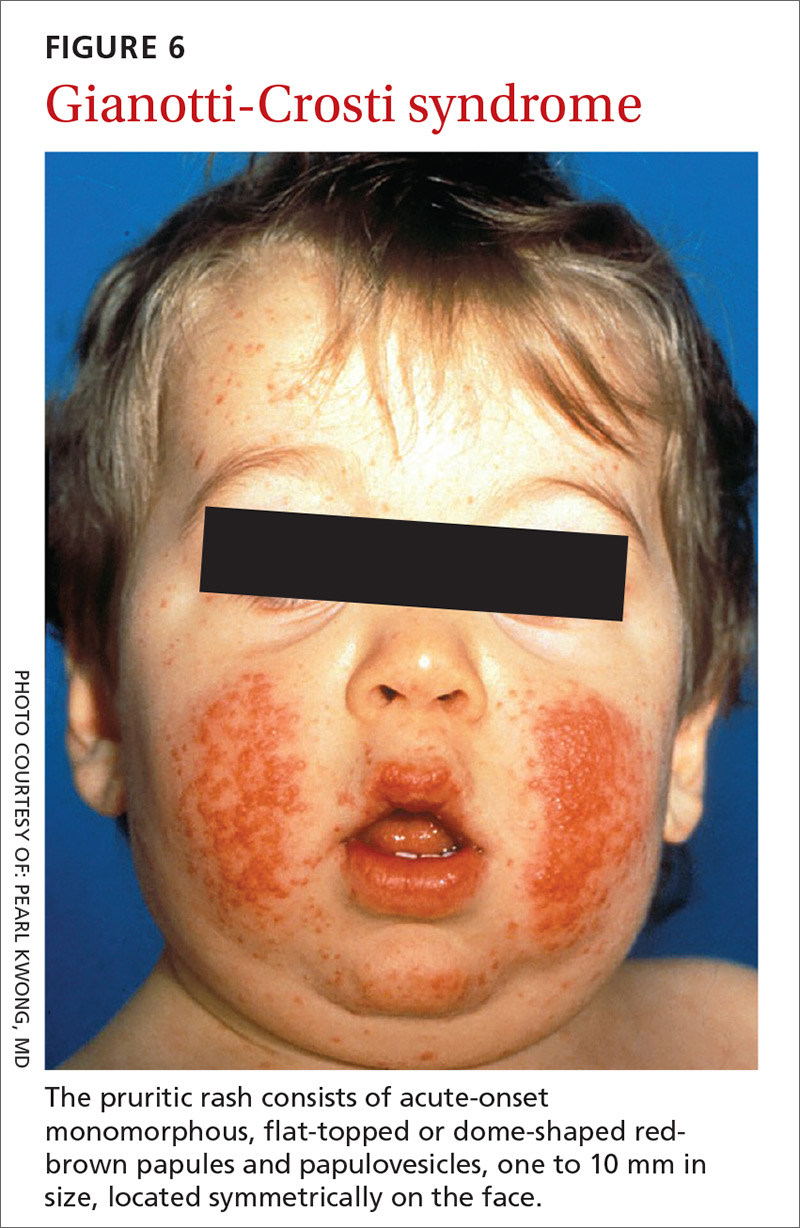
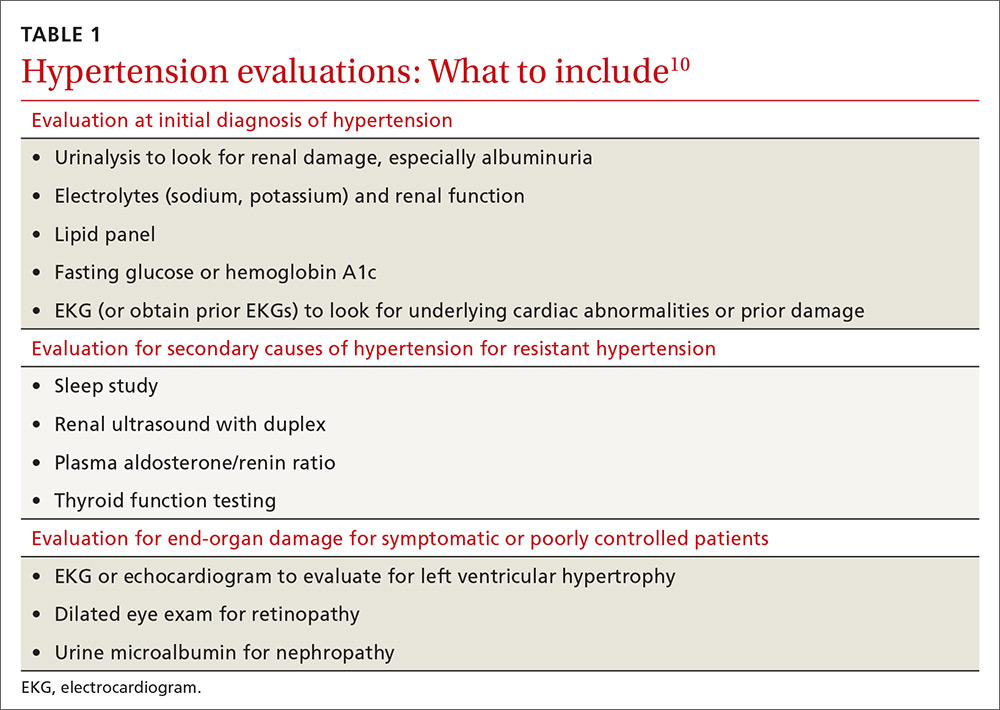
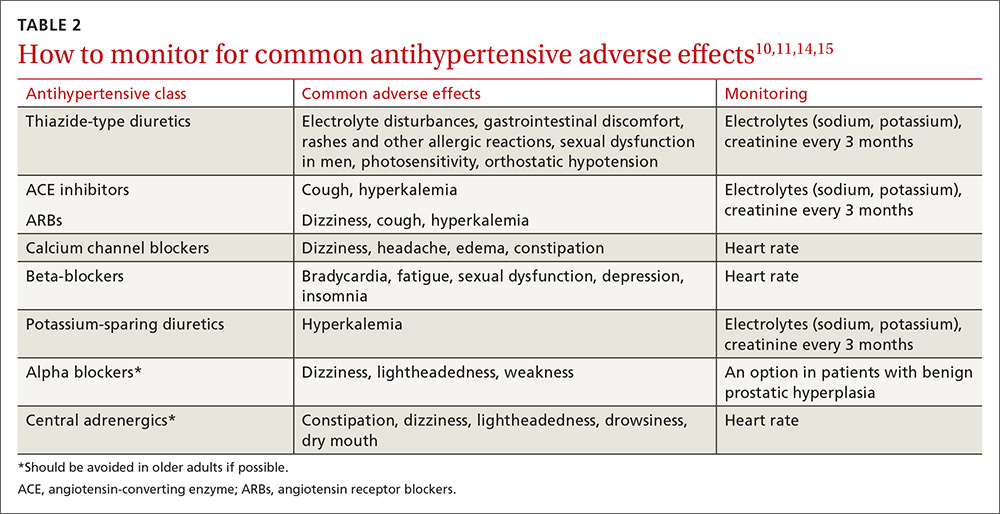
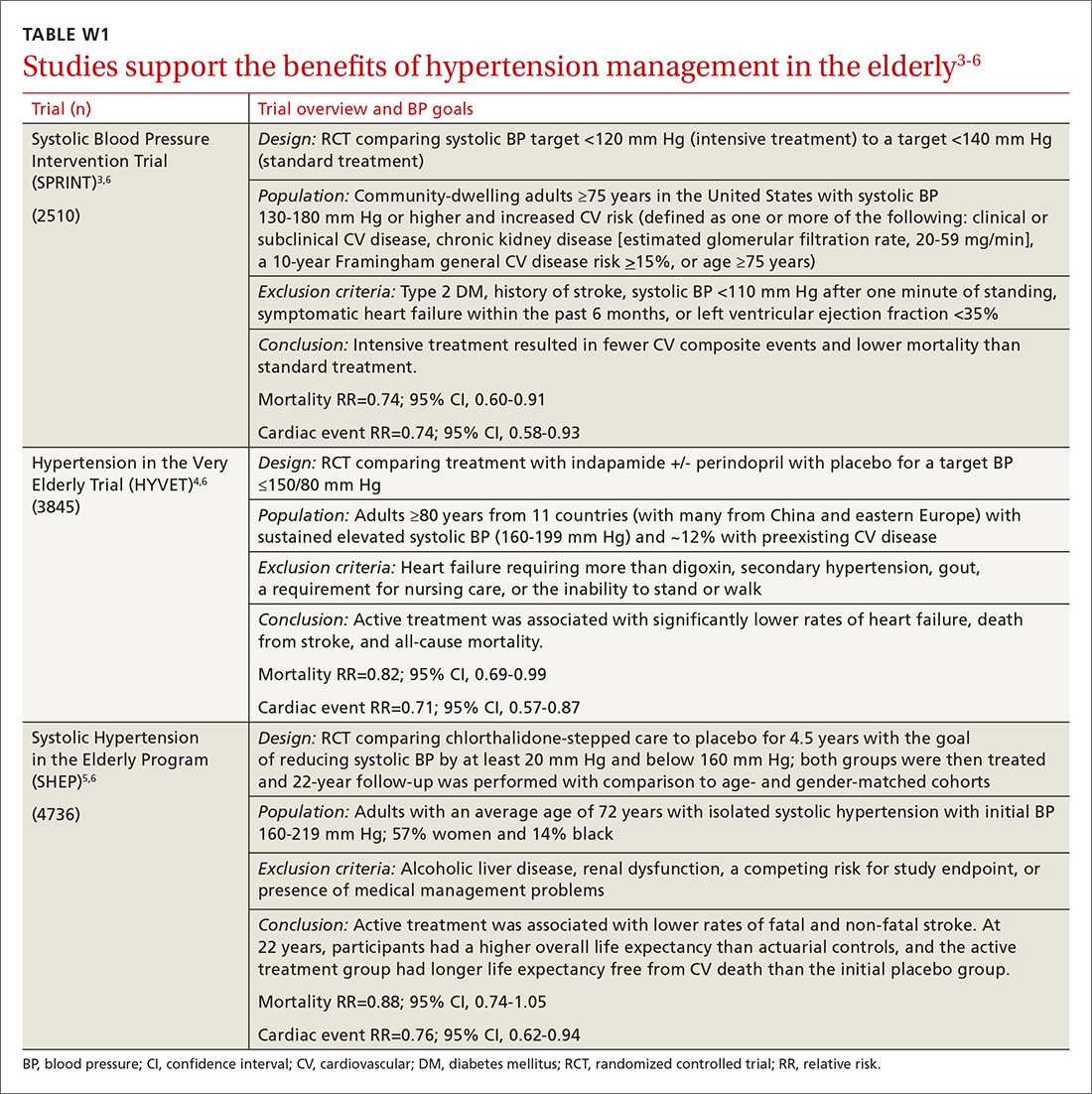
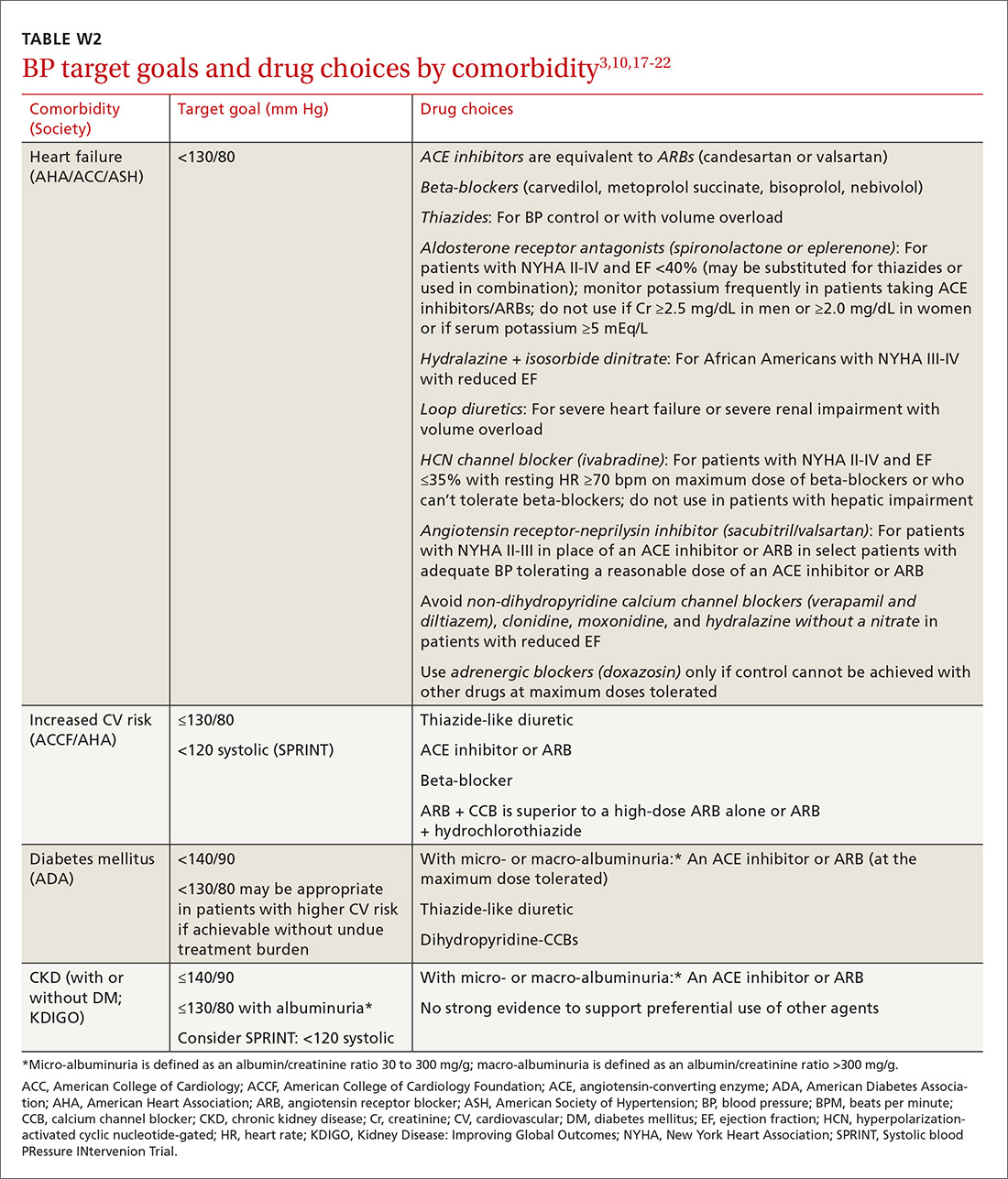
User login
Treating an Alzheimer’s patient? 6 tips from a patient’s spouse
What’s it like to be the caregiver for an Alzheimer’s patient? In my case, it was like being both married and widowed at the same time. Or as a person in my support group once put it: It’s a life filled with grief on the installment plan.
My wife, Clare, struggled for nearly 10 years with Alzheimer’s disease before passing away in April 2016—just one month shy of her 70th birthday and 2 months shy of our 49th wedding anniversary.
Our experience was gut-wrenching, but not unique for families coping with Alzheimer’s disease. Life as a caregiver is one of non-stop daily stress, with much sadness and anxiety, often accompanied by periods of mild or serious depression. Doubt, guilt, frustration, and many other emotions lead many caregivers to take anti-anxiety or antidepressant medication, meet regularly with therapists, take sleeping pills, or experience significant weight gain or loss. Stress drove me to my comfort foods, and I gained nearly 100 pounds while caring for Clare. Only in the last few months have I been able to start taking off that weight.
Helping a loved one who has Alzheimer’s with even the basic activities of daily living—hygiene, dressing, eating—becomes progressively difficult. Caring for a loved one who is confused, no longer remembers your name or who you are, or can occasionally become aggressive, is emotionally painful.
After being Clare’s 24/7 caregiver for 6 years, I agreed that placement in an assisted living facility was in her best interest. My role morphed from primary caregiver to primary care advocate, but the stress did not lessen. I met regularly with facility staff to ensure proper care because many staff members were not sufficiently motivated, educated, or trained to consistently provide proper care for individuals with Alzheimer’s disease.
Financial stress weighs heavily on caregivers. Unless one qualifies for Medicaid, is very wealthy, or is lucky enough to have outstanding long-term health care insurance and prescription drug coverage, caregiving costs can be astronomical. For someone with Alzheimer’s in a community such as Long Island, NY, assisted living facilities charge between $7000 and $10,000 per month, and nursing homes between $15,000 and $18,000 per month. Home health aides working 24/7 also cost around $15,000 per month. Caregiving costs can drain not just the patient’s bank account, but can wipe out the retirement life savings of the surviving caregiver.
Once Clare went into assisted living, I dealt with the daily loneliness and the enormous lifestyle changes. Being alone in my bed those first few nights after placement was painful beyond words, and learning to live alone for the first time after many years of marriage brought incredible sadness. It is no surprise to me that research points to caregiver stress as an independent risk factor for elderly caregiver mortality.1
My experience navigating the health care system with my wife included numerous challenges and instances of unnecessary frustration. My hope in providing the following suggestions is that they will help you help other families like mine.
1. Listen carefully to caregivers
When Clare first exhibited symptoms suggestive of Alzheimer’s, I started logging them and presented written summaries to doctors at each visit. But unless Clare exhibited those same symptoms in the presence of her doctors, my observations were routinely ignored. I’d try to discuss concerns—eg, Clare getting lost while driving to familiar locations, experiencing increased aphasia—but the doctors didn’t read my logs or listen carefully to what I was trying to tell them. The January/February 2017 AARP Bulletin2 noted studies showing that doctors listen for about 23 seconds before interrupting patients, but it also cited a 2001 South Carolina study3 that found patients spoke, uninterrupted, for an average of 12 seconds before being interrupted by a resident.
I eventually did learn that early Alzheimer’s symptoms can be easily misinterpreted as signs of stress, anxiety, or depression. But that underscores the need for doctors to listen carefully to caregivers, especially spouse caregivers who observe behaviors 24/7 that may not be present in a quick office visit or revealed on a brief cognitive screening test.
2. Stay up to date on screening tools that detect Alzheimer’s
The Mini-Mental State Examination, or MMSE, is the most frequently used cognitive screening tool, in part because it can be administered in less than 10 minutes. Although unquestionably valuable, a Cochrane review “did not find evidence supporting a substantial role of MMSE as a stand-alone single-administration test in the identification of MCI [mild-cognitive impairment] patients who could develop dementia.”4
Time-pressured doctors might consider using the AD8 screening interview, an informant questionnaire that takes only 2 to 3 minutes to administer, but has demonstrated superior sensitivity in detecting early dementia compared with the MMSE.5 In addition, a study in the December 2016 issue of the Journal of Alzheimer’s Disease 6 confirmed the usefulness of the Sniffin’ Sticks Odor Identification Test whereby patients try to identify 16 different odors. I can attest to Clare’s rapidly deteriorating senses of taste and smell as her disease progressed.
“Results suggest that a simple odor identification test can be a useful supplementary tool for clinically categorizing MCI and Alzheimer’s, and even for identifying people who are at the highest risk of worsening,” according to principal investigator, David R. Roalf, PhD.7
Prompted by prior studies that have linked a weakening sense of smell to Alzheimer’s, doctors in a few larger dementia clinics have already begun using smell tests in their assessments. One possible reason the practice has not yet become common, however, is that the tests take about 5 to 8 minutes to administer. Roalf and his colleagues are hoping to develop a shorter test that will work as well as the longer ones. “We’re hoping to shorten the Sniffin’ Sticks test … down to 3 minutes or so … We think that will encourage more neurology clinics to do this type of screening.”7
Is 5 minutes too much time to take to administer a valuable screening test?
3. Be candid when speaking with patients and their caregivers
A survey reported in Time magazine on March 24, 2015, found that as many as 64% of doctors do not share a diagnosis of Alzheimer’s with their patients because of “fear of causing emotional distress in their patients” due to a lack of effective treatment or cure, and because of a “lack of time and resources to fully explain what the diagnosis means.”8
But Alzheimer’s patients and their caregivers need as much time as possible to plan accordingly, especially if they have not already discussed and finalized end-of-life planning (will, living will, health care proxy, durable power of attorney), preferences for staying at home with aides or being placed in a facility, or wishes to take final trips or enjoy final activities together before cognitive impairment worsens. Withholding a diagnosis can rob patients and caregivers of that valuable planning time.
4. Connect caregivers to resources and support groups
Information on the stages of the disease, available local support groups, and online resources are extremely helpful. Of the 15 people in my spouse support group, only one or 2 were referred there by a doctor. Become familiar with local support groups because that is where caregivers discuss common needs, learn and share helpful caregiving strategies and techniques, and find emotional support from others walking in similar shoes.
5. Help caregivers take away the car keys
When to take away the car keys is an extremely difficult emotional decision that often leads to heated arguments. People with Alzheimer’s rightfully fear losing their independence and only reluctantly accept they can no longer drive safely. But their caregivers worry about them getting lost or causing an accident or, worse, a death. Even though some people with Alzheimer’s can continue to drive safely for a while, the ever-worsening cognitive decline with the disease sooner or later leads to impaired judgment and the inability to drive safely.
If caregivers have already observed issues with their loved one’s driving ability and ask you to intervene, please help remove a major cause of caregiver stress while also making our roads safer. And please do not routinely refer people with Alzheimer’s to driving test facilities. A person with Alzheimer’s may do very well at the particular moment of the test, yet might fail that same test if it was given an hour earlier or later.
6. Manage expectations of what medications can do
None of the current FDA-approved medications have proven to have any long-term positive effects on Alzheimer’s. Clinical trial data show that these meds may be able to slow the rate of disease progression for some people who take them, but even then the benefit is short-lived. Yet many doctors, year after year, renew these “expensive bottles of hope,” as I call them, when the thousands of dollars needed to buy them could be much better spent on day-care programs or personal aides. A candid disclosure to patients and caregivers would enable better decision-making.
1. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215-2219.
2. Patural A. How to talk so your doctor will listen. AARP Bulletin. January/February 2017. Available at: http://www.aarp.org/health/healthy-living/info-2016/talk-to-doctor-patient-relationship.html. Accessed September 25, 2017.
3. Rhoades DR, McFarland KF, Finch WH, et al. Speaking and interruptions during primary care office visits. Fam Med. 2001;33:528-532.
4. Arevalo-Rodriguez I, Smailagic N, Roque I Figuls M, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;(3):CD010783.
5. Galvin JE, Fagan AM, Holtzman DM, et al. Relationship of dementia screening tests with biomarkers of Alzheimer's disease. Brain. 2010;133:3290-3300.
6. Quarmley M, Moberg PJ, Mechanic-Hamilton D, et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J Alzheimers Dis. 2017;55:1497-1507.
7. Penn study confirms that “sniff test” may be useful in diagnosing early Alzheimer’s disease. December 21, 2016. Available at: http://www.j-alz.com/content/penn-study-confirms-%E2%80%9Csniff-test%E2%80%9D-may-be-useful-diagnosing-early-alzheimer%E2%80%99s-disease. Accessed October 12, 2017.
8. Park A. Many doctors don’t tell patients they have Alzheimer’s. Time. March 24, 2015. Available at: http://time.com/3755176/doctors-diagnose-alzheimers-dont-tell/. Accessed September 25, 2017.
What’s it like to be the caregiver for an Alzheimer’s patient? In my case, it was like being both married and widowed at the same time. Or as a person in my support group once put it: It’s a life filled with grief on the installment plan.
My wife, Clare, struggled for nearly 10 years with Alzheimer’s disease before passing away in April 2016—just one month shy of her 70th birthday and 2 months shy of our 49th wedding anniversary.
Our experience was gut-wrenching, but not unique for families coping with Alzheimer’s disease. Life as a caregiver is one of non-stop daily stress, with much sadness and anxiety, often accompanied by periods of mild or serious depression. Doubt, guilt, frustration, and many other emotions lead many caregivers to take anti-anxiety or antidepressant medication, meet regularly with therapists, take sleeping pills, or experience significant weight gain or loss. Stress drove me to my comfort foods, and I gained nearly 100 pounds while caring for Clare. Only in the last few months have I been able to start taking off that weight.
Helping a loved one who has Alzheimer’s with even the basic activities of daily living—hygiene, dressing, eating—becomes progressively difficult. Caring for a loved one who is confused, no longer remembers your name or who you are, or can occasionally become aggressive, is emotionally painful.
After being Clare’s 24/7 caregiver for 6 years, I agreed that placement in an assisted living facility was in her best interest. My role morphed from primary caregiver to primary care advocate, but the stress did not lessen. I met regularly with facility staff to ensure proper care because many staff members were not sufficiently motivated, educated, or trained to consistently provide proper care for individuals with Alzheimer’s disease.
Financial stress weighs heavily on caregivers. Unless one qualifies for Medicaid, is very wealthy, or is lucky enough to have outstanding long-term health care insurance and prescription drug coverage, caregiving costs can be astronomical. For someone with Alzheimer’s in a community such as Long Island, NY, assisted living facilities charge between $7000 and $10,000 per month, and nursing homes between $15,000 and $18,000 per month. Home health aides working 24/7 also cost around $15,000 per month. Caregiving costs can drain not just the patient’s bank account, but can wipe out the retirement life savings of the surviving caregiver.
Once Clare went into assisted living, I dealt with the daily loneliness and the enormous lifestyle changes. Being alone in my bed those first few nights after placement was painful beyond words, and learning to live alone for the first time after many years of marriage brought incredible sadness. It is no surprise to me that research points to caregiver stress as an independent risk factor for elderly caregiver mortality.1
My experience navigating the health care system with my wife included numerous challenges and instances of unnecessary frustration. My hope in providing the following suggestions is that they will help you help other families like mine.
1. Listen carefully to caregivers
When Clare first exhibited symptoms suggestive of Alzheimer’s, I started logging them and presented written summaries to doctors at each visit. But unless Clare exhibited those same symptoms in the presence of her doctors, my observations were routinely ignored. I’d try to discuss concerns—eg, Clare getting lost while driving to familiar locations, experiencing increased aphasia—but the doctors didn’t read my logs or listen carefully to what I was trying to tell them. The January/February 2017 AARP Bulletin2 noted studies showing that doctors listen for about 23 seconds before interrupting patients, but it also cited a 2001 South Carolina study3 that found patients spoke, uninterrupted, for an average of 12 seconds before being interrupted by a resident.
I eventually did learn that early Alzheimer’s symptoms can be easily misinterpreted as signs of stress, anxiety, or depression. But that underscores the need for doctors to listen carefully to caregivers, especially spouse caregivers who observe behaviors 24/7 that may not be present in a quick office visit or revealed on a brief cognitive screening test.
2. Stay up to date on screening tools that detect Alzheimer’s
The Mini-Mental State Examination, or MMSE, is the most frequently used cognitive screening tool, in part because it can be administered in less than 10 minutes. Although unquestionably valuable, a Cochrane review “did not find evidence supporting a substantial role of MMSE as a stand-alone single-administration test in the identification of MCI [mild-cognitive impairment] patients who could develop dementia.”4
Time-pressured doctors might consider using the AD8 screening interview, an informant questionnaire that takes only 2 to 3 minutes to administer, but has demonstrated superior sensitivity in detecting early dementia compared with the MMSE.5 In addition, a study in the December 2016 issue of the Journal of Alzheimer’s Disease 6 confirmed the usefulness of the Sniffin’ Sticks Odor Identification Test whereby patients try to identify 16 different odors. I can attest to Clare’s rapidly deteriorating senses of taste and smell as her disease progressed.
“Results suggest that a simple odor identification test can be a useful supplementary tool for clinically categorizing MCI and Alzheimer’s, and even for identifying people who are at the highest risk of worsening,” according to principal investigator, David R. Roalf, PhD.7
Prompted by prior studies that have linked a weakening sense of smell to Alzheimer’s, doctors in a few larger dementia clinics have already begun using smell tests in their assessments. One possible reason the practice has not yet become common, however, is that the tests take about 5 to 8 minutes to administer. Roalf and his colleagues are hoping to develop a shorter test that will work as well as the longer ones. “We’re hoping to shorten the Sniffin’ Sticks test … down to 3 minutes or so … We think that will encourage more neurology clinics to do this type of screening.”7
Is 5 minutes too much time to take to administer a valuable screening test?
3. Be candid when speaking with patients and their caregivers
A survey reported in Time magazine on March 24, 2015, found that as many as 64% of doctors do not share a diagnosis of Alzheimer’s with their patients because of “fear of causing emotional distress in their patients” due to a lack of effective treatment or cure, and because of a “lack of time and resources to fully explain what the diagnosis means.”8
But Alzheimer’s patients and their caregivers need as much time as possible to plan accordingly, especially if they have not already discussed and finalized end-of-life planning (will, living will, health care proxy, durable power of attorney), preferences for staying at home with aides or being placed in a facility, or wishes to take final trips or enjoy final activities together before cognitive impairment worsens. Withholding a diagnosis can rob patients and caregivers of that valuable planning time.
4. Connect caregivers to resources and support groups
Information on the stages of the disease, available local support groups, and online resources are extremely helpful. Of the 15 people in my spouse support group, only one or 2 were referred there by a doctor. Become familiar with local support groups because that is where caregivers discuss common needs, learn and share helpful caregiving strategies and techniques, and find emotional support from others walking in similar shoes.
5. Help caregivers take away the car keys
When to take away the car keys is an extremely difficult emotional decision that often leads to heated arguments. People with Alzheimer’s rightfully fear losing their independence and only reluctantly accept they can no longer drive safely. But their caregivers worry about them getting lost or causing an accident or, worse, a death. Even though some people with Alzheimer’s can continue to drive safely for a while, the ever-worsening cognitive decline with the disease sooner or later leads to impaired judgment and the inability to drive safely.
If caregivers have already observed issues with their loved one’s driving ability and ask you to intervene, please help remove a major cause of caregiver stress while also making our roads safer. And please do not routinely refer people with Alzheimer’s to driving test facilities. A person with Alzheimer’s may do very well at the particular moment of the test, yet might fail that same test if it was given an hour earlier or later.
6. Manage expectations of what medications can do
None of the current FDA-approved medications have proven to have any long-term positive effects on Alzheimer’s. Clinical trial data show that these meds may be able to slow the rate of disease progression for some people who take them, but even then the benefit is short-lived. Yet many doctors, year after year, renew these “expensive bottles of hope,” as I call them, when the thousands of dollars needed to buy them could be much better spent on day-care programs or personal aides. A candid disclosure to patients and caregivers would enable better decision-making.
What’s it like to be the caregiver for an Alzheimer’s patient? In my case, it was like being both married and widowed at the same time. Or as a person in my support group once put it: It’s a life filled with grief on the installment plan.
My wife, Clare, struggled for nearly 10 years with Alzheimer’s disease before passing away in April 2016—just one month shy of her 70th birthday and 2 months shy of our 49th wedding anniversary.
Our experience was gut-wrenching, but not unique for families coping with Alzheimer’s disease. Life as a caregiver is one of non-stop daily stress, with much sadness and anxiety, often accompanied by periods of mild or serious depression. Doubt, guilt, frustration, and many other emotions lead many caregivers to take anti-anxiety or antidepressant medication, meet regularly with therapists, take sleeping pills, or experience significant weight gain or loss. Stress drove me to my comfort foods, and I gained nearly 100 pounds while caring for Clare. Only in the last few months have I been able to start taking off that weight.
Helping a loved one who has Alzheimer’s with even the basic activities of daily living—hygiene, dressing, eating—becomes progressively difficult. Caring for a loved one who is confused, no longer remembers your name or who you are, or can occasionally become aggressive, is emotionally painful.
After being Clare’s 24/7 caregiver for 6 years, I agreed that placement in an assisted living facility was in her best interest. My role morphed from primary caregiver to primary care advocate, but the stress did not lessen. I met regularly with facility staff to ensure proper care because many staff members were not sufficiently motivated, educated, or trained to consistently provide proper care for individuals with Alzheimer’s disease.
Financial stress weighs heavily on caregivers. Unless one qualifies for Medicaid, is very wealthy, or is lucky enough to have outstanding long-term health care insurance and prescription drug coverage, caregiving costs can be astronomical. For someone with Alzheimer’s in a community such as Long Island, NY, assisted living facilities charge between $7000 and $10,000 per month, and nursing homes between $15,000 and $18,000 per month. Home health aides working 24/7 also cost around $15,000 per month. Caregiving costs can drain not just the patient’s bank account, but can wipe out the retirement life savings of the surviving caregiver.
Once Clare went into assisted living, I dealt with the daily loneliness and the enormous lifestyle changes. Being alone in my bed those first few nights after placement was painful beyond words, and learning to live alone for the first time after many years of marriage brought incredible sadness. It is no surprise to me that research points to caregiver stress as an independent risk factor for elderly caregiver mortality.1
My experience navigating the health care system with my wife included numerous challenges and instances of unnecessary frustration. My hope in providing the following suggestions is that they will help you help other families like mine.
1. Listen carefully to caregivers
When Clare first exhibited symptoms suggestive of Alzheimer’s, I started logging them and presented written summaries to doctors at each visit. But unless Clare exhibited those same symptoms in the presence of her doctors, my observations were routinely ignored. I’d try to discuss concerns—eg, Clare getting lost while driving to familiar locations, experiencing increased aphasia—but the doctors didn’t read my logs or listen carefully to what I was trying to tell them. The January/February 2017 AARP Bulletin2 noted studies showing that doctors listen for about 23 seconds before interrupting patients, but it also cited a 2001 South Carolina study3 that found patients spoke, uninterrupted, for an average of 12 seconds before being interrupted by a resident.
I eventually did learn that early Alzheimer’s symptoms can be easily misinterpreted as signs of stress, anxiety, or depression. But that underscores the need for doctors to listen carefully to caregivers, especially spouse caregivers who observe behaviors 24/7 that may not be present in a quick office visit or revealed on a brief cognitive screening test.
2. Stay up to date on screening tools that detect Alzheimer’s
The Mini-Mental State Examination, or MMSE, is the most frequently used cognitive screening tool, in part because it can be administered in less than 10 minutes. Although unquestionably valuable, a Cochrane review “did not find evidence supporting a substantial role of MMSE as a stand-alone single-administration test in the identification of MCI [mild-cognitive impairment] patients who could develop dementia.”4
Time-pressured doctors might consider using the AD8 screening interview, an informant questionnaire that takes only 2 to 3 minutes to administer, but has demonstrated superior sensitivity in detecting early dementia compared with the MMSE.5 In addition, a study in the December 2016 issue of the Journal of Alzheimer’s Disease 6 confirmed the usefulness of the Sniffin’ Sticks Odor Identification Test whereby patients try to identify 16 different odors. I can attest to Clare’s rapidly deteriorating senses of taste and smell as her disease progressed.
“Results suggest that a simple odor identification test can be a useful supplementary tool for clinically categorizing MCI and Alzheimer’s, and even for identifying people who are at the highest risk of worsening,” according to principal investigator, David R. Roalf, PhD.7
Prompted by prior studies that have linked a weakening sense of smell to Alzheimer’s, doctors in a few larger dementia clinics have already begun using smell tests in their assessments. One possible reason the practice has not yet become common, however, is that the tests take about 5 to 8 minutes to administer. Roalf and his colleagues are hoping to develop a shorter test that will work as well as the longer ones. “We’re hoping to shorten the Sniffin’ Sticks test … down to 3 minutes or so … We think that will encourage more neurology clinics to do this type of screening.”7
Is 5 minutes too much time to take to administer a valuable screening test?
3. Be candid when speaking with patients and their caregivers
A survey reported in Time magazine on March 24, 2015, found that as many as 64% of doctors do not share a diagnosis of Alzheimer’s with their patients because of “fear of causing emotional distress in their patients” due to a lack of effective treatment or cure, and because of a “lack of time and resources to fully explain what the diagnosis means.”8
But Alzheimer’s patients and their caregivers need as much time as possible to plan accordingly, especially if they have not already discussed and finalized end-of-life planning (will, living will, health care proxy, durable power of attorney), preferences for staying at home with aides or being placed in a facility, or wishes to take final trips or enjoy final activities together before cognitive impairment worsens. Withholding a diagnosis can rob patients and caregivers of that valuable planning time.
4. Connect caregivers to resources and support groups
Information on the stages of the disease, available local support groups, and online resources are extremely helpful. Of the 15 people in my spouse support group, only one or 2 were referred there by a doctor. Become familiar with local support groups because that is where caregivers discuss common needs, learn and share helpful caregiving strategies and techniques, and find emotional support from others walking in similar shoes.
5. Help caregivers take away the car keys
When to take away the car keys is an extremely difficult emotional decision that often leads to heated arguments. People with Alzheimer’s rightfully fear losing their independence and only reluctantly accept they can no longer drive safely. But their caregivers worry about them getting lost or causing an accident or, worse, a death. Even though some people with Alzheimer’s can continue to drive safely for a while, the ever-worsening cognitive decline with the disease sooner or later leads to impaired judgment and the inability to drive safely.
If caregivers have already observed issues with their loved one’s driving ability and ask you to intervene, please help remove a major cause of caregiver stress while also making our roads safer. And please do not routinely refer people with Alzheimer’s to driving test facilities. A person with Alzheimer’s may do very well at the particular moment of the test, yet might fail that same test if it was given an hour earlier or later.
6. Manage expectations of what medications can do
None of the current FDA-approved medications have proven to have any long-term positive effects on Alzheimer’s. Clinical trial data show that these meds may be able to slow the rate of disease progression for some people who take them, but even then the benefit is short-lived. Yet many doctors, year after year, renew these “expensive bottles of hope,” as I call them, when the thousands of dollars needed to buy them could be much better spent on day-care programs or personal aides. A candid disclosure to patients and caregivers would enable better decision-making.
1. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215-2219.
2. Patural A. How to talk so your doctor will listen. AARP Bulletin. January/February 2017. Available at: http://www.aarp.org/health/healthy-living/info-2016/talk-to-doctor-patient-relationship.html. Accessed September 25, 2017.
3. Rhoades DR, McFarland KF, Finch WH, et al. Speaking and interruptions during primary care office visits. Fam Med. 2001;33:528-532.
4. Arevalo-Rodriguez I, Smailagic N, Roque I Figuls M, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;(3):CD010783.
5. Galvin JE, Fagan AM, Holtzman DM, et al. Relationship of dementia screening tests with biomarkers of Alzheimer's disease. Brain. 2010;133:3290-3300.
6. Quarmley M, Moberg PJ, Mechanic-Hamilton D, et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J Alzheimers Dis. 2017;55:1497-1507.
7. Penn study confirms that “sniff test” may be useful in diagnosing early Alzheimer’s disease. December 21, 2016. Available at: http://www.j-alz.com/content/penn-study-confirms-%E2%80%9Csniff-test%E2%80%9D-may-be-useful-diagnosing-early-alzheimer%E2%80%99s-disease. Accessed October 12, 2017.
8. Park A. Many doctors don’t tell patients they have Alzheimer’s. Time. March 24, 2015. Available at: http://time.com/3755176/doctors-diagnose-alzheimers-dont-tell/. Accessed September 25, 2017.
1. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215-2219.
2. Patural A. How to talk so your doctor will listen. AARP Bulletin. January/February 2017. Available at: http://www.aarp.org/health/healthy-living/info-2016/talk-to-doctor-patient-relationship.html. Accessed September 25, 2017.
3. Rhoades DR, McFarland KF, Finch WH, et al. Speaking and interruptions during primary care office visits. Fam Med. 2001;33:528-532.
4. Arevalo-Rodriguez I, Smailagic N, Roque I Figuls M, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;(3):CD010783.
5. Galvin JE, Fagan AM, Holtzman DM, et al. Relationship of dementia screening tests with biomarkers of Alzheimer's disease. Brain. 2010;133:3290-3300.
6. Quarmley M, Moberg PJ, Mechanic-Hamilton D, et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J Alzheimers Dis. 2017;55:1497-1507.
7. Penn study confirms that “sniff test” may be useful in diagnosing early Alzheimer’s disease. December 21, 2016. Available at: http://www.j-alz.com/content/penn-study-confirms-%E2%80%9Csniff-test%E2%80%9D-may-be-useful-diagnosing-early-alzheimer%E2%80%99s-disease. Accessed October 12, 2017.
8. Park A. Many doctors don’t tell patients they have Alzheimer’s. Time. March 24, 2015. Available at: http://time.com/3755176/doctors-diagnose-alzheimers-dont-tell/. Accessed September 25, 2017.
5 drug interactions you don’t want to miss
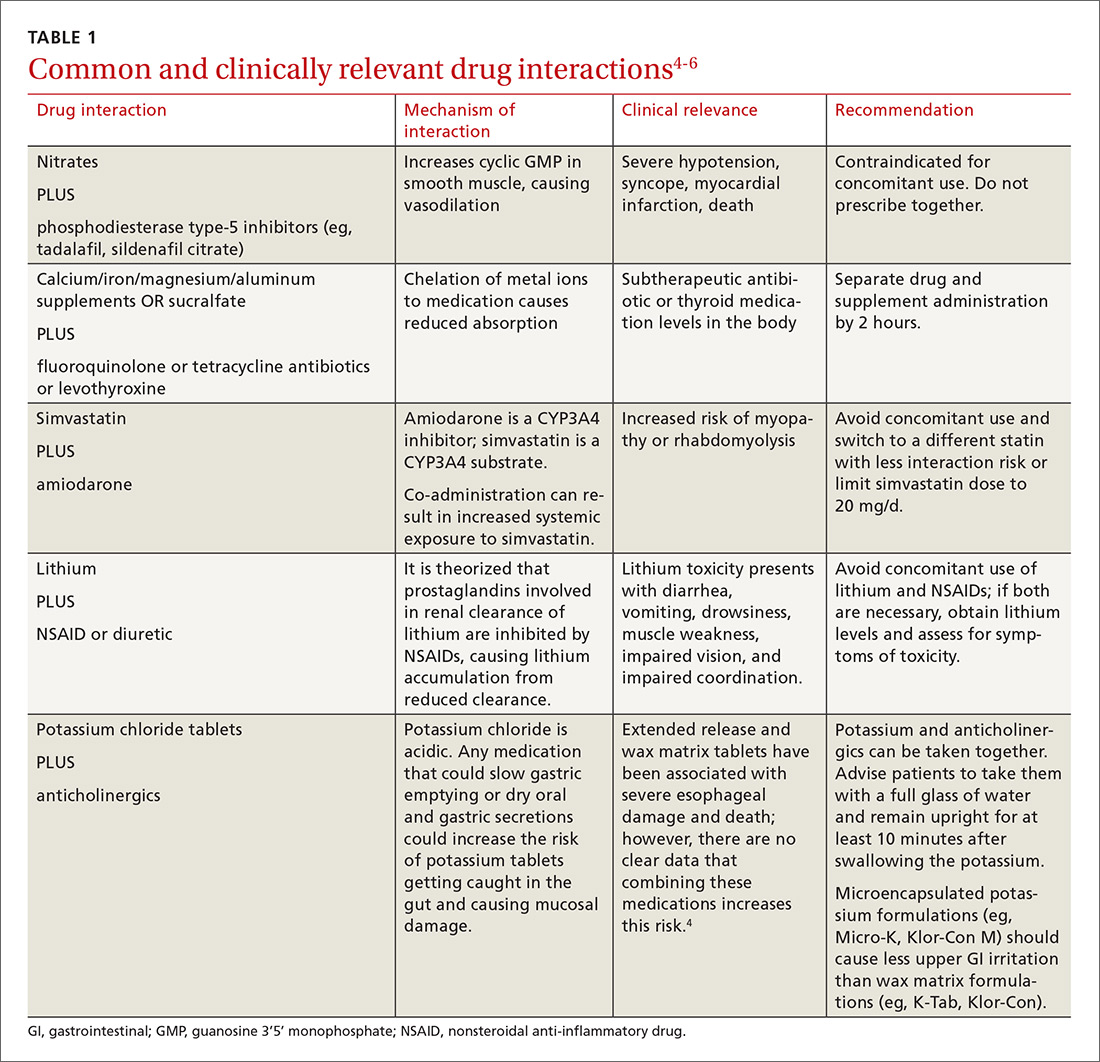
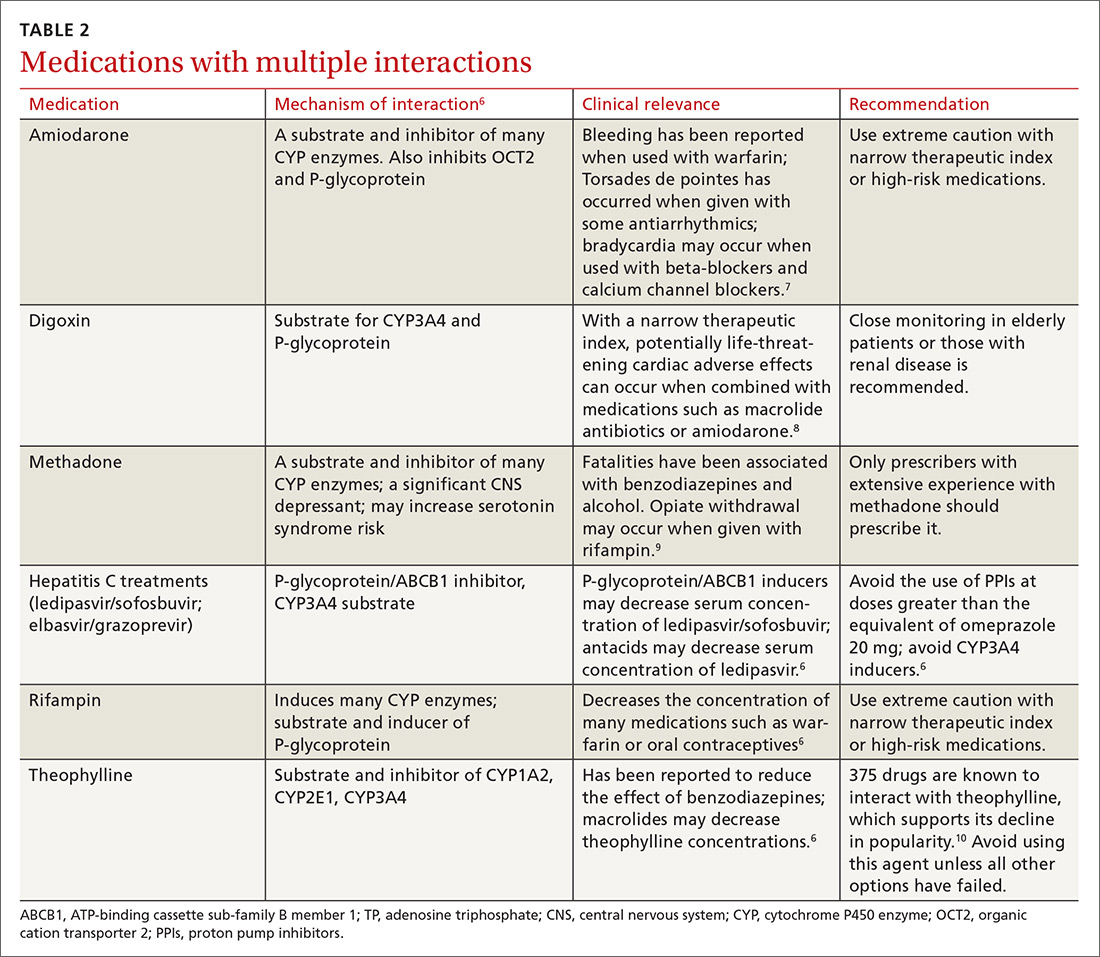
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
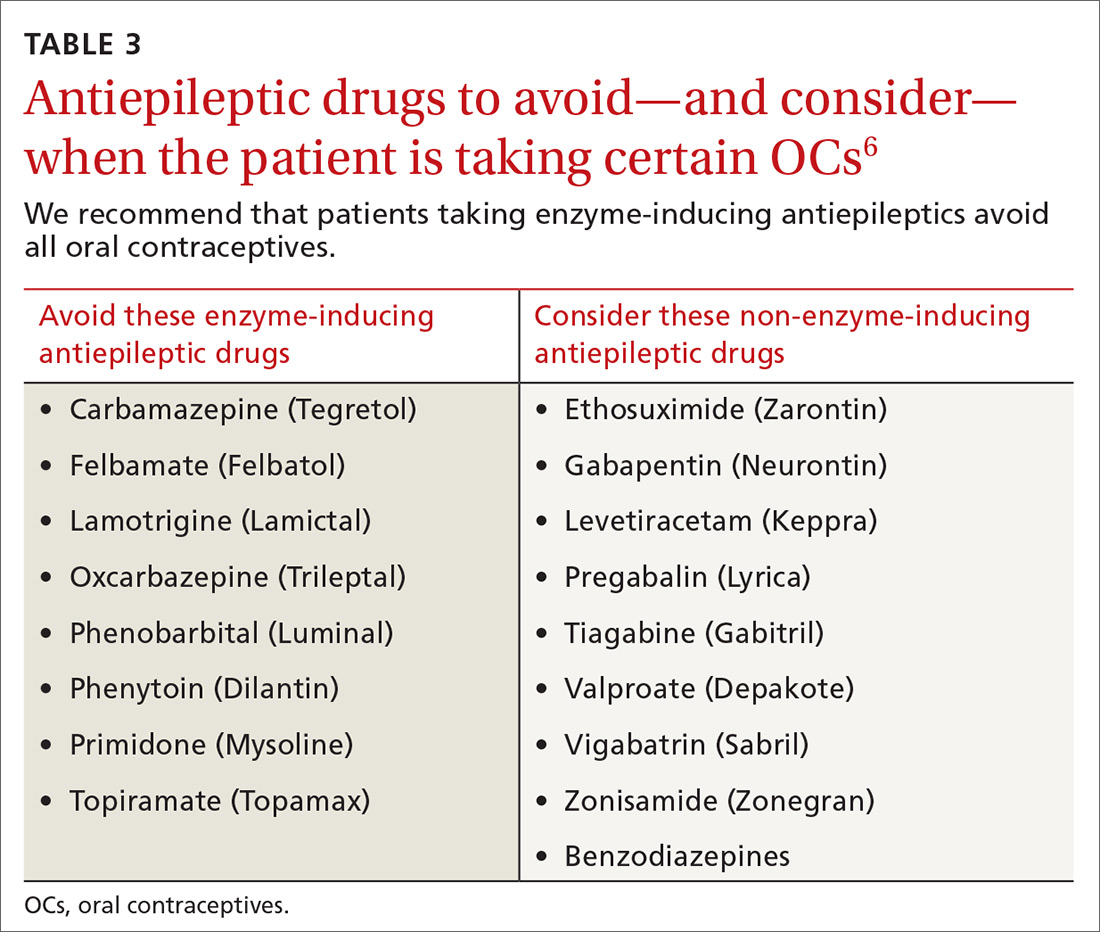
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26
3. Direct oral anticoagulants and antiepileptics
Don’t use DOACs in patients taking certain antiepileptic medications
Drug interactions with anticoagulants, such as warfarin, are well documented and have been publicized for years, but physicians must also be aware of the potential for interaction between the direct oral anticoagulants (DOACs) and AEDs.
Apixaban, rivaroxaban, and dabigatran appear to interact withthe AEDs carbamazepine, phenytoin, and phenobarbital.27,28 These interactions occur due to AED induction of the CYP3A4 enzyme and effects on the P-glycoprotein (P-gp) efflux pump.27,29 When taken together, the AED induces metabolism and elimination of the DOAC medication to occur more quickly than it would normally, resulting in subtherapeutic concentrations of the DOAC. This could theoretically result in a venous thromboembolic event or stroke.
A caveat. One thing to consider is that studies demonstrating interaction between the DOAC and AED drug classes have been performed in healthy volunteers, making it difficult to extrapolate how this interaction may increase the risk for thrombotic events in other patients.
Some studies demonstrated reductions in drug levels of up to 50% with strong CYP3A4 and P-glycoprotein inducers.30 Common inducers include carbamazepine, rifampin, and St. John’s Wort.6 Patients taking such agents could theoretically have decreased exposure to the DOAC, resulting in an increase in thromboembolic risk.31
4. Statins & certain CYP inhibitors
Combining simvastatin with fibrates warrants extra attention
The efficacy of statin medications in the prevention of atherosclerotic cardiovascular disease (ASCVD) is clear. However, the clinical significance of many identified drug interactions involving statins is difficult to interpret. Interactions that cause increased serum concentrations of statins can increase the risk for liver enzyme elevations and skeletal muscle abnormalities (myalgias to rhabdomyolysis).32 Strong inhibitors of CYP3A4 (amiodarone, cyclosporine, ketoconazole, etc.) significantly increase concentrations of lovastatin, simvastatin, and atorvastatin. Pitavastatin, pravastatin, and rosuvastatin are not susceptible to any CYP-mediated drug interactions;33 therefore, rosuvastatin (a high-intensity statin) is usually recommended over other statins for patients taking strong inhibitors of CYP3A4.
When to limit simvastatin. Doses of simvastatin should not exceed 10 mg/d when combined with diltiazem, dronedarone, or verapamil, and doses should not exceed 20 mg/d when used with amiodarone, amlodipine, or ranolazine.6 These recommendations are in response to results from the SEARCH (Study of the Effectiveness of Additional Reductions in cholesterol and homocysteine) trial, which found a higher incidence of myopathies and rhabdomyolysis in patients taking 80 mg of simvastatin compared with those taking 20-mg doses.34 CYP3A4-inducing medications, especially diltiazem, were thought to also contribute to an increased risk.34
Avoid gemfibrozil with statins. Using fibrates with statins is beneficial for some patients; however, gemfibrozil significantly interacts with statins by inhibiting CYP2C8 and organic anion transporting polypeptide 1B1 (OATP1B1).33 The safer choice is fenofibrate because it does not interfere with statin metabolism and can be safely used in combination with statins.6
A retrospective review of the FDA Adverse Event Reporting System (AERS) database found that 88% of fibrate and statin combinations that resulted in rhabdomyolysis were associated with gemfibrozil/cerivastatin (cerivastatin is no longer available in the United States).35
5. One serotonergic drug & another
Serotonin syndrome is associated with more than just SSRIs
Serotonin syndrome is a constellation of symptoms (hyperthermia, hyperreflexia, muscle clonus, tremor and altered mental status) caused by increases in serotonin levels in the central and peripheral nervous systems that can lead to mild or life-threatening complications such as seizures, muscle breakdown, or hyperthermia. Serotonin syndrome is most likely to occur within 24 hours after a dose increase, after starting a new medication that increases serotonin levels, or after a drug overdose.36
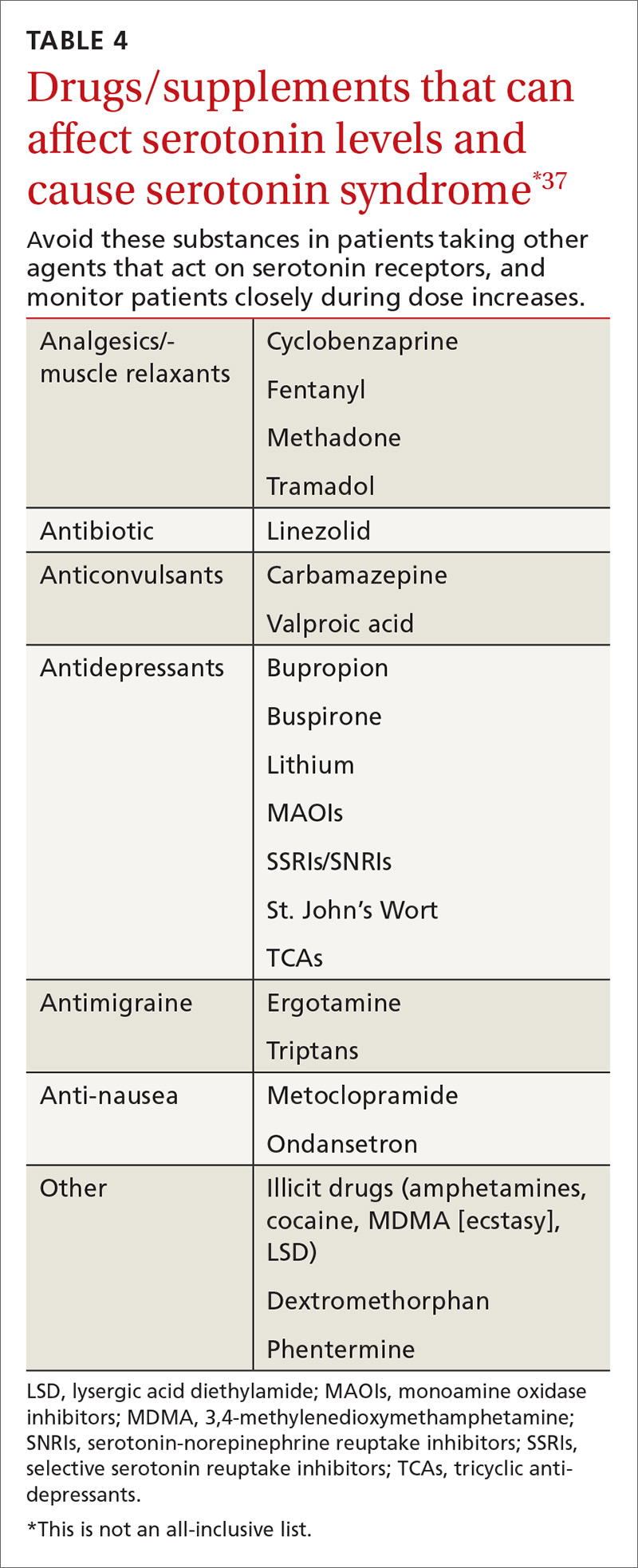
SSRIs are the most commonly reported drug associated with serotonin syndrome; however, other medications (TABLE 437) may be responsible, especially when used in combination with agents that act on serotonin receptors or in patients with impaired metabolism of the drugs being used.37
Other culprits. Serotonergic effects can also be associated with illicit drugs, some nonprescription medications, and supplements. And in March 2016, the FDA issued a warning about the risks of taking opioids with serotonergic medications.38 Although labeling changes have been recommended for all opioids, the cases of serotonin syndrome were reported more often with normal doses of fentanyl and methadone.
There are 2 mechanisms by which drugs may increase a patient’s risk for serotonin syndrome. The first is a pharmacodynamic interaction, which can occur when 2 or more medications act at the same receptor site (serotonin receptors in this example), which may result in an additive or synergistic effect.39
The second mechanism is a pharmacokinetic alteration (an agent alters absorption, distribution, metabolism, or excretion) of CYP enzymes.40 Of the more commonly used antidepressants, citalopram, escitalopram, venlafaxine, and mirtazapine seem to have the least potential for clinically significant pharmacokinetic interactions.41
Guidelines? Currently there are no guidelines for preventing serotonin syndrome. Clinicians should exercise caution in patients at high risk for drug adverse events, such as the elderly, patients taking multiple medications, and patients with comorbidities. Healthy low-risk patients can generally take 2 or 3 serotonergic medications at therapeutic doses without a major risk of harm.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, 191 East Orchard Road, Suite 200, Littleton, CO 80121; [email protected].
1. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
2. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
3. Pharmacist’s Letter. Online continuing medical education and webinars. Drug interaction overload: Problems and solutions for drug interaction alerts. Volume 2012, Course No. 216. Self-Study Course #120216. Available at: http://pharmacistsletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-219&quiz=1. Accessed June 9, 2016.
4. PL Detail-Document, Potassium and Anticholinergic Drug Interaction. Pharmacist’s Letter/Prescriber’s Letter. October 2011.
5. Micromedex Solutions. Available at: http://www.micromedexsolutions.com. Accessed May 3, 2016.
6. Lexi-Comp Online. Available at: http://online.lexi.com/lco/action/home. Accessed May 22, 2016.
7. Marcus FI. Drug interactions with amiodarone. Am Heart J. 1983;106(4 Pt 2):924-930.
8. Digoxin: serious drug interactions. Prescrire Int. 2010;19:68-70.
9. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
10. Drugs.com. Theophylline drug interactions. Available at: https://www.drugs.com/drug-interactions/theophylline.html. Accessed June 23, 2016.
11. Bhakta J, Bainbridge J, Borgelt L. Teratogenic medications and concurrent contraceptive use in women of childbearing ability with epilepsy. Epilepsy Behav. 2015;52(Pt A):212-217.
12. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010;3:183-192.
13. Carl JS, Weaver SP, Tweed E. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
14. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
15. Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17:145-150.
16. Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Antiepileptic drugs and contraception. CEU statement. January 2010. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed April 25, 2016.
17. Perruca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246-255.
18. ACOG practice bulletin. Number 73: Use of hormonal contraception in women with coexisting medical conditions. ACOG Committee on Practice Bulletins-Gynecology. Obstet Gynecol. 2006;107:1453-1472.
19. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement). Neurology. 1998;51:944-948.
20. National Institute for Health and Care Excellence. Do not do recommendation. Available at: https://www.nice.org.uk/donotdo/the-progestogenonly-pill-is-not-recommended-as-reliable-contraception-inwomen-and-girls-taking-enzymeinducing-anti-epileptic-drugs-aeds. Accessed September 21, 2017.
21. Faculty of Sexual and Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed September 21, 2017.
22. de Jong JCF, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55:591-595.
23. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
24. Mort JR, Aparasu RR, Baer RK, et al. Interaction between selective serotonin reuptake inhibitors and nonsteroidal anti-inflammatory drugs: review of the literature. Pharmacotherapy. 2006;26:1307-1313.
25. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27:31-40.
26. Venerito M, Wex T, Malfertheiner P. Nonsteroidal anti-inflammatory drug-induced gastroduodenal bleeding: risk factors and prevention strategies. Pharmaceuticals. 2010;3:2225-2237.
27. Boehringer S, Williams CD, Yawn BP, et al. Managing interactions with direct oral anticoagulants (DOACs). Pharmacist’s Letter. May 2016.
28. Johannessen SI, Landmark CJ. Antiepileptic drug interactions – principles and clinical implications. Curr Neuropharmacol. 2010;8:254-267.
29. Mohrien K, Oliphant CS, Self TH. Drug interactions with novel oral anticoagulants. Consultant. 2013;53:918-919. Available at: http://www.consultant360.com/articles/drug-interactions-novel-oral-anticoagulants. Accessed May 3, 2016.
30. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-e7.
31. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
32. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681-1690.
33. Hirota T, Leiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11:1435-1447.
34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol wih 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial inffarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
35. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120-122.
36. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
37. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
38. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm. Accessed June 15, 2016.
39. Sultana J, Spina E, Trifirò G. Antidepressant use in the elderly: the role of pharmacodynamics and pharmacokinetics in drug safety. Expert Opin Drug Metab Toxicol. 2015;11:883-892.
40. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454-471.
41. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26
3. Direct oral anticoagulants and antiepileptics
Don’t use DOACs in patients taking certain antiepileptic medications
Drug interactions with anticoagulants, such as warfarin, are well documented and have been publicized for years, but physicians must also be aware of the potential for interaction between the direct oral anticoagulants (DOACs) and AEDs.
Apixaban, rivaroxaban, and dabigatran appear to interact withthe AEDs carbamazepine, phenytoin, and phenobarbital.27,28 These interactions occur due to AED induction of the CYP3A4 enzyme and effects on the P-glycoprotein (P-gp) efflux pump.27,29 When taken together, the AED induces metabolism and elimination of the DOAC medication to occur more quickly than it would normally, resulting in subtherapeutic concentrations of the DOAC. This could theoretically result in a venous thromboembolic event or stroke.
A caveat. One thing to consider is that studies demonstrating interaction between the DOAC and AED drug classes have been performed in healthy volunteers, making it difficult to extrapolate how this interaction may increase the risk for thrombotic events in other patients.
Some studies demonstrated reductions in drug levels of up to 50% with strong CYP3A4 and P-glycoprotein inducers.30 Common inducers include carbamazepine, rifampin, and St. John’s Wort.6 Patients taking such agents could theoretically have decreased exposure to the DOAC, resulting in an increase in thromboembolic risk.31
4. Statins & certain CYP inhibitors
Combining simvastatin with fibrates warrants extra attention
The efficacy of statin medications in the prevention of atherosclerotic cardiovascular disease (ASCVD) is clear. However, the clinical significance of many identified drug interactions involving statins is difficult to interpret. Interactions that cause increased serum concentrations of statins can increase the risk for liver enzyme elevations and skeletal muscle abnormalities (myalgias to rhabdomyolysis).32 Strong inhibitors of CYP3A4 (amiodarone, cyclosporine, ketoconazole, etc.) significantly increase concentrations of lovastatin, simvastatin, and atorvastatin. Pitavastatin, pravastatin, and rosuvastatin are not susceptible to any CYP-mediated drug interactions;33 therefore, rosuvastatin (a high-intensity statin) is usually recommended over other statins for patients taking strong inhibitors of CYP3A4.
When to limit simvastatin. Doses of simvastatin should not exceed 10 mg/d when combined with diltiazem, dronedarone, or verapamil, and doses should not exceed 20 mg/d when used with amiodarone, amlodipine, or ranolazine.6 These recommendations are in response to results from the SEARCH (Study of the Effectiveness of Additional Reductions in cholesterol and homocysteine) trial, which found a higher incidence of myopathies and rhabdomyolysis in patients taking 80 mg of simvastatin compared with those taking 20-mg doses.34 CYP3A4-inducing medications, especially diltiazem, were thought to also contribute to an increased risk.34
Avoid gemfibrozil with statins. Using fibrates with statins is beneficial for some patients; however, gemfibrozil significantly interacts with statins by inhibiting CYP2C8 and organic anion transporting polypeptide 1B1 (OATP1B1).33 The safer choice is fenofibrate because it does not interfere with statin metabolism and can be safely used in combination with statins.6
A retrospective review of the FDA Adverse Event Reporting System (AERS) database found that 88% of fibrate and statin combinations that resulted in rhabdomyolysis were associated with gemfibrozil/cerivastatin (cerivastatin is no longer available in the United States).35
5. One serotonergic drug & another
Serotonin syndrome is associated with more than just SSRIs
Serotonin syndrome is a constellation of symptoms (hyperthermia, hyperreflexia, muscle clonus, tremor and altered mental status) caused by increases in serotonin levels in the central and peripheral nervous systems that can lead to mild or life-threatening complications such as seizures, muscle breakdown, or hyperthermia. Serotonin syndrome is most likely to occur within 24 hours after a dose increase, after starting a new medication that increases serotonin levels, or after a drug overdose.36
SSRIs are the most commonly reported drug associated with serotonin syndrome; however, other medications (TABLE 437) may be responsible, especially when used in combination with agents that act on serotonin receptors or in patients with impaired metabolism of the drugs being used.37
Other culprits. Serotonergic effects can also be associated with illicit drugs, some nonprescription medications, and supplements. And in March 2016, the FDA issued a warning about the risks of taking opioids with serotonergic medications.38 Although labeling changes have been recommended for all opioids, the cases of serotonin syndrome were reported more often with normal doses of fentanyl and methadone.
There are 2 mechanisms by which drugs may increase a patient’s risk for serotonin syndrome. The first is a pharmacodynamic interaction, which can occur when 2 or more medications act at the same receptor site (serotonin receptors in this example), which may result in an additive or synergistic effect.39
The second mechanism is a pharmacokinetic alteration (an agent alters absorption, distribution, metabolism, or excretion) of CYP enzymes.40 Of the more commonly used antidepressants, citalopram, escitalopram, venlafaxine, and mirtazapine seem to have the least potential for clinically significant pharmacokinetic interactions.41
Guidelines? Currently there are no guidelines for preventing serotonin syndrome. Clinicians should exercise caution in patients at high risk for drug adverse events, such as the elderly, patients taking multiple medications, and patients with comorbidities. Healthy low-risk patients can generally take 2 or 3 serotonergic medications at therapeutic doses without a major risk of harm.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, 191 East Orchard Road, Suite 200, Littleton, CO 80121; [email protected].
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26
3. Direct oral anticoagulants and antiepileptics
Don’t use DOACs in patients taking certain antiepileptic medications
Drug interactions with anticoagulants, such as warfarin, are well documented and have been publicized for years, but physicians must also be aware of the potential for interaction between the direct oral anticoagulants (DOACs) and AEDs.
Apixaban, rivaroxaban, and dabigatran appear to interact withthe AEDs carbamazepine, phenytoin, and phenobarbital.27,28 These interactions occur due to AED induction of the CYP3A4 enzyme and effects on the P-glycoprotein (P-gp) efflux pump.27,29 When taken together, the AED induces metabolism and elimination of the DOAC medication to occur more quickly than it would normally, resulting in subtherapeutic concentrations of the DOAC. This could theoretically result in a venous thromboembolic event or stroke.
A caveat. One thing to consider is that studies demonstrating interaction between the DOAC and AED drug classes have been performed in healthy volunteers, making it difficult to extrapolate how this interaction may increase the risk for thrombotic events in other patients.
Some studies demonstrated reductions in drug levels of up to 50% with strong CYP3A4 and P-glycoprotein inducers.30 Common inducers include carbamazepine, rifampin, and St. John’s Wort.6 Patients taking such agents could theoretically have decreased exposure to the DOAC, resulting in an increase in thromboembolic risk.31
4. Statins & certain CYP inhibitors
Combining simvastatin with fibrates warrants extra attention
The efficacy of statin medications in the prevention of atherosclerotic cardiovascular disease (ASCVD) is clear. However, the clinical significance of many identified drug interactions involving statins is difficult to interpret. Interactions that cause increased serum concentrations of statins can increase the risk for liver enzyme elevations and skeletal muscle abnormalities (myalgias to rhabdomyolysis).32 Strong inhibitors of CYP3A4 (amiodarone, cyclosporine, ketoconazole, etc.) significantly increase concentrations of lovastatin, simvastatin, and atorvastatin. Pitavastatin, pravastatin, and rosuvastatin are not susceptible to any CYP-mediated drug interactions;33 therefore, rosuvastatin (a high-intensity statin) is usually recommended over other statins for patients taking strong inhibitors of CYP3A4.
When to limit simvastatin. Doses of simvastatin should not exceed 10 mg/d when combined with diltiazem, dronedarone, or verapamil, and doses should not exceed 20 mg/d when used with amiodarone, amlodipine, or ranolazine.6 These recommendations are in response to results from the SEARCH (Study of the Effectiveness of Additional Reductions in cholesterol and homocysteine) trial, which found a higher incidence of myopathies and rhabdomyolysis in patients taking 80 mg of simvastatin compared with those taking 20-mg doses.34 CYP3A4-inducing medications, especially diltiazem, were thought to also contribute to an increased risk.34
Avoid gemfibrozil with statins. Using fibrates with statins is beneficial for some patients; however, gemfibrozil significantly interacts with statins by inhibiting CYP2C8 and organic anion transporting polypeptide 1B1 (OATP1B1).33 The safer choice is fenofibrate because it does not interfere with statin metabolism and can be safely used in combination with statins.6
A retrospective review of the FDA Adverse Event Reporting System (AERS) database found that 88% of fibrate and statin combinations that resulted in rhabdomyolysis were associated with gemfibrozil/cerivastatin (cerivastatin is no longer available in the United States).35
5. One serotonergic drug & another
Serotonin syndrome is associated with more than just SSRIs
Serotonin syndrome is a constellation of symptoms (hyperthermia, hyperreflexia, muscle clonus, tremor and altered mental status) caused by increases in serotonin levels in the central and peripheral nervous systems that can lead to mild or life-threatening complications such as seizures, muscle breakdown, or hyperthermia. Serotonin syndrome is most likely to occur within 24 hours after a dose increase, after starting a new medication that increases serotonin levels, or after a drug overdose.36
SSRIs are the most commonly reported drug associated with serotonin syndrome; however, other medications (TABLE 437) may be responsible, especially when used in combination with agents that act on serotonin receptors or in patients with impaired metabolism of the drugs being used.37
Other culprits. Serotonergic effects can also be associated with illicit drugs, some nonprescription medications, and supplements. And in March 2016, the FDA issued a warning about the risks of taking opioids with serotonergic medications.38 Although labeling changes have been recommended for all opioids, the cases of serotonin syndrome were reported more often with normal doses of fentanyl and methadone.
There are 2 mechanisms by which drugs may increase a patient’s risk for serotonin syndrome. The first is a pharmacodynamic interaction, which can occur when 2 or more medications act at the same receptor site (serotonin receptors in this example), which may result in an additive or synergistic effect.39
The second mechanism is a pharmacokinetic alteration (an agent alters absorption, distribution, metabolism, or excretion) of CYP enzymes.40 Of the more commonly used antidepressants, citalopram, escitalopram, venlafaxine, and mirtazapine seem to have the least potential for clinically significant pharmacokinetic interactions.41
Guidelines? Currently there are no guidelines for preventing serotonin syndrome. Clinicians should exercise caution in patients at high risk for drug adverse events, such as the elderly, patients taking multiple medications, and patients with comorbidities. Healthy low-risk patients can generally take 2 or 3 serotonergic medications at therapeutic doses without a major risk of harm.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, 191 East Orchard Road, Suite 200, Littleton, CO 80121; [email protected].
1. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
2. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
3. Pharmacist’s Letter. Online continuing medical education and webinars. Drug interaction overload: Problems and solutions for drug interaction alerts. Volume 2012, Course No. 216. Self-Study Course #120216. Available at: http://pharmacistsletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-219&quiz=1. Accessed June 9, 2016.
4. PL Detail-Document, Potassium and Anticholinergic Drug Interaction. Pharmacist’s Letter/Prescriber’s Letter. October 2011.
5. Micromedex Solutions. Available at: http://www.micromedexsolutions.com. Accessed May 3, 2016.
6. Lexi-Comp Online. Available at: http://online.lexi.com/lco/action/home. Accessed May 22, 2016.
7. Marcus FI. Drug interactions with amiodarone. Am Heart J. 1983;106(4 Pt 2):924-930.
8. Digoxin: serious drug interactions. Prescrire Int. 2010;19:68-70.
9. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
10. Drugs.com. Theophylline drug interactions. Available at: https://www.drugs.com/drug-interactions/theophylline.html. Accessed June 23, 2016.
11. Bhakta J, Bainbridge J, Borgelt L. Teratogenic medications and concurrent contraceptive use in women of childbearing ability with epilepsy. Epilepsy Behav. 2015;52(Pt A):212-217.
12. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010;3:183-192.
13. Carl JS, Weaver SP, Tweed E. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
14. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
15. Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17:145-150.
16. Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Antiepileptic drugs and contraception. CEU statement. January 2010. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed April 25, 2016.
17. Perruca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246-255.
18. ACOG practice bulletin. Number 73: Use of hormonal contraception in women with coexisting medical conditions. ACOG Committee on Practice Bulletins-Gynecology. Obstet Gynecol. 2006;107:1453-1472.
19. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement). Neurology. 1998;51:944-948.
20. National Institute for Health and Care Excellence. Do not do recommendation. Available at: https://www.nice.org.uk/donotdo/the-progestogenonly-pill-is-not-recommended-as-reliable-contraception-inwomen-and-girls-taking-enzymeinducing-anti-epileptic-drugs-aeds. Accessed September 21, 2017.
21. Faculty of Sexual and Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed September 21, 2017.
22. de Jong JCF, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55:591-595.
23. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
24. Mort JR, Aparasu RR, Baer RK, et al. Interaction between selective serotonin reuptake inhibitors and nonsteroidal anti-inflammatory drugs: review of the literature. Pharmacotherapy. 2006;26:1307-1313.
25. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27:31-40.
26. Venerito M, Wex T, Malfertheiner P. Nonsteroidal anti-inflammatory drug-induced gastroduodenal bleeding: risk factors and prevention strategies. Pharmaceuticals. 2010;3:2225-2237.
27. Boehringer S, Williams CD, Yawn BP, et al. Managing interactions with direct oral anticoagulants (DOACs). Pharmacist’s Letter. May 2016.
28. Johannessen SI, Landmark CJ. Antiepileptic drug interactions – principles and clinical implications. Curr Neuropharmacol. 2010;8:254-267.
29. Mohrien K, Oliphant CS, Self TH. Drug interactions with novel oral anticoagulants. Consultant. 2013;53:918-919. Available at: http://www.consultant360.com/articles/drug-interactions-novel-oral-anticoagulants. Accessed May 3, 2016.
30. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-e7.
31. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
32. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681-1690.
33. Hirota T, Leiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11:1435-1447.
34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol wih 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial inffarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
35. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120-122.
36. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
37. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
38. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm. Accessed June 15, 2016.
39. Sultana J, Spina E, Trifirò G. Antidepressant use in the elderly: the role of pharmacodynamics and pharmacokinetics in drug safety. Expert Opin Drug Metab Toxicol. 2015;11:883-892.
40. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454-471.
41. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
1. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
2. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
3. Pharmacist’s Letter. Online continuing medical education and webinars. Drug interaction overload: Problems and solutions for drug interaction alerts. Volume 2012, Course No. 216. Self-Study Course #120216. Available at: http://pharmacistsletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-219&quiz=1. Accessed June 9, 2016.
4. PL Detail-Document, Potassium and Anticholinergic Drug Interaction. Pharmacist’s Letter/Prescriber’s Letter. October 2011.
5. Micromedex Solutions. Available at: http://www.micromedexsolutions.com. Accessed May 3, 2016.
6. Lexi-Comp Online. Available at: http://online.lexi.com/lco/action/home. Accessed May 22, 2016.
7. Marcus FI. Drug interactions with amiodarone. Am Heart J. 1983;106(4 Pt 2):924-930.
8. Digoxin: serious drug interactions. Prescrire Int. 2010;19:68-70.
9. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
10. Drugs.com. Theophylline drug interactions. Available at: https://www.drugs.com/drug-interactions/theophylline.html. Accessed June 23, 2016.
11. Bhakta J, Bainbridge J, Borgelt L. Teratogenic medications and concurrent contraceptive use in women of childbearing ability with epilepsy. Epilepsy Behav. 2015;52(Pt A):212-217.
12. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010;3:183-192.
13. Carl JS, Weaver SP, Tweed E. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
14. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
15. Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17:145-150.
16. Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Antiepileptic drugs and contraception. CEU statement. January 2010. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed April 25, 2016.
17. Perruca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246-255.
18. ACOG practice bulletin. Number 73: Use of hormonal contraception in women with coexisting medical conditions. ACOG Committee on Practice Bulletins-Gynecology. Obstet Gynecol. 2006;107:1453-1472.
19. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement). Neurology. 1998;51:944-948.
20. National Institute for Health and Care Excellence. Do not do recommendation. Available at: https://www.nice.org.uk/donotdo/the-progestogenonly-pill-is-not-recommended-as-reliable-contraception-inwomen-and-girls-taking-enzymeinducing-anti-epileptic-drugs-aeds. Accessed September 21, 2017.
21. Faculty of Sexual and Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed September 21, 2017.
22. de Jong JCF, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55:591-595.
23. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
24. Mort JR, Aparasu RR, Baer RK, et al. Interaction between selective serotonin reuptake inhibitors and nonsteroidal anti-inflammatory drugs: review of the literature. Pharmacotherapy. 2006;26:1307-1313.
25. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27:31-40.
26. Venerito M, Wex T, Malfertheiner P. Nonsteroidal anti-inflammatory drug-induced gastroduodenal bleeding: risk factors and prevention strategies. Pharmaceuticals. 2010;3:2225-2237.
27. Boehringer S, Williams CD, Yawn BP, et al. Managing interactions with direct oral anticoagulants (DOACs). Pharmacist’s Letter. May 2016.
28. Johannessen SI, Landmark CJ. Antiepileptic drug interactions – principles and clinical implications. Curr Neuropharmacol. 2010;8:254-267.
29. Mohrien K, Oliphant CS, Self TH. Drug interactions with novel oral anticoagulants. Consultant. 2013;53:918-919. Available at: http://www.consultant360.com/articles/drug-interactions-novel-oral-anticoagulants. Accessed May 3, 2016.
30. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-e7.
31. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
32. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681-1690.
33. Hirota T, Leiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11:1435-1447.
34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol wih 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial inffarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
35. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120-122.
36. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
37. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
38. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm. Accessed June 15, 2016.
39. Sultana J, Spina E, Trifirò G. Antidepressant use in the elderly: the role of pharmacodynamics and pharmacokinetics in drug safety. Expert Opin Drug Metab Toxicol. 2015;11:883-892.
40. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454-471.
41. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
PRACTICE RECOMMENDATIONS
› Recommend progesterone-containing intrauterine devices or long-acting progesterone injections for women using antiepileptic drugs. B
› Be aware that there is an increased risk of gastrointestinal bleeding when nonsteroidal anti-inflammatory drugs are used with selective serotonin reuptake inhibitors. A
› Do not prescribe novel oral anticoagulants for patients taking carbamazepine, phenytoin, or phenobarbital. B
› Choose fenofibrate over gemfibrozil when combining a fibrate and a statin. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Contraceptive care best practices
While the unintended pregnancy rate for women ages 15 to 44 years decreased by 18% between 2008 and 2011, almost half of pregnancies in the United States remain unintended.1 On a more positive note, however, women who use birth control consistently and correctly account for only 5% of unintended pregnancies.2 As family physicians (FPs), we can support and facilitate our female patients’ efforts to consistently use highly effective forms of contraception. The 5 initiatives detailed here can help toward that end.
1. Routinely screen patients for their reproductive intentions
All women of reproductive age should be screened routinely for their pregnancy intentions. The American College of Obstetricians and Gynecologists (ACOG) encourages clinicians to ask women about pregnancy intendedness and encourages patients to develop a reproductive life plan, or a set of personal goals about whether or when to have children.3 The Centers for Disease Control and Prevention (CDC) has also developed a reproductive life plan tool for health professionals to encourage women and men to reflect upon their plans.4 So just as we regularly screen and document cigarette use and blood pressure (BP), so too, should we routinely screen women for their reproductive goals.
Ask women this one question. The Oregon Foundation for Reproductive Health launched the One Key Question Initiative, which proposes that the care team ask women ages 18 to 50: “Would you like to become pregnant in the next year?”5 A common workflow includes the medical assistant asking women about pregnancy intentions and providing a preconception and/or contraceptive handout, if appropriate. The physician provides additional counseling as needed. Pilot studies of One Key Question indicate that 30% to 40% of women screened needed follow-up counseling, suggesting the need for clinicians to be proactive in asking about reproductive plans. (Additional information on the Initiative is available on the Foundation’s Web site at http://www.orfrh.org/.)
This approach assumes women feel in control of their reproduction; however, this may not be the reality for many, especially low-income women.6 Additionally, women commonly cite planning a pregnancy as appropriate only when they are in an ideal relationship and when they are living in a financially stable environment—conditions that some women may never achieve.
Another caveat is that women may not have explicit pregnancy intentions, in which case, this particular approach may not be effective. A study of low-income women found only 60% intended to use the method prescribed after contraception counseling, with 37% of those stopping because of adverse effects, 23% saying they wanted another method, and 17% citing method complexity.7
Reproductive coercion from male partners, ranging from pressure to become pregnant to method sabotage, is also common in low-income women.8 Regular conversations that prioritize a woman’s values and experience are needed to promote reproductive autonomy.
2. Decouple provision of contraception from unnecessary exams
Pelvic exams and pap smears should not be required prior to offering patients hormonal contraception, according to the Choosing Wisely campaign of the American Board of Internal Medicine and ACOG.9,10 Hormonal contraception may instead be provided safely based on a medical history and BP assessment. Adolescents, minority groups, obese women, and victims of sexual trauma, in particular may avoid asking about birth control because of anxiety and fear of pain from these exams.11 The American College of Physicians recommends against speculum and bimanual exams in asymptomatic, non-pregnant, adult women.12 Pap smears and sexually transmitted infection (STI) testing should be performed at their normally scheduled intervals as recommended by the US Preventive Services Task Force (USPSTF) and not be tied to contraceptive provision.13
Assess pregnancy status using criteria,rather than a pregnancy text
Use the CDC’s criteria to assess pregnancy status rather than relying on a urine pregnancy test prior to providing contraception. Once you are reasonably sure that a woman is not pregnant (TABLE 114), contraception may be started. Some physicians have traditionally requested that a woman delay starting contraception until the next menses to ensure that she is not already pregnant. However, given the evidence that hormonal contraception does not cause birth defects, such a delay is not warranted and puts the woman at risk of an unintended pregnancy during the gap.15
Furthermore, there is an approximate 2-week window in which a woman could have a negative urine pregnancy test despite being pregnant, so the test alone is not completely reliable. In addition, obese women may experience irregular cycles, further complicating the traditional approach.16
Another largely unnecessary step … The US Selected Practice Recommendations (US SPR) from the CDC notes that additional STI screening prior to an intrauterine device (IUD) insertion is unnecessary for most women if appropriate screening guidelines have been previously followed.14 For those who have not been screened according to guidelines, the CDC recommends same-day screening and IUD insertion. You can then treat an STI without removing the IUD. Women with purulent cervicitis or a current chlamydial or gonorrheal infection should delay IUD insertion until after treatment.
3. Expand long-acting reversible contraception counseling and access
Offer long-acting reversible contraception (LARC), such as IUDs and implants, as first-line options for most women. ACOG endorses LARC as the most effective reversible method for most women, including those who have not given birth and adolescents.17 Unfortunately, a 2012 study found that family physicians were less likely than OB-GYNs to have enough time for contraceptive counseling and fewer than half felt competent inserting IUDs.18 While 79% of OB-GYNs routinely discussed IUDs with their patients, only 47% of family physicians did. In 2014, the American Academy of Pediatrics (AAP) endorsed a LARC-first tiered counseling approach for adolescents.19
A test of LARC-first counseling
The Contraceptive CHOICE project, a St. Louis, Missouri-based initiative, was launched to reduce unintended pregnancies in women ages 14 to 45 years by offering LARC-first counseling and free contraception of their choice.20 This project involved more than 9000 women at high risk for unintended pregnancy. Same-day LARC insertion was available. Seventy-five percent of women chose a LARC method and they reported greater continuation at 12 and 24 months, when compared to women who did not choose a LARC method. LARC users also reported higher satisfaction at one year. Provision of contraception through the project contributed to a reduction in repeat abortions as well as decreased rates of teenage pregnancy, birth, and abortion. Three years after the start of the project, IUDs had continuation rates of nearly 70%, implants of 56%, and non-LARC methods of 31%.21
When counseling women, it’s important to remember that effectiveness may not be the only criterium a woman uses when choosing a method. A 2010 study found that for 91% of women at high risk for unintended pregnancy, no single method possessed all the features they deemed “extremely important.”22 Clinicians should take a patient-centered approach to find birth control that fits each patient’s priorities.
Clinicians need proper training in LARC methods
Only 20% of FPs regularly insert IUDs, and 11% offer contraceptive implants, according to estimates from physicians recertifying with the American Board of Family Medicine in 2014.23 Access to training during residency is a key component to increasing these rates. FPs who practice obstetrics should be trained in postpartum LARC insertion and offer this option prior to hospital discharge as well as during the postpartum office visit.
Performing LARC insertions on the same day as counseling is ideal, and clinics should strive to reduce barriers to same-day procedures. Time constraints may be addressed by shifting tasks among the medical team. In the CHOICE project, contraceptive counselors—half of whom had no clinical experience—were trained to provide tiered counseling to participants. By working with a cross-trained health care team and offering prepared resources, clinicians can save time and improve access.
Physicians may want to incorporate the free online resources Bedsider.org or Stayteen.org to help women learn about contraceptive methods.24 The user-friendly Web sites, operated by the National Campaign to Prevent Teen and Unplanned Pregnancy, describe various forms of contraception and offer text and email reminders. Incorporating Bedsider into the counseling workflow and discussing the various reminder tools available may improve patients’ knowledge and enhance their compliance.
Additional barriers for practices may include high upfront costs associated with stocking devices. Practices that may be unable to sustain the costs surrounding enhanced contraception counseling and provision can collaborate with family planning clinics that are able to offer same-day services. A study of clinics in California found that Title X clinics were more likely to provide on-site LARC services than non-Title X public and private providers.25
4. Follow CDC guidelines for initiating and continuing contraception
Follow the US SPR for guidance on initiating and continuing contraceptive methods.14 The CDC’s Medical Eligibility Criteria for Contraceptive Use is another vital resource, providing recommendations for contraceptive methods to patients who have specific medical conditions or characteristics.26
Utilize the “quick start” method for hormonal contraception, where birth control is started on the same day as its prescription regardless of timing of the menstrual cycle. If you can’t be reasonably certain that a woman is not pregnant based on the criteria listed in TABLE 1,14 conduct a pregnancy test (while recognizing the aforementioned 2-week window of limitations) and counsel the patient to use back-up protection for the first 7 days along with repeating a pregnancy test in 2 weeks’ time.
The quick start method may lead to higher adherence than delayed initiation.27 Differences in continuation rates between women who use the quick start method and those who follow the delayed approach may disappear over time.28
Prescribe and provide a year’s supply of oral contraceptive pills (OCPs) as recommended by the CDC US SPR.14 It is important to note that pharmacists are usually restricted by insurance companies to only fill a one or 3 month’s supply.
In January 2016, Oregon began requiring private and state health insurance providers to reimburse for a year’s supply of prescription contraception; in January 2017, insurers in Washington, DC, were also required to offer women a year’s supply of prescription contraception.29,30 Several other states have followed suit. The California Health Benefits Review Program estimates a savings of $42.8 million a year from fewer office visits and 15,000 fewer unintended pregnancies if their state enacts a similar policy.31
Pharmacist initiatives are worth watching. In January 2016, Oregon pharmacists with additional training were allowed to prescribe OCs and hormonal patches to women 18 years and older.32 In April 2016, a similar law went into effect in California, but without a minimum age requirement and with the additional coverage of vaginal rings and Depo-Provera (depo) injections.33 Pharmacists in both states must review a health questionnaire completed by the woman and can refer to a physician as necessary.
The CDC recommends that clinicians extend the allowed window for repeat depo injections to 15 weeks.14 Common institutional protocol is to give repeat injections every 11 to 13 weeks. If past that window, protocol often dictates the woman abstain from unprotected sex for 2 weeks and then return for a negative pregnancy test (or await menses) before the next injection. However, the CDC notes that depo is effective for longer than the 13-week period.14 No additional birth control or pregnancy testing is needed and the woman can receive the next depo shot if she is up to 15 weeks from the previous shot.
One study found no additional pregnancy risks for those who were up to 4 weeks “late” for their next shot, suggesting there is potential for an even larger grace period.34 The World Health Organization advises allowing a repeat injection up to 4 weeks late.35 We encourage institutions to change their policies to comply with the CDC’s 15-week window.
Another initiative is over-the-counter (OTC) access to OCs, which the American Academy of Family Physicians (AAFP) and ACOG support.36,37 ACOG notes that “no drug or intervention is completely without risk of harm” and that the risk of venous thromboembolism for OC users is lower than the risk of pregnancy.37 Women can successfully self-screen for contraindications using a checklist. Concerns about women potentially being less adherent or less likely to choose LARCs are not reasons to preclude access to other methods. The AAFP supports insurance coverage of OCs, regardless of prescription status.36
5. Routinely counsel about, and advance-prescribe, emergency contraception pills
Physicians should counsel and advance-prescribe emergency contraception pills (ECPs) to women, including adolescents, using less reliable contraception, as recommended by ACOG, AAP, and the CDC.14,37,38 It’s also important to provide information on the copper IUD as the most effective method of emergency contraception, with nearly 100% efficacy if placed within 5 days.39 An easy-to-read patient hand-out in English and Spanish on EC options can be found at http://beyondthepill.ucsf.edu/tools-materials.
Only 3% of respondents participating in the 2006-2010 National Survey of Family Growth received counseling about emergency contraception in the past year.40 ECPs are most effective when used within 24 hours but have some efficacy up to 5 days.37 Due to the Affordable Care Act, most insurance plans will cover ECPs if purchased with a prescription, but coverage varies by state.41 Ulipristal acetate (UPA) ECP is only available with a prescription. Advance prescriptions can alleviate financial burdens on women when they need to access ECPs quickly.
Women should wait at least 5 days before resuming or starting hormonal contraception after taking UPA-based ECP, as it may reduce the ovulation-delaying effect of the ECP.14 For IUDs, implants, and depo, which require a visit to a health care provider, physicians evaluating earlier provision should consider the risks of reduced efficacy against the many barriers to access.
UPA-based ECPs (such as ella) may be more effective for overweight and obese women than levonorgestrel-based ECPs (such as Plan B and Next Choice).14 Consider advance-prescribing UPA ECPs to women with a body mass index (BMI) >25 kg/m2.42 Such considerations are important as the prevalence of obesity in women between 2013 and 2014 was 40.4%.43
In May 2016, the FDA noted that while current data are insufficient regarding whether the effectiveness of levonorgestrel ECPs is reduced in overweight or obese women, there are no safety concerns regarding their use in this population.44 Therefore, a woman with a BMI >25 kg/m2 should use UPA ECPs if available; but if not, she can still use levonorgestrel ECPs. One study, however, has found that UPA ECPs are only as effective as a placebo when BMI is ≥35 kg/m2, at which point a copper IUD may be the only effective form of emergency contraception.45
Transitioning from customary practices to best practices
Following these practical steps, FPs can improve contraceptive care for women. However, to make a significant impact, clinicians must be willing to change customary practices that are based on tradition, routines, or outdated protocols in favor of those based on current evidence.
One good place to start the transition to best practices is to familiarize yourself with the 2016 US Medical Eligibility Criteria for Contraceptive Use26 and Selected Practice Recommendations for Contraceptive Use.14 TABLES 214,26,46,47 and 3 offer additional resources that can enhance contraceptive counseling and further promote access to contraceptive care.
The contraceptive coverage guarantee under the Affordable Care Act has allowed many women to make contraceptive choices based on personal needs and preferences rather than cost. The new contraceptive coverage exemptions issued under the Trump administration will bring cost back as the driving decision factor for women whose employers choose not to provide contraceptive coverage. Providers should be aware of the typical costs associated with the various contraceptive options offered in their practice and community.
CORRESPONDENCE
Jessica Dalby, MD, Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, 1102 South Park St, Suite 100, Madison, WI 53715; [email protected].
1. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016; 374:843-852.
2. Sonfield A, Hasstedt K, Gold RB. Moving Forward: Family Planning in the Era of Health Reform. New York: Guttmacher Institute. 2014. Available at: https://www.guttmacher.org/report/moving-forward-family-planning-era-health-reform. Accessed October 5, 2017.
3. Committee on Health Care for Underserved Women. Reproductive Life Planning to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2016. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Reproductive-Life-Planning-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
4. Centers for Disease Control and Prevention. Reproductive Life Plan Tool for Health Care Providers. 2016. Available at: http://www.cdc.gov/preconception/rlptool.html. Accessed August 31, 2016.
5. Oregon Health Authority. Effective Contraceptive Use among Women at Risk of Unintended Pregnancy Guidance Document. 2014. Available at: http://www.oregon.gov/oha/HPA/ANALYTICS/CCOData/Effective%20Contraceptive%20Use%20Guidance%20Document.pdf. Accessed October 5, 2017.
6. Borrero S, Nikolajski C, Steinberg JR, et al. “It just happens”: a qualitative study exploring low-income women’s perspectives on pregnancy intention and planning. Contraception. 2015;91:150-156.
7. Yee LM, Farner KC, King E, et al. What do women want? Experiences of low-income women with postpartum contraception and contraceptive counseling. J Pregnancy Child Health. 2015;2.
8. Kalichman SC, Williams EA, Cherry C, et al. Sexual coercion, domestic violence, and negotiating condom use among low-income African American women. J Womens Health. 1998;7:371-378.
9. ABIM Foundation. Pelvic Exams, Pap Tests and Oral Contraceptives. 2016. Available at: http://www.choosingwisely.org/patient-resources/pelvic-exams-pap-tests-and-oral-contraceptives/. Accessed May 31, 2016.
10. Committee on Health Care for Underserved Women. Access to Contraception: American College of Obstetricians and Gynecologists. 2015. Number 615. Available at: https://www.acog.org/-/media/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/co615.pdf?dmc=1&ts=201710. Accessed October 5, 2017.
11. Bates CK, Carroll N, Potter J. The challenging pelvic examination. J Gen Intern Med. 2011;26:651-657.
12. Qaseem A, Humphrey LL, Harris R, et al. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:67-72.
13. U.S. Preventive Services Task Force. Cervical Cancer: Screening. 2012. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Accessed May 25, 2016.
14. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-66.
15. Lesnewski R, Prine L. Initiating hormonal contraception. Am Fam Physician. 2006;74:105-112.
16. Jacobsen BK, Knutsen SF, Oda K, et al. Obesity at age 20 and the risk of miscarriages, irregular periods and reported problems of becoming pregnant: the Adventist Health Study-2. Eur J Epidemiol. 2012; 27:923-931.
17. Committee on Gynecologic Practice. Increasing Access to Contraceptive Implants and Intrauterine Devices to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2015. Number 642. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Increasing-Access-to-Contraceptive-Implants-and-Intrauterine-Devices-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
18. Harper CC, Henderson JT, Raine TR, et al. Evidence-based IUD practice: family physicians and obstetrician-gynecologists. Fam Med. 2012;44:637-645.
19. American Academy of Pediatrics, Committee on Adolescence. Policy statement: Contraception for Adolescents. 2014. Available at: http://pediatrics.aappublications.org/content/pediatrics/early/2014/09/24/peds.2014-2299.full.pdf. Accessed October 5, 2017.
20. Birgisson NE, Zhao Q, Secura GM, et al. Preventing unintended pregnancy: The Contraceptive CHOICE Project in review. J Womens Health (Larchmt). 2015;24:349-353.
21. Diedrich JT, Zhao Q, Madden T, et al. Three-year continuation of reversible contraception. Am J Obstet Gynecol. 2015;213:662.e1-e8.
22. Lessard LN, Karasek D, Ma S, et al. Contraceptive features preferred by women at high risk of unintended pregnancy. Perspect Sex Reprod Health. 2012;44:194-200.
23. Nisen MB, Peterson LE, Cochrane A, et al. US family physicians’ intrauterine and implantable contraception provision: results from a national survey. Contraception. 2016;93:432-437.
24. National Campaign to Prevent Teen and Unplanned Pregnancy. Bedsider. Available at: https://bedsider.org/. Accessed June 14, 2016.
25. Park HY, Rodriguez MI, Hulett D, et al. Long-acting reversible contraception method use among Title X providers and non-Title X providers in California. Contraception. 2012;86:557-561.
26. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-103.
27. Westhoff C, Kerns J, Morroni C, et al. Quick start: novel oral contraceptive initiation method. Contraception. 2002;66:141-145.
28. Brahmi D, Curtis KM. When can a woman start combined hormonal contraceptives (CHCs)? A systematic review. Contraception. 2013;87:524-538.
29. Lachman S. Oregon To Require Insurers To Cover A Year’s Supply Of Birth Control. Huffington Post. June 11, 2015. Available at: https://www.huffingtonpost.com/2015/06/11/oregon-birth-control-_n_7564712.html. Accessed October 16, 2017.
30. Andrews M. D.C. Women To Get Access To Full Year’s Worth Of Contraceptives. Kaiser Health News. September 25, 2015. Available at: https://khn.org/news/d-c-women-to-get-access-to-full-years-worth-of-contraceptives/. Accessed October 16, 2017.
31. Analysis of California Senate Bill (SB) 999 Contraceptives: Annual Supply: A Report to the 2015-2016 California State Legislature: California Health Benefits Review Program. 2016. Available at: http://chbrp.ucop.edu/index.php?action=read&bill_id=195&doc_type=1000. Accessed October 5, 2017.
32. Frazier A. Pharmacist-prescribed birth control in effect Jan 1. KOIN News. December 30, 2015. Available at: http://koin.com/2015/12/30/pharmacist-provided-birth-control-in-effect-jan-1/. Accessed October 5, 2017.
33. Karlamangla S. Birth control pills without prescriptions, coming soon to California under new law. Los Angeles Times. February 14, 2016. Available at: http://www.latimes.com/health/la-me-birth-control-pharmacies-20160214-story.html. Accessed October 16, 2017.
34. Steiner MJ, Kwok C, Stanback J, et al. Injectable contraception: what should the longest interval be for reinjections? Contraception. 2008;77:410-414.
35. World Health Organization. Family Planning: A Global Handbook for Providers. 2011. Available at: http://apps.who.int/iris/bitstream/10665/44028/1/9780978856373_eng.pdf. Accessed October 5, 2017.
36. American Academy of Family Physicians. Over-the-Counter Oral Contraceptives. 2014; Available at: http://www.aafp.org/about/policies/all/otc-oral-contraceptives.html. Accessed June 2, 2016.
37. Committee on Gynecologic Practice. Over-the-Counter Access to Oral Contraceptives: American College of Obstetricians and Gynecologists. 2012. Number 544. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Over-the-Counter-Access-to-Oral-Contraceptives. Accessed October 5, 2017.
38. Committee on Adolescence. Emergency contraception. Pediatrics. 2012;130:1174-1182.
39. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
40. Martinez G, Chandra A, Febo-Vazquez I, et al. Use of Family Planning and Related Medical Services Among Women Aged 15–44 in the United States: National Survey of Family Growth, 2006–2010: National Center for Health Statistics, Centers for Disease Control and Prevention. 2013. Available at: https://www.cdc.gov/nchs/data/nhsr/nhsr068.pdf. Accessed October 5, 2017.
41. Guttmacher Institute. Insurance Coverage of Contraceptives: Guttmacher Institute;2017. Available at: https://www.guttmacher.org/state-policy/explore/insurance-coverage-contraceptives Accessed October 7, 2017.
42. Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363-367.
43. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284-2291.
44. US Food & Drug Administration. Postmarket Drug Safety Information for Patients and Providers - Plan B (0.75mg levonorgestrel) and Plan B One-Step (1.5 mg levonorgestrel) Tablets Information. 2016; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109775.htm. Accessed May 25, 2016.
45. Simmons KB, Edelman AB. Contraception and sexual health in obese women. Best Pract Res Clin Obstet Gynaecol. 2015;29:466-478.
46. Centers for Disease Control and Prevention. Providing quality family planning services: recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recomm Rep. 2014;63:1-29.
47. LARC FIRST. Available at: http://www.larcfirst.com/index.html. Accessed May 2016.
While the unintended pregnancy rate for women ages 15 to 44 years decreased by 18% between 2008 and 2011, almost half of pregnancies in the United States remain unintended.1 On a more positive note, however, women who use birth control consistently and correctly account for only 5% of unintended pregnancies.2 As family physicians (FPs), we can support and facilitate our female patients’ efforts to consistently use highly effective forms of contraception. The 5 initiatives detailed here can help toward that end.
1. Routinely screen patients for their reproductive intentions
All women of reproductive age should be screened routinely for their pregnancy intentions. The American College of Obstetricians and Gynecologists (ACOG) encourages clinicians to ask women about pregnancy intendedness and encourages patients to develop a reproductive life plan, or a set of personal goals about whether or when to have children.3 The Centers for Disease Control and Prevention (CDC) has also developed a reproductive life plan tool for health professionals to encourage women and men to reflect upon their plans.4 So just as we regularly screen and document cigarette use and blood pressure (BP), so too, should we routinely screen women for their reproductive goals.
Ask women this one question. The Oregon Foundation for Reproductive Health launched the One Key Question Initiative, which proposes that the care team ask women ages 18 to 50: “Would you like to become pregnant in the next year?”5 A common workflow includes the medical assistant asking women about pregnancy intentions and providing a preconception and/or contraceptive handout, if appropriate. The physician provides additional counseling as needed. Pilot studies of One Key Question indicate that 30% to 40% of women screened needed follow-up counseling, suggesting the need for clinicians to be proactive in asking about reproductive plans. (Additional information on the Initiative is available on the Foundation’s Web site at http://www.orfrh.org/.)
This approach assumes women feel in control of their reproduction; however, this may not be the reality for many, especially low-income women.6 Additionally, women commonly cite planning a pregnancy as appropriate only when they are in an ideal relationship and when they are living in a financially stable environment—conditions that some women may never achieve.
Another caveat is that women may not have explicit pregnancy intentions, in which case, this particular approach may not be effective. A study of low-income women found only 60% intended to use the method prescribed after contraception counseling, with 37% of those stopping because of adverse effects, 23% saying they wanted another method, and 17% citing method complexity.7
Reproductive coercion from male partners, ranging from pressure to become pregnant to method sabotage, is also common in low-income women.8 Regular conversations that prioritize a woman’s values and experience are needed to promote reproductive autonomy.
2. Decouple provision of contraception from unnecessary exams
Pelvic exams and pap smears should not be required prior to offering patients hormonal contraception, according to the Choosing Wisely campaign of the American Board of Internal Medicine and ACOG.9,10 Hormonal contraception may instead be provided safely based on a medical history and BP assessment. Adolescents, minority groups, obese women, and victims of sexual trauma, in particular may avoid asking about birth control because of anxiety and fear of pain from these exams.11 The American College of Physicians recommends against speculum and bimanual exams in asymptomatic, non-pregnant, adult women.12 Pap smears and sexually transmitted infection (STI) testing should be performed at their normally scheduled intervals as recommended by the US Preventive Services Task Force (USPSTF) and not be tied to contraceptive provision.13
Assess pregnancy status using criteria,rather than a pregnancy text
Use the CDC’s criteria to assess pregnancy status rather than relying on a urine pregnancy test prior to providing contraception. Once you are reasonably sure that a woman is not pregnant (TABLE 114), contraception may be started. Some physicians have traditionally requested that a woman delay starting contraception until the next menses to ensure that she is not already pregnant. However, given the evidence that hormonal contraception does not cause birth defects, such a delay is not warranted and puts the woman at risk of an unintended pregnancy during the gap.15
Furthermore, there is an approximate 2-week window in which a woman could have a negative urine pregnancy test despite being pregnant, so the test alone is not completely reliable. In addition, obese women may experience irregular cycles, further complicating the traditional approach.16
Another largely unnecessary step … The US Selected Practice Recommendations (US SPR) from the CDC notes that additional STI screening prior to an intrauterine device (IUD) insertion is unnecessary for most women if appropriate screening guidelines have been previously followed.14 For those who have not been screened according to guidelines, the CDC recommends same-day screening and IUD insertion. You can then treat an STI without removing the IUD. Women with purulent cervicitis or a current chlamydial or gonorrheal infection should delay IUD insertion until after treatment.
3. Expand long-acting reversible contraception counseling and access
Offer long-acting reversible contraception (LARC), such as IUDs and implants, as first-line options for most women. ACOG endorses LARC as the most effective reversible method for most women, including those who have not given birth and adolescents.17 Unfortunately, a 2012 study found that family physicians were less likely than OB-GYNs to have enough time for contraceptive counseling and fewer than half felt competent inserting IUDs.18 While 79% of OB-GYNs routinely discussed IUDs with their patients, only 47% of family physicians did. In 2014, the American Academy of Pediatrics (AAP) endorsed a LARC-first tiered counseling approach for adolescents.19
A test of LARC-first counseling
The Contraceptive CHOICE project, a St. Louis, Missouri-based initiative, was launched to reduce unintended pregnancies in women ages 14 to 45 years by offering LARC-first counseling and free contraception of their choice.20 This project involved more than 9000 women at high risk for unintended pregnancy. Same-day LARC insertion was available. Seventy-five percent of women chose a LARC method and they reported greater continuation at 12 and 24 months, when compared to women who did not choose a LARC method. LARC users also reported higher satisfaction at one year. Provision of contraception through the project contributed to a reduction in repeat abortions as well as decreased rates of teenage pregnancy, birth, and abortion. Three years after the start of the project, IUDs had continuation rates of nearly 70%, implants of 56%, and non-LARC methods of 31%.21
When counseling women, it’s important to remember that effectiveness may not be the only criterium a woman uses when choosing a method. A 2010 study found that for 91% of women at high risk for unintended pregnancy, no single method possessed all the features they deemed “extremely important.”22 Clinicians should take a patient-centered approach to find birth control that fits each patient’s priorities.
Clinicians need proper training in LARC methods
Only 20% of FPs regularly insert IUDs, and 11% offer contraceptive implants, according to estimates from physicians recertifying with the American Board of Family Medicine in 2014.23 Access to training during residency is a key component to increasing these rates. FPs who practice obstetrics should be trained in postpartum LARC insertion and offer this option prior to hospital discharge as well as during the postpartum office visit.
Performing LARC insertions on the same day as counseling is ideal, and clinics should strive to reduce barriers to same-day procedures. Time constraints may be addressed by shifting tasks among the medical team. In the CHOICE project, contraceptive counselors—half of whom had no clinical experience—were trained to provide tiered counseling to participants. By working with a cross-trained health care team and offering prepared resources, clinicians can save time and improve access.
Physicians may want to incorporate the free online resources Bedsider.org or Stayteen.org to help women learn about contraceptive methods.24 The user-friendly Web sites, operated by the National Campaign to Prevent Teen and Unplanned Pregnancy, describe various forms of contraception and offer text and email reminders. Incorporating Bedsider into the counseling workflow and discussing the various reminder tools available may improve patients’ knowledge and enhance their compliance.
Additional barriers for practices may include high upfront costs associated with stocking devices. Practices that may be unable to sustain the costs surrounding enhanced contraception counseling and provision can collaborate with family planning clinics that are able to offer same-day services. A study of clinics in California found that Title X clinics were more likely to provide on-site LARC services than non-Title X public and private providers.25
4. Follow CDC guidelines for initiating and continuing contraception
Follow the US SPR for guidance on initiating and continuing contraceptive methods.14 The CDC’s Medical Eligibility Criteria for Contraceptive Use is another vital resource, providing recommendations for contraceptive methods to patients who have specific medical conditions or characteristics.26
Utilize the “quick start” method for hormonal contraception, where birth control is started on the same day as its prescription regardless of timing of the menstrual cycle. If you can’t be reasonably certain that a woman is not pregnant based on the criteria listed in TABLE 1,14 conduct a pregnancy test (while recognizing the aforementioned 2-week window of limitations) and counsel the patient to use back-up protection for the first 7 days along with repeating a pregnancy test in 2 weeks’ time.
The quick start method may lead to higher adherence than delayed initiation.27 Differences in continuation rates between women who use the quick start method and those who follow the delayed approach may disappear over time.28
Prescribe and provide a year’s supply of oral contraceptive pills (OCPs) as recommended by the CDC US SPR.14 It is important to note that pharmacists are usually restricted by insurance companies to only fill a one or 3 month’s supply.
In January 2016, Oregon began requiring private and state health insurance providers to reimburse for a year’s supply of prescription contraception; in January 2017, insurers in Washington, DC, were also required to offer women a year’s supply of prescription contraception.29,30 Several other states have followed suit. The California Health Benefits Review Program estimates a savings of $42.8 million a year from fewer office visits and 15,000 fewer unintended pregnancies if their state enacts a similar policy.31
Pharmacist initiatives are worth watching. In January 2016, Oregon pharmacists with additional training were allowed to prescribe OCs and hormonal patches to women 18 years and older.32 In April 2016, a similar law went into effect in California, but without a minimum age requirement and with the additional coverage of vaginal rings and Depo-Provera (depo) injections.33 Pharmacists in both states must review a health questionnaire completed by the woman and can refer to a physician as necessary.
The CDC recommends that clinicians extend the allowed window for repeat depo injections to 15 weeks.14 Common institutional protocol is to give repeat injections every 11 to 13 weeks. If past that window, protocol often dictates the woman abstain from unprotected sex for 2 weeks and then return for a negative pregnancy test (or await menses) before the next injection. However, the CDC notes that depo is effective for longer than the 13-week period.14 No additional birth control or pregnancy testing is needed and the woman can receive the next depo shot if she is up to 15 weeks from the previous shot.
One study found no additional pregnancy risks for those who were up to 4 weeks “late” for their next shot, suggesting there is potential for an even larger grace period.34 The World Health Organization advises allowing a repeat injection up to 4 weeks late.35 We encourage institutions to change their policies to comply with the CDC’s 15-week window.
Another initiative is over-the-counter (OTC) access to OCs, which the American Academy of Family Physicians (AAFP) and ACOG support.36,37 ACOG notes that “no drug or intervention is completely without risk of harm” and that the risk of venous thromboembolism for OC users is lower than the risk of pregnancy.37 Women can successfully self-screen for contraindications using a checklist. Concerns about women potentially being less adherent or less likely to choose LARCs are not reasons to preclude access to other methods. The AAFP supports insurance coverage of OCs, regardless of prescription status.36
5. Routinely counsel about, and advance-prescribe, emergency contraception pills
Physicians should counsel and advance-prescribe emergency contraception pills (ECPs) to women, including adolescents, using less reliable contraception, as recommended by ACOG, AAP, and the CDC.14,37,38 It’s also important to provide information on the copper IUD as the most effective method of emergency contraception, with nearly 100% efficacy if placed within 5 days.39 An easy-to-read patient hand-out in English and Spanish on EC options can be found at http://beyondthepill.ucsf.edu/tools-materials.
Only 3% of respondents participating in the 2006-2010 National Survey of Family Growth received counseling about emergency contraception in the past year.40 ECPs are most effective when used within 24 hours but have some efficacy up to 5 days.37 Due to the Affordable Care Act, most insurance plans will cover ECPs if purchased with a prescription, but coverage varies by state.41 Ulipristal acetate (UPA) ECP is only available with a prescription. Advance prescriptions can alleviate financial burdens on women when they need to access ECPs quickly.
Women should wait at least 5 days before resuming or starting hormonal contraception after taking UPA-based ECP, as it may reduce the ovulation-delaying effect of the ECP.14 For IUDs, implants, and depo, which require a visit to a health care provider, physicians evaluating earlier provision should consider the risks of reduced efficacy against the many barriers to access.
UPA-based ECPs (such as ella) may be more effective for overweight and obese women than levonorgestrel-based ECPs (such as Plan B and Next Choice).14 Consider advance-prescribing UPA ECPs to women with a body mass index (BMI) >25 kg/m2.42 Such considerations are important as the prevalence of obesity in women between 2013 and 2014 was 40.4%.43
In May 2016, the FDA noted that while current data are insufficient regarding whether the effectiveness of levonorgestrel ECPs is reduced in overweight or obese women, there are no safety concerns regarding their use in this population.44 Therefore, a woman with a BMI >25 kg/m2 should use UPA ECPs if available; but if not, she can still use levonorgestrel ECPs. One study, however, has found that UPA ECPs are only as effective as a placebo when BMI is ≥35 kg/m2, at which point a copper IUD may be the only effective form of emergency contraception.45
Transitioning from customary practices to best practices
Following these practical steps, FPs can improve contraceptive care for women. However, to make a significant impact, clinicians must be willing to change customary practices that are based on tradition, routines, or outdated protocols in favor of those based on current evidence.
One good place to start the transition to best practices is to familiarize yourself with the 2016 US Medical Eligibility Criteria for Contraceptive Use26 and Selected Practice Recommendations for Contraceptive Use.14 TABLES 214,26,46,47 and 3 offer additional resources that can enhance contraceptive counseling and further promote access to contraceptive care.
The contraceptive coverage guarantee under the Affordable Care Act has allowed many women to make contraceptive choices based on personal needs and preferences rather than cost. The new contraceptive coverage exemptions issued under the Trump administration will bring cost back as the driving decision factor for women whose employers choose not to provide contraceptive coverage. Providers should be aware of the typical costs associated with the various contraceptive options offered in their practice and community.
CORRESPONDENCE
Jessica Dalby, MD, Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, 1102 South Park St, Suite 100, Madison, WI 53715; [email protected].
While the unintended pregnancy rate for women ages 15 to 44 years decreased by 18% between 2008 and 2011, almost half of pregnancies in the United States remain unintended.1 On a more positive note, however, women who use birth control consistently and correctly account for only 5% of unintended pregnancies.2 As family physicians (FPs), we can support and facilitate our female patients’ efforts to consistently use highly effective forms of contraception. The 5 initiatives detailed here can help toward that end.
1. Routinely screen patients for their reproductive intentions
All women of reproductive age should be screened routinely for their pregnancy intentions. The American College of Obstetricians and Gynecologists (ACOG) encourages clinicians to ask women about pregnancy intendedness and encourages patients to develop a reproductive life plan, or a set of personal goals about whether or when to have children.3 The Centers for Disease Control and Prevention (CDC) has also developed a reproductive life plan tool for health professionals to encourage women and men to reflect upon their plans.4 So just as we regularly screen and document cigarette use and blood pressure (BP), so too, should we routinely screen women for their reproductive goals.
Ask women this one question. The Oregon Foundation for Reproductive Health launched the One Key Question Initiative, which proposes that the care team ask women ages 18 to 50: “Would you like to become pregnant in the next year?”5 A common workflow includes the medical assistant asking women about pregnancy intentions and providing a preconception and/or contraceptive handout, if appropriate. The physician provides additional counseling as needed. Pilot studies of One Key Question indicate that 30% to 40% of women screened needed follow-up counseling, suggesting the need for clinicians to be proactive in asking about reproductive plans. (Additional information on the Initiative is available on the Foundation’s Web site at http://www.orfrh.org/.)
This approach assumes women feel in control of their reproduction; however, this may not be the reality for many, especially low-income women.6 Additionally, women commonly cite planning a pregnancy as appropriate only when they are in an ideal relationship and when they are living in a financially stable environment—conditions that some women may never achieve.
Another caveat is that women may not have explicit pregnancy intentions, in which case, this particular approach may not be effective. A study of low-income women found only 60% intended to use the method prescribed after contraception counseling, with 37% of those stopping because of adverse effects, 23% saying they wanted another method, and 17% citing method complexity.7
Reproductive coercion from male partners, ranging from pressure to become pregnant to method sabotage, is also common in low-income women.8 Regular conversations that prioritize a woman’s values and experience are needed to promote reproductive autonomy.
2. Decouple provision of contraception from unnecessary exams
Pelvic exams and pap smears should not be required prior to offering patients hormonal contraception, according to the Choosing Wisely campaign of the American Board of Internal Medicine and ACOG.9,10 Hormonal contraception may instead be provided safely based on a medical history and BP assessment. Adolescents, minority groups, obese women, and victims of sexual trauma, in particular may avoid asking about birth control because of anxiety and fear of pain from these exams.11 The American College of Physicians recommends against speculum and bimanual exams in asymptomatic, non-pregnant, adult women.12 Pap smears and sexually transmitted infection (STI) testing should be performed at their normally scheduled intervals as recommended by the US Preventive Services Task Force (USPSTF) and not be tied to contraceptive provision.13
Assess pregnancy status using criteria,rather than a pregnancy text
Use the CDC’s criteria to assess pregnancy status rather than relying on a urine pregnancy test prior to providing contraception. Once you are reasonably sure that a woman is not pregnant (TABLE 114), contraception may be started. Some physicians have traditionally requested that a woman delay starting contraception until the next menses to ensure that she is not already pregnant. However, given the evidence that hormonal contraception does not cause birth defects, such a delay is not warranted and puts the woman at risk of an unintended pregnancy during the gap.15
Furthermore, there is an approximate 2-week window in which a woman could have a negative urine pregnancy test despite being pregnant, so the test alone is not completely reliable. In addition, obese women may experience irregular cycles, further complicating the traditional approach.16
Another largely unnecessary step … The US Selected Practice Recommendations (US SPR) from the CDC notes that additional STI screening prior to an intrauterine device (IUD) insertion is unnecessary for most women if appropriate screening guidelines have been previously followed.14 For those who have not been screened according to guidelines, the CDC recommends same-day screening and IUD insertion. You can then treat an STI without removing the IUD. Women with purulent cervicitis or a current chlamydial or gonorrheal infection should delay IUD insertion until after treatment.
3. Expand long-acting reversible contraception counseling and access
Offer long-acting reversible contraception (LARC), such as IUDs and implants, as first-line options for most women. ACOG endorses LARC as the most effective reversible method for most women, including those who have not given birth and adolescents.17 Unfortunately, a 2012 study found that family physicians were less likely than OB-GYNs to have enough time for contraceptive counseling and fewer than half felt competent inserting IUDs.18 While 79% of OB-GYNs routinely discussed IUDs with their patients, only 47% of family physicians did. In 2014, the American Academy of Pediatrics (AAP) endorsed a LARC-first tiered counseling approach for adolescents.19
A test of LARC-first counseling
The Contraceptive CHOICE project, a St. Louis, Missouri-based initiative, was launched to reduce unintended pregnancies in women ages 14 to 45 years by offering LARC-first counseling and free contraception of their choice.20 This project involved more than 9000 women at high risk for unintended pregnancy. Same-day LARC insertion was available. Seventy-five percent of women chose a LARC method and they reported greater continuation at 12 and 24 months, when compared to women who did not choose a LARC method. LARC users also reported higher satisfaction at one year. Provision of contraception through the project contributed to a reduction in repeat abortions as well as decreased rates of teenage pregnancy, birth, and abortion. Three years after the start of the project, IUDs had continuation rates of nearly 70%, implants of 56%, and non-LARC methods of 31%.21
When counseling women, it’s important to remember that effectiveness may not be the only criterium a woman uses when choosing a method. A 2010 study found that for 91% of women at high risk for unintended pregnancy, no single method possessed all the features they deemed “extremely important.”22 Clinicians should take a patient-centered approach to find birth control that fits each patient’s priorities.
Clinicians need proper training in LARC methods
Only 20% of FPs regularly insert IUDs, and 11% offer contraceptive implants, according to estimates from physicians recertifying with the American Board of Family Medicine in 2014.23 Access to training during residency is a key component to increasing these rates. FPs who practice obstetrics should be trained in postpartum LARC insertion and offer this option prior to hospital discharge as well as during the postpartum office visit.
Performing LARC insertions on the same day as counseling is ideal, and clinics should strive to reduce barriers to same-day procedures. Time constraints may be addressed by shifting tasks among the medical team. In the CHOICE project, contraceptive counselors—half of whom had no clinical experience—were trained to provide tiered counseling to participants. By working with a cross-trained health care team and offering prepared resources, clinicians can save time and improve access.
Physicians may want to incorporate the free online resources Bedsider.org or Stayteen.org to help women learn about contraceptive methods.24 The user-friendly Web sites, operated by the National Campaign to Prevent Teen and Unplanned Pregnancy, describe various forms of contraception and offer text and email reminders. Incorporating Bedsider into the counseling workflow and discussing the various reminder tools available may improve patients’ knowledge and enhance their compliance.
Additional barriers for practices may include high upfront costs associated with stocking devices. Practices that may be unable to sustain the costs surrounding enhanced contraception counseling and provision can collaborate with family planning clinics that are able to offer same-day services. A study of clinics in California found that Title X clinics were more likely to provide on-site LARC services than non-Title X public and private providers.25
4. Follow CDC guidelines for initiating and continuing contraception
Follow the US SPR for guidance on initiating and continuing contraceptive methods.14 The CDC’s Medical Eligibility Criteria for Contraceptive Use is another vital resource, providing recommendations for contraceptive methods to patients who have specific medical conditions or characteristics.26
Utilize the “quick start” method for hormonal contraception, where birth control is started on the same day as its prescription regardless of timing of the menstrual cycle. If you can’t be reasonably certain that a woman is not pregnant based on the criteria listed in TABLE 1,14 conduct a pregnancy test (while recognizing the aforementioned 2-week window of limitations) and counsel the patient to use back-up protection for the first 7 days along with repeating a pregnancy test in 2 weeks’ time.
The quick start method may lead to higher adherence than delayed initiation.27 Differences in continuation rates between women who use the quick start method and those who follow the delayed approach may disappear over time.28
Prescribe and provide a year’s supply of oral contraceptive pills (OCPs) as recommended by the CDC US SPR.14 It is important to note that pharmacists are usually restricted by insurance companies to only fill a one or 3 month’s supply.
In January 2016, Oregon began requiring private and state health insurance providers to reimburse for a year’s supply of prescription contraception; in January 2017, insurers in Washington, DC, were also required to offer women a year’s supply of prescription contraception.29,30 Several other states have followed suit. The California Health Benefits Review Program estimates a savings of $42.8 million a year from fewer office visits and 15,000 fewer unintended pregnancies if their state enacts a similar policy.31
Pharmacist initiatives are worth watching. In January 2016, Oregon pharmacists with additional training were allowed to prescribe OCs and hormonal patches to women 18 years and older.32 In April 2016, a similar law went into effect in California, but without a minimum age requirement and with the additional coverage of vaginal rings and Depo-Provera (depo) injections.33 Pharmacists in both states must review a health questionnaire completed by the woman and can refer to a physician as necessary.
The CDC recommends that clinicians extend the allowed window for repeat depo injections to 15 weeks.14 Common institutional protocol is to give repeat injections every 11 to 13 weeks. If past that window, protocol often dictates the woman abstain from unprotected sex for 2 weeks and then return for a negative pregnancy test (or await menses) before the next injection. However, the CDC notes that depo is effective for longer than the 13-week period.14 No additional birth control or pregnancy testing is needed and the woman can receive the next depo shot if she is up to 15 weeks from the previous shot.
One study found no additional pregnancy risks for those who were up to 4 weeks “late” for their next shot, suggesting there is potential for an even larger grace period.34 The World Health Organization advises allowing a repeat injection up to 4 weeks late.35 We encourage institutions to change their policies to comply with the CDC’s 15-week window.
Another initiative is over-the-counter (OTC) access to OCs, which the American Academy of Family Physicians (AAFP) and ACOG support.36,37 ACOG notes that “no drug or intervention is completely without risk of harm” and that the risk of venous thromboembolism for OC users is lower than the risk of pregnancy.37 Women can successfully self-screen for contraindications using a checklist. Concerns about women potentially being less adherent or less likely to choose LARCs are not reasons to preclude access to other methods. The AAFP supports insurance coverage of OCs, regardless of prescription status.36
5. Routinely counsel about, and advance-prescribe, emergency contraception pills
Physicians should counsel and advance-prescribe emergency contraception pills (ECPs) to women, including adolescents, using less reliable contraception, as recommended by ACOG, AAP, and the CDC.14,37,38 It’s also important to provide information on the copper IUD as the most effective method of emergency contraception, with nearly 100% efficacy if placed within 5 days.39 An easy-to-read patient hand-out in English and Spanish on EC options can be found at http://beyondthepill.ucsf.edu/tools-materials.
Only 3% of respondents participating in the 2006-2010 National Survey of Family Growth received counseling about emergency contraception in the past year.40 ECPs are most effective when used within 24 hours but have some efficacy up to 5 days.37 Due to the Affordable Care Act, most insurance plans will cover ECPs if purchased with a prescription, but coverage varies by state.41 Ulipristal acetate (UPA) ECP is only available with a prescription. Advance prescriptions can alleviate financial burdens on women when they need to access ECPs quickly.
Women should wait at least 5 days before resuming or starting hormonal contraception after taking UPA-based ECP, as it may reduce the ovulation-delaying effect of the ECP.14 For IUDs, implants, and depo, which require a visit to a health care provider, physicians evaluating earlier provision should consider the risks of reduced efficacy against the many barriers to access.
UPA-based ECPs (such as ella) may be more effective for overweight and obese women than levonorgestrel-based ECPs (such as Plan B and Next Choice).14 Consider advance-prescribing UPA ECPs to women with a body mass index (BMI) >25 kg/m2.42 Such considerations are important as the prevalence of obesity in women between 2013 and 2014 was 40.4%.43
In May 2016, the FDA noted that while current data are insufficient regarding whether the effectiveness of levonorgestrel ECPs is reduced in overweight or obese women, there are no safety concerns regarding their use in this population.44 Therefore, a woman with a BMI >25 kg/m2 should use UPA ECPs if available; but if not, she can still use levonorgestrel ECPs. One study, however, has found that UPA ECPs are only as effective as a placebo when BMI is ≥35 kg/m2, at which point a copper IUD may be the only effective form of emergency contraception.45
Transitioning from customary practices to best practices
Following these practical steps, FPs can improve contraceptive care for women. However, to make a significant impact, clinicians must be willing to change customary practices that are based on tradition, routines, or outdated protocols in favor of those based on current evidence.
One good place to start the transition to best practices is to familiarize yourself with the 2016 US Medical Eligibility Criteria for Contraceptive Use26 and Selected Practice Recommendations for Contraceptive Use.14 TABLES 214,26,46,47 and 3 offer additional resources that can enhance contraceptive counseling and further promote access to contraceptive care.
The contraceptive coverage guarantee under the Affordable Care Act has allowed many women to make contraceptive choices based on personal needs and preferences rather than cost. The new contraceptive coverage exemptions issued under the Trump administration will bring cost back as the driving decision factor for women whose employers choose not to provide contraceptive coverage. Providers should be aware of the typical costs associated with the various contraceptive options offered in their practice and community.
CORRESPONDENCE
Jessica Dalby, MD, Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, 1102 South Park St, Suite 100, Madison, WI 53715; [email protected].
1. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016; 374:843-852.
2. Sonfield A, Hasstedt K, Gold RB. Moving Forward: Family Planning in the Era of Health Reform. New York: Guttmacher Institute. 2014. Available at: https://www.guttmacher.org/report/moving-forward-family-planning-era-health-reform. Accessed October 5, 2017.
3. Committee on Health Care for Underserved Women. Reproductive Life Planning to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2016. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Reproductive-Life-Planning-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
4. Centers for Disease Control and Prevention. Reproductive Life Plan Tool for Health Care Providers. 2016. Available at: http://www.cdc.gov/preconception/rlptool.html. Accessed August 31, 2016.
5. Oregon Health Authority. Effective Contraceptive Use among Women at Risk of Unintended Pregnancy Guidance Document. 2014. Available at: http://www.oregon.gov/oha/HPA/ANALYTICS/CCOData/Effective%20Contraceptive%20Use%20Guidance%20Document.pdf. Accessed October 5, 2017.
6. Borrero S, Nikolajski C, Steinberg JR, et al. “It just happens”: a qualitative study exploring low-income women’s perspectives on pregnancy intention and planning. Contraception. 2015;91:150-156.
7. Yee LM, Farner KC, King E, et al. What do women want? Experiences of low-income women with postpartum contraception and contraceptive counseling. J Pregnancy Child Health. 2015;2.
8. Kalichman SC, Williams EA, Cherry C, et al. Sexual coercion, domestic violence, and negotiating condom use among low-income African American women. J Womens Health. 1998;7:371-378.
9. ABIM Foundation. Pelvic Exams, Pap Tests and Oral Contraceptives. 2016. Available at: http://www.choosingwisely.org/patient-resources/pelvic-exams-pap-tests-and-oral-contraceptives/. Accessed May 31, 2016.
10. Committee on Health Care for Underserved Women. Access to Contraception: American College of Obstetricians and Gynecologists. 2015. Number 615. Available at: https://www.acog.org/-/media/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/co615.pdf?dmc=1&ts=201710. Accessed October 5, 2017.
11. Bates CK, Carroll N, Potter J. The challenging pelvic examination. J Gen Intern Med. 2011;26:651-657.
12. Qaseem A, Humphrey LL, Harris R, et al. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:67-72.
13. U.S. Preventive Services Task Force. Cervical Cancer: Screening. 2012. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Accessed May 25, 2016.
14. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-66.
15. Lesnewski R, Prine L. Initiating hormonal contraception. Am Fam Physician. 2006;74:105-112.
16. Jacobsen BK, Knutsen SF, Oda K, et al. Obesity at age 20 and the risk of miscarriages, irregular periods and reported problems of becoming pregnant: the Adventist Health Study-2. Eur J Epidemiol. 2012; 27:923-931.
17. Committee on Gynecologic Practice. Increasing Access to Contraceptive Implants and Intrauterine Devices to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2015. Number 642. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Increasing-Access-to-Contraceptive-Implants-and-Intrauterine-Devices-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
18. Harper CC, Henderson JT, Raine TR, et al. Evidence-based IUD practice: family physicians and obstetrician-gynecologists. Fam Med. 2012;44:637-645.
19. American Academy of Pediatrics, Committee on Adolescence. Policy statement: Contraception for Adolescents. 2014. Available at: http://pediatrics.aappublications.org/content/pediatrics/early/2014/09/24/peds.2014-2299.full.pdf. Accessed October 5, 2017.
20. Birgisson NE, Zhao Q, Secura GM, et al. Preventing unintended pregnancy: The Contraceptive CHOICE Project in review. J Womens Health (Larchmt). 2015;24:349-353.
21. Diedrich JT, Zhao Q, Madden T, et al. Three-year continuation of reversible contraception. Am J Obstet Gynecol. 2015;213:662.e1-e8.
22. Lessard LN, Karasek D, Ma S, et al. Contraceptive features preferred by women at high risk of unintended pregnancy. Perspect Sex Reprod Health. 2012;44:194-200.
23. Nisen MB, Peterson LE, Cochrane A, et al. US family physicians’ intrauterine and implantable contraception provision: results from a national survey. Contraception. 2016;93:432-437.
24. National Campaign to Prevent Teen and Unplanned Pregnancy. Bedsider. Available at: https://bedsider.org/. Accessed June 14, 2016.
25. Park HY, Rodriguez MI, Hulett D, et al. Long-acting reversible contraception method use among Title X providers and non-Title X providers in California. Contraception. 2012;86:557-561.
26. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-103.
27. Westhoff C, Kerns J, Morroni C, et al. Quick start: novel oral contraceptive initiation method. Contraception. 2002;66:141-145.
28. Brahmi D, Curtis KM. When can a woman start combined hormonal contraceptives (CHCs)? A systematic review. Contraception. 2013;87:524-538.
29. Lachman S. Oregon To Require Insurers To Cover A Year’s Supply Of Birth Control. Huffington Post. June 11, 2015. Available at: https://www.huffingtonpost.com/2015/06/11/oregon-birth-control-_n_7564712.html. Accessed October 16, 2017.
30. Andrews M. D.C. Women To Get Access To Full Year’s Worth Of Contraceptives. Kaiser Health News. September 25, 2015. Available at: https://khn.org/news/d-c-women-to-get-access-to-full-years-worth-of-contraceptives/. Accessed October 16, 2017.
31. Analysis of California Senate Bill (SB) 999 Contraceptives: Annual Supply: A Report to the 2015-2016 California State Legislature: California Health Benefits Review Program. 2016. Available at: http://chbrp.ucop.edu/index.php?action=read&bill_id=195&doc_type=1000. Accessed October 5, 2017.
32. Frazier A. Pharmacist-prescribed birth control in effect Jan 1. KOIN News. December 30, 2015. Available at: http://koin.com/2015/12/30/pharmacist-provided-birth-control-in-effect-jan-1/. Accessed October 5, 2017.
33. Karlamangla S. Birth control pills without prescriptions, coming soon to California under new law. Los Angeles Times. February 14, 2016. Available at: http://www.latimes.com/health/la-me-birth-control-pharmacies-20160214-story.html. Accessed October 16, 2017.
34. Steiner MJ, Kwok C, Stanback J, et al. Injectable contraception: what should the longest interval be for reinjections? Contraception. 2008;77:410-414.
35. World Health Organization. Family Planning: A Global Handbook for Providers. 2011. Available at: http://apps.who.int/iris/bitstream/10665/44028/1/9780978856373_eng.pdf. Accessed October 5, 2017.
36. American Academy of Family Physicians. Over-the-Counter Oral Contraceptives. 2014; Available at: http://www.aafp.org/about/policies/all/otc-oral-contraceptives.html. Accessed June 2, 2016.
37. Committee on Gynecologic Practice. Over-the-Counter Access to Oral Contraceptives: American College of Obstetricians and Gynecologists. 2012. Number 544. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Over-the-Counter-Access-to-Oral-Contraceptives. Accessed October 5, 2017.
38. Committee on Adolescence. Emergency contraception. Pediatrics. 2012;130:1174-1182.
39. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
40. Martinez G, Chandra A, Febo-Vazquez I, et al. Use of Family Planning and Related Medical Services Among Women Aged 15–44 in the United States: National Survey of Family Growth, 2006–2010: National Center for Health Statistics, Centers for Disease Control and Prevention. 2013. Available at: https://www.cdc.gov/nchs/data/nhsr/nhsr068.pdf. Accessed October 5, 2017.
41. Guttmacher Institute. Insurance Coverage of Contraceptives: Guttmacher Institute;2017. Available at: https://www.guttmacher.org/state-policy/explore/insurance-coverage-contraceptives Accessed October 7, 2017.
42. Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363-367.
43. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284-2291.
44. US Food & Drug Administration. Postmarket Drug Safety Information for Patients and Providers - Plan B (0.75mg levonorgestrel) and Plan B One-Step (1.5 mg levonorgestrel) Tablets Information. 2016; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109775.htm. Accessed May 25, 2016.
45. Simmons KB, Edelman AB. Contraception and sexual health in obese women. Best Pract Res Clin Obstet Gynaecol. 2015;29:466-478.
46. Centers for Disease Control and Prevention. Providing quality family planning services: recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recomm Rep. 2014;63:1-29.
47. LARC FIRST. Available at: http://www.larcfirst.com/index.html. Accessed May 2016.
1. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016; 374:843-852.
2. Sonfield A, Hasstedt K, Gold RB. Moving Forward: Family Planning in the Era of Health Reform. New York: Guttmacher Institute. 2014. Available at: https://www.guttmacher.org/report/moving-forward-family-planning-era-health-reform. Accessed October 5, 2017.
3. Committee on Health Care for Underserved Women. Reproductive Life Planning to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2016. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Reproductive-Life-Planning-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
4. Centers for Disease Control and Prevention. Reproductive Life Plan Tool for Health Care Providers. 2016. Available at: http://www.cdc.gov/preconception/rlptool.html. Accessed August 31, 2016.
5. Oregon Health Authority. Effective Contraceptive Use among Women at Risk of Unintended Pregnancy Guidance Document. 2014. Available at: http://www.oregon.gov/oha/HPA/ANALYTICS/CCOData/Effective%20Contraceptive%20Use%20Guidance%20Document.pdf. Accessed October 5, 2017.
6. Borrero S, Nikolajski C, Steinberg JR, et al. “It just happens”: a qualitative study exploring low-income women’s perspectives on pregnancy intention and planning. Contraception. 2015;91:150-156.
7. Yee LM, Farner KC, King E, et al. What do women want? Experiences of low-income women with postpartum contraception and contraceptive counseling. J Pregnancy Child Health. 2015;2.
8. Kalichman SC, Williams EA, Cherry C, et al. Sexual coercion, domestic violence, and negotiating condom use among low-income African American women. J Womens Health. 1998;7:371-378.
9. ABIM Foundation. Pelvic Exams, Pap Tests and Oral Contraceptives. 2016. Available at: http://www.choosingwisely.org/patient-resources/pelvic-exams-pap-tests-and-oral-contraceptives/. Accessed May 31, 2016.
10. Committee on Health Care for Underserved Women. Access to Contraception: American College of Obstetricians and Gynecologists. 2015. Number 615. Available at: https://www.acog.org/-/media/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/co615.pdf?dmc=1&ts=201710. Accessed October 5, 2017.
11. Bates CK, Carroll N, Potter J. The challenging pelvic examination. J Gen Intern Med. 2011;26:651-657.
12. Qaseem A, Humphrey LL, Harris R, et al. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:67-72.
13. U.S. Preventive Services Task Force. Cervical Cancer: Screening. 2012. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Accessed May 25, 2016.
14. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-66.
15. Lesnewski R, Prine L. Initiating hormonal contraception. Am Fam Physician. 2006;74:105-112.
16. Jacobsen BK, Knutsen SF, Oda K, et al. Obesity at age 20 and the risk of miscarriages, irregular periods and reported problems of becoming pregnant: the Adventist Health Study-2. Eur J Epidemiol. 2012; 27:923-931.
17. Committee on Gynecologic Practice. Increasing Access to Contraceptive Implants and Intrauterine Devices to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2015. Number 642. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Increasing-Access-to-Contraceptive-Implants-and-Intrauterine-Devices-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
18. Harper CC, Henderson JT, Raine TR, et al. Evidence-based IUD practice: family physicians and obstetrician-gynecologists. Fam Med. 2012;44:637-645.
19. American Academy of Pediatrics, Committee on Adolescence. Policy statement: Contraception for Adolescents. 2014. Available at: http://pediatrics.aappublications.org/content/pediatrics/early/2014/09/24/peds.2014-2299.full.pdf. Accessed October 5, 2017.
20. Birgisson NE, Zhao Q, Secura GM, et al. Preventing unintended pregnancy: The Contraceptive CHOICE Project in review. J Womens Health (Larchmt). 2015;24:349-353.
21. Diedrich JT, Zhao Q, Madden T, et al. Three-year continuation of reversible contraception. Am J Obstet Gynecol. 2015;213:662.e1-e8.
22. Lessard LN, Karasek D, Ma S, et al. Contraceptive features preferred by women at high risk of unintended pregnancy. Perspect Sex Reprod Health. 2012;44:194-200.
23. Nisen MB, Peterson LE, Cochrane A, et al. US family physicians’ intrauterine and implantable contraception provision: results from a national survey. Contraception. 2016;93:432-437.
24. National Campaign to Prevent Teen and Unplanned Pregnancy. Bedsider. Available at: https://bedsider.org/. Accessed June 14, 2016.
25. Park HY, Rodriguez MI, Hulett D, et al. Long-acting reversible contraception method use among Title X providers and non-Title X providers in California. Contraception. 2012;86:557-561.
26. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-103.
27. Westhoff C, Kerns J, Morroni C, et al. Quick start: novel oral contraceptive initiation method. Contraception. 2002;66:141-145.
28. Brahmi D, Curtis KM. When can a woman start combined hormonal contraceptives (CHCs)? A systematic review. Contraception. 2013;87:524-538.
29. Lachman S. Oregon To Require Insurers To Cover A Year’s Supply Of Birth Control. Huffington Post. June 11, 2015. Available at: https://www.huffingtonpost.com/2015/06/11/oregon-birth-control-_n_7564712.html. Accessed October 16, 2017.
30. Andrews M. D.C. Women To Get Access To Full Year’s Worth Of Contraceptives. Kaiser Health News. September 25, 2015. Available at: https://khn.org/news/d-c-women-to-get-access-to-full-years-worth-of-contraceptives/. Accessed October 16, 2017.
31. Analysis of California Senate Bill (SB) 999 Contraceptives: Annual Supply: A Report to the 2015-2016 California State Legislature: California Health Benefits Review Program. 2016. Available at: http://chbrp.ucop.edu/index.php?action=read&bill_id=195&doc_type=1000. Accessed October 5, 2017.
32. Frazier A. Pharmacist-prescribed birth control in effect Jan 1. KOIN News. December 30, 2015. Available at: http://koin.com/2015/12/30/pharmacist-provided-birth-control-in-effect-jan-1/. Accessed October 5, 2017.
33. Karlamangla S. Birth control pills without prescriptions, coming soon to California under new law. Los Angeles Times. February 14, 2016. Available at: http://www.latimes.com/health/la-me-birth-control-pharmacies-20160214-story.html. Accessed October 16, 2017.
34. Steiner MJ, Kwok C, Stanback J, et al. Injectable contraception: what should the longest interval be for reinjections? Contraception. 2008;77:410-414.
35. World Health Organization. Family Planning: A Global Handbook for Providers. 2011. Available at: http://apps.who.int/iris/bitstream/10665/44028/1/9780978856373_eng.pdf. Accessed October 5, 2017.
36. American Academy of Family Physicians. Over-the-Counter Oral Contraceptives. 2014; Available at: http://www.aafp.org/about/policies/all/otc-oral-contraceptives.html. Accessed June 2, 2016.
37. Committee on Gynecologic Practice. Over-the-Counter Access to Oral Contraceptives: American College of Obstetricians and Gynecologists. 2012. Number 544. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Over-the-Counter-Access-to-Oral-Contraceptives. Accessed October 5, 2017.
38. Committee on Adolescence. Emergency contraception. Pediatrics. 2012;130:1174-1182.
39. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
40. Martinez G, Chandra A, Febo-Vazquez I, et al. Use of Family Planning and Related Medical Services Among Women Aged 15–44 in the United States: National Survey of Family Growth, 2006–2010: National Center for Health Statistics, Centers for Disease Control and Prevention. 2013. Available at: https://www.cdc.gov/nchs/data/nhsr/nhsr068.pdf. Accessed October 5, 2017.
41. Guttmacher Institute. Insurance Coverage of Contraceptives: Guttmacher Institute;2017. Available at: https://www.guttmacher.org/state-policy/explore/insurance-coverage-contraceptives Accessed October 7, 2017.
42. Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363-367.
43. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284-2291.
44. US Food & Drug Administration. Postmarket Drug Safety Information for Patients and Providers - Plan B (0.75mg levonorgestrel) and Plan B One-Step (1.5 mg levonorgestrel) Tablets Information. 2016; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109775.htm. Accessed May 25, 2016.
45. Simmons KB, Edelman AB. Contraception and sexual health in obese women. Best Pract Res Clin Obstet Gynaecol. 2015;29:466-478.
46. Centers for Disease Control and Prevention. Providing quality family planning services: recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recomm Rep. 2014;63:1-29.
47. LARC FIRST. Available at: http://www.larcfirst.com/index.html. Accessed May 2016.
Managing atraumatic meniscal tears in middle-aged patients
Meniscectomy is the most common orthopedic procedure performed in the United States with 700,000 meniscectomies performed every year.1 More than half of these procedures are performed in patients ≥45 years of age,2 giving rise to the question: Does arthroscopic surgery have a role in the treatment of patients who may have osteoarthritis (OA) and another knee condition, such as a symptomatic meniscal tear? Determining the answer is especially important when you consider that the number of true and incidental tears diagnosed on magnetic resonance imaging (MRI) has been on the rise3—a result of the routine use of MRIs to identify the cause of patients’ chronic knee pain.
At a cost of roughly $5,000 per procedure, some experts have suggested that at least a portion of the approximately $4 billion annual direct medical costs associated with meniscectomy could be put to better use.4,5 This prompted us to wonder what the literature tells us about the management of degenerative meniscus tears in middle-aged patients with OA and whether these patients would benefit from nonoperative management with optimized physical and medical therapy as a first-line approach. Our findings follow.
But first, a word about the connection between OA and meniscal tears.
What we’ve learned about meniscal damage
Research has shown that over one-third of individuals >50 years of age and three-quarters of people with knee OA have degenerative meniscal tears.6 In the past, the relative paucity of epidemiologic data on the prevalence of meniscal tears in the general population made it difficult to interpret the diagnostic information provided by MRI.
More recently, experts found that meniscal damage is especially prevalent among individuals with OA, and they began treating with arthroscopic partial meniscectomy (APM), as the meniscal damage was thought to be the anatomical foundation for the complaint of knee pain.7
However, researchers then began realizing that many patients with findings of a meniscal tear visualized on MRI reported no knee symptoms. In one study, adults in a large community-based sample found to have a meniscal tear on MRI were no more likely to have knee pain than subjects without a meniscal tear.6 Similarly, subjects with a meniscal tear and OA had no more severe pain than subjects with OA and no meniscal tear.6
In addition, the landmark Fairbank study from 19488 and others since have shown that meniscectomy can lead to other problems. Removal of meniscal tissue decreases the contact stress area, which increases stress on the articular cartilage, and inevitably leads to degeneration of the involved joint.9 Researchers have shown that even partial meniscectomy produces late articular cartilage changes.10
Which interventions and when?
We conducted an in-depth literature review to determine which approaches were best for the treatment of OA and meniscal tears, and summarize our findings below, according to OA severity. (For details of how the literature review was conducted and an at-a-glance summary of the key findings, see the TABLE.4,5,11-17) Of note: All of the studies reviewed here included patients with chronic knee pain and excluded patients with sudden onset pain from a single physical event.
The findings: Early OA and meniscal tears
The first 2 studies we identified in the literature, both published by Herrlin et al,11,12 examined the efficacy of APM in middle-aged patients with early OA (≤grade 1), according to the Ahlbäck classification.
In the first Herrlin study, a 6-month prospective randomized trial, 90 middle-aged patients with a medial meniscal tear (without traumatic history) were assigned to either APM followed by supervised exercise or 8 weeks of supervised exercise alone.11 Exercise consisted of activities for improving muscle strength, endurance, and flexibility, as well as for balance and proprioception. The authors concluded that a combination of APM and supervised exercise did not lead to greater improvements in knee function compared with supervised exercise alone.11
In the second Herrlin study, a prospective randomized study involving 96 middle-aged patients with an MRI-verified medial meniscal tear and radiographic OA, the authors concluded that arthroscopic surgery followed by exercise therapy was not superior to the same exercise therapy alone.12 The results were gleaned from both patient-reported outcomes and radiographic assessment at 2 and 5 years. Both groups reported significant improvements at 5 years, but participants did not reach the level of fitness and quality of life of similarly-aged healthy controls.
Perhaps one of the most interesting aspects of this study was that approximately one-third of patients from the exercise-only group still had disabling knee symptoms after exercise therapy, but improved to the same level as the rest of the patients after crossing over and undergoing APM.12 Part of the observed benefit of arthroscopy in these patients has the potential to be explained by the placebo effect, especially given that invasive procedures have a stronger placebo effect than do noninvasive ones, and due to the lack of blinding.18 Additionally, limitations of the above studies include small sample sizes, lack of a control group, and short-term follow-up.
Next, a 2013 study by Yim et al looked specifically at APM vs nonoperative treatment with strengthening exercises.13 A total of 102 patients with an average age of 53.8 years, a Kellgren-Lawrence Classification of Osteoarthritis of <2, and an MRI-confirmed degenerative horizontal tear of the posterior horn of the medial meniscus were randomized to the 2 intervention groups. The 2 groups were highly comparable, giving the study high internal validity. These patients were then assessed at 3 months, one year, and at 2 years after treatment.
Although most patients at the outset of the study had intense knee pain with mechanical symptoms, both groups reported a decrease in experienced knee pain, improved function, and a high level of satisfaction with their prescribed treatment, with no significant difference in any of these values after 2 years of follow-up.13 A limitation of the study was that it used subjective questionnaires to assess pain, swelling, and activities of daily living (ADLs).
A fourth study, a 2013 multicenter, randomized, sham-controlled trial, looked at 146 patients ages 35 to 65 years who had knee symptoms consistent with a degenerative medial meniscus tear (confirmed by MRI and arthroscopic evaluation) and no knee arthritis.4 The subjects were assigned to either APM or sham surgery (skin incisions only). The results showed that APM was not superior to sham surgery with regard to outcomes assessed during a 12-month follow-up period.4
Most recently (2016), Kise et al14 published the results of a randomized controlled superiority trial conducted in Norway comparing 12 weeks of supervised exercise therapy with APM for patients with degenerative meniscus tears. Their study included 140 patients ages 36 to 60 years. Notably, most (96%), but not all, of their patients had no radiographic evidence of OA.
At the 2-year follow-up, there were no differences in patient-reported pain or functional outcomes, with all patients improving significantly from baseline. Muscle strength was also measured and found to be significantly greater at 3 and 12 months in the exercise group. Limitations of this study were a lack of patient blinding and a 19% crossover from the exercise to the APM group.
The findings: Mild to moderate OA and meniscal tears
The authors of a 2002 double-blind, placebo-controlled trial randomly assigned 180 patients with degenerative meniscus tears and knee OA to either arthroscopic debridement, arthroscopic lavage, or placebo surgery consisting of superficial skin incisions without insertion of an arthroscope.5 Patients were eligible if they were ≤75 years of age and had not undergone arthroscopy of the knee within the previous 2 years. Arthritis was graded utilizing the Kellgren-Lawrence scale (0-4) and by calculating a severity score (0-12) by adding the individual scores for each of the 3 compartments of the knee; patients were excluded if they had a severity grade ≥9. One-quarter of the participants had severe arthritis, with scores of 7 or 8. Outcomes were measured over a 24-month period. At no point did either of the intervention groups report less pain or better function than the sham-surgery group.5
Additionally, in a single-center RCT involving 178 patients with mild to severe OA, subjects were randomly assigned to surgical lavage and arthroscopic debridement together with optimized physical and medical therapy or to treatment with physical and medical therapy alone.15 Patients were excluded if they had “bucket handle” meniscal tears detected by physical exam or by MRI, previous arthroscopic surgery, previous major knee trauma, or steroid injections in the last 3 months. All participants were required to have Kellgren-Lawrence grade 2, 3, or 4 arthritis.
The researchers used 2 validated outcome measures to evaluate pain, symptoms, and functional ability and followed the patients for 2 years after the initiation of treatment. This study failed to show that arthroscopic surgery provided any additional benefit to optimized physical and medical therapy.15
A 2013 multicenter, RCT randomly assigned 351 patients ≥45 years with a meniscal tear and radiographic evidence of mild to moderate OA to either surgery and postoperative physical therapy or to a standardized physical therapy regimen.16 Patients were required to have at least one symptom that was consistent with a meniscal tear for approximately one month. About 30% of the patients who were assigned to physical therapy alone underwent surgery within 6 months. There were no significant differences between the 2 groups in the magnitude of improvement in functional status or pain at 6 or 12 months.
Finally, a prospective Scottish study consisting of 270 patients with everything from no signs of OA to advanced OA who underwent APM were sent preoperative and 6-month postop questionnaires evaluating ADLs, pain, symptoms, quality of life, and body mass index (BMI).17 Their OA was graded via preop MRI or radiographs and confirmed by the operating surgeon. The investigators were unable to demonstrate any significant benefit associated with arthroscopic meniscectomy, and, therefore, could not recommend the procedure for patients with moderate to advanced OA.
However, in their analysis, 3 subgroup populations were found to benefit from APM: those with greater body habitus (BMI >30 kg/m2), those without signs of OA, and those with early OA. Limitations of this study included the lack of randomization, blinding, a control, and long-term follow up, and that the authors didn’t use established OA grading criteria.17
The bottom line: Nonoperative treatment benefits most patients
Physical therapy is an appropriate first-line treatment for degenerative meniscus tears in middle-aged patients. In fact, a trial of nonoperative treatment is likely to benefit the majority of patients. In addition, avoiding surgery eliminates surgical complications and decreases overall health care costs.
Reserve APM for those patients without significant OA who fail to improve after physical therapy, who have mechanical symptoms, or who have intra-articular loose bodies.
In addition, exercise therapy is an effective treatment for patients with knee OA. It improves function and limits joint pain in both acute arthritic flares and more long-term, chronic situations. There is strong evidence that strengthening plays a critical role in reducing symptoms and improving muscle strength, physical ability, and quality of life.19 It has been suggested that physical exercise 3 times a week for 4 months could lead to >35% improvement of knee function.20 In contrast, other studies reported that while 91% of patients 11.5 years after APM considered their knees “normal or almost normal,” patients actually experienced a reduction in postoperative physical activity and quality of life.21
The most recent American Academy of Orthopaedic Surgeons (AAOS) guidelines22 do not recommend for or against arthroscopic partial meniscectomy in patients with knee OA and a torn meniscus. In middle-aged patients, MRI abnormalities of the meniscus do not consistently correlate with symptoms. Many meniscus lesions are asymptomatic or not the primary source of pain in the setting of OA.3
Potential harms and considerations. Deep venous thrombosis is the most frequently reported adverse event of arthroscopic surgery, with an approximate incidence of 4.13 per 1000 procedures, followed by less frequent complications such as infection, pulmonary embolism, and death.18
It is important to note that the degenerative meniscus tears that occur in middle age and that are associated with OA are not the same as acute, traumatic meniscus tears. All of the studies discussed here included patients with chronic knee pain and excluded patients with sudden onset pain from a single physical event. Many of the studies excluded patients with bucket-handle tears or severe mechanical symptoms (ie, locking). APM may be indicated for these meniscus tears regardless of age or OA status.
Making a sensible choice. Ultimately, physicians and their patients must use the best evidence available to make sensible clinical decisions. The ability to retain native meniscal tissue is of utmost importance to maintaining the longevity of their knee. According to previous studies, OA progression is more likely to occur after meniscectomy than after nonoperative treatment.23
CORRESPONDENCE
William Bassett, MD, Department of Orthopedics, Rutgers-Robert Wood Johnson Medical School, 51 French St., PO Box 19, New Brunswick, NJ 08903; [email protected].
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11:1-25.
2. Hall MJ, Lawrence L. Ambulatory surgery in the United States, 1996. Advance data from vital and health statistics of the Centers for Disease Control and Prevention/National Center for Health Statistics. No. 300. August 12, 1998. Available at: https://cdc.gov/nchs/data/ad/ad300.pdf. Accessed October 11, 2017.
3. Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108-1115.
4. Sihvonen R, Paavola M, Malmivaara A, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515-2524.
5. Moseley JB, O’Malley KO, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
6. Bhattacharyya T, Gale D, Dewire P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg. 2003;85:4-9.
7. Paxton ES, Stock MV, Brophy RH. Meniscal repair versus partial meniscectomy: a systematic review comparing reoperation rates and clinical outcomes. Arthroscopy. 2011;27:1275-1288.
8. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B:664-670.
9. Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90-95.
10. Morgan CD, Wojtys EM, Casscells CD, et al. Arthroscopic meniscal repair evaluated by second-look arthroscopy. Am J Sports Med. 1991;19:632-638.
11. Herrlin S, Hållander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393-401.
12. Herrlin SV, Wange PO, Lapidus G, et al. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2013;21:358-364.
13. Yim JH, Seon JK, Song EK, et al. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. Am J Sports Med. 2013;41:1565-1570.
14. Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ. 2016;354:i3740.
15. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097-1107.
16. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
17. Bailey O, Gronkowski K, Leach WJ. Effect of body mass index and osteoarthritis on outcomes following arthroscopic meniscectomy: A prospective nationwide study. The Knee. 2015;22:95-99.
18. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
19. Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scan J Med Sci Sports. 2006;16(Suppl1):3-63.
20. Mangione KK, McCully K, Gloviak A, et al. The effects of high-intensity and low-intensity cycle ergometry in older adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 1999;54:M184-190.
21. Chatain F, Robinson AHN, Adeleine P, et al. The natural history of the knee following arthroscopic medial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2001;9:15-18.
22. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee. Evidence-based guideline. 2nd ed. May 18, 2013. Available at: https://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Accessed October 11, 2017.
23. Lohmander LS, Thorlund JB, Roos EM. Routine knee arthroscopic surgery for the painful knee in middle-aged and old patients—time to abandon ship. Acta Orthop. 2016;87:2-4.
Meniscectomy is the most common orthopedic procedure performed in the United States with 700,000 meniscectomies performed every year.1 More than half of these procedures are performed in patients ≥45 years of age,2 giving rise to the question: Does arthroscopic surgery have a role in the treatment of patients who may have osteoarthritis (OA) and another knee condition, such as a symptomatic meniscal tear? Determining the answer is especially important when you consider that the number of true and incidental tears diagnosed on magnetic resonance imaging (MRI) has been on the rise3—a result of the routine use of MRIs to identify the cause of patients’ chronic knee pain.
At a cost of roughly $5,000 per procedure, some experts have suggested that at least a portion of the approximately $4 billion annual direct medical costs associated with meniscectomy could be put to better use.4,5 This prompted us to wonder what the literature tells us about the management of degenerative meniscus tears in middle-aged patients with OA and whether these patients would benefit from nonoperative management with optimized physical and medical therapy as a first-line approach. Our findings follow.
But first, a word about the connection between OA and meniscal tears.
What we’ve learned about meniscal damage
Research has shown that over one-third of individuals >50 years of age and three-quarters of people with knee OA have degenerative meniscal tears.6 In the past, the relative paucity of epidemiologic data on the prevalence of meniscal tears in the general population made it difficult to interpret the diagnostic information provided by MRI.
More recently, experts found that meniscal damage is especially prevalent among individuals with OA, and they began treating with arthroscopic partial meniscectomy (APM), as the meniscal damage was thought to be the anatomical foundation for the complaint of knee pain.7
However, researchers then began realizing that many patients with findings of a meniscal tear visualized on MRI reported no knee symptoms. In one study, adults in a large community-based sample found to have a meniscal tear on MRI were no more likely to have knee pain than subjects without a meniscal tear.6 Similarly, subjects with a meniscal tear and OA had no more severe pain than subjects with OA and no meniscal tear.6
In addition, the landmark Fairbank study from 19488 and others since have shown that meniscectomy can lead to other problems. Removal of meniscal tissue decreases the contact stress area, which increases stress on the articular cartilage, and inevitably leads to degeneration of the involved joint.9 Researchers have shown that even partial meniscectomy produces late articular cartilage changes.10
Which interventions and when?
We conducted an in-depth literature review to determine which approaches were best for the treatment of OA and meniscal tears, and summarize our findings below, according to OA severity. (For details of how the literature review was conducted and an at-a-glance summary of the key findings, see the TABLE.4,5,11-17) Of note: All of the studies reviewed here included patients with chronic knee pain and excluded patients with sudden onset pain from a single physical event.
The findings: Early OA and meniscal tears
The first 2 studies we identified in the literature, both published by Herrlin et al,11,12 examined the efficacy of APM in middle-aged patients with early OA (≤grade 1), according to the Ahlbäck classification.
In the first Herrlin study, a 6-month prospective randomized trial, 90 middle-aged patients with a medial meniscal tear (without traumatic history) were assigned to either APM followed by supervised exercise or 8 weeks of supervised exercise alone.11 Exercise consisted of activities for improving muscle strength, endurance, and flexibility, as well as for balance and proprioception. The authors concluded that a combination of APM and supervised exercise did not lead to greater improvements in knee function compared with supervised exercise alone.11
In the second Herrlin study, a prospective randomized study involving 96 middle-aged patients with an MRI-verified medial meniscal tear and radiographic OA, the authors concluded that arthroscopic surgery followed by exercise therapy was not superior to the same exercise therapy alone.12 The results were gleaned from both patient-reported outcomes and radiographic assessment at 2 and 5 years. Both groups reported significant improvements at 5 years, but participants did not reach the level of fitness and quality of life of similarly-aged healthy controls.
Perhaps one of the most interesting aspects of this study was that approximately one-third of patients from the exercise-only group still had disabling knee symptoms after exercise therapy, but improved to the same level as the rest of the patients after crossing over and undergoing APM.12 Part of the observed benefit of arthroscopy in these patients has the potential to be explained by the placebo effect, especially given that invasive procedures have a stronger placebo effect than do noninvasive ones, and due to the lack of blinding.18 Additionally, limitations of the above studies include small sample sizes, lack of a control group, and short-term follow-up.
Next, a 2013 study by Yim et al looked specifically at APM vs nonoperative treatment with strengthening exercises.13 A total of 102 patients with an average age of 53.8 years, a Kellgren-Lawrence Classification of Osteoarthritis of <2, and an MRI-confirmed degenerative horizontal tear of the posterior horn of the medial meniscus were randomized to the 2 intervention groups. The 2 groups were highly comparable, giving the study high internal validity. These patients were then assessed at 3 months, one year, and at 2 years after treatment.
Although most patients at the outset of the study had intense knee pain with mechanical symptoms, both groups reported a decrease in experienced knee pain, improved function, and a high level of satisfaction with their prescribed treatment, with no significant difference in any of these values after 2 years of follow-up.13 A limitation of the study was that it used subjective questionnaires to assess pain, swelling, and activities of daily living (ADLs).
A fourth study, a 2013 multicenter, randomized, sham-controlled trial, looked at 146 patients ages 35 to 65 years who had knee symptoms consistent with a degenerative medial meniscus tear (confirmed by MRI and arthroscopic evaluation) and no knee arthritis.4 The subjects were assigned to either APM or sham surgery (skin incisions only). The results showed that APM was not superior to sham surgery with regard to outcomes assessed during a 12-month follow-up period.4
Most recently (2016), Kise et al14 published the results of a randomized controlled superiority trial conducted in Norway comparing 12 weeks of supervised exercise therapy with APM for patients with degenerative meniscus tears. Their study included 140 patients ages 36 to 60 years. Notably, most (96%), but not all, of their patients had no radiographic evidence of OA.
At the 2-year follow-up, there were no differences in patient-reported pain or functional outcomes, with all patients improving significantly from baseline. Muscle strength was also measured and found to be significantly greater at 3 and 12 months in the exercise group. Limitations of this study were a lack of patient blinding and a 19% crossover from the exercise to the APM group.
The findings: Mild to moderate OA and meniscal tears
The authors of a 2002 double-blind, placebo-controlled trial randomly assigned 180 patients with degenerative meniscus tears and knee OA to either arthroscopic debridement, arthroscopic lavage, or placebo surgery consisting of superficial skin incisions without insertion of an arthroscope.5 Patients were eligible if they were ≤75 years of age and had not undergone arthroscopy of the knee within the previous 2 years. Arthritis was graded utilizing the Kellgren-Lawrence scale (0-4) and by calculating a severity score (0-12) by adding the individual scores for each of the 3 compartments of the knee; patients were excluded if they had a severity grade ≥9. One-quarter of the participants had severe arthritis, with scores of 7 or 8. Outcomes were measured over a 24-month period. At no point did either of the intervention groups report less pain or better function than the sham-surgery group.5
Additionally, in a single-center RCT involving 178 patients with mild to severe OA, subjects were randomly assigned to surgical lavage and arthroscopic debridement together with optimized physical and medical therapy or to treatment with physical and medical therapy alone.15 Patients were excluded if they had “bucket handle” meniscal tears detected by physical exam or by MRI, previous arthroscopic surgery, previous major knee trauma, or steroid injections in the last 3 months. All participants were required to have Kellgren-Lawrence grade 2, 3, or 4 arthritis.
The researchers used 2 validated outcome measures to evaluate pain, symptoms, and functional ability and followed the patients for 2 years after the initiation of treatment. This study failed to show that arthroscopic surgery provided any additional benefit to optimized physical and medical therapy.15
A 2013 multicenter, RCT randomly assigned 351 patients ≥45 years with a meniscal tear and radiographic evidence of mild to moderate OA to either surgery and postoperative physical therapy or to a standardized physical therapy regimen.16 Patients were required to have at least one symptom that was consistent with a meniscal tear for approximately one month. About 30% of the patients who were assigned to physical therapy alone underwent surgery within 6 months. There were no significant differences between the 2 groups in the magnitude of improvement in functional status or pain at 6 or 12 months.
Finally, a prospective Scottish study consisting of 270 patients with everything from no signs of OA to advanced OA who underwent APM were sent preoperative and 6-month postop questionnaires evaluating ADLs, pain, symptoms, quality of life, and body mass index (BMI).17 Their OA was graded via preop MRI or radiographs and confirmed by the operating surgeon. The investigators were unable to demonstrate any significant benefit associated with arthroscopic meniscectomy, and, therefore, could not recommend the procedure for patients with moderate to advanced OA.
However, in their analysis, 3 subgroup populations were found to benefit from APM: those with greater body habitus (BMI >30 kg/m2), those without signs of OA, and those with early OA. Limitations of this study included the lack of randomization, blinding, a control, and long-term follow up, and that the authors didn’t use established OA grading criteria.17
The bottom line: Nonoperative treatment benefits most patients
Physical therapy is an appropriate first-line treatment for degenerative meniscus tears in middle-aged patients. In fact, a trial of nonoperative treatment is likely to benefit the majority of patients. In addition, avoiding surgery eliminates surgical complications and decreases overall health care costs.
Reserve APM for those patients without significant OA who fail to improve after physical therapy, who have mechanical symptoms, or who have intra-articular loose bodies.
In addition, exercise therapy is an effective treatment for patients with knee OA. It improves function and limits joint pain in both acute arthritic flares and more long-term, chronic situations. There is strong evidence that strengthening plays a critical role in reducing symptoms and improving muscle strength, physical ability, and quality of life.19 It has been suggested that physical exercise 3 times a week for 4 months could lead to >35% improvement of knee function.20 In contrast, other studies reported that while 91% of patients 11.5 years after APM considered their knees “normal or almost normal,” patients actually experienced a reduction in postoperative physical activity and quality of life.21
The most recent American Academy of Orthopaedic Surgeons (AAOS) guidelines22 do not recommend for or against arthroscopic partial meniscectomy in patients with knee OA and a torn meniscus. In middle-aged patients, MRI abnormalities of the meniscus do not consistently correlate with symptoms. Many meniscus lesions are asymptomatic or not the primary source of pain in the setting of OA.3
Potential harms and considerations. Deep venous thrombosis is the most frequently reported adverse event of arthroscopic surgery, with an approximate incidence of 4.13 per 1000 procedures, followed by less frequent complications such as infection, pulmonary embolism, and death.18
It is important to note that the degenerative meniscus tears that occur in middle age and that are associated with OA are not the same as acute, traumatic meniscus tears. All of the studies discussed here included patients with chronic knee pain and excluded patients with sudden onset pain from a single physical event. Many of the studies excluded patients with bucket-handle tears or severe mechanical symptoms (ie, locking). APM may be indicated for these meniscus tears regardless of age or OA status.
Making a sensible choice. Ultimately, physicians and their patients must use the best evidence available to make sensible clinical decisions. The ability to retain native meniscal tissue is of utmost importance to maintaining the longevity of their knee. According to previous studies, OA progression is more likely to occur after meniscectomy than after nonoperative treatment.23
CORRESPONDENCE
William Bassett, MD, Department of Orthopedics, Rutgers-Robert Wood Johnson Medical School, 51 French St., PO Box 19, New Brunswick, NJ 08903; [email protected].
Meniscectomy is the most common orthopedic procedure performed in the United States with 700,000 meniscectomies performed every year.1 More than half of these procedures are performed in patients ≥45 years of age,2 giving rise to the question: Does arthroscopic surgery have a role in the treatment of patients who may have osteoarthritis (OA) and another knee condition, such as a symptomatic meniscal tear? Determining the answer is especially important when you consider that the number of true and incidental tears diagnosed on magnetic resonance imaging (MRI) has been on the rise3—a result of the routine use of MRIs to identify the cause of patients’ chronic knee pain.
At a cost of roughly $5,000 per procedure, some experts have suggested that at least a portion of the approximately $4 billion annual direct medical costs associated with meniscectomy could be put to better use.4,5 This prompted us to wonder what the literature tells us about the management of degenerative meniscus tears in middle-aged patients with OA and whether these patients would benefit from nonoperative management with optimized physical and medical therapy as a first-line approach. Our findings follow.
But first, a word about the connection between OA and meniscal tears.
What we’ve learned about meniscal damage
Research has shown that over one-third of individuals >50 years of age and three-quarters of people with knee OA have degenerative meniscal tears.6 In the past, the relative paucity of epidemiologic data on the prevalence of meniscal tears in the general population made it difficult to interpret the diagnostic information provided by MRI.
More recently, experts found that meniscal damage is especially prevalent among individuals with OA, and they began treating with arthroscopic partial meniscectomy (APM), as the meniscal damage was thought to be the anatomical foundation for the complaint of knee pain.7
However, researchers then began realizing that many patients with findings of a meniscal tear visualized on MRI reported no knee symptoms. In one study, adults in a large community-based sample found to have a meniscal tear on MRI were no more likely to have knee pain than subjects without a meniscal tear.6 Similarly, subjects with a meniscal tear and OA had no more severe pain than subjects with OA and no meniscal tear.6
In addition, the landmark Fairbank study from 19488 and others since have shown that meniscectomy can lead to other problems. Removal of meniscal tissue decreases the contact stress area, which increases stress on the articular cartilage, and inevitably leads to degeneration of the involved joint.9 Researchers have shown that even partial meniscectomy produces late articular cartilage changes.10
Which interventions and when?
We conducted an in-depth literature review to determine which approaches were best for the treatment of OA and meniscal tears, and summarize our findings below, according to OA severity. (For details of how the literature review was conducted and an at-a-glance summary of the key findings, see the TABLE.4,5,11-17) Of note: All of the studies reviewed here included patients with chronic knee pain and excluded patients with sudden onset pain from a single physical event.
The findings: Early OA and meniscal tears
The first 2 studies we identified in the literature, both published by Herrlin et al,11,12 examined the efficacy of APM in middle-aged patients with early OA (≤grade 1), according to the Ahlbäck classification.
In the first Herrlin study, a 6-month prospective randomized trial, 90 middle-aged patients with a medial meniscal tear (without traumatic history) were assigned to either APM followed by supervised exercise or 8 weeks of supervised exercise alone.11 Exercise consisted of activities for improving muscle strength, endurance, and flexibility, as well as for balance and proprioception. The authors concluded that a combination of APM and supervised exercise did not lead to greater improvements in knee function compared with supervised exercise alone.11
In the second Herrlin study, a prospective randomized study involving 96 middle-aged patients with an MRI-verified medial meniscal tear and radiographic OA, the authors concluded that arthroscopic surgery followed by exercise therapy was not superior to the same exercise therapy alone.12 The results were gleaned from both patient-reported outcomes and radiographic assessment at 2 and 5 years. Both groups reported significant improvements at 5 years, but participants did not reach the level of fitness and quality of life of similarly-aged healthy controls.
Perhaps one of the most interesting aspects of this study was that approximately one-third of patients from the exercise-only group still had disabling knee symptoms after exercise therapy, but improved to the same level as the rest of the patients after crossing over and undergoing APM.12 Part of the observed benefit of arthroscopy in these patients has the potential to be explained by the placebo effect, especially given that invasive procedures have a stronger placebo effect than do noninvasive ones, and due to the lack of blinding.18 Additionally, limitations of the above studies include small sample sizes, lack of a control group, and short-term follow-up.
Next, a 2013 study by Yim et al looked specifically at APM vs nonoperative treatment with strengthening exercises.13 A total of 102 patients with an average age of 53.8 years, a Kellgren-Lawrence Classification of Osteoarthritis of <2, and an MRI-confirmed degenerative horizontal tear of the posterior horn of the medial meniscus were randomized to the 2 intervention groups. The 2 groups were highly comparable, giving the study high internal validity. These patients were then assessed at 3 months, one year, and at 2 years after treatment.
Although most patients at the outset of the study had intense knee pain with mechanical symptoms, both groups reported a decrease in experienced knee pain, improved function, and a high level of satisfaction with their prescribed treatment, with no significant difference in any of these values after 2 years of follow-up.13 A limitation of the study was that it used subjective questionnaires to assess pain, swelling, and activities of daily living (ADLs).
A fourth study, a 2013 multicenter, randomized, sham-controlled trial, looked at 146 patients ages 35 to 65 years who had knee symptoms consistent with a degenerative medial meniscus tear (confirmed by MRI and arthroscopic evaluation) and no knee arthritis.4 The subjects were assigned to either APM or sham surgery (skin incisions only). The results showed that APM was not superior to sham surgery with regard to outcomes assessed during a 12-month follow-up period.4
Most recently (2016), Kise et al14 published the results of a randomized controlled superiority trial conducted in Norway comparing 12 weeks of supervised exercise therapy with APM for patients with degenerative meniscus tears. Their study included 140 patients ages 36 to 60 years. Notably, most (96%), but not all, of their patients had no radiographic evidence of OA.
At the 2-year follow-up, there were no differences in patient-reported pain or functional outcomes, with all patients improving significantly from baseline. Muscle strength was also measured and found to be significantly greater at 3 and 12 months in the exercise group. Limitations of this study were a lack of patient blinding and a 19% crossover from the exercise to the APM group.
The findings: Mild to moderate OA and meniscal tears
The authors of a 2002 double-blind, placebo-controlled trial randomly assigned 180 patients with degenerative meniscus tears and knee OA to either arthroscopic debridement, arthroscopic lavage, or placebo surgery consisting of superficial skin incisions without insertion of an arthroscope.5 Patients were eligible if they were ≤75 years of age and had not undergone arthroscopy of the knee within the previous 2 years. Arthritis was graded utilizing the Kellgren-Lawrence scale (0-4) and by calculating a severity score (0-12) by adding the individual scores for each of the 3 compartments of the knee; patients were excluded if they had a severity grade ≥9. One-quarter of the participants had severe arthritis, with scores of 7 or 8. Outcomes were measured over a 24-month period. At no point did either of the intervention groups report less pain or better function than the sham-surgery group.5
Additionally, in a single-center RCT involving 178 patients with mild to severe OA, subjects were randomly assigned to surgical lavage and arthroscopic debridement together with optimized physical and medical therapy or to treatment with physical and medical therapy alone.15 Patients were excluded if they had “bucket handle” meniscal tears detected by physical exam or by MRI, previous arthroscopic surgery, previous major knee trauma, or steroid injections in the last 3 months. All participants were required to have Kellgren-Lawrence grade 2, 3, or 4 arthritis.
The researchers used 2 validated outcome measures to evaluate pain, symptoms, and functional ability and followed the patients for 2 years after the initiation of treatment. This study failed to show that arthroscopic surgery provided any additional benefit to optimized physical and medical therapy.15
A 2013 multicenter, RCT randomly assigned 351 patients ≥45 years with a meniscal tear and radiographic evidence of mild to moderate OA to either surgery and postoperative physical therapy or to a standardized physical therapy regimen.16 Patients were required to have at least one symptom that was consistent with a meniscal tear for approximately one month. About 30% of the patients who were assigned to physical therapy alone underwent surgery within 6 months. There were no significant differences between the 2 groups in the magnitude of improvement in functional status or pain at 6 or 12 months.
Finally, a prospective Scottish study consisting of 270 patients with everything from no signs of OA to advanced OA who underwent APM were sent preoperative and 6-month postop questionnaires evaluating ADLs, pain, symptoms, quality of life, and body mass index (BMI).17 Their OA was graded via preop MRI or radiographs and confirmed by the operating surgeon. The investigators were unable to demonstrate any significant benefit associated with arthroscopic meniscectomy, and, therefore, could not recommend the procedure for patients with moderate to advanced OA.
However, in their analysis, 3 subgroup populations were found to benefit from APM: those with greater body habitus (BMI >30 kg/m2), those without signs of OA, and those with early OA. Limitations of this study included the lack of randomization, blinding, a control, and long-term follow up, and that the authors didn’t use established OA grading criteria.17
The bottom line: Nonoperative treatment benefits most patients
Physical therapy is an appropriate first-line treatment for degenerative meniscus tears in middle-aged patients. In fact, a trial of nonoperative treatment is likely to benefit the majority of patients. In addition, avoiding surgery eliminates surgical complications and decreases overall health care costs.
Reserve APM for those patients without significant OA who fail to improve after physical therapy, who have mechanical symptoms, or who have intra-articular loose bodies.
In addition, exercise therapy is an effective treatment for patients with knee OA. It improves function and limits joint pain in both acute arthritic flares and more long-term, chronic situations. There is strong evidence that strengthening plays a critical role in reducing symptoms and improving muscle strength, physical ability, and quality of life.19 It has been suggested that physical exercise 3 times a week for 4 months could lead to >35% improvement of knee function.20 In contrast, other studies reported that while 91% of patients 11.5 years after APM considered their knees “normal or almost normal,” patients actually experienced a reduction in postoperative physical activity and quality of life.21
The most recent American Academy of Orthopaedic Surgeons (AAOS) guidelines22 do not recommend for or against arthroscopic partial meniscectomy in patients with knee OA and a torn meniscus. In middle-aged patients, MRI abnormalities of the meniscus do not consistently correlate with symptoms. Many meniscus lesions are asymptomatic or not the primary source of pain in the setting of OA.3
Potential harms and considerations. Deep venous thrombosis is the most frequently reported adverse event of arthroscopic surgery, with an approximate incidence of 4.13 per 1000 procedures, followed by less frequent complications such as infection, pulmonary embolism, and death.18
It is important to note that the degenerative meniscus tears that occur in middle age and that are associated with OA are not the same as acute, traumatic meniscus tears. All of the studies discussed here included patients with chronic knee pain and excluded patients with sudden onset pain from a single physical event. Many of the studies excluded patients with bucket-handle tears or severe mechanical symptoms (ie, locking). APM may be indicated for these meniscus tears regardless of age or OA status.
Making a sensible choice. Ultimately, physicians and their patients must use the best evidence available to make sensible clinical decisions. The ability to retain native meniscal tissue is of utmost importance to maintaining the longevity of their knee. According to previous studies, OA progression is more likely to occur after meniscectomy than after nonoperative treatment.23
CORRESPONDENCE
William Bassett, MD, Department of Orthopedics, Rutgers-Robert Wood Johnson Medical School, 51 French St., PO Box 19, New Brunswick, NJ 08903; [email protected].
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11:1-25.
2. Hall MJ, Lawrence L. Ambulatory surgery in the United States, 1996. Advance data from vital and health statistics of the Centers for Disease Control and Prevention/National Center for Health Statistics. No. 300. August 12, 1998. Available at: https://cdc.gov/nchs/data/ad/ad300.pdf. Accessed October 11, 2017.
3. Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108-1115.
4. Sihvonen R, Paavola M, Malmivaara A, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515-2524.
5. Moseley JB, O’Malley KO, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
6. Bhattacharyya T, Gale D, Dewire P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg. 2003;85:4-9.
7. Paxton ES, Stock MV, Brophy RH. Meniscal repair versus partial meniscectomy: a systematic review comparing reoperation rates and clinical outcomes. Arthroscopy. 2011;27:1275-1288.
8. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B:664-670.
9. Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90-95.
10. Morgan CD, Wojtys EM, Casscells CD, et al. Arthroscopic meniscal repair evaluated by second-look arthroscopy. Am J Sports Med. 1991;19:632-638.
11. Herrlin S, Hållander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393-401.
12. Herrlin SV, Wange PO, Lapidus G, et al. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2013;21:358-364.
13. Yim JH, Seon JK, Song EK, et al. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. Am J Sports Med. 2013;41:1565-1570.
14. Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ. 2016;354:i3740.
15. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097-1107.
16. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
17. Bailey O, Gronkowski K, Leach WJ. Effect of body mass index and osteoarthritis on outcomes following arthroscopic meniscectomy: A prospective nationwide study. The Knee. 2015;22:95-99.
18. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
19. Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scan J Med Sci Sports. 2006;16(Suppl1):3-63.
20. Mangione KK, McCully K, Gloviak A, et al. The effects of high-intensity and low-intensity cycle ergometry in older adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 1999;54:M184-190.
21. Chatain F, Robinson AHN, Adeleine P, et al. The natural history of the knee following arthroscopic medial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2001;9:15-18.
22. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee. Evidence-based guideline. 2nd ed. May 18, 2013. Available at: https://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Accessed October 11, 2017.
23. Lohmander LS, Thorlund JB, Roos EM. Routine knee arthroscopic surgery for the painful knee in middle-aged and old patients—time to abandon ship. Acta Orthop. 2016;87:2-4.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11:1-25.
2. Hall MJ, Lawrence L. Ambulatory surgery in the United States, 1996. Advance data from vital and health statistics of the Centers for Disease Control and Prevention/National Center for Health Statistics. No. 300. August 12, 1998. Available at: https://cdc.gov/nchs/data/ad/ad300.pdf. Accessed October 11, 2017.
3. Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108-1115.
4. Sihvonen R, Paavola M, Malmivaara A, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515-2524.
5. Moseley JB, O’Malley KO, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
6. Bhattacharyya T, Gale D, Dewire P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg. 2003;85:4-9.
7. Paxton ES, Stock MV, Brophy RH. Meniscal repair versus partial meniscectomy: a systematic review comparing reoperation rates and clinical outcomes. Arthroscopy. 2011;27:1275-1288.
8. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B:664-670.
9. Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90-95.
10. Morgan CD, Wojtys EM, Casscells CD, et al. Arthroscopic meniscal repair evaluated by second-look arthroscopy. Am J Sports Med. 1991;19:632-638.
11. Herrlin S, Hållander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393-401.
12. Herrlin SV, Wange PO, Lapidus G, et al. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2013;21:358-364.
13. Yim JH, Seon JK, Song EK, et al. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. Am J Sports Med. 2013;41:1565-1570.
14. Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ. 2016;354:i3740.
15. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097-1107.
16. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
17. Bailey O, Gronkowski K, Leach WJ. Effect of body mass index and osteoarthritis on outcomes following arthroscopic meniscectomy: A prospective nationwide study. The Knee. 2015;22:95-99.
18. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
19. Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scan J Med Sci Sports. 2006;16(Suppl1):3-63.
20. Mangione KK, McCully K, Gloviak A, et al. The effects of high-intensity and low-intensity cycle ergometry in older adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 1999;54:M184-190.
21. Chatain F, Robinson AHN, Adeleine P, et al. The natural history of the knee following arthroscopic medial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2001;9:15-18.
22. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee. Evidence-based guideline. 2nd ed. May 18, 2013. Available at: https://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Accessed October 11, 2017.
23. Lohmander LS, Thorlund JB, Roos EM. Routine knee arthroscopic surgery for the painful knee in middle-aged and old patients—time to abandon ship. Acta Orthop. 2016;87:2-4.
From The Journal of Family Practice | 2017;66(11):E1-E6.
PRACTICE RECOMMENDATIONS
› Start middle-aged patients with knee pain and a degenerative meniscal tear on a regimen of strengthening-based physical therapy. A
› Limit meniscectomy to patients who either have no preoperative osteoarthritis (OA) or early-stage OA, unless there is evidence of mechanical locking or intra-articular loose bodies. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Obesity: When to consider medication
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4-6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14-39). These medications have the potential to not only limit weight gain, but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—namely family practitioners—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehensive treatment plan that includes diet, physical activity, and behavioral modification.
[polldaddy:9840472]
CASE 1 Melissa C, a 27-year-old woman with obesity (BMI 33 kg/m2), hyperlipidemia, and migraine headaches, presents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she’s taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for Ms. C?
Discussion
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .”
SIDEBAR
Before prescribing antiobesity medication . . .Have a frank discussion with the patient and be sure to cover the following points:
- The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
- Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
- Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
- Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30-34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24-26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Norman S, a 52-year-old overweight man (BMI 29 kg/m2) with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma, has recently hit a plateau with his weight loss. He lost 45 pounds secondary to diet and exercise, but hasn’t been able to lose any more. He also struggles with constant hunger. His medications include metformin 1000 mg bid, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until he undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
Mr. S is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
Discussion
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. He has worked hard to lose a significant number of pounds, and is now at high risk of regaining them. That’s because his appetite has increased with his new exercise regimen, but his energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of his opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
He could try orlistat, especially if he struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for his diabetes and may also promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOM-DM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of his initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomic testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 Kathryn M, a 38-year-old woman with obesity (BMI 42 kg/m2), obstructive sleep apnea, gastroesophageal reflux disease, and depression, is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
Ms. M smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discussion
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like Ms. M, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to Ms. M that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for Ms. M because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion could also help Ms. M quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses Ms. M’s problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg could also be used and should certainly be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 William P, a 65-year-old man with obesity (BMI 39 kg/m2) who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about his weight. He lost 100 lbs following surgery and maintained his weight for 3 years, but then regained 30 lbs. He comes in for an office visit because he’s concerned about his increasing blood sugar and wants to prevent further weight gain. His medications include metformin 1000 mg bid, lisinopril 5 mg/d, carvedilol 12.5 mg bid, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. He denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for Mr. P?
Discussion
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of his heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given his need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide;48 however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37-39 Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
CORRESPONDENCE
Katherine H. Saunders, MD, DABOM, Comprehensive Weight Control Center, Division of Endocrinology, Diabetes and Metabolism, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; [email protected].
1. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
2. Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
3. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023.
4. Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
5. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
6. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24:1612-1619.
7. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
8. Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
9. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
10. US Food and Drug Administration. Drug approval package. Qsymia. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
11. Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQ® (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. Available at: http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
12. Drugs.com. Contrave approval history. Available at: https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
13. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
14. Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
15. Adipex-P package insert. Available at: http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
16. Ionamin package insert. Available at: http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
17. Lomaira package insert. Available at: https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
18. Suprenza package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
19. Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
20. Alli package labeling. Available at: http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
21. Xenical package insert. Available at: https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28, 2017.
22. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
23. Qsymia package insert. Available at: https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
24. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
25. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
26. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
27. Belviq package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
28. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
29. Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
30. O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
31. Contrave package insert. Available at: https://contrave.com/wp-content/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
32. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
33. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
34. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
35. Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
36. Saxenda package insert. Available at: http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
37. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
38. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
39. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
40. Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. [Epub ahead of print]
41. Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
42. Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. Available at: http://www.qsymiarems.com. Accessed January 16, 2017.
43. Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
44. US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
45. Belviq XR package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
46. Smith SR, O’Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
47. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
48. Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
49. Fujioka K, O’Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4-6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14-39). These medications have the potential to not only limit weight gain, but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—namely family practitioners—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehensive treatment plan that includes diet, physical activity, and behavioral modification.
[polldaddy:9840472]
CASE 1 Melissa C, a 27-year-old woman with obesity (BMI 33 kg/m2), hyperlipidemia, and migraine headaches, presents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she’s taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for Ms. C?
Discussion
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .”
SIDEBAR
Before prescribing antiobesity medication . . .Have a frank discussion with the patient and be sure to cover the following points:
- The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
- Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
- Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
- Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30-34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24-26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Norman S, a 52-year-old overweight man (BMI 29 kg/m2) with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma, has recently hit a plateau with his weight loss. He lost 45 pounds secondary to diet and exercise, but hasn’t been able to lose any more. He also struggles with constant hunger. His medications include metformin 1000 mg bid, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until he undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
Mr. S is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
Discussion
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. He has worked hard to lose a significant number of pounds, and is now at high risk of regaining them. That’s because his appetite has increased with his new exercise regimen, but his energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of his opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
He could try orlistat, especially if he struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for his diabetes and may also promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOM-DM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of his initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomic testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 Kathryn M, a 38-year-old woman with obesity (BMI 42 kg/m2), obstructive sleep apnea, gastroesophageal reflux disease, and depression, is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
Ms. M smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discussion
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like Ms. M, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to Ms. M that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for Ms. M because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion could also help Ms. M quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses Ms. M’s problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg could also be used and should certainly be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 William P, a 65-year-old man with obesity (BMI 39 kg/m2) who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about his weight. He lost 100 lbs following surgery and maintained his weight for 3 years, but then regained 30 lbs. He comes in for an office visit because he’s concerned about his increasing blood sugar and wants to prevent further weight gain. His medications include metformin 1000 mg bid, lisinopril 5 mg/d, carvedilol 12.5 mg bid, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. He denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for Mr. P?
Discussion
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of his heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given his need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide;48 however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37-39 Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
CORRESPONDENCE
Katherine H. Saunders, MD, DABOM, Comprehensive Weight Control Center, Division of Endocrinology, Diabetes and Metabolism, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; [email protected].
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4-6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14-39). These medications have the potential to not only limit weight gain, but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—namely family practitioners—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehensive treatment plan that includes diet, physical activity, and behavioral modification.
[polldaddy:9840472]
CASE 1 Melissa C, a 27-year-old woman with obesity (BMI 33 kg/m2), hyperlipidemia, and migraine headaches, presents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she’s taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for Ms. C?
Discussion
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .”
SIDEBAR
Before prescribing antiobesity medication . . .Have a frank discussion with the patient and be sure to cover the following points:
- The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
- Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
- Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
- Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30-34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24-26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Norman S, a 52-year-old overweight man (BMI 29 kg/m2) with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma, has recently hit a plateau with his weight loss. He lost 45 pounds secondary to diet and exercise, but hasn’t been able to lose any more. He also struggles with constant hunger. His medications include metformin 1000 mg bid, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until he undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
Mr. S is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
Discussion
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. He has worked hard to lose a significant number of pounds, and is now at high risk of regaining them. That’s because his appetite has increased with his new exercise regimen, but his energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of his opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
He could try orlistat, especially if he struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for his diabetes and may also promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOM-DM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of his initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomic testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 Kathryn M, a 38-year-old woman with obesity (BMI 42 kg/m2), obstructive sleep apnea, gastroesophageal reflux disease, and depression, is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
Ms. M smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discussion
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like Ms. M, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to Ms. M that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for Ms. M because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion could also help Ms. M quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses Ms. M’s problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg could also be used and should certainly be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 William P, a 65-year-old man with obesity (BMI 39 kg/m2) who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about his weight. He lost 100 lbs following surgery and maintained his weight for 3 years, but then regained 30 lbs. He comes in for an office visit because he’s concerned about his increasing blood sugar and wants to prevent further weight gain. His medications include metformin 1000 mg bid, lisinopril 5 mg/d, carvedilol 12.5 mg bid, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. He denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for Mr. P?
Discussion
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of his heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given his need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide;48 however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37-39 Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
CORRESPONDENCE
Katherine H. Saunders, MD, DABOM, Comprehensive Weight Control Center, Division of Endocrinology, Diabetes and Metabolism, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; [email protected].
1. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
2. Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
3. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023.
4. Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
5. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
6. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24:1612-1619.
7. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
8. Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
9. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
10. US Food and Drug Administration. Drug approval package. Qsymia. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
11. Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQ® (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. Available at: http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
12. Drugs.com. Contrave approval history. Available at: https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
13. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
14. Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
15. Adipex-P package insert. Available at: http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
16. Ionamin package insert. Available at: http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
17. Lomaira package insert. Available at: https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
18. Suprenza package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
19. Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
20. Alli package labeling. Available at: http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
21. Xenical package insert. Available at: https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28, 2017.
22. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
23. Qsymia package insert. Available at: https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
24. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
25. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
26. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
27. Belviq package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
28. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
29. Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
30. O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
31. Contrave package insert. Available at: https://contrave.com/wp-content/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
32. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
33. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
34. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
35. Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
36. Saxenda package insert. Available at: http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
37. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
38. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
39. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
40. Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. [Epub ahead of print]
41. Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
42. Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. Available at: http://www.qsymiarems.com. Accessed January 16, 2017.
43. Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
44. US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
45. Belviq XR package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
46. Smith SR, O’Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
47. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
48. Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
49. Fujioka K, O’Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
1. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
2. Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
3. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023.
4. Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
5. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
6. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24:1612-1619.
7. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
8. Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
9. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
10. US Food and Drug Administration. Drug approval package. Qsymia. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
11. Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQ® (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. Available at: http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
12. Drugs.com. Contrave approval history. Available at: https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
13. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
14. Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
15. Adipex-P package insert. Available at: http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
16. Ionamin package insert. Available at: http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
17. Lomaira package insert. Available at: https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
18. Suprenza package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
19. Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
20. Alli package labeling. Available at: http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
21. Xenical package insert. Available at: https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28, 2017.
22. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
23. Qsymia package insert. Available at: https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
24. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
25. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
26. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
27. Belviq package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
28. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
29. Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
30. O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
31. Contrave package insert. Available at: https://contrave.com/wp-content/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
32. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
33. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
34. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
35. Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
36. Saxenda package insert. Available at: http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
37. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
38. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
39. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
40. Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. [Epub ahead of print]
41. Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
42. Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. Available at: http://www.qsymiarems.com. Accessed January 16, 2017.
43. Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
44. US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
45. Belviq XR package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
46. Smith SR, O’Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
47. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
48. Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
49. Fujioka K, O’Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
From The Journal of Family Practice | 2017;66(10):608-616.
PRACTICE RECOMMENDATIONS
For patients with a body mass index (BMI) ≥30 kg/m2 or BMI ≥27 kg/m2 with weight-related comorbidities:
› Consider antiobesity pharmacotherapy when diet, exercise, and behavior modification do not produce sufficient weight loss. A
› Continue an antiobesity medication if it is deemed effective and well tolerated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Effectively engaging patients in everyday health-care decisions
The discipline of family medicine is committed to providing patient-centered care through recommendations that are grounded both in evidence and in patients’ personal values.1,2 The current health care environment, however, often demands heavy reliance on outcome-based performance metrics that can be insensitive to patient preferences.3 This tension necessitates models of decision-making that maximize reliance on measured performance, yet fulfill the clinician’s fiduciary responsibility to prioritize patients’ interests. The philosophy and practice of shared decision-making (SDM) can facilitate these aims.
The 3 elements of shared decision-making
SDM provides a framework for offering everyday medical advice and facilitating informed consent.4 Its 3 elements are:
- discussing with patients relevant information about their health conditions, possible treatments, and likely outcomes,
- clarifying and understanding a patient’s unique values and priorities and how they relate to the treatment options, and
- enabling a patient to select a care plan that is in keeping with his or her personal goals.5
This model is significant not only from a theoretical perspective, but also from a practical one. Studies have shown that both health outcomes and patient satisfaction improve when patients participate more actively in health care decision-making.6,7
Unfortunately, there is evidence that some decision-making practices in primary care settings remain inadequate. For example, unlike the standard disclosure of procedure risks in surgical settings, the burdens of cancer screening are frequently omitted from primary care discussions.8 Moreover, agreement about what should be disclosed, as well as how to disclose it, is still not sufficient. The following 3 recommendations, one for each element of SDM, aim to help clinicians effectively engage patients in everyday decision-making.
1. Provide patients with relevant information
The first element of SDM requires discussing the health-related information that is relevant to the patient’s decision-making process. The literature about informed consent supports explaining the risks that are common, as well as those that are particularly dangerous, and the likely benefits of recommended treatment, nontreatment, and alternative treatments.9 Moreover, adequate informed consent requires identifying what a reasonable person in a particular patient’s position would want to know.10
Accounting for “a patient’s position” is significant because it signals that personal factors (eg, the individual’s beliefs, goals, and familial responsibilities) are as important as the patient’s external clinical situation and what can be known by reviewing medical evidence. Incorporating a patient’s particular circumstances distinguishes patient-centered care from the mechanical application of generic best practices. This is the standard for what information should be provided.
When evidence is lacking. Clinicians facilitating decisions for which data is lacking should convey the best available evidence, including the inherent uncertainties. Like evidence-based medicine (EBM), the principles of SDM should be at work in most clinical encounters. The extent to which one engages in SDM depends upon the seriousness of the proposed interventions, the degree to which the decision is preference-sensitive, and the availability of evidence.
Statistics: Explain absolute and baseline risk
It is generally better to provide absolute risk rather than relative risk, because people perceive absolute risk reductions more accurately.11 Presentation of risks, in terms of relative risk or relative risk reduction, typically exaggerates the benefits of treatment, especially when the risk is small. This exaggeration makes it more likely that patients will accept interventions they might otherwise have rejected after reviewing the data more fully. Furthermore, relative risk statistics can impact clinicians’ perceptions, leading them to recommend an intervention more often than they might when absolute risk statistics are discussed.12
But even absolute risk, if presented on its own, can be misleading. A reasonable person trying to determine the value of an intervention needs to know his or her baseline risk of an event, in addition to his or her absolute risk with the intervention. For example, a hypothetical absolute risk reduction associated with a breast cancer treatment of 10% has different meanings, depending upon whether the baseline risk is 10% or 80%. A reduction from 10% to 0% would be a miraculous cure, while going from 80% to 70% may be viewed as only a slight improvement. Try as we might to present a single neat statistic, presenting both baseline risk and absolute risk with intervention is often necessary.13
How to effectively communicate medical information
Many patients struggle with processing information that is expressed as a probability.14 Patients process frequencies (eg, 10 in 100) better than probabilities (eg, 10%), and there is evidence that they understand best when decision aids are used.15 Decision aids, such as pictographs (FIGURE16), are supplementary, evidence-based tools for effectively communicating with patients and their families in a way that facilitates comparison between available options. Such aids are readily available online for many conditions or can be created using various software tools.16,17
Pictographs reveal that there is a values-sensitive decision to be made and visually demonstrate the outcomes associated with each option. Both pictographs and bar graphs have been shown to improve patient understanding and satisfaction.11 The benefit of pictographs is their ability to effectively, and simultaneously, convey both the numerator and the denominator in frequency statistics.12,18
There is high-quality evidence demonstrating that decision aids enhance an individual’s knowledge about the treatment and screening options available to them. A 2014 Cochrane review of the effects of decision aids found that they increased average knowledge scores when compared to usual care.15 Decision aids also improved accurate perception of risk.15 It is our belief that one of the reasons pictographs work so well is that they combine the salience of absolute risks with and without intervention.12,13
Beyond increased understanding, the Cochrane review also found high-quality evidence indicating that people who make decisions using decision aids feel less decisional conflict when compared to usual care.15 Moreover, in the context of SDM, decisional conflict may contribute to patients passing the decision-making responsibility to their clinician.19 And finally, there is moderate-quality evidence that patients are more likely to participate in decision-making when given tools such as pictographs.15
A potential barrier to putting pictographs into practice concerns perceptions that decision aids increase the length of office visits. Indeed, previous studies have identified perceived time constraints as one of the major barriers to enacting SDM in clinical settings.20 On this topic, the Cochrane review offers variable yet potentially promising results: Studies of the effects on appointment length ranged from a decrease of 8 minutes to an increase of 23 minutes.15 These results suggest that, under the right circumstances, pictographs can be used to facilitate SDM within the constraints of current clinical practice. More research is needed to determine the optimal circumstances that promote efficient SDM.
2. Elicit the patient’s unique values and priorities
Formalized approaches to building rapport with patients have been popular for more than 2 decades,21,22 and they are now routinely part of medical training. Nevertheless, there is always room for improvement when it comes to aligning treatment and screening recommendations with patient values. Some decision aids are designed to offer the added benefit of clarifying individual values and, thus, increase the likelihood that patients will make decisions that are more in line with their goals.15
When decision aids are not available to elicit patient values, clinicians can integrate preference-clarifying questions as part of the standard patient encounter.23 These questions are aimed at surfacing the values underlying what the patient wants, what the patient does not want, and most importantly, why.
“Why” matters because it ultimately helps the clinician understand the patient’s mindset, enabling the clinician to help the patient make choices that serve his or her values.24 Eliciting values not only promotes patient well-being and self-determination, but also facilitates the development of empathic patient-clinician partnerships.
Categorizing decisions. Regardless of the particular method chosen to elicit patient values, the underlying questions faced by many patients often fit into one of 2 categories: 1) Do I prefer quality of life over length of life? or 2) Am I willing to be inconvenienced now to prevent more severe illness later? Clarifying the category into which a decision falls may open the conversation and help to explore patients’ values and priorities. Alternatively, asking questions such as, “Thinking about this decision, what is the most important aspect for you to consider?”25 may facilitate the conversation.
Much of the research on techniques geared to elicit values comes from the palliative care and oncology literature.26 Although this research generally focuses on decisions about serious illness or end-of-life preferences, preference-sensitive decisions in primary care settings create a need for clinicians who are effective in eliciting patient values.
The more serious and preference-sensitive the decision, the deeper the clinician needs to explore the patient’s personal goals. Despite scant literature about seemingly innocuous decisions, we recommend that clinicians elicit from their patients a brief, but overt, acknowledgement of the values guiding their choice for most preference-sensitive decisions.
3. Offer a professional recommendation
Once clinicians have a sense of an individual’s values and priorities, they are positioned to make a professional recommendation that aligns with these values and priorities, and leaves room for the patient to reach a decision. Historically, one of the clinician’s major roles was to provide advice and recommendations to patients. For a long time, this was done without the patient’s involvement in the decision-making.27
With an increasing emphasis on patient self-determination over the last 50 years, there has been some concern that the pendulum is swinging too far in the opposite direction, with clinicians shying away from providing specific recommendations.28 Although this line of thinking acknowledges the power of the clinician to influence patients, it falls short of distinguishing between a personal recommendation and a professional one. While personal recommendations have no place in medical decision-making, clinicians should offer patients a professional recommendation, along with their rationale.
How do personal and professional recommendations differ?
Personal recommendations arise from clinicians considering what they themselves might decide if they were in the patient’s position. Such recommendations are inappropriate because every person has unique values and priorities.
In contrast, professional recommendations stem from the clinician’s knowledge of the best available evidence, his or her understanding of the patient’s values, and his or her weaving of these pieces together in the context of the patient’s specific clinical presentation. Experienced clinicians bring all 3 elements of SDM to bear in making professional recommendations, even if these recommendations are at odds with what they might choose for themselves.
EBM and SDM: Not so different after all?
Another way to understand the legitimacy of a professional recommendation is to view the parallels between SDM and EBM. From the outset, EBM positioned itself as arising from the best available evidence, the patient’s values, and clinical expertise29—elements that are strikingly similar to the components of SDM.
Although commonly overlooked, the concept of EBM recognizes that established evidence alone is not sufficient for decision-making.30 Additionally, EBM allows for making a recommendation that may not appear to be guideline-based, because guidelines typically do not take into account individual patient preferences.30-32 What’s more, both EBM and SDM highlight the essential contribution of the clinician’s judgment about his or her patient’s unique presentation.
Thus, both EBM and SDM are dependent on the professional communicating a recommendation to the patient. This communication involves not only making clear what one recommends, but also why one recommends it. For example, a clinician might say the following to a patient with worsening asthma symptoms:
“The asthma guidelines give us 2 treatment options. We can either double the dose of your inhaled corticosteroid, or start a 5-day course of corticosteroid pills. Given your concerns about the adverse effects of the pills, and the moderate severity of this exacerbation, I recommend doubling the dose of your inhaled corticosteroid. We can reconsider the pills if your symptoms worsen or if you don’t improve within the next week. How does that sound to you?”
An informed choice. Explaining the evidence, articulating the patient’s values, and summarizing the clinical elements that went into the clinician’s recommendation clarifies and signals to the patient that this is a professional recommendation. Ultimately, the process of SDM concludes with the patient considering the clinician’s recommendation and making an informed choice from the available options.
CORRESPONDENCE
David J. Satin, MD, 2020 E 28th St., Minneapolis, MN 55407; [email protected].
1. Medalie JH. Family Medicine: Principles and Applications. Baltimore, MD: Williams and Wilkins; 1978.
2. Philips RL Jr, Brundgardt S, Lesko SE, et al. The future role of the family physician in the United States: a rigorous exercise in definition. Ann Fam Med. 2014;12:250-255.
3. Berwick DM. Era 3 for medicine and health care. JAMA. 2016;315:1329-1330.
4. Edwards A, Elwyn G. Shared decision making in health care: achieving evidence-based patient choice. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2009.
5. American Medical Association. Shared decision making H-373.997. Available at: https://policysearch.ama-assn.org/policyfinder/detail/H-373.997%20?uri=%2FAMADoc%2FHOD.xml-0-3162.xml. Accessed September 6, 2017.
6. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410-417.
8. Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215-1221.
9. Spatz ES, Krumhoiz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA. 2016;315:2063-2064.
10. Faden RR, Becker C, Lewis C, et al. Disclosure of information to patients in medical care. Med Care. 1981;19:718-733.
11. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270-280.
12. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436-1443.
13. Stovitz SD, Shrier I. Medical decision making and the importance of baseline risk. Br J Gen Pract. 2013;63:e795-e797.
14. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37-44.
15. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011:CD001431.
16. Mayo Clinic. Statin choice decision aid. Available at: https://statindecisionaid.mayoclinic.org/index.php/statin/index. Accessed September 6, 2017.
17. Risk Science Center and Center for Bioethics and Social Sciences in Medicine, University of Michigan. Icon Array. Available at: http://www.iconarray.com/. Accessed September 6, 2017.
18. Price M, Cameron R, Butow P. Communicating risk information: the influence of graphical display format on quantitative information perception–accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121-128.
19. Kon AA. The shared decision making continuum. JAMA. 2010;304:903-904.
20. Légaré F1, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535.
21. Haidet P, Paterniti DA. “Building” a history rather than “taking” one: a perspective on information sharing during the medical interview. Arch Intern Med. 2003;163:1134-1140.
22. Frankel RM, Stein R. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16:184-194.
23. Delbanco TL. Enriching the doctor-patient relationship by inviting the patient’s perspective. Ann Intern Med. 1992;116:414-418.
24. Doukas DJ, McCullough LB. The values history: the evaluation of the patient’s values and advance directives. J Fam Pract. 1991;32:145-153.
25. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
26. Bernacki RE, Block SD, American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994-2003.
27. Katz J. The Silent World of Doctor and Patient. Baltimore, MD, and London, England: The Johns Hopkins University Press; 1984.
28. Baylis F, Downie J. Professional recommendations: disclosing facts and values. J Med Ethics. 2001;27:20-24.
29. Sackett DL, Rosenburg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
30. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2053-2054.
31. Mora S, Ames JM, Manson JE. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA. 2016;316:709-710.
32. Stovitz SD, Satin D, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276.
The discipline of family medicine is committed to providing patient-centered care through recommendations that are grounded both in evidence and in patients’ personal values.1,2 The current health care environment, however, often demands heavy reliance on outcome-based performance metrics that can be insensitive to patient preferences.3 This tension necessitates models of decision-making that maximize reliance on measured performance, yet fulfill the clinician’s fiduciary responsibility to prioritize patients’ interests. The philosophy and practice of shared decision-making (SDM) can facilitate these aims.
The 3 elements of shared decision-making
SDM provides a framework for offering everyday medical advice and facilitating informed consent.4 Its 3 elements are:
- discussing with patients relevant information about their health conditions, possible treatments, and likely outcomes,
- clarifying and understanding a patient’s unique values and priorities and how they relate to the treatment options, and
- enabling a patient to select a care plan that is in keeping with his or her personal goals.5
This model is significant not only from a theoretical perspective, but also from a practical one. Studies have shown that both health outcomes and patient satisfaction improve when patients participate more actively in health care decision-making.6,7
Unfortunately, there is evidence that some decision-making practices in primary care settings remain inadequate. For example, unlike the standard disclosure of procedure risks in surgical settings, the burdens of cancer screening are frequently omitted from primary care discussions.8 Moreover, agreement about what should be disclosed, as well as how to disclose it, is still not sufficient. The following 3 recommendations, one for each element of SDM, aim to help clinicians effectively engage patients in everyday decision-making.
1. Provide patients with relevant information
The first element of SDM requires discussing the health-related information that is relevant to the patient’s decision-making process. The literature about informed consent supports explaining the risks that are common, as well as those that are particularly dangerous, and the likely benefits of recommended treatment, nontreatment, and alternative treatments.9 Moreover, adequate informed consent requires identifying what a reasonable person in a particular patient’s position would want to know.10
Accounting for “a patient’s position” is significant because it signals that personal factors (eg, the individual’s beliefs, goals, and familial responsibilities) are as important as the patient’s external clinical situation and what can be known by reviewing medical evidence. Incorporating a patient’s particular circumstances distinguishes patient-centered care from the mechanical application of generic best practices. This is the standard for what information should be provided.
When evidence is lacking. Clinicians facilitating decisions for which data is lacking should convey the best available evidence, including the inherent uncertainties. Like evidence-based medicine (EBM), the principles of SDM should be at work in most clinical encounters. The extent to which one engages in SDM depends upon the seriousness of the proposed interventions, the degree to which the decision is preference-sensitive, and the availability of evidence.
Statistics: Explain absolute and baseline risk
It is generally better to provide absolute risk rather than relative risk, because people perceive absolute risk reductions more accurately.11 Presentation of risks, in terms of relative risk or relative risk reduction, typically exaggerates the benefits of treatment, especially when the risk is small. This exaggeration makes it more likely that patients will accept interventions they might otherwise have rejected after reviewing the data more fully. Furthermore, relative risk statistics can impact clinicians’ perceptions, leading them to recommend an intervention more often than they might when absolute risk statistics are discussed.12
But even absolute risk, if presented on its own, can be misleading. A reasonable person trying to determine the value of an intervention needs to know his or her baseline risk of an event, in addition to his or her absolute risk with the intervention. For example, a hypothetical absolute risk reduction associated with a breast cancer treatment of 10% has different meanings, depending upon whether the baseline risk is 10% or 80%. A reduction from 10% to 0% would be a miraculous cure, while going from 80% to 70% may be viewed as only a slight improvement. Try as we might to present a single neat statistic, presenting both baseline risk and absolute risk with intervention is often necessary.13
How to effectively communicate medical information
Many patients struggle with processing information that is expressed as a probability.14 Patients process frequencies (eg, 10 in 100) better than probabilities (eg, 10%), and there is evidence that they understand best when decision aids are used.15 Decision aids, such as pictographs (FIGURE16), are supplementary, evidence-based tools for effectively communicating with patients and their families in a way that facilitates comparison between available options. Such aids are readily available online for many conditions or can be created using various software tools.16,17
Pictographs reveal that there is a values-sensitive decision to be made and visually demonstrate the outcomes associated with each option. Both pictographs and bar graphs have been shown to improve patient understanding and satisfaction.11 The benefit of pictographs is their ability to effectively, and simultaneously, convey both the numerator and the denominator in frequency statistics.12,18
There is high-quality evidence demonstrating that decision aids enhance an individual’s knowledge about the treatment and screening options available to them. A 2014 Cochrane review of the effects of decision aids found that they increased average knowledge scores when compared to usual care.15 Decision aids also improved accurate perception of risk.15 It is our belief that one of the reasons pictographs work so well is that they combine the salience of absolute risks with and without intervention.12,13
Beyond increased understanding, the Cochrane review also found high-quality evidence indicating that people who make decisions using decision aids feel less decisional conflict when compared to usual care.15 Moreover, in the context of SDM, decisional conflict may contribute to patients passing the decision-making responsibility to their clinician.19 And finally, there is moderate-quality evidence that patients are more likely to participate in decision-making when given tools such as pictographs.15
A potential barrier to putting pictographs into practice concerns perceptions that decision aids increase the length of office visits. Indeed, previous studies have identified perceived time constraints as one of the major barriers to enacting SDM in clinical settings.20 On this topic, the Cochrane review offers variable yet potentially promising results: Studies of the effects on appointment length ranged from a decrease of 8 minutes to an increase of 23 minutes.15 These results suggest that, under the right circumstances, pictographs can be used to facilitate SDM within the constraints of current clinical practice. More research is needed to determine the optimal circumstances that promote efficient SDM.
2. Elicit the patient’s unique values and priorities
Formalized approaches to building rapport with patients have been popular for more than 2 decades,21,22 and they are now routinely part of medical training. Nevertheless, there is always room for improvement when it comes to aligning treatment and screening recommendations with patient values. Some decision aids are designed to offer the added benefit of clarifying individual values and, thus, increase the likelihood that patients will make decisions that are more in line with their goals.15
When decision aids are not available to elicit patient values, clinicians can integrate preference-clarifying questions as part of the standard patient encounter.23 These questions are aimed at surfacing the values underlying what the patient wants, what the patient does not want, and most importantly, why.
“Why” matters because it ultimately helps the clinician understand the patient’s mindset, enabling the clinician to help the patient make choices that serve his or her values.24 Eliciting values not only promotes patient well-being and self-determination, but also facilitates the development of empathic patient-clinician partnerships.
Categorizing decisions. Regardless of the particular method chosen to elicit patient values, the underlying questions faced by many patients often fit into one of 2 categories: 1) Do I prefer quality of life over length of life? or 2) Am I willing to be inconvenienced now to prevent more severe illness later? Clarifying the category into which a decision falls may open the conversation and help to explore patients’ values and priorities. Alternatively, asking questions such as, “Thinking about this decision, what is the most important aspect for you to consider?”25 may facilitate the conversation.
Much of the research on techniques geared to elicit values comes from the palliative care and oncology literature.26 Although this research generally focuses on decisions about serious illness or end-of-life preferences, preference-sensitive decisions in primary care settings create a need for clinicians who are effective in eliciting patient values.
The more serious and preference-sensitive the decision, the deeper the clinician needs to explore the patient’s personal goals. Despite scant literature about seemingly innocuous decisions, we recommend that clinicians elicit from their patients a brief, but overt, acknowledgement of the values guiding their choice for most preference-sensitive decisions.
3. Offer a professional recommendation
Once clinicians have a sense of an individual’s values and priorities, they are positioned to make a professional recommendation that aligns with these values and priorities, and leaves room for the patient to reach a decision. Historically, one of the clinician’s major roles was to provide advice and recommendations to patients. For a long time, this was done without the patient’s involvement in the decision-making.27
With an increasing emphasis on patient self-determination over the last 50 years, there has been some concern that the pendulum is swinging too far in the opposite direction, with clinicians shying away from providing specific recommendations.28 Although this line of thinking acknowledges the power of the clinician to influence patients, it falls short of distinguishing between a personal recommendation and a professional one. While personal recommendations have no place in medical decision-making, clinicians should offer patients a professional recommendation, along with their rationale.
How do personal and professional recommendations differ?
Personal recommendations arise from clinicians considering what they themselves might decide if they were in the patient’s position. Such recommendations are inappropriate because every person has unique values and priorities.
In contrast, professional recommendations stem from the clinician’s knowledge of the best available evidence, his or her understanding of the patient’s values, and his or her weaving of these pieces together in the context of the patient’s specific clinical presentation. Experienced clinicians bring all 3 elements of SDM to bear in making professional recommendations, even if these recommendations are at odds with what they might choose for themselves.
EBM and SDM: Not so different after all?
Another way to understand the legitimacy of a professional recommendation is to view the parallels between SDM and EBM. From the outset, EBM positioned itself as arising from the best available evidence, the patient’s values, and clinical expertise29—elements that are strikingly similar to the components of SDM.
Although commonly overlooked, the concept of EBM recognizes that established evidence alone is not sufficient for decision-making.30 Additionally, EBM allows for making a recommendation that may not appear to be guideline-based, because guidelines typically do not take into account individual patient preferences.30-32 What’s more, both EBM and SDM highlight the essential contribution of the clinician’s judgment about his or her patient’s unique presentation.
Thus, both EBM and SDM are dependent on the professional communicating a recommendation to the patient. This communication involves not only making clear what one recommends, but also why one recommends it. For example, a clinician might say the following to a patient with worsening asthma symptoms:
“The asthma guidelines give us 2 treatment options. We can either double the dose of your inhaled corticosteroid, or start a 5-day course of corticosteroid pills. Given your concerns about the adverse effects of the pills, and the moderate severity of this exacerbation, I recommend doubling the dose of your inhaled corticosteroid. We can reconsider the pills if your symptoms worsen or if you don’t improve within the next week. How does that sound to you?”
An informed choice. Explaining the evidence, articulating the patient’s values, and summarizing the clinical elements that went into the clinician’s recommendation clarifies and signals to the patient that this is a professional recommendation. Ultimately, the process of SDM concludes with the patient considering the clinician’s recommendation and making an informed choice from the available options.
CORRESPONDENCE
David J. Satin, MD, 2020 E 28th St., Minneapolis, MN 55407; [email protected].
The discipline of family medicine is committed to providing patient-centered care through recommendations that are grounded both in evidence and in patients’ personal values.1,2 The current health care environment, however, often demands heavy reliance on outcome-based performance metrics that can be insensitive to patient preferences.3 This tension necessitates models of decision-making that maximize reliance on measured performance, yet fulfill the clinician’s fiduciary responsibility to prioritize patients’ interests. The philosophy and practice of shared decision-making (SDM) can facilitate these aims.
The 3 elements of shared decision-making
SDM provides a framework for offering everyday medical advice and facilitating informed consent.4 Its 3 elements are:
- discussing with patients relevant information about their health conditions, possible treatments, and likely outcomes,
- clarifying and understanding a patient’s unique values and priorities and how they relate to the treatment options, and
- enabling a patient to select a care plan that is in keeping with his or her personal goals.5
This model is significant not only from a theoretical perspective, but also from a practical one. Studies have shown that both health outcomes and patient satisfaction improve when patients participate more actively in health care decision-making.6,7
Unfortunately, there is evidence that some decision-making practices in primary care settings remain inadequate. For example, unlike the standard disclosure of procedure risks in surgical settings, the burdens of cancer screening are frequently omitted from primary care discussions.8 Moreover, agreement about what should be disclosed, as well as how to disclose it, is still not sufficient. The following 3 recommendations, one for each element of SDM, aim to help clinicians effectively engage patients in everyday decision-making.
1. Provide patients with relevant information
The first element of SDM requires discussing the health-related information that is relevant to the patient’s decision-making process. The literature about informed consent supports explaining the risks that are common, as well as those that are particularly dangerous, and the likely benefits of recommended treatment, nontreatment, and alternative treatments.9 Moreover, adequate informed consent requires identifying what a reasonable person in a particular patient’s position would want to know.10
Accounting for “a patient’s position” is significant because it signals that personal factors (eg, the individual’s beliefs, goals, and familial responsibilities) are as important as the patient’s external clinical situation and what can be known by reviewing medical evidence. Incorporating a patient’s particular circumstances distinguishes patient-centered care from the mechanical application of generic best practices. This is the standard for what information should be provided.
When evidence is lacking. Clinicians facilitating decisions for which data is lacking should convey the best available evidence, including the inherent uncertainties. Like evidence-based medicine (EBM), the principles of SDM should be at work in most clinical encounters. The extent to which one engages in SDM depends upon the seriousness of the proposed interventions, the degree to which the decision is preference-sensitive, and the availability of evidence.
Statistics: Explain absolute and baseline risk
It is generally better to provide absolute risk rather than relative risk, because people perceive absolute risk reductions more accurately.11 Presentation of risks, in terms of relative risk or relative risk reduction, typically exaggerates the benefits of treatment, especially when the risk is small. This exaggeration makes it more likely that patients will accept interventions they might otherwise have rejected after reviewing the data more fully. Furthermore, relative risk statistics can impact clinicians’ perceptions, leading them to recommend an intervention more often than they might when absolute risk statistics are discussed.12
But even absolute risk, if presented on its own, can be misleading. A reasonable person trying to determine the value of an intervention needs to know his or her baseline risk of an event, in addition to his or her absolute risk with the intervention. For example, a hypothetical absolute risk reduction associated with a breast cancer treatment of 10% has different meanings, depending upon whether the baseline risk is 10% or 80%. A reduction from 10% to 0% would be a miraculous cure, while going from 80% to 70% may be viewed as only a slight improvement. Try as we might to present a single neat statistic, presenting both baseline risk and absolute risk with intervention is often necessary.13
How to effectively communicate medical information
Many patients struggle with processing information that is expressed as a probability.14 Patients process frequencies (eg, 10 in 100) better than probabilities (eg, 10%), and there is evidence that they understand best when decision aids are used.15 Decision aids, such as pictographs (FIGURE16), are supplementary, evidence-based tools for effectively communicating with patients and their families in a way that facilitates comparison between available options. Such aids are readily available online for many conditions or can be created using various software tools.16,17
Pictographs reveal that there is a values-sensitive decision to be made and visually demonstrate the outcomes associated with each option. Both pictographs and bar graphs have been shown to improve patient understanding and satisfaction.11 The benefit of pictographs is their ability to effectively, and simultaneously, convey both the numerator and the denominator in frequency statistics.12,18
There is high-quality evidence demonstrating that decision aids enhance an individual’s knowledge about the treatment and screening options available to them. A 2014 Cochrane review of the effects of decision aids found that they increased average knowledge scores when compared to usual care.15 Decision aids also improved accurate perception of risk.15 It is our belief that one of the reasons pictographs work so well is that they combine the salience of absolute risks with and without intervention.12,13
Beyond increased understanding, the Cochrane review also found high-quality evidence indicating that people who make decisions using decision aids feel less decisional conflict when compared to usual care.15 Moreover, in the context of SDM, decisional conflict may contribute to patients passing the decision-making responsibility to their clinician.19 And finally, there is moderate-quality evidence that patients are more likely to participate in decision-making when given tools such as pictographs.15
A potential barrier to putting pictographs into practice concerns perceptions that decision aids increase the length of office visits. Indeed, previous studies have identified perceived time constraints as one of the major barriers to enacting SDM in clinical settings.20 On this topic, the Cochrane review offers variable yet potentially promising results: Studies of the effects on appointment length ranged from a decrease of 8 minutes to an increase of 23 minutes.15 These results suggest that, under the right circumstances, pictographs can be used to facilitate SDM within the constraints of current clinical practice. More research is needed to determine the optimal circumstances that promote efficient SDM.
2. Elicit the patient’s unique values and priorities
Formalized approaches to building rapport with patients have been popular for more than 2 decades,21,22 and they are now routinely part of medical training. Nevertheless, there is always room for improvement when it comes to aligning treatment and screening recommendations with patient values. Some decision aids are designed to offer the added benefit of clarifying individual values and, thus, increase the likelihood that patients will make decisions that are more in line with their goals.15
When decision aids are not available to elicit patient values, clinicians can integrate preference-clarifying questions as part of the standard patient encounter.23 These questions are aimed at surfacing the values underlying what the patient wants, what the patient does not want, and most importantly, why.
“Why” matters because it ultimately helps the clinician understand the patient’s mindset, enabling the clinician to help the patient make choices that serve his or her values.24 Eliciting values not only promotes patient well-being and self-determination, but also facilitates the development of empathic patient-clinician partnerships.
Categorizing decisions. Regardless of the particular method chosen to elicit patient values, the underlying questions faced by many patients often fit into one of 2 categories: 1) Do I prefer quality of life over length of life? or 2) Am I willing to be inconvenienced now to prevent more severe illness later? Clarifying the category into which a decision falls may open the conversation and help to explore patients’ values and priorities. Alternatively, asking questions such as, “Thinking about this decision, what is the most important aspect for you to consider?”25 may facilitate the conversation.
Much of the research on techniques geared to elicit values comes from the palliative care and oncology literature.26 Although this research generally focuses on decisions about serious illness or end-of-life preferences, preference-sensitive decisions in primary care settings create a need for clinicians who are effective in eliciting patient values.
The more serious and preference-sensitive the decision, the deeper the clinician needs to explore the patient’s personal goals. Despite scant literature about seemingly innocuous decisions, we recommend that clinicians elicit from their patients a brief, but overt, acknowledgement of the values guiding their choice for most preference-sensitive decisions.
3. Offer a professional recommendation
Once clinicians have a sense of an individual’s values and priorities, they are positioned to make a professional recommendation that aligns with these values and priorities, and leaves room for the patient to reach a decision. Historically, one of the clinician’s major roles was to provide advice and recommendations to patients. For a long time, this was done without the patient’s involvement in the decision-making.27
With an increasing emphasis on patient self-determination over the last 50 years, there has been some concern that the pendulum is swinging too far in the opposite direction, with clinicians shying away from providing specific recommendations.28 Although this line of thinking acknowledges the power of the clinician to influence patients, it falls short of distinguishing between a personal recommendation and a professional one. While personal recommendations have no place in medical decision-making, clinicians should offer patients a professional recommendation, along with their rationale.
How do personal and professional recommendations differ?
Personal recommendations arise from clinicians considering what they themselves might decide if they were in the patient’s position. Such recommendations are inappropriate because every person has unique values and priorities.
In contrast, professional recommendations stem from the clinician’s knowledge of the best available evidence, his or her understanding of the patient’s values, and his or her weaving of these pieces together in the context of the patient’s specific clinical presentation. Experienced clinicians bring all 3 elements of SDM to bear in making professional recommendations, even if these recommendations are at odds with what they might choose for themselves.
EBM and SDM: Not so different after all?
Another way to understand the legitimacy of a professional recommendation is to view the parallels between SDM and EBM. From the outset, EBM positioned itself as arising from the best available evidence, the patient’s values, and clinical expertise29—elements that are strikingly similar to the components of SDM.
Although commonly overlooked, the concept of EBM recognizes that established evidence alone is not sufficient for decision-making.30 Additionally, EBM allows for making a recommendation that may not appear to be guideline-based, because guidelines typically do not take into account individual patient preferences.30-32 What’s more, both EBM and SDM highlight the essential contribution of the clinician’s judgment about his or her patient’s unique presentation.
Thus, both EBM and SDM are dependent on the professional communicating a recommendation to the patient. This communication involves not only making clear what one recommends, but also why one recommends it. For example, a clinician might say the following to a patient with worsening asthma symptoms:
“The asthma guidelines give us 2 treatment options. We can either double the dose of your inhaled corticosteroid, or start a 5-day course of corticosteroid pills. Given your concerns about the adverse effects of the pills, and the moderate severity of this exacerbation, I recommend doubling the dose of your inhaled corticosteroid. We can reconsider the pills if your symptoms worsen or if you don’t improve within the next week. How does that sound to you?”
An informed choice. Explaining the evidence, articulating the patient’s values, and summarizing the clinical elements that went into the clinician’s recommendation clarifies and signals to the patient that this is a professional recommendation. Ultimately, the process of SDM concludes with the patient considering the clinician’s recommendation and making an informed choice from the available options.
CORRESPONDENCE
David J. Satin, MD, 2020 E 28th St., Minneapolis, MN 55407; [email protected].
1. Medalie JH. Family Medicine: Principles and Applications. Baltimore, MD: Williams and Wilkins; 1978.
2. Philips RL Jr, Brundgardt S, Lesko SE, et al. The future role of the family physician in the United States: a rigorous exercise in definition. Ann Fam Med. 2014;12:250-255.
3. Berwick DM. Era 3 for medicine and health care. JAMA. 2016;315:1329-1330.
4. Edwards A, Elwyn G. Shared decision making in health care: achieving evidence-based patient choice. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2009.
5. American Medical Association. Shared decision making H-373.997. Available at: https://policysearch.ama-assn.org/policyfinder/detail/H-373.997%20?uri=%2FAMADoc%2FHOD.xml-0-3162.xml. Accessed September 6, 2017.
6. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410-417.
8. Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215-1221.
9. Spatz ES, Krumhoiz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA. 2016;315:2063-2064.
10. Faden RR, Becker C, Lewis C, et al. Disclosure of information to patients in medical care. Med Care. 1981;19:718-733.
11. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270-280.
12. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436-1443.
13. Stovitz SD, Shrier I. Medical decision making and the importance of baseline risk. Br J Gen Pract. 2013;63:e795-e797.
14. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37-44.
15. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011:CD001431.
16. Mayo Clinic. Statin choice decision aid. Available at: https://statindecisionaid.mayoclinic.org/index.php/statin/index. Accessed September 6, 2017.
17. Risk Science Center and Center for Bioethics and Social Sciences in Medicine, University of Michigan. Icon Array. Available at: http://www.iconarray.com/. Accessed September 6, 2017.
18. Price M, Cameron R, Butow P. Communicating risk information: the influence of graphical display format on quantitative information perception–accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121-128.
19. Kon AA. The shared decision making continuum. JAMA. 2010;304:903-904.
20. Légaré F1, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535.
21. Haidet P, Paterniti DA. “Building” a history rather than “taking” one: a perspective on information sharing during the medical interview. Arch Intern Med. 2003;163:1134-1140.
22. Frankel RM, Stein R. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16:184-194.
23. Delbanco TL. Enriching the doctor-patient relationship by inviting the patient’s perspective. Ann Intern Med. 1992;116:414-418.
24. Doukas DJ, McCullough LB. The values history: the evaluation of the patient’s values and advance directives. J Fam Pract. 1991;32:145-153.
25. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
26. Bernacki RE, Block SD, American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994-2003.
27. Katz J. The Silent World of Doctor and Patient. Baltimore, MD, and London, England: The Johns Hopkins University Press; 1984.
28. Baylis F, Downie J. Professional recommendations: disclosing facts and values. J Med Ethics. 2001;27:20-24.
29. Sackett DL, Rosenburg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
30. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2053-2054.
31. Mora S, Ames JM, Manson JE. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA. 2016;316:709-710.
32. Stovitz SD, Satin D, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276.
1. Medalie JH. Family Medicine: Principles and Applications. Baltimore, MD: Williams and Wilkins; 1978.
2. Philips RL Jr, Brundgardt S, Lesko SE, et al. The future role of the family physician in the United States: a rigorous exercise in definition. Ann Fam Med. 2014;12:250-255.
3. Berwick DM. Era 3 for medicine and health care. JAMA. 2016;315:1329-1330.
4. Edwards A, Elwyn G. Shared decision making in health care: achieving evidence-based patient choice. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2009.
5. American Medical Association. Shared decision making H-373.997. Available at: https://policysearch.ama-assn.org/policyfinder/detail/H-373.997%20?uri=%2FAMADoc%2FHOD.xml-0-3162.xml. Accessed September 6, 2017.
6. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410-417.
8. Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215-1221.
9. Spatz ES, Krumhoiz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA. 2016;315:2063-2064.
10. Faden RR, Becker C, Lewis C, et al. Disclosure of information to patients in medical care. Med Care. 1981;19:718-733.
11. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270-280.
12. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436-1443.
13. Stovitz SD, Shrier I. Medical decision making and the importance of baseline risk. Br J Gen Pract. 2013;63:e795-e797.
14. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37-44.
15. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011:CD001431.
16. Mayo Clinic. Statin choice decision aid. Available at: https://statindecisionaid.mayoclinic.org/index.php/statin/index. Accessed September 6, 2017.
17. Risk Science Center and Center for Bioethics and Social Sciences in Medicine, University of Michigan. Icon Array. Available at: http://www.iconarray.com/. Accessed September 6, 2017.
18. Price M, Cameron R, Butow P. Communicating risk information: the influence of graphical display format on quantitative information perception–accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121-128.
19. Kon AA. The shared decision making continuum. JAMA. 2010;304:903-904.
20. Légaré F1, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535.
21. Haidet P, Paterniti DA. “Building” a history rather than “taking” one: a perspective on information sharing during the medical interview. Arch Intern Med. 2003;163:1134-1140.
22. Frankel RM, Stein R. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16:184-194.
23. Delbanco TL. Enriching the doctor-patient relationship by inviting the patient’s perspective. Ann Intern Med. 1992;116:414-418.
24. Doukas DJ, McCullough LB. The values history: the evaluation of the patient’s values and advance directives. J Fam Pract. 1991;32:145-153.
25. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
26. Bernacki RE, Block SD, American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994-2003.
27. Katz J. The Silent World of Doctor and Patient. Baltimore, MD, and London, England: The Johns Hopkins University Press; 1984.
28. Baylis F, Downie J. Professional recommendations: disclosing facts and values. J Med Ethics. 2001;27:20-24.
29. Sackett DL, Rosenburg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
30. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2053-2054.
31. Mora S, Ames JM, Manson JE. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA. 2016;316:709-710.
32. Stovitz SD, Satin D, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276.
PRACTICE RECOMMENDATIONS
› Provide patients with information in terms of absolute and baseline risks, ideally using pictograph decision aids. A
› Elicit the patient’s values and priorities by categorizing decisions and asking broad open-ended questions. C
› Offer patients a professional (not a personal) recommendation, including your rationale. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
8 viral exanthems of childhood
Family physicians encounter skin rashes on a daily basis. First steps in making the diagnosis include identifying the characteristics of the rash and determining whether the eruption is accompanied by fever or any other symptoms. In the article that follows, we review 8 viral exanthems of childhood that range from the common (chickenpox) to the not-so-common (Gianotti-Crosti syndrome).
Varicella-zoster virus
Varicella-zoster virus (VZV) is a human neurotropic alphaherpesvirus that causes a primary infection commonly known as chickenpox (varicella).1 This disease is usually mild and resolves spontaneously.
This highly contagious virus is transmitted by directly touching the blisters, saliva, or mucus of an infected person. It is also transmitted through the air by coughing and sneezing. VZV initiates primary infection by inoculating the respiratory mucosa. It then establishes a lifelong presence in the sensory ganglionic neurons and, thus, can reactivate later in life causing herpes zoster (shingles), which can affect cranial, thoracic, and lumbosacral dermatomes. Acute or chronic neurologic disorders, including cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis, and multiple ocular disorders, have been reported after VZV reactivation resulting in herpes-zoster.1
Presentation. With varicella, an extremely pruritic rash follows a brief prodromal stage consisting of a low-grade fever, upper respiratory tract symptoms, tiredness, and fatigue. This exanthem develops rapidly, often beginning on the chest, trunk, or scalp and then spreading to the arms and legs (centrifugally) (FIGURE 1). Varicella also affects mucosal areas of the body, such as the conjunctiva, mouth, rectum, and vagina.
The lesions are papules that rapidly become vesicular with clear fluid inside. Subsequently, the lesions begin to crust. Scabbing occurs within 10 to 14 days. A sure sign of chickenpox is the presence of papules, vesicles, and crusting lesions in close proximity.
Complications. The most common complications of chickenpox—especially in children—are invasive streptococcal and staphylococcal infections.2 The most serious complication occurs when the virus invades the spinal cord, causing myelitis or affecting the cerebral arteries, leading to vasculopathy. Diagnosis of VZV in the central nervous system is based on isolation of the virus in cerebral spinal fluid by polymerase chain reaction (PCR). Early diagnosis is important to minimize morbidity and mortality.
Reactivation is sometimes associated with post-herpetic neuralgia (PHN), a severe neuropathic pain syndrome isolated to the dermatomes affected by VZV. PHN can cause pain and suffering for years after shingles resolves, and sometimes is refractory to treatment. PHN may reflect a chronic varicella virus ganglionitis.
A number of treatment choices exist for shingles, but not so much for varicella
Oral treatment. Oral medications such as acyclovir and its prodrug valacyclovir are the current gold standards for the treatment of VZV.3
Famciclovir, the prodrug of penciclovir, is more effective than valacyclovir at resolving acute herpes zoster rash and shortening the duration of PHN.4 Gabapentinoids (eg, pregabalin) are the only oral medications approved by the US Food and Drug Administration (FDA) to treat PHN.5
Topical medications can also be used. Lidocaine 5% is favored as first-line therapy for the amelioration of pain due to shingles, as it provides modest pain relief with a better safety and tolerability profile than capsaicin 8% patch, which is a second-line choice. The latter must be applied multiple times daily, has minimal analgesic efficacy, and frequently causes initial pain upon application.
Gabapentinoids and topical analgesics can be used in combination due to the low propensity for drug interactions.6,7 The treatment of choice for focal vasculopathy is intravenous acyclovir, usually for 14 days, although immunocompromised patients should be treated for a longer period of time. Also consider 5 days of steroid therapy for patients with VZV vasculopathy.8
Non-FDA approved treatments include tricyclic antidepressants (TCA), such as amitriptyline, nortriptyline, and desipramine, which are sometimes used as first-line therapy for shingles. TCAs may not work well in patients with burning pain, and can have significant adverse effects, including possible cardiotoxicity.9
Opioids, including oxycodone, morphine, methadone, and tramadol, are sometimes used in pain management, but concern exists for abuse. Because patients may develop physical dependence, use opioids with considerable caution.10
Prevention. The United States became the first country to institute a routine varicella immunization program after a varicella vaccine (Varivax) was licensed in 1995.11 The vaccine has reduced the number of varicella infection cases dramatically.11 Vaccine effectiveness is high, and protective herd immunity is obtained after 2 doses.11-13 The vaccine is administered to children after one year of age with a booster dose administered after the fourth birthday.
A live, attenuated VZV vaccine (Zostavax) is given to individuals ≥60 years of age to prevent or attenuate herpes zoster infection. This vaccine is used to boost VZV-specific cell-mediated immunity in adults, thereby decreasing the burden of herpes zoster and the pain associated with PHN.14
Roseola
Roseola infantum, also known as exanthema subitum and sixth disease, is a common mild acute febrile illness of childhood caused by infection with human herpesvirus (HHV) 6 (the primary agent causing roseola) or 7 (a secondary causal agent for roseola).15 HHV-6 has 2 variants (HHV-6a and HHV-6b). Roseola infantum is mostly associated with the HHV-6b variant, which predominantly affects children 6 to 36 months of age.16
The virus replicates in the salivary glands and is shed through saliva, which is the route of transmission. After a 10- to 15-day incubation period, it remains latent in lymphocytes and monocytes, thus persisting in cells and tissues. It may reactivate late in life, particularly in immunosuppressed individuals. Reactivated infection in immunocompromised patients may be associated with serious illness such as encephalitis/encephalopathy. In patients who have received a bone marrow transplant, it can induce graft vs host disease.17
Presentation. The virus causes a 5- to 6-day illness characterized by high fever (temperature as high as 105°-106° F), miringitis (inflammation of tympanic membranes), and irritability. Once defervescence occurs, an erythematous morbilliform exanthem appears.The rash, which has a discrete macular/papular soft-pink appearance, starts on the trunk and spreads centrifugally to the extremities, neck, and face (FIGURE 2). It usually resolves within one to 2 days.
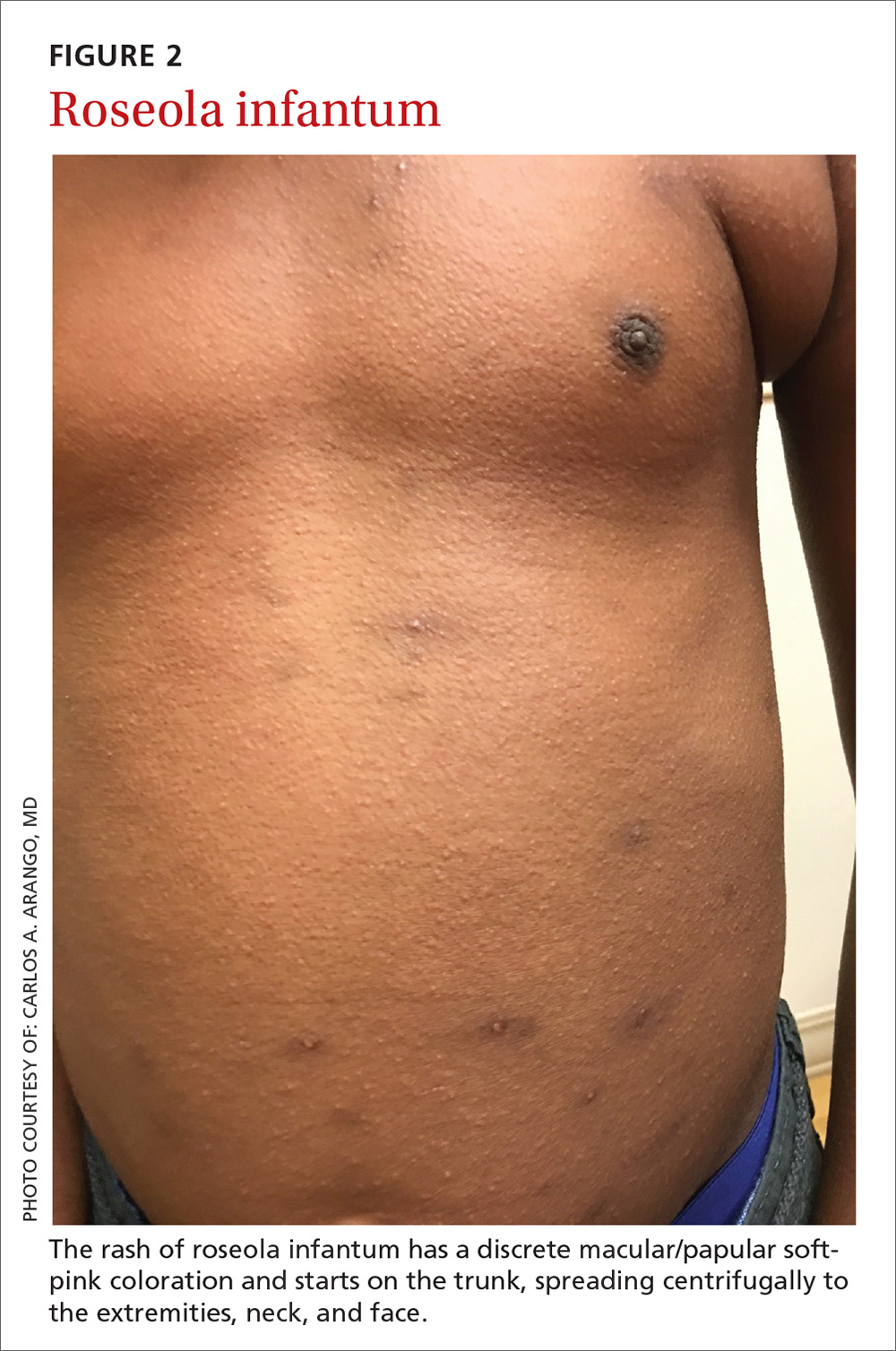
Complications. The most common complication of roseola is febrile seizures.17 Less common ones include encephalitis, encephalopathy, fatal hemophagocytic syndrome,18 or fulminant hepatitis.19
Treatment and prevention. Treatment depends on symptoms and may include antipyretics for fever management and liquids to maintain hydration. Recovery is usually complete with no significant sequelae. If a child develops a seizure, no antiepileptic drugs are recommended. No vaccine exists.
Fifth disease
Human parvovirus B19, a minute ssDNA virus, was first associated with human disease in 1981, when it was linked to an aplastic crisis in a patient with sickle cell disease.20 Since then researchers have determined that it is also the cause of erythema infectiosum or fifth disease of childhood. The B19 virus can also cause anemia in the fetus as well as hydrops fetalis. It has been linked to arthralgia and arthritis (especially in adults). There is an association with autoimmune diseases with characteristics similar to rheumatoid arthritis.20
The B19 virus is transmitted via aerosolized respiratory secretions, contaminated blood, or the placenta. The virus replicates in erythroid cells in bone marrow and peripheral blood, thus inhibiting erythropoiesis.21 Once the rash appears, the virus is no longer infectious.22 Seasonal peaks occur in the late winter and spring, with sporadic infections throughout the year.23 More than 70% of the adult population is seropositive for this virus.20
Presentation. Erythema infectiosum is a mild illness in childhood with an incubation period of 6 to 18 days. It presents with a characteristic malar rash on the face that gives patients a slapped cheek appearance (FIGURE 3A). A softer pink-colored “lacy” reticulated rash that blanches when touched may appear on the trunk, arms, and legs (FIGURE 3B).
Another presentation, which involves the hands and feet (glove and sock syndrome) (FIGURES 3C and 3D), consists of a purpuric eruption with painful edema and numerous small confluent petechiae.22,24 A majority of patients present with inflammatory symptoms that tend to resolve without sequelae within 3 weeks of infection.23
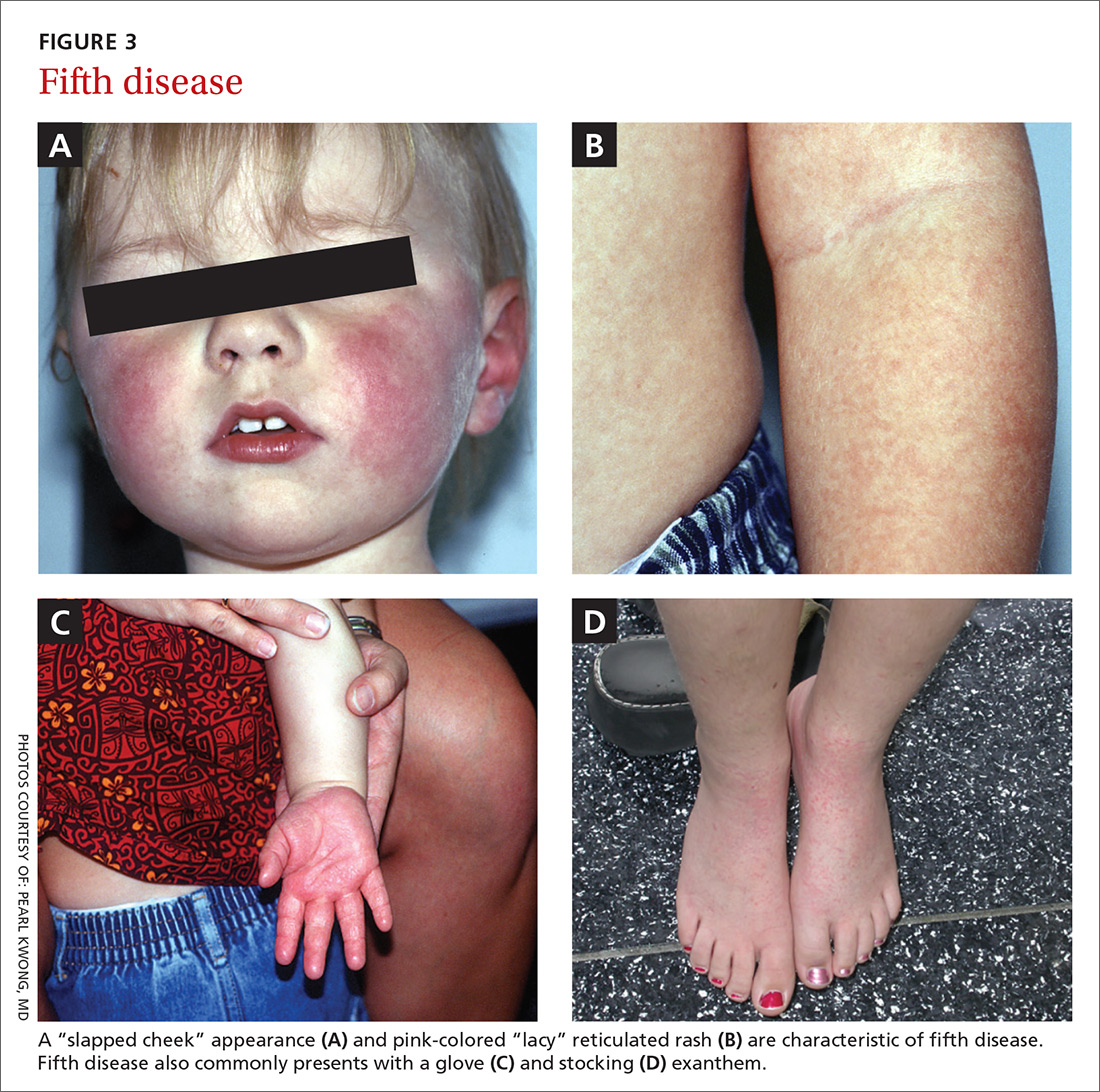
A rash is not as prevalent in adults as in children. Adults often present with more systemic systems, such as a debilitating influenza-like illness, arthropathy, transient aplastic anemia in sickle cell-affected individuals, and persistent viral suppression of erythrocyte production in immunocompromised patients and organ-transplant recipients.
Complications. The B19 virus can cause spontaneous abortion in pregnant women and anemia and hydrops fetalis in the fetus.22 Arthritis can occur in children, but is more common in adults, especially in women. The arthritis tends to be symmetrical and affects small joints such as the hands, wrists, and knees.
In one study of parvovirus B19 involving 633 children with sickle cell disease, 68 children developed transient red cell aplasia, 19% of them developed splenic sequestration, and 12% developed acute chest syndrome, a lung-related complication of sickle cell disease that can lower the level of oxygen in the blood and can be fatal.25
Treatment and prevention. Treatment of B19 infection is symptomatic; for example, nonsteroidal anti-inflammatory drugs (NSAIDs) are used if joint pain develops. No vaccine exists.
Hand, foot, and mouth disease
Hand, foot, and mouth disease (HFMD) is caused by the picornavirus family, including the Coxsackievirus, Enterovirus, and Echovirus. Infections commonly occur in the spring, summer, and fall. The virus primarily affects infants and children <10 years of age with the infection typically lasting 7 to 10 days.26
Presentation. The disease usually presents with a febrile episode, progressing to nasal congestion and malaise. One to 2 days later, the classic rash appears. Patients with HFMD usually present with papular or vesicular lesions on the hands and feet and painful oral lesions (FIGURES 4A, 4B, and 4C). The rash may also affect other parts of the body including the legs and buttocks. Desquamation of nails may occur up to one month after the HFM infection.27 Most cases are diagnosed by clinical presentation, but infection can be confirmed by PCR of vesicular lesion fluid.
Complications. In addition to being caused by Coxsackievirus, HFMD may be caused by human Enterovirus A serotype 71 (HEVA-71), which is associated with a high prevalence of acute neurologic disease including aseptic meningitis, poliomyelitis-like paralysis, and encephalitis.26 Of 764 HFMD patients enrolled in a prospective study, 214 cases were associated with Coxsackievirus A 16 (CVA16) infection and 173 cases were associated with HEVA-71 infection. Rare cases of HFMD have led to encephalitis, meningitis, flaccid paralysis, and death.26
Treatment and prevention. HFMD is usually self-limited, and treatment is supportive. There has been interest in developing an HFMD vaccine, but no products are as yet commercially available.
Rubella
Rubella, also known as the German measles or the 3-day measles, is caused by the rubella virus, which is transmitted via respiratory droplets. Up to half of rubella infections are asymptomatic.28-30
Presentation. Rubella typically has an incubation period of 12 to 24 days, with a 5-day prodromal period characterized by fever, headache, and other symptoms typical of an upper respiratory infection, including sore throat, arthralgia, and tender lymphadenopathy.28
The rash often starts as erythematous or as rose-pink macules on the face that progress down the body. The rash can cover the trunk and extremities within 24 hours. (For photos, see https://www.cdc.gov/rubella/about/photos.html.)
Patients are infectious from 7 days prior to the appearance of the rash to 7 days after resolution of the rash. Given the potentially prolonged infectious period, patients hospitalized for rubella infection should be placed on droplet precautions, and children should be kept from day care and school for 7 days after the appearance of the rash.28
Rubella is typically a mild disease in immunocompetent patients; however, immunocompromised patients may develop pneumonia, coagulopathies, or neurologic sequelae including encephalitis.
Complications. Rubella infection, especially during the first trimester, can lead to spontaneous abortion, stillbirth, or congenital rubella syndrome (CRS), a condition characterized by congenital cataracts and “blueberry muffin” skin lesions.31 Infants affected by CRS can also have heart defects, intellectual disabilities, deafness, and low birth weight. Diagnosis of primary maternal infection should be made with serologic tests. Fetal infection can be determined by detection of fetal serum IgM after 22 to 24 weeks of gestation or with viral culture of amniotic fluid.31
Treatment and prevention. Currently, no antiviral treatments are available; however, vaccines are highly effective at preventing infection. Rubella vaccine is usually given as part of the measles, mumps, rubella (MMR) vaccine, which is administered at age 12 to 15 months and again between 4 and 6 years of age.
Measles
Measles is a highly contagious disease caused by a virus that belongs to the Morbillivirus genus of the family Paramyxoviridae. Infection occurs through inhalation of, or direct contact with, respiratory droplets from infected people.32
Presentation. People with measles often present with what starts as a macular rash on the face that then spreads downward to the neck, trunk, and extremities (for photos, see http://www.cdc.gov/measles/hcp/index.html). As the disease progresses, papules can develop and the lesions may coalesce.
The rash is often preceded by 3 to 4 days of malaise and fever (temperature often greater than 104° F), along with the classic symptom triad of cough, coryza, and conjunctivitis. Koplik spots—clustered white lesions on the buccal mucosa—are often present prior to the measles rash and are pathognomonic for measles infection.33
Because the symptoms of measles are easily confused with other viral infections, suspected cases of measles should be confirmed via IgG and IgM antibody tests, by reverse transcription-PCR, or both.34,35 For limited and unusual cases, the Centers for Disease Control and Prevention can perform a plaque reduction neutralization assay.35
Complications. Measles infection is self-limited in immunocompetent patients. The most common complications are diarrhea and ear infections, but more serious complications, such as pneumonia, hearing loss, and encephalitis, can occur. Children <2 years of age, particularly boys, are at an increased risk of developing subacute sclerosing panencephalitis, a fatal neurologic disorder that can develop years after the initial measles infection.33,36
Treatment and prevention. Treatment is supportive and usually consists of acetaminophen or NSAIDs and fluids.
A live attenuated version of the measles vaccine is highly effective against the measles virus and has greatly reduced the number of measles cases globally.37 The measles vaccine is usually given in 2 doses—the first one after one year of age, and the second one before entering kindergarten. The most common adverse reactions to the vaccine are pain at the injection site and fever. Despite the fact that the MMR vaccine is effective and relatively benign, measles outbreaks continue to occur, as some parents forego routine childhood immunizations because of religious or other personal beliefs or safety concerns.38
Molluscum contagiosum
Molluscum contagiosum (MC) is caused by the MC virus, a member of the poxvirus family. The virus is transmitted by direct contact with the skin lesions. This skin condition is seen mainly in children, although it can occur in adults.
A study conducted in England and Wales that obtained information from the Royal College of General Practitioners reported an incidence of 15.0-17.2/1000 population over a 10-year period (1994-2003) with no variation between sexes.39 There is an association between atopic dermatitis and MC; 24% of children with atopic dermatitis develop MC.40 There might also be an association between recent swimming in a public pool and development of MC lesions.41
Presentation. Lesions caused by MC are small, discrete, waxy dome-shaped papules with central umbilication that are usually 3 to 5 mm in diameter (FIGURE 5).42,43 In immunocompetent patients, there are usually fewer than 20 lesions, which resolve within a year. However, in immunocompromised patients, the number of lesions is usually higher, and the diameter of each may be greater than 1 cm.42
Complications. The lesions are usually self-limited, but on occasion can become secondarily infected, usually with gram-positive organisms such as Staphylococcus aureus. Very rarely, abscesses develop requiring topical and/or systemic antimicrobials and perhaps incision and drainage.44
Treatment and prevention. Because the infection is often self-limited and benign, the preferred therapeutic modality is watchful waiting. Other treatments for MC include curettage, chemical agents, immune modulators, and antiviral drugs. A 2009 Cochran review of 11 studies involving 495 patients found “no single intervention to be convincingly effective in the treatment of molluscum contagiosum.”45 And no vaccine exists.
Gianotti-Crosti syndrome
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis of childhood, is a relatively rare, self-limited exanthema that usually affects infants and children 6 months to 12 years of age (peak occurrence is in one- to 6-year-olds). Although there have been reports of adults with this syndrome, it is unusual in this age group.
Pathogenesis is still unknown. Although GCS itself isn’t contagious, the viruses that can cause it may be. Initially, researchers believed that GCS was linked to acute hepatitis B virus infection, but more recently other viral and bacterial infections have been associated with the condition.46
The most commonly associated virus in the United States is Epstein-Barr virus; other viruses include hepatitis A virus, cytomegalovirus, coxsackievirus, respiratory syncytial virus, parainfluenza virus, rotavirus, the mumps virus, parvovirus, and molluscum contagiosum.
Bacterial infections, such as those caused by Bartonella henselae, Mycoplasma pneumoniae, and group A streptococci may trigger GCS.47-49
Vaccines that have been implicated in GCS include those for polio, diphtheria/pertussis/tetanus, MMR, hepatitis A and B, as well as the influenza vaccine.48-51
Presentation. While GCS is relatively rare, its presentation is classic, making it easy to diagnose once it’s included in the differential. The pruritic rash usually consists of acute-onset monomorphous, flat-topped or dome-shaped red-brown papules and papulovesicles, one to 10 mm in size, located symmetrically on the face (FIGURE 6), the extensor surfaces of the arms and legs, and, less commonly, the buttocks. It rarely affects other parts of the body.48
The diagnosis is usually based on the characteristic rash and the benign nature of the condition; other than the rash, patients are typically asymptomatic and healthy. Sometimes a biopsy is performed and it reveals a dense lichenoid lymphohistiocytic infiltrate with a strong cytoplasmic immunopositivity for beta-defensin-4 in the stratum corneum, granulosum, and spinosum.52
The lesions spontaneously resolve within 8 to 12 weeks. GCS usually presents during spring and early summer and affects both sexes equally.46
Treatment and prevention. Treatment is usually symptomatic, with the use of oral antihistamines if the lesions become pruritic. Topical steroids may be used, and, in a few cases, oral corticosteroids may be considered. No vaccine exists.
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; [email protected].
1. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
2. Blumental S, Sabbe M, Lepage P, the Belgian Group for Varicella. Varicella paediatric hospitalisations in Belgium: a 1-year national survey. Arch Dis Child. 2016;101:16-22.
3. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clinic Proc. 2009;84:274-280.
4. Ono F, Yasumoto S, Furumura M, et al. Comparison between famciclovir and valacyclovir for acute pain in adult Japanese immunocompetent patients with herpes zoster. J Dermatol. 2012;39:902-908.
5. Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of post-herpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125-132.
6. Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs & Aging. 2012;29:863-869.
7. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164.
8. Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;9:731-740.
9. Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and post herpetic neuralgia. Am Fam Physician. 2000;61:2437-2444.
10. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3-S14.
11. Thomas CA, Shwe T, Bixler D, et al. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J. 2014;33:1164-1168.
12. Helmuth IG, Poulsen A, Suppli CH, et al. Varicella in Europe-a review of the epidemiology and experience with vaccination. Vaccine. 2015;33:2406-2413.
13. Marin M, Marti M, Kambhampati A, et al. Global varicella vaccine effectiveness: a metanalysis. Pediatrics. 2016;137:e20153741.
14. Centers for Disease Control and Prevention. What everyone should know about shingles vaccine. Available at: www.cdc.gov/vaccines/vpd/shingles/public/index.html. Accessed September 12, 2017.
15. Tanaka K, Kondo T, Torigoe S, et al. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J Pediatr. 1994;125:1-5.
16. Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33:829-833.
17. Koch WC. Fifth (human parvovirus) and sixth (herpesvirus 6) diseases. Curr Opin Infect Dis. 2001;14:343-356.
18. Marabelle A, Bergeron C, Billaud G, et al. Hemophagocytic syndrome revealing primary HHV-6 infection. J Pediatr. 2010;157:511.
19. Charnot-Katsikasa A, Baewer D, Cook L, et al. Fulminant hepatic failure attributed to infection with human herpesvirus 6 (HHV-6) in an immunocompetent woman: a case report and review of the literature. J Clin Virol. 2016;75:27-32.
20. Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol. 2004;53(Pt 6):459-475.
21. Dolin R. Parvovirus erythema infectiousum, Aplastic anemia. In: Mandell, Douglas, Bennett’s Principles and Practice of Infectious Diseases. 3rd ed. New York, NY: Churchill Livingstone Inc; 1990:1231-1232.
22. Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. 2007;75:373-376.
23. Martin DR, Schlott DW, Flynn JA. Clinical problem-solving. No respecter of age. N Engl J Med. 2007;357:1856-1859.
24. Ozaydin V, Eceviz A, Sari Dogan F, et al. An adult patient who presented to emergency service with a papular purpuric gloves and socks syndrome: a case report. Turk J Emerg Med. 2014;14:179-181.
25. Smith-Whitley K, Zhao H, Hodinka RL, et al. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood. 2004;103:422-427.
26. Tu PV, Thao NT, Perera D, et al. Epidemiologic and virologic investigation of hand, foot, and mouth disease, Southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733-1741.
27. Ferrari B, Taliercio V, Hornos L, et al. Onychomadesis associated with mouth, hand and foot disease. Arch Argent Pediatr. 2013;111:e148-e151.
28. Alter SJ, Bennett JS, Koranyi K, et al. Common childhood viral infections. Curr Probl Pediatr Adolesc Health Care. 2015;45:21-53.
29. Lambert N, Strebel P, Orenstein W, et al. Rubella. Lancet. 2015;385:2297-2307.
30. Silasi M, Cardenas I, Kwon JY, et al. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73:199-213.
31. Tang JW, Aarons E, Hesketh LM, et al. Prenatal diagnosis of congenital rubella infection in the second trimester of pregnancy Prenat Diagn. 2003;23:509-512.
32. Naim HY. Measles virus. Hum Vaccin Immunother. 2015;11:21-26.
33. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: http://www.cdc.gov/measles/hcp/index.html. Accessed April 28, 2016.
34. Takao S, Shigemoto N, Shimazu Y, et al. Detection of exanthematic viruses using a TaqMan real-time PCR assay panel in patients with clinically diagnosed or suspected measles. Jpn J Infect Dis. 2012;65:444-448.
35. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: https://www.cdc.gov/measles/lab-tools/rt-pcr.html. Accessed April 28, 2016.
36. Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012;36:649-662.
37. Centers for Disease Control and Prevention. Measles, vaccination. Available at: https://www.cdc.gov/measles/vaccination.html. Accessed April 28, 2016.
38. Campos-Outcalt D. Measles: Why it’s still a threat. J Fam Pract. 2017;66:446-449.
39. Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
40. Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
41. Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
42. Martin P. Interventions for molluscum contagiosum in people infected with human immunodeficiency virus: a systematic review. Int J Dermatol. 2016;55:956-966.
43. Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13:877-888.
44. Lacour M, Posfay-Barbe KM, La Scala GC. Staphylococcus lugdunensis abscesses complicating molluscum contagiosum in two children. Pediatr Dermatol. 2015;32:289-291.
45. van der Wouden JC, van der Sande R, van Suijlekom-Smit LW, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2009;CD004767.
46. Tagawa C, Speakman M. Photo quiz. Papular rash in a child after a fever. Gianotti-Crosti syndrome. Am Fam Physician. 2013;87:59-60.
47. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
48. Retrouvey M, Koch LH, Williams JV. Gianotti-Crosti syndrome following childhood vaccinations. Pediatr Dermatol. 2013;30:137-138.
49. Velangi SS, Tidman MJ. Gianotti-Crosti syndrome after measles, mumps, and rubella vaccination. Br J Dermatol. 1998;139:1122-1123.
50. Lacour M, Harms M. Gianotti-Crosti syndrome as a result of vaccination and Epstein-Barr virus infection. Eur J Pediatr. 1995;154:688-689.
51. Kroeskop A, Lewis AB, Barril FA, et al. Gianotti-Crosti syndrome after H1N1-influenza vaccine. Pediatr Dermatol. 2011;28:595-596.
52. Caltabiano R, Vecchio GM, De Pasquale R, et al. Human beta-defensin 4 expression in Gianotti-Crosti. Acta Dermatovenerol Croat. 2013;21:43-47.
Family physicians encounter skin rashes on a daily basis. First steps in making the diagnosis include identifying the characteristics of the rash and determining whether the eruption is accompanied by fever or any other symptoms. In the article that follows, we review 8 viral exanthems of childhood that range from the common (chickenpox) to the not-so-common (Gianotti-Crosti syndrome).
Varicella-zoster virus
Varicella-zoster virus (VZV) is a human neurotropic alphaherpesvirus that causes a primary infection commonly known as chickenpox (varicella).1 This disease is usually mild and resolves spontaneously.
This highly contagious virus is transmitted by directly touching the blisters, saliva, or mucus of an infected person. It is also transmitted through the air by coughing and sneezing. VZV initiates primary infection by inoculating the respiratory mucosa. It then establishes a lifelong presence in the sensory ganglionic neurons and, thus, can reactivate later in life causing herpes zoster (shingles), which can affect cranial, thoracic, and lumbosacral dermatomes. Acute or chronic neurologic disorders, including cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis, and multiple ocular disorders, have been reported after VZV reactivation resulting in herpes-zoster.1
Presentation. With varicella, an extremely pruritic rash follows a brief prodromal stage consisting of a low-grade fever, upper respiratory tract symptoms, tiredness, and fatigue. This exanthem develops rapidly, often beginning on the chest, trunk, or scalp and then spreading to the arms and legs (centrifugally) (FIGURE 1). Varicella also affects mucosal areas of the body, such as the conjunctiva, mouth, rectum, and vagina.
The lesions are papules that rapidly become vesicular with clear fluid inside. Subsequently, the lesions begin to crust. Scabbing occurs within 10 to 14 days. A sure sign of chickenpox is the presence of papules, vesicles, and crusting lesions in close proximity.
Complications. The most common complications of chickenpox—especially in children—are invasive streptococcal and staphylococcal infections.2 The most serious complication occurs when the virus invades the spinal cord, causing myelitis or affecting the cerebral arteries, leading to vasculopathy. Diagnosis of VZV in the central nervous system is based on isolation of the virus in cerebral spinal fluid by polymerase chain reaction (PCR). Early diagnosis is important to minimize morbidity and mortality.
Reactivation is sometimes associated with post-herpetic neuralgia (PHN), a severe neuropathic pain syndrome isolated to the dermatomes affected by VZV. PHN can cause pain and suffering for years after shingles resolves, and sometimes is refractory to treatment. PHN may reflect a chronic varicella virus ganglionitis.
A number of treatment choices exist for shingles, but not so much for varicella
Oral treatment. Oral medications such as acyclovir and its prodrug valacyclovir are the current gold standards for the treatment of VZV.3
Famciclovir, the prodrug of penciclovir, is more effective than valacyclovir at resolving acute herpes zoster rash and shortening the duration of PHN.4 Gabapentinoids (eg, pregabalin) are the only oral medications approved by the US Food and Drug Administration (FDA) to treat PHN.5
Topical medications can also be used. Lidocaine 5% is favored as first-line therapy for the amelioration of pain due to shingles, as it provides modest pain relief with a better safety and tolerability profile than capsaicin 8% patch, which is a second-line choice. The latter must be applied multiple times daily, has minimal analgesic efficacy, and frequently causes initial pain upon application.
Gabapentinoids and topical analgesics can be used in combination due to the low propensity for drug interactions.6,7 The treatment of choice for focal vasculopathy is intravenous acyclovir, usually for 14 days, although immunocompromised patients should be treated for a longer period of time. Also consider 5 days of steroid therapy for patients with VZV vasculopathy.8
Non-FDA approved treatments include tricyclic antidepressants (TCA), such as amitriptyline, nortriptyline, and desipramine, which are sometimes used as first-line therapy for shingles. TCAs may not work well in patients with burning pain, and can have significant adverse effects, including possible cardiotoxicity.9
Opioids, including oxycodone, morphine, methadone, and tramadol, are sometimes used in pain management, but concern exists for abuse. Because patients may develop physical dependence, use opioids with considerable caution.10
Prevention. The United States became the first country to institute a routine varicella immunization program after a varicella vaccine (Varivax) was licensed in 1995.11 The vaccine has reduced the number of varicella infection cases dramatically.11 Vaccine effectiveness is high, and protective herd immunity is obtained after 2 doses.11-13 The vaccine is administered to children after one year of age with a booster dose administered after the fourth birthday.
A live, attenuated VZV vaccine (Zostavax) is given to individuals ≥60 years of age to prevent or attenuate herpes zoster infection. This vaccine is used to boost VZV-specific cell-mediated immunity in adults, thereby decreasing the burden of herpes zoster and the pain associated with PHN.14
Roseola
Roseola infantum, also known as exanthema subitum and sixth disease, is a common mild acute febrile illness of childhood caused by infection with human herpesvirus (HHV) 6 (the primary agent causing roseola) or 7 (a secondary causal agent for roseola).15 HHV-6 has 2 variants (HHV-6a and HHV-6b). Roseola infantum is mostly associated with the HHV-6b variant, which predominantly affects children 6 to 36 months of age.16
The virus replicates in the salivary glands and is shed through saliva, which is the route of transmission. After a 10- to 15-day incubation period, it remains latent in lymphocytes and monocytes, thus persisting in cells and tissues. It may reactivate late in life, particularly in immunosuppressed individuals. Reactivated infection in immunocompromised patients may be associated with serious illness such as encephalitis/encephalopathy. In patients who have received a bone marrow transplant, it can induce graft vs host disease.17
Presentation. The virus causes a 5- to 6-day illness characterized by high fever (temperature as high as 105°-106° F), miringitis (inflammation of tympanic membranes), and irritability. Once defervescence occurs, an erythematous morbilliform exanthem appears.The rash, which has a discrete macular/papular soft-pink appearance, starts on the trunk and spreads centrifugally to the extremities, neck, and face (FIGURE 2). It usually resolves within one to 2 days.

Complications. The most common complication of roseola is febrile seizures.17 Less common ones include encephalitis, encephalopathy, fatal hemophagocytic syndrome,18 or fulminant hepatitis.19
Treatment and prevention. Treatment depends on symptoms and may include antipyretics for fever management and liquids to maintain hydration. Recovery is usually complete with no significant sequelae. If a child develops a seizure, no antiepileptic drugs are recommended. No vaccine exists.
Fifth disease
Human parvovirus B19, a minute ssDNA virus, was first associated with human disease in 1981, when it was linked to an aplastic crisis in a patient with sickle cell disease.20 Since then researchers have determined that it is also the cause of erythema infectiosum or fifth disease of childhood. The B19 virus can also cause anemia in the fetus as well as hydrops fetalis. It has been linked to arthralgia and arthritis (especially in adults). There is an association with autoimmune diseases with characteristics similar to rheumatoid arthritis.20
The B19 virus is transmitted via aerosolized respiratory secretions, contaminated blood, or the placenta. The virus replicates in erythroid cells in bone marrow and peripheral blood, thus inhibiting erythropoiesis.21 Once the rash appears, the virus is no longer infectious.22 Seasonal peaks occur in the late winter and spring, with sporadic infections throughout the year.23 More than 70% of the adult population is seropositive for this virus.20
Presentation. Erythema infectiosum is a mild illness in childhood with an incubation period of 6 to 18 days. It presents with a characteristic malar rash on the face that gives patients a slapped cheek appearance (FIGURE 3A). A softer pink-colored “lacy” reticulated rash that blanches when touched may appear on the trunk, arms, and legs (FIGURE 3B).
Another presentation, which involves the hands and feet (glove and sock syndrome) (FIGURES 3C and 3D), consists of a purpuric eruption with painful edema and numerous small confluent petechiae.22,24 A majority of patients present with inflammatory symptoms that tend to resolve without sequelae within 3 weeks of infection.23

A rash is not as prevalent in adults as in children. Adults often present with more systemic systems, such as a debilitating influenza-like illness, arthropathy, transient aplastic anemia in sickle cell-affected individuals, and persistent viral suppression of erythrocyte production in immunocompromised patients and organ-transplant recipients.
Complications. The B19 virus can cause spontaneous abortion in pregnant women and anemia and hydrops fetalis in the fetus.22 Arthritis can occur in children, but is more common in adults, especially in women. The arthritis tends to be symmetrical and affects small joints such as the hands, wrists, and knees.
In one study of parvovirus B19 involving 633 children with sickle cell disease, 68 children developed transient red cell aplasia, 19% of them developed splenic sequestration, and 12% developed acute chest syndrome, a lung-related complication of sickle cell disease that can lower the level of oxygen in the blood and can be fatal.25
Treatment and prevention. Treatment of B19 infection is symptomatic; for example, nonsteroidal anti-inflammatory drugs (NSAIDs) are used if joint pain develops. No vaccine exists.
Hand, foot, and mouth disease
Hand, foot, and mouth disease (HFMD) is caused by the picornavirus family, including the Coxsackievirus, Enterovirus, and Echovirus. Infections commonly occur in the spring, summer, and fall. The virus primarily affects infants and children <10 years of age with the infection typically lasting 7 to 10 days.26
Presentation. The disease usually presents with a febrile episode, progressing to nasal congestion and malaise. One to 2 days later, the classic rash appears. Patients with HFMD usually present with papular or vesicular lesions on the hands and feet and painful oral lesions (FIGURES 4A, 4B, and 4C). The rash may also affect other parts of the body including the legs and buttocks. Desquamation of nails may occur up to one month after the HFM infection.27 Most cases are diagnosed by clinical presentation, but infection can be confirmed by PCR of vesicular lesion fluid.
Complications. In addition to being caused by Coxsackievirus, HFMD may be caused by human Enterovirus A serotype 71 (HEVA-71), which is associated with a high prevalence of acute neurologic disease including aseptic meningitis, poliomyelitis-like paralysis, and encephalitis.26 Of 764 HFMD patients enrolled in a prospective study, 214 cases were associated with Coxsackievirus A 16 (CVA16) infection and 173 cases were associated with HEVA-71 infection. Rare cases of HFMD have led to encephalitis, meningitis, flaccid paralysis, and death.26
Treatment and prevention. HFMD is usually self-limited, and treatment is supportive. There has been interest in developing an HFMD vaccine, but no products are as yet commercially available.
Rubella
Rubella, also known as the German measles or the 3-day measles, is caused by the rubella virus, which is transmitted via respiratory droplets. Up to half of rubella infections are asymptomatic.28-30
Presentation. Rubella typically has an incubation period of 12 to 24 days, with a 5-day prodromal period characterized by fever, headache, and other symptoms typical of an upper respiratory infection, including sore throat, arthralgia, and tender lymphadenopathy.28
The rash often starts as erythematous or as rose-pink macules on the face that progress down the body. The rash can cover the trunk and extremities within 24 hours. (For photos, see https://www.cdc.gov/rubella/about/photos.html.)
Patients are infectious from 7 days prior to the appearance of the rash to 7 days after resolution of the rash. Given the potentially prolonged infectious period, patients hospitalized for rubella infection should be placed on droplet precautions, and children should be kept from day care and school for 7 days after the appearance of the rash.28
Rubella is typically a mild disease in immunocompetent patients; however, immunocompromised patients may develop pneumonia, coagulopathies, or neurologic sequelae including encephalitis.
Complications. Rubella infection, especially during the first trimester, can lead to spontaneous abortion, stillbirth, or congenital rubella syndrome (CRS), a condition characterized by congenital cataracts and “blueberry muffin” skin lesions.31 Infants affected by CRS can also have heart defects, intellectual disabilities, deafness, and low birth weight. Diagnosis of primary maternal infection should be made with serologic tests. Fetal infection can be determined by detection of fetal serum IgM after 22 to 24 weeks of gestation or with viral culture of amniotic fluid.31
Treatment and prevention. Currently, no antiviral treatments are available; however, vaccines are highly effective at preventing infection. Rubella vaccine is usually given as part of the measles, mumps, rubella (MMR) vaccine, which is administered at age 12 to 15 months and again between 4 and 6 years of age.
Measles
Measles is a highly contagious disease caused by a virus that belongs to the Morbillivirus genus of the family Paramyxoviridae. Infection occurs through inhalation of, or direct contact with, respiratory droplets from infected people.32
Presentation. People with measles often present with what starts as a macular rash on the face that then spreads downward to the neck, trunk, and extremities (for photos, see http://www.cdc.gov/measles/hcp/index.html). As the disease progresses, papules can develop and the lesions may coalesce.
The rash is often preceded by 3 to 4 days of malaise and fever (temperature often greater than 104° F), along with the classic symptom triad of cough, coryza, and conjunctivitis. Koplik spots—clustered white lesions on the buccal mucosa—are often present prior to the measles rash and are pathognomonic for measles infection.33
Because the symptoms of measles are easily confused with other viral infections, suspected cases of measles should be confirmed via IgG and IgM antibody tests, by reverse transcription-PCR, or both.34,35 For limited and unusual cases, the Centers for Disease Control and Prevention can perform a plaque reduction neutralization assay.35
Complications. Measles infection is self-limited in immunocompetent patients. The most common complications are diarrhea and ear infections, but more serious complications, such as pneumonia, hearing loss, and encephalitis, can occur. Children <2 years of age, particularly boys, are at an increased risk of developing subacute sclerosing panencephalitis, a fatal neurologic disorder that can develop years after the initial measles infection.33,36
Treatment and prevention. Treatment is supportive and usually consists of acetaminophen or NSAIDs and fluids.
A live attenuated version of the measles vaccine is highly effective against the measles virus and has greatly reduced the number of measles cases globally.37 The measles vaccine is usually given in 2 doses—the first one after one year of age, and the second one before entering kindergarten. The most common adverse reactions to the vaccine are pain at the injection site and fever. Despite the fact that the MMR vaccine is effective and relatively benign, measles outbreaks continue to occur, as some parents forego routine childhood immunizations because of religious or other personal beliefs or safety concerns.38
Molluscum contagiosum
Molluscum contagiosum (MC) is caused by the MC virus, a member of the poxvirus family. The virus is transmitted by direct contact with the skin lesions. This skin condition is seen mainly in children, although it can occur in adults.
A study conducted in England and Wales that obtained information from the Royal College of General Practitioners reported an incidence of 15.0-17.2/1000 population over a 10-year period (1994-2003) with no variation between sexes.39 There is an association between atopic dermatitis and MC; 24% of children with atopic dermatitis develop MC.40 There might also be an association between recent swimming in a public pool and development of MC lesions.41
Presentation. Lesions caused by MC are small, discrete, waxy dome-shaped papules with central umbilication that are usually 3 to 5 mm in diameter (FIGURE 5).42,43 In immunocompetent patients, there are usually fewer than 20 lesions, which resolve within a year. However, in immunocompromised patients, the number of lesions is usually higher, and the diameter of each may be greater than 1 cm.42
Complications. The lesions are usually self-limited, but on occasion can become secondarily infected, usually with gram-positive organisms such as Staphylococcus aureus. Very rarely, abscesses develop requiring topical and/or systemic antimicrobials and perhaps incision and drainage.44
Treatment and prevention. Because the infection is often self-limited and benign, the preferred therapeutic modality is watchful waiting. Other treatments for MC include curettage, chemical agents, immune modulators, and antiviral drugs. A 2009 Cochran review of 11 studies involving 495 patients found “no single intervention to be convincingly effective in the treatment of molluscum contagiosum.”45 And no vaccine exists.
Gianotti-Crosti syndrome
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis of childhood, is a relatively rare, self-limited exanthema that usually affects infants and children 6 months to 12 years of age (peak occurrence is in one- to 6-year-olds). Although there have been reports of adults with this syndrome, it is unusual in this age group.
Pathogenesis is still unknown. Although GCS itself isn’t contagious, the viruses that can cause it may be. Initially, researchers believed that GCS was linked to acute hepatitis B virus infection, but more recently other viral and bacterial infections have been associated with the condition.46
The most commonly associated virus in the United States is Epstein-Barr virus; other viruses include hepatitis A virus, cytomegalovirus, coxsackievirus, respiratory syncytial virus, parainfluenza virus, rotavirus, the mumps virus, parvovirus, and molluscum contagiosum.
Bacterial infections, such as those caused by Bartonella henselae, Mycoplasma pneumoniae, and group A streptococci may trigger GCS.47-49
Vaccines that have been implicated in GCS include those for polio, diphtheria/pertussis/tetanus, MMR, hepatitis A and B, as well as the influenza vaccine.48-51
Presentation. While GCS is relatively rare, its presentation is classic, making it easy to diagnose once it’s included in the differential. The pruritic rash usually consists of acute-onset monomorphous, flat-topped or dome-shaped red-brown papules and papulovesicles, one to 10 mm in size, located symmetrically on the face (FIGURE 6), the extensor surfaces of the arms and legs, and, less commonly, the buttocks. It rarely affects other parts of the body.48
The diagnosis is usually based on the characteristic rash and the benign nature of the condition; other than the rash, patients are typically asymptomatic and healthy. Sometimes a biopsy is performed and it reveals a dense lichenoid lymphohistiocytic infiltrate with a strong cytoplasmic immunopositivity for beta-defensin-4 in the stratum corneum, granulosum, and spinosum.52
The lesions spontaneously resolve within 8 to 12 weeks. GCS usually presents during spring and early summer and affects both sexes equally.46
Treatment and prevention. Treatment is usually symptomatic, with the use of oral antihistamines if the lesions become pruritic. Topical steroids may be used, and, in a few cases, oral corticosteroids may be considered. No vaccine exists.
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; [email protected].
Family physicians encounter skin rashes on a daily basis. First steps in making the diagnosis include identifying the characteristics of the rash and determining whether the eruption is accompanied by fever or any other symptoms. In the article that follows, we review 8 viral exanthems of childhood that range from the common (chickenpox) to the not-so-common (Gianotti-Crosti syndrome).
Varicella-zoster virus
Varicella-zoster virus (VZV) is a human neurotropic alphaherpesvirus that causes a primary infection commonly known as chickenpox (varicella).1 This disease is usually mild and resolves spontaneously.
This highly contagious virus is transmitted by directly touching the blisters, saliva, or mucus of an infected person. It is also transmitted through the air by coughing and sneezing. VZV initiates primary infection by inoculating the respiratory mucosa. It then establishes a lifelong presence in the sensory ganglionic neurons and, thus, can reactivate later in life causing herpes zoster (shingles), which can affect cranial, thoracic, and lumbosacral dermatomes. Acute or chronic neurologic disorders, including cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis, and multiple ocular disorders, have been reported after VZV reactivation resulting in herpes-zoster.1
Presentation. With varicella, an extremely pruritic rash follows a brief prodromal stage consisting of a low-grade fever, upper respiratory tract symptoms, tiredness, and fatigue. This exanthem develops rapidly, often beginning on the chest, trunk, or scalp and then spreading to the arms and legs (centrifugally) (FIGURE 1). Varicella also affects mucosal areas of the body, such as the conjunctiva, mouth, rectum, and vagina.
The lesions are papules that rapidly become vesicular with clear fluid inside. Subsequently, the lesions begin to crust. Scabbing occurs within 10 to 14 days. A sure sign of chickenpox is the presence of papules, vesicles, and crusting lesions in close proximity.
Complications. The most common complications of chickenpox—especially in children—are invasive streptococcal and staphylococcal infections.2 The most serious complication occurs when the virus invades the spinal cord, causing myelitis or affecting the cerebral arteries, leading to vasculopathy. Diagnosis of VZV in the central nervous system is based on isolation of the virus in cerebral spinal fluid by polymerase chain reaction (PCR). Early diagnosis is important to minimize morbidity and mortality.
Reactivation is sometimes associated with post-herpetic neuralgia (PHN), a severe neuropathic pain syndrome isolated to the dermatomes affected by VZV. PHN can cause pain and suffering for years after shingles resolves, and sometimes is refractory to treatment. PHN may reflect a chronic varicella virus ganglionitis.
A number of treatment choices exist for shingles, but not so much for varicella
Oral treatment. Oral medications such as acyclovir and its prodrug valacyclovir are the current gold standards for the treatment of VZV.3
Famciclovir, the prodrug of penciclovir, is more effective than valacyclovir at resolving acute herpes zoster rash and shortening the duration of PHN.4 Gabapentinoids (eg, pregabalin) are the only oral medications approved by the US Food and Drug Administration (FDA) to treat PHN.5
Topical medications can also be used. Lidocaine 5% is favored as first-line therapy for the amelioration of pain due to shingles, as it provides modest pain relief with a better safety and tolerability profile than capsaicin 8% patch, which is a second-line choice. The latter must be applied multiple times daily, has minimal analgesic efficacy, and frequently causes initial pain upon application.
Gabapentinoids and topical analgesics can be used in combination due to the low propensity for drug interactions.6,7 The treatment of choice for focal vasculopathy is intravenous acyclovir, usually for 14 days, although immunocompromised patients should be treated for a longer period of time. Also consider 5 days of steroid therapy for patients with VZV vasculopathy.8
Non-FDA approved treatments include tricyclic antidepressants (TCA), such as amitriptyline, nortriptyline, and desipramine, which are sometimes used as first-line therapy for shingles. TCAs may not work well in patients with burning pain, and can have significant adverse effects, including possible cardiotoxicity.9
Opioids, including oxycodone, morphine, methadone, and tramadol, are sometimes used in pain management, but concern exists for abuse. Because patients may develop physical dependence, use opioids with considerable caution.10
Prevention. The United States became the first country to institute a routine varicella immunization program after a varicella vaccine (Varivax) was licensed in 1995.11 The vaccine has reduced the number of varicella infection cases dramatically.11 Vaccine effectiveness is high, and protective herd immunity is obtained after 2 doses.11-13 The vaccine is administered to children after one year of age with a booster dose administered after the fourth birthday.
A live, attenuated VZV vaccine (Zostavax) is given to individuals ≥60 years of age to prevent or attenuate herpes zoster infection. This vaccine is used to boost VZV-specific cell-mediated immunity in adults, thereby decreasing the burden of herpes zoster and the pain associated with PHN.14
Roseola
Roseola infantum, also known as exanthema subitum and sixth disease, is a common mild acute febrile illness of childhood caused by infection with human herpesvirus (HHV) 6 (the primary agent causing roseola) or 7 (a secondary causal agent for roseola).15 HHV-6 has 2 variants (HHV-6a and HHV-6b). Roseola infantum is mostly associated with the HHV-6b variant, which predominantly affects children 6 to 36 months of age.16
The virus replicates in the salivary glands and is shed through saliva, which is the route of transmission. After a 10- to 15-day incubation period, it remains latent in lymphocytes and monocytes, thus persisting in cells and tissues. It may reactivate late in life, particularly in immunosuppressed individuals. Reactivated infection in immunocompromised patients may be associated with serious illness such as encephalitis/encephalopathy. In patients who have received a bone marrow transplant, it can induce graft vs host disease.17
Presentation. The virus causes a 5- to 6-day illness characterized by high fever (temperature as high as 105°-106° F), miringitis (inflammation of tympanic membranes), and irritability. Once defervescence occurs, an erythematous morbilliform exanthem appears.The rash, which has a discrete macular/papular soft-pink appearance, starts on the trunk and spreads centrifugally to the extremities, neck, and face (FIGURE 2). It usually resolves within one to 2 days.

Complications. The most common complication of roseola is febrile seizures.17 Less common ones include encephalitis, encephalopathy, fatal hemophagocytic syndrome,18 or fulminant hepatitis.19
Treatment and prevention. Treatment depends on symptoms and may include antipyretics for fever management and liquids to maintain hydration. Recovery is usually complete with no significant sequelae. If a child develops a seizure, no antiepileptic drugs are recommended. No vaccine exists.
Fifth disease
Human parvovirus B19, a minute ssDNA virus, was first associated with human disease in 1981, when it was linked to an aplastic crisis in a patient with sickle cell disease.20 Since then researchers have determined that it is also the cause of erythema infectiosum or fifth disease of childhood. The B19 virus can also cause anemia in the fetus as well as hydrops fetalis. It has been linked to arthralgia and arthritis (especially in adults). There is an association with autoimmune diseases with characteristics similar to rheumatoid arthritis.20
The B19 virus is transmitted via aerosolized respiratory secretions, contaminated blood, or the placenta. The virus replicates in erythroid cells in bone marrow and peripheral blood, thus inhibiting erythropoiesis.21 Once the rash appears, the virus is no longer infectious.22 Seasonal peaks occur in the late winter and spring, with sporadic infections throughout the year.23 More than 70% of the adult population is seropositive for this virus.20
Presentation. Erythema infectiosum is a mild illness in childhood with an incubation period of 6 to 18 days. It presents with a characteristic malar rash on the face that gives patients a slapped cheek appearance (FIGURE 3A). A softer pink-colored “lacy” reticulated rash that blanches when touched may appear on the trunk, arms, and legs (FIGURE 3B).
Another presentation, which involves the hands and feet (glove and sock syndrome) (FIGURES 3C and 3D), consists of a purpuric eruption with painful edema and numerous small confluent petechiae.22,24 A majority of patients present with inflammatory symptoms that tend to resolve without sequelae within 3 weeks of infection.23

A rash is not as prevalent in adults as in children. Adults often present with more systemic systems, such as a debilitating influenza-like illness, arthropathy, transient aplastic anemia in sickle cell-affected individuals, and persistent viral suppression of erythrocyte production in immunocompromised patients and organ-transplant recipients.
Complications. The B19 virus can cause spontaneous abortion in pregnant women and anemia and hydrops fetalis in the fetus.22 Arthritis can occur in children, but is more common in adults, especially in women. The arthritis tends to be symmetrical and affects small joints such as the hands, wrists, and knees.
In one study of parvovirus B19 involving 633 children with sickle cell disease, 68 children developed transient red cell aplasia, 19% of them developed splenic sequestration, and 12% developed acute chest syndrome, a lung-related complication of sickle cell disease that can lower the level of oxygen in the blood and can be fatal.25
Treatment and prevention. Treatment of B19 infection is symptomatic; for example, nonsteroidal anti-inflammatory drugs (NSAIDs) are used if joint pain develops. No vaccine exists.
Hand, foot, and mouth disease
Hand, foot, and mouth disease (HFMD) is caused by the picornavirus family, including the Coxsackievirus, Enterovirus, and Echovirus. Infections commonly occur in the spring, summer, and fall. The virus primarily affects infants and children <10 years of age with the infection typically lasting 7 to 10 days.26
Presentation. The disease usually presents with a febrile episode, progressing to nasal congestion and malaise. One to 2 days later, the classic rash appears. Patients with HFMD usually present with papular or vesicular lesions on the hands and feet and painful oral lesions (FIGURES 4A, 4B, and 4C). The rash may also affect other parts of the body including the legs and buttocks. Desquamation of nails may occur up to one month after the HFM infection.27 Most cases are diagnosed by clinical presentation, but infection can be confirmed by PCR of vesicular lesion fluid.
Complications. In addition to being caused by Coxsackievirus, HFMD may be caused by human Enterovirus A serotype 71 (HEVA-71), which is associated with a high prevalence of acute neurologic disease including aseptic meningitis, poliomyelitis-like paralysis, and encephalitis.26 Of 764 HFMD patients enrolled in a prospective study, 214 cases were associated with Coxsackievirus A 16 (CVA16) infection and 173 cases were associated with HEVA-71 infection. Rare cases of HFMD have led to encephalitis, meningitis, flaccid paralysis, and death.26
Treatment and prevention. HFMD is usually self-limited, and treatment is supportive. There has been interest in developing an HFMD vaccine, but no products are as yet commercially available.
Rubella
Rubella, also known as the German measles or the 3-day measles, is caused by the rubella virus, which is transmitted via respiratory droplets. Up to half of rubella infections are asymptomatic.28-30
Presentation. Rubella typically has an incubation period of 12 to 24 days, with a 5-day prodromal period characterized by fever, headache, and other symptoms typical of an upper respiratory infection, including sore throat, arthralgia, and tender lymphadenopathy.28
The rash often starts as erythematous or as rose-pink macules on the face that progress down the body. The rash can cover the trunk and extremities within 24 hours. (For photos, see https://www.cdc.gov/rubella/about/photos.html.)
Patients are infectious from 7 days prior to the appearance of the rash to 7 days after resolution of the rash. Given the potentially prolonged infectious period, patients hospitalized for rubella infection should be placed on droplet precautions, and children should be kept from day care and school for 7 days after the appearance of the rash.28
Rubella is typically a mild disease in immunocompetent patients; however, immunocompromised patients may develop pneumonia, coagulopathies, or neurologic sequelae including encephalitis.
Complications. Rubella infection, especially during the first trimester, can lead to spontaneous abortion, stillbirth, or congenital rubella syndrome (CRS), a condition characterized by congenital cataracts and “blueberry muffin” skin lesions.31 Infants affected by CRS can also have heart defects, intellectual disabilities, deafness, and low birth weight. Diagnosis of primary maternal infection should be made with serologic tests. Fetal infection can be determined by detection of fetal serum IgM after 22 to 24 weeks of gestation or with viral culture of amniotic fluid.31
Treatment and prevention. Currently, no antiviral treatments are available; however, vaccines are highly effective at preventing infection. Rubella vaccine is usually given as part of the measles, mumps, rubella (MMR) vaccine, which is administered at age 12 to 15 months and again between 4 and 6 years of age.
Measles
Measles is a highly contagious disease caused by a virus that belongs to the Morbillivirus genus of the family Paramyxoviridae. Infection occurs through inhalation of, or direct contact with, respiratory droplets from infected people.32
Presentation. People with measles often present with what starts as a macular rash on the face that then spreads downward to the neck, trunk, and extremities (for photos, see http://www.cdc.gov/measles/hcp/index.html). As the disease progresses, papules can develop and the lesions may coalesce.
The rash is often preceded by 3 to 4 days of malaise and fever (temperature often greater than 104° F), along with the classic symptom triad of cough, coryza, and conjunctivitis. Koplik spots—clustered white lesions on the buccal mucosa—are often present prior to the measles rash and are pathognomonic for measles infection.33
Because the symptoms of measles are easily confused with other viral infections, suspected cases of measles should be confirmed via IgG and IgM antibody tests, by reverse transcription-PCR, or both.34,35 For limited and unusual cases, the Centers for Disease Control and Prevention can perform a plaque reduction neutralization assay.35
Complications. Measles infection is self-limited in immunocompetent patients. The most common complications are diarrhea and ear infections, but more serious complications, such as pneumonia, hearing loss, and encephalitis, can occur. Children <2 years of age, particularly boys, are at an increased risk of developing subacute sclerosing panencephalitis, a fatal neurologic disorder that can develop years after the initial measles infection.33,36
Treatment and prevention. Treatment is supportive and usually consists of acetaminophen or NSAIDs and fluids.
A live attenuated version of the measles vaccine is highly effective against the measles virus and has greatly reduced the number of measles cases globally.37 The measles vaccine is usually given in 2 doses—the first one after one year of age, and the second one before entering kindergarten. The most common adverse reactions to the vaccine are pain at the injection site and fever. Despite the fact that the MMR vaccine is effective and relatively benign, measles outbreaks continue to occur, as some parents forego routine childhood immunizations because of religious or other personal beliefs or safety concerns.38
Molluscum contagiosum
Molluscum contagiosum (MC) is caused by the MC virus, a member of the poxvirus family. The virus is transmitted by direct contact with the skin lesions. This skin condition is seen mainly in children, although it can occur in adults.
A study conducted in England and Wales that obtained information from the Royal College of General Practitioners reported an incidence of 15.0-17.2/1000 population over a 10-year period (1994-2003) with no variation between sexes.39 There is an association between atopic dermatitis and MC; 24% of children with atopic dermatitis develop MC.40 There might also be an association between recent swimming in a public pool and development of MC lesions.41
Presentation. Lesions caused by MC are small, discrete, waxy dome-shaped papules with central umbilication that are usually 3 to 5 mm in diameter (FIGURE 5).42,43 In immunocompetent patients, there are usually fewer than 20 lesions, which resolve within a year. However, in immunocompromised patients, the number of lesions is usually higher, and the diameter of each may be greater than 1 cm.42
Complications. The lesions are usually self-limited, but on occasion can become secondarily infected, usually with gram-positive organisms such as Staphylococcus aureus. Very rarely, abscesses develop requiring topical and/or systemic antimicrobials and perhaps incision and drainage.44
Treatment and prevention. Because the infection is often self-limited and benign, the preferred therapeutic modality is watchful waiting. Other treatments for MC include curettage, chemical agents, immune modulators, and antiviral drugs. A 2009 Cochran review of 11 studies involving 495 patients found “no single intervention to be convincingly effective in the treatment of molluscum contagiosum.”45 And no vaccine exists.
Gianotti-Crosti syndrome
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis of childhood, is a relatively rare, self-limited exanthema that usually affects infants and children 6 months to 12 years of age (peak occurrence is in one- to 6-year-olds). Although there have been reports of adults with this syndrome, it is unusual in this age group.
Pathogenesis is still unknown. Although GCS itself isn’t contagious, the viruses that can cause it may be. Initially, researchers believed that GCS was linked to acute hepatitis B virus infection, but more recently other viral and bacterial infections have been associated with the condition.46
The most commonly associated virus in the United States is Epstein-Barr virus; other viruses include hepatitis A virus, cytomegalovirus, coxsackievirus, respiratory syncytial virus, parainfluenza virus, rotavirus, the mumps virus, parvovirus, and molluscum contagiosum.
Bacterial infections, such as those caused by Bartonella henselae, Mycoplasma pneumoniae, and group A streptococci may trigger GCS.47-49
Vaccines that have been implicated in GCS include those for polio, diphtheria/pertussis/tetanus, MMR, hepatitis A and B, as well as the influenza vaccine.48-51
Presentation. While GCS is relatively rare, its presentation is classic, making it easy to diagnose once it’s included in the differential. The pruritic rash usually consists of acute-onset monomorphous, flat-topped or dome-shaped red-brown papules and papulovesicles, one to 10 mm in size, located symmetrically on the face (FIGURE 6), the extensor surfaces of the arms and legs, and, less commonly, the buttocks. It rarely affects other parts of the body.48
The diagnosis is usually based on the characteristic rash and the benign nature of the condition; other than the rash, patients are typically asymptomatic and healthy. Sometimes a biopsy is performed and it reveals a dense lichenoid lymphohistiocytic infiltrate with a strong cytoplasmic immunopositivity for beta-defensin-4 in the stratum corneum, granulosum, and spinosum.52
The lesions spontaneously resolve within 8 to 12 weeks. GCS usually presents during spring and early summer and affects both sexes equally.46
Treatment and prevention. Treatment is usually symptomatic, with the use of oral antihistamines if the lesions become pruritic. Topical steroids may be used, and, in a few cases, oral corticosteroids may be considered. No vaccine exists.
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; [email protected].
1. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
2. Blumental S, Sabbe M, Lepage P, the Belgian Group for Varicella. Varicella paediatric hospitalisations in Belgium: a 1-year national survey. Arch Dis Child. 2016;101:16-22.
3. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clinic Proc. 2009;84:274-280.
4. Ono F, Yasumoto S, Furumura M, et al. Comparison between famciclovir and valacyclovir for acute pain in adult Japanese immunocompetent patients with herpes zoster. J Dermatol. 2012;39:902-908.
5. Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of post-herpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125-132.
6. Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs & Aging. 2012;29:863-869.
7. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164.
8. Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;9:731-740.
9. Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and post herpetic neuralgia. Am Fam Physician. 2000;61:2437-2444.
10. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3-S14.
11. Thomas CA, Shwe T, Bixler D, et al. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J. 2014;33:1164-1168.
12. Helmuth IG, Poulsen A, Suppli CH, et al. Varicella in Europe-a review of the epidemiology and experience with vaccination. Vaccine. 2015;33:2406-2413.
13. Marin M, Marti M, Kambhampati A, et al. Global varicella vaccine effectiveness: a metanalysis. Pediatrics. 2016;137:e20153741.
14. Centers for Disease Control and Prevention. What everyone should know about shingles vaccine. Available at: www.cdc.gov/vaccines/vpd/shingles/public/index.html. Accessed September 12, 2017.
15. Tanaka K, Kondo T, Torigoe S, et al. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J Pediatr. 1994;125:1-5.
16. Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33:829-833.
17. Koch WC. Fifth (human parvovirus) and sixth (herpesvirus 6) diseases. Curr Opin Infect Dis. 2001;14:343-356.
18. Marabelle A, Bergeron C, Billaud G, et al. Hemophagocytic syndrome revealing primary HHV-6 infection. J Pediatr. 2010;157:511.
19. Charnot-Katsikasa A, Baewer D, Cook L, et al. Fulminant hepatic failure attributed to infection with human herpesvirus 6 (HHV-6) in an immunocompetent woman: a case report and review of the literature. J Clin Virol. 2016;75:27-32.
20. Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol. 2004;53(Pt 6):459-475.
21. Dolin R. Parvovirus erythema infectiousum, Aplastic anemia. In: Mandell, Douglas, Bennett’s Principles and Practice of Infectious Diseases. 3rd ed. New York, NY: Churchill Livingstone Inc; 1990:1231-1232.
22. Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. 2007;75:373-376.
23. Martin DR, Schlott DW, Flynn JA. Clinical problem-solving. No respecter of age. N Engl J Med. 2007;357:1856-1859.
24. Ozaydin V, Eceviz A, Sari Dogan F, et al. An adult patient who presented to emergency service with a papular purpuric gloves and socks syndrome: a case report. Turk J Emerg Med. 2014;14:179-181.
25. Smith-Whitley K, Zhao H, Hodinka RL, et al. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood. 2004;103:422-427.
26. Tu PV, Thao NT, Perera D, et al. Epidemiologic and virologic investigation of hand, foot, and mouth disease, Southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733-1741.
27. Ferrari B, Taliercio V, Hornos L, et al. Onychomadesis associated with mouth, hand and foot disease. Arch Argent Pediatr. 2013;111:e148-e151.
28. Alter SJ, Bennett JS, Koranyi K, et al. Common childhood viral infections. Curr Probl Pediatr Adolesc Health Care. 2015;45:21-53.
29. Lambert N, Strebel P, Orenstein W, et al. Rubella. Lancet. 2015;385:2297-2307.
30. Silasi M, Cardenas I, Kwon JY, et al. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73:199-213.
31. Tang JW, Aarons E, Hesketh LM, et al. Prenatal diagnosis of congenital rubella infection in the second trimester of pregnancy Prenat Diagn. 2003;23:509-512.
32. Naim HY. Measles virus. Hum Vaccin Immunother. 2015;11:21-26.
33. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: http://www.cdc.gov/measles/hcp/index.html. Accessed April 28, 2016.
34. Takao S, Shigemoto N, Shimazu Y, et al. Detection of exanthematic viruses using a TaqMan real-time PCR assay panel in patients with clinically diagnosed or suspected measles. Jpn J Infect Dis. 2012;65:444-448.
35. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: https://www.cdc.gov/measles/lab-tools/rt-pcr.html. Accessed April 28, 2016.
36. Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012;36:649-662.
37. Centers for Disease Control and Prevention. Measles, vaccination. Available at: https://www.cdc.gov/measles/vaccination.html. Accessed April 28, 2016.
38. Campos-Outcalt D. Measles: Why it’s still a threat. J Fam Pract. 2017;66:446-449.
39. Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
40. Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
41. Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
42. Martin P. Interventions for molluscum contagiosum in people infected with human immunodeficiency virus: a systematic review. Int J Dermatol. 2016;55:956-966.
43. Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13:877-888.
44. Lacour M, Posfay-Barbe KM, La Scala GC. Staphylococcus lugdunensis abscesses complicating molluscum contagiosum in two children. Pediatr Dermatol. 2015;32:289-291.
45. van der Wouden JC, van der Sande R, van Suijlekom-Smit LW, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2009;CD004767.
46. Tagawa C, Speakman M. Photo quiz. Papular rash in a child after a fever. Gianotti-Crosti syndrome. Am Fam Physician. 2013;87:59-60.
47. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
48. Retrouvey M, Koch LH, Williams JV. Gianotti-Crosti syndrome following childhood vaccinations. Pediatr Dermatol. 2013;30:137-138.
49. Velangi SS, Tidman MJ. Gianotti-Crosti syndrome after measles, mumps, and rubella vaccination. Br J Dermatol. 1998;139:1122-1123.
50. Lacour M, Harms M. Gianotti-Crosti syndrome as a result of vaccination and Epstein-Barr virus infection. Eur J Pediatr. 1995;154:688-689.
51. Kroeskop A, Lewis AB, Barril FA, et al. Gianotti-Crosti syndrome after H1N1-influenza vaccine. Pediatr Dermatol. 2011;28:595-596.
52. Caltabiano R, Vecchio GM, De Pasquale R, et al. Human beta-defensin 4 expression in Gianotti-Crosti. Acta Dermatovenerol Croat. 2013;21:43-47.
1. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
2. Blumental S, Sabbe M, Lepage P, the Belgian Group for Varicella. Varicella paediatric hospitalisations in Belgium: a 1-year national survey. Arch Dis Child. 2016;101:16-22.
3. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clinic Proc. 2009;84:274-280.
4. Ono F, Yasumoto S, Furumura M, et al. Comparison between famciclovir and valacyclovir for acute pain in adult Japanese immunocompetent patients with herpes zoster. J Dermatol. 2012;39:902-908.
5. Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of post-herpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125-132.
6. Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs & Aging. 2012;29:863-869.
7. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164.
8. Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;9:731-740.
9. Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and post herpetic neuralgia. Am Fam Physician. 2000;61:2437-2444.
10. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3-S14.
11. Thomas CA, Shwe T, Bixler D, et al. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J. 2014;33:1164-1168.
12. Helmuth IG, Poulsen A, Suppli CH, et al. Varicella in Europe-a review of the epidemiology and experience with vaccination. Vaccine. 2015;33:2406-2413.
13. Marin M, Marti M, Kambhampati A, et al. Global varicella vaccine effectiveness: a metanalysis. Pediatrics. 2016;137:e20153741.
14. Centers for Disease Control and Prevention. What everyone should know about shingles vaccine. Available at: www.cdc.gov/vaccines/vpd/shingles/public/index.html. Accessed September 12, 2017.
15. Tanaka K, Kondo T, Torigoe S, et al. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J Pediatr. 1994;125:1-5.
16. Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33:829-833.
17. Koch WC. Fifth (human parvovirus) and sixth (herpesvirus 6) diseases. Curr Opin Infect Dis. 2001;14:343-356.
18. Marabelle A, Bergeron C, Billaud G, et al. Hemophagocytic syndrome revealing primary HHV-6 infection. J Pediatr. 2010;157:511.
19. Charnot-Katsikasa A, Baewer D, Cook L, et al. Fulminant hepatic failure attributed to infection with human herpesvirus 6 (HHV-6) in an immunocompetent woman: a case report and review of the literature. J Clin Virol. 2016;75:27-32.
20. Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol. 2004;53(Pt 6):459-475.
21. Dolin R. Parvovirus erythema infectiousum, Aplastic anemia. In: Mandell, Douglas, Bennett’s Principles and Practice of Infectious Diseases. 3rd ed. New York, NY: Churchill Livingstone Inc; 1990:1231-1232.
22. Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. 2007;75:373-376.
23. Martin DR, Schlott DW, Flynn JA. Clinical problem-solving. No respecter of age. N Engl J Med. 2007;357:1856-1859.
24. Ozaydin V, Eceviz A, Sari Dogan F, et al. An adult patient who presented to emergency service with a papular purpuric gloves and socks syndrome: a case report. Turk J Emerg Med. 2014;14:179-181.
25. Smith-Whitley K, Zhao H, Hodinka RL, et al. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood. 2004;103:422-427.
26. Tu PV, Thao NT, Perera D, et al. Epidemiologic and virologic investigation of hand, foot, and mouth disease, Southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733-1741.
27. Ferrari B, Taliercio V, Hornos L, et al. Onychomadesis associated with mouth, hand and foot disease. Arch Argent Pediatr. 2013;111:e148-e151.
28. Alter SJ, Bennett JS, Koranyi K, et al. Common childhood viral infections. Curr Probl Pediatr Adolesc Health Care. 2015;45:21-53.
29. Lambert N, Strebel P, Orenstein W, et al. Rubella. Lancet. 2015;385:2297-2307.
30. Silasi M, Cardenas I, Kwon JY, et al. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73:199-213.
31. Tang JW, Aarons E, Hesketh LM, et al. Prenatal diagnosis of congenital rubella infection in the second trimester of pregnancy Prenat Diagn. 2003;23:509-512.
32. Naim HY. Measles virus. Hum Vaccin Immunother. 2015;11:21-26.
33. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: http://www.cdc.gov/measles/hcp/index.html. Accessed April 28, 2016.
34. Takao S, Shigemoto N, Shimazu Y, et al. Detection of exanthematic viruses using a TaqMan real-time PCR assay panel in patients with clinically diagnosed or suspected measles. Jpn J Infect Dis. 2012;65:444-448.
35. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: https://www.cdc.gov/measles/lab-tools/rt-pcr.html. Accessed April 28, 2016.
36. Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012;36:649-662.
37. Centers for Disease Control and Prevention. Measles, vaccination. Available at: https://www.cdc.gov/measles/vaccination.html. Accessed April 28, 2016.
38. Campos-Outcalt D. Measles: Why it’s still a threat. J Fam Pract. 2017;66:446-449.
39. Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
40. Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
41. Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
42. Martin P. Interventions for molluscum contagiosum in people infected with human immunodeficiency virus: a systematic review. Int J Dermatol. 2016;55:956-966.
43. Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13:877-888.
44. Lacour M, Posfay-Barbe KM, La Scala GC. Staphylococcus lugdunensis abscesses complicating molluscum contagiosum in two children. Pediatr Dermatol. 2015;32:289-291.
45. van der Wouden JC, van der Sande R, van Suijlekom-Smit LW, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2009;CD004767.
46. Tagawa C, Speakman M. Photo quiz. Papular rash in a child after a fever. Gianotti-Crosti syndrome. Am Fam Physician. 2013;87:59-60.
47. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
48. Retrouvey M, Koch LH, Williams JV. Gianotti-Crosti syndrome following childhood vaccinations. Pediatr Dermatol. 2013;30:137-138.
49. Velangi SS, Tidman MJ. Gianotti-Crosti syndrome after measles, mumps, and rubella vaccination. Br J Dermatol. 1998;139:1122-1123.
50. Lacour M, Harms M. Gianotti-Crosti syndrome as a result of vaccination and Epstein-Barr virus infection. Eur J Pediatr. 1995;154:688-689.
51. Kroeskop A, Lewis AB, Barril FA, et al. Gianotti-Crosti syndrome after H1N1-influenza vaccine. Pediatr Dermatol. 2011;28:595-596.
52. Caltabiano R, Vecchio GM, De Pasquale R, et al. Human beta-defensin 4 expression in Gianotti-Crosti. Acta Dermatovenerol Croat. 2013;21:43-47.
From The Journal of Family Practice | 2017;66(10):598-606.
PRACTICE RECOMMENDATIONS
› Administer the varicella-zoster vaccine to all adults ≥60 years of age to prevent or attenuate herpes zoster infection. A
› Avoid congenital rubella syndrome by vaccinating all at-risk pregnant women. A
› Administer 2 doses of the measles vaccine (one at 12-15 months of age and one at 4-6 years of age) to all children to avoid a resurgence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hypertension treatment strategies for older adults
CASE 1 An 82-year-old black woman comes in for an annual exam. She has no medical concerns. She volunteers at a hospice, walks daily, and maintains a healthy diet. Her past medical history (PMH) includes osteopenia and osteoarthritis, and her medications include acetaminophen as needed and vitamin D. She has no drug allergies. Her exam reveals a blood pressure (BP) of 148/70 mm Hg, a body mass index of 31, and a heart rate (HR) of 71 beats per minute (bpm). Cardiac and pulmonary exams are normal, and she shows no signs of peripheral edema.
CASE 2 An 88-year-old white man presents to the office for a 3-month follow-up of his hypertension. His systolic BP at home has ranged from 140 to 170 mm Hg. He denies chest pain, shortness of breath, or lower extremity edema. He lives with his wife and frequently swims for exercise. His PMH is significant for depression and degenerative disc disease. His medications include hydrochlorothiazide 12.5 mg/d, sertraline 50 mg/d, and naproxen 250 mg bid. His BP is 160/80 mm Hg and his HR is 70 bpm with normal cardiovascular (CV) and pulmonary exams.
CASE 3 An 80-year-old white man with diabetes mellitus (DM), hypertension, and chronic kidney disease (CKD) presents for a 3-month follow-up visit. His home systolic BP has been in the 140s to 150s. He is functional in all of his activities of daily living (ADLs), but is starting to require assistance with medications, finances, and transportation. He takes aspirin 81 mg/d, chlorthalidone 25 mg/d, and atenolol 50 mg/d. Remarkable laboratory test results include a hemoglobin A1c of 8.6%, a serum creatinine of 1.9 mg/dL (normal range: 0.6-1.2 mg/dL), and an albumin-creatinine ratio of 250 mg/g (normal range: <30 mg/g). During the exam, his BP is 143/70 mm Hg, his HR is 70 bpm, he is alert and oriented to person, place, and time, and he has normal CV and pulmonary exams with no signs of peripheral edema. He has decreased sensation in his feet, but normal reflexes.
How would you proceed with the care of these 3 patients?
Hypertension is the most common diagnosis made during physician office visits in the United States.1 Nearly one-third of the population has hypertension, and its prevalence increases with age, such that 67% of men and 79% of women ≥75 years of age have the condition.2
Evidence indicates that hypertension is a modifiable risk factor for CV and all-cause mortality (TABLE W13-6). All adults ≥75 years of age are at increased CV risk based on Framingham criteria,7 making hypertension management paramount. Complicating the situation are findings that indicate nearly half of adults with hypertension have inadequate BP control.2
Clinicians require clear direction about optimal BP targets, how best to adjust antihypertensive medications for comorbidities, and how to incorporate frailty and cognitive impairment into management strategies. This article presents recommendations derived from recent evidence and consensus guidelines regarding the management of hypertension in adults ≥75 years of age.
[polldaddy:9818133]
Diagnosing hypertension
According to the seventh report of the Joint National Committee (JNC 7), hypertension is defined as a systolic BP ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg.8 The JNC’s more recent report (JNC 8), however, does not define hypertension; instead, it sets forth treatment thresholds (eg, that there is strong evidence to support treating individuals ≥60 years of age when BP ≥150/90 mm Hg).9
It starts with an accurate BP measurement. Ensuring the accuracy of a BP measurement requires multiple readings over time. White coat hypertension and masked hypertension can complicate BP measurement. Home measurements better correlate with atherosclerotic cardiovascular disease (ASCVD) risk than do office measurements.10-12 In fact, the US Preventive Services Task Force recommends obtaining measurements outside of the clinic setting prior to initiating treatment for hypertension.13
Educate staff on the proper technique for obtaining BP measurements in the office (ie, taking measurements using an appropriately sized cuff when patients have been seated for at least 5 minutes with feet uncrossed and with their arm supported at heart level). Cold temperatures, coffee consumption, talking, and recent tobacco use can transiently raise BP. TABLE 110 outlines the initial work-up after confirming the diagnosis of hypertension. No other routine tests are recommended for the management of hypertension except those associated with medication monitoring (outlined in TABLE 210,11,14,15).
What’s the optimal BP target for older patients? No consensus exists on an optimal BP target for older patients. JNC 8 recommends a target BP <150/90 mm Hg in patients ≥60 years of age.9 The American College of Physicians recommends a systolic BP target <140 mm Hg in patients ≥60 years of age with increased stroke or CV risk.11 A subgroup analysis of patients ≥75 years of age from the Systolic BP Intervention Trial (SPRINT)3 was stopped early because of the clear composite CV and mortality benefits associated with targeting a systolic BP <120 mm Hg as compared with <140 mm Hg (TABLE W13-6). Although a criticism of this trial and its results is that the researchers included only adults with high CV risk, all adults ≥75 years of age are considered to have high CV risk by the SPRINT study.3 Another criticism is that early suspension of the trial may have exaggerated treatment effects.6
Lastly, results were seemingly discrepant from previous trials, most notably, the Action to Control CV Risk in Diabetes (ACCORD) trial.6,16 However, on closer review, the ACCORD trial16 included only patients with DM, while the SPRINT3 trial excluded patients with DM, and ACCORD comprised a younger population than the SPRINT subgroup analysis. Also, the ACCORD trial did demonstrate stroke reduction and non-significant reduction in CV events, albeit, at the cost of increased adverse events, such as hypotension, bradycardia, and hypokalemia, with tighter BP control.16
Population differences presumably explain the discrepancy in results, and a systolic BP target of <120 mm Hg is appropriate in community-dwelling, non-diabetic adults ≥75 years of age. If this target goal cannot be achieved without undue burden (ie, without syncope, hypotension, bradycardia, electrolyte disturbance, renal impairment, or substantial medication burden), a recent meta-analysis found evidence that a systolic BP goal <140 mm Hg also provides benefit.6
Initiate treatment, watch for age-related changes
Lifestyle modifications (including appropriate weight loss; reduced caffeine, salt, and alcohol intake; increased physical activity; and smoking cessation) are important in the initial and ongoing management of hypertension.10,11,17,18 JNC 8 recommends initial treatment with a thiazide-type diuretic, calcium channel blocker (CCB), angiotensin converting enzyme (ACE) inhibitor, or angiotensin receptor blocker (ARB) in the nonblack population, and a CCB or thiazide diuretic in the black population.9 Specific initial medication choices for comorbid conditions are outlined in TABLE W23,10,17-22. JNC 8 recommends against the use of a beta-blocker or alpha blocker for initial treatment of hypertension.9
Start a second drug instead of maximizing the dose of the first
If the target BP cannot be achieved within one month of initiating medication, JNC 8 recommends increasing the dose of the initial drug or adding a second drug without preference for one strategy over the other.9 However, a meta-analysis demonstrates that approximately 80% of the antihypertensive effect of a drug can be achieved with half of the standard dose of the medication; this is true for thiazide-type diuretics, ACE inhibitors/ARBs, beta-blockers, and CCBs.23
Furthermore, due to fewer adverse effects and positive synergies, studies show that combining low doses of 2 medications is more beneficial than high-dose monotherapy.19,23,24 Prescribing combination pills can be helpful to limit pill burden. It is appropriate to combine any of the 4 classes of medications recommended as initial therapy by JNC 8 except for an ACE inhibitor combined with an ARB. If the target BP cannot be achieved with 3 drugs in those classes, other medications such as potassium-sparing diuretics or beta-blockers can be added.9
Changes associated with aging
Changes associated with aging include atherosclerosis and stiffening of blood vessels, increased systolic BP, widened pulse pressure, reduced glomerular filtration rate, reduced sodium elimination and volume expansion, sinoatrial node cellular dropout, and decreased sensitivity of baroreceptors.10 Because of these alterations, antihypertensive requirements may change, and resistant hypertension may develop. In addition, older patients may be more susceptible to orthostatic hypotension, heart block, electrolyte derangements, and other antihypertensive adverse effects.
When hypertension is difficult to control. Resistant hypertension is defined as hypertension that cannot be controlled with 3 drugs from 3 different antihypertensive classes, one of which is a diuretic. Cognitive impairment, polypharmacy, and multimorbidity may contribute to difficult-to-control hypertension in older adults and should be assessed prior to work-up for other secondary causes of poorly controlled hypertension.
- Cognitive impairment is often unrecognized and may impact medication adherence, which can masquerade as treatment failure. Assess for cognitive impairment on an ongoing basis with the aging patient, especially when medication adherence appears poor.
- Polypharmacy may also contribute to uncontrolled BP. Common pharmacotherapeutic contributors to uncontrolled BP include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, high-dose decongestants, and selective norepinephrine reuptake inhibitors.25
- Multimorbidity describes 2 or more chronic medical conditions in one patient. These patients are medically complex. Comorbidities can increase pill burden and make medication adherence difficult for patients. Other poorly controlled disease states can worsen hypertension (eg, renal dysfunction secondary to diabetes). Optimize treatment of comorbid conditions.
Secondary causes. If resistant hypertension persists despite confirming medication adherence and eliminating offending medications, a work-up should ensue for secondary causes of hypertension, as well as end-organ damage. Causes of secondary hypertension include sleep apnea (see this month's HelpDesk), renal dysfunction (renal artery stenosis), aldosterone-mediated hypertension (often with hypokalemia), and thyroid disease. Evaluation for secondary causes of hypertension and end-organ damage is outlined in TABLE 1.10 Patients with well-controlled hypertension do not require repeated assessments for end-organ damage unless new symptoms—such as chest pain or edema—develop.
Consider comorbidities
Clinical trials implicitly or explicitly exclude patients with multiple comorbidities. JNC 8 provided minimal guidance for adjusting BP targets based on comorbidity with only nondiabetic CKD and DM specifically addressed.9 Guidelines from specialty organizations and recent trials provide some additional guidance in these situations and are outlined in TABLE W23,10,17-22.
Heart failure. Hypertension is a major risk factor for heart failure. Long-term treatment of systolic and diastolic hypertension can reduce the incidence of heart failure by approximately half with increased benefit in patients with prior myocardial infarction.22 Research demonstrates clear mortality benefits of certain antihypertensive drug classes, including diuretics, beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, combination hydralazine and nitrates, and angiotensin receptor-neprilysin inhibitors.21,22 The overall treatment goal in heart failure is to optimize drugs with mortality benefit, while lowering BP to a goal <130/80 mm Hg in patients ≥75 years of age.22
Increased risk for CV disease. The SPRINT trial3 defined high risk of CV disease as clinical or subclinical CV disease, CKD, 10-year ASCVD risk of ≥15%, or age ≥75 years. SPRINT supports a systolic BP goal <120 mm Hg, but, as a reminder, SPRINT excluded patients with diabetes. The American College of Cardiology Foundation Task Force and the American Heart Association define high CV risk as a 10-year ASCVD risk ≥10% and recommend a BP goal <130/80 mm Hg.10
Diabetes mellitus. A BP >115/75 mm Hg is associated with increased CV events and mortality in patients with DM.18 The American Diabetes Association (ADA) and JNC 8 recommend a BP target <140/90 mm Hg.9,18 ADA suggests a lower target of 130/80 mm Hg in patients with high CV risk if it is achievable without undue burden.18
Studies show increased mortality associated with initiating additional treatment once a systolic goal <140 mm Hg has been achieved in patients with DM.26 The ACCORD trial found increased adverse events with aggressive BP lowering to <120/80 mm Hg.16
For patients with DM requiring more than one antihypertensive agent, there are CV mortality benefits associated with administering at least one antihypertensive drug at night, likely related to the beneficial effect of physiologic nocturnal dipping.27
Chronic kidney disease. JNC 8 specifically recommends an ACE inhibitor or ARB for initial or add-on treatment in patients with CKD and a BP goal <140/90 mm Hg.9 The Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group recommends a BP target ≤140/90 mm Hg in patients without albuminuria and ≤130/80 mm Hg in patients with albuminuria to protect against the progression of nephropathy.17 The SPRINT trial3 included patients with CKD, and KDIGO has not yet updated its guidelines to reflect SPRINT.
Frailty is a clinical syndrome that has been defined as a state of increased vulnerability that is associated with a decline in reserve and function.28 The largest hypertension studies in older adults address frailty, although often the most frail patients are excluded from these studies (TABLE W13-6).
The Hypertension in the Very Elderly Trial (HYVET) categorized patients as frail, pre-frail, or robust and found a consistent benefit of antihypertensive treatment on stroke, CV events, and total mortality—regardless of baseline frailty status.29 The SPRINT trial included only community-dwelling adults.3 Other studies suggest that hypertension actually has a protective effect by lowering overall mortality in frail older adults, especially in the frailest and oldest nursing home populations.30,31
Although there is a paucity of data to direct the management of hypertension in frail older patients, physicians should prioritize the condition and focus on adverse events from antihypertensives and on slow titration of medications. The JNC 8 BP target of <150/90 mm Hg is a reasonable BP goal in this population, given the lack of evidence for lower or higher targets.9 Many frail patients have one or more of the comorbidities described earlier, and it is reasonable to strive for the comorbidity-specific target, provided it can be achieved without undue burden.
Cognitive impairment and dementia. The association between hypertension and dementia/cognitive impairment is evolving. Hypertension may impact various forms of dementia, such as Alzheimer’s disease (AD) or vascular dementia, differently. There is evidence linking hypertension to AD.32 The relationship between BP and brain perfusion is complex with the potential existence of an age-adjusted relationship such that mid-life hypertension may increase the risk of dementia while late-life hypertension may not.33
A number of studies reveal the evolving nature of our understanding of these 2 conditions:
- A recent systematic review and meta-analysis examining intensive BP treatments in older adults demonstrated that lower BP targets did not increase cognitive decline.6
- HYVET’s cognitive function assessment did not find a significant reduction in the incidence of dementia with BP reduction over a short follow-up period, but when results were combined in a meta-analysis with other placebo-controlled, double-blind trials of antihypertensive treatments, there was significant reduction in incident dementia in patients randomized to antihypertensive treatment.34
- The ACCORD Memory in Diabetes trial (ACCORD-MIND) had the unexpected outcome that intensive lowering of systolic BP to a target <120 mm Hg resulted in a greater decline in total brain volume, compared with the standard BP goal <140 mm Hg. This was measured with magnetic resonance imaging in older adults with type 2 DM.35
- Results from the SPRINT sub-analysis Memory and Cognition in Decreased Hypertension trial are forthcoming and aim to determine the effects of BP reduction on dementia.36
The JNC 89 BP target <150/90 mm Hg or a comorbidity-specific target, if achievable without undue burden, is reasonable in patients with dementia. In a systematic review of observational studies in patients with hypertension and dementia, diuretics, CCBs, ACE inhibitors/ARBs, and beta-blockers were commonly used medications with a trend toward prescribing CCBs and ACE inhibitors/ARBs.37
As previously highlighted, cognitive impairment may lead to problems with medication adherence and even inadvertent improper medication use, potentially resulting in adverse events from antihypertensives. If cognitive impairment or dementia is suspected, ensure additional measures (such as medication assistance or supervision) are in place before prescribing antihypertensives.
Certain diseases, such as Parkinson’s-related dementia and multiple system atrophy, can cause autonomic instability, which can increase the risk of falls and complicate hypertension management. Carefully monitor patients for signs of orthostasis.
CASE 1 Repeat the BP measurement in the office once the patient has been seated for ≥5 minutes, and have the patient monitor her BP at home; schedule a follow-up visit in 2 weeks. If hypertension is confirmed with home measurements, then, in addition to lifestyle modifications, initiate treatment with a CCB or thiazide diuretic to achieve a systolic BP goal <120 mm Hg. Titrate medications slowly while monitoring for adverse effects.
CASE 2 Consistent with the office measurement, the patient has home BP readings that are above the BP target (<120 mm Hg systolic). He has been taking a single antihypertensive for longer than one month. Discontinue his NSAID prior to adding any new medications. If his BP is still above target without NSAIDs, then add a second agent, such as a low dose of an ACE inhibitor, ARB, or CCB, rather than maximizing the dose of hydrochlorothiazide.
CASE 3 Given the patient’s diabetes, CKD, and albuminuria, a target BP goal <130/80 mm Hg is reasonable. An ACE inhibitor or ARB is a better medication choice than atenolol in this patient with albuminuria. Because of the deterioration in his ADLs, careful assessment of mobility, functionality, comorbidities, frailty, and cognitive function should take place at each office visit and inform adjustments to the patient’s BP target. Employ cautious medication titration with monitoring for adverse effects, especially hypotension and syncope. If his functional status declines, adverse effects develop, or the medication regimen becomes burdensome, relax the target BP goal to 150/90 mm Hg.
CORRESPONDENCE
Julienne K. Kirk, PharmD, Family and Community Medicine, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1084; [email protected].
1. National Ambulatory Medical Care Survey: 2013 State and National Summary Tables. Available at: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2013_namcs_web_tables.pdf. Accessed May 29, 2017.
2. Centers for Disease Control and Prevention. High blood pressure facts. Available at: https://cdc.gov/bloodpressure/facts.htm. Accessed May 29, 2017.
3. Williamson JD, Suplano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
4. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
5. Kostis WJ, Cabrera J, Messerli FH, et al. Competing cardiovascular and noncardiovascular risks and longevity in the systolic hypertension in the elderly program. Am J Cardiol. 2014;113:676-681.
6. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
7. Framingham Heart Study. Available at: https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php. Accessed May 29, 2017.
8. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
9. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
10. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037-2114.
11. Qaseem A, Wilt TJ, Rich R, et al. Pharmacological treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
12. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777-1783.
13. US Preventive Services Task Force. Final recommendation statement: high blood pressure in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 29, 2017.
14. Steinman MA, Miao Y, Boscardin WJ, et al. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29:1379-1386.
15. Schwartz JB. Primary prevention: do the very elderly require a different approach. Trends Cardiovasc Med. 2015:25:228-239.
16. Accord Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2012;2:337-414.
18. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1-S135.
19. Ogawa H, Kim-Mitsuyama S, Matsui K, et al, OSCAR Study Group. Angiotensin II receptor blocker-based therapy in Japanese elderly, high-risk, hypertensive patients. Am J Med. 2012;125:981-990.
20. Rosendorff C, Lackland DT, Allison M, et al. AHA/ACC/ASH Scientific Statement. Treatment of hypertension in patients with coronary heart disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension. 2015;65:1372-1407.
21. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
22. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137-e161.
23. Law MR, Wald MJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427.
24. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290-300.
25. Mukete BN, Ferdinand KC. Polypharmacy in older adults with hypertension: a comprehensive review. J Clin Hypertens (Greenwich). 2016;18:10-18.
26. Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
27. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diab Care. 2011;34:1270-1276.
28. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15.
29. Warwick J, Falashcetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78.
30. Zhang XE, Cheng B, Wang Q. Relationship between high blood pressure and cardiovascular outcomes in elderly frail patients: a systematic review and meta-analysis. Geriatric Nurs. 2016;37:385-392.
31. Benetos A, Rossignol P, Cherbuini A, et al. Polypharmacy in the aging patient: management of hypertension in octogenarians. JAMA. 2015;314:170-180.
32. de Bruijn R, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12:130.
33. Joas E, Bäckman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796-801.
34. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lanc Neurol. 2008;7:683-689.
35. Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324-333.
36. Tom Wade, MD. Methods of the SPRINT MIND Trial—how they did it + why it matters to primary care physicians. Available at: /www.tomwademd.net/methods-of-the-sprint-mind-trial-how-they-did-it-why-it-matters-to-primary-care-physicians/. Accessed August 11, 2017.
37. Welsh TJ, Gladman JR, Gordon AL. The treatment of hypertension in people with dementia: a systematic review of observational studies. BMC Geriatr. 2014;14:19.
CASE 1 An 82-year-old black woman comes in for an annual exam. She has no medical concerns. She volunteers at a hospice, walks daily, and maintains a healthy diet. Her past medical history (PMH) includes osteopenia and osteoarthritis, and her medications include acetaminophen as needed and vitamin D. She has no drug allergies. Her exam reveals a blood pressure (BP) of 148/70 mm Hg, a body mass index of 31, and a heart rate (HR) of 71 beats per minute (bpm). Cardiac and pulmonary exams are normal, and she shows no signs of peripheral edema.
CASE 2 An 88-year-old white man presents to the office for a 3-month follow-up of his hypertension. His systolic BP at home has ranged from 140 to 170 mm Hg. He denies chest pain, shortness of breath, or lower extremity edema. He lives with his wife and frequently swims for exercise. His PMH is significant for depression and degenerative disc disease. His medications include hydrochlorothiazide 12.5 mg/d, sertraline 50 mg/d, and naproxen 250 mg bid. His BP is 160/80 mm Hg and his HR is 70 bpm with normal cardiovascular (CV) and pulmonary exams.
CASE 3 An 80-year-old white man with diabetes mellitus (DM), hypertension, and chronic kidney disease (CKD) presents for a 3-month follow-up visit. His home systolic BP has been in the 140s to 150s. He is functional in all of his activities of daily living (ADLs), but is starting to require assistance with medications, finances, and transportation. He takes aspirin 81 mg/d, chlorthalidone 25 mg/d, and atenolol 50 mg/d. Remarkable laboratory test results include a hemoglobin A1c of 8.6%, a serum creatinine of 1.9 mg/dL (normal range: 0.6-1.2 mg/dL), and an albumin-creatinine ratio of 250 mg/g (normal range: <30 mg/g). During the exam, his BP is 143/70 mm Hg, his HR is 70 bpm, he is alert and oriented to person, place, and time, and he has normal CV and pulmonary exams with no signs of peripheral edema. He has decreased sensation in his feet, but normal reflexes.
How would you proceed with the care of these 3 patients?
Hypertension is the most common diagnosis made during physician office visits in the United States.1 Nearly one-third of the population has hypertension, and its prevalence increases with age, such that 67% of men and 79% of women ≥75 years of age have the condition.2
Evidence indicates that hypertension is a modifiable risk factor for CV and all-cause mortality (TABLE W13-6). All adults ≥75 years of age are at increased CV risk based on Framingham criteria,7 making hypertension management paramount. Complicating the situation are findings that indicate nearly half of adults with hypertension have inadequate BP control.2
Clinicians require clear direction about optimal BP targets, how best to adjust antihypertensive medications for comorbidities, and how to incorporate frailty and cognitive impairment into management strategies. This article presents recommendations derived from recent evidence and consensus guidelines regarding the management of hypertension in adults ≥75 years of age.
[polldaddy:9818133]
Diagnosing hypertension
According to the seventh report of the Joint National Committee (JNC 7), hypertension is defined as a systolic BP ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg.8 The JNC’s more recent report (JNC 8), however, does not define hypertension; instead, it sets forth treatment thresholds (eg, that there is strong evidence to support treating individuals ≥60 years of age when BP ≥150/90 mm Hg).9
It starts with an accurate BP measurement. Ensuring the accuracy of a BP measurement requires multiple readings over time. White coat hypertension and masked hypertension can complicate BP measurement. Home measurements better correlate with atherosclerotic cardiovascular disease (ASCVD) risk than do office measurements.10-12 In fact, the US Preventive Services Task Force recommends obtaining measurements outside of the clinic setting prior to initiating treatment for hypertension.13
Educate staff on the proper technique for obtaining BP measurements in the office (ie, taking measurements using an appropriately sized cuff when patients have been seated for at least 5 minutes with feet uncrossed and with their arm supported at heart level). Cold temperatures, coffee consumption, talking, and recent tobacco use can transiently raise BP. TABLE 110 outlines the initial work-up after confirming the diagnosis of hypertension. No other routine tests are recommended for the management of hypertension except those associated with medication monitoring (outlined in TABLE 210,11,14,15).
What’s the optimal BP target for older patients? No consensus exists on an optimal BP target for older patients. JNC 8 recommends a target BP <150/90 mm Hg in patients ≥60 years of age.9 The American College of Physicians recommends a systolic BP target <140 mm Hg in patients ≥60 years of age with increased stroke or CV risk.11 A subgroup analysis of patients ≥75 years of age from the Systolic BP Intervention Trial (SPRINT)3 was stopped early because of the clear composite CV and mortality benefits associated with targeting a systolic BP <120 mm Hg as compared with <140 mm Hg (TABLE W13-6). Although a criticism of this trial and its results is that the researchers included only adults with high CV risk, all adults ≥75 years of age are considered to have high CV risk by the SPRINT study.3 Another criticism is that early suspension of the trial may have exaggerated treatment effects.6
Lastly, results were seemingly discrepant from previous trials, most notably, the Action to Control CV Risk in Diabetes (ACCORD) trial.6,16 However, on closer review, the ACCORD trial16 included only patients with DM, while the SPRINT3 trial excluded patients with DM, and ACCORD comprised a younger population than the SPRINT subgroup analysis. Also, the ACCORD trial did demonstrate stroke reduction and non-significant reduction in CV events, albeit, at the cost of increased adverse events, such as hypotension, bradycardia, and hypokalemia, with tighter BP control.16
Population differences presumably explain the discrepancy in results, and a systolic BP target of <120 mm Hg is appropriate in community-dwelling, non-diabetic adults ≥75 years of age. If this target goal cannot be achieved without undue burden (ie, without syncope, hypotension, bradycardia, electrolyte disturbance, renal impairment, or substantial medication burden), a recent meta-analysis found evidence that a systolic BP goal <140 mm Hg also provides benefit.6
Initiate treatment, watch for age-related changes
Lifestyle modifications (including appropriate weight loss; reduced caffeine, salt, and alcohol intake; increased physical activity; and smoking cessation) are important in the initial and ongoing management of hypertension.10,11,17,18 JNC 8 recommends initial treatment with a thiazide-type diuretic, calcium channel blocker (CCB), angiotensin converting enzyme (ACE) inhibitor, or angiotensin receptor blocker (ARB) in the nonblack population, and a CCB or thiazide diuretic in the black population.9 Specific initial medication choices for comorbid conditions are outlined in TABLE W23,10,17-22. JNC 8 recommends against the use of a beta-blocker or alpha blocker for initial treatment of hypertension.9
Start a second drug instead of maximizing the dose of the first
If the target BP cannot be achieved within one month of initiating medication, JNC 8 recommends increasing the dose of the initial drug or adding a second drug without preference for one strategy over the other.9 However, a meta-analysis demonstrates that approximately 80% of the antihypertensive effect of a drug can be achieved with half of the standard dose of the medication; this is true for thiazide-type diuretics, ACE inhibitors/ARBs, beta-blockers, and CCBs.23
Furthermore, due to fewer adverse effects and positive synergies, studies show that combining low doses of 2 medications is more beneficial than high-dose monotherapy.19,23,24 Prescribing combination pills can be helpful to limit pill burden. It is appropriate to combine any of the 4 classes of medications recommended as initial therapy by JNC 8 except for an ACE inhibitor combined with an ARB. If the target BP cannot be achieved with 3 drugs in those classes, other medications such as potassium-sparing diuretics or beta-blockers can be added.9
Changes associated with aging
Changes associated with aging include atherosclerosis and stiffening of blood vessels, increased systolic BP, widened pulse pressure, reduced glomerular filtration rate, reduced sodium elimination and volume expansion, sinoatrial node cellular dropout, and decreased sensitivity of baroreceptors.10 Because of these alterations, antihypertensive requirements may change, and resistant hypertension may develop. In addition, older patients may be more susceptible to orthostatic hypotension, heart block, electrolyte derangements, and other antihypertensive adverse effects.
When hypertension is difficult to control. Resistant hypertension is defined as hypertension that cannot be controlled with 3 drugs from 3 different antihypertensive classes, one of which is a diuretic. Cognitive impairment, polypharmacy, and multimorbidity may contribute to difficult-to-control hypertension in older adults and should be assessed prior to work-up for other secondary causes of poorly controlled hypertension.
- Cognitive impairment is often unrecognized and may impact medication adherence, which can masquerade as treatment failure. Assess for cognitive impairment on an ongoing basis with the aging patient, especially when medication adherence appears poor.
- Polypharmacy may also contribute to uncontrolled BP. Common pharmacotherapeutic contributors to uncontrolled BP include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, high-dose decongestants, and selective norepinephrine reuptake inhibitors.25
- Multimorbidity describes 2 or more chronic medical conditions in one patient. These patients are medically complex. Comorbidities can increase pill burden and make medication adherence difficult for patients. Other poorly controlled disease states can worsen hypertension (eg, renal dysfunction secondary to diabetes). Optimize treatment of comorbid conditions.
Secondary causes. If resistant hypertension persists despite confirming medication adherence and eliminating offending medications, a work-up should ensue for secondary causes of hypertension, as well as end-organ damage. Causes of secondary hypertension include sleep apnea (see this month's HelpDesk), renal dysfunction (renal artery stenosis), aldosterone-mediated hypertension (often with hypokalemia), and thyroid disease. Evaluation for secondary causes of hypertension and end-organ damage is outlined in TABLE 1.10 Patients with well-controlled hypertension do not require repeated assessments for end-organ damage unless new symptoms—such as chest pain or edema—develop.
Consider comorbidities
Clinical trials implicitly or explicitly exclude patients with multiple comorbidities. JNC 8 provided minimal guidance for adjusting BP targets based on comorbidity with only nondiabetic CKD and DM specifically addressed.9 Guidelines from specialty organizations and recent trials provide some additional guidance in these situations and are outlined in TABLE W23,10,17-22.
Heart failure. Hypertension is a major risk factor for heart failure. Long-term treatment of systolic and diastolic hypertension can reduce the incidence of heart failure by approximately half with increased benefit in patients with prior myocardial infarction.22 Research demonstrates clear mortality benefits of certain antihypertensive drug classes, including diuretics, beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, combination hydralazine and nitrates, and angiotensin receptor-neprilysin inhibitors.21,22 The overall treatment goal in heart failure is to optimize drugs with mortality benefit, while lowering BP to a goal <130/80 mm Hg in patients ≥75 years of age.22
Increased risk for CV disease. The SPRINT trial3 defined high risk of CV disease as clinical or subclinical CV disease, CKD, 10-year ASCVD risk of ≥15%, or age ≥75 years. SPRINT supports a systolic BP goal <120 mm Hg, but, as a reminder, SPRINT excluded patients with diabetes. The American College of Cardiology Foundation Task Force and the American Heart Association define high CV risk as a 10-year ASCVD risk ≥10% and recommend a BP goal <130/80 mm Hg.10
Diabetes mellitus. A BP >115/75 mm Hg is associated with increased CV events and mortality in patients with DM.18 The American Diabetes Association (ADA) and JNC 8 recommend a BP target <140/90 mm Hg.9,18 ADA suggests a lower target of 130/80 mm Hg in patients with high CV risk if it is achievable without undue burden.18
Studies show increased mortality associated with initiating additional treatment once a systolic goal <140 mm Hg has been achieved in patients with DM.26 The ACCORD trial found increased adverse events with aggressive BP lowering to <120/80 mm Hg.16
For patients with DM requiring more than one antihypertensive agent, there are CV mortality benefits associated with administering at least one antihypertensive drug at night, likely related to the beneficial effect of physiologic nocturnal dipping.27
Chronic kidney disease. JNC 8 specifically recommends an ACE inhibitor or ARB for initial or add-on treatment in patients with CKD and a BP goal <140/90 mm Hg.9 The Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group recommends a BP target ≤140/90 mm Hg in patients without albuminuria and ≤130/80 mm Hg in patients with albuminuria to protect against the progression of nephropathy.17 The SPRINT trial3 included patients with CKD, and KDIGO has not yet updated its guidelines to reflect SPRINT.
Frailty is a clinical syndrome that has been defined as a state of increased vulnerability that is associated with a decline in reserve and function.28 The largest hypertension studies in older adults address frailty, although often the most frail patients are excluded from these studies (TABLE W13-6).
The Hypertension in the Very Elderly Trial (HYVET) categorized patients as frail, pre-frail, or robust and found a consistent benefit of antihypertensive treatment on stroke, CV events, and total mortality—regardless of baseline frailty status.29 The SPRINT trial included only community-dwelling adults.3 Other studies suggest that hypertension actually has a protective effect by lowering overall mortality in frail older adults, especially in the frailest and oldest nursing home populations.30,31
Although there is a paucity of data to direct the management of hypertension in frail older patients, physicians should prioritize the condition and focus on adverse events from antihypertensives and on slow titration of medications. The JNC 8 BP target of <150/90 mm Hg is a reasonable BP goal in this population, given the lack of evidence for lower or higher targets.9 Many frail patients have one or more of the comorbidities described earlier, and it is reasonable to strive for the comorbidity-specific target, provided it can be achieved without undue burden.
Cognitive impairment and dementia. The association between hypertension and dementia/cognitive impairment is evolving. Hypertension may impact various forms of dementia, such as Alzheimer’s disease (AD) or vascular dementia, differently. There is evidence linking hypertension to AD.32 The relationship between BP and brain perfusion is complex with the potential existence of an age-adjusted relationship such that mid-life hypertension may increase the risk of dementia while late-life hypertension may not.33
A number of studies reveal the evolving nature of our understanding of these 2 conditions:
- A recent systematic review and meta-analysis examining intensive BP treatments in older adults demonstrated that lower BP targets did not increase cognitive decline.6
- HYVET’s cognitive function assessment did not find a significant reduction in the incidence of dementia with BP reduction over a short follow-up period, but when results were combined in a meta-analysis with other placebo-controlled, double-blind trials of antihypertensive treatments, there was significant reduction in incident dementia in patients randomized to antihypertensive treatment.34
- The ACCORD Memory in Diabetes trial (ACCORD-MIND) had the unexpected outcome that intensive lowering of systolic BP to a target <120 mm Hg resulted in a greater decline in total brain volume, compared with the standard BP goal <140 mm Hg. This was measured with magnetic resonance imaging in older adults with type 2 DM.35
- Results from the SPRINT sub-analysis Memory and Cognition in Decreased Hypertension trial are forthcoming and aim to determine the effects of BP reduction on dementia.36
The JNC 89 BP target <150/90 mm Hg or a comorbidity-specific target, if achievable without undue burden, is reasonable in patients with dementia. In a systematic review of observational studies in patients with hypertension and dementia, diuretics, CCBs, ACE inhibitors/ARBs, and beta-blockers were commonly used medications with a trend toward prescribing CCBs and ACE inhibitors/ARBs.37
As previously highlighted, cognitive impairment may lead to problems with medication adherence and even inadvertent improper medication use, potentially resulting in adverse events from antihypertensives. If cognitive impairment or dementia is suspected, ensure additional measures (such as medication assistance or supervision) are in place before prescribing antihypertensives.
Certain diseases, such as Parkinson’s-related dementia and multiple system atrophy, can cause autonomic instability, which can increase the risk of falls and complicate hypertension management. Carefully monitor patients for signs of orthostasis.
CASE 1 Repeat the BP measurement in the office once the patient has been seated for ≥5 minutes, and have the patient monitor her BP at home; schedule a follow-up visit in 2 weeks. If hypertension is confirmed with home measurements, then, in addition to lifestyle modifications, initiate treatment with a CCB or thiazide diuretic to achieve a systolic BP goal <120 mm Hg. Titrate medications slowly while monitoring for adverse effects.
CASE 2 Consistent with the office measurement, the patient has home BP readings that are above the BP target (<120 mm Hg systolic). He has been taking a single antihypertensive for longer than one month. Discontinue his NSAID prior to adding any new medications. If his BP is still above target without NSAIDs, then add a second agent, such as a low dose of an ACE inhibitor, ARB, or CCB, rather than maximizing the dose of hydrochlorothiazide.
CASE 3 Given the patient’s diabetes, CKD, and albuminuria, a target BP goal <130/80 mm Hg is reasonable. An ACE inhibitor or ARB is a better medication choice than atenolol in this patient with albuminuria. Because of the deterioration in his ADLs, careful assessment of mobility, functionality, comorbidities, frailty, and cognitive function should take place at each office visit and inform adjustments to the patient’s BP target. Employ cautious medication titration with monitoring for adverse effects, especially hypotension and syncope. If his functional status declines, adverse effects develop, or the medication regimen becomes burdensome, relax the target BP goal to 150/90 mm Hg.
CORRESPONDENCE
Julienne K. Kirk, PharmD, Family and Community Medicine, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1084; [email protected].
CASE 1 An 82-year-old black woman comes in for an annual exam. She has no medical concerns. She volunteers at a hospice, walks daily, and maintains a healthy diet. Her past medical history (PMH) includes osteopenia and osteoarthritis, and her medications include acetaminophen as needed and vitamin D. She has no drug allergies. Her exam reveals a blood pressure (BP) of 148/70 mm Hg, a body mass index of 31, and a heart rate (HR) of 71 beats per minute (bpm). Cardiac and pulmonary exams are normal, and she shows no signs of peripheral edema.
CASE 2 An 88-year-old white man presents to the office for a 3-month follow-up of his hypertension. His systolic BP at home has ranged from 140 to 170 mm Hg. He denies chest pain, shortness of breath, or lower extremity edema. He lives with his wife and frequently swims for exercise. His PMH is significant for depression and degenerative disc disease. His medications include hydrochlorothiazide 12.5 mg/d, sertraline 50 mg/d, and naproxen 250 mg bid. His BP is 160/80 mm Hg and his HR is 70 bpm with normal cardiovascular (CV) and pulmonary exams.
CASE 3 An 80-year-old white man with diabetes mellitus (DM), hypertension, and chronic kidney disease (CKD) presents for a 3-month follow-up visit. His home systolic BP has been in the 140s to 150s. He is functional in all of his activities of daily living (ADLs), but is starting to require assistance with medications, finances, and transportation. He takes aspirin 81 mg/d, chlorthalidone 25 mg/d, and atenolol 50 mg/d. Remarkable laboratory test results include a hemoglobin A1c of 8.6%, a serum creatinine of 1.9 mg/dL (normal range: 0.6-1.2 mg/dL), and an albumin-creatinine ratio of 250 mg/g (normal range: <30 mg/g). During the exam, his BP is 143/70 mm Hg, his HR is 70 bpm, he is alert and oriented to person, place, and time, and he has normal CV and pulmonary exams with no signs of peripheral edema. He has decreased sensation in his feet, but normal reflexes.
How would you proceed with the care of these 3 patients?
Hypertension is the most common diagnosis made during physician office visits in the United States.1 Nearly one-third of the population has hypertension, and its prevalence increases with age, such that 67% of men and 79% of women ≥75 years of age have the condition.2
Evidence indicates that hypertension is a modifiable risk factor for CV and all-cause mortality (TABLE W13-6). All adults ≥75 years of age are at increased CV risk based on Framingham criteria,7 making hypertension management paramount. Complicating the situation are findings that indicate nearly half of adults with hypertension have inadequate BP control.2
Clinicians require clear direction about optimal BP targets, how best to adjust antihypertensive medications for comorbidities, and how to incorporate frailty and cognitive impairment into management strategies. This article presents recommendations derived from recent evidence and consensus guidelines regarding the management of hypertension in adults ≥75 years of age.
[polldaddy:9818133]
Diagnosing hypertension
According to the seventh report of the Joint National Committee (JNC 7), hypertension is defined as a systolic BP ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg.8 The JNC’s more recent report (JNC 8), however, does not define hypertension; instead, it sets forth treatment thresholds (eg, that there is strong evidence to support treating individuals ≥60 years of age when BP ≥150/90 mm Hg).9
It starts with an accurate BP measurement. Ensuring the accuracy of a BP measurement requires multiple readings over time. White coat hypertension and masked hypertension can complicate BP measurement. Home measurements better correlate with atherosclerotic cardiovascular disease (ASCVD) risk than do office measurements.10-12 In fact, the US Preventive Services Task Force recommends obtaining measurements outside of the clinic setting prior to initiating treatment for hypertension.13
Educate staff on the proper technique for obtaining BP measurements in the office (ie, taking measurements using an appropriately sized cuff when patients have been seated for at least 5 minutes with feet uncrossed and with their arm supported at heart level). Cold temperatures, coffee consumption, talking, and recent tobacco use can transiently raise BP. TABLE 110 outlines the initial work-up after confirming the diagnosis of hypertension. No other routine tests are recommended for the management of hypertension except those associated with medication monitoring (outlined in TABLE 210,11,14,15).
What’s the optimal BP target for older patients? No consensus exists on an optimal BP target for older patients. JNC 8 recommends a target BP <150/90 mm Hg in patients ≥60 years of age.9 The American College of Physicians recommends a systolic BP target <140 mm Hg in patients ≥60 years of age with increased stroke or CV risk.11 A subgroup analysis of patients ≥75 years of age from the Systolic BP Intervention Trial (SPRINT)3 was stopped early because of the clear composite CV and mortality benefits associated with targeting a systolic BP <120 mm Hg as compared with <140 mm Hg (TABLE W13-6). Although a criticism of this trial and its results is that the researchers included only adults with high CV risk, all adults ≥75 years of age are considered to have high CV risk by the SPRINT study.3 Another criticism is that early suspension of the trial may have exaggerated treatment effects.6
Lastly, results were seemingly discrepant from previous trials, most notably, the Action to Control CV Risk in Diabetes (ACCORD) trial.6,16 However, on closer review, the ACCORD trial16 included only patients with DM, while the SPRINT3 trial excluded patients with DM, and ACCORD comprised a younger population than the SPRINT subgroup analysis. Also, the ACCORD trial did demonstrate stroke reduction and non-significant reduction in CV events, albeit, at the cost of increased adverse events, such as hypotension, bradycardia, and hypokalemia, with tighter BP control.16
Population differences presumably explain the discrepancy in results, and a systolic BP target of <120 mm Hg is appropriate in community-dwelling, non-diabetic adults ≥75 years of age. If this target goal cannot be achieved without undue burden (ie, without syncope, hypotension, bradycardia, electrolyte disturbance, renal impairment, or substantial medication burden), a recent meta-analysis found evidence that a systolic BP goal <140 mm Hg also provides benefit.6
Initiate treatment, watch for age-related changes
Lifestyle modifications (including appropriate weight loss; reduced caffeine, salt, and alcohol intake; increased physical activity; and smoking cessation) are important in the initial and ongoing management of hypertension.10,11,17,18 JNC 8 recommends initial treatment with a thiazide-type diuretic, calcium channel blocker (CCB), angiotensin converting enzyme (ACE) inhibitor, or angiotensin receptor blocker (ARB) in the nonblack population, and a CCB or thiazide diuretic in the black population.9 Specific initial medication choices for comorbid conditions are outlined in TABLE W23,10,17-22. JNC 8 recommends against the use of a beta-blocker or alpha blocker for initial treatment of hypertension.9
Start a second drug instead of maximizing the dose of the first
If the target BP cannot be achieved within one month of initiating medication, JNC 8 recommends increasing the dose of the initial drug or adding a second drug without preference for one strategy over the other.9 However, a meta-analysis demonstrates that approximately 80% of the antihypertensive effect of a drug can be achieved with half of the standard dose of the medication; this is true for thiazide-type diuretics, ACE inhibitors/ARBs, beta-blockers, and CCBs.23
Furthermore, due to fewer adverse effects and positive synergies, studies show that combining low doses of 2 medications is more beneficial than high-dose monotherapy.19,23,24 Prescribing combination pills can be helpful to limit pill burden. It is appropriate to combine any of the 4 classes of medications recommended as initial therapy by JNC 8 except for an ACE inhibitor combined with an ARB. If the target BP cannot be achieved with 3 drugs in those classes, other medications such as potassium-sparing diuretics or beta-blockers can be added.9
Changes associated with aging
Changes associated with aging include atherosclerosis and stiffening of blood vessels, increased systolic BP, widened pulse pressure, reduced glomerular filtration rate, reduced sodium elimination and volume expansion, sinoatrial node cellular dropout, and decreased sensitivity of baroreceptors.10 Because of these alterations, antihypertensive requirements may change, and resistant hypertension may develop. In addition, older patients may be more susceptible to orthostatic hypotension, heart block, electrolyte derangements, and other antihypertensive adverse effects.
When hypertension is difficult to control. Resistant hypertension is defined as hypertension that cannot be controlled with 3 drugs from 3 different antihypertensive classes, one of which is a diuretic. Cognitive impairment, polypharmacy, and multimorbidity may contribute to difficult-to-control hypertension in older adults and should be assessed prior to work-up for other secondary causes of poorly controlled hypertension.
- Cognitive impairment is often unrecognized and may impact medication adherence, which can masquerade as treatment failure. Assess for cognitive impairment on an ongoing basis with the aging patient, especially when medication adherence appears poor.
- Polypharmacy may also contribute to uncontrolled BP. Common pharmacotherapeutic contributors to uncontrolled BP include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, high-dose decongestants, and selective norepinephrine reuptake inhibitors.25
- Multimorbidity describes 2 or more chronic medical conditions in one patient. These patients are medically complex. Comorbidities can increase pill burden and make medication adherence difficult for patients. Other poorly controlled disease states can worsen hypertension (eg, renal dysfunction secondary to diabetes). Optimize treatment of comorbid conditions.
Secondary causes. If resistant hypertension persists despite confirming medication adherence and eliminating offending medications, a work-up should ensue for secondary causes of hypertension, as well as end-organ damage. Causes of secondary hypertension include sleep apnea (see this month's HelpDesk), renal dysfunction (renal artery stenosis), aldosterone-mediated hypertension (often with hypokalemia), and thyroid disease. Evaluation for secondary causes of hypertension and end-organ damage is outlined in TABLE 1.10 Patients with well-controlled hypertension do not require repeated assessments for end-organ damage unless new symptoms—such as chest pain or edema—develop.
Consider comorbidities
Clinical trials implicitly or explicitly exclude patients with multiple comorbidities. JNC 8 provided minimal guidance for adjusting BP targets based on comorbidity with only nondiabetic CKD and DM specifically addressed.9 Guidelines from specialty organizations and recent trials provide some additional guidance in these situations and are outlined in TABLE W23,10,17-22.
Heart failure. Hypertension is a major risk factor for heart failure. Long-term treatment of systolic and diastolic hypertension can reduce the incidence of heart failure by approximately half with increased benefit in patients with prior myocardial infarction.22 Research demonstrates clear mortality benefits of certain antihypertensive drug classes, including diuretics, beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, combination hydralazine and nitrates, and angiotensin receptor-neprilysin inhibitors.21,22 The overall treatment goal in heart failure is to optimize drugs with mortality benefit, while lowering BP to a goal <130/80 mm Hg in patients ≥75 years of age.22
Increased risk for CV disease. The SPRINT trial3 defined high risk of CV disease as clinical or subclinical CV disease, CKD, 10-year ASCVD risk of ≥15%, or age ≥75 years. SPRINT supports a systolic BP goal <120 mm Hg, but, as a reminder, SPRINT excluded patients with diabetes. The American College of Cardiology Foundation Task Force and the American Heart Association define high CV risk as a 10-year ASCVD risk ≥10% and recommend a BP goal <130/80 mm Hg.10
Diabetes mellitus. A BP >115/75 mm Hg is associated with increased CV events and mortality in patients with DM.18 The American Diabetes Association (ADA) and JNC 8 recommend a BP target <140/90 mm Hg.9,18 ADA suggests a lower target of 130/80 mm Hg in patients with high CV risk if it is achievable without undue burden.18
Studies show increased mortality associated with initiating additional treatment once a systolic goal <140 mm Hg has been achieved in patients with DM.26 The ACCORD trial found increased adverse events with aggressive BP lowering to <120/80 mm Hg.16
For patients with DM requiring more than one antihypertensive agent, there are CV mortality benefits associated with administering at least one antihypertensive drug at night, likely related to the beneficial effect of physiologic nocturnal dipping.27
Chronic kidney disease. JNC 8 specifically recommends an ACE inhibitor or ARB for initial or add-on treatment in patients with CKD and a BP goal <140/90 mm Hg.9 The Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group recommends a BP target ≤140/90 mm Hg in patients without albuminuria and ≤130/80 mm Hg in patients with albuminuria to protect against the progression of nephropathy.17 The SPRINT trial3 included patients with CKD, and KDIGO has not yet updated its guidelines to reflect SPRINT.
Frailty is a clinical syndrome that has been defined as a state of increased vulnerability that is associated with a decline in reserve and function.28 The largest hypertension studies in older adults address frailty, although often the most frail patients are excluded from these studies (TABLE W13-6).
The Hypertension in the Very Elderly Trial (HYVET) categorized patients as frail, pre-frail, or robust and found a consistent benefit of antihypertensive treatment on stroke, CV events, and total mortality—regardless of baseline frailty status.29 The SPRINT trial included only community-dwelling adults.3 Other studies suggest that hypertension actually has a protective effect by lowering overall mortality in frail older adults, especially in the frailest and oldest nursing home populations.30,31
Although there is a paucity of data to direct the management of hypertension in frail older patients, physicians should prioritize the condition and focus on adverse events from antihypertensives and on slow titration of medications. The JNC 8 BP target of <150/90 mm Hg is a reasonable BP goal in this population, given the lack of evidence for lower or higher targets.9 Many frail patients have one or more of the comorbidities described earlier, and it is reasonable to strive for the comorbidity-specific target, provided it can be achieved without undue burden.
Cognitive impairment and dementia. The association between hypertension and dementia/cognitive impairment is evolving. Hypertension may impact various forms of dementia, such as Alzheimer’s disease (AD) or vascular dementia, differently. There is evidence linking hypertension to AD.32 The relationship between BP and brain perfusion is complex with the potential existence of an age-adjusted relationship such that mid-life hypertension may increase the risk of dementia while late-life hypertension may not.33
A number of studies reveal the evolving nature of our understanding of these 2 conditions:
- A recent systematic review and meta-analysis examining intensive BP treatments in older adults demonstrated that lower BP targets did not increase cognitive decline.6
- HYVET’s cognitive function assessment did not find a significant reduction in the incidence of dementia with BP reduction over a short follow-up period, but when results were combined in a meta-analysis with other placebo-controlled, double-blind trials of antihypertensive treatments, there was significant reduction in incident dementia in patients randomized to antihypertensive treatment.34
- The ACCORD Memory in Diabetes trial (ACCORD-MIND) had the unexpected outcome that intensive lowering of systolic BP to a target <120 mm Hg resulted in a greater decline in total brain volume, compared with the standard BP goal <140 mm Hg. This was measured with magnetic resonance imaging in older adults with type 2 DM.35
- Results from the SPRINT sub-analysis Memory and Cognition in Decreased Hypertension trial are forthcoming and aim to determine the effects of BP reduction on dementia.36
The JNC 89 BP target <150/90 mm Hg or a comorbidity-specific target, if achievable without undue burden, is reasonable in patients with dementia. In a systematic review of observational studies in patients with hypertension and dementia, diuretics, CCBs, ACE inhibitors/ARBs, and beta-blockers were commonly used medications with a trend toward prescribing CCBs and ACE inhibitors/ARBs.37
As previously highlighted, cognitive impairment may lead to problems with medication adherence and even inadvertent improper medication use, potentially resulting in adverse events from antihypertensives. If cognitive impairment or dementia is suspected, ensure additional measures (such as medication assistance or supervision) are in place before prescribing antihypertensives.
Certain diseases, such as Parkinson’s-related dementia and multiple system atrophy, can cause autonomic instability, which can increase the risk of falls and complicate hypertension management. Carefully monitor patients for signs of orthostasis.
CASE 1 Repeat the BP measurement in the office once the patient has been seated for ≥5 minutes, and have the patient monitor her BP at home; schedule a follow-up visit in 2 weeks. If hypertension is confirmed with home measurements, then, in addition to lifestyle modifications, initiate treatment with a CCB or thiazide diuretic to achieve a systolic BP goal <120 mm Hg. Titrate medications slowly while monitoring for adverse effects.
CASE 2 Consistent with the office measurement, the patient has home BP readings that are above the BP target (<120 mm Hg systolic). He has been taking a single antihypertensive for longer than one month. Discontinue his NSAID prior to adding any new medications. If his BP is still above target without NSAIDs, then add a second agent, such as a low dose of an ACE inhibitor, ARB, or CCB, rather than maximizing the dose of hydrochlorothiazide.
CASE 3 Given the patient’s diabetes, CKD, and albuminuria, a target BP goal <130/80 mm Hg is reasonable. An ACE inhibitor or ARB is a better medication choice than atenolol in this patient with albuminuria. Because of the deterioration in his ADLs, careful assessment of mobility, functionality, comorbidities, frailty, and cognitive function should take place at each office visit and inform adjustments to the patient’s BP target. Employ cautious medication titration with monitoring for adverse effects, especially hypotension and syncope. If his functional status declines, adverse effects develop, or the medication regimen becomes burdensome, relax the target BP goal to 150/90 mm Hg.
CORRESPONDENCE
Julienne K. Kirk, PharmD, Family and Community Medicine, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1084; [email protected].
1. National Ambulatory Medical Care Survey: 2013 State and National Summary Tables. Available at: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2013_namcs_web_tables.pdf. Accessed May 29, 2017.
2. Centers for Disease Control and Prevention. High blood pressure facts. Available at: https://cdc.gov/bloodpressure/facts.htm. Accessed May 29, 2017.
3. Williamson JD, Suplano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
4. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
5. Kostis WJ, Cabrera J, Messerli FH, et al. Competing cardiovascular and noncardiovascular risks and longevity in the systolic hypertension in the elderly program. Am J Cardiol. 2014;113:676-681.
6. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
7. Framingham Heart Study. Available at: https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php. Accessed May 29, 2017.
8. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
9. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
10. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037-2114.
11. Qaseem A, Wilt TJ, Rich R, et al. Pharmacological treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
12. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777-1783.
13. US Preventive Services Task Force. Final recommendation statement: high blood pressure in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 29, 2017.
14. Steinman MA, Miao Y, Boscardin WJ, et al. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29:1379-1386.
15. Schwartz JB. Primary prevention: do the very elderly require a different approach. Trends Cardiovasc Med. 2015:25:228-239.
16. Accord Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2012;2:337-414.
18. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1-S135.
19. Ogawa H, Kim-Mitsuyama S, Matsui K, et al, OSCAR Study Group. Angiotensin II receptor blocker-based therapy in Japanese elderly, high-risk, hypertensive patients. Am J Med. 2012;125:981-990.
20. Rosendorff C, Lackland DT, Allison M, et al. AHA/ACC/ASH Scientific Statement. Treatment of hypertension in patients with coronary heart disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension. 2015;65:1372-1407.
21. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
22. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137-e161.
23. Law MR, Wald MJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427.
24. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290-300.
25. Mukete BN, Ferdinand KC. Polypharmacy in older adults with hypertension: a comprehensive review. J Clin Hypertens (Greenwich). 2016;18:10-18.
26. Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
27. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diab Care. 2011;34:1270-1276.
28. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15.
29. Warwick J, Falashcetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78.
30. Zhang XE, Cheng B, Wang Q. Relationship between high blood pressure and cardiovascular outcomes in elderly frail patients: a systematic review and meta-analysis. Geriatric Nurs. 2016;37:385-392.
31. Benetos A, Rossignol P, Cherbuini A, et al. Polypharmacy in the aging patient: management of hypertension in octogenarians. JAMA. 2015;314:170-180.
32. de Bruijn R, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12:130.
33. Joas E, Bäckman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796-801.
34. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lanc Neurol. 2008;7:683-689.
35. Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324-333.
36. Tom Wade, MD. Methods of the SPRINT MIND Trial—how they did it + why it matters to primary care physicians. Available at: /www.tomwademd.net/methods-of-the-sprint-mind-trial-how-they-did-it-why-it-matters-to-primary-care-physicians/. Accessed August 11, 2017.
37. Welsh TJ, Gladman JR, Gordon AL. The treatment of hypertension in people with dementia: a systematic review of observational studies. BMC Geriatr. 2014;14:19.
1. National Ambulatory Medical Care Survey: 2013 State and National Summary Tables. Available at: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2013_namcs_web_tables.pdf. Accessed May 29, 2017.
2. Centers for Disease Control and Prevention. High blood pressure facts. Available at: https://cdc.gov/bloodpressure/facts.htm. Accessed May 29, 2017.
3. Williamson JD, Suplano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
4. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
5. Kostis WJ, Cabrera J, Messerli FH, et al. Competing cardiovascular and noncardiovascular risks and longevity in the systolic hypertension in the elderly program. Am J Cardiol. 2014;113:676-681.
6. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
7. Framingham Heart Study. Available at: https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php. Accessed May 29, 2017.
8. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
9. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
10. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037-2114.
11. Qaseem A, Wilt TJ, Rich R, et al. Pharmacological treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
12. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777-1783.
13. US Preventive Services Task Force. Final recommendation statement: high blood pressure in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 29, 2017.
14. Steinman MA, Miao Y, Boscardin WJ, et al. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29:1379-1386.
15. Schwartz JB. Primary prevention: do the very elderly require a different approach. Trends Cardiovasc Med. 2015:25:228-239.
16. Accord Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2012;2:337-414.
18. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1-S135.
19. Ogawa H, Kim-Mitsuyama S, Matsui K, et al, OSCAR Study Group. Angiotensin II receptor blocker-based therapy in Japanese elderly, high-risk, hypertensive patients. Am J Med. 2012;125:981-990.
20. Rosendorff C, Lackland DT, Allison M, et al. AHA/ACC/ASH Scientific Statement. Treatment of hypertension in patients with coronary heart disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension. 2015;65:1372-1407.
21. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
22. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137-e161.
23. Law MR, Wald MJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427.
24. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290-300.
25. Mukete BN, Ferdinand KC. Polypharmacy in older adults with hypertension: a comprehensive review. J Clin Hypertens (Greenwich). 2016;18:10-18.
26. Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
27. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diab Care. 2011;34:1270-1276.
28. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15.
29. Warwick J, Falashcetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78.
30. Zhang XE, Cheng B, Wang Q. Relationship between high blood pressure and cardiovascular outcomes in elderly frail patients: a systematic review and meta-analysis. Geriatric Nurs. 2016;37:385-392.
31. Benetos A, Rossignol P, Cherbuini A, et al. Polypharmacy in the aging patient: management of hypertension in octogenarians. JAMA. 2015;314:170-180.
32. de Bruijn R, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12:130.
33. Joas E, Bäckman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796-801.
34. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lanc Neurol. 2008;7:683-689.
35. Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324-333.
36. Tom Wade, MD. Methods of the SPRINT MIND Trial—how they did it + why it matters to primary care physicians. Available at: /www.tomwademd.net/methods-of-the-sprint-mind-trial-how-they-did-it-why-it-matters-to-primary-care-physicians/. Accessed August 11, 2017.
37. Welsh TJ, Gladman JR, Gordon AL. The treatment of hypertension in people with dementia: a systematic review of observational studies. BMC Geriatr. 2014;14:19.
From The Journal of Family Practice | 2017;66(9):546-548,550-554.
PRACTICE RECOMMENDATIONS
› Target a systolic blood pressure (BP) <120 mm Hg in community-dwelling, non-diabetic patients ≥75 years of age if it is achievable without undue burden. A
› Combine low doses of 2 medications, rather than increase the dose of a single agent, to achieve the desired BP target. A
› Consider cognitive function, polypharmacy, multimorbidity, and frailty when assessing and treating hypertension in older patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series