than the routinely recommended age of 12 to 15 months. Adolescents and adults without evidence of immunity against measles should get 2 doses of MMR separated by at least 28 days.11 Acceptable presumptive evidence of immunity against measles includes written documentation of adequate vaccination, laboratory evidence of immunity, laboratory confirmation of measles, or birth before 1957.
Varicella vaccine. Children, adolescents, and young adults who have received only one dose of varicella should get a second dose prior to departure. For children 7 to 12 years, the recommended minimum interval between doses is 3 months. For individuals 13 years or older, the minimum interval is 4 weeks.7,8
Influenza vaccine is routinely recommended for all travelers 6 months of age or older, as flu season varies geographically. Flu season in the Northern Hemisphere may begin as early as October and can extend until May. In the Southern Hemisphere, it may begin in April and last through September. Travelers should be vaccinated at least 2 weeks before travel in order to develop adequate immunity.12,13
Required vaccinations: Proof is needed before traveling
Yellow fever (YF) is a mosquito-borne viral illness characterized by fever, chills, headache, myalgia, and vomiting. The disease can progress to coagulopathy, shock, and multisystem organ failure.14 YF vaccine is recommended for individuals 9 months or older who are traveling to or living in areas of South America or Africa where YF virus transmission is common (map: http://www.cdc.gov/yellowfever/maps/).
YF vaccine is a live-attenuated virus formulation and, therefore, should not be given to individuals with primary immunodeficiencies, transplant recipients or patients on immunosuppressive and immunomodulatory therapies, or patients with human immunodeficiency virus (HIV) whose CD4 count is below 200/mL. Other contraindications to YF vaccine are age younger than 6 months, allergy to a vaccine component, and thymic disorders. Serious adverse reactions to the vaccine are rare, but include 2 syndromes: YF-associated neurotropic disease and YF vaccine-associated viscerotropic disease.15
In many YF-endemic countries, vaccination is legally required for entry, and proof of vaccination must be documented on an International Certificate of Vaccination or Prophylaxis (ICVP). Additionally, some countries may require proof of vaccination before allowing travel through an endemic region, to prevent introduction of the disease elsewhere. Travelers with a specific contraindication to YF vaccine should obtain a waiver from a physician before traveling to a country requiring vaccination.16
The vaccination certificate is valid beginning 10 days after administration of YF vaccine. Immunity after a single dose is long lasting and may provide lifetime protection. Previously, re-vaccination was required every 10 years; however, in February 2015, ACIP approved a new recommendation stating a single dose of YF vaccine is adequate for most travelers.1
Although ACIP no longer recommends booster doses of YF vaccine for most travelers, clinicians and travelers should review the entry requirements for destination countries because changes to the International Health Regulations have not yet been fully implemented. Once this change is instituted, a completed ICVP will be valid for the lifetime of the vaccine.18,19 Country-specific requirements for YF can be found at http://www.cdc.gov/yellowfever/maps/. (Click on the link below the appropriate map.) In the United States, the YF vaccine is distributed only through approved vaccination centers. These designated clinics are listed in a registry on the CDC travel Web site at https://wwwnc.cdc.gov/travel/yellow-fever-vaccination-clinics/search.
Meningococcal disease. ACIP recommends routine vaccination against meningococcal disease for people 11 to 18 years of age and for individuals with persistent complement component deficiency, functional or anatomic asplenia, and HIV. Vaccination is recommended for travelers who visit or reside in areas where meningococcal disease is hyperendemic or epidemic, such as the meningitis belt of sub-Saharan Africa during the dry season of December to June (map: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/meningococcal-disease). Travelers to Saudi Arabia during the annual Hajj and Umrah pilgrimages are required to have a certificate of vaccination with quadrivalent (serogroups A, C, Y, W-135) meningococcal vaccine issued within 3 years (and not less than 10 days) before entry.
Several meningococcal vaccines are available in the United States. The quadrivalent vaccines are Menactra (MenACWY-D, Sanofi Pasteur) and Menveo (MenACWY-CRM, GSK). A bivalent (serogroups C and Y) conjugate vaccine MenHibrix (Hib-MenCY-TT, GSK) is also licensed for use in the United States, but infants traveling to areas with high endemic rates of meningococcal disease who received this vaccine are not protected against serogroups A and W and should receive quadrivalent meningococcal conjugate vaccine. Serogroup B vaccination is not routinely recommended for travelers. Approximately 7 to 10 days are required after vaccination for the development of protective antibody levels.7,8,20,21
Polio. Although polio has been nearly eradicated, as of the time this article was written, the disease has not been eliminated in Afghanistan, Guinea, Laos, Nigeria, or Pakistan. Other countries, such as Cameroon, Chad, and Ukraine remain vulnerable to international transmission.22 The CDC recommends that adults who are traveling to areas where wild polio virus (WPV) has circulated in the last 12 months and who are unvaccinated, incompletely vaccinated, or whose vaccination status is unknown should receive a series of 3 doses of IPV to prevent ongoing spread.23 Adults who completed the polio vaccine series as children and are traveling to areas where WPV has circulated in the last 12 months should receive a one-time booster dose of IPV.23
Infants and children in the United States should be vaccinated against polio as part of a routine age-appropriate series. If a child cannot complete the routine series before departure and is traveling to an area where WPV has circulated in the last 12 months, an accelerated schedule is recommended. Vaccination should be documented on the ICVP, as countries with active spread of poliovirus may require proof of polio vaccination upon exit. A list of the countries where the polio virus is currently circulating is available at http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/.
Both routine and accelerated vaccination schedules for children and adults are published annually by the CDC and are available at http://www.cdc.gov/vaccines/schedules/hcp/index.html.
Recommended vaccines
Japanese encephalitis (JE) is endemic throughout most of Asia and parts of the Western Pacific region (map: http://www.cdc.gov/japaneseencephalitis/maps/). JE vaccine is recommended for travelers who plan to spend more than a month in endemic areas during the JE virus transmission season. (In temperate areas of Asia, JE virus transmission is seasonal and usually peaks in the summer and fall. In the subtropics and tropics, transmission can occur year-round, often with a peak during the rainy season.)
This recommendation includes recurrent travelers or expatriates who are likely to visit endemic rural or agricultural areas during a high-risk period of JE virus transmission. Risk is low for travelers who spend less than a month in endemic areas and for those who confine their travel to urban centers. Nevertheless, vaccination should be considered if travel is planned for outside an urban area and includes such activities as camping, hiking, trekking, biking, fishing, hunting, or farming. Inactivated Vero cell culture-derived vaccine (Ixiaro) is the only JE vaccine licensed and available in the United States. Ixiaro is given as a 2-dose series, with the doses spaced 28 days apart. The last dose should be given at least one week before travel.24
Typhoid fever. Vaccination against typhoid fever is recommended for travelers to highly endemic areas such as the Indian subcontinent, Africa, and Central and South America. Two typhoid vaccines are available: Vi capsular polysaccharide vaccine (ViCPS) administered intramuscularly (IM), and oral live attenuated vaccine (Ty21a). Ty21a is a live vaccine and should not be given to immunocompromised people or those taking antibiotics, as it may reduce immunogenicity. Ty21a must be kept refrigerated at 35.6° F to 46.4° F (2° C - 8° C) and administered with cool liquid no warmer than 98.6° F (37° C). Both vaccines are only 50% to 80% efficacious, making access to clean food and water essential.3,5,25
Hepatitis A vaccine should be given to all children older than one year traveling to areas where there is an intermediate or high risk of the disease. Children younger than one year who are traveling to high-risk areas can receive a single dose of immunoglobulin (IG) 0.02 mL/kg IM, which provides protection for up to 3 months. One 0.06 mL/kg-dose IM provides protection for 3 to 5 months.
If travel continues, children should receive a second dose after 5 months. IG does not interfere with the response to YF vaccine, but can interfere with the response to other live injected vaccines (such as MMR and varicella).26
Hepatitis B vaccination should be administered to all unvaccinated travelers who plan to visit an area with intermediate to high prevalence of chronic hepatitis B (HBV surface antigen prevalence ≥2%). Unvaccinated travelers who may engage in high-risk sexual activity or injection drug use should receive hepatitis B vaccine regardless of destination. Additionally, travelers who access medical care for injury or illness while abroad may also be at risk of acquiring hepatitis B via contaminated blood products or medical equipment.27
Serologic testing and booster vaccination are not recommended before travel for immunocompetent adults who have been previously vaccinated. The combined hepatitis A and B vaccine provides effective and convenient dual protection for travelers and can be administered with an accelerated 0-, 7-, and 21-day schedule for last-minute travelers.7,8
Rabies remains endemic in developing countries of Africa and Asia, where appropriate post-exposure prophylaxis is limited or non-existent.28 Consider pre-exposure rabies prophylaxis for traveling patients based on the availability of rabies vaccine and immunoglobulin in their destination area, planned duration of stay, and the likelihood of animal exposure (eg, veterinarians, animal handlers, cavers, missionaries). Advise travelers who decline vaccination to avoid or minimize animal contact during travel. In the event the traveler sustains an animal bite or scratch, immediate cleansing of the wound substantially reduces the risk of infection, especially when followed by timely administration of post-exposure prophylaxis.
Post-exposure prophylaxis for unvaccinated individuals consists of local infiltration of rabies immunoglobulin at the site of the bite and a series of 4 injections of rabies vaccine over 14 days, or 5 doses over one month for immunosuppressed patients. The first dose of the 4-dose course should be administered as soon as possible after exposure. Two vaccines are licensed for use in the United States: human diploid cell vaccine (HDCV, Imovax Rabies, Sanofi Pasteur) and purified chick embryo cell vaccine (PCECV, RabAvert, Novartis Vaccines and Diagnostics). The vaccine should never be administered in the gluteal area, as this may result in lower antibody titers.29
Additionally, promising new vaccines against malaria and dengue fever are under clinical development and may be available in the near future.
CORRESPONDENCE
Vini Vijayan, MD, Division of Infectious Diseases, Arkansas Children's Hospital, 1 Children's Way, Slot 512-11, Little Rock, AR 72202; [email protected].
1. U.S. Department of Commerce, International Trade Administration, National Travel and Tourism Office (NTTO). 2015. Available at: http://travel.trade.gov/view/m-2015-O-001/index.html. Accessed July 12, 2017.
2. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499-1539.
3. Centers for Disease Control and Prevention. The pre-travel consultation. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/the-pre-travel-consultation. Accessed June 20, 2017.
4. Hochberg NS, Barnett ED, Chen LH, et al. International travel by persons with medical comorbidities: understanding risks and providing advice. Mayo Clin Proc. 2013;88:1231-1240.
5. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44-e100.
6. Centers for Disease Control and Prevention. Yellow Book table of contents: Chapter 3. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/table-of-contents. Accessed July 21, 2017.
7. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65;86-87.
8. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:88-90.
9. Boggild AK, Castelli F, Gautret P, et al. Vaccine preventable diseases in returned international travelers: results from the GeoSentinel Surveillance Network. Vaccine. 2010;28:7389-7395.
10. Sotir MJ, Esposito DH, Barnett ED, et al. Measles in the 21st century, a continuing preventable risk to travelers: data from the GeoSentinel Global Network. Clin Infect Dis. 2016;62:210-212.
11. Measles. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015:535-546.
12. Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
13. Marti F, Steffen R, Mutsch M. Influenza vaccine: a travelers’ vaccine? Expert Rev Vaccines. 2008;7:679-687.
14. Monath T, Gershman MD, Staples JE, et al. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 6th ed. London, England: W.B. Saunders; 2013:870-968.
15. Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-27.
16. World Health Organization. International Health Regulations. 2nd ed. Geneva, Switzerland: World Health Organization; 2005. Available at: http://whqlibdoc.who.int/publications/2008/9789241580410_eng.pdf. Accessed June 20, 2017.
17. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices: summary report. February 26, 2015. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2015-02.pdf. Accessed July 20, 2017.
18. Staples JE, Bocchini JA Jr, Rubin L, et al. Yellow fever vaccine booster doses: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:647-650.
19. World Health Organization. International travel and health: World–yellow fever vaccination booster. Geneva, Switzerland: World Health Organization; 2014. Available at: http://www.who.int/ith/updates/20140605/en. Accessed June 20, 2017.
20. Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1-28.
21. Memish ZA, Stephens GM, Steffen R, et al. Emergence of medicine for mass gatherings: lessons from the Hajj. Lancet Infect Dis. 2012;12:56-65.
22. World Health Organization. Twelfth meeting of the Emergency Committee under the International Health Regulations (2015) regarding the international spread of poliovirus. Available at: http://www.who.int/mediacentre/news/statements/2017/poliovirus-twelfth-ec/en/. Accessed June 21, 2017.
23. Centers for Disease Control and Prevention. Interim CDC Guidance for Travel to and from Countries Affected by the New Polio Vaccine Requirements. Available at http://wwwnc.cdc.gov/travel/news-announcements/polio-guidance-new-requirements. Accessed August 1, 2017.
24. Centers for Disease Control and Prevention. Use of Japanese encephalitis vaccine in children: recommendations of the advisory committee on immunization practices, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:898-900.
25. Mahon BE, Newton AE, Mintz ED. Effectiveness of typhoid vaccination in US travelers. Vaccine. 2014;32:3577-3579.
26. Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55:1-23.
27. Vivancos R, Abubakar I, Hunter PR. Foreign travel, casual sex, and sexually transmitted infections: systematic review and meta-analysis. Int J Infect Dis. 2010;14:e842-e851.
28. Gautret P, Harvey K, Pandey P, et al for the GeoSentinel Surveillance Network. Animal-associated exposure to rabies virus among travelers, 1997-2012. Emerg Infect Dis. 2015;21:569-577.
29. Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2010;59:1-9.
1. U.S. Department of Commerce, International Trade Administration, National Travel and Tourism Office (NTTO). 2015. Available at: http://travel.trade.gov/view/m-2015-O-001/index.html. Accessed July 12, 2017.
2. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499-1539.
3. Centers for Disease Control and Prevention. The pre-travel consultation. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/the-pre-travel-consultation. Accessed June 20, 2017.
4. Hochberg NS, Barnett ED, Chen LH, et al. International travel by persons with medical comorbidities: understanding risks and providing advice. Mayo Clin Proc. 2013;88:1231-1240.
5. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44-e100.
6. Centers for Disease Control and Prevention. Yellow Book table of contents: Chapter 3. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/table-of-contents. Accessed July 21, 2017.
7. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65;86-87.
8. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:88-90.
9. Boggild AK, Castelli F, Gautret P, et al. Vaccine preventable diseases in returned international travelers: results from the GeoSentinel Surveillance Network. Vaccine. 2010;28:7389-7395.
10. Sotir MJ, Esposito DH, Barnett ED, et al. Measles in the 21st century, a continuing preventable risk to travelers: data from the GeoSentinel Global Network. Clin Infect Dis. 2016;62:210-212.
11. Measles. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015:535-546.
12. Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
13. Marti F, Steffen R, Mutsch M. Influenza vaccine: a travelers’ vaccine? Expert Rev Vaccines. 2008;7:679-687.
14. Monath T, Gershman MD, Staples JE, et al. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 6th ed. London, England: W.B. Saunders; 2013:870-968.
15. Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-27.
16. World Health Organization. International Health Regulations. 2nd ed. Geneva, Switzerland: World Health Organization; 2005. Available at: http://whqlibdoc.who.int/publications/2008/9789241580410_eng.pdf. Accessed June 20, 2017.
17. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices: summary report. February 26, 2015. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2015-02.pdf. Accessed July 20, 2017.
18. Staples JE, Bocchini JA Jr, Rubin L, et al. Yellow fever vaccine booster doses: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:647-650.
19. World Health Organization. International travel and health: World–yellow fever vaccination booster. Geneva, Switzerland: World Health Organization; 2014. Available at: http://www.who.int/ith/updates/20140605/en. Accessed June 20, 2017.
20. Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1-28.
21. Memish ZA, Stephens GM, Steffen R, et al. Emergence of medicine for mass gatherings: lessons from the Hajj. Lancet Infect Dis. 2012;12:56-65.
22. World Health Organization. Twelfth meeting of the Emergency Committee under the International Health Regulations (2015) regarding the international spread of poliovirus. Available at: http://www.who.int/mediacentre/news/statements/2017/poliovirus-twelfth-ec/en/. Accessed June 21, 2017.
23. Centers for Disease Control and Prevention. Interim CDC Guidance for Travel to and from Countries Affected by the New Polio Vaccine Requirements. Available at http://wwwnc.cdc.gov/travel/news-announcements/polio-guidance-new-requirements. Accessed August 1, 2017.
24. Centers for Disease Control and Prevention. Use of Japanese encephalitis vaccine in children: recommendations of the advisory committee on immunization practices, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:898-900.
25. Mahon BE, Newton AE, Mintz ED. Effectiveness of typhoid vaccination in US travelers. Vaccine. 2014;32:3577-3579.
26. Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55:1-23.
27. Vivancos R, Abubakar I, Hunter PR. Foreign travel, casual sex, and sexually transmitted infections: systematic review and meta-analysis. Int J Infect Dis. 2010;14:e842-e851.
28. Gautret P, Harvey K, Pandey P, et al for the GeoSentinel Surveillance Network. Animal-associated exposure to rabies virus among travelers, 1997-2012. Emerg Infect Dis. 2015;21:569-577.
29. Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2010;59:1-9.
PRACTICE RECOMMENDATIONS
› Recommend immunizations and safety precautions to international travelers based on their destinations, previous immunizations, health status and anticipated activities, and time available before departure. C
› Consider accelerating routine immunizations for children who may be traveling abroad. C
› Refer immunocompromised or pregnant patients to a travel medicine clinic for consultation before departure. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Autonomic dysfunction: A guide for FPs
Signs and symptoms of autonomic dysfunction commonly present in the primary care setting. Potential causes of dysfunction include certain medications and age-related changes in physiology, as well as conditions such as diabetes mellitus, multiple sclerosis, and Parkinson’s disease (TABLE1). This evidence-based review details common manifestations of autonomic dysfunction, provides a streamlined approach to patients presenting with symptoms, and reviews appropriate step-wise management.

When a delicate balance is disrupted
The autonomic nervous system provides brisk physiologic adjustments necessary to maintain homeostasis. Physiologic functions impacted by the central nervous system include: heart rate, blood pressure (BP), tone of the bladder sphincter and detrusor muscle, bowel motility, bronchodilation and constriction, pupillary dilation and constriction, sweating, catecholamine release, erection, ejaculation and orgasm, tearing, and salivation.1
Disorders of the autonomic system may result from pathologies of the central or peripheral nervous system or from medications including some antihypertensives, selective serotonin-reuptake inhibitors (SSRIs), and opioids.1 Such disorders tend to be grouped into one of 3 categories: those involving the brain, those involving the spinal cord, and autonomic neuropathies.1
The source of dysautonomia can often be determined by clinical context, coexisting neurologic abnormalities, targeted testing of the autonomic nervous system, and neuroimaging.1
Worrisome symptoms prompt a visit
A thorough history is critical to zeroing in on a patient’s complaints and ultimately providing treatment that will help manage symptoms.
When patient complaints are suggestive of autonomic dysfunction, a review of systems should include inquiry about lightheadedness, abnormal salivation, temperature changes of the extremities, gastrointestinal issues (vomiting, constipation, or diarrhea), and symptoms of presyncope/syncope or urinary or sexual dysfunction.1 The physical exam should include recordings of BP and heart rate in the supine and standing positions and a complete neurologic examination.1 Findings will typically point to one or more common complications.
Common complications of autonomic dysfunction
Complications of autonomic dysfunction include impotence, bladder dysfunction, gastrointestinal (GI) dysfunction, and orthostatic hypotension and vasomotor abnormalities. A less common condition—autonomic dysreflexia, which is a distinct type of autonomic dysfunction, and a true medical emergency—is also important to keep in mind.
Impotence
Autonomic neuropathy is a common cause of impotence and retrograde ejaculation. Loss of early morning erections and complete loss of nocturnal erections often have an etiology related to vascular disease and/or autonomic neuropathy. In addition, poor glycemic control and vascular risk factors appear to be associated with the development of diabetic autonomic neuropathy.2
Development of an erection requires an increase in parasympathetic activity and a decrease in sympathetic output. Nocturnal penile tumescence testing has been used to infer parasympathetic damage to the penis in men with diabetes who do not have vascular disease.3
First- and second-line agents. Phosphodiesterase-5 inhibitors (eg, sildenafil, tadalafil, vardenafil) have demonstrated efficacy in improving the ability to achieve and maintain erections in patients with autonomic dysfunction, including diabetic autonomic neuropathy.4-6 Second-line therapies with proven efficacy include intraurethral application and intracavernosal injections of alprostadil.7,8
Bladder dysfunction
Sympathetic activity increases bladder sphincter tone and inhibits detrusor activity, while the parasympathetic nervous system increases detrusor activity and decreases sphincter tone to aid in voiding.1 Disrupted autonomic activity can lead to urinary frequency, retention, and hesitancy; overactive bladder; and incontinence.1 Brain and spinal cord disease above the level of the lumbar spine results in urinary frequency and small bladder volumes, whereas diseases involving autonomic nerve fibers to and from the bladder result in large bladder volumes and overflow incontinence.9
Patients presenting with lower urinary tract symptoms require a comprehensive evaluation to rule out other pathologies, as the differential for such symptoms is broad and includes infection, malignancies, interstitial cystitis, and bladder stones. The initial evaluation of lower urinary tract symptoms should include a history and physical exam including that of the abdomen, pelvis, and neurologic system. Lab work should assess renal function and blood glucose, and should include urinalysis and culture to rule out infection and/or hematuria. A prostate-specific antigen (PSA) test may be appropriate in men with a life expectancy >10 years, after counseling regarding the risks and benefits of screening.
Anticholinergic drugs with antimuscarinic effects, such as oxybutynin, may be used to treat symptoms of urge incontinence and overactive bladder. They work to suppress involuntary contractions of the bladder’s smooth muscle by blocking the release of acetylcholine. These medications relax the bladder’s outer layer of muscle—the detrusor. Such medications often have a number of anticholinergic adverse effects, such as dry mouth and constipation, sometimes leading to discontinuation. A post-void residual (PVR) test may be helpful in guiding management. For example, caution should be used in patients with elevated PVRs, as anticholinergics can worsen urinary retention.
Beta-3 agonists (eg, mirabegron) are a novel class of medications used to treat overactive bladder. These medications act to increase sympathetic tone in the bladder. Because they have the potential to raise BP, monitor BP in patients taking these agents. In addition, monitor patients taking antimuscarinics or beta-3 agonists for the development of urinary retention.
Other tests, treatments. Urodynamic testing is recommended for patients who fail to respond to treatment. Combining behavioral therapy with medication has been shown to be effective in patients with urge incontinence.10 Botulinum toxin type A, injected directly into the detrusor muscle, can be as effective as medication in patients with urinary urge incontinence.11
Detrusor underactivity is defined as contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying within a normal timespan.12 This diagnosis is typically made using urodynamic testing.13 PVRs ≥150 mL are considered evidence of urinary retention. Overflow incontinence can result from detrusor underactivity.
Consider a trial of a cholinergic agonist, such as bethanechol, in patients with urinary retention. Some patients will require intermittent straight catheterization or chronic indwelling foley or suprapubic catheters to void.
Gastrointestinal dysfunction
In patients with diabetes, GI autonomic neuropathy can result in altered esophageal motility leading to gastroesophageal reflux disease (GERD) or dysphagia, gastroparesis, or diabetic enteropathy.14 Gastroparesis often presents as nausea, vomiting, and bloating.1 It may be diagnosed via gastric emptying studies (scintigraphy), and often requires a multidimensional approach to treatment.
Management. Food may be chopped or pureed to aid in digestion. Metoclopramide is the most commonly used prokinetic agent, but avoid its use in patients with parkinsonism. In more severe cases, consider adding domperidone and erythromycin as prokinetic agents. Recommend antiemetics, such as diphenhydramine, ondansetron, and prochlorperazine for management of nausea and vomiting. Severe cases of gastroparesis may merit a venting gastrostomy tube for decompression and/or feeding via a jejunostomy tube.15 Impaired intestinal mobility may lead to stasis syndrome, causing diarrhea.
Hypermobility caused by decreased sympathetic inhibition can also contribute to diarrhea. Altered anal sphincter function tone may contribute to fecal incontinence. Management should focus on balancing electrolytes, maintaining adequate fluid intake, and relieving symptoms. Consider antidiarrheals such as loperamide, but use them with caution to avoid toxic megacolon.16
Constipation. Another common manifestation of autonomic dysfunction in the GI tract is severe constipation.1 This may be managed conservatively with hydration, increased activity, and increased fiber intake. If such measures prove inadequate, consider stool softeners and laxatives.
Patients with constipation due to spinal cord lesions may benefit from a routine bowel regimen. To provide predictable defecation, advise patients to begin by inserting a stimulant rectal suppository. Follow with gentle digital stimulation of the distal rectum for one minute or less. They’ll need to repeat the process every 5 to 10 minutes until stool evacuation is complete. A forward-leaning position may assist with evacuation. It is helpful to perform this routine at the same time each day.17
Orthostatic (postural) hypotension
The autonomic nervous system plays an important role in maintaining BP during positional changes. The sympathetic nervous system adjusts the tone in arteries, veins, and the heart. Baroreceptors located primarily in the carotid arteries and aorta, are highly sensitive to changes in BP. When the baroreceptors sense the slightest drop in pressure, a coordinated increase in sympathetic outflow occurs. Arteries constrict to increase peripheral resistance and BP, and heart rate and contractility increase, all in an attempt to maintain BP and perfusion.18
The most common causes of orthostatic hypotension are not neurologic in origin,9 but rather involve medications, hypovolemia, and impaired autonomic reflexes. The condition is common in the elderly, with one study demonstrating a prevalence of 18.2% in those ≥65 years.19
Orthostatic hypotension may present with dimming or loss of vision, lightheadedness, diaphoresis, diminished hearing, pallor, and weakness. As a result, it is a risk factor for falls. Syncope results when the drop in BP impairs cerebral perfusion. Signs of impaired baroreflexes are supine hypertension, a heart rate that is fixed regardless of posture (the heart rate should increase upon standing), postprandial hypotension, and an excessively high nocturnal BP.1
Orthostatic hypotension is diagnosed when, within 3 minutes of quiet standing after a 5-minute period of supine rest, one or both of the following is present: at least a 20 mm Hg-fall in systolic pressure or at least a 10 mm Hg-fall in diastolic pressure.20 Soysal et al demonstrated that such a drop in BP, measured one minute after standing, is adequate and effective for diagnosing orthostatic hypotension in the elderly.21
Nonpharmacologic management. Recognition and removal of medications that can exacerbate orthostatic hypotension is the first step in managing the condition. Such medications include diuretics, beta-blockers, alpha adrenergic blockers, vasodilators, antipsychotics, antidepressants (SSRIs, trazodone, monoamine oxidase inhibitors, and tricyclic antidepressants), phosphodiesterase inhibitors, narcotics, and antiparkinsonian medications.22
Lifestyle interventions, such as having the patient arise slowly and maintain good hydration, can be helpful. Eating smaller, more frequent meals may also help if the orthostatic hypotension is triggered postprandially. Compressive stockings can help limit venous pooling in the lower extremities and improve venous return. Tensing the legs by crossing them while standing on both feet has been shown to increase cardiac output and BP.23 An aerobic exercise regimen of walking or stair climbing 30 to 45 minutes/day 3 days/week for 6 months was shown to eliminate symptoms of orthostasis on tilt table testing in elderly patients with cardiac deconditioning, as opposed to chronic autonomic failure.24
The reduction in central blood volume associated with autonomic insufficiency (due to increased urinary sodium and water excretion) can be lessened by increasing sodium and water intake.25-27
Pharmacotherapy. Fludrocortisone acetate, a synthetic mineralocorticoid, is the medication of first choice for most patients with orthostatic hypotension whose symptoms are not adequately controlled using nonpharmacologic measures,28 but keep in mind that treating orthostatic hypotension with fludrocortisones is an off-label use of the medication.
Monitor patients taking fludrocortisone for worsened supine hypertension and edema. Also, check their serum potassium levels one to 2 weeks after initiation of therapy and after dose increases. Frequent home monitoring of BP in sitting, standing, and supine positions may be helpful in assessing response to therapy.
If the patient remains symptomatic despite therapy with fludrocortisone, consider adding an alpha-1 adrenergic agonist, such as midodrine. Avoid prescribing midodrine, however, for patients with advanced cardiovascular disease, urinary retention, or uncontrolled hypertension.29
Autonomic dysreflexia: A medical emergency
Autonomic dysreflexia, a medical emergency that must be recognized immediately, is a distinct type of autonomic dysfunction seen in patients with spinal cord injury at or above the T6 level.30 It is a condition of uncontrolled sympathetic response secondary to an underlying condition such as infection, urinary retention, or rectal distention.30
Common symptoms include headache, significant hypertension, flushing of the skin, and diaphoresis above the level of injury.2 In addition, a review of systems should screen for fever, visual changes, abnormalities of the cardiovascular system, syncope, bowel and bladder symptoms, and sexual dysfunction.
Patients demonstrating autonomic dysreflexia should be placed in the upright position to produce an orthostatic decrease in BP.30 Patients should be evaluated to identify any reversible precipitants, such as urinary retention or fecal impaction. Severe attacks involving hypertensive crisis require prompt transfer to the emergency department. Sublingual nifedipine or an intravenous agent, such as hydralazine, may be used to lower BP.31
CORRESPONDENCE
Kristen Thornton, MD, 777 South Clinton Ave., Rochester, NY 14620; [email protected]
1. Low PA, Engstrom JW. Disorders of the autonomic nervous system. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015. Available at: http://accessmedicine.mhmedical.com/content.aspx?bookid=1130&Sectionid=79755967. Accessed May 15, 2016.
2. Ko SH, Park SA, Cho JH, et al. Progression of cardiovascular dysfunction in patients with type 2 diabetes: a 7 year follow-up study. Diabetes Care. 2008;31:1832-1836.
3. Brown JS, Wessells H, Chancellor MB, et al. Urologic complications of diabetes. Diabetes Care. 2005;28:177-185.
4. Rendell MS, Rajfer J, Wicker PA, et al. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421-426.
5. Goldstein I, Young JM, Fischer J, et al. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care. 2003;26:777-783.
6. Sáenz de Tejada I, Anglin G, Knight JR, et al. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002;25:2159-2164.
7. Padma-Nathan H, Hellstrom WJ, Kaiser FE, et al. Treatment of men with erectile dysfunction with transurethral alprostadil. Medicated Urethral System for Erection (MUSE) Study Group. N Engl J Med. 1997;336:1-7.
8. Linet OI, Ogrinc FG. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction. The Alprostadil Study Group. N Engl J Med. 1996;334:873-877.
9. Engstrom JW, Maring JB. Disorders of the autonomic nervous system. In: Braunwald E, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York, NY: McGraw Hill; 2001.
10. Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370-374.
11. Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs. onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367:1803-1813.
12. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
13. Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65:389-398.
14. Kempler P, Amarenco G, Freeman R, et al. Management strategies for gastrointestinal, erectile, bladder, and sudomotor dysfunction in patients with diabetes. Diabetes Metab Res Rev. 2011;27:665-677.
15. Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820-829.
16. Shakil A, Church RJ, Rao SS. Gastrointestinal complications of diabetes. Am Fam Physician. 2008;77:1697-1702.
17. Krassioukov A, Eng JJ, Claxton G, et al. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord. 2010;48:718-733.
18. Bradley JG, Davis K. Orthostatic hypotension. Am Fam Physician. 2003;68:2393-2399.
19. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19(6 Pt 1):508-519.
20. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
21. Soysal P, Aydin AE, Koc Okudur S, et al. When should orthostatic BP changes be evaluated in elderly: 1st, 3rd or 5th minute? Arch Gerontol Geriatr. 2016;65:199-203.
22. Perlmuter LC, Sarda G, Casavant V, et al. A review of the etiology, associated comorbidities, and treatment of orthostatic hypotension. Am J Ther. 2013;20:279-291.
23. Ten Harkel ADJ, van Lieshout JJ, Wieling W. Effects of leg muscle pumping and tensing on orthostatic arterial pressure: a study in normal subjects and patients with autonomic failure. Clin Sci. 1994;87:553-558.
24. Carroll JF, Wood CE, Pollock ML, et al. Hormonal responses in elders experiencing pre-syncopal symptoms during head-up tilt before and after exercise training. J Gerontol A Biol Sci Med Sci. 1995;50:M324-M329.
25. Shannon JR, Diedrich A, Biaggioni I, et al. Water drinking as a treatment for orthostatic syndromes. Am J Med. 2002;112:355-360.
26. Young T, Mathias C. The effects of water ingestion on orthostatic hypotension in two groups of chronic autonomic failure: multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry. 2004;75:1737-1741.
27. Humm AM, Mason LM, Mathias CJ. Effects of water drinking on cardiovascular responses to supine exercise and on orthostatic hypotension after exercise in pure autonomic failure. J Neurol Neurosurg Psychiatry. 2008;79:1160-1164.
28. Campbell IW, Ewing DJ, Clarke BF. 9-Alpha-fluorohydrocortisone in the treatment of postural hypotension in diabetic autonomic neuropathy. Diabetes. 1975;24:381-384.
29. Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis. 2013;55:425-433.
30. Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999;37:383-391.
31. Bycroft J, Shergill IS, Choong EAL, et al. Autonomic dysreflexia: a medical emergency. Postgrad Med J. 2005;81:232-235.
Signs and symptoms of autonomic dysfunction commonly present in the primary care setting. Potential causes of dysfunction include certain medications and age-related changes in physiology, as well as conditions such as diabetes mellitus, multiple sclerosis, and Parkinson’s disease (TABLE1). This evidence-based review details common manifestations of autonomic dysfunction, provides a streamlined approach to patients presenting with symptoms, and reviews appropriate step-wise management.

When a delicate balance is disrupted
The autonomic nervous system provides brisk physiologic adjustments necessary to maintain homeostasis. Physiologic functions impacted by the central nervous system include: heart rate, blood pressure (BP), tone of the bladder sphincter and detrusor muscle, bowel motility, bronchodilation and constriction, pupillary dilation and constriction, sweating, catecholamine release, erection, ejaculation and orgasm, tearing, and salivation.1
Disorders of the autonomic system may result from pathologies of the central or peripheral nervous system or from medications including some antihypertensives, selective serotonin-reuptake inhibitors (SSRIs), and opioids.1 Such disorders tend to be grouped into one of 3 categories: those involving the brain, those involving the spinal cord, and autonomic neuropathies.1
The source of dysautonomia can often be determined by clinical context, coexisting neurologic abnormalities, targeted testing of the autonomic nervous system, and neuroimaging.1
Worrisome symptoms prompt a visit
A thorough history is critical to zeroing in on a patient’s complaints and ultimately providing treatment that will help manage symptoms.
When patient complaints are suggestive of autonomic dysfunction, a review of systems should include inquiry about lightheadedness, abnormal salivation, temperature changes of the extremities, gastrointestinal issues (vomiting, constipation, or diarrhea), and symptoms of presyncope/syncope or urinary or sexual dysfunction.1 The physical exam should include recordings of BP and heart rate in the supine and standing positions and a complete neurologic examination.1 Findings will typically point to one or more common complications.
Common complications of autonomic dysfunction
Complications of autonomic dysfunction include impotence, bladder dysfunction, gastrointestinal (GI) dysfunction, and orthostatic hypotension and vasomotor abnormalities. A less common condition—autonomic dysreflexia, which is a distinct type of autonomic dysfunction, and a true medical emergency—is also important to keep in mind.
Impotence
Autonomic neuropathy is a common cause of impotence and retrograde ejaculation. Loss of early morning erections and complete loss of nocturnal erections often have an etiology related to vascular disease and/or autonomic neuropathy. In addition, poor glycemic control and vascular risk factors appear to be associated with the development of diabetic autonomic neuropathy.2
Development of an erection requires an increase in parasympathetic activity and a decrease in sympathetic output. Nocturnal penile tumescence testing has been used to infer parasympathetic damage to the penis in men with diabetes who do not have vascular disease.3
First- and second-line agents. Phosphodiesterase-5 inhibitors (eg, sildenafil, tadalafil, vardenafil) have demonstrated efficacy in improving the ability to achieve and maintain erections in patients with autonomic dysfunction, including diabetic autonomic neuropathy.4-6 Second-line therapies with proven efficacy include intraurethral application and intracavernosal injections of alprostadil.7,8
Bladder dysfunction
Sympathetic activity increases bladder sphincter tone and inhibits detrusor activity, while the parasympathetic nervous system increases detrusor activity and decreases sphincter tone to aid in voiding.1 Disrupted autonomic activity can lead to urinary frequency, retention, and hesitancy; overactive bladder; and incontinence.1 Brain and spinal cord disease above the level of the lumbar spine results in urinary frequency and small bladder volumes, whereas diseases involving autonomic nerve fibers to and from the bladder result in large bladder volumes and overflow incontinence.9
Patients presenting with lower urinary tract symptoms require a comprehensive evaluation to rule out other pathologies, as the differential for such symptoms is broad and includes infection, malignancies, interstitial cystitis, and bladder stones. The initial evaluation of lower urinary tract symptoms should include a history and physical exam including that of the abdomen, pelvis, and neurologic system. Lab work should assess renal function and blood glucose, and should include urinalysis and culture to rule out infection and/or hematuria. A prostate-specific antigen (PSA) test may be appropriate in men with a life expectancy >10 years, after counseling regarding the risks and benefits of screening.
Anticholinergic drugs with antimuscarinic effects, such as oxybutynin, may be used to treat symptoms of urge incontinence and overactive bladder. They work to suppress involuntary contractions of the bladder’s smooth muscle by blocking the release of acetylcholine. These medications relax the bladder’s outer layer of muscle—the detrusor. Such medications often have a number of anticholinergic adverse effects, such as dry mouth and constipation, sometimes leading to discontinuation. A post-void residual (PVR) test may be helpful in guiding management. For example, caution should be used in patients with elevated PVRs, as anticholinergics can worsen urinary retention.
Beta-3 agonists (eg, mirabegron) are a novel class of medications used to treat overactive bladder. These medications act to increase sympathetic tone in the bladder. Because they have the potential to raise BP, monitor BP in patients taking these agents. In addition, monitor patients taking antimuscarinics or beta-3 agonists for the development of urinary retention.
Other tests, treatments. Urodynamic testing is recommended for patients who fail to respond to treatment. Combining behavioral therapy with medication has been shown to be effective in patients with urge incontinence.10 Botulinum toxin type A, injected directly into the detrusor muscle, can be as effective as medication in patients with urinary urge incontinence.11
Detrusor underactivity is defined as contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying within a normal timespan.12 This diagnosis is typically made using urodynamic testing.13 PVRs ≥150 mL are considered evidence of urinary retention. Overflow incontinence can result from detrusor underactivity.
Consider a trial of a cholinergic agonist, such as bethanechol, in patients with urinary retention. Some patients will require intermittent straight catheterization or chronic indwelling foley or suprapubic catheters to void.
Gastrointestinal dysfunction
In patients with diabetes, GI autonomic neuropathy can result in altered esophageal motility leading to gastroesophageal reflux disease (GERD) or dysphagia, gastroparesis, or diabetic enteropathy.14 Gastroparesis often presents as nausea, vomiting, and bloating.1 It may be diagnosed via gastric emptying studies (scintigraphy), and often requires a multidimensional approach to treatment.
Management. Food may be chopped or pureed to aid in digestion. Metoclopramide is the most commonly used prokinetic agent, but avoid its use in patients with parkinsonism. In more severe cases, consider adding domperidone and erythromycin as prokinetic agents. Recommend antiemetics, such as diphenhydramine, ondansetron, and prochlorperazine for management of nausea and vomiting. Severe cases of gastroparesis may merit a venting gastrostomy tube for decompression and/or feeding via a jejunostomy tube.15 Impaired intestinal mobility may lead to stasis syndrome, causing diarrhea.
Hypermobility caused by decreased sympathetic inhibition can also contribute to diarrhea. Altered anal sphincter function tone may contribute to fecal incontinence. Management should focus on balancing electrolytes, maintaining adequate fluid intake, and relieving symptoms. Consider antidiarrheals such as loperamide, but use them with caution to avoid toxic megacolon.16
Constipation. Another common manifestation of autonomic dysfunction in the GI tract is severe constipation.1 This may be managed conservatively with hydration, increased activity, and increased fiber intake. If such measures prove inadequate, consider stool softeners and laxatives.
Patients with constipation due to spinal cord lesions may benefit from a routine bowel regimen. To provide predictable defecation, advise patients to begin by inserting a stimulant rectal suppository. Follow with gentle digital stimulation of the distal rectum for one minute or less. They’ll need to repeat the process every 5 to 10 minutes until stool evacuation is complete. A forward-leaning position may assist with evacuation. It is helpful to perform this routine at the same time each day.17
Orthostatic (postural) hypotension
The autonomic nervous system plays an important role in maintaining BP during positional changes. The sympathetic nervous system adjusts the tone in arteries, veins, and the heart. Baroreceptors located primarily in the carotid arteries and aorta, are highly sensitive to changes in BP. When the baroreceptors sense the slightest drop in pressure, a coordinated increase in sympathetic outflow occurs. Arteries constrict to increase peripheral resistance and BP, and heart rate and contractility increase, all in an attempt to maintain BP and perfusion.18
The most common causes of orthostatic hypotension are not neurologic in origin,9 but rather involve medications, hypovolemia, and impaired autonomic reflexes. The condition is common in the elderly, with one study demonstrating a prevalence of 18.2% in those ≥65 years.19
Orthostatic hypotension may present with dimming or loss of vision, lightheadedness, diaphoresis, diminished hearing, pallor, and weakness. As a result, it is a risk factor for falls. Syncope results when the drop in BP impairs cerebral perfusion. Signs of impaired baroreflexes are supine hypertension, a heart rate that is fixed regardless of posture (the heart rate should increase upon standing), postprandial hypotension, and an excessively high nocturnal BP.1
Orthostatic hypotension is diagnosed when, within 3 minutes of quiet standing after a 5-minute period of supine rest, one or both of the following is present: at least a 20 mm Hg-fall in systolic pressure or at least a 10 mm Hg-fall in diastolic pressure.20 Soysal et al demonstrated that such a drop in BP, measured one minute after standing, is adequate and effective for diagnosing orthostatic hypotension in the elderly.21
Nonpharmacologic management. Recognition and removal of medications that can exacerbate orthostatic hypotension is the first step in managing the condition. Such medications include diuretics, beta-blockers, alpha adrenergic blockers, vasodilators, antipsychotics, antidepressants (SSRIs, trazodone, monoamine oxidase inhibitors, and tricyclic antidepressants), phosphodiesterase inhibitors, narcotics, and antiparkinsonian medications.22
Lifestyle interventions, such as having the patient arise slowly and maintain good hydration, can be helpful. Eating smaller, more frequent meals may also help if the orthostatic hypotension is triggered postprandially. Compressive stockings can help limit venous pooling in the lower extremities and improve venous return. Tensing the legs by crossing them while standing on both feet has been shown to increase cardiac output and BP.23 An aerobic exercise regimen of walking or stair climbing 30 to 45 minutes/day 3 days/week for 6 months was shown to eliminate symptoms of orthostasis on tilt table testing in elderly patients with cardiac deconditioning, as opposed to chronic autonomic failure.24
The reduction in central blood volume associated with autonomic insufficiency (due to increased urinary sodium and water excretion) can be lessened by increasing sodium and water intake.25-27
Pharmacotherapy. Fludrocortisone acetate, a synthetic mineralocorticoid, is the medication of first choice for most patients with orthostatic hypotension whose symptoms are not adequately controlled using nonpharmacologic measures,28 but keep in mind that treating orthostatic hypotension with fludrocortisones is an off-label use of the medication.
Monitor patients taking fludrocortisone for worsened supine hypertension and edema. Also, check their serum potassium levels one to 2 weeks after initiation of therapy and after dose increases. Frequent home monitoring of BP in sitting, standing, and supine positions may be helpful in assessing response to therapy.
If the patient remains symptomatic despite therapy with fludrocortisone, consider adding an alpha-1 adrenergic agonist, such as midodrine. Avoid prescribing midodrine, however, for patients with advanced cardiovascular disease, urinary retention, or uncontrolled hypertension.29
Autonomic dysreflexia: A medical emergency
Autonomic dysreflexia, a medical emergency that must be recognized immediately, is a distinct type of autonomic dysfunction seen in patients with spinal cord injury at or above the T6 level.30 It is a condition of uncontrolled sympathetic response secondary to an underlying condition such as infection, urinary retention, or rectal distention.30
Common symptoms include headache, significant hypertension, flushing of the skin, and diaphoresis above the level of injury.2 In addition, a review of systems should screen for fever, visual changes, abnormalities of the cardiovascular system, syncope, bowel and bladder symptoms, and sexual dysfunction.
Patients demonstrating autonomic dysreflexia should be placed in the upright position to produce an orthostatic decrease in BP.30 Patients should be evaluated to identify any reversible precipitants, such as urinary retention or fecal impaction. Severe attacks involving hypertensive crisis require prompt transfer to the emergency department. Sublingual nifedipine or an intravenous agent, such as hydralazine, may be used to lower BP.31
CORRESPONDENCE
Kristen Thornton, MD, 777 South Clinton Ave., Rochester, NY 14620; [email protected]
Signs and symptoms of autonomic dysfunction commonly present in the primary care setting. Potential causes of dysfunction include certain medications and age-related changes in physiology, as well as conditions such as diabetes mellitus, multiple sclerosis, and Parkinson’s disease (TABLE1). This evidence-based review details common manifestations of autonomic dysfunction, provides a streamlined approach to patients presenting with symptoms, and reviews appropriate step-wise management.

When a delicate balance is disrupted
The autonomic nervous system provides brisk physiologic adjustments necessary to maintain homeostasis. Physiologic functions impacted by the central nervous system include: heart rate, blood pressure (BP), tone of the bladder sphincter and detrusor muscle, bowel motility, bronchodilation and constriction, pupillary dilation and constriction, sweating, catecholamine release, erection, ejaculation and orgasm, tearing, and salivation.1
Disorders of the autonomic system may result from pathologies of the central or peripheral nervous system or from medications including some antihypertensives, selective serotonin-reuptake inhibitors (SSRIs), and opioids.1 Such disorders tend to be grouped into one of 3 categories: those involving the brain, those involving the spinal cord, and autonomic neuropathies.1
The source of dysautonomia can often be determined by clinical context, coexisting neurologic abnormalities, targeted testing of the autonomic nervous system, and neuroimaging.1
Worrisome symptoms prompt a visit
A thorough history is critical to zeroing in on a patient’s complaints and ultimately providing treatment that will help manage symptoms.
When patient complaints are suggestive of autonomic dysfunction, a review of systems should include inquiry about lightheadedness, abnormal salivation, temperature changes of the extremities, gastrointestinal issues (vomiting, constipation, or diarrhea), and symptoms of presyncope/syncope or urinary or sexual dysfunction.1 The physical exam should include recordings of BP and heart rate in the supine and standing positions and a complete neurologic examination.1 Findings will typically point to one or more common complications.
Common complications of autonomic dysfunction
Complications of autonomic dysfunction include impotence, bladder dysfunction, gastrointestinal (GI) dysfunction, and orthostatic hypotension and vasomotor abnormalities. A less common condition—autonomic dysreflexia, which is a distinct type of autonomic dysfunction, and a true medical emergency—is also important to keep in mind.
Impotence
Autonomic neuropathy is a common cause of impotence and retrograde ejaculation. Loss of early morning erections and complete loss of nocturnal erections often have an etiology related to vascular disease and/or autonomic neuropathy. In addition, poor glycemic control and vascular risk factors appear to be associated with the development of diabetic autonomic neuropathy.2
Development of an erection requires an increase in parasympathetic activity and a decrease in sympathetic output. Nocturnal penile tumescence testing has been used to infer parasympathetic damage to the penis in men with diabetes who do not have vascular disease.3
First- and second-line agents. Phosphodiesterase-5 inhibitors (eg, sildenafil, tadalafil, vardenafil) have demonstrated efficacy in improving the ability to achieve and maintain erections in patients with autonomic dysfunction, including diabetic autonomic neuropathy.4-6 Second-line therapies with proven efficacy include intraurethral application and intracavernosal injections of alprostadil.7,8
Bladder dysfunction
Sympathetic activity increases bladder sphincter tone and inhibits detrusor activity, while the parasympathetic nervous system increases detrusor activity and decreases sphincter tone to aid in voiding.1 Disrupted autonomic activity can lead to urinary frequency, retention, and hesitancy; overactive bladder; and incontinence.1 Brain and spinal cord disease above the level of the lumbar spine results in urinary frequency and small bladder volumes, whereas diseases involving autonomic nerve fibers to and from the bladder result in large bladder volumes and overflow incontinence.9
Patients presenting with lower urinary tract symptoms require a comprehensive evaluation to rule out other pathologies, as the differential for such symptoms is broad and includes infection, malignancies, interstitial cystitis, and bladder stones. The initial evaluation of lower urinary tract symptoms should include a history and physical exam including that of the abdomen, pelvis, and neurologic system. Lab work should assess renal function and blood glucose, and should include urinalysis and culture to rule out infection and/or hematuria. A prostate-specific antigen (PSA) test may be appropriate in men with a life expectancy >10 years, after counseling regarding the risks and benefits of screening.
Anticholinergic drugs with antimuscarinic effects, such as oxybutynin, may be used to treat symptoms of urge incontinence and overactive bladder. They work to suppress involuntary contractions of the bladder’s smooth muscle by blocking the release of acetylcholine. These medications relax the bladder’s outer layer of muscle—the detrusor. Such medications often have a number of anticholinergic adverse effects, such as dry mouth and constipation, sometimes leading to discontinuation. A post-void residual (PVR) test may be helpful in guiding management. For example, caution should be used in patients with elevated PVRs, as anticholinergics can worsen urinary retention.
Beta-3 agonists (eg, mirabegron) are a novel class of medications used to treat overactive bladder. These medications act to increase sympathetic tone in the bladder. Because they have the potential to raise BP, monitor BP in patients taking these agents. In addition, monitor patients taking antimuscarinics or beta-3 agonists for the development of urinary retention.
Other tests, treatments. Urodynamic testing is recommended for patients who fail to respond to treatment. Combining behavioral therapy with medication has been shown to be effective in patients with urge incontinence.10 Botulinum toxin type A, injected directly into the detrusor muscle, can be as effective as medication in patients with urinary urge incontinence.11
Detrusor underactivity is defined as contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying within a normal timespan.12 This diagnosis is typically made using urodynamic testing.13 PVRs ≥150 mL are considered evidence of urinary retention. Overflow incontinence can result from detrusor underactivity.
Consider a trial of a cholinergic agonist, such as bethanechol, in patients with urinary retention. Some patients will require intermittent straight catheterization or chronic indwelling foley or suprapubic catheters to void.
Gastrointestinal dysfunction
In patients with diabetes, GI autonomic neuropathy can result in altered esophageal motility leading to gastroesophageal reflux disease (GERD) or dysphagia, gastroparesis, or diabetic enteropathy.14 Gastroparesis often presents as nausea, vomiting, and bloating.1 It may be diagnosed via gastric emptying studies (scintigraphy), and often requires a multidimensional approach to treatment.
Management. Food may be chopped or pureed to aid in digestion. Metoclopramide is the most commonly used prokinetic agent, but avoid its use in patients with parkinsonism. In more severe cases, consider adding domperidone and erythromycin as prokinetic agents. Recommend antiemetics, such as diphenhydramine, ondansetron, and prochlorperazine for management of nausea and vomiting. Severe cases of gastroparesis may merit a venting gastrostomy tube for decompression and/or feeding via a jejunostomy tube.15 Impaired intestinal mobility may lead to stasis syndrome, causing diarrhea.
Hypermobility caused by decreased sympathetic inhibition can also contribute to diarrhea. Altered anal sphincter function tone may contribute to fecal incontinence. Management should focus on balancing electrolytes, maintaining adequate fluid intake, and relieving symptoms. Consider antidiarrheals such as loperamide, but use them with caution to avoid toxic megacolon.16
Constipation. Another common manifestation of autonomic dysfunction in the GI tract is severe constipation.1 This may be managed conservatively with hydration, increased activity, and increased fiber intake. If such measures prove inadequate, consider stool softeners and laxatives.
Patients with constipation due to spinal cord lesions may benefit from a routine bowel regimen. To provide predictable defecation, advise patients to begin by inserting a stimulant rectal suppository. Follow with gentle digital stimulation of the distal rectum for one minute or less. They’ll need to repeat the process every 5 to 10 minutes until stool evacuation is complete. A forward-leaning position may assist with evacuation. It is helpful to perform this routine at the same time each day.17
Orthostatic (postural) hypotension
The autonomic nervous system plays an important role in maintaining BP during positional changes. The sympathetic nervous system adjusts the tone in arteries, veins, and the heart. Baroreceptors located primarily in the carotid arteries and aorta, are highly sensitive to changes in BP. When the baroreceptors sense the slightest drop in pressure, a coordinated increase in sympathetic outflow occurs. Arteries constrict to increase peripheral resistance and BP, and heart rate and contractility increase, all in an attempt to maintain BP and perfusion.18
The most common causes of orthostatic hypotension are not neurologic in origin,9 but rather involve medications, hypovolemia, and impaired autonomic reflexes. The condition is common in the elderly, with one study demonstrating a prevalence of 18.2% in those ≥65 years.19
Orthostatic hypotension may present with dimming or loss of vision, lightheadedness, diaphoresis, diminished hearing, pallor, and weakness. As a result, it is a risk factor for falls. Syncope results when the drop in BP impairs cerebral perfusion. Signs of impaired baroreflexes are supine hypertension, a heart rate that is fixed regardless of posture (the heart rate should increase upon standing), postprandial hypotension, and an excessively high nocturnal BP.1
Orthostatic hypotension is diagnosed when, within 3 minutes of quiet standing after a 5-minute period of supine rest, one or both of the following is present: at least a 20 mm Hg-fall in systolic pressure or at least a 10 mm Hg-fall in diastolic pressure.20 Soysal et al demonstrated that such a drop in BP, measured one minute after standing, is adequate and effective for diagnosing orthostatic hypotension in the elderly.21
Nonpharmacologic management. Recognition and removal of medications that can exacerbate orthostatic hypotension is the first step in managing the condition. Such medications include diuretics, beta-blockers, alpha adrenergic blockers, vasodilators, antipsychotics, antidepressants (SSRIs, trazodone, monoamine oxidase inhibitors, and tricyclic antidepressants), phosphodiesterase inhibitors, narcotics, and antiparkinsonian medications.22
Lifestyle interventions, such as having the patient arise slowly and maintain good hydration, can be helpful. Eating smaller, more frequent meals may also help if the orthostatic hypotension is triggered postprandially. Compressive stockings can help limit venous pooling in the lower extremities and improve venous return. Tensing the legs by crossing them while standing on both feet has been shown to increase cardiac output and BP.23 An aerobic exercise regimen of walking or stair climbing 30 to 45 minutes/day 3 days/week for 6 months was shown to eliminate symptoms of orthostasis on tilt table testing in elderly patients with cardiac deconditioning, as opposed to chronic autonomic failure.24
The reduction in central blood volume associated with autonomic insufficiency (due to increased urinary sodium and water excretion) can be lessened by increasing sodium and water intake.25-27
Pharmacotherapy. Fludrocortisone acetate, a synthetic mineralocorticoid, is the medication of first choice for most patients with orthostatic hypotension whose symptoms are not adequately controlled using nonpharmacologic measures,28 but keep in mind that treating orthostatic hypotension with fludrocortisones is an off-label use of the medication.
Monitor patients taking fludrocortisone for worsened supine hypertension and edema. Also, check their serum potassium levels one to 2 weeks after initiation of therapy and after dose increases. Frequent home monitoring of BP in sitting, standing, and supine positions may be helpful in assessing response to therapy.
If the patient remains symptomatic despite therapy with fludrocortisone, consider adding an alpha-1 adrenergic agonist, such as midodrine. Avoid prescribing midodrine, however, for patients with advanced cardiovascular disease, urinary retention, or uncontrolled hypertension.29
Autonomic dysreflexia: A medical emergency
Autonomic dysreflexia, a medical emergency that must be recognized immediately, is a distinct type of autonomic dysfunction seen in patients with spinal cord injury at or above the T6 level.30 It is a condition of uncontrolled sympathetic response secondary to an underlying condition such as infection, urinary retention, or rectal distention.30
Common symptoms include headache, significant hypertension, flushing of the skin, and diaphoresis above the level of injury.2 In addition, a review of systems should screen for fever, visual changes, abnormalities of the cardiovascular system, syncope, bowel and bladder symptoms, and sexual dysfunction.
Patients demonstrating autonomic dysreflexia should be placed in the upright position to produce an orthostatic decrease in BP.30 Patients should be evaluated to identify any reversible precipitants, such as urinary retention or fecal impaction. Severe attacks involving hypertensive crisis require prompt transfer to the emergency department. Sublingual nifedipine or an intravenous agent, such as hydralazine, may be used to lower BP.31
CORRESPONDENCE
Kristen Thornton, MD, 777 South Clinton Ave., Rochester, NY 14620; [email protected]
1. Low PA, Engstrom JW. Disorders of the autonomic nervous system. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015. Available at: http://accessmedicine.mhmedical.com/content.aspx?bookid=1130&Sectionid=79755967. Accessed May 15, 2016.
2. Ko SH, Park SA, Cho JH, et al. Progression of cardiovascular dysfunction in patients with type 2 diabetes: a 7 year follow-up study. Diabetes Care. 2008;31:1832-1836.
3. Brown JS, Wessells H, Chancellor MB, et al. Urologic complications of diabetes. Diabetes Care. 2005;28:177-185.
4. Rendell MS, Rajfer J, Wicker PA, et al. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421-426.
5. Goldstein I, Young JM, Fischer J, et al. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care. 2003;26:777-783.
6. Sáenz de Tejada I, Anglin G, Knight JR, et al. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002;25:2159-2164.
7. Padma-Nathan H, Hellstrom WJ, Kaiser FE, et al. Treatment of men with erectile dysfunction with transurethral alprostadil. Medicated Urethral System for Erection (MUSE) Study Group. N Engl J Med. 1997;336:1-7.
8. Linet OI, Ogrinc FG. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction. The Alprostadil Study Group. N Engl J Med. 1996;334:873-877.
9. Engstrom JW, Maring JB. Disorders of the autonomic nervous system. In: Braunwald E, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York, NY: McGraw Hill; 2001.
10. Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370-374.
11. Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs. onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367:1803-1813.
12. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
13. Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65:389-398.
14. Kempler P, Amarenco G, Freeman R, et al. Management strategies for gastrointestinal, erectile, bladder, and sudomotor dysfunction in patients with diabetes. Diabetes Metab Res Rev. 2011;27:665-677.
15. Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820-829.
16. Shakil A, Church RJ, Rao SS. Gastrointestinal complications of diabetes. Am Fam Physician. 2008;77:1697-1702.
17. Krassioukov A, Eng JJ, Claxton G, et al. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord. 2010;48:718-733.
18. Bradley JG, Davis K. Orthostatic hypotension. Am Fam Physician. 2003;68:2393-2399.
19. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19(6 Pt 1):508-519.
20. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
21. Soysal P, Aydin AE, Koc Okudur S, et al. When should orthostatic BP changes be evaluated in elderly: 1st, 3rd or 5th minute? Arch Gerontol Geriatr. 2016;65:199-203.
22. Perlmuter LC, Sarda G, Casavant V, et al. A review of the etiology, associated comorbidities, and treatment of orthostatic hypotension. Am J Ther. 2013;20:279-291.
23. Ten Harkel ADJ, van Lieshout JJ, Wieling W. Effects of leg muscle pumping and tensing on orthostatic arterial pressure: a study in normal subjects and patients with autonomic failure. Clin Sci. 1994;87:553-558.
24. Carroll JF, Wood CE, Pollock ML, et al. Hormonal responses in elders experiencing pre-syncopal symptoms during head-up tilt before and after exercise training. J Gerontol A Biol Sci Med Sci. 1995;50:M324-M329.
25. Shannon JR, Diedrich A, Biaggioni I, et al. Water drinking as a treatment for orthostatic syndromes. Am J Med. 2002;112:355-360.
26. Young T, Mathias C. The effects of water ingestion on orthostatic hypotension in two groups of chronic autonomic failure: multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry. 2004;75:1737-1741.
27. Humm AM, Mason LM, Mathias CJ. Effects of water drinking on cardiovascular responses to supine exercise and on orthostatic hypotension after exercise in pure autonomic failure. J Neurol Neurosurg Psychiatry. 2008;79:1160-1164.
28. Campbell IW, Ewing DJ, Clarke BF. 9-Alpha-fluorohydrocortisone in the treatment of postural hypotension in diabetic autonomic neuropathy. Diabetes. 1975;24:381-384.
29. Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis. 2013;55:425-433.
30. Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999;37:383-391.
31. Bycroft J, Shergill IS, Choong EAL, et al. Autonomic dysreflexia: a medical emergency. Postgrad Med J. 2005;81:232-235.
1. Low PA, Engstrom JW. Disorders of the autonomic nervous system. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015. Available at: http://accessmedicine.mhmedical.com/content.aspx?bookid=1130&Sectionid=79755967. Accessed May 15, 2016.
2. Ko SH, Park SA, Cho JH, et al. Progression of cardiovascular dysfunction in patients with type 2 diabetes: a 7 year follow-up study. Diabetes Care. 2008;31:1832-1836.
3. Brown JS, Wessells H, Chancellor MB, et al. Urologic complications of diabetes. Diabetes Care. 2005;28:177-185.
4. Rendell MS, Rajfer J, Wicker PA, et al. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421-426.
5. Goldstein I, Young JM, Fischer J, et al. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care. 2003;26:777-783.
6. Sáenz de Tejada I, Anglin G, Knight JR, et al. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002;25:2159-2164.
7. Padma-Nathan H, Hellstrom WJ, Kaiser FE, et al. Treatment of men with erectile dysfunction with transurethral alprostadil. Medicated Urethral System for Erection (MUSE) Study Group. N Engl J Med. 1997;336:1-7.
8. Linet OI, Ogrinc FG. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction. The Alprostadil Study Group. N Engl J Med. 1996;334:873-877.
9. Engstrom JW, Maring JB. Disorders of the autonomic nervous system. In: Braunwald E, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York, NY: McGraw Hill; 2001.
10. Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370-374.
11. Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs. onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367:1803-1813.
12. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
13. Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65:389-398.
14. Kempler P, Amarenco G, Freeman R, et al. Management strategies for gastrointestinal, erectile, bladder, and sudomotor dysfunction in patients with diabetes. Diabetes Metab Res Rev. 2011;27:665-677.
15. Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820-829.
16. Shakil A, Church RJ, Rao SS. Gastrointestinal complications of diabetes. Am Fam Physician. 2008;77:1697-1702.
17. Krassioukov A, Eng JJ, Claxton G, et al. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord. 2010;48:718-733.
18. Bradley JG, Davis K. Orthostatic hypotension. Am Fam Physician. 2003;68:2393-2399.
19. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19(6 Pt 1):508-519.
20. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
21. Soysal P, Aydin AE, Koc Okudur S, et al. When should orthostatic BP changes be evaluated in elderly: 1st, 3rd or 5th minute? Arch Gerontol Geriatr. 2016;65:199-203.
22. Perlmuter LC, Sarda G, Casavant V, et al. A review of the etiology, associated comorbidities, and treatment of orthostatic hypotension. Am J Ther. 2013;20:279-291.
23. Ten Harkel ADJ, van Lieshout JJ, Wieling W. Effects of leg muscle pumping and tensing on orthostatic arterial pressure: a study in normal subjects and patients with autonomic failure. Clin Sci. 1994;87:553-558.
24. Carroll JF, Wood CE, Pollock ML, et al. Hormonal responses in elders experiencing pre-syncopal symptoms during head-up tilt before and after exercise training. J Gerontol A Biol Sci Med Sci. 1995;50:M324-M329.
25. Shannon JR, Diedrich A, Biaggioni I, et al. Water drinking as a treatment for orthostatic syndromes. Am J Med. 2002;112:355-360.
26. Young T, Mathias C. The effects of water ingestion on orthostatic hypotension in two groups of chronic autonomic failure: multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry. 2004;75:1737-1741.
27. Humm AM, Mason LM, Mathias CJ. Effects of water drinking on cardiovascular responses to supine exercise and on orthostatic hypotension after exercise in pure autonomic failure. J Neurol Neurosurg Psychiatry. 2008;79:1160-1164.
28. Campbell IW, Ewing DJ, Clarke BF. 9-Alpha-fluorohydrocortisone in the treatment of postural hypotension in diabetic autonomic neuropathy. Diabetes. 1975;24:381-384.
29. Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis. 2013;55:425-433.
30. Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999;37:383-391.
31. Bycroft J, Shergill IS, Choong EAL, et al. Autonomic dysreflexia: a medical emergency. Postgrad Med J. 2005;81:232-235.
PRACTICE RECOMMENDATIONS
› Begin a trial of an antimuscarinic if initial nonpharmacologic treatment of urge incontinence or overactive bladder is ineffective. B
› Start step-wise treatment beginning with metoclopramide A, followed by domperidone, and, finally, oral erythromycin B in patients with gastroparesis who have failed conservative measures.
› Employ step-wise pharmacologic treatment, starting with fludrocortisone, for patients with disabling symptoms of orthostatic hypotension who fail to respond to nonpharmacologic measures. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hand and arm pain: A pictorial guide to injections
Primary care physicians are frequently the first to evaluate hand, wrist, and forearm pain in patients, making knowledge of the symptoms, causes, and treatment of common diagnoses in the upper extremities imperative. Primary symptoms usually include pain and/or swelling. While most tendon disorders originating in the hand and wrist are idiopathic in nature, some patients occasionally report having recently performed unusual manual activity or having experienced trauma to the area days or weeks prior. A significant portion of patients are injured as a result of chronic repetitive activities at work.1
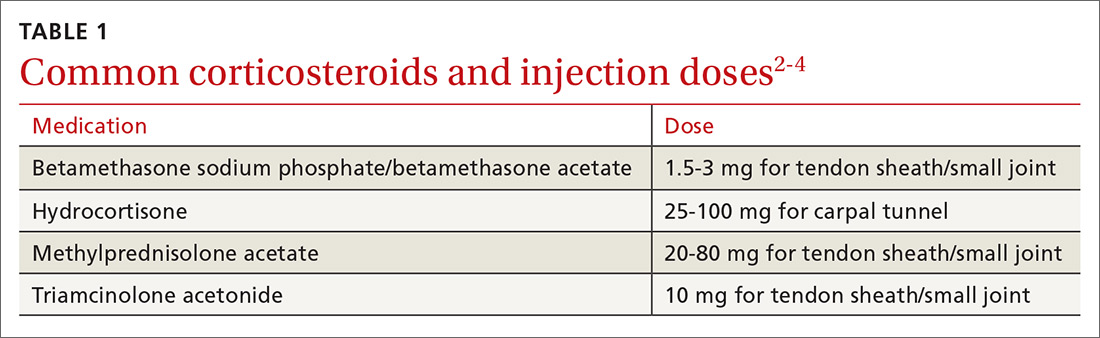
Most diagnoses can be made by pairing your knowledge of hand and forearm anatomy with an understanding of which tender points are indicative of which common conditions. (Care, of course, must be taken to ensure that there is no underlying infection.) Common conditions can often be treated nonsurgically with conservative treatments such as physical therapy, bracing/splinting, nonsteroidal anti-inflammatory drugs (NSAIDs), and injections of corticosteroids (eg, betamethasone, hydrocortisone, methylprednisolone, and triamcinolone) (TABLE 12-4) with or without the use of ultrasound. The benefits of corticosteroid injections for these conditions are well studied and documented in the literature, although physicians should always warn patients of the possible adverse effects prior to injection3,5 (TABLE 24).

To help you refine your skills, we review some of the more common hand and forearm conditions you are likely to encounter in the office and provide photos that reveal underlying anatomy so that you can administer injections without, in many cases, the need for ultrasound.
Trigger finger/thumb: New pathophysiologic findings?
Trigger finger most commonly occurs in the dominant hand. It is also more common in women, patients in their 50s, and in individuals with diabetes.6 Trigger finger/thumb is caused by inflammation and constriction of the flexor tendon sheath, which carries the flexor tendons through the palm and into the fingers and thumb. This, in turn, causes irritation of the tendons, sometimes via the formation of tendinous nodules, which may impinge upon the sheath’s “pulley system.”

When the “pulley” is compromised. The retinacular sheath is composed of 5 annular ligaments, or pulleys, that hold the tendons of the fingers close to the bone and allow the fingers to flex properly. The A1 pulley, at the level of the metacarpal head, is the first part of the sheath and is subject to the highest force; high forces may subsequently lead to the finger becoming locked in a flexed, or trigger, position.6 Patients may experience pain in the distal palm at the level of the A1 pulley and clicking of the finger.6
Additionally . . . recent studies show discrete histologic changes in trigger finger tendons, similar to findings with Achilles tendinosis and tendinopathy.7 In trigger finger tendons, collagen type 1A1 and 3A1, aggrecan, and biglycan are up-regulated, while metalloproteinase inhibitor 3 (TIMP-3) and matrix metallopeptidase3 (MMP-3) are down-regulated, a situation also described in Achilles tendinosis.7 This similarity in conditions provides new insight into the pathophysiology of the condition and may help provide future treatments.
Making the Dx: Look for swelling, check for carpal tunnel
During the examination, first look at both hands for swelling, arthropathy, or injury, and note the presence of any joint contractures. Next, examine all of the digits in flexion and extension while noting which ones are triggering, as the problem can occur in multiple digits on one hand. Then palpate the palms over the patient’s metacarpal heads, feeling for tender nodules.
Finally, examine the patient for carpal tunnel syndrome (CTS). A positive Tinel’s sign (shooting pain into the hand when the median nerve in the wrist is percussed), a positive Phalen maneuver (numbness or pain, usually within one minute of full wrist flexion), or thenar muscle wasting are highly indicative of CTS (compression of the median nerve at the transverse carpal ligament in the carpal tunnel). It is important to check for CTS when examining a patient for trigger finger because the 2 conditions frequently co-occur.6 (For more on CTS, see here.)
Treatment: Consider corticosteroids first
First-line treatment for patients with trigger finger or thumb is a corticosteroid injection into the subcutaneous tissue around the tendon sheath (FIGURES 1 and 2). (For this indication and for the others discussed throughout the article, there isn’t tremendous evidence for one particular type of corticosteroid over another; see TABLE 12-4 for choices.) Up to 57% of cases resolve with one injection, and 86% resolve with 2,8 but keep in mind that it may take up to 2 weeks to achieve the full clinical benefit.
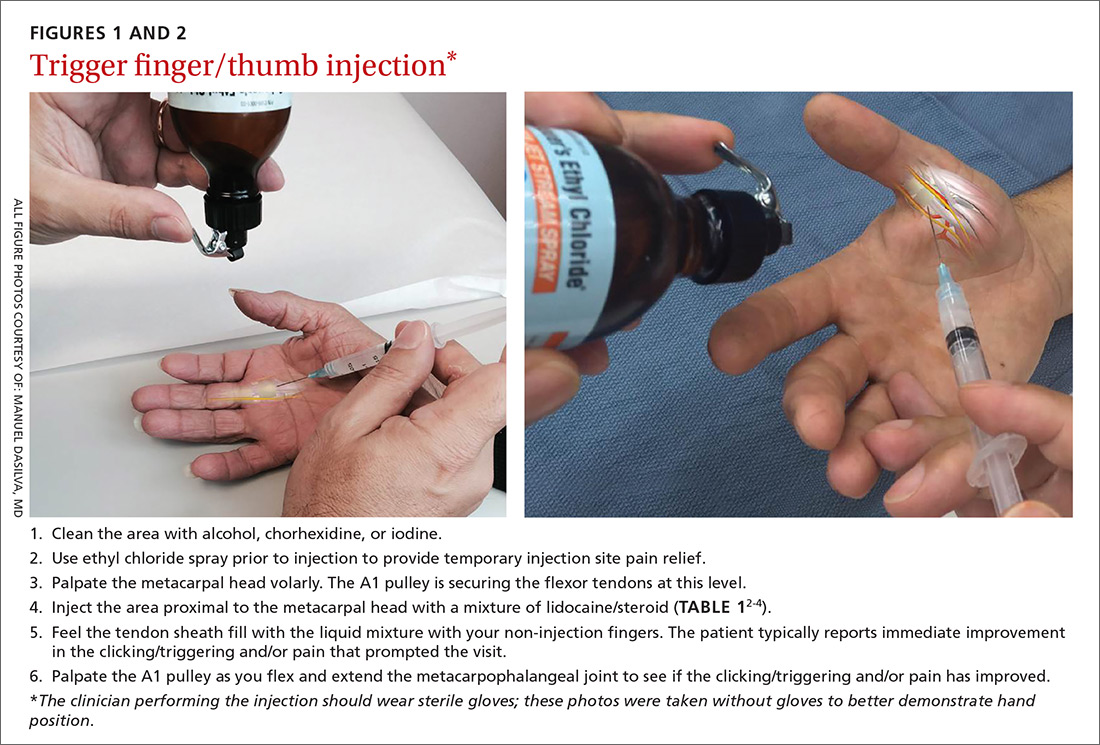
Patients with multiple trigger fingers can be treated with oral corticosteroids (eg, a methylprednisolone dose pack). Peters-Veluthamaningal et al performed a systematic review in 2009 and found 2 randomized controlled trials involving 63 patients (34 received injections of a corticosteroid [either methylprednisolone or betamethasone] and lidocaine and 29 received lidocaine only).2 The corticosteroid/lidocaine combination was more effective at 4 weeks (relative risk [RR]=3.15; 95% confidence interval [CI], 1.34 to 7.40).2
If 2 corticosteroid injections 6 weeks apart fail to provide benefit, or the finger is irreversibly locked in flexion, surgical release of the pulley is required and is performed through a palmar incision at the level of the A1 pulley. Complications from this surgery, including nerve damage, are exceedingly rare, but injury can occur, given the proximity of the digital nerves to the A1 pulley.
Patient is a child? Refer children with trigger finger or thumb to a hand surgeon for evaluation and management because the indications for nonoperative treatment in the pediatric population are unclear.9
Carpometacarpal arthritis: Common, with many causes
Osteoarthritis of the first carpometacarpal (CMC) joint is the most common site of arthritis in the hand/wrist region, affecting up to 11% of men and 33% of women in their 50s and 60s.10 Because the CMC joint lacks a bony restraint, it relies on a number of ligaments for stability—the strongest and most important of which is the palmar oblique “beak” ligament.11 A major cause of degenerative arthritis of this joint is attenuation and laxity of these ligaments, leading to abnormal and increased stress loads, which, in turn, can lead to loss of cartilage and bony impingement. While the exact mechanism of this process is not fully understood,10,12 acute or chronic trauma, advanced age, hormonal factors, and genetic factors seem to play a role.11
Many believe there is a relationship between a patient's occupation and the development of CMC arthritis, but studies are inconclusive.13 At risk are secretarial workers, tailors, domestic helpers/cleaners, and individuals whose jobs involve repetitive thumb use and/or insufficient rest of the joint throughout the day.
Making the Dx: Perform the Grind test
A detailed patient history (which is usually void of trauma to the hand) and physical examination are the keys to making the diagnosis of CMC arthritis. A history of pain at the base of the thumb during pinching and gripping tasks is often elucidated. Classically, patients describe pain upon turning keys, opening jars, and gripping doorknobs.11
It's important to focus on the dorsoradial aspect of the thumb during the physical exam and to rule out other causes of pain, such as de Quervain’s tenosynovitis, flexor carpi radialis tendinitis, CTS, and trigger thumb.11 Typical findings include pain with palpation directly over the dorsoradial aspect of the CMC joint and pain with axial loading and upon circumduction during a Grind test of the CMC joint. (The Grind test is performed by moving the metacarpal bone of the thumb in a circle and loading it with gentle axial forces. People with thumb joint arthritis generally experience sudden sharp pain at the CMC joint.)
Radiographic findings can be useful as a diagnostic adjunct, with staging of the disease, and in determining who can benefit from conservative management.11
Treatment: Start with NSAIDs and splinting
Depending on the degree of arthritis, management may include both conservative and surgical options.10 Patient education describing activity modification is useful during all stages of CMC arthritis. Research has shown that avoiding inciting activities, such as key turning, pinching, and grasping, helps to alleviate symptoms.14 Patients may also obtain relief from NSAIDs, especially when they are used in conjunction with activity modification and splinting. NSAIDs, however, do not halt or reverse the disease process; they only reduce inflammation, synovitis, and pain.11
Splinting. Studies have shown splinting of the thumb CMC joint to provide pain relief and to potentially slow disease progression.15 Because splints decrease motion and increase joint stability, they are especially useful for patients with joint hypermobility. The long opponens thumb spica splint is commonly used; it immobilizes the wrist and CMC, while leaving the thumb interphalangeal joint free. Short thumb spica and neoprene splints are also commercially available, and studies have shown that they provide good results.15 Splinting is most beneficial in patients with early-stage disease and may be used for either short-term flares or long-term treatment.11
Cortisone injections. For those patients who do not respond to activity modification, NSAIDs, and/or splinting, consider cortisone injections (FIGURE 3). Intra-articular cortisone injections can decrease inflammation and provide good pain relief, especially in patients with early-stage disease. The effectiveness of cortisone injections in patients with more advanced disease is not clear; no benefit has been shown in studies to date.16 Equally unclear is the long-term benefit of injections.11 Patients who do not respond to conservative treatments will often require surgical care.
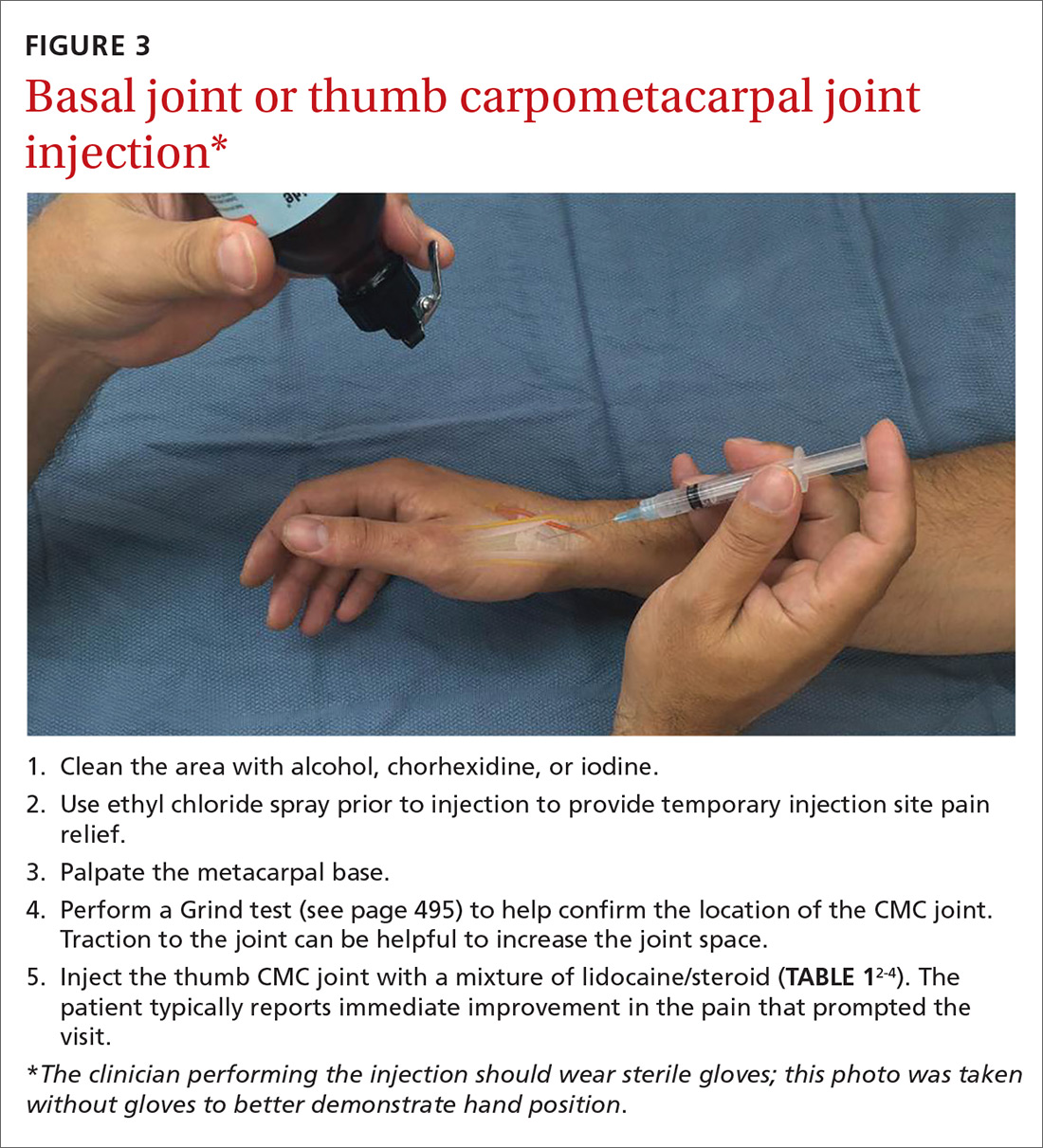
Carpal tunnel syndrome: Moving slower to surgery
CTS is one of the most common conditions of the upper extremities. Researchers estimate that 491 women per 100,000 person-years and 258 men per 100,000 person-years will develop CTS, with 109 per 100,000 person-years receiving carpal tunnel release surgery.17 Risk factors for the development of CTS include diabetes, hypothyroidism, rheumatoid arthritis, pregnancy, obesity, family history, trauma, and occupations that involve repetitive tasks or long hours working at a computer.18
CTS is caused by compression of the median nerve as it passes through the carpal tunnel.19 The elevated pressure in the carpal tunnel restricts epineural blood flow and supply, causing the pain felt with CTS.20 Even after surgical decompression, recurrent or persistent CTS can be a problem.21
Making the Dx: Perform the Phalen maneuver, Durkan’s test
Patients typically present with complaints of weakness, pain, and/or numbness in at least 2 of 4 radial digits (thumb, index, middle, ring).19,22 The most common time of day for patients to have symptoms is at night.21
The diagnostic tools. Tinel’s sign is a useful diagnostic tool when you suspect carpal tunnel syndrome. Tinel’s sign is positive if percussion over the median nerve at the carpal tunnel elicits pain or paresthesia.18
When employing the Phalen maneuver, be certain to have the patient flex his/her wrist to 90 degrees and to document the number of seconds it takes for numbness to present in the fingers. Pain or paresthesia should occur in <60 seconds for the test to be positive.18
Median nerve compression over the carpal tunnel, also known as Durkan’s test, may also elicit symptoms. With Durkan’s test, you apply direct pressure over the transverse carpal ligament. If pain or paresthesia occurs in <30 seconds, the test is positive.18 Often clinicians will combine the Phalen maneuver and Durkan’s test to increase sensitivity and specificity.18 Nerve conduction studies are often performed to confirm the clinical diagnosis.
Is more than one condition at play? It is important to determine whether cervical spine disease and/or peripheral neuropathy is contributing to the patient’s symptoms, along with CTS; patients may have more than one condition contributing to their pain. We routinely check cervical spine motion, tenderness, and nerve compression as part of the exam on a patient with suspected CTS. In the office, a monofilament test or 2-point discrimination test can help make the clinical diagnosis by uncovering decreased sensation in the thumb, index, and/or middle fingers.23
The 5.07 monofilament test is performed with the clinician applying the monofilament to different dermatomal or sensory distributions while the patient has his/her eyes closed. The 2-point discrimination test is performed with a caliper device that measures the distance at which the patient can feel 2 separate stimuli. Often electromyography or nerve conduction studies are necessary.18
Treatment: Pursue nonoperative approaches
A survey of the membership of the American Society for Surgery of the Hand revealed that surgeons are utilizing nonoperative treatments for a longer duration of time and are employing narrowed surgical indications.24 Thus, clinicians are more likely to try splints and steroid injections before proceeding to operative release.24
Nonsurgical management. In our practice, we commonly recommend corticosteroid injections (TABLE 12-4) into the carpal tunnel (FIGURES 4 and 5) to patients who are poor candidates for surgery (ie, those who have too many medical comorbidities or wound healing concerns). This is one indication for which you may want to consider ultrasound-guided injections because the improved accuracy may provide symptom relief faster than “blind” or palpation-guided injections.25
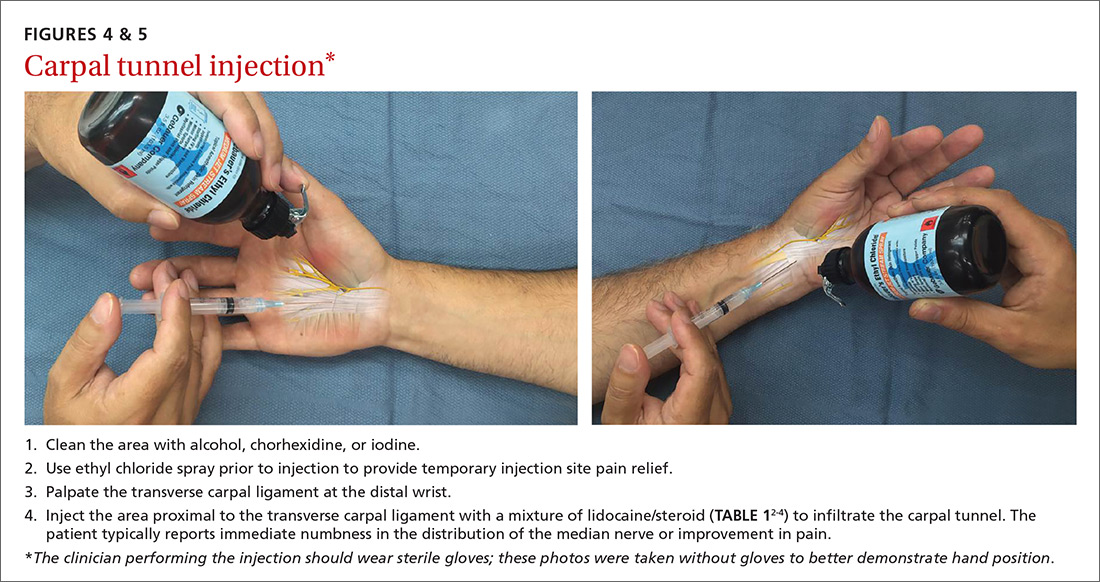
A recent randomized controlled trial from Sweden showed that injections of methylprednisolone relieved symptoms in patients with mild to moderate CTS at 10 weeks and reduced the rate of surgery one year after treatment; however, 3 out of 4 patients still went on to have surgery within a year.22 Patients in the study had failed a 2-month trial of splinting and were given either 80 mg or 40 mg of methylprednisolone or saline. There was no statistical difference between the doses of methylprednisolone in preventing surgery at one year. Compared to placebo, the 80-mg methylprednisolone group was less likely to have surgery with an odds ratio of 0.24 (P=.042).22
There is evidence that oral steroids, injected steroids, ultrasound, electromagnetic field therapy, nocturnal splinting, and use of ergonomic keyboards are effective nonoperative modalities in the short term, but evidence is sparse for mid- or long-term use.19 In addition, at least one randomized trial found traditional cupping therapy applied around the shoulder alleviated carpal tunnel symptoms in the short-term.26 Other nonoperative therapies include rest, NSAIDs, extracorporeal shock wave therapy, and activity modification.19,27
Surgical outcomes by either endoscopic, mini-open, or open surgical techniques are typically good.20,21 Surgical release involves cutting the transverse carpal ligament over the carpal tunnel to decompress the median nerve.24 You should inform patients of the risks and inconveniences associated with surgery, including the cost, absence from work, infection, and chronic pain. Patients who have recurrent or persistent symptoms after surgery may have had an incompletely released transverse carpal ligament or there may be no identifiable cause.21 Overall, surgical treatment, combined with physical therapy, seems to be more effective than splinting or NSAIDs for mid- and long-term treatment of CTS.28
De Quervain’s tenosynovitis: Common during pregnancy
De Quervain’s tenosynovitis (radial styloid tenosynovitis) involves painful inflammation of the 2 tendons in the first dorsal compartment of the wrist—the abductor pollicis longus (APL) and the extensor pollicis brevis (EPB). The tendons comprise the radial border of the anatomic snuffbox.
The APL abducts and extends the thumb at the CMC joint, while the EPB extends the thumb proximal phalanx at the metacarpophalangeal joint. These tendons are contained in a synovial sheath that is subject to inflammation and constriction and subsequent wear and damage.29 In addition, the extensor retinaculum in patients with de Quervain’s disease demonstrates increased vascularity and deposition of dense fibrous tissue resulting in thickening of the tendon up to 5 times its normal width.30
As a result, degeneration and thickening of the tendon sheath, as well as radial-sided wrist pain elicited at the first dorsal compartment, are common pathophysiologic and clinical findings.31 Pain is often accompanied by the build-up of protuberances and nodulations of the tendon sheath.
De Quervain’s disease commonly occurs during and after pregnancy.32 Other risk factors include racquet sports, golfing, wrist trauma, and other activities involving repetitive hand and wrist motions.33 Often, however, de Quervain’s is idiopathic.
Making the Dx: Perform a Finkelstein's test
The major finding in patients with de Quervain’s tenosynovitis is a positive Finkelstein's test. To perform Finkelstein's test (FIGURE 6), ask the patient to oppose the thumb into the palm and flex the fingers of the same hand over the thumb. Holding the patient’s fingers around the thumb, ulnarly deviate the wrist. Finkelstein's test puts strain on the APL and EPB, causing pain along the radial border of the wrist and forearm in patients with de Quervain’s tenosynovitis. Since the maneuver can be uncomfortable, complete the exam on the unaffected side for comparison.
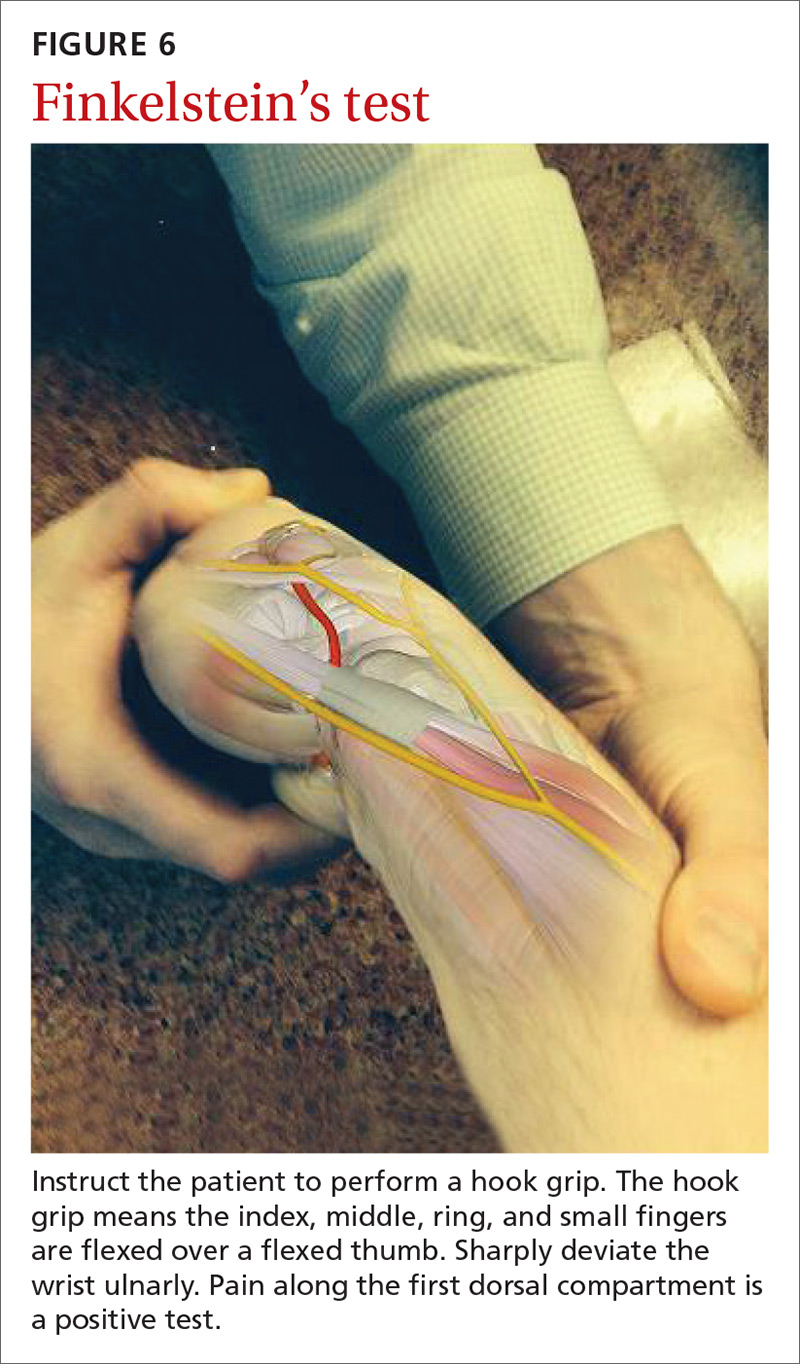
Stenosis of the tendon sheath may lead to crepitus over the first dorsal wrist compartment. This should be distinguished from intersection syndrome (tenosynovitis at the intersection of the first and second extensor compartments), which can also present with forearm and wrist crepitus. Patients usually have swelling of the wrist with marked discomfort upon palpation of the radial tendons. An x-ray can be useful to evaluate for CMC or radiocarpal arthritis, which may be an underlying cause.
Treatment: Select an approach based on symptom severity
In a retrospective analysis, Lane et al concluded that classification of patients with de Quervain’s disease based on pretreatment symptoms may assist physicians in selecting the most efficacious treatment and in providing prognostic information to their patients (TABLE 334). Patients with mild to moderate (Types 1 and 2) de Quervain’s may benefit from immobilization in a thumb spica splint, rest, NSAIDs, and physical or occupational therapy. If work conditions played a role in causing the symptoms, they need to be addressed to improve outcomes. Types 2 and 3 can be initially treated with a corticosteroid injection, but may eventually require surgery.33
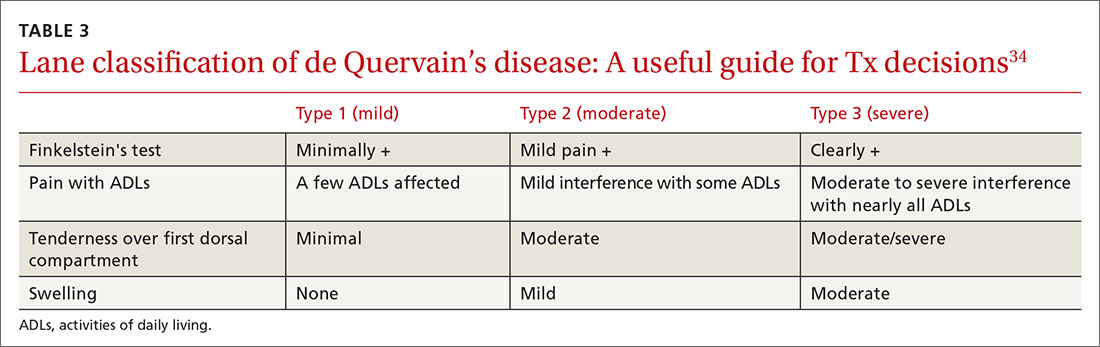
Treatment with NSAIDs or corticosteroid injections (see TABLE 12-4 for choices) in the first compartment of the extensor retinaculum (FIGURE 7) is usually adequate to provide relief. Peters-Veluthamaningal et al performed a systematic review in 2009 and found only one controlled trial of 18 participants (all pregnant or lactating women) who were either injected with corticosteroids or given a thumb spica splint.35 All 9 patients in the injection group had complete pain relief, whereas no one in the splint group had complete resolution of symptoms.35 Typical anatomic placement of corticosteroid injections is shown in FIGURE 7.
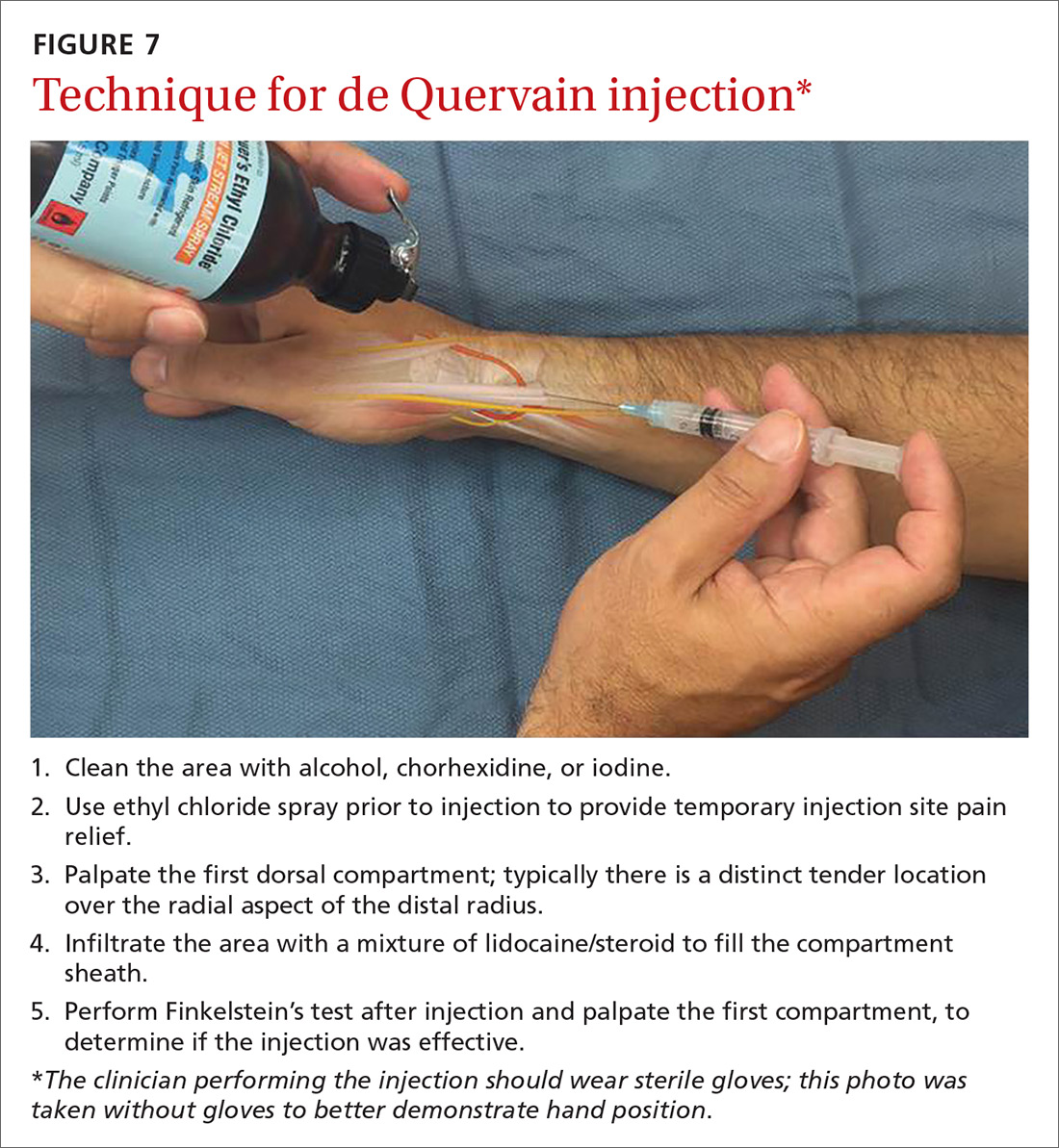
More complicated injection methods have been described, but injecting the first dorsal compartment is usually satisfactory. Patients will feel the tendon sheath filling with the injection material. The 2-point technique, implemented by Sawaizumi et al, which involves injecting corticosteroid into 2 points over the EPB and APL tendon in the area of maximum pain and soft tissue thickening, is more effective than the 1-point injection technique.36
Severe, recalcitrant cases. Professional and college athletes may be prone to recalcitrant de Quervain’s tenosynovitis. A 2010 study by Pagonis et al showed that recurrent symptomatic episodes commonly occur in athletes who engage in high-resistance, intense athletic training. In these severe cases, a 4-point injection technique offers better distribution of corticosteroid solution to the first extensor compartment than other methods.37 Consider referring severe cases to a hand surgeon.
Surgical release of the first dorsal compartmental sheath around the tendons serves as a final option for patients who fail conservative treatment. Care should be taken to release both tendons completely, as there may be at least 2 tendon slips of the APL or there may be a distinct EPB sheath dorsally.38
“Tennis elbow”— you don’t have to play tennis to have it
Lateral epicondylitis (tennis elbow) is a painful condition involving microtears within the extensor carpi radialis brevis muscle and the subsequent development of angiofibroblastic dysplasia.39 According to Regan et al who studied the histopathologic features of 11 patients with lateral epicondylitis, the underlying cause of recalcitrant lateral epicondylitis is, in fact, degenerative, rather than inflammatory.40
Although the condition has been nicknamed “tennis elbow,” only about 5% of tennis players have the condition.41 In tennis players, males are more often affected than females, whereas in the general population, incidence is approximately equal in men and women.41 Lateral epicondylitis occurs between 4 and 7 times more frequently than medial-sided elbow pain.42
Making the Dx: Look for localized pain, normal ROM
The diagnosis of lateral epicondylitis is based upon a history of pain over the lateral epicondyle and findings on physical examination, including local tenderness directly over the lateral epicondyle,43 pain aggravated by resisted wrist extension and radial deviation, pain with resisted middle finger extension, and decreased grip strength or pain aggravated by strong gripping. These findings typically occur in the presence of normal elbow range of motion.
Treatment: Choose from a range of options
Since lateral epicondylitis was first described, researchers have proposed a wide variety of treatments as initial interventions including rest, activity, equipment modification, NSAIDs, wrist bracing/elbow straps, and physical therapy. If initial treatment does not produce the desired effect, second-line treatments include corticosteroid injections (FIGURE 8), prolotherapy (injection of an irritant, often dextrose; see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768), autologous blood injections, platelet-rich plasma injections (see “Is platelet-rich plasma right for your patient?” J Fam Pract. 2016;65:319-328), and needling of the extensor tendon origin. Refer patients who do not improve after one corticosteroid injection to an orthopedic surgeon for consideration of open or arthroscopic treatment.
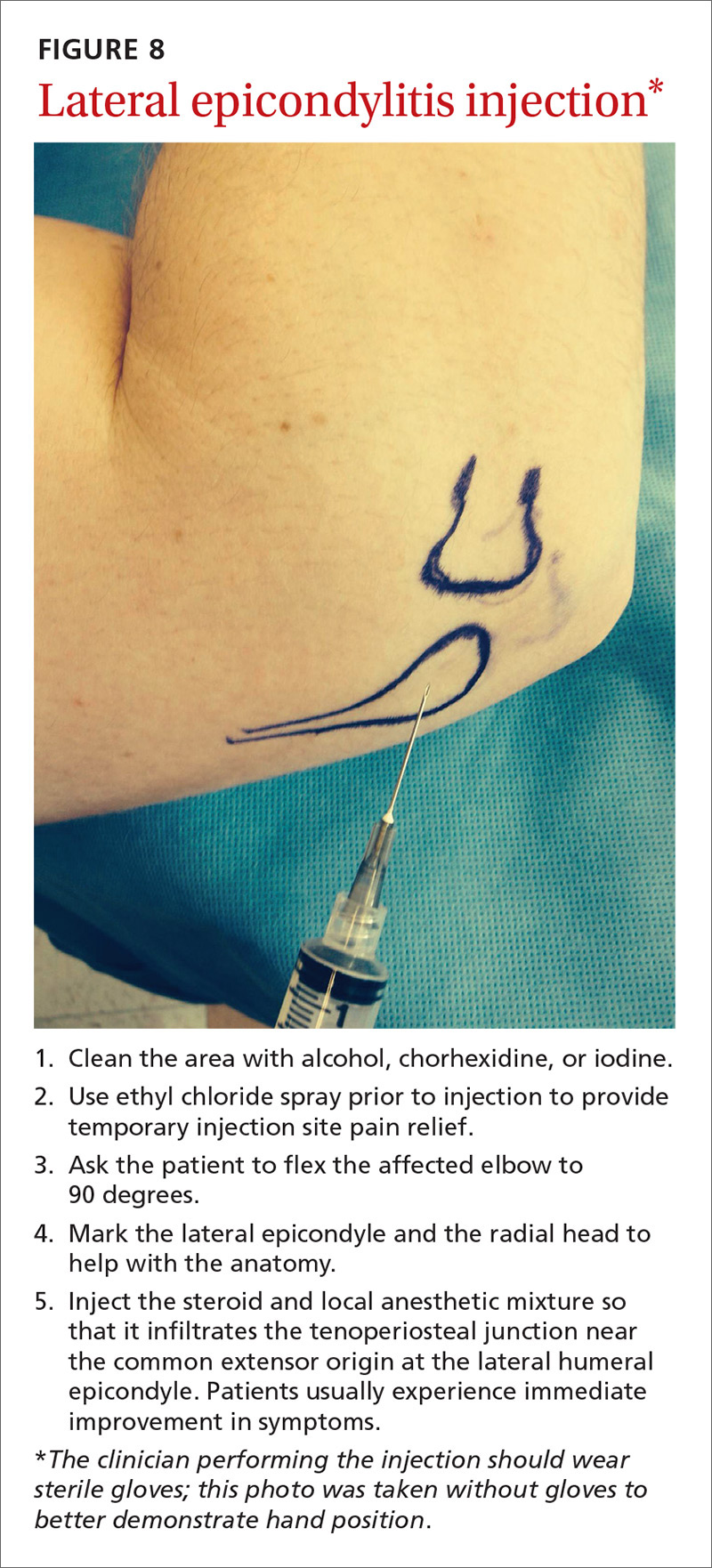
CORRESPONDENCE
Gregory R. Waryasz, MD, Rhode Island Hospital, Department of Orthopaedic Surgery, 593 Eddy St., Providence, RI 02903; [email protected].
1. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775.
2. Peters-Veluthamaningal C, Van der Windt DA, Winters JC, et al. Corticosteroid injection for trigger finger in adults. Cochrane Database Syst Rev. 2009:CD005617.
3. Cheng J, Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11:141-147.
4. Waryasz GR, Tambone R, Borenstein TR, et al. A review of anatomical placement of corticosteroid injections for uncommon hand, wrist, and elbow pathologies. R I Med J. 2017;100:31-34.
5. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1:396-404.
6. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743.
7. Lundin AC, Aspenberg P, Eliasson P. Trigger finger, tendinosis, and intratendinous gene expression. Scand J Med Sci Sports. 2014;24:363-368.
8. Sato ES, Gomes Dos Santos JB, Belloti JC, et al. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology (Oxford). 2012;51:93-99.
9. Baek GH, Kim JH, Chug MS, et al. The natural history of pediatric trigger thumb. J Bone Joint Surg Am. 2008;90:980-985.
10. Gillis J, Calder K, Williams J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can J Plast Surg. 2011;19:134-138.
11. Yao J, Park MJ. Early treatment of degenerative arthritis of the thumb carpometacarpal joint. Hand Clin. 2008;24:251-261.
12. Ladd AL, Weiss AP, Crisco JJ, et al. The thumb carpometacarpal joint: anatomy, hormones, and biomechanics. Instr Course Lect. 2013;62:165-179.
13. Fontana L, Neel S, Claise JM, et al. Osteoarthritis of the thumb carpometacarpal joint in women and occupational risk factors: a case-control study. J Hand Surg Am. 2007;32:459-465.
14. Stamm TA, Machold KP, Smolen JS, et al. Joint protection and home hand exercises improve hand function in patients with hand osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2002;47:44-49.
15. Weiss S, LaStayo P, Mills A, et al. Prospective analysis of splinting the first carpometacarpal joint: an objective, subjective, and radiographic assessment. J Hand Ther. 2000;13:218-226.
16. Day CS, Gelberman R, Patel AA, et al. Basal joint osteoarthritis of the thumb: a prospective trial of steroid injection and splinting. J Hand Surg Am. 2004;29:247-251.
17. Gelfman R, Melton LJ 3rd, Yawn BP, et al. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33-41.
18. Wipperman J, Potter L. Carpal tunnel syndrome-try these diagnostic maneuvers. J Fam Pract. 2012;61:726-732.
19. Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981-1004.
20. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43:431-437.
21. Soltani AM, Allan BJ, Best MJ, et al. A systematic review of the literature on the outcomes of treatment for recurrent and persistent carpal tunnel syndrome. Plast Reconstr Surg. 2013;132:114-121.
22. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
23. Raji P, Ansari NN, Naghdi S, et al. Relationship between Semmes-Weinstein Monofilaments perception test and sensory nerve conduction studies in carpal tunnel syndrome. NeuroRehabilitation. 2014;35:543-552.
24. Leinberry CF, Rivlin M, Maltenfort M, et al. Treatment of carpal tunnel syndrome by members of the American Society for Surgery of the Hand: a 25-year perspective. J Hand Surg Am. 2012;37:1997-2003.e3.
25. Ustün N, Tok F, Yagz AE, et al. Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: a single-blind randomized prospective study. Am J Phys Med Rehabil. 2013;92:999-1004.
26. Michalsen A, Bock S, Lüdtke R, et al. Effects of traditional cupping therapy in patients with carpal tunnel syndrome: a randomized controlled trial. J Pain. 2009;10:601-608.
27. Seok H, Kim SH. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil. 2013;92:327-334.
28. Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024.
29. Shehab R, Mirabelli MH. Evaluation and diagnosis of wrist pain: a case-based approach. Am Fam Physician. 2013;87:568-573.
30. Clarke MT, Lyall HA, Grant JW, et al. The histopathology of de Quervain’s disease. J Hand Surg Br. 1998;23:732-734.
31. Zychowicz MA. A closer look at hand and wrist complaints. Nurse Pract. 2013;38:46-53.
32. Avci S, Yilmaz C, Sayli U. Comparison of nonsurgical treatment measures for de Quervain’s disease of pregnancy and lactation. J Hand Surg Am. 2002;27:322-324.
33. Mani L, Gerr F. Work-related upper extremity musculoskeletal disorders. Prim Care. 2000;27:845-864.
34. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
35. Peters-Veluthamaningal C, Van der Windt JC, Winters JC, et al. Corticosteroid injection for de Quervain’s tenosynovitis. Cochrane Database Syst Rev. 2009;8:CD005616.
36. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
37. Pagonis T, Ditsios K, Toli P, et al. Improved corticosteroid treatment of recalcitrant de Quervain tenosynovitis with a novel 4-point injection technique. Am J Sports Med. 2011;39:398-403.
38. Scheller A, Schuh R, Hönle W, et al. Long-term results of surgical release of de Quervain’s stenosing tenosynovitis. Int Orthop. 2009;33:1301-1303.
39. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832-839.
40. Regan W, Wold LE, Coonrad R, et al. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992;20:746-749.
41. Van Hofwegen C, Baker CL 3rd, Baker CL Jr. Epicondylitis in the athlete’s elbow. Clin Sports Med. 2010;29:577-597.
42. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6:259-272.
43. Weerakul S, Galassi M. Randomized controlled trial local injection for treatment of lateral epicondylitis, 5 and 10 mg triamcinolone compared. J Med Assoc Thai. 2012;95 Supp 10:S184-188.
Primary care physicians are frequently the first to evaluate hand, wrist, and forearm pain in patients, making knowledge of the symptoms, causes, and treatment of common diagnoses in the upper extremities imperative. Primary symptoms usually include pain and/or swelling. While most tendon disorders originating in the hand and wrist are idiopathic in nature, some patients occasionally report having recently performed unusual manual activity or having experienced trauma to the area days or weeks prior. A significant portion of patients are injured as a result of chronic repetitive activities at work.1

Most diagnoses can be made by pairing your knowledge of hand and forearm anatomy with an understanding of which tender points are indicative of which common conditions. (Care, of course, must be taken to ensure that there is no underlying infection.) Common conditions can often be treated nonsurgically with conservative treatments such as physical therapy, bracing/splinting, nonsteroidal anti-inflammatory drugs (NSAIDs), and injections of corticosteroids (eg, betamethasone, hydrocortisone, methylprednisolone, and triamcinolone) (TABLE 12-4) with or without the use of ultrasound. The benefits of corticosteroid injections for these conditions are well studied and documented in the literature, although physicians should always warn patients of the possible adverse effects prior to injection3,5 (TABLE 24).

To help you refine your skills, we review some of the more common hand and forearm conditions you are likely to encounter in the office and provide photos that reveal underlying anatomy so that you can administer injections without, in many cases, the need for ultrasound.
Trigger finger/thumb: New pathophysiologic findings?
Trigger finger most commonly occurs in the dominant hand. It is also more common in women, patients in their 50s, and in individuals with diabetes.6 Trigger finger/thumb is caused by inflammation and constriction of the flexor tendon sheath, which carries the flexor tendons through the palm and into the fingers and thumb. This, in turn, causes irritation of the tendons, sometimes via the formation of tendinous nodules, which may impinge upon the sheath’s “pulley system.”

When the “pulley” is compromised. The retinacular sheath is composed of 5 annular ligaments, or pulleys, that hold the tendons of the fingers close to the bone and allow the fingers to flex properly. The A1 pulley, at the level of the metacarpal head, is the first part of the sheath and is subject to the highest force; high forces may subsequently lead to the finger becoming locked in a flexed, or trigger, position.6 Patients may experience pain in the distal palm at the level of the A1 pulley and clicking of the finger.6
Additionally . . . recent studies show discrete histologic changes in trigger finger tendons, similar to findings with Achilles tendinosis and tendinopathy.7 In trigger finger tendons, collagen type 1A1 and 3A1, aggrecan, and biglycan are up-regulated, while metalloproteinase inhibitor 3 (TIMP-3) and matrix metallopeptidase3 (MMP-3) are down-regulated, a situation also described in Achilles tendinosis.7 This similarity in conditions provides new insight into the pathophysiology of the condition and may help provide future treatments.
Making the Dx: Look for swelling, check for carpal tunnel
During the examination, first look at both hands for swelling, arthropathy, or injury, and note the presence of any joint contractures. Next, examine all of the digits in flexion and extension while noting which ones are triggering, as the problem can occur in multiple digits on one hand. Then palpate the palms over the patient’s metacarpal heads, feeling for tender nodules.
Finally, examine the patient for carpal tunnel syndrome (CTS). A positive Tinel’s sign (shooting pain into the hand when the median nerve in the wrist is percussed), a positive Phalen maneuver (numbness or pain, usually within one minute of full wrist flexion), or thenar muscle wasting are highly indicative of CTS (compression of the median nerve at the transverse carpal ligament in the carpal tunnel). It is important to check for CTS when examining a patient for trigger finger because the 2 conditions frequently co-occur.6 (For more on CTS, see here.)
Treatment: Consider corticosteroids first
First-line treatment for patients with trigger finger or thumb is a corticosteroid injection into the subcutaneous tissue around the tendon sheath (FIGURES 1 and 2). (For this indication and for the others discussed throughout the article, there isn’t tremendous evidence for one particular type of corticosteroid over another; see TABLE 12-4 for choices.) Up to 57% of cases resolve with one injection, and 86% resolve with 2,8 but keep in mind that it may take up to 2 weeks to achieve the full clinical benefit.

Patients with multiple trigger fingers can be treated with oral corticosteroids (eg, a methylprednisolone dose pack). Peters-Veluthamaningal et al performed a systematic review in 2009 and found 2 randomized controlled trials involving 63 patients (34 received injections of a corticosteroid [either methylprednisolone or betamethasone] and lidocaine and 29 received lidocaine only).2 The corticosteroid/lidocaine combination was more effective at 4 weeks (relative risk [RR]=3.15; 95% confidence interval [CI], 1.34 to 7.40).2
If 2 corticosteroid injections 6 weeks apart fail to provide benefit, or the finger is irreversibly locked in flexion, surgical release of the pulley is required and is performed through a palmar incision at the level of the A1 pulley. Complications from this surgery, including nerve damage, are exceedingly rare, but injury can occur, given the proximity of the digital nerves to the A1 pulley.
Patient is a child? Refer children with trigger finger or thumb to a hand surgeon for evaluation and management because the indications for nonoperative treatment in the pediatric population are unclear.9
Carpometacarpal arthritis: Common, with many causes
Osteoarthritis of the first carpometacarpal (CMC) joint is the most common site of arthritis in the hand/wrist region, affecting up to 11% of men and 33% of women in their 50s and 60s.10 Because the CMC joint lacks a bony restraint, it relies on a number of ligaments for stability—the strongest and most important of which is the palmar oblique “beak” ligament.11 A major cause of degenerative arthritis of this joint is attenuation and laxity of these ligaments, leading to abnormal and increased stress loads, which, in turn, can lead to loss of cartilage and bony impingement. While the exact mechanism of this process is not fully understood,10,12 acute or chronic trauma, advanced age, hormonal factors, and genetic factors seem to play a role.11
Many believe there is a relationship between a patient's occupation and the development of CMC arthritis, but studies are inconclusive.13 At risk are secretarial workers, tailors, domestic helpers/cleaners, and individuals whose jobs involve repetitive thumb use and/or insufficient rest of the joint throughout the day.
Making the Dx: Perform the Grind test
A detailed patient history (which is usually void of trauma to the hand) and physical examination are the keys to making the diagnosis of CMC arthritis. A history of pain at the base of the thumb during pinching and gripping tasks is often elucidated. Classically, patients describe pain upon turning keys, opening jars, and gripping doorknobs.11
It's important to focus on the dorsoradial aspect of the thumb during the physical exam and to rule out other causes of pain, such as de Quervain’s tenosynovitis, flexor carpi radialis tendinitis, CTS, and trigger thumb.11 Typical findings include pain with palpation directly over the dorsoradial aspect of the CMC joint and pain with axial loading and upon circumduction during a Grind test of the CMC joint. (The Grind test is performed by moving the metacarpal bone of the thumb in a circle and loading it with gentle axial forces. People with thumb joint arthritis generally experience sudden sharp pain at the CMC joint.)
Radiographic findings can be useful as a diagnostic adjunct, with staging of the disease, and in determining who can benefit from conservative management.11
Treatment: Start with NSAIDs and splinting
Depending on the degree of arthritis, management may include both conservative and surgical options.10 Patient education describing activity modification is useful during all stages of CMC arthritis. Research has shown that avoiding inciting activities, such as key turning, pinching, and grasping, helps to alleviate symptoms.14 Patients may also obtain relief from NSAIDs, especially when they are used in conjunction with activity modification and splinting. NSAIDs, however, do not halt or reverse the disease process; they only reduce inflammation, synovitis, and pain.11
Splinting. Studies have shown splinting of the thumb CMC joint to provide pain relief and to potentially slow disease progression.15 Because splints decrease motion and increase joint stability, they are especially useful for patients with joint hypermobility. The long opponens thumb spica splint is commonly used; it immobilizes the wrist and CMC, while leaving the thumb interphalangeal joint free. Short thumb spica and neoprene splints are also commercially available, and studies have shown that they provide good results.15 Splinting is most beneficial in patients with early-stage disease and may be used for either short-term flares or long-term treatment.11
Cortisone injections. For those patients who do not respond to activity modification, NSAIDs, and/or splinting, consider cortisone injections (FIGURE 3). Intra-articular cortisone injections can decrease inflammation and provide good pain relief, especially in patients with early-stage disease. The effectiveness of cortisone injections in patients with more advanced disease is not clear; no benefit has been shown in studies to date.16 Equally unclear is the long-term benefit of injections.11 Patients who do not respond to conservative treatments will often require surgical care.

Carpal tunnel syndrome: Moving slower to surgery
CTS is one of the most common conditions of the upper extremities. Researchers estimate that 491 women per 100,000 person-years and 258 men per 100,000 person-years will develop CTS, with 109 per 100,000 person-years receiving carpal tunnel release surgery.17 Risk factors for the development of CTS include diabetes, hypothyroidism, rheumatoid arthritis, pregnancy, obesity, family history, trauma, and occupations that involve repetitive tasks or long hours working at a computer.18
CTS is caused by compression of the median nerve as it passes through the carpal tunnel.19 The elevated pressure in the carpal tunnel restricts epineural blood flow and supply, causing the pain felt with CTS.20 Even after surgical decompression, recurrent or persistent CTS can be a problem.21
Making the Dx: Perform the Phalen maneuver, Durkan’s test
Patients typically present with complaints of weakness, pain, and/or numbness in at least 2 of 4 radial digits (thumb, index, middle, ring).19,22 The most common time of day for patients to have symptoms is at night.21
The diagnostic tools. Tinel’s sign is a useful diagnostic tool when you suspect carpal tunnel syndrome. Tinel’s sign is positive if percussion over the median nerve at the carpal tunnel elicits pain or paresthesia.18
When employing the Phalen maneuver, be certain to have the patient flex his/her wrist to 90 degrees and to document the number of seconds it takes for numbness to present in the fingers. Pain or paresthesia should occur in <60 seconds for the test to be positive.18
Median nerve compression over the carpal tunnel, also known as Durkan’s test, may also elicit symptoms. With Durkan’s test, you apply direct pressure over the transverse carpal ligament. If pain or paresthesia occurs in <30 seconds, the test is positive.18 Often clinicians will combine the Phalen maneuver and Durkan’s test to increase sensitivity and specificity.18 Nerve conduction studies are often performed to confirm the clinical diagnosis.
Is more than one condition at play? It is important to determine whether cervical spine disease and/or peripheral neuropathy is contributing to the patient’s symptoms, along with CTS; patients may have more than one condition contributing to their pain. We routinely check cervical spine motion, tenderness, and nerve compression as part of the exam on a patient with suspected CTS. In the office, a monofilament test or 2-point discrimination test can help make the clinical diagnosis by uncovering decreased sensation in the thumb, index, and/or middle fingers.23
The 5.07 monofilament test is performed with the clinician applying the monofilament to different dermatomal or sensory distributions while the patient has his/her eyes closed. The 2-point discrimination test is performed with a caliper device that measures the distance at which the patient can feel 2 separate stimuli. Often electromyography or nerve conduction studies are necessary.18
Treatment: Pursue nonoperative approaches
A survey of the membership of the American Society for Surgery of the Hand revealed that surgeons are utilizing nonoperative treatments for a longer duration of time and are employing narrowed surgical indications.24 Thus, clinicians are more likely to try splints and steroid injections before proceeding to operative release.24
Nonsurgical management. In our practice, we commonly recommend corticosteroid injections (TABLE 12-4) into the carpal tunnel (FIGURES 4 and 5) to patients who are poor candidates for surgery (ie, those who have too many medical comorbidities or wound healing concerns). This is one indication for which you may want to consider ultrasound-guided injections because the improved accuracy may provide symptom relief faster than “blind” or palpation-guided injections.25

A recent randomized controlled trial from Sweden showed that injections of methylprednisolone relieved symptoms in patients with mild to moderate CTS at 10 weeks and reduced the rate of surgery one year after treatment; however, 3 out of 4 patients still went on to have surgery within a year.22 Patients in the study had failed a 2-month trial of splinting and were given either 80 mg or 40 mg of methylprednisolone or saline. There was no statistical difference between the doses of methylprednisolone in preventing surgery at one year. Compared to placebo, the 80-mg methylprednisolone group was less likely to have surgery with an odds ratio of 0.24 (P=.042).22
There is evidence that oral steroids, injected steroids, ultrasound, electromagnetic field therapy, nocturnal splinting, and use of ergonomic keyboards are effective nonoperative modalities in the short term, but evidence is sparse for mid- or long-term use.19 In addition, at least one randomized trial found traditional cupping therapy applied around the shoulder alleviated carpal tunnel symptoms in the short-term.26 Other nonoperative therapies include rest, NSAIDs, extracorporeal shock wave therapy, and activity modification.19,27
Surgical outcomes by either endoscopic, mini-open, or open surgical techniques are typically good.20,21 Surgical release involves cutting the transverse carpal ligament over the carpal tunnel to decompress the median nerve.24 You should inform patients of the risks and inconveniences associated with surgery, including the cost, absence from work, infection, and chronic pain. Patients who have recurrent or persistent symptoms after surgery may have had an incompletely released transverse carpal ligament or there may be no identifiable cause.21 Overall, surgical treatment, combined with physical therapy, seems to be more effective than splinting or NSAIDs for mid- and long-term treatment of CTS.28
De Quervain’s tenosynovitis: Common during pregnancy
De Quervain’s tenosynovitis (radial styloid tenosynovitis) involves painful inflammation of the 2 tendons in the first dorsal compartment of the wrist—the abductor pollicis longus (APL) and the extensor pollicis brevis (EPB). The tendons comprise the radial border of the anatomic snuffbox.
The APL abducts and extends the thumb at the CMC joint, while the EPB extends the thumb proximal phalanx at the metacarpophalangeal joint. These tendons are contained in a synovial sheath that is subject to inflammation and constriction and subsequent wear and damage.29 In addition, the extensor retinaculum in patients with de Quervain’s disease demonstrates increased vascularity and deposition of dense fibrous tissue resulting in thickening of the tendon up to 5 times its normal width.30
As a result, degeneration and thickening of the tendon sheath, as well as radial-sided wrist pain elicited at the first dorsal compartment, are common pathophysiologic and clinical findings.31 Pain is often accompanied by the build-up of protuberances and nodulations of the tendon sheath.
De Quervain’s disease commonly occurs during and after pregnancy.32 Other risk factors include racquet sports, golfing, wrist trauma, and other activities involving repetitive hand and wrist motions.33 Often, however, de Quervain’s is idiopathic.
Making the Dx: Perform a Finkelstein's test
The major finding in patients with de Quervain’s tenosynovitis is a positive Finkelstein's test. To perform Finkelstein's test (FIGURE 6), ask the patient to oppose the thumb into the palm and flex the fingers of the same hand over the thumb. Holding the patient’s fingers around the thumb, ulnarly deviate the wrist. Finkelstein's test puts strain on the APL and EPB, causing pain along the radial border of the wrist and forearm in patients with de Quervain’s tenosynovitis. Since the maneuver can be uncomfortable, complete the exam on the unaffected side for comparison.

Stenosis of the tendon sheath may lead to crepitus over the first dorsal wrist compartment. This should be distinguished from intersection syndrome (tenosynovitis at the intersection of the first and second extensor compartments), which can also present with forearm and wrist crepitus. Patients usually have swelling of the wrist with marked discomfort upon palpation of the radial tendons. An x-ray can be useful to evaluate for CMC or radiocarpal arthritis, which may be an underlying cause.
Treatment: Select an approach based on symptom severity
In a retrospective analysis, Lane et al concluded that classification of patients with de Quervain’s disease based on pretreatment symptoms may assist physicians in selecting the most efficacious treatment and in providing prognostic information to their patients (TABLE 334). Patients with mild to moderate (Types 1 and 2) de Quervain’s may benefit from immobilization in a thumb spica splint, rest, NSAIDs, and physical or occupational therapy. If work conditions played a role in causing the symptoms, they need to be addressed to improve outcomes. Types 2 and 3 can be initially treated with a corticosteroid injection, but may eventually require surgery.33

Treatment with NSAIDs or corticosteroid injections (see TABLE 12-4 for choices) in the first compartment of the extensor retinaculum (FIGURE 7) is usually adequate to provide relief. Peters-Veluthamaningal et al performed a systematic review in 2009 and found only one controlled trial of 18 participants (all pregnant or lactating women) who were either injected with corticosteroids or given a thumb spica splint.35 All 9 patients in the injection group had complete pain relief, whereas no one in the splint group had complete resolution of symptoms.35 Typical anatomic placement of corticosteroid injections is shown in FIGURE 7.

More complicated injection methods have been described, but injecting the first dorsal compartment is usually satisfactory. Patients will feel the tendon sheath filling with the injection material. The 2-point technique, implemented by Sawaizumi et al, which involves injecting corticosteroid into 2 points over the EPB and APL tendon in the area of maximum pain and soft tissue thickening, is more effective than the 1-point injection technique.36
Severe, recalcitrant cases. Professional and college athletes may be prone to recalcitrant de Quervain’s tenosynovitis. A 2010 study by Pagonis et al showed that recurrent symptomatic episodes commonly occur in athletes who engage in high-resistance, intense athletic training. In these severe cases, a 4-point injection technique offers better distribution of corticosteroid solution to the first extensor compartment than other methods.37 Consider referring severe cases to a hand surgeon.
Surgical release of the first dorsal compartmental sheath around the tendons serves as a final option for patients who fail conservative treatment. Care should be taken to release both tendons completely, as there may be at least 2 tendon slips of the APL or there may be a distinct EPB sheath dorsally.38
“Tennis elbow”— you don’t have to play tennis to have it
Lateral epicondylitis (tennis elbow) is a painful condition involving microtears within the extensor carpi radialis brevis muscle and the subsequent development of angiofibroblastic dysplasia.39 According to Regan et al who studied the histopathologic features of 11 patients with lateral epicondylitis, the underlying cause of recalcitrant lateral epicondylitis is, in fact, degenerative, rather than inflammatory.40
Although the condition has been nicknamed “tennis elbow,” only about 5% of tennis players have the condition.41 In tennis players, males are more often affected than females, whereas in the general population, incidence is approximately equal in men and women.41 Lateral epicondylitis occurs between 4 and 7 times more frequently than medial-sided elbow pain.42
Making the Dx: Look for localized pain, normal ROM
The diagnosis of lateral epicondylitis is based upon a history of pain over the lateral epicondyle and findings on physical examination, including local tenderness directly over the lateral epicondyle,43 pain aggravated by resisted wrist extension and radial deviation, pain with resisted middle finger extension, and decreased grip strength or pain aggravated by strong gripping. These findings typically occur in the presence of normal elbow range of motion.
Treatment: Choose from a range of options
Since lateral epicondylitis was first described, researchers have proposed a wide variety of treatments as initial interventions including rest, activity, equipment modification, NSAIDs, wrist bracing/elbow straps, and physical therapy. If initial treatment does not produce the desired effect, second-line treatments include corticosteroid injections (FIGURE 8), prolotherapy (injection of an irritant, often dextrose; see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768), autologous blood injections, platelet-rich plasma injections (see “Is platelet-rich plasma right for your patient?” J Fam Pract. 2016;65:319-328), and needling of the extensor tendon origin. Refer patients who do not improve after one corticosteroid injection to an orthopedic surgeon for consideration of open or arthroscopic treatment.

CORRESPONDENCE
Gregory R. Waryasz, MD, Rhode Island Hospital, Department of Orthopaedic Surgery, 593 Eddy St., Providence, RI 02903; [email protected].
Primary care physicians are frequently the first to evaluate hand, wrist, and forearm pain in patients, making knowledge of the symptoms, causes, and treatment of common diagnoses in the upper extremities imperative. Primary symptoms usually include pain and/or swelling. While most tendon disorders originating in the hand and wrist are idiopathic in nature, some patients occasionally report having recently performed unusual manual activity or having experienced trauma to the area days or weeks prior. A significant portion of patients are injured as a result of chronic repetitive activities at work.1

Most diagnoses can be made by pairing your knowledge of hand and forearm anatomy with an understanding of which tender points are indicative of which common conditions. (Care, of course, must be taken to ensure that there is no underlying infection.) Common conditions can often be treated nonsurgically with conservative treatments such as physical therapy, bracing/splinting, nonsteroidal anti-inflammatory drugs (NSAIDs), and injections of corticosteroids (eg, betamethasone, hydrocortisone, methylprednisolone, and triamcinolone) (TABLE 12-4) with or without the use of ultrasound. The benefits of corticosteroid injections for these conditions are well studied and documented in the literature, although physicians should always warn patients of the possible adverse effects prior to injection3,5 (TABLE 24).

To help you refine your skills, we review some of the more common hand and forearm conditions you are likely to encounter in the office and provide photos that reveal underlying anatomy so that you can administer injections without, in many cases, the need for ultrasound.
Trigger finger/thumb: New pathophysiologic findings?
Trigger finger most commonly occurs in the dominant hand. It is also more common in women, patients in their 50s, and in individuals with diabetes.6 Trigger finger/thumb is caused by inflammation and constriction of the flexor tendon sheath, which carries the flexor tendons through the palm and into the fingers and thumb. This, in turn, causes irritation of the tendons, sometimes via the formation of tendinous nodules, which may impinge upon the sheath’s “pulley system.”

When the “pulley” is compromised. The retinacular sheath is composed of 5 annular ligaments, or pulleys, that hold the tendons of the fingers close to the bone and allow the fingers to flex properly. The A1 pulley, at the level of the metacarpal head, is the first part of the sheath and is subject to the highest force; high forces may subsequently lead to the finger becoming locked in a flexed, or trigger, position.6 Patients may experience pain in the distal palm at the level of the A1 pulley and clicking of the finger.6
Additionally . . . recent studies show discrete histologic changes in trigger finger tendons, similar to findings with Achilles tendinosis and tendinopathy.7 In trigger finger tendons, collagen type 1A1 and 3A1, aggrecan, and biglycan are up-regulated, while metalloproteinase inhibitor 3 (TIMP-3) and matrix metallopeptidase3 (MMP-3) are down-regulated, a situation also described in Achilles tendinosis.7 This similarity in conditions provides new insight into the pathophysiology of the condition and may help provide future treatments.
Making the Dx: Look for swelling, check for carpal tunnel
During the examination, first look at both hands for swelling, arthropathy, or injury, and note the presence of any joint contractures. Next, examine all of the digits in flexion and extension while noting which ones are triggering, as the problem can occur in multiple digits on one hand. Then palpate the palms over the patient’s metacarpal heads, feeling for tender nodules.
Finally, examine the patient for carpal tunnel syndrome (CTS). A positive Tinel’s sign (shooting pain into the hand when the median nerve in the wrist is percussed), a positive Phalen maneuver (numbness or pain, usually within one minute of full wrist flexion), or thenar muscle wasting are highly indicative of CTS (compression of the median nerve at the transverse carpal ligament in the carpal tunnel). It is important to check for CTS when examining a patient for trigger finger because the 2 conditions frequently co-occur.6 (For more on CTS, see here.)
Treatment: Consider corticosteroids first
First-line treatment for patients with trigger finger or thumb is a corticosteroid injection into the subcutaneous tissue around the tendon sheath (FIGURES 1 and 2). (For this indication and for the others discussed throughout the article, there isn’t tremendous evidence for one particular type of corticosteroid over another; see TABLE 12-4 for choices.) Up to 57% of cases resolve with one injection, and 86% resolve with 2,8 but keep in mind that it may take up to 2 weeks to achieve the full clinical benefit.

Patients with multiple trigger fingers can be treated with oral corticosteroids (eg, a methylprednisolone dose pack). Peters-Veluthamaningal et al performed a systematic review in 2009 and found 2 randomized controlled trials involving 63 patients (34 received injections of a corticosteroid [either methylprednisolone or betamethasone] and lidocaine and 29 received lidocaine only).2 The corticosteroid/lidocaine combination was more effective at 4 weeks (relative risk [RR]=3.15; 95% confidence interval [CI], 1.34 to 7.40).2
If 2 corticosteroid injections 6 weeks apart fail to provide benefit, or the finger is irreversibly locked in flexion, surgical release of the pulley is required and is performed through a palmar incision at the level of the A1 pulley. Complications from this surgery, including nerve damage, are exceedingly rare, but injury can occur, given the proximity of the digital nerves to the A1 pulley.
Patient is a child? Refer children with trigger finger or thumb to a hand surgeon for evaluation and management because the indications for nonoperative treatment in the pediatric population are unclear.9
Carpometacarpal arthritis: Common, with many causes
Osteoarthritis of the first carpometacarpal (CMC) joint is the most common site of arthritis in the hand/wrist region, affecting up to 11% of men and 33% of women in their 50s and 60s.10 Because the CMC joint lacks a bony restraint, it relies on a number of ligaments for stability—the strongest and most important of which is the palmar oblique “beak” ligament.11 A major cause of degenerative arthritis of this joint is attenuation and laxity of these ligaments, leading to abnormal and increased stress loads, which, in turn, can lead to loss of cartilage and bony impingement. While the exact mechanism of this process is not fully understood,10,12 acute or chronic trauma, advanced age, hormonal factors, and genetic factors seem to play a role.11
Many believe there is a relationship between a patient's occupation and the development of CMC arthritis, but studies are inconclusive.13 At risk are secretarial workers, tailors, domestic helpers/cleaners, and individuals whose jobs involve repetitive thumb use and/or insufficient rest of the joint throughout the day.
Making the Dx: Perform the Grind test
A detailed patient history (which is usually void of trauma to the hand) and physical examination are the keys to making the diagnosis of CMC arthritis. A history of pain at the base of the thumb during pinching and gripping tasks is often elucidated. Classically, patients describe pain upon turning keys, opening jars, and gripping doorknobs.11
It's important to focus on the dorsoradial aspect of the thumb during the physical exam and to rule out other causes of pain, such as de Quervain’s tenosynovitis, flexor carpi radialis tendinitis, CTS, and trigger thumb.11 Typical findings include pain with palpation directly over the dorsoradial aspect of the CMC joint and pain with axial loading and upon circumduction during a Grind test of the CMC joint. (The Grind test is performed by moving the metacarpal bone of the thumb in a circle and loading it with gentle axial forces. People with thumb joint arthritis generally experience sudden sharp pain at the CMC joint.)
Radiographic findings can be useful as a diagnostic adjunct, with staging of the disease, and in determining who can benefit from conservative management.11
Treatment: Start with NSAIDs and splinting
Depending on the degree of arthritis, management may include both conservative and surgical options.10 Patient education describing activity modification is useful during all stages of CMC arthritis. Research has shown that avoiding inciting activities, such as key turning, pinching, and grasping, helps to alleviate symptoms.14 Patients may also obtain relief from NSAIDs, especially when they are used in conjunction with activity modification and splinting. NSAIDs, however, do not halt or reverse the disease process; they only reduce inflammation, synovitis, and pain.11
Splinting. Studies have shown splinting of the thumb CMC joint to provide pain relief and to potentially slow disease progression.15 Because splints decrease motion and increase joint stability, they are especially useful for patients with joint hypermobility. The long opponens thumb spica splint is commonly used; it immobilizes the wrist and CMC, while leaving the thumb interphalangeal joint free. Short thumb spica and neoprene splints are also commercially available, and studies have shown that they provide good results.15 Splinting is most beneficial in patients with early-stage disease and may be used for either short-term flares or long-term treatment.11
Cortisone injections. For those patients who do not respond to activity modification, NSAIDs, and/or splinting, consider cortisone injections (FIGURE 3). Intra-articular cortisone injections can decrease inflammation and provide good pain relief, especially in patients with early-stage disease. The effectiveness of cortisone injections in patients with more advanced disease is not clear; no benefit has been shown in studies to date.16 Equally unclear is the long-term benefit of injections.11 Patients who do not respond to conservative treatments will often require surgical care.

Carpal tunnel syndrome: Moving slower to surgery
CTS is one of the most common conditions of the upper extremities. Researchers estimate that 491 women per 100,000 person-years and 258 men per 100,000 person-years will develop CTS, with 109 per 100,000 person-years receiving carpal tunnel release surgery.17 Risk factors for the development of CTS include diabetes, hypothyroidism, rheumatoid arthritis, pregnancy, obesity, family history, trauma, and occupations that involve repetitive tasks or long hours working at a computer.18
CTS is caused by compression of the median nerve as it passes through the carpal tunnel.19 The elevated pressure in the carpal tunnel restricts epineural blood flow and supply, causing the pain felt with CTS.20 Even after surgical decompression, recurrent or persistent CTS can be a problem.21
Making the Dx: Perform the Phalen maneuver, Durkan’s test
Patients typically present with complaints of weakness, pain, and/or numbness in at least 2 of 4 radial digits (thumb, index, middle, ring).19,22 The most common time of day for patients to have symptoms is at night.21
The diagnostic tools. Tinel’s sign is a useful diagnostic tool when you suspect carpal tunnel syndrome. Tinel’s sign is positive if percussion over the median nerve at the carpal tunnel elicits pain or paresthesia.18
When employing the Phalen maneuver, be certain to have the patient flex his/her wrist to 90 degrees and to document the number of seconds it takes for numbness to present in the fingers. Pain or paresthesia should occur in <60 seconds for the test to be positive.18
Median nerve compression over the carpal tunnel, also known as Durkan’s test, may also elicit symptoms. With Durkan’s test, you apply direct pressure over the transverse carpal ligament. If pain or paresthesia occurs in <30 seconds, the test is positive.18 Often clinicians will combine the Phalen maneuver and Durkan’s test to increase sensitivity and specificity.18 Nerve conduction studies are often performed to confirm the clinical diagnosis.
Is more than one condition at play? It is important to determine whether cervical spine disease and/or peripheral neuropathy is contributing to the patient’s symptoms, along with CTS; patients may have more than one condition contributing to their pain. We routinely check cervical spine motion, tenderness, and nerve compression as part of the exam on a patient with suspected CTS. In the office, a monofilament test or 2-point discrimination test can help make the clinical diagnosis by uncovering decreased sensation in the thumb, index, and/or middle fingers.23
The 5.07 monofilament test is performed with the clinician applying the monofilament to different dermatomal or sensory distributions while the patient has his/her eyes closed. The 2-point discrimination test is performed with a caliper device that measures the distance at which the patient can feel 2 separate stimuli. Often electromyography or nerve conduction studies are necessary.18
Treatment: Pursue nonoperative approaches
A survey of the membership of the American Society for Surgery of the Hand revealed that surgeons are utilizing nonoperative treatments for a longer duration of time and are employing narrowed surgical indications.24 Thus, clinicians are more likely to try splints and steroid injections before proceeding to operative release.24
Nonsurgical management. In our practice, we commonly recommend corticosteroid injections (TABLE 12-4) into the carpal tunnel (FIGURES 4 and 5) to patients who are poor candidates for surgery (ie, those who have too many medical comorbidities or wound healing concerns). This is one indication for which you may want to consider ultrasound-guided injections because the improved accuracy may provide symptom relief faster than “blind” or palpation-guided injections.25

A recent randomized controlled trial from Sweden showed that injections of methylprednisolone relieved symptoms in patients with mild to moderate CTS at 10 weeks and reduced the rate of surgery one year after treatment; however, 3 out of 4 patients still went on to have surgery within a year.22 Patients in the study had failed a 2-month trial of splinting and were given either 80 mg or 40 mg of methylprednisolone or saline. There was no statistical difference between the doses of methylprednisolone in preventing surgery at one year. Compared to placebo, the 80-mg methylprednisolone group was less likely to have surgery with an odds ratio of 0.24 (P=.042).22
There is evidence that oral steroids, injected steroids, ultrasound, electromagnetic field therapy, nocturnal splinting, and use of ergonomic keyboards are effective nonoperative modalities in the short term, but evidence is sparse for mid- or long-term use.19 In addition, at least one randomized trial found traditional cupping therapy applied around the shoulder alleviated carpal tunnel symptoms in the short-term.26 Other nonoperative therapies include rest, NSAIDs, extracorporeal shock wave therapy, and activity modification.19,27
Surgical outcomes by either endoscopic, mini-open, or open surgical techniques are typically good.20,21 Surgical release involves cutting the transverse carpal ligament over the carpal tunnel to decompress the median nerve.24 You should inform patients of the risks and inconveniences associated with surgery, including the cost, absence from work, infection, and chronic pain. Patients who have recurrent or persistent symptoms after surgery may have had an incompletely released transverse carpal ligament or there may be no identifiable cause.21 Overall, surgical treatment, combined with physical therapy, seems to be more effective than splinting or NSAIDs for mid- and long-term treatment of CTS.28
De Quervain’s tenosynovitis: Common during pregnancy
De Quervain’s tenosynovitis (radial styloid tenosynovitis) involves painful inflammation of the 2 tendons in the first dorsal compartment of the wrist—the abductor pollicis longus (APL) and the extensor pollicis brevis (EPB). The tendons comprise the radial border of the anatomic snuffbox.
The APL abducts and extends the thumb at the CMC joint, while the EPB extends the thumb proximal phalanx at the metacarpophalangeal joint. These tendons are contained in a synovial sheath that is subject to inflammation and constriction and subsequent wear and damage.29 In addition, the extensor retinaculum in patients with de Quervain’s disease demonstrates increased vascularity and deposition of dense fibrous tissue resulting in thickening of the tendon up to 5 times its normal width.30
As a result, degeneration and thickening of the tendon sheath, as well as radial-sided wrist pain elicited at the first dorsal compartment, are common pathophysiologic and clinical findings.31 Pain is often accompanied by the build-up of protuberances and nodulations of the tendon sheath.
De Quervain’s disease commonly occurs during and after pregnancy.32 Other risk factors include racquet sports, golfing, wrist trauma, and other activities involving repetitive hand and wrist motions.33 Often, however, de Quervain’s is idiopathic.
Making the Dx: Perform a Finkelstein's test
The major finding in patients with de Quervain’s tenosynovitis is a positive Finkelstein's test. To perform Finkelstein's test (FIGURE 6), ask the patient to oppose the thumb into the palm and flex the fingers of the same hand over the thumb. Holding the patient’s fingers around the thumb, ulnarly deviate the wrist. Finkelstein's test puts strain on the APL and EPB, causing pain along the radial border of the wrist and forearm in patients with de Quervain’s tenosynovitis. Since the maneuver can be uncomfortable, complete the exam on the unaffected side for comparison.

Stenosis of the tendon sheath may lead to crepitus over the first dorsal wrist compartment. This should be distinguished from intersection syndrome (tenosynovitis at the intersection of the first and second extensor compartments), which can also present with forearm and wrist crepitus. Patients usually have swelling of the wrist with marked discomfort upon palpation of the radial tendons. An x-ray can be useful to evaluate for CMC or radiocarpal arthritis, which may be an underlying cause.
Treatment: Select an approach based on symptom severity
In a retrospective analysis, Lane et al concluded that classification of patients with de Quervain’s disease based on pretreatment symptoms may assist physicians in selecting the most efficacious treatment and in providing prognostic information to their patients (TABLE 334). Patients with mild to moderate (Types 1 and 2) de Quervain’s may benefit from immobilization in a thumb spica splint, rest, NSAIDs, and physical or occupational therapy. If work conditions played a role in causing the symptoms, they need to be addressed to improve outcomes. Types 2 and 3 can be initially treated with a corticosteroid injection, but may eventually require surgery.33

Treatment with NSAIDs or corticosteroid injections (see TABLE 12-4 for choices) in the first compartment of the extensor retinaculum (FIGURE 7) is usually adequate to provide relief. Peters-Veluthamaningal et al performed a systematic review in 2009 and found only one controlled trial of 18 participants (all pregnant or lactating women) who were either injected with corticosteroids or given a thumb spica splint.35 All 9 patients in the injection group had complete pain relief, whereas no one in the splint group had complete resolution of symptoms.35 Typical anatomic placement of corticosteroid injections is shown in FIGURE 7.

More complicated injection methods have been described, but injecting the first dorsal compartment is usually satisfactory. Patients will feel the tendon sheath filling with the injection material. The 2-point technique, implemented by Sawaizumi et al, which involves injecting corticosteroid into 2 points over the EPB and APL tendon in the area of maximum pain and soft tissue thickening, is more effective than the 1-point injection technique.36
Severe, recalcitrant cases. Professional and college athletes may be prone to recalcitrant de Quervain’s tenosynovitis. A 2010 study by Pagonis et al showed that recurrent symptomatic episodes commonly occur in athletes who engage in high-resistance, intense athletic training. In these severe cases, a 4-point injection technique offers better distribution of corticosteroid solution to the first extensor compartment than other methods.37 Consider referring severe cases to a hand surgeon.
Surgical release of the first dorsal compartmental sheath around the tendons serves as a final option for patients who fail conservative treatment. Care should be taken to release both tendons completely, as there may be at least 2 tendon slips of the APL or there may be a distinct EPB sheath dorsally.38
“Tennis elbow”— you don’t have to play tennis to have it
Lateral epicondylitis (tennis elbow) is a painful condition involving microtears within the extensor carpi radialis brevis muscle and the subsequent development of angiofibroblastic dysplasia.39 According to Regan et al who studied the histopathologic features of 11 patients with lateral epicondylitis, the underlying cause of recalcitrant lateral epicondylitis is, in fact, degenerative, rather than inflammatory.40
Although the condition has been nicknamed “tennis elbow,” only about 5% of tennis players have the condition.41 In tennis players, males are more often affected than females, whereas in the general population, incidence is approximately equal in men and women.41 Lateral epicondylitis occurs between 4 and 7 times more frequently than medial-sided elbow pain.42
Making the Dx: Look for localized pain, normal ROM
The diagnosis of lateral epicondylitis is based upon a history of pain over the lateral epicondyle and findings on physical examination, including local tenderness directly over the lateral epicondyle,43 pain aggravated by resisted wrist extension and radial deviation, pain with resisted middle finger extension, and decreased grip strength or pain aggravated by strong gripping. These findings typically occur in the presence of normal elbow range of motion.
Treatment: Choose from a range of options
Since lateral epicondylitis was first described, researchers have proposed a wide variety of treatments as initial interventions including rest, activity, equipment modification, NSAIDs, wrist bracing/elbow straps, and physical therapy. If initial treatment does not produce the desired effect, second-line treatments include corticosteroid injections (FIGURE 8), prolotherapy (injection of an irritant, often dextrose; see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768), autologous blood injections, platelet-rich plasma injections (see “Is platelet-rich plasma right for your patient?” J Fam Pract. 2016;65:319-328), and needling of the extensor tendon origin. Refer patients who do not improve after one corticosteroid injection to an orthopedic surgeon for consideration of open or arthroscopic treatment.

CORRESPONDENCE
Gregory R. Waryasz, MD, Rhode Island Hospital, Department of Orthopaedic Surgery, 593 Eddy St., Providence, RI 02903; [email protected].
1. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775.
2. Peters-Veluthamaningal C, Van der Windt DA, Winters JC, et al. Corticosteroid injection for trigger finger in adults. Cochrane Database Syst Rev. 2009:CD005617.
3. Cheng J, Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11:141-147.
4. Waryasz GR, Tambone R, Borenstein TR, et al. A review of anatomical placement of corticosteroid injections for uncommon hand, wrist, and elbow pathologies. R I Med J. 2017;100:31-34.
5. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1:396-404.
6. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743.
7. Lundin AC, Aspenberg P, Eliasson P. Trigger finger, tendinosis, and intratendinous gene expression. Scand J Med Sci Sports. 2014;24:363-368.
8. Sato ES, Gomes Dos Santos JB, Belloti JC, et al. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology (Oxford). 2012;51:93-99.
9. Baek GH, Kim JH, Chug MS, et al. The natural history of pediatric trigger thumb. J Bone Joint Surg Am. 2008;90:980-985.
10. Gillis J, Calder K, Williams J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can J Plast Surg. 2011;19:134-138.
11. Yao J, Park MJ. Early treatment of degenerative arthritis of the thumb carpometacarpal joint. Hand Clin. 2008;24:251-261.
12. Ladd AL, Weiss AP, Crisco JJ, et al. The thumb carpometacarpal joint: anatomy, hormones, and biomechanics. Instr Course Lect. 2013;62:165-179.
13. Fontana L, Neel S, Claise JM, et al. Osteoarthritis of the thumb carpometacarpal joint in women and occupational risk factors: a case-control study. J Hand Surg Am. 2007;32:459-465.
14. Stamm TA, Machold KP, Smolen JS, et al. Joint protection and home hand exercises improve hand function in patients with hand osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2002;47:44-49.
15. Weiss S, LaStayo P, Mills A, et al. Prospective analysis of splinting the first carpometacarpal joint: an objective, subjective, and radiographic assessment. J Hand Ther. 2000;13:218-226.
16. Day CS, Gelberman R, Patel AA, et al. Basal joint osteoarthritis of the thumb: a prospective trial of steroid injection and splinting. J Hand Surg Am. 2004;29:247-251.
17. Gelfman R, Melton LJ 3rd, Yawn BP, et al. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33-41.
18. Wipperman J, Potter L. Carpal tunnel syndrome-try these diagnostic maneuvers. J Fam Pract. 2012;61:726-732.
19. Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981-1004.
20. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43:431-437.
21. Soltani AM, Allan BJ, Best MJ, et al. A systematic review of the literature on the outcomes of treatment for recurrent and persistent carpal tunnel syndrome. Plast Reconstr Surg. 2013;132:114-121.
22. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
23. Raji P, Ansari NN, Naghdi S, et al. Relationship between Semmes-Weinstein Monofilaments perception test and sensory nerve conduction studies in carpal tunnel syndrome. NeuroRehabilitation. 2014;35:543-552.
24. Leinberry CF, Rivlin M, Maltenfort M, et al. Treatment of carpal tunnel syndrome by members of the American Society for Surgery of the Hand: a 25-year perspective. J Hand Surg Am. 2012;37:1997-2003.e3.
25. Ustün N, Tok F, Yagz AE, et al. Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: a single-blind randomized prospective study. Am J Phys Med Rehabil. 2013;92:999-1004.
26. Michalsen A, Bock S, Lüdtke R, et al. Effects of traditional cupping therapy in patients with carpal tunnel syndrome: a randomized controlled trial. J Pain. 2009;10:601-608.
27. Seok H, Kim SH. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil. 2013;92:327-334.
28. Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024.
29. Shehab R, Mirabelli MH. Evaluation and diagnosis of wrist pain: a case-based approach. Am Fam Physician. 2013;87:568-573.
30. Clarke MT, Lyall HA, Grant JW, et al. The histopathology of de Quervain’s disease. J Hand Surg Br. 1998;23:732-734.
31. Zychowicz MA. A closer look at hand and wrist complaints. Nurse Pract. 2013;38:46-53.
32. Avci S, Yilmaz C, Sayli U. Comparison of nonsurgical treatment measures for de Quervain’s disease of pregnancy and lactation. J Hand Surg Am. 2002;27:322-324.
33. Mani L, Gerr F. Work-related upper extremity musculoskeletal disorders. Prim Care. 2000;27:845-864.
34. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
35. Peters-Veluthamaningal C, Van der Windt JC, Winters JC, et al. Corticosteroid injection for de Quervain’s tenosynovitis. Cochrane Database Syst Rev. 2009;8:CD005616.
36. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
37. Pagonis T, Ditsios K, Toli P, et al. Improved corticosteroid treatment of recalcitrant de Quervain tenosynovitis with a novel 4-point injection technique. Am J Sports Med. 2011;39:398-403.
38. Scheller A, Schuh R, Hönle W, et al. Long-term results of surgical release of de Quervain’s stenosing tenosynovitis. Int Orthop. 2009;33:1301-1303.
39. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832-839.
40. Regan W, Wold LE, Coonrad R, et al. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992;20:746-749.
41. Van Hofwegen C, Baker CL 3rd, Baker CL Jr. Epicondylitis in the athlete’s elbow. Clin Sports Med. 2010;29:577-597.
42. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6:259-272.
43. Weerakul S, Galassi M. Randomized controlled trial local injection for treatment of lateral epicondylitis, 5 and 10 mg triamcinolone compared. J Med Assoc Thai. 2012;95 Supp 10:S184-188.
1. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775.
2. Peters-Veluthamaningal C, Van der Windt DA, Winters JC, et al. Corticosteroid injection for trigger finger in adults. Cochrane Database Syst Rev. 2009:CD005617.
3. Cheng J, Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11:141-147.
4. Waryasz GR, Tambone R, Borenstein TR, et al. A review of anatomical placement of corticosteroid injections for uncommon hand, wrist, and elbow pathologies. R I Med J. 2017;100:31-34.
5. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1:396-404.
6. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743.
7. Lundin AC, Aspenberg P, Eliasson P. Trigger finger, tendinosis, and intratendinous gene expression. Scand J Med Sci Sports. 2014;24:363-368.
8. Sato ES, Gomes Dos Santos JB, Belloti JC, et al. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology (Oxford). 2012;51:93-99.
9. Baek GH, Kim JH, Chug MS, et al. The natural history of pediatric trigger thumb. J Bone Joint Surg Am. 2008;90:980-985.
10. Gillis J, Calder K, Williams J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can J Plast Surg. 2011;19:134-138.
11. Yao J, Park MJ. Early treatment of degenerative arthritis of the thumb carpometacarpal joint. Hand Clin. 2008;24:251-261.
12. Ladd AL, Weiss AP, Crisco JJ, et al. The thumb carpometacarpal joint: anatomy, hormones, and biomechanics. Instr Course Lect. 2013;62:165-179.
13. Fontana L, Neel S, Claise JM, et al. Osteoarthritis of the thumb carpometacarpal joint in women and occupational risk factors: a case-control study. J Hand Surg Am. 2007;32:459-465.
14. Stamm TA, Machold KP, Smolen JS, et al. Joint protection and home hand exercises improve hand function in patients with hand osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2002;47:44-49.
15. Weiss S, LaStayo P, Mills A, et al. Prospective analysis of splinting the first carpometacarpal joint: an objective, subjective, and radiographic assessment. J Hand Ther. 2000;13:218-226.
16. Day CS, Gelberman R, Patel AA, et al. Basal joint osteoarthritis of the thumb: a prospective trial of steroid injection and splinting. J Hand Surg Am. 2004;29:247-251.
17. Gelfman R, Melton LJ 3rd, Yawn BP, et al. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33-41.
18. Wipperman J, Potter L. Carpal tunnel syndrome-try these diagnostic maneuvers. J Fam Pract. 2012;61:726-732.
19. Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981-1004.
20. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43:431-437.
21. Soltani AM, Allan BJ, Best MJ, et al. A systematic review of the literature on the outcomes of treatment for recurrent and persistent carpal tunnel syndrome. Plast Reconstr Surg. 2013;132:114-121.
22. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
23. Raji P, Ansari NN, Naghdi S, et al. Relationship between Semmes-Weinstein Monofilaments perception test and sensory nerve conduction studies in carpal tunnel syndrome. NeuroRehabilitation. 2014;35:543-552.
24. Leinberry CF, Rivlin M, Maltenfort M, et al. Treatment of carpal tunnel syndrome by members of the American Society for Surgery of the Hand: a 25-year perspective. J Hand Surg Am. 2012;37:1997-2003.e3.
25. Ustün N, Tok F, Yagz AE, et al. Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: a single-blind randomized prospective study. Am J Phys Med Rehabil. 2013;92:999-1004.
26. Michalsen A, Bock S, Lüdtke R, et al. Effects of traditional cupping therapy in patients with carpal tunnel syndrome: a randomized controlled trial. J Pain. 2009;10:601-608.
27. Seok H, Kim SH. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil. 2013;92:327-334.
28. Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024.
29. Shehab R, Mirabelli MH. Evaluation and diagnosis of wrist pain: a case-based approach. Am Fam Physician. 2013;87:568-573.
30. Clarke MT, Lyall HA, Grant JW, et al. The histopathology of de Quervain’s disease. J Hand Surg Br. 1998;23:732-734.
31. Zychowicz MA. A closer look at hand and wrist complaints. Nurse Pract. 2013;38:46-53.
32. Avci S, Yilmaz C, Sayli U. Comparison of nonsurgical treatment measures for de Quervain’s disease of pregnancy and lactation. J Hand Surg Am. 2002;27:322-324.
33. Mani L, Gerr F. Work-related upper extremity musculoskeletal disorders. Prim Care. 2000;27:845-864.
34. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
35. Peters-Veluthamaningal C, Van der Windt JC, Winters JC, et al. Corticosteroid injection for de Quervain’s tenosynovitis. Cochrane Database Syst Rev. 2009;8:CD005616.
36. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
37. Pagonis T, Ditsios K, Toli P, et al. Improved corticosteroid treatment of recalcitrant de Quervain tenosynovitis with a novel 4-point injection technique. Am J Sports Med. 2011;39:398-403.
38. Scheller A, Schuh R, Hönle W, et al. Long-term results of surgical release of de Quervain’s stenosing tenosynovitis. Int Orthop. 2009;33:1301-1303.
39. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832-839.
40. Regan W, Wold LE, Coonrad R, et al. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992;20:746-749.
41. Van Hofwegen C, Baker CL 3rd, Baker CL Jr. Epicondylitis in the athlete’s elbow. Clin Sports Med. 2010;29:577-597.
42. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6:259-272.
43. Weerakul S, Galassi M. Randomized controlled trial local injection for treatment of lateral epicondylitis, 5 and 10 mg triamcinolone compared. J Med Assoc Thai. 2012;95 Supp 10:S184-188.
PRACTICE RECOMMENDATIONS
› Diagnose common upper extremity conditions based on anatomic relationships. B
› Refer patients who do not respond to splinting, corticosteroid injections, or other conservative therapies to a surgeon for evaluation. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Radiation therapy: Managing GI tract complications
CASE A 57-year-old man presented for evaluation of painless, intermittent passage of bright red blood per rectum for several months. His bowel habits were otherwise unchanged, averaging 2 soft bowel movements daily without straining. His medical history was significant for radiation therapy for prostate cancer 18 months earlier and a recent finding of mild microcytic anemia. A colonoscopy 7 years ago was negative for polyps, diverticula, or other lesions. He denied any family history of colon cancer or other gastrointestinal disorders. He wanted to know what he could do to stop the bleeding or if further testing would be needed.
Next steps?
Radiation therapy and its effect on the GI tract
In 1895, Dr. Wilhelm Roentgen first introduced the use of x-rays for diagnostic radiographic purposes. A year later, Dr. Emil Gruble made the first attempt to use radiation therapy (XRT) to treat cancer. In 1897, Dr. David Walsh described the first case of XRT-induced tissue injury in the British Medical Journal.1
Since then, XRT has been used extensively to treat cancer, and its delivery techniques have improved and diversified. Like chemotherapy, XRT has its greatest effect on rapidly dividing cells, but as a result, the adverse effects of therapy are also greatest on rapidly dividing normal tissues, as well as others in the radiation field.
A large proportion of cancer patients will receive XRT, yet XRT-related costs account for less than 5% of total cancer care expenditure, suggesting cost effectiveness.2,3 However, even with the great progress achieved in the delivery of XRT, it continues to have its share of acute and chronic complications, among the most common of which is gastrointestinal (GI) tract toxicity. These adverse effects are often first reported to, diagnosed, or treated by the primary care provider, who frequently remains pivotally involved in the patient’s longitudinal care.
Approximately 50% to 75% of patients undergoing XRT will have some degree of GI symptoms of acute injury, but the majority will recover fully within a few weeks following completion of treatment.4-6 However, in about 5% of patients,4-6 there will be long-term consequences of varying degrees that may develop as soon as one year or as long as 10 years after XRT. These can pose substantial challenges for patients, as well as both the primary care provider and consulting specialists.
In the review that follows, we detail the potential acute and chronic complications of XRT on the GI tract and how best to manage them. But first, a word about the related terminology.
Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.
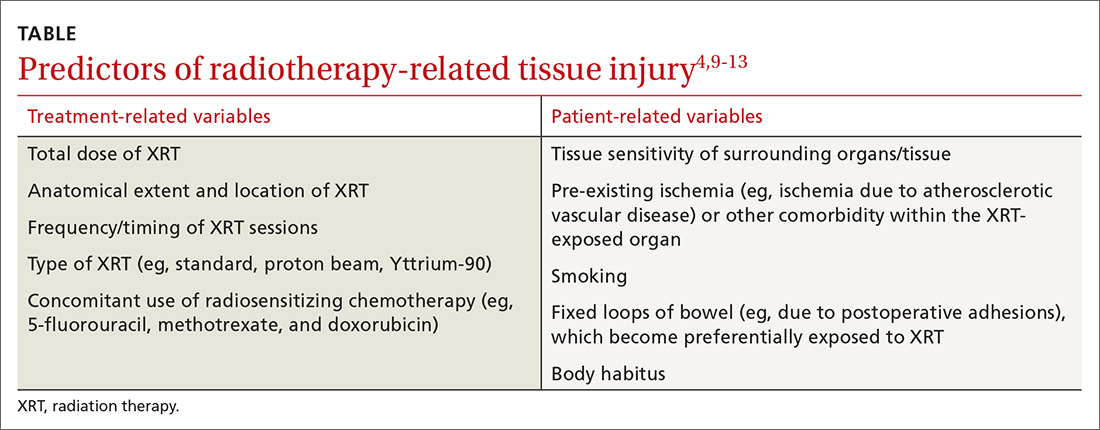
Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.
The stomach
The stomach is relatively resistant to XRT injury. Although XRT therapy can cause a transient decrease in acid output, there are rarely significant short- or long-term consequences with conventional therapeutic dosing (less than 50 gray).11
The liver
Hepatic resistance to radiation is relatively high; however, liver toxicity has been reported at low doses, an effect that is seen largely following bone marrow transplantation.24 Acute histologic XRT-related liver injury changes consist of severe pan-lobar congestion leading to hemorrhagic necrosis, cell atrophy, and perivascular fibrosis, as well as sclerosis of central and sublobular hepatic veins. The majority of patients will show reversal of the histologic changes within 3 months; however, approximately 25% to 40% of patients,25 depending on total XRT dose to the liver and other technical factors, will experience progressive and chronic changes resulting in liver atrophy, severe perivascular injury, and fibrosis of the portal vein or bile ducts.
The clinical symptoms of acute liver injury may include right upper quadrant pain, ascites, jaundice, veno-occlusive disease, or Budd-Chiari syndrome.25 The major chronic complication of XRT-related liver injury is progressive fibrosis, which may advance to cirrhosis.
Small bowel
The small bowel is the most radiosensitive GI tract organ due to high cell turnover, which makes it very susceptible to XRT-related injury.4,8,10,26-28 Under 3 gray, ≤20% of patients will develop radiation enteropathy, while at >5 gray, the incidence rises progressively with dose, and a majority of patients will be symptomatic.29 The degree to which the bowel is healthy before XRT can be an important factor in developing enteropathy. Parenthetically, treatment with a full bladder may also help displace some of the loops from the field of XRT and decrease injury.
Acute XRT-related injury of the small bowel includes mucosal necrosis (ie, direct cell death) and ulcerations that may present as diarrhea, pain, malabsorption, weight loss, bleeding, and perforation.4,8,10,26-28 Fortunately, in most patients, these are self-limited and can be managed symptomatically. Loperamide is the first-line medication for diarrhea, although Lomotil (diphenoxylate/atropine) may also be used if necessary.4,8,10,26-28 Nutrition may be challenging in severe cases, and if dietary modifications and supplementation do not prove sufficient, home parenteral nutrition is required.
Over time, chronic small bowel pathology may develop, including strictures in 3% to 15%, fistulae in 0.6% to 4.8%, secondary neoplasia in up to 10%, dysmotility- or adhesion-related small intestinal bacterial overgrowth in up to 45%, and malabsorption with associated nutritional deficiency in up to 63%.26-28 Other common XRT-related complications are chronic pain, which could be due to adhesions or ischemia, small intestinal bacterial overgrowth, or partial bowel obstruction, and telangiectasias that result with acute or chronic blood loss.13
Imaging of small bowel disease to diagnose the various manifestations of radiation enteropathy is challenging. Conventional X-rays may be difficult to interpret. Therefore, computerized tomography or magnetic resonance enterography, capsule endoscopy, or balloon-assisted enteroscopy is preferred—depending on availability, local expertise, and the suspected pre-procedure diagnosis.
Telangiectasias are not seen on cross-sectional imaging but can be seen with capsule endoscopy (which should not be ordered if stricture is suspected unless a patency capsule has been tried). Single or double balloon enteroscopy (specialized endoscopes intended for reaching the mid and distal ileum), which has been used to treat strictures or telangiectasia in healthy tissues,29 can be difficult or impossible in post-XRT patients because adhesions may limit progress of the scope to the area of interest, and forceful advancement of the scope increases the risk of perforation.
Small bowel telangiectasias can cause chronic occult blood loss, which often requires iron supplementation; acute bleeding may require blood transfusion and hospitalization. Of note, choosing an iron formulation that is well tolerated is critical to avoid (additional) unpleasant GI tract adverse effects. We typically recommend elemental iron with Vitamin C to augment absorption or ferrous gluconate; some patients will require intravenous iron infusion.
Surgery may be advisable to address complications such as fistulous tracts, complex strictures, or bowel obstruction; how-ever, operating on radiated abdominal tissues and ischemic bowel is associated with high morbidity and mortality.4,25,28,30 The surgeon may encounter dense adhesions that make an otherwise “simple” surgery problematic.
For example, it may be difficult to access the desired region and determine the borders of healthy tissue; wide excisions are, thus, often performed, which may result in small bowel failure (ie, short gut syndrome) and a mortality rate in excess of 30%.31 In addition, the ischemic post-XRT tissues may not heal well even if the intended surgery is completed; indeed, anastomotic leaks, failures, and infections are not uncommon. Moreover, another 30% will have other postoperative complications, 40% to 60% may require more than one laparotomy, and 50% of those who recover from the initial surgery will develop recurrence of the fistulous tract or stricture.4,25,28,30
No drug therapy has proven effective for prevention or mechanistically-driven treatment of XRT-induced small bowel injury. Hyperbaric oxygen therapy may be the most promising medical treatment, with early response in 53% of cases and long-term response of 66% to 73% for global symptomatic relief.32 It has been used successfully for treatment of pain, diarrhea, malabsorption, and hemorrhage from mucosal ulcerations, stenosis, and fistulous tracts. When available, it should be considered as a potential therapeutic intervention.
Colon
Injury to the colon is seen in 10% to 20% of patients following XRT for prostate, bladder, cervical, or uterine cancer.33 The maximum tolerated dose of the colon is slightly higher than for the small intestine.34 The rectosigmoid area is the area most commonly implicated, but depending on the field of radiation, injury can be more extensive/proximal.
Acute XRT injury of the colon produces acute mucosal necrosis, which may manifest as bowel dysmotility, diarrhea, cramps, tenesmus, or hematochezia. Sigmoidoscopy or colonoscopy will show mucosal edema, erosions, and ulcerations with a purplish/red discoloration. A barium enema will show spasm of the affected area with so-called “thumbprinting,” which indicates mucosal edema. The onset of symptoms is generally within 3 weeks of XRT initiation; symptoms are self-limited in most cases. Management is centered on symptom relief; loperamide and Lomotil are first-line agents for diarrheal symptoms.
Chronic XRT-related colopathy is the result of chronic tissue ischemia and fibrosis. This may lead to dysmotility resulting in abnormal bowel habits (ranging from constipation to diarrhea) or sigmoid stenosis/stricture resulting in an inability to evacuate the bowel. For the latter, it is important to note that fiber supplementation may not be optimal, since increasing the fecal caliber makes it more difficult to pass through the stenotic, colonic segment.
Emollients such as small doses of mineral oil will not increase the fecal caliber, but will soften fecal matter so that it can be passed with greater ease. MiraLAX may be effective, as well, but can increase the sense of urgency and contribute to incontinence in some. Lactulose can be effective, but it causes excessive gassiness/bloating that may result in abdominal pain and episodes of incontinence.
Bleeding from telangiectasias is another chronic complication of XRT-related colonic injury. Argon plasma coagulation (APC) via flexible sigmoidoscopy or colonoscopy is typically the primary therapeutic approach, reported to have a success rate of up to 90% in healthy tissues.33,35 Even with endoscopic treatment, as mentioned earlier in the context of small bowel XRT-related telangiectasias, iron supplementation is often needed to replete stores, and choice of iron agent is important.
Furthermore, it is essential to recognize that repeat endoscopic sessions may be needed to fully treat telangiectasias, and recrudescence of bleeding months or years later should raise suspicion for recurrent telangiectasia formation (and need for repeat treatment). As with other organs, there may be a role for hyperbaric oxygen, even in difficult-to-treat cases.36,37
Colonic fibrosis/stenosis and fistulous tract formation, as in the small bowel, are also seen in this population of patients. Endoscopic dilation can be considered, and stenting may be reasonable for short and/or distal strictures. Surgical approaches for fistulous tracts and strictures can be high-risk and associated with poor outcomes, mostly because of the underlying chronic tissue ischemia and fibrosis,4,8,27,30,34 as discussed in the small bowel section.
Rectum
The rectum has tolerance to XRT similar to the colon,38 but because of its anatomical location, rectal radiation injury is more common, and is typically seen after XRT for prostate, bladder, cervical, or uterine cancer. Acute rectal radiation injury is seen in 50% to 78% of patients,36 and symptoms are similar to that of injury to the sigmoid (eg, tenesmus, loose evacuations, hematochezia), all of which are consequences of direct radiation injury to the mucosa.
Chronic rectal radiation injury may present in a variety of ways. Tenesmus and incontinence are seen in 8% to 20% of patients, frequent defecation in 50%, urgency in 47%, and rectal cancer in up to 2% to 3% after 10 years.36,37 Other complications include anorectal strictures, fissures, fistulae, and bleeding from rectal telangiectasias. While anoscopy can diagnose many of these, flexible sigmoidoscopy is needed to examine more proximal rectal sites as well as for treatment. Treatment of these chronic complications of XRT is analogous to those of the colon7 with the following exceptions:
- Anorectal strictures. In contrast to sigmoid strictures, these are generally more amenable to dilatation. If symptoms recur frequently, patients may be instructed on self-dilatations at home.
- Bleeding from rectal telangiectasias. In the rare cases where endoscopic APC is not feasible or successful, an alternative treatment would be radiofrequency ablation or the application of 2% to 10% formalin intra-rectally. This is reported to have up to a 93% success rate;37 however, because formalin can also cause rectal pain, spasm, ulcerations, or stenosis, it is not a first-line therapy.
- Tenesmus, urgency, and incontinence. These represent a therapeutic challenge, often with no satisfactory outcomes. An array of empiric treatments may be used for symptomatic relief, including but not limited to, a trial of loperamide or fiber supplementation, which may be helpful for frequent evacuation.
- Fistulous tracts associated with rectal radiation. Endoscopic clip closure of XRT-related and other fistulous tracts is an option. This has been attempted via a variety of techniques, but results depend on the size and location of the fistulous tract, as well as other characteristics of the fistula and its surrounding tissue.7,38,39 Use of mesenchymal stem cells has also been described for rectal and other fistulae,40 but its indications have yet to be elucidated, and current use is mostly experimental.
CASE The patient’s recent-onset symptoms and clinical history were most suggestive of radiation proctopathy; a shared decision was made to pursue endoscopic evaluation with possible therapeutic intervention.
Given that data were not available about the quality of the colon preparation during the exam 7 years earlier, and to rule out a more proximal colonic lesion, the patient was scheduled for colonoscopy. This revealed numerous telangiectasias and moderate friability involving the distal third of the rectum, consistent with radiation proctopathy. The telangiectasias were treated with APC. Follow-up flexible sigmoidoscopy 2 months later showed a few remaining scattered telangiectasias, which were also treated with APC.
The patient has been clinically well, without evidence of bleeding for 6 months and with resolution of anemia.
CORRESPONDENCE
James H. Tabibian, Division of Gastroenterology, Department of Medicine, 14445 Olive View Dr., 2B-182, Sylmar, CA 91342; [email protected].
1. Walsh D. Deep tissue traumatism from roentgen ray exposure. Brit Med J. 1897;2:272-273.
2. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11:403-409.
3. Leung HWC, Chan ALF. Direct medical cost of radiation therapy for cancer patients in Taiwan. SciRes. 2013;5:989-993.
4. Andreyev HJ. GI consequences of cancer treatment: a clinical perspective. Radiat Res. 2016;185:341-348.
5. Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the Vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92:1663-1670.
6. Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016 Aug 5;8:CD003034.
7. ASGE. The role of endoscopy in patients with anorectal disorders. Gastrointest Endosc. 2010;72:1117-1123.
8. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014:5:15-29.
9. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-143.
10. Theiss VS, Sripadam R, Ramani V, et al. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83.
11. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-384.
12. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198.
13. Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: a practical review of an often overlooked entity. Am Med Surg (Lond). 2017;15:9-13.
14. Baumann J, Lin M, Patel C. An unusual case of gastritis and duodenitis after yttrium 90-microsphere selective internal radiation. Clin Gastroenterol Hepatol. 2015;13:xxiii-xxiv.
15. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016 Apr 28;4:CD005005.
16. Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54-59.
17. Marshall GT, Thirlby RC, Bredfelt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
18. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473.
19. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-120.
20. Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812.
21. Agarwalla A, Small AJ, Mendelson AH, et al. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912.
22. Kaasa S, Mastekaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol. 1988;27:343-349.
23. Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822-828.
24. Lewin K, Mills RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21-26.
25. Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128-134.
26. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361-365.
27. Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Phys. 2005;62:1464-1471.
28. Zimmer T, Böcker U, Wang F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? A short review. Z Gastroenterol. 2008;46:441-448.
29. Kita H, Yamamoto H, Yano T, et al. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small bowel intestinal disorders. Inflammopharmacology. 2007;15:74-77.
30. Girvent M, Carlson GL, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198-201.
31. Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89.
32. Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
33. Chun M, Kang S, Kil HJ, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Phys. 2004;58:98-105.
34. Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85-91.
35. Villavicencia RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
36. Dall’Era MA, Hampson NB, His RA, et al. Hyperbaric oxygen therapy for radiation-induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
37. Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359-365.
38. Gilinsky NH, Kottler RE. Idiopathic obstructive eosinophilic enteritis with raised IgE: response to oral disodium cromoglycate. Postgrad Med J. 1982;58:239-243.
39. Tabibian JH, Kochman ML. Over-the-wire technique to facilitate over-the-scope clip closure of fistulae. Gastrointest Endosc. 2017;85:454-455.
40. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140.
CASE A 57-year-old man presented for evaluation of painless, intermittent passage of bright red blood per rectum for several months. His bowel habits were otherwise unchanged, averaging 2 soft bowel movements daily without straining. His medical history was significant for radiation therapy for prostate cancer 18 months earlier and a recent finding of mild microcytic anemia. A colonoscopy 7 years ago was negative for polyps, diverticula, or other lesions. He denied any family history of colon cancer or other gastrointestinal disorders. He wanted to know what he could do to stop the bleeding or if further testing would be needed.
Next steps?
Radiation therapy and its effect on the GI tract
In 1895, Dr. Wilhelm Roentgen first introduced the use of x-rays for diagnostic radiographic purposes. A year later, Dr. Emil Gruble made the first attempt to use radiation therapy (XRT) to treat cancer. In 1897, Dr. David Walsh described the first case of XRT-induced tissue injury in the British Medical Journal.1
Since then, XRT has been used extensively to treat cancer, and its delivery techniques have improved and diversified. Like chemotherapy, XRT has its greatest effect on rapidly dividing cells, but as a result, the adverse effects of therapy are also greatest on rapidly dividing normal tissues, as well as others in the radiation field.
A large proportion of cancer patients will receive XRT, yet XRT-related costs account for less than 5% of total cancer care expenditure, suggesting cost effectiveness.2,3 However, even with the great progress achieved in the delivery of XRT, it continues to have its share of acute and chronic complications, among the most common of which is gastrointestinal (GI) tract toxicity. These adverse effects are often first reported to, diagnosed, or treated by the primary care provider, who frequently remains pivotally involved in the patient’s longitudinal care.
Approximately 50% to 75% of patients undergoing XRT will have some degree of GI symptoms of acute injury, but the majority will recover fully within a few weeks following completion of treatment.4-6 However, in about 5% of patients,4-6 there will be long-term consequences of varying degrees that may develop as soon as one year or as long as 10 years after XRT. These can pose substantial challenges for patients, as well as both the primary care provider and consulting specialists.
In the review that follows, we detail the potential acute and chronic complications of XRT on the GI tract and how best to manage them. But first, a word about the related terminology.
Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.

Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.
The stomach
The stomach is relatively resistant to XRT injury. Although XRT therapy can cause a transient decrease in acid output, there are rarely significant short- or long-term consequences with conventional therapeutic dosing (less than 50 gray).11
The liver
Hepatic resistance to radiation is relatively high; however, liver toxicity has been reported at low doses, an effect that is seen largely following bone marrow transplantation.24 Acute histologic XRT-related liver injury changes consist of severe pan-lobar congestion leading to hemorrhagic necrosis, cell atrophy, and perivascular fibrosis, as well as sclerosis of central and sublobular hepatic veins. The majority of patients will show reversal of the histologic changes within 3 months; however, approximately 25% to 40% of patients,25 depending on total XRT dose to the liver and other technical factors, will experience progressive and chronic changes resulting in liver atrophy, severe perivascular injury, and fibrosis of the portal vein or bile ducts.
The clinical symptoms of acute liver injury may include right upper quadrant pain, ascites, jaundice, veno-occlusive disease, or Budd-Chiari syndrome.25 The major chronic complication of XRT-related liver injury is progressive fibrosis, which may advance to cirrhosis.
Small bowel
The small bowel is the most radiosensitive GI tract organ due to high cell turnover, which makes it very susceptible to XRT-related injury.4,8,10,26-28 Under 3 gray, ≤20% of patients will develop radiation enteropathy, while at >5 gray, the incidence rises progressively with dose, and a majority of patients will be symptomatic.29 The degree to which the bowel is healthy before XRT can be an important factor in developing enteropathy. Parenthetically, treatment with a full bladder may also help displace some of the loops from the field of XRT and decrease injury.
Acute XRT-related injury of the small bowel includes mucosal necrosis (ie, direct cell death) and ulcerations that may present as diarrhea, pain, malabsorption, weight loss, bleeding, and perforation.4,8,10,26-28 Fortunately, in most patients, these are self-limited and can be managed symptomatically. Loperamide is the first-line medication for diarrhea, although Lomotil (diphenoxylate/atropine) may also be used if necessary.4,8,10,26-28 Nutrition may be challenging in severe cases, and if dietary modifications and supplementation do not prove sufficient, home parenteral nutrition is required.
Over time, chronic small bowel pathology may develop, including strictures in 3% to 15%, fistulae in 0.6% to 4.8%, secondary neoplasia in up to 10%, dysmotility- or adhesion-related small intestinal bacterial overgrowth in up to 45%, and malabsorption with associated nutritional deficiency in up to 63%.26-28 Other common XRT-related complications are chronic pain, which could be due to adhesions or ischemia, small intestinal bacterial overgrowth, or partial bowel obstruction, and telangiectasias that result with acute or chronic blood loss.13
Imaging of small bowel disease to diagnose the various manifestations of radiation enteropathy is challenging. Conventional X-rays may be difficult to interpret. Therefore, computerized tomography or magnetic resonance enterography, capsule endoscopy, or balloon-assisted enteroscopy is preferred—depending on availability, local expertise, and the suspected pre-procedure diagnosis.
Telangiectasias are not seen on cross-sectional imaging but can be seen with capsule endoscopy (which should not be ordered if stricture is suspected unless a patency capsule has been tried). Single or double balloon enteroscopy (specialized endoscopes intended for reaching the mid and distal ileum), which has been used to treat strictures or telangiectasia in healthy tissues,29 can be difficult or impossible in post-XRT patients because adhesions may limit progress of the scope to the area of interest, and forceful advancement of the scope increases the risk of perforation.
Small bowel telangiectasias can cause chronic occult blood loss, which often requires iron supplementation; acute bleeding may require blood transfusion and hospitalization. Of note, choosing an iron formulation that is well tolerated is critical to avoid (additional) unpleasant GI tract adverse effects. We typically recommend elemental iron with Vitamin C to augment absorption or ferrous gluconate; some patients will require intravenous iron infusion.
Surgery may be advisable to address complications such as fistulous tracts, complex strictures, or bowel obstruction; how-ever, operating on radiated abdominal tissues and ischemic bowel is associated with high morbidity and mortality.4,25,28,30 The surgeon may encounter dense adhesions that make an otherwise “simple” surgery problematic.
For example, it may be difficult to access the desired region and determine the borders of healthy tissue; wide excisions are, thus, often performed, which may result in small bowel failure (ie, short gut syndrome) and a mortality rate in excess of 30%.31 In addition, the ischemic post-XRT tissues may not heal well even if the intended surgery is completed; indeed, anastomotic leaks, failures, and infections are not uncommon. Moreover, another 30% will have other postoperative complications, 40% to 60% may require more than one laparotomy, and 50% of those who recover from the initial surgery will develop recurrence of the fistulous tract or stricture.4,25,28,30
No drug therapy has proven effective for prevention or mechanistically-driven treatment of XRT-induced small bowel injury. Hyperbaric oxygen therapy may be the most promising medical treatment, with early response in 53% of cases and long-term response of 66% to 73% for global symptomatic relief.32 It has been used successfully for treatment of pain, diarrhea, malabsorption, and hemorrhage from mucosal ulcerations, stenosis, and fistulous tracts. When available, it should be considered as a potential therapeutic intervention.
Colon
Injury to the colon is seen in 10% to 20% of patients following XRT for prostate, bladder, cervical, or uterine cancer.33 The maximum tolerated dose of the colon is slightly higher than for the small intestine.34 The rectosigmoid area is the area most commonly implicated, but depending on the field of radiation, injury can be more extensive/proximal.
Acute XRT injury of the colon produces acute mucosal necrosis, which may manifest as bowel dysmotility, diarrhea, cramps, tenesmus, or hematochezia. Sigmoidoscopy or colonoscopy will show mucosal edema, erosions, and ulcerations with a purplish/red discoloration. A barium enema will show spasm of the affected area with so-called “thumbprinting,” which indicates mucosal edema. The onset of symptoms is generally within 3 weeks of XRT initiation; symptoms are self-limited in most cases. Management is centered on symptom relief; loperamide and Lomotil are first-line agents for diarrheal symptoms.
Chronic XRT-related colopathy is the result of chronic tissue ischemia and fibrosis. This may lead to dysmotility resulting in abnormal bowel habits (ranging from constipation to diarrhea) or sigmoid stenosis/stricture resulting in an inability to evacuate the bowel. For the latter, it is important to note that fiber supplementation may not be optimal, since increasing the fecal caliber makes it more difficult to pass through the stenotic, colonic segment.
Emollients such as small doses of mineral oil will not increase the fecal caliber, but will soften fecal matter so that it can be passed with greater ease. MiraLAX may be effective, as well, but can increase the sense of urgency and contribute to incontinence in some. Lactulose can be effective, but it causes excessive gassiness/bloating that may result in abdominal pain and episodes of incontinence.
Bleeding from telangiectasias is another chronic complication of XRT-related colonic injury. Argon plasma coagulation (APC) via flexible sigmoidoscopy or colonoscopy is typically the primary therapeutic approach, reported to have a success rate of up to 90% in healthy tissues.33,35 Even with endoscopic treatment, as mentioned earlier in the context of small bowel XRT-related telangiectasias, iron supplementation is often needed to replete stores, and choice of iron agent is important.
Furthermore, it is essential to recognize that repeat endoscopic sessions may be needed to fully treat telangiectasias, and recrudescence of bleeding months or years later should raise suspicion for recurrent telangiectasia formation (and need for repeat treatment). As with other organs, there may be a role for hyperbaric oxygen, even in difficult-to-treat cases.36,37
Colonic fibrosis/stenosis and fistulous tract formation, as in the small bowel, are also seen in this population of patients. Endoscopic dilation can be considered, and stenting may be reasonable for short and/or distal strictures. Surgical approaches for fistulous tracts and strictures can be high-risk and associated with poor outcomes, mostly because of the underlying chronic tissue ischemia and fibrosis,4,8,27,30,34 as discussed in the small bowel section.
Rectum
The rectum has tolerance to XRT similar to the colon,38 but because of its anatomical location, rectal radiation injury is more common, and is typically seen after XRT for prostate, bladder, cervical, or uterine cancer. Acute rectal radiation injury is seen in 50% to 78% of patients,36 and symptoms are similar to that of injury to the sigmoid (eg, tenesmus, loose evacuations, hematochezia), all of which are consequences of direct radiation injury to the mucosa.
Chronic rectal radiation injury may present in a variety of ways. Tenesmus and incontinence are seen in 8% to 20% of patients, frequent defecation in 50%, urgency in 47%, and rectal cancer in up to 2% to 3% after 10 years.36,37 Other complications include anorectal strictures, fissures, fistulae, and bleeding from rectal telangiectasias. While anoscopy can diagnose many of these, flexible sigmoidoscopy is needed to examine more proximal rectal sites as well as for treatment. Treatment of these chronic complications of XRT is analogous to those of the colon7 with the following exceptions:
- Anorectal strictures. In contrast to sigmoid strictures, these are generally more amenable to dilatation. If symptoms recur frequently, patients may be instructed on self-dilatations at home.
- Bleeding from rectal telangiectasias. In the rare cases where endoscopic APC is not feasible or successful, an alternative treatment would be radiofrequency ablation or the application of 2% to 10% formalin intra-rectally. This is reported to have up to a 93% success rate;37 however, because formalin can also cause rectal pain, spasm, ulcerations, or stenosis, it is not a first-line therapy.
- Tenesmus, urgency, and incontinence. These represent a therapeutic challenge, often with no satisfactory outcomes. An array of empiric treatments may be used for symptomatic relief, including but not limited to, a trial of loperamide or fiber supplementation, which may be helpful for frequent evacuation.
- Fistulous tracts associated with rectal radiation. Endoscopic clip closure of XRT-related and other fistulous tracts is an option. This has been attempted via a variety of techniques, but results depend on the size and location of the fistulous tract, as well as other characteristics of the fistula and its surrounding tissue.7,38,39 Use of mesenchymal stem cells has also been described for rectal and other fistulae,40 but its indications have yet to be elucidated, and current use is mostly experimental.
CASE The patient’s recent-onset symptoms and clinical history were most suggestive of radiation proctopathy; a shared decision was made to pursue endoscopic evaluation with possible therapeutic intervention.
Given that data were not available about the quality of the colon preparation during the exam 7 years earlier, and to rule out a more proximal colonic lesion, the patient was scheduled for colonoscopy. This revealed numerous telangiectasias and moderate friability involving the distal third of the rectum, consistent with radiation proctopathy. The telangiectasias were treated with APC. Follow-up flexible sigmoidoscopy 2 months later showed a few remaining scattered telangiectasias, which were also treated with APC.
The patient has been clinically well, without evidence of bleeding for 6 months and with resolution of anemia.
CORRESPONDENCE
James H. Tabibian, Division of Gastroenterology, Department of Medicine, 14445 Olive View Dr., 2B-182, Sylmar, CA 91342; [email protected].
CASE A 57-year-old man presented for evaluation of painless, intermittent passage of bright red blood per rectum for several months. His bowel habits were otherwise unchanged, averaging 2 soft bowel movements daily without straining. His medical history was significant for radiation therapy for prostate cancer 18 months earlier and a recent finding of mild microcytic anemia. A colonoscopy 7 years ago was negative for polyps, diverticula, or other lesions. He denied any family history of colon cancer or other gastrointestinal disorders. He wanted to know what he could do to stop the bleeding or if further testing would be needed.
Next steps?
Radiation therapy and its effect on the GI tract
In 1895, Dr. Wilhelm Roentgen first introduced the use of x-rays for diagnostic radiographic purposes. A year later, Dr. Emil Gruble made the first attempt to use radiation therapy (XRT) to treat cancer. In 1897, Dr. David Walsh described the first case of XRT-induced tissue injury in the British Medical Journal.1
Since then, XRT has been used extensively to treat cancer, and its delivery techniques have improved and diversified. Like chemotherapy, XRT has its greatest effect on rapidly dividing cells, but as a result, the adverse effects of therapy are also greatest on rapidly dividing normal tissues, as well as others in the radiation field.
A large proportion of cancer patients will receive XRT, yet XRT-related costs account for less than 5% of total cancer care expenditure, suggesting cost effectiveness.2,3 However, even with the great progress achieved in the delivery of XRT, it continues to have its share of acute and chronic complications, among the most common of which is gastrointestinal (GI) tract toxicity. These adverse effects are often first reported to, diagnosed, or treated by the primary care provider, who frequently remains pivotally involved in the patient’s longitudinal care.
Approximately 50% to 75% of patients undergoing XRT will have some degree of GI symptoms of acute injury, but the majority will recover fully within a few weeks following completion of treatment.4-6 However, in about 5% of patients,4-6 there will be long-term consequences of varying degrees that may develop as soon as one year or as long as 10 years after XRT. These can pose substantial challenges for patients, as well as both the primary care provider and consulting specialists.
In the review that follows, we detail the potential acute and chronic complications of XRT on the GI tract and how best to manage them. But first, a word about the related terminology.
Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.

Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.
The stomach
The stomach is relatively resistant to XRT injury. Although XRT therapy can cause a transient decrease in acid output, there are rarely significant short- or long-term consequences with conventional therapeutic dosing (less than 50 gray).11
The liver
Hepatic resistance to radiation is relatively high; however, liver toxicity has been reported at low doses, an effect that is seen largely following bone marrow transplantation.24 Acute histologic XRT-related liver injury changes consist of severe pan-lobar congestion leading to hemorrhagic necrosis, cell atrophy, and perivascular fibrosis, as well as sclerosis of central and sublobular hepatic veins. The majority of patients will show reversal of the histologic changes within 3 months; however, approximately 25% to 40% of patients,25 depending on total XRT dose to the liver and other technical factors, will experience progressive and chronic changes resulting in liver atrophy, severe perivascular injury, and fibrosis of the portal vein or bile ducts.
The clinical symptoms of acute liver injury may include right upper quadrant pain, ascites, jaundice, veno-occlusive disease, or Budd-Chiari syndrome.25 The major chronic complication of XRT-related liver injury is progressive fibrosis, which may advance to cirrhosis.
Small bowel
The small bowel is the most radiosensitive GI tract organ due to high cell turnover, which makes it very susceptible to XRT-related injury.4,8,10,26-28 Under 3 gray, ≤20% of patients will develop radiation enteropathy, while at >5 gray, the incidence rises progressively with dose, and a majority of patients will be symptomatic.29 The degree to which the bowel is healthy before XRT can be an important factor in developing enteropathy. Parenthetically, treatment with a full bladder may also help displace some of the loops from the field of XRT and decrease injury.
Acute XRT-related injury of the small bowel includes mucosal necrosis (ie, direct cell death) and ulcerations that may present as diarrhea, pain, malabsorption, weight loss, bleeding, and perforation.4,8,10,26-28 Fortunately, in most patients, these are self-limited and can be managed symptomatically. Loperamide is the first-line medication for diarrhea, although Lomotil (diphenoxylate/atropine) may also be used if necessary.4,8,10,26-28 Nutrition may be challenging in severe cases, and if dietary modifications and supplementation do not prove sufficient, home parenteral nutrition is required.
Over time, chronic small bowel pathology may develop, including strictures in 3% to 15%, fistulae in 0.6% to 4.8%, secondary neoplasia in up to 10%, dysmotility- or adhesion-related small intestinal bacterial overgrowth in up to 45%, and malabsorption with associated nutritional deficiency in up to 63%.26-28 Other common XRT-related complications are chronic pain, which could be due to adhesions or ischemia, small intestinal bacterial overgrowth, or partial bowel obstruction, and telangiectasias that result with acute or chronic blood loss.13
Imaging of small bowel disease to diagnose the various manifestations of radiation enteropathy is challenging. Conventional X-rays may be difficult to interpret. Therefore, computerized tomography or magnetic resonance enterography, capsule endoscopy, or balloon-assisted enteroscopy is preferred—depending on availability, local expertise, and the suspected pre-procedure diagnosis.
Telangiectasias are not seen on cross-sectional imaging but can be seen with capsule endoscopy (which should not be ordered if stricture is suspected unless a patency capsule has been tried). Single or double balloon enteroscopy (specialized endoscopes intended for reaching the mid and distal ileum), which has been used to treat strictures or telangiectasia in healthy tissues,29 can be difficult or impossible in post-XRT patients because adhesions may limit progress of the scope to the area of interest, and forceful advancement of the scope increases the risk of perforation.
Small bowel telangiectasias can cause chronic occult blood loss, which often requires iron supplementation; acute bleeding may require blood transfusion and hospitalization. Of note, choosing an iron formulation that is well tolerated is critical to avoid (additional) unpleasant GI tract adverse effects. We typically recommend elemental iron with Vitamin C to augment absorption or ferrous gluconate; some patients will require intravenous iron infusion.
Surgery may be advisable to address complications such as fistulous tracts, complex strictures, or bowel obstruction; how-ever, operating on radiated abdominal tissues and ischemic bowel is associated with high morbidity and mortality.4,25,28,30 The surgeon may encounter dense adhesions that make an otherwise “simple” surgery problematic.
For example, it may be difficult to access the desired region and determine the borders of healthy tissue; wide excisions are, thus, often performed, which may result in small bowel failure (ie, short gut syndrome) and a mortality rate in excess of 30%.31 In addition, the ischemic post-XRT tissues may not heal well even if the intended surgery is completed; indeed, anastomotic leaks, failures, and infections are not uncommon. Moreover, another 30% will have other postoperative complications, 40% to 60% may require more than one laparotomy, and 50% of those who recover from the initial surgery will develop recurrence of the fistulous tract or stricture.4,25,28,30
No drug therapy has proven effective for prevention or mechanistically-driven treatment of XRT-induced small bowel injury. Hyperbaric oxygen therapy may be the most promising medical treatment, with early response in 53% of cases and long-term response of 66% to 73% for global symptomatic relief.32 It has been used successfully for treatment of pain, diarrhea, malabsorption, and hemorrhage from mucosal ulcerations, stenosis, and fistulous tracts. When available, it should be considered as a potential therapeutic intervention.
Colon
Injury to the colon is seen in 10% to 20% of patients following XRT for prostate, bladder, cervical, or uterine cancer.33 The maximum tolerated dose of the colon is slightly higher than for the small intestine.34 The rectosigmoid area is the area most commonly implicated, but depending on the field of radiation, injury can be more extensive/proximal.
Acute XRT injury of the colon produces acute mucosal necrosis, which may manifest as bowel dysmotility, diarrhea, cramps, tenesmus, or hematochezia. Sigmoidoscopy or colonoscopy will show mucosal edema, erosions, and ulcerations with a purplish/red discoloration. A barium enema will show spasm of the affected area with so-called “thumbprinting,” which indicates mucosal edema. The onset of symptoms is generally within 3 weeks of XRT initiation; symptoms are self-limited in most cases. Management is centered on symptom relief; loperamide and Lomotil are first-line agents for diarrheal symptoms.
Chronic XRT-related colopathy is the result of chronic tissue ischemia and fibrosis. This may lead to dysmotility resulting in abnormal bowel habits (ranging from constipation to diarrhea) or sigmoid stenosis/stricture resulting in an inability to evacuate the bowel. For the latter, it is important to note that fiber supplementation may not be optimal, since increasing the fecal caliber makes it more difficult to pass through the stenotic, colonic segment.
Emollients such as small doses of mineral oil will not increase the fecal caliber, but will soften fecal matter so that it can be passed with greater ease. MiraLAX may be effective, as well, but can increase the sense of urgency and contribute to incontinence in some. Lactulose can be effective, but it causes excessive gassiness/bloating that may result in abdominal pain and episodes of incontinence.
Bleeding from telangiectasias is another chronic complication of XRT-related colonic injury. Argon plasma coagulation (APC) via flexible sigmoidoscopy or colonoscopy is typically the primary therapeutic approach, reported to have a success rate of up to 90% in healthy tissues.33,35 Even with endoscopic treatment, as mentioned earlier in the context of small bowel XRT-related telangiectasias, iron supplementation is often needed to replete stores, and choice of iron agent is important.
Furthermore, it is essential to recognize that repeat endoscopic sessions may be needed to fully treat telangiectasias, and recrudescence of bleeding months or years later should raise suspicion for recurrent telangiectasia formation (and need for repeat treatment). As with other organs, there may be a role for hyperbaric oxygen, even in difficult-to-treat cases.36,37
Colonic fibrosis/stenosis and fistulous tract formation, as in the small bowel, are also seen in this population of patients. Endoscopic dilation can be considered, and stenting may be reasonable for short and/or distal strictures. Surgical approaches for fistulous tracts and strictures can be high-risk and associated with poor outcomes, mostly because of the underlying chronic tissue ischemia and fibrosis,4,8,27,30,34 as discussed in the small bowel section.
Rectum
The rectum has tolerance to XRT similar to the colon,38 but because of its anatomical location, rectal radiation injury is more common, and is typically seen after XRT for prostate, bladder, cervical, or uterine cancer. Acute rectal radiation injury is seen in 50% to 78% of patients,36 and symptoms are similar to that of injury to the sigmoid (eg, tenesmus, loose evacuations, hematochezia), all of which are consequences of direct radiation injury to the mucosa.
Chronic rectal radiation injury may present in a variety of ways. Tenesmus and incontinence are seen in 8% to 20% of patients, frequent defecation in 50%, urgency in 47%, and rectal cancer in up to 2% to 3% after 10 years.36,37 Other complications include anorectal strictures, fissures, fistulae, and bleeding from rectal telangiectasias. While anoscopy can diagnose many of these, flexible sigmoidoscopy is needed to examine more proximal rectal sites as well as for treatment. Treatment of these chronic complications of XRT is analogous to those of the colon7 with the following exceptions:
- Anorectal strictures. In contrast to sigmoid strictures, these are generally more amenable to dilatation. If symptoms recur frequently, patients may be instructed on self-dilatations at home.
- Bleeding from rectal telangiectasias. In the rare cases where endoscopic APC is not feasible or successful, an alternative treatment would be radiofrequency ablation or the application of 2% to 10% formalin intra-rectally. This is reported to have up to a 93% success rate;37 however, because formalin can also cause rectal pain, spasm, ulcerations, or stenosis, it is not a first-line therapy.
- Tenesmus, urgency, and incontinence. These represent a therapeutic challenge, often with no satisfactory outcomes. An array of empiric treatments may be used for symptomatic relief, including but not limited to, a trial of loperamide or fiber supplementation, which may be helpful for frequent evacuation.
- Fistulous tracts associated with rectal radiation. Endoscopic clip closure of XRT-related and other fistulous tracts is an option. This has been attempted via a variety of techniques, but results depend on the size and location of the fistulous tract, as well as other characteristics of the fistula and its surrounding tissue.7,38,39 Use of mesenchymal stem cells has also been described for rectal and other fistulae,40 but its indications have yet to be elucidated, and current use is mostly experimental.
CASE The patient’s recent-onset symptoms and clinical history were most suggestive of radiation proctopathy; a shared decision was made to pursue endoscopic evaluation with possible therapeutic intervention.
Given that data were not available about the quality of the colon preparation during the exam 7 years earlier, and to rule out a more proximal colonic lesion, the patient was scheduled for colonoscopy. This revealed numerous telangiectasias and moderate friability involving the distal third of the rectum, consistent with radiation proctopathy. The telangiectasias were treated with APC. Follow-up flexible sigmoidoscopy 2 months later showed a few remaining scattered telangiectasias, which were also treated with APC.
The patient has been clinically well, without evidence of bleeding for 6 months and with resolution of anemia.
CORRESPONDENCE
James H. Tabibian, Division of Gastroenterology, Department of Medicine, 14445 Olive View Dr., 2B-182, Sylmar, CA 91342; [email protected].
1. Walsh D. Deep tissue traumatism from roentgen ray exposure. Brit Med J. 1897;2:272-273.
2. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11:403-409.
3. Leung HWC, Chan ALF. Direct medical cost of radiation therapy for cancer patients in Taiwan. SciRes. 2013;5:989-993.
4. Andreyev HJ. GI consequences of cancer treatment: a clinical perspective. Radiat Res. 2016;185:341-348.
5. Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the Vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92:1663-1670.
6. Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016 Aug 5;8:CD003034.
7. ASGE. The role of endoscopy in patients with anorectal disorders. Gastrointest Endosc. 2010;72:1117-1123.
8. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014:5:15-29.
9. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-143.
10. Theiss VS, Sripadam R, Ramani V, et al. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83.
11. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-384.
12. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198.
13. Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: a practical review of an often overlooked entity. Am Med Surg (Lond). 2017;15:9-13.
14. Baumann J, Lin M, Patel C. An unusual case of gastritis and duodenitis after yttrium 90-microsphere selective internal radiation. Clin Gastroenterol Hepatol. 2015;13:xxiii-xxiv.
15. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016 Apr 28;4:CD005005.
16. Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54-59.
17. Marshall GT, Thirlby RC, Bredfelt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
18. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473.
19. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-120.
20. Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812.
21. Agarwalla A, Small AJ, Mendelson AH, et al. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912.
22. Kaasa S, Mastekaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol. 1988;27:343-349.
23. Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822-828.
24. Lewin K, Mills RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21-26.
25. Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128-134.
26. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361-365.
27. Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Phys. 2005;62:1464-1471.
28. Zimmer T, Böcker U, Wang F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? A short review. Z Gastroenterol. 2008;46:441-448.
29. Kita H, Yamamoto H, Yano T, et al. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small bowel intestinal disorders. Inflammopharmacology. 2007;15:74-77.
30. Girvent M, Carlson GL, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198-201.
31. Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89.
32. Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
33. Chun M, Kang S, Kil HJ, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Phys. 2004;58:98-105.
34. Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85-91.
35. Villavicencia RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
36. Dall’Era MA, Hampson NB, His RA, et al. Hyperbaric oxygen therapy for radiation-induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
37. Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359-365.
38. Gilinsky NH, Kottler RE. Idiopathic obstructive eosinophilic enteritis with raised IgE: response to oral disodium cromoglycate. Postgrad Med J. 1982;58:239-243.
39. Tabibian JH, Kochman ML. Over-the-wire technique to facilitate over-the-scope clip closure of fistulae. Gastrointest Endosc. 2017;85:454-455.
40. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140.
1. Walsh D. Deep tissue traumatism from roentgen ray exposure. Brit Med J. 1897;2:272-273.
2. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11:403-409.
3. Leung HWC, Chan ALF. Direct medical cost of radiation therapy for cancer patients in Taiwan. SciRes. 2013;5:989-993.
4. Andreyev HJ. GI consequences of cancer treatment: a clinical perspective. Radiat Res. 2016;185:341-348.
5. Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the Vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92:1663-1670.
6. Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016 Aug 5;8:CD003034.
7. ASGE. The role of endoscopy in patients with anorectal disorders. Gastrointest Endosc. 2010;72:1117-1123.
8. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014:5:15-29.
9. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-143.
10. Theiss VS, Sripadam R, Ramani V, et al. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83.
11. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-384.
12. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198.
13. Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: a practical review of an often overlooked entity. Am Med Surg (Lond). 2017;15:9-13.
14. Baumann J, Lin M, Patel C. An unusual case of gastritis and duodenitis after yttrium 90-microsphere selective internal radiation. Clin Gastroenterol Hepatol. 2015;13:xxiii-xxiv.
15. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016 Apr 28;4:CD005005.
16. Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54-59.
17. Marshall GT, Thirlby RC, Bredfelt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
18. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473.
19. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-120.
20. Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812.
21. Agarwalla A, Small AJ, Mendelson AH, et al. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912.
22. Kaasa S, Mastekaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol. 1988;27:343-349.
23. Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822-828.
24. Lewin K, Mills RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21-26.
25. Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128-134.
26. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361-365.
27. Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Phys. 2005;62:1464-1471.
28. Zimmer T, Böcker U, Wang F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? A short review. Z Gastroenterol. 2008;46:441-448.
29. Kita H, Yamamoto H, Yano T, et al. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small bowel intestinal disorders. Inflammopharmacology. 2007;15:74-77.
30. Girvent M, Carlson GL, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198-201.
31. Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89.
32. Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
33. Chun M, Kang S, Kil HJ, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Phys. 2004;58:98-105.
34. Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85-91.
35. Villavicencia RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
36. Dall’Era MA, Hampson NB, His RA, et al. Hyperbaric oxygen therapy for radiation-induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
37. Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359-365.
38. Gilinsky NH, Kottler RE. Idiopathic obstructive eosinophilic enteritis with raised IgE: response to oral disodium cromoglycate. Postgrad Med J. 1982;58:239-243.
39. Tabibian JH, Kochman ML. Over-the-wire technique to facilitate over-the-scope clip closure of fistulae. Gastrointest Endosc. 2017;85:454-455.
40. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140.
PRACTICE RECOMMENDATIONS
› Correlate the patient’s symptoms with the radiation therapy history to determine if the onset, anatomical location, and nature of the symptoms suggest a (causal) relationship. B
› Refer patients for radiographic, endoscopic, or other diagnostic modalities according to the suspected pathology and treat (eg, pharmacologically, endoscopically, or surgically) when possible. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Advance care planning: Making it easier for patients (and you)
With the number of aging Americans projected to grow dramatically in the next several years, the need for primary palliative care and advance care planning (ACP) is more important than ever. Patients and their families want and expect palliative care when needed, but initial conversations about ACP can be difficult for them. Appropriate timing in raising this subject and clear communication can give patients the opportunity, while they are still independent, to set their goals for medical care.
For the past several decades, political decisions and judicial cases have shaped palliative care as we know it today. And its shape is still evolving. In support of ACP, advocacy groups at a national level are developing models that practitioners can use to engage patients in setting goals. And Medicare is now reimbursing primary care providers for this work that they have been doing for years (although many still may not be billing for the service).
Finally, the busy primary care office may have its own set of challenges in addressing ACP. Our aim in this review is to identify the barriers we face and the solutions we can implement to make a difference in our patients’ end-of-life care planning.
[polldaddy:9795119]
Landmark events have defined advance care planning today
In 1969, Luis Kutner, an Illinois attorney, proposed the idea of a “living will,” envisioned as a document specifying the types of treatment a person would be willing to receive were they unable at a later time to participate in making a decision.1 In 1976, California became the first state to give living wills the power of the law through the Natural Death Act.2
Throughout the 1970s and '80s, several high-profile court cases brought this idea into the national spotlight. In 1975, the New Jersey Supreme Court granted the parents of 21-year-old Karen Ann Quinlan the right to discontinue the treatment sustaining her in a persistent vegetative state. Ms. Quinlan was removed from the ventilator and lived 9 more months before dying in a nursing home.
A few years later, Nancy Cruzan, a 32-year-old woman involved in a 1983 motor vehicle accident, was also in a persistent vegetative state and remained so until 1988 when her parents asked that her feeding tube be removed. The hospital refused, indicating that it would lead to her death. The family sued and the case eventually went to the US Supreme Court in 1989.
In a 5-to-4 decision, the Supreme Court ruled that a state was legally able to require “clear and convincing evidence” of a patient’s wish for removal of life-sustaining therapies. Cruzan’s family was able to provide such evidence and her artificial nutrition was withheld. She died 12 days later.
The Cruzan case was instrumental in furthering ACP, leading to the passage of the Patient Self Determination Act (PSDA) by Congress in 1990. All federally funded health care facilities were now required to educate patients of their rights in determining their medical care and to ask about advance directives.3 The ACP movement gained additional momentum from the landmark SUPPORT study that documented shortcomings in communication between physicians and patients/families about treatment preferences and end-of-life care in US hospitals.4
In the Terri Schiavo case, the patient’s husband disagreed with the life-sustaining decisions of his wife’s parents given her persistent vegetative state and the fact that she had no chance of meaningful recovery. After a prolonged national debate, it was ultimately decided that the husband could elect to withhold artificial nutrition. (She died in 2005.) The Schiavo case, as well as the Institute of Medicine’s report on “Dying in America,”5 influenced Congress in 2016 to pass legislation funding ACP conversations.
The demonstrated benefits of advance care planning
When done comprehensively, ACP yields many benefits for patients and families and for the health care system. A systematic review demonstrated that, despite the few studies examining the economic cost of ACP, the process may lead to decreased health care costs in certain populations (nursing home residents, community dwelling adults with dementia, and those living in high health care spending regions) and at the very least does not increase health care costs.6 ACP has increased the number of do-not-resuscitate orders7 and has decreased hospitalizations,8 admissions to intensive care units,7,8 and rates of cardiopulmonary resuscitation,7,8 mechanical ventilation,7,8 and use of tube feeding.8
More noteworthy than the decrease in resource utilization and potential cost savings is the impact that ACP can have on a patient’s quality of life. Patients who receive aggressive care at the end of life tend to experience decreased quality of life compared with those receiving hospice care.7 Quality-of-life scores for patients in hospice improved with the length of enrollment in that care.7 When ACP discussions have taken place, the care patients receive at the end of life tends to conform more closely to their wishes and to increase family satisfaction.9-11
One reason that practitioners often give for not completing ACP is the fear of increasing patient or family anxiety or post-traumatic stress disorder (PTSD). However, studies have shown this concern to be unfounded.7,12 While ACP studies have not shown a decrease in rates of anxiety or PTSD, no study has shown an increase in these psychological morbidities.8
Caveats to keep in mind. Not all studies have shown unambiguous benefits related to ACP. Among the systematic reviews previously noted, there was significant variability in quality of data. Additionally, some experts argue that the traditional view of ACP (ie, completion of a single advance directive/living will) is outdated and should be replaced with a method that prepares patients and families to anticipate “in-the-moment decision making.”13 While we still believe that completion of an advance directive is useful, the experts’ point is well taken, especially since many patients change their preferences over time (and typically towards more aggressive care).14,15 While the advance directive serves a role, it is more important to help patients recognize their goals and preferences and to facilitate ongoing discussions between the patient and their families/surrogate decision maker and providers.
A snapshot of participation in advance care planning
Despite the ACP movement and the likely benefits associated with it, most individuals have not participated. Rates of completion do seem to be rising, but there is still room for improvement. Among all individuals older than 18 years, only 26.3% have an advance directive.16 In a cohort of older patients seen in an emergency department, only 40% had a living will, while nearly 54% had a designated health care power of attorney.17 Perhaps more alarming is the lack of ACP for those patients almost all physicians would agree need it—the long-term care population. The National Center for Health Statistics has reported that only 28% of home health care patients, 65% of nursing home residents, and 88% of hospice patients have an advance directive on file.18
Physician and patient barriers to advance care planning
If ACP can decrease resource utilization and improve caregiver compliance with a patient’s wishes for end of life, the obvious question is: Why isn’t it done more often? A longstanding barrier for physicians has been that these types of discussions are time intensive and have not been billable. However, since January 1, 2016, we are now able to bill for these discussions. (More on this in a bit.) Physicians do cite other barriers, though.
A recent systematic review showed that ACP is hindered by time constraints imposed by other clinical and administrative tasks that are heavily monitored.19 Barriers to engaging in ACP reported by patients include a reluctance to think about dying, a belief that family or physicians will know what to do, difficulty understanding ACP forms, and the absence of a person who can serve as a surrogate decision-maker.20,21
There are national models to help with implementation
The percentage of individuals with an advance directive in the United States has not increased significantly over the past decade.22 The lack of traction in completion and use of advance directives has lead several authors to question the utility of this older model of ACP.22 Several experts in the field believe that more robust, ongoing goals-of-care conversations between patients, families, and providers are equally, or even more, important than the completion of actual advance directive documents.23,24
National models such as the POLST (Physician Orders for Life-Sustaining Treatment) paradigm have become popular in several states (http://www.polst.org). Intended for those with estimated life expectancy of less than one year, POLST is not an advance directive but a physician order for these seriously ill patients. Emergency medical service workers are legally able to follow a POLST document but not a living will or advance directive—a significant reason for those with end-stage illness to consider completing a POLST document with their health care provider. Programs such as, “Respecting Choices,” have incorporated POLST documentation as part of ongoing goals-of-care conversations between patients and health care providers (http://www.gundersenhealth.org/respecting-choices).
Many groups have developed products to encourage patients and their families to initiate conversations at home. An example is the Conversation Project, a free online resource available in multiple languages that can help break the ice for patients and get them talking about their wishes for end-of-life care (http://www.theconversationproject.org). It poses simple stimulating questions such as: “What kind of role do you want to have in the decision-making process?” and “What are your concerns about treatment?”
How-to tips for advance care planning in the outpatient setting
When approaching the topic of ACP with patients, it’s important to do so over time, starting as soon as possible with older patients and those with chronic illness conferring a high risk of significant morbidity or mortality. Assess each patient’s understanding of ACP and readiness to discuss the topic. Many patients think of ACP in the context of a document (eg, living will), so asking about the existence of a living will may help to start the conversation. Alternatively, consider inquiring about whether the patient has had experience with family or friends at the end of life or during a difficult medical situation, and whether the patient has thought about making personal plans for such a situation.25
When a patient is ready to have this conversation, your goal should be three-fold: 26
- Help the patient articulate personal values, goals, and preferences.
- Ask the patient
to formally assign health care power of attorney (POA) to a trusted individual or to name a surrogate decision-maker. Document this decision in the medical record. - Help the patient translate expressed values into specific medical care plans, if applicable.
Because ACP conversations are often time consuming, it’s a good idea to schedule separate appointments to focus on this alone. If, however, a patient is unable to return for a dedicated ACP visit, a first step that can be completed in a reasonably short period would be choosing a surrogate decision-maker.
Helping a patient articulate personal values may be eased by asking such questions as: “Have you ever thought about what kind of care you would want if the time came when you could not make your own decisions?” or “What worries you the most about possibly not being able to make your own decisions?”27 If the patient is able to identify a surrogate decision maker before the ACP appointment, ask that this person attend. A family member or close friend may remember instances in which the patient expressed health care preferences, and their presence can help to minimize gaps in communication.
Once the patient’s preferences are clear, document them in the medical record. Some preferences may be suitable for translation into POLST orders or an advance directive, but this is less important than the overall discussion. ACP should be an ongoing conversation, since a patient’s goals may change over time. And encourage the patient to share any desired change in plans with their surrogate decision-
Be sure to bill for advance care planning services
To encourage office-based providers to conduct ACP, CMS implemented payment for CPT codes 99497 and 99498.
CPT code 99497 covers the first 30 minutes of face-to-face time with patients or their family members or medical decision-makers. This time can be used to discuss living wills or advance directives.
CPT code 99498 can be applied to each additional 30 minutes of ACP services. Typically, this billing code would be used as an add-on for a particular diagnosis such as heart failure, chronic obstructive pulmonary disease, or pancreatic cancer.
CPT Code 99497 equates to 2.40 relative-value units (RVU) with an estimated payment of $85.99, while CPT code 99498 equates to 2.09 RVU with an estimated payment of $74.88.28
According to CMS, there is no annual limit to the number of times the ACP codes can be billed for a particular patient. And there are no restrictions regarding location of service, meaning a provider could perform this in an outpatient setting, an inpatient setting, or a long-term care facility. Both physicians and non-physician practitioners are allowed to bill with this code. Also worth noting: You don’t need to complete any particular documentation for a visit to be billed as an ACP service. CMS provides a helpful Q & A at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-Advance-Care-Planning.pdf.
CORRESPONDENCE
John Liantonio, MD, Thomas Jefferson University Hospital, Department of Family and Community Medicine, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected]
1. Kutner L. Due process of euthanasia: the living will, a proposal. Indiana Law J. 1969;44:539-554.
2. California Law Revision Commission. 2000 Health Care Decisions Law and Revised Power of Attorney Law. Available at: http://www.clrc.ca.gov/pub/Printed-Reports/Pub208.pdf. Accessed May 15, 2017.
3. H.R. 5067 - 101st Congress. Patient Self Determination Act of 1990. Available at: https://www.govtrack.us/congress/bills/101/hr5067. Accessed November 16, 2016
4. The SUPPORT Principle Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274:1591-1598.
5. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015.
6. Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: systematic review of evidence. Palliat Med. 2015;29:869-884.
7. Wright AA, Ray A, Mack JW, et al. Associations between end-of-life discussions, patient mental-health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
8. Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28:1000-1025.
9. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345.
10. Morrison RS, Chichin E, Carter J, et al. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatr Soc. 2005;53:290-294.
11. Schamp R, Tenkku L. Managed death in a PACE: pathways in present and advance directives. J Am Med Dir Assoc. 2006;7:339-344.
12. Walczak A, Butow PN, Bu S, et al. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Educ Couns. 2016;99:3-16.
13. Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153:256-261.
14. Straton JB, Wang NY, Meoni LA, et al. Physical functioning, depression, and preferences for treatment at the end of life: the Johns Hopkins Precursors study. J Am Geriatr Soc. 2004;52:577-582.
15. Fried TR, Byers AL, Gallo WT, et al. Prospective study of health status preferences and changes in preferences over time in older adults. Arch Intern Med. 2006;166:890-895.
16. Rao JK, Anderson LA, Lin F, et al. Completion of advance directives among U.S. consumers. Am J Prev Med. 2014;46:65-70.
17. Grudzen CR, Buonocore P, Steinberg J, et al; AAHPM Research Committee Writing Group. Concordance of advance care plans with inpatient directives in the electronic medical record for older patients admitted from the emergency department. J Pain Symptom Manage. 2016;51:647-651.
18. Jones AL, Moss AJ, Harris-Kojetin LD. Use of advance directives in long-term care populations. NCHS Data Brief. 2011;(54):1-8.
19. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10:e0116629.
20. Fried TR, Bullock K, Iannone L, et al. Understanding advance care planning as a process of health behavior change. J Am Geriatr Soc. 2009;57:1547-1555.
21. Schickedanz AD, Schillinger D, Landefeld CS, et al. A clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57:31-39.
22. Winter L, Parks SM, Diamond JJ. Ask a different question, get a different answer: why living wills are poor guides to care preferences at the end of life. J Pall Med. 2010;13:567-572.
23. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Available at: https://www.nap.edu/read/18748/chapter/1. Accessed May 15, 2017.
24. Sudore RL, Schickedanz AD, Landefeld CS, et al. Engagement in multiple steps of the advance care planning process: a descriptive study of diverse older adults. J Am Geriatr Soc. 2008;56:1006-1013.
25. McMahan RD, Knight SJ, Fried TR, et al. Advance care planning beyond advance directives: perspectives from patients and surrogates. J Pain Symptom Manage. 2013;46:355-365.
26. Lum HD, Sudore RL, Bekelman DB. Advance care planning in the elderly. Med Clin North Am. 2015;99:391-403.
27. Lum HD, Sudore RL. Advance care planning and goals of care communication in older adults with cardiovascular disease and multi-morbidity. Clin Geriatr Med. 2016;32:247-260.
28. American College of Physicians. Advanced Care Planning: Implementation for practices. Available at: https://www.acponline.org/system/files/documents/practice-resources/business-resources/payment/advance_care_planning_toolkit.pdf. Accessed May 15, 2017.
With the number of aging Americans projected to grow dramatically in the next several years, the need for primary palliative care and advance care planning (ACP) is more important than ever. Patients and their families want and expect palliative care when needed, but initial conversations about ACP can be difficult for them. Appropriate timing in raising this subject and clear communication can give patients the opportunity, while they are still independent, to set their goals for medical care.
For the past several decades, political decisions and judicial cases have shaped palliative care as we know it today. And its shape is still evolving. In support of ACP, advocacy groups at a national level are developing models that practitioners can use to engage patients in setting goals. And Medicare is now reimbursing primary care providers for this work that they have been doing for years (although many still may not be billing for the service).
Finally, the busy primary care office may have its own set of challenges in addressing ACP. Our aim in this review is to identify the barriers we face and the solutions we can implement to make a difference in our patients’ end-of-life care planning.
[polldaddy:9795119]
Landmark events have defined advance care planning today
In 1969, Luis Kutner, an Illinois attorney, proposed the idea of a “living will,” envisioned as a document specifying the types of treatment a person would be willing to receive were they unable at a later time to participate in making a decision.1 In 1976, California became the first state to give living wills the power of the law through the Natural Death Act.2
Throughout the 1970s and '80s, several high-profile court cases brought this idea into the national spotlight. In 1975, the New Jersey Supreme Court granted the parents of 21-year-old Karen Ann Quinlan the right to discontinue the treatment sustaining her in a persistent vegetative state. Ms. Quinlan was removed from the ventilator and lived 9 more months before dying in a nursing home.
A few years later, Nancy Cruzan, a 32-year-old woman involved in a 1983 motor vehicle accident, was also in a persistent vegetative state and remained so until 1988 when her parents asked that her feeding tube be removed. The hospital refused, indicating that it would lead to her death. The family sued and the case eventually went to the US Supreme Court in 1989.
In a 5-to-4 decision, the Supreme Court ruled that a state was legally able to require “clear and convincing evidence” of a patient’s wish for removal of life-sustaining therapies. Cruzan’s family was able to provide such evidence and her artificial nutrition was withheld. She died 12 days later.
The Cruzan case was instrumental in furthering ACP, leading to the passage of the Patient Self Determination Act (PSDA) by Congress in 1990. All federally funded health care facilities were now required to educate patients of their rights in determining their medical care and to ask about advance directives.3 The ACP movement gained additional momentum from the landmark SUPPORT study that documented shortcomings in communication between physicians and patients/families about treatment preferences and end-of-life care in US hospitals.4
In the Terri Schiavo case, the patient’s husband disagreed with the life-sustaining decisions of his wife’s parents given her persistent vegetative state and the fact that she had no chance of meaningful recovery. After a prolonged national debate, it was ultimately decided that the husband could elect to withhold artificial nutrition. (She died in 2005.) The Schiavo case, as well as the Institute of Medicine’s report on “Dying in America,”5 influenced Congress in 2016 to pass legislation funding ACP conversations.
The demonstrated benefits of advance care planning
When done comprehensively, ACP yields many benefits for patients and families and for the health care system. A systematic review demonstrated that, despite the few studies examining the economic cost of ACP, the process may lead to decreased health care costs in certain populations (nursing home residents, community dwelling adults with dementia, and those living in high health care spending regions) and at the very least does not increase health care costs.6 ACP has increased the number of do-not-resuscitate orders7 and has decreased hospitalizations,8 admissions to intensive care units,7,8 and rates of cardiopulmonary resuscitation,7,8 mechanical ventilation,7,8 and use of tube feeding.8
More noteworthy than the decrease in resource utilization and potential cost savings is the impact that ACP can have on a patient’s quality of life. Patients who receive aggressive care at the end of life tend to experience decreased quality of life compared with those receiving hospice care.7 Quality-of-life scores for patients in hospice improved with the length of enrollment in that care.7 When ACP discussions have taken place, the care patients receive at the end of life tends to conform more closely to their wishes and to increase family satisfaction.9-11
One reason that practitioners often give for not completing ACP is the fear of increasing patient or family anxiety or post-traumatic stress disorder (PTSD). However, studies have shown this concern to be unfounded.7,12 While ACP studies have not shown a decrease in rates of anxiety or PTSD, no study has shown an increase in these psychological morbidities.8
Caveats to keep in mind. Not all studies have shown unambiguous benefits related to ACP. Among the systematic reviews previously noted, there was significant variability in quality of data. Additionally, some experts argue that the traditional view of ACP (ie, completion of a single advance directive/living will) is outdated and should be replaced with a method that prepares patients and families to anticipate “in-the-moment decision making.”13 While we still believe that completion of an advance directive is useful, the experts’ point is well taken, especially since many patients change their preferences over time (and typically towards more aggressive care).14,15 While the advance directive serves a role, it is more important to help patients recognize their goals and preferences and to facilitate ongoing discussions between the patient and their families/surrogate decision maker and providers.
A snapshot of participation in advance care planning
Despite the ACP movement and the likely benefits associated with it, most individuals have not participated. Rates of completion do seem to be rising, but there is still room for improvement. Among all individuals older than 18 years, only 26.3% have an advance directive.16 In a cohort of older patients seen in an emergency department, only 40% had a living will, while nearly 54% had a designated health care power of attorney.17 Perhaps more alarming is the lack of ACP for those patients almost all physicians would agree need it—the long-term care population. The National Center for Health Statistics has reported that only 28% of home health care patients, 65% of nursing home residents, and 88% of hospice patients have an advance directive on file.18
Physician and patient barriers to advance care planning
If ACP can decrease resource utilization and improve caregiver compliance with a patient’s wishes for end of life, the obvious question is: Why isn’t it done more often? A longstanding barrier for physicians has been that these types of discussions are time intensive and have not been billable. However, since January 1, 2016, we are now able to bill for these discussions. (More on this in a bit.) Physicians do cite other barriers, though.
A recent systematic review showed that ACP is hindered by time constraints imposed by other clinical and administrative tasks that are heavily monitored.19 Barriers to engaging in ACP reported by patients include a reluctance to think about dying, a belief that family or physicians will know what to do, difficulty understanding ACP forms, and the absence of a person who can serve as a surrogate decision-maker.20,21
There are national models to help with implementation
The percentage of individuals with an advance directive in the United States has not increased significantly over the past decade.22 The lack of traction in completion and use of advance directives has lead several authors to question the utility of this older model of ACP.22 Several experts in the field believe that more robust, ongoing goals-of-care conversations between patients, families, and providers are equally, or even more, important than the completion of actual advance directive documents.23,24
National models such as the POLST (Physician Orders for Life-Sustaining Treatment) paradigm have become popular in several states (http://www.polst.org). Intended for those with estimated life expectancy of less than one year, POLST is not an advance directive but a physician order for these seriously ill patients. Emergency medical service workers are legally able to follow a POLST document but not a living will or advance directive—a significant reason for those with end-stage illness to consider completing a POLST document with their health care provider. Programs such as, “Respecting Choices,” have incorporated POLST documentation as part of ongoing goals-of-care conversations between patients and health care providers (http://www.gundersenhealth.org/respecting-choices).
Many groups have developed products to encourage patients and their families to initiate conversations at home. An example is the Conversation Project, a free online resource available in multiple languages that can help break the ice for patients and get them talking about their wishes for end-of-life care (http://www.theconversationproject.org). It poses simple stimulating questions such as: “What kind of role do you want to have in the decision-making process?” and “What are your concerns about treatment?”
How-to tips for advance care planning in the outpatient setting
When approaching the topic of ACP with patients, it’s important to do so over time, starting as soon as possible with older patients and those with chronic illness conferring a high risk of significant morbidity or mortality. Assess each patient’s understanding of ACP and readiness to discuss the topic. Many patients think of ACP in the context of a document (eg, living will), so asking about the existence of a living will may help to start the conversation. Alternatively, consider inquiring about whether the patient has had experience with family or friends at the end of life or during a difficult medical situation, and whether the patient has thought about making personal plans for such a situation.25
When a patient is ready to have this conversation, your goal should be three-fold: 26
- Help the patient articulate personal values, goals, and preferences.
- Ask the patient
to formally assign health care power of attorney (POA) to a trusted individual or to name a surrogate decision-maker. Document this decision in the medical record. - Help the patient translate expressed values into specific medical care plans, if applicable.
Because ACP conversations are often time consuming, it’s a good idea to schedule separate appointments to focus on this alone. If, however, a patient is unable to return for a dedicated ACP visit, a first step that can be completed in a reasonably short period would be choosing a surrogate decision-maker.
Helping a patient articulate personal values may be eased by asking such questions as: “Have you ever thought about what kind of care you would want if the time came when you could not make your own decisions?” or “What worries you the most about possibly not being able to make your own decisions?”27 If the patient is able to identify a surrogate decision maker before the ACP appointment, ask that this person attend. A family member or close friend may remember instances in which the patient expressed health care preferences, and their presence can help to minimize gaps in communication.
Once the patient’s preferences are clear, document them in the medical record. Some preferences may be suitable for translation into POLST orders or an advance directive, but this is less important than the overall discussion. ACP should be an ongoing conversation, since a patient’s goals may change over time. And encourage the patient to share any desired change in plans with their surrogate decision-
Be sure to bill for advance care planning services
To encourage office-based providers to conduct ACP, CMS implemented payment for CPT codes 99497 and 99498.
CPT code 99497 covers the first 30 minutes of face-to-face time with patients or their family members or medical decision-makers. This time can be used to discuss living wills or advance directives.
CPT code 99498 can be applied to each additional 30 minutes of ACP services. Typically, this billing code would be used as an add-on for a particular diagnosis such as heart failure, chronic obstructive pulmonary disease, or pancreatic cancer.
CPT Code 99497 equates to 2.40 relative-value units (RVU) with an estimated payment of $85.99, while CPT code 99498 equates to 2.09 RVU with an estimated payment of $74.88.28
According to CMS, there is no annual limit to the number of times the ACP codes can be billed for a particular patient. And there are no restrictions regarding location of service, meaning a provider could perform this in an outpatient setting, an inpatient setting, or a long-term care facility. Both physicians and non-physician practitioners are allowed to bill with this code. Also worth noting: You don’t need to complete any particular documentation for a visit to be billed as an ACP service. CMS provides a helpful Q & A at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-Advance-Care-Planning.pdf.
CORRESPONDENCE
John Liantonio, MD, Thomas Jefferson University Hospital, Department of Family and Community Medicine, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected]
With the number of aging Americans projected to grow dramatically in the next several years, the need for primary palliative care and advance care planning (ACP) is more important than ever. Patients and their families want and expect palliative care when needed, but initial conversations about ACP can be difficult for them. Appropriate timing in raising this subject and clear communication can give patients the opportunity, while they are still independent, to set their goals for medical care.
For the past several decades, political decisions and judicial cases have shaped palliative care as we know it today. And its shape is still evolving. In support of ACP, advocacy groups at a national level are developing models that practitioners can use to engage patients in setting goals. And Medicare is now reimbursing primary care providers for this work that they have been doing for years (although many still may not be billing for the service).
Finally, the busy primary care office may have its own set of challenges in addressing ACP. Our aim in this review is to identify the barriers we face and the solutions we can implement to make a difference in our patients’ end-of-life care planning.
[polldaddy:9795119]
Landmark events have defined advance care planning today
In 1969, Luis Kutner, an Illinois attorney, proposed the idea of a “living will,” envisioned as a document specifying the types of treatment a person would be willing to receive were they unable at a later time to participate in making a decision.1 In 1976, California became the first state to give living wills the power of the law through the Natural Death Act.2
Throughout the 1970s and '80s, several high-profile court cases brought this idea into the national spotlight. In 1975, the New Jersey Supreme Court granted the parents of 21-year-old Karen Ann Quinlan the right to discontinue the treatment sustaining her in a persistent vegetative state. Ms. Quinlan was removed from the ventilator and lived 9 more months before dying in a nursing home.
A few years later, Nancy Cruzan, a 32-year-old woman involved in a 1983 motor vehicle accident, was also in a persistent vegetative state and remained so until 1988 when her parents asked that her feeding tube be removed. The hospital refused, indicating that it would lead to her death. The family sued and the case eventually went to the US Supreme Court in 1989.
In a 5-to-4 decision, the Supreme Court ruled that a state was legally able to require “clear and convincing evidence” of a patient’s wish for removal of life-sustaining therapies. Cruzan’s family was able to provide such evidence and her artificial nutrition was withheld. She died 12 days later.
The Cruzan case was instrumental in furthering ACP, leading to the passage of the Patient Self Determination Act (PSDA) by Congress in 1990. All federally funded health care facilities were now required to educate patients of their rights in determining their medical care and to ask about advance directives.3 The ACP movement gained additional momentum from the landmark SUPPORT study that documented shortcomings in communication between physicians and patients/families about treatment preferences and end-of-life care in US hospitals.4
In the Terri Schiavo case, the patient’s husband disagreed with the life-sustaining decisions of his wife’s parents given her persistent vegetative state and the fact that she had no chance of meaningful recovery. After a prolonged national debate, it was ultimately decided that the husband could elect to withhold artificial nutrition. (She died in 2005.) The Schiavo case, as well as the Institute of Medicine’s report on “Dying in America,”5 influenced Congress in 2016 to pass legislation funding ACP conversations.
The demonstrated benefits of advance care planning
When done comprehensively, ACP yields many benefits for patients and families and for the health care system. A systematic review demonstrated that, despite the few studies examining the economic cost of ACP, the process may lead to decreased health care costs in certain populations (nursing home residents, community dwelling adults with dementia, and those living in high health care spending regions) and at the very least does not increase health care costs.6 ACP has increased the number of do-not-resuscitate orders7 and has decreased hospitalizations,8 admissions to intensive care units,7,8 and rates of cardiopulmonary resuscitation,7,8 mechanical ventilation,7,8 and use of tube feeding.8
More noteworthy than the decrease in resource utilization and potential cost savings is the impact that ACP can have on a patient’s quality of life. Patients who receive aggressive care at the end of life tend to experience decreased quality of life compared with those receiving hospice care.7 Quality-of-life scores for patients in hospice improved with the length of enrollment in that care.7 When ACP discussions have taken place, the care patients receive at the end of life tends to conform more closely to their wishes and to increase family satisfaction.9-11
One reason that practitioners often give for not completing ACP is the fear of increasing patient or family anxiety or post-traumatic stress disorder (PTSD). However, studies have shown this concern to be unfounded.7,12 While ACP studies have not shown a decrease in rates of anxiety or PTSD, no study has shown an increase in these psychological morbidities.8
Caveats to keep in mind. Not all studies have shown unambiguous benefits related to ACP. Among the systematic reviews previously noted, there was significant variability in quality of data. Additionally, some experts argue that the traditional view of ACP (ie, completion of a single advance directive/living will) is outdated and should be replaced with a method that prepares patients and families to anticipate “in-the-moment decision making.”13 While we still believe that completion of an advance directive is useful, the experts’ point is well taken, especially since many patients change their preferences over time (and typically towards more aggressive care).14,15 While the advance directive serves a role, it is more important to help patients recognize their goals and preferences and to facilitate ongoing discussions between the patient and their families/surrogate decision maker and providers.
A snapshot of participation in advance care planning
Despite the ACP movement and the likely benefits associated with it, most individuals have not participated. Rates of completion do seem to be rising, but there is still room for improvement. Among all individuals older than 18 years, only 26.3% have an advance directive.16 In a cohort of older patients seen in an emergency department, only 40% had a living will, while nearly 54% had a designated health care power of attorney.17 Perhaps more alarming is the lack of ACP for those patients almost all physicians would agree need it—the long-term care population. The National Center for Health Statistics has reported that only 28% of home health care patients, 65% of nursing home residents, and 88% of hospice patients have an advance directive on file.18
Physician and patient barriers to advance care planning
If ACP can decrease resource utilization and improve caregiver compliance with a patient’s wishes for end of life, the obvious question is: Why isn’t it done more often? A longstanding barrier for physicians has been that these types of discussions are time intensive and have not been billable. However, since January 1, 2016, we are now able to bill for these discussions. (More on this in a bit.) Physicians do cite other barriers, though.
A recent systematic review showed that ACP is hindered by time constraints imposed by other clinical and administrative tasks that are heavily monitored.19 Barriers to engaging in ACP reported by patients include a reluctance to think about dying, a belief that family or physicians will know what to do, difficulty understanding ACP forms, and the absence of a person who can serve as a surrogate decision-maker.20,21
There are national models to help with implementation
The percentage of individuals with an advance directive in the United States has not increased significantly over the past decade.22 The lack of traction in completion and use of advance directives has lead several authors to question the utility of this older model of ACP.22 Several experts in the field believe that more robust, ongoing goals-of-care conversations between patients, families, and providers are equally, or even more, important than the completion of actual advance directive documents.23,24
National models such as the POLST (Physician Orders for Life-Sustaining Treatment) paradigm have become popular in several states (http://www.polst.org). Intended for those with estimated life expectancy of less than one year, POLST is not an advance directive but a physician order for these seriously ill patients. Emergency medical service workers are legally able to follow a POLST document but not a living will or advance directive—a significant reason for those with end-stage illness to consider completing a POLST document with their health care provider. Programs such as, “Respecting Choices,” have incorporated POLST documentation as part of ongoing goals-of-care conversations between patients and health care providers (http://www.gundersenhealth.org/respecting-choices).
Many groups have developed products to encourage patients and their families to initiate conversations at home. An example is the Conversation Project, a free online resource available in multiple languages that can help break the ice for patients and get them talking about their wishes for end-of-life care (http://www.theconversationproject.org). It poses simple stimulating questions such as: “What kind of role do you want to have in the decision-making process?” and “What are your concerns about treatment?”
How-to tips for advance care planning in the outpatient setting
When approaching the topic of ACP with patients, it’s important to do so over time, starting as soon as possible with older patients and those with chronic illness conferring a high risk of significant morbidity or mortality. Assess each patient’s understanding of ACP and readiness to discuss the topic. Many patients think of ACP in the context of a document (eg, living will), so asking about the existence of a living will may help to start the conversation. Alternatively, consider inquiring about whether the patient has had experience with family or friends at the end of life or during a difficult medical situation, and whether the patient has thought about making personal plans for such a situation.25
When a patient is ready to have this conversation, your goal should be three-fold: 26
- Help the patient articulate personal values, goals, and preferences.
- Ask the patient
to formally assign health care power of attorney (POA) to a trusted individual or to name a surrogate decision-maker. Document this decision in the medical record. - Help the patient translate expressed values into specific medical care plans, if applicable.
Because ACP conversations are often time consuming, it’s a good idea to schedule separate appointments to focus on this alone. If, however, a patient is unable to return for a dedicated ACP visit, a first step that can be completed in a reasonably short period would be choosing a surrogate decision-maker.
Helping a patient articulate personal values may be eased by asking such questions as: “Have you ever thought about what kind of care you would want if the time came when you could not make your own decisions?” or “What worries you the most about possibly not being able to make your own decisions?”27 If the patient is able to identify a surrogate decision maker before the ACP appointment, ask that this person attend. A family member or close friend may remember instances in which the patient expressed health care preferences, and their presence can help to minimize gaps in communication.
Once the patient’s preferences are clear, document them in the medical record. Some preferences may be suitable for translation into POLST orders or an advance directive, but this is less important than the overall discussion. ACP should be an ongoing conversation, since a patient’s goals may change over time. And encourage the patient to share any desired change in plans with their surrogate decision-
Be sure to bill for advance care planning services
To encourage office-based providers to conduct ACP, CMS implemented payment for CPT codes 99497 and 99498.
CPT code 99497 covers the first 30 minutes of face-to-face time with patients or their family members or medical decision-makers. This time can be used to discuss living wills or advance directives.
CPT code 99498 can be applied to each additional 30 minutes of ACP services. Typically, this billing code would be used as an add-on for a particular diagnosis such as heart failure, chronic obstructive pulmonary disease, or pancreatic cancer.
CPT Code 99497 equates to 2.40 relative-value units (RVU) with an estimated payment of $85.99, while CPT code 99498 equates to 2.09 RVU with an estimated payment of $74.88.28
According to CMS, there is no annual limit to the number of times the ACP codes can be billed for a particular patient. And there are no restrictions regarding location of service, meaning a provider could perform this in an outpatient setting, an inpatient setting, or a long-term care facility. Both physicians and non-physician practitioners are allowed to bill with this code. Also worth noting: You don’t need to complete any particular documentation for a visit to be billed as an ACP service. CMS provides a helpful Q & A at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-Advance-Care-Planning.pdf.
CORRESPONDENCE
John Liantonio, MD, Thomas Jefferson University Hospital, Department of Family and Community Medicine, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected]
1. Kutner L. Due process of euthanasia: the living will, a proposal. Indiana Law J. 1969;44:539-554.
2. California Law Revision Commission. 2000 Health Care Decisions Law and Revised Power of Attorney Law. Available at: http://www.clrc.ca.gov/pub/Printed-Reports/Pub208.pdf. Accessed May 15, 2017.
3. H.R. 5067 - 101st Congress. Patient Self Determination Act of 1990. Available at: https://www.govtrack.us/congress/bills/101/hr5067. Accessed November 16, 2016
4. The SUPPORT Principle Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274:1591-1598.
5. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015.
6. Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: systematic review of evidence. Palliat Med. 2015;29:869-884.
7. Wright AA, Ray A, Mack JW, et al. Associations between end-of-life discussions, patient mental-health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
8. Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28:1000-1025.
9. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345.
10. Morrison RS, Chichin E, Carter J, et al. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatr Soc. 2005;53:290-294.
11. Schamp R, Tenkku L. Managed death in a PACE: pathways in present and advance directives. J Am Med Dir Assoc. 2006;7:339-344.
12. Walczak A, Butow PN, Bu S, et al. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Educ Couns. 2016;99:3-16.
13. Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153:256-261.
14. Straton JB, Wang NY, Meoni LA, et al. Physical functioning, depression, and preferences for treatment at the end of life: the Johns Hopkins Precursors study. J Am Geriatr Soc. 2004;52:577-582.
15. Fried TR, Byers AL, Gallo WT, et al. Prospective study of health status preferences and changes in preferences over time in older adults. Arch Intern Med. 2006;166:890-895.
16. Rao JK, Anderson LA, Lin F, et al. Completion of advance directives among U.S. consumers. Am J Prev Med. 2014;46:65-70.
17. Grudzen CR, Buonocore P, Steinberg J, et al; AAHPM Research Committee Writing Group. Concordance of advance care plans with inpatient directives in the electronic medical record for older patients admitted from the emergency department. J Pain Symptom Manage. 2016;51:647-651.
18. Jones AL, Moss AJ, Harris-Kojetin LD. Use of advance directives in long-term care populations. NCHS Data Brief. 2011;(54):1-8.
19. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10:e0116629.
20. Fried TR, Bullock K, Iannone L, et al. Understanding advance care planning as a process of health behavior change. J Am Geriatr Soc. 2009;57:1547-1555.
21. Schickedanz AD, Schillinger D, Landefeld CS, et al. A clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57:31-39.
22. Winter L, Parks SM, Diamond JJ. Ask a different question, get a different answer: why living wills are poor guides to care preferences at the end of life. J Pall Med. 2010;13:567-572.
23. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Available at: https://www.nap.edu/read/18748/chapter/1. Accessed May 15, 2017.
24. Sudore RL, Schickedanz AD, Landefeld CS, et al. Engagement in multiple steps of the advance care planning process: a descriptive study of diverse older adults. J Am Geriatr Soc. 2008;56:1006-1013.
25. McMahan RD, Knight SJ, Fried TR, et al. Advance care planning beyond advance directives: perspectives from patients and surrogates. J Pain Symptom Manage. 2013;46:355-365.
26. Lum HD, Sudore RL, Bekelman DB. Advance care planning in the elderly. Med Clin North Am. 2015;99:391-403.
27. Lum HD, Sudore RL. Advance care planning and goals of care communication in older adults with cardiovascular disease and multi-morbidity. Clin Geriatr Med. 2016;32:247-260.
28. American College of Physicians. Advanced Care Planning: Implementation for practices. Available at: https://www.acponline.org/system/files/documents/practice-resources/business-resources/payment/advance_care_planning_toolkit.pdf. Accessed May 15, 2017.
1. Kutner L. Due process of euthanasia: the living will, a proposal. Indiana Law J. 1969;44:539-554.
2. California Law Revision Commission. 2000 Health Care Decisions Law and Revised Power of Attorney Law. Available at: http://www.clrc.ca.gov/pub/Printed-Reports/Pub208.pdf. Accessed May 15, 2017.
3. H.R. 5067 - 101st Congress. Patient Self Determination Act of 1990. Available at: https://www.govtrack.us/congress/bills/101/hr5067. Accessed November 16, 2016
4. The SUPPORT Principle Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274:1591-1598.
5. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015.
6. Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: systematic review of evidence. Palliat Med. 2015;29:869-884.
7. Wright AA, Ray A, Mack JW, et al. Associations between end-of-life discussions, patient mental-health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
8. Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28:1000-1025.
9. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345.
10. Morrison RS, Chichin E, Carter J, et al. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatr Soc. 2005;53:290-294.
11. Schamp R, Tenkku L. Managed death in a PACE: pathways in present and advance directives. J Am Med Dir Assoc. 2006;7:339-344.
12. Walczak A, Butow PN, Bu S, et al. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Educ Couns. 2016;99:3-16.
13. Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153:256-261.
14. Straton JB, Wang NY, Meoni LA, et al. Physical functioning, depression, and preferences for treatment at the end of life: the Johns Hopkins Precursors study. J Am Geriatr Soc. 2004;52:577-582.
15. Fried TR, Byers AL, Gallo WT, et al. Prospective study of health status preferences and changes in preferences over time in older adults. Arch Intern Med. 2006;166:890-895.
16. Rao JK, Anderson LA, Lin F, et al. Completion of advance directives among U.S. consumers. Am J Prev Med. 2014;46:65-70.
17. Grudzen CR, Buonocore P, Steinberg J, et al; AAHPM Research Committee Writing Group. Concordance of advance care plans with inpatient directives in the electronic medical record for older patients admitted from the emergency department. J Pain Symptom Manage. 2016;51:647-651.
18. Jones AL, Moss AJ, Harris-Kojetin LD. Use of advance directives in long-term care populations. NCHS Data Brief. 2011;(54):1-8.
19. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10:e0116629.
20. Fried TR, Bullock K, Iannone L, et al. Understanding advance care planning as a process of health behavior change. J Am Geriatr Soc. 2009;57:1547-1555.
21. Schickedanz AD, Schillinger D, Landefeld CS, et al. A clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57:31-39.
22. Winter L, Parks SM, Diamond JJ. Ask a different question, get a different answer: why living wills are poor guides to care preferences at the end of life. J Pall Med. 2010;13:567-572.
23. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Available at: https://www.nap.edu/read/18748/chapter/1. Accessed May 15, 2017.
24. Sudore RL, Schickedanz AD, Landefeld CS, et al. Engagement in multiple steps of the advance care planning process: a descriptive study of diverse older adults. J Am Geriatr Soc. 2008;56:1006-1013.
25. McMahan RD, Knight SJ, Fried TR, et al. Advance care planning beyond advance directives: perspectives from patients and surrogates. J Pain Symptom Manage. 2013;46:355-365.
26. Lum HD, Sudore RL, Bekelman DB. Advance care planning in the elderly. Med Clin North Am. 2015;99:391-403.
27. Lum HD, Sudore RL. Advance care planning and goals of care communication in older adults with cardiovascular disease and multi-morbidity. Clin Geriatr Med. 2016;32:247-260.
28. American College of Physicians. Advanced Care Planning: Implementation for practices. Available at: https://www.acponline.org/system/files/documents/practice-resources/business-resources/payment/advance_care_planning_toolkit.pdf. Accessed May 15, 2017.
PRACTICE RECOMMENDATIONS
› Schedule visits dedicated to advance care planning (ACP) to remove time barriers and ensure that ACP is completed. C
› Give priority to identifying a health care representative. C
› Bill Centers for Medicare and Medicaid Services (CMS) for primary care ACP visits with CPT codes 99497 and 99498. Most private insurers are following CMS recommendations. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Deprescribing: A simple method for reducing polypharmacy
CASE An 82-year-old woman with a history of hypertension, diabetes, hyperlipidemia, stage 3 chronic kidney disease, anxiety, urge urinary incontinence, constipation, and bilateral knee osteoarthritis presents to her primary care physician’s office after a fall. She reports that she visited the emergency department (ED) a week ago after falling in the middle of the night on her way to the bathroom. This is the third fall she’s had this year. On chart review, she had a blood pressure (BP) of 112/60 mm Hg and a blood glucose level of 65 mg/dL in the ED. All other testing (head imaging, chest x-ray, urinalysis) was normal. The ED physician recommended that she stop taking her lisinopril-hydrochlorothiazide (HCTZ) and glipizide extended release (XL) until her follow-up appointment. Today, she asks about the need to restart these medications.
Polypharmacy is common among older adults due to a high prevalence of chronic conditions that often require multiple medications for optimal management. Cut points of 5 or 9 medications are frequently used to define polypharmacy. However, some define polypharmacy as taking a medication that lacks an indication, is ineffective, or is duplicating treatment provided by another medication.
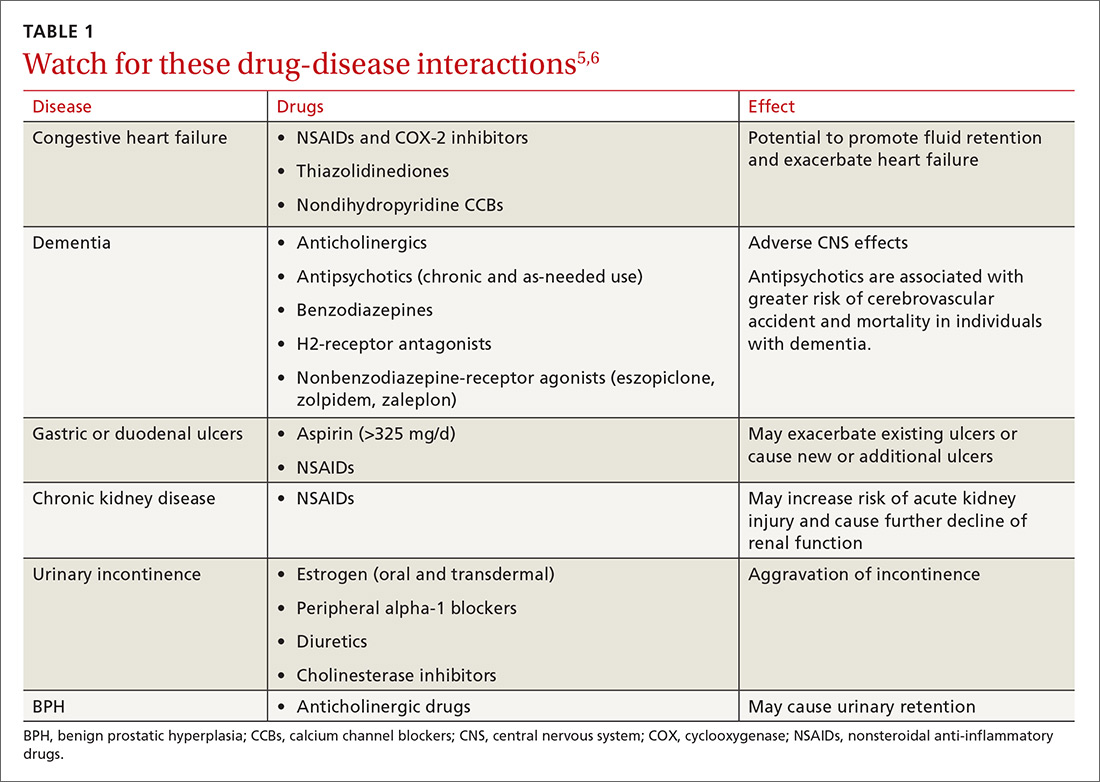
Either way, polypharmacy is associated with multiple negative consequences, including an increased risk for adverse drug events (ADEs),1-4 drug-drug and drug-disease interactions (TABLE 15,6),7 reduced functional capacity,8 multiple geriatric syndromes (TABLE 25,9-12), medication non-adherence,13 and increased mortality.14 Polypharmacy also contributes to increased health care costs for both the patient and the health care system.15

Taking a step back. Polypharmacy often results from prescribing cascades, which occur when an adverse drug effect is misinterpreted as a new medical problem, leading to the prescribing of more medication to treat the initial drug-induced symptom. Potentially inappropriate medications (PIMs), which are medications that should be avoided in older adults and in those with certain conditions, are also more likely to be prescribed in the setting of polypharmacy.16
Deprescribing is the process of identifying and discontinuing medications that are unnecessary, ineffective, and/or inappropriate in order to reduce polypharmacy and improve health outcomes. Deprescribing is a collaborative process that involves weighing the benefits and harms of medications in the context of a patient’s care goals, current level of functioning, life expectancy, values, and preferences. This article reviews polypharmacy and discusses safe and effective deprescribing strategies for older adults in the primary care setting.
[polldaddy:9781245]
How many people on how many meds?
According to a 2016 study, 36% of community-dwelling older adults (ages 62-85 years) were taking 5 or more prescription medications in 2010 to 2011—up from 31% in 2005 to 2006.17 When one narrows the population to older adults in the United States who are hospitalized, almost half (46%) take 7 or more medications.18 Among frail, older US veterans at hospital discharge, 40% were prescribed 9 or more medications, with 44% of these patients receiving at least one unnecessary drug.19
The challenges of multimorbidity
In the United States, 80% of those 65 and older have 2 or more chronic conditions, or multimorbidity.20 Clinical practice guidelines making recommendations for the management of single conditions, such as heart failure, hypertension, or diabetes, often suggest the use of 2 or more medications to achieve optimal management and fail to provide guidance in the setting of multimorbidity. Following treatment recommendations for multiple conditions predictably leads to polypharmacy, with complicated, costly, and burdensome regimens.
Further, the research contributing to the development of clinical practice guidelines frequently excludes older adults and those with multimorbidity, reducing applicability in this population. As a result, many treatment recommendations have uncertain benefit and may be harmful in the multimorbid older patient.21
CASE In addition to the patient’s multimorbidity, she had a stroke at age 73 and has some mild residual left-sided weakness. Functionally, she is independent and able to perform her activities of daily living and her instrumental activities of daily living. She lives alone, quit smoking at age 65, and has an occasional glass of wine during family parties. The patient’s daughter and granddaughter live 2 blocks away.
Her current medications include glipizide XL 10 mg/d and lisinopril-HCTZ 20-25 mg/d, which she has temporarily discontinued at the ED doctor’s recommendation, as well as: amlodipine 10 mg/d, metformin 1000 mg BID, senna 8.6 mg/d, docusate 100 mg BID, furosemide 40 mg/d, and ibuprofen 600 mg/d (for knee pain). She reports taking omeprazole 20 mg/d “for almost 20 years,” even though she has not had any reflux symptoms in recent memory. After her stroke, she began taking atorvastatin 10 mg/d, aspirin 81 mg/d, and clopidogrel 75 mg/d, which she continues to take today. About a year ago, she started oxybutynin 5 mg/d for urinary incontinence, but she has not noticed significant relief. Additionally, she takes lorazepam 1 mg for insomnia most nights of the week.

A review of systems reveals issues with chronic constipation and intermittent dizziness, but is otherwise negative. The physical examination reveals a well-appearing woman with a body mass index of 26. Her temperature is 98.5° F, her heart rate is 78 beats/min and regular, her respirations are 14 breaths/min, and her BP is 117/65 mm Hg. Orthostatic testing is negative. Her heart, lung, and abdominal exams are within normal limits. Her timed up and go test is 14 seconds. Her blood glucose level today in the office after eating breakfast 2 hours ago is 135 mg/dL (normal: <140 mg/dL). Laboratory tests performed at the time of the ED visit show a creatinine level of 1.2 mg/dL (normal range: 0.6 to 1.1 mg/dL), a glomerular filtration rate (GFR) of 44 units (normal range: >60 units), a hemoglobin level of 9.8 g/dL (normal range: 12-15.5 g/dL), and a thyroid stimulating hormone level of 1.4 mIU/L (normal range: 0.5-8.9 mIU/L). A recent hemoglobin A1C is 6.8% (normal: <5.7%), low-density lipoprotein (LDL) level is 103 mg/dL (optimal <100 mg/dL), and high-density lipoprotein (HDL) level is 65 mg/dL (optimal >60 mg/dL). An echocardiogram performed a year ago showed mild aortic stenosis with normal systolic and diastolic function.
Starting the deprescribing process: Several approaches to choose from
The goal of deprescribing is to reduce polypharmacy and improve health outcomes. It is a process defined as, “reviewing all current medications; identifying medications to be ceased, substituted, or reduced; planning a deprescribing regimen in partnership with the patient; and frequently reviewing and supporting the patient.”22 A medication review should include prescription, over-the-counter (OTC), and complementary/alternative medicine (CAM) agents.
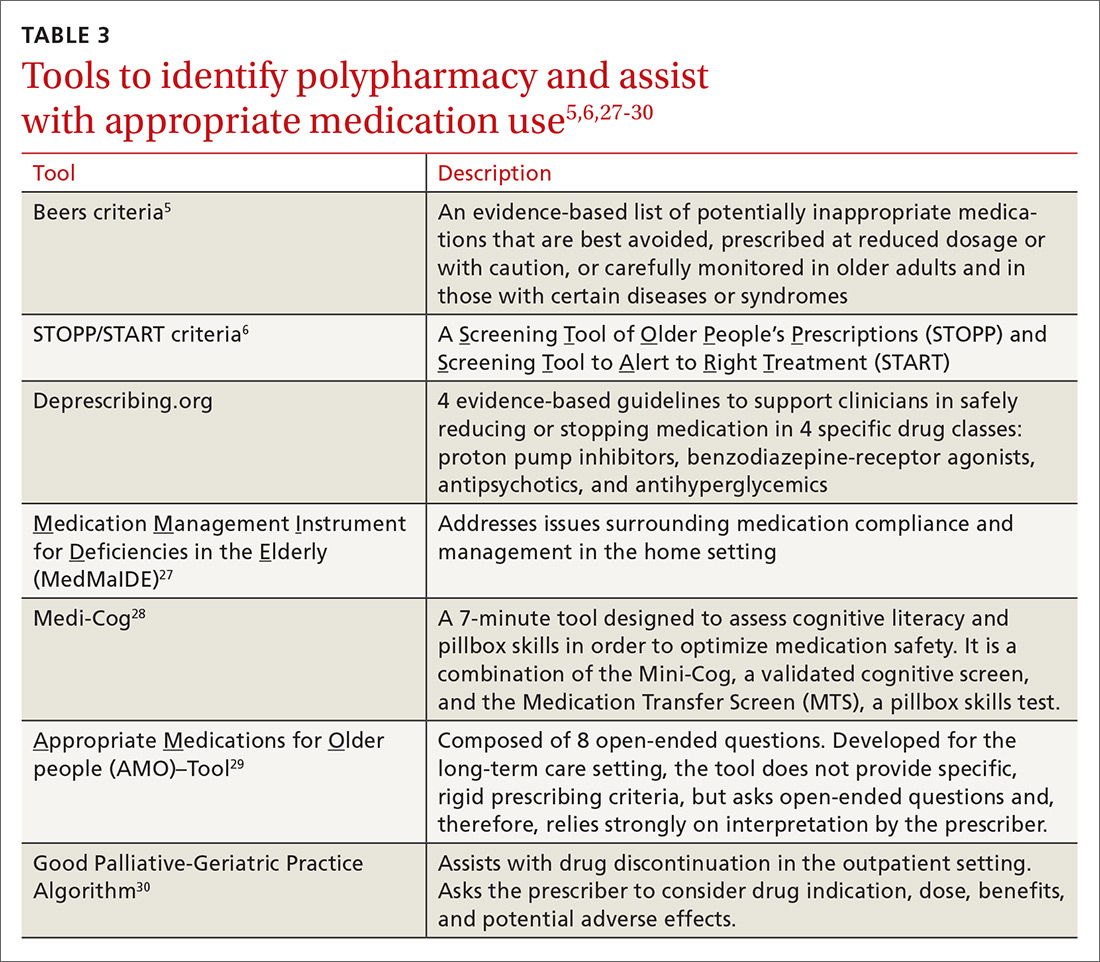
Until recently, studies evaluating the process of deprescribing across drug classes and disease conditions were limited, but new research is beginning to show its potential impact. After deprescribing, patients experience fewer falls and show improvements in cognition.23 While there have not yet been large randomized trials to evaluate deprescribing, a recent systematic review and meta-analysis showed that use of patient-specific deprescribing interventions is associated with improved survival.24 Importantly, there have been no reported adverse drug withdrawal events or deaths associated with deprescribing.23

Smaller studies have reported additional benefits including decreases in health care costs, reductions in drug-drug interactions and PIMs, improvements in medication adherence, and increases in patient satisfaction.25 In addition, the removal of unnecessary medications may allow for increased consideration of prescribing appropriate medications with known benefit.25
Practically speaking, every encounter between a patient and health care provider is an opportunity to reduce unnecessary medications. Electronic alert systems at pharmacies and those embedded within electronic health record (EHR) systems can also prompt a medication review and an effort to deprescribe.26 Evidence-based tools to identify polypharmacy and guide appropriate medication use are listed in TABLE 3.5,6,27-30 In addition, suggested approaches to beginning the deprescribing process are included in TABLE 4.5,31-33 And a medication class-based approach to deprescribing is provided in TABLE 5.5,34-45
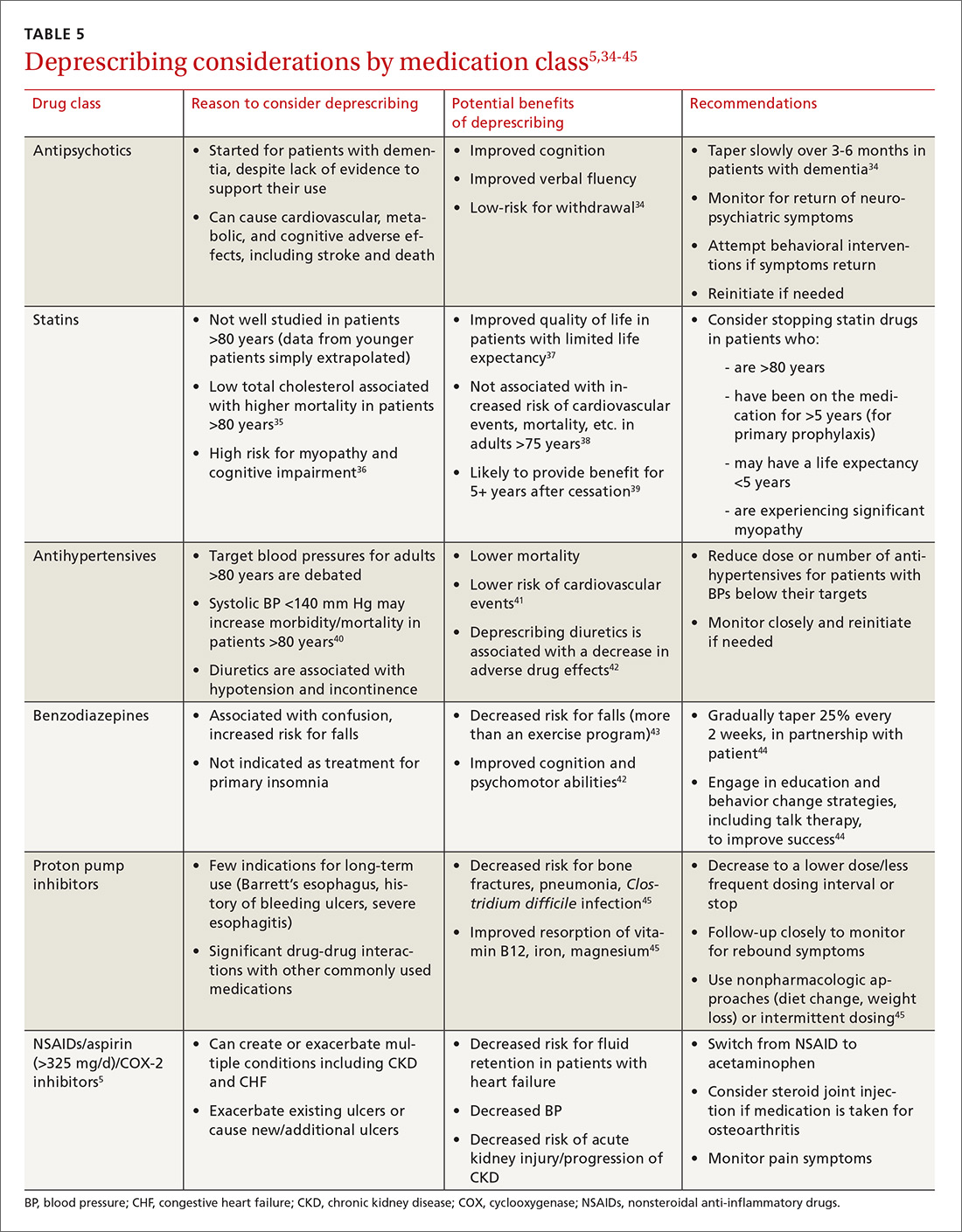
Although no gold standard process exists for deprescribing, experts suggest that any deprescribing protocol should include the following steps:32,46
1. Start with a “brown bag” review of the patient’s medications.
Have the patient bring all of his/her medications in a bag to the visit; review them together or have the medication history taken by a pharmacist. Determine and discuss the indication for each medication and its effectiveness for that indication. Consider the potential benefits and harms of each medication in the context of the patient’s care goals and preferences. Assess whether the patient is taking all of the medications that have been prescribed, and identify any reasons for missed pills (eg, adverse effects, dosing regimens, understanding, cognitive issues).
2. Talk to the patient about the deprescribing process.
Talk with the patient about the risks and benefits of deprescribing, and prioritize which medications to address in the process. Prioritize the medications by balancing patient preferences with available pharmacologic evidence. If there is a lack of evidence supporting the benefits for a particular medication, consider known or suspected adverse effects, the ease or burden of the dosing regimen, the patient’s preferences and goals of care, remaining life expectancy, the time until drug benefit is appreciated, and the length of drug benefit after discontinuation.
3. Deprescribe medications.
If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a condition.
Acknowledging potential barriers to deprescribing may help structure conversations and provide anticipatory guidance to patients and their families. Working to overcome these barriers will help maximize the benefits of deprescribing and help to build trust with patients.
Patient-driven barriers include fear of a condition worsening or returning, lack of a suitable alternative, lack of ongoing support to manage a particular condition, a previous bad experience with medication cessation, and influence from other care providers (eg, family, home caregivers, nurses, specialists, friends). Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementia.47 Utilizing a team-based and stepwise patient approach to deprescribing aims to provide hesitant patients with appropriate amounts of education and support to begin to reduce unnecessary medicines.
Provider-driven barriers include feeling uneasy about contradicting a specialist’s recommendations for initiation/continuation of specific medications, fear of causing withdrawal symptoms or disease relapse, and lack of specific data to adequately understand and assess benefits and harms in the older adult population. Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or “downgraded.”48 Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue them.
One way to overcome some of these concerns is to consider working with a clinical pharmacist. By gaining information regarding medication-specific factors, such as half-life and expected withdrawal patterns, you can feel more confident deprescribing or continuing medications.
Additionally, communicating closely with specialists, ideally with the help of an integrated EHR, can allow you to discuss indications for particular medications or concerns about adverse effects, limited benefits, or difficulty with compliance, so that you can develop a collaborative, cohesive, and patient-centered plan. This, in turn, may improve patient understanding and compliance.
4. Create a follow-up plan.
At the time of deprescribing a medication, develop a plan with the patient for monitoring and assessment. Ensure that the patient understands which symptoms may occur in the event of drug withdrawal and which symptoms may suggest the return of a condition. Make sure that other supports are in place if needed (eg, cognitive behavioral therapy, physical therapy, social support or assistance) to help ensure that medication cessation is successful.
CASE During the office visit, you advise the patient that her BP looks normal, her blood sugar is within an appropriate range, and she is lucky to have not sustained any injuries after her most recent fall. In addition to discussing the benefits of some outpatient physical therapy to help with her balance, you ask if she would like to discuss reducing her medications. She is agreeable and asks for your recommendations.
You are aware of several resources that can help you with your recommendations, among them the STOPP/START6 and Beers criteria,5 as well as the Good Geriatric-Palliative Algorithm.30
If you were to use the STOPP/START and Beers criteria, you might consider stopping:
- lorazepam, which increases the risk of falls and confusion.
- ibuprofen, since this patient has only mild osteoarthritis pain, and ibuprofen has the potential for renal, cardiac, and gastrointestinal toxicities.
- oxybutynin, because it could be contributing to the patient’s constipation and cause confusion and falls.
- furosemide, since the patient has no clinical heart failure.
- omeprazole, since the indication is unknown and the patient has no history of ulceration, esophagitis, or symptomatic gastroesophageal reflux disease.
After reviewing the Good Geriatric-Palliative Algorithm,30 you might consider stopping:
- clopidogrel, as there is no clear indication for this medication in combination with aspirin in this patient.
- glipizide XL, as this patient’s A1c is below goal and this medication puts her at risk of hypoglycemia and its associated morbidities.
- metformin, as it increases her risk of lactic acidosis because her GFR is <45 units.
- docusate, as the evidence to show clear benefit in improving chronic constipation in older adults is lacking.
You tell your patient that there are multiple medications to consider stopping. In order to monitor any symptoms of withdrawal or return of a condition, it would be best to stop one at a time and follow-up closely. Since she has done well for the past week without the glipizide and lisinopril-HCTZ combination, she can remain off the glipizide and the HCTZ. Lisinopril, however, may provide renal protection in the setting of diabetes and will be continued at this time.
You ask her about adverse effects from her other medications. She indicates that the furosemide makes her run to the bathroom all the time, so she would like to try stopping it. You agree and make a plan for her to monitor her weight, watch for edema, and return in 4 weeks for a follow-up visit.
On follow-up, she is feeling well, has no edema on exam, and is happy to report her urinary incontinence has resolved. You therefore suggest her next deprescribing trial be discontinuation of her oxybutynin. She thanks you for your recommendations about her medications and heads off to her physical therapy appointment.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Division of Geriatric Medicine and Palliative Care, Thomas Jefferson University, 2422 S Broad St, 2nd Floor, Philadelphia, PA 19145; [email protected].
1. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901-910.
2. Nair NP, Chalmers L, Peterson GM, et al. Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin Interv Aging. 2016;11:497-506.
3. Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006; 4:36-41.
4. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666-671.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218.
7. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186.
8. Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470-484.
9. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
10. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-595.
11. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician. 1998;57:2675-2694.
12. Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129:753, E1-E6.
13. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
14. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170-175.
15. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010; 8:146-160.
16. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-1523.
17. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
18. Flaherty JH, Perry HM 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:554-559.
19. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
20. Gerteis J, Izrael D, Deitz D, et al. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality. 2014.
21. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1-E25.
22. Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323-328.
23. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
24. Page AT, Clifford RM, Potter K, et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:583-623.
25. Reeve E, Shakib S, Hendrix I, et al. The benefits and harms of deprescribing. Med J Aust. 2014;201:386-389.
26. Walsh K, Kwan D, Marr P, et al. Deprescribing in a family health team: a study of chronic proton pump inhibitor use. J Prim Health Care. 2016;8:164-171.
27. Orwig D, Brandt N, Gruber-Baldini AL. Medication management assessment for older adults in the community. Gerontologist. 2006;46:661-668.
28. Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23:459-472.
29. Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8-14.
30. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9:430-434.
31. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175:701-702.
32. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
33. Guirguis-Blake JM, Evans CV,Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
34. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
35. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing. 2010;39:674-680.
36. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396-399.
37. Goldstein MR, Mascitelli L, Pezzetta F. Statin therapy in the elderly: misconceptions. J Am Geriatr Soc. 2008;56:1365.
38. Han BH, Sutin D, Williamson JD, et al, for the ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. Published online May 22, 2017.
39. Sever PS, Chang CL, Gupta AK, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525-2532.
40. Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719-726.
41. Ekbom T, Lindholm LH, Oden A, et al. A 5‐year prospective, observational study of the withdrawal of antihypertensive treatment in elderly people. J Intern Med. 1994;235:581-588.
42. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging. 2008;25:1021-1031.
43. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850-853.
44. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16:19.
45. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Can Fam Phys. 2017; 63:354-364.
46. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24:37-42.
47. Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56.
48. Scott I, Anderson K, Freeman CR, et al. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201:390-392.
CASE An 82-year-old woman with a history of hypertension, diabetes, hyperlipidemia, stage 3 chronic kidney disease, anxiety, urge urinary incontinence, constipation, and bilateral knee osteoarthritis presents to her primary care physician’s office after a fall. She reports that she visited the emergency department (ED) a week ago after falling in the middle of the night on her way to the bathroom. This is the third fall she’s had this year. On chart review, she had a blood pressure (BP) of 112/60 mm Hg and a blood glucose level of 65 mg/dL in the ED. All other testing (head imaging, chest x-ray, urinalysis) was normal. The ED physician recommended that she stop taking her lisinopril-hydrochlorothiazide (HCTZ) and glipizide extended release (XL) until her follow-up appointment. Today, she asks about the need to restart these medications.
Polypharmacy is common among older adults due to a high prevalence of chronic conditions that often require multiple medications for optimal management. Cut points of 5 or 9 medications are frequently used to define polypharmacy. However, some define polypharmacy as taking a medication that lacks an indication, is ineffective, or is duplicating treatment provided by another medication.

Either way, polypharmacy is associated with multiple negative consequences, including an increased risk for adverse drug events (ADEs),1-4 drug-drug and drug-disease interactions (TABLE 15,6),7 reduced functional capacity,8 multiple geriatric syndromes (TABLE 25,9-12), medication non-adherence,13 and increased mortality.14 Polypharmacy also contributes to increased health care costs for both the patient and the health care system.15

Taking a step back. Polypharmacy often results from prescribing cascades, which occur when an adverse drug effect is misinterpreted as a new medical problem, leading to the prescribing of more medication to treat the initial drug-induced symptom. Potentially inappropriate medications (PIMs), which are medications that should be avoided in older adults and in those with certain conditions, are also more likely to be prescribed in the setting of polypharmacy.16
Deprescribing is the process of identifying and discontinuing medications that are unnecessary, ineffective, and/or inappropriate in order to reduce polypharmacy and improve health outcomes. Deprescribing is a collaborative process that involves weighing the benefits and harms of medications in the context of a patient’s care goals, current level of functioning, life expectancy, values, and preferences. This article reviews polypharmacy and discusses safe and effective deprescribing strategies for older adults in the primary care setting.
[polldaddy:9781245]
How many people on how many meds?
According to a 2016 study, 36% of community-dwelling older adults (ages 62-85 years) were taking 5 or more prescription medications in 2010 to 2011—up from 31% in 2005 to 2006.17 When one narrows the population to older adults in the United States who are hospitalized, almost half (46%) take 7 or more medications.18 Among frail, older US veterans at hospital discharge, 40% were prescribed 9 or more medications, with 44% of these patients receiving at least one unnecessary drug.19
The challenges of multimorbidity
In the United States, 80% of those 65 and older have 2 or more chronic conditions, or multimorbidity.20 Clinical practice guidelines making recommendations for the management of single conditions, such as heart failure, hypertension, or diabetes, often suggest the use of 2 or more medications to achieve optimal management and fail to provide guidance in the setting of multimorbidity. Following treatment recommendations for multiple conditions predictably leads to polypharmacy, with complicated, costly, and burdensome regimens.
Further, the research contributing to the development of clinical practice guidelines frequently excludes older adults and those with multimorbidity, reducing applicability in this population. As a result, many treatment recommendations have uncertain benefit and may be harmful in the multimorbid older patient.21
CASE In addition to the patient’s multimorbidity, she had a stroke at age 73 and has some mild residual left-sided weakness. Functionally, she is independent and able to perform her activities of daily living and her instrumental activities of daily living. She lives alone, quit smoking at age 65, and has an occasional glass of wine during family parties. The patient’s daughter and granddaughter live 2 blocks away.
Her current medications include glipizide XL 10 mg/d and lisinopril-HCTZ 20-25 mg/d, which she has temporarily discontinued at the ED doctor’s recommendation, as well as: amlodipine 10 mg/d, metformin 1000 mg BID, senna 8.6 mg/d, docusate 100 mg BID, furosemide 40 mg/d, and ibuprofen 600 mg/d (for knee pain). She reports taking omeprazole 20 mg/d “for almost 20 years,” even though she has not had any reflux symptoms in recent memory. After her stroke, she began taking atorvastatin 10 mg/d, aspirin 81 mg/d, and clopidogrel 75 mg/d, which she continues to take today. About a year ago, she started oxybutynin 5 mg/d for urinary incontinence, but she has not noticed significant relief. Additionally, she takes lorazepam 1 mg for insomnia most nights of the week.

A review of systems reveals issues with chronic constipation and intermittent dizziness, but is otherwise negative. The physical examination reveals a well-appearing woman with a body mass index of 26. Her temperature is 98.5° F, her heart rate is 78 beats/min and regular, her respirations are 14 breaths/min, and her BP is 117/65 mm Hg. Orthostatic testing is negative. Her heart, lung, and abdominal exams are within normal limits. Her timed up and go test is 14 seconds. Her blood glucose level today in the office after eating breakfast 2 hours ago is 135 mg/dL (normal: <140 mg/dL). Laboratory tests performed at the time of the ED visit show a creatinine level of 1.2 mg/dL (normal range: 0.6 to 1.1 mg/dL), a glomerular filtration rate (GFR) of 44 units (normal range: >60 units), a hemoglobin level of 9.8 g/dL (normal range: 12-15.5 g/dL), and a thyroid stimulating hormone level of 1.4 mIU/L (normal range: 0.5-8.9 mIU/L). A recent hemoglobin A1C is 6.8% (normal: <5.7%), low-density lipoprotein (LDL) level is 103 mg/dL (optimal <100 mg/dL), and high-density lipoprotein (HDL) level is 65 mg/dL (optimal >60 mg/dL). An echocardiogram performed a year ago showed mild aortic stenosis with normal systolic and diastolic function.
Starting the deprescribing process: Several approaches to choose from
The goal of deprescribing is to reduce polypharmacy and improve health outcomes. It is a process defined as, “reviewing all current medications; identifying medications to be ceased, substituted, or reduced; planning a deprescribing regimen in partnership with the patient; and frequently reviewing and supporting the patient.”22 A medication review should include prescription, over-the-counter (OTC), and complementary/alternative medicine (CAM) agents.

Until recently, studies evaluating the process of deprescribing across drug classes and disease conditions were limited, but new research is beginning to show its potential impact. After deprescribing, patients experience fewer falls and show improvements in cognition.23 While there have not yet been large randomized trials to evaluate deprescribing, a recent systematic review and meta-analysis showed that use of patient-specific deprescribing interventions is associated with improved survival.24 Importantly, there have been no reported adverse drug withdrawal events or deaths associated with deprescribing.23

Smaller studies have reported additional benefits including decreases in health care costs, reductions in drug-drug interactions and PIMs, improvements in medication adherence, and increases in patient satisfaction.25 In addition, the removal of unnecessary medications may allow for increased consideration of prescribing appropriate medications with known benefit.25
Practically speaking, every encounter between a patient and health care provider is an opportunity to reduce unnecessary medications. Electronic alert systems at pharmacies and those embedded within electronic health record (EHR) systems can also prompt a medication review and an effort to deprescribe.26 Evidence-based tools to identify polypharmacy and guide appropriate medication use are listed in TABLE 3.5,6,27-30 In addition, suggested approaches to beginning the deprescribing process are included in TABLE 4.5,31-33 And a medication class-based approach to deprescribing is provided in TABLE 5.5,34-45

Although no gold standard process exists for deprescribing, experts suggest that any deprescribing protocol should include the following steps:32,46
1. Start with a “brown bag” review of the patient’s medications.
Have the patient bring all of his/her medications in a bag to the visit; review them together or have the medication history taken by a pharmacist. Determine and discuss the indication for each medication and its effectiveness for that indication. Consider the potential benefits and harms of each medication in the context of the patient’s care goals and preferences. Assess whether the patient is taking all of the medications that have been prescribed, and identify any reasons for missed pills (eg, adverse effects, dosing regimens, understanding, cognitive issues).
2. Talk to the patient about the deprescribing process.
Talk with the patient about the risks and benefits of deprescribing, and prioritize which medications to address in the process. Prioritize the medications by balancing patient preferences with available pharmacologic evidence. If there is a lack of evidence supporting the benefits for a particular medication, consider known or suspected adverse effects, the ease or burden of the dosing regimen, the patient’s preferences and goals of care, remaining life expectancy, the time until drug benefit is appreciated, and the length of drug benefit after discontinuation.
3. Deprescribe medications.
If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a condition.
Acknowledging potential barriers to deprescribing may help structure conversations and provide anticipatory guidance to patients and their families. Working to overcome these barriers will help maximize the benefits of deprescribing and help to build trust with patients.
Patient-driven barriers include fear of a condition worsening or returning, lack of a suitable alternative, lack of ongoing support to manage a particular condition, a previous bad experience with medication cessation, and influence from other care providers (eg, family, home caregivers, nurses, specialists, friends). Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementia.47 Utilizing a team-based and stepwise patient approach to deprescribing aims to provide hesitant patients with appropriate amounts of education and support to begin to reduce unnecessary medicines.
Provider-driven barriers include feeling uneasy about contradicting a specialist’s recommendations for initiation/continuation of specific medications, fear of causing withdrawal symptoms or disease relapse, and lack of specific data to adequately understand and assess benefits and harms in the older adult population. Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or “downgraded.”48 Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue them.
One way to overcome some of these concerns is to consider working with a clinical pharmacist. By gaining information regarding medication-specific factors, such as half-life and expected withdrawal patterns, you can feel more confident deprescribing or continuing medications.
Additionally, communicating closely with specialists, ideally with the help of an integrated EHR, can allow you to discuss indications for particular medications or concerns about adverse effects, limited benefits, or difficulty with compliance, so that you can develop a collaborative, cohesive, and patient-centered plan. This, in turn, may improve patient understanding and compliance.
4. Create a follow-up plan.
At the time of deprescribing a medication, develop a plan with the patient for monitoring and assessment. Ensure that the patient understands which symptoms may occur in the event of drug withdrawal and which symptoms may suggest the return of a condition. Make sure that other supports are in place if needed (eg, cognitive behavioral therapy, physical therapy, social support or assistance) to help ensure that medication cessation is successful.
CASE During the office visit, you advise the patient that her BP looks normal, her blood sugar is within an appropriate range, and she is lucky to have not sustained any injuries after her most recent fall. In addition to discussing the benefits of some outpatient physical therapy to help with her balance, you ask if she would like to discuss reducing her medications. She is agreeable and asks for your recommendations.
You are aware of several resources that can help you with your recommendations, among them the STOPP/START6 and Beers criteria,5 as well as the Good Geriatric-Palliative Algorithm.30
If you were to use the STOPP/START and Beers criteria, you might consider stopping:
- lorazepam, which increases the risk of falls and confusion.
- ibuprofen, since this patient has only mild osteoarthritis pain, and ibuprofen has the potential for renal, cardiac, and gastrointestinal toxicities.
- oxybutynin, because it could be contributing to the patient’s constipation and cause confusion and falls.
- furosemide, since the patient has no clinical heart failure.
- omeprazole, since the indication is unknown and the patient has no history of ulceration, esophagitis, or symptomatic gastroesophageal reflux disease.
After reviewing the Good Geriatric-Palliative Algorithm,30 you might consider stopping:
- clopidogrel, as there is no clear indication for this medication in combination with aspirin in this patient.
- glipizide XL, as this patient’s A1c is below goal and this medication puts her at risk of hypoglycemia and its associated morbidities.
- metformin, as it increases her risk of lactic acidosis because her GFR is <45 units.
- docusate, as the evidence to show clear benefit in improving chronic constipation in older adults is lacking.
You tell your patient that there are multiple medications to consider stopping. In order to monitor any symptoms of withdrawal or return of a condition, it would be best to stop one at a time and follow-up closely. Since she has done well for the past week without the glipizide and lisinopril-HCTZ combination, she can remain off the glipizide and the HCTZ. Lisinopril, however, may provide renal protection in the setting of diabetes and will be continued at this time.
You ask her about adverse effects from her other medications. She indicates that the furosemide makes her run to the bathroom all the time, so she would like to try stopping it. You agree and make a plan for her to monitor her weight, watch for edema, and return in 4 weeks for a follow-up visit.
On follow-up, she is feeling well, has no edema on exam, and is happy to report her urinary incontinence has resolved. You therefore suggest her next deprescribing trial be discontinuation of her oxybutynin. She thanks you for your recommendations about her medications and heads off to her physical therapy appointment.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Division of Geriatric Medicine and Palliative Care, Thomas Jefferson University, 2422 S Broad St, 2nd Floor, Philadelphia, PA 19145; [email protected].
CASE An 82-year-old woman with a history of hypertension, diabetes, hyperlipidemia, stage 3 chronic kidney disease, anxiety, urge urinary incontinence, constipation, and bilateral knee osteoarthritis presents to her primary care physician’s office after a fall. She reports that she visited the emergency department (ED) a week ago after falling in the middle of the night on her way to the bathroom. This is the third fall she’s had this year. On chart review, she had a blood pressure (BP) of 112/60 mm Hg and a blood glucose level of 65 mg/dL in the ED. All other testing (head imaging, chest x-ray, urinalysis) was normal. The ED physician recommended that she stop taking her lisinopril-hydrochlorothiazide (HCTZ) and glipizide extended release (XL) until her follow-up appointment. Today, she asks about the need to restart these medications.
Polypharmacy is common among older adults due to a high prevalence of chronic conditions that often require multiple medications for optimal management. Cut points of 5 or 9 medications are frequently used to define polypharmacy. However, some define polypharmacy as taking a medication that lacks an indication, is ineffective, or is duplicating treatment provided by another medication.

Either way, polypharmacy is associated with multiple negative consequences, including an increased risk for adverse drug events (ADEs),1-4 drug-drug and drug-disease interactions (TABLE 15,6),7 reduced functional capacity,8 multiple geriatric syndromes (TABLE 25,9-12), medication non-adherence,13 and increased mortality.14 Polypharmacy also contributes to increased health care costs for both the patient and the health care system.15

Taking a step back. Polypharmacy often results from prescribing cascades, which occur when an adverse drug effect is misinterpreted as a new medical problem, leading to the prescribing of more medication to treat the initial drug-induced symptom. Potentially inappropriate medications (PIMs), which are medications that should be avoided in older adults and in those with certain conditions, are also more likely to be prescribed in the setting of polypharmacy.16
Deprescribing is the process of identifying and discontinuing medications that are unnecessary, ineffective, and/or inappropriate in order to reduce polypharmacy and improve health outcomes. Deprescribing is a collaborative process that involves weighing the benefits and harms of medications in the context of a patient’s care goals, current level of functioning, life expectancy, values, and preferences. This article reviews polypharmacy and discusses safe and effective deprescribing strategies for older adults in the primary care setting.
[polldaddy:9781245]
How many people on how many meds?
According to a 2016 study, 36% of community-dwelling older adults (ages 62-85 years) were taking 5 or more prescription medications in 2010 to 2011—up from 31% in 2005 to 2006.17 When one narrows the population to older adults in the United States who are hospitalized, almost half (46%) take 7 or more medications.18 Among frail, older US veterans at hospital discharge, 40% were prescribed 9 or more medications, with 44% of these patients receiving at least one unnecessary drug.19
The challenges of multimorbidity
In the United States, 80% of those 65 and older have 2 or more chronic conditions, or multimorbidity.20 Clinical practice guidelines making recommendations for the management of single conditions, such as heart failure, hypertension, or diabetes, often suggest the use of 2 or more medications to achieve optimal management and fail to provide guidance in the setting of multimorbidity. Following treatment recommendations for multiple conditions predictably leads to polypharmacy, with complicated, costly, and burdensome regimens.
Further, the research contributing to the development of clinical practice guidelines frequently excludes older adults and those with multimorbidity, reducing applicability in this population. As a result, many treatment recommendations have uncertain benefit and may be harmful in the multimorbid older patient.21
CASE In addition to the patient’s multimorbidity, she had a stroke at age 73 and has some mild residual left-sided weakness. Functionally, she is independent and able to perform her activities of daily living and her instrumental activities of daily living. She lives alone, quit smoking at age 65, and has an occasional glass of wine during family parties. The patient’s daughter and granddaughter live 2 blocks away.
Her current medications include glipizide XL 10 mg/d and lisinopril-HCTZ 20-25 mg/d, which she has temporarily discontinued at the ED doctor’s recommendation, as well as: amlodipine 10 mg/d, metformin 1000 mg BID, senna 8.6 mg/d, docusate 100 mg BID, furosemide 40 mg/d, and ibuprofen 600 mg/d (for knee pain). She reports taking omeprazole 20 mg/d “for almost 20 years,” even though she has not had any reflux symptoms in recent memory. After her stroke, she began taking atorvastatin 10 mg/d, aspirin 81 mg/d, and clopidogrel 75 mg/d, which she continues to take today. About a year ago, she started oxybutynin 5 mg/d for urinary incontinence, but she has not noticed significant relief. Additionally, she takes lorazepam 1 mg for insomnia most nights of the week.

A review of systems reveals issues with chronic constipation and intermittent dizziness, but is otherwise negative. The physical examination reveals a well-appearing woman with a body mass index of 26. Her temperature is 98.5° F, her heart rate is 78 beats/min and regular, her respirations are 14 breaths/min, and her BP is 117/65 mm Hg. Orthostatic testing is negative. Her heart, lung, and abdominal exams are within normal limits. Her timed up and go test is 14 seconds. Her blood glucose level today in the office after eating breakfast 2 hours ago is 135 mg/dL (normal: <140 mg/dL). Laboratory tests performed at the time of the ED visit show a creatinine level of 1.2 mg/dL (normal range: 0.6 to 1.1 mg/dL), a glomerular filtration rate (GFR) of 44 units (normal range: >60 units), a hemoglobin level of 9.8 g/dL (normal range: 12-15.5 g/dL), and a thyroid stimulating hormone level of 1.4 mIU/L (normal range: 0.5-8.9 mIU/L). A recent hemoglobin A1C is 6.8% (normal: <5.7%), low-density lipoprotein (LDL) level is 103 mg/dL (optimal <100 mg/dL), and high-density lipoprotein (HDL) level is 65 mg/dL (optimal >60 mg/dL). An echocardiogram performed a year ago showed mild aortic stenosis with normal systolic and diastolic function.
Starting the deprescribing process: Several approaches to choose from
The goal of deprescribing is to reduce polypharmacy and improve health outcomes. It is a process defined as, “reviewing all current medications; identifying medications to be ceased, substituted, or reduced; planning a deprescribing regimen in partnership with the patient; and frequently reviewing and supporting the patient.”22 A medication review should include prescription, over-the-counter (OTC), and complementary/alternative medicine (CAM) agents.

Until recently, studies evaluating the process of deprescribing across drug classes and disease conditions were limited, but new research is beginning to show its potential impact. After deprescribing, patients experience fewer falls and show improvements in cognition.23 While there have not yet been large randomized trials to evaluate deprescribing, a recent systematic review and meta-analysis showed that use of patient-specific deprescribing interventions is associated with improved survival.24 Importantly, there have been no reported adverse drug withdrawal events or deaths associated with deprescribing.23

Smaller studies have reported additional benefits including decreases in health care costs, reductions in drug-drug interactions and PIMs, improvements in medication adherence, and increases in patient satisfaction.25 In addition, the removal of unnecessary medications may allow for increased consideration of prescribing appropriate medications with known benefit.25
Practically speaking, every encounter between a patient and health care provider is an opportunity to reduce unnecessary medications. Electronic alert systems at pharmacies and those embedded within electronic health record (EHR) systems can also prompt a medication review and an effort to deprescribe.26 Evidence-based tools to identify polypharmacy and guide appropriate medication use are listed in TABLE 3.5,6,27-30 In addition, suggested approaches to beginning the deprescribing process are included in TABLE 4.5,31-33 And a medication class-based approach to deprescribing is provided in TABLE 5.5,34-45

Although no gold standard process exists for deprescribing, experts suggest that any deprescribing protocol should include the following steps:32,46
1. Start with a “brown bag” review of the patient’s medications.
Have the patient bring all of his/her medications in a bag to the visit; review them together or have the medication history taken by a pharmacist. Determine and discuss the indication for each medication and its effectiveness for that indication. Consider the potential benefits and harms of each medication in the context of the patient’s care goals and preferences. Assess whether the patient is taking all of the medications that have been prescribed, and identify any reasons for missed pills (eg, adverse effects, dosing regimens, understanding, cognitive issues).
2. Talk to the patient about the deprescribing process.
Talk with the patient about the risks and benefits of deprescribing, and prioritize which medications to address in the process. Prioritize the medications by balancing patient preferences with available pharmacologic evidence. If there is a lack of evidence supporting the benefits for a particular medication, consider known or suspected adverse effects, the ease or burden of the dosing regimen, the patient’s preferences and goals of care, remaining life expectancy, the time until drug benefit is appreciated, and the length of drug benefit after discontinuation.
3. Deprescribe medications.
If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a condition.
Acknowledging potential barriers to deprescribing may help structure conversations and provide anticipatory guidance to patients and their families. Working to overcome these barriers will help maximize the benefits of deprescribing and help to build trust with patients.
Patient-driven barriers include fear of a condition worsening or returning, lack of a suitable alternative, lack of ongoing support to manage a particular condition, a previous bad experience with medication cessation, and influence from other care providers (eg, family, home caregivers, nurses, specialists, friends). Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementia.47 Utilizing a team-based and stepwise patient approach to deprescribing aims to provide hesitant patients with appropriate amounts of education and support to begin to reduce unnecessary medicines.
Provider-driven barriers include feeling uneasy about contradicting a specialist’s recommendations for initiation/continuation of specific medications, fear of causing withdrawal symptoms or disease relapse, and lack of specific data to adequately understand and assess benefits and harms in the older adult population. Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or “downgraded.”48 Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue them.
One way to overcome some of these concerns is to consider working with a clinical pharmacist. By gaining information regarding medication-specific factors, such as half-life and expected withdrawal patterns, you can feel more confident deprescribing or continuing medications.
Additionally, communicating closely with specialists, ideally with the help of an integrated EHR, can allow you to discuss indications for particular medications or concerns about adverse effects, limited benefits, or difficulty with compliance, so that you can develop a collaborative, cohesive, and patient-centered plan. This, in turn, may improve patient understanding and compliance.
4. Create a follow-up plan.
At the time of deprescribing a medication, develop a plan with the patient for monitoring and assessment. Ensure that the patient understands which symptoms may occur in the event of drug withdrawal and which symptoms may suggest the return of a condition. Make sure that other supports are in place if needed (eg, cognitive behavioral therapy, physical therapy, social support or assistance) to help ensure that medication cessation is successful.
CASE During the office visit, you advise the patient that her BP looks normal, her blood sugar is within an appropriate range, and she is lucky to have not sustained any injuries after her most recent fall. In addition to discussing the benefits of some outpatient physical therapy to help with her balance, you ask if she would like to discuss reducing her medications. She is agreeable and asks for your recommendations.
You are aware of several resources that can help you with your recommendations, among them the STOPP/START6 and Beers criteria,5 as well as the Good Geriatric-Palliative Algorithm.30
If you were to use the STOPP/START and Beers criteria, you might consider stopping:
- lorazepam, which increases the risk of falls and confusion.
- ibuprofen, since this patient has only mild osteoarthritis pain, and ibuprofen has the potential for renal, cardiac, and gastrointestinal toxicities.
- oxybutynin, because it could be contributing to the patient’s constipation and cause confusion and falls.
- furosemide, since the patient has no clinical heart failure.
- omeprazole, since the indication is unknown and the patient has no history of ulceration, esophagitis, or symptomatic gastroesophageal reflux disease.
After reviewing the Good Geriatric-Palliative Algorithm,30 you might consider stopping:
- clopidogrel, as there is no clear indication for this medication in combination with aspirin in this patient.
- glipizide XL, as this patient’s A1c is below goal and this medication puts her at risk of hypoglycemia and its associated morbidities.
- metformin, as it increases her risk of lactic acidosis because her GFR is <45 units.
- docusate, as the evidence to show clear benefit in improving chronic constipation in older adults is lacking.
You tell your patient that there are multiple medications to consider stopping. In order to monitor any symptoms of withdrawal or return of a condition, it would be best to stop one at a time and follow-up closely. Since she has done well for the past week without the glipizide and lisinopril-HCTZ combination, she can remain off the glipizide and the HCTZ. Lisinopril, however, may provide renal protection in the setting of diabetes and will be continued at this time.
You ask her about adverse effects from her other medications. She indicates that the furosemide makes her run to the bathroom all the time, so she would like to try stopping it. You agree and make a plan for her to monitor her weight, watch for edema, and return in 4 weeks for a follow-up visit.
On follow-up, she is feeling well, has no edema on exam, and is happy to report her urinary incontinence has resolved. You therefore suggest her next deprescribing trial be discontinuation of her oxybutynin. She thanks you for your recommendations about her medications and heads off to her physical therapy appointment.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Division of Geriatric Medicine and Palliative Care, Thomas Jefferson University, 2422 S Broad St, 2nd Floor, Philadelphia, PA 19145; [email protected].
1. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901-910.
2. Nair NP, Chalmers L, Peterson GM, et al. Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin Interv Aging. 2016;11:497-506.
3. Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006; 4:36-41.
4. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666-671.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218.
7. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186.
8. Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470-484.
9. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
10. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-595.
11. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician. 1998;57:2675-2694.
12. Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129:753, E1-E6.
13. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
14. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170-175.
15. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010; 8:146-160.
16. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-1523.
17. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
18. Flaherty JH, Perry HM 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:554-559.
19. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
20. Gerteis J, Izrael D, Deitz D, et al. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality. 2014.
21. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1-E25.
22. Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323-328.
23. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
24. Page AT, Clifford RM, Potter K, et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:583-623.
25. Reeve E, Shakib S, Hendrix I, et al. The benefits and harms of deprescribing. Med J Aust. 2014;201:386-389.
26. Walsh K, Kwan D, Marr P, et al. Deprescribing in a family health team: a study of chronic proton pump inhibitor use. J Prim Health Care. 2016;8:164-171.
27. Orwig D, Brandt N, Gruber-Baldini AL. Medication management assessment for older adults in the community. Gerontologist. 2006;46:661-668.
28. Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23:459-472.
29. Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8-14.
30. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9:430-434.
31. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175:701-702.
32. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
33. Guirguis-Blake JM, Evans CV,Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
34. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
35. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing. 2010;39:674-680.
36. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396-399.
37. Goldstein MR, Mascitelli L, Pezzetta F. Statin therapy in the elderly: misconceptions. J Am Geriatr Soc. 2008;56:1365.
38. Han BH, Sutin D, Williamson JD, et al, for the ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. Published online May 22, 2017.
39. Sever PS, Chang CL, Gupta AK, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525-2532.
40. Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719-726.
41. Ekbom T, Lindholm LH, Oden A, et al. A 5‐year prospective, observational study of the withdrawal of antihypertensive treatment in elderly people. J Intern Med. 1994;235:581-588.
42. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging. 2008;25:1021-1031.
43. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850-853.
44. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16:19.
45. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Can Fam Phys. 2017; 63:354-364.
46. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24:37-42.
47. Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56.
48. Scott I, Anderson K, Freeman CR, et al. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201:390-392.
1. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901-910.
2. Nair NP, Chalmers L, Peterson GM, et al. Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin Interv Aging. 2016;11:497-506.
3. Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006; 4:36-41.
4. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666-671.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218.
7. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186.
8. Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470-484.
9. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
10. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-595.
11. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician. 1998;57:2675-2694.
12. Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129:753, E1-E6.
13. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
14. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170-175.
15. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010; 8:146-160.
16. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-1523.
17. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
18. Flaherty JH, Perry HM 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:554-559.
19. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
20. Gerteis J, Izrael D, Deitz D, et al. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality. 2014.
21. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1-E25.
22. Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323-328.
23. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
24. Page AT, Clifford RM, Potter K, et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:583-623.
25. Reeve E, Shakib S, Hendrix I, et al. The benefits and harms of deprescribing. Med J Aust. 2014;201:386-389.
26. Walsh K, Kwan D, Marr P, et al. Deprescribing in a family health team: a study of chronic proton pump inhibitor use. J Prim Health Care. 2016;8:164-171.
27. Orwig D, Brandt N, Gruber-Baldini AL. Medication management assessment for older adults in the community. Gerontologist. 2006;46:661-668.
28. Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23:459-472.
29. Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8-14.
30. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9:430-434.
31. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175:701-702.
32. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
33. Guirguis-Blake JM, Evans CV,Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
34. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
35. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing. 2010;39:674-680.
36. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396-399.
37. Goldstein MR, Mascitelli L, Pezzetta F. Statin therapy in the elderly: misconceptions. J Am Geriatr Soc. 2008;56:1365.
38. Han BH, Sutin D, Williamson JD, et al, for the ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. Published online May 22, 2017.
39. Sever PS, Chang CL, Gupta AK, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525-2532.
40. Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719-726.
41. Ekbom T, Lindholm LH, Oden A, et al. A 5‐year prospective, observational study of the withdrawal of antihypertensive treatment in elderly people. J Intern Med. 1994;235:581-588.
42. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging. 2008;25:1021-1031.
43. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850-853.
44. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16:19.
45. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Can Fam Phys. 2017; 63:354-364.
46. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24:37-42.
47. Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56.
48. Scott I, Anderson K, Freeman CR, et al. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201:390-392.
From The Journal of Family Practice | 2017;66(7):436-445.
PRACTICE RECOMMENDATIONS
› Avoid medications that are inappropriate for older adults because of adverse effects, lack of efficacy, and/or potential for interactions. A
› Discontinue medications when the harms outweigh the benefits in the context of the patient’s care goals, life expectancy, and/or preferences. C
› Utilize resources such as the STOPP/START and Beers criteria to help you decide where to begin the deprescribing process. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke: Secondary prevention of ischemic events
Patients who suffer a stroke rarely have just one vascular risk factor. Therefore, the approach to secondary stroke prevention must be multifactorial. In fact, it has been estimated that 80% of recurrent strokes could be prevented through the application of a comprehensive, multifactorial approach that includes lifestyle modification and optimal medical management.1 Such an achievement would save millions of people from disability and functional decline, as well as millions of dollars in related medical costs.
The initial approach to patients with stroke is focused on stabilization and a rapid work-up to identify the most likely etiology. Common causes of stroke include large artery atherosclerosis, cardiac emboli, and small vessel disease; less common causes include dissection, aortic emboli, and non-atherosclerotic vascular disease. If a complete diagnostic work-up is unrevealing, the stroke is said to be cryptogenic. Determining the correct etiology of a stroke is paramount to preventing secondary stroke (FIGURE2-13).
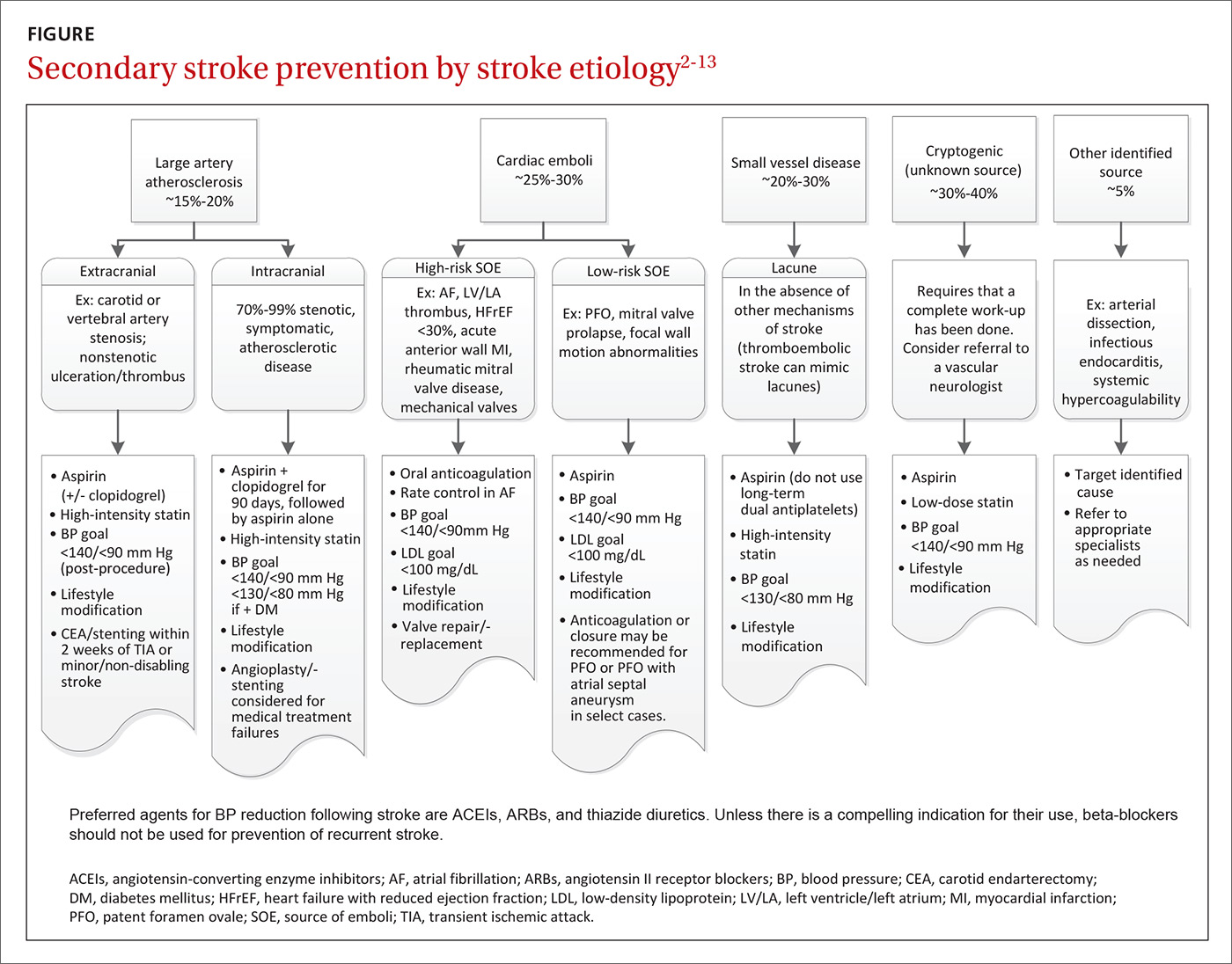
Effective secondary prevention strategies designed to prevent a stroke or transient ischemic attack (TIA) in a patient with a known history of either event include lifestyle modifications, medications, and when appropriate, mechanical interventions. As a primary care physician (PCP), you are uniquely positioned to spearhead the prevention of secondary strokes: Not only are you at the forefront of prevention and the use of techniques such as motivational interviewing, but you also have longstanding relationships with many of your patients. In fact, the success of many interventions is improved by the informed, enduring, and trusting nature of relationships between patients and their PCPs.
In the first part of this 2-part series, we focused on subacute stroke management and outlined the recommended work-up for subacute stroke/TIA (see “Stroke: A road map to subacute management,” 2017;66:366-374). In this part, we focus on secondary prevention. The more common modifiable conditions encountered in primary care are discussed here, while many of the more rare etiologies (hypercoagulable states, sickle cell disease, and vasculitis) are outside the scope of this article.
Lifestyle interventions: Target tobacco use, obesity, alcohol intake
Lifestyle modifications can have a positive impact on many of America’s most prevalent diseases, and stroke is no exception.14 Many of the disease states identified as risk factors for stroke (type 2 diabetes, hypertension, dyslipidemia) are exacerbated by tobacco use, obesity, and excessive alcohol intake.
Does your patient smoke? Up to 25% of all strokes are directly attributable to cigarette smoking.15 Smoking raises an individual’s risk for stroke in a dose-dependent fashion.15,16 One study demonstrated that, compared to never-smokers, women ages 15 to 49 years who smoked a half-pack per day had an odds ratio for ischemic stroke of 2.2; those who smoked 2 packs per day had an odds ratio of 9.1.17 After cessation, stroke risk generally returns to baseline within 5 years.16 Thus, smoking cessation is among the most significant steps a patient can take to reduce the risk of both primary and secondary stroke.
Is your patient overweight? While obesity in and of itself is a risk factor for stroke, a focus on nutrition and physical activity as mechanisms for weight loss is far superior to focusing on either element alone. Physical activity—consisting of at least 40 minutes of moderate intensity aerobic exercise 3 to 4 times per week—and a diet that emphasizes fruits and vegetables, whole grains, and healthy fats, have both independently demonstrated benefits in secondary stroke prevention and are important parts of American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.2,3
The Mediterranean Diet, which emphasizes consumption of fruits and vegetables, legumes, tree nuts, olive oil, and lean protein, has long been associated with cardiovascular benefit.18 One prospective, randomized, single-blinded trial involving approximately 600 patients that looked at secondary prevention of coronary heart disease found that following the diet significantly reduced mortality compared with a usual prudent post-infarct diet (number needed to treat [NNT]=30 over 4 years).19
Is alcohol consumption an issue? Chronic heavy alcohol intake contributes to the development of hemorrhagic and ischemic stroke through multiple mechanisms, including alcohol-induced hypertension, alcoholic cardiomyopathy, and atrial fibrillation (AF). Light or moderate alcohol consumption has a paradoxical mild protective effect on ischemic stroke, thought to possibly be mediated by an increase in high-density lipoprotein (HDL) level and mild antiplatelet effect.3
AHA/American Stroke Association (ASA) guidelines indicate that no more than one standard drink per day for women and 2 drinks per day for men is reasonable.3 Counsel patients who drink in excess of this about the benefits of decreasing alcohol intake or abstaining altogether.
Choosing medications to manage BP, cholesterol, and clotting
Optimize blood pressure control. Blood pressure (BP) plays a critical role in both the management and prevention of stroke and is considered to be the most important modifiable risk factor in both primary and secondary stroke prevention.20 In the first 24 to 48 hours following a cerebral ischemic event that is not eligible for thrombolysis, permissive hypertension (treating BP only if it exceeds 220/120 mm Hg unless there is a concurrent medical illness that requires you do so) is appropriate, as hypotension or rapid fluctuations in BP can be harmful.21
This flexibility does not continue into the subacute phase of management (at a minimum, after the initial 48 hours) or into secondary prevention. Initiation and titration of oral agents to gradually achieve a BP <140/90 mm Hg or a reduction of 10/5 mm Hg for patients already within optimal range are the most widely recognized goals.3,20 Patients with stroke secondary to small vessel disease may benefit from an even lower goal of <130/<80 mm Hg.11 Encourage patients to monitor their BP at home for added accuracy and consistency.22
Pharmacologic BP management is appropriate for patients who are consistently above optimal range despite attempting recommended lifestyle modifications. The data are relatively consistent with respect to the effects of different drug classes after a stroke: beta-blockers have no effect on any outcome; thiazide diuretics significantly reduce stroke and total vascular events; angiotensin-converting enzyme (ACE) inhibitors significantly reduce myocardial infarction (MI); and the combination of an ACE inhibitor and thiazide diuretic reduces stroke, MI, and combined vascular events.4
This has led many stroke specialists to recommend the combination of an ACE inhibitor or angiotensin II receptor blocker (ARB) and a thiazide diuretic as a first-line approach to secondary stroke prevention rather than a beta-blocker (assuming there is no additional indication for a beta-blocker). Similarly, there is ample evidence to show that the magnitude of BP reduction is proportional to the reduction in recurrent vascular events.3
Make use of statin therapy—regardless of LDL. The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial5 explored the potential role of statin medication for secondary stroke prevention. Researchers randomly assigned almost 5000 participants who’d had a stroke or TIA one to 6 months before study entry (but had no known history of coronary artery disease) to placebo or a high-intensity statin (80 mg/d atorvastatin). The statin group demonstrated a 4.9-year absolute risk reduction in fatal or nonfatal recurrent stroke of 1.9% (NNT=53).
Given these findings and those from other studies, the AHA and ASA recommend treating patients with stroke or TIA presumed to be of atherosclerotic origin with high-intensity statin therapy, regardless of low-density lipoprotein (LDL) level.3 Of note, statins are not indicated for the secondary prevention of hemorrhagic stroke.
Select antiplatelet therapy based on ischemic stroke subtype. Investigators are still trying to determine the optimal antiplatelet for secondary stroke prevention; it is likely that the ideal choice depends largely on the etiology of the stroke. Trials that did not select patients based on subtype of ischemic stroke have not shown a long-term benefit from dual antiplatelet therapy (clopidogrel and aspirin),23,24 and one double-blind, multicenter trial involving more than 3000 patients with recent stroke secondary to small vessel disease demonstrated harm from such therapy in terms of a significantly increased risk of bleeding and death.6
However, a 2011 study compared aggressive medical management (aspirin 325 mg/d plus clopidogrel 75 mg/d for 90 days) alone to aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). The study involved almost 500 patients who'd had a recent TIA or stroke attributed to intracranial atherosclerotic stenosis. The authors found that the 30-day rate of stroke or death was 14.7% in the PTAS group vs 5.8% in the medical management group.25
Similarly, a randomized double-blind, placebo-controlled trial published in 2013 involving over 5000 patients in China found that short-term use of dual antiplatelets (clopidogrel and aspirin for the first 21 days after an ischemic event, followed by aspirin monotherapy for 90 days) had an absolute risk reduction of 3.5% without increasing the risk of major bleeding in patients with high-risk TIA or minor stroke.26
All stroke patients who do not have an indication for oral anticoagulation should be placed on long-term daily aspirin (75-325 mg); research has shown that lower doses are as effective as higher doses but with a lower risk of adverse gastrointestinal effects, including bleeding.3,20 Aspirin 81 mg/d is a common effective dose.
For patients who cannot tolerate aspirin due to allergy, clopidogrel 75 mg/d is a reasonable alternative. Long-term studies of aspirin vs clopidogrel7 and clopidogrel vs extended-release dipyridamole8 showed no difference in secondary stroke prevention. The International Stroke Trial27 and Chinese Acute Stroke Trial28 both indicate that aspirin should be started as soon as possible after the onset of an acute stroke.
This special population should probably get antiplatelets, too. One recent study explored the use of an antiplatelet vs anticoagulation therapy for stroke patients with carotid artery dissection. The CADISS (Cervical Artery Dissection in Stroke Study) trial29 randomized 250 patients with extracranial carotid and vertebral artery dissection with onset of symptoms within the previous 7 days to either antiplatelet or anticoagulation therapy and found no difference in the primary outcomes of recurrent stroke or death. The study also demonstrated a low risk of recurrent stroke in this population, which was 2% at 3-month follow-up.
Most patients with cervical artery dissection, therefore, are now treated with antiplatelet therapy. That said, situations may still arise in which anticoagulation can be considered, and consultation with a neurologist for guidance on choice of therapy is recommended.
Is an anticoagulant in order? Which agent, when
The most common cause of cardioembolic stroke is AF, which accounts for at least 15% of ischemic strokes, a number that rises in those over the age of 80.20,30,31 A meta-analysis of more than 28,000 patients with non-valvular AF demonstrated that warfarin reduced the risk of stroke by 64%.32
The rate of intracerebral hemorrhages during oral anticoagulation ranges from 0.3% to 0.6% per year.33 The risk of bleeding complications can be mitigated by keeping international normalized ratios ≤3.0, maintaining good BP control, and avoiding concurrent use of antiplatelets in the absence of a clear indication for them.33
Several risk assessment scores, such as the HAS-BLED,34 can help with estimating the risk of hemorrhagic complications, although these scores have their limitations.35,36 Even in an older population (mean age 83 years) with a high risk for falls, warfarin provided a net benefit in a composite endpoint of out-of-hospital death or hospitalization for stroke, MI, or hemorrhage in a retrospective study of over 1200 Medicare beneficiaries.37
AF is not the only cause of cardioembolic stroke to consider. Additional high-risk factors warranting anticoagulation include rheumatic mitral valve disease, the presence of mechanical aortic or mitral valves, known mural thrombus, and acute anterior ST segment elevation myocardial infarctions (STEMIs) with resulting anterior apical dyskinesis/akinesis and concurrent ischemic stroke/TIA.3 (The specific management of each of these situations is beyond the scope of this paper.)
The choice of anticoagulation agent is based on multiple factors, including cost, risk of non-reversible bleeding, drug interactions, renal function, and patient preference. Approved options currently include warfarin/vitamin K antagonist therapy, apixaban, rivaroxaban, dabigatran and edoxaban.3 Choice of therapy will continue to evolve as reversal agents, such as idarucizumab, are developed. Idarucizumab, a reversal agent for dabigatran, received approval from the US Food and Drug Administration in October 2015.38
When to start anticoagulation. There are limited data regarding the optimal timing of initiation of anticoagulation following a stroke; however, a recent multicenter prospective study supported the common practice of initiating anticoagulation therapy within 4 to 14 days of the event.39 Individual patient factors must be taken into consideration, including the size of the stroke (the larger the stroke, the higher the risk for hemorrhagic transformation), BP control, any additional risk factors for bleeding, and the estimated risk of early recurrent stroke.
Bridging patients onto anticoagulation with unfractionated or low-molecular-weight heparin in the setting of acute stroke is not recommended.40 Results from randomized controlled trials involving unfractionated heparin, heparinoids, and low-molecular-weight heparin have not reported any benefit to these agents over aspirin at preventing early stroke recurrence.27,41,42
For immobile or hospitalized patients. Subcutaneous heparin for the prevention of deep vein thrombosis (DVT) during immobility and hospitalization is recommended.43 Patients who cannot tolerate anticoagulation should be maintained on low-dose antiplatelet therapy. Experts do not recommend dual treatment with aspirin and anticoagulation in most cases. However, recent coronary artery stent placement does require temporary dual treatment, with duration dependent on the type of stent placed.
A role for glycemic control? Still to be determined
The specific role of diabetic management in secondary stroke prevention remains unclear. The 2008 ACCORD trial,44 a randomized study involving over 10,000 patients with a median glycated hemoglobin level of 8.1%, investigated intensive hyperglycemic control (targeting a glycated hemoglobin level <6.0% vs <7.9%) as a means of decreasing cardiovascular risk. However, the trial ended 17 months early because of an increase in all-cause mortality in the intensive treatment arm compared with the standard management group. The same trial was also unable to demonstrate a decrease in stroke risk with a decrease in A1c.44
More recently, the IRIS (Insulin Resistance Intervention after Stroke) trial45 (2016) found a 2.8% absolute risk reduction in stroke or MI among participants who had a stroke or TIA in the previous 6 months who were treated with pioglitazone vs placebo over 4.8 years (NNT=36). Participants were required to have insulin resistance, but were excluded if they had diabetes. The authors did, however, report a notable increase in the risk of bone fractures requiring surgery or hospitalization in the pioglitazone arm (5.1% vs 3.2%; number needed to harm [NNH]=53).
The impact this single study should have on standard secondary prevention is not yet clear. The authors concluded, “It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy.”45
Determining whether mechanical interventions are needed
Almost all conditions leading to stroke warrant active medical management, but a few benefit from procedural intervention, as well.
Extracranial carotid atherosclerosis. Carotid endarterectomy or carotid artery stenting is recommended as secondary prevention for patients with a history of stroke or TIA who have ipsilateral high-grade extracranial carotid stenosis of 70% to 99% and, in some cases, 50% to 69%.3,9,20 In patients with mild non-disabling stroke, the optimal timing for these procedures is within 2 weeks of the ischemic event. A delay of 6 weeks is generally preferred for moderate or larger strokes to allow for some healing of the injured brain.
The choice of procedure is based on risk profile, with the most important factor being age. For patients >70 years, endarterectomy is preferred because stenting is associated with an increased risk of stroke.3,9,10 Experts do not recommend either procedure for patients who have had a severe disabling stroke. Generally speaking, these procedures have higher rates of success when they are performed in centers that perform a higher number of these procedures.10
Vertebrobasilar atherosclerosis. Due to generally good compensatory blood flow of the contralateral vertebral artery in the setting of vertebral artery stenosis, and an unacceptably high complication rate of angioplasty and stenting in the basilar artery, medical management is typically the first-line approach. If a patient has recurrent symptoms in the setting of optimal medical management and a focal lesion that is amenable to an endovascular intervention (most commonly a vertebral artery origin high-grade stenosis), angioplasty and stenting may be considered.10
Intracranial atherosclerosis. Similarly, medical management is the preferred strategy for intracranial atherosclerosis. Angioplasty and/or stenting are reserved for complex cases or recurrence despite adherence to secondary stroke prevention measures. Ideally, these patients should be managed with long-term aspirin 81 mg/d, adjunctive clopidogrel 75 mg/d for 90 days post stroke, a high-intensity statin, BP optimization, and any relevant lifestyle interventions.13
Patent foramen ovale. Research to date has not shown that closure of a patent foramen ovale (PFO) is superior to medical therapy for secondary stroke prevention in patients <60 years with cryptogenic stroke.12,46,47 The decision to anticoagulate these patients should be based on the presence or absence of a DVT and not on a PFO alone. In patients with an identified DVT and a contraindication to oral anticoagulation, inferior vena cava filter placement should be considered. For patients with ongoing prothrombotic risk thought to increase the chances of future paradoxical embolism, closure of the PFO may be considered.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881-1885.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
3. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748.
5. Amarenco P, Bogousslavsky J, Callahan A, et al, for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
6. Benavente OR, Hart RG, McClure LA, et al, for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
8. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
9. Diethrich EB, N’diaye M, Reid DB. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): implications for clinical practice. In: Henry M, Diethrich EB, Polydorou A, eds. The Carotid and Supra-Aortic Trunks: Diagnosis, Angioplasty and Stenting. 2nd ed. Oxford, UK: Wiley-Blackwell; 2011.
10. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489-532.
11. SPS3 Study Group. Blood pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507-515.
12. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
13. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
14. Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287-303.
15. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207-211.
16. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917-932.
17. Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439-2443.
18. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a Mediterranean-style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007-2011.
19. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
20. Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914-1922.
21. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
22. Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40-41.
23. Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364:331-337.
24. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
25. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
26. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
27. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
28. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
29. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361-367.
30. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
31. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
32. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med. 2007;146:857-867.
33. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Recent data and ideas. Stroke. 2005;36:1588-1593.
34. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
35. Quinn GR, Singer DE, Chang Y, et al. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. 2016;118:697-699.
36. Gorman EW, Perkel D, Dennis D, et al. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation. 2016;9:1461.
37. Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
38. US Food and Drug Administration. FDA approves Praxbind, the first reversal agent for the anticoagulant Pradaxa. October 16, 2015. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm467300.htm. Accessed May 26, 2017.
39. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175-2182.
40. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;3:CD000024.
41. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702-710.
42. Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205-1210.
43. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
44. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
45. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321-1331.
46. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
47. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
Patients who suffer a stroke rarely have just one vascular risk factor. Therefore, the approach to secondary stroke prevention must be multifactorial. In fact, it has been estimated that 80% of recurrent strokes could be prevented through the application of a comprehensive, multifactorial approach that includes lifestyle modification and optimal medical management.1 Such an achievement would save millions of people from disability and functional decline, as well as millions of dollars in related medical costs.
The initial approach to patients with stroke is focused on stabilization and a rapid work-up to identify the most likely etiology. Common causes of stroke include large artery atherosclerosis, cardiac emboli, and small vessel disease; less common causes include dissection, aortic emboli, and non-atherosclerotic vascular disease. If a complete diagnostic work-up is unrevealing, the stroke is said to be cryptogenic. Determining the correct etiology of a stroke is paramount to preventing secondary stroke (FIGURE2-13).

Effective secondary prevention strategies designed to prevent a stroke or transient ischemic attack (TIA) in a patient with a known history of either event include lifestyle modifications, medications, and when appropriate, mechanical interventions. As a primary care physician (PCP), you are uniquely positioned to spearhead the prevention of secondary strokes: Not only are you at the forefront of prevention and the use of techniques such as motivational interviewing, but you also have longstanding relationships with many of your patients. In fact, the success of many interventions is improved by the informed, enduring, and trusting nature of relationships between patients and their PCPs.
In the first part of this 2-part series, we focused on subacute stroke management and outlined the recommended work-up for subacute stroke/TIA (see “Stroke: A road map to subacute management,” 2017;66:366-374). In this part, we focus on secondary prevention. The more common modifiable conditions encountered in primary care are discussed here, while many of the more rare etiologies (hypercoagulable states, sickle cell disease, and vasculitis) are outside the scope of this article.
Lifestyle interventions: Target tobacco use, obesity, alcohol intake
Lifestyle modifications can have a positive impact on many of America’s most prevalent diseases, and stroke is no exception.14 Many of the disease states identified as risk factors for stroke (type 2 diabetes, hypertension, dyslipidemia) are exacerbated by tobacco use, obesity, and excessive alcohol intake.
Does your patient smoke? Up to 25% of all strokes are directly attributable to cigarette smoking.15 Smoking raises an individual’s risk for stroke in a dose-dependent fashion.15,16 One study demonstrated that, compared to never-smokers, women ages 15 to 49 years who smoked a half-pack per day had an odds ratio for ischemic stroke of 2.2; those who smoked 2 packs per day had an odds ratio of 9.1.17 After cessation, stroke risk generally returns to baseline within 5 years.16 Thus, smoking cessation is among the most significant steps a patient can take to reduce the risk of both primary and secondary stroke.
Is your patient overweight? While obesity in and of itself is a risk factor for stroke, a focus on nutrition and physical activity as mechanisms for weight loss is far superior to focusing on either element alone. Physical activity—consisting of at least 40 minutes of moderate intensity aerobic exercise 3 to 4 times per week—and a diet that emphasizes fruits and vegetables, whole grains, and healthy fats, have both independently demonstrated benefits in secondary stroke prevention and are important parts of American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.2,3
The Mediterranean Diet, which emphasizes consumption of fruits and vegetables, legumes, tree nuts, olive oil, and lean protein, has long been associated with cardiovascular benefit.18 One prospective, randomized, single-blinded trial involving approximately 600 patients that looked at secondary prevention of coronary heart disease found that following the diet significantly reduced mortality compared with a usual prudent post-infarct diet (number needed to treat [NNT]=30 over 4 years).19
Is alcohol consumption an issue? Chronic heavy alcohol intake contributes to the development of hemorrhagic and ischemic stroke through multiple mechanisms, including alcohol-induced hypertension, alcoholic cardiomyopathy, and atrial fibrillation (AF). Light or moderate alcohol consumption has a paradoxical mild protective effect on ischemic stroke, thought to possibly be mediated by an increase in high-density lipoprotein (HDL) level and mild antiplatelet effect.3
AHA/American Stroke Association (ASA) guidelines indicate that no more than one standard drink per day for women and 2 drinks per day for men is reasonable.3 Counsel patients who drink in excess of this about the benefits of decreasing alcohol intake or abstaining altogether.
Choosing medications to manage BP, cholesterol, and clotting
Optimize blood pressure control. Blood pressure (BP) plays a critical role in both the management and prevention of stroke and is considered to be the most important modifiable risk factor in both primary and secondary stroke prevention.20 In the first 24 to 48 hours following a cerebral ischemic event that is not eligible for thrombolysis, permissive hypertension (treating BP only if it exceeds 220/120 mm Hg unless there is a concurrent medical illness that requires you do so) is appropriate, as hypotension or rapid fluctuations in BP can be harmful.21
This flexibility does not continue into the subacute phase of management (at a minimum, after the initial 48 hours) or into secondary prevention. Initiation and titration of oral agents to gradually achieve a BP <140/90 mm Hg or a reduction of 10/5 mm Hg for patients already within optimal range are the most widely recognized goals.3,20 Patients with stroke secondary to small vessel disease may benefit from an even lower goal of <130/<80 mm Hg.11 Encourage patients to monitor their BP at home for added accuracy and consistency.22
Pharmacologic BP management is appropriate for patients who are consistently above optimal range despite attempting recommended lifestyle modifications. The data are relatively consistent with respect to the effects of different drug classes after a stroke: beta-blockers have no effect on any outcome; thiazide diuretics significantly reduce stroke and total vascular events; angiotensin-converting enzyme (ACE) inhibitors significantly reduce myocardial infarction (MI); and the combination of an ACE inhibitor and thiazide diuretic reduces stroke, MI, and combined vascular events.4
This has led many stroke specialists to recommend the combination of an ACE inhibitor or angiotensin II receptor blocker (ARB) and a thiazide diuretic as a first-line approach to secondary stroke prevention rather than a beta-blocker (assuming there is no additional indication for a beta-blocker). Similarly, there is ample evidence to show that the magnitude of BP reduction is proportional to the reduction in recurrent vascular events.3
Make use of statin therapy—regardless of LDL. The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial5 explored the potential role of statin medication for secondary stroke prevention. Researchers randomly assigned almost 5000 participants who’d had a stroke or TIA one to 6 months before study entry (but had no known history of coronary artery disease) to placebo or a high-intensity statin (80 mg/d atorvastatin). The statin group demonstrated a 4.9-year absolute risk reduction in fatal or nonfatal recurrent stroke of 1.9% (NNT=53).
Given these findings and those from other studies, the AHA and ASA recommend treating patients with stroke or TIA presumed to be of atherosclerotic origin with high-intensity statin therapy, regardless of low-density lipoprotein (LDL) level.3 Of note, statins are not indicated for the secondary prevention of hemorrhagic stroke.
Select antiplatelet therapy based on ischemic stroke subtype. Investigators are still trying to determine the optimal antiplatelet for secondary stroke prevention; it is likely that the ideal choice depends largely on the etiology of the stroke. Trials that did not select patients based on subtype of ischemic stroke have not shown a long-term benefit from dual antiplatelet therapy (clopidogrel and aspirin),23,24 and one double-blind, multicenter trial involving more than 3000 patients with recent stroke secondary to small vessel disease demonstrated harm from such therapy in terms of a significantly increased risk of bleeding and death.6
However, a 2011 study compared aggressive medical management (aspirin 325 mg/d plus clopidogrel 75 mg/d for 90 days) alone to aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). The study involved almost 500 patients who'd had a recent TIA or stroke attributed to intracranial atherosclerotic stenosis. The authors found that the 30-day rate of stroke or death was 14.7% in the PTAS group vs 5.8% in the medical management group.25
Similarly, a randomized double-blind, placebo-controlled trial published in 2013 involving over 5000 patients in China found that short-term use of dual antiplatelets (clopidogrel and aspirin for the first 21 days after an ischemic event, followed by aspirin monotherapy for 90 days) had an absolute risk reduction of 3.5% without increasing the risk of major bleeding in patients with high-risk TIA or minor stroke.26
All stroke patients who do not have an indication for oral anticoagulation should be placed on long-term daily aspirin (75-325 mg); research has shown that lower doses are as effective as higher doses but with a lower risk of adverse gastrointestinal effects, including bleeding.3,20 Aspirin 81 mg/d is a common effective dose.
For patients who cannot tolerate aspirin due to allergy, clopidogrel 75 mg/d is a reasonable alternative. Long-term studies of aspirin vs clopidogrel7 and clopidogrel vs extended-release dipyridamole8 showed no difference in secondary stroke prevention. The International Stroke Trial27 and Chinese Acute Stroke Trial28 both indicate that aspirin should be started as soon as possible after the onset of an acute stroke.
This special population should probably get antiplatelets, too. One recent study explored the use of an antiplatelet vs anticoagulation therapy for stroke patients with carotid artery dissection. The CADISS (Cervical Artery Dissection in Stroke Study) trial29 randomized 250 patients with extracranial carotid and vertebral artery dissection with onset of symptoms within the previous 7 days to either antiplatelet or anticoagulation therapy and found no difference in the primary outcomes of recurrent stroke or death. The study also demonstrated a low risk of recurrent stroke in this population, which was 2% at 3-month follow-up.
Most patients with cervical artery dissection, therefore, are now treated with antiplatelet therapy. That said, situations may still arise in which anticoagulation can be considered, and consultation with a neurologist for guidance on choice of therapy is recommended.
Is an anticoagulant in order? Which agent, when
The most common cause of cardioembolic stroke is AF, which accounts for at least 15% of ischemic strokes, a number that rises in those over the age of 80.20,30,31 A meta-analysis of more than 28,000 patients with non-valvular AF demonstrated that warfarin reduced the risk of stroke by 64%.32
The rate of intracerebral hemorrhages during oral anticoagulation ranges from 0.3% to 0.6% per year.33 The risk of bleeding complications can be mitigated by keeping international normalized ratios ≤3.0, maintaining good BP control, and avoiding concurrent use of antiplatelets in the absence of a clear indication for them.33
Several risk assessment scores, such as the HAS-BLED,34 can help with estimating the risk of hemorrhagic complications, although these scores have their limitations.35,36 Even in an older population (mean age 83 years) with a high risk for falls, warfarin provided a net benefit in a composite endpoint of out-of-hospital death or hospitalization for stroke, MI, or hemorrhage in a retrospective study of over 1200 Medicare beneficiaries.37
AF is not the only cause of cardioembolic stroke to consider. Additional high-risk factors warranting anticoagulation include rheumatic mitral valve disease, the presence of mechanical aortic or mitral valves, known mural thrombus, and acute anterior ST segment elevation myocardial infarctions (STEMIs) with resulting anterior apical dyskinesis/akinesis and concurrent ischemic stroke/TIA.3 (The specific management of each of these situations is beyond the scope of this paper.)
The choice of anticoagulation agent is based on multiple factors, including cost, risk of non-reversible bleeding, drug interactions, renal function, and patient preference. Approved options currently include warfarin/vitamin K antagonist therapy, apixaban, rivaroxaban, dabigatran and edoxaban.3 Choice of therapy will continue to evolve as reversal agents, such as idarucizumab, are developed. Idarucizumab, a reversal agent for dabigatran, received approval from the US Food and Drug Administration in October 2015.38
When to start anticoagulation. There are limited data regarding the optimal timing of initiation of anticoagulation following a stroke; however, a recent multicenter prospective study supported the common practice of initiating anticoagulation therapy within 4 to 14 days of the event.39 Individual patient factors must be taken into consideration, including the size of the stroke (the larger the stroke, the higher the risk for hemorrhagic transformation), BP control, any additional risk factors for bleeding, and the estimated risk of early recurrent stroke.
Bridging patients onto anticoagulation with unfractionated or low-molecular-weight heparin in the setting of acute stroke is not recommended.40 Results from randomized controlled trials involving unfractionated heparin, heparinoids, and low-molecular-weight heparin have not reported any benefit to these agents over aspirin at preventing early stroke recurrence.27,41,42
For immobile or hospitalized patients. Subcutaneous heparin for the prevention of deep vein thrombosis (DVT) during immobility and hospitalization is recommended.43 Patients who cannot tolerate anticoagulation should be maintained on low-dose antiplatelet therapy. Experts do not recommend dual treatment with aspirin and anticoagulation in most cases. However, recent coronary artery stent placement does require temporary dual treatment, with duration dependent on the type of stent placed.
A role for glycemic control? Still to be determined
The specific role of diabetic management in secondary stroke prevention remains unclear. The 2008 ACCORD trial,44 a randomized study involving over 10,000 patients with a median glycated hemoglobin level of 8.1%, investigated intensive hyperglycemic control (targeting a glycated hemoglobin level <6.0% vs <7.9%) as a means of decreasing cardiovascular risk. However, the trial ended 17 months early because of an increase in all-cause mortality in the intensive treatment arm compared with the standard management group. The same trial was also unable to demonstrate a decrease in stroke risk with a decrease in A1c.44
More recently, the IRIS (Insulin Resistance Intervention after Stroke) trial45 (2016) found a 2.8% absolute risk reduction in stroke or MI among participants who had a stroke or TIA in the previous 6 months who were treated with pioglitazone vs placebo over 4.8 years (NNT=36). Participants were required to have insulin resistance, but were excluded if they had diabetes. The authors did, however, report a notable increase in the risk of bone fractures requiring surgery or hospitalization in the pioglitazone arm (5.1% vs 3.2%; number needed to harm [NNH]=53).
The impact this single study should have on standard secondary prevention is not yet clear. The authors concluded, “It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy.”45
Determining whether mechanical interventions are needed
Almost all conditions leading to stroke warrant active medical management, but a few benefit from procedural intervention, as well.
Extracranial carotid atherosclerosis. Carotid endarterectomy or carotid artery stenting is recommended as secondary prevention for patients with a history of stroke or TIA who have ipsilateral high-grade extracranial carotid stenosis of 70% to 99% and, in some cases, 50% to 69%.3,9,20 In patients with mild non-disabling stroke, the optimal timing for these procedures is within 2 weeks of the ischemic event. A delay of 6 weeks is generally preferred for moderate or larger strokes to allow for some healing of the injured brain.
The choice of procedure is based on risk profile, with the most important factor being age. For patients >70 years, endarterectomy is preferred because stenting is associated with an increased risk of stroke.3,9,10 Experts do not recommend either procedure for patients who have had a severe disabling stroke. Generally speaking, these procedures have higher rates of success when they are performed in centers that perform a higher number of these procedures.10
Vertebrobasilar atherosclerosis. Due to generally good compensatory blood flow of the contralateral vertebral artery in the setting of vertebral artery stenosis, and an unacceptably high complication rate of angioplasty and stenting in the basilar artery, medical management is typically the first-line approach. If a patient has recurrent symptoms in the setting of optimal medical management and a focal lesion that is amenable to an endovascular intervention (most commonly a vertebral artery origin high-grade stenosis), angioplasty and stenting may be considered.10
Intracranial atherosclerosis. Similarly, medical management is the preferred strategy for intracranial atherosclerosis. Angioplasty and/or stenting are reserved for complex cases or recurrence despite adherence to secondary stroke prevention measures. Ideally, these patients should be managed with long-term aspirin 81 mg/d, adjunctive clopidogrel 75 mg/d for 90 days post stroke, a high-intensity statin, BP optimization, and any relevant lifestyle interventions.13
Patent foramen ovale. Research to date has not shown that closure of a patent foramen ovale (PFO) is superior to medical therapy for secondary stroke prevention in patients <60 years with cryptogenic stroke.12,46,47 The decision to anticoagulate these patients should be based on the presence or absence of a DVT and not on a PFO alone. In patients with an identified DVT and a contraindication to oral anticoagulation, inferior vena cava filter placement should be considered. For patients with ongoing prothrombotic risk thought to increase the chances of future paradoxical embolism, closure of the PFO may be considered.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
Patients who suffer a stroke rarely have just one vascular risk factor. Therefore, the approach to secondary stroke prevention must be multifactorial. In fact, it has been estimated that 80% of recurrent strokes could be prevented through the application of a comprehensive, multifactorial approach that includes lifestyle modification and optimal medical management.1 Such an achievement would save millions of people from disability and functional decline, as well as millions of dollars in related medical costs.
The initial approach to patients with stroke is focused on stabilization and a rapid work-up to identify the most likely etiology. Common causes of stroke include large artery atherosclerosis, cardiac emboli, and small vessel disease; less common causes include dissection, aortic emboli, and non-atherosclerotic vascular disease. If a complete diagnostic work-up is unrevealing, the stroke is said to be cryptogenic. Determining the correct etiology of a stroke is paramount to preventing secondary stroke (FIGURE2-13).

Effective secondary prevention strategies designed to prevent a stroke or transient ischemic attack (TIA) in a patient with a known history of either event include lifestyle modifications, medications, and when appropriate, mechanical interventions. As a primary care physician (PCP), you are uniquely positioned to spearhead the prevention of secondary strokes: Not only are you at the forefront of prevention and the use of techniques such as motivational interviewing, but you also have longstanding relationships with many of your patients. In fact, the success of many interventions is improved by the informed, enduring, and trusting nature of relationships between patients and their PCPs.
In the first part of this 2-part series, we focused on subacute stroke management and outlined the recommended work-up for subacute stroke/TIA (see “Stroke: A road map to subacute management,” 2017;66:366-374). In this part, we focus on secondary prevention. The more common modifiable conditions encountered in primary care are discussed here, while many of the more rare etiologies (hypercoagulable states, sickle cell disease, and vasculitis) are outside the scope of this article.
Lifestyle interventions: Target tobacco use, obesity, alcohol intake
Lifestyle modifications can have a positive impact on many of America’s most prevalent diseases, and stroke is no exception.14 Many of the disease states identified as risk factors for stroke (type 2 diabetes, hypertension, dyslipidemia) are exacerbated by tobacco use, obesity, and excessive alcohol intake.
Does your patient smoke? Up to 25% of all strokes are directly attributable to cigarette smoking.15 Smoking raises an individual’s risk for stroke in a dose-dependent fashion.15,16 One study demonstrated that, compared to never-smokers, women ages 15 to 49 years who smoked a half-pack per day had an odds ratio for ischemic stroke of 2.2; those who smoked 2 packs per day had an odds ratio of 9.1.17 After cessation, stroke risk generally returns to baseline within 5 years.16 Thus, smoking cessation is among the most significant steps a patient can take to reduce the risk of both primary and secondary stroke.
Is your patient overweight? While obesity in and of itself is a risk factor for stroke, a focus on nutrition and physical activity as mechanisms for weight loss is far superior to focusing on either element alone. Physical activity—consisting of at least 40 minutes of moderate intensity aerobic exercise 3 to 4 times per week—and a diet that emphasizes fruits and vegetables, whole grains, and healthy fats, have both independently demonstrated benefits in secondary stroke prevention and are important parts of American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.2,3
The Mediterranean Diet, which emphasizes consumption of fruits and vegetables, legumes, tree nuts, olive oil, and lean protein, has long been associated with cardiovascular benefit.18 One prospective, randomized, single-blinded trial involving approximately 600 patients that looked at secondary prevention of coronary heart disease found that following the diet significantly reduced mortality compared with a usual prudent post-infarct diet (number needed to treat [NNT]=30 over 4 years).19
Is alcohol consumption an issue? Chronic heavy alcohol intake contributes to the development of hemorrhagic and ischemic stroke through multiple mechanisms, including alcohol-induced hypertension, alcoholic cardiomyopathy, and atrial fibrillation (AF). Light or moderate alcohol consumption has a paradoxical mild protective effect on ischemic stroke, thought to possibly be mediated by an increase in high-density lipoprotein (HDL) level and mild antiplatelet effect.3
AHA/American Stroke Association (ASA) guidelines indicate that no more than one standard drink per day for women and 2 drinks per day for men is reasonable.3 Counsel patients who drink in excess of this about the benefits of decreasing alcohol intake or abstaining altogether.
Choosing medications to manage BP, cholesterol, and clotting
Optimize blood pressure control. Blood pressure (BP) plays a critical role in both the management and prevention of stroke and is considered to be the most important modifiable risk factor in both primary and secondary stroke prevention.20 In the first 24 to 48 hours following a cerebral ischemic event that is not eligible for thrombolysis, permissive hypertension (treating BP only if it exceeds 220/120 mm Hg unless there is a concurrent medical illness that requires you do so) is appropriate, as hypotension or rapid fluctuations in BP can be harmful.21
This flexibility does not continue into the subacute phase of management (at a minimum, after the initial 48 hours) or into secondary prevention. Initiation and titration of oral agents to gradually achieve a BP <140/90 mm Hg or a reduction of 10/5 mm Hg for patients already within optimal range are the most widely recognized goals.3,20 Patients with stroke secondary to small vessel disease may benefit from an even lower goal of <130/<80 mm Hg.11 Encourage patients to monitor their BP at home for added accuracy and consistency.22
Pharmacologic BP management is appropriate for patients who are consistently above optimal range despite attempting recommended lifestyle modifications. The data are relatively consistent with respect to the effects of different drug classes after a stroke: beta-blockers have no effect on any outcome; thiazide diuretics significantly reduce stroke and total vascular events; angiotensin-converting enzyme (ACE) inhibitors significantly reduce myocardial infarction (MI); and the combination of an ACE inhibitor and thiazide diuretic reduces stroke, MI, and combined vascular events.4
This has led many stroke specialists to recommend the combination of an ACE inhibitor or angiotensin II receptor blocker (ARB) and a thiazide diuretic as a first-line approach to secondary stroke prevention rather than a beta-blocker (assuming there is no additional indication for a beta-blocker). Similarly, there is ample evidence to show that the magnitude of BP reduction is proportional to the reduction in recurrent vascular events.3
Make use of statin therapy—regardless of LDL. The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial5 explored the potential role of statin medication for secondary stroke prevention. Researchers randomly assigned almost 5000 participants who’d had a stroke or TIA one to 6 months before study entry (but had no known history of coronary artery disease) to placebo or a high-intensity statin (80 mg/d atorvastatin). The statin group demonstrated a 4.9-year absolute risk reduction in fatal or nonfatal recurrent stroke of 1.9% (NNT=53).
Given these findings and those from other studies, the AHA and ASA recommend treating patients with stroke or TIA presumed to be of atherosclerotic origin with high-intensity statin therapy, regardless of low-density lipoprotein (LDL) level.3 Of note, statins are not indicated for the secondary prevention of hemorrhagic stroke.
Select antiplatelet therapy based on ischemic stroke subtype. Investigators are still trying to determine the optimal antiplatelet for secondary stroke prevention; it is likely that the ideal choice depends largely on the etiology of the stroke. Trials that did not select patients based on subtype of ischemic stroke have not shown a long-term benefit from dual antiplatelet therapy (clopidogrel and aspirin),23,24 and one double-blind, multicenter trial involving more than 3000 patients with recent stroke secondary to small vessel disease demonstrated harm from such therapy in terms of a significantly increased risk of bleeding and death.6
However, a 2011 study compared aggressive medical management (aspirin 325 mg/d plus clopidogrel 75 mg/d for 90 days) alone to aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). The study involved almost 500 patients who'd had a recent TIA or stroke attributed to intracranial atherosclerotic stenosis. The authors found that the 30-day rate of stroke or death was 14.7% in the PTAS group vs 5.8% in the medical management group.25
Similarly, a randomized double-blind, placebo-controlled trial published in 2013 involving over 5000 patients in China found that short-term use of dual antiplatelets (clopidogrel and aspirin for the first 21 days after an ischemic event, followed by aspirin monotherapy for 90 days) had an absolute risk reduction of 3.5% without increasing the risk of major bleeding in patients with high-risk TIA or minor stroke.26
All stroke patients who do not have an indication for oral anticoagulation should be placed on long-term daily aspirin (75-325 mg); research has shown that lower doses are as effective as higher doses but with a lower risk of adverse gastrointestinal effects, including bleeding.3,20 Aspirin 81 mg/d is a common effective dose.
For patients who cannot tolerate aspirin due to allergy, clopidogrel 75 mg/d is a reasonable alternative. Long-term studies of aspirin vs clopidogrel7 and clopidogrel vs extended-release dipyridamole8 showed no difference in secondary stroke prevention. The International Stroke Trial27 and Chinese Acute Stroke Trial28 both indicate that aspirin should be started as soon as possible after the onset of an acute stroke.
This special population should probably get antiplatelets, too. One recent study explored the use of an antiplatelet vs anticoagulation therapy for stroke patients with carotid artery dissection. The CADISS (Cervical Artery Dissection in Stroke Study) trial29 randomized 250 patients with extracranial carotid and vertebral artery dissection with onset of symptoms within the previous 7 days to either antiplatelet or anticoagulation therapy and found no difference in the primary outcomes of recurrent stroke or death. The study also demonstrated a low risk of recurrent stroke in this population, which was 2% at 3-month follow-up.
Most patients with cervical artery dissection, therefore, are now treated with antiplatelet therapy. That said, situations may still arise in which anticoagulation can be considered, and consultation with a neurologist for guidance on choice of therapy is recommended.
Is an anticoagulant in order? Which agent, when
The most common cause of cardioembolic stroke is AF, which accounts for at least 15% of ischemic strokes, a number that rises in those over the age of 80.20,30,31 A meta-analysis of more than 28,000 patients with non-valvular AF demonstrated that warfarin reduced the risk of stroke by 64%.32
The rate of intracerebral hemorrhages during oral anticoagulation ranges from 0.3% to 0.6% per year.33 The risk of bleeding complications can be mitigated by keeping international normalized ratios ≤3.0, maintaining good BP control, and avoiding concurrent use of antiplatelets in the absence of a clear indication for them.33
Several risk assessment scores, such as the HAS-BLED,34 can help with estimating the risk of hemorrhagic complications, although these scores have their limitations.35,36 Even in an older population (mean age 83 years) with a high risk for falls, warfarin provided a net benefit in a composite endpoint of out-of-hospital death or hospitalization for stroke, MI, or hemorrhage in a retrospective study of over 1200 Medicare beneficiaries.37
AF is not the only cause of cardioembolic stroke to consider. Additional high-risk factors warranting anticoagulation include rheumatic mitral valve disease, the presence of mechanical aortic or mitral valves, known mural thrombus, and acute anterior ST segment elevation myocardial infarctions (STEMIs) with resulting anterior apical dyskinesis/akinesis and concurrent ischemic stroke/TIA.3 (The specific management of each of these situations is beyond the scope of this paper.)
The choice of anticoagulation agent is based on multiple factors, including cost, risk of non-reversible bleeding, drug interactions, renal function, and patient preference. Approved options currently include warfarin/vitamin K antagonist therapy, apixaban, rivaroxaban, dabigatran and edoxaban.3 Choice of therapy will continue to evolve as reversal agents, such as idarucizumab, are developed. Idarucizumab, a reversal agent for dabigatran, received approval from the US Food and Drug Administration in October 2015.38
When to start anticoagulation. There are limited data regarding the optimal timing of initiation of anticoagulation following a stroke; however, a recent multicenter prospective study supported the common practice of initiating anticoagulation therapy within 4 to 14 days of the event.39 Individual patient factors must be taken into consideration, including the size of the stroke (the larger the stroke, the higher the risk for hemorrhagic transformation), BP control, any additional risk factors for bleeding, and the estimated risk of early recurrent stroke.
Bridging patients onto anticoagulation with unfractionated or low-molecular-weight heparin in the setting of acute stroke is not recommended.40 Results from randomized controlled trials involving unfractionated heparin, heparinoids, and low-molecular-weight heparin have not reported any benefit to these agents over aspirin at preventing early stroke recurrence.27,41,42
For immobile or hospitalized patients. Subcutaneous heparin for the prevention of deep vein thrombosis (DVT) during immobility and hospitalization is recommended.43 Patients who cannot tolerate anticoagulation should be maintained on low-dose antiplatelet therapy. Experts do not recommend dual treatment with aspirin and anticoagulation in most cases. However, recent coronary artery stent placement does require temporary dual treatment, with duration dependent on the type of stent placed.
A role for glycemic control? Still to be determined
The specific role of diabetic management in secondary stroke prevention remains unclear. The 2008 ACCORD trial,44 a randomized study involving over 10,000 patients with a median glycated hemoglobin level of 8.1%, investigated intensive hyperglycemic control (targeting a glycated hemoglobin level <6.0% vs <7.9%) as a means of decreasing cardiovascular risk. However, the trial ended 17 months early because of an increase in all-cause mortality in the intensive treatment arm compared with the standard management group. The same trial was also unable to demonstrate a decrease in stroke risk with a decrease in A1c.44
More recently, the IRIS (Insulin Resistance Intervention after Stroke) trial45 (2016) found a 2.8% absolute risk reduction in stroke or MI among participants who had a stroke or TIA in the previous 6 months who were treated with pioglitazone vs placebo over 4.8 years (NNT=36). Participants were required to have insulin resistance, but were excluded if they had diabetes. The authors did, however, report a notable increase in the risk of bone fractures requiring surgery or hospitalization in the pioglitazone arm (5.1% vs 3.2%; number needed to harm [NNH]=53).
The impact this single study should have on standard secondary prevention is not yet clear. The authors concluded, “It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy.”45
Determining whether mechanical interventions are needed
Almost all conditions leading to stroke warrant active medical management, but a few benefit from procedural intervention, as well.
Extracranial carotid atherosclerosis. Carotid endarterectomy or carotid artery stenting is recommended as secondary prevention for patients with a history of stroke or TIA who have ipsilateral high-grade extracranial carotid stenosis of 70% to 99% and, in some cases, 50% to 69%.3,9,20 In patients with mild non-disabling stroke, the optimal timing for these procedures is within 2 weeks of the ischemic event. A delay of 6 weeks is generally preferred for moderate or larger strokes to allow for some healing of the injured brain.
The choice of procedure is based on risk profile, with the most important factor being age. For patients >70 years, endarterectomy is preferred because stenting is associated with an increased risk of stroke.3,9,10 Experts do not recommend either procedure for patients who have had a severe disabling stroke. Generally speaking, these procedures have higher rates of success when they are performed in centers that perform a higher number of these procedures.10
Vertebrobasilar atherosclerosis. Due to generally good compensatory blood flow of the contralateral vertebral artery in the setting of vertebral artery stenosis, and an unacceptably high complication rate of angioplasty and stenting in the basilar artery, medical management is typically the first-line approach. If a patient has recurrent symptoms in the setting of optimal medical management and a focal lesion that is amenable to an endovascular intervention (most commonly a vertebral artery origin high-grade stenosis), angioplasty and stenting may be considered.10
Intracranial atherosclerosis. Similarly, medical management is the preferred strategy for intracranial atherosclerosis. Angioplasty and/or stenting are reserved for complex cases or recurrence despite adherence to secondary stroke prevention measures. Ideally, these patients should be managed with long-term aspirin 81 mg/d, adjunctive clopidogrel 75 mg/d for 90 days post stroke, a high-intensity statin, BP optimization, and any relevant lifestyle interventions.13
Patent foramen ovale. Research to date has not shown that closure of a patent foramen ovale (PFO) is superior to medical therapy for secondary stroke prevention in patients <60 years with cryptogenic stroke.12,46,47 The decision to anticoagulate these patients should be based on the presence or absence of a DVT and not on a PFO alone. In patients with an identified DVT and a contraindication to oral anticoagulation, inferior vena cava filter placement should be considered. For patients with ongoing prothrombotic risk thought to increase the chances of future paradoxical embolism, closure of the PFO may be considered.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881-1885.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
3. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748.
5. Amarenco P, Bogousslavsky J, Callahan A, et al, for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
6. Benavente OR, Hart RG, McClure LA, et al, for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
8. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
9. Diethrich EB, N’diaye M, Reid DB. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): implications for clinical practice. In: Henry M, Diethrich EB, Polydorou A, eds. The Carotid and Supra-Aortic Trunks: Diagnosis, Angioplasty and Stenting. 2nd ed. Oxford, UK: Wiley-Blackwell; 2011.
10. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489-532.
11. SPS3 Study Group. Blood pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507-515.
12. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
13. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
14. Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287-303.
15. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207-211.
16. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917-932.
17. Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439-2443.
18. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a Mediterranean-style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007-2011.
19. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
20. Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914-1922.
21. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
22. Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40-41.
23. Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364:331-337.
24. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
25. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
26. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
27. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
28. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
29. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361-367.
30. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
31. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
32. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med. 2007;146:857-867.
33. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Recent data and ideas. Stroke. 2005;36:1588-1593.
34. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
35. Quinn GR, Singer DE, Chang Y, et al. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. 2016;118:697-699.
36. Gorman EW, Perkel D, Dennis D, et al. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation. 2016;9:1461.
37. Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
38. US Food and Drug Administration. FDA approves Praxbind, the first reversal agent for the anticoagulant Pradaxa. October 16, 2015. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm467300.htm. Accessed May 26, 2017.
39. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175-2182.
40. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;3:CD000024.
41. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702-710.
42. Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205-1210.
43. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
44. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
45. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321-1331.
46. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
47. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
1. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881-1885.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
3. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748.
5. Amarenco P, Bogousslavsky J, Callahan A, et al, for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
6. Benavente OR, Hart RG, McClure LA, et al, for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
8. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
9. Diethrich EB, N’diaye M, Reid DB. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): implications for clinical practice. In: Henry M, Diethrich EB, Polydorou A, eds. The Carotid and Supra-Aortic Trunks: Diagnosis, Angioplasty and Stenting. 2nd ed. Oxford, UK: Wiley-Blackwell; 2011.
10. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489-532.
11. SPS3 Study Group. Blood pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507-515.
12. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
13. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
14. Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287-303.
15. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207-211.
16. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917-932.
17. Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439-2443.
18. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a Mediterranean-style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007-2011.
19. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
20. Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914-1922.
21. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
22. Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40-41.
23. Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364:331-337.
24. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
25. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
26. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
27. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
28. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
29. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361-367.
30. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
31. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
32. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med. 2007;146:857-867.
33. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Recent data and ideas. Stroke. 2005;36:1588-1593.
34. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
35. Quinn GR, Singer DE, Chang Y, et al. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. 2016;118:697-699.
36. Gorman EW, Perkel D, Dennis D, et al. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation. 2016;9:1461.
37. Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
38. US Food and Drug Administration. FDA approves Praxbind, the first reversal agent for the anticoagulant Pradaxa. October 16, 2015. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm467300.htm. Accessed May 26, 2017.
39. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175-2182.
40. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;3:CD000024.
41. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702-710.
42. Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205-1210.
43. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
44. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
45. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321-1331.
46. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
47. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
From The Journal of Family Practice | 2017;66(7):420-422,424-427.
PRACTICE RECOMMENDATIONS
› Encourage lifestyle modifications, including smoking cessation, alcohol moderation, appropriate diet, and exercise to reduce the risk of recurrent stroke. A
› Optimize blood pressure control using an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker and a thiazide diuretic. A
› Only use beta-blockers if there is another indication for them. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series