User login
Mammography at age 40? A risk-based strategy
› Recommend that women consider having a single mammogram at age 40 as a baseline so that breast density can be included in the assessment of risk. minor MRSA skin lesions in children with mupirocin. C
› Advise women with low breast density and no other significant risk factors that they are at lower than average risk for breast cancer and should consider this when discussing when to begin routine screening with their physician. C
› Recommend that women with a 2-fold increased risk for breast cancer begin regular screening in their 40s. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, when should I start having mammograms?” That’s a question you’re apt to hear again and again from women in their early 40s. It’s also a question with no easy answer.
While deaths from breast cancer are declining, it remains the most commonly diagnosed cancer among US women. In 2012, approximately 229,060 new cases of breast cancer were detected and an estimated 39,920 women died from breast cancer1—about 10% of them in their 40s.2
Based on these numbers alone, it would seem that every woman should begin regular screening at age 40. Yet there are many other issues to consider, namely the high rate of false positives, as well as the overdiagnosis and overtreatment associated with such screening. Further complicating matters is the fact that there is no consensus as to whether screening mammography should be recommended—and if so, how often—for women ages 40 to 49 years who are at average risk.
In light of this, we offer a risk-based strategy to mammography for younger women, which we’ve distilled into an ALGORITHM. But first, let’s look at the evidence and what the US Preventive Services Task Force (USPSTF) and major medical groups have to say.
To screen or not to screen? A look at the evidence
A decision to perform screening mammography in premenopausal women should be made by weighing benefits vs harms. Benefits include diagnosis of breast cancer when it’s in an early stage and a reduction in death. Meta-analyses have consistently shown that routine screening mammograms for women in their 40s can reduce mortality from breast cancer by 15% to 20%.3-5 As noted by Cochrane reviewers in a meta-analysis of 7 randomized controlled studies of breast cancer screening in younger women, a 15% relative risk (RR) reduction represents an absolute risk reduction of 0.05%.5
Potential harms include the financial cost; the screening regimen itself, which includes radiation exposure, pain, inconvenience, and anxiety; the ensuing diagnostic workup in the case of false positive results; and overdiagnosis—ie, detection of lowgrade cancer that would not have otherwise become clinically evident—and subsequent overtreatment.6 Diagnosis of ductal carcinoma in situ (DCIS) was rare before the advent of screening mammography. Now, DCIS accounts for 25% of all breast cancer diagnoses, and more than 90% of cases are detected only by imaging.6 A large epidemiologic review published in 2012 suggested that the increase in breast cancer survival over the last 30 years is due to improved treatment regimens, not early detection.7
Recommendations are equivocal
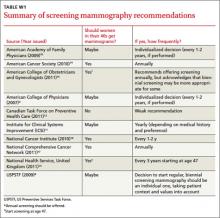
Groups like the USPSTF, the American College of Obstetricians and Gynecologists, and the American Cancer Society, among others (See TABLE W1,8-17 at the end of this article), recognize that women in their 40s may benefit from screening mammography. They generally acknowledge, however, that, the evidence is not strong enough to definitely recommend routine screening mammograms due to the higher risk of false positives and the lower overall incidence of breast cancer in this age group.
The USPSTF set off a firestorm in 2009 with its initial recommendation against routine screening for women in their 40s.8 Shortly after, the group issued an update to “clarify their ... intent,” stating that the decision to start regular screening mammography before age 50 should be an individual one based on patient values as well as an assessment of benefits and risks.8
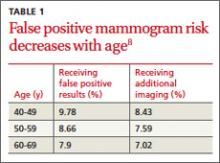
False positives decline with age
The risk of having a false positive result on a screening mammography decreases with increasing age, as the incidence of breast cancer rises (TABLE 1).8 More than 1900 women in their 40s need to undergo screening mammography in order to prevent just one death from breast cancer in 11 years of follow-up,8 with a direct cost of more than 20,000 visits for breast imaging and approximately 2000 false positive mammograms. In contrast, fewer than 400 women in their 60s would need to be screened in order to prevent one breast cancer death in 13 years of follow-up.18 A large prospective cohort study (N=169,456) found that women who started annual screening at age 40 had a 61% chance of receiving at least one false positive mammogram result over the course of 10 years; the chance of a false positive dropped to 41.6% with biennial screening.19
The impact of a false positive lingers. A cohort study that followed 454 women for 3 years after they received a false positive mammogram result found that it continued to have a negative psychological impact on them.20
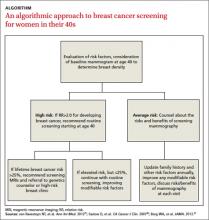
A risk-based screening approach
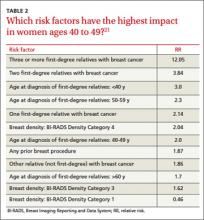
With no clear consensus on when to begin screening, primary care physicians and their patients would be wise to adopt a risk-based approach. Risk-based screening would focus efforts on women ages 40 to 49 who are more likely to benefit from screening mammography, which would represent a more effective use of resources.2 To implement such an approach, it is critical to know the magnitude of risk reduction that would tip the balance of benefits and harms in favor of early screening, and which risk factors are associated with such an elevated risk (TABLE 2).21
A recent comparative modeling study found that for women with a 2-fold increased risk for breast cancer, the benefits and risks of starting biennial screening at age 40 are about the same as that of women at average risk who start biennial screening at age 50. As biennial screening at age 50 is widely recommended, the results of this study suggest that ≥2-fold risk is a useful threshold in determining when to start mammography screening for women in their 40s.21
The traditional counseling of women about breast cancer risks focuses on parity and age of first delivery, breastfeeding, obesity, and alcohol use, in addition to family history. However, none of these has an RR >1.5.22
Two risk factors are associated with ≥2-fold RR for breast cancer:
• having one or more first-degree relatives with breast cancer
• having extremely dense breasts.
A prior breast biopsy is also associated with a high RR (1.87).21
Does your patient have dense breasts? A baseline mammogram is necessary to determine a woman’s breast density. The American College of Radiology developed BI-RADS (Breast Imaging Reporting and Data System) to standardize the reporting of density on mammograms.23 BI-RADS has 4 categories of breast density:
1. Breast tissue is almost entirely fatty. (Adipose tissue is radiolucent and makes the mammogram easier to read.)
2. There are scattered fibroglandular densities in the breast.
3. The breasts are heterogeneously dense.
4. The breasts are extremely dense.
When there is a discrepancy between the density of the left and right breasts, radiologists are instructed to use the higher density.23 Another method of documenting density assesses the percentage of the breast tissue that is dense as compared to fatty tissue.
Increased density (BI-RADS category 3 or 4) likely accounts for a sizeable proportion of nonfamilial breast cancers.24 In a large case control study (N=1112), density in ≥75% of the breast was associated with 26% of all breast cancers diagnosed in women under 56 years.25 While a number of other risk factors for breast cancer are related to breast density (nulliparity, positive family history of breast cancer, and hormone therapy), higher density is associated with large increased risks of breast cancer independent of the other factors.24
Initiate regular screening for women at high risk
Most high-risk women should have regular screening beginning at age 40. The American Cancer Society recommends screening with magnetic resonance imaging (MRI) as opposed to mammography for women with ≥20% lifetime risk of developing breast cancer.26
Adding an annual ultrasound to mammography may be another method of screening for high-risk women. A study of 2809 women with elevated breast cancer risk and dense breasts demonstrated that the addition of annual screening with either ultrasound or MRI detected an additional 3.7 cancers per 1000 women per year beyond mammography alone.27 In that study, however, there was a significant number of false positive results, as well.
MRI is not indicated for women with a 15% to 20% lifetime risk. These women will benefit from routine screening starting at age 40, as well as genetic counseling if they have a family history of breast cancer. Increased breast density can also make mammograms harder to read, and there is concern that density can mask an early cancer. In fact, multiple studies have refuted that claim.28 Breast density does tend to decrease with age, but the relationship between increased density and elevated risk of breast cancer persists through all age groups.
Get a baseline mammogram for those at lower risk
One approach to risk-based screening is to recommend that all women at average risk have an initial screening mammogram at age 40 to determine breast density and discuss other pertinent risk factors. If they are found to have BI-RADS density category 3 or 4, regular screening mammography throughout their 40s is a reasonable approach.
For those at low or average risk, things are less clear, and a discussion to determine the appropriate course of screening is needed. Some women with no family history of breast cancer will elect to wait until age 50 to start screening mammography; others may not be comfortable doing so. It is important to point out to patients with very low density (BI-RADS density category 1) breasts that their risk for breast cancer is very low (RR=0.46) and that waiting until age 50 to start regular screening mammography would be a reasonable decision.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2012;62:10-29.
2. Brawley OW. Risk-based mammography screening: an effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156:662-663.
3. Hendrick RE, Smith RA, Rutledge JH 3rd, et al. Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
4. Kerlikowske K, Grady D, Ernster V. Benefit of mammography screening in women ages 40-49 years: current evidence from randomized controlled trials. Cancer. 1995;76:1679-1681.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.
6. Warner E. Breast-cancer screening. N Engl J Med. 2011;365:1025-1032.
7. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
8. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
9. Qaseem A, Snow V, Sherif K, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 146:511-515.
10. American Academy of Family Physicians. AAFP screening recommendation. Breast cancer, mammography before age 50. Available at: http://www.aafp.org/online/en/home/clinical/exam/ae.html. Accessed September 25, 2012.
11. Institute for Clinical Systems Improvement (ICSI) Breast cancer screening recommendations. Available at http://www.icsi.org/breast_disease_diagnosis/diagnosis_of_breast_disease_2.html. Accessed September 25, 2012.
12. Canadian Task Force on Preventive Health Care. Screening for breast cancer, 2011. Available at: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html. Accessed September 25, 2012.
13. National Health Service,. Breast cancer screening. Available at: http://www.screening.nhs.uk/professionals. Accessed September 25, 2012.
14. American Cancer Society. Guidelines for the early detection of cancer, breast cancer screening. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-ofcancer. Accessed October 1, 2012.
15. American College of Obstetricians and Gynecologists (ACOG). Breast cancer screening. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2011 Aug. 11 p. (ACOG practice bulletin; no. 122).
16. National Cancer Institute. Breast cancer screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/breast/healthprofessional/page1. Accessed September 25, 2012.
17. National Comprehensive Cancer Institute. Guidelines for the detection of breast cancer. Available at: http://www.nccn.org. Accessed October 1, 2012.
18. Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010;363 :1076-1079.
19. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
20. Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106-115.
21. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Int Med. 2012;156:609-617.
22. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and metaanalysis. Ann Intern Med. 2012;156:635-648.
23. D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR Bi-RADS Mammography. 4th ed. Reston, VA: American College of Radiology; 2003.
24. Gierach GL, Ichikawa L, Kerikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218-1227.
25. Boyd NJ, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
26. Saslow D, Boetets C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
27. Berg WA, Zhang A, Lehrer D, et al; ACRINN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
28. McCormark VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Epidemol Biomarkers Prev. 2006;15:1159-1169.
› Recommend that women consider having a single mammogram at age 40 as a baseline so that breast density can be included in the assessment of risk. minor MRSA skin lesions in children with mupirocin. C
› Advise women with low breast density and no other significant risk factors that they are at lower than average risk for breast cancer and should consider this when discussing when to begin routine screening with their physician. C
› Recommend that women with a 2-fold increased risk for breast cancer begin regular screening in their 40s. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, when should I start having mammograms?” That’s a question you’re apt to hear again and again from women in their early 40s. It’s also a question with no easy answer.
While deaths from breast cancer are declining, it remains the most commonly diagnosed cancer among US women. In 2012, approximately 229,060 new cases of breast cancer were detected and an estimated 39,920 women died from breast cancer1—about 10% of them in their 40s.2
Based on these numbers alone, it would seem that every woman should begin regular screening at age 40. Yet there are many other issues to consider, namely the high rate of false positives, as well as the overdiagnosis and overtreatment associated with such screening. Further complicating matters is the fact that there is no consensus as to whether screening mammography should be recommended—and if so, how often—for women ages 40 to 49 years who are at average risk.
In light of this, we offer a risk-based strategy to mammography for younger women, which we’ve distilled into an ALGORITHM. But first, let’s look at the evidence and what the US Preventive Services Task Force (USPSTF) and major medical groups have to say.
To screen or not to screen? A look at the evidence
A decision to perform screening mammography in premenopausal women should be made by weighing benefits vs harms. Benefits include diagnosis of breast cancer when it’s in an early stage and a reduction in death. Meta-analyses have consistently shown that routine screening mammograms for women in their 40s can reduce mortality from breast cancer by 15% to 20%.3-5 As noted by Cochrane reviewers in a meta-analysis of 7 randomized controlled studies of breast cancer screening in younger women, a 15% relative risk (RR) reduction represents an absolute risk reduction of 0.05%.5
Potential harms include the financial cost; the screening regimen itself, which includes radiation exposure, pain, inconvenience, and anxiety; the ensuing diagnostic workup in the case of false positive results; and overdiagnosis—ie, detection of lowgrade cancer that would not have otherwise become clinically evident—and subsequent overtreatment.6 Diagnosis of ductal carcinoma in situ (DCIS) was rare before the advent of screening mammography. Now, DCIS accounts for 25% of all breast cancer diagnoses, and more than 90% of cases are detected only by imaging.6 A large epidemiologic review published in 2012 suggested that the increase in breast cancer survival over the last 30 years is due to improved treatment regimens, not early detection.7
Recommendations are equivocal
Groups like the USPSTF, the American College of Obstetricians and Gynecologists, and the American Cancer Society, among others (See TABLE W1,8-17 at the end of this article), recognize that women in their 40s may benefit from screening mammography. They generally acknowledge, however, that, the evidence is not strong enough to definitely recommend routine screening mammograms due to the higher risk of false positives and the lower overall incidence of breast cancer in this age group.
The USPSTF set off a firestorm in 2009 with its initial recommendation against routine screening for women in their 40s.8 Shortly after, the group issued an update to “clarify their ... intent,” stating that the decision to start regular screening mammography before age 50 should be an individual one based on patient values as well as an assessment of benefits and risks.8
False positives decline with age
The risk of having a false positive result on a screening mammography decreases with increasing age, as the incidence of breast cancer rises (TABLE 1).8 More than 1900 women in their 40s need to undergo screening mammography in order to prevent just one death from breast cancer in 11 years of follow-up,8 with a direct cost of more than 20,000 visits for breast imaging and approximately 2000 false positive mammograms. In contrast, fewer than 400 women in their 60s would need to be screened in order to prevent one breast cancer death in 13 years of follow-up.18 A large prospective cohort study (N=169,456) found that women who started annual screening at age 40 had a 61% chance of receiving at least one false positive mammogram result over the course of 10 years; the chance of a false positive dropped to 41.6% with biennial screening.19
The impact of a false positive lingers. A cohort study that followed 454 women for 3 years after they received a false positive mammogram result found that it continued to have a negative psychological impact on them.20
A risk-based screening approach
With no clear consensus on when to begin screening, primary care physicians and their patients would be wise to adopt a risk-based approach. Risk-based screening would focus efforts on women ages 40 to 49 who are more likely to benefit from screening mammography, which would represent a more effective use of resources.2 To implement such an approach, it is critical to know the magnitude of risk reduction that would tip the balance of benefits and harms in favor of early screening, and which risk factors are associated with such an elevated risk (TABLE 2).21
A recent comparative modeling study found that for women with a 2-fold increased risk for breast cancer, the benefits and risks of starting biennial screening at age 40 are about the same as that of women at average risk who start biennial screening at age 50. As biennial screening at age 50 is widely recommended, the results of this study suggest that ≥2-fold risk is a useful threshold in determining when to start mammography screening for women in their 40s.21
The traditional counseling of women about breast cancer risks focuses on parity and age of first delivery, breastfeeding, obesity, and alcohol use, in addition to family history. However, none of these has an RR >1.5.22
Two risk factors are associated with ≥2-fold RR for breast cancer:
• having one or more first-degree relatives with breast cancer
• having extremely dense breasts.
A prior breast biopsy is also associated with a high RR (1.87).21
Does your patient have dense breasts? A baseline mammogram is necessary to determine a woman’s breast density. The American College of Radiology developed BI-RADS (Breast Imaging Reporting and Data System) to standardize the reporting of density on mammograms.23 BI-RADS has 4 categories of breast density:
1. Breast tissue is almost entirely fatty. (Adipose tissue is radiolucent and makes the mammogram easier to read.)
2. There are scattered fibroglandular densities in the breast.
3. The breasts are heterogeneously dense.
4. The breasts are extremely dense.
When there is a discrepancy between the density of the left and right breasts, radiologists are instructed to use the higher density.23 Another method of documenting density assesses the percentage of the breast tissue that is dense as compared to fatty tissue.
Increased density (BI-RADS category 3 or 4) likely accounts for a sizeable proportion of nonfamilial breast cancers.24 In a large case control study (N=1112), density in ≥75% of the breast was associated with 26% of all breast cancers diagnosed in women under 56 years.25 While a number of other risk factors for breast cancer are related to breast density (nulliparity, positive family history of breast cancer, and hormone therapy), higher density is associated with large increased risks of breast cancer independent of the other factors.24
Initiate regular screening for women at high risk
Most high-risk women should have regular screening beginning at age 40. The American Cancer Society recommends screening with magnetic resonance imaging (MRI) as opposed to mammography for women with ≥20% lifetime risk of developing breast cancer.26
Adding an annual ultrasound to mammography may be another method of screening for high-risk women. A study of 2809 women with elevated breast cancer risk and dense breasts demonstrated that the addition of annual screening with either ultrasound or MRI detected an additional 3.7 cancers per 1000 women per year beyond mammography alone.27 In that study, however, there was a significant number of false positive results, as well.
MRI is not indicated for women with a 15% to 20% lifetime risk. These women will benefit from routine screening starting at age 40, as well as genetic counseling if they have a family history of breast cancer. Increased breast density can also make mammograms harder to read, and there is concern that density can mask an early cancer. In fact, multiple studies have refuted that claim.28 Breast density does tend to decrease with age, but the relationship between increased density and elevated risk of breast cancer persists through all age groups.
Get a baseline mammogram for those at lower risk
One approach to risk-based screening is to recommend that all women at average risk have an initial screening mammogram at age 40 to determine breast density and discuss other pertinent risk factors. If they are found to have BI-RADS density category 3 or 4, regular screening mammography throughout their 40s is a reasonable approach.
For those at low or average risk, things are less clear, and a discussion to determine the appropriate course of screening is needed. Some women with no family history of breast cancer will elect to wait until age 50 to start screening mammography; others may not be comfortable doing so. It is important to point out to patients with very low density (BI-RADS density category 1) breasts that their risk for breast cancer is very low (RR=0.46) and that waiting until age 50 to start regular screening mammography would be a reasonable decision.
› Recommend that women consider having a single mammogram at age 40 as a baseline so that breast density can be included in the assessment of risk. minor MRSA skin lesions in children with mupirocin. C
› Advise women with low breast density and no other significant risk factors that they are at lower than average risk for breast cancer and should consider this when discussing when to begin routine screening with their physician. C
› Recommend that women with a 2-fold increased risk for breast cancer begin regular screening in their 40s. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, when should I start having mammograms?” That’s a question you’re apt to hear again and again from women in their early 40s. It’s also a question with no easy answer.
While deaths from breast cancer are declining, it remains the most commonly diagnosed cancer among US women. In 2012, approximately 229,060 new cases of breast cancer were detected and an estimated 39,920 women died from breast cancer1—about 10% of them in their 40s.2
Based on these numbers alone, it would seem that every woman should begin regular screening at age 40. Yet there are many other issues to consider, namely the high rate of false positives, as well as the overdiagnosis and overtreatment associated with such screening. Further complicating matters is the fact that there is no consensus as to whether screening mammography should be recommended—and if so, how often—for women ages 40 to 49 years who are at average risk.
In light of this, we offer a risk-based strategy to mammography for younger women, which we’ve distilled into an ALGORITHM. But first, let’s look at the evidence and what the US Preventive Services Task Force (USPSTF) and major medical groups have to say.
To screen or not to screen? A look at the evidence
A decision to perform screening mammography in premenopausal women should be made by weighing benefits vs harms. Benefits include diagnosis of breast cancer when it’s in an early stage and a reduction in death. Meta-analyses have consistently shown that routine screening mammograms for women in their 40s can reduce mortality from breast cancer by 15% to 20%.3-5 As noted by Cochrane reviewers in a meta-analysis of 7 randomized controlled studies of breast cancer screening in younger women, a 15% relative risk (RR) reduction represents an absolute risk reduction of 0.05%.5
Potential harms include the financial cost; the screening regimen itself, which includes radiation exposure, pain, inconvenience, and anxiety; the ensuing diagnostic workup in the case of false positive results; and overdiagnosis—ie, detection of lowgrade cancer that would not have otherwise become clinically evident—and subsequent overtreatment.6 Diagnosis of ductal carcinoma in situ (DCIS) was rare before the advent of screening mammography. Now, DCIS accounts for 25% of all breast cancer diagnoses, and more than 90% of cases are detected only by imaging.6 A large epidemiologic review published in 2012 suggested that the increase in breast cancer survival over the last 30 years is due to improved treatment regimens, not early detection.7
Recommendations are equivocal
Groups like the USPSTF, the American College of Obstetricians and Gynecologists, and the American Cancer Society, among others (See TABLE W1,8-17 at the end of this article), recognize that women in their 40s may benefit from screening mammography. They generally acknowledge, however, that, the evidence is not strong enough to definitely recommend routine screening mammograms due to the higher risk of false positives and the lower overall incidence of breast cancer in this age group.
The USPSTF set off a firestorm in 2009 with its initial recommendation against routine screening for women in their 40s.8 Shortly after, the group issued an update to “clarify their ... intent,” stating that the decision to start regular screening mammography before age 50 should be an individual one based on patient values as well as an assessment of benefits and risks.8
False positives decline with age
The risk of having a false positive result on a screening mammography decreases with increasing age, as the incidence of breast cancer rises (TABLE 1).8 More than 1900 women in their 40s need to undergo screening mammography in order to prevent just one death from breast cancer in 11 years of follow-up,8 with a direct cost of more than 20,000 visits for breast imaging and approximately 2000 false positive mammograms. In contrast, fewer than 400 women in their 60s would need to be screened in order to prevent one breast cancer death in 13 years of follow-up.18 A large prospective cohort study (N=169,456) found that women who started annual screening at age 40 had a 61% chance of receiving at least one false positive mammogram result over the course of 10 years; the chance of a false positive dropped to 41.6% with biennial screening.19
The impact of a false positive lingers. A cohort study that followed 454 women for 3 years after they received a false positive mammogram result found that it continued to have a negative psychological impact on them.20
A risk-based screening approach
With no clear consensus on when to begin screening, primary care physicians and their patients would be wise to adopt a risk-based approach. Risk-based screening would focus efforts on women ages 40 to 49 who are more likely to benefit from screening mammography, which would represent a more effective use of resources.2 To implement such an approach, it is critical to know the magnitude of risk reduction that would tip the balance of benefits and harms in favor of early screening, and which risk factors are associated with such an elevated risk (TABLE 2).21
A recent comparative modeling study found that for women with a 2-fold increased risk for breast cancer, the benefits and risks of starting biennial screening at age 40 are about the same as that of women at average risk who start biennial screening at age 50. As biennial screening at age 50 is widely recommended, the results of this study suggest that ≥2-fold risk is a useful threshold in determining when to start mammography screening for women in their 40s.21
The traditional counseling of women about breast cancer risks focuses on parity and age of first delivery, breastfeeding, obesity, and alcohol use, in addition to family history. However, none of these has an RR >1.5.22
Two risk factors are associated with ≥2-fold RR for breast cancer:
• having one or more first-degree relatives with breast cancer
• having extremely dense breasts.
A prior breast biopsy is also associated with a high RR (1.87).21
Does your patient have dense breasts? A baseline mammogram is necessary to determine a woman’s breast density. The American College of Radiology developed BI-RADS (Breast Imaging Reporting and Data System) to standardize the reporting of density on mammograms.23 BI-RADS has 4 categories of breast density:
1. Breast tissue is almost entirely fatty. (Adipose tissue is radiolucent and makes the mammogram easier to read.)
2. There are scattered fibroglandular densities in the breast.
3. The breasts are heterogeneously dense.
4. The breasts are extremely dense.
When there is a discrepancy between the density of the left and right breasts, radiologists are instructed to use the higher density.23 Another method of documenting density assesses the percentage of the breast tissue that is dense as compared to fatty tissue.
Increased density (BI-RADS category 3 or 4) likely accounts for a sizeable proportion of nonfamilial breast cancers.24 In a large case control study (N=1112), density in ≥75% of the breast was associated with 26% of all breast cancers diagnosed in women under 56 years.25 While a number of other risk factors for breast cancer are related to breast density (nulliparity, positive family history of breast cancer, and hormone therapy), higher density is associated with large increased risks of breast cancer independent of the other factors.24
Initiate regular screening for women at high risk
Most high-risk women should have regular screening beginning at age 40. The American Cancer Society recommends screening with magnetic resonance imaging (MRI) as opposed to mammography for women with ≥20% lifetime risk of developing breast cancer.26
Adding an annual ultrasound to mammography may be another method of screening for high-risk women. A study of 2809 women with elevated breast cancer risk and dense breasts demonstrated that the addition of annual screening with either ultrasound or MRI detected an additional 3.7 cancers per 1000 women per year beyond mammography alone.27 In that study, however, there was a significant number of false positive results, as well.
MRI is not indicated for women with a 15% to 20% lifetime risk. These women will benefit from routine screening starting at age 40, as well as genetic counseling if they have a family history of breast cancer. Increased breast density can also make mammograms harder to read, and there is concern that density can mask an early cancer. In fact, multiple studies have refuted that claim.28 Breast density does tend to decrease with age, but the relationship between increased density and elevated risk of breast cancer persists through all age groups.
Get a baseline mammogram for those at lower risk
One approach to risk-based screening is to recommend that all women at average risk have an initial screening mammogram at age 40 to determine breast density and discuss other pertinent risk factors. If they are found to have BI-RADS density category 3 or 4, regular screening mammography throughout their 40s is a reasonable approach.
For those at low or average risk, things are less clear, and a discussion to determine the appropriate course of screening is needed. Some women with no family history of breast cancer will elect to wait until age 50 to start screening mammography; others may not be comfortable doing so. It is important to point out to patients with very low density (BI-RADS density category 1) breasts that their risk for breast cancer is very low (RR=0.46) and that waiting until age 50 to start regular screening mammography would be a reasonable decision.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2012;62:10-29.
2. Brawley OW. Risk-based mammography screening: an effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156:662-663.
3. Hendrick RE, Smith RA, Rutledge JH 3rd, et al. Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
4. Kerlikowske K, Grady D, Ernster V. Benefit of mammography screening in women ages 40-49 years: current evidence from randomized controlled trials. Cancer. 1995;76:1679-1681.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.
6. Warner E. Breast-cancer screening. N Engl J Med. 2011;365:1025-1032.
7. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
8. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
9. Qaseem A, Snow V, Sherif K, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 146:511-515.
10. American Academy of Family Physicians. AAFP screening recommendation. Breast cancer, mammography before age 50. Available at: http://www.aafp.org/online/en/home/clinical/exam/ae.html. Accessed September 25, 2012.
11. Institute for Clinical Systems Improvement (ICSI) Breast cancer screening recommendations. Available at http://www.icsi.org/breast_disease_diagnosis/diagnosis_of_breast_disease_2.html. Accessed September 25, 2012.
12. Canadian Task Force on Preventive Health Care. Screening for breast cancer, 2011. Available at: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html. Accessed September 25, 2012.
13. National Health Service,. Breast cancer screening. Available at: http://www.screening.nhs.uk/professionals. Accessed September 25, 2012.
14. American Cancer Society. Guidelines for the early detection of cancer, breast cancer screening. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-ofcancer. Accessed October 1, 2012.
15. American College of Obstetricians and Gynecologists (ACOG). Breast cancer screening. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2011 Aug. 11 p. (ACOG practice bulletin; no. 122).
16. National Cancer Institute. Breast cancer screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/breast/healthprofessional/page1. Accessed September 25, 2012.
17. National Comprehensive Cancer Institute. Guidelines for the detection of breast cancer. Available at: http://www.nccn.org. Accessed October 1, 2012.
18. Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010;363 :1076-1079.
19. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
20. Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106-115.
21. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Int Med. 2012;156:609-617.
22. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and metaanalysis. Ann Intern Med. 2012;156:635-648.
23. D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR Bi-RADS Mammography. 4th ed. Reston, VA: American College of Radiology; 2003.
24. Gierach GL, Ichikawa L, Kerikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218-1227.
25. Boyd NJ, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
26. Saslow D, Boetets C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
27. Berg WA, Zhang A, Lehrer D, et al; ACRINN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
28. McCormark VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Epidemol Biomarkers Prev. 2006;15:1159-1169.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2012;62:10-29.
2. Brawley OW. Risk-based mammography screening: an effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156:662-663.
3. Hendrick RE, Smith RA, Rutledge JH 3rd, et al. Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
4. Kerlikowske K, Grady D, Ernster V. Benefit of mammography screening in women ages 40-49 years: current evidence from randomized controlled trials. Cancer. 1995;76:1679-1681.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.
6. Warner E. Breast-cancer screening. N Engl J Med. 2011;365:1025-1032.
7. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
8. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
9. Qaseem A, Snow V, Sherif K, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 146:511-515.
10. American Academy of Family Physicians. AAFP screening recommendation. Breast cancer, mammography before age 50. Available at: http://www.aafp.org/online/en/home/clinical/exam/ae.html. Accessed September 25, 2012.
11. Institute for Clinical Systems Improvement (ICSI) Breast cancer screening recommendations. Available at http://www.icsi.org/breast_disease_diagnosis/diagnosis_of_breast_disease_2.html. Accessed September 25, 2012.
12. Canadian Task Force on Preventive Health Care. Screening for breast cancer, 2011. Available at: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html. Accessed September 25, 2012.
13. National Health Service,. Breast cancer screening. Available at: http://www.screening.nhs.uk/professionals. Accessed September 25, 2012.
14. American Cancer Society. Guidelines for the early detection of cancer, breast cancer screening. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-ofcancer. Accessed October 1, 2012.
15. American College of Obstetricians and Gynecologists (ACOG). Breast cancer screening. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2011 Aug. 11 p. (ACOG practice bulletin; no. 122).
16. National Cancer Institute. Breast cancer screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/breast/healthprofessional/page1. Accessed September 25, 2012.
17. National Comprehensive Cancer Institute. Guidelines for the detection of breast cancer. Available at: http://www.nccn.org. Accessed October 1, 2012.
18. Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010;363 :1076-1079.
19. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
20. Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106-115.
21. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Int Med. 2012;156:609-617.
22. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and metaanalysis. Ann Intern Med. 2012;156:635-648.
23. D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR Bi-RADS Mammography. 4th ed. Reston, VA: American College of Radiology; 2003.
24. Gierach GL, Ichikawa L, Kerikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218-1227.
25. Boyd NJ, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
26. Saslow D, Boetets C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
27. Berg WA, Zhang A, Lehrer D, et al; ACRINN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
28. McCormark VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Epidemol Biomarkers Prev. 2006;15:1159-1169.
A practical guide to community-acquired MRSA
› Treat a simple cutaneous abscess from a methicillin-resistant Staphylococcus
aureus (MRSA) infection with incision and drainage alone. A
› Treat minor MRSA skin lesions in children with mupirocin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
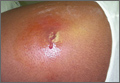
CASE › A 21-year-old man seeks care at his family physician (FP)’s office for a painful, draining lesion that’s been on his left thigh for 5 days. He reports that he was bitten by a spider during one of his weekly football games with his fraternity brothers, but doesn’t recall seeing a spider. He has been applying an over-the-counter topical antibiotic without any improvement, and reports that the area of redness has tripled in size within the last 24 hours.
His past medical history is unremarkable except for an allergy to sulfa drugs that was discovered during treatment for a skin infection 2 years ago. He takes no regular medications.
The patient is afebrile and in no distress. The skin overlying his left thigh has a 1 cm oozing lesion with pus evident and surrounding erythema. The wound is warm and tender to the touch.
Based on the patient’s history, the FP suspects methicillin-resistant Staphylococcus aureus (MRSA) and obtains a swab for bacterial culture and sensitivities.
What the surveillance data tell us about MRSA
MRSA infections—a subset of staph infections that are resistant to beta-lactam antibiotics and cephalosporins—continue to be a major source of infection in the community.1 The prevalence of both community-acquired (CA) and hospital acquired (HA) MRSA infections worldwide has continued to grow despite improvements in controlling nosocomial spread.2,3 Data from the early 2000s found an approximate 1% to 2% MRSA colonization rate in the United States, but other countries have had rates as high as 50%.3 Rates across countries have consistently been higher in children and adolescents. A recent surveillance study suggests a decline in the incidence of the most serious types of MRSA infections in major metropolitan areas, the significance of which is still under investigation.4
CA-MRSA and HA-MRSA have different in vitro sensitivities to antimicrobials, different virulence factors, and different epidemiologic profiles.
CA-MRSA occurs in people who have not been recently hospitalized or had any recent medical procedures. These infections usually develop on skin and soft tissue. CA-MRSA typically contains the genes for the Panton-Valentine Leukocidin (PVL) toxin, which is a virulence factor that leads to increased interleukin-8 secretion and skin necrosis.5,6 In addition, CA-MRSA usually does not have genes associated with multidrug-resistant strains.
HA-MRSA occurs in people who have recently been hospitalized, had recent medical procedures, or have been treated in a longterm care setting. HA-MRSA is associated with multidrug-resistant strains; however, it usually does not have the genes for PVL toxin.7
Factors that put patients at risk for CA-MRSA skin infections
As many as 90% of CA-MRSA infections present as skin and soft tissue infections (SSTIs) that have the potential to become invasive if not managed appropriately.3,8 There are a number of factors that put patients at risk for these SSTIs (TABLE 1)7-9—chief among them, intrafamilial or close contact transmission. People living in close quarters with colonized individuals are 14 times more likely to be carriers than a matched unexposed population.9 Similarly, other environments that typically involve close quarters or overcrowding, including military installations, prisons, long-term care facilities, and daycare centers, generally see higher rates of MRSA colonization.10
Researchers also hypothesize that repeated skin trauma is another risk factor for CA-MRSA SSTIs. This may explain the increased rates of CA-MRSA infections seen in athletes and military recruits undergoing basic training, as they are prone to skin abrasions.8 Certain ethnic groups have a higher prevalence of CA-MRSA infections as well, including Native Americans, Pacific Islanders, and African Americans.9,11 It is not entirely clear if there is anything unique predisposing these populations to MRSA or if this might be attributed to living in tight communities with close household contacts.
High-risk groups that have elevated rates of CA-MRSA SSTIs and are more likely to be carriers include intravenous drug users, men who have sex with men, immunocompromised individuals (including those with human immunodeficiency virus), and the homeless.8,9 Several studies have also reported higher rates of MRSA colonization and CA-MRSA infections in individuals who have come into contact with the health care system. A meta-analysis indicated that nasal swabs taken from patients at health care facilities were 2.35 times more likely to be positive for MRSA than those taken from individuals as nonhealth care locations.9 Risk factors such as antibiotic use or one or more physician visits in the past year have been associated with higher rates of infections, as well.2
Diagnosis requires careful history and exam
Community-acquired MRSA SSTIs are often diagnosed based on the patient’s history and risk factors, along with physical exam findings. Common findings include single or multiple erythematous pustules, furuncles, carbuncles, cellulitis, and abscesses. Patients may confuse the initial lesion with an insect or spider bite,12 as occurred with the patient in our opening scenario.
If purulent drainage is present, perform a swab for culture and sensitivity to confirm the diagnosis of MRSA and assist in antibiotic selection.13 If a patient with an SSTI does not respond to beta-lactam antibiotics, consider MRSA until proven otherwise.
Once confirmed by microbiological assessment, follow Centers for Disease Control and Prevention guidelines to differentiate CA-MRSA from HA-MRSA.14 It is important to distinguish between the two, as patients with HA-MRSA are at greater risk for antibiotic failure and progression to more invasive infections.
Confirmation of a CA-MRSA infection requires all of the following criteria:14
• Diagnosis was made in the outpatient setting or by positive culture within 48 hours of admission to the hospital
• No history of MRSA infection or colonization
• No history in the past year of hospitalization; admission to a skilled nursing facility or hospice; dialysis; or surgery
• No permanent indwelling catheters or medical devices that go through the skin.
Confirmation of an HA-MRSA infection requires at least one of the following criteria:14
• It occurs more than 48 hours after admission to the hospital.
• There is a prior history of MRSA infection or colonization.
• There is a history in the past year of hospitalization; admission to a skilled
nursing facility, nursing home, or hospice; dialysis; or surgery.
• The presence of any indwelling catheter or medical device that passes through the skin into the body.
Rely on evidence-based treatment protocols
In 2011, the Infectious Disease Society of America (IDSA) published consensus guidelines for CA-MRSA to assist with evidencebased decision making. Primary treatment of cutaneous abscesses remains incision and drainage alone without antibiotics, except under certain circumstances (TABLE 2).13 The guidelines recommend antibiotic treatment with empiric coverage for CA-MRSA when purulent cellulitis exists. However, if cellulitis exists without purulence or abscess formation, antibiotic coverage for CA-MRSA is not encouraged for initial treatment.13
Empiric antibiotic coverage for uncomplicated CA-MRSA SSTIs managed in the outpatient setting should include clindamycin, trimethoprim/sulfamethoxazole (TMPSMX), a tetracycline, or linezolid for 5 to 10 days. Factors including cost, patient age and comorbidities, drug allergies, and local resistance patterns should guide the initial antibiotic you choose. TMP-SMX and tetracyclines are relatively inexpensive (<$20), clindamycin is more costly, and linezolid is often costprohibitive as standard treatment.
Do not prescribe tetracyclines for children younger than 8 years of age because of the risk of permanent tooth discoloration. Clindamycin is the only category “B” antibiotic in the group that can be used during pregnancy, and renal impairment needs to be taken into consideration with TMP-SMX and linezolid. For very minor skin lesions in children, mupirocin 2% topical ointment appears to be the therapy of choice.13
Although IDSA has not provided strong recommendations about treatment of asymptomatic close household contacts, this may be considered as another means of attempting to control the spread of MRSA in the community.
Provide patient education. Patient education is paramount to successful treatment and prevention. Explain to patients that people living in close quarters with individuals who already have the bacteria on the skin are far more likely to be carriers. Overcrowding situations that pose a risk include military installations, prisons, long-term care facilities, athletic teams, and daycare centers.
To prevent MRSA, encourage patients to wash their hands often and shower regularly, especially after exercise. Also keep cuts, scrapes, and wounds clean and covered until they heal. Avoid sharing personal items, such as towel and razors.
Finally, remind patients to get care early if they think they may be infected. Symptoms suggestive of MRSA include a red, swollen, painful area on the skin that may look like a spider bite. The area may contain pus and be accompanied by a fever.
Provide close outpatient follow-up within a few days of initiating therapy to document clinical response and aid in further decision-making. Signs of more extensive cellulitis may signal a need for a change of antibiotics or parenteral therapy. Similarly, joint or bone pain underlying areas of cellulitis, significant myalgias or muscle pain out of proportion to exam, or systemic symptoms such as fever, chills, nausea, or lethargy might indicate more extensive infection requiring inpatient treatment.
How to manage recurrent infections
Management of recurrent CA-MRSA SSTIs in the outpatient setting poses a challenge. Following successful treatment of active infection, you may want to attempt decolonization in select patients. Those with repeated MRSA infections despite adequate hygiene measures or with a high probability of reexposure to colonized close contacts may be treated, although the evidence supporting such protocols is lacking.8,13
Acceptable procedures described by IDSA include nasal mupirocin twice daily for 5 to 10 days, mupirocin plus topical antiseptic solution (eg, chlorhexidine, triclosan, or povidone-iodine) for 5 to 14 days, or mupirocin plus dilute bleach baths (1 teaspoon bleach/gallon of water) twice weekly for 15 minutes over 3 months.13 Although antibiotics are generally only recommended for active infection, the combination of rifampin and an antibiotic with MRSA coverage may be used for 1 to 2 weeks in cases of recurrent infections despite recommended hygiene and topical decolonization measures.13,15,16 Rifampin is not recommended as monotherapy for MRSA infection or decolonization.13
Unfortunately, even in cases where eradication is initially successful, about half of those with subsequent negative MRSA cultures will test positive before the end of a year.16 With recurrent or severe infections or immunocompromised patients, it’s advisable to consider an infectious disease consult.
CASE › The patient’s lesion did not require incision as it was already draining. He received a prescription for doxycycline hyclate 100 mg BID for 7 days (since there was evidence of rapidly progressing cellulitis) and was instructed to return to the clinic in 48 hours.
When he returned to the clinic, the patient stated that the pain had improved and the wound was no longer oozing. Culture results confirmed MR SA sensitive to TMP-SMX, doxycycline, and clindamycin. Examination showed improved erythema, a dry wound, and no pain on palpation. He was given a patient information handout on MRSA infection and advised to return to the clinic if the wound did not completely heal within the next 7 days.
1. Özel G, Aslan V, Bahar Erdem G, et al. Comparison of oxacillin, cefoxitin, ceftizoxime, and moxalactam disk diffusion methods for detection of methicillin susceptibility in staphylococci. Mikrobiyol Bul. 2011;45:258-265.
2. Crum NF, Lee RU, Thornton SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943-951.
3. Skov R, Christiansen K, Dancer SJ, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents. 2012;39:193-200.
4. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. September 16, 2013. Available at: http://archinte.jamanetwork.com/article.aspx?articleid=1738718. Accessed October 15, 2013.
5. Kawaguchiya M, Urushibara N, Kuwahara O, et al. Molecular characteristics of community-acquired methicillin-resistant Staphylococcus aureus in Hokkaido, northern main island of Japan: identification of sequence types 6 and 59 Panton-Valentine leucocidin-positive community-acquired methicillin-resistant Staphylococcus aureus. Microb Drug Resist. 2011;17:241-250.
6. Wiener-Kronish JP, Pittet JF. Therapies against virulence products of Staphylococcus aureus and Pseudomonas aeruginosa. Semin Respir Crit Care Med. 2011;32:228-235.
7. Hansra NK, Shinkai K. Cutaneous community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus. Dermatol Ther. 2011;24:263-272.
8. Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Am Acad Dermatol. 2007;56:1-16; quiz 17-20.
9. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-139.
10. Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:1-7.
11. Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core Surveillance (ABCs) MRSA Investigators. Invasive methicillinresistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
12. NeVille-Swensen M, Clayton M. Outpatient management of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infection. J Pediatr Health Care. 2011;25:308-315.
13. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
14. Diagnosis and testing for MRSA infections. Centers for Disease Control and Infection Web site. Available at: http://www.cdc.gov/mrsa/diagnosis/index.html. Accessed September 30, 2013.
15. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29:510-516.
16. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44:178-185.
› Treat a simple cutaneous abscess from a methicillin-resistant Staphylococcus
aureus (MRSA) infection with incision and drainage alone. A
› Treat minor MRSA skin lesions in children with mupirocin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 21-year-old man seeks care at his family physician (FP)’s office for a painful, draining lesion that’s been on his left thigh for 5 days. He reports that he was bitten by a spider during one of his weekly football games with his fraternity brothers, but doesn’t recall seeing a spider. He has been applying an over-the-counter topical antibiotic without any improvement, and reports that the area of redness has tripled in size within the last 24 hours.
His past medical history is unremarkable except for an allergy to sulfa drugs that was discovered during treatment for a skin infection 2 years ago. He takes no regular medications.
The patient is afebrile and in no distress. The skin overlying his left thigh has a 1 cm oozing lesion with pus evident and surrounding erythema. The wound is warm and tender to the touch.
Based on the patient’s history, the FP suspects methicillin-resistant Staphylococcus aureus (MRSA) and obtains a swab for bacterial culture and sensitivities.
What the surveillance data tell us about MRSA
MRSA infections—a subset of staph infections that are resistant to beta-lactam antibiotics and cephalosporins—continue to be a major source of infection in the community.1 The prevalence of both community-acquired (CA) and hospital acquired (HA) MRSA infections worldwide has continued to grow despite improvements in controlling nosocomial spread.2,3 Data from the early 2000s found an approximate 1% to 2% MRSA colonization rate in the United States, but other countries have had rates as high as 50%.3 Rates across countries have consistently been higher in children and adolescents. A recent surveillance study suggests a decline in the incidence of the most serious types of MRSA infections in major metropolitan areas, the significance of which is still under investigation.4
CA-MRSA and HA-MRSA have different in vitro sensitivities to antimicrobials, different virulence factors, and different epidemiologic profiles.
CA-MRSA occurs in people who have not been recently hospitalized or had any recent medical procedures. These infections usually develop on skin and soft tissue. CA-MRSA typically contains the genes for the Panton-Valentine Leukocidin (PVL) toxin, which is a virulence factor that leads to increased interleukin-8 secretion and skin necrosis.5,6 In addition, CA-MRSA usually does not have genes associated with multidrug-resistant strains.
HA-MRSA occurs in people who have recently been hospitalized, had recent medical procedures, or have been treated in a longterm care setting. HA-MRSA is associated with multidrug-resistant strains; however, it usually does not have the genes for PVL toxin.7
Factors that put patients at risk for CA-MRSA skin infections
As many as 90% of CA-MRSA infections present as skin and soft tissue infections (SSTIs) that have the potential to become invasive if not managed appropriately.3,8 There are a number of factors that put patients at risk for these SSTIs (TABLE 1)7-9—chief among them, intrafamilial or close contact transmission. People living in close quarters with colonized individuals are 14 times more likely to be carriers than a matched unexposed population.9 Similarly, other environments that typically involve close quarters or overcrowding, including military installations, prisons, long-term care facilities, and daycare centers, generally see higher rates of MRSA colonization.10
Researchers also hypothesize that repeated skin trauma is another risk factor for CA-MRSA SSTIs. This may explain the increased rates of CA-MRSA infections seen in athletes and military recruits undergoing basic training, as they are prone to skin abrasions.8 Certain ethnic groups have a higher prevalence of CA-MRSA infections as well, including Native Americans, Pacific Islanders, and African Americans.9,11 It is not entirely clear if there is anything unique predisposing these populations to MRSA or if this might be attributed to living in tight communities with close household contacts.
High-risk groups that have elevated rates of CA-MRSA SSTIs and are more likely to be carriers include intravenous drug users, men who have sex with men, immunocompromised individuals (including those with human immunodeficiency virus), and the homeless.8,9 Several studies have also reported higher rates of MRSA colonization and CA-MRSA infections in individuals who have come into contact with the health care system. A meta-analysis indicated that nasal swabs taken from patients at health care facilities were 2.35 times more likely to be positive for MRSA than those taken from individuals as nonhealth care locations.9 Risk factors such as antibiotic use or one or more physician visits in the past year have been associated with higher rates of infections, as well.2
Diagnosis requires careful history and exam
Community-acquired MRSA SSTIs are often diagnosed based on the patient’s history and risk factors, along with physical exam findings. Common findings include single or multiple erythematous pustules, furuncles, carbuncles, cellulitis, and abscesses. Patients may confuse the initial lesion with an insect or spider bite,12 as occurred with the patient in our opening scenario.
If purulent drainage is present, perform a swab for culture and sensitivity to confirm the diagnosis of MRSA and assist in antibiotic selection.13 If a patient with an SSTI does not respond to beta-lactam antibiotics, consider MRSA until proven otherwise.
Once confirmed by microbiological assessment, follow Centers for Disease Control and Prevention guidelines to differentiate CA-MRSA from HA-MRSA.14 It is important to distinguish between the two, as patients with HA-MRSA are at greater risk for antibiotic failure and progression to more invasive infections.
Confirmation of a CA-MRSA infection requires all of the following criteria:14
• Diagnosis was made in the outpatient setting or by positive culture within 48 hours of admission to the hospital
• No history of MRSA infection or colonization
• No history in the past year of hospitalization; admission to a skilled nursing facility or hospice; dialysis; or surgery
• No permanent indwelling catheters or medical devices that go through the skin.
Confirmation of an HA-MRSA infection requires at least one of the following criteria:14
• It occurs more than 48 hours after admission to the hospital.
• There is a prior history of MRSA infection or colonization.
• There is a history in the past year of hospitalization; admission to a skilled
nursing facility, nursing home, or hospice; dialysis; or surgery.
• The presence of any indwelling catheter or medical device that passes through the skin into the body.
Rely on evidence-based treatment protocols
In 2011, the Infectious Disease Society of America (IDSA) published consensus guidelines for CA-MRSA to assist with evidencebased decision making. Primary treatment of cutaneous abscesses remains incision and drainage alone without antibiotics, except under certain circumstances (TABLE 2).13 The guidelines recommend antibiotic treatment with empiric coverage for CA-MRSA when purulent cellulitis exists. However, if cellulitis exists without purulence or abscess formation, antibiotic coverage for CA-MRSA is not encouraged for initial treatment.13
Empiric antibiotic coverage for uncomplicated CA-MRSA SSTIs managed in the outpatient setting should include clindamycin, trimethoprim/sulfamethoxazole (TMPSMX), a tetracycline, or linezolid for 5 to 10 days. Factors including cost, patient age and comorbidities, drug allergies, and local resistance patterns should guide the initial antibiotic you choose. TMP-SMX and tetracyclines are relatively inexpensive (<$20), clindamycin is more costly, and linezolid is often costprohibitive as standard treatment.
Do not prescribe tetracyclines for children younger than 8 years of age because of the risk of permanent tooth discoloration. Clindamycin is the only category “B” antibiotic in the group that can be used during pregnancy, and renal impairment needs to be taken into consideration with TMP-SMX and linezolid. For very minor skin lesions in children, mupirocin 2% topical ointment appears to be the therapy of choice.13
Although IDSA has not provided strong recommendations about treatment of asymptomatic close household contacts, this may be considered as another means of attempting to control the spread of MRSA in the community.
Provide patient education. Patient education is paramount to successful treatment and prevention. Explain to patients that people living in close quarters with individuals who already have the bacteria on the skin are far more likely to be carriers. Overcrowding situations that pose a risk include military installations, prisons, long-term care facilities, athletic teams, and daycare centers.
To prevent MRSA, encourage patients to wash their hands often and shower regularly, especially after exercise. Also keep cuts, scrapes, and wounds clean and covered until they heal. Avoid sharing personal items, such as towel and razors.
Finally, remind patients to get care early if they think they may be infected. Symptoms suggestive of MRSA include a red, swollen, painful area on the skin that may look like a spider bite. The area may contain pus and be accompanied by a fever.
Provide close outpatient follow-up within a few days of initiating therapy to document clinical response and aid in further decision-making. Signs of more extensive cellulitis may signal a need for a change of antibiotics or parenteral therapy. Similarly, joint or bone pain underlying areas of cellulitis, significant myalgias or muscle pain out of proportion to exam, or systemic symptoms such as fever, chills, nausea, or lethargy might indicate more extensive infection requiring inpatient treatment.
How to manage recurrent infections
Management of recurrent CA-MRSA SSTIs in the outpatient setting poses a challenge. Following successful treatment of active infection, you may want to attempt decolonization in select patients. Those with repeated MRSA infections despite adequate hygiene measures or with a high probability of reexposure to colonized close contacts may be treated, although the evidence supporting such protocols is lacking.8,13
Acceptable procedures described by IDSA include nasal mupirocin twice daily for 5 to 10 days, mupirocin plus topical antiseptic solution (eg, chlorhexidine, triclosan, or povidone-iodine) for 5 to 14 days, or mupirocin plus dilute bleach baths (1 teaspoon bleach/gallon of water) twice weekly for 15 minutes over 3 months.13 Although antibiotics are generally only recommended for active infection, the combination of rifampin and an antibiotic with MRSA coverage may be used for 1 to 2 weeks in cases of recurrent infections despite recommended hygiene and topical decolonization measures.13,15,16 Rifampin is not recommended as monotherapy for MRSA infection or decolonization.13
Unfortunately, even in cases where eradication is initially successful, about half of those with subsequent negative MRSA cultures will test positive before the end of a year.16 With recurrent or severe infections or immunocompromised patients, it’s advisable to consider an infectious disease consult.
CASE › The patient’s lesion did not require incision as it was already draining. He received a prescription for doxycycline hyclate 100 mg BID for 7 days (since there was evidence of rapidly progressing cellulitis) and was instructed to return to the clinic in 48 hours.
When he returned to the clinic, the patient stated that the pain had improved and the wound was no longer oozing. Culture results confirmed MR SA sensitive to TMP-SMX, doxycycline, and clindamycin. Examination showed improved erythema, a dry wound, and no pain on palpation. He was given a patient information handout on MRSA infection and advised to return to the clinic if the wound did not completely heal within the next 7 days.
› Treat a simple cutaneous abscess from a methicillin-resistant Staphylococcus
aureus (MRSA) infection with incision and drainage alone. A
› Treat minor MRSA skin lesions in children with mupirocin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 21-year-old man seeks care at his family physician (FP)’s office for a painful, draining lesion that’s been on his left thigh for 5 days. He reports that he was bitten by a spider during one of his weekly football games with his fraternity brothers, but doesn’t recall seeing a spider. He has been applying an over-the-counter topical antibiotic without any improvement, and reports that the area of redness has tripled in size within the last 24 hours.
His past medical history is unremarkable except for an allergy to sulfa drugs that was discovered during treatment for a skin infection 2 years ago. He takes no regular medications.
The patient is afebrile and in no distress. The skin overlying his left thigh has a 1 cm oozing lesion with pus evident and surrounding erythema. The wound is warm and tender to the touch.
Based on the patient’s history, the FP suspects methicillin-resistant Staphylococcus aureus (MRSA) and obtains a swab for bacterial culture and sensitivities.
What the surveillance data tell us about MRSA
MRSA infections—a subset of staph infections that are resistant to beta-lactam antibiotics and cephalosporins—continue to be a major source of infection in the community.1 The prevalence of both community-acquired (CA) and hospital acquired (HA) MRSA infections worldwide has continued to grow despite improvements in controlling nosocomial spread.2,3 Data from the early 2000s found an approximate 1% to 2% MRSA colonization rate in the United States, but other countries have had rates as high as 50%.3 Rates across countries have consistently been higher in children and adolescents. A recent surveillance study suggests a decline in the incidence of the most serious types of MRSA infections in major metropolitan areas, the significance of which is still under investigation.4
CA-MRSA and HA-MRSA have different in vitro sensitivities to antimicrobials, different virulence factors, and different epidemiologic profiles.
CA-MRSA occurs in people who have not been recently hospitalized or had any recent medical procedures. These infections usually develop on skin and soft tissue. CA-MRSA typically contains the genes for the Panton-Valentine Leukocidin (PVL) toxin, which is a virulence factor that leads to increased interleukin-8 secretion and skin necrosis.5,6 In addition, CA-MRSA usually does not have genes associated with multidrug-resistant strains.
HA-MRSA occurs in people who have recently been hospitalized, had recent medical procedures, or have been treated in a longterm care setting. HA-MRSA is associated with multidrug-resistant strains; however, it usually does not have the genes for PVL toxin.7
Factors that put patients at risk for CA-MRSA skin infections
As many as 90% of CA-MRSA infections present as skin and soft tissue infections (SSTIs) that have the potential to become invasive if not managed appropriately.3,8 There are a number of factors that put patients at risk for these SSTIs (TABLE 1)7-9—chief among them, intrafamilial or close contact transmission. People living in close quarters with colonized individuals are 14 times more likely to be carriers than a matched unexposed population.9 Similarly, other environments that typically involve close quarters or overcrowding, including military installations, prisons, long-term care facilities, and daycare centers, generally see higher rates of MRSA colonization.10
Researchers also hypothesize that repeated skin trauma is another risk factor for CA-MRSA SSTIs. This may explain the increased rates of CA-MRSA infections seen in athletes and military recruits undergoing basic training, as they are prone to skin abrasions.8 Certain ethnic groups have a higher prevalence of CA-MRSA infections as well, including Native Americans, Pacific Islanders, and African Americans.9,11 It is not entirely clear if there is anything unique predisposing these populations to MRSA or if this might be attributed to living in tight communities with close household contacts.
High-risk groups that have elevated rates of CA-MRSA SSTIs and are more likely to be carriers include intravenous drug users, men who have sex with men, immunocompromised individuals (including those with human immunodeficiency virus), and the homeless.8,9 Several studies have also reported higher rates of MRSA colonization and CA-MRSA infections in individuals who have come into contact with the health care system. A meta-analysis indicated that nasal swabs taken from patients at health care facilities were 2.35 times more likely to be positive for MRSA than those taken from individuals as nonhealth care locations.9 Risk factors such as antibiotic use or one or more physician visits in the past year have been associated with higher rates of infections, as well.2
Diagnosis requires careful history and exam
Community-acquired MRSA SSTIs are often diagnosed based on the patient’s history and risk factors, along with physical exam findings. Common findings include single or multiple erythematous pustules, furuncles, carbuncles, cellulitis, and abscesses. Patients may confuse the initial lesion with an insect or spider bite,12 as occurred with the patient in our opening scenario.
If purulent drainage is present, perform a swab for culture and sensitivity to confirm the diagnosis of MRSA and assist in antibiotic selection.13 If a patient with an SSTI does not respond to beta-lactam antibiotics, consider MRSA until proven otherwise.
Once confirmed by microbiological assessment, follow Centers for Disease Control and Prevention guidelines to differentiate CA-MRSA from HA-MRSA.14 It is important to distinguish between the two, as patients with HA-MRSA are at greater risk for antibiotic failure and progression to more invasive infections.
Confirmation of a CA-MRSA infection requires all of the following criteria:14
• Diagnosis was made in the outpatient setting or by positive culture within 48 hours of admission to the hospital
• No history of MRSA infection or colonization
• No history in the past year of hospitalization; admission to a skilled nursing facility or hospice; dialysis; or surgery
• No permanent indwelling catheters or medical devices that go through the skin.
Confirmation of an HA-MRSA infection requires at least one of the following criteria:14
• It occurs more than 48 hours after admission to the hospital.
• There is a prior history of MRSA infection or colonization.
• There is a history in the past year of hospitalization; admission to a skilled
nursing facility, nursing home, or hospice; dialysis; or surgery.
• The presence of any indwelling catheter or medical device that passes through the skin into the body.
Rely on evidence-based treatment protocols
In 2011, the Infectious Disease Society of America (IDSA) published consensus guidelines for CA-MRSA to assist with evidencebased decision making. Primary treatment of cutaneous abscesses remains incision and drainage alone without antibiotics, except under certain circumstances (TABLE 2).13 The guidelines recommend antibiotic treatment with empiric coverage for CA-MRSA when purulent cellulitis exists. However, if cellulitis exists without purulence or abscess formation, antibiotic coverage for CA-MRSA is not encouraged for initial treatment.13
Empiric antibiotic coverage for uncomplicated CA-MRSA SSTIs managed in the outpatient setting should include clindamycin, trimethoprim/sulfamethoxazole (TMPSMX), a tetracycline, or linezolid for 5 to 10 days. Factors including cost, patient age and comorbidities, drug allergies, and local resistance patterns should guide the initial antibiotic you choose. TMP-SMX and tetracyclines are relatively inexpensive (<$20), clindamycin is more costly, and linezolid is often costprohibitive as standard treatment.
Do not prescribe tetracyclines for children younger than 8 years of age because of the risk of permanent tooth discoloration. Clindamycin is the only category “B” antibiotic in the group that can be used during pregnancy, and renal impairment needs to be taken into consideration with TMP-SMX and linezolid. For very minor skin lesions in children, mupirocin 2% topical ointment appears to be the therapy of choice.13
Although IDSA has not provided strong recommendations about treatment of asymptomatic close household contacts, this may be considered as another means of attempting to control the spread of MRSA in the community.
Provide patient education. Patient education is paramount to successful treatment and prevention. Explain to patients that people living in close quarters with individuals who already have the bacteria on the skin are far more likely to be carriers. Overcrowding situations that pose a risk include military installations, prisons, long-term care facilities, athletic teams, and daycare centers.
To prevent MRSA, encourage patients to wash their hands often and shower regularly, especially after exercise. Also keep cuts, scrapes, and wounds clean and covered until they heal. Avoid sharing personal items, such as towel and razors.
Finally, remind patients to get care early if they think they may be infected. Symptoms suggestive of MRSA include a red, swollen, painful area on the skin that may look like a spider bite. The area may contain pus and be accompanied by a fever.
Provide close outpatient follow-up within a few days of initiating therapy to document clinical response and aid in further decision-making. Signs of more extensive cellulitis may signal a need for a change of antibiotics or parenteral therapy. Similarly, joint or bone pain underlying areas of cellulitis, significant myalgias or muscle pain out of proportion to exam, or systemic symptoms such as fever, chills, nausea, or lethargy might indicate more extensive infection requiring inpatient treatment.
How to manage recurrent infections
Management of recurrent CA-MRSA SSTIs in the outpatient setting poses a challenge. Following successful treatment of active infection, you may want to attempt decolonization in select patients. Those with repeated MRSA infections despite adequate hygiene measures or with a high probability of reexposure to colonized close contacts may be treated, although the evidence supporting such protocols is lacking.8,13
Acceptable procedures described by IDSA include nasal mupirocin twice daily for 5 to 10 days, mupirocin plus topical antiseptic solution (eg, chlorhexidine, triclosan, or povidone-iodine) for 5 to 14 days, or mupirocin plus dilute bleach baths (1 teaspoon bleach/gallon of water) twice weekly for 15 minutes over 3 months.13 Although antibiotics are generally only recommended for active infection, the combination of rifampin and an antibiotic with MRSA coverage may be used for 1 to 2 weeks in cases of recurrent infections despite recommended hygiene and topical decolonization measures.13,15,16 Rifampin is not recommended as monotherapy for MRSA infection or decolonization.13
Unfortunately, even in cases where eradication is initially successful, about half of those with subsequent negative MRSA cultures will test positive before the end of a year.16 With recurrent or severe infections or immunocompromised patients, it’s advisable to consider an infectious disease consult.
CASE › The patient’s lesion did not require incision as it was already draining. He received a prescription for doxycycline hyclate 100 mg BID for 7 days (since there was evidence of rapidly progressing cellulitis) and was instructed to return to the clinic in 48 hours.
When he returned to the clinic, the patient stated that the pain had improved and the wound was no longer oozing. Culture results confirmed MR SA sensitive to TMP-SMX, doxycycline, and clindamycin. Examination showed improved erythema, a dry wound, and no pain on palpation. He was given a patient information handout on MRSA infection and advised to return to the clinic if the wound did not completely heal within the next 7 days.
1. Özel G, Aslan V, Bahar Erdem G, et al. Comparison of oxacillin, cefoxitin, ceftizoxime, and moxalactam disk diffusion methods for detection of methicillin susceptibility in staphylococci. Mikrobiyol Bul. 2011;45:258-265.
2. Crum NF, Lee RU, Thornton SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943-951.
3. Skov R, Christiansen K, Dancer SJ, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents. 2012;39:193-200.
4. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. September 16, 2013. Available at: http://archinte.jamanetwork.com/article.aspx?articleid=1738718. Accessed October 15, 2013.
5. Kawaguchiya M, Urushibara N, Kuwahara O, et al. Molecular characteristics of community-acquired methicillin-resistant Staphylococcus aureus in Hokkaido, northern main island of Japan: identification of sequence types 6 and 59 Panton-Valentine leucocidin-positive community-acquired methicillin-resistant Staphylococcus aureus. Microb Drug Resist. 2011;17:241-250.
6. Wiener-Kronish JP, Pittet JF. Therapies against virulence products of Staphylococcus aureus and Pseudomonas aeruginosa. Semin Respir Crit Care Med. 2011;32:228-235.
7. Hansra NK, Shinkai K. Cutaneous community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus. Dermatol Ther. 2011;24:263-272.
8. Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Am Acad Dermatol. 2007;56:1-16; quiz 17-20.
9. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-139.
10. Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:1-7.
11. Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core Surveillance (ABCs) MRSA Investigators. Invasive methicillinresistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
12. NeVille-Swensen M, Clayton M. Outpatient management of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infection. J Pediatr Health Care. 2011;25:308-315.
13. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
14. Diagnosis and testing for MRSA infections. Centers for Disease Control and Infection Web site. Available at: http://www.cdc.gov/mrsa/diagnosis/index.html. Accessed September 30, 2013.
15. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29:510-516.
16. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44:178-185.
1. Özel G, Aslan V, Bahar Erdem G, et al. Comparison of oxacillin, cefoxitin, ceftizoxime, and moxalactam disk diffusion methods for detection of methicillin susceptibility in staphylococci. Mikrobiyol Bul. 2011;45:258-265.
2. Crum NF, Lee RU, Thornton SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943-951.
3. Skov R, Christiansen K, Dancer SJ, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents. 2012;39:193-200.
4. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. September 16, 2013. Available at: http://archinte.jamanetwork.com/article.aspx?articleid=1738718. Accessed October 15, 2013.
5. Kawaguchiya M, Urushibara N, Kuwahara O, et al. Molecular characteristics of community-acquired methicillin-resistant Staphylococcus aureus in Hokkaido, northern main island of Japan: identification of sequence types 6 and 59 Panton-Valentine leucocidin-positive community-acquired methicillin-resistant Staphylococcus aureus. Microb Drug Resist. 2011;17:241-250.
6. Wiener-Kronish JP, Pittet JF. Therapies against virulence products of Staphylococcus aureus and Pseudomonas aeruginosa. Semin Respir Crit Care Med. 2011;32:228-235.
7. Hansra NK, Shinkai K. Cutaneous community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus. Dermatol Ther. 2011;24:263-272.
8. Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Am Acad Dermatol. 2007;56:1-16; quiz 17-20.
9. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-139.
10. Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:1-7.
11. Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core Surveillance (ABCs) MRSA Investigators. Invasive methicillinresistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
12. NeVille-Swensen M, Clayton M. Outpatient management of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infection. J Pediatr Health Care. 2011;25:308-315.
13. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
14. Diagnosis and testing for MRSA infections. Centers for Disease Control and Infection Web site. Available at: http://www.cdc.gov/mrsa/diagnosis/index.html. Accessed September 30, 2013.
15. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29:510-516.
16. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44:178-185.
Oral contraceptives: Does formulation matter?
• Consider prescribing monophasic pills as the first choice for women starting oral contraceptives (OCs) given the lack of advantage in using multiphasic formulations, and the larger number of studies showing the safety and efficacy of monophasic pills.
• Avoid prescribing OCs with estrogen—even with ultra-low estrogen—to women at high risk for venous thromboembolism, given that there are no studies that show differences in low (25-35 mcg) ethinyl estradiol vs ultra-low doses (10 mcg) formulas.
Strength of recommendation (SOR)
• Good-quality patient-oriented evidence
• Inconsistent or limited-quality patient-oriented evidence
• Consensus, usual practice, opinion, disease-oriented evidence, case series
For a healthy woman interested in contraception, there are multiple oral contraceptive (OC) formulations on the market from which to choose. But are there any significant differences in their effectiveness or safety profiles that make one formulation superior?
Comparative trials of OCs have attempted to answer these questions by evaluating formulations that contain the synthetic components: ethinyl estradiol, norethindrone, levonorgestrel, desogestrel, norgestimate, gestodene, and drospirenone.
Unfortunately, many studies that have evaluated OCs have had methodological weaknesses, making their clinical significance confusing. Few randomized controlled trials (RCTs) have been double blinded or powered to find infrequent outcomes like pregnancy or adverse events. Trials are rarely reproduced by other researchers and many have been funded by pharmaceutical companies with conflicts of interest. Despite these shortcomings, it is possible to glean valuable data from existing studies.
With that in mind, our purpose here is to review whether there are significant differences in effectiveness, cycle control (bleeding), side effects, or satisfaction that may help physicians and patients select the appropriate formulation.
Comparing OC Effectiveness
OC effectiveness is determined by the inherent properties to prevent ovulation, conception, and/or implantation when the formulation is used correctly.1,2 and during typical inconsistent use in the population (ie, adherence).3 Effectiveness is also measured by whether the method is discontinued and there is a gap in contraception allowing pregnancy to occur.
There is no evidence that any combined or progesterone-only hormonal formulation is inherently better at preventing ovulation, conception, or implantation. (For more on combined OCs, see “A closer look at combined OCs,” on page E3.) Theoretically, progestins with longer half-lives may be more effective at preventing ovulation if a pill is not taken the same time each day, and extended cycle pills provide more continuous suppression of ovulation. But, no studies have found any formulation to be more effective.
A 2004 Cochrane review4 compared progestins in OCs by examining 22 different trials with various study protocols. The review found a lower rate of discontinuation in patients taking OCs with second generation progestins compared with first generation progestins (relative risk [RR]=0.79; 95% Extended-cycle OCs have a greater risk of breakthrough bleeding, which can decrease adherence and increase discontinuation, thus increasing the risk of pregnancy. confidence interval [CI] 0.61-0.91), and an even lower rate of discontinuation with third generation OCs. Additionally, cycle control was better in second generation progestin OCs compared with first generation progestin OCs. Rates of effectiveness, cycle control and side effects were similar between drospirenone and desogestrel. The review concluded that second and third generation progestins are preferred over first generation progestins in combined OCs,4 although the evidence is not strong.
What about generics? To be considered an FDA-approved bioequivalent generic to a brand name formulation, pharmacokinetic studies must demonstrate that a product provides equivalent serum levels. There are no studies evaluating differences in effectiveness of generic vs brand name OCs. Generic medications typically cost about 50% less than brand name OCs.5 The Society of Obstetricians and Gynaecologists of Canada supports generic formulations “providing increased choice and less expensive options.”6
What are the differences among the options?
While OCs, in general, have a reputation of causing side effects, when compared with a placebo, no significant findings have been noted in the frequency of headache, nausea, vomiting, breast pain, or weight gain.7,8 That being the case, it is unlikely there are differences among formulations.
Ultra-low estrogen. Estrogen in OCs has been reduced to 10 to 35 mcg to minimize side effects and adverse events, yet remain at a level sufficient to provide menstrual cycle control with minimal breakthrough bleeding. Advantages of ultra-low estrogen 10 mcg products include reduction of estrogen, side effects9 but the disadvantage includes breakthrough bleeding, which can negatively affect adherence.10 In a double-blind RCT of 649 women comparing OCs with gestodene 75 mcg and either 20 mcg or 30 mcg ethinyl estradiol (EE), more intermenstrual breakthrough bleeding occurred with the 20 mcg group (P<0.05). This difference was not enough to cause an increased discontinuation rate in the 20-mcg EE group.11
Progestin-only pills (POPs) are recommended for women who cannot or should not take estrogen in OCs, and women who are breastfeeding. The advantages of POPs include a simplified and fixed regimen. Disadvantages include irregular bleeding and menstrual cycle length. A 2010 Cochrane review examined various POP formulations in 6 different trials and concluded that there is not sufficient research to compare POPs in terms of efficacy, acceptability, and continuation rates.12
Monophasic vs multiphasic OCs. Biphasic and triphasic OCs were introduced in an effort to decrease the amount of hormone and the side effects. Their phasic nature also attempts to mimic the pattern of rising and falling estrogen and progesterone levels seen during a normal menstrual cycle. Cochrane reviews in 200913 and 201014 compared the cycle control and side effects of biphasic vs monophasic, and triphasic vs monophasic formulations of OCs, respectively. The 2009 review comparing biphasic and monophasic OCPs was limited to one study of 533 women using biphasic pills and 481 women using monophasic pills. No differences were found in intermenstrual bleeding, amenorrhea, or discontinuation due to intermenstrual bleeding.
The 2011 review comparing triphasic and monophasic OCs included 21 studies, and found no significant difference in discontinuation due to medical reasons, cycle disturbance, intermenstrual bleeding, or adverse events. Both of the Cochrane reviews concluded that monophasic pills should be the first choice for women starting OCs given the lack of advantage in using multiphasic formulations, and the larger number of studies showing the safety and efficacy of monophasic pills.
The 2009 Cochrane review compared biphasic and triphasic OCPs in terms of cycle control and side effects.13The first trial examined in this review included 458 women and compared 2 biphasic pills and one triphasic pill, all containing LNG and EE. It found no important differences between all 3 formulations, but found that 252 women of the initial 458 (55%) discontinued the study for various reasons.
The second trial included 469 women (169 of whom withdrew from the study or 36%), and compared a biphasic pill containing norethindrone with 2 triphasic pills, one containing LNG and the other containing norethindrone. This study showed no differences between the biphasic and triphasic pills containing norethindrone, but inferior cycle control in the biphasic pill containing norethindrone compared with the triphasic containing LNG. The review concluded that the choice of progestin type (LNG preferred over norethindrone) might be more important than the choice of phasic regimen.13
Monthly vs extended cycle OCs. When OCs were first introduced, researchers believed that women would prefer a 21-day formulation followed by a 7-day pill free time that mimicked an average woman’s menstrual cycle because the withdrawal bleeding would be an indicator that she was not pregnant. Extending the time between menses has garnered increased interest. Extended-cycle preparations are available for durations ranging from 84 to 365 days.15
A study of 99 women evaluated the impact of omitting the first 3 combined OC pills (second and third generation) on ovulation during a 28-day cycle. While none of the women experienced ovulation, follicle-stimulating hormone reached a maximal serum concentration in most women during the first 7 pill-free days, indicating complete pituitary recovery. Additionally, the researchers detected increases in serum estradiol, indicating that follicular growth up to preovulatory size is common in women missing the first one to 3 pills of their contraceptive cycle.16 Non-adherence often occurs during transitions between successive packs of Ocs.17 It has been reported that 47% of women using OCPs miss one pill and 22% miss 2 pills per cycle.18 Ovulation and pregnancy are more likely to occur if pills are missed in the first week after menses.
Extended-cycle OCs prevent hormonal fluctuations and provide continuous suppression of follicle stimulating hormone (FSH) and luteinizing hormone (LH), decreasing the likelihood of ovulation and, therefore, pregnancy. Since the extended-cycle regimen decreases the number of transitions between packs of OCs, one might expect a reduction in the risk associated with non-adherence at the beginning of a cycle. However, extended cycles have a greater risk of breakthrough bleeding, which can decrease adherence and increase discontinuation of the method and, thereby, increase the risk of pregnancy.
A multicenter RCT of 682 women examined the efficacy and safety of the extended-cycle OC Seasonale (30 mcg EE/150 mcg LNG) compared with a traditional cycle OC Nordette-28 (30 mcg EE/150 mcg LNG). Women received either 4, 91-day extended cycles (n=456) or 13, 28-day regular cycle (n=226) regimens over the course of one year. On average, 38% of women in the extended cycle group reported unscheduled (breakthrough) bleeding, while 18% of women in the conventional cycle group reported unscheduled bleeding. Breakthrough bleeding decreased with each successive cycle of the extended regimen, from a median of 12 days with the first cycle, to a median of 4 days during the fourth and final cycle. This study also reported no significant differences in side effects between the extended and traditional cycle regimens, including changes in lipids, body weight, blood pressure, or endometrial hyperplasia.19
Another RCT examined the difference in bleeding patterns, side effects, and acceptability between a standard 28-day cycle OC and an extended regimen 168-day cycle OC in 32 women. Both OCs contained 20 mcg EE and 100 mcg LNG, and the study was conducted over 6 months. Women in the extended cycle regimen reported significantly fewer days of bloating (0.7 vs 11.1 days; P=0.04), and menstrual pain (1.9 vs 13.3 days; P<0.01). There was no significant difference in reported headache, breast tenderness, nausea, depression, or premenstrual symptoms. Women in the extended cycle group also reported significantly fewer bleeding days that required sanitary pads (18.4 vs 33.8 days; P<0.01). However, there was no statistically significant difference in the total number of days where any degree of bleeding occurred (34.9 days in the monthly cycle group, 25.9 days in the extended cycle group; P=0.33).20
In a study of 126-day extended-cycle OCs with 30 mcg EE and 3 mg drospirenone, the bleeding profile improved over time and endometrial biopsies revealed no hyperplasia.21 Another benefit of the extended cycle is personal preference, ie, controlling the timing of one’s menses,22 for example, in athletes during training and competition.
Continuous use of OCs prevents the cyclic fluctuations of serum levels of EE and progestogen and, hence, the cyclic variations of related serum-based metabolic parameters. Extended cycle OCs can make it easier to titrate other medications affected by hormonal fluctuations. Another study of extended cycle drospirenone OCs compared with monthly OCs over 6 months showed no difference in lipid, carbohydrate, and coagulation markers.23
Six RCTs were reviewed in a Cochrane review of monthly vs extended cycle combined pills. It found no significant differences in efficacy, adherence, discontinuation rates, and patient satisfaction. The only difference was improvement of menstrual-associated symptoms of “headaches, genital irritation, tiredness, bloating and menstrual pain” with the extended cycle regimen.24
OCs effect on weight, BP, and premenstrual symptoms
Weight gain. A 2008 Cochrane review examined 3 placebo-controlled RCTs and concluded that the available evidence was insufficient to determine the effect of combined hormonal contraceptives on weight, and that larger doses of estrogen were not shown to cause larger weight gain.25
One RCT examined the effects of OCs on variations of total body water, fat mass, and fat-free mass throughout the menstrual cycle to determine if different doses of estrogen (15 mcg vs 30 mcg EE) or different types of progestins (gestodene 60 mcg vs drospirenone 3 mg) impact weight gain. This study only included 80 women randomized to the 2 treatment groups and an additional control group using male condoms. No differences were found in total body water or fat mass. There was, however, a significant increase in the fat-free mass in women of the EE/gestodene group when compared to controls, indicating a possible effect of the androgenic properties of gestodene compared with drospirenone (which has anti-androgen properties) in increasing muscle mass.26
In a 6 month study of drospirenone compared with LNG, mean body weight fell by 0.8 to 1.7 kg in women treated with drospirenone compared with a 0.7 kg weight gain in the LNG group (P < 0.05).27 A multicenter RCT comparing OCs with EE 30 mcg/drospirenone 3 mg, and EE 30 mcg/desogestrel 150 mcg, concluded that EE/drospirenone has a more favorable effect on body weight than EE/desogestrel. This finding may have resulted from the antimineralocorticoid, mild diuretic effects of drospirenone.28
Hypertension. In a review of progestin-only OCs in normotensive women, the authors could find no evidence to show a statistically significant increase in blood pressure.29
In a study of 120 women randomized to drospirenone/EE or LNG/EE, the drospirenone group had a mean decrease in systolic blood pressure from 107 to 103 mm Hg, and a significantly lower group mean blood pressure compared with the LNG group.30 Another study of 80 women over 6 months randomized into 3groups each having 3 mg of drospirenone with either a 30-, 20-, or 15-mcg dose of EE found that systolic blood pressure decreased by 1 to 4 mm Hg compared with an elevation of blood pressure of 4 mm Hg in the LNG/EE group.27
In women with well-controlled blood pressure who were less than 35 years old, non-smokers and otherwise healthy, the American College of Obstetricians and Gynecologists (ACOG) recommends31 a trial of OCs with monitoring of their blood pressure.
Acne. One Cochrane review looked at studies that compared combined OCs to placebo, and found OCs improved the condition. However, there was insufficient evidence regarding the difference in effectiveness of various formulations of OCs in treating the disease.32 There was no difference between first and second generation progestins,33 between second and third generations,34 or third generation vs drospirenone.35
Premenstrual symptoms. A 2005 open-label RCT compared the effects of DRSP/20 mcg EE with the second-generation progestin LNG/30 mcg EE on premenstrual symptoms after 6 menstrual cycles. In the premenstrual phase, the DRSP/EE group showed less negative mood and weight gain.36
A 2012 Cochrane review examined the effects of OCs containing DRSP on premenstrual dysphoric disorder (PMDD) vs placebo and other OC formulations. The review included 5 trials and found that DRSP is associated with significantly greater improvements than placebo in symptoms of PMDD but was inconclusive on whether DRSP formulations have greater effects on PMDD than other OC formulations.37
Dysmenorrhea. A 2009 Cochrane review compared 10 studies examining the role of different formulations of combined OCs in management of dysmenorrhea and concluded there is no difference in improvement between different OC preparations.38
OCs and coronary heart disease
Estrogen has several favorable effects on circulating lipoproteins, including increasing high-density lipoprotein (HDL), and increasing low-density lipoprotein (LDL) receptor activity, thereby enhancing removal of LDL.
Women using a 20 mcg EE/100-mcg LNG OCP experienced reductions in HDL and small increases in LDL and triglycerides compared with a 30 mcg EE/150-mcg LNG OCP.39 A study of gestodene 75 mcg with either EE 20 mcg or 30 mcg for 13 cycles, found that there was a greater increase in triglyceride levels in the formulation with a higher dose of estrogen (p = 0.029).40
Barkfeldt and colleagues41 conducted a double-blind RCT that evaluated the effects of lipid metabolism on 98 women who received 2 different types of progestin-only pills, desogestrel 75 mcg/day vs LNG 30 mcg/day. There were minimal changes in the lipid profile except for decreasing trends in levels of HDL, its subfractions, and apolipoprotein-I and -II. No differences were observed between the 2 formulations despite the higher progestin dose found in desogestrel, including no changes in LDL or apolipoprotein-B.41
Third generation progestins with “lesser androgenicity” may allow more “expression” of the effects of estrogen on lipids. A prospective study of 66 women over 9 months comparing either desogestrel (50/100/150 mcg) and EE (35/30/30 mcg), or LNG (50/100/150 mcg) and EE (30/40/30 mcg), showed that the desogestrel formulation increased HDL whereas LNG decreased HDL.42 Another study compared monophasic desogestrel/EE with triphasic LNG/EE in 37 healthy young women. While both preparations led to an increase in total cholesterol, the desogestrel formulation led to a reduction in the LDL.43 A 1995 study of drospirenone (DRSP) compared with LNG for 6 months, showed that HDL increased in the DRSP group (P < 0.05) but triglyceride levels showed a greater increase in the DRSP (P <0.05).27
The use of OCs in the absence of risk factors does not appear to promote CAD and there is no reason to withhold OCs from dyslipidemic women. In women with LDL greater than 160 mg/dL or multiple cardiac risk factors, ACOG recommends an alternative non-hormonal method such as an intrauterine device (IUD).31
OCs and glucose metabolism, thromboembolism
Glucose metabolism. Oelkers and colleagues27 studied glucose levels in 80 healthy women assigned to 4 equal groups who received 3 mg of drospirenone combined with 30-, 20-, and 15-mcg doses of EE or LNG/30-mcg EE. Each woman performed oral glucose tolerance tests at pre-treatment and at the end of the 6-month OCP cycle. On treatment, fasting glucose was unchanged for all groups, but the area under the curve for the glucose tolerance increased for all formulations. Although not statistically significant between groups, the drospirenone/30-mcg EE group had a 19% worsening of glucose tolerance.27 This research suggests that women with Type 1 or 2 diabetes who are otherwise healthy, non-smokers and younger than 35 years of age can safely use OCs.
Thromboembolism. Estrogen has been known to increase the risk of venous thromboembolism (VTE) by increasing prothrombin and decreasing antithrombin III.44 In OC users, the incidence of VTE is increased by a factor of 3 to 5.45 While several studies have compared high-dose estrogen (50 mcg) with low-dose (35 mcg or less) OCs46,47 there is no information about any differences in low (25-35 mcg) EE vs ultra-low doses (10 mcg). ACOG recommends a nonestrogen hormonal alternative such as progestin-only pills or an IUD, for obese women.
Third generation desogestrel-containing OCs have a slightly increased risk of VTE compared with second generation pills48unexplained by bias and confounding factors.49,50 It has been estimated that 25 additional cases of VTE occur every year among 100,000 women using thirrd generation OCs compared with 10 additional cases per 100,000 women using second generation OCs.51 A meta-analysis that included 9 case control and 3 cohort studies estimated an odds ratio for third vs second generation OCs of 1.7 (95% CI, 1.4 to 2.0).52 A 2010 meta-analysis refutes these finding and found no differences in third generation gestodene progestin vs other OCs.53 Because obesity (BMI >30 kg/m2) is an independent risk factor for VTE, ACOG recommends an alternative non-estrogen hormonal method such as progestin-only pills or IUD in obese women.31
Bone mineral density (BMD). A 2000 study compared 2 OCs with the same dose of progestin (gestodene 75 mcg) and 2 doses of EE (20 vs 30 mcg) to determine if there was a correlation between dose of estrogen and loss of BMD in young post-adolescent women taking OCs. It concluded that pills with 20 and 30 mcg of estrogen were associated with the same reduction in BMD.54
However a 2009 Cochrane review concluded that combined OCs do not affect bone health, ie, fracture rate, BMD, or biochemical markers of bone change. Thirteen RCTs were reviewed and researchers concluded that the relationship between OC use and fracture risk cannot be determined from the limited data currently available.55
Cancer. Research does not support the notion that OCs contribute to cancer. In fact, reduced endometrial and ovarian cancers have been shown among users of OCs containing 50 mcg EE.56-58 Low-dose formulations (≤35 mg EE) have been less studied but also confer a substantial risk reduction.59
Data are conflicting regarding a slight increase in risk for breast cancer in current or recent users of OC from older, higher-estrogen doses; that risk returns to normal over time.60 The World Health Organization recognizes this slight risk, but has concluded that the benefits of OCs outweigh the risks. 61
Evidence-based guidelines are lacking
There is a paucity of RCTs with sufficient duration and sample size that compare different OC formulations to provide evidence-based guidance for physicians. While some pharmaceutical companies market their product for particular benefits, these finding too often come from non-comparative trials, ie, their product vs placebo.
So here’s what we know...
No OC formulation is more effective at preventing pregnancy than any others. Cycle control, ie, less intermenstrual bleeding, is improved with 30 to 35 mcg EE formulations compared with ultra-low dose (20 mcg) EE. There are no advantages to choosing a multiphasic formulation over a monophasic OC. While extended-cycle formulations have more breakthrough bleeding than monthly cycles, overall they have fewer days of menstrual bleeding, which tend to decrease even further in successive cycles. Extended-cycle formulations have decreased days of bloating and menstrual cramping.
There is no evidence that different doses of estrogen or progestin affect weight gain or total body water. DRSP leads to a more favorable lean body mass profile than LNG and desogestrel, which may be related to its anti-mineralocorticoid effect. While both second and third generation progestin formulations have been shown to improve acne, there is no evidence to indicate a preference.
There is also little evidence to recommend a particular OC to avoid adverse events such as CAD or VTE; in fact the evidence is often contradictory. Epidemiologic studies confirm that venous thromboembolic disease is similar for 20 and 30 mcg EE. There may be an increase in VTE with desogestrel, but recent evidence finds no significant increase. The clinical significance that DRSP increases triglyceride levels while it decreases LDL and HDL, and the significance of LDL reduction by desogestrel requires further investigation.
There is no evidence that OCs affect bone health indices such as fracture rate, BMD, or biochemical markers of bone change. OC formulations with While extended-cycle formulations have more breakthrough bleeding than monthly cycles, they have overall fewer days of menstrual bleeding.higher doses of estrogen have been shown to reduce ovarian and endometrial cancer, presumably due to fewer ovulatory cycles. However, similar reductions should therefore be observed with lower EE dose formulations as well.
Clearly, the literature indicates that there is little evidence to recommend one OC formulation over another. All currently marketed OCs have low dose EE. However, when counseling patients, keep in mind that extended cycle formulations decrease some side effects and generic formulations reduce costs.
CORRESPONDENCE
Eric A. Schaff, MD, Philadelphia Women’s Center, 777 Appletree Street, #7, Philadelphia, PA 19106; [email protected]
1. Chandra A, Martinez GM, Mosher WD, et al. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;25:1-160.
2. Keam SJ, Wagstaff AJ. Ethinyl estradiol/drospirenone: a review of its use as an oral contraceptive. Treatments Endocrinol. 2003;2:49-70.
3. Krattenmacher R. Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception. 2000;62:29-38.
4. Maitra NN, Kulier R, Bloemenkamp K, et al. Progestogens in combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2004, Issue 3.
5. Oral Contraceptives at Drugstore.com. http://www.drugstore.com/pharmacy/drugindex/rxsearch.asp?search=oral+contraceptive [accessed January 24, 2011]
6. Society of Obstetricians and Gynaecologists of Canada Statement on Generic Contraceptions. J Obstet Gynecol Canada. 2008;30:271-272.
7. Coney P, Washenik K, Langley RG, et al. Weight change and adverse event incidence with a low-dose oral contraceptive: two randomized, placebo-controlled trials. Contraception. 2001;63:297-302.
8. O’Connell K, Davis AR, Kerns J. Oral contraceptives: side effects and depression in adolescent girls. Contraception. 2007;75:299-304.
9. Redmond GP, Olson WH, Lippman JS, et al. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo-controlled trial. Obstet Gynecol. 1997;89:615-622.
10. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355-360.
11. Endrikat J, Muller U, Dusterberg B. A twelve-month comparative clinical investigation of two low-dose oral contraceptives containing 20 micrograms ethinylestradiol/75 micrograms gestodene and 30 micrograms ethinylestradiol/75 micrograms gestodene, with respect to efficacy, cycle control, and tolerance. Contraception. 1997;55:131-137.
12. Grimes DA, Lopez LM, O’Brien PA, et al. Progestin-only pills for contraception. Cochrane Database of Systematic Reviews 2010, Issue 1.
13. Van Vliet HAAM, Grimes DA, Helmerhorst FM, et al. Biphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2009, Issue 2.
14. Grimes DA, Lopez LM, Schulz KF et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2009, Issue 2.
15. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
16. Elomaa K, Rolland R, Brosens I, et al. Omitting the first oral contraceptive pills of the cycle does not automatically lead to ovulation. Am J Obstet Gynecol. 1998;179:41-46.
17. Adams Hillard PJ. Oral contraception noncompliance: The extent of the problem. Adv Contracept. 1992;8(suppl 1):13-20.
18. Rosenberg M, Waugh MS. Causes and consequences of oral contraceptive noncompliance. Am J Obstet Gynecol. 1999;180:S276-279.
19. Anderson, FD, Hait, H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
20. Kwiecien M, Edelman A, Nichols MD, et al. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67:9-13.
21. Foidard JM, Sulak PJ, Schellschmidt I, et al. The Yasmin Extended Regimen Study Group. The use of an oral contraceptive containing ethinylestradiol and drospirenone in an extended regimen over 126 days. Contraception. 2006;73:34-40.
22. Shakespeare J, Neve E, Hodder E. Is norethisterone a lifestyle drug? Results of database analysis. BMJ. 2000;320:291.
23. Machado RB, de Melo NR, Maia Jr. H, et al. Effect of a continuous regimen of contraceptive combination of ethinylestradiol and drospirenone on lipid, carbohydrate and coagulation profiles. Contraception. 2010;81:102-106.
24. Edelman A, Gallo MF, Nichols, MD, et al. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Human Reprod. 2006;21:573-578.
25. Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008, Issue 4.
26. Machado RB, Tachotti F, Cavenague G, et al. Effects of two different oral contraceptives on total body water: a randomized study. Contraception. 2006;73:344-347.
27. Oelkers W, Foidart JM, Dombrovicz N, et al. Effects of a new oral contraceptive containing an anti-mineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J Clinical Endo Metabolism. 1995;80:1816-1821.
28. Foidart JM, Wuttke W, Bouw GM, et al. A comparative investigation of contraceptive reliability, cycle control, and tolerance of two monophasic oral contraceptives containing either drospirenone or desogestrel. Eur J Contracept Reprod Health Care. 2000;5:124-134.
29. Hussain, SF. Progestogen-only pills and high blood pressure: is there an association? A literature review. Contraception. 2004;69:89-97.
30. Suthipongse W, Taneepanichskul S. An open-label randomized comparative study of oral contraceptives between medications containing 3 mg drospirenone/30 mcg ethinylestradiol and 150 mcg levonorgestrel/30 mcg ethinylestradiol in Thai women. Contraception. 2004;69:23-26.
31. ACOG practice bulletin. No. 73. Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
32. Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2009, Issue 3.
33. Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262.
34. Rosen, MP, Breitkopf, DM, Nagamani, M. A randomized controlled trial of second- versus third-generation oral contraceptives in the treatment of acne vulgaris. Am J Obstet Gynecol. 2003;188:1158-1160.
35. Huber J, Foidart JM, Wuttke W, et al. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. Eur J Contracept Reprod Health Care. 2000;5:25-34.
36. Sangthawan, M, Taneepanichskul, S. A comparative study of monophasic oral contraceptives containing either drospirenone 3 mg or levonorgestrel 150 mcg on premenstrual symptoms. Contraception. 2005;71:1-77.
37. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database of Systematic Reviews. 2009, Issue 2.
38. Wong CL, Farquhar C, Roberts H, et al. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev. 2009, Issue 4.
39. Endrikat J, Klipping C, Cronin M, et al. An open label, comparative study of the effects of a dose-reduced oral contraceptive containing 20 mcg ethinyl estradiol and 100 mcg levonorgestrel on hemostatic, lipids, and carbohydrate metabolism variables. Contraception. 2002;65:215-221.
40. Brill K, Then A, Beisiegel U, et al. Investigation of the influence of two low-dose monophasic oral contraceptives containing 20 micrograms ethinylestradiol/75 micrograms gestodene and 30 micrograms ethinylestradiol/75 micrograms gestodene, on lipid metabolism in an open randomized trial. Contraception. 1996;54:291-297.
41. Barkfeldt J, Virkkunen A, Dieben T. The effects of two progestogen-only pills containing either desogestrel (75 mcg/day) or levonorgestrel (30 mcg/day) on lipid metabolism. Contraception. 2001; 64:295-299.
42. Knopp RH, Broyles FE, Cheung M, et al. Comparison of the lipoprotein, carbohydrate, and hemostatic effects of phasic oral contraceptives containing desogestrel or levonorgestrel. Contraception. 2001;63:1-11.
43. Foulon T, Payen N, Laporte F, et al., Effects of two low-dose oral contraceptives containing ethinylestradiol and either desogestrel of levonorgestrel on serum lipids and lipoproteins with particular regards to LDL size. Contraception. 2001;64:11-16.
44. Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system: lessons learned and unanswered questions. J Am Coll Cardiol. 2006;47:1741-1753.
45. Martinelli I. Risk factors in venous thromboembolism. Thromb Haemost. 2001;86:395-403.
46. Vessey M, Mant D, Smith A, et al. Oral contraceptives and venous thromboembolism: findings in a large prospective study. BMJ. 1986;292:526.
47. Gerstman BB, Piper TM, Tomita DK, et al. Oral contraceptive estrogen dose and the risk of venous thromboembolic disease. Am J Epidemiol. 1991;133:32-137.
48. Rosendaal FR, Van Hylckama Vlieg A, et al. Estrogens, progestogens and thrombosis. J Thromb Haemost. 2003;1:1371-1380.
49. Farley TM, Meirik O, Collins J. Cardiovascular disease and combined oral contraceptives: reviewing the evidence and balancing risks. Hum Reprod Update. 1999;5:721-735.
50. Vandenbroucke JP, Helmerhorst FM, Bloemenkamp KW, et al. Third generation oral contraceptive and deep venous thrombosis: from epidemiologic controversy to new insight in coagulation. Am J Obstet Gynecol. 1997;177:887-891.
51. Hannaford P. Health consequences of oral combined oral contraceptives. Br Med Bull. 2000;56:749-760.
52. Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. BMJ. 2001;323:131-134.
53. Heinemann LA, Dinger JC, Assmann A, et al. Use of oral contraceptives containing gestodene and risk of venous thromboembolism: outlook 10 years after the third-generation “pill scare”. Contraception. 2010;81:401-407.
54. Paoletti AM, Orru M, Floris S, et al. Evidence that treatment with monophasic oral contraceptive formulations containing ethinylestradiol plus gestodene reduces bone reabsorption in young women. Contraception. 2000;61:259-263.
55. Lopez LM, Grimes DA, Schulz KF, et al. Steroidal contraceptives: effect on bone fractures in women. Cochrane Database Syst Rev. 2009, Issue 2.
56. Schlesselman JJ. Oral contraceptives and neoplasia of the uterine corpus. Contraception. 1991;43:557-580.
57. Hankinson SE, Colditz GA, Hunter DJ, et al. A quantitative assessment of oral contraceptive use and risk of ovarian cancer. Obstet Gynecol. 1992;80:708-714.
58. LaVecchia C, Franceschi S, Decarli A. Oral contraceptive use and the risk of epithelial ovarian cancer. Br J Cancer. 1984;50:31-34.
59. Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95:370-374.
60. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 347:1713-27, 1996
61. World Health Organization. Carcinogenicity of combined hormonal contraceptives and combined menopausal treatment. September 2005. http://www.who.int/reproductivehealth/topics/ageing/cocs_hrt_statement.pdf. Accessed Aug 28, 2013.
• Consider prescribing monophasic pills as the first choice for women starting oral contraceptives (OCs) given the lack of advantage in using multiphasic formulations, and the larger number of studies showing the safety and efficacy of monophasic pills.
• Avoid prescribing OCs with estrogen—even with ultra-low estrogen—to women at high risk for venous thromboembolism, given that there are no studies that show differences in low (25-35 mcg) ethinyl estradiol vs ultra-low doses (10 mcg) formulas.
Strength of recommendation (SOR)
• Good-quality patient-oriented evidence
• Inconsistent or limited-quality patient-oriented evidence
• Consensus, usual practice, opinion, disease-oriented evidence, case series
For a healthy woman interested in contraception, there are multiple oral contraceptive (OC) formulations on the market from which to choose. But are there any significant differences in their effectiveness or safety profiles that make one formulation superior?
Comparative trials of OCs have attempted to answer these questions by evaluating formulations that contain the synthetic components: ethinyl estradiol, norethindrone, levonorgestrel, desogestrel, norgestimate, gestodene, and drospirenone.
Unfortunately, many studies that have evaluated OCs have had methodological weaknesses, making their clinical significance confusing. Few randomized controlled trials (RCTs) have been double blinded or powered to find infrequent outcomes like pregnancy or adverse events. Trials are rarely reproduced by other researchers and many have been funded by pharmaceutical companies with conflicts of interest. Despite these shortcomings, it is possible to glean valuable data from existing studies.
With that in mind, our purpose here is to review whether there are significant differences in effectiveness, cycle control (bleeding), side effects, or satisfaction that may help physicians and patients select the appropriate formulation.
Comparing OC Effectiveness
OC effectiveness is determined by the inherent properties to prevent ovulation, conception, and/or implantation when the formulation is used correctly.1,2 and during typical inconsistent use in the population (ie, adherence).3 Effectiveness is also measured by whether the method is discontinued and there is a gap in contraception allowing pregnancy to occur.
There is no evidence that any combined or progesterone-only hormonal formulation is inherently better at preventing ovulation, conception, or implantation. (For more on combined OCs, see “A closer look at combined OCs,” on page E3.) Theoretically, progestins with longer half-lives may be more effective at preventing ovulation if a pill is not taken the same time each day, and extended cycle pills provide more continuous suppression of ovulation. But, no studies have found any formulation to be more effective.
A 2004 Cochrane review4 compared progestins in OCs by examining 22 different trials with various study protocols. The review found a lower rate of discontinuation in patients taking OCs with second generation progestins compared with first generation progestins (relative risk [RR]=0.79; 95% Extended-cycle OCs have a greater risk of breakthrough bleeding, which can decrease adherence and increase discontinuation, thus increasing the risk of pregnancy. confidence interval [CI] 0.61-0.91), and an even lower rate of discontinuation with third generation OCs. Additionally, cycle control was better in second generation progestin OCs compared with first generation progestin OCs. Rates of effectiveness, cycle control and side effects were similar between drospirenone and desogestrel. The review concluded that second and third generation progestins are preferred over first generation progestins in combined OCs,4 although the evidence is not strong.
What about generics? To be considered an FDA-approved bioequivalent generic to a brand name formulation, pharmacokinetic studies must demonstrate that a product provides equivalent serum levels. There are no studies evaluating differences in effectiveness of generic vs brand name OCs. Generic medications typically cost about 50% less than brand name OCs.5 The Society of Obstetricians and Gynaecologists of Canada supports generic formulations “providing increased choice and less expensive options.”6
What are the differences among the options?
While OCs, in general, have a reputation of causing side effects, when compared with a placebo, no significant findings have been noted in the frequency of headache, nausea, vomiting, breast pain, or weight gain.7,8 That being the case, it is unlikely there are differences among formulations.
Ultra-low estrogen. Estrogen in OCs has been reduced to 10 to 35 mcg to minimize side effects and adverse events, yet remain at a level sufficient to provide menstrual cycle control with minimal breakthrough bleeding. Advantages of ultra-low estrogen 10 mcg products include reduction of estrogen, side effects9 but the disadvantage includes breakthrough bleeding, which can negatively affect adherence.10 In a double-blind RCT of 649 women comparing OCs with gestodene 75 mcg and either 20 mcg or 30 mcg ethinyl estradiol (EE), more intermenstrual breakthrough bleeding occurred with the 20 mcg group (P<0.05). This difference was not enough to cause an increased discontinuation rate in the 20-mcg EE group.11
Progestin-only pills (POPs) are recommended for women who cannot or should not take estrogen in OCs, and women who are breastfeeding. The advantages of POPs include a simplified and fixed regimen. Disadvantages include irregular bleeding and menstrual cycle length. A 2010 Cochrane review examined various POP formulations in 6 different trials and concluded that there is not sufficient research to compare POPs in terms of efficacy, acceptability, and continuation rates.12
Monophasic vs multiphasic OCs. Biphasic and triphasic OCs were introduced in an effort to decrease the amount of hormone and the side effects. Their phasic nature also attempts to mimic the pattern of rising and falling estrogen and progesterone levels seen during a normal menstrual cycle. Cochrane reviews in 200913 and 201014 compared the cycle control and side effects of biphasic vs monophasic, and triphasic vs monophasic formulations of OCs, respectively. The 2009 review comparing biphasic and monophasic OCPs was limited to one study of 533 women using biphasic pills and 481 women using monophasic pills. No differences were found in intermenstrual bleeding, amenorrhea, or discontinuation due to intermenstrual bleeding.
The 2011 review comparing triphasic and monophasic OCs included 21 studies, and found no significant difference in discontinuation due to medical reasons, cycle disturbance, intermenstrual bleeding, or adverse events. Both of the Cochrane reviews concluded that monophasic pills should be the first choice for women starting OCs given the lack of advantage in using multiphasic formulations, and the larger number of studies showing the safety and efficacy of monophasic pills.
The 2009 Cochrane review compared biphasic and triphasic OCPs in terms of cycle control and side effects.13The first trial examined in this review included 458 women and compared 2 biphasic pills and one triphasic pill, all containing LNG and EE. It found no important differences between all 3 formulations, but found that 252 women of the initial 458 (55%) discontinued the study for various reasons.
The second trial included 469 women (169 of whom withdrew from the study or 36%), and compared a biphasic pill containing norethindrone with 2 triphasic pills, one containing LNG and the other containing norethindrone. This study showed no differences between the biphasic and triphasic pills containing norethindrone, but inferior cycle control in the biphasic pill containing norethindrone compared with the triphasic containing LNG. The review concluded that the choice of progestin type (LNG preferred over norethindrone) might be more important than the choice of phasic regimen.13
Monthly vs extended cycle OCs. When OCs were first introduced, researchers believed that women would prefer a 21-day formulation followed by a 7-day pill free time that mimicked an average woman’s menstrual cycle because the withdrawal bleeding would be an indicator that she was not pregnant. Extending the time between menses has garnered increased interest. Extended-cycle preparations are available for durations ranging from 84 to 365 days.15
A study of 99 women evaluated the impact of omitting the first 3 combined OC pills (second and third generation) on ovulation during a 28-day cycle. While none of the women experienced ovulation, follicle-stimulating hormone reached a maximal serum concentration in most women during the first 7 pill-free days, indicating complete pituitary recovery. Additionally, the researchers detected increases in serum estradiol, indicating that follicular growth up to preovulatory size is common in women missing the first one to 3 pills of their contraceptive cycle.16 Non-adherence often occurs during transitions between successive packs of Ocs.17 It has been reported that 47% of women using OCPs miss one pill and 22% miss 2 pills per cycle.18 Ovulation and pregnancy are more likely to occur if pills are missed in the first week after menses.
Extended-cycle OCs prevent hormonal fluctuations and provide continuous suppression of follicle stimulating hormone (FSH) and luteinizing hormone (LH), decreasing the likelihood of ovulation and, therefore, pregnancy. Since the extended-cycle regimen decreases the number of transitions between packs of OCs, one might expect a reduction in the risk associated with non-adherence at the beginning of a cycle. However, extended cycles have a greater risk of breakthrough bleeding, which can decrease adherence and increase discontinuation of the method and, thereby, increase the risk of pregnancy.
A multicenter RCT of 682 women examined the efficacy and safety of the extended-cycle OC Seasonale (30 mcg EE/150 mcg LNG) compared with a traditional cycle OC Nordette-28 (30 mcg EE/150 mcg LNG). Women received either 4, 91-day extended cycles (n=456) or 13, 28-day regular cycle (n=226) regimens over the course of one year. On average, 38% of women in the extended cycle group reported unscheduled (breakthrough) bleeding, while 18% of women in the conventional cycle group reported unscheduled bleeding. Breakthrough bleeding decreased with each successive cycle of the extended regimen, from a median of 12 days with the first cycle, to a median of 4 days during the fourth and final cycle. This study also reported no significant differences in side effects between the extended and traditional cycle regimens, including changes in lipids, body weight, blood pressure, or endometrial hyperplasia.19
Another RCT examined the difference in bleeding patterns, side effects, and acceptability between a standard 28-day cycle OC and an extended regimen 168-day cycle OC in 32 women. Both OCs contained 20 mcg EE and 100 mcg LNG, and the study was conducted over 6 months. Women in the extended cycle regimen reported significantly fewer days of bloating (0.7 vs 11.1 days; P=0.04), and menstrual pain (1.9 vs 13.3 days; P<0.01). There was no significant difference in reported headache, breast tenderness, nausea, depression, or premenstrual symptoms. Women in the extended cycle group also reported significantly fewer bleeding days that required sanitary pads (18.4 vs 33.8 days; P<0.01). However, there was no statistically significant difference in the total number of days where any degree of bleeding occurred (34.9 days in the monthly cycle group, 25.9 days in the extended cycle group; P=0.33).20
In a study of 126-day extended-cycle OCs with 30 mcg EE and 3 mg drospirenone, the bleeding profile improved over time and endometrial biopsies revealed no hyperplasia.21 Another benefit of the extended cycle is personal preference, ie, controlling the timing of one’s menses,22 for example, in athletes during training and competition.
Continuous use of OCs prevents the cyclic fluctuations of serum levels of EE and progestogen and, hence, the cyclic variations of related serum-based metabolic parameters. Extended cycle OCs can make it easier to titrate other medications affected by hormonal fluctuations. Another study of extended cycle drospirenone OCs compared with monthly OCs over 6 months showed no difference in lipid, carbohydrate, and coagulation markers.23
Six RCTs were reviewed in a Cochrane review of monthly vs extended cycle combined pills. It found no significant differences in efficacy, adherence, discontinuation rates, and patient satisfaction. The only difference was improvement of menstrual-associated symptoms of “headaches, genital irritation, tiredness, bloating and menstrual pain” with the extended cycle regimen.24
OCs effect on weight, BP, and premenstrual symptoms
Weight gain. A 2008 Cochrane review examined 3 placebo-controlled RCTs and concluded that the available evidence was insufficient to determine the effect of combined hormonal contraceptives on weight, and that larger doses of estrogen were not shown to cause larger weight gain.25
One RCT examined the effects of OCs on variations of total body water, fat mass, and fat-free mass throughout the menstrual cycle to determine if different doses of estrogen (15 mcg vs 30 mcg EE) or different types of progestins (gestodene 60 mcg vs drospirenone 3 mg) impact weight gain. This study only included 80 women randomized to the 2 treatment groups and an additional control group using male condoms. No differences were found in total body water or fat mass. There was, however, a significant increase in the fat-free mass in women of the EE/gestodene group when compared to controls, indicating a possible effect of the androgenic properties of gestodene compared with drospirenone (which has anti-androgen properties) in increasing muscle mass.26
In a 6 month study of drospirenone compared with LNG, mean body weight fell by 0.8 to 1.7 kg in women treated with drospirenone compared with a 0.7 kg weight gain in the LNG group (P < 0.05).27 A multicenter RCT comparing OCs with EE 30 mcg/drospirenone 3 mg, and EE 30 mcg/desogestrel 150 mcg, concluded that EE/drospirenone has a more favorable effect on body weight than EE/desogestrel. This finding may have resulted from the antimineralocorticoid, mild diuretic effects of drospirenone.28
Hypertension. In a review of progestin-only OCs in normotensive women, the authors could find no evidence to show a statistically significant increase in blood pressure.29
In a study of 120 women randomized to drospirenone/EE or LNG/EE, the drospirenone group had a mean decrease in systolic blood pressure from 107 to 103 mm Hg, and a significantly lower group mean blood pressure compared with the LNG group.30 Another study of 80 women over 6 months randomized into 3groups each having 3 mg of drospirenone with either a 30-, 20-, or 15-mcg dose of EE found that systolic blood pressure decreased by 1 to 4 mm Hg compared with an elevation of blood pressure of 4 mm Hg in the LNG/EE group.27
In women with well-controlled blood pressure who were less than 35 years old, non-smokers and otherwise healthy, the American College of Obstetricians and Gynecologists (ACOG) recommends31 a trial of OCs with monitoring of their blood pressure.
Acne. One Cochrane review looked at studies that compared combined OCs to placebo, and found OCs improved the condition. However, there was insufficient evidence regarding the difference in effectiveness of various formulations of OCs in treating the disease.32 There was no difference between first and second generation progestins,33 between second and third generations,34 or third generation vs drospirenone.35
Premenstrual symptoms. A 2005 open-label RCT compared the effects of DRSP/20 mcg EE with the second-generation progestin LNG/30 mcg EE on premenstrual symptoms after 6 menstrual cycles. In the premenstrual phase, the DRSP/EE group showed less negative mood and weight gain.36
A 2012 Cochrane review examined the effects of OCs containing DRSP on premenstrual dysphoric disorder (PMDD) vs placebo and other OC formulations. The review included 5 trials and found that DRSP is associated with significantly greater improvements than placebo in symptoms of PMDD but was inconclusive on whether DRSP formulations have greater effects on PMDD than other OC formulations.37
Dysmenorrhea. A 2009 Cochrane review compared 10 studies examining the role of different formulations of combined OCs in management of dysmenorrhea and concluded there is no difference in improvement between different OC preparations.38
OCs and coronary heart disease
Estrogen has several favorable effects on circulating lipoproteins, including increasing high-density lipoprotein (HDL), and increasing low-density lipoprotein (LDL) receptor activity, thereby enhancing removal of LDL.
Women using a 20 mcg EE/100-mcg LNG OCP experienced reductions in HDL and small increases in LDL and triglycerides compared with a 30 mcg EE/150-mcg LNG OCP.39 A study of gestodene 75 mcg with either EE 20 mcg or 30 mcg for 13 cycles, found that there was a greater increase in triglyceride levels in the formulation with a higher dose of estrogen (p = 0.029).40
Barkfeldt and colleagues41 conducted a double-blind RCT that evaluated the effects of lipid metabolism on 98 women who received 2 different types of progestin-only pills, desogestrel 75 mcg/day vs LNG 30 mcg/day. There were minimal changes in the lipid profile except for decreasing trends in levels of HDL, its subfractions, and apolipoprotein-I and -II. No differences were observed between the 2 formulations despite the higher progestin dose found in desogestrel, including no changes in LDL or apolipoprotein-B.41
Third generation progestins with “lesser androgenicity” may allow more “expression” of the effects of estrogen on lipids. A prospective study of 66 women over 9 months comparing either desogestrel (50/100/150 mcg) and EE (35/30/30 mcg), or LNG (50/100/150 mcg) and EE (30/40/30 mcg), showed that the desogestrel formulation increased HDL whereas LNG decreased HDL.42 Another study compared monophasic desogestrel/EE with triphasic LNG/EE in 37 healthy young women. While both preparations led to an increase in total cholesterol, the desogestrel formulation led to a reduction in the LDL.43 A 1995 study of drospirenone (DRSP) compared with LNG for 6 months, showed that HDL increased in the DRSP group (P < 0.05) but triglyceride levels showed a greater increase in the DRSP (P <0.05).27
The use of OCs in the absence of risk factors does not appear to promote CAD and there is no reason to withhold OCs from dyslipidemic women. In women with LDL greater than 160 mg/dL or multiple cardiac risk factors, ACOG recommends an alternative non-hormonal method such as an intrauterine device (IUD).31
OCs and glucose metabolism, thromboembolism
Glucose metabolism. Oelkers and colleagues27 studied glucose levels in 80 healthy women assigned to 4 equal groups who received 3 mg of drospirenone combined with 30-, 20-, and 15-mcg doses of EE or LNG/30-mcg EE. Each woman performed oral glucose tolerance tests at pre-treatment and at the end of the 6-month OCP cycle. On treatment, fasting glucose was unchanged for all groups, but the area under the curve for the glucose tolerance increased for all formulations. Although not statistically significant between groups, the drospirenone/30-mcg EE group had a 19% worsening of glucose tolerance.27 This research suggests that women with Type 1 or 2 diabetes who are otherwise healthy, non-smokers and younger than 35 years of age can safely use OCs.
Thromboembolism. Estrogen has been known to increase the risk of venous thromboembolism (VTE) by increasing prothrombin and decreasing antithrombin III.44 In OC users, the incidence of VTE is increased by a factor of 3 to 5.45 While several studies have compared high-dose estrogen (50 mcg) with low-dose (35 mcg or less) OCs46,47 there is no information about any differences in low (25-35 mcg) EE vs ultra-low doses (10 mcg). ACOG recommends a nonestrogen hormonal alternative such as progestin-only pills or an IUD, for obese women.
Third generation desogestrel-containing OCs have a slightly increased risk of VTE compared with second generation pills48unexplained by bias and confounding factors.49,50 It has been estimated that 25 additional cases of VTE occur every year among 100,000 women using thirrd generation OCs compared with 10 additional cases per 100,000 women using second generation OCs.51 A meta-analysis that included 9 case control and 3 cohort studies estimated an odds ratio for third vs second generation OCs of 1.7 (95% CI, 1.4 to 2.0).52 A 2010 meta-analysis refutes these finding and found no differences in third generation gestodene progestin vs other OCs.53 Because obesity (BMI >30 kg/m2) is an independent risk factor for VTE, ACOG recommends an alternative non-estrogen hormonal method such as progestin-only pills or IUD in obese women.31
Bone mineral density (BMD). A 2000 study compared 2 OCs with the same dose of progestin (gestodene 75 mcg) and 2 doses of EE (20 vs 30 mcg) to determine if there was a correlation between dose of estrogen and loss of BMD in young post-adolescent women taking OCs. It concluded that pills with 20 and 30 mcg of estrogen were associated with the same reduction in BMD.54
However a 2009 Cochrane review concluded that combined OCs do not affect bone health, ie, fracture rate, BMD, or biochemical markers of bone change. Thirteen RCTs were reviewed and researchers concluded that the relationship between OC use and fracture risk cannot be determined from the limited data currently available.55
Cancer. Research does not support the notion that OCs contribute to cancer. In fact, reduced endometrial and ovarian cancers have been shown among users of OCs containing 50 mcg EE.56-58 Low-dose formulations (≤35 mg EE) have been less studied but also confer a substantial risk reduction.59
Data are conflicting regarding a slight increase in risk for breast cancer in current or recent users of OC from older, higher-estrogen doses; that risk returns to normal over time.60 The World Health Organization recognizes this slight risk, but has concluded that the benefits of OCs outweigh the risks. 61
Evidence-based guidelines are lacking
There is a paucity of RCTs with sufficient duration and sample size that compare different OC formulations to provide evidence-based guidance for physicians. While some pharmaceutical companies market their product for particular benefits, these finding too often come from non-comparative trials, ie, their product vs placebo.
So here’s what we know...
No OC formulation is more effective at preventing pregnancy than any others. Cycle control, ie, less intermenstrual bleeding, is improved with 30 to 35 mcg EE formulations compared with ultra-low dose (20 mcg) EE. There are no advantages to choosing a multiphasic formulation over a monophasic OC. While extended-cycle formulations have more breakthrough bleeding than monthly cycles, overall they have fewer days of menstrual bleeding, which tend to decrease even further in successive cycles. Extended-cycle formulations have decreased days of bloating and menstrual cramping.
There is no evidence that different doses of estrogen or progestin affect weight gain or total body water. DRSP leads to a more favorable lean body mass profile than LNG and desogestrel, which may be related to its anti-mineralocorticoid effect. While both second and third generation progestin formulations have been shown to improve acne, there is no evidence to indicate a preference.
There is also little evidence to recommend a particular OC to avoid adverse events such as CAD or VTE; in fact the evidence is often contradictory. Epidemiologic studies confirm that venous thromboembolic disease is similar for 20 and 30 mcg EE. There may be an increase in VTE with desogestrel, but recent evidence finds no significant increase. The clinical significance that DRSP increases triglyceride levels while it decreases LDL and HDL, and the significance of LDL reduction by desogestrel requires further investigation.
There is no evidence that OCs affect bone health indices such as fracture rate, BMD, or biochemical markers of bone change. OC formulations with While extended-cycle formulations have more breakthrough bleeding than monthly cycles, they have overall fewer days of menstrual bleeding.higher doses of estrogen have been shown to reduce ovarian and endometrial cancer, presumably due to fewer ovulatory cycles. However, similar reductions should therefore be observed with lower EE dose formulations as well.
Clearly, the literature indicates that there is little evidence to recommend one OC formulation over another. All currently marketed OCs have low dose EE. However, when counseling patients, keep in mind that extended cycle formulations decrease some side effects and generic formulations reduce costs.
CORRESPONDENCE
Eric A. Schaff, MD, Philadelphia Women’s Center, 777 Appletree Street, #7, Philadelphia, PA 19106; [email protected]
• Consider prescribing monophasic pills as the first choice for women starting oral contraceptives (OCs) given the lack of advantage in using multiphasic formulations, and the larger number of studies showing the safety and efficacy of monophasic pills.
• Avoid prescribing OCs with estrogen—even with ultra-low estrogen—to women at high risk for venous thromboembolism, given that there are no studies that show differences in low (25-35 mcg) ethinyl estradiol vs ultra-low doses (10 mcg) formulas.
Strength of recommendation (SOR)
• Good-quality patient-oriented evidence
• Inconsistent or limited-quality patient-oriented evidence
• Consensus, usual practice, opinion, disease-oriented evidence, case series
For a healthy woman interested in contraception, there are multiple oral contraceptive (OC) formulations on the market from which to choose. But are there any significant differences in their effectiveness or safety profiles that make one formulation superior?
Comparative trials of OCs have attempted to answer these questions by evaluating formulations that contain the synthetic components: ethinyl estradiol, norethindrone, levonorgestrel, desogestrel, norgestimate, gestodene, and drospirenone.
Unfortunately, many studies that have evaluated OCs have had methodological weaknesses, making their clinical significance confusing. Few randomized controlled trials (RCTs) have been double blinded or powered to find infrequent outcomes like pregnancy or adverse events. Trials are rarely reproduced by other researchers and many have been funded by pharmaceutical companies with conflicts of interest. Despite these shortcomings, it is possible to glean valuable data from existing studies.
With that in mind, our purpose here is to review whether there are significant differences in effectiveness, cycle control (bleeding), side effects, or satisfaction that may help physicians and patients select the appropriate formulation.
Comparing OC Effectiveness
OC effectiveness is determined by the inherent properties to prevent ovulation, conception, and/or implantation when the formulation is used correctly.1,2 and during typical inconsistent use in the population (ie, adherence).3 Effectiveness is also measured by whether the method is discontinued and there is a gap in contraception allowing pregnancy to occur.
There is no evidence that any combined or progesterone-only hormonal formulation is inherently better at preventing ovulation, conception, or implantation. (For more on combined OCs, see “A closer look at combined OCs,” on page E3.) Theoretically, progestins with longer half-lives may be more effective at preventing ovulation if a pill is not taken the same time each day, and extended cycle pills provide more continuous suppression of ovulation. But, no studies have found any formulation to be more effective.
A 2004 Cochrane review4 compared progestins in OCs by examining 22 different trials with various study protocols. The review found a lower rate of discontinuation in patients taking OCs with second generation progestins compared with first generation progestins (relative risk [RR]=0.79; 95% Extended-cycle OCs have a greater risk of breakthrough bleeding, which can decrease adherence and increase discontinuation, thus increasing the risk of pregnancy. confidence interval [CI] 0.61-0.91), and an even lower rate of discontinuation with third generation OCs. Additionally, cycle control was better in second generation progestin OCs compared with first generation progestin OCs. Rates of effectiveness, cycle control and side effects were similar between drospirenone and desogestrel. The review concluded that second and third generation progestins are preferred over first generation progestins in combined OCs,4 although the evidence is not strong.
What about generics? To be considered an FDA-approved bioequivalent generic to a brand name formulation, pharmacokinetic studies must demonstrate that a product provides equivalent serum levels. There are no studies evaluating differences in effectiveness of generic vs brand name OCs. Generic medications typically cost about 50% less than brand name OCs.5 The Society of Obstetricians and Gynaecologists of Canada supports generic formulations “providing increased choice and less expensive options.”6
What are the differences among the options?
While OCs, in general, have a reputation of causing side effects, when compared with a placebo, no significant findings have been noted in the frequency of headache, nausea, vomiting, breast pain, or weight gain.7,8 That being the case, it is unlikely there are differences among formulations.
Ultra-low estrogen. Estrogen in OCs has been reduced to 10 to 35 mcg to minimize side effects and adverse events, yet remain at a level sufficient to provide menstrual cycle control with minimal breakthrough bleeding. Advantages of ultra-low estrogen 10 mcg products include reduction of estrogen, side effects9 but the disadvantage includes breakthrough bleeding, which can negatively affect adherence.10 In a double-blind RCT of 649 women comparing OCs with gestodene 75 mcg and either 20 mcg or 30 mcg ethinyl estradiol (EE), more intermenstrual breakthrough bleeding occurred with the 20 mcg group (P<0.05). This difference was not enough to cause an increased discontinuation rate in the 20-mcg EE group.11
Progestin-only pills (POPs) are recommended for women who cannot or should not take estrogen in OCs, and women who are breastfeeding. The advantages of POPs include a simplified and fixed regimen. Disadvantages include irregular bleeding and menstrual cycle length. A 2010 Cochrane review examined various POP formulations in 6 different trials and concluded that there is not sufficient research to compare POPs in terms of efficacy, acceptability, and continuation rates.12
Monophasic vs multiphasic OCs. Biphasic and triphasic OCs were introduced in an effort to decrease the amount of hormone and the side effects. Their phasic nature also attempts to mimic the pattern of rising and falling estrogen and progesterone levels seen during a normal menstrual cycle. Cochrane reviews in 200913 and 201014 compared the cycle control and side effects of biphasic vs monophasic, and triphasic vs monophasic formulations of OCs, respectively. The 2009 review comparing biphasic and monophasic OCPs was limited to one study of 533 women using biphasic pills and 481 women using monophasic pills. No differences were found in intermenstrual bleeding, amenorrhea, or discontinuation due to intermenstrual bleeding.
The 2011 review comparing triphasic and monophasic OCs included 21 studies, and found no significant difference in discontinuation due to medical reasons, cycle disturbance, intermenstrual bleeding, or adverse events. Both of the Cochrane reviews concluded that monophasic pills should be the first choice for women starting OCs given the lack of advantage in using multiphasic formulations, and the larger number of studies showing the safety and efficacy of monophasic pills.
The 2009 Cochrane review compared biphasic and triphasic OCPs in terms of cycle control and side effects.13The first trial examined in this review included 458 women and compared 2 biphasic pills and one triphasic pill, all containing LNG and EE. It found no important differences between all 3 formulations, but found that 252 women of the initial 458 (55%) discontinued the study for various reasons.
The second trial included 469 women (169 of whom withdrew from the study or 36%), and compared a biphasic pill containing norethindrone with 2 triphasic pills, one containing LNG and the other containing norethindrone. This study showed no differences between the biphasic and triphasic pills containing norethindrone, but inferior cycle control in the biphasic pill containing norethindrone compared with the triphasic containing LNG. The review concluded that the choice of progestin type (LNG preferred over norethindrone) might be more important than the choice of phasic regimen.13
Monthly vs extended cycle OCs. When OCs were first introduced, researchers believed that women would prefer a 21-day formulation followed by a 7-day pill free time that mimicked an average woman’s menstrual cycle because the withdrawal bleeding would be an indicator that she was not pregnant. Extending the time between menses has garnered increased interest. Extended-cycle preparations are available for durations ranging from 84 to 365 days.15
A study of 99 women evaluated the impact of omitting the first 3 combined OC pills (second and third generation) on ovulation during a 28-day cycle. While none of the women experienced ovulation, follicle-stimulating hormone reached a maximal serum concentration in most women during the first 7 pill-free days, indicating complete pituitary recovery. Additionally, the researchers detected increases in serum estradiol, indicating that follicular growth up to preovulatory size is common in women missing the first one to 3 pills of their contraceptive cycle.16 Non-adherence often occurs during transitions between successive packs of Ocs.17 It has been reported that 47% of women using OCPs miss one pill and 22% miss 2 pills per cycle.18 Ovulation and pregnancy are more likely to occur if pills are missed in the first week after menses.
Extended-cycle OCs prevent hormonal fluctuations and provide continuous suppression of follicle stimulating hormone (FSH) and luteinizing hormone (LH), decreasing the likelihood of ovulation and, therefore, pregnancy. Since the extended-cycle regimen decreases the number of transitions between packs of OCs, one might expect a reduction in the risk associated with non-adherence at the beginning of a cycle. However, extended cycles have a greater risk of breakthrough bleeding, which can decrease adherence and increase discontinuation of the method and, thereby, increase the risk of pregnancy.
A multicenter RCT of 682 women examined the efficacy and safety of the extended-cycle OC Seasonale (30 mcg EE/150 mcg LNG) compared with a traditional cycle OC Nordette-28 (30 mcg EE/150 mcg LNG). Women received either 4, 91-day extended cycles (n=456) or 13, 28-day regular cycle (n=226) regimens over the course of one year. On average, 38% of women in the extended cycle group reported unscheduled (breakthrough) bleeding, while 18% of women in the conventional cycle group reported unscheduled bleeding. Breakthrough bleeding decreased with each successive cycle of the extended regimen, from a median of 12 days with the first cycle, to a median of 4 days during the fourth and final cycle. This study also reported no significant differences in side effects between the extended and traditional cycle regimens, including changes in lipids, body weight, blood pressure, or endometrial hyperplasia.19
Another RCT examined the difference in bleeding patterns, side effects, and acceptability between a standard 28-day cycle OC and an extended regimen 168-day cycle OC in 32 women. Both OCs contained 20 mcg EE and 100 mcg LNG, and the study was conducted over 6 months. Women in the extended cycle regimen reported significantly fewer days of bloating (0.7 vs 11.1 days; P=0.04), and menstrual pain (1.9 vs 13.3 days; P<0.01). There was no significant difference in reported headache, breast tenderness, nausea, depression, or premenstrual symptoms. Women in the extended cycle group also reported significantly fewer bleeding days that required sanitary pads (18.4 vs 33.8 days; P<0.01). However, there was no statistically significant difference in the total number of days where any degree of bleeding occurred (34.9 days in the monthly cycle group, 25.9 days in the extended cycle group; P=0.33).20
In a study of 126-day extended-cycle OCs with 30 mcg EE and 3 mg drospirenone, the bleeding profile improved over time and endometrial biopsies revealed no hyperplasia.21 Another benefit of the extended cycle is personal preference, ie, controlling the timing of one’s menses,22 for example, in athletes during training and competition.
Continuous use of OCs prevents the cyclic fluctuations of serum levels of EE and progestogen and, hence, the cyclic variations of related serum-based metabolic parameters. Extended cycle OCs can make it easier to titrate other medications affected by hormonal fluctuations. Another study of extended cycle drospirenone OCs compared with monthly OCs over 6 months showed no difference in lipid, carbohydrate, and coagulation markers.23
Six RCTs were reviewed in a Cochrane review of monthly vs extended cycle combined pills. It found no significant differences in efficacy, adherence, discontinuation rates, and patient satisfaction. The only difference was improvement of menstrual-associated symptoms of “headaches, genital irritation, tiredness, bloating and menstrual pain” with the extended cycle regimen.24
OCs effect on weight, BP, and premenstrual symptoms
Weight gain. A 2008 Cochrane review examined 3 placebo-controlled RCTs and concluded that the available evidence was insufficient to determine the effect of combined hormonal contraceptives on weight, and that larger doses of estrogen were not shown to cause larger weight gain.25
One RCT examined the effects of OCs on variations of total body water, fat mass, and fat-free mass throughout the menstrual cycle to determine if different doses of estrogen (15 mcg vs 30 mcg EE) or different types of progestins (gestodene 60 mcg vs drospirenone 3 mg) impact weight gain. This study only included 80 women randomized to the 2 treatment groups and an additional control group using male condoms. No differences were found in total body water or fat mass. There was, however, a significant increase in the fat-free mass in women of the EE/gestodene group when compared to controls, indicating a possible effect of the androgenic properties of gestodene compared with drospirenone (which has anti-androgen properties) in increasing muscle mass.26
In a 6 month study of drospirenone compared with LNG, mean body weight fell by 0.8 to 1.7 kg in women treated with drospirenone compared with a 0.7 kg weight gain in the LNG group (P < 0.05).27 A multicenter RCT comparing OCs with EE 30 mcg/drospirenone 3 mg, and EE 30 mcg/desogestrel 150 mcg, concluded that EE/drospirenone has a more favorable effect on body weight than EE/desogestrel. This finding may have resulted from the antimineralocorticoid, mild diuretic effects of drospirenone.28
Hypertension. In a review of progestin-only OCs in normotensive women, the authors could find no evidence to show a statistically significant increase in blood pressure.29
In a study of 120 women randomized to drospirenone/EE or LNG/EE, the drospirenone group had a mean decrease in systolic blood pressure from 107 to 103 mm Hg, and a significantly lower group mean blood pressure compared with the LNG group.30 Another study of 80 women over 6 months randomized into 3groups each having 3 mg of drospirenone with either a 30-, 20-, or 15-mcg dose of EE found that systolic blood pressure decreased by 1 to 4 mm Hg compared with an elevation of blood pressure of 4 mm Hg in the LNG/EE group.27
In women with well-controlled blood pressure who were less than 35 years old, non-smokers and otherwise healthy, the American College of Obstetricians and Gynecologists (ACOG) recommends31 a trial of OCs with monitoring of their blood pressure.
Acne. One Cochrane review looked at studies that compared combined OCs to placebo, and found OCs improved the condition. However, there was insufficient evidence regarding the difference in effectiveness of various formulations of OCs in treating the disease.32 There was no difference between first and second generation progestins,33 between second and third generations,34 or third generation vs drospirenone.35
Premenstrual symptoms. A 2005 open-label RCT compared the effects of DRSP/20 mcg EE with the second-generation progestin LNG/30 mcg EE on premenstrual symptoms after 6 menstrual cycles. In the premenstrual phase, the DRSP/EE group showed less negative mood and weight gain.36
A 2012 Cochrane review examined the effects of OCs containing DRSP on premenstrual dysphoric disorder (PMDD) vs placebo and other OC formulations. The review included 5 trials and found that DRSP is associated with significantly greater improvements than placebo in symptoms of PMDD but was inconclusive on whether DRSP formulations have greater effects on PMDD than other OC formulations.37
Dysmenorrhea. A 2009 Cochrane review compared 10 studies examining the role of different formulations of combined OCs in management of dysmenorrhea and concluded there is no difference in improvement between different OC preparations.38
OCs and coronary heart disease
Estrogen has several favorable effects on circulating lipoproteins, including increasing high-density lipoprotein (HDL), and increasing low-density lipoprotein (LDL) receptor activity, thereby enhancing removal of LDL.
Women using a 20 mcg EE/100-mcg LNG OCP experienced reductions in HDL and small increases in LDL and triglycerides compared with a 30 mcg EE/150-mcg LNG OCP.39 A study of gestodene 75 mcg with either EE 20 mcg or 30 mcg for 13 cycles, found that there was a greater increase in triglyceride levels in the formulation with a higher dose of estrogen (p = 0.029).40
Barkfeldt and colleagues41 conducted a double-blind RCT that evaluated the effects of lipid metabolism on 98 women who received 2 different types of progestin-only pills, desogestrel 75 mcg/day vs LNG 30 mcg/day. There were minimal changes in the lipid profile except for decreasing trends in levels of HDL, its subfractions, and apolipoprotein-I and -II. No differences were observed between the 2 formulations despite the higher progestin dose found in desogestrel, including no changes in LDL or apolipoprotein-B.41
Third generation progestins with “lesser androgenicity” may allow more “expression” of the effects of estrogen on lipids. A prospective study of 66 women over 9 months comparing either desogestrel (50/100/150 mcg) and EE (35/30/30 mcg), or LNG (50/100/150 mcg) and EE (30/40/30 mcg), showed that the desogestrel formulation increased HDL whereas LNG decreased HDL.42 Another study compared monophasic desogestrel/EE with triphasic LNG/EE in 37 healthy young women. While both preparations led to an increase in total cholesterol, the desogestrel formulation led to a reduction in the LDL.43 A 1995 study of drospirenone (DRSP) compared with LNG for 6 months, showed that HDL increased in the DRSP group (P < 0.05) but triglyceride levels showed a greater increase in the DRSP (P <0.05).27
The use of OCs in the absence of risk factors does not appear to promote CAD and there is no reason to withhold OCs from dyslipidemic women. In women with LDL greater than 160 mg/dL or multiple cardiac risk factors, ACOG recommends an alternative non-hormonal method such as an intrauterine device (IUD).31
OCs and glucose metabolism, thromboembolism
Glucose metabolism. Oelkers and colleagues27 studied glucose levels in 80 healthy women assigned to 4 equal groups who received 3 mg of drospirenone combined with 30-, 20-, and 15-mcg doses of EE or LNG/30-mcg EE. Each woman performed oral glucose tolerance tests at pre-treatment and at the end of the 6-month OCP cycle. On treatment, fasting glucose was unchanged for all groups, but the area under the curve for the glucose tolerance increased for all formulations. Although not statistically significant between groups, the drospirenone/30-mcg EE group had a 19% worsening of glucose tolerance.27 This research suggests that women with Type 1 or 2 diabetes who are otherwise healthy, non-smokers and younger than 35 years of age can safely use OCs.
Thromboembolism. Estrogen has been known to increase the risk of venous thromboembolism (VTE) by increasing prothrombin and decreasing antithrombin III.44 In OC users, the incidence of VTE is increased by a factor of 3 to 5.45 While several studies have compared high-dose estrogen (50 mcg) with low-dose (35 mcg or less) OCs46,47 there is no information about any differences in low (25-35 mcg) EE vs ultra-low doses (10 mcg). ACOG recommends a nonestrogen hormonal alternative such as progestin-only pills or an IUD, for obese women.
Third generation desogestrel-containing OCs have a slightly increased risk of VTE compared with second generation pills48unexplained by bias and confounding factors.49,50 It has been estimated that 25 additional cases of VTE occur every year among 100,000 women using thirrd generation OCs compared with 10 additional cases per 100,000 women using second generation OCs.51 A meta-analysis that included 9 case control and 3 cohort studies estimated an odds ratio for third vs second generation OCs of 1.7 (95% CI, 1.4 to 2.0).52 A 2010 meta-analysis refutes these finding and found no differences in third generation gestodene progestin vs other OCs.53 Because obesity (BMI >30 kg/m2) is an independent risk factor for VTE, ACOG recommends an alternative non-estrogen hormonal method such as progestin-only pills or IUD in obese women.31
Bone mineral density (BMD). A 2000 study compared 2 OCs with the same dose of progestin (gestodene 75 mcg) and 2 doses of EE (20 vs 30 mcg) to determine if there was a correlation between dose of estrogen and loss of BMD in young post-adolescent women taking OCs. It concluded that pills with 20 and 30 mcg of estrogen were associated with the same reduction in BMD.54
However a 2009 Cochrane review concluded that combined OCs do not affect bone health, ie, fracture rate, BMD, or biochemical markers of bone change. Thirteen RCTs were reviewed and researchers concluded that the relationship between OC use and fracture risk cannot be determined from the limited data currently available.55
Cancer. Research does not support the notion that OCs contribute to cancer. In fact, reduced endometrial and ovarian cancers have been shown among users of OCs containing 50 mcg EE.56-58 Low-dose formulations (≤35 mg EE) have been less studied but also confer a substantial risk reduction.59
Data are conflicting regarding a slight increase in risk for breast cancer in current or recent users of OC from older, higher-estrogen doses; that risk returns to normal over time.60 The World Health Organization recognizes this slight risk, but has concluded that the benefits of OCs outweigh the risks. 61
Evidence-based guidelines are lacking
There is a paucity of RCTs with sufficient duration and sample size that compare different OC formulations to provide evidence-based guidance for physicians. While some pharmaceutical companies market their product for particular benefits, these finding too often come from non-comparative trials, ie, their product vs placebo.
So here’s what we know...
No OC formulation is more effective at preventing pregnancy than any others. Cycle control, ie, less intermenstrual bleeding, is improved with 30 to 35 mcg EE formulations compared with ultra-low dose (20 mcg) EE. There are no advantages to choosing a multiphasic formulation over a monophasic OC. While extended-cycle formulations have more breakthrough bleeding than monthly cycles, overall they have fewer days of menstrual bleeding, which tend to decrease even further in successive cycles. Extended-cycle formulations have decreased days of bloating and menstrual cramping.
There is no evidence that different doses of estrogen or progestin affect weight gain or total body water. DRSP leads to a more favorable lean body mass profile than LNG and desogestrel, which may be related to its anti-mineralocorticoid effect. While both second and third generation progestin formulations have been shown to improve acne, there is no evidence to indicate a preference.
There is also little evidence to recommend a particular OC to avoid adverse events such as CAD or VTE; in fact the evidence is often contradictory. Epidemiologic studies confirm that venous thromboembolic disease is similar for 20 and 30 mcg EE. There may be an increase in VTE with desogestrel, but recent evidence finds no significant increase. The clinical significance that DRSP increases triglyceride levels while it decreases LDL and HDL, and the significance of LDL reduction by desogestrel requires further investigation.
There is no evidence that OCs affect bone health indices such as fracture rate, BMD, or biochemical markers of bone change. OC formulations with While extended-cycle formulations have more breakthrough bleeding than monthly cycles, they have overall fewer days of menstrual bleeding.higher doses of estrogen have been shown to reduce ovarian and endometrial cancer, presumably due to fewer ovulatory cycles. However, similar reductions should therefore be observed with lower EE dose formulations as well.
Clearly, the literature indicates that there is little evidence to recommend one OC formulation over another. All currently marketed OCs have low dose EE. However, when counseling patients, keep in mind that extended cycle formulations decrease some side effects and generic formulations reduce costs.
CORRESPONDENCE
Eric A. Schaff, MD, Philadelphia Women’s Center, 777 Appletree Street, #7, Philadelphia, PA 19106; [email protected]
1. Chandra A, Martinez GM, Mosher WD, et al. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;25:1-160.
2. Keam SJ, Wagstaff AJ. Ethinyl estradiol/drospirenone: a review of its use as an oral contraceptive. Treatments Endocrinol. 2003;2:49-70.
3. Krattenmacher R. Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception. 2000;62:29-38.
4. Maitra NN, Kulier R, Bloemenkamp K, et al. Progestogens in combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2004, Issue 3.
5. Oral Contraceptives at Drugstore.com. http://www.drugstore.com/pharmacy/drugindex/rxsearch.asp?search=oral+contraceptive [accessed January 24, 2011]
6. Society of Obstetricians and Gynaecologists of Canada Statement on Generic Contraceptions. J Obstet Gynecol Canada. 2008;30:271-272.
7. Coney P, Washenik K, Langley RG, et al. Weight change and adverse event incidence with a low-dose oral contraceptive: two randomized, placebo-controlled trials. Contraception. 2001;63:297-302.
8. O’Connell K, Davis AR, Kerns J. Oral contraceptives: side effects and depression in adolescent girls. Contraception. 2007;75:299-304.
9. Redmond GP, Olson WH, Lippman JS, et al. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo-controlled trial. Obstet Gynecol. 1997;89:615-622.
10. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355-360.
11. Endrikat J, Muller U, Dusterberg B. A twelve-month comparative clinical investigation of two low-dose oral contraceptives containing 20 micrograms ethinylestradiol/75 micrograms gestodene and 30 micrograms ethinylestradiol/75 micrograms gestodene, with respect to efficacy, cycle control, and tolerance. Contraception. 1997;55:131-137.
12. Grimes DA, Lopez LM, O’Brien PA, et al. Progestin-only pills for contraception. Cochrane Database of Systematic Reviews 2010, Issue 1.
13. Van Vliet HAAM, Grimes DA, Helmerhorst FM, et al. Biphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2009, Issue 2.
14. Grimes DA, Lopez LM, Schulz KF et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2009, Issue 2.
15. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
16. Elomaa K, Rolland R, Brosens I, et al. Omitting the first oral contraceptive pills of the cycle does not automatically lead to ovulation. Am J Obstet Gynecol. 1998;179:41-46.
17. Adams Hillard PJ. Oral contraception noncompliance: The extent of the problem. Adv Contracept. 1992;8(suppl 1):13-20.
18. Rosenberg M, Waugh MS. Causes and consequences of oral contraceptive noncompliance. Am J Obstet Gynecol. 1999;180:S276-279.
19. Anderson, FD, Hait, H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
20. Kwiecien M, Edelman A, Nichols MD, et al. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67:9-13.
21. Foidard JM, Sulak PJ, Schellschmidt I, et al. The Yasmin Extended Regimen Study Group. The use of an oral contraceptive containing ethinylestradiol and drospirenone in an extended regimen over 126 days. Contraception. 2006;73:34-40.
22. Shakespeare J, Neve E, Hodder E. Is norethisterone a lifestyle drug? Results of database analysis. BMJ. 2000;320:291.
23. Machado RB, de Melo NR, Maia Jr. H, et al. Effect of a continuous regimen of contraceptive combination of ethinylestradiol and drospirenone on lipid, carbohydrate and coagulation profiles. Contraception. 2010;81:102-106.
24. Edelman A, Gallo MF, Nichols, MD, et al. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Human Reprod. 2006;21:573-578.
25. Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008, Issue 4.
26. Machado RB, Tachotti F, Cavenague G, et al. Effects of two different oral contraceptives on total body water: a randomized study. Contraception. 2006;73:344-347.
27. Oelkers W, Foidart JM, Dombrovicz N, et al. Effects of a new oral contraceptive containing an anti-mineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J Clinical Endo Metabolism. 1995;80:1816-1821.
28. Foidart JM, Wuttke W, Bouw GM, et al. A comparative investigation of contraceptive reliability, cycle control, and tolerance of two monophasic oral contraceptives containing either drospirenone or desogestrel. Eur J Contracept Reprod Health Care. 2000;5:124-134.
29. Hussain, SF. Progestogen-only pills and high blood pressure: is there an association? A literature review. Contraception. 2004;69:89-97.
30. Suthipongse W, Taneepanichskul S. An open-label randomized comparative study of oral contraceptives between medications containing 3 mg drospirenone/30 mcg ethinylestradiol and 150 mcg levonorgestrel/30 mcg ethinylestradiol in Thai women. Contraception. 2004;69:23-26.
31. ACOG practice bulletin. No. 73. Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
32. Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2009, Issue 3.
33. Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262.
34. Rosen, MP, Breitkopf, DM, Nagamani, M. A randomized controlled trial of second- versus third-generation oral contraceptives in the treatment of acne vulgaris. Am J Obstet Gynecol. 2003;188:1158-1160.
35. Huber J, Foidart JM, Wuttke W, et al. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. Eur J Contracept Reprod Health Care. 2000;5:25-34.
36. Sangthawan, M, Taneepanichskul, S. A comparative study of monophasic oral contraceptives containing either drospirenone 3 mg or levonorgestrel 150 mcg on premenstrual symptoms. Contraception. 2005;71:1-77.
37. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database of Systematic Reviews. 2009, Issue 2.
38. Wong CL, Farquhar C, Roberts H, et al. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev. 2009, Issue 4.
39. Endrikat J, Klipping C, Cronin M, et al. An open label, comparative study of the effects of a dose-reduced oral contraceptive containing 20 mcg ethinyl estradiol and 100 mcg levonorgestrel on hemostatic, lipids, and carbohydrate metabolism variables. Contraception. 2002;65:215-221.
40. Brill K, Then A, Beisiegel U, et al. Investigation of the influence of two low-dose monophasic oral contraceptives containing 20 micrograms ethinylestradiol/75 micrograms gestodene and 30 micrograms ethinylestradiol/75 micrograms gestodene, on lipid metabolism in an open randomized trial. Contraception. 1996;54:291-297.
41. Barkfeldt J, Virkkunen A, Dieben T. The effects of two progestogen-only pills containing either desogestrel (75 mcg/day) or levonorgestrel (30 mcg/day) on lipid metabolism. Contraception. 2001; 64:295-299.
42. Knopp RH, Broyles FE, Cheung M, et al. Comparison of the lipoprotein, carbohydrate, and hemostatic effects of phasic oral contraceptives containing desogestrel or levonorgestrel. Contraception. 2001;63:1-11.
43. Foulon T, Payen N, Laporte F, et al., Effects of two low-dose oral contraceptives containing ethinylestradiol and either desogestrel of levonorgestrel on serum lipids and lipoproteins with particular regards to LDL size. Contraception. 2001;64:11-16.
44. Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system: lessons learned and unanswered questions. J Am Coll Cardiol. 2006;47:1741-1753.
45. Martinelli I. Risk factors in venous thromboembolism. Thromb Haemost. 2001;86:395-403.
46. Vessey M, Mant D, Smith A, et al. Oral contraceptives and venous thromboembolism: findings in a large prospective study. BMJ. 1986;292:526.
47. Gerstman BB, Piper TM, Tomita DK, et al. Oral contraceptive estrogen dose and the risk of venous thromboembolic disease. Am J Epidemiol. 1991;133:32-137.
48. Rosendaal FR, Van Hylckama Vlieg A, et al. Estrogens, progestogens and thrombosis. J Thromb Haemost. 2003;1:1371-1380.
49. Farley TM, Meirik O, Collins J. Cardiovascular disease and combined oral contraceptives: reviewing the evidence and balancing risks. Hum Reprod Update. 1999;5:721-735.
50. Vandenbroucke JP, Helmerhorst FM, Bloemenkamp KW, et al. Third generation oral contraceptive and deep venous thrombosis: from epidemiologic controversy to new insight in coagulation. Am J Obstet Gynecol. 1997;177:887-891.
51. Hannaford P. Health consequences of oral combined oral contraceptives. Br Med Bull. 2000;56:749-760.
52. Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. BMJ. 2001;323:131-134.
53. Heinemann LA, Dinger JC, Assmann A, et al. Use of oral contraceptives containing gestodene and risk of venous thromboembolism: outlook 10 years after the third-generation “pill scare”. Contraception. 2010;81:401-407.
54. Paoletti AM, Orru M, Floris S, et al. Evidence that treatment with monophasic oral contraceptive formulations containing ethinylestradiol plus gestodene reduces bone reabsorption in young women. Contraception. 2000;61:259-263.
55. Lopez LM, Grimes DA, Schulz KF, et al. Steroidal contraceptives: effect on bone fractures in women. Cochrane Database Syst Rev. 2009, Issue 2.
56. Schlesselman JJ. Oral contraceptives and neoplasia of the uterine corpus. Contraception. 1991;43:557-580.
57. Hankinson SE, Colditz GA, Hunter DJ, et al. A quantitative assessment of oral contraceptive use and risk of ovarian cancer. Obstet Gynecol. 1992;80:708-714.
58. LaVecchia C, Franceschi S, Decarli A. Oral contraceptive use and the risk of epithelial ovarian cancer. Br J Cancer. 1984;50:31-34.
59. Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95:370-374.
60. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 347:1713-27, 1996
61. World Health Organization. Carcinogenicity of combined hormonal contraceptives and combined menopausal treatment. September 2005. http://www.who.int/reproductivehealth/topics/ageing/cocs_hrt_statement.pdf. Accessed Aug 28, 2013.
1. Chandra A, Martinez GM, Mosher WD, et al. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;25:1-160.
2. Keam SJ, Wagstaff AJ. Ethinyl estradiol/drospirenone: a review of its use as an oral contraceptive. Treatments Endocrinol. 2003;2:49-70.
3. Krattenmacher R. Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception. 2000;62:29-38.
4. Maitra NN, Kulier R, Bloemenkamp K, et al. Progestogens in combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2004, Issue 3.
5. Oral Contraceptives at Drugstore.com. http://www.drugstore.com/pharmacy/drugindex/rxsearch.asp?search=oral+contraceptive [accessed January 24, 2011]
6. Society of Obstetricians and Gynaecologists of Canada Statement on Generic Contraceptions. J Obstet Gynecol Canada. 2008;30:271-272.
7. Coney P, Washenik K, Langley RG, et al. Weight change and adverse event incidence with a low-dose oral contraceptive: two randomized, placebo-controlled trials. Contraception. 2001;63:297-302.
8. O’Connell K, Davis AR, Kerns J. Oral contraceptives: side effects and depression in adolescent girls. Contraception. 2007;75:299-304.
9. Redmond GP, Olson WH, Lippman JS, et al. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo-controlled trial. Obstet Gynecol. 1997;89:615-622.
10. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355-360.
11. Endrikat J, Muller U, Dusterberg B. A twelve-month comparative clinical investigation of two low-dose oral contraceptives containing 20 micrograms ethinylestradiol/75 micrograms gestodene and 30 micrograms ethinylestradiol/75 micrograms gestodene, with respect to efficacy, cycle control, and tolerance. Contraception. 1997;55:131-137.
12. Grimes DA, Lopez LM, O’Brien PA, et al. Progestin-only pills for contraception. Cochrane Database of Systematic Reviews 2010, Issue 1.
13. Van Vliet HAAM, Grimes DA, Helmerhorst FM, et al. Biphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2009, Issue 2.
14. Grimes DA, Lopez LM, Schulz KF et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2009, Issue 2.
15. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
16. Elomaa K, Rolland R, Brosens I, et al. Omitting the first oral contraceptive pills of the cycle does not automatically lead to ovulation. Am J Obstet Gynecol. 1998;179:41-46.
17. Adams Hillard PJ. Oral contraception noncompliance: The extent of the problem. Adv Contracept. 1992;8(suppl 1):13-20.
18. Rosenberg M, Waugh MS. Causes and consequences of oral contraceptive noncompliance. Am J Obstet Gynecol. 1999;180:S276-279.
19. Anderson, FD, Hait, H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
20. Kwiecien M, Edelman A, Nichols MD, et al. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67:9-13.
21. Foidard JM, Sulak PJ, Schellschmidt I, et al. The Yasmin Extended Regimen Study Group. The use of an oral contraceptive containing ethinylestradiol and drospirenone in an extended regimen over 126 days. Contraception. 2006;73:34-40.
22. Shakespeare J, Neve E, Hodder E. Is norethisterone a lifestyle drug? Results of database analysis. BMJ. 2000;320:291.
23. Machado RB, de Melo NR, Maia Jr. H, et al. Effect of a continuous regimen of contraceptive combination of ethinylestradiol and drospirenone on lipid, carbohydrate and coagulation profiles. Contraception. 2010;81:102-106.
24. Edelman A, Gallo MF, Nichols, MD, et al. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Human Reprod. 2006;21:573-578.
25. Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008, Issue 4.
26. Machado RB, Tachotti F, Cavenague G, et al. Effects of two different oral contraceptives on total body water: a randomized study. Contraception. 2006;73:344-347.
27. Oelkers W, Foidart JM, Dombrovicz N, et al. Effects of a new oral contraceptive containing an anti-mineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J Clinical Endo Metabolism. 1995;80:1816-1821.
28. Foidart JM, Wuttke W, Bouw GM, et al. A comparative investigation of contraceptive reliability, cycle control, and tolerance of two monophasic oral contraceptives containing either drospirenone or desogestrel. Eur J Contracept Reprod Health Care. 2000;5:124-134.
29. Hussain, SF. Progestogen-only pills and high blood pressure: is there an association? A literature review. Contraception. 2004;69:89-97.
30. Suthipongse W, Taneepanichskul S. An open-label randomized comparative study of oral contraceptives between medications containing 3 mg drospirenone/30 mcg ethinylestradiol and 150 mcg levonorgestrel/30 mcg ethinylestradiol in Thai women. Contraception. 2004;69:23-26.
31. ACOG practice bulletin. No. 73. Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
32. Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2009, Issue 3.
33. Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262.
34. Rosen, MP, Breitkopf, DM, Nagamani, M. A randomized controlled trial of second- versus third-generation oral contraceptives in the treatment of acne vulgaris. Am J Obstet Gynecol. 2003;188:1158-1160.
35. Huber J, Foidart JM, Wuttke W, et al. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. Eur J Contracept Reprod Health Care. 2000;5:25-34.
36. Sangthawan, M, Taneepanichskul, S. A comparative study of monophasic oral contraceptives containing either drospirenone 3 mg or levonorgestrel 150 mcg on premenstrual symptoms. Contraception. 2005;71:1-77.
37. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database of Systematic Reviews. 2009, Issue 2.
38. Wong CL, Farquhar C, Roberts H, et al. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev. 2009, Issue 4.
39. Endrikat J, Klipping C, Cronin M, et al. An open label, comparative study of the effects of a dose-reduced oral contraceptive containing 20 mcg ethinyl estradiol and 100 mcg levonorgestrel on hemostatic, lipids, and carbohydrate metabolism variables. Contraception. 2002;65:215-221.
40. Brill K, Then A, Beisiegel U, et al. Investigation of the influence of two low-dose monophasic oral contraceptives containing 20 micrograms ethinylestradiol/75 micrograms gestodene and 30 micrograms ethinylestradiol/75 micrograms gestodene, on lipid metabolism in an open randomized trial. Contraception. 1996;54:291-297.
41. Barkfeldt J, Virkkunen A, Dieben T. The effects of two progestogen-only pills containing either desogestrel (75 mcg/day) or levonorgestrel (30 mcg/day) on lipid metabolism. Contraception. 2001; 64:295-299.
42. Knopp RH, Broyles FE, Cheung M, et al. Comparison of the lipoprotein, carbohydrate, and hemostatic effects of phasic oral contraceptives containing desogestrel or levonorgestrel. Contraception. 2001;63:1-11.
43. Foulon T, Payen N, Laporte F, et al., Effects of two low-dose oral contraceptives containing ethinylestradiol and either desogestrel of levonorgestrel on serum lipids and lipoproteins with particular regards to LDL size. Contraception. 2001;64:11-16.
44. Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system: lessons learned and unanswered questions. J Am Coll Cardiol. 2006;47:1741-1753.
45. Martinelli I. Risk factors in venous thromboembolism. Thromb Haemost. 2001;86:395-403.
46. Vessey M, Mant D, Smith A, et al. Oral contraceptives and venous thromboembolism: findings in a large prospective study. BMJ. 1986;292:526.
47. Gerstman BB, Piper TM, Tomita DK, et al. Oral contraceptive estrogen dose and the risk of venous thromboembolic disease. Am J Epidemiol. 1991;133:32-137.
48. Rosendaal FR, Van Hylckama Vlieg A, et al. Estrogens, progestogens and thrombosis. J Thromb Haemost. 2003;1:1371-1380.
49. Farley TM, Meirik O, Collins J. Cardiovascular disease and combined oral contraceptives: reviewing the evidence and balancing risks. Hum Reprod Update. 1999;5:721-735.
50. Vandenbroucke JP, Helmerhorst FM, Bloemenkamp KW, et al. Third generation oral contraceptive and deep venous thrombosis: from epidemiologic controversy to new insight in coagulation. Am J Obstet Gynecol. 1997;177:887-891.
51. Hannaford P. Health consequences of oral combined oral contraceptives. Br Med Bull. 2000;56:749-760.
52. Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. BMJ. 2001;323:131-134.
53. Heinemann LA, Dinger JC, Assmann A, et al. Use of oral contraceptives containing gestodene and risk of venous thromboembolism: outlook 10 years after the third-generation “pill scare”. Contraception. 2010;81:401-407.
54. Paoletti AM, Orru M, Floris S, et al. Evidence that treatment with monophasic oral contraceptive formulations containing ethinylestradiol plus gestodene reduces bone reabsorption in young women. Contraception. 2000;61:259-263.
55. Lopez LM, Grimes DA, Schulz KF, et al. Steroidal contraceptives: effect on bone fractures in women. Cochrane Database Syst Rev. 2009, Issue 2.
56. Schlesselman JJ. Oral contraceptives and neoplasia of the uterine corpus. Contraception. 1991;43:557-580.
57. Hankinson SE, Colditz GA, Hunter DJ, et al. A quantitative assessment of oral contraceptive use and risk of ovarian cancer. Obstet Gynecol. 1992;80:708-714.
58. LaVecchia C, Franceschi S, Decarli A. Oral contraceptive use and the risk of epithelial ovarian cancer. Br J Cancer. 1984;50:31-34.
59. Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95:370-374.
60. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 347:1713-27, 1996
61. World Health Organization. Carcinogenicity of combined hormonal contraceptives and combined menopausal treatment. September 2005. http://www.who.int/reproductivehealth/topics/ageing/cocs_hrt_statement.pdf. Accessed Aug 28, 2013.
A nondrug approach to dementia
› Attempt nonpharmacologic treatment for dementia behavioral problems before moving on to medications, which are of questionable efficacy for symptoms other than aggression and psychosis. A
› Obtain informed consent from patients and/or their caregivers if you plan to use antipsychotic medications because their use increases morbidity and mortality in the elderly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Ms. M, 86 years old, lives with her daughter, son-in-law, and granddaughter. For several years she has been forgetful, but she has never had a formal work-up for dementia. Her daughter finally brings her to their primary care physician because she was refusing to take showers, was increasingly irritable, and had tried to hit her daughter’s husband.
In the office, however, Ms. M is calm and pleasant. The family says that most nights Ms. M gets up and wanders around the house. She denies feeling depressed or anxious, but her Folstein Mini-Mental State Exam score is 22/30, indicating moderate dementia. (For more on assessment, see “Tools for assessing patients with dementia—and their caregivers” on page 552.)
The physician offers a trial of risperidone 0.25 mg at bedtime to assist with sleep and behavior.
Was this prescription a wise decision? What other questions should this physician have asked?
Understanding the behavioral symptoms
Noncognitive symptoms of dementia, sometimes referred to as behavioral and psychological symptoms, are common, affecting almost 90% of patients with dementia,1-3 which itself can be classified as early, intermediate, and late.
In early dementia, sociability is usually not affected, but patients may repeat questions, misplace items, use poor judgment, and begin to have difficulty with more complex daily tasks like finances and driving.
In intermediate dementia, basic activities of daily living become impaired and normal social and environmental cues may not register.
In late dementia, patients become entirely dependent on others; they may lose the ability to speak, walk, and eventually, eat. Long- and short-term memory is lost.
Behavioral symptoms most often occur when the condition enters the intermediate phase, but they may occur at any time during the course of the disease.4 Behaviors may include refusal of care, yelling, aggressive behavior, agitation, restlessness, reversal of the normal sleep-wake cycle, wandering, hoarding, sexual disinhibition, culturally inappropriate behaviors, hallucinations, delusions, anxiety, depression, apathy, and psychosis.2,5
Behavioral disturbances often overwhelm families, and lack of treatment increases patient morbidity, may result in physical harm, and almost always precipitates institutionalization.2 Dementia-related behavioral disturbances also increase the risk of caregiver burnout and depression.2
These symptoms are difficult to treat with medications or nonpharmacologic therapy and strong evidence for most therapies is lacking. Physicians have historically prescribed either typical or atypical antipsychotics in an attempt to control these behaviors. In fact, medication is often still considered first-line therapy.6,7
CASE Ms. M’s daughter calls the clinic 2 weeks after the initial visit to tell the physician that her mother has been sleeping much better, but had a fall and was admitted to the hospital for a hip fracture. That’s not surprising; typical and atypical antipsychotics increase the risk of falls in the elderly.8
The risks associated with the use of antipsychotics
In 2005, the US Food and Drug Administration (FDA) issued a black box warning for atypical antipsychotics because they were found to increase mortality in the elderly. The increased mortality is due to cardiac events or infection.9,10 In 2008, the FDA warning was added to typical antipsychotics, as well.11,12 Both typical and atypical antipsychotics have been found to increase the risk of falls and strokes in the elderly,8,13 and their efficacy in treating the behavioral and psychological symptoms of dementia has recently been questioned.13-16
Trazodone and medications approved for the specific treatment of cognitive decline, such as donepezil or memantine, are also prescribed for behavioral disturbances, but evidence to support their efficacy is limited.14,17-20 More recently, a meta-analysis of selective serotonin reuptake inhibitors (SSRIs) suggests that they may be effective for treating agitation associated with dementia.21 However, SSRIs may also contribute to falls and to hyponatremia in the elderly.22,23
Pharmacologic Tx is not your only option
Considering the questionable safety and efficacy of pharmacologic treatment, physicians should consider nondrug therapies first, or at least concurrently with medication.2,15,16,24
But before you get started, be sure to look for and treat medical conditions that cause or contribute to behavioral disturbances, including infection, pain, and adverse effects of medication.6,7,25 Similarly, it is essential that unmet needs, such as hunger, thirst, or desire for attention or socialization, be addressed.6,7,26 Also, discuss disturbing environmental factors, including loud noises, poorly lit quarters, and strong smells, with patients and their caregivers.5-7 In complex situations, you may need to seek assistance from a geriatrician, neurologist, geropsychiatrist, or psychologist, although their availability may be limited.6
CASE Ms. M becomes markedly delirious while in the hospital after hip surgery, and a geriatrics consultation is requested. This is not surprising, given that underlying dementia increases a patient’s risk of delirium in the hospital.27 The geriatrician recommends several measures to reduce the likelihood of delirium—providing good pain control, minimizing night time wake-ups, minimizing Foley catheter use, Hep-locking the IV to encourage mobility, and having staff reorient her frequently by referring to a large print clock and calendar on the wall.
Specific interventions
Most specific nonpharmacologic therapies have not been robustly studied in randomized controlled trials. But a series of smaller studies have been evaluated in systematic reviews. The level of evidence for each intervention is summarized in TABLE 1.28-40
As you review the options that follow, keep 2 things in mind: (1) It is important to set realistic expectations when considering these approaches (as well as pharmacologic ones). Reducing the frequency or severity of problematic behaviors may be more reasonable than their total elimination.6,25 (2) Consider targeting specific symptoms when treating behavioral Behavioral symptoms most often occur when dementia enters the intermediate phase, but they may occur at any time during the course of the disease. disturbances.2,24,41 Such targeting allows physicians and families to better evaluate the effectiveness of interventions because it helps to focus the discussion of the patient’s progress at follow-up visits.
Massage/touch therapy. A 2006 Cochrane review concluded that improvement in nutritional intake and hand massage, when combined with positive encouragement during a meal, may produce a short-term positive effect on agitation.29 Similarly, a meta-analysis of randomized controlled and randomized crossover studies found a statistically significant improvement in agitation with hand massage, although this finding was based on the same single study referenced in the 2006 review.30 Opinions differ among 5 high-quality guidelines included in the systematic review by Azermai et al regarding the value of massage, with 2 of the 5 practice guidelines recommending its use.28
Aromatherapy. Several trials suggest that aroma therapy may reduce agitated behaviors. Lemon balm and lavender oils have been the most commonly studied agents. Two systematic reviews cite the same 2002 randomized controlled trial, which found a reduction in behavioral problems in people who received arm massage with lemon balm compared with those who received arm massage with an odorless cream.30,31 A systematic review by Holt et al also cites a study that found lavender oil placed in a sachet on each side of the pillow for at least one hour during sleep seemed to reduce problem behaviors.31 Several evidence-based guidelines have concluded that aromatherapy may be helpful, and 2 of the 5 practice guidelines reviewed by Azermai et al recommend it.28
Exercise has been shown to benefit patients of all ages, even those with terminal diseases.42 Some studies have indicated a positive effect of physical activities on behaviors ranging from wandering to aggression and agitation. Activities have included group gentle stretches, indoor exercises, and a volunteer-led walking program that encouraged hand holding and singing.34 However, a 2008 Cochrane review concluded that the effect of exercise on behavioral disturbances in dementia has not been adequately studied.35
Music therapy. Numerous types of music therapy have been studied, including listening to music picked out by a patient’s family based on known patient preference, classical music, pleasant sounds such as ocean waves, and even stories and comforting prayer recorded by family members. While most of these smaller studies yielded positive results,34 a 2003 Cochrane review concluded there is not enough evidence to recommend for or against music therapy.43 A more recent meta-analysis suggests that music may be effective for agitation.30 A systematic review of quality guidelines also indicates that most of these guidelines rate the evidence as moderate to high in favor of music and 3 of 5 practice guidelines recommend it.28
Nonphysical barriers have long been used as a creative nonrestraining method of preventing wandering. They include such tricks as camouflaging exits by painting them to look like bookcases, painting a black square in front of an elevator to make it look like a hole, and placing a thin Velcro strip across doorways. Although it would appear from a limited number of small studies and anecdotal evidence that nonphysical barriers work, a Cochrane review concluded that they have not been studied enough to perform a meta-analysis.36
Cognitive stimulation typically consists of activities such as reviewing current events, promoting sensory awareness, drawing, associating words, discussion of hobbies, and planning daily activities. This type of therapy has been shown to improve cognition in patients with dementia, as well as well-being and quality of life. It does not improve behavioral problems, per se.37
Reminiscence therapy is a popular modality that involves stimulating memories of the past by looking at personal photos and newspaper clippings and discussing the past. It is well received by patients and caregivers. It has been shown to improve mood in elderly patients without dementia, but studies of reminiscence therapy have been too dissimilar to draw conclusions regarding its effect on behavioral disturbances in patients with dementia.38
Other therapies that are common in dementia care, such as respite care and specialized dementia units, have simply not been studied well enough to provide any conclusions as to their effectiveness.39,40
CASE When Ms. M is discharged from the hospital, her family enrolls her in an adult day care program, where Ms. M will be able to participate in social activities, exercise, and communal meals. Her daughter asks the family physician what other steps they can take in the home to make things easier on her mother. And as an aside, the daughter admits that while she is glad that she and her family can “be there” for her mother, there have been times when she has simply not felt up to the task.
Help family members care for the patient—and themselves
A recent meta-analysis suggests that caregiver interventions have a positive effect on behavioral problems in patients with dementia.32 Successful programs are tailored to the individual needs of the patient and caregiver and delivered over multiple sessions. Unfortunately, the aforementioned meta-analysis did not provide evidenced-based interventions for specific problems.32 With this in mind, the following are some practical caregiver “do’s and don’ts” that are based on reviews and consensus guidelines.
Don’t take it personally. It is extremely important to help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder.25 Caregivers also need to appreciate that hallucinations are normal in these patients and do not require medications if they don’t disturb the patient or place the patient or anyone else at risk.
Don’t try to reason with the patient; redirect him or her instead. Clinicians should offer caregivers suggestions for reassuring, redirecting, or distracting agitated patients rather than trying to reason with them. Encourage caregivers to develop and maintain routines and consistency.6,25 Using a calm, low tone of voice, giving very simple instructions, and leaving and then reattempting care that is refused the first time may also be effective.5 Some experts have suggested techniques such as giving positive rewards for desired behaviors and not rewarding negative behaviors.6,26
Do create a safe environment. Recommend that caregivers create a safe environment. Make sure that they lock up all guns. Also, encourage them to use locks, alarms, or ID bracelets when patients are prone to wandering.25
Do consider a caregiver support program. Caregivers can make a big difference in the lives of patients with dementia, Help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder. but only if they have support, as well.
A recent meta-analysis concluded that active involvement of caregivers in making choices about treatments distinguishes effective from ineffective support programs, decreases the odds of institutionalization, and may lengthen time to institutionalization.33 To ease caregiver strain and depression, encourage them to make use of resources such as nursing home respite care and community agencies that include the Alzheimer’s Association (http://www.alz.org).6,44,45
CASE Ms. M’s daughter joins a local support group for families of patients with dementia, where she learns redirection techniques to try when her mother refuses care. The exercise and daytime social stimulation that Ms. M receives through the adult day care program helps her to sleep at night. When Ms. M refuses to take a shower—a challenge the family had before her hospitalization—the daughter does not argue with her. Instead, she returns 10 to 20 minutes later and asks again, or tries a bedside sponge bath with a lavender soap that Ms. M seems to like.
Ms. M’s nighttime wandering is markedly reduced and the family no longer uses any antipsychotic medications. The family physician counsels them, however, about the progressive nature of the disease and encourages them to set up periodic follow-up visits, so that he can see how everyone—patient and caregivers alike—are doing.
Welcoming the reprieves, recognizing the realities
The behavioral and psychological symptoms of dementia are the most challenging aspect of dementia care. Unacceptable behaviors sometimes persist even when aggressively addressing modifiable factors and attempting behavioral interventions (TABLE 2).2,5-7,15,16,24-26,32,41,44-46 Patients with behavioral disturbances frequently require a pharmacologic agent or transfer to a different care setting.
But clinicians need to use psychotropic medications with informed patient and/or caregiver consent.7 On a case-by-case basis, a trial of antipsychotics is often justified, despite the black box warning. A family may choose to try an antipsychotic despite the risk to help manage the patient at home in the hope of delaying or preventing institutionalization.
However, even with good home support, in conjunction with nonpharmacologic and/or pharmacologic therapies, most patients with dementia will eventually require institutionalization.47 Because patients and families often rely on family physicians to guide them through these difficult challenges and decisions, you’ll need to remain well versed on the available On a case-by- case basis, a trial of antipsychotics is often justified, despite the black box warning. treatments for the psychological and behavioral symptoms of dementia, as well as the resources available in your community.
CORRESPONDENCE
Jaqueline Raetz, MD, 331 NE Thornton Place, Seattle, WA 98125; [email protected]
1. Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130-135.
2. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S293-S301.
3. Hort J, O’Brien JT, Gainotti G, et al. EFNS guidelines for the diagnosis and management of Alzheimer’s disease. Eur J Neurol. 2010;17:1236-1248.
4. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475-1483.
5. Omelan C. Approach to managing behavioural disturbances in dementia. Can Fam Physician. 2006;52:191-199.
6. Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350-366.
7. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
8. Hill KD, Wee R. Psychotropic drug-induced falls in older people: a review of interventions aimed at reducing the problem. Drugs Aging. 2012;29:15-30.
9. US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm. Accessed September 16, 2013.
10. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943.
11. US Food and Drug Administration. Antipsychotics, conventional and atypical. June 16, 2008. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm110212.htm. Accessed September 16, 2013.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335-2341.
13. Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476.
14. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596-608.
15. Lonergan E, Luxenberg J, Colford JM. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
16. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
17. Martinón-Torres G, Fioravanti M, Grimley EJ. Trazodone for agitation in dementia. Cochrane Database Syst Rev. 2004;(4):CD004990.
18. Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD001190.
19. McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154.
20. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379-397.
21. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2): CD008191.
22. Sterke CS, Ziere G, van Beeck EF, et al. Dose-response relationship between selective serotonin re-uptake inhibitors and injurious falls: a study in nursing home residents with dementia. Br J Clin Pharmacol. 2012;73:812-820.
23. Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother. 2006;40:1618-1622.
24. Segal-Gidan F, Cherry D, Jones R, et al. Alzheimer’s disease management guideline: update 2008. Alzheimers Dement. 2011;7:e51-e59.
25. Rayner A, O’Brien J, Shoenbachler B. Behavior disorders of dementia: recognition and treatment. Am Fam Physician. 2006;73:647-652.
26. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med. 2006;166:2182-2188.
27. Elie M, Cole MG, Primeau FJ, et al. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13:204-212.
28. Azermai M, Petrovic M, Elseviers MM, et al. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Aging Res Rev. 2012;11:78-86.
29. Viggo Hansen N, Jørgensen T, Ørtenblad L. Massage and touch for dementia. Cochrane Database Syst Rev. 2006;(4):CD004989.
30. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13:512-520.
31. Thorgrimsen LM, Spector A, Wiles A, et al. Aroma therapy for dementia. Cochrane Database Syst Rev. 2003;(3):CD003150.
32. Brodaty H, Arasaratnam C. Review of meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012;169:946-953.
33. Spijker A, Vernooij-Dassen M, Vasse E, et al. Effectiveness of nonpharmacological interventions in delaying the institutionalization of patients with dementia: A meta-analysis. J Am Geriatr Soc. 2008;56:1116-1128.
34. Opie J, Rosewarne R, O’Connor DW. The efficacy of psychosocial approaches to behaviour disorders in dementia: a systematic literature review. Aust N Z J Psychiatry. 1999;33:789-799.
35. Forbes D, Forbes S, Morgan DG, et al. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;(3):CD006489.
36. Price JD, Hermans DG, Grimley Evans J. Subjective barriers to prevent wandering of cognitively impaired people. Cochrane Database Syst Rev. 2000;(4):CD001932.
37. Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;(2):CD005562.
38. Woods B, Spector A, Jones C, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;(2):CD001120.
39. Lee H, Cameron M. Respite care for people with dementia and their carers. Cochrane Database Syst Rev. 2004;(2):CD004396.
40. Lai CK, Yeung JH, Mok V, et al. Special care units for dementia individuals with behavioural problems. Cochrane Database Syst Rev. 2009;(4):CD006470.
41. Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1-e26.
42. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: a phase II study. J Pain Symptom Manage. 2006;31:421-430.
43. Vink AC, Birks JS, Bruinsma MS, et al. Music therapy for people with dementia. Cochrane Database Syst Rev. 2004;(3):CD003477.
44. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020-2029.
45. Bass DM, Clark PA, Looman WJ, et al. The Cleveland Alzheimer’s managed care demonstration: outcomes after 12 months of implementation. Gerontologist. 2003;43:73-85.
46. Gitlin LN, Winter L, Dennis MP, et al.. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010;304:983-991.
47. Smith GE, Kokmen E, O’Brien PC. Risk factors for nursing home placement in a population-based dementia cohort. J Am Geriatr Soc. 2000;48:519-525.
› Attempt nonpharmacologic treatment for dementia behavioral problems before moving on to medications, which are of questionable efficacy for symptoms other than aggression and psychosis. A
› Obtain informed consent from patients and/or their caregivers if you plan to use antipsychotic medications because their use increases morbidity and mortality in the elderly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Ms. M, 86 years old, lives with her daughter, son-in-law, and granddaughter. For several years she has been forgetful, but she has never had a formal work-up for dementia. Her daughter finally brings her to their primary care physician because she was refusing to take showers, was increasingly irritable, and had tried to hit her daughter’s husband.
In the office, however, Ms. M is calm and pleasant. The family says that most nights Ms. M gets up and wanders around the house. She denies feeling depressed or anxious, but her Folstein Mini-Mental State Exam score is 22/30, indicating moderate dementia. (For more on assessment, see “Tools for assessing patients with dementia—and their caregivers” on page 552.)
The physician offers a trial of risperidone 0.25 mg at bedtime to assist with sleep and behavior.
Was this prescription a wise decision? What other questions should this physician have asked?
Understanding the behavioral symptoms
Noncognitive symptoms of dementia, sometimes referred to as behavioral and psychological symptoms, are common, affecting almost 90% of patients with dementia,1-3 which itself can be classified as early, intermediate, and late.
In early dementia, sociability is usually not affected, but patients may repeat questions, misplace items, use poor judgment, and begin to have difficulty with more complex daily tasks like finances and driving.
In intermediate dementia, basic activities of daily living become impaired and normal social and environmental cues may not register.
In late dementia, patients become entirely dependent on others; they may lose the ability to speak, walk, and eventually, eat. Long- and short-term memory is lost.
Behavioral symptoms most often occur when the condition enters the intermediate phase, but they may occur at any time during the course of the disease.4 Behaviors may include refusal of care, yelling, aggressive behavior, agitation, restlessness, reversal of the normal sleep-wake cycle, wandering, hoarding, sexual disinhibition, culturally inappropriate behaviors, hallucinations, delusions, anxiety, depression, apathy, and psychosis.2,5
Behavioral disturbances often overwhelm families, and lack of treatment increases patient morbidity, may result in physical harm, and almost always precipitates institutionalization.2 Dementia-related behavioral disturbances also increase the risk of caregiver burnout and depression.2
These symptoms are difficult to treat with medications or nonpharmacologic therapy and strong evidence for most therapies is lacking. Physicians have historically prescribed either typical or atypical antipsychotics in an attempt to control these behaviors. In fact, medication is often still considered first-line therapy.6,7
CASE Ms. M’s daughter calls the clinic 2 weeks after the initial visit to tell the physician that her mother has been sleeping much better, but had a fall and was admitted to the hospital for a hip fracture. That’s not surprising; typical and atypical antipsychotics increase the risk of falls in the elderly.8
The risks associated with the use of antipsychotics
In 2005, the US Food and Drug Administration (FDA) issued a black box warning for atypical antipsychotics because they were found to increase mortality in the elderly. The increased mortality is due to cardiac events or infection.9,10 In 2008, the FDA warning was added to typical antipsychotics, as well.11,12 Both typical and atypical antipsychotics have been found to increase the risk of falls and strokes in the elderly,8,13 and their efficacy in treating the behavioral and psychological symptoms of dementia has recently been questioned.13-16
Trazodone and medications approved for the specific treatment of cognitive decline, such as donepezil or memantine, are also prescribed for behavioral disturbances, but evidence to support their efficacy is limited.14,17-20 More recently, a meta-analysis of selective serotonin reuptake inhibitors (SSRIs) suggests that they may be effective for treating agitation associated with dementia.21 However, SSRIs may also contribute to falls and to hyponatremia in the elderly.22,23
Pharmacologic Tx is not your only option
Considering the questionable safety and efficacy of pharmacologic treatment, physicians should consider nondrug therapies first, or at least concurrently with medication.2,15,16,24
But before you get started, be sure to look for and treat medical conditions that cause or contribute to behavioral disturbances, including infection, pain, and adverse effects of medication.6,7,25 Similarly, it is essential that unmet needs, such as hunger, thirst, or desire for attention or socialization, be addressed.6,7,26 Also, discuss disturbing environmental factors, including loud noises, poorly lit quarters, and strong smells, with patients and their caregivers.5-7 In complex situations, you may need to seek assistance from a geriatrician, neurologist, geropsychiatrist, or psychologist, although their availability may be limited.6
CASE Ms. M becomes markedly delirious while in the hospital after hip surgery, and a geriatrics consultation is requested. This is not surprising, given that underlying dementia increases a patient’s risk of delirium in the hospital.27 The geriatrician recommends several measures to reduce the likelihood of delirium—providing good pain control, minimizing night time wake-ups, minimizing Foley catheter use, Hep-locking the IV to encourage mobility, and having staff reorient her frequently by referring to a large print clock and calendar on the wall.
Specific interventions
Most specific nonpharmacologic therapies have not been robustly studied in randomized controlled trials. But a series of smaller studies have been evaluated in systematic reviews. The level of evidence for each intervention is summarized in TABLE 1.28-40
As you review the options that follow, keep 2 things in mind: (1) It is important to set realistic expectations when considering these approaches (as well as pharmacologic ones). Reducing the frequency or severity of problematic behaviors may be more reasonable than their total elimination.6,25 (2) Consider targeting specific symptoms when treating behavioral Behavioral symptoms most often occur when dementia enters the intermediate phase, but they may occur at any time during the course of the disease. disturbances.2,24,41 Such targeting allows physicians and families to better evaluate the effectiveness of interventions because it helps to focus the discussion of the patient’s progress at follow-up visits.
Massage/touch therapy. A 2006 Cochrane review concluded that improvement in nutritional intake and hand massage, when combined with positive encouragement during a meal, may produce a short-term positive effect on agitation.29 Similarly, a meta-analysis of randomized controlled and randomized crossover studies found a statistically significant improvement in agitation with hand massage, although this finding was based on the same single study referenced in the 2006 review.30 Opinions differ among 5 high-quality guidelines included in the systematic review by Azermai et al regarding the value of massage, with 2 of the 5 practice guidelines recommending its use.28
Aromatherapy. Several trials suggest that aroma therapy may reduce agitated behaviors. Lemon balm and lavender oils have been the most commonly studied agents. Two systematic reviews cite the same 2002 randomized controlled trial, which found a reduction in behavioral problems in people who received arm massage with lemon balm compared with those who received arm massage with an odorless cream.30,31 A systematic review by Holt et al also cites a study that found lavender oil placed in a sachet on each side of the pillow for at least one hour during sleep seemed to reduce problem behaviors.31 Several evidence-based guidelines have concluded that aromatherapy may be helpful, and 2 of the 5 practice guidelines reviewed by Azermai et al recommend it.28
Exercise has been shown to benefit patients of all ages, even those with terminal diseases.42 Some studies have indicated a positive effect of physical activities on behaviors ranging from wandering to aggression and agitation. Activities have included group gentle stretches, indoor exercises, and a volunteer-led walking program that encouraged hand holding and singing.34 However, a 2008 Cochrane review concluded that the effect of exercise on behavioral disturbances in dementia has not been adequately studied.35
Music therapy. Numerous types of music therapy have been studied, including listening to music picked out by a patient’s family based on known patient preference, classical music, pleasant sounds such as ocean waves, and even stories and comforting prayer recorded by family members. While most of these smaller studies yielded positive results,34 a 2003 Cochrane review concluded there is not enough evidence to recommend for or against music therapy.43 A more recent meta-analysis suggests that music may be effective for agitation.30 A systematic review of quality guidelines also indicates that most of these guidelines rate the evidence as moderate to high in favor of music and 3 of 5 practice guidelines recommend it.28
Nonphysical barriers have long been used as a creative nonrestraining method of preventing wandering. They include such tricks as camouflaging exits by painting them to look like bookcases, painting a black square in front of an elevator to make it look like a hole, and placing a thin Velcro strip across doorways. Although it would appear from a limited number of small studies and anecdotal evidence that nonphysical barriers work, a Cochrane review concluded that they have not been studied enough to perform a meta-analysis.36
Cognitive stimulation typically consists of activities such as reviewing current events, promoting sensory awareness, drawing, associating words, discussion of hobbies, and planning daily activities. This type of therapy has been shown to improve cognition in patients with dementia, as well as well-being and quality of life. It does not improve behavioral problems, per se.37
Reminiscence therapy is a popular modality that involves stimulating memories of the past by looking at personal photos and newspaper clippings and discussing the past. It is well received by patients and caregivers. It has been shown to improve mood in elderly patients without dementia, but studies of reminiscence therapy have been too dissimilar to draw conclusions regarding its effect on behavioral disturbances in patients with dementia.38
Other therapies that are common in dementia care, such as respite care and specialized dementia units, have simply not been studied well enough to provide any conclusions as to their effectiveness.39,40
CASE When Ms. M is discharged from the hospital, her family enrolls her in an adult day care program, where Ms. M will be able to participate in social activities, exercise, and communal meals. Her daughter asks the family physician what other steps they can take in the home to make things easier on her mother. And as an aside, the daughter admits that while she is glad that she and her family can “be there” for her mother, there have been times when she has simply not felt up to the task.
Help family members care for the patient—and themselves
A recent meta-analysis suggests that caregiver interventions have a positive effect on behavioral problems in patients with dementia.32 Successful programs are tailored to the individual needs of the patient and caregiver and delivered over multiple sessions. Unfortunately, the aforementioned meta-analysis did not provide evidenced-based interventions for specific problems.32 With this in mind, the following are some practical caregiver “do’s and don’ts” that are based on reviews and consensus guidelines.
Don’t take it personally. It is extremely important to help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder.25 Caregivers also need to appreciate that hallucinations are normal in these patients and do not require medications if they don’t disturb the patient or place the patient or anyone else at risk.
Don’t try to reason with the patient; redirect him or her instead. Clinicians should offer caregivers suggestions for reassuring, redirecting, or distracting agitated patients rather than trying to reason with them. Encourage caregivers to develop and maintain routines and consistency.6,25 Using a calm, low tone of voice, giving very simple instructions, and leaving and then reattempting care that is refused the first time may also be effective.5 Some experts have suggested techniques such as giving positive rewards for desired behaviors and not rewarding negative behaviors.6,26
Do create a safe environment. Recommend that caregivers create a safe environment. Make sure that they lock up all guns. Also, encourage them to use locks, alarms, or ID bracelets when patients are prone to wandering.25
Do consider a caregiver support program. Caregivers can make a big difference in the lives of patients with dementia, Help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder. but only if they have support, as well.
A recent meta-analysis concluded that active involvement of caregivers in making choices about treatments distinguishes effective from ineffective support programs, decreases the odds of institutionalization, and may lengthen time to institutionalization.33 To ease caregiver strain and depression, encourage them to make use of resources such as nursing home respite care and community agencies that include the Alzheimer’s Association (http://www.alz.org).6,44,45
CASE Ms. M’s daughter joins a local support group for families of patients with dementia, where she learns redirection techniques to try when her mother refuses care. The exercise and daytime social stimulation that Ms. M receives through the adult day care program helps her to sleep at night. When Ms. M refuses to take a shower—a challenge the family had before her hospitalization—the daughter does not argue with her. Instead, she returns 10 to 20 minutes later and asks again, or tries a bedside sponge bath with a lavender soap that Ms. M seems to like.
Ms. M’s nighttime wandering is markedly reduced and the family no longer uses any antipsychotic medications. The family physician counsels them, however, about the progressive nature of the disease and encourages them to set up periodic follow-up visits, so that he can see how everyone—patient and caregivers alike—are doing.
Welcoming the reprieves, recognizing the realities
The behavioral and psychological symptoms of dementia are the most challenging aspect of dementia care. Unacceptable behaviors sometimes persist even when aggressively addressing modifiable factors and attempting behavioral interventions (TABLE 2).2,5-7,15,16,24-26,32,41,44-46 Patients with behavioral disturbances frequently require a pharmacologic agent or transfer to a different care setting.
But clinicians need to use psychotropic medications with informed patient and/or caregiver consent.7 On a case-by-case basis, a trial of antipsychotics is often justified, despite the black box warning. A family may choose to try an antipsychotic despite the risk to help manage the patient at home in the hope of delaying or preventing institutionalization.
However, even with good home support, in conjunction with nonpharmacologic and/or pharmacologic therapies, most patients with dementia will eventually require institutionalization.47 Because patients and families often rely on family physicians to guide them through these difficult challenges and decisions, you’ll need to remain well versed on the available On a case-by- case basis, a trial of antipsychotics is often justified, despite the black box warning. treatments for the psychological and behavioral symptoms of dementia, as well as the resources available in your community.
CORRESPONDENCE
Jaqueline Raetz, MD, 331 NE Thornton Place, Seattle, WA 98125; [email protected]
› Attempt nonpharmacologic treatment for dementia behavioral problems before moving on to medications, which are of questionable efficacy for symptoms other than aggression and psychosis. A
› Obtain informed consent from patients and/or their caregivers if you plan to use antipsychotic medications because their use increases morbidity and mortality in the elderly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Ms. M, 86 years old, lives with her daughter, son-in-law, and granddaughter. For several years she has been forgetful, but she has never had a formal work-up for dementia. Her daughter finally brings her to their primary care physician because she was refusing to take showers, was increasingly irritable, and had tried to hit her daughter’s husband.
In the office, however, Ms. M is calm and pleasant. The family says that most nights Ms. M gets up and wanders around the house. She denies feeling depressed or anxious, but her Folstein Mini-Mental State Exam score is 22/30, indicating moderate dementia. (For more on assessment, see “Tools for assessing patients with dementia—and their caregivers” on page 552.)
The physician offers a trial of risperidone 0.25 mg at bedtime to assist with sleep and behavior.
Was this prescription a wise decision? What other questions should this physician have asked?
Understanding the behavioral symptoms
Noncognitive symptoms of dementia, sometimes referred to as behavioral and psychological symptoms, are common, affecting almost 90% of patients with dementia,1-3 which itself can be classified as early, intermediate, and late.
In early dementia, sociability is usually not affected, but patients may repeat questions, misplace items, use poor judgment, and begin to have difficulty with more complex daily tasks like finances and driving.
In intermediate dementia, basic activities of daily living become impaired and normal social and environmental cues may not register.
In late dementia, patients become entirely dependent on others; they may lose the ability to speak, walk, and eventually, eat. Long- and short-term memory is lost.
Behavioral symptoms most often occur when the condition enters the intermediate phase, but they may occur at any time during the course of the disease.4 Behaviors may include refusal of care, yelling, aggressive behavior, agitation, restlessness, reversal of the normal sleep-wake cycle, wandering, hoarding, sexual disinhibition, culturally inappropriate behaviors, hallucinations, delusions, anxiety, depression, apathy, and psychosis.2,5
Behavioral disturbances often overwhelm families, and lack of treatment increases patient morbidity, may result in physical harm, and almost always precipitates institutionalization.2 Dementia-related behavioral disturbances also increase the risk of caregiver burnout and depression.2
These symptoms are difficult to treat with medications or nonpharmacologic therapy and strong evidence for most therapies is lacking. Physicians have historically prescribed either typical or atypical antipsychotics in an attempt to control these behaviors. In fact, medication is often still considered first-line therapy.6,7
CASE Ms. M’s daughter calls the clinic 2 weeks after the initial visit to tell the physician that her mother has been sleeping much better, but had a fall and was admitted to the hospital for a hip fracture. That’s not surprising; typical and atypical antipsychotics increase the risk of falls in the elderly.8
The risks associated with the use of antipsychotics
In 2005, the US Food and Drug Administration (FDA) issued a black box warning for atypical antipsychotics because they were found to increase mortality in the elderly. The increased mortality is due to cardiac events or infection.9,10 In 2008, the FDA warning was added to typical antipsychotics, as well.11,12 Both typical and atypical antipsychotics have been found to increase the risk of falls and strokes in the elderly,8,13 and their efficacy in treating the behavioral and psychological symptoms of dementia has recently been questioned.13-16
Trazodone and medications approved for the specific treatment of cognitive decline, such as donepezil or memantine, are also prescribed for behavioral disturbances, but evidence to support their efficacy is limited.14,17-20 More recently, a meta-analysis of selective serotonin reuptake inhibitors (SSRIs) suggests that they may be effective for treating agitation associated with dementia.21 However, SSRIs may also contribute to falls and to hyponatremia in the elderly.22,23
Pharmacologic Tx is not your only option
Considering the questionable safety and efficacy of pharmacologic treatment, physicians should consider nondrug therapies first, or at least concurrently with medication.2,15,16,24
But before you get started, be sure to look for and treat medical conditions that cause or contribute to behavioral disturbances, including infection, pain, and adverse effects of medication.6,7,25 Similarly, it is essential that unmet needs, such as hunger, thirst, or desire for attention or socialization, be addressed.6,7,26 Also, discuss disturbing environmental factors, including loud noises, poorly lit quarters, and strong smells, with patients and their caregivers.5-7 In complex situations, you may need to seek assistance from a geriatrician, neurologist, geropsychiatrist, or psychologist, although their availability may be limited.6
CASE Ms. M becomes markedly delirious while in the hospital after hip surgery, and a geriatrics consultation is requested. This is not surprising, given that underlying dementia increases a patient’s risk of delirium in the hospital.27 The geriatrician recommends several measures to reduce the likelihood of delirium—providing good pain control, minimizing night time wake-ups, minimizing Foley catheter use, Hep-locking the IV to encourage mobility, and having staff reorient her frequently by referring to a large print clock and calendar on the wall.
Specific interventions
Most specific nonpharmacologic therapies have not been robustly studied in randomized controlled trials. But a series of smaller studies have been evaluated in systematic reviews. The level of evidence for each intervention is summarized in TABLE 1.28-40
As you review the options that follow, keep 2 things in mind: (1) It is important to set realistic expectations when considering these approaches (as well as pharmacologic ones). Reducing the frequency or severity of problematic behaviors may be more reasonable than their total elimination.6,25 (2) Consider targeting specific symptoms when treating behavioral Behavioral symptoms most often occur when dementia enters the intermediate phase, but they may occur at any time during the course of the disease. disturbances.2,24,41 Such targeting allows physicians and families to better evaluate the effectiveness of interventions because it helps to focus the discussion of the patient’s progress at follow-up visits.
Massage/touch therapy. A 2006 Cochrane review concluded that improvement in nutritional intake and hand massage, when combined with positive encouragement during a meal, may produce a short-term positive effect on agitation.29 Similarly, a meta-analysis of randomized controlled and randomized crossover studies found a statistically significant improvement in agitation with hand massage, although this finding was based on the same single study referenced in the 2006 review.30 Opinions differ among 5 high-quality guidelines included in the systematic review by Azermai et al regarding the value of massage, with 2 of the 5 practice guidelines recommending its use.28
Aromatherapy. Several trials suggest that aroma therapy may reduce agitated behaviors. Lemon balm and lavender oils have been the most commonly studied agents. Two systematic reviews cite the same 2002 randomized controlled trial, which found a reduction in behavioral problems in people who received arm massage with lemon balm compared with those who received arm massage with an odorless cream.30,31 A systematic review by Holt et al also cites a study that found lavender oil placed in a sachet on each side of the pillow for at least one hour during sleep seemed to reduce problem behaviors.31 Several evidence-based guidelines have concluded that aromatherapy may be helpful, and 2 of the 5 practice guidelines reviewed by Azermai et al recommend it.28
Exercise has been shown to benefit patients of all ages, even those with terminal diseases.42 Some studies have indicated a positive effect of physical activities on behaviors ranging from wandering to aggression and agitation. Activities have included group gentle stretches, indoor exercises, and a volunteer-led walking program that encouraged hand holding and singing.34 However, a 2008 Cochrane review concluded that the effect of exercise on behavioral disturbances in dementia has not been adequately studied.35
Music therapy. Numerous types of music therapy have been studied, including listening to music picked out by a patient’s family based on known patient preference, classical music, pleasant sounds such as ocean waves, and even stories and comforting prayer recorded by family members. While most of these smaller studies yielded positive results,34 a 2003 Cochrane review concluded there is not enough evidence to recommend for or against music therapy.43 A more recent meta-analysis suggests that music may be effective for agitation.30 A systematic review of quality guidelines also indicates that most of these guidelines rate the evidence as moderate to high in favor of music and 3 of 5 practice guidelines recommend it.28
Nonphysical barriers have long been used as a creative nonrestraining method of preventing wandering. They include such tricks as camouflaging exits by painting them to look like bookcases, painting a black square in front of an elevator to make it look like a hole, and placing a thin Velcro strip across doorways. Although it would appear from a limited number of small studies and anecdotal evidence that nonphysical barriers work, a Cochrane review concluded that they have not been studied enough to perform a meta-analysis.36
Cognitive stimulation typically consists of activities such as reviewing current events, promoting sensory awareness, drawing, associating words, discussion of hobbies, and planning daily activities. This type of therapy has been shown to improve cognition in patients with dementia, as well as well-being and quality of life. It does not improve behavioral problems, per se.37
Reminiscence therapy is a popular modality that involves stimulating memories of the past by looking at personal photos and newspaper clippings and discussing the past. It is well received by patients and caregivers. It has been shown to improve mood in elderly patients without dementia, but studies of reminiscence therapy have been too dissimilar to draw conclusions regarding its effect on behavioral disturbances in patients with dementia.38
Other therapies that are common in dementia care, such as respite care and specialized dementia units, have simply not been studied well enough to provide any conclusions as to their effectiveness.39,40
CASE When Ms. M is discharged from the hospital, her family enrolls her in an adult day care program, where Ms. M will be able to participate in social activities, exercise, and communal meals. Her daughter asks the family physician what other steps they can take in the home to make things easier on her mother. And as an aside, the daughter admits that while she is glad that she and her family can “be there” for her mother, there have been times when she has simply not felt up to the task.
Help family members care for the patient—and themselves
A recent meta-analysis suggests that caregiver interventions have a positive effect on behavioral problems in patients with dementia.32 Successful programs are tailored to the individual needs of the patient and caregiver and delivered over multiple sessions. Unfortunately, the aforementioned meta-analysis did not provide evidenced-based interventions for specific problems.32 With this in mind, the following are some practical caregiver “do’s and don’ts” that are based on reviews and consensus guidelines.
Don’t take it personally. It is extremely important to help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder.25 Caregivers also need to appreciate that hallucinations are normal in these patients and do not require medications if they don’t disturb the patient or place the patient or anyone else at risk.
Don’t try to reason with the patient; redirect him or her instead. Clinicians should offer caregivers suggestions for reassuring, redirecting, or distracting agitated patients rather than trying to reason with them. Encourage caregivers to develop and maintain routines and consistency.6,25 Using a calm, low tone of voice, giving very simple instructions, and leaving and then reattempting care that is refused the first time may also be effective.5 Some experts have suggested techniques such as giving positive rewards for desired behaviors and not rewarding negative behaviors.6,26
Do create a safe environment. Recommend that caregivers create a safe environment. Make sure that they lock up all guns. Also, encourage them to use locks, alarms, or ID bracelets when patients are prone to wandering.25
Do consider a caregiver support program. Caregivers can make a big difference in the lives of patients with dementia, Help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder. but only if they have support, as well.
A recent meta-analysis concluded that active involvement of caregivers in making choices about treatments distinguishes effective from ineffective support programs, decreases the odds of institutionalization, and may lengthen time to institutionalization.33 To ease caregiver strain and depression, encourage them to make use of resources such as nursing home respite care and community agencies that include the Alzheimer’s Association (http://www.alz.org).6,44,45
CASE Ms. M’s daughter joins a local support group for families of patients with dementia, where she learns redirection techniques to try when her mother refuses care. The exercise and daytime social stimulation that Ms. M receives through the adult day care program helps her to sleep at night. When Ms. M refuses to take a shower—a challenge the family had before her hospitalization—the daughter does not argue with her. Instead, she returns 10 to 20 minutes later and asks again, or tries a bedside sponge bath with a lavender soap that Ms. M seems to like.
Ms. M’s nighttime wandering is markedly reduced and the family no longer uses any antipsychotic medications. The family physician counsels them, however, about the progressive nature of the disease and encourages them to set up periodic follow-up visits, so that he can see how everyone—patient and caregivers alike—are doing.
Welcoming the reprieves, recognizing the realities
The behavioral and psychological symptoms of dementia are the most challenging aspect of dementia care. Unacceptable behaviors sometimes persist even when aggressively addressing modifiable factors and attempting behavioral interventions (TABLE 2).2,5-7,15,16,24-26,32,41,44-46 Patients with behavioral disturbances frequently require a pharmacologic agent or transfer to a different care setting.
But clinicians need to use psychotropic medications with informed patient and/or caregiver consent.7 On a case-by-case basis, a trial of antipsychotics is often justified, despite the black box warning. A family may choose to try an antipsychotic despite the risk to help manage the patient at home in the hope of delaying or preventing institutionalization.
However, even with good home support, in conjunction with nonpharmacologic and/or pharmacologic therapies, most patients with dementia will eventually require institutionalization.47 Because patients and families often rely on family physicians to guide them through these difficult challenges and decisions, you’ll need to remain well versed on the available On a case-by- case basis, a trial of antipsychotics is often justified, despite the black box warning. treatments for the psychological and behavioral symptoms of dementia, as well as the resources available in your community.
CORRESPONDENCE
Jaqueline Raetz, MD, 331 NE Thornton Place, Seattle, WA 98125; [email protected]
1. Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130-135.
2. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S293-S301.
3. Hort J, O’Brien JT, Gainotti G, et al. EFNS guidelines for the diagnosis and management of Alzheimer’s disease. Eur J Neurol. 2010;17:1236-1248.
4. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475-1483.
5. Omelan C. Approach to managing behavioural disturbances in dementia. Can Fam Physician. 2006;52:191-199.
6. Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350-366.
7. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
8. Hill KD, Wee R. Psychotropic drug-induced falls in older people: a review of interventions aimed at reducing the problem. Drugs Aging. 2012;29:15-30.
9. US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm. Accessed September 16, 2013.
10. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943.
11. US Food and Drug Administration. Antipsychotics, conventional and atypical. June 16, 2008. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm110212.htm. Accessed September 16, 2013.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335-2341.
13. Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476.
14. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596-608.
15. Lonergan E, Luxenberg J, Colford JM. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
16. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
17. Martinón-Torres G, Fioravanti M, Grimley EJ. Trazodone for agitation in dementia. Cochrane Database Syst Rev. 2004;(4):CD004990.
18. Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD001190.
19. McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154.
20. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379-397.
21. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2): CD008191.
22. Sterke CS, Ziere G, van Beeck EF, et al. Dose-response relationship between selective serotonin re-uptake inhibitors and injurious falls: a study in nursing home residents with dementia. Br J Clin Pharmacol. 2012;73:812-820.
23. Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother. 2006;40:1618-1622.
24. Segal-Gidan F, Cherry D, Jones R, et al. Alzheimer’s disease management guideline: update 2008. Alzheimers Dement. 2011;7:e51-e59.
25. Rayner A, O’Brien J, Shoenbachler B. Behavior disorders of dementia: recognition and treatment. Am Fam Physician. 2006;73:647-652.
26. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med. 2006;166:2182-2188.
27. Elie M, Cole MG, Primeau FJ, et al. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13:204-212.
28. Azermai M, Petrovic M, Elseviers MM, et al. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Aging Res Rev. 2012;11:78-86.
29. Viggo Hansen N, Jørgensen T, Ørtenblad L. Massage and touch for dementia. Cochrane Database Syst Rev. 2006;(4):CD004989.
30. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13:512-520.
31. Thorgrimsen LM, Spector A, Wiles A, et al. Aroma therapy for dementia. Cochrane Database Syst Rev. 2003;(3):CD003150.
32. Brodaty H, Arasaratnam C. Review of meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012;169:946-953.
33. Spijker A, Vernooij-Dassen M, Vasse E, et al. Effectiveness of nonpharmacological interventions in delaying the institutionalization of patients with dementia: A meta-analysis. J Am Geriatr Soc. 2008;56:1116-1128.
34. Opie J, Rosewarne R, O’Connor DW. The efficacy of psychosocial approaches to behaviour disorders in dementia: a systematic literature review. Aust N Z J Psychiatry. 1999;33:789-799.
35. Forbes D, Forbes S, Morgan DG, et al. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;(3):CD006489.
36. Price JD, Hermans DG, Grimley Evans J. Subjective barriers to prevent wandering of cognitively impaired people. Cochrane Database Syst Rev. 2000;(4):CD001932.
37. Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;(2):CD005562.
38. Woods B, Spector A, Jones C, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;(2):CD001120.
39. Lee H, Cameron M. Respite care for people with dementia and their carers. Cochrane Database Syst Rev. 2004;(2):CD004396.
40. Lai CK, Yeung JH, Mok V, et al. Special care units for dementia individuals with behavioural problems. Cochrane Database Syst Rev. 2009;(4):CD006470.
41. Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1-e26.
42. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: a phase II study. J Pain Symptom Manage. 2006;31:421-430.
43. Vink AC, Birks JS, Bruinsma MS, et al. Music therapy for people with dementia. Cochrane Database Syst Rev. 2004;(3):CD003477.
44. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020-2029.
45. Bass DM, Clark PA, Looman WJ, et al. The Cleveland Alzheimer’s managed care demonstration: outcomes after 12 months of implementation. Gerontologist. 2003;43:73-85.
46. Gitlin LN, Winter L, Dennis MP, et al.. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010;304:983-991.
47. Smith GE, Kokmen E, O’Brien PC. Risk factors for nursing home placement in a population-based dementia cohort. J Am Geriatr Soc. 2000;48:519-525.
1. Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130-135.
2. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S293-S301.
3. Hort J, O’Brien JT, Gainotti G, et al. EFNS guidelines for the diagnosis and management of Alzheimer’s disease. Eur J Neurol. 2010;17:1236-1248.
4. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475-1483.
5. Omelan C. Approach to managing behavioural disturbances in dementia. Can Fam Physician. 2006;52:191-199.
6. Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350-366.
7. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
8. Hill KD, Wee R. Psychotropic drug-induced falls in older people: a review of interventions aimed at reducing the problem. Drugs Aging. 2012;29:15-30.
9. US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm. Accessed September 16, 2013.
10. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943.
11. US Food and Drug Administration. Antipsychotics, conventional and atypical. June 16, 2008. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm110212.htm. Accessed September 16, 2013.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335-2341.
13. Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476.
14. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596-608.
15. Lonergan E, Luxenberg J, Colford JM. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
16. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
17. Martinón-Torres G, Fioravanti M, Grimley EJ. Trazodone for agitation in dementia. Cochrane Database Syst Rev. 2004;(4):CD004990.
18. Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD001190.
19. McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154.
20. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379-397.
21. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2): CD008191.
22. Sterke CS, Ziere G, van Beeck EF, et al. Dose-response relationship between selective serotonin re-uptake inhibitors and injurious falls: a study in nursing home residents with dementia. Br J Clin Pharmacol. 2012;73:812-820.
23. Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother. 2006;40:1618-1622.
24. Segal-Gidan F, Cherry D, Jones R, et al. Alzheimer’s disease management guideline: update 2008. Alzheimers Dement. 2011;7:e51-e59.
25. Rayner A, O’Brien J, Shoenbachler B. Behavior disorders of dementia: recognition and treatment. Am Fam Physician. 2006;73:647-652.
26. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med. 2006;166:2182-2188.
27. Elie M, Cole MG, Primeau FJ, et al. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13:204-212.
28. Azermai M, Petrovic M, Elseviers MM, et al. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Aging Res Rev. 2012;11:78-86.
29. Viggo Hansen N, Jørgensen T, Ørtenblad L. Massage and touch for dementia. Cochrane Database Syst Rev. 2006;(4):CD004989.
30. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13:512-520.
31. Thorgrimsen LM, Spector A, Wiles A, et al. Aroma therapy for dementia. Cochrane Database Syst Rev. 2003;(3):CD003150.
32. Brodaty H, Arasaratnam C. Review of meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012;169:946-953.
33. Spijker A, Vernooij-Dassen M, Vasse E, et al. Effectiveness of nonpharmacological interventions in delaying the institutionalization of patients with dementia: A meta-analysis. J Am Geriatr Soc. 2008;56:1116-1128.
34. Opie J, Rosewarne R, O’Connor DW. The efficacy of psychosocial approaches to behaviour disorders in dementia: a systematic literature review. Aust N Z J Psychiatry. 1999;33:789-799.
35. Forbes D, Forbes S, Morgan DG, et al. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;(3):CD006489.
36. Price JD, Hermans DG, Grimley Evans J. Subjective barriers to prevent wandering of cognitively impaired people. Cochrane Database Syst Rev. 2000;(4):CD001932.
37. Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;(2):CD005562.
38. Woods B, Spector A, Jones C, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;(2):CD001120.
39. Lee H, Cameron M. Respite care for people with dementia and their carers. Cochrane Database Syst Rev. 2004;(2):CD004396.
40. Lai CK, Yeung JH, Mok V, et al. Special care units for dementia individuals with behavioural problems. Cochrane Database Syst Rev. 2009;(4):CD006470.
41. Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1-e26.
42. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: a phase II study. J Pain Symptom Manage. 2006;31:421-430.
43. Vink AC, Birks JS, Bruinsma MS, et al. Music therapy for people with dementia. Cochrane Database Syst Rev. 2004;(3):CD003477.
44. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020-2029.
45. Bass DM, Clark PA, Looman WJ, et al. The Cleveland Alzheimer’s managed care demonstration: outcomes after 12 months of implementation. Gerontologist. 2003;43:73-85.
46. Gitlin LN, Winter L, Dennis MP, et al.. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010;304:983-991.
47. Smith GE, Kokmen E, O’Brien PC. Risk factors for nursing home placement in a population-based dementia cohort. J Am Geriatr Soc. 2000;48:519-525.
When to consider Mohs surgery
› Consider recommending Mohs surgery for cancerous lesions that are long-standing or when there is a high risk of local recurrence or metastasis.
› Consider the procedure for the resection of tumors in cosmetically sensitive areas.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 64-year-old white woman with no personal or family history of skin cancer came to our practice complaining of a lesion on her right cheek (FIGURE 1) that had been present for at least 9 months. The lesion had the appearance of a “rodent bite” ulcer that the patient said bled easily when scratched and occasionally drained clear fluid. She had no other complaints. Biopsy confirmed a nodular, infiltrative basal cell carcinoma (BCC). How would you proceed?
BCC is the most common cutaneous malignancy, with an incidence of more than 1 million cases each year in the United States.1 BCCs occur more commonly in men than in women, usually on the head or neck in both sexes.2,3
Specifying BCC subtype has treatment implications. As the terminology indicates, these lesions arise from the basal cell layer of the epidermis, and they can be further defined histologically as superficial, nodular, micronodular, infiltrating, or other subtypes.
Treatment options for BCCs
Selecting a treatment modality from among the many options depends on a lesion’s subtype and its location. Comorbidity can also influence the decision, favoring nonsurgical intervention if an acute or chronic medical condition or overall health status makes a patient a poor surgical candidate.
Surgical options have the highest clearance rates with the fewest recurrences. Mohs micrographic surgery (MMS) has a cure rate of 99% for primary BCCs and 94% for recurrent lesions.4 Standard excision with appropriate margins yields cure rates of 90% and 83%, respectively.4
Superficial destructive options are typically reserved for superficial BCCs. One example, curettage with electrodessication, cures 92% of primary lesions but just 60% of recurrences.4
Additionally, noninvasive modalities such as cryosurgery, laser ablation, radiotherapy, and photodynamic therapy have varying clearance rates. Topical applications of immune system modulators and chemotherapeutic agents including imiquimod and 5-fluorouracil are also available.5 Target lesions for these modalities may include cancers located on surfaces in which surgical excision would result in unacceptable amounts of tissue loss, such as some periocular BCCs.5
CASE Given our patient’s tumor location (adjacent to the lower eyelid) and its nodular and infiltrating histologic subtype, MMS was the best treatment choice to minimize the chance of recurrence and to achieve an acceptable cosmetic outcome.
A tissue-sparing approach
MMS is a tissue-sparing cutaneous surgical technique first described by Dr. Frederick Mohs in 1941.6 The procedure uses real-time microscopic examination of all removed tissue margins, offering maximal tissue preservation and the highest cure rates of all BCC treatments.7
Compared with other surgical techniques, MMS is unique in that the surgeon also serves as the pathologist and performs reconstruction. After clearing tumor margins of all malignant tissue, the surgeon closes the wound using complex techniques such as tissue flaps and grafts that should be avoided with standard excision due to its inadequate real-time margin control.
When MMS would be the treatment of choice. While MMS may be appropriate for a number of situations, common indications include tumors that are long-standing or have a high risk of local recurrence or metastasis; affected areas where tissue preservation is important such as the face and genitalia; and patients who are immunosuppressed.7
Basics of the technique. To systematically visualize and clear 100% of tumor margins, MMS uses a cyclical process of tumor excision, pathology assessment of specimens with microscopy, and mapping of any remaining positive tissue margins noted.4 These cycles, or stages, are repeated until all excised margins are confirmed cancer free. The ability to establish this outcome with certainty is what permits Mohs surgeons to close surgical wounds with flaps, grafts, and other complex closures.
Advantages of MMS over excision alone. Recurrence rates of BCC after MMS are lower than those seen with excision alone. The 5-year cure rate in the treatment of primary tumors with all non-Mohs modalities combined is 91%, whereas the 5-year cure rate with Mohs surgery is 99%.8 This finding is believed to reflect the difference in the methods used to assess excised specimens histologically. In standard surgical excision, the specimen is examined using the “bread loaf” technique in which the surgical margins are examined in consecutive vertical sections (FIGURE 2).4 Because not all of the surgical margins are directly visualized with this technique, it can increase the rate of false-negative results. In contrast, specimens removed by MMS are examined in horizontal sections, and all surgical margins are directly visualized.9
Aesthetic results are another strong point of MMS. For tumor resection in cosmetically sensitive areas, MMS is the standard of care. The Mohs surgeon is trained to use closures that result in less noticeable scars and minimize distortion of surrounding tissue.
CASE With our patient, we circumscribed the clinical margins of the tumor (FIGURE 3A) before performing Mohs surgery. Two procedural stages were needed to clear all surgical margins, leaving a residual defect (FIGURE 3B). We used a cheek advancement flap to repair the wound (FIGURE 3C).
At 4 months postop, the patient was pleased with the cosmetic result (FIGURE 3D).
CORRESPONDENCE
Matthew Morrissey, MD, Wilford Hall Ambulatory Surgical Center, 2200 Bergquist Drive, Lackland AFB, TX 78236; [email protected]
1. Feldman S, Pearce DJ, Williford PM. Surgical decision making for basal cell carcinoma of the face. Lancet Oncol. 2008;9:1119-1120.
2. Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location, and histopathological subtype. Br J Dermatol. 2002;147:41-47.
3. Harris RB, Griffith K, Moon TE. Trends in the incidence of nonmelanoma skin cancers in southeastern Arizona, 1985-1996. J Am Acad Dermatol. 2001;45:528-536.
4. Snow SN, Mikhail GR. Mohs Micrographic Surgery. 2nd ed. Madison, Wisc: University of Wisconsin Press; 2005.
5. Smith V, Walton S. Treatment of facial basal cell carcinoma: a review. J Skin Cancer. 2011;2011:380371.
6. Mohs RE, Chemosurgery. A microscopically controlled method of cancer excision. Arch Surg. 1941;42:279-295.
7. Drake LA, Dineheart SM, Goltz RW, et al. Guidelines of care for Mohs micrographic surgery. American Academy of Dermatology. J Am Acad Dermatol. 1995;33:271-278.
8. Snow SN, Gunkel J. Mohs surgery. In: Bolongia JI, et al, eds. Dermatology. 3rd ed. Philadelphia, Pa: Saunders; 2012:2445-2457.
9. Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs micrographic surgery for primary and recurrent basal cell carcinoma of the face: a prospective randomized controlled trial with 5 years follow up. Lancet Oncol. 2008;9:1149-1156.
› Consider recommending Mohs surgery for cancerous lesions that are long-standing or when there is a high risk of local recurrence or metastasis.
› Consider the procedure for the resection of tumors in cosmetically sensitive areas.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 64-year-old white woman with no personal or family history of skin cancer came to our practice complaining of a lesion on her right cheek (FIGURE 1) that had been present for at least 9 months. The lesion had the appearance of a “rodent bite” ulcer that the patient said bled easily when scratched and occasionally drained clear fluid. She had no other complaints. Biopsy confirmed a nodular, infiltrative basal cell carcinoma (BCC). How would you proceed?
BCC is the most common cutaneous malignancy, with an incidence of more than 1 million cases each year in the United States.1 BCCs occur more commonly in men than in women, usually on the head or neck in both sexes.2,3
Specifying BCC subtype has treatment implications. As the terminology indicates, these lesions arise from the basal cell layer of the epidermis, and they can be further defined histologically as superficial, nodular, micronodular, infiltrating, or other subtypes.
Treatment options for BCCs
Selecting a treatment modality from among the many options depends on a lesion’s subtype and its location. Comorbidity can also influence the decision, favoring nonsurgical intervention if an acute or chronic medical condition or overall health status makes a patient a poor surgical candidate.
Surgical options have the highest clearance rates with the fewest recurrences. Mohs micrographic surgery (MMS) has a cure rate of 99% for primary BCCs and 94% for recurrent lesions.4 Standard excision with appropriate margins yields cure rates of 90% and 83%, respectively.4
Superficial destructive options are typically reserved for superficial BCCs. One example, curettage with electrodessication, cures 92% of primary lesions but just 60% of recurrences.4
Additionally, noninvasive modalities such as cryosurgery, laser ablation, radiotherapy, and photodynamic therapy have varying clearance rates. Topical applications of immune system modulators and chemotherapeutic agents including imiquimod and 5-fluorouracil are also available.5 Target lesions for these modalities may include cancers located on surfaces in which surgical excision would result in unacceptable amounts of tissue loss, such as some periocular BCCs.5
CASE Given our patient’s tumor location (adjacent to the lower eyelid) and its nodular and infiltrating histologic subtype, MMS was the best treatment choice to minimize the chance of recurrence and to achieve an acceptable cosmetic outcome.
A tissue-sparing approach
MMS is a tissue-sparing cutaneous surgical technique first described by Dr. Frederick Mohs in 1941.6 The procedure uses real-time microscopic examination of all removed tissue margins, offering maximal tissue preservation and the highest cure rates of all BCC treatments.7
Compared with other surgical techniques, MMS is unique in that the surgeon also serves as the pathologist and performs reconstruction. After clearing tumor margins of all malignant tissue, the surgeon closes the wound using complex techniques such as tissue flaps and grafts that should be avoided with standard excision due to its inadequate real-time margin control.
When MMS would be the treatment of choice. While MMS may be appropriate for a number of situations, common indications include tumors that are long-standing or have a high risk of local recurrence or metastasis; affected areas where tissue preservation is important such as the face and genitalia; and patients who are immunosuppressed.7
Basics of the technique. To systematically visualize and clear 100% of tumor margins, MMS uses a cyclical process of tumor excision, pathology assessment of specimens with microscopy, and mapping of any remaining positive tissue margins noted.4 These cycles, or stages, are repeated until all excised margins are confirmed cancer free. The ability to establish this outcome with certainty is what permits Mohs surgeons to close surgical wounds with flaps, grafts, and other complex closures.
Advantages of MMS over excision alone. Recurrence rates of BCC after MMS are lower than those seen with excision alone. The 5-year cure rate in the treatment of primary tumors with all non-Mohs modalities combined is 91%, whereas the 5-year cure rate with Mohs surgery is 99%.8 This finding is believed to reflect the difference in the methods used to assess excised specimens histologically. In standard surgical excision, the specimen is examined using the “bread loaf” technique in which the surgical margins are examined in consecutive vertical sections (FIGURE 2).4 Because not all of the surgical margins are directly visualized with this technique, it can increase the rate of false-negative results. In contrast, specimens removed by MMS are examined in horizontal sections, and all surgical margins are directly visualized.9
Aesthetic results are another strong point of MMS. For tumor resection in cosmetically sensitive areas, MMS is the standard of care. The Mohs surgeon is trained to use closures that result in less noticeable scars and minimize distortion of surrounding tissue.
CASE With our patient, we circumscribed the clinical margins of the tumor (FIGURE 3A) before performing Mohs surgery. Two procedural stages were needed to clear all surgical margins, leaving a residual defect (FIGURE 3B). We used a cheek advancement flap to repair the wound (FIGURE 3C).
At 4 months postop, the patient was pleased with the cosmetic result (FIGURE 3D).
CORRESPONDENCE
Matthew Morrissey, MD, Wilford Hall Ambulatory Surgical Center, 2200 Bergquist Drive, Lackland AFB, TX 78236; [email protected]
› Consider recommending Mohs surgery for cancerous lesions that are long-standing or when there is a high risk of local recurrence or metastasis.
› Consider the procedure for the resection of tumors in cosmetically sensitive areas.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 64-year-old white woman with no personal or family history of skin cancer came to our practice complaining of a lesion on her right cheek (FIGURE 1) that had been present for at least 9 months. The lesion had the appearance of a “rodent bite” ulcer that the patient said bled easily when scratched and occasionally drained clear fluid. She had no other complaints. Biopsy confirmed a nodular, infiltrative basal cell carcinoma (BCC). How would you proceed?
BCC is the most common cutaneous malignancy, with an incidence of more than 1 million cases each year in the United States.1 BCCs occur more commonly in men than in women, usually on the head or neck in both sexes.2,3
Specifying BCC subtype has treatment implications. As the terminology indicates, these lesions arise from the basal cell layer of the epidermis, and they can be further defined histologically as superficial, nodular, micronodular, infiltrating, or other subtypes.
Treatment options for BCCs
Selecting a treatment modality from among the many options depends on a lesion’s subtype and its location. Comorbidity can also influence the decision, favoring nonsurgical intervention if an acute or chronic medical condition or overall health status makes a patient a poor surgical candidate.
Surgical options have the highest clearance rates with the fewest recurrences. Mohs micrographic surgery (MMS) has a cure rate of 99% for primary BCCs and 94% for recurrent lesions.4 Standard excision with appropriate margins yields cure rates of 90% and 83%, respectively.4
Superficial destructive options are typically reserved for superficial BCCs. One example, curettage with electrodessication, cures 92% of primary lesions but just 60% of recurrences.4
Additionally, noninvasive modalities such as cryosurgery, laser ablation, radiotherapy, and photodynamic therapy have varying clearance rates. Topical applications of immune system modulators and chemotherapeutic agents including imiquimod and 5-fluorouracil are also available.5 Target lesions for these modalities may include cancers located on surfaces in which surgical excision would result in unacceptable amounts of tissue loss, such as some periocular BCCs.5
CASE Given our patient’s tumor location (adjacent to the lower eyelid) and its nodular and infiltrating histologic subtype, MMS was the best treatment choice to minimize the chance of recurrence and to achieve an acceptable cosmetic outcome.
A tissue-sparing approach
MMS is a tissue-sparing cutaneous surgical technique first described by Dr. Frederick Mohs in 1941.6 The procedure uses real-time microscopic examination of all removed tissue margins, offering maximal tissue preservation and the highest cure rates of all BCC treatments.7
Compared with other surgical techniques, MMS is unique in that the surgeon also serves as the pathologist and performs reconstruction. After clearing tumor margins of all malignant tissue, the surgeon closes the wound using complex techniques such as tissue flaps and grafts that should be avoided with standard excision due to its inadequate real-time margin control.
When MMS would be the treatment of choice. While MMS may be appropriate for a number of situations, common indications include tumors that are long-standing or have a high risk of local recurrence or metastasis; affected areas where tissue preservation is important such as the face and genitalia; and patients who are immunosuppressed.7
Basics of the technique. To systematically visualize and clear 100% of tumor margins, MMS uses a cyclical process of tumor excision, pathology assessment of specimens with microscopy, and mapping of any remaining positive tissue margins noted.4 These cycles, or stages, are repeated until all excised margins are confirmed cancer free. The ability to establish this outcome with certainty is what permits Mohs surgeons to close surgical wounds with flaps, grafts, and other complex closures.
Advantages of MMS over excision alone. Recurrence rates of BCC after MMS are lower than those seen with excision alone. The 5-year cure rate in the treatment of primary tumors with all non-Mohs modalities combined is 91%, whereas the 5-year cure rate with Mohs surgery is 99%.8 This finding is believed to reflect the difference in the methods used to assess excised specimens histologically. In standard surgical excision, the specimen is examined using the “bread loaf” technique in which the surgical margins are examined in consecutive vertical sections (FIGURE 2).4 Because not all of the surgical margins are directly visualized with this technique, it can increase the rate of false-negative results. In contrast, specimens removed by MMS are examined in horizontal sections, and all surgical margins are directly visualized.9
Aesthetic results are another strong point of MMS. For tumor resection in cosmetically sensitive areas, MMS is the standard of care. The Mohs surgeon is trained to use closures that result in less noticeable scars and minimize distortion of surrounding tissue.
CASE With our patient, we circumscribed the clinical margins of the tumor (FIGURE 3A) before performing Mohs surgery. Two procedural stages were needed to clear all surgical margins, leaving a residual defect (FIGURE 3B). We used a cheek advancement flap to repair the wound (FIGURE 3C).
At 4 months postop, the patient was pleased with the cosmetic result (FIGURE 3D).
CORRESPONDENCE
Matthew Morrissey, MD, Wilford Hall Ambulatory Surgical Center, 2200 Bergquist Drive, Lackland AFB, TX 78236; [email protected]
1. Feldman S, Pearce DJ, Williford PM. Surgical decision making for basal cell carcinoma of the face. Lancet Oncol. 2008;9:1119-1120.
2. Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location, and histopathological subtype. Br J Dermatol. 2002;147:41-47.
3. Harris RB, Griffith K, Moon TE. Trends in the incidence of nonmelanoma skin cancers in southeastern Arizona, 1985-1996. J Am Acad Dermatol. 2001;45:528-536.
4. Snow SN, Mikhail GR. Mohs Micrographic Surgery. 2nd ed. Madison, Wisc: University of Wisconsin Press; 2005.
5. Smith V, Walton S. Treatment of facial basal cell carcinoma: a review. J Skin Cancer. 2011;2011:380371.
6. Mohs RE, Chemosurgery. A microscopically controlled method of cancer excision. Arch Surg. 1941;42:279-295.
7. Drake LA, Dineheart SM, Goltz RW, et al. Guidelines of care for Mohs micrographic surgery. American Academy of Dermatology. J Am Acad Dermatol. 1995;33:271-278.
8. Snow SN, Gunkel J. Mohs surgery. In: Bolongia JI, et al, eds. Dermatology. 3rd ed. Philadelphia, Pa: Saunders; 2012:2445-2457.
9. Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs micrographic surgery for primary and recurrent basal cell carcinoma of the face: a prospective randomized controlled trial with 5 years follow up. Lancet Oncol. 2008;9:1149-1156.
1. Feldman S, Pearce DJ, Williford PM. Surgical decision making for basal cell carcinoma of the face. Lancet Oncol. 2008;9:1119-1120.
2. Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location, and histopathological subtype. Br J Dermatol. 2002;147:41-47.
3. Harris RB, Griffith K, Moon TE. Trends in the incidence of nonmelanoma skin cancers in southeastern Arizona, 1985-1996. J Am Acad Dermatol. 2001;45:528-536.
4. Snow SN, Mikhail GR. Mohs Micrographic Surgery. 2nd ed. Madison, Wisc: University of Wisconsin Press; 2005.
5. Smith V, Walton S. Treatment of facial basal cell carcinoma: a review. J Skin Cancer. 2011;2011:380371.
6. Mohs RE, Chemosurgery. A microscopically controlled method of cancer excision. Arch Surg. 1941;42:279-295.
7. Drake LA, Dineheart SM, Goltz RW, et al. Guidelines of care for Mohs micrographic surgery. American Academy of Dermatology. J Am Acad Dermatol. 1995;33:271-278.
8. Snow SN, Gunkel J. Mohs surgery. In: Bolongia JI, et al, eds. Dermatology. 3rd ed. Philadelphia, Pa: Saunders; 2012:2445-2457.
9. Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs micrographic surgery for primary and recurrent basal cell carcinoma of the face: a prospective randomized controlled trial with 5 years follow up. Lancet Oncol. 2008;9:1149-1156.
Peripheral neuropathy linked to obstructive sleep apnea?
CASE A 57-year-old white woman presented with symptoms of bilateral “stocking-like numbness” and the sensation of “wearing socks for a few weeks” but denied any injury, previous chemotherapy, or diabetes. Her medical history was positive for untreated obstructive sleep apnea (OSA), obesity (body mass index, 36 kg/m2), osteoarthritis in various joints, impaired fasting glucose with normal glycosylated hemoglobin (HbA1c), hypertension, gastroesophageal reflux disease, hypothyroidism, hypercholesterolemia, and osteoporosis.
Our initial examination revealed decreased sensation to light palpation and pin prick over the distal portion of her lower extremities in a stocking-like fashion. Proprioception was decreased at the distal joint of the big toe. Her deep tendon reflex pattern was symmetric with 2+ at the knees, ankles, and toes. The rest of her lower extremity exam was within normal limits and there were no obvious vascular abnormalities.
Given the suspicion of peripheral neuropathy, the patient underwent laboratory tests and a nerve conduction study. Vitamin B12, vitamin B1, methylmalonic acid (MMA), thyroid function, thyroid peroxidase (TPO), serum protein electrophoresis (SPEP), rapid plasma reagin (RPR), sedimentation rate, vitamin D, complete blood count, and chemistry profile 24 were all negative. The antinuclear antibody test revealed a homogenous 1:80 titer with a negative nuclear deoxyribonucleic acid. Her fasting glucose had been elevated between 107 to 117 mg/dL in the last 5 years but HbA1c was normal (5.8%). The patient had not been diagnosed with diabetes and her latest glucose values had been stable.
However, electromyography and a nerve conduction study were abnormal, with electrophysiological evidence of mild axonal polyneuropathy. During the month prior to her presentation, she had developed burning pain in addition to the numbness/stocking sensation. Pregabalin, gabapentin, duloxetine, celecoxib, hydrocodone, methadone, and other medications were ineffective. Eventually the foot pain became so severe—she described it as “walking on tacks”—that she was unable to walk.
Our team decided to do a nerve block to relieve the pain. Initially she underwent right and later left peroneal and posterior tibial nerve blocks, which gave her immediate relief that lasted about 2 months.
Relief from the pain, but what about the OSA symptoms?
In the meantime, our patient developed increasing OSA symptoms, including snoring, nonrestorative sleep, daytime somnolence, and fatigue. (To learn more about OSA, see “Obstructive sleep apnea: A diagnostic and treatment guide” on page 565.)
Her history of mild-to-moderate OSA dated back 2 years, and included an apnea-hypopnea index (AHI) of 20 events per hour and 133 episodes of oxygen desaturation with a low O2 desaturation of 83%. The patient had never been treated, however, because she felt that she couldn’t tolerate the continuous positive airway pressure (CPAP) mask.
The patient finally agreed to a CPAP titration study. Her AHI improved from 20 to <2 events per hour; the oxygen desaturation dropped from 133 to 104 episodes; and the lowest O2 desaturation went from 83% to 85%.
When we initially started CPAP, our patient did not tolerate it very well. However, after consulting with our sleep clinic, she was placed on bilevel positive airway pressure, which she did tolerate. Surprisingly, she also noticed immediate improvement of the neuropathic foot pain; after a few weeks it resolved completely.
Still no foot pain…We continue to follow the patient’s progress and, after 3 years, she remains free of foot pain. Her initial numbness remains, however. She has not developed diabetes, with similar fasting sugar levels and an HbA1c of 5.4%. She is not taking any medication for neuropathic pain, but remains on methadone for unrelated severe intractable osteoarthritic pain of the lumbar spine, bilateral knee joints, and left hip.
The link between sleep apnea and neuropathy
Our case report suggests that clinicians should consider OSA as a cause of neuropathic pain. A recent review of the literature supports the relationship between the 2 conditions.
The prevalence of neuropathy in the general population is 2.4%, rising to 8% with advancing age.1 Many different types of peripheral neuropathy have been described; they have different symptoms and characteristics, depending on the specific part of the nervous system that is affected.2
The literature reveals a strong association between OSA and peripheral neuropathy and sight-threatening retinopathy.3 One study found that nearly 60% of patients with diabetes and OSA also have peripheral neuropathy.4 Another report found that OSA is an independent risk factor for axonal damage of peripheral nerves.5 Furthermore, a case-control study revealed that the impaired neural function is at least partly reversible with treatment for sleep apnea.4 Finally, Tahrani et al6 have found that “neuropathy prevalence was higher in patients with OSA than those without” (60% vs 27%; P<.001), which supports our case finding.
The specific mechanism linking OSA and neuropathy remains elusive, but the evidence suggests that peripheral nervous tissue is affected by chronic endoneural hypoxia in this patient population.7 In patients with OSA, 2 types of nerve dysfunction are apparent: ischemia-related axonal degeneration and resistance to ischemic nerve failure.8
An approach worth considering. While nerve blocks did provide some relief for our patient, they are not a long-term solution. To our knowledge, this case report is the first one published in the United States describing resolution of neuropathic pain by treatment of OSA. This approach is certainly worth considering in patients who have not responded to more traditional therapy.
Correspondence
Fong Wong, DDS, MS, Associate Professor, Department of Restorative Dental Sciences, College of Dentistry, University of Florida, 1395 Center Drive, PO Box 100435, Gainesville, FL 32610; [email protected]
1. Martyn C, Hughes R. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310-318.
2. NINDS peripheral neuropathy information page. Available at: http://www.ninds.nih.gov/disorders/peripheralneuropathy/peripheralneuropathy.htm. Last updated September 19, 2012. Accessed September 16, 2013.
3. Waller E, Bendel R, Kaplan J. Sleep disorders and the eye. Mayo Clin Proc. 2008;83:1251-1261.
4. Dziewas R, Schilling M, Engel P, et al. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatry. 2007;78:295-297.
5. Ludemann P, Dziewas R, Soros P, et al. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatry. 2001;70:685-687.
6. Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434-441.
7. Pfeiffer G, Kunze K, Bruch M, et al. Polyneuropathy associated with chronic hypoxaemia: prevalence in patients with chronic obstructive pulmonary disease. J Neurol. 1990;237:230-233.
8. Mayer P, Dematteis M, Pepin J, et al. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:213-219.
CASE A 57-year-old white woman presented with symptoms of bilateral “stocking-like numbness” and the sensation of “wearing socks for a few weeks” but denied any injury, previous chemotherapy, or diabetes. Her medical history was positive for untreated obstructive sleep apnea (OSA), obesity (body mass index, 36 kg/m2), osteoarthritis in various joints, impaired fasting glucose with normal glycosylated hemoglobin (HbA1c), hypertension, gastroesophageal reflux disease, hypothyroidism, hypercholesterolemia, and osteoporosis.
Our initial examination revealed decreased sensation to light palpation and pin prick over the distal portion of her lower extremities in a stocking-like fashion. Proprioception was decreased at the distal joint of the big toe. Her deep tendon reflex pattern was symmetric with 2+ at the knees, ankles, and toes. The rest of her lower extremity exam was within normal limits and there were no obvious vascular abnormalities.
Given the suspicion of peripheral neuropathy, the patient underwent laboratory tests and a nerve conduction study. Vitamin B12, vitamin B1, methylmalonic acid (MMA), thyroid function, thyroid peroxidase (TPO), serum protein electrophoresis (SPEP), rapid plasma reagin (RPR), sedimentation rate, vitamin D, complete blood count, and chemistry profile 24 were all negative. The antinuclear antibody test revealed a homogenous 1:80 titer with a negative nuclear deoxyribonucleic acid. Her fasting glucose had been elevated between 107 to 117 mg/dL in the last 5 years but HbA1c was normal (5.8%). The patient had not been diagnosed with diabetes and her latest glucose values had been stable.
However, electromyography and a nerve conduction study were abnormal, with electrophysiological evidence of mild axonal polyneuropathy. During the month prior to her presentation, she had developed burning pain in addition to the numbness/stocking sensation. Pregabalin, gabapentin, duloxetine, celecoxib, hydrocodone, methadone, and other medications were ineffective. Eventually the foot pain became so severe—she described it as “walking on tacks”—that she was unable to walk.
Our team decided to do a nerve block to relieve the pain. Initially she underwent right and later left peroneal and posterior tibial nerve blocks, which gave her immediate relief that lasted about 2 months.
Relief from the pain, but what about the OSA symptoms?
In the meantime, our patient developed increasing OSA symptoms, including snoring, nonrestorative sleep, daytime somnolence, and fatigue. (To learn more about OSA, see “Obstructive sleep apnea: A diagnostic and treatment guide” on page 565.)
Her history of mild-to-moderate OSA dated back 2 years, and included an apnea-hypopnea index (AHI) of 20 events per hour and 133 episodes of oxygen desaturation with a low O2 desaturation of 83%. The patient had never been treated, however, because she felt that she couldn’t tolerate the continuous positive airway pressure (CPAP) mask.
The patient finally agreed to a CPAP titration study. Her AHI improved from 20 to <2 events per hour; the oxygen desaturation dropped from 133 to 104 episodes; and the lowest O2 desaturation went from 83% to 85%.
When we initially started CPAP, our patient did not tolerate it very well. However, after consulting with our sleep clinic, she was placed on bilevel positive airway pressure, which she did tolerate. Surprisingly, she also noticed immediate improvement of the neuropathic foot pain; after a few weeks it resolved completely.
Still no foot pain…We continue to follow the patient’s progress and, after 3 years, she remains free of foot pain. Her initial numbness remains, however. She has not developed diabetes, with similar fasting sugar levels and an HbA1c of 5.4%. She is not taking any medication for neuropathic pain, but remains on methadone for unrelated severe intractable osteoarthritic pain of the lumbar spine, bilateral knee joints, and left hip.
The link between sleep apnea and neuropathy
Our case report suggests that clinicians should consider OSA as a cause of neuropathic pain. A recent review of the literature supports the relationship between the 2 conditions.
The prevalence of neuropathy in the general population is 2.4%, rising to 8% with advancing age.1 Many different types of peripheral neuropathy have been described; they have different symptoms and characteristics, depending on the specific part of the nervous system that is affected.2
The literature reveals a strong association between OSA and peripheral neuropathy and sight-threatening retinopathy.3 One study found that nearly 60% of patients with diabetes and OSA also have peripheral neuropathy.4 Another report found that OSA is an independent risk factor for axonal damage of peripheral nerves.5 Furthermore, a case-control study revealed that the impaired neural function is at least partly reversible with treatment for sleep apnea.4 Finally, Tahrani et al6 have found that “neuropathy prevalence was higher in patients with OSA than those without” (60% vs 27%; P<.001), which supports our case finding.
The specific mechanism linking OSA and neuropathy remains elusive, but the evidence suggests that peripheral nervous tissue is affected by chronic endoneural hypoxia in this patient population.7 In patients with OSA, 2 types of nerve dysfunction are apparent: ischemia-related axonal degeneration and resistance to ischemic nerve failure.8
An approach worth considering. While nerve blocks did provide some relief for our patient, they are not a long-term solution. To our knowledge, this case report is the first one published in the United States describing resolution of neuropathic pain by treatment of OSA. This approach is certainly worth considering in patients who have not responded to more traditional therapy.
Correspondence
Fong Wong, DDS, MS, Associate Professor, Department of Restorative Dental Sciences, College of Dentistry, University of Florida, 1395 Center Drive, PO Box 100435, Gainesville, FL 32610; [email protected]
CASE A 57-year-old white woman presented with symptoms of bilateral “stocking-like numbness” and the sensation of “wearing socks for a few weeks” but denied any injury, previous chemotherapy, or diabetes. Her medical history was positive for untreated obstructive sleep apnea (OSA), obesity (body mass index, 36 kg/m2), osteoarthritis in various joints, impaired fasting glucose with normal glycosylated hemoglobin (HbA1c), hypertension, gastroesophageal reflux disease, hypothyroidism, hypercholesterolemia, and osteoporosis.
Our initial examination revealed decreased sensation to light palpation and pin prick over the distal portion of her lower extremities in a stocking-like fashion. Proprioception was decreased at the distal joint of the big toe. Her deep tendon reflex pattern was symmetric with 2+ at the knees, ankles, and toes. The rest of her lower extremity exam was within normal limits and there were no obvious vascular abnormalities.
Given the suspicion of peripheral neuropathy, the patient underwent laboratory tests and a nerve conduction study. Vitamin B12, vitamin B1, methylmalonic acid (MMA), thyroid function, thyroid peroxidase (TPO), serum protein electrophoresis (SPEP), rapid plasma reagin (RPR), sedimentation rate, vitamin D, complete blood count, and chemistry profile 24 were all negative. The antinuclear antibody test revealed a homogenous 1:80 titer with a negative nuclear deoxyribonucleic acid. Her fasting glucose had been elevated between 107 to 117 mg/dL in the last 5 years but HbA1c was normal (5.8%). The patient had not been diagnosed with diabetes and her latest glucose values had been stable.
However, electromyography and a nerve conduction study were abnormal, with electrophysiological evidence of mild axonal polyneuropathy. During the month prior to her presentation, she had developed burning pain in addition to the numbness/stocking sensation. Pregabalin, gabapentin, duloxetine, celecoxib, hydrocodone, methadone, and other medications were ineffective. Eventually the foot pain became so severe—she described it as “walking on tacks”—that she was unable to walk.
Our team decided to do a nerve block to relieve the pain. Initially she underwent right and later left peroneal and posterior tibial nerve blocks, which gave her immediate relief that lasted about 2 months.
Relief from the pain, but what about the OSA symptoms?
In the meantime, our patient developed increasing OSA symptoms, including snoring, nonrestorative sleep, daytime somnolence, and fatigue. (To learn more about OSA, see “Obstructive sleep apnea: A diagnostic and treatment guide” on page 565.)
Her history of mild-to-moderate OSA dated back 2 years, and included an apnea-hypopnea index (AHI) of 20 events per hour and 133 episodes of oxygen desaturation with a low O2 desaturation of 83%. The patient had never been treated, however, because she felt that she couldn’t tolerate the continuous positive airway pressure (CPAP) mask.
The patient finally agreed to a CPAP titration study. Her AHI improved from 20 to <2 events per hour; the oxygen desaturation dropped from 133 to 104 episodes; and the lowest O2 desaturation went from 83% to 85%.
When we initially started CPAP, our patient did not tolerate it very well. However, after consulting with our sleep clinic, she was placed on bilevel positive airway pressure, which she did tolerate. Surprisingly, she also noticed immediate improvement of the neuropathic foot pain; after a few weeks it resolved completely.
Still no foot pain…We continue to follow the patient’s progress and, after 3 years, she remains free of foot pain. Her initial numbness remains, however. She has not developed diabetes, with similar fasting sugar levels and an HbA1c of 5.4%. She is not taking any medication for neuropathic pain, but remains on methadone for unrelated severe intractable osteoarthritic pain of the lumbar spine, bilateral knee joints, and left hip.
The link between sleep apnea and neuropathy
Our case report suggests that clinicians should consider OSA as a cause of neuropathic pain. A recent review of the literature supports the relationship between the 2 conditions.
The prevalence of neuropathy in the general population is 2.4%, rising to 8% with advancing age.1 Many different types of peripheral neuropathy have been described; they have different symptoms and characteristics, depending on the specific part of the nervous system that is affected.2
The literature reveals a strong association between OSA and peripheral neuropathy and sight-threatening retinopathy.3 One study found that nearly 60% of patients with diabetes and OSA also have peripheral neuropathy.4 Another report found that OSA is an independent risk factor for axonal damage of peripheral nerves.5 Furthermore, a case-control study revealed that the impaired neural function is at least partly reversible with treatment for sleep apnea.4 Finally, Tahrani et al6 have found that “neuropathy prevalence was higher in patients with OSA than those without” (60% vs 27%; P<.001), which supports our case finding.
The specific mechanism linking OSA and neuropathy remains elusive, but the evidence suggests that peripheral nervous tissue is affected by chronic endoneural hypoxia in this patient population.7 In patients with OSA, 2 types of nerve dysfunction are apparent: ischemia-related axonal degeneration and resistance to ischemic nerve failure.8
An approach worth considering. While nerve blocks did provide some relief for our patient, they are not a long-term solution. To our knowledge, this case report is the first one published in the United States describing resolution of neuropathic pain by treatment of OSA. This approach is certainly worth considering in patients who have not responded to more traditional therapy.
Correspondence
Fong Wong, DDS, MS, Associate Professor, Department of Restorative Dental Sciences, College of Dentistry, University of Florida, 1395 Center Drive, PO Box 100435, Gainesville, FL 32610; [email protected]
1. Martyn C, Hughes R. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310-318.
2. NINDS peripheral neuropathy information page. Available at: http://www.ninds.nih.gov/disorders/peripheralneuropathy/peripheralneuropathy.htm. Last updated September 19, 2012. Accessed September 16, 2013.
3. Waller E, Bendel R, Kaplan J. Sleep disorders and the eye. Mayo Clin Proc. 2008;83:1251-1261.
4. Dziewas R, Schilling M, Engel P, et al. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatry. 2007;78:295-297.
5. Ludemann P, Dziewas R, Soros P, et al. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatry. 2001;70:685-687.
6. Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434-441.
7. Pfeiffer G, Kunze K, Bruch M, et al. Polyneuropathy associated with chronic hypoxaemia: prevalence in patients with chronic obstructive pulmonary disease. J Neurol. 1990;237:230-233.
8. Mayer P, Dematteis M, Pepin J, et al. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:213-219.
1. Martyn C, Hughes R. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310-318.
2. NINDS peripheral neuropathy information page. Available at: http://www.ninds.nih.gov/disorders/peripheralneuropathy/peripheralneuropathy.htm. Last updated September 19, 2012. Accessed September 16, 2013.
3. Waller E, Bendel R, Kaplan J. Sleep disorders and the eye. Mayo Clin Proc. 2008;83:1251-1261.
4. Dziewas R, Schilling M, Engel P, et al. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatry. 2007;78:295-297.
5. Ludemann P, Dziewas R, Soros P, et al. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatry. 2001;70:685-687.
6. Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434-441.
7. Pfeiffer G, Kunze K, Bruch M, et al. Polyneuropathy associated with chronic hypoxaemia: prevalence in patients with chronic obstructive pulmonary disease. J Neurol. 1990;237:230-233.
8. Mayer P, Dematteis M, Pepin J, et al. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:213-219.
Obstructive sleep apnea: A diagnostic and treatment guide
CASE 1 Mr. M, age 59, has diabetes, hypertension, hyperlipidemia, and obesity, with a body mass index (BMI) of 37 kg/m2. His hyperlipidemia is well controlled with atorvastatin. He also takes hydrochlorothiazide, lisinopril, metformin, and glyburide, but neither his hypertension nor his glucose levels are well controlled. Mr. M does not exercise, and acknowledges eating a high-calorie diet.
Mr. M reports that his wife has begun complaining about his loud snoring, and that he’s been falling asleep at work. He says he has 2 to 3 alcoholic drinks per week, and doesn’t smoke or take any other drugs.
CASE 2 Ms. C, age 35, is seeking treatment for early morning headaches that began several months ago. She describes a constant dull pain over the frontal area that occurs almost every day and typically resolves in 2 to 3 hours. The pain is not affected by postural changes, she says.
She also reports fatigue, daytime sleepiness, and anxiety. Ms. C has a normal BMI (24 kg/m2), takes no medication, and has no prior history of migraine or tension headaches. She denies any alcohol or recreational drug use.
If Mr. M and Ms. C were your patients, what would your next step be?
Obstructive sleep apnea (OSA) is characterized by repeated cycles of partial airway obstruction, hypoxemia, sympathetic discharge, and arousal to restore ventilation. In addition to fragmented sleep and daytime sleepiness, this common sleep disorder is associated with a decreased functional capacity1-3 and an increased risk for accidents, arrhythmias, myocardial infarction (MI), stroke, and death.4-6 In the Wisconsin Sleep Cohort, an 18-year follow-up study, the estimated hazard ratio for patients with severe OSA was 3.0 for all-cause mortality (95% CI; P-trend <.008) and 5.2 for cardiovascular death (95% CI; P=.003).7
The prevalence of OSA in the United States is 3% to 7% for males and 2% to 5% for females, with higher rates among those older than 65 years. But these figures apply only to those with an OSA diagnosis. An additional 5% of the general population is believed to have undiagnosed OSA.4,8
Arriving at an OSA diagnosis may not be difficult with a patient like Mr. M, who is obese and has classic symptoms. But it is important to consider OSA in patients who, like Ms. C, are not overweight and whose chief complaint appears to be unrelated to sleep.
This review—of risk factors, common (and uncommon) symptoms, diagnostic criteria, and treatment for OSA—highlights key indicators and optimal therapies.
The OSA profile: Risk factors and presenting symptoms
Obesity is perhaps the best-known risk factor for OSA: About 70% of patients with OSA are obese, and 40% of obese individuals have OSA. Approximately 60% of those with OSA have metabolic syndrome.9-12 But it is important to remember that individuals with normal or even low BMI may develop OSA, as well.
Other possible contributing factors include alcohol use, smoking, nasal congestion, menopause, polycystic ovary disease, and a genetic predisposition. And OSA may cause or worsen a wide range of conditions, including hypertension, congestive heart failure, atrial fibrillation, stroke, and nocturnal arrhythmias.8,13
Symptoms may be classic—or not
A bed partner’s complaint about a patient’s snoring, typically followed by arousal and gasping for air, is often the first indication of OSA. Loud snoring and daytime sleepiness are the most common symptoms. But not all patients present with these classic symptoms. Some may complain of irritability, difficulty concentrating, daily headaches, and nocturia.
Because the symptoms of OSA are not highly specific, a wide differential diagnosis must be considered, including numerous causes of excessive daytime sleepiness and conditions, such as panic attacks, pulmonary disease, and gastroesophageal reflux disease, which can interfere with sleep (TABLE 1).8,13
What to include in the medical history and work-up
When you suspect OSA or another sleep disorder, the medical history should include a complete review of systems. The social history needs to include information regarding alcohol intake, use of sedatives, and recreational drugs. If possible, interview the patient’s bed partner, as well.
Examine upper airway anatomy. In performing a physical exam, pay special attention to blood pressure, BMI, and upper airway anatomy. Assess for conditions that can cause obstruction, such as nasal congestion, septal deviation, retrognathia (malocclusion of the mouth due to an abnormal posterior position of the maxilla or mandible), macroglossia (an unusually large tongue), dental malocclusion, enlarged lymphoid tissue and tonsils, large uvula, low hanging soft palate, and a large neck circumference (>40 cm for females and >43 cm for males).13
Order blood tests. Routine blood tests do not support an OSA diagnosis, but they help rule out or identify other conditions associated or mimicking OSA, such as anemia, hypothyroidism, diabetes, liver disease, and kidney disease. Suggested labs include a comprehensive metabolic profile, complete blood count, and thyroid-stimulating hormone test.
Sleep studies are needed for a definitive diagnosis
Conducted overnight in a sleep lab, polysomnography (PSG) uses respiratory effort, respiratory air flow, and peripheral oximetry to identify and quantify episodes of apnea and hypopnea (reported as the apnea-hypopnea index, or AHI). PSG also records brain electrical activity (electroencephalogram), heart rhythm (electrocardiogram), eye movement (electro-oculogram), and muscle activation (electromyogram)—studies used to identify other sleep disorders, such as restless leg syndrome, narcolepsy, parasomnias, and disturbances in rapid-eye movement
(REM) sleep.
Home monitoring. For patients who are unable or unwilling to undergo an overnight sleep study, home portable monitoring is a less expensive alternative. The monitor—a small wireless device—provides data for calculating the AHI and the presence and degree of oxygen desaturation.14 Unlike PSG, which can identify the amount of pressure needed for continuous positive airway pressure (CPAP) therapy, findings from a portable monitor are not sufficient to rule out other sleep disorders to determine whether CPAP is required.15
Evaluating daytime sleepiness. Several tools have been used to evaluate daytime sleepiness. The Epworth Sleepiness Scale (ESS), a quick 8-item screening questionnaire, determines the average person’s level of sleepiness during the day. It ranges from 0 to 24 points, with 10 being normal. Although the ESS has been used extensively in OSA research, recent studies found that it has a low sensitivity (54%) and specificity (57%) for scores >10 and does not correlate well with hypopnea and apnea measurements.16,17
Two additional tools, the Berlin and STOP questionnaires, can also be used to screen for OSA. Both questionnaires have about a 50% positive predictive value and a 70% negative predictive value.18,19
Diagnosing and classifying OSA
Diagnostic criteria developed by the American Academy of Sleep Medicine (AASM) are based on reported and observed symptoms and PSG recordings of hypopnea and apneic episodes. Of the 4 criteria (A through D), patients must meet either A, B, and D or C and D (TABLE 2).15
The AASM further classifies OSA as mild, moderate, or severe (TABLE 3)15 based on the AHI as well as on clinical findings, including oxygen desaturation and arrhythmias. Patients with severe OSA have excessive daytime sleepiness (EDS) that interferes with their normal activities, 15 as well as severe oxygen desaturation, moderate to severe cardiac arrhythmias, and significant risk for hypertension, MI, stroke, and cor pulmonale.
CASE 1 An examination of Mr. M’s upper airway anatomy reveals a neck circumference of 44 cm and normal oropharynx. The results of his lab tests were only significant for elevated blood sugar (234 mg/dL) and glycosylated hemoglobin (9.2%). Because he presents with classic symptoms of OSA, he receives a referral for PSG. He is found to have an AHI of 49, consistent with severe sleep apnea.
CASE 2 A system review of Ms. C finds no fever, nausea, vomiting, weakness, vision changes, or neurological symptoms. A Patient Health Questionnaire-9 (depression screen) is normal, as are her lab tests and a brain MRI with and without contrast. After an extensive work-up for headaches finds nothing, OSA is considered, in light of her daytime sleepiness—and she, too, is referred for PSG. This patient has moderate OSA, with an AHI of 27.
Initiating treatment: What’s best?
Ideally, treatment of OSA would reverse EDS and fatigue, restore full cognitive function, reduce the risk of accidents associated with OSA, and minimize its harmful cardiovascular and pulmonary effects. In fact, while OSA can be managed and its effects ameliorated, all available treatments have limitations and a cure remains elusive.
Let patients know that our understanding of OSA is limited, that treatment may not reverse or eliminate all the risks associated with this condition, and that compliance can be challenging. You can also tell them that, while more and better studies are needed, several modalities have been found to successfully treat OSA.
What to expect from lifestyle modification
Recommend lifestyle changes, such as weight loss, regular exercise early in the day, greater emphasis on sleep hygiene (eg, using the bed only for sleeping and sexual activity), and avoidance of sedating drugs and alcohol for patients with OSA.20,21
The beneficial effect of weight loss on OSA has been demonstrated in studies of both bariatric surgery and conventional weight loss therapies.21-23 While early studies of bariatric surgery were often limited by small size, ambiguous classification of OSA, and selection and follow-up biases, more recent trials show that while OSA symptoms frequently improve postoperatively, the disorder typically persists despite significant reductions in both BMI and AHI.24 Weight reduction should be strongly encouraged for obese patients, however, not only to improve OSA symptoms, but also to reduce the risk for other diseases.
Avoidance of alcohol. Alcohol has adverse effects on sleep: It shortens sleep latency, increases slow-wave sleep, suppresses REM and parasympathetic nerve activity,25 and can exacerbate OSA. Driving simulation studies have found that, compared with healthy individuals, those with untreated OSA are more susceptible to the effects of alcohol and at higher risk for accidents after just one drink.26
CPAP improves sleep, but some problems persist
CPAP supplies a flow of positive air pressure, adjusted to the level needed to keep the airway open, delivered through a facial device best suited to the patient’s anatomy, physiology, and comfort.
Multiple studies have demonstrated the effectiveness of CPAP in reducing symptoms of moderate to severe OSA, compared with placebo and other treatment modalities such as oral devices, surgical procedures, and medications. CPAP reduces AHI, blood pressure, and cardiac arrhythmias. It improves sleep efficiency, oxygen saturation, and self-reported sleep and well-being.27-30 While it ameliorates many of the harmful effects of OSA, it does not improve or reverse all of them. (See “Peripheral neuropathy linked to obstructive sleep apnea?”)
A Cochrane review of 36 randomized controlled trials with a combined total of more than 1700 patients demonstrated the superiority of CPAP vs control in several measures, such as subjective daytime sleepiness, quality of life, cognitive function, and blood pressure.28 On specific parameters of OSA, such as snoring and EDS, studies yielded mixed results.
CPAP has been found to decrease work-related injuries and morbidity and mortality associated with motor vehicle accidents linked to EDS.30-32 However, no study of CPAP has demonstrated a long-term reduction in morbidity and mortality. And no standards define the minimum number of sleep hours and/or frequency of CPAP use that is required to obtain specific benefits.
Patient compliance is poor. Part of the problem is that CPAP is difficult to use, which affects compliance. Poor patient compliance is a major barrier to evaluating its long-term benefits. (The video below, "CPAP Patients Tips from the FDA", can help ensure that patients use CPAP safely and effectively.) Studies estimate that 65% to 89% of patients with CPAP devices use them for at least 4 hours a night for 70% of nights, but that about half of those for whom CPAP is prescribed stop using it after 2 to 3 years.33-36 Several risk factors and comorbid conditions, including advanced age, diabetes, obesity, smoking, and especially, depression, are associated with decreased compliance.13
Improving CPAP compliance continues to be a challenge, highlighting the importance of treating not only OSA but all comorbidities, particularly depression. Short-term studies have found behavioral modification to be a promising means of improving CPAP compliance.33-36
Although not a first-line therapy, bilevel positive airway pressure (BiPAP), which delivers both inspiratory and expiratory pressure via a face device, can be tried in patients unable to tolerate CPAP.37 Studies are limited and it has been used in patients with complex OSA.
Oral appliances are a CPAP alternative
Mandibular repositioning devices (MRDs) and tongue-retaining devices are alternatives to CPAP.38 Although both types of oral appliance are beneficial, they are less effective than CPAP.28
MRDs, which are more commonly used than tongue-retaining devices, are available in several models. An MRD can be custom-made to hold the lower jaw in a forward position during sleep, enlarge the space behind the tongue, and put tension on the walls of the pharynx and the palate to reduce collapse.38
Tongue-retaining devices—splints that hold the tongue in place to keep the airway open—can be used for mild to moderate OSA, and for patients unable to tolerate CPAP.38,39
Studies comparing MRDs and tongue-retaining devices found no statistically significant difference in their ability to reduce AHI, but patients tolerated MRDs better.38,39 Nonetheless, both devices can cause dental discomfort, temporomandibular joint pain, dry mouth or excessive salivation, gum irritation, bruxism, and long-term occlusal changes.38
Is surgery an option?
Numerous surgical techniques are available for treating OSA, all aimed at relieving the obstruction by removing or bypassing it or increasing airway size. These include uvulopalatopharyngoplasty, which resects the uvula, retrolingual, and palatine tonsillar tissue; septoplasty; rhinoplasty; nasal turbinate reduction; nasal polypectomy palatal advancement pharyngoplasty; tonsillectomy; adenoidectomy; palatal implants; tongue reduction; genioglossus advancement; and maxillomandibular advancement.
The choice of modality depends on the patient’s anatomy and physiology, and is selected only after a full evaluation by a head and neck surgeon who specializes in surgical treatment of OSA.40,41 There is a paucity of reliable studies on the results of such procedures, but a Cochrane review of the existing literature concluded that surgery is only indicated for severe cases of sleep apnea in patients who have an anatomic obstruction.40
Too little evidence of medications’ efficacy
Numerous drugs have been tested for the treatment of OSA. Two Cochrane reviews looked at multiple trials of more than 20 drugs, in meta-analyses encompassing more than 500 patients.42,43 Ten drugs—eszopiclone, paroxetine, acetazolamide, ondansetron-fluoxetine combination, naltrexone, and fluticasone nasal spray among them—showed a statistically significant reduction in AHI, and a few showed a subjective benefit in daytime sleepiness. However, the studies were very small and of short duration and the reviewers concluded that evidence is insufficient to recommend drug therapy for OSA.
CASE 1 CPAP was prescribed for Mr. M. He tolerated it well and after 2 weeks, he reported feeling refreshed upon awakening, having less daytime somnolence and being better able to concentrate at work. His hypertension and glucose control improved, but he continues to struggle with his weight.
CASE 2 Ms. C started CPAP and within a week, her morning headaches and irritability resolved.
CORRESPONDENCE
Cecilia Gutierrez, MD, 200 West Arbor Drive, Mail Code 8809, San Diego, CA 92103; [email protected]
1. Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112.
2. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199-204.
3. Satela MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
4. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
5. Yaggi HK, Concato J, Kernan W, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
7. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;3:1071-1078.
8. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
9. Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741.
10. Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5-7.
11. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
12. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013-2016.
14. Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-base diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257-263.
15. Iber C, Ancoli-Israel S, Chesson AL Jr, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. Sensitivity and specificity of the multiple sleep maintenance tests, the maintenance of wakefulness tests and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5-11.
17. Hesselbacher S, Subramanian S, Allen J, et al. Body mass index, gender, and ethnic variations alter the clinical implication of the Epworth sleepiness scale in patients with suspected of obstructive sleep apnea. Open Respir Med J. 2012;6:2020-2027.
18. Ahmadi N, Chung S, Gibbs A, et al. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:38-45.
19. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
20. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875.
21. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as treatment for obstructive sleep apnea. Sleep Med Rev. 2000;4:435-452.
22. Buchwald H, Avidor H, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
23. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142-1149.
24. Greenburg A, Lettieri C, Arn E. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542.
25. Sagawa Y, Kondo H, Matsubuchi N. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093-2100.
26. Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447-455.
27. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565-571.
28. Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106.
29. Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070-1076.
30. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430-434.
31. Findley L, Smith C, Hooper J, et al. Treatment with nasal CPAP decreases automobile accidents in patient with sleep apnea. Am J Respir Crit Care Med. 2000;161:857-859.
32. Barbe F, Sunyer J, de la Pena A, et al. Effects of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44-49.
33. Sin DD, Mayers I, Man GC, et al. Long Term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430-435.
34. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265-1270.
35. Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553-560.
36. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea-hypopnea syndrome (SAHS). Sleep Med. 2003;7:81-99.
37. Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath. 2012;16:773-739.
38. Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693-699.
39. Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240-243.
40. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4):CD001004.
41. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
42. Smith I, Lasserson TJ, Wright JJ. Drug therapy for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006;():CD003002. Review.
43. Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5): CD003002.
CASE 1 Mr. M, age 59, has diabetes, hypertension, hyperlipidemia, and obesity, with a body mass index (BMI) of 37 kg/m2. His hyperlipidemia is well controlled with atorvastatin. He also takes hydrochlorothiazide, lisinopril, metformin, and glyburide, but neither his hypertension nor his glucose levels are well controlled. Mr. M does not exercise, and acknowledges eating a high-calorie diet.
Mr. M reports that his wife has begun complaining about his loud snoring, and that he’s been falling asleep at work. He says he has 2 to 3 alcoholic drinks per week, and doesn’t smoke or take any other drugs.
CASE 2 Ms. C, age 35, is seeking treatment for early morning headaches that began several months ago. She describes a constant dull pain over the frontal area that occurs almost every day and typically resolves in 2 to 3 hours. The pain is not affected by postural changes, she says.
She also reports fatigue, daytime sleepiness, and anxiety. Ms. C has a normal BMI (24 kg/m2), takes no medication, and has no prior history of migraine or tension headaches. She denies any alcohol or recreational drug use.
If Mr. M and Ms. C were your patients, what would your next step be?
Obstructive sleep apnea (OSA) is characterized by repeated cycles of partial airway obstruction, hypoxemia, sympathetic discharge, and arousal to restore ventilation. In addition to fragmented sleep and daytime sleepiness, this common sleep disorder is associated with a decreased functional capacity1-3 and an increased risk for accidents, arrhythmias, myocardial infarction (MI), stroke, and death.4-6 In the Wisconsin Sleep Cohort, an 18-year follow-up study, the estimated hazard ratio for patients with severe OSA was 3.0 for all-cause mortality (95% CI; P-trend <.008) and 5.2 for cardiovascular death (95% CI; P=.003).7
The prevalence of OSA in the United States is 3% to 7% for males and 2% to 5% for females, with higher rates among those older than 65 years. But these figures apply only to those with an OSA diagnosis. An additional 5% of the general population is believed to have undiagnosed OSA.4,8
Arriving at an OSA diagnosis may not be difficult with a patient like Mr. M, who is obese and has classic symptoms. But it is important to consider OSA in patients who, like Ms. C, are not overweight and whose chief complaint appears to be unrelated to sleep.
This review—of risk factors, common (and uncommon) symptoms, diagnostic criteria, and treatment for OSA—highlights key indicators and optimal therapies.
The OSA profile: Risk factors and presenting symptoms
Obesity is perhaps the best-known risk factor for OSA: About 70% of patients with OSA are obese, and 40% of obese individuals have OSA. Approximately 60% of those with OSA have metabolic syndrome.9-12 But it is important to remember that individuals with normal or even low BMI may develop OSA, as well.
Other possible contributing factors include alcohol use, smoking, nasal congestion, menopause, polycystic ovary disease, and a genetic predisposition. And OSA may cause or worsen a wide range of conditions, including hypertension, congestive heart failure, atrial fibrillation, stroke, and nocturnal arrhythmias.8,13
Symptoms may be classic—or not
A bed partner’s complaint about a patient’s snoring, typically followed by arousal and gasping for air, is often the first indication of OSA. Loud snoring and daytime sleepiness are the most common symptoms. But not all patients present with these classic symptoms. Some may complain of irritability, difficulty concentrating, daily headaches, and nocturia.
Because the symptoms of OSA are not highly specific, a wide differential diagnosis must be considered, including numerous causes of excessive daytime sleepiness and conditions, such as panic attacks, pulmonary disease, and gastroesophageal reflux disease, which can interfere with sleep (TABLE 1).8,13
What to include in the medical history and work-up
When you suspect OSA or another sleep disorder, the medical history should include a complete review of systems. The social history needs to include information regarding alcohol intake, use of sedatives, and recreational drugs. If possible, interview the patient’s bed partner, as well.
Examine upper airway anatomy. In performing a physical exam, pay special attention to blood pressure, BMI, and upper airway anatomy. Assess for conditions that can cause obstruction, such as nasal congestion, septal deviation, retrognathia (malocclusion of the mouth due to an abnormal posterior position of the maxilla or mandible), macroglossia (an unusually large tongue), dental malocclusion, enlarged lymphoid tissue and tonsils, large uvula, low hanging soft palate, and a large neck circumference (>40 cm for females and >43 cm for males).13
Order blood tests. Routine blood tests do not support an OSA diagnosis, but they help rule out or identify other conditions associated or mimicking OSA, such as anemia, hypothyroidism, diabetes, liver disease, and kidney disease. Suggested labs include a comprehensive metabolic profile, complete blood count, and thyroid-stimulating hormone test.
Sleep studies are needed for a definitive diagnosis
Conducted overnight in a sleep lab, polysomnography (PSG) uses respiratory effort, respiratory air flow, and peripheral oximetry to identify and quantify episodes of apnea and hypopnea (reported as the apnea-hypopnea index, or AHI). PSG also records brain electrical activity (electroencephalogram), heart rhythm (electrocardiogram), eye movement (electro-oculogram), and muscle activation (electromyogram)—studies used to identify other sleep disorders, such as restless leg syndrome, narcolepsy, parasomnias, and disturbances in rapid-eye movement
(REM) sleep.
Home monitoring. For patients who are unable or unwilling to undergo an overnight sleep study, home portable monitoring is a less expensive alternative. The monitor—a small wireless device—provides data for calculating the AHI and the presence and degree of oxygen desaturation.14 Unlike PSG, which can identify the amount of pressure needed for continuous positive airway pressure (CPAP) therapy, findings from a portable monitor are not sufficient to rule out other sleep disorders to determine whether CPAP is required.15
Evaluating daytime sleepiness. Several tools have been used to evaluate daytime sleepiness. The Epworth Sleepiness Scale (ESS), a quick 8-item screening questionnaire, determines the average person’s level of sleepiness during the day. It ranges from 0 to 24 points, with 10 being normal. Although the ESS has been used extensively in OSA research, recent studies found that it has a low sensitivity (54%) and specificity (57%) for scores >10 and does not correlate well with hypopnea and apnea measurements.16,17
Two additional tools, the Berlin and STOP questionnaires, can also be used to screen for OSA. Both questionnaires have about a 50% positive predictive value and a 70% negative predictive value.18,19
Diagnosing and classifying OSA
Diagnostic criteria developed by the American Academy of Sleep Medicine (AASM) are based on reported and observed symptoms and PSG recordings of hypopnea and apneic episodes. Of the 4 criteria (A through D), patients must meet either A, B, and D or C and D (TABLE 2).15
The AASM further classifies OSA as mild, moderate, or severe (TABLE 3)15 based on the AHI as well as on clinical findings, including oxygen desaturation and arrhythmias. Patients with severe OSA have excessive daytime sleepiness (EDS) that interferes with their normal activities, 15 as well as severe oxygen desaturation, moderate to severe cardiac arrhythmias, and significant risk for hypertension, MI, stroke, and cor pulmonale.
CASE 1 An examination of Mr. M’s upper airway anatomy reveals a neck circumference of 44 cm and normal oropharynx. The results of his lab tests were only significant for elevated blood sugar (234 mg/dL) and glycosylated hemoglobin (9.2%). Because he presents with classic symptoms of OSA, he receives a referral for PSG. He is found to have an AHI of 49, consistent with severe sleep apnea.
CASE 2 A system review of Ms. C finds no fever, nausea, vomiting, weakness, vision changes, or neurological symptoms. A Patient Health Questionnaire-9 (depression screen) is normal, as are her lab tests and a brain MRI with and without contrast. After an extensive work-up for headaches finds nothing, OSA is considered, in light of her daytime sleepiness—and she, too, is referred for PSG. This patient has moderate OSA, with an AHI of 27.
Initiating treatment: What’s best?
Ideally, treatment of OSA would reverse EDS and fatigue, restore full cognitive function, reduce the risk of accidents associated with OSA, and minimize its harmful cardiovascular and pulmonary effects. In fact, while OSA can be managed and its effects ameliorated, all available treatments have limitations and a cure remains elusive.
Let patients know that our understanding of OSA is limited, that treatment may not reverse or eliminate all the risks associated with this condition, and that compliance can be challenging. You can also tell them that, while more and better studies are needed, several modalities have been found to successfully treat OSA.
What to expect from lifestyle modification
Recommend lifestyle changes, such as weight loss, regular exercise early in the day, greater emphasis on sleep hygiene (eg, using the bed only for sleeping and sexual activity), and avoidance of sedating drugs and alcohol for patients with OSA.20,21
The beneficial effect of weight loss on OSA has been demonstrated in studies of both bariatric surgery and conventional weight loss therapies.21-23 While early studies of bariatric surgery were often limited by small size, ambiguous classification of OSA, and selection and follow-up biases, more recent trials show that while OSA symptoms frequently improve postoperatively, the disorder typically persists despite significant reductions in both BMI and AHI.24 Weight reduction should be strongly encouraged for obese patients, however, not only to improve OSA symptoms, but also to reduce the risk for other diseases.
Avoidance of alcohol. Alcohol has adverse effects on sleep: It shortens sleep latency, increases slow-wave sleep, suppresses REM and parasympathetic nerve activity,25 and can exacerbate OSA. Driving simulation studies have found that, compared with healthy individuals, those with untreated OSA are more susceptible to the effects of alcohol and at higher risk for accidents after just one drink.26
CPAP improves sleep, but some problems persist
CPAP supplies a flow of positive air pressure, adjusted to the level needed to keep the airway open, delivered through a facial device best suited to the patient’s anatomy, physiology, and comfort.
Multiple studies have demonstrated the effectiveness of CPAP in reducing symptoms of moderate to severe OSA, compared with placebo and other treatment modalities such as oral devices, surgical procedures, and medications. CPAP reduces AHI, blood pressure, and cardiac arrhythmias. It improves sleep efficiency, oxygen saturation, and self-reported sleep and well-being.27-30 While it ameliorates many of the harmful effects of OSA, it does not improve or reverse all of them. (See “Peripheral neuropathy linked to obstructive sleep apnea?”)
A Cochrane review of 36 randomized controlled trials with a combined total of more than 1700 patients demonstrated the superiority of CPAP vs control in several measures, such as subjective daytime sleepiness, quality of life, cognitive function, and blood pressure.28 On specific parameters of OSA, such as snoring and EDS, studies yielded mixed results.
CPAP has been found to decrease work-related injuries and morbidity and mortality associated with motor vehicle accidents linked to EDS.30-32 However, no study of CPAP has demonstrated a long-term reduction in morbidity and mortality. And no standards define the minimum number of sleep hours and/or frequency of CPAP use that is required to obtain specific benefits.
Patient compliance is poor. Part of the problem is that CPAP is difficult to use, which affects compliance. Poor patient compliance is a major barrier to evaluating its long-term benefits. (The video below, "CPAP Patients Tips from the FDA", can help ensure that patients use CPAP safely and effectively.) Studies estimate that 65% to 89% of patients with CPAP devices use them for at least 4 hours a night for 70% of nights, but that about half of those for whom CPAP is prescribed stop using it after 2 to 3 years.33-36 Several risk factors and comorbid conditions, including advanced age, diabetes, obesity, smoking, and especially, depression, are associated with decreased compliance.13
Improving CPAP compliance continues to be a challenge, highlighting the importance of treating not only OSA but all comorbidities, particularly depression. Short-term studies have found behavioral modification to be a promising means of improving CPAP compliance.33-36
Although not a first-line therapy, bilevel positive airway pressure (BiPAP), which delivers both inspiratory and expiratory pressure via a face device, can be tried in patients unable to tolerate CPAP.37 Studies are limited and it has been used in patients with complex OSA.
Oral appliances are a CPAP alternative
Mandibular repositioning devices (MRDs) and tongue-retaining devices are alternatives to CPAP.38 Although both types of oral appliance are beneficial, they are less effective than CPAP.28
MRDs, which are more commonly used than tongue-retaining devices, are available in several models. An MRD can be custom-made to hold the lower jaw in a forward position during sleep, enlarge the space behind the tongue, and put tension on the walls of the pharynx and the palate to reduce collapse.38
Tongue-retaining devices—splints that hold the tongue in place to keep the airway open—can be used for mild to moderate OSA, and for patients unable to tolerate CPAP.38,39
Studies comparing MRDs and tongue-retaining devices found no statistically significant difference in their ability to reduce AHI, but patients tolerated MRDs better.38,39 Nonetheless, both devices can cause dental discomfort, temporomandibular joint pain, dry mouth or excessive salivation, gum irritation, bruxism, and long-term occlusal changes.38
Is surgery an option?
Numerous surgical techniques are available for treating OSA, all aimed at relieving the obstruction by removing or bypassing it or increasing airway size. These include uvulopalatopharyngoplasty, which resects the uvula, retrolingual, and palatine tonsillar tissue; septoplasty; rhinoplasty; nasal turbinate reduction; nasal polypectomy palatal advancement pharyngoplasty; tonsillectomy; adenoidectomy; palatal implants; tongue reduction; genioglossus advancement; and maxillomandibular advancement.
The choice of modality depends on the patient’s anatomy and physiology, and is selected only after a full evaluation by a head and neck surgeon who specializes in surgical treatment of OSA.40,41 There is a paucity of reliable studies on the results of such procedures, but a Cochrane review of the existing literature concluded that surgery is only indicated for severe cases of sleep apnea in patients who have an anatomic obstruction.40
Too little evidence of medications’ efficacy
Numerous drugs have been tested for the treatment of OSA. Two Cochrane reviews looked at multiple trials of more than 20 drugs, in meta-analyses encompassing more than 500 patients.42,43 Ten drugs—eszopiclone, paroxetine, acetazolamide, ondansetron-fluoxetine combination, naltrexone, and fluticasone nasal spray among them—showed a statistically significant reduction in AHI, and a few showed a subjective benefit in daytime sleepiness. However, the studies were very small and of short duration and the reviewers concluded that evidence is insufficient to recommend drug therapy for OSA.
CASE 1 CPAP was prescribed for Mr. M. He tolerated it well and after 2 weeks, he reported feeling refreshed upon awakening, having less daytime somnolence and being better able to concentrate at work. His hypertension and glucose control improved, but he continues to struggle with his weight.
CASE 2 Ms. C started CPAP and within a week, her morning headaches and irritability resolved.
CORRESPONDENCE
Cecilia Gutierrez, MD, 200 West Arbor Drive, Mail Code 8809, San Diego, CA 92103; [email protected]
CASE 1 Mr. M, age 59, has diabetes, hypertension, hyperlipidemia, and obesity, with a body mass index (BMI) of 37 kg/m2. His hyperlipidemia is well controlled with atorvastatin. He also takes hydrochlorothiazide, lisinopril, metformin, and glyburide, but neither his hypertension nor his glucose levels are well controlled. Mr. M does not exercise, and acknowledges eating a high-calorie diet.
Mr. M reports that his wife has begun complaining about his loud snoring, and that he’s been falling asleep at work. He says he has 2 to 3 alcoholic drinks per week, and doesn’t smoke or take any other drugs.
CASE 2 Ms. C, age 35, is seeking treatment for early morning headaches that began several months ago. She describes a constant dull pain over the frontal area that occurs almost every day and typically resolves in 2 to 3 hours. The pain is not affected by postural changes, she says.
She also reports fatigue, daytime sleepiness, and anxiety. Ms. C has a normal BMI (24 kg/m2), takes no medication, and has no prior history of migraine or tension headaches. She denies any alcohol or recreational drug use.
If Mr. M and Ms. C were your patients, what would your next step be?
Obstructive sleep apnea (OSA) is characterized by repeated cycles of partial airway obstruction, hypoxemia, sympathetic discharge, and arousal to restore ventilation. In addition to fragmented sleep and daytime sleepiness, this common sleep disorder is associated with a decreased functional capacity1-3 and an increased risk for accidents, arrhythmias, myocardial infarction (MI), stroke, and death.4-6 In the Wisconsin Sleep Cohort, an 18-year follow-up study, the estimated hazard ratio for patients with severe OSA was 3.0 for all-cause mortality (95% CI; P-trend <.008) and 5.2 for cardiovascular death (95% CI; P=.003).7
The prevalence of OSA in the United States is 3% to 7% for males and 2% to 5% for females, with higher rates among those older than 65 years. But these figures apply only to those with an OSA diagnosis. An additional 5% of the general population is believed to have undiagnosed OSA.4,8
Arriving at an OSA diagnosis may not be difficult with a patient like Mr. M, who is obese and has classic symptoms. But it is important to consider OSA in patients who, like Ms. C, are not overweight and whose chief complaint appears to be unrelated to sleep.
This review—of risk factors, common (and uncommon) symptoms, diagnostic criteria, and treatment for OSA—highlights key indicators and optimal therapies.
The OSA profile: Risk factors and presenting symptoms
Obesity is perhaps the best-known risk factor for OSA: About 70% of patients with OSA are obese, and 40% of obese individuals have OSA. Approximately 60% of those with OSA have metabolic syndrome.9-12 But it is important to remember that individuals with normal or even low BMI may develop OSA, as well.
Other possible contributing factors include alcohol use, smoking, nasal congestion, menopause, polycystic ovary disease, and a genetic predisposition. And OSA may cause or worsen a wide range of conditions, including hypertension, congestive heart failure, atrial fibrillation, stroke, and nocturnal arrhythmias.8,13
Symptoms may be classic—or not
A bed partner’s complaint about a patient’s snoring, typically followed by arousal and gasping for air, is often the first indication of OSA. Loud snoring and daytime sleepiness are the most common symptoms. But not all patients present with these classic symptoms. Some may complain of irritability, difficulty concentrating, daily headaches, and nocturia.
Because the symptoms of OSA are not highly specific, a wide differential diagnosis must be considered, including numerous causes of excessive daytime sleepiness and conditions, such as panic attacks, pulmonary disease, and gastroesophageal reflux disease, which can interfere with sleep (TABLE 1).8,13
What to include in the medical history and work-up
When you suspect OSA or another sleep disorder, the medical history should include a complete review of systems. The social history needs to include information regarding alcohol intake, use of sedatives, and recreational drugs. If possible, interview the patient’s bed partner, as well.
Examine upper airway anatomy. In performing a physical exam, pay special attention to blood pressure, BMI, and upper airway anatomy. Assess for conditions that can cause obstruction, such as nasal congestion, septal deviation, retrognathia (malocclusion of the mouth due to an abnormal posterior position of the maxilla or mandible), macroglossia (an unusually large tongue), dental malocclusion, enlarged lymphoid tissue and tonsils, large uvula, low hanging soft palate, and a large neck circumference (>40 cm for females and >43 cm for males).13
Order blood tests. Routine blood tests do not support an OSA diagnosis, but they help rule out or identify other conditions associated or mimicking OSA, such as anemia, hypothyroidism, diabetes, liver disease, and kidney disease. Suggested labs include a comprehensive metabolic profile, complete blood count, and thyroid-stimulating hormone test.
Sleep studies are needed for a definitive diagnosis
Conducted overnight in a sleep lab, polysomnography (PSG) uses respiratory effort, respiratory air flow, and peripheral oximetry to identify and quantify episodes of apnea and hypopnea (reported as the apnea-hypopnea index, or AHI). PSG also records brain electrical activity (electroencephalogram), heart rhythm (electrocardiogram), eye movement (electro-oculogram), and muscle activation (electromyogram)—studies used to identify other sleep disorders, such as restless leg syndrome, narcolepsy, parasomnias, and disturbances in rapid-eye movement
(REM) sleep.
Home monitoring. For patients who are unable or unwilling to undergo an overnight sleep study, home portable monitoring is a less expensive alternative. The monitor—a small wireless device—provides data for calculating the AHI and the presence and degree of oxygen desaturation.14 Unlike PSG, which can identify the amount of pressure needed for continuous positive airway pressure (CPAP) therapy, findings from a portable monitor are not sufficient to rule out other sleep disorders to determine whether CPAP is required.15
Evaluating daytime sleepiness. Several tools have been used to evaluate daytime sleepiness. The Epworth Sleepiness Scale (ESS), a quick 8-item screening questionnaire, determines the average person’s level of sleepiness during the day. It ranges from 0 to 24 points, with 10 being normal. Although the ESS has been used extensively in OSA research, recent studies found that it has a low sensitivity (54%) and specificity (57%) for scores >10 and does not correlate well with hypopnea and apnea measurements.16,17
Two additional tools, the Berlin and STOP questionnaires, can also be used to screen for OSA. Both questionnaires have about a 50% positive predictive value and a 70% negative predictive value.18,19
Diagnosing and classifying OSA
Diagnostic criteria developed by the American Academy of Sleep Medicine (AASM) are based on reported and observed symptoms and PSG recordings of hypopnea and apneic episodes. Of the 4 criteria (A through D), patients must meet either A, B, and D or C and D (TABLE 2).15
The AASM further classifies OSA as mild, moderate, or severe (TABLE 3)15 based on the AHI as well as on clinical findings, including oxygen desaturation and arrhythmias. Patients with severe OSA have excessive daytime sleepiness (EDS) that interferes with their normal activities, 15 as well as severe oxygen desaturation, moderate to severe cardiac arrhythmias, and significant risk for hypertension, MI, stroke, and cor pulmonale.
CASE 1 An examination of Mr. M’s upper airway anatomy reveals a neck circumference of 44 cm and normal oropharynx. The results of his lab tests were only significant for elevated blood sugar (234 mg/dL) and glycosylated hemoglobin (9.2%). Because he presents with classic symptoms of OSA, he receives a referral for PSG. He is found to have an AHI of 49, consistent with severe sleep apnea.
CASE 2 A system review of Ms. C finds no fever, nausea, vomiting, weakness, vision changes, or neurological symptoms. A Patient Health Questionnaire-9 (depression screen) is normal, as are her lab tests and a brain MRI with and without contrast. After an extensive work-up for headaches finds nothing, OSA is considered, in light of her daytime sleepiness—and she, too, is referred for PSG. This patient has moderate OSA, with an AHI of 27.
Initiating treatment: What’s best?
Ideally, treatment of OSA would reverse EDS and fatigue, restore full cognitive function, reduce the risk of accidents associated with OSA, and minimize its harmful cardiovascular and pulmonary effects. In fact, while OSA can be managed and its effects ameliorated, all available treatments have limitations and a cure remains elusive.
Let patients know that our understanding of OSA is limited, that treatment may not reverse or eliminate all the risks associated with this condition, and that compliance can be challenging. You can also tell them that, while more and better studies are needed, several modalities have been found to successfully treat OSA.
What to expect from lifestyle modification
Recommend lifestyle changes, such as weight loss, regular exercise early in the day, greater emphasis on sleep hygiene (eg, using the bed only for sleeping and sexual activity), and avoidance of sedating drugs and alcohol for patients with OSA.20,21
The beneficial effect of weight loss on OSA has been demonstrated in studies of both bariatric surgery and conventional weight loss therapies.21-23 While early studies of bariatric surgery were often limited by small size, ambiguous classification of OSA, and selection and follow-up biases, more recent trials show that while OSA symptoms frequently improve postoperatively, the disorder typically persists despite significant reductions in both BMI and AHI.24 Weight reduction should be strongly encouraged for obese patients, however, not only to improve OSA symptoms, but also to reduce the risk for other diseases.
Avoidance of alcohol. Alcohol has adverse effects on sleep: It shortens sleep latency, increases slow-wave sleep, suppresses REM and parasympathetic nerve activity,25 and can exacerbate OSA. Driving simulation studies have found that, compared with healthy individuals, those with untreated OSA are more susceptible to the effects of alcohol and at higher risk for accidents after just one drink.26
CPAP improves sleep, but some problems persist
CPAP supplies a flow of positive air pressure, adjusted to the level needed to keep the airway open, delivered through a facial device best suited to the patient’s anatomy, physiology, and comfort.
Multiple studies have demonstrated the effectiveness of CPAP in reducing symptoms of moderate to severe OSA, compared with placebo and other treatment modalities such as oral devices, surgical procedures, and medications. CPAP reduces AHI, blood pressure, and cardiac arrhythmias. It improves sleep efficiency, oxygen saturation, and self-reported sleep and well-being.27-30 While it ameliorates many of the harmful effects of OSA, it does not improve or reverse all of them. (See “Peripheral neuropathy linked to obstructive sleep apnea?”)
A Cochrane review of 36 randomized controlled trials with a combined total of more than 1700 patients demonstrated the superiority of CPAP vs control in several measures, such as subjective daytime sleepiness, quality of life, cognitive function, and blood pressure.28 On specific parameters of OSA, such as snoring and EDS, studies yielded mixed results.
CPAP has been found to decrease work-related injuries and morbidity and mortality associated with motor vehicle accidents linked to EDS.30-32 However, no study of CPAP has demonstrated a long-term reduction in morbidity and mortality. And no standards define the minimum number of sleep hours and/or frequency of CPAP use that is required to obtain specific benefits.
Patient compliance is poor. Part of the problem is that CPAP is difficult to use, which affects compliance. Poor patient compliance is a major barrier to evaluating its long-term benefits. (The video below, "CPAP Patients Tips from the FDA", can help ensure that patients use CPAP safely and effectively.) Studies estimate that 65% to 89% of patients with CPAP devices use them for at least 4 hours a night for 70% of nights, but that about half of those for whom CPAP is prescribed stop using it after 2 to 3 years.33-36 Several risk factors and comorbid conditions, including advanced age, diabetes, obesity, smoking, and especially, depression, are associated with decreased compliance.13
Improving CPAP compliance continues to be a challenge, highlighting the importance of treating not only OSA but all comorbidities, particularly depression. Short-term studies have found behavioral modification to be a promising means of improving CPAP compliance.33-36
Although not a first-line therapy, bilevel positive airway pressure (BiPAP), which delivers both inspiratory and expiratory pressure via a face device, can be tried in patients unable to tolerate CPAP.37 Studies are limited and it has been used in patients with complex OSA.
Oral appliances are a CPAP alternative
Mandibular repositioning devices (MRDs) and tongue-retaining devices are alternatives to CPAP.38 Although both types of oral appliance are beneficial, they are less effective than CPAP.28
MRDs, which are more commonly used than tongue-retaining devices, are available in several models. An MRD can be custom-made to hold the lower jaw in a forward position during sleep, enlarge the space behind the tongue, and put tension on the walls of the pharynx and the palate to reduce collapse.38
Tongue-retaining devices—splints that hold the tongue in place to keep the airway open—can be used for mild to moderate OSA, and for patients unable to tolerate CPAP.38,39
Studies comparing MRDs and tongue-retaining devices found no statistically significant difference in their ability to reduce AHI, but patients tolerated MRDs better.38,39 Nonetheless, both devices can cause dental discomfort, temporomandibular joint pain, dry mouth or excessive salivation, gum irritation, bruxism, and long-term occlusal changes.38
Is surgery an option?
Numerous surgical techniques are available for treating OSA, all aimed at relieving the obstruction by removing or bypassing it or increasing airway size. These include uvulopalatopharyngoplasty, which resects the uvula, retrolingual, and palatine tonsillar tissue; septoplasty; rhinoplasty; nasal turbinate reduction; nasal polypectomy palatal advancement pharyngoplasty; tonsillectomy; adenoidectomy; palatal implants; tongue reduction; genioglossus advancement; and maxillomandibular advancement.
The choice of modality depends on the patient’s anatomy and physiology, and is selected only after a full evaluation by a head and neck surgeon who specializes in surgical treatment of OSA.40,41 There is a paucity of reliable studies on the results of such procedures, but a Cochrane review of the existing literature concluded that surgery is only indicated for severe cases of sleep apnea in patients who have an anatomic obstruction.40
Too little evidence of medications’ efficacy
Numerous drugs have been tested for the treatment of OSA. Two Cochrane reviews looked at multiple trials of more than 20 drugs, in meta-analyses encompassing more than 500 patients.42,43 Ten drugs—eszopiclone, paroxetine, acetazolamide, ondansetron-fluoxetine combination, naltrexone, and fluticasone nasal spray among them—showed a statistically significant reduction in AHI, and a few showed a subjective benefit in daytime sleepiness. However, the studies were very small and of short duration and the reviewers concluded that evidence is insufficient to recommend drug therapy for OSA.
CASE 1 CPAP was prescribed for Mr. M. He tolerated it well and after 2 weeks, he reported feeling refreshed upon awakening, having less daytime somnolence and being better able to concentrate at work. His hypertension and glucose control improved, but he continues to struggle with his weight.
CASE 2 Ms. C started CPAP and within a week, her morning headaches and irritability resolved.
CORRESPONDENCE
Cecilia Gutierrez, MD, 200 West Arbor Drive, Mail Code 8809, San Diego, CA 92103; [email protected]
1. Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112.
2. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199-204.
3. Satela MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
4. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
5. Yaggi HK, Concato J, Kernan W, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
7. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;3:1071-1078.
8. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
9. Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741.
10. Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5-7.
11. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
12. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013-2016.
14. Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-base diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257-263.
15. Iber C, Ancoli-Israel S, Chesson AL Jr, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. Sensitivity and specificity of the multiple sleep maintenance tests, the maintenance of wakefulness tests and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5-11.
17. Hesselbacher S, Subramanian S, Allen J, et al. Body mass index, gender, and ethnic variations alter the clinical implication of the Epworth sleepiness scale in patients with suspected of obstructive sleep apnea. Open Respir Med J. 2012;6:2020-2027.
18. Ahmadi N, Chung S, Gibbs A, et al. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:38-45.
19. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
20. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875.
21. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as treatment for obstructive sleep apnea. Sleep Med Rev. 2000;4:435-452.
22. Buchwald H, Avidor H, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
23. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142-1149.
24. Greenburg A, Lettieri C, Arn E. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542.
25. Sagawa Y, Kondo H, Matsubuchi N. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093-2100.
26. Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447-455.
27. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565-571.
28. Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106.
29. Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070-1076.
30. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430-434.
31. Findley L, Smith C, Hooper J, et al. Treatment with nasal CPAP decreases automobile accidents in patient with sleep apnea. Am J Respir Crit Care Med. 2000;161:857-859.
32. Barbe F, Sunyer J, de la Pena A, et al. Effects of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44-49.
33. Sin DD, Mayers I, Man GC, et al. Long Term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430-435.
34. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265-1270.
35. Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553-560.
36. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea-hypopnea syndrome (SAHS). Sleep Med. 2003;7:81-99.
37. Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath. 2012;16:773-739.
38. Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693-699.
39. Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240-243.
40. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4):CD001004.
41. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
42. Smith I, Lasserson TJ, Wright JJ. Drug therapy for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006;():CD003002. Review.
43. Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5): CD003002.
1. Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112.
2. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199-204.
3. Satela MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
4. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
5. Yaggi HK, Concato J, Kernan W, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
7. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;3:1071-1078.
8. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
9. Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741.
10. Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5-7.
11. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
12. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013-2016.
14. Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-base diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257-263.
15. Iber C, Ancoli-Israel S, Chesson AL Jr, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. Sensitivity and specificity of the multiple sleep maintenance tests, the maintenance of wakefulness tests and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5-11.
17. Hesselbacher S, Subramanian S, Allen J, et al. Body mass index, gender, and ethnic variations alter the clinical implication of the Epworth sleepiness scale in patients with suspected of obstructive sleep apnea. Open Respir Med J. 2012;6:2020-2027.
18. Ahmadi N, Chung S, Gibbs A, et al. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:38-45.
19. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
20. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875.
21. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as treatment for obstructive sleep apnea. Sleep Med Rev. 2000;4:435-452.
22. Buchwald H, Avidor H, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
23. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142-1149.
24. Greenburg A, Lettieri C, Arn E. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542.
25. Sagawa Y, Kondo H, Matsubuchi N. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093-2100.
26. Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447-455.
27. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565-571.
28. Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106.
29. Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070-1076.
30. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430-434.
31. Findley L, Smith C, Hooper J, et al. Treatment with nasal CPAP decreases automobile accidents in patient with sleep apnea. Am J Respir Crit Care Med. 2000;161:857-859.
32. Barbe F, Sunyer J, de la Pena A, et al. Effects of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44-49.
33. Sin DD, Mayers I, Man GC, et al. Long Term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430-435.
34. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265-1270.
35. Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553-560.
36. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea-hypopnea syndrome (SAHS). Sleep Med. 2003;7:81-99.
37. Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath. 2012;16:773-739.
38. Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693-699.
39. Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240-243.
40. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4):CD001004.
41. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
42. Smith I, Lasserson TJ, Wright JJ. Drug therapy for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006;():CD003002. Review.
43. Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5): CD003002.
Diabetes: 8 Strategies to put into Practice
› Develop a diabetes registry and use it to identify patients in need of intervention. B
› Adopt routine depression screening for patients with diabetes. A
› Individualize HbA1c targets based on the patient’s comorbidities and duration of diabetes, among other patient factors. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The prevalence of diabetes, particularly type 2 (T2D), continues to grow at an unprecedented rate,1 largely because Americans are eating more than in years past and exercising less. At the same time, improvements in treatment are resulting in lower rates of cardiovascular (CV) comorbidities and increased longevity for those with T2D.2,3
Most patients with diabetes are cared for in a primary care setting. With more than a quarter of those who have diabetes (an estimated 7 million Americans) unaware that they have it,4,5 primary care physicians typically see many patients with undiagnosed T2D, as well.
Diabetes care is extremely costly; approximately 20 cents of every health care dollar is spent on those with the disease.6 As a result of this expenditure and increasing adherence to annually updated evidence-based guidelines,7 control is improving, but slowly: Between 2007 and 2010, only 18.8% of patients with diabetes achieved all 3 American Diabetes Association (ADA) goals—for glycemia, blood pressure, and low-density lipoprotein (LDL) cholesterol.8
Part of the problem, experts agree, is that the US health care system is not well suited to manage chronic conditions. This has prompted efforts to develop enhanced delivery modes like the Chronic Care Model and the Patient-Centered Medical Home,9,10 but none has been widely adopted. While groups that have implemented such changes have had significant success,11,12 practices already operating at full capacity often find the work of practice transformation to be daunting.
Difficult as the task may be, we’ve been able to identify—and follow—a number of strategies that serve us well in caring for patients with diabetes. Whether you have the resources to undertake a major practice transformation or simply wish to sharpen your focus, adopting any (or all) of the strategies detailed here will help you optimize diabetes care.
1. Develop a diabetes registry
To have the greatest possible impact on a particular type of patient, you need a way to reliably identify those with a specific condition or set of symptoms. A diabetes registry—a database that starts with basic demographic information for all the patients in your practice with a diabetes diagnosis and is populated with relevant lab results and dates, immunization status, and date of last visit—serves such a function. Some EHRs have this built-in functionality, but most spreadsheet software packages, such as Excel, have the necessary features, as well.
The ideal registry is accurate and up to date, comprehensive, sortable by any of the parameters, and easily accessed, ideally at the point of care. In addition to being able to generate reports for individual patients, the registry should have the ability to track providers—showing, for example, how many (or what percentage) of a provider’s patients have had a diabetes foot exam within the past 12 months. A registry should also be able to pull such statistics for the practice as a whole.
Population management, in which the same standards are applied to all the patients in your practice with a particular diagnosis, is made possible by a registry. Because the registry can be searched by any of the parameters, office staff can use it to identify patients in need of interventions—eg, because of an HbA1c >8%, LDL cholesterol >100 mg/dL, or no recent visit. Medical assistants can then reach out to such patients to ensure that they receive the interventions they need.
Often the greatest challenge associated with the creation of a useful registry is the ability to populate it with accurate and continuously updated data. Using the presence of diabetes on the problem list is a reasonable place to start. But this can create difficulties if any clinicians in the practice have coded for T2D when they were simply testing for it. To avoid such problems, develop clearly defined inclusion criteria before trying to populate the registry.
2. Analyze (and streamline) workflow
Practices that undertake a critical analysis of their workflow often find, as we did, that some staff members are not working to the full capacity of their license. Medical assistants, for example, could give routine immunizations following protocols and standing orders.
To learn more about the workflow in your practice, consider establishing a “change team,” with at least one Teams that conduct “waste walks” to analyze practice workflow often find that some staff members are not working to the full capacity of their license. representative from each position (eg, front office clerk, medical assistant, RN, physician assistant, and family physician). The team can then conduct “waste walks”—literally walking through the workspace to assess office processes from a fresh perspective.
Typically, such teams identify potentially wasteful activities—duplication of efforts or steps that can be done in a more efficient way, or eliminated completely, without ill effect. Medical assistants could stop reconciling medications, for instance, if the primary care providers in the practice are already doing this, and use the time saved to screen patients for depression or neuropathy.
In our experience, physicians who undertake workflow analyses are often surprised to find that members of their staff have many ideas about practice improvements—and are happy to take on more work if they see that doing so would improve patient care. When our staff was reminded of how important blood pressure control is for patients with diabetes, for example, they started placing a sticky note with out-of-range numbers on the computer monitor in the exam room to ensure that this important finding would not be missed.
3. Build a multidisciplinary team
Properly managing a disorder as complex as diabetes requires a team approach. The team might include diabetes educators, nutritionists, behavioral counselors, diabetologists, ophthalmologists, nephrologists, cardiologists, and podiatrists, as well as primary care physicians and family members. Whenever possible, such team members should be integrated within the practice. When this is not the case, transparent communication is critical. A shared EHR can facilitate this.
And while sharing—and gathering—patient data such as blood glucose levels, carbohydrates consumed, intensity and duration of exercise, and daily medications can be time consuming, it is critical to do so. Data review makes it Office staff can be trained to give patients with diabetes a brief depression screen, such as the Patient Health Questionnaire (PHQ-9), at least once a year. possible to prevent acute complications associated with glucose levels that are too high or too low, for example, or to uncover patterns, such as increasing weight or abnormally low (or high) blood sugar at a particular time of day, and take timely corrective actions.
Whether such data analysis occurs within your practice or in the office of a nutritionist or other specialist, diabetes management in a primary care setting benefits from teamwork, too. Medical assistants can administer monofilament tests for neuropathy at each visit, for example, to ensure that this important screening isn’t missed. Office staff can flag the charts of patients in need of additional screening and review before the end of the visit to ensure that the requisite testing has been done. They can also facilitate previsit labs (see “Previsit labs: A simple but effective practice change” on page 546), eliminating the need to contact patients in the days after the visit to review findings and make recommendations that could have been done during the visit.
4. Screen for depression
Patients with diabetes are more likely than those who do not have diabetes to suffer from depression.13 It has also been shown that those who are depressed are less likely to have their diabetes under control than those who are not depressed,14 in part because depressed patients are not as likely to adhere to a medication regimen.15
In some cases, identifying and treating depression can be the key intervention that leads to improved diabetes management. A recent randomized controlled trial found that an integrated approach to managing diabetes and depression resulted in improvements in both glycemic control and depression.16
Without a good screening program, clinicians typically fail to identify depression in a substantial number of their patients.17 A brief screening tool, such as the Patient Health Questionnaire (PHQ-9), can reliably identify depression. Office staff can be trained to give patients with diabetes a depression screen at least once a year when they check in.
5. Screen for undiagnosed diabetes and prediabetes
The ADA recommends screening all patients ages 45 years or older for diabetes, as well as overweight adults (BMI ≥25 kg/m2) with one or more additional risk factors (TABLE).7
Screening for diabetes is effective because:
- Diabetes is prevalent (affecting nearly 26 million US residents).5
- The disease is often asymptomatic and many patients do not recognize or acknowledge their symptoms (more than a quarter of those with diabetes are undiagnosed).5
- Accurate, reliable, and inexpensive screening tests are available.
- Early identification provides opportunities for useful interventions.
Traditionally, diabetes was diagnosed by fasting plasma glucose, oral glucose tolerance tests, or—in symptomatic individuals—random glucose elevations. HbA1c was added as a recommended diagnostic test in 2009 and endorsed by the ADA in 2010, with a threshold for diagnosis of ≥6.5%.18
Patients with an HbA1c between 5.7% and 6.4% are considered to have prediabetes, according to the ADA, and have a greater risk for developing both diabetes and CV complications at the higher end of this range. Such patients should be counseled regarding diet, exercise, and other lifestyle issues; metformin should be considered, as well, for those at particularly high risk.7
6. Individualize HbA1c targets
Recent large clinical trials have indicated that no single A1c target is appropriate for all patients.19 Generally speaking, more aggressive, lower targets (eg, <7%) are appropriate for younger patients; recently diagnosed patients who do not have significant CV disease; and those who are highly motivated and have adequate resources and support.20
Higher targets (8% or even higher for some patients) may be appropriate for those who do not fit the above profile. The ADA suggests a fundamentally patient-centered approach to determine an individual’s A1c target, noting that “the desires and values of the patient should be considered, since the achievement of any degree of glucose control requires active participation and commitment.”21
7. Do more to engage patients
Achieving glycemic control and optimizing CV risk factors requires tremendous effort on the part of patients, and sometimes by their families, as well. Unilateral efforts by a physician, no matter how robust and determinedly implemented, nearly always fall short.
Given the complexities associated with managing diabetes, nearly all patients diagnosed with T2D will benefit from education. While some physicians and other health care team members have become very adept at this, there is almost always a role for certified diabetes educators. The ADA recommends that patients receive diabetes self-management education (DSME) according to national standards when their diabetes is diagnosed and as needed thereafter.7
While education is necessary, it is not sufficient. Success occurs only when the patient is educated, engaged, and activated—having the knowledge, skills, and confidence to play the key role in his or her own health care. A recent study with more than 5000 participants found that among those seeing the same physician, patients at higher levels of activation had better health care experiences than those who were less activated.22
Recommendations to patients with diabetes typically require that they carefully obtain, prepare, and consume a particular diet; exercise regularly; manage multiple medications; keep their health care appointments; and engage in regular monitoring of glucose levels and other parameters. Although the health care team may be the source of these recommendations, in every case, it is the patient who must carry them out.
For most patients, the greatest challenge lies in getting and staying motivated to implement all the recommended interventions. Motivational interviewing can be a powerful technique (http://www.motivationalinterview.org/ to learn more). But most patients require a multifaceted approach. Evidence-based principles for promoting and supporting optimal self-management in primary care—including the use of a collaborative, nonjudgmental approach and the support of diverse providers—were identified in a recent publication.23
Many practices have adopted protocols that make it possible for patients with diabetes to have laboratory testing done prior to each visit—a change that benefits both patients and clinicians. office staff can be trained to do the work that this entails, which includes:
- following a protocol to determine which lab tests are indicated
- ensuring that patients have the appropriate lab order for testing before they leave
- contacting patients before their next appointment to ensure that the tests are done and the results available at the time of the visit.
In practices that have adopted such protocols, most visits conclude with the physician giving the patient the requisite lab order. Previsit lab results make an office visit more productive, as they allow for more targeted patient education and counseling, as well as any medication adjustment that is indicated.
This practice also increases efficiency, reducing the time and effort spent trying to communicate with patients after their visit regarding test results and new recommendations. more importantly, it makes it possible for physician and patient to negotiate and reach a consensus about any new interventions during a face-to-face encounter. even accounting for the extra effort of a separate visit for lab testing, we’ve found that most patients appreciate the added value of this approach and are happy to make the effort.
At our facility (UMass Memorial Health Care), primary care providers and diabetes specialists are working together to develop ways to more fully engage patients with diabetes. One such initiative, the Diabetes Scorecard, is delivered to each patient during check-in. Featuring patient-friendly language and simple graphics, the scorecard is automatically populated by data from our EHR, providing an at-a-glance summary that is useful to clinicians and patients alike.
To promote patient self-management and the ability of patients and clinicians to access and carefully review various parameters, several manufacturers of blood glucose meters have developed systems that allow patients to upload the data to a secure, Web-based database that both patients and providers can access and review. We have found, however, that only a few patients—and even fewer clinicians—consistently use them.
Presuming that simple inconvenience is at least part of the reason for such limited use, UMass Memorial has implemented a new system (MyCareTeam™), which works with our EHR provider, Allscripts. This system, which has been shown to improve patient outcomes in other clinical settings, can be launched with a single mouse click from within our EHR. It works with most commercially available glucometers used by our patients, has a user-friendly interface, and provides access to educational resources designed to promote patient engagement. Our goal is to make it easy for patients to upload their own data from a desktop computer, or eventually from mobile devices. Like other systems that electronically capture glucose readings, it prevents patients from excluding any of the results.
At some facilities, physicians “prescribe” apps that patients can use to track chronic diseases on their smartphone or tablet and transmit data, such as glucose readings, to their clinician. Highly rated diabetes apps include Glooko Logbook, Glucose Buddy, and OnTrack Diabetes, to name a few.24
8. Learn more about b-cell function
As the medications available to manage glucose levels have increased in number, it has become more important for clinicians to understand T2D pathophysiology and how various pharmaceutical agents affect it. Central to this understanding are the individual’s sensitivity to insulin’s action and the status of his or her pancreatic b-cell function.
The b-cell dysfunction underlying T2D appears to respond, often dramatically, to even modest weight loss or increased physical activity. Thus, all patients with T2D should be encouraged to pursue daily physical activity and adhere to a diet designed to promote moderate weight loss; any discussion of pharmaceutical approaches should begin with mention of exercise and diet.
For patients whose diabetes is inadequately controlled by lifestyle interventions, medication should be chosen based on an understanding of the pathophysiology and disease state, and particularly, on the patient’s remaining b-cell function. Other considerations include comorbidities, anticipated efficacy, cost, mode of administration, and patient preferences.
Early in the T2D disease process, insulin resistance typically predominates, and b-cell dysfunction is mild. At our facility, we emphasize agents that help restore insulin sensitivity, especially metformin. Patients who are not achieving their glycemic target with lifestyle changes and metformin will benefit from the addition of a secretagogue, which provides a complementary mechanism of action.
Sulfonylureas are inexpensive and effective but potentially problematic because they may cause hypoglycemia and contribute to b-cell exhaustion. This is because they stimulate insulin secretion independent of circulating glucose levels. Glinides work in a similar manner, but have a more rapid onset and a shorter duration of action than sulfonylureas. Thus, they can be effective at mitigating prandial hyperglycemia, but require dosing with meals.
Newer secretagogues such as GLP-1 agonists and DPP-4 inhibitors stimulate secretion of insulin in response to hyperglycemia, reducing the risk of hypoglycemia and ultimately preserving some b-cell function. These newer agents, as well as glinides, are significantly more expensive than sulfony-lureas. GLP-1 agonists have the additional disadvantage of requiring injection. Patients who are Our Diabetes Scorecard, which is automatically populated by data from our EHR, provides an at-a-glance summary that is useful to clinicians and patients alike. far from their glycemic target will benefit from the addition of insulin.
CORRESPONDENCE
Ronald N. Adler, MD, UMass Memorial Medical Center, 279 Lincoln Street, Worcester, MA 01605; [email protected]
1. Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes preva- lence since 1980. Lancet. 2011;378:31-40.
2. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653.
3. Ford ES. Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the U.S.: findings from the National Health and Nutrition Examination Survey, 1999- 2008. Diabetes Care. 2011;34:1337-1343.
4. Golden SH. Emerging therapeutic approaches for the manage- ment of diabetes mellitus and macrovascular complications. Am J Cardiol. 2011;108(3 suppl):59B-67B.
5. American Diabetes Association. Diabetes statistics. Edited Au- gust 20, 2013. Available at: http://www.diabetes.org/diabetes- basics/diabetes-statistics/. Accessed September 11, 2013.
6. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596-615.
7. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
8. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The preva- lence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36:2271- 2279.
9. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2-4.
10. Wagner EH, Austin BT, Davis C, et al. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20:64-78.
11. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millennium. Health Aff (Millwood). 2009;28:75-85.
12. Bojadzievski T, Gabbay RA. Patient-centered medical home and diabetes. Diabetes Care. 2011;34:1047-1053.
13. Anderson RJ, Freedland KE, Clouse RE, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069-1078.
14. Katon W, Von KM, Ciechanowski P, et al. Behavioral and clini- cal factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914-920.
15. Katon W, Russo J, Lin EH, et al. Diabetes and poor disease control: is comorbid depression associated with poor medica- tion adherence or lack of treatment intensification? Psychosom Med. 2009;71:965-972.
16. Bogner HR, Morales KH, de Vries HF, et al. Integrated manage- ment of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med. 2012;10:15-22.
17. Pignone MP, Gaynes BN, Rushton JL, et al. Screening for de- pression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:765- 776.
18. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
19. Executive summary: standards of medical care in diabe- tes—2013. Diabetes Care. 2013;36(suppl 1):S4-S10.
20. Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med. 2012;366:1319-1327.
21. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hy- perglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
22. Greene J, Hibbard JH, Sacks R, et al. When seeing the same physician, highly activated patients have better experi- ences than less activated patients. Health Aff (Millwood). 2013;32:1299-1305.
23. Battersby M, Von KM, Schaefer J, et al. Twelve evidence-based principles for implementing self-management support in pri- mary care. Jt Comm J Qual Patient Saf. 2010;36:561-570.
24. Watson S. The 13 best diabetes iPhone & Android apps of 2013. Healthline Web site. Available at: http://www.healthline.com/ health-slideshow/top-iphone-android-apps-diabetes. Published August 8, 2013. Accessed September 16, 2013.
› Develop a diabetes registry and use it to identify patients in need of intervention. B
› Adopt routine depression screening for patients with diabetes. A
› Individualize HbA1c targets based on the patient’s comorbidities and duration of diabetes, among other patient factors. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The prevalence of diabetes, particularly type 2 (T2D), continues to grow at an unprecedented rate,1 largely because Americans are eating more than in years past and exercising less. At the same time, improvements in treatment are resulting in lower rates of cardiovascular (CV) comorbidities and increased longevity for those with T2D.2,3
Most patients with diabetes are cared for in a primary care setting. With more than a quarter of those who have diabetes (an estimated 7 million Americans) unaware that they have it,4,5 primary care physicians typically see many patients with undiagnosed T2D, as well.
Diabetes care is extremely costly; approximately 20 cents of every health care dollar is spent on those with the disease.6 As a result of this expenditure and increasing adherence to annually updated evidence-based guidelines,7 control is improving, but slowly: Between 2007 and 2010, only 18.8% of patients with diabetes achieved all 3 American Diabetes Association (ADA) goals—for glycemia, blood pressure, and low-density lipoprotein (LDL) cholesterol.8
Part of the problem, experts agree, is that the US health care system is not well suited to manage chronic conditions. This has prompted efforts to develop enhanced delivery modes like the Chronic Care Model and the Patient-Centered Medical Home,9,10 but none has been widely adopted. While groups that have implemented such changes have had significant success,11,12 practices already operating at full capacity often find the work of practice transformation to be daunting.
Difficult as the task may be, we’ve been able to identify—and follow—a number of strategies that serve us well in caring for patients with diabetes. Whether you have the resources to undertake a major practice transformation or simply wish to sharpen your focus, adopting any (or all) of the strategies detailed here will help you optimize diabetes care.
1. Develop a diabetes registry
To have the greatest possible impact on a particular type of patient, you need a way to reliably identify those with a specific condition or set of symptoms. A diabetes registry—a database that starts with basic demographic information for all the patients in your practice with a diabetes diagnosis and is populated with relevant lab results and dates, immunization status, and date of last visit—serves such a function. Some EHRs have this built-in functionality, but most spreadsheet software packages, such as Excel, have the necessary features, as well.
The ideal registry is accurate and up to date, comprehensive, sortable by any of the parameters, and easily accessed, ideally at the point of care. In addition to being able to generate reports for individual patients, the registry should have the ability to track providers—showing, for example, how many (or what percentage) of a provider’s patients have had a diabetes foot exam within the past 12 months. A registry should also be able to pull such statistics for the practice as a whole.
Population management, in which the same standards are applied to all the patients in your practice with a particular diagnosis, is made possible by a registry. Because the registry can be searched by any of the parameters, office staff can use it to identify patients in need of interventions—eg, because of an HbA1c >8%, LDL cholesterol >100 mg/dL, or no recent visit. Medical assistants can then reach out to such patients to ensure that they receive the interventions they need.
Often the greatest challenge associated with the creation of a useful registry is the ability to populate it with accurate and continuously updated data. Using the presence of diabetes on the problem list is a reasonable place to start. But this can create difficulties if any clinicians in the practice have coded for T2D when they were simply testing for it. To avoid such problems, develop clearly defined inclusion criteria before trying to populate the registry.
2. Analyze (and streamline) workflow
Practices that undertake a critical analysis of their workflow often find, as we did, that some staff members are not working to the full capacity of their license. Medical assistants, for example, could give routine immunizations following protocols and standing orders.
To learn more about the workflow in your practice, consider establishing a “change team,” with at least one Teams that conduct “waste walks” to analyze practice workflow often find that some staff members are not working to the full capacity of their license. representative from each position (eg, front office clerk, medical assistant, RN, physician assistant, and family physician). The team can then conduct “waste walks”—literally walking through the workspace to assess office processes from a fresh perspective.
Typically, such teams identify potentially wasteful activities—duplication of efforts or steps that can be done in a more efficient way, or eliminated completely, without ill effect. Medical assistants could stop reconciling medications, for instance, if the primary care providers in the practice are already doing this, and use the time saved to screen patients for depression or neuropathy.
In our experience, physicians who undertake workflow analyses are often surprised to find that members of their staff have many ideas about practice improvements—and are happy to take on more work if they see that doing so would improve patient care. When our staff was reminded of how important blood pressure control is for patients with diabetes, for example, they started placing a sticky note with out-of-range numbers on the computer monitor in the exam room to ensure that this important finding would not be missed.
3. Build a multidisciplinary team
Properly managing a disorder as complex as diabetes requires a team approach. The team might include diabetes educators, nutritionists, behavioral counselors, diabetologists, ophthalmologists, nephrologists, cardiologists, and podiatrists, as well as primary care physicians and family members. Whenever possible, such team members should be integrated within the practice. When this is not the case, transparent communication is critical. A shared EHR can facilitate this.
And while sharing—and gathering—patient data such as blood glucose levels, carbohydrates consumed, intensity and duration of exercise, and daily medications can be time consuming, it is critical to do so. Data review makes it Office staff can be trained to give patients with diabetes a brief depression screen, such as the Patient Health Questionnaire (PHQ-9), at least once a year. possible to prevent acute complications associated with glucose levels that are too high or too low, for example, or to uncover patterns, such as increasing weight or abnormally low (or high) blood sugar at a particular time of day, and take timely corrective actions.
Whether such data analysis occurs within your practice or in the office of a nutritionist or other specialist, diabetes management in a primary care setting benefits from teamwork, too. Medical assistants can administer monofilament tests for neuropathy at each visit, for example, to ensure that this important screening isn’t missed. Office staff can flag the charts of patients in need of additional screening and review before the end of the visit to ensure that the requisite testing has been done. They can also facilitate previsit labs (see “Previsit labs: A simple but effective practice change” on page 546), eliminating the need to contact patients in the days after the visit to review findings and make recommendations that could have been done during the visit.
4. Screen for depression
Patients with diabetes are more likely than those who do not have diabetes to suffer from depression.13 It has also been shown that those who are depressed are less likely to have their diabetes under control than those who are not depressed,14 in part because depressed patients are not as likely to adhere to a medication regimen.15
In some cases, identifying and treating depression can be the key intervention that leads to improved diabetes management. A recent randomized controlled trial found that an integrated approach to managing diabetes and depression resulted in improvements in both glycemic control and depression.16
Without a good screening program, clinicians typically fail to identify depression in a substantial number of their patients.17 A brief screening tool, such as the Patient Health Questionnaire (PHQ-9), can reliably identify depression. Office staff can be trained to give patients with diabetes a depression screen at least once a year when they check in.
5. Screen for undiagnosed diabetes and prediabetes
The ADA recommends screening all patients ages 45 years or older for diabetes, as well as overweight adults (BMI ≥25 kg/m2) with one or more additional risk factors (TABLE).7
Screening for diabetes is effective because:
- Diabetes is prevalent (affecting nearly 26 million US residents).5
- The disease is often asymptomatic and many patients do not recognize or acknowledge their symptoms (more than a quarter of those with diabetes are undiagnosed).5
- Accurate, reliable, and inexpensive screening tests are available.
- Early identification provides opportunities for useful interventions.
Traditionally, diabetes was diagnosed by fasting plasma glucose, oral glucose tolerance tests, or—in symptomatic individuals—random glucose elevations. HbA1c was added as a recommended diagnostic test in 2009 and endorsed by the ADA in 2010, with a threshold for diagnosis of ≥6.5%.18
Patients with an HbA1c between 5.7% and 6.4% are considered to have prediabetes, according to the ADA, and have a greater risk for developing both diabetes and CV complications at the higher end of this range. Such patients should be counseled regarding diet, exercise, and other lifestyle issues; metformin should be considered, as well, for those at particularly high risk.7
6. Individualize HbA1c targets
Recent large clinical trials have indicated that no single A1c target is appropriate for all patients.19 Generally speaking, more aggressive, lower targets (eg, <7%) are appropriate for younger patients; recently diagnosed patients who do not have significant CV disease; and those who are highly motivated and have adequate resources and support.20
Higher targets (8% or even higher for some patients) may be appropriate for those who do not fit the above profile. The ADA suggests a fundamentally patient-centered approach to determine an individual’s A1c target, noting that “the desires and values of the patient should be considered, since the achievement of any degree of glucose control requires active participation and commitment.”21
7. Do more to engage patients
Achieving glycemic control and optimizing CV risk factors requires tremendous effort on the part of patients, and sometimes by their families, as well. Unilateral efforts by a physician, no matter how robust and determinedly implemented, nearly always fall short.
Given the complexities associated with managing diabetes, nearly all patients diagnosed with T2D will benefit from education. While some physicians and other health care team members have become very adept at this, there is almost always a role for certified diabetes educators. The ADA recommends that patients receive diabetes self-management education (DSME) according to national standards when their diabetes is diagnosed and as needed thereafter.7
While education is necessary, it is not sufficient. Success occurs only when the patient is educated, engaged, and activated—having the knowledge, skills, and confidence to play the key role in his or her own health care. A recent study with more than 5000 participants found that among those seeing the same physician, patients at higher levels of activation had better health care experiences than those who were less activated.22
Recommendations to patients with diabetes typically require that they carefully obtain, prepare, and consume a particular diet; exercise regularly; manage multiple medications; keep their health care appointments; and engage in regular monitoring of glucose levels and other parameters. Although the health care team may be the source of these recommendations, in every case, it is the patient who must carry them out.
For most patients, the greatest challenge lies in getting and staying motivated to implement all the recommended interventions. Motivational interviewing can be a powerful technique (http://www.motivationalinterview.org/ to learn more). But most patients require a multifaceted approach. Evidence-based principles for promoting and supporting optimal self-management in primary care—including the use of a collaborative, nonjudgmental approach and the support of diverse providers—were identified in a recent publication.23
Many practices have adopted protocols that make it possible for patients with diabetes to have laboratory testing done prior to each visit—a change that benefits both patients and clinicians. office staff can be trained to do the work that this entails, which includes:
- following a protocol to determine which lab tests are indicated
- ensuring that patients have the appropriate lab order for testing before they leave
- contacting patients before their next appointment to ensure that the tests are done and the results available at the time of the visit.
In practices that have adopted such protocols, most visits conclude with the physician giving the patient the requisite lab order. Previsit lab results make an office visit more productive, as they allow for more targeted patient education and counseling, as well as any medication adjustment that is indicated.
This practice also increases efficiency, reducing the time and effort spent trying to communicate with patients after their visit regarding test results and new recommendations. more importantly, it makes it possible for physician and patient to negotiate and reach a consensus about any new interventions during a face-to-face encounter. even accounting for the extra effort of a separate visit for lab testing, we’ve found that most patients appreciate the added value of this approach and are happy to make the effort.
At our facility (UMass Memorial Health Care), primary care providers and diabetes specialists are working together to develop ways to more fully engage patients with diabetes. One such initiative, the Diabetes Scorecard, is delivered to each patient during check-in. Featuring patient-friendly language and simple graphics, the scorecard is automatically populated by data from our EHR, providing an at-a-glance summary that is useful to clinicians and patients alike.
To promote patient self-management and the ability of patients and clinicians to access and carefully review various parameters, several manufacturers of blood glucose meters have developed systems that allow patients to upload the data to a secure, Web-based database that both patients and providers can access and review. We have found, however, that only a few patients—and even fewer clinicians—consistently use them.
Presuming that simple inconvenience is at least part of the reason for such limited use, UMass Memorial has implemented a new system (MyCareTeam™), which works with our EHR provider, Allscripts. This system, which has been shown to improve patient outcomes in other clinical settings, can be launched with a single mouse click from within our EHR. It works with most commercially available glucometers used by our patients, has a user-friendly interface, and provides access to educational resources designed to promote patient engagement. Our goal is to make it easy for patients to upload their own data from a desktop computer, or eventually from mobile devices. Like other systems that electronically capture glucose readings, it prevents patients from excluding any of the results.
At some facilities, physicians “prescribe” apps that patients can use to track chronic diseases on their smartphone or tablet and transmit data, such as glucose readings, to their clinician. Highly rated diabetes apps include Glooko Logbook, Glucose Buddy, and OnTrack Diabetes, to name a few.24
8. Learn more about b-cell function
As the medications available to manage glucose levels have increased in number, it has become more important for clinicians to understand T2D pathophysiology and how various pharmaceutical agents affect it. Central to this understanding are the individual’s sensitivity to insulin’s action and the status of his or her pancreatic b-cell function.
The b-cell dysfunction underlying T2D appears to respond, often dramatically, to even modest weight loss or increased physical activity. Thus, all patients with T2D should be encouraged to pursue daily physical activity and adhere to a diet designed to promote moderate weight loss; any discussion of pharmaceutical approaches should begin with mention of exercise and diet.
For patients whose diabetes is inadequately controlled by lifestyle interventions, medication should be chosen based on an understanding of the pathophysiology and disease state, and particularly, on the patient’s remaining b-cell function. Other considerations include comorbidities, anticipated efficacy, cost, mode of administration, and patient preferences.
Early in the T2D disease process, insulin resistance typically predominates, and b-cell dysfunction is mild. At our facility, we emphasize agents that help restore insulin sensitivity, especially metformin. Patients who are not achieving their glycemic target with lifestyle changes and metformin will benefit from the addition of a secretagogue, which provides a complementary mechanism of action.
Sulfonylureas are inexpensive and effective but potentially problematic because they may cause hypoglycemia and contribute to b-cell exhaustion. This is because they stimulate insulin secretion independent of circulating glucose levels. Glinides work in a similar manner, but have a more rapid onset and a shorter duration of action than sulfonylureas. Thus, they can be effective at mitigating prandial hyperglycemia, but require dosing with meals.
Newer secretagogues such as GLP-1 agonists and DPP-4 inhibitors stimulate secretion of insulin in response to hyperglycemia, reducing the risk of hypoglycemia and ultimately preserving some b-cell function. These newer agents, as well as glinides, are significantly more expensive than sulfony-lureas. GLP-1 agonists have the additional disadvantage of requiring injection. Patients who are Our Diabetes Scorecard, which is automatically populated by data from our EHR, provides an at-a-glance summary that is useful to clinicians and patients alike. far from their glycemic target will benefit from the addition of insulin.
CORRESPONDENCE
Ronald N. Adler, MD, UMass Memorial Medical Center, 279 Lincoln Street, Worcester, MA 01605; [email protected]
› Develop a diabetes registry and use it to identify patients in need of intervention. B
› Adopt routine depression screening for patients with diabetes. A
› Individualize HbA1c targets based on the patient’s comorbidities and duration of diabetes, among other patient factors. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The prevalence of diabetes, particularly type 2 (T2D), continues to grow at an unprecedented rate,1 largely because Americans are eating more than in years past and exercising less. At the same time, improvements in treatment are resulting in lower rates of cardiovascular (CV) comorbidities and increased longevity for those with T2D.2,3
Most patients with diabetes are cared for in a primary care setting. With more than a quarter of those who have diabetes (an estimated 7 million Americans) unaware that they have it,4,5 primary care physicians typically see many patients with undiagnosed T2D, as well.
Diabetes care is extremely costly; approximately 20 cents of every health care dollar is spent on those with the disease.6 As a result of this expenditure and increasing adherence to annually updated evidence-based guidelines,7 control is improving, but slowly: Between 2007 and 2010, only 18.8% of patients with diabetes achieved all 3 American Diabetes Association (ADA) goals—for glycemia, blood pressure, and low-density lipoprotein (LDL) cholesterol.8
Part of the problem, experts agree, is that the US health care system is not well suited to manage chronic conditions. This has prompted efforts to develop enhanced delivery modes like the Chronic Care Model and the Patient-Centered Medical Home,9,10 but none has been widely adopted. While groups that have implemented such changes have had significant success,11,12 practices already operating at full capacity often find the work of practice transformation to be daunting.
Difficult as the task may be, we’ve been able to identify—and follow—a number of strategies that serve us well in caring for patients with diabetes. Whether you have the resources to undertake a major practice transformation or simply wish to sharpen your focus, adopting any (or all) of the strategies detailed here will help you optimize diabetes care.
1. Develop a diabetes registry
To have the greatest possible impact on a particular type of patient, you need a way to reliably identify those with a specific condition or set of symptoms. A diabetes registry—a database that starts with basic demographic information for all the patients in your practice with a diabetes diagnosis and is populated with relevant lab results and dates, immunization status, and date of last visit—serves such a function. Some EHRs have this built-in functionality, but most spreadsheet software packages, such as Excel, have the necessary features, as well.
The ideal registry is accurate and up to date, comprehensive, sortable by any of the parameters, and easily accessed, ideally at the point of care. In addition to being able to generate reports for individual patients, the registry should have the ability to track providers—showing, for example, how many (or what percentage) of a provider’s patients have had a diabetes foot exam within the past 12 months. A registry should also be able to pull such statistics for the practice as a whole.
Population management, in which the same standards are applied to all the patients in your practice with a particular diagnosis, is made possible by a registry. Because the registry can be searched by any of the parameters, office staff can use it to identify patients in need of interventions—eg, because of an HbA1c >8%, LDL cholesterol >100 mg/dL, or no recent visit. Medical assistants can then reach out to such patients to ensure that they receive the interventions they need.
Often the greatest challenge associated with the creation of a useful registry is the ability to populate it with accurate and continuously updated data. Using the presence of diabetes on the problem list is a reasonable place to start. But this can create difficulties if any clinicians in the practice have coded for T2D when they were simply testing for it. To avoid such problems, develop clearly defined inclusion criteria before trying to populate the registry.
2. Analyze (and streamline) workflow
Practices that undertake a critical analysis of their workflow often find, as we did, that some staff members are not working to the full capacity of their license. Medical assistants, for example, could give routine immunizations following protocols and standing orders.
To learn more about the workflow in your practice, consider establishing a “change team,” with at least one Teams that conduct “waste walks” to analyze practice workflow often find that some staff members are not working to the full capacity of their license. representative from each position (eg, front office clerk, medical assistant, RN, physician assistant, and family physician). The team can then conduct “waste walks”—literally walking through the workspace to assess office processes from a fresh perspective.
Typically, such teams identify potentially wasteful activities—duplication of efforts or steps that can be done in a more efficient way, or eliminated completely, without ill effect. Medical assistants could stop reconciling medications, for instance, if the primary care providers in the practice are already doing this, and use the time saved to screen patients for depression or neuropathy.
In our experience, physicians who undertake workflow analyses are often surprised to find that members of their staff have many ideas about practice improvements—and are happy to take on more work if they see that doing so would improve patient care. When our staff was reminded of how important blood pressure control is for patients with diabetes, for example, they started placing a sticky note with out-of-range numbers on the computer monitor in the exam room to ensure that this important finding would not be missed.
3. Build a multidisciplinary team
Properly managing a disorder as complex as diabetes requires a team approach. The team might include diabetes educators, nutritionists, behavioral counselors, diabetologists, ophthalmologists, nephrologists, cardiologists, and podiatrists, as well as primary care physicians and family members. Whenever possible, such team members should be integrated within the practice. When this is not the case, transparent communication is critical. A shared EHR can facilitate this.
And while sharing—and gathering—patient data such as blood glucose levels, carbohydrates consumed, intensity and duration of exercise, and daily medications can be time consuming, it is critical to do so. Data review makes it Office staff can be trained to give patients with diabetes a brief depression screen, such as the Patient Health Questionnaire (PHQ-9), at least once a year. possible to prevent acute complications associated with glucose levels that are too high or too low, for example, or to uncover patterns, such as increasing weight or abnormally low (or high) blood sugar at a particular time of day, and take timely corrective actions.
Whether such data analysis occurs within your practice or in the office of a nutritionist or other specialist, diabetes management in a primary care setting benefits from teamwork, too. Medical assistants can administer monofilament tests for neuropathy at each visit, for example, to ensure that this important screening isn’t missed. Office staff can flag the charts of patients in need of additional screening and review before the end of the visit to ensure that the requisite testing has been done. They can also facilitate previsit labs (see “Previsit labs: A simple but effective practice change” on page 546), eliminating the need to contact patients in the days after the visit to review findings and make recommendations that could have been done during the visit.
4. Screen for depression
Patients with diabetes are more likely than those who do not have diabetes to suffer from depression.13 It has also been shown that those who are depressed are less likely to have their diabetes under control than those who are not depressed,14 in part because depressed patients are not as likely to adhere to a medication regimen.15
In some cases, identifying and treating depression can be the key intervention that leads to improved diabetes management. A recent randomized controlled trial found that an integrated approach to managing diabetes and depression resulted in improvements in both glycemic control and depression.16
Without a good screening program, clinicians typically fail to identify depression in a substantial number of their patients.17 A brief screening tool, such as the Patient Health Questionnaire (PHQ-9), can reliably identify depression. Office staff can be trained to give patients with diabetes a depression screen at least once a year when they check in.
5. Screen for undiagnosed diabetes and prediabetes
The ADA recommends screening all patients ages 45 years or older for diabetes, as well as overweight adults (BMI ≥25 kg/m2) with one or more additional risk factors (TABLE).7
Screening for diabetes is effective because:
- Diabetes is prevalent (affecting nearly 26 million US residents).5
- The disease is often asymptomatic and many patients do not recognize or acknowledge their symptoms (more than a quarter of those with diabetes are undiagnosed).5
- Accurate, reliable, and inexpensive screening tests are available.
- Early identification provides opportunities for useful interventions.
Traditionally, diabetes was diagnosed by fasting plasma glucose, oral glucose tolerance tests, or—in symptomatic individuals—random glucose elevations. HbA1c was added as a recommended diagnostic test in 2009 and endorsed by the ADA in 2010, with a threshold for diagnosis of ≥6.5%.18
Patients with an HbA1c between 5.7% and 6.4% are considered to have prediabetes, according to the ADA, and have a greater risk for developing both diabetes and CV complications at the higher end of this range. Such patients should be counseled regarding diet, exercise, and other lifestyle issues; metformin should be considered, as well, for those at particularly high risk.7
6. Individualize HbA1c targets
Recent large clinical trials have indicated that no single A1c target is appropriate for all patients.19 Generally speaking, more aggressive, lower targets (eg, <7%) are appropriate for younger patients; recently diagnosed patients who do not have significant CV disease; and those who are highly motivated and have adequate resources and support.20
Higher targets (8% or even higher for some patients) may be appropriate for those who do not fit the above profile. The ADA suggests a fundamentally patient-centered approach to determine an individual’s A1c target, noting that “the desires and values of the patient should be considered, since the achievement of any degree of glucose control requires active participation and commitment.”21
7. Do more to engage patients
Achieving glycemic control and optimizing CV risk factors requires tremendous effort on the part of patients, and sometimes by their families, as well. Unilateral efforts by a physician, no matter how robust and determinedly implemented, nearly always fall short.
Given the complexities associated with managing diabetes, nearly all patients diagnosed with T2D will benefit from education. While some physicians and other health care team members have become very adept at this, there is almost always a role for certified diabetes educators. The ADA recommends that patients receive diabetes self-management education (DSME) according to national standards when their diabetes is diagnosed and as needed thereafter.7
While education is necessary, it is not sufficient. Success occurs only when the patient is educated, engaged, and activated—having the knowledge, skills, and confidence to play the key role in his or her own health care. A recent study with more than 5000 participants found that among those seeing the same physician, patients at higher levels of activation had better health care experiences than those who were less activated.22
Recommendations to patients with diabetes typically require that they carefully obtain, prepare, and consume a particular diet; exercise regularly; manage multiple medications; keep their health care appointments; and engage in regular monitoring of glucose levels and other parameters. Although the health care team may be the source of these recommendations, in every case, it is the patient who must carry them out.
For most patients, the greatest challenge lies in getting and staying motivated to implement all the recommended interventions. Motivational interviewing can be a powerful technique (http://www.motivationalinterview.org/ to learn more). But most patients require a multifaceted approach. Evidence-based principles for promoting and supporting optimal self-management in primary care—including the use of a collaborative, nonjudgmental approach and the support of diverse providers—were identified in a recent publication.23
Many practices have adopted protocols that make it possible for patients with diabetes to have laboratory testing done prior to each visit—a change that benefits both patients and clinicians. office staff can be trained to do the work that this entails, which includes:
- following a protocol to determine which lab tests are indicated
- ensuring that patients have the appropriate lab order for testing before they leave
- contacting patients before their next appointment to ensure that the tests are done and the results available at the time of the visit.
In practices that have adopted such protocols, most visits conclude with the physician giving the patient the requisite lab order. Previsit lab results make an office visit more productive, as they allow for more targeted patient education and counseling, as well as any medication adjustment that is indicated.
This practice also increases efficiency, reducing the time and effort spent trying to communicate with patients after their visit regarding test results and new recommendations. more importantly, it makes it possible for physician and patient to negotiate and reach a consensus about any new interventions during a face-to-face encounter. even accounting for the extra effort of a separate visit for lab testing, we’ve found that most patients appreciate the added value of this approach and are happy to make the effort.
At our facility (UMass Memorial Health Care), primary care providers and diabetes specialists are working together to develop ways to more fully engage patients with diabetes. One such initiative, the Diabetes Scorecard, is delivered to each patient during check-in. Featuring patient-friendly language and simple graphics, the scorecard is automatically populated by data from our EHR, providing an at-a-glance summary that is useful to clinicians and patients alike.
To promote patient self-management and the ability of patients and clinicians to access and carefully review various parameters, several manufacturers of blood glucose meters have developed systems that allow patients to upload the data to a secure, Web-based database that both patients and providers can access and review. We have found, however, that only a few patients—and even fewer clinicians—consistently use them.
Presuming that simple inconvenience is at least part of the reason for such limited use, UMass Memorial has implemented a new system (MyCareTeam™), which works with our EHR provider, Allscripts. This system, which has been shown to improve patient outcomes in other clinical settings, can be launched with a single mouse click from within our EHR. It works with most commercially available glucometers used by our patients, has a user-friendly interface, and provides access to educational resources designed to promote patient engagement. Our goal is to make it easy for patients to upload their own data from a desktop computer, or eventually from mobile devices. Like other systems that electronically capture glucose readings, it prevents patients from excluding any of the results.
At some facilities, physicians “prescribe” apps that patients can use to track chronic diseases on their smartphone or tablet and transmit data, such as glucose readings, to their clinician. Highly rated diabetes apps include Glooko Logbook, Glucose Buddy, and OnTrack Diabetes, to name a few.24
8. Learn more about b-cell function
As the medications available to manage glucose levels have increased in number, it has become more important for clinicians to understand T2D pathophysiology and how various pharmaceutical agents affect it. Central to this understanding are the individual’s sensitivity to insulin’s action and the status of his or her pancreatic b-cell function.
The b-cell dysfunction underlying T2D appears to respond, often dramatically, to even modest weight loss or increased physical activity. Thus, all patients with T2D should be encouraged to pursue daily physical activity and adhere to a diet designed to promote moderate weight loss; any discussion of pharmaceutical approaches should begin with mention of exercise and diet.
For patients whose diabetes is inadequately controlled by lifestyle interventions, medication should be chosen based on an understanding of the pathophysiology and disease state, and particularly, on the patient’s remaining b-cell function. Other considerations include comorbidities, anticipated efficacy, cost, mode of administration, and patient preferences.
Early in the T2D disease process, insulin resistance typically predominates, and b-cell dysfunction is mild. At our facility, we emphasize agents that help restore insulin sensitivity, especially metformin. Patients who are not achieving their glycemic target with lifestyle changes and metformin will benefit from the addition of a secretagogue, which provides a complementary mechanism of action.
Sulfonylureas are inexpensive and effective but potentially problematic because they may cause hypoglycemia and contribute to b-cell exhaustion. This is because they stimulate insulin secretion independent of circulating glucose levels. Glinides work in a similar manner, but have a more rapid onset and a shorter duration of action than sulfonylureas. Thus, they can be effective at mitigating prandial hyperglycemia, but require dosing with meals.
Newer secretagogues such as GLP-1 agonists and DPP-4 inhibitors stimulate secretion of insulin in response to hyperglycemia, reducing the risk of hypoglycemia and ultimately preserving some b-cell function. These newer agents, as well as glinides, are significantly more expensive than sulfony-lureas. GLP-1 agonists have the additional disadvantage of requiring injection. Patients who are Our Diabetes Scorecard, which is automatically populated by data from our EHR, provides an at-a-glance summary that is useful to clinicians and patients alike. far from their glycemic target will benefit from the addition of insulin.
CORRESPONDENCE
Ronald N. Adler, MD, UMass Memorial Medical Center, 279 Lincoln Street, Worcester, MA 01605; [email protected]
1. Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes preva- lence since 1980. Lancet. 2011;378:31-40.
2. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653.
3. Ford ES. Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the U.S.: findings from the National Health and Nutrition Examination Survey, 1999- 2008. Diabetes Care. 2011;34:1337-1343.
4. Golden SH. Emerging therapeutic approaches for the manage- ment of diabetes mellitus and macrovascular complications. Am J Cardiol. 2011;108(3 suppl):59B-67B.
5. American Diabetes Association. Diabetes statistics. Edited Au- gust 20, 2013. Available at: http://www.diabetes.org/diabetes- basics/diabetes-statistics/. Accessed September 11, 2013.
6. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596-615.
7. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
8. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The preva- lence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36:2271- 2279.
9. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2-4.
10. Wagner EH, Austin BT, Davis C, et al. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20:64-78.
11. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millennium. Health Aff (Millwood). 2009;28:75-85.
12. Bojadzievski T, Gabbay RA. Patient-centered medical home and diabetes. Diabetes Care. 2011;34:1047-1053.
13. Anderson RJ, Freedland KE, Clouse RE, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069-1078.
14. Katon W, Von KM, Ciechanowski P, et al. Behavioral and clini- cal factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914-920.
15. Katon W, Russo J, Lin EH, et al. Diabetes and poor disease control: is comorbid depression associated with poor medica- tion adherence or lack of treatment intensification? Psychosom Med. 2009;71:965-972.
16. Bogner HR, Morales KH, de Vries HF, et al. Integrated manage- ment of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med. 2012;10:15-22.
17. Pignone MP, Gaynes BN, Rushton JL, et al. Screening for de- pression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:765- 776.
18. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
19. Executive summary: standards of medical care in diabe- tes—2013. Diabetes Care. 2013;36(suppl 1):S4-S10.
20. Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med. 2012;366:1319-1327.
21. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hy- perglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
22. Greene J, Hibbard JH, Sacks R, et al. When seeing the same physician, highly activated patients have better experi- ences than less activated patients. Health Aff (Millwood). 2013;32:1299-1305.
23. Battersby M, Von KM, Schaefer J, et al. Twelve evidence-based principles for implementing self-management support in pri- mary care. Jt Comm J Qual Patient Saf. 2010;36:561-570.
24. Watson S. The 13 best diabetes iPhone & Android apps of 2013. Healthline Web site. Available at: http://www.healthline.com/ health-slideshow/top-iphone-android-apps-diabetes. Published August 8, 2013. Accessed September 16, 2013.
1. Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes preva- lence since 1980. Lancet. 2011;378:31-40.
2. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653.
3. Ford ES. Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the U.S.: findings from the National Health and Nutrition Examination Survey, 1999- 2008. Diabetes Care. 2011;34:1337-1343.
4. Golden SH. Emerging therapeutic approaches for the manage- ment of diabetes mellitus and macrovascular complications. Am J Cardiol. 2011;108(3 suppl):59B-67B.
5. American Diabetes Association. Diabetes statistics. Edited Au- gust 20, 2013. Available at: http://www.diabetes.org/diabetes- basics/diabetes-statistics/. Accessed September 11, 2013.
6. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596-615.
7. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
8. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The preva- lence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36:2271- 2279.
9. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2-4.
10. Wagner EH, Austin BT, Davis C, et al. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20:64-78.
11. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millennium. Health Aff (Millwood). 2009;28:75-85.
12. Bojadzievski T, Gabbay RA. Patient-centered medical home and diabetes. Diabetes Care. 2011;34:1047-1053.
13. Anderson RJ, Freedland KE, Clouse RE, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069-1078.
14. Katon W, Von KM, Ciechanowski P, et al. Behavioral and clini- cal factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914-920.
15. Katon W, Russo J, Lin EH, et al. Diabetes and poor disease control: is comorbid depression associated with poor medica- tion adherence or lack of treatment intensification? Psychosom Med. 2009;71:965-972.
16. Bogner HR, Morales KH, de Vries HF, et al. Integrated manage- ment of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med. 2012;10:15-22.
17. Pignone MP, Gaynes BN, Rushton JL, et al. Screening for de- pression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:765- 776.
18. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
19. Executive summary: standards of medical care in diabe- tes—2013. Diabetes Care. 2013;36(suppl 1):S4-S10.
20. Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med. 2012;366:1319-1327.
21. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hy- perglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
22. Greene J, Hibbard JH, Sacks R, et al. When seeing the same physician, highly activated patients have better experi- ences than less activated patients. Health Aff (Millwood). 2013;32:1299-1305.
23. Battersby M, Von KM, Schaefer J, et al. Twelve evidence-based principles for implementing self-management support in pri- mary care. Jt Comm J Qual Patient Saf. 2010;36:561-570.
24. Watson S. The 13 best diabetes iPhone & Android apps of 2013. Healthline Web site. Available at: http://www.healthline.com/ health-slideshow/top-iphone-android-apps-diabetes. Published August 8, 2013. Accessed September 16, 2013.
When to worry about incidental renal and adrenal masses
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.
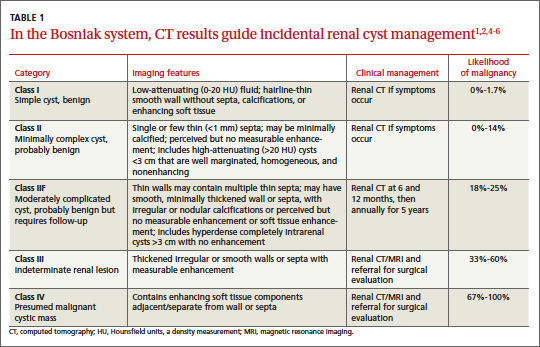
Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
Plantar fasciitis: How best to treat?
› Use plantar fascia specific stretching to decrease pain in patients with plantar fasciitis. A
› Consider recommending prefabricated orthoses, including night splints, to decrease pain. A
› Consider using extracorporeal shock wave therapy for plantar fascial pain. A
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 43-year-old obese woman seeks advice for left heel pain she has had for 2 months. Before the onset of pain, her activity level had increased as part of a weight loss program. Her pain is at its worst in the morning, with her first few steps; it decreases with continued walking and intensifies again after being on her feet all day. There is no history of trauma, and she reports no paresthesias or radiation of the pain. Her medical history is otherwise unremarkable. She has used ibuprofen sparingly, with limited relief.
If you were this patient’s physician, how would you proceed with her care?
Plantar fasciitis (PF) is a common cause of heel pain that affects up to 10% of the US population and accounts for approximately 600,000 outpatient visits annually.1 The plantar fascia is a dense, fibrous membrane spanning the length of the foot. It originates at the medial calcaneal tubercle, attaches to the phalanges, and provides stability and arch support to the foot. The etiology of PF is unknown, but predisposing factors include overtraining, obesity, pes planus, decreased ankle dorsiflexion, and inappropriate footwear.2 Limited dorsiflexion due to tightness of the Achilles tendon strains the plantar fascia and can lead to PF. Histology shows minimal inflammatory changes, and some experts advocate the term plantar fasciosis to counter the misperception that it is primarily an inflammatory condition.3
A patient’s history and physical exam findings are the basis for confirming or dismissing a diagnosis of PF. Radiologic studies, used judiciously, can rule out important alternative diagnoses that should not be overlooked. Multiple treatment options range from conservative to surgical interventions, although studies of the effectiveness of each modality have had conflicting results. Clinical practice guidelines generally advocate a stepwise approach to treatment.
Diagnosis
The differential diagnosis of PF (TABLE) includes significant disorders such as calcaneal stress fracture, entrapment neuropathies (eg, tarsal tunnel syndrome), calcaneal tumor, Paget’s disease, and systemic arthritidies.4,5
What to look for in the history and physical exam
Severe heel pain upon initial weight bearing in the morning or after prolonged periods of inactivity is pathognomonic for PF.2 Initially the pain presents diffusely, but over time it localizes to the area of the medial calcaneal tubercle. Pain typically subsides with activity but may return with prolonged weight bearing, as it did with the patient in the opening case.
Test range of motion of the foot and ankle. Although this is not needed for diagnosing PF, some patients will exhibit limited ankle dorsiflexion, a predisposing factor for PF.4,6 Look for heel pad swelling, inflammation, or atrophy, and palpate the heel, plantar fascia, and calcaneal tubercle. Lastly, evaluate for gait abnormalities and the presence of sensory deficits or hypesthesias.4
The most common exam finding in PF is pain at the medial calcaneal tubercle, which may be exacerbated with passive ankle dorsiflexion or first digit extension.2,4 If paresthesias occur with percussion inferior to the medial malleolus, suspect possible nerve entrapment or tarsal tunnel syndrome. Tenderness with heel compression (squeeze test) may indicate a calcaneal fracture or apophysitis.
Imaging is useful to rule out alternative disorders
Radiologic studies generally do not contribute to the diagnosis or management of PF, but they can assist in ruling out alternative causes of heel pain or in reevaluation if symptoms of PF persist after 3 to 6 months of treatment.
Plain films lack the sensitivity to detect plantar fasciitis. While a plantar calcaneal spur is often seen on radiography, it does not confirm the diagnosis, correlate with severity of symptoms, or predict prognosis.4 Despite this deficiency, plain radiography remains the initial choice of imaging modalities, particularly to rule out other conditions.
Ultrasound accurately diagnoses plantar fasciitis. Plantar fascia thickness of more than 4.0 mm is diagnostic of PF.7 Additionally, a decrease in plantar fascia thickness correlates with a decrease in pain levels, and thus ultrasound can aid in monitoring treatment progress.8 If results of plain films and ultrasound are inconclusive and clinical concern for an alternative diagnosis warrants additional expense, consider arranging for magnetic resonance imaging.9
Noninvasive treatments
Conservative therapies remain the preferred approach to treating PF, successfully managing 85% to 90% of cases.10,11 A 2010 clinical practice guideline from the American College of Foot and Ankle Surgeons recommends conservative treatments such as nonsteroidal inflammatory drugs (NSAIDs), stretching, and prefabricated orthotics for the initial management of plantar heel pain.4 Emphasize to patients that it may take 6 to 12 months for symptoms to resolve.4
Stretching and trigger-point manual therapy are effective
The traditional primary treatment modality for PF has been early initiation of an Achilles-soleus (heel-cord) muscle–stretching program. However, studies have shown that plantar fascia–specific stretching (PFSS) (FIGURE) significantly diminishes or eliminates heel pain when compared with traditional stretching movements, and is useful in treating chronic recalcitrant heel pain.12,13 PFSS has also yielded results superior to low-dose shock wave therapy.14
In a 2011 study, adding myofascial trigger-point manual therapy to a PFSS routine improved self-reported physical function and pain vs stretching alone.15 This manual therapy technique is specialized and should be administered only by trained physical therapists. Data are limited and mixed regarding the effectiveness of deep tissue massage, iontophoresis, or eccentric stretching of the plantar fascia to alleviate plantar fascial pain. Support for therapies such as rest, ice, heat, and massage has largely been anecdotal.
NSAIDs for PF lack good evidence
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often prescribed to treat PF, despite a lack of evidence supporting their use. A small randomized, placebo-controlled double-blind study established a trend toward improvement in pain and disability scores with the use of NSAIDs. However, no statistically significant difference was noted in the measures between the NSAID and placebo groups at 1, 2, and 6 months.16 We found no studies that demonstrate a significant reduction in pain or improvement in function with the use of NSAIDs alone.
Although NSAIDs carry their own risks, they may work for some patients. And studies showing a lack of significant pain reduction may have been underpowered. If patients are willing to accept the risks of NSAID use, it would be reasonable to prescribe a therapeutic trial.
Orthotics and night splints can help, depending on comfort and compliance
Foot orthotics help prevent overpronation and attenuate tensile forces on the plantar fascia. A 2009 meta-analysis confirmed that both prefabricated and custom-made foot orthotics can decrease pain.17 One prospective study showed that 95% of patients had improvement in PF symptoms after 8 weeks of treatment with prefabricated orthotics.18 A Cochrane review found no difference in pain reduction between custom and prefabricated foot orthotics.19 A recent study demonstrated that rocker sole shoes—a type of therapeutic footwear with a more rounded outsole contour—combined with custom orthotics significantly reduced pain during walking compared with either modality alone.20 More research needs to be conducted into the use of rocker sole shoes before recommending them to PF patients.
Night splints help keep the foot and ankle in a neutral position, or slightly dorsiflexed, while patients sleep. Several studies have shown a reduction in pain with the use of night splints alone.17,21,22 Patient comfort and compliance tend to be the limiting factors in their use. Anterior splints are better tolerated than posterior splints.23
Shock wave therapy has better long-term results than steroid injections
Shock waves used to treat PF are thought to invoke extracellular responses that cause neovascularization and induce tissue repair and regeneration. A 2012 review article concluded that most research confirms that extracorporeal shock wave therapy (ESWT) reduces PF pain and improves function in 34% to 88% of cases.24 ESWT is comparable to surgical plantar fasciotomy without the operative risks, and yields better long-term effects in recalcitrant PF compared with corticosteroid injections (CSI).24 Many studies are underway to validate the effectiveness of ESWT. Currently, expense or lack of availability limits its use in some communities.
Invasive treatments
Corticosteroid injections may be used for more than just refractory pain
CSI have historically been reserved for recalcitrant heel pain. However, one systematic review cites evidence in support of CSI for the short-term management of plantar fascia pain.25 Compared with placebo, CSI reduces pain at both 6 and 12 weeks and decreases plantar fascia thickness.26 Additionally, the American College of Foot and Ankle Surgeons lists CSI as an acceptable first-line treatment for PF.4
The most common complication of CSI is postinjection pain. Other complications, such as fat pad atrophy, rarely occur.27 While the evidence is limited, CSI may be part of an initial approach to treating PF in addition to heel-cord or plantar fascia-specific stretching, particularly for patients who desire an expedited return to normal activity.
Platelet-rich plasma therapy holds promise
Platelet-rich plasma (PRP) has been gaining popularity as a treatment for PF pain. PRP is a component of whole blood that is centrifuged to a concentrated state, treated with an activating agent, and injected into the affected area. Theoretically, injected PRP increases the release of reparative growth factors, enhancing the healing process.28 PRP has been shown to be as effective in reducing pain scores as CSI at 3 weeks and 6 months.29 PRP also decreases plantar fascia thickness and improves pain scores and functional ability.30
To date, no trials have compared PRP with placebo injections. Postprocedural pain is the most common risk with PRP. While limited evidence exists, PRP seems to be a relatively safe and effective therapeutic alternative for treating chronic PF.
Surgery only when conservative measures fail
Reserve surgery for those who have not responded adequately after 6 to 12 months of conservative therapy.5 Endoscopic plantar fascia release is superior to traditional open surgery.31 Heel spur resection is no longer routinely practiced. Patients undergoing surgery should expect a return to normal activity in approximately 2 to 3 months, and up to 35% of patients may continue to have symptoms after surgical intervention.2,31
Treatment options in perspective
Treat conservatively at first. Stretching the plantar fascia and heel cord, using prefabricated orthotics, and wearing night splints are backed by firm clinical evidence of benefit. Acute treatment of PF may also include CSI, especially for patients who are athletic or otherwise active and wish to return to full function as soon as possible, and are willing to accept the risks associated with CSI.
ESWT improves pain and function scores and may also relieve pain in patients with recalcitrant PF pain. PRP has limited but promising evidence for patients with chronic PF pain. Surgical intervention remains the last line of therapy and is not always effective at reducing pain.
CASE You prescribe a conservative treatment program of plantar fascia–specific stretches and prefabricated orthoses for the patient in the opening scenario. At one month, her pain drops by 30%. At 6 months, her pain disappears, and she resumes a daily aerobic exercise program to assist in weight loss.
CORRESPONDENCE
Carlton J. Covey, MD, Nellis Family Medicine Residency, 99MDOS/SGOF, 4700 Las Vegas Boulevard N, Las Vegas, NV 89191;
[email protected]
1. Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25: 303-310.
2. Glazer JL. An approach to the diagnosis and treatment of plantar fasciitis. Phys Sportsmed. 2009;37:74-79.
3. Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93:234-237.
4. Thomas JL, Christensen JC, Kravitz SR, et al. The diagnosis and treatment of heal pain: a clinical practice guideline – revision 2010. J Foot Ankle Surg. 2010;49(suppl):S1-S19.
5. Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg. 2008;16:338-346.
6. Singh D, Angel J, Bentley G, et al. Fortnightly review: plantar fasciitis. BMJ. 1997;315:172-175.
7. McMillan AM, Landorf KB, Barrett JT, et al. Diagnostic imaging for chronic plantar heel pain: a systematic review and metaanalysis. J Foot Ankle Res. 2009;2:32.
8. Mahowald S, Legge BS, Grady JF. The correlation between plantar fascia thickness and symptoms of plantar fasciitis. J Am Podiatr Med Assoc. 2011;101:385-389.
9. American College of Radiology. ACR appropriateness criteria. Chronic foot pain. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/ChronicFootPain.pdf. Accessed November 11, 2012.
10. Gill LH. Plantar fasciitis: diagnosis and conservative treatment. J Am Acad Orthop Surg. 1997;5:109-117.
11. Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int. 1998;19:803-811.
12. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fasciaspecific stretching exercise improves outcomes in patients with chronic plantar fasciitis: a prospective clinic trial with two-year follow up. J Bone Joint Surg Am. 2006;88:1775-1781.
13. Sweeting D, Parish B, Hooper L, et al. The effectiveness of manual stretching in the treatment of plantar heel pain: a systemic review. J Foot Ankle Res. 2011;4:1-13.
14. Rompe JD, Cacchio A, Lowell W, et al. Plantar fascia-specific stretching versus radial shock-wave therapy as initial treatment of plantar fasciopathy. J Bone Joint Surg Am. 2010;92:2514-2522.
15. Renan-Ordine R, Alburquerque-Sendin F, Rodriques De Souza DP, et al. Effectiveness of myofascial trigger point manual therapy combined with a self stretching protocol for the management of plantar heel pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2011;41:43-50.
16. Donley BG, Moore T, Sferra J, et al. The efficacy of oral nonsteroidal anti-inflammatory medication (NSAID) in the treatment of plantar fasciitis: a randomized, prospective, placebo-controlled study. Foot Ankle Int. 2007;28:20-23.
17. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve selfreported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
18. Pfeffer G, Bacchetti P, Deland J, et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999;20:214-221.
19. Hawke F, Burns J, Radford JA, et al. Custom-made foot orthoses for the treatment of foot pain. Cochrane Database Syst Rev. 2008;(3):CD006801.
20. Fong DT, Pang KY, Chung MM, et al. Evaluation of combined prescription of rocker sole shoes and custom-made foot orthoses for the treatment of plantar fasciitis. Clin Biomech. 2012;27: 1072-1077.
21. Berlet GC, Anderson RB, Davis H. A prospective trial of night splinting in the treatment of recalcitrant plantar fasciitis: the Ankle Dorsiflexion Dynasplint. Orthopedics. 2002;25: 1273-1275.
22. Roos E, Engstrom M, Soderberg B. Foot orthoses for the treatment of plantar fasciitis. Foot Ankle Int. 2006;27:606-611.
23. Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician. 2011;84:676-682.
24. Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11.
25. Landorf KB, Menz HB. Plantar heel pain and fasciitis. Clin Evid (Online). 2008;2008:1111.
26. Ball EM, McKeeman HM, Patterson C, et al. Steroid injection for inferior heel pain: a randomized controlled trial. Ann Rheum Dis. 2013;72:996-1002.
27. Uden H, Boesch E, Kumar S. Plantar fasciitis – to jab or support? A systematic review of the current best evidence. J Multidiscip Healthc. 2011;4:155-164.
28. Shetty VD. Platelet-rich plasma: a ‘feeling’ and ‘hope’ ailing athletes. Br J Sports Med. 2010;44(suppl 1):i1-i82.
29. Aksahin E, Dogruyol D, Yüksel HY, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:781-785.
30. Ragab EM, Othman AM. Platelets rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:1065-1070.
31. Saxena A. Uniportal endoscopic plantar fasciotomy: a prospective study on athletic patients. Foot Ankle Int. 2004;25:882-889.
› Use plantar fascia specific stretching to decrease pain in patients with plantar fasciitis. A
› Consider recommending prefabricated orthoses, including night splints, to decrease pain. A
› Consider using extracorporeal shock wave therapy for plantar fascial pain. A
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 43-year-old obese woman seeks advice for left heel pain she has had for 2 months. Before the onset of pain, her activity level had increased as part of a weight loss program. Her pain is at its worst in the morning, with her first few steps; it decreases with continued walking and intensifies again after being on her feet all day. There is no history of trauma, and she reports no paresthesias or radiation of the pain. Her medical history is otherwise unremarkable. She has used ibuprofen sparingly, with limited relief.
If you were this patient’s physician, how would you proceed with her care?
Plantar fasciitis (PF) is a common cause of heel pain that affects up to 10% of the US population and accounts for approximately 600,000 outpatient visits annually.1 The plantar fascia is a dense, fibrous membrane spanning the length of the foot. It originates at the medial calcaneal tubercle, attaches to the phalanges, and provides stability and arch support to the foot. The etiology of PF is unknown, but predisposing factors include overtraining, obesity, pes planus, decreased ankle dorsiflexion, and inappropriate footwear.2 Limited dorsiflexion due to tightness of the Achilles tendon strains the plantar fascia and can lead to PF. Histology shows minimal inflammatory changes, and some experts advocate the term plantar fasciosis to counter the misperception that it is primarily an inflammatory condition.3
A patient’s history and physical exam findings are the basis for confirming or dismissing a diagnosis of PF. Radiologic studies, used judiciously, can rule out important alternative diagnoses that should not be overlooked. Multiple treatment options range from conservative to surgical interventions, although studies of the effectiveness of each modality have had conflicting results. Clinical practice guidelines generally advocate a stepwise approach to treatment.
Diagnosis
The differential diagnosis of PF (TABLE) includes significant disorders such as calcaneal stress fracture, entrapment neuropathies (eg, tarsal tunnel syndrome), calcaneal tumor, Paget’s disease, and systemic arthritidies.4,5
What to look for in the history and physical exam
Severe heel pain upon initial weight bearing in the morning or after prolonged periods of inactivity is pathognomonic for PF.2 Initially the pain presents diffusely, but over time it localizes to the area of the medial calcaneal tubercle. Pain typically subsides with activity but may return with prolonged weight bearing, as it did with the patient in the opening case.
Test range of motion of the foot and ankle. Although this is not needed for diagnosing PF, some patients will exhibit limited ankle dorsiflexion, a predisposing factor for PF.4,6 Look for heel pad swelling, inflammation, or atrophy, and palpate the heel, plantar fascia, and calcaneal tubercle. Lastly, evaluate for gait abnormalities and the presence of sensory deficits or hypesthesias.4
The most common exam finding in PF is pain at the medial calcaneal tubercle, which may be exacerbated with passive ankle dorsiflexion or first digit extension.2,4 If paresthesias occur with percussion inferior to the medial malleolus, suspect possible nerve entrapment or tarsal tunnel syndrome. Tenderness with heel compression (squeeze test) may indicate a calcaneal fracture or apophysitis.
Imaging is useful to rule out alternative disorders
Radiologic studies generally do not contribute to the diagnosis or management of PF, but they can assist in ruling out alternative causes of heel pain or in reevaluation if symptoms of PF persist after 3 to 6 months of treatment.
Plain films lack the sensitivity to detect plantar fasciitis. While a plantar calcaneal spur is often seen on radiography, it does not confirm the diagnosis, correlate with severity of symptoms, or predict prognosis.4 Despite this deficiency, plain radiography remains the initial choice of imaging modalities, particularly to rule out other conditions.
Ultrasound accurately diagnoses plantar fasciitis. Plantar fascia thickness of more than 4.0 mm is diagnostic of PF.7 Additionally, a decrease in plantar fascia thickness correlates with a decrease in pain levels, and thus ultrasound can aid in monitoring treatment progress.8 If results of plain films and ultrasound are inconclusive and clinical concern for an alternative diagnosis warrants additional expense, consider arranging for magnetic resonance imaging.9
Noninvasive treatments
Conservative therapies remain the preferred approach to treating PF, successfully managing 85% to 90% of cases.10,11 A 2010 clinical practice guideline from the American College of Foot and Ankle Surgeons recommends conservative treatments such as nonsteroidal inflammatory drugs (NSAIDs), stretching, and prefabricated orthotics for the initial management of plantar heel pain.4 Emphasize to patients that it may take 6 to 12 months for symptoms to resolve.4
Stretching and trigger-point manual therapy are effective
The traditional primary treatment modality for PF has been early initiation of an Achilles-soleus (heel-cord) muscle–stretching program. However, studies have shown that plantar fascia–specific stretching (PFSS) (FIGURE) significantly diminishes or eliminates heel pain when compared with traditional stretching movements, and is useful in treating chronic recalcitrant heel pain.12,13 PFSS has also yielded results superior to low-dose shock wave therapy.14
In a 2011 study, adding myofascial trigger-point manual therapy to a PFSS routine improved self-reported physical function and pain vs stretching alone.15 This manual therapy technique is specialized and should be administered only by trained physical therapists. Data are limited and mixed regarding the effectiveness of deep tissue massage, iontophoresis, or eccentric stretching of the plantar fascia to alleviate plantar fascial pain. Support for therapies such as rest, ice, heat, and massage has largely been anecdotal.
NSAIDs for PF lack good evidence
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often prescribed to treat PF, despite a lack of evidence supporting their use. A small randomized, placebo-controlled double-blind study established a trend toward improvement in pain and disability scores with the use of NSAIDs. However, no statistically significant difference was noted in the measures between the NSAID and placebo groups at 1, 2, and 6 months.16 We found no studies that demonstrate a significant reduction in pain or improvement in function with the use of NSAIDs alone.
Although NSAIDs carry their own risks, they may work for some patients. And studies showing a lack of significant pain reduction may have been underpowered. If patients are willing to accept the risks of NSAID use, it would be reasonable to prescribe a therapeutic trial.
Orthotics and night splints can help, depending on comfort and compliance
Foot orthotics help prevent overpronation and attenuate tensile forces on the plantar fascia. A 2009 meta-analysis confirmed that both prefabricated and custom-made foot orthotics can decrease pain.17 One prospective study showed that 95% of patients had improvement in PF symptoms after 8 weeks of treatment with prefabricated orthotics.18 A Cochrane review found no difference in pain reduction between custom and prefabricated foot orthotics.19 A recent study demonstrated that rocker sole shoes—a type of therapeutic footwear with a more rounded outsole contour—combined with custom orthotics significantly reduced pain during walking compared with either modality alone.20 More research needs to be conducted into the use of rocker sole shoes before recommending them to PF patients.
Night splints help keep the foot and ankle in a neutral position, or slightly dorsiflexed, while patients sleep. Several studies have shown a reduction in pain with the use of night splints alone.17,21,22 Patient comfort and compliance tend to be the limiting factors in their use. Anterior splints are better tolerated than posterior splints.23
Shock wave therapy has better long-term results than steroid injections
Shock waves used to treat PF are thought to invoke extracellular responses that cause neovascularization and induce tissue repair and regeneration. A 2012 review article concluded that most research confirms that extracorporeal shock wave therapy (ESWT) reduces PF pain and improves function in 34% to 88% of cases.24 ESWT is comparable to surgical plantar fasciotomy without the operative risks, and yields better long-term effects in recalcitrant PF compared with corticosteroid injections (CSI).24 Many studies are underway to validate the effectiveness of ESWT. Currently, expense or lack of availability limits its use in some communities.
Invasive treatments
Corticosteroid injections may be used for more than just refractory pain
CSI have historically been reserved for recalcitrant heel pain. However, one systematic review cites evidence in support of CSI for the short-term management of plantar fascia pain.25 Compared with placebo, CSI reduces pain at both 6 and 12 weeks and decreases plantar fascia thickness.26 Additionally, the American College of Foot and Ankle Surgeons lists CSI as an acceptable first-line treatment for PF.4
The most common complication of CSI is postinjection pain. Other complications, such as fat pad atrophy, rarely occur.27 While the evidence is limited, CSI may be part of an initial approach to treating PF in addition to heel-cord or plantar fascia-specific stretching, particularly for patients who desire an expedited return to normal activity.
Platelet-rich plasma therapy holds promise
Platelet-rich plasma (PRP) has been gaining popularity as a treatment for PF pain. PRP is a component of whole blood that is centrifuged to a concentrated state, treated with an activating agent, and injected into the affected area. Theoretically, injected PRP increases the release of reparative growth factors, enhancing the healing process.28 PRP has been shown to be as effective in reducing pain scores as CSI at 3 weeks and 6 months.29 PRP also decreases plantar fascia thickness and improves pain scores and functional ability.30
To date, no trials have compared PRP with placebo injections. Postprocedural pain is the most common risk with PRP. While limited evidence exists, PRP seems to be a relatively safe and effective therapeutic alternative for treating chronic PF.
Surgery only when conservative measures fail
Reserve surgery for those who have not responded adequately after 6 to 12 months of conservative therapy.5 Endoscopic plantar fascia release is superior to traditional open surgery.31 Heel spur resection is no longer routinely practiced. Patients undergoing surgery should expect a return to normal activity in approximately 2 to 3 months, and up to 35% of patients may continue to have symptoms after surgical intervention.2,31
Treatment options in perspective
Treat conservatively at first. Stretching the plantar fascia and heel cord, using prefabricated orthotics, and wearing night splints are backed by firm clinical evidence of benefit. Acute treatment of PF may also include CSI, especially for patients who are athletic or otherwise active and wish to return to full function as soon as possible, and are willing to accept the risks associated with CSI.
ESWT improves pain and function scores and may also relieve pain in patients with recalcitrant PF pain. PRP has limited but promising evidence for patients with chronic PF pain. Surgical intervention remains the last line of therapy and is not always effective at reducing pain.
CASE You prescribe a conservative treatment program of plantar fascia–specific stretches and prefabricated orthoses for the patient in the opening scenario. At one month, her pain drops by 30%. At 6 months, her pain disappears, and she resumes a daily aerobic exercise program to assist in weight loss.
CORRESPONDENCE
Carlton J. Covey, MD, Nellis Family Medicine Residency, 99MDOS/SGOF, 4700 Las Vegas Boulevard N, Las Vegas, NV 89191;
[email protected]
› Use plantar fascia specific stretching to decrease pain in patients with plantar fasciitis. A
› Consider recommending prefabricated orthoses, including night splints, to decrease pain. A
› Consider using extracorporeal shock wave therapy for plantar fascial pain. A
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 43-year-old obese woman seeks advice for left heel pain she has had for 2 months. Before the onset of pain, her activity level had increased as part of a weight loss program. Her pain is at its worst in the morning, with her first few steps; it decreases with continued walking and intensifies again after being on her feet all day. There is no history of trauma, and she reports no paresthesias or radiation of the pain. Her medical history is otherwise unremarkable. She has used ibuprofen sparingly, with limited relief.
If you were this patient’s physician, how would you proceed with her care?
Plantar fasciitis (PF) is a common cause of heel pain that affects up to 10% of the US population and accounts for approximately 600,000 outpatient visits annually.1 The plantar fascia is a dense, fibrous membrane spanning the length of the foot. It originates at the medial calcaneal tubercle, attaches to the phalanges, and provides stability and arch support to the foot. The etiology of PF is unknown, but predisposing factors include overtraining, obesity, pes planus, decreased ankle dorsiflexion, and inappropriate footwear.2 Limited dorsiflexion due to tightness of the Achilles tendon strains the plantar fascia and can lead to PF. Histology shows minimal inflammatory changes, and some experts advocate the term plantar fasciosis to counter the misperception that it is primarily an inflammatory condition.3
A patient’s history and physical exam findings are the basis for confirming or dismissing a diagnosis of PF. Radiologic studies, used judiciously, can rule out important alternative diagnoses that should not be overlooked. Multiple treatment options range from conservative to surgical interventions, although studies of the effectiveness of each modality have had conflicting results. Clinical practice guidelines generally advocate a stepwise approach to treatment.
Diagnosis
The differential diagnosis of PF (TABLE) includes significant disorders such as calcaneal stress fracture, entrapment neuropathies (eg, tarsal tunnel syndrome), calcaneal tumor, Paget’s disease, and systemic arthritidies.4,5
What to look for in the history and physical exam
Severe heel pain upon initial weight bearing in the morning or after prolonged periods of inactivity is pathognomonic for PF.2 Initially the pain presents diffusely, but over time it localizes to the area of the medial calcaneal tubercle. Pain typically subsides with activity but may return with prolonged weight bearing, as it did with the patient in the opening case.
Test range of motion of the foot and ankle. Although this is not needed for diagnosing PF, some patients will exhibit limited ankle dorsiflexion, a predisposing factor for PF.4,6 Look for heel pad swelling, inflammation, or atrophy, and palpate the heel, plantar fascia, and calcaneal tubercle. Lastly, evaluate for gait abnormalities and the presence of sensory deficits or hypesthesias.4
The most common exam finding in PF is pain at the medial calcaneal tubercle, which may be exacerbated with passive ankle dorsiflexion or first digit extension.2,4 If paresthesias occur with percussion inferior to the medial malleolus, suspect possible nerve entrapment or tarsal tunnel syndrome. Tenderness with heel compression (squeeze test) may indicate a calcaneal fracture or apophysitis.
Imaging is useful to rule out alternative disorders
Radiologic studies generally do not contribute to the diagnosis or management of PF, but they can assist in ruling out alternative causes of heel pain or in reevaluation if symptoms of PF persist after 3 to 6 months of treatment.
Plain films lack the sensitivity to detect plantar fasciitis. While a plantar calcaneal spur is often seen on radiography, it does not confirm the diagnosis, correlate with severity of symptoms, or predict prognosis.4 Despite this deficiency, plain radiography remains the initial choice of imaging modalities, particularly to rule out other conditions.
Ultrasound accurately diagnoses plantar fasciitis. Plantar fascia thickness of more than 4.0 mm is diagnostic of PF.7 Additionally, a decrease in plantar fascia thickness correlates with a decrease in pain levels, and thus ultrasound can aid in monitoring treatment progress.8 If results of plain films and ultrasound are inconclusive and clinical concern for an alternative diagnosis warrants additional expense, consider arranging for magnetic resonance imaging.9
Noninvasive treatments
Conservative therapies remain the preferred approach to treating PF, successfully managing 85% to 90% of cases.10,11 A 2010 clinical practice guideline from the American College of Foot and Ankle Surgeons recommends conservative treatments such as nonsteroidal inflammatory drugs (NSAIDs), stretching, and prefabricated orthotics for the initial management of plantar heel pain.4 Emphasize to patients that it may take 6 to 12 months for symptoms to resolve.4
Stretching and trigger-point manual therapy are effective
The traditional primary treatment modality for PF has been early initiation of an Achilles-soleus (heel-cord) muscle–stretching program. However, studies have shown that plantar fascia–specific stretching (PFSS) (FIGURE) significantly diminishes or eliminates heel pain when compared with traditional stretching movements, and is useful in treating chronic recalcitrant heel pain.12,13 PFSS has also yielded results superior to low-dose shock wave therapy.14
In a 2011 study, adding myofascial trigger-point manual therapy to a PFSS routine improved self-reported physical function and pain vs stretching alone.15 This manual therapy technique is specialized and should be administered only by trained physical therapists. Data are limited and mixed regarding the effectiveness of deep tissue massage, iontophoresis, or eccentric stretching of the plantar fascia to alleviate plantar fascial pain. Support for therapies such as rest, ice, heat, and massage has largely been anecdotal.
NSAIDs for PF lack good evidence
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often prescribed to treat PF, despite a lack of evidence supporting their use. A small randomized, placebo-controlled double-blind study established a trend toward improvement in pain and disability scores with the use of NSAIDs. However, no statistically significant difference was noted in the measures between the NSAID and placebo groups at 1, 2, and 6 months.16 We found no studies that demonstrate a significant reduction in pain or improvement in function with the use of NSAIDs alone.
Although NSAIDs carry their own risks, they may work for some patients. And studies showing a lack of significant pain reduction may have been underpowered. If patients are willing to accept the risks of NSAID use, it would be reasonable to prescribe a therapeutic trial.
Orthotics and night splints can help, depending on comfort and compliance
Foot orthotics help prevent overpronation and attenuate tensile forces on the plantar fascia. A 2009 meta-analysis confirmed that both prefabricated and custom-made foot orthotics can decrease pain.17 One prospective study showed that 95% of patients had improvement in PF symptoms after 8 weeks of treatment with prefabricated orthotics.18 A Cochrane review found no difference in pain reduction between custom and prefabricated foot orthotics.19 A recent study demonstrated that rocker sole shoes—a type of therapeutic footwear with a more rounded outsole contour—combined with custom orthotics significantly reduced pain during walking compared with either modality alone.20 More research needs to be conducted into the use of rocker sole shoes before recommending them to PF patients.
Night splints help keep the foot and ankle in a neutral position, or slightly dorsiflexed, while patients sleep. Several studies have shown a reduction in pain with the use of night splints alone.17,21,22 Patient comfort and compliance tend to be the limiting factors in their use. Anterior splints are better tolerated than posterior splints.23
Shock wave therapy has better long-term results than steroid injections
Shock waves used to treat PF are thought to invoke extracellular responses that cause neovascularization and induce tissue repair and regeneration. A 2012 review article concluded that most research confirms that extracorporeal shock wave therapy (ESWT) reduces PF pain and improves function in 34% to 88% of cases.24 ESWT is comparable to surgical plantar fasciotomy without the operative risks, and yields better long-term effects in recalcitrant PF compared with corticosteroid injections (CSI).24 Many studies are underway to validate the effectiveness of ESWT. Currently, expense or lack of availability limits its use in some communities.
Invasive treatments
Corticosteroid injections may be used for more than just refractory pain
CSI have historically been reserved for recalcitrant heel pain. However, one systematic review cites evidence in support of CSI for the short-term management of plantar fascia pain.25 Compared with placebo, CSI reduces pain at both 6 and 12 weeks and decreases plantar fascia thickness.26 Additionally, the American College of Foot and Ankle Surgeons lists CSI as an acceptable first-line treatment for PF.4
The most common complication of CSI is postinjection pain. Other complications, such as fat pad atrophy, rarely occur.27 While the evidence is limited, CSI may be part of an initial approach to treating PF in addition to heel-cord or plantar fascia-specific stretching, particularly for patients who desire an expedited return to normal activity.
Platelet-rich plasma therapy holds promise
Platelet-rich plasma (PRP) has been gaining popularity as a treatment for PF pain. PRP is a component of whole blood that is centrifuged to a concentrated state, treated with an activating agent, and injected into the affected area. Theoretically, injected PRP increases the release of reparative growth factors, enhancing the healing process.28 PRP has been shown to be as effective in reducing pain scores as CSI at 3 weeks and 6 months.29 PRP also decreases plantar fascia thickness and improves pain scores and functional ability.30
To date, no trials have compared PRP with placebo injections. Postprocedural pain is the most common risk with PRP. While limited evidence exists, PRP seems to be a relatively safe and effective therapeutic alternative for treating chronic PF.
Surgery only when conservative measures fail
Reserve surgery for those who have not responded adequately after 6 to 12 months of conservative therapy.5 Endoscopic plantar fascia release is superior to traditional open surgery.31 Heel spur resection is no longer routinely practiced. Patients undergoing surgery should expect a return to normal activity in approximately 2 to 3 months, and up to 35% of patients may continue to have symptoms after surgical intervention.2,31
Treatment options in perspective
Treat conservatively at first. Stretching the plantar fascia and heel cord, using prefabricated orthotics, and wearing night splints are backed by firm clinical evidence of benefit. Acute treatment of PF may also include CSI, especially for patients who are athletic or otherwise active and wish to return to full function as soon as possible, and are willing to accept the risks associated with CSI.
ESWT improves pain and function scores and may also relieve pain in patients with recalcitrant PF pain. PRP has limited but promising evidence for patients with chronic PF pain. Surgical intervention remains the last line of therapy and is not always effective at reducing pain.
CASE You prescribe a conservative treatment program of plantar fascia–specific stretches and prefabricated orthoses for the patient in the opening scenario. At one month, her pain drops by 30%. At 6 months, her pain disappears, and she resumes a daily aerobic exercise program to assist in weight loss.
CORRESPONDENCE
Carlton J. Covey, MD, Nellis Family Medicine Residency, 99MDOS/SGOF, 4700 Las Vegas Boulevard N, Las Vegas, NV 89191;
[email protected]
1. Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25: 303-310.
2. Glazer JL. An approach to the diagnosis and treatment of plantar fasciitis. Phys Sportsmed. 2009;37:74-79.
3. Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93:234-237.
4. Thomas JL, Christensen JC, Kravitz SR, et al. The diagnosis and treatment of heal pain: a clinical practice guideline – revision 2010. J Foot Ankle Surg. 2010;49(suppl):S1-S19.
5. Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg. 2008;16:338-346.
6. Singh D, Angel J, Bentley G, et al. Fortnightly review: plantar fasciitis. BMJ. 1997;315:172-175.
7. McMillan AM, Landorf KB, Barrett JT, et al. Diagnostic imaging for chronic plantar heel pain: a systematic review and metaanalysis. J Foot Ankle Res. 2009;2:32.
8. Mahowald S, Legge BS, Grady JF. The correlation between plantar fascia thickness and symptoms of plantar fasciitis. J Am Podiatr Med Assoc. 2011;101:385-389.
9. American College of Radiology. ACR appropriateness criteria. Chronic foot pain. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/ChronicFootPain.pdf. Accessed November 11, 2012.
10. Gill LH. Plantar fasciitis: diagnosis and conservative treatment. J Am Acad Orthop Surg. 1997;5:109-117.
11. Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int. 1998;19:803-811.
12. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fasciaspecific stretching exercise improves outcomes in patients with chronic plantar fasciitis: a prospective clinic trial with two-year follow up. J Bone Joint Surg Am. 2006;88:1775-1781.
13. Sweeting D, Parish B, Hooper L, et al. The effectiveness of manual stretching in the treatment of plantar heel pain: a systemic review. J Foot Ankle Res. 2011;4:1-13.
14. Rompe JD, Cacchio A, Lowell W, et al. Plantar fascia-specific stretching versus radial shock-wave therapy as initial treatment of plantar fasciopathy. J Bone Joint Surg Am. 2010;92:2514-2522.
15. Renan-Ordine R, Alburquerque-Sendin F, Rodriques De Souza DP, et al. Effectiveness of myofascial trigger point manual therapy combined with a self stretching protocol for the management of plantar heel pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2011;41:43-50.
16. Donley BG, Moore T, Sferra J, et al. The efficacy of oral nonsteroidal anti-inflammatory medication (NSAID) in the treatment of plantar fasciitis: a randomized, prospective, placebo-controlled study. Foot Ankle Int. 2007;28:20-23.
17. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve selfreported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
18. Pfeffer G, Bacchetti P, Deland J, et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999;20:214-221.
19. Hawke F, Burns J, Radford JA, et al. Custom-made foot orthoses for the treatment of foot pain. Cochrane Database Syst Rev. 2008;(3):CD006801.
20. Fong DT, Pang KY, Chung MM, et al. Evaluation of combined prescription of rocker sole shoes and custom-made foot orthoses for the treatment of plantar fasciitis. Clin Biomech. 2012;27: 1072-1077.
21. Berlet GC, Anderson RB, Davis H. A prospective trial of night splinting in the treatment of recalcitrant plantar fasciitis: the Ankle Dorsiflexion Dynasplint. Orthopedics. 2002;25: 1273-1275.
22. Roos E, Engstrom M, Soderberg B. Foot orthoses for the treatment of plantar fasciitis. Foot Ankle Int. 2006;27:606-611.
23. Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician. 2011;84:676-682.
24. Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11.
25. Landorf KB, Menz HB. Plantar heel pain and fasciitis. Clin Evid (Online). 2008;2008:1111.
26. Ball EM, McKeeman HM, Patterson C, et al. Steroid injection for inferior heel pain: a randomized controlled trial. Ann Rheum Dis. 2013;72:996-1002.
27. Uden H, Boesch E, Kumar S. Plantar fasciitis – to jab or support? A systematic review of the current best evidence. J Multidiscip Healthc. 2011;4:155-164.
28. Shetty VD. Platelet-rich plasma: a ‘feeling’ and ‘hope’ ailing athletes. Br J Sports Med. 2010;44(suppl 1):i1-i82.
29. Aksahin E, Dogruyol D, Yüksel HY, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:781-785.
30. Ragab EM, Othman AM. Platelets rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:1065-1070.
31. Saxena A. Uniportal endoscopic plantar fasciotomy: a prospective study on athletic patients. Foot Ankle Int. 2004;25:882-889.
1. Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25: 303-310.
2. Glazer JL. An approach to the diagnosis and treatment of plantar fasciitis. Phys Sportsmed. 2009;37:74-79.
3. Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93:234-237.
4. Thomas JL, Christensen JC, Kravitz SR, et al. The diagnosis and treatment of heal pain: a clinical practice guideline – revision 2010. J Foot Ankle Surg. 2010;49(suppl):S1-S19.
5. Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg. 2008;16:338-346.
6. Singh D, Angel J, Bentley G, et al. Fortnightly review: plantar fasciitis. BMJ. 1997;315:172-175.
7. McMillan AM, Landorf KB, Barrett JT, et al. Diagnostic imaging for chronic plantar heel pain: a systematic review and metaanalysis. J Foot Ankle Res. 2009;2:32.
8. Mahowald S, Legge BS, Grady JF. The correlation between plantar fascia thickness and symptoms of plantar fasciitis. J Am Podiatr Med Assoc. 2011;101:385-389.
9. American College of Radiology. ACR appropriateness criteria. Chronic foot pain. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/ChronicFootPain.pdf. Accessed November 11, 2012.
10. Gill LH. Plantar fasciitis: diagnosis and conservative treatment. J Am Acad Orthop Surg. 1997;5:109-117.
11. Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int. 1998;19:803-811.
12. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fasciaspecific stretching exercise improves outcomes in patients with chronic plantar fasciitis: a prospective clinic trial with two-year follow up. J Bone Joint Surg Am. 2006;88:1775-1781.
13. Sweeting D, Parish B, Hooper L, et al. The effectiveness of manual stretching in the treatment of plantar heel pain: a systemic review. J Foot Ankle Res. 2011;4:1-13.
14. Rompe JD, Cacchio A, Lowell W, et al. Plantar fascia-specific stretching versus radial shock-wave therapy as initial treatment of plantar fasciopathy. J Bone Joint Surg Am. 2010;92:2514-2522.
15. Renan-Ordine R, Alburquerque-Sendin F, Rodriques De Souza DP, et al. Effectiveness of myofascial trigger point manual therapy combined with a self stretching protocol for the management of plantar heel pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2011;41:43-50.
16. Donley BG, Moore T, Sferra J, et al. The efficacy of oral nonsteroidal anti-inflammatory medication (NSAID) in the treatment of plantar fasciitis: a randomized, prospective, placebo-controlled study. Foot Ankle Int. 2007;28:20-23.
17. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve selfreported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
18. Pfeffer G, Bacchetti P, Deland J, et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999;20:214-221.
19. Hawke F, Burns J, Radford JA, et al. Custom-made foot orthoses for the treatment of foot pain. Cochrane Database Syst Rev. 2008;(3):CD006801.
20. Fong DT, Pang KY, Chung MM, et al. Evaluation of combined prescription of rocker sole shoes and custom-made foot orthoses for the treatment of plantar fasciitis. Clin Biomech. 2012;27: 1072-1077.
21. Berlet GC, Anderson RB, Davis H. A prospective trial of night splinting in the treatment of recalcitrant plantar fasciitis: the Ankle Dorsiflexion Dynasplint. Orthopedics. 2002;25: 1273-1275.
22. Roos E, Engstrom M, Soderberg B. Foot orthoses for the treatment of plantar fasciitis. Foot Ankle Int. 2006;27:606-611.
23. Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician. 2011;84:676-682.
24. Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11.
25. Landorf KB, Menz HB. Plantar heel pain and fasciitis. Clin Evid (Online). 2008;2008:1111.
26. Ball EM, McKeeman HM, Patterson C, et al. Steroid injection for inferior heel pain: a randomized controlled trial. Ann Rheum Dis. 2013;72:996-1002.
27. Uden H, Boesch E, Kumar S. Plantar fasciitis – to jab or support? A systematic review of the current best evidence. J Multidiscip Healthc. 2011;4:155-164.
28. Shetty VD. Platelet-rich plasma: a ‘feeling’ and ‘hope’ ailing athletes. Br J Sports Med. 2010;44(suppl 1):i1-i82.
29. Aksahin E, Dogruyol D, Yüksel HY, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:781-785.
30. Ragab EM, Othman AM. Platelets rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:1065-1070.
31. Saxena A. Uniportal endoscopic plantar fasciotomy: a prospective study on athletic patients. Foot Ankle Int. 2004;25:882-889.