User login
Tinnitus: Steps to take, drugs to avoid
› Advise patients to wear earplugs or earmuffs to prevent hearing loss and tinnitus when exposed to excessively loud sounds (>80dB). C
› Avoid prescribing benzodiazepines, antidepressants, or gabapentin for the treatment of tinnitus; little evidence supports routine use of these agents. B
› Consider referring patients for cognitive-behavioral therapy or tinnitus retraining therapy, each of which can reduce the bothersome nature of tinnitus. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Mr. L is a 47-year-old construction worker who comes to the clinic with a 3-month history of bothersome, constant, high-pitched ringing in his ears that is worse on his right side. He also reports mild hearing loss. Mr. L notes that he keeps a busy schedule, working weekends at a local shooting range. He is a 30-pack-year smoker and takes sumatriptan and nasal fluticasone spray as needed for migraines and allergies. On inspection, his ears appear normal.
CASE 2 › Ms. B, age 68 years, seeks treatment for a constant pulsatile noise in her left ear, which has been bothering her for the past 5 months. She lives alone and says this noise is worse when the house is quiet. She takes chlorthalidone for hypertension and prophylactic aspirin. She indicates that she has no problems with her hearing. On exam, the noise synchronizes with her pulse.
If Mr. L and Ms. B were your patients, what would your next steps be?
An estimated 50 million people in the United States experience some form of tinnitus,1 and the incidence is on the rise, which some have attributed to the increased use of personal music devices.2,3 Patients often describe tinnitus as a ringing noise, but it also can be perceived as buzzing, chirping, hissing, whistling, humming, or other sound. It is more often bilateral than unilateral4 and more often intermittent than continuous.1
Tinnitus may be present in childhood, but the prevalence increases with age. Surveys show that approximately 25% of adults experience symptoms and one-fourth of these patients report that it interferes with daily activities.1,2 The prevalence peaks at 31% in patients between the ages of 60 and 69 years.1
The severity of the condition ranges from causing patients to merely be aware of the noise to having substantial adverse effects on their quality of life. Because not all patients will report tinnitus symptoms, it is important to be aware of risk factors, which include advanced age, male sex, history of military service, and a work history that includes exposure to loud noise.1,2 Smoking and hypertension also are associated with higher rates of tinnitus, as is living in the southern United States.1
A subjective, or objective case of tinnitus?
While subjective tinnitus consists of noises only the patient can hear, objective tinnitus refers to noises, including somatosounds such as turbulent blood flow or palatal myoclonus, that a physician could at least theoretically detect by auscultation or with an amplifying device. Objective tinnitus is less common than subjective tinnitus and more often has an identifiable and correctable source,5 though it may herald a serious underlying condition. When tinnitus is pulsatile or rhythmic, it may be the result of an arteriovenous fistula, arteriovenous malformation, cerebral aneurysm, arterial bruit, or other vascular lesion, such as a glomus tumor.6 Nonvascular conditions like palatal myoclonus present with clicking or low-pitched buzzing and may be a result of multiple sclerosis.7
The causes of both subjective and objective tinnitus are detailed in TABLE 1.2,6,7
Tinnitus and hearing loss: The connection
Most tinnitus is associated with hearing loss and probably results from a disruption in the normal suppression of neuronal activity in the central nervous system.2,8
Conductive hearing loss can be caused by cerumen impaction, otosclerosis, or cholesteatoma. Sensorineural hearing loss (SNHL), which is more common than conductive hearing loss, often is irreversible. The damage typically occurs in the stereocilia cells of the cochlea. These cells trigger the release of neurotransmitters that activate the eighth cranial nerve and cause abnormal excitation along the auditory pathway, giving the perception of sound in a quiet environment.2
Patients with SNHL usually have a history of prolonged exposure to loud noise (eg, heavy machinery, firearms, personal musical devices such as an iPod, or musical instruments) and often describe their tinnitus as a bilateral, high-pitched, continuous ringing. The other major category of SNHL that causes tinnitus is presbycusis—the hearing loss associated with aging—which has clinical features similar to noise-induced hearing loss.8
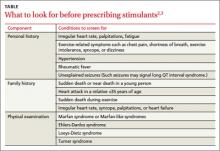
What to look for
Evaluation of tinnitus begins with a thorough history and physical exam (FIGURE 1).2,6,7,9 Key components of the exam include inspecting the ears, nose, and throat and evaluating cranial nerve function. Weber and Rinne tuning fork testing can help to confirm a conductive hearing loss. When evaluating a patient who reports pulsatile tinnitus, perform auscultation over vascular structures in the neck, temple, and around the ear.
Obtain targeted laboratory studies if there is a suggested metabolic etiology for tinnitus (TABLE 1).2,6,7 Handheld tympanometry that is flatlined or fluctuates with breathing can help support the diagnosis of a subtle middle ear effusion or patulous eustachian tube, respectively.
It is important to quantify how tinnitus affects a patient’s mood, including irritability and concentration. Tinnitus can be measured on several scales, including the Tinnitus Functional Index (TFI),10 which is easily completed in the office. It has been validated to quantify the severity of symptoms and can be used to monitor a patient’s progress. A copy of the TFI and its scoring instructions are available at http://www.ohsu.edu/xd/health/services/ent/services/tinnitus-clinic/tinnitus-functional-index.cfm.
Refer most patients to audiology. Patient’s symptoms often correlate poorly with acoustic functioning.6 Unless you find simple, reversible causes of tinnitus on history and physical, a comprehensive audiologic evaluation is essential. Components of these evaluations include pure-tone thresholds, tympanometry, speech thresholds, and speech discrimination testing.7
Image when necessary. If audiometric testing indicates cochlear damage, imaging generally is unnecessary because SNHL has been confirmed.7 However, if a retrocochlear hearing deficit is detected, auditory brainstem response testing is useful to help locate the lesion. Gadolinium-enhanced magnetic resonance imaging of the internal auditory canals also can be performed to evaluate for central nervous system lesions.2,7 This will detect vestibular schwannoma, which is the most frequent cause of tinnitus apparent on imaging.11
Pulsatility is the one true red flag feature of tinnitus and regardless of audiometry, patients with pulsatile tinnitus require imaging to rule out vascular lesions. The petrous carotid system is a common culprit; therefore, contrast-enhanced high-resolution computed tomography (CT) of the temporal bone is a reasonable initial study since it also will detect osseous abnormalities of the inner ear. However, angiography often is eventually necessary (conventional, magnetic resonance, or CT) to exclude a dural arteriovenous fistula or malformation-the most common cause of objective, pulsatile tinnitus.11 When tinnitus is pulsatile, unilateral, atypical in nature, or associated with deafness, imaging plus referral to a neurologist or otolaryngologist is advisable.6
Medications, and other factors to consider
Many different types of medications and substances can have ototoxic effects, mainly on the cochlear hair cells (TABLE 2).12 The damage may be reversible or irreversible. When doing so would be clinically prudent, consider tapering a patient off a drug that may be causing tinnitus.7
Other causes to consider. Pain in the jaw or neck may be due to a temporomandibular joint disorder or a cervical spine problem like whiplash; these conditions are associated with tinnitus and vertigo.7,13 The combination of low-pitched tinnitus, vertigo, aural fullness, and hearing loss often signifies Meniere’s disease—especially if symptoms are episodic.
Address mood disorders. Although insomnia, anxiety, depression, and posttraumatic stress disorder generally are not considered causes, these conditions are associated with tinnitus and can exacerbate the condition. Tinnitus can trigger depression, and vice versa. Optimizing treatment for these common problems can significantly reduce suffering.6,7
For most patients, you'll focus on prevention, rather than Tx
Treatment for tinnitus (which we’ll describe in a bit) is necessary only for patients for whom the condition has substantially affected the quality of their life.2 Greater emphasis should be placed on prevention.
Most tinnitus originates from the auditory system and is considered irreversible, but up to 25% of patients with chronic tinnitus report an increase in severity over time.14 Therefore, prevention can be beneficial not only for patients at risk of developing tinnitus, but also for those already affected by it. Prevention efforts should focus on protecting hearing by reducing noise levels and exposure time to certain noise thresholds.
The decibel (dB) scale is logarithmic; perception of sound loudness doubles every 10 dB. The sound of a vacuum cleaner is approximately 70 dB; the average human pain threshold is roughly 110 dB, which is the loudness of live rock music. Eardrum rupture occurs at approximately 150 dB—the equivalent of hearing a jet take off at 25 meters.
Talk to patients about hearing protection devices. The US Environmental Protection Agency monitors all hearing protection devices and assigns them a Noise Reduction Rating (NRR). The adequacy of single vs double hearing protection depends on the dB exposure level, duration of exposure, and NRR for the protective device(s). In general, recommend single hearing protection (ear plugs, which are inserted in the ear canal, or ear muffs, which fit around the ears) to patients exposed to >80 dB and dual hearing protection (ear plugs and muffs) to those exposed to >95 dB. More guidance on single or dual hearing protection can be obtained from a local occupational health physician or from https://www.osha.gov/dts/osta/otm/noise/hcp/attenuation_estimation.html.
There are drawbacks to using certain forms of ear protection. Regular use can increase the likelihood of cerumen impaction or otitis externa, both of which can actually cause tinnitus. Proper training on how to use hearing protection devices and routine otologic examinations are advisable for patients who frequently use ear protection.
Techniques that can help patients to better cope
The most common therapies used to treat tinnitus are cognitive-behavioral therapy (CBT) and tinnitus retraining therapy (TRT). Both are techniques of habituation designed to change the way patients think about, and emotionally respond to, tinnitus.15,16
CBT is administered by a skilled therapist and employs relaxation exercises, coping strategies, and deconditioning techniques.16 The goal of CBT is to reduce arousal levels and reverse negative thoughts about tinnitus.16 A recent Cochrane review found that although CBT does not subjectively reduce the loudness of tinnitus, it does significantly improve quality of life and depression caused by tinnitus.17 CBT’s benefits also extend to other common comorbidities such as SNHL, insomnia, depression, and anxiety.16 Up to 75% of patients experience improvement in their score on the standardized Tinnitus Handicap Questionnaire one year after completing therapy.16
TRT combines counseling, education, and acoustic therapy—using soft music or a sound machine—to minimize the bothersome nature of the condition.15 TRT is delivered by a team of physicians, audiologists, and psychologists and requires commitment from patients because most therapies are performed at a specialized tinnitus center over the course of up to 2 years.15 Retrospective trials of TRT have generally been positive, finding that this approach minimizes the annoyance patients experience.15
Even in the absence of a formal TRT protocol, patients can take advantage of acoustic therapy. Patients should be advised to add pleasant noise to quiet environments with soft music or sound machines. “Masking” devices are also an option. These commercially available sound generators fit in the ear and may lessen patients’ perception of tinnitus.2,18
Evidence supporting medications is weak
Though many medications have been investigated for treating tinnitus, most have been studied in small clinical trials and none is FDA-approved for tinnitus.
Acamprosate, which is FDA-approved for maintaining alcohol abstinence in alcohol-dependent patients, is a relatively new tinnitus treatment option. In small randomized, double-blinded, placebo controlled trials, approximately 90% of patients treated with acamprosate experienced improvement in tinnitus severity and quality of life.19 Larger studies will be necessary to determine if frequent adverse effects (including depression, anxiety, diarrhea, and drowsiness) will hamper its usefulness.
Benzodiazepines (mainly alprazolam) tend to reduce tinnitus-associated anxiety and also may decrease tinnitus intensity via central suppression of the auditory pathway. However, because evidence is limited to small trials with methodological flaws, and because benzodiazepines have the potential for dependence, the risks and benefits of these agents must be weighed carefully.7,16
Lidocaine has a long history of use for tinnitus, by both intravenous and intratympanic routes. Its benefits are unclear. In some trials, lidocaine was moderately effective in the short term, whereas in others, it appeared to make tinnitus worse.7,20
Oral misoprostol also may be an option, according to a series of placebo-controlled trials.20 But the benefit of this medication may be limited to the perception of loudness, and not other tinnitus measures, such as improved sleep and concentration.20
Antidepressants can have a profound positive effect on tinnitus in patients with severe depression but do not have the same effect on patients who do not suffer from depression.20 Anticonvulsants such as gabapentin have not been found to be effective for tinnitus.21
Researchers are investigating centrally acting agents, such as the N-methyl-D-aspartate antagonist neramexane, for the treatment of tinnitus. With safety and tolerability now established, neramexane is in European phase III trials.22
The jury is out on alternative therapies
With data lacking for prescription medications, patients may look into complementary and alternative therapies. However, consistent evidence is lacking for these therapies, as well. Ginkgo biloba has been found to reduce tinnitus severity and loudness in limited studies,23 although some preparations may be superior to others.14 In a double-blind, randomized controlled trial, melatonin decreased tinnitus intensity significantly, particularly for men and for patients with severe symptoms or a history of noise exposure.24 Zinc supplements may improve tinnitus in patients with zinc deficiency.20,25 Acupuncture and electromagnetic stimulation have not proven efficacious in the treatment of tinnitus.21
Additional steps that your patient can take
A trial of a hearing aid is often worthwhile as a noninvasive, first-line intervention for patients with tinnitus and SNHL. Hearing aids reduce the perception of tinnitus by amplifying ambient sounds.8 Some hearing aids also incorporate masking devices and are used to treat tinnitus in patients with hearing loss. Cochlear implants also are an option for certain patients with confirmed severe SNHL. One study found that tinnitus intensity and awareness were reduced in up to 86% of patients who received cochlear implants.26
The American Tinnitus Association also advises patients with tinnitus to eliminate potential aggravating factors, including salt, artificial sweeteners, sugar, alcohol, tobacco, and caffeine.27
CASE 1 › Mr. L was referred for audiometric testing, which revealed severe high-frequency SNHL that was worse in the right ear. His symptoms improved slightly following a trial of a combination hearing aid/masking device and participation in TRT. He was counseled to quit smoking and use dual hearing protection for future high-noise exposure.
CASE 2 › Ms. B had normal audiometric testing and was referred for angiography. This revealed a dural arteriovenous fistula that was categorized as type III (draining directly into subarachnoid veins) with a small adjacent aneurysm. She underwent a successful clipping of the draining vein to prevent future hemorrhage. Her tinnitus subsequently resolved. Her aspirin use was not modified because it was low dose.
CORRESPONDENCE
Ethan Zimmerman, MD, Mike O’Callaghan Federal Hospital,
4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711-718.
2. Folmer RL, Martin WH, Shi Y. Tinnitus: questions to reveal the cause, answers to provide relief. J Fam Pract. 2004;53:532-540.
3. Figueiredo RR, Azevedo AA, Oliveira PM, et al. Incidence of tinnitus in mp3 player users. Braz J Otorhinolaryngol. 2011;77:293-298.
4. Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Disord. 1990;55:439-453.
5. Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am. 2003;36:239-248.
6. Crummer RW, Hassan GA. Diagnostic approach to tinnitus. Am Fam Physician. 2004;69:120-126.
7. Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904-910.
8. Bauer CA. Tinnitus and hyperacusis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia: Mosby Elsevier; 2010: 2131-2139.
9. ACR Appropriateness Criteria®: vertigo and hearing loss. American College of Radiology Web site. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/HearingLossVertigo.pdf. Accessed January 7, 2014.
10. Meikle MB, Henry JA, Griest SE, et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33:153-176.
11. Kang M, Escott E. Imaging of tinnitus. Otolaryngol Clin North Am. 2008;41:179-193.
12. Seligmann H, Podoshin L, Ben-David J, et al. Drug-induced tinnitus and other hearing disorders. Drug Saf. 1996;14:198-212.
13. Bernhardt O, Mundt T, Welk A, et al. Signs and symptoms of temporomandibular disorders and the incidence of tinnitus. J Oral Rehabil. 2011;38:891-901.
14. von Boetticher A. Ginkgo biloba extract in the treatment of tinnitus: a systematic review. Neuropsychiatr Dis Treat. 2011;7:441-447.
15. Bauer CA, Brozoski TJ. Effect of tinnitus retraining therapy on the loudness and annoyance of tinnitus: a controlled trial. Ear Hear. 2011;32:145-155.
16. Fioretti A, Eibenstein A, Fusetti M. New trends in tinnitus management. Open Neurol J. 2011;5:12-17.
17. Martinez-Devesa P, Perera R, Theodoulou M, et al. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233.
18. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371.
19. Sharma DK, Kaur S, Singh J, et al. Role of acamprosate in sensorineural tinnitus. Indian J Pharmacol. 2012;44:93-96.
20. Salvi R, Lobarinas E, Sun W. Pharmacological treatments for tinnitus: new and old. Drugs Future. 2009;34:381-400.
21. Savage J, Waddell A. Tinnitus. Clin Evid (Online). 2012;pii:0506.
22. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
23. Ernst E, Stevinson C. Ginkgo biloba for tinnitus: a review. Clin Otolaryngol. 1999;24:164-167.
24. Hurtuk A, Dome C, Holloman CH, et al. Melatonin: can it stop the ringing? Ann Otol Rhinol Laryngol. 2011;120:433-440.
25. Arda HN, Tuncel U, Akdogan O, et al. The role of zinc in the treatment of tinnitus. Otol Neurotol. 2003;24:86-89.
26. Quaranta N, Wagstaff S, Baguley DM. Tinnitus and cochlear implantation. Int J Audiol. 2004;43:245-251.
27. Management tips. American Tinnitus Association Web site. Available at: http://www.ata.org/for-patients/tips. Accessed January 13, 2014.
› Advise patients to wear earplugs or earmuffs to prevent hearing loss and tinnitus when exposed to excessively loud sounds (>80dB). C
› Avoid prescribing benzodiazepines, antidepressants, or gabapentin for the treatment of tinnitus; little evidence supports routine use of these agents. B
› Consider referring patients for cognitive-behavioral therapy or tinnitus retraining therapy, each of which can reduce the bothersome nature of tinnitus. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Mr. L is a 47-year-old construction worker who comes to the clinic with a 3-month history of bothersome, constant, high-pitched ringing in his ears that is worse on his right side. He also reports mild hearing loss. Mr. L notes that he keeps a busy schedule, working weekends at a local shooting range. He is a 30-pack-year smoker and takes sumatriptan and nasal fluticasone spray as needed for migraines and allergies. On inspection, his ears appear normal.
CASE 2 › Ms. B, age 68 years, seeks treatment for a constant pulsatile noise in her left ear, which has been bothering her for the past 5 months. She lives alone and says this noise is worse when the house is quiet. She takes chlorthalidone for hypertension and prophylactic aspirin. She indicates that she has no problems with her hearing. On exam, the noise synchronizes with her pulse.
If Mr. L and Ms. B were your patients, what would your next steps be?
An estimated 50 million people in the United States experience some form of tinnitus,1 and the incidence is on the rise, which some have attributed to the increased use of personal music devices.2,3 Patients often describe tinnitus as a ringing noise, but it also can be perceived as buzzing, chirping, hissing, whistling, humming, or other sound. It is more often bilateral than unilateral4 and more often intermittent than continuous.1
Tinnitus may be present in childhood, but the prevalence increases with age. Surveys show that approximately 25% of adults experience symptoms and one-fourth of these patients report that it interferes with daily activities.1,2 The prevalence peaks at 31% in patients between the ages of 60 and 69 years.1
The severity of the condition ranges from causing patients to merely be aware of the noise to having substantial adverse effects on their quality of life. Because not all patients will report tinnitus symptoms, it is important to be aware of risk factors, which include advanced age, male sex, history of military service, and a work history that includes exposure to loud noise.1,2 Smoking and hypertension also are associated with higher rates of tinnitus, as is living in the southern United States.1
A subjective, or objective case of tinnitus?
While subjective tinnitus consists of noises only the patient can hear, objective tinnitus refers to noises, including somatosounds such as turbulent blood flow or palatal myoclonus, that a physician could at least theoretically detect by auscultation or with an amplifying device. Objective tinnitus is less common than subjective tinnitus and more often has an identifiable and correctable source,5 though it may herald a serious underlying condition. When tinnitus is pulsatile or rhythmic, it may be the result of an arteriovenous fistula, arteriovenous malformation, cerebral aneurysm, arterial bruit, or other vascular lesion, such as a glomus tumor.6 Nonvascular conditions like palatal myoclonus present with clicking or low-pitched buzzing and may be a result of multiple sclerosis.7
The causes of both subjective and objective tinnitus are detailed in TABLE 1.2,6,7
Tinnitus and hearing loss: The connection
Most tinnitus is associated with hearing loss and probably results from a disruption in the normal suppression of neuronal activity in the central nervous system.2,8
Conductive hearing loss can be caused by cerumen impaction, otosclerosis, or cholesteatoma. Sensorineural hearing loss (SNHL), which is more common than conductive hearing loss, often is irreversible. The damage typically occurs in the stereocilia cells of the cochlea. These cells trigger the release of neurotransmitters that activate the eighth cranial nerve and cause abnormal excitation along the auditory pathway, giving the perception of sound in a quiet environment.2
Patients with SNHL usually have a history of prolonged exposure to loud noise (eg, heavy machinery, firearms, personal musical devices such as an iPod, or musical instruments) and often describe their tinnitus as a bilateral, high-pitched, continuous ringing. The other major category of SNHL that causes tinnitus is presbycusis—the hearing loss associated with aging—which has clinical features similar to noise-induced hearing loss.8
What to look for
Evaluation of tinnitus begins with a thorough history and physical exam (FIGURE 1).2,6,7,9 Key components of the exam include inspecting the ears, nose, and throat and evaluating cranial nerve function. Weber and Rinne tuning fork testing can help to confirm a conductive hearing loss. When evaluating a patient who reports pulsatile tinnitus, perform auscultation over vascular structures in the neck, temple, and around the ear.
Obtain targeted laboratory studies if there is a suggested metabolic etiology for tinnitus (TABLE 1).2,6,7 Handheld tympanometry that is flatlined or fluctuates with breathing can help support the diagnosis of a subtle middle ear effusion or patulous eustachian tube, respectively.
It is important to quantify how tinnitus affects a patient’s mood, including irritability and concentration. Tinnitus can be measured on several scales, including the Tinnitus Functional Index (TFI),10 which is easily completed in the office. It has been validated to quantify the severity of symptoms and can be used to monitor a patient’s progress. A copy of the TFI and its scoring instructions are available at http://www.ohsu.edu/xd/health/services/ent/services/tinnitus-clinic/tinnitus-functional-index.cfm.
Refer most patients to audiology. Patient’s symptoms often correlate poorly with acoustic functioning.6 Unless you find simple, reversible causes of tinnitus on history and physical, a comprehensive audiologic evaluation is essential. Components of these evaluations include pure-tone thresholds, tympanometry, speech thresholds, and speech discrimination testing.7
Image when necessary. If audiometric testing indicates cochlear damage, imaging generally is unnecessary because SNHL has been confirmed.7 However, if a retrocochlear hearing deficit is detected, auditory brainstem response testing is useful to help locate the lesion. Gadolinium-enhanced magnetic resonance imaging of the internal auditory canals also can be performed to evaluate for central nervous system lesions.2,7 This will detect vestibular schwannoma, which is the most frequent cause of tinnitus apparent on imaging.11
Pulsatility is the one true red flag feature of tinnitus and regardless of audiometry, patients with pulsatile tinnitus require imaging to rule out vascular lesions. The petrous carotid system is a common culprit; therefore, contrast-enhanced high-resolution computed tomography (CT) of the temporal bone is a reasonable initial study since it also will detect osseous abnormalities of the inner ear. However, angiography often is eventually necessary (conventional, magnetic resonance, or CT) to exclude a dural arteriovenous fistula or malformation-the most common cause of objective, pulsatile tinnitus.11 When tinnitus is pulsatile, unilateral, atypical in nature, or associated with deafness, imaging plus referral to a neurologist or otolaryngologist is advisable.6
Medications, and other factors to consider
Many different types of medications and substances can have ototoxic effects, mainly on the cochlear hair cells (TABLE 2).12 The damage may be reversible or irreversible. When doing so would be clinically prudent, consider tapering a patient off a drug that may be causing tinnitus.7
Other causes to consider. Pain in the jaw or neck may be due to a temporomandibular joint disorder or a cervical spine problem like whiplash; these conditions are associated with tinnitus and vertigo.7,13 The combination of low-pitched tinnitus, vertigo, aural fullness, and hearing loss often signifies Meniere’s disease—especially if symptoms are episodic.
Address mood disorders. Although insomnia, anxiety, depression, and posttraumatic stress disorder generally are not considered causes, these conditions are associated with tinnitus and can exacerbate the condition. Tinnitus can trigger depression, and vice versa. Optimizing treatment for these common problems can significantly reduce suffering.6,7
For most patients, you'll focus on prevention, rather than Tx
Treatment for tinnitus (which we’ll describe in a bit) is necessary only for patients for whom the condition has substantially affected the quality of their life.2 Greater emphasis should be placed on prevention.
Most tinnitus originates from the auditory system and is considered irreversible, but up to 25% of patients with chronic tinnitus report an increase in severity over time.14 Therefore, prevention can be beneficial not only for patients at risk of developing tinnitus, but also for those already affected by it. Prevention efforts should focus on protecting hearing by reducing noise levels and exposure time to certain noise thresholds.
The decibel (dB) scale is logarithmic; perception of sound loudness doubles every 10 dB. The sound of a vacuum cleaner is approximately 70 dB; the average human pain threshold is roughly 110 dB, which is the loudness of live rock music. Eardrum rupture occurs at approximately 150 dB—the equivalent of hearing a jet take off at 25 meters.
Talk to patients about hearing protection devices. The US Environmental Protection Agency monitors all hearing protection devices and assigns them a Noise Reduction Rating (NRR). The adequacy of single vs double hearing protection depends on the dB exposure level, duration of exposure, and NRR for the protective device(s). In general, recommend single hearing protection (ear plugs, which are inserted in the ear canal, or ear muffs, which fit around the ears) to patients exposed to >80 dB and dual hearing protection (ear plugs and muffs) to those exposed to >95 dB. More guidance on single or dual hearing protection can be obtained from a local occupational health physician or from https://www.osha.gov/dts/osta/otm/noise/hcp/attenuation_estimation.html.
There are drawbacks to using certain forms of ear protection. Regular use can increase the likelihood of cerumen impaction or otitis externa, both of which can actually cause tinnitus. Proper training on how to use hearing protection devices and routine otologic examinations are advisable for patients who frequently use ear protection.
Techniques that can help patients to better cope
The most common therapies used to treat tinnitus are cognitive-behavioral therapy (CBT) and tinnitus retraining therapy (TRT). Both are techniques of habituation designed to change the way patients think about, and emotionally respond to, tinnitus.15,16
CBT is administered by a skilled therapist and employs relaxation exercises, coping strategies, and deconditioning techniques.16 The goal of CBT is to reduce arousal levels and reverse negative thoughts about tinnitus.16 A recent Cochrane review found that although CBT does not subjectively reduce the loudness of tinnitus, it does significantly improve quality of life and depression caused by tinnitus.17 CBT’s benefits also extend to other common comorbidities such as SNHL, insomnia, depression, and anxiety.16 Up to 75% of patients experience improvement in their score on the standardized Tinnitus Handicap Questionnaire one year after completing therapy.16
TRT combines counseling, education, and acoustic therapy—using soft music or a sound machine—to minimize the bothersome nature of the condition.15 TRT is delivered by a team of physicians, audiologists, and psychologists and requires commitment from patients because most therapies are performed at a specialized tinnitus center over the course of up to 2 years.15 Retrospective trials of TRT have generally been positive, finding that this approach minimizes the annoyance patients experience.15
Even in the absence of a formal TRT protocol, patients can take advantage of acoustic therapy. Patients should be advised to add pleasant noise to quiet environments with soft music or sound machines. “Masking” devices are also an option. These commercially available sound generators fit in the ear and may lessen patients’ perception of tinnitus.2,18
Evidence supporting medications is weak
Though many medications have been investigated for treating tinnitus, most have been studied in small clinical trials and none is FDA-approved for tinnitus.
Acamprosate, which is FDA-approved for maintaining alcohol abstinence in alcohol-dependent patients, is a relatively new tinnitus treatment option. In small randomized, double-blinded, placebo controlled trials, approximately 90% of patients treated with acamprosate experienced improvement in tinnitus severity and quality of life.19 Larger studies will be necessary to determine if frequent adverse effects (including depression, anxiety, diarrhea, and drowsiness) will hamper its usefulness.
Benzodiazepines (mainly alprazolam) tend to reduce tinnitus-associated anxiety and also may decrease tinnitus intensity via central suppression of the auditory pathway. However, because evidence is limited to small trials with methodological flaws, and because benzodiazepines have the potential for dependence, the risks and benefits of these agents must be weighed carefully.7,16
Lidocaine has a long history of use for tinnitus, by both intravenous and intratympanic routes. Its benefits are unclear. In some trials, lidocaine was moderately effective in the short term, whereas in others, it appeared to make tinnitus worse.7,20
Oral misoprostol also may be an option, according to a series of placebo-controlled trials.20 But the benefit of this medication may be limited to the perception of loudness, and not other tinnitus measures, such as improved sleep and concentration.20
Antidepressants can have a profound positive effect on tinnitus in patients with severe depression but do not have the same effect on patients who do not suffer from depression.20 Anticonvulsants such as gabapentin have not been found to be effective for tinnitus.21
Researchers are investigating centrally acting agents, such as the N-methyl-D-aspartate antagonist neramexane, for the treatment of tinnitus. With safety and tolerability now established, neramexane is in European phase III trials.22
The jury is out on alternative therapies
With data lacking for prescription medications, patients may look into complementary and alternative therapies. However, consistent evidence is lacking for these therapies, as well. Ginkgo biloba has been found to reduce tinnitus severity and loudness in limited studies,23 although some preparations may be superior to others.14 In a double-blind, randomized controlled trial, melatonin decreased tinnitus intensity significantly, particularly for men and for patients with severe symptoms or a history of noise exposure.24 Zinc supplements may improve tinnitus in patients with zinc deficiency.20,25 Acupuncture and electromagnetic stimulation have not proven efficacious in the treatment of tinnitus.21
Additional steps that your patient can take
A trial of a hearing aid is often worthwhile as a noninvasive, first-line intervention for patients with tinnitus and SNHL. Hearing aids reduce the perception of tinnitus by amplifying ambient sounds.8 Some hearing aids also incorporate masking devices and are used to treat tinnitus in patients with hearing loss. Cochlear implants also are an option for certain patients with confirmed severe SNHL. One study found that tinnitus intensity and awareness were reduced in up to 86% of patients who received cochlear implants.26
The American Tinnitus Association also advises patients with tinnitus to eliminate potential aggravating factors, including salt, artificial sweeteners, sugar, alcohol, tobacco, and caffeine.27
CASE 1 › Mr. L was referred for audiometric testing, which revealed severe high-frequency SNHL that was worse in the right ear. His symptoms improved slightly following a trial of a combination hearing aid/masking device and participation in TRT. He was counseled to quit smoking and use dual hearing protection for future high-noise exposure.
CASE 2 › Ms. B had normal audiometric testing and was referred for angiography. This revealed a dural arteriovenous fistula that was categorized as type III (draining directly into subarachnoid veins) with a small adjacent aneurysm. She underwent a successful clipping of the draining vein to prevent future hemorrhage. Her tinnitus subsequently resolved. Her aspirin use was not modified because it was low dose.
CORRESPONDENCE
Ethan Zimmerman, MD, Mike O’Callaghan Federal Hospital,
4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
› Advise patients to wear earplugs or earmuffs to prevent hearing loss and tinnitus when exposed to excessively loud sounds (>80dB). C
› Avoid prescribing benzodiazepines, antidepressants, or gabapentin for the treatment of tinnitus; little evidence supports routine use of these agents. B
› Consider referring patients for cognitive-behavioral therapy or tinnitus retraining therapy, each of which can reduce the bothersome nature of tinnitus. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Mr. L is a 47-year-old construction worker who comes to the clinic with a 3-month history of bothersome, constant, high-pitched ringing in his ears that is worse on his right side. He also reports mild hearing loss. Mr. L notes that he keeps a busy schedule, working weekends at a local shooting range. He is a 30-pack-year smoker and takes sumatriptan and nasal fluticasone spray as needed for migraines and allergies. On inspection, his ears appear normal.
CASE 2 › Ms. B, age 68 years, seeks treatment for a constant pulsatile noise in her left ear, which has been bothering her for the past 5 months. She lives alone and says this noise is worse when the house is quiet. She takes chlorthalidone for hypertension and prophylactic aspirin. She indicates that she has no problems with her hearing. On exam, the noise synchronizes with her pulse.
If Mr. L and Ms. B were your patients, what would your next steps be?
An estimated 50 million people in the United States experience some form of tinnitus,1 and the incidence is on the rise, which some have attributed to the increased use of personal music devices.2,3 Patients often describe tinnitus as a ringing noise, but it also can be perceived as buzzing, chirping, hissing, whistling, humming, or other sound. It is more often bilateral than unilateral4 and more often intermittent than continuous.1
Tinnitus may be present in childhood, but the prevalence increases with age. Surveys show that approximately 25% of adults experience symptoms and one-fourth of these patients report that it interferes with daily activities.1,2 The prevalence peaks at 31% in patients between the ages of 60 and 69 years.1
The severity of the condition ranges from causing patients to merely be aware of the noise to having substantial adverse effects on their quality of life. Because not all patients will report tinnitus symptoms, it is important to be aware of risk factors, which include advanced age, male sex, history of military service, and a work history that includes exposure to loud noise.1,2 Smoking and hypertension also are associated with higher rates of tinnitus, as is living in the southern United States.1
A subjective, or objective case of tinnitus?
While subjective tinnitus consists of noises only the patient can hear, objective tinnitus refers to noises, including somatosounds such as turbulent blood flow or palatal myoclonus, that a physician could at least theoretically detect by auscultation or with an amplifying device. Objective tinnitus is less common than subjective tinnitus and more often has an identifiable and correctable source,5 though it may herald a serious underlying condition. When tinnitus is pulsatile or rhythmic, it may be the result of an arteriovenous fistula, arteriovenous malformation, cerebral aneurysm, arterial bruit, or other vascular lesion, such as a glomus tumor.6 Nonvascular conditions like palatal myoclonus present with clicking or low-pitched buzzing and may be a result of multiple sclerosis.7
The causes of both subjective and objective tinnitus are detailed in TABLE 1.2,6,7
Tinnitus and hearing loss: The connection
Most tinnitus is associated with hearing loss and probably results from a disruption in the normal suppression of neuronal activity in the central nervous system.2,8
Conductive hearing loss can be caused by cerumen impaction, otosclerosis, or cholesteatoma. Sensorineural hearing loss (SNHL), which is more common than conductive hearing loss, often is irreversible. The damage typically occurs in the stereocilia cells of the cochlea. These cells trigger the release of neurotransmitters that activate the eighth cranial nerve and cause abnormal excitation along the auditory pathway, giving the perception of sound in a quiet environment.2
Patients with SNHL usually have a history of prolonged exposure to loud noise (eg, heavy machinery, firearms, personal musical devices such as an iPod, or musical instruments) and often describe their tinnitus as a bilateral, high-pitched, continuous ringing. The other major category of SNHL that causes tinnitus is presbycusis—the hearing loss associated with aging—which has clinical features similar to noise-induced hearing loss.8
What to look for
Evaluation of tinnitus begins with a thorough history and physical exam (FIGURE 1).2,6,7,9 Key components of the exam include inspecting the ears, nose, and throat and evaluating cranial nerve function. Weber and Rinne tuning fork testing can help to confirm a conductive hearing loss. When evaluating a patient who reports pulsatile tinnitus, perform auscultation over vascular structures in the neck, temple, and around the ear.
Obtain targeted laboratory studies if there is a suggested metabolic etiology for tinnitus (TABLE 1).2,6,7 Handheld tympanometry that is flatlined or fluctuates with breathing can help support the diagnosis of a subtle middle ear effusion or patulous eustachian tube, respectively.
It is important to quantify how tinnitus affects a patient’s mood, including irritability and concentration. Tinnitus can be measured on several scales, including the Tinnitus Functional Index (TFI),10 which is easily completed in the office. It has been validated to quantify the severity of symptoms and can be used to monitor a patient’s progress. A copy of the TFI and its scoring instructions are available at http://www.ohsu.edu/xd/health/services/ent/services/tinnitus-clinic/tinnitus-functional-index.cfm.
Refer most patients to audiology. Patient’s symptoms often correlate poorly with acoustic functioning.6 Unless you find simple, reversible causes of tinnitus on history and physical, a comprehensive audiologic evaluation is essential. Components of these evaluations include pure-tone thresholds, tympanometry, speech thresholds, and speech discrimination testing.7
Image when necessary. If audiometric testing indicates cochlear damage, imaging generally is unnecessary because SNHL has been confirmed.7 However, if a retrocochlear hearing deficit is detected, auditory brainstem response testing is useful to help locate the lesion. Gadolinium-enhanced magnetic resonance imaging of the internal auditory canals also can be performed to evaluate for central nervous system lesions.2,7 This will detect vestibular schwannoma, which is the most frequent cause of tinnitus apparent on imaging.11
Pulsatility is the one true red flag feature of tinnitus and regardless of audiometry, patients with pulsatile tinnitus require imaging to rule out vascular lesions. The petrous carotid system is a common culprit; therefore, contrast-enhanced high-resolution computed tomography (CT) of the temporal bone is a reasonable initial study since it also will detect osseous abnormalities of the inner ear. However, angiography often is eventually necessary (conventional, magnetic resonance, or CT) to exclude a dural arteriovenous fistula or malformation-the most common cause of objective, pulsatile tinnitus.11 When tinnitus is pulsatile, unilateral, atypical in nature, or associated with deafness, imaging plus referral to a neurologist or otolaryngologist is advisable.6
Medications, and other factors to consider
Many different types of medications and substances can have ototoxic effects, mainly on the cochlear hair cells (TABLE 2).12 The damage may be reversible or irreversible. When doing so would be clinically prudent, consider tapering a patient off a drug that may be causing tinnitus.7
Other causes to consider. Pain in the jaw or neck may be due to a temporomandibular joint disorder or a cervical spine problem like whiplash; these conditions are associated with tinnitus and vertigo.7,13 The combination of low-pitched tinnitus, vertigo, aural fullness, and hearing loss often signifies Meniere’s disease—especially if symptoms are episodic.
Address mood disorders. Although insomnia, anxiety, depression, and posttraumatic stress disorder generally are not considered causes, these conditions are associated with tinnitus and can exacerbate the condition. Tinnitus can trigger depression, and vice versa. Optimizing treatment for these common problems can significantly reduce suffering.6,7
For most patients, you'll focus on prevention, rather than Tx
Treatment for tinnitus (which we’ll describe in a bit) is necessary only for patients for whom the condition has substantially affected the quality of their life.2 Greater emphasis should be placed on prevention.
Most tinnitus originates from the auditory system and is considered irreversible, but up to 25% of patients with chronic tinnitus report an increase in severity over time.14 Therefore, prevention can be beneficial not only for patients at risk of developing tinnitus, but also for those already affected by it. Prevention efforts should focus on protecting hearing by reducing noise levels and exposure time to certain noise thresholds.
The decibel (dB) scale is logarithmic; perception of sound loudness doubles every 10 dB. The sound of a vacuum cleaner is approximately 70 dB; the average human pain threshold is roughly 110 dB, which is the loudness of live rock music. Eardrum rupture occurs at approximately 150 dB—the equivalent of hearing a jet take off at 25 meters.
Talk to patients about hearing protection devices. The US Environmental Protection Agency monitors all hearing protection devices and assigns them a Noise Reduction Rating (NRR). The adequacy of single vs double hearing protection depends on the dB exposure level, duration of exposure, and NRR for the protective device(s). In general, recommend single hearing protection (ear plugs, which are inserted in the ear canal, or ear muffs, which fit around the ears) to patients exposed to >80 dB and dual hearing protection (ear plugs and muffs) to those exposed to >95 dB. More guidance on single or dual hearing protection can be obtained from a local occupational health physician or from https://www.osha.gov/dts/osta/otm/noise/hcp/attenuation_estimation.html.
There are drawbacks to using certain forms of ear protection. Regular use can increase the likelihood of cerumen impaction or otitis externa, both of which can actually cause tinnitus. Proper training on how to use hearing protection devices and routine otologic examinations are advisable for patients who frequently use ear protection.
Techniques that can help patients to better cope
The most common therapies used to treat tinnitus are cognitive-behavioral therapy (CBT) and tinnitus retraining therapy (TRT). Both are techniques of habituation designed to change the way patients think about, and emotionally respond to, tinnitus.15,16
CBT is administered by a skilled therapist and employs relaxation exercises, coping strategies, and deconditioning techniques.16 The goal of CBT is to reduce arousal levels and reverse negative thoughts about tinnitus.16 A recent Cochrane review found that although CBT does not subjectively reduce the loudness of tinnitus, it does significantly improve quality of life and depression caused by tinnitus.17 CBT’s benefits also extend to other common comorbidities such as SNHL, insomnia, depression, and anxiety.16 Up to 75% of patients experience improvement in their score on the standardized Tinnitus Handicap Questionnaire one year after completing therapy.16
TRT combines counseling, education, and acoustic therapy—using soft music or a sound machine—to minimize the bothersome nature of the condition.15 TRT is delivered by a team of physicians, audiologists, and psychologists and requires commitment from patients because most therapies are performed at a specialized tinnitus center over the course of up to 2 years.15 Retrospective trials of TRT have generally been positive, finding that this approach minimizes the annoyance patients experience.15
Even in the absence of a formal TRT protocol, patients can take advantage of acoustic therapy. Patients should be advised to add pleasant noise to quiet environments with soft music or sound machines. “Masking” devices are also an option. These commercially available sound generators fit in the ear and may lessen patients’ perception of tinnitus.2,18
Evidence supporting medications is weak
Though many medications have been investigated for treating tinnitus, most have been studied in small clinical trials and none is FDA-approved for tinnitus.
Acamprosate, which is FDA-approved for maintaining alcohol abstinence in alcohol-dependent patients, is a relatively new tinnitus treatment option. In small randomized, double-blinded, placebo controlled trials, approximately 90% of patients treated with acamprosate experienced improvement in tinnitus severity and quality of life.19 Larger studies will be necessary to determine if frequent adverse effects (including depression, anxiety, diarrhea, and drowsiness) will hamper its usefulness.
Benzodiazepines (mainly alprazolam) tend to reduce tinnitus-associated anxiety and also may decrease tinnitus intensity via central suppression of the auditory pathway. However, because evidence is limited to small trials with methodological flaws, and because benzodiazepines have the potential for dependence, the risks and benefits of these agents must be weighed carefully.7,16
Lidocaine has a long history of use for tinnitus, by both intravenous and intratympanic routes. Its benefits are unclear. In some trials, lidocaine was moderately effective in the short term, whereas in others, it appeared to make tinnitus worse.7,20
Oral misoprostol also may be an option, according to a series of placebo-controlled trials.20 But the benefit of this medication may be limited to the perception of loudness, and not other tinnitus measures, such as improved sleep and concentration.20
Antidepressants can have a profound positive effect on tinnitus in patients with severe depression but do not have the same effect on patients who do not suffer from depression.20 Anticonvulsants such as gabapentin have not been found to be effective for tinnitus.21
Researchers are investigating centrally acting agents, such as the N-methyl-D-aspartate antagonist neramexane, for the treatment of tinnitus. With safety and tolerability now established, neramexane is in European phase III trials.22
The jury is out on alternative therapies
With data lacking for prescription medications, patients may look into complementary and alternative therapies. However, consistent evidence is lacking for these therapies, as well. Ginkgo biloba has been found to reduce tinnitus severity and loudness in limited studies,23 although some preparations may be superior to others.14 In a double-blind, randomized controlled trial, melatonin decreased tinnitus intensity significantly, particularly for men and for patients with severe symptoms or a history of noise exposure.24 Zinc supplements may improve tinnitus in patients with zinc deficiency.20,25 Acupuncture and electromagnetic stimulation have not proven efficacious in the treatment of tinnitus.21
Additional steps that your patient can take
A trial of a hearing aid is often worthwhile as a noninvasive, first-line intervention for patients with tinnitus and SNHL. Hearing aids reduce the perception of tinnitus by amplifying ambient sounds.8 Some hearing aids also incorporate masking devices and are used to treat tinnitus in patients with hearing loss. Cochlear implants also are an option for certain patients with confirmed severe SNHL. One study found that tinnitus intensity and awareness were reduced in up to 86% of patients who received cochlear implants.26
The American Tinnitus Association also advises patients with tinnitus to eliminate potential aggravating factors, including salt, artificial sweeteners, sugar, alcohol, tobacco, and caffeine.27
CASE 1 › Mr. L was referred for audiometric testing, which revealed severe high-frequency SNHL that was worse in the right ear. His symptoms improved slightly following a trial of a combination hearing aid/masking device and participation in TRT. He was counseled to quit smoking and use dual hearing protection for future high-noise exposure.
CASE 2 › Ms. B had normal audiometric testing and was referred for angiography. This revealed a dural arteriovenous fistula that was categorized as type III (draining directly into subarachnoid veins) with a small adjacent aneurysm. She underwent a successful clipping of the draining vein to prevent future hemorrhage. Her tinnitus subsequently resolved. Her aspirin use was not modified because it was low dose.
CORRESPONDENCE
Ethan Zimmerman, MD, Mike O’Callaghan Federal Hospital,
4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711-718.
2. Folmer RL, Martin WH, Shi Y. Tinnitus: questions to reveal the cause, answers to provide relief. J Fam Pract. 2004;53:532-540.
3. Figueiredo RR, Azevedo AA, Oliveira PM, et al. Incidence of tinnitus in mp3 player users. Braz J Otorhinolaryngol. 2011;77:293-298.
4. Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Disord. 1990;55:439-453.
5. Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am. 2003;36:239-248.
6. Crummer RW, Hassan GA. Diagnostic approach to tinnitus. Am Fam Physician. 2004;69:120-126.
7. Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904-910.
8. Bauer CA. Tinnitus and hyperacusis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia: Mosby Elsevier; 2010: 2131-2139.
9. ACR Appropriateness Criteria®: vertigo and hearing loss. American College of Radiology Web site. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/HearingLossVertigo.pdf. Accessed January 7, 2014.
10. Meikle MB, Henry JA, Griest SE, et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33:153-176.
11. Kang M, Escott E. Imaging of tinnitus. Otolaryngol Clin North Am. 2008;41:179-193.
12. Seligmann H, Podoshin L, Ben-David J, et al. Drug-induced tinnitus and other hearing disorders. Drug Saf. 1996;14:198-212.
13. Bernhardt O, Mundt T, Welk A, et al. Signs and symptoms of temporomandibular disorders and the incidence of tinnitus. J Oral Rehabil. 2011;38:891-901.
14. von Boetticher A. Ginkgo biloba extract in the treatment of tinnitus: a systematic review. Neuropsychiatr Dis Treat. 2011;7:441-447.
15. Bauer CA, Brozoski TJ. Effect of tinnitus retraining therapy on the loudness and annoyance of tinnitus: a controlled trial. Ear Hear. 2011;32:145-155.
16. Fioretti A, Eibenstein A, Fusetti M. New trends in tinnitus management. Open Neurol J. 2011;5:12-17.
17. Martinez-Devesa P, Perera R, Theodoulou M, et al. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233.
18. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371.
19. Sharma DK, Kaur S, Singh J, et al. Role of acamprosate in sensorineural tinnitus. Indian J Pharmacol. 2012;44:93-96.
20. Salvi R, Lobarinas E, Sun W. Pharmacological treatments for tinnitus: new and old. Drugs Future. 2009;34:381-400.
21. Savage J, Waddell A. Tinnitus. Clin Evid (Online). 2012;pii:0506.
22. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
23. Ernst E, Stevinson C. Ginkgo biloba for tinnitus: a review. Clin Otolaryngol. 1999;24:164-167.
24. Hurtuk A, Dome C, Holloman CH, et al. Melatonin: can it stop the ringing? Ann Otol Rhinol Laryngol. 2011;120:433-440.
25. Arda HN, Tuncel U, Akdogan O, et al. The role of zinc in the treatment of tinnitus. Otol Neurotol. 2003;24:86-89.
26. Quaranta N, Wagstaff S, Baguley DM. Tinnitus and cochlear implantation. Int J Audiol. 2004;43:245-251.
27. Management tips. American Tinnitus Association Web site. Available at: http://www.ata.org/for-patients/tips. Accessed January 13, 2014.
1. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711-718.
2. Folmer RL, Martin WH, Shi Y. Tinnitus: questions to reveal the cause, answers to provide relief. J Fam Pract. 2004;53:532-540.
3. Figueiredo RR, Azevedo AA, Oliveira PM, et al. Incidence of tinnitus in mp3 player users. Braz J Otorhinolaryngol. 2011;77:293-298.
4. Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Disord. 1990;55:439-453.
5. Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am. 2003;36:239-248.
6. Crummer RW, Hassan GA. Diagnostic approach to tinnitus. Am Fam Physician. 2004;69:120-126.
7. Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904-910.
8. Bauer CA. Tinnitus and hyperacusis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Philadelphia: Mosby Elsevier; 2010: 2131-2139.
9. ACR Appropriateness Criteria®: vertigo and hearing loss. American College of Radiology Web site. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/HearingLossVertigo.pdf. Accessed January 7, 2014.
10. Meikle MB, Henry JA, Griest SE, et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33:153-176.
11. Kang M, Escott E. Imaging of tinnitus. Otolaryngol Clin North Am. 2008;41:179-193.
12. Seligmann H, Podoshin L, Ben-David J, et al. Drug-induced tinnitus and other hearing disorders. Drug Saf. 1996;14:198-212.
13. Bernhardt O, Mundt T, Welk A, et al. Signs and symptoms of temporomandibular disorders and the incidence of tinnitus. J Oral Rehabil. 2011;38:891-901.
14. von Boetticher A. Ginkgo biloba extract in the treatment of tinnitus: a systematic review. Neuropsychiatr Dis Treat. 2011;7:441-447.
15. Bauer CA, Brozoski TJ. Effect of tinnitus retraining therapy on the loudness and annoyance of tinnitus: a controlled trial. Ear Hear. 2011;32:145-155.
16. Fioretti A, Eibenstein A, Fusetti M. New trends in tinnitus management. Open Neurol J. 2011;5:12-17.
17. Martinez-Devesa P, Perera R, Theodoulou M, et al. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233.
18. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371.
19. Sharma DK, Kaur S, Singh J, et al. Role of acamprosate in sensorineural tinnitus. Indian J Pharmacol. 2012;44:93-96.
20. Salvi R, Lobarinas E, Sun W. Pharmacological treatments for tinnitus: new and old. Drugs Future. 2009;34:381-400.
21. Savage J, Waddell A. Tinnitus. Clin Evid (Online). 2012;pii:0506.
22. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
23. Ernst E, Stevinson C. Ginkgo biloba for tinnitus: a review. Clin Otolaryngol. 1999;24:164-167.
24. Hurtuk A, Dome C, Holloman CH, et al. Melatonin: can it stop the ringing? Ann Otol Rhinol Laryngol. 2011;120:433-440.
25. Arda HN, Tuncel U, Akdogan O, et al. The role of zinc in the treatment of tinnitus. Otol Neurotol. 2003;24:86-89.
26. Quaranta N, Wagstaff S, Baguley DM. Tinnitus and cochlear implantation. Int J Audiol. 2004;43:245-251.
27. Management tips. American Tinnitus Association Web site. Available at: http://www.ata.org/for-patients/tips. Accessed January 13, 2014.
Insulin for type 2 diabetes: How and when to get started
› Initiate insulin for patients whose hemoglobin A1c ≥8% despite taking 2 or more oral agents. C
› Prescribe insulin for patients who have not reached their goal one year after diagnosis and initiation of oral therapy. C
› Consider reducing—but do not discontinue—oral agents, such as sulfonylureas and meglitinides, when you initiate insulin therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With type 2 diabetes now affecting 8.3% of the US population, most primary care physicians see patients with this disorder every day.1 Based on the concurrent obesity epidemic, aging population, and emergence of type 2 diabetes in children and adolescents, it is estimated that by 2050, the prevalence will have risen from one in 12 Americans to one in 3.1
Type 2 diabetes is a progressive disorder, with a relentless decline in beta cells. By the time of diagnosis, patients typically have lost at least 50% of insulin secretion; within 6 years of diagnosis, insulin secretion decreases to less than 25%.2
The American Association of Clinical Endocrinologists (AACE)3 and the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD)4 have recently published guidelines for the management of type 2 diabetes. While the AACE’s guidelines (available at https://www.aace.com/files/aace_algorithm.pdf) focus on different treatments at different stages of disease and both glycemic and nonglycemic benefits of treatment,3 the ADA/EASD’s guidelines (see http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) emphasize a patient-centered approach, shared decision making, and individualization of treatment goals based on both patient preference and comorbid disease states.4
One thing both sets of guidelines have in common is a purposeful intensification of therapy every 2 to 3 months, as needed, and the introduction of insulin one year after diagnosis if the patient is still not at goal.3,4 But all too often, this does not occur, particularly in primary care settings.
This article will review the “when” and “how” of insulin initiation. But first, a look at barriers to insulin therapy and evidence in support of earlier use.
Clinical inertia and patient fear are associated with delays
Both the AACE and the ADA/EASD guidelines agree that metformin is best used as early as possible.5,6 With typical use, however, metformin fails to prevent the progression of diabetes, as measured by the climb of hemoglobin A1c (HbA1c), at a failure rate of about 17% of patients per year.5 Physicians have been slow to intensify treatment for type 2 diabetes6—a phenomenon referred to as clinical inertia.
Typically, physicians adopt a stepwise approach, which often results in patients spending more than 10 years with an HbA1c >7% and 5 years with an HbA1c >8% before insulin is started.5 In a recent Veterans Administration study, patients were out of control, with an HbA1c >8%, for an average of 4.6 years before insulin was initiated.7
Both patient and physician factors contribute to the delay. Patient factors include the fear of injection, the belief that insulin will interfere with their lifestyle, and the idea that the use of insulin signifies impending complications or even death.8 But such beliefs are starting to change. In a recent multinational study of patients with type 2 diabetes, less than 20% stated they were unwilling to start insulin.9
For their part, primary care physicians are much less likely to prescribe insulin than clinicians specializing in diabetes.6 Physician-reported barriers to insulin initiation include the time required to train patients to use it correctly; the lack of support, including access to diabetes educators; and the absence of clear guidelines on the use of insulin.10
A case for earlier insulin
There has been recent momentum in favor of earlier initiation of insulin. In fact, some researchers regard intensive insulin as an excellent first treatment for type 2 diabetes,11 based on the belief that early insulin (used for a brief time) can provide not only immediate improvement in glucose control, but also a lasting “legacy” effect. The ADA/EASD guidelines support the use of insulin as a first-line treatment for patients with symptoms of insulin deficiency,4 but do not recommend it for everyone with newly diagnosed type 2 diabetes.
There have also been a number of advances in insulin therapy over the past 2 decades. These include insulin analogs with physiologic profiles that better match daily schedules, as well as improvements in the way insulin is delivered. Insulin pens, smaller needles, disposable devices, and insulin pumps have made it easier to administer and fine-tune insulin delivery. Despite these improvements and recommendations for earlier implementation, the use of insulin in type 2 diabetes is significantly lower today than in the 1990s.12
When to introduce insulin
Insulin is indicated for patients with type 2 diabetes whose disease is not easily controlled. That includes individuals with decompensated type 2 diabetes, those whose HbA1c remains high even with 2 or more oral agents, and individuals who have not reached goal after a year of treatment.
Glucose toxicity. It is generally agreed that insulin is the most effective treatment for patients who present with decompensated type 2 diabetes4—ie, with significant hyperglycemia and catabolic symptoms such as polydipsia, polyuria, and weight loss. Initiation of insulin promotes reversal of glucose toxicity and stabilization of metabolic status. In such cases, insulin can be started at a low dose to expose the patient to the complexities of injection therapy (more about this in a bit), then titrated as needed for stabilization.
HbA1c ≥8% even with 2 or more drugs. In my experience, an oral diabetes drug will lead to a drop in HbA1c of about one percentage point. Generally, the further from goal the patient is, the greater the effect the medication will have. As HbA1c inches closer to 7%, the effect diminishes. And when 2 oral agents fail to lower a patient’s HbA1c adequately, the incremental change expected from the addition of a third, fourth, or fifth agent is small.
Thus, in a patient with an HbA1c ≥8%, there is still a significant fasting hyperglycemic component. In such a case, a basal insulin is likely the best treatment option.
Not at goal at one year. Both the AACE and the ADA/EASD guidelines agree that treatment titration should be considered every 2 to 3 months to achieve metabolic control and that if a patient is not at goal after a year, insulin should be started.3,4 However, traditionally this is not done. The delayed implementation of this recommendation is an example of clinical inertia, which can contribute to further misunderstandings about the role and effect of insulin therapy.
Getting started with basal insulin
Most patients who are started on insulin have global hyperglycemia. But because fasting hyperglycemia can affect pancreatic insulin secretion, it is important to get control of the fasting glucose first. This can often be done with insulin sensitizers (metformin, thiazolidinediones, and incretin-based agents).
Suppression of excessive hepatic glucose production, which is very common in type 2 diabetes, is one of the biggest challenges in normalizing fasting glucose. This is well managed with a basal insulin. When starting basal insulin, however, it is critical that current treatments not be stopped. Oral agents such as sulfonylureas and meglitinides can be reduced to lower the risk of hypoglycemia, but stopping them altogether will only prolong the time it takes to get to goal.
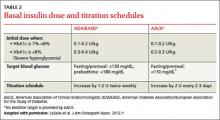
There are 3 insulin formulations that can serve as basal insulin (TABLE 1).13 Neutral protamine Hagedorn (NPH) is a human insulin that can be used 2 to 3 times daily to provide basal insulin coverage. But long-acting basal analog insulins glargine and detemir, typically administered once a day when used by patients with type 2 diabetes, are a better option.14
While all 3 formulations have similar efficacy for lowering HbA1c, the analog basal insulins have numerous advantages: less weight gain, less hypoglycemia for the same level of glucose control, and less frequent dosing. In addition, glargine and detemir are available in a pen or vial, while generic NPH is available only in a vial. The primary disadvantage of the analogs is cost: A month’s supply—one vial—of NPH sells for approximately $25 (generic) or $94 (brand name); in comparison, a month’s supply (one box of 5 3-mL pens) of detemir and glargine costs about $300 and $320, respectively.15 (Humulin N, a brand-name NPH, is available in a pen, at a cost of approximately $315 per box.)
Use a weight-based initial dose
The recommended starting dose is 0.1 to 0.2 U/kg daily for patients with an HbA1c <8%. If HbA1c is ≥8%, the ADA/EASD guidelines recommend a starting dose of 0.3 to 0.4 U/kg daily4(TABLE 2).3,4,16 While basal insulin is most commonly dosed at bedtime, in fact, basal analog insulins can be given at any time that’s convenient for the patient. Morning dosing may be preferable for individuals with a significant fear of hypoglycemia—a phobia that sometimes causes patients to skip insulin doses and engage in “defensive eating” (ie, eating in an attempt to prevent hypoglycemia rather than because of hunger or the need for nutrition).
Teach injection technique
It is critically important that patients get the first shot in the office, guided by a clinician who can teach proper injection technique. This also helps to dispel the apprehension of self-injection.
In addition to being surprised at how easy and painless injection can be, patients have the opportunity to observe the results and gain confidence in insulin’s efficacy. And, in my experience, adherence to an insulin regimen is much greater if the first injection is administered in an office setting.
(Tech-savvy patients may find it helpful to use a smartphone app, such as Glucose Buddy or Dbees.com, to help manage their diabetes. See “The 13 best diabetes iPhone & Android apps of 2013” at http://www.healthline.com/health-slideshow/top-iphone-android-apps-diabetes.)
Establish a titration schedule
It is important, too, to teach the patient how to titrate the insulin dose from the start, rather than waiting until the next visit to address this. Patient titration—facilitated by a clinician-provided titration schedule (available from the AACE and the ADA/EASD3,4)—has been shown to achieve target glucose levels faster than physician titration.17
I usually suggest that patients increase the basal insulin dose by 3 units every 3 days, with an upper limit of 0.5 U/kg/d, until fasting glucose is consistently between 100 and 150 mg/dL. I advise every patient who starts taking insulin to track morning readings and titrate the dose until one of 3 things occurs:
1) the 0.5 U/kg/d limit is reached;
2) the patient has a glucose reading <100 mg/dL; or
3) the patient achieves his or her HbA1c target (<7% for most patients).
In every case, I recommend that the patient call my office for further instruction.
If the patient has any low glucose readings, I reduce the basal insulin by 5 U/kg/d. If he or she is still above goal, I advise the patient to continue titration, but more slowly. If the patient is at goal, I advise continuing at the current dose.
Basal titration vs mealtime coverage. Most people with type 2 diabetes require between 0.2 and 1 U/kg of basal insulin daily. It is currently recommended that when a patient has titrated to a dose of 0.5 U/kg/d, it is time to look at the glucose pattern to determine whether further titrating basal insulin or addressing prandial hyperglycemia should be the next step.4,18 This requires a change in fingerstick pattern.
The patient can stop the first morning glucose check and start checking before meals and 90 to 120 minutes postmeal. This allows for exploration of the mealtime excursion. Generally, a difference of <50 mg/dL is preferred. If the morning glucose level is at target but HbA1c is high, it is likely that postprandial glucose is contributing to this difference. This is particularly true when the HbA1c is between 7% and 8%. If the glucose pattern shows high postmeal glucose readings, it is much safer to address mealtime insulin (not discussed in this article) than to continue to titrate the basal insulin.4,18
Avoid “overbasalization”—ie, titrating basal insulin beyond its normal role to suppress hepatic glucose production and get the fasting glucose to goal. Doing so puts the patient at risk for unexpected hypoglycemia, as the insulin will now try to overcome hyperglycemia with meals, as well. Basal insulins are not designed to meet insulin requirements at meals. If a patient misses a meal yet continues the same dose of basal insulin, the risk of a hypoglycemic episode increases substantially.
In the pipeline. There are a number of new basal insulins in development, including one that has a prolonged duration of action and the potential for every-other-day injections19 and another that uses an attached polyethylene glycol moiety to slow absorption and prolong its effect.20
The nuts and bolts of insulin prescribing
When you prescribe insulin, there are a number of components to consider.
Pen or vial? In addition to deciding whether to order pen or vial, it is essential to consider the volume of insulin needed. Glargine, detemir, and Humulin N are available in 10 mL vials (100 U/mL) and in 3 mL pens (100 U/mL). (Generic NPH is available in vials only.) Most patients prefer insulin pens, which are more convenient and easier to use than a vial and syringe.
The choice also depends on the dosage, however. A patient on a daily dose of 45 units would need one box of 5 pens (each prefilled pen has a 3 mL, or 300 unit, capacity) to have sufficient insulin for a month. Vials would be preferable for an individual who requires a larger single dose than a pen can dispense at one time (80 units of glargine, 60 units of detemir).
Syringe and needle size. If you are ordering insulin vials, you will also need to specify the correct syringe—available in 0.3 mL (which holds 30 units), 0.5 mL (50 units), and 1 mL (100 units) sizes. If the patient requires <50 units, order a small syringe to ensure that the unit markings are clear; a 1 mL syringe is preferable for those using a larger volume of insulin. Order the smallest syringe, which also has half-unit markings, if the patient is a child.
All needles are fine, with a 29 to 31 gauge, and available in regular (12.7 mm), short (8 mm), mini (5 mm), and nano (4 mm). Recent studies have shown that absorption, safety, and adverse events are similar for all needle lengths across a variety of patient factors,21 but patients generally prefer shorter needles.
Remember, too, to specify the maximum daily dose of insulin—a consideration that will be more important when prescribing mealtime insulin but is worth mentioning here.
Finally, tell patients who are getting started on insulin about www.accurateinsulin.org. Hosted by The Endocrine Society in partnership with the American Association of Diabetes Educators, ADA, American Pharmacists Association, and American College of Osteopathic Family Physicians, among other clinical groups, the Web site is designed to help patients (as well as providers) navigate the initiation and adjustment of insulin.18
CORRESPONDENCE
Jay Shubrook, DO, FAAFP, FACOFP, The Diabetes Institute at Ohio University, Athens, OH 45701; [email protected]
1. 2011 CDC National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. Available at http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed January 3, 2014.
2. UKPDS Group. Intensive blood glucose control with sulphonylurea or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
4. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
5. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
6. Shah BR, Hux JE, Laupacis A, et al. Clinical inertia in response to inadequate glycemic control. Diabetes Care. 2005;28:600-606.
7. Parchman ML, Wang CP. Initiation of insulin among veterans with type 2 diabetes and sustained elevation of HbA1c. Primary Care Diabetes. 2012;6:19-25.
8. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
9. Polonsky WH, Hajos TR, Dain MP, et al. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large international trial. Curr Med Res Opin. 2011;27:1169-1174.
10. Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;(164):6-10.
11. Presswala LS, Shubrook JH. Intensive insulin therapy as the primary treatment of type 2 diabetes. Clin Diabetes. 2011;29:151-153.
12. Li, C, Ford ES, Zhao G, et al. Trends of insulin use among US adults with type 2 diabetes: the Behavioral Risk Factor Surveillance System, 1995-2007. J Diabetes Complications. 2012;12:17-22.
13. Monthly Prescribing Reference (MPR). Insulin. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
14. Monami M, Marchionni N, Mannucci E. Long acting insulin analogs vs. NPH human insulin in Type 1 diabetes. A metaanalysis. Diabetes Obes Metab. 2009;11:372-378.
15. Goodrx Web site. Available at: www.goodrx.com. Accessed January 4, 2014.
16. LaSalle JR, Berria R. Insulin therapy in type 2 diabetes: a practical approach for primary care physicians and other health professionals. J Am Osteopath Assoc. 2013;113:152-162.
17. Davies M, Storms F, Shutler S, et al; ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282-1288.
18. Accurate Insulin Decisions. The Endocrine Society Web site. Available at: http://www.accurateinsulin.org/. Accessed January 4, 2014.
19. Keating GM. Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs. 2013;73:575-593.
20. Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once –daily LY2605541, a novel long acting basal insulin, versus insulin glargine in basal Insulin treated with patients in type 2 diabetes. Diabetes Care. 2012;35:2140-2147.
21. Hirsch LJ, Gibney MA, Li L, et al. Glycemic control, reported pain and leakage with a 4 mm × 32 G pen needle in obese and non-obese adults with diabetes: a post hoc analysis. Curr Med Res Opin. 2012;28:1305-1311.
› Initiate insulin for patients whose hemoglobin A1c ≥8% despite taking 2 or more oral agents. C
› Prescribe insulin for patients who have not reached their goal one year after diagnosis and initiation of oral therapy. C
› Consider reducing—but do not discontinue—oral agents, such as sulfonylureas and meglitinides, when you initiate insulin therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With type 2 diabetes now affecting 8.3% of the US population, most primary care physicians see patients with this disorder every day.1 Based on the concurrent obesity epidemic, aging population, and emergence of type 2 diabetes in children and adolescents, it is estimated that by 2050, the prevalence will have risen from one in 12 Americans to one in 3.1
Type 2 diabetes is a progressive disorder, with a relentless decline in beta cells. By the time of diagnosis, patients typically have lost at least 50% of insulin secretion; within 6 years of diagnosis, insulin secretion decreases to less than 25%.2
The American Association of Clinical Endocrinologists (AACE)3 and the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD)4 have recently published guidelines for the management of type 2 diabetes. While the AACE’s guidelines (available at https://www.aace.com/files/aace_algorithm.pdf) focus on different treatments at different stages of disease and both glycemic and nonglycemic benefits of treatment,3 the ADA/EASD’s guidelines (see http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) emphasize a patient-centered approach, shared decision making, and individualization of treatment goals based on both patient preference and comorbid disease states.4
One thing both sets of guidelines have in common is a purposeful intensification of therapy every 2 to 3 months, as needed, and the introduction of insulin one year after diagnosis if the patient is still not at goal.3,4 But all too often, this does not occur, particularly in primary care settings.
This article will review the “when” and “how” of insulin initiation. But first, a look at barriers to insulin therapy and evidence in support of earlier use.
Clinical inertia and patient fear are associated with delays
Both the AACE and the ADA/EASD guidelines agree that metformin is best used as early as possible.5,6 With typical use, however, metformin fails to prevent the progression of diabetes, as measured by the climb of hemoglobin A1c (HbA1c), at a failure rate of about 17% of patients per year.5 Physicians have been slow to intensify treatment for type 2 diabetes6—a phenomenon referred to as clinical inertia.
Typically, physicians adopt a stepwise approach, which often results in patients spending more than 10 years with an HbA1c >7% and 5 years with an HbA1c >8% before insulin is started.5 In a recent Veterans Administration study, patients were out of control, with an HbA1c >8%, for an average of 4.6 years before insulin was initiated.7
Both patient and physician factors contribute to the delay. Patient factors include the fear of injection, the belief that insulin will interfere with their lifestyle, and the idea that the use of insulin signifies impending complications or even death.8 But such beliefs are starting to change. In a recent multinational study of patients with type 2 diabetes, less than 20% stated they were unwilling to start insulin.9
For their part, primary care physicians are much less likely to prescribe insulin than clinicians specializing in diabetes.6 Physician-reported barriers to insulin initiation include the time required to train patients to use it correctly; the lack of support, including access to diabetes educators; and the absence of clear guidelines on the use of insulin.10
A case for earlier insulin
There has been recent momentum in favor of earlier initiation of insulin. In fact, some researchers regard intensive insulin as an excellent first treatment for type 2 diabetes,11 based on the belief that early insulin (used for a brief time) can provide not only immediate improvement in glucose control, but also a lasting “legacy” effect. The ADA/EASD guidelines support the use of insulin as a first-line treatment for patients with symptoms of insulin deficiency,4 but do not recommend it for everyone with newly diagnosed type 2 diabetes.
There have also been a number of advances in insulin therapy over the past 2 decades. These include insulin analogs with physiologic profiles that better match daily schedules, as well as improvements in the way insulin is delivered. Insulin pens, smaller needles, disposable devices, and insulin pumps have made it easier to administer and fine-tune insulin delivery. Despite these improvements and recommendations for earlier implementation, the use of insulin in type 2 diabetes is significantly lower today than in the 1990s.12
When to introduce insulin
Insulin is indicated for patients with type 2 diabetes whose disease is not easily controlled. That includes individuals with decompensated type 2 diabetes, those whose HbA1c remains high even with 2 or more oral agents, and individuals who have not reached goal after a year of treatment.
Glucose toxicity. It is generally agreed that insulin is the most effective treatment for patients who present with decompensated type 2 diabetes4—ie, with significant hyperglycemia and catabolic symptoms such as polydipsia, polyuria, and weight loss. Initiation of insulin promotes reversal of glucose toxicity and stabilization of metabolic status. In such cases, insulin can be started at a low dose to expose the patient to the complexities of injection therapy (more about this in a bit), then titrated as needed for stabilization.
HbA1c ≥8% even with 2 or more drugs. In my experience, an oral diabetes drug will lead to a drop in HbA1c of about one percentage point. Generally, the further from goal the patient is, the greater the effect the medication will have. As HbA1c inches closer to 7%, the effect diminishes. And when 2 oral agents fail to lower a patient’s HbA1c adequately, the incremental change expected from the addition of a third, fourth, or fifth agent is small.
Thus, in a patient with an HbA1c ≥8%, there is still a significant fasting hyperglycemic component. In such a case, a basal insulin is likely the best treatment option.
Not at goal at one year. Both the AACE and the ADA/EASD guidelines agree that treatment titration should be considered every 2 to 3 months to achieve metabolic control and that if a patient is not at goal after a year, insulin should be started.3,4 However, traditionally this is not done. The delayed implementation of this recommendation is an example of clinical inertia, which can contribute to further misunderstandings about the role and effect of insulin therapy.
Getting started with basal insulin
Most patients who are started on insulin have global hyperglycemia. But because fasting hyperglycemia can affect pancreatic insulin secretion, it is important to get control of the fasting glucose first. This can often be done with insulin sensitizers (metformin, thiazolidinediones, and incretin-based agents).
Suppression of excessive hepatic glucose production, which is very common in type 2 diabetes, is one of the biggest challenges in normalizing fasting glucose. This is well managed with a basal insulin. When starting basal insulin, however, it is critical that current treatments not be stopped. Oral agents such as sulfonylureas and meglitinides can be reduced to lower the risk of hypoglycemia, but stopping them altogether will only prolong the time it takes to get to goal.
There are 3 insulin formulations that can serve as basal insulin (TABLE 1).13 Neutral protamine Hagedorn (NPH) is a human insulin that can be used 2 to 3 times daily to provide basal insulin coverage. But long-acting basal analog insulins glargine and detemir, typically administered once a day when used by patients with type 2 diabetes, are a better option.14
While all 3 formulations have similar efficacy for lowering HbA1c, the analog basal insulins have numerous advantages: less weight gain, less hypoglycemia for the same level of glucose control, and less frequent dosing. In addition, glargine and detemir are available in a pen or vial, while generic NPH is available only in a vial. The primary disadvantage of the analogs is cost: A month’s supply—one vial—of NPH sells for approximately $25 (generic) or $94 (brand name); in comparison, a month’s supply (one box of 5 3-mL pens) of detemir and glargine costs about $300 and $320, respectively.15 (Humulin N, a brand-name NPH, is available in a pen, at a cost of approximately $315 per box.)
Use a weight-based initial dose
The recommended starting dose is 0.1 to 0.2 U/kg daily for patients with an HbA1c <8%. If HbA1c is ≥8%, the ADA/EASD guidelines recommend a starting dose of 0.3 to 0.4 U/kg daily4(TABLE 2).3,4,16 While basal insulin is most commonly dosed at bedtime, in fact, basal analog insulins can be given at any time that’s convenient for the patient. Morning dosing may be preferable for individuals with a significant fear of hypoglycemia—a phobia that sometimes causes patients to skip insulin doses and engage in “defensive eating” (ie, eating in an attempt to prevent hypoglycemia rather than because of hunger or the need for nutrition).
Teach injection technique
It is critically important that patients get the first shot in the office, guided by a clinician who can teach proper injection technique. This also helps to dispel the apprehension of self-injection.
In addition to being surprised at how easy and painless injection can be, patients have the opportunity to observe the results and gain confidence in insulin’s efficacy. And, in my experience, adherence to an insulin regimen is much greater if the first injection is administered in an office setting.
(Tech-savvy patients may find it helpful to use a smartphone app, such as Glucose Buddy or Dbees.com, to help manage their diabetes. See “The 13 best diabetes iPhone & Android apps of 2013” at http://www.healthline.com/health-slideshow/top-iphone-android-apps-diabetes.)
Establish a titration schedule
It is important, too, to teach the patient how to titrate the insulin dose from the start, rather than waiting until the next visit to address this. Patient titration—facilitated by a clinician-provided titration schedule (available from the AACE and the ADA/EASD3,4)—has been shown to achieve target glucose levels faster than physician titration.17
I usually suggest that patients increase the basal insulin dose by 3 units every 3 days, with an upper limit of 0.5 U/kg/d, until fasting glucose is consistently between 100 and 150 mg/dL. I advise every patient who starts taking insulin to track morning readings and titrate the dose until one of 3 things occurs:
1) the 0.5 U/kg/d limit is reached;
2) the patient has a glucose reading <100 mg/dL; or
3) the patient achieves his or her HbA1c target (<7% for most patients).
In every case, I recommend that the patient call my office for further instruction.
If the patient has any low glucose readings, I reduce the basal insulin by 5 U/kg/d. If he or she is still above goal, I advise the patient to continue titration, but more slowly. If the patient is at goal, I advise continuing at the current dose.
Basal titration vs mealtime coverage. Most people with type 2 diabetes require between 0.2 and 1 U/kg of basal insulin daily. It is currently recommended that when a patient has titrated to a dose of 0.5 U/kg/d, it is time to look at the glucose pattern to determine whether further titrating basal insulin or addressing prandial hyperglycemia should be the next step.4,18 This requires a change in fingerstick pattern.
The patient can stop the first morning glucose check and start checking before meals and 90 to 120 minutes postmeal. This allows for exploration of the mealtime excursion. Generally, a difference of <50 mg/dL is preferred. If the morning glucose level is at target but HbA1c is high, it is likely that postprandial glucose is contributing to this difference. This is particularly true when the HbA1c is between 7% and 8%. If the glucose pattern shows high postmeal glucose readings, it is much safer to address mealtime insulin (not discussed in this article) than to continue to titrate the basal insulin.4,18
Avoid “overbasalization”—ie, titrating basal insulin beyond its normal role to suppress hepatic glucose production and get the fasting glucose to goal. Doing so puts the patient at risk for unexpected hypoglycemia, as the insulin will now try to overcome hyperglycemia with meals, as well. Basal insulins are not designed to meet insulin requirements at meals. If a patient misses a meal yet continues the same dose of basal insulin, the risk of a hypoglycemic episode increases substantially.
In the pipeline. There are a number of new basal insulins in development, including one that has a prolonged duration of action and the potential for every-other-day injections19 and another that uses an attached polyethylene glycol moiety to slow absorption and prolong its effect.20
The nuts and bolts of insulin prescribing
When you prescribe insulin, there are a number of components to consider.
Pen or vial? In addition to deciding whether to order pen or vial, it is essential to consider the volume of insulin needed. Glargine, detemir, and Humulin N are available in 10 mL vials (100 U/mL) and in 3 mL pens (100 U/mL). (Generic NPH is available in vials only.) Most patients prefer insulin pens, which are more convenient and easier to use than a vial and syringe.
The choice also depends on the dosage, however. A patient on a daily dose of 45 units would need one box of 5 pens (each prefilled pen has a 3 mL, or 300 unit, capacity) to have sufficient insulin for a month. Vials would be preferable for an individual who requires a larger single dose than a pen can dispense at one time (80 units of glargine, 60 units of detemir).
Syringe and needle size. If you are ordering insulin vials, you will also need to specify the correct syringe—available in 0.3 mL (which holds 30 units), 0.5 mL (50 units), and 1 mL (100 units) sizes. If the patient requires <50 units, order a small syringe to ensure that the unit markings are clear; a 1 mL syringe is preferable for those using a larger volume of insulin. Order the smallest syringe, which also has half-unit markings, if the patient is a child.
All needles are fine, with a 29 to 31 gauge, and available in regular (12.7 mm), short (8 mm), mini (5 mm), and nano (4 mm). Recent studies have shown that absorption, safety, and adverse events are similar for all needle lengths across a variety of patient factors,21 but patients generally prefer shorter needles.
Remember, too, to specify the maximum daily dose of insulin—a consideration that will be more important when prescribing mealtime insulin but is worth mentioning here.
Finally, tell patients who are getting started on insulin about www.accurateinsulin.org. Hosted by The Endocrine Society in partnership with the American Association of Diabetes Educators, ADA, American Pharmacists Association, and American College of Osteopathic Family Physicians, among other clinical groups, the Web site is designed to help patients (as well as providers) navigate the initiation and adjustment of insulin.18
CORRESPONDENCE
Jay Shubrook, DO, FAAFP, FACOFP, The Diabetes Institute at Ohio University, Athens, OH 45701; [email protected]
› Initiate insulin for patients whose hemoglobin A1c ≥8% despite taking 2 or more oral agents. C
› Prescribe insulin for patients who have not reached their goal one year after diagnosis and initiation of oral therapy. C
› Consider reducing—but do not discontinue—oral agents, such as sulfonylureas and meglitinides, when you initiate insulin therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With type 2 diabetes now affecting 8.3% of the US population, most primary care physicians see patients with this disorder every day.1 Based on the concurrent obesity epidemic, aging population, and emergence of type 2 diabetes in children and adolescents, it is estimated that by 2050, the prevalence will have risen from one in 12 Americans to one in 3.1
Type 2 diabetes is a progressive disorder, with a relentless decline in beta cells. By the time of diagnosis, patients typically have lost at least 50% of insulin secretion; within 6 years of diagnosis, insulin secretion decreases to less than 25%.2
The American Association of Clinical Endocrinologists (AACE)3 and the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD)4 have recently published guidelines for the management of type 2 diabetes. While the AACE’s guidelines (available at https://www.aace.com/files/aace_algorithm.pdf) focus on different treatments at different stages of disease and both glycemic and nonglycemic benefits of treatment,3 the ADA/EASD’s guidelines (see http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) emphasize a patient-centered approach, shared decision making, and individualization of treatment goals based on both patient preference and comorbid disease states.4
One thing both sets of guidelines have in common is a purposeful intensification of therapy every 2 to 3 months, as needed, and the introduction of insulin one year after diagnosis if the patient is still not at goal.3,4 But all too often, this does not occur, particularly in primary care settings.
This article will review the “when” and “how” of insulin initiation. But first, a look at barriers to insulin therapy and evidence in support of earlier use.
Clinical inertia and patient fear are associated with delays
Both the AACE and the ADA/EASD guidelines agree that metformin is best used as early as possible.5,6 With typical use, however, metformin fails to prevent the progression of diabetes, as measured by the climb of hemoglobin A1c (HbA1c), at a failure rate of about 17% of patients per year.5 Physicians have been slow to intensify treatment for type 2 diabetes6—a phenomenon referred to as clinical inertia.
Typically, physicians adopt a stepwise approach, which often results in patients spending more than 10 years with an HbA1c >7% and 5 years with an HbA1c >8% before insulin is started.5 In a recent Veterans Administration study, patients were out of control, with an HbA1c >8%, for an average of 4.6 years before insulin was initiated.7
Both patient and physician factors contribute to the delay. Patient factors include the fear of injection, the belief that insulin will interfere with their lifestyle, and the idea that the use of insulin signifies impending complications or even death.8 But such beliefs are starting to change. In a recent multinational study of patients with type 2 diabetes, less than 20% stated they were unwilling to start insulin.9
For their part, primary care physicians are much less likely to prescribe insulin than clinicians specializing in diabetes.6 Physician-reported barriers to insulin initiation include the time required to train patients to use it correctly; the lack of support, including access to diabetes educators; and the absence of clear guidelines on the use of insulin.10
A case for earlier insulin
There has been recent momentum in favor of earlier initiation of insulin. In fact, some researchers regard intensive insulin as an excellent first treatment for type 2 diabetes,11 based on the belief that early insulin (used for a brief time) can provide not only immediate improvement in glucose control, but also a lasting “legacy” effect. The ADA/EASD guidelines support the use of insulin as a first-line treatment for patients with symptoms of insulin deficiency,4 but do not recommend it for everyone with newly diagnosed type 2 diabetes.
There have also been a number of advances in insulin therapy over the past 2 decades. These include insulin analogs with physiologic profiles that better match daily schedules, as well as improvements in the way insulin is delivered. Insulin pens, smaller needles, disposable devices, and insulin pumps have made it easier to administer and fine-tune insulin delivery. Despite these improvements and recommendations for earlier implementation, the use of insulin in type 2 diabetes is significantly lower today than in the 1990s.12
When to introduce insulin
Insulin is indicated for patients with type 2 diabetes whose disease is not easily controlled. That includes individuals with decompensated type 2 diabetes, those whose HbA1c remains high even with 2 or more oral agents, and individuals who have not reached goal after a year of treatment.
Glucose toxicity. It is generally agreed that insulin is the most effective treatment for patients who present with decompensated type 2 diabetes4—ie, with significant hyperglycemia and catabolic symptoms such as polydipsia, polyuria, and weight loss. Initiation of insulin promotes reversal of glucose toxicity and stabilization of metabolic status. In such cases, insulin can be started at a low dose to expose the patient to the complexities of injection therapy (more about this in a bit), then titrated as needed for stabilization.
HbA1c ≥8% even with 2 or more drugs. In my experience, an oral diabetes drug will lead to a drop in HbA1c of about one percentage point. Generally, the further from goal the patient is, the greater the effect the medication will have. As HbA1c inches closer to 7%, the effect diminishes. And when 2 oral agents fail to lower a patient’s HbA1c adequately, the incremental change expected from the addition of a third, fourth, or fifth agent is small.
Thus, in a patient with an HbA1c ≥8%, there is still a significant fasting hyperglycemic component. In such a case, a basal insulin is likely the best treatment option.
Not at goal at one year. Both the AACE and the ADA/EASD guidelines agree that treatment titration should be considered every 2 to 3 months to achieve metabolic control and that if a patient is not at goal after a year, insulin should be started.3,4 However, traditionally this is not done. The delayed implementation of this recommendation is an example of clinical inertia, which can contribute to further misunderstandings about the role and effect of insulin therapy.
Getting started with basal insulin
Most patients who are started on insulin have global hyperglycemia. But because fasting hyperglycemia can affect pancreatic insulin secretion, it is important to get control of the fasting glucose first. This can often be done with insulin sensitizers (metformin, thiazolidinediones, and incretin-based agents).
Suppression of excessive hepatic glucose production, which is very common in type 2 diabetes, is one of the biggest challenges in normalizing fasting glucose. This is well managed with a basal insulin. When starting basal insulin, however, it is critical that current treatments not be stopped. Oral agents such as sulfonylureas and meglitinides can be reduced to lower the risk of hypoglycemia, but stopping them altogether will only prolong the time it takes to get to goal.
There are 3 insulin formulations that can serve as basal insulin (TABLE 1).13 Neutral protamine Hagedorn (NPH) is a human insulin that can be used 2 to 3 times daily to provide basal insulin coverage. But long-acting basal analog insulins glargine and detemir, typically administered once a day when used by patients with type 2 diabetes, are a better option.14
While all 3 formulations have similar efficacy for lowering HbA1c, the analog basal insulins have numerous advantages: less weight gain, less hypoglycemia for the same level of glucose control, and less frequent dosing. In addition, glargine and detemir are available in a pen or vial, while generic NPH is available only in a vial. The primary disadvantage of the analogs is cost: A month’s supply—one vial—of NPH sells for approximately $25 (generic) or $94 (brand name); in comparison, a month’s supply (one box of 5 3-mL pens) of detemir and glargine costs about $300 and $320, respectively.15 (Humulin N, a brand-name NPH, is available in a pen, at a cost of approximately $315 per box.)
Use a weight-based initial dose
The recommended starting dose is 0.1 to 0.2 U/kg daily for patients with an HbA1c <8%. If HbA1c is ≥8%, the ADA/EASD guidelines recommend a starting dose of 0.3 to 0.4 U/kg daily4(TABLE 2).3,4,16 While basal insulin is most commonly dosed at bedtime, in fact, basal analog insulins can be given at any time that’s convenient for the patient. Morning dosing may be preferable for individuals with a significant fear of hypoglycemia—a phobia that sometimes causes patients to skip insulin doses and engage in “defensive eating” (ie, eating in an attempt to prevent hypoglycemia rather than because of hunger or the need for nutrition).
Teach injection technique
It is critically important that patients get the first shot in the office, guided by a clinician who can teach proper injection technique. This also helps to dispel the apprehension of self-injection.
In addition to being surprised at how easy and painless injection can be, patients have the opportunity to observe the results and gain confidence in insulin’s efficacy. And, in my experience, adherence to an insulin regimen is much greater if the first injection is administered in an office setting.
(Tech-savvy patients may find it helpful to use a smartphone app, such as Glucose Buddy or Dbees.com, to help manage their diabetes. See “The 13 best diabetes iPhone & Android apps of 2013” at http://www.healthline.com/health-slideshow/top-iphone-android-apps-diabetes.)
Establish a titration schedule
It is important, too, to teach the patient how to titrate the insulin dose from the start, rather than waiting until the next visit to address this. Patient titration—facilitated by a clinician-provided titration schedule (available from the AACE and the ADA/EASD3,4)—has been shown to achieve target glucose levels faster than physician titration.17
I usually suggest that patients increase the basal insulin dose by 3 units every 3 days, with an upper limit of 0.5 U/kg/d, until fasting glucose is consistently between 100 and 150 mg/dL. I advise every patient who starts taking insulin to track morning readings and titrate the dose until one of 3 things occurs:
1) the 0.5 U/kg/d limit is reached;
2) the patient has a glucose reading <100 mg/dL; or
3) the patient achieves his or her HbA1c target (<7% for most patients).
In every case, I recommend that the patient call my office for further instruction.
If the patient has any low glucose readings, I reduce the basal insulin by 5 U/kg/d. If he or she is still above goal, I advise the patient to continue titration, but more slowly. If the patient is at goal, I advise continuing at the current dose.
Basal titration vs mealtime coverage. Most people with type 2 diabetes require between 0.2 and 1 U/kg of basal insulin daily. It is currently recommended that when a patient has titrated to a dose of 0.5 U/kg/d, it is time to look at the glucose pattern to determine whether further titrating basal insulin or addressing prandial hyperglycemia should be the next step.4,18 This requires a change in fingerstick pattern.
The patient can stop the first morning glucose check and start checking before meals and 90 to 120 minutes postmeal. This allows for exploration of the mealtime excursion. Generally, a difference of <50 mg/dL is preferred. If the morning glucose level is at target but HbA1c is high, it is likely that postprandial glucose is contributing to this difference. This is particularly true when the HbA1c is between 7% and 8%. If the glucose pattern shows high postmeal glucose readings, it is much safer to address mealtime insulin (not discussed in this article) than to continue to titrate the basal insulin.4,18
Avoid “overbasalization”—ie, titrating basal insulin beyond its normal role to suppress hepatic glucose production and get the fasting glucose to goal. Doing so puts the patient at risk for unexpected hypoglycemia, as the insulin will now try to overcome hyperglycemia with meals, as well. Basal insulins are not designed to meet insulin requirements at meals. If a patient misses a meal yet continues the same dose of basal insulin, the risk of a hypoglycemic episode increases substantially.
In the pipeline. There are a number of new basal insulins in development, including one that has a prolonged duration of action and the potential for every-other-day injections19 and another that uses an attached polyethylene glycol moiety to slow absorption and prolong its effect.20
The nuts and bolts of insulin prescribing
When you prescribe insulin, there are a number of components to consider.
Pen or vial? In addition to deciding whether to order pen or vial, it is essential to consider the volume of insulin needed. Glargine, detemir, and Humulin N are available in 10 mL vials (100 U/mL) and in 3 mL pens (100 U/mL). (Generic NPH is available in vials only.) Most patients prefer insulin pens, which are more convenient and easier to use than a vial and syringe.
The choice also depends on the dosage, however. A patient on a daily dose of 45 units would need one box of 5 pens (each prefilled pen has a 3 mL, or 300 unit, capacity) to have sufficient insulin for a month. Vials would be preferable for an individual who requires a larger single dose than a pen can dispense at one time (80 units of glargine, 60 units of detemir).
Syringe and needle size. If you are ordering insulin vials, you will also need to specify the correct syringe—available in 0.3 mL (which holds 30 units), 0.5 mL (50 units), and 1 mL (100 units) sizes. If the patient requires <50 units, order a small syringe to ensure that the unit markings are clear; a 1 mL syringe is preferable for those using a larger volume of insulin. Order the smallest syringe, which also has half-unit markings, if the patient is a child.
All needles are fine, with a 29 to 31 gauge, and available in regular (12.7 mm), short (8 mm), mini (5 mm), and nano (4 mm). Recent studies have shown that absorption, safety, and adverse events are similar for all needle lengths across a variety of patient factors,21 but patients generally prefer shorter needles.
Remember, too, to specify the maximum daily dose of insulin—a consideration that will be more important when prescribing mealtime insulin but is worth mentioning here.
Finally, tell patients who are getting started on insulin about www.accurateinsulin.org. Hosted by The Endocrine Society in partnership with the American Association of Diabetes Educators, ADA, American Pharmacists Association, and American College of Osteopathic Family Physicians, among other clinical groups, the Web site is designed to help patients (as well as providers) navigate the initiation and adjustment of insulin.18
CORRESPONDENCE
Jay Shubrook, DO, FAAFP, FACOFP, The Diabetes Institute at Ohio University, Athens, OH 45701; [email protected]
1. 2011 CDC National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. Available at http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed January 3, 2014.
2. UKPDS Group. Intensive blood glucose control with sulphonylurea or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
4. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
5. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
6. Shah BR, Hux JE, Laupacis A, et al. Clinical inertia in response to inadequate glycemic control. Diabetes Care. 2005;28:600-606.
7. Parchman ML, Wang CP. Initiation of insulin among veterans with type 2 diabetes and sustained elevation of HbA1c. Primary Care Diabetes. 2012;6:19-25.
8. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
9. Polonsky WH, Hajos TR, Dain MP, et al. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large international trial. Curr Med Res Opin. 2011;27:1169-1174.
10. Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;(164):6-10.
11. Presswala LS, Shubrook JH. Intensive insulin therapy as the primary treatment of type 2 diabetes. Clin Diabetes. 2011;29:151-153.
12. Li, C, Ford ES, Zhao G, et al. Trends of insulin use among US adults with type 2 diabetes: the Behavioral Risk Factor Surveillance System, 1995-2007. J Diabetes Complications. 2012;12:17-22.
13. Monthly Prescribing Reference (MPR). Insulin. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
14. Monami M, Marchionni N, Mannucci E. Long acting insulin analogs vs. NPH human insulin in Type 1 diabetes. A metaanalysis. Diabetes Obes Metab. 2009;11:372-378.
15. Goodrx Web site. Available at: www.goodrx.com. Accessed January 4, 2014.
16. LaSalle JR, Berria R. Insulin therapy in type 2 diabetes: a practical approach for primary care physicians and other health professionals. J Am Osteopath Assoc. 2013;113:152-162.
17. Davies M, Storms F, Shutler S, et al; ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282-1288.
18. Accurate Insulin Decisions. The Endocrine Society Web site. Available at: http://www.accurateinsulin.org/. Accessed January 4, 2014.
19. Keating GM. Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs. 2013;73:575-593.
20. Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once –daily LY2605541, a novel long acting basal insulin, versus insulin glargine in basal Insulin treated with patients in type 2 diabetes. Diabetes Care. 2012;35:2140-2147.
21. Hirsch LJ, Gibney MA, Li L, et al. Glycemic control, reported pain and leakage with a 4 mm × 32 G pen needle in obese and non-obese adults with diabetes: a post hoc analysis. Curr Med Res Opin. 2012;28:1305-1311.
1. 2011 CDC National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. Available at http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed January 3, 2014.
2. UKPDS Group. Intensive blood glucose control with sulphonylurea or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
4. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
5. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
6. Shah BR, Hux JE, Laupacis A, et al. Clinical inertia in response to inadequate glycemic control. Diabetes Care. 2005;28:600-606.
7. Parchman ML, Wang CP. Initiation of insulin among veterans with type 2 diabetes and sustained elevation of HbA1c. Primary Care Diabetes. 2012;6:19-25.
8. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
9. Polonsky WH, Hajos TR, Dain MP, et al. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large international trial. Curr Med Res Opin. 2011;27:1169-1174.
10. Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;(164):6-10.
11. Presswala LS, Shubrook JH. Intensive insulin therapy as the primary treatment of type 2 diabetes. Clin Diabetes. 2011;29:151-153.
12. Li, C, Ford ES, Zhao G, et al. Trends of insulin use among US adults with type 2 diabetes: the Behavioral Risk Factor Surveillance System, 1995-2007. J Diabetes Complications. 2012;12:17-22.
13. Monthly Prescribing Reference (MPR). Insulin. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
14. Monami M, Marchionni N, Mannucci E. Long acting insulin analogs vs. NPH human insulin in Type 1 diabetes. A metaanalysis. Diabetes Obes Metab. 2009;11:372-378.
15. Goodrx Web site. Available at: www.goodrx.com. Accessed January 4, 2014.
16. LaSalle JR, Berria R. Insulin therapy in type 2 diabetes: a practical approach for primary care physicians and other health professionals. J Am Osteopath Assoc. 2013;113:152-162.
17. Davies M, Storms F, Shutler S, et al; ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282-1288.
18. Accurate Insulin Decisions. The Endocrine Society Web site. Available at: http://www.accurateinsulin.org/. Accessed January 4, 2014.
19. Keating GM. Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs. 2013;73:575-593.
20. Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once –daily LY2605541, a novel long acting basal insulin, versus insulin glargine in basal Insulin treated with patients in type 2 diabetes. Diabetes Care. 2012;35:2140-2147.
21. Hirsch LJ, Gibney MA, Li L, et al. Glycemic control, reported pain and leakage with a 4 mm × 32 G pen needle in obese and non-obese adults with diabetes: a post hoc analysis. Curr Med Res Opin. 2012;28:1305-1311.
Stimulants for kids with ADHD—how to proceed safely
› Complete a thorough, cardiac-focused history and physical examination before starting stimulants for attention deficit hyperactivity disorder (ADHD) in a child or adolescent. C
› Avoid using stimulants in children or adolescents with comorbid conditions associated with sudden cardiac death, including hypertrophic cardiomyopathy, long QT interval syndrome, and preexcitation syndromes such as Wolff-Parkinson-White syndrome. C
› Monitor all children and adolescents who are taking stimulants for tachycardia, hypertension, palpitations, and chest pain. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A young patient has been struggling in school. His worried mother, having had several conferences with the child’s teachers, brings him to the family physician (FP), where he is given a diagnosis of attention deficit hyperactivity disorder (ADHD). The FP considers prescribing a stimulant medication, but first plans on conducting a more thorough family history and exam. She also debates the merits of ordering an electrocardiogram (EKG) to screen for conditions that could lead to sudden cardiac death.
If you were caring for this patient, how would you proceed?
That’s a good question, given the debate that has surrounded this subject since the US Food and Drug Administration (FDA) first learned of 25 cases of sudden death that were linked to stimulant medications.1 The majority of the cases, which were reported to the FDA’s Adverse Event Reporting System between 1999 and 2003, involved amphetamines or methylphenidate in patients under the age of 19.1 In 2008, the American Heart Association (AHA) issued a scientific statement advocating that physicians perform a proper family history and physical exam that includes blood pressure (BP) and an EKG before prescribing a stimulant for children and adolescents.2 The inclusion of EKG screening was intended to increase the likelihood of identifying patients with potentially life-threatening conditions that could lead to sudden cardiac death (SCD).2
Not everyone, however, agreed.
Later that year, the American Academy of Pediatrics (AAP) challenged the routine use of EKGs in this screening process, citing a lack of evidence between stimulant use and the induction of potentially lethal arrhythmias.3 And in 2011, the European Guideline Group also concluded that there was no evidence to suggest an incremental benefit for routine EKG assessment of ADHD patients before initiation of medication.4
Underscoring the uncertainty surrounding the subject are the findings of a 2012 survey of 525 randomly selected US pediatricians.5 Nearly a quarter of the respondents expressed concerns over the risk for SCD in children receiving stimulants for ADHD, and a slightly higher number—30%—worried that the risks for legal liability were high enough to warrant cardiac assessment.5
So how should the prudent FP proceed? In this review, we will describe how to thoroughly screen children and adolescents for their risk of SCD before prescribing stimulants for ADHD. We’ll also summarize what the evidence tells us about whether—and when—you should order an EKG. But first, a word about the pharmacology of stimulants.
How stimulants might increase SCD risk
Stimulants have been used to treat ADHD for more than 40 years6 and are a first-line of therapy for children with ADHD. Stimulants increase attention span by releasing dopamine and norepinephrine at synapses in the frontal cortex, brain stem, and midbrain.
The effect on heart rate and BP. In clinical trials with small samples sizes, children and adolescents receiving stimulants to treat ADHD experienced a minimal rise in heart rate and BP. As measured by 24-hour ambulatory BP monitoring, 13 subjects in a double-blind, randomized, placebo/stimulant crossover trial had slightly elevated total diastolic BP (69.7 vs 65.8 mm Hg; P=.02), waking diastolic BP (75.5 vs 72.3 mm Hg; P=.03), and total heart rate (85.5 vs 79.9 beats per minutes [bpm]; P=.004) while receiving stimulants.7 Other investigators noted similar findings among 17 boys ages 7 to 11 years.8
Whether prolonged childhood exposure to stimulants increases the risk for developing hypertension or tachycardia is unknown. A 10-year follow-up study of 579 children between the ages of 7 to 9 years found stimulants had no effect on systolic or diastolic BP.9 Stimulants use did, however, lead to a higher heart rate (84.2±12.4 vs 79.1±12.0 bpm) during treatment.9 No stimulant-related QT interval changes—which some have proposed might explain SCD in ADHD patients—have been reported in pediatric patients.10 Researchers have noted small increases in mean QTc intervals in adults treated with stimulants for ADHD, but none were >480 msec.11
Steps you should always take before prescribing a stimulant
Before prescribing stimulants to children or adolescents with ADHD, complete an in-depth cardiac history and physical examination, as recommended by the AHA and AAP (TABLE),2,3 to identify conditions that increase the likelihood of SCD, such as hypertrophic cardiomyopathy (HCM), long QT interval syndrome (LQTS), and preexcitation syndromes such as Wolff-Parkinson-White syndrome (WPW).
Confirm, for instance, that your patient has a normal heart rate, rhythm, and BP, and no pathological murmurs. In a survey of families with a child or young adult who had sudden cardiac arrest, 72% reported the patient had at least one cardiovascular symptom within 19 to 71 months of SCD, and 27% reported having a family member with a history of SCD before age 50.12 For patients with no such complaints or family history, the news is good. Two large studies found that in the absence of any suspected or overt cardiac disease, children with ADHD who were receiving stimulant therapy had no increased risk of SCD.13,14
What about patients with this common heart problem? Physicians face a dilemma when a stimulant is needed and the patient has a common acyanotic congenital heart lesion, such as a small atrial or ventricular septal defect, which is considered nonlethal. Based on limited data, there is no evidence that the risk of SCD is higher when these patients take stimulants.15
Should you order that EKG—or not?
Currently, the AHA still favors an EKG, though in a correction to its original statement, it adjusted the language to say that EKG could be “useful,” in addition to an in-depth cardiac history and physical examination.16
Opposition to routine EKG screening in these patients stems from the procedure’s extremely low yield and relatively high false positive findings, which may result in higher financial and psychological burdens for patients and families. Thomas et al17 reported that at a single center, the number of EKGs ordered with an indication of “stimulant medication screening” quadrupled during 2009, the year after the AHA published its recommendations. Of 372 patients referred for EKG, 24 (6.4%) had abnormal findings and 18 were referred for further evaluation, but none were found to have cardiac disease. ADHD therapy was delayed in 6 patients because of the EKG.
In a similar evaluation of 1470 ADHD patients ages 21 years and younger, Mahle et al18 noted that 119 patients (8.1%) had an abnormal EKG, 78 of whom (65%) were already receiving stimulants. Five patients had cardiac disease, including 2 who had a preexcitation syndrome. Overall, the positive predictive value was low (4.2%).18 Other research, including a study lead by one of this article’s authors (SKM), has found similar increases in the number of EKGs ordered for patients with ADHD.19
Cost vs benefit. In the Mahle et al18 study described above, the mean cost of EKG screening, including further testing for patients with abnormal initial results, was $58 per child. The mean cost to identify a true-positive result was $17,162.18
In 2012, Leslie et al20 used simulation models to estimate the societal cost of routine EKG screening to prevent SCD in children with ADHD. Their findings: The cost would be high relative to its health benefits—approximately $91,000 to $204,000 per life year saved. Furthermore, these researchers found that ordering an EKG to screen for 3 common cardiac conditions linked to SCD (HCM, WPW, and LQTS) would add <2 days to a patient’s projected life expectancy.20
Our recommendations
We believe stimulants can safely be used in the treatment of children and adolescents with ADHD, given the evidence that suggests a low risk of SCD. That said, it is prudent to avoid prescribing stimulants for children who have an underlying condition that may deteriorate secondary to increased blood pressure or heart rate.
We agree with the current AHA and AAP recommendations that physicians should obtain an in-depth cardiac history and physical examination, with emphasis on screening for cardiac disorders that may put a child at risk for SCD, such as HCM, LQTS, and preexcitation syndromes. For instance, a history of a family member with palpitations should prompt an EKG, which may reveal familial preexcitation syndrome. Similarly, an EKG is in order if you suspect LQTS based on a parent’s description of a family member’s death after hearing a loud noise, such as fireworks.
It often takes active probing to uncover a history of sudden death in the family that a parent may not consider relevant. For example, one of the authors (SKM) cared for a 6-year-old boy who presented with a history of syncope after his hand got caught in a door jam. On further probing, his mother revealed that her father had died at age 30 while he was taking astemizole, an allergy drug known to prolong the QT interval. Subsequent EKGs revealed that both the boy and his mother had LQTS.
For patients already taking stimulants, we recommend monitoring BP and heart rate and ordering an EKG only if the patient exhibits cardiac symptoms or there are concerns based on follow-up history and physical examination. Should a patient develop palpitations while taking a therapeutic dose of stimulants, a detailed history of the onset and duration of symptoms is important. For example, tachycardia that has a gradual onset and occurs with exercise is suggestive of physiological sinus tachycardia. In our judgment, most patients who experience symptoms that suggest sinus tachycardia simply require downward readjustment of their medication or a switch to a nonstimulant.
However, if the patient or family history prompts you to suspect other arrhythmias such as ectopic beats or supraventricular tachycardia, immediate assessment either in an emergency department or in the physician’s office may be required, because obtaining an EKG during symptoms is crucial for the diagnosis. Similarly, unexplained exercise intolerance or the onset of chest pain associated with exercise, dizziness, syncope, seizures, or dyspnea requires immediate cardiovascular assessment.
And finally, whether your patient has just started taking medication for his or her ADHD or has been on the medication for some time, it’s important to periodically reassess the need to continue the stimulant therapy; ADHD symptoms may decrease during mid- to late adolescence and into adulthood.21
CASE › The FP completed a thorough physical exam and found no evidence of any conditions that would increase the likelihood of SCD in the young patient. There was no history of SCD in the boy’s family, either. Based on these findings, the FP opted to forgo an EKG. She prescribed lisdexamfetamine, starting with 20 mg/d (the lowest dose available) and then monitored his course by telephone. Eventually, 30 mg was found to be an effective dose. At a 6-week follow-up visit, the boy’s ADHD symptoms were substantially reduced, without any adverse effects—cardiac or otherwise.
CORRESPONDENCE
Sudhir Ken Mehta, Cleveland Clinic Children’s Hospital, 9500 Euclid Avenue, Cleveland, OH 44111; [email protected]
1. Safety review: Follow up review of AERS search identifying cases of sudden death occurring with drugs used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). US Food and Drug Administration Web site. Available at: http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4210b_07_01_safetyreview.pdf. Accessed January 17, 2014.
2. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117:2407-2423.
3. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
4. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20:17-37.
5. Leslie LK, Rodday AM, Saunders TS, et al. Cardiac screening prior to stimulant treatment of ADHD: a survey of US-based pediatricians. Pediatrics. 2012;129:222-230.
6. Conners CK. Symposium: behavior modification by drugs. II. Psychological effects of stimulant drugs in children with minimal brain dysfunction. Pediatrics. 1972;49:702-708.
7. Samuels JA, Franco K, Wan F, et al. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95.
8. Stowe CD, Gardner SF, Gist CC, et al. 24-hour ambulatory blood pressure monitoring in male children receiving stimulant therapy. Ann Pharmacother. 2002;36:1142-1149.
9. Vitiello B, Elliott GR, Swanson JM, et al. Blood pressure and heart rate over 10 years in the multimodal treatment study of children with ADHD. Am J Psychiatry. 2012;169:167-177.
10. Hammerness P, Wilens T, Mick E, et al. Cardiovascular effects of longer-term, high-dose OROS methylphenidate in adolescents with attention deficit hyperactivity disorder. J Pediatr. 2009;155:84-89,89.e1.
11. Weisler RH, Biederman J, Spencer TJ, et al. Long-term cardiovascular effects of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr. 2005;10(suppl 20):35-43.
12. Drezner JA, Fudge J, Harmon KG, et al. Warning symptoms and family history in children and young adults with sudden cardiac arrest. J Am Board Fam Med. 2012;25:408-415.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896-1904.
14. Schelleman H, Bilker WB, Strom BL, et al. Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics. 2011;127:1102-1110.
15. Winterstein AG, Gerhard T, Kubilis P, et al. Cardiovascular safety of central nervous system stimulants in children and adolescents: population based cohort study. BMJ. 2012;345:e4627.
16. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [published correction appears in Circulation. 2009;120:e55-e59]. Circulation. 2008;117:2407-2423.
17. Thomas PE, Carlo WF, Decker JA, et al. Impact of the American Heart Association scientific statement on screening electrocardiograms and stimulant medications. Arch Pediatr Adolesc Med. 2011;165:166-170.
18. Mahle WT, Hebson C, Strieper MJ. Electrocardiographic screening in children with attention-deficit hyperactivity disorder. Am J Cardiol. 2009;104:1296-1299.
19. Mehta SK, Richards N, Jacobs I. Children and adolescents with attention deficit hyperactivity disorder in a pediatric cardiology office. Cardiol Young. 2010;20(suppl 3):167.
20. Leslie LK, Cohen JT, Newburger JW, et al. Costs and benefits of targeted screening for causes of sudden cardiac death in children and adolescents. Circulation. 2012;125:2621-2629.
21. Mannuzza S, Klein RG, Bessler A, et al. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155:493-488.
› Complete a thorough, cardiac-focused history and physical examination before starting stimulants for attention deficit hyperactivity disorder (ADHD) in a child or adolescent. C
› Avoid using stimulants in children or adolescents with comorbid conditions associated with sudden cardiac death, including hypertrophic cardiomyopathy, long QT interval syndrome, and preexcitation syndromes such as Wolff-Parkinson-White syndrome. C
› Monitor all children and adolescents who are taking stimulants for tachycardia, hypertension, palpitations, and chest pain. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A young patient has been struggling in school. His worried mother, having had several conferences with the child’s teachers, brings him to the family physician (FP), where he is given a diagnosis of attention deficit hyperactivity disorder (ADHD). The FP considers prescribing a stimulant medication, but first plans on conducting a more thorough family history and exam. She also debates the merits of ordering an electrocardiogram (EKG) to screen for conditions that could lead to sudden cardiac death.
If you were caring for this patient, how would you proceed?
That’s a good question, given the debate that has surrounded this subject since the US Food and Drug Administration (FDA) first learned of 25 cases of sudden death that were linked to stimulant medications.1 The majority of the cases, which were reported to the FDA’s Adverse Event Reporting System between 1999 and 2003, involved amphetamines or methylphenidate in patients under the age of 19.1 In 2008, the American Heart Association (AHA) issued a scientific statement advocating that physicians perform a proper family history and physical exam that includes blood pressure (BP) and an EKG before prescribing a stimulant for children and adolescents.2 The inclusion of EKG screening was intended to increase the likelihood of identifying patients with potentially life-threatening conditions that could lead to sudden cardiac death (SCD).2
Not everyone, however, agreed.
Later that year, the American Academy of Pediatrics (AAP) challenged the routine use of EKGs in this screening process, citing a lack of evidence between stimulant use and the induction of potentially lethal arrhythmias.3 And in 2011, the European Guideline Group also concluded that there was no evidence to suggest an incremental benefit for routine EKG assessment of ADHD patients before initiation of medication.4
Underscoring the uncertainty surrounding the subject are the findings of a 2012 survey of 525 randomly selected US pediatricians.5 Nearly a quarter of the respondents expressed concerns over the risk for SCD in children receiving stimulants for ADHD, and a slightly higher number—30%—worried that the risks for legal liability were high enough to warrant cardiac assessment.5
So how should the prudent FP proceed? In this review, we will describe how to thoroughly screen children and adolescents for their risk of SCD before prescribing stimulants for ADHD. We’ll also summarize what the evidence tells us about whether—and when—you should order an EKG. But first, a word about the pharmacology of stimulants.
How stimulants might increase SCD risk
Stimulants have been used to treat ADHD for more than 40 years6 and are a first-line of therapy for children with ADHD. Stimulants increase attention span by releasing dopamine and norepinephrine at synapses in the frontal cortex, brain stem, and midbrain.
The effect on heart rate and BP. In clinical trials with small samples sizes, children and adolescents receiving stimulants to treat ADHD experienced a minimal rise in heart rate and BP. As measured by 24-hour ambulatory BP monitoring, 13 subjects in a double-blind, randomized, placebo/stimulant crossover trial had slightly elevated total diastolic BP (69.7 vs 65.8 mm Hg; P=.02), waking diastolic BP (75.5 vs 72.3 mm Hg; P=.03), and total heart rate (85.5 vs 79.9 beats per minutes [bpm]; P=.004) while receiving stimulants.7 Other investigators noted similar findings among 17 boys ages 7 to 11 years.8
Whether prolonged childhood exposure to stimulants increases the risk for developing hypertension or tachycardia is unknown. A 10-year follow-up study of 579 children between the ages of 7 to 9 years found stimulants had no effect on systolic or diastolic BP.9 Stimulants use did, however, lead to a higher heart rate (84.2±12.4 vs 79.1±12.0 bpm) during treatment.9 No stimulant-related QT interval changes—which some have proposed might explain SCD in ADHD patients—have been reported in pediatric patients.10 Researchers have noted small increases in mean QTc intervals in adults treated with stimulants for ADHD, but none were >480 msec.11
Steps you should always take before prescribing a stimulant
Before prescribing stimulants to children or adolescents with ADHD, complete an in-depth cardiac history and physical examination, as recommended by the AHA and AAP (TABLE),2,3 to identify conditions that increase the likelihood of SCD, such as hypertrophic cardiomyopathy (HCM), long QT interval syndrome (LQTS), and preexcitation syndromes such as Wolff-Parkinson-White syndrome (WPW).
Confirm, for instance, that your patient has a normal heart rate, rhythm, and BP, and no pathological murmurs. In a survey of families with a child or young adult who had sudden cardiac arrest, 72% reported the patient had at least one cardiovascular symptom within 19 to 71 months of SCD, and 27% reported having a family member with a history of SCD before age 50.12 For patients with no such complaints or family history, the news is good. Two large studies found that in the absence of any suspected or overt cardiac disease, children with ADHD who were receiving stimulant therapy had no increased risk of SCD.13,14
What about patients with this common heart problem? Physicians face a dilemma when a stimulant is needed and the patient has a common acyanotic congenital heart lesion, such as a small atrial or ventricular septal defect, which is considered nonlethal. Based on limited data, there is no evidence that the risk of SCD is higher when these patients take stimulants.15
Should you order that EKG—or not?
Currently, the AHA still favors an EKG, though in a correction to its original statement, it adjusted the language to say that EKG could be “useful,” in addition to an in-depth cardiac history and physical examination.16
Opposition to routine EKG screening in these patients stems from the procedure’s extremely low yield and relatively high false positive findings, which may result in higher financial and psychological burdens for patients and families. Thomas et al17 reported that at a single center, the number of EKGs ordered with an indication of “stimulant medication screening” quadrupled during 2009, the year after the AHA published its recommendations. Of 372 patients referred for EKG, 24 (6.4%) had abnormal findings and 18 were referred for further evaluation, but none were found to have cardiac disease. ADHD therapy was delayed in 6 patients because of the EKG.
In a similar evaluation of 1470 ADHD patients ages 21 years and younger, Mahle et al18 noted that 119 patients (8.1%) had an abnormal EKG, 78 of whom (65%) were already receiving stimulants. Five patients had cardiac disease, including 2 who had a preexcitation syndrome. Overall, the positive predictive value was low (4.2%).18 Other research, including a study lead by one of this article’s authors (SKM), has found similar increases in the number of EKGs ordered for patients with ADHD.19
Cost vs benefit. In the Mahle et al18 study described above, the mean cost of EKG screening, including further testing for patients with abnormal initial results, was $58 per child. The mean cost to identify a true-positive result was $17,162.18
In 2012, Leslie et al20 used simulation models to estimate the societal cost of routine EKG screening to prevent SCD in children with ADHD. Their findings: The cost would be high relative to its health benefits—approximately $91,000 to $204,000 per life year saved. Furthermore, these researchers found that ordering an EKG to screen for 3 common cardiac conditions linked to SCD (HCM, WPW, and LQTS) would add <2 days to a patient’s projected life expectancy.20
Our recommendations
We believe stimulants can safely be used in the treatment of children and adolescents with ADHD, given the evidence that suggests a low risk of SCD. That said, it is prudent to avoid prescribing stimulants for children who have an underlying condition that may deteriorate secondary to increased blood pressure or heart rate.
We agree with the current AHA and AAP recommendations that physicians should obtain an in-depth cardiac history and physical examination, with emphasis on screening for cardiac disorders that may put a child at risk for SCD, such as HCM, LQTS, and preexcitation syndromes. For instance, a history of a family member with palpitations should prompt an EKG, which may reveal familial preexcitation syndrome. Similarly, an EKG is in order if you suspect LQTS based on a parent’s description of a family member’s death after hearing a loud noise, such as fireworks.
It often takes active probing to uncover a history of sudden death in the family that a parent may not consider relevant. For example, one of the authors (SKM) cared for a 6-year-old boy who presented with a history of syncope after his hand got caught in a door jam. On further probing, his mother revealed that her father had died at age 30 while he was taking astemizole, an allergy drug known to prolong the QT interval. Subsequent EKGs revealed that both the boy and his mother had LQTS.
For patients already taking stimulants, we recommend monitoring BP and heart rate and ordering an EKG only if the patient exhibits cardiac symptoms or there are concerns based on follow-up history and physical examination. Should a patient develop palpitations while taking a therapeutic dose of stimulants, a detailed history of the onset and duration of symptoms is important. For example, tachycardia that has a gradual onset and occurs with exercise is suggestive of physiological sinus tachycardia. In our judgment, most patients who experience symptoms that suggest sinus tachycardia simply require downward readjustment of their medication or a switch to a nonstimulant.
However, if the patient or family history prompts you to suspect other arrhythmias such as ectopic beats or supraventricular tachycardia, immediate assessment either in an emergency department or in the physician’s office may be required, because obtaining an EKG during symptoms is crucial for the diagnosis. Similarly, unexplained exercise intolerance or the onset of chest pain associated with exercise, dizziness, syncope, seizures, or dyspnea requires immediate cardiovascular assessment.
And finally, whether your patient has just started taking medication for his or her ADHD or has been on the medication for some time, it’s important to periodically reassess the need to continue the stimulant therapy; ADHD symptoms may decrease during mid- to late adolescence and into adulthood.21
CASE › The FP completed a thorough physical exam and found no evidence of any conditions that would increase the likelihood of SCD in the young patient. There was no history of SCD in the boy’s family, either. Based on these findings, the FP opted to forgo an EKG. She prescribed lisdexamfetamine, starting with 20 mg/d (the lowest dose available) and then monitored his course by telephone. Eventually, 30 mg was found to be an effective dose. At a 6-week follow-up visit, the boy’s ADHD symptoms were substantially reduced, without any adverse effects—cardiac or otherwise.
CORRESPONDENCE
Sudhir Ken Mehta, Cleveland Clinic Children’s Hospital, 9500 Euclid Avenue, Cleveland, OH 44111; [email protected]
› Complete a thorough, cardiac-focused history and physical examination before starting stimulants for attention deficit hyperactivity disorder (ADHD) in a child or adolescent. C
› Avoid using stimulants in children or adolescents with comorbid conditions associated with sudden cardiac death, including hypertrophic cardiomyopathy, long QT interval syndrome, and preexcitation syndromes such as Wolff-Parkinson-White syndrome. C
› Monitor all children and adolescents who are taking stimulants for tachycardia, hypertension, palpitations, and chest pain. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A young patient has been struggling in school. His worried mother, having had several conferences with the child’s teachers, brings him to the family physician (FP), where he is given a diagnosis of attention deficit hyperactivity disorder (ADHD). The FP considers prescribing a stimulant medication, but first plans on conducting a more thorough family history and exam. She also debates the merits of ordering an electrocardiogram (EKG) to screen for conditions that could lead to sudden cardiac death.
If you were caring for this patient, how would you proceed?
That’s a good question, given the debate that has surrounded this subject since the US Food and Drug Administration (FDA) first learned of 25 cases of sudden death that were linked to stimulant medications.1 The majority of the cases, which were reported to the FDA’s Adverse Event Reporting System between 1999 and 2003, involved amphetamines or methylphenidate in patients under the age of 19.1 In 2008, the American Heart Association (AHA) issued a scientific statement advocating that physicians perform a proper family history and physical exam that includes blood pressure (BP) and an EKG before prescribing a stimulant for children and adolescents.2 The inclusion of EKG screening was intended to increase the likelihood of identifying patients with potentially life-threatening conditions that could lead to sudden cardiac death (SCD).2
Not everyone, however, agreed.
Later that year, the American Academy of Pediatrics (AAP) challenged the routine use of EKGs in this screening process, citing a lack of evidence between stimulant use and the induction of potentially lethal arrhythmias.3 And in 2011, the European Guideline Group also concluded that there was no evidence to suggest an incremental benefit for routine EKG assessment of ADHD patients before initiation of medication.4
Underscoring the uncertainty surrounding the subject are the findings of a 2012 survey of 525 randomly selected US pediatricians.5 Nearly a quarter of the respondents expressed concerns over the risk for SCD in children receiving stimulants for ADHD, and a slightly higher number—30%—worried that the risks for legal liability were high enough to warrant cardiac assessment.5
So how should the prudent FP proceed? In this review, we will describe how to thoroughly screen children and adolescents for their risk of SCD before prescribing stimulants for ADHD. We’ll also summarize what the evidence tells us about whether—and when—you should order an EKG. But first, a word about the pharmacology of stimulants.
How stimulants might increase SCD risk
Stimulants have been used to treat ADHD for more than 40 years6 and are a first-line of therapy for children with ADHD. Stimulants increase attention span by releasing dopamine and norepinephrine at synapses in the frontal cortex, brain stem, and midbrain.
The effect on heart rate and BP. In clinical trials with small samples sizes, children and adolescents receiving stimulants to treat ADHD experienced a minimal rise in heart rate and BP. As measured by 24-hour ambulatory BP monitoring, 13 subjects in a double-blind, randomized, placebo/stimulant crossover trial had slightly elevated total diastolic BP (69.7 vs 65.8 mm Hg; P=.02), waking diastolic BP (75.5 vs 72.3 mm Hg; P=.03), and total heart rate (85.5 vs 79.9 beats per minutes [bpm]; P=.004) while receiving stimulants.7 Other investigators noted similar findings among 17 boys ages 7 to 11 years.8
Whether prolonged childhood exposure to stimulants increases the risk for developing hypertension or tachycardia is unknown. A 10-year follow-up study of 579 children between the ages of 7 to 9 years found stimulants had no effect on systolic or diastolic BP.9 Stimulants use did, however, lead to a higher heart rate (84.2±12.4 vs 79.1±12.0 bpm) during treatment.9 No stimulant-related QT interval changes—which some have proposed might explain SCD in ADHD patients—have been reported in pediatric patients.10 Researchers have noted small increases in mean QTc intervals in adults treated with stimulants for ADHD, but none were >480 msec.11
Steps you should always take before prescribing a stimulant
Before prescribing stimulants to children or adolescents with ADHD, complete an in-depth cardiac history and physical examination, as recommended by the AHA and AAP (TABLE),2,3 to identify conditions that increase the likelihood of SCD, such as hypertrophic cardiomyopathy (HCM), long QT interval syndrome (LQTS), and preexcitation syndromes such as Wolff-Parkinson-White syndrome (WPW).
Confirm, for instance, that your patient has a normal heart rate, rhythm, and BP, and no pathological murmurs. In a survey of families with a child or young adult who had sudden cardiac arrest, 72% reported the patient had at least one cardiovascular symptom within 19 to 71 months of SCD, and 27% reported having a family member with a history of SCD before age 50.12 For patients with no such complaints or family history, the news is good. Two large studies found that in the absence of any suspected or overt cardiac disease, children with ADHD who were receiving stimulant therapy had no increased risk of SCD.13,14
What about patients with this common heart problem? Physicians face a dilemma when a stimulant is needed and the patient has a common acyanotic congenital heart lesion, such as a small atrial or ventricular septal defect, which is considered nonlethal. Based on limited data, there is no evidence that the risk of SCD is higher when these patients take stimulants.15
Should you order that EKG—or not?
Currently, the AHA still favors an EKG, though in a correction to its original statement, it adjusted the language to say that EKG could be “useful,” in addition to an in-depth cardiac history and physical examination.16
Opposition to routine EKG screening in these patients stems from the procedure’s extremely low yield and relatively high false positive findings, which may result in higher financial and psychological burdens for patients and families. Thomas et al17 reported that at a single center, the number of EKGs ordered with an indication of “stimulant medication screening” quadrupled during 2009, the year after the AHA published its recommendations. Of 372 patients referred for EKG, 24 (6.4%) had abnormal findings and 18 were referred for further evaluation, but none were found to have cardiac disease. ADHD therapy was delayed in 6 patients because of the EKG.
In a similar evaluation of 1470 ADHD patients ages 21 years and younger, Mahle et al18 noted that 119 patients (8.1%) had an abnormal EKG, 78 of whom (65%) were already receiving stimulants. Five patients had cardiac disease, including 2 who had a preexcitation syndrome. Overall, the positive predictive value was low (4.2%).18 Other research, including a study lead by one of this article’s authors (SKM), has found similar increases in the number of EKGs ordered for patients with ADHD.19
Cost vs benefit. In the Mahle et al18 study described above, the mean cost of EKG screening, including further testing for patients with abnormal initial results, was $58 per child. The mean cost to identify a true-positive result was $17,162.18
In 2012, Leslie et al20 used simulation models to estimate the societal cost of routine EKG screening to prevent SCD in children with ADHD. Their findings: The cost would be high relative to its health benefits—approximately $91,000 to $204,000 per life year saved. Furthermore, these researchers found that ordering an EKG to screen for 3 common cardiac conditions linked to SCD (HCM, WPW, and LQTS) would add <2 days to a patient’s projected life expectancy.20
Our recommendations
We believe stimulants can safely be used in the treatment of children and adolescents with ADHD, given the evidence that suggests a low risk of SCD. That said, it is prudent to avoid prescribing stimulants for children who have an underlying condition that may deteriorate secondary to increased blood pressure or heart rate.
We agree with the current AHA and AAP recommendations that physicians should obtain an in-depth cardiac history and physical examination, with emphasis on screening for cardiac disorders that may put a child at risk for SCD, such as HCM, LQTS, and preexcitation syndromes. For instance, a history of a family member with palpitations should prompt an EKG, which may reveal familial preexcitation syndrome. Similarly, an EKG is in order if you suspect LQTS based on a parent’s description of a family member’s death after hearing a loud noise, such as fireworks.
It often takes active probing to uncover a history of sudden death in the family that a parent may not consider relevant. For example, one of the authors (SKM) cared for a 6-year-old boy who presented with a history of syncope after his hand got caught in a door jam. On further probing, his mother revealed that her father had died at age 30 while he was taking astemizole, an allergy drug known to prolong the QT interval. Subsequent EKGs revealed that both the boy and his mother had LQTS.
For patients already taking stimulants, we recommend monitoring BP and heart rate and ordering an EKG only if the patient exhibits cardiac symptoms or there are concerns based on follow-up history and physical examination. Should a patient develop palpitations while taking a therapeutic dose of stimulants, a detailed history of the onset and duration of symptoms is important. For example, tachycardia that has a gradual onset and occurs with exercise is suggestive of physiological sinus tachycardia. In our judgment, most patients who experience symptoms that suggest sinus tachycardia simply require downward readjustment of their medication or a switch to a nonstimulant.
However, if the patient or family history prompts you to suspect other arrhythmias such as ectopic beats or supraventricular tachycardia, immediate assessment either in an emergency department or in the physician’s office may be required, because obtaining an EKG during symptoms is crucial for the diagnosis. Similarly, unexplained exercise intolerance or the onset of chest pain associated with exercise, dizziness, syncope, seizures, or dyspnea requires immediate cardiovascular assessment.
And finally, whether your patient has just started taking medication for his or her ADHD or has been on the medication for some time, it’s important to periodically reassess the need to continue the stimulant therapy; ADHD symptoms may decrease during mid- to late adolescence and into adulthood.21
CASE › The FP completed a thorough physical exam and found no evidence of any conditions that would increase the likelihood of SCD in the young patient. There was no history of SCD in the boy’s family, either. Based on these findings, the FP opted to forgo an EKG. She prescribed lisdexamfetamine, starting with 20 mg/d (the lowest dose available) and then monitored his course by telephone. Eventually, 30 mg was found to be an effective dose. At a 6-week follow-up visit, the boy’s ADHD symptoms were substantially reduced, without any adverse effects—cardiac or otherwise.
CORRESPONDENCE
Sudhir Ken Mehta, Cleveland Clinic Children’s Hospital, 9500 Euclid Avenue, Cleveland, OH 44111; [email protected]
1. Safety review: Follow up review of AERS search identifying cases of sudden death occurring with drugs used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). US Food and Drug Administration Web site. Available at: http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4210b_07_01_safetyreview.pdf. Accessed January 17, 2014.
2. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117:2407-2423.
3. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
4. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20:17-37.
5. Leslie LK, Rodday AM, Saunders TS, et al. Cardiac screening prior to stimulant treatment of ADHD: a survey of US-based pediatricians. Pediatrics. 2012;129:222-230.
6. Conners CK. Symposium: behavior modification by drugs. II. Psychological effects of stimulant drugs in children with minimal brain dysfunction. Pediatrics. 1972;49:702-708.
7. Samuels JA, Franco K, Wan F, et al. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95.
8. Stowe CD, Gardner SF, Gist CC, et al. 24-hour ambulatory blood pressure monitoring in male children receiving stimulant therapy. Ann Pharmacother. 2002;36:1142-1149.
9. Vitiello B, Elliott GR, Swanson JM, et al. Blood pressure and heart rate over 10 years in the multimodal treatment study of children with ADHD. Am J Psychiatry. 2012;169:167-177.
10. Hammerness P, Wilens T, Mick E, et al. Cardiovascular effects of longer-term, high-dose OROS methylphenidate in adolescents with attention deficit hyperactivity disorder. J Pediatr. 2009;155:84-89,89.e1.
11. Weisler RH, Biederman J, Spencer TJ, et al. Long-term cardiovascular effects of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr. 2005;10(suppl 20):35-43.
12. Drezner JA, Fudge J, Harmon KG, et al. Warning symptoms and family history in children and young adults with sudden cardiac arrest. J Am Board Fam Med. 2012;25:408-415.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896-1904.
14. Schelleman H, Bilker WB, Strom BL, et al. Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics. 2011;127:1102-1110.
15. Winterstein AG, Gerhard T, Kubilis P, et al. Cardiovascular safety of central nervous system stimulants in children and adolescents: population based cohort study. BMJ. 2012;345:e4627.
16. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [published correction appears in Circulation. 2009;120:e55-e59]. Circulation. 2008;117:2407-2423.
17. Thomas PE, Carlo WF, Decker JA, et al. Impact of the American Heart Association scientific statement on screening electrocardiograms and stimulant medications. Arch Pediatr Adolesc Med. 2011;165:166-170.
18. Mahle WT, Hebson C, Strieper MJ. Electrocardiographic screening in children with attention-deficit hyperactivity disorder. Am J Cardiol. 2009;104:1296-1299.
19. Mehta SK, Richards N, Jacobs I. Children and adolescents with attention deficit hyperactivity disorder in a pediatric cardiology office. Cardiol Young. 2010;20(suppl 3):167.
20. Leslie LK, Cohen JT, Newburger JW, et al. Costs and benefits of targeted screening for causes of sudden cardiac death in children and adolescents. Circulation. 2012;125:2621-2629.
21. Mannuzza S, Klein RG, Bessler A, et al. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155:493-488.
1. Safety review: Follow up review of AERS search identifying cases of sudden death occurring with drugs used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). US Food and Drug Administration Web site. Available at: http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4210b_07_01_safetyreview.pdf. Accessed January 17, 2014.
2. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117:2407-2423.
3. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
4. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20:17-37.
5. Leslie LK, Rodday AM, Saunders TS, et al. Cardiac screening prior to stimulant treatment of ADHD: a survey of US-based pediatricians. Pediatrics. 2012;129:222-230.
6. Conners CK. Symposium: behavior modification by drugs. II. Psychological effects of stimulant drugs in children with minimal brain dysfunction. Pediatrics. 1972;49:702-708.
7. Samuels JA, Franco K, Wan F, et al. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95.
8. Stowe CD, Gardner SF, Gist CC, et al. 24-hour ambulatory blood pressure monitoring in male children receiving stimulant therapy. Ann Pharmacother. 2002;36:1142-1149.
9. Vitiello B, Elliott GR, Swanson JM, et al. Blood pressure and heart rate over 10 years in the multimodal treatment study of children with ADHD. Am J Psychiatry. 2012;169:167-177.
10. Hammerness P, Wilens T, Mick E, et al. Cardiovascular effects of longer-term, high-dose OROS methylphenidate in adolescents with attention deficit hyperactivity disorder. J Pediatr. 2009;155:84-89,89.e1.
11. Weisler RH, Biederman J, Spencer TJ, et al. Long-term cardiovascular effects of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr. 2005;10(suppl 20):35-43.
12. Drezner JA, Fudge J, Harmon KG, et al. Warning symptoms and family history in children and young adults with sudden cardiac arrest. J Am Board Fam Med. 2012;25:408-415.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896-1904.
14. Schelleman H, Bilker WB, Strom BL, et al. Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics. 2011;127:1102-1110.
15. Winterstein AG, Gerhard T, Kubilis P, et al. Cardiovascular safety of central nervous system stimulants in children and adolescents: population based cohort study. BMJ. 2012;345:e4627.
16. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [published correction appears in Circulation. 2009;120:e55-e59]. Circulation. 2008;117:2407-2423.
17. Thomas PE, Carlo WF, Decker JA, et al. Impact of the American Heart Association scientific statement on screening electrocardiograms and stimulant medications. Arch Pediatr Adolesc Med. 2011;165:166-170.
18. Mahle WT, Hebson C, Strieper MJ. Electrocardiographic screening in children with attention-deficit hyperactivity disorder. Am J Cardiol. 2009;104:1296-1299.
19. Mehta SK, Richards N, Jacobs I. Children and adolescents with attention deficit hyperactivity disorder in a pediatric cardiology office. Cardiol Young. 2010;20(suppl 3):167.
20. Leslie LK, Cohen JT, Newburger JW, et al. Costs and benefits of targeted screening for causes of sudden cardiac death in children and adolescents. Circulation. 2012;125:2621-2629.
21. Mannuzza S, Klein RG, Bessler A, et al. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155:493-488.
Caring for Asian immigrants: Tips on culture that can enhance patient care
› Ask Asian immigrants open-ended questions and encourage them to share their use of alternative remedies. C
› Consider providing an interpretation service for patients not proficient in English, as opposed to asking family members to help. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though often considered a “model minority,” Asian immigrants pose significant challenges for Western health care providers, including radically different ideas of disease causation, differing communication styles, and somatic presentations of mental illness. Asian diversity is tremendous, but several cultural trends are held in common: strong family structures, respect, adaptability, and, for first generation immigrants, widespread use of traditional therapies.1
While Asians and Pacific Islanders (APIs) represent only 5.6% of the US population, or 17.3 million people, that figure represents a 46% increase between 2000 and 2010, the most rapid for any ethnic group.2 A 79% increase is anticipated by 2050, bringing Asians to 9.3% of the US population. In order of population, API subpopulations include Chinese, Filipinos, Asian Indians, Vietnamese, Koreans, and Japanese.2 More than half of Asian Americans reside in the states of California, New York, and Hawaii, although enclaves exist in most major cities.3
Addressing the health needs of Asian immigrants in an increasingly diverse society mandates that US physicians develop the necessary skills to communicate, even when expectations for care may be very different. Fortunately, excellent resources are available (TABLE 1).
Barriers to good healthcare
The most formidable obstacle is limited English proficiency of patients, making them significantly less likely to seek care.4 They often struggle to arrange an appointment, although they arrive on time.5
Inadequate interpretation services. Frequently family members must interpret for patients, despite a federal mandate (Title VI of the 1964 Civil Rights Act) requiring professional services be provided at no charge if there is a federal payer (Medicare or Medicaid) involved.6 Unfortunately, these services are not currently reimbursable. Use of family or friends as interpreters, while convenient, results in far less accurate interpretation, frequent embarrassment, and loss of patient confidentiality. Trained medical interpreters or even telephone services are preferable, as they are much more accurate. Interviews involve a triad comprised of provider, patient, and interpreter, with the provider speaking directly to the patient using first-person address at all times. The interpreter should sit to the side or slightly behind the patient. All communication should be interpreted sentence by sentence so everyone is able to understand the entire conversation. It is well documented that proper interpretive services vastly improve the quality of care.7
Patient illiteracy. Health care illiteracy leads to medication errors due to the inability to understand instructions.8 Some immigrants have the added disadvantage of being illiterate both in English and in their native tongue.4 If not remedied, these situations easily lead to drug overdoses or missed allergies.9 Older immigrants neither understand the intricacies of the US health care system nor possess the language skills to master it.4
Stereotyping by caregivers must be surmounted if patients are to receive quality care. Many Asian patients report that physicians fail to understand them as unique individuals apart from their ethnic identity. Others feel excluded from the decision-making process or find culturally sensitive treatment options lacking.10
Subtleties of relational interaction. Asian culture has been defined as possessing a high Power Distance Index (PDI).11 The PDI refers to the distance or level of respect which an individual must afford to a superior, and this ideal is reflected in Asian conformance to a strict social hierarchy. Thus, physicians are viewed as authority figures and it is proper to nod or smile to indicate polite deference.12 However, showing respect and “buying in” to treatment recommendations are entirely different matters. Cultural factors make it difficult for patients to openly disagree with physician recommendations without feeling as though they have been disrespectful.12 Asian cultures are also “high context” cultures, having far more unwritten rules for conduct and communication that often prove baffling to westerners from “lower context” cultures.
Financial limitations. Socioeconomic influences also play a role. Although Asians have a higher income than other minority groups, 12.5% of Asians still live in poverty and 17.2% lack health insurance.2 Lack of coverage makes many Asians reluctant to seek regular medical care.13
Special medical concerns
Asian-Americans face a variety of challenging medical issues, including disproportionately high rates of tuberculosis (TB) and hepatitis B.
TB. Although rates of TB infection in the United States are low, rates in Asian immigrants are up to 100 times greater than that of the general population, more than any other immigrant group.14 Screening with interferon gamma release assays (IGRAs), such as T-SPOT TB, should be routine for Asian immigrants, since IGRAs do not cross-react with the bacillus Calmette-Guérin (BCG) vaccine. The Centers for Disease Control and Prevention now recommends IGRA blood testing in lieu of tuberculin skin testing (TST) for immigrants who received BCG in infancy, with the exception of children <5 years, for whom the TST is still preferable.15 Patients with positive IGRA tests are also more likely to be amenable to treatment.
Chronic hepatitis B infection in Asian immigrants is often due to perinatal transmission in their home countries. Rates of hepatitis B virus (HBV) infection in the United States have been steadily declining since vaccination began in 1981. However, chronic HBV infection in Asian immigrants approaches 10% of that population.16 An estimated 15% to 20% of patients with chronic HBV develop cirrhosis within 5 years and are at high risk for hepatocellular carcinoma (HCC).17,18
Evaluate HBV carriers annually with liver function testing (LFTs), hepatitis B surface Antigen (HBsAg), and hepatitis B e-Antigen (HBeAg). A positive HBsAg result indicates the virus is present; a positive HBeAg result indicates the virus is actively replicating. LFT elevation (AST >200 IU/L) or a positive HBeAg test should prompt referral to a gastroenterologist for liver biopsy and therapy. Screening for HCC with alpha-fetoprotein (AFP) levels and liver ultrasounds every 6 to 12 months has been recommended for all chronic HBV carriers,19 but this interval remains controversial. Screen men ≥40 years and women ≥50, or anyone who has had HBV infection >10 years, every 6 months.19,20 More recent recommendations favor ultrasound over AFP for screening, as the latter test lacks adequate sensitivity and specificity.20 Test partners and family members of HBV patients, and vaccinate them against HBV if not already immune.
Other medical issues. Asians from the Indian subcontinent have a significantly elevated risk of heart disease, in part due to low HDL-cholesterol levels.21 South Asian immigrant populations have a 3- to 5-fold increased risk of myocardial infarction and cardiovascular death compared with other ethnicities, and often exhibit coronary disease before the age of 40.22 The recent adoption of a Western diet and sedentary lifestyle has provoked an epidemic of diabetes throughout urban Asia and in Asians living abroad. This may be related to the “thrifty gene hypothesis,” which suggests that genes which evolved early in human history to facilitate storage of fat for periods of famine are detrimental in modern society where food is plentiful. A study of Asian Indian immigrants in Atlanta demonstrated an 18.3% prevalence rate for diabetes, higher than any other ethnic group.23 Tobacco and its causal relationship with lung cancer and heart disease only adds to this concern. Southeast Asians in particular demonstrate alarmingly high rates of tobacco consumption, and lung cancer is the leading cause of death among Asian Americans.12,24
Recently, a new acquired immune deficiency syndrome has been described in East Asia. In this syndrome, interferon–gamma (IFN–gamma) is blocked by an auto-antibody. Although not communicable like human immunodeficiency virus, this autoimmune syndrome may lead to similar opportunistic infections such as atypical mycobacterial infections.25
Mental health concerns
Perhaps no topic deserves more emphasis than that of mental health. In the aftermath of the war in Vietnam, Southeast Asian immigrants suffered a great deal from traumatic immigration experiences, with severe adjustment reactions.26 A high incidence of posttraumatic stress disorder (PTSD) among the Hmong in particular reflects their turbulent national history.27
While the incidence of mental illness among Asians is comparable to that of the general population, Asian Americans are less likely to report such problems or to use mental health services26 due to the stigmatization of mental illness in Asian culture.28 Consequently, these symptoms may be subconsciously converted into the more socially acceptable medium of physical illness, which “saves face” and preserves family honor.29 In many ways, Asian culture still perceives mental illness as personal weakness.30 In Hmong culture, inability to speak about being depressed stems not just from cultural bias but from linguistic constraints—the language simply lacks a word for depression.31 Even Asian cultures recognizing mental illness deem depression to be more dependent on circumstances than on the psyche.31 A first-generation immigrant with mental illness is therefore more likely to present with somatic symptoms than a mood disturbance, and is likely to be resistant to counseling or medication for depression. (See “Cultural influence on self-perception: A case ”).
Asian health care beliefs and illnesses
Asian culture substantially influences the ways in which an individual perceives disease, experiences illness, and copes with the phenomenon of sickness. The interplay between illness, disease, and sickness was first elucidated by the pioneering work of Arthur Kleinman, MD, in 1978.32 The term disease denotes a pathological process, while illness describes the subjective impact of disease in a patient’s life. Sickness is the sum of both, as it relates to the total picture of biological and social disruption. The culturally competent physician must understand not only the patient’s disease but also his experience of illness. Asking Dr. Kleinman’s questions will help physicians understand their patient’s perception of illness (TABLE 2).32
Traditional Chinese medicine
Many East Asians derive their conception of illness from traditional Chinese medicine (TCM), a broad range of therapies including herbs, acupuncture, massage (tuina), and diet that has been used for millennia. Similarly, South Asians are influenced by the Ayurvedic or Unani traditions. TCM views the body as an energy system, rather than a machine, through which the life force, or chi, flows. Health is not just the absence of disease, but a proper balance of the antithetic forces, yin and yang, maintained by herbs, diet, and acupuncture.33 TCM is often preferred for treating chronic conditions and viral syndromes, or as a substitute for Western medications with adverse effects.34 It is most popular among newly arrived immigrants, those with low literacy, and those with limited access to conventional medical treatment.34 Most Western physicians know little about TCM, feel uncomfortable when their patients use it, and fail to recognize its popularity.34,35 Likewise, most Asian patients are reluctant to discuss their use of TCM unless questioned about it in a nonjudgmental manner.
Many East Asian cultures practice a distinct form of folk healing known as “coining,” in which a coin dipped in cold oil or Tiger Balm is rubbed against the skin, enabling “wind illness” to escape the body (FIGURE 1). Linear bands of painless petechiae develop. The more extensive the bruising, the more illness is thought to be released. Failure to expel “wind-cold” from the body is believed to account for many ailments. Other traditions are moxibustion and cupping. In moxibustion, a smoldering plug of dried artemisia herb (moxi) is either impaled upon an acupuncture needle or placed directly on the skin to create a burn (FIGURE 2). In cupping, a flame is quickly passed through a glass bowl which is then placed against the skin. The resulting suction creates a circular bruise and draws blood to the area (FIGURE 3). Many folk remedies have been mistaken for child abuse by individuals unfamiliar with such practices.36 Occasionally TCM may result in harm from burns, unsterilized acupuncture needles, or (most commonly) adulterated herbal formulations.37
Culture-bound syndromes
Asian folk illnesses usually go unrecognized by western practitioners, and many of these are somatic presentations of mental illness or stress (TABLE 3). A classic example is the Korean folk condition Hwa-byung, which may include the sensation of an abdominal mass. US practitioners might pursue a fruitless abdominal workup before suspecting a psychiatric condition, even though a careful history would likely elicit other anxiety symptoms and loss of sleep and appetite.26
Asian social conventions
Asian cultural conventions often create considerable confusion. In India, head waggling (shaking the head back and forth) is equivalent to nodding in conversation, indicating an acknowledgement of communication. To western eyes, it appears that the patient is resisting advice rather than welcoming it. In East Asians, smiling expresses a variety of emotions, including polite disagreement. Acute embarrassment may provoke giggling. Eye contact is usually for social equals; avoiding it, especially between the sexes, is the norm. Only the right hand should be used when giving patients a prescription; the left hand is considered unclean. A patient’s head should only be touched with advance permission, as it is viewed as the seat of the soul and is therefore sacred. Under no circumstances should a patient ever see the bottom of the practitioner’s feet or be touched by them.38 Demonstrating respect (especially for older Asians) and preserving modesty are essential when examining patients.
Naming conventions can also be confusing. In China and much of Southeast Asia, it is customary for the surname to precede the given name, often with the 2 run together, rather than the other way round. It is best to ask how a patient would prefer to be addressed, regardless of how the name appears on the medical chart.38
Cultivating knowledge of Asian culture provides a framework from which practitioners can better understand and treat their patients. By asking respectful, open ended questions and encouraging patients to take an active role in their own treatment, physicians become therapeutic allies actively engaged in the healing process. Asking patients to share their use of alternative remedies allows the option of rationally integrating those most meaningful for the patient.
The cross-cultural interview
It is helpful to have a specific approach in mind when interviewing patients from other cultures. A number of mnemonic techniques exist.39-41 Perhaps the most useful of these is the LEARN model, which stands for Listen, Explain, Acknowledge, Recommend, and Negotiate.39 The physician first listens carefully to the patient’s perception of his illness before explaining any medical (disease) issues. This exchange is followed by acknowledging differences and similarities between the 2 viewpoints. Finally, the physician recommends a treatment plan and negotiates patient agreement.39 Negotiation implies flexibility and willingness to compromise with reasonable cultural demands, without compromising patient care. Use of the LEARN model aids in the identification and resolution of any cultural conflicts that might arise during the course of the clinical interview.
Teach back and patient activation
An extremely useful technique for all cultures is termed “teach back” or “show me,” which involves asking patients to repeat their care instructions at the end of the visit. This extra step provides an opportunity to correct errors that might have occurred during the transmission of instructions.42 Caregivers should also encourage or “activate” patients to become more involved in managing their own health care. Patient activation measures may be assessed on a one-to-4 point scale.43 Using both of these techniques combats passivity, promotes patient acceptance, and improves outcomes.
A caring environment
There are various strategies and approaches that can help make a medical practice more immigrant friendly (TABLE 4).44,45 Instructing office staff to assist patients in getting to the clinic is critical for those with limited mobility or who lack English proficiency. Adding evening hours that can also accommodate walk-ins helps working patients. For practices with larger immigrant populations, recognizing Asian holidays like Chinese New Year, Diwali, or Tet will be well received. These practices have been directly correlated with more positive health outcomes and better patient satisfaction.44
Conveying complex instructions to patients with little English takes effort for even the most unflappable providers. While written follow-up instructions in English could be interpreted by a more fluent family member, the ideal solution would be to have materials available in the native language. Fortunately, several Web sites, such as SPIRAL (Selective Patient Information in Asian Languages) provide downloadable Asian language instructions.46
Physicians should try to implement the Culturally & Linguistically Appropriate Services (CLAS) guidelines and mandates from the Office of Minority Health (http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15).6 They go far towards providing optimal care for patients of all cultures. Cultural competence does not imply being an expert in all cultures, let alone those of Asia. However, health care providers can develop the skills necessary for effective cross-cultural communication, which, to be most effective, must be accompanied by a caring attitude and respectful practice environment.
CORRESPONDENCE
Gregory Juckett, MD, MPH, West Virginia University School of Medicine, Box 9247, Robert C. Byrd Health Sciences Center, Morgantown, West Virginia, 26506; [email protected]
1. Min PG, ed. Asian Americans: Contemporary Trends and Issues. 2nd ed. Thousand Oaks, California: Pine Forge Press; 2006.
2. Ortman JM, Guarneri CE; National Census Bureau. United States population projections: 2000 to 2050. Available at: http://www.census.gov/population/projections/files/analytical-document09.pdf. Accessed February 20, 2012.
3. Barnes JS, Bennett CE; US Census Bureau Web site. The Asian population: 2000. Available at: http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed February 2, 2012.
4. Kim G, Worley CB, Allen RS, et al. Vulnerability of older Latino and Asian immigrants with limited English proficiency. J Am Geriatr Soc. 2011;59:1246-1252.
5. Silver D, Blustein J, Weitzman BC. Transportation to clinic: findings from a pilot clinic-based survey of low-income suburbanites. J Immigr Minor Health. 2012;14:350-355.
6. US Department of Health and Human Services Office of Minority Health Web site. The National CLAS Standards. Available at: http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15. Updated May 3, 2013. Accessed June 1, 2013.
7. Karliner LS, Jacobs EA, Chen AH, et al. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42:727-754.
8. Wilson E, Chen AH, Grumbach K, et al. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med. 2005;20:800-806.
9. Ku L, Flores G. Pay now or pay later: providing interpreter services in health care. Health Aff (Millwood). 2005;24:435-444.
10. Ngo-Metzger Q, Massagali MP, Clarridge BR, et al. Linguistic and cultural barriers to care. J Gen Intern Med. 2003;18:44-52.
11. Basabe N, Ros M. Cultural dimensions and social behavior correlates: individualism-collectivism and power distance. Revue Internationale De Pscyhologie Sociale. 2005;17:189-225.
12. Ngo-Metzger Q, Legedza AT, Phillips RS. Asian Americans’ reports of their health care experiences. Results of a national survey. J Gen Intern Med. 2004;19:111-119.
13. Collins KS, Hughes DL, Doty MM, et al; The Commonwealth Fund. Diverse communities, common concerns: assessing health care quality for minority Americans. Available at: http://www.commonwealthfund.org/Publications/Fund-Reports/2002/Mar/Diverse-Communities--Common-Concerns--Assessing-Health-Care-Quality-for-Minority-Americans.aspx. Published March 2002. Accessed December 20, 2013.
14. Houston HR, Harada N, Makinodan T. Development of a culturally sensitive educational intervention program to reduce high incidence of tuberculosis among foreign-born Vietnamese. Ethn Health. 2002;7:255-265.
15. Mazurel GH, Jereb J, Vernon A, et al; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
16. Hutton DW, Tan D, So SK, et al. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147:460-469.
17. Fattovich G, Brollo L, Giustina G, et al. Natural history and prognostic factors in chronic hepatitis B. Gut. 1991;32:294-298.
18. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956.
19. Smith C. Managing Adult Patients with Chronic HBV. Hepatitis B Foundation. Accessed February 15, 2012, at http://www.hepb.org/professionals/management_guidelines.htm.
20. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
21. Hamaad A, Lip G. Assessing heart disease in your ethnic patients. Pulse. 2003;63:48-49.
22. Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113:e924-e929.
23. Venkataraman R, Nanda NC, Beweja G, et al. Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States. Am J Cardiol. 2004;94:977-980.
24. Nishtar S. Prevention of coronary heart disease in south Asia. Lancet. 2002;360:1015-1018.
25. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725-734.
26. Sorkin DH, Nguyen H, Ngo-Metzger Q. Assessing the mental health needs and barriers to care among a diverse sample of Asian American older adults. J Gen Intern Med. 2011;26:595-602.
27. PTSD, depression epidemic among Cambodian immigrants [press release]. Bethesda, MD: National Institutes of Health; August 2, 2005.
28. Sue S, Sue DW, Sue L, et al. Psychopathology among Asian Americans: a model minority? Cult Divers Ment Health. 1995;1:39-51.
29. Parker G, Cheah YC, Roy K. Do the Chinese somaticize depression? A cross-cultural study. Soc Psychiatry Psychiatr Epidemiol. 2001;36:287-293.
30. Sribney W, Elliot K, Aguilar-Gaxiola S, et al. The role of nonmedical human services and alternative medicine. In: Ruiz P, Primm A, eds. Disparities in Psychiatric Care. Baltimore, MD: Lippincott, Williams & Wilkins; 2010:274-289.
31. Lee HY, Lytle K, Yang PN, et al. Mental health literacy in Hmong and Cambodian elderly refugees: a barrier to understanding, recognizing, and responding to depression. Int J Aging Hum Dev. 2010;71:323-344.
32. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthroplologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
33. Patwardhan B, Warude D, Pushpangadan P, et al. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2:465-473.
34. Wu AP, Burke A, LeBaron S. Use of traditional medicine by immigrant Chinese patients. Fam Med. 2007;39:195-200.
35. Nguyen G, Bowman M. Culture, language, and health literacy: communicating about health with Asians and Pacific Islanders. Fam Med. 2007;39:208-210.
36. Oates RK. Overturning the diagnosis of child abuse. Arch Dis Child. 1984;59:665-666.
37. Efferth T, Kaina B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr Drug Metab. 2011;12:989-996.
38. Galanti G. Communication and time orientation. In: Caring for Patients from Different Cultures. 4th ed. Philadelphia, PA: University of Pennsylvania Press; 2008:27-51.
39. Berlin E, Fowkes WC Jr. A teaching framework for cross-cultural health care: application in family practice. West J Med. 1983;139:934-938.
40. Stuart MR, Lieberman JA III, eds. The Fifteen Minute Hour: Applied Psychotherapy for the Primary Care Physician. 2nd ed. Westport, CT: Praeger; 1993:101-183.
41. Kobylarz FA, Heath JM, Like RC. The ETHNIC(S) mnemonic: a clinical tool for ethnogeriatric education. J Am Geriat Soc. 2002;50:1582-1589.
42. Kountz DS. Strategies for improving low health literacy. Postgrad Med. 2009;121:171-177.
43. Patient Activation Measure Assessment. Insignia Health Web site. Available at: http://www.insigniahealth.com/solutions/patientactivation-measure. Accessed February 20, 2012.
44. Glenn-Vega A. Achieving a more minority-friendly practice. Fam Pract Manag. 2002;9:39-43.
45. Galanti G. Making a Difference. In: Caring for Patients from Different Cultures. 3rd ed. Philadelphia, PA: University of Pennsylvania Press; 2003:1222-1229.
46. SPIRAL: Selected Patient Information in Asian Languages. Tufts University Hirsh Health Sciences Web site. Available at: http://spiral.tufts.edu/topic.shtml. Accessed February 10, 2012.
› Ask Asian immigrants open-ended questions and encourage them to share their use of alternative remedies. C
› Consider providing an interpretation service for patients not proficient in English, as opposed to asking family members to help. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though often considered a “model minority,” Asian immigrants pose significant challenges for Western health care providers, including radically different ideas of disease causation, differing communication styles, and somatic presentations of mental illness. Asian diversity is tremendous, but several cultural trends are held in common: strong family structures, respect, adaptability, and, for first generation immigrants, widespread use of traditional therapies.1
While Asians and Pacific Islanders (APIs) represent only 5.6% of the US population, or 17.3 million people, that figure represents a 46% increase between 2000 and 2010, the most rapid for any ethnic group.2 A 79% increase is anticipated by 2050, bringing Asians to 9.3% of the US population. In order of population, API subpopulations include Chinese, Filipinos, Asian Indians, Vietnamese, Koreans, and Japanese.2 More than half of Asian Americans reside in the states of California, New York, and Hawaii, although enclaves exist in most major cities.3
Addressing the health needs of Asian immigrants in an increasingly diverse society mandates that US physicians develop the necessary skills to communicate, even when expectations for care may be very different. Fortunately, excellent resources are available (TABLE 1).
Barriers to good healthcare
The most formidable obstacle is limited English proficiency of patients, making them significantly less likely to seek care.4 They often struggle to arrange an appointment, although they arrive on time.5
Inadequate interpretation services. Frequently family members must interpret for patients, despite a federal mandate (Title VI of the 1964 Civil Rights Act) requiring professional services be provided at no charge if there is a federal payer (Medicare or Medicaid) involved.6 Unfortunately, these services are not currently reimbursable. Use of family or friends as interpreters, while convenient, results in far less accurate interpretation, frequent embarrassment, and loss of patient confidentiality. Trained medical interpreters or even telephone services are preferable, as they are much more accurate. Interviews involve a triad comprised of provider, patient, and interpreter, with the provider speaking directly to the patient using first-person address at all times. The interpreter should sit to the side or slightly behind the patient. All communication should be interpreted sentence by sentence so everyone is able to understand the entire conversation. It is well documented that proper interpretive services vastly improve the quality of care.7
Patient illiteracy. Health care illiteracy leads to medication errors due to the inability to understand instructions.8 Some immigrants have the added disadvantage of being illiterate both in English and in their native tongue.4 If not remedied, these situations easily lead to drug overdoses or missed allergies.9 Older immigrants neither understand the intricacies of the US health care system nor possess the language skills to master it.4
Stereotyping by caregivers must be surmounted if patients are to receive quality care. Many Asian patients report that physicians fail to understand them as unique individuals apart from their ethnic identity. Others feel excluded from the decision-making process or find culturally sensitive treatment options lacking.10
Subtleties of relational interaction. Asian culture has been defined as possessing a high Power Distance Index (PDI).11 The PDI refers to the distance or level of respect which an individual must afford to a superior, and this ideal is reflected in Asian conformance to a strict social hierarchy. Thus, physicians are viewed as authority figures and it is proper to nod or smile to indicate polite deference.12 However, showing respect and “buying in” to treatment recommendations are entirely different matters. Cultural factors make it difficult for patients to openly disagree with physician recommendations without feeling as though they have been disrespectful.12 Asian cultures are also “high context” cultures, having far more unwritten rules for conduct and communication that often prove baffling to westerners from “lower context” cultures.
Financial limitations. Socioeconomic influences also play a role. Although Asians have a higher income than other minority groups, 12.5% of Asians still live in poverty and 17.2% lack health insurance.2 Lack of coverage makes many Asians reluctant to seek regular medical care.13
Special medical concerns
Asian-Americans face a variety of challenging medical issues, including disproportionately high rates of tuberculosis (TB) and hepatitis B.
TB. Although rates of TB infection in the United States are low, rates in Asian immigrants are up to 100 times greater than that of the general population, more than any other immigrant group.14 Screening with interferon gamma release assays (IGRAs), such as T-SPOT TB, should be routine for Asian immigrants, since IGRAs do not cross-react with the bacillus Calmette-Guérin (BCG) vaccine. The Centers for Disease Control and Prevention now recommends IGRA blood testing in lieu of tuberculin skin testing (TST) for immigrants who received BCG in infancy, with the exception of children <5 years, for whom the TST is still preferable.15 Patients with positive IGRA tests are also more likely to be amenable to treatment.
Chronic hepatitis B infection in Asian immigrants is often due to perinatal transmission in their home countries. Rates of hepatitis B virus (HBV) infection in the United States have been steadily declining since vaccination began in 1981. However, chronic HBV infection in Asian immigrants approaches 10% of that population.16 An estimated 15% to 20% of patients with chronic HBV develop cirrhosis within 5 years and are at high risk for hepatocellular carcinoma (HCC).17,18
Evaluate HBV carriers annually with liver function testing (LFTs), hepatitis B surface Antigen (HBsAg), and hepatitis B e-Antigen (HBeAg). A positive HBsAg result indicates the virus is present; a positive HBeAg result indicates the virus is actively replicating. LFT elevation (AST >200 IU/L) or a positive HBeAg test should prompt referral to a gastroenterologist for liver biopsy and therapy. Screening for HCC with alpha-fetoprotein (AFP) levels and liver ultrasounds every 6 to 12 months has been recommended for all chronic HBV carriers,19 but this interval remains controversial. Screen men ≥40 years and women ≥50, or anyone who has had HBV infection >10 years, every 6 months.19,20 More recent recommendations favor ultrasound over AFP for screening, as the latter test lacks adequate sensitivity and specificity.20 Test partners and family members of HBV patients, and vaccinate them against HBV if not already immune.
Other medical issues. Asians from the Indian subcontinent have a significantly elevated risk of heart disease, in part due to low HDL-cholesterol levels.21 South Asian immigrant populations have a 3- to 5-fold increased risk of myocardial infarction and cardiovascular death compared with other ethnicities, and often exhibit coronary disease before the age of 40.22 The recent adoption of a Western diet and sedentary lifestyle has provoked an epidemic of diabetes throughout urban Asia and in Asians living abroad. This may be related to the “thrifty gene hypothesis,” which suggests that genes which evolved early in human history to facilitate storage of fat for periods of famine are detrimental in modern society where food is plentiful. A study of Asian Indian immigrants in Atlanta demonstrated an 18.3% prevalence rate for diabetes, higher than any other ethnic group.23 Tobacco and its causal relationship with lung cancer and heart disease only adds to this concern. Southeast Asians in particular demonstrate alarmingly high rates of tobacco consumption, and lung cancer is the leading cause of death among Asian Americans.12,24
Recently, a new acquired immune deficiency syndrome has been described in East Asia. In this syndrome, interferon–gamma (IFN–gamma) is blocked by an auto-antibody. Although not communicable like human immunodeficiency virus, this autoimmune syndrome may lead to similar opportunistic infections such as atypical mycobacterial infections.25
Mental health concerns
Perhaps no topic deserves more emphasis than that of mental health. In the aftermath of the war in Vietnam, Southeast Asian immigrants suffered a great deal from traumatic immigration experiences, with severe adjustment reactions.26 A high incidence of posttraumatic stress disorder (PTSD) among the Hmong in particular reflects their turbulent national history.27
While the incidence of mental illness among Asians is comparable to that of the general population, Asian Americans are less likely to report such problems or to use mental health services26 due to the stigmatization of mental illness in Asian culture.28 Consequently, these symptoms may be subconsciously converted into the more socially acceptable medium of physical illness, which “saves face” and preserves family honor.29 In many ways, Asian culture still perceives mental illness as personal weakness.30 In Hmong culture, inability to speak about being depressed stems not just from cultural bias but from linguistic constraints—the language simply lacks a word for depression.31 Even Asian cultures recognizing mental illness deem depression to be more dependent on circumstances than on the psyche.31 A first-generation immigrant with mental illness is therefore more likely to present with somatic symptoms than a mood disturbance, and is likely to be resistant to counseling or medication for depression. (See “Cultural influence on self-perception: A case ”).
Asian health care beliefs and illnesses
Asian culture substantially influences the ways in which an individual perceives disease, experiences illness, and copes with the phenomenon of sickness. The interplay between illness, disease, and sickness was first elucidated by the pioneering work of Arthur Kleinman, MD, in 1978.32 The term disease denotes a pathological process, while illness describes the subjective impact of disease in a patient’s life. Sickness is the sum of both, as it relates to the total picture of biological and social disruption. The culturally competent physician must understand not only the patient’s disease but also his experience of illness. Asking Dr. Kleinman’s questions will help physicians understand their patient’s perception of illness (TABLE 2).32
Traditional Chinese medicine
Many East Asians derive their conception of illness from traditional Chinese medicine (TCM), a broad range of therapies including herbs, acupuncture, massage (tuina), and diet that has been used for millennia. Similarly, South Asians are influenced by the Ayurvedic or Unani traditions. TCM views the body as an energy system, rather than a machine, through which the life force, or chi, flows. Health is not just the absence of disease, but a proper balance of the antithetic forces, yin and yang, maintained by herbs, diet, and acupuncture.33 TCM is often preferred for treating chronic conditions and viral syndromes, or as a substitute for Western medications with adverse effects.34 It is most popular among newly arrived immigrants, those with low literacy, and those with limited access to conventional medical treatment.34 Most Western physicians know little about TCM, feel uncomfortable when their patients use it, and fail to recognize its popularity.34,35 Likewise, most Asian patients are reluctant to discuss their use of TCM unless questioned about it in a nonjudgmental manner.
Many East Asian cultures practice a distinct form of folk healing known as “coining,” in which a coin dipped in cold oil or Tiger Balm is rubbed against the skin, enabling “wind illness” to escape the body (FIGURE 1). Linear bands of painless petechiae develop. The more extensive the bruising, the more illness is thought to be released. Failure to expel “wind-cold” from the body is believed to account for many ailments. Other traditions are moxibustion and cupping. In moxibustion, a smoldering plug of dried artemisia herb (moxi) is either impaled upon an acupuncture needle or placed directly on the skin to create a burn (FIGURE 2). In cupping, a flame is quickly passed through a glass bowl which is then placed against the skin. The resulting suction creates a circular bruise and draws blood to the area (FIGURE 3). Many folk remedies have been mistaken for child abuse by individuals unfamiliar with such practices.36 Occasionally TCM may result in harm from burns, unsterilized acupuncture needles, or (most commonly) adulterated herbal formulations.37
Culture-bound syndromes
Asian folk illnesses usually go unrecognized by western practitioners, and many of these are somatic presentations of mental illness or stress (TABLE 3). A classic example is the Korean folk condition Hwa-byung, which may include the sensation of an abdominal mass. US practitioners might pursue a fruitless abdominal workup before suspecting a psychiatric condition, even though a careful history would likely elicit other anxiety symptoms and loss of sleep and appetite.26
Asian social conventions
Asian cultural conventions often create considerable confusion. In India, head waggling (shaking the head back and forth) is equivalent to nodding in conversation, indicating an acknowledgement of communication. To western eyes, it appears that the patient is resisting advice rather than welcoming it. In East Asians, smiling expresses a variety of emotions, including polite disagreement. Acute embarrassment may provoke giggling. Eye contact is usually for social equals; avoiding it, especially between the sexes, is the norm. Only the right hand should be used when giving patients a prescription; the left hand is considered unclean. A patient’s head should only be touched with advance permission, as it is viewed as the seat of the soul and is therefore sacred. Under no circumstances should a patient ever see the bottom of the practitioner’s feet or be touched by them.38 Demonstrating respect (especially for older Asians) and preserving modesty are essential when examining patients.
Naming conventions can also be confusing. In China and much of Southeast Asia, it is customary for the surname to precede the given name, often with the 2 run together, rather than the other way round. It is best to ask how a patient would prefer to be addressed, regardless of how the name appears on the medical chart.38
Cultivating knowledge of Asian culture provides a framework from which practitioners can better understand and treat their patients. By asking respectful, open ended questions and encouraging patients to take an active role in their own treatment, physicians become therapeutic allies actively engaged in the healing process. Asking patients to share their use of alternative remedies allows the option of rationally integrating those most meaningful for the patient.
The cross-cultural interview
It is helpful to have a specific approach in mind when interviewing patients from other cultures. A number of mnemonic techniques exist.39-41 Perhaps the most useful of these is the LEARN model, which stands for Listen, Explain, Acknowledge, Recommend, and Negotiate.39 The physician first listens carefully to the patient’s perception of his illness before explaining any medical (disease) issues. This exchange is followed by acknowledging differences and similarities between the 2 viewpoints. Finally, the physician recommends a treatment plan and negotiates patient agreement.39 Negotiation implies flexibility and willingness to compromise with reasonable cultural demands, without compromising patient care. Use of the LEARN model aids in the identification and resolution of any cultural conflicts that might arise during the course of the clinical interview.
Teach back and patient activation
An extremely useful technique for all cultures is termed “teach back” or “show me,” which involves asking patients to repeat their care instructions at the end of the visit. This extra step provides an opportunity to correct errors that might have occurred during the transmission of instructions.42 Caregivers should also encourage or “activate” patients to become more involved in managing their own health care. Patient activation measures may be assessed on a one-to-4 point scale.43 Using both of these techniques combats passivity, promotes patient acceptance, and improves outcomes.
A caring environment
There are various strategies and approaches that can help make a medical practice more immigrant friendly (TABLE 4).44,45 Instructing office staff to assist patients in getting to the clinic is critical for those with limited mobility or who lack English proficiency. Adding evening hours that can also accommodate walk-ins helps working patients. For practices with larger immigrant populations, recognizing Asian holidays like Chinese New Year, Diwali, or Tet will be well received. These practices have been directly correlated with more positive health outcomes and better patient satisfaction.44
Conveying complex instructions to patients with little English takes effort for even the most unflappable providers. While written follow-up instructions in English could be interpreted by a more fluent family member, the ideal solution would be to have materials available in the native language. Fortunately, several Web sites, such as SPIRAL (Selective Patient Information in Asian Languages) provide downloadable Asian language instructions.46
Physicians should try to implement the Culturally & Linguistically Appropriate Services (CLAS) guidelines and mandates from the Office of Minority Health (http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15).6 They go far towards providing optimal care for patients of all cultures. Cultural competence does not imply being an expert in all cultures, let alone those of Asia. However, health care providers can develop the skills necessary for effective cross-cultural communication, which, to be most effective, must be accompanied by a caring attitude and respectful practice environment.
CORRESPONDENCE
Gregory Juckett, MD, MPH, West Virginia University School of Medicine, Box 9247, Robert C. Byrd Health Sciences Center, Morgantown, West Virginia, 26506; [email protected]
› Ask Asian immigrants open-ended questions and encourage them to share their use of alternative remedies. C
› Consider providing an interpretation service for patients not proficient in English, as opposed to asking family members to help. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though often considered a “model minority,” Asian immigrants pose significant challenges for Western health care providers, including radically different ideas of disease causation, differing communication styles, and somatic presentations of mental illness. Asian diversity is tremendous, but several cultural trends are held in common: strong family structures, respect, adaptability, and, for first generation immigrants, widespread use of traditional therapies.1
While Asians and Pacific Islanders (APIs) represent only 5.6% of the US population, or 17.3 million people, that figure represents a 46% increase between 2000 and 2010, the most rapid for any ethnic group.2 A 79% increase is anticipated by 2050, bringing Asians to 9.3% of the US population. In order of population, API subpopulations include Chinese, Filipinos, Asian Indians, Vietnamese, Koreans, and Japanese.2 More than half of Asian Americans reside in the states of California, New York, and Hawaii, although enclaves exist in most major cities.3
Addressing the health needs of Asian immigrants in an increasingly diverse society mandates that US physicians develop the necessary skills to communicate, even when expectations for care may be very different. Fortunately, excellent resources are available (TABLE 1).
Barriers to good healthcare
The most formidable obstacle is limited English proficiency of patients, making them significantly less likely to seek care.4 They often struggle to arrange an appointment, although they arrive on time.5
Inadequate interpretation services. Frequently family members must interpret for patients, despite a federal mandate (Title VI of the 1964 Civil Rights Act) requiring professional services be provided at no charge if there is a federal payer (Medicare or Medicaid) involved.6 Unfortunately, these services are not currently reimbursable. Use of family or friends as interpreters, while convenient, results in far less accurate interpretation, frequent embarrassment, and loss of patient confidentiality. Trained medical interpreters or even telephone services are preferable, as they are much more accurate. Interviews involve a triad comprised of provider, patient, and interpreter, with the provider speaking directly to the patient using first-person address at all times. The interpreter should sit to the side or slightly behind the patient. All communication should be interpreted sentence by sentence so everyone is able to understand the entire conversation. It is well documented that proper interpretive services vastly improve the quality of care.7
Patient illiteracy. Health care illiteracy leads to medication errors due to the inability to understand instructions.8 Some immigrants have the added disadvantage of being illiterate both in English and in their native tongue.4 If not remedied, these situations easily lead to drug overdoses or missed allergies.9 Older immigrants neither understand the intricacies of the US health care system nor possess the language skills to master it.4
Stereotyping by caregivers must be surmounted if patients are to receive quality care. Many Asian patients report that physicians fail to understand them as unique individuals apart from their ethnic identity. Others feel excluded from the decision-making process or find culturally sensitive treatment options lacking.10
Subtleties of relational interaction. Asian culture has been defined as possessing a high Power Distance Index (PDI).11 The PDI refers to the distance or level of respect which an individual must afford to a superior, and this ideal is reflected in Asian conformance to a strict social hierarchy. Thus, physicians are viewed as authority figures and it is proper to nod or smile to indicate polite deference.12 However, showing respect and “buying in” to treatment recommendations are entirely different matters. Cultural factors make it difficult for patients to openly disagree with physician recommendations without feeling as though they have been disrespectful.12 Asian cultures are also “high context” cultures, having far more unwritten rules for conduct and communication that often prove baffling to westerners from “lower context” cultures.
Financial limitations. Socioeconomic influences also play a role. Although Asians have a higher income than other minority groups, 12.5% of Asians still live in poverty and 17.2% lack health insurance.2 Lack of coverage makes many Asians reluctant to seek regular medical care.13
Special medical concerns
Asian-Americans face a variety of challenging medical issues, including disproportionately high rates of tuberculosis (TB) and hepatitis B.
TB. Although rates of TB infection in the United States are low, rates in Asian immigrants are up to 100 times greater than that of the general population, more than any other immigrant group.14 Screening with interferon gamma release assays (IGRAs), such as T-SPOT TB, should be routine for Asian immigrants, since IGRAs do not cross-react with the bacillus Calmette-Guérin (BCG) vaccine. The Centers for Disease Control and Prevention now recommends IGRA blood testing in lieu of tuberculin skin testing (TST) for immigrants who received BCG in infancy, with the exception of children <5 years, for whom the TST is still preferable.15 Patients with positive IGRA tests are also more likely to be amenable to treatment.
Chronic hepatitis B infection in Asian immigrants is often due to perinatal transmission in their home countries. Rates of hepatitis B virus (HBV) infection in the United States have been steadily declining since vaccination began in 1981. However, chronic HBV infection in Asian immigrants approaches 10% of that population.16 An estimated 15% to 20% of patients with chronic HBV develop cirrhosis within 5 years and are at high risk for hepatocellular carcinoma (HCC).17,18
Evaluate HBV carriers annually with liver function testing (LFTs), hepatitis B surface Antigen (HBsAg), and hepatitis B e-Antigen (HBeAg). A positive HBsAg result indicates the virus is present; a positive HBeAg result indicates the virus is actively replicating. LFT elevation (AST >200 IU/L) or a positive HBeAg test should prompt referral to a gastroenterologist for liver biopsy and therapy. Screening for HCC with alpha-fetoprotein (AFP) levels and liver ultrasounds every 6 to 12 months has been recommended for all chronic HBV carriers,19 but this interval remains controversial. Screen men ≥40 years and women ≥50, or anyone who has had HBV infection >10 years, every 6 months.19,20 More recent recommendations favor ultrasound over AFP for screening, as the latter test lacks adequate sensitivity and specificity.20 Test partners and family members of HBV patients, and vaccinate them against HBV if not already immune.
Other medical issues. Asians from the Indian subcontinent have a significantly elevated risk of heart disease, in part due to low HDL-cholesterol levels.21 South Asian immigrant populations have a 3- to 5-fold increased risk of myocardial infarction and cardiovascular death compared with other ethnicities, and often exhibit coronary disease before the age of 40.22 The recent adoption of a Western diet and sedentary lifestyle has provoked an epidemic of diabetes throughout urban Asia and in Asians living abroad. This may be related to the “thrifty gene hypothesis,” which suggests that genes which evolved early in human history to facilitate storage of fat for periods of famine are detrimental in modern society where food is plentiful. A study of Asian Indian immigrants in Atlanta demonstrated an 18.3% prevalence rate for diabetes, higher than any other ethnic group.23 Tobacco and its causal relationship with lung cancer and heart disease only adds to this concern. Southeast Asians in particular demonstrate alarmingly high rates of tobacco consumption, and lung cancer is the leading cause of death among Asian Americans.12,24
Recently, a new acquired immune deficiency syndrome has been described in East Asia. In this syndrome, interferon–gamma (IFN–gamma) is blocked by an auto-antibody. Although not communicable like human immunodeficiency virus, this autoimmune syndrome may lead to similar opportunistic infections such as atypical mycobacterial infections.25
Mental health concerns
Perhaps no topic deserves more emphasis than that of mental health. In the aftermath of the war in Vietnam, Southeast Asian immigrants suffered a great deal from traumatic immigration experiences, with severe adjustment reactions.26 A high incidence of posttraumatic stress disorder (PTSD) among the Hmong in particular reflects their turbulent national history.27
While the incidence of mental illness among Asians is comparable to that of the general population, Asian Americans are less likely to report such problems or to use mental health services26 due to the stigmatization of mental illness in Asian culture.28 Consequently, these symptoms may be subconsciously converted into the more socially acceptable medium of physical illness, which “saves face” and preserves family honor.29 In many ways, Asian culture still perceives mental illness as personal weakness.30 In Hmong culture, inability to speak about being depressed stems not just from cultural bias but from linguistic constraints—the language simply lacks a word for depression.31 Even Asian cultures recognizing mental illness deem depression to be more dependent on circumstances than on the psyche.31 A first-generation immigrant with mental illness is therefore more likely to present with somatic symptoms than a mood disturbance, and is likely to be resistant to counseling or medication for depression. (See “Cultural influence on self-perception: A case ”).
Asian health care beliefs and illnesses
Asian culture substantially influences the ways in which an individual perceives disease, experiences illness, and copes with the phenomenon of sickness. The interplay between illness, disease, and sickness was first elucidated by the pioneering work of Arthur Kleinman, MD, in 1978.32 The term disease denotes a pathological process, while illness describes the subjective impact of disease in a patient’s life. Sickness is the sum of both, as it relates to the total picture of biological and social disruption. The culturally competent physician must understand not only the patient’s disease but also his experience of illness. Asking Dr. Kleinman’s questions will help physicians understand their patient’s perception of illness (TABLE 2).32
Traditional Chinese medicine
Many East Asians derive their conception of illness from traditional Chinese medicine (TCM), a broad range of therapies including herbs, acupuncture, massage (tuina), and diet that has been used for millennia. Similarly, South Asians are influenced by the Ayurvedic or Unani traditions. TCM views the body as an energy system, rather than a machine, through which the life force, or chi, flows. Health is not just the absence of disease, but a proper balance of the antithetic forces, yin and yang, maintained by herbs, diet, and acupuncture.33 TCM is often preferred for treating chronic conditions and viral syndromes, or as a substitute for Western medications with adverse effects.34 It is most popular among newly arrived immigrants, those with low literacy, and those with limited access to conventional medical treatment.34 Most Western physicians know little about TCM, feel uncomfortable when their patients use it, and fail to recognize its popularity.34,35 Likewise, most Asian patients are reluctant to discuss their use of TCM unless questioned about it in a nonjudgmental manner.
Many East Asian cultures practice a distinct form of folk healing known as “coining,” in which a coin dipped in cold oil or Tiger Balm is rubbed against the skin, enabling “wind illness” to escape the body (FIGURE 1). Linear bands of painless petechiae develop. The more extensive the bruising, the more illness is thought to be released. Failure to expel “wind-cold” from the body is believed to account for many ailments. Other traditions are moxibustion and cupping. In moxibustion, a smoldering plug of dried artemisia herb (moxi) is either impaled upon an acupuncture needle or placed directly on the skin to create a burn (FIGURE 2). In cupping, a flame is quickly passed through a glass bowl which is then placed against the skin. The resulting suction creates a circular bruise and draws blood to the area (FIGURE 3). Many folk remedies have been mistaken for child abuse by individuals unfamiliar with such practices.36 Occasionally TCM may result in harm from burns, unsterilized acupuncture needles, or (most commonly) adulterated herbal formulations.37
Culture-bound syndromes
Asian folk illnesses usually go unrecognized by western practitioners, and many of these are somatic presentations of mental illness or stress (TABLE 3). A classic example is the Korean folk condition Hwa-byung, which may include the sensation of an abdominal mass. US practitioners might pursue a fruitless abdominal workup before suspecting a psychiatric condition, even though a careful history would likely elicit other anxiety symptoms and loss of sleep and appetite.26
Asian social conventions
Asian cultural conventions often create considerable confusion. In India, head waggling (shaking the head back and forth) is equivalent to nodding in conversation, indicating an acknowledgement of communication. To western eyes, it appears that the patient is resisting advice rather than welcoming it. In East Asians, smiling expresses a variety of emotions, including polite disagreement. Acute embarrassment may provoke giggling. Eye contact is usually for social equals; avoiding it, especially between the sexes, is the norm. Only the right hand should be used when giving patients a prescription; the left hand is considered unclean. A patient’s head should only be touched with advance permission, as it is viewed as the seat of the soul and is therefore sacred. Under no circumstances should a patient ever see the bottom of the practitioner’s feet or be touched by them.38 Demonstrating respect (especially for older Asians) and preserving modesty are essential when examining patients.
Naming conventions can also be confusing. In China and much of Southeast Asia, it is customary for the surname to precede the given name, often with the 2 run together, rather than the other way round. It is best to ask how a patient would prefer to be addressed, regardless of how the name appears on the medical chart.38
Cultivating knowledge of Asian culture provides a framework from which practitioners can better understand and treat their patients. By asking respectful, open ended questions and encouraging patients to take an active role in their own treatment, physicians become therapeutic allies actively engaged in the healing process. Asking patients to share their use of alternative remedies allows the option of rationally integrating those most meaningful for the patient.
The cross-cultural interview
It is helpful to have a specific approach in mind when interviewing patients from other cultures. A number of mnemonic techniques exist.39-41 Perhaps the most useful of these is the LEARN model, which stands for Listen, Explain, Acknowledge, Recommend, and Negotiate.39 The physician first listens carefully to the patient’s perception of his illness before explaining any medical (disease) issues. This exchange is followed by acknowledging differences and similarities between the 2 viewpoints. Finally, the physician recommends a treatment plan and negotiates patient agreement.39 Negotiation implies flexibility and willingness to compromise with reasonable cultural demands, without compromising patient care. Use of the LEARN model aids in the identification and resolution of any cultural conflicts that might arise during the course of the clinical interview.
Teach back and patient activation
An extremely useful technique for all cultures is termed “teach back” or “show me,” which involves asking patients to repeat their care instructions at the end of the visit. This extra step provides an opportunity to correct errors that might have occurred during the transmission of instructions.42 Caregivers should also encourage or “activate” patients to become more involved in managing their own health care. Patient activation measures may be assessed on a one-to-4 point scale.43 Using both of these techniques combats passivity, promotes patient acceptance, and improves outcomes.
A caring environment
There are various strategies and approaches that can help make a medical practice more immigrant friendly (TABLE 4).44,45 Instructing office staff to assist patients in getting to the clinic is critical for those with limited mobility or who lack English proficiency. Adding evening hours that can also accommodate walk-ins helps working patients. For practices with larger immigrant populations, recognizing Asian holidays like Chinese New Year, Diwali, or Tet will be well received. These practices have been directly correlated with more positive health outcomes and better patient satisfaction.44
Conveying complex instructions to patients with little English takes effort for even the most unflappable providers. While written follow-up instructions in English could be interpreted by a more fluent family member, the ideal solution would be to have materials available in the native language. Fortunately, several Web sites, such as SPIRAL (Selective Patient Information in Asian Languages) provide downloadable Asian language instructions.46
Physicians should try to implement the Culturally & Linguistically Appropriate Services (CLAS) guidelines and mandates from the Office of Minority Health (http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15).6 They go far towards providing optimal care for patients of all cultures. Cultural competence does not imply being an expert in all cultures, let alone those of Asia. However, health care providers can develop the skills necessary for effective cross-cultural communication, which, to be most effective, must be accompanied by a caring attitude and respectful practice environment.
CORRESPONDENCE
Gregory Juckett, MD, MPH, West Virginia University School of Medicine, Box 9247, Robert C. Byrd Health Sciences Center, Morgantown, West Virginia, 26506; [email protected]
1. Min PG, ed. Asian Americans: Contemporary Trends and Issues. 2nd ed. Thousand Oaks, California: Pine Forge Press; 2006.
2. Ortman JM, Guarneri CE; National Census Bureau. United States population projections: 2000 to 2050. Available at: http://www.census.gov/population/projections/files/analytical-document09.pdf. Accessed February 20, 2012.
3. Barnes JS, Bennett CE; US Census Bureau Web site. The Asian population: 2000. Available at: http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed February 2, 2012.
4. Kim G, Worley CB, Allen RS, et al. Vulnerability of older Latino and Asian immigrants with limited English proficiency. J Am Geriatr Soc. 2011;59:1246-1252.
5. Silver D, Blustein J, Weitzman BC. Transportation to clinic: findings from a pilot clinic-based survey of low-income suburbanites. J Immigr Minor Health. 2012;14:350-355.
6. US Department of Health and Human Services Office of Minority Health Web site. The National CLAS Standards. Available at: http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15. Updated May 3, 2013. Accessed June 1, 2013.
7. Karliner LS, Jacobs EA, Chen AH, et al. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42:727-754.
8. Wilson E, Chen AH, Grumbach K, et al. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med. 2005;20:800-806.
9. Ku L, Flores G. Pay now or pay later: providing interpreter services in health care. Health Aff (Millwood). 2005;24:435-444.
10. Ngo-Metzger Q, Massagali MP, Clarridge BR, et al. Linguistic and cultural barriers to care. J Gen Intern Med. 2003;18:44-52.
11. Basabe N, Ros M. Cultural dimensions and social behavior correlates: individualism-collectivism and power distance. Revue Internationale De Pscyhologie Sociale. 2005;17:189-225.
12. Ngo-Metzger Q, Legedza AT, Phillips RS. Asian Americans’ reports of their health care experiences. Results of a national survey. J Gen Intern Med. 2004;19:111-119.
13. Collins KS, Hughes DL, Doty MM, et al; The Commonwealth Fund. Diverse communities, common concerns: assessing health care quality for minority Americans. Available at: http://www.commonwealthfund.org/Publications/Fund-Reports/2002/Mar/Diverse-Communities--Common-Concerns--Assessing-Health-Care-Quality-for-Minority-Americans.aspx. Published March 2002. Accessed December 20, 2013.
14. Houston HR, Harada N, Makinodan T. Development of a culturally sensitive educational intervention program to reduce high incidence of tuberculosis among foreign-born Vietnamese. Ethn Health. 2002;7:255-265.
15. Mazurel GH, Jereb J, Vernon A, et al; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
16. Hutton DW, Tan D, So SK, et al. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147:460-469.
17. Fattovich G, Brollo L, Giustina G, et al. Natural history and prognostic factors in chronic hepatitis B. Gut. 1991;32:294-298.
18. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956.
19. Smith C. Managing Adult Patients with Chronic HBV. Hepatitis B Foundation. Accessed February 15, 2012, at http://www.hepb.org/professionals/management_guidelines.htm.
20. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
21. Hamaad A, Lip G. Assessing heart disease in your ethnic patients. Pulse. 2003;63:48-49.
22. Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113:e924-e929.
23. Venkataraman R, Nanda NC, Beweja G, et al. Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States. Am J Cardiol. 2004;94:977-980.
24. Nishtar S. Prevention of coronary heart disease in south Asia. Lancet. 2002;360:1015-1018.
25. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725-734.
26. Sorkin DH, Nguyen H, Ngo-Metzger Q. Assessing the mental health needs and barriers to care among a diverse sample of Asian American older adults. J Gen Intern Med. 2011;26:595-602.
27. PTSD, depression epidemic among Cambodian immigrants [press release]. Bethesda, MD: National Institutes of Health; August 2, 2005.
28. Sue S, Sue DW, Sue L, et al. Psychopathology among Asian Americans: a model minority? Cult Divers Ment Health. 1995;1:39-51.
29. Parker G, Cheah YC, Roy K. Do the Chinese somaticize depression? A cross-cultural study. Soc Psychiatry Psychiatr Epidemiol. 2001;36:287-293.
30. Sribney W, Elliot K, Aguilar-Gaxiola S, et al. The role of nonmedical human services and alternative medicine. In: Ruiz P, Primm A, eds. Disparities in Psychiatric Care. Baltimore, MD: Lippincott, Williams & Wilkins; 2010:274-289.
31. Lee HY, Lytle K, Yang PN, et al. Mental health literacy in Hmong and Cambodian elderly refugees: a barrier to understanding, recognizing, and responding to depression. Int J Aging Hum Dev. 2010;71:323-344.
32. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthroplologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
33. Patwardhan B, Warude D, Pushpangadan P, et al. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2:465-473.
34. Wu AP, Burke A, LeBaron S. Use of traditional medicine by immigrant Chinese patients. Fam Med. 2007;39:195-200.
35. Nguyen G, Bowman M. Culture, language, and health literacy: communicating about health with Asians and Pacific Islanders. Fam Med. 2007;39:208-210.
36. Oates RK. Overturning the diagnosis of child abuse. Arch Dis Child. 1984;59:665-666.
37. Efferth T, Kaina B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr Drug Metab. 2011;12:989-996.
38. Galanti G. Communication and time orientation. In: Caring for Patients from Different Cultures. 4th ed. Philadelphia, PA: University of Pennsylvania Press; 2008:27-51.
39. Berlin E, Fowkes WC Jr. A teaching framework for cross-cultural health care: application in family practice. West J Med. 1983;139:934-938.
40. Stuart MR, Lieberman JA III, eds. The Fifteen Minute Hour: Applied Psychotherapy for the Primary Care Physician. 2nd ed. Westport, CT: Praeger; 1993:101-183.
41. Kobylarz FA, Heath JM, Like RC. The ETHNIC(S) mnemonic: a clinical tool for ethnogeriatric education. J Am Geriat Soc. 2002;50:1582-1589.
42. Kountz DS. Strategies for improving low health literacy. Postgrad Med. 2009;121:171-177.
43. Patient Activation Measure Assessment. Insignia Health Web site. Available at: http://www.insigniahealth.com/solutions/patientactivation-measure. Accessed February 20, 2012.
44. Glenn-Vega A. Achieving a more minority-friendly practice. Fam Pract Manag. 2002;9:39-43.
45. Galanti G. Making a Difference. In: Caring for Patients from Different Cultures. 3rd ed. Philadelphia, PA: University of Pennsylvania Press; 2003:1222-1229.
46. SPIRAL: Selected Patient Information in Asian Languages. Tufts University Hirsh Health Sciences Web site. Available at: http://spiral.tufts.edu/topic.shtml. Accessed February 10, 2012.
1. Min PG, ed. Asian Americans: Contemporary Trends and Issues. 2nd ed. Thousand Oaks, California: Pine Forge Press; 2006.
2. Ortman JM, Guarneri CE; National Census Bureau. United States population projections: 2000 to 2050. Available at: http://www.census.gov/population/projections/files/analytical-document09.pdf. Accessed February 20, 2012.
3. Barnes JS, Bennett CE; US Census Bureau Web site. The Asian population: 2000. Available at: http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed February 2, 2012.
4. Kim G, Worley CB, Allen RS, et al. Vulnerability of older Latino and Asian immigrants with limited English proficiency. J Am Geriatr Soc. 2011;59:1246-1252.
5. Silver D, Blustein J, Weitzman BC. Transportation to clinic: findings from a pilot clinic-based survey of low-income suburbanites. J Immigr Minor Health. 2012;14:350-355.
6. US Department of Health and Human Services Office of Minority Health Web site. The National CLAS Standards. Available at: http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15. Updated May 3, 2013. Accessed June 1, 2013.
7. Karliner LS, Jacobs EA, Chen AH, et al. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42:727-754.
8. Wilson E, Chen AH, Grumbach K, et al. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med. 2005;20:800-806.
9. Ku L, Flores G. Pay now or pay later: providing interpreter services in health care. Health Aff (Millwood). 2005;24:435-444.
10. Ngo-Metzger Q, Massagali MP, Clarridge BR, et al. Linguistic and cultural barriers to care. J Gen Intern Med. 2003;18:44-52.
11. Basabe N, Ros M. Cultural dimensions and social behavior correlates: individualism-collectivism and power distance. Revue Internationale De Pscyhologie Sociale. 2005;17:189-225.
12. Ngo-Metzger Q, Legedza AT, Phillips RS. Asian Americans’ reports of their health care experiences. Results of a national survey. J Gen Intern Med. 2004;19:111-119.
13. Collins KS, Hughes DL, Doty MM, et al; The Commonwealth Fund. Diverse communities, common concerns: assessing health care quality for minority Americans. Available at: http://www.commonwealthfund.org/Publications/Fund-Reports/2002/Mar/Diverse-Communities--Common-Concerns--Assessing-Health-Care-Quality-for-Minority-Americans.aspx. Published March 2002. Accessed December 20, 2013.
14. Houston HR, Harada N, Makinodan T. Development of a culturally sensitive educational intervention program to reduce high incidence of tuberculosis among foreign-born Vietnamese. Ethn Health. 2002;7:255-265.
15. Mazurel GH, Jereb J, Vernon A, et al; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
16. Hutton DW, Tan D, So SK, et al. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147:460-469.
17. Fattovich G, Brollo L, Giustina G, et al. Natural history and prognostic factors in chronic hepatitis B. Gut. 1991;32:294-298.
18. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956.
19. Smith C. Managing Adult Patients with Chronic HBV. Hepatitis B Foundation. Accessed February 15, 2012, at http://www.hepb.org/professionals/management_guidelines.htm.
20. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
21. Hamaad A, Lip G. Assessing heart disease in your ethnic patients. Pulse. 2003;63:48-49.
22. Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113:e924-e929.
23. Venkataraman R, Nanda NC, Beweja G, et al. Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States. Am J Cardiol. 2004;94:977-980.
24. Nishtar S. Prevention of coronary heart disease in south Asia. Lancet. 2002;360:1015-1018.
25. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725-734.
26. Sorkin DH, Nguyen H, Ngo-Metzger Q. Assessing the mental health needs and barriers to care among a diverse sample of Asian American older adults. J Gen Intern Med. 2011;26:595-602.
27. PTSD, depression epidemic among Cambodian immigrants [press release]. Bethesda, MD: National Institutes of Health; August 2, 2005.
28. Sue S, Sue DW, Sue L, et al. Psychopathology among Asian Americans: a model minority? Cult Divers Ment Health. 1995;1:39-51.
29. Parker G, Cheah YC, Roy K. Do the Chinese somaticize depression? A cross-cultural study. Soc Psychiatry Psychiatr Epidemiol. 2001;36:287-293.
30. Sribney W, Elliot K, Aguilar-Gaxiola S, et al. The role of nonmedical human services and alternative medicine. In: Ruiz P, Primm A, eds. Disparities in Psychiatric Care. Baltimore, MD: Lippincott, Williams & Wilkins; 2010:274-289.
31. Lee HY, Lytle K, Yang PN, et al. Mental health literacy in Hmong and Cambodian elderly refugees: a barrier to understanding, recognizing, and responding to depression. Int J Aging Hum Dev. 2010;71:323-344.
32. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthroplologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
33. Patwardhan B, Warude D, Pushpangadan P, et al. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2:465-473.
34. Wu AP, Burke A, LeBaron S. Use of traditional medicine by immigrant Chinese patients. Fam Med. 2007;39:195-200.
35. Nguyen G, Bowman M. Culture, language, and health literacy: communicating about health with Asians and Pacific Islanders. Fam Med. 2007;39:208-210.
36. Oates RK. Overturning the diagnosis of child abuse. Arch Dis Child. 1984;59:665-666.
37. Efferth T, Kaina B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr Drug Metab. 2011;12:989-996.
38. Galanti G. Communication and time orientation. In: Caring for Patients from Different Cultures. 4th ed. Philadelphia, PA: University of Pennsylvania Press; 2008:27-51.
39. Berlin E, Fowkes WC Jr. A teaching framework for cross-cultural health care: application in family practice. West J Med. 1983;139:934-938.
40. Stuart MR, Lieberman JA III, eds. The Fifteen Minute Hour: Applied Psychotherapy for the Primary Care Physician. 2nd ed. Westport, CT: Praeger; 1993:101-183.
41. Kobylarz FA, Heath JM, Like RC. The ETHNIC(S) mnemonic: a clinical tool for ethnogeriatric education. J Am Geriat Soc. 2002;50:1582-1589.
42. Kountz DS. Strategies for improving low health literacy. Postgrad Med. 2009;121:171-177.
43. Patient Activation Measure Assessment. Insignia Health Web site. Available at: http://www.insigniahealth.com/solutions/patientactivation-measure. Accessed February 20, 2012.
44. Glenn-Vega A. Achieving a more minority-friendly practice. Fam Pract Manag. 2002;9:39-43.
45. Galanti G. Making a Difference. In: Caring for Patients from Different Cultures. 3rd ed. Philadelphia, PA: University of Pennsylvania Press; 2003:1222-1229.
46. SPIRAL: Selected Patient Information in Asian Languages. Tufts University Hirsh Health Sciences Web site. Available at: http://spiral.tufts.edu/topic.shtml. Accessed February 10, 2012.
Is a novel anticoagulant right for your patient?
› Consider novel oral anticoagualants (NOACs) for patients who have normal renal function, are comlpiant with medication regimens, and have no history of peptic ulcer or gastrointestinal bleeding. B
› Avoid overlapping warfarin with rivaroxaban or apixaban when transitioning a patient from one anticoagulant to the other, as both agents prolong prothrombin time. B
› When initiating a NOAC, it is not necessary to overlap with a parenteral anticoagulant. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Sally J is a 72-year-old Caucasian woman who comes to your clinic after being diagnosed with atrial fibrillation (AF). The patient has a 10-year history of type 2 diabetes; she also has a history of hypertension and chronic kidney disease (CKD), with a baseline creatinine clearance of approximately 40 mL/min. Ms. J tells you she knows people who take warfarin and really dislike it. She asks for your opinion of the new anticoagulants she’s seen advertised on TV, and wonders whether one of them would be right for her.
CASE 2 Bobby W, a 35-year-old African American man, was recently diagnosed with deep vein thrombosis (DVT). This was his second clot in 5 years, and occurred after a long flight home from Europe. The patient explains that he leads a very active lifestyle and doesn’t have the time to come in for the monthly international normalized ratio (INR) checks that warfarin requires. What would you recommend for these patients?
Troubled by warfarin’s narrow therapeutic index, numerous medication and dietary interactions, and need for frequent monitoring, patients requiring long-term oral anticoagulation therapy have been seeking alternatives for years. Finally, they have a choice. The US Food and Drug Administration (FDA) approved 3 oral anticoagulants—dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—in less than 4 years. Known as novel oral anticoagulants (NOACs), they are the first such drugs to enter the market in more than 50 years.1,2
While warfarin inhibits a wide range of clotting factors (including II, VII, IX, and X), NOACs work further down the clotting cascade (TABLE 1).1,3-7 Dabigatran, a direct thrombin inhibitor, only inhibits factor IIa.3,5 Rivaroxaban and apixaban directly inhibit factor Xa and indirectly inhibit factor IIa.3,6,7
There are notable advantages to these newer agents, but some disadvantages that must be considered, as well. Appropriate patient selection, guided by a thorough understanding of the benefits and risks of NOACs, is key.
Stroke prevention in atrial fibrillation
All 3 NOACs are approved for stroke prevention in patients with nonvalvular atrial fibrillation (AF). The approvals are based on a small number of well-designed trials: RE-LY (dabigatran), ROCKET-AF (rivaroxaban), and ARISTOTLE (apixaban).8-10 Compared with warfarin, dabigatran is the only oral anticoagulant with a lower rate of both hemorrhagic and ischemic stroke.8 Both rivaroxaban and apixaban were found to decrease overall stroke risk relative to warfarin, but the difference was driven by a lower risk for hemorrhagic, not ischemic, stroke.9,10
In these trials, overall rates of major bleeding were similar to that of warfarin.8-10 Patients taking warfarin generally experienced higher rates of intracranial hemorrhage but lower rates of gastrointestinal (GI) bleeding than those on NOACs. Relative to warfarin, apixaban was the only NOAC that did not have a higher rate of GI bleeding and the only one with a lower rate of major bleeding.8-10 In addition, apixaban remains the only NOAC found to have a statistically significant decrease in all-cause mortality compared with warfarin.10 Although dabigatran and rivaroxaban were associated with a strong trend towards decreased mortality, both studies were underpowered for this secondary outcome.8,9
Adding NOACs to stroke guidelines. The role of NOACs in the prevention of stroke in patients with nonvalvular AF is beginning to be reflected in newer guidelines. The American College of Chest Physicians (ACCP)’s 2012 guidelines recommend dabigatran over warfarin (grade 2B—weak recommendation; moderate quality evidence) unless the patient is well controlled on warfarin.11 The European Society of Cardiology (ESC)’s 2012 guidelines recommend dabigatran, apixaban, and rivaroxaban as broadly preferable to warfarin, while noting that experience with these agents is limited and appropriate patient selection is important.12
Anticoagulation to treat—and prevent—VTE
The standard of care for acute venous thromboembolism (VTE) is to initiate warfarin along with a parenteral anticoagulant, such as unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.13 Due to warfarin’s slow onset to peak effect, a parenteral anticoagulant is overlapped for ≥5 days—until warfarin reaches a therapeutic level and can be continued as monotherapy.13 But many patients find subcutaneous delivery of LMWH disagreeable and costly and frequent INR monitoring inconvenient, so the new agents offer notable advantages.
In well-designed studies, dabigatran, rivaroxaban, and apixaban have all been shown to be noninferior to warfarin in the initial treatment of acute DVT and pulmonary embolism (PE).14-17 All 3 agents were also shown to have lower rates of major bleeding than warfarin. Rivaroxaban and apixaban were also superior to warfarin with regard to bleeding events, and dabigatran was noninferior to warfarin for this outcome.14-17
NOACs help prevent recurrence
All 3 NOACs have been studied for long-term prevention of recurrent VTE after 3 to 18 months of anticoagulation, as well. Dabigatran was found in the RE-MEDY trial to be noninferior to warfarin for the risk of recurrent VTE, and to have lower rates of bleeding.18 In separate trials, all 3 agents were superior to placebo in preventing recurrent VTE. Rates of long-term major bleeding were significantly higher than placebo with rivaroxaban and dabigatran, but not with apixaban.15,18,19
Rivaroxaban is the only NOAC to be FDA approved for the treatment of acute DVT and PE, and the ACCP’s 2012 guidelines list it as a viable alternative to parenteral anticoagulation when initiating treatment for acute VTE.6,13 When treating VTE long term, the guidelines continue to recommend warfarin or LMWH rather than dabigatran or rivaroxaban.13 Recommendations may change in coming years, as physicians gain more experience with NOACs and more clinical trials are published.16-19
Starting or converting to NOAC therapy
In patients who have not been on anticoagulant therapy, any NOAC can be initiated immediately, with no need for parenteral, or “bridge” therapy. This is because of the rapid onset of action of the NOACs.12
To transition a patient from warfarin to a NOAC, it is necessary to discontinue warfarin therapy completely and closely monitor INR, then initiate NOAC therapy when INR≤2. No parenteral anticoagulation is necessary (TABLE 2).5-7
If it is necessary to transition a patient from a NOAC to warfarin, the protocol depends on the agent. Because dabigatran has no significant impact on prolongation of prothrombin time (PT), it can be overlapped with warfarin. Rivaroxaban and apixaban have a significant impact on PT prolongation, however, and overlapping either agent with warfarin is not recommended.4 Keep in mind that the recommended dosages for the NOACs are not standardized, and can differ drastically depending on the indication for use as well as on patient-specific factors, including renal function, body weight, and age.
Laboratory monitoring is not required
While warfarin has a great deal of interpatient variability and requires frequent lab monitoring, an oft-cited advantage of the NOACs is that they do not require regular monitoring. However, that also has a downside (TABLE 3).1,3-10,12,14-17,20-22 Monitoring INR in patients on warfarin allows physicians to assess patient compliance. And, if a patient on warfarin requires an invasive procedure, coagulation status and bleeding risk can easily be determined. That is not the case with the NOACs.
While some routine laboratory tests may be elevated in a patient taking a NOAC, the degree of elevation does not correlate well with anticoagulant concentration. And, because each NOAC has a different mechanism of action, different measures will be elevated in a patient taking dabigatran vs apixaban or rivaroxaban.4
Activated partial thromboplastin time (aPTT) is the most readily available lab test to assess the presence or absence of dabigatran.4 A normal aPTT indicates that there is little to no dabigatran present.4 But, while an elevated aPTT suggests the presence of dabigatran, it provides little information about how much.4
PT is a useful test to assess coagulation status in patients on either rivaroxaban or apixaban. A normal PT suggests that minimal amounts (or none) of the NOAC are present in the plasma.4 (A direct thrombin inhibitor assay, calibrated to more accurately assess dabigatran concentration, is being developed for clinical use, but is currently available only for research purposes in the United States; a chromogenic antifactor Xa test specific to apixaban and rivaroxaban is also being developed, but is not yet commercially available.4)
What to do when NOAC reversal is required
Patients often need to stop taking an oral anticoagulation in the days leading up to a planned invasive procedure. In an individual with normal renal function who will undergo a procedure with a standard bleeding risk, a NOAC would generally need to be withheld for one to 2 days prior to surgery, given the relatively short half-life. If a patient has acute renal failure or CKD, however, dabigatran may need to be withheld for a prolonged period (3-6 days) in order to safely proceed to surgery.4,23 NOACs may also need to be withheld for 2 to 6 days prior to any surgery with a high risk for bleeding.4
When speed is of the essence
There is no known antidote to aid in the reversal of dabigatran, rivaroxaban, or apixaban.4 Because of their relatively short half-lives, withholding the medication and providing supportive care is generally sufficient to ensure adequate hemostasis in cases of mild to moderate bleeding.4 If a patient presents with acute ingestion or an overdose, activated charcoal should be administered if the ingestion has occurred within the past 3 hours.4,24 The lack of a clear-cut reversal strategy can be extremely problematic in cases of trauma or life-threatening bleeding, however. (Fresh frozen plasma has not been shown to be effective at reversing NOACs’ effects.4)
In instances of severe bleeding or the need for urgent surgery, a more aggressive approach may be needed. Hemodialysis can be used to assist in the removal of dabigatran, but not rivaroxaban or apixaban.4 However, evidence suggests that the most effective therapy for patients who need rapid reversal of any NOAC is to administer 75 to 80 units/kg of activated prothrombin complex concentrate (aPCC).4,25-27 Recombinant factor VIIa has shown some promise in reversing the anticoagulant effects of these novel agents, but evidence is insufficient to recommend it as first-line therapy at this time.26, 27
Patients are more satisfied
The most obvious advantage of the NOACs as a group compared with warfarin is the lack of need for laboratory monitoring or continuous dose titration. Reliably stable pharmacokinetics make once or twice daily dosing possible. A rapid onset of action negates the need for bridging therapy with parenteral anticoagulants in patients at high risk of thrombosis. This may improve compliance, as many patients are averse to the use of subcutaneous injections or need extensive education before they can safely self-inject. The incidence of heparin-induced thrombocytopenia may also be decreased if unfractionated heparin and LMWH are used less frequently.
NOACs also appear to improve patient satisfaction.20-22 In one study that included patients with AF on dabigatran or warfarin, satisfaction was higher in those taking dabigatran, particularly among those who did not experience significant GI adverse effects.20 Another study showed improved patient satisfaction with rivaroxaban compared with LMWH following lower extremity joint replacement, which led to significantly higher rates of compliance.22
… but problems and pitfalls remain
In addition to the lack of a readily available and clinically validated reversal agent, the absence of a lab test that reliably measures the concentration of NOACs makes it difficult to determine whether patients are following their prescribed regimen.3,4
Medication compliance must be assessed when considering a transition from warfarin to a NOAC. Switching patients with poor INR control on warfarin to a NOAC should be done only after determining that the poor control is not the result of nonadherence. Because of the NOACs’ shorter half-life, patients who don’t take them regularly may be at higher risk for thromboembolic events.1,12
Cost is a serious consideration. While there are some costs associated with the monitoring warfarin requires, the medication itself has been generic for several decades and can be found on many “$4 lists” at pharmacies nationwide. In contrast, all 3 NOACs are available only as branded drugs, and can cost a patient with limited drug coverage anywhere from $250 to $350 per month28—a serious concern, given that the likelihood of noncompliance increases as out-of-pocket costs rise. This was highlighted in a recent study that found patients were twice as likely to discontinue their cholesterol-lowering medication if 100% of the cost was out of pocket, compared with patients who had no prescription copay.29 From the perspective of the US health care system, however, NOACs have been found to be cost effective compared with warfarin, mostly due to the lack of laboratory monitoring.30-32
Adverse effects. The risk of GI bleeds has been shown to be higher in patients taking rivaroxaban and dabigatran vs warfarin.8,9 Dabigatran has also been associated with a significant risk for dyspepsia.5,8,14 In clinical trials, the reported rate for dyspepsia in patients taking dabigatran was 3% to 11%; subsequent investigations have found the incidence to be far higher (33%).8,14,33
Drug interactions. Warfarin has a large number of drug interactions, of course, but because of its long history, these interactions are well established. NOACs also have a number of drug interactions, but the true clinical impact has not yet been established. All 3 agents are substrates for the P-glycoprotein (P-gp) transport system, so any known inhibitors or inducers of the P-gp system should be used cautiously in patients on NOACs.1,3-7,12 Rivaroxaban and apixaban are also substrates for the CYP3A4 hepatic enzyme system, so any drugs known to inhibit or induce this system require caution, as well.1,3-7,12
Who should not take a NOAC?
NOACs should not be prescribed for patients with mechanical heart valves.34 Dabigatran is the only NOAC to have been studied in this patient population, and the phase II trial was stopped prematurely due to increased risk for both bleeding and stroke in patients on dabigatran compared with warfarin.34
Renal impairment must be considered, as well. Do a baseline assessment of renal function in all patients before transitioning them to a NOAC, and periodic reassessment during therapy. While this is important for patients on rivaroxaban and apixaban, it is essential for those on dabigatran, as 80% of the drug is excreted by the kidneys.1,5,12 NOACs have not been adequately assessed in patients with severe renal dysfunction and should be avoided in this patient population. Caution should be exercised in patients with moderate renal dysfunction, as well.5-10,14-19 Apixaban appears to be the safest NOAC for patients with moderate renal dysfunction, as it has the least renal clearance.1,12
Who should take a NOAC?
No well-established criteria for patient selection for NOACs exist, yet appropriate patient selection is crucial. Evidence suggests that NOAC therapy is best suited to those who:
• are relatively young (<65 years)
• have normal renal function
• have poorly controlled INR with warfarin that is unrelated to noncompliance
• are unable to have regular INR monitoring.
Patients best suited for continued use of warfarin would be those whose INR is well controlled, those who have higher goal INR ranges (eg, because of the presence of mechanical heart valves), patients with significant renal dysfunction, and individuals with a history of peptic ulcer disease or GI bleeding. Warfarin may also be the best option for patients with a history of noncompliance and for uninsured or underinsured patients.
CASE 1 Warfarin and any of the NOACs were all feasible options for Ms. J, but apixaban was deemed to be the safest because of her moderate renal dysfunction. However, after she was told that apixaban has little “real world” clinical data, no effective antidote if bleeding were to occur, and a much higher cost than warfarin, she opted for warfarin therapy, despite the laboratory monitoring required.
CASE 2 Mr. W was excited to learn that there were new alternatives to warfarin; he had taken warfarin for 6 months after his last DVT and had a hard time coming in for INR checks. The patient reported that he had no history of bleeding and was compliant with medications. Rivaroxaban was the best option for Mr. W, as it is the only NOAC with FDA approval for the treatment of acute VTE.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, Swedish Medical Center, Room 3260, 501 East Hampden Avenue, Englewood, CO 80013; [email protected]
1. Wittkowsky AK. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy. 2011;31:1175-1191.
2. Gums JG. Place of dabigatran in contemporary pharmacotherapy. Pharmacotherapy. 2011;31:335-337.
3. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e44S-e88S.
4. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34:489-496.
5. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2010.
6. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011.
7. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
8. Connolly SJ, Zekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
9. Patel MR, Mahaffery KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
10. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
11. You JJ, Singer DE, Howard P, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S-e575S.
12. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719-2747.
13. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e419S-e494S.
14. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
15. Bauersachs R, Berkowitz SD, Brenner B, et al. Rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
16. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
17. Agnelli A, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808.
18. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709-718.
19. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699-708.
20. Michel J, Mundell D, Boga T, et al. Dabigatran for anticoagulation in atrial fibrillation–Early clinical experience in a hospital population and comparison to trial data. Heart Lung Circ. 2013;22:50-55.
21. Kendoff D, Perka C, Fritsche HM, et al. Oral thromboprophylaxis following total hip or knee replacement: review and multicentre experience with dabigatran etexilate. Open Orthop J. 2011;5:395-399.
22. Rogers BA, Phillip S, Foote J, et al. Is there adequate provision of venous thromboembolism prophylaxis following hip arthroplasty? An audit and international survey. Ann R Coll Surg Engl. 2010;92:668-672.
23. Healey JS, Eikelboom J, Douketis J, at al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126:343-348.
24. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate: a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127.
25. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573-1579.
26. Marlu R, Hodaj E, Paris A, et al. Effect of nonspecific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217-224.
27. Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, et al. Reversal of apixaban induced alterations of hemostasis by different coagulation factor concentrates: studies in vitro with circulating human blood. PLOS ONE. 2013;8:e78696.
28. Drug pricing information. Costco Pharmacy Web site. Available at: http://www2.costco.com/Pharmacy/DrugInformation.aspx?p=1. Accessed December 9, 2013.
29. Schneeweiss S, Patrick AR, Maclure M, et al. Adherence to statin therapy under drug cost sharing in patients with and without myocardial infarction: a population-based natural experiment. Circulation. 2007;115:2128-2135.
30. McKeage K. Dabigatran etexilate: a pharmacoeconomic review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Pharmacoeconomics. 2012;30:841-55.
31. Lee S, Anglade MW, Pham D, et al. Cost–Effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110:845-851.
32. Kamel H, Easton JD, Johnston SC, et al. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79:1428-1434.
33. Schulman S, Shortt B, Robinson M, et al. Adherence to anticoagulant treatment with dabigatran in a real-world setting. J Thromb Haemost. 2013; 11:1295-1299
34. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-1214.
› Consider novel oral anticoagualants (NOACs) for patients who have normal renal function, are comlpiant with medication regimens, and have no history of peptic ulcer or gastrointestinal bleeding. B
› Avoid overlapping warfarin with rivaroxaban or apixaban when transitioning a patient from one anticoagulant to the other, as both agents prolong prothrombin time. B
› When initiating a NOAC, it is not necessary to overlap with a parenteral anticoagulant. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Sally J is a 72-year-old Caucasian woman who comes to your clinic after being diagnosed with atrial fibrillation (AF). The patient has a 10-year history of type 2 diabetes; she also has a history of hypertension and chronic kidney disease (CKD), with a baseline creatinine clearance of approximately 40 mL/min. Ms. J tells you she knows people who take warfarin and really dislike it. She asks for your opinion of the new anticoagulants she’s seen advertised on TV, and wonders whether one of them would be right for her.
CASE 2 Bobby W, a 35-year-old African American man, was recently diagnosed with deep vein thrombosis (DVT). This was his second clot in 5 years, and occurred after a long flight home from Europe. The patient explains that he leads a very active lifestyle and doesn’t have the time to come in for the monthly international normalized ratio (INR) checks that warfarin requires. What would you recommend for these patients?
Troubled by warfarin’s narrow therapeutic index, numerous medication and dietary interactions, and need for frequent monitoring, patients requiring long-term oral anticoagulation therapy have been seeking alternatives for years. Finally, they have a choice. The US Food and Drug Administration (FDA) approved 3 oral anticoagulants—dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—in less than 4 years. Known as novel oral anticoagulants (NOACs), they are the first such drugs to enter the market in more than 50 years.1,2
While warfarin inhibits a wide range of clotting factors (including II, VII, IX, and X), NOACs work further down the clotting cascade (TABLE 1).1,3-7 Dabigatran, a direct thrombin inhibitor, only inhibits factor IIa.3,5 Rivaroxaban and apixaban directly inhibit factor Xa and indirectly inhibit factor IIa.3,6,7
There are notable advantages to these newer agents, but some disadvantages that must be considered, as well. Appropriate patient selection, guided by a thorough understanding of the benefits and risks of NOACs, is key.
Stroke prevention in atrial fibrillation
All 3 NOACs are approved for stroke prevention in patients with nonvalvular atrial fibrillation (AF). The approvals are based on a small number of well-designed trials: RE-LY (dabigatran), ROCKET-AF (rivaroxaban), and ARISTOTLE (apixaban).8-10 Compared with warfarin, dabigatran is the only oral anticoagulant with a lower rate of both hemorrhagic and ischemic stroke.8 Both rivaroxaban and apixaban were found to decrease overall stroke risk relative to warfarin, but the difference was driven by a lower risk for hemorrhagic, not ischemic, stroke.9,10
In these trials, overall rates of major bleeding were similar to that of warfarin.8-10 Patients taking warfarin generally experienced higher rates of intracranial hemorrhage but lower rates of gastrointestinal (GI) bleeding than those on NOACs. Relative to warfarin, apixaban was the only NOAC that did not have a higher rate of GI bleeding and the only one with a lower rate of major bleeding.8-10 In addition, apixaban remains the only NOAC found to have a statistically significant decrease in all-cause mortality compared with warfarin.10 Although dabigatran and rivaroxaban were associated with a strong trend towards decreased mortality, both studies were underpowered for this secondary outcome.8,9
Adding NOACs to stroke guidelines. The role of NOACs in the prevention of stroke in patients with nonvalvular AF is beginning to be reflected in newer guidelines. The American College of Chest Physicians (ACCP)’s 2012 guidelines recommend dabigatran over warfarin (grade 2B—weak recommendation; moderate quality evidence) unless the patient is well controlled on warfarin.11 The European Society of Cardiology (ESC)’s 2012 guidelines recommend dabigatran, apixaban, and rivaroxaban as broadly preferable to warfarin, while noting that experience with these agents is limited and appropriate patient selection is important.12
Anticoagulation to treat—and prevent—VTE
The standard of care for acute venous thromboembolism (VTE) is to initiate warfarin along with a parenteral anticoagulant, such as unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.13 Due to warfarin’s slow onset to peak effect, a parenteral anticoagulant is overlapped for ≥5 days—until warfarin reaches a therapeutic level and can be continued as monotherapy.13 But many patients find subcutaneous delivery of LMWH disagreeable and costly and frequent INR monitoring inconvenient, so the new agents offer notable advantages.
In well-designed studies, dabigatran, rivaroxaban, and apixaban have all been shown to be noninferior to warfarin in the initial treatment of acute DVT and pulmonary embolism (PE).14-17 All 3 agents were also shown to have lower rates of major bleeding than warfarin. Rivaroxaban and apixaban were also superior to warfarin with regard to bleeding events, and dabigatran was noninferior to warfarin for this outcome.14-17
NOACs help prevent recurrence
All 3 NOACs have been studied for long-term prevention of recurrent VTE after 3 to 18 months of anticoagulation, as well. Dabigatran was found in the RE-MEDY trial to be noninferior to warfarin for the risk of recurrent VTE, and to have lower rates of bleeding.18 In separate trials, all 3 agents were superior to placebo in preventing recurrent VTE. Rates of long-term major bleeding were significantly higher than placebo with rivaroxaban and dabigatran, but not with apixaban.15,18,19
Rivaroxaban is the only NOAC to be FDA approved for the treatment of acute DVT and PE, and the ACCP’s 2012 guidelines list it as a viable alternative to parenteral anticoagulation when initiating treatment for acute VTE.6,13 When treating VTE long term, the guidelines continue to recommend warfarin or LMWH rather than dabigatran or rivaroxaban.13 Recommendations may change in coming years, as physicians gain more experience with NOACs and more clinical trials are published.16-19
Starting or converting to NOAC therapy
In patients who have not been on anticoagulant therapy, any NOAC can be initiated immediately, with no need for parenteral, or “bridge” therapy. This is because of the rapid onset of action of the NOACs.12
To transition a patient from warfarin to a NOAC, it is necessary to discontinue warfarin therapy completely and closely monitor INR, then initiate NOAC therapy when INR≤2. No parenteral anticoagulation is necessary (TABLE 2).5-7
If it is necessary to transition a patient from a NOAC to warfarin, the protocol depends on the agent. Because dabigatran has no significant impact on prolongation of prothrombin time (PT), it can be overlapped with warfarin. Rivaroxaban and apixaban have a significant impact on PT prolongation, however, and overlapping either agent with warfarin is not recommended.4 Keep in mind that the recommended dosages for the NOACs are not standardized, and can differ drastically depending on the indication for use as well as on patient-specific factors, including renal function, body weight, and age.
Laboratory monitoring is not required
While warfarin has a great deal of interpatient variability and requires frequent lab monitoring, an oft-cited advantage of the NOACs is that they do not require regular monitoring. However, that also has a downside (TABLE 3).1,3-10,12,14-17,20-22 Monitoring INR in patients on warfarin allows physicians to assess patient compliance. And, if a patient on warfarin requires an invasive procedure, coagulation status and bleeding risk can easily be determined. That is not the case with the NOACs.
While some routine laboratory tests may be elevated in a patient taking a NOAC, the degree of elevation does not correlate well with anticoagulant concentration. And, because each NOAC has a different mechanism of action, different measures will be elevated in a patient taking dabigatran vs apixaban or rivaroxaban.4
Activated partial thromboplastin time (aPTT) is the most readily available lab test to assess the presence or absence of dabigatran.4 A normal aPTT indicates that there is little to no dabigatran present.4 But, while an elevated aPTT suggests the presence of dabigatran, it provides little information about how much.4
PT is a useful test to assess coagulation status in patients on either rivaroxaban or apixaban. A normal PT suggests that minimal amounts (or none) of the NOAC are present in the plasma.4 (A direct thrombin inhibitor assay, calibrated to more accurately assess dabigatran concentration, is being developed for clinical use, but is currently available only for research purposes in the United States; a chromogenic antifactor Xa test specific to apixaban and rivaroxaban is also being developed, but is not yet commercially available.4)
What to do when NOAC reversal is required
Patients often need to stop taking an oral anticoagulation in the days leading up to a planned invasive procedure. In an individual with normal renal function who will undergo a procedure with a standard bleeding risk, a NOAC would generally need to be withheld for one to 2 days prior to surgery, given the relatively short half-life. If a patient has acute renal failure or CKD, however, dabigatran may need to be withheld for a prolonged period (3-6 days) in order to safely proceed to surgery.4,23 NOACs may also need to be withheld for 2 to 6 days prior to any surgery with a high risk for bleeding.4
When speed is of the essence
There is no known antidote to aid in the reversal of dabigatran, rivaroxaban, or apixaban.4 Because of their relatively short half-lives, withholding the medication and providing supportive care is generally sufficient to ensure adequate hemostasis in cases of mild to moderate bleeding.4 If a patient presents with acute ingestion or an overdose, activated charcoal should be administered if the ingestion has occurred within the past 3 hours.4,24 The lack of a clear-cut reversal strategy can be extremely problematic in cases of trauma or life-threatening bleeding, however. (Fresh frozen plasma has not been shown to be effective at reversing NOACs’ effects.4)
In instances of severe bleeding or the need for urgent surgery, a more aggressive approach may be needed. Hemodialysis can be used to assist in the removal of dabigatran, but not rivaroxaban or apixaban.4 However, evidence suggests that the most effective therapy for patients who need rapid reversal of any NOAC is to administer 75 to 80 units/kg of activated prothrombin complex concentrate (aPCC).4,25-27 Recombinant factor VIIa has shown some promise in reversing the anticoagulant effects of these novel agents, but evidence is insufficient to recommend it as first-line therapy at this time.26, 27
Patients are more satisfied
The most obvious advantage of the NOACs as a group compared with warfarin is the lack of need for laboratory monitoring or continuous dose titration. Reliably stable pharmacokinetics make once or twice daily dosing possible. A rapid onset of action negates the need for bridging therapy with parenteral anticoagulants in patients at high risk of thrombosis. This may improve compliance, as many patients are averse to the use of subcutaneous injections or need extensive education before they can safely self-inject. The incidence of heparin-induced thrombocytopenia may also be decreased if unfractionated heparin and LMWH are used less frequently.
NOACs also appear to improve patient satisfaction.20-22 In one study that included patients with AF on dabigatran or warfarin, satisfaction was higher in those taking dabigatran, particularly among those who did not experience significant GI adverse effects.20 Another study showed improved patient satisfaction with rivaroxaban compared with LMWH following lower extremity joint replacement, which led to significantly higher rates of compliance.22
… but problems and pitfalls remain
In addition to the lack of a readily available and clinically validated reversal agent, the absence of a lab test that reliably measures the concentration of NOACs makes it difficult to determine whether patients are following their prescribed regimen.3,4
Medication compliance must be assessed when considering a transition from warfarin to a NOAC. Switching patients with poor INR control on warfarin to a NOAC should be done only after determining that the poor control is not the result of nonadherence. Because of the NOACs’ shorter half-life, patients who don’t take them regularly may be at higher risk for thromboembolic events.1,12
Cost is a serious consideration. While there are some costs associated with the monitoring warfarin requires, the medication itself has been generic for several decades and can be found on many “$4 lists” at pharmacies nationwide. In contrast, all 3 NOACs are available only as branded drugs, and can cost a patient with limited drug coverage anywhere from $250 to $350 per month28—a serious concern, given that the likelihood of noncompliance increases as out-of-pocket costs rise. This was highlighted in a recent study that found patients were twice as likely to discontinue their cholesterol-lowering medication if 100% of the cost was out of pocket, compared with patients who had no prescription copay.29 From the perspective of the US health care system, however, NOACs have been found to be cost effective compared with warfarin, mostly due to the lack of laboratory monitoring.30-32
Adverse effects. The risk of GI bleeds has been shown to be higher in patients taking rivaroxaban and dabigatran vs warfarin.8,9 Dabigatran has also been associated with a significant risk for dyspepsia.5,8,14 In clinical trials, the reported rate for dyspepsia in patients taking dabigatran was 3% to 11%; subsequent investigations have found the incidence to be far higher (33%).8,14,33
Drug interactions. Warfarin has a large number of drug interactions, of course, but because of its long history, these interactions are well established. NOACs also have a number of drug interactions, but the true clinical impact has not yet been established. All 3 agents are substrates for the P-glycoprotein (P-gp) transport system, so any known inhibitors or inducers of the P-gp system should be used cautiously in patients on NOACs.1,3-7,12 Rivaroxaban and apixaban are also substrates for the CYP3A4 hepatic enzyme system, so any drugs known to inhibit or induce this system require caution, as well.1,3-7,12
Who should not take a NOAC?
NOACs should not be prescribed for patients with mechanical heart valves.34 Dabigatran is the only NOAC to have been studied in this patient population, and the phase II trial was stopped prematurely due to increased risk for both bleeding and stroke in patients on dabigatran compared with warfarin.34
Renal impairment must be considered, as well. Do a baseline assessment of renal function in all patients before transitioning them to a NOAC, and periodic reassessment during therapy. While this is important for patients on rivaroxaban and apixaban, it is essential for those on dabigatran, as 80% of the drug is excreted by the kidneys.1,5,12 NOACs have not been adequately assessed in patients with severe renal dysfunction and should be avoided in this patient population. Caution should be exercised in patients with moderate renal dysfunction, as well.5-10,14-19 Apixaban appears to be the safest NOAC for patients with moderate renal dysfunction, as it has the least renal clearance.1,12
Who should take a NOAC?
No well-established criteria for patient selection for NOACs exist, yet appropriate patient selection is crucial. Evidence suggests that NOAC therapy is best suited to those who:
• are relatively young (<65 years)
• have normal renal function
• have poorly controlled INR with warfarin that is unrelated to noncompliance
• are unable to have regular INR monitoring.
Patients best suited for continued use of warfarin would be those whose INR is well controlled, those who have higher goal INR ranges (eg, because of the presence of mechanical heart valves), patients with significant renal dysfunction, and individuals with a history of peptic ulcer disease or GI bleeding. Warfarin may also be the best option for patients with a history of noncompliance and for uninsured or underinsured patients.
CASE 1 Warfarin and any of the NOACs were all feasible options for Ms. J, but apixaban was deemed to be the safest because of her moderate renal dysfunction. However, after she was told that apixaban has little “real world” clinical data, no effective antidote if bleeding were to occur, and a much higher cost than warfarin, she opted for warfarin therapy, despite the laboratory monitoring required.
CASE 2 Mr. W was excited to learn that there were new alternatives to warfarin; he had taken warfarin for 6 months after his last DVT and had a hard time coming in for INR checks. The patient reported that he had no history of bleeding and was compliant with medications. Rivaroxaban was the best option for Mr. W, as it is the only NOAC with FDA approval for the treatment of acute VTE.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, Swedish Medical Center, Room 3260, 501 East Hampden Avenue, Englewood, CO 80013; [email protected]
› Consider novel oral anticoagualants (NOACs) for patients who have normal renal function, are comlpiant with medication regimens, and have no history of peptic ulcer or gastrointestinal bleeding. B
› Avoid overlapping warfarin with rivaroxaban or apixaban when transitioning a patient from one anticoagulant to the other, as both agents prolong prothrombin time. B
› When initiating a NOAC, it is not necessary to overlap with a parenteral anticoagulant. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Sally J is a 72-year-old Caucasian woman who comes to your clinic after being diagnosed with atrial fibrillation (AF). The patient has a 10-year history of type 2 diabetes; she also has a history of hypertension and chronic kidney disease (CKD), with a baseline creatinine clearance of approximately 40 mL/min. Ms. J tells you she knows people who take warfarin and really dislike it. She asks for your opinion of the new anticoagulants she’s seen advertised on TV, and wonders whether one of them would be right for her.
CASE 2 Bobby W, a 35-year-old African American man, was recently diagnosed with deep vein thrombosis (DVT). This was his second clot in 5 years, and occurred after a long flight home from Europe. The patient explains that he leads a very active lifestyle and doesn’t have the time to come in for the monthly international normalized ratio (INR) checks that warfarin requires. What would you recommend for these patients?
Troubled by warfarin’s narrow therapeutic index, numerous medication and dietary interactions, and need for frequent monitoring, patients requiring long-term oral anticoagulation therapy have been seeking alternatives for years. Finally, they have a choice. The US Food and Drug Administration (FDA) approved 3 oral anticoagulants—dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—in less than 4 years. Known as novel oral anticoagulants (NOACs), they are the first such drugs to enter the market in more than 50 years.1,2
While warfarin inhibits a wide range of clotting factors (including II, VII, IX, and X), NOACs work further down the clotting cascade (TABLE 1).1,3-7 Dabigatran, a direct thrombin inhibitor, only inhibits factor IIa.3,5 Rivaroxaban and apixaban directly inhibit factor Xa and indirectly inhibit factor IIa.3,6,7
There are notable advantages to these newer agents, but some disadvantages that must be considered, as well. Appropriate patient selection, guided by a thorough understanding of the benefits and risks of NOACs, is key.
Stroke prevention in atrial fibrillation
All 3 NOACs are approved for stroke prevention in patients with nonvalvular atrial fibrillation (AF). The approvals are based on a small number of well-designed trials: RE-LY (dabigatran), ROCKET-AF (rivaroxaban), and ARISTOTLE (apixaban).8-10 Compared with warfarin, dabigatran is the only oral anticoagulant with a lower rate of both hemorrhagic and ischemic stroke.8 Both rivaroxaban and apixaban were found to decrease overall stroke risk relative to warfarin, but the difference was driven by a lower risk for hemorrhagic, not ischemic, stroke.9,10
In these trials, overall rates of major bleeding were similar to that of warfarin.8-10 Patients taking warfarin generally experienced higher rates of intracranial hemorrhage but lower rates of gastrointestinal (GI) bleeding than those on NOACs. Relative to warfarin, apixaban was the only NOAC that did not have a higher rate of GI bleeding and the only one with a lower rate of major bleeding.8-10 In addition, apixaban remains the only NOAC found to have a statistically significant decrease in all-cause mortality compared with warfarin.10 Although dabigatran and rivaroxaban were associated with a strong trend towards decreased mortality, both studies were underpowered for this secondary outcome.8,9
Adding NOACs to stroke guidelines. The role of NOACs in the prevention of stroke in patients with nonvalvular AF is beginning to be reflected in newer guidelines. The American College of Chest Physicians (ACCP)’s 2012 guidelines recommend dabigatran over warfarin (grade 2B—weak recommendation; moderate quality evidence) unless the patient is well controlled on warfarin.11 The European Society of Cardiology (ESC)’s 2012 guidelines recommend dabigatran, apixaban, and rivaroxaban as broadly preferable to warfarin, while noting that experience with these agents is limited and appropriate patient selection is important.12
Anticoagulation to treat—and prevent—VTE
The standard of care for acute venous thromboembolism (VTE) is to initiate warfarin along with a parenteral anticoagulant, such as unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.13 Due to warfarin’s slow onset to peak effect, a parenteral anticoagulant is overlapped for ≥5 days—until warfarin reaches a therapeutic level and can be continued as monotherapy.13 But many patients find subcutaneous delivery of LMWH disagreeable and costly and frequent INR monitoring inconvenient, so the new agents offer notable advantages.
In well-designed studies, dabigatran, rivaroxaban, and apixaban have all been shown to be noninferior to warfarin in the initial treatment of acute DVT and pulmonary embolism (PE).14-17 All 3 agents were also shown to have lower rates of major bleeding than warfarin. Rivaroxaban and apixaban were also superior to warfarin with regard to bleeding events, and dabigatran was noninferior to warfarin for this outcome.14-17
NOACs help prevent recurrence
All 3 NOACs have been studied for long-term prevention of recurrent VTE after 3 to 18 months of anticoagulation, as well. Dabigatran was found in the RE-MEDY trial to be noninferior to warfarin for the risk of recurrent VTE, and to have lower rates of bleeding.18 In separate trials, all 3 agents were superior to placebo in preventing recurrent VTE. Rates of long-term major bleeding were significantly higher than placebo with rivaroxaban and dabigatran, but not with apixaban.15,18,19
Rivaroxaban is the only NOAC to be FDA approved for the treatment of acute DVT and PE, and the ACCP’s 2012 guidelines list it as a viable alternative to parenteral anticoagulation when initiating treatment for acute VTE.6,13 When treating VTE long term, the guidelines continue to recommend warfarin or LMWH rather than dabigatran or rivaroxaban.13 Recommendations may change in coming years, as physicians gain more experience with NOACs and more clinical trials are published.16-19
Starting or converting to NOAC therapy
In patients who have not been on anticoagulant therapy, any NOAC can be initiated immediately, with no need for parenteral, or “bridge” therapy. This is because of the rapid onset of action of the NOACs.12
To transition a patient from warfarin to a NOAC, it is necessary to discontinue warfarin therapy completely and closely monitor INR, then initiate NOAC therapy when INR≤2. No parenteral anticoagulation is necessary (TABLE 2).5-7
If it is necessary to transition a patient from a NOAC to warfarin, the protocol depends on the agent. Because dabigatran has no significant impact on prolongation of prothrombin time (PT), it can be overlapped with warfarin. Rivaroxaban and apixaban have a significant impact on PT prolongation, however, and overlapping either agent with warfarin is not recommended.4 Keep in mind that the recommended dosages for the NOACs are not standardized, and can differ drastically depending on the indication for use as well as on patient-specific factors, including renal function, body weight, and age.
Laboratory monitoring is not required
While warfarin has a great deal of interpatient variability and requires frequent lab monitoring, an oft-cited advantage of the NOACs is that they do not require regular monitoring. However, that also has a downside (TABLE 3).1,3-10,12,14-17,20-22 Monitoring INR in patients on warfarin allows physicians to assess patient compliance. And, if a patient on warfarin requires an invasive procedure, coagulation status and bleeding risk can easily be determined. That is not the case with the NOACs.
While some routine laboratory tests may be elevated in a patient taking a NOAC, the degree of elevation does not correlate well with anticoagulant concentration. And, because each NOAC has a different mechanism of action, different measures will be elevated in a patient taking dabigatran vs apixaban or rivaroxaban.4
Activated partial thromboplastin time (aPTT) is the most readily available lab test to assess the presence or absence of dabigatran.4 A normal aPTT indicates that there is little to no dabigatran present.4 But, while an elevated aPTT suggests the presence of dabigatran, it provides little information about how much.4
PT is a useful test to assess coagulation status in patients on either rivaroxaban or apixaban. A normal PT suggests that minimal amounts (or none) of the NOAC are present in the plasma.4 (A direct thrombin inhibitor assay, calibrated to more accurately assess dabigatran concentration, is being developed for clinical use, but is currently available only for research purposes in the United States; a chromogenic antifactor Xa test specific to apixaban and rivaroxaban is also being developed, but is not yet commercially available.4)
What to do when NOAC reversal is required
Patients often need to stop taking an oral anticoagulation in the days leading up to a planned invasive procedure. In an individual with normal renal function who will undergo a procedure with a standard bleeding risk, a NOAC would generally need to be withheld for one to 2 days prior to surgery, given the relatively short half-life. If a patient has acute renal failure or CKD, however, dabigatran may need to be withheld for a prolonged period (3-6 days) in order to safely proceed to surgery.4,23 NOACs may also need to be withheld for 2 to 6 days prior to any surgery with a high risk for bleeding.4
When speed is of the essence
There is no known antidote to aid in the reversal of dabigatran, rivaroxaban, or apixaban.4 Because of their relatively short half-lives, withholding the medication and providing supportive care is generally sufficient to ensure adequate hemostasis in cases of mild to moderate bleeding.4 If a patient presents with acute ingestion or an overdose, activated charcoal should be administered if the ingestion has occurred within the past 3 hours.4,24 The lack of a clear-cut reversal strategy can be extremely problematic in cases of trauma or life-threatening bleeding, however. (Fresh frozen plasma has not been shown to be effective at reversing NOACs’ effects.4)
In instances of severe bleeding or the need for urgent surgery, a more aggressive approach may be needed. Hemodialysis can be used to assist in the removal of dabigatran, but not rivaroxaban or apixaban.4 However, evidence suggests that the most effective therapy for patients who need rapid reversal of any NOAC is to administer 75 to 80 units/kg of activated prothrombin complex concentrate (aPCC).4,25-27 Recombinant factor VIIa has shown some promise in reversing the anticoagulant effects of these novel agents, but evidence is insufficient to recommend it as first-line therapy at this time.26, 27
Patients are more satisfied
The most obvious advantage of the NOACs as a group compared with warfarin is the lack of need for laboratory monitoring or continuous dose titration. Reliably stable pharmacokinetics make once or twice daily dosing possible. A rapid onset of action negates the need for bridging therapy with parenteral anticoagulants in patients at high risk of thrombosis. This may improve compliance, as many patients are averse to the use of subcutaneous injections or need extensive education before they can safely self-inject. The incidence of heparin-induced thrombocytopenia may also be decreased if unfractionated heparin and LMWH are used less frequently.
NOACs also appear to improve patient satisfaction.20-22 In one study that included patients with AF on dabigatran or warfarin, satisfaction was higher in those taking dabigatran, particularly among those who did not experience significant GI adverse effects.20 Another study showed improved patient satisfaction with rivaroxaban compared with LMWH following lower extremity joint replacement, which led to significantly higher rates of compliance.22
… but problems and pitfalls remain
In addition to the lack of a readily available and clinically validated reversal agent, the absence of a lab test that reliably measures the concentration of NOACs makes it difficult to determine whether patients are following their prescribed regimen.3,4
Medication compliance must be assessed when considering a transition from warfarin to a NOAC. Switching patients with poor INR control on warfarin to a NOAC should be done only after determining that the poor control is not the result of nonadherence. Because of the NOACs’ shorter half-life, patients who don’t take them regularly may be at higher risk for thromboembolic events.1,12
Cost is a serious consideration. While there are some costs associated with the monitoring warfarin requires, the medication itself has been generic for several decades and can be found on many “$4 lists” at pharmacies nationwide. In contrast, all 3 NOACs are available only as branded drugs, and can cost a patient with limited drug coverage anywhere from $250 to $350 per month28—a serious concern, given that the likelihood of noncompliance increases as out-of-pocket costs rise. This was highlighted in a recent study that found patients were twice as likely to discontinue their cholesterol-lowering medication if 100% of the cost was out of pocket, compared with patients who had no prescription copay.29 From the perspective of the US health care system, however, NOACs have been found to be cost effective compared with warfarin, mostly due to the lack of laboratory monitoring.30-32
Adverse effects. The risk of GI bleeds has been shown to be higher in patients taking rivaroxaban and dabigatran vs warfarin.8,9 Dabigatran has also been associated with a significant risk for dyspepsia.5,8,14 In clinical trials, the reported rate for dyspepsia in patients taking dabigatran was 3% to 11%; subsequent investigations have found the incidence to be far higher (33%).8,14,33
Drug interactions. Warfarin has a large number of drug interactions, of course, but because of its long history, these interactions are well established. NOACs also have a number of drug interactions, but the true clinical impact has not yet been established. All 3 agents are substrates for the P-glycoprotein (P-gp) transport system, so any known inhibitors or inducers of the P-gp system should be used cautiously in patients on NOACs.1,3-7,12 Rivaroxaban and apixaban are also substrates for the CYP3A4 hepatic enzyme system, so any drugs known to inhibit or induce this system require caution, as well.1,3-7,12
Who should not take a NOAC?
NOACs should not be prescribed for patients with mechanical heart valves.34 Dabigatran is the only NOAC to have been studied in this patient population, and the phase II trial was stopped prematurely due to increased risk for both bleeding and stroke in patients on dabigatran compared with warfarin.34
Renal impairment must be considered, as well. Do a baseline assessment of renal function in all patients before transitioning them to a NOAC, and periodic reassessment during therapy. While this is important for patients on rivaroxaban and apixaban, it is essential for those on dabigatran, as 80% of the drug is excreted by the kidneys.1,5,12 NOACs have not been adequately assessed in patients with severe renal dysfunction and should be avoided in this patient population. Caution should be exercised in patients with moderate renal dysfunction, as well.5-10,14-19 Apixaban appears to be the safest NOAC for patients with moderate renal dysfunction, as it has the least renal clearance.1,12
Who should take a NOAC?
No well-established criteria for patient selection for NOACs exist, yet appropriate patient selection is crucial. Evidence suggests that NOAC therapy is best suited to those who:
• are relatively young (<65 years)
• have normal renal function
• have poorly controlled INR with warfarin that is unrelated to noncompliance
• are unable to have regular INR monitoring.
Patients best suited for continued use of warfarin would be those whose INR is well controlled, those who have higher goal INR ranges (eg, because of the presence of mechanical heart valves), patients with significant renal dysfunction, and individuals with a history of peptic ulcer disease or GI bleeding. Warfarin may also be the best option for patients with a history of noncompliance and for uninsured or underinsured patients.
CASE 1 Warfarin and any of the NOACs were all feasible options for Ms. J, but apixaban was deemed to be the safest because of her moderate renal dysfunction. However, after she was told that apixaban has little “real world” clinical data, no effective antidote if bleeding were to occur, and a much higher cost than warfarin, she opted for warfarin therapy, despite the laboratory monitoring required.
CASE 2 Mr. W was excited to learn that there were new alternatives to warfarin; he had taken warfarin for 6 months after his last DVT and had a hard time coming in for INR checks. The patient reported that he had no history of bleeding and was compliant with medications. Rivaroxaban was the best option for Mr. W, as it is the only NOAC with FDA approval for the treatment of acute VTE.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, Swedish Medical Center, Room 3260, 501 East Hampden Avenue, Englewood, CO 80013; [email protected]
1. Wittkowsky AK. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy. 2011;31:1175-1191.
2. Gums JG. Place of dabigatran in contemporary pharmacotherapy. Pharmacotherapy. 2011;31:335-337.
3. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e44S-e88S.
4. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34:489-496.
5. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2010.
6. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011.
7. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
8. Connolly SJ, Zekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
9. Patel MR, Mahaffery KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
10. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
11. You JJ, Singer DE, Howard P, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S-e575S.
12. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719-2747.
13. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e419S-e494S.
14. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
15. Bauersachs R, Berkowitz SD, Brenner B, et al. Rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
16. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
17. Agnelli A, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808.
18. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709-718.
19. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699-708.
20. Michel J, Mundell D, Boga T, et al. Dabigatran for anticoagulation in atrial fibrillation–Early clinical experience in a hospital population and comparison to trial data. Heart Lung Circ. 2013;22:50-55.
21. Kendoff D, Perka C, Fritsche HM, et al. Oral thromboprophylaxis following total hip or knee replacement: review and multicentre experience with dabigatran etexilate. Open Orthop J. 2011;5:395-399.
22. Rogers BA, Phillip S, Foote J, et al. Is there adequate provision of venous thromboembolism prophylaxis following hip arthroplasty? An audit and international survey. Ann R Coll Surg Engl. 2010;92:668-672.
23. Healey JS, Eikelboom J, Douketis J, at al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126:343-348.
24. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate: a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127.
25. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573-1579.
26. Marlu R, Hodaj E, Paris A, et al. Effect of nonspecific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217-224.
27. Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, et al. Reversal of apixaban induced alterations of hemostasis by different coagulation factor concentrates: studies in vitro with circulating human blood. PLOS ONE. 2013;8:e78696.
28. Drug pricing information. Costco Pharmacy Web site. Available at: http://www2.costco.com/Pharmacy/DrugInformation.aspx?p=1. Accessed December 9, 2013.
29. Schneeweiss S, Patrick AR, Maclure M, et al. Adherence to statin therapy under drug cost sharing in patients with and without myocardial infarction: a population-based natural experiment. Circulation. 2007;115:2128-2135.
30. McKeage K. Dabigatran etexilate: a pharmacoeconomic review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Pharmacoeconomics. 2012;30:841-55.
31. Lee S, Anglade MW, Pham D, et al. Cost–Effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110:845-851.
32. Kamel H, Easton JD, Johnston SC, et al. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79:1428-1434.
33. Schulman S, Shortt B, Robinson M, et al. Adherence to anticoagulant treatment with dabigatran in a real-world setting. J Thromb Haemost. 2013; 11:1295-1299
34. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-1214.
1. Wittkowsky AK. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy. 2011;31:1175-1191.
2. Gums JG. Place of dabigatran in contemporary pharmacotherapy. Pharmacotherapy. 2011;31:335-337.
3. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e44S-e88S.
4. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34:489-496.
5. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2010.
6. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011.
7. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
8. Connolly SJ, Zekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
9. Patel MR, Mahaffery KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
10. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
11. You JJ, Singer DE, Howard P, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S-e575S.
12. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719-2747.
13. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e419S-e494S.
14. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
15. Bauersachs R, Berkowitz SD, Brenner B, et al. Rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
16. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
17. Agnelli A, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808.
18. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709-718.
19. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699-708.
20. Michel J, Mundell D, Boga T, et al. Dabigatran for anticoagulation in atrial fibrillation–Early clinical experience in a hospital population and comparison to trial data. Heart Lung Circ. 2013;22:50-55.
21. Kendoff D, Perka C, Fritsche HM, et al. Oral thromboprophylaxis following total hip or knee replacement: review and multicentre experience with dabigatran etexilate. Open Orthop J. 2011;5:395-399.
22. Rogers BA, Phillip S, Foote J, et al. Is there adequate provision of venous thromboembolism prophylaxis following hip arthroplasty? An audit and international survey. Ann R Coll Surg Engl. 2010;92:668-672.
23. Healey JS, Eikelboom J, Douketis J, at al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126:343-348.
24. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate: a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127.
25. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573-1579.
26. Marlu R, Hodaj E, Paris A, et al. Effect of nonspecific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217-224.
27. Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, et al. Reversal of apixaban induced alterations of hemostasis by different coagulation factor concentrates: studies in vitro with circulating human blood. PLOS ONE. 2013;8:e78696.
28. Drug pricing information. Costco Pharmacy Web site. Available at: http://www2.costco.com/Pharmacy/DrugInformation.aspx?p=1. Accessed December 9, 2013.
29. Schneeweiss S, Patrick AR, Maclure M, et al. Adherence to statin therapy under drug cost sharing in patients with and without myocardial infarction: a population-based natural experiment. Circulation. 2007;115:2128-2135.
30. McKeage K. Dabigatran etexilate: a pharmacoeconomic review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Pharmacoeconomics. 2012;30:841-55.
31. Lee S, Anglade MW, Pham D, et al. Cost–Effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110:845-851.
32. Kamel H, Easton JD, Johnston SC, et al. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79:1428-1434.
33. Schulman S, Shortt B, Robinson M, et al. Adherence to anticoagulant treatment with dabigatran in a real-world setting. J Thromb Haemost. 2013; 11:1295-1299
34. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-1214.
Abnormal bleeding in your female patient? Consider a progestin IUD
› Recommend the 52 mg levonorgestrel-releasing intrauterine device (LNG-IUD) as a first-line treatment for heavy menstrual bleeding. A
› Advise patients with dysmenorrhea that the 52 mg LNG-IUD is an effective nonsurgical treatment. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane K, a 40-year-old multiparous woman, is seeking treatment for heavy menstrual bleeding and cramping, both of which have troubled her for 4 years. Another physician had given her oral contraceptive pills (OCPs) to decrease the pain and bleeding, she reports, but she has difficulty remembering to take a pill every day.
On physical exam, you note an enlarged uterus (approximately 16-week size). A pregnancy test is negative, her thyroidstimulating hormone level is normal, and her hemoglobin is 9 g/dL. Transvaginal ultrasound reveals multiple fibroids.
What can you offer her?
At some point in their reproductive years, 10% to 15% of women experience heavy menstrual bleeding (HMB), or menorrhagia.1,2 In fact, HMB and dysmenorrhea are among the most common reasons for office visits and missed work among women in this age group.3,4 In addition to having a negative impact on quality of life, HMB can cause severe anemia.5
All too often, the suggested solution is a hysterectomy. In fact, 90% of the more than 600,000 hysterectomies performed annually in the United States are for benign disease.6,7 Yet many women with HMB want nonsurgical treatments, and some seek to preserve their fertility. A progestin IUD can often fulfill both of these desires.
An IUD containing 52 mg levonorgestrel (Mirena) has US Food and Drug Administration (FDA) approval both for use as a contraceptive and for the treatment of HMB in women who want intrauterine contraception.8 Recent studies have confirmed its efficacy in treating a wide variety of conditions associated with menorrhagia and dysmenorrhea. In 2013, a smaller, lower dose (13.5 mg) levonorgestrel-releasing IUD (Skyla) received FDA approval as a contraceptive.9 But because this device has been neither tested nor approved for other applications, the following review applies only to Mirena—referred to throughout this article as the LNG-IUD.
A proven (and often superior) treatment for menorrhagia
Leiomyomas, or fibroids—the most common benign tumor of the female genital tract—are a frequent cause of menorrhagia.10 OCPs are often used for the treatment of symptomatic fibroids for women who wish to avoid surgery. When compared with low-dose OCPs for the treatment of menorrhagia secondary to fibroids, however, the LNG-IUD resulted in a significantly greater reduction in blood loss.11
While fibroid size does not appear to decrease significantly after insertion of the device, a systematic review found that menstrual bleeding lessens and hemoglobin levels improve in women with symptomatic fibroids.12,13 The LNG-IUD has also been shown to improve symptoms in women with dysmenorrhea secondary to fibroids.14
Hemostatic disorders. Anticoagulants are vital for women with hemostatic disorders such as Von Willebrand disease, immune thrombocytopenia, or a clotting factor deficiency (See “Is a novel anticoagulant right for your patient?”), but their use may cause or worsen menorrhagia. In such cases, the LNG-IUD appears to be an effective treatment.
In a retrospective case review of 28 women with menorrhagia secondary to various hemostatic disorders, 68% experienced improvement after insertion of the IUD.15 Another study compared women on anticoagulant therapy for cardiac valve disease (N=40) with and without the LNG-IUD. Compared with the control group, those with the IUD were found to have significant increases in hemoglobin levels 3 months after insertion.16
Obesity-related uterine bleeding. Obese women are at higher risk for excessive uterine bleeding, the result of increased conversion of plasma androstenedione to estrogen in adipose tissue. In one study evaluating the use of the LNG-IUD in this patient population, 75% of participants experienced a reduction in bleeding.17
Idiopathic HMB. The IUD is as good as, or better than, other treatments for idiopathic menorrhagia. It results in a significantly higher reduction in both blood loss and days out of work than OCPs.1 The device also reduces blood loss more effectively than oral medroxyprogesterone,18 another common approach to idiopathic HMB; and, compared with hysterectomy, it results in similar patient satisfaction—but lower costs and complication rates.19
In a randomized controlled trial that compared the LNG-IUD with tranexamic acid, mefenamic acid, combined estrogenprogesterone, or progesterone alone over a 2-year period, scores on a menorrhagia symptom scale were significantly higher (indicating greater improvement) in the LNG-IUD group.20
Medical management of endometrial proliferation
Endometrial hyperplasia, often found in women with abnormal uterine bleeding patterns and recurrent anovulatory cycles,21 is sometimes treated with supplemental progesterone. According to the Centers for Disease Control and Prevention (CDC)’s US Medical Eligibility Criteria for Contraceptive Use, the LNG-IUD can be used without restriction in this patient population, as well.22
A systematic review of 9 studies of women with endometrial hyperplasia without atypia found the LNG-IUD to be both safe and effective. In 7 of the 9 studies, 100% of participants experienced disease regression; regression rates for the other 2 studies were 90% and 67%.23 The only caveat: Both endometrial atypia and endometrial cancer should be excluded prior to IUD insertion.
Adenomyosis is caused by the presence of ectopic endometrial glands and stroma within the myometrium and frequently results in pelvic pain, menorrhagia, and dysmenorrhea.10 Hysterectomy is often regarded as the mainstay of treatment. But medical management with the LNG-IUD is also an option, as it has demonstrated similar improvements to hysterectomy both in hemoglobin levels and quality of life.24 Three years after insertion of the LNG-IUD to treat moderate or severe adenomyosis-associated dysmenorrhea, one study found, women reported significant improvement in their symptoms—and 73% were satisfied with their treatment.25 The LNG-IUD also appears to decrease uterine volume, although this effect may begin to decrease 2 years after insertion.26
Endometriosis, another common cause of dysmenorrhea and chronic pelvic pain,27 can also be treated with the LNG-IUD. The local progestin administration to pelvic structures that the device provides has been found to significantly decrease both endometrial proliferation and monthly blood flow.28 Additional studies of the LNG-IUD as a treatment for endometriosis and pelvic pain are ongoing and encouraging. After surgery for endometriosis, a Cochrane review found, women who had the LNG-IUD inserted had a lower rate of recurrence of dysmenorrhea than those without it.29
Helping women through perimenopause
Compared with oral or intramuscular progesterone therapy, the LNG-IUD has been found to be superior for the treatment of perimenopausal symptoms.30 Two years after insertion, one study found, perimenopausal women had a 95% reduction in blood loss and a 63% decrease in dysmenorrhea.31 The LNG-IUD also provides reliable endometrial protection for women receiving estrogen therapy32 and for those who are taking adjuvant tamoxifen because of a history of breast cancer.33,34
IUD insertion is a safe office procedure
The Society of Teachers of Family Medicine cites IUD insertion in its core list of routine procedures to be included in family medicine residency programs.35 The risk associated with insertion is small—uterine perforation occurs in about 2.6/1000 insertions36—and there is a small and transient increase in the risk of IUD-related infection in the first few weeks to months after insertion. IUD insertion does not increase the overall risk of pelvic inflammatory disease in women at low risk for sexually transmitted infections.37,38
Who is not a candidate?
While IUDs are safe for most women, there are several absolute contraindications to the LNG-IUD:
• current breast, cervical, or endometrial cancer
• current pelvic inflammatory disease, cervicitis, chlamydia, or gonorrhea
• having just had a septic abortion.38
Teach patients about the benefits and adverse effects
For women who are potential candidates for the LNG-IUD, education is vital. Evidence suggests that satisfaction levels are very high, provided patients receive adequate counseling about the benefits and adverse effects. Risks (of uterine perforation and infection) are small, as noted earlier.39
Contraceptive efficacy, of course, is a major benefit, and has been well documented: The LNG-IUD has an estimated failure rate of just 0.2%.40 Unlike user-dependent methods such as OCPs, the patch, and the ring, the IUD has a perfect-use failure rate that is the same as the typical use rate. Thus, it is an excellent choice for women who want to preserve their fertility yet avoid an unintended pregnancy. For women approaching menopause—a time when estrogen may be contraindicated—the LNG-IUD can safely protect women against unwanted pregnancy.
Lower cost, less invasive. The ability to treat HMB and dysmennorhea with an IUD inserted in a family practice setting, without referrals to specialists for additional invasive treatments, increases cost savings.19 In addition, the LNG-IUD is less invasive and generally more acceptable to women than hysterectomy, endometrial ablation, uterine artery embolization, and myomectomy.18,41 It leads to a greater reduction in menstrual bleeding than OCPs, oral progestins, tranexamic acid, and oral mefenamic acid.41 And, unlike some progestational agents, there is no evidence that the LNG-IUD has any adverse effects on bone density, vaginal tone, or urinary continence.42
Adverse effects. Vaginal spotting is the most commonly reported adverse effect associated with the LNG-IUD, particularly in the first 3 to 6 months postinsertion.42 The other common adverse effect is increased cramping, which some women experience in the first few months after insertion. Rarely women may experience ovarian cysts from unruptured follicles, which will regress on their own. Another potential problem is expulsion, which is more common in women who are using the device to control heavy bleeding (8.9%-13.6%).42 After the device is in place for several years, many women experience amenorrhea—a side effect that patients who have suffered from HMB and dysmenorrhea may consider a benefit.
CASE After counseling regarding her treatment options, Ms. K decides on the LNG-IUD, which her family physician inserts. At 3-month follow-up, she reports significantly less bleeding and decreased perimenstrual discomfort. If her workup had revealed adenomyosis or a hemostatic disorder, the LNG-IUD would still have been a first-line option.
CORRESPONDENCE
Erin Hendriks, MD, Wayne State University Family Medicine, 1135 West University Drive, Suite 250, Rochester Hills, MI 48307; [email protected]
1. Shabaan MM, Zakherah MS, El-Nashar SA, et al. Levonorgestrelreleasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomized clinical trial. Contraception. 2011;83:48-54.
2. Blumenthal PD, Dawson L, Hurskainen R. Cost-effectiveness and quality of life associated with heavy menstrual bleeding among women using the levonorgestrel-releasing intrauterine system. Int J Gynaecol Obstet. 2011;112:171-178.
3. de Souza S, Camargos A, Ferreira M, et al. Hemoglobin levels predict quality of life in women with heavy menstrual bleeding. Arch Gynecol Obstet. 2010;281:895-900.
4. Shankar M, Chi C, Kadir RA. Review of quality of life: menorrhagia in women with or without inherited bleeding disorders. Haemophilia. 2008;14:15-20.
5. Royal College of Obstetricians and Gynaecologists, London, National Collaborating Centre for Women’s and Children’s Health, Clinical guideline. Heavy menstrual bleeding. 2007. Available at: http://www.nice.org.uk/nicemedia/pdf/CG44NICEGuideline.pdf. Accessed December 11, 2013.
6. Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110:1091-1095.
7. Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monitor. 2008;14:CR24-CR31.
8. Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2013.
9. Skyla [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2013.
10. Ascher SM, Jha RC, Reinhold C. Benign myometrial conditions: leiomyomas and adenomyosis. Top Magn Reson Imaging. 2003;14:281-304.
11. Sayed GH, Zakherah MS, El-Nashar SA, et al. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. Int J Gynecol Obstet. 2011;112:126-130.
12. Heikinheimo O, Gemzell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system (LNG-IUS). Acta Obstet Gynecol Scand. 2012;91:3-9.
13. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55.
14. Wildemeersch D, Schacht E, Wildemeersch P. Treatment of primary and secondary dysmenorrhea with a novel ‘frameless’ intrauterine levonorgestrel-releasing drug delivery system: a pilot study. Eur J Contracept Reprod Health Care. 2001;6:192-198.
15. Schaedel ZE, Dolan G, Powell MC. The use of the levonorgestrelreleasing intrauterine system in the management of menorrhagia in women with hemostatic disorders. Am J Obstet Gynecol. 2005;193:1361-1363.
16. Kilic S, Yuksel B, Doganay M, et al. The effect of levonorgestrelreleasing intrauterine device on menorrhagia in women taking anticoagulant medication after cardiac valve replacement. Contraception. 2009;80:152-157.
17. Vilos GA, Marks J, Tureanu V, et al. The levonorgestrel intrauterine system is an effective treatment in selected obese women with abnormal uterine bleeding. J Minim Invasive Gynecol. 2011;18:75-80.
18. Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
19. Hurskainen R, Teperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463.
20. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med. 2013;368:128-137.
21. Sweet MG, Schmidt-Dalton TA, Weiss PM, et al. Evaluation and management of abnormal uterine bleeding in premenopausal women. Am Fam Physician. 2012;85:35-43.
22. United States Medical Eligibility Criteria (USMEC) for Contraceptive Use. Centers for Disease Control and Prevention Web site. 2010. Available at: http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/USMEC.htm. Updated September 18, 2013. Accessed April, 2012.
23. Whiteman MK, Zapata LB, Tepper NK, et al. Use of contraceptive methods among women with endometrial hyperplasia: a systematic review. Contraception. 2010;82:56-63.
24. Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95:497-502.
25. Sheng J, Zhang WY, Zhang JP, et al. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79:189-193.
26. Cho SH, Nam A, Kim HY, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198:373.e1-373.e7.
27. Anpalagan A, Condous G. Is there a role for use of levonorgestrel intrauterine system in women with chronic pelvic pain? J Minim Invasive Gynecol. 2008;15:663-666.
28. Vercellini P, Viganò P, Somigliana E. The role of the levonorgestrel-releasing intrauterine device in the management of symptomatic endometriosis. Curr Opinion Obstet Gynecol. 2005;17:359-365.
29. Abou-Setta AM, Al-Inany HG, Farquhar CM. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(4):CD005072.
30. Küçük T, Ertan K. Continuous oral or intramuscular medroxyprogesterone acetate versus the levonorgestrel releasing intrauterine system in the treatment of perimenopausal menorrhagia: a randomized, prospective, controlled clinical trial in female smokers. Clin Exp Obstet Gynecol. 2008;35:57-60.
31. Yoo HJ, Lee MA, Ko YB, et al. The efficacy of the levonorgestrel-releasing intrauterine system in perimenopausal women with menorrhagia or dysmenorrhea. Arch Gynecol Obstet. 2012;285:161-166.
32. Sitruk-Ware R. The levonorgestrel intrauterine system for use in peri- and postmenopausal women. Contraception. 2007;75(6 suppl):S155-S160.
33. Somboonporn W, Panna S, Temtanakitpaisan T, et al. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18:1060-1066.
34. Chin J, Konje JC, Hickey M. Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. Cochrane Database Syst Rev. 2009;(4):CD007245.
35. Nothnagle M, Sicilia JM, Forman S, et al; STFM Group on Hosptial Medicine and Procedural Training. Required procedural training in family medicine residency: a consensus statement. Fam Med. 2008;40:248-252.
36. Van Houdenhoven K, van Kaam KJ, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
37. Grimes DA, Schulz KF. Antibiotic prophylaxis for intrauterine contraceptive device insertion. Cochrane Database Syst Rev. 2001;(2):CD001327.
38. Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
39. Backman T, Huhtala S, Luoto R, et al. Advance information improves user satisfaction with the levonorgestrel intrauterine system. Obstet Gynecol. 2002;99:608-613.
40. Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397-404.
41. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
42. Zieman M, Hatcher RA, Cwiak C, et al. Managing Contraception for Your Pocket 2010-2012. 10th ed. New York, NY: Ardent Media; 2010.
› Recommend the 52 mg levonorgestrel-releasing intrauterine device (LNG-IUD) as a first-line treatment for heavy menstrual bleeding. A
› Advise patients with dysmenorrhea that the 52 mg LNG-IUD is an effective nonsurgical treatment. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane K, a 40-year-old multiparous woman, is seeking treatment for heavy menstrual bleeding and cramping, both of which have troubled her for 4 years. Another physician had given her oral contraceptive pills (OCPs) to decrease the pain and bleeding, she reports, but she has difficulty remembering to take a pill every day.
On physical exam, you note an enlarged uterus (approximately 16-week size). A pregnancy test is negative, her thyroidstimulating hormone level is normal, and her hemoglobin is 9 g/dL. Transvaginal ultrasound reveals multiple fibroids.
What can you offer her?
At some point in their reproductive years, 10% to 15% of women experience heavy menstrual bleeding (HMB), or menorrhagia.1,2 In fact, HMB and dysmenorrhea are among the most common reasons for office visits and missed work among women in this age group.3,4 In addition to having a negative impact on quality of life, HMB can cause severe anemia.5
All too often, the suggested solution is a hysterectomy. In fact, 90% of the more than 600,000 hysterectomies performed annually in the United States are for benign disease.6,7 Yet many women with HMB want nonsurgical treatments, and some seek to preserve their fertility. A progestin IUD can often fulfill both of these desires.
An IUD containing 52 mg levonorgestrel (Mirena) has US Food and Drug Administration (FDA) approval both for use as a contraceptive and for the treatment of HMB in women who want intrauterine contraception.8 Recent studies have confirmed its efficacy in treating a wide variety of conditions associated with menorrhagia and dysmenorrhea. In 2013, a smaller, lower dose (13.5 mg) levonorgestrel-releasing IUD (Skyla) received FDA approval as a contraceptive.9 But because this device has been neither tested nor approved for other applications, the following review applies only to Mirena—referred to throughout this article as the LNG-IUD.
A proven (and often superior) treatment for menorrhagia
Leiomyomas, or fibroids—the most common benign tumor of the female genital tract—are a frequent cause of menorrhagia.10 OCPs are often used for the treatment of symptomatic fibroids for women who wish to avoid surgery. When compared with low-dose OCPs for the treatment of menorrhagia secondary to fibroids, however, the LNG-IUD resulted in a significantly greater reduction in blood loss.11
While fibroid size does not appear to decrease significantly after insertion of the device, a systematic review found that menstrual bleeding lessens and hemoglobin levels improve in women with symptomatic fibroids.12,13 The LNG-IUD has also been shown to improve symptoms in women with dysmenorrhea secondary to fibroids.14
Hemostatic disorders. Anticoagulants are vital for women with hemostatic disorders such as Von Willebrand disease, immune thrombocytopenia, or a clotting factor deficiency (See “Is a novel anticoagulant right for your patient?”), but their use may cause or worsen menorrhagia. In such cases, the LNG-IUD appears to be an effective treatment.
In a retrospective case review of 28 women with menorrhagia secondary to various hemostatic disorders, 68% experienced improvement after insertion of the IUD.15 Another study compared women on anticoagulant therapy for cardiac valve disease (N=40) with and without the LNG-IUD. Compared with the control group, those with the IUD were found to have significant increases in hemoglobin levels 3 months after insertion.16
Obesity-related uterine bleeding. Obese women are at higher risk for excessive uterine bleeding, the result of increased conversion of plasma androstenedione to estrogen in adipose tissue. In one study evaluating the use of the LNG-IUD in this patient population, 75% of participants experienced a reduction in bleeding.17
Idiopathic HMB. The IUD is as good as, or better than, other treatments for idiopathic menorrhagia. It results in a significantly higher reduction in both blood loss and days out of work than OCPs.1 The device also reduces blood loss more effectively than oral medroxyprogesterone,18 another common approach to idiopathic HMB; and, compared with hysterectomy, it results in similar patient satisfaction—but lower costs and complication rates.19
In a randomized controlled trial that compared the LNG-IUD with tranexamic acid, mefenamic acid, combined estrogenprogesterone, or progesterone alone over a 2-year period, scores on a menorrhagia symptom scale were significantly higher (indicating greater improvement) in the LNG-IUD group.20
Medical management of endometrial proliferation
Endometrial hyperplasia, often found in women with abnormal uterine bleeding patterns and recurrent anovulatory cycles,21 is sometimes treated with supplemental progesterone. According to the Centers for Disease Control and Prevention (CDC)’s US Medical Eligibility Criteria for Contraceptive Use, the LNG-IUD can be used without restriction in this patient population, as well.22
A systematic review of 9 studies of women with endometrial hyperplasia without atypia found the LNG-IUD to be both safe and effective. In 7 of the 9 studies, 100% of participants experienced disease regression; regression rates for the other 2 studies were 90% and 67%.23 The only caveat: Both endometrial atypia and endometrial cancer should be excluded prior to IUD insertion.
Adenomyosis is caused by the presence of ectopic endometrial glands and stroma within the myometrium and frequently results in pelvic pain, menorrhagia, and dysmenorrhea.10 Hysterectomy is often regarded as the mainstay of treatment. But medical management with the LNG-IUD is also an option, as it has demonstrated similar improvements to hysterectomy both in hemoglobin levels and quality of life.24 Three years after insertion of the LNG-IUD to treat moderate or severe adenomyosis-associated dysmenorrhea, one study found, women reported significant improvement in their symptoms—and 73% were satisfied with their treatment.25 The LNG-IUD also appears to decrease uterine volume, although this effect may begin to decrease 2 years after insertion.26
Endometriosis, another common cause of dysmenorrhea and chronic pelvic pain,27 can also be treated with the LNG-IUD. The local progestin administration to pelvic structures that the device provides has been found to significantly decrease both endometrial proliferation and monthly blood flow.28 Additional studies of the LNG-IUD as a treatment for endometriosis and pelvic pain are ongoing and encouraging. After surgery for endometriosis, a Cochrane review found, women who had the LNG-IUD inserted had a lower rate of recurrence of dysmenorrhea than those without it.29
Helping women through perimenopause
Compared with oral or intramuscular progesterone therapy, the LNG-IUD has been found to be superior for the treatment of perimenopausal symptoms.30 Two years after insertion, one study found, perimenopausal women had a 95% reduction in blood loss and a 63% decrease in dysmenorrhea.31 The LNG-IUD also provides reliable endometrial protection for women receiving estrogen therapy32 and for those who are taking adjuvant tamoxifen because of a history of breast cancer.33,34
IUD insertion is a safe office procedure
The Society of Teachers of Family Medicine cites IUD insertion in its core list of routine procedures to be included in family medicine residency programs.35 The risk associated with insertion is small—uterine perforation occurs in about 2.6/1000 insertions36—and there is a small and transient increase in the risk of IUD-related infection in the first few weeks to months after insertion. IUD insertion does not increase the overall risk of pelvic inflammatory disease in women at low risk for sexually transmitted infections.37,38
Who is not a candidate?
While IUDs are safe for most women, there are several absolute contraindications to the LNG-IUD:
• current breast, cervical, or endometrial cancer
• current pelvic inflammatory disease, cervicitis, chlamydia, or gonorrhea
• having just had a septic abortion.38
Teach patients about the benefits and adverse effects
For women who are potential candidates for the LNG-IUD, education is vital. Evidence suggests that satisfaction levels are very high, provided patients receive adequate counseling about the benefits and adverse effects. Risks (of uterine perforation and infection) are small, as noted earlier.39
Contraceptive efficacy, of course, is a major benefit, and has been well documented: The LNG-IUD has an estimated failure rate of just 0.2%.40 Unlike user-dependent methods such as OCPs, the patch, and the ring, the IUD has a perfect-use failure rate that is the same as the typical use rate. Thus, it is an excellent choice for women who want to preserve their fertility yet avoid an unintended pregnancy. For women approaching menopause—a time when estrogen may be contraindicated—the LNG-IUD can safely protect women against unwanted pregnancy.
Lower cost, less invasive. The ability to treat HMB and dysmennorhea with an IUD inserted in a family practice setting, without referrals to specialists for additional invasive treatments, increases cost savings.19 In addition, the LNG-IUD is less invasive and generally more acceptable to women than hysterectomy, endometrial ablation, uterine artery embolization, and myomectomy.18,41 It leads to a greater reduction in menstrual bleeding than OCPs, oral progestins, tranexamic acid, and oral mefenamic acid.41 And, unlike some progestational agents, there is no evidence that the LNG-IUD has any adverse effects on bone density, vaginal tone, or urinary continence.42
Adverse effects. Vaginal spotting is the most commonly reported adverse effect associated with the LNG-IUD, particularly in the first 3 to 6 months postinsertion.42 The other common adverse effect is increased cramping, which some women experience in the first few months after insertion. Rarely women may experience ovarian cysts from unruptured follicles, which will regress on their own. Another potential problem is expulsion, which is more common in women who are using the device to control heavy bleeding (8.9%-13.6%).42 After the device is in place for several years, many women experience amenorrhea—a side effect that patients who have suffered from HMB and dysmenorrhea may consider a benefit.
CASE After counseling regarding her treatment options, Ms. K decides on the LNG-IUD, which her family physician inserts. At 3-month follow-up, she reports significantly less bleeding and decreased perimenstrual discomfort. If her workup had revealed adenomyosis or a hemostatic disorder, the LNG-IUD would still have been a first-line option.
CORRESPONDENCE
Erin Hendriks, MD, Wayne State University Family Medicine, 1135 West University Drive, Suite 250, Rochester Hills, MI 48307; [email protected]
› Recommend the 52 mg levonorgestrel-releasing intrauterine device (LNG-IUD) as a first-line treatment for heavy menstrual bleeding. A
› Advise patients with dysmenorrhea that the 52 mg LNG-IUD is an effective nonsurgical treatment. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane K, a 40-year-old multiparous woman, is seeking treatment for heavy menstrual bleeding and cramping, both of which have troubled her for 4 years. Another physician had given her oral contraceptive pills (OCPs) to decrease the pain and bleeding, she reports, but she has difficulty remembering to take a pill every day.
On physical exam, you note an enlarged uterus (approximately 16-week size). A pregnancy test is negative, her thyroidstimulating hormone level is normal, and her hemoglobin is 9 g/dL. Transvaginal ultrasound reveals multiple fibroids.
What can you offer her?
At some point in their reproductive years, 10% to 15% of women experience heavy menstrual bleeding (HMB), or menorrhagia.1,2 In fact, HMB and dysmenorrhea are among the most common reasons for office visits and missed work among women in this age group.3,4 In addition to having a negative impact on quality of life, HMB can cause severe anemia.5
All too often, the suggested solution is a hysterectomy. In fact, 90% of the more than 600,000 hysterectomies performed annually in the United States are for benign disease.6,7 Yet many women with HMB want nonsurgical treatments, and some seek to preserve their fertility. A progestin IUD can often fulfill both of these desires.
An IUD containing 52 mg levonorgestrel (Mirena) has US Food and Drug Administration (FDA) approval both for use as a contraceptive and for the treatment of HMB in women who want intrauterine contraception.8 Recent studies have confirmed its efficacy in treating a wide variety of conditions associated with menorrhagia and dysmenorrhea. In 2013, a smaller, lower dose (13.5 mg) levonorgestrel-releasing IUD (Skyla) received FDA approval as a contraceptive.9 But because this device has been neither tested nor approved for other applications, the following review applies only to Mirena—referred to throughout this article as the LNG-IUD.
A proven (and often superior) treatment for menorrhagia
Leiomyomas, or fibroids—the most common benign tumor of the female genital tract—are a frequent cause of menorrhagia.10 OCPs are often used for the treatment of symptomatic fibroids for women who wish to avoid surgery. When compared with low-dose OCPs for the treatment of menorrhagia secondary to fibroids, however, the LNG-IUD resulted in a significantly greater reduction in blood loss.11
While fibroid size does not appear to decrease significantly after insertion of the device, a systematic review found that menstrual bleeding lessens and hemoglobin levels improve in women with symptomatic fibroids.12,13 The LNG-IUD has also been shown to improve symptoms in women with dysmenorrhea secondary to fibroids.14
Hemostatic disorders. Anticoagulants are vital for women with hemostatic disorders such as Von Willebrand disease, immune thrombocytopenia, or a clotting factor deficiency (See “Is a novel anticoagulant right for your patient?”), but their use may cause or worsen menorrhagia. In such cases, the LNG-IUD appears to be an effective treatment.
In a retrospective case review of 28 women with menorrhagia secondary to various hemostatic disorders, 68% experienced improvement after insertion of the IUD.15 Another study compared women on anticoagulant therapy for cardiac valve disease (N=40) with and without the LNG-IUD. Compared with the control group, those with the IUD were found to have significant increases in hemoglobin levels 3 months after insertion.16
Obesity-related uterine bleeding. Obese women are at higher risk for excessive uterine bleeding, the result of increased conversion of plasma androstenedione to estrogen in adipose tissue. In one study evaluating the use of the LNG-IUD in this patient population, 75% of participants experienced a reduction in bleeding.17
Idiopathic HMB. The IUD is as good as, or better than, other treatments for idiopathic menorrhagia. It results in a significantly higher reduction in both blood loss and days out of work than OCPs.1 The device also reduces blood loss more effectively than oral medroxyprogesterone,18 another common approach to idiopathic HMB; and, compared with hysterectomy, it results in similar patient satisfaction—but lower costs and complication rates.19
In a randomized controlled trial that compared the LNG-IUD with tranexamic acid, mefenamic acid, combined estrogenprogesterone, or progesterone alone over a 2-year period, scores on a menorrhagia symptom scale were significantly higher (indicating greater improvement) in the LNG-IUD group.20
Medical management of endometrial proliferation
Endometrial hyperplasia, often found in women with abnormal uterine bleeding patterns and recurrent anovulatory cycles,21 is sometimes treated with supplemental progesterone. According to the Centers for Disease Control and Prevention (CDC)’s US Medical Eligibility Criteria for Contraceptive Use, the LNG-IUD can be used without restriction in this patient population, as well.22
A systematic review of 9 studies of women with endometrial hyperplasia without atypia found the LNG-IUD to be both safe and effective. In 7 of the 9 studies, 100% of participants experienced disease regression; regression rates for the other 2 studies were 90% and 67%.23 The only caveat: Both endometrial atypia and endometrial cancer should be excluded prior to IUD insertion.
Adenomyosis is caused by the presence of ectopic endometrial glands and stroma within the myometrium and frequently results in pelvic pain, menorrhagia, and dysmenorrhea.10 Hysterectomy is often regarded as the mainstay of treatment. But medical management with the LNG-IUD is also an option, as it has demonstrated similar improvements to hysterectomy both in hemoglobin levels and quality of life.24 Three years after insertion of the LNG-IUD to treat moderate or severe adenomyosis-associated dysmenorrhea, one study found, women reported significant improvement in their symptoms—and 73% were satisfied with their treatment.25 The LNG-IUD also appears to decrease uterine volume, although this effect may begin to decrease 2 years after insertion.26
Endometriosis, another common cause of dysmenorrhea and chronic pelvic pain,27 can also be treated with the LNG-IUD. The local progestin administration to pelvic structures that the device provides has been found to significantly decrease both endometrial proliferation and monthly blood flow.28 Additional studies of the LNG-IUD as a treatment for endometriosis and pelvic pain are ongoing and encouraging. After surgery for endometriosis, a Cochrane review found, women who had the LNG-IUD inserted had a lower rate of recurrence of dysmenorrhea than those without it.29
Helping women through perimenopause
Compared with oral or intramuscular progesterone therapy, the LNG-IUD has been found to be superior for the treatment of perimenopausal symptoms.30 Two years after insertion, one study found, perimenopausal women had a 95% reduction in blood loss and a 63% decrease in dysmenorrhea.31 The LNG-IUD also provides reliable endometrial protection for women receiving estrogen therapy32 and for those who are taking adjuvant tamoxifen because of a history of breast cancer.33,34
IUD insertion is a safe office procedure
The Society of Teachers of Family Medicine cites IUD insertion in its core list of routine procedures to be included in family medicine residency programs.35 The risk associated with insertion is small—uterine perforation occurs in about 2.6/1000 insertions36—and there is a small and transient increase in the risk of IUD-related infection in the first few weeks to months after insertion. IUD insertion does not increase the overall risk of pelvic inflammatory disease in women at low risk for sexually transmitted infections.37,38
Who is not a candidate?
While IUDs are safe for most women, there are several absolute contraindications to the LNG-IUD:
• current breast, cervical, or endometrial cancer
• current pelvic inflammatory disease, cervicitis, chlamydia, or gonorrhea
• having just had a septic abortion.38
Teach patients about the benefits and adverse effects
For women who are potential candidates for the LNG-IUD, education is vital. Evidence suggests that satisfaction levels are very high, provided patients receive adequate counseling about the benefits and adverse effects. Risks (of uterine perforation and infection) are small, as noted earlier.39
Contraceptive efficacy, of course, is a major benefit, and has been well documented: The LNG-IUD has an estimated failure rate of just 0.2%.40 Unlike user-dependent methods such as OCPs, the patch, and the ring, the IUD has a perfect-use failure rate that is the same as the typical use rate. Thus, it is an excellent choice for women who want to preserve their fertility yet avoid an unintended pregnancy. For women approaching menopause—a time when estrogen may be contraindicated—the LNG-IUD can safely protect women against unwanted pregnancy.
Lower cost, less invasive. The ability to treat HMB and dysmennorhea with an IUD inserted in a family practice setting, without referrals to specialists for additional invasive treatments, increases cost savings.19 In addition, the LNG-IUD is less invasive and generally more acceptable to women than hysterectomy, endometrial ablation, uterine artery embolization, and myomectomy.18,41 It leads to a greater reduction in menstrual bleeding than OCPs, oral progestins, tranexamic acid, and oral mefenamic acid.41 And, unlike some progestational agents, there is no evidence that the LNG-IUD has any adverse effects on bone density, vaginal tone, or urinary continence.42
Adverse effects. Vaginal spotting is the most commonly reported adverse effect associated with the LNG-IUD, particularly in the first 3 to 6 months postinsertion.42 The other common adverse effect is increased cramping, which some women experience in the first few months after insertion. Rarely women may experience ovarian cysts from unruptured follicles, which will regress on their own. Another potential problem is expulsion, which is more common in women who are using the device to control heavy bleeding (8.9%-13.6%).42 After the device is in place for several years, many women experience amenorrhea—a side effect that patients who have suffered from HMB and dysmenorrhea may consider a benefit.
CASE After counseling regarding her treatment options, Ms. K decides on the LNG-IUD, which her family physician inserts. At 3-month follow-up, she reports significantly less bleeding and decreased perimenstrual discomfort. If her workup had revealed adenomyosis or a hemostatic disorder, the LNG-IUD would still have been a first-line option.
CORRESPONDENCE
Erin Hendriks, MD, Wayne State University Family Medicine, 1135 West University Drive, Suite 250, Rochester Hills, MI 48307; [email protected]
1. Shabaan MM, Zakherah MS, El-Nashar SA, et al. Levonorgestrelreleasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomized clinical trial. Contraception. 2011;83:48-54.
2. Blumenthal PD, Dawson L, Hurskainen R. Cost-effectiveness and quality of life associated with heavy menstrual bleeding among women using the levonorgestrel-releasing intrauterine system. Int J Gynaecol Obstet. 2011;112:171-178.
3. de Souza S, Camargos A, Ferreira M, et al. Hemoglobin levels predict quality of life in women with heavy menstrual bleeding. Arch Gynecol Obstet. 2010;281:895-900.
4. Shankar M, Chi C, Kadir RA. Review of quality of life: menorrhagia in women with or without inherited bleeding disorders. Haemophilia. 2008;14:15-20.
5. Royal College of Obstetricians and Gynaecologists, London, National Collaborating Centre for Women’s and Children’s Health, Clinical guideline. Heavy menstrual bleeding. 2007. Available at: http://www.nice.org.uk/nicemedia/pdf/CG44NICEGuideline.pdf. Accessed December 11, 2013.
6. Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110:1091-1095.
7. Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monitor. 2008;14:CR24-CR31.
8. Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2013.
9. Skyla [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2013.
10. Ascher SM, Jha RC, Reinhold C. Benign myometrial conditions: leiomyomas and adenomyosis. Top Magn Reson Imaging. 2003;14:281-304.
11. Sayed GH, Zakherah MS, El-Nashar SA, et al. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. Int J Gynecol Obstet. 2011;112:126-130.
12. Heikinheimo O, Gemzell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system (LNG-IUS). Acta Obstet Gynecol Scand. 2012;91:3-9.
13. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55.
14. Wildemeersch D, Schacht E, Wildemeersch P. Treatment of primary and secondary dysmenorrhea with a novel ‘frameless’ intrauterine levonorgestrel-releasing drug delivery system: a pilot study. Eur J Contracept Reprod Health Care. 2001;6:192-198.
15. Schaedel ZE, Dolan G, Powell MC. The use of the levonorgestrelreleasing intrauterine system in the management of menorrhagia in women with hemostatic disorders. Am J Obstet Gynecol. 2005;193:1361-1363.
16. Kilic S, Yuksel B, Doganay M, et al. The effect of levonorgestrelreleasing intrauterine device on menorrhagia in women taking anticoagulant medication after cardiac valve replacement. Contraception. 2009;80:152-157.
17. Vilos GA, Marks J, Tureanu V, et al. The levonorgestrel intrauterine system is an effective treatment in selected obese women with abnormal uterine bleeding. J Minim Invasive Gynecol. 2011;18:75-80.
18. Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
19. Hurskainen R, Teperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463.
20. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med. 2013;368:128-137.
21. Sweet MG, Schmidt-Dalton TA, Weiss PM, et al. Evaluation and management of abnormal uterine bleeding in premenopausal women. Am Fam Physician. 2012;85:35-43.
22. United States Medical Eligibility Criteria (USMEC) for Contraceptive Use. Centers for Disease Control and Prevention Web site. 2010. Available at: http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/USMEC.htm. Updated September 18, 2013. Accessed April, 2012.
23. Whiteman MK, Zapata LB, Tepper NK, et al. Use of contraceptive methods among women with endometrial hyperplasia: a systematic review. Contraception. 2010;82:56-63.
24. Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95:497-502.
25. Sheng J, Zhang WY, Zhang JP, et al. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79:189-193.
26. Cho SH, Nam A, Kim HY, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198:373.e1-373.e7.
27. Anpalagan A, Condous G. Is there a role for use of levonorgestrel intrauterine system in women with chronic pelvic pain? J Minim Invasive Gynecol. 2008;15:663-666.
28. Vercellini P, Viganò P, Somigliana E. The role of the levonorgestrel-releasing intrauterine device in the management of symptomatic endometriosis. Curr Opinion Obstet Gynecol. 2005;17:359-365.
29. Abou-Setta AM, Al-Inany HG, Farquhar CM. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(4):CD005072.
30. Küçük T, Ertan K. Continuous oral or intramuscular medroxyprogesterone acetate versus the levonorgestrel releasing intrauterine system in the treatment of perimenopausal menorrhagia: a randomized, prospective, controlled clinical trial in female smokers. Clin Exp Obstet Gynecol. 2008;35:57-60.
31. Yoo HJ, Lee MA, Ko YB, et al. The efficacy of the levonorgestrel-releasing intrauterine system in perimenopausal women with menorrhagia or dysmenorrhea. Arch Gynecol Obstet. 2012;285:161-166.
32. Sitruk-Ware R. The levonorgestrel intrauterine system for use in peri- and postmenopausal women. Contraception. 2007;75(6 suppl):S155-S160.
33. Somboonporn W, Panna S, Temtanakitpaisan T, et al. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18:1060-1066.
34. Chin J, Konje JC, Hickey M. Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. Cochrane Database Syst Rev. 2009;(4):CD007245.
35. Nothnagle M, Sicilia JM, Forman S, et al; STFM Group on Hosptial Medicine and Procedural Training. Required procedural training in family medicine residency: a consensus statement. Fam Med. 2008;40:248-252.
36. Van Houdenhoven K, van Kaam KJ, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
37. Grimes DA, Schulz KF. Antibiotic prophylaxis for intrauterine contraceptive device insertion. Cochrane Database Syst Rev. 2001;(2):CD001327.
38. Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
39. Backman T, Huhtala S, Luoto R, et al. Advance information improves user satisfaction with the levonorgestrel intrauterine system. Obstet Gynecol. 2002;99:608-613.
40. Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397-404.
41. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
42. Zieman M, Hatcher RA, Cwiak C, et al. Managing Contraception for Your Pocket 2010-2012. 10th ed. New York, NY: Ardent Media; 2010.
1. Shabaan MM, Zakherah MS, El-Nashar SA, et al. Levonorgestrelreleasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomized clinical trial. Contraception. 2011;83:48-54.
2. Blumenthal PD, Dawson L, Hurskainen R. Cost-effectiveness and quality of life associated with heavy menstrual bleeding among women using the levonorgestrel-releasing intrauterine system. Int J Gynaecol Obstet. 2011;112:171-178.
3. de Souza S, Camargos A, Ferreira M, et al. Hemoglobin levels predict quality of life in women with heavy menstrual bleeding. Arch Gynecol Obstet. 2010;281:895-900.
4. Shankar M, Chi C, Kadir RA. Review of quality of life: menorrhagia in women with or without inherited bleeding disorders. Haemophilia. 2008;14:15-20.
5. Royal College of Obstetricians and Gynaecologists, London, National Collaborating Centre for Women’s and Children’s Health, Clinical guideline. Heavy menstrual bleeding. 2007. Available at: http://www.nice.org.uk/nicemedia/pdf/CG44NICEGuideline.pdf. Accessed December 11, 2013.
6. Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110:1091-1095.
7. Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monitor. 2008;14:CR24-CR31.
8. Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2013.
9. Skyla [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2013.
10. Ascher SM, Jha RC, Reinhold C. Benign myometrial conditions: leiomyomas and adenomyosis. Top Magn Reson Imaging. 2003;14:281-304.
11. Sayed GH, Zakherah MS, El-Nashar SA, et al. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. Int J Gynecol Obstet. 2011;112:126-130.
12. Heikinheimo O, Gemzell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system (LNG-IUS). Acta Obstet Gynecol Scand. 2012;91:3-9.
13. Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55.
14. Wildemeersch D, Schacht E, Wildemeersch P. Treatment of primary and secondary dysmenorrhea with a novel ‘frameless’ intrauterine levonorgestrel-releasing drug delivery system: a pilot study. Eur J Contracept Reprod Health Care. 2001;6:192-198.
15. Schaedel ZE, Dolan G, Powell MC. The use of the levonorgestrelreleasing intrauterine system in the management of menorrhagia in women with hemostatic disorders. Am J Obstet Gynecol. 2005;193:1361-1363.
16. Kilic S, Yuksel B, Doganay M, et al. The effect of levonorgestrelreleasing intrauterine device on menorrhagia in women taking anticoagulant medication after cardiac valve replacement. Contraception. 2009;80:152-157.
17. Vilos GA, Marks J, Tureanu V, et al. The levonorgestrel intrauterine system is an effective treatment in selected obese women with abnormal uterine bleeding. J Minim Invasive Gynecol. 2011;18:75-80.
18. Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
19. Hurskainen R, Teperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463.
20. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med. 2013;368:128-137.
21. Sweet MG, Schmidt-Dalton TA, Weiss PM, et al. Evaluation and management of abnormal uterine bleeding in premenopausal women. Am Fam Physician. 2012;85:35-43.
22. United States Medical Eligibility Criteria (USMEC) for Contraceptive Use. Centers for Disease Control and Prevention Web site. 2010. Available at: http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/USMEC.htm. Updated September 18, 2013. Accessed April, 2012.
23. Whiteman MK, Zapata LB, Tepper NK, et al. Use of contraceptive methods among women with endometrial hyperplasia: a systematic review. Contraception. 2010;82:56-63.
24. Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95:497-502.
25. Sheng J, Zhang WY, Zhang JP, et al. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79:189-193.
26. Cho SH, Nam A, Kim HY, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198:373.e1-373.e7.
27. Anpalagan A, Condous G. Is there a role for use of levonorgestrel intrauterine system in women with chronic pelvic pain? J Minim Invasive Gynecol. 2008;15:663-666.
28. Vercellini P, Viganò P, Somigliana E. The role of the levonorgestrel-releasing intrauterine device in the management of symptomatic endometriosis. Curr Opinion Obstet Gynecol. 2005;17:359-365.
29. Abou-Setta AM, Al-Inany HG, Farquhar CM. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(4):CD005072.
30. Küçük T, Ertan K. Continuous oral or intramuscular medroxyprogesterone acetate versus the levonorgestrel releasing intrauterine system in the treatment of perimenopausal menorrhagia: a randomized, prospective, controlled clinical trial in female smokers. Clin Exp Obstet Gynecol. 2008;35:57-60.
31. Yoo HJ, Lee MA, Ko YB, et al. The efficacy of the levonorgestrel-releasing intrauterine system in perimenopausal women with menorrhagia or dysmenorrhea. Arch Gynecol Obstet. 2012;285:161-166.
32. Sitruk-Ware R. The levonorgestrel intrauterine system for use in peri- and postmenopausal women. Contraception. 2007;75(6 suppl):S155-S160.
33. Somboonporn W, Panna S, Temtanakitpaisan T, et al. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18:1060-1066.
34. Chin J, Konje JC, Hickey M. Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. Cochrane Database Syst Rev. 2009;(4):CD007245.
35. Nothnagle M, Sicilia JM, Forman S, et al; STFM Group on Hosptial Medicine and Procedural Training. Required procedural training in family medicine residency: a consensus statement. Fam Med. 2008;40:248-252.
36. Van Houdenhoven K, van Kaam KJ, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
37. Grimes DA, Schulz KF. Antibiotic prophylaxis for intrauterine contraceptive device insertion. Cochrane Database Syst Rev. 2001;(2):CD001327.
38. Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
39. Backman T, Huhtala S, Luoto R, et al. Advance information improves user satisfaction with the levonorgestrel intrauterine system. Obstet Gynecol. 2002;99:608-613.
40. Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397-404.
41. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
42. Zieman M, Hatcher RA, Cwiak C, et al. Managing Contraception for Your Pocket 2010-2012. 10th ed. New York, NY: Ardent Media; 2010.
Neuroendocrine dysfunction following mild TBI: When to screen for it
› Consider neuroendocrine dysfunction (NED) following confirmed traumatic brain injury of any severity when symptoms suggestive of NED persist for >3 months after injury. A
› Order blood studies to detect deficiencies in pituitary and other key hormones when NED is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Centers for Disease Control and Prevention (CDC) reports that >1.7 million cases of traumatic brain injury (TBI) occur annually in the United States.1 More than 266,000 military service members sustained at least one TBI from 2000 to 2012.2 Most TBIs (80%-85%), military and civilian, are classified as mild (mTBI), and most mTBI patients (80%-85%) experience a complete functional recovery within 3 months of injury.1,3 The remaining 15% to 20% of mTBI patients experience persistent symptoms and difficulty in rehabilitation, particularly if there are concomitant disorders, such as post-traumatic stress disorder (PTSD), sleep disorders, acute stress disorder, substance abuse disorder, and depression.4,5 Symptoms that mTBI and these other disorders have in common can make differential diagnosis difficult, requiring a high degree of clinical awareness by primary care providers. An additional concern following mTBI is neuroendocrine dysfunction (NED). This association has not been widely discussed and therefore may go largely undiagnosed.6 Consider NED in the setting of prolonged symptoms or in patients experiencing difficulty with rehabilitation following mTBI.7,8
NED following mTBI is more common than once thought
The term “neuroendocrine dysfunction,” as discussed in this article, refers to a variety of conditions caused by imbalances in the body’s hormone production directly related to the pituitary, hypothalamus, and their axes following TBI. Until the past decade, the incidence of TBI-associated pituitary dysfunction was thought to be an uncommon event, usually associated with catastrophic head injuries. Studies of NED in TBI patients focused primarily on moderate or severe TBI, usually from motor vehicle incidents, falls, and assaults.7 Other research has since shown that NED occurs more commonly than once believed.9 And while the risk of NED may be higher for patients who sustain more severe brain injuries, NED also occurs in mTBI.7,9,10,11 Interestingly, a recent literature review indicated that the incidence of NED in mTBI was 16.8%, while the incidence with moderate TBI was reported at 10.9%.7 Other research has noted that the incidence of NED in mTBI may be as high as 42%.9,12 No evidence suggests that the severity of NED is related to a specific hormonal dysfunction, nor is there evidence that NED may be associated with a specific mechanism of injury.
Pituitary anatomy is susceptible to injury and dysfunction
The anatomic and physiologic complexities of the hypothalamus and pituitary gland increase their susceptibility to injury from TBI. The pituitary gland is connected to the hypothalamus by a blood vessel-containing stalk, making the pituitary gland—particularly the anterior portion—susceptible to damage during a head injury.13 The hypothalamus secretes thyrotropin-releasing hormone (TRH) and luteinizing-releasing hormone (LRH) to stimulate or suppress the production of anterior pituitary gland hormones, which in turn stimulate the release of hormones and other substances from target organs. Anterior pituitary hormones are growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), and prolactin (PRL). The posterior pituitary secretes oxytocin and vasopressin, also known as antidiuretic hormone (ADH).13
Impact from a direct blow with an object or from a concussive blast can cause focal trauma or rotational shearing of tissue internally. Resultant vascular injury, rupture, cerebral edema, vasospasm, pituitary swelling, or inflammation may then initiate an endocrine response that drives a cascade of complex hormonal processes.5,7,8 Anterior pituitary deficiencies account for the majority of chronic neuroendocrine disorders following mTBI. GH and gonadotropin deficiencies are the most common, but TSH deficiency (secondary hypothyroidism) and ACTH deficiency (adrenal insufficiency) may occur as well, although in <10% of cases with TBI associated NED.12
Clinical features of NED mimic those of other conditions
The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances (TABLE).7,12,14-17 Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, postconcussive syndrome (PCS), and memory and attention difficulties.18 The onset of NED may be immediate (eg, in diabetes insipidus [DI] or syndrome of inappropriate antidiuretic hormone [SIADH], which are very rare in mTBI) and potentially life-threatening (eg, in sodium and potassium imbalances), or may be nonspecific and take years to manifest.6,10,15,19 Additionally, symptoms of NED may spontaneously resolve or persist. Studies have demonstrated pituitary dysfunction in the acute postinjury phase as well as its development as late as 2 to 3 years after injury.7,8,11,20
Due to the range of symptoms related to the combinations of possible hormonal derangements, NED can be an elusive diagnosis and may have a deleterious effect on individuals who sustain TBI.12 For example, an undiagnosed GH deficiency—which can result in increased abdominal fat mass and decreased lean muscle mass as well as impaired cardiac function, dyslipidemia, and insulin resistance—makes it more difficult for an affected individual either to recover from additional injuries or to maintain fitness. Considering NED may avoid a delay in diagnosis and improve prognosis.7,8,20
Findings leading to recommendations on diagnosis
Primary care providers, military and civilian alike, can benefit from the findings and recommendations of an expert panel assembled by the Defense Centers of Excellence (DCoE) for Psychological Health and Traumatic Brain Injury to address NED in mTBI. The panel that convened in December 2010 included experts representing the military services, the Department of Veterans Affairs, DCoE, and civilian sectors. Based on the group’s recommendations combined with literature review findings, the DCoE developed a clinical recommendation to encourage primary care providers to consider screening for NED in patients with persistent symptoms following mTBI.21 Key findings and issues identified by the group included the following:
• The most frequent mechanism of injury in the military deployed population is blast-induced TBI. Such injury could occur in the civilian population at construction blast sites or in factories producing or using highly flammable substances.
• The prevalence of any anterior pituitary hormone deficiency is as high as 30% to hormone deficiency is as high as 30% to 80% at 24 to 36 months post injury.
• The prevalence of posterior pituitary hormone deficiency is as high as 4% to 7% at 12 months post injury.
• The anterior pituitary hormones most frequently affected in survivors of TBI are ACTH, gonadotropin, prolactin, and GH.
• In 2004 Agha et al,22 reported >28% of survivors of TBI had at least one anterior pituitary hormone deficiency.
• According to research by Agha et al23 in 2005, >20% of survivors of TBI developed DI; those who developed DI, either acutely or permanently, were more likely to have sustained a severe TBI.
• The development of pituitary dysfunction is independent of the severity of TBI.
• In 2005, civilian guidelines4 recommended screening for pituitary dysfunction in all patients who sustained a moderate to severe TBI.
• In 2010, civilian guidelines7 recommended screening for pituitary dysfunction in patients who sustained a mild TBI.
When to screen for NED after TBI
Given the complexities described—including the similarity of NED to other post-mTBI medical diagnoses and such concomitant disorders as a sleep disorder, memory difficulties, depression, PTSD, and PCS24—consider NED in the primary care setting following confirmed TBI of any severity level when symptoms suggestive of NED persist for >3 months following injury or appear up to 36 months later.7,8,12,20
Order a lab evaluation of blood levels for cortisol (drawn at 8 am), LH, FSH, PRL, insulin-like growth factor-1, TSH, free thyroxine-4, and testosterone for men (8 am) and estradiol for women (8 am). With frankly abnormal lab results or with borderline results and strong clinical suspicion for NED, refer for further endocrinology workup (FIGURE).21 Earlier diagnosis of NED results in more rapid improvement of symptoms and an improved prognosis.7,8,20 Postinjury screening for NED should be one component of a thorough clinical evaluation by a qualified provider, and not used in isolation for clinical decision making. NED screening should not be routinely ordered during the early stages of mTBI, defined as <3 months postinjury.
Provider awareness and willingness to include NED screening in a timely manner, and to refer to specialty services as indicated for symptoms that may be sleep related or psychiatric in nature, may increase the opportunities for early treatment, better rehabilitation outcomes, and better overall quality of life.
Looking ahead
While the DCoE expert group made recommendations on screening for NED in the military combat population, they also acknowledged that NED diagnosis and treatment would benefit from additional areas of research:
• the effect of GH replacement (for GH-deficient patients or as prophylaxis for all TBI patients) on rehabilitation response and quality of life
• the role of multiple TBIs on long-term cognition and possible premature aging
• the role of NED over time
• biomarkers for diagnosis
• factors affecting resiliency
• resiliency in the context of increased or decreased susceptibility to the development of an acute clinical syndrome, as well as susceptibility in developing the spectrum of consequences of TBI.
The research areas given the highest priority by the group were incidence and prevalence studies of pituitary dysfunction after TBI in the combat military population, including pre- and postdeployment rates of dysfunction and the incidence of comorbidities. Also of benefit would be a retrospective study of the consequences of pituitary dysfunction that additionally addresses the effects of comorbid conditions commonly associated with TBI. Considering the rapid expansion in the field of mTBI, additional research and provider awareness concerning early identification and treatments may improve the outcomes for those with persistent mTBI symptoms.
CORRESPONDENCE
Theres A. West, DNP, APN, BC, Defense and Veterans Brain Injury Center, 1335 East West Highway, 6th floor, Silver Spring, MD 20910; [email protected]
1. Injury prevention & control: Traumatic brain injury national TBI estimates. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed August 12, 2013.
2. Armed Forces Surveillance Center. DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Centers Web site. Available at: www.dvbic.org/dod-worldwide-Numbers-tbi. Accessed August 12, 2013.
3. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury (mTBI). Available at: http://www.healthquality.va.gov/management_of_concussion_mtbi.asp. Published April 2009. Accessed August 12, 2013.
4. Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711-724.
5. Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hyperphysical axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581-584.
6. Behan LA, Phillips J, Thompson CJ, et al. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753-759.
7. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-153.
8. Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679-691.
9. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-hormone abnormalities after blast related mild traumatic brain injury. Front Neurol. 2012;3:11.
10. Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687-1697.
11. Benvenga S, Campenmi A, Ruggeri R, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353-1361.
12. Schneider H, Kreitschman-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429-1438.
13. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11-23.
14. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: Pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827-831.
15. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-696.
16. Klose M, Watt T, Brennum J, et al. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life in 12 months after injury. J Clin Endocrinol Metab. 2007;92:3861-3868.
17. Aimeretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitaryism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085-6092.
18. Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury. Br J Neurosurg. 2007;21:210-216.
19. Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358-2366.
20. Rothman MS, Arciniegas DS, Filley CM, et al. The neuroendocrine effects of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:363-372.
21. Defense Centers of Excellence. Neuroendocrine screening post mild TBI clinical recommendation. Available at: http://www.dcoe.mil/Content/Navigation/Documents/DCoE_TBI_NED_Reference_Card.pdf. Published February 2012. Accessed August 12, 2013.
22. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60:584-591.
23. Agha A, Phillips J, O’Kelly P, et al. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118:1416.
24. Guerrero AF, Alfonso A. Traumatic brain injury related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574-580.
› Consider neuroendocrine dysfunction (NED) following confirmed traumatic brain injury of any severity when symptoms suggestive of NED persist for >3 months after injury. A
› Order blood studies to detect deficiencies in pituitary and other key hormones when NED is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Centers for Disease Control and Prevention (CDC) reports that >1.7 million cases of traumatic brain injury (TBI) occur annually in the United States.1 More than 266,000 military service members sustained at least one TBI from 2000 to 2012.2 Most TBIs (80%-85%), military and civilian, are classified as mild (mTBI), and most mTBI patients (80%-85%) experience a complete functional recovery within 3 months of injury.1,3 The remaining 15% to 20% of mTBI patients experience persistent symptoms and difficulty in rehabilitation, particularly if there are concomitant disorders, such as post-traumatic stress disorder (PTSD), sleep disorders, acute stress disorder, substance abuse disorder, and depression.4,5 Symptoms that mTBI and these other disorders have in common can make differential diagnosis difficult, requiring a high degree of clinical awareness by primary care providers. An additional concern following mTBI is neuroendocrine dysfunction (NED). This association has not been widely discussed and therefore may go largely undiagnosed.6 Consider NED in the setting of prolonged symptoms or in patients experiencing difficulty with rehabilitation following mTBI.7,8
NED following mTBI is more common than once thought
The term “neuroendocrine dysfunction,” as discussed in this article, refers to a variety of conditions caused by imbalances in the body’s hormone production directly related to the pituitary, hypothalamus, and their axes following TBI. Until the past decade, the incidence of TBI-associated pituitary dysfunction was thought to be an uncommon event, usually associated with catastrophic head injuries. Studies of NED in TBI patients focused primarily on moderate or severe TBI, usually from motor vehicle incidents, falls, and assaults.7 Other research has since shown that NED occurs more commonly than once believed.9 And while the risk of NED may be higher for patients who sustain more severe brain injuries, NED also occurs in mTBI.7,9,10,11 Interestingly, a recent literature review indicated that the incidence of NED in mTBI was 16.8%, while the incidence with moderate TBI was reported at 10.9%.7 Other research has noted that the incidence of NED in mTBI may be as high as 42%.9,12 No evidence suggests that the severity of NED is related to a specific hormonal dysfunction, nor is there evidence that NED may be associated with a specific mechanism of injury.
Pituitary anatomy is susceptible to injury and dysfunction
The anatomic and physiologic complexities of the hypothalamus and pituitary gland increase their susceptibility to injury from TBI. The pituitary gland is connected to the hypothalamus by a blood vessel-containing stalk, making the pituitary gland—particularly the anterior portion—susceptible to damage during a head injury.13 The hypothalamus secretes thyrotropin-releasing hormone (TRH) and luteinizing-releasing hormone (LRH) to stimulate or suppress the production of anterior pituitary gland hormones, which in turn stimulate the release of hormones and other substances from target organs. Anterior pituitary hormones are growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), and prolactin (PRL). The posterior pituitary secretes oxytocin and vasopressin, also known as antidiuretic hormone (ADH).13
Impact from a direct blow with an object or from a concussive blast can cause focal trauma or rotational shearing of tissue internally. Resultant vascular injury, rupture, cerebral edema, vasospasm, pituitary swelling, or inflammation may then initiate an endocrine response that drives a cascade of complex hormonal processes.5,7,8 Anterior pituitary deficiencies account for the majority of chronic neuroendocrine disorders following mTBI. GH and gonadotropin deficiencies are the most common, but TSH deficiency (secondary hypothyroidism) and ACTH deficiency (adrenal insufficiency) may occur as well, although in <10% of cases with TBI associated NED.12
Clinical features of NED mimic those of other conditions
The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances (TABLE).7,12,14-17 Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, postconcussive syndrome (PCS), and memory and attention difficulties.18 The onset of NED may be immediate (eg, in diabetes insipidus [DI] or syndrome of inappropriate antidiuretic hormone [SIADH], which are very rare in mTBI) and potentially life-threatening (eg, in sodium and potassium imbalances), or may be nonspecific and take years to manifest.6,10,15,19 Additionally, symptoms of NED may spontaneously resolve or persist. Studies have demonstrated pituitary dysfunction in the acute postinjury phase as well as its development as late as 2 to 3 years after injury.7,8,11,20
Due to the range of symptoms related to the combinations of possible hormonal derangements, NED can be an elusive diagnosis and may have a deleterious effect on individuals who sustain TBI.12 For example, an undiagnosed GH deficiency—which can result in increased abdominal fat mass and decreased lean muscle mass as well as impaired cardiac function, dyslipidemia, and insulin resistance—makes it more difficult for an affected individual either to recover from additional injuries or to maintain fitness. Considering NED may avoid a delay in diagnosis and improve prognosis.7,8,20
Findings leading to recommendations on diagnosis
Primary care providers, military and civilian alike, can benefit from the findings and recommendations of an expert panel assembled by the Defense Centers of Excellence (DCoE) for Psychological Health and Traumatic Brain Injury to address NED in mTBI. The panel that convened in December 2010 included experts representing the military services, the Department of Veterans Affairs, DCoE, and civilian sectors. Based on the group’s recommendations combined with literature review findings, the DCoE developed a clinical recommendation to encourage primary care providers to consider screening for NED in patients with persistent symptoms following mTBI.21 Key findings and issues identified by the group included the following:
• The most frequent mechanism of injury in the military deployed population is blast-induced TBI. Such injury could occur in the civilian population at construction blast sites or in factories producing or using highly flammable substances.
• The prevalence of any anterior pituitary hormone deficiency is as high as 30% to hormone deficiency is as high as 30% to 80% at 24 to 36 months post injury.
• The prevalence of posterior pituitary hormone deficiency is as high as 4% to 7% at 12 months post injury.
• The anterior pituitary hormones most frequently affected in survivors of TBI are ACTH, gonadotropin, prolactin, and GH.
• In 2004 Agha et al,22 reported >28% of survivors of TBI had at least one anterior pituitary hormone deficiency.
• According to research by Agha et al23 in 2005, >20% of survivors of TBI developed DI; those who developed DI, either acutely or permanently, were more likely to have sustained a severe TBI.
• The development of pituitary dysfunction is independent of the severity of TBI.
• In 2005, civilian guidelines4 recommended screening for pituitary dysfunction in all patients who sustained a moderate to severe TBI.
• In 2010, civilian guidelines7 recommended screening for pituitary dysfunction in patients who sustained a mild TBI.
When to screen for NED after TBI
Given the complexities described—including the similarity of NED to other post-mTBI medical diagnoses and such concomitant disorders as a sleep disorder, memory difficulties, depression, PTSD, and PCS24—consider NED in the primary care setting following confirmed TBI of any severity level when symptoms suggestive of NED persist for >3 months following injury or appear up to 36 months later.7,8,12,20
Order a lab evaluation of blood levels for cortisol (drawn at 8 am), LH, FSH, PRL, insulin-like growth factor-1, TSH, free thyroxine-4, and testosterone for men (8 am) and estradiol for women (8 am). With frankly abnormal lab results or with borderline results and strong clinical suspicion for NED, refer for further endocrinology workup (FIGURE).21 Earlier diagnosis of NED results in more rapid improvement of symptoms and an improved prognosis.7,8,20 Postinjury screening for NED should be one component of a thorough clinical evaluation by a qualified provider, and not used in isolation for clinical decision making. NED screening should not be routinely ordered during the early stages of mTBI, defined as <3 months postinjury.
Provider awareness and willingness to include NED screening in a timely manner, and to refer to specialty services as indicated for symptoms that may be sleep related or psychiatric in nature, may increase the opportunities for early treatment, better rehabilitation outcomes, and better overall quality of life.
Looking ahead
While the DCoE expert group made recommendations on screening for NED in the military combat population, they also acknowledged that NED diagnosis and treatment would benefit from additional areas of research:
• the effect of GH replacement (for GH-deficient patients or as prophylaxis for all TBI patients) on rehabilitation response and quality of life
• the role of multiple TBIs on long-term cognition and possible premature aging
• the role of NED over time
• biomarkers for diagnosis
• factors affecting resiliency
• resiliency in the context of increased or decreased susceptibility to the development of an acute clinical syndrome, as well as susceptibility in developing the spectrum of consequences of TBI.
The research areas given the highest priority by the group were incidence and prevalence studies of pituitary dysfunction after TBI in the combat military population, including pre- and postdeployment rates of dysfunction and the incidence of comorbidities. Also of benefit would be a retrospective study of the consequences of pituitary dysfunction that additionally addresses the effects of comorbid conditions commonly associated with TBI. Considering the rapid expansion in the field of mTBI, additional research and provider awareness concerning early identification and treatments may improve the outcomes for those with persistent mTBI symptoms.
CORRESPONDENCE
Theres A. West, DNP, APN, BC, Defense and Veterans Brain Injury Center, 1335 East West Highway, 6th floor, Silver Spring, MD 20910; [email protected]
› Consider neuroendocrine dysfunction (NED) following confirmed traumatic brain injury of any severity when symptoms suggestive of NED persist for >3 months after injury. A
› Order blood studies to detect deficiencies in pituitary and other key hormones when NED is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Centers for Disease Control and Prevention (CDC) reports that >1.7 million cases of traumatic brain injury (TBI) occur annually in the United States.1 More than 266,000 military service members sustained at least one TBI from 2000 to 2012.2 Most TBIs (80%-85%), military and civilian, are classified as mild (mTBI), and most mTBI patients (80%-85%) experience a complete functional recovery within 3 months of injury.1,3 The remaining 15% to 20% of mTBI patients experience persistent symptoms and difficulty in rehabilitation, particularly if there are concomitant disorders, such as post-traumatic stress disorder (PTSD), sleep disorders, acute stress disorder, substance abuse disorder, and depression.4,5 Symptoms that mTBI and these other disorders have in common can make differential diagnosis difficult, requiring a high degree of clinical awareness by primary care providers. An additional concern following mTBI is neuroendocrine dysfunction (NED). This association has not been widely discussed and therefore may go largely undiagnosed.6 Consider NED in the setting of prolonged symptoms or in patients experiencing difficulty with rehabilitation following mTBI.7,8
NED following mTBI is more common than once thought
The term “neuroendocrine dysfunction,” as discussed in this article, refers to a variety of conditions caused by imbalances in the body’s hormone production directly related to the pituitary, hypothalamus, and their axes following TBI. Until the past decade, the incidence of TBI-associated pituitary dysfunction was thought to be an uncommon event, usually associated with catastrophic head injuries. Studies of NED in TBI patients focused primarily on moderate or severe TBI, usually from motor vehicle incidents, falls, and assaults.7 Other research has since shown that NED occurs more commonly than once believed.9 And while the risk of NED may be higher for patients who sustain more severe brain injuries, NED also occurs in mTBI.7,9,10,11 Interestingly, a recent literature review indicated that the incidence of NED in mTBI was 16.8%, while the incidence with moderate TBI was reported at 10.9%.7 Other research has noted that the incidence of NED in mTBI may be as high as 42%.9,12 No evidence suggests that the severity of NED is related to a specific hormonal dysfunction, nor is there evidence that NED may be associated with a specific mechanism of injury.
Pituitary anatomy is susceptible to injury and dysfunction
The anatomic and physiologic complexities of the hypothalamus and pituitary gland increase their susceptibility to injury from TBI. The pituitary gland is connected to the hypothalamus by a blood vessel-containing stalk, making the pituitary gland—particularly the anterior portion—susceptible to damage during a head injury.13 The hypothalamus secretes thyrotropin-releasing hormone (TRH) and luteinizing-releasing hormone (LRH) to stimulate or suppress the production of anterior pituitary gland hormones, which in turn stimulate the release of hormones and other substances from target organs. Anterior pituitary hormones are growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), and prolactin (PRL). The posterior pituitary secretes oxytocin and vasopressin, also known as antidiuretic hormone (ADH).13
Impact from a direct blow with an object or from a concussive blast can cause focal trauma or rotational shearing of tissue internally. Resultant vascular injury, rupture, cerebral edema, vasospasm, pituitary swelling, or inflammation may then initiate an endocrine response that drives a cascade of complex hormonal processes.5,7,8 Anterior pituitary deficiencies account for the majority of chronic neuroendocrine disorders following mTBI. GH and gonadotropin deficiencies are the most common, but TSH deficiency (secondary hypothyroidism) and ACTH deficiency (adrenal insufficiency) may occur as well, although in <10% of cases with TBI associated NED.12
Clinical features of NED mimic those of other conditions
The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances (TABLE).7,12,14-17 Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, postconcussive syndrome (PCS), and memory and attention difficulties.18 The onset of NED may be immediate (eg, in diabetes insipidus [DI] or syndrome of inappropriate antidiuretic hormone [SIADH], which are very rare in mTBI) and potentially life-threatening (eg, in sodium and potassium imbalances), or may be nonspecific and take years to manifest.6,10,15,19 Additionally, symptoms of NED may spontaneously resolve or persist. Studies have demonstrated pituitary dysfunction in the acute postinjury phase as well as its development as late as 2 to 3 years after injury.7,8,11,20
Due to the range of symptoms related to the combinations of possible hormonal derangements, NED can be an elusive diagnosis and may have a deleterious effect on individuals who sustain TBI.12 For example, an undiagnosed GH deficiency—which can result in increased abdominal fat mass and decreased lean muscle mass as well as impaired cardiac function, dyslipidemia, and insulin resistance—makes it more difficult for an affected individual either to recover from additional injuries or to maintain fitness. Considering NED may avoid a delay in diagnosis and improve prognosis.7,8,20
Findings leading to recommendations on diagnosis
Primary care providers, military and civilian alike, can benefit from the findings and recommendations of an expert panel assembled by the Defense Centers of Excellence (DCoE) for Psychological Health and Traumatic Brain Injury to address NED in mTBI. The panel that convened in December 2010 included experts representing the military services, the Department of Veterans Affairs, DCoE, and civilian sectors. Based on the group’s recommendations combined with literature review findings, the DCoE developed a clinical recommendation to encourage primary care providers to consider screening for NED in patients with persistent symptoms following mTBI.21 Key findings and issues identified by the group included the following:
• The most frequent mechanism of injury in the military deployed population is blast-induced TBI. Such injury could occur in the civilian population at construction blast sites or in factories producing or using highly flammable substances.
• The prevalence of any anterior pituitary hormone deficiency is as high as 30% to hormone deficiency is as high as 30% to 80% at 24 to 36 months post injury.
• The prevalence of posterior pituitary hormone deficiency is as high as 4% to 7% at 12 months post injury.
• The anterior pituitary hormones most frequently affected in survivors of TBI are ACTH, gonadotropin, prolactin, and GH.
• In 2004 Agha et al,22 reported >28% of survivors of TBI had at least one anterior pituitary hormone deficiency.
• According to research by Agha et al23 in 2005, >20% of survivors of TBI developed DI; those who developed DI, either acutely or permanently, were more likely to have sustained a severe TBI.
• The development of pituitary dysfunction is independent of the severity of TBI.
• In 2005, civilian guidelines4 recommended screening for pituitary dysfunction in all patients who sustained a moderate to severe TBI.
• In 2010, civilian guidelines7 recommended screening for pituitary dysfunction in patients who sustained a mild TBI.
When to screen for NED after TBI
Given the complexities described—including the similarity of NED to other post-mTBI medical diagnoses and such concomitant disorders as a sleep disorder, memory difficulties, depression, PTSD, and PCS24—consider NED in the primary care setting following confirmed TBI of any severity level when symptoms suggestive of NED persist for >3 months following injury or appear up to 36 months later.7,8,12,20
Order a lab evaluation of blood levels for cortisol (drawn at 8 am), LH, FSH, PRL, insulin-like growth factor-1, TSH, free thyroxine-4, and testosterone for men (8 am) and estradiol for women (8 am). With frankly abnormal lab results or with borderline results and strong clinical suspicion for NED, refer for further endocrinology workup (FIGURE).21 Earlier diagnosis of NED results in more rapid improvement of symptoms and an improved prognosis.7,8,20 Postinjury screening for NED should be one component of a thorough clinical evaluation by a qualified provider, and not used in isolation for clinical decision making. NED screening should not be routinely ordered during the early stages of mTBI, defined as <3 months postinjury.
Provider awareness and willingness to include NED screening in a timely manner, and to refer to specialty services as indicated for symptoms that may be sleep related or psychiatric in nature, may increase the opportunities for early treatment, better rehabilitation outcomes, and better overall quality of life.
Looking ahead
While the DCoE expert group made recommendations on screening for NED in the military combat population, they also acknowledged that NED diagnosis and treatment would benefit from additional areas of research:
• the effect of GH replacement (for GH-deficient patients or as prophylaxis for all TBI patients) on rehabilitation response and quality of life
• the role of multiple TBIs on long-term cognition and possible premature aging
• the role of NED over time
• biomarkers for diagnosis
• factors affecting resiliency
• resiliency in the context of increased or decreased susceptibility to the development of an acute clinical syndrome, as well as susceptibility in developing the spectrum of consequences of TBI.
The research areas given the highest priority by the group were incidence and prevalence studies of pituitary dysfunction after TBI in the combat military population, including pre- and postdeployment rates of dysfunction and the incidence of comorbidities. Also of benefit would be a retrospective study of the consequences of pituitary dysfunction that additionally addresses the effects of comorbid conditions commonly associated with TBI. Considering the rapid expansion in the field of mTBI, additional research and provider awareness concerning early identification and treatments may improve the outcomes for those with persistent mTBI symptoms.
CORRESPONDENCE
Theres A. West, DNP, APN, BC, Defense and Veterans Brain Injury Center, 1335 East West Highway, 6th floor, Silver Spring, MD 20910; [email protected]
1. Injury prevention & control: Traumatic brain injury national TBI estimates. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed August 12, 2013.
2. Armed Forces Surveillance Center. DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Centers Web site. Available at: www.dvbic.org/dod-worldwide-Numbers-tbi. Accessed August 12, 2013.
3. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury (mTBI). Available at: http://www.healthquality.va.gov/management_of_concussion_mtbi.asp. Published April 2009. Accessed August 12, 2013.
4. Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711-724.
5. Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hyperphysical axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581-584.
6. Behan LA, Phillips J, Thompson CJ, et al. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753-759.
7. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-153.
8. Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679-691.
9. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-hormone abnormalities after blast related mild traumatic brain injury. Front Neurol. 2012;3:11.
10. Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687-1697.
11. Benvenga S, Campenmi A, Ruggeri R, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353-1361.
12. Schneider H, Kreitschman-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429-1438.
13. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11-23.
14. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: Pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827-831.
15. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-696.
16. Klose M, Watt T, Brennum J, et al. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life in 12 months after injury. J Clin Endocrinol Metab. 2007;92:3861-3868.
17. Aimeretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitaryism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085-6092.
18. Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury. Br J Neurosurg. 2007;21:210-216.
19. Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358-2366.
20. Rothman MS, Arciniegas DS, Filley CM, et al. The neuroendocrine effects of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:363-372.
21. Defense Centers of Excellence. Neuroendocrine screening post mild TBI clinical recommendation. Available at: http://www.dcoe.mil/Content/Navigation/Documents/DCoE_TBI_NED_Reference_Card.pdf. Published February 2012. Accessed August 12, 2013.
22. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60:584-591.
23. Agha A, Phillips J, O’Kelly P, et al. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118:1416.
24. Guerrero AF, Alfonso A. Traumatic brain injury related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574-580.
1. Injury prevention & control: Traumatic brain injury national TBI estimates. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed August 12, 2013.
2. Armed Forces Surveillance Center. DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Centers Web site. Available at: www.dvbic.org/dod-worldwide-Numbers-tbi. Accessed August 12, 2013.
3. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury (mTBI). Available at: http://www.healthquality.va.gov/management_of_concussion_mtbi.asp. Published April 2009. Accessed August 12, 2013.
4. Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711-724.
5. Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hyperphysical axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581-584.
6. Behan LA, Phillips J, Thompson CJ, et al. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753-759.
7. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-153.
8. Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679-691.
9. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-hormone abnormalities after blast related mild traumatic brain injury. Front Neurol. 2012;3:11.
10. Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687-1697.
11. Benvenga S, Campenmi A, Ruggeri R, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353-1361.
12. Schneider H, Kreitschman-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429-1438.
13. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11-23.
14. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: Pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827-831.
15. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-696.
16. Klose M, Watt T, Brennum J, et al. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life in 12 months after injury. J Clin Endocrinol Metab. 2007;92:3861-3868.
17. Aimeretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitaryism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085-6092.
18. Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury. Br J Neurosurg. 2007;21:210-216.
19. Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358-2366.
20. Rothman MS, Arciniegas DS, Filley CM, et al. The neuroendocrine effects of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:363-372.
21. Defense Centers of Excellence. Neuroendocrine screening post mild TBI clinical recommendation. Available at: http://www.dcoe.mil/Content/Navigation/Documents/DCoE_TBI_NED_Reference_Card.pdf. Published February 2012. Accessed August 12, 2013.
22. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60:584-591.
23. Agha A, Phillips J, O’Kelly P, et al. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118:1416.
24. Guerrero AF, Alfonso A. Traumatic brain injury related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574-580.
Statin therapy: When to think twice
› Avoid adding niacin to statin therapy, as it does not appear to provide any added benefit and may increase the risk of stroke. B
› Continue statin therapy in a patient who has chronic kidney disease progressing to end-stage renal disease, but do not initiate it in patients on dialysis. B
› Do not add statins to the medication regimen of patients with heart failure; focus on optimizing therapies known to reduce mortality in this patient population instead. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Morbidity and mortality from atherosclerotic disease have decreased significantly in the last several decades, in large part because of advances in therapies targeting serum lipids—including statins.1 Since their introduction in 1987, HMG Coenzyme A inhibitors have been intensively studied, and their use has increased dramatically. A recent report from the National Center for Health Statistics reveals that in the years 1999 to 2002, 26% of men ages 65 to 74 years were taking statins; several years later (2005-2008), that number had soared to 50%. In the same time frame, statin use among women ages 65 to 74 went from 24% to 36%.2
Statins lower serum low-density lipoprotein cholesterol (LDL-C) and triglycerides, raise high-density lipoprotein cholesterol (HDL-C), and improve surrogate markers for cardiovascular events. Most importantly, statins reduce the risk for major cardiovascular events, such as myocardial infarction (MI) and death from cardiovascular disease (CVD), in select populations. Yet doubts about the benefits of statins, alone or in combination with other lipid-lowering agents, for certain patient populations remain.
Primary care physicians need to know when, or whether, to add a second lipid-lowering agent to the drug regimen of patients whose response to a statin is less than hoped for, and which patient populations and clinical indicators statins have not been found to help. You’ll find answers, the results of the latest studies, and details of the 2013 cholesterol guideline released last month by the American Heart Association/American College of Cardiology and in this evidence-based update.
A statin is not enough? Don't add these drugs
In patients with very elevated LDL-C or mixed dyslipidemias that fail to reach the desired lipid levels on statin monotherapy, other classes of lipid-modifying agents are often added in an attempt to improve clinical outcomes.3 Fibrates, extended release (niacin, and n-3 polyunsaturated fatty acids have been frequently used for this purpose. But it is only in the last several years that large, well-designed studies have looked closely at patient-oriented outcomes associated with statins in combination with other lipid-modifying drugs.4-9
Fenofibrate + a statin yields little benefit
The ACCORD lipid placebo-controlled trial studied fenofibrate as a simvastatin add-on in patients with diabetes.4 Its findings? While the lipid levels of patients receiving this drug combination improved significantly, the primary endpoint (MI, stroke, or death from cardiovascular causes) was no different from that of the controls, who were taking the statin alone.
Sub-group analysis suggested that the simvastatin-fenofibrate combination benefitted only one particular group: patients with high triglyceride levels (≥204 mg/dL) and low HDL-C (≤34 mg/dL). This finding prompted the US Food and Drug Administration to call for an additional clinical trial to evaluate the effectiveness of add-on therapy with fenofibrate in patients who meet this criteria.10 The status of such a study is uncertain.
Adding niacin to a statin does more harm than good
The HATS trial, published in 2001, found the addition of niacin to a statin regimen to be beneficial.7 But because of the wide confidence interval associated with the clinical endpoints and the small number of subjects (N=160) in that study, larger trials were needed to confirm the positive results. In fact, they found the opposite.
Niacin increases stroke risk. In both the AIM-HIGH5 (N=3414) and HPS2-THRIVE6 (N=25,673) trials, the addition of extended release niacin not only failed to reduce the risk of major cardiovascular events, it was shown to increase the risk of stroke.
n-3 polyunsaturated fatty acids don’t help much
Studies evaluating the addition of n-3 polyunsaturated fatty acids to statin therapy have had mixed results. The JELIS8 trial had more than 18,000 participants, 20% of whom had known coronary artery disease. All were taking statins and randomized to either open-label eicosapentaenoic acid (EPA) 600 mg 3 times daily or placebo. The primary endpoint, a composite of sudden cardiac death, fatal or nonfatal MI, unstable angina, angioplasty, and stenting or coronary artery bypass grafting, was lower in the intervention group: (2.8% vs 3.5%; number needed to treat [NNT]: 143).
It is important to note, however, that only one of the individual components of the primary endpoint—unstable angina—was significantly reduced by EPA (2.1% vs 1.6%; P=.014).8 In the Alpha Omega trial,9 various n-3 polyunsaturated fatty acids were tested in combination with statins. None was found to be superior to placebo in reducing cardiovascular outcomes.
Based on the evidence, the new cholesterol guideline does not support the routine use of these agents to reduce atherosclerotic CVD (See “The new cholesterol guideline: Beyond the headlines”.)11
Statins and kidney disease: Factors to consider
More than half of the deaths in patients with end-stage renal disease (ESRD) are from cardiovascular causes.12 The relationship between renal dysfunction and cardiovascular events is independent of other risk factors, including a history of CVD. Risk rises with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2, with a sharp increase when the rate <45 mL/min/1.73m.2 Thus, strategies known to reduce major cardiovascular events in the general population, including statins, have the potential to offer substantial benefit for patients with chronic kidney disease (CKD).
Statin use in patients with CKD has been evaluated in post-hoc and subgroup analyses of large clinical trials and, more recently, in RCTs targeting patients with both moderate and end-stage disease (TABLE).13-18
Three post-hoc analyses of large multicenter, double-blind RCTs13-15,19 compared patients with normal renal function with those with CKD. All 3 found that moderate or high-intensity statin therapy significantly reduced the incidence of the primary outcome—a composite of major cardiovascular events—compared with either placebo or a lower-intensity statin.
For patients with CKD, drug combo lowered the risk
The SHARP trial17 (N=9270) was the first large prospective, double-blind, multicenter RCT to compare the effect of a statin plus a second lipid-lowering drug (simvastatin plus ezetimibe) vs placebo in patients with CKD. A third of the participants were on dialysis at the start of the trial (ESRD was defined as starting long-term dialysis or requiring kidney transplantation).
Patients in the intervention group were significantly (17%) less likely to experience a major atherosclerotic event compared with those on placebo. This translated into an NNT of 47 over a period of 4.9 years. (Since no group received only simvastatin, it is not known what role ezetimibe had in the reduction of cardiovascular events.) Although no difference in outcomes was found when the results were stratified based on whether participants were on dialysis, this trial was not adequately powered for this subgroup analysis.17
Little benefit from statins in patients with end-stage disease
Two major prospective randomized, double-blinded, placebo-controlled, multicenter trials evaluating the effects of statin use on cardiovascular outcomes in ESRD patients on dialysis have been published.18,19 Both found a significant decline in LDL-C in patients receiving statin therapy. But neither found a significant difference in mortality rates in the statin vs placebo groups.
One group of researchers speculated that the lack of effect may be due to a difference in the pathogenesis of vascular events in patients with and without ESRD. Delayed use of statins until patients have ESRD will offer limited benefit, they concluded, and recommended against routine statin treatment in an attempt to reduce the incidence of CVD in this patient population.18 Based on these results, the new cholesterol guideline indicates that this group of patients may not benefit from statin therapy.
For patients with heart failure, statins offer limited benefit
More than half of the heart failure (HF) in the United States is caused by ischemic heart disease.20 Improvements in post-MI survival have increased the prevalence of chronic HF.
Statins have a well-established role in the prevention and treatment of atherosclerosis because of their ability to modify the natural course of the disease and reduce major adverse cardiovascular events. Thus, it seems reasonable to assume that, in patients who have or are at high risk for coronary heart disease, statins would help to prevent the occurrence or slow the progression of HF.
Early studies of statins either excluded patients with HF or enrolled so few HF patients that no conclusions could be reached regarding the safety or efficacy of statin use in this population.21-25 More recently, 2 large RCTs have studied the effect of statins in patients with HF. Both have found them to be ineffective.26,27
The CORONA trial enrolled elderly patients with HF of ischemic causes and ejection fraction ≤40% (≤35% in patients with New York Heart Association [NYHA] Class II), randomized to either rosuvastatin 10 mg/d or placebo.25 More than 40% of the participants had a history of MI, and more than 60% were NYHA Class III or IV. HF medications were well-managed; more than 90% of the patients were being treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; 75%, with beta-blockers; and 39%, with aldosterone antagonists.
The researchers found no significant difference between the rosuvastatin and placebo groups in the primary outcome of death from cardiovascular causes, nonfatal MI, or nonfatal stroke (11.4% in the rosuvastatin group vs 12.3% among those on placebo; 95% confidence interval, 0.83-1.02; P=.12). No difference was found in patients with a history of MI (13.9% placebo event rate vs 12.7% rosuvastatin arm; P=not significant [NS]). Neither death from worsening HF nor sudden death was reduced.26
There were fewer hospitalizations among those taking rosuvastatin, however (NNT=17 per year). And there was a trend towards a benefit among those with more advanced HF (NYHA III/IV), with primary outcome rates of 12.7% for those in the rosuvastatin group vs 14.2% in the placebo group. (P=NS). Rosuvastatin was safe for HF patients, as most types of adverse events were more common in the placebo group. Assessments of muscle toxicity were similar in both groups.
The GISSI-HF study, another RCT of rosuvastatin vs placebo in HF patients, also showed a lack of benefit from statin treatment.27 Researchers enrolled more than 4500 patients with NYHA Class II to IV HF, from both ischemic and nonischemic causes.26 Similar to the findings in the CORONA trial, LDL-C was substantially reduced by rosuvastatin 10 mg/d (-32% vs no change for those in the placebo group), but this did not translate into clinically relevant endpoints. After 3.9 years of therapy, the primary endpoints of time to death and time to death or hospitalization for cardiovascular causes were not significantly reduced, nor were any of the secondary endpoints.26
The similar lack of benefit in these 2 trials is striking in view of the benefit of statins in patients with coronary heart disease but without HF. Given these findings, focusing on optimizing therapies known to reduce mortality in patients with HF rather than adding a statin in an attempt to alter the atherosclerotic process appears to be a better approach. Thus, the recently published cholesterol guideline does not advocate the initiation or continuation of statin therapy in patients with NYHA Class II-IV HF.
1. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2010 update. Circulation. 2010;121:948-954.
2. US Department of Health and Human Services. Health, United States, 2010. Available at http://www.cdc.gov/nchs/data/hus/hus10.pdf. Accessed July 22, 2011.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
5. AIM-HIGH Investigators; Boden WE, Probsfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. .N Engl J Med. 2011;365:2255-2267.
6. Niacin causes serious unexpected side-effects, but no worthwhile benefits for patients who are at increased risk of heart attacks and strokes [press release]. HPS2-THRIVE: University of Oxford; March 9, 2013. Available at: www.thrivestudy.org/press_release.htm. Accessed July 3, 2013.
7. Brown BG, Zhao X, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583-1592.
8. Yokyama M, Origasa H, Matsuzaki M, et al; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients. Lancet. 2007;369:1090-1098.
9. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015-2026.
10. The Endocrinologic and Metabolic Drugs Advisory Committee of the FDA, Center for Drug Evaluation and Research. Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM261162.pdf. Published June 24, 2011. Accessed July 3, 2013.
11. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Associaton task force on practice guidelines . Circulation. 2013 Nov 12 [Epub ahead of print.]
12. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305.
13. Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557-1563.
14. Shepherd J, Kastelein JJ, Bittner V, et al; TNT (Treating to New Targets) Investigators. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease. J Am Coll Cardiol. 2008;51:1448-1454.
15. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes. Am J Kidney Dis. 2009;54:810-819.
16. Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomized placebo-controlled trial. Lancet. 2011;377:2181-2192.
17. Wanner C, Krane V, Màrz W, et al; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248.
18. Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-1407.
19. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Lancet. 2004;364:685-696.
20. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:e1-e90.
21. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001-1009.
22. Downs JR, Clearfield M, Weis S, et al; AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279:1615-1622.
23. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301-1307.
24. The Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994;344:1383-1389.
25. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). Lancet. 2002;360:1623-1630.
26. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248-2261.
27. GISSI-HF Investigators; Tavozzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial). Lancet. 2008;372:1231-1239.
› Avoid adding niacin to statin therapy, as it does not appear to provide any added benefit and may increase the risk of stroke. B
› Continue statin therapy in a patient who has chronic kidney disease progressing to end-stage renal disease, but do not initiate it in patients on dialysis. B
› Do not add statins to the medication regimen of patients with heart failure; focus on optimizing therapies known to reduce mortality in this patient population instead. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Morbidity and mortality from atherosclerotic disease have decreased significantly in the last several decades, in large part because of advances in therapies targeting serum lipids—including statins.1 Since their introduction in 1987, HMG Coenzyme A inhibitors have been intensively studied, and their use has increased dramatically. A recent report from the National Center for Health Statistics reveals that in the years 1999 to 2002, 26% of men ages 65 to 74 years were taking statins; several years later (2005-2008), that number had soared to 50%. In the same time frame, statin use among women ages 65 to 74 went from 24% to 36%.2
Statins lower serum low-density lipoprotein cholesterol (LDL-C) and triglycerides, raise high-density lipoprotein cholesterol (HDL-C), and improve surrogate markers for cardiovascular events. Most importantly, statins reduce the risk for major cardiovascular events, such as myocardial infarction (MI) and death from cardiovascular disease (CVD), in select populations. Yet doubts about the benefits of statins, alone or in combination with other lipid-lowering agents, for certain patient populations remain.
Primary care physicians need to know when, or whether, to add a second lipid-lowering agent to the drug regimen of patients whose response to a statin is less than hoped for, and which patient populations and clinical indicators statins have not been found to help. You’ll find answers, the results of the latest studies, and details of the 2013 cholesterol guideline released last month by the American Heart Association/American College of Cardiology and in this evidence-based update.
A statin is not enough? Don't add these drugs
In patients with very elevated LDL-C or mixed dyslipidemias that fail to reach the desired lipid levels on statin monotherapy, other classes of lipid-modifying agents are often added in an attempt to improve clinical outcomes.3 Fibrates, extended release (niacin, and n-3 polyunsaturated fatty acids have been frequently used for this purpose. But it is only in the last several years that large, well-designed studies have looked closely at patient-oriented outcomes associated with statins in combination with other lipid-modifying drugs.4-9
Fenofibrate + a statin yields little benefit
The ACCORD lipid placebo-controlled trial studied fenofibrate as a simvastatin add-on in patients with diabetes.4 Its findings? While the lipid levels of patients receiving this drug combination improved significantly, the primary endpoint (MI, stroke, or death from cardiovascular causes) was no different from that of the controls, who were taking the statin alone.
Sub-group analysis suggested that the simvastatin-fenofibrate combination benefitted only one particular group: patients with high triglyceride levels (≥204 mg/dL) and low HDL-C (≤34 mg/dL). This finding prompted the US Food and Drug Administration to call for an additional clinical trial to evaluate the effectiveness of add-on therapy with fenofibrate in patients who meet this criteria.10 The status of such a study is uncertain.
Adding niacin to a statin does more harm than good
The HATS trial, published in 2001, found the addition of niacin to a statin regimen to be beneficial.7 But because of the wide confidence interval associated with the clinical endpoints and the small number of subjects (N=160) in that study, larger trials were needed to confirm the positive results. In fact, they found the opposite.
Niacin increases stroke risk. In both the AIM-HIGH5 (N=3414) and HPS2-THRIVE6 (N=25,673) trials, the addition of extended release niacin not only failed to reduce the risk of major cardiovascular events, it was shown to increase the risk of stroke.
n-3 polyunsaturated fatty acids don’t help much
Studies evaluating the addition of n-3 polyunsaturated fatty acids to statin therapy have had mixed results. The JELIS8 trial had more than 18,000 participants, 20% of whom had known coronary artery disease. All were taking statins and randomized to either open-label eicosapentaenoic acid (EPA) 600 mg 3 times daily or placebo. The primary endpoint, a composite of sudden cardiac death, fatal or nonfatal MI, unstable angina, angioplasty, and stenting or coronary artery bypass grafting, was lower in the intervention group: (2.8% vs 3.5%; number needed to treat [NNT]: 143).
It is important to note, however, that only one of the individual components of the primary endpoint—unstable angina—was significantly reduced by EPA (2.1% vs 1.6%; P=.014).8 In the Alpha Omega trial,9 various n-3 polyunsaturated fatty acids were tested in combination with statins. None was found to be superior to placebo in reducing cardiovascular outcomes.
Based on the evidence, the new cholesterol guideline does not support the routine use of these agents to reduce atherosclerotic CVD (See “The new cholesterol guideline: Beyond the headlines”.)11
Statins and kidney disease: Factors to consider
More than half of the deaths in patients with end-stage renal disease (ESRD) are from cardiovascular causes.12 The relationship between renal dysfunction and cardiovascular events is independent of other risk factors, including a history of CVD. Risk rises with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2, with a sharp increase when the rate <45 mL/min/1.73m.2 Thus, strategies known to reduce major cardiovascular events in the general population, including statins, have the potential to offer substantial benefit for patients with chronic kidney disease (CKD).
Statin use in patients with CKD has been evaluated in post-hoc and subgroup analyses of large clinical trials and, more recently, in RCTs targeting patients with both moderate and end-stage disease (TABLE).13-18
Three post-hoc analyses of large multicenter, double-blind RCTs13-15,19 compared patients with normal renal function with those with CKD. All 3 found that moderate or high-intensity statin therapy significantly reduced the incidence of the primary outcome—a composite of major cardiovascular events—compared with either placebo or a lower-intensity statin.
For patients with CKD, drug combo lowered the risk
The SHARP trial17 (N=9270) was the first large prospective, double-blind, multicenter RCT to compare the effect of a statin plus a second lipid-lowering drug (simvastatin plus ezetimibe) vs placebo in patients with CKD. A third of the participants were on dialysis at the start of the trial (ESRD was defined as starting long-term dialysis or requiring kidney transplantation).
Patients in the intervention group were significantly (17%) less likely to experience a major atherosclerotic event compared with those on placebo. This translated into an NNT of 47 over a period of 4.9 years. (Since no group received only simvastatin, it is not known what role ezetimibe had in the reduction of cardiovascular events.) Although no difference in outcomes was found when the results were stratified based on whether participants were on dialysis, this trial was not adequately powered for this subgroup analysis.17
Little benefit from statins in patients with end-stage disease
Two major prospective randomized, double-blinded, placebo-controlled, multicenter trials evaluating the effects of statin use on cardiovascular outcomes in ESRD patients on dialysis have been published.18,19 Both found a significant decline in LDL-C in patients receiving statin therapy. But neither found a significant difference in mortality rates in the statin vs placebo groups.
One group of researchers speculated that the lack of effect may be due to a difference in the pathogenesis of vascular events in patients with and without ESRD. Delayed use of statins until patients have ESRD will offer limited benefit, they concluded, and recommended against routine statin treatment in an attempt to reduce the incidence of CVD in this patient population.18 Based on these results, the new cholesterol guideline indicates that this group of patients may not benefit from statin therapy.
For patients with heart failure, statins offer limited benefit
More than half of the heart failure (HF) in the United States is caused by ischemic heart disease.20 Improvements in post-MI survival have increased the prevalence of chronic HF.
Statins have a well-established role in the prevention and treatment of atherosclerosis because of their ability to modify the natural course of the disease and reduce major adverse cardiovascular events. Thus, it seems reasonable to assume that, in patients who have or are at high risk for coronary heart disease, statins would help to prevent the occurrence or slow the progression of HF.
Early studies of statins either excluded patients with HF or enrolled so few HF patients that no conclusions could be reached regarding the safety or efficacy of statin use in this population.21-25 More recently, 2 large RCTs have studied the effect of statins in patients with HF. Both have found them to be ineffective.26,27
The CORONA trial enrolled elderly patients with HF of ischemic causes and ejection fraction ≤40% (≤35% in patients with New York Heart Association [NYHA] Class II), randomized to either rosuvastatin 10 mg/d or placebo.25 More than 40% of the participants had a history of MI, and more than 60% were NYHA Class III or IV. HF medications were well-managed; more than 90% of the patients were being treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; 75%, with beta-blockers; and 39%, with aldosterone antagonists.
The researchers found no significant difference between the rosuvastatin and placebo groups in the primary outcome of death from cardiovascular causes, nonfatal MI, or nonfatal stroke (11.4% in the rosuvastatin group vs 12.3% among those on placebo; 95% confidence interval, 0.83-1.02; P=.12). No difference was found in patients with a history of MI (13.9% placebo event rate vs 12.7% rosuvastatin arm; P=not significant [NS]). Neither death from worsening HF nor sudden death was reduced.26
There were fewer hospitalizations among those taking rosuvastatin, however (NNT=17 per year). And there was a trend towards a benefit among those with more advanced HF (NYHA III/IV), with primary outcome rates of 12.7% for those in the rosuvastatin group vs 14.2% in the placebo group. (P=NS). Rosuvastatin was safe for HF patients, as most types of adverse events were more common in the placebo group. Assessments of muscle toxicity were similar in both groups.
The GISSI-HF study, another RCT of rosuvastatin vs placebo in HF patients, also showed a lack of benefit from statin treatment.27 Researchers enrolled more than 4500 patients with NYHA Class II to IV HF, from both ischemic and nonischemic causes.26 Similar to the findings in the CORONA trial, LDL-C was substantially reduced by rosuvastatin 10 mg/d (-32% vs no change for those in the placebo group), but this did not translate into clinically relevant endpoints. After 3.9 years of therapy, the primary endpoints of time to death and time to death or hospitalization for cardiovascular causes were not significantly reduced, nor were any of the secondary endpoints.26
The similar lack of benefit in these 2 trials is striking in view of the benefit of statins in patients with coronary heart disease but without HF. Given these findings, focusing on optimizing therapies known to reduce mortality in patients with HF rather than adding a statin in an attempt to alter the atherosclerotic process appears to be a better approach. Thus, the recently published cholesterol guideline does not advocate the initiation or continuation of statin therapy in patients with NYHA Class II-IV HF.
› Avoid adding niacin to statin therapy, as it does not appear to provide any added benefit and may increase the risk of stroke. B
› Continue statin therapy in a patient who has chronic kidney disease progressing to end-stage renal disease, but do not initiate it in patients on dialysis. B
› Do not add statins to the medication regimen of patients with heart failure; focus on optimizing therapies known to reduce mortality in this patient population instead. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Morbidity and mortality from atherosclerotic disease have decreased significantly in the last several decades, in large part because of advances in therapies targeting serum lipids—including statins.1 Since their introduction in 1987, HMG Coenzyme A inhibitors have been intensively studied, and their use has increased dramatically. A recent report from the National Center for Health Statistics reveals that in the years 1999 to 2002, 26% of men ages 65 to 74 years were taking statins; several years later (2005-2008), that number had soared to 50%. In the same time frame, statin use among women ages 65 to 74 went from 24% to 36%.2
Statins lower serum low-density lipoprotein cholesterol (LDL-C) and triglycerides, raise high-density lipoprotein cholesterol (HDL-C), and improve surrogate markers for cardiovascular events. Most importantly, statins reduce the risk for major cardiovascular events, such as myocardial infarction (MI) and death from cardiovascular disease (CVD), in select populations. Yet doubts about the benefits of statins, alone or in combination with other lipid-lowering agents, for certain patient populations remain.
Primary care physicians need to know when, or whether, to add a second lipid-lowering agent to the drug regimen of patients whose response to a statin is less than hoped for, and which patient populations and clinical indicators statins have not been found to help. You’ll find answers, the results of the latest studies, and details of the 2013 cholesterol guideline released last month by the American Heart Association/American College of Cardiology and in this evidence-based update.
A statin is not enough? Don't add these drugs
In patients with very elevated LDL-C or mixed dyslipidemias that fail to reach the desired lipid levels on statin monotherapy, other classes of lipid-modifying agents are often added in an attempt to improve clinical outcomes.3 Fibrates, extended release (niacin, and n-3 polyunsaturated fatty acids have been frequently used for this purpose. But it is only in the last several years that large, well-designed studies have looked closely at patient-oriented outcomes associated with statins in combination with other lipid-modifying drugs.4-9
Fenofibrate + a statin yields little benefit
The ACCORD lipid placebo-controlled trial studied fenofibrate as a simvastatin add-on in patients with diabetes.4 Its findings? While the lipid levels of patients receiving this drug combination improved significantly, the primary endpoint (MI, stroke, or death from cardiovascular causes) was no different from that of the controls, who were taking the statin alone.
Sub-group analysis suggested that the simvastatin-fenofibrate combination benefitted only one particular group: patients with high triglyceride levels (≥204 mg/dL) and low HDL-C (≤34 mg/dL). This finding prompted the US Food and Drug Administration to call for an additional clinical trial to evaluate the effectiveness of add-on therapy with fenofibrate in patients who meet this criteria.10 The status of such a study is uncertain.
Adding niacin to a statin does more harm than good
The HATS trial, published in 2001, found the addition of niacin to a statin regimen to be beneficial.7 But because of the wide confidence interval associated with the clinical endpoints and the small number of subjects (N=160) in that study, larger trials were needed to confirm the positive results. In fact, they found the opposite.
Niacin increases stroke risk. In both the AIM-HIGH5 (N=3414) and HPS2-THRIVE6 (N=25,673) trials, the addition of extended release niacin not only failed to reduce the risk of major cardiovascular events, it was shown to increase the risk of stroke.
n-3 polyunsaturated fatty acids don’t help much
Studies evaluating the addition of n-3 polyunsaturated fatty acids to statin therapy have had mixed results. The JELIS8 trial had more than 18,000 participants, 20% of whom had known coronary artery disease. All were taking statins and randomized to either open-label eicosapentaenoic acid (EPA) 600 mg 3 times daily or placebo. The primary endpoint, a composite of sudden cardiac death, fatal or nonfatal MI, unstable angina, angioplasty, and stenting or coronary artery bypass grafting, was lower in the intervention group: (2.8% vs 3.5%; number needed to treat [NNT]: 143).
It is important to note, however, that only one of the individual components of the primary endpoint—unstable angina—was significantly reduced by EPA (2.1% vs 1.6%; P=.014).8 In the Alpha Omega trial,9 various n-3 polyunsaturated fatty acids were tested in combination with statins. None was found to be superior to placebo in reducing cardiovascular outcomes.
Based on the evidence, the new cholesterol guideline does not support the routine use of these agents to reduce atherosclerotic CVD (See “The new cholesterol guideline: Beyond the headlines”.)11
Statins and kidney disease: Factors to consider
More than half of the deaths in patients with end-stage renal disease (ESRD) are from cardiovascular causes.12 The relationship between renal dysfunction and cardiovascular events is independent of other risk factors, including a history of CVD. Risk rises with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2, with a sharp increase when the rate <45 mL/min/1.73m.2 Thus, strategies known to reduce major cardiovascular events in the general population, including statins, have the potential to offer substantial benefit for patients with chronic kidney disease (CKD).
Statin use in patients with CKD has been evaluated in post-hoc and subgroup analyses of large clinical trials and, more recently, in RCTs targeting patients with both moderate and end-stage disease (TABLE).13-18
Three post-hoc analyses of large multicenter, double-blind RCTs13-15,19 compared patients with normal renal function with those with CKD. All 3 found that moderate or high-intensity statin therapy significantly reduced the incidence of the primary outcome—a composite of major cardiovascular events—compared with either placebo or a lower-intensity statin.
For patients with CKD, drug combo lowered the risk
The SHARP trial17 (N=9270) was the first large prospective, double-blind, multicenter RCT to compare the effect of a statin plus a second lipid-lowering drug (simvastatin plus ezetimibe) vs placebo in patients with CKD. A third of the participants were on dialysis at the start of the trial (ESRD was defined as starting long-term dialysis or requiring kidney transplantation).
Patients in the intervention group were significantly (17%) less likely to experience a major atherosclerotic event compared with those on placebo. This translated into an NNT of 47 over a period of 4.9 years. (Since no group received only simvastatin, it is not known what role ezetimibe had in the reduction of cardiovascular events.) Although no difference in outcomes was found when the results were stratified based on whether participants were on dialysis, this trial was not adequately powered for this subgroup analysis.17
Little benefit from statins in patients with end-stage disease
Two major prospective randomized, double-blinded, placebo-controlled, multicenter trials evaluating the effects of statin use on cardiovascular outcomes in ESRD patients on dialysis have been published.18,19 Both found a significant decline in LDL-C in patients receiving statin therapy. But neither found a significant difference in mortality rates in the statin vs placebo groups.
One group of researchers speculated that the lack of effect may be due to a difference in the pathogenesis of vascular events in patients with and without ESRD. Delayed use of statins until patients have ESRD will offer limited benefit, they concluded, and recommended against routine statin treatment in an attempt to reduce the incidence of CVD in this patient population.18 Based on these results, the new cholesterol guideline indicates that this group of patients may not benefit from statin therapy.
For patients with heart failure, statins offer limited benefit
More than half of the heart failure (HF) in the United States is caused by ischemic heart disease.20 Improvements in post-MI survival have increased the prevalence of chronic HF.
Statins have a well-established role in the prevention and treatment of atherosclerosis because of their ability to modify the natural course of the disease and reduce major adverse cardiovascular events. Thus, it seems reasonable to assume that, in patients who have or are at high risk for coronary heart disease, statins would help to prevent the occurrence or slow the progression of HF.
Early studies of statins either excluded patients with HF or enrolled so few HF patients that no conclusions could be reached regarding the safety or efficacy of statin use in this population.21-25 More recently, 2 large RCTs have studied the effect of statins in patients with HF. Both have found them to be ineffective.26,27
The CORONA trial enrolled elderly patients with HF of ischemic causes and ejection fraction ≤40% (≤35% in patients with New York Heart Association [NYHA] Class II), randomized to either rosuvastatin 10 mg/d or placebo.25 More than 40% of the participants had a history of MI, and more than 60% were NYHA Class III or IV. HF medications were well-managed; more than 90% of the patients were being treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; 75%, with beta-blockers; and 39%, with aldosterone antagonists.
The researchers found no significant difference between the rosuvastatin and placebo groups in the primary outcome of death from cardiovascular causes, nonfatal MI, or nonfatal stroke (11.4% in the rosuvastatin group vs 12.3% among those on placebo; 95% confidence interval, 0.83-1.02; P=.12). No difference was found in patients with a history of MI (13.9% placebo event rate vs 12.7% rosuvastatin arm; P=not significant [NS]). Neither death from worsening HF nor sudden death was reduced.26
There were fewer hospitalizations among those taking rosuvastatin, however (NNT=17 per year). And there was a trend towards a benefit among those with more advanced HF (NYHA III/IV), with primary outcome rates of 12.7% for those in the rosuvastatin group vs 14.2% in the placebo group. (P=NS). Rosuvastatin was safe for HF patients, as most types of adverse events were more common in the placebo group. Assessments of muscle toxicity were similar in both groups.
The GISSI-HF study, another RCT of rosuvastatin vs placebo in HF patients, also showed a lack of benefit from statin treatment.27 Researchers enrolled more than 4500 patients with NYHA Class II to IV HF, from both ischemic and nonischemic causes.26 Similar to the findings in the CORONA trial, LDL-C was substantially reduced by rosuvastatin 10 mg/d (-32% vs no change for those in the placebo group), but this did not translate into clinically relevant endpoints. After 3.9 years of therapy, the primary endpoints of time to death and time to death or hospitalization for cardiovascular causes were not significantly reduced, nor were any of the secondary endpoints.26
The similar lack of benefit in these 2 trials is striking in view of the benefit of statins in patients with coronary heart disease but without HF. Given these findings, focusing on optimizing therapies known to reduce mortality in patients with HF rather than adding a statin in an attempt to alter the atherosclerotic process appears to be a better approach. Thus, the recently published cholesterol guideline does not advocate the initiation or continuation of statin therapy in patients with NYHA Class II-IV HF.
1. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2010 update. Circulation. 2010;121:948-954.
2. US Department of Health and Human Services. Health, United States, 2010. Available at http://www.cdc.gov/nchs/data/hus/hus10.pdf. Accessed July 22, 2011.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
5. AIM-HIGH Investigators; Boden WE, Probsfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. .N Engl J Med. 2011;365:2255-2267.
6. Niacin causes serious unexpected side-effects, but no worthwhile benefits for patients who are at increased risk of heart attacks and strokes [press release]. HPS2-THRIVE: University of Oxford; March 9, 2013. Available at: www.thrivestudy.org/press_release.htm. Accessed July 3, 2013.
7. Brown BG, Zhao X, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583-1592.
8. Yokyama M, Origasa H, Matsuzaki M, et al; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients. Lancet. 2007;369:1090-1098.
9. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015-2026.
10. The Endocrinologic and Metabolic Drugs Advisory Committee of the FDA, Center for Drug Evaluation and Research. Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM261162.pdf. Published June 24, 2011. Accessed July 3, 2013.
11. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Associaton task force on practice guidelines . Circulation. 2013 Nov 12 [Epub ahead of print.]
12. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305.
13. Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557-1563.
14. Shepherd J, Kastelein JJ, Bittner V, et al; TNT (Treating to New Targets) Investigators. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease. J Am Coll Cardiol. 2008;51:1448-1454.
15. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes. Am J Kidney Dis. 2009;54:810-819.
16. Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomized placebo-controlled trial. Lancet. 2011;377:2181-2192.
17. Wanner C, Krane V, Màrz W, et al; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248.
18. Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-1407.
19. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Lancet. 2004;364:685-696.
20. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:e1-e90.
21. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001-1009.
22. Downs JR, Clearfield M, Weis S, et al; AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279:1615-1622.
23. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301-1307.
24. The Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994;344:1383-1389.
25. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). Lancet. 2002;360:1623-1630.
26. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248-2261.
27. GISSI-HF Investigators; Tavozzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial). Lancet. 2008;372:1231-1239.
1. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2010 update. Circulation. 2010;121:948-954.
2. US Department of Health and Human Services. Health, United States, 2010. Available at http://www.cdc.gov/nchs/data/hus/hus10.pdf. Accessed July 22, 2011.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
5. AIM-HIGH Investigators; Boden WE, Probsfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. .N Engl J Med. 2011;365:2255-2267.
6. Niacin causes serious unexpected side-effects, but no worthwhile benefits for patients who are at increased risk of heart attacks and strokes [press release]. HPS2-THRIVE: University of Oxford; March 9, 2013. Available at: www.thrivestudy.org/press_release.htm. Accessed July 3, 2013.
7. Brown BG, Zhao X, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583-1592.
8. Yokyama M, Origasa H, Matsuzaki M, et al; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients. Lancet. 2007;369:1090-1098.
9. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015-2026.
10. The Endocrinologic and Metabolic Drugs Advisory Committee of the FDA, Center for Drug Evaluation and Research. Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM261162.pdf. Published June 24, 2011. Accessed July 3, 2013.
11. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Associaton task force on practice guidelines . Circulation. 2013 Nov 12 [Epub ahead of print.]
12. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305.
13. Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557-1563.
14. Shepherd J, Kastelein JJ, Bittner V, et al; TNT (Treating to New Targets) Investigators. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease. J Am Coll Cardiol. 2008;51:1448-1454.
15. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes. Am J Kidney Dis. 2009;54:810-819.
16. Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomized placebo-controlled trial. Lancet. 2011;377:2181-2192.
17. Wanner C, Krane V, Màrz W, et al; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248.
18. Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-1407.
19. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Lancet. 2004;364:685-696.
20. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:e1-e90.
21. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001-1009.
22. Downs JR, Clearfield M, Weis S, et al; AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279:1615-1622.
23. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301-1307.
24. The Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994;344:1383-1389.
25. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). Lancet. 2002;360:1623-1630.
26. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248-2261.
27. GISSI-HF Investigators; Tavozzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial). Lancet. 2008;372:1231-1239.
An algorithmic approach to otitis media with effusion
› Perform a hearing test when otitis media with effusion is present for 3 months or longer, or whenever you suspect a language delay, learning problems, or a significant hearing loss. C
› Use the results of the hearing test to guide management decisions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A mother brings in her 3-year-old son for a regular check-up. Her only concern is that for the past 2 weeks, he has not been sleeping through the night. She indicates that the sleeping problem began after he was diagnosed with and treated for an ear infection. Fortunately, this hasn’t affected his daily activity or energy, she says.
The child’s appetite is good and he speaks clearly, in 5-word sentences. He is meeting his developmental milestones, and appears well—sitting in his mother’s lap and playing with her smartphone. His head, eyes, ears, nose, and throat exam only turns up fluid behind his left tympanic membrane, which is not red or bulging. The right membrane appears normal, and he has no cervical lymphadenopathy. The rest of his exam is normal. How would you manage a patient like this?
"Glue ear" is often asymptomatic
Otitis media with effusion (OME) is defined as middle-ear effusion (MEE) in the absence of acute signs of infection. In children, OME—also referred to as “glue ear”—most often arises after acute otitis media (AOM). In adults, it often occurs in association with eustachian tube dysfunction, although OME is a separate diagnosis. (To learn more, see “What about OME in adults?”1,2.)
Experts have found it difficult to determine the exact incidence of OME because it is often asymptomatic. In addition, many cases quickly resolve on their own, making it challenging to diagnose. A 2-year prospective study of 2- to 6-year-old preschoolers revealed that MEE, diagnosed via monthly otoscopy and tympanometry, occurred at least once in 53% of the children in the first year and in 61% of the children in the second year.3 A second study followed 7-year-olds monthly for one year and found a 31% incidence of MEE using tympanometry.4 In the 25% of children found to have persistent MEE, the researchers noted spontaneous recovery after an average of 2 months.
We believe that nearly all children have experienced one episode of OME by the age of 3 years, but the prevalence of OME varies with age and the time of year. It is more prevalent in the winter than the summer months.5 OME is more common in Caucasian children than in African American or Asian children.6
Etiology remains elusive
Risk factors for children include a family history of OME, bottle-feeding, day care attendance, exposure to tobacco smoke, and a personal history of allergies.7,8 One study conducted on mice suggested that inherited structural abnormalities of the middle ear and eustachian tube may play a role as well.9 Some have suggested that effusions of OME in children result from chronic inflammation, for example, after AOM, and that the effusions are sterile; however, recent studies have demonstrated that a biofilm is formed by bacterial otopathogens in the effusion.10-12 The common pathogens found include nontypeable Haemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis. Inflammatory exudate or neutrophil infiltration is rare in the fluid, however.
The contribution of allergies to OME in children remains somewhat controversial. A retrospective review from the United Kingdom of 209 children with OME found a history of allergic rhinitis, asthma, and eczema in 89%, 36%, and 24%, respectively.13 However, this study was done at an allergy clinic, and it is possible that the data from the clinic’s specialized patient population are not generalizable. Gastroesophageal reflux may also be associated with OME in children. However, studies measuring the concentration of pepsin and pepsinogen in middle-ear fluid have provided conflicting results.14,15
Look for these signs and symptoms
OME is often asymptomatic. If a patient has clinical signs of an acute illness, including fever and an erythematous tympanic membrane, it’s important to evaluate for another cause. OME can present with hearing loss or a sense of fullness in the ear. While an infant cannot express the hearing loss, the parent may detect it when observing and interacting mwith the child. Parents are also likely to report that the child is experiencing sleep disturbances.16
Vertigo may occur with OME, although not often. It may manifest itself if the child stumbles or falls. An older child or adult with vertigo may say that it feels like the room is spinning.
Diagnosis relies on pneumatic otoscopy
On physical exam, the patient will likely appear well. Otoscopic examination reveals fluid behind a normal or retracted tympanic membrane; the fluid is often clear or yellowish in color.
A subcommittee comprised of members of the American Academy of Pediatrics, American Academy of Family Physicians, and the American Academy of Otolaryngology-Head and Neck Surgery (AAP/AAFP/AAOHNS) published a clinical practice guideline in 2004 that delineates the current diagnosis and management of children between 2 months and 12 years of age with OME.17
Pneumatic otoscopy, which can reveal decreased or absent movement of the tympanic membrane (the result of fluid behind the membrane), is the primary diagnostic method recommended by the guideline. Tympanometry and acoustic reflectometry may also be used to make the diagnosis, especially when the presence of MEE is difficult to determine using pneumatic otoscopy.
CASE › Upon further discussion with the patient’s mother, you learn that the boy goes to day care 3 days a week and stays with his grandmother 2 days a week. His grandmother smokes outside of the home when he is staying with her.
After reviewing these risk factors with the mother (including the importance of smoking cessation for the grandmother), the mother asks if he needs antibiotics or a referral to a specialist for treating his OME.
How best to approach treatment
There are several management options to choose from, including watchful waiting, medication, and/or surgery. (Another option, autoinflation, which has shown some short-term benefits, is described in “Should you recommend autoinflation?”17-19.)
The goals of management are to resolve the effusion, restore normal hearing (if diminished secondary to the effusion), and prevent future episodes or sequelae. The most significant complication of OME is permanent conductive hearing loss, but tinnitus, cholesteatoma, or tympanosclerosis may also occur.
In most patients, OME resolves without medical intervention. If additional action is required, however, the following options may be explored.
Medication. While the AAP/AAFP/AAOHNS guideline recommends against routine antibiotics for OME,17 it does note that a short course may provide short-term benefit to some patients (eg, those for whom a specialist referral or surgery is being considered).
A separate meta-analysis found that antibiotics improve clearance of the effusion within the first month after treatment (rate difference [RD]=0.16; 95% confidence interval [CI], 0.03-0.29 in 12 studies analyzed), but effusion relapses were common, and no significant benefit was noted past the first month (RD=0.06; 95% CI, -0.03 to 0.14 in 8 studies).20
If you do use antibiotics, a 10- to 14-day course is preferred.17 Amoxicillin, amoxicillin-clavulanate and ceftibuten have been evaluated in separate clinical trials, but none has been clearly shown to have significant advantage over any other.21,22
Antihistamines, decongestants, and oral and intranasal corticosteroids have little effect on OME in children and are not recommended.17 A Cochrane review including 16 studies found that children receiving antihistamines and decongestants are unlikely to see their symptoms improve significantly, and many patients experience adverse effects from the medications23 (number needed to harm=9).
A randomized, double-blind trial involving 144 children <9 years of age with OME for at least 2 months evaluated 4 regimens involving amoxicillin alone or in combination with prednisolone. Children in the amoxicillin+prednisolone arms were significantly more likely to clear their effusions at 2 weeks (number needed to treat=6; P=.03), but not at 4 weeks (P=.12). At 4-month follow-up, effusions had recurred in 68.4% and 69.2% of those receiving amoxicillin+prednisolone and those receiving amoxicillin alone, respectively (P= .94).24
Surgery—or not? The AAP/AAFP/AAOHNS guideline recommends physicians perform hearing testing when OME is present for 3 months or longer, or at any time if language delay, learning problems, or a significant hearing loss is suspected in a child with OME. The results of the hearing test can help determine how to proceed, based on the hearing level noted for the better hearing ear.
You can manage children with hearing loss ≤20 dB and without speech, language, or developmental problems with watchful waiting. Children with hearing loss of 21 to 39 dB can be managed with watchful waiting or referred for surgery. If watchful waiting is pursued, there are interventions at home and at school that can help. These include speaking near the child, facing the child when speaking, and providing accommodations in school so the child sits closer to the teacher. Consider re-examination and repeat hearing tests every 3 to 6 months until the effusion has resolved or the child develops symptoms indicating surgical referral.
When hearing loss is ≥40 dB, the AAP/AAFP/AAOHNS guideline recommends that you make a referral for surgical evaluation (ALGORITHM).17
Other indications for referral to a surgeon for evaluation of tympanostomy tube placement include situations in which there is:
• structural damage to the tympanic membrane or middle ear (prompt referral is recommended)
• OME of ≥4 months’ duration with persistent hearing loss (≥40 dB) or other signs or symptoms related to the effusion
• bilateral OME for ≥3 months, unilateral OME ≥6 months, or total duration of any degree of OME ≥12 months.17
Any decision regarding surgery should involve an otolaryngologist, the primary care provider, and the patient and/or family. The AAP/AAFP/AAOHNS guideline recommends against adenoidectomy in children with persistent OME without an indication for the procedure other than OME (eg, chronic sinusitis or nasal obstruction).17
Keep in mind that evidence of lasting benefit (>12 months) is limited for surgery in most patients, and the surgical and anesthetic risks must be considered before moving forward.17 (For more on the evidence regarding surgery, see “Cochrane weighs in on tympanostomy tubes”.25) Tonsillectomy also does not appear to affect outcomes and is not advised.17
When a referral is always needed. Regardless of hearing status, promptly refer children with recurrent or persistent OME who are at risk of speech, language, or learning problems (including those with autism spectrum disorder, developmental delay, Down’s syndrome, diagnosed speech or language delay, or craniofacial disorders such as cleft palate) to a specialist.17
CASE › You tell your young patient’s mother that watchful waiting is appropriate at this point, since his acute otitis media was only 2 weeks ago, and his OME likely started after the acute infection. Given that his speech is clear and he is otherwise meeting his milestones, you tell her that he does not need a referral at this time, but that she should bring him back in 4 weeks for reassessment. At the next visit, his effusion has resolved, and his mother reports he is sleeping well through the night again.
1. Lesinskas E. Factors affecting the results of nonsurgical treatment of secretory otitis media in adults. Auris Nasus Larynx. 2003:30:7-14.
2. Chole RA, HH Sudhoff. Chronic otitis media, mastoiditis, and petrosis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Maryland Heights, MO. Mosby;2010:chap 139.
3. Casselbrant ML, Brostoff LM, Cantekin EI, et al. Otitis media with effusion in preschool children. Laryngoscope. 1985;95:428-436.
4. Lous J, Fiellau-Nikolajsen M. Epidemiology of middle-ear effusion and tubal dysfunction: a one year prospective study comprising monthly tympanometry in 387 non-selected seven-yearold children. Int J Pediatr Otorhinolaryngol. 1981;3:303-317.
5. Tos M, Holm-Jensen S, Sørensen CH. Changes in prevalence of secretory otitis from summer to winter in four-year-old children. Am J Otol. 1981;2:324-327.
6. Vernacchio L, Lesko SM, Vezina RM, et al. Racial/ethnic disparities in the diagnosis of otitis media in infancy. Int J Pediatr Otorhinolaryngol. 2004;68:795-804.
7. Owen MJ, Baldwin CD, Swank PR, et al. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J Pediatr. 1993;123:702-711.
8. Gultekin E, Develio˘gu ON, Yener M, et al. Prevalence and risk factors for persistent otitis media with effusion in primary school children in Istanbul, Turkey. Auris Nasus Larynx. 2010;37:145-149.
9. Depreux FF, Darrow K, Conner DA, et al. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008;118:651-658.
10. Poetker DM, Lindstrom DR, Edmiston CE, et al. Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol. 2005;69:799-804.
11. Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202-211.
12. Brook I, Yocum P, Shah K, et al. Microbiology of serous otitis media in children: correlation with age and length of effusion. Ann Otol Rhinol Laryngol. 2001;110:87-90.
13. Alles R, Parikh A, Hawk L, et al. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol. 2001;12:102-106.
14. Lieu JE, Muthappan PG, Uppaluri R. Association of reflux with otitis media in children. Otolaryngol Head Neck Surg. 2005;133:357-361.
15. O’Reilly RC, He Z, Bloedon E, et al. The role of extraesophageal reflux in otitis media in infants and children. Laryngoscope. 2008;118 (7 part 2, suppl 116):S1-S9.
16. Rosenfeld RM, Goldsmith AJ, Tetlus L, et al. Quality of life for children with otitis media. Arch Otolaryngol Head Neck Surg. 1997;123:1049-1054.
17. American Academy of Family Physicians, American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Otitis media with effusion. Pediatrics. 2004;113:1412-1429.
18. Perera R, Glasziou PP, Heneghan CJ, et al Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev. 2013;5:CD006285.
19. Stangerup SE, Sederberg-Olsen J, Balle V. Autoinflation as a treatment of secretory otitis media. A randomized controlled study. Arch Otolaryngol Head Neck Surg. 1992;118:149-152.
20. Williams RL, Chalmers TC, Stange KC, et al. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA. 1993;270:1344-1351.
21. Mandel EM, Casselbrant ML, Kurs-Lasky M, et al. Efficacy of ceftibuten compared with amoxicillin for otitis media with effusion in infants and children. Pediatr Infect Dis J. 1996;15:409-414.
22. Chan KH, Mandel EM, Rockette HE, et al. A comparative study of amoxicillin-clavulanate and amoxicillin. Treatment of otitis media with effusion. Arch Otolaryngol Head Neck Surg. 1988; 114:142-146.
23. Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2011;(9):CD003423.
24. Mandel EM, Casselbrant ML, Rockette HE, et al. Systemic steroid for chronic otitis media with effusion in children. Pediatrics. 2002;110:1071-1080.
25. Browning GG, Rovers MM, Williamson I, et al. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;(10):CD001801.
› Perform a hearing test when otitis media with effusion is present for 3 months or longer, or whenever you suspect a language delay, learning problems, or a significant hearing loss. C
› Use the results of the hearing test to guide management decisions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A mother brings in her 3-year-old son for a regular check-up. Her only concern is that for the past 2 weeks, he has not been sleeping through the night. She indicates that the sleeping problem began after he was diagnosed with and treated for an ear infection. Fortunately, this hasn’t affected his daily activity or energy, she says.
The child’s appetite is good and he speaks clearly, in 5-word sentences. He is meeting his developmental milestones, and appears well—sitting in his mother’s lap and playing with her smartphone. His head, eyes, ears, nose, and throat exam only turns up fluid behind his left tympanic membrane, which is not red or bulging. The right membrane appears normal, and he has no cervical lymphadenopathy. The rest of his exam is normal. How would you manage a patient like this?
"Glue ear" is often asymptomatic
Otitis media with effusion (OME) is defined as middle-ear effusion (MEE) in the absence of acute signs of infection. In children, OME—also referred to as “glue ear”—most often arises after acute otitis media (AOM). In adults, it often occurs in association with eustachian tube dysfunction, although OME is a separate diagnosis. (To learn more, see “What about OME in adults?”1,2.)
Experts have found it difficult to determine the exact incidence of OME because it is often asymptomatic. In addition, many cases quickly resolve on their own, making it challenging to diagnose. A 2-year prospective study of 2- to 6-year-old preschoolers revealed that MEE, diagnosed via monthly otoscopy and tympanometry, occurred at least once in 53% of the children in the first year and in 61% of the children in the second year.3 A second study followed 7-year-olds monthly for one year and found a 31% incidence of MEE using tympanometry.4 In the 25% of children found to have persistent MEE, the researchers noted spontaneous recovery after an average of 2 months.
We believe that nearly all children have experienced one episode of OME by the age of 3 years, but the prevalence of OME varies with age and the time of year. It is more prevalent in the winter than the summer months.5 OME is more common in Caucasian children than in African American or Asian children.6
Etiology remains elusive
Risk factors for children include a family history of OME, bottle-feeding, day care attendance, exposure to tobacco smoke, and a personal history of allergies.7,8 One study conducted on mice suggested that inherited structural abnormalities of the middle ear and eustachian tube may play a role as well.9 Some have suggested that effusions of OME in children result from chronic inflammation, for example, after AOM, and that the effusions are sterile; however, recent studies have demonstrated that a biofilm is formed by bacterial otopathogens in the effusion.10-12 The common pathogens found include nontypeable Haemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis. Inflammatory exudate or neutrophil infiltration is rare in the fluid, however.
The contribution of allergies to OME in children remains somewhat controversial. A retrospective review from the United Kingdom of 209 children with OME found a history of allergic rhinitis, asthma, and eczema in 89%, 36%, and 24%, respectively.13 However, this study was done at an allergy clinic, and it is possible that the data from the clinic’s specialized patient population are not generalizable. Gastroesophageal reflux may also be associated with OME in children. However, studies measuring the concentration of pepsin and pepsinogen in middle-ear fluid have provided conflicting results.14,15
Look for these signs and symptoms
OME is often asymptomatic. If a patient has clinical signs of an acute illness, including fever and an erythematous tympanic membrane, it’s important to evaluate for another cause. OME can present with hearing loss or a sense of fullness in the ear. While an infant cannot express the hearing loss, the parent may detect it when observing and interacting mwith the child. Parents are also likely to report that the child is experiencing sleep disturbances.16
Vertigo may occur with OME, although not often. It may manifest itself if the child stumbles or falls. An older child or adult with vertigo may say that it feels like the room is spinning.
Diagnosis relies on pneumatic otoscopy
On physical exam, the patient will likely appear well. Otoscopic examination reveals fluid behind a normal or retracted tympanic membrane; the fluid is often clear or yellowish in color.
A subcommittee comprised of members of the American Academy of Pediatrics, American Academy of Family Physicians, and the American Academy of Otolaryngology-Head and Neck Surgery (AAP/AAFP/AAOHNS) published a clinical practice guideline in 2004 that delineates the current diagnosis and management of children between 2 months and 12 years of age with OME.17
Pneumatic otoscopy, which can reveal decreased or absent movement of the tympanic membrane (the result of fluid behind the membrane), is the primary diagnostic method recommended by the guideline. Tympanometry and acoustic reflectometry may also be used to make the diagnosis, especially when the presence of MEE is difficult to determine using pneumatic otoscopy.
CASE › Upon further discussion with the patient’s mother, you learn that the boy goes to day care 3 days a week and stays with his grandmother 2 days a week. His grandmother smokes outside of the home when he is staying with her.
After reviewing these risk factors with the mother (including the importance of smoking cessation for the grandmother), the mother asks if he needs antibiotics or a referral to a specialist for treating his OME.
How best to approach treatment
There are several management options to choose from, including watchful waiting, medication, and/or surgery. (Another option, autoinflation, which has shown some short-term benefits, is described in “Should you recommend autoinflation?”17-19.)
The goals of management are to resolve the effusion, restore normal hearing (if diminished secondary to the effusion), and prevent future episodes or sequelae. The most significant complication of OME is permanent conductive hearing loss, but tinnitus, cholesteatoma, or tympanosclerosis may also occur.
In most patients, OME resolves without medical intervention. If additional action is required, however, the following options may be explored.
Medication. While the AAP/AAFP/AAOHNS guideline recommends against routine antibiotics for OME,17 it does note that a short course may provide short-term benefit to some patients (eg, those for whom a specialist referral or surgery is being considered).
A separate meta-analysis found that antibiotics improve clearance of the effusion within the first month after treatment (rate difference [RD]=0.16; 95% confidence interval [CI], 0.03-0.29 in 12 studies analyzed), but effusion relapses were common, and no significant benefit was noted past the first month (RD=0.06; 95% CI, -0.03 to 0.14 in 8 studies).20
If you do use antibiotics, a 10- to 14-day course is preferred.17 Amoxicillin, amoxicillin-clavulanate and ceftibuten have been evaluated in separate clinical trials, but none has been clearly shown to have significant advantage over any other.21,22
Antihistamines, decongestants, and oral and intranasal corticosteroids have little effect on OME in children and are not recommended.17 A Cochrane review including 16 studies found that children receiving antihistamines and decongestants are unlikely to see their symptoms improve significantly, and many patients experience adverse effects from the medications23 (number needed to harm=9).
A randomized, double-blind trial involving 144 children <9 years of age with OME for at least 2 months evaluated 4 regimens involving amoxicillin alone or in combination with prednisolone. Children in the amoxicillin+prednisolone arms were significantly more likely to clear their effusions at 2 weeks (number needed to treat=6; P=.03), but not at 4 weeks (P=.12). At 4-month follow-up, effusions had recurred in 68.4% and 69.2% of those receiving amoxicillin+prednisolone and those receiving amoxicillin alone, respectively (P= .94).24
Surgery—or not? The AAP/AAFP/AAOHNS guideline recommends physicians perform hearing testing when OME is present for 3 months or longer, or at any time if language delay, learning problems, or a significant hearing loss is suspected in a child with OME. The results of the hearing test can help determine how to proceed, based on the hearing level noted for the better hearing ear.
You can manage children with hearing loss ≤20 dB and without speech, language, or developmental problems with watchful waiting. Children with hearing loss of 21 to 39 dB can be managed with watchful waiting or referred for surgery. If watchful waiting is pursued, there are interventions at home and at school that can help. These include speaking near the child, facing the child when speaking, and providing accommodations in school so the child sits closer to the teacher. Consider re-examination and repeat hearing tests every 3 to 6 months until the effusion has resolved or the child develops symptoms indicating surgical referral.
When hearing loss is ≥40 dB, the AAP/AAFP/AAOHNS guideline recommends that you make a referral for surgical evaluation (ALGORITHM).17
Other indications for referral to a surgeon for evaluation of tympanostomy tube placement include situations in which there is:
• structural damage to the tympanic membrane or middle ear (prompt referral is recommended)
• OME of ≥4 months’ duration with persistent hearing loss (≥40 dB) or other signs or symptoms related to the effusion
• bilateral OME for ≥3 months, unilateral OME ≥6 months, or total duration of any degree of OME ≥12 months.17
Any decision regarding surgery should involve an otolaryngologist, the primary care provider, and the patient and/or family. The AAP/AAFP/AAOHNS guideline recommends against adenoidectomy in children with persistent OME without an indication for the procedure other than OME (eg, chronic sinusitis or nasal obstruction).17
Keep in mind that evidence of lasting benefit (>12 months) is limited for surgery in most patients, and the surgical and anesthetic risks must be considered before moving forward.17 (For more on the evidence regarding surgery, see “Cochrane weighs in on tympanostomy tubes”.25) Tonsillectomy also does not appear to affect outcomes and is not advised.17
When a referral is always needed. Regardless of hearing status, promptly refer children with recurrent or persistent OME who are at risk of speech, language, or learning problems (including those with autism spectrum disorder, developmental delay, Down’s syndrome, diagnosed speech or language delay, or craniofacial disorders such as cleft palate) to a specialist.17
CASE › You tell your young patient’s mother that watchful waiting is appropriate at this point, since his acute otitis media was only 2 weeks ago, and his OME likely started after the acute infection. Given that his speech is clear and he is otherwise meeting his milestones, you tell her that he does not need a referral at this time, but that she should bring him back in 4 weeks for reassessment. At the next visit, his effusion has resolved, and his mother reports he is sleeping well through the night again.
› Perform a hearing test when otitis media with effusion is present for 3 months or longer, or whenever you suspect a language delay, learning problems, or a significant hearing loss. C
› Use the results of the hearing test to guide management decisions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A mother brings in her 3-year-old son for a regular check-up. Her only concern is that for the past 2 weeks, he has not been sleeping through the night. She indicates that the sleeping problem began after he was diagnosed with and treated for an ear infection. Fortunately, this hasn’t affected his daily activity or energy, she says.
The child’s appetite is good and he speaks clearly, in 5-word sentences. He is meeting his developmental milestones, and appears well—sitting in his mother’s lap and playing with her smartphone. His head, eyes, ears, nose, and throat exam only turns up fluid behind his left tympanic membrane, which is not red or bulging. The right membrane appears normal, and he has no cervical lymphadenopathy. The rest of his exam is normal. How would you manage a patient like this?
"Glue ear" is often asymptomatic
Otitis media with effusion (OME) is defined as middle-ear effusion (MEE) in the absence of acute signs of infection. In children, OME—also referred to as “glue ear”—most often arises after acute otitis media (AOM). In adults, it often occurs in association with eustachian tube dysfunction, although OME is a separate diagnosis. (To learn more, see “What about OME in adults?”1,2.)
Experts have found it difficult to determine the exact incidence of OME because it is often asymptomatic. In addition, many cases quickly resolve on their own, making it challenging to diagnose. A 2-year prospective study of 2- to 6-year-old preschoolers revealed that MEE, diagnosed via monthly otoscopy and tympanometry, occurred at least once in 53% of the children in the first year and in 61% of the children in the second year.3 A second study followed 7-year-olds monthly for one year and found a 31% incidence of MEE using tympanometry.4 In the 25% of children found to have persistent MEE, the researchers noted spontaneous recovery after an average of 2 months.
We believe that nearly all children have experienced one episode of OME by the age of 3 years, but the prevalence of OME varies with age and the time of year. It is more prevalent in the winter than the summer months.5 OME is more common in Caucasian children than in African American or Asian children.6
Etiology remains elusive
Risk factors for children include a family history of OME, bottle-feeding, day care attendance, exposure to tobacco smoke, and a personal history of allergies.7,8 One study conducted on mice suggested that inherited structural abnormalities of the middle ear and eustachian tube may play a role as well.9 Some have suggested that effusions of OME in children result from chronic inflammation, for example, after AOM, and that the effusions are sterile; however, recent studies have demonstrated that a biofilm is formed by bacterial otopathogens in the effusion.10-12 The common pathogens found include nontypeable Haemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis. Inflammatory exudate or neutrophil infiltration is rare in the fluid, however.
The contribution of allergies to OME in children remains somewhat controversial. A retrospective review from the United Kingdom of 209 children with OME found a history of allergic rhinitis, asthma, and eczema in 89%, 36%, and 24%, respectively.13 However, this study was done at an allergy clinic, and it is possible that the data from the clinic’s specialized patient population are not generalizable. Gastroesophageal reflux may also be associated with OME in children. However, studies measuring the concentration of pepsin and pepsinogen in middle-ear fluid have provided conflicting results.14,15
Look for these signs and symptoms
OME is often asymptomatic. If a patient has clinical signs of an acute illness, including fever and an erythematous tympanic membrane, it’s important to evaluate for another cause. OME can present with hearing loss or a sense of fullness in the ear. While an infant cannot express the hearing loss, the parent may detect it when observing and interacting mwith the child. Parents are also likely to report that the child is experiencing sleep disturbances.16
Vertigo may occur with OME, although not often. It may manifest itself if the child stumbles or falls. An older child or adult with vertigo may say that it feels like the room is spinning.
Diagnosis relies on pneumatic otoscopy
On physical exam, the patient will likely appear well. Otoscopic examination reveals fluid behind a normal or retracted tympanic membrane; the fluid is often clear or yellowish in color.
A subcommittee comprised of members of the American Academy of Pediatrics, American Academy of Family Physicians, and the American Academy of Otolaryngology-Head and Neck Surgery (AAP/AAFP/AAOHNS) published a clinical practice guideline in 2004 that delineates the current diagnosis and management of children between 2 months and 12 years of age with OME.17
Pneumatic otoscopy, which can reveal decreased or absent movement of the tympanic membrane (the result of fluid behind the membrane), is the primary diagnostic method recommended by the guideline. Tympanometry and acoustic reflectometry may also be used to make the diagnosis, especially when the presence of MEE is difficult to determine using pneumatic otoscopy.
CASE › Upon further discussion with the patient’s mother, you learn that the boy goes to day care 3 days a week and stays with his grandmother 2 days a week. His grandmother smokes outside of the home when he is staying with her.
After reviewing these risk factors with the mother (including the importance of smoking cessation for the grandmother), the mother asks if he needs antibiotics or a referral to a specialist for treating his OME.
How best to approach treatment
There are several management options to choose from, including watchful waiting, medication, and/or surgery. (Another option, autoinflation, which has shown some short-term benefits, is described in “Should you recommend autoinflation?”17-19.)
The goals of management are to resolve the effusion, restore normal hearing (if diminished secondary to the effusion), and prevent future episodes or sequelae. The most significant complication of OME is permanent conductive hearing loss, but tinnitus, cholesteatoma, or tympanosclerosis may also occur.
In most patients, OME resolves without medical intervention. If additional action is required, however, the following options may be explored.
Medication. While the AAP/AAFP/AAOHNS guideline recommends against routine antibiotics for OME,17 it does note that a short course may provide short-term benefit to some patients (eg, those for whom a specialist referral or surgery is being considered).
A separate meta-analysis found that antibiotics improve clearance of the effusion within the first month after treatment (rate difference [RD]=0.16; 95% confidence interval [CI], 0.03-0.29 in 12 studies analyzed), but effusion relapses were common, and no significant benefit was noted past the first month (RD=0.06; 95% CI, -0.03 to 0.14 in 8 studies).20
If you do use antibiotics, a 10- to 14-day course is preferred.17 Amoxicillin, amoxicillin-clavulanate and ceftibuten have been evaluated in separate clinical trials, but none has been clearly shown to have significant advantage over any other.21,22
Antihistamines, decongestants, and oral and intranasal corticosteroids have little effect on OME in children and are not recommended.17 A Cochrane review including 16 studies found that children receiving antihistamines and decongestants are unlikely to see their symptoms improve significantly, and many patients experience adverse effects from the medications23 (number needed to harm=9).
A randomized, double-blind trial involving 144 children <9 years of age with OME for at least 2 months evaluated 4 regimens involving amoxicillin alone or in combination with prednisolone. Children in the amoxicillin+prednisolone arms were significantly more likely to clear their effusions at 2 weeks (number needed to treat=6; P=.03), but not at 4 weeks (P=.12). At 4-month follow-up, effusions had recurred in 68.4% and 69.2% of those receiving amoxicillin+prednisolone and those receiving amoxicillin alone, respectively (P= .94).24
Surgery—or not? The AAP/AAFP/AAOHNS guideline recommends physicians perform hearing testing when OME is present for 3 months or longer, or at any time if language delay, learning problems, or a significant hearing loss is suspected in a child with OME. The results of the hearing test can help determine how to proceed, based on the hearing level noted for the better hearing ear.
You can manage children with hearing loss ≤20 dB and without speech, language, or developmental problems with watchful waiting. Children with hearing loss of 21 to 39 dB can be managed with watchful waiting or referred for surgery. If watchful waiting is pursued, there are interventions at home and at school that can help. These include speaking near the child, facing the child when speaking, and providing accommodations in school so the child sits closer to the teacher. Consider re-examination and repeat hearing tests every 3 to 6 months until the effusion has resolved or the child develops symptoms indicating surgical referral.
When hearing loss is ≥40 dB, the AAP/AAFP/AAOHNS guideline recommends that you make a referral for surgical evaluation (ALGORITHM).17
Other indications for referral to a surgeon for evaluation of tympanostomy tube placement include situations in which there is:
• structural damage to the tympanic membrane or middle ear (prompt referral is recommended)
• OME of ≥4 months’ duration with persistent hearing loss (≥40 dB) or other signs or symptoms related to the effusion
• bilateral OME for ≥3 months, unilateral OME ≥6 months, or total duration of any degree of OME ≥12 months.17
Any decision regarding surgery should involve an otolaryngologist, the primary care provider, and the patient and/or family. The AAP/AAFP/AAOHNS guideline recommends against adenoidectomy in children with persistent OME without an indication for the procedure other than OME (eg, chronic sinusitis or nasal obstruction).17
Keep in mind that evidence of lasting benefit (>12 months) is limited for surgery in most patients, and the surgical and anesthetic risks must be considered before moving forward.17 (For more on the evidence regarding surgery, see “Cochrane weighs in on tympanostomy tubes”.25) Tonsillectomy also does not appear to affect outcomes and is not advised.17
When a referral is always needed. Regardless of hearing status, promptly refer children with recurrent or persistent OME who are at risk of speech, language, or learning problems (including those with autism spectrum disorder, developmental delay, Down’s syndrome, diagnosed speech or language delay, or craniofacial disorders such as cleft palate) to a specialist.17
CASE › You tell your young patient’s mother that watchful waiting is appropriate at this point, since his acute otitis media was only 2 weeks ago, and his OME likely started after the acute infection. Given that his speech is clear and he is otherwise meeting his milestones, you tell her that he does not need a referral at this time, but that she should bring him back in 4 weeks for reassessment. At the next visit, his effusion has resolved, and his mother reports he is sleeping well through the night again.
1. Lesinskas E. Factors affecting the results of nonsurgical treatment of secretory otitis media in adults. Auris Nasus Larynx. 2003:30:7-14.
2. Chole RA, HH Sudhoff. Chronic otitis media, mastoiditis, and petrosis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Maryland Heights, MO. Mosby;2010:chap 139.
3. Casselbrant ML, Brostoff LM, Cantekin EI, et al. Otitis media with effusion in preschool children. Laryngoscope. 1985;95:428-436.
4. Lous J, Fiellau-Nikolajsen M. Epidemiology of middle-ear effusion and tubal dysfunction: a one year prospective study comprising monthly tympanometry in 387 non-selected seven-yearold children. Int J Pediatr Otorhinolaryngol. 1981;3:303-317.
5. Tos M, Holm-Jensen S, Sørensen CH. Changes in prevalence of secretory otitis from summer to winter in four-year-old children. Am J Otol. 1981;2:324-327.
6. Vernacchio L, Lesko SM, Vezina RM, et al. Racial/ethnic disparities in the diagnosis of otitis media in infancy. Int J Pediatr Otorhinolaryngol. 2004;68:795-804.
7. Owen MJ, Baldwin CD, Swank PR, et al. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J Pediatr. 1993;123:702-711.
8. Gultekin E, Develio˘gu ON, Yener M, et al. Prevalence and risk factors for persistent otitis media with effusion in primary school children in Istanbul, Turkey. Auris Nasus Larynx. 2010;37:145-149.
9. Depreux FF, Darrow K, Conner DA, et al. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008;118:651-658.
10. Poetker DM, Lindstrom DR, Edmiston CE, et al. Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol. 2005;69:799-804.
11. Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202-211.
12. Brook I, Yocum P, Shah K, et al. Microbiology of serous otitis media in children: correlation with age and length of effusion. Ann Otol Rhinol Laryngol. 2001;110:87-90.
13. Alles R, Parikh A, Hawk L, et al. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol. 2001;12:102-106.
14. Lieu JE, Muthappan PG, Uppaluri R. Association of reflux with otitis media in children. Otolaryngol Head Neck Surg. 2005;133:357-361.
15. O’Reilly RC, He Z, Bloedon E, et al. The role of extraesophageal reflux in otitis media in infants and children. Laryngoscope. 2008;118 (7 part 2, suppl 116):S1-S9.
16. Rosenfeld RM, Goldsmith AJ, Tetlus L, et al. Quality of life for children with otitis media. Arch Otolaryngol Head Neck Surg. 1997;123:1049-1054.
17. American Academy of Family Physicians, American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Otitis media with effusion. Pediatrics. 2004;113:1412-1429.
18. Perera R, Glasziou PP, Heneghan CJ, et al Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev. 2013;5:CD006285.
19. Stangerup SE, Sederberg-Olsen J, Balle V. Autoinflation as a treatment of secretory otitis media. A randomized controlled study. Arch Otolaryngol Head Neck Surg. 1992;118:149-152.
20. Williams RL, Chalmers TC, Stange KC, et al. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA. 1993;270:1344-1351.
21. Mandel EM, Casselbrant ML, Kurs-Lasky M, et al. Efficacy of ceftibuten compared with amoxicillin for otitis media with effusion in infants and children. Pediatr Infect Dis J. 1996;15:409-414.
22. Chan KH, Mandel EM, Rockette HE, et al. A comparative study of amoxicillin-clavulanate and amoxicillin. Treatment of otitis media with effusion. Arch Otolaryngol Head Neck Surg. 1988; 114:142-146.
23. Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2011;(9):CD003423.
24. Mandel EM, Casselbrant ML, Rockette HE, et al. Systemic steroid for chronic otitis media with effusion in children. Pediatrics. 2002;110:1071-1080.
25. Browning GG, Rovers MM, Williamson I, et al. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;(10):CD001801.
1. Lesinskas E. Factors affecting the results of nonsurgical treatment of secretory otitis media in adults. Auris Nasus Larynx. 2003:30:7-14.
2. Chole RA, HH Sudhoff. Chronic otitis media, mastoiditis, and petrosis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 5th ed. Maryland Heights, MO. Mosby;2010:chap 139.
3. Casselbrant ML, Brostoff LM, Cantekin EI, et al. Otitis media with effusion in preschool children. Laryngoscope. 1985;95:428-436.
4. Lous J, Fiellau-Nikolajsen M. Epidemiology of middle-ear effusion and tubal dysfunction: a one year prospective study comprising monthly tympanometry in 387 non-selected seven-yearold children. Int J Pediatr Otorhinolaryngol. 1981;3:303-317.
5. Tos M, Holm-Jensen S, Sørensen CH. Changes in prevalence of secretory otitis from summer to winter in four-year-old children. Am J Otol. 1981;2:324-327.
6. Vernacchio L, Lesko SM, Vezina RM, et al. Racial/ethnic disparities in the diagnosis of otitis media in infancy. Int J Pediatr Otorhinolaryngol. 2004;68:795-804.
7. Owen MJ, Baldwin CD, Swank PR, et al. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J Pediatr. 1993;123:702-711.
8. Gultekin E, Develio˘gu ON, Yener M, et al. Prevalence and risk factors for persistent otitis media with effusion in primary school children in Istanbul, Turkey. Auris Nasus Larynx. 2010;37:145-149.
9. Depreux FF, Darrow K, Conner DA, et al. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008;118:651-658.
10. Poetker DM, Lindstrom DR, Edmiston CE, et al. Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol. 2005;69:799-804.
11. Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202-211.
12. Brook I, Yocum P, Shah K, et al. Microbiology of serous otitis media in children: correlation with age and length of effusion. Ann Otol Rhinol Laryngol. 2001;110:87-90.
13. Alles R, Parikh A, Hawk L, et al. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol. 2001;12:102-106.
14. Lieu JE, Muthappan PG, Uppaluri R. Association of reflux with otitis media in children. Otolaryngol Head Neck Surg. 2005;133:357-361.
15. O’Reilly RC, He Z, Bloedon E, et al. The role of extraesophageal reflux in otitis media in infants and children. Laryngoscope. 2008;118 (7 part 2, suppl 116):S1-S9.
16. Rosenfeld RM, Goldsmith AJ, Tetlus L, et al. Quality of life for children with otitis media. Arch Otolaryngol Head Neck Surg. 1997;123:1049-1054.
17. American Academy of Family Physicians, American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Otitis media with effusion. Pediatrics. 2004;113:1412-1429.
18. Perera R, Glasziou PP, Heneghan CJ, et al Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev. 2013;5:CD006285.
19. Stangerup SE, Sederberg-Olsen J, Balle V. Autoinflation as a treatment of secretory otitis media. A randomized controlled study. Arch Otolaryngol Head Neck Surg. 1992;118:149-152.
20. Williams RL, Chalmers TC, Stange KC, et al. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA. 1993;270:1344-1351.
21. Mandel EM, Casselbrant ML, Kurs-Lasky M, et al. Efficacy of ceftibuten compared with amoxicillin for otitis media with effusion in infants and children. Pediatr Infect Dis J. 1996;15:409-414.
22. Chan KH, Mandel EM, Rockette HE, et al. A comparative study of amoxicillin-clavulanate and amoxicillin. Treatment of otitis media with effusion. Arch Otolaryngol Head Neck Surg. 1988; 114:142-146.
23. Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2011;(9):CD003423.
24. Mandel EM, Casselbrant ML, Rockette HE, et al. Systemic steroid for chronic otitis media with effusion in children. Pediatrics. 2002;110:1071-1080.
25. Browning GG, Rovers MM, Williamson I, et al. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;(10):CD001801.
Fecal incontinence: Help for patients who suffer silently
› Consider adding a question about fecal incontinence—a condition often unreported by patients and undetected by physicians—to your medical intake form. C
› Use bowel diaries and fecal incontinence grading systems, as needed, to better understand the extent of the problem and assess the effects of treatment. C
› Consider sacral nerve stimulation, the first-line surgical treatment for fecal incontinence, for those who fail to respond to medical therapies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimates suggest that about 18 million adults in the United States suffer from fecal incontinence.1 But because the condition often goes unreported by patients and undetected by physicians, the actual prevalence is not known—and may be considerably higher.
What is known is that fecal incontinence carries a substantial socioeconomic burden. The average annual per patient cost is estimated at $4110.2 But fecal incontinence also exacts a heavy personal toll, and is one of the main reasons elderly individuals are placed in nursing homes.3
But it’s not just the elderly who are affected. A recent study of women ages 45 years and older found that nearly one in 5 had an episode of fecal incontinence at least once a year, and for nearly half, the frequency was once a month or more.4 Less than 3 in 10 reported their symptoms to a clinician, but those who did were most likely to have confided in their primary care physician.5
Fortunately, recent developments—most notably, sacral nerve stimulation, a minimally invasive surgical technique with a high success rate—have changed the outlook for patients with fecal incontinence. Here’s what you need to know to help patients who suffer from this embarrassing condition achieve optimal outcomes.
Risk factors and key causes
Maintaining fecal continence involves a complex series of events, both voluntary and involuntary. Problems at various levels—stool consistency, anatomic and neurologic abnormalities, and psychological problems among them—can disrupt the process.
Those at high risk for fecal incontinence, in addition to the elderly, include patients who are mentally ill and institutionalized, individuals with neurologic disorders, patients who have had anorectal surgery, and women who have had vaginal deliveries.6-8 Obstetric and operative injuries account for most cases of fecal incontinence.9-10
Sphincter defects including attenuation and scarring (shown here), are commonly caused by obstetric and operative injuries.
Risks of vaginal delivery
As many as 25% of women report some degree of fecal incontinence—although often confined to loss of control of flatus—3 months after giving birth.11 Stool incontinence is more frequent among women who sustained third- or fourth-degree perineal tears. Obstetrical risk factors include first vaginal birth, median episiotomy, forceps delivery, vacuum-assisted delivery, and a prolonged second stage of labor.
Asymptomatic sphincter defects. Studies in which women underwent endosonographic examination of the sphincter complex both before and after vaginal delivery have found sphincter defects in anywhere from 7% to 41% of new mothers.12-14 It is important to note, however, that as many as 70% of those with defects detected by sonogram were asymptomatic.15 (Despite the risk of sphincter injury during vaginal delivery and evidence suggesting that the risk of fecal incontinence increases with additional deliveries after a previous perineal tear, prophylactic cesarean section is not recommended.)
Fistula surgery and postop incontinence
Fistula surgery is the primary cause of postoperative incontinence, typically resulting from inadvertent injury to either the internal or external sphincter muscle.16 Other relatively common causes of fecal incontinence are rectal prolapse, trauma, irradiation, neurologic and demyelinating disorders such as multiple sclerosis, neoplasms, stroke, infection (eg, of a perineal wound), and diabetes.17 As diagnostic modalities have improved, much of what was previously termed idiopathic incontinence has been found to have identifiable underlying pathology, such as pudendal and inferior hypogastric neuropathies.18-20
Identifying fecal incontinence starts with a single question
As already noted, most patients with symptoms of bowel leakage do not voluntarily mention it to their physician. Many are likely to acknowledge the problem, however, if they’re specifically asked. While little has been written about how best to screen for fecal incontinence, simply adding it to the checklist on your medical intake form may be a good starting point.
Follow up with a targeted history and physical
When a patient checks fecal incontinence on a form or broaches the subject, it is important to question him or her about medical conditions that may be related. These include urinary incontinence, prolapsing tissue, diabetes, and a history of radiation, as well as childbirth. A medication history is also needed, as certain drugs—including some antacids and laxatives—have been implicated in fecal incontinence.21
Physical assessment should include a general neurologic exam as well as a perineal exam, to look for prolapsing tissue and evidence of scars from prior surgery or obstetrical trauma. Check the anocutaneous reflex by stroking the perianal skin. Absence of the anal wink in a younger patient is likely associated with nerve damage; in an older patient, it may simply indicate muscle weakness. Perform a digital rectal exam to assess for normal resting tone and augmentation with squeeze, regardless of the patient’s age.
Use tools to assess the severity
Anal incontinence can be broadly characterized as complete or partial. Numerous other systems have been proposed for classifying severity, the simplest of which has the following 4 components:
A: Continent of solid, liquid, and flatus (complete continence)
B: Continent of solid and liquid, but not flatus
C: Continent of solid, but not liquid or flatus
D: Continued fecal leakage (complete incontinence).22
Although this classification system may be helpful, it yields little information about the significance of the problem from the patient’s perspective.23 Thus, scales that take into account both the frequency of incontinence episodes and the extent of both the mental and physical impact are used more frequently.
One of the most widely used scales is the Cleveland Clinic Fecal Incontinence Score (TABLE),24 which quantifies both the frequency and type of incontinence and scores the level of severity. Fecal incontinence quality of life scales are available, as well, and include questions about the impact on the patient’s lifestyle, coping behavior, mood, and level of embarrassment.25
Even without a quality of life scale, a couple of targeted questions—(eg, Are you ever afraid to go out? Do you worry about others smelling stool on you?)—will give you an idea of how great an impact fecal incontinence is having on your patient’s life. Asking patients to keep bowel diaries can also be helpful in assessing the extent of the problem and the effect of treatment.
Next steps: Start with modifiable risks
While there are numerous diagnostic tests for fecal incontinence (more about these in a bit), none is necessary for initial treatment, which starts with modifiable risks. Chief among them is smoking.
Smoking cessation. Nicotine is believed to have a direct effect on colonic transit and rectal compliance.26 Thus, smoking is associated with an increased risk for fecal incontinence, independent of chronic cough or chronic obstructive pulmonary disease. Patients should be advised to quit smoking and referred to a smoking cessation program.
Dietary fiber. Diet may be a factor in fecal incontinence, as well. Ask patients to record everything they eat, and advise those with a low intake of dietary fiber to eat more fruits, vegetables, whole grains, and other high-fiber food. Recommend that they avoid caffeine and alcohol, as well.
Some medications may also affect stool form and frequency, and precipitate fecal incontinence. Common offenders, in addition to laxatives and antacids, include antibiotics, proton pump inhibitors, and senna-based colon cleansers.27 Consider a switch to another drug class. A trial with a drug thought to improve bowel continence is recommended, as well.
Prescribe pharmacologic treatment
Kaolin, pectin, bulking agents, bismuth salts, anticholinergics, opium derivatives, diphenoxylate/atropine, and loperamide have all been used to treat fecal incontinence, with variable success. Loperamide, the drug most extensively studied for this purpose, has been found to increase resting anal pressure and improve anal sphincter function and continence by acting directly on the circular and longitudinal muscles of the bowel.28
Amitriptyline has also been used empirically, with some success. It is believed to work by decreasing the frequency and amplitude of rectal motor complexes.29 Clonidine in the form of a transdermal patch has been shown to increase the number of problem-free days and overall quality of life for patients with fecal incontinence.30
Consider biofeedback
Biofeedback training is often the next step after pharmacologic treatment. It has been investigated for the treatment of fecal incontinence, and many patients—particularly if they are highly motivated—have reported improvement.31 Therapy generally has 3 components: exercising the external sphincter complex, training in the discrimination of rectal sensations, and developing synchrony of the internal and external sphincter responses during rectal distension.
The goal is for the patient to learn to contract the sphincter in response to small amounts of rectal distension.
But a significant time commitment on the part of the patient and sophisticated apparatus are necessary to carry out such therapy, and only a few randomized controlled trials (RCTs) have evaluated the effect. The largest RCT had 4 arms: a standard care group; standard care plus instruction on sphincter exercises; standard care with sphincter exercises and biofeedback; and standard care with sphincter exercises, biofeedback, and training at home.32
All 4 groups had similar improvement in symptoms, raising questions about the therapeutic value of biofeedback.32 Long-term studies have found that 60% to 80% of patients will continue to have episodes of incontinence after undergoing biofeedback. A Cochrane review of RCTs concluded that there is not enough evidence to judge whether sphincter exercises and biofeedback are effective in reducing fecal incontinence.33
Still no relief? Order tests and consider surgery
For patients with fecal incontinence refractory to conservative management, more sophisticated diagnostic studies can provide invaluable information for guiding further treatment.
Endoanal ultrasound is considered the gold standard diagnostic test for fecal incontinence. It is superior to electromyography in terms of availability, patient tolerance, and ability to assess the internal anal sphincter, except in cases in which nerve injury is suspected.34
Other tests sometimes used to pinpoint the cause of fecal incontinence include an enema challenge (which can differentiate between liquid and solid incontinence) and anal manometry (which can quantify anal sphincter tone). Defecography (which makes it possible to visualize the rectal emptying process) can be helpful if a diagnosis of rectal prolapse is being considered.
Magnetic resonance imaging is among the most costly diagnostic studies associated with fecal incontinence. But it is the only modality that can depict the morphology of the external sphincter and the presence of muscle atrophy—providing information that has been shown to significantly improve the likelihood of successful sphincter repair.35
A wider range of surgical options
When medical therapy and biofeedback fail to produce adequate results, referral to a colorectal surgeon is appropriate. (Although conservative management is frequently unsuccessful, health plans typically require that they be attempted before surgical intervention is considered.)
Sphincteroplasty, or anterior anal sphincter repair, addresses the most common cause of fecal incontinence—and is still a common surgical procedure.36 Sphincteroplasty generally has good to excellent results, providing there is sufficient muscle mass for a successful repair.37,38
The procedure involves dissecting the sphincter complex from the surrounding anoderm, then overlapping the edges of the sphincter muscle and suturing them together. Continence has been reported nearly 80% of the time, although a longer duration of fecal incontinence and incontinence secondary to operative injury of the sphincter are risk factors for poorer outcomes.39,40
Recent studies have called into question the durability of anterior sphincter repair. A systematic review of 16 studies reporting short- and long-term outcomes for more than 900 patients found that all but one of the studies showed a decline over time in the number of patients who were happy with the outcome.39
Sacral nerve stimulation is first-line surgical treatment
Sacral nerve stimulation (SNS) is the most promising development in the treatment of fecal incontinence. In the last decade, SNS has become the first-line surgical treatment for patients for whom medical and behavioral therapy are unsuccessful.40
A minimally invasive procedure that involves an implantable device, SNS is always preceded by an effectiveness trial in which a finder needle is percutaneously inserted into the third sacral foramen. Stimulation should result in immediate contraction of the pelvic floor and external sphincter and plantar flexion of the big toe.
The next step is the insertion of a temporary stimulator lead, which remains in place for a 2- to 3-week test of low-frequency stimulation. If significant reduction in the number of incontinence episodes during the trial period occurs, the device is inserted (See “Sacral nerve stimulation: A case study” above).
Improvement in fecal continence has been reported to be as high as 100% in some cases, with up to 75% of patients achieving complete continence.41 While the mechanism involved remains unclear, multiple studies have confirmed its effectiveness.42,43
Posterior tibial nerve stimulation is another recent development, in which a small, thin lead is placed at the posterior tibial nerve, then connected to a temporary stimulator. Less data are available for this treatment, but a recent review summarized the findings of 8 published studies and found success rates ranging from 30% to 83%.44
The Secca procedure—a relatively new therapy that delivers radiofrequency energy to the anal sphincter—is another option, believed to work by reducing compliance of the sphincter complex and the level of tolerable rectal distension.45 Procedures using injectable bulking materials and fat grafting around the sphincter complex have demonstrated some promise, as well.46
A number of other surgical modalities are available, and often effective under certain circumstances. Among them are rotational and free muscle transfers, used only in cases in which the bulk of the sphincter complex has been destroyed.47,48 Implantable anal sphincters (made from human muscle and nerve cells) are occasionally used, as well, but frequently need to be removed because of infection.49-51
Regardless of the type of treatment they receive, patients often do not achieve total continence. Anyone who continues to have occasional episodes of fecal incontinence or leakage should be advised to wear incontinence pads, as needed.
Consider colostomy when incontinence is severe
For patients with fecal incontinence severe enough to be disabling—often as a result of irradiation—colostomy remains a tried and true treatment. The rectum can either be left intact or a proctectomy performed in concert with ostomy creation. Most studies evaluating colostomy for the treatment of incontinence have found that it significantly improves the quality of life and that most patients say they would choose to undergo the procedure again.52
1. Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
2. Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586-598.
3. Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the impact of fecal incontinence on nursing home referral. J Am Geriatr Soc. 2010;58:1058-1062.
4. Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66:1101–1108.
5. Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int Clin Pract. 2012;66:1109–1116.
6. Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113-119.
7. Sundquist JC. Long-term outcome after obstetric injury: a retrospective study. Acta Obstet Gynecol Scand. 2012 Jun;91:715-718.
8. Planting A, Phang PT, Raval MJ, et al. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248.
9. Ctercteko GC, Fazio VW, Jagelman DG, et al. Anal sphincter repair: a report of 60 cases and review of the literature. Aust N Z J Surg. 1988;58:703–710.
10. Keighley MRB, Fielding JWL. Management of faecal incontinence and results of surgical treatment. Br J Surg. 1983;70: 463–468.
11. Eason E, Labrecque M, Marcoux S, et al. Anal incontinence after childbirth. CMAJ. 2002;166:326–330.
12. Rieger N, Schloithe A, Saccone G, et al. A prospective study of analsphincter injury due to childbirth. Scand J Gastroenterol. 1998;33:950–955.
13. Zetterstrom J, Mellgren A, Jensen LL, et al. Effect of delivery on anal sphinctermorphology and function. Dis Colon Rectum. 1999;42:1253–1260.
14. Varma A, Gunn J, Gardiner A, et al. Obstetric anal sphincter injury: prospective evaluation of incidence. Dis Colon Rectum. 1999;42:1537–1543.
15. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333–1337.
16. Lindsey I, Jones OM, Smilgin-Humphreys MM, et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47:1643–1649.
17. National Digestive Diseases Information Clearinghouse. Fecal
incontinence. Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/fecalincontinence. Accessed October 20, 2013.
18. Roig JV, Villoslada C, Lledo S, et al. Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum. 1995;38:952–958.
19. Swash M, Gray A, Lubowski DZ, et al. Ultrastructural changes in internal sphincter in neurogenic incontinence. Gut. 1988;29:1692–1698.
20. Rogers J, Henry MM, Misiewicz JJ. Combined sensory and motor deficit in primary fecal incontinence. Gut. 1988;29:5–9.
21. Medline Plus Web site. Bowel incontinence. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003135.htm. Accessed October 20, 2013.
22. Browning GP, Parks AG. Post anal repair for neuropathic fecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg. 1983;70:101–104.
23. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal incontinence. Dis Colon Rectum. 2003;46:1591–1605.
24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
25. American Society of Colon & Rectal Surgeons Web site. Fecal incontinence quality of life scale. Available at: http://www.fascrs.org/physicians/Fecal_Incontinence_Quality_of_Life_Scale/. Accessed October 20, 2013.
26. Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factor for late onset fecal incontinence: a population based case-control study in women. Gastroenterology. 2010;139:1559-1566.
27. MedlinePlus Web site. Drug-induced diarrhea. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000293.htm. Accessed October 21, 2013.
28. Hallgren T, Fasth S, Delbro DS, et al. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612-2618.
29. Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676-1681.
30. Bharucha AE, Seide BM, Zinsmeister AR, et al. The effects of clonodine on symptoms and anorectal sensoriomotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681-688.
31. Engel BT, Nikoomnesh P, Schuster MM. Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence. N Engl J Med. 1974;290:646-649.
32. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329.
33. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of fecal incontinence in adults. Cochrane Database Syst Rev. 2006;(3):CD002111.
34. Sultan AH, Nicholls RJ, Kamm MA, et al. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993; 80:508–511.
35. Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal MRI adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327.
36. Goetz LH, Lowry AC. Overlapping sphincteroplasty: is it the standard of care? Clin Colon Rectal Surg. 2005;18:22-31.
37. El-Gazzazz G, Zutshi M, Hannaway C, et al. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55:256-261.
38. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482-490.
39. Lehto K, Hyoty M, Collin P, et al. Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis. 2013;5:653-658.
40. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302-306.
41. Jarrett MED, Mowatt G, Glazener CMA, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569.
42. Melenhorst J, Koch SM, Uludag O, et al. Is a morphologically intact anal sphincter necessary for success with sacral nerve modulation in patients with faecal incontinence? Colorectal Dis. 2008;10:257-262.
43. Dudding TC, Pares D, Vaizey CJ, et al. Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: a 10-year cohort analysis. Colorectal Dis. 2008;10:294-256.
44. Findlay JM, Mawell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26:265-273.
45. Feretis C, Benakis P, Dailianas A, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609.
46. Kenefick NJ, Vaizey CJ, Malouf AJ, et al. Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut. 2002;55:225–228.
47. Konsten J, Baeten CG, Spaans F, et al. Follow-up of anal dynamic graciloplasty for fecal continence. World J Surg. 1993;17:404–409.
48. Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence following previously implanted gracilis muscle: report of a case. Dis Colon Rectum. 1988;31:134–137.
49. Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773-779.
50. Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum 2003;46:722–729.
51. Takahashi T, Garcia-Osogobio S, Valdovinos MA, et al. Extended two-year results of radio-frequency energy delivery for the treatment of fecal incontinence (Secca procedure). Dis Colon Rectum. 2003;46:711–715.
52. Norton C, Burch J, Kamm MA. Patient’s views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062.
› Consider adding a question about fecal incontinence—a condition often unreported by patients and undetected by physicians—to your medical intake form. C
› Use bowel diaries and fecal incontinence grading systems, as needed, to better understand the extent of the problem and assess the effects of treatment. C
› Consider sacral nerve stimulation, the first-line surgical treatment for fecal incontinence, for those who fail to respond to medical therapies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimates suggest that about 18 million adults in the United States suffer from fecal incontinence.1 But because the condition often goes unreported by patients and undetected by physicians, the actual prevalence is not known—and may be considerably higher.
What is known is that fecal incontinence carries a substantial socioeconomic burden. The average annual per patient cost is estimated at $4110.2 But fecal incontinence also exacts a heavy personal toll, and is one of the main reasons elderly individuals are placed in nursing homes.3
But it’s not just the elderly who are affected. A recent study of women ages 45 years and older found that nearly one in 5 had an episode of fecal incontinence at least once a year, and for nearly half, the frequency was once a month or more.4 Less than 3 in 10 reported their symptoms to a clinician, but those who did were most likely to have confided in their primary care physician.5
Fortunately, recent developments—most notably, sacral nerve stimulation, a minimally invasive surgical technique with a high success rate—have changed the outlook for patients with fecal incontinence. Here’s what you need to know to help patients who suffer from this embarrassing condition achieve optimal outcomes.
Risk factors and key causes
Maintaining fecal continence involves a complex series of events, both voluntary and involuntary. Problems at various levels—stool consistency, anatomic and neurologic abnormalities, and psychological problems among them—can disrupt the process.
Those at high risk for fecal incontinence, in addition to the elderly, include patients who are mentally ill and institutionalized, individuals with neurologic disorders, patients who have had anorectal surgery, and women who have had vaginal deliveries.6-8 Obstetric and operative injuries account for most cases of fecal incontinence.9-10
Sphincter defects including attenuation and scarring (shown here), are commonly caused by obstetric and operative injuries.
Risks of vaginal delivery
As many as 25% of women report some degree of fecal incontinence—although often confined to loss of control of flatus—3 months after giving birth.11 Stool incontinence is more frequent among women who sustained third- or fourth-degree perineal tears. Obstetrical risk factors include first vaginal birth, median episiotomy, forceps delivery, vacuum-assisted delivery, and a prolonged second stage of labor.
Asymptomatic sphincter defects. Studies in which women underwent endosonographic examination of the sphincter complex both before and after vaginal delivery have found sphincter defects in anywhere from 7% to 41% of new mothers.12-14 It is important to note, however, that as many as 70% of those with defects detected by sonogram were asymptomatic.15 (Despite the risk of sphincter injury during vaginal delivery and evidence suggesting that the risk of fecal incontinence increases with additional deliveries after a previous perineal tear, prophylactic cesarean section is not recommended.)
Fistula surgery and postop incontinence
Fistula surgery is the primary cause of postoperative incontinence, typically resulting from inadvertent injury to either the internal or external sphincter muscle.16 Other relatively common causes of fecal incontinence are rectal prolapse, trauma, irradiation, neurologic and demyelinating disorders such as multiple sclerosis, neoplasms, stroke, infection (eg, of a perineal wound), and diabetes.17 As diagnostic modalities have improved, much of what was previously termed idiopathic incontinence has been found to have identifiable underlying pathology, such as pudendal and inferior hypogastric neuropathies.18-20
Identifying fecal incontinence starts with a single question
As already noted, most patients with symptoms of bowel leakage do not voluntarily mention it to their physician. Many are likely to acknowledge the problem, however, if they’re specifically asked. While little has been written about how best to screen for fecal incontinence, simply adding it to the checklist on your medical intake form may be a good starting point.
Follow up with a targeted history and physical
When a patient checks fecal incontinence on a form or broaches the subject, it is important to question him or her about medical conditions that may be related. These include urinary incontinence, prolapsing tissue, diabetes, and a history of radiation, as well as childbirth. A medication history is also needed, as certain drugs—including some antacids and laxatives—have been implicated in fecal incontinence.21
Physical assessment should include a general neurologic exam as well as a perineal exam, to look for prolapsing tissue and evidence of scars from prior surgery or obstetrical trauma. Check the anocutaneous reflex by stroking the perianal skin. Absence of the anal wink in a younger patient is likely associated with nerve damage; in an older patient, it may simply indicate muscle weakness. Perform a digital rectal exam to assess for normal resting tone and augmentation with squeeze, regardless of the patient’s age.
Use tools to assess the severity
Anal incontinence can be broadly characterized as complete or partial. Numerous other systems have been proposed for classifying severity, the simplest of which has the following 4 components:
A: Continent of solid, liquid, and flatus (complete continence)
B: Continent of solid and liquid, but not flatus
C: Continent of solid, but not liquid or flatus
D: Continued fecal leakage (complete incontinence).22
Although this classification system may be helpful, it yields little information about the significance of the problem from the patient’s perspective.23 Thus, scales that take into account both the frequency of incontinence episodes and the extent of both the mental and physical impact are used more frequently.
One of the most widely used scales is the Cleveland Clinic Fecal Incontinence Score (TABLE),24 which quantifies both the frequency and type of incontinence and scores the level of severity. Fecal incontinence quality of life scales are available, as well, and include questions about the impact on the patient’s lifestyle, coping behavior, mood, and level of embarrassment.25
Even without a quality of life scale, a couple of targeted questions—(eg, Are you ever afraid to go out? Do you worry about others smelling stool on you?)—will give you an idea of how great an impact fecal incontinence is having on your patient’s life. Asking patients to keep bowel diaries can also be helpful in assessing the extent of the problem and the effect of treatment.
Next steps: Start with modifiable risks
While there are numerous diagnostic tests for fecal incontinence (more about these in a bit), none is necessary for initial treatment, which starts with modifiable risks. Chief among them is smoking.
Smoking cessation. Nicotine is believed to have a direct effect on colonic transit and rectal compliance.26 Thus, smoking is associated with an increased risk for fecal incontinence, independent of chronic cough or chronic obstructive pulmonary disease. Patients should be advised to quit smoking and referred to a smoking cessation program.
Dietary fiber. Diet may be a factor in fecal incontinence, as well. Ask patients to record everything they eat, and advise those with a low intake of dietary fiber to eat more fruits, vegetables, whole grains, and other high-fiber food. Recommend that they avoid caffeine and alcohol, as well.
Some medications may also affect stool form and frequency, and precipitate fecal incontinence. Common offenders, in addition to laxatives and antacids, include antibiotics, proton pump inhibitors, and senna-based colon cleansers.27 Consider a switch to another drug class. A trial with a drug thought to improve bowel continence is recommended, as well.
Prescribe pharmacologic treatment
Kaolin, pectin, bulking agents, bismuth salts, anticholinergics, opium derivatives, diphenoxylate/atropine, and loperamide have all been used to treat fecal incontinence, with variable success. Loperamide, the drug most extensively studied for this purpose, has been found to increase resting anal pressure and improve anal sphincter function and continence by acting directly on the circular and longitudinal muscles of the bowel.28
Amitriptyline has also been used empirically, with some success. It is believed to work by decreasing the frequency and amplitude of rectal motor complexes.29 Clonidine in the form of a transdermal patch has been shown to increase the number of problem-free days and overall quality of life for patients with fecal incontinence.30
Consider biofeedback
Biofeedback training is often the next step after pharmacologic treatment. It has been investigated for the treatment of fecal incontinence, and many patients—particularly if they are highly motivated—have reported improvement.31 Therapy generally has 3 components: exercising the external sphincter complex, training in the discrimination of rectal sensations, and developing synchrony of the internal and external sphincter responses during rectal distension.
The goal is for the patient to learn to contract the sphincter in response to small amounts of rectal distension.
But a significant time commitment on the part of the patient and sophisticated apparatus are necessary to carry out such therapy, and only a few randomized controlled trials (RCTs) have evaluated the effect. The largest RCT had 4 arms: a standard care group; standard care plus instruction on sphincter exercises; standard care with sphincter exercises and biofeedback; and standard care with sphincter exercises, biofeedback, and training at home.32
All 4 groups had similar improvement in symptoms, raising questions about the therapeutic value of biofeedback.32 Long-term studies have found that 60% to 80% of patients will continue to have episodes of incontinence after undergoing biofeedback. A Cochrane review of RCTs concluded that there is not enough evidence to judge whether sphincter exercises and biofeedback are effective in reducing fecal incontinence.33
Still no relief? Order tests and consider surgery
For patients with fecal incontinence refractory to conservative management, more sophisticated diagnostic studies can provide invaluable information for guiding further treatment.
Endoanal ultrasound is considered the gold standard diagnostic test for fecal incontinence. It is superior to electromyography in terms of availability, patient tolerance, and ability to assess the internal anal sphincter, except in cases in which nerve injury is suspected.34
Other tests sometimes used to pinpoint the cause of fecal incontinence include an enema challenge (which can differentiate between liquid and solid incontinence) and anal manometry (which can quantify anal sphincter tone). Defecography (which makes it possible to visualize the rectal emptying process) can be helpful if a diagnosis of rectal prolapse is being considered.
Magnetic resonance imaging is among the most costly diagnostic studies associated with fecal incontinence. But it is the only modality that can depict the morphology of the external sphincter and the presence of muscle atrophy—providing information that has been shown to significantly improve the likelihood of successful sphincter repair.35
A wider range of surgical options
When medical therapy and biofeedback fail to produce adequate results, referral to a colorectal surgeon is appropriate. (Although conservative management is frequently unsuccessful, health plans typically require that they be attempted before surgical intervention is considered.)
Sphincteroplasty, or anterior anal sphincter repair, addresses the most common cause of fecal incontinence—and is still a common surgical procedure.36 Sphincteroplasty generally has good to excellent results, providing there is sufficient muscle mass for a successful repair.37,38
The procedure involves dissecting the sphincter complex from the surrounding anoderm, then overlapping the edges of the sphincter muscle and suturing them together. Continence has been reported nearly 80% of the time, although a longer duration of fecal incontinence and incontinence secondary to operative injury of the sphincter are risk factors for poorer outcomes.39,40
Recent studies have called into question the durability of anterior sphincter repair. A systematic review of 16 studies reporting short- and long-term outcomes for more than 900 patients found that all but one of the studies showed a decline over time in the number of patients who were happy with the outcome.39
Sacral nerve stimulation is first-line surgical treatment
Sacral nerve stimulation (SNS) is the most promising development in the treatment of fecal incontinence. In the last decade, SNS has become the first-line surgical treatment for patients for whom medical and behavioral therapy are unsuccessful.40
A minimally invasive procedure that involves an implantable device, SNS is always preceded by an effectiveness trial in which a finder needle is percutaneously inserted into the third sacral foramen. Stimulation should result in immediate contraction of the pelvic floor and external sphincter and plantar flexion of the big toe.
The next step is the insertion of a temporary stimulator lead, which remains in place for a 2- to 3-week test of low-frequency stimulation. If significant reduction in the number of incontinence episodes during the trial period occurs, the device is inserted (See “Sacral nerve stimulation: A case study” above).
Improvement in fecal continence has been reported to be as high as 100% in some cases, with up to 75% of patients achieving complete continence.41 While the mechanism involved remains unclear, multiple studies have confirmed its effectiveness.42,43
Posterior tibial nerve stimulation is another recent development, in which a small, thin lead is placed at the posterior tibial nerve, then connected to a temporary stimulator. Less data are available for this treatment, but a recent review summarized the findings of 8 published studies and found success rates ranging from 30% to 83%.44
The Secca procedure—a relatively new therapy that delivers radiofrequency energy to the anal sphincter—is another option, believed to work by reducing compliance of the sphincter complex and the level of tolerable rectal distension.45 Procedures using injectable bulking materials and fat grafting around the sphincter complex have demonstrated some promise, as well.46
A number of other surgical modalities are available, and often effective under certain circumstances. Among them are rotational and free muscle transfers, used only in cases in which the bulk of the sphincter complex has been destroyed.47,48 Implantable anal sphincters (made from human muscle and nerve cells) are occasionally used, as well, but frequently need to be removed because of infection.49-51
Regardless of the type of treatment they receive, patients often do not achieve total continence. Anyone who continues to have occasional episodes of fecal incontinence or leakage should be advised to wear incontinence pads, as needed.
Consider colostomy when incontinence is severe
For patients with fecal incontinence severe enough to be disabling—often as a result of irradiation—colostomy remains a tried and true treatment. The rectum can either be left intact or a proctectomy performed in concert with ostomy creation. Most studies evaluating colostomy for the treatment of incontinence have found that it significantly improves the quality of life and that most patients say they would choose to undergo the procedure again.52
› Consider adding a question about fecal incontinence—a condition often unreported by patients and undetected by physicians—to your medical intake form. C
› Use bowel diaries and fecal incontinence grading systems, as needed, to better understand the extent of the problem and assess the effects of treatment. C
› Consider sacral nerve stimulation, the first-line surgical treatment for fecal incontinence, for those who fail to respond to medical therapies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimates suggest that about 18 million adults in the United States suffer from fecal incontinence.1 But because the condition often goes unreported by patients and undetected by physicians, the actual prevalence is not known—and may be considerably higher.
What is known is that fecal incontinence carries a substantial socioeconomic burden. The average annual per patient cost is estimated at $4110.2 But fecal incontinence also exacts a heavy personal toll, and is one of the main reasons elderly individuals are placed in nursing homes.3
But it’s not just the elderly who are affected. A recent study of women ages 45 years and older found that nearly one in 5 had an episode of fecal incontinence at least once a year, and for nearly half, the frequency was once a month or more.4 Less than 3 in 10 reported their symptoms to a clinician, but those who did were most likely to have confided in their primary care physician.5
Fortunately, recent developments—most notably, sacral nerve stimulation, a minimally invasive surgical technique with a high success rate—have changed the outlook for patients with fecal incontinence. Here’s what you need to know to help patients who suffer from this embarrassing condition achieve optimal outcomes.
Risk factors and key causes
Maintaining fecal continence involves a complex series of events, both voluntary and involuntary. Problems at various levels—stool consistency, anatomic and neurologic abnormalities, and psychological problems among them—can disrupt the process.
Those at high risk for fecal incontinence, in addition to the elderly, include patients who are mentally ill and institutionalized, individuals with neurologic disorders, patients who have had anorectal surgery, and women who have had vaginal deliveries.6-8 Obstetric and operative injuries account for most cases of fecal incontinence.9-10
Sphincter defects including attenuation and scarring (shown here), are commonly caused by obstetric and operative injuries.
Risks of vaginal delivery
As many as 25% of women report some degree of fecal incontinence—although often confined to loss of control of flatus—3 months after giving birth.11 Stool incontinence is more frequent among women who sustained third- or fourth-degree perineal tears. Obstetrical risk factors include first vaginal birth, median episiotomy, forceps delivery, vacuum-assisted delivery, and a prolonged second stage of labor.
Asymptomatic sphincter defects. Studies in which women underwent endosonographic examination of the sphincter complex both before and after vaginal delivery have found sphincter defects in anywhere from 7% to 41% of new mothers.12-14 It is important to note, however, that as many as 70% of those with defects detected by sonogram were asymptomatic.15 (Despite the risk of sphincter injury during vaginal delivery and evidence suggesting that the risk of fecal incontinence increases with additional deliveries after a previous perineal tear, prophylactic cesarean section is not recommended.)
Fistula surgery and postop incontinence
Fistula surgery is the primary cause of postoperative incontinence, typically resulting from inadvertent injury to either the internal or external sphincter muscle.16 Other relatively common causes of fecal incontinence are rectal prolapse, trauma, irradiation, neurologic and demyelinating disorders such as multiple sclerosis, neoplasms, stroke, infection (eg, of a perineal wound), and diabetes.17 As diagnostic modalities have improved, much of what was previously termed idiopathic incontinence has been found to have identifiable underlying pathology, such as pudendal and inferior hypogastric neuropathies.18-20
Identifying fecal incontinence starts with a single question
As already noted, most patients with symptoms of bowel leakage do not voluntarily mention it to their physician. Many are likely to acknowledge the problem, however, if they’re specifically asked. While little has been written about how best to screen for fecal incontinence, simply adding it to the checklist on your medical intake form may be a good starting point.
Follow up with a targeted history and physical
When a patient checks fecal incontinence on a form or broaches the subject, it is important to question him or her about medical conditions that may be related. These include urinary incontinence, prolapsing tissue, diabetes, and a history of radiation, as well as childbirth. A medication history is also needed, as certain drugs—including some antacids and laxatives—have been implicated in fecal incontinence.21
Physical assessment should include a general neurologic exam as well as a perineal exam, to look for prolapsing tissue and evidence of scars from prior surgery or obstetrical trauma. Check the anocutaneous reflex by stroking the perianal skin. Absence of the anal wink in a younger patient is likely associated with nerve damage; in an older patient, it may simply indicate muscle weakness. Perform a digital rectal exam to assess for normal resting tone and augmentation with squeeze, regardless of the patient’s age.
Use tools to assess the severity
Anal incontinence can be broadly characterized as complete or partial. Numerous other systems have been proposed for classifying severity, the simplest of which has the following 4 components:
A: Continent of solid, liquid, and flatus (complete continence)
B: Continent of solid and liquid, but not flatus
C: Continent of solid, but not liquid or flatus
D: Continued fecal leakage (complete incontinence).22
Although this classification system may be helpful, it yields little information about the significance of the problem from the patient’s perspective.23 Thus, scales that take into account both the frequency of incontinence episodes and the extent of both the mental and physical impact are used more frequently.
One of the most widely used scales is the Cleveland Clinic Fecal Incontinence Score (TABLE),24 which quantifies both the frequency and type of incontinence and scores the level of severity. Fecal incontinence quality of life scales are available, as well, and include questions about the impact on the patient’s lifestyle, coping behavior, mood, and level of embarrassment.25
Even without a quality of life scale, a couple of targeted questions—(eg, Are you ever afraid to go out? Do you worry about others smelling stool on you?)—will give you an idea of how great an impact fecal incontinence is having on your patient’s life. Asking patients to keep bowel diaries can also be helpful in assessing the extent of the problem and the effect of treatment.
Next steps: Start with modifiable risks
While there are numerous diagnostic tests for fecal incontinence (more about these in a bit), none is necessary for initial treatment, which starts with modifiable risks. Chief among them is smoking.
Smoking cessation. Nicotine is believed to have a direct effect on colonic transit and rectal compliance.26 Thus, smoking is associated with an increased risk for fecal incontinence, independent of chronic cough or chronic obstructive pulmonary disease. Patients should be advised to quit smoking and referred to a smoking cessation program.
Dietary fiber. Diet may be a factor in fecal incontinence, as well. Ask patients to record everything they eat, and advise those with a low intake of dietary fiber to eat more fruits, vegetables, whole grains, and other high-fiber food. Recommend that they avoid caffeine and alcohol, as well.
Some medications may also affect stool form and frequency, and precipitate fecal incontinence. Common offenders, in addition to laxatives and antacids, include antibiotics, proton pump inhibitors, and senna-based colon cleansers.27 Consider a switch to another drug class. A trial with a drug thought to improve bowel continence is recommended, as well.
Prescribe pharmacologic treatment
Kaolin, pectin, bulking agents, bismuth salts, anticholinergics, opium derivatives, diphenoxylate/atropine, and loperamide have all been used to treat fecal incontinence, with variable success. Loperamide, the drug most extensively studied for this purpose, has been found to increase resting anal pressure and improve anal sphincter function and continence by acting directly on the circular and longitudinal muscles of the bowel.28
Amitriptyline has also been used empirically, with some success. It is believed to work by decreasing the frequency and amplitude of rectal motor complexes.29 Clonidine in the form of a transdermal patch has been shown to increase the number of problem-free days and overall quality of life for patients with fecal incontinence.30
Consider biofeedback
Biofeedback training is often the next step after pharmacologic treatment. It has been investigated for the treatment of fecal incontinence, and many patients—particularly if they are highly motivated—have reported improvement.31 Therapy generally has 3 components: exercising the external sphincter complex, training in the discrimination of rectal sensations, and developing synchrony of the internal and external sphincter responses during rectal distension.
The goal is for the patient to learn to contract the sphincter in response to small amounts of rectal distension.
But a significant time commitment on the part of the patient and sophisticated apparatus are necessary to carry out such therapy, and only a few randomized controlled trials (RCTs) have evaluated the effect. The largest RCT had 4 arms: a standard care group; standard care plus instruction on sphincter exercises; standard care with sphincter exercises and biofeedback; and standard care with sphincter exercises, biofeedback, and training at home.32
All 4 groups had similar improvement in symptoms, raising questions about the therapeutic value of biofeedback.32 Long-term studies have found that 60% to 80% of patients will continue to have episodes of incontinence after undergoing biofeedback. A Cochrane review of RCTs concluded that there is not enough evidence to judge whether sphincter exercises and biofeedback are effective in reducing fecal incontinence.33
Still no relief? Order tests and consider surgery
For patients with fecal incontinence refractory to conservative management, more sophisticated diagnostic studies can provide invaluable information for guiding further treatment.
Endoanal ultrasound is considered the gold standard diagnostic test for fecal incontinence. It is superior to electromyography in terms of availability, patient tolerance, and ability to assess the internal anal sphincter, except in cases in which nerve injury is suspected.34
Other tests sometimes used to pinpoint the cause of fecal incontinence include an enema challenge (which can differentiate between liquid and solid incontinence) and anal manometry (which can quantify anal sphincter tone). Defecography (which makes it possible to visualize the rectal emptying process) can be helpful if a diagnosis of rectal prolapse is being considered.
Magnetic resonance imaging is among the most costly diagnostic studies associated with fecal incontinence. But it is the only modality that can depict the morphology of the external sphincter and the presence of muscle atrophy—providing information that has been shown to significantly improve the likelihood of successful sphincter repair.35
A wider range of surgical options
When medical therapy and biofeedback fail to produce adequate results, referral to a colorectal surgeon is appropriate. (Although conservative management is frequently unsuccessful, health plans typically require that they be attempted before surgical intervention is considered.)
Sphincteroplasty, or anterior anal sphincter repair, addresses the most common cause of fecal incontinence—and is still a common surgical procedure.36 Sphincteroplasty generally has good to excellent results, providing there is sufficient muscle mass for a successful repair.37,38
The procedure involves dissecting the sphincter complex from the surrounding anoderm, then overlapping the edges of the sphincter muscle and suturing them together. Continence has been reported nearly 80% of the time, although a longer duration of fecal incontinence and incontinence secondary to operative injury of the sphincter are risk factors for poorer outcomes.39,40
Recent studies have called into question the durability of anterior sphincter repair. A systematic review of 16 studies reporting short- and long-term outcomes for more than 900 patients found that all but one of the studies showed a decline over time in the number of patients who were happy with the outcome.39
Sacral nerve stimulation is first-line surgical treatment
Sacral nerve stimulation (SNS) is the most promising development in the treatment of fecal incontinence. In the last decade, SNS has become the first-line surgical treatment for patients for whom medical and behavioral therapy are unsuccessful.40
A minimally invasive procedure that involves an implantable device, SNS is always preceded by an effectiveness trial in which a finder needle is percutaneously inserted into the third sacral foramen. Stimulation should result in immediate contraction of the pelvic floor and external sphincter and plantar flexion of the big toe.
The next step is the insertion of a temporary stimulator lead, which remains in place for a 2- to 3-week test of low-frequency stimulation. If significant reduction in the number of incontinence episodes during the trial period occurs, the device is inserted (See “Sacral nerve stimulation: A case study” above).
Improvement in fecal continence has been reported to be as high as 100% in some cases, with up to 75% of patients achieving complete continence.41 While the mechanism involved remains unclear, multiple studies have confirmed its effectiveness.42,43
Posterior tibial nerve stimulation is another recent development, in which a small, thin lead is placed at the posterior tibial nerve, then connected to a temporary stimulator. Less data are available for this treatment, but a recent review summarized the findings of 8 published studies and found success rates ranging from 30% to 83%.44
The Secca procedure—a relatively new therapy that delivers radiofrequency energy to the anal sphincter—is another option, believed to work by reducing compliance of the sphincter complex and the level of tolerable rectal distension.45 Procedures using injectable bulking materials and fat grafting around the sphincter complex have demonstrated some promise, as well.46
A number of other surgical modalities are available, and often effective under certain circumstances. Among them are rotational and free muscle transfers, used only in cases in which the bulk of the sphincter complex has been destroyed.47,48 Implantable anal sphincters (made from human muscle and nerve cells) are occasionally used, as well, but frequently need to be removed because of infection.49-51
Regardless of the type of treatment they receive, patients often do not achieve total continence. Anyone who continues to have occasional episodes of fecal incontinence or leakage should be advised to wear incontinence pads, as needed.
Consider colostomy when incontinence is severe
For patients with fecal incontinence severe enough to be disabling—often as a result of irradiation—colostomy remains a tried and true treatment. The rectum can either be left intact or a proctectomy performed in concert with ostomy creation. Most studies evaluating colostomy for the treatment of incontinence have found that it significantly improves the quality of life and that most patients say they would choose to undergo the procedure again.52
1. Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
2. Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586-598.
3. Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the impact of fecal incontinence on nursing home referral. J Am Geriatr Soc. 2010;58:1058-1062.
4. Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66:1101–1108.
5. Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int Clin Pract. 2012;66:1109–1116.
6. Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113-119.
7. Sundquist JC. Long-term outcome after obstetric injury: a retrospective study. Acta Obstet Gynecol Scand. 2012 Jun;91:715-718.
8. Planting A, Phang PT, Raval MJ, et al. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248.
9. Ctercteko GC, Fazio VW, Jagelman DG, et al. Anal sphincter repair: a report of 60 cases and review of the literature. Aust N Z J Surg. 1988;58:703–710.
10. Keighley MRB, Fielding JWL. Management of faecal incontinence and results of surgical treatment. Br J Surg. 1983;70: 463–468.
11. Eason E, Labrecque M, Marcoux S, et al. Anal incontinence after childbirth. CMAJ. 2002;166:326–330.
12. Rieger N, Schloithe A, Saccone G, et al. A prospective study of analsphincter injury due to childbirth. Scand J Gastroenterol. 1998;33:950–955.
13. Zetterstrom J, Mellgren A, Jensen LL, et al. Effect of delivery on anal sphinctermorphology and function. Dis Colon Rectum. 1999;42:1253–1260.
14. Varma A, Gunn J, Gardiner A, et al. Obstetric anal sphincter injury: prospective evaluation of incidence. Dis Colon Rectum. 1999;42:1537–1543.
15. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333–1337.
16. Lindsey I, Jones OM, Smilgin-Humphreys MM, et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47:1643–1649.
17. National Digestive Diseases Information Clearinghouse. Fecal
incontinence. Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/fecalincontinence. Accessed October 20, 2013.
18. Roig JV, Villoslada C, Lledo S, et al. Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum. 1995;38:952–958.
19. Swash M, Gray A, Lubowski DZ, et al. Ultrastructural changes in internal sphincter in neurogenic incontinence. Gut. 1988;29:1692–1698.
20. Rogers J, Henry MM, Misiewicz JJ. Combined sensory and motor deficit in primary fecal incontinence. Gut. 1988;29:5–9.
21. Medline Plus Web site. Bowel incontinence. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003135.htm. Accessed October 20, 2013.
22. Browning GP, Parks AG. Post anal repair for neuropathic fecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg. 1983;70:101–104.
23. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal incontinence. Dis Colon Rectum. 2003;46:1591–1605.
24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
25. American Society of Colon & Rectal Surgeons Web site. Fecal incontinence quality of life scale. Available at: http://www.fascrs.org/physicians/Fecal_Incontinence_Quality_of_Life_Scale/. Accessed October 20, 2013.
26. Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factor for late onset fecal incontinence: a population based case-control study in women. Gastroenterology. 2010;139:1559-1566.
27. MedlinePlus Web site. Drug-induced diarrhea. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000293.htm. Accessed October 21, 2013.
28. Hallgren T, Fasth S, Delbro DS, et al. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612-2618.
29. Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676-1681.
30. Bharucha AE, Seide BM, Zinsmeister AR, et al. The effects of clonodine on symptoms and anorectal sensoriomotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681-688.
31. Engel BT, Nikoomnesh P, Schuster MM. Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence. N Engl J Med. 1974;290:646-649.
32. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329.
33. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of fecal incontinence in adults. Cochrane Database Syst Rev. 2006;(3):CD002111.
34. Sultan AH, Nicholls RJ, Kamm MA, et al. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993; 80:508–511.
35. Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal MRI adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327.
36. Goetz LH, Lowry AC. Overlapping sphincteroplasty: is it the standard of care? Clin Colon Rectal Surg. 2005;18:22-31.
37. El-Gazzazz G, Zutshi M, Hannaway C, et al. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55:256-261.
38. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482-490.
39. Lehto K, Hyoty M, Collin P, et al. Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis. 2013;5:653-658.
40. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302-306.
41. Jarrett MED, Mowatt G, Glazener CMA, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569.
42. Melenhorst J, Koch SM, Uludag O, et al. Is a morphologically intact anal sphincter necessary for success with sacral nerve modulation in patients with faecal incontinence? Colorectal Dis. 2008;10:257-262.
43. Dudding TC, Pares D, Vaizey CJ, et al. Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: a 10-year cohort analysis. Colorectal Dis. 2008;10:294-256.
44. Findlay JM, Mawell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26:265-273.
45. Feretis C, Benakis P, Dailianas A, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609.
46. Kenefick NJ, Vaizey CJ, Malouf AJ, et al. Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut. 2002;55:225–228.
47. Konsten J, Baeten CG, Spaans F, et al. Follow-up of anal dynamic graciloplasty for fecal continence. World J Surg. 1993;17:404–409.
48. Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence following previously implanted gracilis muscle: report of a case. Dis Colon Rectum. 1988;31:134–137.
49. Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773-779.
50. Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum 2003;46:722–729.
51. Takahashi T, Garcia-Osogobio S, Valdovinos MA, et al. Extended two-year results of radio-frequency energy delivery for the treatment of fecal incontinence (Secca procedure). Dis Colon Rectum. 2003;46:711–715.
52. Norton C, Burch J, Kamm MA. Patient’s views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062.
1. Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
2. Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586-598.
3. Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the impact of fecal incontinence on nursing home referral. J Am Geriatr Soc. 2010;58:1058-1062.
4. Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66:1101–1108.
5. Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int Clin Pract. 2012;66:1109–1116.
6. Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113-119.
7. Sundquist JC. Long-term outcome after obstetric injury: a retrospective study. Acta Obstet Gynecol Scand. 2012 Jun;91:715-718.
8. Planting A, Phang PT, Raval MJ, et al. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248.
9. Ctercteko GC, Fazio VW, Jagelman DG, et al. Anal sphincter repair: a report of 60 cases and review of the literature. Aust N Z J Surg. 1988;58:703–710.
10. Keighley MRB, Fielding JWL. Management of faecal incontinence and results of surgical treatment. Br J Surg. 1983;70: 463–468.
11. Eason E, Labrecque M, Marcoux S, et al. Anal incontinence after childbirth. CMAJ. 2002;166:326–330.
12. Rieger N, Schloithe A, Saccone G, et al. A prospective study of analsphincter injury due to childbirth. Scand J Gastroenterol. 1998;33:950–955.
13. Zetterstrom J, Mellgren A, Jensen LL, et al. Effect of delivery on anal sphinctermorphology and function. Dis Colon Rectum. 1999;42:1253–1260.
14. Varma A, Gunn J, Gardiner A, et al. Obstetric anal sphincter injury: prospective evaluation of incidence. Dis Colon Rectum. 1999;42:1537–1543.
15. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333–1337.
16. Lindsey I, Jones OM, Smilgin-Humphreys MM, et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47:1643–1649.
17. National Digestive Diseases Information Clearinghouse. Fecal
incontinence. Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/fecalincontinence. Accessed October 20, 2013.
18. Roig JV, Villoslada C, Lledo S, et al. Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum. 1995;38:952–958.
19. Swash M, Gray A, Lubowski DZ, et al. Ultrastructural changes in internal sphincter in neurogenic incontinence. Gut. 1988;29:1692–1698.
20. Rogers J, Henry MM, Misiewicz JJ. Combined sensory and motor deficit in primary fecal incontinence. Gut. 1988;29:5–9.
21. Medline Plus Web site. Bowel incontinence. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003135.htm. Accessed October 20, 2013.
22. Browning GP, Parks AG. Post anal repair for neuropathic fecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg. 1983;70:101–104.
23. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal incontinence. Dis Colon Rectum. 2003;46:1591–1605.
24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
25. American Society of Colon & Rectal Surgeons Web site. Fecal incontinence quality of life scale. Available at: http://www.fascrs.org/physicians/Fecal_Incontinence_Quality_of_Life_Scale/. Accessed October 20, 2013.
26. Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factor for late onset fecal incontinence: a population based case-control study in women. Gastroenterology. 2010;139:1559-1566.
27. MedlinePlus Web site. Drug-induced diarrhea. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000293.htm. Accessed October 21, 2013.
28. Hallgren T, Fasth S, Delbro DS, et al. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612-2618.
29. Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676-1681.
30. Bharucha AE, Seide BM, Zinsmeister AR, et al. The effects of clonodine on symptoms and anorectal sensoriomotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681-688.
31. Engel BT, Nikoomnesh P, Schuster MM. Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence. N Engl J Med. 1974;290:646-649.
32. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329.
33. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of fecal incontinence in adults. Cochrane Database Syst Rev. 2006;(3):CD002111.
34. Sultan AH, Nicholls RJ, Kamm MA, et al. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993; 80:508–511.
35. Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal MRI adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327.
36. Goetz LH, Lowry AC. Overlapping sphincteroplasty: is it the standard of care? Clin Colon Rectal Surg. 2005;18:22-31.
37. El-Gazzazz G, Zutshi M, Hannaway C, et al. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55:256-261.
38. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482-490.
39. Lehto K, Hyoty M, Collin P, et al. Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis. 2013;5:653-658.
40. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302-306.
41. Jarrett MED, Mowatt G, Glazener CMA, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569.
42. Melenhorst J, Koch SM, Uludag O, et al. Is a morphologically intact anal sphincter necessary for success with sacral nerve modulation in patients with faecal incontinence? Colorectal Dis. 2008;10:257-262.
43. Dudding TC, Pares D, Vaizey CJ, et al. Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: a 10-year cohort analysis. Colorectal Dis. 2008;10:294-256.
44. Findlay JM, Mawell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26:265-273.
45. Feretis C, Benakis P, Dailianas A, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609.
46. Kenefick NJ, Vaizey CJ, Malouf AJ, et al. Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut. 2002;55:225–228.
47. Konsten J, Baeten CG, Spaans F, et al. Follow-up of anal dynamic graciloplasty for fecal continence. World J Surg. 1993;17:404–409.
48. Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence following previously implanted gracilis muscle: report of a case. Dis Colon Rectum. 1988;31:134–137.
49. Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773-779.
50. Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum 2003;46:722–729.
51. Takahashi T, Garcia-Osogobio S, Valdovinos MA, et al. Extended two-year results of radio-frequency energy delivery for the treatment of fecal incontinence (Secca procedure). Dis Colon Rectum. 2003;46:711–715.
52. Norton C, Burch J, Kamm MA. Patient’s views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062.