User login
Stroke: A road map for subacute management
CASE › A 68-year-old woman with hypertension and hyperlipidemia comes into your office for evaluation of a 30-minute episode of sudden-onset right-hand weakness and difficulty speaking that occurred 4 days earlier. The patient, who is also a smoker, has come in at the insistence of her daughter. On examination, her blood pressure (BP) is 145/88 mm Hg and her heart rate is 76 beats/minute and regular. She appears well and her language function is normal. The rest of her examination is normal. How would you proceed?
Stroke—the death of nerve cells due to a lack of blood supply from either infarction or hemorrhage—strikes nearly 800,000 people in the United States every year.1,2 Of these events, 130,000 are fatal, making stroke the fifth leading cause of death.3 Effective, early evaluation and cause-specific treatment are crucial parts of stroke care.
Research has helped to clarify the critical role primary care physicians play in recognizing, triaging, and managing stroke and transient ischemic attacks (TIA). This article reviews what we know about the different ways that a stroke and a TIA can present, the appropriate diagnostic work-up for patients presenting with symptoms of either event, and management strategies for subacute care (24 hours to up to 14 days after a stroke has occurred).4,5 Unless otherwise specified, this review will focus on ischemic stroke because 87% of strokes are attributable to ischemia.1
A follow-up to this article on secondary stroke prevention will appear in the journal next month.
Look to onset more than type of symptoms for clues
Stroke presents as a sudden onset of neurologic deficits (language, motor, sensory, cerebellar, or brainstem functions) (TABLE 14). Because presenting symptoms can vary widely, sudden onset, rather than particular symptoms, should raise a red flag for potential stroke.
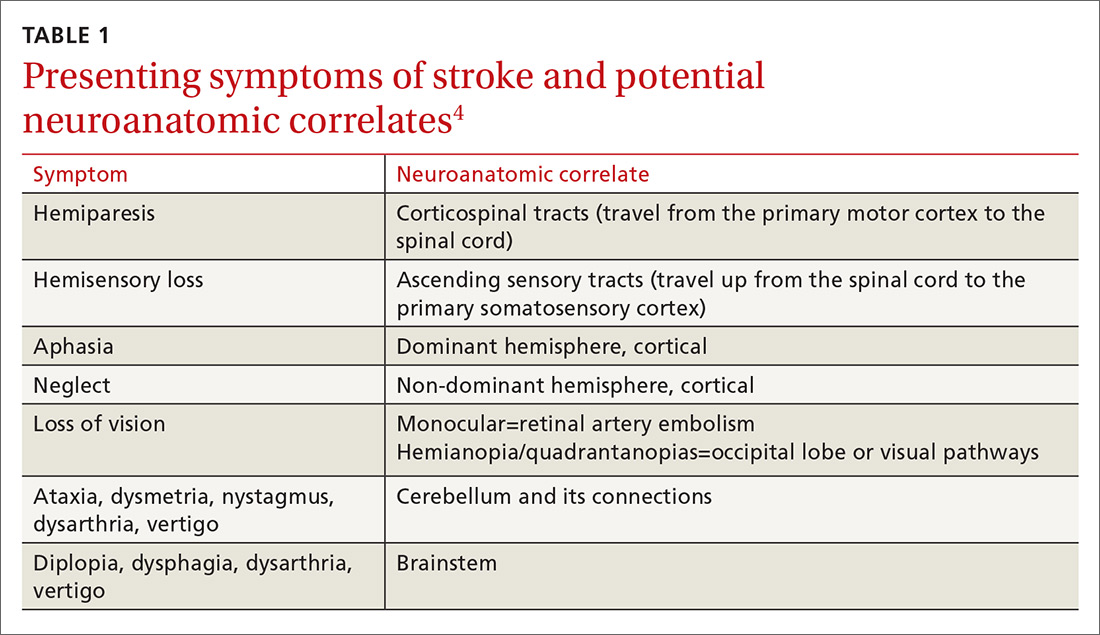
The differential diagnosis includes: seizure, complex migraine, medication effect (eg, slurred speech or confusion after taking a central nervous system [CNS] depressant), toxin exposure, electrolyte abnormalities (especially hypoglycemia), concussion/trauma, infection of the CNS, peripheral vertigo, demyelination, intracranial mass, Bell’s palsy, and psychogenic disorders. The history and physical, along with laboratory findings and brain imaging (detailed later in this article), will guide the FP toward (or away from) these various etiologies.
Optimal triage is a subject of ongoing interest and research
If stroke or TIA remains a possibility after an initial assessment, it’s time to stratify patients by risk.
One of the most widely accepted tools is the ABCD2 score (see TABLE 26). Clinicians can employ the ABCD2 risk stratification tool when trying to determine whether it is reasonable to pursue an expedited work-up (ie, <1 day) in the outpatient setting or recommend that the patient be evaluated in an emergency department (ED). The 90-day stroke rate following a TIA ranges from 3% with an ABCD2 score of 0 to 3 to 18% with a score of 6 or 7. A score of 0 to 3 is considered relatively low risk; in the absence of other compelling factors, rapid outpatient evaluation is appropriate. For patients with an ABCD2 score ≥4, referral to the ED or direct admission to the hospital is advised.
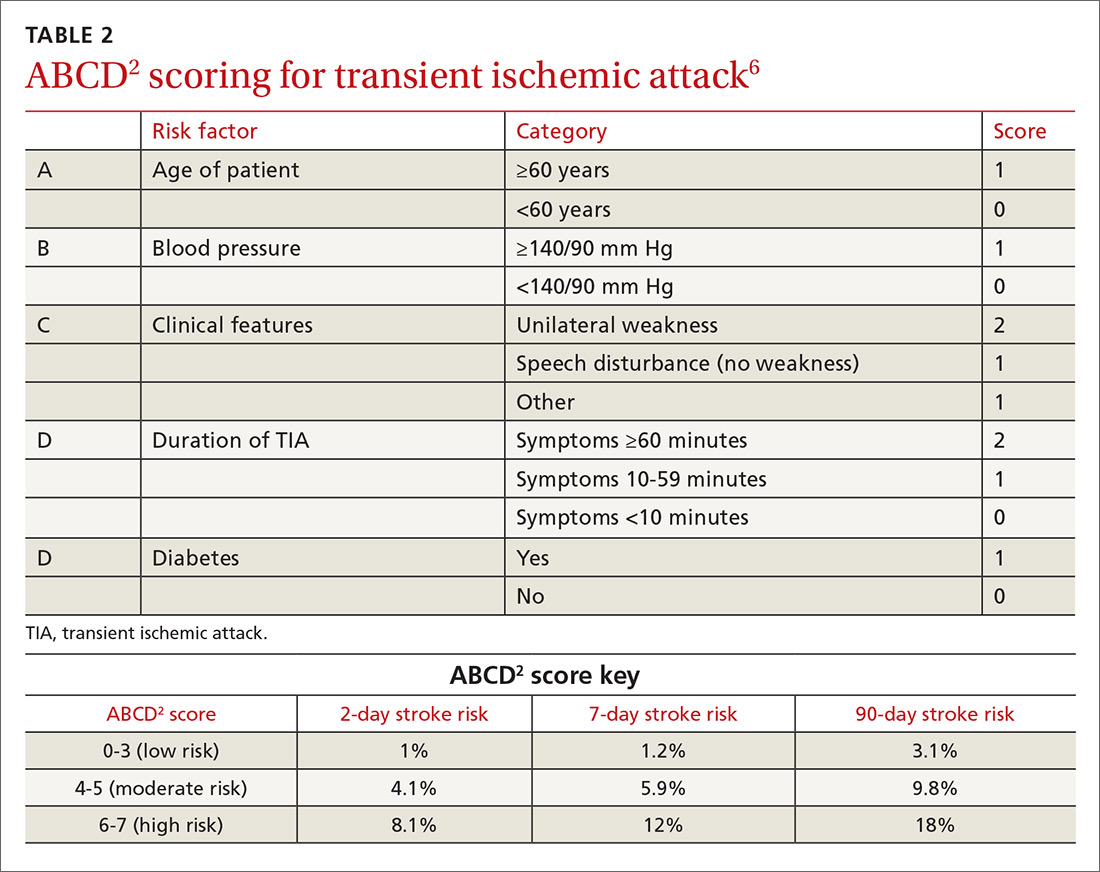
The validity of the ABCD2 score for risk stratification has been studied extensively with conflicting results.7-10 As with any assessment tool, it should be used as a guide, and should not supplant a full assessment of the patient or the judgment of the examining physician. In making the decision regarding inpatient or outpatient evaluation, it’s also important to consider available resources, access to specialists, and patient preference.
In a 2016 population-based study, the 30-day recurrent stroke/TIA rate for patients hospitalized for TIA was 3% compared with 10.7% for those discharged from the ED with referral to a stroke clinic and 10.6% for those discharged from the ED without a referral to a stroke clinic.11 These data suggest that only patients for whom you have a low clinical suspicion of stroke/TIA should be worked up as outpatients, and that hospital admission is advised in moderate- and high-risk cases. The findings also highlight the critical role that primary care physicians can play in triaging and managing these patients for secondary prevention.
CASE › This patient’s recent history of sudden-onset right-sided weakness and expressive language dysfunction is suspicious for left hemispheric ischemia. She has several risk factors for stroke, and her ABCD2 score is 5 (hypertension, age ≥60 years, unilateral weakness, and duration 10-59 min), which places her at moderate risk. Thus, the recommendation would be to have her go directly to an ED for rapid evaluation.
The diagnostic work-up
Even when a patient is sent to the ED, the FP plays a critical role in his or her continuing care. FPs will often coordinate with inpatient care and manage transition of care to the outpatient setting. (And in many communities, the ED or hospital physicians may themselves be family practitioners.)
In terms of care, not even an aspirin should be administered in a case like this because the patient has not yet had any neuroimaging, and differentiation of ischemic from hemorrhagic stroke cannot be made on clinical grounds alone. Once an ischemic stroke is confirmed, determining the etiology is critical given the significant management differences between the different types of stroke (atherosclerotic, cardioembolic, lacunar, or other).
Which imaging method, and when?
While a computerized tomography (CT) scan is the preferred initial imaging strategy for acute stroke to discern the ischemic type from the hemorrhagic, MRI is preferred for the evaluation of acute ischemic stroke because the method has a higher sensitivity for infarction and a greater ability to identify findings (such as demyelination) that would suggest an alternative diagnosis.
In addition to evaluating the brain parenchyma, physicians must also assess the cerebral vasculature. CT angiography (CTA) or MR angiography (MRA) of the head and neck are preferred over carotid ultrasound because they are capable of evaluating the entire cerebrovascular system12,13 and can be instrumental in identifying potential causes of stroke, as well as guiding therapeutic decisions. Carotid ultrasound is a reasonable alternative for patients presenting with symptoms indicative of anterior circulation involvement when CTA and MRA are unavailable or contraindicated, but it will not identify intracranial vascular disease, proximal common carotid disease, or vertebrobasilar disease.
Getting to the cause of suspected stroke: Labs and other diagnostic tests
A routine work-up includes BP checks, routine labs (complete blood count, complete metabolic panel, coagulation profile, and troponin), an electrocardiogram (EKG), a transthoracic echocardiogram (TTE) with bubble study if possible, and a minimum of 24 to 48 hours of cardiac rhythm monitoring. Cardiac rhythm monitoring should be extended in the setting of clinical concern for unidentified paroxysmal atrial fibrillation, such as an embolism without a proximal vascular source, multiple embolic infarcts in different vascular territories, a dilated left atrium, or other risk factors for atrial fibrillation that include smoking, systolic hypertension, diabetes, and heart failure (see TABLE 312,13,17,18).14-16 This standard diagnostic work-up will identify the cause of stroke in 70% to 80% of patients.19
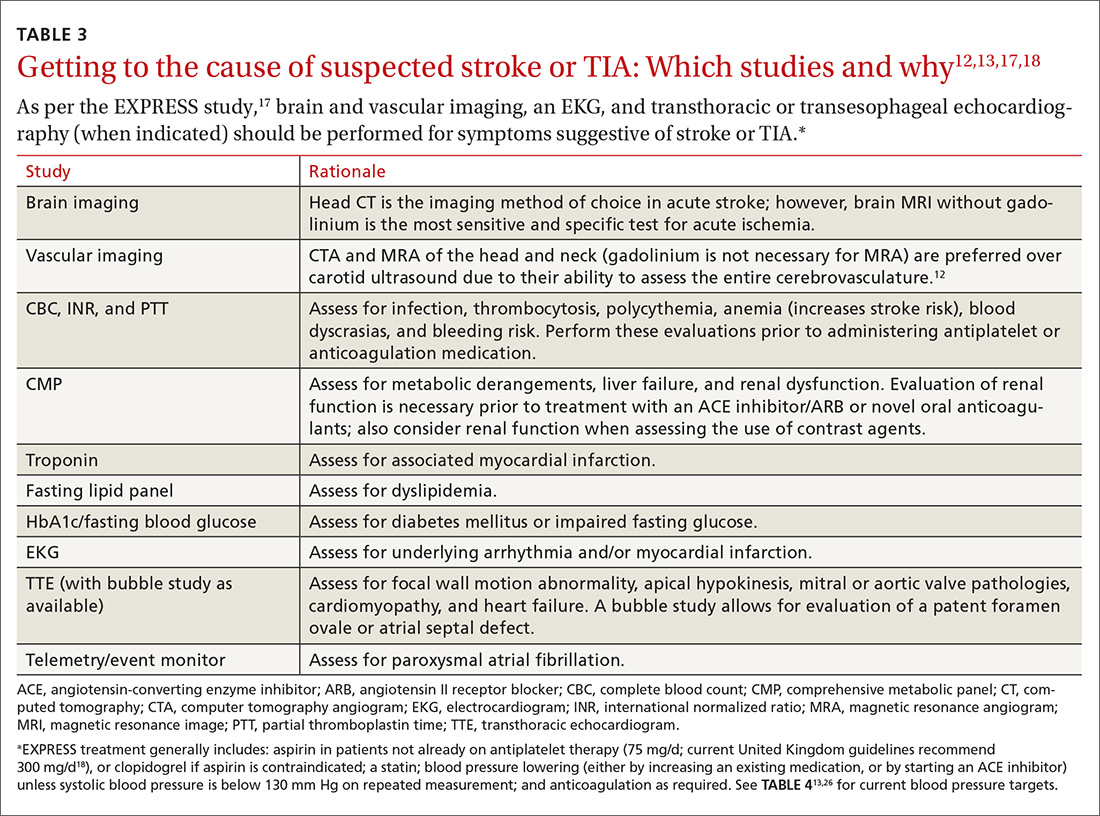
Additional investigations to consider if the etiology is not yet elucidated include a transesophageal echocardiogram (TEE), cerebral angiography, a coagulopathy evaluation, a lumbar puncture, and a vasculitis work-up. If available, consultation with a neurologist is appropriate for any patient who has had a stroke or TIA. Patients with unclear etiologies or for whom there are questions concerning strategies for preventing secondary stroke should be referred to Neurology and preferably a stroke specialist.
Timing matters, even when symptoms have resolved (ie, TIA).11,20 The EXPRESS trial17 (the Early use of eXisting PREventive Strategies for Stroke) looked at the effect of urgent assessment and treatment (≤1 day) of patients presenting with a TIA or minor stroke on the risk of recurrent stroke within 90 days. The diagnostic work-up included brain and vascular imaging together with an EKG. This intensive approach led to an absolute risk reduction of 8.2% (from 10.3% to 2.1%) in the risk of recurrent stroke at 90 days (number needed to treat [NNT]=12).17
Expedited work-up and treatment was also recently evaluated in a non-trial, real-world setting and was associated with reducing recurrent stroke by more than half the rate reported in older studies.20 Overall, the data suggest that evaluation within 24 hours confers substantial benefit, and that this evaluation can happen in an outpatient setting.21-23
Acute management: Use of tPA
Once imaging rules out intracranial hemorrhage, patients should be treated with tissue plasminogen activator (tPA) or an endovascular intervention as per guidelines.24 For patients with ischemic stroke ineligible for tPA or endovascular treatments, the initial focus is to determine the etiology of the symptoms so that the best strategies for prevention of secondary stroke may be employed.
Aspirin should be provided within 24 to 48 hours to all patients after intracranial hemorrhage is ruled out. Aspirin should be delayed for 24 hours in those given thrombolytics. The initial recommended dose of aspirin is 325 mg with continued low-dose (81 mg) aspirin daily.13 The addition of clopidogrel to aspirin within 24 hours of an event and continued for 21 days, followed by aspirin alone, was shown to be beneficial in a Chinese population with high-risk TIA (ABCD2 score ≥4) or minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3).25 Anticoagulation with heparin, warfarin, or a novel oral anticoagulant is generally not indicated in the acute setting due to the risk of hemorrhagic transformation.
Acute BP management depends upon the type of stroke (ischemic or hemorrhagic), eligibility for thrombolytics, timing of presentation, and possible comorbidities such as myocardial infarction or aortic dissection (see TABLE 413,26). In the absence of contraindications, high-intensity statins should be initiated in all patients able to take oral medications.
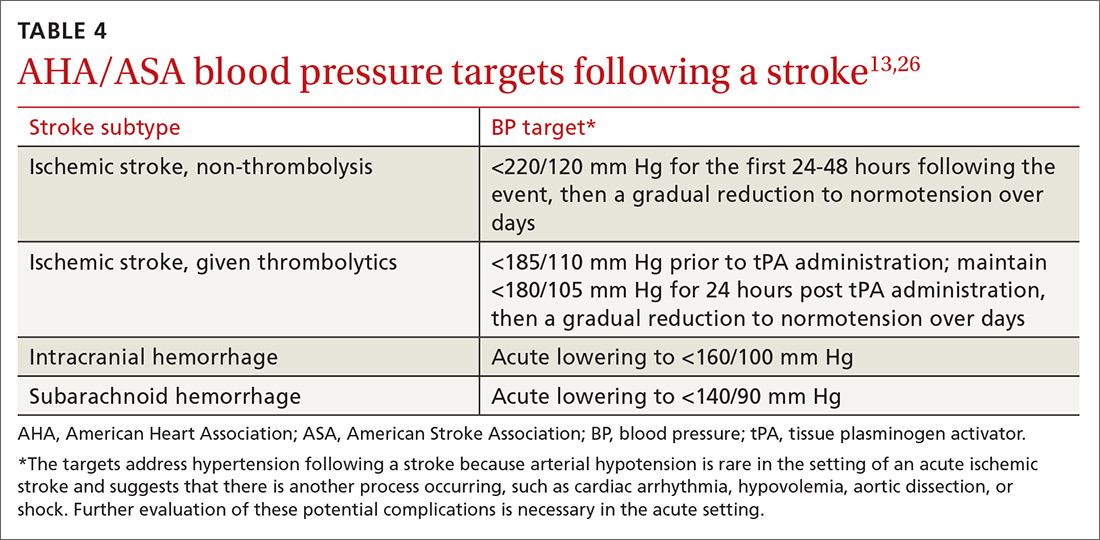
CASE › You appropriately referred your patient to the local ED. A head CT with head and neck CTA was performed. While the head CT did not show any abnormalities, the CTA demonstrated high-grade left internal carotid artery stenosis. The patient was given an initial dose of aspirin 325 mg and a high-intensity statin and admitted for further management. An MRI revealed a small shower of emboli in the left hemisphere, confirming the diagnosis of stroke over TIA. Labs were marginally remarkable with a low-density lipoprotein level of 115 mg/dL and an HbA1c of 6.2. Telemetry monitoring did not reveal any arrhythmias, and TTE was normal. BP remained in the high-normal to low-hypertensive range.
A Vascular Surgery consultation was obtained and the patient underwent a left carotid endarterectomy the following day. She did well without surgical complications. Her BP medications were adjusted; a combination of an angiotensin-converting enzyme inhibitor and a thiazide diuretic achieved a goal BP <140/90 mm Hg.
Permissive hypertension was not indicated due to her presentation >48 hours beyond the acute event. Low-dose aspirin and a high-intensity statin were continued, for secondary stroke prevention in the setting of atherosclerotic disease. She received smoking cessation counseling, which will continue.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
3. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db178.pdf. Accessed June 5, 2016.
4. Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457-2460.
5. Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096-3098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18688003. Accessed June 5, 2016.
6. Hankey GJ. The ABCD, California, and unified ABCD2 risk scores predicted stroke within 2, 7, and 90 days after TIA. Evid Based Med. 2007;12:88.
7. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844-850.
8. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005; 366:29-36.
9. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892-2897.
10. Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45:418-425.
11. Sacco RL, Rundek T. The value of urgent specialized care for TIA and minor stroke. N Engl J Med. 2016;374:1577-1579.
12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47:273-281.
13. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
14. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
16. Christophersen IE, Yin X, Larson MG, et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation ni the Framingham Heart Study. Am Heart J. 2016;178:45-54.
17. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442.
18. National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available at: https://www.nice.org.uk/guidance/cg68. Published 2008. Accessed February 5, 2017.
19. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438.
20. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
21. Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology. 2011;77:2082-2088.
22. Mijalski C, Silver B. TIA management: should TIA patients be admitted? should TIA patients get combination antiplatelet therapy? The Neurohospitalist. 2015;5:151-160.
23. Silver B, Adeoye O. Management of patients with transient ischemic attack in the emergency department. Neurology. 2016;86:1568-1569.
24. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641.
25. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
26. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032-2060.
CASE › A 68-year-old woman with hypertension and hyperlipidemia comes into your office for evaluation of a 30-minute episode of sudden-onset right-hand weakness and difficulty speaking that occurred 4 days earlier. The patient, who is also a smoker, has come in at the insistence of her daughter. On examination, her blood pressure (BP) is 145/88 mm Hg and her heart rate is 76 beats/minute and regular. She appears well and her language function is normal. The rest of her examination is normal. How would you proceed?
Stroke—the death of nerve cells due to a lack of blood supply from either infarction or hemorrhage—strikes nearly 800,000 people in the United States every year.1,2 Of these events, 130,000 are fatal, making stroke the fifth leading cause of death.3 Effective, early evaluation and cause-specific treatment are crucial parts of stroke care.
Research has helped to clarify the critical role primary care physicians play in recognizing, triaging, and managing stroke and transient ischemic attacks (TIA). This article reviews what we know about the different ways that a stroke and a TIA can present, the appropriate diagnostic work-up for patients presenting with symptoms of either event, and management strategies for subacute care (24 hours to up to 14 days after a stroke has occurred).4,5 Unless otherwise specified, this review will focus on ischemic stroke because 87% of strokes are attributable to ischemia.1
A follow-up to this article on secondary stroke prevention will appear in the journal next month.

Look to onset more than type of symptoms for clues
Stroke presents as a sudden onset of neurologic deficits (language, motor, sensory, cerebellar, or brainstem functions) (TABLE 14). Because presenting symptoms can vary widely, sudden onset, rather than particular symptoms, should raise a red flag for potential stroke.

The differential diagnosis includes: seizure, complex migraine, medication effect (eg, slurred speech or confusion after taking a central nervous system [CNS] depressant), toxin exposure, electrolyte abnormalities (especially hypoglycemia), concussion/trauma, infection of the CNS, peripheral vertigo, demyelination, intracranial mass, Bell’s palsy, and psychogenic disorders. The history and physical, along with laboratory findings and brain imaging (detailed later in this article), will guide the FP toward (or away from) these various etiologies.
Optimal triage is a subject of ongoing interest and research
If stroke or TIA remains a possibility after an initial assessment, it’s time to stratify patients by risk.
One of the most widely accepted tools is the ABCD2 score (see TABLE 26). Clinicians can employ the ABCD2 risk stratification tool when trying to determine whether it is reasonable to pursue an expedited work-up (ie, <1 day) in the outpatient setting or recommend that the patient be evaluated in an emergency department (ED). The 90-day stroke rate following a TIA ranges from 3% with an ABCD2 score of 0 to 3 to 18% with a score of 6 or 7. A score of 0 to 3 is considered relatively low risk; in the absence of other compelling factors, rapid outpatient evaluation is appropriate. For patients with an ABCD2 score ≥4, referral to the ED or direct admission to the hospital is advised.

The validity of the ABCD2 score for risk stratification has been studied extensively with conflicting results.7-10 As with any assessment tool, it should be used as a guide, and should not supplant a full assessment of the patient or the judgment of the examining physician. In making the decision regarding inpatient or outpatient evaluation, it’s also important to consider available resources, access to specialists, and patient preference.
In a 2016 population-based study, the 30-day recurrent stroke/TIA rate for patients hospitalized for TIA was 3% compared with 10.7% for those discharged from the ED with referral to a stroke clinic and 10.6% for those discharged from the ED without a referral to a stroke clinic.11 These data suggest that only patients for whom you have a low clinical suspicion of stroke/TIA should be worked up as outpatients, and that hospital admission is advised in moderate- and high-risk cases. The findings also highlight the critical role that primary care physicians can play in triaging and managing these patients for secondary prevention.
CASE › This patient’s recent history of sudden-onset right-sided weakness and expressive language dysfunction is suspicious for left hemispheric ischemia. She has several risk factors for stroke, and her ABCD2 score is 5 (hypertension, age ≥60 years, unilateral weakness, and duration 10-59 min), which places her at moderate risk. Thus, the recommendation would be to have her go directly to an ED for rapid evaluation.
The diagnostic work-up
Even when a patient is sent to the ED, the FP plays a critical role in his or her continuing care. FPs will often coordinate with inpatient care and manage transition of care to the outpatient setting. (And in many communities, the ED or hospital physicians may themselves be family practitioners.)
In terms of care, not even an aspirin should be administered in a case like this because the patient has not yet had any neuroimaging, and differentiation of ischemic from hemorrhagic stroke cannot be made on clinical grounds alone. Once an ischemic stroke is confirmed, determining the etiology is critical given the significant management differences between the different types of stroke (atherosclerotic, cardioembolic, lacunar, or other).
Which imaging method, and when?
While a computerized tomography (CT) scan is the preferred initial imaging strategy for acute stroke to discern the ischemic type from the hemorrhagic, MRI is preferred for the evaluation of acute ischemic stroke because the method has a higher sensitivity for infarction and a greater ability to identify findings (such as demyelination) that would suggest an alternative diagnosis.
In addition to evaluating the brain parenchyma, physicians must also assess the cerebral vasculature. CT angiography (CTA) or MR angiography (MRA) of the head and neck are preferred over carotid ultrasound because they are capable of evaluating the entire cerebrovascular system12,13 and can be instrumental in identifying potential causes of stroke, as well as guiding therapeutic decisions. Carotid ultrasound is a reasonable alternative for patients presenting with symptoms indicative of anterior circulation involvement when CTA and MRA are unavailable or contraindicated, but it will not identify intracranial vascular disease, proximal common carotid disease, or vertebrobasilar disease.
Getting to the cause of suspected stroke: Labs and other diagnostic tests
A routine work-up includes BP checks, routine labs (complete blood count, complete metabolic panel, coagulation profile, and troponin), an electrocardiogram (EKG), a transthoracic echocardiogram (TTE) with bubble study if possible, and a minimum of 24 to 48 hours of cardiac rhythm monitoring. Cardiac rhythm monitoring should be extended in the setting of clinical concern for unidentified paroxysmal atrial fibrillation, such as an embolism without a proximal vascular source, multiple embolic infarcts in different vascular territories, a dilated left atrium, or other risk factors for atrial fibrillation that include smoking, systolic hypertension, diabetes, and heart failure (see TABLE 312,13,17,18).14-16 This standard diagnostic work-up will identify the cause of stroke in 70% to 80% of patients.19

Additional investigations to consider if the etiology is not yet elucidated include a transesophageal echocardiogram (TEE), cerebral angiography, a coagulopathy evaluation, a lumbar puncture, and a vasculitis work-up. If available, consultation with a neurologist is appropriate for any patient who has had a stroke or TIA. Patients with unclear etiologies or for whom there are questions concerning strategies for preventing secondary stroke should be referred to Neurology and preferably a stroke specialist.
Timing matters, even when symptoms have resolved (ie, TIA).11,20 The EXPRESS trial17 (the Early use of eXisting PREventive Strategies for Stroke) looked at the effect of urgent assessment and treatment (≤1 day) of patients presenting with a TIA or minor stroke on the risk of recurrent stroke within 90 days. The diagnostic work-up included brain and vascular imaging together with an EKG. This intensive approach led to an absolute risk reduction of 8.2% (from 10.3% to 2.1%) in the risk of recurrent stroke at 90 days (number needed to treat [NNT]=12).17
Expedited work-up and treatment was also recently evaluated in a non-trial, real-world setting and was associated with reducing recurrent stroke by more than half the rate reported in older studies.20 Overall, the data suggest that evaluation within 24 hours confers substantial benefit, and that this evaluation can happen in an outpatient setting.21-23
Acute management: Use of tPA
Once imaging rules out intracranial hemorrhage, patients should be treated with tissue plasminogen activator (tPA) or an endovascular intervention as per guidelines.24 For patients with ischemic stroke ineligible for tPA or endovascular treatments, the initial focus is to determine the etiology of the symptoms so that the best strategies for prevention of secondary stroke may be employed.
Aspirin should be provided within 24 to 48 hours to all patients after intracranial hemorrhage is ruled out. Aspirin should be delayed for 24 hours in those given thrombolytics. The initial recommended dose of aspirin is 325 mg with continued low-dose (81 mg) aspirin daily.13 The addition of clopidogrel to aspirin within 24 hours of an event and continued for 21 days, followed by aspirin alone, was shown to be beneficial in a Chinese population with high-risk TIA (ABCD2 score ≥4) or minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3).25 Anticoagulation with heparin, warfarin, or a novel oral anticoagulant is generally not indicated in the acute setting due to the risk of hemorrhagic transformation.
Acute BP management depends upon the type of stroke (ischemic or hemorrhagic), eligibility for thrombolytics, timing of presentation, and possible comorbidities such as myocardial infarction or aortic dissection (see TABLE 413,26). In the absence of contraindications, high-intensity statins should be initiated in all patients able to take oral medications.

CASE › You appropriately referred your patient to the local ED. A head CT with head and neck CTA was performed. While the head CT did not show any abnormalities, the CTA demonstrated high-grade left internal carotid artery stenosis. The patient was given an initial dose of aspirin 325 mg and a high-intensity statin and admitted for further management. An MRI revealed a small shower of emboli in the left hemisphere, confirming the diagnosis of stroke over TIA. Labs were marginally remarkable with a low-density lipoprotein level of 115 mg/dL and an HbA1c of 6.2. Telemetry monitoring did not reveal any arrhythmias, and TTE was normal. BP remained in the high-normal to low-hypertensive range.
A Vascular Surgery consultation was obtained and the patient underwent a left carotid endarterectomy the following day. She did well without surgical complications. Her BP medications were adjusted; a combination of an angiotensin-converting enzyme inhibitor and a thiazide diuretic achieved a goal BP <140/90 mm Hg.
Permissive hypertension was not indicated due to her presentation >48 hours beyond the acute event. Low-dose aspirin and a high-intensity statin were continued, for secondary stroke prevention in the setting of atherosclerotic disease. She received smoking cessation counseling, which will continue.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
CASE › A 68-year-old woman with hypertension and hyperlipidemia comes into your office for evaluation of a 30-minute episode of sudden-onset right-hand weakness and difficulty speaking that occurred 4 days earlier. The patient, who is also a smoker, has come in at the insistence of her daughter. On examination, her blood pressure (BP) is 145/88 mm Hg and her heart rate is 76 beats/minute and regular. She appears well and her language function is normal. The rest of her examination is normal. How would you proceed?
Stroke—the death of nerve cells due to a lack of blood supply from either infarction or hemorrhage—strikes nearly 800,000 people in the United States every year.1,2 Of these events, 130,000 are fatal, making stroke the fifth leading cause of death.3 Effective, early evaluation and cause-specific treatment are crucial parts of stroke care.
Research has helped to clarify the critical role primary care physicians play in recognizing, triaging, and managing stroke and transient ischemic attacks (TIA). This article reviews what we know about the different ways that a stroke and a TIA can present, the appropriate diagnostic work-up for patients presenting with symptoms of either event, and management strategies for subacute care (24 hours to up to 14 days after a stroke has occurred).4,5 Unless otherwise specified, this review will focus on ischemic stroke because 87% of strokes are attributable to ischemia.1
A follow-up to this article on secondary stroke prevention will appear in the journal next month.

Look to onset more than type of symptoms for clues
Stroke presents as a sudden onset of neurologic deficits (language, motor, sensory, cerebellar, or brainstem functions) (TABLE 14). Because presenting symptoms can vary widely, sudden onset, rather than particular symptoms, should raise a red flag for potential stroke.

The differential diagnosis includes: seizure, complex migraine, medication effect (eg, slurred speech or confusion after taking a central nervous system [CNS] depressant), toxin exposure, electrolyte abnormalities (especially hypoglycemia), concussion/trauma, infection of the CNS, peripheral vertigo, demyelination, intracranial mass, Bell’s palsy, and psychogenic disorders. The history and physical, along with laboratory findings and brain imaging (detailed later in this article), will guide the FP toward (or away from) these various etiologies.
Optimal triage is a subject of ongoing interest and research
If stroke or TIA remains a possibility after an initial assessment, it’s time to stratify patients by risk.
One of the most widely accepted tools is the ABCD2 score (see TABLE 26). Clinicians can employ the ABCD2 risk stratification tool when trying to determine whether it is reasonable to pursue an expedited work-up (ie, <1 day) in the outpatient setting or recommend that the patient be evaluated in an emergency department (ED). The 90-day stroke rate following a TIA ranges from 3% with an ABCD2 score of 0 to 3 to 18% with a score of 6 or 7. A score of 0 to 3 is considered relatively low risk; in the absence of other compelling factors, rapid outpatient evaluation is appropriate. For patients with an ABCD2 score ≥4, referral to the ED or direct admission to the hospital is advised.

The validity of the ABCD2 score for risk stratification has been studied extensively with conflicting results.7-10 As with any assessment tool, it should be used as a guide, and should not supplant a full assessment of the patient or the judgment of the examining physician. In making the decision regarding inpatient or outpatient evaluation, it’s also important to consider available resources, access to specialists, and patient preference.
In a 2016 population-based study, the 30-day recurrent stroke/TIA rate for patients hospitalized for TIA was 3% compared with 10.7% for those discharged from the ED with referral to a stroke clinic and 10.6% for those discharged from the ED without a referral to a stroke clinic.11 These data suggest that only patients for whom you have a low clinical suspicion of stroke/TIA should be worked up as outpatients, and that hospital admission is advised in moderate- and high-risk cases. The findings also highlight the critical role that primary care physicians can play in triaging and managing these patients for secondary prevention.
CASE › This patient’s recent history of sudden-onset right-sided weakness and expressive language dysfunction is suspicious for left hemispheric ischemia. She has several risk factors for stroke, and her ABCD2 score is 5 (hypertension, age ≥60 years, unilateral weakness, and duration 10-59 min), which places her at moderate risk. Thus, the recommendation would be to have her go directly to an ED for rapid evaluation.
The diagnostic work-up
Even when a patient is sent to the ED, the FP plays a critical role in his or her continuing care. FPs will often coordinate with inpatient care and manage transition of care to the outpatient setting. (And in many communities, the ED or hospital physicians may themselves be family practitioners.)
In terms of care, not even an aspirin should be administered in a case like this because the patient has not yet had any neuroimaging, and differentiation of ischemic from hemorrhagic stroke cannot be made on clinical grounds alone. Once an ischemic stroke is confirmed, determining the etiology is critical given the significant management differences between the different types of stroke (atherosclerotic, cardioembolic, lacunar, or other).
Which imaging method, and when?
While a computerized tomography (CT) scan is the preferred initial imaging strategy for acute stroke to discern the ischemic type from the hemorrhagic, MRI is preferred for the evaluation of acute ischemic stroke because the method has a higher sensitivity for infarction and a greater ability to identify findings (such as demyelination) that would suggest an alternative diagnosis.
In addition to evaluating the brain parenchyma, physicians must also assess the cerebral vasculature. CT angiography (CTA) or MR angiography (MRA) of the head and neck are preferred over carotid ultrasound because they are capable of evaluating the entire cerebrovascular system12,13 and can be instrumental in identifying potential causes of stroke, as well as guiding therapeutic decisions. Carotid ultrasound is a reasonable alternative for patients presenting with symptoms indicative of anterior circulation involvement when CTA and MRA are unavailable or contraindicated, but it will not identify intracranial vascular disease, proximal common carotid disease, or vertebrobasilar disease.
Getting to the cause of suspected stroke: Labs and other diagnostic tests
A routine work-up includes BP checks, routine labs (complete blood count, complete metabolic panel, coagulation profile, and troponin), an electrocardiogram (EKG), a transthoracic echocardiogram (TTE) with bubble study if possible, and a minimum of 24 to 48 hours of cardiac rhythm monitoring. Cardiac rhythm monitoring should be extended in the setting of clinical concern for unidentified paroxysmal atrial fibrillation, such as an embolism without a proximal vascular source, multiple embolic infarcts in different vascular territories, a dilated left atrium, or other risk factors for atrial fibrillation that include smoking, systolic hypertension, diabetes, and heart failure (see TABLE 312,13,17,18).14-16 This standard diagnostic work-up will identify the cause of stroke in 70% to 80% of patients.19

Additional investigations to consider if the etiology is not yet elucidated include a transesophageal echocardiogram (TEE), cerebral angiography, a coagulopathy evaluation, a lumbar puncture, and a vasculitis work-up. If available, consultation with a neurologist is appropriate for any patient who has had a stroke or TIA. Patients with unclear etiologies or for whom there are questions concerning strategies for preventing secondary stroke should be referred to Neurology and preferably a stroke specialist.
Timing matters, even when symptoms have resolved (ie, TIA).11,20 The EXPRESS trial17 (the Early use of eXisting PREventive Strategies for Stroke) looked at the effect of urgent assessment and treatment (≤1 day) of patients presenting with a TIA or minor stroke on the risk of recurrent stroke within 90 days. The diagnostic work-up included brain and vascular imaging together with an EKG. This intensive approach led to an absolute risk reduction of 8.2% (from 10.3% to 2.1%) in the risk of recurrent stroke at 90 days (number needed to treat [NNT]=12).17
Expedited work-up and treatment was also recently evaluated in a non-trial, real-world setting and was associated with reducing recurrent stroke by more than half the rate reported in older studies.20 Overall, the data suggest that evaluation within 24 hours confers substantial benefit, and that this evaluation can happen in an outpatient setting.21-23
Acute management: Use of tPA
Once imaging rules out intracranial hemorrhage, patients should be treated with tissue plasminogen activator (tPA) or an endovascular intervention as per guidelines.24 For patients with ischemic stroke ineligible for tPA or endovascular treatments, the initial focus is to determine the etiology of the symptoms so that the best strategies for prevention of secondary stroke may be employed.
Aspirin should be provided within 24 to 48 hours to all patients after intracranial hemorrhage is ruled out. Aspirin should be delayed for 24 hours in those given thrombolytics. The initial recommended dose of aspirin is 325 mg with continued low-dose (81 mg) aspirin daily.13 The addition of clopidogrel to aspirin within 24 hours of an event and continued for 21 days, followed by aspirin alone, was shown to be beneficial in a Chinese population with high-risk TIA (ABCD2 score ≥4) or minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3).25 Anticoagulation with heparin, warfarin, or a novel oral anticoagulant is generally not indicated in the acute setting due to the risk of hemorrhagic transformation.
Acute BP management depends upon the type of stroke (ischemic or hemorrhagic), eligibility for thrombolytics, timing of presentation, and possible comorbidities such as myocardial infarction or aortic dissection (see TABLE 413,26). In the absence of contraindications, high-intensity statins should be initiated in all patients able to take oral medications.

CASE › You appropriately referred your patient to the local ED. A head CT with head and neck CTA was performed. While the head CT did not show any abnormalities, the CTA demonstrated high-grade left internal carotid artery stenosis. The patient was given an initial dose of aspirin 325 mg and a high-intensity statin and admitted for further management. An MRI revealed a small shower of emboli in the left hemisphere, confirming the diagnosis of stroke over TIA. Labs were marginally remarkable with a low-density lipoprotein level of 115 mg/dL and an HbA1c of 6.2. Telemetry monitoring did not reveal any arrhythmias, and TTE was normal. BP remained in the high-normal to low-hypertensive range.
A Vascular Surgery consultation was obtained and the patient underwent a left carotid endarterectomy the following day. She did well without surgical complications. Her BP medications were adjusted; a combination of an angiotensin-converting enzyme inhibitor and a thiazide diuretic achieved a goal BP <140/90 mm Hg.
Permissive hypertension was not indicated due to her presentation >48 hours beyond the acute event. Low-dose aspirin and a high-intensity statin were continued, for secondary stroke prevention in the setting of atherosclerotic disease. She received smoking cessation counseling, which will continue.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
3. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db178.pdf. Accessed June 5, 2016.
4. Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457-2460.
5. Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096-3098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18688003. Accessed June 5, 2016.
6. Hankey GJ. The ABCD, California, and unified ABCD2 risk scores predicted stroke within 2, 7, and 90 days after TIA. Evid Based Med. 2007;12:88.
7. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844-850.
8. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005; 366:29-36.
9. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892-2897.
10. Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45:418-425.
11. Sacco RL, Rundek T. The value of urgent specialized care for TIA and minor stroke. N Engl J Med. 2016;374:1577-1579.
12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47:273-281.
13. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
14. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
16. Christophersen IE, Yin X, Larson MG, et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation ni the Framingham Heart Study. Am Heart J. 2016;178:45-54.
17. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442.
18. National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available at: https://www.nice.org.uk/guidance/cg68. Published 2008. Accessed February 5, 2017.
19. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438.
20. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
21. Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology. 2011;77:2082-2088.
22. Mijalski C, Silver B. TIA management: should TIA patients be admitted? should TIA patients get combination antiplatelet therapy? The Neurohospitalist. 2015;5:151-160.
23. Silver B, Adeoye O. Management of patients with transient ischemic attack in the emergency department. Neurology. 2016;86:1568-1569.
24. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641.
25. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
26. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032-2060.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
3. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db178.pdf. Accessed June 5, 2016.
4. Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457-2460.
5. Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096-3098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18688003. Accessed June 5, 2016.
6. Hankey GJ. The ABCD, California, and unified ABCD2 risk scores predicted stroke within 2, 7, and 90 days after TIA. Evid Based Med. 2007;12:88.
7. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844-850.
8. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005; 366:29-36.
9. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892-2897.
10. Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45:418-425.
11. Sacco RL, Rundek T. The value of urgent specialized care for TIA and minor stroke. N Engl J Med. 2016;374:1577-1579.
12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47:273-281.
13. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
14. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
16. Christophersen IE, Yin X, Larson MG, et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation ni the Framingham Heart Study. Am Heart J. 2016;178:45-54.
17. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442.
18. National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available at: https://www.nice.org.uk/guidance/cg68. Published 2008. Accessed February 5, 2017.
19. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438.
20. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
21. Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology. 2011;77:2082-2088.
22. Mijalski C, Silver B. TIA management: should TIA patients be admitted? should TIA patients get combination antiplatelet therapy? The Neurohospitalist. 2015;5:151-160.
23. Silver B, Adeoye O. Management of patients with transient ischemic attack in the emergency department. Neurology. 2016;86:1568-1569.
24. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641.
25. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
26. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032-2060.
PRACTICE RECOMMENDATIONS
› Perform an urgent work-up on patients presenting with symptoms of a transient ischemic attack or stroke. A
› Employ the ABCD2 risk stratification tool when determining whether it is reasonable to pursue an expedited work-up in the outpatient setting or recommend that a patient be evaluated in an emergency department. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Post-bariatric surgery patients: Your role in their long-term care
More than one-third of American adults and approximately 17% of children and adolescents between the ages of 2 and 19 years are obese.1,2 Poor diet coupled with a sedentary lifestyle is the highest ranked cause of non-communicable disease and a leading cause of preventable death, according to the National Research Council.3
Bariatric surgery (BS) is a viable therapeutic option for obese patients who do not respond to conventional lifestyle interventions for losing weight. There are multiple gastrointestinal (GI) procedures available that are classified as either malabsorptive (Roux-en-Y gastric bypass [RYGB] and biliopancreatic diversion [BPD] with or without duodenal switch) or restrictive (laparoscopic adjustable gastric banding [LAGB] and vertical sleeve gastrectomy [VSG]).
Approximately half of the 196,000 bariatric procedures performed in the United States in 2015 were of the sleeve variety, another 23% were RYGB, and the remaining percentage was divided among the other types.4 Postoperative risks include nutritional deficiencies, decreased bone mineral density (BMD), dumping syndrome (when food rapidly dumps from the stomach to the intestine), and gastroesophageal reflux disease (GERD) with possible ulceration.
Despite these potential complications, a systematic review and meta-analysis found that obese people who underwent BS (gastric banding or gastric bypass) had significantly reduced risks of global, non-cardiovascular (CV), and CV mortality compared with obese controls.5 Helping patients to realize these benefits requires that the entire health care team—especially the family physician—is aware of the special considerations for this population.
To that end, this article reviews the details of diagnosing and managing post-surgical complications. It also addresses issues unique to managing certain subpopulations, such as post-BS patients who require revision surgery or who want to pursue body contouring surgery; adolescents who undergo BS surgery; and women who want to get pregnant postoperatively.
Monitor patients for these post-surgery complications
Postoperative BS follow-up varies depending on location, surgeon preference, and availability of multidisciplinary resources. At our institution, patients have a minimum of 3 follow-up visits with their surgeon (during hospitalization and 2 weeks and 2 months postoperatively). This is followed by visits with Endocrinology 6 months after surgery and annually thereafter. Given the variability of follow-up, family physicians should coordinate with specialists where appropriate and be aware of postoperative complications and monitoring since it is likely they will have the most frequent contact with these patients.
Nutritional deficiencies are common and require lifelong screening
Nutritional deficiencies are the most common complications of malabsorptive BS. Guidelines from the Endocrine Society, as well as guidelines from the American Association of Clinical Endocrinologists (AACE), The Obesity Society (TOS), and the American Society for Metabolic and Bariatric Surgery (ASMBS), recommend routine lifetime screening for deficiencies after surgery.6,7 Complete blood cell count, electrolytes, glucose, creatinine, and liver function tests should be obtained at one, 3, 6, 12, 18, and 24 months following surgery and annually thereafter.6
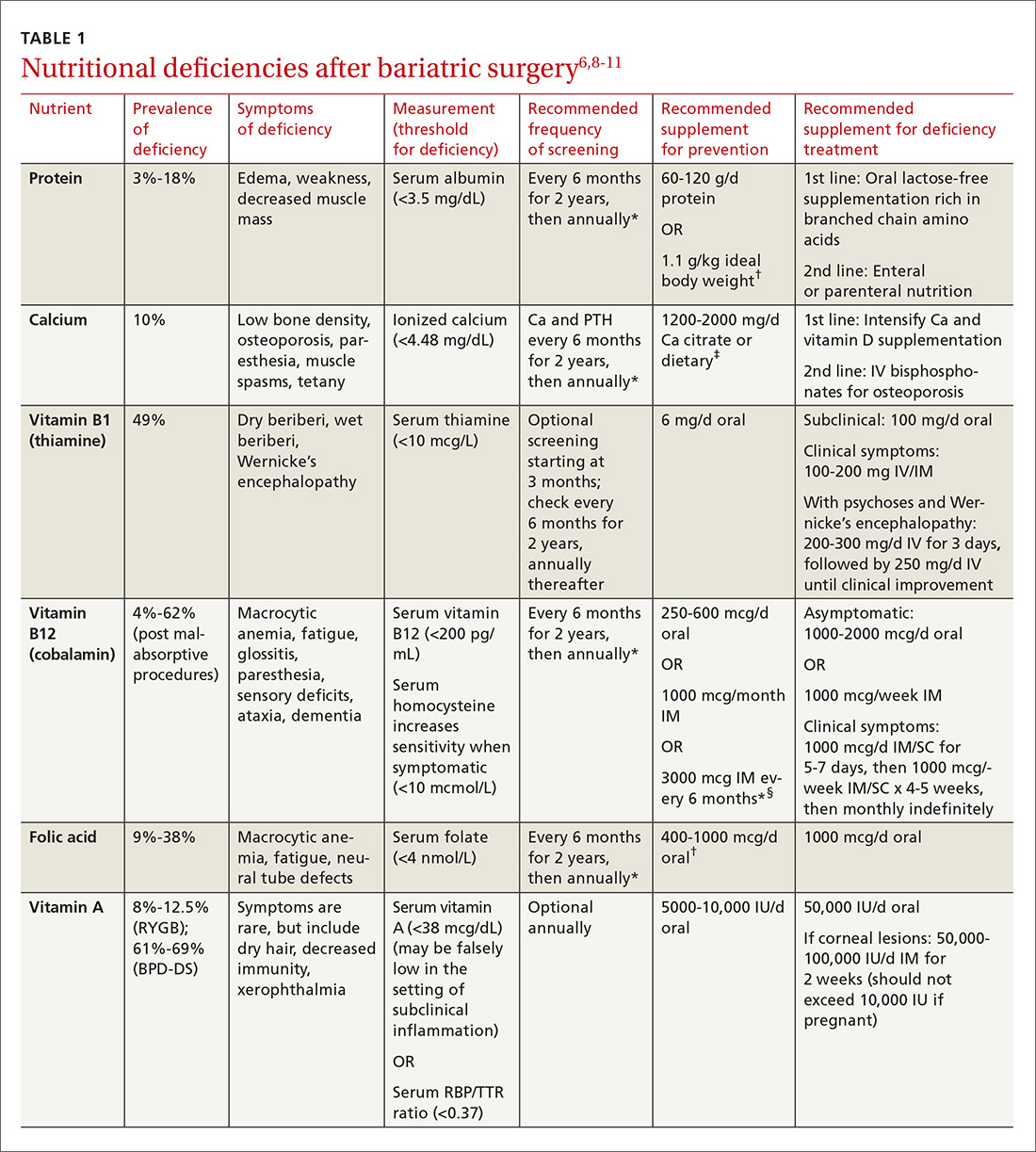
Multiple factors contribute to nutritional and micronutrient deficiencies, including reduced oral intake of food, decreased GI absorption, food intolerance, nausea/vomiting, and nonadherence with dietary supplements.8 Oral supplementation should be in chewable, powder, or liquid form because pill and capsule absorption may be altered.8,9 Over-the-counter multivitamins may not contain the requisite daily doses recommended after BS.9 Patients and physicians should evaluate supplements together to ensure appropriate nutritional and micronutrient supplementation (TABLE 16,8-11).
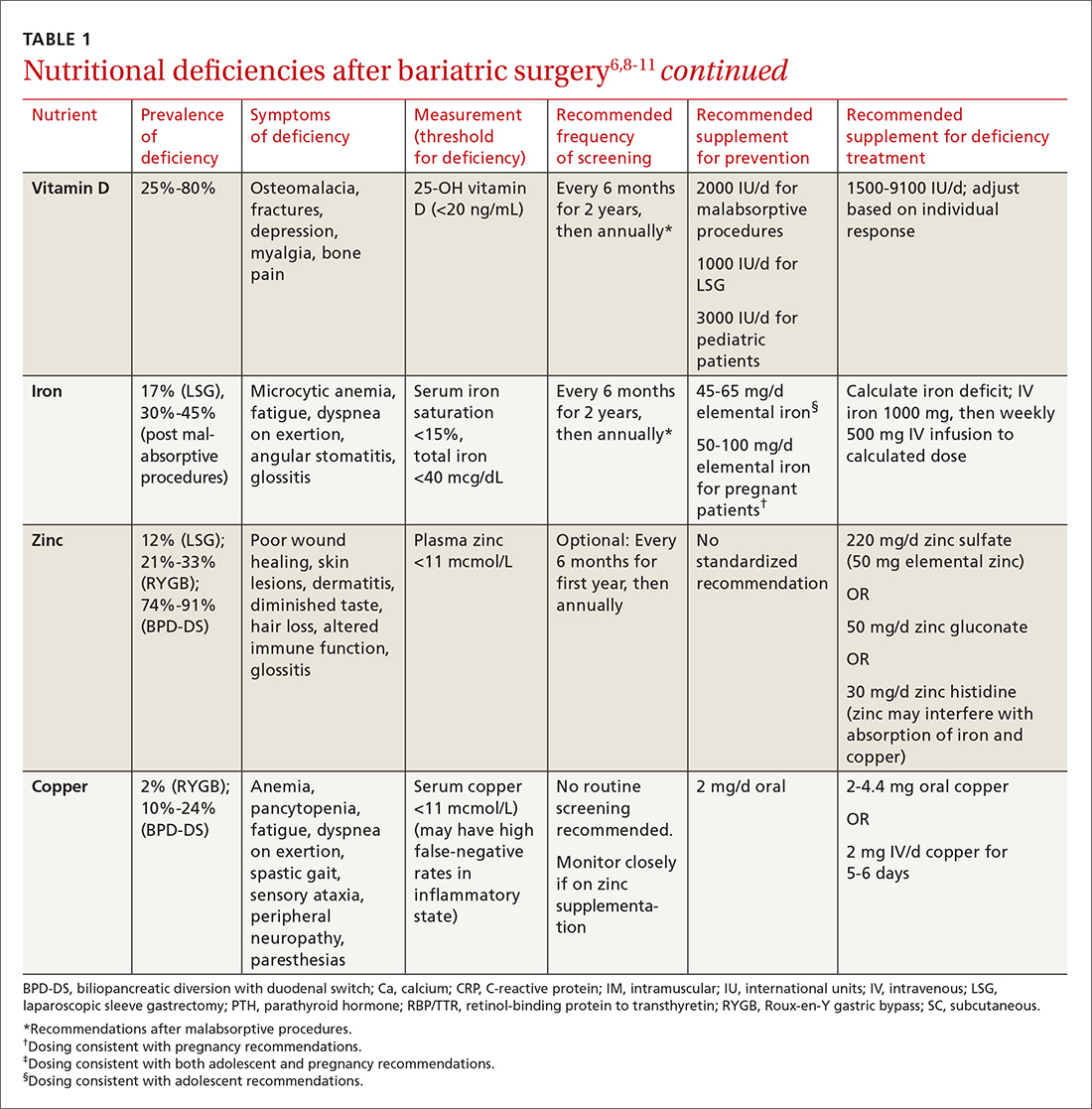
Bone mineral density can start to decrease soon after surgery
Studies evaluating BMD after BS have produced variable findings. In obese patients, dual-energy x-ray absorptiometry (DEXA) measurements may not be accurate due to adipose tissue artifact and table weight limits. In addition, limited data exist on the incidence of fractures after BS. Of 2 notable studies, only one, a population-based study involving 258 Minnesota residents who underwent a first bariatric surgery between 1985 and 2004, demonstrated a significantly increased incidence of fractures.12,13
In addition, studies show bone turnover markers, including C-terminal telopeptide, increase as early as 3 months after BS.14 Several guidelines recommend routine BMD screening after BS (TABLE 2).6,7 The mechanism of bone demineralization is likely multifactorial—a function of the magnitude of the weight loss and skeletal unloading, calcium and vitamin D deficiencies, and associated secondary hyperparathyroidism.15 Treatment for secondary hyperparathyroidism is adequate supplementation with vitamin D and calcium.

Optimal dosing for vitamin D has not been determined. One recent systematic review suggests routine prophylaxis with at least 2000 international units (IU)/d and found the greatest improvement for known deficiency with doses of 1500-9100 IU/d following malabsorptive surgeries.11 After laparoscopic sleeve gastrectomy, at least 1000 IU/d vitamin D is recommended.11
Overall, high variability exists among patients, and an individualized approach for dosing is recommended.11 Vitamin D levels should be monitored 2 and 4 weeks after initiation of treatment and every 3 months thereafter.11 Normal levels of serum calcium, 25-OH vitamin D, bone-specific alkaline phosphatase, and 24-hour urinary calcium excretion indicate adequate calcium and vitamin D supplementation.6
Dumping syndrome can lead to hypoglycemia
Dumping syndrome is a common complication following BS, with prevalence ranging from 25% to 75%, depending upon the type of procedure performed.16,17 There are 2 types: early and late. Early dumping syndrome occurs within 30 minutes of eating. Symptoms are related to the robust release of gastrointestinal hormones caused by rapid gastric emptying. Symptoms include nausea, abdominal pain, diarrhea, flushing, hypotension, and tachycardia.
Late dumping is characterized as postprandial hypoglycemia occurring one to 3 hours after eating. Late dumping is likely caused by a combination of changes within the pancreatic beta cells and abnormal insulin response to glucose.16-18 Rapid gastric emptying leads to rapid release of glucose in the gut, which, in turn, leads to brisk insulin secretion. Since glucose is absorbed faster than insulin’s half-life, the resulting (relatively) high levels of insulin may cause hypoglycemia.16-18
Sigstad’s scoring system can be used to confirm suspected cases of dumping syndrome (TABLE 316,17,19). A diagnosis can also be made with an oral glucose challenge in which pulse, blood pressure, glucose, and hematocrit are measured after ingestion of 50 g glucose. The test is positive if heart rate increases by 10 beats per minute, hematocrit increases by 3% 30 minutes after ingestion, or glucose falls below 60 mg/dL 2 to 3 hours after ingestion.17
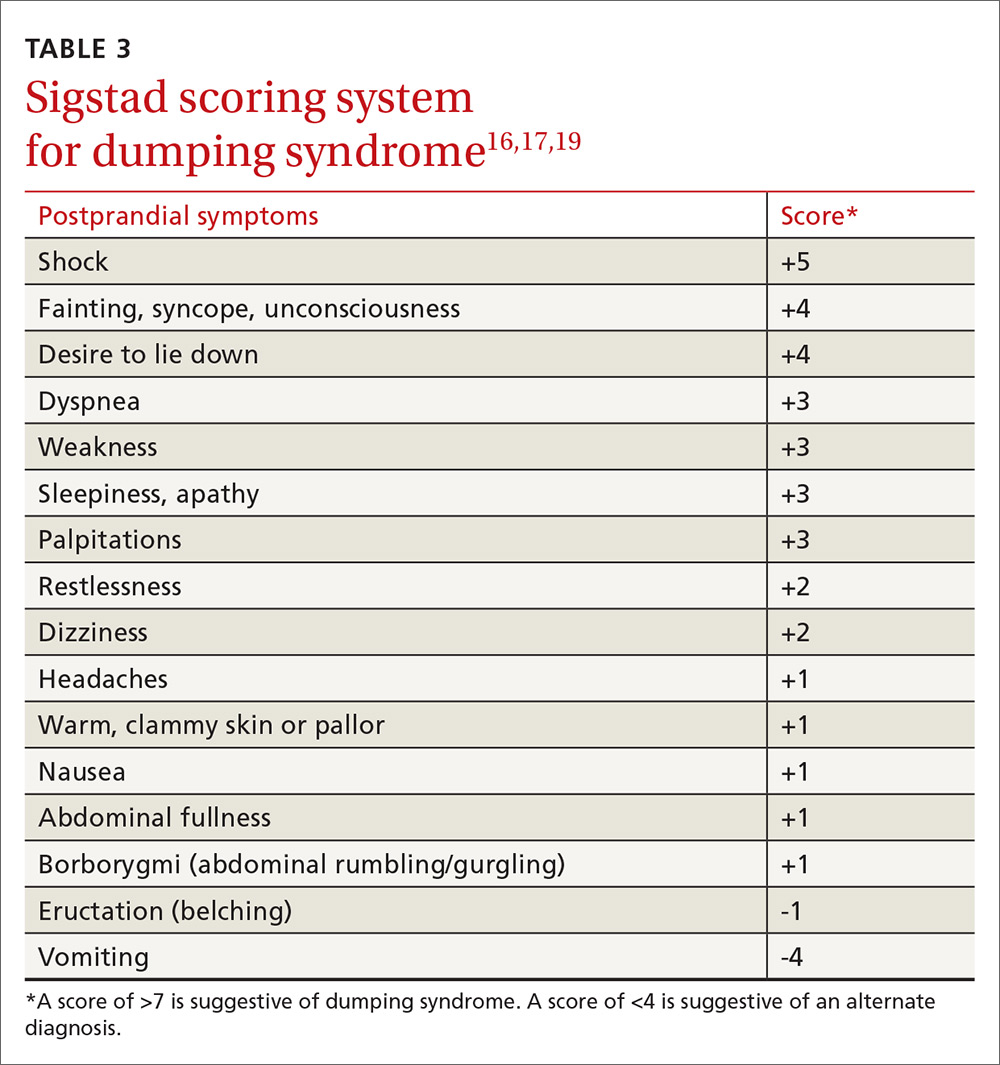
First-line treatment of dumping syndrome consists of dietary modifications. The goal is to slow the rate of gastric emptying by eating smaller, more frequent meals; separating beverages from food; decreasing carbohydrates; and increasing fiber and protein content.
If results are suboptimal after dietary changes, medications can be prescribed including acarbose to prevent postprandial hypoglycemia; anticholinergics such as dicyclomine to slow gastric emptying; and somatostatin to decrease gastric emptying and inhibit GI hormone release.17 Lastly, for resistant and severe postprandial hypoglycemia, a few patients have undergone pancreatectomy, but only about 65% experienced improvement in symptoms and 12% developed diabetes post-surgically.20
Gout attacks may initially increase, but then decrease
BS affects the incidence of gout attacks in patients with a history of gout. One comparative study of approximately 150 patients demonstrated that those with a history of gout had significantly more gout attacks in the first month after BS compared with obese patients with a history of gout undergoing other upper GI surgeries.21 There was no difference between malabsorptive and restrictive procedures. But after the first month, BS patients had significantly fewer gout attacks and lower uric acid levels than their obese counterparts.21
Protein rich diets, catabolism potentiated by aggressive caloric restriction following BS, and dehydration contribute to the initial increase. Therefore, patients who have had at least one gout attack in the year prior to surgery or who are on hypouricemic medication may benefit from at least one month of prophylactic therapy (eg, allopurinol and colchicine) after surgery.
GERD and ulceration: How to respond
Obesity is a known risk factor for GERD, but the effect of BS on GERD is uncertain and seems to vary with the procedure performed. RYGB decreases GERD and is, therefore, used as both a secondary treatment in those not responding to medications and a revision treatment for fundoplication and other types of BSs. Sleeve gastrectomy and adjustable gastric banding have mixed effects on GERD. A systematic review by de Jong et al revealed a decreased prevalence of reflux symptoms and GERD medication use after LAGB; however, during longer follow-up, 15% of previously unaffected patients reported experiencing GERD.22 The 2011 International Sleeve Gastrectomy Expert Panel Consensus Statement retrospectively noted a postoperative incidence of GERD as high as 31%.23
BS patients with GERD should be treated with a proton pump inhibitor. If this fails, refer patients to a gastroenterologist for further evaluation.24
Ulcers after BS may be an indication for revision surgery. Data are mixed regarding increased risk of marginal ulceration from nonsteroidal anti-inflammatory drug (NSAID) use, but NSAIDs have been linked to an increased risk of anastomotic leakage.25-28 Thus, it seems prudent to avoid NSAIDs in people who have undergone BS.
Keeping watch over psychiatric comorbidities
A recent meta-analysis by Dawes et al29 showed that about 23% of patients pursuing BS have a comorbid mood disorder. Specifically, the preoperative prevalence of depression (19%) and binge-eating disorder (17%) were found to be higher than rates in the general population.29 The meta-analysis found improvement in the prevalence of depression with fewer symptoms and less antidepressant medication use in the first 3 years after surgery and a decrease in the rate of binge-eating disorder, although there were fewer supporting data for the latter. These findings were observed with both restrictive and malabsorptive procedures.
The data are mixed regarding rates of alcohol abuse and suicide. Further research is necessary in this field. Patients who have had BS should receive ongoing psychiatric and psychological care from a multidisciplinary team as a matter of course.
Will a second surgery be needed?
Revision surgery. In 2015, about 14% of the almost 200,000 BSs performed were revisions.4 Revision surgery is indicated in BS patients with weight regain, recurrent comorbid diseases (eg, diabetes, hypertension), or complications of primary BS. Restrictive procedures have a higher revision rate than malabsorptive procedures, primarily due to a higher rate of weight regain.6,30
Because revision surgery is associated with more complications and possibly longer hospital stays than primary BS, it should be performed by a bariatric surgeon with extensive experience.30,31 Restrictive revisions are typically converted to malabsorptive procedures. Cost is a limiting factor as many patients’ insurance coverage is limited to one BS per lifetime.
Body contouring. Body contouring surgery (BCS) can improve physical and mental well-being and may be a protective factor for weight regain after bariatric surgery.32 Despite its desirability—particularly to women, adolescents, and those with large decreases in body mass index (BMI)—few patients can afford BCS since it is rarely covered by insurance.
Complications of BCS vary, but are most commonly infection and wound dehiscence. This is, in part, due to poorer wound healing in BS patients compared to those with nonsurgical massive weight loss. The cause of poor wound healing is thought to be secondary to nutritional deficiencies and the catabolic state induced by post-surgical weight loss. Recommendations for BCS include weight stability for more than one year after BS, age >16 years, excess skin causing significant functional impairment, non-smoking status, and presence of good social support.33
Bariatric surgery in adolescents is on the rise
Children in the highest body mass index quartile have more than twice the death rate of those in the lowest BMI quartile.34 Thus, it is not surprising that the rate of BS in adolescents is increasing.7 BS in this age group is successful for weight loss and improvement of comorbid conditions, with relatively low complication rates.35 Options include malabsorptive and restrictive procedures, although gastric banding has not been approved by the US Food and Drug Administration for patients under the age of 18 years.
After BS, adolescent girls should be counseled regarding the possibility of pregnancy (restoration of fertility) and appropriate contraception. Adolescent patients require nutritional supplementation after BS as indicated in TABLE 1.6,8-11
When determining which adolescents to refer for BS, we recommend the following criteria: 35-38
- failure of a minimum 6-month trial of a staged treatment approach, as recommended by Barlow et al,36 including diet, exercise, and pharmacologic treatment
- BMI ≥35 with type 2 diabetes or severe sleep apnea (apnea hypopnea index [AHI] >15)37
- BMI ≥40 with mild sleep apnea (AHI >5), hypertension, or pre-diabetes37
- Tanner stage IV or V
- at least 95% skeletal growth (for malabsorptive surgery).37 This can be determined using an estimated adult height from mid-parental height formula and assessing growth plate closure with hand radiographs for bone age
- appropriate maturity level permitting adherence
- good psychological support
- a multidisciplinary team for postoperative and long-term follow-up care.
Planning for the future: Exploring the possibility of pregnancy
Obesity is the primary cause of maternal and fetal morbidity during pregnancy. It is associated with increased rates of early miscarriage, congenital defects, macrosomia, and fetal death. Maternal risks of obesity include: gestational hypertension, gestational diabetes mellitus (GDM), and pre-eclampsia. Obese mothers also have a higher incidence of failed induction, caesarean section, and breastfeeding failure.10,39 Given that half of all BSs are performed in women of reproductive age, this population deserves special consideration.10
A recent meta-analysis by Galazis et al40 concluded that BS performed prior to pregnancy led to decreased rates of preeclampsia, GDM, large neonates, preterm birth, and neonatal intensive care unit admission. Perinatal mortality did not increase after BS. However, BS led to higher rates of maternal anemia. There was no significant difference between groups in incidence of cesarean section.
The post BS female patient should be advised to use a reliable form of contraception for a minimum of 12 to 18 months after surgery.6,10,39 Involve high-risk obstetric specialists during pregnancies. Diet should be supplemented as indicated in TABLE 1.6,8-11
CORRESPONDENCE
Amy Rothberg, MD, PhD, Domino’s Farms, Lobby G, Suite 1500, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48106; [email protected].
1. Centers for Disease Control and Prevention. Overweight and obesity. Adult obesity facts. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed April 5, 2017.
2. Centers for Disease Control and Prevention. Overweight and obesity. Childhood obesity facts. Available at: https://www.cdc.gov/obesity/data/childhood.html. Accessed April 5, 2017.
3. McGinnis JM. Actual causes of death, 1990-2010. Workshop on Determinants of Premature Mortality, September 18, 2013, National Research Council, Washington, DC.
4. American Society of Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2015. Available at: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed April 5, 2017.
5. Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253:484-487.
6. Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4823-4843.
7. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:S1-S27.
8. Stein J, Stier C, Raab H, et al. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582-609.
9. Boyce SG, Goriparthi R, Clark J, et al. Can composite nutritional supplement based on the current guidelines prevent vitamin and mineral deficiency after weight loss surgery? Obes Surg. 2016;26:966-971.
10. Beard JH, Bell RL, Duffy AJ. Reproductive considerations and pregnancy after bariatric surgery: current evidence and recommendations. Obes Surg. 2008;18:1023-1027.
11. Chakhtoura MT, Nakhoul NN, Shawwa K, et al. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2016;65:574-585.
12. Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporosis Int. 2014;25:151-158.
13. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population-based, retrospective cohort study. BMJ. 2012;345:e5085.
14. Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061-1065.
15. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences and management. Lancet Diabetes Endocrinol. 2014;2:165-174.
16. Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol. 2014;28:741-749.
17. Berg P, McCallum R. Dumping syndrome: a review of the current concepts of pathophysiology, diagnosis, and treatment. Dig Dis Sci. 2016;61:11-18.
18. Ritz P, Vaurs C, Barigou M, et al. Hypoglycaemia after gastric bypass: mechanisms and treatment. Diabetes Obes Metab. 2016;18:217-223.
19. Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand. 1970;188:479-486.
20. Mala T. Postprandial hyperinsulinemic hypoglycaemia after gastric bypass surgical treatment. Surg Obes Relat Dis. 2014;10:1220-1225.
21. Romero-Talamás H, Daigle CR, Aminian A. The effect of bariatric surgery on gout: a comparative study. Surg Obes Relat Dis. 2014;10:1161-1165.
22. de Jong JR, Besselink MG, van Ramshorst B, et al. Effects of adjustable gastric banding on gastroesophageal reflux and esophageal motility: a systematic review. Obes Rev. 2010;11:297-305.
23. Rosenthal RJ; International Sleeve Gastrectomy Expert Panel. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8-19.
24. Altieri MS, Pryor AD. Gastroesophageal reflux disease after bariatric procedures. Surg Clin North Am. 2015;95:579-591.
25. Hakkarainen TW, Steele SR, Bastaworous A, et al. Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg. 2015;150:223-228.
26. El-Hayek K, Timratana P, Shimizu H, et al. Marginal ulcer after Roux-en-Y gastric bypass: what have we really learned? Surg Endosc. 2012;26:2789-2796.
27. Sverdén E, Mattsson F, Sondén AM, et al. Risk factors for marginal ulcer after gastric bypass surgery for obesity: a population-based cohort study. Ann Surg. 2016;263:733-737.
28. Azagury DE, Abu Dayyeh BK, Greenwalt IT, et al. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950-954.
29. Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. JAMA. 2016;315:150-163.
30. Ferrer-Márquez MP, Belda-Lozano R, Solvas-Salmerón MJ, et al. Revisional surgery after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2015;25:6-9.
31. Sanchez H, Cabrera A, Cabrera K, et al. Laparoscopic Roux-en-Y gastric bypass as a revision procedure after restrictive bariatric surgery. Obes Surg. 2008;18:1539-1543.
32. van der Beek ES, Te Riele W, Specken TF, et al. The impact of reconstructive procedures following bariatric surgery on patient well-being and quality of life. Obes Surg. 2010;20:36-41.
33. Ellison JM, Steffen KJ, Sarwer DB. Body contouring after bariatric surgery. Eur Eat Disord Rev. 2015;23:479-487.
34. Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. NEJM. 2010;362:485-489.
35. Gravelle BL, Broyles M. Interventions of weight reduction and prevention in children and adolescents: update. Am J Ther. 2015;22:159-166.
36. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164-S192.
37. Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity. 2009;17:901-910.
38. Nogueira I, Hrovat K. Adolescent bariatric surgery: review on nutrition considerations. Nutr Clin Pract. 2014;29:740-746.
39. Nicklas JM, Barbour LA. Optimizing weight for maternal and infant health: tenable, or too late? Expert Rev Endocrinol Metab. 2015;10:227-242.
40. Galazis N, Docheva N, Simillis C, et al. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;181:45-53.
More than one-third of American adults and approximately 17% of children and adolescents between the ages of 2 and 19 years are obese.1,2 Poor diet coupled with a sedentary lifestyle is the highest ranked cause of non-communicable disease and a leading cause of preventable death, according to the National Research Council.3
Bariatric surgery (BS) is a viable therapeutic option for obese patients who do not respond to conventional lifestyle interventions for losing weight. There are multiple gastrointestinal (GI) procedures available that are classified as either malabsorptive (Roux-en-Y gastric bypass [RYGB] and biliopancreatic diversion [BPD] with or without duodenal switch) or restrictive (laparoscopic adjustable gastric banding [LAGB] and vertical sleeve gastrectomy [VSG]).
Approximately half of the 196,000 bariatric procedures performed in the United States in 2015 were of the sleeve variety, another 23% were RYGB, and the remaining percentage was divided among the other types.4 Postoperative risks include nutritional deficiencies, decreased bone mineral density (BMD), dumping syndrome (when food rapidly dumps from the stomach to the intestine), and gastroesophageal reflux disease (GERD) with possible ulceration.
Despite these potential complications, a systematic review and meta-analysis found that obese people who underwent BS (gastric banding or gastric bypass) had significantly reduced risks of global, non-cardiovascular (CV), and CV mortality compared with obese controls.5 Helping patients to realize these benefits requires that the entire health care team—especially the family physician—is aware of the special considerations for this population.
To that end, this article reviews the details of diagnosing and managing post-surgical complications. It also addresses issues unique to managing certain subpopulations, such as post-BS patients who require revision surgery or who want to pursue body contouring surgery; adolescents who undergo BS surgery; and women who want to get pregnant postoperatively.
Monitor patients for these post-surgery complications
Postoperative BS follow-up varies depending on location, surgeon preference, and availability of multidisciplinary resources. At our institution, patients have a minimum of 3 follow-up visits with their surgeon (during hospitalization and 2 weeks and 2 months postoperatively). This is followed by visits with Endocrinology 6 months after surgery and annually thereafter. Given the variability of follow-up, family physicians should coordinate with specialists where appropriate and be aware of postoperative complications and monitoring since it is likely they will have the most frequent contact with these patients.
Nutritional deficiencies are common and require lifelong screening
Nutritional deficiencies are the most common complications of malabsorptive BS. Guidelines from the Endocrine Society, as well as guidelines from the American Association of Clinical Endocrinologists (AACE), The Obesity Society (TOS), and the American Society for Metabolic and Bariatric Surgery (ASMBS), recommend routine lifetime screening for deficiencies after surgery.6,7 Complete blood cell count, electrolytes, glucose, creatinine, and liver function tests should be obtained at one, 3, 6, 12, 18, and 24 months following surgery and annually thereafter.6

Multiple factors contribute to nutritional and micronutrient deficiencies, including reduced oral intake of food, decreased GI absorption, food intolerance, nausea/vomiting, and nonadherence with dietary supplements.8 Oral supplementation should be in chewable, powder, or liquid form because pill and capsule absorption may be altered.8,9 Over-the-counter multivitamins may not contain the requisite daily doses recommended after BS.9 Patients and physicians should evaluate supplements together to ensure appropriate nutritional and micronutrient supplementation (TABLE 16,8-11).

Bone mineral density can start to decrease soon after surgery
Studies evaluating BMD after BS have produced variable findings. In obese patients, dual-energy x-ray absorptiometry (DEXA) measurements may not be accurate due to adipose tissue artifact and table weight limits. In addition, limited data exist on the incidence of fractures after BS. Of 2 notable studies, only one, a population-based study involving 258 Minnesota residents who underwent a first bariatric surgery between 1985 and 2004, demonstrated a significantly increased incidence of fractures.12,13
In addition, studies show bone turnover markers, including C-terminal telopeptide, increase as early as 3 months after BS.14 Several guidelines recommend routine BMD screening after BS (TABLE 2).6,7 The mechanism of bone demineralization is likely multifactorial—a function of the magnitude of the weight loss and skeletal unloading, calcium and vitamin D deficiencies, and associated secondary hyperparathyroidism.15 Treatment for secondary hyperparathyroidism is adequate supplementation with vitamin D and calcium.

Optimal dosing for vitamin D has not been determined. One recent systematic review suggests routine prophylaxis with at least 2000 international units (IU)/d and found the greatest improvement for known deficiency with doses of 1500-9100 IU/d following malabsorptive surgeries.11 After laparoscopic sleeve gastrectomy, at least 1000 IU/d vitamin D is recommended.11
Overall, high variability exists among patients, and an individualized approach for dosing is recommended.11 Vitamin D levels should be monitored 2 and 4 weeks after initiation of treatment and every 3 months thereafter.11 Normal levels of serum calcium, 25-OH vitamin D, bone-specific alkaline phosphatase, and 24-hour urinary calcium excretion indicate adequate calcium and vitamin D supplementation.6
Dumping syndrome can lead to hypoglycemia
Dumping syndrome is a common complication following BS, with prevalence ranging from 25% to 75%, depending upon the type of procedure performed.16,17 There are 2 types: early and late. Early dumping syndrome occurs within 30 minutes of eating. Symptoms are related to the robust release of gastrointestinal hormones caused by rapid gastric emptying. Symptoms include nausea, abdominal pain, diarrhea, flushing, hypotension, and tachycardia.
Late dumping is characterized as postprandial hypoglycemia occurring one to 3 hours after eating. Late dumping is likely caused by a combination of changes within the pancreatic beta cells and abnormal insulin response to glucose.16-18 Rapid gastric emptying leads to rapid release of glucose in the gut, which, in turn, leads to brisk insulin secretion. Since glucose is absorbed faster than insulin’s half-life, the resulting (relatively) high levels of insulin may cause hypoglycemia.16-18
Sigstad’s scoring system can be used to confirm suspected cases of dumping syndrome (TABLE 316,17,19). A diagnosis can also be made with an oral glucose challenge in which pulse, blood pressure, glucose, and hematocrit are measured after ingestion of 50 g glucose. The test is positive if heart rate increases by 10 beats per minute, hematocrit increases by 3% 30 minutes after ingestion, or glucose falls below 60 mg/dL 2 to 3 hours after ingestion.17

First-line treatment of dumping syndrome consists of dietary modifications. The goal is to slow the rate of gastric emptying by eating smaller, more frequent meals; separating beverages from food; decreasing carbohydrates; and increasing fiber and protein content.
If results are suboptimal after dietary changes, medications can be prescribed including acarbose to prevent postprandial hypoglycemia; anticholinergics such as dicyclomine to slow gastric emptying; and somatostatin to decrease gastric emptying and inhibit GI hormone release.17 Lastly, for resistant and severe postprandial hypoglycemia, a few patients have undergone pancreatectomy, but only about 65% experienced improvement in symptoms and 12% developed diabetes post-surgically.20
Gout attacks may initially increase, but then decrease
BS affects the incidence of gout attacks in patients with a history of gout. One comparative study of approximately 150 patients demonstrated that those with a history of gout had significantly more gout attacks in the first month after BS compared with obese patients with a history of gout undergoing other upper GI surgeries.21 There was no difference between malabsorptive and restrictive procedures. But after the first month, BS patients had significantly fewer gout attacks and lower uric acid levels than their obese counterparts.21
Protein rich diets, catabolism potentiated by aggressive caloric restriction following BS, and dehydration contribute to the initial increase. Therefore, patients who have had at least one gout attack in the year prior to surgery or who are on hypouricemic medication may benefit from at least one month of prophylactic therapy (eg, allopurinol and colchicine) after surgery.
GERD and ulceration: How to respond
Obesity is a known risk factor for GERD, but the effect of BS on GERD is uncertain and seems to vary with the procedure performed. RYGB decreases GERD and is, therefore, used as both a secondary treatment in those not responding to medications and a revision treatment for fundoplication and other types of BSs. Sleeve gastrectomy and adjustable gastric banding have mixed effects on GERD. A systematic review by de Jong et al revealed a decreased prevalence of reflux symptoms and GERD medication use after LAGB; however, during longer follow-up, 15% of previously unaffected patients reported experiencing GERD.22 The 2011 International Sleeve Gastrectomy Expert Panel Consensus Statement retrospectively noted a postoperative incidence of GERD as high as 31%.23
BS patients with GERD should be treated with a proton pump inhibitor. If this fails, refer patients to a gastroenterologist for further evaluation.24
Ulcers after BS may be an indication for revision surgery. Data are mixed regarding increased risk of marginal ulceration from nonsteroidal anti-inflammatory drug (NSAID) use, but NSAIDs have been linked to an increased risk of anastomotic leakage.25-28 Thus, it seems prudent to avoid NSAIDs in people who have undergone BS.
Keeping watch over psychiatric comorbidities
A recent meta-analysis by Dawes et al29 showed that about 23% of patients pursuing BS have a comorbid mood disorder. Specifically, the preoperative prevalence of depression (19%) and binge-eating disorder (17%) were found to be higher than rates in the general population.29 The meta-analysis found improvement in the prevalence of depression with fewer symptoms and less antidepressant medication use in the first 3 years after surgery and a decrease in the rate of binge-eating disorder, although there were fewer supporting data for the latter. These findings were observed with both restrictive and malabsorptive procedures.
The data are mixed regarding rates of alcohol abuse and suicide. Further research is necessary in this field. Patients who have had BS should receive ongoing psychiatric and psychological care from a multidisciplinary team as a matter of course.
Will a second surgery be needed?
Revision surgery. In 2015, about 14% of the almost 200,000 BSs performed were revisions.4 Revision surgery is indicated in BS patients with weight regain, recurrent comorbid diseases (eg, diabetes, hypertension), or complications of primary BS. Restrictive procedures have a higher revision rate than malabsorptive procedures, primarily due to a higher rate of weight regain.6,30
Because revision surgery is associated with more complications and possibly longer hospital stays than primary BS, it should be performed by a bariatric surgeon with extensive experience.30,31 Restrictive revisions are typically converted to malabsorptive procedures. Cost is a limiting factor as many patients’ insurance coverage is limited to one BS per lifetime.
Body contouring. Body contouring surgery (BCS) can improve physical and mental well-being and may be a protective factor for weight regain after bariatric surgery.32 Despite its desirability—particularly to women, adolescents, and those with large decreases in body mass index (BMI)—few patients can afford BCS since it is rarely covered by insurance.
Complications of BCS vary, but are most commonly infection and wound dehiscence. This is, in part, due to poorer wound healing in BS patients compared to those with nonsurgical massive weight loss. The cause of poor wound healing is thought to be secondary to nutritional deficiencies and the catabolic state induced by post-surgical weight loss. Recommendations for BCS include weight stability for more than one year after BS, age >16 years, excess skin causing significant functional impairment, non-smoking status, and presence of good social support.33
Bariatric surgery in adolescents is on the rise
Children in the highest body mass index quartile have more than twice the death rate of those in the lowest BMI quartile.34 Thus, it is not surprising that the rate of BS in adolescents is increasing.7 BS in this age group is successful for weight loss and improvement of comorbid conditions, with relatively low complication rates.35 Options include malabsorptive and restrictive procedures, although gastric banding has not been approved by the US Food and Drug Administration for patients under the age of 18 years.
After BS, adolescent girls should be counseled regarding the possibility of pregnancy (restoration of fertility) and appropriate contraception. Adolescent patients require nutritional supplementation after BS as indicated in TABLE 1.6,8-11
When determining which adolescents to refer for BS, we recommend the following criteria: 35-38
- failure of a minimum 6-month trial of a staged treatment approach, as recommended by Barlow et al,36 including diet, exercise, and pharmacologic treatment
- BMI ≥35 with type 2 diabetes or severe sleep apnea (apnea hypopnea index [AHI] >15)37
- BMI ≥40 with mild sleep apnea (AHI >5), hypertension, or pre-diabetes37
- Tanner stage IV or V
- at least 95% skeletal growth (for malabsorptive surgery).37 This can be determined using an estimated adult height from mid-parental height formula and assessing growth plate closure with hand radiographs for bone age
- appropriate maturity level permitting adherence
- good psychological support
- a multidisciplinary team for postoperative and long-term follow-up care.
Planning for the future: Exploring the possibility of pregnancy
Obesity is the primary cause of maternal and fetal morbidity during pregnancy. It is associated with increased rates of early miscarriage, congenital defects, macrosomia, and fetal death. Maternal risks of obesity include: gestational hypertension, gestational diabetes mellitus (GDM), and pre-eclampsia. Obese mothers also have a higher incidence of failed induction, caesarean section, and breastfeeding failure.10,39 Given that half of all BSs are performed in women of reproductive age, this population deserves special consideration.10
A recent meta-analysis by Galazis et al40 concluded that BS performed prior to pregnancy led to decreased rates of preeclampsia, GDM, large neonates, preterm birth, and neonatal intensive care unit admission. Perinatal mortality did not increase after BS. However, BS led to higher rates of maternal anemia. There was no significant difference between groups in incidence of cesarean section.
The post BS female patient should be advised to use a reliable form of contraception for a minimum of 12 to 18 months after surgery.6,10,39 Involve high-risk obstetric specialists during pregnancies. Diet should be supplemented as indicated in TABLE 1.6,8-11
CORRESPONDENCE
Amy Rothberg, MD, PhD, Domino’s Farms, Lobby G, Suite 1500, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48106; [email protected].
More than one-third of American adults and approximately 17% of children and adolescents between the ages of 2 and 19 years are obese.1,2 Poor diet coupled with a sedentary lifestyle is the highest ranked cause of non-communicable disease and a leading cause of preventable death, according to the National Research Council.3
Bariatric surgery (BS) is a viable therapeutic option for obese patients who do not respond to conventional lifestyle interventions for losing weight. There are multiple gastrointestinal (GI) procedures available that are classified as either malabsorptive (Roux-en-Y gastric bypass [RYGB] and biliopancreatic diversion [BPD] with or without duodenal switch) or restrictive (laparoscopic adjustable gastric banding [LAGB] and vertical sleeve gastrectomy [VSG]).
Approximately half of the 196,000 bariatric procedures performed in the United States in 2015 were of the sleeve variety, another 23% were RYGB, and the remaining percentage was divided among the other types.4 Postoperative risks include nutritional deficiencies, decreased bone mineral density (BMD), dumping syndrome (when food rapidly dumps from the stomach to the intestine), and gastroesophageal reflux disease (GERD) with possible ulceration.
Despite these potential complications, a systematic review and meta-analysis found that obese people who underwent BS (gastric banding or gastric bypass) had significantly reduced risks of global, non-cardiovascular (CV), and CV mortality compared with obese controls.5 Helping patients to realize these benefits requires that the entire health care team—especially the family physician—is aware of the special considerations for this population.
To that end, this article reviews the details of diagnosing and managing post-surgical complications. It also addresses issues unique to managing certain subpopulations, such as post-BS patients who require revision surgery or who want to pursue body contouring surgery; adolescents who undergo BS surgery; and women who want to get pregnant postoperatively.
Monitor patients for these post-surgery complications
Postoperative BS follow-up varies depending on location, surgeon preference, and availability of multidisciplinary resources. At our institution, patients have a minimum of 3 follow-up visits with their surgeon (during hospitalization and 2 weeks and 2 months postoperatively). This is followed by visits with Endocrinology 6 months after surgery and annually thereafter. Given the variability of follow-up, family physicians should coordinate with specialists where appropriate and be aware of postoperative complications and monitoring since it is likely they will have the most frequent contact with these patients.
Nutritional deficiencies are common and require lifelong screening
Nutritional deficiencies are the most common complications of malabsorptive BS. Guidelines from the Endocrine Society, as well as guidelines from the American Association of Clinical Endocrinologists (AACE), The Obesity Society (TOS), and the American Society for Metabolic and Bariatric Surgery (ASMBS), recommend routine lifetime screening for deficiencies after surgery.6,7 Complete blood cell count, electrolytes, glucose, creatinine, and liver function tests should be obtained at one, 3, 6, 12, 18, and 24 months following surgery and annually thereafter.6

Multiple factors contribute to nutritional and micronutrient deficiencies, including reduced oral intake of food, decreased GI absorption, food intolerance, nausea/vomiting, and nonadherence with dietary supplements.8 Oral supplementation should be in chewable, powder, or liquid form because pill and capsule absorption may be altered.8,9 Over-the-counter multivitamins may not contain the requisite daily doses recommended after BS.9 Patients and physicians should evaluate supplements together to ensure appropriate nutritional and micronutrient supplementation (TABLE 16,8-11).

Bone mineral density can start to decrease soon after surgery
Studies evaluating BMD after BS have produced variable findings. In obese patients, dual-energy x-ray absorptiometry (DEXA) measurements may not be accurate due to adipose tissue artifact and table weight limits. In addition, limited data exist on the incidence of fractures after BS. Of 2 notable studies, only one, a population-based study involving 258 Minnesota residents who underwent a first bariatric surgery between 1985 and 2004, demonstrated a significantly increased incidence of fractures.12,13
In addition, studies show bone turnover markers, including C-terminal telopeptide, increase as early as 3 months after BS.14 Several guidelines recommend routine BMD screening after BS (TABLE 2).6,7 The mechanism of bone demineralization is likely multifactorial—a function of the magnitude of the weight loss and skeletal unloading, calcium and vitamin D deficiencies, and associated secondary hyperparathyroidism.15 Treatment for secondary hyperparathyroidism is adequate supplementation with vitamin D and calcium.

Optimal dosing for vitamin D has not been determined. One recent systematic review suggests routine prophylaxis with at least 2000 international units (IU)/d and found the greatest improvement for known deficiency with doses of 1500-9100 IU/d following malabsorptive surgeries.11 After laparoscopic sleeve gastrectomy, at least 1000 IU/d vitamin D is recommended.11
Overall, high variability exists among patients, and an individualized approach for dosing is recommended.11 Vitamin D levels should be monitored 2 and 4 weeks after initiation of treatment and every 3 months thereafter.11 Normal levels of serum calcium, 25-OH vitamin D, bone-specific alkaline phosphatase, and 24-hour urinary calcium excretion indicate adequate calcium and vitamin D supplementation.6
Dumping syndrome can lead to hypoglycemia
Dumping syndrome is a common complication following BS, with prevalence ranging from 25% to 75%, depending upon the type of procedure performed.16,17 There are 2 types: early and late. Early dumping syndrome occurs within 30 minutes of eating. Symptoms are related to the robust release of gastrointestinal hormones caused by rapid gastric emptying. Symptoms include nausea, abdominal pain, diarrhea, flushing, hypotension, and tachycardia.
Late dumping is characterized as postprandial hypoglycemia occurring one to 3 hours after eating. Late dumping is likely caused by a combination of changes within the pancreatic beta cells and abnormal insulin response to glucose.16-18 Rapid gastric emptying leads to rapid release of glucose in the gut, which, in turn, leads to brisk insulin secretion. Since glucose is absorbed faster than insulin’s half-life, the resulting (relatively) high levels of insulin may cause hypoglycemia.16-18
Sigstad’s scoring system can be used to confirm suspected cases of dumping syndrome (TABLE 316,17,19). A diagnosis can also be made with an oral glucose challenge in which pulse, blood pressure, glucose, and hematocrit are measured after ingestion of 50 g glucose. The test is positive if heart rate increases by 10 beats per minute, hematocrit increases by 3% 30 minutes after ingestion, or glucose falls below 60 mg/dL 2 to 3 hours after ingestion.17

First-line treatment of dumping syndrome consists of dietary modifications. The goal is to slow the rate of gastric emptying by eating smaller, more frequent meals; separating beverages from food; decreasing carbohydrates; and increasing fiber and protein content.
If results are suboptimal after dietary changes, medications can be prescribed including acarbose to prevent postprandial hypoglycemia; anticholinergics such as dicyclomine to slow gastric emptying; and somatostatin to decrease gastric emptying and inhibit GI hormone release.17 Lastly, for resistant and severe postprandial hypoglycemia, a few patients have undergone pancreatectomy, but only about 65% experienced improvement in symptoms and 12% developed diabetes post-surgically.20
Gout attacks may initially increase, but then decrease
BS affects the incidence of gout attacks in patients with a history of gout. One comparative study of approximately 150 patients demonstrated that those with a history of gout had significantly more gout attacks in the first month after BS compared with obese patients with a history of gout undergoing other upper GI surgeries.21 There was no difference between malabsorptive and restrictive procedures. But after the first month, BS patients had significantly fewer gout attacks and lower uric acid levels than their obese counterparts.21
Protein rich diets, catabolism potentiated by aggressive caloric restriction following BS, and dehydration contribute to the initial increase. Therefore, patients who have had at least one gout attack in the year prior to surgery or who are on hypouricemic medication may benefit from at least one month of prophylactic therapy (eg, allopurinol and colchicine) after surgery.
GERD and ulceration: How to respond
Obesity is a known risk factor for GERD, but the effect of BS on GERD is uncertain and seems to vary with the procedure performed. RYGB decreases GERD and is, therefore, used as both a secondary treatment in those not responding to medications and a revision treatment for fundoplication and other types of BSs. Sleeve gastrectomy and adjustable gastric banding have mixed effects on GERD. A systematic review by de Jong et al revealed a decreased prevalence of reflux symptoms and GERD medication use after LAGB; however, during longer follow-up, 15% of previously unaffected patients reported experiencing GERD.22 The 2011 International Sleeve Gastrectomy Expert Panel Consensus Statement retrospectively noted a postoperative incidence of GERD as high as 31%.23
BS patients with GERD should be treated with a proton pump inhibitor. If this fails, refer patients to a gastroenterologist for further evaluation.24
Ulcers after BS may be an indication for revision surgery. Data are mixed regarding increased risk of marginal ulceration from nonsteroidal anti-inflammatory drug (NSAID) use, but NSAIDs have been linked to an increased risk of anastomotic leakage.25-28 Thus, it seems prudent to avoid NSAIDs in people who have undergone BS.
Keeping watch over psychiatric comorbidities
A recent meta-analysis by Dawes et al29 showed that about 23% of patients pursuing BS have a comorbid mood disorder. Specifically, the preoperative prevalence of depression (19%) and binge-eating disorder (17%) were found to be higher than rates in the general population.29 The meta-analysis found improvement in the prevalence of depression with fewer symptoms and less antidepressant medication use in the first 3 years after surgery and a decrease in the rate of binge-eating disorder, although there were fewer supporting data for the latter. These findings were observed with both restrictive and malabsorptive procedures.
The data are mixed regarding rates of alcohol abuse and suicide. Further research is necessary in this field. Patients who have had BS should receive ongoing psychiatric and psychological care from a multidisciplinary team as a matter of course.
Will a second surgery be needed?
Revision surgery. In 2015, about 14% of the almost 200,000 BSs performed were revisions.4 Revision surgery is indicated in BS patients with weight regain, recurrent comorbid diseases (eg, diabetes, hypertension), or complications of primary BS. Restrictive procedures have a higher revision rate than malabsorptive procedures, primarily due to a higher rate of weight regain.6,30
Because revision surgery is associated with more complications and possibly longer hospital stays than primary BS, it should be performed by a bariatric surgeon with extensive experience.30,31 Restrictive revisions are typically converted to malabsorptive procedures. Cost is a limiting factor as many patients’ insurance coverage is limited to one BS per lifetime.
Body contouring. Body contouring surgery (BCS) can improve physical and mental well-being and may be a protective factor for weight regain after bariatric surgery.32 Despite its desirability—particularly to women, adolescents, and those with large decreases in body mass index (BMI)—few patients can afford BCS since it is rarely covered by insurance.
Complications of BCS vary, but are most commonly infection and wound dehiscence. This is, in part, due to poorer wound healing in BS patients compared to those with nonsurgical massive weight loss. The cause of poor wound healing is thought to be secondary to nutritional deficiencies and the catabolic state induced by post-surgical weight loss. Recommendations for BCS include weight stability for more than one year after BS, age >16 years, excess skin causing significant functional impairment, non-smoking status, and presence of good social support.33
Bariatric surgery in adolescents is on the rise
Children in the highest body mass index quartile have more than twice the death rate of those in the lowest BMI quartile.34 Thus, it is not surprising that the rate of BS in adolescents is increasing.7 BS in this age group is successful for weight loss and improvement of comorbid conditions, with relatively low complication rates.35 Options include malabsorptive and restrictive procedures, although gastric banding has not been approved by the US Food and Drug Administration for patients under the age of 18 years.
After BS, adolescent girls should be counseled regarding the possibility of pregnancy (restoration of fertility) and appropriate contraception. Adolescent patients require nutritional supplementation after BS as indicated in TABLE 1.6,8-11
When determining which adolescents to refer for BS, we recommend the following criteria: 35-38
- failure of a minimum 6-month trial of a staged treatment approach, as recommended by Barlow et al,36 including diet, exercise, and pharmacologic treatment
- BMI ≥35 with type 2 diabetes or severe sleep apnea (apnea hypopnea index [AHI] >15)37
- BMI ≥40 with mild sleep apnea (AHI >5), hypertension, or pre-diabetes37
- Tanner stage IV or V
- at least 95% skeletal growth (for malabsorptive surgery).37 This can be determined using an estimated adult height from mid-parental height formula and assessing growth plate closure with hand radiographs for bone age
- appropriate maturity level permitting adherence
- good psychological support
- a multidisciplinary team for postoperative and long-term follow-up care.
Planning for the future: Exploring the possibility of pregnancy
Obesity is the primary cause of maternal and fetal morbidity during pregnancy. It is associated with increased rates of early miscarriage, congenital defects, macrosomia, and fetal death. Maternal risks of obesity include: gestational hypertension, gestational diabetes mellitus (GDM), and pre-eclampsia. Obese mothers also have a higher incidence of failed induction, caesarean section, and breastfeeding failure.10,39 Given that half of all BSs are performed in women of reproductive age, this population deserves special consideration.10
A recent meta-analysis by Galazis et al40 concluded that BS performed prior to pregnancy led to decreased rates of preeclampsia, GDM, large neonates, preterm birth, and neonatal intensive care unit admission. Perinatal mortality did not increase after BS. However, BS led to higher rates of maternal anemia. There was no significant difference between groups in incidence of cesarean section.
The post BS female patient should be advised to use a reliable form of contraception for a minimum of 12 to 18 months after surgery.6,10,39 Involve high-risk obstetric specialists during pregnancies. Diet should be supplemented as indicated in TABLE 1.6,8-11
CORRESPONDENCE
Amy Rothberg, MD, PhD, Domino’s Farms, Lobby G, Suite 1500, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48106; [email protected].
1. Centers for Disease Control and Prevention. Overweight and obesity. Adult obesity facts. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed April 5, 2017.
2. Centers for Disease Control and Prevention. Overweight and obesity. Childhood obesity facts. Available at: https://www.cdc.gov/obesity/data/childhood.html. Accessed April 5, 2017.
3. McGinnis JM. Actual causes of death, 1990-2010. Workshop on Determinants of Premature Mortality, September 18, 2013, National Research Council, Washington, DC.
4. American Society of Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2015. Available at: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed April 5, 2017.
5. Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253:484-487.
6. Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4823-4843.
7. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:S1-S27.
8. Stein J, Stier C, Raab H, et al. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582-609.
9. Boyce SG, Goriparthi R, Clark J, et al. Can composite nutritional supplement based on the current guidelines prevent vitamin and mineral deficiency after weight loss surgery? Obes Surg. 2016;26:966-971.
10. Beard JH, Bell RL, Duffy AJ. Reproductive considerations and pregnancy after bariatric surgery: current evidence and recommendations. Obes Surg. 2008;18:1023-1027.
11. Chakhtoura MT, Nakhoul NN, Shawwa K, et al. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2016;65:574-585.
12. Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporosis Int. 2014;25:151-158.
13. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population-based, retrospective cohort study. BMJ. 2012;345:e5085.
14. Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061-1065.
15. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences and management. Lancet Diabetes Endocrinol. 2014;2:165-174.
16. Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol. 2014;28:741-749.
17. Berg P, McCallum R. Dumping syndrome: a review of the current concepts of pathophysiology, diagnosis, and treatment. Dig Dis Sci. 2016;61:11-18.
18. Ritz P, Vaurs C, Barigou M, et al. Hypoglycaemia after gastric bypass: mechanisms and treatment. Diabetes Obes Metab. 2016;18:217-223.
19. Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand. 1970;188:479-486.
20. Mala T. Postprandial hyperinsulinemic hypoglycaemia after gastric bypass surgical treatment. Surg Obes Relat Dis. 2014;10:1220-1225.
21. Romero-Talamás H, Daigle CR, Aminian A. The effect of bariatric surgery on gout: a comparative study. Surg Obes Relat Dis. 2014;10:1161-1165.
22. de Jong JR, Besselink MG, van Ramshorst B, et al. Effects of adjustable gastric banding on gastroesophageal reflux and esophageal motility: a systematic review. Obes Rev. 2010;11:297-305.
23. Rosenthal RJ; International Sleeve Gastrectomy Expert Panel. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8-19.
24. Altieri MS, Pryor AD. Gastroesophageal reflux disease after bariatric procedures. Surg Clin North Am. 2015;95:579-591.
25. Hakkarainen TW, Steele SR, Bastaworous A, et al. Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg. 2015;150:223-228.
26. El-Hayek K, Timratana P, Shimizu H, et al. Marginal ulcer after Roux-en-Y gastric bypass: what have we really learned? Surg Endosc. 2012;26:2789-2796.
27. Sverdén E, Mattsson F, Sondén AM, et al. Risk factors for marginal ulcer after gastric bypass surgery for obesity: a population-based cohort study. Ann Surg. 2016;263:733-737.
28. Azagury DE, Abu Dayyeh BK, Greenwalt IT, et al. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950-954.
29. Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. JAMA. 2016;315:150-163.
30. Ferrer-Márquez MP, Belda-Lozano R, Solvas-Salmerón MJ, et al. Revisional surgery after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2015;25:6-9.
31. Sanchez H, Cabrera A, Cabrera K, et al. Laparoscopic Roux-en-Y gastric bypass as a revision procedure after restrictive bariatric surgery. Obes Surg. 2008;18:1539-1543.
32. van der Beek ES, Te Riele W, Specken TF, et al. The impact of reconstructive procedures following bariatric surgery on patient well-being and quality of life. Obes Surg. 2010;20:36-41.
33. Ellison JM, Steffen KJ, Sarwer DB. Body contouring after bariatric surgery. Eur Eat Disord Rev. 2015;23:479-487.
34. Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. NEJM. 2010;362:485-489.
35. Gravelle BL, Broyles M. Interventions of weight reduction and prevention in children and adolescents: update. Am J Ther. 2015;22:159-166.
36. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164-S192.
37. Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity. 2009;17:901-910.
38. Nogueira I, Hrovat K. Adolescent bariatric surgery: review on nutrition considerations. Nutr Clin Pract. 2014;29:740-746.
39. Nicklas JM, Barbour LA. Optimizing weight for maternal and infant health: tenable, or too late? Expert Rev Endocrinol Metab. 2015;10:227-242.
40. Galazis N, Docheva N, Simillis C, et al. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;181:45-53.
1. Centers for Disease Control and Prevention. Overweight and obesity. Adult obesity facts. Available at: https://www.cdc.gov/obesity/data/adult.html. Accessed April 5, 2017.
2. Centers for Disease Control and Prevention. Overweight and obesity. Childhood obesity facts. Available at: https://www.cdc.gov/obesity/data/childhood.html. Accessed April 5, 2017.
3. McGinnis JM. Actual causes of death, 1990-2010. Workshop on Determinants of Premature Mortality, September 18, 2013, National Research Council, Washington, DC.
4. American Society of Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2015. Available at: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed April 5, 2017.
5. Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253:484-487.
6. Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4823-4843.
7. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:S1-S27.
8. Stein J, Stier C, Raab H, et al. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582-609.
9. Boyce SG, Goriparthi R, Clark J, et al. Can composite nutritional supplement based on the current guidelines prevent vitamin and mineral deficiency after weight loss surgery? Obes Surg. 2016;26:966-971.
10. Beard JH, Bell RL, Duffy AJ. Reproductive considerations and pregnancy after bariatric surgery: current evidence and recommendations. Obes Surg. 2008;18:1023-1027.
11. Chakhtoura MT, Nakhoul NN, Shawwa K, et al. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2016;65:574-585.
12. Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporosis Int. 2014;25:151-158.
13. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population-based, retrospective cohort study. BMJ. 2012;345:e5085.
14. Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061-1065.
15. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences and management. Lancet Diabetes Endocrinol. 2014;2:165-174.
16. Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol. 2014;28:741-749.
17. Berg P, McCallum R. Dumping syndrome: a review of the current concepts of pathophysiology, diagnosis, and treatment. Dig Dis Sci. 2016;61:11-18.
18. Ritz P, Vaurs C, Barigou M, et al. Hypoglycaemia after gastric bypass: mechanisms and treatment. Diabetes Obes Metab. 2016;18:217-223.
19. Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand. 1970;188:479-486.
20. Mala T. Postprandial hyperinsulinemic hypoglycaemia after gastric bypass surgical treatment. Surg Obes Relat Dis. 2014;10:1220-1225.
21. Romero-Talamás H, Daigle CR, Aminian A. The effect of bariatric surgery on gout: a comparative study. Surg Obes Relat Dis. 2014;10:1161-1165.
22. de Jong JR, Besselink MG, van Ramshorst B, et al. Effects of adjustable gastric banding on gastroesophageal reflux and esophageal motility: a systematic review. Obes Rev. 2010;11:297-305.
23. Rosenthal RJ; International Sleeve Gastrectomy Expert Panel. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8-19.
24. Altieri MS, Pryor AD. Gastroesophageal reflux disease after bariatric procedures. Surg Clin North Am. 2015;95:579-591.
25. Hakkarainen TW, Steele SR, Bastaworous A, et al. Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg. 2015;150:223-228.
26. El-Hayek K, Timratana P, Shimizu H, et al. Marginal ulcer after Roux-en-Y gastric bypass: what have we really learned? Surg Endosc. 2012;26:2789-2796.
27. Sverdén E, Mattsson F, Sondén AM, et al. Risk factors for marginal ulcer after gastric bypass surgery for obesity: a population-based cohort study. Ann Surg. 2016;263:733-737.
28. Azagury DE, Abu Dayyeh BK, Greenwalt IT, et al. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950-954.
29. Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. JAMA. 2016;315:150-163.
30. Ferrer-Márquez MP, Belda-Lozano R, Solvas-Salmerón MJ, et al. Revisional surgery after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2015;25:6-9.
31. Sanchez H, Cabrera A, Cabrera K, et al. Laparoscopic Roux-en-Y gastric bypass as a revision procedure after restrictive bariatric surgery. Obes Surg. 2008;18:1539-1543.
32. van der Beek ES, Te Riele W, Specken TF, et al. The impact of reconstructive procedures following bariatric surgery on patient well-being and quality of life. Obes Surg. 2010;20:36-41.
33. Ellison JM, Steffen KJ, Sarwer DB. Body contouring after bariatric surgery. Eur Eat Disord Rev. 2015;23:479-487.
34. Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. NEJM. 2010;362:485-489.
35. Gravelle BL, Broyles M. Interventions of weight reduction and prevention in children and adolescents: update. Am J Ther. 2015;22:159-166.
36. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164-S192.
37. Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity. 2009;17:901-910.
38. Nogueira I, Hrovat K. Adolescent bariatric surgery: review on nutrition considerations. Nutr Clin Pract. 2014;29:740-746.
39. Nicklas JM, Barbour LA. Optimizing weight for maternal and infant health: tenable, or too late? Expert Rev Endocrinol Metab. 2015;10:227-242.
40. Galazis N, Docheva N, Simillis C, et al. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;181:45-53.
From The Journal of Family Practice | 2017;66(6):356-363.
PRACTICE RECOMMENDATIONS
› Routinely screen bariatric surgery patients for nutritional deficiencies throughout their life. A
› Avoid the use of nonsteroidal anti-inflammatory medications in patients who have had bariatric surgery becauseof the risk of anastomotic ulceration and leakage. A
› Consider revision surgery for bariatric surgery patients with weight regain, recurrent comorbid diseases, or surgical complications. B
› Counsel obese women who want to become pregnant that bariatric surgery decreases rates of future pregnancy complications. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
These 3 tools can help you streamline management of IBS
CASE › Amber S,* a 33-year-old woman who works on the production line at a bread factory, sought care at my health center with a several month history of non-bloody diarrhea that was increasing in frequency and urgency and was accompanied by painful abdominal bloating and cramping. She said that these symptoms were negatively impacting her interpersonal relationships, as well as her productivity at work. She reported that “almost everything” she ate upset her stomach and “goes right through her,” including fruits, vegetables, and meat, as well as greasy fast food. She had researched her symptoms on the Internet and was worried that she might have something serious like inflammatory bowel disease or cancer.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that negatively impacts the quality of life (QOL) of millions of people worldwide.1 In fact, one study of 179 people with IBS found that 76% of survey respondents reported some degree of IBS-related impairment in at least 5 domains of daily life: daily activities, comorbid psychiatric diagnoses, symptom severity, QOL, and symptom-specific cognitive affective factors related to IBS.2
Estimating prevalence and incidence is a formidable challenge given various diagnostic criteria, the influence of population selection, inclusion or exclusion of non-GI comorbidities, and various cultural influences.3 That said, it’s estimated that IBS impacts approximately 11% of the world’s population, and approximately 30% of these individuals seek treatment.1,4 While there are no significant differences in GI symptoms between those who consult physicians and those who do not, those who do seek treatment report higher pain scores, greater levels of anxiety, and a greater reduction in QOL.5
All ages affected. IBS has been reported in patients of all ages, including children and the elderly, with no definable difference reported in the frequency of subtypes (diarrhea- or constipation-predominant).
This article reviews the latest explanations, diagnostic criteria, and treatment guidelines for this challenging condition so that you can offer your patients confident care without needless testing or referral.
[polldaddy:9755564]
A lack of consensus among practicing physicians
Historically, IBS has been regarded by many primary care physicians (PCPs) as a diagnosis of exclusion. Lab tests would be ordered, nothing significant would be found, and the patient would be referred to the gastroenterologist for a definitive diagnosis.
Perceptions and misconceptions about IBS continue to abound to this day. Many are neither completely right nor wrong partly because so many triggers for IBS exist and partly because of the heretofore lack of simple, standardized criteria to diagnose the condition. Other factors contributing to the confusion are that the diagnosis of IBS is purely symptom-based and that proposals of its pathophysiology have traditionally been complex.
For example, a 2006 survey-based study of PCPs and gastroenterologists found that PCPs were less likely than gastroenterologists to believe that IBS was related to prior physical or sexual abuse, previous infection, or learned behavior, but were more likely to associate dietary factors or a linkable genetic etiology with IBS.6 Both sets of beliefs, however, may be considered correct.
Similarly, a 2009 qualitative study conducted in the Netherlands found that general practitioners (GPs) considered smoking, caffeine, diet, “hasty lifestyle,” and lack of exercise as potential triggers for IBS symptoms, while PCPs in the United Kingdom considered diet, infection, and travel to be possible triggers.7 Again, all play a role.
While GPs reported that patients should take responsibility for managing their IBS and for minimizing its impact on their daily lives, they admitted limited awareness of the extent to which IBS affected their patients’ daily living.7
A 2013 survey-based study in England determined that GPs understand the relationship between IBS and psychological symptoms including anxiety and stress, and posited that the majority of patients could be managed within primary care without referral for psychological interventions.8 Moreover, they reported that a dedicated risk assessment tool for patients with IBS would be helpful to stratify severity of disease. The study concluded that the reluctance of GPs to refer patients for evidence-based psychological treatments may prevent them from obtaining appropriate services and care.
Newer explanatory model shines light on IBS
A newer explanation that is based on 3 main hypotheses is elucidating the true nature of IBS and providing a pragmatic model for the clinical setting (FIGURE 1).9 According to the model, IBS entails the following 3 elements, which combined lead to the symptoms of IBS:
- Altered or abnormal peripheral regulation of gut function (including sensory and secretory mechanisms)
- Altered brain-gut signaling (including visceral hypersensitivity)
- Psychological distress.
It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).

Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3
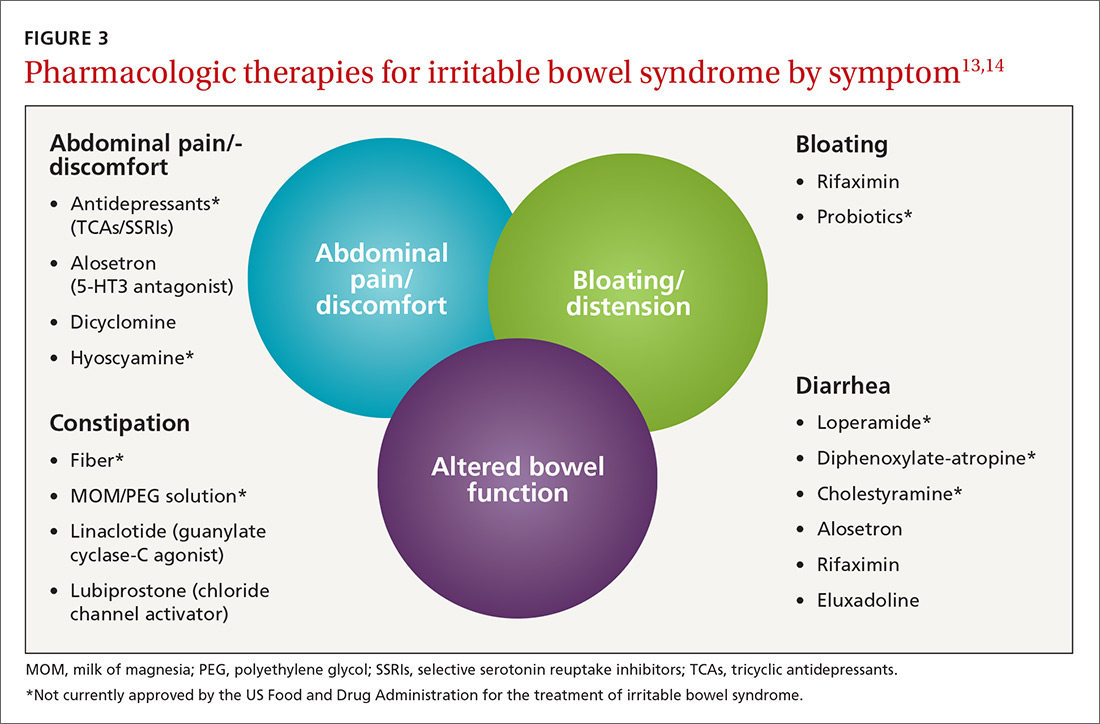
Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.
Pharmacologic therapies for IBS-C
Linaclotide is a guanylate cyclase-C agonist with an indication for treatment of IBS-C. A double-blind, parallel-group, placebo-controlled trial found that the percentage of patients who experienced a decrease in abdominal pain was nearly 25%, with statistically significant improvements in bloating, straining, and stool consistency over a 26-week period.20 In a report on 2 phase 3 trials, researchers found that linaclotide improved global symptom scores and significantly decreased abdominal bloating and fullness, pain, cramping, and discomfort vs placebo. Diarrhea was the most commonly reported adverse event in patients with severe abdominal symptoms (18.8%-21%).21
Lubiprostone is a prostaglandin E1 analogue that activates type-2-chloride channels on the apical membrane of epithelial cells in the intestine. In a combined analysis of 2 phase 3 randomized trials, lubiprostone was administered twice daily for 12 weeks vs placebo and patients were asked to describe how they felt after the trial period. Survey responders reported significant improvements in global IBS-C symptoms (17.9% vs 10.1%; P=.001).22 A meta-analysis of studies on lubiprostone found that diarrhea, nausea, and abdominal pain were the most common adverse effects, but their occurrence was not that much greater than with placebo.23
Diet and probiotics can play a significant role
The role of dietary components in the treatment of IBS is gaining increasing attention. Such components can have a direct effect on gastric and intestinal motility, visceral sensation, immune activation, brain-gut interactions, and the microbiome. Current evidence suggests that targeted carbohydrate and gluten exclusion plays a favorable role in the treatment and symptomatic improvement of patients with IBS.24
A 2014 study conducted in Australia showed that a diet low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), which is characterized by avoiding foods containing gluten and those that are high in fructose, reduced overall GI symptom scores (including scores involving abdominal bloating, pain, and flatus) in patients with IBS compared to those consuming a normal Australian diet.25 The International Foundation for Functional Gastrointestinal Disorders’ Web site provides a detailed guide to low FODMAP foods and can be found at: http://www.aboutibs.org/low-fodmap-diet.html.
Probiotics are now commonly used in the symptomatic treatment of many upper and lower GI disorders. While much anecdotal evidence exists to support their benefit, there is a paucity of large-scale and rigorous research to provide substantial outcomes-based evidence. The theory for their use is that they support regulation of the gut microbiome, which in turn improves the imbalance between the intestinal microbiome and a dysfunctional intestinal barrier.
A 2014 randomized, double-blind, placebo-controlled trial involving multispecies probiotics (a mixture of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus) found that patients who received probiotics had significantly reduced symptoms of IBS after 4 weeks compared with placebo, and modest improvement in abdominal pain and discomfort as well as bloating.26 One study involving 122 patients from 2011 found that B. bifidum MIMBb75 reduced the global assessment of IBS symptoms by -88 points (95% CI, -1.07 to -0.69) when compared with only -0.16 (95% CI, -.32 to 0.00) points in the placebo group (P<.0001).27 MIMBb75 also significantly improved the IBS symptoms of pain/discomfort, distension/bloating, urgency, and digestive disorder. And one randomized, double-blind, placebo-controlled study involving 67 patients found that QOL scores improved two-fold when patients took Saccharomyces boulardii (15.4% vs 7.0%; P<.05).28
Dried plums or prunes have been used successfully for decades for the symptomatic treatment of constipation. A single-blinded, randomized, cross-over study compared prunes 50 g/d to psyllium fiber 11 g/d and found that prunes were more efficacious (P<.05) with spontaneous bowel movements and stool consistency scores.29
Peppermint oil has been studied as an alternative therapy for symptoms of IBS, but efficacy and tolerability are concerns. A meta-analysis of randomized controlled trials with a minimum duration of 2 weeks found that compared with placebo, peppermint oil provided improvement in abdominal pain, bloating, and global symptoms, but some patients reported transient heartburn.30 A 4-week, randomized, double-blind, placebo-controlled clinical trial sponsored by IM HealthScience found a novel oral formulation of triple-enteric-coated sustained-release peppermint oil microspheres caused less heartburn than was reported in the previous study, but still significantly improved abdominal symptoms and lessened pain on defecation and fecal urgency.31
CASE › Suspecting IBS-D, the FP ordered a complete blood count, tissue transglutaminase antibodies, and a stool culture, all of which were unremarkable. Ms. S has been trying to follow a low FODMAP diet and has been taking some over-the-counter probiotics with only minimal relief of abdominal bloating and cramping and no improvement in stool consistency. Her FP started her on eluxadoline 100 mg twice daily with food. After 12 weeks of therapy, she reports significant improvement in global IBS symptoms and nearly complete resolution of her diarrhea.
*Amber S is a real patient in my practice. Her name has been changed to protect her identity.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected].
1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
2. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29. Epub 2016 Oct 25.
3. Heidelbaugh J, Hungin P, eds. ROME IV: Functional Gastrointestinal Disorders for Primary Care and Non-GI Clinicians. 1st ed. Raleigh, NC: Rome Foundation, Inc.; 2016.
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
5. Lee V, Guthrie E, Robinson A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008;64:129-138.
6. Lacy BE, Rosemore J, Robertson D, et al. Physicians’ attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892-902.
7. Casiday RE, Hungin AP, Cornford CS, et al. GPs’ explanatory models for irritable bowel syndrome: a mismatch with patient models? J Fam Pract. 2009;26:34-39.
8. Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome—an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
9. Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: an integrated explanatory model for clinical practice. Neurogastroenterol Motil. 2015;27:750-753.
10. Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterol. 2016;150:1393-1407.
11. Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
12. Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438.
13. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
14. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
15. Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem Pharmacol. 2014;92:448-456.
16. Pimentel M, Lembo A, Chey WD, et al, for the TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
17. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
18. Lewis JH. Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol. 2010;4:13-29.
19. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6:344-357.
20. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712.
21. Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623.
22. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341.
23. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009;10:143-152.
24. Spencer M, Chey WD, Eswaran S. Dietary Renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12:424-440.
25. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.
26. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59.
27. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
28. Choi CH, Jo SY, Park HJ, et al. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679-683.
29. Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822-828.
30. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
31. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
CASE › Amber S,* a 33-year-old woman who works on the production line at a bread factory, sought care at my health center with a several month history of non-bloody diarrhea that was increasing in frequency and urgency and was accompanied by painful abdominal bloating and cramping. She said that these symptoms were negatively impacting her interpersonal relationships, as well as her productivity at work. She reported that “almost everything” she ate upset her stomach and “goes right through her,” including fruits, vegetables, and meat, as well as greasy fast food. She had researched her symptoms on the Internet and was worried that she might have something serious like inflammatory bowel disease or cancer.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that negatively impacts the quality of life (QOL) of millions of people worldwide.1 In fact, one study of 179 people with IBS found that 76% of survey respondents reported some degree of IBS-related impairment in at least 5 domains of daily life: daily activities, comorbid psychiatric diagnoses, symptom severity, QOL, and symptom-specific cognitive affective factors related to IBS.2
Estimating prevalence and incidence is a formidable challenge given various diagnostic criteria, the influence of population selection, inclusion or exclusion of non-GI comorbidities, and various cultural influences.3 That said, it’s estimated that IBS impacts approximately 11% of the world’s population, and approximately 30% of these individuals seek treatment.1,4 While there are no significant differences in GI symptoms between those who consult physicians and those who do not, those who do seek treatment report higher pain scores, greater levels of anxiety, and a greater reduction in QOL.5
All ages affected. IBS has been reported in patients of all ages, including children and the elderly, with no definable difference reported in the frequency of subtypes (diarrhea- or constipation-predominant).
This article reviews the latest explanations, diagnostic criteria, and treatment guidelines for this challenging condition so that you can offer your patients confident care without needless testing or referral.
[polldaddy:9755564]
A lack of consensus among practicing physicians
Historically, IBS has been regarded by many primary care physicians (PCPs) as a diagnosis of exclusion. Lab tests would be ordered, nothing significant would be found, and the patient would be referred to the gastroenterologist for a definitive diagnosis.
Perceptions and misconceptions about IBS continue to abound to this day. Many are neither completely right nor wrong partly because so many triggers for IBS exist and partly because of the heretofore lack of simple, standardized criteria to diagnose the condition. Other factors contributing to the confusion are that the diagnosis of IBS is purely symptom-based and that proposals of its pathophysiology have traditionally been complex.
For example, a 2006 survey-based study of PCPs and gastroenterologists found that PCPs were less likely than gastroenterologists to believe that IBS was related to prior physical or sexual abuse, previous infection, or learned behavior, but were more likely to associate dietary factors or a linkable genetic etiology with IBS.6 Both sets of beliefs, however, may be considered correct.
Similarly, a 2009 qualitative study conducted in the Netherlands found that general practitioners (GPs) considered smoking, caffeine, diet, “hasty lifestyle,” and lack of exercise as potential triggers for IBS symptoms, while PCPs in the United Kingdom considered diet, infection, and travel to be possible triggers.7 Again, all play a role.
While GPs reported that patients should take responsibility for managing their IBS and for minimizing its impact on their daily lives, they admitted limited awareness of the extent to which IBS affected their patients’ daily living.7
A 2013 survey-based study in England determined that GPs understand the relationship between IBS and psychological symptoms including anxiety and stress, and posited that the majority of patients could be managed within primary care without referral for psychological interventions.8 Moreover, they reported that a dedicated risk assessment tool for patients with IBS would be helpful to stratify severity of disease. The study concluded that the reluctance of GPs to refer patients for evidence-based psychological treatments may prevent them from obtaining appropriate services and care.
Newer explanatory model shines light on IBS
A newer explanation that is based on 3 main hypotheses is elucidating the true nature of IBS and providing a pragmatic model for the clinical setting (FIGURE 1).9 According to the model, IBS entails the following 3 elements, which combined lead to the symptoms of IBS:
- Altered or abnormal peripheral regulation of gut function (including sensory and secretory mechanisms)
- Altered brain-gut signaling (including visceral hypersensitivity)
- Psychological distress.

It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).

Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3

Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.
Pharmacologic therapies for IBS-C
Linaclotide is a guanylate cyclase-C agonist with an indication for treatment of IBS-C. A double-blind, parallel-group, placebo-controlled trial found that the percentage of patients who experienced a decrease in abdominal pain was nearly 25%, with statistically significant improvements in bloating, straining, and stool consistency over a 26-week period.20 In a report on 2 phase 3 trials, researchers found that linaclotide improved global symptom scores and significantly decreased abdominal bloating and fullness, pain, cramping, and discomfort vs placebo. Diarrhea was the most commonly reported adverse event in patients with severe abdominal symptoms (18.8%-21%).21
Lubiprostone is a prostaglandin E1 analogue that activates type-2-chloride channels on the apical membrane of epithelial cells in the intestine. In a combined analysis of 2 phase 3 randomized trials, lubiprostone was administered twice daily for 12 weeks vs placebo and patients were asked to describe how they felt after the trial period. Survey responders reported significant improvements in global IBS-C symptoms (17.9% vs 10.1%; P=.001).22 A meta-analysis of studies on lubiprostone found that diarrhea, nausea, and abdominal pain were the most common adverse effects, but their occurrence was not that much greater than with placebo.23
Diet and probiotics can play a significant role
The role of dietary components in the treatment of IBS is gaining increasing attention. Such components can have a direct effect on gastric and intestinal motility, visceral sensation, immune activation, brain-gut interactions, and the microbiome. Current evidence suggests that targeted carbohydrate and gluten exclusion plays a favorable role in the treatment and symptomatic improvement of patients with IBS.24
A 2014 study conducted in Australia showed that a diet low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), which is characterized by avoiding foods containing gluten and those that are high in fructose, reduced overall GI symptom scores (including scores involving abdominal bloating, pain, and flatus) in patients with IBS compared to those consuming a normal Australian diet.25 The International Foundation for Functional Gastrointestinal Disorders’ Web site provides a detailed guide to low FODMAP foods and can be found at: http://www.aboutibs.org/low-fodmap-diet.html.
Probiotics are now commonly used in the symptomatic treatment of many upper and lower GI disorders. While much anecdotal evidence exists to support their benefit, there is a paucity of large-scale and rigorous research to provide substantial outcomes-based evidence. The theory for their use is that they support regulation of the gut microbiome, which in turn improves the imbalance between the intestinal microbiome and a dysfunctional intestinal barrier.
A 2014 randomized, double-blind, placebo-controlled trial involving multispecies probiotics (a mixture of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus) found that patients who received probiotics had significantly reduced symptoms of IBS after 4 weeks compared with placebo, and modest improvement in abdominal pain and discomfort as well as bloating.26 One study involving 122 patients from 2011 found that B. bifidum MIMBb75 reduced the global assessment of IBS symptoms by -88 points (95% CI, -1.07 to -0.69) when compared with only -0.16 (95% CI, -.32 to 0.00) points in the placebo group (P<.0001).27 MIMBb75 also significantly improved the IBS symptoms of pain/discomfort, distension/bloating, urgency, and digestive disorder. And one randomized, double-blind, placebo-controlled study involving 67 patients found that QOL scores improved two-fold when patients took Saccharomyces boulardii (15.4% vs 7.0%; P<.05).28
Dried plums or prunes have been used successfully for decades for the symptomatic treatment of constipation. A single-blinded, randomized, cross-over study compared prunes 50 g/d to psyllium fiber 11 g/d and found that prunes were more efficacious (P<.05) with spontaneous bowel movements and stool consistency scores.29
Peppermint oil has been studied as an alternative therapy for symptoms of IBS, but efficacy and tolerability are concerns. A meta-analysis of randomized controlled trials with a minimum duration of 2 weeks found that compared with placebo, peppermint oil provided improvement in abdominal pain, bloating, and global symptoms, but some patients reported transient heartburn.30 A 4-week, randomized, double-blind, placebo-controlled clinical trial sponsored by IM HealthScience found a novel oral formulation of triple-enteric-coated sustained-release peppermint oil microspheres caused less heartburn than was reported in the previous study, but still significantly improved abdominal symptoms and lessened pain on defecation and fecal urgency.31
CASE › Suspecting IBS-D, the FP ordered a complete blood count, tissue transglutaminase antibodies, and a stool culture, all of which were unremarkable. Ms. S has been trying to follow a low FODMAP diet and has been taking some over-the-counter probiotics with only minimal relief of abdominal bloating and cramping and no improvement in stool consistency. Her FP started her on eluxadoline 100 mg twice daily with food. After 12 weeks of therapy, she reports significant improvement in global IBS symptoms and nearly complete resolution of her diarrhea.
*Amber S is a real patient in my practice. Her name has been changed to protect her identity.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected].
CASE › Amber S,* a 33-year-old woman who works on the production line at a bread factory, sought care at my health center with a several month history of non-bloody diarrhea that was increasing in frequency and urgency and was accompanied by painful abdominal bloating and cramping. She said that these symptoms were negatively impacting her interpersonal relationships, as well as her productivity at work. She reported that “almost everything” she ate upset her stomach and “goes right through her,” including fruits, vegetables, and meat, as well as greasy fast food. She had researched her symptoms on the Internet and was worried that she might have something serious like inflammatory bowel disease or cancer.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that negatively impacts the quality of life (QOL) of millions of people worldwide.1 In fact, one study of 179 people with IBS found that 76% of survey respondents reported some degree of IBS-related impairment in at least 5 domains of daily life: daily activities, comorbid psychiatric diagnoses, symptom severity, QOL, and symptom-specific cognitive affective factors related to IBS.2
Estimating prevalence and incidence is a formidable challenge given various diagnostic criteria, the influence of population selection, inclusion or exclusion of non-GI comorbidities, and various cultural influences.3 That said, it’s estimated that IBS impacts approximately 11% of the world’s population, and approximately 30% of these individuals seek treatment.1,4 While there are no significant differences in GI symptoms between those who consult physicians and those who do not, those who do seek treatment report higher pain scores, greater levels of anxiety, and a greater reduction in QOL.5
All ages affected. IBS has been reported in patients of all ages, including children and the elderly, with no definable difference reported in the frequency of subtypes (diarrhea- or constipation-predominant).
This article reviews the latest explanations, diagnostic criteria, and treatment guidelines for this challenging condition so that you can offer your patients confident care without needless testing or referral.
[polldaddy:9755564]
A lack of consensus among practicing physicians
Historically, IBS has been regarded by many primary care physicians (PCPs) as a diagnosis of exclusion. Lab tests would be ordered, nothing significant would be found, and the patient would be referred to the gastroenterologist for a definitive diagnosis.
Perceptions and misconceptions about IBS continue to abound to this day. Many are neither completely right nor wrong partly because so many triggers for IBS exist and partly because of the heretofore lack of simple, standardized criteria to diagnose the condition. Other factors contributing to the confusion are that the diagnosis of IBS is purely symptom-based and that proposals of its pathophysiology have traditionally been complex.
For example, a 2006 survey-based study of PCPs and gastroenterologists found that PCPs were less likely than gastroenterologists to believe that IBS was related to prior physical or sexual abuse, previous infection, or learned behavior, but were more likely to associate dietary factors or a linkable genetic etiology with IBS.6 Both sets of beliefs, however, may be considered correct.
Similarly, a 2009 qualitative study conducted in the Netherlands found that general practitioners (GPs) considered smoking, caffeine, diet, “hasty lifestyle,” and lack of exercise as potential triggers for IBS symptoms, while PCPs in the United Kingdom considered diet, infection, and travel to be possible triggers.7 Again, all play a role.
While GPs reported that patients should take responsibility for managing their IBS and for minimizing its impact on their daily lives, they admitted limited awareness of the extent to which IBS affected their patients’ daily living.7
A 2013 survey-based study in England determined that GPs understand the relationship between IBS and psychological symptoms including anxiety and stress, and posited that the majority of patients could be managed within primary care without referral for psychological interventions.8 Moreover, they reported that a dedicated risk assessment tool for patients with IBS would be helpful to stratify severity of disease. The study concluded that the reluctance of GPs to refer patients for evidence-based psychological treatments may prevent them from obtaining appropriate services and care.
Newer explanatory model shines light on IBS
A newer explanation that is based on 3 main hypotheses is elucidating the true nature of IBS and providing a pragmatic model for the clinical setting (FIGURE 1).9 According to the model, IBS entails the following 3 elements, which combined lead to the symptoms of IBS:
- Altered or abnormal peripheral regulation of gut function (including sensory and secretory mechanisms)
- Altered brain-gut signaling (including visceral hypersensitivity)
- Psychological distress.

It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).

Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3

Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.
Pharmacologic therapies for IBS-C
Linaclotide is a guanylate cyclase-C agonist with an indication for treatment of IBS-C. A double-blind, parallel-group, placebo-controlled trial found that the percentage of patients who experienced a decrease in abdominal pain was nearly 25%, with statistically significant improvements in bloating, straining, and stool consistency over a 26-week period.20 In a report on 2 phase 3 trials, researchers found that linaclotide improved global symptom scores and significantly decreased abdominal bloating and fullness, pain, cramping, and discomfort vs placebo. Diarrhea was the most commonly reported adverse event in patients with severe abdominal symptoms (18.8%-21%).21
Lubiprostone is a prostaglandin E1 analogue that activates type-2-chloride channels on the apical membrane of epithelial cells in the intestine. In a combined analysis of 2 phase 3 randomized trials, lubiprostone was administered twice daily for 12 weeks vs placebo and patients were asked to describe how they felt after the trial period. Survey responders reported significant improvements in global IBS-C symptoms (17.9% vs 10.1%; P=.001).22 A meta-analysis of studies on lubiprostone found that diarrhea, nausea, and abdominal pain were the most common adverse effects, but their occurrence was not that much greater than with placebo.23
Diet and probiotics can play a significant role
The role of dietary components in the treatment of IBS is gaining increasing attention. Such components can have a direct effect on gastric and intestinal motility, visceral sensation, immune activation, brain-gut interactions, and the microbiome. Current evidence suggests that targeted carbohydrate and gluten exclusion plays a favorable role in the treatment and symptomatic improvement of patients with IBS.24
A 2014 study conducted in Australia showed that a diet low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), which is characterized by avoiding foods containing gluten and those that are high in fructose, reduced overall GI symptom scores (including scores involving abdominal bloating, pain, and flatus) in patients with IBS compared to those consuming a normal Australian diet.25 The International Foundation for Functional Gastrointestinal Disorders’ Web site provides a detailed guide to low FODMAP foods and can be found at: http://www.aboutibs.org/low-fodmap-diet.html.
Probiotics are now commonly used in the symptomatic treatment of many upper and lower GI disorders. While much anecdotal evidence exists to support their benefit, there is a paucity of large-scale and rigorous research to provide substantial outcomes-based evidence. The theory for their use is that they support regulation of the gut microbiome, which in turn improves the imbalance between the intestinal microbiome and a dysfunctional intestinal barrier.
A 2014 randomized, double-blind, placebo-controlled trial involving multispecies probiotics (a mixture of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus) found that patients who received probiotics had significantly reduced symptoms of IBS after 4 weeks compared with placebo, and modest improvement in abdominal pain and discomfort as well as bloating.26 One study involving 122 patients from 2011 found that B. bifidum MIMBb75 reduced the global assessment of IBS symptoms by -88 points (95% CI, -1.07 to -0.69) when compared with only -0.16 (95% CI, -.32 to 0.00) points in the placebo group (P<.0001).27 MIMBb75 also significantly improved the IBS symptoms of pain/discomfort, distension/bloating, urgency, and digestive disorder. And one randomized, double-blind, placebo-controlled study involving 67 patients found that QOL scores improved two-fold when patients took Saccharomyces boulardii (15.4% vs 7.0%; P<.05).28
Dried plums or prunes have been used successfully for decades for the symptomatic treatment of constipation. A single-blinded, randomized, cross-over study compared prunes 50 g/d to psyllium fiber 11 g/d and found that prunes were more efficacious (P<.05) with spontaneous bowel movements and stool consistency scores.29
Peppermint oil has been studied as an alternative therapy for symptoms of IBS, but efficacy and tolerability are concerns. A meta-analysis of randomized controlled trials with a minimum duration of 2 weeks found that compared with placebo, peppermint oil provided improvement in abdominal pain, bloating, and global symptoms, but some patients reported transient heartburn.30 A 4-week, randomized, double-blind, placebo-controlled clinical trial sponsored by IM HealthScience found a novel oral formulation of triple-enteric-coated sustained-release peppermint oil microspheres caused less heartburn than was reported in the previous study, but still significantly improved abdominal symptoms and lessened pain on defecation and fecal urgency.31
CASE › Suspecting IBS-D, the FP ordered a complete blood count, tissue transglutaminase antibodies, and a stool culture, all of which were unremarkable. Ms. S has been trying to follow a low FODMAP diet and has been taking some over-the-counter probiotics with only minimal relief of abdominal bloating and cramping and no improvement in stool consistency. Her FP started her on eluxadoline 100 mg twice daily with food. After 12 weeks of therapy, she reports significant improvement in global IBS symptoms and nearly complete resolution of her diarrhea.
*Amber S is a real patient in my practice. Her name has been changed to protect her identity.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected].
1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
2. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29. Epub 2016 Oct 25.
3. Heidelbaugh J, Hungin P, eds. ROME IV: Functional Gastrointestinal Disorders for Primary Care and Non-GI Clinicians. 1st ed. Raleigh, NC: Rome Foundation, Inc.; 2016.
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
5. Lee V, Guthrie E, Robinson A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008;64:129-138.
6. Lacy BE, Rosemore J, Robertson D, et al. Physicians’ attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892-902.
7. Casiday RE, Hungin AP, Cornford CS, et al. GPs’ explanatory models for irritable bowel syndrome: a mismatch with patient models? J Fam Pract. 2009;26:34-39.
8. Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome—an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
9. Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: an integrated explanatory model for clinical practice. Neurogastroenterol Motil. 2015;27:750-753.
10. Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterol. 2016;150:1393-1407.
11. Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
12. Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438.
13. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
14. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
15. Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem Pharmacol. 2014;92:448-456.
16. Pimentel M, Lembo A, Chey WD, et al, for the TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
17. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
18. Lewis JH. Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol. 2010;4:13-29.
19. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6:344-357.
20. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712.
21. Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623.
22. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341.
23. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009;10:143-152.
24. Spencer M, Chey WD, Eswaran S. Dietary Renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12:424-440.
25. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.
26. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59.
27. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
28. Choi CH, Jo SY, Park HJ, et al. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679-683.
29. Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822-828.
30. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
31. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
2. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29. Epub 2016 Oct 25.
3. Heidelbaugh J, Hungin P, eds. ROME IV: Functional Gastrointestinal Disorders for Primary Care and Non-GI Clinicians. 1st ed. Raleigh, NC: Rome Foundation, Inc.; 2016.
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
5. Lee V, Guthrie E, Robinson A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008;64:129-138.
6. Lacy BE, Rosemore J, Robertson D, et al. Physicians’ attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892-902.
7. Casiday RE, Hungin AP, Cornford CS, et al. GPs’ explanatory models for irritable bowel syndrome: a mismatch with patient models? J Fam Pract. 2009;26:34-39.
8. Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome—an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
9. Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: an integrated explanatory model for clinical practice. Neurogastroenterol Motil. 2015;27:750-753.
10. Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterol. 2016;150:1393-1407.
11. Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
12. Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438.
13. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
14. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
15. Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem Pharmacol. 2014;92:448-456.
16. Pimentel M, Lembo A, Chey WD, et al, for the TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
17. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
18. Lewis JH. Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol. 2010;4:13-29.
19. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6:344-357.
20. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712.
21. Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623.
22. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341.
23. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009;10:143-152.
24. Spencer M, Chey WD, Eswaran S. Dietary Renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12:424-440.
25. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.
26. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59.
27. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
28. Choi CH, Jo SY, Park HJ, et al. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679-683.
29. Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822-828.
30. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
31. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
PRACTICE RECOMMENDATIONS
› Prescribe eluxadoline, rifaximin, or alosetron for diarrhea-predominant IBS because all 3 have proven efficacy with this diagnosis. A
› Prescribe linaclotide or lubiprostone for constipation-predominant IBS, as both have proven efficacy with this condition. A
› Suggest that patients with IBS follow a low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet; probiotics, prunes, and peppermint oil may also offer some improvement of IBS symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Reducing CV risk in diabetes: An ADA update
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.

Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
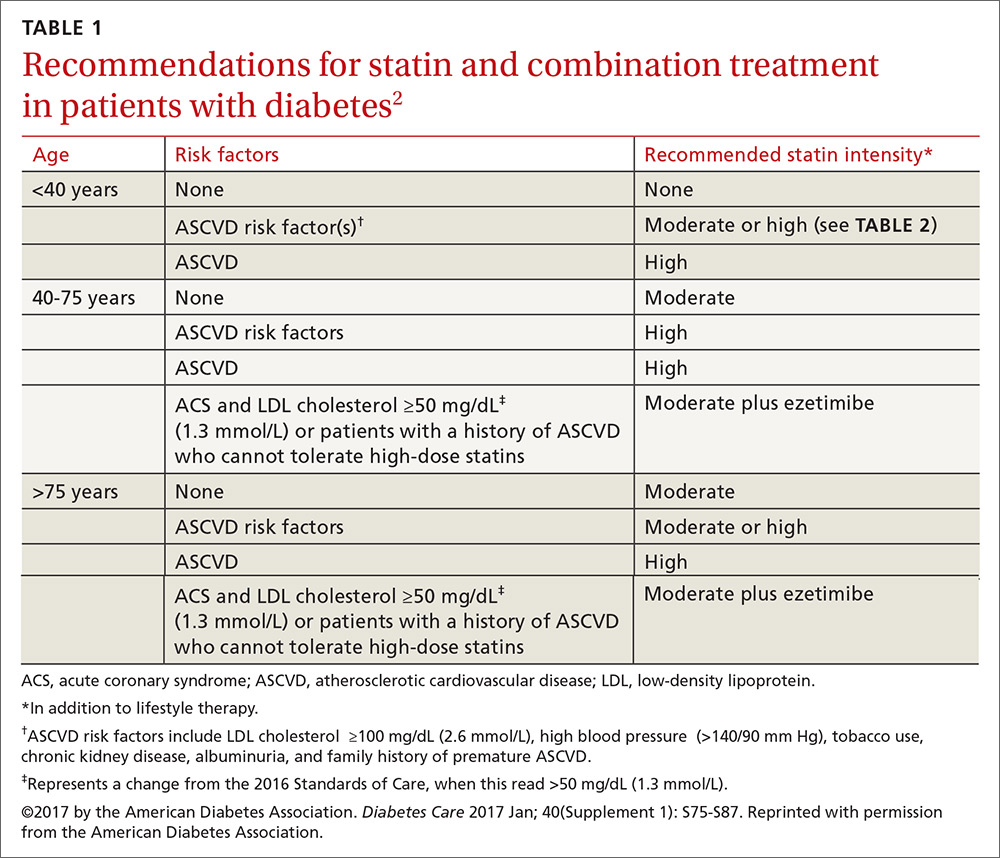
Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
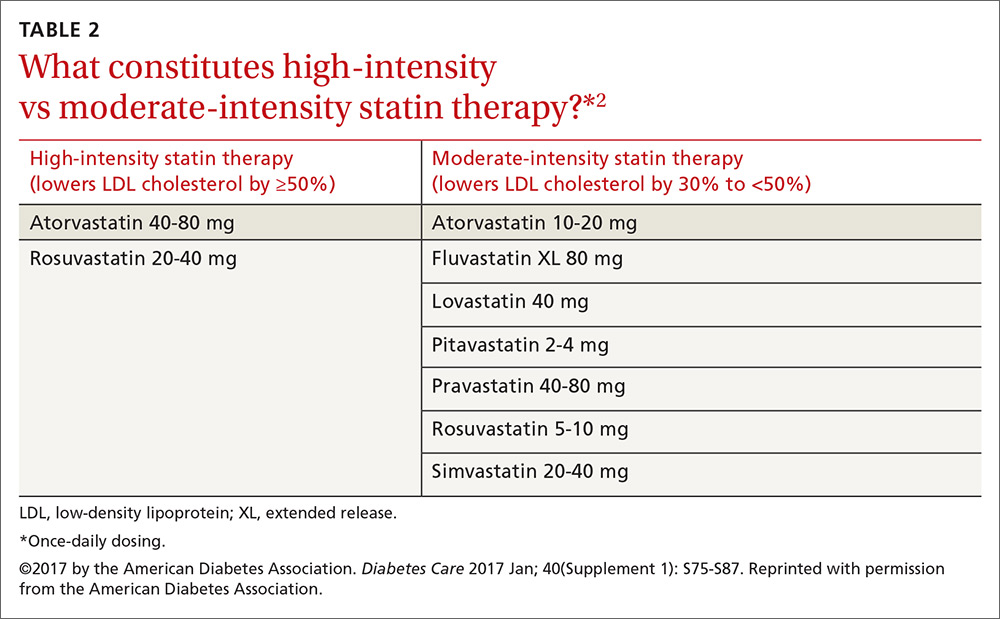
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; [email protected].
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.

Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.

Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37

For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; [email protected].
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.

Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.

Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37

For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; [email protected].
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
Assessment steps and treatment tips for ankle arthritis
CASE › A 57-year-old man had been experiencing intermittent pain in his left ankle for the past 2.5 years. About 6 weeks before coming to our clinic, his symptoms became significantly worse after playing a pickup game of basketball. At the clinic visit, he reported no other recent injury or trauma to the leg. However, 15 years earlier he had fractured his left ankle and was treated conservatively with a short period in a cast followed by a course of physical therapy. After completing the physical therapy, he noted significant improvement, although he continued to have minor episodes of pain. He felt no instability or mechanical locking but did note a decreased ability to move the ankle. And it felt much stiffer than his right ankle.
Examination of his left ankle revealed tenderness over the anterior aspect at the tibiotalar joint. He also exhibited decreased dorsiflexion and was unable to perform a toe raise. There was no tenderness over the major ligaments, and results of anterior drawer and talar tilt tests were normal. X-rays revealed tibiotalar joint arthritis (FIGURE).
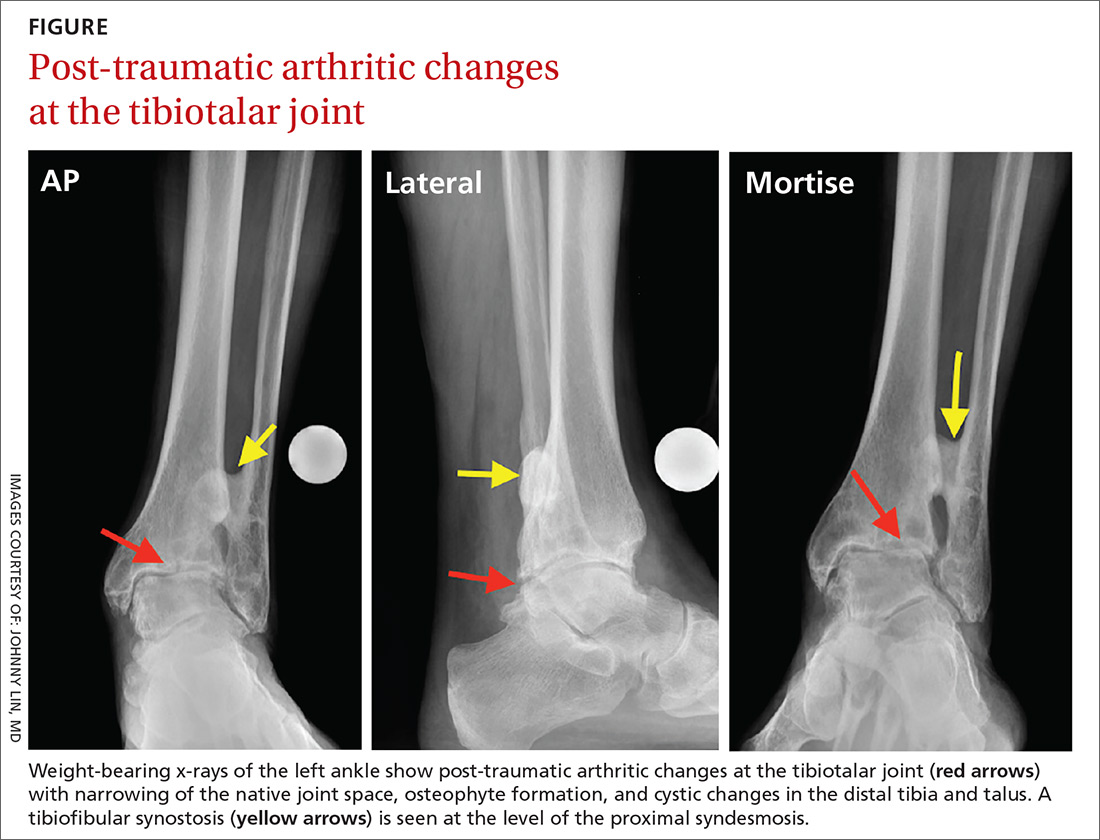
How would you proceed if this were your patient?
Arthritis of the tibiotalar joint, which has an estimated prevalence of approximately 1%, occurs much less frequently than arthritis of the knee or hip joints.1 This low prevalence is primarily due to the ankle joint’s unique biomechanics and the features of the cartilage within the joint, including its thickness.2
Specifically, the hip and knee joints have greater degrees of freedom than the tibiotalar articulation, which is significantly constrained. The bony congruity between the talus, tibia, and fibula provides inherent stability to the ankle joint, thus protecting against primary osteoarthritis (OA).
Additionally, the large number of ligamentous structures and overall strength of the ligaments provide significant supplemental stability to the ankle joint articulation. Articular cartilage within the ankle joint is thicker than that of the knee and hip (1-1.7 mm). This cartilage also tends to retain its tensile strength with age, unlike cartilage in the hip; the ankle is therefore more resistant to age-related degeneration.3
Metabolic factors also protect against arthritis. Chondrocytes in the ankle are less responsive to inflammatory mediators, including interleukin-1 (IL-1), and therefore produce fewer matrix metalloproteinases.1,2,4 There are also fewer IL-1 receptors on ankle chondrocytes.
The role of trauma in ankle OA. Given the ankle joint’s inherent stability, the most common cause of ankle OA is trauma,4 mainly ankle fracture and, less commonly, ligamentous injury.5,6 Other rarer causes of ankle arthritis include primary OA, crystalline arthropathy, inflammatory disease, septic arthritis, neuroarthropathy, hemochromatosis, and ochronosis.
The ankle’s characteristics that protect it against primary OA may facilitate the pathogenesis of post-traumatic OA through 2 main mechanisms. First, direct trauma to the chondral surfaces can hasten the onset of progressive degeneration. Second, articular incongruity from a fracture can lead to insidious deterioration. The stiffer cartilage layer may be less adaptable to malalignment, and incongruity may cause secondary instability and chronic overloading. Ultimately, the joint breaks down with associated cartilage wear.6,7
The importance of the normal ankle’s congruity and stability became clear in the landmark study by Ramsey and colleagues,8 showing that the contact area between the talus and the tibia decreases as talar displacement increases laterally. This innate stability explains why the contact area of the ankle joint can bear loads similar to those of the hip and knee, yet does not experience primary OA nearly as often.
A stepwise diagnostic appraisal
Ask these questions. Since most ankle pain results from trauma, ask about any recent or remote injury to the affected ankle. Knowing the type of injury that occurred and the exact treatment, if received, may shed light on the relationship between the injury and current symptoms. Acute traumatic events can cause fractures or injury to various soft-tissue structures traversing the ankle joint. Ankle ligament sprains or tendon strains may result after abnormal rotation of the foot. Alternatively, chronic overuse injuries may lead to tendinopathy in any of the tendons that control motion throughout the foot and ankle or degenerative changes within the tibiotalar joint. Knowing the exact location of pain may also help identify the pathology (TABLE 1).
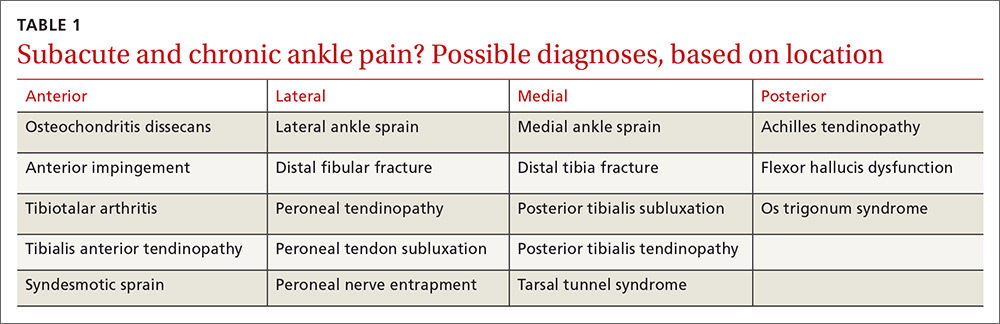
The patient in our case had not suffered a recent injury, so it was important to learn as much as possible about his prior fracture. Was the injury treated conservatively or surgically? If management was conservative, the type and duration of treatment could offer clues to the mechanism underlying symptoms. If a patient has undergone surgery, knowledge of the exact procedure could suggest specific problems. For example, surgical fixation would likely indicate there was ankle instability, thus altering the normal biomechanics in the injured tibiotalar joint.
Other key questions to ask. Most patients with ankle pain also complain of limitations in their usual activities. Ask about the duration and type of pain and other symptoms. Also ask about the position of the foot and ankle when the pain is at its greatest, which will provide insight into likely areas of pathology. For example, if pain arises when the patient navigates uneven ground, subtalar pathology is highly likely. If the patient complains of pain while walking down stairs, suspect injury to the posterior (plantar flexed) ankle; pain while walking up stairs more likely indicates anterior (dorsiflexed) pathology.
Finally, ask about nonorthopedic medical problems and all medications being taken. Systemic conditions, too, can lead to ankle pain—eg, inflammatory arthropathies, infections, and crystalline arthropathy.
Physical examination. Observe the patient’s gait to assess any functional or range-of-motion limitations or abnormal loading throughout the foot and ankle.9 With the patient standing, evaluate any malalignment from the foot through the knees and to the hips. Evaluate the skin for any lesions, wounds, or evidence of trauma or surgery. Next, with the patient seated, examine carefully for neuropathy or vascular abnormalities. Evaluate the ankle’s range of motion and assess for any mechanical locking, clicking, or crepitus. Palpate all bony and ligamentous landmarks to reveal areas of tenderness or swelling. Perform anterior drawer and varus tilt tests to determine overall ligamentous stability of the ankle, and compare your findings with test results of the opposite, uninjured ankle.
Diagnostic imaging. Order weight-bearing radiographs of the foot and ankle. Including the foot allows you to identify additional potential concerns such as malalignment, deformity, or adjacent joint arthritis. Look particularly for joint space narrowing, malalignment, post-traumatic changes, or implanted hardware. Advanced imaging studies—computerized tomography, magnetic resonance imaging, bone scan—are reserved for cases that necessitate ruling out alternative diagnoses, or for preoperative evaluation by an orthopedic surgeon.
Management: Make use of multiple modalities
Conservative management options for ankle OA are limited, and high-quality evidence of efficacy is lacking. Surgical alternatives, however, are invasive and yield modest outcomes. Therefore, unless specific indications for surgery are present, exhaust conservative options (TABLE 2) before considering referral.
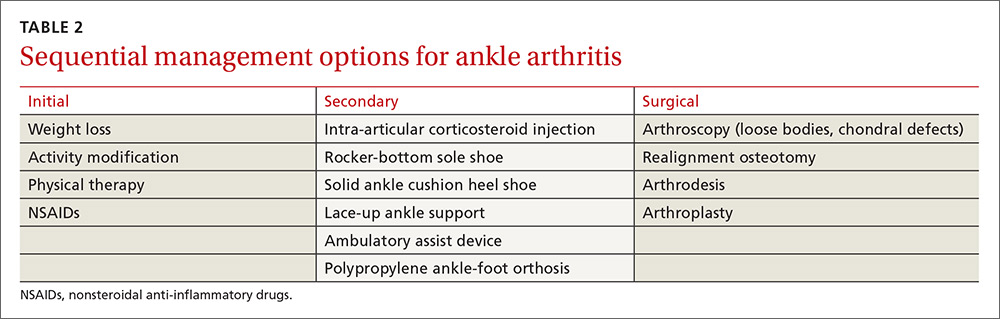
Weight loss is important for those who are overweight—as with knee OA management—to decrease the reactive forces within the ankle joint and to decrease pain. Weight loss will also enhance the outcomes of other treatment modalities and improve overall health.10,11
Activity modification is usually required, even though this may make weight loss more difficult. Avoiding vigorous activities, restricting work-related movements that place high-impact stress on the ankle, and decreasing overall walking time often reduce the severity of symptoms and improve functioning in other activities. Use of assistive-devices, such as a cane, can decrease the weight-bearing load on the affected joint.10,11
Physical therapy has not been shown to alleviate pain in ankle arthritis, although stretching, joint mobilization, and gait training may help prevent further progression of arthritis and improve function.11 The strength of dorsiflexion and plantar-flexion muscles is often decreased in individuals with ankle arthritis. Strengthening exercises may be indicated in individuals exhibiting deficits.
Prescriptive conservative management. Begin with a combination, as needed, of anti-inflammatory medications, orthotic devices, and footwear modifications.
Nonsteroidal anti-inflammatory agents are generally safe, but long-term use requires monitoring. Intra-articular steroid injections have some supporting evidence of effectiveness, but any benefit is short-lived.12 Glucosamine and chondroitin, although unlikely to cause harm, are not supported by the evidence for use in ankle arthritis. Intra-articular viscosupplementation is controversial, and evidence is limited regarding its efficacy.1
Adding a rocker-bottom sole and a solid ankle cushion heel to a shoe helps decrease heel strike impact in individuals with decreased ankle motion, and they aid in the transition from the heel strike to the push-off during level walking.11 If the arthritic joint is unstable, a lace-up ankle support may help with proprioception and stability. A polypropylene ankle-foot orthosis, custom leather ankle corset, or a double-upright brace with a patellar-tendon-bearing support are options to restrict ankle motion and decrease weight-bearing forces.10,11
Immobilization is not recommended except for short-term use during an arthritic flare. Limiting ankle motion reduces pain, but the downside tradeoff is acquired stiffness and weakness that accompanies prolonged periods of immobilization. A controlled ankle motion walking boot or walking plaster cast are both reasonable options for the short term.
Consider surgical referral for specific indications such as osteophytes, loose bodies, and chondral defects, which may be treated with arthroscopy. Patients with large areas of exposed chondral bone or rapid onset of degeneration have poorer outcomes with conservative management and should also be referred to a surgeon earlier. Otherwise, consider surgical referral only after a full trial of conservative management.11
Surgical options vary in scope and effectiveness and include osteotomy, arthrodesis, and arthroplasty. Osteotomies can be performed in early OA to correct bony alignment deformities. Arthrodesis in neutral dorsiflexion with roughly 5 degrees of external rotation is reserved for end-stage ankle OA to allow for near normal gait and pain relief. Total ankle arthroplasty is an emerging option for severe ankle OA, resulting in improved pain relief, gait, and patient satisfaction, but potentially has a higher reoperation rate when compared with arthrodesis.1,2
CASE › We prescribed short-term immobilization with a controlled ankle motion boot and administered an intra-articular corticosteroid injection. At the patient’s follow-up visit 6 weeks later, he reported only moderate improvement in pain. We then advised physical therapy at a specialty ankle rehabilitation program to focus on mobilization, strengthening, and gait training. Nearly one year after his initial visit to our clinic, he is doing well. He understands, however, that the nature of his ankle arthrosis may necessitate surgical intervention in the future.
CORRESPONDENCE
Adam Bitterman, DO, Department of Orthopedic Surgery, Hofstra Northwell School of Medicine at Huntington Hospital, 155 East Main Street, Huntington, NY 11743; [email protected].
1. Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806.
2. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
3. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223-230.
4. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
5. Brown T, Johnston R, Saltzman C, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
6. Barg A, Pagenstert G, Hügle T, et al. Ankle osteoarthritis etiology, diagnostics and classification. Foot Ankle Clin. 2013;18:411-426.
7. Schenker M, Mauck R, Ahn J, et al. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20-28.
8. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-357.
9. Hayes BJ, Gonzalez T, Smith JT, et al. Ankle arthritis: you can’t always replace it. J Am Acad Orthop Surg. 2016;24:e29-e38.
10. Thomas R, Daniels T. Current concepts review ankle arthritis. J Bone Joint Surg Am. 2003;85A:923-936.
11. Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. J Orthop Sports Phys Ther. 2007:37:253-259.
12. Pekarek B, Osher L, Buck S, et al. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21:66-70.
13. Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle arthritis: why evidence of efficacy is still lacking? Clin Exp Rheumatol. 2012;30:277-281.
14. Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643.
15. Rao S, Ellis SJ, Deland JT, et al. Nonmedicinal therapy in the management of ankle arthritis. Curr Opin Rheumatol. 2010;22:223-228.
CASE › A 57-year-old man had been experiencing intermittent pain in his left ankle for the past 2.5 years. About 6 weeks before coming to our clinic, his symptoms became significantly worse after playing a pickup game of basketball. At the clinic visit, he reported no other recent injury or trauma to the leg. However, 15 years earlier he had fractured his left ankle and was treated conservatively with a short period in a cast followed by a course of physical therapy. After completing the physical therapy, he noted significant improvement, although he continued to have minor episodes of pain. He felt no instability or mechanical locking but did note a decreased ability to move the ankle. And it felt much stiffer than his right ankle.
Examination of his left ankle revealed tenderness over the anterior aspect at the tibiotalar joint. He also exhibited decreased dorsiflexion and was unable to perform a toe raise. There was no tenderness over the major ligaments, and results of anterior drawer and talar tilt tests were normal. X-rays revealed tibiotalar joint arthritis (FIGURE).

How would you proceed if this were your patient?
Arthritis of the tibiotalar joint, which has an estimated prevalence of approximately 1%, occurs much less frequently than arthritis of the knee or hip joints.1 This low prevalence is primarily due to the ankle joint’s unique biomechanics and the features of the cartilage within the joint, including its thickness.2
Specifically, the hip and knee joints have greater degrees of freedom than the tibiotalar articulation, which is significantly constrained. The bony congruity between the talus, tibia, and fibula provides inherent stability to the ankle joint, thus protecting against primary osteoarthritis (OA).
Additionally, the large number of ligamentous structures and overall strength of the ligaments provide significant supplemental stability to the ankle joint articulation. Articular cartilage within the ankle joint is thicker than that of the knee and hip (1-1.7 mm). This cartilage also tends to retain its tensile strength with age, unlike cartilage in the hip; the ankle is therefore more resistant to age-related degeneration.3
Metabolic factors also protect against arthritis. Chondrocytes in the ankle are less responsive to inflammatory mediators, including interleukin-1 (IL-1), and therefore produce fewer matrix metalloproteinases.1,2,4 There are also fewer IL-1 receptors on ankle chondrocytes.
The role of trauma in ankle OA. Given the ankle joint’s inherent stability, the most common cause of ankle OA is trauma,4 mainly ankle fracture and, less commonly, ligamentous injury.5,6 Other rarer causes of ankle arthritis include primary OA, crystalline arthropathy, inflammatory disease, septic arthritis, neuroarthropathy, hemochromatosis, and ochronosis.
The ankle’s characteristics that protect it against primary OA may facilitate the pathogenesis of post-traumatic OA through 2 main mechanisms. First, direct trauma to the chondral surfaces can hasten the onset of progressive degeneration. Second, articular incongruity from a fracture can lead to insidious deterioration. The stiffer cartilage layer may be less adaptable to malalignment, and incongruity may cause secondary instability and chronic overloading. Ultimately, the joint breaks down with associated cartilage wear.6,7
The importance of the normal ankle’s congruity and stability became clear in the landmark study by Ramsey and colleagues,8 showing that the contact area between the talus and the tibia decreases as talar displacement increases laterally. This innate stability explains why the contact area of the ankle joint can bear loads similar to those of the hip and knee, yet does not experience primary OA nearly as often.
A stepwise diagnostic appraisal
Ask these questions. Since most ankle pain results from trauma, ask about any recent or remote injury to the affected ankle. Knowing the type of injury that occurred and the exact treatment, if received, may shed light on the relationship between the injury and current symptoms. Acute traumatic events can cause fractures or injury to various soft-tissue structures traversing the ankle joint. Ankle ligament sprains or tendon strains may result after abnormal rotation of the foot. Alternatively, chronic overuse injuries may lead to tendinopathy in any of the tendons that control motion throughout the foot and ankle or degenerative changes within the tibiotalar joint. Knowing the exact location of pain may also help identify the pathology (TABLE 1).

The patient in our case had not suffered a recent injury, so it was important to learn as much as possible about his prior fracture. Was the injury treated conservatively or surgically? If management was conservative, the type and duration of treatment could offer clues to the mechanism underlying symptoms. If a patient has undergone surgery, knowledge of the exact procedure could suggest specific problems. For example, surgical fixation would likely indicate there was ankle instability, thus altering the normal biomechanics in the injured tibiotalar joint.
Other key questions to ask. Most patients with ankle pain also complain of limitations in their usual activities. Ask about the duration and type of pain and other symptoms. Also ask about the position of the foot and ankle when the pain is at its greatest, which will provide insight into likely areas of pathology. For example, if pain arises when the patient navigates uneven ground, subtalar pathology is highly likely. If the patient complains of pain while walking down stairs, suspect injury to the posterior (plantar flexed) ankle; pain while walking up stairs more likely indicates anterior (dorsiflexed) pathology.
Finally, ask about nonorthopedic medical problems and all medications being taken. Systemic conditions, too, can lead to ankle pain—eg, inflammatory arthropathies, infections, and crystalline arthropathy.
Physical examination. Observe the patient’s gait to assess any functional or range-of-motion limitations or abnormal loading throughout the foot and ankle.9 With the patient standing, evaluate any malalignment from the foot through the knees and to the hips. Evaluate the skin for any lesions, wounds, or evidence of trauma or surgery. Next, with the patient seated, examine carefully for neuropathy or vascular abnormalities. Evaluate the ankle’s range of motion and assess for any mechanical locking, clicking, or crepitus. Palpate all bony and ligamentous landmarks to reveal areas of tenderness or swelling. Perform anterior drawer and varus tilt tests to determine overall ligamentous stability of the ankle, and compare your findings with test results of the opposite, uninjured ankle.
Diagnostic imaging. Order weight-bearing radiographs of the foot and ankle. Including the foot allows you to identify additional potential concerns such as malalignment, deformity, or adjacent joint arthritis. Look particularly for joint space narrowing, malalignment, post-traumatic changes, or implanted hardware. Advanced imaging studies—computerized tomography, magnetic resonance imaging, bone scan—are reserved for cases that necessitate ruling out alternative diagnoses, or for preoperative evaluation by an orthopedic surgeon.
Management: Make use of multiple modalities
Conservative management options for ankle OA are limited, and high-quality evidence of efficacy is lacking. Surgical alternatives, however, are invasive and yield modest outcomes. Therefore, unless specific indications for surgery are present, exhaust conservative options (TABLE 2) before considering referral.

Weight loss is important for those who are overweight—as with knee OA management—to decrease the reactive forces within the ankle joint and to decrease pain. Weight loss will also enhance the outcomes of other treatment modalities and improve overall health.10,11
Activity modification is usually required, even though this may make weight loss more difficult. Avoiding vigorous activities, restricting work-related movements that place high-impact stress on the ankle, and decreasing overall walking time often reduce the severity of symptoms and improve functioning in other activities. Use of assistive-devices, such as a cane, can decrease the weight-bearing load on the affected joint.10,11
Physical therapy has not been shown to alleviate pain in ankle arthritis, although stretching, joint mobilization, and gait training may help prevent further progression of arthritis and improve function.11 The strength of dorsiflexion and plantar-flexion muscles is often decreased in individuals with ankle arthritis. Strengthening exercises may be indicated in individuals exhibiting deficits.
Prescriptive conservative management. Begin with a combination, as needed, of anti-inflammatory medications, orthotic devices, and footwear modifications.
Nonsteroidal anti-inflammatory agents are generally safe, but long-term use requires monitoring. Intra-articular steroid injections have some supporting evidence of effectiveness, but any benefit is short-lived.12 Glucosamine and chondroitin, although unlikely to cause harm, are not supported by the evidence for use in ankle arthritis. Intra-articular viscosupplementation is controversial, and evidence is limited regarding its efficacy.1
Adding a rocker-bottom sole and a solid ankle cushion heel to a shoe helps decrease heel strike impact in individuals with decreased ankle motion, and they aid in the transition from the heel strike to the push-off during level walking.11 If the arthritic joint is unstable, a lace-up ankle support may help with proprioception and stability. A polypropylene ankle-foot orthosis, custom leather ankle corset, or a double-upright brace with a patellar-tendon-bearing support are options to restrict ankle motion and decrease weight-bearing forces.10,11
Immobilization is not recommended except for short-term use during an arthritic flare. Limiting ankle motion reduces pain, but the downside tradeoff is acquired stiffness and weakness that accompanies prolonged periods of immobilization. A controlled ankle motion walking boot or walking plaster cast are both reasonable options for the short term.
Consider surgical referral for specific indications such as osteophytes, loose bodies, and chondral defects, which may be treated with arthroscopy. Patients with large areas of exposed chondral bone or rapid onset of degeneration have poorer outcomes with conservative management and should also be referred to a surgeon earlier. Otherwise, consider surgical referral only after a full trial of conservative management.11
Surgical options vary in scope and effectiveness and include osteotomy, arthrodesis, and arthroplasty. Osteotomies can be performed in early OA to correct bony alignment deformities. Arthrodesis in neutral dorsiflexion with roughly 5 degrees of external rotation is reserved for end-stage ankle OA to allow for near normal gait and pain relief. Total ankle arthroplasty is an emerging option for severe ankle OA, resulting in improved pain relief, gait, and patient satisfaction, but potentially has a higher reoperation rate when compared with arthrodesis.1,2
CASE › We prescribed short-term immobilization with a controlled ankle motion boot and administered an intra-articular corticosteroid injection. At the patient’s follow-up visit 6 weeks later, he reported only moderate improvement in pain. We then advised physical therapy at a specialty ankle rehabilitation program to focus on mobilization, strengthening, and gait training. Nearly one year after his initial visit to our clinic, he is doing well. He understands, however, that the nature of his ankle arthrosis may necessitate surgical intervention in the future.
CORRESPONDENCE
Adam Bitterman, DO, Department of Orthopedic Surgery, Hofstra Northwell School of Medicine at Huntington Hospital, 155 East Main Street, Huntington, NY 11743; [email protected].
CASE › A 57-year-old man had been experiencing intermittent pain in his left ankle for the past 2.5 years. About 6 weeks before coming to our clinic, his symptoms became significantly worse after playing a pickup game of basketball. At the clinic visit, he reported no other recent injury or trauma to the leg. However, 15 years earlier he had fractured his left ankle and was treated conservatively with a short period in a cast followed by a course of physical therapy. After completing the physical therapy, he noted significant improvement, although he continued to have minor episodes of pain. He felt no instability or mechanical locking but did note a decreased ability to move the ankle. And it felt much stiffer than his right ankle.
Examination of his left ankle revealed tenderness over the anterior aspect at the tibiotalar joint. He also exhibited decreased dorsiflexion and was unable to perform a toe raise. There was no tenderness over the major ligaments, and results of anterior drawer and talar tilt tests were normal. X-rays revealed tibiotalar joint arthritis (FIGURE).

How would you proceed if this were your patient?
Arthritis of the tibiotalar joint, which has an estimated prevalence of approximately 1%, occurs much less frequently than arthritis of the knee or hip joints.1 This low prevalence is primarily due to the ankle joint’s unique biomechanics and the features of the cartilage within the joint, including its thickness.2
Specifically, the hip and knee joints have greater degrees of freedom than the tibiotalar articulation, which is significantly constrained. The bony congruity between the talus, tibia, and fibula provides inherent stability to the ankle joint, thus protecting against primary osteoarthritis (OA).
Additionally, the large number of ligamentous structures and overall strength of the ligaments provide significant supplemental stability to the ankle joint articulation. Articular cartilage within the ankle joint is thicker than that of the knee and hip (1-1.7 mm). This cartilage also tends to retain its tensile strength with age, unlike cartilage in the hip; the ankle is therefore more resistant to age-related degeneration.3
Metabolic factors also protect against arthritis. Chondrocytes in the ankle are less responsive to inflammatory mediators, including interleukin-1 (IL-1), and therefore produce fewer matrix metalloproteinases.1,2,4 There are also fewer IL-1 receptors on ankle chondrocytes.
The role of trauma in ankle OA. Given the ankle joint’s inherent stability, the most common cause of ankle OA is trauma,4 mainly ankle fracture and, less commonly, ligamentous injury.5,6 Other rarer causes of ankle arthritis include primary OA, crystalline arthropathy, inflammatory disease, septic arthritis, neuroarthropathy, hemochromatosis, and ochronosis.
The ankle’s characteristics that protect it against primary OA may facilitate the pathogenesis of post-traumatic OA through 2 main mechanisms. First, direct trauma to the chondral surfaces can hasten the onset of progressive degeneration. Second, articular incongruity from a fracture can lead to insidious deterioration. The stiffer cartilage layer may be less adaptable to malalignment, and incongruity may cause secondary instability and chronic overloading. Ultimately, the joint breaks down with associated cartilage wear.6,7
The importance of the normal ankle’s congruity and stability became clear in the landmark study by Ramsey and colleagues,8 showing that the contact area between the talus and the tibia decreases as talar displacement increases laterally. This innate stability explains why the contact area of the ankle joint can bear loads similar to those of the hip and knee, yet does not experience primary OA nearly as often.
A stepwise diagnostic appraisal
Ask these questions. Since most ankle pain results from trauma, ask about any recent or remote injury to the affected ankle. Knowing the type of injury that occurred and the exact treatment, if received, may shed light on the relationship between the injury and current symptoms. Acute traumatic events can cause fractures or injury to various soft-tissue structures traversing the ankle joint. Ankle ligament sprains or tendon strains may result after abnormal rotation of the foot. Alternatively, chronic overuse injuries may lead to tendinopathy in any of the tendons that control motion throughout the foot and ankle or degenerative changes within the tibiotalar joint. Knowing the exact location of pain may also help identify the pathology (TABLE 1).

The patient in our case had not suffered a recent injury, so it was important to learn as much as possible about his prior fracture. Was the injury treated conservatively or surgically? If management was conservative, the type and duration of treatment could offer clues to the mechanism underlying symptoms. If a patient has undergone surgery, knowledge of the exact procedure could suggest specific problems. For example, surgical fixation would likely indicate there was ankle instability, thus altering the normal biomechanics in the injured tibiotalar joint.
Other key questions to ask. Most patients with ankle pain also complain of limitations in their usual activities. Ask about the duration and type of pain and other symptoms. Also ask about the position of the foot and ankle when the pain is at its greatest, which will provide insight into likely areas of pathology. For example, if pain arises when the patient navigates uneven ground, subtalar pathology is highly likely. If the patient complains of pain while walking down stairs, suspect injury to the posterior (plantar flexed) ankle; pain while walking up stairs more likely indicates anterior (dorsiflexed) pathology.
Finally, ask about nonorthopedic medical problems and all medications being taken. Systemic conditions, too, can lead to ankle pain—eg, inflammatory arthropathies, infections, and crystalline arthropathy.
Physical examination. Observe the patient’s gait to assess any functional or range-of-motion limitations or abnormal loading throughout the foot and ankle.9 With the patient standing, evaluate any malalignment from the foot through the knees and to the hips. Evaluate the skin for any lesions, wounds, or evidence of trauma or surgery. Next, with the patient seated, examine carefully for neuropathy or vascular abnormalities. Evaluate the ankle’s range of motion and assess for any mechanical locking, clicking, or crepitus. Palpate all bony and ligamentous landmarks to reveal areas of tenderness or swelling. Perform anterior drawer and varus tilt tests to determine overall ligamentous stability of the ankle, and compare your findings with test results of the opposite, uninjured ankle.
Diagnostic imaging. Order weight-bearing radiographs of the foot and ankle. Including the foot allows you to identify additional potential concerns such as malalignment, deformity, or adjacent joint arthritis. Look particularly for joint space narrowing, malalignment, post-traumatic changes, or implanted hardware. Advanced imaging studies—computerized tomography, magnetic resonance imaging, bone scan—are reserved for cases that necessitate ruling out alternative diagnoses, or for preoperative evaluation by an orthopedic surgeon.
Management: Make use of multiple modalities
Conservative management options for ankle OA are limited, and high-quality evidence of efficacy is lacking. Surgical alternatives, however, are invasive and yield modest outcomes. Therefore, unless specific indications for surgery are present, exhaust conservative options (TABLE 2) before considering referral.

Weight loss is important for those who are overweight—as with knee OA management—to decrease the reactive forces within the ankle joint and to decrease pain. Weight loss will also enhance the outcomes of other treatment modalities and improve overall health.10,11
Activity modification is usually required, even though this may make weight loss more difficult. Avoiding vigorous activities, restricting work-related movements that place high-impact stress on the ankle, and decreasing overall walking time often reduce the severity of symptoms and improve functioning in other activities. Use of assistive-devices, such as a cane, can decrease the weight-bearing load on the affected joint.10,11
Physical therapy has not been shown to alleviate pain in ankle arthritis, although stretching, joint mobilization, and gait training may help prevent further progression of arthritis and improve function.11 The strength of dorsiflexion and plantar-flexion muscles is often decreased in individuals with ankle arthritis. Strengthening exercises may be indicated in individuals exhibiting deficits.
Prescriptive conservative management. Begin with a combination, as needed, of anti-inflammatory medications, orthotic devices, and footwear modifications.
Nonsteroidal anti-inflammatory agents are generally safe, but long-term use requires monitoring. Intra-articular steroid injections have some supporting evidence of effectiveness, but any benefit is short-lived.12 Glucosamine and chondroitin, although unlikely to cause harm, are not supported by the evidence for use in ankle arthritis. Intra-articular viscosupplementation is controversial, and evidence is limited regarding its efficacy.1
Adding a rocker-bottom sole and a solid ankle cushion heel to a shoe helps decrease heel strike impact in individuals with decreased ankle motion, and they aid in the transition from the heel strike to the push-off during level walking.11 If the arthritic joint is unstable, a lace-up ankle support may help with proprioception and stability. A polypropylene ankle-foot orthosis, custom leather ankle corset, or a double-upright brace with a patellar-tendon-bearing support are options to restrict ankle motion and decrease weight-bearing forces.10,11
Immobilization is not recommended except for short-term use during an arthritic flare. Limiting ankle motion reduces pain, but the downside tradeoff is acquired stiffness and weakness that accompanies prolonged periods of immobilization. A controlled ankle motion walking boot or walking plaster cast are both reasonable options for the short term.
Consider surgical referral for specific indications such as osteophytes, loose bodies, and chondral defects, which may be treated with arthroscopy. Patients with large areas of exposed chondral bone or rapid onset of degeneration have poorer outcomes with conservative management and should also be referred to a surgeon earlier. Otherwise, consider surgical referral only after a full trial of conservative management.11
Surgical options vary in scope and effectiveness and include osteotomy, arthrodesis, and arthroplasty. Osteotomies can be performed in early OA to correct bony alignment deformities. Arthrodesis in neutral dorsiflexion with roughly 5 degrees of external rotation is reserved for end-stage ankle OA to allow for near normal gait and pain relief. Total ankle arthroplasty is an emerging option for severe ankle OA, resulting in improved pain relief, gait, and patient satisfaction, but potentially has a higher reoperation rate when compared with arthrodesis.1,2
CASE › We prescribed short-term immobilization with a controlled ankle motion boot and administered an intra-articular corticosteroid injection. At the patient’s follow-up visit 6 weeks later, he reported only moderate improvement in pain. We then advised physical therapy at a specialty ankle rehabilitation program to focus on mobilization, strengthening, and gait training. Nearly one year after his initial visit to our clinic, he is doing well. He understands, however, that the nature of his ankle arthrosis may necessitate surgical intervention in the future.
CORRESPONDENCE
Adam Bitterman, DO, Department of Orthopedic Surgery, Hofstra Northwell School of Medicine at Huntington Hospital, 155 East Main Street, Huntington, NY 11743; [email protected].
1. Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806.
2. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
3. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223-230.
4. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
5. Brown T, Johnston R, Saltzman C, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
6. Barg A, Pagenstert G, Hügle T, et al. Ankle osteoarthritis etiology, diagnostics and classification. Foot Ankle Clin. 2013;18:411-426.
7. Schenker M, Mauck R, Ahn J, et al. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20-28.
8. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-357.
9. Hayes BJ, Gonzalez T, Smith JT, et al. Ankle arthritis: you can’t always replace it. J Am Acad Orthop Surg. 2016;24:e29-e38.
10. Thomas R, Daniels T. Current concepts review ankle arthritis. J Bone Joint Surg Am. 2003;85A:923-936.
11. Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. J Orthop Sports Phys Ther. 2007:37:253-259.
12. Pekarek B, Osher L, Buck S, et al. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21:66-70.
13. Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle arthritis: why evidence of efficacy is still lacking? Clin Exp Rheumatol. 2012;30:277-281.
14. Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643.
15. Rao S, Ellis SJ, Deland JT, et al. Nonmedicinal therapy in the management of ankle arthritis. Curr Opin Rheumatol. 2010;22:223-228.
1. Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806.
2. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
3. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223-230.
4. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
5. Brown T, Johnston R, Saltzman C, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
6. Barg A, Pagenstert G, Hügle T, et al. Ankle osteoarthritis etiology, diagnostics and classification. Foot Ankle Clin. 2013;18:411-426.
7. Schenker M, Mauck R, Ahn J, et al. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20-28.
8. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-357.
9. Hayes BJ, Gonzalez T, Smith JT, et al. Ankle arthritis: you can’t always replace it. J Am Acad Orthop Surg. 2016;24:e29-e38.
10. Thomas R, Daniels T. Current concepts review ankle arthritis. J Bone Joint Surg Am. 2003;85A:923-936.
11. Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. J Orthop Sports Phys Ther. 2007:37:253-259.
12. Pekarek B, Osher L, Buck S, et al. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21:66-70.
13. Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle arthritis: why evidence of efficacy is still lacking? Clin Exp Rheumatol. 2012;30:277-281.
14. Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643.
15. Rao S, Ellis SJ, Deland JT, et al. Nonmedicinal therapy in the management of ankle arthritis. Curr Opin Rheumatol. 2010;22:223-228.
PRACTICE RECOMMENDATIONS
› Always ask that the foot be included in ankle x-rays to aid in identifying malalignment, deformity, or joint arthritis. C
› Use anti-inflammatory medications, orthotic devices, and footwear modifications, as needed, for ankle osteoarthritis. C
› Avoid ankle immobilization except, perhaps, during arthritic flare. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A stepwise approach to pediatric asthma
Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.
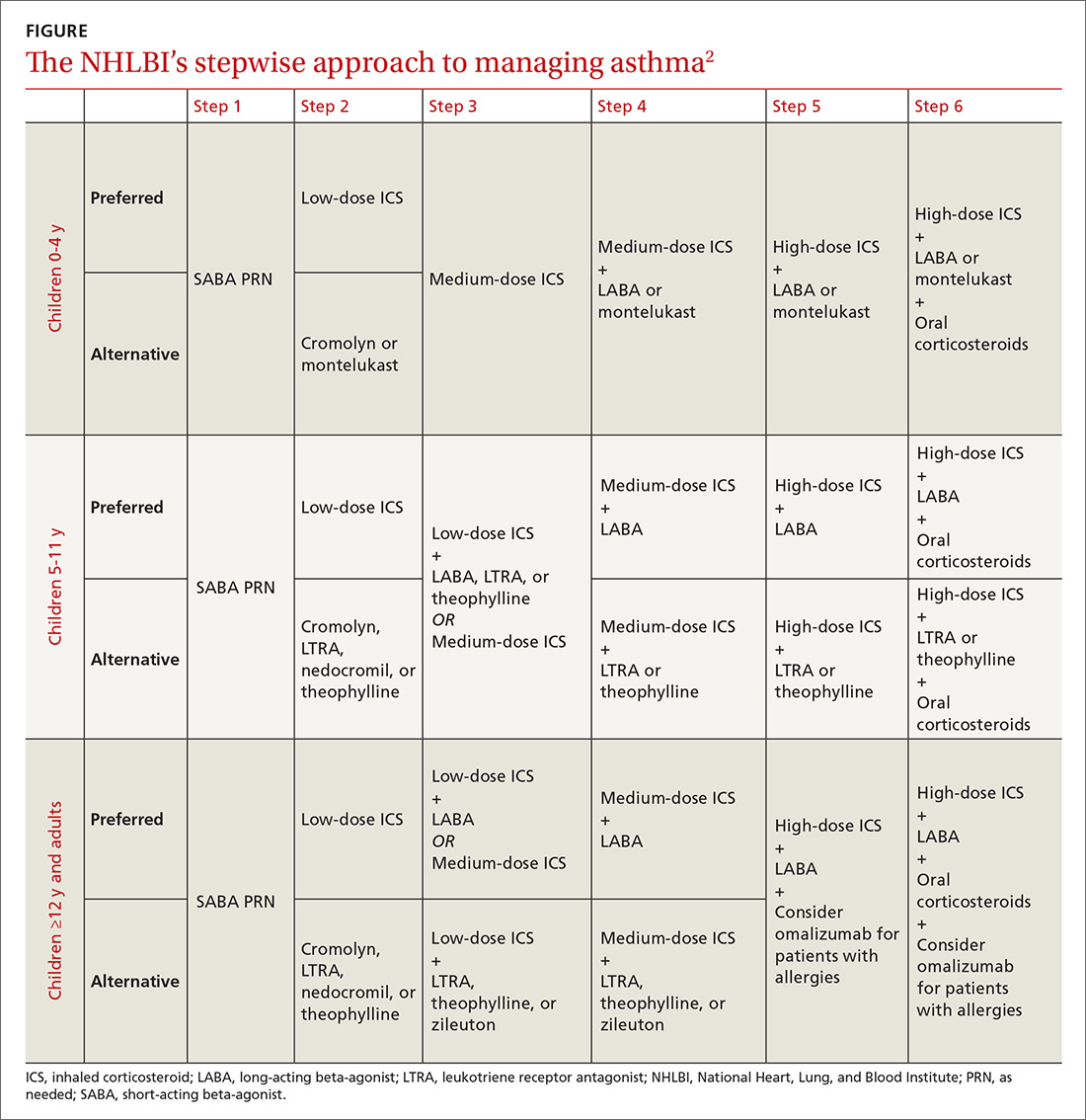
The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12
Another recent study demonstrated that the combination of ICSs and LABAs was noninferior to ICSs alone in preventing hospitalizations, intubations, and deaths.13 There are limited data on whether patients already on LABAs and ICSs should be continued on dual medications. Additionally, there is no clear method describing how to de-escalate therapy for those patients who are well controlled on ICSs and LABAs. A reasonable approach is to reduce doses of both medications and discontinue the LABA if tolerated.14,15 The US Food and Drug Administration has issued a black box warning that LABAs should not be used as a single controller medication because patients may be at increased risk of asthma-related deaths.16,17
What role for leukotriene receptor antagonists?
According to NHLBI guidelines, LTRAs can be considered as an alternative to ICSs when starting a control medication for mild persistent asthma.2 A recent meta-analysis, however, showed that there were increased rates of hospitalizations with LTRAs alone when compared to ICSs.18 The NHLBI guidelines also suggest that LTRAs can be used as adjunctive medication for those patients not well controlled on ICSs rather than increasing the ICS dose or adding a LABA.2
A 2011 clinical trial found no difference in quality of life measures between LTRAs and LABAs as adjunctive therapy at 2 months, but LABAs were more effective when patients were reassessed in 2 years.19 Similarly, the same study also found that adding LTRAs to low-dose ICSs rather than increasing the ICS dose was equivalent in the short term but not at 2 years.19
2 other adjunctive therapy options: Xanthines, cromolyn
Similar to LTRAs, xanthines can be considered as adjunctive therapy for children older than 5 years who are not well controlled on a low-dose ICS. Although xanthines decrease asthma symptoms when compared to placebo alone, they are not more effective than ICSs alone and should be considered only as adjunctive therapy.2,20 There have been few studies comparing xanthines to other adjunctive medications.21
Cromolyn is another adjunctive medication cited in the NHLBI guidelines for escalation of therapy.2 Although the medication has few adverse effects, its use is generally limited in the United States because data supporting its efficacy are lacking.
Omalizumab for allergy-related asthma exacerbations
Omalizumab, an anti-IgE antibody injected every 2 to 4 weeks, is available for children older than 6 years with moderate to severe asthma that is not responsive to ICSs and LABAs.22 The medication is effective in reducing allergy-related asthma exacerbations and hospitalizations, but data comparing it to other adjunctive medications are limited.22 Due to their significant systemic effects, the role of oral steroids as control medications is reserved for patients with severe asthma who are refractory to other medications. Children should be placed on oral steroids for the least amount of time required to achieve symptom control.
Acute exacerbation treatment: What to consider
Although there is no agreed-upon definition for an acute asthma exacerbation, the American Thoracic Society defines it as "an event characterized by a change from the patient’s previous status."23 All patients should be given an asthma action plan that clearly delineates the escalation of therapy in the event of an exacerbation, although only half of all patients report experiencing one.24 Symptom-based plans may prevent more acute care visits when compared to plans that use peak-flow measurements, although children on peak-flow plans may have fewer symptomatic days.25
Start with short-acting beta-agonists
The SABAs serve as the initial treatment of choice for management of asthma exacerbations. In young children (0-3 years), SABAs delivered by MDI with a spacer were more effective in reducing admission rates (11.3% vs 21.7%) when compared to SABAs delivered by nebulizers, resulting in a number needed to treat to prevent one admission of 10.26
In older children (3-18 years), SABAs delivered via spacer reduced ED length of stay, but did not significantly affect hospitalization rates. Additionally, SABAs administered with anticholinergics such as ipratropium bromide were more effective than SABAs alone in reducing admissions (16.9% vs 23.2%), particularly in older children with moderate to severe asthma, while also minimizing adverse effects.8,26
Corticosteroids: A mainstay in the ED
In addition to albuterol administration, corticosteroids remain the mainstay of ED management for asthma exacerbations. Administration of systemic steroids has been shown to reduce hospitalizations in children under 6 years, although, paradoxically, studies examining outpatient administration have demonstrated an increase in hospitalizations when compared to placebo.27
Dexamethasone and prednisone are the 2 most commonly used systemic steroids, and studies haven't indicated superiority of either.28,29 There is no difference in efficacy between oral and intravenous steroids.30 A recent clinical trial found a 2-day course of dexamethasone (0.6 mg/kg) had similar efficacy with fewer adverse effects when compared to a 5-day course of prednisone (1-2 mg/kg/day).28
Patient isn’t responding? Try IV magnesium
For patients who don't respond to corticosteroids and albuterol treatments, IV magnesium sulfate (usual dose, 25-75 mg/kg/d; maximum dose, 2000 mg/d) has been shown to improve respiratory function, but not necessarily decrease admission rates.31 Inhaled magnesium sulfate hasn't been shown to be more effective than IV administration and isn'trecommended.32
Evidence doesn’t support use of heliox
Heliox, which consists of 80% helium, is theorized to be effective in the treatment of asthma by increasing laminar flow and increasing the delivery of medications to the alveoli.32 Overall, the evidence does not support the use of heliox, which is typically restricted to patients with severe asthma exacerbations.33
Is it worth considering noninvasive positive pressure ventilation?
If patients don't improve with medical treatment, noninvasive positive pressure ventilation can be considered. A recent meta-analysis suggests that there is no definitive benefit or harm from this treatment, although several studies have indicated a decrease in symptom severity.34
Intubation should be considered for hypoxemia unresponsive to medications, or in cases of exhaustion, worsening mental status, or respiratory acidosis unresponsive to medication. Ventilation should allow for a permissive respiratory acidosis (pH, 7.2), while maintaining adequate oxygenation.35
Reducing the burden of asthma
Due to the complex task of reducing triggers and providing effective controller medications, working with parents and children is integral to improving the quality of life for patients with asthma. Although there is an obvious genetic predisposition, family physicians can help reduce the risk of developing asthma by encouraging healthy behaviors at home before the child is born. In the prenatal period, this includes avoiding tobacco-smoke exposure, lessening maternal obesity, decreasing maternal antibiotic and acetaminophen use, and curtailing stress.
Evidence suggests that after birth, breastfeeding and reducing childhood obesity can help lower the risk of asthma.36 Atopic disease, in general, can be reduced by breastfeeding until at least 4 months, as well as encouraging a varied diet that does not restrict potential allergens during pregnancy or lactation, and introducing foods (including potential allergens) after the age of 4 months.
The risk of atopic disease can also be lowered by lessening potential triggers at home. These include restricting exposure to cats (but not dogs), reducing home mold by decreasing humidity and ensuring adequate ventilation, avoiding volatile organic compounds, such as chlorine, and curtailing exposure to vehicle emissions. Although often marketed to be effective in reducing allergies, dust-mite covers and soy-based formulas don't prevent or minimize allergies and are often costly.37,38 In addition, there is no evidence that vaccinations areassociated with allergies.39
CORRESPONDENCE
Douglas M. Maurer, 9040A Jackson Ave, Joint-Base Lewis-McChord, WA 98431; [email protected].
1. US Department of Health and Human Services. Centers for Disease Control and Prevention. Asthma and Schools. Available at: www.cdc.gov/healthyschools/asthma/index.htm. Updated June 17, 2015. Accessed September 28, 2016.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Report No.:07-4051.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available at: www.ginasthma.org. Accessed April 11, 2017.
4. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695-703.
5. Ko FW, Hui DS, Leung TS, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17: 370-378.
6. Sudhanthar S, Thakur K, Sigal Y, et al. Improving asthma severity and control screening in a primary care pediatric practice. BMJ Qual Improv Rep. 2016;5
7. Wilkinson M, Bulloch B, Garcia-Filion P, et al. Efficacy of racemic albuterol versus levalbuterol used as a continuous nebulization for the treatment of acute asthma exacerbations: a randomized, double-blind, clinical trial. J Asthma. 2011;48:188-193.
8. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;19.
9. Delgado A, Chou KJ, Silver EJ, et al. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76-80.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
11. Loke YK, Blanco P, Thavarajh M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS ONE. 2015;10:e0133428.
12. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24:CD007949.
13. Stempel DA, Szefler SJ, Pedersen S, et al; VESTRI Investigators. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840-849.
14. Blair MM. PL Detail-Document, Safety of long-acting beta-agonists in asthma. Pharmacist’s Letter/Prescriber’s Letter. November 2012.
15. Kew KM, Beggs S, Ahmad S. Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015;5:CD011316.
16. Nelson HS, Weiss ST, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
17. US Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed December 23, 2016.
18. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
19. Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695-1707.
20. Seddon P, Bara A, Ducharme FM, et al. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database Syst Rev. 2006;1:CD002885.
21. van der Mark LB, Lyklema PHE, Geskus RB, et al. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm Med. 2012;12:63.
22. Normansell R, Walker S, Milan SJ. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
23. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society Statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59-99.
24. Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2-17 years of age, United States, 2002-2013. J Pediatr. 2016;171:283-289.
25. Bhogal SK, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3: CD005306.
26. Pollock M, Sinha IP, Hartling L, et al. Inhaled short-acting bronchodilators for managing emergency childhood asthma: an overview of reviews. Allergy. 2017;72:183-200.
27. Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51:868-876.
28. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493-499.
29. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD011801.
30. Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
31. Rowe BH, Bretzlaff J, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD001490.
32. Powell C, Dwan K, Milan SJ, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898.
33. Rodrigo GJ, Pollack CV, Rodrigo C, et al. Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev. 2006;4:CD002884.
34. Korang SK, Feinberg J, Wetterslev J, et al. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database System Rev. 2016;9:CD012067.
35. Kline-Krammes S, Patel NH, Robinson S. Childhood asthma: a guide for pediatric emergency medicine providers. Emerg Med Clin North Am. 2013;31:705-732.
36. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4:1111-1122.
37. Gøtzsche P, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;2:1465-1858.
38. Osborn DA, Sinn JKH. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev. 2006;4:1465-1858.
39. Schäfer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German society for Pediatric and Adolescent Medicine (DGKJ). Allegro J Int. 2014;23:186-199.
Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.

The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12
Another recent study demonstrated that the combination of ICSs and LABAs was noninferior to ICSs alone in preventing hospitalizations, intubations, and deaths.13 There are limited data on whether patients already on LABAs and ICSs should be continued on dual medications. Additionally, there is no clear method describing how to de-escalate therapy for those patients who are well controlled on ICSs and LABAs. A reasonable approach is to reduce doses of both medications and discontinue the LABA if tolerated.14,15 The US Food and Drug Administration has issued a black box warning that LABAs should not be used as a single controller medication because patients may be at increased risk of asthma-related deaths.16,17
What role for leukotriene receptor antagonists?
According to NHLBI guidelines, LTRAs can be considered as an alternative to ICSs when starting a control medication for mild persistent asthma.2 A recent meta-analysis, however, showed that there were increased rates of hospitalizations with LTRAs alone when compared to ICSs.18 The NHLBI guidelines also suggest that LTRAs can be used as adjunctive medication for those patients not well controlled on ICSs rather than increasing the ICS dose or adding a LABA.2
A 2011 clinical trial found no difference in quality of life measures between LTRAs and LABAs as adjunctive therapy at 2 months, but LABAs were more effective when patients were reassessed in 2 years.19 Similarly, the same study also found that adding LTRAs to low-dose ICSs rather than increasing the ICS dose was equivalent in the short term but not at 2 years.19
2 other adjunctive therapy options: Xanthines, cromolyn
Similar to LTRAs, xanthines can be considered as adjunctive therapy for children older than 5 years who are not well controlled on a low-dose ICS. Although xanthines decrease asthma symptoms when compared to placebo alone, they are not more effective than ICSs alone and should be considered only as adjunctive therapy.2,20 There have been few studies comparing xanthines to other adjunctive medications.21
Cromolyn is another adjunctive medication cited in the NHLBI guidelines for escalation of therapy.2 Although the medication has few adverse effects, its use is generally limited in the United States because data supporting its efficacy are lacking.
Omalizumab for allergy-related asthma exacerbations
Omalizumab, an anti-IgE antibody injected every 2 to 4 weeks, is available for children older than 6 years with moderate to severe asthma that is not responsive to ICSs and LABAs.22 The medication is effective in reducing allergy-related asthma exacerbations and hospitalizations, but data comparing it to other adjunctive medications are limited.22 Due to their significant systemic effects, the role of oral steroids as control medications is reserved for patients with severe asthma who are refractory to other medications. Children should be placed on oral steroids for the least amount of time required to achieve symptom control.
Acute exacerbation treatment: What to consider
Although there is no agreed-upon definition for an acute asthma exacerbation, the American Thoracic Society defines it as "an event characterized by a change from the patient’s previous status."23 All patients should be given an asthma action plan that clearly delineates the escalation of therapy in the event of an exacerbation, although only half of all patients report experiencing one.24 Symptom-based plans may prevent more acute care visits when compared to plans that use peak-flow measurements, although children on peak-flow plans may have fewer symptomatic days.25
Start with short-acting beta-agonists
The SABAs serve as the initial treatment of choice for management of asthma exacerbations. In young children (0-3 years), SABAs delivered by MDI with a spacer were more effective in reducing admission rates (11.3% vs 21.7%) when compared to SABAs delivered by nebulizers, resulting in a number needed to treat to prevent one admission of 10.26
In older children (3-18 years), SABAs delivered via spacer reduced ED length of stay, but did not significantly affect hospitalization rates. Additionally, SABAs administered with anticholinergics such as ipratropium bromide were more effective than SABAs alone in reducing admissions (16.9% vs 23.2%), particularly in older children with moderate to severe asthma, while also minimizing adverse effects.8,26
Corticosteroids: A mainstay in the ED
In addition to albuterol administration, corticosteroids remain the mainstay of ED management for asthma exacerbations. Administration of systemic steroids has been shown to reduce hospitalizations in children under 6 years, although, paradoxically, studies examining outpatient administration have demonstrated an increase in hospitalizations when compared to placebo.27
Dexamethasone and prednisone are the 2 most commonly used systemic steroids, and studies haven't indicated superiority of either.28,29 There is no difference in efficacy between oral and intravenous steroids.30 A recent clinical trial found a 2-day course of dexamethasone (0.6 mg/kg) had similar efficacy with fewer adverse effects when compared to a 5-day course of prednisone (1-2 mg/kg/day).28
Patient isn’t responding? Try IV magnesium
For patients who don't respond to corticosteroids and albuterol treatments, IV magnesium sulfate (usual dose, 25-75 mg/kg/d; maximum dose, 2000 mg/d) has been shown to improve respiratory function, but not necessarily decrease admission rates.31 Inhaled magnesium sulfate hasn't been shown to be more effective than IV administration and isn'trecommended.32
Evidence doesn’t support use of heliox
Heliox, which consists of 80% helium, is theorized to be effective in the treatment of asthma by increasing laminar flow and increasing the delivery of medications to the alveoli.32 Overall, the evidence does not support the use of heliox, which is typically restricted to patients with severe asthma exacerbations.33
Is it worth considering noninvasive positive pressure ventilation?
If patients don't improve with medical treatment, noninvasive positive pressure ventilation can be considered. A recent meta-analysis suggests that there is no definitive benefit or harm from this treatment, although several studies have indicated a decrease in symptom severity.34
Intubation should be considered for hypoxemia unresponsive to medications, or in cases of exhaustion, worsening mental status, or respiratory acidosis unresponsive to medication. Ventilation should allow for a permissive respiratory acidosis (pH, 7.2), while maintaining adequate oxygenation.35
Reducing the burden of asthma
Due to the complex task of reducing triggers and providing effective controller medications, working with parents and children is integral to improving the quality of life for patients with asthma. Although there is an obvious genetic predisposition, family physicians can help reduce the risk of developing asthma by encouraging healthy behaviors at home before the child is born. In the prenatal period, this includes avoiding tobacco-smoke exposure, lessening maternal obesity, decreasing maternal antibiotic and acetaminophen use, and curtailing stress.
Evidence suggests that after birth, breastfeeding and reducing childhood obesity can help lower the risk of asthma.36 Atopic disease, in general, can be reduced by breastfeeding until at least 4 months, as well as encouraging a varied diet that does not restrict potential allergens during pregnancy or lactation, and introducing foods (including potential allergens) after the age of 4 months.
The risk of atopic disease can also be lowered by lessening potential triggers at home. These include restricting exposure to cats (but not dogs), reducing home mold by decreasing humidity and ensuring adequate ventilation, avoiding volatile organic compounds, such as chlorine, and curtailing exposure to vehicle emissions. Although often marketed to be effective in reducing allergies, dust-mite covers and soy-based formulas don't prevent or minimize allergies and are often costly.37,38 In addition, there is no evidence that vaccinations areassociated with allergies.39
CORRESPONDENCE
Douglas M. Maurer, 9040A Jackson Ave, Joint-Base Lewis-McChord, WA 98431; [email protected].
Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.

The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12
Another recent study demonstrated that the combination of ICSs and LABAs was noninferior to ICSs alone in preventing hospitalizations, intubations, and deaths.13 There are limited data on whether patients already on LABAs and ICSs should be continued on dual medications. Additionally, there is no clear method describing how to de-escalate therapy for those patients who are well controlled on ICSs and LABAs. A reasonable approach is to reduce doses of both medications and discontinue the LABA if tolerated.14,15 The US Food and Drug Administration has issued a black box warning that LABAs should not be used as a single controller medication because patients may be at increased risk of asthma-related deaths.16,17
What role for leukotriene receptor antagonists?
According to NHLBI guidelines, LTRAs can be considered as an alternative to ICSs when starting a control medication for mild persistent asthma.2 A recent meta-analysis, however, showed that there were increased rates of hospitalizations with LTRAs alone when compared to ICSs.18 The NHLBI guidelines also suggest that LTRAs can be used as adjunctive medication for those patients not well controlled on ICSs rather than increasing the ICS dose or adding a LABA.2
A 2011 clinical trial found no difference in quality of life measures between LTRAs and LABAs as adjunctive therapy at 2 months, but LABAs were more effective when patients were reassessed in 2 years.19 Similarly, the same study also found that adding LTRAs to low-dose ICSs rather than increasing the ICS dose was equivalent in the short term but not at 2 years.19
2 other adjunctive therapy options: Xanthines, cromolyn
Similar to LTRAs, xanthines can be considered as adjunctive therapy for children older than 5 years who are not well controlled on a low-dose ICS. Although xanthines decrease asthma symptoms when compared to placebo alone, they are not more effective than ICSs alone and should be considered only as adjunctive therapy.2,20 There have been few studies comparing xanthines to other adjunctive medications.21
Cromolyn is another adjunctive medication cited in the NHLBI guidelines for escalation of therapy.2 Although the medication has few adverse effects, its use is generally limited in the United States because data supporting its efficacy are lacking.
Omalizumab for allergy-related asthma exacerbations
Omalizumab, an anti-IgE antibody injected every 2 to 4 weeks, is available for children older than 6 years with moderate to severe asthma that is not responsive to ICSs and LABAs.22 The medication is effective in reducing allergy-related asthma exacerbations and hospitalizations, but data comparing it to other adjunctive medications are limited.22 Due to their significant systemic effects, the role of oral steroids as control medications is reserved for patients with severe asthma who are refractory to other medications. Children should be placed on oral steroids for the least amount of time required to achieve symptom control.
Acute exacerbation treatment: What to consider
Although there is no agreed-upon definition for an acute asthma exacerbation, the American Thoracic Society defines it as "an event characterized by a change from the patient’s previous status."23 All patients should be given an asthma action plan that clearly delineates the escalation of therapy in the event of an exacerbation, although only half of all patients report experiencing one.24 Symptom-based plans may prevent more acute care visits when compared to plans that use peak-flow measurements, although children on peak-flow plans may have fewer symptomatic days.25
Start with short-acting beta-agonists
The SABAs serve as the initial treatment of choice for management of asthma exacerbations. In young children (0-3 years), SABAs delivered by MDI with a spacer were more effective in reducing admission rates (11.3% vs 21.7%) when compared to SABAs delivered by nebulizers, resulting in a number needed to treat to prevent one admission of 10.26
In older children (3-18 years), SABAs delivered via spacer reduced ED length of stay, but did not significantly affect hospitalization rates. Additionally, SABAs administered with anticholinergics such as ipratropium bromide were more effective than SABAs alone in reducing admissions (16.9% vs 23.2%), particularly in older children with moderate to severe asthma, while also minimizing adverse effects.8,26
Corticosteroids: A mainstay in the ED
In addition to albuterol administration, corticosteroids remain the mainstay of ED management for asthma exacerbations. Administration of systemic steroids has been shown to reduce hospitalizations in children under 6 years, although, paradoxically, studies examining outpatient administration have demonstrated an increase in hospitalizations when compared to placebo.27
Dexamethasone and prednisone are the 2 most commonly used systemic steroids, and studies haven't indicated superiority of either.28,29 There is no difference in efficacy between oral and intravenous steroids.30 A recent clinical trial found a 2-day course of dexamethasone (0.6 mg/kg) had similar efficacy with fewer adverse effects when compared to a 5-day course of prednisone (1-2 mg/kg/day).28
Patient isn’t responding? Try IV magnesium
For patients who don't respond to corticosteroids and albuterol treatments, IV magnesium sulfate (usual dose, 25-75 mg/kg/d; maximum dose, 2000 mg/d) has been shown to improve respiratory function, but not necessarily decrease admission rates.31 Inhaled magnesium sulfate hasn't been shown to be more effective than IV administration and isn'trecommended.32
Evidence doesn’t support use of heliox
Heliox, which consists of 80% helium, is theorized to be effective in the treatment of asthma by increasing laminar flow and increasing the delivery of medications to the alveoli.32 Overall, the evidence does not support the use of heliox, which is typically restricted to patients with severe asthma exacerbations.33
Is it worth considering noninvasive positive pressure ventilation?
If patients don't improve with medical treatment, noninvasive positive pressure ventilation can be considered. A recent meta-analysis suggests that there is no definitive benefit or harm from this treatment, although several studies have indicated a decrease in symptom severity.34
Intubation should be considered for hypoxemia unresponsive to medications, or in cases of exhaustion, worsening mental status, or respiratory acidosis unresponsive to medication. Ventilation should allow for a permissive respiratory acidosis (pH, 7.2), while maintaining adequate oxygenation.35
Reducing the burden of asthma
Due to the complex task of reducing triggers and providing effective controller medications, working with parents and children is integral to improving the quality of life for patients with asthma. Although there is an obvious genetic predisposition, family physicians can help reduce the risk of developing asthma by encouraging healthy behaviors at home before the child is born. In the prenatal period, this includes avoiding tobacco-smoke exposure, lessening maternal obesity, decreasing maternal antibiotic and acetaminophen use, and curtailing stress.
Evidence suggests that after birth, breastfeeding and reducing childhood obesity can help lower the risk of asthma.36 Atopic disease, in general, can be reduced by breastfeeding until at least 4 months, as well as encouraging a varied diet that does not restrict potential allergens during pregnancy or lactation, and introducing foods (including potential allergens) after the age of 4 months.
The risk of atopic disease can also be lowered by lessening potential triggers at home. These include restricting exposure to cats (but not dogs), reducing home mold by decreasing humidity and ensuring adequate ventilation, avoiding volatile organic compounds, such as chlorine, and curtailing exposure to vehicle emissions. Although often marketed to be effective in reducing allergies, dust-mite covers and soy-based formulas don't prevent or minimize allergies and are often costly.37,38 In addition, there is no evidence that vaccinations areassociated with allergies.39
CORRESPONDENCE
Douglas M. Maurer, 9040A Jackson Ave, Joint-Base Lewis-McChord, WA 98431; [email protected].
1. US Department of Health and Human Services. Centers for Disease Control and Prevention. Asthma and Schools. Available at: www.cdc.gov/healthyschools/asthma/index.htm. Updated June 17, 2015. Accessed September 28, 2016.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Report No.:07-4051.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available at: www.ginasthma.org. Accessed April 11, 2017.
4. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695-703.
5. Ko FW, Hui DS, Leung TS, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17: 370-378.
6. Sudhanthar S, Thakur K, Sigal Y, et al. Improving asthma severity and control screening in a primary care pediatric practice. BMJ Qual Improv Rep. 2016;5
7. Wilkinson M, Bulloch B, Garcia-Filion P, et al. Efficacy of racemic albuterol versus levalbuterol used as a continuous nebulization for the treatment of acute asthma exacerbations: a randomized, double-blind, clinical trial. J Asthma. 2011;48:188-193.
8. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;19.
9. Delgado A, Chou KJ, Silver EJ, et al. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76-80.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
11. Loke YK, Blanco P, Thavarajh M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS ONE. 2015;10:e0133428.
12. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24:CD007949.
13. Stempel DA, Szefler SJ, Pedersen S, et al; VESTRI Investigators. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840-849.
14. Blair MM. PL Detail-Document, Safety of long-acting beta-agonists in asthma. Pharmacist’s Letter/Prescriber’s Letter. November 2012.
15. Kew KM, Beggs S, Ahmad S. Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015;5:CD011316.
16. Nelson HS, Weiss ST, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
17. US Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed December 23, 2016.
18. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
19. Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695-1707.
20. Seddon P, Bara A, Ducharme FM, et al. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database Syst Rev. 2006;1:CD002885.
21. van der Mark LB, Lyklema PHE, Geskus RB, et al. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm Med. 2012;12:63.
22. Normansell R, Walker S, Milan SJ. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
23. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society Statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59-99.
24. Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2-17 years of age, United States, 2002-2013. J Pediatr. 2016;171:283-289.
25. Bhogal SK, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3: CD005306.
26. Pollock M, Sinha IP, Hartling L, et al. Inhaled short-acting bronchodilators for managing emergency childhood asthma: an overview of reviews. Allergy. 2017;72:183-200.
27. Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51:868-876.
28. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493-499.
29. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD011801.
30. Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
31. Rowe BH, Bretzlaff J, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD001490.
32. Powell C, Dwan K, Milan SJ, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898.
33. Rodrigo GJ, Pollack CV, Rodrigo C, et al. Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev. 2006;4:CD002884.
34. Korang SK, Feinberg J, Wetterslev J, et al. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database System Rev. 2016;9:CD012067.
35. Kline-Krammes S, Patel NH, Robinson S. Childhood asthma: a guide for pediatric emergency medicine providers. Emerg Med Clin North Am. 2013;31:705-732.
36. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4:1111-1122.
37. Gøtzsche P, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;2:1465-1858.
38. Osborn DA, Sinn JKH. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev. 2006;4:1465-1858.
39. Schäfer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German society for Pediatric and Adolescent Medicine (DGKJ). Allegro J Int. 2014;23:186-199.
1. US Department of Health and Human Services. Centers for Disease Control and Prevention. Asthma and Schools. Available at: www.cdc.gov/healthyschools/asthma/index.htm. Updated June 17, 2015. Accessed September 28, 2016.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Report No.:07-4051.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available at: www.ginasthma.org. Accessed April 11, 2017.
4. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695-703.
5. Ko FW, Hui DS, Leung TS, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17: 370-378.
6. Sudhanthar S, Thakur K, Sigal Y, et al. Improving asthma severity and control screening in a primary care pediatric practice. BMJ Qual Improv Rep. 2016;5
7. Wilkinson M, Bulloch B, Garcia-Filion P, et al. Efficacy of racemic albuterol versus levalbuterol used as a continuous nebulization for the treatment of acute asthma exacerbations: a randomized, double-blind, clinical trial. J Asthma. 2011;48:188-193.
8. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;19.
9. Delgado A, Chou KJ, Silver EJ, et al. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76-80.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
11. Loke YK, Blanco P, Thavarajh M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS ONE. 2015;10:e0133428.
12. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24:CD007949.
13. Stempel DA, Szefler SJ, Pedersen S, et al; VESTRI Investigators. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840-849.
14. Blair MM. PL Detail-Document, Safety of long-acting beta-agonists in asthma. Pharmacist’s Letter/Prescriber’s Letter. November 2012.
15. Kew KM, Beggs S, Ahmad S. Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015;5:CD011316.
16. Nelson HS, Weiss ST, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
17. US Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed December 23, 2016.
18. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
19. Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695-1707.
20. Seddon P, Bara A, Ducharme FM, et al. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database Syst Rev. 2006;1:CD002885.
21. van der Mark LB, Lyklema PHE, Geskus RB, et al. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm Med. 2012;12:63.
22. Normansell R, Walker S, Milan SJ. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
23. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society Statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59-99.
24. Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2-17 years of age, United States, 2002-2013. J Pediatr. 2016;171:283-289.
25. Bhogal SK, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3: CD005306.
26. Pollock M, Sinha IP, Hartling L, et al. Inhaled short-acting bronchodilators for managing emergency childhood asthma: an overview of reviews. Allergy. 2017;72:183-200.
27. Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51:868-876.
28. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493-499.
29. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD011801.
30. Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
31. Rowe BH, Bretzlaff J, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD001490.
32. Powell C, Dwan K, Milan SJ, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898.
33. Rodrigo GJ, Pollack CV, Rodrigo C, et al. Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev. 2006;4:CD002884.
34. Korang SK, Feinberg J, Wetterslev J, et al. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database System Rev. 2016;9:CD012067.
35. Kline-Krammes S, Patel NH, Robinson S. Childhood asthma: a guide for pediatric emergency medicine providers. Emerg Med Clin North Am. 2013;31:705-732.
36. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4:1111-1122.
37. Gøtzsche P, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;2:1465-1858.
38. Osborn DA, Sinn JKH. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev. 2006;4:1465-1858.
39. Schäfer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German society for Pediatric and Adolescent Medicine (DGKJ). Allegro J Int. 2014;23:186-199.
PRACTICE RECOMMENDATIONS
› Reassure parents that metered-dose inhalers are as effective as nebulizers for asthma exacerbations. A
› Use a 2-day course of systemic steroids for asthma exacerbations rather than extended regimens. B
› Develop an asthma action plan for every patient with asthma to decrease acute care visits. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Insomnia: Getting to the cause, facilitating relief
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.
Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.
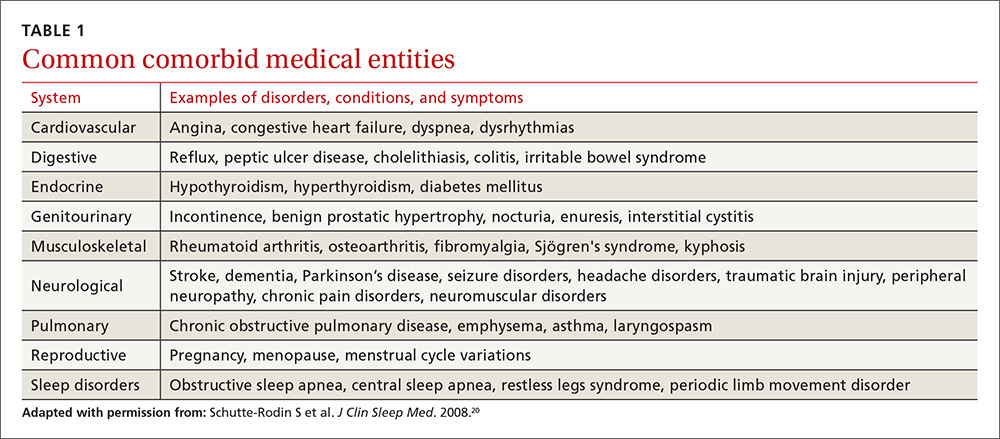
Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.
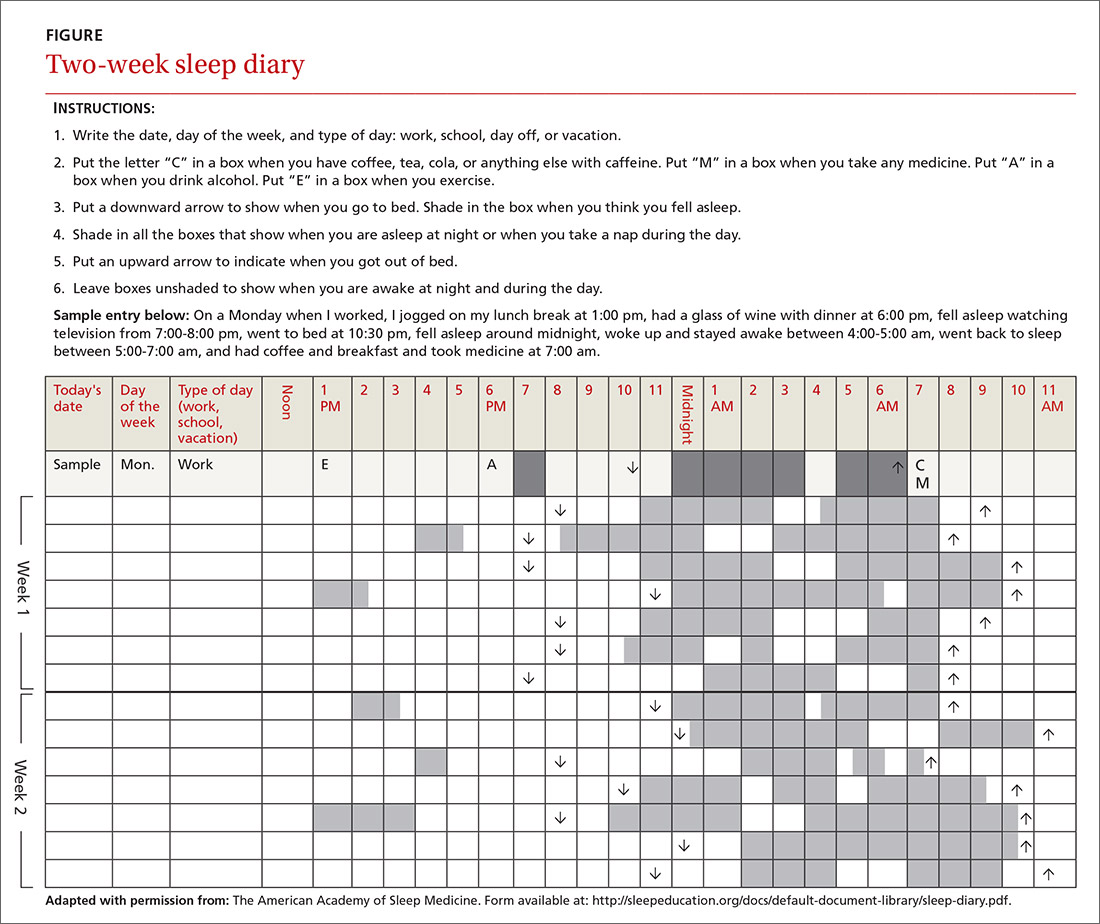
Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20
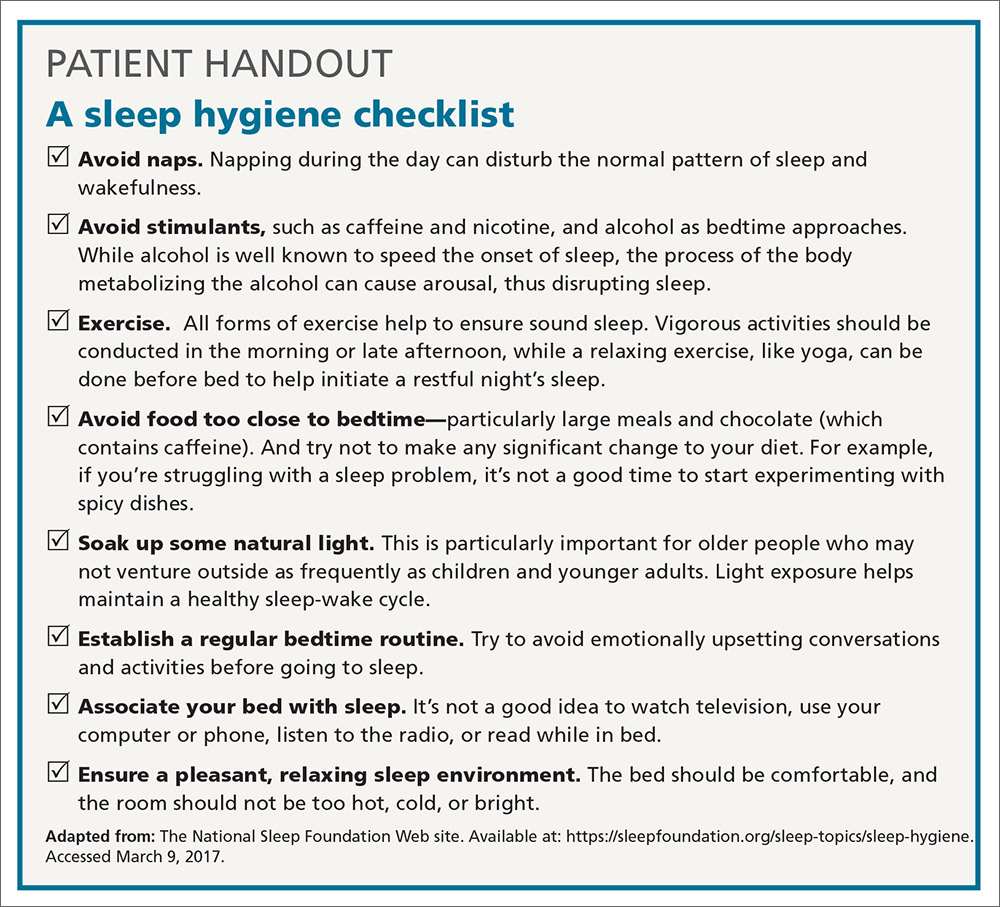
CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.
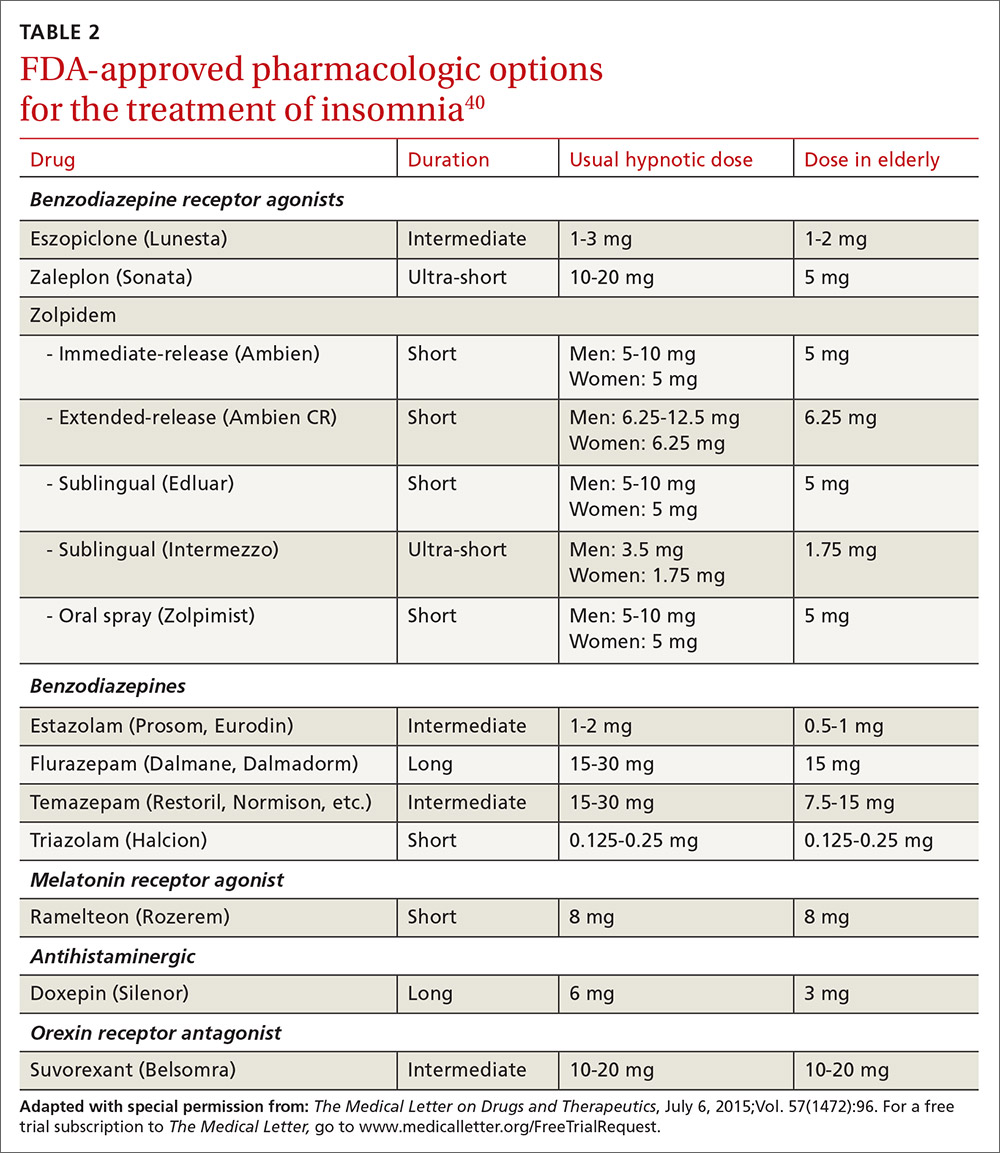
Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; [email protected].
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.

Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.

Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.

Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20

CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.

Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; [email protected].
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.

Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.

Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.

Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20

CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.

Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; [email protected].
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
PRACTICE RECOMMENDATIONS
› Recommend that patients try cognitive behavioral therapy for insomnia (CBT-I), as it is highly effective and some of its techniques can be employed in a busy family medicine clinic with little time commitment. B
› Consider pharmacotherapy for patients with chronic insomnia that persists despite CBT-I, as long as they are properly screened and followed regularly. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series