User login
Hip fracture in older patients: Tips and tools to speed recovery
› Ensure that surgical stabilization of hip fracture is performed as soon as possible—ideally within 48 hours of injury. A
› To reduce the risk of delirium, orient the patient frequently; get her out of bed as soon as possible, and avoid prolonged catheter use. A
› Order protein supplements for patients recovering from hip fracture and take steps to facilitate an early return to eating. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
The patient and family request a consultation with Ms. J’s primary care physician. If you were her physician, what would you advise?
Hip fracture in a frail elderly patient is an injury that, while common, can be difficult to manage. With good reason. Geriatric hip fracture is associated with increased morbidity, functional decline, and use of nursing home services, as well as a higher mortality rate: One in 5 hip fracture patients dies within a year of the injury.1
As the population ages, we are seeing more hip fractures in the “oldest old” those who, like Ms. J, are older than 85. While the incidence increases exponentially with age in both men and women, women are 3 times more likely than men to sustain a hip fracture.2 White women ages 85 to 95 face the highest risk, with an incidence of more than 3%.3
In addition to managing the acute phase of hip fracture and helping patients and families make decisions about optimal treatment, there is much you can do to boost the likelihood of a rapid rehabilitation and a successful outcome.
What type of fracture? How best to treat it?
Two types of hip fractures are responsible for the vast majority of cases: About 45% of hip fractures are intracapsular, involving the femoral head and neck; another 45% are intertrochanteric fractures. Both usually involve low-energy trauma, such as a fall from a chair or tripping over a rug. Intertrochanteric and subtrochanteric fractures (the latter accounting for the remaining 10%) are extracapsular.2,4,5
Typically associated with high-energy trauma such as a motor vehicle accident, or with metastatic lesions, subtrochanteric fractures have a bimodal distribution: They are most common in individuals between the ages of 20 and 40 and those older than 60.2
Fractures involving the femoral neck can disrupt the vascular supply to the femoral head and result in avascular necrosis (AVN) or nonunion.2,4,5 A meta-analysis of the outcome of displaced femoral neck fractures found the rates of osteonecrosis and nonunion to be as high as 20% to 30%.5 Intertrochanteric fractures rarely lead to AVN or nonunion, but patients may develop complications associated with degenerative changes.2,4,5 Nonunion is a potential complication of subtrochanteric fracture.2
For most patients, surgical management is preferred
The main goals of treatment are to stabilize the hip, decrease pain and restore the level of prefracture function. Surgery is the preferred treatment for hip fracture because it provides stable fixation, facilitating full weight bearing and decreasing the risk of complications. Surgery is also associated with a shorter stay in the hospital and improved rehabilitation and recovery.6
Surgical stabilization should be performed as soon as possible—ideally, within 48 hours.5 A recent study found conflicting evidence of the effect of delayed surgery on mortality, but demonstrated that surgery within 24 hours of injury minimizes the rate of chest infections, urinary tract infections, and pressure sores, as well as the duration of the hospital stay.7 (To learn more about surgical stabilization of hip fracture, see “What type of surgery? Age is just one consideration” 5,8-10 below.)
When surgery is contraindicated
Nonoperative management is reserved for patients who stand to gain only minimal function from surgical stabilization, because they either were not ambulatory to begin with or have severe dementia. In addition, medical management is used for patients with contraindications to anesthesia, those who delay seeking medical care until the fracture has begun to heal, and patients who refuse surgical fixation.5,11
The choice of surgical intervention depends on multiple factors, including the:
- type and severity of the fracture
- preference of the orthopedic surgeon
- age of the patient
- comorbid conditions
- prognosis.
For femoral neck fractures, patients younger than 65 years are candidates for internal fixation; for older individuals and those who already had limited mobility, arthroplasty should be considered.5 Studies of pain and functional outcomes show a modest tendency for total hip arthroplasty to have better results than internal fixation in patients older than 65.8
Intertrochanteric fractures can be treated with either sliding hip screws or
intramedullary nails. Intramedullary nail implants are done percutaneously, resulting in a shorter duration for surgery, less blood loss, and an earlier return to full weight bearing.5 A recent study suggests that intramedullary nails result in more reoperations than hip screws.9 No evidence is conclusive about the superiority of either type of hardware.
Subtrochanteric fractures are typically repaired by hemiarthroplasty.
A Cochrane review of randomized controlled trials found insufficient evidence to determine whether replacement arthroplasty has any advantage over internal fixation for extracapsular hip fractures.10
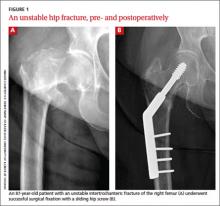
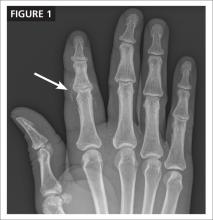
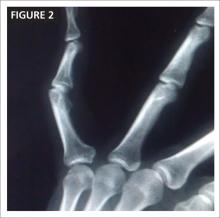
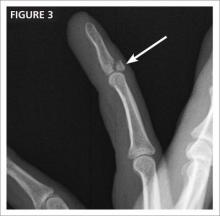
CASE After a careful review of Ms. J’s health status, radiographs of the fracture (FIGURE 1A), and consultation with an orthopedic surgeon and a geriatrician, you recommend surgery as soon as the patient is fully stabilized. Without it, she would be at high risk for urinary tract infection, pressure sores, and thromboembolism associated with long-term immobility.
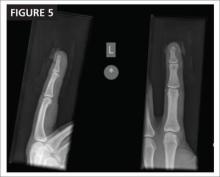
The next day, Ms. J undergoes surgical fixation with a sliding hip screw (FIGURE 1B). Her Foley catheter is removed the same day, and physical therapy is begun the following day. On postoperative day 4 she is discharged to an in patient rehabilitation facility.
Begin rehabilitation without delay
Whether a patient has surgery or is treated nonoperatively for hip fracture, the goal of rehabilitation is the same—to restore mobility as quickly as possible. A clinical review found no significant difference in mortality rates between those who underwent surgical fixation and those who were treated medically with early mobilization, consisting of immediate bed-to-chair transfer (with assistance), followed by progression to ambulation as tolerated.12
For patients who undergo surgery for hip fracture, increased immobility is linked to poorer functioning in the areas of self-care and transfers at 2 months and to higher mortality rates at 6 months.13 Physical therapy should be initiated on the first postoperative day and should start with bed mobility range of motion, followed by independent transfers from bed to chair, and ultimately achieving full weight bearing.5
Many complications are predictable, and often preventable
The term “hip fracture syndrome”4 is often used in reference to a cluster of common (and often preventable) complications of hip fracture, with delirium, venous thromboembolism (VTE), and malnutrition foremost among them.
Take steps to prevent—or treat—delirium
Delirium is among the most common complication, occurring in up to 62% of older patients with hip fracture.4 The highest predictor of delirium is preexisting cognitive impairment.
Other risk factors for delirium include advanced age, vision or hearing impairment, concurrent alcohol abuse, malnutrition, comorbidity, and polypharmacy.4,14 Delirium is associated with increased morbidity and mortality, decreased rehabilitation potential, and poor functional recovery independent of prior frailty.4,15,16
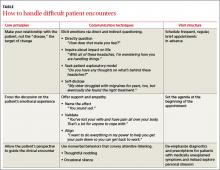
Hypoactive delirium is easily missed. While agitated, or hyperactive, delirium is more easily recognized, it is crucial to be aware of hypoactive delirium, as well. Patients with hypoactive delirium tend to become more withdrawn and their delirium is easily missed, leading to worse outcomes.15 The Confusion Assessment Method (TABLE 1)17 is an easy-to-use validated tool developed to aid in the diagnosis of delirium at the bedside.
Many factors contribute to the development of delirium. Medical complications, such as infection, electrolyte and volume imbalances, hypoxia, and myocardial infarction, are obvious precipitants.15 Disturbances in sleep-wake cycles, an unfamiliar environment, physical restraints, and the use of Foley catheters—all of which can impair an older patient’s sensory awareness—are less well-known contributing factors.
Tips for preventing delirium. Early mobilization, in addition to boosting physical recovery, can help prevent delirium.
Other tips:
- discontinue catheterization as soon as possible; this may help prevent delirium, and lessen the risk of urinary tract infection.
- remind nurses and family members to continuously reorient patients to their surroundings.
- treat pain aggressively.
- consult a geriatrician early on.
While opioids are often thought to cause delirium, several studies have shown an inverse relationship—that is, hip fracture patients who were given opioids for pain were actually less likely to develop delirium than those who did not receive opioids. This raises 2 important points:
1. untreated pain may itself be a significant risk factor for delirium,15,18 and
2. delirium itself is not a contraindication to opioids.18
CASE In her first week at the inpatient rehabilitation center, Ms. J requires slightly more narcotic medication for pain control. The staff notices increased confusion and a decrease in the number of bowel movements. Ms. J is started on a regimen of sennosides and docusate twice daily. Her mental status improves quickly and she has no further complications while at the rehab center.
Nonopioid pain medications such as acetaminophen should be scheduled at appropriate doses (eg, 1 g tid). Ensure that patients recovering from hip fracture are not given benzodiazepines, anticholinergics, or antihistamines15— which are sometimes included in a facility’s PRN protocol. In clinical trials, prophylactic administration of antipsychotics or anticholinesterase therapy to high-risk patients has had conflicting results.19,20
Arrange for a geriatric consult before problems occur. Several studies have shown that a geriatric consultation and concurrent management by a geriatrician using structured protocols to evaluate for common risk factors known to precipitate delirium (eg, pain, bowel/bladder function, nutrition, mobilization) can reduce the risk of delirium.16
Provide supportive care. Although treatment of the underlying cause is the definitive treatment for delirium, there are times when supportive care is all that’s needed. Reassurance from family members or staff is the recommended first step. Physical restraints should be avoided unless patient safety is threatened despite attempts to provide supportive care.
If treatment for delirium is needed, lowdose antipsychotics are recommended. The most studied agent is haloperidol, which can be administered intravenously (IV), intramuscularly (IM), or orally. Monitoring the corrected QT (QTc) interval is recommended for patients taking haloperidol, and discontinuation of the drug—or a cardiology consult— is recommended if the QTc interval is prolonged (>450 ms or >25% of baseline).21
There is a slightly higher risk of cardiac arrhythmias with IV administration of haloperidol compared with IM or oral dosing. Despite this risk, haloperidol IV is the treatment of choice for delirium.21 Newer atypical antipsychotics have also been used to treat delirium, but data are limited.21
Guard against VTE
Studies have shown rates of VTE to be as high as 40% to 60% after orthopedic procedures, and prophylaxis has long been the standard of care.22 In its 2012 consensus guidelines for antithrombotic therapy, the American College of Chest Physicians (ACCP) recommends fondiparinux, apixaban, rivaroxaban, dabigatran, low-molecular-weight heparin (LMWH), low-dose unfractionated heparin, aspirin, warfarin, or an intermittent pneumatic compression device (IPCD) as prophylaxis.23 Portable battery-powered IPCDs are recommended for 18 hours postop.23
The guideline authors prefer LMWH to the other treatments, and recommend dual prophylaxis with an IPCD and an antithrombotic agent while the patient is in the hospital and for a minimum of 10 to 14 days (and up to 35 days) after discharge. If surgery for hip fracture is delayed, the ACCP recommends that LMWH be administered after admission, but withheld for at least 12 hours before surgery. In patients with a high risk of bleeding, the ACCP recommends either an IPCD alone or no prophylaxis and notes that inferior vena cava filters should not be placed in high-risk patients.23
Take steps to ensure ample protein intake
Malnourishment is another common complication, affecting up to 20% of hip fracture patients.24 In many cases, a catabolic state predisposes patients to protein depletion, leading to decreased wound healing and an increase in other postop complications.24,25 Protein supplementation is associated with decreased length of stay and a reduction in postop complications.26
This complication can often be avoided by encouraging an early return to eating. Specific steps: Ensure that patients have their dentures available and are able to use them; are positioned properly for eating; and receive high-caloric supplemental drinks. Nutritional assessments should also be done to ensure that their intake of calcium and vitamin D is sufficient to prevent future falls and reduce fracture risk. (For more information, see “Vitamin D: When it helps, when it harms” [J Fam Pract. 2013;62:368-370.])
Combat hip fracture by stressing avoidance
Prevention of hip fracture, of course, is the ideal way to reduce the burden of disease for older patients. Along these lines, there are many ways you can help.
Start with fall reduction
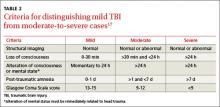
Hip fracture is associated with a fall 90% of the time,27 and care for older patients should be focused on reducing the risk for falls and improving bone health and muscular function. While a complete review of preventive measures is beyond the scope of this article, we offer some highlights here and in TABLE 2.
Encourage physical activity In addition to helping to reduce falls, physical activity—particularly repetitive weight-bearing exercise—can help maintain bone density and improve muscle mass, strength, and balance.28
Rather than focus on a single exercise, however, a combination of activities—Tai Chi and walking, for instance, or weight lifting and cycling —appears to have the best likelihood of fall reduction.29 Whenever possible, physical activity for older patients should include challenges in executive function, as well. In a recent study comparing regular walking with trail-walking between sequentially marked flags, participants in the more complex activity had a greater decrease in fall rates.30
Review vitamin D and calcium intake. Elderly patients with low levels of vitamin D are at increased risk of muscle mass decline, and therefore increased risk of fracture.31 A systematic review and meta-analysis of vitamin D supplementation in older adults found the relative risk of falling was 0.86 (95% confidence interval [CI], 0.79-0.93) for those assigned to vitamin D therapy compared with those on placebo. Risk reduction was greater in groups taking 800 IU or more of vitamin D daily and those taking adjunctive calcium supplementation.32
Maximizing vitamin D for falls reduction is supported by the American Geriatrics Society, 33 the Agency for Healthcare Research and Quality (AHRQ),34 and the US Preventive Services Task Force (USPSTF).35 The USPSTF recently released a recommendation for exercise or physical therapy and vitamin D supplementation (800 IU) to prevent falls in community-dwelling adults ages 65 and over who are at an increased risk for falls.36
However, the USPSTF advises against daily supplementation with vitamin D and calcium at doses ?400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women for primary fracture prevention. Calcium supplementation has not been shown to reduce hip fractures, but has been found to improve hip bone density.37
Consider bisphosphonates. Order a dual energy x-ray absorptiometry (DEXA) scan for older patients to identify osteoporosis. Most hip fractures are osteoporotic, and patients should be started on bisphosphonates within 2 to 12 weeks of injury38 to reduce the risk of mortality associated with hip fracture.39 The most studied bisphosphonates in geriatric hip fracture are alendronate, risedronate, and zoledronate; all were found to have a number needed to treat of 91 to prevent one hip fracture.40
Focus on the home environment. In addition to addressing the bone and muscular health of older patients, focus should be placed on the home environment. A Cochrane review of fall prevention for those living in the community found that home safety interventions reduced the risk of falls, but only for those with severe vision impairment and a high risk of falls.29 A 2010 American Geriatric Society (AGS) and British Geriatric Society (BGS) review of fall prevention gave an A recommendation—the highest rating— to home assessment and intervention by a health care professional to identify home hazards and promote safe performance of daily activities.33
Conduct brown-bag reviews. Polypharmacy is a well-documented (and growing) problem among the elderly.41 Both the AGS and BGS encourage a review of medications (including over-the-counter products) and interactions at each office visit,33 with specific attention paid to drugs that may cause dizziness, drowsiness, and near syncopal or syncopal episodes.
To reduce the risk of medication interactions and adverse effects, look for opportunities to reduce the number of drugs your elderly patients are taking. Consider involving a clinical pharmacist in medication reviews—an intervention that has been shown to be cost effective and lead to better patient outcomes.42
CASE After 4 weeks, Ms. J is ready to return home. Rather than a return to independent living, however, her children convince her to move to an assisted living facility—a move you strongly support. You schedule a visit in 2 weeks.
CORRESPONDENCE
Jeremy D. Close, MD, Department of Family and Community Medicine, Thomas Jefferson University, 833 Chestnut Street #301, Philadelphia, PA 19107; [email protected]
1. Leibson CL, Toteson ANA, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-50.
2. Brunner LC, Eshilian-Oates L, Kuo TY. Hip fractures in adults. Am Fam Physician. 2003;67:537-542.
3. Jacobsen SJ, Goldberg J, Miles TP, et al. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871-873.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24:701-719.
5. Jackman JM, Watson JT. Hip fractures in older men. Clin Geriatr Med. 2010;26:311-329.
6. Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;(3):CD000337.
7. Leung F, Lau W, Kwan K, et al. Does timing of surgery matter in fragility hip fractures? Osteoporos Int. 2010; 21(suppl 4):S529-S534.
8. Butler M, Forte ML, Joglekar SB, et al. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104-1115.
9. Matre K, Havelin LI, Gjertsen JE, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res. 2013;471: 1379-1386.
10. Parker MJ, Handoll HH. Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2006;(2):CD000086.
11. Cummings-Vaughn LA, Gammack JK. Falls, osteoporosis, and hip fractures. Med Clin North Am. 2011;95:495-506.
12. Jain R, Basinski A, Kreder HJ. Nonoperative treatment of hip fractures. Int Orthop. 2003;27:11-17.
13. Siu A, Penrod J, Boockvar K, et al. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766-771.
14. Juliebø V, Bjøro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361.
15. Flinn DR, Deihl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg. 2009;209:261-268.
16. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
18. Sieber FE, Mears S, Lee H, et al. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256-2262.
19. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for Rather than focus on a single exercise, a combination of activities—eg, Tai Chi and walking, or weight lifting and cycling—have the greatest likelihood of fall reduction. prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714-719.
20. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343-349.
21. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
22. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference of Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
23. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):7S-47S.
24. Garcia Lazaro M, Montero Perez-Barquero M, Carpintero Benitez P. The role of malnutrition and other medical factors in the evolution of patients with hip fracture [article in Spanish]. An Med Interna. 2004;21:557-563.
25. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18:274-278.
26. Huddleston JM, Whitford KJ. Medical care of elderly patients with hip fractures. Mayo Clin Proc. 2001;76:295-298.
27. Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
28. Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures a randomized controlled trial. JAMA. 1994;272:1909-1914.
29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
30. Yamada M, Tanaka B, Nagai K, et al. Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc. 2010;58:1946-1951.
31. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772.
32. Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: systematic review and metaanalysis. J Am Geriatr Soc. 2010;58:1299-1310.
33. The American Geriatrics Society. Prevention of falls in older persons [clinical practice guideline]. 2010. Available at: http:// www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/ 2010/. Accessed August 16, 2013.
34. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235.
35. Michael YL, Whitlock EP, Lin JS, et al. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815-825.
36. USPSTF. Prevention of falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. May 2012. Available at: www.uspreventiveservices taskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed August 19, 2013.
37. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
38. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; for the HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
39. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983-991.
40. Ringe, JD, Doherty, JG. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010;30:863-869.
41. Veehof L, Stewart R, Haaijer-Ruskamp F, et al. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261-267.
42. Choe HM, Farris KB, Stevenson JG, et al. Patient-centered medical home: developing, expanding, and sustaining a role for pharmacists. Am J Health Syst Pharm. 2012;69:1063-1071.
› Ensure that surgical stabilization of hip fracture is performed as soon as possible—ideally within 48 hours of injury. A
› To reduce the risk of delirium, orient the patient frequently; get her out of bed as soon as possible, and avoid prolonged catheter use. A
› Order protein supplements for patients recovering from hip fracture and take steps to facilitate an early return to eating. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
The patient and family request a consultation with Ms. J’s primary care physician. If you were her physician, what would you advise?
Hip fracture in a frail elderly patient is an injury that, while common, can be difficult to manage. With good reason. Geriatric hip fracture is associated with increased morbidity, functional decline, and use of nursing home services, as well as a higher mortality rate: One in 5 hip fracture patients dies within a year of the injury.1
As the population ages, we are seeing more hip fractures in the “oldest old” those who, like Ms. J, are older than 85. While the incidence increases exponentially with age in both men and women, women are 3 times more likely than men to sustain a hip fracture.2 White women ages 85 to 95 face the highest risk, with an incidence of more than 3%.3
In addition to managing the acute phase of hip fracture and helping patients and families make decisions about optimal treatment, there is much you can do to boost the likelihood of a rapid rehabilitation and a successful outcome.
What type of fracture? How best to treat it?
Two types of hip fractures are responsible for the vast majority of cases: About 45% of hip fractures are intracapsular, involving the femoral head and neck; another 45% are intertrochanteric fractures. Both usually involve low-energy trauma, such as a fall from a chair or tripping over a rug. Intertrochanteric and subtrochanteric fractures (the latter accounting for the remaining 10%) are extracapsular.2,4,5
Typically associated with high-energy trauma such as a motor vehicle accident, or with metastatic lesions, subtrochanteric fractures have a bimodal distribution: They are most common in individuals between the ages of 20 and 40 and those older than 60.2
Fractures involving the femoral neck can disrupt the vascular supply to the femoral head and result in avascular necrosis (AVN) or nonunion.2,4,5 A meta-analysis of the outcome of displaced femoral neck fractures found the rates of osteonecrosis and nonunion to be as high as 20% to 30%.5 Intertrochanteric fractures rarely lead to AVN or nonunion, but patients may develop complications associated with degenerative changes.2,4,5 Nonunion is a potential complication of subtrochanteric fracture.2
For most patients, surgical management is preferred
The main goals of treatment are to stabilize the hip, decrease pain and restore the level of prefracture function. Surgery is the preferred treatment for hip fracture because it provides stable fixation, facilitating full weight bearing and decreasing the risk of complications. Surgery is also associated with a shorter stay in the hospital and improved rehabilitation and recovery.6
Surgical stabilization should be performed as soon as possible—ideally, within 48 hours.5 A recent study found conflicting evidence of the effect of delayed surgery on mortality, but demonstrated that surgery within 24 hours of injury minimizes the rate of chest infections, urinary tract infections, and pressure sores, as well as the duration of the hospital stay.7 (To learn more about surgical stabilization of hip fracture, see “What type of surgery? Age is just one consideration” 5,8-10 below.)
When surgery is contraindicated
Nonoperative management is reserved for patients who stand to gain only minimal function from surgical stabilization, because they either were not ambulatory to begin with or have severe dementia. In addition, medical management is used for patients with contraindications to anesthesia, those who delay seeking medical care until the fracture has begun to heal, and patients who refuse surgical fixation.5,11
The choice of surgical intervention depends on multiple factors, including the:
- type and severity of the fracture
- preference of the orthopedic surgeon
- age of the patient
- comorbid conditions
- prognosis.
For femoral neck fractures, patients younger than 65 years are candidates for internal fixation; for older individuals and those who already had limited mobility, arthroplasty should be considered.5 Studies of pain and functional outcomes show a modest tendency for total hip arthroplasty to have better results than internal fixation in patients older than 65.8
Intertrochanteric fractures can be treated with either sliding hip screws or
intramedullary nails. Intramedullary nail implants are done percutaneously, resulting in a shorter duration for surgery, less blood loss, and an earlier return to full weight bearing.5 A recent study suggests that intramedullary nails result in more reoperations than hip screws.9 No evidence is conclusive about the superiority of either type of hardware.
Subtrochanteric fractures are typically repaired by hemiarthroplasty.
A Cochrane review of randomized controlled trials found insufficient evidence to determine whether replacement arthroplasty has any advantage over internal fixation for extracapsular hip fractures.10
CASE After a careful review of Ms. J’s health status, radiographs of the fracture (FIGURE 1A), and consultation with an orthopedic surgeon and a geriatrician, you recommend surgery as soon as the patient is fully stabilized. Without it, she would be at high risk for urinary tract infection, pressure sores, and thromboembolism associated with long-term immobility.
The next day, Ms. J undergoes surgical fixation with a sliding hip screw (FIGURE 1B). Her Foley catheter is removed the same day, and physical therapy is begun the following day. On postoperative day 4 she is discharged to an in patient rehabilitation facility.
Begin rehabilitation without delay
Whether a patient has surgery or is treated nonoperatively for hip fracture, the goal of rehabilitation is the same—to restore mobility as quickly as possible. A clinical review found no significant difference in mortality rates between those who underwent surgical fixation and those who were treated medically with early mobilization, consisting of immediate bed-to-chair transfer (with assistance), followed by progression to ambulation as tolerated.12
For patients who undergo surgery for hip fracture, increased immobility is linked to poorer functioning in the areas of self-care and transfers at 2 months and to higher mortality rates at 6 months.13 Physical therapy should be initiated on the first postoperative day and should start with bed mobility range of motion, followed by independent transfers from bed to chair, and ultimately achieving full weight bearing.5
Many complications are predictable, and often preventable
The term “hip fracture syndrome”4 is often used in reference to a cluster of common (and often preventable) complications of hip fracture, with delirium, venous thromboembolism (VTE), and malnutrition foremost among them.
Take steps to prevent—or treat—delirium
Delirium is among the most common complication, occurring in up to 62% of older patients with hip fracture.4 The highest predictor of delirium is preexisting cognitive impairment.
Other risk factors for delirium include advanced age, vision or hearing impairment, concurrent alcohol abuse, malnutrition, comorbidity, and polypharmacy.4,14 Delirium is associated with increased morbidity and mortality, decreased rehabilitation potential, and poor functional recovery independent of prior frailty.4,15,16
Hypoactive delirium is easily missed. While agitated, or hyperactive, delirium is more easily recognized, it is crucial to be aware of hypoactive delirium, as well. Patients with hypoactive delirium tend to become more withdrawn and their delirium is easily missed, leading to worse outcomes.15 The Confusion Assessment Method (TABLE 1)17 is an easy-to-use validated tool developed to aid in the diagnosis of delirium at the bedside.
Many factors contribute to the development of delirium. Medical complications, such as infection, electrolyte and volume imbalances, hypoxia, and myocardial infarction, are obvious precipitants.15 Disturbances in sleep-wake cycles, an unfamiliar environment, physical restraints, and the use of Foley catheters—all of which can impair an older patient’s sensory awareness—are less well-known contributing factors.
Tips for preventing delirium. Early mobilization, in addition to boosting physical recovery, can help prevent delirium.
Other tips:
- discontinue catheterization as soon as possible; this may help prevent delirium, and lessen the risk of urinary tract infection.
- remind nurses and family members to continuously reorient patients to their surroundings.
- treat pain aggressively.
- consult a geriatrician early on.
While opioids are often thought to cause delirium, several studies have shown an inverse relationship—that is, hip fracture patients who were given opioids for pain were actually less likely to develop delirium than those who did not receive opioids. This raises 2 important points:
1. untreated pain may itself be a significant risk factor for delirium,15,18 and
2. delirium itself is not a contraindication to opioids.18
CASE In her first week at the inpatient rehabilitation center, Ms. J requires slightly more narcotic medication for pain control. The staff notices increased confusion and a decrease in the number of bowel movements. Ms. J is started on a regimen of sennosides and docusate twice daily. Her mental status improves quickly and she has no further complications while at the rehab center.
Nonopioid pain medications such as acetaminophen should be scheduled at appropriate doses (eg, 1 g tid). Ensure that patients recovering from hip fracture are not given benzodiazepines, anticholinergics, or antihistamines15— which are sometimes included in a facility’s PRN protocol. In clinical trials, prophylactic administration of antipsychotics or anticholinesterase therapy to high-risk patients has had conflicting results.19,20
Arrange for a geriatric consult before problems occur. Several studies have shown that a geriatric consultation and concurrent management by a geriatrician using structured protocols to evaluate for common risk factors known to precipitate delirium (eg, pain, bowel/bladder function, nutrition, mobilization) can reduce the risk of delirium.16
Provide supportive care. Although treatment of the underlying cause is the definitive treatment for delirium, there are times when supportive care is all that’s needed. Reassurance from family members or staff is the recommended first step. Physical restraints should be avoided unless patient safety is threatened despite attempts to provide supportive care.
If treatment for delirium is needed, lowdose antipsychotics are recommended. The most studied agent is haloperidol, which can be administered intravenously (IV), intramuscularly (IM), or orally. Monitoring the corrected QT (QTc) interval is recommended for patients taking haloperidol, and discontinuation of the drug—or a cardiology consult— is recommended if the QTc interval is prolonged (>450 ms or >25% of baseline).21
There is a slightly higher risk of cardiac arrhythmias with IV administration of haloperidol compared with IM or oral dosing. Despite this risk, haloperidol IV is the treatment of choice for delirium.21 Newer atypical antipsychotics have also been used to treat delirium, but data are limited.21
Guard against VTE
Studies have shown rates of VTE to be as high as 40% to 60% after orthopedic procedures, and prophylaxis has long been the standard of care.22 In its 2012 consensus guidelines for antithrombotic therapy, the American College of Chest Physicians (ACCP) recommends fondiparinux, apixaban, rivaroxaban, dabigatran, low-molecular-weight heparin (LMWH), low-dose unfractionated heparin, aspirin, warfarin, or an intermittent pneumatic compression device (IPCD) as prophylaxis.23 Portable battery-powered IPCDs are recommended for 18 hours postop.23
The guideline authors prefer LMWH to the other treatments, and recommend dual prophylaxis with an IPCD and an antithrombotic agent while the patient is in the hospital and for a minimum of 10 to 14 days (and up to 35 days) after discharge. If surgery for hip fracture is delayed, the ACCP recommends that LMWH be administered after admission, but withheld for at least 12 hours before surgery. In patients with a high risk of bleeding, the ACCP recommends either an IPCD alone or no prophylaxis and notes that inferior vena cava filters should not be placed in high-risk patients.23
Take steps to ensure ample protein intake
Malnourishment is another common complication, affecting up to 20% of hip fracture patients.24 In many cases, a catabolic state predisposes patients to protein depletion, leading to decreased wound healing and an increase in other postop complications.24,25 Protein supplementation is associated with decreased length of stay and a reduction in postop complications.26
This complication can often be avoided by encouraging an early return to eating. Specific steps: Ensure that patients have their dentures available and are able to use them; are positioned properly for eating; and receive high-caloric supplemental drinks. Nutritional assessments should also be done to ensure that their intake of calcium and vitamin D is sufficient to prevent future falls and reduce fracture risk. (For more information, see “Vitamin D: When it helps, when it harms” [J Fam Pract. 2013;62:368-370.])
Combat hip fracture by stressing avoidance
Prevention of hip fracture, of course, is the ideal way to reduce the burden of disease for older patients. Along these lines, there are many ways you can help.
Start with fall reduction
Hip fracture is associated with a fall 90% of the time,27 and care for older patients should be focused on reducing the risk for falls and improving bone health and muscular function. While a complete review of preventive measures is beyond the scope of this article, we offer some highlights here and in TABLE 2.
Encourage physical activity In addition to helping to reduce falls, physical activity—particularly repetitive weight-bearing exercise—can help maintain bone density and improve muscle mass, strength, and balance.28
Rather than focus on a single exercise, however, a combination of activities—Tai Chi and walking, for instance, or weight lifting and cycling —appears to have the best likelihood of fall reduction.29 Whenever possible, physical activity for older patients should include challenges in executive function, as well. In a recent study comparing regular walking with trail-walking between sequentially marked flags, participants in the more complex activity had a greater decrease in fall rates.30
Review vitamin D and calcium intake. Elderly patients with low levels of vitamin D are at increased risk of muscle mass decline, and therefore increased risk of fracture.31 A systematic review and meta-analysis of vitamin D supplementation in older adults found the relative risk of falling was 0.86 (95% confidence interval [CI], 0.79-0.93) for those assigned to vitamin D therapy compared with those on placebo. Risk reduction was greater in groups taking 800 IU or more of vitamin D daily and those taking adjunctive calcium supplementation.32
Maximizing vitamin D for falls reduction is supported by the American Geriatrics Society, 33 the Agency for Healthcare Research and Quality (AHRQ),34 and the US Preventive Services Task Force (USPSTF).35 The USPSTF recently released a recommendation for exercise or physical therapy and vitamin D supplementation (800 IU) to prevent falls in community-dwelling adults ages 65 and over who are at an increased risk for falls.36
However, the USPSTF advises against daily supplementation with vitamin D and calcium at doses ?400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women for primary fracture prevention. Calcium supplementation has not been shown to reduce hip fractures, but has been found to improve hip bone density.37
Consider bisphosphonates. Order a dual energy x-ray absorptiometry (DEXA) scan for older patients to identify osteoporosis. Most hip fractures are osteoporotic, and patients should be started on bisphosphonates within 2 to 12 weeks of injury38 to reduce the risk of mortality associated with hip fracture.39 The most studied bisphosphonates in geriatric hip fracture are alendronate, risedronate, and zoledronate; all were found to have a number needed to treat of 91 to prevent one hip fracture.40
Focus on the home environment. In addition to addressing the bone and muscular health of older patients, focus should be placed on the home environment. A Cochrane review of fall prevention for those living in the community found that home safety interventions reduced the risk of falls, but only for those with severe vision impairment and a high risk of falls.29 A 2010 American Geriatric Society (AGS) and British Geriatric Society (BGS) review of fall prevention gave an A recommendation—the highest rating— to home assessment and intervention by a health care professional to identify home hazards and promote safe performance of daily activities.33
Conduct brown-bag reviews. Polypharmacy is a well-documented (and growing) problem among the elderly.41 Both the AGS and BGS encourage a review of medications (including over-the-counter products) and interactions at each office visit,33 with specific attention paid to drugs that may cause dizziness, drowsiness, and near syncopal or syncopal episodes.
To reduce the risk of medication interactions and adverse effects, look for opportunities to reduce the number of drugs your elderly patients are taking. Consider involving a clinical pharmacist in medication reviews—an intervention that has been shown to be cost effective and lead to better patient outcomes.42
CASE After 4 weeks, Ms. J is ready to return home. Rather than a return to independent living, however, her children convince her to move to an assisted living facility—a move you strongly support. You schedule a visit in 2 weeks.
CORRESPONDENCE
Jeremy D. Close, MD, Department of Family and Community Medicine, Thomas Jefferson University, 833 Chestnut Street #301, Philadelphia, PA 19107; [email protected]
› Ensure that surgical stabilization of hip fracture is performed as soon as possible—ideally within 48 hours of injury. A
› To reduce the risk of delirium, orient the patient frequently; get her out of bed as soon as possible, and avoid prolonged catheter use. A
› Order protein supplements for patients recovering from hip fracture and take steps to facilitate an early return to eating. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
The patient and family request a consultation with Ms. J’s primary care physician. If you were her physician, what would you advise?
Hip fracture in a frail elderly patient is an injury that, while common, can be difficult to manage. With good reason. Geriatric hip fracture is associated with increased morbidity, functional decline, and use of nursing home services, as well as a higher mortality rate: One in 5 hip fracture patients dies within a year of the injury.1
As the population ages, we are seeing more hip fractures in the “oldest old” those who, like Ms. J, are older than 85. While the incidence increases exponentially with age in both men and women, women are 3 times more likely than men to sustain a hip fracture.2 White women ages 85 to 95 face the highest risk, with an incidence of more than 3%.3
In addition to managing the acute phase of hip fracture and helping patients and families make decisions about optimal treatment, there is much you can do to boost the likelihood of a rapid rehabilitation and a successful outcome.
What type of fracture? How best to treat it?
Two types of hip fractures are responsible for the vast majority of cases: About 45% of hip fractures are intracapsular, involving the femoral head and neck; another 45% are intertrochanteric fractures. Both usually involve low-energy trauma, such as a fall from a chair or tripping over a rug. Intertrochanteric and subtrochanteric fractures (the latter accounting for the remaining 10%) are extracapsular.2,4,5
Typically associated with high-energy trauma such as a motor vehicle accident, or with metastatic lesions, subtrochanteric fractures have a bimodal distribution: They are most common in individuals between the ages of 20 and 40 and those older than 60.2
Fractures involving the femoral neck can disrupt the vascular supply to the femoral head and result in avascular necrosis (AVN) or nonunion.2,4,5 A meta-analysis of the outcome of displaced femoral neck fractures found the rates of osteonecrosis and nonunion to be as high as 20% to 30%.5 Intertrochanteric fractures rarely lead to AVN or nonunion, but patients may develop complications associated with degenerative changes.2,4,5 Nonunion is a potential complication of subtrochanteric fracture.2
For most patients, surgical management is preferred
The main goals of treatment are to stabilize the hip, decrease pain and restore the level of prefracture function. Surgery is the preferred treatment for hip fracture because it provides stable fixation, facilitating full weight bearing and decreasing the risk of complications. Surgery is also associated with a shorter stay in the hospital and improved rehabilitation and recovery.6
Surgical stabilization should be performed as soon as possible—ideally, within 48 hours.5 A recent study found conflicting evidence of the effect of delayed surgery on mortality, but demonstrated that surgery within 24 hours of injury minimizes the rate of chest infections, urinary tract infections, and pressure sores, as well as the duration of the hospital stay.7 (To learn more about surgical stabilization of hip fracture, see “What type of surgery? Age is just one consideration” 5,8-10 below.)
When surgery is contraindicated
Nonoperative management is reserved for patients who stand to gain only minimal function from surgical stabilization, because they either were not ambulatory to begin with or have severe dementia. In addition, medical management is used for patients with contraindications to anesthesia, those who delay seeking medical care until the fracture has begun to heal, and patients who refuse surgical fixation.5,11
The choice of surgical intervention depends on multiple factors, including the:
- type and severity of the fracture
- preference of the orthopedic surgeon
- age of the patient
- comorbid conditions
- prognosis.
For femoral neck fractures, patients younger than 65 years are candidates for internal fixation; for older individuals and those who already had limited mobility, arthroplasty should be considered.5 Studies of pain and functional outcomes show a modest tendency for total hip arthroplasty to have better results than internal fixation in patients older than 65.8
Intertrochanteric fractures can be treated with either sliding hip screws or
intramedullary nails. Intramedullary nail implants are done percutaneously, resulting in a shorter duration for surgery, less blood loss, and an earlier return to full weight bearing.5 A recent study suggests that intramedullary nails result in more reoperations than hip screws.9 No evidence is conclusive about the superiority of either type of hardware.
Subtrochanteric fractures are typically repaired by hemiarthroplasty.
A Cochrane review of randomized controlled trials found insufficient evidence to determine whether replacement arthroplasty has any advantage over internal fixation for extracapsular hip fractures.10
CASE After a careful review of Ms. J’s health status, radiographs of the fracture (FIGURE 1A), and consultation with an orthopedic surgeon and a geriatrician, you recommend surgery as soon as the patient is fully stabilized. Without it, she would be at high risk for urinary tract infection, pressure sores, and thromboembolism associated with long-term immobility.
The next day, Ms. J undergoes surgical fixation with a sliding hip screw (FIGURE 1B). Her Foley catheter is removed the same day, and physical therapy is begun the following day. On postoperative day 4 she is discharged to an in patient rehabilitation facility.
Begin rehabilitation without delay
Whether a patient has surgery or is treated nonoperatively for hip fracture, the goal of rehabilitation is the same—to restore mobility as quickly as possible. A clinical review found no significant difference in mortality rates between those who underwent surgical fixation and those who were treated medically with early mobilization, consisting of immediate bed-to-chair transfer (with assistance), followed by progression to ambulation as tolerated.12
For patients who undergo surgery for hip fracture, increased immobility is linked to poorer functioning in the areas of self-care and transfers at 2 months and to higher mortality rates at 6 months.13 Physical therapy should be initiated on the first postoperative day and should start with bed mobility range of motion, followed by independent transfers from bed to chair, and ultimately achieving full weight bearing.5
Many complications are predictable, and often preventable
The term “hip fracture syndrome”4 is often used in reference to a cluster of common (and often preventable) complications of hip fracture, with delirium, venous thromboembolism (VTE), and malnutrition foremost among them.
Take steps to prevent—or treat—delirium
Delirium is among the most common complication, occurring in up to 62% of older patients with hip fracture.4 The highest predictor of delirium is preexisting cognitive impairment.
Other risk factors for delirium include advanced age, vision or hearing impairment, concurrent alcohol abuse, malnutrition, comorbidity, and polypharmacy.4,14 Delirium is associated with increased morbidity and mortality, decreased rehabilitation potential, and poor functional recovery independent of prior frailty.4,15,16
Hypoactive delirium is easily missed. While agitated, or hyperactive, delirium is more easily recognized, it is crucial to be aware of hypoactive delirium, as well. Patients with hypoactive delirium tend to become more withdrawn and their delirium is easily missed, leading to worse outcomes.15 The Confusion Assessment Method (TABLE 1)17 is an easy-to-use validated tool developed to aid in the diagnosis of delirium at the bedside.
Many factors contribute to the development of delirium. Medical complications, such as infection, electrolyte and volume imbalances, hypoxia, and myocardial infarction, are obvious precipitants.15 Disturbances in sleep-wake cycles, an unfamiliar environment, physical restraints, and the use of Foley catheters—all of which can impair an older patient’s sensory awareness—are less well-known contributing factors.
Tips for preventing delirium. Early mobilization, in addition to boosting physical recovery, can help prevent delirium.
Other tips:
- discontinue catheterization as soon as possible; this may help prevent delirium, and lessen the risk of urinary tract infection.
- remind nurses and family members to continuously reorient patients to their surroundings.
- treat pain aggressively.
- consult a geriatrician early on.
While opioids are often thought to cause delirium, several studies have shown an inverse relationship—that is, hip fracture patients who were given opioids for pain were actually less likely to develop delirium than those who did not receive opioids. This raises 2 important points:
1. untreated pain may itself be a significant risk factor for delirium,15,18 and
2. delirium itself is not a contraindication to opioids.18
CASE In her first week at the inpatient rehabilitation center, Ms. J requires slightly more narcotic medication for pain control. The staff notices increased confusion and a decrease in the number of bowel movements. Ms. J is started on a regimen of sennosides and docusate twice daily. Her mental status improves quickly and she has no further complications while at the rehab center.
Nonopioid pain medications such as acetaminophen should be scheduled at appropriate doses (eg, 1 g tid). Ensure that patients recovering from hip fracture are not given benzodiazepines, anticholinergics, or antihistamines15— which are sometimes included in a facility’s PRN protocol. In clinical trials, prophylactic administration of antipsychotics or anticholinesterase therapy to high-risk patients has had conflicting results.19,20
Arrange for a geriatric consult before problems occur. Several studies have shown that a geriatric consultation and concurrent management by a geriatrician using structured protocols to evaluate for common risk factors known to precipitate delirium (eg, pain, bowel/bladder function, nutrition, mobilization) can reduce the risk of delirium.16
Provide supportive care. Although treatment of the underlying cause is the definitive treatment for delirium, there are times when supportive care is all that’s needed. Reassurance from family members or staff is the recommended first step. Physical restraints should be avoided unless patient safety is threatened despite attempts to provide supportive care.
If treatment for delirium is needed, lowdose antipsychotics are recommended. The most studied agent is haloperidol, which can be administered intravenously (IV), intramuscularly (IM), or orally. Monitoring the corrected QT (QTc) interval is recommended for patients taking haloperidol, and discontinuation of the drug—or a cardiology consult— is recommended if the QTc interval is prolonged (>450 ms or >25% of baseline).21
There is a slightly higher risk of cardiac arrhythmias with IV administration of haloperidol compared with IM or oral dosing. Despite this risk, haloperidol IV is the treatment of choice for delirium.21 Newer atypical antipsychotics have also been used to treat delirium, but data are limited.21
Guard against VTE
Studies have shown rates of VTE to be as high as 40% to 60% after orthopedic procedures, and prophylaxis has long been the standard of care.22 In its 2012 consensus guidelines for antithrombotic therapy, the American College of Chest Physicians (ACCP) recommends fondiparinux, apixaban, rivaroxaban, dabigatran, low-molecular-weight heparin (LMWH), low-dose unfractionated heparin, aspirin, warfarin, or an intermittent pneumatic compression device (IPCD) as prophylaxis.23 Portable battery-powered IPCDs are recommended for 18 hours postop.23
The guideline authors prefer LMWH to the other treatments, and recommend dual prophylaxis with an IPCD and an antithrombotic agent while the patient is in the hospital and for a minimum of 10 to 14 days (and up to 35 days) after discharge. If surgery for hip fracture is delayed, the ACCP recommends that LMWH be administered after admission, but withheld for at least 12 hours before surgery. In patients with a high risk of bleeding, the ACCP recommends either an IPCD alone or no prophylaxis and notes that inferior vena cava filters should not be placed in high-risk patients.23
Take steps to ensure ample protein intake
Malnourishment is another common complication, affecting up to 20% of hip fracture patients.24 In many cases, a catabolic state predisposes patients to protein depletion, leading to decreased wound healing and an increase in other postop complications.24,25 Protein supplementation is associated with decreased length of stay and a reduction in postop complications.26
This complication can often be avoided by encouraging an early return to eating. Specific steps: Ensure that patients have their dentures available and are able to use them; are positioned properly for eating; and receive high-caloric supplemental drinks. Nutritional assessments should also be done to ensure that their intake of calcium and vitamin D is sufficient to prevent future falls and reduce fracture risk. (For more information, see “Vitamin D: When it helps, when it harms” [J Fam Pract. 2013;62:368-370.])
Combat hip fracture by stressing avoidance
Prevention of hip fracture, of course, is the ideal way to reduce the burden of disease for older patients. Along these lines, there are many ways you can help.
Start with fall reduction
Hip fracture is associated with a fall 90% of the time,27 and care for older patients should be focused on reducing the risk for falls and improving bone health and muscular function. While a complete review of preventive measures is beyond the scope of this article, we offer some highlights here and in TABLE 2.
Encourage physical activity In addition to helping to reduce falls, physical activity—particularly repetitive weight-bearing exercise—can help maintain bone density and improve muscle mass, strength, and balance.28
Rather than focus on a single exercise, however, a combination of activities—Tai Chi and walking, for instance, or weight lifting and cycling —appears to have the best likelihood of fall reduction.29 Whenever possible, physical activity for older patients should include challenges in executive function, as well. In a recent study comparing regular walking with trail-walking between sequentially marked flags, participants in the more complex activity had a greater decrease in fall rates.30
Review vitamin D and calcium intake. Elderly patients with low levels of vitamin D are at increased risk of muscle mass decline, and therefore increased risk of fracture.31 A systematic review and meta-analysis of vitamin D supplementation in older adults found the relative risk of falling was 0.86 (95% confidence interval [CI], 0.79-0.93) for those assigned to vitamin D therapy compared with those on placebo. Risk reduction was greater in groups taking 800 IU or more of vitamin D daily and those taking adjunctive calcium supplementation.32
Maximizing vitamin D for falls reduction is supported by the American Geriatrics Society, 33 the Agency for Healthcare Research and Quality (AHRQ),34 and the US Preventive Services Task Force (USPSTF).35 The USPSTF recently released a recommendation for exercise or physical therapy and vitamin D supplementation (800 IU) to prevent falls in community-dwelling adults ages 65 and over who are at an increased risk for falls.36
However, the USPSTF advises against daily supplementation with vitamin D and calcium at doses ?400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women for primary fracture prevention. Calcium supplementation has not been shown to reduce hip fractures, but has been found to improve hip bone density.37
Consider bisphosphonates. Order a dual energy x-ray absorptiometry (DEXA) scan for older patients to identify osteoporosis. Most hip fractures are osteoporotic, and patients should be started on bisphosphonates within 2 to 12 weeks of injury38 to reduce the risk of mortality associated with hip fracture.39 The most studied bisphosphonates in geriatric hip fracture are alendronate, risedronate, and zoledronate; all were found to have a number needed to treat of 91 to prevent one hip fracture.40
Focus on the home environment. In addition to addressing the bone and muscular health of older patients, focus should be placed on the home environment. A Cochrane review of fall prevention for those living in the community found that home safety interventions reduced the risk of falls, but only for those with severe vision impairment and a high risk of falls.29 A 2010 American Geriatric Society (AGS) and British Geriatric Society (BGS) review of fall prevention gave an A recommendation—the highest rating— to home assessment and intervention by a health care professional to identify home hazards and promote safe performance of daily activities.33
Conduct brown-bag reviews. Polypharmacy is a well-documented (and growing) problem among the elderly.41 Both the AGS and BGS encourage a review of medications (including over-the-counter products) and interactions at each office visit,33 with specific attention paid to drugs that may cause dizziness, drowsiness, and near syncopal or syncopal episodes.
To reduce the risk of medication interactions and adverse effects, look for opportunities to reduce the number of drugs your elderly patients are taking. Consider involving a clinical pharmacist in medication reviews—an intervention that has been shown to be cost effective and lead to better patient outcomes.42
CASE After 4 weeks, Ms. J is ready to return home. Rather than a return to independent living, however, her children convince her to move to an assisted living facility—a move you strongly support. You schedule a visit in 2 weeks.
CORRESPONDENCE
Jeremy D. Close, MD, Department of Family and Community Medicine, Thomas Jefferson University, 833 Chestnut Street #301, Philadelphia, PA 19107; [email protected]
1. Leibson CL, Toteson ANA, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-50.
2. Brunner LC, Eshilian-Oates L, Kuo TY. Hip fractures in adults. Am Fam Physician. 2003;67:537-542.
3. Jacobsen SJ, Goldberg J, Miles TP, et al. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871-873.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24:701-719.
5. Jackman JM, Watson JT. Hip fractures in older men. Clin Geriatr Med. 2010;26:311-329.
6. Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;(3):CD000337.
7. Leung F, Lau W, Kwan K, et al. Does timing of surgery matter in fragility hip fractures? Osteoporos Int. 2010; 21(suppl 4):S529-S534.
8. Butler M, Forte ML, Joglekar SB, et al. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104-1115.
9. Matre K, Havelin LI, Gjertsen JE, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res. 2013;471: 1379-1386.
10. Parker MJ, Handoll HH. Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2006;(2):CD000086.
11. Cummings-Vaughn LA, Gammack JK. Falls, osteoporosis, and hip fractures. Med Clin North Am. 2011;95:495-506.
12. Jain R, Basinski A, Kreder HJ. Nonoperative treatment of hip fractures. Int Orthop. 2003;27:11-17.
13. Siu A, Penrod J, Boockvar K, et al. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766-771.
14. Juliebø V, Bjøro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361.
15. Flinn DR, Deihl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg. 2009;209:261-268.
16. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
18. Sieber FE, Mears S, Lee H, et al. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256-2262.
19. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for Rather than focus on a single exercise, a combination of activities—eg, Tai Chi and walking, or weight lifting and cycling—have the greatest likelihood of fall reduction. prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714-719.
20. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343-349.
21. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
22. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference of Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
23. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):7S-47S.
24. Garcia Lazaro M, Montero Perez-Barquero M, Carpintero Benitez P. The role of malnutrition and other medical factors in the evolution of patients with hip fracture [article in Spanish]. An Med Interna. 2004;21:557-563.
25. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18:274-278.
26. Huddleston JM, Whitford KJ. Medical care of elderly patients with hip fractures. Mayo Clin Proc. 2001;76:295-298.
27. Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
28. Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures a randomized controlled trial. JAMA. 1994;272:1909-1914.
29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
30. Yamada M, Tanaka B, Nagai K, et al. Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc. 2010;58:1946-1951.
31. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772.
32. Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: systematic review and metaanalysis. J Am Geriatr Soc. 2010;58:1299-1310.
33. The American Geriatrics Society. Prevention of falls in older persons [clinical practice guideline]. 2010. Available at: http:// www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/ 2010/. Accessed August 16, 2013.
34. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235.
35. Michael YL, Whitlock EP, Lin JS, et al. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815-825.
36. USPSTF. Prevention of falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. May 2012. Available at: www.uspreventiveservices taskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed August 19, 2013.
37. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
38. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; for the HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
39. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983-991.
40. Ringe, JD, Doherty, JG. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010;30:863-869.
41. Veehof L, Stewart R, Haaijer-Ruskamp F, et al. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261-267.
42. Choe HM, Farris KB, Stevenson JG, et al. Patient-centered medical home: developing, expanding, and sustaining a role for pharmacists. Am J Health Syst Pharm. 2012;69:1063-1071.
1. Leibson CL, Toteson ANA, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-50.
2. Brunner LC, Eshilian-Oates L, Kuo TY. Hip fractures in adults. Am Fam Physician. 2003;67:537-542.
3. Jacobsen SJ, Goldberg J, Miles TP, et al. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871-873.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24:701-719.
5. Jackman JM, Watson JT. Hip fractures in older men. Clin Geriatr Med. 2010;26:311-329.
6. Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;(3):CD000337.
7. Leung F, Lau W, Kwan K, et al. Does timing of surgery matter in fragility hip fractures? Osteoporos Int. 2010; 21(suppl 4):S529-S534.
8. Butler M, Forte ML, Joglekar SB, et al. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104-1115.
9. Matre K, Havelin LI, Gjertsen JE, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res. 2013;471: 1379-1386.
10. Parker MJ, Handoll HH. Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2006;(2):CD000086.
11. Cummings-Vaughn LA, Gammack JK. Falls, osteoporosis, and hip fractures. Med Clin North Am. 2011;95:495-506.
12. Jain R, Basinski A, Kreder HJ. Nonoperative treatment of hip fractures. Int Orthop. 2003;27:11-17.
13. Siu A, Penrod J, Boockvar K, et al. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766-771.
14. Juliebø V, Bjøro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361.
15. Flinn DR, Deihl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg. 2009;209:261-268.
16. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
18. Sieber FE, Mears S, Lee H, et al. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256-2262.
19. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for Rather than focus on a single exercise, a combination of activities—eg, Tai Chi and walking, or weight lifting and cycling—have the greatest likelihood of fall reduction. prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714-719.
20. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343-349.
21. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
22. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference of Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
23. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):7S-47S.
24. Garcia Lazaro M, Montero Perez-Barquero M, Carpintero Benitez P. The role of malnutrition and other medical factors in the evolution of patients with hip fracture [article in Spanish]. An Med Interna. 2004;21:557-563.
25. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18:274-278.
26. Huddleston JM, Whitford KJ. Medical care of elderly patients with hip fractures. Mayo Clin Proc. 2001;76:295-298.
27. Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
28. Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures a randomized controlled trial. JAMA. 1994;272:1909-1914.
29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
30. Yamada M, Tanaka B, Nagai K, et al. Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc. 2010;58:1946-1951.
31. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772.
32. Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: systematic review and metaanalysis. J Am Geriatr Soc. 2010;58:1299-1310.
33. The American Geriatrics Society. Prevention of falls in older persons [clinical practice guideline]. 2010. Available at: http:// www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/ 2010/. Accessed August 16, 2013.
34. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235.
35. Michael YL, Whitlock EP, Lin JS, et al. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815-825.
36. USPSTF. Prevention of falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. May 2012. Available at: www.uspreventiveservices taskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed August 19, 2013.
37. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
38. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; for the HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
39. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983-991.
40. Ringe, JD, Doherty, JG. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010;30:863-869.
41. Veehof L, Stewart R, Haaijer-Ruskamp F, et al. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261-267.
42. Choe HM, Farris KB, Stevenson JG, et al. Patient-centered medical home: developing, expanding, and sustaining a role for pharmacists. Am J Health Syst Pharm. 2012;69:1063-1071.
When war follows combat veterans home
› Ask, “Have you or a loved one ever served in the military?” as a way to uncover service-related concerns. C
› Conduct a thorough neurological evaluation with suspected mild traumatic brain injury, including vestibular, vision, postural, and neuro-cognitive assessments. C
› Use the Post-Traumatic Checklist–Military to assess individuals with possible post-traumatic stress disorder. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 37-year-old white woman presents for an employment physical. Your nurse reports that she also has a complaint of headaches, that she scored an 8 on the Alcohol Use Disorders identification Test-consumption (AUDiT-c), and that the result on her patient health Questionnaire (phQ-2) suggests a depressive disorder. You ask the patient whether she has served in the military and discover that, in the last 4 years, she served 2 year-long tours in Afghanistan with her Army reserve unit, returning home 6 months ago. Since her return, she has lost her job due to chronic tardiness (sleeping through her alarm, she says) and admits she has “started drinking again.” Her visit with you this day is only to undergo the physical exam required by her new employer. What are your next steps with this patient? What resources can you use to help her?
As long as human beings have engaged in combat, there have often been extraordinarily damaging psychiatric1 injuries among those who survive. Combat survivability today is 84% to 90%, the highest in the history of armed conflict,2,3 thanks to improvements in personal protective gear, vehicle armor, rapid casualty evacuation, and surgical resuscitation and stabilization that is “far forward” on the battlefield. These survivors are subsequently at high risk for a host of other medical conditions, which commonly include traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), depression, suicide, and substance abuse.4-8
Family physicians—both civilian and uniformed—may be the first to encounter these individuals. Of the more than 2.4 million US service members who have been deployed to Afghanistan or Iraq in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), nearly 60% are no longer on active duty.
Among this group, only half receive care from the US Department of Veterans Affairs (VA).9 Despite a concerted effort on the part of the Department of Defense (DoD) and the VA to develop and distribute effective, evidenced-based treatment protocols for veterans with combat-related conditions, major gaps remain in the care provided to combat veterans.10
This article seeks to help fill that gap by providing the information you need to recognize and treat common combat-related illness, as well as resources to help improve the quality of life for veterans and their families (TABLE 1).
Initial roadblocks to care
One of the biggest challenges in treating veterans with behavioral health issues is the fact that only 23% to 40% of those with mental illness seek care.11 Among the reasons veterans have offered for avoiding behavioral health care are a fear of the stigma associated with mental illness, concern that treatment will negatively affect their career, lack of comfort with mental health professionals, and the perception that mental health treatment is a “last resort.”12 Unfortunately, efforts by the DoD leadership to overcome these inherent biases have been largely unsuccessful13 and much work is still required to see that service members get the care they need.
Due to low rates of self-reporting, effective screening is essential. With this in mind, the DoD has implemented the deployment health assessment program (DHAP), which requires service members to be screened for common conditions within 60 days of deployment, within 30 days of returning, and again at 90 to 180 days after their return.
While the long-term effects of this program are yet to be determined, results to date are promising. Since the DHAP was implemented, there has been a significant decrease in occupationally impairing mental health problems and suicidal ideation requiring medical evacuation from a combat theater.14
FPs should begin with a simple question. Many of the 20+ million veterans living in the United States will not be wearing a uniform when they enter your office. Simply asking all of your patients, “Have you or a loved one ever served in the military?” may help you discover service-related questions or concerns.15,16 Underscoring the importance of such screening is the recent decision by the American Academy of Family Physicians to partner with First Lady Michelle Obama and Dr. Jill Biden in a new campaign called “Joining Forces,” which aims to support veterans and their families.16
Mild traumatic brain injury: Common—though overlooked
A TBI is any temporary or permanent neurologic dysfunction after a blow to the head.10,17 TBI is classified based on severity and mechanism (direct blow to the head or exposure to blast waves). Mild TBI (mTBI) is commonly referred to as a concussion and usually is not associated with loss of consciousness or altered mental status. Brain imaging results are also normal with mTBI. Severe TBI, on the other hand, is associated with prolonged loss of consciousness, altered mental status, and abnormal brain imaging results (TABLE 2).17
A unique obstacle to accurate evaluation in the field. It is important to emphasize that mTBI is a clinical diagnosis, and its detection requires honest patient communication. This can be problematic with motivated soldiers who are anxious to continue the mission and fear that any admission of symptoms might delay a return to their unit. As with a concussed athlete eager to return to the field of play, the clinical diagnosis of mTBI requires a high index of clinical suspicion and constant vigilance by the health care provider. Despite being the most common combat- related injury, mTBI is often overlooked due to the absence of obvious physical injuries.4 Recent data suggest that 28% to 60% of ser- vice members evacuated from combat have a TBI. Most of these injuries (77%) are mTBI.18-20 Improved personal protective equipment (including Kevlar helmets and body armor) and the high number of blast-related injuries are likely responsible for the high incidence of mTBI among OEF/OIF veterans.8,21,22 The prevalence of mTBI among service members not evacuated is estimated to be 20% to 30%.20
Symptoms can persist. Most patients with mTBI completely recover within 30 days of the injury. Unfortunately, 10% to 15% of mTBI patients develop chronic problems lasting months to years.4 Residual symptoms most commonly include headache, irritability, depression, sleep disturbance, impaired reasoning, memory problems, and difficulty concentrating. These symptoms are not unique to mTBI and overlap with comorbid combat diagnoses like PTSD, depression, and sleep deprivation.10 The following tools can help physicians determine whether mTBI is present.
Checking for possible mtBi. In the field, patients with possible mTBI can be screened rapidly using the Military Acute Concussion Evaluation (MACE, found at www.dvbic.org), a modification of the validated and widely used Sideline Assessment of Concussion (SAC) tool. More challenging is evaluating potential mTBI patients who present weeks or months after a traumatic event, for which there are no simple confirmatory tests. In this event, conduct a thorough neurological evaluation that includes vestibular, vision, postural, and neurocognitive assessments. For patients with persistent symptoms or possible anatomic brain abnormalities, magnetic resonance imaging (MRI) is the imaging modality of choice. Patients with complications or a questionable diagnosis are best managed in consultation with a neurologist.
Initial treatment of mtBi is symptom-based. When practical, try nonpharmacologic interventions first (TABLE 3).10 In particular, have the patient avoid further high-risk exposures that could lead to second impact syndrome (an issue increasingly recognized in contact sports). Also critical are physical and cognitive rest and the restoration of sleep until the patient is completely asymptomatic.
If the patient exhibits irritability and depression, selective serotonin reuptake inhibitors (SSRIs) are first-line treatment. Avoid narcotics and sedative-hypnotic sleep medications if treating comorbidities such as pain and sleep deprivation. The VA/DoD guideline on managing concussion and mTBI provides additional detailed, evidence-based treatment recommendations.17
Reliving the horror again and again: PTSD
PTSD is a persistent and, at times, debilitating clinical syndrome that develops after exposure to a psychologically traumatic event. It’s the second most common illness among OEF/OIF combat veterans, with an estimated prevalence of 3% to 20%, a finding consistent with prior wars.6,23-25 In the case of combat veterans, the inciting event usually involves an actual or perceived risk of death or serious injury. The individual’s response to the event involves intense fear, helplessness, or horror. The traumatic event is persistently re-experienced through intrusive and disturbing recollections or dreams that cause intense psychological distress. This, in turn, leads to a state of persistent sympathetic arousal. As symptoms are often triggered by specific cues, individuals with PTSD actively seek to avoid thoughts, situations, or stimuli associated with the event.23,26
Symptoms commonly associated with PTSD include difficulty falling or staying asleep, recurrent nightmares, hypervigilance, and an exaggerated startle response. Individuals with PTSD also have a poorer sense of well-being, a higher rate of work absenteeism, and significantly more somatic complaints than age-matched peers.27 For symptoms to be attributable to PTSD, their onset must follow a recent inciting event and must also cause clinically significant distress or impairment in social, occupational, or other areas of daily living. Common comorbid illnesses include mTBI, depression, and substance abuse. As with mTBI, the presence of multiple comorbidities in patients with PTSD can complicate evaluation, diagnosis, and treatment.
Diagnosis. PTSD is subdivided into acute (symptoms lasting more than one month but less than 3 months after the traumatic event) and chronic (symptoms lasting longer than 3 months after the traumatic event).28 The distinction of acute or chronic does not affect treatment, but it is useful information for the patient to have regarding prognosis and eventual outcome. Like mTBI, PTSD is a clinical diagnosis made only after a thorough, structured diagnostic interview. The use of a validated, self-administered checklist, such as the Post-Traumatic Checklist-Military (PCL-M), allows for an efficient review of a patient’s symptoms and a reliable way to track treatment progress (http://www.ptsd.va.gov/professional/ pages/assessments/ptsd-checklist.asp).
Treatment Options. Effective evidence-based treatments for PTSD are cognitive behavioral therapy, eye movement desensitization and reprocessing (EMDR), and pharmacotherapy. SSRIs and serotonin- norepinephrine reuptake inhibitors (SNRIs) have the strongest evidence for pharmacologic benefit in the treatment of PTSD.28,29 Other helpful medications are prazosin for nightmares and trazodone for sleep. Family physicians can use these medications as part of a patient-centered collaboration with the rest of the integrated care team, to offer the best chance for treatment success.10,28,30
Depression: Vets are reluctant to self-report
Combat experience is a significant risk factor for major depression. Estimates of the lifetime prevalence of depression in the general US population vary from 9% to 25% in women and 5% to 12% in men. By contrast, the prevalence of depression in OIF/OEF veterans ranges from 2% to 37%.24,31,32
Screening can yield false negatives. Many combat veterans are reluctant to self-report behavioral conditions, including depression. Screening, therefore, is important to identify potential depression and allow for intervention. Validated screening tools for depression include the PHQ-2 and PHQ-9, which are easy to use in the office setting. (See http://www.cqaimh.org/pdf/ tool_phq2.pdf [PHQ-2] and http://www. integration.samhsa.gov/images/res/ PHQ%20-%20Questions.pdf [PHQ-9]). Importantly, some veterans will have a negative depression screen on return from deployment, and then test positive 6 to 12 months later.24 Explanations for the early false-negative results include the excitement of being home and patients intentionally answering questions inaccurately to avoid excessive screening at their home base.11
Treatment is most effective with a combination approach. As with most cases of depression, combining psychotherapy and psychopharmacology appears to be most effective for treating depression related to combat experience.33,34 While SSRIs and SNRIs are typical first-line pharmacologic agents, combat veterans often have comorbid mTBI, PTSD, or substance abuse issues that may influence the initial choice of therapy35 (TABLE 3).10
Suicide is on the rise in the military
Historically, the incidence of suicide has been 25% lower in military personnel than in civilian peers.36 However, between 2005 and 2009, the incidence of suicide in the Marine Corps and Army almost doubled.37 While the exact reasons remain unknown, it is likely due to prolonged and repeated deployments to a combat environment.12 While the incidence of suicide has been particularly high in the Army (22 per 100,000 active-duty and reserve personnel per year), all services have been affected. In fact, since 2009, the number of suicides among active duty service members exceeds those killed in action.37
Consider all veterans to be at risk for suicide, and screen accordingly. An effective screening tool is the Columbia-Suicide Severity Rating Scale (C-SSRS), which is able to predict those most at risk for an impending suicide attempt.34 Service members identified as high risk for suicide require unhindered access to care. The VA has worked to improve access to care and provide evidence-based point-of-care treatment strategies.38 Available resources can be found in TABLE 1.
Unfortunately, even with effective screening and treatment, not all suicides can be prevented. Studies have demonstrated that approximately 65% of service members who commit suicide had no known history of communicating their suicidal intent. Sadly, 25% of service members who committed suicide had seen a mental health provider within the previous 30 days.39
Alcohol abuse is common; opioids present a unique risk
Excessive use of alcohol and recreational and prescription drugs is common among OEF/ OIF veterans, especially those with comorbid mental health disorders. Retrospective cross-sectional studies show that 11% to 20% of OEF/OIF veterans met DSM-IV-TR diagnostic criteria for substance use disorders.40-42 At highest risk are single enlisted men under the age of 24 in the Army or Marine Corps who serve in a combat-specific capacity. Interestingly, the prevalence of substance use disorders among OEF/OIF veterans closely mirrors that reported in epidemiologic studies of Vietnam veterans (11%-14%).41 This similarity, combined with the 39% lifetime prevalence of substance use disorders among Vietnam veterans, may foreshadow a similar lifetime prevalence of substance use disorders among OEF/OIF veterans.41
Most-abused substances. Alcohol is the most commonly abused substance among OEF/OIF veterans (10%-20%).40,41,43-45 Other abused substances include opioids (prescribed or illicitly obtained), synthetic marijuana (“Spice” and “K2”), and “bath salts” (synthetic stimulants) (W.M. Sauve, MD, personal communication, August 27, 2012).
OEF/OIF veterans seem to be at particular risk for developing problems related to opioid use. A 2012 retrospective cohort study showed that veterans with non–cancer- related pain diagnoses treated with opioid analgesics had an increased risk for adverse clinical outcomes compared with those not treated with opioid analgesics (9.5% vs 4.1%; relative risk [RR]=2.33; 95% confidence interval [CI], 2.20-2.46). These outcomes included traumatic accidents, overdoses, self-inflicted injuries, and injuries related to violence. This study also demonstrated that, compared with veterans without mental illness, veterans with mental illness (particularly PTSD) and non–cancer-related pain were significantly more likely to receive opioids to treat their pain and had a higher risk of adverse clinical outcomes, including overdose.46,47
Recreational use of synthetic marijuana and “bath salts” has increased in recent years. These substances are commonly labeled “not for human consumption,” which allows them to remain outside US Food and Drug Administration (FDA) regulation and be sold legally in the United States. Efforts to prohibit the sale or possession of these drugs, including the Federal Synthetic Bath Salt Ban in 2012, have fallen short, often due to creative product ”re-engineering.”33 Synthetic marijuana and stimulants are inexpensive, readily available, and perceived by users to be safe. Health care providers are often unaware that their patients are using these products. Adverse health outcomes associated with the use of these synthetic drugs include memory loss, depression, and psychosis.
These alcohol and drug screens can help
One efficient screening tool to identify veterans at risk for alcohol abuse is the AUDIT-C, developed by the World Health Organization. This brief 3-question test identifies past-year hazardous drinking and alcohol abuse or dependence with >79% sensitivity and >56% specificity in male veterans, and >66% sensitivity and >87% specificity in female veterans. These numbers are similar to those provided by the full 10-question AUDIT.48,49 The Drug Abuse Screen Test-10 (DAST-10) provides a similar screening instrument for other substances. Condensed from the original DAST-28 instrument, the DAST-10 identifies high-risk substance abuse with 74% to 94% sensitivity and 68% to 88% specificity.3
Screen for comorbidities. When you see veterans with a diagnosis of substance abuse, also evaluate for comorbid disease. Most veterans with substance use disorders (82%-93%) have at least one other mental health diagnosis (a 45% greater risk than that of civilians with substance abuse disorders),50 most commonly PTSD, depression, anxiety, and adjustment disorders.41,44,45 A number of hypotheses exist to explain the association between substance use disorders and other mental health diagnoses (“dual diagnoses”). The prevailing theory, in both veteran and civilian populations, is that substance abuse is an attempt to self-treat mental illness. Other evidence suggests that substance abuse promotes the development of mental illness, either by leading to a higher risk for traumatic experiences (increasing the chance of developing PTSD) or through a direct biochemical mechanism. Finally, con- current substance use disorder and mental illness may be due to an undefined genetic or biological vulnerability.38,44 This complicated relationship between substance abuse and behavioral health reinforces the need for screening, early diagnosis, and a comprehensive, multidisciplinary approach to treatment.
Treatment options. Office-based treatment options for narcotic and alcohol abuse and dependency are available to family physicians. Methadone has been used since the 1950s to treat opioid addiction and remains one of the mainstays of outpatient treatment.47,51 Originally, methadone was restricted to detoxification and maintenance treatment in narcotic addiction treatment programs approved by the FDA. In 1976, this restriction was lifted, and all physicians registered with the Drug Enforcement Agency (DEA) were permitted to prescribe methadone for analgesia.
In 2002, the FDA approved buprenorphine monotherapy and the combination product buprenorphine/naloxone for the treatment of opioid addiction. The prescribing of buprenorphine products requires physicians to undergo extra training, declare to the DEA their intent to prescribe buprenorphine, and obtain a special DEA identification number.52,53 Physicians interested in finding out more about buprenorphine treatment and prescribing requirements can go to the Substance Abuse and Mental Health Services Administration (SAMHSA) Web page at http://samhsa.gov.
Naltrexone is an opioid receptor agonist that is used primarily to treat alcohol dependency, and is thought to work by reducing the craving for alcohol. Multiple studies have proven the efficacy of naltrexone in an outpatient setting when used alone or in combination with psychotherapy.54,55 If you are uncomfortable or unfamiliar with the use or prescribing of these medications, referral to a substance abuse clinic specializing in dual-diagnosis treatment (TABLE 1) may offer optimal outcomes for patients with substance abuse disorders and other mental illness.
Cognitive behavioral therapy—including coping skills training, relapse prevention, contingency management, and behavioral couples’ therapy—and 12-step treatment programs are evidence-based options for the treatment of substance abuse disorders. Behavioral counseling interventions in the primary care setting (typically lasting 5-15 minutes) result in decreases in alcohol consumption, heavy drinking episodes, drinking above recommended amounts, and the number of days spent in the hospital, but have not been demonstrated to affect mortality, alcohol-related liver problems, outpatient visits, legal problems, or quality of life.56 Resources can be found at www.niaaa.nih.gov. For patients with dual diagnoses, it is not yet known whether sequential therapy (in which substance abuse is treated first, followed by treatment of the comorbid mental illness) or concurrent therapy results in better outcomes.57
CASE Your patient’s history of recent combat service, acknowledgement of employment and behavioral difficulties, and initial screening results lead you to diagnose alcoholism and depression. Additionally, she denies any suicidal ideation, but admits to experimenting with synthetic marijuana. After some discussion, she agrees to see your clinic’s social worker, and you start her on an SSri with scheduled follow-up.
CORRESPONDENCE
Shawn Kane, MD, USASoc, Attn: Surgeon (AomD), 2929 Desert Storm Drive, Ft. Bragg, NC 28310, or PO Box 3639 Pinehurst, NC 28374; [email protected]
1. Wessely S. Risk, psychiatry and the military. Br J Psychiatry. 2005;186:459-466.
2. Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
3. Kotwal RS, Montgomery HR, Kotwal BM, et al. Eliminating pre- ventable death on the battlefield. Arch Surg. 2011;146:1350-1358.
4. Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration’s (VHA’s) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J Head Trauma Rehabil. 2009;24:4-13.
5. Galarneau MR, Woodruff SI, Dye JL, et al. Traumatic brain in- jury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950-957.
6. Hermann BA, Shiner B, Friedman MJ. Epidemiology and preven- tion of combat-related post-traumatic stress in OEF/OIF/OND service members. Mil Med. 2012;177:1-6.
7. Uomoto JM. Best practices in veteran traumatic brain injury care. J Head Trauma Rehabil. 2012;27:241-243.
8. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
9. Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346.
10. Quinlan JD, Guaron MR, Deschere BR, et al. Care of the returning veteran. Am Fam Physician. 2010;82:43-49.
11. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13-22.
12. Hoge CW, Castro CA. Preventing suicides in US service mem- bers and veterans: concerns after a decade of war. JAMA. 2012;308:671-672.
13. Jaffe G. New name for PTSD could mean less stigma. The Washington Post. May 5, 2012. Available at: http://articles. washingtonpost.com/2012-05-05/world/35454931_1_ptsd-post- traumatic-stress-psychiatrists. Accessed June 19, 2013.
14. Warner CH, Appenzeller GN, Parker JR, et al. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am J Psychiatry. 2011;168:378-385.
15. United States Census Bureau. Sex by age by veteran sta- tus for civilian population 18 years and over. 2010 American community survey 1-year estimates. Available at: https:// d3gqux9sl0z33u.cloudfront.net/AA/AT/gambillingonjustice- com/downloads/206273/ACS_10_1YR_B21001A.pdf. Accessed June 19, 2013.
16. American Academy of Family Physicians. Joining forces. Avail- able at: http://www.aafp.org/online/en/home/membership/ initiatives/joiningforces.html. Accessed June 19, 2013.
17. Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. April 2009. Available at: http://www. healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf. Accessed June 19, 2013.
18. Lew HL, Poole JH, Alvarez S, et al. Soldiers with occult traumatic brain injury. Am J Phys Med Rehabil. 2005;84:393-398.
19. Marshall KR, Holland SL, Meyer KS, et al. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177:67- 75.
20. Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14-23.
21. Mossadegh S, Tai N, Midwinter M, et al. Improvised explosive de- vice related pelvi-perineal trauma: anatomic injuries and surgical management. J Trauma Acute Care Surg. 2012;73:S24-S31.
22. Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
23. Espinoza JM. Posttraumatic stress disorder and the perceived consequences of seeking therapy among US Army special forces operators exposed to combat. J Psychol Issues Organ Culture. 2010;1:6-28.
24. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress dis- order and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
25. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023-1032.
26. Adler AB, Wright KM, Bliese PD, et al. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J Trauma Stress. 2008;21:301-308.
27. Hoge CW, Terhakopian A, Castro CA, et al. Association of post- traumatic stress disorder with somatic symptoms, health care vis- its, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164:150-153.
28. Department of Veterans Affairs and Department of Defense. Clin- ical Practice Guideline for Management of Post-Traumatic Stress. October 2010. Available at: http://www.healthquality.va.gov/ ptsd/cpg_PTSD-FULL-201011612.pdf. Accessed June 19, 2013.
29. Alexander W. Pharmacotherapy for post-traumatic stress disor- der in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37:32-38.
30. Wisco BE, Marx BP, Keane TM. Screening, diagnosis, and treat- ment of post-traumatic stress disorder. Mil Med. 2012;177:7-13.
31. Gadermann AM, Engel CC, Naifeh JA, et al. Prevalence of DSM-IV major depression among U.S. military personnel: meta-analysis and simulation. Mil Med. 2012;177:47-59.
32. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
33. Perrone M. Many drugs remain legal after ‘bath salts’ ban. Boston. com. July 25, 2012. Available at: http://articles.boston.com/2012- 07-25/lifestyle/32850962_1_bath-salts-mdpv-synthetic-drugs. Accessed June 19, 2013.
34. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Se- verity Rating Scale: initial validity and internal consistency find- ings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
35. Greenberg J, Tesfazion AA, Robinson CS. Screening, diagnosis, and treatment of depression. Mil Med. 2012;177:60-66.
36. Eaton KM, Messer SC, Garvey Wilson AL, et al. Strengthening the validity of population-based suicide rate comparisons: an il- lustration using U.S. military and civilian data. Suicide Life Threat Behav. 2006;36:182-191.
37. Miller M, Azrael D, Barber C, et al. A call to link data to answer pressing questions about suicide risk among veterans. Am J Pub Health. 2012;102(suppl 1):S20-S22.
38. Department of Veterans Affairs. Report of the Blue Ribbon Work Group on suicide prevention in the veteran population. June 2008. Available at: http://www.mentalhealth.va.gov/suicide_ prevention/Blue_Ribbon_Report-FINAL_June-30-08.pdf. Accessed July 18, 2013.
39. Kinn JT, Luxton DD, Reger MA, et al. Department of Defense sui- cide event report: calendar year 2010 annual report. September 2011. Available at: http://t2health.org/sites/default/files/dodser/ DoDSER_2010_Annual_Report.pdf. Accessed June 19, 2013.
40. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196:513-521.
41. Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93-101.
42. Mirza RA, Eick-Cost A, Otto JL. The risk of mental health disor- ders among U.S. military personnel infected with human immu- nodeficiency virus, active component, U.S. Armed Forces, 2000- 2011. MSMR. 2012;19:10-13.
43. Bohnert AS, Ilgen MA, Bossarte RM, et al. Veteran status and alco- hol use in men in the United States. Mil Med. 2012;177:198-203.
44. Erbes CR, Kaler ME, Schult T, et al. Mental health diagnosis and occupational functioning in National Guard/Reserve veterans re- turning from Iraq. J Rehabil Res Dev. 2011;48:1159-1170.
45. Stecker T, Fortney J, Owen R, et al. Co-occurring medical, psychi- atric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51:503-507.
46. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
47. Praveen KT, Law F, O’Shea J, et al. Opioid dependence. Am Fam Physician. 2012;86:565-566.
48. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-829.
49. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
50. Farrell M, Howes S, Taylor C, et al. Substance misuse and psychi- atric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict Behav. 1998;23:909-918.
51. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
52. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) series 40. DHHS pub- lication (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Available at: http:// buprenorphine.samhsa.gov/Bup_Guidelines.pdf. Accessed June 19, 2013.
53. U.S.DepartmentofHealthandHumanServices,SubstanceAbuse and Mental Health Services Administration Web site. About buprenorphine therapy. Available at: http://buprenorphine. samhsa.gov/about.html. Accessed June 19, 2013.
54. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
55. O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treat- ment of alcoholism: a clinical review. Alcohol. 1996;13:35-39.
56. Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157:645-654.
57. van Dam D, Vedel E, Ehring T, et al. Psychological treatments for concurrent posttraumatic stress disorder and substance use dis- order: a systematic review. Clin Psychol Rev. 2012;32:202-214.
› Ask, “Have you or a loved one ever served in the military?” as a way to uncover service-related concerns. C
› Conduct a thorough neurological evaluation with suspected mild traumatic brain injury, including vestibular, vision, postural, and neuro-cognitive assessments. C
› Use the Post-Traumatic Checklist–Military to assess individuals with possible post-traumatic stress disorder. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 37-year-old white woman presents for an employment physical. Your nurse reports that she also has a complaint of headaches, that she scored an 8 on the Alcohol Use Disorders identification Test-consumption (AUDiT-c), and that the result on her patient health Questionnaire (phQ-2) suggests a depressive disorder. You ask the patient whether she has served in the military and discover that, in the last 4 years, she served 2 year-long tours in Afghanistan with her Army reserve unit, returning home 6 months ago. Since her return, she has lost her job due to chronic tardiness (sleeping through her alarm, she says) and admits she has “started drinking again.” Her visit with you this day is only to undergo the physical exam required by her new employer. What are your next steps with this patient? What resources can you use to help her?
As long as human beings have engaged in combat, there have often been extraordinarily damaging psychiatric1 injuries among those who survive. Combat survivability today is 84% to 90%, the highest in the history of armed conflict,2,3 thanks to improvements in personal protective gear, vehicle armor, rapid casualty evacuation, and surgical resuscitation and stabilization that is “far forward” on the battlefield. These survivors are subsequently at high risk for a host of other medical conditions, which commonly include traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), depression, suicide, and substance abuse.4-8
Family physicians—both civilian and uniformed—may be the first to encounter these individuals. Of the more than 2.4 million US service members who have been deployed to Afghanistan or Iraq in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), nearly 60% are no longer on active duty.
Among this group, only half receive care from the US Department of Veterans Affairs (VA).9 Despite a concerted effort on the part of the Department of Defense (DoD) and the VA to develop and distribute effective, evidenced-based treatment protocols for veterans with combat-related conditions, major gaps remain in the care provided to combat veterans.10
This article seeks to help fill that gap by providing the information you need to recognize and treat common combat-related illness, as well as resources to help improve the quality of life for veterans and their families (TABLE 1).
Initial roadblocks to care
One of the biggest challenges in treating veterans with behavioral health issues is the fact that only 23% to 40% of those with mental illness seek care.11 Among the reasons veterans have offered for avoiding behavioral health care are a fear of the stigma associated with mental illness, concern that treatment will negatively affect their career, lack of comfort with mental health professionals, and the perception that mental health treatment is a “last resort.”12 Unfortunately, efforts by the DoD leadership to overcome these inherent biases have been largely unsuccessful13 and much work is still required to see that service members get the care they need.
Due to low rates of self-reporting, effective screening is essential. With this in mind, the DoD has implemented the deployment health assessment program (DHAP), which requires service members to be screened for common conditions within 60 days of deployment, within 30 days of returning, and again at 90 to 180 days after their return.
While the long-term effects of this program are yet to be determined, results to date are promising. Since the DHAP was implemented, there has been a significant decrease in occupationally impairing mental health problems and suicidal ideation requiring medical evacuation from a combat theater.14
FPs should begin with a simple question. Many of the 20+ million veterans living in the United States will not be wearing a uniform when they enter your office. Simply asking all of your patients, “Have you or a loved one ever served in the military?” may help you discover service-related questions or concerns.15,16 Underscoring the importance of such screening is the recent decision by the American Academy of Family Physicians to partner with First Lady Michelle Obama and Dr. Jill Biden in a new campaign called “Joining Forces,” which aims to support veterans and their families.16
Mild traumatic brain injury: Common—though overlooked
A TBI is any temporary or permanent neurologic dysfunction after a blow to the head.10,17 TBI is classified based on severity and mechanism (direct blow to the head or exposure to blast waves). Mild TBI (mTBI) is commonly referred to as a concussion and usually is not associated with loss of consciousness or altered mental status. Brain imaging results are also normal with mTBI. Severe TBI, on the other hand, is associated with prolonged loss of consciousness, altered mental status, and abnormal brain imaging results (TABLE 2).17
A unique obstacle to accurate evaluation in the field. It is important to emphasize that mTBI is a clinical diagnosis, and its detection requires honest patient communication. This can be problematic with motivated soldiers who are anxious to continue the mission and fear that any admission of symptoms might delay a return to their unit. As with a concussed athlete eager to return to the field of play, the clinical diagnosis of mTBI requires a high index of clinical suspicion and constant vigilance by the health care provider. Despite being the most common combat- related injury, mTBI is often overlooked due to the absence of obvious physical injuries.4 Recent data suggest that 28% to 60% of ser- vice members evacuated from combat have a TBI. Most of these injuries (77%) are mTBI.18-20 Improved personal protective equipment (including Kevlar helmets and body armor) and the high number of blast-related injuries are likely responsible for the high incidence of mTBI among OEF/OIF veterans.8,21,22 The prevalence of mTBI among service members not evacuated is estimated to be 20% to 30%.20
Symptoms can persist. Most patients with mTBI completely recover within 30 days of the injury. Unfortunately, 10% to 15% of mTBI patients develop chronic problems lasting months to years.4 Residual symptoms most commonly include headache, irritability, depression, sleep disturbance, impaired reasoning, memory problems, and difficulty concentrating. These symptoms are not unique to mTBI and overlap with comorbid combat diagnoses like PTSD, depression, and sleep deprivation.10 The following tools can help physicians determine whether mTBI is present.
Checking for possible mtBi. In the field, patients with possible mTBI can be screened rapidly using the Military Acute Concussion Evaluation (MACE, found at www.dvbic.org), a modification of the validated and widely used Sideline Assessment of Concussion (SAC) tool. More challenging is evaluating potential mTBI patients who present weeks or months after a traumatic event, for which there are no simple confirmatory tests. In this event, conduct a thorough neurological evaluation that includes vestibular, vision, postural, and neurocognitive assessments. For patients with persistent symptoms or possible anatomic brain abnormalities, magnetic resonance imaging (MRI) is the imaging modality of choice. Patients with complications or a questionable diagnosis are best managed in consultation with a neurologist.
Initial treatment of mtBi is symptom-based. When practical, try nonpharmacologic interventions first (TABLE 3).10 In particular, have the patient avoid further high-risk exposures that could lead to second impact syndrome (an issue increasingly recognized in contact sports). Also critical are physical and cognitive rest and the restoration of sleep until the patient is completely asymptomatic.
If the patient exhibits irritability and depression, selective serotonin reuptake inhibitors (SSRIs) are first-line treatment. Avoid narcotics and sedative-hypnotic sleep medications if treating comorbidities such as pain and sleep deprivation. The VA/DoD guideline on managing concussion and mTBI provides additional detailed, evidence-based treatment recommendations.17
Reliving the horror again and again: PTSD
PTSD is a persistent and, at times, debilitating clinical syndrome that develops after exposure to a psychologically traumatic event. It’s the second most common illness among OEF/OIF combat veterans, with an estimated prevalence of 3% to 20%, a finding consistent with prior wars.6,23-25 In the case of combat veterans, the inciting event usually involves an actual or perceived risk of death or serious injury. The individual’s response to the event involves intense fear, helplessness, or horror. The traumatic event is persistently re-experienced through intrusive and disturbing recollections or dreams that cause intense psychological distress. This, in turn, leads to a state of persistent sympathetic arousal. As symptoms are often triggered by specific cues, individuals with PTSD actively seek to avoid thoughts, situations, or stimuli associated with the event.23,26
Symptoms commonly associated with PTSD include difficulty falling or staying asleep, recurrent nightmares, hypervigilance, and an exaggerated startle response. Individuals with PTSD also have a poorer sense of well-being, a higher rate of work absenteeism, and significantly more somatic complaints than age-matched peers.27 For symptoms to be attributable to PTSD, their onset must follow a recent inciting event and must also cause clinically significant distress or impairment in social, occupational, or other areas of daily living. Common comorbid illnesses include mTBI, depression, and substance abuse. As with mTBI, the presence of multiple comorbidities in patients with PTSD can complicate evaluation, diagnosis, and treatment.
Diagnosis. PTSD is subdivided into acute (symptoms lasting more than one month but less than 3 months after the traumatic event) and chronic (symptoms lasting longer than 3 months after the traumatic event).28 The distinction of acute or chronic does not affect treatment, but it is useful information for the patient to have regarding prognosis and eventual outcome. Like mTBI, PTSD is a clinical diagnosis made only after a thorough, structured diagnostic interview. The use of a validated, self-administered checklist, such as the Post-Traumatic Checklist-Military (PCL-M), allows for an efficient review of a patient’s symptoms and a reliable way to track treatment progress (http://www.ptsd.va.gov/professional/ pages/assessments/ptsd-checklist.asp).
Treatment Options. Effective evidence-based treatments for PTSD are cognitive behavioral therapy, eye movement desensitization and reprocessing (EMDR), and pharmacotherapy. SSRIs and serotonin- norepinephrine reuptake inhibitors (SNRIs) have the strongest evidence for pharmacologic benefit in the treatment of PTSD.28,29 Other helpful medications are prazosin for nightmares and trazodone for sleep. Family physicians can use these medications as part of a patient-centered collaboration with the rest of the integrated care team, to offer the best chance for treatment success.10,28,30
Depression: Vets are reluctant to self-report
Combat experience is a significant risk factor for major depression. Estimates of the lifetime prevalence of depression in the general US population vary from 9% to 25% in women and 5% to 12% in men. By contrast, the prevalence of depression in OIF/OEF veterans ranges from 2% to 37%.24,31,32
Screening can yield false negatives. Many combat veterans are reluctant to self-report behavioral conditions, including depression. Screening, therefore, is important to identify potential depression and allow for intervention. Validated screening tools for depression include the PHQ-2 and PHQ-9, which are easy to use in the office setting. (See http://www.cqaimh.org/pdf/ tool_phq2.pdf [PHQ-2] and http://www. integration.samhsa.gov/images/res/ PHQ%20-%20Questions.pdf [PHQ-9]). Importantly, some veterans will have a negative depression screen on return from deployment, and then test positive 6 to 12 months later.24 Explanations for the early false-negative results include the excitement of being home and patients intentionally answering questions inaccurately to avoid excessive screening at their home base.11
Treatment is most effective with a combination approach. As with most cases of depression, combining psychotherapy and psychopharmacology appears to be most effective for treating depression related to combat experience.33,34 While SSRIs and SNRIs are typical first-line pharmacologic agents, combat veterans often have comorbid mTBI, PTSD, or substance abuse issues that may influence the initial choice of therapy35 (TABLE 3).10
Suicide is on the rise in the military
Historically, the incidence of suicide has been 25% lower in military personnel than in civilian peers.36 However, between 2005 and 2009, the incidence of suicide in the Marine Corps and Army almost doubled.37 While the exact reasons remain unknown, it is likely due to prolonged and repeated deployments to a combat environment.12 While the incidence of suicide has been particularly high in the Army (22 per 100,000 active-duty and reserve personnel per year), all services have been affected. In fact, since 2009, the number of suicides among active duty service members exceeds those killed in action.37
Consider all veterans to be at risk for suicide, and screen accordingly. An effective screening tool is the Columbia-Suicide Severity Rating Scale (C-SSRS), which is able to predict those most at risk for an impending suicide attempt.34 Service members identified as high risk for suicide require unhindered access to care. The VA has worked to improve access to care and provide evidence-based point-of-care treatment strategies.38 Available resources can be found in TABLE 1.
Unfortunately, even with effective screening and treatment, not all suicides can be prevented. Studies have demonstrated that approximately 65% of service members who commit suicide had no known history of communicating their suicidal intent. Sadly, 25% of service members who committed suicide had seen a mental health provider within the previous 30 days.39
Alcohol abuse is common; opioids present a unique risk
Excessive use of alcohol and recreational and prescription drugs is common among OEF/ OIF veterans, especially those with comorbid mental health disorders. Retrospective cross-sectional studies show that 11% to 20% of OEF/OIF veterans met DSM-IV-TR diagnostic criteria for substance use disorders.40-42 At highest risk are single enlisted men under the age of 24 in the Army or Marine Corps who serve in a combat-specific capacity. Interestingly, the prevalence of substance use disorders among OEF/OIF veterans closely mirrors that reported in epidemiologic studies of Vietnam veterans (11%-14%).41 This similarity, combined with the 39% lifetime prevalence of substance use disorders among Vietnam veterans, may foreshadow a similar lifetime prevalence of substance use disorders among OEF/OIF veterans.41
Most-abused substances. Alcohol is the most commonly abused substance among OEF/OIF veterans (10%-20%).40,41,43-45 Other abused substances include opioids (prescribed or illicitly obtained), synthetic marijuana (“Spice” and “K2”), and “bath salts” (synthetic stimulants) (W.M. Sauve, MD, personal communication, August 27, 2012).
OEF/OIF veterans seem to be at particular risk for developing problems related to opioid use. A 2012 retrospective cohort study showed that veterans with non–cancer- related pain diagnoses treated with opioid analgesics had an increased risk for adverse clinical outcomes compared with those not treated with opioid analgesics (9.5% vs 4.1%; relative risk [RR]=2.33; 95% confidence interval [CI], 2.20-2.46). These outcomes included traumatic accidents, overdoses, self-inflicted injuries, and injuries related to violence. This study also demonstrated that, compared with veterans without mental illness, veterans with mental illness (particularly PTSD) and non–cancer-related pain were significantly more likely to receive opioids to treat their pain and had a higher risk of adverse clinical outcomes, including overdose.46,47
Recreational use of synthetic marijuana and “bath salts” has increased in recent years. These substances are commonly labeled “not for human consumption,” which allows them to remain outside US Food and Drug Administration (FDA) regulation and be sold legally in the United States. Efforts to prohibit the sale or possession of these drugs, including the Federal Synthetic Bath Salt Ban in 2012, have fallen short, often due to creative product ”re-engineering.”33 Synthetic marijuana and stimulants are inexpensive, readily available, and perceived by users to be safe. Health care providers are often unaware that their patients are using these products. Adverse health outcomes associated with the use of these synthetic drugs include memory loss, depression, and psychosis.
These alcohol and drug screens can help
One efficient screening tool to identify veterans at risk for alcohol abuse is the AUDIT-C, developed by the World Health Organization. This brief 3-question test identifies past-year hazardous drinking and alcohol abuse or dependence with >79% sensitivity and >56% specificity in male veterans, and >66% sensitivity and >87% specificity in female veterans. These numbers are similar to those provided by the full 10-question AUDIT.48,49 The Drug Abuse Screen Test-10 (DAST-10) provides a similar screening instrument for other substances. Condensed from the original DAST-28 instrument, the DAST-10 identifies high-risk substance abuse with 74% to 94% sensitivity and 68% to 88% specificity.3
Screen for comorbidities. When you see veterans with a diagnosis of substance abuse, also evaluate for comorbid disease. Most veterans with substance use disorders (82%-93%) have at least one other mental health diagnosis (a 45% greater risk than that of civilians with substance abuse disorders),50 most commonly PTSD, depression, anxiety, and adjustment disorders.41,44,45 A number of hypotheses exist to explain the association between substance use disorders and other mental health diagnoses (“dual diagnoses”). The prevailing theory, in both veteran and civilian populations, is that substance abuse is an attempt to self-treat mental illness. Other evidence suggests that substance abuse promotes the development of mental illness, either by leading to a higher risk for traumatic experiences (increasing the chance of developing PTSD) or through a direct biochemical mechanism. Finally, con- current substance use disorder and mental illness may be due to an undefined genetic or biological vulnerability.38,44 This complicated relationship between substance abuse and behavioral health reinforces the need for screening, early diagnosis, and a comprehensive, multidisciplinary approach to treatment.
Treatment options. Office-based treatment options for narcotic and alcohol abuse and dependency are available to family physicians. Methadone has been used since the 1950s to treat opioid addiction and remains one of the mainstays of outpatient treatment.47,51 Originally, methadone was restricted to detoxification and maintenance treatment in narcotic addiction treatment programs approved by the FDA. In 1976, this restriction was lifted, and all physicians registered with the Drug Enforcement Agency (DEA) were permitted to prescribe methadone for analgesia.
In 2002, the FDA approved buprenorphine monotherapy and the combination product buprenorphine/naloxone for the treatment of opioid addiction. The prescribing of buprenorphine products requires physicians to undergo extra training, declare to the DEA their intent to prescribe buprenorphine, and obtain a special DEA identification number.52,53 Physicians interested in finding out more about buprenorphine treatment and prescribing requirements can go to the Substance Abuse and Mental Health Services Administration (SAMHSA) Web page at http://samhsa.gov.
Naltrexone is an opioid receptor agonist that is used primarily to treat alcohol dependency, and is thought to work by reducing the craving for alcohol. Multiple studies have proven the efficacy of naltrexone in an outpatient setting when used alone or in combination with psychotherapy.54,55 If you are uncomfortable or unfamiliar with the use or prescribing of these medications, referral to a substance abuse clinic specializing in dual-diagnosis treatment (TABLE 1) may offer optimal outcomes for patients with substance abuse disorders and other mental illness.
Cognitive behavioral therapy—including coping skills training, relapse prevention, contingency management, and behavioral couples’ therapy—and 12-step treatment programs are evidence-based options for the treatment of substance abuse disorders. Behavioral counseling interventions in the primary care setting (typically lasting 5-15 minutes) result in decreases in alcohol consumption, heavy drinking episodes, drinking above recommended amounts, and the number of days spent in the hospital, but have not been demonstrated to affect mortality, alcohol-related liver problems, outpatient visits, legal problems, or quality of life.56 Resources can be found at www.niaaa.nih.gov. For patients with dual diagnoses, it is not yet known whether sequential therapy (in which substance abuse is treated first, followed by treatment of the comorbid mental illness) or concurrent therapy results in better outcomes.57
CASE Your patient’s history of recent combat service, acknowledgement of employment and behavioral difficulties, and initial screening results lead you to diagnose alcoholism and depression. Additionally, she denies any suicidal ideation, but admits to experimenting with synthetic marijuana. After some discussion, she agrees to see your clinic’s social worker, and you start her on an SSri with scheduled follow-up.
CORRESPONDENCE
Shawn Kane, MD, USASoc, Attn: Surgeon (AomD), 2929 Desert Storm Drive, Ft. Bragg, NC 28310, or PO Box 3639 Pinehurst, NC 28374; [email protected]
› Ask, “Have you or a loved one ever served in the military?” as a way to uncover service-related concerns. C
› Conduct a thorough neurological evaluation with suspected mild traumatic brain injury, including vestibular, vision, postural, and neuro-cognitive assessments. C
› Use the Post-Traumatic Checklist–Military to assess individuals with possible post-traumatic stress disorder. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 37-year-old white woman presents for an employment physical. Your nurse reports that she also has a complaint of headaches, that she scored an 8 on the Alcohol Use Disorders identification Test-consumption (AUDiT-c), and that the result on her patient health Questionnaire (phQ-2) suggests a depressive disorder. You ask the patient whether she has served in the military and discover that, in the last 4 years, she served 2 year-long tours in Afghanistan with her Army reserve unit, returning home 6 months ago. Since her return, she has lost her job due to chronic tardiness (sleeping through her alarm, she says) and admits she has “started drinking again.” Her visit with you this day is only to undergo the physical exam required by her new employer. What are your next steps with this patient? What resources can you use to help her?
As long as human beings have engaged in combat, there have often been extraordinarily damaging psychiatric1 injuries among those who survive. Combat survivability today is 84% to 90%, the highest in the history of armed conflict,2,3 thanks to improvements in personal protective gear, vehicle armor, rapid casualty evacuation, and surgical resuscitation and stabilization that is “far forward” on the battlefield. These survivors are subsequently at high risk for a host of other medical conditions, which commonly include traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), depression, suicide, and substance abuse.4-8
Family physicians—both civilian and uniformed—may be the first to encounter these individuals. Of the more than 2.4 million US service members who have been deployed to Afghanistan or Iraq in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), nearly 60% are no longer on active duty.
Among this group, only half receive care from the US Department of Veterans Affairs (VA).9 Despite a concerted effort on the part of the Department of Defense (DoD) and the VA to develop and distribute effective, evidenced-based treatment protocols for veterans with combat-related conditions, major gaps remain in the care provided to combat veterans.10
This article seeks to help fill that gap by providing the information you need to recognize and treat common combat-related illness, as well as resources to help improve the quality of life for veterans and their families (TABLE 1).
Initial roadblocks to care
One of the biggest challenges in treating veterans with behavioral health issues is the fact that only 23% to 40% of those with mental illness seek care.11 Among the reasons veterans have offered for avoiding behavioral health care are a fear of the stigma associated with mental illness, concern that treatment will negatively affect their career, lack of comfort with mental health professionals, and the perception that mental health treatment is a “last resort.”12 Unfortunately, efforts by the DoD leadership to overcome these inherent biases have been largely unsuccessful13 and much work is still required to see that service members get the care they need.
Due to low rates of self-reporting, effective screening is essential. With this in mind, the DoD has implemented the deployment health assessment program (DHAP), which requires service members to be screened for common conditions within 60 days of deployment, within 30 days of returning, and again at 90 to 180 days after their return.
While the long-term effects of this program are yet to be determined, results to date are promising. Since the DHAP was implemented, there has been a significant decrease in occupationally impairing mental health problems and suicidal ideation requiring medical evacuation from a combat theater.14
FPs should begin with a simple question. Many of the 20+ million veterans living in the United States will not be wearing a uniform when they enter your office. Simply asking all of your patients, “Have you or a loved one ever served in the military?” may help you discover service-related questions or concerns.15,16 Underscoring the importance of such screening is the recent decision by the American Academy of Family Physicians to partner with First Lady Michelle Obama and Dr. Jill Biden in a new campaign called “Joining Forces,” which aims to support veterans and their families.16
Mild traumatic brain injury: Common—though overlooked
A TBI is any temporary or permanent neurologic dysfunction after a blow to the head.10,17 TBI is classified based on severity and mechanism (direct blow to the head or exposure to blast waves). Mild TBI (mTBI) is commonly referred to as a concussion and usually is not associated with loss of consciousness or altered mental status. Brain imaging results are also normal with mTBI. Severe TBI, on the other hand, is associated with prolonged loss of consciousness, altered mental status, and abnormal brain imaging results (TABLE 2).17
A unique obstacle to accurate evaluation in the field. It is important to emphasize that mTBI is a clinical diagnosis, and its detection requires honest patient communication. This can be problematic with motivated soldiers who are anxious to continue the mission and fear that any admission of symptoms might delay a return to their unit. As with a concussed athlete eager to return to the field of play, the clinical diagnosis of mTBI requires a high index of clinical suspicion and constant vigilance by the health care provider. Despite being the most common combat- related injury, mTBI is often overlooked due to the absence of obvious physical injuries.4 Recent data suggest that 28% to 60% of ser- vice members evacuated from combat have a TBI. Most of these injuries (77%) are mTBI.18-20 Improved personal protective equipment (including Kevlar helmets and body armor) and the high number of blast-related injuries are likely responsible for the high incidence of mTBI among OEF/OIF veterans.8,21,22 The prevalence of mTBI among service members not evacuated is estimated to be 20% to 30%.20
Symptoms can persist. Most patients with mTBI completely recover within 30 days of the injury. Unfortunately, 10% to 15% of mTBI patients develop chronic problems lasting months to years.4 Residual symptoms most commonly include headache, irritability, depression, sleep disturbance, impaired reasoning, memory problems, and difficulty concentrating. These symptoms are not unique to mTBI and overlap with comorbid combat diagnoses like PTSD, depression, and sleep deprivation.10 The following tools can help physicians determine whether mTBI is present.
Checking for possible mtBi. In the field, patients with possible mTBI can be screened rapidly using the Military Acute Concussion Evaluation (MACE, found at www.dvbic.org), a modification of the validated and widely used Sideline Assessment of Concussion (SAC) tool. More challenging is evaluating potential mTBI patients who present weeks or months after a traumatic event, for which there are no simple confirmatory tests. In this event, conduct a thorough neurological evaluation that includes vestibular, vision, postural, and neurocognitive assessments. For patients with persistent symptoms or possible anatomic brain abnormalities, magnetic resonance imaging (MRI) is the imaging modality of choice. Patients with complications or a questionable diagnosis are best managed in consultation with a neurologist.
Initial treatment of mtBi is symptom-based. When practical, try nonpharmacologic interventions first (TABLE 3).10 In particular, have the patient avoid further high-risk exposures that could lead to second impact syndrome (an issue increasingly recognized in contact sports). Also critical are physical and cognitive rest and the restoration of sleep until the patient is completely asymptomatic.
If the patient exhibits irritability and depression, selective serotonin reuptake inhibitors (SSRIs) are first-line treatment. Avoid narcotics and sedative-hypnotic sleep medications if treating comorbidities such as pain and sleep deprivation. The VA/DoD guideline on managing concussion and mTBI provides additional detailed, evidence-based treatment recommendations.17
Reliving the horror again and again: PTSD
PTSD is a persistent and, at times, debilitating clinical syndrome that develops after exposure to a psychologically traumatic event. It’s the second most common illness among OEF/OIF combat veterans, with an estimated prevalence of 3% to 20%, a finding consistent with prior wars.6,23-25 In the case of combat veterans, the inciting event usually involves an actual or perceived risk of death or serious injury. The individual’s response to the event involves intense fear, helplessness, or horror. The traumatic event is persistently re-experienced through intrusive and disturbing recollections or dreams that cause intense psychological distress. This, in turn, leads to a state of persistent sympathetic arousal. As symptoms are often triggered by specific cues, individuals with PTSD actively seek to avoid thoughts, situations, or stimuli associated with the event.23,26
Symptoms commonly associated with PTSD include difficulty falling or staying asleep, recurrent nightmares, hypervigilance, and an exaggerated startle response. Individuals with PTSD also have a poorer sense of well-being, a higher rate of work absenteeism, and significantly more somatic complaints than age-matched peers.27 For symptoms to be attributable to PTSD, their onset must follow a recent inciting event and must also cause clinically significant distress or impairment in social, occupational, or other areas of daily living. Common comorbid illnesses include mTBI, depression, and substance abuse. As with mTBI, the presence of multiple comorbidities in patients with PTSD can complicate evaluation, diagnosis, and treatment.
Diagnosis. PTSD is subdivided into acute (symptoms lasting more than one month but less than 3 months after the traumatic event) and chronic (symptoms lasting longer than 3 months after the traumatic event).28 The distinction of acute or chronic does not affect treatment, but it is useful information for the patient to have regarding prognosis and eventual outcome. Like mTBI, PTSD is a clinical diagnosis made only after a thorough, structured diagnostic interview. The use of a validated, self-administered checklist, such as the Post-Traumatic Checklist-Military (PCL-M), allows for an efficient review of a patient’s symptoms and a reliable way to track treatment progress (http://www.ptsd.va.gov/professional/ pages/assessments/ptsd-checklist.asp).
Treatment Options. Effective evidence-based treatments for PTSD are cognitive behavioral therapy, eye movement desensitization and reprocessing (EMDR), and pharmacotherapy. SSRIs and serotonin- norepinephrine reuptake inhibitors (SNRIs) have the strongest evidence for pharmacologic benefit in the treatment of PTSD.28,29 Other helpful medications are prazosin for nightmares and trazodone for sleep. Family physicians can use these medications as part of a patient-centered collaboration with the rest of the integrated care team, to offer the best chance for treatment success.10,28,30
Depression: Vets are reluctant to self-report
Combat experience is a significant risk factor for major depression. Estimates of the lifetime prevalence of depression in the general US population vary from 9% to 25% in women and 5% to 12% in men. By contrast, the prevalence of depression in OIF/OEF veterans ranges from 2% to 37%.24,31,32
Screening can yield false negatives. Many combat veterans are reluctant to self-report behavioral conditions, including depression. Screening, therefore, is important to identify potential depression and allow for intervention. Validated screening tools for depression include the PHQ-2 and PHQ-9, which are easy to use in the office setting. (See http://www.cqaimh.org/pdf/ tool_phq2.pdf [PHQ-2] and http://www. integration.samhsa.gov/images/res/ PHQ%20-%20Questions.pdf [PHQ-9]). Importantly, some veterans will have a negative depression screen on return from deployment, and then test positive 6 to 12 months later.24 Explanations for the early false-negative results include the excitement of being home and patients intentionally answering questions inaccurately to avoid excessive screening at their home base.11
Treatment is most effective with a combination approach. As with most cases of depression, combining psychotherapy and psychopharmacology appears to be most effective for treating depression related to combat experience.33,34 While SSRIs and SNRIs are typical first-line pharmacologic agents, combat veterans often have comorbid mTBI, PTSD, or substance abuse issues that may influence the initial choice of therapy35 (TABLE 3).10
Suicide is on the rise in the military
Historically, the incidence of suicide has been 25% lower in military personnel than in civilian peers.36 However, between 2005 and 2009, the incidence of suicide in the Marine Corps and Army almost doubled.37 While the exact reasons remain unknown, it is likely due to prolonged and repeated deployments to a combat environment.12 While the incidence of suicide has been particularly high in the Army (22 per 100,000 active-duty and reserve personnel per year), all services have been affected. In fact, since 2009, the number of suicides among active duty service members exceeds those killed in action.37
Consider all veterans to be at risk for suicide, and screen accordingly. An effective screening tool is the Columbia-Suicide Severity Rating Scale (C-SSRS), which is able to predict those most at risk for an impending suicide attempt.34 Service members identified as high risk for suicide require unhindered access to care. The VA has worked to improve access to care and provide evidence-based point-of-care treatment strategies.38 Available resources can be found in TABLE 1.
Unfortunately, even with effective screening and treatment, not all suicides can be prevented. Studies have demonstrated that approximately 65% of service members who commit suicide had no known history of communicating their suicidal intent. Sadly, 25% of service members who committed suicide had seen a mental health provider within the previous 30 days.39
Alcohol abuse is common; opioids present a unique risk
Excessive use of alcohol and recreational and prescription drugs is common among OEF/ OIF veterans, especially those with comorbid mental health disorders. Retrospective cross-sectional studies show that 11% to 20% of OEF/OIF veterans met DSM-IV-TR diagnostic criteria for substance use disorders.40-42 At highest risk are single enlisted men under the age of 24 in the Army or Marine Corps who serve in a combat-specific capacity. Interestingly, the prevalence of substance use disorders among OEF/OIF veterans closely mirrors that reported in epidemiologic studies of Vietnam veterans (11%-14%).41 This similarity, combined with the 39% lifetime prevalence of substance use disorders among Vietnam veterans, may foreshadow a similar lifetime prevalence of substance use disorders among OEF/OIF veterans.41
Most-abused substances. Alcohol is the most commonly abused substance among OEF/OIF veterans (10%-20%).40,41,43-45 Other abused substances include opioids (prescribed or illicitly obtained), synthetic marijuana (“Spice” and “K2”), and “bath salts” (synthetic stimulants) (W.M. Sauve, MD, personal communication, August 27, 2012).
OEF/OIF veterans seem to be at particular risk for developing problems related to opioid use. A 2012 retrospective cohort study showed that veterans with non–cancer- related pain diagnoses treated with opioid analgesics had an increased risk for adverse clinical outcomes compared with those not treated with opioid analgesics (9.5% vs 4.1%; relative risk [RR]=2.33; 95% confidence interval [CI], 2.20-2.46). These outcomes included traumatic accidents, overdoses, self-inflicted injuries, and injuries related to violence. This study also demonstrated that, compared with veterans without mental illness, veterans with mental illness (particularly PTSD) and non–cancer-related pain were significantly more likely to receive opioids to treat their pain and had a higher risk of adverse clinical outcomes, including overdose.46,47
Recreational use of synthetic marijuana and “bath salts” has increased in recent years. These substances are commonly labeled “not for human consumption,” which allows them to remain outside US Food and Drug Administration (FDA) regulation and be sold legally in the United States. Efforts to prohibit the sale or possession of these drugs, including the Federal Synthetic Bath Salt Ban in 2012, have fallen short, often due to creative product ”re-engineering.”33 Synthetic marijuana and stimulants are inexpensive, readily available, and perceived by users to be safe. Health care providers are often unaware that their patients are using these products. Adverse health outcomes associated with the use of these synthetic drugs include memory loss, depression, and psychosis.
These alcohol and drug screens can help
One efficient screening tool to identify veterans at risk for alcohol abuse is the AUDIT-C, developed by the World Health Organization. This brief 3-question test identifies past-year hazardous drinking and alcohol abuse or dependence with >79% sensitivity and >56% specificity in male veterans, and >66% sensitivity and >87% specificity in female veterans. These numbers are similar to those provided by the full 10-question AUDIT.48,49 The Drug Abuse Screen Test-10 (DAST-10) provides a similar screening instrument for other substances. Condensed from the original DAST-28 instrument, the DAST-10 identifies high-risk substance abuse with 74% to 94% sensitivity and 68% to 88% specificity.3
Screen for comorbidities. When you see veterans with a diagnosis of substance abuse, also evaluate for comorbid disease. Most veterans with substance use disorders (82%-93%) have at least one other mental health diagnosis (a 45% greater risk than that of civilians with substance abuse disorders),50 most commonly PTSD, depression, anxiety, and adjustment disorders.41,44,45 A number of hypotheses exist to explain the association between substance use disorders and other mental health diagnoses (“dual diagnoses”). The prevailing theory, in both veteran and civilian populations, is that substance abuse is an attempt to self-treat mental illness. Other evidence suggests that substance abuse promotes the development of mental illness, either by leading to a higher risk for traumatic experiences (increasing the chance of developing PTSD) or through a direct biochemical mechanism. Finally, con- current substance use disorder and mental illness may be due to an undefined genetic or biological vulnerability.38,44 This complicated relationship between substance abuse and behavioral health reinforces the need for screening, early diagnosis, and a comprehensive, multidisciplinary approach to treatment.
Treatment options. Office-based treatment options for narcotic and alcohol abuse and dependency are available to family physicians. Methadone has been used since the 1950s to treat opioid addiction and remains one of the mainstays of outpatient treatment.47,51 Originally, methadone was restricted to detoxification and maintenance treatment in narcotic addiction treatment programs approved by the FDA. In 1976, this restriction was lifted, and all physicians registered with the Drug Enforcement Agency (DEA) were permitted to prescribe methadone for analgesia.
In 2002, the FDA approved buprenorphine monotherapy and the combination product buprenorphine/naloxone for the treatment of opioid addiction. The prescribing of buprenorphine products requires physicians to undergo extra training, declare to the DEA their intent to prescribe buprenorphine, and obtain a special DEA identification number.52,53 Physicians interested in finding out more about buprenorphine treatment and prescribing requirements can go to the Substance Abuse and Mental Health Services Administration (SAMHSA) Web page at http://samhsa.gov.
Naltrexone is an opioid receptor agonist that is used primarily to treat alcohol dependency, and is thought to work by reducing the craving for alcohol. Multiple studies have proven the efficacy of naltrexone in an outpatient setting when used alone or in combination with psychotherapy.54,55 If you are uncomfortable or unfamiliar with the use or prescribing of these medications, referral to a substance abuse clinic specializing in dual-diagnosis treatment (TABLE 1) may offer optimal outcomes for patients with substance abuse disorders and other mental illness.
Cognitive behavioral therapy—including coping skills training, relapse prevention, contingency management, and behavioral couples’ therapy—and 12-step treatment programs are evidence-based options for the treatment of substance abuse disorders. Behavioral counseling interventions in the primary care setting (typically lasting 5-15 minutes) result in decreases in alcohol consumption, heavy drinking episodes, drinking above recommended amounts, and the number of days spent in the hospital, but have not been demonstrated to affect mortality, alcohol-related liver problems, outpatient visits, legal problems, or quality of life.56 Resources can be found at www.niaaa.nih.gov. For patients with dual diagnoses, it is not yet known whether sequential therapy (in which substance abuse is treated first, followed by treatment of the comorbid mental illness) or concurrent therapy results in better outcomes.57
CASE Your patient’s history of recent combat service, acknowledgement of employment and behavioral difficulties, and initial screening results lead you to diagnose alcoholism and depression. Additionally, she denies any suicidal ideation, but admits to experimenting with synthetic marijuana. After some discussion, she agrees to see your clinic’s social worker, and you start her on an SSri with scheduled follow-up.
CORRESPONDENCE
Shawn Kane, MD, USASoc, Attn: Surgeon (AomD), 2929 Desert Storm Drive, Ft. Bragg, NC 28310, or PO Box 3639 Pinehurst, NC 28374; [email protected]
1. Wessely S. Risk, psychiatry and the military. Br J Psychiatry. 2005;186:459-466.
2. Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
3. Kotwal RS, Montgomery HR, Kotwal BM, et al. Eliminating pre- ventable death on the battlefield. Arch Surg. 2011;146:1350-1358.
4. Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration’s (VHA’s) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J Head Trauma Rehabil. 2009;24:4-13.
5. Galarneau MR, Woodruff SI, Dye JL, et al. Traumatic brain in- jury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950-957.
6. Hermann BA, Shiner B, Friedman MJ. Epidemiology and preven- tion of combat-related post-traumatic stress in OEF/OIF/OND service members. Mil Med. 2012;177:1-6.
7. Uomoto JM. Best practices in veteran traumatic brain injury care. J Head Trauma Rehabil. 2012;27:241-243.
8. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
9. Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346.
10. Quinlan JD, Guaron MR, Deschere BR, et al. Care of the returning veteran. Am Fam Physician. 2010;82:43-49.
11. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13-22.
12. Hoge CW, Castro CA. Preventing suicides in US service mem- bers and veterans: concerns after a decade of war. JAMA. 2012;308:671-672.
13. Jaffe G. New name for PTSD could mean less stigma. The Washington Post. May 5, 2012. Available at: http://articles. washingtonpost.com/2012-05-05/world/35454931_1_ptsd-post- traumatic-stress-psychiatrists. Accessed June 19, 2013.
14. Warner CH, Appenzeller GN, Parker JR, et al. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am J Psychiatry. 2011;168:378-385.
15. United States Census Bureau. Sex by age by veteran sta- tus for civilian population 18 years and over. 2010 American community survey 1-year estimates. Available at: https:// d3gqux9sl0z33u.cloudfront.net/AA/AT/gambillingonjustice- com/downloads/206273/ACS_10_1YR_B21001A.pdf. Accessed June 19, 2013.
16. American Academy of Family Physicians. Joining forces. Avail- able at: http://www.aafp.org/online/en/home/membership/ initiatives/joiningforces.html. Accessed June 19, 2013.
17. Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. April 2009. Available at: http://www. healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf. Accessed June 19, 2013.
18. Lew HL, Poole JH, Alvarez S, et al. Soldiers with occult traumatic brain injury. Am J Phys Med Rehabil. 2005;84:393-398.
19. Marshall KR, Holland SL, Meyer KS, et al. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177:67- 75.
20. Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14-23.
21. Mossadegh S, Tai N, Midwinter M, et al. Improvised explosive de- vice related pelvi-perineal trauma: anatomic injuries and surgical management. J Trauma Acute Care Surg. 2012;73:S24-S31.
22. Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
23. Espinoza JM. Posttraumatic stress disorder and the perceived consequences of seeking therapy among US Army special forces operators exposed to combat. J Psychol Issues Organ Culture. 2010;1:6-28.
24. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress dis- order and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
25. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023-1032.
26. Adler AB, Wright KM, Bliese PD, et al. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J Trauma Stress. 2008;21:301-308.
27. Hoge CW, Terhakopian A, Castro CA, et al. Association of post- traumatic stress disorder with somatic symptoms, health care vis- its, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164:150-153.
28. Department of Veterans Affairs and Department of Defense. Clin- ical Practice Guideline for Management of Post-Traumatic Stress. October 2010. Available at: http://www.healthquality.va.gov/ ptsd/cpg_PTSD-FULL-201011612.pdf. Accessed June 19, 2013.
29. Alexander W. Pharmacotherapy for post-traumatic stress disor- der in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37:32-38.
30. Wisco BE, Marx BP, Keane TM. Screening, diagnosis, and treat- ment of post-traumatic stress disorder. Mil Med. 2012;177:7-13.
31. Gadermann AM, Engel CC, Naifeh JA, et al. Prevalence of DSM-IV major depression among U.S. military personnel: meta-analysis and simulation. Mil Med. 2012;177:47-59.
32. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
33. Perrone M. Many drugs remain legal after ‘bath salts’ ban. Boston. com. July 25, 2012. Available at: http://articles.boston.com/2012- 07-25/lifestyle/32850962_1_bath-salts-mdpv-synthetic-drugs. Accessed June 19, 2013.
34. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Se- verity Rating Scale: initial validity and internal consistency find- ings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
35. Greenberg J, Tesfazion AA, Robinson CS. Screening, diagnosis, and treatment of depression. Mil Med. 2012;177:60-66.
36. Eaton KM, Messer SC, Garvey Wilson AL, et al. Strengthening the validity of population-based suicide rate comparisons: an il- lustration using U.S. military and civilian data. Suicide Life Threat Behav. 2006;36:182-191.
37. Miller M, Azrael D, Barber C, et al. A call to link data to answer pressing questions about suicide risk among veterans. Am J Pub Health. 2012;102(suppl 1):S20-S22.
38. Department of Veterans Affairs. Report of the Blue Ribbon Work Group on suicide prevention in the veteran population. June 2008. Available at: http://www.mentalhealth.va.gov/suicide_ prevention/Blue_Ribbon_Report-FINAL_June-30-08.pdf. Accessed July 18, 2013.
39. Kinn JT, Luxton DD, Reger MA, et al. Department of Defense sui- cide event report: calendar year 2010 annual report. September 2011. Available at: http://t2health.org/sites/default/files/dodser/ DoDSER_2010_Annual_Report.pdf. Accessed June 19, 2013.
40. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196:513-521.
41. Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93-101.
42. Mirza RA, Eick-Cost A, Otto JL. The risk of mental health disor- ders among U.S. military personnel infected with human immu- nodeficiency virus, active component, U.S. Armed Forces, 2000- 2011. MSMR. 2012;19:10-13.
43. Bohnert AS, Ilgen MA, Bossarte RM, et al. Veteran status and alco- hol use in men in the United States. Mil Med. 2012;177:198-203.
44. Erbes CR, Kaler ME, Schult T, et al. Mental health diagnosis and occupational functioning in National Guard/Reserve veterans re- turning from Iraq. J Rehabil Res Dev. 2011;48:1159-1170.
45. Stecker T, Fortney J, Owen R, et al. Co-occurring medical, psychi- atric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51:503-507.
46. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
47. Praveen KT, Law F, O’Shea J, et al. Opioid dependence. Am Fam Physician. 2012;86:565-566.
48. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-829.
49. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
50. Farrell M, Howes S, Taylor C, et al. Substance misuse and psychi- atric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict Behav. 1998;23:909-918.
51. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
52. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) series 40. DHHS pub- lication (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Available at: http:// buprenorphine.samhsa.gov/Bup_Guidelines.pdf. Accessed June 19, 2013.
53. U.S.DepartmentofHealthandHumanServices,SubstanceAbuse and Mental Health Services Administration Web site. About buprenorphine therapy. Available at: http://buprenorphine. samhsa.gov/about.html. Accessed June 19, 2013.
54. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
55. O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treat- ment of alcoholism: a clinical review. Alcohol. 1996;13:35-39.
56. Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157:645-654.
57. van Dam D, Vedel E, Ehring T, et al. Psychological treatments for concurrent posttraumatic stress disorder and substance use dis- order: a systematic review. Clin Psychol Rev. 2012;32:202-214.
1. Wessely S. Risk, psychiatry and the military. Br J Psychiatry. 2005;186:459-466.
2. Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
3. Kotwal RS, Montgomery HR, Kotwal BM, et al. Eliminating pre- ventable death on the battlefield. Arch Surg. 2011;146:1350-1358.
4. Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration’s (VHA’s) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J Head Trauma Rehabil. 2009;24:4-13.
5. Galarneau MR, Woodruff SI, Dye JL, et al. Traumatic brain in- jury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950-957.
6. Hermann BA, Shiner B, Friedman MJ. Epidemiology and preven- tion of combat-related post-traumatic stress in OEF/OIF/OND service members. Mil Med. 2012;177:1-6.
7. Uomoto JM. Best practices in veteran traumatic brain injury care. J Head Trauma Rehabil. 2012;27:241-243.
8. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
9. Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346.
10. Quinlan JD, Guaron MR, Deschere BR, et al. Care of the returning veteran. Am Fam Physician. 2010;82:43-49.
11. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13-22.
12. Hoge CW, Castro CA. Preventing suicides in US service mem- bers and veterans: concerns after a decade of war. JAMA. 2012;308:671-672.
13. Jaffe G. New name for PTSD could mean less stigma. The Washington Post. May 5, 2012. Available at: http://articles. washingtonpost.com/2012-05-05/world/35454931_1_ptsd-post- traumatic-stress-psychiatrists. Accessed June 19, 2013.
14. Warner CH, Appenzeller GN, Parker JR, et al. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am J Psychiatry. 2011;168:378-385.
15. United States Census Bureau. Sex by age by veteran sta- tus for civilian population 18 years and over. 2010 American community survey 1-year estimates. Available at: https:// d3gqux9sl0z33u.cloudfront.net/AA/AT/gambillingonjustice- com/downloads/206273/ACS_10_1YR_B21001A.pdf. Accessed June 19, 2013.
16. American Academy of Family Physicians. Joining forces. Avail- able at: http://www.aafp.org/online/en/home/membership/ initiatives/joiningforces.html. Accessed June 19, 2013.
17. Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. April 2009. Available at: http://www. healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf. Accessed June 19, 2013.
18. Lew HL, Poole JH, Alvarez S, et al. Soldiers with occult traumatic brain injury. Am J Phys Med Rehabil. 2005;84:393-398.
19. Marshall KR, Holland SL, Meyer KS, et al. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177:67- 75.
20. Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14-23.
21. Mossadegh S, Tai N, Midwinter M, et al. Improvised explosive de- vice related pelvi-perineal trauma: anatomic injuries and surgical management. J Trauma Acute Care Surg. 2012;73:S24-S31.
22. Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
23. Espinoza JM. Posttraumatic stress disorder and the perceived consequences of seeking therapy among US Army special forces operators exposed to combat. J Psychol Issues Organ Culture. 2010;1:6-28.
24. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress dis- order and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
25. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023-1032.
26. Adler AB, Wright KM, Bliese PD, et al. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J Trauma Stress. 2008;21:301-308.
27. Hoge CW, Terhakopian A, Castro CA, et al. Association of post- traumatic stress disorder with somatic symptoms, health care vis- its, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164:150-153.
28. Department of Veterans Affairs and Department of Defense. Clin- ical Practice Guideline for Management of Post-Traumatic Stress. October 2010. Available at: http://www.healthquality.va.gov/ ptsd/cpg_PTSD-FULL-201011612.pdf. Accessed June 19, 2013.
29. Alexander W. Pharmacotherapy for post-traumatic stress disor- der in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37:32-38.
30. Wisco BE, Marx BP, Keane TM. Screening, diagnosis, and treat- ment of post-traumatic stress disorder. Mil Med. 2012;177:7-13.
31. Gadermann AM, Engel CC, Naifeh JA, et al. Prevalence of DSM-IV major depression among U.S. military personnel: meta-analysis and simulation. Mil Med. 2012;177:47-59.
32. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
33. Perrone M. Many drugs remain legal after ‘bath salts’ ban. Boston. com. July 25, 2012. Available at: http://articles.boston.com/2012- 07-25/lifestyle/32850962_1_bath-salts-mdpv-synthetic-drugs. Accessed June 19, 2013.
34. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Se- verity Rating Scale: initial validity and internal consistency find- ings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
35. Greenberg J, Tesfazion AA, Robinson CS. Screening, diagnosis, and treatment of depression. Mil Med. 2012;177:60-66.
36. Eaton KM, Messer SC, Garvey Wilson AL, et al. Strengthening the validity of population-based suicide rate comparisons: an il- lustration using U.S. military and civilian data. Suicide Life Threat Behav. 2006;36:182-191.
37. Miller M, Azrael D, Barber C, et al. A call to link data to answer pressing questions about suicide risk among veterans. Am J Pub Health. 2012;102(suppl 1):S20-S22.
38. Department of Veterans Affairs. Report of the Blue Ribbon Work Group on suicide prevention in the veteran population. June 2008. Available at: http://www.mentalhealth.va.gov/suicide_ prevention/Blue_Ribbon_Report-FINAL_June-30-08.pdf. Accessed July 18, 2013.
39. Kinn JT, Luxton DD, Reger MA, et al. Department of Defense sui- cide event report: calendar year 2010 annual report. September 2011. Available at: http://t2health.org/sites/default/files/dodser/ DoDSER_2010_Annual_Report.pdf. Accessed June 19, 2013.
40. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196:513-521.
41. Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93-101.
42. Mirza RA, Eick-Cost A, Otto JL. The risk of mental health disor- ders among U.S. military personnel infected with human immu- nodeficiency virus, active component, U.S. Armed Forces, 2000- 2011. MSMR. 2012;19:10-13.
43. Bohnert AS, Ilgen MA, Bossarte RM, et al. Veteran status and alco- hol use in men in the United States. Mil Med. 2012;177:198-203.
44. Erbes CR, Kaler ME, Schult T, et al. Mental health diagnosis and occupational functioning in National Guard/Reserve veterans re- turning from Iraq. J Rehabil Res Dev. 2011;48:1159-1170.
45. Stecker T, Fortney J, Owen R, et al. Co-occurring medical, psychi- atric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51:503-507.
46. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
47. Praveen KT, Law F, O’Shea J, et al. Opioid dependence. Am Fam Physician. 2012;86:565-566.
48. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-829.
49. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
50. Farrell M, Howes S, Taylor C, et al. Substance misuse and psychi- atric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict Behav. 1998;23:909-918.
51. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
52. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) series 40. DHHS pub- lication (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Available at: http:// buprenorphine.samhsa.gov/Bup_Guidelines.pdf. Accessed June 19, 2013.
53. U.S.DepartmentofHealthandHumanServices,SubstanceAbuse and Mental Health Services Administration Web site. About buprenorphine therapy. Available at: http://buprenorphine. samhsa.gov/about.html. Accessed June 19, 2013.
54. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
55. O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treat- ment of alcoholism: a clinical review. Alcohol. 1996;13:35-39.
56. Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157:645-654.
57. van Dam D, Vedel E, Ehring T, et al. Psychological treatments for concurrent posttraumatic stress disorder and substance use dis- order: a systematic review. Clin Psychol Rev. 2012;32:202-214.
How can we better manage difficult patient encounters?
It’s a scenario all office-based physicians are familiar with: Your day has been going well, and all your appointments have been (relatively) on time. But when you scan your afternoon schedule your mood shifts from buoyant to crestfallen. The reason: Three patients whom you find particularly frustrating are slotted for the last appointments of the day.
CASE 1 Mr. E, age 44, a high-powered attorney, has chronic headache that he has complained off and on about for several years. He has no other past medical history. Physical examination strongly suggests that Mr. E suffers from tension-type headaches, but he continues to demand magnetic resonance imaging (MRI) of the brain. “I think there’s something wrong in there,” he has stated repeatedly. “If my last doctor, who is one of the best in the country, ordered an MRI for me, why can’t you?”
CASE 2 Mr. A is a 37-year-old man who lost his home in a hurricane 3 years ago. Although he sustained only minor physical injuries, Mr. A appears to have lost his sense of well-being. He has developed chronic—and debilitating— musculoskeletal pain in his neck and low back in the aftermath of the storm and has been unable to work since then.
At his last 2 visits, Mr. A requested increasing doses of oxycodone, insisting that nothing else alleviates the pain. When you suggested a nonopioid analgesic, he broke down in tears. “Nobody takes my injuries seriously! My insurance doesn’t want to compensate me for my losses. Now you don’t even believe I’m in pain.”
CASE 3 Ms. S is a 65-year-old socially isolated widow who lost her husband several years ago. She has a history of multiple somatic complaints, including fatigue, abdominal pain, back pain, joint pain, and dizziness. As a result, she has undergone numerous diagnostic procedures, including esophagastroduodenoscopy, colonoscopy, and various blood tests, all of which have been negative. Ms. S consistently requires longer than the usual 20-minute visit. When you try to end an appointment, she typically brings up new issues. “Two days ago, i had this pain by my belly button and left shoulder blade. i think that there must be something wrong with me. Can you examine me?” she asked toward the end of her last visit 6 weeks ago.
The burden of difficult patient encounters
Cases such as these are frustrating, not so much for their clinical complexity, but rather because of the elusiveness of satisfying doctor-patient interactions. Besides a litany of physical complaints, such patients typically present with anxiety, depression, and other psychiatric symptoms; express dissatisfaction with the care they are receiving; and repeatedly request tests and interventions that are not medically indicated.1
From a primary care perspective, such cases can be frustrating and time- consuming, significantly contribute to exhaustion and burnout, and result in unnecessary health care expenditures.1 (See “Unexplained complaints in primary care: Evidence of action bias” also in this issue to learn more.) Studies suggest that family physicians see such patients on a daily basis, and rate about one patient in 6 as a “difficult” case.2,3
Physician attitudes, training play a role
Research has established other critical spheres of influence that conspire to create difficult or frustrating patient encounters, including “system” factors (ie, reduced duration of visits and interrupted visits)4 and physician factors. In fact, physicians’ negative attitude toward psychosocial aspects of patient care may be a more potent factor in shaping difficult encounters than any patient characteristic.3,5
Consider the following statements:
- “Talking about psychosocial issues causes more trouble than it is worth.”
- “I do not focus on psychosocial problems until I have ruled out organic disease.”
- “I am too pressed for time to routinely investigate psychosocial issues.”
Such sentiments, which have been associated with difficult encounters, are part of the 32-item Physician Belief Scale, developed 30 years ago and still used to assess beliefs about psychosocial aspects of patient care held by primary care physicians.6
Lack of training is a potential problem, as well. In one survey, more than half of directors of family medicine programs agreed that training in mental health is inadequate.7Thus, family physicians often respond to patients like Mr. E, Mr. A, and Ms. S by becoming irritated or avoiding further interaction. A more appropriate response is for the physician to self-acknowledge his or her emotions, then to engage in an empathic interaction in keeping with patient expectations.4
As mental health treatment becomes more integrated within family medicine,8 pointers for handling difficult patient encounters can be gleaned from the traditional psychiatric approach to difficult or frustrating cases. Indeed, we believe that what is now known as a “patient-centered approach” is rooted in traditions and techniques that psychiatrists and psychologists have used for decades.9
Core principles for handling frustrating cases
A useful approach to the difficult patient encounter, detailed in the TABLE that we created, is based on 3 key principles:
- The doctor-patient relationship should be the target for change.
- The patient’s emotional experience should be an explicit focus of the clinical interaction.
- The patient’s perspective should guide the clinical encounter.
In our experience, when the interaction between patient and doctor shifts from searching for specific pathologies to building a collaborative relationship, previously recalcitrant symptoms often improve.
Use these communication techniques
There are 2 main ways to elicit a conversation about personal issues, including emotions, with patients. One is to directly ask patients to describe the distress they are experiencing and elicit the emotions connected to this distress. The other is to invite a discussion about emotional issues indirectly, by asking patients how the symptoms affect their lives and what they think is causing the problem or by selectively sharing an emotional experience of your own.
Once a patient has shared emotions, you will need to show support and empathy in order to build an alliance. There is more than one way to do this, and methods can be used alone or in combination, depending on the particular situation. (You’ll find examples in the TABLE.)
Name the affect. The simple act of naming the patient’s affect or emotional expression (eg, “You sound sad”) is surprisingly helpful, as it lets patients know they have been “heard.”
Validate. You can also validate patients like Mr. E, Mr. A, and Ms. S by stating that their emotional reactions are legitimate, praising them for how well they have coped with difficult symptoms, and acknowledging the seriousness and complexity of their situations.
Align. Once a patient expresses his or her interests and goals, aligning yourself with them (eg, “I want to do everything in my power to help you reduce your pain...”) will elicit hope and improve patient satisfaction.
And 2 mnemonic devices—detailed in the box 10,11—can help you improve the way you communicate with patients.
Communication can also be nonverbal, such as thoughtful nodding or a timely therapeutic silence. The former is characterized by slow, steady, and purposeful movement accompanied by eye contact; in the latter case, you simply resist the urge to immediately respond after a patient has revealed something emotionally laden, and wait a few seconds to take in what has been said.
NURS is a reminder to:
Name the patient’s emotion (“you say that these constant headaches really get on your nerves.”)
Understand (“i can see why you feel this way.”)
Respect (“you’ve been through a lot and that takes a lot of courage.”)
Support (“i want to help you get better.”)10
Background (“What has been going on in your life?”)
Affect (“how do you feel about that?”)
Trouble (“What troubles you the most about this situation?”)
Handling (“how are you handling this?”)
Empathy (“That must be difficult.”)11
How to provide therapeutic structure
Family physicians can further manage patient behaviors that they find bothersome by implementing changes in the way they organize and conduct patient visits. Studies of patients with complex somatic symptoms offer additional hints for the management of frustrating cases. The following strategies can lead to positive outcomes, including a decrease in disability and health care costs.12
Schedule regular brief visits. Mr. E, Mr. A, and Ms. S should have frequent and regular, but brief, appointments (eg, 15 minutes every 2 weeks for 2 months). Proactively schedule return visits, rather than waiting for the patient to call for an appointment PRN.11 Sharing this kind of plan gives such patients a concrete time line and clear evidence of support. Avoid the temptation to schedule difficult cases for the last time slot of the day, as going over the allotted time can insidiously give some patients the expectation of progressively longer visits.
Set the agenda. To prevent “doorknob questions” like Ms. S’s new symptoms, reported just as you’re about to leave the exam room, the agenda must be set at the outset of the visit. This can be done by asking, “What did you want to discuss today?”, “Is there anything else you want to address today?”, or “What else did you need taken care of?”13 Explicitly inquiring about patient expectations at the start of the visit lets patients like Ms. S know that they are being taken seriously. If the agenda still balloons, you can simply state, “You deserve more than 15 minutes for all these issues. Let’s pick the top 2 for today and tackle others at our next visit in 2 weeks.” To further save time, you can ask the patient to bring a symptom diary or written agenda to the appointment. We’ve found that many anxious patients benefit from this exercise.
Avoid the urge to act. When a patient suffers from unexplained symptoms, effective interventions require physicians to avoid certain “reflex” behaviors—repeatedly performing diagnostic tests, prescribing medications for symptoms of unknown etiology, insinuating that the problem is “in your head,” formulating ambiguous diagnoses, and repeating physical exams.12 Such physician behaviors tend to reinforce the pathology in patients with unexplained symptoms. The time saved avoiding these pitfalls is better invested in exploring personal issues and stressors.
The point is that such patients should be reassured via discussion, rather than with dubious diagnostic labels and potentially dangerous drugs. This approach has been shown to improve patients’ physical functioning while reducing medical expenditures.12
Into action with our 3 cases
CASE 1 Given these principles, how would you handle Mr. E, the patient who is demanding an MRI for a simple tension headache? Although placating him by ordering the test, providing a referral to a specialist, or defending your recommendation through medical reasoning may seem to be more intuitive (or a “quick fix”), these strategies often lead to excessive medical spending, transfer of the burden to a specialist colleague, and ongoing frustration and dissatisfaction on the part of the patient. In this case, validation may be a more useful approach.
“I can totally understand why you’re frustrated that we disagree,” you might say to Mr. E. “But you’re right! you definitely deserve the best care. That’s why I’m recommending against the MRI, as I feel that would be a suboptimal approach.”
Often patients like Mr. E will require repeated validation of their suffering and frustration. The key is to be persistent in validating their feelings without compromising your own principles in providing optimal medical care.
CASE 2 Let’s turn now to Mr. A, who is requesting escalating doses of opioids. Some physicians might write the prescription for the dose he’s insisting on, while others draw a hard boundary by refusing to prescribe above a certain dose or beyond a specific time frame. Both strategies may compromise optimal care or endanger the doctor-patient alliance. Another quick solution would be to provide a referral to a psychiatrist, without further discussion.
In cases like that of Mr. A, however, the patient’s demands are often a sign of more complicated emotions and dynamics below the surface. So you might respond by stating, “I’m sorry to hear that things haven’t been going well. How are you feeling about these things? How does the oxycodone help you? In what way doesn’t it help?”
It is important here to understand how the medication serves the patient—in addition to the ways it hurts him—in order for him to feel understood. Inviting Mr. A to have an open-ended discussion may allow him to reveal what is the real source of his distress—losing his job and his home.
CASE 3 Now let’s turn to Ms. S, who is convinced that she has a physical malady despite negative exams and tests. In truth, she may be depressed or anxious over her husband’s death. One way to address this is to confront the patient directly by suggesting that she has depression triggered by her husband’s death. But this strategy—if used too early—may feel like an accusation, make her angry, and jeopardize your relationship with her.
An alternative approach would be to say, “I think your problems are long-standing and could require a while to treat. Let’s see each other every 2 weeks for the next 2 months so we get adequate time to work on them.” This would be an example of structuring more frequent visits, while also validating the distressing nature of her symptoms.
These strategies are evidence-based
These techniques, while easily adaptable to primary care, are grounded in psychotherapy theory and are evidence-based. A seminal randomized controlled trial conducted more than 30 years ago showed that a patient-centered interview incorporating a number of these techniques bolstered physicians’ knowledge, interviewing skills, attitudes, and ability to manage patients with unexplained complaints.14
A multicenter study analyzed audio recordings of strategies used by primary care physicians to deny patient requests for a particular medication. It revealed that explanations based on patient perspectives were significantly more likely to result in excellent patient satisfaction than biomedical explanations or other explanatory approaches.15 Research has also shown that agenda-setting improves both patient and provider satisfaction.16
Some cases will still be frustrating, and some “difficult” patients will still need a psychiatric referral at some point—ideally, to a psychiatric or psychological consultant who collaborates closely with the primary care clinic.17,18
Family physicians sometimes worry that the communication techniques we’ve outlined cannot be incorporated into an already harried primary care visit. Many may think it’s better not ask at all than risk opening a Pandora’s box. We urge you to reconsider. Although the techniques we’ve outlined certainly require practice, they need not be time-consuming.19 By embracing this management approach, you can improve patient satisfaction while enhancing your own repertoire of doctoring skills.
CORRESPONDENCE
Alan R. Teo, MD, MS, 3710 SW US Veterans Hospital Road, Portland, OR 97239; [email protected]
ACKNOWLEDGEMENT
The authors thank Drs. Michael Fetters and Rod Hayward for their help in the development of the manuscript.
1. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: preva- lence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1-8.
2. An PG, Rabatin JS, Manwell LB, et al. Burden of difficult encoun- ters in primary care: data From the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169:410-414.
3. Hinchey SA, Jackson JL. A cohort study assessing difficult patient encoun- ters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588-594.
4. Haas LJ, Leiser JP, Magill MK, et al. Management of the difficult patient. Am Fam Physician. 2005;72:2063-2068.
5. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159:1069- 1075.
6. Ashworth CD, Williamson P, Montano D. A scale to measure physi- cian beliefs about psychosocial aspects of patient care. Soc Sci Med. 1984;19:1235-1238.
7. Leigh H, Stewart D, Mallios R. Mental health and psychiatry training in primary care residency programs. Gen Hosp Psychiatry. 2006;28:189-194.
8. Katon W, Unützer J. Collaborative care models for depression: time to move from evidence to practice. Arch Intern Med. 2006;166:2304-2306.
9. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298:883- 887.
10. Fortin AH, Dwamena FC, Frankel RM, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 3rd ed. New York, NY: McGraw-Hill; 2012.
11. Stuart MR, Lieberman JA. The Fifteen Minute Hour: Therapeutic Talk in Primary Care. 4th ed. Milton Keynes, UK: Radcliffe Publishing; 2008.
12. Smith GR Jr, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry. 1995;52:238-243.
13. Baker LH, O’Connell D, Platt FW. “What else?” Setting the agenda for the clinical interview. Ann Intern Med. 2005;143:766 -770.
14. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
15. Paterniti DA, Fancher TL, Cipri CS, et al. Getting to “no”: strategies pri- mary care physicians use to deny patient requests. Arch Intern Med. 2010;170:381-388.
16. Kroenke K. Unburdening the difficult clinical encounter. Arch Intern Med. 2009;169:333-334.
17. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32:456-464.
18. Williams M, Angstman K, Johnson I, et al. Implementation of a care man- agement model for depression at two primary care clinics. J Ambul Care Manage. 2011;34:163-173.
19. Lieberman JA III, Stuart MR. The BATHE method: incorporating counsel- ing and psychotherapy into the everyday management of patients. Prim Care Companion J Clin Psychiatry. 1999;1:35-38.
It’s a scenario all office-based physicians are familiar with: Your day has been going well, and all your appointments have been (relatively) on time. But when you scan your afternoon schedule your mood shifts from buoyant to crestfallen. The reason: Three patients whom you find particularly frustrating are slotted for the last appointments of the day.
CASE 1 Mr. E, age 44, a high-powered attorney, has chronic headache that he has complained off and on about for several years. He has no other past medical history. Physical examination strongly suggests that Mr. E suffers from tension-type headaches, but he continues to demand magnetic resonance imaging (MRI) of the brain. “I think there’s something wrong in there,” he has stated repeatedly. “If my last doctor, who is one of the best in the country, ordered an MRI for me, why can’t you?”
CASE 2 Mr. A is a 37-year-old man who lost his home in a hurricane 3 years ago. Although he sustained only minor physical injuries, Mr. A appears to have lost his sense of well-being. He has developed chronic—and debilitating— musculoskeletal pain in his neck and low back in the aftermath of the storm and has been unable to work since then.
At his last 2 visits, Mr. A requested increasing doses of oxycodone, insisting that nothing else alleviates the pain. When you suggested a nonopioid analgesic, he broke down in tears. “Nobody takes my injuries seriously! My insurance doesn’t want to compensate me for my losses. Now you don’t even believe I’m in pain.”
CASE 3 Ms. S is a 65-year-old socially isolated widow who lost her husband several years ago. She has a history of multiple somatic complaints, including fatigue, abdominal pain, back pain, joint pain, and dizziness. As a result, she has undergone numerous diagnostic procedures, including esophagastroduodenoscopy, colonoscopy, and various blood tests, all of which have been negative. Ms. S consistently requires longer than the usual 20-minute visit. When you try to end an appointment, she typically brings up new issues. “Two days ago, i had this pain by my belly button and left shoulder blade. i think that there must be something wrong with me. Can you examine me?” she asked toward the end of her last visit 6 weeks ago.
The burden of difficult patient encounters
Cases such as these are frustrating, not so much for their clinical complexity, but rather because of the elusiveness of satisfying doctor-patient interactions. Besides a litany of physical complaints, such patients typically present with anxiety, depression, and other psychiatric symptoms; express dissatisfaction with the care they are receiving; and repeatedly request tests and interventions that are not medically indicated.1
From a primary care perspective, such cases can be frustrating and time- consuming, significantly contribute to exhaustion and burnout, and result in unnecessary health care expenditures.1 (See “Unexplained complaints in primary care: Evidence of action bias” also in this issue to learn more.) Studies suggest that family physicians see such patients on a daily basis, and rate about one patient in 6 as a “difficult” case.2,3
Physician attitudes, training play a role
Research has established other critical spheres of influence that conspire to create difficult or frustrating patient encounters, including “system” factors (ie, reduced duration of visits and interrupted visits)4 and physician factors. In fact, physicians’ negative attitude toward psychosocial aspects of patient care may be a more potent factor in shaping difficult encounters than any patient characteristic.3,5
Consider the following statements:
- “Talking about psychosocial issues causes more trouble than it is worth.”
- “I do not focus on psychosocial problems until I have ruled out organic disease.”
- “I am too pressed for time to routinely investigate psychosocial issues.”
Such sentiments, which have been associated with difficult encounters, are part of the 32-item Physician Belief Scale, developed 30 years ago and still used to assess beliefs about psychosocial aspects of patient care held by primary care physicians.6
Lack of training is a potential problem, as well. In one survey, more than half of directors of family medicine programs agreed that training in mental health is inadequate.7Thus, family physicians often respond to patients like Mr. E, Mr. A, and Ms. S by becoming irritated or avoiding further interaction. A more appropriate response is for the physician to self-acknowledge his or her emotions, then to engage in an empathic interaction in keeping with patient expectations.4
As mental health treatment becomes more integrated within family medicine,8 pointers for handling difficult patient encounters can be gleaned from the traditional psychiatric approach to difficult or frustrating cases. Indeed, we believe that what is now known as a “patient-centered approach” is rooted in traditions and techniques that psychiatrists and psychologists have used for decades.9
Core principles for handling frustrating cases
A useful approach to the difficult patient encounter, detailed in the TABLE that we created, is based on 3 key principles:
- The doctor-patient relationship should be the target for change.
- The patient’s emotional experience should be an explicit focus of the clinical interaction.
- The patient’s perspective should guide the clinical encounter.
In our experience, when the interaction between patient and doctor shifts from searching for specific pathologies to building a collaborative relationship, previously recalcitrant symptoms often improve.
Use these communication techniques
There are 2 main ways to elicit a conversation about personal issues, including emotions, with patients. One is to directly ask patients to describe the distress they are experiencing and elicit the emotions connected to this distress. The other is to invite a discussion about emotional issues indirectly, by asking patients how the symptoms affect their lives and what they think is causing the problem or by selectively sharing an emotional experience of your own.
Once a patient has shared emotions, you will need to show support and empathy in order to build an alliance. There is more than one way to do this, and methods can be used alone or in combination, depending on the particular situation. (You’ll find examples in the TABLE.)
Name the affect. The simple act of naming the patient’s affect or emotional expression (eg, “You sound sad”) is surprisingly helpful, as it lets patients know they have been “heard.”
Validate. You can also validate patients like Mr. E, Mr. A, and Ms. S by stating that their emotional reactions are legitimate, praising them for how well they have coped with difficult symptoms, and acknowledging the seriousness and complexity of their situations.
Align. Once a patient expresses his or her interests and goals, aligning yourself with them (eg, “I want to do everything in my power to help you reduce your pain...”) will elicit hope and improve patient satisfaction.
And 2 mnemonic devices—detailed in the box 10,11—can help you improve the way you communicate with patients.
Communication can also be nonverbal, such as thoughtful nodding or a timely therapeutic silence. The former is characterized by slow, steady, and purposeful movement accompanied by eye contact; in the latter case, you simply resist the urge to immediately respond after a patient has revealed something emotionally laden, and wait a few seconds to take in what has been said.
NURS is a reminder to:
Name the patient’s emotion (“you say that these constant headaches really get on your nerves.”)
Understand (“i can see why you feel this way.”)
Respect (“you’ve been through a lot and that takes a lot of courage.”)
Support (“i want to help you get better.”)10
Background (“What has been going on in your life?”)
Affect (“how do you feel about that?”)
Trouble (“What troubles you the most about this situation?”)
Handling (“how are you handling this?”)
Empathy (“That must be difficult.”)11
How to provide therapeutic structure
Family physicians can further manage patient behaviors that they find bothersome by implementing changes in the way they organize and conduct patient visits. Studies of patients with complex somatic symptoms offer additional hints for the management of frustrating cases. The following strategies can lead to positive outcomes, including a decrease in disability and health care costs.12
Schedule regular brief visits. Mr. E, Mr. A, and Ms. S should have frequent and regular, but brief, appointments (eg, 15 minutes every 2 weeks for 2 months). Proactively schedule return visits, rather than waiting for the patient to call for an appointment PRN.11 Sharing this kind of plan gives such patients a concrete time line and clear evidence of support. Avoid the temptation to schedule difficult cases for the last time slot of the day, as going over the allotted time can insidiously give some patients the expectation of progressively longer visits.
Set the agenda. To prevent “doorknob questions” like Ms. S’s new symptoms, reported just as you’re about to leave the exam room, the agenda must be set at the outset of the visit. This can be done by asking, “What did you want to discuss today?”, “Is there anything else you want to address today?”, or “What else did you need taken care of?”13 Explicitly inquiring about patient expectations at the start of the visit lets patients like Ms. S know that they are being taken seriously. If the agenda still balloons, you can simply state, “You deserve more than 15 minutes for all these issues. Let’s pick the top 2 for today and tackle others at our next visit in 2 weeks.” To further save time, you can ask the patient to bring a symptom diary or written agenda to the appointment. We’ve found that many anxious patients benefit from this exercise.
Avoid the urge to act. When a patient suffers from unexplained symptoms, effective interventions require physicians to avoid certain “reflex” behaviors—repeatedly performing diagnostic tests, prescribing medications for symptoms of unknown etiology, insinuating that the problem is “in your head,” formulating ambiguous diagnoses, and repeating physical exams.12 Such physician behaviors tend to reinforce the pathology in patients with unexplained symptoms. The time saved avoiding these pitfalls is better invested in exploring personal issues and stressors.
The point is that such patients should be reassured via discussion, rather than with dubious diagnostic labels and potentially dangerous drugs. This approach has been shown to improve patients’ physical functioning while reducing medical expenditures.12
Into action with our 3 cases
CASE 1 Given these principles, how would you handle Mr. E, the patient who is demanding an MRI for a simple tension headache? Although placating him by ordering the test, providing a referral to a specialist, or defending your recommendation through medical reasoning may seem to be more intuitive (or a “quick fix”), these strategies often lead to excessive medical spending, transfer of the burden to a specialist colleague, and ongoing frustration and dissatisfaction on the part of the patient. In this case, validation may be a more useful approach.
“I can totally understand why you’re frustrated that we disagree,” you might say to Mr. E. “But you’re right! you definitely deserve the best care. That’s why I’m recommending against the MRI, as I feel that would be a suboptimal approach.”
Often patients like Mr. E will require repeated validation of their suffering and frustration. The key is to be persistent in validating their feelings without compromising your own principles in providing optimal medical care.
CASE 2 Let’s turn now to Mr. A, who is requesting escalating doses of opioids. Some physicians might write the prescription for the dose he’s insisting on, while others draw a hard boundary by refusing to prescribe above a certain dose or beyond a specific time frame. Both strategies may compromise optimal care or endanger the doctor-patient alliance. Another quick solution would be to provide a referral to a psychiatrist, without further discussion.
In cases like that of Mr. A, however, the patient’s demands are often a sign of more complicated emotions and dynamics below the surface. So you might respond by stating, “I’m sorry to hear that things haven’t been going well. How are you feeling about these things? How does the oxycodone help you? In what way doesn’t it help?”
It is important here to understand how the medication serves the patient—in addition to the ways it hurts him—in order for him to feel understood. Inviting Mr. A to have an open-ended discussion may allow him to reveal what is the real source of his distress—losing his job and his home.
CASE 3 Now let’s turn to Ms. S, who is convinced that she has a physical malady despite negative exams and tests. In truth, she may be depressed or anxious over her husband’s death. One way to address this is to confront the patient directly by suggesting that she has depression triggered by her husband’s death. But this strategy—if used too early—may feel like an accusation, make her angry, and jeopardize your relationship with her.
An alternative approach would be to say, “I think your problems are long-standing and could require a while to treat. Let’s see each other every 2 weeks for the next 2 months so we get adequate time to work on them.” This would be an example of structuring more frequent visits, while also validating the distressing nature of her symptoms.
These strategies are evidence-based
These techniques, while easily adaptable to primary care, are grounded in psychotherapy theory and are evidence-based. A seminal randomized controlled trial conducted more than 30 years ago showed that a patient-centered interview incorporating a number of these techniques bolstered physicians’ knowledge, interviewing skills, attitudes, and ability to manage patients with unexplained complaints.14
A multicenter study analyzed audio recordings of strategies used by primary care physicians to deny patient requests for a particular medication. It revealed that explanations based on patient perspectives were significantly more likely to result in excellent patient satisfaction than biomedical explanations or other explanatory approaches.15 Research has also shown that agenda-setting improves both patient and provider satisfaction.16
Some cases will still be frustrating, and some “difficult” patients will still need a psychiatric referral at some point—ideally, to a psychiatric or psychological consultant who collaborates closely with the primary care clinic.17,18
Family physicians sometimes worry that the communication techniques we’ve outlined cannot be incorporated into an already harried primary care visit. Many may think it’s better not ask at all than risk opening a Pandora’s box. We urge you to reconsider. Although the techniques we’ve outlined certainly require practice, they need not be time-consuming.19 By embracing this management approach, you can improve patient satisfaction while enhancing your own repertoire of doctoring skills.
CORRESPONDENCE
Alan R. Teo, MD, MS, 3710 SW US Veterans Hospital Road, Portland, OR 97239; [email protected]
ACKNOWLEDGEMENT
The authors thank Drs. Michael Fetters and Rod Hayward for their help in the development of the manuscript.
It’s a scenario all office-based physicians are familiar with: Your day has been going well, and all your appointments have been (relatively) on time. But when you scan your afternoon schedule your mood shifts from buoyant to crestfallen. The reason: Three patients whom you find particularly frustrating are slotted for the last appointments of the day.
CASE 1 Mr. E, age 44, a high-powered attorney, has chronic headache that he has complained off and on about for several years. He has no other past medical history. Physical examination strongly suggests that Mr. E suffers from tension-type headaches, but he continues to demand magnetic resonance imaging (MRI) of the brain. “I think there’s something wrong in there,” he has stated repeatedly. “If my last doctor, who is one of the best in the country, ordered an MRI for me, why can’t you?”
CASE 2 Mr. A is a 37-year-old man who lost his home in a hurricane 3 years ago. Although he sustained only minor physical injuries, Mr. A appears to have lost his sense of well-being. He has developed chronic—and debilitating— musculoskeletal pain in his neck and low back in the aftermath of the storm and has been unable to work since then.
At his last 2 visits, Mr. A requested increasing doses of oxycodone, insisting that nothing else alleviates the pain. When you suggested a nonopioid analgesic, he broke down in tears. “Nobody takes my injuries seriously! My insurance doesn’t want to compensate me for my losses. Now you don’t even believe I’m in pain.”
CASE 3 Ms. S is a 65-year-old socially isolated widow who lost her husband several years ago. She has a history of multiple somatic complaints, including fatigue, abdominal pain, back pain, joint pain, and dizziness. As a result, she has undergone numerous diagnostic procedures, including esophagastroduodenoscopy, colonoscopy, and various blood tests, all of which have been negative. Ms. S consistently requires longer than the usual 20-minute visit. When you try to end an appointment, she typically brings up new issues. “Two days ago, i had this pain by my belly button and left shoulder blade. i think that there must be something wrong with me. Can you examine me?” she asked toward the end of her last visit 6 weeks ago.
The burden of difficult patient encounters
Cases such as these are frustrating, not so much for their clinical complexity, but rather because of the elusiveness of satisfying doctor-patient interactions. Besides a litany of physical complaints, such patients typically present with anxiety, depression, and other psychiatric symptoms; express dissatisfaction with the care they are receiving; and repeatedly request tests and interventions that are not medically indicated.1
From a primary care perspective, such cases can be frustrating and time- consuming, significantly contribute to exhaustion and burnout, and result in unnecessary health care expenditures.1 (See “Unexplained complaints in primary care: Evidence of action bias” also in this issue to learn more.) Studies suggest that family physicians see such patients on a daily basis, and rate about one patient in 6 as a “difficult” case.2,3
Physician attitudes, training play a role
Research has established other critical spheres of influence that conspire to create difficult or frustrating patient encounters, including “system” factors (ie, reduced duration of visits and interrupted visits)4 and physician factors. In fact, physicians’ negative attitude toward psychosocial aspects of patient care may be a more potent factor in shaping difficult encounters than any patient characteristic.3,5
Consider the following statements:
- “Talking about psychosocial issues causes more trouble than it is worth.”
- “I do not focus on psychosocial problems until I have ruled out organic disease.”
- “I am too pressed for time to routinely investigate psychosocial issues.”
Such sentiments, which have been associated with difficult encounters, are part of the 32-item Physician Belief Scale, developed 30 years ago and still used to assess beliefs about psychosocial aspects of patient care held by primary care physicians.6
Lack of training is a potential problem, as well. In one survey, more than half of directors of family medicine programs agreed that training in mental health is inadequate.7Thus, family physicians often respond to patients like Mr. E, Mr. A, and Ms. S by becoming irritated or avoiding further interaction. A more appropriate response is for the physician to self-acknowledge his or her emotions, then to engage in an empathic interaction in keeping with patient expectations.4
As mental health treatment becomes more integrated within family medicine,8 pointers for handling difficult patient encounters can be gleaned from the traditional psychiatric approach to difficult or frustrating cases. Indeed, we believe that what is now known as a “patient-centered approach” is rooted in traditions and techniques that psychiatrists and psychologists have used for decades.9
Core principles for handling frustrating cases
A useful approach to the difficult patient encounter, detailed in the TABLE that we created, is based on 3 key principles:
- The doctor-patient relationship should be the target for change.
- The patient’s emotional experience should be an explicit focus of the clinical interaction.
- The patient’s perspective should guide the clinical encounter.
In our experience, when the interaction between patient and doctor shifts from searching for specific pathologies to building a collaborative relationship, previously recalcitrant symptoms often improve.
Use these communication techniques
There are 2 main ways to elicit a conversation about personal issues, including emotions, with patients. One is to directly ask patients to describe the distress they are experiencing and elicit the emotions connected to this distress. The other is to invite a discussion about emotional issues indirectly, by asking patients how the symptoms affect their lives and what they think is causing the problem or by selectively sharing an emotional experience of your own.
Once a patient has shared emotions, you will need to show support and empathy in order to build an alliance. There is more than one way to do this, and methods can be used alone or in combination, depending on the particular situation. (You’ll find examples in the TABLE.)
Name the affect. The simple act of naming the patient’s affect or emotional expression (eg, “You sound sad”) is surprisingly helpful, as it lets patients know they have been “heard.”
Validate. You can also validate patients like Mr. E, Mr. A, and Ms. S by stating that their emotional reactions are legitimate, praising them for how well they have coped with difficult symptoms, and acknowledging the seriousness and complexity of their situations.
Align. Once a patient expresses his or her interests and goals, aligning yourself with them (eg, “I want to do everything in my power to help you reduce your pain...”) will elicit hope and improve patient satisfaction.
And 2 mnemonic devices—detailed in the box 10,11—can help you improve the way you communicate with patients.
Communication can also be nonverbal, such as thoughtful nodding or a timely therapeutic silence. The former is characterized by slow, steady, and purposeful movement accompanied by eye contact; in the latter case, you simply resist the urge to immediately respond after a patient has revealed something emotionally laden, and wait a few seconds to take in what has been said.
NURS is a reminder to:
Name the patient’s emotion (“you say that these constant headaches really get on your nerves.”)
Understand (“i can see why you feel this way.”)
Respect (“you’ve been through a lot and that takes a lot of courage.”)
Support (“i want to help you get better.”)10
Background (“What has been going on in your life?”)
Affect (“how do you feel about that?”)
Trouble (“What troubles you the most about this situation?”)
Handling (“how are you handling this?”)
Empathy (“That must be difficult.”)11
How to provide therapeutic structure
Family physicians can further manage patient behaviors that they find bothersome by implementing changes in the way they organize and conduct patient visits. Studies of patients with complex somatic symptoms offer additional hints for the management of frustrating cases. The following strategies can lead to positive outcomes, including a decrease in disability and health care costs.12
Schedule regular brief visits. Mr. E, Mr. A, and Ms. S should have frequent and regular, but brief, appointments (eg, 15 minutes every 2 weeks for 2 months). Proactively schedule return visits, rather than waiting for the patient to call for an appointment PRN.11 Sharing this kind of plan gives such patients a concrete time line and clear evidence of support. Avoid the temptation to schedule difficult cases for the last time slot of the day, as going over the allotted time can insidiously give some patients the expectation of progressively longer visits.
Set the agenda. To prevent “doorknob questions” like Ms. S’s new symptoms, reported just as you’re about to leave the exam room, the agenda must be set at the outset of the visit. This can be done by asking, “What did you want to discuss today?”, “Is there anything else you want to address today?”, or “What else did you need taken care of?”13 Explicitly inquiring about patient expectations at the start of the visit lets patients like Ms. S know that they are being taken seriously. If the agenda still balloons, you can simply state, “You deserve more than 15 minutes for all these issues. Let’s pick the top 2 for today and tackle others at our next visit in 2 weeks.” To further save time, you can ask the patient to bring a symptom diary or written agenda to the appointment. We’ve found that many anxious patients benefit from this exercise.
Avoid the urge to act. When a patient suffers from unexplained symptoms, effective interventions require physicians to avoid certain “reflex” behaviors—repeatedly performing diagnostic tests, prescribing medications for symptoms of unknown etiology, insinuating that the problem is “in your head,” formulating ambiguous diagnoses, and repeating physical exams.12 Such physician behaviors tend to reinforce the pathology in patients with unexplained symptoms. The time saved avoiding these pitfalls is better invested in exploring personal issues and stressors.
The point is that such patients should be reassured via discussion, rather than with dubious diagnostic labels and potentially dangerous drugs. This approach has been shown to improve patients’ physical functioning while reducing medical expenditures.12
Into action with our 3 cases
CASE 1 Given these principles, how would you handle Mr. E, the patient who is demanding an MRI for a simple tension headache? Although placating him by ordering the test, providing a referral to a specialist, or defending your recommendation through medical reasoning may seem to be more intuitive (or a “quick fix”), these strategies often lead to excessive medical spending, transfer of the burden to a specialist colleague, and ongoing frustration and dissatisfaction on the part of the patient. In this case, validation may be a more useful approach.
“I can totally understand why you’re frustrated that we disagree,” you might say to Mr. E. “But you’re right! you definitely deserve the best care. That’s why I’m recommending against the MRI, as I feel that would be a suboptimal approach.”
Often patients like Mr. E will require repeated validation of their suffering and frustration. The key is to be persistent in validating their feelings without compromising your own principles in providing optimal medical care.
CASE 2 Let’s turn now to Mr. A, who is requesting escalating doses of opioids. Some physicians might write the prescription for the dose he’s insisting on, while others draw a hard boundary by refusing to prescribe above a certain dose or beyond a specific time frame. Both strategies may compromise optimal care or endanger the doctor-patient alliance. Another quick solution would be to provide a referral to a psychiatrist, without further discussion.
In cases like that of Mr. A, however, the patient’s demands are often a sign of more complicated emotions and dynamics below the surface. So you might respond by stating, “I’m sorry to hear that things haven’t been going well. How are you feeling about these things? How does the oxycodone help you? In what way doesn’t it help?”
It is important here to understand how the medication serves the patient—in addition to the ways it hurts him—in order for him to feel understood. Inviting Mr. A to have an open-ended discussion may allow him to reveal what is the real source of his distress—losing his job and his home.
CASE 3 Now let’s turn to Ms. S, who is convinced that she has a physical malady despite negative exams and tests. In truth, she may be depressed or anxious over her husband’s death. One way to address this is to confront the patient directly by suggesting that she has depression triggered by her husband’s death. But this strategy—if used too early—may feel like an accusation, make her angry, and jeopardize your relationship with her.
An alternative approach would be to say, “I think your problems are long-standing and could require a while to treat. Let’s see each other every 2 weeks for the next 2 months so we get adequate time to work on them.” This would be an example of structuring more frequent visits, while also validating the distressing nature of her symptoms.
These strategies are evidence-based
These techniques, while easily adaptable to primary care, are grounded in psychotherapy theory and are evidence-based. A seminal randomized controlled trial conducted more than 30 years ago showed that a patient-centered interview incorporating a number of these techniques bolstered physicians’ knowledge, interviewing skills, attitudes, and ability to manage patients with unexplained complaints.14
A multicenter study analyzed audio recordings of strategies used by primary care physicians to deny patient requests for a particular medication. It revealed that explanations based on patient perspectives were significantly more likely to result in excellent patient satisfaction than biomedical explanations or other explanatory approaches.15 Research has also shown that agenda-setting improves both patient and provider satisfaction.16
Some cases will still be frustrating, and some “difficult” patients will still need a psychiatric referral at some point—ideally, to a psychiatric or psychological consultant who collaborates closely with the primary care clinic.17,18
Family physicians sometimes worry that the communication techniques we’ve outlined cannot be incorporated into an already harried primary care visit. Many may think it’s better not ask at all than risk opening a Pandora’s box. We urge you to reconsider. Although the techniques we’ve outlined certainly require practice, they need not be time-consuming.19 By embracing this management approach, you can improve patient satisfaction while enhancing your own repertoire of doctoring skills.
CORRESPONDENCE
Alan R. Teo, MD, MS, 3710 SW US Veterans Hospital Road, Portland, OR 97239; [email protected]
ACKNOWLEDGEMENT
The authors thank Drs. Michael Fetters and Rod Hayward for their help in the development of the manuscript.
1. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: preva- lence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1-8.
2. An PG, Rabatin JS, Manwell LB, et al. Burden of difficult encoun- ters in primary care: data From the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169:410-414.
3. Hinchey SA, Jackson JL. A cohort study assessing difficult patient encoun- ters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588-594.
4. Haas LJ, Leiser JP, Magill MK, et al. Management of the difficult patient. Am Fam Physician. 2005;72:2063-2068.
5. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159:1069- 1075.
6. Ashworth CD, Williamson P, Montano D. A scale to measure physi- cian beliefs about psychosocial aspects of patient care. Soc Sci Med. 1984;19:1235-1238.
7. Leigh H, Stewart D, Mallios R. Mental health and psychiatry training in primary care residency programs. Gen Hosp Psychiatry. 2006;28:189-194.
8. Katon W, Unützer J. Collaborative care models for depression: time to move from evidence to practice. Arch Intern Med. 2006;166:2304-2306.
9. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298:883- 887.
10. Fortin AH, Dwamena FC, Frankel RM, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 3rd ed. New York, NY: McGraw-Hill; 2012.
11. Stuart MR, Lieberman JA. The Fifteen Minute Hour: Therapeutic Talk in Primary Care. 4th ed. Milton Keynes, UK: Radcliffe Publishing; 2008.
12. Smith GR Jr, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry. 1995;52:238-243.
13. Baker LH, O’Connell D, Platt FW. “What else?” Setting the agenda for the clinical interview. Ann Intern Med. 2005;143:766 -770.
14. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
15. Paterniti DA, Fancher TL, Cipri CS, et al. Getting to “no”: strategies pri- mary care physicians use to deny patient requests. Arch Intern Med. 2010;170:381-388.
16. Kroenke K. Unburdening the difficult clinical encounter. Arch Intern Med. 2009;169:333-334.
17. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32:456-464.
18. Williams M, Angstman K, Johnson I, et al. Implementation of a care man- agement model for depression at two primary care clinics. J Ambul Care Manage. 2011;34:163-173.
19. Lieberman JA III, Stuart MR. The BATHE method: incorporating counsel- ing and psychotherapy into the everyday management of patients. Prim Care Companion J Clin Psychiatry. 1999;1:35-38.
1. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: preva- lence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1-8.
2. An PG, Rabatin JS, Manwell LB, et al. Burden of difficult encoun- ters in primary care: data From the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169:410-414.
3. Hinchey SA, Jackson JL. A cohort study assessing difficult patient encoun- ters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588-594.
4. Haas LJ, Leiser JP, Magill MK, et al. Management of the difficult patient. Am Fam Physician. 2005;72:2063-2068.
5. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159:1069- 1075.
6. Ashworth CD, Williamson P, Montano D. A scale to measure physi- cian beliefs about psychosocial aspects of patient care. Soc Sci Med. 1984;19:1235-1238.
7. Leigh H, Stewart D, Mallios R. Mental health and psychiatry training in primary care residency programs. Gen Hosp Psychiatry. 2006;28:189-194.
8. Katon W, Unützer J. Collaborative care models for depression: time to move from evidence to practice. Arch Intern Med. 2006;166:2304-2306.
9. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298:883- 887.
10. Fortin AH, Dwamena FC, Frankel RM, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 3rd ed. New York, NY: McGraw-Hill; 2012.
11. Stuart MR, Lieberman JA. The Fifteen Minute Hour: Therapeutic Talk in Primary Care. 4th ed. Milton Keynes, UK: Radcliffe Publishing; 2008.
12. Smith GR Jr, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry. 1995;52:238-243.
13. Baker LH, O’Connell D, Platt FW. “What else?” Setting the agenda for the clinical interview. Ann Intern Med. 2005;143:766 -770.
14. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
15. Paterniti DA, Fancher TL, Cipri CS, et al. Getting to “no”: strategies pri- mary care physicians use to deny patient requests. Arch Intern Med. 2010;170:381-388.
16. Kroenke K. Unburdening the difficult clinical encounter. Arch Intern Med. 2009;169:333-334.
17. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32:456-464.
18. Williams M, Angstman K, Johnson I, et al. Implementation of a care man- agement model for depression at two primary care clinics. J Ambul Care Manage. 2011;34:163-173.
19. Lieberman JA III, Stuart MR. The BATHE method: incorporating counsel- ing and psychotherapy into the everyday management of patients. Prim Care Companion J Clin Psychiatry. 1999;1:35-38.
Elevated APTT? How best to follow up
CASE During an office visit, 23-year-old John K tells you that he recently experienced excessive bleeding after a dental extraction. He says that he has no personal or family history of a bleeding disorder and is not taking any medication that might have contributed to the problem.
His lab work shows a normal complete blood count and international normalized ratio (INR). His activated partial thromboplastin time (APTT), however, is 67 seconds (normal=24-37 seconds).
How would you manage his care?
Correct interpretation of abnormal APTT readings requires an understanding of the clinical context in which the test is ordered and the test’s limitations. In cases like Mr. K’s, the top priorities are taking a careful history and performing a thorough physical examination.
Specifically, you need to ask about any personal or family history of spontaneous mucosal bleeding, menorrhagia, hemostatic difficulties with previous surgeries or dental extractions, medication use (including anticoagulants), and alcohol use. Find out whether there is a history of liver disease or malnutrition/malabsorption. Does the patient bruise easily? Ask patients with an elevated APTT about a personal or family history of spontaneous mucosal bleeding, menorrhagia, or hemostatic difficulties with previous surgeries or dental extractions.
As you would expect, such signposts warrant further investigations. And if the personal or family history does suggest a bleeding disorder, the patient should be referred to a hematologist.
CASE Mr. K’s physical examination is unremarkable and his renal and liver panel—including serum albumin—are within the normal range. As noted earlier, he had no history of bleeding before the tooth extraction; his family history is negative for excessive bleeding, as well.
Consider an artifactual cause
It’s important to rule out artifactual causes of an abnormal APTT before undertaking a more detailed investigation. Although not applicable in this case, unfractionated heparin in a central venous or arterial catheter can prolong APTT.
A high hematocrit value will give falsely prolonged APTT. This is due to the increased concentration of citrate relative to the small volume of plasma.1 The lab should be alerted if the hematocrit is high so that the volume of anticoagulant in the collection tube may be adjusted for more reliable results.
Lipemic, hemolyzed, or icteric plasma specimens interfere with the optical system of the instruments and may also give false results.2
Delays and extreme temperatures can also alter results. The specimen should not be delayed for more than 4 hours as factor VIII is labile and longer time will result in an artifactually prolonged APTT.3 (See “APTT: Understanding the test”.) In addition, prolonged exposure to high temperatures may enhance degradation of factors V and VIII, whereas prolonged exposure to cold temperatures may activate factor VII.4
Activated partial thromboplastin time (APTT) measures the integrity of the intrinsic and common pathways of the coagulation cascade. Any deficiency or inhibitor of the clotting factors within the intrinsic or common pathways will result in a prolonged APTT. The factors involved in the intrinsic and common pathways are II (prothrombin), V, VIII, IX, X, XI, XII, and fibrinogen (factor I).
CASE Because there is no physical evidence of liver, renal, or connective tissue disease in Mr. K’s case and artifactual causes are not at work, the next logical step is a mixing study.
Does the mixing study correct the prolonged APTT?
A mixing study with normal plasma will differentiate between a coagulation factor deficiency and the presence of an inhibitor. Correction of the prolonged APTT to the reference range after mixing a sample of the patient’s blood with normal plasma in a 1:1 ratio implies a deficiency of a clotting factor in the intrinsic or common final pathway of the coagulation cascade. The deficiency may involve one or more of the following: factors VIII, IX, XI, or XII; high-molecular-weight kininogen (HMWK); and prekallikrein (PK). A clotting factor assay will identify the deficient factor. A mixing study with normal plasma will differentiate between a coagulation factor deficiency and the presence of an inhibitor.
Congenital or acquired? Assuming that a clotting factor deficiency exists, the next step is to determine the nature of the deficiency in terms of congenital or acquired defects. Congenital causes of factor VIII, IX, and XI deficiency are hemophilia A, B, and C, respectively. Hemophilia affects one in 5000 males born in the United States;5 about 9 out of 10 have hemophilia A.6
Acquired causes of factor deficiencies are liver disease, warfarin use, disseminated intravascular coagulation, and vitamin K deficiency due to malabsorption or malnutrition.
Is an inhibitor at work? The presence of an inhibitor is suggested if the mixing studies do not result in correction of the prolonged APTT to normal range.
If factitious use of heparin or thrombin inhibitors is suspected, thrombin time (TT) and reptilase time help determine if heparin or direct thrombin inhibitor is present. TT is prolonged by both heparin and thrombin inhibitors, but reptilase time is not affected by either of the drugs.
Lupus anticoagulant should be tested if there is no history of treatment with unfractionated heparin or direct thrombin inhibitors such as lepirudin and argatroban. However, antiphospholipid antibodies are estimated to have a prevalence of 1.0% to 5.6% in healthy populations.7 Moreover, lupus anticoagulant is transient in most cases and is most often of no clinical significance. It is therefore important to interpret the lupus anticoagulant in the context of the illness.
If the inhibitor is still a mystery, you will need to test for specific factor inhibitors like factor VIII inhibitor (acquired hemophilia). Inhibitors are alloantibodies, which develop, for example, in patients with hemophilia who are treated with blood products such as fresh frozen plasma (FFP), cryoprecipitate, or factor VIII concentrate.
When an elevated number doesn't mean what you think
There are certain limitations of APTT in identifying the deficient factors because the sensitivity of APTT to identify deficient common pathway factors is low.8 (There may be deficiency of a common pathway factor but a normal APTT). Composition of the partial thromboplastin reagent can result in marked differences in the sensitivity of the test to coagulation factor deficiencies.
In addition, factor VIII levels may give false-negative results in pregnancy and in response to physical stress and trauma, as factor VIII rises markedly in both cases. Thus, it may mask a mild deficiency.
Deficiencies of factor XII, HMWK, or PK do not result in a bleeding disorder despite prolonging the APTT markedly. This is important since factor XII deficiency has been found to be among the most common causes of unexpected prolongation of APTT.9
CASE You exclude an acquired bleeding disorder based on Mr. K’s history, physical exam, and normal results on his liver panel and a complete blood count. However, a repeat APTT is prolonged and a mixing study with normal plasma corrects the APTT. Further testing demonstrates normal factors IX and XI, but his level of factor VIII is only 10% of the reference range, confirming a diagnosis of hemophilia A.
You advise Mr. K to get vaccinated against hepatitis A and B. You also discuss available treatment options and their indications. Recombinant factor VIII is the treatment of choice for bleeding episodes. Prophylactic treatment is given before surgical procedures or activities that carry a high risk of provoking a bleed.
Factor VIII infusion 3 times a week to prevent hemarthrosis in severely affected patients is gaining acceptance. Desmopressin acetate (DDAVP) is the drug of choice for treatment of patients with mild hemophilia (factor VIII activity >5%).10 Antifibrinolytic agents like ε-aminocaproic acid and tranexamic acid can be used for mucosal oral or dental bleeds. You also make sure Mr. K obtains a medical alert bracelet.
1. Adcock DM, Kressin DC, Marlar RA. Minimum specimen volume requirements for routine coagulation testing: dependence on citrate concentration. Am J Clin Pathol. 1998;109:595–599.
2. Laga AC, Cheves TA, Sweeney JD. The effect of specimen hemolysis on coagulation best results. Am J Clin Pathol. 2006;126:748–755.
3. Kamal A, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82:864–873.
4. McCraw A, Hillarp A, Echenagucia M. Considerations in the laboratory assessment of haemostatsis. Haemophilia. 2010;16(suppl 5):74–78.
5. Centers for Disease Control and Prevention. Hemophilia. Data & Statistics. Updated May 7, 2013. Available at: http://www.cdc.gov/ncbddd/hemophilia/data.html. Accessed May 28, 2013.
6. National Heart, Lung, and Blood Institute. What is hemophilia? July 1, 2011. Available at: http://www.nhlbi.nih.gov/health/health-topics/topics/hemophilia/. Accessed May 28, 2013.
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145–151.
8. Hillman C, Lusher JM. Tests of blood coagulation technical points of clinical relevance. In: Lusher JM, Barhart MI, eds. Acquired Bleeding Disorders in Children. Vol 2. New York, NY: Masson; 1981:107.
9. Halbmayer WM, Haushofer A, Schön R, et al. The prevalence of moderate and severe FXII (Hageman factor) deficiency among the normal population: evaluation of the incidence of FXII deficiency among 300 healthy blood donors. Thromb Haemost. 1994;71:68–72.
10. Hemophilia and von Willebrand’s disease: 2. Management. Association of Hemophilia Clinic Directors of Canada. CMAJ. 1995;153:147–157.
CASE During an office visit, 23-year-old John K tells you that he recently experienced excessive bleeding after a dental extraction. He says that he has no personal or family history of a bleeding disorder and is not taking any medication that might have contributed to the problem.
His lab work shows a normal complete blood count and international normalized ratio (INR). His activated partial thromboplastin time (APTT), however, is 67 seconds (normal=24-37 seconds).
How would you manage his care?
Correct interpretation of abnormal APTT readings requires an understanding of the clinical context in which the test is ordered and the test’s limitations. In cases like Mr. K’s, the top priorities are taking a careful history and performing a thorough physical examination.
Specifically, you need to ask about any personal or family history of spontaneous mucosal bleeding, menorrhagia, hemostatic difficulties with previous surgeries or dental extractions, medication use (including anticoagulants), and alcohol use. Find out whether there is a history of liver disease or malnutrition/malabsorption. Does the patient bruise easily? Ask patients with an elevated APTT about a personal or family history of spontaneous mucosal bleeding, menorrhagia, or hemostatic difficulties with previous surgeries or dental extractions.
As you would expect, such signposts warrant further investigations. And if the personal or family history does suggest a bleeding disorder, the patient should be referred to a hematologist.
CASE Mr. K’s physical examination is unremarkable and his renal and liver panel—including serum albumin—are within the normal range. As noted earlier, he had no history of bleeding before the tooth extraction; his family history is negative for excessive bleeding, as well.
Consider an artifactual cause
It’s important to rule out artifactual causes of an abnormal APTT before undertaking a more detailed investigation. Although not applicable in this case, unfractionated heparin in a central venous or arterial catheter can prolong APTT.
A high hematocrit value will give falsely prolonged APTT. This is due to the increased concentration of citrate relative to the small volume of plasma.1 The lab should be alerted if the hematocrit is high so that the volume of anticoagulant in the collection tube may be adjusted for more reliable results.
Lipemic, hemolyzed, or icteric plasma specimens interfere with the optical system of the instruments and may also give false results.2
Delays and extreme temperatures can also alter results. The specimen should not be delayed for more than 4 hours as factor VIII is labile and longer time will result in an artifactually prolonged APTT.3 (See “APTT: Understanding the test”.) In addition, prolonged exposure to high temperatures may enhance degradation of factors V and VIII, whereas prolonged exposure to cold temperatures may activate factor VII.4
Activated partial thromboplastin time (APTT) measures the integrity of the intrinsic and common pathways of the coagulation cascade. Any deficiency or inhibitor of the clotting factors within the intrinsic or common pathways will result in a prolonged APTT. The factors involved in the intrinsic and common pathways are II (prothrombin), V, VIII, IX, X, XI, XII, and fibrinogen (factor I).
CASE Because there is no physical evidence of liver, renal, or connective tissue disease in Mr. K’s case and artifactual causes are not at work, the next logical step is a mixing study.
Does the mixing study correct the prolonged APTT?
A mixing study with normal plasma will differentiate between a coagulation factor deficiency and the presence of an inhibitor. Correction of the prolonged APTT to the reference range after mixing a sample of the patient’s blood with normal plasma in a 1:1 ratio implies a deficiency of a clotting factor in the intrinsic or common final pathway of the coagulation cascade. The deficiency may involve one or more of the following: factors VIII, IX, XI, or XII; high-molecular-weight kininogen (HMWK); and prekallikrein (PK). A clotting factor assay will identify the deficient factor. A mixing study with normal plasma will differentiate between a coagulation factor deficiency and the presence of an inhibitor.
Congenital or acquired? Assuming that a clotting factor deficiency exists, the next step is to determine the nature of the deficiency in terms of congenital or acquired defects. Congenital causes of factor VIII, IX, and XI deficiency are hemophilia A, B, and C, respectively. Hemophilia affects one in 5000 males born in the United States;5 about 9 out of 10 have hemophilia A.6
Acquired causes of factor deficiencies are liver disease, warfarin use, disseminated intravascular coagulation, and vitamin K deficiency due to malabsorption or malnutrition.
Is an inhibitor at work? The presence of an inhibitor is suggested if the mixing studies do not result in correction of the prolonged APTT to normal range.
If factitious use of heparin or thrombin inhibitors is suspected, thrombin time (TT) and reptilase time help determine if heparin or direct thrombin inhibitor is present. TT is prolonged by both heparin and thrombin inhibitors, but reptilase time is not affected by either of the drugs.
Lupus anticoagulant should be tested if there is no history of treatment with unfractionated heparin or direct thrombin inhibitors such as lepirudin and argatroban. However, antiphospholipid antibodies are estimated to have a prevalence of 1.0% to 5.6% in healthy populations.7 Moreover, lupus anticoagulant is transient in most cases and is most often of no clinical significance. It is therefore important to interpret the lupus anticoagulant in the context of the illness.
If the inhibitor is still a mystery, you will need to test for specific factor inhibitors like factor VIII inhibitor (acquired hemophilia). Inhibitors are alloantibodies, which develop, for example, in patients with hemophilia who are treated with blood products such as fresh frozen plasma (FFP), cryoprecipitate, or factor VIII concentrate.
When an elevated number doesn't mean what you think
There are certain limitations of APTT in identifying the deficient factors because the sensitivity of APTT to identify deficient common pathway factors is low.8 (There may be deficiency of a common pathway factor but a normal APTT). Composition of the partial thromboplastin reagent can result in marked differences in the sensitivity of the test to coagulation factor deficiencies.
In addition, factor VIII levels may give false-negative results in pregnancy and in response to physical stress and trauma, as factor VIII rises markedly in both cases. Thus, it may mask a mild deficiency.
Deficiencies of factor XII, HMWK, or PK do not result in a bleeding disorder despite prolonging the APTT markedly. This is important since factor XII deficiency has been found to be among the most common causes of unexpected prolongation of APTT.9
CASE You exclude an acquired bleeding disorder based on Mr. K’s history, physical exam, and normal results on his liver panel and a complete blood count. However, a repeat APTT is prolonged and a mixing study with normal plasma corrects the APTT. Further testing demonstrates normal factors IX and XI, but his level of factor VIII is only 10% of the reference range, confirming a diagnosis of hemophilia A.
You advise Mr. K to get vaccinated against hepatitis A and B. You also discuss available treatment options and their indications. Recombinant factor VIII is the treatment of choice for bleeding episodes. Prophylactic treatment is given before surgical procedures or activities that carry a high risk of provoking a bleed.
Factor VIII infusion 3 times a week to prevent hemarthrosis in severely affected patients is gaining acceptance. Desmopressin acetate (DDAVP) is the drug of choice for treatment of patients with mild hemophilia (factor VIII activity >5%).10 Antifibrinolytic agents like ε-aminocaproic acid and tranexamic acid can be used for mucosal oral or dental bleeds. You also make sure Mr. K obtains a medical alert bracelet.
CASE During an office visit, 23-year-old John K tells you that he recently experienced excessive bleeding after a dental extraction. He says that he has no personal or family history of a bleeding disorder and is not taking any medication that might have contributed to the problem.
His lab work shows a normal complete blood count and international normalized ratio (INR). His activated partial thromboplastin time (APTT), however, is 67 seconds (normal=24-37 seconds).
How would you manage his care?
Correct interpretation of abnormal APTT readings requires an understanding of the clinical context in which the test is ordered and the test’s limitations. In cases like Mr. K’s, the top priorities are taking a careful history and performing a thorough physical examination.
Specifically, you need to ask about any personal or family history of spontaneous mucosal bleeding, menorrhagia, hemostatic difficulties with previous surgeries or dental extractions, medication use (including anticoagulants), and alcohol use. Find out whether there is a history of liver disease or malnutrition/malabsorption. Does the patient bruise easily? Ask patients with an elevated APTT about a personal or family history of spontaneous mucosal bleeding, menorrhagia, or hemostatic difficulties with previous surgeries or dental extractions.
As you would expect, such signposts warrant further investigations. And if the personal or family history does suggest a bleeding disorder, the patient should be referred to a hematologist.
CASE Mr. K’s physical examination is unremarkable and his renal and liver panel—including serum albumin—are within the normal range. As noted earlier, he had no history of bleeding before the tooth extraction; his family history is negative for excessive bleeding, as well.
Consider an artifactual cause
It’s important to rule out artifactual causes of an abnormal APTT before undertaking a more detailed investigation. Although not applicable in this case, unfractionated heparin in a central venous or arterial catheter can prolong APTT.
A high hematocrit value will give falsely prolonged APTT. This is due to the increased concentration of citrate relative to the small volume of plasma.1 The lab should be alerted if the hematocrit is high so that the volume of anticoagulant in the collection tube may be adjusted for more reliable results.
Lipemic, hemolyzed, or icteric plasma specimens interfere with the optical system of the instruments and may also give false results.2
Delays and extreme temperatures can also alter results. The specimen should not be delayed for more than 4 hours as factor VIII is labile and longer time will result in an artifactually prolonged APTT.3 (See “APTT: Understanding the test”.) In addition, prolonged exposure to high temperatures may enhance degradation of factors V and VIII, whereas prolonged exposure to cold temperatures may activate factor VII.4
Activated partial thromboplastin time (APTT) measures the integrity of the intrinsic and common pathways of the coagulation cascade. Any deficiency or inhibitor of the clotting factors within the intrinsic or common pathways will result in a prolonged APTT. The factors involved in the intrinsic and common pathways are II (prothrombin), V, VIII, IX, X, XI, XII, and fibrinogen (factor I).
CASE Because there is no physical evidence of liver, renal, or connective tissue disease in Mr. K’s case and artifactual causes are not at work, the next logical step is a mixing study.
Does the mixing study correct the prolonged APTT?
A mixing study with normal plasma will differentiate between a coagulation factor deficiency and the presence of an inhibitor. Correction of the prolonged APTT to the reference range after mixing a sample of the patient’s blood with normal plasma in a 1:1 ratio implies a deficiency of a clotting factor in the intrinsic or common final pathway of the coagulation cascade. The deficiency may involve one or more of the following: factors VIII, IX, XI, or XII; high-molecular-weight kininogen (HMWK); and prekallikrein (PK). A clotting factor assay will identify the deficient factor. A mixing study with normal plasma will differentiate between a coagulation factor deficiency and the presence of an inhibitor.
Congenital or acquired? Assuming that a clotting factor deficiency exists, the next step is to determine the nature of the deficiency in terms of congenital or acquired defects. Congenital causes of factor VIII, IX, and XI deficiency are hemophilia A, B, and C, respectively. Hemophilia affects one in 5000 males born in the United States;5 about 9 out of 10 have hemophilia A.6
Acquired causes of factor deficiencies are liver disease, warfarin use, disseminated intravascular coagulation, and vitamin K deficiency due to malabsorption or malnutrition.
Is an inhibitor at work? The presence of an inhibitor is suggested if the mixing studies do not result in correction of the prolonged APTT to normal range.
If factitious use of heparin or thrombin inhibitors is suspected, thrombin time (TT) and reptilase time help determine if heparin or direct thrombin inhibitor is present. TT is prolonged by both heparin and thrombin inhibitors, but reptilase time is not affected by either of the drugs.
Lupus anticoagulant should be tested if there is no history of treatment with unfractionated heparin or direct thrombin inhibitors such as lepirudin and argatroban. However, antiphospholipid antibodies are estimated to have a prevalence of 1.0% to 5.6% in healthy populations.7 Moreover, lupus anticoagulant is transient in most cases and is most often of no clinical significance. It is therefore important to interpret the lupus anticoagulant in the context of the illness.
If the inhibitor is still a mystery, you will need to test for specific factor inhibitors like factor VIII inhibitor (acquired hemophilia). Inhibitors are alloantibodies, which develop, for example, in patients with hemophilia who are treated with blood products such as fresh frozen plasma (FFP), cryoprecipitate, or factor VIII concentrate.
When an elevated number doesn't mean what you think
There are certain limitations of APTT in identifying the deficient factors because the sensitivity of APTT to identify deficient common pathway factors is low.8 (There may be deficiency of a common pathway factor but a normal APTT). Composition of the partial thromboplastin reagent can result in marked differences in the sensitivity of the test to coagulation factor deficiencies.
In addition, factor VIII levels may give false-negative results in pregnancy and in response to physical stress and trauma, as factor VIII rises markedly in both cases. Thus, it may mask a mild deficiency.
Deficiencies of factor XII, HMWK, or PK do not result in a bleeding disorder despite prolonging the APTT markedly. This is important since factor XII deficiency has been found to be among the most common causes of unexpected prolongation of APTT.9
CASE You exclude an acquired bleeding disorder based on Mr. K’s history, physical exam, and normal results on his liver panel and a complete blood count. However, a repeat APTT is prolonged and a mixing study with normal plasma corrects the APTT. Further testing demonstrates normal factors IX and XI, but his level of factor VIII is only 10% of the reference range, confirming a diagnosis of hemophilia A.
You advise Mr. K to get vaccinated against hepatitis A and B. You also discuss available treatment options and their indications. Recombinant factor VIII is the treatment of choice for bleeding episodes. Prophylactic treatment is given before surgical procedures or activities that carry a high risk of provoking a bleed.
Factor VIII infusion 3 times a week to prevent hemarthrosis in severely affected patients is gaining acceptance. Desmopressin acetate (DDAVP) is the drug of choice for treatment of patients with mild hemophilia (factor VIII activity >5%).10 Antifibrinolytic agents like ε-aminocaproic acid and tranexamic acid can be used for mucosal oral or dental bleeds. You also make sure Mr. K obtains a medical alert bracelet.
1. Adcock DM, Kressin DC, Marlar RA. Minimum specimen volume requirements for routine coagulation testing: dependence on citrate concentration. Am J Clin Pathol. 1998;109:595–599.
2. Laga AC, Cheves TA, Sweeney JD. The effect of specimen hemolysis on coagulation best results. Am J Clin Pathol. 2006;126:748–755.
3. Kamal A, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82:864–873.
4. McCraw A, Hillarp A, Echenagucia M. Considerations in the laboratory assessment of haemostatsis. Haemophilia. 2010;16(suppl 5):74–78.
5. Centers for Disease Control and Prevention. Hemophilia. Data & Statistics. Updated May 7, 2013. Available at: http://www.cdc.gov/ncbddd/hemophilia/data.html. Accessed May 28, 2013.
6. National Heart, Lung, and Blood Institute. What is hemophilia? July 1, 2011. Available at: http://www.nhlbi.nih.gov/health/health-topics/topics/hemophilia/. Accessed May 28, 2013.
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145–151.
8. Hillman C, Lusher JM. Tests of blood coagulation technical points of clinical relevance. In: Lusher JM, Barhart MI, eds. Acquired Bleeding Disorders in Children. Vol 2. New York, NY: Masson; 1981:107.
9. Halbmayer WM, Haushofer A, Schön R, et al. The prevalence of moderate and severe FXII (Hageman factor) deficiency among the normal population: evaluation of the incidence of FXII deficiency among 300 healthy blood donors. Thromb Haemost. 1994;71:68–72.
10. Hemophilia and von Willebrand’s disease: 2. Management. Association of Hemophilia Clinic Directors of Canada. CMAJ. 1995;153:147–157.
1. Adcock DM, Kressin DC, Marlar RA. Minimum specimen volume requirements for routine coagulation testing: dependence on citrate concentration. Am J Clin Pathol. 1998;109:595–599.
2. Laga AC, Cheves TA, Sweeney JD. The effect of specimen hemolysis on coagulation best results. Am J Clin Pathol. 2006;126:748–755.
3. Kamal A, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82:864–873.
4. McCraw A, Hillarp A, Echenagucia M. Considerations in the laboratory assessment of haemostatsis. Haemophilia. 2010;16(suppl 5):74–78.
5. Centers for Disease Control and Prevention. Hemophilia. Data & Statistics. Updated May 7, 2013. Available at: http://www.cdc.gov/ncbddd/hemophilia/data.html. Accessed May 28, 2013.
6. National Heart, Lung, and Blood Institute. What is hemophilia? July 1, 2011. Available at: http://www.nhlbi.nih.gov/health/health-topics/topics/hemophilia/. Accessed May 28, 2013.
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145–151.
8. Hillman C, Lusher JM. Tests of blood coagulation technical points of clinical relevance. In: Lusher JM, Barhart MI, eds. Acquired Bleeding Disorders in Children. Vol 2. New York, NY: Masson; 1981:107.
9. Halbmayer WM, Haushofer A, Schön R, et al. The prevalence of moderate and severe FXII (Hageman factor) deficiency among the normal population: evaluation of the incidence of FXII deficiency among 300 healthy blood donors. Thromb Haemost. 1994;71:68–72.
10. Hemophilia and von Willebrand’s disease: 2. Management. Association of Hemophilia Clinic Directors of Canada. CMAJ. 1995;153:147–157.
A differential guide to 5 eye complaints
- Provide an urgent ophthalmology referral for any patient with a sudden decrease in visual acuity. C
- Record bilateral pupil size as part of a comprehensive eye exam, and provide an urgent referral for a patient whose pupils are of unequal size. C
- Involve an ophthalmologist or other specialist in the management of eye conditions caused by systemic diseases such as stroke or giant cell arteritis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Knowing how to respond when patients present with problems involving the eye is crucial for family physicians. Yet it is often difficult to know whether to treat or refer and which signs and symptoms are indicative of an ophthalmologic emergency with the potential to cause loss of sight.
Categorizing ophthalmologic conditions based on patients’ chief complaints, we have found, can help to narrow the differential diagnosis and home in on emergent signs and symptoms. Thus, we’ve used that approach in this review.
In the pages that follow, common complaints like “I can’t see,” “I’m seeing things,” and “My eye hurts” are used to highlight disorders—both benign and emergent—associated with each. You’ll also find an at-a-glance table listing the differential diagnosis for each presentation, and a mnemonic to guide you through the elements of a comprehensive eye exam.
1) "I can't see"
Patients may use words like “cloudy vision,“ “a veil over my eyes,” or “fuzziness” to describe diminished vision. Some will report black areas within their visual field; others will have a loss of peripheral vision or total vision loss in one eye, or possibly even both. Some causes of vision problems, such as cataracts, are not emergencies. Causes of more severe (but painless) vision loss include central retinal artery occlusion (CRAO) or vein occlusion (CRVO), giant cell arteritis (GCA), stroke or transient ischemic attack (TIA), nonarteritic anterior ischemic optic neuropathy (NAION), and nonorganic (functional) vision loss (TABLE).1-11
When the cause is ischemic
Patients with CRAO experience acute loss of vision in one eye, usually occurring within seconds to minutes. Most patients with CRVO will have a similar presentation, depending on the presence or absence of ischemia and involvement of the macula. Those with branch retinal vein occlusion may have no vision loss
at all.1-3
Risk factors for CRAO include cardiovascular disease, hypertension, diabetes, and other disorders associated with systemic inflammation. In patients older than 60 years, it is also important to consider GCA, which we’ll talk more about shortly, as a cause of CRAO.
In patients with CRAO, an eye exam will show profoundly decreased visual acuity, and the swinging light test (see “Use this mnemonic to ensure a comprehensive eye exam” on page 348) will reveal a relative afferent pupillary defect (RAPD). Fundoscopy is diagnostic, revealing a pale retina due to decreased blood flow.4 Emergent referral to ophthalmology is indicated to establish a definitive diagnosis and initiate treatment based on the cause of the occlusion. If emergency care is not immediately available, massaging the eye globe through closed lids, then releasing, in 10- to 15-second cycles, may be helpful.5
Use this mnemonic to ensure a comprehensive eye exam. In a potential emergency, an eye exam needs to be quick and thorough. To ensure that all the key elements are included, use the mnemonic VVEEPP (Visual acuity, Visual fields, External exam, Extraocular movements, Pupillary exam, and Pressure) as a guide.1Visual acuity. Check distance vision, with the patient wearing his or her corrective lenses, if possible. If not, substitute pinhole testing, which can function like corrective lenses and eliminate refractive error.2
Begin with distance charts. If the patient can’t see the charts, hold up fingers and ask whether the patient is able to count them. If not, try hand motion—or, if the patient can’t see that, try testing the patient’s ability to see light. Swing a light between the eyes. Paradoxical dilation of the affected eye when directly exposed to the light is evidence of a relative afferent pupillary defect (RAPD).2
Visual fields. Examine visual fields by using the standard confrontation technique—ie, asking the patient to cover one eye at a time while you move your hand in and out of his or her visual field.
External exam and extraocular movements. Use a penlight to inspect the eyelids, conjunctiva, sclera, cornea, and anterior chamber of the eye and to assess extraocular movements.1
Pupillary examination and pressure. Observe bilateral pupil size and swing a light between the eyes to test pupillary response to direct and consensual light (and to rule out an RAPD).2 If available, measure eye pressure, as well.1
Fundoscopy should be performed to complete the examination—along with a slit lamp evaluation, if possible.1
Risk factors for CRVO include age older than 65 and a number of chronic conditions. One analysis attributed 48% of cases to hypertension, 20% to hyperlipidemia, and 5% to diabetes.3 Fundoscopy will reveal dilated veins, retinal hemorrhages, and cotton wool spots, which look like puffy white patches on the retina.6
As with CRAO, an urgent ophthalmology referral is critical to establish the diagnosis and develop a treatment plan. Outcomes are poor in patients with visual acuity of 20/200 or worse at the time of diagnosis.7,8
GCA. Patients with GCA may develop arteritic ischemic optic neuropathy, resulting in vision loss in one or both eyes. Risk factors for GCA include age (>50 years), polymyalgia rheumatica, Caucasian race, and female sex. Systemic symptoms include fever, muscle aches, headache, jaw claudication, and scalp pain.6
The swinging light test will reveal an RAPD;1,2 fundoscopy findings typically include disk edema and disk hemorrhages, or a pale retina if GCA is associated with CRAO.6 Testing, including an erythrocyte sedimentation rate and a C-reactive protein, will provide supportive evidence, and biopsy of the temporal artery will confirm the diagnosis.4
Blindness from GCA is often profound. Bilateral disease is treated immediately with high-dose corticosteroids; when just one eye is affected, high-dose steroids should also be started right away to prevent vision loss in the other eye. Whenever GCA is suspected, initiate treatment and provide an urgent referral to an ophthalmologist for biopsy and further treatment.6
Strokes and TIAs that affect vision may be a result of ischemia of the visual cortex, or the eye itself. Visual cortex ischemia will present as a homonymous visual field cut between the eyes; TIAs that affect only one eye (known as amaurosis fugax) are associated with ischemia to the optic nerve or retina.
Patients with amaurosis fugax will experience unilateral loss of vision that extends like a dark shade from the top or bottom periphery to the center of vision. When a TIA is the cause, vision will return to normal within minutes. The underlying pathology is usually carotid artery atherosclerosis. If left untreated, evidence suggests that 30% to 50% of patients will have a stroke within a month.9
Visual acuity may or may not be decreased, depending on whether the ischemia involves the macula. Symptoms suggestive of amaurosis fugax should prompt an urgent ophthalmology referral, while patients with persistent vision loss or visual field deficit require urgent referral to a stroke treatment center.9
NAION is also associated with acute monocular vision loss, particularly in older patients.10 Visual acuity will be markedly decreased, and fundoscopic exam will show a swollen and hemorrhagic optic disc. The vision loss can be profound, and is usually permanent; neither medical nor surgical treatment has been shown to improve outcomes.10
When the cause is functional
Functional (nonorganic) visual disturbances should also be considered when sudden blindness is reported. Nonorganic vision loss has a number of causes, and patients present with a range of chief complaints, making diagnosis complex. Because some patients will have organic disease with a component of functional vision loss, it is best to refer individuals whom you suspect of having functional vision loss to an ophthalmologist for testing and a definitive diagnosis. Treatment includes psychological support and reassurance that vision will return.11
2) "I'm seeing things"
Patients with this problem often use words like “flashes,” “floaters” “worms,” or “lights,” and various colors and unusual shapes to describe what they see. When this phenomenon is accompanied by decreased visual acuity, emergent or urgent referral is required. Normal vision in a patient who reports “seeing things” calls for careful consideration of the etiology, and referral if the diagnosis is uncertain or the suspected disorder is sight-threatening (TABLE).4,12-14 Migraine and psychiatric disorders should be considered if suggested by history. (Patients with ocular migraine—which may or may not be associated with a headache—may also report seeing light patterns off to one side, typically lasting 20-45 minutes.)
Vitreous or retinal detachment
Patients with vitreous detachment, which is far more common and less serious than retinal detachment, report seeing new floaters or peripheral flashing lights in one eye. Risks for vitreous detachment include myopia, older age, eye trauma, and previous eye surgery.4 Physical examination and visual acuity will be normal unless there is an accompanying retinal detachment.12
Patients who have decreased visual acuity or a visual field defect, or who describe a “curtain of darkness” are at risk for retinal detachment (shown above) and require a same-day referral.
A full ophthalmologic evaluation is indicated to detect or rule out a retinal detachment or tear—which has been found to co-occur with acute vitreous detachment in 14% of cases.13 Those who present with decreased visual acuity or a visual field defect or describe a “curtain of darkness” are at risk for retinal detachment and require a same-day referral.13
Like patients with vitreous detachment, those with a retinal detachment will report new floaters or peripheral flashing lights.12 The presence of vitreous hemorrhage or pigment, which can be seen in a slit lamp exam, is associated with increased risk for retinal detachment, as is a subjective report of vision loss.13
When retinal detachment is suspected, immediate referral to an ophthalmologist is needed.13 Reattachment surgery has good outcomes, especially if it is performed prior to macular involvement or within the first 3 days of macular detachment.14
3) "My eye hurts and is red"
Patients with painful, red eyes are at risk for a variety of sight-threatening conditions, iritis (anterior uveitis), keratitis, and acute angle closure glaucoma, as well as eye trauma, among them (TABLE).1,2,4,12,15-27 Decreased visual acuity in a patient with painful, red eyes warrants an urgent or emergent ophthalmologic referral.
When to suspect iritis
Patients with iritis will complain of vision loss, pain, photophobia, and redness. An eye exam will reveal injection of the conjunctiva around the cornea. Visual acuity is often decreased. Pupillary reaction may be sluggish, and the pupil may be smaller or larger than the other eye,4 but a normal pupil size does not exclude iritis in a patient with unilateral eye pain and ciliary injection.15
Iritis is often idiopathic, but risk factors include chronic inflammatory conditions such as ankylosing spondylitis, ulcerative colitis, and Crohn’s disease.16
Treatment with topical steroids is recommended.16 Urgent referral for long-term management of iritis is needed.17
Keratitis has varied causes
Patients with keratitis present with eye pain or foreign body sensation, redness, blurred vision, and photophobia. Examination of the eye will show injection of the conjunctiva surrounding the cornea, and possible corneal defects or opacities; visual acuity may be normal or decreased. The cause varies, based on whether keratitis is bacterial, viral, or noninfectious.
Risk factors for bacterial keratitis include extended wear of contact lenses, eye trauma, eye surgery, and systemic disease such as diabetes mellitus, while viral keratitis often follows a case of viral conjunctivitis and herpes simplex keratitis often involves reactivation of the virus. Causes of noninfectious keratitis include flash burns, dry eye or blepharitis, snow blindness, and sunburn.18
Treatment with topical antibiotics is effective for bacterial keratitis, but follow-up referral is needed because the infection could lead to loss of sight.19 Herpes simplex keratitis, which may appear as a mild corneal ulcer (a slit lamp examination will show the classic branching dendritic lesion), can be managed with topical antiviral medications,20 but here, too, an ophthalmologic referral is recommended to look for deeper corneal infiltrates that could lead to vision loss.20,21 Topical numbing medications should not be prescribed for patients with eye problems, as their extended use can lead to infection, corneal thinning, or even perforation of the cornea.22
Blurred vision, pain suggest acute angle closure glaucoma
Patients with acute angle closure glaucoma present with blurred vision, deep eye pain or brow ache, and frequently, nausea and vomiting.23 Some patients report seeing halos around lights, as well.
Risk factors for acute angle closure glaucoma include older age, Asian descent, farsightedness, family history, and female sex. Attacks are commonly idiopathic, but some are associated with routine pupillary dilation during eye exams.24
On examination, the cornea will be cloudy due to edema and the pupil will be mid-dilated and fixed.12 Typically, intraocular pressure in the affected eye will be elevated, an indication that the nausea and vomiting are associated with this disorder rather than a gastrointestinal condition.23 Emergent referral is needed to preserve vision.25
Eye trauma: What you’ll see, when to act Hyphema. In patients with a hyphema—typically the result of eye trauma—you’ll usually see a meniscus of blood in front of the iris in the anterior chamber. If the patient was supine before the evaluation, however, you’ll see red discoloration of the iris. Hyphemas can be a threat to vision, mostly due to potential elevated pressure. Because they are often associated with more extensive ocular injuries that are not always immediately evident, urgent referral is required.26
Hyphema—What you'll see
Courtesy of: Eye Teachers of America Foundation
More significant blunt trauma can cause globe rupture, resulting in both eye pain and loss of vision. Flooding the eye with fluorescein before examining it may make it possible to see a dark or green stream from the ruptured globe.
If you suspect a globe rupture, immediately stop your exam. Do not touch the eye. Instead, protect the eye—with a metal or plastic shield and an antiemetic to prevent pressure and Valsalva strain—and obtain an emergency ophthalmology consult.2,4
Chemical burns. Patients who incur chemical burns of the eye should irrigate the injured eye right away. The physical exam should be delayed until irrigation reaches an endpoint of neutral pH, as measured with Nitrazine paper.4,27 Alkali burns are particularly destructive to the eye and require longer irrigation.27
An emergent ophthalmology referral is needed for all alkali burns of the eye, as well as for any patient whose visual acuity does not return to baseline after irrigation. Slit lamp examination showing a deep corneal injury is also reason for an ophthalmology referral.1,2
4) "My eye is red" (but pain free)
When a patient seeks care for a red eye that’s not painful, the history and physical will help you determine whether the condition is benign or emergent. Orbital cellulitis, which we’ll discuss shortly, is the most dangerous condition related to this presentation (TABLE),4,9,28-32 requiring inpatient management and ophthalmology referral.
Conjunctivitis. The entire conjunctiva will be red and discharge will be present, but visual acuity will be normal.
Conjunctivitis can be viral or bacterial; office-based testing is now available for viral conjunctivitis caused by adenovirus. Treating bacterial conjunctivitis with antibiotic drops or ointment speeds recovery.29 When the cause is viral, standard treatment is supportive, with emphasis on preventing viral spread. Some antiviral preparations are being investigated as potential treatments for adenovirus conjunctivitis.28
Periorbital and orbital cellulitis. Redness surrounding the eye can be caused by preseptal (commonly called periorbital) or orbital cellulitis. The clinical presentation of these 2 conditions is similar, including redness, lid edema, and tenderness. However, periorbital cellulitis is more commonly seen after minor trauma to the eyelid skin or related to a stye or chalazion. Orbital cellulitis, which is considerably more serious, is typically associated with sinus disease or abscess.30
Patients with orbital cellulitis will present with restricted eye movements, decreased visual acuity, proptosis, and possibly an RAPD. These patients will often have pain as well. A fine-cut computed tomography of the orbits aids in diagnosis.31
Care for each is different. Oral antibiotics are usually sufficient for patients with periorbital cellulitis, but for orbital cellulitis, a same-day ophthalmology referral and hospitalization for treatment with parenteral antibiotics is required.9,32
Subconjunctival hemorrhage—dramatic but harmless
While dramatic in appearance, subconjunctival hemorrhage generally does not affect vision. It may be the result of trauma to the globe, but can also occur spontaneously.
On physical exam, you’ll see bleeding into the conjunctiva that stops at the edge of the cornea. Visual acuity will be normal, as will the remainder of the eye examination. Abnormal vision, pain, or significant or recurrent bleeding should prompt a search for an alternative diagnosis. No treatment is needed for a simple subconjunctival hemorrhage.4
5) "My eye hurts"
Patients complaining of eye pain with or without vision changes—and without redness—usually have a medical history that leads to the diagnosis (TABLE).1,2,4,33-38 Physical exam findings are compatible with the history.
Optic neuritis. Patients with optic neuritis have acute to subacute vision loss, usually in one eye but sometimes bilaterally, lasting hours to days. Optic neuritis is more common in women and in those ages 15 to 45 years, with an incidence of 5 in 100,000 among Caucasians.33 Pain with eye movement is present in more than 90% of adults with optic neuritis,34 and is also common in children.35
In addition to vision loss, patients will report decreased detection of light and color,6 and examination will reveal an RAPD.1,2 Vision returns without treatment to the same extent as with treatment, but treatment will speed recovery.36 Patients with optic neuritis require an urgent referral to an ophthalmologist or neurologist to evaluate for multiple sclerosis, which develops in about 30% of those with optic neuritis.4,33
Corneal abrasion. Pain, localized to the surface of the eye, will be the primary complaint of patients with a corneal abrasion, who may or may not have loss of vision. Larger and deeper abrasions are extremely painful, while smaller corneal abrasions may be experienced as a foreign body sensation. The typical patient with a corneal abrasion is likely to have had trauma to the eye.37
Fluorescein is used to examine the patient with a suspected abrasion to highlight the epithelial defect.1 Visual acuity needs to be tested, and checked using a pinhole if it is below baseline.37 Treatment protocols range from artificial tears to antibiotic drops or ointments. Topical steroids should be given to patients only by an ophthalmologist.4
Is patching necessary? In a systematic review comparing outcomes based on the use of patching vs not patching on the first day of injury, patients who were not given patches fared the same or better than those whose eyes were patched, both in terms of healing time and pain relief. Primary care physicians can treat most corneal abrasions, and symptoms typically resolve in 2 days.38
1. Wright JL, Wightman JM. Red and painful eye. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, Pa: Mosby Elsevier; 2009:chap 32.
2. Knoop KJ, Dennis WR, Hedges JR. Ophthalmologic procedures. In: Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 5th ed. Philadelphia, Pa: Saunders Elsevier;2009:chap 63.
3. Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56:281-299.
4. Sharma R, Brunette DD. Ophthalmology. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, Pa: Mosby Elsevier; 2009:chap 69.
5. Cugati S, Varma DD, Chen CS, et al. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15:63-77.
6. Matson M, Fujimoto L. Bilateral arteritic anterior ischemic optic neuropathy. Optometry. 2011;82:622-631.
7. McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1113-1123.
8. Wong TY, Scott IU. Retinal-vein occlusion. N Engl J Med. 2010;363:2135-2144.
9. Crouch ER, Crouch ER, Grant T. Ophthalmology. In: Rakel RE, ed. Textbook of Family Medicine. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 41.
10. Dickersin K, Manheimer E, Li T. Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev. 2012;(1):CD001538.
11. Thurtell MJ, Tomsak RL. Neuro-ophthalmology: afferent visual system. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Los Angeles, Calif: Saunders Elsevier; 2012:chap 36.
12. Yanoff M, Cameron D. Diseases of the visual system. In: Goldman L, Schafer AI, eds. Cecil Medicine. 24th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 431.
13. Hollands H, Johnson D, Brox A, et al. Acute-onset floaters and flashes: is this patient at risk for retinal detachment? JAMA. 2009;302:2243-2249.
14. D’Amico DJ. Primary retinal detachment. N Engl J Med. 2008;359:2346-2354.
15. Hunsley T, Lee C. Does a normal-shaped pupil exclude the diagnosis of iritis? Best evidence topic reports. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. Emerg Med J. 2006;23:872-877.
16. Islam N, Pavesio C. Uveitis (acute anterior). Clin Evid. 2010;4:705.
17. Grunwald L, Newcomb CW, Daniel E, et al. Risk of relapse in primary acute anterior uveitis. Ophthalmology. 2011;118:1911-1915.
18. Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20:129-141.
19. Suwan-Apichon O, Reyes JM, Herretes S, et al. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst Rev. 2007;(4):CD005430.
20. Morris D, Latham E. Ulcers in the eye. J Emerg Med. 2012;42:62-64.
21. Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2010;(12):CD002898.
22. Yagci A, Bozkurt B, Egrilmez S, et al. Topical anesthetic abuse keratopathy: a commonly overlooked health care problem.Cornea. 2011;30:571-575.
23. Cholongitas E, Pipili C, Dasenaki M. Acute angle closure glaucoma presented with nausea and epigastric pain. Dig Dis Sci. 2008;53:1430-1431.
24. White J. Diagnosis and management of acute angle-closure glaucoma. Emerg Nurse. 2011;19:27.
25. Lama DSC, Thama CCY, Laia JSM, et al. Current approaches to the management of acute primary angle closure. Curr Opin Ophthalmol. 2007;18:146-151.
26. Gharaibeh A, Savage HI, Scherer RW, et al. Medical interventions for traumatic hyphema. Cochrane Database Syst Rev. 2011;(1):CD005431.
27. Connor AJ, Severn P. Use of a control test to aid pH assessment of chemical eye injuries. Emerg Med J. 2009;26:811-812.
28. Sambursky R, Trattler W, Tauber S, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013;131:17-22.
29. Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006;(2):CD001211.
30. Papier A, Tuttle DJ, Mahara TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
31. Howe L, Jones NS. Guidelines for the management of periorbital cellulitis/abscess. Clin Otolaryngol. 2004;29:725-728.
32. Mahalingam-Dhingra A, Lander L, Preciado DA, et al. Orbital and periorbital infections: a national perspective. Arch Otolaryngol Head Neck Surg. 2011;137:769-773.
33. Germann CA, Baumann MR, Hamzavi S. Ophthalmic diagnoses in the ED: optic neuritis. Am J Emerg Med. 2007;25:834-837.
34. Balcer LJ. Optic neuritis. N Engl J Med. 2006;354:1273-1280.
35. Olitsky SE, Hug D, Plummer L, et al. Abnormalities of the optic nerve. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds.Nelson Textbook of Pediatrics. 19th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 623.
36. Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2012;(4):CD001430.
37. Aslam SA, Sheth HG, Vaughan AJ. Emergency management of corneal injuries. Injury. 2007;38:594-597.
38. Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006;(2):CD004764.
- Provide an urgent ophthalmology referral for any patient with a sudden decrease in visual acuity. C
- Record bilateral pupil size as part of a comprehensive eye exam, and provide an urgent referral for a patient whose pupils are of unequal size. C
- Involve an ophthalmologist or other specialist in the management of eye conditions caused by systemic diseases such as stroke or giant cell arteritis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Knowing how to respond when patients present with problems involving the eye is crucial for family physicians. Yet it is often difficult to know whether to treat or refer and which signs and symptoms are indicative of an ophthalmologic emergency with the potential to cause loss of sight.
Categorizing ophthalmologic conditions based on patients’ chief complaints, we have found, can help to narrow the differential diagnosis and home in on emergent signs and symptoms. Thus, we’ve used that approach in this review.
In the pages that follow, common complaints like “I can’t see,” “I’m seeing things,” and “My eye hurts” are used to highlight disorders—both benign and emergent—associated with each. You’ll also find an at-a-glance table listing the differential diagnosis for each presentation, and a mnemonic to guide you through the elements of a comprehensive eye exam.
1) "I can't see"
Patients may use words like “cloudy vision,“ “a veil over my eyes,” or “fuzziness” to describe diminished vision. Some will report black areas within their visual field; others will have a loss of peripheral vision or total vision loss in one eye, or possibly even both. Some causes of vision problems, such as cataracts, are not emergencies. Causes of more severe (but painless) vision loss include central retinal artery occlusion (CRAO) or vein occlusion (CRVO), giant cell arteritis (GCA), stroke or transient ischemic attack (TIA), nonarteritic anterior ischemic optic neuropathy (NAION), and nonorganic (functional) vision loss (TABLE).1-11
When the cause is ischemic
Patients with CRAO experience acute loss of vision in one eye, usually occurring within seconds to minutes. Most patients with CRVO will have a similar presentation, depending on the presence or absence of ischemia and involvement of the macula. Those with branch retinal vein occlusion may have no vision loss
at all.1-3
Risk factors for CRAO include cardiovascular disease, hypertension, diabetes, and other disorders associated with systemic inflammation. In patients older than 60 years, it is also important to consider GCA, which we’ll talk more about shortly, as a cause of CRAO.
In patients with CRAO, an eye exam will show profoundly decreased visual acuity, and the swinging light test (see “Use this mnemonic to ensure a comprehensive eye exam” on page 348) will reveal a relative afferent pupillary defect (RAPD). Fundoscopy is diagnostic, revealing a pale retina due to decreased blood flow.4 Emergent referral to ophthalmology is indicated to establish a definitive diagnosis and initiate treatment based on the cause of the occlusion. If emergency care is not immediately available, massaging the eye globe through closed lids, then releasing, in 10- to 15-second cycles, may be helpful.5
Use this mnemonic to ensure a comprehensive eye exam. In a potential emergency, an eye exam needs to be quick and thorough. To ensure that all the key elements are included, use the mnemonic VVEEPP (Visual acuity, Visual fields, External exam, Extraocular movements, Pupillary exam, and Pressure) as a guide.1Visual acuity. Check distance vision, with the patient wearing his or her corrective lenses, if possible. If not, substitute pinhole testing, which can function like corrective lenses and eliminate refractive error.2
Begin with distance charts. If the patient can’t see the charts, hold up fingers and ask whether the patient is able to count them. If not, try hand motion—or, if the patient can’t see that, try testing the patient’s ability to see light. Swing a light between the eyes. Paradoxical dilation of the affected eye when directly exposed to the light is evidence of a relative afferent pupillary defect (RAPD).2
Visual fields. Examine visual fields by using the standard confrontation technique—ie, asking the patient to cover one eye at a time while you move your hand in and out of his or her visual field.
External exam and extraocular movements. Use a penlight to inspect the eyelids, conjunctiva, sclera, cornea, and anterior chamber of the eye and to assess extraocular movements.1
Pupillary examination and pressure. Observe bilateral pupil size and swing a light between the eyes to test pupillary response to direct and consensual light (and to rule out an RAPD).2 If available, measure eye pressure, as well.1
Fundoscopy should be performed to complete the examination—along with a slit lamp evaluation, if possible.1
Risk factors for CRVO include age older than 65 and a number of chronic conditions. One analysis attributed 48% of cases to hypertension, 20% to hyperlipidemia, and 5% to diabetes.3 Fundoscopy will reveal dilated veins, retinal hemorrhages, and cotton wool spots, which look like puffy white patches on the retina.6
As with CRAO, an urgent ophthalmology referral is critical to establish the diagnosis and develop a treatment plan. Outcomes are poor in patients with visual acuity of 20/200 or worse at the time of diagnosis.7,8
GCA. Patients with GCA may develop arteritic ischemic optic neuropathy, resulting in vision loss in one or both eyes. Risk factors for GCA include age (>50 years), polymyalgia rheumatica, Caucasian race, and female sex. Systemic symptoms include fever, muscle aches, headache, jaw claudication, and scalp pain.6
The swinging light test will reveal an RAPD;1,2 fundoscopy findings typically include disk edema and disk hemorrhages, or a pale retina if GCA is associated with CRAO.6 Testing, including an erythrocyte sedimentation rate and a C-reactive protein, will provide supportive evidence, and biopsy of the temporal artery will confirm the diagnosis.4
Blindness from GCA is often profound. Bilateral disease is treated immediately with high-dose corticosteroids; when just one eye is affected, high-dose steroids should also be started right away to prevent vision loss in the other eye. Whenever GCA is suspected, initiate treatment and provide an urgent referral to an ophthalmologist for biopsy and further treatment.6
Strokes and TIAs that affect vision may be a result of ischemia of the visual cortex, or the eye itself. Visual cortex ischemia will present as a homonymous visual field cut between the eyes; TIAs that affect only one eye (known as amaurosis fugax) are associated with ischemia to the optic nerve or retina.
Patients with amaurosis fugax will experience unilateral loss of vision that extends like a dark shade from the top or bottom periphery to the center of vision. When a TIA is the cause, vision will return to normal within minutes. The underlying pathology is usually carotid artery atherosclerosis. If left untreated, evidence suggests that 30% to 50% of patients will have a stroke within a month.9
Visual acuity may or may not be decreased, depending on whether the ischemia involves the macula. Symptoms suggestive of amaurosis fugax should prompt an urgent ophthalmology referral, while patients with persistent vision loss or visual field deficit require urgent referral to a stroke treatment center.9
NAION is also associated with acute monocular vision loss, particularly in older patients.10 Visual acuity will be markedly decreased, and fundoscopic exam will show a swollen and hemorrhagic optic disc. The vision loss can be profound, and is usually permanent; neither medical nor surgical treatment has been shown to improve outcomes.10
When the cause is functional
Functional (nonorganic) visual disturbances should also be considered when sudden blindness is reported. Nonorganic vision loss has a number of causes, and patients present with a range of chief complaints, making diagnosis complex. Because some patients will have organic disease with a component of functional vision loss, it is best to refer individuals whom you suspect of having functional vision loss to an ophthalmologist for testing and a definitive diagnosis. Treatment includes psychological support and reassurance that vision will return.11
2) "I'm seeing things"
Patients with this problem often use words like “flashes,” “floaters” “worms,” or “lights,” and various colors and unusual shapes to describe what they see. When this phenomenon is accompanied by decreased visual acuity, emergent or urgent referral is required. Normal vision in a patient who reports “seeing things” calls for careful consideration of the etiology, and referral if the diagnosis is uncertain or the suspected disorder is sight-threatening (TABLE).4,12-14 Migraine and psychiatric disorders should be considered if suggested by history. (Patients with ocular migraine—which may or may not be associated with a headache—may also report seeing light patterns off to one side, typically lasting 20-45 minutes.)
Vitreous or retinal detachment
Patients with vitreous detachment, which is far more common and less serious than retinal detachment, report seeing new floaters or peripheral flashing lights in one eye. Risks for vitreous detachment include myopia, older age, eye trauma, and previous eye surgery.4 Physical examination and visual acuity will be normal unless there is an accompanying retinal detachment.12
Patients who have decreased visual acuity or a visual field defect, or who describe a “curtain of darkness” are at risk for retinal detachment (shown above) and require a same-day referral.
A full ophthalmologic evaluation is indicated to detect or rule out a retinal detachment or tear—which has been found to co-occur with acute vitreous detachment in 14% of cases.13 Those who present with decreased visual acuity or a visual field defect or describe a “curtain of darkness” are at risk for retinal detachment and require a same-day referral.13
Like patients with vitreous detachment, those with a retinal detachment will report new floaters or peripheral flashing lights.12 The presence of vitreous hemorrhage or pigment, which can be seen in a slit lamp exam, is associated with increased risk for retinal detachment, as is a subjective report of vision loss.13
When retinal detachment is suspected, immediate referral to an ophthalmologist is needed.13 Reattachment surgery has good outcomes, especially if it is performed prior to macular involvement or within the first 3 days of macular detachment.14
3) "My eye hurts and is red"
Patients with painful, red eyes are at risk for a variety of sight-threatening conditions, iritis (anterior uveitis), keratitis, and acute angle closure glaucoma, as well as eye trauma, among them (TABLE).1,2,4,12,15-27 Decreased visual acuity in a patient with painful, red eyes warrants an urgent or emergent ophthalmologic referral.
When to suspect iritis
Patients with iritis will complain of vision loss, pain, photophobia, and redness. An eye exam will reveal injection of the conjunctiva around the cornea. Visual acuity is often decreased. Pupillary reaction may be sluggish, and the pupil may be smaller or larger than the other eye,4 but a normal pupil size does not exclude iritis in a patient with unilateral eye pain and ciliary injection.15
Iritis is often idiopathic, but risk factors include chronic inflammatory conditions such as ankylosing spondylitis, ulcerative colitis, and Crohn’s disease.16
Treatment with topical steroids is recommended.16 Urgent referral for long-term management of iritis is needed.17
Keratitis has varied causes
Patients with keratitis present with eye pain or foreign body sensation, redness, blurred vision, and photophobia. Examination of the eye will show injection of the conjunctiva surrounding the cornea, and possible corneal defects or opacities; visual acuity may be normal or decreased. The cause varies, based on whether keratitis is bacterial, viral, or noninfectious.
Risk factors for bacterial keratitis include extended wear of contact lenses, eye trauma, eye surgery, and systemic disease such as diabetes mellitus, while viral keratitis often follows a case of viral conjunctivitis and herpes simplex keratitis often involves reactivation of the virus. Causes of noninfectious keratitis include flash burns, dry eye or blepharitis, snow blindness, and sunburn.18
Treatment with topical antibiotics is effective for bacterial keratitis, but follow-up referral is needed because the infection could lead to loss of sight.19 Herpes simplex keratitis, which may appear as a mild corneal ulcer (a slit lamp examination will show the classic branching dendritic lesion), can be managed with topical antiviral medications,20 but here, too, an ophthalmologic referral is recommended to look for deeper corneal infiltrates that could lead to vision loss.20,21 Topical numbing medications should not be prescribed for patients with eye problems, as their extended use can lead to infection, corneal thinning, or even perforation of the cornea.22
Blurred vision, pain suggest acute angle closure glaucoma
Patients with acute angle closure glaucoma present with blurred vision, deep eye pain or brow ache, and frequently, nausea and vomiting.23 Some patients report seeing halos around lights, as well.
Risk factors for acute angle closure glaucoma include older age, Asian descent, farsightedness, family history, and female sex. Attacks are commonly idiopathic, but some are associated with routine pupillary dilation during eye exams.24
On examination, the cornea will be cloudy due to edema and the pupil will be mid-dilated and fixed.12 Typically, intraocular pressure in the affected eye will be elevated, an indication that the nausea and vomiting are associated with this disorder rather than a gastrointestinal condition.23 Emergent referral is needed to preserve vision.25
Eye trauma: What you’ll see, when to act Hyphema. In patients with a hyphema—typically the result of eye trauma—you’ll usually see a meniscus of blood in front of the iris in the anterior chamber. If the patient was supine before the evaluation, however, you’ll see red discoloration of the iris. Hyphemas can be a threat to vision, mostly due to potential elevated pressure. Because they are often associated with more extensive ocular injuries that are not always immediately evident, urgent referral is required.26
Hyphema—What you'll see
Courtesy of: Eye Teachers of America Foundation
More significant blunt trauma can cause globe rupture, resulting in both eye pain and loss of vision. Flooding the eye with fluorescein before examining it may make it possible to see a dark or green stream from the ruptured globe.
If you suspect a globe rupture, immediately stop your exam. Do not touch the eye. Instead, protect the eye—with a metal or plastic shield and an antiemetic to prevent pressure and Valsalva strain—and obtain an emergency ophthalmology consult.2,4
Chemical burns. Patients who incur chemical burns of the eye should irrigate the injured eye right away. The physical exam should be delayed until irrigation reaches an endpoint of neutral pH, as measured with Nitrazine paper.4,27 Alkali burns are particularly destructive to the eye and require longer irrigation.27
An emergent ophthalmology referral is needed for all alkali burns of the eye, as well as for any patient whose visual acuity does not return to baseline after irrigation. Slit lamp examination showing a deep corneal injury is also reason for an ophthalmology referral.1,2
4) "My eye is red" (but pain free)
When a patient seeks care for a red eye that’s not painful, the history and physical will help you determine whether the condition is benign or emergent. Orbital cellulitis, which we’ll discuss shortly, is the most dangerous condition related to this presentation (TABLE),4,9,28-32 requiring inpatient management and ophthalmology referral.
Conjunctivitis. The entire conjunctiva will be red and discharge will be present, but visual acuity will be normal.
Conjunctivitis can be viral or bacterial; office-based testing is now available for viral conjunctivitis caused by adenovirus. Treating bacterial conjunctivitis with antibiotic drops or ointment speeds recovery.29 When the cause is viral, standard treatment is supportive, with emphasis on preventing viral spread. Some antiviral preparations are being investigated as potential treatments for adenovirus conjunctivitis.28
Periorbital and orbital cellulitis. Redness surrounding the eye can be caused by preseptal (commonly called periorbital) or orbital cellulitis. The clinical presentation of these 2 conditions is similar, including redness, lid edema, and tenderness. However, periorbital cellulitis is more commonly seen after minor trauma to the eyelid skin or related to a stye or chalazion. Orbital cellulitis, which is considerably more serious, is typically associated with sinus disease or abscess.30
Patients with orbital cellulitis will present with restricted eye movements, decreased visual acuity, proptosis, and possibly an RAPD. These patients will often have pain as well. A fine-cut computed tomography of the orbits aids in diagnosis.31
Care for each is different. Oral antibiotics are usually sufficient for patients with periorbital cellulitis, but for orbital cellulitis, a same-day ophthalmology referral and hospitalization for treatment with parenteral antibiotics is required.9,32
Subconjunctival hemorrhage—dramatic but harmless
While dramatic in appearance, subconjunctival hemorrhage generally does not affect vision. It may be the result of trauma to the globe, but can also occur spontaneously.
On physical exam, you’ll see bleeding into the conjunctiva that stops at the edge of the cornea. Visual acuity will be normal, as will the remainder of the eye examination. Abnormal vision, pain, or significant or recurrent bleeding should prompt a search for an alternative diagnosis. No treatment is needed for a simple subconjunctival hemorrhage.4
5) "My eye hurts"
Patients complaining of eye pain with or without vision changes—and without redness—usually have a medical history that leads to the diagnosis (TABLE).1,2,4,33-38 Physical exam findings are compatible with the history.
Optic neuritis. Patients with optic neuritis have acute to subacute vision loss, usually in one eye but sometimes bilaterally, lasting hours to days. Optic neuritis is more common in women and in those ages 15 to 45 years, with an incidence of 5 in 100,000 among Caucasians.33 Pain with eye movement is present in more than 90% of adults with optic neuritis,34 and is also common in children.35
In addition to vision loss, patients will report decreased detection of light and color,6 and examination will reveal an RAPD.1,2 Vision returns without treatment to the same extent as with treatment, but treatment will speed recovery.36 Patients with optic neuritis require an urgent referral to an ophthalmologist or neurologist to evaluate for multiple sclerosis, which develops in about 30% of those with optic neuritis.4,33
Corneal abrasion. Pain, localized to the surface of the eye, will be the primary complaint of patients with a corneal abrasion, who may or may not have loss of vision. Larger and deeper abrasions are extremely painful, while smaller corneal abrasions may be experienced as a foreign body sensation. The typical patient with a corneal abrasion is likely to have had trauma to the eye.37
Fluorescein is used to examine the patient with a suspected abrasion to highlight the epithelial defect.1 Visual acuity needs to be tested, and checked using a pinhole if it is below baseline.37 Treatment protocols range from artificial tears to antibiotic drops or ointments. Topical steroids should be given to patients only by an ophthalmologist.4
Is patching necessary? In a systematic review comparing outcomes based on the use of patching vs not patching on the first day of injury, patients who were not given patches fared the same or better than those whose eyes were patched, both in terms of healing time and pain relief. Primary care physicians can treat most corneal abrasions, and symptoms typically resolve in 2 days.38
- Provide an urgent ophthalmology referral for any patient with a sudden decrease in visual acuity. C
- Record bilateral pupil size as part of a comprehensive eye exam, and provide an urgent referral for a patient whose pupils are of unequal size. C
- Involve an ophthalmologist or other specialist in the management of eye conditions caused by systemic diseases such as stroke or giant cell arteritis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Knowing how to respond when patients present with problems involving the eye is crucial for family physicians. Yet it is often difficult to know whether to treat or refer and which signs and symptoms are indicative of an ophthalmologic emergency with the potential to cause loss of sight.
Categorizing ophthalmologic conditions based on patients’ chief complaints, we have found, can help to narrow the differential diagnosis and home in on emergent signs and symptoms. Thus, we’ve used that approach in this review.
In the pages that follow, common complaints like “I can’t see,” “I’m seeing things,” and “My eye hurts” are used to highlight disorders—both benign and emergent—associated with each. You’ll also find an at-a-glance table listing the differential diagnosis for each presentation, and a mnemonic to guide you through the elements of a comprehensive eye exam.
1) "I can't see"
Patients may use words like “cloudy vision,“ “a veil over my eyes,” or “fuzziness” to describe diminished vision. Some will report black areas within their visual field; others will have a loss of peripheral vision or total vision loss in one eye, or possibly even both. Some causes of vision problems, such as cataracts, are not emergencies. Causes of more severe (but painless) vision loss include central retinal artery occlusion (CRAO) or vein occlusion (CRVO), giant cell arteritis (GCA), stroke or transient ischemic attack (TIA), nonarteritic anterior ischemic optic neuropathy (NAION), and nonorganic (functional) vision loss (TABLE).1-11
When the cause is ischemic
Patients with CRAO experience acute loss of vision in one eye, usually occurring within seconds to minutes. Most patients with CRVO will have a similar presentation, depending on the presence or absence of ischemia and involvement of the macula. Those with branch retinal vein occlusion may have no vision loss
at all.1-3
Risk factors for CRAO include cardiovascular disease, hypertension, diabetes, and other disorders associated with systemic inflammation. In patients older than 60 years, it is also important to consider GCA, which we’ll talk more about shortly, as a cause of CRAO.
In patients with CRAO, an eye exam will show profoundly decreased visual acuity, and the swinging light test (see “Use this mnemonic to ensure a comprehensive eye exam” on page 348) will reveal a relative afferent pupillary defect (RAPD). Fundoscopy is diagnostic, revealing a pale retina due to decreased blood flow.4 Emergent referral to ophthalmology is indicated to establish a definitive diagnosis and initiate treatment based on the cause of the occlusion. If emergency care is not immediately available, massaging the eye globe through closed lids, then releasing, in 10- to 15-second cycles, may be helpful.5
Use this mnemonic to ensure a comprehensive eye exam. In a potential emergency, an eye exam needs to be quick and thorough. To ensure that all the key elements are included, use the mnemonic VVEEPP (Visual acuity, Visual fields, External exam, Extraocular movements, Pupillary exam, and Pressure) as a guide.1Visual acuity. Check distance vision, with the patient wearing his or her corrective lenses, if possible. If not, substitute pinhole testing, which can function like corrective lenses and eliminate refractive error.2
Begin with distance charts. If the patient can’t see the charts, hold up fingers and ask whether the patient is able to count them. If not, try hand motion—or, if the patient can’t see that, try testing the patient’s ability to see light. Swing a light between the eyes. Paradoxical dilation of the affected eye when directly exposed to the light is evidence of a relative afferent pupillary defect (RAPD).2
Visual fields. Examine visual fields by using the standard confrontation technique—ie, asking the patient to cover one eye at a time while you move your hand in and out of his or her visual field.
External exam and extraocular movements. Use a penlight to inspect the eyelids, conjunctiva, sclera, cornea, and anterior chamber of the eye and to assess extraocular movements.1
Pupillary examination and pressure. Observe bilateral pupil size and swing a light between the eyes to test pupillary response to direct and consensual light (and to rule out an RAPD).2 If available, measure eye pressure, as well.1
Fundoscopy should be performed to complete the examination—along with a slit lamp evaluation, if possible.1
Risk factors for CRVO include age older than 65 and a number of chronic conditions. One analysis attributed 48% of cases to hypertension, 20% to hyperlipidemia, and 5% to diabetes.3 Fundoscopy will reveal dilated veins, retinal hemorrhages, and cotton wool spots, which look like puffy white patches on the retina.6
As with CRAO, an urgent ophthalmology referral is critical to establish the diagnosis and develop a treatment plan. Outcomes are poor in patients with visual acuity of 20/200 or worse at the time of diagnosis.7,8
GCA. Patients with GCA may develop arteritic ischemic optic neuropathy, resulting in vision loss in one or both eyes. Risk factors for GCA include age (>50 years), polymyalgia rheumatica, Caucasian race, and female sex. Systemic symptoms include fever, muscle aches, headache, jaw claudication, and scalp pain.6
The swinging light test will reveal an RAPD;1,2 fundoscopy findings typically include disk edema and disk hemorrhages, or a pale retina if GCA is associated with CRAO.6 Testing, including an erythrocyte sedimentation rate and a C-reactive protein, will provide supportive evidence, and biopsy of the temporal artery will confirm the diagnosis.4
Blindness from GCA is often profound. Bilateral disease is treated immediately with high-dose corticosteroids; when just one eye is affected, high-dose steroids should also be started right away to prevent vision loss in the other eye. Whenever GCA is suspected, initiate treatment and provide an urgent referral to an ophthalmologist for biopsy and further treatment.6
Strokes and TIAs that affect vision may be a result of ischemia of the visual cortex, or the eye itself. Visual cortex ischemia will present as a homonymous visual field cut between the eyes; TIAs that affect only one eye (known as amaurosis fugax) are associated with ischemia to the optic nerve or retina.
Patients with amaurosis fugax will experience unilateral loss of vision that extends like a dark shade from the top or bottom periphery to the center of vision. When a TIA is the cause, vision will return to normal within minutes. The underlying pathology is usually carotid artery atherosclerosis. If left untreated, evidence suggests that 30% to 50% of patients will have a stroke within a month.9
Visual acuity may or may not be decreased, depending on whether the ischemia involves the macula. Symptoms suggestive of amaurosis fugax should prompt an urgent ophthalmology referral, while patients with persistent vision loss or visual field deficit require urgent referral to a stroke treatment center.9
NAION is also associated with acute monocular vision loss, particularly in older patients.10 Visual acuity will be markedly decreased, and fundoscopic exam will show a swollen and hemorrhagic optic disc. The vision loss can be profound, and is usually permanent; neither medical nor surgical treatment has been shown to improve outcomes.10
When the cause is functional
Functional (nonorganic) visual disturbances should also be considered when sudden blindness is reported. Nonorganic vision loss has a number of causes, and patients present with a range of chief complaints, making diagnosis complex. Because some patients will have organic disease with a component of functional vision loss, it is best to refer individuals whom you suspect of having functional vision loss to an ophthalmologist for testing and a definitive diagnosis. Treatment includes psychological support and reassurance that vision will return.11
2) "I'm seeing things"
Patients with this problem often use words like “flashes,” “floaters” “worms,” or “lights,” and various colors and unusual shapes to describe what they see. When this phenomenon is accompanied by decreased visual acuity, emergent or urgent referral is required. Normal vision in a patient who reports “seeing things” calls for careful consideration of the etiology, and referral if the diagnosis is uncertain or the suspected disorder is sight-threatening (TABLE).4,12-14 Migraine and psychiatric disorders should be considered if suggested by history. (Patients with ocular migraine—which may or may not be associated with a headache—may also report seeing light patterns off to one side, typically lasting 20-45 minutes.)
Vitreous or retinal detachment
Patients with vitreous detachment, which is far more common and less serious than retinal detachment, report seeing new floaters or peripheral flashing lights in one eye. Risks for vitreous detachment include myopia, older age, eye trauma, and previous eye surgery.4 Physical examination and visual acuity will be normal unless there is an accompanying retinal detachment.12
Patients who have decreased visual acuity or a visual field defect, or who describe a “curtain of darkness” are at risk for retinal detachment (shown above) and require a same-day referral.
A full ophthalmologic evaluation is indicated to detect or rule out a retinal detachment or tear—which has been found to co-occur with acute vitreous detachment in 14% of cases.13 Those who present with decreased visual acuity or a visual field defect or describe a “curtain of darkness” are at risk for retinal detachment and require a same-day referral.13
Like patients with vitreous detachment, those with a retinal detachment will report new floaters or peripheral flashing lights.12 The presence of vitreous hemorrhage or pigment, which can be seen in a slit lamp exam, is associated with increased risk for retinal detachment, as is a subjective report of vision loss.13
When retinal detachment is suspected, immediate referral to an ophthalmologist is needed.13 Reattachment surgery has good outcomes, especially if it is performed prior to macular involvement or within the first 3 days of macular detachment.14
3) "My eye hurts and is red"
Patients with painful, red eyes are at risk for a variety of sight-threatening conditions, iritis (anterior uveitis), keratitis, and acute angle closure glaucoma, as well as eye trauma, among them (TABLE).1,2,4,12,15-27 Decreased visual acuity in a patient with painful, red eyes warrants an urgent or emergent ophthalmologic referral.
When to suspect iritis
Patients with iritis will complain of vision loss, pain, photophobia, and redness. An eye exam will reveal injection of the conjunctiva around the cornea. Visual acuity is often decreased. Pupillary reaction may be sluggish, and the pupil may be smaller or larger than the other eye,4 but a normal pupil size does not exclude iritis in a patient with unilateral eye pain and ciliary injection.15
Iritis is often idiopathic, but risk factors include chronic inflammatory conditions such as ankylosing spondylitis, ulcerative colitis, and Crohn’s disease.16
Treatment with topical steroids is recommended.16 Urgent referral for long-term management of iritis is needed.17
Keratitis has varied causes
Patients with keratitis present with eye pain or foreign body sensation, redness, blurred vision, and photophobia. Examination of the eye will show injection of the conjunctiva surrounding the cornea, and possible corneal defects or opacities; visual acuity may be normal or decreased. The cause varies, based on whether keratitis is bacterial, viral, or noninfectious.
Risk factors for bacterial keratitis include extended wear of contact lenses, eye trauma, eye surgery, and systemic disease such as diabetes mellitus, while viral keratitis often follows a case of viral conjunctivitis and herpes simplex keratitis often involves reactivation of the virus. Causes of noninfectious keratitis include flash burns, dry eye or blepharitis, snow blindness, and sunburn.18
Treatment with topical antibiotics is effective for bacterial keratitis, but follow-up referral is needed because the infection could lead to loss of sight.19 Herpes simplex keratitis, which may appear as a mild corneal ulcer (a slit lamp examination will show the classic branching dendritic lesion), can be managed with topical antiviral medications,20 but here, too, an ophthalmologic referral is recommended to look for deeper corneal infiltrates that could lead to vision loss.20,21 Topical numbing medications should not be prescribed for patients with eye problems, as their extended use can lead to infection, corneal thinning, or even perforation of the cornea.22
Blurred vision, pain suggest acute angle closure glaucoma
Patients with acute angle closure glaucoma present with blurred vision, deep eye pain or brow ache, and frequently, nausea and vomiting.23 Some patients report seeing halos around lights, as well.
Risk factors for acute angle closure glaucoma include older age, Asian descent, farsightedness, family history, and female sex. Attacks are commonly idiopathic, but some are associated with routine pupillary dilation during eye exams.24
On examination, the cornea will be cloudy due to edema and the pupil will be mid-dilated and fixed.12 Typically, intraocular pressure in the affected eye will be elevated, an indication that the nausea and vomiting are associated with this disorder rather than a gastrointestinal condition.23 Emergent referral is needed to preserve vision.25
Eye trauma: What you’ll see, when to act Hyphema. In patients with a hyphema—typically the result of eye trauma—you’ll usually see a meniscus of blood in front of the iris in the anterior chamber. If the patient was supine before the evaluation, however, you’ll see red discoloration of the iris. Hyphemas can be a threat to vision, mostly due to potential elevated pressure. Because they are often associated with more extensive ocular injuries that are not always immediately evident, urgent referral is required.26
Hyphema—What you'll see
Courtesy of: Eye Teachers of America Foundation
More significant blunt trauma can cause globe rupture, resulting in both eye pain and loss of vision. Flooding the eye with fluorescein before examining it may make it possible to see a dark or green stream from the ruptured globe.
If you suspect a globe rupture, immediately stop your exam. Do not touch the eye. Instead, protect the eye—with a metal or plastic shield and an antiemetic to prevent pressure and Valsalva strain—and obtain an emergency ophthalmology consult.2,4
Chemical burns. Patients who incur chemical burns of the eye should irrigate the injured eye right away. The physical exam should be delayed until irrigation reaches an endpoint of neutral pH, as measured with Nitrazine paper.4,27 Alkali burns are particularly destructive to the eye and require longer irrigation.27
An emergent ophthalmology referral is needed for all alkali burns of the eye, as well as for any patient whose visual acuity does not return to baseline after irrigation. Slit lamp examination showing a deep corneal injury is also reason for an ophthalmology referral.1,2
4) "My eye is red" (but pain free)
When a patient seeks care for a red eye that’s not painful, the history and physical will help you determine whether the condition is benign or emergent. Orbital cellulitis, which we’ll discuss shortly, is the most dangerous condition related to this presentation (TABLE),4,9,28-32 requiring inpatient management and ophthalmology referral.
Conjunctivitis. The entire conjunctiva will be red and discharge will be present, but visual acuity will be normal.
Conjunctivitis can be viral or bacterial; office-based testing is now available for viral conjunctivitis caused by adenovirus. Treating bacterial conjunctivitis with antibiotic drops or ointment speeds recovery.29 When the cause is viral, standard treatment is supportive, with emphasis on preventing viral spread. Some antiviral preparations are being investigated as potential treatments for adenovirus conjunctivitis.28
Periorbital and orbital cellulitis. Redness surrounding the eye can be caused by preseptal (commonly called periorbital) or orbital cellulitis. The clinical presentation of these 2 conditions is similar, including redness, lid edema, and tenderness. However, periorbital cellulitis is more commonly seen after minor trauma to the eyelid skin or related to a stye or chalazion. Orbital cellulitis, which is considerably more serious, is typically associated with sinus disease or abscess.30
Patients with orbital cellulitis will present with restricted eye movements, decreased visual acuity, proptosis, and possibly an RAPD. These patients will often have pain as well. A fine-cut computed tomography of the orbits aids in diagnosis.31
Care for each is different. Oral antibiotics are usually sufficient for patients with periorbital cellulitis, but for orbital cellulitis, a same-day ophthalmology referral and hospitalization for treatment with parenteral antibiotics is required.9,32
Subconjunctival hemorrhage—dramatic but harmless
While dramatic in appearance, subconjunctival hemorrhage generally does not affect vision. It may be the result of trauma to the globe, but can also occur spontaneously.
On physical exam, you’ll see bleeding into the conjunctiva that stops at the edge of the cornea. Visual acuity will be normal, as will the remainder of the eye examination. Abnormal vision, pain, or significant or recurrent bleeding should prompt a search for an alternative diagnosis. No treatment is needed for a simple subconjunctival hemorrhage.4
5) "My eye hurts"
Patients complaining of eye pain with or without vision changes—and without redness—usually have a medical history that leads to the diagnosis (TABLE).1,2,4,33-38 Physical exam findings are compatible with the history.
Optic neuritis. Patients with optic neuritis have acute to subacute vision loss, usually in one eye but sometimes bilaterally, lasting hours to days. Optic neuritis is more common in women and in those ages 15 to 45 years, with an incidence of 5 in 100,000 among Caucasians.33 Pain with eye movement is present in more than 90% of adults with optic neuritis,34 and is also common in children.35
In addition to vision loss, patients will report decreased detection of light and color,6 and examination will reveal an RAPD.1,2 Vision returns without treatment to the same extent as with treatment, but treatment will speed recovery.36 Patients with optic neuritis require an urgent referral to an ophthalmologist or neurologist to evaluate for multiple sclerosis, which develops in about 30% of those with optic neuritis.4,33
Corneal abrasion. Pain, localized to the surface of the eye, will be the primary complaint of patients with a corneal abrasion, who may or may not have loss of vision. Larger and deeper abrasions are extremely painful, while smaller corneal abrasions may be experienced as a foreign body sensation. The typical patient with a corneal abrasion is likely to have had trauma to the eye.37
Fluorescein is used to examine the patient with a suspected abrasion to highlight the epithelial defect.1 Visual acuity needs to be tested, and checked using a pinhole if it is below baseline.37 Treatment protocols range from artificial tears to antibiotic drops or ointments. Topical steroids should be given to patients only by an ophthalmologist.4
Is patching necessary? In a systematic review comparing outcomes based on the use of patching vs not patching on the first day of injury, patients who were not given patches fared the same or better than those whose eyes were patched, both in terms of healing time and pain relief. Primary care physicians can treat most corneal abrasions, and symptoms typically resolve in 2 days.38
1. Wright JL, Wightman JM. Red and painful eye. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, Pa: Mosby Elsevier; 2009:chap 32.
2. Knoop KJ, Dennis WR, Hedges JR. Ophthalmologic procedures. In: Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 5th ed. Philadelphia, Pa: Saunders Elsevier;2009:chap 63.
3. Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56:281-299.
4. Sharma R, Brunette DD. Ophthalmology. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, Pa: Mosby Elsevier; 2009:chap 69.
5. Cugati S, Varma DD, Chen CS, et al. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15:63-77.
6. Matson M, Fujimoto L. Bilateral arteritic anterior ischemic optic neuropathy. Optometry. 2011;82:622-631.
7. McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1113-1123.
8. Wong TY, Scott IU. Retinal-vein occlusion. N Engl J Med. 2010;363:2135-2144.
9. Crouch ER, Crouch ER, Grant T. Ophthalmology. In: Rakel RE, ed. Textbook of Family Medicine. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 41.
10. Dickersin K, Manheimer E, Li T. Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev. 2012;(1):CD001538.
11. Thurtell MJ, Tomsak RL. Neuro-ophthalmology: afferent visual system. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Los Angeles, Calif: Saunders Elsevier; 2012:chap 36.
12. Yanoff M, Cameron D. Diseases of the visual system. In: Goldman L, Schafer AI, eds. Cecil Medicine. 24th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 431.
13. Hollands H, Johnson D, Brox A, et al. Acute-onset floaters and flashes: is this patient at risk for retinal detachment? JAMA. 2009;302:2243-2249.
14. D’Amico DJ. Primary retinal detachment. N Engl J Med. 2008;359:2346-2354.
15. Hunsley T, Lee C. Does a normal-shaped pupil exclude the diagnosis of iritis? Best evidence topic reports. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. Emerg Med J. 2006;23:872-877.
16. Islam N, Pavesio C. Uveitis (acute anterior). Clin Evid. 2010;4:705.
17. Grunwald L, Newcomb CW, Daniel E, et al. Risk of relapse in primary acute anterior uveitis. Ophthalmology. 2011;118:1911-1915.
18. Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20:129-141.
19. Suwan-Apichon O, Reyes JM, Herretes S, et al. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst Rev. 2007;(4):CD005430.
20. Morris D, Latham E. Ulcers in the eye. J Emerg Med. 2012;42:62-64.
21. Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2010;(12):CD002898.
22. Yagci A, Bozkurt B, Egrilmez S, et al. Topical anesthetic abuse keratopathy: a commonly overlooked health care problem.Cornea. 2011;30:571-575.
23. Cholongitas E, Pipili C, Dasenaki M. Acute angle closure glaucoma presented with nausea and epigastric pain. Dig Dis Sci. 2008;53:1430-1431.
24. White J. Diagnosis and management of acute angle-closure glaucoma. Emerg Nurse. 2011;19:27.
25. Lama DSC, Thama CCY, Laia JSM, et al. Current approaches to the management of acute primary angle closure. Curr Opin Ophthalmol. 2007;18:146-151.
26. Gharaibeh A, Savage HI, Scherer RW, et al. Medical interventions for traumatic hyphema. Cochrane Database Syst Rev. 2011;(1):CD005431.
27. Connor AJ, Severn P. Use of a control test to aid pH assessment of chemical eye injuries. Emerg Med J. 2009;26:811-812.
28. Sambursky R, Trattler W, Tauber S, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013;131:17-22.
29. Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006;(2):CD001211.
30. Papier A, Tuttle DJ, Mahara TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
31. Howe L, Jones NS. Guidelines for the management of periorbital cellulitis/abscess. Clin Otolaryngol. 2004;29:725-728.
32. Mahalingam-Dhingra A, Lander L, Preciado DA, et al. Orbital and periorbital infections: a national perspective. Arch Otolaryngol Head Neck Surg. 2011;137:769-773.
33. Germann CA, Baumann MR, Hamzavi S. Ophthalmic diagnoses in the ED: optic neuritis. Am J Emerg Med. 2007;25:834-837.
34. Balcer LJ. Optic neuritis. N Engl J Med. 2006;354:1273-1280.
35. Olitsky SE, Hug D, Plummer L, et al. Abnormalities of the optic nerve. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds.Nelson Textbook of Pediatrics. 19th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 623.
36. Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2012;(4):CD001430.
37. Aslam SA, Sheth HG, Vaughan AJ. Emergency management of corneal injuries. Injury. 2007;38:594-597.
38. Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006;(2):CD004764.
1. Wright JL, Wightman JM. Red and painful eye. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, Pa: Mosby Elsevier; 2009:chap 32.
2. Knoop KJ, Dennis WR, Hedges JR. Ophthalmologic procedures. In: Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 5th ed. Philadelphia, Pa: Saunders Elsevier;2009:chap 63.
3. Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56:281-299.
4. Sharma R, Brunette DD. Ophthalmology. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, Pa: Mosby Elsevier; 2009:chap 69.
5. Cugati S, Varma DD, Chen CS, et al. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15:63-77.
6. Matson M, Fujimoto L. Bilateral arteritic anterior ischemic optic neuropathy. Optometry. 2011;82:622-631.
7. McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1113-1123.
8. Wong TY, Scott IU. Retinal-vein occlusion. N Engl J Med. 2010;363:2135-2144.
9. Crouch ER, Crouch ER, Grant T. Ophthalmology. In: Rakel RE, ed. Textbook of Family Medicine. 8th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 41.
10. Dickersin K, Manheimer E, Li T. Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev. 2012;(1):CD001538.
11. Thurtell MJ, Tomsak RL. Neuro-ophthalmology: afferent visual system. In: Daroff RB, Fenichel GM, Jankovic J, et al, eds. Bradley’s Neurology in Clinical Practice. 6th ed. Los Angeles, Calif: Saunders Elsevier; 2012:chap 36.
12. Yanoff M, Cameron D. Diseases of the visual system. In: Goldman L, Schafer AI, eds. Cecil Medicine. 24th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 431.
13. Hollands H, Johnson D, Brox A, et al. Acute-onset floaters and flashes: is this patient at risk for retinal detachment? JAMA. 2009;302:2243-2249.
14. D’Amico DJ. Primary retinal detachment. N Engl J Med. 2008;359:2346-2354.
15. Hunsley T, Lee C. Does a normal-shaped pupil exclude the diagnosis of iritis? Best evidence topic reports. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. Emerg Med J. 2006;23:872-877.
16. Islam N, Pavesio C. Uveitis (acute anterior). Clin Evid. 2010;4:705.
17. Grunwald L, Newcomb CW, Daniel E, et al. Risk of relapse in primary acute anterior uveitis. Ophthalmology. 2011;118:1911-1915.
18. Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20:129-141.
19. Suwan-Apichon O, Reyes JM, Herretes S, et al. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst Rev. 2007;(4):CD005430.
20. Morris D, Latham E. Ulcers in the eye. J Emerg Med. 2012;42:62-64.
21. Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2010;(12):CD002898.
22. Yagci A, Bozkurt B, Egrilmez S, et al. Topical anesthetic abuse keratopathy: a commonly overlooked health care problem.Cornea. 2011;30:571-575.
23. Cholongitas E, Pipili C, Dasenaki M. Acute angle closure glaucoma presented with nausea and epigastric pain. Dig Dis Sci. 2008;53:1430-1431.
24. White J. Diagnosis and management of acute angle-closure glaucoma. Emerg Nurse. 2011;19:27.
25. Lama DSC, Thama CCY, Laia JSM, et al. Current approaches to the management of acute primary angle closure. Curr Opin Ophthalmol. 2007;18:146-151.
26. Gharaibeh A, Savage HI, Scherer RW, et al. Medical interventions for traumatic hyphema. Cochrane Database Syst Rev. 2011;(1):CD005431.
27. Connor AJ, Severn P. Use of a control test to aid pH assessment of chemical eye injuries. Emerg Med J. 2009;26:811-812.
28. Sambursky R, Trattler W, Tauber S, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013;131:17-22.
29. Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006;(2):CD001211.
30. Papier A, Tuttle DJ, Mahara TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
31. Howe L, Jones NS. Guidelines for the management of periorbital cellulitis/abscess. Clin Otolaryngol. 2004;29:725-728.
32. Mahalingam-Dhingra A, Lander L, Preciado DA, et al. Orbital and periorbital infections: a national perspective. Arch Otolaryngol Head Neck Surg. 2011;137:769-773.
33. Germann CA, Baumann MR, Hamzavi S. Ophthalmic diagnoses in the ED: optic neuritis. Am J Emerg Med. 2007;25:834-837.
34. Balcer LJ. Optic neuritis. N Engl J Med. 2006;354:1273-1280.
35. Olitsky SE, Hug D, Plummer L, et al. Abnormalities of the optic nerve. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds.Nelson Textbook of Pediatrics. 19th ed. Philadelphia, Pa: Saunders Elsevier; 2011:chap 623.
36. Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2012;(4):CD001430.
37. Aslam SA, Sheth HG, Vaughan AJ. Emergency management of corneal injuries. Injury. 2007;38:594-597.
38. Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006;(2):CD004764.
Finger injuries: 5 cases to test your skills
Some finger injuries require little more than icing; others are more serious, often emergent, conditions with outcomes that are dependent on an accurate diagnosis and rapid initiation of treatment.
The 5 cases that follow describe injuries with varying degrees of severity. Read each case and select the multiple-choice answer you think is most appropriate. Then read on to find out if you were right—and to learn more about the clinical presentation, diagnosis, and treatment for each type of injury.
CASE 1 A 45-year-old auto body worker walks into your office at 5:30 pm, just as your staff is closing up for the day. A few hours ago, he reports, he was spray-painting a car with a paint gun when he felt a sudden pain in his right index finger. His immediate thought was that he had torn something, but the pain quickly subsided. So he continued to work—until about 45 minutes ago, when the pain became so intense that he knew he needed medical care right away.
Examination reveals redness and increased skin temperature on the radial palmar side of the proximal interphalangeal (PIP) joint of the index finger. Two-point discrimination is decreased to 10 mm, vs 5 mm on the same finger of the opposite hand. The patient can flex his PIP and distal interphalangeal (DIP) joints but complains of pain and stiffness. You obtain x-rays of the injured
finger (FIGURE 1).
WHAT'S YOUR NEXT STEP?
B. Update the patient’s tetanus immunization and start him on a broad-spectrum antibiotic.
C. Put a dorsal splint on the injured finger in the “safe hand” position and schedule a return visit in one week.
D. “Buddy tape” the index and long fingers and refer the patient to a hand surgeon.
Answer: Send the auto-body worker to the nearest ED and call ahead (A).
This patient sustained a high-pressure injection injury to the PIP joint of his right index finger. The patient’s description of how the injury occurred suggested this, and the radiograph confirmed it by showing some paint under the skin (See arrow, FIGURE 1). Such injuries occur when a high pressure (typically from a hose) forces air or a substance—eg, diesel fuel, paint, or solvent—through the skin into the finger.
Although high-pressure injection injury often has a benign presentation, it is actually a medical emergency. If aggressive surgical debridement does not occur within a 6-hour window, the patient runs a high risk for amputation of the digit.1 A hand surgeon should be contacted as soon as possible.
The severity of the injury varies, depending on the amount of pressure (amputation rates are as high as 43% when the pressure per square inch >1000), the type of material injected (diesel fuel is the most toxic), and the location.1,2
Instruct the patient to remove any jewelry, such as a wedding band or watch, on the affected hand or wrist, and to keep the hand elevated. Broad-spectrum antibiotics should be started right away, and a tetanus booster given, if needed. Do not apply heat or use local anesthesia, as both can increase the swelling.2
CASE 2 A 17-year-old cheerleader comes to see you on Monday afternoon, after injuring her left pinky during a Friday night game. The patient, who is right-handed, points to the left PIP joint when you ask where it hurts, and tells you that the finger is stiff. She has been icing it since the injury occurred, to make sure she is ready to cheer by next weekend.
The injury occurred when she was spotting another cheerleader during a routine, the patient reports, adding that the pinky was “dislocated.” The coach “popped” it back in place and buddy-taped the injured finger to her ring finger.
The patient is able to flex and extend the DIP joint on the pinky when the PIP joint is stabilized. She can also flex the PIP joint unassisted, but has difficulty extending it. The digit demonstrates slight flexion of the PIP joint. You note tenderness over both collateral ligaments and the dorsum of the PIP joint, but not over the volar aspect of the injured finger, and order x-rays (FIGURE 2).
WHAT'S YOUR NEXT STEP?
B. Refer the patient to a hand surgeon.
C. Apply an extension block splint so the patient can flex the finger but not extend it, and schedule a follow-up appointment in one to 2 weeks.
D. Apply an aluminum dorsal splint, allowing the DIP joint to be flexed and keeping the PIP joint in full extension for 4 weeks.
Answer: Refer the cheerleader to a hand surgeon (B).
This patient has a rupture of the central extensor tendon of the pinky finger at the PIP joint. The mechanism of injury and her inability to completely extend the injured finger at the PIP joint alert you to this type of injury. An x-ray may sometimes be normal but in this case, it shows the flexion of the PIP joint. Surgical repair of the rupture should be scheduled without delay.3
Most injuries at this joint occur from forced extension, not flexion, and result in a volar plate rupture.4 If swelling and pain make evaluation of an acute dislocation injury difficult, splinting in the “safe hand” position for 72 hours while icing the injured finger will make it possible to do a more detailed follow-up exam.3
Extended periods of splinting can make the PIP joint very stiff, however—and harder to treat than the original injury.5 If the rupture of the central extensor tendon is undetected or simply not treated, a Boutonniere deformity, in which the PIP joint is flexed and the DIP joint is hyperextended, is the likely result.3
CASE 3 A 24-year-old man “jammed” his right ring finger while trying to catch a ball that was passed to him during a pick-up basketball game. He has rested and iced the finger for a couple of days, but it’s still painful and hard to move. He has no significant medical history and has been taking only acetaminophen for the pain.
Examination reveals that the injured finger has good capillary refill, 2-point discrimination is intact at 5 mm, and the other fingers on his right hand have no deformities and a normal range of motion. On the injured finger, however, the DIP joint is swollen and tender; it cannot be fully extended (FiGURE 3).
WHAT'S YOUR DIAGNOSIS?
B. Distal tuft fracture.
C. Mallet finger.
D. Finger sprain.
E. Trigger finger.
Answer: The basketball player has mallet finger (C).
Mallet finger typically occurs on the dominant hand. The key physical finding is that the joint is “stuck” in flexion, which is evident during an exam and on x-ray. Although the DIP joint may be passively fully extended, the patient with mallet finger is unable to actively extend it.
Mallet injuries, which are common in sports and associated with minor trauma, are typically caused by sudden forced flexion of the DIP joint during active extension of the finger. This can either stretch or tear the extensor tendon or lead to avulsion of the tendon insertion from the dorsum of the distal phalanx, with or without a fragment of bone. The injury is called a “soft” mallet finger when there is no bone involvement and a “bony” mallet finger when an avulsion is present, like the one that is evident on the FIGURE 3 x-ray (see arrow).
On clinical examination, the finger may or may not have an obvious deformity; similarly, you won’t always see bruising, swelling, or tenderness over the DIP joint.6 The work-up should include posterior/anterior, oblique, and lateral x-rays, followed by an examination of the soft tissue and a range-of-motion evaluation of the metacarpophalangeal and PIP joints. In acute injuries, tenderness is elicited with palpation over the dorsal aspect of the DIP joint. Although most patients develop an extensor lag at the DIP joint immediately after injury, the deformity may be delayed by a few hours or even days.6,7
Nonsurgical management is the standard of care for most mallet injuries, including mallet fractures involving less than one-third of the articular surface with no associated DIP joint subluxation.7
If there is no displacement, round-the-clock splinting to keep the joint in extension for a minimum of 6 weeks is indicated, followed by 2 to 3 weeks of nighttime splinting. It is important that the splinting allow for complete extension of the DIP joint but flexion of the PIP joint. Keeping the PIP in extension for prolonged periods can lead to permanent stiffness of the joint, while failure to provide any immobilization may lead to permanent deformity.
Surgery is indicated for a fracture fragment involving >30% of the joint surface (as demonstrated in the radiograph), volar subluxation, or a swan neck deformity—and when conservative therapy fails.7
CASE 4 An 18-year-old high school football player presents with pain and swelling at the tip of his right ring finger from an injury that occurred a week ago. When the player he was trying to tackle broke away, the patient says, he immediately felt pain and a “pop” in the finger.
The DIP joint of his right ring finger is swollen (FIGURE 4), but appears normal otherwise. When you isolate the joint, however, the patient is unable to flex it. You can palpate a stump on the volar surface of the finger.
What’s your next step?
B. Treat with splinting, RICE (rest, ice, compression, and elevation), and nonsteroidal anti-inflammatory drugs.
C. Order an ultrasound of the finger and palm.
D. Order magnetic resonance imaging (MRI) of the hand.
Answer: Order an ultrasound of the football player’s finger and palm (C).
This patient has Jersey finger, caused by a traumatic avulsion of the flexor digitorum profundus (FDP) from the distal phalanx and diagnosed based on the mechanism of injury and the patient’s inability to flex the DIP joint. The injury often does not show on x-rays, and the diagnosis may be missed for several weeks.
Jersey finger usually happens in sports like football or rugby, where players tackle each other, and involves forced, passive extension of the DIP joint at a time of active flexion. Management of Jersey finger starts with splinting, with both the DIP and PIP in slight flexion. Surgical reattachment of the flexor tendon is needed, with best results when it is done within 7 to 10 days of injury.4
You may be able to palpate the tendon stump in the palm or along the digit; bony avulsions can be trapped at the flexor sheath. Soft tissue swelling can be misleading, however, and the point of maximal tenderness is not an accurate means of identifying the avulsed tendon stump.8
Ultrasound is effective in differentiating between a partial and full thickness rupture and in localizing the distal tendon stump.8 MRI is usually reserved for precise evaluation of the tendon edges, to aid in operative planning. If the tendon is retracted to the palm, scarring may be irreversible because of the lack of blood supply.
Athletes typically return to play 12 weeks after injury, starting with protected activity and progressing to full gripping/grasping. Physical therapy and/or occupational therapy will be needed after the surgical wound has healed.
CASE 5 A 40-year-old construction worker who smashed his left index finger with a hammer one day ago presents with severe pain in his fingertip, which he is unable to move. On examination, you find that the distal finger is swollen and there is extensive ecchymosis and swelling underneath the nail. The finger has normal sensation, but you are unable to see capillary refill due to a large hematoma.
X-rays (FIGURE 5) reveal a distal tuft fracture. The patient’s main concern is the pain, and he asks what you can do to relieve it.
What’s your next step?
B. Perform fenestration of the nail.
C. Refer the patient to a hand surgeon.
D. Order computed tomography of the hand.
Answer: Perform fenestration of the construction worker’s nail (B).
This patient has a closed fracture of the distal phalanx, called a tuft fracture, and a sub-ungual hematoma, evident from the x-ray and the physical presentation.
Subungual hematoma requires fenestration with a needle to create small holes in the nail. If the nail bed is lacerated, the nail is removed and the injured nail bed repaired with sutures.
Tuft fractures sometimes require reduction. More often, they are stable and minimally displaced and can be managed conservatively, with splinting with a padded aluminum splint or a fingertip guard (Stax splint) for 3 to 4 weeks. Antibiotics are not indicated unless there is suspicion of an overlying or secondary infection. Referral to a hand surgeon is required for severe crush injuries, avulsion of the nail matrix, and open fractures of the distal phalanx.5,6
1. Hogan CJ, Ruland RT. High-pressure injection injuries to the upper extremity: a review of the literature. J Orthop Trauma. 2006;20:503-511.
2. Gonzalez R, Kasdan ML. High pressure injection injuries of the hand. Clin Occup Environ Med. 2006;5:407-411.
3. Freiberg A. Management of proximal interphalangeal joint injuries. Can J Plast Surg. 2007;15:199-203.
4. Perron AD, Brady WJ, Keats TE, et al. Orthopedic pitfalls in the emergency department: closed tendon injuries of the hand. Am J Emerg Med. 2001;19:76-80.
5. Oetgen ME, Dodds SD. Non-operative treatment of common finger injuries. Curr Rev Musculoskel Med. 2008;1:97-102.
6. Anderson D. Mallet finger. Aust Fam Physician. 2011;40:91-92.
7. Smit JM, Beets MR. Treatment options for mallet finger: a review. Plast Reconstr Surg. 2010;126:1624-1629.
8. Goodson A, Morgan M. Current management of Jersey finger in rugby players: cases series and literature review. Hand Surg. 2010;15:103-107.
Some finger injuries require little more than icing; others are more serious, often emergent, conditions with outcomes that are dependent on an accurate diagnosis and rapid initiation of treatment.
The 5 cases that follow describe injuries with varying degrees of severity. Read each case and select the multiple-choice answer you think is most appropriate. Then read on to find out if you were right—and to learn more about the clinical presentation, diagnosis, and treatment for each type of injury.
CASE 1 A 45-year-old auto body worker walks into your office at 5:30 pm, just as your staff is closing up for the day. A few hours ago, he reports, he was spray-painting a car with a paint gun when he felt a sudden pain in his right index finger. His immediate thought was that he had torn something, but the pain quickly subsided. So he continued to work—until about 45 minutes ago, when the pain became so intense that he knew he needed medical care right away.
Examination reveals redness and increased skin temperature on the radial palmar side of the proximal interphalangeal (PIP) joint of the index finger. Two-point discrimination is decreased to 10 mm, vs 5 mm on the same finger of the opposite hand. The patient can flex his PIP and distal interphalangeal (DIP) joints but complains of pain and stiffness. You obtain x-rays of the injured
finger (FIGURE 1).
WHAT'S YOUR NEXT STEP?
B. Update the patient’s tetanus immunization and start him on a broad-spectrum antibiotic.
C. Put a dorsal splint on the injured finger in the “safe hand” position and schedule a return visit in one week.
D. “Buddy tape” the index and long fingers and refer the patient to a hand surgeon.
Answer: Send the auto-body worker to the nearest ED and call ahead (A).
This patient sustained a high-pressure injection injury to the PIP joint of his right index finger. The patient’s description of how the injury occurred suggested this, and the radiograph confirmed it by showing some paint under the skin (See arrow, FIGURE 1). Such injuries occur when a high pressure (typically from a hose) forces air or a substance—eg, diesel fuel, paint, or solvent—through the skin into the finger.
Although high-pressure injection injury often has a benign presentation, it is actually a medical emergency. If aggressive surgical debridement does not occur within a 6-hour window, the patient runs a high risk for amputation of the digit.1 A hand surgeon should be contacted as soon as possible.
The severity of the injury varies, depending on the amount of pressure (amputation rates are as high as 43% when the pressure per square inch >1000), the type of material injected (diesel fuel is the most toxic), and the location.1,2
Instruct the patient to remove any jewelry, such as a wedding band or watch, on the affected hand or wrist, and to keep the hand elevated. Broad-spectrum antibiotics should be started right away, and a tetanus booster given, if needed. Do not apply heat or use local anesthesia, as both can increase the swelling.2
CASE 2 A 17-year-old cheerleader comes to see you on Monday afternoon, after injuring her left pinky during a Friday night game. The patient, who is right-handed, points to the left PIP joint when you ask where it hurts, and tells you that the finger is stiff. She has been icing it since the injury occurred, to make sure she is ready to cheer by next weekend.
The injury occurred when she was spotting another cheerleader during a routine, the patient reports, adding that the pinky was “dislocated.” The coach “popped” it back in place and buddy-taped the injured finger to her ring finger.
The patient is able to flex and extend the DIP joint on the pinky when the PIP joint is stabilized. She can also flex the PIP joint unassisted, but has difficulty extending it. The digit demonstrates slight flexion of the PIP joint. You note tenderness over both collateral ligaments and the dorsum of the PIP joint, but not over the volar aspect of the injured finger, and order x-rays (FIGURE 2).
WHAT'S YOUR NEXT STEP?
B. Refer the patient to a hand surgeon.
C. Apply an extension block splint so the patient can flex the finger but not extend it, and schedule a follow-up appointment in one to 2 weeks.
D. Apply an aluminum dorsal splint, allowing the DIP joint to be flexed and keeping the PIP joint in full extension for 4 weeks.
Answer: Refer the cheerleader to a hand surgeon (B).
This patient has a rupture of the central extensor tendon of the pinky finger at the PIP joint. The mechanism of injury and her inability to completely extend the injured finger at the PIP joint alert you to this type of injury. An x-ray may sometimes be normal but in this case, it shows the flexion of the PIP joint. Surgical repair of the rupture should be scheduled without delay.3
Most injuries at this joint occur from forced extension, not flexion, and result in a volar plate rupture.4 If swelling and pain make evaluation of an acute dislocation injury difficult, splinting in the “safe hand” position for 72 hours while icing the injured finger will make it possible to do a more detailed follow-up exam.3
Extended periods of splinting can make the PIP joint very stiff, however—and harder to treat than the original injury.5 If the rupture of the central extensor tendon is undetected or simply not treated, a Boutonniere deformity, in which the PIP joint is flexed and the DIP joint is hyperextended, is the likely result.3
CASE 3 A 24-year-old man “jammed” his right ring finger while trying to catch a ball that was passed to him during a pick-up basketball game. He has rested and iced the finger for a couple of days, but it’s still painful and hard to move. He has no significant medical history and has been taking only acetaminophen for the pain.
Examination reveals that the injured finger has good capillary refill, 2-point discrimination is intact at 5 mm, and the other fingers on his right hand have no deformities and a normal range of motion. On the injured finger, however, the DIP joint is swollen and tender; it cannot be fully extended (FiGURE 3).
WHAT'S YOUR DIAGNOSIS?
B. Distal tuft fracture.
C. Mallet finger.
D. Finger sprain.
E. Trigger finger.
Answer: The basketball player has mallet finger (C).
Mallet finger typically occurs on the dominant hand. The key physical finding is that the joint is “stuck” in flexion, which is evident during an exam and on x-ray. Although the DIP joint may be passively fully extended, the patient with mallet finger is unable to actively extend it.
Mallet injuries, which are common in sports and associated with minor trauma, are typically caused by sudden forced flexion of the DIP joint during active extension of the finger. This can either stretch or tear the extensor tendon or lead to avulsion of the tendon insertion from the dorsum of the distal phalanx, with or without a fragment of bone. The injury is called a “soft” mallet finger when there is no bone involvement and a “bony” mallet finger when an avulsion is present, like the one that is evident on the FIGURE 3 x-ray (see arrow).
On clinical examination, the finger may or may not have an obvious deformity; similarly, you won’t always see bruising, swelling, or tenderness over the DIP joint.6 The work-up should include posterior/anterior, oblique, and lateral x-rays, followed by an examination of the soft tissue and a range-of-motion evaluation of the metacarpophalangeal and PIP joints. In acute injuries, tenderness is elicited with palpation over the dorsal aspect of the DIP joint. Although most patients develop an extensor lag at the DIP joint immediately after injury, the deformity may be delayed by a few hours or even days.6,7
Nonsurgical management is the standard of care for most mallet injuries, including mallet fractures involving less than one-third of the articular surface with no associated DIP joint subluxation.7
If there is no displacement, round-the-clock splinting to keep the joint in extension for a minimum of 6 weeks is indicated, followed by 2 to 3 weeks of nighttime splinting. It is important that the splinting allow for complete extension of the DIP joint but flexion of the PIP joint. Keeping the PIP in extension for prolonged periods can lead to permanent stiffness of the joint, while failure to provide any immobilization may lead to permanent deformity.
Surgery is indicated for a fracture fragment involving >30% of the joint surface (as demonstrated in the radiograph), volar subluxation, or a swan neck deformity—and when conservative therapy fails.7
CASE 4 An 18-year-old high school football player presents with pain and swelling at the tip of his right ring finger from an injury that occurred a week ago. When the player he was trying to tackle broke away, the patient says, he immediately felt pain and a “pop” in the finger.
The DIP joint of his right ring finger is swollen (FIGURE 4), but appears normal otherwise. When you isolate the joint, however, the patient is unable to flex it. You can palpate a stump on the volar surface of the finger.
What’s your next step?
B. Treat with splinting, RICE (rest, ice, compression, and elevation), and nonsteroidal anti-inflammatory drugs.
C. Order an ultrasound of the finger and palm.
D. Order magnetic resonance imaging (MRI) of the hand.
Answer: Order an ultrasound of the football player’s finger and palm (C).
This patient has Jersey finger, caused by a traumatic avulsion of the flexor digitorum profundus (FDP) from the distal phalanx and diagnosed based on the mechanism of injury and the patient’s inability to flex the DIP joint. The injury often does not show on x-rays, and the diagnosis may be missed for several weeks.
Jersey finger usually happens in sports like football or rugby, where players tackle each other, and involves forced, passive extension of the DIP joint at a time of active flexion. Management of Jersey finger starts with splinting, with both the DIP and PIP in slight flexion. Surgical reattachment of the flexor tendon is needed, with best results when it is done within 7 to 10 days of injury.4
You may be able to palpate the tendon stump in the palm or along the digit; bony avulsions can be trapped at the flexor sheath. Soft tissue swelling can be misleading, however, and the point of maximal tenderness is not an accurate means of identifying the avulsed tendon stump.8
Ultrasound is effective in differentiating between a partial and full thickness rupture and in localizing the distal tendon stump.8 MRI is usually reserved for precise evaluation of the tendon edges, to aid in operative planning. If the tendon is retracted to the palm, scarring may be irreversible because of the lack of blood supply.
Athletes typically return to play 12 weeks after injury, starting with protected activity and progressing to full gripping/grasping. Physical therapy and/or occupational therapy will be needed after the surgical wound has healed.
CASE 5 A 40-year-old construction worker who smashed his left index finger with a hammer one day ago presents with severe pain in his fingertip, which he is unable to move. On examination, you find that the distal finger is swollen and there is extensive ecchymosis and swelling underneath the nail. The finger has normal sensation, but you are unable to see capillary refill due to a large hematoma.
X-rays (FIGURE 5) reveal a distal tuft fracture. The patient’s main concern is the pain, and he asks what you can do to relieve it.
What’s your next step?
B. Perform fenestration of the nail.
C. Refer the patient to a hand surgeon.
D. Order computed tomography of the hand.
Answer: Perform fenestration of the construction worker’s nail (B).
This patient has a closed fracture of the distal phalanx, called a tuft fracture, and a sub-ungual hematoma, evident from the x-ray and the physical presentation.
Subungual hematoma requires fenestration with a needle to create small holes in the nail. If the nail bed is lacerated, the nail is removed and the injured nail bed repaired with sutures.
Tuft fractures sometimes require reduction. More often, they are stable and minimally displaced and can be managed conservatively, with splinting with a padded aluminum splint or a fingertip guard (Stax splint) for 3 to 4 weeks. Antibiotics are not indicated unless there is suspicion of an overlying or secondary infection. Referral to a hand surgeon is required for severe crush injuries, avulsion of the nail matrix, and open fractures of the distal phalanx.5,6
Some finger injuries require little more than icing; others are more serious, often emergent, conditions with outcomes that are dependent on an accurate diagnosis and rapid initiation of treatment.
The 5 cases that follow describe injuries with varying degrees of severity. Read each case and select the multiple-choice answer you think is most appropriate. Then read on to find out if you were right—and to learn more about the clinical presentation, diagnosis, and treatment for each type of injury.
CASE 1 A 45-year-old auto body worker walks into your office at 5:30 pm, just as your staff is closing up for the day. A few hours ago, he reports, he was spray-painting a car with a paint gun when he felt a sudden pain in his right index finger. His immediate thought was that he had torn something, but the pain quickly subsided. So he continued to work—until about 45 minutes ago, when the pain became so intense that he knew he needed medical care right away.
Examination reveals redness and increased skin temperature on the radial palmar side of the proximal interphalangeal (PIP) joint of the index finger. Two-point discrimination is decreased to 10 mm, vs 5 mm on the same finger of the opposite hand. The patient can flex his PIP and distal interphalangeal (DIP) joints but complains of pain and stiffness. You obtain x-rays of the injured
finger (FIGURE 1).
WHAT'S YOUR NEXT STEP?
B. Update the patient’s tetanus immunization and start him on a broad-spectrum antibiotic.
C. Put a dorsal splint on the injured finger in the “safe hand” position and schedule a return visit in one week.
D. “Buddy tape” the index and long fingers and refer the patient to a hand surgeon.
Answer: Send the auto-body worker to the nearest ED and call ahead (A).
This patient sustained a high-pressure injection injury to the PIP joint of his right index finger. The patient’s description of how the injury occurred suggested this, and the radiograph confirmed it by showing some paint under the skin (See arrow, FIGURE 1). Such injuries occur when a high pressure (typically from a hose) forces air or a substance—eg, diesel fuel, paint, or solvent—through the skin into the finger.
Although high-pressure injection injury often has a benign presentation, it is actually a medical emergency. If aggressive surgical debridement does not occur within a 6-hour window, the patient runs a high risk for amputation of the digit.1 A hand surgeon should be contacted as soon as possible.
The severity of the injury varies, depending on the amount of pressure (amputation rates are as high as 43% when the pressure per square inch >1000), the type of material injected (diesel fuel is the most toxic), and the location.1,2
Instruct the patient to remove any jewelry, such as a wedding band or watch, on the affected hand or wrist, and to keep the hand elevated. Broad-spectrum antibiotics should be started right away, and a tetanus booster given, if needed. Do not apply heat or use local anesthesia, as both can increase the swelling.2
CASE 2 A 17-year-old cheerleader comes to see you on Monday afternoon, after injuring her left pinky during a Friday night game. The patient, who is right-handed, points to the left PIP joint when you ask where it hurts, and tells you that the finger is stiff. She has been icing it since the injury occurred, to make sure she is ready to cheer by next weekend.
The injury occurred when she was spotting another cheerleader during a routine, the patient reports, adding that the pinky was “dislocated.” The coach “popped” it back in place and buddy-taped the injured finger to her ring finger.
The patient is able to flex and extend the DIP joint on the pinky when the PIP joint is stabilized. She can also flex the PIP joint unassisted, but has difficulty extending it. The digit demonstrates slight flexion of the PIP joint. You note tenderness over both collateral ligaments and the dorsum of the PIP joint, but not over the volar aspect of the injured finger, and order x-rays (FIGURE 2).
WHAT'S YOUR NEXT STEP?
B. Refer the patient to a hand surgeon.
C. Apply an extension block splint so the patient can flex the finger but not extend it, and schedule a follow-up appointment in one to 2 weeks.
D. Apply an aluminum dorsal splint, allowing the DIP joint to be flexed and keeping the PIP joint in full extension for 4 weeks.
Answer: Refer the cheerleader to a hand surgeon (B).
This patient has a rupture of the central extensor tendon of the pinky finger at the PIP joint. The mechanism of injury and her inability to completely extend the injured finger at the PIP joint alert you to this type of injury. An x-ray may sometimes be normal but in this case, it shows the flexion of the PIP joint. Surgical repair of the rupture should be scheduled without delay.3
Most injuries at this joint occur from forced extension, not flexion, and result in a volar plate rupture.4 If swelling and pain make evaluation of an acute dislocation injury difficult, splinting in the “safe hand” position for 72 hours while icing the injured finger will make it possible to do a more detailed follow-up exam.3
Extended periods of splinting can make the PIP joint very stiff, however—and harder to treat than the original injury.5 If the rupture of the central extensor tendon is undetected or simply not treated, a Boutonniere deformity, in which the PIP joint is flexed and the DIP joint is hyperextended, is the likely result.3
CASE 3 A 24-year-old man “jammed” his right ring finger while trying to catch a ball that was passed to him during a pick-up basketball game. He has rested and iced the finger for a couple of days, but it’s still painful and hard to move. He has no significant medical history and has been taking only acetaminophen for the pain.
Examination reveals that the injured finger has good capillary refill, 2-point discrimination is intact at 5 mm, and the other fingers on his right hand have no deformities and a normal range of motion. On the injured finger, however, the DIP joint is swollen and tender; it cannot be fully extended (FiGURE 3).
WHAT'S YOUR DIAGNOSIS?
B. Distal tuft fracture.
C. Mallet finger.
D. Finger sprain.
E. Trigger finger.
Answer: The basketball player has mallet finger (C).
Mallet finger typically occurs on the dominant hand. The key physical finding is that the joint is “stuck” in flexion, which is evident during an exam and on x-ray. Although the DIP joint may be passively fully extended, the patient with mallet finger is unable to actively extend it.
Mallet injuries, which are common in sports and associated with minor trauma, are typically caused by sudden forced flexion of the DIP joint during active extension of the finger. This can either stretch or tear the extensor tendon or lead to avulsion of the tendon insertion from the dorsum of the distal phalanx, with or without a fragment of bone. The injury is called a “soft” mallet finger when there is no bone involvement and a “bony” mallet finger when an avulsion is present, like the one that is evident on the FIGURE 3 x-ray (see arrow).
On clinical examination, the finger may or may not have an obvious deformity; similarly, you won’t always see bruising, swelling, or tenderness over the DIP joint.6 The work-up should include posterior/anterior, oblique, and lateral x-rays, followed by an examination of the soft tissue and a range-of-motion evaluation of the metacarpophalangeal and PIP joints. In acute injuries, tenderness is elicited with palpation over the dorsal aspect of the DIP joint. Although most patients develop an extensor lag at the DIP joint immediately after injury, the deformity may be delayed by a few hours or even days.6,7
Nonsurgical management is the standard of care for most mallet injuries, including mallet fractures involving less than one-third of the articular surface with no associated DIP joint subluxation.7
If there is no displacement, round-the-clock splinting to keep the joint in extension for a minimum of 6 weeks is indicated, followed by 2 to 3 weeks of nighttime splinting. It is important that the splinting allow for complete extension of the DIP joint but flexion of the PIP joint. Keeping the PIP in extension for prolonged periods can lead to permanent stiffness of the joint, while failure to provide any immobilization may lead to permanent deformity.
Surgery is indicated for a fracture fragment involving >30% of the joint surface (as demonstrated in the radiograph), volar subluxation, or a swan neck deformity—and when conservative therapy fails.7
CASE 4 An 18-year-old high school football player presents with pain and swelling at the tip of his right ring finger from an injury that occurred a week ago. When the player he was trying to tackle broke away, the patient says, he immediately felt pain and a “pop” in the finger.
The DIP joint of his right ring finger is swollen (FIGURE 4), but appears normal otherwise. When you isolate the joint, however, the patient is unable to flex it. You can palpate a stump on the volar surface of the finger.
What’s your next step?
B. Treat with splinting, RICE (rest, ice, compression, and elevation), and nonsteroidal anti-inflammatory drugs.
C. Order an ultrasound of the finger and palm.
D. Order magnetic resonance imaging (MRI) of the hand.
Answer: Order an ultrasound of the football player’s finger and palm (C).
This patient has Jersey finger, caused by a traumatic avulsion of the flexor digitorum profundus (FDP) from the distal phalanx and diagnosed based on the mechanism of injury and the patient’s inability to flex the DIP joint. The injury often does not show on x-rays, and the diagnosis may be missed for several weeks.
Jersey finger usually happens in sports like football or rugby, where players tackle each other, and involves forced, passive extension of the DIP joint at a time of active flexion. Management of Jersey finger starts with splinting, with both the DIP and PIP in slight flexion. Surgical reattachment of the flexor tendon is needed, with best results when it is done within 7 to 10 days of injury.4
You may be able to palpate the tendon stump in the palm or along the digit; bony avulsions can be trapped at the flexor sheath. Soft tissue swelling can be misleading, however, and the point of maximal tenderness is not an accurate means of identifying the avulsed tendon stump.8
Ultrasound is effective in differentiating between a partial and full thickness rupture and in localizing the distal tendon stump.8 MRI is usually reserved for precise evaluation of the tendon edges, to aid in operative planning. If the tendon is retracted to the palm, scarring may be irreversible because of the lack of blood supply.
Athletes typically return to play 12 weeks after injury, starting with protected activity and progressing to full gripping/grasping. Physical therapy and/or occupational therapy will be needed after the surgical wound has healed.
CASE 5 A 40-year-old construction worker who smashed his left index finger with a hammer one day ago presents with severe pain in his fingertip, which he is unable to move. On examination, you find that the distal finger is swollen and there is extensive ecchymosis and swelling underneath the nail. The finger has normal sensation, but you are unable to see capillary refill due to a large hematoma.
X-rays (FIGURE 5) reveal a distal tuft fracture. The patient’s main concern is the pain, and he asks what you can do to relieve it.
What’s your next step?
B. Perform fenestration of the nail.
C. Refer the patient to a hand surgeon.
D. Order computed tomography of the hand.
Answer: Perform fenestration of the construction worker’s nail (B).
This patient has a closed fracture of the distal phalanx, called a tuft fracture, and a sub-ungual hematoma, evident from the x-ray and the physical presentation.
Subungual hematoma requires fenestration with a needle to create small holes in the nail. If the nail bed is lacerated, the nail is removed and the injured nail bed repaired with sutures.
Tuft fractures sometimes require reduction. More often, they are stable and minimally displaced and can be managed conservatively, with splinting with a padded aluminum splint or a fingertip guard (Stax splint) for 3 to 4 weeks. Antibiotics are not indicated unless there is suspicion of an overlying or secondary infection. Referral to a hand surgeon is required for severe crush injuries, avulsion of the nail matrix, and open fractures of the distal phalanx.5,6
1. Hogan CJ, Ruland RT. High-pressure injection injuries to the upper extremity: a review of the literature. J Orthop Trauma. 2006;20:503-511.
2. Gonzalez R, Kasdan ML. High pressure injection injuries of the hand. Clin Occup Environ Med. 2006;5:407-411.
3. Freiberg A. Management of proximal interphalangeal joint injuries. Can J Plast Surg. 2007;15:199-203.
4. Perron AD, Brady WJ, Keats TE, et al. Orthopedic pitfalls in the emergency department: closed tendon injuries of the hand. Am J Emerg Med. 2001;19:76-80.
5. Oetgen ME, Dodds SD. Non-operative treatment of common finger injuries. Curr Rev Musculoskel Med. 2008;1:97-102.
6. Anderson D. Mallet finger. Aust Fam Physician. 2011;40:91-92.
7. Smit JM, Beets MR. Treatment options for mallet finger: a review. Plast Reconstr Surg. 2010;126:1624-1629.
8. Goodson A, Morgan M. Current management of Jersey finger in rugby players: cases series and literature review. Hand Surg. 2010;15:103-107.
1. Hogan CJ, Ruland RT. High-pressure injection injuries to the upper extremity: a review of the literature. J Orthop Trauma. 2006;20:503-511.
2. Gonzalez R, Kasdan ML. High pressure injection injuries of the hand. Clin Occup Environ Med. 2006;5:407-411.
3. Freiberg A. Management of proximal interphalangeal joint injuries. Can J Plast Surg. 2007;15:199-203.
4. Perron AD, Brady WJ, Keats TE, et al. Orthopedic pitfalls in the emergency department: closed tendon injuries of the hand. Am J Emerg Med. 2001;19:76-80.
5. Oetgen ME, Dodds SD. Non-operative treatment of common finger injuries. Curr Rev Musculoskel Med. 2008;1:97-102.
6. Anderson D. Mallet finger. Aust Fam Physician. 2011;40:91-92.
7. Smit JM, Beets MR. Treatment options for mallet finger: a review. Plast Reconstr Surg. 2010;126:1624-1629.
8. Goodson A, Morgan M. Current management of Jersey finger in rugby players: cases series and literature review. Hand Surg. 2010;15:103-107.
Should you clear a child with a URI for surgery?
• Consult the anesthesiologist if a pediatric patient is about to undergo an elective surgical procedure and is febrile or coughing—especially if the child has significant comorbidities. These conditions may warrant postponing the procedure. A
• Avoid surgery in a child with cardiac disease who has inflammatory respiratory disease—especially if he or she has had palliative procedures for cyanotic lesions or has a hypoplastic right or left heart. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE You are seeing a 2-year-old boy with a runny nose in your busy clinic. He was sent to you by a local surgeon who plans to repair a hernia 3 days from now. Other than the upper respiratory tract infection, the child is healthy. The surgeon wants you to clear the boy for surgery to avoid the possibility of the anesthesiologist canceling on the day of the procedure.
What are your next steps?
In our experience, children are regularly brought to the ambulatory surgery suite after having seen their family physician or pediatrician. To better equip you for such visits, we’ve put together the following summary of the risks for a child with an upper respiratory infection (URI) who is about to undergo surgery. We’ve also detailed some of the reasoning and evidence behind the decisions that anesthesiologists make in cases like this.
Making decisions in the absence of consensus
While the American Society of Anesthesiologists has a consensus statement on preoperative fasting to reduce the risk of pulmonary aspiration,1 there is no consensus on how to manage a child scheduled for elective surgery who develops a URI.
Historically, any child with a current or recent URI would not be considered a candidate for elective surgery due to the potential for respiratory complications caused by airway irritability.2 These complications can include bronchospasm, laryngospasm, hypoxemia, croup, pulmonary shunting, atelectasis, postoperative apnea, negative pressure pulmonary edema, and airway or endotracheal tube obstruction from increased secretions.3
This concern has been based on the clinical observation that children with URI-related airway irritability are at a greater risk of having such events during the perioperative period.4 In fact, pulmonary function studies reveal an increase in airway irritability for as long as 6 weeks after a significant URI.
Many children with a URI will have airway edema and increased secretions in the upper nasopharynx and the posterior oropharynx down to the level of the vocal cords. In addition, patients with some viral infections—including respiratory syncytial virus—may experience increased edema in the larynx, trachea, and small and large bronchi. The presence of airway inflammation increases mucus production, which is normally coughed out in an awake patient.
The period between the awake state and surgical anesthesia—referred to as Stage 2— is the time of highest risk for the development of laryngospasm. Stage 2 occurs both during the induction of and the emergence from general anesthesia. Children who develop laryngospasm may be difficult to ventilate by mask, and tracheal intubation can be difficult through the closed glottis. In these clinically emergent situations, patients become hypoxemic rapidly. Ventilation may be possible only if the vocal cords are relaxed with agents such as succinylcholine.5
If the anesthesia team cannot quickly treat such laryngospasm, it can lead to postobstructive pulmonary edema. Negative pressure developed in the thorax during spontaneous ventilation against a closed glottis causes a pressure gradient across the alveolar-capillary membrane, leading to movement of fluid into the alveoli, characterized by a typically pink, frothy transudate. Hypoxia may ensue, and the chest x-ray will reveal pulmonary edema. Mild forms may respond to an increase in ambient oxygen alone, but severe cases may require intubation, ventilation, and diuretics to restore the child to a normal state.6
Certain anesthetic agents may be problematic
Unfortunately, airway irritability is only one of many problems to contend with. Inhalational anesthetic agents have an adverse effect on the mucociliary elevator, as well.7,8 Cilia on the surface of epithelial cells lining the trachea and bronchi act to move mucus from the distal to the proximal airway so that it can be coughed out. Failure of this mechanism in a child with an inflammatory condition in the airway increases the risk of atelectasis from thickened secretions and occasionally from pneumonia.
Most of the potent general anesthetic agents have significant bronchodilatory properties. But desflurane, a commonly used agent, causes bronchoconstriction when used in a patient with an irritated, infected airway.9This agent will produce predictable wheezing from bronchospasm, especially in patients who have confounding pulmonary disease such as asthma.
Talk to the anesthesiologist. With these concerns in mind, clinicians must consider the type of anesthetic and the nature of the surgical procedure and discuss these issues with the anesthesiologist in the preoperative period. Some anesthetic agents and techniques are less irritating to airways.2,3 Avoidance of both desflurane and endotracheal intubation, for instance, will minimize airway irritation.
Brief procedures that do not involve major body cavities (eg, abdominal, thoracic, and intracranial) may be done without instrumenting the trachea. Face masks and laryngeal mask airways have been shown to decrease the incidence of adverse reactions because these forms of airway management are less invasive and physiologically insulting than direct laryngoscopy and endotracheal intubation.
Clinical observations suggest that endotracheal intubation increases pulmonary risks for the child with a URI.10,11 Long procedures, a patient position that limits access to the airway, the anticipated need to use muscle relaxants, airway surgery, and surgery in major cavities all require intubation for airway management. In these circumstances, it’s best to plan the perioperative care of a child suffering from a URI with an anesthesiologist who is comfortable caring for pediatric patients.12
Proceeding with surgery despite the risks
During emergency procedures on infants and children, the anesthesiologist has to do the best possible job under less than ideal conditions. Bowel obstruction, an incarcerated inguinal hernia, or a foreign body in the airway can all be life-threatening. In these cases, the anesthesiologist will counsel the surgeon and parent on the risks of the anesthetic. They likely will proceed with the knowledge that the usual methods of anesthetizing a child may have to be altered to provide the safest possible conditions.
But even certain nonemergent procedures may require taking some risks. Anesthesiologists are likely to anesthetize a child for placement of pressure-equalizing (PE) tubes, for instance, even with a mild infection in the upper oropharynx. This is because the possibility is high that the patient will be infected throughout the winter season, and waiting for a URI-free period might mean that the child would not get the PE tubes at all. Furthermore, PE tube placement is performed very quickly, with no instrumentation of the airway necessary. The anesthesiologist performs a mask anesthetic, always has control and access to the airway, and the procedure can be aborted at any time, with no incision to close.
How long should you wait it a URI is serious?
As mentioned earlier, there is no consensus on how long to wait, but clinical studies have suggested delaying surgery for as long as 6 weeks after the acute episode.4 The thinking was that this long period allowed time for the inflammatory response to dissipate completely. Unfortunately, in the middle of the winter, it’s likely that the child will be exposed to another viral strain and develop yet another URI. Clinical judgment plays a pivotal role here; it is always best to establish a relationship with an anesthesiologist in your community and call him or her with questions about individual patients.
Before you sign off on surgery
There are several other circumstances to consider when approving a child with a URI for surgery.
Children with cardiac disease, especially those who have had palliative procedures for cyanotic lesions or who have a hypoplastic right or left heart, are characteristically unstable in the face of inflammatory respiratory disease. Unless the surgical procedure is an emergency, such patients should not be considered for general anesthesia if they have a URI.13 As an example, bronchiolitis plus cyanotic heart disease can be rapidly fatal, requiring prolonged ventilation or extracorporeal membrane oxygenation in order to save the patient.
Intensive care nursery “graduates” may present to your office for preoperative assessment. Many of these infants and children will have marginally compensated lung disease, some with substantial pulmonary hypertension. Their respiratory function will continue to improve, some until the age of 7 to 10 years. In the meantime, they, too, are at high risk for complications from general anesthesia if they have a URI, and the decision to take them to the operating room should be discussed with other care providers and the parents.
Children with fever, mucopurulent discharge, wheezing, lethargy, and cough are at high risk for complications during the perioperative period, regardless of any comorbidities. Many anesthesiologists would cancel surgery in these circumstances, even if the patient has been seen recently by his or her primary care physician and is taking antibiotics for coverage of a potential bacterial infection.
Other indicators of increased risk of pulmonary complications include a history of reactive airway disease, exposure to tobacco smoke, snoring, nasal congestion, the need for endotracheal intubation, and surgery on the airway.14
CASE You evaluate the 2-year-old and note that he has a history of mucopurulent nasal discharge and a productive cough. The child’s temperature in the clinic is 99.8°F and his chest x-ray is consistent with bronchitis. After talking with a local anesthesiologist and the surgeon, you all agree that the boy’s surgery should be postponed for a month.
1. American Society of Anesthesiologists Task Force on Preoperative Fasting. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 1999;90:896-905.
2. Parnis SJ, Barker DS, Van Der Walt JH. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11:29-40.
3. Cote CJ. The upper respiratory tract infection URI dilemma: fear of a complication or litigation? Anesthesiology. 2001;95:283-285.
4. Nandwani N, Raphael JH, Langton JA. Effect of an upper respiratory tract infection on airway reactivity. Br J Anaesth. 1997;
78:352-355.
5. Hampson-Evans D, Morgan P, Farrar M. Pediatric laryngospasm. Pediatr Anesth. 2008;18:303-307.
6. Krodel DJ, Bittner BA, Abdulnour R, et al. Case scenario: acute negative pressure pulmonary edema. Anesthesiology. 2010;113: 200-207.
7. Forbes AR. Halothane depresses mucociliary flow in the trachea. Anesthesiology. 1976;45:59-63.
8. Dikmen Y, Eminoglu E, Salihoglu Z, et al. Pulmonary mechanics during isoflurane, sevoflurane, and desflurane anaesthesia.
Anaesthesia. 2003;58:745-748.
9. Forbes AR, Horrigan RW. Mucociliary flow in the trachea during anesthesia with enflurane, ether, nitrous oxide and morphine. Anesthesiology. 1977;46:319-321.
10. Tait AR, Pandit UA, Voepel-Lewis T, et al. Use of the laryngeal mask airway in children with upper respiratory infections: a comparison with endotracheal intubation. Anesth Analg. 1998;
86:701-711.
11. Tait AR, Malviya S, Voepel-Lewis T, et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299-306.
12. Von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in pediatric anaesthesia: a prospective cohort study. Lancet. 2010;376:773-783.
13. Malviya S, Voepel-Lewis T, Siewert M, et al. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628-632.
14. Tait AR, Malviya S. Anesthesia for the child with an upper respiratory infection: still a dilemma? Anesth Analg. 2005;100:59-65.
• Consult the anesthesiologist if a pediatric patient is about to undergo an elective surgical procedure and is febrile or coughing—especially if the child has significant comorbidities. These conditions may warrant postponing the procedure. A
• Avoid surgery in a child with cardiac disease who has inflammatory respiratory disease—especially if he or she has had palliative procedures for cyanotic lesions or has a hypoplastic right or left heart. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE You are seeing a 2-year-old boy with a runny nose in your busy clinic. He was sent to you by a local surgeon who plans to repair a hernia 3 days from now. Other than the upper respiratory tract infection, the child is healthy. The surgeon wants you to clear the boy for surgery to avoid the possibility of the anesthesiologist canceling on the day of the procedure.
What are your next steps?
In our experience, children are regularly brought to the ambulatory surgery suite after having seen their family physician or pediatrician. To better equip you for such visits, we’ve put together the following summary of the risks for a child with an upper respiratory infection (URI) who is about to undergo surgery. We’ve also detailed some of the reasoning and evidence behind the decisions that anesthesiologists make in cases like this.
Making decisions in the absence of consensus
While the American Society of Anesthesiologists has a consensus statement on preoperative fasting to reduce the risk of pulmonary aspiration,1 there is no consensus on how to manage a child scheduled for elective surgery who develops a URI.
Historically, any child with a current or recent URI would not be considered a candidate for elective surgery due to the potential for respiratory complications caused by airway irritability.2 These complications can include bronchospasm, laryngospasm, hypoxemia, croup, pulmonary shunting, atelectasis, postoperative apnea, negative pressure pulmonary edema, and airway or endotracheal tube obstruction from increased secretions.3
This concern has been based on the clinical observation that children with URI-related airway irritability are at a greater risk of having such events during the perioperative period.4 In fact, pulmonary function studies reveal an increase in airway irritability for as long as 6 weeks after a significant URI.
Many children with a URI will have airway edema and increased secretions in the upper nasopharynx and the posterior oropharynx down to the level of the vocal cords. In addition, patients with some viral infections—including respiratory syncytial virus—may experience increased edema in the larynx, trachea, and small and large bronchi. The presence of airway inflammation increases mucus production, which is normally coughed out in an awake patient.
The period between the awake state and surgical anesthesia—referred to as Stage 2— is the time of highest risk for the development of laryngospasm. Stage 2 occurs both during the induction of and the emergence from general anesthesia. Children who develop laryngospasm may be difficult to ventilate by mask, and tracheal intubation can be difficult through the closed glottis. In these clinically emergent situations, patients become hypoxemic rapidly. Ventilation may be possible only if the vocal cords are relaxed with agents such as succinylcholine.5
If the anesthesia team cannot quickly treat such laryngospasm, it can lead to postobstructive pulmonary edema. Negative pressure developed in the thorax during spontaneous ventilation against a closed glottis causes a pressure gradient across the alveolar-capillary membrane, leading to movement of fluid into the alveoli, characterized by a typically pink, frothy transudate. Hypoxia may ensue, and the chest x-ray will reveal pulmonary edema. Mild forms may respond to an increase in ambient oxygen alone, but severe cases may require intubation, ventilation, and diuretics to restore the child to a normal state.6
Certain anesthetic agents may be problematic
Unfortunately, airway irritability is only one of many problems to contend with. Inhalational anesthetic agents have an adverse effect on the mucociliary elevator, as well.7,8 Cilia on the surface of epithelial cells lining the trachea and bronchi act to move mucus from the distal to the proximal airway so that it can be coughed out. Failure of this mechanism in a child with an inflammatory condition in the airway increases the risk of atelectasis from thickened secretions and occasionally from pneumonia.
Most of the potent general anesthetic agents have significant bronchodilatory properties. But desflurane, a commonly used agent, causes bronchoconstriction when used in a patient with an irritated, infected airway.9This agent will produce predictable wheezing from bronchospasm, especially in patients who have confounding pulmonary disease such as asthma.
Talk to the anesthesiologist. With these concerns in mind, clinicians must consider the type of anesthetic and the nature of the surgical procedure and discuss these issues with the anesthesiologist in the preoperative period. Some anesthetic agents and techniques are less irritating to airways.2,3 Avoidance of both desflurane and endotracheal intubation, for instance, will minimize airway irritation.
Brief procedures that do not involve major body cavities (eg, abdominal, thoracic, and intracranial) may be done without instrumenting the trachea. Face masks and laryngeal mask airways have been shown to decrease the incidence of adverse reactions because these forms of airway management are less invasive and physiologically insulting than direct laryngoscopy and endotracheal intubation.
Clinical observations suggest that endotracheal intubation increases pulmonary risks for the child with a URI.10,11 Long procedures, a patient position that limits access to the airway, the anticipated need to use muscle relaxants, airway surgery, and surgery in major cavities all require intubation for airway management. In these circumstances, it’s best to plan the perioperative care of a child suffering from a URI with an anesthesiologist who is comfortable caring for pediatric patients.12
Proceeding with surgery despite the risks
During emergency procedures on infants and children, the anesthesiologist has to do the best possible job under less than ideal conditions. Bowel obstruction, an incarcerated inguinal hernia, or a foreign body in the airway can all be life-threatening. In these cases, the anesthesiologist will counsel the surgeon and parent on the risks of the anesthetic. They likely will proceed with the knowledge that the usual methods of anesthetizing a child may have to be altered to provide the safest possible conditions.
But even certain nonemergent procedures may require taking some risks. Anesthesiologists are likely to anesthetize a child for placement of pressure-equalizing (PE) tubes, for instance, even with a mild infection in the upper oropharynx. This is because the possibility is high that the patient will be infected throughout the winter season, and waiting for a URI-free period might mean that the child would not get the PE tubes at all. Furthermore, PE tube placement is performed very quickly, with no instrumentation of the airway necessary. The anesthesiologist performs a mask anesthetic, always has control and access to the airway, and the procedure can be aborted at any time, with no incision to close.
How long should you wait it a URI is serious?
As mentioned earlier, there is no consensus on how long to wait, but clinical studies have suggested delaying surgery for as long as 6 weeks after the acute episode.4 The thinking was that this long period allowed time for the inflammatory response to dissipate completely. Unfortunately, in the middle of the winter, it’s likely that the child will be exposed to another viral strain and develop yet another URI. Clinical judgment plays a pivotal role here; it is always best to establish a relationship with an anesthesiologist in your community and call him or her with questions about individual patients.
Before you sign off on surgery
There are several other circumstances to consider when approving a child with a URI for surgery.
Children with cardiac disease, especially those who have had palliative procedures for cyanotic lesions or who have a hypoplastic right or left heart, are characteristically unstable in the face of inflammatory respiratory disease. Unless the surgical procedure is an emergency, such patients should not be considered for general anesthesia if they have a URI.13 As an example, bronchiolitis plus cyanotic heart disease can be rapidly fatal, requiring prolonged ventilation or extracorporeal membrane oxygenation in order to save the patient.
Intensive care nursery “graduates” may present to your office for preoperative assessment. Many of these infants and children will have marginally compensated lung disease, some with substantial pulmonary hypertension. Their respiratory function will continue to improve, some until the age of 7 to 10 years. In the meantime, they, too, are at high risk for complications from general anesthesia if they have a URI, and the decision to take them to the operating room should be discussed with other care providers and the parents.
Children with fever, mucopurulent discharge, wheezing, lethargy, and cough are at high risk for complications during the perioperative period, regardless of any comorbidities. Many anesthesiologists would cancel surgery in these circumstances, even if the patient has been seen recently by his or her primary care physician and is taking antibiotics for coverage of a potential bacterial infection.
Other indicators of increased risk of pulmonary complications include a history of reactive airway disease, exposure to tobacco smoke, snoring, nasal congestion, the need for endotracheal intubation, and surgery on the airway.14
CASE You evaluate the 2-year-old and note that he has a history of mucopurulent nasal discharge and a productive cough. The child’s temperature in the clinic is 99.8°F and his chest x-ray is consistent with bronchitis. After talking with a local anesthesiologist and the surgeon, you all agree that the boy’s surgery should be postponed for a month.
• Consult the anesthesiologist if a pediatric patient is about to undergo an elective surgical procedure and is febrile or coughing—especially if the child has significant comorbidities. These conditions may warrant postponing the procedure. A
• Avoid surgery in a child with cardiac disease who has inflammatory respiratory disease—especially if he or she has had palliative procedures for cyanotic lesions or has a hypoplastic right or left heart. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE You are seeing a 2-year-old boy with a runny nose in your busy clinic. He was sent to you by a local surgeon who plans to repair a hernia 3 days from now. Other than the upper respiratory tract infection, the child is healthy. The surgeon wants you to clear the boy for surgery to avoid the possibility of the anesthesiologist canceling on the day of the procedure.
What are your next steps?
In our experience, children are regularly brought to the ambulatory surgery suite after having seen their family physician or pediatrician. To better equip you for such visits, we’ve put together the following summary of the risks for a child with an upper respiratory infection (URI) who is about to undergo surgery. We’ve also detailed some of the reasoning and evidence behind the decisions that anesthesiologists make in cases like this.
Making decisions in the absence of consensus
While the American Society of Anesthesiologists has a consensus statement on preoperative fasting to reduce the risk of pulmonary aspiration,1 there is no consensus on how to manage a child scheduled for elective surgery who develops a URI.
Historically, any child with a current or recent URI would not be considered a candidate for elective surgery due to the potential for respiratory complications caused by airway irritability.2 These complications can include bronchospasm, laryngospasm, hypoxemia, croup, pulmonary shunting, atelectasis, postoperative apnea, negative pressure pulmonary edema, and airway or endotracheal tube obstruction from increased secretions.3
This concern has been based on the clinical observation that children with URI-related airway irritability are at a greater risk of having such events during the perioperative period.4 In fact, pulmonary function studies reveal an increase in airway irritability for as long as 6 weeks after a significant URI.
Many children with a URI will have airway edema and increased secretions in the upper nasopharynx and the posterior oropharynx down to the level of the vocal cords. In addition, patients with some viral infections—including respiratory syncytial virus—may experience increased edema in the larynx, trachea, and small and large bronchi. The presence of airway inflammation increases mucus production, which is normally coughed out in an awake patient.
The period between the awake state and surgical anesthesia—referred to as Stage 2— is the time of highest risk for the development of laryngospasm. Stage 2 occurs both during the induction of and the emergence from general anesthesia. Children who develop laryngospasm may be difficult to ventilate by mask, and tracheal intubation can be difficult through the closed glottis. In these clinically emergent situations, patients become hypoxemic rapidly. Ventilation may be possible only if the vocal cords are relaxed with agents such as succinylcholine.5
If the anesthesia team cannot quickly treat such laryngospasm, it can lead to postobstructive pulmonary edema. Negative pressure developed in the thorax during spontaneous ventilation against a closed glottis causes a pressure gradient across the alveolar-capillary membrane, leading to movement of fluid into the alveoli, characterized by a typically pink, frothy transudate. Hypoxia may ensue, and the chest x-ray will reveal pulmonary edema. Mild forms may respond to an increase in ambient oxygen alone, but severe cases may require intubation, ventilation, and diuretics to restore the child to a normal state.6
Certain anesthetic agents may be problematic
Unfortunately, airway irritability is only one of many problems to contend with. Inhalational anesthetic agents have an adverse effect on the mucociliary elevator, as well.7,8 Cilia on the surface of epithelial cells lining the trachea and bronchi act to move mucus from the distal to the proximal airway so that it can be coughed out. Failure of this mechanism in a child with an inflammatory condition in the airway increases the risk of atelectasis from thickened secretions and occasionally from pneumonia.
Most of the potent general anesthetic agents have significant bronchodilatory properties. But desflurane, a commonly used agent, causes bronchoconstriction when used in a patient with an irritated, infected airway.9This agent will produce predictable wheezing from bronchospasm, especially in patients who have confounding pulmonary disease such as asthma.
Talk to the anesthesiologist. With these concerns in mind, clinicians must consider the type of anesthetic and the nature of the surgical procedure and discuss these issues with the anesthesiologist in the preoperative period. Some anesthetic agents and techniques are less irritating to airways.2,3 Avoidance of both desflurane and endotracheal intubation, for instance, will minimize airway irritation.
Brief procedures that do not involve major body cavities (eg, abdominal, thoracic, and intracranial) may be done without instrumenting the trachea. Face masks and laryngeal mask airways have been shown to decrease the incidence of adverse reactions because these forms of airway management are less invasive and physiologically insulting than direct laryngoscopy and endotracheal intubation.
Clinical observations suggest that endotracheal intubation increases pulmonary risks for the child with a URI.10,11 Long procedures, a patient position that limits access to the airway, the anticipated need to use muscle relaxants, airway surgery, and surgery in major cavities all require intubation for airway management. In these circumstances, it’s best to plan the perioperative care of a child suffering from a URI with an anesthesiologist who is comfortable caring for pediatric patients.12
Proceeding with surgery despite the risks
During emergency procedures on infants and children, the anesthesiologist has to do the best possible job under less than ideal conditions. Bowel obstruction, an incarcerated inguinal hernia, or a foreign body in the airway can all be life-threatening. In these cases, the anesthesiologist will counsel the surgeon and parent on the risks of the anesthetic. They likely will proceed with the knowledge that the usual methods of anesthetizing a child may have to be altered to provide the safest possible conditions.
But even certain nonemergent procedures may require taking some risks. Anesthesiologists are likely to anesthetize a child for placement of pressure-equalizing (PE) tubes, for instance, even with a mild infection in the upper oropharynx. This is because the possibility is high that the patient will be infected throughout the winter season, and waiting for a URI-free period might mean that the child would not get the PE tubes at all. Furthermore, PE tube placement is performed very quickly, with no instrumentation of the airway necessary. The anesthesiologist performs a mask anesthetic, always has control and access to the airway, and the procedure can be aborted at any time, with no incision to close.
How long should you wait it a URI is serious?
As mentioned earlier, there is no consensus on how long to wait, but clinical studies have suggested delaying surgery for as long as 6 weeks after the acute episode.4 The thinking was that this long period allowed time for the inflammatory response to dissipate completely. Unfortunately, in the middle of the winter, it’s likely that the child will be exposed to another viral strain and develop yet another URI. Clinical judgment plays a pivotal role here; it is always best to establish a relationship with an anesthesiologist in your community and call him or her with questions about individual patients.
Before you sign off on surgery
There are several other circumstances to consider when approving a child with a URI for surgery.
Children with cardiac disease, especially those who have had palliative procedures for cyanotic lesions or who have a hypoplastic right or left heart, are characteristically unstable in the face of inflammatory respiratory disease. Unless the surgical procedure is an emergency, such patients should not be considered for general anesthesia if they have a URI.13 As an example, bronchiolitis plus cyanotic heart disease can be rapidly fatal, requiring prolonged ventilation or extracorporeal membrane oxygenation in order to save the patient.
Intensive care nursery “graduates” may present to your office for preoperative assessment. Many of these infants and children will have marginally compensated lung disease, some with substantial pulmonary hypertension. Their respiratory function will continue to improve, some until the age of 7 to 10 years. In the meantime, they, too, are at high risk for complications from general anesthesia if they have a URI, and the decision to take them to the operating room should be discussed with other care providers and the parents.
Children with fever, mucopurulent discharge, wheezing, lethargy, and cough are at high risk for complications during the perioperative period, regardless of any comorbidities. Many anesthesiologists would cancel surgery in these circumstances, even if the patient has been seen recently by his or her primary care physician and is taking antibiotics for coverage of a potential bacterial infection.
Other indicators of increased risk of pulmonary complications include a history of reactive airway disease, exposure to tobacco smoke, snoring, nasal congestion, the need for endotracheal intubation, and surgery on the airway.14
CASE You evaluate the 2-year-old and note that he has a history of mucopurulent nasal discharge and a productive cough. The child’s temperature in the clinic is 99.8°F and his chest x-ray is consistent with bronchitis. After talking with a local anesthesiologist and the surgeon, you all agree that the boy’s surgery should be postponed for a month.
1. American Society of Anesthesiologists Task Force on Preoperative Fasting. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 1999;90:896-905.
2. Parnis SJ, Barker DS, Van Der Walt JH. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11:29-40.
3. Cote CJ. The upper respiratory tract infection URI dilemma: fear of a complication or litigation? Anesthesiology. 2001;95:283-285.
4. Nandwani N, Raphael JH, Langton JA. Effect of an upper respiratory tract infection on airway reactivity. Br J Anaesth. 1997;
78:352-355.
5. Hampson-Evans D, Morgan P, Farrar M. Pediatric laryngospasm. Pediatr Anesth. 2008;18:303-307.
6. Krodel DJ, Bittner BA, Abdulnour R, et al. Case scenario: acute negative pressure pulmonary edema. Anesthesiology. 2010;113: 200-207.
7. Forbes AR. Halothane depresses mucociliary flow in the trachea. Anesthesiology. 1976;45:59-63.
8. Dikmen Y, Eminoglu E, Salihoglu Z, et al. Pulmonary mechanics during isoflurane, sevoflurane, and desflurane anaesthesia.
Anaesthesia. 2003;58:745-748.
9. Forbes AR, Horrigan RW. Mucociliary flow in the trachea during anesthesia with enflurane, ether, nitrous oxide and morphine. Anesthesiology. 1977;46:319-321.
10. Tait AR, Pandit UA, Voepel-Lewis T, et al. Use of the laryngeal mask airway in children with upper respiratory infections: a comparison with endotracheal intubation. Anesth Analg. 1998;
86:701-711.
11. Tait AR, Malviya S, Voepel-Lewis T, et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299-306.
12. Von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in pediatric anaesthesia: a prospective cohort study. Lancet. 2010;376:773-783.
13. Malviya S, Voepel-Lewis T, Siewert M, et al. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628-632.
14. Tait AR, Malviya S. Anesthesia for the child with an upper respiratory infection: still a dilemma? Anesth Analg. 2005;100:59-65.
1. American Society of Anesthesiologists Task Force on Preoperative Fasting. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 1999;90:896-905.
2. Parnis SJ, Barker DS, Van Der Walt JH. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11:29-40.
3. Cote CJ. The upper respiratory tract infection URI dilemma: fear of a complication or litigation? Anesthesiology. 2001;95:283-285.
4. Nandwani N, Raphael JH, Langton JA. Effect of an upper respiratory tract infection on airway reactivity. Br J Anaesth. 1997;
78:352-355.
5. Hampson-Evans D, Morgan P, Farrar M. Pediatric laryngospasm. Pediatr Anesth. 2008;18:303-307.
6. Krodel DJ, Bittner BA, Abdulnour R, et al. Case scenario: acute negative pressure pulmonary edema. Anesthesiology. 2010;113: 200-207.
7. Forbes AR. Halothane depresses mucociliary flow in the trachea. Anesthesiology. 1976;45:59-63.
8. Dikmen Y, Eminoglu E, Salihoglu Z, et al. Pulmonary mechanics during isoflurane, sevoflurane, and desflurane anaesthesia.
Anaesthesia. 2003;58:745-748.
9. Forbes AR, Horrigan RW. Mucociliary flow in the trachea during anesthesia with enflurane, ether, nitrous oxide and morphine. Anesthesiology. 1977;46:319-321.
10. Tait AR, Pandit UA, Voepel-Lewis T, et al. Use of the laryngeal mask airway in children with upper respiratory infections: a comparison with endotracheal intubation. Anesth Analg. 1998;
86:701-711.
11. Tait AR, Malviya S, Voepel-Lewis T, et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299-306.
12. Von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in pediatric anaesthesia: a prospective cohort study. Lancet. 2010;376:773-783.
13. Malviya S, Voepel-Lewis T, Siewert M, et al. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628-632.
14. Tait AR, Malviya S. Anesthesia for the child with an upper respiratory infection: still a dilemma? Anesth Analg. 2005;100:59-65.
Travelers' diarrhea: Prevention, treatment, and post-trip evaluation
1. Recommend antibiotic chemoprophylaxis for travelers at high risk for travelers’ diarrhea (TD) and those at high risk for complications. It is also appropriate for travelers who have an inflexible itinerary. B
2. Recommend bismuth subsalicylate chemoprophylaxis for travelers at high risk for TD who are willing to comply with the regimen and want to avoid antibiotic prophylaxis. B
3. Advise travelers to initiate self-treatment for TD with a fluoroquinolone (or azithromycin, if in South or Southeast Asia) at the onset of diarrhea if it is bloody or accompanied by fever. A
NOTE: This practice recommendation in the print version of this article stated that travelers should also take loperamide; however, both the Centers for Disease Control and Prevention and the Infectious Diseases Society of America advise against the use of loperamide by travelers with fever or bloody diarrhea [corrected August 27, 2013].
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 40-year-old female patient, a childhood immigrant from India, is seeking advice regarding her upcoming 2-week trip to Mumbai. She is taking her 2 children, ages 16 years and 16 months, to visit their grandparents for the first time. She has made this trip alone a few times and has invariably experienced short bouts of self-limited diarrheal illness. She wonders what she might do to prevent travelers’ diarrhea. Her only medical problem is rheumatoid arthritis, which has been well controlled with methotrexate. Her children are healthy. What would you recommend?
Recommendations regarding travelers’ diarrhea (TD) address prevention and management. Prevention encompasses advice about personal behaviors and the use of chemoprophylaxis (antimicrobial and non-antimicrobial) and vaccinations. Since international travelers are known to treat themselves for diarrheal illnesses during their trips,1 recommendations regarding management should assume self-treatment and include the use of both antibiotics and non-antibiotic remedies. Pretravel recommendations will of course be most effective if they account for the individual’s risk for TD.
Innate patient susceptibility, destination, and dietary choices determine TD risk
TD is generally defined as the passage of 3 of more loose stools in a 24-hour period, with associated symptoms of enteric infection—eg, fever, nausea, vomiting, or abdominal cramping. Defined in this manner, TD is thought to occur in 60% to 70% of individuals who travel from developed countries to less-developed countries.2,4 Risk of TD is influenced both by intrinsic personal factors and by factors specific to the trip.
Personal risk factorsIndividual variation in susceptibility to TD might result from a genetic predisposition arising from single nucleotide polymorphisms governing various inflammatory marker proteins.5 A history of multiple episodes of TD, especially if fellow travelers were spared, can suggest this kind of individual susceptibility. Other factors that increase vulnerability to TD are immunodeficiency, achlorhydric states such as atrophic gastritis, and chronic use of proton pump inhibitors.6,7 However, the trip itself is much more important in assessing risk for TD.
Trip-related risk factors
The destination. The most salient risk factor for TD is the geographic destination. Regions of the world can be divided into TD risk strata:2
- Very high: South Asia
- High: South America, Sub-Saharan Africa
- Medium: Central America, Mexico, Caribbean, Middle East, North Africa, Southeast Asia, Oceania
- Low: Europe, North America (excluding Mexico), Australasia, Northeast Asia.
Particularly notable countries, in descending order of risk, are Nepal, India, Myanmar, Bolivia, Sri Lanka, Ecuador, Peru, Kenya, and Guatemala.2
Dietary choices. Additionally, since travelers acquire TD by ingesting food or beverages contaminated with pathogenic fecal microbes, dietary behaviors during the trip affect their susceptibility. At least risk are business travelers and tourists who confine their activities to more affluent settings in which food and beverages are prepared and stored hygienically.1,4,8,9 At greater risk are travelers who immerse themselves in local culture, visiting locations that are more impoverished and not as well equipped with sanitation systems, especially if their stay is at least 2 to 3 weeks.1,4,8,9
Also, the older a traveler is, the lower his or her risk of TD.1,9 An exception to this might be infants whose diet consists solely of breast milk or formula prepared under sanitary conditions.
Mandates and options for preventing TD
Emphasize food and beverage precautions
It might be reasonable to expect that travelers who are circumspect about their food and beverage choices on trips will be able to avoid TD. Indeed, this is the basis for the aphorism, “Boil it, peel it, or forget it.” Guidelines routinely recommend that travelers restrict their selection of foods to those that have been well cooked and are served while still very hot, and to fruits and vegetables that they peel themselves. Likewise, they should drink only beverages that have been boiled or are in sealed bottles or under carbonation and served without ice.10-12 Many travelers might find these recommendations too restrictive to follow faithfully. Moreover, studies suggest it may not be possible for even the most assiduous traveler to fully avoid the risk of TD.13,14 The hygienic characteristics of the travel destination may be more determinative, as illustrated by the successful reduction of TD rates in Jamaica by improving sanitation in tourist resorts.15
Antibiotic chemoprophylaxis: A debated practice with limited consensusThe etiologic agents of TD are multiple and vary somewhat in predominance according to geographic region.3,16,17 TABLE 1 depicts variance by region.16 The most common pathogens are strains of the bacterium Escherichia coli, particularly enterotoxigenic (ETEC), enteroaggregative (EAEC), and enteropathogenic (EPEC) strains.16 Other bacteria of importance are Campylobacter, Salmonella, and Shigella. Viruses, particularly norovirus (notably connected with cruise ships), can also cause TD, although it is implicated in no more than 17% of cases.18 Parasitic pathogens are even less common causes of TD (4%-10%) and mainly involve the protozoa, Giardia lamblia, and, to a lesser extent, Entamoeba histolytica and Cryptosporidium.
Although some pathogens often have a characteristic presentation—such as frothy, greasy diarrhea in the case of G lamblia—they generally cannot be reliably distinguished from one another clinically. Notably, up to 50% of stool samples from TD patients do not yield any pathogen,16 raising the suspicion that current diagnostic technology is not sufficiently sensitive to routinely identify certain bacteria.
There is no consensus on recommending antibiotic chemoprophylaxis against TD.
Opponents of this practice10-12,19,20 point out that TD is generally a brief (3-5 days), self-limited illness. Moreover, concerns about antibiotic resistance have come to pass. Previously used agents, trimethoprim-sulfamethoxazole and doxycycline, are no longer effective in preventing or treating TD. In addition, antibiotic use carries the risk of allergic reactions as well as other adverse effects including, ironically, the development of antibiotic-associated diarrhea and Clostridium difficile diarrhea.
Proponents of antibiotic chemoprophylaxis21,22 point to its demonstrated efficacy in reducing the risk of TD by 4% to 40%.11 They also argue that at least 20% to 25% of travelers who get TD must significantly curtail their activities for a day or more.1,23 This change in travel plans is associated not only with significant personal loss but also imposes a financial burden.23 Furthermore, TD is known to have longer-term effects. Up to 10% of sufferers develop postinfectious irritable bowel syndrome (PI-IBS) that can last for 5 or 6 years.21,22,24,25 It is not known, however, whether the use of antibiotic chemoprophylaxis significantly reduces the incidence of PI-IBS.
Finally, the luminal antibiotic, rifaximin, nonabsorbable as it is, is very well-tolerated and holds promise for not inciting antibiotic resistance.22 However, while its efficacy in preventing TD has been demonstrated in various settings,22,26,27 it is not approved by the US Food and Drug Administration for this indication. Also, concerns persist that it might not be effective in preventing TD caused by invasive pathogens.19
Indications on which all agree. Even opponents of antibiotic chemoprophylaxis grant that it is probably warranted for 2 groups of travelers.10-12 The first is those whose trip schedule is of such importance that any deviation would be intolerable. The second is travelers with comorbidities that would render them at high risk for serious inconvenience or illness if they developed TD. Examples of the latter include patients with enterostomies, mobility impairments, immune suppression, inflammatory bowel disease, and renal or metabolic diseases.
Chemoprophylaxis regimens. If you prescribe an antibiotic prophylactically, consider daily doses of a fluoroquinolone (eg, ciprofloxacin 500 mg orally once daily, not twice daily as for treatment) or rifaximin 200 mg orally once or twice a day, for no longer than 2 to 3 weeks.10
Non-antimicrobial chemoprophylaxis
Bismuth subsalicylate has reduced the incidence of TD from 40% to just 14% when taken in doses of 2 chewable tablets or 60 mL of liquid 4 times daily. 11,19,22 However, the dosing frequency can hinder adherence. Moreover, the relatively high doses required raise the risk of adverse drug reactions such as blackening of the tongue and stool, nausea, constipation, Reye syndrome (in children under 12 years), and possibly tinnitus. The salicylate component of the drug poses a threat to patients with aspirin allergy, renal disease, and those taking anticoagulants. Drug interactions with probenecid and methotrexate are also possible. Bismuth is not recommended for use for longer than 3 weeks, or for children younger than 3 years or pregnant women in their third trimester.
Other non-antimicrobial chemoprophylaxis agents include probiotics such as Lactobacillus andSaccharomyces. These preparations of bacteria and fungi are marketed either singly or in blends of varying composition and proportion. The evidence is divided on their efficacy, and even though some meta-analyses have concluded probiotics such as Saccharomyces boulardii are useful in preventing TD, endorsement in clinical guidelines is muted.10-12,28-30
Immunizations have limited value so farNatural immunity to E coli gastrointestinal infection among indigenous people in less developed countries has raised the possibility of a role for vaccines in preventing TD. Some strains of ETEC produce a heat-labile toxin (LT) that bears significant resemblance to the toxin produced by Vibrio cholerae. Therefore, the oral cholera vaccine, Dukoral, has been marketed outside the United States for the prevention of TD.19,22 However, only ≤7% of TD cases worldwide would be prevented by routine use of this vaccine.31 A transdermal LT vaccine, which involves the antigen-presenting Langerhans cells in the superficial skin layers, is promising but not yet available for routine use.19,22
Treating TD and associated symptoms
Antibiotic treatment
Given that most cases of TD are caused by bacterial pathogens, antibiotics are considered the mainstay of treatment. Concerns about the ill effects of antibiotic use in the case of enterohemorrhagic E coli(EHEC O157:H7) can be allayed because this strain is rarely a cause of TD.9Patient factors that increase vulnerability to TD are immunodeficiency, achlorhydric states such as atrophic gastritis, and chronic use of proton pump inhibitors.
Consider local resistance patterns and risk of invasive infection. Which antibiotic to recommend is governed by the antibiotic resistance patterns prevalent in the travel destinations and by the risk of infection by invasive pathogens. Invasive TD is generally caused by Campylobacter, Shigella, or Salmonella and manifests clinically with bloody diarrhea, fever, or both. Rifaximin at a dose of 200 mg orally 3 times daily is effective for noninvasive TD.31,32 However, travelers who develop invasive TD need an alternative to rifaximin. (Those who advocate reserving antibiotic treatment only for invasive diarrhea will not see a role for rifaximin in the first place.) In most invasive cases, a fluoroquinolone will suffice.10-12,19,32 However, increasing prevalence of fluoroquinolone-resistantCampylobacter species has been reported in South and Southeast Asia. In those locations, azithromycin is an effective alternative, albeit with risk of nausea.33TABLE 212 provides details of recommended antibiotic dosages for adults and children. The duration of treatment is generally 1 day unless symptoms persist, in which case a 3-day course is recommended.10-12,19,32 If the traveler experiences persistent, new, or worsening symptoms beyond this point, immediate evaluation by a physician is required.
Non-antibiotic treatment
The antimotility agent loperamide is a well-established antidiarrheal agent. Its effective and safe use as an adjunct to antibiotics in the treatment of TD has been demonstrated in several studies.10-12,19,32,34 It is generally not used to treat children with TD9
No other non-antibiotic treatment for TD has significant guideline or clinical trial support. Bismuth subsalicylate can be helpful as an antidiarrheal agent,35 but is not often recommended because the regimen makes adherence difficult and because antibiotics and loperamide are effective.
Oral rehydration is usually a mainstay of treating gastrointestinal disease among infants and children. However, it, too, has a limited role in cases of TD because dehydration is not usually a significant part of the clinical presentation, perhaps because vomiting is not often prominent.
CASE Advice regarding safe food and beverage choices is essential for the patient and her children. Despite the increased risk for TD due to her history and her use of the immunosuppressant methotrexate, she decides not to pursue antibiotic prophylaxis. Bismuth is also contraindicated because of the methotrexate. Her teenage daughter declines bismuth prophylaxis, and her toddler is too young for it.
The patient does accept a prescription for azithromycin for her and her daughters in case they experience TD. This choice is appropriate given the destination of India and concern about Campylobacterresistance to fluoroquinolones. You also recommend loperamide for use by the mother and older child, in conjunction with the antibiotic.
Two weeks after their trip abroad, the travelers return for an office visit. On the trip, the mother and toddler suffered diarrhea, which responded well to your recommended management. The older child was well during the trip, but she developed diarrhea, abdominal pain, and anorexia one week after returning to the United States. These symptoms have persisted despite a 3-day course of azithromycin and loperamide.
Post-travel evaluation
TD generally occurs within one to 2 weeks of arrival at the travel destination and usually lasts no longer than 4 to 5 days.19 This scenario is typical of a bacterial infection. When it occurs later or lasts longer, or both, consider several alternative possibilities.19,36 First, the likelihood of a protozoal parasitic infection is increased. Although giardiasis is most likely, other protozoa such as Entamoeba, Cyclospora, Isospora, and Cryptosporidium are also possibilities. Second, if diarrhea persists, it might be due, not to continued infection, but to a self-limited post-infectious enteropathy or to PI-IBS. Third, TD is known to precipitate the clinical manifestation of underlying gastrointestinal disorders such as inflammatory bowel disease (IBD), celiac disease, or even cancer.37
With an atypical disease course, it’s advisable to send 3 stool samples for laboratory evaluation for ova and parasites and for antigen assays for Giardia. If results of these tests are negative, given the difficulty inherent in diagnosing Giardia, consider empiric treatment with metronidazole in lieu of duodenal sampling.36 If the diarrhea persists, investigate serologic markers for celiac disease and IBD. If these are not revealing, referral for colonoscopy is prudent.
CASE The teenager’s 3 stool samples were negative for ova and parasites and for Giardia antigen. Following empirical treatment with oral metronidazole 250 mg, 3 times daily for 7 days, the diarrhea resolved.
CORRESPONDENCE Dilip Nair, MD, Joan C. Edwards School of Medicine at Marshall University, 1600 Medical Center Drive, Suite 1500, Huntington, WV 25701; [email protected]
1. Hill DR. Occurrence and self-treatment of diarrhea in a large cohort of Americans traveling to developing countries. Am J Trop Med Hyg. 2000;62:585–589.
2. Greenwood Z, Black J, Weld L, et al. for the GeoSentinel Surveillance Network. Gastrointestinal infection among international travelers globally. J Travel Med. 2008;15:221–228.
3. DuPont HL. Systematic review: the epidemiology and clinical features of travellers’ diarrhoea. Aliment Pharmacol Ther. 2009;30:187–196.
4. Steffen R, Tornieporth N, Clemens SA, et al. Epidemiology of travelers’ diarrhea: details of a global survey. J Travel Med. 2004;11:231–237.
5. de la Cabada Bauche J, DuPont HL. New developments in traveler’s diarrhea. Gastroenterol Hepatol. 2011;7:88–95.
6. Cabada MM, White AC. Travelers’ diarrhea: an update on susceptibility, prevention, and treatment. Curr Gastroenterol Rep. 2008;10:473–479.
7. Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents. 2003;21:181–188.
8. Cabada MM, Maldonado F, Quispe W, et al. Risk factors associated with diarrhea among international visitors to Cuzco, Peru. Am J Trop Med Hyg. 2006;75:968–972.
9. Mackell S. Traveler’s diarrhea in the pediatric population: etiology and impact. Clin Infect Dis. 2005;41(suppl 8):S547–S552.
10. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499–1539.
11. Connor BA. Travelers’ diarrhea. Available at:http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-2-the-pre-travel-consultation/travelers-diarrhea.htm. Accessed August 20, 2012.
12. Advice for travelers. Treat Guidel Med Lett. 2012;10:45–56.
13. Shlim DR. Looking for evidence that personal hygiene precautions prevent travelers’ diarrhea. Clin Infect Dis. 2005;41(suppl 8):S531–S535.
14. Laverone E, Boccalini S, Bechini A, et al. Travelers’ compliance to prophylactic measures and behavior during stay abroad: results of a retrospective study of subjects returning to a travel medicine center in Italy. J Travel Med. 2006;13:338–344.
15. Ashley DV, Walters C, Dockery-Brown C, et al. Interventions to prevent and control food-borne diseases associated with a reduction in traveler’s diarrhea in tourists to Jamaica. J Travel Med. 2004;11:364–367.
16. Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609–614.
17. Riddle MS, Sanders JW, Putnam SD, et al. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74:891–900.
18. Koo HL, Ajami NJ, Jiang ZD, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol. 2010;48:1673–1676.
19. Hill DR, Ryan ET. Management of travellers’ diarrhoea. BMJ. 2008;337:863–867.
20. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628–633.
21. Pimentel M, Riddle MS. Prevention of traveler’s diarrhea: a call to reconvene. Clin Infect Dis. 2008;46:151–152.
22. DuPont HL. Systematic review: prevention of travellers’ diarrhoea. Aliment Pharmacol Ther. 2008;27:741–751.
23. Wang M, Szucs TD, Steffen R. Economic aspects of travelers’ diarrhea. J Travel Med. 2008;15:110–118.
24. Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410–413.
25. Tornblom H, Holmvall P, Svenungsson B, et al. Gastrointestinal symptoms after infectious diarrhea: a five-year follow-up in a Swedish cohort of adults. Clin Gastroenterol Hepatol. 2007;5:461–464.
26. DuPont HL, Jiang ZD, Okhuysen PC, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med. 2005;142:805–812.
27. Taylor DN, McKenzie R, Durbin A, et al. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin Infect Dis. 2006;42:1283–1288.
28. Sazawal S, Hiremath G, Dhingra U, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374–382.
29. Bri V, Buffet P, Genty S, et al. Absence of efficacy of nonviable Lactobacillus acidophilus for the prevention of traveler’s diarrhea: a randomized, double-blind, controlled study. Clin Infect Dis. 2006;43:1170–1175.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines—use in clinical practice. Lancet Infect Dis. 2006;6:361–373.
31. Taylor DN, Bourgeois AL, Ericsson CD, et al. A randomized double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74:1060–1066.
32. DuPont HL, Ericsson CD, Farthing MJG, et al. Expert review of the evidence base for self-therapy of travelers’ diarrhea. J Travel Med. 2009;16:161–171.
33. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338–346.
34. Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in travelers’ diarrhea: a systematic review and meta-analysis. Clin Infect Dis. 2008;47:1007–1014.
35. Steffen R. Worldwide efficacy of bismuth subsalicylate in the treatment of travelers’ diarrhea. Rev Infect Dis. 1990;12(suppl 1):S80–S86.
36. Connor BA. Persistent travelers’ diarrhea. Available at:http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-5-post-travel-evaluation/persistent-travelers-diarrhea.htm. Accessed August 20, 2012.
37.Landzberg BR, Connor BA. Persistent diarrhea in the returning traveler: think beyond persistent infection. Scand J Gastroenterol. 2005;40:112–114.
1. Recommend antibiotic chemoprophylaxis for travelers at high risk for travelers’ diarrhea (TD) and those at high risk for complications. It is also appropriate for travelers who have an inflexible itinerary. B
2. Recommend bismuth subsalicylate chemoprophylaxis for travelers at high risk for TD who are willing to comply with the regimen and want to avoid antibiotic prophylaxis. B
3. Advise travelers to initiate self-treatment for TD with a fluoroquinolone (or azithromycin, if in South or Southeast Asia) at the onset of diarrhea if it is bloody or accompanied by fever. A
NOTE: This practice recommendation in the print version of this article stated that travelers should also take loperamide; however, both the Centers for Disease Control and Prevention and the Infectious Diseases Society of America advise against the use of loperamide by travelers with fever or bloody diarrhea [corrected August 27, 2013].
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 40-year-old female patient, a childhood immigrant from India, is seeking advice regarding her upcoming 2-week trip to Mumbai. She is taking her 2 children, ages 16 years and 16 months, to visit their grandparents for the first time. She has made this trip alone a few times and has invariably experienced short bouts of self-limited diarrheal illness. She wonders what she might do to prevent travelers’ diarrhea. Her only medical problem is rheumatoid arthritis, which has been well controlled with methotrexate. Her children are healthy. What would you recommend?
Recommendations regarding travelers’ diarrhea (TD) address prevention and management. Prevention encompasses advice about personal behaviors and the use of chemoprophylaxis (antimicrobial and non-antimicrobial) and vaccinations. Since international travelers are known to treat themselves for diarrheal illnesses during their trips,1 recommendations regarding management should assume self-treatment and include the use of both antibiotics and non-antibiotic remedies. Pretravel recommendations will of course be most effective if they account for the individual’s risk for TD.
Innate patient susceptibility, destination, and dietary choices determine TD risk
TD is generally defined as the passage of 3 of more loose stools in a 24-hour period, with associated symptoms of enteric infection—eg, fever, nausea, vomiting, or abdominal cramping. Defined in this manner, TD is thought to occur in 60% to 70% of individuals who travel from developed countries to less-developed countries.2,4 Risk of TD is influenced both by intrinsic personal factors and by factors specific to the trip.
Personal risk factorsIndividual variation in susceptibility to TD might result from a genetic predisposition arising from single nucleotide polymorphisms governing various inflammatory marker proteins.5 A history of multiple episodes of TD, especially if fellow travelers were spared, can suggest this kind of individual susceptibility. Other factors that increase vulnerability to TD are immunodeficiency, achlorhydric states such as atrophic gastritis, and chronic use of proton pump inhibitors.6,7 However, the trip itself is much more important in assessing risk for TD.
Trip-related risk factors
The destination. The most salient risk factor for TD is the geographic destination. Regions of the world can be divided into TD risk strata:2
- Very high: South Asia
- High: South America, Sub-Saharan Africa
- Medium: Central America, Mexico, Caribbean, Middle East, North Africa, Southeast Asia, Oceania
- Low: Europe, North America (excluding Mexico), Australasia, Northeast Asia.
Particularly notable countries, in descending order of risk, are Nepal, India, Myanmar, Bolivia, Sri Lanka, Ecuador, Peru, Kenya, and Guatemala.2
Dietary choices. Additionally, since travelers acquire TD by ingesting food or beverages contaminated with pathogenic fecal microbes, dietary behaviors during the trip affect their susceptibility. At least risk are business travelers and tourists who confine their activities to more affluent settings in which food and beverages are prepared and stored hygienically.1,4,8,9 At greater risk are travelers who immerse themselves in local culture, visiting locations that are more impoverished and not as well equipped with sanitation systems, especially if their stay is at least 2 to 3 weeks.1,4,8,9
Also, the older a traveler is, the lower his or her risk of TD.1,9 An exception to this might be infants whose diet consists solely of breast milk or formula prepared under sanitary conditions.
Mandates and options for preventing TD
Emphasize food and beverage precautions
It might be reasonable to expect that travelers who are circumspect about their food and beverage choices on trips will be able to avoid TD. Indeed, this is the basis for the aphorism, “Boil it, peel it, or forget it.” Guidelines routinely recommend that travelers restrict their selection of foods to those that have been well cooked and are served while still very hot, and to fruits and vegetables that they peel themselves. Likewise, they should drink only beverages that have been boiled or are in sealed bottles or under carbonation and served without ice.10-12 Many travelers might find these recommendations too restrictive to follow faithfully. Moreover, studies suggest it may not be possible for even the most assiduous traveler to fully avoid the risk of TD.13,14 The hygienic characteristics of the travel destination may be more determinative, as illustrated by the successful reduction of TD rates in Jamaica by improving sanitation in tourist resorts.15
Antibiotic chemoprophylaxis: A debated practice with limited consensusThe etiologic agents of TD are multiple and vary somewhat in predominance according to geographic region.3,16,17 TABLE 1 depicts variance by region.16 The most common pathogens are strains of the bacterium Escherichia coli, particularly enterotoxigenic (ETEC), enteroaggregative (EAEC), and enteropathogenic (EPEC) strains.16 Other bacteria of importance are Campylobacter, Salmonella, and Shigella. Viruses, particularly norovirus (notably connected with cruise ships), can also cause TD, although it is implicated in no more than 17% of cases.18 Parasitic pathogens are even less common causes of TD (4%-10%) and mainly involve the protozoa, Giardia lamblia, and, to a lesser extent, Entamoeba histolytica and Cryptosporidium.
Although some pathogens often have a characteristic presentation—such as frothy, greasy diarrhea in the case of G lamblia—they generally cannot be reliably distinguished from one another clinically. Notably, up to 50% of stool samples from TD patients do not yield any pathogen,16 raising the suspicion that current diagnostic technology is not sufficiently sensitive to routinely identify certain bacteria.
There is no consensus on recommending antibiotic chemoprophylaxis against TD.
Opponents of this practice10-12,19,20 point out that TD is generally a brief (3-5 days), self-limited illness. Moreover, concerns about antibiotic resistance have come to pass. Previously used agents, trimethoprim-sulfamethoxazole and doxycycline, are no longer effective in preventing or treating TD. In addition, antibiotic use carries the risk of allergic reactions as well as other adverse effects including, ironically, the development of antibiotic-associated diarrhea and Clostridium difficile diarrhea.
Proponents of antibiotic chemoprophylaxis21,22 point to its demonstrated efficacy in reducing the risk of TD by 4% to 40%.11 They also argue that at least 20% to 25% of travelers who get TD must significantly curtail their activities for a day or more.1,23 This change in travel plans is associated not only with significant personal loss but also imposes a financial burden.23 Furthermore, TD is known to have longer-term effects. Up to 10% of sufferers develop postinfectious irritable bowel syndrome (PI-IBS) that can last for 5 or 6 years.21,22,24,25 It is not known, however, whether the use of antibiotic chemoprophylaxis significantly reduces the incidence of PI-IBS.
Finally, the luminal antibiotic, rifaximin, nonabsorbable as it is, is very well-tolerated and holds promise for not inciting antibiotic resistance.22 However, while its efficacy in preventing TD has been demonstrated in various settings,22,26,27 it is not approved by the US Food and Drug Administration for this indication. Also, concerns persist that it might not be effective in preventing TD caused by invasive pathogens.19
Indications on which all agree. Even opponents of antibiotic chemoprophylaxis grant that it is probably warranted for 2 groups of travelers.10-12 The first is those whose trip schedule is of such importance that any deviation would be intolerable. The second is travelers with comorbidities that would render them at high risk for serious inconvenience or illness if they developed TD. Examples of the latter include patients with enterostomies, mobility impairments, immune suppression, inflammatory bowel disease, and renal or metabolic diseases.
Chemoprophylaxis regimens. If you prescribe an antibiotic prophylactically, consider daily doses of a fluoroquinolone (eg, ciprofloxacin 500 mg orally once daily, not twice daily as for treatment) or rifaximin 200 mg orally once or twice a day, for no longer than 2 to 3 weeks.10
Non-antimicrobial chemoprophylaxis
Bismuth subsalicylate has reduced the incidence of TD from 40% to just 14% when taken in doses of 2 chewable tablets or 60 mL of liquid 4 times daily. 11,19,22 However, the dosing frequency can hinder adherence. Moreover, the relatively high doses required raise the risk of adverse drug reactions such as blackening of the tongue and stool, nausea, constipation, Reye syndrome (in children under 12 years), and possibly tinnitus. The salicylate component of the drug poses a threat to patients with aspirin allergy, renal disease, and those taking anticoagulants. Drug interactions with probenecid and methotrexate are also possible. Bismuth is not recommended for use for longer than 3 weeks, or for children younger than 3 years or pregnant women in their third trimester.
Other non-antimicrobial chemoprophylaxis agents include probiotics such as Lactobacillus andSaccharomyces. These preparations of bacteria and fungi are marketed either singly or in blends of varying composition and proportion. The evidence is divided on their efficacy, and even though some meta-analyses have concluded probiotics such as Saccharomyces boulardii are useful in preventing TD, endorsement in clinical guidelines is muted.10-12,28-30
Immunizations have limited value so farNatural immunity to E coli gastrointestinal infection among indigenous people in less developed countries has raised the possibility of a role for vaccines in preventing TD. Some strains of ETEC produce a heat-labile toxin (LT) that bears significant resemblance to the toxin produced by Vibrio cholerae. Therefore, the oral cholera vaccine, Dukoral, has been marketed outside the United States for the prevention of TD.19,22 However, only ≤7% of TD cases worldwide would be prevented by routine use of this vaccine.31 A transdermal LT vaccine, which involves the antigen-presenting Langerhans cells in the superficial skin layers, is promising but not yet available for routine use.19,22
Treating TD and associated symptoms
Antibiotic treatment
Given that most cases of TD are caused by bacterial pathogens, antibiotics are considered the mainstay of treatment. Concerns about the ill effects of antibiotic use in the case of enterohemorrhagic E coli(EHEC O157:H7) can be allayed because this strain is rarely a cause of TD.9Patient factors that increase vulnerability to TD are immunodeficiency, achlorhydric states such as atrophic gastritis, and chronic use of proton pump inhibitors.
Consider local resistance patterns and risk of invasive infection. Which antibiotic to recommend is governed by the antibiotic resistance patterns prevalent in the travel destinations and by the risk of infection by invasive pathogens. Invasive TD is generally caused by Campylobacter, Shigella, or Salmonella and manifests clinically with bloody diarrhea, fever, or both. Rifaximin at a dose of 200 mg orally 3 times daily is effective for noninvasive TD.31,32 However, travelers who develop invasive TD need an alternative to rifaximin. (Those who advocate reserving antibiotic treatment only for invasive diarrhea will not see a role for rifaximin in the first place.) In most invasive cases, a fluoroquinolone will suffice.10-12,19,32 However, increasing prevalence of fluoroquinolone-resistantCampylobacter species has been reported in South and Southeast Asia. In those locations, azithromycin is an effective alternative, albeit with risk of nausea.33TABLE 212 provides details of recommended antibiotic dosages for adults and children. The duration of treatment is generally 1 day unless symptoms persist, in which case a 3-day course is recommended.10-12,19,32 If the traveler experiences persistent, new, or worsening symptoms beyond this point, immediate evaluation by a physician is required.
Non-antibiotic treatment
The antimotility agent loperamide is a well-established antidiarrheal agent. Its effective and safe use as an adjunct to antibiotics in the treatment of TD has been demonstrated in several studies.10-12,19,32,34 It is generally not used to treat children with TD9
No other non-antibiotic treatment for TD has significant guideline or clinical trial support. Bismuth subsalicylate can be helpful as an antidiarrheal agent,35 but is not often recommended because the regimen makes adherence difficult and because antibiotics and loperamide are effective.
Oral rehydration is usually a mainstay of treating gastrointestinal disease among infants and children. However, it, too, has a limited role in cases of TD because dehydration is not usually a significant part of the clinical presentation, perhaps because vomiting is not often prominent.
CASE Advice regarding safe food and beverage choices is essential for the patient and her children. Despite the increased risk for TD due to her history and her use of the immunosuppressant methotrexate, she decides not to pursue antibiotic prophylaxis. Bismuth is also contraindicated because of the methotrexate. Her teenage daughter declines bismuth prophylaxis, and her toddler is too young for it.
The patient does accept a prescription for azithromycin for her and her daughters in case they experience TD. This choice is appropriate given the destination of India and concern about Campylobacterresistance to fluoroquinolones. You also recommend loperamide for use by the mother and older child, in conjunction with the antibiotic.
Two weeks after their trip abroad, the travelers return for an office visit. On the trip, the mother and toddler suffered diarrhea, which responded well to your recommended management. The older child was well during the trip, but she developed diarrhea, abdominal pain, and anorexia one week after returning to the United States. These symptoms have persisted despite a 3-day course of azithromycin and loperamide.
Post-travel evaluation
TD generally occurs within one to 2 weeks of arrival at the travel destination and usually lasts no longer than 4 to 5 days.19 This scenario is typical of a bacterial infection. When it occurs later or lasts longer, or both, consider several alternative possibilities.19,36 First, the likelihood of a protozoal parasitic infection is increased. Although giardiasis is most likely, other protozoa such as Entamoeba, Cyclospora, Isospora, and Cryptosporidium are also possibilities. Second, if diarrhea persists, it might be due, not to continued infection, but to a self-limited post-infectious enteropathy or to PI-IBS. Third, TD is known to precipitate the clinical manifestation of underlying gastrointestinal disorders such as inflammatory bowel disease (IBD), celiac disease, or even cancer.37
With an atypical disease course, it’s advisable to send 3 stool samples for laboratory evaluation for ova and parasites and for antigen assays for Giardia. If results of these tests are negative, given the difficulty inherent in diagnosing Giardia, consider empiric treatment with metronidazole in lieu of duodenal sampling.36 If the diarrhea persists, investigate serologic markers for celiac disease and IBD. If these are not revealing, referral for colonoscopy is prudent.
CASE The teenager’s 3 stool samples were negative for ova and parasites and for Giardia antigen. Following empirical treatment with oral metronidazole 250 mg, 3 times daily for 7 days, the diarrhea resolved.
CORRESPONDENCE Dilip Nair, MD, Joan C. Edwards School of Medicine at Marshall University, 1600 Medical Center Drive, Suite 1500, Huntington, WV 25701; [email protected]
1. Recommend antibiotic chemoprophylaxis for travelers at high risk for travelers’ diarrhea (TD) and those at high risk for complications. It is also appropriate for travelers who have an inflexible itinerary. B
2. Recommend bismuth subsalicylate chemoprophylaxis for travelers at high risk for TD who are willing to comply with the regimen and want to avoid antibiotic prophylaxis. B
3. Advise travelers to initiate self-treatment for TD with a fluoroquinolone (or azithromycin, if in South or Southeast Asia) at the onset of diarrhea if it is bloody or accompanied by fever. A
NOTE: This practice recommendation in the print version of this article stated that travelers should also take loperamide; however, both the Centers for Disease Control and Prevention and the Infectious Diseases Society of America advise against the use of loperamide by travelers with fever or bloody diarrhea [corrected August 27, 2013].
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 40-year-old female patient, a childhood immigrant from India, is seeking advice regarding her upcoming 2-week trip to Mumbai. She is taking her 2 children, ages 16 years and 16 months, to visit their grandparents for the first time. She has made this trip alone a few times and has invariably experienced short bouts of self-limited diarrheal illness. She wonders what she might do to prevent travelers’ diarrhea. Her only medical problem is rheumatoid arthritis, which has been well controlled with methotrexate. Her children are healthy. What would you recommend?
Recommendations regarding travelers’ diarrhea (TD) address prevention and management. Prevention encompasses advice about personal behaviors and the use of chemoprophylaxis (antimicrobial and non-antimicrobial) and vaccinations. Since international travelers are known to treat themselves for diarrheal illnesses during their trips,1 recommendations regarding management should assume self-treatment and include the use of both antibiotics and non-antibiotic remedies. Pretravel recommendations will of course be most effective if they account for the individual’s risk for TD.
Innate patient susceptibility, destination, and dietary choices determine TD risk
TD is generally defined as the passage of 3 of more loose stools in a 24-hour period, with associated symptoms of enteric infection—eg, fever, nausea, vomiting, or abdominal cramping. Defined in this manner, TD is thought to occur in 60% to 70% of individuals who travel from developed countries to less-developed countries.2,4 Risk of TD is influenced both by intrinsic personal factors and by factors specific to the trip.
Personal risk factorsIndividual variation in susceptibility to TD might result from a genetic predisposition arising from single nucleotide polymorphisms governing various inflammatory marker proteins.5 A history of multiple episodes of TD, especially if fellow travelers were spared, can suggest this kind of individual susceptibility. Other factors that increase vulnerability to TD are immunodeficiency, achlorhydric states such as atrophic gastritis, and chronic use of proton pump inhibitors.6,7 However, the trip itself is much more important in assessing risk for TD.
Trip-related risk factors
The destination. The most salient risk factor for TD is the geographic destination. Regions of the world can be divided into TD risk strata:2
- Very high: South Asia
- High: South America, Sub-Saharan Africa
- Medium: Central America, Mexico, Caribbean, Middle East, North Africa, Southeast Asia, Oceania
- Low: Europe, North America (excluding Mexico), Australasia, Northeast Asia.
Particularly notable countries, in descending order of risk, are Nepal, India, Myanmar, Bolivia, Sri Lanka, Ecuador, Peru, Kenya, and Guatemala.2
Dietary choices. Additionally, since travelers acquire TD by ingesting food or beverages contaminated with pathogenic fecal microbes, dietary behaviors during the trip affect their susceptibility. At least risk are business travelers and tourists who confine their activities to more affluent settings in which food and beverages are prepared and stored hygienically.1,4,8,9 At greater risk are travelers who immerse themselves in local culture, visiting locations that are more impoverished and not as well equipped with sanitation systems, especially if their stay is at least 2 to 3 weeks.1,4,8,9
Also, the older a traveler is, the lower his or her risk of TD.1,9 An exception to this might be infants whose diet consists solely of breast milk or formula prepared under sanitary conditions.
Mandates and options for preventing TD
Emphasize food and beverage precautions
It might be reasonable to expect that travelers who are circumspect about their food and beverage choices on trips will be able to avoid TD. Indeed, this is the basis for the aphorism, “Boil it, peel it, or forget it.” Guidelines routinely recommend that travelers restrict their selection of foods to those that have been well cooked and are served while still very hot, and to fruits and vegetables that they peel themselves. Likewise, they should drink only beverages that have been boiled or are in sealed bottles or under carbonation and served without ice.10-12 Many travelers might find these recommendations too restrictive to follow faithfully. Moreover, studies suggest it may not be possible for even the most assiduous traveler to fully avoid the risk of TD.13,14 The hygienic characteristics of the travel destination may be more determinative, as illustrated by the successful reduction of TD rates in Jamaica by improving sanitation in tourist resorts.15
Antibiotic chemoprophylaxis: A debated practice with limited consensusThe etiologic agents of TD are multiple and vary somewhat in predominance according to geographic region.3,16,17 TABLE 1 depicts variance by region.16 The most common pathogens are strains of the bacterium Escherichia coli, particularly enterotoxigenic (ETEC), enteroaggregative (EAEC), and enteropathogenic (EPEC) strains.16 Other bacteria of importance are Campylobacter, Salmonella, and Shigella. Viruses, particularly norovirus (notably connected with cruise ships), can also cause TD, although it is implicated in no more than 17% of cases.18 Parasitic pathogens are even less common causes of TD (4%-10%) and mainly involve the protozoa, Giardia lamblia, and, to a lesser extent, Entamoeba histolytica and Cryptosporidium.
Although some pathogens often have a characteristic presentation—such as frothy, greasy diarrhea in the case of G lamblia—they generally cannot be reliably distinguished from one another clinically. Notably, up to 50% of stool samples from TD patients do not yield any pathogen,16 raising the suspicion that current diagnostic technology is not sufficiently sensitive to routinely identify certain bacteria.
There is no consensus on recommending antibiotic chemoprophylaxis against TD.
Opponents of this practice10-12,19,20 point out that TD is generally a brief (3-5 days), self-limited illness. Moreover, concerns about antibiotic resistance have come to pass. Previously used agents, trimethoprim-sulfamethoxazole and doxycycline, are no longer effective in preventing or treating TD. In addition, antibiotic use carries the risk of allergic reactions as well as other adverse effects including, ironically, the development of antibiotic-associated diarrhea and Clostridium difficile diarrhea.
Proponents of antibiotic chemoprophylaxis21,22 point to its demonstrated efficacy in reducing the risk of TD by 4% to 40%.11 They also argue that at least 20% to 25% of travelers who get TD must significantly curtail their activities for a day or more.1,23 This change in travel plans is associated not only with significant personal loss but also imposes a financial burden.23 Furthermore, TD is known to have longer-term effects. Up to 10% of sufferers develop postinfectious irritable bowel syndrome (PI-IBS) that can last for 5 or 6 years.21,22,24,25 It is not known, however, whether the use of antibiotic chemoprophylaxis significantly reduces the incidence of PI-IBS.
Finally, the luminal antibiotic, rifaximin, nonabsorbable as it is, is very well-tolerated and holds promise for not inciting antibiotic resistance.22 However, while its efficacy in preventing TD has been demonstrated in various settings,22,26,27 it is not approved by the US Food and Drug Administration for this indication. Also, concerns persist that it might not be effective in preventing TD caused by invasive pathogens.19
Indications on which all agree. Even opponents of antibiotic chemoprophylaxis grant that it is probably warranted for 2 groups of travelers.10-12 The first is those whose trip schedule is of such importance that any deviation would be intolerable. The second is travelers with comorbidities that would render them at high risk for serious inconvenience or illness if they developed TD. Examples of the latter include patients with enterostomies, mobility impairments, immune suppression, inflammatory bowel disease, and renal or metabolic diseases.
Chemoprophylaxis regimens. If you prescribe an antibiotic prophylactically, consider daily doses of a fluoroquinolone (eg, ciprofloxacin 500 mg orally once daily, not twice daily as for treatment) or rifaximin 200 mg orally once or twice a day, for no longer than 2 to 3 weeks.10
Non-antimicrobial chemoprophylaxis
Bismuth subsalicylate has reduced the incidence of TD from 40% to just 14% when taken in doses of 2 chewable tablets or 60 mL of liquid 4 times daily. 11,19,22 However, the dosing frequency can hinder adherence. Moreover, the relatively high doses required raise the risk of adverse drug reactions such as blackening of the tongue and stool, nausea, constipation, Reye syndrome (in children under 12 years), and possibly tinnitus. The salicylate component of the drug poses a threat to patients with aspirin allergy, renal disease, and those taking anticoagulants. Drug interactions with probenecid and methotrexate are also possible. Bismuth is not recommended for use for longer than 3 weeks, or for children younger than 3 years or pregnant women in their third trimester.
Other non-antimicrobial chemoprophylaxis agents include probiotics such as Lactobacillus andSaccharomyces. These preparations of bacteria and fungi are marketed either singly or in blends of varying composition and proportion. The evidence is divided on their efficacy, and even though some meta-analyses have concluded probiotics such as Saccharomyces boulardii are useful in preventing TD, endorsement in clinical guidelines is muted.10-12,28-30
Immunizations have limited value so farNatural immunity to E coli gastrointestinal infection among indigenous people in less developed countries has raised the possibility of a role for vaccines in preventing TD. Some strains of ETEC produce a heat-labile toxin (LT) that bears significant resemblance to the toxin produced by Vibrio cholerae. Therefore, the oral cholera vaccine, Dukoral, has been marketed outside the United States for the prevention of TD.19,22 However, only ≤7% of TD cases worldwide would be prevented by routine use of this vaccine.31 A transdermal LT vaccine, which involves the antigen-presenting Langerhans cells in the superficial skin layers, is promising but not yet available for routine use.19,22
Treating TD and associated symptoms
Antibiotic treatment
Given that most cases of TD are caused by bacterial pathogens, antibiotics are considered the mainstay of treatment. Concerns about the ill effects of antibiotic use in the case of enterohemorrhagic E coli(EHEC O157:H7) can be allayed because this strain is rarely a cause of TD.9Patient factors that increase vulnerability to TD are immunodeficiency, achlorhydric states such as atrophic gastritis, and chronic use of proton pump inhibitors.
Consider local resistance patterns and risk of invasive infection. Which antibiotic to recommend is governed by the antibiotic resistance patterns prevalent in the travel destinations and by the risk of infection by invasive pathogens. Invasive TD is generally caused by Campylobacter, Shigella, or Salmonella and manifests clinically with bloody diarrhea, fever, or both. Rifaximin at a dose of 200 mg orally 3 times daily is effective for noninvasive TD.31,32 However, travelers who develop invasive TD need an alternative to rifaximin. (Those who advocate reserving antibiotic treatment only for invasive diarrhea will not see a role for rifaximin in the first place.) In most invasive cases, a fluoroquinolone will suffice.10-12,19,32 However, increasing prevalence of fluoroquinolone-resistantCampylobacter species has been reported in South and Southeast Asia. In those locations, azithromycin is an effective alternative, albeit with risk of nausea.33TABLE 212 provides details of recommended antibiotic dosages for adults and children. The duration of treatment is generally 1 day unless symptoms persist, in which case a 3-day course is recommended.10-12,19,32 If the traveler experiences persistent, new, or worsening symptoms beyond this point, immediate evaluation by a physician is required.
Non-antibiotic treatment
The antimotility agent loperamide is a well-established antidiarrheal agent. Its effective and safe use as an adjunct to antibiotics in the treatment of TD has been demonstrated in several studies.10-12,19,32,34 It is generally not used to treat children with TD9
No other non-antibiotic treatment for TD has significant guideline or clinical trial support. Bismuth subsalicylate can be helpful as an antidiarrheal agent,35 but is not often recommended because the regimen makes adherence difficult and because antibiotics and loperamide are effective.
Oral rehydration is usually a mainstay of treating gastrointestinal disease among infants and children. However, it, too, has a limited role in cases of TD because dehydration is not usually a significant part of the clinical presentation, perhaps because vomiting is not often prominent.
CASE Advice regarding safe food and beverage choices is essential for the patient and her children. Despite the increased risk for TD due to her history and her use of the immunosuppressant methotrexate, she decides not to pursue antibiotic prophylaxis. Bismuth is also contraindicated because of the methotrexate. Her teenage daughter declines bismuth prophylaxis, and her toddler is too young for it.
The patient does accept a prescription for azithromycin for her and her daughters in case they experience TD. This choice is appropriate given the destination of India and concern about Campylobacterresistance to fluoroquinolones. You also recommend loperamide for use by the mother and older child, in conjunction with the antibiotic.
Two weeks after their trip abroad, the travelers return for an office visit. On the trip, the mother and toddler suffered diarrhea, which responded well to your recommended management. The older child was well during the trip, but she developed diarrhea, abdominal pain, and anorexia one week after returning to the United States. These symptoms have persisted despite a 3-day course of azithromycin and loperamide.
Post-travel evaluation
TD generally occurs within one to 2 weeks of arrival at the travel destination and usually lasts no longer than 4 to 5 days.19 This scenario is typical of a bacterial infection. When it occurs later or lasts longer, or both, consider several alternative possibilities.19,36 First, the likelihood of a protozoal parasitic infection is increased. Although giardiasis is most likely, other protozoa such as Entamoeba, Cyclospora, Isospora, and Cryptosporidium are also possibilities. Second, if diarrhea persists, it might be due, not to continued infection, but to a self-limited post-infectious enteropathy or to PI-IBS. Third, TD is known to precipitate the clinical manifestation of underlying gastrointestinal disorders such as inflammatory bowel disease (IBD), celiac disease, or even cancer.37
With an atypical disease course, it’s advisable to send 3 stool samples for laboratory evaluation for ova and parasites and for antigen assays for Giardia. If results of these tests are negative, given the difficulty inherent in diagnosing Giardia, consider empiric treatment with metronidazole in lieu of duodenal sampling.36 If the diarrhea persists, investigate serologic markers for celiac disease and IBD. If these are not revealing, referral for colonoscopy is prudent.
CASE The teenager’s 3 stool samples were negative for ova and parasites and for Giardia antigen. Following empirical treatment with oral metronidazole 250 mg, 3 times daily for 7 days, the diarrhea resolved.
CORRESPONDENCE Dilip Nair, MD, Joan C. Edwards School of Medicine at Marshall University, 1600 Medical Center Drive, Suite 1500, Huntington, WV 25701; [email protected]
1. Hill DR. Occurrence and self-treatment of diarrhea in a large cohort of Americans traveling to developing countries. Am J Trop Med Hyg. 2000;62:585–589.
2. Greenwood Z, Black J, Weld L, et al. for the GeoSentinel Surveillance Network. Gastrointestinal infection among international travelers globally. J Travel Med. 2008;15:221–228.
3. DuPont HL. Systematic review: the epidemiology and clinical features of travellers’ diarrhoea. Aliment Pharmacol Ther. 2009;30:187–196.
4. Steffen R, Tornieporth N, Clemens SA, et al. Epidemiology of travelers’ diarrhea: details of a global survey. J Travel Med. 2004;11:231–237.
5. de la Cabada Bauche J, DuPont HL. New developments in traveler’s diarrhea. Gastroenterol Hepatol. 2011;7:88–95.
6. Cabada MM, White AC. Travelers’ diarrhea: an update on susceptibility, prevention, and treatment. Curr Gastroenterol Rep. 2008;10:473–479.
7. Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents. 2003;21:181–188.
8. Cabada MM, Maldonado F, Quispe W, et al. Risk factors associated with diarrhea among international visitors to Cuzco, Peru. Am J Trop Med Hyg. 2006;75:968–972.
9. Mackell S. Traveler’s diarrhea in the pediatric population: etiology and impact. Clin Infect Dis. 2005;41(suppl 8):S547–S552.
10. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499–1539.
11. Connor BA. Travelers’ diarrhea. Available at:http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-2-the-pre-travel-consultation/travelers-diarrhea.htm. Accessed August 20, 2012.
12. Advice for travelers. Treat Guidel Med Lett. 2012;10:45–56.
13. Shlim DR. Looking for evidence that personal hygiene precautions prevent travelers’ diarrhea. Clin Infect Dis. 2005;41(suppl 8):S531–S535.
14. Laverone E, Boccalini S, Bechini A, et al. Travelers’ compliance to prophylactic measures and behavior during stay abroad: results of a retrospective study of subjects returning to a travel medicine center in Italy. J Travel Med. 2006;13:338–344.
15. Ashley DV, Walters C, Dockery-Brown C, et al. Interventions to prevent and control food-borne diseases associated with a reduction in traveler’s diarrhea in tourists to Jamaica. J Travel Med. 2004;11:364–367.
16. Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609–614.
17. Riddle MS, Sanders JW, Putnam SD, et al. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74:891–900.
18. Koo HL, Ajami NJ, Jiang ZD, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol. 2010;48:1673–1676.
19. Hill DR, Ryan ET. Management of travellers’ diarrhoea. BMJ. 2008;337:863–867.
20. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628–633.
21. Pimentel M, Riddle MS. Prevention of traveler’s diarrhea: a call to reconvene. Clin Infect Dis. 2008;46:151–152.
22. DuPont HL. Systematic review: prevention of travellers’ diarrhoea. Aliment Pharmacol Ther. 2008;27:741–751.
23. Wang M, Szucs TD, Steffen R. Economic aspects of travelers’ diarrhea. J Travel Med. 2008;15:110–118.
24. Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410–413.
25. Tornblom H, Holmvall P, Svenungsson B, et al. Gastrointestinal symptoms after infectious diarrhea: a five-year follow-up in a Swedish cohort of adults. Clin Gastroenterol Hepatol. 2007;5:461–464.
26. DuPont HL, Jiang ZD, Okhuysen PC, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med. 2005;142:805–812.
27. Taylor DN, McKenzie R, Durbin A, et al. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin Infect Dis. 2006;42:1283–1288.
28. Sazawal S, Hiremath G, Dhingra U, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374–382.
29. Bri V, Buffet P, Genty S, et al. Absence of efficacy of nonviable Lactobacillus acidophilus for the prevention of traveler’s diarrhea: a randomized, double-blind, controlled study. Clin Infect Dis. 2006;43:1170–1175.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines—use in clinical practice. Lancet Infect Dis. 2006;6:361–373.
31. Taylor DN, Bourgeois AL, Ericsson CD, et al. A randomized double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74:1060–1066.
32. DuPont HL, Ericsson CD, Farthing MJG, et al. Expert review of the evidence base for self-therapy of travelers’ diarrhea. J Travel Med. 2009;16:161–171.
33. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338–346.
34. Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in travelers’ diarrhea: a systematic review and meta-analysis. Clin Infect Dis. 2008;47:1007–1014.
35. Steffen R. Worldwide efficacy of bismuth subsalicylate in the treatment of travelers’ diarrhea. Rev Infect Dis. 1990;12(suppl 1):S80–S86.
36. Connor BA. Persistent travelers’ diarrhea. Available at:http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-5-post-travel-evaluation/persistent-travelers-diarrhea.htm. Accessed August 20, 2012.
37.Landzberg BR, Connor BA. Persistent diarrhea in the returning traveler: think beyond persistent infection. Scand J Gastroenterol. 2005;40:112–114.
1. Hill DR. Occurrence and self-treatment of diarrhea in a large cohort of Americans traveling to developing countries. Am J Trop Med Hyg. 2000;62:585–589.
2. Greenwood Z, Black J, Weld L, et al. for the GeoSentinel Surveillance Network. Gastrointestinal infection among international travelers globally. J Travel Med. 2008;15:221–228.
3. DuPont HL. Systematic review: the epidemiology and clinical features of travellers’ diarrhoea. Aliment Pharmacol Ther. 2009;30:187–196.
4. Steffen R, Tornieporth N, Clemens SA, et al. Epidemiology of travelers’ diarrhea: details of a global survey. J Travel Med. 2004;11:231–237.
5. de la Cabada Bauche J, DuPont HL. New developments in traveler’s diarrhea. Gastroenterol Hepatol. 2011;7:88–95.
6. Cabada MM, White AC. Travelers’ diarrhea: an update on susceptibility, prevention, and treatment. Curr Gastroenterol Rep. 2008;10:473–479.
7. Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents. 2003;21:181–188.
8. Cabada MM, Maldonado F, Quispe W, et al. Risk factors associated with diarrhea among international visitors to Cuzco, Peru. Am J Trop Med Hyg. 2006;75:968–972.
9. Mackell S. Traveler’s diarrhea in the pediatric population: etiology and impact. Clin Infect Dis. 2005;41(suppl 8):S547–S552.
10. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499–1539.
11. Connor BA. Travelers’ diarrhea. Available at:http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-2-the-pre-travel-consultation/travelers-diarrhea.htm. Accessed August 20, 2012.
12. Advice for travelers. Treat Guidel Med Lett. 2012;10:45–56.
13. Shlim DR. Looking for evidence that personal hygiene precautions prevent travelers’ diarrhea. Clin Infect Dis. 2005;41(suppl 8):S531–S535.
14. Laverone E, Boccalini S, Bechini A, et al. Travelers’ compliance to prophylactic measures and behavior during stay abroad: results of a retrospective study of subjects returning to a travel medicine center in Italy. J Travel Med. 2006;13:338–344.
15. Ashley DV, Walters C, Dockery-Brown C, et al. Interventions to prevent and control food-borne diseases associated with a reduction in traveler’s diarrhea in tourists to Jamaica. J Travel Med. 2004;11:364–367.
16. Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609–614.
17. Riddle MS, Sanders JW, Putnam SD, et al. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74:891–900.
18. Koo HL, Ajami NJ, Jiang ZD, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol. 2010;48:1673–1676.
19. Hill DR, Ryan ET. Management of travellers’ diarrhoea. BMJ. 2008;337:863–867.
20. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628–633.
21. Pimentel M, Riddle MS. Prevention of traveler’s diarrhea: a call to reconvene. Clin Infect Dis. 2008;46:151–152.
22. DuPont HL. Systematic review: prevention of travellers’ diarrhoea. Aliment Pharmacol Ther. 2008;27:741–751.
23. Wang M, Szucs TD, Steffen R. Economic aspects of travelers’ diarrhea. J Travel Med. 2008;15:110–118.
24. Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410–413.
25. Tornblom H, Holmvall P, Svenungsson B, et al. Gastrointestinal symptoms after infectious diarrhea: a five-year follow-up in a Swedish cohort of adults. Clin Gastroenterol Hepatol. 2007;5:461–464.
26. DuPont HL, Jiang ZD, Okhuysen PC, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med. 2005;142:805–812.
27. Taylor DN, McKenzie R, Durbin A, et al. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin Infect Dis. 2006;42:1283–1288.
28. Sazawal S, Hiremath G, Dhingra U, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374–382.
29. Bri V, Buffet P, Genty S, et al. Absence of efficacy of nonviable Lactobacillus acidophilus for the prevention of traveler’s diarrhea: a randomized, double-blind, controlled study. Clin Infect Dis. 2006;43:1170–1175.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines—use in clinical practice. Lancet Infect Dis. 2006;6:361–373.
31. Taylor DN, Bourgeois AL, Ericsson CD, et al. A randomized double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74:1060–1066.
32. DuPont HL, Ericsson CD, Farthing MJG, et al. Expert review of the evidence base for self-therapy of travelers’ diarrhea. J Travel Med. 2009;16:161–171.
33. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338–346.
34. Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in travelers’ diarrhea: a systematic review and meta-analysis. Clin Infect Dis. 2008;47:1007–1014.
35. Steffen R. Worldwide efficacy of bismuth subsalicylate in the treatment of travelers’ diarrhea. Rev Infect Dis. 1990;12(suppl 1):S80–S86.
36. Connor BA. Persistent travelers’ diarrhea. Available at:http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-5-post-travel-evaluation/persistent-travelers-diarrhea.htm. Accessed August 20, 2012.
37.Landzberg BR, Connor BA. Persistent diarrhea in the returning traveler: think beyond persistent infection. Scand J Gastroenterol. 2005;40:112–114.
How apps are changing family medicine
In April, hundreds of attendees at TEDMED, a conference on medical innovation, waited in line for a “smartphone physical.” Curated by Shiv Gaglani, a medical student and an editor at the medical technology journal Medgadget, the exam involved 10 apps that turn an ordinary smartphone into a medical device (TABLE 1).1 Among them were the AliveCor Heart Monitor (pictured at right), which produces a one-lead EKG in seconds when a patient’s fingers or chest are pressed against the electrodes embedded in the back of what is essentially a phone case2; a pulse oximeter, and an ultrasound that can capture images of the carotid arteries.1
All but one of the apps is paired with a physical component, such as an ultrasound wand or otoscope. The exception is SpiroSmart, an app that uses the phone’s The AliveCor app and Heart Monitor—a smartphone case fitted with sensors—can generate a one-lead EKG tracing in seconds.built-in microphone and lip reverberations to assess lung function. Shwetak Patel, PhD, of the University of Washington, one of its developers, told JFP that the accuracy of SpiroSmart has been found to be within 5% of traditional spirometry results.3
While smartphone physicals are not likely to be integrated into family practice for some time to come, Glen Stream, MD, board chair of the American Academy of Family Physicians, predicts that integration of some of their features is not too far away. “The spirometry application is an especially good one; it addresses one of the top 5 chronic conditions that contribute to health care costs,” Dr. Stream said. The apps will be beneficial, he added, as long as they “are used in a way that contributes, to, rather than detracts from, collaboration between patients and physicians.”
For now, Dr. Stream and many of his fellow FPs use mobile devices and medical apps primarily to access reference materials, both in and out of the exam room. Some have begun “prescribing” apps to tech-savvy patients. Still others have never used a medical app, either because they prefer a desktop or laptop computer to a smartphone or tablet or because, as one FP put it, "I have a dumb phone."
Wherever you fall on the spectrum, it’s a safe bet that you’re going to be increasingly inundated by the many manifestations of mobile health (mHealth).
Epocrates is No. 1 reference app
The number of medical/health apps for smart-phones or tablets is difficult to pin down; estimates range from 17,000 to more than 40,000, and growing.4 More is known about physician use of smartphones and tablets.
A March 2013 survey of nearly 3000 physicians found that 74% use smartphones at work and 43% use them to look up drug information.5 The favorite tool? A 2012 survey conducted by the University of Pennsylvania’s Perelman School of Medicine to identify the best medical apps put Epocrates at the top of the list (TABLE 2).6 Epocrates was the very first app cited by virtually all the FPs interviewed for this article, as well.
Other drug references cited tend to be patient-specific. Colan Kennelly, MD, a clinical educator at the Good Samaritan Family Medicine Residency in Phoenix, finds LactMed particularly useful. Developed by the National Library of Medicine and part of its Toxicology Data Network, the app lets you pull up medications quickly and see whether and how they will affect breastfeeding.
Another favorite of Kennelly’s is the Agency for Healthcare Research and Quality’s ePSS (electronic Preventive Services Selector) app designed to help primary care clinicians identify the preventive services that are appropriate for their patients. “You just plug in a patient’s age and sex”—(pregnancy, tobacco use, and whether the patient is sexually active are also considered)—“and it tells you what you should be checking for,” Dr. Kennelly said.
The benefits of mobile textbooks
Textbook apps and online texts are slowly gaining in popularity. A recent survey by Manhattan Research found that in 2013 for the first time, usage of electronic medical texts surpassed that of print editions.7 Part of the appeal is that mobile texts are easy to tote. “Apps make it possible to carry around information from a number of textbooks with no added weight,” said Richard Usatine, MD, of the University of Texas Health Science Center at San Antonio and editor of JFP’s Photo Rounds column. Dr. Usatine is also a principal of Usatine Media, which turns medical reference materials into apps.
Dr. Usatine’s own experience is a case in point. He recently used a textbook app to prepare to take his boards (for the fifth time). “I’ve brushed up each time,” he said, “but this time I really studied because it was fun.
“With a print textbook you have to cover up the answers so you don’t see them. Here, you don’t get to see the answer until you commit to one of the multiple choice answers. Then you get told what the correct answer is and why you got it right or wrong,” Dr. Usatine said. Interactivity, including the opportunity to watch a video, say, of a procedure to review how it’s done before embarking on it yourself, is a big part of the value of apps, he said.
Rx: App
In January, Eric Topol, MD, a prominent cardiologist and chief academic officer of Scripps Health in La Jolla, Calif., demonstrated the AliveCor heart monitor and other mobile devices on NBC’s Rock Center.8 In March, he went on The Colbert Report and examined Stephen Colbert’s ear with an otoscope smartphone accessory (CellScope) like the one used in Gaglani’s smartphone physical.9 Dr. Topol’s use of the mobile heart monitor to assess an airplane passenger in distress midflight also received widespread news coverage.
In response to an interviewer’s question, Dr. Topol said he is now more likely to prescribe an app than a drug.8 While it’s unlikely that any FP could make such a claim, many have begun recommending apps to tech-savvy patients.
Smartphones as symptom trackers
A January 2013 Pew Internet study found that 7 in 10 US adults track at least one health indicator, for themselves or a loved one. Six in 10 reported tracking weight, diet, or exercise, and one in 3 said they track indicators of medical problems, such as blood pressure, glucose levels, headaches, or sleep patterns—usually without the aid of a smartphone.10
In fact, half of those who report tracking health measures said they keep the information “in their head,” and a third still use pencil and paper.10 That could change, of course, if their physicians suggest they do otherwise (TABLE 3).
Kelly M. Latimer, MD, an FP in the Navy stationed in Djibouti, Africa, routinely asks patients whether they have a smartphone and often recommends apps to those who do.
“It sounds like you have a lot of different symptoms,” she might say to a patient who complains of frequent headaches. “It will help me if you keep a headache diary.”
She used to give such patients paper and pen, Dr. Latimer noted. “Now I ask them to download the app (iHeadache, in this case) right then and there and do a quick review” so they’re ready to use it at home.
Apps are also a good way to help people with anxiety, Dr. Latimer has found. She frequently recommends apps like Relaxation Techniques and Breathe2Relax, and often suggests apps like Calorie Count and MyFitnessPal to boost patients’ efforts to lose weight and get in shape.
Abigail Lowther, MD, an FP at the University of Michigan in Ann Arbor, also recommends apps frequently. But she typically broaches the subject only with patients who have their smartphones out when she walks into the room.
Among the apps Dr. Lowther prescribes are myPause to track menopausal symptoms and Bladder Pal, a voiding diary for women struggling with incontinence. She advises women taking oral contraceptives to use the timer function on their phone to remember to take a pill at the same time every day. But there are apps (myPill, for one) that do that, too.
The upside of patient apps. A smartphone is ideal for keeping a symptom diary because it’s something that most people are never without. Anyone can use the notes function on a phone or tablet to jot down details about exacerbations, but those using disease-specific apps tend to capture more precise information. Some patients print out the information they’ve gathered and bring a hard copy to an office visit, while others simply show their physician what’s on their smartphone.
Can apps affect outcomes? There are few high-quality studies and the jury is still out, but “the smartphone has a very bright future in the world of medicine,” the authors of a review of smartphones in the medical arena concluded. After examining the use of apps to track (literally) wandering dementia patients; calculate and recommend insulin dosages for patients with type 1 diabetes; and teach yoga, to name a few, the researchers concluded that “the smartphone may one day be recognized as a diagnostic and therapeutic tool…as irreplaceable as the stethoscope.”11
Dr. Lowther recalls an obese patient who found MyFitnessPal to be helpful where other, more traditional diet programs had failed. The reason? He was less than truthful with the people overseeing the weight loss programs about what he’d eaten when he tried—and failed—to follow diets like Weight Watchers. He then ended up feeling so guilty that he abandoned the effort entirely. But, he told her, he “wouldn’t lie” to an app.
…and the downside. Even physicians who haven’t begun A weight loss app would be more likely to help this patient reach his goal than other diet programs because he "wouldn't lie" to an app.recommending apps to patients are aware that carefully tracking measures related to chronic conditions like hypertension or diabetes often results in better control. But in some cases, there may be too much of a good thing. Evidence suggests that for some patients with type 2 diabetes, glucose self-monitoring is associated with depression and may do more harm than good.12
Dr. Lowther has witnessed a similar phenomenon in patients using disease-tracking apps. “Sometimes people get too focused on the problem and drive themselves crazy,” she observed, adding that those with high blood pressure are particularly at risk. “I think sometimes it’s hard for patients to understand the concept of an average value and normal fluctuation,” Dr. Lowther said. When that happens, “I have to tell them to back off.”
Who's minding the (app) store?
The mHealth arena has been called “the wild West.”13 With at least one app for virtually every aspect of health and medicine you can think of, it’s not hard to understand why.
In an article on the use of symptom diaries in outpatient care, Bryan Hodge, DO, an FP in Hendersonville, NC, mentions mobile self-tracking apps as one of a number of ways for patients to keep symptom diaries.14 Given the fact that few of these apps have been validated, Dr. Hodge writes, “The best approach is to familiarize yourself with a few options that you can offer to your patients.”14
That depends on the nature of the app. An app that tracks calories consumed or simply keeps an organized file of patient symptoms may do little harm; an app that conveys physical measurements that a patient or physician may act on or calculates medication dosages requires a higher level of vigilance.
A recent study of smartphone apps that calculate opioid dosage conversion, for example, found a lack of consistency that raised a red flag about the reliability of information provided by unvalidated apps. Better regulation of medical apps is crucial to ensure that patient safety is maintained, the authors concluded.15
The FDA’s role
The US Food and Drug Administration, which has approved more than 75 medical apps, issued a proposed approach to its oversight of the apps in 2011.16
Under the proposed rules, the agency would regulate mobile apps that were either used as an accessory to a medical device already regulated by the FDA or that transform a smartphone or tablet into a regulated medical device. A final rule has not yet been issued, but a spokesperson told Congress that it will be forthcoming before the end of the year.17
False claims are a target of federal regulation, as well. In 2011, the Federal Trade Commission pulled 2 acne apps off the market because both advertised—without scientific evidence—that the light emitted by smartphones equipped with the apps could treat acne. “Smartphones make our lives easier in countless ways, but unfortunately, when it comes to curing acne, there’s no app for that,” the FTC chairman stated in a press release.18
In May 2013, the FDA sent an “It has come to our attention letter” to Biosense Technologies regarding its uChek urine analyzer app. The problem, the letter stated, is that the dipsticks that the app allows a mobile phone to analyze are cleared by the FDA only when interpreted by direct visual reading. But the phone and device together function “as an automated strip reader”—a urinalysis test system for which new FDA Smartphones make our lives easier in countless ways, but unfortunately, when it comes to curing acne, there's no app for that," the chairman of the Federal Trade Commission stated in a press release. clearance is required.19
Other ways of evaluating apps
Happtique, a mobile health solutions company, recently announced the launch of its Health App Certification Program—a voluntary program designed to help clinicians and patients easily identify apps that are credible and safe.20 “We will be certifying medical, health, and fitness apps, Corey Ackerman, president and CEO of Happtique, told JFP. The program is currently accepting medical education and nursing apps for review, and “discussions are underway with numerous other organizations that will provide experts for apps in additional subject matter areas,” Mr. Ackerman said.
There are other means of evaluating mobile medical apps that fall outside of the medical device realm, of course—starting by perusing the reviews posted at the app stores. Exchanging information with other clinicians using an app you’re interested in is another way to learn more about its efficacy. (Yes, there’s an app for that, too: Doximity, the professional network for clinicians.)
Other suggestions for safe use of apps:
- Peruse iMedicalApps (imedicalapps.com), the self-described leading physician publication on mobile medicine. Its physician editors and team of clinicians research and review medical apps.
- Consider the source. An app that has been developed by a medical society, federal agency, or prestigious medical school, for example, is more trustworthy than one from an unknown source (a point you would be wise to pass on to your patients).
- Try the app yourself before you recommend it to a patient.
Finally, keep the privacy provision in the Health Insurance Portability and Accountability Act in mind. Before using any app through which private patient health information can be transmitted or stored, ensure that the data will be encrypted and that your mobile device is password-protected, advises mHIMSS, the mobile branch of the Healthcare Information and Management Systems Society.21
1. TEDMED. The smartphone physical. Available at: http://www.smartphonephysical.org/tedmed.html. Accessed June 14, 2013.
2. AliveCor. AliveCor heart monitor. Available at: http://www.alivecor.com/. Accessed June 14, 2013.
3. Ubiquitous Computing Lab, University of Washington. Mobile phone spirometry. Available at: http://ubicomplab.cs.washington.edu/wiki/SpiroSmart. Accessed June 19, 2013.
4. Association of American Medical Colleges. Explosive growth in health care apps raises oversight questions. Available at: https://www.aamc.org/newsroom/reporter/october2012/308516/health-care-apps.html. Accessed June 14, 2013.
5. Alvarez A. How are physicians using smartphones for professional purposes? April 22, 2013. Available at: www.kantarmedia-healthcare.com/how-are-physicians-using-smartphones-for-professional-purposes. Accessed June 14, 2013.
6. Penn Medical Student Government. 2012 Medical app survey results. February 9, 2013. Available at: http://msg.med.upenn.edu/?p=17784. Accessed June 19, 2013.
7. Comstock J. Manhattan: 72% of physicians have tablets. April 18, 2013. Available at: http://mobihealthnews.com/21733/manhattan-72-percent-of-physicians-have-tablets/. Accessed June 19, 2013.
8. Dr. Eric Topol on NBC’s Rock Center. January 24, 2013. Available at: http://www.youtube.com/watch?v=0B-jUOOrtks. Accessed June 14, 2013.
9. Comstock J. Topol turns Colbert around on digital health. March 27, 2013. Available at: http://mobihealthnews.com/21263/topol-turns-colbert-around-on-digital-health/.Accessed June 14,2013.
10. Pew Research Center. Tracking for health. January 28, 2013. Available at: http://pewinternet.org/Press-Releases/2013/Tracking-for-health. Accessed June 14, 2013.
11. Ozdalga E, Ozdalga A, Ahuja N. The smartphone in medicine: a review of current and potential use among physicians and students. J Med Internet Res. 2012;14:e128.
12. Mendoza M, Rosenberg T. Self-management of type 2 diabetes: a good idea or not? J Fam Pract. 2013;62:244-248.
13. McMillan R. iPad: ‘Wild West’ of medical apps seeks sheriff. December 12, 2011. Available at: http://www.wired.com/wiredenterprise/2011/12/fda_apps/. Accessed June 14, 2013.
14. Hodge B. The use of symptom diaries in outpatient care. Fam Pract Manag. 2013;20:24-28.
15. Haffey F, Brady RR, Maxwell S. A comparison of the reliability of smartphone apps for opioid conversion. Drug Saf. 2013;36:111-117.
16. US Food and Drug Administration. FDA proposes health “app” guidelines. July 19, 2011. Available at: http://www.fda.gov/forconsumers/consumerupdates/ucm263332.htm. Accessed June 14, 2013.
17. Pavlovic P. 10 issues that mobile medical app developers should keep in mind. April 18, 2013. Available at: http://www.mhimss.org/news/10-issues-mobile-medical-app-developers-should-keep-mind. Accessed June 14, 2013.
18. Federal Trade Commission. “Acne cure” mobile app marketers will drop baseless claims under FTC settlements. September 8, 2011. Available at: http://www.ftc.gov/opa/2011/09/acnecure.shtm. Accessed June 14, 2013.
19. FDA. Letter to Biosense Technologies Private Limited concerning the uChek urine analyzer. Available at: http://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm353513.htm. Accessed June 14, 2013.
20. Happtique publishes final standards for mobile health app certification program. February 27, 2013. Available at: http://www.happtique.com/happtique-publishes-final-standards-for-mobile-health-app-certification-program/. Accessed June 19, 2013.
21. mHIMSS. Privacy and security. Available at: http://www.mhimss.org/resource-topics/privacy-security. Accessed June 14, 2013.
In April, hundreds of attendees at TEDMED, a conference on medical innovation, waited in line for a “smartphone physical.” Curated by Shiv Gaglani, a medical student and an editor at the medical technology journal Medgadget, the exam involved 10 apps that turn an ordinary smartphone into a medical device (TABLE 1).1 Among them were the AliveCor Heart Monitor (pictured at right), which produces a one-lead EKG in seconds when a patient’s fingers or chest are pressed against the electrodes embedded in the back of what is essentially a phone case2; a pulse oximeter, and an ultrasound that can capture images of the carotid arteries.1
All but one of the apps is paired with a physical component, such as an ultrasound wand or otoscope. The exception is SpiroSmart, an app that uses the phone’s The AliveCor app and Heart Monitor—a smartphone case fitted with sensors—can generate a one-lead EKG tracing in seconds.built-in microphone and lip reverberations to assess lung function. Shwetak Patel, PhD, of the University of Washington, one of its developers, told JFP that the accuracy of SpiroSmart has been found to be within 5% of traditional spirometry results.3
While smartphone physicals are not likely to be integrated into family practice for some time to come, Glen Stream, MD, board chair of the American Academy of Family Physicians, predicts that integration of some of their features is not too far away. “The spirometry application is an especially good one; it addresses one of the top 5 chronic conditions that contribute to health care costs,” Dr. Stream said. The apps will be beneficial, he added, as long as they “are used in a way that contributes, to, rather than detracts from, collaboration between patients and physicians.”
For now, Dr. Stream and many of his fellow FPs use mobile devices and medical apps primarily to access reference materials, both in and out of the exam room. Some have begun “prescribing” apps to tech-savvy patients. Still others have never used a medical app, either because they prefer a desktop or laptop computer to a smartphone or tablet or because, as one FP put it, "I have a dumb phone."
Wherever you fall on the spectrum, it’s a safe bet that you’re going to be increasingly inundated by the many manifestations of mobile health (mHealth).
Epocrates is No. 1 reference app
The number of medical/health apps for smart-phones or tablets is difficult to pin down; estimates range from 17,000 to more than 40,000, and growing.4 More is known about physician use of smartphones and tablets.
A March 2013 survey of nearly 3000 physicians found that 74% use smartphones at work and 43% use them to look up drug information.5 The favorite tool? A 2012 survey conducted by the University of Pennsylvania’s Perelman School of Medicine to identify the best medical apps put Epocrates at the top of the list (TABLE 2).6 Epocrates was the very first app cited by virtually all the FPs interviewed for this article, as well.
Other drug references cited tend to be patient-specific. Colan Kennelly, MD, a clinical educator at the Good Samaritan Family Medicine Residency in Phoenix, finds LactMed particularly useful. Developed by the National Library of Medicine and part of its Toxicology Data Network, the app lets you pull up medications quickly and see whether and how they will affect breastfeeding.
Another favorite of Kennelly’s is the Agency for Healthcare Research and Quality’s ePSS (electronic Preventive Services Selector) app designed to help primary care clinicians identify the preventive services that are appropriate for their patients. “You just plug in a patient’s age and sex”—(pregnancy, tobacco use, and whether the patient is sexually active are also considered)—“and it tells you what you should be checking for,” Dr. Kennelly said.
The benefits of mobile textbooks
Textbook apps and online texts are slowly gaining in popularity. A recent survey by Manhattan Research found that in 2013 for the first time, usage of electronic medical texts surpassed that of print editions.7 Part of the appeal is that mobile texts are easy to tote. “Apps make it possible to carry around information from a number of textbooks with no added weight,” said Richard Usatine, MD, of the University of Texas Health Science Center at San Antonio and editor of JFP’s Photo Rounds column. Dr. Usatine is also a principal of Usatine Media, which turns medical reference materials into apps.
Dr. Usatine’s own experience is a case in point. He recently used a textbook app to prepare to take his boards (for the fifth time). “I’ve brushed up each time,” he said, “but this time I really studied because it was fun.
“With a print textbook you have to cover up the answers so you don’t see them. Here, you don’t get to see the answer until you commit to one of the multiple choice answers. Then you get told what the correct answer is and why you got it right or wrong,” Dr. Usatine said. Interactivity, including the opportunity to watch a video, say, of a procedure to review how it’s done before embarking on it yourself, is a big part of the value of apps, he said.
Rx: App
In January, Eric Topol, MD, a prominent cardiologist and chief academic officer of Scripps Health in La Jolla, Calif., demonstrated the AliveCor heart monitor and other mobile devices on NBC’s Rock Center.8 In March, he went on The Colbert Report and examined Stephen Colbert’s ear with an otoscope smartphone accessory (CellScope) like the one used in Gaglani’s smartphone physical.9 Dr. Topol’s use of the mobile heart monitor to assess an airplane passenger in distress midflight also received widespread news coverage.
In response to an interviewer’s question, Dr. Topol said he is now more likely to prescribe an app than a drug.8 While it’s unlikely that any FP could make such a claim, many have begun recommending apps to tech-savvy patients.
Smartphones as symptom trackers
A January 2013 Pew Internet study found that 7 in 10 US adults track at least one health indicator, for themselves or a loved one. Six in 10 reported tracking weight, diet, or exercise, and one in 3 said they track indicators of medical problems, such as blood pressure, glucose levels, headaches, or sleep patterns—usually without the aid of a smartphone.10
In fact, half of those who report tracking health measures said they keep the information “in their head,” and a third still use pencil and paper.10 That could change, of course, if their physicians suggest they do otherwise (TABLE 3).
Kelly M. Latimer, MD, an FP in the Navy stationed in Djibouti, Africa, routinely asks patients whether they have a smartphone and often recommends apps to those who do.
“It sounds like you have a lot of different symptoms,” she might say to a patient who complains of frequent headaches. “It will help me if you keep a headache diary.”
She used to give such patients paper and pen, Dr. Latimer noted. “Now I ask them to download the app (iHeadache, in this case) right then and there and do a quick review” so they’re ready to use it at home.
Apps are also a good way to help people with anxiety, Dr. Latimer has found. She frequently recommends apps like Relaxation Techniques and Breathe2Relax, and often suggests apps like Calorie Count and MyFitnessPal to boost patients’ efforts to lose weight and get in shape.
Abigail Lowther, MD, an FP at the University of Michigan in Ann Arbor, also recommends apps frequently. But she typically broaches the subject only with patients who have their smartphones out when she walks into the room.
Among the apps Dr. Lowther prescribes are myPause to track menopausal symptoms and Bladder Pal, a voiding diary for women struggling with incontinence. She advises women taking oral contraceptives to use the timer function on their phone to remember to take a pill at the same time every day. But there are apps (myPill, for one) that do that, too.
The upside of patient apps. A smartphone is ideal for keeping a symptom diary because it’s something that most people are never without. Anyone can use the notes function on a phone or tablet to jot down details about exacerbations, but those using disease-specific apps tend to capture more precise information. Some patients print out the information they’ve gathered and bring a hard copy to an office visit, while others simply show their physician what’s on their smartphone.
Can apps affect outcomes? There are few high-quality studies and the jury is still out, but “the smartphone has a very bright future in the world of medicine,” the authors of a review of smartphones in the medical arena concluded. After examining the use of apps to track (literally) wandering dementia patients; calculate and recommend insulin dosages for patients with type 1 diabetes; and teach yoga, to name a few, the researchers concluded that “the smartphone may one day be recognized as a diagnostic and therapeutic tool…as irreplaceable as the stethoscope.”11
Dr. Lowther recalls an obese patient who found MyFitnessPal to be helpful where other, more traditional diet programs had failed. The reason? He was less than truthful with the people overseeing the weight loss programs about what he’d eaten when he tried—and failed—to follow diets like Weight Watchers. He then ended up feeling so guilty that he abandoned the effort entirely. But, he told her, he “wouldn’t lie” to an app.
…and the downside. Even physicians who haven’t begun A weight loss app would be more likely to help this patient reach his goal than other diet programs because he "wouldn't lie" to an app.recommending apps to patients are aware that carefully tracking measures related to chronic conditions like hypertension or diabetes often results in better control. But in some cases, there may be too much of a good thing. Evidence suggests that for some patients with type 2 diabetes, glucose self-monitoring is associated with depression and may do more harm than good.12
Dr. Lowther has witnessed a similar phenomenon in patients using disease-tracking apps. “Sometimes people get too focused on the problem and drive themselves crazy,” she observed, adding that those with high blood pressure are particularly at risk. “I think sometimes it’s hard for patients to understand the concept of an average value and normal fluctuation,” Dr. Lowther said. When that happens, “I have to tell them to back off.”
Who's minding the (app) store?
The mHealth arena has been called “the wild West.”13 With at least one app for virtually every aspect of health and medicine you can think of, it’s not hard to understand why.
In an article on the use of symptom diaries in outpatient care, Bryan Hodge, DO, an FP in Hendersonville, NC, mentions mobile self-tracking apps as one of a number of ways for patients to keep symptom diaries.14 Given the fact that few of these apps have been validated, Dr. Hodge writes, “The best approach is to familiarize yourself with a few options that you can offer to your patients.”14
That depends on the nature of the app. An app that tracks calories consumed or simply keeps an organized file of patient symptoms may do little harm; an app that conveys physical measurements that a patient or physician may act on or calculates medication dosages requires a higher level of vigilance.
A recent study of smartphone apps that calculate opioid dosage conversion, for example, found a lack of consistency that raised a red flag about the reliability of information provided by unvalidated apps. Better regulation of medical apps is crucial to ensure that patient safety is maintained, the authors concluded.15
The FDA’s role
The US Food and Drug Administration, which has approved more than 75 medical apps, issued a proposed approach to its oversight of the apps in 2011.16
Under the proposed rules, the agency would regulate mobile apps that were either used as an accessory to a medical device already regulated by the FDA or that transform a smartphone or tablet into a regulated medical device. A final rule has not yet been issued, but a spokesperson told Congress that it will be forthcoming before the end of the year.17
False claims are a target of federal regulation, as well. In 2011, the Federal Trade Commission pulled 2 acne apps off the market because both advertised—without scientific evidence—that the light emitted by smartphones equipped with the apps could treat acne. “Smartphones make our lives easier in countless ways, but unfortunately, when it comes to curing acne, there’s no app for that,” the FTC chairman stated in a press release.18
In May 2013, the FDA sent an “It has come to our attention letter” to Biosense Technologies regarding its uChek urine analyzer app. The problem, the letter stated, is that the dipsticks that the app allows a mobile phone to analyze are cleared by the FDA only when interpreted by direct visual reading. But the phone and device together function “as an automated strip reader”—a urinalysis test system for which new FDA Smartphones make our lives easier in countless ways, but unfortunately, when it comes to curing acne, there's no app for that," the chairman of the Federal Trade Commission stated in a press release. clearance is required.19
Other ways of evaluating apps
Happtique, a mobile health solutions company, recently announced the launch of its Health App Certification Program—a voluntary program designed to help clinicians and patients easily identify apps that are credible and safe.20 “We will be certifying medical, health, and fitness apps, Corey Ackerman, president and CEO of Happtique, told JFP. The program is currently accepting medical education and nursing apps for review, and “discussions are underway with numerous other organizations that will provide experts for apps in additional subject matter areas,” Mr. Ackerman said.
There are other means of evaluating mobile medical apps that fall outside of the medical device realm, of course—starting by perusing the reviews posted at the app stores. Exchanging information with other clinicians using an app you’re interested in is another way to learn more about its efficacy. (Yes, there’s an app for that, too: Doximity, the professional network for clinicians.)
Other suggestions for safe use of apps:
- Peruse iMedicalApps (imedicalapps.com), the self-described leading physician publication on mobile medicine. Its physician editors and team of clinicians research and review medical apps.
- Consider the source. An app that has been developed by a medical society, federal agency, or prestigious medical school, for example, is more trustworthy than one from an unknown source (a point you would be wise to pass on to your patients).
- Try the app yourself before you recommend it to a patient.
Finally, keep the privacy provision in the Health Insurance Portability and Accountability Act in mind. Before using any app through which private patient health information can be transmitted or stored, ensure that the data will be encrypted and that your mobile device is password-protected, advises mHIMSS, the mobile branch of the Healthcare Information and Management Systems Society.21
In April, hundreds of attendees at TEDMED, a conference on medical innovation, waited in line for a “smartphone physical.” Curated by Shiv Gaglani, a medical student and an editor at the medical technology journal Medgadget, the exam involved 10 apps that turn an ordinary smartphone into a medical device (TABLE 1).1 Among them were the AliveCor Heart Monitor (pictured at right), which produces a one-lead EKG in seconds when a patient’s fingers or chest are pressed against the electrodes embedded in the back of what is essentially a phone case2; a pulse oximeter, and an ultrasound that can capture images of the carotid arteries.1
All but one of the apps is paired with a physical component, such as an ultrasound wand or otoscope. The exception is SpiroSmart, an app that uses the phone’s The AliveCor app and Heart Monitor—a smartphone case fitted with sensors—can generate a one-lead EKG tracing in seconds.built-in microphone and lip reverberations to assess lung function. Shwetak Patel, PhD, of the University of Washington, one of its developers, told JFP that the accuracy of SpiroSmart has been found to be within 5% of traditional spirometry results.3
While smartphone physicals are not likely to be integrated into family practice for some time to come, Glen Stream, MD, board chair of the American Academy of Family Physicians, predicts that integration of some of their features is not too far away. “The spirometry application is an especially good one; it addresses one of the top 5 chronic conditions that contribute to health care costs,” Dr. Stream said. The apps will be beneficial, he added, as long as they “are used in a way that contributes, to, rather than detracts from, collaboration between patients and physicians.”
For now, Dr. Stream and many of his fellow FPs use mobile devices and medical apps primarily to access reference materials, both in and out of the exam room. Some have begun “prescribing” apps to tech-savvy patients. Still others have never used a medical app, either because they prefer a desktop or laptop computer to a smartphone or tablet or because, as one FP put it, "I have a dumb phone."
Wherever you fall on the spectrum, it’s a safe bet that you’re going to be increasingly inundated by the many manifestations of mobile health (mHealth).
Epocrates is No. 1 reference app
The number of medical/health apps for smart-phones or tablets is difficult to pin down; estimates range from 17,000 to more than 40,000, and growing.4 More is known about physician use of smartphones and tablets.
A March 2013 survey of nearly 3000 physicians found that 74% use smartphones at work and 43% use them to look up drug information.5 The favorite tool? A 2012 survey conducted by the University of Pennsylvania’s Perelman School of Medicine to identify the best medical apps put Epocrates at the top of the list (TABLE 2).6 Epocrates was the very first app cited by virtually all the FPs interviewed for this article, as well.
Other drug references cited tend to be patient-specific. Colan Kennelly, MD, a clinical educator at the Good Samaritan Family Medicine Residency in Phoenix, finds LactMed particularly useful. Developed by the National Library of Medicine and part of its Toxicology Data Network, the app lets you pull up medications quickly and see whether and how they will affect breastfeeding.
Another favorite of Kennelly’s is the Agency for Healthcare Research and Quality’s ePSS (electronic Preventive Services Selector) app designed to help primary care clinicians identify the preventive services that are appropriate for their patients. “You just plug in a patient’s age and sex”—(pregnancy, tobacco use, and whether the patient is sexually active are also considered)—“and it tells you what you should be checking for,” Dr. Kennelly said.
The benefits of mobile textbooks
Textbook apps and online texts are slowly gaining in popularity. A recent survey by Manhattan Research found that in 2013 for the first time, usage of electronic medical texts surpassed that of print editions.7 Part of the appeal is that mobile texts are easy to tote. “Apps make it possible to carry around information from a number of textbooks with no added weight,” said Richard Usatine, MD, of the University of Texas Health Science Center at San Antonio and editor of JFP’s Photo Rounds column. Dr. Usatine is also a principal of Usatine Media, which turns medical reference materials into apps.
Dr. Usatine’s own experience is a case in point. He recently used a textbook app to prepare to take his boards (for the fifth time). “I’ve brushed up each time,” he said, “but this time I really studied because it was fun.
“With a print textbook you have to cover up the answers so you don’t see them. Here, you don’t get to see the answer until you commit to one of the multiple choice answers. Then you get told what the correct answer is and why you got it right or wrong,” Dr. Usatine said. Interactivity, including the opportunity to watch a video, say, of a procedure to review how it’s done before embarking on it yourself, is a big part of the value of apps, he said.
Rx: App
In January, Eric Topol, MD, a prominent cardiologist and chief academic officer of Scripps Health in La Jolla, Calif., demonstrated the AliveCor heart monitor and other mobile devices on NBC’s Rock Center.8 In March, he went on The Colbert Report and examined Stephen Colbert’s ear with an otoscope smartphone accessory (CellScope) like the one used in Gaglani’s smartphone physical.9 Dr. Topol’s use of the mobile heart monitor to assess an airplane passenger in distress midflight also received widespread news coverage.
In response to an interviewer’s question, Dr. Topol said he is now more likely to prescribe an app than a drug.8 While it’s unlikely that any FP could make such a claim, many have begun recommending apps to tech-savvy patients.
Smartphones as symptom trackers
A January 2013 Pew Internet study found that 7 in 10 US adults track at least one health indicator, for themselves or a loved one. Six in 10 reported tracking weight, diet, or exercise, and one in 3 said they track indicators of medical problems, such as blood pressure, glucose levels, headaches, or sleep patterns—usually without the aid of a smartphone.10
In fact, half of those who report tracking health measures said they keep the information “in their head,” and a third still use pencil and paper.10 That could change, of course, if their physicians suggest they do otherwise (TABLE 3).
Kelly M. Latimer, MD, an FP in the Navy stationed in Djibouti, Africa, routinely asks patients whether they have a smartphone and often recommends apps to those who do.
“It sounds like you have a lot of different symptoms,” she might say to a patient who complains of frequent headaches. “It will help me if you keep a headache diary.”
She used to give such patients paper and pen, Dr. Latimer noted. “Now I ask them to download the app (iHeadache, in this case) right then and there and do a quick review” so they’re ready to use it at home.
Apps are also a good way to help people with anxiety, Dr. Latimer has found. She frequently recommends apps like Relaxation Techniques and Breathe2Relax, and often suggests apps like Calorie Count and MyFitnessPal to boost patients’ efforts to lose weight and get in shape.
Abigail Lowther, MD, an FP at the University of Michigan in Ann Arbor, also recommends apps frequently. But she typically broaches the subject only with patients who have their smartphones out when she walks into the room.
Among the apps Dr. Lowther prescribes are myPause to track menopausal symptoms and Bladder Pal, a voiding diary for women struggling with incontinence. She advises women taking oral contraceptives to use the timer function on their phone to remember to take a pill at the same time every day. But there are apps (myPill, for one) that do that, too.
The upside of patient apps. A smartphone is ideal for keeping a symptom diary because it’s something that most people are never without. Anyone can use the notes function on a phone or tablet to jot down details about exacerbations, but those using disease-specific apps tend to capture more precise information. Some patients print out the information they’ve gathered and bring a hard copy to an office visit, while others simply show their physician what’s on their smartphone.
Can apps affect outcomes? There are few high-quality studies and the jury is still out, but “the smartphone has a very bright future in the world of medicine,” the authors of a review of smartphones in the medical arena concluded. After examining the use of apps to track (literally) wandering dementia patients; calculate and recommend insulin dosages for patients with type 1 diabetes; and teach yoga, to name a few, the researchers concluded that “the smartphone may one day be recognized as a diagnostic and therapeutic tool…as irreplaceable as the stethoscope.”11
Dr. Lowther recalls an obese patient who found MyFitnessPal to be helpful where other, more traditional diet programs had failed. The reason? He was less than truthful with the people overseeing the weight loss programs about what he’d eaten when he tried—and failed—to follow diets like Weight Watchers. He then ended up feeling so guilty that he abandoned the effort entirely. But, he told her, he “wouldn’t lie” to an app.
…and the downside. Even physicians who haven’t begun A weight loss app would be more likely to help this patient reach his goal than other diet programs because he "wouldn't lie" to an app.recommending apps to patients are aware that carefully tracking measures related to chronic conditions like hypertension or diabetes often results in better control. But in some cases, there may be too much of a good thing. Evidence suggests that for some patients with type 2 diabetes, glucose self-monitoring is associated with depression and may do more harm than good.12
Dr. Lowther has witnessed a similar phenomenon in patients using disease-tracking apps. “Sometimes people get too focused on the problem and drive themselves crazy,” she observed, adding that those with high blood pressure are particularly at risk. “I think sometimes it’s hard for patients to understand the concept of an average value and normal fluctuation,” Dr. Lowther said. When that happens, “I have to tell them to back off.”
Who's minding the (app) store?
The mHealth arena has been called “the wild West.”13 With at least one app for virtually every aspect of health and medicine you can think of, it’s not hard to understand why.
In an article on the use of symptom diaries in outpatient care, Bryan Hodge, DO, an FP in Hendersonville, NC, mentions mobile self-tracking apps as one of a number of ways for patients to keep symptom diaries.14 Given the fact that few of these apps have been validated, Dr. Hodge writes, “The best approach is to familiarize yourself with a few options that you can offer to your patients.”14
That depends on the nature of the app. An app that tracks calories consumed or simply keeps an organized file of patient symptoms may do little harm; an app that conveys physical measurements that a patient or physician may act on or calculates medication dosages requires a higher level of vigilance.
A recent study of smartphone apps that calculate opioid dosage conversion, for example, found a lack of consistency that raised a red flag about the reliability of information provided by unvalidated apps. Better regulation of medical apps is crucial to ensure that patient safety is maintained, the authors concluded.15
The FDA’s role
The US Food and Drug Administration, which has approved more than 75 medical apps, issued a proposed approach to its oversight of the apps in 2011.16
Under the proposed rules, the agency would regulate mobile apps that were either used as an accessory to a medical device already regulated by the FDA or that transform a smartphone or tablet into a regulated medical device. A final rule has not yet been issued, but a spokesperson told Congress that it will be forthcoming before the end of the year.17
False claims are a target of federal regulation, as well. In 2011, the Federal Trade Commission pulled 2 acne apps off the market because both advertised—without scientific evidence—that the light emitted by smartphones equipped with the apps could treat acne. “Smartphones make our lives easier in countless ways, but unfortunately, when it comes to curing acne, there’s no app for that,” the FTC chairman stated in a press release.18
In May 2013, the FDA sent an “It has come to our attention letter” to Biosense Technologies regarding its uChek urine analyzer app. The problem, the letter stated, is that the dipsticks that the app allows a mobile phone to analyze are cleared by the FDA only when interpreted by direct visual reading. But the phone and device together function “as an automated strip reader”—a urinalysis test system for which new FDA Smartphones make our lives easier in countless ways, but unfortunately, when it comes to curing acne, there's no app for that," the chairman of the Federal Trade Commission stated in a press release. clearance is required.19
Other ways of evaluating apps
Happtique, a mobile health solutions company, recently announced the launch of its Health App Certification Program—a voluntary program designed to help clinicians and patients easily identify apps that are credible and safe.20 “We will be certifying medical, health, and fitness apps, Corey Ackerman, president and CEO of Happtique, told JFP. The program is currently accepting medical education and nursing apps for review, and “discussions are underway with numerous other organizations that will provide experts for apps in additional subject matter areas,” Mr. Ackerman said.
There are other means of evaluating mobile medical apps that fall outside of the medical device realm, of course—starting by perusing the reviews posted at the app stores. Exchanging information with other clinicians using an app you’re interested in is another way to learn more about its efficacy. (Yes, there’s an app for that, too: Doximity, the professional network for clinicians.)
Other suggestions for safe use of apps:
- Peruse iMedicalApps (imedicalapps.com), the self-described leading physician publication on mobile medicine. Its physician editors and team of clinicians research and review medical apps.
- Consider the source. An app that has been developed by a medical society, federal agency, or prestigious medical school, for example, is more trustworthy than one from an unknown source (a point you would be wise to pass on to your patients).
- Try the app yourself before you recommend it to a patient.
Finally, keep the privacy provision in the Health Insurance Portability and Accountability Act in mind. Before using any app through which private patient health information can be transmitted or stored, ensure that the data will be encrypted and that your mobile device is password-protected, advises mHIMSS, the mobile branch of the Healthcare Information and Management Systems Society.21
1. TEDMED. The smartphone physical. Available at: http://www.smartphonephysical.org/tedmed.html. Accessed June 14, 2013.
2. AliveCor. AliveCor heart monitor. Available at: http://www.alivecor.com/. Accessed June 14, 2013.
3. Ubiquitous Computing Lab, University of Washington. Mobile phone spirometry. Available at: http://ubicomplab.cs.washington.edu/wiki/SpiroSmart. Accessed June 19, 2013.
4. Association of American Medical Colleges. Explosive growth in health care apps raises oversight questions. Available at: https://www.aamc.org/newsroom/reporter/october2012/308516/health-care-apps.html. Accessed June 14, 2013.
5. Alvarez A. How are physicians using smartphones for professional purposes? April 22, 2013. Available at: www.kantarmedia-healthcare.com/how-are-physicians-using-smartphones-for-professional-purposes. Accessed June 14, 2013.
6. Penn Medical Student Government. 2012 Medical app survey results. February 9, 2013. Available at: http://msg.med.upenn.edu/?p=17784. Accessed June 19, 2013.
7. Comstock J. Manhattan: 72% of physicians have tablets. April 18, 2013. Available at: http://mobihealthnews.com/21733/manhattan-72-percent-of-physicians-have-tablets/. Accessed June 19, 2013.
8. Dr. Eric Topol on NBC’s Rock Center. January 24, 2013. Available at: http://www.youtube.com/watch?v=0B-jUOOrtks. Accessed June 14, 2013.
9. Comstock J. Topol turns Colbert around on digital health. March 27, 2013. Available at: http://mobihealthnews.com/21263/topol-turns-colbert-around-on-digital-health/.Accessed June 14,2013.
10. Pew Research Center. Tracking for health. January 28, 2013. Available at: http://pewinternet.org/Press-Releases/2013/Tracking-for-health. Accessed June 14, 2013.
11. Ozdalga E, Ozdalga A, Ahuja N. The smartphone in medicine: a review of current and potential use among physicians and students. J Med Internet Res. 2012;14:e128.
12. Mendoza M, Rosenberg T. Self-management of type 2 diabetes: a good idea or not? J Fam Pract. 2013;62:244-248.
13. McMillan R. iPad: ‘Wild West’ of medical apps seeks sheriff. December 12, 2011. Available at: http://www.wired.com/wiredenterprise/2011/12/fda_apps/. Accessed June 14, 2013.
14. Hodge B. The use of symptom diaries in outpatient care. Fam Pract Manag. 2013;20:24-28.
15. Haffey F, Brady RR, Maxwell S. A comparison of the reliability of smartphone apps for opioid conversion. Drug Saf. 2013;36:111-117.
16. US Food and Drug Administration. FDA proposes health “app” guidelines. July 19, 2011. Available at: http://www.fda.gov/forconsumers/consumerupdates/ucm263332.htm. Accessed June 14, 2013.
17. Pavlovic P. 10 issues that mobile medical app developers should keep in mind. April 18, 2013. Available at: http://www.mhimss.org/news/10-issues-mobile-medical-app-developers-should-keep-mind. Accessed June 14, 2013.
18. Federal Trade Commission. “Acne cure” mobile app marketers will drop baseless claims under FTC settlements. September 8, 2011. Available at: http://www.ftc.gov/opa/2011/09/acnecure.shtm. Accessed June 14, 2013.
19. FDA. Letter to Biosense Technologies Private Limited concerning the uChek urine analyzer. Available at: http://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm353513.htm. Accessed June 14, 2013.
20. Happtique publishes final standards for mobile health app certification program. February 27, 2013. Available at: http://www.happtique.com/happtique-publishes-final-standards-for-mobile-health-app-certification-program/. Accessed June 19, 2013.
21. mHIMSS. Privacy and security. Available at: http://www.mhimss.org/resource-topics/privacy-security. Accessed June 14, 2013.
1. TEDMED. The smartphone physical. Available at: http://www.smartphonephysical.org/tedmed.html. Accessed June 14, 2013.
2. AliveCor. AliveCor heart monitor. Available at: http://www.alivecor.com/. Accessed June 14, 2013.
3. Ubiquitous Computing Lab, University of Washington. Mobile phone spirometry. Available at: http://ubicomplab.cs.washington.edu/wiki/SpiroSmart. Accessed June 19, 2013.
4. Association of American Medical Colleges. Explosive growth in health care apps raises oversight questions. Available at: https://www.aamc.org/newsroom/reporter/october2012/308516/health-care-apps.html. Accessed June 14, 2013.
5. Alvarez A. How are physicians using smartphones for professional purposes? April 22, 2013. Available at: www.kantarmedia-healthcare.com/how-are-physicians-using-smartphones-for-professional-purposes. Accessed June 14, 2013.
6. Penn Medical Student Government. 2012 Medical app survey results. February 9, 2013. Available at: http://msg.med.upenn.edu/?p=17784. Accessed June 19, 2013.
7. Comstock J. Manhattan: 72% of physicians have tablets. April 18, 2013. Available at: http://mobihealthnews.com/21733/manhattan-72-percent-of-physicians-have-tablets/. Accessed June 19, 2013.
8. Dr. Eric Topol on NBC’s Rock Center. January 24, 2013. Available at: http://www.youtube.com/watch?v=0B-jUOOrtks. Accessed June 14, 2013.
9. Comstock J. Topol turns Colbert around on digital health. March 27, 2013. Available at: http://mobihealthnews.com/21263/topol-turns-colbert-around-on-digital-health/.Accessed June 14,2013.
10. Pew Research Center. Tracking for health. January 28, 2013. Available at: http://pewinternet.org/Press-Releases/2013/Tracking-for-health. Accessed June 14, 2013.
11. Ozdalga E, Ozdalga A, Ahuja N. The smartphone in medicine: a review of current and potential use among physicians and students. J Med Internet Res. 2012;14:e128.
12. Mendoza M, Rosenberg T. Self-management of type 2 diabetes: a good idea or not? J Fam Pract. 2013;62:244-248.
13. McMillan R. iPad: ‘Wild West’ of medical apps seeks sheriff. December 12, 2011. Available at: http://www.wired.com/wiredenterprise/2011/12/fda_apps/. Accessed June 14, 2013.
14. Hodge B. The use of symptom diaries in outpatient care. Fam Pract Manag. 2013;20:24-28.
15. Haffey F, Brady RR, Maxwell S. A comparison of the reliability of smartphone apps for opioid conversion. Drug Saf. 2013;36:111-117.
16. US Food and Drug Administration. FDA proposes health “app” guidelines. July 19, 2011. Available at: http://www.fda.gov/forconsumers/consumerupdates/ucm263332.htm. Accessed June 14, 2013.
17. Pavlovic P. 10 issues that mobile medical app developers should keep in mind. April 18, 2013. Available at: http://www.mhimss.org/news/10-issues-mobile-medical-app-developers-should-keep-mind. Accessed June 14, 2013.
18. Federal Trade Commission. “Acne cure” mobile app marketers will drop baseless claims under FTC settlements. September 8, 2011. Available at: http://www.ftc.gov/opa/2011/09/acnecure.shtm. Accessed June 14, 2013.
19. FDA. Letter to Biosense Technologies Private Limited concerning the uChek urine analyzer. Available at: http://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm353513.htm. Accessed June 14, 2013.
20. Happtique publishes final standards for mobile health app certification program. February 27, 2013. Available at: http://www.happtique.com/happtique-publishes-final-standards-for-mobile-health-app-certification-program/. Accessed June 19, 2013.
21. mHIMSS. Privacy and security. Available at: http://www.mhimss.org/resource-topics/privacy-security. Accessed June 14, 2013.
Targeting diabetes: The benefits of an integrative approach
- Tell patients with type 2 diabetes mellitus (T2DM) that chromium and fiber appear to have a beneficial effect on glycemic control, while the benefits of other dietary supplements are not known. C
- Recommend acupuncture for patients with T2DM and peripheral neuropathy, bladder dysfunction, or symptoms of other comorbidities that have not fully responded to conventional therapy. B
- Advise patients that biofeedback and meditation are more likely than other types of stress reduction to improve glycemic control. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Integrative medicine—an approach that combines conventional and alternative therapies with an emphasis on natural, less invasive evidence-based options—is well suited to the management of complex chronic diseases like type 2 diabetes mellitus (T2DM). And we’ve found that patients with T2DM are increasingly interested in integrative strategies, most of which involve self-management and lifestyle changes. They’re often motivated by the desire to limit the number of medications they’re taking or to avoid diabetes drugs entirely. In many cases, patients also hope to alleviate symptoms of comorbidities that have not fully responded to conventional treatment, such as peripheral neuropathy, bladder dysfunction, and gastroparesis.
As a family physician, you’re likely to be asked about unconventional approaches to diabetes and to be in a position to recommend alternative therapies in conjunction with pharmaceutical management of T2DM. In both cases, you need to know which integrative strategies have evidence to support their use. We created the text and tables that follow with this in mind.
Nutrition and weight loss: What works?
To reduce cardiovascular disease risk factors, patients with T2DM are advised to eliminate trans fats and to limit saturated fat to <7% of total caloric intake. A moderate weight loss (5% of body weight) has been found to improve insulin action, decrease fasting blood glucose (FBG) concentrations, and reduce the need for diabetes medications.1,2 One small retrospective cohort study (N=72) found that a 10% weight loss was associated with a reduction in glycosylated hemoglobin (HbA1c) of 0.81 percentage points.3 Weight maintenance is also an important element of diabetes management, even for patients who have not been able to lose their excess weight.2
American Diabetes Association (ADA) guidelines do not endorse a specific diet. But patients often ask for dietary advice and want to know how specific diet plans and food choices will affect glycemic control and comorbid conditions. You can present the evidence (TABLE 1)4-6 of the effectiveness of a low glycemic index (GI) diet, a Mediterranean diet, and a vegetarian diet and point out that the best diet for a particular patient is the one best suited to his or her lifestyle and dietary goals.
Low glycemic index diet. The 2013 ADA guidelines acknowledge that a dietary regimen with a low glycemic index (GI) may be more beneficial for glycemic control than a diet based on total carbohydrate count alone.7
A Cochrane review of 11 small studies found a reduction in HbA1c of 0.5 (95% confidence interval [CI], -0.9 to -0.1; P=.02) in patients with T2DM who followed a low GI diet for 4 weeks or more. Patients on this diet also had a significant reduction in hypoglycemic events compared with those on other dietary regimens;4 one of the studies included in the meta-analysis also found a statistically significant increase in high-density lipoprotein cholesterol (HDL-C) in those on the low
GI diet.8
Mediterranean diet. Rich in fruits and vegetables and unsaturated fats, fish, and grains with a low GI, the Mediterranean diet has a higher carbohydrate and fat content than a portion-controlled diet formerly recommended by the ADA. (As noted earlier, current ADA guidelines do not recommend a particular diet).
A systematic review of 5 randomized controlled trials (RCTs) (N=1077) found improved glycemic control in patients on a Mediterranean diet compared with those on other commonly used diets, such as low fat and portion-controlled regimens. Fasting blood glucose (FBG) fell between 7 and 40 mg/dL and HbA1c by 0.1 to 0.6 in studies that ranged from 6 months to 2 years.5 The effectiveness of the Mediterranean diet, despite its higher carbohydrate content, suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia. The effectiveness of the Mediterranean diet--despite its higher carbohydrate content--suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia.
Vegetarian diet. Vegetarian and vegan diets also offer potential benefits in the management of T2DM—including the fact that they tend to be lower in calories than other dietary regimens and therefore more likely to promote weight loss. In one 22-week RCT (N=99), those who were randomized to a low-fat vegan diet lost more weight (6.5 vs 3.1 kg; P<.001) and had a larger decrease in HbA1c (-1.23 vs -0.38; P<.01) and low density lipoprotein cholesterol (LDL-C) (-22.6 vs -10.7 mg/dL; P=.02) compared with those following a portion-controlled ADA diet. Glycemic change correlated with the change in body weight.6
Choose carefully among the supplements
Metformin was derived from Galega officinalis—a plant (sometimes called goat’s rue, or French lilac) used by Europeans as a traditional treatment for diabetes since the Middle Ages.9 More than 400 dietary supplements have been reported to have beneficial effects for patients with diabetes, including plant-based products such as fenugreek, prickly pear, and ginseng; vitamins, and minerals. But in most cases, the evidence is of poor quality (TABLE 2). Only one—cinnamon—has Level I evidence (ie, evidence derived from at least one well-designed RCT).
Two recent meta-analyses of cinnamon supplementation for patients with diabetes—one from 2012 and the other from 2011— yielded different results. The 2012 evidence comes from a Cochrane review showing that 3 types of cinnamon—true cinnamon (Cinnamomum zeylanicum), Chinese cinnamon (C cassia), and Indonesian cinnamon (C burmanni)—had no effect on HbA1c in patients with type 1 or T2DM and a borderline effect on FBG.10 However, the 2011 meta-analysis found that cinnamon supplementation led to a significant improvement in FBG.11
The bottom line? The evidence is inconclusive.
Fiber and chromium stand out
Fiber. Whether taken as a supplement or in foods such as chickpeas, beans, peas, and lentils, fiber has been shown to improve glycemic control.12,13 The ADA recommends 25 to 35 g of dietary fiber daily.13 The American Diabetes Association recommends 25 to 35 g of dietary fiber daily.
Chromium, a trace mineral thought to be a necessary cofactor for insulin regulation and glucose metabolism, is present in many foods, especially brewer’s yeast, liver, carrots, potatoes, broccoli, and spinach. Notably, however, refining grains and processing foods removes most absorbable chromium. Patients with T2DM may therefore benefit from chromium supplementation: A 2007 systematic review found that it lowered their HbA1c by an average of 0.6.14
What about other supplements?
The ADA does not generally support the use of micronutrient supplements for patients with diabetes, but notes that individuals at increased risk for deficiencies (eg, those following very-low-calorie diets, the elderly, and strict vegetarians) may benefit from multivitamin supplements.
Magnesium. Dietary sources of magnesium, which is involved in insulin secretion, binding, and activity, include whole grains, leafy green vegetables, legumes, and nuts. A deficiency is associated with decreased absorption (in patients with diets high in processed food, for example) or increased elimination (in those who ingest large quantities of alcohol or caffeine or take diuretics or birth control pills). A 2006 meta-analysis found that magnesium supplements led to an improvement in FBG, but did not significantly lower HbA1c in patients with T2DM.15
Vitamin D. Although individuals with the highest vitamin D levels (>25 ng/mL) have a 43% lower risk of developing diabetes,16 it is not known whether vitamin D supplements are beneficial to patients with T2DM. (For more on vitamin D supplementation, see the Practice Alert in this issue.)
Vitamin E. A fat-soluble antioxidant found in vegetable oil, nuts, and green leafy vegetables, vitamin E’s best-studied component is alpha-tocopherol. A 2011 meta-analysis found vitamin E supplementation to have a beneficial effect in patients with T2DM—but only for the subset of patients who had both low serum vitamin E levels and an HbA1c >8.0%.17 Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
What about omega-3 PUFAs?
The role of omega-3 polyunsaturated fatty acids (PUFAs) in the prevention and treatment of diseases related to inflammation has garnered much attention for years. But there is no evidence that taking omega-3 PUFAs lowers the risk of macrovascular outcomes or mortality for patients with T2DM.
Alpha lipoic acid (ALA), an antioxidant made by the body and found in very small amounts in foods, is widely used in Europe, and has shown promise in the treatment of diabetic neuropathy. Small studies have found that ALA may reduce oxidative stress and improve insulin sensitivity in patients with diabetes,19 and a recent small RCT showed a statistically significant decrease in FBG and postprandial glucose after 8 weeks of taking it.20
How to get patients moving
The ADA recommends that patients with T2DM get 150 minutes per week of moderate intensity aerobic activity (at 50%-70% of maximum heart rate), spread over at least 3 days a week and with no more than 2 consecutive days without activity. Resistance training provides additional benefit; the ADA recommends that patients engage in resistance training at least twice a week, using 5 major muscle groups.7 Regular exercise helps both weight reduction and glucose uptake, but simply pointing that out to patients with T2DM is not enough.
What works? A large meta-analysis found that structured exercise training (in most studies, this consisted of 2-5 supervised sessions weekly for 12-16 weeks) led to a decrease in HbA1c (-0.67; 95% CI, -0.84 to -0.49). When the structured exercise was aerobic, HbA1c declined by 0.73 (95% CI, -1.06 to -0.40); when it was resistance training, HbA1c fell by 0.57 (95% CI, -1.14 to -0.01).21 Simply advising a patient to be physically active—without involvement in both the planning and supervision—led to statistically significant reductions in HbA1c only when the advice was combined with dietary recommendations. Giving patients both exercise advice and dietary recommendations led to an HbA1c reduction of 0.58 (95% CI, -0.74 to -0.43).21
The duration of the structured exercise mattered, too, of course, with those who exercised more than 150 minutes per week achieving a larger reduction in HbA1c than those who exercised 150 minutes or less (-0.89; 95% CI, -1.26 to -0.50 vs -0.36; 95% CI, -0.50 to -0.23). Higher intensity activity did not improve glycemic For patients with type 2 diabetes, higher intensity activity does not appear to lead to greater improvement in glycemic control compared with moderate intensity activity. control any more than moderate intensity exercise.21
Mind-body stress relievers
The National Health Interview Survey (NHIS) estimated that in 2007 (the most recent survey that addressed mind-body modalities), 19% of US adults used at least one mind-body modality in the previous 12 months.22 Modalities included in the NHIS were biofeedback and yoga, body interventions best studied for diabetes management. Here’s what the evidence shows:
Biofeedback. In a small RCT (N=30 patients with T2DM) comparing biofeedback-assisted relaxation training (10 weekly 45-minute sessions) with education alone, the treatment group had significant improvement in HbA1c levels (which went from 7.4% to 6.8%) and in average blood glucose values that persisted at 3-month follow-up.23
Biofeedback can also produce clinically significant toe temperature elevations. In patients with T2DM, volitional warming has been associated with increased circulation, improvement or elimination of intermittent claudication pain, more rapid healing of diabetic ulcers, and improved functional status.24
Yoga. Two systematic reviews concluded that yoga is likely to benefit patients with T2DM, leading to lower blood sugar, LDL-C levels, triglycerides, body weight, waist-to hip ratio, and HbA1c, and higher HDL-C.25,26 Additionally, yoga appears to have a beneficial effect on the blood pressure, heart rate, oxidative stress, sympathetic activation, catecholamine levels, coronary stenosis, coagulation profiles, and pulmonary function of patients with T2DM, and is associated with reductions in the amount of medication needed and in psychosocial risk factors. (Because of the heterogeneous nature of the studies reviewed, however, no statistical analyses were reported.)
A third systematic review, which included only 5 studies, found that yoga yielded a short-term improvement in FBG and lipids, but no statistically significant improvement in long-term outcomes of body mass index, body weight, or HbA1c.27 All 5 studies noted that there were methodological problems and uncertainty about the generalizability of the findings to Western culture.
Meditation. The regular practice of transcendental meditation (TM) is associated with a reduction of catecholamine levels, a study comparing meditators with a control group found.28 A study examining the relationship between depression and diabetes found compelling evidence of an association between mental stress and hypothalamic-pituitary-adrenal axis hyperactivity,29and another comparing meditators with controls found the regular practice of TM to be associated with a reduction in catecholamine levels.30 As increased catecholamine levels affect glucose transport and insulin resistance, this finding suggests that reducing stress levels through meditation might lead to improved glycemic control. Transcendental meditation has been found to reduce mean arterial pressure, insulin resistance, and insulin levels.
One RCT comparing diabetes education alone with education plus stress management (progressive muscle relaxation, deep breathing, and mental imagery) found that HbA1c levels decreased by 0.5 in the latter group at one year.31In a single blinded randomized study, the TM group had a statistically significant reduction in mean arterial pressure, insulin resistance, and insulin levels compared with those who received diabetes education alone.32
Qigong. The effectiveness of Qigong systems such as Tai Chi—which integrate physical postures, breathing techniques, and focused attention—is difficult to determine because of methodological challenges in design and variability in practice. Authors of a systematic review of Tai Chi and diabetes found only 2 RCTs and 3 nonrandomized clinical trials and concluded that there was no convincing evidence that the practice aids in glucose control.33 Two other systematic reviews of Qigong for T2DM reported some improvement in glucose control, but limited study quality prevented definite
conclusions.34,35
When to consider "manual medicine"
An integrative approach to health also includes a number of modalities collectively known as manual medicine: acupuncture, massage/energy therapy, acupressure, and chiropractic and other forms of manipulation. Evidence on these modalities for the treatment of diabetes and diabetic complications is limited.
Acupuncture. Although acupuncture has long been reported to improve glycemic control in patients with diabetes and prediabetes, the evidence is limited and of poor quality.36,37
In recent years, 2 small RCTs have found that acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol.38,39 In one of the studies, 87.5% of participants randomized to acupuncture had symptom improvement, compared with 63.6% of those in the oral inositol group. In fact, marked symptom relief after 3 months of treatment was reported by 50% of those Acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol. who had acupuncture, compared with 21% of those who did not.39
In a small 2-week RCT, patients randomized to acupuncture vs sham acupuncture for diabetic bladder dysfunction showed statistically significant improvements in both subjective symptoms and urodynamic measurements.40 And a study comparing patients receiving electroacupuncture—in which an electric current is transmitted between 2 needles placed in the muscles—vs sham acupuncture found nonstatistically significant improvements in symptomatic
gastroparesis.41
Massage/energy therapy. Massage has been shown in several studies to reduce glucose levels,42-44 although no reductions in glucose levels were found in one small RCT.45 Connective tissue reflex massage led to improved lower limb blood flow in patients with diabetes and peripheral artery disease in another study, but the clinical significance is uncertain.46 Studies of reflexology and acupressure are similarly limited to small experimental and observational studies.47 No RCTs of chiropractic treatment for diabetes were found.
CORRESPONDENCE
Jacqueline Redmer, MD, MPH, Department of Family Medicine, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
ACKNOWLEDGEMENTS
The authors thank Drs. Sarina Schrager and Mindy Smith for their manuscript assistance. The work presented here was carried out while Drs. Longmier and Wedel were Primary Care Research Fellows supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine.
1. Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2003;26(suppl 1):S51–S61.
2. Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care. 2004;27:2067–2073.
3. Shantha GP, Kumar AA, Kahan S, et al. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ. 2012;38:417–426.
4. Thomas D, Elliott EJ. Low glycaemic index or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;(1):CD006296.
5. Esposito K, Maiorino MI, Ceriello A, et al. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract. 2010;89:97–102.
6. Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29:1777–1783.
7. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11–S66.
8. Jenkins DJA, Kendall CWC, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–2753.
9. Hadden DR. Goat’s rue-French lilac-Italian fitch-Spanish sainfoin: Gallega officinalis and metformin: the Edinburgh connection. J R Coll Physicians Edinb. 2005;35:258–260.
10. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
11. Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14:884–889.
12. Post RE, Mainous AG 3rd, King DE, et al. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25:16–23.
13. Sievenpiper JL, Kendall CWC, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495.
14. Balk EM, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163.
15. Song Y, He K, Levitan EB, et al. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes. Diabet Med. 2006;23:1050–1056.
16. Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015.
17. Suksomboon N, Poolsup N, Sinprasert S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. J Clin Pharm Ther. 2011;36:53–63.
18. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46.
19. Poh ZX, Goh KP. A current update on the use of alpha lipoic acid in the management of type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009;9:392–398.
20. Ansar H, Mazloom Z, Kazemi F, et al. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32:584–588.
21. Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes. JAMA. 2011;305:1790–1799.
22. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States 2007. Natl Health Stat Rep. 2008;(12):1–23.
23. McGinnis RA, McGrady A, Cox SA, et al. Biofeedback-assisted relaxation in type 2 diabetes. Diabetes Care. 2005;28:2145–2149.
24. Galper DI, Taylor AG, Cox DJ. Current status of mind-body interventions for vascular complications of diabetes. Fam Community Health. 2003;26:34–40.
25. Alexander GK, Taylor AG, Innes KE, et al. Contextualizing the effects of yoga therapy on diabetes management. Fam Community Health. 2008;31:228–239.
26. Innes KE, Vincent HK. The influence of yoga-based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. Evid Based Complement Alternat Med. 2007;4:469–486.
27. Aljasir B, Bryson M, Al-Shehri B. Yoga practice for the management of type II diabetes mellitus in adults: a systematic review. Evid Based Complement Alternat Med. 2010;7:399–408.
28. Infante JR, Torres-Avisbal M, Pinel P, et al. Catecholamine levels in practitioners of the transcendental meditation technique. Physiol Behav. 2001;72:141–146.
29. Dusek JA, Benson H. Mind-body medicine: a model of the comparative clinical impact of the acute stress and relaxation responses. Minn Med. 2009;92:47–50.
30. Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology. 2011;36:1276–1286.
31. Surwit RS, Van Tilburg MAL, Zucker N, et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25:30–34.
32. Paul-Labrador M, Polk D, Dwyer JH, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med. 2006;166:1218–1224.
33. Lee MS, Pittler MH, Kim M-S, et al. Tai chi for type 2 diabetes: a systematic review. Diabet Med. 2008;25:240–241.
34. Chen KW, Liu T, Zhang H, et al. An analytical review of the Chinese literature on Qigong therapy for diabetes mellitus. Am J Chin Med. 2009;37:439–457.
35. Lee MS, Chen KW, Choi T-Y, et al. Qigong for type 2 diabetes care: a systematic review. Complement Ther Med. 2009;17:236–242.
36. Hu H. A review of treatment of diabetes by acupuncture during the past forty years. J Tradit Chin Med. 1995;15:145–154.
37. Liang F, Koya D. Acupuncture: is it effective for treatment of insulin resistance? Diabetes Obes Metab. 2010;12:555–569.
38. Tong Y, Guo H, Han B. Fifteen-day acupuncture treatment relieves diabetic peripheral neuropathy. J Acupunct Meridian Stud. 2010;3:95–103.
39. Zhang C, Ma Y-X, Yan Y. Clinical effects of acupuncture for diabetic peripheral neuropathy. J Tradit Chin Med. 2010;30:13–14.
40. Tong Y, Jia Q, Sun Y, et al. Acupuncture in the treatment of diabetic bladder dysfunction. J Alternat Complement Med. 2009;15:905–909.
41. Wang C-P, Kao C-H, Chen W-K, et al. A single-blinded, randomized pilot study evaluating effects of electroacupuncture in diabetic patients with symptoms suggestive of gastroparesis. J Alternat Complement Med. 2008;14:833–839.
42. Guthrie DW, Gamble M. Energy therapies and diabetes mellitus. Diabetes Spectr. 2001;14:149–153.
43. Ezzo J, Donner T, Nickols D, et al. Is massage useful in the management of diabetes? A systematic review. Diabetes Spectr. 2001;14:218–224.
44. Sajedi F, Kashaninia Z, Hoseinzadeh S, et al. How effective is Swedish massage on blood glucose level in children with diabetes mellitus? Acta Med Iran. 2011;49:592–597.
45. Wändell PE, Carlsson AC, Gåfvels C, et al. Measuring possible effect on health-related quality of life by tactile massage or relaxation in patients with type 2 diabetes. Complement Ther Med. 2012;20:8–15.
46. Castro-Sánchez AM, Moreno-Lorenzo C, Matarán-Peñarrocha GA, et al. Connective tissue reflex massage for type 2 diabetic patients with peripheral arterial disease: randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:1–12.
47. Pilkington K, Stenhouse E, Kirkwood G, et al. Diabetes and complementary therapies: mapping the evidence. Pract Diab Int. 2007;24:371–376.
- Tell patients with type 2 diabetes mellitus (T2DM) that chromium and fiber appear to have a beneficial effect on glycemic control, while the benefits of other dietary supplements are not known. C
- Recommend acupuncture for patients with T2DM and peripheral neuropathy, bladder dysfunction, or symptoms of other comorbidities that have not fully responded to conventional therapy. B
- Advise patients that biofeedback and meditation are more likely than other types of stress reduction to improve glycemic control. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Integrative medicine—an approach that combines conventional and alternative therapies with an emphasis on natural, less invasive evidence-based options—is well suited to the management of complex chronic diseases like type 2 diabetes mellitus (T2DM). And we’ve found that patients with T2DM are increasingly interested in integrative strategies, most of which involve self-management and lifestyle changes. They’re often motivated by the desire to limit the number of medications they’re taking or to avoid diabetes drugs entirely. In many cases, patients also hope to alleviate symptoms of comorbidities that have not fully responded to conventional treatment, such as peripheral neuropathy, bladder dysfunction, and gastroparesis.
As a family physician, you’re likely to be asked about unconventional approaches to diabetes and to be in a position to recommend alternative therapies in conjunction with pharmaceutical management of T2DM. In both cases, you need to know which integrative strategies have evidence to support their use. We created the text and tables that follow with this in mind.
Nutrition and weight loss: What works?
To reduce cardiovascular disease risk factors, patients with T2DM are advised to eliminate trans fats and to limit saturated fat to <7% of total caloric intake. A moderate weight loss (5% of body weight) has been found to improve insulin action, decrease fasting blood glucose (FBG) concentrations, and reduce the need for diabetes medications.1,2 One small retrospective cohort study (N=72) found that a 10% weight loss was associated with a reduction in glycosylated hemoglobin (HbA1c) of 0.81 percentage points.3 Weight maintenance is also an important element of diabetes management, even for patients who have not been able to lose their excess weight.2
American Diabetes Association (ADA) guidelines do not endorse a specific diet. But patients often ask for dietary advice and want to know how specific diet plans and food choices will affect glycemic control and comorbid conditions. You can present the evidence (TABLE 1)4-6 of the effectiveness of a low glycemic index (GI) diet, a Mediterranean diet, and a vegetarian diet and point out that the best diet for a particular patient is the one best suited to his or her lifestyle and dietary goals.
Low glycemic index diet. The 2013 ADA guidelines acknowledge that a dietary regimen with a low glycemic index (GI) may be more beneficial for glycemic control than a diet based on total carbohydrate count alone.7
A Cochrane review of 11 small studies found a reduction in HbA1c of 0.5 (95% confidence interval [CI], -0.9 to -0.1; P=.02) in patients with T2DM who followed a low GI diet for 4 weeks or more. Patients on this diet also had a significant reduction in hypoglycemic events compared with those on other dietary regimens;4 one of the studies included in the meta-analysis also found a statistically significant increase in high-density lipoprotein cholesterol (HDL-C) in those on the low
GI diet.8
Mediterranean diet. Rich in fruits and vegetables and unsaturated fats, fish, and grains with a low GI, the Mediterranean diet has a higher carbohydrate and fat content than a portion-controlled diet formerly recommended by the ADA. (As noted earlier, current ADA guidelines do not recommend a particular diet).
A systematic review of 5 randomized controlled trials (RCTs) (N=1077) found improved glycemic control in patients on a Mediterranean diet compared with those on other commonly used diets, such as low fat and portion-controlled regimens. Fasting blood glucose (FBG) fell between 7 and 40 mg/dL and HbA1c by 0.1 to 0.6 in studies that ranged from 6 months to 2 years.5 The effectiveness of the Mediterranean diet, despite its higher carbohydrate content, suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia. The effectiveness of the Mediterranean diet--despite its higher carbohydrate content--suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia.
Vegetarian diet. Vegetarian and vegan diets also offer potential benefits in the management of T2DM—including the fact that they tend to be lower in calories than other dietary regimens and therefore more likely to promote weight loss. In one 22-week RCT (N=99), those who were randomized to a low-fat vegan diet lost more weight (6.5 vs 3.1 kg; P<.001) and had a larger decrease in HbA1c (-1.23 vs -0.38; P<.01) and low density lipoprotein cholesterol (LDL-C) (-22.6 vs -10.7 mg/dL; P=.02) compared with those following a portion-controlled ADA diet. Glycemic change correlated with the change in body weight.6
Choose carefully among the supplements
Metformin was derived from Galega officinalis—a plant (sometimes called goat’s rue, or French lilac) used by Europeans as a traditional treatment for diabetes since the Middle Ages.9 More than 400 dietary supplements have been reported to have beneficial effects for patients with diabetes, including plant-based products such as fenugreek, prickly pear, and ginseng; vitamins, and minerals. But in most cases, the evidence is of poor quality (TABLE 2). Only one—cinnamon—has Level I evidence (ie, evidence derived from at least one well-designed RCT).
Two recent meta-analyses of cinnamon supplementation for patients with diabetes—one from 2012 and the other from 2011— yielded different results. The 2012 evidence comes from a Cochrane review showing that 3 types of cinnamon—true cinnamon (Cinnamomum zeylanicum), Chinese cinnamon (C cassia), and Indonesian cinnamon (C burmanni)—had no effect on HbA1c in patients with type 1 or T2DM and a borderline effect on FBG.10 However, the 2011 meta-analysis found that cinnamon supplementation led to a significant improvement in FBG.11
The bottom line? The evidence is inconclusive.
Fiber and chromium stand out
Fiber. Whether taken as a supplement or in foods such as chickpeas, beans, peas, and lentils, fiber has been shown to improve glycemic control.12,13 The ADA recommends 25 to 35 g of dietary fiber daily.13 The American Diabetes Association recommends 25 to 35 g of dietary fiber daily.
Chromium, a trace mineral thought to be a necessary cofactor for insulin regulation and glucose metabolism, is present in many foods, especially brewer’s yeast, liver, carrots, potatoes, broccoli, and spinach. Notably, however, refining grains and processing foods removes most absorbable chromium. Patients with T2DM may therefore benefit from chromium supplementation: A 2007 systematic review found that it lowered their HbA1c by an average of 0.6.14
What about other supplements?
The ADA does not generally support the use of micronutrient supplements for patients with diabetes, but notes that individuals at increased risk for deficiencies (eg, those following very-low-calorie diets, the elderly, and strict vegetarians) may benefit from multivitamin supplements.
Magnesium. Dietary sources of magnesium, which is involved in insulin secretion, binding, and activity, include whole grains, leafy green vegetables, legumes, and nuts. A deficiency is associated with decreased absorption (in patients with diets high in processed food, for example) or increased elimination (in those who ingest large quantities of alcohol or caffeine or take diuretics or birth control pills). A 2006 meta-analysis found that magnesium supplements led to an improvement in FBG, but did not significantly lower HbA1c in patients with T2DM.15
Vitamin D. Although individuals with the highest vitamin D levels (>25 ng/mL) have a 43% lower risk of developing diabetes,16 it is not known whether vitamin D supplements are beneficial to patients with T2DM. (For more on vitamin D supplementation, see the Practice Alert in this issue.)
Vitamin E. A fat-soluble antioxidant found in vegetable oil, nuts, and green leafy vegetables, vitamin E’s best-studied component is alpha-tocopherol. A 2011 meta-analysis found vitamin E supplementation to have a beneficial effect in patients with T2DM—but only for the subset of patients who had both low serum vitamin E levels and an HbA1c >8.0%.17 Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
What about omega-3 PUFAs?
The role of omega-3 polyunsaturated fatty acids (PUFAs) in the prevention and treatment of diseases related to inflammation has garnered much attention for years. But there is no evidence that taking omega-3 PUFAs lowers the risk of macrovascular outcomes or mortality for patients with T2DM.
Alpha lipoic acid (ALA), an antioxidant made by the body and found in very small amounts in foods, is widely used in Europe, and has shown promise in the treatment of diabetic neuropathy. Small studies have found that ALA may reduce oxidative stress and improve insulin sensitivity in patients with diabetes,19 and a recent small RCT showed a statistically significant decrease in FBG and postprandial glucose after 8 weeks of taking it.20
How to get patients moving
The ADA recommends that patients with T2DM get 150 minutes per week of moderate intensity aerobic activity (at 50%-70% of maximum heart rate), spread over at least 3 days a week and with no more than 2 consecutive days without activity. Resistance training provides additional benefit; the ADA recommends that patients engage in resistance training at least twice a week, using 5 major muscle groups.7 Regular exercise helps both weight reduction and glucose uptake, but simply pointing that out to patients with T2DM is not enough.
What works? A large meta-analysis found that structured exercise training (in most studies, this consisted of 2-5 supervised sessions weekly for 12-16 weeks) led to a decrease in HbA1c (-0.67; 95% CI, -0.84 to -0.49). When the structured exercise was aerobic, HbA1c declined by 0.73 (95% CI, -1.06 to -0.40); when it was resistance training, HbA1c fell by 0.57 (95% CI, -1.14 to -0.01).21 Simply advising a patient to be physically active—without involvement in both the planning and supervision—led to statistically significant reductions in HbA1c only when the advice was combined with dietary recommendations. Giving patients both exercise advice and dietary recommendations led to an HbA1c reduction of 0.58 (95% CI, -0.74 to -0.43).21
The duration of the structured exercise mattered, too, of course, with those who exercised more than 150 minutes per week achieving a larger reduction in HbA1c than those who exercised 150 minutes or less (-0.89; 95% CI, -1.26 to -0.50 vs -0.36; 95% CI, -0.50 to -0.23). Higher intensity activity did not improve glycemic For patients with type 2 diabetes, higher intensity activity does not appear to lead to greater improvement in glycemic control compared with moderate intensity activity. control any more than moderate intensity exercise.21
Mind-body stress relievers
The National Health Interview Survey (NHIS) estimated that in 2007 (the most recent survey that addressed mind-body modalities), 19% of US adults used at least one mind-body modality in the previous 12 months.22 Modalities included in the NHIS were biofeedback and yoga, body interventions best studied for diabetes management. Here’s what the evidence shows:
Biofeedback. In a small RCT (N=30 patients with T2DM) comparing biofeedback-assisted relaxation training (10 weekly 45-minute sessions) with education alone, the treatment group had significant improvement in HbA1c levels (which went from 7.4% to 6.8%) and in average blood glucose values that persisted at 3-month follow-up.23
Biofeedback can also produce clinically significant toe temperature elevations. In patients with T2DM, volitional warming has been associated with increased circulation, improvement or elimination of intermittent claudication pain, more rapid healing of diabetic ulcers, and improved functional status.24
Yoga. Two systematic reviews concluded that yoga is likely to benefit patients with T2DM, leading to lower blood sugar, LDL-C levels, triglycerides, body weight, waist-to hip ratio, and HbA1c, and higher HDL-C.25,26 Additionally, yoga appears to have a beneficial effect on the blood pressure, heart rate, oxidative stress, sympathetic activation, catecholamine levels, coronary stenosis, coagulation profiles, and pulmonary function of patients with T2DM, and is associated with reductions in the amount of medication needed and in psychosocial risk factors. (Because of the heterogeneous nature of the studies reviewed, however, no statistical analyses were reported.)
A third systematic review, which included only 5 studies, found that yoga yielded a short-term improvement in FBG and lipids, but no statistically significant improvement in long-term outcomes of body mass index, body weight, or HbA1c.27 All 5 studies noted that there were methodological problems and uncertainty about the generalizability of the findings to Western culture.
Meditation. The regular practice of transcendental meditation (TM) is associated with a reduction of catecholamine levels, a study comparing meditators with a control group found.28 A study examining the relationship between depression and diabetes found compelling evidence of an association between mental stress and hypothalamic-pituitary-adrenal axis hyperactivity,29and another comparing meditators with controls found the regular practice of TM to be associated with a reduction in catecholamine levels.30 As increased catecholamine levels affect glucose transport and insulin resistance, this finding suggests that reducing stress levels through meditation might lead to improved glycemic control. Transcendental meditation has been found to reduce mean arterial pressure, insulin resistance, and insulin levels.
One RCT comparing diabetes education alone with education plus stress management (progressive muscle relaxation, deep breathing, and mental imagery) found that HbA1c levels decreased by 0.5 in the latter group at one year.31In a single blinded randomized study, the TM group had a statistically significant reduction in mean arterial pressure, insulin resistance, and insulin levels compared with those who received diabetes education alone.32
Qigong. The effectiveness of Qigong systems such as Tai Chi—which integrate physical postures, breathing techniques, and focused attention—is difficult to determine because of methodological challenges in design and variability in practice. Authors of a systematic review of Tai Chi and diabetes found only 2 RCTs and 3 nonrandomized clinical trials and concluded that there was no convincing evidence that the practice aids in glucose control.33 Two other systematic reviews of Qigong for T2DM reported some improvement in glucose control, but limited study quality prevented definite
conclusions.34,35
When to consider "manual medicine"
An integrative approach to health also includes a number of modalities collectively known as manual medicine: acupuncture, massage/energy therapy, acupressure, and chiropractic and other forms of manipulation. Evidence on these modalities for the treatment of diabetes and diabetic complications is limited.
Acupuncture. Although acupuncture has long been reported to improve glycemic control in patients with diabetes and prediabetes, the evidence is limited and of poor quality.36,37
In recent years, 2 small RCTs have found that acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol.38,39 In one of the studies, 87.5% of participants randomized to acupuncture had symptom improvement, compared with 63.6% of those in the oral inositol group. In fact, marked symptom relief after 3 months of treatment was reported by 50% of those Acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol. who had acupuncture, compared with 21% of those who did not.39
In a small 2-week RCT, patients randomized to acupuncture vs sham acupuncture for diabetic bladder dysfunction showed statistically significant improvements in both subjective symptoms and urodynamic measurements.40 And a study comparing patients receiving electroacupuncture—in which an electric current is transmitted between 2 needles placed in the muscles—vs sham acupuncture found nonstatistically significant improvements in symptomatic
gastroparesis.41
Massage/energy therapy. Massage has been shown in several studies to reduce glucose levels,42-44 although no reductions in glucose levels were found in one small RCT.45 Connective tissue reflex massage led to improved lower limb blood flow in patients with diabetes and peripheral artery disease in another study, but the clinical significance is uncertain.46 Studies of reflexology and acupressure are similarly limited to small experimental and observational studies.47 No RCTs of chiropractic treatment for diabetes were found.
CORRESPONDENCE
Jacqueline Redmer, MD, MPH, Department of Family Medicine, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
ACKNOWLEDGEMENTS
The authors thank Drs. Sarina Schrager and Mindy Smith for their manuscript assistance. The work presented here was carried out while Drs. Longmier and Wedel were Primary Care Research Fellows supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine.
- Tell patients with type 2 diabetes mellitus (T2DM) that chromium and fiber appear to have a beneficial effect on glycemic control, while the benefits of other dietary supplements are not known. C
- Recommend acupuncture for patients with T2DM and peripheral neuropathy, bladder dysfunction, or symptoms of other comorbidities that have not fully responded to conventional therapy. B
- Advise patients that biofeedback and meditation are more likely than other types of stress reduction to improve glycemic control. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Integrative medicine—an approach that combines conventional and alternative therapies with an emphasis on natural, less invasive evidence-based options—is well suited to the management of complex chronic diseases like type 2 diabetes mellitus (T2DM). And we’ve found that patients with T2DM are increasingly interested in integrative strategies, most of which involve self-management and lifestyle changes. They’re often motivated by the desire to limit the number of medications they’re taking or to avoid diabetes drugs entirely. In many cases, patients also hope to alleviate symptoms of comorbidities that have not fully responded to conventional treatment, such as peripheral neuropathy, bladder dysfunction, and gastroparesis.
As a family physician, you’re likely to be asked about unconventional approaches to diabetes and to be in a position to recommend alternative therapies in conjunction with pharmaceutical management of T2DM. In both cases, you need to know which integrative strategies have evidence to support their use. We created the text and tables that follow with this in mind.
Nutrition and weight loss: What works?
To reduce cardiovascular disease risk factors, patients with T2DM are advised to eliminate trans fats and to limit saturated fat to <7% of total caloric intake. A moderate weight loss (5% of body weight) has been found to improve insulin action, decrease fasting blood glucose (FBG) concentrations, and reduce the need for diabetes medications.1,2 One small retrospective cohort study (N=72) found that a 10% weight loss was associated with a reduction in glycosylated hemoglobin (HbA1c) of 0.81 percentage points.3 Weight maintenance is also an important element of diabetes management, even for patients who have not been able to lose their excess weight.2
American Diabetes Association (ADA) guidelines do not endorse a specific diet. But patients often ask for dietary advice and want to know how specific diet plans and food choices will affect glycemic control and comorbid conditions. You can present the evidence (TABLE 1)4-6 of the effectiveness of a low glycemic index (GI) diet, a Mediterranean diet, and a vegetarian diet and point out that the best diet for a particular patient is the one best suited to his or her lifestyle and dietary goals.
Low glycemic index diet. The 2013 ADA guidelines acknowledge that a dietary regimen with a low glycemic index (GI) may be more beneficial for glycemic control than a diet based on total carbohydrate count alone.7
A Cochrane review of 11 small studies found a reduction in HbA1c of 0.5 (95% confidence interval [CI], -0.9 to -0.1; P=.02) in patients with T2DM who followed a low GI diet for 4 weeks or more. Patients on this diet also had a significant reduction in hypoglycemic events compared with those on other dietary regimens;4 one of the studies included in the meta-analysis also found a statistically significant increase in high-density lipoprotein cholesterol (HDL-C) in those on the low
GI diet.8
Mediterranean diet. Rich in fruits and vegetables and unsaturated fats, fish, and grains with a low GI, the Mediterranean diet has a higher carbohydrate and fat content than a portion-controlled diet formerly recommended by the ADA. (As noted earlier, current ADA guidelines do not recommend a particular diet).
A systematic review of 5 randomized controlled trials (RCTs) (N=1077) found improved glycemic control in patients on a Mediterranean diet compared with those on other commonly used diets, such as low fat and portion-controlled regimens. Fasting blood glucose (FBG) fell between 7 and 40 mg/dL and HbA1c by 0.1 to 0.6 in studies that ranged from 6 months to 2 years.5 The effectiveness of the Mediterranean diet, despite its higher carbohydrate content, suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia. The effectiveness of the Mediterranean diet--despite its higher carbohydrate content--suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia.
Vegetarian diet. Vegetarian and vegan diets also offer potential benefits in the management of T2DM—including the fact that they tend to be lower in calories than other dietary regimens and therefore more likely to promote weight loss. In one 22-week RCT (N=99), those who were randomized to a low-fat vegan diet lost more weight (6.5 vs 3.1 kg; P<.001) and had a larger decrease in HbA1c (-1.23 vs -0.38; P<.01) and low density lipoprotein cholesterol (LDL-C) (-22.6 vs -10.7 mg/dL; P=.02) compared with those following a portion-controlled ADA diet. Glycemic change correlated with the change in body weight.6
Choose carefully among the supplements
Metformin was derived from Galega officinalis—a plant (sometimes called goat’s rue, or French lilac) used by Europeans as a traditional treatment for diabetes since the Middle Ages.9 More than 400 dietary supplements have been reported to have beneficial effects for patients with diabetes, including plant-based products such as fenugreek, prickly pear, and ginseng; vitamins, and minerals. But in most cases, the evidence is of poor quality (TABLE 2). Only one—cinnamon—has Level I evidence (ie, evidence derived from at least one well-designed RCT).
Two recent meta-analyses of cinnamon supplementation for patients with diabetes—one from 2012 and the other from 2011— yielded different results. The 2012 evidence comes from a Cochrane review showing that 3 types of cinnamon—true cinnamon (Cinnamomum zeylanicum), Chinese cinnamon (C cassia), and Indonesian cinnamon (C burmanni)—had no effect on HbA1c in patients with type 1 or T2DM and a borderline effect on FBG.10 However, the 2011 meta-analysis found that cinnamon supplementation led to a significant improvement in FBG.11
The bottom line? The evidence is inconclusive.
Fiber and chromium stand out
Fiber. Whether taken as a supplement or in foods such as chickpeas, beans, peas, and lentils, fiber has been shown to improve glycemic control.12,13 The ADA recommends 25 to 35 g of dietary fiber daily.13 The American Diabetes Association recommends 25 to 35 g of dietary fiber daily.
Chromium, a trace mineral thought to be a necessary cofactor for insulin regulation and glucose metabolism, is present in many foods, especially brewer’s yeast, liver, carrots, potatoes, broccoli, and spinach. Notably, however, refining grains and processing foods removes most absorbable chromium. Patients with T2DM may therefore benefit from chromium supplementation: A 2007 systematic review found that it lowered their HbA1c by an average of 0.6.14
What about other supplements?
The ADA does not generally support the use of micronutrient supplements for patients with diabetes, but notes that individuals at increased risk for deficiencies (eg, those following very-low-calorie diets, the elderly, and strict vegetarians) may benefit from multivitamin supplements.
Magnesium. Dietary sources of magnesium, which is involved in insulin secretion, binding, and activity, include whole grains, leafy green vegetables, legumes, and nuts. A deficiency is associated with decreased absorption (in patients with diets high in processed food, for example) or increased elimination (in those who ingest large quantities of alcohol or caffeine or take diuretics or birth control pills). A 2006 meta-analysis found that magnesium supplements led to an improvement in FBG, but did not significantly lower HbA1c in patients with T2DM.15
Vitamin D. Although individuals with the highest vitamin D levels (>25 ng/mL) have a 43% lower risk of developing diabetes,16 it is not known whether vitamin D supplements are beneficial to patients with T2DM. (For more on vitamin D supplementation, see the Practice Alert in this issue.)
Vitamin E. A fat-soluble antioxidant found in vegetable oil, nuts, and green leafy vegetables, vitamin E’s best-studied component is alpha-tocopherol. A 2011 meta-analysis found vitamin E supplementation to have a beneficial effect in patients with T2DM—but only for the subset of patients who had both low serum vitamin E levels and an HbA1c >8.0%.17 Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
What about omega-3 PUFAs?
The role of omega-3 polyunsaturated fatty acids (PUFAs) in the prevention and treatment of diseases related to inflammation has garnered much attention for years. But there is no evidence that taking omega-3 PUFAs lowers the risk of macrovascular outcomes or mortality for patients with T2DM.
Alpha lipoic acid (ALA), an antioxidant made by the body and found in very small amounts in foods, is widely used in Europe, and has shown promise in the treatment of diabetic neuropathy. Small studies have found that ALA may reduce oxidative stress and improve insulin sensitivity in patients with diabetes,19 and a recent small RCT showed a statistically significant decrease in FBG and postprandial glucose after 8 weeks of taking it.20
How to get patients moving
The ADA recommends that patients with T2DM get 150 minutes per week of moderate intensity aerobic activity (at 50%-70% of maximum heart rate), spread over at least 3 days a week and with no more than 2 consecutive days without activity. Resistance training provides additional benefit; the ADA recommends that patients engage in resistance training at least twice a week, using 5 major muscle groups.7 Regular exercise helps both weight reduction and glucose uptake, but simply pointing that out to patients with T2DM is not enough.
What works? A large meta-analysis found that structured exercise training (in most studies, this consisted of 2-5 supervised sessions weekly for 12-16 weeks) led to a decrease in HbA1c (-0.67; 95% CI, -0.84 to -0.49). When the structured exercise was aerobic, HbA1c declined by 0.73 (95% CI, -1.06 to -0.40); when it was resistance training, HbA1c fell by 0.57 (95% CI, -1.14 to -0.01).21 Simply advising a patient to be physically active—without involvement in both the planning and supervision—led to statistically significant reductions in HbA1c only when the advice was combined with dietary recommendations. Giving patients both exercise advice and dietary recommendations led to an HbA1c reduction of 0.58 (95% CI, -0.74 to -0.43).21
The duration of the structured exercise mattered, too, of course, with those who exercised more than 150 minutes per week achieving a larger reduction in HbA1c than those who exercised 150 minutes or less (-0.89; 95% CI, -1.26 to -0.50 vs -0.36; 95% CI, -0.50 to -0.23). Higher intensity activity did not improve glycemic For patients with type 2 diabetes, higher intensity activity does not appear to lead to greater improvement in glycemic control compared with moderate intensity activity. control any more than moderate intensity exercise.21
Mind-body stress relievers
The National Health Interview Survey (NHIS) estimated that in 2007 (the most recent survey that addressed mind-body modalities), 19% of US adults used at least one mind-body modality in the previous 12 months.22 Modalities included in the NHIS were biofeedback and yoga, body interventions best studied for diabetes management. Here’s what the evidence shows:
Biofeedback. In a small RCT (N=30 patients with T2DM) comparing biofeedback-assisted relaxation training (10 weekly 45-minute sessions) with education alone, the treatment group had significant improvement in HbA1c levels (which went from 7.4% to 6.8%) and in average blood glucose values that persisted at 3-month follow-up.23
Biofeedback can also produce clinically significant toe temperature elevations. In patients with T2DM, volitional warming has been associated with increased circulation, improvement or elimination of intermittent claudication pain, more rapid healing of diabetic ulcers, and improved functional status.24
Yoga. Two systematic reviews concluded that yoga is likely to benefit patients with T2DM, leading to lower blood sugar, LDL-C levels, triglycerides, body weight, waist-to hip ratio, and HbA1c, and higher HDL-C.25,26 Additionally, yoga appears to have a beneficial effect on the blood pressure, heart rate, oxidative stress, sympathetic activation, catecholamine levels, coronary stenosis, coagulation profiles, and pulmonary function of patients with T2DM, and is associated with reductions in the amount of medication needed and in psychosocial risk factors. (Because of the heterogeneous nature of the studies reviewed, however, no statistical analyses were reported.)
A third systematic review, which included only 5 studies, found that yoga yielded a short-term improvement in FBG and lipids, but no statistically significant improvement in long-term outcomes of body mass index, body weight, or HbA1c.27 All 5 studies noted that there were methodological problems and uncertainty about the generalizability of the findings to Western culture.
Meditation. The regular practice of transcendental meditation (TM) is associated with a reduction of catecholamine levels, a study comparing meditators with a control group found.28 A study examining the relationship between depression and diabetes found compelling evidence of an association between mental stress and hypothalamic-pituitary-adrenal axis hyperactivity,29and another comparing meditators with controls found the regular practice of TM to be associated with a reduction in catecholamine levels.30 As increased catecholamine levels affect glucose transport and insulin resistance, this finding suggests that reducing stress levels through meditation might lead to improved glycemic control. Transcendental meditation has been found to reduce mean arterial pressure, insulin resistance, and insulin levels.
One RCT comparing diabetes education alone with education plus stress management (progressive muscle relaxation, deep breathing, and mental imagery) found that HbA1c levels decreased by 0.5 in the latter group at one year.31In a single blinded randomized study, the TM group had a statistically significant reduction in mean arterial pressure, insulin resistance, and insulin levels compared with those who received diabetes education alone.32
Qigong. The effectiveness of Qigong systems such as Tai Chi—which integrate physical postures, breathing techniques, and focused attention—is difficult to determine because of methodological challenges in design and variability in practice. Authors of a systematic review of Tai Chi and diabetes found only 2 RCTs and 3 nonrandomized clinical trials and concluded that there was no convincing evidence that the practice aids in glucose control.33 Two other systematic reviews of Qigong for T2DM reported some improvement in glucose control, but limited study quality prevented definite
conclusions.34,35
When to consider "manual medicine"
An integrative approach to health also includes a number of modalities collectively known as manual medicine: acupuncture, massage/energy therapy, acupressure, and chiropractic and other forms of manipulation. Evidence on these modalities for the treatment of diabetes and diabetic complications is limited.
Acupuncture. Although acupuncture has long been reported to improve glycemic control in patients with diabetes and prediabetes, the evidence is limited and of poor quality.36,37
In recent years, 2 small RCTs have found that acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol.38,39 In one of the studies, 87.5% of participants randomized to acupuncture had symptom improvement, compared with 63.6% of those in the oral inositol group. In fact, marked symptom relief after 3 months of treatment was reported by 50% of those Acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol. who had acupuncture, compared with 21% of those who did not.39
In a small 2-week RCT, patients randomized to acupuncture vs sham acupuncture for diabetic bladder dysfunction showed statistically significant improvements in both subjective symptoms and urodynamic measurements.40 And a study comparing patients receiving electroacupuncture—in which an electric current is transmitted between 2 needles placed in the muscles—vs sham acupuncture found nonstatistically significant improvements in symptomatic
gastroparesis.41
Massage/energy therapy. Massage has been shown in several studies to reduce glucose levels,42-44 although no reductions in glucose levels were found in one small RCT.45 Connective tissue reflex massage led to improved lower limb blood flow in patients with diabetes and peripheral artery disease in another study, but the clinical significance is uncertain.46 Studies of reflexology and acupressure are similarly limited to small experimental and observational studies.47 No RCTs of chiropractic treatment for diabetes were found.
CORRESPONDENCE
Jacqueline Redmer, MD, MPH, Department of Family Medicine, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
ACKNOWLEDGEMENTS
The authors thank Drs. Sarina Schrager and Mindy Smith for their manuscript assistance. The work presented here was carried out while Drs. Longmier and Wedel were Primary Care Research Fellows supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine.
1. Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2003;26(suppl 1):S51–S61.
2. Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care. 2004;27:2067–2073.
3. Shantha GP, Kumar AA, Kahan S, et al. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ. 2012;38:417–426.
4. Thomas D, Elliott EJ. Low glycaemic index or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;(1):CD006296.
5. Esposito K, Maiorino MI, Ceriello A, et al. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract. 2010;89:97–102.
6. Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29:1777–1783.
7. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11–S66.
8. Jenkins DJA, Kendall CWC, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–2753.
9. Hadden DR. Goat’s rue-French lilac-Italian fitch-Spanish sainfoin: Gallega officinalis and metformin: the Edinburgh connection. J R Coll Physicians Edinb. 2005;35:258–260.
10. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
11. Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14:884–889.
12. Post RE, Mainous AG 3rd, King DE, et al. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25:16–23.
13. Sievenpiper JL, Kendall CWC, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495.
14. Balk EM, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163.
15. Song Y, He K, Levitan EB, et al. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes. Diabet Med. 2006;23:1050–1056.
16. Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015.
17. Suksomboon N, Poolsup N, Sinprasert S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. J Clin Pharm Ther. 2011;36:53–63.
18. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46.
19. Poh ZX, Goh KP. A current update on the use of alpha lipoic acid in the management of type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009;9:392–398.
20. Ansar H, Mazloom Z, Kazemi F, et al. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32:584–588.
21. Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes. JAMA. 2011;305:1790–1799.
22. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States 2007. Natl Health Stat Rep. 2008;(12):1–23.
23. McGinnis RA, McGrady A, Cox SA, et al. Biofeedback-assisted relaxation in type 2 diabetes. Diabetes Care. 2005;28:2145–2149.
24. Galper DI, Taylor AG, Cox DJ. Current status of mind-body interventions for vascular complications of diabetes. Fam Community Health. 2003;26:34–40.
25. Alexander GK, Taylor AG, Innes KE, et al. Contextualizing the effects of yoga therapy on diabetes management. Fam Community Health. 2008;31:228–239.
26. Innes KE, Vincent HK. The influence of yoga-based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. Evid Based Complement Alternat Med. 2007;4:469–486.
27. Aljasir B, Bryson M, Al-Shehri B. Yoga practice for the management of type II diabetes mellitus in adults: a systematic review. Evid Based Complement Alternat Med. 2010;7:399–408.
28. Infante JR, Torres-Avisbal M, Pinel P, et al. Catecholamine levels in practitioners of the transcendental meditation technique. Physiol Behav. 2001;72:141–146.
29. Dusek JA, Benson H. Mind-body medicine: a model of the comparative clinical impact of the acute stress and relaxation responses. Minn Med. 2009;92:47–50.
30. Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology. 2011;36:1276–1286.
31. Surwit RS, Van Tilburg MAL, Zucker N, et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25:30–34.
32. Paul-Labrador M, Polk D, Dwyer JH, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med. 2006;166:1218–1224.
33. Lee MS, Pittler MH, Kim M-S, et al. Tai chi for type 2 diabetes: a systematic review. Diabet Med. 2008;25:240–241.
34. Chen KW, Liu T, Zhang H, et al. An analytical review of the Chinese literature on Qigong therapy for diabetes mellitus. Am J Chin Med. 2009;37:439–457.
35. Lee MS, Chen KW, Choi T-Y, et al. Qigong for type 2 diabetes care: a systematic review. Complement Ther Med. 2009;17:236–242.
36. Hu H. A review of treatment of diabetes by acupuncture during the past forty years. J Tradit Chin Med. 1995;15:145–154.
37. Liang F, Koya D. Acupuncture: is it effective for treatment of insulin resistance? Diabetes Obes Metab. 2010;12:555–569.
38. Tong Y, Guo H, Han B. Fifteen-day acupuncture treatment relieves diabetic peripheral neuropathy. J Acupunct Meridian Stud. 2010;3:95–103.
39. Zhang C, Ma Y-X, Yan Y. Clinical effects of acupuncture for diabetic peripheral neuropathy. J Tradit Chin Med. 2010;30:13–14.
40. Tong Y, Jia Q, Sun Y, et al. Acupuncture in the treatment of diabetic bladder dysfunction. J Alternat Complement Med. 2009;15:905–909.
41. Wang C-P, Kao C-H, Chen W-K, et al. A single-blinded, randomized pilot study evaluating effects of electroacupuncture in diabetic patients with symptoms suggestive of gastroparesis. J Alternat Complement Med. 2008;14:833–839.
42. Guthrie DW, Gamble M. Energy therapies and diabetes mellitus. Diabetes Spectr. 2001;14:149–153.
43. Ezzo J, Donner T, Nickols D, et al. Is massage useful in the management of diabetes? A systematic review. Diabetes Spectr. 2001;14:218–224.
44. Sajedi F, Kashaninia Z, Hoseinzadeh S, et al. How effective is Swedish massage on blood glucose level in children with diabetes mellitus? Acta Med Iran. 2011;49:592–597.
45. Wändell PE, Carlsson AC, Gåfvels C, et al. Measuring possible effect on health-related quality of life by tactile massage or relaxation in patients with type 2 diabetes. Complement Ther Med. 2012;20:8–15.
46. Castro-Sánchez AM, Moreno-Lorenzo C, Matarán-Peñarrocha GA, et al. Connective tissue reflex massage for type 2 diabetic patients with peripheral arterial disease: randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:1–12.
47. Pilkington K, Stenhouse E, Kirkwood G, et al. Diabetes and complementary therapies: mapping the evidence. Pract Diab Int. 2007;24:371–376.
1. Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2003;26(suppl 1):S51–S61.
2. Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care. 2004;27:2067–2073.
3. Shantha GP, Kumar AA, Kahan S, et al. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ. 2012;38:417–426.
4. Thomas D, Elliott EJ. Low glycaemic index or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;(1):CD006296.
5. Esposito K, Maiorino MI, Ceriello A, et al. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract. 2010;89:97–102.
6. Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29:1777–1783.
7. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11–S66.
8. Jenkins DJA, Kendall CWC, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–2753.
9. Hadden DR. Goat’s rue-French lilac-Italian fitch-Spanish sainfoin: Gallega officinalis and metformin: the Edinburgh connection. J R Coll Physicians Edinb. 2005;35:258–260.
10. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
11. Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14:884–889.
12. Post RE, Mainous AG 3rd, King DE, et al. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25:16–23.
13. Sievenpiper JL, Kendall CWC, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495.
14. Balk EM, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163.
15. Song Y, He K, Levitan EB, et al. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes. Diabet Med. 2006;23:1050–1056.
16. Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015.
17. Suksomboon N, Poolsup N, Sinprasert S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. J Clin Pharm Ther. 2011;36:53–63.
18. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46.
19. Poh ZX, Goh KP. A current update on the use of alpha lipoic acid in the management of type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009;9:392–398.
20. Ansar H, Mazloom Z, Kazemi F, et al. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32:584–588.
21. Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes. JAMA. 2011;305:1790–1799.
22. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States 2007. Natl Health Stat Rep. 2008;(12):1–23.
23. McGinnis RA, McGrady A, Cox SA, et al. Biofeedback-assisted relaxation in type 2 diabetes. Diabetes Care. 2005;28:2145–2149.
24. Galper DI, Taylor AG, Cox DJ. Current status of mind-body interventions for vascular complications of diabetes. Fam Community Health. 2003;26:34–40.
25. Alexander GK, Taylor AG, Innes KE, et al. Contextualizing the effects of yoga therapy on diabetes management. Fam Community Health. 2008;31:228–239.
26. Innes KE, Vincent HK. The influence of yoga-based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. Evid Based Complement Alternat Med. 2007;4:469–486.
27. Aljasir B, Bryson M, Al-Shehri B. Yoga practice for the management of type II diabetes mellitus in adults: a systematic review. Evid Based Complement Alternat Med. 2010;7:399–408.
28. Infante JR, Torres-Avisbal M, Pinel P, et al. Catecholamine levels in practitioners of the transcendental meditation technique. Physiol Behav. 2001;72:141–146.
29. Dusek JA, Benson H. Mind-body medicine: a model of the comparative clinical impact of the acute stress and relaxation responses. Minn Med. 2009;92:47–50.
30. Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology. 2011;36:1276–1286.
31. Surwit RS, Van Tilburg MAL, Zucker N, et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25:30–34.
32. Paul-Labrador M, Polk D, Dwyer JH, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med. 2006;166:1218–1224.
33. Lee MS, Pittler MH, Kim M-S, et al. Tai chi for type 2 diabetes: a systematic review. Diabet Med. 2008;25:240–241.
34. Chen KW, Liu T, Zhang H, et al. An analytical review of the Chinese literature on Qigong therapy for diabetes mellitus. Am J Chin Med. 2009;37:439–457.
35. Lee MS, Chen KW, Choi T-Y, et al. Qigong for type 2 diabetes care: a systematic review. Complement Ther Med. 2009;17:236–242.
36. Hu H. A review of treatment of diabetes by acupuncture during the past forty years. J Tradit Chin Med. 1995;15:145–154.
37. Liang F, Koya D. Acupuncture: is it effective for treatment of insulin resistance? Diabetes Obes Metab. 2010;12:555–569.
38. Tong Y, Guo H, Han B. Fifteen-day acupuncture treatment relieves diabetic peripheral neuropathy. J Acupunct Meridian Stud. 2010;3:95–103.
39. Zhang C, Ma Y-X, Yan Y. Clinical effects of acupuncture for diabetic peripheral neuropathy. J Tradit Chin Med. 2010;30:13–14.
40. Tong Y, Jia Q, Sun Y, et al. Acupuncture in the treatment of diabetic bladder dysfunction. J Alternat Complement Med. 2009;15:905–909.
41. Wang C-P, Kao C-H, Chen W-K, et al. A single-blinded, randomized pilot study evaluating effects of electroacupuncture in diabetic patients with symptoms suggestive of gastroparesis. J Alternat Complement Med. 2008;14:833–839.
42. Guthrie DW, Gamble M. Energy therapies and diabetes mellitus. Diabetes Spectr. 2001;14:149–153.
43. Ezzo J, Donner T, Nickols D, et al. Is massage useful in the management of diabetes? A systematic review. Diabetes Spectr. 2001;14:218–224.
44. Sajedi F, Kashaninia Z, Hoseinzadeh S, et al. How effective is Swedish massage on blood glucose level in children with diabetes mellitus? Acta Med Iran. 2011;49:592–597.
45. Wändell PE, Carlsson AC, Gåfvels C, et al. Measuring possible effect on health-related quality of life by tactile massage or relaxation in patients with type 2 diabetes. Complement Ther Med. 2012;20:8–15.
46. Castro-Sánchez AM, Moreno-Lorenzo C, Matarán-Peñarrocha GA, et al. Connective tissue reflex massage for type 2 diabetic patients with peripheral arterial disease: randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:1–12.
47. Pilkington K, Stenhouse E, Kirkwood G, et al. Diabetes and complementary therapies: mapping the evidence. Pract Diab Int. 2007;24:371–376.